Abstract
Pembrolizumab is an anti-programmed cell death-1 (PD-1) antibody used to treat various cancer types. Treatments with such immune checkpoint inhibitors cause immune-related adverse events. However, airway inflammation caused by immune-related adverse events has rarely been reported. A 54-year-old woman with endometrial cancer experienced asthma exacerbation, and increased blood eosinophil counts 3 months after pembrolizumab administration. Although asthma exacerbation improved, the resumption of pembrolizumab caused the recurrence of dry cough and hypereosinophilia. The discontinuation of pembrolizumab improved her symptoms. Serum interleukin-5 levels increased during pembrolizumab treatment but decreased upon discontinuation. The blockade of PD-1 and its ligand may exacerbate asthma through eosinophilic inflammation.
Keywords: Asthma, Eosinophil, Immune checkpoint inhibitor, Immune-related adverse events, Pembrolizumab
Abbreviations
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death ligands 1
- irAEs
Immune-related adverse events
- IL-4
Interleukin-5, IL-5
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- CT
Computed tomography
- FeNO
Fraction of exhaled nitric oxide
- FF/VI
Fluticasone furoate and vilanterol trifenatate
- FP/FOR
Fluticasone propionate/formoterol fumarate
- ILC2
Innate lymphoid cell
1. Introduction
Pembrolizumab is an anti-programmed cell death-1 (PD-1) antibody that inhibits PD-1 binding to programmed cell death ligands 1 (PD-L1) and PD-L2. In cancer cells, PD-L1 expression sends inhibitory signals that suppress T-cell activation through PD-1, allowing tumor cells to evade the immune system. The anti-PD-1 antibody activates tumor-specific CD8+ T cells. Pembrolizumab is used to treat several types of cancer. However, widespread immune activation, responsible for its anti-tumor activity, can also result in autoimmune phenomena called immune-related adverse events (irAEs) [1]. Immune-related eosinophil-induced organ damage is rare in patients treated with anti-PD-1 antibodies against typical irAEs, including endocrine disorders, rashes, liver dysfunction, and interstitial pneumonia. To the best of our knowledge, no study has measured the cytokine levels in cases of immune-related eosinophilia.
Herein, we report the case of a patient with endometrial cancer who experienced severe asthma exacerbation and severe eosinophilia after pembrolizumab administration, which was attributed to elevated interleukin-4 (IL-4) and interleukin-5 (IL-5) levels.
2. Case presentation
A 54-year-old woman with a history of asthma was diagnosed with endometrium cancer. The patient had no history of smoking. She did not receive any medication for asthma because of the absence of symptoms. She had no asthma exacerbation in the previous year or a history of intubation for asthma exacerbation. The initial stage of endometrial cancer was IIIC; therefore, the patient underwent a hysterectomy but showed an invasion of the greater omentum and cardinal ligament. She experienced stump recurrence and the development of distal metastasis of endometrial cancer to the lungs after 1 month. Although the patient was administered carboplatin and paclitaxel for endometrial cancer recurrence, the tumor enlarged. She was administered 200 mg pembrolizumab intravenously every 3 weeks.
