Abstract
Cryptogenic stroke refers to a stroke of undetermined etiology. It accounts for approximately one-fifth of ischemic strokes and has a higher prevalence in younger patients. Embolic stroke of undetermined source (ESUS) refers to a subgroup of patients with nonlacunar cryptogenic strokes in whom embolism is the suspected stroke mechanism. Under the classifications of cryptogenic stroke or ESUS, there is wide heterogeneity in possible stroke mechanisms. In the absence of a confirmed stroke etiology, there is no established treatment for secondary prevention of stroke in patients experiencing cryptogenic stroke or ESUS, despite several clinical trials, leaving physicians with a clinical dilemma. Both conventional and advanced MRI techniques are available in clinical practice to identify differentiating features and stroke patterns and to determine or infer the underlying etiologic cause, such as atherosclerotic plaques and cardiogenic or paradoxical embolism due to occult pelvic venous thrombi. The aim of this review is to highlight the diagnostic utility of various MRI techniques in patients with cryptogenic stroke or ESUS. Future trends in technological advancement for promoting the adoption of MRI in such a special clinical application are also discussed.
© RSNA, 2024
Summary
MRI is a valuable modality for the etiologic work up in patients with cryptogenic stroke and embolic stroke of undetermined source.
Essentials
■ The diagnosis of cryptogenic stroke or embolic stroke of undetermined source is common in patients with ischemic stroke after routine work up but is insufficient for secondary prevention.
■ Both conventional and advanced MRI techniques are widely used in various organs and the circulatory system to determine or infer stroke etiology.
■ Technological development aiming to provide more information with improved image quality and shorter scan times could expand and strengthen the role of MRI in the diagnosis and management of cryptogenic stroke.
Introduction
The terminology of cryptogenic stroke or stroke of undetermined origin was established in 1993 using the Trial of Org 10172 in Acute Stroke Treatment classification system (1) and refers to an ischemic stroke that cannot be conclusively attributed to large artery atherosclerosis (LAA), small vessel disease, cardioembolism, or other etiologies. With the recognition of patent foramen ovale (PFO)–associated stroke, cryptogenic stroke now accounts for an estimated 21% of all ischemic strokes (2).
The concept of embolic stroke of undetermined source (ESUS) was proposed in 2014 to refer to a subgroup of patients with nonlacunar cryptogenic stroke and without proximal arterial stenosis or confirmed cardioembolic sources (3). ESUS accounts for approximately 17% of all ischemic strokes (4).
Failure to determine the cause of a stroke can result in prolonged diagnostic evaluation and increased morbidity. Additionally, it can create a clinical dilemma as strokes without a confirmed etiology lack established therapeutic options. Two randomized multicenter clinical trials, NAVIGATE ESUS (ClinicalTrials.gov registration no. NCT02313909 [5]) and RE-SPECT ESUS (ClinicalTrials.gov registration no. NCT02239120 [6]), were conducted to investigate the use of oral anticoagulant therapy for stroke prevention after ESUS, but both concluded that anticoagulant medication was not superior to aspirin in preventing recurrent stroke. Given the lack of therapeutic options, determining the etiology of stroke via imaging is critical.
When routine work up fails to identify the stroke mechanism, advanced diagnostic imaging is often pursued (7). MRI is frequently used to help identify stroke etiologies because it can assess multiple organ systems (Fig 1). The purpose of this article is to review different MRI techniques and their value in diagnosing cryptogenic stroke or ESUS.
Figure 1:
Illustration shows MRI applications in different areas of the body for determining the etiology of stroke. ASL = arterial spin labeling, CMR = cardiac MRI, DSC = dynamic susceptibility contrast, DWI = diffusion-weighted imaging, FLAIR = fluid-attenuated inversion recovery, MRA = MR angiography, MRV = MR venography, SWI = susceptibility-weighted imaging, T2WI = T2-weighted imaging, T2*WI = T2*-weighted imaging, VWI = vessel wall imaging. Illustration was created using BioRender.
Brain Structural Imaging
T2-weighted imaging, diffusion-weighted imaging (DWI), and fluid-attenuated inversion recovery are usually part of the standard brain MRI work up for stroke to determine the presence, burden, and spatial distribution of ischemic lesions. Ischemic lesions are commonly captured with DWI during the hyperacute phase of stroke (≤24 hours), showing increased signal intensity and decreased apparent diffusion coefficient values (8). In the acute phase (≤1 week), both DWI and T2-weighted imaging show hyperintensity, whereas T1-weighted imaging shows hypointensity. One week after stroke occurrence, apparent diffusion coefficient values begin to normalize and show increasing hyperintensity throughout the subacute and chronic phases. The signal intensity remains high on T2-weighted images and low on T1-weighted images (Fig 2).
Figure 2:
Brain MRI scans show example findings at different stages of ischemic stroke, with arrows indicating ischemic lesions on various contrast-weighted images. ADC = apparent diffusion coefficient, DWI = diffusion-weighted imaging, FLAIR = fluid-attenuated inversion recovery, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging.
A single acute lacunar ischemic lesion is usually caused by small vessel disease, whereas multiple acute lesions in one vascular territory suggests embolism from a large artery (9). If more than one vascular territory is involved, a cardiac or systemic origin should be considered (Fig 3). The ischemic lesion pattern of cryptogenic stroke and ESUS can belong to any of these categories. As shown in a substudy of the NAVIGATE ESUS trial in 855 patients with visible brain infarcts, 24% had a single infarct, 29% had multiple infarcts in one territory, and 47% had infarcts in multiple territories (10).
Figure 3:
Example diffusion-weighted imaging (DWI) and MR angiography (MRA) in patients with stroke. (A) Images in a 54-year-old male patient diagnosed with cryptogenic stroke show multiple bilateral cortical-subcortical and periventricular infarcts on the DWI scan (arrows, left), while MRA (right) and transesophageal echocardiography were unrevealing. (B) Images in a 44-year-old male patient with stroke attributed to large artery atherosclerosis show multiple unilateral small subcortical infarcts on the DWI scan (arrows, left) and severe focal stenosis of the ipsilateral middle cerebral artery on the MR angiogram (arrowhead, right). (C) Images in an 82-year-old male patient with stroke attributed to a cardioembolic source show large territorial infarcts on the DWI scan (arrows, left) and occlusion of the left posterior cerebral artery on the MR angiogram (arrowhead, right).
Ischemic lesion patterns on MRI scans may provide clues about stroke etiology. A study in 832 patients with acute middle cerebral artery territory infarcts demonstrated that lesion patterns on DWI scans in patients with cryptogenic stroke (n = 115) were different from those of patients with cardioembolic stroke (n = 133) or small arterial occlusion (n = 182), but did not differ from patients with LAA stroke (n = 402) (11). Deep nonlacunar infarcts and superficial perforator infarcts were found to be independently associated with cryptogenic stroke. The findings suggest that LAA is a potential source of cryptogenic stroke, including artery-to-artery embolism from nonstenotic plaques. However, a definitive etiologic diagnosis for each patient cannot be established.
The relationship between arrhythmias and ischemic lesion patterns has also been explored. Vollmuth et al (12) enrolled patients with cryptogenic stroke who recently received implantable cardiac monitors for atrial fibrillation detection. Over a 1-year surveillance period, occult atrial fibrillation was detected in 20 of 95 patients (21%), whereas 75 patients remained in sinus rhythm. No difference in the infarction size, vascular distribution, or number of affected territories on DWI, T2-weighted, and fluid-attenuated inversion recovery images were observed between these two groups and a reference group of patients with cardioembolic stroke. In a study by Gürdoğan et al (13), patients with cryptogenic stroke were divided into two groups based on the involvement of single or multiple vascular territories on DWI scans. The total premature atrial contraction count and short atrial run count, which are associated with atrial fibrillation, were found to be independently associated with infarcts in multiple vascular territories, suggesting a possible cardioembolic etiology.
