Abstract
Complications associated with Type 1 diabetes (T1D) have complex origins that revolve around chronic hyperglycemia; these complications involve hemostasis disorders, coagulopathies, and vascular damage. Our study aims to develop innovative approaches to minimize these complications and to compare the outcomes of the new approach with those of traditional methods. To achieve our objective, we designed novel nanoparticles comprising covalent organic frameworks (nCOF) loaded with insulin, termed nCOF/Insulin, and compared it to subcutaneous insulin to elucidate the influence of insulin delivery methods on various parameters, including bleeding time, coagulation factors, platelet counts, cortisol plasma levels, lipid profiles, and oxidative stress parameters. Traditional subcutaneous insulin injections exacerbated hemostasis disorder and vascular injuries in streptozotocin (STZ)-induced diabetic rats through increasing plasma triglycerides and lipid peroxidation. Conversely, oral delivery of nCOF/Insulin ameliorated hemostatic disorders and restored the endothelial oxidant/antioxidant balance by reducing lipid peroxidation and enhancing the lipid profile. Our study pioneers the understanding of how STZ-induced diabetes disrupts bleeding time, induces a hypercoagulable state, and causes vascular damage through lipid peroxidation. Additionally, it provides the first evidence for the involvement of subcutaneous insulin treatment in exacerbating vascular and hemostasis disorders in type 1 diabetes (T1D). Introducing an innovative oral insulin delivery via the nCOF approach represents a potential paradigm shift in diabetes management and patient care and promises to improve treatment strategies for type 1 Diabetes.
Keywords: Covalent organic framework nanoparticles (nCOF), Diabetes complications, Hemostasis disorders, Insulin delivery, Type 1 diabetes
Graphical abstract
Highlights
-
•
Subcutaneous injected insulin exacerbates diabetic hemostasis disorders.
-
•
Subcutaneous injected insulin exacerbates diabetes-related vasculopathy.
-
•
Oral insulin improves diabetic hemostasis disorders.
-
•
Oral insulin improves diabetes-related vasculopathy.
-
•
Lipid peroxidation is the leading cause of the onset of diabetic hemostasis disorder.
Abbreviations
- APTT
Activated Partial Thromboplastin Time
- C
Control Rats
- DC
Diabetic Rats
- DFP
2,6-Diformylpyridine
- ELISA
Enzyme-Linked Immunosorbent Assay
- GSH
Glutathione
- HDL-C:
High-Density Lipoprotein Cholesterol
- IACUC
Animal Care and Use Committee
- K2-EDTA
Potassium Ethylenediaminetetraacetic Acid
- LDL-C:
Low-Density Lipoprotein Cholesterol
- MDA
Malondialdehyde
- NBT
Nitroblue Tetrazolium
- nCOF
Covalent Organic Framework Nanoparticles
- NF-κB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- NPs
Nanoparticles
- O2•–
Superoxide
- OG Ins
Oral Gavage of nCOF/Insulin
- Oral nCOF/Insulin
Oral Insulin delivered by Covalent Organic Framework nanoparticles
- PBS Buffer
Phosphate Buffered Saline
- PPP
Platelet-poor Plasma
- PT
Prothrombin Time
- RAGE
Receptor for Advanced Glycation End-products
- SC Ins
Subcutaneous Insulin
- STZ
Streptozotocin
- T1D
Type 1 Diabetes
- TBA
Thiobarbituric Acid
- TBARS
Thiobarbituric Acid Reactive Substances
- TTA
4,4′,4''-(1,3,5-triazine-2,4,6-triyl)trianiline
1. Introduction
Complications associated with type 1 diabetes (T1D), encompassing both microvascular and macrovascular issues, remain the foremost contributors to morbidity and mortality among affected individuals. Patients with T1D often exhibit a proinflammatory and pro-coagulant state, characterized by endothelial injury, heightened platelet adhesion, and reduced plasma fibrinolytic potential [1,2].
Chronic hyperglycemia, while a significant contributor to these complications, represents only part of the multifaceted problem. Other factors, such as alterations in lipid metabolism [3] and the oxidative stress associated with diabetes [4,5], play equally important roles. Specifically, lipid peroxidation can intensify the pro-coagulant state by inhibiting the natural anti-coagulant defences, disrupting anti-thrombotic mechanisms [6], and inducing platelet hyperactivation [7,8]. Additionally, the combination of hyperglycemia and dyslipidemia elevates plasma concentrations of pro-coagulant factors [9] while reducing levels of anticoagulant proteins [10].
This interplay of factors does not act in isolation; rather, they collectively contribute to the complex network of complications associated with T1D. Chronic hyperglycemia further compounds the situation by inducing endothelial damage through two primary mechanisms [11]. First, glucose permeates endothelial cells independently of insulin, triggering multiple signaling pathways, including the generation of reactive oxygen species such as superoxide (O2•). This disrupts the delicate equilibrium between vasodilators and vasoconstrictors within the vasculature. Second, hyperglycemia directly initiates vascular inflammation [12], worsening endothelial dysfunction. Elevated stress hormone levels, such as cortisol, further contribute to the intensification of endothelial dysfunction [11].
From a therapeutic perspective, the impact of traditional subcutaneous insulin injections on hemostasis-related complications in T1D has not been extensively explored. Moreover, while certain studies suggest that intensive insulin therapy may provide cardiovascular benefits in diabetic patients [13] or animals treated with streptozotocin [14], it is essential to consider the existing controversy surrounding this approach due to the associated risk of hypoglycemia [15,16].
Furthermore, the management of diabetic patients extends beyond addressing hyperglycemia; it also involves effectively managing its complications. In this context, the field of nanomedicine offers innovative avenues that can enhance treatment effectiveness [[17], [18], [19]] while concurrently reducing the risk of complications [20,21].
In our previous study, our pharmacokinetic analysis showed that the glycemic response to nCOF/insulin is balanced, so that blood glucose levels can be maintained within a normal range without causing extreme fluctuations. In contrast, with subcutaneous insulin, a hypoglycemic spike was observed within 1 h of injection, followed by a rapid return to a hyperglycemic state characteristic of diabetes. These results suggest that nCOF/insulin provides more stable glycemic control compared to subcutaneous insulin. In addition, our observations showed different insulinemic profiles for the two insulins. In fasting rats, nCOF/insulin led to a gradual increase in blood insulin concentration, whereas subcutaneous insulin led to a hyperinsulinemic peak after injection. This difference in insulin distribution and the organism's response to administration leads to different biological responses. Thus, our previous study suggests that nCOF/insulin may prevent hepatic and renal complications associated with diabetes compared to conventional subcutaneous insulin injection [21].
Building upon these findings, our current research assesses the impact of administering insulin through oral nanoparticle delivery on key parameters such as lipid profile, lipid peroxidation, bleeding time, vascular damage, and coagulation-fibrinolysis processes, all intertwined with diabetes. To achieve this, we conducted a comparative analysis between the outcomes of subcutaneous insulin injections and oral nCOF/Insulin treatments in rats with experimentally induced T1D.
To assess platelet activity, we measured bleeding time and platelet count. Furthermore, we delved into the potential vascular alterations triggered by diabetes by evaluating the equilibrium between prooxidants and antioxidants. This equilibrium was precisely determined through the measurement of O2•– and glutathione (GSH) levels in the abdominal aortas of the experimental rats. Our investigation of blood coagulation encompassed a comprehensive approach. We employed global tests such as activated partial thromboplastin time (APTT) and prothrombin time (PT). Additionally, we performed specialized tests designed to gauge the coagulant activities of specific factors, including II, V, VII, VIII, IX, and X. In order to evaluate the hemostatic profile, we analysed fibrinogen levels to assess acute phase response, providing insights into inflammation. Furthermore, we examined d-dimer levels as an indirect indicator of fibrinolysis and cortisol levels to reflect stress levels. In parallel, we conducted a lipid profile analysis, measuring plasma levels of total cholesterol (TC), High-Density Lipoprotein Cholesterol (HDL-C), and triglycerides (TG), as well as determining Low-Density Lipoprotein Cholesterol (LDL-C). Finally, lipid peroxidation was evaluated by measuring Malondialdehyde (MDA) levels.
