Graphical abstract
Keywords: Cocoa by-products, Sonoextraction, Antioxidants, Response surface methodology, Extraction optimization, Metabolites
Highlights
-
•
Sonotrode-based extraction was used for the recovery of bioactive compounds from CBS and CPH.
-
•
The RSM coupled to BBD was used to optimize the CBS and CPH extraction.
-
•
Optimum conditions were 15 min, 80 % amplitude, and 50 % ethanol.
-
•
Identification of 39 compounds in CBS and 40 compounds in CPH by LC-ESI-qTOF-MS.
-
•
The main classes of bioactive compounds were organic acids, fatty acids, glycosides, flavonoids and procyanidins.
Abstract
Cocoa pod husk (CPH) and cocoa bean shell (CBS) are by-products obtained during pre-processing and processing of cocoa beans. Several bioactive compounds have been identified in these by-products that can be used for commercial applications as a way to promote the circular economy. Therefore, the objective of this paper was to recover bioactive compounds from CPH and CBS by sonoextraction process, to determine the type, content, and antioxidant activity in optimized extracts. To achieve our purpose, an optimization strategy using Box-Behnken Design coupled response surface methodology (MRS) was applied. The extraction conditions were optimized. The results obtained for CBS were: TPC (193 mg GAE/g), TEAC (1.02 mmol TE/g), FRAP (1.02 mmol FeSO4/g) and ORAC (2.6 mmol TE/g), while for CPH, the reported values were: TPC (48 mg GAE/g), TEAC (0.30 mmol TE/g), FRAP (0.35 mmol FeSO4/g) and ORAC (0.43 mmol TE/g) under the optimized conditions: Time (XA): 15 min, Amplitude (XB): 80 %, Ethanol (XC): 50 %. The LC-ESI-qTOF-MS analysis results allowed the identification of 79 compounds, of which 39 represent the CBS extract, while 40 compounds were identified in CPH extract. To conclude, sonotrode based extraction could be considered as an efficient and fast alternative for the recovery of bioactive substances from CBS and CPH.
1. Introduction
Cocoa (Theobroma cacao) is an exotic fruit from the tropical regions of the American continent. This fruit is grown in several regions of the world, with the African continent being the largest production of cocoa beans [1]. Cocoa beans constitute a food matrix widely used for the chocolate production process. About 80 % of the cocoa fruit is represented by the pod, while the difference is made from pulp, seeds, veins, and other constituents. Cocoa bean shell (CBS) and cocoa pod husk (CPH) present several phytochemicals of interest (organic acids, fatty acids, procyanidins, flavonols, flavanols, phenolic acids, amino acids, and alkaloids) [2], [3], which provide excellent health benefits, for example, cocoa flavanols have been shown to be associated with a lower incidence of stroke, lower risk for heart attack, reduction in the risk of type 2 diabetes, lipid oxidation, insulin resistance and systemic Inflammation [4], [5]. The CBS of Forastero and Trinitario genotypes originating from Peru contain between 26 and 43 mg/g of phenolic compounds. Among the bioactive compounds with the greatest presence are theobromine (764 to 9028 mg/kg), caffeine (150 to 3370 mg/kg), epicatechin (4 to 748 mg/kg), catechin (12 to 180 mg/kg), and other minor compounds such as procyanidin type A and type B [6]. In the CPH of CCN51 genotype, around 111 mg/g of phenolic compounds have been found, and the antioxidant activity using the ABTS radical was 155 µmol of Trolox equivalent per grams of sample [3].Recently, in an in silico study, it has been reported that epicatechin and catechin from CPH are highly associated with the inhibitory activity of histone deacetylase 6 in HT-29 colorectal cancer cells [7], and epicatechin has also been shown to promote the inhibition of fibroblast growth factor receptor 3 (FGFR3) gene signalling pathways by inducing changes in elongation primary cilium of chondrocytes and promote femur lengthening [8].
Based on several investigations with satisfactory results, the recovery of bioactive compounds is promoted for novel applications in food systems that allow improving chemical and sensory characteristics and provide better functional and healthy benefits [9]. For this reason, several green extraction systems have been proposed for the recovery of bioactive compounds from food industry processing by-products as a measure in the “zero waste economy”. Among the advanced technologies through a sustainable approach are pressurized liquid extraction, ultrasound-assisted extraction, microwave-assisted extraction, supercritical fluid extraction with cosolvent, high hydrostatic pressure-assisted extraction [10] and systems extraction using natural deep eutectic solvents [11]. Several green extraction technology proposals for the recovery of phenolic compounds, carotenoids, alkaloids, pectins in CBS and CPH have been developed. In addition, microwave-assisted extraction and supercritical CO2 extraction have greater use in this type of by-products [12]. Of all the systems mentioned, ultrasound-assisted extraction offers certain advantages by consuming less time, energy and solvents compared to other techniques. In several by-products such as orange and mango peel, the sonoextraction process has been used to recover phenolic compounds. In these studies, the effect of independent variables such as ethanol/water ratio (%, v/v), extraction time (min), amplitude (%) or power (W), and pulse (%) and their influence on response variables such as total polyphenol content and antioxidant activity have been considered [13], [14]. In this context, it has been established that there are several factors that affect sonoextraction such as sonication time, power, nature of sample, which includes particle size, solid/liquid ratio, and type of solvent [15].
Response surface methodology (RSM) has been used as an efficient statistical tool to optimize extraction processes of bioactive compounds from food industry processing by-products. The RSM approach is based on the determination of optimal conditions from a set of experiments that are affected by the conditions of the independent variables and impact the characteristics or contents of the response variables. For example, the extraction conditions of phenolic compounds from CBS using RSM and artificial neural networks with different factors such as temperature, extraction time, % acidity and solid/liquid ratio and how these factors influence the polyphenol content, flavonoids, flavanols, and antioxidant activity [16].
Therefore, the present study aimed to apply RSM to optimize the extraction conditions (extraction time, amplitude, and solvent concentration) in CBS and CPH through sonoextraction process. The optimal extract was analyzed according to the content of total polyphenols, antioxidant activity by in vitro Trolox equivalent antioxidant capacity (TEAC), Ferric Reducing Antioxidant Power (FRAP) and Oxygen Radical Antioxidant Capacity (ORAC) assays. The metabolite profile of the optimal extract was investigated by LC-ESI-qTOF-MS.
2. Materials and methods
2.1. Sample material and treatment
Cocoa by-products were obtained from the Cooperativa Agroindustrial Cacao Alto Huallaga (Castillo Grande District, province of Leoncio Prado, Peru). Samples were previously dehydrated using a vacuum oven (VO 400, Memmert, Schwabach, Germany), for CBS at a temperature of 50 °C for 3 h and 100 mbar, while CPH was at 60 °C for 8 h and 50 mbar [17]. The dehydrated byproducts were pulverized (GM 200, Retsch, Haan, Germany), vacuum packed, and stored at − 20 °C before analyses.
2.2. Experimental design and sonoextraction process
In order to optimize the extraction of CBS and CPH, a Box-Behnken design with response surface methodology was used. For optimization, three factors and three levels (−1, 0, 1) were considered. The extraction time was 1, 8, and 15 min, while the pulse amplitude was 10, 45, and 80 %, with pulse intermittency of 5 s on and 5 s off (Sonopuls, Bandelin, Berlin, Germany). The extraction solvent was a mixture of ethanol/water that varied between 0 to 100 %, the selection was based on previous work [18]. A total of fifteen experiments that were composed of twelve midpoints of the edges and three replicates at the central point were executed to optimize the sonoextraction conditions. The experiment was previously randomized.
Approximately 500 mg of sample was mixed with 50 mL of solvent (0 to 100 %) and sonicated with an amplitude adjustment range of 10 to 80 % (ultrasonic frequency: 20 kHz ± 500 Hz). A VS 70 T probe (Ø 13 mm) connected to a standard SH 70 G horn (Bandelin, Berlin, Germany) was used for the HD 2070.2 ultrasound homogenizer. The ratio between sample and solvent was maintained at 10 g/L. To avoid a high increase in temperature, an ice bath was used, which was placed around the beaker containing the sample, solvent and sonotrode. After the application of ultrasound, the extractions were filtered using filter paper (Filtros Anoia S.A., Barcelona, Spain) and collected in 50 mL plastic centrifuge tubes. The extracts were centrifuged at 4000 rpm for 10 min and then filtered with RC membrane 0.45 μm pore size, 13 mm circle (PhenexTM). The extracts were stored at −20 °C until use.
2.3. Analysis of total polyphenol content (TPC)
The determination of TPC was carried out according to the procedure described by Rojas-García et al. [19]. A previously diluted aliquot of extract was reacted with 50 μL of the Folin-Ciocalteu reagent (2.0 N), after 10 min, 150 μL of sodium carbonate (20 %) was added. Subsequently, the reaction was left for 2 h in the dark. Absorbance readings were recorded at 760 nm using a multi-mode microplate reader with monochromator-based optics (Synergy H1, BioTek, Winooski, VT, USA). TPC results were expressed in mg gallic acid equivalent per g sample (mg GAE/g).
2.4. Trolox equivalent antioxidant capacity (TEAC)
The TEAC assay was measured by the decolorization of the ABTS•+ cation based on the methodology described by Re et al. [20]. Previously, the ABTS•+ radical was formed by the reaction of potassium persulfate/2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) that reached an absorbance of 0.7 ± 0.02 (an aliquot of 500 μL of ABTS•+ and 40 mL H2O). The reaction between the radical and the extract was developed by adding 300 μL of ABTS•+ and 30 μL of the previously diluted extract. Absorbance readings were recorded at 734 nm. TEAC values were expressed in mmol of Trolox equivalent per g of sample (mmol TE/g).
