Abstract
Recent advancements in nanotechnology have introduced a myriad of potential applications in dentistry, with nanomaterials playing an increasing role in endodontics. These nanomaterials exhibit distinctive mechanical and chemical properties, rendering them suitable for various dental applications in endodontics, including obturating materials, sealers, retro-filling agents, and root-repair materials. Certain nanomaterials demonstrate versatile functionalities in endodontics, such as antimicrobial properties that bolster the eradication of bacteria within root canals during endodontic procedures. Moreover, they offer promise in drug delivery, facilitating targeted and controlled release of therapeutic agents to enhance tissue regeneration and repair, which can be used for endodontic tissue repair or regeneration. This review outlines the diverse applications of nanomaterials in endodontics, encompassing endodontic medicaments, irrigants, obturating materials, sealers, retro-filling agents, root-repair materials, as well as pulpal repair and regeneration. The integration of nanomaterials into endodontics stands poised to revolutionize treatment methodologies, presenting substantial potential advancements in the field. Our review aims to provide guidance for the effective translation of nanotechnologies into endodontic practice, serving as an invaluable resource for researchers, clinicians, and professionals in the fields of materials science and dentistry.
Introduction
Nanotechnology has experienced notable advancements, capturing the attention of various scientific disciplines including dentistry [1]. In endodontics, the application of nanomaterials is reflected in the growing body of research literature [2–4]. As for the definition of nanomaterials, the European Commission recommends an updated definition, proposing that nanomaterials encompass solid particles of natural, incidental, or manufactured origin and at least 50% of their number-based size distribution falls within the range of 1 to 100 nm [5]. Alternatively, nanomaterials may exhibit an elongated shape, with two external dimensions smaller than 1 nm and one dimension larger than 100 nm. They can also adopt a plate-like configuration, with one external dimension smaller than 1 nm and the remaining dimensions larger than 100 nm [5]. For the United States Food and Drug Administration (FDA), nanomaterials are defined as materials or products at the atomic, molecular, or macromolecular level, with at least one dimension influencing their functional behavior within a length scale of approximately 1 to 100 nm. The implementation of a functional nano-system encompasses the design, fabrication, and deployment of structures and devices that exhibit novel properties and functions due to their small size, along with the control or manipulation of the product at the atomic level [6].
The properties of materials, including shape, texture, and size, exert a profound influence on the biological functions. At the nanoscale, materials exhibit distinct and innovative properties that are not observed in their atomic or macroscopic bulk states. This phenomenon, known as the “size effect,” arises from the nanoscale dimensions of nanomaterials. Such dimensions contribute to a large proportion of surface atoms, elevated surface energy, spatial confinement, and a decrease in structural imperfections, forming characteristics that diverge markedly from those found in bulk materials [7]. For example, nanoparticles (NPs), a type of nanomaterial characterized by a spherical morphology and nanometer-scale size, exert antibacterial effects through mechanisms distinct from traditional antimicrobial therapies [8,9]. NPs with positive charges and larger specific surface areas effectively interact with negatively charged bacteria, leading to heightened antibacterial activity compared to bulk materials [10,11].
Owing to their superior properties and advantages over conventional materials, nanomaterials have gained remarkable traction in endodontics (Fig. 1). Recent endeavors have explored the application of NPs capable of releasing reactive oxygen species (ROS) for the remediation of discolored teeth, a methodology elaborated by Kim et al. [12]. Prolonged and intensive research has been dedicated to amalgamating endodontics with nanotechnology, with a specific focus on integrating nanoscale materials into restorative resin composites and mineralization agents, as detailed in [13–18]. Moreover, nanomaterials offer substantial enhancements to root canal procedures by augmenting the efficacy of antimicrobial strategies and by improving the quality of obturation materials [19].
Fig. 1.
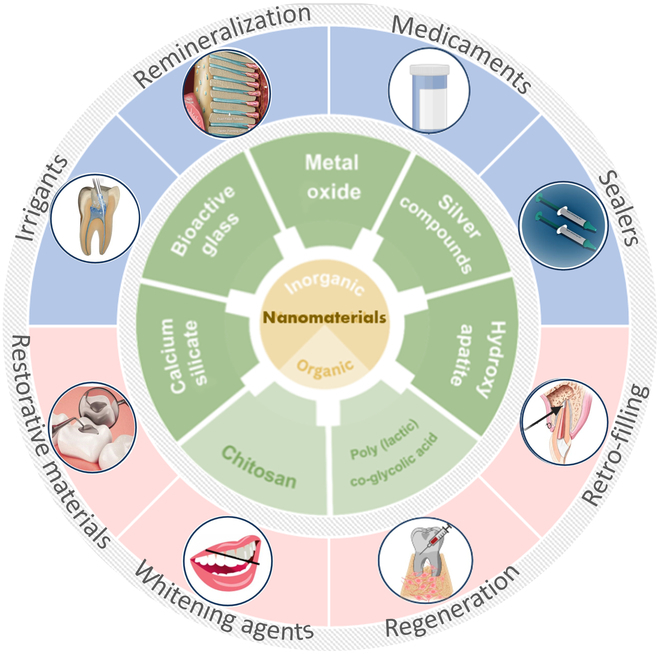
Utilization of nanomaterials in endodontic applications.
Additionally, contemporary studies have proposed nanomaterials as a viable alternative to antibiotics, aiming to mitigate the burgeoning issue of antibiotic resistance [20]. In the realm of tissue engineering, the strategic integration of nanomaterials and nanofeatures into scaffolds promises to revolutionize regenerative approaches, enabling the comprehensive restoration of the dentin–pulp complex in therapeutic settings [19]. In conclusion, nanotechnology’s integration into endodontics signifies a leap toward refining techniques, materials, and the endodontic armamentariums, with the ultimate objective of achieving superior treatment outcomes [21].
This review article provides a comprehensive overview of the diverse applications of nanomaterials in endodontics. We systematically outline their utilization across various domains including medicaments, irrigation solutions, obturation, sealing, retro-filling, root repair, and pulpal regeneration. We underscore their capacity to augment antimicrobial efficacy, enhance mechanical attributes of endodontic materials, and foster tissue regeneration. Furthermore, we offer insights into prospective research trajectories within this domain, pinpointing areas ripe for exploration and advancement. Our endeavor is to furnish a roadmap for materials scientists, engineers, biologists, dentists, and endodontists, facilitating the seamless integration of nanomaterials into the spectrum of endodontic.
Nanomaterials Used in Endodontic Medicaments
The objective of current endodontic treatment includes the thorough removal of bacteria, their byproducts, and pulpal remnants from infected root canals. Endodontic medicaments are considered a crucial step in effectively eradicating bacteria within the root canals. The most commonly used medication in endodontics is calcium hydroxide [Ca(OH)₂], which is effective due to its alkalinity [22–24]. The released hydroxyl ions cause cell membrane distortion, protein denaturation, and DNA damage [25]. However, the effectiveness of Ca(OH)₂ depends on the formulation, which in turn affects the pH value and the extent of penetration through dentine tubules [24]. Additionally, water-based Ca(OH)2 medicaments may not be able to effectively eliminate bacteria within biofilms. This was shown in a recent study for biofilms of Enterococcus faecalis [25].
Another challenge in achieving effective endodontic disinfection is to deal with biofilms that are located in regions that are difficult to access. During instrumentation of the root canal, some root canal surfaces remain untouched. Bacteria may persist in apical portions of prepared root canals even after irrigation procedures [26]. Additionally, microbial leakage can occur over time since the root canal has been filled [27]. Several nanomaterials, including organic NPs (mainly chitosan NPs) and inorganic NPs such as metal/metal oxide NPs and calcium-silicate NPs, have been utilized as endodontic medicaments to enhance the ability to eliminate endodontic biofilms. These are summarized below.
Chitosan NPs
Chitosan is a cationic polymer derived from chitin, and is one of the most abundant biopolymers in nature [28]. Chitosan is biocompatible and biodegradable. It can exert antimicrobial actions and can bind to calcium within dentine [29,30]. The antimicrobial actions vary according to the molecular weight and pH, with lower pH values enhancing this activity [29–31]. Positively charged chitosan can bind to negatively charged bacteria, causing membrane damage, increased membrane permeability, osmotic leakage, and ultimately bacterial cell death [29]. In addition, chitosan can prevent bacteria from adhering to dentine [32].
Incorporating chitosan NPs into Ca(OH)2 products can enhance their antimicrobial activity and reduce the likelihood of recolonization of dentine with bacteria following endodontic treatment [33]. Chitosan can also be used in a standalone medicament without Ca(OH)2 [34], but may be most useful when used with it. A study that investigated combining Ca(OH)2 with chitosan NPs, chlorhexidine gluconate (CHX), or glycerine, for use as an intracanal medicament, showed the highest antibacterial efficacy against E. faecalis for chitosan NPs. This combination also had the highest pH and released the most calcium ions over 14 days [35]. Similar results were observed in another study that assessed calcium ion release and changes in pH, where chitosan NPs helped maintain an alkaline pH and facilitated sustained calcium ion release within the root canal [36]. Chitosan NPs can exert antimicrobial actions against E. faecalis biofilms, as shown in studies using these NPs at concentrations of 250 μg/ml [37]. Not only capable of inhibiting the growth of E. faecalis and Streptococcus mutans, chitosan NPs are also able to exert fungicidal activity against Candida albicans [34]. Moreover, chitosan may support or reinforce dentinal collagen by binding to it through its many amino and free hydroxyl groups, and this may enhance root fracture resistance [38]. However, the penetration of chitosan into dentine can be limited due to its tendency to agglomerate, even in nanosize form.
Metal/metal oxide NPs
Silver NPs (Ag NPs) can cause damage to bacterial cells walls, and also penetrate to reach the cytoplasm, where they interact with biomolecules containing sulfur and phosphorus, such as nucleic acids [4]. In addition, Ag NPs release Ag ions that further disrupt bacterial metabolic activity [4]. Ag NPs can be delivered in various vehicles, including hydroxyethyl cellulose polymer, polyethylene glycol, and carbomer polymer. When assessed for antibacterial effects against S. mutans, E. faecalis, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus, the hydroxyethyl cellulose polymer gel gave the best handling characteristics and had superior performance as an antibacterial agent [4]. The surface charge (zeta potential) of Ag NPs affects their antibacterial activity and their interactions with human cells. In one study, Ag NPs with a positive charge displayed antimicrobial activity against E. faecalis and good levels of biocompatibility with L929 fibroblast cell lines [39]. Other studies have demonstrated that Ag NPs give sustained release of Ag+ ions, which drive persisting antimicrobial effect against E. faecalis biofilms over 9 days [40].
As well as being used alone, Ag NPs can be added into other intracanal medicaments to enhance their effectiveness. A useful combination is Ag NPs with Ca(OH)2, which can significantly impair the growth of E. faecalis biofilms [26]. This combination also shows enhanced anti-inflammatory actions [41] and antioxidant activity [41], as well as antibiofilm actions, even when used for short-term applications [42]. It is essential that, when Ag NPs are combined with other materials, the carrier is able to release the Ag NPs. This problem has been seen with methyl cellulose gels, where release of Ag NPs is minimal [43].
Little is known about the mechanisms of adaptation to Ag NPs by endodontic bacteria, which may include intrinsic (efflux pumps, down-regulation of porins, chromosomal resistance genes) or extrinsic (point and adaptive mutations, plasmids with resistance genes) systems [44]. A recent report of silver resistance genes in endodontic pathogens raises the concern for endodontic use of Ag NPs. Bacteria present in secondary endodontic infections frequently have plasmids that carry genes for antibiotic resistance, and these same plasmids are now also recognized to also carry genes for resistance to certain metals such as silver. Due to the presence of such resistance genes, the use of metal NPs (especially Ag NPs) in endodontic treatment needs careful assessment in the context of resistance [45].
Similar to silver, copper NPs (Cu NPs) also exert antimicrobial actions against Gram-positive and Gram-negative bacteria as well as fungi [46]. They penetrate bacterial cell walls and then bind to sulfhydryl (-SH groups), causing protein denaturation and enzyme inactivation. Cu NPs also indirectly impair the synthesis of DNA and proteins in bacteria [47]. Besides an immediate action, Cu NPs also exert a longer-term antimicrobial action over 7 days. This makes them of interest against multi-species biofilms [47]. Cu NPs cause greater generation of ROS than ZnO NPs, making them superior against many strains of bacteria [48]. The released ROS penetrate through biofilms, causing inactivation of bacteria even in the deeper layers [49].
In addition to metal, zinc oxide NPs (ZnO NPs) exert antimicrobial actions against a wide range of microorganisms through the generation of ROS and penetration of NPs through the outer cell membrane or into the cytoplasm. ZnO NPs can inhibit the growth of C. albicans [50] as well as Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans [51], and E. faecalis [52]. ZnO NPs are superior to microparticles of ZnO when added to Ca(OH)2, giving enhanced release of hydroxyl ions and improved antibacterial activity against E. faecalis [53]. The addition of ZnO NPs to Ca(OH)2 makes such combination medicaments of interest as intracanal medicaments [54]; however, adding ZnO NPs may not be as useful as adding other materials, such as chlorhexidine [55].
Calcium-silicate NPs
Calcium-silicate NPs such as Ca(OH)₂ NPs, bioactive glass, and mesoporous silica NPs are also used for endodontic medicaments. Compared with conventional micrometer-sized particles, Ca(OH)2 NPs (<100 nm) are considered to penetrate deeper into dentinal tubules, and this gives improved antimicrobial actions against E. faecalis at depths up to 400 μm within dentinal tubules [56]. These NPs when in the size range of 80 to 115 nm did not cause any change in fracture resistance of the teeth over 1 month [38]. NPs of Ca(OH)2 are more effective at eliminating E. faecalis than NPs of calcium oxide, when tested in blocks of dentine [57]. Ca(OH)2 NPs appear to be a safe and effective alternative to conventional Ca(OH)2 and cause no significant adverse effects on dentine microhardness [58], but will cause subtle changes in the chemical structure of superficial layers of dentine, as evidenced by an increase in the phosphate/amide I ratio [58].
Bioactive glass contains SiO2, CaO, Na2O, and P2O5 and is reported to have antimicrobial actions. Antimicrobial effects of bioglass NPs on E. faecalis biofilms are superior to those of larger particles of bioglass [59]. Mesoporous bioactive glass (MBG) is a new class of bioglass with a well-ordered mesoporous channel structure and pores that can be loaded with various materials to enhance the biological activity of the NPs [60,61]. Silver-loaded MBG NPs give sustained release of Ag ions, making them effective against planktonic bacteria [62] as well as biofilms [62]. In addition to MBG NPs, mesoporous silica NPs were also reported to be loaded with CHX, and under alkaline conditions, they can release CHX, as well as calcium and silicate ions, in a sustained manner. Such NPs can exhibit strong antimicrobial actions against E. faecalis. They can also promote mineral formation [63]. In a similar manner, mesoporous calcium-silicate NPs can also be loaded with silver to give sustained release of Ag+, Ca2+, and SiO32− ions. The released silver ions can then interfere with the growth of endodontic bacteria, such as E. faecalis [64].
Nanomaterials Used as Endodontic Irrigants
Irrigation is a key aspect of endodontic disinfection as it can reach, inactivate, and dislodge bacteria attached to dentine walls that have not been reached by instruments. Thus, irrigation facilitates both the killing and removal of microbes, as well as the removal of remnants of necrotic tissues and dentine debris [65]. To date, common choices for irrigants with antimicrobial activity for use in root canal treatment are sodium hypochlorite (NaOCl), CHX, and hydrogen peroxide [66]. CHX is less irritant than NaOCl should accidental extrusion occur; however, it cannot dissolve organic matter. Its lack of proteolytic action necessitates the use of NaOCl as the main irrigant.
