Highlights
-
•
Hepatocellular carcinoma (HCC) is a malignant liver disease.
-
•
Conventional therapies have faced failure in HCC therapy.
-
•
Liposomes have been emerged as biocompatible nanostructures in cancer therapy.
-
•
Liposomes provide targeted delivery of drugs and genes in HCC suppression.
-
•
Liposome-mediated phototherapy and stimuli-responsive ones suppress tumorigenesis.
Keywords: Liposomes, Hepatocellular carcinoma, Drug delivery, Gene delivery, Stimuli-responsive nanoparticles
Abstract
Hepatocellular carcinoma (HCC) is the most prevalent type of liver cancer, mainly occurring in Asian countries with an increased incidence rate globally. Currently, several kinds of therapies have been deployed for HCC therapy including surgical resection, chemotherapy, radiotherapy and immunotherapy. However, this tumor is still incurable, requiring novel strategies for its treatment. The nanomedicine has provided the new insights regarding the treatment of cancer that liposomes as lipid-based nanoparticles, have been widely applied in cancer therapy due to their biocompaitiblity, high drug loading and ease of synthesis and modification. The current review evaluates the application of liposomes for the HCC therapy. The drugs and genes lack targeting ability into tumor tissues and cells. Therefore, loading drugs or genes on liposomes can increase their accumulation in tumor site for HCC suppression. Moreover, the stimuli-responsive liposomes including pH-, redox- and light-sensitive liposomes are able to deliver drug into tumor microenvironment to improve therapeutic index. Since a number of receptors upregulate on HCC cells, the functionalization of liposomes with lactoferrin and peptides can promote the targeting ability towards HCC cells. Moreover, phototherapy can be induced by liposomes through loading phtoosensitizers to stimulate photothermal- and photodynamic-driven ablation of HCC cells. Overall, the findings are in line with the fact that liposomes are promising nanocarriers for the treatment of HCC.
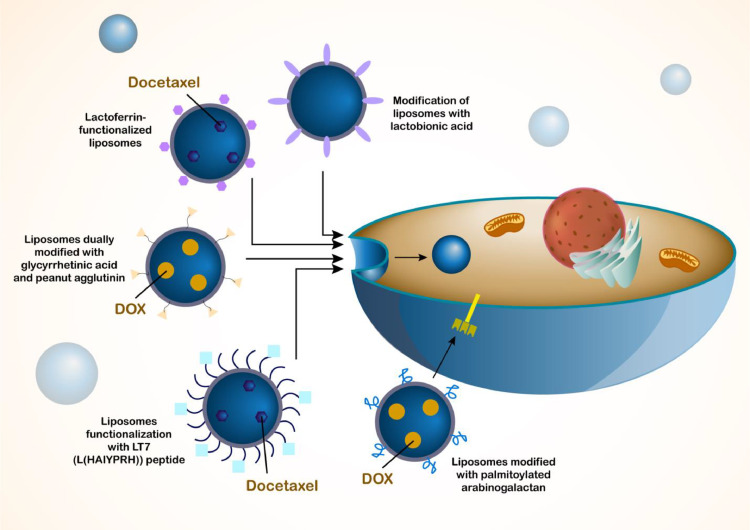
Graphical abstract
Introduction
Nanocarriers are usually considered as structures with particle size of 1–100 nm and they can be used for drug delivery by loading drugs into them or conjugation to their surface to increase therapeutic index of drugs [1,2]. Nanostructures can be utilized for both purposes of cancer diagnosis and therapy, and moreover, they display long-acting property and show high cellular uptake in cells. Furthermore, the release rate of nanostructures can be altered and therefore, their adverse impacts on normal cells can be reduced [3]. Micelles, dendrimers, protein-based nanostructures and liposomes can be considered as four main kinds of nanostructures; however, there are also other kinds of nanoparticles including carbon- and metal-based nanoparticles with important functions in disease therapy. The application of nanoparticles in pharmaceutical industry has been of importance and they have been used in a wide area from drug and gene delivery to other applications including disease diagnosis. Furthermore, recent decade has witnessed an explosion in using nanostructures in cancer therapy that these structures can promote therapeutic index of drugs and enhance potential in tumor suppression [4], [5], [6], [7]. Since drug resistance and dysregulation of biological mechanisms have been challenging in the treatment of cancer [8], [9], [10], nanoparticles for overcoming drug resistance are highly suggested [11,12]. More than five decades ago, Alec Bangham observed that phospholipids can generate closed bilayer structures in aqueous system that are known as liposomes and a long way has been passed in which liposomes have been used in pharmaceutical industry for practical applications [13]. The history of liposome development and application can be divided into four periods in which in 1960s, the discovery and initial exploration of liposomes was performed by Alec Bangham and colleagues, and found that during hydration of phospholipids, the bilayered vesicles are generated known as liposomes. During 