DWI and fluid-attenuated inversion recovery are useful for stroke follow-up and prognostication. A 3-year study followed 272 patients with cryptogenic stroke and found that patients with multiple lesions in multiple vascular territories were at a higher risk for recurrent stroke than patients with single or scattered lesions in one vascular territory (14). Multiple infarcts on DWI scans were independently associated with recurrent stroke and all-cause mortality. A study using fluid-attenuated inversion recovery in 235 patients with cryptogenic stroke found that severe white matter hyperintensity independently predicted poor outcomes at 3 months and was associated with long-term mortality in patients younger than 65 years (15). The association of severe white matter hyperintensity with recurrent stroke and new-onset atrial fibrillation has also been reported in patients with ESUS (16).
Brain Perfusion Imaging
Perfusion imaging can play an important role in the diagnosis and monitoring of the extent and configuration of ischemic lesions and provide prognostic information through assessment of collateral blood supply. Dynamic susceptibility contrast MRI is a commonly used contrast-enhanced perfusion technique (17). Based on patterns observed on DWI scans and time-to-peak maps generated from dynamic susceptibility contrast MRI, Restrepo et al (18) divided patients with acute brain ischemia into three groups as follows: (a) mismatch (perfusion imaging defect volume ≥25% of DWI defect volume), (b) reverse mismatch (DWI defect volume >25% of perfusion imaging defect volume), and (c) matched (<25% difference between the two volumes). The mismatch pattern was associated with LAA, whereas the reverse mismatch pattern was associated with cryptogenic stroke, indicating that perfusion information adds value to the diagnostic work up.
Other perfusion techniques, including arterial spin labeling and intravoxel incoherent motion imaging, have been used as noncontrast approaches to assess ischemic stroke (19,20). Future study is warranted to investigate their roles in cryptogenic stroke.
Intracranial Vessel Imaging
MR Angiography
Time-of-flight (typically a three-dimensional [3D] flow-compensated gradient-echo sequence) or contrast-enhanced (typically 3D T1-weighted spoiled gradient-echo sequence) MR angiography (MRA) is a well-established technique to evaluate luminal stenosis (21). A vessel lumen reduction greater than 50% is commonly used to define a lesion at high risk for causing stroke (1). However, less than or equal to 50% stenosis at intracranial MRA is a common finding in patients with cryptogenic stroke (22).
The utility of MRA beyond stenosis detection has also been investigated. Arterial tortuosity, which could increase in underrecognized causes of stroke such as genetic connective tissue disorders and arterial dissection (23), can be measured with MRA (as the ratio of the actual vessel length to the shortest linear distance between two points). In a pediatric study, the arterial tortuosity quantified in patients with cryptogenic stroke was similar to that in patients with stroke with nontraumatic intracranial dissection (24). When compared with the control group, patients with cryptogenic stroke showed increased arterial tortuosity at the distal extracranial vertebral arteries, basilar artery, and intracranial internal carotid artery, and a decrease in the intracranial vertebral arteries. Similar studies have not been performed in adult patients.
Vessel Wall Imaging
Vessel wall imaging (VWI) is a high-spatial-resolution blood-suppressed MRI technique for directly assessing arterial walls and identifying vessel wall lesions regardless of the degree of luminal stenosis (Fig 4) (25,26). The most commonly used VWI sequence is 3D turbo spin echo with a long series of variable flip angle refocusing pulses (27). (For technical details, readers are referred to reference 28.) Using this method, Tao et al (22) found that patients with ESUS had a higher prevalence of intracranial atherosclerotic plaque on the ipsilateral side of stroke than on the contralateral side, which was not seen in patients with small vessel disease. A larger normalized wall index ([vessel area − luminal area/vessel area at the maximal luminal narrowing site] × 100%), remodeling index (vessel area at the maximal luminal narrowing site divided by the reference vessel area), positive remodeling (a remodeling index >1.05), discontinuity of plaque surface, and complicated plaque (presence of plaque surface irregularity and/or intraplaque hemorrhage) were more frequently found ipsilateral to the stroke. Moreover, the remodeling index was independently associated with ESUS. In another study evaluating the same group of patients, atrial cardiopathy was more prevalent in patients with ESUS than in those with small vessel disease, especially in patients without ipsilateral nonstenotic complicated plaque (29). These studies suggest that VWI helps narrow down the possible etiologies of ESUS.
Figure 4:
MRI in a 58-year-old female patient initially diagnosed with cryptogenic stroke. (A) Diffusion-weighted image shows multiple unilateral infarcts in the distribution of the left middle cerebral artery. (B) MR angiogram shows a failure to identify stenosis. (C) Precontrast vessel wall images (parallel and perpendicular views) show a nonstenotic plaque (arrowhead) in the left middle cerebral artery, with focal eccentric wall thickening and positive remodeling (corresponding to the arrow in B). (D) Postcontrast vessel wall images show the plaque with strong enhancement (arrowhead). The etiology was subsequently reclassified as intracranial large artery atherosclerosis.
VWI has shown promise for determining stroke etiology, especially when vessel wall pathologies have not yet caused stenosis detectable with conventional imaging (30,31). In a retrospective study in 205 consecutive patients with ischemic stroke or transient ischemic attack (32), using VWI at 3 T decreased the number of patients classified as having “intracranial arteriopathy not otherwise specified” from 64 to nine and increased the number of patients classified as having “intracranial atherosclerotic disease” from 48 to 116. Furthermore, VWI helped reduce the number of patients classified as having “etiology undetermined due to two or more potential causes” from nine to two and those classified as having “small vessel disease” from seven to one. In another retrospective study, use of VWI resulted in 20 patients having the diagnosis changed from cryptogenic stroke to intracranial vasculitis, with concentric contrast enhancement of arterial walls and involvement of multiple intracranial arteries seen in all these patients (33). In addition to the 3-T VWI studies, a 7-T VWI study in 34 patients initially diagnosed with cryptogenic stroke reported the stroke etiology as intracranial atherosclerotic disease in 25 patients and arterial dissection in three patients (34). Compared with 3 T, 7 T yields a higher signal to noise ratio, tradable for higher spatial resolution or improved quality of vessel wall delineation at the same spatial resolution (35). However, due to its limited availability in clinical practice, more investigations of its added value in assessing cryptogenic stroke are warranted.
Thrombus Imaging
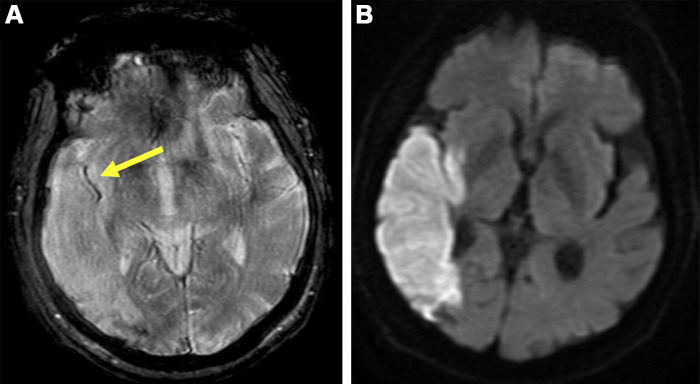
Information regarding the composition of occlusive thrombus can help identify its source. T2*-weighted imaging delineates deoxyhemoglobin, commonly seen in thromboembolism (36). The “susceptibility vessel sign” on T2*-weighted images, appearing as a dark blooming artifact (Fig 5), indicates “red” thrombi rich in erythrocytes. Fibrin-rich, platelet-rich, and erythrocyte-poor “white” thrombi lack this sign. In a study in 37 patients undergoing T2*-weighted imaging followed by thrombectomy and histopathologic analysis, red clots producing a susceptibility vessel sign occurred more often with cardioembolic stroke, and white clots invisible on MRI scans occurred more often with LAA (37). Alhazmi et al (38) found that patients with LAA stroke had a longer susceptibility vessel sign (18.3 mm) than patients with ESUS (11.3 mm) or cardioembolic stroke (11.0 mm). A 14.55-mm sign length showed a sensitivity of 79.7% and specificity of 72.7% for differentiating cardioembolic stroke and LAA stroke. However, the length did not differ between the ESUS and cardioembolic groups, suggesting potential thrombus source overlap in patients classified as having ESUS and cardioembolic stroke.
Figure 5:
Brain MRI in a 71-year-old male patient diagnosed with cryptogenic stroke. (A) T2*-weighted image shows the susceptibility vessel sign (arrow) in the M2 segment of the right middle cerebral artery. (B) Diffusion-weighted image shows the corresponding ischemic area.