In an ideal scenario, a nontoxic or low-toxic irrigant with sufficient antimicrobial ability would address concerns about adverse reactions caused by inadvertent extrusion. NPs have been explored as possible alternatives, especially Ag NPs [4,8,67]. NPs have a high surface area-to-volume ratio that increases the interactions with microbes, and can be designed to have particular sizes and shapes that enhance their antimicrobial actions [68]. It was reported that Ag NPs have high biocompatibility for human tissues, but are highly toxic for bacteria, including E. faecalis [69,70]. Moreover, as shown by optical coherence tomography, Ag NPs can reach the apical third of the root canal when used in endodontic irrigants [71]. Ag NPs (1 to 2 nm in size) were found to penetrate the smear layer and form a barrier film on the dentinal surface, which would potentially lower the risk of later bacterial invasion [72], without harming the physical structure of the dentine itself [73,74].
An important challenge is the delivery of irrigants into regions of the root canal system with complex anatomy [75]. Chitosan will form deposits on dentine surfaces [76]. To drive penetration further into the root canal, low-level electric fields have been used to facilitate the movement of cationic chitosan NPs. This approach causes chitosan NPs to form a thin homogeneous layer in the apical 2 mm of the root canal system, with an associated reduction in bacterial load [77]. This concept has been extended to include actively propelled NPs to target locations that are hard to reach. One example includes using oscillating magnetic fields to propel helical nanobots with an iron-cored silica blob-head to travel through dentinal tubules, with magnetic hyperthermia being used to kill bacteria [78].
Moreover, NPs can be used in conjunction with various activation methods to enhance bactericidal effects against E. faecalis and other prominent pathogens featured with high resistance to disinfectants. It has been reported that activation methods including manual dynamic activation (MDA), passive ultrasonic irrigation (PUI), and sonic irrigation can improve irrigant penetration across different regions of the root canal [79]. Ag NPs-graphene oxide irrigants under ultrasonic activation exhibited improved efficacy against multispecies biofilm compared to syringe-mediated irrigation and XP Endo Finisher file-mediated irrigation [80]. Another promising way to optimize irrigation is to introduce laser-assisted activation. Near-infrared (NIR) laser energy itself can have disinfecting actions deep in dentine and impedes the re-colonization of biofilm due to its capability to occlude dentinal tubules [81]. Nanocurcumin photosensitizers and light-emitting diode (LED) were combined as a means of photodynamic therapies (PDTs) to eliminate E. faecalis from the root canal system. Along with the shock wave enhanced emission photoacoustic streaming technique, this combination therapy had improved efficacy against E. faecalis [82]. Diode lasers (DLs) have been combined with chitosan NPs to achieve bacterial eradication [83]. Ag NPs, together with Nd:YAG lasers, showed greatest reduction of E. faecalis, giving superior effects to using Ag NPs alone [84]. Another study compared the effectiveness of Ag NPs, an 810-nm DL, conventional PDT with indocyanine green (ICG) photosensitizer, modified PDT with Ag NPs in disinfecting root canals infected with E. faecalis, and found significant reductions in bacterial colony counts across all groups, with the greatest reduction observed in the Ag NPs/ICG/810-nm DL group, suggesting the potential use of PDT with Ag NPs, DL, and Ag NPs as an adjunct for root canal disinfection [85]. Although diverse lasers are available, the wide range of laser parameters reported in various studies leads to a dilemma where there is no universally agreed standardized disinfecting protocol at the present time [86]. One recent study further compared the efficacy of different systems to activate Ag NP irrigant for eliminating E. faecalis, and results showed that photon-induced photoacoustic streaming and passive ultrasonic irrigation led to higher antibacterial efficacy when compared with DL-activated irrigation and MDA [87]. Further investigations are necessary to identify whether and what kind of activations along with nanomaterials have an indispensable role in elimination of microorganisms from the root canal system.
NPs may also be of use for smear layer removal. Chitosan is a metal chelator, even at concentrations as low as 0.2% by weight [88]. Using chitosan may enhance the penetration of root canal sealers into dentinal tubules [89]. The smear layer removal capabilities of chitosan NPs are now attracting considerable interest [32]. In one study, chitosan NPs (0.2%) were as effective for eliminating smear layer as 17% EDTA, but caused less erosion, leaving the canal walls with greater microhardness and lower surface roughness [90]. In another study, chitosan NPs (0.5%) were superior to both 17% EDTA and 10% citric acid for removal of smear layer in the apical third [91]. However, this capability of smear layer elimination was inhibited at the apical third when chitosan NPs were at a higher concentration (2%).
In addition, NP-based irrigants can potentially improve the fracture resistance of endodontically treated teeth [92]. Chitosan NPs inhibit the enzymatic degradation of collagen, which can thus help to conserve the physical properties of dentine [93], and stabilize the structural integrity of the root over the longer term [94,95]. This adds to the previously mentioned benefits of using chitosan NPs in irrigation fluids to lower bacterial loads and remove smear layer.
Nanomaterials Used as Obturating Materials
Obturating materials are important in endodontics to fill the root canal. Gutta-percha is the most commonly used root filling material [96]. It contains both organic and inorganic components. The organic components, such as natural latex isoprene polymers and waxes, provide flexibility and compressibility to the material. The inorganic components, such as zinc oxide, provide radiopacity and serve as fillers; however, they also make the material more brittle [97]. Despite its widespread use, gutta-percha has several shortcomings, notably the absence of antibacterial properties. Consequently, there is a growing interest in incorporating NPs into gutta-percha points to address these limitations [98]. Lee et al. [99] developed gutta-percha incorporating nanodiamond–amoxicillin conjugates, where the nanodiamond particles (4 to 6 nm) exhibit inherent antibacterial properties and adsorb antibiotics, facilitating the elimination of persistent bacteria. This nanodiamond–gutta-percha composition can lower the risk of re-infection and concurrently enhance the mechanical strength of gutta-percha points, thereby improving their clinical manageability (Fig. 2).
Fig. 2.
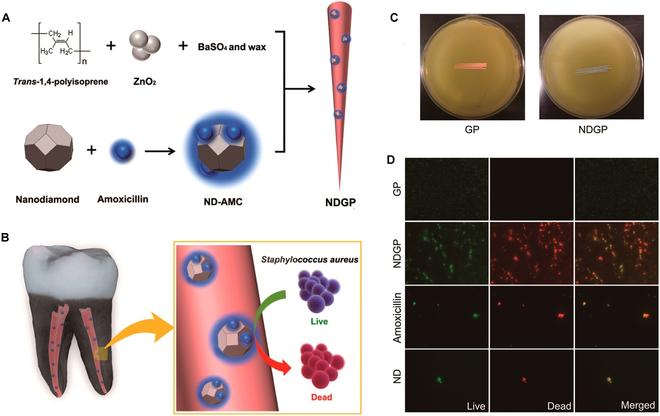
Nanodiamond incorporated gutta-percha for root canal filling. (A and B) Schematic drawing showing the synthesis and structure of nanodiamond incorporated gutta-percha, amoxicil. (C and D) Antibacterial properties of nanodiamond-incorporated gutta-percha. The image is reproduced from Lee et al. [99] with the permission of the American Chemical Society.
Ag NPs can be incorporated into gutta-percha to achieve sustained release of silver ions, imparting antibacterial properties. Diverse techniques have been employed for coating Ag NPs onto gutta-percha points, with one approach involving the use of a plasticizer [100]. The Ag NPs-coated points have low toxicity to human cells and good biocompatibility [101]. The coating exerts a worthwhile antibacterial effect against C. albicans as well as against several bacteria, including E. faecalis, S. aureus, and E. coli [102]. The treated points inhibited the growth of E. faecalis in a dose-related manner. An alternative approach used polyethylene glycol and polyvinylpyrrolidone as adhesives to bind silver/curcumin NPs to gutta-percha [103]. This gave antibacterial activity against E. faecalis, E. coli, and S. aureus. Issues with coating points are that binders may cause allergic reactions or inflammation, while water-soluble polymers may dissolve in body fluids. In addition, most Ag NPs will be embedded in the binder matrix rather than on the surface, which limits their activity [104,105]. To overcome these issues, an in situ method has been used to establish a layer of Ag NPs on the surface to give strong adhesion of the Ag NPs without the need for a binder. A higher loading of particles on the surface gave greater effectiveness against E. faecalis and E. coli [3].
Bioceramic calcium silicate NPs can be coated onto or incorporated into gutta-percha points to improve the quality of obturation. These points are used with hydrophilic bioceramic sealers, which react with moisture. The setting reaction of the sealer causes a slight expansion, which enhances the seal [98]. In one study, gutta-percha points were modified by incorporating bioceramic calcium silicate NPs both inside, and by also coating their surface. Using these modified points with BS or with an epoxy resin sealer (AH Plus) to fill root canals increased the resistance of roots to fracture of the prepared roots when compared to roots that were prepared but not filled to reach the level of intact sound roots. The treated points gave better obturation, and there was improved sealer penetration, as well as enhanced push-out bond strength [106].
Nanomaterials as Endodontic Sealers
Root canal sealers are an integral part of the obturation process since they fill the space between bulk fillers (such as gutta-percha points) and dentinal walls, and should flow into and occlude lateral and accessory canals, thus ensuring a complete seal of the root canal system in three dimensions [107]. To ensure the long-term success of root canal treatment, endodontic sealers should provide a hermetic seal and have a stable volume over time, exert bacteriostatic or bactericidal properties while remaining biocompatible to periapical tissues, and remain insoluble in tissue fluids after setting yet be capable of being removed when necessary, in line with the specifications of ISO 6876 and ANSI/ADA 57.
Over recent decades, many sealers have been developed. Based on their prime constituent and/or reaction system, endodontic sealers can be categorized as zinc oxide-eugenol, Ca(OH)₂, calcium phosphate, glass ionomer, calcium silicate, salicylate, methacrylate resin, silicone, epoxy resin, and others [108]. No existing sealers fulfill all the requirements stated by Komabayashi et al. [109]. Hence, there is interest in incorporating nanomaterials to overcome these deficits. Nanomaterials themselves might possess innate functionalities to enhance the performance of certain sealers. In addition to providing sustained antimicrobial actions, they could act as carriers for the therapeutic payloads and surface modifiers [110]. NPs could also enhance the sealing ability and the process of micromechanical adhesion to dentine. They could also enhance the bioactivity in terms of mineralization. A summary of past work involving adding nanomaterials to sealers is presented in Table 1.
Table 1.
Recent advances in nanomaterials used in endodontic sealers
| Type | Base materials | Nanomaterials | Properties assessed | Main findings | Year | References |
|---|---|---|---|---|---|---|
| Zinc oxide-eugenol (ZOE) | Endomethasone N (Septodont, France) | AgVO3 nanomaterial | Biocompatibility (human gingival fibroblasts) | A reduction in cell viability due to AgVO3. | 2021 | [229,230] |
| Lab-made experimental materials* | Nano-ZOE | Biocompatibility (L929 cells), sealing ability, and post-treatment pain control | The biocompatibility of the nano-ZOE was comparable to Pulpdent but lower than AH-26. In addition, nano-ZOE showed less leakage than AH-26 and commercial ZOE. However, almost half of the subjects treated with nano-ZOE reported severe to very severe pain in the first 6 h of follow-up. | 2015, 2013, 2020 | [231–233] | |
| Lab-made experimental materials | ZnO NPs | Physiochemical properties | Addition of 25% ZnO NPs significantly improved dimensional stability. | 2016 | [130] | |
| Calcium hydroxide | Apexit Plus (Ivoclar Vivadent, Liechtenstein) | Chitosan NPs and ZnO NPs | Antimicrobial activity (E. faecalis) | ZnO NPs had superior antibacterial activity to chitosan NPs. | 2018 | [159] |
| Calcium silicate | Lab-made experimental materials (β-dicalcium silicate) | Attapulgite (ATT) nanofibers | Physiochemical properties | ATT (1–4%) enhanced the compressive strength and retarded bio-dissolution. | 2022 | [152] |
| BioRoot RCS (Septodont, France) | Multi-walled carbon nanotubes, titanium carbide nanopowder, and boron nitride nanotubes | Physiochemical properties | The incorporation of nanomaterials enhanced compressive strength and shortened the setting time. | 2021, 2020 | [153,234] | |
| EndoSequence BC Sealer (Brasseler, USA) | Chitosan-hydroxyapatite nanocomplexes | Physiochemical properties | Addition of nanocomplexes increased nanohardness and elastic modulus. | 2022 | [163] | |
| Bright Endo MTA Sealer (Genoss, Korea) | Bioactive glass NPs | Bioactivity | Osteogenic differentiation was stimulated by the addition of bioactive glass NPs (0.5% and 1%). | 2022 | [140] | |
| Lab-made experimental materials* | Silver/zinc-loaded mesoporous Ca–Si nanoparticles (MCSNs) | Antimicrobial activity (E. faecalis and animal root canal infection model) and biocompatibility (MC3T3-E1 cells) | Ag/Zn ratios influenced antibacterial activity and cytotoxicity. Ag/Zn-MCSNs demonstrated strong impacts against bacteria in vitro and in vivo, without compromising biocompatibility and hardness of materials. | 2021, 2020 | [20,186] | |
| Epoxy resin | AH 26 (Dentsply Sirona, Germany) | Chitosan NPs | Antimicrobial activity (E. faecalis) | Incorporation of chitosan NPs (10, 20, and 30%) produced a larger inhibition zone with improved cytocompatibility. | 2022 | [160] |
| AH Plus (Dentsply Sirona, Germany) | Bismuth lipophilic (BisBAL) NPs | Antimicrobial activity (E. faecalis) | Incorporation of BisBAL NPs produced a larger inhibition zone and inhibited biofilm formation completely on both the E. faecalis ATCC strain and the clinical isolates from endodontic patients. | 2022 | [235] | |
| AH Plus (Dentsply Sirona, Germany) | Ag NPs | Antimicrobial activity (Klebsiella and E. coli, and E. faecalis) | The incorporated Ag NPs did not prevent apical bacterial leakage after 3 months. Ag NPs-loaded sealers exhibited comparable antibacterial activity to those added with chitosan NPs. | 2021, 2019 | [114,120] | |
| AH Plus (Dentsply Sirona, Germany) | Chitosan NPs | Antimicrobial activity (E. faecalis) | Chitosan NP-loaded sealers had comparable antibacterial activity to those with Ag NPs. | 2019 | [114] | |
| ADSEAL (META BIOMED, Korea) | Chlorhexidine (CHX)/Ag NPs @ multi-walled carbon nanotubes (CNTs) | Antimicrobial activity (E. faecalis, C. albicans and S. aureus) | Addition of CNTs incorporating CHX and Ag NPs enhanced antibacterial and antifungal efficacy. | 2021 | [180] | |
| AH Plus (Dentsply Sirona, Germany) | Quaternary ammonium polyethyleneimine NPs | Antimicrobial activity (E. faecalis) | The incorporation of quaternary ammonium polyethyleneimine NPs significantly improved antibacterial activity. | 2015 | [173] | |
| AH Plus (Dentsply Sirona, Germany) | AgVO3 nanomaterials | Biocompatibility (human gingival fibroblast) | A reduction in cell viability was due to AgVO3. | 2021 | [229,230] | |
| Sealer 26 (containing calcium hydroxide; Dentsply Sirona, Brazil) | AgVO3 nanomaterials | Biocompatibility (human gingival fibroblast) | A reduction in cell viability was due to AgVO3. | 2021 | [229,230] | |
| AH Plus (Dentsply Sirona, Germany) | Mg(OH)2 NPs | Biocompatibility (MC3T3-E1 cells), bioactivity and antimicrobial activity (S. mutans) | The addition of 3% Mg(OH)2 NPs promoted cell proliferation and osteogenic differentiation. 5% and 7% Mg(OH)2 NPs enhanced the antibacterial function of AH Plus in the fresh state. | 2020 | [146,147] | |
| AH Plus (Dentsply Maillefer, USA) | β-TCP nanocrystals | Biocompatibility (human periodontal ligament fibroblasts), antimicrobial activity (E. faecalis, C. albicans, E. coli) and physiochemical property | The addition of β-TCP did not affect antibacterial activity, but supported higher cell viability as well as increased adhesiveness to root canal walls. | 2019 | [236] | |