2.5. Ferric reducing antioxidant power assay (FRAP)
FRAP method was determined according to the methodology modified by Rojas-García et al. [21]. A 250 μL aliquot of the Fe (III)-2,4,6-tripyridyl-s-triazine (TPTZ) complex (prepared from 5 mL of 10 mM TPTZ in acidic medium (pH 3.6) with 5 mL of 20 mM ferric chloride) was reacted with 40 μL of the previously diluted extract. Absorbance readings were measured at 593 nm. FRAP results were expressed in mmol FeSO4 equivalent per g of sample (mmol FeSO4/g).
2.6. Oxygen radical absorbance capacity (ORAC)
ORAC assay was determined following the method proposed by Huang et al. [22] with some modifications. The reaction was performed in a 96-well microplate reader at a final volume of 210 μL. The reaction mixture contained 150 μL of fluorescein (40 nM) and 30 μL of extract or standard (0.5 to 15 μM Trolox in PBS at pH 7.4). Before the addition of 2,2′azobis (2methylpropionamidine) dyhydrochloride-AAPH (30 μL of 19 mM AAPH in PBS at pH 7.4), the mixture was tempered at 37 °C for 15 min in the microplate reader.
2.7. LC-ESI-qTOF-MS analysis
Cocoa shell and pod optimal extracts (5 mg/mL) were analysed by high performance liquid chromatography (HPLC) using an Agilent 1290 HPLC platform (Agilent Technologies, Palo Alto, CA, USA) coupled to mass spectrometry with a quadrupole time-of-flight analyser (ESI-qTOF-MS, Agilent 6545 qTOF Ultra High Definition, Agilent Technologies, Palo Alto, CA, USA). Chromatographic analysis was carried out in reversed phase with a C18 ACQUITY UPLC BEH column (1.7 µm, 2.1 mm, 150 mm, 130 Å, Waters Corporation). The selected mobile phases were (A) acidified water with 0.1 % of formic acid (v/v), and (B) acetonitrile. The following multistep linear gradient was used in order to achieve efficient separation: 0.00 min [A:B 100/0], 5 min [A:B 90/10], 18 min [A:B 15/85], 24 min [A:B 0/100], 25.5 min [A:B 0/100], 26.5 min [A:B 95/5] and 32.5 min [A:B 95/5]. A mobile phase flow rate of 0.4 mL/min and an injection volume of 5 µL was used.
MS acquisition was performed in electrospray negative ionisation (ESI) mode in a mass range between 50–1200 m/z. Other parameters were stablished as follows: gas flow rate 10 L/min; gas temperature 200 °C; nebuliser 20 psig, enveloping gas temperature 350 °C, enveloping gas flow rate 12 l/min, VCap 4000 V, nozzle voltage 500 V.
For the treatment of the data obtained, free software Mzmine and Sirius were used. For the characterisation, experimental data (retention time, m/z, and molecular formula) were consulted in the literature and several databases such as SciFinder, MassBank of North America (MoNa) or Human Metabolome Database (HMDB).
2.8. RSM and statistical analysis
The response variables considered in the optimization process were: TPC (mg GAE/g dw); TEAC (mmol TE/g dry weight) and FRAP (mmol FeSO4/g dw). Response surface models were fitted to the second-order polynomial equation to respond to the response variables according to the following equation (Eq. (1).
| (1) |
Where: was the response variable; , , were the regression coefficients; was the intercept coefficient.
Data analysis, analysis of variance (ANOVA), models, coefficient estimates were done using STATISTICA version 14.0.1.25 (TIBCO Software Inc.).
3. Results and discussion
3.1. Optimization of sonoextraction conditions in CBS
Table 1 summarizes the results of experimental measurements of response variables based on the Box-Behnken Design for the 15 experiments. The effect of the experimental model has a significant impact (p < 0.05) on TPC, TEAC and FRAP assays. When considering cocoa bean shell (CBS), TPC varied from 8.12 to 263.69 mg GAE/g. At the same time, antioxidant activity measured by TEAC ranged from 0.03 to 0.31 mmol TE/g of sample, and the ferric reducing antioxidant power (FRAP) oscillated between 0.02 to 0.41 mmol FeSO4/g of sample. Experiments 7 (XA, 1 min; XB, 45 %; XC, 100 %), 11 (XA, 8 min; XB, 10 %; XC, 100 %), 5 (XA, 8 min; XB, 80 %; XC, 100 %), and 6 (XA, 15 min; XB, 45 %; XC, 100 %) showed lower TPC with values between 8.12 and 11.17 mg GAE/g, corresponding to extraction systems with 100 % ethanol. These results were similar to those reported by Gil-Martínez et al. [23], who reported low recoverability of phenolic compounds using 100 % ethanol in Vaccinium myrtillus leaves. On the other hand, experiments 4 (XA, 8 min; XB, 45 %; XC, 50 %) and 15 (XA, 15 min; XB, 80 %; XC, 50 %) showed higher values of 254.80 and 263.69 mg GAE/g, respectively. Regarding antioxidant activity through TEAC and FRAP, experiments 15 and 4 exhibited the highest activity (Table 1). Several reports mention that the recovery of phenolic compounds through sonoextraction has been considered as independent variables such as extraction time (XA), ultrasonic amplitude (XB), and sample-solvent ratio (XC) [15], [24]. In these reports, XB was found to be the most influential variable for total polyphenol extraction, with the 50 % ethanol proportion showing the highest recovery capacity [13]. When considering experiments 1, 3, 9, and 15 (0 % ethanol), TPC, TEAC, and FRAP presented average values of 151.39 mg GAE/g, 0.10 mmol TE/g, and 0.16 mmol FeSO4/g, while experiments 5, 6, 7, and 11 (100 % ethanol) were 9.72 mg GAE/g, 0.04 mmol TE/g, and 0.02 mmol FeSO4/g, respectively. Experiments (2, 4, 8, 10, 12, 14, and 15) containing 50 % ethanol had an average of 185 mg GAE/g, 0.15 mmol TE/g, and 0.18 mmol FeSO4/g. These results are consistent with Grassia et al. [25], who reported that the highest yield during polyphenol recovery in CBS was with a 50 % v/v ethanolic solution (TPC between 0.80 and 1.20 g/100 g of sample), while in 25 % and 75 % ethanolic solutions, TPC was around 0.40 g/100 g of the sample.
Table 1.
Box-Behnken design (BBD) with responses of the dependent variables obtained by sonoextraction from cocoa bean shell (CBS) and cocoa pod husk (CPH).
| Runs | Experimental domains |
Experimental responses |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| XA | XB | XC | CBS |
CPH |
|||||
| TPC | TEAC | FRAP | TPC | TEAC | FRAP | ||||
| 1 | 8 | 10 | 0 | 146.16 h | 0.09f | 0.16e | 30.81i | 0.02 h | 0.04ef |
| 2 | 8 | 45 | 50 | 213.19c | 0.15bc | 0.23bc | 172.84c | 0.14b | 0.25a |
| 3 | 15 | 45 | 0 | 209.93d | 0.14 cd | 0.23c | 43.15 h | 0.03 g | 0.04e |
| 4 | 8 | 45 | 50 | 254.80b | 0.21a | 0.26a | 153.92e | 0.12c | 0.23b |
| 5 | 8 | 80 | 100 | 10.15 l | 0.04 g | 0.02i | 4.29j | 0.04f | 0.01 g |
| 6 | 15 | 45 | 100 | 11.17 l | 0.08f | 0.03i | 5.53j | 0.04f | 0.01 g |
| 7 | 1 | 45 | 100 | 8.12 l | 0.03 g | 0.02i | 2.25j | 0.02 h | 0.004 g |
| 8 | 1 | 80 | 50 | 124.10i | 0.08f | 0.10 g | 132.53f | 0.10d | 0.17d |
| 9 | 1 | 45 | 0 | 62.61 k | 0.04 g | 0.08 h | 33.30i | 0.09e | 0.03f |
| 10 | 8 | 45 | 50 | 173.66f | 0.11e | 0.19d | 193.68a | 0.14b | 0.24a |
| 11 | 8 | 10 | 100 | 9.43 l | 0.03 g | 0.02i | 2.68j | 0.02 g | 0.005 g |
| 12 | 1 | 10 | 50 | 109.73j | 0.07f | 0.11 g | 131.92f | 0.10d | 0.17d |
| 13 | 8 | 80 | 0 | 186.88e | 0.13d | 0.18d | 50.61 g | 0.03 g | 0.04e |
| 14 | 15 | 10 | 50 | 155.21 g | 0.16b | 0.15f | 158.04d | 0.12c | 0.19c |
| 15 | 15 | 80 | 50 | 263.69a | 0.22a | 0.24b | 190.42b | 0.15a | 0.25a |
XA (Time, min), XB (Amplitude, %), XC (Ethanol, %), TPC (mg GAE/g sample), TEAC (mmol TE/g sample), FRAP (mmol FeSO4/g sample). The different letters next to the means are significantly different (p < 0.05) according to Duncan’s multiple range test.
