Abstract
IgG4 related disease (IgG4-RD) is a multisystem inflammatory disease and can affect several organs including salivary glands, orbits, lungs, pancreas, kidneys and lymph nodes. Up to 40 % of patients have allergic manifestations including asthma, chronic rhinosinusitis, eczema and asthma. Commonly pulmonary manifestations include pulmonary nodules ranging from <1 to 5 cm in diameter, interstitial opacities and mediastinal lymphadenopathy. Rarely, IgG4-RD presents as isolated tracheal disease. Symptoms include dyspnea and stridor due to airway narrowing. Diagnosis of IgG4-RD including tracheal IgG4-RD requires a biopsy. The histologic specimen is characterized by lymphoplasmacytic infiltrate with high density of IgG4 positive plasma cells, and storiform fibrosis (a cartwheel appearance of fibroblasts and inflammatory cells). Up to 30 % of patients with IgG4-RD have normal serum IgG4 levels. The mainstay of therapy is glucocorticoids for those with systemic disease. Rituximab is an alternative for those who cannot tolerate glucocorticoids or those with disease recurrence. Patients with tracheal disease often require balloon dilation. Recurrence is common in patients and up to two thirds of patients have residual disease despite treatment. These patients often require surgical resection of affected area for symptomatic relief.
Keywords: Chronic cough, IgG4-related disease, Stridor, Trachea
1. Introduction
IgG4 related disease (IgG4-RD) is a multisystem disease often manifesting as a mass in the involved organ including the lung [1]. Common lung manifestations include lymphadenopathy and reticular opacities. Uncommon manifestations of lung IgG-RD include small and large airway involvement. Furthermore, isolated tracheal involvement without any parenchymal and lymphadenopathy is very rare. Our case describes a rare manifestation of case of IgG4-RD isolated to the trachea only.
2. Case presentation
46-year-old woman with history of gastrointestinal reflux disease, fibromyalgia, and chronic rhinosinusitis status post unspecified nasal sinus surgery was referred to otolaryngology for dyspnea, chronic non-productive cough and stridor. She described a six-year history of aforementioned symptoms with significant worsening 12 months prior to the clinic visit. In addition, she reported scant hemoptysis, globus sensation, and heartburn. She denied a history of tracheal procedures, voicing problems, anterior neck or throat pain with voicing, odynophagia, dysphagia, otalgia, nasal crusting, nasal pain or nasal obstruction, epistaxis, sinus pain or dental pain. She denied fevers, weight loss, night sweats, malaise, skin lesions or rashes, hematuria, and abdominal complaints. She had no history of tobacco use or illicit drug use. Family history was negative for any airway or lung disorders. Prior to the visit she had received a diagnosis of asthma but had minimal improvement with bronchodilators and daily inhaled corticosteroids.
On physical examination she appeared comfortable and vitals were within normal limits. However, there was audible stridor with talking. Nasal examination showed an intact septum and no lesions. Her oropharynx revealed no masses and neck examination showed a normal thyroid. Respiratory examination was significant for upper airway stridor and normal breath sounds in the upper and lower lung fields. The remainder of her physical examination was normal. Laboratory testing showed normal renal function, complement 3 (C3) and C4, erythrocyte sedimentation rate, c-reactive protein, immunoglobulin E levels and blood counts including eosinophils and negative anti-neutrophilic cytoplasmic antibody, anti-nuclear antibody, anti-SSA and SSB. Spirometry demonstrated normal flow values, but the inspiratory and expiratory flow-volume curves exhibited flattening deemed consistent with a fixed upper airway obstruction (Fig. 1).
Fig. 1.
Flow volume loop demonstrating both inspiratory and expiratory limb flattening suggestive of fixed upper airway obstruction.
