Abstract
Type 2 diabetes disparities in the USA persist in both the prevalence of disease and diabetes-related complications. We conducted a literature review related to diabetes prevention, management, and complications across racial and ethnic groups in the USA. The objective of this review is to summarise the current understanding of diabetes disparities by examining differences between and within racial and ethnic groups and among young people (aged <18 years). We also examine the pathophysiology of diabetes as it relates to race and ethnic differences. We use a conceptual framework built on the socioecological model to categorise the causes of diabetes disparities across the lifespan looking at factors in five domains of health behaviours and social norms, public awareness, structural racism, economic development, and access to high-quality care. The range of disparities in diabetes prevalence and management in the USA calls for a community-engaged and multidisciplinary approach that must involve community partners, researchers, practitioners, health system administrators, and policy makers. We offer recommendations for each of these groups to help to promote equity in diabetes prevention and care in the USA.
Introduction
Type 2 diabetes is a highly prevalent, costly, chronic disease that poses a serious threat to both domestic and global health. The International Diabetes Federation reports that 10% of adults (aged 20–79 years) have type 2 diabetes worldwide, with North America reporting 51 million adults living with this disease.1 In the USA specifically, an estimated 37·3 million (11·1% of the population) had type 2 diabetes in 2020.2 Type 2 diabetes is currently one of the leading causes of death in the USA with a crude death rate of 26·7 per 100 000 people.2 Type 2 diabetes prevalence varies between states in the USA, ranging from 3·3% (95% CI 2·2–4·5) in Teton County, WY, to 19·5% (17·2–22·0) in Oglala Lakota County, SD, in 2017.3 There is also 7-fold to 8-fold variation in incidence of age-standardised diagnosed diabetes across US counties.4 A common and potentially preventable disease, type 2 diabetes imposes substantial health and economic burdens in the USA, where the medical costs and lost work wages of people diagnosed with the disease total an estimated US$327 billion annually.5
The burden of type 2 diabetes in the USA is not shared equally between different racial and ethnic groups. Several reviews from the past 5 years have discussed disparities in the prevalence of diabetes and diabetes-related complications by racial and ethnic groups in the USA. For example, Cheng and colleagues’ 2019 review6 of the literature showed persistent disparity in the prevalence of diabetes in the USA with Hispanic American adults (aged ≥20 years) having the highest prevalence (22·1%) followed by non-Hispanic Black adults (20·4%), and non-Hispanic Asian American adults (19·1%) compared with the non-Hispanic White American adult population (12·1%). In their 2021 study, Wang and colleagues7 showed that there continues to be poor control of risk factors such as blood sugar levels, blood pressure, cholesterol concentrations, and smoking among individuals with type 2 diabetes. Moreover, non-Hispanic Black individuals were significantly less likely than non-Hispanic White adults to have these risk factors controlled.7 In 2021, Ezzatvar and colleagues8 described the persistent disparities in diabetes-related complications and all-cause mortality that are in part a consequence of poorly controlled risk factors. In 2022, El Hussein and colleagues9 described disparities in the prescribing of new diabetes medication (eg, GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT2 inhibitors), with Black (hazard ratio 0·81, 95% CI 0·71–0·94) and American Indian and Native American individuals (0·51, 0·26–0·99) less likely to be prescribed these medications than White individuals.
The limitation of many of these reviews is that they analyse racial and ethnic groups as one homogeneous entity. However, there is growing realisation of the importance of examining within-group differences to address diabetes disparities.10,11 Furthermore, focusing solely on adults with diabetes overlooks the degree to which child and adolescent onset type 2 diabetes is contributing to the growing burden of disease and worsening disparities. Not only has the prevalence of type 2 diabetes among young people (aged 10–19 years) nearly doubled in the past two decades (from 34 per 100 000 in 2001 to 67 per 100 000 in 2017), but the highest number of young people living with type 2 diabetes per 1000 individuals is seen among Black or American Indian populations.12 The story of disparities in diabetes in the USA is, therefore, incomplete if not considering the whole lifespan.
Our objective in this Series paper is to compile and summarise key literature examining diabetes disparities across the lifespan and within racial and ethnic groups. We then provide a summary of more recent literature looking at the role of genetics and emerging views on pathophysiological basis of different diabetes phenotypes. Finally, we provide a framework to summarise the multilevel contributors to diabetes disparities in the USA. Leveraging this more informative view of disparities, we recommend a path forward to address knowledge gaps and an implementation and policy agenda to promote diabetes health equity.
Definition of racial and ethnic groups
We define racial and ethnic groups in this Series paper using the Office of Management and Budget Standards (OMB) definition.13 The racial groups are American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, and White. The ethnic groups are Hispanic and non-Hispanic. From here on, we will refer to non-Hispanic Black as Black, and non-Hispanic White as White. American Indian or Alaska Native might also be referred to as Native American on the basis of study descriptions.
Conceptual framework
We consider the contributors to diabetes disparities across the lifespan, within, and between racial and ethnic groups using a modified version of the conceptual framework proposed by Agarwal and colleagues in this Series paper.14 We consider the domains of health behaviours and social norms, structural racism, access to high-quality care, economic development, and public awareness. We discuss how these domains differentially exert their influence at the individual, interpersonal, community, and societal levels across the lifespan (figure 1).
Figure 1: Conceptual framework for diabetes disparities in the USA (adapted from Agarwal et al14).
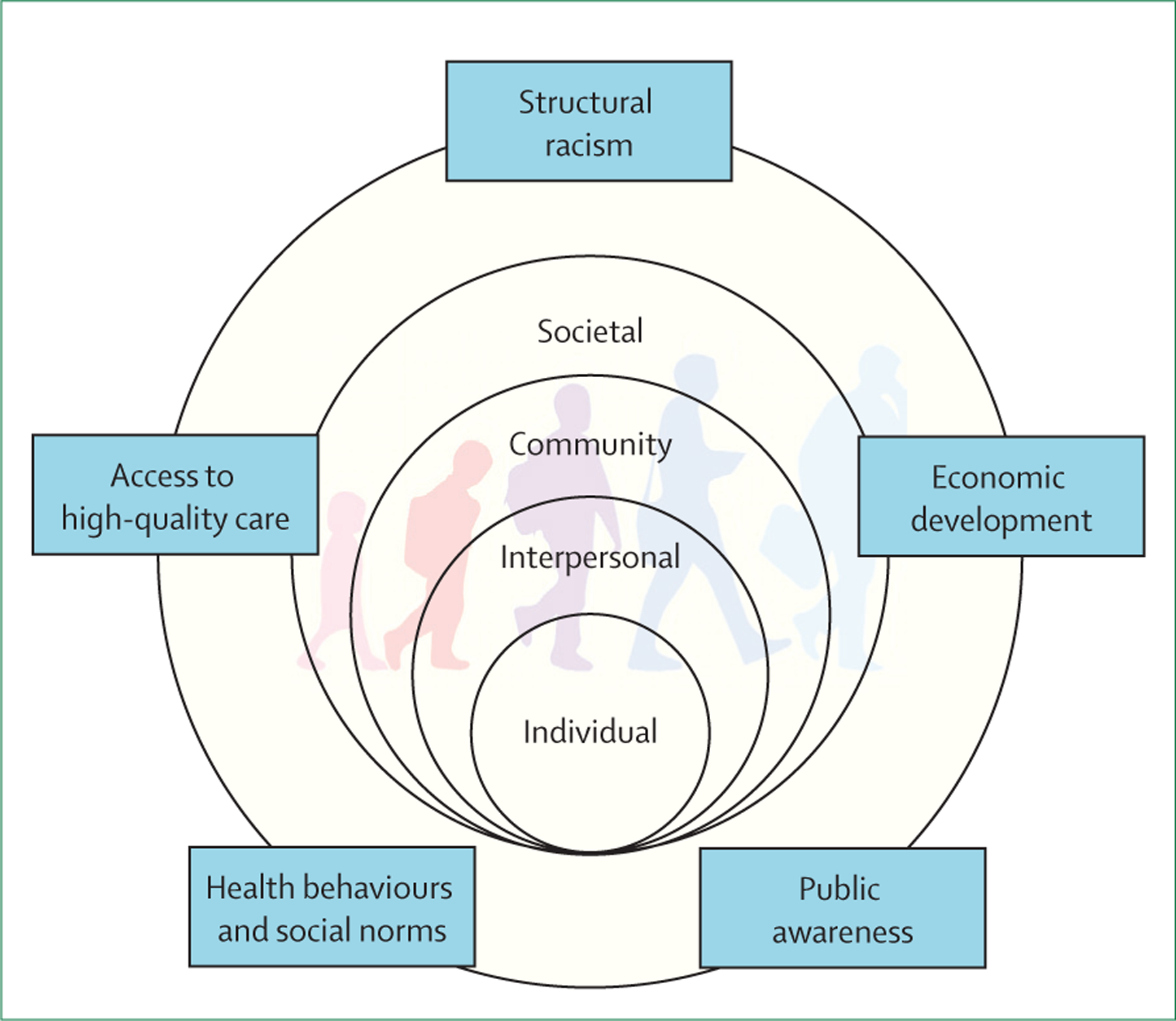
The burden of diabetes-related disparities in the USA
Differences in diabetes burden and diabetes-related complications by race or ethnicity in young people
Parallel to the growing epidemic of childhood obesity, prevalence and incidence of type 2 diabetes in children and adolescents has increased since the turn of the 21st century, with continued and persistent earlier onset of diagnosis, which has been exacerbated by the COVID-19 pandemic.15 The burden of type 1 and type 2 diabetes varies by race and ethnicity, as reported in the SEARCH for Diabetes in Youth Study (SEARCH; figure 1). Although this Series paper is focused on type 2 diabetes, type 1 diabetes continues to play an important role in diabetes in young people. We note here that data in young people are limited by racial and ethnic group differences and not within racial and ethnic groups; therefore, this topic will not be discussed in the young people section (figure 2).
Figure 2: Type 1 and type 2 diabetes incidence per 100 000 person-years (2014–15) and prevalence per 100 000 individuals (2017): results from the SEARCH for Diabetes in Youth Study12,16.