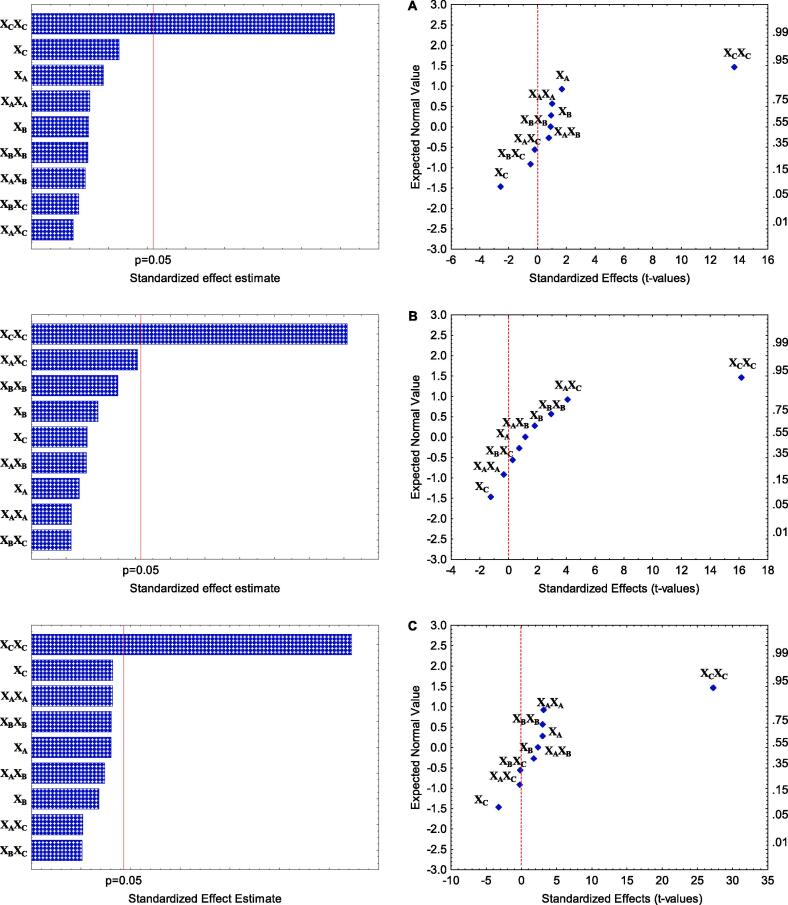
Furthermore, when response variables (TPC, TEAC, and FRAP) underwent analysis of variance for statistical modeling, the ANOVA shown in Table 2 indicates that CBS presented significant results for TPC (F-value = 13.06**) and FRAP (F-value = 16.42***). In contrast, the TEAC variable was insignificant (F-value = 4.36 ns). The factors with values below (p < 0.05) were the sample-solvent ratio, both linear and quadratic (XC) and (XCXC) for TPC and FRAP (Fig. 1A and C), while extraction time (XA), ultrasonic power (XB), and interactions did not seem to affect the recovery of phenolic compounds and subsequently antioxidant activity. Additionally, normal probability plots indicate that response variables (TPC and FRAP) against standardized factor effects (XCXC and XC) showed positive and negative effects, respectively. On the other hand, antioxidant activity through TEAC was not influenced by any of the factors, as observed in the Pareto plots of standardized effects (Fig. 1B).
Table 2.
Analysis of variance for the statistical modeling of TPC, TEAC, and FRAP from cocoa bean shell (CBS) and cocoa pod husk (CPH).
| Source | TPC |
TEAC |
FRAP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of squares |
F-value |
Sum of squares |
F-value |
Sum of squares |
F-value |
|||||||
| CBS | CPH | CBS | CPH | CBS | CPH | CBS | CPH | CBS | CPH | CBS | CPH | |
| Model | 111025.4 | 78677.1 | 13.06** | 30.36*** | 0.0478 | 0.0323 | 4.36 ns | 8.58** | 0.1818 | 0.1464 | 16.42*** | 53.70*** |
| XA | 14065.4 | 1179.80 | 8.54 | 2.98 | 0.0165 | 0.0001 | 7.42 | 0.58 | 0.0132 | 0.0017 | 10.61 | 9.22 |
| XB | 3373.3 | 370.07 | 2.04 | 0.93 | 0.0014 | 0.0003 | 0.63 | 3.37 | 0.0014 | 0.0006 | 1.18 | 3.30 |
| XC | 40144.0 | 2560.09 | 24.38* | 6.47 | 0.0066 | 0.0002 | 2.98 | 1.47 | 0.0382 | 0.0019 | 30.55* | 10.23 |
| XAXB | 2213.6 | 252.39 | 1.34 | 0.63 | 0.0004 | 0.0002 | 0.19 | 1.37 | 0.0029 | 0.0010 | 2.37 | 5.77 |
| XAXC | 5203.1 | 10.77 | 3.16 | 0.027 | 0.0005 | 0.0018 | 0.25 | 16.93 | 0.0054 | 0.0000 | 4.33 | 0.033 |
| XBXC | 400.0 | 82.77 | 0.24 | 0.20 | 0.0003 | 0.0000 | 0.15 | 0.086 | 0.0001 | 0.0000 | 0.057 | 0.013 |
| XAXA | 4033.2 | 418.64 | 2.45 | 1.05 | 0.0007 | 0.0000 | 0.33 | 0.092 | 0.0061 | 0.0019 | 4.88 | 10.14 |
| XBXB | 1176.7 | 340.59 | 0.71 | 0.86 | 0.0007 | 0.0009 | 0.31 | 8.90 | 0.0042 | 0.0017 | 3.41 | 9.35 |
| XCXC | 43127.8 | 74214.9 | 26.20* | 187.72** | 0.0213 | 0.0290 | 9.57 | 262.17** | 0.0340 | 0.1407 | 27.16* | 744.20** |
| Residual | 4724.3 | 1439.58 | 0.0061 | 0.0021 | 0.0034 | 0.0015 | ||||||
| LoF | 1432.4 | 648.89 | 0.29 ns | 0.54 ns | 0.0016 | 0.0018 | 0.24 ns | 5.63 ns | 0.0009 | 0.0011 | 0.25 ns | 2.01 ns |
| PE | 3291.9 | 790.69 | 0.0044 | 0.0002 | 0.0025 | 0.0003 | ||||||
| Total | 115749.8 | 80116.7 | 0.0538 | 0.0344 | 0.1052 | 0.1479 | ||||||
| Pred. R2 | 0.9592 | 0.9820 | 0.8870 | 0.9391 | 0.9672 | 0.9897 | ||||||
| Adj. R2 | 0.8857 | 0.9496 | 0.6836 | 0.8297 | 0.9083 | 0.9713 | ||||||
XA (Time, min), XB (Amplitude, %), XC (Ethanol, %). ns not significant (p > 0.05), * p < 0.05, ** p < 0.01, *** p < 0.001.
Fig. 1.
Pareto charts and normal probability plot of standardized effects of three-factor Box-Behnken design on the extraction of (A) TPC, (B) TEAC, and (C) FRAP from cocoa bean shell (CBS).
In all cases, the difference between predicted R2 and adjusted R2 was less than or equal to 20 %. The adjusted R2 statistic for FRAP, TPC, and TEAC were 90.83 %, 88.53 %, and 68.36 %, respectively. These results indicate that response variables can be explained by the experimental domains affected by sonoextraction processes. Lack-of-fit was not significant for TPC (F-value = 0.29 ns), TEAC (F-value = 0.24 ns) and FRAP (F-value = 0.25 ns) (Table 2). The regression models to predict TPC (mg GAE/g), TEAC (mmol TE/g), and FRAP (mmol FeSO4/g) as a function of the impact of factors, XA (time, min), XB (amplitude, %), XC (ethanol, %) are summarized in the following equations (Eq. 2, 3, and 4).