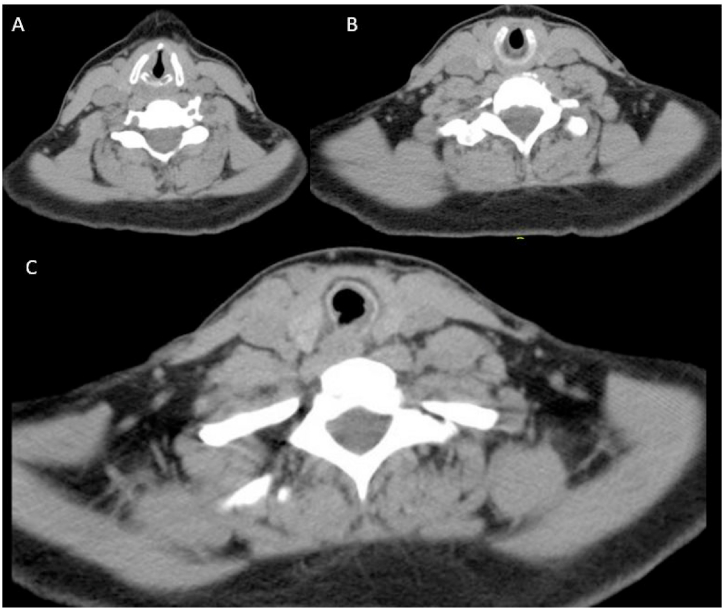
Given the concern for an airway mass, further diagnostic work-up was pursued. Laryngoscopy showed a spiral-shaped soft tissue narrowing 1.5 cm below the true vocal cords consistent with membranous subglottic and tracheal stenosis with no tracheal cartilage involvement. A nasal septal perforation was also observed. Computed tomography (CT) of the neck was obtained which showed tracheal wall thickening with associated tracheal stenosis (Fig. 2). At its narrowest section, the trachea measured 10 × 8 mm in the anterior-posterior (AP) to transverse plane (TP), respectively, with no cartilaginous involvement.
Fig. 2.
Computed tomography of the neck at the initial visit showing tracheal thickening at the A) and B) level of cricotracheal cartilage and C) below the level of the cricotracheal cartilage.
Differentials for tracheal thickening and narrowing included several possibilities such as sarcoidosis, granulomatosis with polyangiitis, relapsing polychondritis, amyloidosis and malignancy (Table 1). In addition, it is crucial to rule out other conditions associated with elevated IgG4 levels such as connective tissue diseases, Castleman's disease, malignancies and sarcoidosis. Infections were less likely due to the lack of systemic symptoms.
Table 1.
Differentials for tracheal thickening.
| Condition | Clinical | Lung Imaging | Bronchoscopy |
|---|---|---|---|
| Relapsing polychondritis | Inflammation of cartilaginous structures; ears and nose are most frequently affected; other sites include costal cartilage, tracheal cartilage; mitral and/or aortic valvular regurgitation in 7 % of patients. | Airway thickening, airway stenosis, tracheomalacia, and airway calcification in 30 % of patients; mediastinal adenopathy in 50 % of patients [2]. | Airway stenosis; in late stages, protrusion of cartilage into the tracheal lumen. |
| Tracheopathia osteochondroplastica | Occurs in men. Rarely symptomatic unless in late stages. | Calcified nodules protruding in to the tracheal lumen and decreased lateral diameter [3]. | Presence of osseous and cartilaginous nodules on the tracheal cartilage with sparing of the posterior membranous trachea. Airway narrowing. |
| Granulomatosis polyangiitis | Rhinosinusitis, cough, dyspnea, stridor and hemoptysis. Subglottic stenosis is the most common finding in tracheobronchial GPA and occurs in approximately 60 % of patients [4]. Saddle nose deformity may occur due to destruction of cartilage. Other symptoms include joint pain and purpuric rash. | Circumferential wall thickenings due to tracheal or bronchial stenosis. More common findings include multiple pulmonary cavitary nodules and consolidative opacities. | Bronchial ulcers and tracheal narrowing are rare observations. |
| Sarcoidosis | Symptoms due to large airway narrowing is uncommon. | Airway manifestations include bronchial stenosis in 2 %–8 % of patients [5]. | Bronchial thickening, mucosal nodules and plaques and stenosis are uncommon manifestations [6]. |