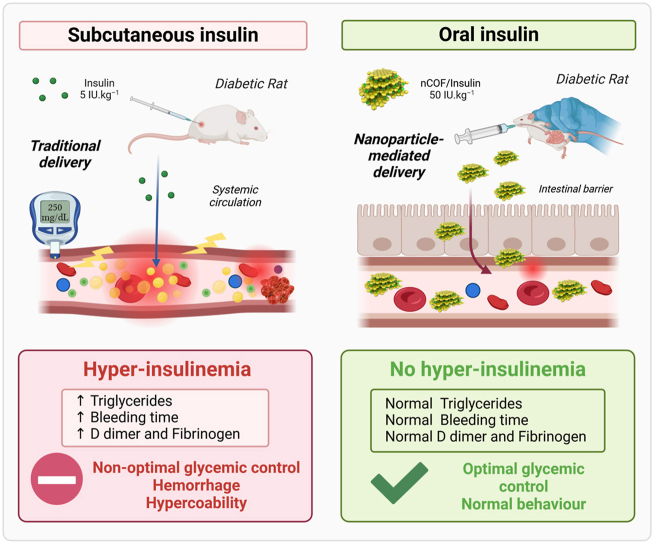
Our findings reveal that subcutaneous insulin injections intensify hemostasis disorders and vascular damage in diabetic rats. This aggravation primarily manifests through elevated plasma triglycerides and increased lipid peroxidation levels. Conversely, the oral administration of nCOF/Insulin enhances the coagulation profile, fibrinolysis, and bleeding time of diabetic rats while also restoring the equilibrium between oxidants and antioxidants by mitigating lipid peroxidation and enhancing the overall lipid profile.
Our study is among the first to clearly demonstrate how STZ-induced diabetes in rats leads to significant disruption of the lipid profile, increased lipid peroxidation, and prolonged bleeding times, culminating in a hypercoagulable state that increases oxidative stress within the endothelium. The introduction of oral insulin delivery via the nCOF approach effectively addresses vascular injury and corrects hemostatic disorders in diabetic rats. It improves bleeding time, coagulation profile, and fibrinolysis. Additionally, it mitigates dyslipidemia, suppresses lipid peroxidation, and prevents superoxide accumulation in the aorta of diabetic rats.
Overall, our research brings significant advances in our understanding of diabetes-related vascular complications and opens new avenues for safer and more effective insulin therapy methods.
2. Materials and methods
2.1. Synthesis of insulin loaded nCOF nanoparticles (nCOF/Insulin)
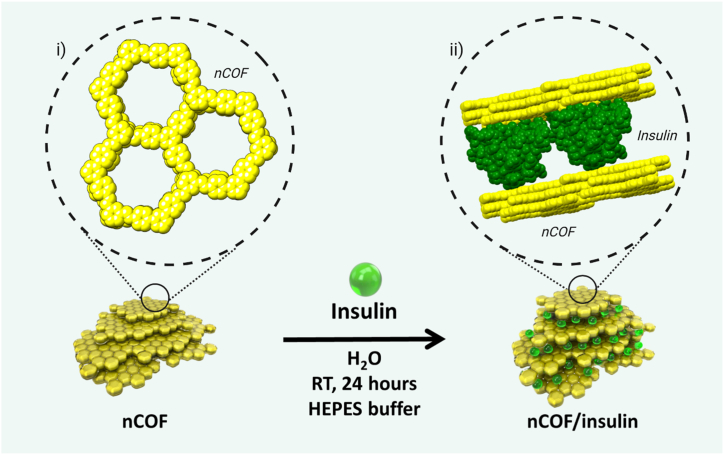
nCOFs nanoparticles were synthesized by co-condensation of 2,6-diformylpyridine (DFP, 21 mg, 0.15 mmol, 5 equivalents) and 4,4′,4''-(1,3,5-triazine-2,4,6-triyl)trianiline (TTA, 12 mg, 0.03 mmol, 1 equivalent), in 3 mL of anhydrous 1,4-dioxane in the presence of 0.5 mL of acetic acid (13 M, [acetic acid]final = 4.0 M) at room temperature for 10 min (Fig. 1). The solution was cleaned using dialysis in H2O to obtain a stable colloidal suspension. Characterization revealed nanoparticles with an average diameter of 123.7 nm, a crystalline structure with distinct PXRD peaks, and a significant BET surface area indicating permanent porosity.
Fig. 1.
Schematic representation of the encapsulation of insulin between the layers of the nCOF. Cartoon representation (green spheres) represents the insulin. Inset i) chemical structure of the nCOF. Inset ii) van der Waals representation of the optimized location of insulin monomer molecule intercalated between nCOF layers.
Insulin was loaded into nCOF by a simple impregnation method. nCOF (5 mg) was suspended in 2 mL HEPES buffer. A HEPES-buffered aqueous insulin solution ([insulin] = 10 mg mL−1, 1 mL) was added (nCOF:Insulin ratio = 1:2). The solution (pH 7.4) was stirred overnight at room temperature, cleaned with water several times by centrifugation, and finally washed with deionized H2O to remove unloaded insulin molecules. The nCOF/Insulin nanoparticles exhibited a high insulin-loading capacity (64.6 ± 1.7 wt%) and there is evidence that the insulin is located between the layers of the nCOF nanosheets (Fig. 1). Stability assessments showed the nanoparticles are resilient under simulated gastrointestinal and bloodstream conditions. Advanced characterization techniques, including FTIR and XPS analyses, confirmed the encapsulation of insulin and the presence of specific chemical interactions between insulin and the nCOF structure. Additionally, computational simulations supported the experimental loading data, suggesting insulin intercalation between the nCOF layers. This comprehensive preparation and characterization makes the nCOF system a promising candidate for oral insulin delivery.
The efficacy and release mechanism of nCOF/insulin FITC nanoparticles were investigated in vitro. This showed minimal insulin release in simulated gastric and intestinal fluids, so that the structure of the insulin was preserved under acidic, stomach-like conditions. Dynamic light scattering and TEM confirmed the stability and unchanged morphology of the nanoparticles in these environments. Remarkably, the system showed glucose-responsive insulin release, especially under hyperglycemic conditions. Nearly 100 % release was achieved, with a pulsatile release pattern observed between normal and hyperglycemic conditions. This glucose-triggered release mechanism is favored by the smaller size of the glucose molecules, which allows them to penetrate the nCOF structure and displace insulin. Studies in different solutions showed that insulin release is specifically triggered by glucose and not by other sugars or amino acids, underlining the potential of the system for targeted diabetes therapy. The maintained structural integrity of the insulin after release confirms the clinical relevance of this nCOF system for diabetes treatment.
In vitro and in vivo studies confirmed the biocompatibility and efficacy of nCOF/insulin as an oral insulin delivery system. In vitro studies on the viability of several cell lines showed no cytotoxic effects, indicating the suitability of the system for oral use. TEM analysis showed that nCOF/insulin did not alter cellular ultrastructure, suggesting safe endocytosis and internal cellular processing. Hemolytic assays also showed that it is not immunotoxic to human erythrocytes, highlighting its safety for entry into the bloodstream. Ex vivo experiments have demonstrated that the nanoparticles can effectively cross the intestinal barrier, which increases the permeability of insulin and represents a promising approach for the treatment of diabetes. Oral administration to diabetic rats (T1D) resulted in a significant reduction in blood glucose levels without any organ damage or changes in kidney and liver functions being observed. This demonstrates the potential of nCOF/insulin to safely maintain glucose homeostasis and replace subcutaneous injections [21].
2.2. Animals
Male and female Wistar rats (12 weeks, 200 g ± 20) from Pasteur Institute were used for this study. Rats were housed individually in wood-chip bedded plastic cages at constant temperature (25 °C), maintained on a 12:12 h light/dark cycle, and fed with a standard pellet diet with water ad libitum. The study was conducted following the policies of the University of Tlemcen Institutional Animal Care and Use Committee (IACUC) (accreditation number: D01N01UN130120150006)) and complies with the ARRIVE guidelines.
2.3. T1D induction
T1D was induced via a single intraperitoneal injection of streptozotocin (STZ, dissolved in 10 mM citrate buffer at pH 4.5) at an STZ dose of 45 mg kg−1 of body weight. Rats were returned to their cages and given food and water till the onset of diabetes. Blood glucose levels were monitored using a blood glucose monitoring system (AccuChek Performa, Hoffman-La Roche) by taking samples from a rat tail vein. Rats showing fasting blood glucose levels ≥250 mg/dL (13.7 mmol.L−1) were considered diabetic and selected for the study (N = 20).