| TPC (mg GAE/g) = 10.3366 + 17.6135*XA − 0.674494*XA2 + 1.41584*XB −0.014573*XB2 + 3.9878*XC − 0.0432304*XC2 + 0.0960182*XA*XB − 0.103046*XA*XC − 0.00571424*XB*XC(2) |
| TEAC (mmol TE/g) = − 0.00633342 + 0.0109074*XA −0.000288265*XA2 + 0.00132434*XB − 1.12857e-05*XB2 + 0.00297993*XC − 3.042e-05*XC2 + 4.2449e-05*XA*XB − 3.40714e-05*XA*XC − 5.38571e-06*XB*XC(3) |
| FRAP (mmol FeSO4/g) = 0.0184737 + 0.0193717*XA − 0.000830867*XA2 + 0.00211862*XB − 2.77653e-05*XB2 + 0.00340807*XC − 3.8395e-05*XC2 + 0.000111327*XA*XB − 0.000105286*XA*XC − 2.42857e-06*XB*XC(4) |
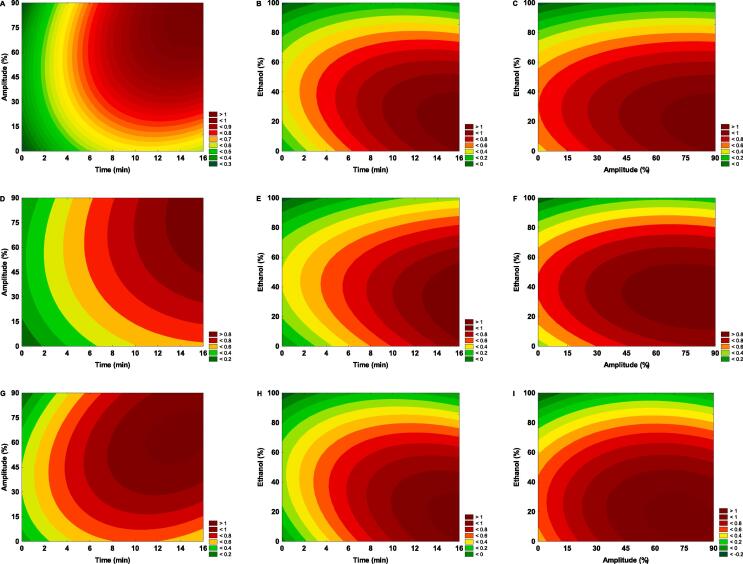
Following the response surface contour plot to predict the desirable optimal parameters of factors influencing the sonoextraction process, as depicted in Fig. 2A–I, the optimal extraction conditions for obtaining higher total polyphenol content (TPC), TEAC, and FRAP in cocoa bean shell are observed to be 15 min of extraction time, an ultrasonic amplitude of 80 %, using an ethanol proportion of 50 %. Non-significant differences were found between predicted and experimental values for TEAC (p = 0.299) and FRAP (p = 0.061), while for TPC, the difference was significant with a p-value of 0.001. The predicted values were 280.60 mg GAE/g for TPC, 0.209 mmol TE/g for TEAC, and 0.264 mmol FeSO4/g for FRAP. Regarding TPC, TEAC, and FRAP, the experimental values for the optimized extract were 267.25 mg GAE/g, 0.199 mmol TE/g, and 0.214 mmol FeSO4/g. The TPC obtained through sonoextraction of the optimized extract was superior compared to the extraction system used by Rojo-Poveda et al. [6], who used a solid–liquid extraction (50 % ethanol) with orbital agitation for 2 h at 25 °C. The TPC using this extraction system ranged from 3 to 43 mg GAE/g in CBS samples from different geographical origins and genotypes.
Fig. 2.
MRS contour plots showing the interaction effect of experimental domains, XA (time, min), XB (amplitude, %), XC (ethanol, %) on the TPC (A-C), TEAC (D-F) and FRAP (G-I) during the sonoextraction process from cocoa bean shell (CBS).
3.2. Optimization of sonoextraction conditions in CPH
Table 1 summarizes the effect of the experimental model on TPC and antioxidant activity (TEAC and FRAP). When considering all experiments for CPH, TPC ranged from 2.25 to 193.68 mg GAE/g, while antioxidant activity through TEAC for all experiments varied between 0.02 to 0.15 mmol TE/g of the sample and FRAP ranged from 0.004 to 0.25 mmol FeSO4/g of the sample. Experiments with lower total polyphenol content were 7, 11, 5, and 6, representing a 100 % ethanol extraction system with TPC values between 2.25 and 5.53 mg GAE/g. The impact of the recoverability of polyphenols using 100 % ethanol in CPH was very similar to CBS; in both cases, lower TPC was observed. Experiments with higher recoverability of polyphenols were 12, 8, 4, 14, 2, 15, and 10. The impact of factors on this group of experiments ranged from 131.92 to 193.68 mg GAE/g. Experiments 15 (XA, 15 min; XB, 80 %; XC, 50 %) and 10 (XA, 8 min; XB, 45 %; XC, 50 %) were the most effective. Regarding antioxidant activity through TEAC and FRAP, experiments 15 and 10 also exhibited the highest activity (Table 1). Similar to CBS, the highest polyphenol content and antioxidant activity developed in experiments where the ethanol percentage was 50 %. As in the case of CBS, the most influential factor was variable XB. For instance, Shepherd [26] mentions that the apple pomace extract (var. Gala) with higher recoverability of phloretin, phloridzin, TPC, and better in vitro antioxidant activity (DPPH, ABTS, and FRAP) was achieved using 50 % ethanol. Similar results were obtained by Boateng et al. [27], who reported higher contents of TPC, total anthocyanins, and condensed tannins in purple corn pericarp extracts under optimized conditions with 50 % ethanol. Considering the ethanol proportion, experiments 1, 3, 9, and 13 (0 % ethanol) presented average values of 39.47 mg GAE/g, 0.04 mmol TE/g, and 0.04 mmol FeSO4/g, while experiments 5, 6, 7, and 11 (100 % ethanol) were 3.69 mg GAE/g, 0.03 mmol TE/g, and 0.01 mmol FeSO4/g, respectively. Meanwhile, experiments (2, 4, 8, 10, 12, 14, and 15) containing 50 % ethanol had an average of 161.91 mg GAE/g, 0.12 mmol TE/g, and 0.21 mmol FeSO4/g. Considering the CPH extraction conditions, TPC was higher compared to those reported by Delgado-Ospina et al. [28], which presented values between 5.4 to 16.6 mg GAE/g of the sample, while under optimization conditions with 80 % methanol, extraction time of 9.35 h, and extraction temperature of 49 °C, a TPC of 115.16 mg GAE/g of sample was achieved [29].
Table 2 presents the findings of the regression model applied to response variables; the obtained ANOVA indicates that CPH presented significant values for TPC (F-value = 30.36***), TEAC (F-value = 8.58**) and FRAP (F-value = 53.70***). The sample-solvent ratio was the only parameter that significantly affected (p < 0.05) all responses quadratically (XCXC) for TPC, TEAC, and FRAP. Moreover, factors XA, XB, and interactions did not show a significant effect on the variables. The normal probability plot diagram showed that response variables against standardized factor effects (XCXC) exhibited a positive effect (Fig. 3A–C).
Fig. 3.
Pareto charts and normal probability plot of standardized effects of three-factor Box-Behnken design on the extraction of (A) TPC, (B) TEAC, and (C) FRAP from cocoa pod husk (CPH).
In all cases, it is observed that the coefficient values (R2) for TEAC, TPC, and FRAP varied between 0.9391 and 0.9897, while adjusted R2 varied between 0.8297 and 0.9713. These values indicate a high correlation between variables of the experimental domain and response variables. Lack-of-fit was not significant (p > 0.05) for TPC (F-value = 0.54 ns), TEAC (F-value = 5.63 ns) and FRAP (F-value = 2.01 ns) (Table 2). When p-values are not significant [30], it indicates that the data fit well to a second-order quadratic polynomial model. The regression equations describing each variable are shown in the following equations (Eq. 5, 6, and 7).
| TPC (mg GAE/g) = 1.13325 + 3.98724*XA − 0.217308*XA2 + 0.770546*XB − 0.00784032*XB2 + 5.46766*XC − 0.0567096*XC2 + 0.0324217*XA*XB − 0.004689*XA*XC −0.00259931*XB*XC(5) |
| TEAC (mmol TE/g) = 0.0468023 − 0.00436454*XA + 3.39286e-05*XA2 + 0.00114974*XB − 1.33367e-05*XB2 + 0.00292129*XC − 3.5465e-05*XC2 + 2.52041e-05*XA*XB + 6.18571e-05*XA*XC + 8.85714e-07*XB*XC(6) |
| FRAP (mmol FeSO4/g) = − 0.0116166 + 0.00669311*XA −0.000465051*XA2 + 0.00134383*XB − 1.78673e-05*XB2 + 0.00754664*XC − 7.8085e-05*XC2 + 6.7449e-05*XA*XB −3.57143e-06*XA*XC − 4.57143e-07*XB*XC(7) |
According to the response surface contour plot (Fig. 4A–I), optimal and desirable parameters are observed for predicting the factors XA (time, min), XB (amplitude, %), and XC (ethanol, %) that influence the sonoextraction process. Based on the Box-Behnken design approach, the optimal extraction conditions recorded a drying time (XA), amplitude (XB), and an ethanol concentration (XC) of 15 min, 80 %, and 50 % EtOH, respectively. Significant differences were found between the predicted and experimental values for the variables TPC (p = 0.0188) and FRAP (p = 0.0158), while no significant differences were observed for the TEAC variable (p = 0.8604). Regarding the predicted values, TPC was observed to be 180.11 mg GAE/g, TEAC was 0.138 mmol TE/g, while FRAP was 0.245 mmol FeSO4/g. On the other hand, the experimental values were 193.70 mg GAE/g for TPC, 0.139 mmol TE/g for TEAC, and 0.174 mmol FeSO4/g for FRAP. The TPC obtained through sonoextraction of CPH exceeded that reported by Lu et al. [31], who mentioned phenolic content ranging from ∼ 46 to ∼ 69 mg GAE/g. Furthermore, Oñate-Gutiérrez et al. [32] reported the TPC of lyophilized extracts from the epicarp of cocoa fruits of different colors (yellow, red, and purple samples) that used an ultrasound-assisted extraction system with EtOH/H2O solvent (80:20 v/v), with values ranging from 153.9 to 167.9 mg GAE/g.
Fig. 4.
MRS contour plots showing the interaction effect of experimental domains, XA (time, min), XB (amplitude, %), XC (ethanol, %) on the TPC (A-C), TEAC (D-F) and FRAP (G-I) during the sonoextraction process from cocoa pod husk (CPH).