| AH Plus (Dentsply Sirona, Germany) | Fluoridated bioactive glass NPs (F-nBG) | Physiochemical property | F-nBG incorporated sealers released fluoride and gave enhanced bond strengths. | 2020 | [142] | |
| AH 26 (Dentsply Sirona, Germany) | Fluoridated hydroxyapatite NPs, hydroxyapatite NPs and bioactive glass NPs | Physiochemical property and bioactivity | BAG and HA NPs enhanced the in vitro apatite-forming ability and did not alter the physical performance of AH 26. However, fluoridated HA NPs did not improve the apatitic layer formation. | 2019 | [139] | |
| AH Plus (Dentsply Sirona, Germany) | ZnO NPs | Penetrability to dentinal tubule | Addition of ZnO NPs significantly enhanced tubular sealer penetration depth. | 2020 | [131] | |
| Methacrylate resin | Lab-made experimental materials | Ag@SiO NPs | Antimicrobial activity (E. faecalis) | The addition of Ag@SiO up to 10 wt % did not affect the biocompatibility, radiopacity, flow, film thickness, and showed an immediate and long-term (9 months) antibacterial effect. | 2023 | [2] |
| Lab-made experimental materials | Calcium hydroxide-containing halloysite nanotube (HNT_CaOH2) and β-tricalcium phosphate-containing nanotube (HNT_β-TCP) | Antimicrobial activity (unknown species) | The incorporation of HNT_CaOH2 or HNT_β-TCP reduced the bacterial count. | 2022 | [178] | |
| Lab-made experimental materials | Halloysite nanotubes (HNT) doped with alkyl trimethyl ammonium bromide(ATAB) | Antimicrobial activity (E. faecalis) | The incorporation of ATAB/HNT enhanced antibacterial activity against biofilm and planktonic E. faecalis. | 2019 | [177] | |
| Lab-made experimental materials | ZnO NPs with needle-like nanostructure | Antimicrobial activity (E. faecalis) | ZnO NPs improved the antibacterial effect without a significant detrimental impact on the chemical and physical properties. | 2020 | [124] | |
| EndoREZ (Ultradent, USA) with 2.5% quaternary ammonium salt | Magnetic nanoparticles or Fe3O4 NPs | Penetrability to dentinal tubule and antimicrobial activity (E. faecalis) | Fe3O4 NPs could penetrate into dentinal tubes under a magnetic field to kill bacteria embedded in the deeper dentinal tubules in vitro and in vivo. | 2022 | [132,237] | |
| Lab-made experimental materials | Hydroxyapatite NPs | Bioactivity | Hydroxyapatite NPs were inferior to α-TCP in terms of stimulating mineralized nodule formation. | 2021 | [137] | |
| Lab-made experimental materials | Amorphous calcium phosphate NPs (ACP NPs) | Mineralization | The incorporation of ACP NPs increased the release of Ca and P ions at pH levels below 7. | 2019, 2017 | [148–150] | |
| Others | Lab-made experimental materials (polyurethane base) | nano-ZnO and nano-hydroxyapatite | Antimicrobial activity (S. mutans, S. aureus, and E. faecalis) and biocompatibility (L929 cells) | Zn-containing sealers exhibited more robust and long-lasting antibacterial activity and lower cytotoxicity than the Ag-containing sealers. Nano-HA exhibited stable antibacterial activity. | 2021, 2019 | [126,127] |
| Lab-made experimental materials (urethane-acrylate base) | Nanoscale silicate platelets (NSPs) immobilized with Ag NPs and/or ZnO NPs (Ag@NSP, ZnO@NSP, or Ag/ZnO@NSP) | Antimicrobial activity (E. faecalis) and biocompatibility (3T3 cells) | Simultaneous immobilization of Ag NPs and ZnO NPs on silicate platelets enhanced the antibacterial activities, and also reduced the dose of Ag NPs needed, resulting in acceptable cytotoxicity. | 2020 | [125] | |
| Lab-made experimental materials* | Monodispersed silica-based bioactive glass NPs (SBG-NS) grafted with quaternary ammonium polymethacrylate (QAPM) | Antimicrobial activity (E. faecalis, S. mutans, and S. sanguis) and biocompatibility (periodontal ligament stem cells, calvarial implantation model) | SBG-QAPM had the strongest long-term antibacterial effect and lowest inflammatory reaction when compared with ProRoot MTA, Endomethasone C, and AH Plus. | 2017 | [151] | |
| Lab-made experimental materials* | Polymeric PLGA NPs loaded with Egyptian propolis extract (ProE) | Biocompatibility (subcutaneous implantation model) and sealing ability | ProE-loaded PLGA NPs caused milder inflammatory reactions and gave comparable sealing ability when compared with AH Plus. | 2020 | [188] |
*The main compositions of sealers are listed nanomaterials.
Metal/metal oxide NPs as endodontic sealers
The rationale for including Ag NPs into endodontic sealers is that they exert antifungal actions against C. albicans and antibacterial actions against many bacterial species, including S. aureus, S. mutans, Streptococcus pyogenes, P. vulgaris, E. coli, and E. faecalis [111–113]. The approach of adding Ag NPs (54.2 nm) into endodontic sealers to enhance their antibacterial activity against E. faecalis has been effective for multiple sealers (AH Plus, Endosequence, MTA Fillapex, Sealapex and Tubliseal) [114]. Also, both Ag NPs (20 nm) and other antimicrobial agents such as dimethylaminohexadecyl methacrylate (DMAHDM) have been incorporated into a commercial sealer (AH Plus) [115,116]. A silicone-based (polydimethylsiloxane) sealer has been modified with nanosilver (NanoSeal-S). This gives apical sealing comparable to AH Plus, but its bond strength and sealing ability are inferior to BioRoot RCS [117–119]. Nevertheless, even sealers with Ag NPs will not block bacterial leakage over long timeframes such as 3 months [120].
Since sealers may potentially come into contact with periapical tissues, issues of irritation and toxicity must be considered, e.g., Ag NPs could trigger the generation of ROS and thus cause nonspecific oxidative damage [121]. Silver ions that are released could also influence bone-derived cells, as shown in cell culture experiments with Ag NPs using osteoblastic MC3TC E1 cells, in a dose-dependent manner [20], and likewise for AgVO3 NPs when tested using human gingival fibroblasts [122].
Zinc oxide NPs exert antibacterial actions by disrupting bacterial cell membranes [14]. Compared to its bulk state, ZnO at nanoscale displays superior bactericidal effects, because of the large surface area of the NPs [122]. ZnO NPs exhibited stronger antimicrobial actions than NPs of other metal oxides (e.g., MgO, TiO2, Al2O3, CuO, and CeO2) [123]. ZnO NPs (~40 nm) have been incorporated into methacrylate resin-based sealers, and this inhibits the growth of planktonic E. faecalis without compromising the physical performance of the original sealers [124]. Interestingly, materials with ZnO NPs (~30 nm) appear to be less cytotoxic to fibroblasts than their Ag NPs-containing counterparts [125–127]. Adding Ag NPs may reduce the viability of MC3T3 E1 cells in culture after 3 days, while in contrast adding ZnO NPs maintains or slightly promotes cell proliferation in culture. If both types of NPs are used together, it may be possible to optimize the antibacterial effects and cytocompatibility by adjusting the ratio between Ag NPs and ZnO NPs [20]. However, ZnO NPs (<50 nm) could be toxic for osteoblasts, whereas no greater than ZnO microparticles [128]. In general, cytotoxicity varies based on NP shape and size, and the type of cell that is being exposed to the NPs [129]. Therefore, it is logical to consider host cells that reside in the periapical area, including periodontal ligament cells, alveolar bone cells, and immune cells when designing endodontic products.
Adding NPs to sealers may also enhance their physical properties. One study showed that replacing ZnO powder with ZnO NPs (~20 nm) significantly improved the physicochemical properties of conventional Grossman sealer in terms of flow, setting time, dimensional stability, and radiopacity [130]. Likewise, adding ZnO NPs into AH Plus enhanced the flow characteristics and favors deeper penetration into dentinal tubules [131]. Other metal or metal oxide NPs are also of interest as materials that could be added into existing root canal sealers. One recent study incorporated ferrimagnetic magnetite (Fe3O4) NPs (50 to 100 nm) and a quaternary ammonium salt (QAS) into EndoREZ sealer [132]. By applying an external magnetic field, dentinal tubule penetration was enhanced.
Bioceramic nanomaterials as endodontic sealers
Ceramics are inorganic nonmetallic solids that exist in amorphous or crystalline forms. Their elemental composition includes primarily calcium, silica, phosphorous, and aluminum [133]. A consideration with endodontic sealers is that when extruded they will come into contact with periradicular tissues. Many commercially available sealers are based on epoxy resins or methacrylate resins and do not have the ability to drive hard tissue formation because they lack bioactive components. More recently, research into nanophase bioceramics has been carried out to identify their prospects in optimization of endodontic sealers [134]. It was reported that the addition of nano-hydroxyapatite (nHA) (~26.8 nm) in methacrylate-based root canal sealers, even up to 40%, had no negative effects on radiopacity, flow, and film thickness [135]. Furthermore, there is a consensus among the literatures that nHA could enhance in vitro apatite-forming ability of pristine endodontic sealers [136–138]. Apart from nHA, nanostructured bioactive glass or bioglass (nBAG), an amorphous silicate-based compound, also has exhibited excellent capability to induce apatite formation and osteogenic differentiation [139–141]; moreover, the sealing ability of certain endodontic sealer could be promoted by fluoridated nBAG [142]. However, previous data suggested the inferiority of weak antibacterial effect of nHA and nBAG [140,143,144]. It would thus be of clinical interest to converge desired properties for endodontic sealers, i.e., bioactivity and microbicidal effect, in cases of severe pulpal and periapical inflammation [145]. Recently, nano-magnesium hydroxide containing AH Plus sealers were shown to exhibit improved bioactivity and antibacterial effects against S. mutans [146,147]. Beside incorporating nanobioceramic alone, some researchers also attempted to introduce effective antibacterial component 12-methacryloyloxydodecylpyridinium bromide (MDPB) and/or DMAHDM together with amorphous calcium phosphate (ACP) NPs to develop novel bioactive endodontic sealers with mineralization and antibiofilm properties [148–150]. The results demonstrated bioactivity of modified sealers to release abundant Ca and P ion, and the antibacterial activity to inhibit E. faecalis biofilm as well as multi-species biofilm. Similarly, new strategy to permanently copolymerize antimicrobial quaternary ammonium methacrylate salt (QAMS) with endodontic sealers based on monodispersed silica-based bioactive glass (SBG) nanospheres has also been reported [151]. SBG sealers covalently grafted with QAMS demonstrated excellent stability after 6-month immersion in phosphate-buffered saline (PBS) and a level of biocompatibility that was comparable to ProRoot MTA, and superior to Endomethasone C and AH Plus.
With bioceramic sealers turning into the focal point, more efforts were made to provide an added value for them, by adequate modification [134]. To address the limitation of slow hydration nature of dicalcium silicate-based sealers, Zheng et al. [152] explored the influence of attapulgite (ATT) nanofiber, a clay that belongs to hydrous Mg–Al–silicate minerals, on the self-curing of prime cement sealers. Data obtained showed that ATT nanofiber with low content (≤4%) not only contributed to structural densification and favored self-curing property but also enhanced compressive strength and improved anti-microleakage ability. Baghdadi et al. [153] in their study evaluated the reinforcement of 1 and 2 wt % multi-walled carbon nanotubes (MWCNTs), titanium carbide (TiC), and boron nitride (BN) separately in BioRoot RCS, a typical calcium silicate-based root canal sealer. The resultant sealer demonstrated a shorter setting time, lower elution profiles, and high alkalinity with a pH ≥10 after 24 h, which are beneficial for minimizing microleakage and inhibiting microbes. Further scanning electron microscopy (SEM) analysis showed that nanomaterials with the lower percentage (1%) were more homogeneously distributed when compared to the ones with higher concentration (2%). Besides, different radiopacifiers at nanoscale such as zirconium oxide (ZrO2) and niobium oxide (Nb2O5) were also utilized to improve the overall physicochemical properties and bioactivity of root canal sealers [154,155].
Organic NPs as endodontic sealers
Organic NPs such as chitosan NPs have attracted considerable attention in endodontic therapy because of their notable biocompatibility and broad-spectrum antimicrobial activities [156]. Around the early 2010s, their antibacterial properties and in vitro studies to simulate clinical scenario for endodontic sealer application began to emerge linking [157]. It was reported that incorporating chitosan NPs into epoxy-based sealers could significantly enhance the antibacterial properties, as a result of both direct-contact and membrane-restricted antibacterial assays [158]. Furthermore, the addition of chitosan NPs prolonged the antibacterial efficacy of commercial endodontic sealers even after a 1-month duration by reducing total and viable biovolume of E. faecalis. To evaluate the significance of chitosan NPs in terms of antimicrobial effectiveness, researchers incorporated chitosan NPs alone or together with CHX in various conventional endodontic sealers, and further compared them with those containing Ag NPs or CHX [114]. Data from bacteriostasis circle and SEM micrographs have identified that the synergistic bactericidal activity of chitosan NPs and CHX had the strongest inhibition against E. faecalis, irrespective of the types of sealers. However, one study by Nair et al. [159] showed that the incorporation of chitosan NPs in Ca(OH)2 sealer (i.e., Apexit Plus) failed to improve its antibacterial properties against E. faecalis strain OG1RF. Moreover, Ratih et al. [160] found that the increment of chitosan NP concentration from 10% to 30% in epoxy resin-based sealer (AH-26) did not significantly enlarge E. faecalis inhibition zone, indicating the need for more comprehensive investigations to optimize the antimicrobial effects of chitosan NPs.
Achieving an ideal endodontic sealer involves addressing the challenge of preventing collagen degradation, which can compromise interfacial adhesion, promote microbial recolonization, and reduce instrumented dentin resistance to fracture. Persadmehr et al. [93] demonstrated that collagen gels treated with 1% chitosan NPs exhibited reduced hydroxyproline release in the presence of collagenase, suggesting chitosan NPs’ potential to protect collagen from enzymatic degradation. Further SEM analysis indicated the intimate association of chitosan NPs with collagen fibrils [161]. Recent evidence highlights the ability of chitosan NPs-containing nanocomplexes, such as carboxymethyl chitosan/ACP nanocomplexes (<40 nm), to achieve intrafibrillar mineralization of collagen, enabling dentine repair. Employing this biomimetic approach, chitosan/HA nanocomposites blended with tricalcium silicate sealer improved the physico-mechanical properties of endodontic sealers and enhanced root dentin fracture resistance [162,163].
Furthermore, quaternary ammonium compound (QAC) NPs [164,165], such as quaternary ammonium polyethylenimine (QPEI) NPs [166], exhibit robust antibacterial properties. QPEI NPs, immobilized on resinous material surfaces, demonstrate enduring antimicrobial function without leaching [167]. Studies by Beyth et al. [168] reveal that QPEI NPs enhance antibacterial activity against E. faecalis without compromising physical properties or setting time. QPEI NP modification in commercial endodontic sealers, including AH Plus, GuttaFlow, and Pulp Canal Sealer EWT, also improves antibacterial effects [169–171]. In addition, Shvero et al. [167] introduced an antigravitational test using endodontic sealers and a bacterial pellet to investigate the cellular behavior of anionic E. faecalis on cationic QPEI NPs-modified sealer surfaces. The study revealed that QPEI NPs attracted E. faecalis through electrostatic interactions, leading to dead cells observed throughout all layers of the formed biofilm, suggesting both direct and indirect mechanisms for QPEI NPs in bacterial eradication.