1970s-1980s, the refinement and application of liposomes were performed that the size and compositions of liposomes were modified to enhance their stability and promote the delivery of drugs into the targeted site in the body. Among these modification, addition of cholesterol into the membrane of liposome was performed. The last period from 1990s until now is the therapeutic and diagnostic application of liposomes, since the liposomes were approved by FDA in 1990s. Due to milestone progresses that have been made in recent years, a number of liposomal drugs have been approved for being used in disease therapy and there are emergence of different biomedical products and technologies based on liposomes. Currently, there is high interest towards liposomes that is obvious from high research in this field. Since liposomal nanostructures have demonstrated high success and achievements in treatment of diseases, their clinical application has been also performed. One of the most important clinical application of liposomes is their use for treatment of cancer patients that PEGylated liposomes can be utilized for delivery of doxorubicin (DOX) in suppressing metastasis in breast cancer, leading to significant improvement in prognosis and survival rate of patients [14], [15], [16]. Furthermore, liposomal nanocarriers have been utilized for co-deliver of DOX and paclitaxel [17] or Caelyx and carboplatin [18] in cancer therapy. Caelyx is DOX in PEGylated liposomes that has been used in clinical level (phase II) in suppressing progression of head and neck cancer [19] and ovarian tumor . Moreover, the results of clinical studies were promising showing that DOX-loaded PEGylated liposomes are able to suppress hepatocellular carcinoma [20] and other kinds of tumors including lymphoma [21] and sarcoma [22]. Interestingly, in drug resistant-tumor cells, their delivery by liposomes has shown promising results; delivery of lurtotecan by liposomes has been beneficial in promoting sensitivity of ovarian tumor cells to chemotherapy [23]. Therefore, liposomes are ideal candidates in cancer therapy, even in clinical studies.
Liposomes are considered as predominant kinds of nano-scale delivery systems that their application for clinical level has been approved by FDA [24,25]. The interest towards liposomes emanates from a number of their characteristics including ease of fabrication, high biocompatibility and safety profile, and ability in loading hydrophobic and hydrophilic drugs [26]. In spite of significant advantageous, there are some challenges related to liposomes such as instability of unmodified liposomes that they can release drug after exposure to low pH level of gastric acid. For example, liposomes can display low response to stimuli and rapid clearance at physiological pH levels [27], [28], [29], [30], [31]. In order to solver aforementioned difficulties, modification of liposomes with biopolymers has been conducted and synthetic and natural products have been employed to modify surface of liposomes [32,33]. The structural features of liposomes have been beneficial in the encapsulation of drugs for delivery applications. The phospholipids applied in the structure of liposomes have both hydrophilic and hydrophobic features, allowing encapsulation of drugs with various characteristics. Moreover, the liposomes possess a bilayer flexibility providing liposomes with ability of putting the drugs in the aquous core or in the lipid bilayer. The hydrophilic drugs are loaded in the aqueous interior of liposomes while hydrophobic drugs are loaded into phospholipid bilayer of liposomes. The size of liposomes is different that can change the drug loading efficiency and biodistribution. Moreover, lamellarity or number of bilayers can chance the release kinetics and drug encapsulation by the liposomal nanostructures. The liposomes can undergo modification by PEGylation (beneficial in enhancing the blood circulation) or with ligands to increase their specificity in cancer cell targeting. Therefore, structural features of liposomes can determine their drug loading and taregtign ability.