3.3. Optimal conditions for sonoextraction process variables
To achieve maximum levels of TPC sonoextraction efficiency and its antioxidant activity through TEAC and FRAP, an experimental method involving the factors XA (time, min), XB (amplitude, %), and XC (ethanol, %) was considered. The optimal conditions determined through the response surface methodology with Box-Behnken design show values within the experimental domains: XA (15 min), XB (80 %), and XC (50 %) for CBS and CPH. The dried extract, weighed and dissolved at a concentration of 5 mg/mL, was utilized for TPC, TEAC, FRAP, ORAC assays, and LC-ESI-qTOF-MS analysis. Results obtained for CBS were: TPC (193 ± 9 mg GAE/g), TEAC (1.02 ± 0.02 mmol TE/g), FRAP (1.02 ± 0.06 mmol FeSO4/g), and ORAC (2.6 ± 0.2 mmol TE/g). Under these optimized conditions, TPC was markedly higher compared to previous works where phenolic content ranged from ∼ 4.9 to ∼ 22.2 mg GAE/g for different cocoa genotypes [2]. Regarding CPH, the obtained results were: TPC (48 ± 1 mg GAE/g), TEAC (0.30 ± 0.007 mmol TE/g), FRAP (0.35 ± 0.009 mmol FeSO4/g), and ORAC (0.43 ± 0.003 mmol TE/g). These results were higher compared to other by-products such as chirimoya peel and seed, which presented total polyphenol values of 28.7 and 30.4 mg GAE/g of dry extract, respectively [19]. However, these results are comparable to avocado seed and peel, which showed values of 60 to 190 mg GAE/g of dry extract [21].
Characterization of the phytochemical profile of CBS and CPH is summarized in Table 3, Table 4. The base peak chromatograms of both extracts are visualized in Supplementary Material Fig. S1 and S2. A total of 79 compounds were identified in CBS and CPH by ESI-qTOF-MS. Of these identified phytochemical compounds, 39 were representative of the CBS extract, mainly including organic acids, fatty acids, glycosides, flavonoids, procyanidins, aspartic acid derivatives, jasmonates, benzoic acid derivatives, amino acids and peptides, coumestan derivatives, and sugar alcohols (Supplementary Material Fig. S3). Several of these compounds were previously identified in CBS [2], [33], [34]. On the other hand, some compounds not identified in previous works were iditol, hydroxyjasmonic acid sulfate (isomer 1 and 2), dihydrojasmonic acid sulfate, norwedelolactone, and hedysarimcoumestan F (Table 3). The negative ionization pattern indicated that peak 1 was tentatively identified as iditol, with a molecular ion [M − H] − at m/z 181.0735, corresponding to the molecular formula C6H14O6, confirmed by characteristic ions (m/z 163, 101, 89, 73, and 59), also detected in Inonotus hispidus mushroom [35], and Daucus carota. In addition, Megías-Pérez et al. [36] noted that the cocoa bean fermentation process is five to six days, and an extended time could lead to the development of undesirable molds. For instance, Saccharomyces cerevisiae can also produce this compound [37]. Peaks 31, 32, and 33 correspond to three jasmonate derivatives. Compounds 31 and 32 exhibit a precursor ion of m/z 305.06 and a molecular formula of C12H18SO7 [38], while peak 33 has a molecular ion [M − H] − at m/z 307.0834. The ion produced at m/z 225 [M − H − 80] − is due to the loss of sulfur trioxide. The fragment with a molecular mass of m/z 225 + H corresponds to the reduced form of 12-hydroxyjasmonic acid (C12H18O4). The ion produced at m/z 225 → 59 is characteristic of jasmonic acid [39]. Peak 42 (demethylwedelolactone or norwedelolactone) produces an ion [M − H] − at m/z 299.0181 (corresponding to a molecular formula of C15H8O7) with a retention time of 12.12 min in the total ion chromatogram. According to the ion fragmentation pattern of m/z 299 → 271, it is characterized by the loss of carbon monoxide (–CO) [M − H − 28]− [40]. This compound was characterized in the extract of Ecliptae Herba (Eclipta prostrata L.) [41], and Sphagneticola calendulacea, plants widely used in traditional Chinese medicine. Peak 44 showed a parent ion [M − H] − at m/z 327.0507 (corresponding to a molecular formula of C17H12O7), tentatively identified as hedysarimcoumestan F (1,7-dihydroxy-3,9-dimethoxycoumestan) [42]. The daughter ions produced were m/z 327, 217, and 109. This compound preferred to lose –OCH3 groups (m/z 31), –OH (m/z 17), and –CH3 (m/z 15). Wang et al. [43] reported that the infrared spectrum showed the presence of hydroxyl, lactone carbonyl, and phenyl groups. This type of coumestan was isolated from ethanolic extracts of Hedysarum multijugum roots.
Table 3.
Identification of phytochemical compounds in CBS extract by HPLC-ESI-qTOF-MS.
| Peak | RT (min) | [M−H] -- | Mol. Formula | Proposed Compound | Fragments |
|---|---|---|---|---|---|
| 1 | 0.96 | 181.0735 | C6H14O6 | Iditol | 89/101/163/73/59 |
| 2 | 1.01 | 195.0541 | C6H12O7 | Gluconic acid | 129/75/99/59 |
| 3 | 1.1 | 133.0157 | C4H6O5 | Malic acid | 89/73/133/71/115 |
| 4 | 1.17 | 191.0217 | C6H8O7 | Citric acid isomer 1 | 111/87/85/67/57 |
| 5 | 1.32 | 191.0213 | C6H8O7 | Citric acid isomer 2 | 111/87/85/67/57 |
| 6 | 1.7 | 96.9614 | − | * | − |
| 7 | 2.67 | 117.0546 | C5H10O3 | Butyric acid derivative | 71/117 |
| 8 | 3.01 | 153.0183 | C7H6O4 | Protocatechuic acid | 108/109/91/65/53 |
| 9 | 3.39 | 181.0495 | C9H10O4 | Propanoic acid derivative | 163/135/119/73/136/134/164 |
| 10 | 3.7 | 175.0587 | C7H12O5 | Isopropyl malic acid | 157/131/85/113/175/115 |
| 11 | 3.88 | 308.1352 | C14H15NO7 | N-Feruloylaspartic acid | 308/149/114/115/264/246 |
| 12 | 4.66 | 233.0671 | C9H14O7 | Isopropyl citric acid | 101/113/59/87/103 |
| 13 | 4.85 | 292.0443 | C13H11NO7 | Unknown 1 | − |
| 14 | 4.99 | 294.0533 | C13H13NO7 | N-Caffeoylaspartic acid | 160/135/71/88/134/179 |
| 15 | 5.04 | 589.1371 | C26H27N2O14 | N-Caffeoylaspartic acid dimer | 294/179/132/135 |
| 16 | 5.27 | 210.0758 | C10H13NO4 | Methoxytyrosine | 124/94/125/66/126/106 |
| 17 | 5.43 | 131.0707 | C6H12O3 | Leucinic Acid | 85/69 |
| 18 | 5.89 | 289.0693 | C15H14O6 | (Epi)catechin isomer 1 | 245/205/179/125/109/123 |
| 19 | 6.16 | 241.1191 | − | Unknown 2 | 82/141/112/70/130/58/197 |
| 20 | 6.46 | 409.1712 | C19H22O10 | Unknown 3 isomer 1 | 409/265/307/347/247/365 |
| 21 | 6.62 | 427.1811 | C17H32O12 | Glucopyranoside derivative | 59/71/55/73/85/101/113/197 |
| 22 | 6.81 | 278.0656 | C13H13NO6 | N-hydroxycinnamoyl aspartic acid | 119/114/163/162/216/234 |
| 23 | 7 | 165.0551 | C9H10O3 | Phenyllactic acid | 147/103/119/73 |
| 24 | 7.16 | 409.1735 | C19H22O10 | Unknown 3 isomer 2 | 409/265/307/347/247/365 |
| 25 | 7.58 | 289.0698 | C15H14O6 | (Epi)catechin isomer 2 | 245/205/179/125/109/123 |
| 26 | 7.77 | 275.1045 | C14H16N2O4 | Lactoyl-tryptophan | 275/127/231/109 |
| 27 | 7.92 | 577.1351 | C30H26O12 | Procyanidin B | 289/407/125/245/408/161 |
| 28 | 8.31 | 329.0901 | C14H18O9 | Vanillic acid glycoside | 329/59/241/71 |
| 29 | 8.54 | 437.1959 | C19H34O11 | Ebracteatoside D | 391/437/59/331/349 |
| 30 | 8.73 | 865.1958 | C45H38O18 | Procyanidin C | − |
| 31 | 9.08 | 305.0699 | C12H18SO7 | Hydroxyjasmonic acid sulfate isomer 1 | 225/59/147 |
| 32 | 9.27 | 305.0718 | C12H18SO7 | Hydroxyjasmonic acid sulfate isomer 2 | 225/59/147 |
| 33 | 9.61 | 307.0834 | C12H20SO7 | Dihydrojasmonic acid sulfate | 227 |
| 34 | 9.67 | 737.1635 | C36H34O17 | Procyanidin A hexoside | 449/611/539/289/737/125 |
| 35 | 9.75 | 427.1588 | C17H32O12 | Glycosidic derivative | 59/71/55/73/101/179 |
| 36 | 9.81 | 463.0838 | C21H20O12 | Quercetin glucoside | 300/301/302/151/179 |
| 37 | 9.9 | 707.1526 | C35H32O16 | Procyanidin A pentoside | 449/539/581/289/707/125 |
| 38 | 10 | 516.2378 | − | Unknown 4 | 184/516/164/127 |
| 39 | 10.23 | 433.0727 | C20H18O11 | Quercetin derivative | 300/301/271/255/243 |
| 40 | 10.46 | 238.1107 | − | Unknown 5 | 94/66/164/238 |
| 41 | 11.07 | 421.2063 | C19H34O10 | Unknown 6 | 421/277/319/359 |
| 42 | 12.12 | 299.0181 | C15H8O7 | Norwedelolactone | 299/271/151 |
| 43 | 12.46 | 329.2338 | C18H34O5 | Trihydroxy octadecenoic acid | 329/201/171 |
| 44 | 12.65 | 327.0507 | C17H12O7 | Hedysarimcoumestan F | 327/217/109 |
| 45 | 12.95 | 357.1217 | C16H22O9 | Sweroside | 357/241/139/59/151 |
| 46 | 17.83 | 271.2272 | C16H32O3 | Hydroxy hexadecanoic acid isomer 1 | 59 |
| 47 | 18.48 | 271.2277 | C16H32O3 | Hydroxy hexadecanoic acid isomer 2 | 225/223/226/55/197 |
*Peaks considered as residues because of the equipment, mobile phases and blanks used during the study.