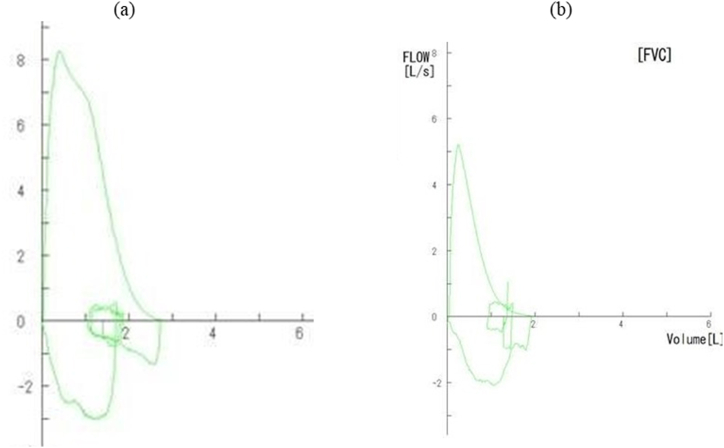
After 3 months, she developed a dry cough accompanied by wheezing without upper respiratory infection symptoms. Five months after receiving the first dose of pembrolizumab, the patient was referred to our department and was admitted because of asthma exacerbation. On admission, her body temperature was 36.8 °C, her blood pressure was 146/72 mmHg, her pulse rate was 110 beats/min, and the oxygen saturation in the room air was 94 %. Her spirometry values at the time of admission were as follows: forced expiratory volume in 1 s (FEV1), 1.36 L (54.6 % of predicted); forced vital capacity (FVC), 2.04 L (65.4 % of predicted); FEV1/FVC, 66.7 % (Fig. 1).
Fig. 1.
Pulmonary function was declined after pembrolizumab administration.
Flow volume curve (a) before pembrolizumab administration: FVC 2.94 L, %FVC 92.5 %, FEV1 2.45 L, %FEV1 96.5 %, FEV1/FVC 83.3 % and (b) five months after pembrolizumab administration: FVC 2.04 L, %FVC 65.4 %, FEV1 1.36 L, %FEV1 54.6 %, FEV1/FVC 66.7%
FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s.
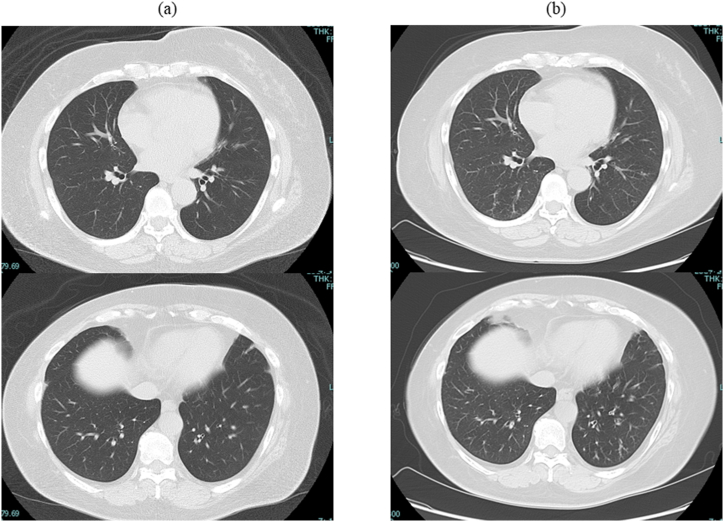
Chest computed tomography (CT) revealed bilateral linear shadows and thickened bronchial walls that had not been detected before pembrolizumab administration (Fig. 2). Laboratory findings revealed the white blood cell count was 6400/μL (neutrophils 2496/μL, lymphocyte 640/μL, and eosinophil 2560/μL). The serum IgE level was 395 IU/mL, and the C-reactive protein level was 1.66 mg/dL. Test results for antinuclear antibody and myeloperoxidase-antineutrophil cytoplasmic antibody were negative. The fraction of exhaled nitric oxide (FeNO) was 136 ppb (the normal range is <37 ppb).
Fig. 2.
Chest CT showed bilateral linear shadows and thickened bronchial walls after pembrolizumab administration.
Chest CT scans (a) before pembrolizumab administration and (b) five months after pembrolizumab administration.
CT, computed tomography.
Despite treatment with 40 mg methylprednisolone three times daily for 6 days, montelukast, inhalation of procaterol, intravenous theophylline, and compound of fluticasone furoate (100 μg) and vilanterol trifenatate (25 μg) (FF/VI), her wheezing did not resolve. After switching from methylprednisolone to 4 mg betamethasone twice daily and FF/VI, the dry powder inhaler, to fluticasone propionate/formoterol fumarate (FP/FOR), a pressurized metered-dose inhaler, gradually improved and resolved by day 14. Betamethasone was discontinued on day 14.