To better understand latent toxicity of QPEI NPs on periapical tissues, Barros et al. [172] in their study evaluated the proliferation, apoptosis, and phenotype differentiation of human osteoblastic and osteoclastic cells, which had been exposed to extracts from pristine and QPEI NP-containing AH Plus and Pulp Canal Sealer EWT. Additionally, confocal laser scanning microscopy (CLSM) results from evaluating AH Plus sealers containing 1% QPEI NPs in an infectious root canal model demonstrated successful bacterial infiltration in dentinal tubules and reduced bacterial viability upon root canal obturation with 1% QPEI NPs-containing endodontic sealers [173]. Furthermore, to increase the affinity of QACs with epoxy resin-based AH Plus, Gong et al. [174] synthesized quaternary ammonium epoxy silicate (QAES) NPs, which enables copolymerization of these NPs with epoxy resin-based materials. Direct-contact test and CLSM observations indicated that even after 4 weeks of water aging, QAES NPs-incorporated endodontic sealers possessed antibacterial activity against E. faecalis in the form of both plankton and biofilm, suggesting their superiority over unmodified controls in the long term.
Moreover, QACs, including QPEI NPs, exhibit the ability to inhibit matrix metalloproteinases (MMPs) impact on dentin collagen when incorporated into dental materials as polymerizable quaternary ammonium methacrylates, offering a sustained anti-MMPs effect [175]. Molecular interrelationships between QACs and MMPs catalytic sites hinder collagen matrix hydrolysis [164]. Considering the crucial role of MMPs in controlling infections, QAC NPs may be advantageous for reducing periapical inflammation. Comprehensive studies are needed to further understand the value of QAC NPs-containing endodontic sealers in inhibiting bacteria, sealing root canals, and promoting periapical inflammation healing.
Nanomaterials as nanocarriers for sealers
Nanomaterials, such as halloysite nanotubes (HNTs) and MWCNTs, serve as promising nanocarriers for endodontic sealers. HNTs, with their unique tubular structure [176], successfully encapsulate antimicrobial compounds, enhancing the antibacterial efficacy of methacrylate resin-based root canal sealers [177]. Additionally, the incorporation of calcium-loaded HNTs demonstrates a significant reduction of E. faecalis when compared with AH Plus and pristine experimental sealers, highlighting the potential of clay nanotubes in endodontic applications [178]. Meanwhile, MWCNTs, explored for physical reinforcement in BioRoot RCS [179] and as carriers for CHX and Ag NPs in ADSEAL [180,181], exhibit uniform distribution and promising long-term antimicrobial effects. Specifically, even after 7-day setting, CHX and Ag NPs-encapsulated MWCNTs exhibited satisfactory antimicrobial effects, with the maximum efficacy against E. faecalis, followed by S. aureus and C. albicans. However, the impact of different CNTs on endodontic bacterial inactivation and sealer physical properties requires further investigation.
Mesoporous silica nanoparticles (MSNs) are extensively studied as nanocargoes due to their remarkable biocompatibility, large drug-loading capacity, and customizable physicochemical properties [182]. Li et al. [183] demonstrated that MSNs with a diameter below 100 nm effectively infiltrate dentin tubules, showcasing potential for delivering antimicrobial and mineralization agents in endodontic sealers. Similarly, mesoporous calcium-silicate NPs (MCS NPs) exhibit promise for infiltrating dentinal tubules [184]. Continuous release of Ca2+ and SiO44− creates a microenvironment inhibiting microbes [20,185]. In vivo root canal infection model further illustrated that MCS NPs loaded with nano-silver and nano-zinc could effectively reduce inflammatory area in the periapical tissue, suggesting their prospects to be utilized in endodontic sealers [186]. Rucker et al. [2] extended the antimicrobial effect of endodontic sealers by incorporating Ag@SiO2 NPs, confirming reduced E. faecalis viability while maintaining sealer physicochemical properties. More recently, nanoscale silicate platelets (NSPs) immobilized with Ag NPs and/or ZnO NPs were prepared to render homogeneous dispersion of NPs while maintaining the microbicidal efficacy of Ag NPs and ZnO NPs in resin-based endodontic sealers [125].
Polymer-based NPs, such as polylactic-co-glycolic acid (PLGA) NPs, enhance pharmaceutical delivery and release. Pagonis et al. [187] found PLGA NPs concentrated on E. faecalis cell walls, developing a nanoplatform for PDT in infected root canals. Raheem et al. [188] introduced propolis-loaded PLGA NPs with prolonged propolis release, exhibiting satisfactory cytocompatibility and robust antimicrobial activity against E. faecalis, S. mutans, and C. albicans.
Nanomaterials Used as Retro-Filling and Root-Repair Materials
Several studies have emphasized the significance of placing root-end fillings during periapical surgery [189,190]. The material chosen for root-end filling significantly impacts procedural success, aiming to seal the root end effectively [191]. Mineral trioxide aggregate (MTA) remains the most widely used and is still considered the gold standard for comparison with new materials [192]. However, MTA has drawbacks, including handling difficulties, a prolonged setting time, high cost, a lack of antimicrobial properties, and susceptibility to dissolution in acidic pH [193]. Incorporating NPs into MTA and other bioactive endodontic cements offers the potential to enhance their mechanical, physical, and biological properties.
Incorporating Ag NPs into bioactive endodontic cements yields multifaceted improvements. First, it substantially enhances antimicrobial activity in materials like MTA and calcium-enriched mixture, effectively combating bacteria linked to dental infections without compromising the cements’ physical properties [194]. Furthermore, it demonstrates excellent tissue reaction and biocompatibility, as observed in a rat model, with no significant adverse effects in subcutaneous tissue compared to control implants without Ag NPs [195]. Additionally, Ag NPs addition increases calcium ion release from MTA, crucial for promoting new bone tissue growth and expediting the healing process [196].
Moreover, incorporating Ag NPs is reported to reduce the setting time of MTA and accelerate silicates’ hydration and potentially enhance the material’s utility [197]. It is also reported to improve dimensional stability, vital for proper tissue regeneration, and serves as an excellent radiopacifier, overcoming MTA’s low radiopacity for clearer radiographic observations [196,198].
Various other NPs have also been investigated for their potential to enhance endodontic cements. The introduction of bismuth lipophilic NPs into MTA has shown a significant improvement in antimicrobial and antibiofilm activities without compromising physical properties, promising enhanced clinical outcomes [199]. Silver-zeolite NPs, known for their porous structure releasing silver ions over time, augment the antibacterial properties of MTA against diverse microorganisms. However, potential drawbacks such as altered solubility and pH changes necessitate careful consideration for clinical applications [200–204]. The incorporation of TiO2 NPs into MTA at 1 wt % demonstrates favorable biocompatibility without adverse effects. This addition improves flexural and compressive strength, showcasing potential material property enhancements [205–207]. ZnO NPs combined with Portland cement (PC), alongside zirconium oxide, exhibit antibiofilm activity and radiopacity. However, a cautious approach is essential, as higher concentrations of ZnO NPs may compromise compressive strength, demanding a thoughtful material selection process in various clinical scenarios [208]. Incorporating hydroxyapatite NPs negatively affects compressive strength and solubility but enhances radiopacity, final setting time, and antibiofilm activity. These trade-offs require careful consideration, particularly regarding water requirements and clinical acceptability [209].
Bond strength is a critical determinant in endodontic repair materials. Notably, the incorporation of TiO2 and Ag NPs into MTA has been found to enhance push-out bond strength, whereas the introduction of silicon dioxide NPs demonstrates no significant impact [210]. A comparable investigation reveals that the bond strength of calcium enriched mixture (CEM) cement remains unaffected by the addition of TiO2, whereas the inclusion of TiO2 in MTA and PC leads to an increase in push-out bond strength for these materials [211].
Nanomaterials Used for Pulpal Repair and Regeneration
Pulpal regeneration procedures, which aim to biologically and functionally repair the pulp–dentin complex, require appropriate disinfection of the root canal space, followed by pulp tissue repair [212]. Regenerative medicine is based upon three key elements: stem cells, natural/synthetic scaffolds, and growth factors (bioactive molecules). Stem cells from different sources have been studied for the purpose of regenerating pulp and dentin. These sources include dental pulp stem cells, stem cells from the apical papilla, stem cells of human exfoliated teeth, and periodontal ligament stem cells [213]. Scaffolds, resembling the structure and complexity of the extracellular matrix in the pulp, offer mechanical support, facilitate cell attachment, and stimulate the growth and differentiation of stem cells. Bioactive molecules are signaling compounds that play a crucial role in initiating and sustaining cellular interactions necessary for the regeneration of damaged pulp tissues. To facilitate tissue regeneration, these molecules rely on a suitable microenvironment and an appropriate scaffold [214].
Nanofibrous-based scaffolds for pulpal repair and regeneration
In order to regenerate pulp and dentin successfully, scaffolds should mimic the extracellular matrix, extracellular fibers, and proteins of pulp and dentin; collagen, which constitutes the majority of dentin, is an excellent natural scaffold [215]. Nanofibrous scaffolds have been found to promote positive interactions between cells and the extracellular matrix, enhance cell proliferation, maintain cell phenotype, facilitate stem cell differentiation, and activate cell-signaling pathways through physical and chemical stimuli [216,217].
Several mechanisms have been proposed to explain the positive biological effects induced by nanofibers. These mechanisms include increased protein adsorption, enhanced integrin expression, and altered signaling pathways. It is suggested that nanofibers promote initial cell attachment by selectively absorbing extracellular matrix proteins such as fibronectin, vitronectin, and laminin. This increased protein adsorption may subsequently lead to higher expression of integrins, which are proteins involved in cell–extracellular matrix attachment [217]. Studies have shown that nanofibrous matrices can up-regulate the expression of specific integrins in cells, which has been linked to enhanced differentiation into mesodermal and osteogenic lineages. Integrins may activate proteins like paxillin and focal adhesion kinase, which play roles in differentiation pathways. Additionally, the expression of RhoA and Rac, which regulate cytoskeletal organization and other cellular behaviors, has been associated with differentiation, morphology, and adhesion responses [217].
NP-based systems for pulpal repair and regeneration
Nanotechnology offers a promising avenue for developing scaffolds composed of fibers containing bioactive NPs in the context of regenerative endodontics [218]. These NPs possess unique attributes, such as small size, high surface area, increased solubility, and antibacterial activity, making them valuable for controlled release of bioactive agents [219]. Notably, magnetic NPs, like superparamagnetic iron oxide NPs and quantum dots, serve for long-term tracking of stem cells after transplantation, providing insights into their migration and differentiation in dental pathology and repair [220]. Incorporating iron oxide NPs into a calcium phosphate cement scaffold demonstrated enhanced attachment of human dental stem cells and facilitated bone mineralization [220]. Similarly, titanium dioxide NPs proved beneficial by improving the proliferation and morphology of dental pulp stem cells without causing cytotoxicity, contributing to collagen fiber formation essential for hard tissue development [221].
Hydroxyapatite NPs exhibited positive effects on stem cells from the apical papilla, enhancing adhesion, proliferation, and odontoblast differentiation on scaffolds [222]. Mesoporous bioactive NPs stimulated odontoblast differentiation from rat dental stem cells, showcasing potential applications in dental materials for damaged dentin regeneration [223]. Zinc bioglass NPs in calcium phosphate cement promoted odontoblast differentiation and angiogenesis, suggesting a role in regenerative dentistry [224]. Chitosan NPs, recognized for drug delivery, demonstrated varying impacts on stem cells from the apical papilla, influencing alkaline phosphatase activity and odontogenic differentiation based on the encapsulation or adsorption techniques [225,226].
Furthermore, mesoporous bioglass nanospheres, with the ability to deliver silver ions, antimicrobial drugs, and regenerative ions, present a multifaceted approach for antibacterial and pro-regenerative effects. This NP system, featuring intracellular delivery, ionic doping capabilities, high mesoporosity, degradability, and biocompatibility, holds promise for repairing and regenerating infected tissues [227].
Conclusion and Prospects
In conclusion, the growing body of research exploring nanomaterials in endodontics underscores their potential as valuable tools in combatting root canal infections and promoting endodontic tissue repair. The antibacterial, anti-adhesive, and drug delivery capabilities of NPs present promising avenues for enhancing endodontic treatments. However, it is crucial to acknowledge that the current evidence is primarily based on preliminary reports, warranting additional investigations through animal and in vivo studies. To establish the clinical validity of these findings and ensure the safety of nanomaterial applications, further research efforts should focus on elucidating cytotoxicity profiles and thoroughly assessing the biological effects of these innovative approaches. This ongoing exploration holds the key to realizing the full potential of nanomaterials in advancing the field of endodontics and improving patient outcomes.
Understanding the potential drawbacks and long-term toxicity of nanomaterials is paramount given their diverse pharmacodynamic effects. The escalating utilization of NPs raises concerns about their environmental accumulation and unintended impacts on ecosystems. Cellular repercussions, including apoptosis induction, inhibition of cell growth, genotoxicity, and neurological and respiratory damage, necessitate careful consideration in NP applications. Nanosized particles exhibit heightened inflammatory, oxidative, and cytotoxic effects compared to larger counterparts, owing to increased lung deposition and systemic translocation [8]. Technologically, stability, biodistribution, degradation, and interactions with body fluids and cells are critical considerations. Consequently, strict quality control procedures, comprehensive risk assessments, and utmost vigilance are imperative in the realm of nanotechnology research [228].
Furthermore, the majority of research on nanomaterials’ impact on oral pathogenic biofilms has been conducted in vitro, predominantly focusing on common oral bacteria within single-species biofilm models. This approach overlooks the complexity of biofilms composed of multiple species, including fungi and viruses, which are also present in oral biofilms. Notably, investigations into the effects of NPs on oral pathogenic biofilms have primarily concentrated on bacteria, potentially yielding conclusions that differ from real clinical scenarios. Addressing these limitations is crucial for a more comprehensive understanding of the implications of nanomaterials in the context of oral health [67].
In addition, a substantial challenge in the clinical application of nanomaterials in endodontics lies in the necessity for comprehensive large animal and clinical trials. While initial investigations show promising outcomes in vitro and in smaller-scale studies, the translation of these findings to real-world clinical scenarios demands rigorous evaluation through extensive trials involving larger animal models and human subjects. Large animal trials provide a crucial intermediary step, offering insights into the biological responses, safety profiles, and potential efficacy of nanomaterials in a more complex biological environment. Subsequently, clinical trials are indispensable for validating the feasibility, safety, and efficacy of these nanomaterials in diverse patient populations, ensuring that the proposed innovations meet the stringent standards required for successful integration into routine endodontic practices. The transition from laboratory findings to clinical reality necessitates a meticulous and stepwise approach, emphasizing the importance of robust evidence derived from large-scale trials for the successful implementation of nanomaterials in endodontic care.
Acknowledgments
Competing interests: The authors declare that they have no competing interests.