| Lymphomas | Dyspnea, wheezing and cough may occur. Most lesions occur in the mainstem bronchi as a result of metastatic disease rather than primary tracheobronchial lymphoma. | Focal thickening of the trachea with tracheal and/or endobronchial lesion may be present. Other manifestations such as post-obstructive pneumonia and atelectasis are potential complications of the endobronchial mass. | Endobronchial lesions are the most likely finding [7]. |
| Primary tracheal tumors Squamous cell Adenoid cystic Carcinoid Mucoepidermoid |
SCCA is the most common and occurs in smokers. Symptoms are determined by the size of the tumor and degree of obstruction [8]. | Focal thickening due to tracheal or endobronchial lesion. | Single mass in tracheal lumen or multiples nodules in the trachea. |
| Inflammatory bowel disease | 40 % of patients with IBD may have airway involvement. Productive cough is the most common symptom [9]. | Focal or diffuse circumferential thickening of trachea. Other manifestations include bronchiectasis. | Mucosal irregularity, ulceration, and exudates may be present. Other findings include airway stenosis. |
| Amyloidosis | Rarely symptomatic. Usually occurs in the sixth decade. Males are more commonly affected. Congo red staining of biopsy specimens confirms amyloidosis [10]. | Tracheal thickening may be localized or circumferential with subsequent narrowing. Calcifications may be present. | Localized or diffuse sub-mucosal plaques may be seen. |
| Infection Aspergillus |
Primarily occurs in immunocompromised patients [11,12]. | Circumferential irregular tracheal thickening. | Ulcerations, pseudo-membranes, and tracheal necrosis. |
She then underwent radical incision by CO2 laser, biopsy of the lesion, followed by steroid injection and balloon dilation of the lesion which successfully relieved the narrowing. Pathology showed respiratory and squamous mucosa with surface erosion, sclerosis, and patchy lymphoplasmacellular inflammation with plasma cells expressing CD138 (a marker for terminally differentiated plasma cells) and IgG4 (Fig. 3). There was no evidence of neoplasia or granulomas. She was diagnosed with isolated IgG4-RD of the trachea. Serum IgG and IgG4 levels were within normal limits.
Fig. 3.
A) Trachea with surface erosion, (200x) B) fibrosis, and lymphoplasmacytic inflammation (200x) composed of abundant C) CD138-positive plasma cells (200x), many of which were also D) IgG4-positive (200x).
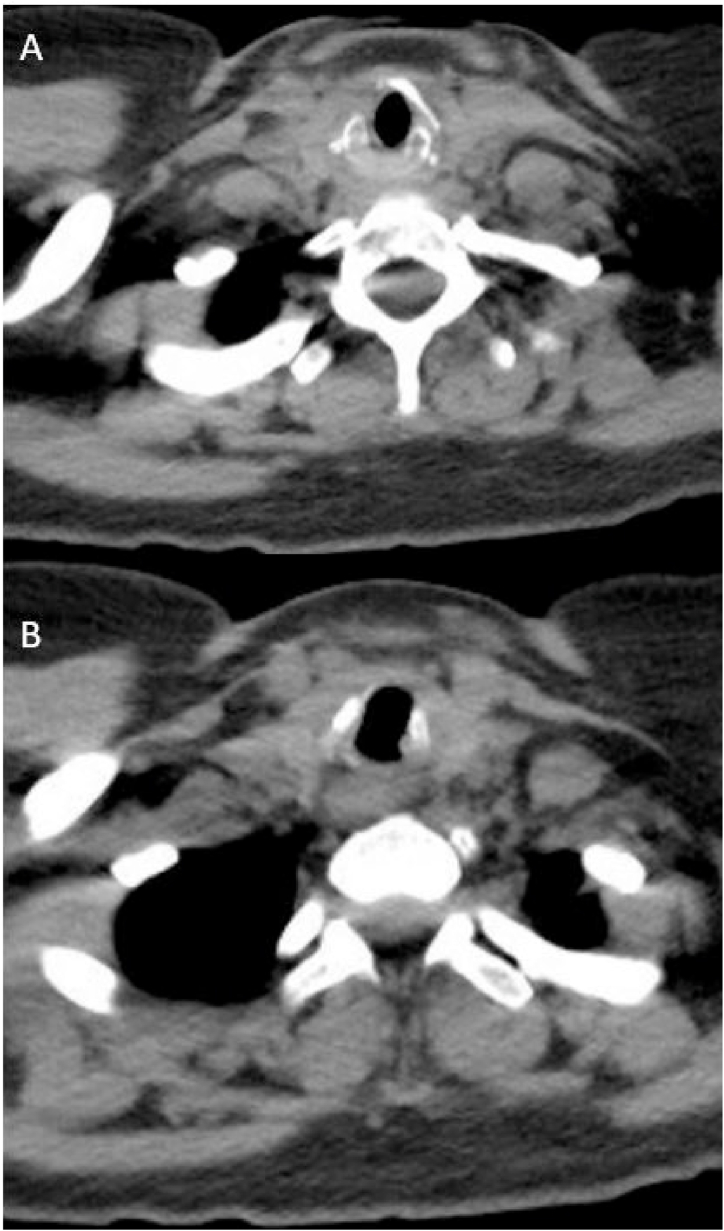
The patient noted improvement of her symptoms immediately after the steroid injection and dilation. Unfortunately, she had a recurrence of her symptoms a year later, a finding reported in other cases of isolated tracheal involvement. She received another steroid injection and balloon dilation. Despite an initial improvement, her symptoms recurred 10 months later. She subsequently underwent tracheal resection and was started on rituximab. At 6 months follow up, there was an improvement in the tracheal diameter with the narrowest area measuring 14.5 × 10 mm in the AP to TP at 6 months follow up (Fig. 4). In addition, her flow volume loops normalized (Fig. 5). The patient remains asymptomatic at 3 year follow up.
