Abstract
The diagnosis and management of chronic nonspinal osteomyelitis can be challenging, and guidelines regarding the appropriateness of performing percutaneous image-guided biopsies to acquire bone samples for microbiological analysis remain limited. An expert panel convened by the Society of Academic Bone Radiologists developed and endorsed consensus statements on the various indications for percutaneous image-guided biopsies to standardize care and eliminate inconsistencies across institutions. The issued statements pertain to several commonly encountered clinical presentations of chronic osteomyelitis and were supported by a literature review. For most patients, MRI can help guide management and effectively rule out osteomyelitis when performed soon after presentation. Additionally, in the appropriate clinical setting, open wounds such as sinus tracts and ulcers, as well as joint fluid aspirates, can be used for microbiological culture to determine the causative microorganism. If MRI findings are positive, surgery is not needed, and alternative sites for microbiological culture are not available, then percutaneous image-guided biopsies can be performed. The expert panel recommends that antibiotics be avoided or discontinued for an optimal period of 2 weeks prior to a biopsy whenever possible. Patients with extensive necrotic decubitus ulcers or other surgical emergencies should not undergo percutaneous image-guided biopsies but rather should be admitted for surgical debridement and intraoperative cultures. Multidisciplinary discussion and approach are crucial to ensure optimal diagnosis and care of patients diagnosed with chronic osteomyelitis.
© RSNA, 2024
Summary
Experts convened by the Society of Academic Bone Radiologists recommend percutaneous image-guided biopsies for managing chronic nonspinal osteomyelitis when MRI findings are positive and surgical or alternative microbiological culture sites are unavailable.
Essentials
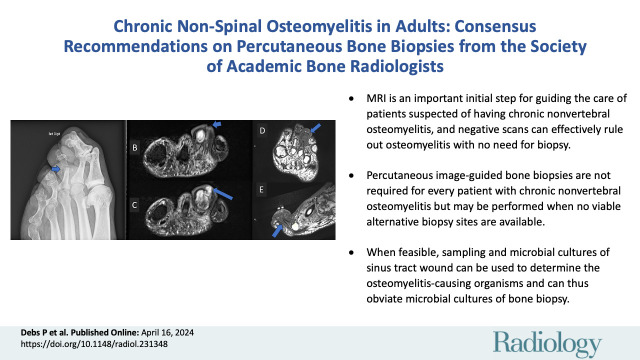
■ MRI is an important initial step for guiding the care of patients suspected of having chronic nonvertebral osteomyelitis, and negative scans can effectively rule out osteomyelitis with no need for biopsy.
■ Percutaneous image-guided bone biopsies are not required for every patient with chronic nonvertebral osteomyelitis but may be performed when no viable alternative biopsy sites are available.
■ When feasible, sampling and microbial cultures of sinus tract wounds can be used to determine the osteomyelitis-causing organisms and can thus obviate the need for microbial cultures of bone biopsy.
Introduction
Osteomyelitis is an acute or chronic inflammatory process of the bone secondary to an infection with microorganisms that can spread through direct inoculation (from chronic wounds or penetrating trauma) or the bloodstream (1). The overall incidence of osteomyelitis is as high as 21.8 cases per 100 000 person-years. These numbers are conceivably increasing with the improved diagnostic test sensitivity and the rising prevalence of common risk factors, such as diabetes (2).
Chronic osteomyelitis can have varying definitions but usually refers to an infection that lasts longer than 4 weeks on average and can result in marked bone destruction, draining sinus tracts, pain, deformity, pathologic fractures, and even limb loss (3). Its chronic nature may manifest as recurrent or intermittent disease, and its symptoms and duration may vary considerably, especially in the presence of extensive soft-tissue ulceration, vascular insufficiency, or comorbidities (diabetes, chronic vascular disease), rendering management challenging and the incidence of relapse high (4,5).
Given the challenging nature of chronic osteomyelitis, a multidisciplinary approach to management involving radiology, infectious disease, and surgery experts is recommended (4). Initial laboratory workup and MRI (the most sensitive test for diagnosing osteomyelitis) (6,7) are often followed by bone biopsies to identify microorganisms and determine appropriate antimicrobial therapy (8). While tissue sampling for suspected vertebral osteomyelitis is recommended, tissue sampling for all types of nonvertebral osteomyelitis has a lower diagnostic yield than sampling performed for vertebral cases, and recommendations for sampling in the workup of chronic osteomyelitis vary across disciplines and between practices and institutions (9–12).
Because of their minimally invasive nature, percutaneous image-guided biopsies are widely preferred over open biopsies for bone sampling (13,14). They are considered a safe technique that considerably reduces the risk of destabilizing an already diseased skeletal segment. Image guidance helps triangulate the needle path to the target area (13,15–17), and whenever possible, multiple samples are obtained to increase the diagnostic yield of the procedure (14).
Presently, recommendations on the appropriateness of performing percutaneous image-guided biopsies for suspected nonvertebral osteomyelitis and its comparability to samples from blood cultures and adjacent wounds are limited. A prior European initiative provided general recommendations for the care of patients suspected of having bone and joint infection without specific recommendations for chronic nonvertebral osteomyelitis (18). Acute osteomyelitis is less challenging to diagnose, given that there are typically abnormal laboratory tests with characteristic local signs and symptoms associated with the infection, and percutaneous sampling is warranted more routinely in the acute setting. In the current initiative, a multidisciplinary group of medical specialists from various disciplines including musculoskeletal radiology, infectious disease, vascular surgery, and orthopedic surgery practicing in the United States was convened to refine the indications for percutaneous image-guided biopsies and their role in managing suspected nonvertebral chronic osteomyelitis. The committee prioritized a patient-centered approach, whereby each statement was believed to have the highest ratio of perceived benefit to potential harm to patients, and elected to present this document as a consensus recommendation. Although these statements are based on current scientific evidence and expert opinions, it is important to recognize that decisions can be influenced by many factors and that this is a dynamic area of ongoing research (19). Consequently, recommendations in this document should be considered in the context of the entire clinical scenario.
Materials and Methods
Expert Multidisciplinary Panel
This consensus statement is based on expert opinions from a panel of radiology, infectious disease, and surgery experts convened by the Society of Academic Bone Radiologists. This multidisciplinary panel included five senior musculoskeletal radiologists (D.B., L.M.F., M.M., R.D.B., and S.E.S., all members of the International Skeletal Society, the Society of Skeletal Radiology, and the Society of Academic Bone Radiologists, with ≥ 20 years of experience in musculoskeletal imaging), one infectious disease specialist (M.B., member of the Musculoskeletal Infection Society, with ≥ 15 years of clinical experience), and one vascular surgeon (V.C., member of the Society for Vascular Surgery, with ≥ 10 years of clinical experience). Panel members were selected due to extensive experience in treating patients with chronic nonvertebral osteomyelitis, often in the setting of multiple comorbidities. The radiologists on the panel had current or prior experience performing at least one to three percutaneous biopsies a week. Surgeons on the panel routinely performed or assisted in open biopsies for numerous indications when a percutaneous biopsy by a radiologist was not an option. The infectious disease specialist routinely worked with radiologists to triage cases for percutaneous biopsy, when necessary.