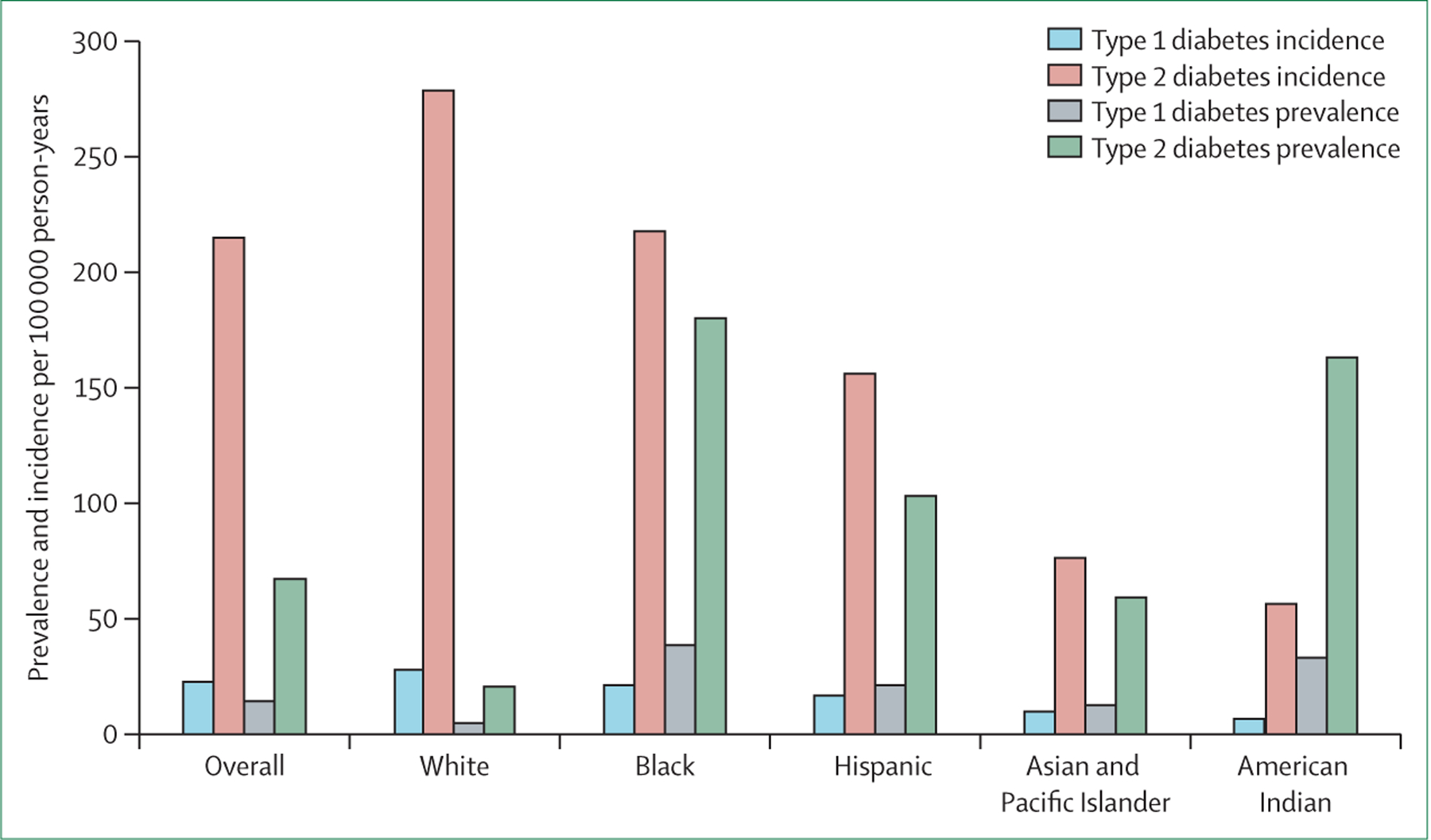
SEARCH has conducted diabetes surveillance in young people in the USA for nearly two decades. In 2001, 6379 individuals aged 0–19 years were identified with diabetes within a population of approximately 3·5 million, with Native American young people having the largest proportion (76%) of type 2 diabetes.17 Population projections from this study estimated roughly 154 000 young people aged 0–19 years were living with diabetes in the USA in 2001. Since 2001, diabetes prevalence in young people has increased among all racial and ethnic groups, with the largest increases among Asian and Pacific Islander (these groups were not separated in SEARCH), Black, and Hispanic children. Between 2009 and 2017, the estimated prevalence of diabetes in Black children increased by 7·1% (95% CI 4·8 to 9·4), compared with 7·3% (2·7 to 12·1) in Asian and Pacific Islander children, 4·8% (0·7 to 9·0) in American Indian children, 3·2% (1·4 to 5·0) in Hispanic children, and 1·4% (–1·2 to 4·1) in White children.12,16 A separate study showed a pronounced increase in diabetes among American Indian and Alaska Native young people (aged 15–19 years), with a 68% increase in cases of diagnosed type 2 diabetes from 1994 to 2004.12,18 The COVID-19 pandemic has been associated with a rise of incident paediatric diabetes cases, with one study finding incident type 2 diabetes cases decreasing moderately by 7·4% in the 2 years before the pandemic and increasing 182% during the pandemic (n=141; 1·45 cases per month; p<0·001). Type 2 diabetes cases increased by more in Black children (76·6%) than in White children (56·7%; p=0·001) and more in boys (58·9%) than in girls (40·4%; p=0·005).19 Among American Indian children and adolescents, metabolic abnormalities led to greater relative risk of diabetes in children (aged 5–11 years) compared with adolescents (aged 12–19 years).20,21 With one in five adolescents in the USA having prediabetes, the problem of diabetes among young people will probably continue to grow.22
Overall, adolescents have more rapid and complete onset of β-cell failure compared with adults, increasing urgency for the few available treatment options.23 Furthermore, the onset of diabetes-related complications in specific racial and ethnic groups is accelerated in young people. The Pediatric Diabetes Consortium Registry reported significant race and ethnic differences in HbA1c and C-peptide levels, with Black young people (aged <18 years) having higher HbA1c and lower C-peptide than White young people. Black children are significantly more likely than White children (odds ratio [OR] 4·85, 95% CI 1·49–15·77) to have poor glycaemic control (HbA1c >9·5%) at 1 year from time of diagnosis.24 Non-alcoholic fatty liver disease was diagnosed in 9% of Hispanic children and 11% of White children, compared with 2% of Black children.25 Similarly, Hispanic children have significantly elevated liver aminotransferases compared with Black children.26
Differences in diabetes burden within racial and ethnic groups
Here, we describe within-group differences in diabetes prevalence, burden, and management among Hispanic, Black, and Asian individuals. Data on differences within White, American Indian, and Pacific Islander populations are scarce. White people are often viewed as one large, homogeneous group; however, many people who identify as White in the USA might represent different and culturally distinct subgroups, such as people born within the USA and those born overseas (eg, Europe or the Middle East), or religious subgroups. Although studies exploring these subgroups are scarce, differences in diabetes prevalence by rural versus urban residency have been found.27,28 Additionally, demographic-adjusted models have shown increased diabetes risk among White populations based on residency in the so-called stroke belt of the southeastern USA (OR 1·22, 95% CI 1·09–1·36) and buckle of eastern North Carolina, South Carolina, and Georgia (1·26, 1·10–1·44), compared with residency in other parts of the USA.29
Within the Hispanic population, the national diabetes statistic report of 2020 highlights differences in age-adjusted prevalence of diagnosed diabetes among Hispanic Americans, with Cuban Americans having the lowest prevalence at 6·5%, compared with 12·4% in Puerto Rican American and 13·4% in Mexican American adults.2 These differences have been shown using different datasets as well, including the nationally representative NESARC-III study.22 The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) corroborates these findings with a population-based cohort. A longitudinal analysis from HCHS/SOL showed persistent differences in diabetes incidence within the Hispanic population with higher incidence among those with Puerto Rican and Mexican backgrounds.30,31
US-born Black individuals and non-US born Black individuals of sub-Saharan African or Caribbean descent are often analysed as one racial group (Black) in the literature, although research disaggregating these groups has shown important differences in diabetes prevalence.32 Several studies have indicated the higher prevalence of diabetes among US-born versus African-born or Caribbean-born Black adults.33,34 Ford and colleagues10 used National Health Interview Survey data to show that Black individuals born in Africa or the Caribbean had significantly lower odds of having diabetes (OR 0·75, 95% CI 0·62–0·89) compared with US-born Black individuals. Within the population of Black individuals born in Africa or the Caribbean, studies have shown little to no difference between those born in the Caribbean and those born in sub-Saharan Africa. Nevertheless, increasing time in the USA and higher levels of acculturation were associated with increased odds of diabetes diagnosis among foreign-born Black individuals.33 This increase has highlighted the importance of furthering our understanding of the differences between Black people born within and outside of the USA, as a unique opportunity to examine genetic differences, sociocultural contexts, migration experience, and population characteristics.35
Asian Americans are a large and diverse population, although they are often grouped into one large racial and ethnic group.36 South Asian Americans have higher age-adjusted prevalence of diabetes and gestational diabetes than other Asian American subgroups.37 Filipino American and Korean American adults have the next highest prevalence of diabetes among Asian Americans;38,39 prevalence of diabetes among Korean Americans was 10% in New York City and 11·9% in California,40,41 with Korean Americans having very low rates of physical activity compared with other Asian subgroups.42,43 Of all Asian American subgroups, Chinese Americans continue to have the lowest rates of diabetes.44
Factoring in within-group differences will allow us to better understand trends in diabetes prevalence epidemiologically. We can better target resources and interventions if we look at subgroups within racial and ethnic groups whose rising rates might be otherwise masked. These described differences within racial and ethnic groups are crucial considerations when designing interventions for diabetes prevention or management.
Disparities in diabetes complications
Disparities in diabetes prevalence are compounded by disparities in diabetes-related complications. Although disparities in diabetes-related complications in young people have been described, there are scarce data on differences in complications within racial and ethnic groups. Diabetes-related microvascular complications include chronic kidney disease, retinopathy, and amputations. Among US adults with diabetes, 39·2% were reported to have chronic kidney disease (stages 1–4). This prevalence was higher among Black adults, with 23·1% having chronic kidney disease stage 3–4, compared with 17·2% of White adults and 8·9% of Hispanic adults with diabetes.45 Diabetes is now the leading cause of new cases of blindness among adults in the USA.46 Diabetic retinopathy is more prevalent in Black individuals (38·8%) than in Hispanic individuals (31·0%) and White individuals (26·4%). Black and Hispanic individuals with diabetic retinopathy are much more likely to progress to visual impairment and blindness than White individuals.46–48 Odds for major amputation were significantly greater in Black (OR 1·44, 95% CI 1·36–1·53), Hispanic (1·33, 1·25–1·41), and Native American (1·47, 1·2–1·8) patients with diabetic foot infections than in White patients.49 Asian patients had a 64% lower rate of major amputation in cases of diabetic foot infections compared with White patients.50
Cardiovascular and cerebrovascular disease are the major macrovascular complications of diabetes. To prevent these complications, individuals with diabetes should meet the recommended ABC metrics for cardiovascular disease risk reduction—ie, HbA1c <7%, blood pressure <130/80 mm Hg, LDL cholesterol <100 mg/dL. Multiple studies have shown that Black Americans and Mexican Americans are less likely to meet all three ABC goals than White individuals.51 Cardiovascular disease is the leading cause of mortality among people with diabetes in the USA.52 Although overall cardiovascular death in the USA decreased significantly between 1988 and 2015, gaps in mortality between racial and ethnic groups have persisted.52
Disparities in diabetes management
Disparities between racial and ethnic groups for diabetes management have received little attention in the past; however, there are important differences between these groups—eg, geographical location (ie, rural and urban), income, and insurance status. Disparities can be seen at the level of diabetes screening as well as management. Large disparities exist in the diagnosis of diabetes with all racial and minority ethnic populations (eg, Black, Hispanic, and Asian) being more likely to have undiagnosed diabetes than White individuals in the USA.53 For screening of diabetes-related complications in the USA, only 33–68% of adults with diabetes obtain an annual dilated eye exam; however, these screening rates are even lower in Black individuals and Latino individuals.48 A 2008 qualitative review of various minority groups, including Black, American Indian, and Latino, reported substantially decreased rates of eye examinations in these populations.54 New modalities for efficient screening using artificial intelligence, machine learning, and telehealth have the potential to increase access to retinopathy screening for minority populations; however, data on their reach and uptake are not yet available. Similarly, data on reach and uptake of new modalities to treat diabetic retinopathy including intravitreal anti-VEGF injections, which present opportunities to prevent blindness, are not yet available.