According to the size and number of bilayers, liposomal nanostructures are categorized into four groups including small unilamellar vesicles (SUV), large unilamellar vesicles (LUV), multilamellar vesicle (MLV), and multivesicular vesicles (MVV) [34]. With increase in particle size of liposomes, their entrapment efficiency enhances and such capacity diminishes with reduction in number of bilayers for hydrophobic compounds [35]. In terms of structures, liposomes are spherical in shape and that can be formed by self-assembly of diacyl-chain phospholipids in aqueous solution [36]. The bilayer phospholipid membrane has been comprised of hydrophobic tail and a hydrophilic head [37,38], resulting in formation of an amphiphilic structure. Both natural and synthetic phospholipids can be utilized for synthesis of liposomes [39]. The particle size, rigidity, fluidity, stability and zeta potential are significantly affected by lipid composition of liposomes [40,41]. For instance, when liposomes are fabricated from natural phospholipids including egg or soybean phosphatidylcholine, the resulting liposomes display low stability, while their permeability is high. However, when liposomes are generated from saturated-phospholipids, they are rigid and their permeability is low [37]. Moreover, the hydrophilic group in lipids may show positive, negative or zwitterionic charge. This charge is of importance for determining stability via electrostatic repels and hydrophilic group pf lipids may show alteration in acyl chain length, symmetry and saturation [42].
Therefore, liposomes have opened their way into pharmaceutical applications and more importantly, the recent studies have highlighted the wide use of liposomes in cancer therapy [43], [44], [45], [46]. Since hepatocellular carcinoma (HCC) is the most common primary liver cancer, the on-time diagnosis and efficient treatment of this malignant tumor are of importance. However, the studies have focused on the application of other kinds of nanoparticles including polymeric nanoparticles [5,47], metal nanoparticles [6] and carbon nanostructures [48,49] in cancer therapy that their clinical application is challenging due to their poor biocompaitiblity and lack of solid evidence regarding the long-term safety of these structures. However, the recently developed vaccine (Pfizer) uses liposomes and the biosafety and tolerance of liposomes in patients have been shown. On the other hand, there is a need for having a comprehensive review evaluating the potential of liposomes as delivery systems, their functionalization, multifunctional liposomes and their application as diagnostic tools that current review will address these topics to shed some light on the utilization of liposomes in HCC diagnosis and therapy.
Liposomes in cancer therapy: a summary
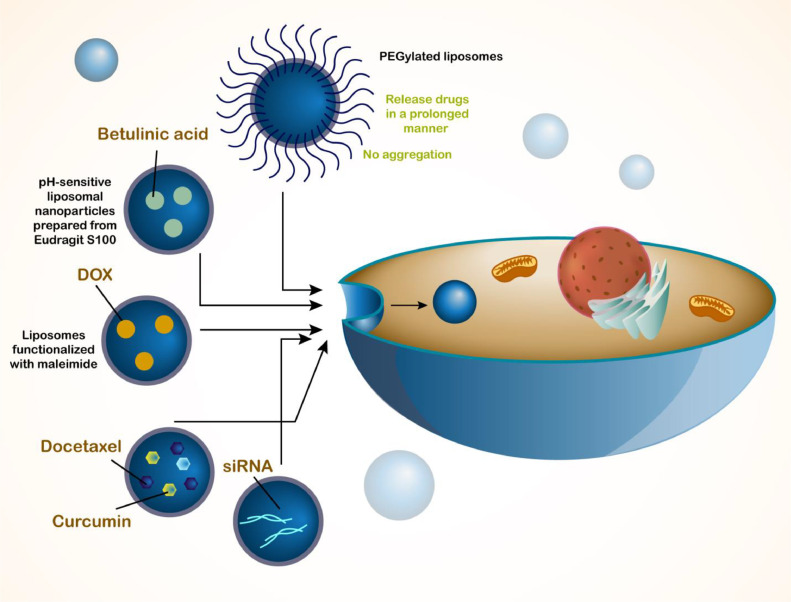