References
- 1.Raura N, Garg A, Arora A, Roma M. Nanoparticle technology and its implications in endodontics: A review. Biomater Res. 2020;24(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rucker VB, Balbinot GS, Collares FM, Araujo Neto VG, Giannini M, Leitune VCB. Synthesis of silver core-shell nanoparticles and their influence on an experimental resin endodontic sealer: An in vitro analysis. Int. Endod. J. 2023;56(2):289–303. [DOI] [PubMed] [Google Scholar]
- 3.Monisha K, Shilpa SA, Hikku GS. In-situ nano-Ag coated gutta percha cones to counteract internal endodontic failures through bacterial infections. Mater. Chem. Phys. 2023;301:127532. [Google Scholar]
- 4.Afkhami F, Forghan P, Gutmann JL, Kishen A. Silver nanoparticles and their therapeutic applications in endodontics: A narrative review. Pharmaceutics. 2023;15(3):715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EuropeanCommission. Commission recommendation of 10 June 2022 on the definition of nanomaterial. Off. J. Eur. Union. 2022;2022(229):1. [Google Scholar]
- 6.Sadrieh N, Espandiari P. Nanotechnology and the FDA: What are the scientific and regulatory considerations for products containing nanomaterials. Nanotech L Bus. 2006;3:339. [Google Scholar]
- 7.Mansfield E, Kaiser DL, Fujita D, Van de Voorde M. Metrology and standardization for nanotechnology: Protocols and industrial innovations. Germany: John Wiley & Sons; 2017.
- 8.Samiei M, Farjami A, Dizaj SM, Lotfipour F. Nanoparticles for antimicrobial purposes in endodontics: A systematic review of in vitro studies. Mater. Sci. Eng. C. 2016;58:1269–1278. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Zhang W, Niu J, Chen Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano. 2012;6(6):5164–5173. [DOI] [PubMed] [Google Scholar]
- 10.Hewakuruppu YL, Dombrovsky LA, Chen C, Timchenko V, Jiang X, Baek S, Taylor RA. Plasmonic “pump–probe” method to study semi-transparent nanofluids. Appl. Optics. 2013;52(24):6041–6050. [DOI] [PubMed] [Google Scholar]
- 11.Blanc W, Martin I, Francois-Saint-Cyr H, Bidault X, Chaussedent S, Hombourger C, Lacomme S, le Coustumer P, Neuville DR, Larson DJ, et al. Compositional changes at the early stages of nanoparticles growth in glasses. J. Phys. Chem. C. 2019;123(47):29008–29014. [Google Scholar]
- 12.Kim DH, Bae J, Heo JH, Park CH, Kim EB, Lee JH. Nanoparticles as next-generation tooth-whitening agents: Progress and perspectives. ACS Nano. 2022;16(7):10042–10065. [DOI] [PubMed] [Google Scholar]
- 13.Hannig M, Hannig C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010;5(8):565–569. [DOI] [PubMed] [Google Scholar]
- 14.Melo MAS, Guedes SFF, Xu HHK, Rodrigues LKA. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013;31(8):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padovani GC, Feitosa VP, Sauro S, Tay FR, Duran G, Paula AJ, Duran N. Advances in dental materials through nanotechnology: Facts, perspectives and toxicological aspects. Trends Biotechnol. 2015;33(11):621–636. [DOI] [PubMed] [Google Scholar]
- 16.Nizami MZI, Xu VW, Yin IX, Yu OY, Chu C-H. Metal and metal oxide nanoparticles in caries prevention: A review. Nanomaterials. 2021;11(12):3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu VW, Nizami MZI, Yin IX, Lung CYK, Yu OY, Chu C-H. Caries management with non-metallic nanomaterials: A systematic review. Int. J. Nanomedicine. 2022;17:5809–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Shi H, Luo J, Yao H, Wang P, Li Z, Wei J. Advanced materials for enamel remineralization. Front. Bioeng. Biotechnol. 2022;10: Article 985881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moussa DG, Aparicio C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019;13(1):58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leng D, Li Y, Zhu J, Liang R, Zhang C, Zhou Y, Li M, Wang Y, Rong D, Wu D, et al. The antibiofilm activity and mechanism of nanosilver-and nanozinc-incorporated mesoporous calcium-silicate nanoparticles. Int. J. Nanomedicine. 2020;15:3921–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishen A. Nanotechnology in endodontics. Switzerland: Springer; 2016.
- 22.Garrocho-Rangel A, Escobar-Garcia DM, Gutierrez-Sanchez M, Herrera-Badillo D, Carranco-Rodriguez F, Flores-Arriaga JC, Pozos-Guillen A. Calcium hydroxide/iodoform nanoparticles as an intracanal filling medication: Synthesis, characterization, and in vitro study using a bovine primary tooth model. Odontology. 2021;109(3):687–695. [DOI] [PubMed] [Google Scholar]
- 23.Elmsmari F, González Sánchez JA, Duran-Sindreu F, Belkadi R, Espina M, García ML, Sánchez-López E. Calcium hydroxide-loaded PLGA biodegradable nanoparticles as an intracanal medicament. Int. Endod. J. 2021;54(11):2086–2098. [DOI] [PubMed] [Google Scholar]
- 24.Nadar A, Muliya VS, Pai S, Pentapati KC. A comparative evaluation of calcium ion release and pH change using calcium hydroxide nanoparticles as intracanal medicament with different vehicles—An in vitro study. J Conserv Dent. 2023;26(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momenijavid M, Salimizand H, Korani A, Dianat O, Nouri B, Ramazanzadeh R, Ahmadi A, Rostamipour J, Khosravi MR. Effect of calcium hydroxide on morphology and physicochemical properties of Enterococcus faecalis biofilm. Sci. Rep. 2022;12(1):7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javidi M, Afkhami F, Zarei M, Ghazvini K, Rajabi O. Efficacy of a combined nanoparticulate/calcium hydroxide root canal medication on elimination of Enterococcus faecalis. Aust. Endod. J. 2014;40(2):61–65. [DOI] [PubMed] [Google Scholar]
- 27.Haapasalo M, Udnæs T, Endal U. Persistent, recurrent, and acquired infection of the root canal system post-treatment. Endod Top. 2003;6(1):29–56. [Google Scholar]
- 28.Vinodh R, Sasikumar Y, Kim H-J, Atchudan R, Yi M. Chitin and chitosan based biopolymer derived electrode materials for supercapacitor applications: A critical review. J Ind Eng Chem. 2021;104:155–171. [Google Scholar]
- 29.Chandrasekaran M, Kim KD, Chun SC. Antibacterial activity of chitosan nanoparticles: A review. Processes. 2020;8(9):1173. [Google Scholar]
- 30.Xu Z, Neoh KG, Lin CC, Kishen A. Biomimetic deposition of calcium phosphate minerals on the surface of partially demineralized dentine modified with phosphorylated chitosan. J. Biomed. Mater. Res. B Appl. Biomater. 2011;98(1):150–159. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Wang L, Fan Y, Feng Q, Cui F-Z. Biocompatibility and toxicity of nanoparticles and nanotubes. J Nanomater. 2012;2012:548389. [Google Scholar]
- 32.Del Carpio-Perochena A, Bramante CM, Duarte MAH, Moura MR, Aouada FA, Kishen A. Chelating and antibacterial properties of chitosan nanoparticles on dentin. Restor Dent Endod. 2015;40(3):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpio-Perochena A, Kishen A, Felitti R, Bhagirath AY, Medapati MR, Lai C, Cunha RS. Antibacterial properties of chitosan nanoparticles and propolis associated with calcium hydroxide against single-and multispecies biofilms: An in vitro and in situ study. J. Endod.. 2017;43(8):1332–1336. [DOI] [PubMed] [Google Scholar]
- 34.Elshinawy MI, Al-Madboly LA, Ghoneim WM, El-Deeb NM. Synergistic effect of newly introduced root canal medicaments; ozonated olive oil and chitosan nanoparticles, against persistent endodontic pathogens. Front. Microbiol. 2018;9:1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratih DN, Mulyawati E, Fajrianti H. Antibacterial efficacy, calcium ion release, and pH using calcium hydroxide with three vehicles. J Conserv Dent. 2022;25(5):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grover C, Shetty N. Evaluation of calcium ion release and change in pH on combining calcium hydroxide with different vehicles. Contemp Clin Dent. 2014;5(4):434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parolia A, Kumar H, Ramamurthy S, Davamani F, Pau A. Effectiveness of chitosan-propolis nanoparticle against Enterococcus faecalis biofilms in the root canal. BMC Oral Health. 2020;20(1):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sireesha A, Jayasree R, Vidhya S, Mahalaxmi S, Sujatha V, Kumar TS. Comparative evaluation of micron-and nano-sized intracanal medicaments on penetration and fracture resistance of root dentin—An in vitro study. Int. J. Biol. Macromol. 2017;104(Pt B):1866–1873. [DOI] [PubMed] [Google Scholar]
- 39.Abbaszadegan A, Nabavizadeh M, Gholami A, Aleyasin Z, Dorostkar S, Saliminasab M, Ghasemi Y, Hemmateenejad B, Sharghi H. Positively charged imidazolium-based ionic liquid-protected silver nanoparticles: A promising disinfectant in root canal treatment. Int. Endod. J. 2015;48(8):790–800. [DOI] [PubMed] [Google Scholar]
- 40.Liu T, Aman A, Ainiwaer M, Ding L, Zhang F, Hu Q, Song Y, Ni Y, Tang X. Evaluation of the anti-biofilm effect of poloxamer-based thermoreversible gel of silver nanoparticles as a potential medication for root canal therapy. Sci. Rep. 2021;11(1):12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nasim I, Shamly M, Jaju K, Vishnupriya V, Jabin Z. Antioxidant and anti-inflammatory activity of a nanoparticle based intracanal drugs. Bioinformation. 2022;18(5):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afkhami F, Pourhashemi SJ, Sadegh M, Salehi Y, Fard MJK. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. J. Dent. 2015;43(12):1573–1579. [DOI] [PubMed] [Google Scholar]
- 43.Mozayeni MA, Haeri A, Dianat O, Jafari AR. Antimicrobial effects of four intracanal medicaments on Enterococcus faecalis: An in vitro study. Iran Endod J. 2014;9(3):195–198. [PMC free article] [PubMed] [Google Scholar]
- 44.Salas-Orozco M, Niño-Martínez N, Martínez-Castañón G-A, Méndez FT, Jasso MEC, Ruiz F. Mechanisms of resistance to silver nanoparticles in endodontic bacteria: A literature review. J Nanomater. 2019;2019:7630316. [Google Scholar]
- 45.Salas-Orozco MF, Martínez NN, Martínez-Castañón G-A, Méndez FT, Patiño-Marín N, Ruiz F. Detection of genes related to resistance to silver nanoparticles in bacteria from secondary endodontic infections. J Nanomater. 2019;2019:8742975. [Google Scholar]
- 46.Shankar S, Rhim J-W. Effect of copper salts and reducing agents on characteristics and antimicrobial activity of copper nanoparticles. Mater. Lett. 2014;132:307–311. [Google Scholar]
- 47.Rojas B, Soto N, Villalba M, Bello-Toledo H, Meléndrez-Castro M, Sánchez-Sanhueza G. Antibacterial activity of copper nanoparticles (CuNPs) against a resistant calcium hydroxide multispecies endodontic biofilm. Nanomaterials. 2021;11(9):2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sacoto-Figueroa FK, Bello-Toledo HM, González-Rocha GE, Luengo Machuca L, Lima CA, Meléndrez-Castro M, Sánchez-Sanhueza GA. Molecular characterization and antibacterial activity of oral antibiotics and copper nanoparticles against endodontic pathogens commonly related to health care-associated infections. Clin. Oral Investig. 2021;25(12):6729–6741. [DOI] [PubMed] [Google Scholar]
- 49.Shrestha A, Kishen A. Antibacterial nanoparticles in endodontics: A review. J. Endod. 2016;42(10):1417–1426. [DOI] [PubMed] [Google Scholar]
- 50.Lipovsky A, Nitzan Y, Gedanken A, Lubart R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology. 2011;22(10): Article 105101. [DOI] [PubMed] [Google Scholar]
- 51.Vargas-Reus MA, Memarzadeh K, Huang J, Ren GG, Allaker RP. Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int. J. Antimicrob. Agents. 2012;40(2):135–139. [DOI] [PubMed] [Google Scholar]
- 52.Shrestha A, Zhilong S, Gee NK, Kishen A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J. Endod. 2010;36(6):1030–1035. [DOI] [PubMed] [Google Scholar]
- 53.Aguiar AS, Guerreiro-Tanomaru JM, Faria G, Leonardo RT, Tanomaru-Filho M. Antimicrobial activity and ph of calcium hydroxide and zinc oxide nanoparticles intracanal medication and association with chlorhexidine. J. Contemp. Dent. Pract. 2015;16(8):624–629. [DOI] [PubMed] [Google Scholar]
- 54.Guerreiro-Tanomaru JM, Figueiredo Pereira K, Almeida Nascimento C, Basso Bernardi MI, Tanomaru-Filho M. Use of nanoparticulate zinc oxide as intracanal medication in endodontics: pH and antimicrobial activity. Acta Odontol. Latinoam. 2013;26(3):144–148. [PubMed] [Google Scholar]
- 55.Samiei M, Torab A, Hosseini O, Abbasi T, Abdollahi AA, Divband B. Antibacterial effect of two nano zinc oxide gel preparations compared to calcium hydroxide and chlorhexidine mixture. Iran Endod J. 2018;13(3):305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dianat O, Saedi S, Kazem M, Alam M. Antimicrobial activity of nanoparticle calcium hydroxide against Enterococcus faecalis: An in vitro study. Iran Endod J. 2015;10(1):39–43. [PMC free article] [PubMed] [Google Scholar]