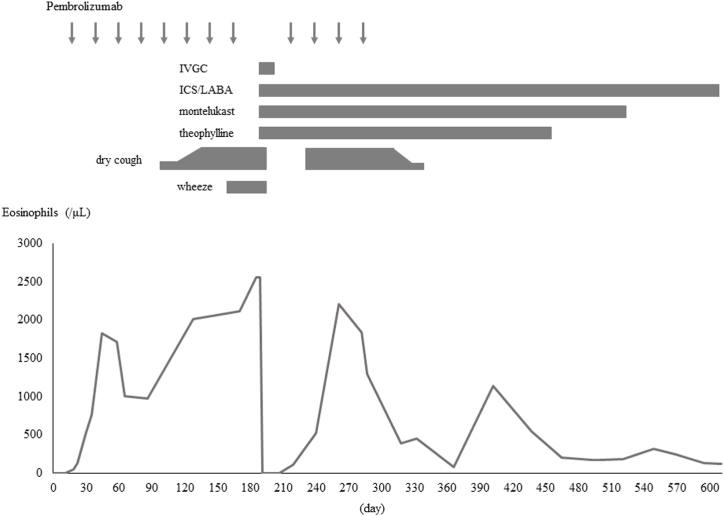
Before pembrolizumab administration, she did not have eosinophilia or obstructive ventilatory impairment; however, her blood eosinophil count gradually increased (Fig. 3), and she developed obstructive ventilatory impairment during treatment. Two weeks after discharge from our hospital, she resumed pembrolizumab treatment, which resulted in the recurrence of hypereosinophilia and dry cough three weeks after the resumption of pembrolizumab. She discontinued pembrolizumab owing to the progression of endometrial cancer, leading to a gradual decrease in blood eosinophil count and improvement of her severe cough, after which she continued to receive only FF/VI and did not experience a relapse of cough or wheezing.
Fig. 3.
Eosinophil counts and clinical presentation after starting pembrolizumab.
IVGC, intravenous glucocorticoid; ICS/LABA, inhaled corticosteroid/long-acting beta-agonist.
Due to the patient's development of hypereosinophilia during pembrolizumab administration, we measured the levels of IL-4, IL-5, FeNO, and IgE after resuming pembrolizumab. After pembrolizumab resumption, the level of IL-4 was 11.0 pg/mL (normal range is <6.0 pg/mL), IL-5 was 11 pg/mL (normal range is under 4 pg/mL), FeNO was 48 ppb, IgE was 410 IU/mL, and eosinophil count was 2208/μL. Her asthma therapy remained unchanged after the resumption and discontinuation of pembrolizumab. Nevertheless, the level of IL-5 and eosinophil count was markedly decreased to <4 pg/mL and 390/μL, while that of IL-4 was slightly increased to 16.1 pg/mL, FeNO was slightly decreased to 38 ppb, and IgE was not changed (412 IU/mL) (Table 1).
Table 1.
Pulmonary function and laboratory findings before, during, after, and at the time of resumption of pembrolizumab therapy.
| Before pembrolizumab therapy | During pembrolizumab therapy | Resumption of pembrolizumab therapy | After pembrolizumab therapy | |
|---|---|---|---|---|
| Eosinophil counts | 52/μL | 2.560/μL | 2.208/μL | 390/μL |
| FEV1 | 2.45 L | 1.36 L | NE | NE |
| % predicted FEV1 | 96.5 % | 54.6 % | NE | NE |
| FVC | 2.94 L | 2.04 L | NE | NE |
| % predicted FVC | 92.5 % | 65.4 % | NE | NE |
| FEV1/FVC | 83.3 % | 66.7 % | NE | NE |
| FeNO | NE | 136 ppb | 48 ppb | 38 ppb |
| IgE | NE | 395 IU/mL | 410 IIU/mL | 412 IU/mL |
| IL-4 | NE | NE | 11.0 pg/mL | 16.1 pg/mL |
| IL-5 | NE | NE | 11 pg/mL | <4 pg/mL |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FeNO, fraction of exhaled nitric oxide; NE, not evaluated.
