Abstract
Background
The purpose of this study was to evaluate the prognostic value of the multigene EndoPredict test in prospectively collected data of patients screened for the randomized, double-blind, phase III UNIRAD trial, which evaluated the addition of everolimus to adjuvant endocrine therapy in high-risk, hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative early breast cancer.
Patients and methods
Patients were classified into low or high risk according to the EPclin score, consisting of a 12-gene molecular score combined with tumor size and nodal status. Association of the EPclin score with disease-free survival (DFS) and distant metastasis-free survival (DMFS) was evaluated using Kaplan–Meier estimates. The independent prognostic added value of EPclin score was tested in a multivariate Cox model after adjusting on tumor characteristics.
Results
EndoPredict test results were available for 768 patients: 663 patients classified as EPclin high risk (EPCH) and 105 patients as EPclin low risk (EPCL). Median follow-up was 70 months (range 1-172 months). For the 429 EPCH randomized patients, there was no significant difference in DFS between treatment arms. The 60-month relapse rate for patients in the EPCL and EPCH groups was 0% and 7%, respectively. Hazard ratio (HR) supposing continuous EPclin score was 1.87 [95% confidence interval (CI) 1.4-2.5, P < 0.0001]. This prognostic effect remained significant when assessed in a Cox model adjusting on tumor size, number of positive nodes and tumor grade (HR 1.52, 95% CI 1.09-2.13, P = 0.0141). The 60-month DMFS for patients in the EPCL and EPCH groups was 100% and 94%, respectively (adjusted HR 8.10, 95% CI 1.1-59.1, P < 0.0001).
Conclusions
The results confirm the value of EPclin score as an independent prognostic parameter in node-positive, hormone receptor-positive, HER2-negative early breast cancer patients receiving standard adjuvant treatment. EPclin score can be used to identify patients at higher risk of recurrence who may warrant additional systemic treatments.
Key words: breast cancer, EndoPredict, risk stratification, endocrine therapy, prognostic biomarker
Highlights
-
•
EndoPredict was prospectively evaluated in node-positive, early breast cancer patients, screened for the UNIRAD trial.
-
•
The independent prognostic value of EPclin score was confirmed.
-
•
EPclin score can be used to identify patients at higher risk of recurrence who may warrant additional systemic treatments.
Introduction
Hormone receptor-positive and human epidermal growth factor receptor 2 (HER2)-negative breast cancer is the most common breast cancer subtype, representing ∼70% of breast cancer.1 Adjuvant endocrine therapies (ETs), which have been shown to substantially reduce the risk of recurrence (ROR) and death,2, 3, 4 are the standard of care. Nevertheless, the ROR persists for up to 20 years after administration of ET, with late recurrence occurring in up to 41% of patients at high clinico-pathological risk.5 In addition to classical risk factors such as age, tumor size and nodal status, genomic signatures have been developed in the past two decades to evaluate the patients’ long-term prognosis and guide treatment decisions.6 Current international guidelines recommend using multigene assays to identify low-risk patients who would benefit from ET alone and those at high risk who would benefit from addition of chemotherapy to ET or extended ET.7,8
The EndoPredict test is a multigene test comprising 12 genes (8 genes linked to proliferation pathways, apoptosis pathways and hormone receptor pathways, as well as 4 control genes). This 12-gene molecular score combined with two risk factors, tumor size and nodal status, gives the EPclin score, which predicts distant recurrence in hormone receptor-positive, HER2-negative disease.9 The prognostic value of Epclin has been retrospectively validated in several independent clinical trials.10, 11, 12, 13, 14, 15
The Epclin score was used to classify patients into low or high risk in the double-blind, multicenter, international, randomized trial that compared the combination of adjuvant everolimus plus standard adjuvant ET with placebo plus ET in women with high-risk, hormone receptor-positive, HER2-negative early breast cancer (ClinicalTrials.gov: NCT0180527116). We report here the results of the non-protocol-defined exploratory sub-analysis that evaluated the prognostic added value of EndoPredict test results on outcomes of patients screened for the UNIRAD study.
Patients and methods
In the UNIRAD trial, patients with estrogen receptor (ER)-positive, HER2-negative breast cancer at high risk of relapse were randomly assigned to receive either everolimus in combination with standard adjuvant ET or ET alone. Patients had to have their primary tumor completely resected, with no clinically or radiologically detectable metastases at the time of inclusion. Patients initiated ET at the same time as the study treatment (everolimus or placebo) or up to 4 years before. Primary endpoint was disease-free survival (DFS) measured from the date of randomization. DFS events were defined as invasive local, regional or metastatic relapse, contralateral breast cancer or death from any cause. Secondary endpoints included overall survival, distant metastasis-free survival (DMFS) defined as metastatic relapse or death from any cause, second malignancies and toxicity. Between June 2013 and March 2020, 1278 patients were included in the trial (637 in the everolimus arm and 641 in the placebo arm). The trial was stopped for futility at the first interim analysis. Three-year DFS did not differ between patients who received ET plus everolimus, 88% [95% confidence interval (CI) 85% to 91%], or ET plus placebo, 89% [95% CI 86% to 91%; hazard ratio (HR) 0.95, 95% CI 0.69-1.32, P = 0.77].16
In the UNIRAD study, high risk was defined as ≥4 positive lymph nodes, ≥1 positive lymph node if surgery was carried out after neoadjuvant chemotherapy or ET administered for ≥3 months; or 1-3 positive lymph nodes at primary surgery and an Epclin score ≥3.3. Patients classified as Epclin low risk (EPCL) were not included in the trial, but were followed up with regard to recurrence and survival.