Diabetes management also refers to medication and equipment that is prescribed. A 2021 review looked at access to GLP-1 receptor agonists and found that Asian, Black, and Hispanic individuals and those with low incomes were less likely to receive treatment with GLP-1 receptor agonists than White individuals and those with better incomes.55 This disparity is important to recognise given the known cardiovascular benefits of GLP-1 receptor agonists. Further disparity can be seen in access to new technology. Data show that for continuous glucose monitoring (CGM), for example, Black individuals have the lowest rates of access across age groups, health insurance coverage, and location.56,57
Pathophysiology of diabetes and diabetes disparities
Increased diabetes risk at low BMI in minority race and ethnic populations
Overweight and obesity are well established risk factors for diabetes. However, evidence suggests that the relationship between bodyweight and diabetes is not linear, and that diabetes at low BMI is considerably more prevalent in minority race and ethnic populations.58 A study from 2022 of diabetes prevalence in US Asian adults by weight found an increased burden of diabetes risk among south Asian Americans and Filipino Americans who had a BMI within a healthy range compared with White Americans and Chinese Americans.59 Additionally, a study examining the prevalence of diabetes by BMI status and place of birth found that the prevalence of diabetes in immigrants with a healthy weight from India and Africa was higher than the prevalence of diabetes in immigrants with obesity from Europe.60 A 2019 study examining racial and ethnic disparities in the prevalence of diabetes by BMI category in six racial and ethnic groups in the USA found that in those with a healthy weight, the prevalence of diabetes was 5·0% in White participants, 10·1% in Asian participants, 9.6% in American Indian and Alaskan Native participants, 13·0% in Hispanic participants, 13·5% in Black participants, and 18·0% in Hawaiian and Pacific Islander participants.61 Furthermore, when examining the relative risks for diabetes for each BMI category across all racial and ethnic groups, White participants showed the greatest sensitivity to changes in BMI followed by Asian, American Indian and Alaskan Native, Hispanic, Hawaiian and Pacific Islander, and Black participants.61 These results do not indicate greater sensitivity to excess adiposity in non-White populations, rather they suggest that factors other than BMI are driving the disproportionate burden of diabetes in non-White populations, both in the USA and globally. Additionally, these data have implications for screening and prevention. Elevated BMI should not necessarily be the first criterion to consider when screening for diabetes in minority race and ethnic populations as it could result in a substantial number of at-risk individuals being missed. Furthermore, prevention strategies that target weight loss could be less effective in minority racial and ethnic groups when diabetes in individuals with a healthy weight is prevalent.
Differences in the contributions of insulin secretion by racial and ethnic group
Traditionally, diabetes pathogenesis has been described as obesity-driven insulin resistance followed by a subsequent decline in insulin secretion, eventually leading to overt type 2 diabetes.62 However, evidence suggests that some racial and ethnic groups might also exhibit early declines in insulin secretion, which can contribute to excess diabetes risk in the absence of obesity. A study comparing the prevalence and associated risk factors of diabetes in four racial and ethnic groups in the USA found that south Asian Americans, Black Americans, Hispanic Americans, and Chinese Americans all had a diabetes prevalence that was at least two-times, if not three-times, higher than that of White Americans.37 Furthermore, these same groups all had worse insulin secretion than the White Americans. Additionally, a 2004 study by Torréns and colleagues63 assessed differences in insulin sensitivity and β-cell function between non-diabetic premenopausal or early perimenopausal non-Hispanic White women and African American, Chinese American, Japanese American, and non-Mexican-American Latino women. Torréns and colleagues found that Japanese American and Chinese American women had worse insulin secretion than non-Hispanic White women, whereas African American women had higher insulin secretion than non-Hispanic White women, and insulin secretion was similar between non-Mexican-American Latino women and non-Hispanic White women, after correcting for confounders.63
The role of ectopic fat in diabetes development
Although obesity is a well known risk factor in diabetes pathogenesis, the pattern and placement of fat deposition is also important as several organs including the muscle, liver, and pancreas are all highly involved in glucose metabolism.62 Notably, hepatic fat content, independent of BMI has been associated with metabolic disturbances even in individuals with a healthy weight and normoglycaemia.64,65 Furthermore, pancreatic fat content has been associated with poor insulin secretion in individuals without diabetes.66,67 Therefore, increased fat deposition in the liver and pancreas could lead to early metabolic disturbances and subsequent diabetes risk. Studies have pointed to large differences in ectopic fat deposition by racial and ethnic group.68,69 There has not been an exploration of how these differences change across the lifespan and if there are any differences within racial and ethnic groups.
The aforementioned studies related to ectopic fat suggest that increased susceptibility to diabetes in some racial and ethnic groups such as Asian American individuals could be associated with ectopic fat deposition contributing to increased insulin resistance and worse insulin secretion. However, in other groups such as Black Americans, the increased risk of diabetes persists despite normal rates of ectopic, particularly hepatic, fat deposition. The combined contributions of hepatic and pancreatic fat to insulin resistance and insulin secretion in various ethnic groups has not been well studied, and could provide target mechanisms for treatment and prevention across populations.
Diabetes subgroups and race and ethnic risk factor burden
Type 2 diabetes risk is multifaceted and there is a need to better classify the heterogeneity in diabetes development as there might be differences in underlying disease aetiology and progression, particularly among different race and ethnic populations. Studies categorising diabetes subgroups have found variations in the distribution of cohort membership by racial and ethnic group. For example, a study seeking to characterise potential diabetes subgroups among race and ethnic populations in the USA identified five distinct subgroups—ie, older age at diabetes onset (mean age 66·8 years [SD 8·3]), severe hyperglycaemia (mean HbA1c 8·3% [1·8]), severe obesity (mean BMI 35·4 kg/m² [6·2]), younger age (mean age 23·8 years [7·7]) at diabetes onset, and insulin use.70 In 2022, a systematic review of 18 studies characterising novel diabetes clusters based on clinical variables found that in Asian populations, the cluster of severe insulin deficiency was more common than severe insulin resistance whereas the cluster of severe insulin resistance was more common in populations with European ancestry.71 Given the differences in diabetes pathophysiology among ethnic groups, there is a need for more targeted diagnosis, prevention, and treatment, as methods traditionally applied to one population might not be applicable to all.
The role of polygenic scores
During the past decade, there have been substantial advances in our understanding of the genetic basis for type 2 diabetes.72 Identified individual genetic variants can only provide a modest effect on risk, but when combined into a polygenic risk score, they provide information on disease predisposition and could be useful for guiding clinical management. These polygenic risk scores are determined using ever-growing genome-wide association studies. Polygenic risk scores have been used to identify subtypes of diabetes, predict risk for type 2 diabetes, and predict effectiveness of interventions. In a diabetes prevention programme post-hoc analysis, polygenic risk scores identified subgroups at high risk for whom successful lifestyle modification was associated with an improved absolute reduction in the risk of incident diabetes.73 As polygenic risk scores become more widely available, it is important to remember that genetics represent only one contributor to individual disease risk and profile.73 Additionally, there is a need to ensure that the benefits of these risk scores are equitably available to all. Equitable access to polygenic risk scores is especially important as much of the genome-wide association studies and sequence data are derived from individuals of European descent. As such, there is an urgency to build a repository for other ethnic groups as well.
Contributors to diabetes disparities in the USA
The factors within domains and across levels of influence that could contribute to diabetes disparities are summarised in the table.
Contributors to diabetes disparities between racial and ethnic groups
The complex interplay of factors across all five domains and all levels of influence contribute to disparities between racial and ethnic groups. Structural racism play a prominent role at all levels. Past policies, laws, and economies contribute to where people live, their access to sufficient and healthy food, and their access to health-care services.106,110,111,113,115 For example, redlining and predatory lending affected where and what type of housing was available to Black individuals, forcing them to live in less healthy and worse resourced neighbourhoods compared with other racial and ethnic groups. Furthermore, these same areas received fewer government and private sector investments, leading to fewer facilities, such as health facilities and food access points.161,162 These factors, stemming largely from structural racism and discrimination, play an important role in driving racial and ethnic inequities in diabetes and diabetes-related complications.163 At the community level, neighbourhoods with primarily Black and Hispanic individuals tend to have little space for physical activity, more food deserts (ie, neighbourhoods of 5000–15 000 people served by two or fewer big supermarkets), and restricted access to health care compared with other neighbourhoods. These factors have been linked to physical inactivity, poor diet quality, and reduced use of health-care services, which in turn have been found to contribute to the development of diabetes and poor diabetes management.112 Additionally, neighbourhoods with Black and Hispanic individuals have higher levels of toxic environmental exposures than other neighbourhoods, and a growing body of evidence indicates that these exposures are associated with the development of diabetes.112 The interpersonal level reflects relationships between individuals in a family, within a social network, or with health-care professionals. The many forms of interpersonal racism and discrimination can play an important role in health disparities including in those with diabetes. For example, perceived racism has been associated with increased incidence of diabetes in Black women.152 This association is hypothesised to be through biological mechanisms including increase in chronic stress. These interpersonal relationships in turn affect the gut microbiome, lifestyle behaviours, mental health, and access to care.156 The determinants are all inter-related and overlapping. The factors within the structural racism domain overlap with the economic development domain and the access to high-quality care domain as outlined.
Native Americans have the highest age-adjusted prevalence of diabetes compared with all other racial and ethnic groups in the USA. Native Americans have 2·0 times higher diabetes prevalence and 1·9 times higher diabetes-related mortality than White Americans.164 The disproportionate prevalence and disease burden of diabetes has been associated with factors in the economic development domain with social disadvantages faced by American Indian and other Native American communities, including high rates of neighbourhood poverty, inadequate access to health services, and long-standing economic adversity. These factors in turn are related to the health behaviours and social norms domain, where they facilitate unhealthy day-to-day living practices that ultimately contribute to disease, and subsequently hinder optimal management of chronic illnesses. The structural racism domain is also relevant in this case as discrimination has been associated with increased prevalence of diabetes among American Indians and other Native Americans. Some findings have shown that microaggressions experienced by American Indians and other Native Americans with diabetes in health-care settings were associated with greater reports of worse physical and mental health, including increased hospitalisations and depressive symptoms within the previous year.156
Although pathophysiology of diabetes is important to consider, and there might be a role of genome-wide association studies, it is important to recognise that biological factors are not a cause of disparities. There is a growing body of evidence on biological and epigenetic differences that contribute to the risk of developing diabetes.165 However, race is a socially constructed variable, not a biologically constructed one.166 Existing literature suggests that acknowledging the admixture of multiple ethnic groups is crucial in efforts to further define any genetic predisposition to diabetes.167 Admixture is also an important consideration when looking at the performance of diagnostic tests such as HbA1c in different racial and ethnic groups.168,169
Contributors to diabetes disparities within racial and ethnic groups
Using the modified framework proposed by Agarwal and colleagues,14 we can see that factors at the individual, interpersonal, community, and societal levels together contribute to differences within racial and ethnic groups (table). The health behaviours and social norms domain plays an important role as a contributor to diabetes disparities. Diversity within racial and ethnic groups in cultural practices, knowledge, and beliefs can contribute to differences in dietary and lifestyle habits that in turn can drive within racial and ethnic group differences.170 Cultural practices, knowledge, and beliefs can also contribute to different relationships with the health-care profession, which might lead to different levels of trust and adherence to medical advice.132–135 Economic development is also important. Previous studies have consistently found that people with lower socioeconomic status are more likely to develop diabetes and experience more diabetes-related complications compared with those with higher socioeconomic status.98 There is also a rural and urban divide that can help explain disparities within groups given rural counties have the highest diabetes-related mortality.171 Examining trends in diabetes-related mortality in urban and rural areas in the USA between 1999 and 2018, Kobo and colleagues found that diabetes-related age-adjusted mortality rates of both Black and White adults decreased in urban but not rural areas.172 The socioeconomic and built environment can also contribute to disparities within racial and ethnic groups. In 2014, Gaskin and colleagues described the “nexus of race, poverty, and place” and showed that both individual poverty and living in a poor neighbourhood increased the odds of having diabetes for Black and White adults.173 In 2019, a systematic review and meta-analysis of longitudinal studies found strong evidence for relationships between neighbourhood walkability and diabetes outcomes.174 Results from an analysis of nationally representative county-level data showed that counties with higher rates of unemployment and poverty had higher incidence rates of diabetes compared with counties with lower levels of unemployment and poverty, whereas counties with greater access to healthy food and more opportunities for exercise had fewer cases of diabetes.4
Table: Contributors (ie, levels of influence and domains of influence over the life course) to diabetes using Agarwal and colleagues’ framework14.