Table 4.
Identification of phytochemical compounds in CPH extract by HPLC-ESI-qTOF-MS.
| Peak | RT (min) | [M−H] -- | Mol. Formula | Proposed Compound | Fragments |
|---|---|---|---|---|---|
| 1 | 0.96 | 179.0568 | C7H8N4O2 | Theobromine | 59/71/89/179 |
| 2 | 1.01 | 195.053 | C6H12O7 | Gluconic acid | 195/75/129/59/99 |
| 3 | 1.04 | 193.0373 | C6H10O7 | Galacturonic acid | 75/59/73/131/85/193 |
| 4 | 1.07 | 149.0108 | C4H6O6 | Tartaric acid isomer 1 | 149/87/73/59/103 |
| 5 | 1.09 | 133.0163 | C4H6O5 | Malic acid | 115/71/133 |
| 6 | 1.14 | 149.0111 | C4H6O6 | Tartaric acid isomer 2 | 149/87/73/59/103 |
| 7 | 1.21 | 191.0214 | C6H8O7 | Citric acid | 111/87/85/67/57 |
| 8 | 1.24 | 203.0218 | C7H8O7 | Daucic acid | 97/141/71/79 |
| 9 | 1.59 | 115.005 | C4H4O4 | Maleic acid | 71/99/73/115/97 |
| 10 | 1.92 | 96.9613 | − | * | − |
| 11 | 2.99 | 329.0864 | C14H18O9 | Vanillic acid glycoside isomer 1 | 167/329/108/152 |
| 12 | 3.71 | 175.0608 | C7H12O5 | 2-isoproylmalic acid | 115/175/85 |
| 13 | 4.66 | 233.0668 | C9H14O7 | 2-isopropyl citrate | 101/59/113/87 |
| 14 | 5.55 | 295.1386 | C19H20O3 | Prenyl resveratrol | 253/94/145/158/173 |
| 15 | 5.78 | 395.1914 | C17H32O10 | 1-hexanol arabinosylglucoside | 349/395/59/89/101 |
| 16 | 6.62 | 427.1846 | C16H30O10 | Glycosidic derivative | 381/249/101 |
| 17 | 6.93 | 427.1803 | C16H30O10 | Glycosidic derivative | 381/249/101 |
| 18 | 7.57 | 289.0721 | C15H14O6 | (Epi)catechin | 289/109/245/123/203 |
| 19 | 7.69 | 381.1737 | C16H30O10 | Everlastoside C isomer 1 | 249/319/119/59 |
| 20 | 7.92 | 577.1358 | C30H26O12 | Procyanidin B | 407/289/125/425 |
| 21 | 8.31 | 329.0903 | C14H18O9 | Vanillic acid glycoside isomer 2 | 329/59/241 |
| 22 | 8.72 | 358.0926 | C18H17NO7 | Clovamide | 222/358/178/135 |
| 23 | 8.73 | 337.148 | C21H22O4 | Hidroxy prenyl methoxystilbene carboxylic acid | 79/117/104/293 |
| 24 | 8.92 | 381.1747 | C16H30O10 | Everlastoside C isomer 2 | 249/319/119/59 |
| 25 | 9 | 381.1781 | C16H30O10 | Everlastoside C isomer 3 | 249/319/119/59 |
| 26 | 9.19 | 431.0962 | C21H20O10 | Vitexin isomer 1 | 431/311/341/283 |
| 27 | 9.58 | 431.0971 | C21H20O10 | Vitexin isomer 2 | 431/311/341/283 |
| 28 | 9.65 | 372.1075 | − | Glucosinolate derivative | 372/178/222/134 |
| 29 | 9.73 | 575.118 | C30H24O12 | Procyanidin A | 575/125/151/289 |
| 30 | 9.84 | 455.2129 | C19H36O12 | Apiosyl glucoside derivative isomer 1 | 409/255 |
| 31 | 9.89 | 423.1867 | C18H32O11 | Grandidentatin isomer 1 | 117/241/59/357/349 |
| 32 | 9.91 | 455.2155 | C19H36O12 | Apiosyl glucoside derivative isomer 2 | 409/255 |
| 33 | 9.96 | 419.1925 | − | Unknown 2 isomer 1 | − |
| 34 | 9.99 | 423.1881 | C18H32O11 | Grandidentatin isomer 2 | 117/241/59/357/349 |
| 35 | 10.08 | 423.1867 | C18H32O11 | Grandidentatin isomer 3 | 117/241/59/357/349 |
| 36 | 10.15 | 323.1711 | − | Unknown 3 | − |
| 37 | 10.26 | 419.1889 | − | Unknown 2 isomer 2 | − |
| 38 | 10.34 | 363.1653 | C20H28O6 | Gibberellin A | 59/101/275/301 |
| 39 | 10.52 | 469.2275 | C22H30O11 | Iridoid derivative | 469/80 |
| 40 | 11 | 463.0908 | C21H20O12 | Glycosylated flavonoid | 420/377/255/236 |
| 41 | 11 | 727.3382 | − | Unknown 4 | − |
| 42 | 11.07 | 365.1772 | C16H30O9 | Glycosidic derivative | 277/365 |
| 43 | 12.65 | 357.1218 | C16H22O9 | Sweroside isomer 1 | 357/241/139/59/151 |
| 44 | 12.95 | 357.1202 | C16H22O9 | Sweroside isomer 2 | 357/241/139/59/151 |
| 45 | 13.09 | 394.2957 | − | * | − |
| 46 | 13.26 | 329.2328 | C18H34O5 | Trihydroxy octadecenoic acid | 329/201/171 |
*Peaks considered as residues because of the equipment, mobile phases and blanks used during the study.
Regarding the CPH extract, around 40 compounds were identified, mainly including alkaloids, organic acids, sugar acids, hydroxybenzoic acids, tricarboxylic acids and derivatives, stilbenoids, amides, glycosides, flavonoids, glucosinolates, procyanidins, hydroxycinnamic acids and derivatives, hormones, iridoids, fatty acids, and fatty acyl glycosides (Supplementary Material Fig. S4). Several of these compounds have been previously identified in CPH [2], [3], [44]. The main classes of antioxidant compounds correspond to peaks 11, 14, 18, 20, 21, 23, 26, 27, 29, 31, 34, 35, and 40. These compounds (peaks 11 and 21) are characteristic of vanillic acid glycoside isomers (m/z 329.08) [45], prenyl resveratrol (m/z 295.1386) [44], hydroxy prenyl methoxystilbene carboxylic acid (m/z 337.1480) [46], grandidentatin isomer (m/z 423.1867) [47]. While peaks 20 and 29 correspond to procyanidin B (m/z 577.1358) [48], and procyanidin A (m/z 575.1180) [2]. In addition to their antioxidant effect, it has been postulated that procyanidins can be very useful for glucose metabolism, promoting the cellular function of pancreatic islets, and reducing the prevalence of complications in diabetes mellitus [49]. Epicatechin was characterized with [M − H] − at m/z 289.0721; according to Cádiz-Gurrea et al. [48], this compound was detected in cocoa beans, CBS, and CPH. Compounds 26 and 27 were identified as vitexin isomers. Both isomers share the same fragmentation pattern at m/z 431.09 and exhibit the same MS/MS fragment ions at m/z 311, 341, and 283 with retention times of 9.19 and 9.58 min. These flavonoids were also identified in mung beans (Vigna radiata L.) [50], buckwheat, flax, linseed, and naked oat [51]. Finally, compound 40 was identified as a glycosylated flavonoid with [M − H] − at m/z 463.0908, with the presence of fragment ions at m/z 420, 377, 255, and 236. This compound corresponds to the molecular formula C21H20O12. Some flavonoids sharing the same fragmentation pattern with the rest of the sugars include quercetin-3-O-glucoside and myricetin 3-O-rhamnoside [52].