Statistical analysis
In this sub-analysis, we evaluated the prognostic added value of EndoPredict test results on outcomes in patients screened for the UNIRAD study. As there was no significant effect of everolimus on DFS, the present analysis was carried out on all patients who were screened for the trial and had an Epclin score, regardless of enrollment in the study or treatment arm (everolimus or placebo). Association of the Epclin score with the primary and secondary endpoints, DFS and DMFS, was evaluated using Kaplan–Meier estimates. The Cox regression was carried out provided the proportional hazards assumption was fulfilled; proportionality was evaluated using test of the interaction between treatment arm and time. DFS and DMFS were estimated from the date of EndoPredict testing. The independent prognostic added value of Epclin score was tested in a multivariate Cox model after adjusting on tumor characteristics (grade, T and N). P values were based on likelihood ratio chi-square test statistics and reported as two-sided. A two-sided P value <0.05 was considered as statistically significant. Estimated risks were reported with 95% log-log CIs. Ninety-five percent CIs were reported with HRs.
Results
Patient characteristics
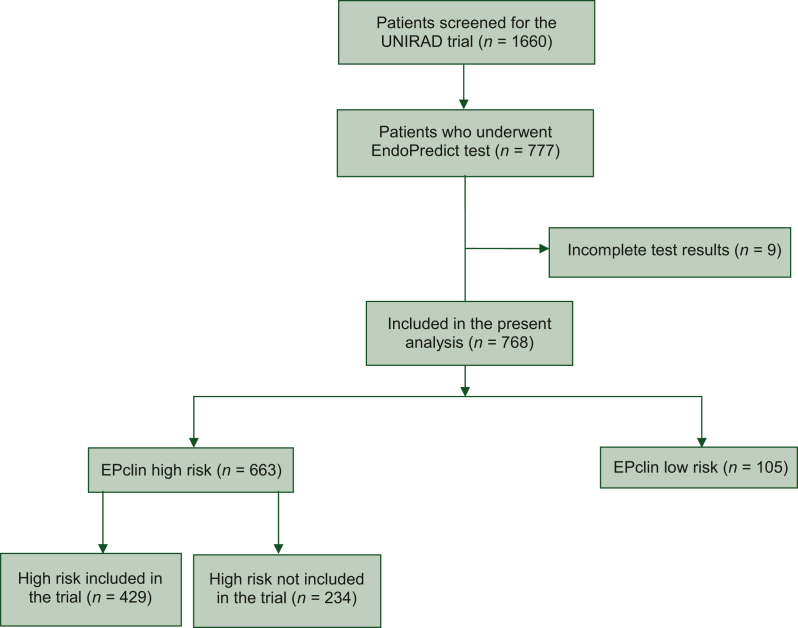
A total of 777 patients with ER-positive, HER2-negative early breast cancer underwent EndoPredict test screening between May 2015 and March 2020, of which 768 (99%) had complete test results and were included in the present analysis (Figure 1). As per the study procedure, patients undergoing EndoPredict test screening had not received neoadjuvant chemotherapy. The cohort comprised 663 (86%) patients who were classified as Epclin high risk (EPCH) (n = 429 included in the trial; n = 234 not included in the trial) and 105 (14%) patients classified as EPCL (none included in the trial). The mean Epclin score of the entire cohort was 4.18 [standard deviation (SD) = 0.83], and median score 4.10 (min; max 2.2; 6.5) [interquartile range (IQR) 3.6-4.8]. The mean score of the EPCH group included in the trial was 4.39 (SD = 0.76) and the median 4.20 (3.3; 6.5) (IQR 3.8-4.9). The mean score of the EPCH group not included in the trial was 4.35 (SD = 0.64) and the median 4.20 (3.4; 6.2) (IQR 3.9-4.8). The mean score of the EPCL group was 2.97 (SD = 0.28) and the median 3.00 (2.2; 3.3) (IQR 2.8-3.2). Baseline patient characteristics are shown in Table 1. Median follow-up of the cohort was 70 months (min = 1; max = 172) from the date of EndoPredict test: that of EPCH patients included in the trial was 79.9 months (10-134 months), that of EPCH patients not included in the trial 47.8 months (2.9-172 months) and that of EPCL patients 68.5 months (9.7-135 months). Two hundred and sixty-nine (35%) patients reported being premenopausal. While the EndoPredict test was only proposed to patients with 1-3 positive nodes as per the study protocol, 8 patients with ≥4 positive nodes and 10 node-negative patients underwent EndoPredict test. For three patients with ≥4 positive nodes, EndoPredict was carried out by error. For the remaining five patients, no explanation could be provided.
Figure 1.
Study flow chart.
Table 1.