Fig. 4.
Computed tomography of the chest at 6 months follow up after tracheal resection and initiation of rituximab at the A) level of cricotracheal cartilage and C) below the level of the cricotracheal cartilage.
Fig. 5.
Normalization of follow volume loop following surgical resection and medical therapy.
3. Discussion
IgG4 related disease (IgG4-RD) is a fibroinflammatory condition which can involve virtually any organ system [1]. Common sites of involvement include the pancreas, biliary tree, salivary glands, thyroid, kidneys, lung, periorbital tissues, uvea, lymph nodes, aorta, breast, pericardium and skin. Patient may present with a sub-acute mass (eg. renal mass, enlarged pancreas). In addition, patients may have allergic conditions such as asthma, eczema, and chronic sinusitis as seen in our patient [13]. Most patients have elevations in serum IgG4; however, approximately 30 % of patients do not as seen in our patient [14]. Other laboratory findings include low C3 and C4. The degree of IgG4 elevation has been shown to correlate with number of organs involved and often decreases with glucocorticoid therapy [15]. In our patient, CT chest, abdomen and pelvis revealed no other sites of involvement. Of note, there is an increased risk of malignancy in the affected organ [16].
Although head and neck manifestations are common, tracheal and laryngeal manifestations have been rarely reported. In one large systematic review which included 730 cases of biopsy-proven IgG4-RD of the head and neck, there were zero cases of upper airway involvement [17]. One case series of previously published literature identified 11 patients with isolated laryngopharyngeal involvement [18]. Of these 11 subjects, only two patients had isolated subglottic stenosis and both had laryngeal involvement.
Common radiographic manifestations of IgG4-RD include nodular lesions, interstitial opacities and mediastinal lymphadenopathy [19]. Mediastinal and hilar adenopathy occurs in 40 %–90 % of patients, respectively and is the most common intra-thoracic finding. Often, these nodes are 18F-fluorodeoxyglucose positron emission tomography (PET) avid. Pulmonary nodules can range from <1 to >5 cm in diameter, be solid or ground-glass in appearance, and can occur in both upper and lower lung fields. Less common presentations include pleural nodules and airway disease manifesting as upper airway masses and diffuse tracheal thickening with resulting tracheobronchial stenosis. Bronchiectasis has also been reported but this occurs more often as a result of fibrotic changes in the lung with resulting traction bronchiectasis. Given that IgG4-RD could involve lung parenchyma and/or airway, patients may have a restrictive or obstructive process or both and have impaired diffusion. No parenchymal, pleural or intra-thoracic adenopathy was present in our patient.