Similar to T2*-weighted imaging, susceptibility-weighted imaging, which is a fully velocity-compensated high-spatial-resolution 3D gradient-echo sequence, is reliable in visualizing thrombus (39). A study in 84 patients with middle cerebral artery occlusions, including 31 patients with cryptogenic stroke, showed that occlusion was detected equivalently with susceptibility-weighted imaging, MRA, and digital subtraction angiography (40). However, susceptibility-weighted imaging was found to be superior at locating the distal end of the thrombus.
Cervical Carotid Imaging
Carotid atherosclerosis, leading to nonstenotic vulnerable lesions, has important clinical implications for stroke (41). The American Heart Association Committee on Vascular Lesions suggested an international histopathologic classification system to differentiate carotid atherosclerotic plaques (42). Among them, type VI lesions are characterized by intraplaque hemorrhage, large lipid-rich necrotic core, or ruptured fibrous cap, and are associated with a high risk of stroke (Fig 6); therefore, many imaging studies have been dedicated to identifying type VI plaque and corresponding high-risk features.
Figure 6:
MR images of carotid plaque show the complicated characteristics of American Heart Association (AHA) type VI plaques. The presence of at least one of the following criteria defines plaques as type VI: intraplaque hemorrhage (red arrowheads, left), ruptured fibrous cap (yellow arrowheads, middle), or mural thrombus indicating juxtaluminal hemorrhage (white arrowheads, right). CE = contrast-enhanced, TOF = time of flight, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging. (Adapted, with permission, from reference 48.)
MR Angiography
Several studies investigated whether MRA can provide arteriopathy-related information in addition to the stenosis degree. Gupta et al (43) enrolled 27 patients with cryptogenic stroke and unilateral anterior circulation infarction without significant stenosis (≤50%) of the cervical carotid artery. Time-of-flight MRA was used to assess bilateral carotid arteries for the presence of intraplaque high signal intensity, an indicator of intraplaque hemorrhage. The authors identified six patients (22%) with intraplaque high signal intensity on the ipsilateral side of the stroke and none on the contralateral side, and no difference in the degree of stenosis was observed between the two sides. In a second study, patients with cryptogenic stroke showed a higher proportion of ipsilateral intraplaque high signal intensity (ipsilateral vs contralateral, 11 of 50 vs 0 of 50) compared with patients with cardioembolic (seven of 37 vs six of 37) or small vessel disease (four of 22 vs three of 22) stroke (44). These findings support the use of MRA in detecting intraplaque hemorrhage in low-grade stenotic plaques responsible for cryptogenic strokes.
Vessel Wall Imaging
Carotid plaque morphologic features and composition can be assessed using VWI, showing good agreement with histologic findings (45). Two studies applied VWI to patients with ESUS and unilateral anterior circulation infarction and both found that intraplaque hemorrhage in carotid plaque was more common on the ipsilateral than on the contralateral side of stroke (46,47), consistent with the findings of Gupta et al (43). Furthermore, patients with intraplaque hemorrhage showed a higher rate of recurrent stroke (16.7%) than those without intraplaque hemorrhage (2.4%) (47). Three other studies in patients with cryptogenic stroke and unilateral anterior circulation infarction found a higher prevalence of type VI plaques on the ipsilateral versus contralateral side of stroke when using VWI and time-of-flight MRA (48–50). Furthermore, patients with cryptogenic stroke showed a higher prevalence of ipsilateral type VI plaques compared with patients with cardioembolic or small vessel disease stroke, and lower prevalence compared with patients with LAA (48). These results suggest that in patients diagnosed with cryptogenic stroke or ESUS, VWI identifies complicated and nonstenotic carotid atherosclerotic plaques potentially responsible for the ischemic events.
Cardiovascular Imaging
Cardiac Imaging
Transthoracic and transesophageal echocardiography are commonly used to identify cardioembolic sources of stroke and, particularly, transesophageal echocardiography serves as the standard of reference for diagnosing left atrial thrombi and PFO. Cardiac MRI (CMR) has emerged as a noninvasive complementary approach with excellent spatial and tissue resolution (51). In a study that included 20 patients with cryptogenic stroke who underwent both echocardiography and CMR, six patients with PFO and one patient with aortic plaque greater than or equal to 4 mm thick were identified with echocardiography, while only one patient with PFO was identified with CMR (52). In another study in 64 patients with cryptogenic stroke, CMR altered the diagnosis for five patients due to the presence of PFO and focal cardiac infarct (n = 1), mitral valve thickening (n = 1), or hypertensive cardiomyopathy (n = 3) (53).
Conventional CMR techniques have shown equivalent or improved diagnostic accuracy in detecting intracardiac thrombi compared with echocardiography (54,55). In a prospective study in patients with acute ischemic stroke and a history of myocardial infarction or a left ventricular ejection fraction less than 50%, contrast-enhanced CMR helped detect left ventricular thrombus in 12 of 60 patients, whereas transthoracic echocardiography enabled detection in only one patient (56). In 14 patients who were originally diagnosed as having ESUS, four had left ventricular thrombus identified at CMR. The application of CMR in addition to transthoracic echocardiography resulted in a 29% relative reduction in the diagnosis of ESUS and a 14% relative increase in the diagnosis of cardioembolic stroke (Fig 7). Neri et al (57) reported similar stroke etiologic reclassifications, finding potential sources of cardioembolism in 17 of 66 patients with ESUS by using CMR. Further studies are needed to verify which patient populations would benefit from the addition of CMR examination.
Figure 7:
Example contrast-enhanced cardiac MRI (CMR) and conventional transthoracic echocardiography for detecting left ventricular (LV) thrombi in patients diagnosed with embolic stroke of undetermined source. (A, B) Images in an 85-year-old male patient show a large apical thrombus (arrows) on both the CMR scan (A) and the transthoracic echocardiogram (B). (C, D) Images in an 82-year-old male patient show a small apical thrombus on the CMR scan (arrow in C) but not on the transthoracic echocardiogram (D) because the apical view image was diminished by an artifact. (E, F) Images in a 74-year-old male patient show a mural thrombus of the posterior wall on the CMR scan (arrows in E) but not on the transthoracic echocardiogram (F). Ao = ascending aorta, LA = left atrium. (Reprinted, with permission, from reference 56.)
Underlying atrial fibrosis has been proposed as an alternative stroke etiology, independent of atrial fibrillation (58), and late-enhanced CMR is the reference standard in assessing myocardial fibrosis. A study investigating left atrial fibrosis using CMR reported that patients with other specific causes of stroke had the lowest percentage of atrial fibrosis, whereas no difference was found between patients with cryptogenic stroke and cardioembolic stroke secondary to atrial fibrillation (59). Two recent studies also found that patients with ESUS had more atrial fibrosis on CMR scans than healthy controls and comparable fibrosis to those with atrial fibrillation (60,61), suggesting that CMR allows effective quantification of atrial fibrosis, one of the etiologies of cardioembolic stroke. Studies of the value of anticoagulant therapy for secondary prevention in this specific patient subgroup may be warranted.
Insight into cardiac blood flow using four-dimensional flow MRI can also assist in etiologic diagnosis by analyzing the atrial flow pattern. Vortical flow is considered the “typical” right atrial flow pattern (62). A small-scale study found that nonvortical flow patterns were more common in cryptogenic stroke with PFO, implying an enhanced risk of paradoxical embolism due to nonvortical flow (63).
Aortic Imaging
Transesophageal echocardiography is the current standard for detecting high-risk aortic sources of stroke. Its diagnostic value is compromised by its invasiveness, air artifacts, and limited coverage. Alternatively, cardiac CT angiography can identify embolic risk sources noninvasively but is limited by iodinated contrast agent usage and radiation exposure (64). To overcome these restrictions, MRI was proposed as a new method for assessing aortic disease as a potential stroke etiology. Harloff et al (65) found that two dissections and six high-risk plaques were identified with aortic MRI in eight of 26 patients with cryptogenic stroke who had a prior nondiagnostic echocardiogram. In a separate study, Harloff et al (66) found that MRI had better image quality and helped detect additional complex plaques (≥4 mm thick, ulcerated, or containing mobile thrombi) in the ascending aorta and aortic arch in 19 of 58 patients with cryptogenic stroke following a complete routine diagnostic work up that included transesophageal echocardiography.