Baseline patient and tumor characteristics
| EPclin subgroup |
All patients |
|||
|---|---|---|---|---|
| High risk included in the trial |
High risk not included in the trial |
Low risk |
||
| n = 429 | n = 234 | n = 105 | n = 768 | |
| Age, years | ||||
| n | 429 | 231 | 104 | 764 |
| Mean (SD) | 54.31 (10.32) | 55.49 (10.99) | 56.95 (10.13) | 55.03 (10.53) |
| Median (min; max) | 52.00 (31.0; 89.0) | 55.00 (31.0; 89.0) | 56.00 (30.0; 78.0) | 54.00 (30.0; 89.0) |
| IQR (Q1-Q3) | (46.0-62.0) | (47.0-64.0) | (49.5-65.0) | (47.0-63.0) |
| Age group, n (%) | ||||
| ≤50 years | 172 (40.1) | 90 (39.0) | 29 (27.9) | 291 (38.1) |
| >50 years | 257 (59.9) | 141 (61.0) | 75 (72.1) | 473 (61.9) |
| Missing | 0 | 3 | 1 | 4 |
| Menopausal status, n (%) | ||||
| No | 150 (35.0) | 90 (38.8) | 29 (27.9) | 269 (35.2) |
| Yes | 279 (65.0) | 142 (61.2) | 75 (72.1) | 496 (64.8) |
| Missing | 0 | 2 | 1 | 3 |
| Bilateral breast cancer, n (%) | ||||
| Yes | 22 (5.1) | 3 (1.3%) | 2 (1.9%) | 27 (3.5%) |
| Pathological size (longest axis), n (%) | ||||
| <10 mm | 10 (2.3) | 12 (5.2) | 18 (17.5) | 40 (5.2) |
| 10-20 mm | 112 (26.2) | 59 (25.4) | 37 (35.9) | 208 (27.3) |
| 20-30 mm | 122 (28.6) | 59 (25.4) | 18 (17.5) | 199 (26.1) |
| 30-50 mm | 99 (23.2) | 47 (20.3) | 14 (13.6) | 160 (21.0) |
| ≥50 mm | 84 (19.7) | 55 (23.7) | 16 (15.5) | 155 (20.3) |
| Missing | 2 | 2 | 2 | 6 |
| Invasive size | ||||
| n | 420 | 211 | 94 | 725 |
| Mean (SD) | 31.21 (23.70) | 27.68 (19.36) | 20.01 (15.64) | 28.73 (21.89) |
| Median (min; max) | 25.00 (2.0; 220.0) | 22.00 (4.0; 150.0) | 16.50 (2.0; 135.0) | 23.00 (2.0; 220.0) |
| IQR (Q1-Q3) | (18.0-35.0) | (16.0-30.0) | (12.0-25.0) | (16.0-33.0) |
| Histological type, n (%) | ||||
| Ductal NOS | 330 (76.9) | 170 (74.2) | 69 (66.3) | 569 (74.7) |
| Lobular | 54 (12.6) | 37 (16.2) | 26 (25.0) | 117 (15.4) |
| Mixed | 27 (6.3) | 10 (4.4) | 5 (4.8) | 42 (5.5) |
| Other | 18 (4.2) | 12 (5.1) | 4 (3.8) | 34 (4.4) |
| Missing | 0 | 5 | 1 | 6 |
| Elston and Ellis grade, n (%) | ||||
| Grade I | 45 (10.8) | 23 (11.1) | 29 (31.2) | 97 (13.5) |
| Grade II | 235 (56.4) | 132 (63.8) | 61 (65.6) | 428 (59.7) |
| Grade III | 137 (32.9) | 52 (25.1) | 3 (3.2) | 192 (26.8) |
| Unknown or missing | 12 | 27 | 12 | 51 |
| Lymphovascular embolism, n (%) | ||||
| No | 223 (55.6) | 115 (59.0) | 71 (78.9) | 409 (59.6) |