2.4. Study design
Rats were randomly divided into four groups (n = 5) as shown in Table 1: standard control (C), diabetic control (DC), diabetic treated with subcutaneous insulin (SC Ins, 5 IU.kg−1), and diabetic treated with oral gavage of nCOF/Insulin (OG Ins, 50 IU.kg−1). In the treated groups, the diabetic rats received a single dose of either SC Ins or OG Ins on day 1, as described in the experimental protocol summarized in Fig. 2. Our decision to use different doses for subcutaneous (5 IU/kg) and oral (50 IU/kg) insulin delivery is based on the difference in bioavailability. Subcutaneous insulin, which has nearly 100 % bioavailability due to bypassing the digestive system and first-pass metabolism, requires lower doses to be effective. Oral insulin faces degradation and significant first-pass metabolism, resulting in lower bioavailability. Therefore, a higher dose is used to compensate for this and ensure sufficient systemic levels.
Table 1.
Rat groups assigned randomly: insulin administration methods and injected concentrations. Wistar rats were randomly divided into 4 groups: normal control (C), diabetic control (DC), diabetic treated with subcutaneous insulin (SC Ins, 5 IU.kg−1), and diabetic treated with Oral nCOF/Insulin (OG Ins, 50 IU.kg−1).
| Group | Insulin Administration Method | Dose | Comments |
|---|---|---|---|
| C (Control) | N/A (Not Applicable) | N/A | Non-diabetic rats serving as a positive control group. |
| DC (Diabetic Control) | N/A (Not Applicable) | N/A | Untreated diabetic rats serving as a negative control group. |
| SC Ins (Subcutaneous insulin) | SC (Subcutaneous injection) | 5 IU.kg−1 | Diabetic rats treated with insulin injections under their skin. |
| OG Ins (Oral nCOF/Insulin) | OG (Oral gavage) | 50 IU.kg−1 | Diabetic rats treated with insulin delivered orally using nCOF nanoparticles. |
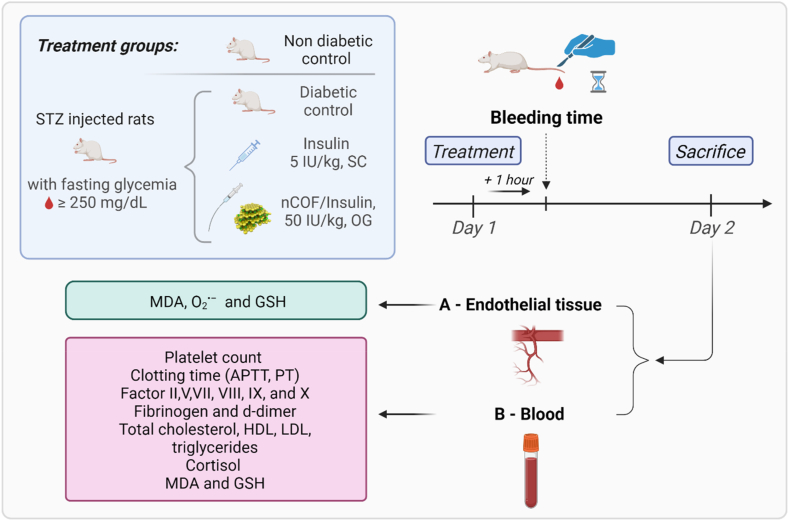
Fig. 2.
Schematic representation of T1D induction and subsequent study protocol in rats. This diagram illustrates the sequential methodology employed in our study. Starting with the induction of T1D using streptozotocin (STZ), we monitored and selected diabetic rats based on specific blood glucose levels (≥250 mg/dL). The study design categorizes rats into four distinct groups, each with varied insulin administration methods, as detailed in Table 1. We then measured bleeding times 1-h post-insulin treatment, employing a precise protocol based on prior research findings. Following overnight fasting and subsequent sacrifice of the rats, various samples were collected. Using these samples, an array of tests was conducted including coagulation profiles, endothelial lysate preparations, thrombocytes counting, cortisol levels, lipid profiling, and markers of oxidative stress. Statistical analyses were performed using SPSS.
2.5. Bleeding time (BT) measurement
Following the administration of the insulin doses, a bleeding time measurements were performed as described by Ayodele et al. [22]. A cut was made on each rat's tail at 1–2 cm proximal from the end, and the stopwatch was started immediately upon the onset of bleeding. At intervals of 15 s, blood spots were made with the bleeding tail on a blotting paper until the bleeding stopped. Bleeding time was recorded as the time taken for bleeding to stop.
Bleeding time measurements were conducted 1 h after insulin administration on day 1. We specifically chose this time point based on our previous findings [21], which showed that 1 h corresponds with the highest insulin levels in the bloodstream following subcutaneous insulin injection, while oral insulin administration effectively regulates blood glucose levels.
2.6. Sample collection
After an overnight fast, the animals were sacrificed on day 2 in deep isoflurane anaesthesia (approximately 2.5 % v/v). Blood samples were collected via cardiac puncture. From each rat, two tubes of blood were collected and treated as follows.
-
•
tube 1 which contained 3.2 % sodium citrate in a ratio of 1:9 anticoagulant: blood
-
•
tube 2 which had K2-EDTA as an anticoagulant.
The abdominal aorta was also removed and cleaned for further analysis (endothelial lysate). All tests were performed immediately following sacrifice.
2.7. Sample preparation
2.7.1. Preparation of platelet-poor plasma samples
Platelet-poor plasma (PPP) was obtained from tube 1 containing 3.2 % sodium citrate by blood centrifugation (3000 rpm, 20 min, room temperature). PPP was used for coagulation tests by performing the following analysis: quick time; activated partial thromboplastin time (APTT); concentration of fibrinogen; concentration of d-dimers and clotting factors (factor II, factor V, factor VII, factor VIII, factor IX, factor X).
2.7.2. K2-EDTA blood samples
Blood from tube 2 containing K2-EDTA as an anticoagulant was used for counting platelets from whole blood. Blood samples were centrifuged (1500 rpm, 15 min, room temperature) to obtain plasma. From the obtained plasma was measured: levels of total cholesterol, triglycerides, high-density lipoproteins (HDL), thiobarbituric acid reactive substances (TBARS), and glutathione (GSH).
2.7.3. Endothelial lysate
Abdominal aorta samples were collected from a descending thoracic fragment. After gentle rinsing and removal of surrounding tissue, the samples were homogenized in pH 7.4 PBS buffer using an ultra-Turrax homogenizer (Bioblock Scientific, Illkirch, France) for three cycles of 10 s each, and subsequently sonicated for 10 s using an ultrasonic homogenizer (SONICS, USA) and then centrifuged (3000 rpm, 5 min). The supernatant was used to estimate GSH and reactive oxygen species superoxide (O2•–).
2.8. Coagulation tests
Quantitative determination of fibrinogen levels in plasma was conducted using the clotting method developed by Clauss [23]. An immunoturbidimetric assay determined d-dimer plasma levels. APTT and Prothrombin Time (PT) were assayed using Stago reagents [24]. Coagulant activities of factors II, V, VII, VIII, IX–X were determined in one-stage clotting assays with factor–deficient plasmas (Stago reagents). All measurements were made on an STA coagulometer Stago (STA Compact Max2).
2.9. Thrombocytes counting
Thrombocytes count from complete blood was performed on an automated hematology analyzer SIEMENS (ADVIA 2120i, France) using well-mixed whole blood with K2-EDTA to prevent blood clotting.
2.10. Cortisol
Cortisol plasma levels were quantified using an enzyme-linked immunosorbent assay (ELISA) kit from Sigma-Aldrich.
2.11. Lipid profile
Plasmatic total cholesterol (TC), High-Density Lipoprotein Cholesterol (HDL-C), and triglyceride (TG) levels were determined using SPINREACT Kit. Low-Density Lipoprotein Cholesterol (LDL-C) plasma level was determined based on Friedewald et al.’s formula [25]:
| LDL – C = TC − HDL − C − TG/5 |
2.12. Thiobarbituric acid reactive substances (TBARS)
Plasma and endothelial thiobarbituric acid reactive substances (TBARS) were estimated according to literature [26,27] and expressed as malondialdehyde (MDA). TBARS were quantitated by their reactivity with thiobarbituric acid (TBA) in acidic conditions to generate a pink-colored chromophore, monitored at 532 nm.