Diagnosis of IgG4 requires sampling of the involved tissue. Pathogenesis is likely an overlap of autoimmune and allergic mechanisms. The typical histologic pattern in IgG4-RD consists of tumefactive lesions with lymphoplasmacytic infiltrate with high density of IgG4 positive plasma cells, and storiform fibrosis (characterized by a cartwheel appearance of fibroblasts and inflammatory cells) [1]. Other suggestive findings include mild tissue eosinophilia and obliterative phlebitis [20]. The pathologic manifestations and criteria for IgG4-RD vary somewhat by organ site, but the overall pattern is relatively similar regardless of tissue type involved [20]. In our case, biopsies of the trachea and subglottic region demonstrated respiratory and squamous mucosa with surface erosion, fibrosis, chronic inflammation, and increased IgG4-positive plasma cells. IgG4+ plasma cells/hpf to IgG plasma cells/hpf ratio >40 % is also suggestive of IgG4-RD in the current clinical context [20]. Our patient had a ratio of 49 %. In addition, depending on the site, the number of IgG4+ cells/hpf may be suggestive of the diagnosis. For example, >10 cells/hpf and >50 cells/hpf in a lung transbronchial biopsy specimen and lung surgical specimen respectively may be suggestive of the diagnosis [21]. The appropriate number of cells in the tracheal specimen is unknown. Our patient had 90 IgG4+ cells/hpf. A resection performed a few months into the patient's second recurrence showed similar histologic features.
The first step prior to initiating therapy is to establish extent of the disease burden. In patients with systemic involvement, the mainstay of treatment is glucocorticoids 0.6 mg/kg/day. In those with contraindications to steroids, rituximab is an option. The optimal treatment for those with isolated tracheal involvement remains unknown. However, in the largest case series of 14 patients with isolated tracheal involvement, majority of them were treated with glucocorticoids [22]. In addition, two-thirds of patients received local steroid injection and balloon dilation. Most patients with systemic involvement respond well to steroids as evidenced by reduction in size of organ involvement. However, recurrence is common upon discontinuation of therapy. PET-CT may be used to monitor disease activity but it's role in management of isolated tracheal disease is unknown. Majority of patients who undergo medial therapy with immunomodulators and bronchoscopic dilation have clinical improvement. In a case series of 14 patients, a third of patients had radiographic improvement of the tracheal diameter [22]. In the remaining two-thirds of patients, majority of the patients achieve partial resolution and only one patient had disease progression. The optimal timing of follow-up imaging studies remains unknown.
4. Conclusion
IgG4-RD is a systemic disease and can affect any organ in the body. Common sites of involvement the pancreas, lymph nodes, salivary glands and eyes. Approximately, 60–90 percent of patients have systemic disease. Isolated tracheal involvement while rare has been reported in a few case reports. Plasma cell infiltration with IgG4 positivity and fibrosis is a hallmark pathologic finding. The lack of elevated serum IgG4 is common and occurs in up to 30 % of patients. The mainstay of treatment for isolated tracheal involvement is dilation and immunomodulators such as glucocorticoids or rituximab. Recurrence of the symptoms and tracheal narrowing are common and up to two-thirds of patients with tracheal involvement may need tracheal resection and reconstruction.
Funding
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Handling Editor: DR AC Amit Chopra
References
- 1.Perugino C.A., Stone J.H. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat. Rev. Rheumatol. 2020;16 doi: 10.1038/s41584-020-0500-7. 702-714. [DOI] [PubMed] [Google Scholar]
- 2.Lin Z.Q., Xu J.R., Chen J.J., et al. Pulmonary CT findings in relapsing polychondritis. Acta Radiol. 2010;51:522–526. doi: 10.3109/02841851003682036. [DOI] [PubMed] [Google Scholar]