Although high-risk aortic pathologies can be detected using MRI, it is uncertain whether lesions in the descending aorta can be a potential cause of stroke. In a prospective study in 63 patients with cryptogenic stroke who were examined with transesophageal echocardiography and multidirectional 3D velocity mapping to assess the extent of retrograde flow, complex descending aortic plaques were found in 33 of 63 patients. Of these 33 patients, 20 had retrograde flow connecting the complex plaque to the left subclavian artery, and four had infarctions in the corresponding region that could not be explained by other sources. These findings were confirmed by Wehrum et al (67), whereby multicontrast plaque imaging and four-dimensional flow MRI were used in 40 patients diagnosed with cryptogenic stroke after routine work up that included transesophageal echocardiography; six patients with type VI plaques with potential retrograde embolization were identified. Markl et al (68) further evaluated the phenomenon, considering differences in cerebrovascular and aortic arch anatomy. Of the 35 patients diagnosed with cryptogenic stroke after a comprehensive work up, retrograde embolization with matching cerebral infarct territory was detected in six patients. In a recent study, aortic wall stiffness measured using 3D dark-blood T1-weighted MRI and retrograde flow measured using four-dimensional flow MRI were higher in patients with cryptogenic stroke than in younger healthy controls, and maximum wall thickness was higher in patients with cryptogenic stroke (mean, 3.1 mm ± 0.7 [SD]) than in younger (mean, 2.2 mm ± 0.2) and age-matched (mean, 2.7 mm ± 0.5) controls (69). The authors also found a positive correlation between measures of aortic wall stiffness and both wall thickness and retrograde flow (Fig 8). These studies provide evidence that descending aortic complex plaque can still be considered a potential stroke mechanism if the retrograde flow is detected at flow MRI.
Figure 8:
(A) Three-dimensional aortic wall image in an 86-year-old male patient with cryptogenic stroke shows the region of atherosclerotic plaque (arrow) in the proximal descending aorta (DAo). (B) Images show four-dimensional flow MRI for assessing aortic flow reversal. The direction of forward and reverse flow is determined for each voxel. Then forward flow, reverse flow, and the flow reversal fraction (FRF) are determined for each voxel during the cardiac cycle and quantified as mean parametric maps. Retrograde flow provides an explanation for how plaque in the descending aorta causes ischemic stroke. Color bars in the forward flow and reverse flow panels indicate the mean flow velocity in each voxel (milliliters per cardiac cycle). The color bar in the flow reversal fraction panel indicates the level of the flow reversal fraction. AAo = ascending aorta. (Adapted, with permission, from reference 69.)
Four-dimensional flow MRI also has potential for monitoring treatment response. In a study using four-dimensional flow MRI to assess 16 patients with cryptogenic stroke during a 6-month follow-up, Soulat et al (70) found decreases in aortic wall stiffness and retrograde flow in patients treated with renin-angiotensin system inhibitors, whereas no changes were observed in the untreated group.
Pulmonary Vessel Imaging
The pulmonary vein can be evaluated for potential sources of emboli (71). An early study employed multibolus, multiphase 3D contrast-enhanced MRA to visualize the pulmonary veins in 18 patients with acute cryptogenic stroke (72). The image quality was good in 14 patients, with poorer visibility of the veins in the left lower lobe compared with other segments due to cardiac motion artifacts and superimposition of the left ventricle. Pulmonary venous thrombosis was not detected in any patients. Given the small sample size, more studies are needed to determine the diagnostic value of pulmonary vessel imaging for thrombus detection.
Pelvic Imaging
Deep venous thrombosis (DVT) could contribute to emboli in the presence of right-to-left shunts, such as PFO, and its identification can indicate patients for treatment options such as PFO closure and anticoagulation (2). DVT is traditionally thought to occur in the large veins of the legs and is commonly diagnosed using Doppler US. However, US evaluation of the pelvic veins is challenging due to body habitus, interference by pelvic bones, or overlapping bowel gas, in which case CT venography is a first-line diagnostic test (73). Considering the radiation exposure and use of iodinated contrast agents in CT, several studies have investigated the prevalence of pelvic DVT using MR venography. Samuel et al (74) recruited 293 patients with suspected ESUS within 48 hours of admission who were screened with pelvic MR venography for DVT in the presence of PFO. The detection of pelvic thrombus in 13 patients led to a diagnosis of paradoxical embolism at the time of discharge. Cramer et al (75) reported that in 30 patients with cryptogenic stroke and right-to-left shunts who underwent MR venography, pelvic DVT was detected in five patients. In a second study, the investigators observed a higher probability of pelvic DVT on MR venographic images in patients with cryptogenic stroke (nine of 46 [20%]) than patients with other determined stroke etiologies (two of 49 [4%]) (76). Furthermore, in a subgroup of patients with PFO or atrial septal defect, the prevalence of pelvic DVT remained higher in patients with cryptogenic stroke versus stroke with determined etiologies (six of 28 vs 0 of nine). In contrast, Liberman et al (77) reported a lower prevalence of pelvic DVT in patients with cryptogenic stroke (two of 98 [2.1%]) with no difference compared with patients with determined etiologies of stroke (0 of 33 [0%]). In that study, contrast-enhanced MR venography was applied to almost all patients, whereas Cramer et al used noncontrast time-of-flight imaging (75,76), which may have contributed to the different reported prevalence of pelvic DVT.
MR venography can also be used to identify May-Thurner syndrome (Fig 9), an anatomic variant characterized by the compression of the left common iliac vein by the right common iliac artery and clinical association with cryptogenic stroke by inducing DVT formation (78). Of 470 consecutive patients with cryptogenic stroke and no evidence of lower extremity DVT who underwent PFO closure, 30 patients were reported to have features consistent with May-Thurner syndrome on MR venographic images (79). A similar study identified four patients with pelvic DVT and five with May-Thurner syndrome among 50 patients with PFO who underwent contrast-enhanced pelvic MR venography for evaluation of cryptogenic stroke (80). In a study that included 214 patients with cryptogenic stroke and 50 controls who underwent abdominopelvic CT, May-Thurner syndrome was present in five in the control group and 66 patients with cryptogenic stroke, with an overall higher degree of left common iliac vein compression (81). These results emphasize the importance of screening the pelvic veins as a potential source of emboli, particularly in the presence of right-to-left shunt and no identifiable cause of stroke.
Figure 9:
(A) Illustration of May-Thurner syndrome, which is clinically associated with cryptogenic stroke, shows the left iliac vein compressed by the right iliac artery. (B) Two-dimensional time-of-flight MR angiographic images in a 30-year-old female patient diagnosed with cryptogenic stroke show narrowing of the left iliac vein caused by the overlying right iliac artery, consistent with May-Thurner syndrome.
Cancer
Both known and occult malignancies are associated with hypercoagulability and clinically relevant thrombosis (82). Cancer is known to increase the risk of ischemic stroke and is considered during cryptogenic stroke and ESUS work up (83). Specific stroke patterns seen on MRI scans might suggest cancer as a contributing factor. In two recent studies of patients with cryptogenic stroke but without a diagnosis of cancer at the time of stroke, infarcts involving multiple vascular territories on DWI scans independently predicted occult cancer (84,85). Achiha et al (86) studied 43 patients with advanced cancer who developed acute ischemic stroke. They found patients with cryptogenic stroke showed accumulations of hyperintense lesions on DWI scans at vascular border zone areas without involving the perforating arterial territories, and their median survival time (96 days) was shorter than that of patients with determined stroke etiologies (570 days).
Imaging Evaluation Considerations
Standard evaluation of ischemic stroke, which includes brain MRI and head and neck MRA, is usually performed within 1 week of symptom onset or during hospitalization. The necessity, selection, and timing of additional advanced evaluation, which includes brain perfusion imaging, head and neck VWI, thrombus imaging, cardiac and great vessels MRI, and pelvic MR venography, depend on the clinical scenario and availability of institutional resources (Table).