- 57.Louwakul P, Saelo A, Khemaleelakul S. Efficacy of calcium oxide and calcium hydroxide nanoparticles on the elimination of Enterococcus faecalis in human root dentin. Clin. Oral Investig. 2017;21:865–871. [DOI] [PubMed] [Google Scholar]
- 58.Naseri M, Eftekhar L, Gholami F, Atai M, Dianat O. The effect of calcium hydroxide and nano–calcium hydroxide on microhardness and superficial chemical structure of root canal dentin: An ex vivo study. J. Endod. 2019;45(9):1148–1154. [DOI] [PubMed] [Google Scholar]
- 59.Obeid MF, El-Batouty KM, Aslam M. The effect of using nanoparticles in bioactive glass on its antimicrobial properties. Restor Dent Endod. 2021;46(4):e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu C, Chang J, Xiao Y. Mesoporous bioactive glasses as drug delivery and bone tissue regeneration platforms. Ther. Deliv. 2011;2(9):1189–1198. [DOI] [PubMed] [Google Scholar]
- 61.Yan X, Yu C, Zhou X, Tang J, Zhao D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004;43(44):5980–5984. [DOI] [PubMed] [Google Scholar]
- 62.Fan W, Wu D, Ma T, Fan B. Ag-loaded mesoporous bioactive glasses against Enterococcus faecalis biofilm in root canal of human teeth. Dent. Mater. J. 2015;34(1):54–60. [DOI] [PubMed] [Google Scholar]
- 63.Fan W, Li Y, Sun Q, Ma T, Fan B. Calcium-silicate mesoporous nanoparticles loaded with chlorhexidine for both anti-Enterococcus faecalis and mineralization properties. J Nanobiotechnology. 2016;14(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan W, Wu Y, Ma T, Li Y, Fan B. Substantivity of Ag–Ca–Si mesoporous nanoparticles on dentin and its ability to inhibit Enterococcus faecalis. J. Mater. Sci. Mater. Med. 2016;27(1):16. [DOI] [PubMed] [Google Scholar]
- 65.Haapasalo M, Shen Y, Wang Z, Gao Y. Irrigation in endodontics. Br. Dent. J. 2014;216(6):299–303. [DOI] [PubMed] [Google Scholar]
- 66.Tsotsis P, Dunlap C, Scott R, Arias A, Peters OA. A survey of current trends in root canal treatment: Access cavity design and cleaning and shaping practices. Aust. Endod. J. 2021;47(1):27–33. [DOI] [PubMed] [Google Scholar]
- 67.Hu C, Wang L-L, Lin Y-Q, Liang H-M, Zhou S-Y, Zheng F, Feng XL, Rui YY, Shao LQ. Nanoparticles for the treatment of oral biofilms: Current state, mechanisms, influencing factors, and prospects. Adv. Healthc. Mater. 2019;8(24):e1901301. [DOI] [PubMed] [Google Scholar]
- 68.Bhandi S, Mehta D, Mashyakhy M, Chohan H, Testarelli L, Thomas J, Dhillon H, Raj AT, Madapusi Balaji T, Varadarajan S, et al. Antimicrobial efficacy of silver nanoparticles as root canal irrigant's: A systematic review. J. Clin. Med. 2021;10(6):1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nabavizadeh M, Ghahramani Y, Abbaszadegan A, Jamshidzadeh A, Jenabi P, Makarempour A. In vivo biocompatibility of an ionic liquid-protected silver nanoparticle solution as root canal irrigant. Iran Endod J. 2018;13(3):293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Fhham BM, Al-Haidar AHM. Evaluation of the antibacterial efficacy of silver nanoparticles as an irrigant against Enterococcus faecalis in vitro study. J Res Med Dent Sci. 2019;7(4):21–27. [Google Scholar]
- 71.Topala F, Nica L-M, Boariu M, Negrutiu ML, Sinescu C, Marinescu A, Cirligeriu L, Stratul SI, Rusu D, Chincia R, et al. En-face optical coherence tomography analysis of gold and silver nanoparticles in endodontic irrigating solutions: An in vitro study. Exp. Ther. Med. 2021;22(3):992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Razumova S, Brago A, Serebrov D, Barakat H, Kozlova Y, Howijieh A, Guryeva Z, Enina Y, Troitskiy V. The application of nano silver argitos as a final root canal irrigation for the treatment of pulpitis and apical periodontitis. In vitro study. Nanomaterials. 2022;12(2):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Umeda Suzuki TY, Gallego J, Assuncao WG, Fraga Briso AL, Santos PH. Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin. PLOS ONE. 2019;14(6):e0217750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suzuki TYU, Pereira MA, Gomes-Filho JE, Wang L, Assuncao WG, Santos PH. Do irrigation solutions influence the bond interface between glass fiber posts and dentin? Braz. Dent. J.. 2019;30(2):106–116. [DOI] [PubMed] [Google Scholar]
- 75.Verhaagen B, Boutsioukis C, Sleutel CP, Kastrinakis E, Sluis LWM, Versluis M. Irrigant transport into dental microchannels. Microfluid Nanofluid. 2014;16(6):1165–1177. [Google Scholar]
- 76.Ururahy MS, Curylofo-Zotti FA, Galo R, Nogueira LFB, Ramos AP, Corona SAM. Wettability and surface morphology of eroded dentin treated with chitosan. Arch. Oral Biol. 2017;75:68–73. [DOI] [PubMed] [Google Scholar]
- 77.Ionescu A, Harris D, Selvaganapathy PR, Kishen A. Electrokinetic transport and distribution of antibacterial nanoparticles for endodontic disinfection. Int. Endod. J. 2020;53(8):1120–1130. [DOI] [PubMed] [Google Scholar]
- 78.Dasgupta D, Peddi S, Saini DK, Ghosh A. Mobile nanobots for prevention of root canal treatment failure. Adv. Healthc. Mater. 2022;11(14):e2200232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Virdee SS, Farnell DJJ, Silva MA, Camilleri J, Cooper PR, Tomson PL. The influence of irrigant activation, concentration and contact time on sodium hypochlorite penetration into root dentine: An ex vivo experiment. Int. Endod. J. 2020;53(7):986–997. [DOI] [PubMed] [Google Scholar]
- 80.Ioannidis K, Niazi S, Mylonas P, Mannocci F, Deb S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent. Mater. 2019;35(11):1614–1629. [DOI] [PubMed] [Google Scholar]
- 81.Stojicic S, Amorim H, Shen Y, Haapasalo M. Ex vivo killing of Enterococcus faecalis and mixed plaque bacteria in planktonic and biofilm culture by modified photoactivated disinfection. Int. Endod. J. 2013;46(7):649–659. [DOI] [PubMed] [Google Scholar]
- 82.Ensafi F, Fazlyab M, Chiniforush N, Akhavan H. Comparative effects of SWEEPS technique and antimicrobial photodynamic therapy by using curcumin and nano-curcumin on Enterococcus faecalis biofilm in root canal treatment. Photodiagnosis Photodyn. Ther. 2022;40:103130. [DOI] [PubMed] [Google Scholar]
- 83.Roshdy NN, Kataia EM, Helmy NA. Assessment of antibacterial activity of 2.5% NaOCl, chitosan nano-particles against Enterococcus faecalis contaminating root canals with and without diode laser irradiation: An in vitro study. Acta Odontol. Scand. 2019;77(1):39–43. [DOI] [PubMed] [Google Scholar]
- 84.Kushwaha V, Yadav R-K, Tikku A-P, Chandra A, Verma P, Gupta P, Shakya VK. Comparative evaluation of antibacterial effect of nanoparticles and lasers against endodontic microbiota: An in vitro study. J Clin Exp Dent. 2018;10(12):e1155–e1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Afkhami F, Akbari S, Chiniforush N. Entrococcus faecalis elimination in root canals using silver nanoparticles, photodynamic therapy, diode laser, or laser-activated nanoparticles: An in vitro study. J. Endod. 2017;43(2):279–282. [DOI] [PubMed] [Google Scholar]
- 86.Kurian AH, Sethi S, Aneja K, Gupta A, Virmani S, Abraham D. Elimination of Enterococcus faecalis from root canal system using laser-activated nanoparticles: A systematic review. Lasers Med. Sci. 2023;38(1):81–81. [DOI] [PubMed] [Google Scholar]
- 87.Afkhami F, Ahmadi P, Chiniforush N, Sooratgar A. Effect of different activations of silver nanoparticle irrigants on the elimination of Enterococcus faecalis. Clin. Oral Investig. 2021;25(12):6893–6899. [DOI] [PubMed] [Google Scholar]
- 88.Silva PV, Guedes DFC, Nakadi FV, Pecora JD, Cruz-Filho AM. Chitosan: A new solution for removal of smear layer after root canal instrumentation. Int. Endod. J. 2013;46(4):332–338. [DOI] [PubMed] [Google Scholar]
- 89.Ozlek E, Rath PP, Kishen A, Neelakantan P. A chitosan-based irrigant improves the dislocation resistance of a mineral trioxide aggregate-resin hybrid root canal sealer. Clin. Oral Investig. 2020;24(1):151–156. [DOI] [PubMed] [Google Scholar]
- 90.Ratih DN, Enggardipta RA, Kartikaningtyas AT. The effect of chitosan nanoparticle as a final irrigation solution on the smear layer removal, micro-hardness and surface roughness of root canal dentin. Open Dent. J. 2020;14(1):19–26. [Google Scholar]
- 91.Hussein ER, Shukri BMS, Ibrahim RH. The effect of chitosan nanoparticle, citric acid, and ethylenediaminetetraacetic acid on dentin smear layer using two different irrigation needles: A scanning electron microscope study. J Conserv Dent. 2022;25(4):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jowkar Z, Hamidi SA, Shafiei F, Ghahramani Y. The effect of silver, zinc oxide, and titanium dioxide nanoparticles used as final irrigation solutions on the fracture resistance of root-filled teeth. Clin Cosmet Investig Dent. 2020;12:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Persadmehr A, Torneck CD, Cvitkovitch DG, Pinto V, Talior I, Kazembe M, Shrestha S, McCulloch CA, Kishen A. Bioactive chitosan nanoparticles and photodynamic therapy inhibit collagen degradation in vitro. J. Endod. 2014;40(5):703–709. [DOI] [PubMed] [Google Scholar]
- 94.Muthalib S, Verma AH, Sundar S, Kumar TSS, Velmurugan N, Krithikadatta J. Evaluation of effect of two different functionalized nanoparticle photodynamic therapy on nanohardness of root dentin—An in vitro study. Photodiagnosis Photodyn. Ther. 2020;31:101856. [DOI] [PubMed] [Google Scholar]
- 95.Mathew SP, Pai VS, Usha G, Nadig RR. Comparative evaluation of smear layer removal by chitosan and ethylenediaminetetraacetic acid when used as irrigant and its effect on root dentine: An in vitro atomic force microscopic and energy-dispersive X-ray analysis. J Conserv Dent. 2017;20(4):245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goodman A, Schilder H, Aldrich W. The thermomechanical properties of gutta-percha: II. The history and molecular chemistry of gutta-percha. Oral Surg. Oral Med. Oral Pathol. 1974;37(6):954–961. [DOI] [PubMed] [Google Scholar]
- 97.Liao SC, Wang HH, Hsu YH, Huang HM, Gutmann JL, Hsieh SC. The investigation of thermal behaviour and physical properties of several types of contemporary gutta-percha points. Int. Endod. J. 2021;54(11):2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vishwanath V, Rao HM. Gutta-percha in endodontics—A comprehensive review of material science. J Conserv Dent. 2019;22(3):216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee D-K, Kim SV, Limansubroto AN, Yen A, Soundia A, Wang C-Y, Shi W, Hong C, Tetradis S, Kim Y, et al. Nanodiamond–gutta percha composite biomaterials for root canal therapy. ACS Nano. 2015;9(11):11490–11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohan A, Dipallini S, Lata S, Mohanty S, Pradhan P, Patel P, Makkar H, Verma SK. Oxidative stress induced antimicrobial efficacy of chitosan and silver nanoparticles coated Gutta-percha for endodontic applications. Mater Today Chem. 2020;17: Article 100299. [Google Scholar]
- 101.Mozayeni MA, Dianat O, Tahvildari S, Mozayani M, Paymanpour P. Subcutaneous reaction of rat tissues to nanosilver coated gutta-percha. Iran Endod J. 2017;12(2):157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shantiaee Y, Maziar F, Dianat O, Mahjour F. Comparing microleakage in root canals obturated with nanosilver coated gutta-percha to standard gutta-percha by two different methods. Iran Endod J. 2011;6(4):140–145. [PMC free article] [PubMed] [Google Scholar]
- 103.Abd El Hamid HM, Abdel-Aziz MS, Abu Naeem FM. Antimicrobial efficacy of nanopropolis coated vs silver-curcumin nanoparticles coated gutta-percha points on various microbial species. A comparative in vitro study. Egypt. Dent. J. 2020;66(3):1893–1902. [Google Scholar]
- 104.Peng S, Chen Y, Jin X, Lu W, Gou M, Wei X, Xie J. Polyimide with half encapsulated silver nanoparticles grafted ceramic composite membrane: Enhanced silver stability and lasting anti–biofouling performance. J. Membr. Sci. 2020;611: Article 118340. [Google Scholar]
- 105.Akter M, Sikder MT, Rahman MM, Ullah AA, Hossain KFB, Banik S, Hosokawa T, Saito T, Kurasaki M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J Adv Res. 2018;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Osiri S, Banomyong D, Sattabanasuk V, Yanpiset K. Root reinforcement after obturation with calcium silicate–based sealer and modified gutta-percha cone. J. Endod. 2018;44(12):1843–1848. [DOI] [PubMed] [Google Scholar]
- 107.Chogle S, Kinaia BM, Goodis HE. Scope of nanotechnology in endodontics. Nanobiomater Clin Dent. 2019:517–539.