Development of Key Questions
Members of the panel were independently tasked with defining and listing important clinical challenges and diagnostic dilemmas they frequently encountered when providing care for patients with suspected nonvertebral osteomyelitis, and responses were provided in the form of independent lists. Two panel members (L.M.F., P.D.) reviewed, analyzed, and compared the provided lists for overlap to identify common themes that were subsequently used to develop key questions pertaining to percutaneous image-guided biopsies and the factors affecting yield. An initial list of key questions was sent back to panel members for any additional input, panel feedback was received and reviewed (L.M.F., P.D.), and questions were adjusted accordingly and sent back to the panel for approval. Consequently, four key questions were collectively agreed on and iteratively generated: (a) Are percutaneous image-guided biopsies warranted in patients with chronic nonspinal osteomyelitis? (b) What factors affect culture positivity and yield of percutaneous image-guided biopsies? What are the effects of preprocedural antibiotic administration? (c) Are nonbone cultures comparable to bone cultures? (d) The case of septic joints with osteomyelitis: Is joint fluid aspirate adequate? The entire panel was convened during five sessions using a live audio and video interface (Zoom Video Communications). Each of the first four sessions was dedicated to one of the four questions developed, and a fifth session was held for final discussion and deliberation of recommendations generated from prior sessions. All sessions were transcribed (P.D.) and moderated (L.M.F.) after an open discussion format whereby all panel members were given equal opportunity to contribute to ongoing discussions. Discussions culminated in the moderator proposing an initial recommendation for further deliberation and modification by the members, and sessions concluded only when generated statements achieved major consensus by the panel with at least 90% agreement on the direction of the recommendation. Note that an additional orthopedic surgeon (C.K., member of the American Academy of Orthopaedic Surgeons, with ≥ 15 years of clinical experience) and an infectious disease expert (E.T., with ≥ 5 years of clinical experience) joined the original panel to further substantiate the final statements and recommendations. One final session was held convening the two experts with members of the original panel, and recommendations were assessed, revisited, and finalized accordingly.
Literature Search and Finalization of Recommendations
The final recommendations were agreed to by all panel members and supported by a comprehensive literature search for relevant articles using PubMed/MEDLINE and Scopus databases. Using the search terms [(osteomyelitis OR bone diseases OR *chronic osteomyelitis* OR *peripheral bone infection) AND (biopsy OR fine-needle biopsy OR large-core needle biopsy OR image-guided biopsy OR endoscopic ultrasound-guided fine needle aspiration OR *percutaneous biopsy*)]," a total of 665 unique articles published between 1978 and 2023 were identified in March 2023. One reviewer (P.D., 5 years of medical training), supervised by a musculoskeletal radiologist (L.M.F., > 20 years of experience) screened citations and selected relevant studies. First, titles and abstracts were reviewed for any relevance to the use of percutaneous biopsies in osteomyelitis. Next, relevant articles were read completely and assessed for applicability to the four identified questions. Studies not relevant to the questions developed by the panel, studies exclusively discussing acute osteomyelitis, studies pertaining to periprosthetic infections, studies limited to patients younger than 18 years of age, and studies not available in English or French were excluded. Of the initially identified articles, 61 articles were retained and assessed for risk of bias, inconsistency, indirectness, insufficient precision, and publication bias. A summary of key findings from these articles was written by the reviewer and presented to the panel prior to the final session, during which recommendations were reassessed by considering the quality of available evidence, the balance of benefits to harms, perceived patient and provider values and preferences, feasibility, acceptability, and resource use when applicable. Consequently, recommendations were appraised using The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) binary classification of strength of recommendations (strong or conditional/weak) (20), whereby strong recommendations were deemed to demonstrate potential benefits that outweighed potential risks. A discussion of the literature is included herein to offer support for the expert panel recommendations.
Key Questions and Recommendations
The Table summarizes the major consensus recommendations on the value of performing percutaneous bone biopsies in adult patients with suspected chronic nonspinal osteomyelitis.
Summary of Recommendations
Are Percutaneous Image-guided Biopsies Warranted in Patients with Chronic Nonspinal Osteomyelitis?
Investigations into this topic have uncovered conflicting results. Some studies suggest that bone biopsies should be performed for suspected nonvertebral osteomyelitis, claiming lower costs than other diagnostic procedures and risks lower than those of long-term uninformed treatment (21,22). The perceived benefit of percutaneous image-guided biopsies is the ability to minimize soft-tissue disruption, definitively diagnose osteomyelitis, isolate the pathogen, and tailor the antibiotic therapy to be administered, all of which can theoretically contribute to higher therapeutic success (23,24). However, multiple investigations report a low rate of culture positivity and low sensitivity for isolating the pathogen, suggesting that percutaneous image-guided biopsies have an overall small impact on patient treatment and recovery (25,26). One study of patients with suspected nonvertebral osteomyelitis found that percutaneous image-guided biopsies did not influence the final treatment plan or improve patient recovery (26), while another study reported only 43% sensitivity for isolating the causative pathogen when compared with the reference standard (25). More recently, the overall yield of percutaneous image-guided biopsies in chronic osteomyelitis at a large tertiary care center was reported as 18%, with the lowest yield for being in the foot (14%); furthermore, antibiotic treatment was modified in only 17% of cases following biopsy (27). Several other studies have reported that bone biopsies, even those with positive results, have little impact on the choice of antibiotics, and results are not used to tailor antimicrobial therapy (28–30).
While high rates of positive cultures have been reported in studies performed outside of the United States and in studies in which surgical methods were used to obtain samples, it should not be assumed that these data are relevant to image-guided bone biopsies performed in the United States (31). Furthermore, image-guided bone biopsies are moderately challenging procedures in some patients and may carry a risk of causing new or worsening infections (31). To address these inconsistencies and try to mitigate the rate of unnecessary biopsies, our panel identified three frequently encountered clinical scenarios with suspected nonvertebral osteomyelitis and developed guidelines on how to navigate them optimally. In each of these scenarios, it was assumed that radiographs had already been obtained by the clinician as part of the initial workup, leading to a suspicion of osteomyelitis (32). If further imaging was needed, then MRI was the recommended modality. The optimal MRI protocol for assessing osteomyelitis is not discussed, noting that recent recommendations from the Society of Skeletal Radiology suggest that noncontrast techniques are generally sufficient for diagnosing osteomyelitis (33). Finally, in circumstances where MRI might be unavailable or contraindicated (32), the following guidelines do not apply, and nuclear medicine studies and CT scans may be helpful alternatives.