| Yes | 178 (44.4) | 80 (41.0) | 19 (21.1) | 277 (40.4) |
| Missing | 28 | 39 | 15 | 82 |
| Associated DCIS, n (%) | ||||
| No | 153 (39.0) | 104 (49.3) | 49 (53.8) | 306 (44.1) |
| Yes | 239 (61.0) | 107 (50.7) | 42 (46.2) | 388 (55.9) |
| Missing | 37 | 23 | 14 | 74 |
| Number of positive nodesa,n (%) | ||||
| 0 | 4 (0.9) | 8 (3.5) | 2 (2.0) | 14 (1.8) |
| 1 | 186 (43.4) | 118 (51.3) | 55 (53.9) | 359 (47.2) |
| 2 | 131 (30.5) | 62 (27.0) | 34 (33.3) | 227 (29.8) |
| 3 | 102 (23.8) | 40 (17.4) | 11 (10.8) | 153 (20.1) |
| >3 | 6 (1.4) | 2 (0.9) | 0 (0.0) | 8 (1.1) |
| Missing | 0 | 4 | 3 | 7 |
| Estrogen receptor status, n (%) | ||||
| Negative | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.1) |
| Positive | 429 (100.0) | 226 (99.6) | 102 (100.0) | 757 (99.9) |
| Missing | 0 | 7 | 3 | 10 |
| Progesterone receptor status, n (%) | ||||
| Negative | 57 (13.5) | 27 (14.4) | 5 (5.7) | 89 (12.8) |
| Positive | 365 (86.5) | 161 (85.6) | 83 (94.3) | 609 (87.2) |
| Missing | 7 | 46 | 17 | 70 |
| FISH result, n (%) | ||||
| Non-amplified | 60 (98.4) | 34 (97.1) | 14 (100.0) | 108 (98.2) |
| Amplified | 1 (1.6) | 1 (2.9) | 0 (0.0) | 2 (1.8) |
| Missing | 368 | 199 | 91 | 658 |
| CISH result, n (%) | ||||
| Non-amplified | 17 (100.0) | 4 (100.0) | 0 | 21 (100.0) |
| Missing | 412 | 230 | 105 | 747 |
| DISH result, n (%) | ||||
| Non-amplified | 9 (100.0) | 4 (100.0) | 0 | 13 (100.0) |
| Missing | 420 | 230 | 105 | 755 |
| Pathological tumor size, n (%) | ||||
| pT0 | 1 (0.2) | 2 (0.9) | 1 (1.0) | 4 (0.5) |
| pT1 | 163 (38.1) | 87 (38.5) | 68 (66.7) | 318 (42.1) |
| pT2 | 213 (49.8) | 115 (50.9) | 32 (31.4) | 360 (47.6) |
| pT3 | 50 (11.7) | 20 (8.8) | 0 (0.0) | 70 (9.3) |
| pT4 | 1 (0.%) | 2 (0.9) | 0 (0.0) | 3 (0.4) |
| I | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (0.1) |
| Missing | 1 | 8 | 3 | 12 |
| Pathological node staging, n (%) | ||||
| pN0 | 4 (0.9) | 5 (2.2) | 1 (1.0) | 10 (1.3) |
| pN1 | 411 (95.8 | 212 (94.2) | 99 (97.1) | 722 (95.5) |
| pN2 | 11 (2.6) | 4 (1.8) | 2 (2.0) | 17 (2.2) |
| pN3 | 3 (0.7) | 2 (0.9) | 0 (0.0) | 5 (0.7) |
| pNX | 0 (0.0) | 2 (0.9) | 0 (0.0) | 2 (0.3) |
| Missing | 0 | 9 | 3 | 12 |
| Distant metastasis, n (%) | ||||
| pM0 | 419 (97.7) | 199 (87.7) | 98 (95.1) | 716 (94.3) |
| pM1 | 1 (0.2) | 4 (1.8) | 0 (0.0) | 5 (0.7) |
| pMX | 9 (2.1) | 24 (10.6) | 5 (4.9) | 38 (5.0) |
| Missing | 0 | 7 | 2 | 9 |