Summary of Different MRI Techniques in the Imaging Evaluation of Cryptogenic Stroke or Embolic Stroke of Undetermined Source
The role of infection in ischemic stroke gained attention during the COVID-19 pandemic (87). Currently, it remains controversial whether stroke occurs more often in patients with COVID-19. Meanwhile, other etiologies, such as LAA, small vessel disease, and cardioembolism, are still common in this patient population. Therefore, the imaging evaluation for stroke etiology will be similar to that of patients without COVID-19.
Future Directions
The role of MRI in identifying causes of cryptogenic stroke and ESUS will be expanded and strengthened by evolving technologies. VWI is one area that has attracted numerous research efforts in technological development and clinical validation. Characterization of vulnerable plaques will be improved by novel VWI sequences focused on achieving sufficient coverage and spatial resolution with a clinically reasonable acquisition time, better suppression of signal from surrounding tissue and blood, and multiple contrast weightings (precontrast T1-weighted, postcontrast T1-weighted, T2-weighted) to identify different components (88–92). Compressed sensing and deep learning are being investigated for imaging acceleration (93–96). Motion-compensated techniques are also useful for preserving image quality (97,98).
Assessing the cardiopulmonary system using CMR techniques is another rapidly emerging field. Efforts have been made to develop protocols with shorter scan times, multiple contrasts, and easier setup processes. Free-running, fully self-gating, 3D whole-heart cine phase-contrast MRI, known as five-dimensional flow MRI, can capture cardiovascular hemodynamics in less than 8 minutes (99). Multicontrast multiphase CMR sequences have achieved high-spatial-resolution images in less than 10 minutes (100–102). Ultrashort echo time MRI (103) has achieved comparable results to CT in detecting pulmonary arteriovenous malformations, sources of right-to-left shunts, and paradoxical embolic stroke (104). The diagnostic value of these newly developed techniques must be clinically validated.
The feasibility and reproducibility of combining different MRI techniques, such as brain MRI, multistation VWI, and CMR, to achieve a one-stop, comprehensive, and noninvasive diagnostic imaging examination are worth investigating, particularly in large patient groups with cryptogenic stroke and ESUS. Further optimization of imaging protocols by including a minimum number of sequences for effectively achieving diagnosis will be an important area of research to promote clinical adoption.
Conclusion
The inability to identify the cause of ischemic stroke using standard diagnostic tests continues to challenge medical providers and impedes optimal patient care. Identifying stroke etiology is critical to reducing the risk of recurrent stroke. Emerging MRI techniques with enhanced image quality and faster acquisition could assist in determining stroke etiology, guiding treatment strategy, and predicting response to therapy.
S.S.S. and Z.F. supported by the National Institutes of Health and National Heart, Lung, and Blood Institute (grant R01HL147355).
Disclosures of conflicts of interest: J.X. No relevant relationships. R.A.P. Grant from the Southern California Clinical and Translational Science Institute (UL1TR001855). A.L. No relevant relationships. P.L.N. No relevant relationships. J.W.S. Recipient of the American Heart Association Career Development Award (938082); associate editor for Radiology Advances. N.S. No relevant relationships. A.G.W. Consulting fees from Canon Medical Images. S.S.S. Grants or contracts from the National Institutes of Health–National Institute of Neurological Disorders and Stroke (StrokeNet grant U19NS115388) and Genentech (protocol no. ML40787); consulting fees from Kandu Health; honorarium for Stroke CME lecture; expert witness provided for stroke clinic patient who was in litigation for workers compensation; patents planned, issued, or pending with Cedars-Sinai Medical Center. P.D.L. Consultant for Apex Innovations. J.L.S. No relevant relationships. B.A.W. No relevant relationships. Z.F. No relevant relationships.
Abbreviations:
- CMR
- cardiac MRI
- DVT
- deep venous thrombosis
- DWI
- diffusion-weighted imaging
- ESUS
- embolic stroke of undetermined source
- LAA
- large artery atherosclerosis
- MRA
- MR angiography
- PFO
- patent foramen ovale
- 3D
- three-dimensional
- VWI
- vessel wall imaging
References
- 1. Adams HP Jr , Bendixen BH , Kappelle LJ , et al . Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment . Stroke 1993. ; 24 ( 1 ): 35 – 41 . [DOI] [PubMed] [Google Scholar]
- 2. Elgendy AY , Saver JL , Amin Z , et al . Proposal for Updated Nomenclature and Classification of Potential Causative Mechanism in Patent Foramen Ovale-Associated Stroke . JAMA Neurol 2020. ; 77 ( 7 ): 878 – 886 . [DOI] [PubMed] [Google Scholar]
- 3. Hart RG , Diener HC , Coutts SB , et al . Embolic strokes of undetermined source: the case for a new clinical construct . Lancet Neurol 2014. ; 13 ( 4 ): 429 – 438 . [DOI] [PubMed] [Google Scholar]
- 4. Ntaios G . Embolic Stroke of Undetermined Source: JACC Review Topic of the Week . J Am Coll Cardiol 2020. ; 75 ( 3 ): 333 – 340 . [DOI] [PubMed] [Google Scholar]
- 5. Hart RG , Sharma M , Mundl H , et al . Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source . N Engl J Med 2018. ; 378 ( 23 ): 2191 – 2201 . [DOI] [PubMed] [Google Scholar]
- 6. Diener HC , Sacco RL , Easton JD , et al . Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source . N Engl J Med 2019. ; 380 ( 20 ): 1906 – 1917 . [DOI] [PubMed] [Google Scholar]
- 7. Saver JL . Clinical Practice. Cryptogenic Stroke . N Engl J Med 2016. ; 374 ( 21 ): 2065 – 2074 . [DOI] [PubMed] [Google Scholar]
- 8. Allen LM , Hasso AN , Handwerker J , Farid H . Sequence-specific MR imaging findings that are useful in dating ischemic stroke . RadioGraphics 2012. ; 32 ( 5 ): 1285 – 1297 ; discussion 1297–1299. [DOI] [PubMed] [Google Scholar]
- 9. Roh JK , Kang DW , Lee SH , Yoon BW , Chang KH . Significance of acute multiple brain infarction on diffusion-weighted imaging . Stroke 2000. ; 31 ( 3 ): 688 – 694 . [DOI] [PubMed] [Google Scholar]
- 10. Sharma M , Smith EE , Pearce LA , et al . Frequency and Patterns of Brain Infarction in Patients With Embolic Stroke of Undetermined Source: NAVIGATE ESUS Trial . Stroke 2022. ; 53 ( 1 ): 45 – 52 . [DOI] [PubMed] [Google Scholar]
- 11. Bang OY , Lee PH , Yeo SH , Kim JW , Joo IS , Huh K . Non-cardioembolic Mechanisms in Cryptogenic Stroke: Clinical and Diffusion-weighted Imaging Features . J Clin Neurol 2005. ; 1 ( 1 ): 50 – 58 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vollmuth C , Stoesser S , Neugebauer H , et al . MR-imaging pattern is not a predictor of occult atrial fibrillation in patients with cryptogenic stroke . J Neurol 2019. ; 266 ( 12 ): 3058 – 3064 . [Published correction appears in J Neurol 2020;267(10):3117.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gürdoğan M , Kehaya S , Korkmaz S , Altay S , Özkan U , Kaya Ç . The Relationship between Diffusion-Weighted Magnetic Resonance Imaging Lesions and 24-Hour Rhythm Holter Findings in Patients with Cryptogenic Stroke . Medicina (Kaunas) 2019. ; 55 ( 2 ): 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nezu T , Mukai T , Uemura J , et al . Multiple Infarcts Are Associated With Long-Term Stroke Recurrence and All-Cause Mortality in Cryptogenic Stroke Patients . Stroke 2016. ; 47 ( 9 ): 2209 – 2215 . [DOI] [PubMed] [Google Scholar]