Chronic osteomyelitis in the setting of a decubitus ulcer.—The expert panel agreed that a wound that is visibly necrotic and extends to the bone should be considered positive for osteomyelitis, and the patient should be referred to a surgeon to assess for surgical debridement and intraoperative cultures. When the extension of the wound into the bone is unclear, MRI should be performed and further steps should be determined based on the result of the scan. A negative MRI result can definitively rule out osteomyelitis and a biopsy is not needed. When the MRI result is positive for osteomyelitis, the need for wound care should then determine further management. Specifically, debridement and intraoperative cultures should only be performed if surgery is deemed necessary. This clinical scenario is summarized in Figure 1.
Figure 1:
Diagram illustrates the first clinical scenario in a patient with a decubitus ulcer and who is suspected of having nonvertebral osteomyelitis.
Chronic osteomyelitis in the diabetic foot—Antibiotic therapy based on the results of bone culture has been shown to be successful in the treatment of diabetic foot infections (34). Additionally, a meta-analysis reported that percutaneous image-guided biopsies may have a high rate of culture positivity (35). Despite these findings, the impact of culture-positive bone samples on treatment has not been demonstrated. Percutaneous image-guided biopsies are not routinely used in diabetic foot care centers, as other diagnostic strategies can be used. When immediate surgery is not required, clinical assessment of infection severity and laboratory measurements of threshold erythrocyte sedimentation rate and C-reactive protein levels can be used to determine the presence of osteomyelitis distinct from soft-tissue infection in the diabetic foot (36,37). In addition, wound samples can be acquired, and cultures of bone samples can be obtained by probing communicating ulcers or after a careful debridement when managing diabetic foot wounds (15). Although there is ongoing debate, some evidence suggests that microorganisms isolated from nonbone specimens, such as sinus tracts and superficial wounds, are generally concordant with those found in bone specimens, obviating the need for percutaneous or open bone biopsies (38–40). One study showed that only 20% of clinicians use percutaneous image-guided biopsies in patients with suspected diabetic foot osteomyelitis, instead opting for an empirical or delayed tailored antibiotic strategy (41).
Recently published guidelines from the International Working Group on the Diabetic Foot advise that bone cultures are not always needed but should be considered (a) when clinical and imaging evaluations result in an uncertain diagnosis, (b) when soft-tissue cultures are inconclusive, (c) when the infection has failed to respond to initial empirical antibiotic therapy, or (d) when considering an antibiotic regimen with a higher potential for selecting resistant organisms (42). Therefore, after radiography and clinical assessment of the wound, the next recommended step for the workup of osteomyelitis in the diabetic foot is MRI, as it offers superior accuracy over radiography, delineates the location of potential fluid collections, and provides necessary anatomic detail for possible surgical planning (7). Negative MRI, defined as the maintenance of intramedullary fat signal intensity and the integrity of cortical signal intensity, effectively rules out osteomyelitis, and a biopsy can be averted. MRI findings revealing a septic joint are to be followed by immediate surgical debridement and intraoperative cultures, as the absence of a protective basement membrane within the joint lining results in bacteremia in around 70% of cases, and the risk of concomitant bacteremia is thus high (43). Otherwise, when MRI findings are positive for osteomyelitis, defined by low signal intensity on T1 images and high signal intensity on fluid-sensitive images (44), the next step in management is dictated by whether an ulcer or sinus tract is associated. When present, wound cultures obtained from the tissues closest to the bone can be performed at the bedside to identify the causative organism. However, wound swab cultures should be avoided due to the possibility of contamination with normal skin microorganisms. When an ulceration or a sinus tract is absent, percutaneous image-guided biopsies may be recommended, and a multidisciplinary discussion is beneficial. This clinical scenario is summarized in Figure 2.
Figure 2:
Diagram illustrates the second clinical scenario in a patient with diabetic foot osteomyelitis.
Chronic osteomyelitis in the setting of a fracture nonunion.—This scenario for a fracture nonunion is similar to the diabetic foot. Following radiography, the next recommended step is MRI. A negative MRI result is used to rule out osteomyelitis, in which case a biopsy is not required. However, imaging revealing a septic joint is to be followed by immediate surgical debridement and intraoperative cultures, as the risk of bacteremia is high. When MRI findings are positive for osteomyelitis, the presence or absence of an ulcer or a sinus tract should determine the next step in management. This clinical scenario is summarized in Figure 3.
Figure 3:
Diagram illustrates the third clinical scenario in a patient with a nonunion fracture and suspected nonvertebral osteomyelitis.
What Factors Affect Culture Positivity and Yield of Percutaneous Image-guided Biopsies? What Are the Effects of Preprocedural Antibiotic Administration?
The evidence on factors that can affect the yield and positivity of bone biopsies in chronic osteomyelitis is very heterogeneous. Intravenous drug use, fever, elevated white blood cell counts, open wounds, and aspiration of fluid at the time of biopsy generally predict a positive biopsy culture, while diabetes does not have an apparent impact on the positivity rate (25,45). However, one retrospective study found that the aspiration of purulent fluid at the time of percutaneous biopsy was associated with a higher rate of positive cultures, whereas fever, elevated white blood cell count, elevated erythrocyte sedimentation rate, elevated C-reactive protein levels, and biopsy needle sizes were not (9). Another retrospective study examining 29 percutaneous image-guided biopsies reported no significant differences in the availability of a fluid aspirate, recent antibiotic use, or elevated white blood cell count between patients who did and those who did not have a positive culture (46).
Whether preprocedural antibiotic administration affects the yield of biopsy cultures and should thus be avoided or discontinued prior to a biopsy is another important clinical query where the evidence is mixed. Several studies have found no significant differences in the frequency of positive and negative cultures after antibiotic administration, thus providing evidence that antibiotic therapy before bone biopsies does not affect the microbiologic yield (9,25,45,47,48). Furthermore, one meta-analysis focusing on the effect of withholding antibiotics prior to bone biopsies in patients with suspected osteomyelitis found no significant differences in bacterial pathogen yield based on antibiotic exposure (49). However, several of these studies do not include data regarding the types of antibiotics administered, the dosage given, or the duration of treatment (9,25,45,48–50). Other studies suggest that the highest diagnostic yield is obtained by performing the biopsy before antibiotic therapy is initiated or after a washout period (23,51). In light of the current conflicting evidence, the lack of robust clinical data, and past experiences, our expert panel recommends deferring antibiotic administration until after the biopsy is completed or discontinuing antibiotics prior to a biopsy for an optimal period of 2 weeks, as this will likely improve the culture yield. The 2-week duration has been decided on by prior and current expert opinion (52,53). As isolated chronic osteomyelitis is not life threatening, suspending antibiotics for 2 weeks is unlikely to pose additional risks to patients or hinder their recovery.