4. Conclusion
CBS and CPH contain different classes of bioactive substances. Several substances isolated from these byproducts have demonstrated potent antioxidant activity and other biological effects both in vitro and in a model food system. Conventional methods for the recovery of bioactive components from CBS and CPH are not sufficient to meet the need of food, cosmetic and pharmaceutical industries. In this regard, we developed an optimized method for the extraction of bioactive substances from CBS and CPH using a sonoextraction process. The application of Box-Behnken Design coupled to MRS, taking into account the influence of the factors (experimental domains), time (XA), amplitude (XB), and ethanol concentration (XC) on the TPC, TEAC, and FRAP was evaluated. Under the conditions optimized in this study: time (XA): 15 min, amplitude (XB): 80 %, ethanol (XC): 50 %, CBS was superior to CPH, with TPC (193 mg GAE/g), TEAC (1.02 mmol TE/g), FRAP (1.02 mmol FeSO4/g) and ORAC (2.6 mmol TE/g). The main classes of bioactive compounds found in CBS were organic acids, fatty acids, glycosides, flavonoids and procyanidins, while in CPH, they were glycosides, organic acids, flavonoids, hydroxycinnamic acids and derivatives and sugar acids. Overall, CBS and CPH can significantly contribute various bioactive constituents as a zero-waste economy alternative for the cocoa processing industry.
Funding
This work was supported by Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica of Perú [184–2020-FONDECYT].
CRediT authorship contribution statement
Fernando Ramos-Escudero: Writing – original draft, Software, Resources, Investigation, Formal analysis. Alejandro Rojas-García: Writing – review & editing, Validation, Methodology, Investigation, Formal analysis. María de la Luz Cádiz-Gurrea: Conceptualization, Methodology, Software, Supervision, Writing – review & editing. Antonio Segura-Carretero: Visualization, Supervision, Resources, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2024.106887.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.ICCO. Quarterly bulletin of cocoa statistics, Vol. XLIX, No.1, Cocoa year 2022/23.
- 2.Cádiz-Gurrea M.L., Fernández-Ochoa Á., Leyva-Jiménez F.J., Guerrero-Muñoz N., Villegas-Aguilar M.D.C., Pimentel-Moral S., Ramos-Escudero F., Segura-Carretero A. LC-MS and spectrophotometric approaches for evaluation of bioactive compounds from Peru cocoa by-products for commercial applications. Molecules. 2020;25(14):3177. doi: 10.3390/molecules25143177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vargas-Arana G., Merino-Zegarra C., Tang M., Pertino M.W., Simirgiotis M.J. UHPLC-MS characterization, and antioxidant and nutritional analysis of cocoa waste flours from the Peruvian Amazon. Antioxidants. 2022;11(3):595. doi: 10.3390/antiox11030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraga C.G., Croft K.D., Kennedy D.O., Tomás-Barberán F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10(2):514–528. doi: 10.1039/C8FO01997E. [DOI] [PubMed] [Google Scholar]
- 5.Rana A., Samtiya M., Dhewa T., Mishra V., Aluko R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022;46(10):e14264. doi: 10.1111/jfbc.14264. [DOI] [PubMed] [Google Scholar]
- 6.Rojo-Poveda O., Zeppa G., Ferrocino I., Stévigny C., Barbosa-Pereira L. Chemometric classification of cocoa bean shells based on their polyphenolic profile determined by RP-HPLC-PDA analysis and spectrophotometric assays. Antioxidants. 2021;10(10):1533. doi: 10.3390/antiox10101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieto-Figueroa K.H., Gaytán-Martínez M., Loarca-Piña M.G.F., Campos-Vega R. Effect of drying method on the production of in vitro short-chain fatty acids and histone deacetylase mediation of cocoa pod husk. J. Food Sci. 2022;87(10):4476–4490. doi: 10.1111/1750-3841.16309. [DOI] [PubMed] [Google Scholar]
- 8.Martin L., Kaci N., Benoist-Lasselin C., Mondoloni M., Decaudaveine S., Estibals V., Cornille M., Loisay L., Flipo J., Demuynck B., Cádiz-Gurrea M.L., Barbault F., Fernández-Arroyo S., Schibler L., Segura-Carretero A., Dambroise E., Legeai-Mallet L. Theobroma cacao improves bone growth by modulating defective ciliogenesis in a mouse model of achondroplasia. Bone Res. 2022;10:8. doi: 10.1038/s41413-021-00177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpentieri S., Larrea-Wachtendorf D., Donsì F., Ferrari G. Functionalization of pasta through the incorporation of bioactive compounds from agri-food by-products: Fundamentals, opportunities, and drawbacks. Trends Food Sci. Technol. 2022;122:49–65. doi: 10.1016/j.tifs.2022.02.011. [DOI] [Google Scholar]
- 10.Cádiz-Gurrea M.L., Villegas-Aguilar M.C., Leyva-Jiménez F.J., Pimentel-Moral S., Fernández-Ochoa Á., Alañón M.E., Segura-Carretero A. Revalorization of bioactive compounds from tropical fruit by-products and industrial applications by means of sustainable approaches. Food Res. Int. 2020;138 doi: 10.1016/j.foodres.2020.109786. [DOI] [PubMed] [Google Scholar]
- 11.Maimulyanti A., Nurhidayati I., Mellisani B., Putri F.A.R., Puspita F., Prihadi A.R. Development of natural deep eutectic solvent (NADES) based on choline chloride as a green solvent to extract phenolic compound from coffee husk waste. Arab. J. Chem. 2023;16(4) doi: 10.1016/j.arabjc.2023.104634. [DOI] [Google Scholar]
- 12.Belwal T., Cravotto C., Ramola S., Thakur M., Chemat F., Cravotto G. Bioactive compounds from cocoa husk: Extraction, analysis and applications in food production chain. Foods. 2022;11(6):798. doi: 10.3390/foods11060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razola-Díaz M.C., Guerra-Hernández E.J., Rodríguez-Pérez C., Gómez-Caravaca A.M., García-Villanova B., Verardo V. Optimization of ultrasound-assisted extraction via sonotrode of phenolic compounds from orange by-products. Foods. 2021;10(5):1120. doi: 10.3390/foods10051120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aznar-Ramos M.J., Razola-Díaz M.C.D., Verardo V., Gómez-Caravaca A.M. Comparison between ultrasonic bath and sonotrode extraction of phenolic compounds from mango peel by-products. Horticulturae. 2022;8(11):1014. doi: 10.3390/horticulturae8111014. [DOI] [Google Scholar]
- 15.Dias M.I., Albiston C., Añibarro-Ortega M., Ferreira I.C.F.R., Pinela J., Barros L. Sonoextraction of phenolic compounds and saponins from Aesculus hippocastanum seed kernels: Modeling and optimization. Ind. Crop. Prod. 2022;185 doi: 10.1016/j.indcrop.2022.115142. [DOI] [Google Scholar]
- 16.Rebollo-Hernanz M., Cañas S., Taladrid D., Segovia A., Bartolomé B., Aguilera Y., Martín-Cabrejas M.A. Extraction of phenolic compounds from cocoa shell: Modeling using response surface methodology and artificial neural networks. Sep. Purif. Technol. 2021;270 doi: 10.1016/j.seppur.2021.118779. [DOI] [Google Scholar]
- 17.F. Ramos-Escudero, S. Casimiro-Gonzales, M.L. Cádiz-Gurrea, K. Cancino Chávez, J. Basilio-Atencio, E.S. Ordoñez, A.M. Muñoz, A. Segura-Carretero, Optimizing vacuum drying process of polyphenols, flavanols and DPPH radical scavenging assay in pod husk and bean shell cocoa, Sci. Rep. 13 (2023) 13900, doi: 10.1038/s41598-023-40815-0. [DOI] [PMC free article] [PubMed]
- 18.Martín-García B., Pimentel-Moral S., Gómez-Caravaca A.M., Arráez-Román D., Segura-Carretero A. Box-Behnken experimental design for a green extraction method of phenolic compounds from olive leaves. Ind. Crop. Prod. 2020;154 doi: 10.1016/j.indcrop.2020.112741. [DOI] [Google Scholar]
- 19.Rojas-García A., Rodríguez L., Cádiz-Gurrea M.L., García-Villegas A., Fuentes E., Villegas-Aguilar M.C., Palomo I., Arráez-Román D., Segura-Carretero A. Determination of the bioactive effect of custard apple by-products by in vitro assays. Int. J. Mol. Sci. 2022;23(16):9238. doi: 10.3390/ijms23169238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 21.Rojas-García A., Fuentes E., Cádiz-Gurrea M.L., Rodríguez L., Villegas-Aguilar M.C., Palomo I., Arráez-Román D., Segura-Carretero A. Biological evaluation of avocado residues as a potential source of bioactive compounds. Antioxidants. 2022;11(6):1049. doi: 10.3390/antiox11061049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D. Huang D, B. Ou, M. Hampsch-Woodill, J.A. Flanagan, R.L. Prior, High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format, J. Agric. Food Chem. 50(16) (2002) 4437-4444, doi: 10.1021/jf0201529. [DOI] [PubMed]