CISH, chromogenic in situ hybridization; DCIS, ductal carcinoma in situ; DISH, dual in situ hybridization; FISH, fluorescence in situ hybridization; IQR, interquartile range; NOS, not otherwise specified; SD, standard deviation.
Ten pN0 and eight pN4+ patients were included despite the non-inclusion criteria.
Prognostic value of EPclin score
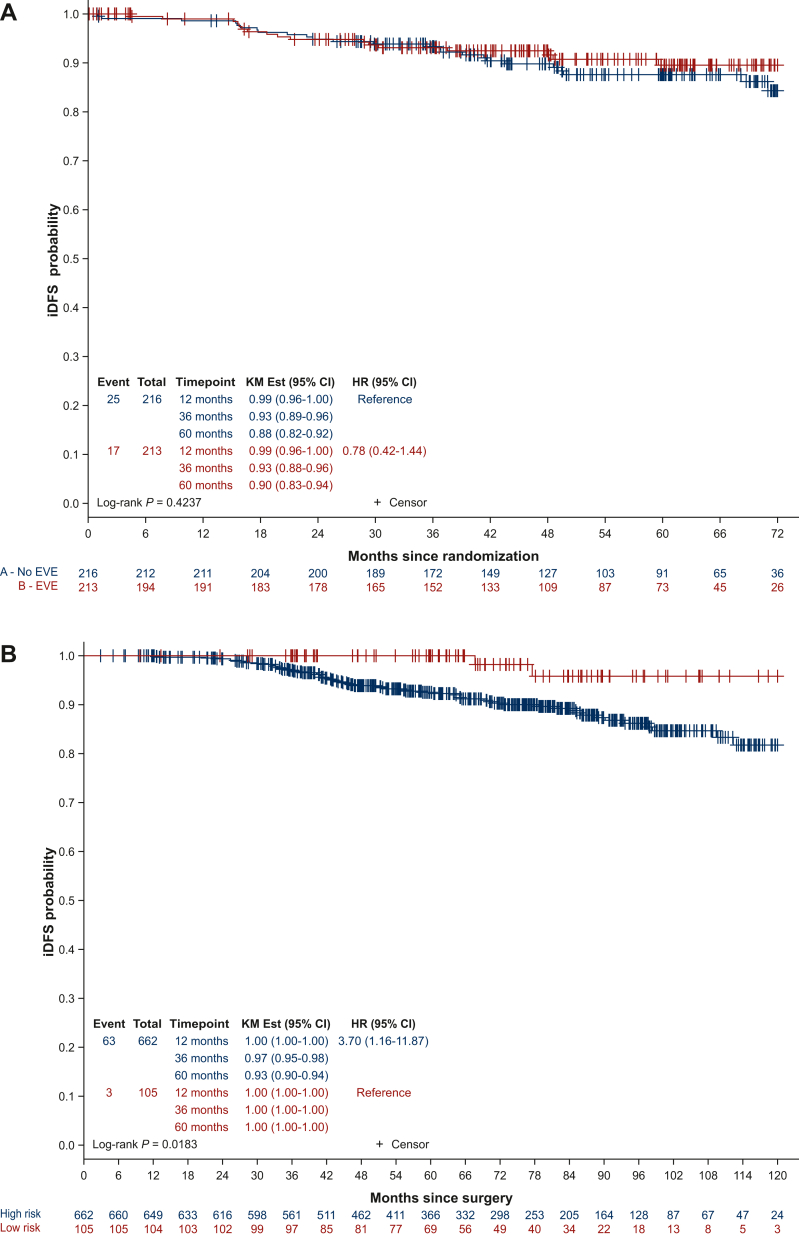
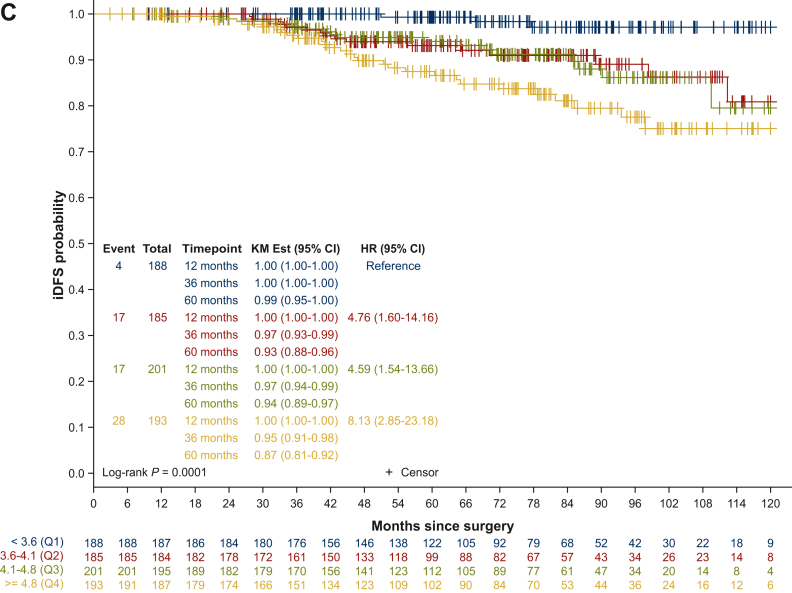
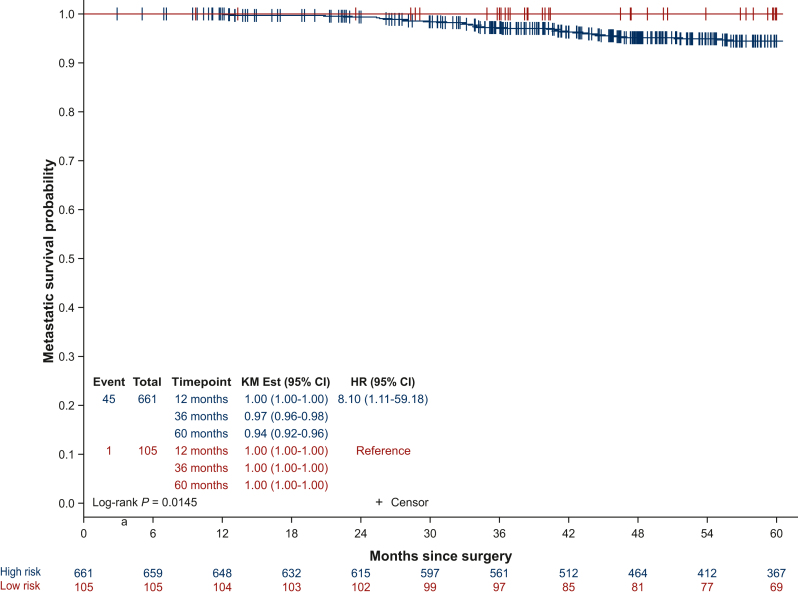
For the 429 EPCH patients included in the trial, there was no significant difference in DFS between treatment arms (n = 213 everolimus arm, n = 216 placebo arm; HR 0.79, 95% CI 0.42-1.44, log-rank P = 0.4237; Figure 2A). Of the 768 patients included in the analysis, one patient was removed from subsequent analyses, as she withdrew her consent 1 day after screening. The independent prognostic added value of EPclin score was therefore analyzed in 767 patients (n = 105 EPCL; n = 662 EPCH). The 60-month relapse rate from testing for patients in the EPCL group and the EPCH group was 0% and 7%, respectively (HR supposing continuous EPclin score 1.87, 95% CI 1.4-2.5, P < 0.0001; log-rank P = 0.0189; Figure 2B). Interestingly, when assessing the prognosis of patients within quartiles of the EPclin score (Q1 <3.6; Q2 3.6-4.1; Q3 4.1-4.8; Q4 ≥4.8), 60-month DFS was 99%, 93%, 94% and 87%, respectively (log-rank P = 0.0001; Figure 2C). This difference remained significant when assessed in a Cox model with tumor size, number of positive nodes and tumor grade (HR 1.73, 95% CI 1.25-2.41, P = 0.0010; Table 2). Age and menopausal status were not significant in univariate analysis (data not shown). Furthermore, EPclin results were independently correlated to DMFS, with 60-month DMFS for patients in the EPCL and EPCH groups of 100% and 94%, respectively (adjusted HR 8.10, 95% CI 1.11-59.1, P < 0.0001; log-rank P = 0.0145; Figure 3). The distribution of the EPclin score of the EPCH patients (EPclin score ≥3.3) who did not relapse is shown in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103443. A wide distribution of the EPclin score was seen (between 3.3 and 6.3), and despite the cut-off value of 3.3, those who did not relapse were not more likely to have a score close to 3.3. On the contrary, patients with relapse or death had an EPclin score between 3.5 and 6.5.