Are Nonbone Cultures Comparable to Bone Cultures?
Because bone biopsy is an invasive tool, several studies have examined the diagnostic value of nonbone cultures obtained noninvasively from superficial wounds, sinus tracts, and deep tissues. Evidence on the comparability of nonbone wound cultures to bone cultures varies depending on factors such as the type of wound examined, its proximity to the bone in question, and the organisms isolated from the specimen. One prospective study of sinus tract cultures compared with intraoperative bone specimens revealed a sensitivity of 50.9%, specificity of 20%, predictive value of 47.5%, and overall concordance of 41.4% (54). Another prospective study of 100 adult patients with chronic osteomyelitis found that nonbone and bone specimen cultures were microbiologically concordant in only 30% of patients, with a slightly higher concordance for infections caused by Staphylococcus aureus (47). Several studies have found better accuracy rates with nonbone versus bone cultures when Staphylococcus aureus is the causative microorganism, even in patients with diabetic foot osteomyelitis (23,24,55,56). A presumptive diagnosis of Staphylococcus aureus osteomyelitis could potentially be justified when the pathogen is isolated from an associated sinus tract (10,34,57–59). Other studies report a poor correlation between wound and bone isolates (60,61). Specifically, one retrospective study comparing sinus tract cultures to bone cultures reported a divergent antibiotic susceptibility pattern even when the same species of microorganism was isolated from both specimens, suggesting that different anatomic regions may be colonized by different microbial strains, though patients with diabetic foot osteomyelitis or decubitus ulcers were excluded, rendering results less generalizable (62). Moreover, no information was provided on whether different antibiotic susceptibility patterns between bone and nonbone cultures had a clinical impact on antibiotic regimens or patient outcomes. In our clinical experience, diverging susceptibility patterns rarely influence the choice of antibiotics.
There are several studies supporting the reliability of nonbone specimens, particularly when the area biopsied is close to the infected bone. A meta-analysis evaluating concordance rates between bone and nonbone specimens found a high positivity rate for sinus tract cultures, concluding that sinus tract cultures have an accuracy comparable to that of bone biopsies (63). In diabetic foot osteomyelitis, deep wound cultures have been reported to correlate well with osseous cultures (64). Another prospective study found that sinus tract cultures in chronic osteomyelitis had a 96% specificity and 90% predictive value compared with operative specimens obtained directly from the infected bone and concluded that sinus tracts are a reliable source for the isolation of all bacteria causing chronic bone infection except Staphylococcus epidermidis (38), a bacterium that is generally considered by infectious disease specialists to cause only hardware-associated infections (65–67).
Nonbone cultures seem to be more reliable when performed twice and when the infection is monomicrobial. For example, taking two consecutive diabetic toe cultures with bone contact has been shown to accurately predict the causative pathogen of diabetic toe osteomyelitis in 90% of patients (68). Furthermore, a prospective study of long bone osteomyelitis found that the accuracy of sinus tract cultures caused by a single organism is higher than that of infections caused by more than one pathogen (39). Finally, a large prospective nonrandomized trial compared two consecutive sinus tract cultures with bone contact taken at different times to surgical bone biopsies and found high concordance between the sinus tract cultures and a high diagnostic accuracy rate when osteomyelitis was monomicrobial (69).
Another sample that could be used for culturing is blood. Indeed, positive blood cultures have been associated with a higher rate of positive biopsy samples in patients with diabetic foot osteomyelitis. However, this is generally seen more in the axial skeleton than in the appendicular skeleton (8,12,25,70). Our expert panel agrees that chronic osteomyelitis alone is not a strong risk factor for bacteremia. Therefore, it is unlikely to routinely lead to positive blood cultures. A negative blood culture cannot rule out the possibility of chronic osteomyelitis. Positive blood cultures encountered in the setting of osteomyelitis are generally indicative of very critical cases where timely intervention is necessary. In such instances, the treatment is immediately initiated without the need for a bone sample.
In light of this mixed evidence and based on experience, our expert panel finds nonbone cultures from the wound to be a practical alternative to bone cultures, especially when bone biopsies cannot be performed, are not available, or pose a grave risk to the patient. Even though wound cultures can be contaminated with skin microorganisms and generally result in a polymicrobial culture (53,71), infectious disease specialists are capable of identifying the causative microorganism in most patients with osteomyelitis, especially if specimens obtained at different times show concordance in culture growth. The recommendation is not for nonbone cultures to completely replace bone biopsies but rather for nonbone cultures to be considered a viable step that can precede and, when successful, obviate the need for a bone biopsy. Figures 4 and 5 present two cases of chronic osteomyelitis where wound sinus tract cultures influenced management.
Figure 4:
Images in a 52-year-old male cancer patient who presented with ulceration of the toe. (A) Oblique radiograph shows destructive osseous changes around the third proximal interphalangeal joint (arrow). (B) Coronal short tau inversion recovery (STIR) image shows superficial ulceration (arrow) leading to osteomyelitis. (C) Coronal STIR image shows small fluid collection (arrow) is also present in the subcutaneous tissues at the infection site. (D) Axial and (E) sagittal T1-images show septic arthritis at the proximal interphalangeal joint with adjacent phalangeal osteomyelitis of the third toe (arrows). MRI was positive for osteomyelitis, so wound culture was performed. The culture was positive for Staphylococcus aureus, and antibiotic treatment with cefazolin was then started. The patient subsequently underwent toe amputation 4 days later. Notably, although bone pathology was positive for osteomyelitis, intraoperative bone culture was negative. This case highlights the effectiveness of wound culture in the workup of chronic osteomyelitis to determine the microbial pathogen. A percutaneous biopsy (given the positive MRI results) would have added no additional information. In addition, the lack of culture positivity from the intraoperative bone sample suggests that preceding antibiotic treatment possibly affected the culture yield.
Figure 5:
Images in a 50-year-old male patient with diabetes and ulceration of the great toe. (A) Axial and (B) sagittal T1-weighted MRI scans show a plantar ulcer contiguous with adjacent first proximal phalanx osteomyelitis (arrows). (C) Coronal T1 and (D) short tau inversion recovery (STIR) images show plantar ulceration and sinus tract (arrows) extending to osteomyelitis. MRI was positive for osteomyelitis, so a wound culture was performed. The culture grew Pseudomonas aeruginosa, and the patient was referred to surgery. Interestingly, an intraoperative bone culture during toe amputation grew methicillin-resistant Staphylococcus aureus. The infectious disease team considered both pathogens important and decided on dual coverage: linezolid to cover methicillin-resistant Staphylococcus aureus and ciprofloxacin to cover Pseudomonas aeruginosa. In this case, it is conceivable that a percutaneous biopsy prior to amputation would have yielded the second pathogen. However, the ultimate treatment (debridement and amputation) would not have changed in this case based on results from the percutaneous biopsy. In patients for whom surgery is not an option after wound culture, multidisciplinary discussion is important to decide on the need for percutaneous bone biopsy, as the infectious disease team may elect to empirically treat for a polymicrobial infection, obtain a second sinus tract sample, or potentially obtain a bone biopsy sample to confirm whether the infection is polymicrobial or monomicrobial.