| Individual | Interpersonal | Community | Societal | |
|---|---|---|---|---|
| Health behaviours and social norms | Health behaviours and coping strategies: smoking and sedentary lifestyle; sociodemographic; cultural identity; response to discrimination; cultural beliefs;* stigma;* acculturation; body image perceptions;* poor English proficiency;* and comorbid health conditions:74 depression,*75 hypertension,* heart disease,* and autoimmune conditions* | Family functioning: parental modelling;† and caregiver–child interaction: gestational diabetes,†76–78 intrauterine exposure to diabetes,†21,79 and preterm birth†80,81 | Community functioning: food eating practices of a community (eg, type of food and communal eating),82,83 acculturation,84 fear of Western medical practices, scarcity of preventive health seeking behaviour,85 social dietary temptations and overcoming temptations,84 apathy (ie, the inevitability of diabetes in the community),86,87 and weight perception88 | Policies and laws: reducing stress exposure via policy,89 association between food prices and insulin resistance,90 mandating diabetes education through policy,91 social norms, societal structural discrimination, smoking as a norm in American Indian and other Native American communities,†92 machismo (ie, masculine ideologies) as driver of health neglect in Mexican men,*93 cultural traditions as a challenge to immigrants managing chronic illness,*94 effect of colonisation on diabetes risk factors in Indigenous communities,95 and education discrimination*96 |
| Economic development | Personal environment:97 income, employment status and job stability, and education level | Household environment: household food insecurity,98 homelessness,*99 housing instability,*100 and energy insecurity;*101 school or work environment; and stress at work102 | Community environment,98 neighbourhood walkability,*103 exposure to greenspace,†104 community resources, poor access to affordable diabetes-friendly food compared with relatively easy access to unhealthy food,105–107 and area deprivation index (housing, income, employment, education)108 | Societal structure: neighbourhood discrimination,106,109 neighbourhood disadvantage contributes to disproportionate diabetes burden,110–112 and food insecurity113–115 |
| Public awareness | Diabetes knowledge and distress,116 diabetes-related knowledge,* and diabetes self-management knowledge* | Social networks, parental and familial modelling,†117 social support;118 family and peer norms and traditional gender roles:†118 familial social interactions;†118 and social norms*119,120 | Community norms; local structural discrimination; diabetes-related social stigma;*121,122 and young adults: community expectations of self-independence, employment, and care of siblings123 | .. |
| Access to high-quality care | Insurance coverage, health literacy, and treatment preferences; regional variation;*124 health literacy and numeracy;*125,126 fear of medications and insulin medication adherence;*127 and cost*128 | Patient–clinician relationship: perceived discrimination,129 communication,*130–133 lack of trust,*132,134,135 physicians’ empathy,*136 medical decision-making, not enough shared decision-making,*132,137 non-adherence labelling,*138,139 discriminatory health-care practices,140,141 and variations in phenotypes not targeted†70,142 | Health-care access, subspecialty care (endocrinologist),*143 safetynet clinics and hospitals,* mental health services,* diabetes self-management education,*144 and transition services for young adults from the paediatrician to adult provider*123 | Health-care affordability and quality, differential access to technology for diabetes management (including telehealth and continous glucose monitoring),145 differential access to new anti-diabetic medication,146 and differential access to subspecialty care147 |
| Structural racism | Sociodemographic, cultural identity, response to discrimination, stigma,* acculturation, poor English proficiency,* chronic stress,148 poor sleep, and obesity149 | Interpersonal discrimination, major experiences of discrimination,†150,151 perceived everyday racism,†152 lifetime racism,†152 racial discrimination,*153–155 and microaggressions*156 | Community environment,98 neighbourhood walkability,*103 exposure to greenspace,†104 community resources, restricted access to affordable diabetes-friendly food compared with relatively easy access to unhealthy food,105–107 area deprivation index (housing, income, employment, education),108 and environmental exposures: air pollution,†157 heat exposure,*158 and endocrine-disrupting chemicals†159 | Societal structure: neighbourhood discrimination,106,109 neighbourhood disadvantage contributes to disproportionate diabetes burden,110–112 and food insecurity;113–115 and exposure: climate-induced extreme weather events (heat, cold, natural disasters);160 and sanitation |
Disparities in diabetes management and control only.
Disparities in diabetes incidence only.
The association between social determinants of health and diabetes differs by ethnicity within the Black population. For example, the association of health insurance, income, and education with diabetes was different among African Americans, African immigrants, and African Caribbeans.34 The differences described within the Hispanic population are most likely multifactorial but are still not fully understood.30 Finally, within the Asian American population, we described how south Asians have the highest prevalence of diabetes. The causes of this are multifactorial and are likely due to not only patterns of immigration and sociodemographic characteristics, but also might be related to distinct cardiometabolic phenotypes that increase the risk of diabetes among south Asians.175
Contributors to diabetes disparities among children and adolescents
Risk factors for type 2 diabetes in children and adolescents (aged <18 years) are similar to those of adults. Studies have found that intergenerational factors, malnutrition, little physical exercise, obesity, family history, and low or very high birthweight elevate diabetes risk in children.176 Poor sleep has also been linked to diabetes risk among both adults and children, with one multiethnic study in the UK finding that children aged 9–10 years had reduced risk of type 2 diabetes for every additional hour of sleep duration.177
Obesity and insulin resistance in children portends an increased risk of developing diabetes. There is now good evidence to show that stress, obesity, and hyperglycaemia in lactating mothers is associated with increased rates of obesity and insulin resistance in their offspring.178 Additionally, overnutrition as a consequence of an infant being born small for gestational age, as well as early weaning from breastmilk are associated with obesity and insulin resistance.178 Lastly, on the other end of the spectrum, a combination of early-life undernutrition and later development of obesity is associated with increased risk of type 2 diabetes in adulthood.179
Economic development also has a role for racial and ethnic disparities in young people. For example, lower socioeconomic status and living in poor areas are associated with higher diabetes risk, a 34% increased likelihood of developing obesity, and a 60% increased likelihood of developing diabetes, when compared with living in high income areas.180,181 Additionally, compared with White women, Asian and Hispanic women have a higher prevalence of gestational diabetes, which has been associated with increased risk in offspring of developing type 2 diabetes later in life.182,183
Diabetes disparities are now evident across the lifespan with the emerging concept of intergenerational risk of developing diabetes.184 Young people who identify as Native American, American Indian, or Alaska Native; Black; and Hispanic have an increased burden of diabetes and obesity. This burden is in part due to social determinants of health within the structural racism domain that are “systemic, population-based, cyclical, and intergenerational.”185 These same social determinants of health are rooted in racism that is internalised, or at the interpersonal, institutional, and societal levels. These factors are experienced by the family (including children) and not only the adult. Additionally, intergenerational poverty makes it harder for children to find a way out of the cycle of intergenerational risk. Although socioeconomic status is complex and multidimensional, all indicators are strongly patterned by racial and ethnic group: “for every dollar of wealth that Whites have, Asian households have 83 cents, Black households have 6 cents, and Hispanic households have 7 cents”.186
Recommendations and future directions
We have provided an overview of the literature from the past decade on disparities in diabetes between and within racial and ethnic groups in the USA across the lifespan. This overview provides a US-focused framing that adds to the other two papers in this Series14,187 and The Lancet Commission on Diabetes.188 This Series paper highlights the importance of considering the complexity of disparities in diabetes and diabetes-related complications beyond racial and ethnic groups to consider within-group differences, how disparities differ in children and adolescents, and the complex pathophysiology. Using a comprehensive framework, this Series paper also emphasises that these disparities arise from many multilevel factors. With these key points in mind, we propose a path forward for providers, researchers, funders, and policy makers to address diabetes disparities (figure 2, panel).
Generate a community-engaged and multidisciplinary research agenda that adopts a life course approach and addresses pathophysiological and multilevel causes of diabetes disparities
This Series paper has thus far highlighted the complexity and multifaceted nature of diabetes disparities. Solutions must, therefore, meet this complexity by ensuring a multidisciplinary approach that carefully engages affected communities in an equitable manner. Increased attention to more detailed diabetes phenotypic structures (see previous sections) is important to guide tailored prevention and management strategies. Understanding diabetes phenotypes and the resulting tailored management strategies can provide the health-care community with the needed information to move towards effective and equitable precision medicine in diabetes care. As we give the pathophysiology of diabetes more attention, researchers should be sure not to confuse social constructs such as race with biological factors such as genetics.189 As we promote ideas around precision medicine for diabetes care, it is crucial to curb any misconceptions of this idea and ensure emergent technologies and advancements overcome current inequities in access. Community-engaged and community-based participatory research is essential for designing acceptable and feasible strategies of promoting uptake of diabetes treatment interventions among disadvantaged groups. These strategies will need to be tailored to groups across the lifespan (strategies related to young people [aged <18 years] will differ from those targeting older people [aged >65 years]). These strategies will also need to be tailored across and within racial and ethnic groups given much of the social and behavioural norms that differ between these groups. To do this well, research needs to continue to disaggregate the large groupings of Black, Asian, and Hispanic individuals.
As we see the growth of the fields of environmental justice, climate, and health, we must pay attention to their effects on diabetes prevalence, management, and thereby disparities. We need more community-engaged research to document the attribution of environmental factors on diabetes risk and management. Moreover, we need community-engaged research to co-design strategies with communities to mitigate the effects of environmental hazardous exposures and climate change on health. Researchers need to apply a health equity lens to ensure that novel therapeutics and diagnostics are implemented without worsening disparities.
The field of implementation science increasingly offers approaches to integrating health equity into intervention design and implementation and should be embraced along with equitable partner and community engagement as the field moves to develop, implement, and scale evidence-based interventions to reduce diabetes disparities.190,191
Principles of equitable, sustainable, and cost-effective research that addresses the multilevel and multidisciplinary behavioural, social, and structural determinants of diabetes disparities must be embraced by the funding community
Funders of community or clinic-based diabetes research should require a health equity plan for all phases of diabetes research—ie, a statement on how the research will promote health equity or at the very least not increase disparities. To achieve this goal, principles of implementation science should be considered even at the effectiveness and clinical trial stages of research to ensure an understanding of how to promote equity as the research progresses. Practically speaking this means evaluating key implementation outcomes such as reach, acceptability, appropriateness, feasibility, and adoption early on. Studying implementation outcomes enables investigators to start to identify early on potential barriers for equitable implementation in the real world. Defining implementation outcomes also provides an opportunity to tailor implementation strategies to age groups and differences within racial and ethnic groups.
Upon review of the multilevel causes of diabetes disparities across the lifespan, it becomes apparent that multilevel and multidisciplinary approaches are crucial to solving the problem of diabetes disparities. Funders should recognise this importance and provide opportunities for multidisciplinary teams to address this need in collaboration with communities. To promote community-based participatory research that addresses these multilevel factors, funders should invest in strengthening capacity among early stage investigators in the intersection of diabetes and health equity with a focus on trainees from under-represented groups. Moreover, given the value of implementation science in improving equity in health service delivery, training, and funding for early-stage investigators to conduct health-equity focused implementation science research could accelerate the implementation and dissemination of evidence-based interventions in routine practice.189 Furthermore, there is a need to provide training opportunities for more experienced scientists to embrace and incorporate principles of community engagement and health equity into existing research programmes.