3. Discussion
Herein, we report a case of asthma exacerbation after pembrolizumab treatment, which resulted in elevated levels of IL-4 and IL-5 following pembrolizumab administration. Asthma is a chronic inflammatory disease of the airways characterized by airflow obstruction and airway hyperresponsiveness. Although the pathogenesis of asthma is heterogeneous and complex, eosinophilic inflammation remains the primary pathological condition. In some cases, asthma develops as an irAE caused by anti-PD-1/PD-L1 antibodies [[2], [3], [4]]. These patients presented with peripheral eosinophilia and elevated FeNO levels similar to those observed in our case (Table 2).
Table 2.
Case series of previously reported asthma exacerbation induced by immune checkpoint inhibitors.
| Case 12) | Case 22) | Case 33) | Case 44) | Present case | |
|---|---|---|---|---|---|
| ICI | Durvalumab | Durvalumab | Pembrolizumab | Nivolumab | Pembrolizumab |
| Type of cancer | NSCLC | NSCLC | Bladder cancer | NSCLC | Endometrial cancer |
| Eosinophil count | About 500/μL | NE | 1.920/μL | 814/μL | 2560/μL |
| FeNO | NE | 39.1 ppb | 131 ppb | 113 ppb | 136 ppb |
| IgE | NE | NE | 291 IU/mL | 863 IU/mL | 395 IU/mL |
| Continuation of ICI | continued | continued | continued | continued | discontinued |
| Outcome of asthma | improved | improved | improved | improved | improved |
ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; FeNO, fraction of exhaled nitric oxide; NE, not evaluated.
Peripheral hypereosinophilia damages the airways and systemic organs, including the skin, lungs, kidneys, heart, and peripheral nerves. Cases of anti-PD-1/PD-L1 antibody-induced allergic diseases with eosinophilia, other than asthma, have been reported. Eosinophilic granulomatosis with polyangiitis induced by nivolumab [5], and allergic bronchopulmonary aspergillosis caused by pembrolizumab [6] are reported. Thirty-three cases of hypereosinophilic syndrome induced by immune checkpoint inhibitors have been described [7], of which 21 had eosinophil-related manifestations and four had eosinophilic pneumonia or bronchiolitis. Corticosteroids were effective in all cases. However, 12 of 37 patients exhibited no eosinophil-related organ damage, suggesting that eosinophilia induced by anti-PD-1/PD-L1 antibodies does not always cause organ damage. A 2.8 % prevalence of immune-related blood eosinophilia has been reported in patients treated with anti-PD1 or anti-PD-L1 drugs, with or without organ damage [8].
However, the relationship between asthma and PD-L1/PD-L2 expression in murine models of asthma remains controversial. The severity of airway hyperreactivity and inflammation was significantly greater in PD-L2−/− mice (this means the knockout mouse that specifically deletes the gene expressing PD-L2) than in wild-type mice (This means mice without genetic modification), whereas the airway hyperreactivity was reduced in PD-L1−/− mice [9]. In addition, the severity of asthma is greatly enhanced in the absence of PD-L2 and PD-L1 deficiency causing reduced airway hyperresponsiveness [10]. Conversely, the PD-1/PD-L1 blockade enhances airway hyperreactivity [11], and low PD-1 expression and circulating CD4+ T cells have been associated with high total and specific IgE concentrations in human allergic asthma [12]. As mentioned above, the association between asthma and PD-1/PD-L1 expression remains controversial. In contrast, histological analysis of pembrolizumab-induced asthma exacerbation and tracheobronchitis showed airway inflammation characterized by the infiltration of CD8+ lymphocytes which are reactivated by immune checkpoint inhibitors [13,14]. This suggests that immune reactivation by immune checkpoint inhibitors affects the airways.