Before discussing role of liposomes in treatment of HCC, it would be beneficial to highlight role of liposomes in treatment of other cancers and provide some rational reasons for using them as nanocarriers in tumor suppression. The first and most common application of liposomes can be considered delivery of drugs in cancer therapy. In spite of progresses in field of drug discovery, there are still big challenges related to current therapeutics for cancer therapy. The most important problem of anti-tumor drugs is lack of specific delivery to tumor tissue. For instance, betulinic acid can be utilized for treatment of colorectal cancer, but when it is loaded in pH-sensitive liposomal nanoparticles prepared from Eudragit S100, its cytotoxicity against tumor cells enhances and based on in vitro and in vivo studies, these drug-loaded liposomes are able to decrease expression levels of NFAT1 and NFAT4, and enhance natural killer (NK) and CD3+ cells in improving anti-tumor immunity against colorectal tumor [50]. The previous study focused on delivery of a naturally occurring anti-tumor compound in cancer therapy and notably, chemotherapy agents such as DOX can be delivered by liposomes in cancer therapy [51]. DOX is an inhibitor of topoisomerase II and due to high cases of chemoresistance, its delivery by nanostructures has been of importance . Application of liposomes functionalized with maleimide is beneficial for promoting retention of DOX at tumor site and enhances its cytotoxicity against breast tumor cells [51]. The more interesting part related to liposomes is their ability in delivery of two anti-tumor compounds. Co-delivery of curcumin and docetaxel by liposomal nanocarriers, increases half-life of these anti-tumor drugs and suppresses tumor growth in animal models [52]. One of the important ways in improving blood circulation time of liposomes is to mediate their PEGylation that can increase their anti-cancer activity. Moreover, PEGylated liposomes can release drugs in a prolonged manner and they have no aggregation [53]. Therefore, liposomes are promising candidates in drug co-delivery and cancer suppression [54]. Another ideal approach in cancer therapy is gene transfection such as using siRNA in cancer suppression, but due to low internalization in cancer cells and degradation by enzymes, delivery of siRNA by nanostructures has been widely investigated . Due to negative charge of siRNA, cationic liposomes can be used for siRNA delivery to make stable complexes and by co-delivery of siRNA and drugs, cationic liposomes can increase their internalization in tumor cells to facilitate process of cancer therapy [55]. Another application of liposomes in phototherapy and also, inhibition of angiogenesis in cancer therapy. The parietin liposomes have been shown to stimulate photodynamic therapy and suppress angiogenesis in breast tumor therapy [56]. Moreover, treatment of skin cancer with phototherapy effect of liposomal nanocarriers can be facilitated [57]. According to the studies, it is highly suggested to use liposomes for purpose of cancer therapy and chemotherapy due to their high potential [58], [59], [60], [61] and in next section, use of liposomes for HCC treatment is discussed (Fig. 1).
Fig. 1.
The schematic representation of liposomes and their application in cancer therapy. The liposomes can be utilized for the delivery of drugs or genes in cancer therapy. The genetic tools, chemotherapy drugs and natural products can be loaded in liposomes for cancer therapy. In case of synergistic HCC therapy, the liposomes can co-deliver drugs including docetaxel and curcumin. Moreover, in order to improve the potential of liposomes in drug delivery and enhance the blood circulation time, the modification of liposomes with PEG has been performed.