Practitioners on the front lines of diabetes prevention and management need to know and understand the multilevel factors contributing to diabetes disparities including those at the provider, community, and health-system levels
Front-line providers play an important role in addressing disparities through their one-on-one interaction with patients, through institutional change, and through community engagement. At an individual level, the role of health-care providers’ implicit bias has long been studied and the effect of training on reducing disparities examined.192 Continued attention to implicit bias with focus on understanding gaps in translational effectiveness is important.193 At an institutional level, front-line practitioners have a role in addressing structural barriers to access to care for their patients. It is crucial to develop evidence-based approaches to address structural barriers to health-care access such as inadequacies of language services, variable dependence on technology, transportation challenges, and financial constraints. Lastly, engaging with community members to actively understand the challenges faced by patients and develop equitable solutions is essential.194–196 We need participatory action research engaging community partners in prioritising and developing approaches for overcoming these health system barriers. This participatory action research provides an opportunity for health providers to understand differences across the lifespan as well as tailoring approaches to within group differences.
In their approach to counselling and management of patients, health providers must learn to recognise differences in phenotypic presentation of diabetes to tailor therapy. Recognising the structural and environmental factors that contribute to diabetes and poor management of diabetes in some communities is imperative as we design therapies, set policy, and provide advice. Training providers on the role of social and environmental determinants of health is crucial to ensuring future practitioners overcome their own and their institution’s contribution to health disparities.
Policy makers must recognise their role in health disparities both based on historical policy decisions as well as current ones to make necessary policy changes to reduce disparities
In this Series paper, we summarised the various ways in which policy decisions now (eg, scarcity of policies to drive investment in disadvantaged neighbourhoods) and in the past (eg, redlining), differentially affect the health of minority race and ethnic communities and, therefore, drive health disparities. Addressing social determinants of health holds great potential for progress towards reducing diabetes disparities.98,197 As health-care quality improvement efforts to address diabetes care advance, there is a need for rigorous studies to design and test delivery models that integrate social care into medical care; for example, through screening of individuals’ social risks and assets and strategies to address or mitigate the adverse effects of social adversity on diabetes management.198 In addition to addressing social determinants of diabetes within the health-care system, there are opportunities to fill gaps in evidence on the effectiveness of large-scale policies and programmes such as food and agricultural policies and programmes in promoting healthy eating and physical activity and thereby contributing to the prevention of diabetes and diabetes-related complications, including their effect on diabetes disparities.199
Recognising the role of institutional racism on existing policies is crucial to revising and developing evidence-based solutions to policy change that can overcome these barriers to care. Further research is needed to inform policies that can address some of the structural and social determinants of health that are the root causes of health disparities, both between and within racial and ethnic groups.200 To achieve this goal, policy and programme monitoring should integrate health equity indicators so that the differential effect of policies can be documented and thus the impact of policy change reported on health disparities. This type of attention would help promote accountability and generate data needed to inform change.
Conclusions
Disparities in the prevalence and management of diabetes in the USA persist today due to multilevel and multidisciplinary factors from the individual level to the systems level. Disparities should be examined beyond differences between racial and ethnic groups to include differences within race and ethnic groups and across the lifespan. Our Review systematically outlines those differences and their drivers and offers actionable recommendations at the researcher, practitioner and health-system, and policy levels to promote health equity in diabetes moving forward.
Supplementary Material
Panel: Recommendations for addressing disparities in diabetes.
Generate a community-engaged and multidisciplinary research agenda that adopts a life course approach and addresses pathophysiological and multilevel causes of diabetes disparities
Community-based participatory research to guide precision medicine approaches
Incorporation of environmental and climate justice principles
Equitable partnerships
Principles of equitable, sustainable, and cost-effective research that addresses the multilevel and multidisciplinary behavioural, social, and structural determinants of diabetes disparities must be embraced by the funding community
Funders should require a health equity plan
Multilevel and multidisciplinary approaches
Strengthening capacity among diverse early-stage investigators
Practitioners on the front lines of diabetes prevention and management need to know and understand the multilevel factors contributing to diabetes disparities including those at the provider, community, and health-system levels
Awareness of implicit bias
Addressing barriers to accessing quality health care
Tailoring therapy to diabetes phenotypes
Policy makers must recognise their role in health disparities both based on historical policy decisions and current ones to make necessary policy changes to reduce disparities
Models of care that integrate social care into medical care
Policies to address social determinants of health within the health-care system
Addressing institutional racism
Search strategy and selection criteria.
We identified references for this Series paper through searches of PubMed for articles published between Jan 1, 2012, and Jan 31, 2022, to reflect the past decade of innovation and discovery in the diabetes disparities literature. Additional articles published before Jan 1, 2012, and after Jan 31, 2022, were included when relevant and important to this Series paper. Our search was designed to identify relevant literature across three main themes: diabetes disparities across the lifespan, diabetes disparities within racial and ethnic groups, and diabetes phenotypes. As this is a narrative review, our objective was to use this search to provide key articles that together could yield a more nuanced understanding of diabetes disparities. A full description of our methods can be found in the appendix.
Acknowledgments
We would like to thank Sara Hendrix and Samaia Hill for their assistance with the literature search. We would like to acknowledge the following funders: SH was supported by a grant from National Institutes of Health (NIH) and National Heart, Lung, and Blood Institute (K23HL152368). RCQ was supported by grants from NIH (1U01DK132737, P30DK111024-06W2, and P30DK111024). KMVN was also supported by a grant from NIH (P30DK111024). MKS is supported in part by the National Institute on Minority Health and Health Disparities (K23 MD015088-04), the Emory School of Medicine Doris Duke Charitable Foundation COVID-19 Fund to Retain Clinical Scientists, and the Georgia Clinical & Translational Science Alliance (UL1-TR002378).
Footnotes
This is the third in a Series of three papers about Global Inequity in Diabetes (papers 1 and 2 appear in The Lancet). All papers in the Series are available at https://www.thelancet.com/series/global-inequity-diabetes
Declaration of interests
We declare no competing interests.
Contributor Information
Saria Hassan, Department of Medicine, Emory Global Diabetes Research Center, Emory University, Atlanta, GA, USA; Hubert Department of Global Health, Rollins School of Public Health, Atlanta, GA, USA.
Unjali P Gujral, Emory Global Diabetes Research Center, Emory University, Atlanta, GA, USA; Hubert Department of Global Health, Rollins School of Public Health, Atlanta, GA, USA.
Prof Rakale C Quarells, Emory Global Diabetes Research Center, Emory University, Atlanta, GA, USA; Department of Community Health and Preventive Medicine, Morehouse School of Medicine, Atlanta, GA, USA.
Elizabeth C Rhodes, Emory Global Diabetes Research Center, Emory University, Atlanta, GA, USA; Hubert Department of Global Health, Rollins School of Public Health, Atlanta, GA, USA.
Megha K Shah, Department of Family and Preventive Medicine, Emory Global Diabetes Research Center, Emory University, Atlanta, GA, USA.
Jane Obi, Emory School of Medicine, and the Nutrition and Health Sciences Doctoral Program, Laney Graduate School, Emory Global Diabetes Research Center, Emory University, Atlanta, GA, USA.
Wei-Hsuan Lee, Department of Medicine, Emory University, Atlanta, GA, USA.
Luwi Shamambo, Department of Medicine, Emory University, Atlanta, GA, USA.
Mary Beth Weber, Emory School of Medicine, and the Nutrition and Health Sciences Doctoral Program, Laney Graduate School, Emory Global Diabetes Research Center, Emory University, Atlanta, GA, USA; Hubert Department of Global Health, Rollins School of Public Health, Atlanta, GA, USA.
Prof K M Venkat Narayan, Department of Medicine, Emory School of Medicine, and the Nutrition and Health Sciences Doctoral Program, Laney Graduate School, Emory Global Diabetes Research Center, Emory University, Atlanta, GA, USA; Hubert Department of Global Health, Rollins School of Public Health, Atlanta, GA, USA.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 10th edn. 2021. https://diabetesatlas.org/atlas/tenth-edition/ (accessed Oct 11, 2022). [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National diabetes statistics report. June 29, 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed Oct 11, 2022). [Google Scholar]
- 3.Centers for Disease Control and Prevention. National and state diabetes trends. May 17, 2022. https://www.cdc.gov/diabetes/library/reports/reportcard/national-state-diabetes-trends.html (accessed Oct 11, 2022). [Google Scholar]
- 4.Cunningham SA, Patel SA, Beckles GL, et al. County-level contextual factors associated with diabetes incidence in the United States. Ann Epidemiol 2018; 28: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorpe KE, Ogden LL, Galactionova K. Chronic conditions account for rise in Medicare spending from 1987 to 2006. Health Aff (Millwood) 2010; 29: 718–24. [DOI] [PubMed] [Google Scholar]
- 6.Cheng YJ, Kanaya AM, Araneta MRG, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011–2016. JAMA 2019; 322: 2389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999–2018. JAMA 2021; 326: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezzatvar Y, Ramírez-Vélez R, Izquierdo M, García-Hermoso A. Racial differences in all-cause mortality and future complications among people with diabetes: a systematic review and meta-analysis of data from more than 2·4 million individuals. Diabetologia 2021; 64: 2389–401. [DOI] [PubMed] [Google Scholar]
- 9.Elhussein A, Anderson A, Bancks MP, et al. Racial/ethnic and socioeconomic disparities in the use of newer diabetes medications in the Look AHEAD study. Lancet Reg Health Am 2022; 6: 100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ND, Narayan KM, Mehta NK. Diabetes among US- and foreign-born blacks in the USA. Ethn Health 2016; 21: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pabon-Nau LP, Cohen A, Meigs JB, Grant RW. Hypertension and diabetes prevalence among U.S. Hispanics by country of origin: the National Health Interview Survey 2000–2005. J Gen Intern Med 2010; 25: 847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence JM, Divers J, Isom S, et al. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001–2017. JAMA 2021; 326: 717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institutes of Health. Office of management and budget (OMB) standards. https://orwh.od.nih.gov/toolkit/other-relevant-federal-policies/OMB-standards (accessed Oct 11, 2022). [Google Scholar]
- 14.Agarwal S, Wade AN, Mbanya JC, et al. The role of structural racism and geographical inequity in diabetes outcomes. Lancet 2023; published online June 22. 10.1016/S0140-6736(23)00909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean HJ, Sellers EA. Children have type 2 diabetes too: an historical perspective. Biochem Cell Biol 2015; 93: 425–29. [DOI] [PubMed] [Google Scholar]