An association between PD-1 and innate lymphoid cells (ILC2) in a humanized mouse model [15], suggests that PD-1 limits the viability of ILC2 and downregulates their effector functions. ILC2s are activated by IL-25 and IL-33, and activated ILC2s produce large amounts of IL-5 and IL-13. IL-5 is a crucial hematopoietic cytokine that induces differentiation, maturation, migration, and activation of eosinophils. Since a relationship between eosinophil count and severe asthma exacerbation or overall asthma control has been reported [16], IL-5 is closely related to asthma control. In this case, we observed increased IL-5 levels during pembrolizumab administration but decreased promptly after the discontinuation of pembrolizumab. This suggests that pembrolizumab-induced asthma exacerbation might be due to increased eosinophil levels through the increased production of IL-5 by activated ILC2s. Although the patient was treated with inhaled glucocorticoid/long-acting beta-agonist, leukotriene receptor antagonist, and theophylline, the symptoms relapsed. Because the eosinophil levels were higher than those in previous cases, more aggressive treatment might have been required to suppress eosinophilic inflammation. Inhibition of IL-5 could be a therapeutic strategy in this case; however, eosinophils are a source of antitumorigenic molecules. Eosinophils have demonstrated antitumor effect in several cancers but have also been linked to poor prognosis [17]. The role of eosinophils in tumors remains controversial.
In the present case, we observed improvement in asthma control after switching from FF/VI to FP/FOR. Pressurized metered-dose inhalers have a smaller particle size and reach drug aerosols in the peripheral airways than dry powder inhaler [18]. Since small airway dysfunction is associated with worse asthma control, increased exacerbation rates, and more severe bronchial hyperresponsiveness [19], switching to pressurized metered-dose inhalers may enhance asthma control.
4. Conclusions
Here, we describe a case of pembrolizumab-induced peripheral eosinophilia and asthma exacerbation in a patient with endometrial cancer. The increase in serum IL-5 levels during pembrolizumab administration reflected the mechanism of allergic inflammation following PD-1 blockade therapy. Although the antitumor effects of eosinophils should be noted, the inhibition of IL-5, such as with mepolizumab and benralizumab could be a potential therapeutic strategy for managing asthma exacerbation during pembrolizumab therapy for cancer.
Take home message.
-
●
Blockade of PD-1/PD-L1 potentially induces eosinophilic inflammation and asthma exacerbation.
-
●
Elevated serum IL-5 levels are the mechanism underlying immune-related eosinophilia and asthma exacerbation after PD-1 blockade.
Funding
There has been no financial support for this work.
Ethical approval
This study was conducted following the principles of the Declaration of Helsinki. The national “Ethical Guidelines for Life Sciences and Medical Research involving Human Subjects” do not require an ethics review committee for case reports. We posted information about case reports to patients who visited our hospital and allowed them to refuse a case report if they disagreed.
CRediT authorship contribution statement
Tomoya Harada: Writing – original draft. Naoki Uetani: Writing – review & editing. Genki Inui: Writing – review & editing. Hiroki Ishikawa: Writing – review & editing. Yoshihiro Funaki: Writing – review & editing. Miki Takata: Writing – review & editing. Ryota Okazaki: Writing – review & editing. Kosuke Yamaguchi: Writing – review & editing. Masato Morita: Writing – review & editing. Shin Kitatani: Writing – review & editing. Akira Yamasaki: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We hank Editage (www.editage.jp) for English language editing.