The Case of Septic Joints with Osteomyelitis: Is Joint Fluid Aspirate Adequate?
Although septic arthritis is a well-known complication of acute osteomyelitis, some cases of septic arthritis caused by chronic osteomyelitis have been described in the literature (72,73). As such, our panel elected to address this question, and recommendations can be followed for all patients with septic joints related to both acute and chronic osteomyelitis.
Evidence on whether a bone biopsy is needed in a patient with septic arthritis is lacking. One retrospective study found that percutaneous CT-guided sacroiliac joint fluid aspiration had a higher sensitivity for detecting suspected infection than a biopsy, and biopsies did not add any additional microbial information (74). Furthermore, a report on one patient with a rare case of Clostridium paraputrificum osteomyelitis of the shoulder and septic arthritis found that the causative agent was detected in both the synovial fluid and the bone culture (75).
Our expert panel agreed that joint aspiration is usually adequate in cases of osteomyelitis with septic arthritis and devised a clinical scenario with a supporting algorithm to help clinicians navigate such situations (Fig 6). When septic arthritis is detected at imaging, the next step depends on the acuity of the case. Patients with surgical emergencies will proceed directly to the operating room, where an intraoperative bone culture is recommended, while nonemergency cases are referred for joint aspiration. A synovial fluid white blood cell count of 50 000 cells/mm3 (50 × 109/L) or higher is suggestive of septic arthritis, but that number is not conclusive and can be influenced by different factors. Alternatively, synovial fluid culture is a definitive test that has been found to demonstrate growth in approximately 80% of all patients with nongonococcal septic arthritis (43). If the joint aspirate yields a positive culture, a biopsy is not usually needed, and providers can proceed with medical treatment or surgery. If, on the other hand, the aspirate is negative, then a percutaneous image-guided biopsy for culture should be considered.
Figure 6:
Diagram illustrates the fourth clinical scenario in a patient with septic arthritis at MRI and suspected osteomyelitis. Indications for emergency surgical debridement vary and are best determined by clinicians at the time of presentation.
Conclusion
The consensus statements are intended to improve and standardize the care and management of patients with suspected chronic osteomyelitis by helping providers determine when to perform a percutaneous image-guided biopsy. These statements represent the collective opinions and perspectives of experts in radiology, infectious diseases, vascular surgery, and orthopedic surgery, who collaborate to care for this patient population. Imaging with radiography and MRI, when feasible, is the first step in the workup of patients with suspected chronic osteomyelitis, except in the setting of a necrotic and extensive decubitus ulcer, for which surgical debridement and intraoperative cultures are recommended. Negative MRI results can rule out osteomyelitis, and positive MRI results help determine how to proceed with management. Cultures from sinus tracts may offer a noninvasive alternative to percutaneous image-guided bone biopsies. If a biopsy is required, then it is recommended that antibiotics be avoided or discontinued for an optimal period of 2 weeks. In the case of septic arthritis, joint aspirations are usually adequate unless negative, in which case a percutaneous image-guided biopsy may be necessary. Finally, it is crucial to note that research was limited to some topics. Thus, some statements were generated using suboptimal evidence and should be considered in the context of the entire clinical scenario. Additionally, adherence to the recommendations presented will not always ensure an accurate diagnosis or a successful outcome for every patient, and providers should follow a reasonable course of action based on the most up-to-date evidence, available resources, and the responsibility to deliver safe and effective medical care. The panel strongly encourages additional high-quality research that further sheds light on the best practices for treating patients with suspected chronic osteomyelitis.
Footnotes
L.M.F. supported by the National Institutes of Health (grants R61 R61AT012279 and U01AR080993-01A), National Therapeutic Acceleration Program, and John Staurulakis Endowed Scholar Award.
Disclosures of conflicts of interest: P.D. No relevant relationships. R.D.B. President-elect, Society of Academic Bone Radiologists. S.E.S. No relevant relationships. M.B. No relevant relationships. D.B. Royalties from Elsevier for a role as an author/editor; payment or honoraria from the American College of Radiology for a speaking role; President, Society of Skeletal Radiology. V.C. No relevant relationships. M.M. No relevant relationships. E.T. No relevant relationships. C.K. Grants from Arthrex, Stryker, Enovis, Zimmer Biomet, and Alafair; royalties or licenses from Arthrex and restor3d; consulting fees from Arthrex and restor3d; payment or honoraria from Arthrex and restor3d; U.S. patent no. 11,737,798; stock options in Arthrex and restor3d;. L.M.F. Johns Hopkins University Discovery Award and Johns Hopkins University Sarcoma Program R61; honorarium for the 30th annual CT/MRI course in the Netherlands, September 2023; support for attending meetings from John Hopkins University; past and present leadership roles in International Skeletal Society, Society of Skeletal Radiology, Stereotactic Ablative Body Radiation Therapy, International Society for Magnetic Resonance in Medicine, and American Roentgen Ray Society.