2.13. Glutathione (GSH)
Estimation of GSH in plasma and endothelial tissue was performed according to Ellman [28]. This method is based on developing a yellow color, monitored at 412 nm when Ellman's reagent (dithionite benzoic acid) is added to compounds containing sulfhydryl groups.
2.14. Reactive oxygen species superoxide (O2•–)
The production of O2•– endothelial tissue was measured at pH 7 and 25 °C in the presence of nitroblue tetrazolium (NBT). The superoxide anion reduces NBT produced by the tissue into blue formazan [29,30], a chromophore that absorbs at 550 nm.
2.15. Statistics
All data were analysed using SPSS (IBM, SPSS Statistics, version 23, USA) and expressed as means ± standard error of the mean (SEM). Data were analysed using one-way ANOVA with post hoc Tukey's tests for multiple comparisons. Pearson correlation coefficient analysis was performed between triglycerides or MDA in plasma levels and the different biomarkers. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Hemostasis analysis
Hemostasis analysis was performed on the four different groups considered in this study (Table 1): control rats (C), diabetic rats (DC), diabetic rats treated with subcutaneous insulin (SC Ins), and diabetic rats treated with oral insulin loaded in nCOFs (OG Ins). Table 2 shows no statistically significant difference between the four groups for Quick time, APTT, factor V, and factor VII plasma levels. However, statistically significant differences were found between the four groups in factors II (p < 0.001), VIII (p < 0.001), IX (p < 0.01), X (p < 0.001), fibrinogen and d-dimers (p < 0.001).
Table 2.
Hemostasis analysis in control rats (C), diabetic rats (DC), diabetic rats treated with subcutaneous insulin (SC Ins), and diabetic rats treated with oral insulin loaded in nCOFs (OG Ins).
| Tests | C | DC | SC Ins | OG Ins |
|---|---|---|---|---|
| Quick time (s) | 15.1 ± 0.5 | 15.2 ± 0.7 | 15.3 ± 0.9 | 14.3 ± 0.6 |
| APTT (s) | 25.3 ± 1.0 | 24.2 ± 1.2 | 23.9 ± 3.1 | 23.9 ± 2.1 |
| Factor II (%) | 97.7 ± 1.1 | 85.6 ± 4.0 | 80.4a* ± 12.7 | 109.9b† ± 11.9 |
| Factor V (%) | 99.0 ± 0.7 | 98.3 ± 2.4 | 99.6 ± 1.1 | 100.4 ± 1.5 |
| Factor VII (%) | 80.0 ± 1.2 | 79.7 ± 1.8 | 79.6 ± 1.1 | 79.8 ± 1.3 |
| Factor VIII (%) | 118.7 ± 32.0 | 294.3a† ± 42.6 | 330.0a† ± 0.0 | 211.5a,b* ± 68.8 |
| Factor IX (%) | 116.4 ± 1.3 | 110.4 ± 12.3 | 203.8a,b* ± 18.8 | 146.7 ± 74.8 |
| Factor X (%) | 86.2 ± 2.4 | 94.2 ± 21.2 | 104.7 ± 3.7 | 150.7a,b† ± 5.5 |
| Fibrinogen (mg/d) | 249.1 ± 28.5 | 369.4a† ± 32.2 | 555.8a,b† ± 47.7 | 289.0b* ± 24.8 |
| d-dimer (μg/ml) | 0.33 ± 0.02 | 0.42a* ± 0.01 | 0.43a* ± 0.08 | 0.32b* ± 0.01 |
Results are expressed as mean ± SEM
(*p < 0.05); (†p < 0.001).
versus C,.
versus DC..
Specifically, we found that factor II plasma levels were higher in OG Ins and lower in SC Ins rats compared to C (p < 0.001 and p < 0.05 respectively). Factor VIII levels were higher in the three diabetic groups compared to the control group (C). Furthermore, diabetic rats treated with subcutaneous insulin (SC Ins) exhibited even higher factor VIII levels compared to untreated diabetic rats (DC), while diabetic rats treated with oral insulin (OG Ins) had lower levels (p < 0.05). Factor IX plasma levels were particularly elevated in diabetic SC Ins (p < 0.05) and those of factor X were elevated in OG Ins (p < 0.0001). Fibrinogen and d-dimer plasma levels were higher in both DC and SC Ins groups when compared to the control group (C), with no statistically significant difference observed in OG Ins group. Additionally, SC Ins exhibited even higher levels of fibrinogen than untreated diabetics rats (DC).
3.2. Lipid profile analysis
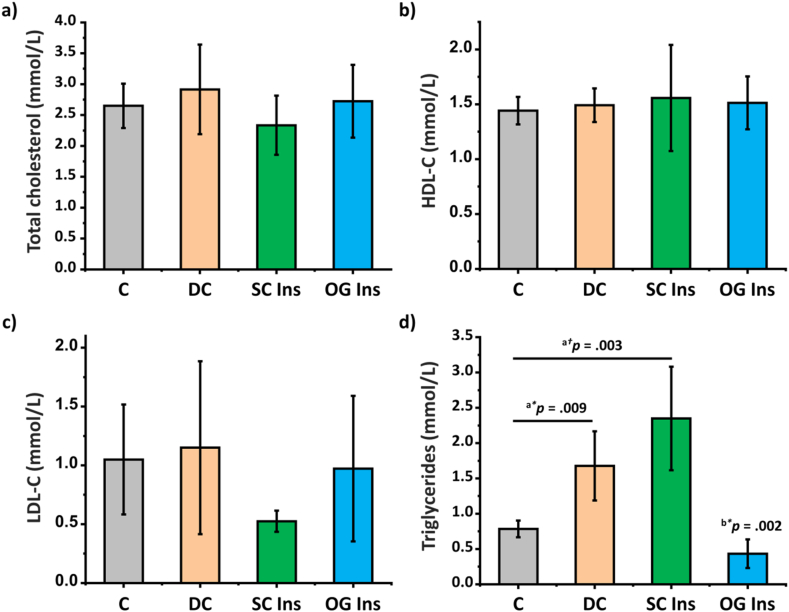
There were no statistically significant differences observed among the four groups in terms of total cholesterol, LDL-C, and HDL-C plasma levels (Fig. 3 a-c). However, a statistically significant difference was evident in the plasma triglyceride levels between groups (Fig. 3d). Specifically, plasma triglyceride levels increased in DC and SC Ins compared to C. In contrast, the oral insulin-treated group (OG Ins) exhibited a significant reduction in plasma triglyceride levels when compared to both the DC and SC Ins groups (p < 0.05).
Fig. 3.
Effect of diabetes and insulin treatments on plasma biochemical parameters. a) Total cholesterol, b) High-Density Lipoprotein Cholesterol (HDL-C), c) Low-Density Lipoprotein Cholesterol (LDL-C) plasma and d) triglycerides levels of control rats (grey, C), diabetic rats (orange, DC), diabetic rats treated with subcutaneous-insulin (green, SC Ins) and diabetic rats treated with oral insulin loaded in nCOFs (blue, OG Ins). Results are expressed as mean ± SEM, aversus C, bversus DC (*p < 0.05); (†p < 0.001).
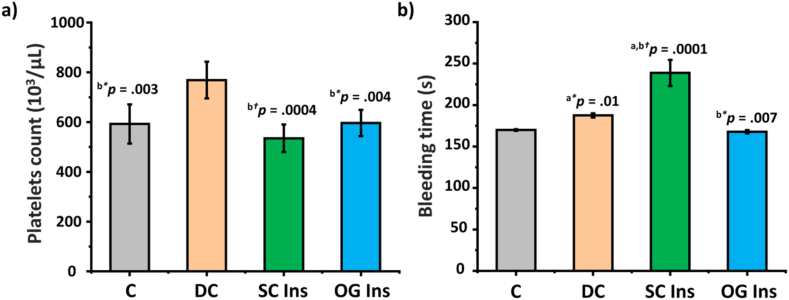
3.3. Platelets activity
As shown in Fig. 4a, DC platelet counts exhibited a significant increase when compared to C, SC Ins, and OG Ins. Additionally, the bleeding time in the DC group was longer in comparison to the C and the OG Ins groups. Notably, the bleeding time was significantly longer in SC Ins when compared to all other groups (p < 0.05) (Fig. 4b).
Fig. 4.
Effect of diabetes and insulin treatments on platelets activity. a) Platelets count and b) bleeding time of control rats (grey, C), diabetic rats (orange, DC), diabetic rats treated with subcutaneous insulin (green, SC Ins), and diabetic rats treated with oral insulin loaded in nCOFs (blue, OG Ins). Results are expressed as mean ± SEM, aversus C, bversus DC (*p < 0.05); (†p < 0.001).