Hepatocellular carcinoma: an overview
The incidence rate of liver cancer has been ascending in recent years and by 2021, it was expected to be 1 million cases annually [62], [63], [64]. Hepatocellular carcinoma (HCC) is a well-known kind of liver cancer comprising 90 % of cases and thanks for targeted treatments and immunotherapy, the life expectancy of HCC patients has been significantly improved [65], [66], [67], [68]. Up to 70 % of HCC cases occur in Asian countries and the worldwide incidence rate of HCC is suggested to be increasing [69]. The diagnosis of HCC at advanced stage is problematic, since the tumor cells are aggressive, increasing the mortality rate with unfavorable clinical outcomes [70]. In spite of understanding the main factors accounting for HCC pathogenesis and focusing on molecular drivers and immune classes [66,71,72], a few actionable mutations are observed in HCC [65,67]. That is why highlighting the tumor-promoting drivers and molecular categories have not been translated into clinic. However, there is no need to be disappointed and due to improvements in results and findings related to immune cells [68], heterogenous nature of cancer [73,74] and some etiological factors [75], there is hope to develop precision medicine for HCC therapy. Chronic liver disease can be defined as one of the reasons for development of HCC and in many cases, it has been reported that incidence rate of HCC is higher in men compared to females (2–4-fold higher) [76,77]. Interestingly, the risk factors involved in development of HCC are different based on geographical changes. The chronic infection with hepatitis B virus (HBV) and aflatoxin B1 lead to HCC development in Eastern Asia and Africa [78], while alcohol consumption and hepatitis C virus (HCV) are common factors involved in HCC development in Europe, Japan and North America [79]. The factors that mediate HCC development will be different in near future in a way that HBV and HCV roles as HCC drivers are not be main reasons in future and metabolic alterations and diseases including obesity, type II diabetes mellitus and non-alcoholic fatty liver disease (NAFLD) will appear as major reasons [77]. Moreover, these aforementioned metabolic changes along with smoking and significant iron intake will cause HCC development [77]. In spite of witnessing significant scientific progresses, the prognosis of HCC patients is not good enough [80]. In early stages of HCC, liver transplantation appears to be promising and moreover, the patients that undergo surgical resection or tumor ablation, display recurrence in majority of cases [81]. Based on the time of HCC diagnosis, the prognosis of patients fluctuates and 5-year survival rate is appeared to be 20 %. Palliative treatments including sorafenib, lenvatinib, regorafenib or cabozantinib can be utilized for HCC patients that do not respond to surgical resection or other therapies [82], [83], [84], [85]. Unfortunately, the aforementioned therapies demonstrate low ability in significantly improving survival and prognosis of patients [86], urging scientists for developing new therapies for HCC. Moreover, in the recent years, immunotherapy has been significantly applied in the treatment of cancer [87,88] that it is of importance for HCC, but the immune evasion is a problem in HCC therapy [89], [90], [91].