References
- 1. Lew DP , Waldvogel FA . Osteomyelitis . Lancet 2004. ; 364 ( 9431 ): 369 – 379 . [DOI] [PubMed] [Google Scholar]
- 2. Kremers HM , Nwojo ME , Ransom JE , Wood-Wentz CM , Melton LJ 3rd , Huddleston PM 3rd . Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009 . J Bone Joint Surg Am 2015. ; 97 ( 10 ): 837 – 845 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Masala IF , Marino F , Sarzi-Puttini P , Atzeni F . Chapter 8 - Bone and joint bacterial infections in patients with rheumatoid arthritis . In: Atzeni F , Galloway JB , Gomez-Reino JJ , Galli M , eds. Handbook of Systemic Autoimmune Diseases . Elsevier; , 2020. ; 167 – 177 . [Google Scholar]
- 4. Panteli M , Giannoudis PV . Chronic osteomyelitis: what the surgeon needs to know . EFORT Open Rev 2017. ; 1 ( 5 ): 128 – 135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newman LG , Waller J , Palestro CJ , et al . Unsuspected osteomyelitis in diabetic foot ulcers. Diagnosis and monitoring by leukocyte scanning with indium in 111 oxyquinoline . JAMA 1991. ; 266 ( 9 ): 1246 – 1251 . [DOI] [PubMed] [Google Scholar]
- 6. Expert Panel on Musculoskeletal Imaging ; Beaman FD , von Herrmann PF , et al . ACR Appropriateness Criteria® Suspected Osteomyelitis, Septic Arthritis, or Soft Tissue Infection (Excluding Spine and Diabetic Foot) . J Am Coll Radiol 2017. ; 14 ( 5S ): S326 – S337 . [DOI] [PubMed] [Google Scholar]
- 7. Llewellyn A , Kraft J , Holton C , Harden M , Simmonds M . Imaging for detection of osteomyelitis in people with diabetic foot ulcers: A systematic review and meta-analysis . Eur J Radiol 2020. ; 131 : 109215 . [DOI] [PubMed] [Google Scholar]
- 8. Hatzenbuehler J , Pulling TJ . Diagnosis and management of osteomyelitis . Am Fam Physician 2011. ; 84 ( 9 ): 1027 – 1033 . [PubMed] [Google Scholar]
- 9. Wu JS , Gorbachova T , Morrison WB , Haims AH . Imaging-guided bone biopsy for osteomyelitis: are there factors associated with positive or negative cultures? AJR Am J Roentgenol 2007. ; 188 ( 6 ): 1529 – 1534 . [DOI] [PubMed] [Google Scholar]
- 10. Senneville E , Morant H , Descamps D , et al . Needle puncture and transcutaneous bone biopsy cultures are inconsistent in patients with diabetes and suspected osteomyelitis of the foot . Clin Infect Dis 2009. ; 48 ( 7 ): 888 – 893 . [DOI] [PubMed] [Google Scholar]
- 11. White LM , Schweitzer ME , Deely DM , Gannon F . Study of osteomyelitis: utility of combined histologic and microbiologic evaluation of percutaneous biopsy samples . Radiology 1995. ; 197 ( 3 ): 840 – 842 . [DOI] [PubMed] [Google Scholar]
- 12. Berbari EF , Kanj SS , Kowalski TJ , et al . 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults . Clin Infect Dis 2015. ; 61 ( 6 ): e26 – e46 . [DOI] [PubMed] [Google Scholar]
- 13. Filippiadis DK , Charalampopoulos G , Mazioti A , Keramida K , Kelekis A . Bone and Soft-Tissue Biopsies: What You Need to Know . Semin Intervent Radiol 2018. ; 35 ( 4 ): 215 – 220 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Neil JT . What Is the Optimal Method to Perform Bone Biopsy (Method, Location, Imaging Use) for Patients With Foot and Ankle Infections? Foot Ankle Int 2019. ; 40 ( 1_suppl ): 38S – 39S . [DOI] [PubMed] [Google Scholar]
- 15. Couturier A , Chabaud A , Desbiez F , et al . Comparison of microbiological results obtained from per-wound bone biopsies versus transcutaneous bone biopsies in diabetic foot osteomyelitis: a prospective cohort study . Eur J Clin Microbiol Infect Dis 2019. ; 38 ( 7 ): 1287 – 1291 . [DOI] [PubMed] [Google Scholar]
- 16. Gogna A , Peh WCG , Munk PL . Image-guided musculoskeletal biopsy . Radiol Clin North Am 2008. ; 46 ( 3 ): 455 – 473, v . [DOI] [PubMed] [Google Scholar]
- 17. Veltri A , Bargellini I , Giorgi L , Almeida PAMS , Akhan O . CIRSE Guidelines on Percutaneous Needle Biopsy (PNB) . Cardiovasc Intervent Radiol 2017. ; 40 ( 10 ): 1501 – 1513 . [DOI] [PubMed] [Google Scholar]
- 18. Glaudemans AWJM , Jutte PC , Cataldo MA , et al . Consensus document for the diagnosis of peripheral bone infection in adults: a joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement) . Eur J Nucl Med Mol Imaging 2019. ; 46 ( 4 ): 957 – 970 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gramberg MCTT , Lagrand RS , Sabelis LWE , et al . Using a BonE BiOPsy (BeBoP) to determine the causative agent in persons with diabetes and foot osteomyelitis: study protocol for a multicentre, randomised controlled trial . Trials 2021. ; 22 ( 1 ): 517 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ 2008;336(7652):1049–1051. [Published correction appears in BMJ 2008;336(7658): doi: 10.1136/bmj.a402.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crisologo PA , Killeen A . Bone Biopsy in Suspected Nonvertebral Osteomyelitis: Point-Let Bone Be the Guide When Treating Nonvertebral Osteomyelitis . AJR Am J Roentgenol 2022. ; 219 ( 2 ): 195 – 196 . [DOI] [PubMed] [Google Scholar]
- 22. Ertugrul MB , Baktiroglu S , Salman S , et al . The diagnosis of osteomyelitis of the foot in diabetes: microbiological examination vs. magnetic resonance imaging and labelled leucocyte scanning . Diabet Med 2006. ; 23 ( 6 ): 649 – 653 . [DOI] [PubMed] [Google Scholar]
- 23. Mandell JC , Khurana B , Smith JT , Czuczman GJ , Ghazikhanian V , Smith SE . Osteomyelitis of the lower extremity: pathophysiology, imaging, and classification, with an emphasis on diabetic foot infection . Emerg Radiol 2018. ; 25 ( 2 ): 175 – 188 . [DOI] [PubMed] [Google Scholar]
- 24. Heidari N , Kwok I , Vris A , Charalambous A . Should Treatment of Diabetic Foot Osteomyelitis Be Based on Bone Biopsies? Foot Ankle Int 2019. ; 40 ( 1_suppl ): 73S – 74S . [DOI] [PubMed] [Google Scholar]