- 15. Jeong SH , Ahn SS , Baik M , et al . Impact of white matter hyperintensities on the prognosis of cryptogenic stroke patients . PLoS ONE 2018. ; 13 ( 4 ): e0196014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kashima S , Shimizu T , Akiyama H , Hasegawa Y . Magnetic Resonance Imaging White Matter Hyperintensity as a Predictor of Stroke Recurrence in Patients with Embolic Stroke of Undetermined Source . J Stroke Cerebrovasc Dis 2018. ; 27 ( 12 ): 3613 – 3620 . [DOI] [PubMed] [Google Scholar]
- 17. Welker K , Boxerman J , Kalnin A , et al . ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain . AJNR Am J Neuroradiol 2015. ; 36 ( 6 ): E41 – E51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Restrepo L , Jacobs MA , Barker PB , et al . Etiology of perfusion-diffusion magnetic resonance imaging mismatch patterns . J Neuroimaging 2005. ; 15 ( 3 ): 254 – 260 . [DOI] [PubMed] [Google Scholar]
- 19. Paschoal AM , Leoni RF , Dos Santos AC , Paiva FF . Intravoxel incoherent motion MRI in neurological and cerebrovascular diseases . Neuroimage Clin 2018. ; 20 : 705 – 714 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haller S , Zaharchuk G , Thomas DL , Lovblad KO , Barkhof F , Golay X . Arterial Spin Labeling Perfusion of the Brain: Emerging Clinical Applications . Radiology 2016. ; 281 ( 2 ): 337 – 356 . [DOI] [PubMed] [Google Scholar]
- 21. Wong KS , Lam WWM , Liang E , Huang YN , Chan YL , Kay R . Variability of magnetic resonance angiography and computed tomography angiography in grading middle cerebral artery stenosis . Stroke 1996. ; 27 ( 6 ): 1084 – 1087 . [DOI] [PubMed] [Google Scholar]
- 22. Tao L , Li XQ , Hou XW , et al . Intracranial Atherosclerotic Plaque as a Potential Cause of Embolic Stroke of Undetermined Source . J Am Coll Cardiol 2021. ; 77 ( 6 ): 680 – 691 . [DOI] [PubMed] [Google Scholar]
- 23. Wei F , Diedrich KT , Fullerton HJ , et al . Arterial Tortuosity: An Imaging Biomarker of Childhood Stroke Pathogenesis? Stroke 2016. ; 47 ( 5 ): 1265 – 1270 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeVela G , Taylor JM , Zhang B , et al . Quantitative Arterial Tortuosity Suggests Arteriopathy in Children With Cryptogenic Stroke . Stroke 2018. ; 49 ( 4 ): 1011 – 1014 . [DOI] [PubMed] [Google Scholar]
- 25. Mattay RR , Saucedo JF , Lehman VT , et al . Current Clinical Applications of Intracranial Vessel Wall MR Imaging . Semin Ultrasound CT MR 2021. ; 42 ( 5 ): 463 – 473 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dieleman N , van der Kolk AG , Zwanenburg JJM , et al . Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions . Circulation 2014. ; 130 ( 2 ): 192 – 201 . [DOI] [PubMed] [Google Scholar]
- 27. Mandell DM , Mossa-Basha M , Qiao Y , et al . Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology . AJNR Am J Neuroradiol 2017. ; 38 ( 2 ): 218 – 229 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song JW , Moon BF , Burke MP , et al . MR Intracranial Vessel Wall Imaging: A Systematic Review . J Neuroimaging 2020. ; 30 ( 4 ): 428 – 442 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tao L , Dai YJ , Shang ZY , et al . Atrial cardiopathy and non-stenotic intracranial complicated atherosclerotic plaque in patients with embolic stroke of undetermined source . J Neurol Neurosurg Psychiatry 2022. ; 93 ( 4 ): 351 – 359 . [DOI] [PubMed] [Google Scholar]
- 30. Kwee RM , Qiao Y , Liu L , Zeiler SR , Wasserman BA . Temporal course and implications of intracranial atherosclerotic plaque enhancement on high-resolution vessel wall MRI . Neuroradiology 2019. ; 61 ( 6 ): 651 – 657 . [DOI] [PubMed] [Google Scholar]
- 31. Qiao Y , Zeiler SR , Mirbagheri S , et al . Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images . Radiology 2014. ; 271 ( 2 ): 534 – 542 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schaafsma JD , Rawal S , Coutinho JM , et al . Diagnostic Impact of Intracranial Vessel Wall MRI in 205 Patients with Ischemic Stroke or TIA . AJNR Am J Neuroradiol 2019. ; 40 ( 10 ): 1701 – 1706 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Destrebecq V , Sadeghi N , Lubicz B , Jodaitis L , Ligot N , Naeije G . Intracranial Vessel Wall MRI in Cryptogenic Stroke and Intracranial Vasculitis . J Stroke Cerebrovasc Dis 2020. ; 29 ( 5 ): 104684 . [DOI] [PubMed] [Google Scholar]
- 34. Fakih R , Roa JA , Bathla G , et al . Detection and Quantification of Symptomatic Atherosclerotic Plaques With High-Resolution Imaging in Cryptogenic Stroke . Stroke 2020. ; 51 ( 12 ): 3623 – 3631 . [DOI] [PubMed] [Google Scholar]
- 35. Zhu C , Haraldsson H , Tian B , et al . High resolution imaging of the intracranial vessel wall at 3 and 7 T using 3D fast spin echo MRI . MAGMA 2016. ; 29 ( 3 ): 559 – 570 . [DOI] [PubMed] [Google Scholar]
- 36. Chavhan GB , Babyn PS , Thomas B , Shroff MM , Haacke EM . Principles, techniques, and applications of T2*-based MR imaging and its special applications . RadioGraphics 2009. ; 29 ( 5 ): 1433 – 1449 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim SK , Yoon W , Kim TS , Kim HS , Heo TW , Park MS . Histologic Analysis of Retrieved Clots in Acute Ischemic Stroke: Correlation with Stroke Etiology and Gradient-Echo MRI . AJNR Am J Neuroradiol 2015. ; 36 ( 9 ): 1756 – 1762 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alhazmi H , Bani-Sadr A , Bochaton T , et al . Large vessel cardioembolic stroke and embolic stroke of undetermined source share a common profile of matrix metalloproteinase-9 level and susceptibility vessel sign length . Eur J Neurol 2021. ; 28 ( 6 ): 1977 – 1983 . [DOI] [PubMed] [Google Scholar]
- 39. Mittal S , Wu Z , Neelavalli J , Haacke EM . Susceptibility-weighted imaging: technical aspects and clinical applications, part 2 . AJNR Am J Neuroradiol 2009. ; 30 ( 2 ): 232 – 252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weisstanner C , Gratz PP , Schroth G , et al . Thrombus imaging in acute stroke: correlation of thrombus length on susceptibility-weighted imaging with endovascular reperfusion success . Eur Radiol 2014. ; 24 ( 8 ): 1735 – 1741 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saba L , Saam T , Jäger HR , et al . Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications . Lancet Neurol 2019. ; 18 ( 6 ): 559 – 572 . [DOI] [PubMed] [Google Scholar]
- 42. Stary HC , Chandler AB , Dinsmore RE , et al . A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association . Circulation 1995. ; 92 ( 5 ): 1355 – 1374 . [DOI] [PubMed] [Google Scholar]
- 43. Gupta A , Gialdini G , Lerario MP , et al . Magnetic resonance angiography detection of abnormal carotid artery plaque in patients with cryptogenic stroke . J Am Heart Assoc 2015. ; 4 ( 6 ): e002012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gupta A , Gialdini G , Giambrone AE , et al . Association Between Nonstenosing Carotid Artery Plaque on MR Angiography and Acute Ischemic Stroke . JACC Cardiovasc Imaging 2016. ; 9 ( 10 ): 1228 – 1229 . [DOI] [PubMed] [Google Scholar]