- 108.Ozdemir O, Kopac T. Cytotoxicity and biocompatibility of root canal sealers: A review on recent studies. J Appl Biomater Funct Mater. 2022;20:22808000221076325. [DOI] [PubMed] [Google Scholar]
- 109.Komabayashi T, Colmenar D, Cvach N, Bhat A, Primus C, Imai Y. Comprehensive review of current endodontic sealers. Dent. Mater. J. 2020;39(5):703–720. [DOI] [PubMed] [Google Scholar]
- 110.Diogo P, Faustino MAF, Palma PJ, Rai A, Neves MGPMS, Miguel Santos J. May carriers at nanoscale improve the endodontic's future? Adv. Drug Deliv. Rev. 2023;195: Article 114731. [DOI] [PubMed] [Google Scholar]
- 111.Abbaszadegan A, Ghahramani Y, Gholami A, Hemmateenejad B, Dorostkar S, Nabavizadeh M, Sharghi H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J Nanomater. 2015;2015:720654. [Google Scholar]
- 112.Oncu A, Celikten B, Aydin B, Amasya G, Acik L, Sevimay FS. Comparative evaluation of the antifungal efficacy of sodium hypochlorite, chlorhexidine, and silver nanoparticles against Candida albicans. Microsc. Res. Tech. 2022;85(12):3755–3760. [DOI] [PubMed] [Google Scholar]
- 113.Nagendrababu V, Meiyazhagan G, Beaula WS, Kalaiselvam R, Subramanian B, Chandrasekar A, Lakshmikanthanbharathi L, Deivanayagam K, Venkatraman G. Comparison of the antibacterial efficacy of silver nanoparticles with chlorhexidine against Enterococcus faecalis biofilm—An in vitro study. J. Nanosci. Nanotechnol. 2017;17(7):4613–4617. [Google Scholar]
- 114.Pablo Loyola-Rodriguez J, Torres-Mendez F, Francisco Espinosa-Cristobal L, Obed Garcia-Cortes J, Loyola-Leyva A, Javier Gonzalez F, Soto-Barreras U, Nieto-Aguilar R, Contreras-Palma G. Antimicrobial activity of endodontic sealers and medications containing chitosan and silver nanoparticles against Enterococcus faecalis. J Appl Biomater Funct Mater. 2019;17(3):2280800019851771. [DOI] [PubMed] [Google Scholar]
- 115.Seung J, Weir MD, Melo MAS, Romberg E, Nosrat A, Xu HHK, Tordik PA. A modified resin sealer: Physical and antibacterial properties. J. Endod. 2018;44(10):1553–1557. [DOI] [PubMed] [Google Scholar]
- 116.Baras BH, Melo MAS, Sun J, Oates TW, Weir MD, Xie X, Bai Y, Xu HHK. Novel endodontic sealer with dual strategies of dimethylaminohexadecyl methacrylate and nanoparticles of silver to inhibit root canal biofilms. Dent. Mater. 2019;35(8):1117–1129. [DOI] [PubMed] [Google Scholar]
- 117.Patil DV, Gaddalay SL, Kabir R. The influence of humidity on bond strength of AH plus, BioRoot RCS, and Nanoseal-S sealers: An in vitro study. Endodontology. 2022;34(3):202–207. [Google Scholar]
- 118.Palanivelu CR, Ravi V, Sivakumar AA, Sivakumar JS, Prasad AS, Arthanari KK. An in vitro comparative evaluation of distribution of three different sealers by single-cone obturation technique. J. Pharm. Bioallied Sci. 2019;11(Suppl 2):S438–S441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rashid DS. Stereomicroscopic evaluation of sealing ability of three different root canal sealers: An in vitro study. Int J Appl Dent Sci. 2021;7(4):166–173. [Google Scholar]
- 120.Afkhami F, Nasri S, Valizadeh S. Bacterial leakage assessment in root canals sealed with AH plus sealer modified with silver nanoparticles. BMC Oral Health. 2021;21(1):577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prasad G, Govula K, Anumula L, Kumar P. Evaluation of the biocompatibility of silver nanoparticles, ascertaining their safety in the field of endodontic therapy. J Int Clin Dent Res Org. 2021;13(2):109–117. [Google Scholar]
- 122.Jiang W, Mashayekhi H, Xing B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009;157(5):1619–1625. [DOI] [PubMed] [Google Scholar]
- 123.Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008;279(1):71–76. [DOI] [PubMed] [Google Scholar]
- 124.Collares FM, Garcia IM, Klein M, Parolo CF, Sanchez FAL, Takimi A, Bergmann CP, Samuel SMW, Melo MA, Leitune VC. Exploring needle-like zinc oxide nanostructures for improving dental resin sealers: Design and evaluation of antibacterial, physical and chemical properties. Polymers. 2020;12(4):789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang H-H, Tseng Y-T, Huang S-W, Kuo Y-F, Yeh C-L, Wu C-H, Huang YC, Jeng RJ, Lin JJ, Lin CP. Evaluation of carbon dioxide-based urethane acrylate composites for sealers of root canal obturation. Polymers. 2020;12(2):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang J, Mei Q, Lin L, Sun F, Li J, Zou Q, Zuo Y, Li Y. A comparison of the characteristics of polyurethane-based sealers including various antimicrobial agents. RSC Adv. 2019;9(13):7043–7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lei X, Wang J, Chen J, Gao J, Zhang J, Zhao Q, Tang J, Fang W, Li J, Li Y, et al. The in vitro evaluation of antibacterial efficacy optimized with cellular apoptosis on multi-functional polyurethane sealers for the root canal treatment. J. Mater. Chem. B. 2021;9(5):1370–1383. [DOI] [PubMed] [Google Scholar]
- 128.Chang MC, Tang CM, Lin YH, Liu HC, Wang TM, Lan WC, Cheng RH, Lin YR, Chang HH, Jeng JH. Toxic mechanisms of Roth801, canals, microparticles and nanoparticles of ZnO on MG-63 osteoblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;119: Article 111635. [DOI] [PubMed] [Google Scholar]
- 129.Lallo da Silva B, Abucafy MP, Berbel Manaia E, Oshiro Junior JA, Chiari-Andreo BG, Pietro RCR, Leitune VC. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomedicine. 2019;14:9395–9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Versiani MA, Abi Rached-Junior FJ, Kishen A, Pecora JD, Silva-Sousa YT, Sousa-Neto MD. Zinc oxide nanoparticles enhance physicochemical characteristics of Grossman sealer. J. Endod.. 2016;42(12):1804–1810. [DOI] [PubMed] [Google Scholar]
- 131.D'Souza VV, Mahaparale RR, Mangala TM, Saraf AA, Mali SR, Pawar S, Mandhane SA, Nahar SR. Comparative evaluation of modified endodontic Sealers' penetration following sonic activation of irrigants: A scanning electron microscope study. J. Clin. Diagn. Res. 2020;14(9):29. [Google Scholar]
- 132.Fan Y, Wang Z, Sun Y, Guo X, Wang H, Xu HHK, Wang S, Zhou X, Li B, Cheng L. Effect of the modified methacrylate-based root canal sealer in single-cone technique. Nanomaterials. 2022;12(21):3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vallet-Regi M, Ruiz-Hernandez E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011;23(44):5177–5218. [DOI] [PubMed] [Google Scholar]
- 134.Al-Haddad A, Che Ab Aziz CA. Bioceramic-based root canal sealers: A review. Int J Biomater. 2016;2016:9753210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Collares FM, Leitune VCB, Rostirolla FV, Trommer RM, Bergmann CP, Samuel SMW. Nanostructured hydroxyapatite as filler for methacrylate-based root canal sealers. Int. Endod. J. 2012;45(1):63–67. [DOI] [PubMed] [Google Scholar]
- 136.Hashmi A, Zhang X, Kishen A. Impact of dentin substrate modification with chitosan-hydroxyapatite precursor nanocomplexes on sealer penetration and tensile strength. J. Endod. 2019;45(7):935–942. [DOI] [PubMed] [Google Scholar]
- 137.Mestieri LB, Collares FM, Zaccara IM, Moreira MSNA, Kopper PMP, Leitune VCB, Grecca FS. Biological properties of experimental methacrylate-based sealers containing calcium phosphates. Braz. Dent. J. 2021;32(1):59–66. [DOI] [PubMed] [Google Scholar]
- 138.Rostirolla FV, Leitune VCB, Bohns FR, Portella FF, Samuel SMW, Collares FM. Calcium phosphates as fillers for methacrylate-based sealer. Clin. Oral Investig. 2019;23(12):4417–4423. [DOI] [PubMed] [Google Scholar]
- 139.Jerri Al-Bakhsh BA, Shafiei F, Hashemian A, Shekofteh K, Bolhari B, Behroozibakhsh M. In-vitro bioactivity evaluation and physical properties of an epoxy-based dental sealer reinforced with synthesized fluorine-substituted hydroxyapatite, hydroxyapatite and bioactive glass nanofillers. Bioact Mater. 2019;4:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jung MK, Park SC, Kim YJ, Park JT, Knowles JC, Park JH, Dashnyam K, Jun SK, Lee HH, Lee JH. Premixed calcium silicate-based root canal sealer reinforced with bioactive glass nanoparticles to improve biological properties. Pharmaceutics. 2022;14(9):1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heid S, Stoessel PR, Tauböck TT, Stark WJ, Zehnder M, Mohn D. Incorporation of particulate bioactive glasses into a dental root canal sealer. Biomed Glasses. 2016;2:29–37. [Google Scholar]
- 142.Almaimouni YK, Hamid SK, Ilyas K, Shah AT, Majeed A, Khan AS. Structural, fluoride release, and 3D interfacial adhesion analysis of bioactive endodontic sealers. Dent. Mater. J. 2020;39(3):483–489. [DOI] [PubMed] [Google Scholar]
- 143.Liu S, Liu Z. In vitro evaluation of the antimicrobial activity of four endodontic sealers. J Mod Stomatol. 2012;26(3):183–185. [Google Scholar]
- 144.Wang J-p, Geogi G. Antimicrobial and sealant properties of nanohydroxyapatite as endodontic sealer. Chin J Tissue Eng Res. 2014;18(21):3350–3354. [Google Scholar]
- 145.Baras BH, Melo MAS, Thumbigere-Math V, Tay FR, Fouad AF, Oates TW, Weir MD, Cheng L, Xu HHK. Novel bioactive and therapeutic root canal sealers with antibacterial and remineralization properties. Materials. 2020;13(5):1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sun X, Sun A, Jia X, Jin S, Zhang D, Xiao K, Wang Q. In vitro bioactivity of AH plus with the addition of nano-magnesium hydroxide. Ann Transl Med. 2020;8(6):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meng Y, Zhang D, Jia X, Xiao K, Lin X, Yang Y, Xu D, Wang Q. Antimicrobial activity of nano-magnesium hydroxide against oral bacteria and application in root canal sealer. Med. Sci. Monit. 2020;26, e922920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang L, Xie X, Li C, Liu H, Zhang K, Zhou Y, Chang X, Xu HHK. Novel bioactive root canal sealer to inhibit endodontic multispecies biofilms with remineralizing calcium phosphate ions. J. Dent. 2017;60:25–35. [DOI] [PubMed] [Google Scholar]
- 149.Baras BH, Wang S, Melo MAS, Tay F, Fouad AF, Arola DD, Weir MD, Xu HHK. Novel bioactive root canal sealer with antibiofilm and remineralization properties. J. Dent. 2019;83:67–76. [DOI] [PubMed] [Google Scholar]
- 150.Baras BH, Sun J, Melo MAS, Tay FR, Oates TW, Zhang K, Weir MD, Xu HHK. Novel root canal sealer with dimethylaminohexadecyl methacrylate, nano-silver and nano-calcium phosphate to kill bacteria inside root dentin and increase dentin hardness. Dent. Mater. 2019;35(10):1479–1489. [DOI] [PubMed] [Google Scholar]
- 151.Cheng X, Qu T, Ma C, Xiang D, Yu Q, Liu X. Bioactive mono-dispersed nanospheres with long-term antibacterial effects for endodontic sealing. J. Mater. Chem. B. 2017;5(6):1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zheng Y, Yang X, Liu S, Xu Y, Bao S, Wang Y, Liu Y, Zhang F, Gou Z. Ball milling medium may tune the self-curing property and root canal microleakage of β-dicalcium silicate-based cement. Materials. 2022;15(14):5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baghdadi I, Zaazou A, Abu Tarboush B, Zakhour M, Ozcan M, Salameh Z. Physiochemical properties of a bioceramic-based root canal sealer reinforced with multi-walled carbon nanotubes, titanium carbide and boron nitride biomaterials. J. Mech. Behav. Biomed. Mater. 2020;110:103892. [DOI] [PubMed] [Google Scholar]
- 154.Viapiana R, Flumignan DL, Guerreiro-Tanomaru JM, Camilleri J, Tanomaru-Filho M. Physicochemical and mechanical properties of zirconium oxide and niobium oxide modified Portland cement-based experimental endodontic sealers. Int. Endod. J. 2014;47(5):437–448. [DOI] [PubMed] [Google Scholar]
- 155.Viapiana R, Guerreiro-Tanomaru JM, Hungaro-Duarte MA, Tanomaru-Filho M, Camilleri J. Chemical characterization and bioactivity of epoxy resin and Portland cement-based sealers with niobium and zirconium oxide radiopacifiers. Dent. Mater. 2014;30(9):1005–1020. [DOI] [PubMed] [Google Scholar]
- 156.Rashki S, Asgarpour K, Tarrahimofrad H, Hashemipour M, Ebrahimi MS, Fathizadeh H, Khorshidi A, Khan H, Marzhoseyni Z, Salavati-Niasari M, et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021;251: Article 117108. [DOI] [PubMed] [Google Scholar]
- 157.DaSilva L, Finer Y, Friedman S, Basrani B, Kishen A. Biofilm formation within the interface of bovine root dentin treated with conjugated chitosan and sealer containing chitosan nanoparticles. J. Endod. 2013;39(2):249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carpio-Perochena A, Kishen A, Shrestha A, Bramante CM. Antibacterial properties associated with chitosan nanoparticle treatment on root dentin and 2 types of endodontic sealers. J. Endod.. 2015;41(8):1353–1358. [DOI] [PubMed] [Google Scholar]
- 159.Nair N, James B, Devadathan A, Johny MK, Mathew J, Jacob J. Comparative evaluation of antibiofilm efficacy of chitosan nanoparticle- and zinc oxide nanoparticle-incorporated calcium hydroxide-based sealer: An in vitro study. Contemp Clin Dent. 2018;9(3):434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ratih DN, Mulyawati E, Santi RK, Kristanti Y. Antibacterial and cytotoxicity of root canal sealer with the addition of chitosan nanoparticle at various concentrations. Eur J Dent. 2023;17(2):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kishen A, Shrestha S, Shrestha A, Cheng C, Goh C. Characterizing the collagen stabilizing effect of crosslinked chitosan nanoparticles against collagenase degradation. Dent. Mater. 2016;32(8):968–977. [DOI] [PubMed] [Google Scholar]
- 162.Chen Z, Cao S, Wang H, Li Y, Kishen A, Deng X, Yang X, Wang Y, Cong C, Wang H, et al. Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries. PLOS ONE. 2015;10(1): Article e0116553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Del Carpio-Perochena A, Nicholson E, Singh CV, Camilleri J, Kishen A. Impact of dentin conditioning and sealer modification with chitosan-hydroxyapatite nanocomplexes on the antibacterial and mechanical characteristics of root dentin. J. Endod. 2022;48(10):1319–1326. [DOI] [PubMed] [Google Scholar]
- 164.Jiao Y, Niu L-n, Ma S, Li J, Tay FR, Chen J-h. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017;71:53–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Makvandi P, Jamaledin R, Jabbari M, Nikfarjam N, Borzacchiello A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018;34(6):851–867. [DOI] [PubMed] [Google Scholar]
- 166.Chrószcz M, Barszczewska-Rybarek I. Nanoparticles of quaternary ammonium polyethylenimine derivatives for application in dental materials. Polymers. 2020;12(11):2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shvero DK, Zaltsman N, Weiss EI, Polak D, Hazan R, Beyth N. Lethal bacterial trap: Cationic surface for endodontic sealing. J. Biomed. Mater. Res. A. 2016;104(2):427–434. [DOI] [PubMed] [Google Scholar]
- 168.Beyth N, Shvero DK, Zaltsman N, Houri-Haddad Y, Abramovitz I, Davidi MP, Weiss EI. Rapid kill-novel endodontic sealer and Enterococcus faecalis. PLOS ONE. 2013;8(11):e78586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shvero DK, Abramovitz I, Zaltsman N, Davidi MP, Weiss EI, Beyth N. Towards antibacterial endodontic sealers using quaternary ammonium nanoparticles. Int. Endod. J. 2013;46(8):747–754. [DOI] [PubMed] [Google Scholar]
- 170.Barros J, Silva MG, Rocas IN, Goncalves LS, Alves FF, Lopes MA, Pina-Vaz I, Siqueira JF Jr. Antibiofilm effects of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. J. Endod. 2014;40(8):1167–1171. [DOI] [PubMed] [Google Scholar]
- 171.Barros J, Silva MG, Rodrigues MA, Alves FRF, Lopes MA, Pina-Vaz I, Siqueira JF Jr. Antibacterial, physicochemical and mechanical properties of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. Int. Endod. J. 2014;47(8):725–734. [DOI] [PubMed] [Google Scholar]
- 172.Barros J, Costa-Rodrigues J, Lopes MA, Pina-Vaz I, Fernandes MH. Response of human osteoblastic and osteoclastic cells to AH Plus and Pulp Canal sealer containing quaternary ammonium polyethylenimine nanoparticles. J. Endod. 2014;40(8):1149–1155. [DOI] [PubMed] [Google Scholar]
- 173.Abramovitz I, Wisblech D, Zaltsman N, Weiss EI, Beyth N, Beyth N. Intratubular antibacterial effect of polyethyleneimine nanoparticles: An ex vivo study in human teeth. J Nanomater. 2015;2015:980529. [Google Scholar]