- 25. Schirò S , Foreman SC , Bucknor M , Chin CT , Joseph GB , Link TM . Diagnostic Performance of CT-Guided Bone Biopsies in Patients with Suspected Osteomyelitis of the Appendicular and Axial Skeleton with a Focus on Clinical and Technical Factors Associated with Positive Microbiology Culture Results . J Vasc Interv Radiol 2020. ; 31 ( 3 ): 464 – 472 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoang D , Fisher S , Oz OK , La Fontaine J , Chhabra A . Percutaneous CT guided bone biopsy for suspected osteomyelitis: Diagnostic yield and impact on patient’s treatment change and recovery . Eur J Radiol 2019. ; 114 : 85 – 91 . [DOI] [PubMed] [Google Scholar]
- 27. Fritz J , Dyer E , Wang J , Abou-Areda M , Ly B , Fayad LM . CT-Guided Bone Biopsy in Chronic Non-spinal Osteomyelitis: Fruitful or Futile? Skeletal Radiol 2019. ; 48 : 479 – 498 . 30569251 [Google Scholar]
- 28. Mammarappallil JG , Seyler TM , Lenchik L , Wuertzer SD , Plate JF . Impact of Fluoroscopically Guided Bone Biopsy on Antibiotic Management of Osteomyelitis in the Lower Extremity . J Surg Orthop Adv 2018. ; 27 ( 4 ): 277 – 280 . [PubMed] [Google Scholar]
- 29. Said N , Chalian M , Fox MG , Nacey NC . Percutaneous image-guided bone biopsy of osteomyelitis in the foot and pelvis has a low impact on guiding antibiotics management: a retrospective analysis of 60 bone biopsies . Skeletal Radiol 2019. ; 48 ( 9 ): 1385 – 1391 . [DOI] [PubMed] [Google Scholar]
- 30. Hirschfeld CB , Kapadia SN , Bryan J , et al . Impact of diagnostic bone biopsies on the management of non-vertebral osteomyelitis: A retrospective cohort study . Medicine (Baltimore) 2019. ; 98 ( 34 ): e16954 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perry MT , Nacey NC . Bone Biopsy in Suspected Nonvertebral Osteomyelitis: Counterpoint-Limited Yield and Clinical Utility of Image-Guided Percutaneous Needle Biopsy . AJR Am J Roentgenol 2022. ; 219 ( 2 ): 197 – 198 . [DOI] [PubMed] [Google Scholar]
- 32. Lee YJ , Sadigh S , Mankad K , Kapse N , Rajeswaran G . The imaging of osteomyelitis . Quant Imaging Med Surg 2016. ; 6 ( 2 ): 184 – 198 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samet JD , Alizai H , Chalian M , et al . Society of skeletal radiology position paper - recommendations for contrast use in musculoskeletal MRI: when is non-contrast imaging enough? Skeletal Radiol 2024. ; 53 ( 1 ): 99 – 115 . [DOI] [PubMed] [Google Scholar]
- 34. Senneville E , Lombart A , Beltrand E , et al . Outcome of diabetic foot osteomyelitis treated nonsurgically: a retrospective cohort study . Diabetes Care 2008. ; 31 ( 4 ): 637 – 642 . [DOI] [PubMed] [Google Scholar]
- 35. Schechter MC , Ali MK , Risk BB , et al . Percutaneous Bone Biopsy for Diabetic Foot Osteomyelitis: A Systematic Review and Meta-Analysis . Open Forum Infect Dis 2020. ; 7 ( 10 ): ofaa393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haghverdian JC , Noori N , Hsu AR . Clinical Pathway for the Management of Diabetic Foot Infections in the Emergency Department . Foot Ankle Orthop 2023. ; 8 ( 1 ): 24730114221148166 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lavery LA , Ahn J , Ryan EC , et al . What are the Optimal Cutoff Values for ESR and CRP to Diagnose Osteomyelitis in Patients with Diabetes-related Foot Infections? Clin Orthop Relat Res 2019. ; 477 ( 7 ): 1594 – 1602 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mousa HA . Evaluation of sinus-track cultures in chronic bone infection . J Bone Joint Surg Br 1997. ; 79 ( 4 ): 567 – 569 . [DOI] [PubMed] [Google Scholar]
- 39. Patzakis MJ , Wilkins J , Kumar J , Holtom P , Greenbaum B , Ressler R . Comparison of the results of bacterial cultures from multiple sites in chronic osteomyelitis of long bones. A prospective study . J Bone Joint Surg Am 1994. ; 76 ( 5 ): 664 – 666 . [DOI] [PubMed] [Google Scholar]
- 40. Perry CR , Pearson RL , Miller GA . Accuracy of cultures of material from swabbing of the superficial aspect of the wound and needle biopsy in the preoperative assessment of osteomyelitis . J Bone Joint Surg Am 1991. ; 73 ( 5 ): 745 – 749 . [PubMed] [Google Scholar]
- 41. Richard JL , Lavigne JP , Got I , et al . Management of patients hospitalized for diabetic foot infection: results of the French OPIDIA study . Diabetes Metab 2011. ; 37 ( 3 ): 208 – 215 . [DOI] [PubMed] [Google Scholar]
- 42. Lipsky BA , Senneville É , Abbas ZG , et al . Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update) . Diabetes Metab Res Rev 2020. ; 36 ( Suppl 1 ): e3280 . [DOI] [PubMed] [Google Scholar]
- 43. Long B , Koyfman A , Gottlieb M . Evaluation and Management of Septic Arthritis and its Mimics in the Emergency Department . West J Emerg Med 2019. ; 20 ( 2 ): 331 – 341 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alaia EF , Chhabra A , Simpfendorfer CS , et al . MRI nomenclature for musculoskeletal infection . Skeletal Radiol 2021. ; 50 ( 12 ): 2319 – 2347 . [Published correction appears in Skeletal Radiol 2022;51(5):1103-1104.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee HT , Sebro R . Predictors of positive bone cultures from CT-guided bone biopsies performed for suspected osteomyelitis . J Med Imaging Radiat Oncol 2020. ; 64 ( 3 ): 313 – 318 . [DOI] [PubMed] [Google Scholar]
- 46. Beroukhim G , Shah R , Bucknor MD . Factors Predicting Positive Culture in CT-Guided Bone Biopsy Performed for Suspected Osteomyelitis . AJR Am J Roentgenol 2019. ; 212 ( 3 ): 620 – 624 . [DOI] [PubMed] [Google Scholar]
- 47. Zuluaga AF , Galvis W , Saldarriaga JG , Agudelo M , Salazar BE , Vesga O . Etiologic diagnosis of chronic osteomyelitis: a prospective study . Arch Intern Med 2006. ; 166 ( 1 ): 95 – 100 . [DOI] [PubMed] [Google Scholar]
- 48. Aragón-Sánchez FJ , Cabrera-Galván JJ , Quintana-Marrero Y , et al . Outcomes of surgical treatment of diabetic foot osteomyelitis: a series of 185 patients with histopathological confirmation of bone involvement . Diabetologia 2008. ; 51 ( 11 ): 1962 – 1970 . [DOI] [PubMed] [Google Scholar]