- 45. Cai JM , Hatsukami TS , Ferguson MS , Small R , Polissar NL , Yuan C . Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging . Circulation 2002. ; 106 ( 11 ): 1368 – 1373 . [DOI] [PubMed] [Google Scholar]
- 46. Singh N , Moody AR , Panzov V , Gladstone DJ . Carotid Intraplaque Hemorrhage in Patients with Embolic Stroke of Undetermined Source . J Stroke Cerebrovasc Dis 2018. ; 27 ( 7 ): 1956 – 1959 . [DOI] [PubMed] [Google Scholar]
- 47. Larson AS , Nasr DM , Rizvi A , et al . Embolic Stroke of Undetermined Source: The Association With Carotid Intraplaque Hemorrhage . JACC Cardiovasc Imaging 2021. ; 14 ( 2 ): 506 – 508 . [DOI] [PubMed] [Google Scholar]
- 48. Kopczak A , Schindler A , Bayer-Karpinska A , et al . Complicated Carotid Artery Plaques as a Cause of Cryptogenic Stroke . J Am Coll Cardiol 2020. ; 76 ( 19 ): 2212 – 2222 . [DOI] [PubMed] [Google Scholar]
- 49. Freilinger TM , Schindler A , Schmidt C , et al . Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke . JACC Cardiovasc Imaging 2012. ; 5 ( 4 ): 397 – 405 . [DOI] [PubMed] [Google Scholar]
- 50. Hyafil F , Schindler A , Sepp D , et al . High-risk plaque features can be detected in non-stenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined (18)F-FDG PET/MR imaging . Eur J Nucl Med Mol Imaging 2016. ; 43 ( 2 ): 270 – 279 . [DOI] [PubMed] [Google Scholar]
- 51. Chang P , Xiao J , Hu Z , Kwan AC , Fan Z . Imaging of left heart intracardiac thrombus: clinical needs, current imaging, and emerging cardiac magnetic resonance techniques . Ther Adv Cardiovasc Dis 2022. ; 16 : 17539447221107737 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zahuranec DB , Mueller GC , Bach DS , et al . Pilot study of cardiac magnetic resonance imaging for detection of embolic source after ischemic stroke . J Stroke Cerebrovasc Dis 2012. ; 21 ( 8 ): 794 – 800 . [DOI] [PubMed] [Google Scholar]
- 53. Liberman AL , Kalani RE , Aw-Zoretic J , et al . Cardiac magnetic resonance imaging has limited additional yield in cryptogenic stroke evaluation after transesophageal echocardiography . Int J Stroke 2017. ; 12 ( 9 ): 946 – 952 . [DOI] [PubMed] [Google Scholar]
- 54. Bulluck H , Chan MHH , Paradies V , et al . Incidence and predictors of left ventricular thrombus by cardiovascular magnetic resonance in acute ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: a meta-analysis . J Cardiovasc Magn Reson 2018. ; 20 ( 1 ): 72 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kitkungvan D , Nabi F , Ghosn MG , et al . Detection of LA and LAA Thrombus by CMR in Patients Referred for Pulmonary Vein Isolation . JACC Cardiovasc Imaging 2016. ; 9 ( 7 ): 809 – 818 . [DOI] [PubMed] [Google Scholar]
- 56. Takasugi J , Yamagami H , Noguchi T , et al . Detection of Left Ventricular Thrombus by Cardiac Magnetic Resonance in Embolic Stroke of Undetermined Source . Stroke 2017. ; 48 ( 9 ): 2434 – 2440 . [DOI] [PubMed] [Google Scholar]
- 57. Neri LR , Torreão JA , Porto LM , et al . Factors associated with abnormal cardiac magnetic resonance imaging in embolic stroke of undetermined source . Int J Stroke 2019. ; 14 ( 4 ): NP6 – NP9 . [DOI] [PubMed] [Google Scholar]
- 58. Peters DC . Association of Left Atrial Fibrosis Detected by Delayed Enhancement Magnetic Resonance Imaging and Risk of Stroke in Patients with Atrial Fibrillation . J Atr Fibrillation 2011. ; 4 ( 2 ): 384 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fonseca AC , Alves P , Inácio N , et al . Patients With Undetermined Stroke Have Increased Atrial Fibrosis: A Cardiac Magnetic Resonance Imaging Study . Stroke 2018. ; 49 ( 3 ): 734 – 737 . [DOI] [PubMed] [Google Scholar]
- 60. Tandon K , Tirschwell D , Longstreth WT Jr , Smith B , Akoum N . Embolic stroke of undetermined source correlates to atrial fibrosis without atrial fibrillation . Neurology 2019. ; 93 ( 4 ): e381 – e387 . [DOI] [PubMed] [Google Scholar]
- 61. Kühnlein P , Mahnkopf C , Majersik JJ , et al . Atrial fibrosis in embolic stroke of undetermined source: A multicenter study . Eur J Neurol 2021. ; 28 ( 11 ): 3634 – 3639 . [DOI] [PubMed] [Google Scholar]
- 62. Kilner PJ , Yang GZ , Wilkes AJ , Mohiaddin RH , Firmin DN , Yacoub MH . Asymmetric redirection of flow through the heart . Nature 2000. ; 404 ( 6779 ): 759 – 761 . [DOI] [PubMed] [Google Scholar]
- 63. Parikh JD , Kakarla J , Keavney B , et al . 4D flow MRI assessment of right atrial flow patterns in the normal heart - influence of caval vein arrangement and implications for the patent foramen ovale . PLoS ONE 2017. ; 12 ( 3 ): e0173046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Holswilder G , Wermer MJ , Holman ER , Kruyt ND , Kroft LJ , van Walderveen MA . CT Angiography of the Heart and Aorta in TIA and Ischaemic Stroke: Cardioembolic Risk Sources and Clinical Implications . J Stroke Cerebrovasc Dis 2020. ; 29 ( 12 ): 105326 . [DOI] [PubMed] [Google Scholar]
- 65. Harloff A , Dudler P , Frydrychowicz A , et al . Reliability of aortic MRI at 3 Tesla in patients with acute cryptogenic stroke . J Neurol Neurosurg Psychiatry 2008. ; 79 ( 5 ): 540 – 546 . [DOI] [PubMed] [Google Scholar]
- 66. Harloff A , Brendecke SM , Simon J , et al . 3D MRI provides improved visualization and detection of aortic arch plaques compared to transesophageal echocardiography . J Magn Reson Imaging 2012. ; 36 ( 3 ): 604 – 611 . [DOI] [PubMed] [Google Scholar]
- 67. Wehrum T , Dragonu I , Strecker C , et al . Aortic atheroma as a source of stroke - assessment of embolization risk using 3D CMR in stroke patients and controls . J Cardiovasc Magn Reson 2017. ; 19 ( 1 ): 67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Markl M , Semaan E , Stromberg L , Carr J , Prabhakaran S , Collins J . Importance of variants in cerebrovascular anatomy for potential retrograde embolization in cryptogenic stroke . Eur Radiol 2017. ; 27 ( 10 ): 4145 – 4152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jarvis K , Soulat G , Scott M , et al . Investigation of Aortic Wall Thickness, Stiffness and Flow Reversal in Patients With Cryptogenic Stroke: A 4D Flow MRI Study . J Magn Reson Imaging 2021. ; 53 ( 3 ): 942 – 952 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Soulat G , Jarvis K , Pathrose A , et al . Renin Angiotensin System Inhibitors Reduce Aortic Stiffness and Flow Reversal After a Cryptogenic Stroke . J Magn Reson Imaging 2021. ; 53 ( 1 ): 213 – 221 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Faughnan ME , Lui YW , Wirth JA , et al . Diffuse pulmonary arteriovenous malformations: characteristics and prognosis . Chest 2000. ; 117 ( 1 ): 31 – 38 . [DOI] [PubMed] [Google Scholar]
- 72. Grau AJ , Schoenberg SO , Lichy C , Buggle F , Bock M , Hacke W . Lack of evidence for pulmonary venous thrombosis in cryptogenic stroke: a magnetic resonance angiography study . Stroke 2002. ; 33 ( 5 ): 1416 – 1419 . [DOI] [PubMed] [Google Scholar]
- 73. Kanne JP , Lalani TA . Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism . Circulation 2004. ; 109 ( 12 Suppl 1 ): I15 – I21 . [DOI] [PubMed] [Google Scholar]
- 74. Samuel S , Reddy ST , Parsha KN , et al . Routine surveillance of pelvic and lower extremity deep vein thrombosis in stroke patients with patent foramen ovale . J Thromb Thrombolysis 2021. ; 51 ( 4 ): 1150 – 1156 . [DOI] [PubMed] [Google Scholar]