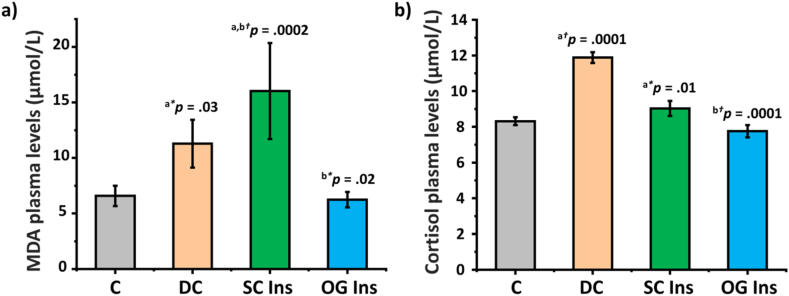
3.4. MDA and cortisol plasma levels
MDA plasma levels exhibited a notable increase in DC and SC Ins (p < 0.05) compared to C and OG Ins (Fig. 5a). Additionally, when comparing SC Ins to DC, a further elevation in MDA plasma levels was observed. As shown in Fig. 5b, cortisol plasma levels increased in DC and SC Ins compared to C. However, no statistically significant difference was observed when comparing the OG Ins group to the DC group.
Fig. 5.
Effect of diabetes and insulin treatments on plasma lipid peroxidation and plasma cortisol levels. a) MDA and b) cortisol plasma levels of control rats (grey, C), diabetic rats (orange, DC), diabetic rats treated with subcutaneous insulin (green, SC Ins), and diabetic rats treated with oral insulin (blue, OG Ins). Results are expressed as mean ± SEM, aversus C, bversus DC (*p < 0.05); (†p < 0.001).
3.5. Pro-oxidant/antioxidant profile analysis in the abdominal aorta
Table 3 reveals significant increases in O2•– content within the abdominal aorta of the DC, OG Ins, and SC Ins groups when compared to the control group. Notably, the increase in O2•– content is more pronounced in the SC Ins group compared to both the C and the OG Ins groups. Conversely, GSH content in the abdominal aorta displayed a reduction in all three diabetic groups when compared to C.
Table 3.
Pro-oxidant/antioxidant profile analysis in the abdominal aorta of control rats (C), diabetic rats (DC), diabetic rats with subcutaneous insulin (SC Ins), and diabetic rats with oral insulin (OG Ins).
| Tests | C | DC | SC Ins | OG Ins |
|---|---|---|---|---|
| O2•– (μmol/g) | 33.0 ± 7.7 | 48.1a* ± 1.3 | 62.9a† ± 3.2 | 47.8a* ± 10.2 |
| GSH (mmol/g) | 2.8 ± 1.0 | 1.0a† ± 0.1 | 1.4a* ± 0.2 | 1.3a* ± 0.2 |
Results are expressed as mean ± SEM
(*p < 0.05); (†p < 0.001).
versus C.
3.6. Correlation analysis
Table 4 presents a comprehensive correlation analysis revealing associations between elevated plasma triglyceride levels and various biomarkers. Notably, high plasma triglyceride levels are positively correlated with increased plasma MDA, fibrinogen, d-dimers, factor VIII, and factor IX levels. Furthermore, elevated plasma triglycerides are associated with longer bleeding times, prolonged clotting times, and higher O2•– content levels in the abdominal aorta. Interestingly, an inverse correlation was observed between plasma factor II concentration and plasma triglyceride levels.
Table 4.
Correlation (r) between triglycerides plasma levels and various biomarkers: plasma MDA, fibrinogen, d-dimers levels, bleeding time, quick time, hemostasis biomarkers, and O2•– in the abdominal aorta.
| Test | r | P |
|---|---|---|
| Plasma MDA | 0.88 | <0.0001 |
| Fibrinogen | 0.80 | <0.0001 |
| d-dimer | 0.79 | <0.0001 |
| Bleeding time | 0.72 | <0.0001 |
| Quick time | 0.61 | <0.01 |
| APTT | −0.21 | N.S. |
| Factor II | −0.81 | <0.0001 |
| Factor V | −0.57 | N.S. |
| Factor VII | −0.15 | N.S. |
| Factor VIII | 0.64 | <0.01 |
| Factor IX | 0.48 | <0.05 |
| Factor X | −0.34 | N.S. |
| O2•– in the abdominal aorta | 0.54 | <0.05 |
N.S., not statistically significant.
Table 5 continues this analysis, demonstrating significant correlations between MDA plasma levels and multiple biomarkers. These include triglyceride levels, fibrinogen plasma levels, bleeding times, and various other factors, such as VIII, IX, d-dimers, and O2•– content in the abdominal aorta. Similarly, an inverse relationship between plasma factor II and MDA plasma levels was noted, mirroring the trend observed with triglyceride in Table 4.
Table 5.
Correlation (r) between MDA plasma levels and various biomarkers: triglycerides, fibrinogen, d-dimers levels, bleeding time, quick time, hemostasis biomarkers, and O2•– in the abdominal aorta.
| Test | r | P |
|---|---|---|
| Triglycerides | 0.88 | <0.0001 |
| Fibrinogen | 0.86 | <0.0001 |
| d-dimer | 0.59 | <0.01 |
| Bleeding time | 0.78 | <0.0001 |
| Quick time | 0.38 | N.S. |
| APTT | −0.40 | N.S. |
| Factor II | −0.81 | <0.0001 |
| Factor V | −0.06 | N.S. |
| Factor VII | −0.19 | N.S. |
| Factor VIII | 0.70 | <0.001 |
| Factor IX | 0.54 | <0.05 |
| Factor X | −0.22 | N.S. |
| O2•– in the abdominal aorta | 0.62 | <0.01 |
N.S., not statistically significant.
4. Discussion
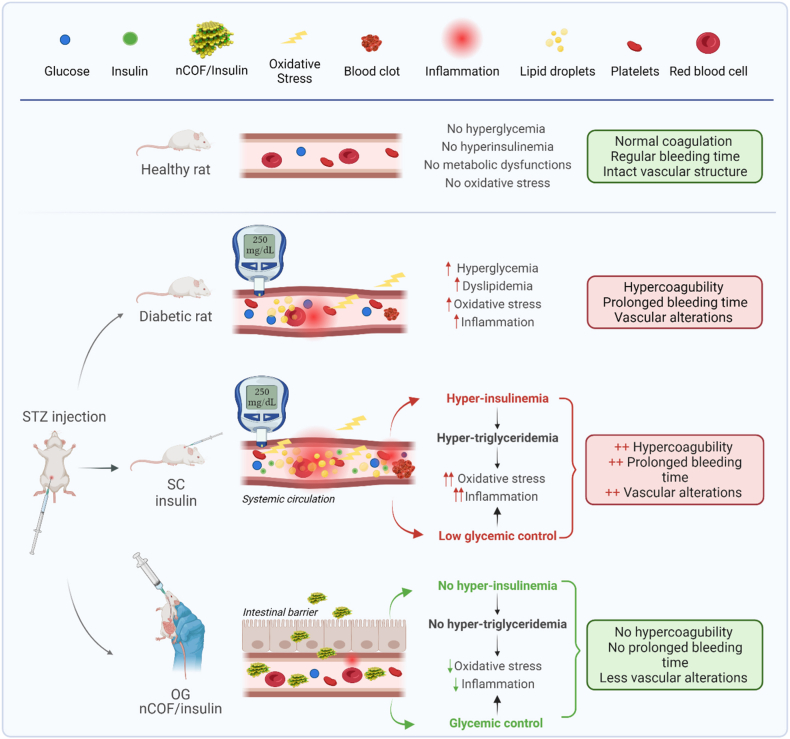
This study delved into the impact of insulin delivery routes, specifically oral and subcutaneous, on various parameters in a T1D rat model, including hemostasis disorders, bleeding time, platelet count, lipid profile, and oxidant/antioxidant balance. Notably, we are the first to demonstrate that subcutaneous insulin injections exacerbate hemostasis disorders and disrupt the oxidant/antioxidant balance in the aortas of diabetic rats. This disturbance primarily results from the deterioration of their lipid profile and the increased levels of lipid peroxidation. In contrast, oral insulin administration improved the coagulation and fibrinolysis as well as the oxidant/antioxidant balance in the abdominal aorta of diabetic rats. This improvement can be attributed to the reduction in lipid peroxidation and the enhancement of their lipid profile. A comprehensive summary of these key findings is presented in Fig. 6.