- 49. Crisologo PA , La Fontaine J , Wukich DK , Kim PJ , Oz OK , Lavery LA . The Effect of Withholding Antibiotics Prior to Bone Biopsy in Patients With Suspected Osteomyelitis: A Meta-analysis of the Literature . Wounds 2019. ; 31 ( 8 ): 205 – 212 . [PubMed] [Google Scholar]
- 50. Chang CY , Pelzl C , Jesse MK , Habibollahi S , Habib U , Gyftopoulos S . Image-Guided Biopsy in Acute Diskitis-Osteomyelitis: A Systematic Review and Meta-Analysis . AJR Am J Roentgenol 2023. ; 220 ( 4 ): 499 – 511 . [DOI] [PubMed] [Google Scholar]
- 51. Lavigne JP , Sotto A . Microbial management of diabetic foot osteomyelitis . Future Microbiol 2017. ; 12 ( 14 ): 1243 – 1246 . [DOI] [PubMed] [Google Scholar]
- 52. Lipsky BA , Berendt AR , Cornia PB , et al . 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections . Clin Infect Dis 2012. ; 54 ( 12 ): e132 – e173 . [DOI] [PubMed] [Google Scholar]
- 53. Vemu L , Sudhaharan S , Mamidi N , Chavali P . Need for appropriate specimen for microbiology diagnosis of chronic osteomyelitis . J Lab Physicians 2018. ; 10 ( 1 ): 21 – 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Akinyoola AL , Adegbehingbe OO , Aboderin AO . Therapeutic decision in chronic osteomyelitis: sinus track culture versus intraoperative bone culture . Arch Orthop Trauma Surg 2009. ; 129 ( 4 ): 449 – 453 . [DOI] [PubMed] [Google Scholar]
- 55. Senneville E , Melliez H , Beltrand E , et al . Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: concordance with ulcer swab cultures . Clin Infect Dis 2006. ; 42 ( 1 ): 57 – 62 . [DOI] [PubMed] [Google Scholar]
- 56. Peters EJ . Pitfalls in diagnosing diabetic foot infections . Diabetes Metab Res Rev 2016. ; 32 ( Suppl 1 ): 254 – 260 . [DOI] [PubMed] [Google Scholar]
- 57. Elamurugan TP , Jagdish S , Kate V , Chandra Parija S . Role of bone biopsy specimen culture in the management of diabetic foot osteomyelitis . Int J Surg 2011. ; 9 ( 3 ): 214 – 216 . [DOI] [PubMed] [Google Scholar]
- 58. Mackowiak PA , Jones SR , Smith JW . Diagnostic value of sinus-tract cultures in chronic osteomyelitis . JAMA 1978. ; 239 ( 26 ): 2772 – 2775 . [DOI] [PubMed] [Google Scholar]
- 59. Senneville EM , Lipsky BA , van Asten SAV , Peters EJ . Diagnosing diabetic foot osteomyelitis . Diabetes Metab Res Rev 2020. ; 36 ( Suppl 1 ): e3250 . [DOI] [PubMed] [Google Scholar]
- 60. Khatri G , Wagner DK , Sohnle PG . Effect of bone biopsy in guiding antimicrobial therapy for osteomyelitis complicating open wounds . Am J Med Sci 2001. ; 321 ( 6 ): 367 – 371 . [DOI] [PubMed] [Google Scholar]
- 61. Chakraborti C , Le C , Yanofsky A . Sensitivity of superficial cultures in lower extremity wounds . J Hosp Med 2010. ; 5 ( 7 ): 415 – 420 . [DOI] [PubMed] [Google Scholar]
- 62. Ulug M , Ayaz C , Celen MK , Geyik MF , Hosoglu S , Necmioglu S . Are sinus-track cultures reliable for identifying the causative agent in chronic osteomyelitis? Arch Orthop Trauma Surg 2009. ; 129 ( 11 ): 1565 – 1570 . [DOI] [PubMed] [Google Scholar]
- 63. Tawfik GM , Dibas M , Dung NM , et al . Concordance of bone and non-bone specimens in microbiological diagnosis of osteomyelitis: A systematic review and meta-analysis . J Infect Public Health 2020. ; 13 ( 11 ): 1682 – 1693 . [DOI] [PubMed] [Google Scholar]
- 64. Malone M , Bowling FL , Gannass A , Jude EB , Boulton AJM . Deep wound cultures correlate well with bone biopsy culture in diabetic foot osteomyelitis . Diabetes Metab Res Rev 2013. ; 29 ( 7 ): 546 – 550 . [DOI] [PubMed] [Google Scholar]
- 65. Kavanagh N , Ryan EJ , Widaa A , et al . Staphylococcal Osteomyelitis: Disease Progression, Treatment Challenges, and Future Directions . Clin Microbiol Rev 2018. ; 31 ( 2 ): e00084 – 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Valour F , Trouillet-Assant S , Rasigade JP , et al . Staphylococcus epidermidis in orthopedic device infections: the role of bacterial internalization in human osteoblasts and biofilm formation . PLoS One 2013. ; 8 ( 6 ): e67240 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Otto M . Staphylococcus epidermidis--the ‘accidental’ pathogen . Nat Rev Microbiol 2009. ; 7 ( 8 ): 555 – 567 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bernard L , Assal M , Garzoni C , Uçkay I . Predicting the pathogen of diabetic toe osteomyelitis by two consecutive ulcer cultures with bone contact . Eur J Clin Microbiol Infect Dis 2011. ; 30 ( 2 ): 279 – 281 . [DOI] [PubMed] [Google Scholar]
- 69. Bernard L , Uçkay I , Vuagnat A , et al . Two consecutive deep sinus tract cultures predict the pathogen of osteomyelitis . Int J Infect Dis 2010. ; 14 ( 5 ): e390 – e393 . [DOI] [PubMed] [Google Scholar]
- 70. Letertre-Gibert P , Desbiez F , Vidal M , et al . Blood cultures after bone biopsy in diabetic foot osteomyelitis . Diagn Microbiol Infect Dis 2017. ; 89 ( 1 ): 78 – 79 . [DOI] [PubMed] [Google Scholar]
- 71. Ertugrul MB , Baktiroglu S , Salman S , et al . Pathogens isolated from deep soft tissue and bone in patients with diabetic foot infections . J Am Podiatr Med Assoc 2008. ; 98 ( 4 ): 290 – 295 . [DOI] [PubMed] [Google Scholar]
- 72. Marsh JL , Watson PA , Crouch CA . Septic arthritis caused by chronic osteomyelitis . Iowa Orthop J 1997. ; 17 : 90 – 95 . [PMC free article] [PubMed] [Google Scholar]
- 73. Le Goff P , Fauchier C , Pennec Y , Schwarzberg C , Le Roy JP , Amouroux J . Subacute monoarthritis of the knee disclosing chronic hematogenous osteomyelitis of the adult. Apropos of 2 cases [in French] . Rev Rhum Mal Osteoartic 1981. ; 48 ( 2 ): 121 – 126 . [PubMed] [Google Scholar]
- 74. Knipp D , Simeone FJ , Nelson SB , Huang AJ , Chang CY . Percutaneous CT-guided sacroiliac joint sampling for infection: aspiration, biopsy, and technique . Skeletal Radiol 2018. ; 47 ( 4 ): 473 – 482 . [DOI] [PubMed] [Google Scholar]
- 75. Vijayvargiya P , Garrigos ZE , Rodino KG , Razonable RR , Abu Saleh OM . Clostridium paraputrificum septic arthritis and osteomyelitis of shoulder: A case report and review of literature . Anaerobe 2020. ; 62 : 102105 . [DOI] [PubMed] [Google Scholar]