- 23.Gil-Martínez L., Aznar-Ramos M.J., Razola-Diaz M.C., Mut-Salud N., Falcón-Piñeiro A., Baños A., Guillamón E., Gómez-Caravaca A.M., Verardo V. Establishment of a sonotrode extraction method and evaluation of the antioxidant, antimicrobial and anticancer potential of an optimized Vaccinium myrtillus L. leaves extract as functional ingredient. Foods. 2023;12(8):1688. doi: 10.3390/foods12081688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macedo C., Silva A.M., Ferreira A.S., Cádiz-Gurrea M.L., Fernández-Ochoa A., Segura-Carretero A., Delerue-Matos C., Costa P., Rodrigues F. Insights into the polyphenols extraction from Actinidia arguta fruit (kiwiberry): A source of pro-healthy compounds. Sci. Hortic. 2023;313 doi: 10.1016/j.scienta.2023.111910. [DOI] [Google Scholar]
- 25.Grassia M., Messia M.C., Marconi E., Şakiyan Demirkol ö., Erdoğdu F., Sarghini F., Cinquanta L., Corona O., Planeta D. Microencapsulation of phenolic extracts from cocoa shells to enrich chocolate bars. Plant Foods Hum. Nutr. 2021;76:449–457. doi: 10.1007/s11130-021-00917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepherd K. Creation of a sonotrode ultrasound-assisted phenolic compound extraction from apple pomace. Appl. Food Sci. J. 2022;6(4):35–36. doi: 10.37532/PULAFS. [DOI] [Google Scholar]
- 27.Boateng I.D., Kumar R., Daubert C.R., Flint-Garcia S., Mustapha A., Kuehnel L., Agliata J., Li Q., Wan C., Somavat P. Sonoprocessing improves phenolics profile, antioxidant capacity, structure, and product qualities of purple corn pericarp extract. Ultrason. Sonochem. 2023;95 doi: 10.1016/j.ultsonch.2023.106418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delgado-Ospina J., Lucas-Gonzalez R., Viuda-Martos M., Fernandez-Lopez J., Pérez-Alvarez J.A., Martuscelli M., Chaves-Lopez C. Bioactive compounds and techno-functional properties of high-fiber co-products of the cacao agro-industrial chain. Heliyon. 2021;7(4):e06799. doi: 10.1016/j.heliyon.2021.e06799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teboukeu G.B., Djikeng F.T., Klang M.J., Ndomou S.H., Karuna M.S.L., Womeni H.M. Polyphenol antioxidants from cocoa pods: Extraction optimization, effect of the optimized extract, and storage time on the stability of palm olein during thermoxidation. J. Food Process. Preserv. 2018;42(5):e13592. [Google Scholar]
- 30.Cannas M., Conte P., Piga A., Del Caro A. Green recovery optimization of phenolic compounds from “Spinoso sardo” globe artichoke by-products using response surface methodology. Front. Sustain. Food Syst. 2023;7:1215809. doi: 10.3389/fsufs.2023.1215809. [DOI] [Google Scholar]
- 31.Lu F., Rodriguez-Garcia J., Van Damme I., Westwood N.J., Shaw L., Robinson J.S., Warren G., Chatzifragkou A., McQueen Mason S., Gomez L., Faas L., Balcombe K., Srinivasan C., Picchioni F., Hadley P., Charalampopoulos D. Valorisation strategies for cocoa pod husk and its fractions. Curr. Opin. Green Sustain. Chem. 2018;14:80–88. doi: 10.1016/j.cogsc.2018.07.007. [DOI] [Google Scholar]
- 32.Oñate-Gutiérrez J.A., Díaz-Sánchez L.M., Urbina D.L., Pinzón J.R., Blanco-Tirado C., Combariza M.Y. Exploring the chemical composition and coloring qualities of cacao fruit epicarp extracts. RSC Adv. 2023;13:12712–12722. doi: 10.1039/D3RA01049J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cádiz-Gurrea M.L., Lozano-Sanchez J., Contreras-Gámez M., Legeai-Mallet L., Fernández-Arroyo S., Segura-Carretero A. Isolation, comprehensive characterization and antioxidant activities of Theobroma cacao extract. J. Funct. Foods. 2014;10:485–498. doi: 10.1016/j.jff.2014.07.016. [DOI] [Google Scholar]
- 34.Barbosa-Pereira L., Belviso S., Ferrocino I., Rojo-Poveda O., G. Zeppa G Characterization and classification of cocoa bean shells from different regions of Venezuela using HPLC-PDA-MS/MS and spectrophotometric techniques coupled to chemometric analysis. Foods. 2021;10(8):1791. doi: 10.3390/foods10081791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z., Bao H. Comparative analysis of metabolic compositions and trace elements of Inonotus hispidus mushroom grown on five different tree species. ACS Omega. 2022;7:9343–9358. doi: 10.1021/acsomega.1c06226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Megías-Pérez R., Grimbs S., D'Souza R.N., Bernaert H., Kuhnert N. Profiling, quantification and classification of cocoa beans based on chemometric analysis of carbohydrates using hydrophilic interaction liquid chromatography coupled to mass spectrometry. Food Chem. 2018;258:284–294. doi: 10.1016/j.foodchem.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 37.NCBI. National Center for Biotechnology Information. PubChem compound summary for CID 5460044, L-Iditol. https://pubchem.ncbi.nlm.nih.gov/compound/L-Iditol (accessed 26 September 2023).
- 38.Patras M.A., Milev B.P., Vrancken G., Kuhnert N. Identification of novel cocoa flavonoids from raw fermented cocoa beans by HPLC-MSn. Food Res. Int. 2014;63:353–359. doi: 10.1016/j.foodres.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 39.Smirnova E., Marquis V., Poirier L., Aubert Y., Zumsteg J., Ménard R., Miesch L., Heitz T. Jasmonic acid oxidase 2 hydroxylates jasmonic acid and represses basal defense and resistance responses against botrytis cinerea infection. Mol. Plant. 2017;10(9):1159–1173. doi: 10.1016/j.molp.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Wang B.E., Zhang L.T., Yang S.B., Xu Z.L. UPLC-MS/MS assay for quantification of wedelolactone and demethylwedelolactone in rat plasma and the application to a preclinical pharmacokinetic study. Comb. Chem. High Throughput Screen. 2022;25(8):1271–1277. doi: 10.2174/1386207324666210520093517. [DOI] [PubMed] [Google Scholar]
- 41.Li M., Sib D., Fu Z., Sang M., Zhang Z., Liu E., Yang W., Gao X., Lifeng H. Enhanced identification of the in vivo metabolites of Ecliptae Herba in rat plasma by integrating untargeted data-dependent MS2 and predictive multiple reaction monitoring-information dependent acquisition-enhanced product ion scan. J. Chromatogr. B. 2019;1109:99–111. doi: 10.1016/j.jchromb.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Buckingham J., Munasinghe V.R.N. Taylor & Francis Group; Boca Raton, FL: 2015. Dictionary of flavonoids with CD-ROM. [Google Scholar]
- 43.Wang W., Zhao Y.-Y., Liang H., Jia Q., Chen H.-B. Coumestans from Hedysarum multijugum. J. Nat. Prod. 2006;69:876–880. doi: 10.1021/np050233+. [DOI] [PubMed] [Google Scholar]
- 44.A. Abdul Karim, A. Azlan, A. Ismail, P. Hashim, S.S.A. Gani, B.H. Zainudin, N.A. Abdullah, Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract, BMC Complement. Altern. Med. 14 (2014) 381, doi: 10.1186/1472-6882-14-381. [DOI] [PMC free article] [PubMed]
- 45.Tahir N.I., Saari K., Abas F., Parveez A., Tarmizi H.A., Ramli U. Identification of oil palm (Elaeis guineensis) spear leaf metabolites using mass spectrometry and neutral loss analysis. J. Oil Palm Res. 2013;25:72–83. [Google Scholar]
- 46.Green P.W., Stevenson P.C., Simmonds M.S., Sharma H.C. Phenolic compounds on the pod-surface of pigeonpea, Cajanus cajan, mediate feeding behavior of Helicoverpa armigera larvae. J. Chem. Ecol. 2003;29(4):811–821. doi: 10.1023/a:1022971430463. [DOI] [PubMed] [Google Scholar]
- 47.Tawfeew N., Sobeh M., Hamdan D.I., Farrag N., Roxo M., El-Shazly A.M., Wink M. Phenolic compounds from Populus alba L. and Salix subserrata Willd. (Salicaceae) counteract oxidative stress in Caenorhabditis elegans. Molecules. 2019;24(10):1999. doi: 10.3390/molecules24101999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cádiz-Gurrea M.L., Borrás-Linares I., Lozano-Sánchez J., Joven J., Fernández-Arroyo S., Segura-Carretero A. Cocoa and grape seed byproducts as a source of antioxidant and anti-inflammatory proanthocyanidins. Int. J. Mol. Sci. 2017;18:376. doi: 10.3390/ijms18020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qaed E., Almoiliqy M., Al-Hamyari B., Qaid A., Alademy H., Al-Maamari A., Alyafeai E., Geng Z., Tang Z., Ma X. Procyanidins: A promising anti-diabetic agent with potential benefits on glucose metabolism and diabetes complications. Wound Repair Regen. 2023;31:688–699. doi: 10.1111/wrr.13115. [DOI] [PubMed] [Google Scholar]
- 50.Hou D., Yousaf L., Xue Y., Hu J., Wu J., Hu X., Feng N., Shen Q. Mung bean (Vigna radiata L.): Bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients. 2019;11:1238. doi: 10.3390/nu11061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalinová J.P., Vrchotová N., Tříska J. Vitexin and isovitexin levels in sprouts of selected plants. J. Food Compos. Anal. 2021;100 doi: 10.1016/j.jfca.2021.103895. [DOI] [Google Scholar]
- 52.Buzgaia N., Lee S.Y., Rukayadi Y., Abas F., Shaari K. Antioxidant activity, α-glucosidase inhibition and UHPLC-ESI-MS/MS profile of shmar (Arbutus pavarii Pamp) Plants. 2021;10:1659. doi: 10.3390/plants10081659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.