- 16.Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths—selected counties and Indian reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep 2020; 69: 161–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liese AD, D’Agostino RB Jr, Hamman RF, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics 2006; 118: 1510–18. [DOI] [PubMed] [Google Scholar]
- 18.Satterfield D, DeBruyn L, Santos M, Alonso L, Frank M. Health promotion and diabetes prevention in American Indian and Alaska Native communities—Traditional Foods Project, 2008–2014. MMWR Suppl 2016; 65: 4–10. [DOI] [PubMed] [Google Scholar]
- 19.Marks BE, Khilnani A, Meyers A, et al. Increase in the diagnosis and severity of presentation of pediatric type 1 and type 2 diabetes during the COVID-19 pandemic. Horm Res Paediatr 2021; 94: 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheelock KM, Sinha M, Knowler WC, Nelson RG, Fufaa GD, Hanson RL. Metabolic risk factors and type 2 diabetes incidence in American Indian children. J Clin Endocrinol Metab 2016; 101: 1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabelea D, Mayer-Davis EJ, Lamichhane AP, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH case-control study. Diabetes Care 2008; 31: 1422–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamody RC, Grilo CM, Vásquez E, Udo T. Diabetes prevalence among diverse Hispanic populations: considering nativity, ethnic discrimination, acculturation, and BMI. Eat Weight Disord 2021; 26: 2673–82. [DOI] [PubMed] [Google Scholar]
- 23.Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012; 366: 2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsen JJ, Black MH, Li BH, Reynolds K, Lawrence JM. Race/ethnicity and measures of glycaemia in the year after diagnosis among youth with type 1 and type 2 diabetes mellitus. J Diabetes Complications 2014; 28: 279–85. [DOI] [PubMed] [Google Scholar]
- 25.Bacha F, Cheng P, Gal RL, et al. Racial and ethnic disparities in comorbidities in youth with type 2 diabetes in the Pediatric Diabetes Consortium (PDC). Diabetes Care 2021; published online Sept 2. https://doi.org/102337/dc21-0143. [DOI] [PubMed] [Google Scholar]
- 26.Hudson OD, Nunez M, Shaibi GQ. Ethnicity and elevated liver transaminases among newly diagnosed children with type 2 diabetes. BMC Pediatr 2012; 12: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis SK, Gebreab S, Quarells R, Gibbons GH. Social determinants of cardiovascular health among black and white women residing in stroke belt and buckle regions of the south. Ethn Dis 2014; 24: 133–43. [PMC free article] [PubMed] [Google Scholar]
- 28.Quarells RC, Liu J, Davis SK. Social determinants of cardiovascular disease risk factor presence among rural and urban Black and White men. J Men’s Health 2012; 9: 120–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voeks JH, McClure LA, Go RC, et al. Regional differences in diabetes as a possible contributor to the geographic disparity in stroke mortality: the REasons for Geographic And Racial Differences in Stroke Study. Stroke 2008; 39: 1675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneiderman N, Llabre M, Cowie CC, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic community health study/study of Latinos (HCHS/SOL). Diabetes Care 2014; 37: 2233–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cordero C, Schneiderman N, Llabre MM, et al. Diabetes incidence among Hispanic/Latino adults in the Hispanic community health study/study of latinos (HCHS/SOL). Diabetes Care 2022; 45: 1482–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oza-Frank R, Chan C, Liu K, Burke G, Kanaya AM. Incidence of type 2 diabetes by place of birth in the Multi-Ethnic Study of Atherosclerosis (MESA). J Immigr Minor Health 2013; 15: 918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukaz DK, Melby MK, Papas MA, Setiloane K, Nmezi NA, Commodore-Mensah Y. Diabetes and acculturation in African immigrants to the United States: analysis of the 2010–2017 National Health Interview Survey (NHIS). Ethn Health 2022; 27: 770–80. [DOI] [PubMed] [Google Scholar]
- 34.Commodore-Mensah Y, Matthie N, Wells J, et al. African Americans, African immigrants, and Afro-Caribbeans differ in social determinants of hypertension and diabetes: evidence from the National Health Interview Survey. J Racial Ethn Health Disparities 2018; 5: 995–1002. [DOI] [PubMed] [Google Scholar]
- 35.Ifatunji MA, Faustin Y, Lee W, Wallace D. Black nativity and health disparities: a research paradigm for understanding the social determinants of health. Int J Environ Res Public Health 2022; 19: 9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vicks WS, Lo JC, Guo L, et al. Prevalence of prediabetes and diabetes vary by ethnicity among U.S. Asian adults at healthy weight, overweight, and obesity ranges: an electronic health record study. BMC Public Health 2022; 22: 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanaya AM, Herrington D, Vittinghoff E, et al. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care 2014; 37: 1621–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: The Diabetes Study of Northern California (DISTANCE). Diabetes Care 2013; 36: 574–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King L, Deng WQ. Epi data brief: health disparities among Asian New Yorkers. March, 2018. https://www.nyc.gov/assets/doh/downloads/pdf/epi/databrief100.pdf (accessed Oct 11, 2022). [Google Scholar]
- 40.Islam NS, Wyatt LC, Kapadia SB, Rey MJ, Trinh-Shevrin C, Kwon SC. Diabetes and associated risk factors among Asian American subgroups in New York City. Diabetes care 2013; 36: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart SL, Dang J, Chen MS Jr. Diabetes prevalence and risk factors in four Asian American communities. J Community Health 2016; 41: 1264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon SC, Wyatt LC, Kum SS, et al. Evaluation of a diabetes prevention intervention for Korean American immigrants at risk for diabetes. Health Equity 2022; 6: 167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han BH, Sadarangani T, Wyatt LC, et al. Correlates of physical activity among middle-aged and older Korean Americans at risk for diabetes. J Nurs Scholarsh 2016; 48: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015; 314: 1021–29. [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Coexisting conditions and complications. Sept 30, 2022. https://www.cdc.gov/diabetes/data/statistics-report/coexisting-conditions-complications.html (accessed Oct 11, 2022). [Google Scholar]
- 46.Barsegian A, Kotlyar B, Lee J, Salifu MO, McFarlane SI. Diabetic retinopathy: focus on minority populations. Int J Clin Endocrinol Metab 2017; 3: 034–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muñoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol 2000; 118: 819–25. [DOI] [PubMed] [Google Scholar]
- 48.Coney JM, Scott AW. Racial disparities in the screening and treatment of diabetic retinopathy. J Natl Med Assoc 2022; 114: 171–81. [DOI] [PubMed] [Google Scholar]
- 49.Tan TW, Shih CD, Concha-Moore KC, et al. Disparities in outcomes of patients admitted with diabetic foot infections. PLoS One 2019; 14: e0211481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gasoyan H, Hussain SR, Wright WG, Sarwer DB. Disparities in diabetes-related lower extremity amputations in the United States: a systematic review. Health Aff (Millwood) 2022; 41: 985–93. [DOI] [PubMed] [Google Scholar]
- 51.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013; 36: 2271–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng YJ, Imperatore G, Geiss LS, et al. Trends and disparities in cardiovascular mortality among U.S. adults with and without self-reported diabetes, 1988–2015. Diabetes Care 2018; 41: 2306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim EJ, Kim T, Conigliaro J, Liebschutz JM, Paasche-Orlow MK, Hanchate AD. Racial and ethnic disparities in diagnosis of chronic medical conditions in the USA. J Gen Intern Med 2018; 33: 1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nsiah-Kumi P, Ortmeier SR, Brown AE. Disparities in diabetic retinopathy screening and disease for racial and ethnic minority populations—a literature review. J Natl Med Assoc 2009; 101: 430–37. [DOI] [PubMed] [Google Scholar]
- 55.Eberly LA, Yang L, Essien UR, et al. Racial, ethnic, and socioeconomic inequities in glucagon-like peptide-1 receptor agonist use among patients with diabetes in the US. JAMA Health Forum 2021; 2: e214182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agarwal S, Simmonds I, Myers AK. The use of diabetes technology to address inequity in health outcomes: limitations and opportunities. Curr Diab Rep 2022; 22: 275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isaacs D, Bellini NJ, Biba U, Cai A, Close KL. Health care disparities in use of continuous glucose monitoring. Diabetes Technol Ther 2021; 23: S81–87. [DOI] [PubMed] [Google Scholar]
- 58.Gujral UP, Weber MB, Staimez LR, Narayan KMV. Diabetes among non-overweight individuals: an emerging public health challenge. Curr Diab Rep 2018; 18: 60. [DOI] [PubMed] [Google Scholar]
- 59.Vicks WS, Lo JC, Guo L, et al. Prevalence of prediabetes and diabetes vary by ethnicity among U.S. Asian adults at healthy weight, overweight, and obesity ranges: an electronic health record study. BMC Public Health 2022; 22: 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oza-Frank R, Narayan KM. Overweight and diabetes prevalence among US immigrants. Am J Public Health 2010; 100: 661–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Y, Sidell MA, Arterburn D, et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI: Patient Outcomes Research To Advance Learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care 2019; 42: 2211–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–46. [DOI] [PubMed] [Google Scholar]
- 63.Torréns JI, Skurnick J, Davidow AL, et al. Ethnic differences in insulin sensitivity and beta-cell function in premenopausal or early perimenopausal women without diabetes: the Study of Women’s health Across the Nation (SWAN). Diabetes Care 2004; 27: 354–61. [DOI] [PubMed] [Google Scholar]
- 64.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 2002; 87: 3023–28. [DOI] [PubMed] [Google Scholar]
- 65.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 1996; 45: 633–38. [DOI] [PubMed] [Google Scholar]
- 66.Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007; 30: 2916–21. [DOI] [PubMed] [Google Scholar]
- 67.Heni M, Machann J, Staiger H, et al. Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev 2010; 26: 200–05. [DOI] [PubMed] [Google Scholar]
- 68.Lê KA, Ventura EE, Fisher JQ, et al. Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care 2011; 34: 485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One 2007; 2: e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bancks MP, Bertoni AG, Carnethon M, et al. Association of diabetes subgroups with race/ethnicity, risk factor burden and complications: the MASALA and MESA studies. J Clin Endocrinol Metab 2021; 106: e2106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varghese JS, Narayan KMV. Ethnic differences between Asians and non-Asians in clustering-based phenotype classification of adult-onset diabetes mellitus: a systematic narrative review. Prim Care Diabetes 2022; 16: 853–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Udler MS, McCarthy MI, Florez JC, Mahajan A. Genetic risk scores for diabetes diagnosis and precision medicine. Endocr Rev 2019; 40: 1500–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raghavan S, Jablonski K, Delahanty LM, et al. Interaction of diabetes genetic risk and successful lifestyle modification in the Diabetes Prevention Programme. Diabetes Obes Metab 2021; 23: 1030–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Committee ADAPP. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care 2022; 45 (suppl 1): S46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bellissimo JL, Holt RM, Maus SM, Marx TL, Schwartz FL, Shubrook JH. Impact of activity participation and depression on glycemic control in older adults with diabetes: glycemic control in nursing homes. Clin Diabetes 2011; 29: 139–44. [Google Scholar]
- 76.Bower JK, Butler BN, Bose-Brill S, Kue J, Wassel CL. Racial/ethnic differences in diabetes screening and hyperglycemia among US women after gestational diabetes. Prev Chronic Dis 2019; 16: E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.You H, Hu J, Liu Y, Luo B, Lei A. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: a systematic review & meta-analysis. Indian J Med Res 2021; 154: 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009; 373: 1773–79. [DOI] [PubMed] [Google Scholar]
- 79.Holder T, Giannini C, Santoro N, et al. A low disposition index in adolescent offspring of mothers with gestational diabetes: a risk marker for the development of impaired glucose tolerance in youth. Diabetologia 2014; 57: 2413–20. [DOI] [PubMed] [Google Scholar]