Figure 2.
Disease-free survival. (A) Disease-free survival in high-risk patients included in the UNIRAD trial, according to treatment arms. (B) Disease-free survival according to EPclin low- and high-risk subgroups. (C) Disease-free survival according to quartiles of the EPclin score.
CI, confidence interval; EVE, everolimus; HR, hazard ratio; iDFS, invasive disease-free survival; KM, Kaplan–Meier.
Table 2.
Multivariate analysis of iDFS with tumor size, number of positive nodes and tumor grade
| Parameter | Class | Events/n | Hazard ratio | 95% CI | P value |
|---|---|---|---|---|---|
| EPclin score | 63/710 | 1.52 | 1.09-2.13 | 0.0141 | |
| Number of positive nodes | 0-1 | 28/338 | 0.9800 | ||
| 2 | 20/219 | 1.009 | 0.56-1.81 | ||
| ≥3 | 15/153 | 0.944 | 0.49-1.81 | ||
| Elston and Ellis grade | Grade I | 4/96 | 0.6582 | ||
| Grade II | 32/423 | 1.31 | 0.45-3.79 | ||
| Grade III | 27/191 | 1.61 | 0.51-5.03 | ||
| Pathological size (longest axis) | <10 mm | 2/36 | 0.2350 | ||
| 10-20 mm | 7/189 | 0.481 | 0.10-2.34 | ||
| 20-30 mm | 18/187 | 1.061 | 0.24-4.64 | ||
| 30-50 mm | 18/148 | 1.213 | 0.27-5.33 | ||
| ≥50 mm | 18/150 | 1.362 | 0.31-5.99 |
CI, confidence interval; iDFS, invasive disease-free survival.
Figure 3.
Distant metastasis-free survival according to EPclin risk category.
CI, confidence interval; HR, hazard ratio; KM, Kaplan–Meier.
The use of the EPclin score to inform treatment decisions
In order to assess whether the EPclin score could help identify patients who would benefit or not from additional systemic treatments, we further compared the eligibility of the patients in the present study and the NATALEE and MonarchE trials, which evaluated the addition of cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) ribociclib and abemaciclib in high-risk, ER-positive, HER2-negative early breast cancer patients,17,18 respectively. The patients in the present study (n = 768) would have all been eligible for the NATALEE study that enrolled patients with stage II or III ER-positive, HER2-negative early breast cancer at ROR, including the 105 EPCL patients. For the MonarchE trial, 308 patients in our study fulfilled the criteria (grade 3 or Ki-67 ≥20%), out of which 302 were EPCH and 6 EPCL. Of note, patients whose tumor was grade 3 or Ki-67 ≥20% were in 98% of the cases EPCH. On the contrary, out of the 484 patients who were grade 2 and Ki-67 <20%, and who would therefore not have been eligible for the MonarchE trial, 406 were EPCH (n = 264 included in the trial; n = 142 not included in the trial) and 78 EPCL. A total of 33 patients relapsed among the EPCH patients and 3 among the EPCL patients.
Discussion
Our results confirm the value of the EPclin score as an independent prognostic parameter in node-positive, ER-positive, HER2-negative early breast cancer patients receiving standard adjuvant treatment. As previously reported, for the patients included in the trial, there was no significant difference in the 12-month or 24-month DFS between treatment arms.16 The analysis of prognosis by quartiles of the EPclin score identified the fourth quartile, with EPclin score ≥4.8, as a very high-risk population. This information would be particularly relevant for the selection of adjuvant interventions, particularly chemotherapy and new targeted therapies. Indeed, several new agents, including CDK4/6i ribociclib17 and abemaciclib,18 have been reported to show benefit in high-risk, ER-positive, HER2-negative early breast cancer patients and olaparib in patients with a germline mutation in BRCA1 or BRCA2 genes.19,20 The very-high-risk population identified by the EPclin score would benefit from CDK4/6i.