- 75. Cramer SC , Maki JH , Waitches GM , et al . Paradoxical emboli from calf and pelvic veins in cryptogenic stroke . J Neuroimaging 2003. ; 13 ( 3 ): 218 – 223 . [PubMed] [Google Scholar]
- 76. Cramer SC , Rordorf G , Maki JH , et al . Increased pelvic vein thrombi in cryptogenic stroke: results of the Paradoxical Emboli from Large Veins in Ischemic Stroke (PELVIS) study . Stroke 2004. ; 35 ( 1 ): 46 – 50 . [DOI] [PubMed] [Google Scholar]
- 77. Liberman AL , Daruwalla VJ , Collins JD , et al . Diagnostic yield of pelvic magnetic resonance venography in patients with cryptogenic stroke and patent foramen ovale . Stroke 2014. ; 45 ( 8 ): 2324 – 2329 . [DOI] [PubMed] [Google Scholar]
- 78. Cockett FB , Thomas ML , Negus D . Iliac vein compression.--Its relation to iliofemoral thrombosis and the post-thrombotic syndrome . BMJ 1967. ; 2 ( 5543 ): 14 – 19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kiernan TJ , Yan BP , Cubeddu RJ , et al . May-Thurner syndrome in patients with cryptogenic stroke and patent foramen ovale: an important clinical association . Stroke 2009. ; 40 ( 4 ): 1502 – 1504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Osgood M , Budman E , Carandang R , Goddeau RP Jr , Henninger N . Prevalence of Pelvic Vein Pathology in Patients with Cryptogenic Stroke and Patent Foramen Ovale Undergoing MRV Pelvis . Cerebrovasc Dis 2015. ; 39 ( 3-4 ): 216 – 223 . [DOI] [PubMed] [Google Scholar]
- 81. Prabhakar AM , Misono AS , Brinegar KN , Khademhosseini A , Oklu R . Use of Magnetic Resonance Venography in Screening Patients With Cryptogenic Stroke for May-Thurner Syndrome . Curr Probl Diagn Radiol 2016. ; 45 ( 6 ): 370 – 372 . [DOI] [PubMed] [Google Scholar]
- 82. Schwarzbach CJ , Schaefer A , Ebert A , et al . Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology . Stroke 2012. ; 43 ( 11 ): 3029 – 3034 . [DOI] [PubMed] [Google Scholar]
- 83. Navi BB , Kasner SE , Elkind MSV , Cushman M , Bang OY , DeAngelis LM . Cancer and Embolic Stroke of Undetermined Source . Stroke 2021. ; 52 ( 3 ): 1121 – 1130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gon Y , Sakaguchi M , Takasugi J , et al . Plasma D-dimer levels and ischaemic lesions in multiple vascular regions can predict occult cancer in patients with cryptogenic stroke . Eur J Neurol 2017. ; 24 ( 3 ): 503 – 508 . [DOI] [PubMed] [Google Scholar]
- 85. Guo L , Wang L , Liu W . Ability of the number of territories involved on DWI-MRI to predict occult systemic malignancy in cryptogenic stroke patients . J Stroke Cerebrovasc Dis 2020. ; 29 ( 7 ): 104823 . [DOI] [PubMed] [Google Scholar]
- 86. Achiha T , Takagaki M , Oe H , et al . Voxel-Based Lesion Mapping of Cryptogenic Stroke in Patients with Advanced Cancer: A Detailed Magnetic Resonance Imaging Analysis of Distribution Pattern . J Stroke Cerebrovasc Dis 2017. ; 26 ( 7 ): 1521 – 1527 . [DOI] [PubMed] [Google Scholar]
- 87. Nannoni S , de Groot R , Bell S , Markus HS . Stroke in COVID-19: A systematic review and meta-analysis . Int J Stroke 2021. ; 16 ( 2 ): 137 – 149 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li H , Li B , Huang W , Dong L , Zhang J . Flow artifact removal in carotid wall imaging based on black and gray-blood dual-contrast images subtraction . Magn Reson Med 2017. ; 77 ( 4 ): 1612 – 1618 . [DOI] [PubMed] [Google Scholar]
- 89. Fan Z , Yang Q , Deng Z , et al . Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo . Magn Reson Med 2017. ; 77 ( 3 ): 1142 – 1150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yang Q , Deng Z , Bi X , et al . Whole-brain vessel wall MRI: A parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo . J Magn Reson Imaging 2017. ; 46 ( 3 ): 751 – 757 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li Y , Chen Q , Wei Z , et al . One-stop MR neurovascular vessel wall imaging with a 48-channel coil system at 3 T . IEEE Trans Biomed Eng 2020. ; 67 ( 8 ): 2317 – 2327 . [DOI] [PubMed] [Google Scholar]
- 92. Zhou Z , Chen S , Balu N , et al . Neural network enhanced 3D turbo spin echo for MR intracranial vessel wall imaging . Magn Reson Imaging 2021. ; 78 : 7 – 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yoneyama M , Nakamura M , Obara M , et al . Hyperecho PROPELLER-MRI: Application to rapid high-resolution motion-insensitive T2 -weighted black-blood imaging of the carotid arterial vessel wall and plaque . J Magn Reson Imaging 2017. ; 45 ( 2 ): 515 – 524 . [DOI] [PubMed] [Google Scholar]
- 94. Eun DI , Jang R , Ha WS , Lee H , Jung SC , Kim N . Deep-learning-based image quality enhancement of compressed sensing magnetic resonance imaging of vessel wall: comparison of self-supervised and unsupervised approaches . Sci Rep 2020. ; 10 ( 1 ): 13950 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jia S , Zhang L , Ren L , et al . Joint intracranial and carotid vessel wall imaging in 5 minutes using compressed sensing accelerated DANTE-SPACE . Eur Radiol 2020. ; 30 ( 1 ): 119 – 127 . [DOI] [PubMed] [Google Scholar]
- 96. Okuchi S , Fushimi Y , Okada T , et al . Visualization of carotid vessel wall and atherosclerotic plaque: T1-SPACE vs. compressed sensing T1-SPACE . Eur Radiol 2019. ; 29 ( 8 ): 4114 – 4122 . [DOI] [PubMed] [Google Scholar]
- 97. Hu Z , van der Kouwe A , Han F , et al . Motion-compensated 3D turbo spin-echo for more robust MR intracranial vessel wall imaging . Magn Reson Med 2021. ; 86 ( 2 ): 637 – 647 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Liu J , Wang C , Wang J , et al . Motion detection and correction for carotid MRI using a markerless optical system . Magn Reson Imaging 2022. ; 94 : 161 – 167 . [DOI] [PubMed] [Google Scholar]
- 99. Ma LE , Yerly J , Piccini D , et al . 5D Flow MRI: A Fully Self-gated, Free-running Framework for Cardiac and Respiratory Motion-resolved 3D Hemodynamics . Radiol Cardiothorac Imaging 2020. ; 2 ( 6 ): e200219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hu Z , Xiao J , Mao X , et al . MR Multitasking-based multi-dimensional assessment of cardiovascular system (MT-MACS) with extended spatial coverage and water-fat separation . Magn Reson Med 2023. ; 89 ( 4 ): 1496 – 1505 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Han F , Rapacchi S , Khan S , et al . Four-dimensional, multiphase, steady-state imaging with contrast enhancement (MUSIC) in the heart: a feasibility study in children . Magn Reson Med 2015. ; 74 ( 4 ): 1042 – 1049 . [DOI] [PubMed] [Google Scholar]
- 102. Ginami G , Neji R , Rashid I , et al . 3D whole-heart phase sensitive inversion recovery CMR for simultaneous black-blood late gadolinium enhancement and bright-blood coronary CMR angiography . J Cardiovasc Magn Reson 2017. ; 19 ( 1 ): 94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hamamoto K , Chiba E , Oyama-Manabe N , Yuzawa H , Shinmoto H . Assessment of pulmonary arteriovenous malformation with ultra-short echo time magnetic resonance imaging . Eur J Radiol 2022. ; 147 : 110144 . [DOI] [PubMed] [Google Scholar]
- 104. Topiwala KK , Patel SD , Saver JL , Streib CD , Shovlin CL . Ischemic Stroke and Pulmonary Arteriovenous Malformations: A Review . Neurology 2022. ; 98 ( 5 ): 188 – 198 . [Published correction appears in Neurology 2022;98(20):864.] [DOI] [PMC free article] [PubMed] [Google Scholar]