Fig. 6.
Overview of vascular changes and treatment effects in normal and STZ-induced diabetic rats. This figure summarizes the key findings of our study: STZ-induced diabetes disrupts the lipid profile, heightening lipid peroxidation and extending bleeding times, leading to a hypercoagulable state and amplified endothelial oxidative stress. Notably, while subcutaneous insulin aggravates these disturbances, oral insulin delivery via nCOF nanoparticles presents a potential solution. This approach mitigates vascular injuries, restores hemostatic balance, averts dyslipidemia, diminishes lipid peroxidation, shields against superoxide buildup in the aorta, and ameliorates the coagulation profile.
To explore platelet function and primary hemostasis in diabetic rats, we conducted bleeding time and platelet count tests [31]. Our findings revealed that diabetes is associated with an extended bleeding time, consistent with previous research suggesting that diabetes alters the kinetics of clot formation [11]. Furthermore, we observed an increased platelet count, indicating that the prolonged bleeding cannot be attributed to a deficiency in platelets. Additional assessments included APTT and PT measurements, indicative of factor deficiencies related to bleeding [32]. However, no significant alterations were noted in these parameters. This shows that the prolonged bleeding time associated with diabetes results from functional alterations in platelet activity rather than quantitative changes, aligning with prior research highlighting discernible platelet changes in diabetes [11,33].
The evaluation of O2•– and GSH levels revealed an imbalance in favor of pro-oxidants within the abdominal aortas of diabetic rats. This imbalance is likely attributed to changes in the lipid profile and increased lipid peroxidation induced by STZ. Streptozotocin-induced diabetes is known to disrupt the lipid profile, elevate lipid peroxidation, and trigger chronic inflammation. These factors, in conjunction with chronic hyperglycemia, collectively contribute to multifactorial vasculopathy [34].
Untreated diabetic rats exhibited a hypercoagulable state, as evidenced by elevated levels of factor VIII and fibrinogen, both considered prothrombotic agents. Additionally, they displayed increased d-dimers levels, a marker associated with reduced fibrinolysis [35]. Furthermore, untreated diabetic rats exhibited elevated cortisol plasma levels, indicating an inflammatory state [11,36,37].
Our results showed that diabetic rats treated with subcutaneous insulin exhibit an extended bleeding time compared to untreated diabetic rats. This observation suggests that insulin injection amplifies platelet alterations induced by diabetes. In a previous study conducted by our team [21], we demonstrated that subcutaneous insulin injections in diabetic rats induce hyper-insulinemia. It is widely accepted that hyper-insulinemia, as with hyperglycemia, can lead to alterations in platelet function [38]. The prolonged bleeding time observed in rats treated with subcutaneous insulin, compared to untreated diabetic rats, is likely due to a combination of factors: the hyperglycemia caused by diabetes and the hyper-insulinemia induced by subcutaneous insulin injection. Furthermore, our research demonstrated that subcutaneous insulin injections exacerbate the levels of plasma factor VIII, d-dimers, and fibrinogen. Additionally, they lead to an increase in factor IX, indicating an aggravation of the overall hemostatic state.
Interestingly, we observed significant correlations between bleeding times, factor IX, factor VIII, fibrinogen, and d-dimer levels with both triglycerides and MDA plasma concentrations. These findings provide strong evidence supporting the notion that subcutaneous insulin injections, which induce peripheral hyperinsulinemia and subsequent hypertriglyceridemia [39], play a critical role in the hemostasis disorder witnessed in T1D patients.
In the diabetic groups, the assessment of intracellular O2•– and GSH concentrations reveals an imbalance in the intracellular antioxidant/pro-oxidant profile, favoring pro-oxidants. Notably, this imbalance is significantly more pronounced in diabetic rats treated with subcutaneous insulin. Previous studies have demonstrated that platelets activated by STZ disrupt vasodilation through the augmentation of oxidative stress, particularly endothelial superoxide production [40]. One plausible explanation for the exacerbated endothelial injuries mediated by oxidative stress in the subcutaneously-treated group is that insulin injections amplify the platelet alterations induced by STZ. This, in turn, intensifies endothelial disturbances by elevating intracellular O2•– concentrations.
Numerous experimental studies have explored the impact of induced hyperglycemia and/or induced hyperinsulinemia on coagulation in healthy human subjects [38,41,42]. These studies have shown several noteworthy findings. Firstly, they have showed that the pro-coagulant effects induced by hyperglycemia remain unaltered even with insulin administration, irrespective of insulin dosage. Additionally, these investigations have highlighted the influence of hyperglycemia and hyperinsulinemia on hemostatic equilibrium [41] and platelet functionality [38]. Furthermore, it has been observed that the impact of hyperglycemia and hyperinsulinemia on the hemostatic response is aggravated during systemic inflammation [42]. These collective outcomes suggest that hyperglycemia, hyperinsulinemia, and inflammation may synergistically contribute to disruptions in hemostasis. However, it is imperative to interpret with caution considering two crucial considerations. Firstly, the studies were conducted on healthy volunteers, which limits their ability to account for other metabolic disorders frequently associated with diabetes, such as dyslipidemia and oxidative stress [43]. Secondly, they inadequately replicate the characteristic episodic peripheral hyperinsulinemia observed in T1D, often accompanied by episodes of hypoglycemia and subsequent hyperglycemia. The robustness of our study lies in its ability to faithfully replicate the authentic conditions of diabetes.
Our investigation unveiled that subcutaneous insulin administration induces hypertriglyceridemia, subsequently increasing lipid peroxidation. This cascade of events triggers an inflammatory response, as evidenced by elevated fibrinogen levels [44]. Importantly, it aggravates the preexisting hemostasis disorder caused by diabetes in STZ-induced diabetic rats. This exacerbation manifests as an extension in bleeding time, indicative of platelet dysfunction, an amplification in coagulation, characterized by increased factor IX levels, which is further potentiated by diabetes-induced factor VIII elevation and heightened fibrinogen levels. Additionally, this subcutaneous insulin-induced milieu stimulates hypo-fibrinolysis, a phenomenon unveiled by elevated d-dimers plasma levels. Simultaneously, it accentuates endothelial injuries mediated by oxidative stress.
Conversely, our findings took a marked turn when insulin was administered orally, employing nanoparticles (NPs) as carriers. Oral insulin delivery not only ameliorated lipid metabolism but also recalibrated plasma lipid peroxidation levels, bringing bleeding time back to normal values. Furthermore, rats treated with oral insulin exhibited clotting times within the normal range, along with regular d-dimer levels and balanced factors II, V, VII, and IX plasma concentrations. Notably, factor VIII plasma levels were reduced compared to untreated diabetic rats and those treated with subcutaneous insulin, signifying a decrease in inflammation. The cortisol levels, fibrinogen plasma levels, and intracellular superoxide concentrations in rats treated orally with insulin mirrored those of the control group. It is worth noting that although factor X concentrations were elevated, they did not exert a significant impact on the coagulation process.
Insulin itself is not the primary cause of hemostasis disorders; instead, it is the resultant hyperinsulinemia that plays a pivotal role. Notably, oral insulin administration, known for its capacity to establish glycemic homeostasis [21], serves as a prophylactic measure against these complications. Administering the same insulin in two different ways - either by direct subcutaneous injection or orally after encapsulation - results in a different insulinemic profile. This difference significantly affects blood glucose levels, as shown in our previous study [21], and lipid metabolism, as shown in the current study. These differences lead to completely different effects on diabetes-induced hemostasis disorders. This finding aligns with prior research, suggesting the protective potential of intensive insulin therapy. This therapeutic approach, characterized by multiple dose-adjusted injections based on blood glucose levels and stringent glycemic control, has demonstrated the ability to minimize vascular events by reducing oxidative stress and inflammation [13]. Further substantiating this concept, Bratseth et al. also observed that children and adolescents undergoing intensive insulin therapy exhibited pro-coagulant activities similar to those of healthy individuals [45]. Importantly, both studies revealed improvements in lipid profiles as a result of intensive therapy. In rodent experimentations, it has been shown that even a low dose of insulin can enhance triglyceride metabolism, glucose metabolism, and oxidative stress. This effect is accompanied by alterations in the expression of key transcription factors, including NF-κB and RAGE, which are implicated in arterial damage [46]. These collective findings emphasize the multifaceted impact of insulin, with hyperinsulinemia emerging as a critical factor influencing hemostasis and overall vascular health.