Recently, nanomedicine has been considered as a new gate in cancer therapy and HCC is not exceptional. Hemoglobin-curcumin nanostructures have been shown to be advantageous in reducing invasion of HCC cells, suppressing vascular mimics and enhancing radio-sensitivity in hypoxic tumor microenvironment via inhibiting growth and DNA damage repair [92]. Trimetylchitosan-camptothecin conjugates can be considered as reduction-sensitive nanostructures in HCC suppression, providing targeted delivery of drug, reducing premature release and inhibiting tumor growth in vitro and in vivo [93]. Hence, nanostructures are promising candidates for sustained delivery of drugs in HCC therapy [94]. pH-sensitive nanostructures can be employed for co-delivery of sorafenib and Tim3-siRNA to provide gene-/chemo-therapy [95]. Increased blood circulation time and promoted retention at tumor tissue are other benefits of using nanoparticles in HCC therapy [96]. Moreover, ferroptosis and apoptosis can be induced upon using nanostructures in HCC therapy [94]. Overall, studies highlight the fact that using nanoparticles in HCC therapy is a new promising approach and this feature should be completely highlighted to direct further studies in using nanostructures in HCC suppression [97], [98], [99].
Liposomes and delivery systems in hepatocellular carcinoma
Drug delivery
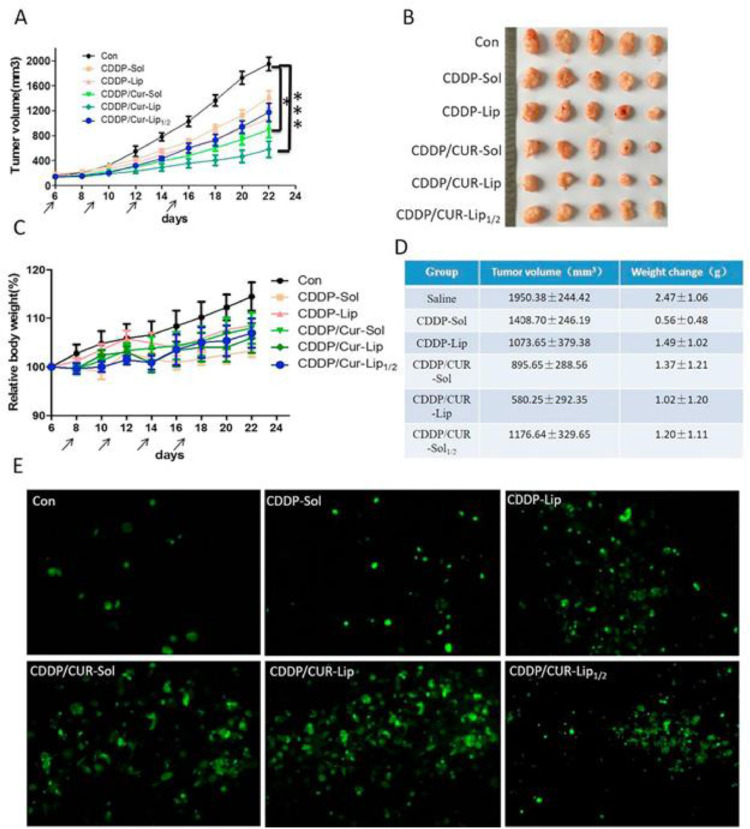
The field of drug discovery has undergone changes due to introduction of nanoparticles for delivery of compounds and drugs with therapeutic features. However, the approaches for treatment of diseases are different and since cancer is a threat and there are various kinds of biological barriers, the therapeutic index of drugs in cancer therapy has been restricted. The bioavailability and blood circulation time of drugs are low and their short half-life has reduced their pharmacological activities. Therefore, improving pharmacokinetic activity of drugs is vital for cancer therapy. In addition to these facts, the concentration of drugs utilized in cancer therapy should be diminished, since they are employed for long-term and their high concentration level can result in chemoresistance development. However, when such drugs for cancer therapy are embedded on nanostructures, low concentration is used and chance of drug resistance reduces significantly. Moreover, nanoparticles are vital for increase in intracellular accumulation of drugs. The purpose of current section is to provide an overview of using liposomes for delivery of drugs in HCC therapy. One of the chemotherapy drugs used in HCC therapy is cisplatin (CP) with ability of DNA intercalation and increasing ROS generation in tumor suppression and triggering cell cycle arrest, apoptosis and decreasing proliferation of cancer cells. However, due to dysregulation of networks in HCC cells, there is chance of CP resistance such as upregulation of circ-MRPS35 that decreases miR-148a expression in increasing degradation of PTEN for promoting CP resistance [100]. Mitochondrial fission factor has ability of promoting Drp1 expression in CP resistance in HCC [101]. The aberrant expression of non-coding RNAs (ncRNAs) can change CP response of HCC cells and ncRNA-based therapeutics have been emerged as new kind of treatments for this disease [102]. Moreover, application of other compounds such as zerumbone can suppress growth and stimulate apoptosis in increasing CP sensitivity in HCC [103]. An experiment has developed liposomes through film dispersion method with 294.6 nm size and then, curcumin and CP have been loaded in nanostructures for combination therapy. They promoted ROS generation in cancer cells and suppressed tumorigenesis in vitro and in vivo, and the benefit of liposomes is to increase the accumulation of drugs in tumor cells (Fig. 2) [104].
Fig. 2.
In vivo antitumor efficacy of different treated groups in BALB/c nude mice implanted with HepG2 cells. (A) Tumor volume growth curve; (B) Photograph of tumors separated from mice at the end of experiment; (C) The weight change of mice during the treatment; (D) Tumor volume and weight change at the end of experiment; (E) TUNEL assay. Reprinted with permission from Elsevier [104].