References
- 1.Ramos-Casals M., Brahmer J.R., Callahan M.K., et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers. 2020;6:38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uemura T., Fukumitsu K., Maeno K., et al. Asthma caused by durvalumab after chemoradiotherapy in two patients with non-small cell lung cancer, Respirol. Case Rep. 2021;9:e0835. doi: 10.1002/rcr2.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamada K., Yoshimura K., Oshinomi K., et al. A case of bronchial asthma as an immune-related adverse event of pembrolizumab treatment for bladder cancer: a case report. Med. (Baltim.). 2022;101 doi: 10.1097/MD.0000000000028339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeno K., Fukuda S., Oguri T., Niimi A. Nivolumab-induced asthma in a patient with non-small-cell lung cancer. Ann. Oncol. 2017;28:2891. doi: 10.1093/annonc/mdx455. [DOI] [PubMed] [Google Scholar]
- 5.Harada M., Naoi H., Yasuda K., et al. Programmed cell death-1 blockade in kidney carcinoma may induce eosinophilic granulomatosis with polyangiitis: a case report. BMC Pulm. Med. 2021;21:6. doi: 10.1186/s12890-020-01375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donato A.A., Krol R. Allergic bronchopulmonary aspergillosis presumably unmasked by PD-1 inhibition. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2018-227814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scanvion Q., Béné J., Gautier S., et al. Moderate-to-severe eosinophilia induced by treatment with immune checkpoint inhibitors: 37 cases from a national reference center for hypereosinophilic syndromes and the French pharmacovigilance database. OncoImmunology. 2020;9 doi: 10.1080/2162402X.2020.1722022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard-Tessier A., Jeanville P., Champiat S., et al. Immune-related eosinophilia induced by anti-programmed death 1 or death-ligand 1 antibodies. Eur. J. Cancer. 2017;81:135–137. doi: 10.1016/j.ejca.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Akbari O., Stock P., Singh A.K., Dekruyff R.H. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3:81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh A.K., Stock P., Akbari O. Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy. 2011;66:155–162. doi: 10.1111/j.1398-9995.2010.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAlees J.W., Lajoie S., Dienger K., et al. Differential control of CD4(+) T-cell subsets by the PD-1/PD-L1 axis in a mouse model of allergic asthma. Eur. J. Immunol. 2015;45:1019–1029. doi: 10.1002/eji.201444778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bratke K., Fritz L., Nokodian F., et al. Differential regulation of PD-1 and its ligands in allergic asthma. Clin. Exp. Allergy. 2017;47:1417–1425. doi: 10.1111/cea.13017. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa T., Miyata J., Maehara J., et al. Fatal airway inflammation induced by pembrolizumab in a patient with NSCLC. J. Thorac. Oncol. 2019;14:e9–e10. doi: 10.1016/j.jtho.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami N., Saito H., Takahashi S., et al. Airway disorders associated with immune checkpoint inhibitor therapy: two case reports and a systematic review. Semin. Oncol. 2022;49:439–455. doi: 10.1053/j.seminoncol.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Helou D.G., Shafiei-Jahani P., Lo R., et al. PD-1 pathway regulates ILC2 metabolism and PD-1 agonist treatment ameliorates airway hyperreactivity. Nat. Commun. 2020;11:3998. doi: 10.1038/s41467-020-17813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price D.B., Rigazio A., Campbell J.D., et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir. Med. 2015;3:849–858. doi: 10.1016/S2213-2600(15)00367-7. [DOI] [PubMed] [Google Scholar]
- 17.Varricchi G., Galdiero M.R., Loffredo S., et al. Eosinophils: the unsung heroes in cancer? Oncoiimunology. 2017;7 doi: 10.1080/2162402X.2017.1393134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holsbeke C.V., Backer J.D., Vos W., et al. Use of functional respiratory imaging to characterize the effect of inhalation profile and particle size on lung deposition of inhaled corticosteroid/long-acting β2-agonists delivered via a pressurized metered-dose inhaler. Ther. Adv. Respir. Dis. 2018;12 doi: 10.1177/1753466618760948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiel E. van der, ten Hacken N.H.T., Postma D.S., et al. Small-airway dysfunction associates with respiratory symptoms and clinical features of asthma: a systematic review. J. Allergy Clin. Immunol. 2013;131:646–657. doi: 10.1016/j.jaci.2012.12.1567. [DOI] [PubMed] [Google Scholar]