5. Conclusion
STZ-induced diabetes in rats disrupts lipid profiles: Our study demonstrates that STZ-induced diabetes in rats disrupts the lipid profile, and oxidative stress within the endothelium. This disruption results in a hypercoagulable state, hypofibrinolysis and prolonged bleeding times.
Detrimental effects of subcutaneous insulin therapy: Our research has shed significant light on the potential detrimental role of subcutaneous insulin therapy in exacerbating these diabetes-related abnormalities. This study highlights, the adverse impact of subcutaneous insulin treatment on amplifying vascular alterations and hemostasis disorder observed in diabetes.
Promising results of oral insulin delivery via nanoparticle carriers: On the other hand, oral delivery of insulin through nanoparticles carriers, in particular the nCOF approach used in our study, represents a promising therapeutic alternative. It effectively counters vascular injuries, corrects hemostatic disorders, alleviates dyslipidemia, suppresses plasma lipid peroxidation, protects against superoxide accumulation in the aorta, and improves the coagulation profile of diabetic rats.
Potential for significant societal and healthcare impact: Our results suggest that the introduction of alternative insulin delivery methods, such as the oral nCOF approach, could lead to a paradigm shift in diabetes care, with far-reaching implications for patient care and research directions.
Foundation for future research and improved patient care: This study not only opens new avenues for insulin-related research but also provides a basis for developing tailored treatment strategies and disease management plans for individuals with T1D, with the goal of improving patient outcomes and quality of life.
Limitations: It is important to note that the sample size of n = 5 per group and the short interval between treatment and subsequent analysis (treatment on day 1 and sacrifice on day 2) were determined based on standard practices for preliminary animal studies and the ethical mandate to minimize harm to the animal. While these choices were supported by established methods for initial investigations [[47], [48]], they may limit the generalizability of our results and highlight the need for further research with larger sample sizes and longer durations to fully understand the long-term effects and efficacy of different insulin administration methods.
Ethical statement
All procedures involving animals were conducted in accordance with ethical principles and regulations. The study was approved by the University of Tlemcen Institutional Animal Care and Use Committee (IACUC) (accreditation number: D01N01UN130120150006) and complies with the ARRIVE guidelines.
The data associated with this study are included in the article.
CRediT authorship contribution statement
Nawel Kaddour: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Farah Benyettou: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Kawtar Moulai: Methodology, Investigation, Formal analysis, Data curation. Abdelouahab Mebarki: Validation, Methodology, Formal analysis, Data curation. Katia Allal-Taouli: Writing – review & editing, Investigation, Formal analysis, Data curation. Rose Ghemrawi: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. Jamie Whelan: Writing – review & editing, Investigation, Formal analysis. Hafida Merzouk: Writing – review & editing, Validation, Methodology, Formal analysis, Data curation. Ali Trabolsi: Writing – review & editing, Writing – original draft, Visualization, Validation, Resources, Investigation, Funding acquisition, Formal analysis, Conceptualization. Nassima Amel Mokhtari-Soulimane: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Ali Trabolsi reports financial support was provided by New York University Abu Dhabi. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
N.M.S., H.M. and N.K. thank the University of Tlemcen, the laboratory PPaBioNut and the Centre Hospital University for their support. A.T., F.B. and J.W. thank New York University Abu Dhabi (NYUAD, UAE) for its generous support of this research. This research was partially carried out using the Core Technology Platforms resources at New York University Abu Dhabi. A.T. and F.B. would like to acknowledge ASPIRE (AARE20-116) for their generous support.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Nawel Kaddour, Email: nawel.kad@yahoo.com.
Ali Trabolsi, Email: ali.trabolsi@nyu.edu.
Nassima Amel Mokhtari-Soulimane, Email: nassima_amel@yahoo.fr, nassima.soulimane@univ-tlemcen.dz.
References
- 1.Targher G., Chonchol M., Zoppini G., Franchini M. Hemostatic disorders in type 1 diabetes mellitus. Semin. Thromb. Hemost. 2011;37(1):58–65. doi: 10.1055/s-0030-1270072. [DOI] [PubMed] [Google Scholar]
- 2.El Khawand C., Jamart J., Donckier J., Chatelain B., Lavenne E., Moriau M., et al. Hemostasis variables in type I diabetic patients without demonstrable vascular complications. Diabetes Care. 1993;16(8):1137–1145. doi: 10.1055/s-0030-1270072. [DOI] [PubMed] [Google Scholar]
- 3.Soulimane-Mokhtari N.A., Guermouche B., Saker M., Merzouk S., Merzouk H., Hichami A., et al. Serum lipoprotein composition, lecithin cholesterol acyltransferase and tissue lipase activities in pregnant diabetic rats and their offspring receiving enriched n-3 PUFA diet. Gen. Physiol. Biophys. 2008;27(1):3–11. [PubMed] [Google Scholar]
- 4.Merzouk S.A., Saker M., Reguig K.B., Soulimane N., Merzouk H., Guermouche B., et al. N-3 polyunsaturated fatty acids modulate in-vitro T cell function in type I diabetic patients. Lipids. 2008;43(6):485–497. doi: 10.1007/s11745-008-3176-3. [DOI] [PubMed] [Google Scholar]
- 5.Schofield J., Ho J., Soran H. Cardiovascular risk in type 1 diabetes mellitus. Diabetes Ther. 2019;10(3):773–789. doi: 10.1007/s13300-019-0612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii H., Tezuka T., Ishikawa H., Takada K., Oida K., Horie S. Oxidized phospholipids in oxidized low-density lipoprotein down-regulate thrombomodulin transcription in vascular endothelial cells through a decrease in the binding of RARβ -RXRα heterodimers and Sp1 and Sp3 to their binding sequences in the TM promote. Blood. 2003;101(12):4765–4774. doi: 10.1182/blood-2002-08-2428. [DOI] [PubMed] [Google Scholar]
- 7.Pratico D., Cyrus T., Li H., Fitzgerald G.A. Endogenous biosynthesis of thromboxane and prostacyclin in 2 distinct murine models of atherosclerosis. Blood. 2000;96(12):3823–3826. doi: 10.1182/blood.V96.12.3823. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y., Ashraf M.Z., Podrez E.A. Scavenger receptor BI modulates platelet reactivity and thrombosis in dyslipidemia. Blood. 2010;116(11):1932–1941. doi: 10.1182/blood-2010-02-268508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atiq F., Wouw J Van De, Merkus D., Duncker D.J., Leebeek F.W.G. Endothelial Dysfunction , Atherosclerosis , and Increase of von Willebrand Factor and Factor VIII : A Randomized Controlled Trial in Swine. Thromb. Haemostasis. 2021;121(5):676–686. doi: 10.1055/s-0040-1722185. [DOI] [PubMed] [Google Scholar]
- 10.Hörber S., Lehmann R., Stefan N., Machann J., Birkenfeld A.L., Wagner R., et al. Hemostatic alterations linked to body fat distribution , fatty liver , and insulin resistance. Mol Metab [Internet] 2021;53(May) doi: 10.1016/j.molmet.2021.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobezak A.I.S., Stewart A.J. Coagulatory defects in type-1 and type-2 diabetes. Int. J. Mol. Sci. 2019;20:1–27. doi: 10.3390/ijms20246345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma H., Lencioni M., Narendran P. Cardiovascular disease in type 1 diabetes. Cardiovasc Endocrinol Metab. 2019;8:28–34. doi: 10.1097/XCE.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gubitosi-Klug R.A., Lachin J.M., Backlund J.Y.C., Lorenzi G.M., Brillon D.J., Orchard T.J. Intensive diabetes treatment and cardiovascular outcomes in type1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39(5):686–693. doi: 10.2337/dc15-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renard C.B., Kramer F., Johansson F., Lamharzi N., Tannock L.R., Von Herrath M.G., et al. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J. Clin. Invest. 2004;114(5):659–668. doi: 10.1172/JCI200417867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griesdale D.E.G., De Souza Rd RJ., Van Dam R.M., Heyland D.K., Cook D.J., Malhotra A., et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. C Can Med Assoc J. 2009;180(8):821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arabi Y.M., Dabbagh O.C., Tamim H.M., Al-Shimemeri A.A., Memish Z.A., Haddad S.H., et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit. Care Med. 2008;36(12):3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 17.Xi Z., Ahmad E., Zhang W., Li J., Wang AFaridoon, et al. Dual-modified nanoparticles overcome sequential absorption barriers for oral insulin delivery. J. Contr. Release. 2022 Feb 1;342:1–13. doi: 10.1016/J.JCONREL.2021.11.045. [DOI] [PubMed] [Google Scholar]