In addition, we confirm in our prospective series a noteworthy lack of events in the low-risk group. Our findings suggest that this patient group may not benefit from the addition of CDK4/6i to standard ET. According to the inclusion criteria, all the patients in the present analysis fulfilled the criteria (n = 768) for the NATALEE trial that evaluated adjuvant ribociclib17 and 308 patients for the MonarchE trial that evaluated abemaciclib18 in high-risk, ER-positive, HER2-negative early breast cancer patients. The EPCL patients (n = 105) in our study had an excellent prognosis (60-month relapse rate of 0%), suggesting that they would not have benefited from the addition of ribociclib. In the case of MonarchE trial, only 6 of the 308 patients who fulfilled the criteria were EPCL and would therefore not have benefited from abemaciclib. Interestingly, 98% of patients whose tumor was grade 3 or Ki-67 ≥20% were EPCH in our patient population, raising the question of the necessity of testing these patients.
This study was a prospective analysis of a cohort from the phase III randomized UNIRAD trial. Our analysis confirms recently published results of retrospective analyses of data from randomized trials. A recent study analyzed 2630 postmenopausal patients with ER-positive, HER2-negative early breast cancer included in three phase III randomized trials (ABCSG-6, ABCSG-8 and TransATAC).21 All the patients had received ET in the absence of chemotherapy. The cohort comprised both node-negative and node-positive patients, although the majority (70%) of patients were node negative. EPclin was prognostic in patients with both invasive ductal carcinoma and invasive lobular carcinoma. A comparison of six prognostic signatures for ER-positive breast cancer in the TransATAC cohort showed that three signatures, the Prosigna ROR, Breast Cancer Index (BCI) and EPclin, were prognostic for overall and late distant recurrence in node-negative disease, whereas EPclin and BCI provided significant but limited prognostic information in node-positive disease.14 Another study on patients enrolled in ABCSG-6 and ABCSG-8 trials also reported that ∼35% of patients with node-positive (1-3 nodes) disease were at low risk for distant recurrence according to the EPclin score and could safely forego chemotherapy or extended ET.15 Furthermore, the GEICAM 9906 trial that included 1246 patients with similar characteristics as the present cohort (lymph node-positive, ER-positive, HER2-negative disease, pre- and postmenopausal, chemotherapy-treated) classified 13% of the patients as low risk according to the EPclin score, and also reported a particularly low rate of distant metastatic events for this subgroup of patients (10-year DMFS of 100%).11 On the basis of currently available evidence, the recent guidelines recommend the use of EndoPredict in postmenopausal women with node-negative or node-positive with 1-3 positive nodes, ER-positive, HER2-negative early breast cancer.8 On the contrary, data are currently estimated insufficient to recommend its use in premenopausal women with 1-3 positive nodes.
Strengths of our analysis include the prospective analysis of patients screened for a randomized trial. We were able to use data from 767 pre- and postmenopausal patients with node-positive, hormone receptor-positive, HER2-negative breast cancer. EndoPredict tests were carried out in the same laboratory. The limitations include the fact that this screening was done for inclusion in the UNIRAD trial, which specifically enrolled high-risk patients.16 As a consequence, our cohort was enriched in patients classified as high risk according to EPclin score (86%). This overrepresentation of high-risk patients compared to the general population is a study bias. It should also be noted that the vast majority of patients included in the UNIRAD trial received chemotherapy.16 Lastly, currently available follow-up data do not allow us yet to evaluate the late recurrences. Patient follow-up will continue for further assessment.
In summary, our results confirm the prognostic value of EndoPredict in women with node-positive, ER-positive, HER2-negative disease receiving standard adjuvant ET. EPclin score can identify low-risk patients who could be candidates for de-escalation studies, and very-high-risk patients who are candidates for optimum adjuvant treatment, including CDK4/6i, and to whom inclusion in trials evaluating new targeted therapies may be recommended. Follow-up will continue to evaluate long-term outcomes.
Acknowledgements
We thank and acknowledge all the patients who participated in UNIRAD trial and their families, and the investigators and staff at all the clinical sites. We are grateful to the Ligue contre le Cancer and Cancer Research-UK for their support.
Funding
This work was supported by a grant from the French Ministry of Health: PHRC 2012 (no grant number) and received funding from La Ligue contre le Cancer, Cancer Research-UK, Myriad Genetics and Novartis (no grant number).