- 3.Restrepo S., Pandit M., Villamil M.A., et al. Tracheobronchopathia osteochondroplastica: helical CT findings in 4 cases. J. Thorac. Imag. 2004;19:112–116. doi: 10.1097/00005382-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Girard C., Charles P., Terrier B., et al. Tracheobronchial stenoses in granulomatosis with polyangiitis (Wegener's): a Report on 26 Cases. Medicine (Baltim.) 2015;94 doi: 10.1097/MD.0000000000001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenique F., Brauner M.W., Grenier P., et al. CT assessment of bronchi in sarcoidosis: endoscopic and pathologic correlations. Radiology. 1995;194:419–423. doi: 10.1148/radiology.194.2.7824721. [DOI] [PubMed] [Google Scholar]
- 6.Polychronopoulos V.S., Prakash U.B.S. Airway involvement in sarcoidosis. Chest. 2009;136:1371–1380. doi: 10.1378/chest.08-2569. [DOI] [PubMed] [Google Scholar]
- 7.Kiani B., Magro C.M., Ross P. Endobronchial presentation of Hodgkin lymphoma: a review of the literature. Ann. Thorac. Surg. 2003;76:967–972. doi: 10.1016/s0003-4975(03)00140-1. [DOI] [PubMed] [Google Scholar]
- 8.Macchiarini P. Primary tracheal tumours. Lancet Oncol. 2006;7:83–91. doi: 10.1016/S1470-2045(05)70541-6. [DOI] [PubMed] [Google Scholar]
- 9.Papanikolaou I., Kagouridis K., Papiris S.A. Patterns of airway involvement in inflammatory bowel diseases. World J. Gastrointest. Pathophysiol. 2014;5:560–569. doi: 10.4291/wjgp.v5.i4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crain M.A., Lakhani D.A., Balar A.B., et al. Tracheobronchial amyloidosis: A case report and review of literature. Radiol Case Rep. 2021;16 doi: 10.1016/j.radcr.2021.05.082. 2399-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaddha U., Yaghmour B., Mahdavi R. A 55-Year-Old Man with a Trachea Undressed. Ann Am Thorac Soc. 2017;14:1212–1215. doi: 10.1513/AnnalsATS.201702-119CC. [DOI] [PubMed] [Google Scholar]
- 12.Katayama S., Tonai K., Minakata D., et al. Pseudomembranous Invasive Tracheobronchial Aspergillosis. Am. J. Respir. Crit. Care Med. 2022;205:e6–e7. doi: 10.1164/rccm.202009-3659IM. [DOI] [PubMed] [Google Scholar]
- 13.Kamisawa T., Anjiki H., Egawa N., et al. Allergic manifestations in autoimmune pancreatitis. Eur. J. Gastroenterol. Hepatol. 2009;21:1136–1139. doi: 10.1097/meg.0b013e3283297417. [DOI] [PubMed] [Google Scholar]
- 14.Sah R.P., Chari S.T. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr. Opin. Rheumatol. 2011;23:108–113. doi: 10.1097/BOR.0b013e3283413469. [DOI] [PubMed] [Google Scholar]
- 15.Kamisawa T., Okamoto A., Funata N. Clinicopathological features of autoimmune pancreatitis in relation to elevation of serum IgG4. Pancreas. 2005;31:28–31. doi: 10.1097/01.mpa.0000167000.11889.3a. [DOI] [PubMed] [Google Scholar]
- 16.Asano J., Watanabe T., Oguchi T., et al. Association Between Immunoglobulin G4-related Disease and Malignancy within 12 Years after Diagnosis: An Analysis after Longterm Followup. J. Rheumatol. 2015;42 doi: 10.3899/jrheum.150436. 2135-2142. [DOI] [PubMed] [Google Scholar]
- 17.Mulholland G.B., Jeffery C.C., Satija P., et al. Immunoglobulin G4-related diseases in the head and neck: a systematic review. J Otolaryngol Head Neck Surg. 2015;44 doi: 10.1186/s40463-015-0071-9. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atienza-Mateo B., Diaz de Teran-Lopez T., Gomez-Roman J., et al. Atypical presentation of immunoglobulin G4-related disease as subglottic stenosis: a case-based review. Rheumatol. Int. 2021;41 doi: 10.1007/s00296-021-04816-4. 1161-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu J.H., Sekiguchi H., Yi E.S. Pulmonary manifestations of immunoglobulin G4-related sclerosing disease. Eur. Respir. J. 2012;39 doi: 10.1183/09031936.00025211. 180-186. [DOI] [PubMed] [Google Scholar]
- 20.Deshpande V., Zen Y., Chan J.K., et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 2012;25 doi: 10.1038/modpathol.2012.72. 1181-1192. [DOI] [PubMed] [Google Scholar]
- 21.Shrestha B., Sekiguchi H., Colby T.V., et al. Distinctive pulmonary histopathology with increased IgG4-positive plasma cells in patients with autoimmune pancreatitis: report of 6 and 12 cases with similar histopathology. Am. J. Surg. Pathol. 2009;33:1450–1462. doi: 10.1097/PAS.0b013e3181ac43b6. [DOI] [PubMed] [Google Scholar]
- 22.Maughan E.F., Michaels J., Miller B., et al. Primary Immunoglobulin G4-Related Laryngeal Disease: A Case Series and Review of Literature. Clin. Med. Insights Case Rep. 2020;13 doi: 10.1177/1179547620960197. 1179547620960197. [DOI] [PMC free article] [PubMed] [Google Scholar]