- 80.Kajantie E, Osmond C, Barker DJP, Eriksson JG. Preterm birth—a risk factor for type 2 diabetes? The Helsinki birth cohort study. Diabetes Care 2010; 33: 2623–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia 2020; 63: 508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kulkarni KD. Food, culture, and diabetes in the United States. Clin Diabetes 2004; 22: 190–92. [Google Scholar]
- 83.Hsu WC, Boyko EJ, Fujimoto WY, et al. Pathophysiologic differences among Asians, native Hawaiians, and other Pacific Islanders and treatment implications. Diabetes Care 2012; 35: 1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li-Geng T, Kilham J, McLeod KM. Cultural influences on dietary self-management of type 2 diabetes in east Asian Americans: a mixed-methods systematic review. Health Equity 2020; 4: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tran P, Tran L, Tran L. Impact of rurality on diabetes screening in the US. BMC Public Health 2019; 19: 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carlson AE, Aronson BD, Unzen M, Lewis M, Benjamin GJ, Walls ML. Apathy and type 2 diabetes among American Indians: exploring the protective effects of traditional cultural involvement. J Health Care Poor Underserved 2017; 28: 770–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Padala PR, Desouza CV, Almeida S, et al. The impact of apathy on glycemic control in diabetes: a cross-sectional study. Diabetes Res Clin Pract 2008; 79: 37–41. [DOI] [PubMed] [Google Scholar]
- 88.Skinner AC, Weinberger M, Mulvaney S, Schlundt D, Rothman RL. Accuracy of perceptions of overweight and relation to self-care behaviors among adolescents with type 2 diabetes and their parents. Diabetes Care 2008; 31: 227–29. [DOI] [PubMed] [Google Scholar]
- 89.Walls ML, Sittner KJ, Aronson BD, Forsberg AK, Whitbeck LB, al’Absi M. Stress exposure and physical, mental, and behavioral health among American Indian adults with type 2 diabetes. Int J Environ Res Public Health 2017; 14: 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kern DM, Auchincloss AH, Stehr MF, et al. Neighborhood price of healthier food relative to unhealthy food and its association with type 2 diabetes and insulin resistance: the multi-ethnic study of atherosclerosis. Prev Med 2018; 106: 122–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Piper CN, Polite-Middleton T, Chalakalal S, Sebastion N, Martin F. Race, socioeconomic status, and rurality influences on type 2 diabetes management among North Carolina adults. Ethn Dis 2015; 25: 46–51. [PubMed] [Google Scholar]
- 92.Walls ML, Hautala D, Gonzalez M, Greenfield B, Aronson BD, Onello E. Perceptions and prevalence of alcohol and cigarette use among American Indian adults with type 2 diabetes. Clin Diabetes 2019; 37: 260–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miranda A, Garcia DO, Sánchez C, Warren C. Health and type 2 diabetes perspectives of at-risk, Mexican-origin males (HD-MxOM): a qualitative study. J Racial Ethn Health Disparities 2021; 8: 1101–11. [DOI] [PubMed] [Google Scholar]
- 94.McConatha JT, Kumar VK, Raymond E, Akwarandu A. Cultural dimensions of diabetes management: a qualitative study of Middle Eastern immigrants in the U.S. J Cross Cult Gerontol 2020; 35: 85–98. [DOI] [PubMed] [Google Scholar]
- 95.Wedekind LE, Mitchell CM, Andersen CC, Knowler WC, Hanson RL. Epidemiology of type 2 diabetes in Indigenous communities in the United States. Curr Diab Rep 2021; 21: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reynolds DB, Walker RJ, Campbell JA, Egede LE. Differential effect of race, education, gender, and language discrimination on glycemic control in adults with type 2 diabetes. Diabetes Technol Ther 2015; 17: 243–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beckles GL, Chou C-F. Disparities in the prevalence of diagnosed diabetes—United States, 1999–2002 and 2011–2014. MMWR Morb Mortal Wkly Rep 2016; 65: 1265–69. [DOI] [PubMed] [Google Scholar]
- 98.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020; 44: 258–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Axon RN, Gebregziabher M, Dismuke CE, et al. Differential impact of homelessness on glycemic control in veterans with type 2 diabetes mellitus. J Gen Intern Med 2016; 31: 1331–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berkowitz SA, Kalkhoran S, Edwards ST, Essien UR, Baggett TP. Unstable housing and diabetes-related emergency department visits and hospitalization: a nationally representative study of safety-net clinic patients. Diabetes Care 2018; 41: 933–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the measuring economic insecurity in diabetes study. JAMA Intern Med 2015; 175: 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol 2007; 165: 175–83. [DOI] [PubMed] [Google Scholar]
- 103.Smalls BL, Gregory CM, Zoller JS, Egede LE. Assessing the relationship between neighborhood factors and diabetes related health outcomes and self-care behaviors. BMC Health Serv Res 2015; 15: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Twohig-Bennett C, Jones A. The health benefits of the great outdoors: a systematic review and meta-analysis of greenspace exposure and health outcomes. Environ Res 2018; 166: 628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.den Braver NR, Lakerveld J, Rutters F, Schoonmade LJ, Brug J, Beulens JWJ. Built environmental characteristics and diabetes: a systematic review and meta-analysis. BMC Med 2018; 16: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gebreab SY, Hickson DA, Sims M, et al. Neighborhood social and physical environments and type 2 diabetes mellitus in African Americans: the Jackson Heart Study. Health Place 2017; 43: 128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Christine PJ, Auchincloss AH, Bertoni AG, et al. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA). JAMA Intern Med 2015; 175: 1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kurani SS, Lampman MA, Funni SA, et al. Association between area-level socioeconomic deprivation and diabetes care quality in US primary care practices. JAMA Netw Open 2021; 4: e2138438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kershaw KN, Pender AE. Racial/ethnic residential segregation, obesity, and diabetes mellitus. Curr Diab Rep 2016; 16: 108. [DOI] [PubMed] [Google Scholar]
- 110.Kowitt SD, Donahue KE, Fisher EB, Mitchell M, Young LA. How is neighborhood social disorganization associated with diabetes outcomes? A multilevel investigation of glycemic control and self-reported use of acute or emergency health care services. Clin Diabetes Endocrinol 2018; 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lynch CP, Gebregziabher M, Axon RN, Hunt KE, Payne E, Egede LE. Geographic and racial/ethnic variations in patterns of multimorbidity burden in patients with type 2 diabetes. J Gen Intern Med 2015; 30: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin KD, Beckles GL, Wu C, et al. Lifecourse socioeconomic position and diabetes incidence in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study, 2003 to 2016. Prev Med 2021; 153: 106848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shalowitz MU, Eng JS, McKinney CO, et al. Food security is related to adult type 2 diabetes control over time in a United States safety net primary care clinic population. Nutr Diabetes 2017; 7: e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Silverman J, Krieger J, Kiefer M, Hebert P, Robinson J, Nelson K. The relationship between food insecurity and depression, diabetes distress and medication adherence among low-income patients with poorly-controlled diabetes. J Gen Intern Med 2015; 30: 1476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steckel RH. The hidden cost of moving up: type 2 diabetes and the escape from persistent poverty in the American South. Am J Hum Biol 2013; 25: 508–15. [DOI] [PubMed] [Google Scholar]
- 116.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev 2007; 64 (suppl): 101S–56S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heuman AN, Scholl JC, Wilkinson K. Rural Hispanic populations at risk in developing diabetes: sociocultural and familial challenges in promoting a healthy diet. Health Commun 2013; 28: 260–74. [DOI] [PubMed] [Google Scholar]
- 118.Soltero EG, Navabi N, Castro FG, et al. Perceptions of family-level social factors that influence health behaviors in Latinx adolescents and young adults at high risk for type 2 diabetes. Children (Basel) 2021; 8: 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mansyur CL, Rustveld LO, Nash SG, Jibaja-Weiss ML. Hispanic acculturation and gender differences in support and self-efficacy for managing diabetes. Diabetes Educ 2016; 42: 315–24. [DOI] [PubMed] [Google Scholar]
- 120.Mansyur CL, Rustveld LO, Nash SG, Jibaja-Weiss ML. Social factors and barriers to self-care adherence in Hispanic men and women with diabetes. Patient Educ Couns 2015; 98: 805–10. [DOI] [PubMed] [Google Scholar]
- 121.Liu NF, Brown AS, Folias AE, et al. Stigma in people with type 1 or type 2 diabetes. Clin Diabetes 2017; 35: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ochieng JM, Crist JD. Social determinants of health and health care delivery: African American women’s T2DM self-management. Clin Nurs Res 2021; 30: 263–72. [DOI] [PubMed] [Google Scholar]
- 123.McCoy RG, Kidney RSM, Holznagel D, Peters T, Madzura V. Challenges for younger adults with diabetes. Minn Med 2019; 102: 34–36. [PMC free article] [PubMed] [Google Scholar]
- 124.Angier H, Ezekiel-Herrera D, Marino M, et al. Racial/ethnic disparities in health insurance and differences in visit type for a population of patients with diabetes after Medicaid expansion. J Health Care Poor Underserved 2019; 30: 116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schillinger D, Grumbach K, Piette J, et al. Association of health literacy with diabetes outcomes. JAMA 2002; 288: 475–82. [DOI] [PubMed] [Google Scholar]
- 126.Osborn CY, Cavanaugh K, Wallston KA, et al. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun 2011; 16 (suppl 3): 268–78 (abstr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peeters B, Van Tongelen I, Boussery K, Mehuys E, Remon JP, Willems S. Factors associated with medication adherence to oral hypoglycaemic agents in different ethnic groups suffering from type 2 diabetes: a systematic literature review and suggestions for further research. Diabet Med 2011; 28: 262–75. [DOI] [PubMed] [Google Scholar]
- 128.Taylor SI. The high cost of diabetes drugs: disparate impact on the most vulnerable patients. Diabetes Care 2020; 43: 2330–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shiyanbola OO, Ward E, Brown C. Sociocultural influences on African Americans’ representations of type 2 diabetes: a qualitative study. Ethn Dis 2018; 28: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Detz A, Mangione CM, Nunez de Jaimes F, et al. Language concordance, interpersonal care, and diabetes self-care in rural Latino patients. J Gen Intern Med 2014; 29: 1650–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reid HW, Lin OM, Fabbro RL, et al. Racial differences in patient perception of interactions with providers are associated with health outcomes in type II diabetes. Patient Educ Couns 2021; 104: 1993–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bauer AM, Parker MM, Schillinger D, et al. Associations between antidepressant adherence and shared decision-making, patient-provider trust, and communication among adults with diabetes: diabetes study of northern California (DISTANCE). J Gen Intern Med 2014; 29: 1139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ratanawongsa N, Karter AJ, Parker MM, et al. Communication and medication refill adherence: the diabetes study of northern California. JAMA Intern Med 2013; 173: 210–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bonds DE, Camacho F, Bell RA, Duren-Winfield VT, Anderson RT, Goff DC. The association of patient trust and self-care among patients with diabetes mellitus. BMC Fam Pract 2004; 5: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martinez-Hollingsworth A, Hamilton N, Choi K, Heilemann M. The secret-self management loop: a grounded theory of provider mistrust among older Latinas with type 2 diabetes and mental health symptoms. Diabetes Res Clin Pract 2021; 175: 108787. [DOI] [PubMed] [Google Scholar]
- 136.Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011; 86: 359–64. [DOI] [PubMed] [Google Scholar]
- 137.Ahmad T, Hari S, Cleary D, Yu C. “I had nobody to represent me”: how perceptions of diabetes health-care providers’ age, gender and ethnicity impact shared decision-making in adults with type 1 and type 2 diabetes. Can J Diabetes 2021; 45: 78–88. [DOI] [PubMed] [Google Scholar]
- 138.Beltrán S, Arenas DJ, López-Hinojosa IJ, Tung EL, Cronholm PF. associations of race, insurance, and zip code-level income with nonadherence diagnoses in primary and specialty diabetes care. J Am Board Fam Med 2021; 34: 891–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beltrán S, Lett E, Cronholm PF. Nonadherence labeling in primary care: bias by race and insurance type for adults with type 2 diabetes. Am J Prev Med 2019; 57: 652–58. [DOI] [PubMed] [Google Scholar]