Disclosure
FPL: advisor for AstraZeneca, Daiichi Sankyo, Genomic Health, GILEAD, GSK, Lilly, Menarini/Stemline, MSD, Myriad, Nanostring, Novartis, Pfizer, Pierre-Fabre, Roche; funding: MSD, Myriad, AstraZeneca, Daiichi Sankyo; travel/expenses: AstraZeneca, BMS, Daiichi Sankyo, MSD, Novartis, Pfizer. FD: advisor for Daichi, Seagen, Novartis, Gilead, Lilly, MSD; travel/expenses: Daichi, Novartis, Pfizer. PC: advisor for Pfizer, Lilly; travel/expenses: Roche, Pfizer, Lilly, Novartis, Sanofi, BMS. JG: advisor for Daiichi Sankyo, Gilead, Pfizer; travel/expenses: Eisai Europe. MC: advisor for Novartis, Sandoz, Accord, Sanofi, Lilly, AstraZeneca, AbbVie, Seattle Genetics, Daiichi Sankyo; consulting fees: Pierre Fabre, Sanofi, Novartis, Servier, Daiichi Sankyo; travel//expenses: Novartis, AstraZeneca, Pfizer, Roche. ACHB: advisor for Novartis, AstraZeneca, Pfizer, Novartis, Clovis Onco, Seattle Genetics, Eisai, Daiichi Sankyo/Astra Zeneca, MSD. SG: advisor for Eisai, Lilly; funding: Merck, AstraZeneca; travel/expenses: Lilly. PB: advisor for Ipsen, BMS, MSD, Pfizer, Merck KGaA, Astellas, Novartis, Gilead, Bayer; travel/expenses: BMS, Pfizer, Janssen-Cilag, Astellas, MSD, Ipsen, Merck. AM: advisor for Pfizer; travel/expenses: AstraZeneca, Pierre Fabre, Lilly. MS: travel/expenses: Eisai Europe. MLT: consulting fees: Myriad Genetics. JB: funding: AstraZeneca, Merck Sharp & Dohme, Puma Biotech, Pfizer, Roche, Lilly, Janssen-Cilag, Clovis Onco; travel/expenses: Pfizer. FA: stock and other ownership interests: PEGASCY; funding: AstraZeneca, Novartis, Pfizer, Lilly, Roche, Daiichi; travel/expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca. TB: advisor for Roche, Novartis, AstraZeneca, Pfizer, Seattle Genetics, MSD; funding: Roche, Novartis, AstraZeneca, Seattle Genetics, Pfizer; travel/expenses: Roche, Pfizer, AstraZeneca. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Parker J.S., Mullins M., Cheang M.C., et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Davies C., Godwin J., Gray R., et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 5.Pan H., Gray R., Braybrooke J., et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krop I., Ismaila N., Andre F., et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2017;35(24):2838–2847. doi: 10.1200/JCO.2017.74.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso F., Kyriakides S., Ohno S., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(10):1674. doi: 10.1093/annonc/mdz189. [DOI] [PubMed] [Google Scholar]
- 8.Andre F., Ismaila N., Allison K.H., et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022;40(16):1816–1837. doi: 10.1200/JCO.22.00069. [DOI] [PubMed] [Google Scholar]
- 9.Filipits M., Rudas M., Jakesz R., et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res. 2011;17(18):6012–6020. doi: 10.1158/1078-0432.CCR-11-0926. [DOI] [PubMed] [Google Scholar]
- 10.Dubsky P., Filipits M., Jakesz R., et al. EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013;24(3):640–647. doi: 10.1093/annonc/mds334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin M., Brase J.C., Calvo L., et al. Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2− breast cancer patients: results from the GEICAM 9906 trial. Breast Cancer Res. 2014;16(2) doi: 10.1186/bcr3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin M., Brase J.C., Ruiz A., et al. Prognostic ability of EndoPredict compared to research-based versions of the PAM50 risk of recurrence (ROR) scores in node-positive, estrogen receptor-positive, and HER2-negative breast cancer. A GEICAM/9906 sub-study. Breast Cancer Res Treat. 2016;156(1):81–89. doi: 10.1007/s10549-016-3725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buus R., Sestak I., Kronenwett R., et al. Comparison of EndoPredict and EPclin with oncotype DX recurrence score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst. 2016;108(11) doi: 10.1093/jnci/djw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sestak I., Buus R., Cuzick J., et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):545–553. doi: 10.1001/jamaoncol.2017.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filipits M., Dubsky P., Rudas M., et al. Prediction of distant recurrence using EndoPredict among women with ER. Clin Cancer Res. 2019;25(13):3865–3872. doi: 10.1158/1078-0432.CCR-19-0376. [DOI] [PubMed] [Google Scholar]
- 16.Bachelot T., Cottu P., Chabaud S., et al. Everolimus added to adjuvant endocrine therapy in patients with high-risk hormone receptor-positive, human epidermal growth factor receptor 2-negative primary breast cancer. J Clin Oncol. 2022;40(32):3699–3708. doi: 10.1200/JCO.21.02179. [DOI] [PubMed] [Google Scholar]
- 17.Slamon D.J., Stroyakovskiy D., Yardley D.A., et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2− early breast cancer: primary results from the phase III NATALEE trial. J Clin Oncol. 2023;41(suppl 17) abstr LBA500. [Google Scholar]
- 18.Johnston S.R.D., Toi M., O’Shaughnessy J., et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomized, open-label, phase 3 trial. Lancet Oncol. 2023;24(1):77–90. doi: 10.1016/S1470-2045(22)00694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tutt A.N.J., Garber J.E., Kaufman B., et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geyer C.E. Jr, Garber J.E., Gelber R.D., et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol. 2022;33(12):1250–1268. doi: 10.1016/j.annonc.2022.09.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sestak I., Filipits M., Buus R., et al. Prognostic value of EndoPredict in women with hormone receptor-positive, HER2-negative invasive lobular breast cancer. Clin Cancer Res. 2020;26(17):4682–4687. doi: 10.1158/1078-0432.CCR-20-0260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.