- 18.Wibel R., Claus V., Spleis H., Federer C., Katrin Z., Laffleur F., et al. Self-emulsifying drug delivery systems (EDDS): in vivo -proof of concept for oral delivery of insulin glargine. Int. J. Pharm. 2023;639(April):10. doi: 10.1016/j.ijpharm.2023.122964. [DOI] [PubMed] [Google Scholar]
- 19.Rehmani S., McLaughlin C.M., Eltaher H.M., Moffett R.C., Flatt P.R., Dixon J.E. Orally-delivered insulin-peptide nanocomplexes enhance transcytosis from cellular depots and improve diabetic blood glucose control. J. Contr. Release. 2023;360(June):93–109. doi: 10.1016/j.jconrel.2023.06.006. [Internet] [DOI] [PubMed] [Google Scholar]
- 20.Elsayed A.M., Al-Remawi M., Jaber N., Khalid M.A.-S. Advances in buccal and oral delivery of insulin. Int. J. Pharm. 2023;633 doi: 10.1016/j.ijpharm.2023.122623. [DOI] [PubMed] [Google Scholar]
- 21.Benyettou F., Kaddour N., Prakasam T., Das G., Sharma S.K., Thomas S.A., et al. In vivo oral insulin delivery via covalent organic frameworks. Chem. Sci. 2021;12:6037. doi: 10.1039/d0sc05328g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayodele O.O., Onajobi F.D., Osoniyi O.R. Modulation of blood coagulation and hematological parameters by crassocephalum crepidioides leaf methanol extract and fractions in STZ-induced diabetes in the rat. Sci. World J. 2020;2020 doi: 10.1155/2020/1036364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clauss A. [Rapid physiological coagulation method in determination of fibrinogen] Acta Haematoogica. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J., Xie Y., Qian X., Jiang R., Song W. Acute effects of fine particles on cardiovascular system: differences between the spontaneously hypertensive rats and wistar kyoto rats. Toxicol Lett [Internet] 2010;193(1):50–60. doi: 10.1016/j.toxlet.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentrationof low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 26.Yagi K. Lipid peroxides and human diseases. Chem. Phys. Lipids. 1987;45(2–4):337–351. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- 27.Genet S., Kale R.K., Baquer N.Z. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues : effect of vanadate and fenugreek (Trigonella foenum graecum) Mol. Cell. Biochem. 2002;236:7–12. doi: 10.1023/A:1016103131408. [DOI] [PubMed] [Google Scholar]
- 28.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1186/1477-3155-8-16. [DOI] [PubMed] [Google Scholar]
- 29.Wang H Di, Pagano P.J., Du Y., Cayatte A.J., Quinn M.T., Brecher P., et al. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ. Res. 1998;82(7):810–818. doi: 10.1161/01.RES.82.7.810. [DOI] [PubMed] [Google Scholar]
- 30.Gheddouchi S., Mokhtari-Soulimane N., Merzouk H., Bekhti F., Soulimane F., Guermouche B., et al. Low SOD activity is associated with overproduction of peroxynitrite and nitric oxide in patients with acute coronary syndrome. Nitric Oxide. 2015;49:40–46. doi: 10.1016/j.niox.2015.05.007. [Internet] [DOI] [PubMed] [Google Scholar]
- 31.Dejana E., Callioni A., Quintana A., Gaetano G. Bleeding time in laboratory animals. A comparison of different assay conditions in rats. Thromb. Res. 1979;15:191–197. doi: 10.1016/0049-3848(79)90064-1. [DOI] [PubMed] [Google Scholar]
- 32.Despotis G.J., Goodnough L.T. Management approaches to platelet-related microvascular bleeding in cardiothoracic surgery. Ann. Thorac. Surg. 2000;70(2 SUPPL.):20–32. doi: 10.1016/S0003-4975(00)01604-0. [DOI] [PubMed] [Google Scholar]
- 33.Flora G., States U., Nayak M., States U., Ghatge M., States U., et al. Mitochondrial pyruvate dehydrogenase kinases contribute to platelet function and thrombosis in mice by regulating aerobic glycolysis. Blood Adv. 2023;27(3):24. doi: 10.1182/bloodadvances.2023010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samarghandian S., Azimi-Nezhad M., Farkhondeh T. Crocin attenuate Tumor Necrosis Factor-alpha (TNF-α) and interleukin-6 (IL-6) in streptozotocin-induced diabetic rat aorta. Cytokine. 2016;88:20–28. doi: 10.1016/j.cyto.2016.08.002. [Internet] [DOI] [PubMed] [Google Scholar]
- 35.Osiński M., Mantaj U., Kędzia M., Gutaj P., Wender-Ożegowska E. Poor glycaemic control contributes to a shift towards prothrombotic and antifibrinolytic state in pregnant women with type 1 diabetes mellitus. PLoS One. 2020;15(10 October):1–15. doi: 10.1371/journal.pone.0237843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luc K., Schramm-Luc A., Guzik T.J., Mikolajczyk T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019;70(6) doi: 10.26402/jpp.2019.6.01. [DOI] [PubMed] [Google Scholar]
- 37.Pouvreau C., Dayre A., Butkowski E.G., De Jong B., Jelinek H.F. Inflammation and oxidative stress markers in diabetes and hypertension. J. Inflamm. Res. 2018;11:61–68. doi: 10.2147/JIR.S148911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao A.K., Freishtat R.J., Jalagadugula G., Singh A., Mao G., Wiles A., et al. Alterations in insulin-signaling and coagulation pathways in platelets during hyperglycemia-hyperinsulinemia in healthy non-diabetic subject. Thromb Res [Internet] 2014;134(3):704–710. doi: 10.1016/j.thromres.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vergès B. Lipid disorders in type 1 diabetes. Diabetes Metab. 2009;35(5):353–360. doi: 10.1016/j.diabet.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Ishida K., Taguchi K., Matsumoto T., Kobayashi T. Activated platelets from diabetic rats cause endothelial dysfunction by decreasing Akt/endothelial NO synthase signaling pathway. PLoS One. 2014;9(7):1–12. doi: 10.1371/journal.pone.0102310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stegenga M.E., Van Der Crabben S.N., Levi M., De Vos A.F., Tanck M.W., Sauerwein H.P., et al. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes. 2006;55(6):1807–1812. doi: 10.2337/db05-1543. [DOI] [PubMed] [Google Scholar]
- 42.Stegenga M.E., Van Der Crabben S.N., Blümer R.M.E., Levi M., Meijers J.C.M., Serlie M.J., et al. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008;112(1):82–89. doi: 10.1182/blood-2007-11-121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amina B.S.G., Nassima M.S., Hafida M., Sid Ahmed M., Ahmed S.B. Elevation of oxidative stress markers in Type 1 diabetic children. J. Diabetes Endocrinol. 2015;6(2):5–11. doi: 10.5897/jde2014.0083. [DOI] [Google Scholar]
- 44.Luyendyk J.P., Schoenecker J.G., Flick M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511–520. doi: 10.1182/blood-2018-07-818211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bratseth V., Margeirsdottir H.D., Heier M., Solheim S., Arnesen H., Dahl-Jørgensen K., et al. Procoagulant activity in children and adolescents on intensive insulin therapy. Pediatr. Diabetes. 2020;21(3):496–504. doi: 10.1111/pedi.12978. [DOI] [PubMed] [Google Scholar]
- 46.Ng A.X.H., Ton S.H., Kadir K.A. Low-dose insulin treatment ameliorate glucose metabolism in type 1 diabetic rats. J. Diabetes Metabol. 2015;7(1):1–8. doi: 10.4172/2155-6156.1000635. [DOI] [Google Scholar]
- 47.Charan J., Kantharia N.D. How to calculate sample size in animal studies. J. Pharmacol. Pharmacother. 2013;4(4):4–7. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.A W.N., Z W.M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. 2017;24(5):101–105. doi: 10.21315/mjms2017.24.5.11. [DOI] [PMC free article] [PubMed] [Google Scholar]