- 140.Perez A, Elrod S, Sanchez J. Differences in the use and quality of antidiabetic therapies in Mexican American and non-Hispanic whites with uncontrolled type 2 diabetes in the US: NHANES 2003–2012. Diabetes Educ 2015; 41: 582–91. [DOI] [PubMed] [Google Scholar]
- 141.Goonesekera SD, Yang MH, Hall SA, Fang SC, Piccolo RS, McKinlay JB. Racial ethnic differences in type 2 diabetes treatment patterns and glycaemic control in the Boston area community health survey. BMJ Open 2015; 5: e007375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bancks MP, Casanova R, Gregg EW, Bertoni AG. Epidemiology of diabetes phenotypes and prevalent cardiovascular risk factors and diabetes complications in the National Health and Nutrition Examination Survey 2003–2014. Diabetes Res Clin Pract 2019; 158: 107915. [DOI] [PubMed] [Google Scholar]
- 143.Lu H, Holt JB, Cheng YJ, Zhang X, Onufrak S, Croft JB. Population-based geographic access to endocrinologists in the United States, 2012. BMC Health Serv Res 2015; 15: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rutledge SA, Masalovich S, Blacher RJ, Saunders MM. Diabetes self-management education programs in nonmetropolitan counties—United States, 2016. MMWR Surveill Summ 2017; 66: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sheon AR, Bolen SD, Callahan B, Shick S, Perzynski AT. Addressing disparities in diabetes management through novel approaches to encourage technology adoption and use. JMIR Diabetes 2017; 2: e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ngo-Metzger Q, Sorkin DH, Billimek J, Greenfield S, Kaplan SH. The effects of financial pressures on adherence and glucose control among racial/ethnically diverse patients with diabetes. J Gen Intern Med 2012; 27: 432–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fisher MA, Ma ZQ. Medicaid-insured and uninsured were more likely to have diabetes emergency/urgent admissions. Am J Manag Care 2015; 21: e312–19. [PubMed] [Google Scholar]
- 148.Ludwig DS, Ebbeling CB, Pereira MA, Pawlak DB. A physiological basis for disparities in diabetes and heart disease risk among racial and ethnic groups. J Nutr 2002; 132: 2492–93. [DOI] [PubMed] [Google Scholar]
- 149.Gregg EW, Cadwell BL, Cheng YJ, et al. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care 2004; 27: 2806–12. [DOI] [PubMed] [Google Scholar]
- 150.Gaston SA, Atere-Roberts J, Ward J, et al. Experiences with everyday and major forms of racial/ethnic discrimination and type 2 diabetes risk among White, Black, and Hispanic/Latina women: findings from the sister study. Am J Epidemiol 2021; 190: 2552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Whitaker KM, Everson-Rose SA, Pankow JS, et al. Experiences of discrimination and incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Epidemiol 2017; 186: 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bacon KL, Stuver SO, Cozier YC, Palmer JR, Rosenberg L, Ruiz-Narváez EA. Perceived racism and incident diabetes in the Black women’s health study. Diabetologia 2017; 60: 2221–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bermudez-Millan A, Schumann KP, Feinn R, Tennen H, Wagner J. Behavioral reactivity to acute stress among Black and White women with type 2 diabetes: the roles of income and racial discrimination. J Health Psychol 2016; 21: 2085–97. [DOI] [PubMed] [Google Scholar]
- 154.Nkwata AK, Song X, Zhang M, Ezeamama AE. Change in quality of life over eight years in a nationally representative sample of US adults with heart disease and type 2 diabetes: minority race and toxic stress as keysocial determinants. BMC Public Health 2020; 20: 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.LeBrón AMW, Spencer M, Kieffer E, Sinco B, Palmisano G. Racial/ethnic discrimination and diabetes-related outcomes among Latinos with type 2 diabetes. J Immigr Minor Health 2019; 21: 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sittner KJ, Greenfield BL, Walls ML. Microaggressions, diabetes distress, and self-care behaviors in a sample of American Indian adults with type 2 diabetes. J Behav Med 2018; 41: 122–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meo SA, Memon AN, Sheikh SA, et al. Effect of environmental air pollution on type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci 2015; 19: 123–28. [PubMed] [Google Scholar]
- 158.Moon J The effect of the heatwave on the morbidity and mortality of diabetes patients; a meta-analysis for the era of the climate crisis. Environ Res 2021; 195: 110762. [DOI] [PubMed] [Google Scholar]
- 159.Ruiz D, Becerra M, Jagai JS, Ard K, Sargis RM. disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care 2018; 41: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vallianou NG, Geladari EV, Kounatidis D, et al. Diabetes mellitus in the era of climate change. Diabetes Metab 2021; 47: 101205. [DOI] [PubMed] [Google Scholar]
- 161.Lee EK, Donley G, Ciesielski TH, et al. Health outcomes in redlined versus non-redlined neighborhoods: a systematic review and meta-analysis. Soc Sci Med 2022; 294: 114696. [DOI] [PubMed] [Google Scholar]
- 162.Linde S, Walker RJ, Campbell JA, Egede LE. Historic residential redlining and present-day diabetes mortality and years of life lost: the persistence of structural racism. Diabetes Care 2022; 45: 1772–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Golden SH, Joseph JJ, Hill-Briggs F. Casting a health equity lens on endocrinology and diabetes. J Clin Endocrinol Metab 2021; 106: e1909–16. [DOI] [PubMed] [Google Scholar]
- 164.Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors—an Endocrine Society scientific statement. J Clin Endocrinol Metab 2012; 97: E1579–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rosen ED, Kaestner KH, Natarajan R, et al. Epigenetics and epigenomics: implications for diabetes and obesity. Diabetes 2018; 67: 1923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Howe CJ, Bailey ZD, Raifman JR, Jackson JW. Recommendations for using causal diagrams to study racial health disparities. Am J Epidemiol 2022; 191: 1981–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep 2013; 13: 814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shoily SS, Ahsan T, Fatema K, Sajib AA. Common genetic variants and pathways in diabetes and associated complications and vulnerability of populations with different ethnic origins. Sci Rep 2021; 11: 7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab 2012; 97: 1067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aguayo-Mazzucato C, Diaque P, Hernandez S, Rosas S, Kostic A, Caballero AE. Understanding the growing epidemic of type 2 diabetes in the Hispanic population living in the United States. Diabetes Metab Res Rev 2019; 35: e3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dugani SB, Wood-Wentz CM, Mielke MM, Bailey KR, Vella A. Assessment of disparities in diabetes mortality in adults in US rural vs nonrural counties, 1999–2018. JAMA Netw Open 2022; 5: e2232318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kobo O, Van Spall HGC, Mamas MA. Urban–rural disparities in diabetes-related mortality in the USA 1999–2019. Diabetologia 2022; 65: 2078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gaskin DJ, Thorpe RJ Jr, McGinty EE, et al. Disparities in diabetes: the nexus of race, poverty, and place. Am J Public Health 2014; 104: 2147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chandrabose M, Rachele JN, Gunn L, et al. Built environment and cardio-metabolic health: systematic review and meta-analysis of longitudinal studies. Obes Rev 2019; 20: 41–54. [DOI] [PubMed] [Google Scholar]
- 175.Narayan KMV, Kanaya AM. Why are south Asians prone to type 2 diabetes? A hypothesis based on underexplored pathways. Diabetologia 2020; 63: 1103–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Savic Hitt TA, Katz LEL. Pediatric type 2 diabetes: not a mini version of adult type 2 diabetes. Endocrinol Metab Clin North Am 2020; 49: 679–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rudnicka AR, Nightingale CM, Donin AS, et al. Sleep duration and risk of type 2 diabetes. Pediatrics 2017; 140: e20170338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jiang X, Ma H, Wang Y, Liu Y. Early life factors and type 2 diabetes mellitus. J Diabetes Res 2013; 2013: 485082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stein AD, Obrutu OE, Behere RV, Yajnik CS. Developmental undernutrition, offspring obesity and type 2 diabetes. Diabetologia 2019; 62: 1773–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Levine JA. Solving obesity without addressing poverty: fat chance. J Hepatol 2015; 63: 1523–24. [DOI] [PubMed] [Google Scholar]
- 181.Habiba NM, Fulda KG, Basha R, et al. Correlation of lipid profile and risk of developing type 2 diabetes mellitus in 10–14 year old children. Cell Physiol Biochem 2016; 39: 1695–704. [DOI] [PubMed] [Google Scholar]
- 182.Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011–2019. JAMA 2021; 326: 660–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Blotsky AL, Rahme E, Dahhou M, Nakhla M, Dasgupta K. Gestational diabetes associated with incident diabetes in childhood and youth: a retrospective cohort study. CMAJ 2019; 191: E410–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ma RC, Popkin BM. Intergenerational diabetes and obesity—a cycle to break? PLoS Med 2017; 14: e1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hill-Briggs F, Ephraim PL, Vrany EA, et al. Social determinants of health, race, and diabetes population health improvement: Black/African Americans as a population exemplar. Curr Diab Rep 2022; 22: 117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol 2016; 35: 407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Walker AF, Graham S, Maple-Brown L, et al. Interventions to address global inequity in diabetes: international progress. Lancet 2023; published online June 22. 10.1016/S0140-6736(23)00914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chan JCN, Lim L-L, Wareham NJ, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet 2021; 396: 2019–82. [DOI] [PubMed] [Google Scholar]
- 189.Sterling MR, Echeverría SE, Commodore-Mensah Y, Breland JY, Nunez-Smith M. Health equity and implementation science in heart, lung, blood, and sleep-related research: emerging themes from the 2018 Saunders-Watkins Leadership Workshop. Circ Cardiovasc Qual Outcomes 2019; 12: e005586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Baumann AA, Cabassa LJ. Reframing implementation science to address inequities in healthcare delivery. BMC Health Serv Res 2020; 20: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Brownson RC, Kumanyika SK, Kreuter MW, Haire-Joshu D. Implementation science should give higher priority to health equity. Implement Sci 2021; 16: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cooper LA, Saha S, van Ryn M, eds. Mandated implicit bias training for health professionals—a step toward equity in health care. JAMA Health Forum 2022; 3: e223250. [DOI] [PubMed] [Google Scholar]
- 193.Devine PG, Forscher PS, Austin AJ, Cox WT. Long-term reduction in implicit race bias: a prejudice habit-breaking intervention. J Exp Soc Psychol 2012; 48: 1267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zestcott CA, Blair IV, Stone J. Examining the presence, consequences, and reduction of implicit bias in health care: a narrative review. Group Process Intergroup Relat 2016; 19: 528–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Stone J, Moskowitz GB, Zestcott CA, Wolsiefer KJ. Testing active learning workshops for reducing implicit stereotyping of Hispanics by majority and minority group medical students. Stigma Health 2020; 5: 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015; 105: e60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Langley W, Braithwaite RL, Blumenthal DS, Akintobi TH. The Morehouse model: how one school fo medicine revolutionized community engagement and health equity. Baltimore, MD: Johns Hopkins University Press, 2020. [Google Scholar]
- 198.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Integrating Social Needs Care into the Delivery of Health Care to Improve the Nation’s Health. Integrating social care into the delivery of health care: moving upstream to improve the nation’s health. Washington, DC: National Academies Press (US), 2019. [PubMed] [Google Scholar]
- 199.Siegel KR, Gregg EW, Duru OK, et al. Time to start addressing (and not just describing) the social determinants of diabetes: results from the NEXT-D 2·0 network. BMJ Open Diabetes Res Care 2021; 9: e002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thorpe LE, Adhikari S, Lopez P, et al. Neighborhood socioeconomic environment and risk of type 2 diabetes: associations and mediation through food environment pathways in three independent study samples. Diabetes Care 2022; 45: 798–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