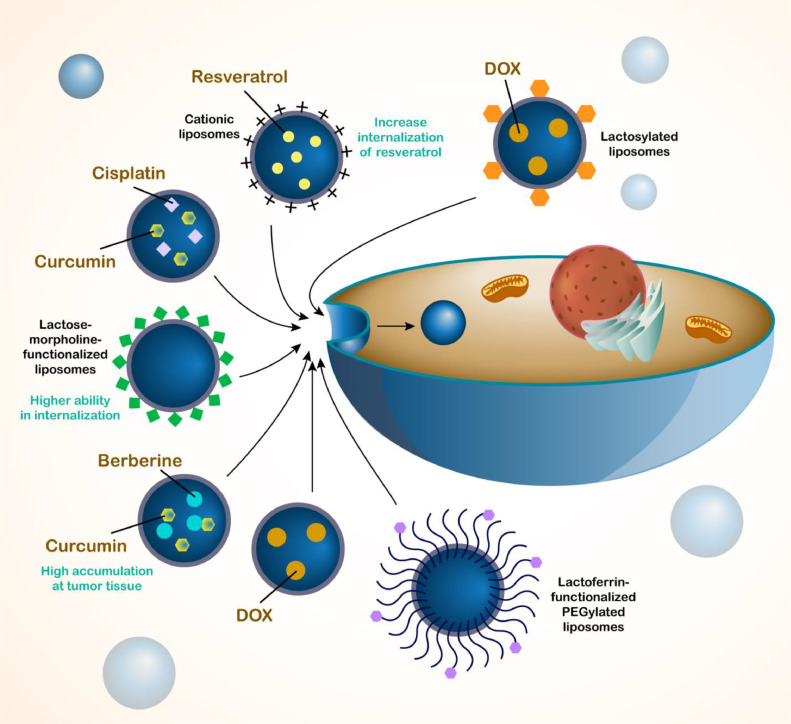
The internalization of liposomes in HCC cells is of importance to mediate high accumulation of drugs in tumor cells. The galactose-morpholine-functionalized liposomes demonstrate higher ability in internalization in HCC cells compared to conventional ones due to cellular uptake by endocytosis that can improve therapeutic index of curcumin [105]. In addition, two natural products can be delivered by liposomes in HCC therapy. Berberine is employed for treatment of HCC and it is capable of reducing inflammation and angiogenesis as a preventative measure for NAFLD-mediated HCC [106]. miR-22–3p expression increases by berberine to reduce expression levels of Bcl-2 as a target of SP1 in HCC therapy [107]. However, function of berberine in HCC suppression can be improved by its delivery through nanocarriers [108,109]. Both curcumin and berberine have been loaded on liposomes and they show high accumulation at tumor tissue. This co-delivery stimulates apoptosis and suppresses proliferation, and one of its benefits is disrupting the interaction of cancer cells and hepatic stellate cells in HCC therapy [110].
Resveratrol is also commonly used in treatment of HCC and it reduces HGF expression in disrupting metastasis [111]. MARCH1 expression is suppressed by resveratrol to increase PTEN expression in Akt inhibition [112]. The levels of CD8+CD122+ Tregs can be reduced by resveratrol in immunotherapy of HCC [113]. However, its delivery by liposomes can increase potential in HCC suppression. The cationic liposomes with 145.78 nm size and 38.03 zeta potential have been employed for delivery of resveratrol in HCC therapy and their entrapment efficiency is 78.14 %. The resveratrol-loaded liposomes display high cellular uptake in HCC cells and they increase internalization of resveratrol up to 3.2- to 2.2-fold [114]. Although the drug-loaded liposomes can be employed for suppressing tumorigenesis in HCC, it has been reported that the therapeutic index can be improved through application of other anti-cancer drugs. Disulfiram enhances ROS generation in HCC, it induces apoptosis and suppresses progression of HCC cells that are associated with increase in potential of doxorubicin-loaded liposomes in HCC therapy [115].
Since HCC is a malignant disease and its treatment has faced problems, different kinds of drugs have been employed for its treatment that doxorubicin is another one. However, lack of response of HCC cells to doxorubicin is problem that icaritin stimulates immunogenic cell death in doxorubicin sensitivity [116]. Moreover, the inhibition of PDCD5’s nuclear transfer [117] and MALAT1 upregulation [118] can lead to doxorubicin resistance in HCC cells. Therefore, one of the strategies in overcoming doxorubicin resistance is development of nanocarriers for its delivery [119]. The lactosylated liposomes have been developed for doxorubicin delivery and they display high cellular uptake in HCC cells by binding to asialoglycoprotein receptors. They demonstrate high blood circulation and they inhibit tumorigenesis in vivo [120]. The benefit of liposomes is their low particle size near to 100 nm and high entrapment efficiency (97 %). The lactoferrin-functionalized PEGylated suppress proliferation and they impair tumorigenesis in vivo [121]. Another benefit is ability of liposomes in prolonged release of drugs in HCC therapy (Fig. 3) [122].
Fig. 3.