- 174.Gong SQ, Huang ZB, Shi W, Ma B, Tay FR, Zhou B. In vitro evaluation of antibacterial effect of AH Plus incorporated with quaternary ammonium epoxy silicate against Enterococcus faecalis. J. Endod. 2014;40(10):1611–1615. [DOI] [PubMed] [Google Scholar]
- 175.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J. Dent. Res. 2011;90(4):535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Santos AC, Pereira I, Reis S, Veiga F, Saleh M, Lvov Y. Biomedical potential of clay nanotube formulations and their toxicity assessment. Expert Opin. Drug Deliv. 2019;16(11):1169–1182. [DOI] [PubMed] [Google Scholar]
- 177.Monteiro JC, Garcia IM, Branco Leitune VC, Visioli F, Balbinot GdS, Werner Samuel SM, Makeeva I, Collares FM, Sauro S. Halloysite nanotubes loaded with alkyl trimethyl ammonium bromide as antibacterial agent for root canal sealers. Dent. Mater. 2019;35(5):789–796. [DOI] [PubMed] [Google Scholar]
- 178.Ribeiro JS, Xavier SR, Cuevas Suarez CE, Pappen FG, Piva E, Lund RG, Bottino MC. Synthesis and characterization of calcium-releasing elastomeric resin-based endodontic sealers. Clin. Oral Investig. 2023;7:3447–3456. [DOI] [PubMed] [Google Scholar]
- 179.Baghdadi I, AbuTarboush BJ, Zaazou A, Skienhe H, Ozcan M, Zakhour M, Salameh Z. Investigation of the structure and compressive strength of a bioceramic root canal sealer reinforced with nanomaterials. J Appl Biomater Funct Mater. 2021;19:22808000211014747. [DOI] [PubMed] [Google Scholar]
- 180.Marica A, Fritea L, Banica F, Sinescu C, Iovan C, Hulka I, Rusu G, Cavalu S. Carbon nanotubes for improved performances of endodontic sealer. Materials. 2021;14(15):4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kang S, Herzberg M, Rodrigues DF, Elimelech M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir. 2008;24(13):6409–6413. [DOI] [PubMed] [Google Scholar]
- 182.Zhuang J, Yu Y, Lu R. Mesoporous silica nanoparticles as carrier to overcome bacterial drug resistant barriers. Int. J. Pharm. 2023;631: Article 122529. [DOI] [PubMed] [Google Scholar]
- 183.Li X, Li X, Wang S, Leung KC-F, Zhang C, Jin L. Infiltration and profiles of mesoporous silica nanoparticles in dentinal tubules. ACS Biomater Sci. Eng. 2018;4(4):1428–1436. [DOI] [PubMed] [Google Scholar]
- 184.Cao Y, Xu C, Wright PP, Liu J, Kong Y, Wang Y, Huang X, Song H, Fu J, Gao F, et al. Calcium-doped silica nanoparticles mixed with phosphate-doped silica nanoparticles for rapid and stable occlusion of dentin tubules. ACS Appl Nano Mater. 2021;4(9):8761–8769. [Google Scholar]
- 185.Zhu J, Liang R, Sun C, Xie L, Wang J, Leng D, Wu D, Liu W. Effects of nanosilver and nanozinc incorporated mesoporous calcium-silicate nanoparticles on the mechanical properties of dentin. PLOS ONE. 2017;12(8): Article e0182583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun Q, Duan M, Fan W, Fan B. Ca-Si mesoporous nanoparticles with the optimal ag-Zn ratio inhibit the Enterococcus faecalis infection of teeth through dentinal tubule infiltration: An in vitro and in vivo study. J. Mater. Chem. B. 2021;9(9):2200–2211. [DOI] [PubMed] [Google Scholar]
- 187.Pagonis TC, Chen J, Fontana CR, Devalapally H, Ruggiero K, Song X, Foschi F, Dunham J, Skobe Z, Yamazaki H, et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J. Endod. 2010;36(2):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Raheem IAA, Razek AA, Elgendy AA, Labah DA, Saleh NM. Egyptian propolis-loaded nanoparticles as a root canal nanosealer: Sealing ability and in vivo biocompatibility. Int. J. Nanomedicine. 2020;15:5265–5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Uğur Aydın Z, Toptaş O, Göller Bulut D, Akay N, Kara T, Akbulut N. Effects of root-end filling on the fractal dimension of the periapical bone after periapical surgery: Retrospective study. Clin. Oral Investig. 2019;23:3645–3651. [DOI] [PubMed] [Google Scholar]
- 190.De Bruyne M, De Moor R. Long-term sealing ability of Resilon apical root-end fillings. Int. Endod. J. 2009;42(10):884–892. [DOI] [PubMed] [Google Scholar]
- 191.Esen E, Yoldas O, Kürkçü M, Doğan MC, Seydaoğlu G. Apical microleakage of root-end cavities prepared by CO2 laser. J. Endod. 2004;30(9):662–664. [DOI] [PubMed] [Google Scholar]
- 192.Torabinejad M, Parirokh M, Dummer PM. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview–part II: Other clinical applications and complications. Int. Endod. J. 2018;51(3):284–317. [DOI] [PubMed] [Google Scholar]
- 193.Suhag A, Chhikara N, Pillania A, Yadav P. Root end filling materials: A review. Int J Appl Dent Sci. 2018;4(2):320–323. [Google Scholar]
- 194.Jonaidi-Jafari N, Izadi M, Javidi P. The effects of silver nanoparticles on antimicrobial activity of ProRoot mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM). J Clin Exp Dent. 2016;8(1):e22–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zand V, Lotfi M, Aghbali A, Mesgariabbasi M, Janani M, Mokhtari H, Tehranchi P, Pakdel SM. Tissue reaction and biocompatibility of implanted mineral trioxide aggregate with silver nanoparticles in a rat model. Iran Endod J. 2016;11(1):13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sheethal Dsouza T, Shetty A, Dsouza N. Evaluation of pH, calcium ion release, and dimensional stability of an experimental silver nanoparticle-incorporated calcium silicate-based cement. Bioinorg Chem Appl. 2021;2021:3919543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vazquez-Garcia F, Tanomaru-Filho M, Chávez-Andrade GM, Bosso-Martelo R, Basso-Bernardi MI, Guerreiro-Tanomaru JM, Universidade Estadual Paulista, Brazil, Universidade Federal da Bahia, Brazil, Universidade de São Paulo, Brazil . Effect of silver nanoparticles on physicochemical and antibacterial properties of calcium silicate cements. Braz. Dent. J. 2016;27:508–514. [DOI] [PubMed] [Google Scholar]
- 198.Mendes MS, Resende LD, Pinto CA, Raldi DP, Cardoso FG, Habitante SM. Radiopacity of mineral trioxide aggregate with and without inclusion of silver nanoparticles. J. Contemp. Dent. Pract. 2017;18(6):448–451. [DOI] [PubMed] [Google Scholar]
- 199.Hernandez-Delgadillo R, Del Angel-Mosqueda C, Solís-Soto JM, Munguia-Moreno S, Pineda-Aguilar N, Sánchez-Nájera RI, Shankararaman C, Cabral-Romero C. Antimicrobial and antibiofilm activities of MTA supplemented with bismuth lipophilic nanoparticles. Dent. Mater. J. 2017;36(4):503–510. [DOI] [PubMed] [Google Scholar]
- 200.Çinar Ç, Odabaş M, Gürel MA, Baldağ I. The effects of incorporation of silver-zeolite on selected properties of mineral trioxide aggregate. Dent. Mater. J. 2013;32(6):872–876. [DOI] [PubMed] [Google Scholar]
- 201.Abe Y, Ishii M, Takeuchi M, Ueshige M, Tanaka S, Akagawa Y. Effect of saliva on an antimicrobial tissue conditioner containing silver–zeolite. J. Oral Rehabil. 2004;31(6):568–573. [DOI] [PubMed] [Google Scholar]
- 202.Odabaş ME, Çinar Ç, Akça G, Araz İ, Ulusu T, Yücel H. Short-term antimicrobial properties of mineral trioxide aggregate with incorporated silver-zeolite. Dent. Traumatol. 2011;27(3):189–194. [DOI] [PubMed] [Google Scholar]
- 203.Ghatole K, Patil A, Giriyappa RH, Singh TV, Jyotsna SV, Rairam S. Evaluation of antibacterial efficacy of MTA with and without additives like silver zeolite and chlorhexidine. J. Clin. Diagn. Res. 2016;10(6):ZC11, ZC14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Samiei M, Ghasemi N, Asl-Aminabadi N, Divband B, Golparvar-Dashti Y, Shirazi S. Zeolite-silver-zinc nanoparticles: Biocompatibility and their effect on the compressive strength of mineral trioxide aggregate. J Clin Exp Dent. 2017;9(3): Article e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Samiei M, Ghasemi N, Aghazadeh M, Divband B, Akbarzadeh F. Biocompatibility of mineral trioxide aggregate with TiO2 nanoparticles on human gingival fibroblasts. J Clin Exp Dent. 2017;9(2): Article e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dsouza T, Hegde MN, Radhakrishna V, Dsouza N, Kumari S. In vitro cytotoxic evaluation of mineral trioxide aggregate with silver and titanium dioxide nanoparticles. World J Dent. 2019. [Google Scholar]
- 207.Samiei M, Janani M, Asl-Aminabadi N, Ghasemi N, Divband B, Shirazi S, Kafili K. Effect of the TiO2 nanoparticles on the selected physical properties of mineral trioxide aggregate. J Clin Exp Dent. 2017;9(2): Article e191–e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Guerreiro-Tanomaru JM, Trindade-Junior A, Cesar Costa B, Silva GF, Drullis Cifali L, Basso Bernardi MI, Tanomaru-Filho M. Effect of zirconium oxide and zinc oxide nanoparticles on physicochemical properties and antibiofilm activity of a calcium silicate-based material. Sci World J. 2014;2014:975213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Guerreiro-Tanomaru JM, Vazquez-Garcia FA, Bosso-Martelo R, Bernardi MIB, Faria G, Tanomaru FM. Effect of addition of nano-hydroxyapatite on physico-chemical and antibiofilm properties of calcium silicate cements. J. Appl. Oral Sci. 2016;24:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bichile ML, Mahaparale R, Mattigatti S, Wahane KD, Raut SV. Push-out bond strength of mineral trioxide aggregate with addition of titanium dioxide, silver, and silicon dioxide nanoparticles: An in vitro comparative study. J Conserv Dent. 2022;25(5):541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shahi S, Samiei M, Bahari M, Yavari H, Rahbar MM. Effect of incorporating titanium dioxide nanoparticles into white portland cement, white mineral trioxide aggregate, and calcium enriched mixture cement on the push-out bond strength to furcal area dentin. J. Dent. 2023;24(4):422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rodríguez CG, Cepeda MAAN, Arana HLM, Lopez JFG, del Perpetuo Socorro Mendiburu Zavala CE, Cuevas RP, Molina BC, Soto DJMS. Current and future options for dental pulp regeneration. Int J Appl Dental Sci. 2023;9(1):178–182. [Google Scholar]
- 213.Morsczeck C, Reichert TE. Dental stem cells in tooth regeneration and repair in the future. Expert Opin. Biol. Ther. 2018;18(2):187–196. [DOI] [PubMed] [Google Scholar]
- 214.Gangolli RA, Devlin SM, Gerstenhaber JA, Lelkes PI, Yang M. A bilayered poly (lactic-co-glycolic acid) scaffold provides differential cues for the differentiation of dental pulp stem cells. Tissue Eng A. 2019;25(3–4):224–233. [DOI] [PubMed] [Google Scholar]
- 215.Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, John AS, George A. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J. Endod. 2008;34(4):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bottino M, Kamocki K, Yassen G, Platt J, Vail M, Ehrlich Y, Spolnik KJ, Gregory RL. Bioactive nanofibrous scaffolds for regenerative endodontics. J. Dent. Res. 2013;92(11):963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gupte M, Ma P. Nanofibrous scaffolds for dental and craniofacial applications. J. Dent. Res. 2012;91(3):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Canales DA, Reyes F, Saavedra M, Peponi L, Leonés A, Palza H, Boccaccini AR, Grünewald A, Zapata PA. Electrospun fibers of poly (lactic acid) containing bioactive glass and magnesium oxide nanoparticles for bone tissue regeneration. Int. J. Biol. Macromol. 2022;210:324–336. [DOI] [PubMed] [Google Scholar]
- 219.Zein N, Harmouch E, Lutz J-C, Fernandez De Grado G, Kuchler-Bopp S, Clauss F, Offner D, Hua G, Benkirane-Jessel N, Fioretti F. Polymer-based instructive scaffolds for endodontic regeneration. Materials. 2019;12(15):2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xia Y, Chen H, Zhang F, Wang L, Chen B, Reynolds MA, Ma J, Schneider A, Gu N, Xu HHK. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif Cells Nanomed Biotechnol. 2018;46(sup1):423–433. [DOI] [PubMed] [Google Scholar]
- 221.Chuang Y-C, Chang C-C, Yang F, Simon M, Rafailovich M. TiO2 nanoparticles synergize with substrate mechanics to improve dental pulp stem cells proliferation and differentiation. Mater. Sci. Eng. C. 2021;118: Article 111366. [DOI] [PubMed] [Google Scholar]
- 222.Yang X, Yang F, Walboomers XF, Bian Z, Fan M, Jansen JA. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J. Biomed. Mater. Res. A. 2010;93(1):247–257. [DOI] [PubMed] [Google Scholar]
- 223.Lee J-H, Kang M-S, Mahapatra C, Kim H-W. Effect of aminated mesoporous bioactive glass nanoparticles on the differentiation of dental pulp stem cells. PLOS ONE. 2016;11(3): Article e0150727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang J, Park Y-D, Bae W-J, El-Fiqi A, Shin S-H, Lee E-J, Kim H-W, Kim E-C. Effects of bioactive cements incorporating zinc-bioglass nanoparticles on odontogenic and angiogenic potential of human dental pulp cells. J. Biomater. Appl. 2015;29(7):954–964. [DOI] [PubMed] [Google Scholar]
- 225.Shrestha S, Diogenes A, Kishen A. Temporal-controlled release of bovine serum albumin from chitosan nanoparticles: Effect on the regulation of alkaline phosphatase activity in stem cells from apical papilla. J. Endod. 2014;40(9):1349–1354. [DOI] [PubMed] [Google Scholar]
- 226.Shrestha S, Diogenes A, Kishen A. Temporal-controlled dexamethasone releasing chitosan nanoparticle system enhances odontogenic differentiation of stem cells from apical papilla. J. Endod. 2015;41(8):1253–1258. [DOI] [PubMed] [Google Scholar]
- 227.Lee J-H, El-Fiqi A, Mandakhbayar N, Lee H-H, Kim H-W. Drug/ion co-delivery multi-functional nanocarrier to regenerate infected tissue defect. Biomaterials. 2017;142:62–76. [DOI] [PubMed] [Google Scholar]
- 228.Sajid M, Ilyas M, Basheer C, Tariq M, Daud M, Baig N, Shehzad F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015;22:4122–4143. [DOI] [PubMed] [Google Scholar]
- 229.Teixeira ABV, Moreira NCS, Takahashi CS, Schiavon MA, Alves OL, Reis AC. Cytotoxic and genotoxic effects in human gingival fibroblast and ions release of endodontic sealers incorporated with nanostructured silver vanadate. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109(9):1380–1388. [DOI] [PubMed] [Google Scholar]
- 230.Teixeira ABV, Castro DT, Schiavon MA, Reis AC. Cytotoxicity and release ions of endodontic sealers incorporated with a silver and vanadium base nanomaterial. Odontology. 2020;108(4):661–668. [DOI] [PubMed] [Google Scholar]
- 231.Javidi M, Zarei M, Omidi S, Ghorbani A, Gharechahi M, Rad MS. Cytotoxicity of a new nano zinc-oxide eugenol sealer on murine fibroblasts. Iran Endod J. 2015;10(4):231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rad MS, Kompany A, Zak AK, Javidi M, Mortazavi SM. Microleakage and antibacterial properties of ZnO and ZnO: Ag nanopowders prepared via a sol-gel method for endodontic sealer application. J. Nanopart. Res. 2013;15:1925. [Google Scholar]
- 233.Javidi M, Zarei M, Ashrafpour E, Gharechahi M, Bagheri H. Post-treatment flare-up incidence after using nano zinc oxide eugenol sealer in mandibular first molars with irreversible pulpitis. J. Dent. 2020;21(4):307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Baghdadi I, AbuTarboush B, Zaazou A, Skienhe H, Ozcan M, Zakhour M, Salameh Z. Effect of sintering temperature on the physiochemical properties, microstructure, and compressive strength of a bioceramic root canal sealer reinforced with multi-walled carbon nanotubes and titanium carbide. J. Mech. Behav. Biomed. Mater. 2021;119: Article 104524. [DOI] [PubMed] [Google Scholar]
- 235.Torres-Betancourt JA, Hernandez-Delgadillo R, Flores-Trevino JJ, Solis-Soto JM, Pineda-Aguilar N, Nakagoshi-Cepeda MAA, Sánchez-Nájera RI, Chellam S, Cabral-Romero C. Antimicrobial potential of AH Plus supplemented with bismuth lipophilic nanoparticles on E. faecalis isolated from clinical isolates. J Appl Biomater Funct Mater. 2022;20:22808000211069221. [DOI] [PubMed] [Google Scholar]
- 236.Ribeiro Camargo CH, Landim Gomes LC, Moreira Franca MC, Bittencourt TS, Valera MC, Camargo SEA, Bottino MC. Incorporating N-acetylcysteine and tricalcium phosphate into epoxy resin-based sealer improved its biocompatibility and adhesiveness to radicular dentine. Dent. Mater. 2019;35(12):1750–1756. [DOI] [PubMed] [Google Scholar]
- 237.Guo X, Sun Y, Wang Z, Ren B, Xu HHK, Peng X, Li M, Wang S, Wang H, Wu Y, et al. The preventive effect of a magnetic nanoparticle-modified root canal sealer on persistent apical periodontitis. Int. J. Mol. Sci. 2022;23(21):13137. [DOI] [PMC free article] [PubMed] [Google Scholar]