Application of liposomes in drug delivery for the treatment of HCC. The liposomes are able to encapsulate both hydrophilic and hydrophobic drugs. A number of drugs have been loaded in the liposomes for the treatment of HCC including cisplatin, resveratrol, curcumin, berberine and doxorubicin. Moreover, the liposomes have been used for the co-delivery of drugs to exert synergistic impact including cisplatin and curcumin or berberine and curcumin. Moreover, for improving the potential of liposomes in targeting HCC cells, the functionalization of liposomes with lactoferrin has been performed.
Gene delivery
Upon the development of cancer resistance into traditional chemotherapy agents, the researchers focused on the application of gene therapy for the treatment of human cancers. Notably, the gene therapy can also exert synergistic impact in enhancing drug sensitivity of cancer cells. The gene therapy is developed to target the specific molecular mechanisms with survival function in human cancers and therefore, their sensitivity to chemotherapy drugs enhances. However, the gene therapy has also its own limitations including degradation by enzymes in the blood stream, off-targeting feature and low accumulation at tumor site. Therefore, it is highly suggested to utilize the nanoparticles that not only improve the targeting and tumor accumulation of genetic tools, but also protect the genes against degradation and prolong their blood circulation time. In the recent years, the nanoparticles have been significantly employed for the delivery of genetic tools in the treatment of human cancers. Notably, the viral structures have been also utilized for the delivery of genes in cancer therapy [123]. However, viral nanoparticles can cause immunogenotoxicity and therefore, application of non-viral vectors in cancer therapy for gene deliveyr is suggested. In an experiment, a polycationic polymer, N,N'-bis(cystamine)acrylamide-buformin (CBA-Bu) has been developed containing multiple biodegradable disulphide bonds and buformin-mimicking side chains and small size (less than 200 nm) to deliver and condense plasmid DNA that are resistant to heparin and DNase I to significantly increase the potential of gene therapy and promote the internalization in cancer cells for stimulation of AMPK and downregulation of mTOR in cancer therapy [124]. Both in vitro and in vivo studies have shown that application of nanoparticles for the delivery of genes can suppress tumorigenesis [125,126]. The nanoparticles increase the gene transfection up to 2.3 and 2.1 times compared to normal groups [127]. Therefore, the application of nanoparticles for the gene delivery is suggested that is aim of current review.
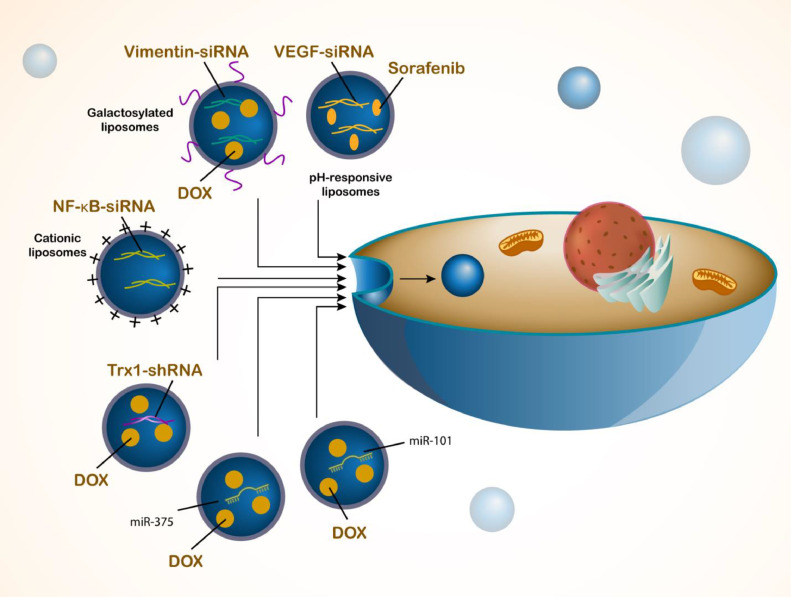
The process of HCC treatment is complicated more than simple application of drugs and their delivery by liposomes. The gene therapy is replacement of mutated genes or changing expression levels of genes in a way to suppress tumorigenesis. Gene therapy has been emerged as a new kind of method for HCC. Small interfering RNA (siRNA) is used for regulating expression level of genes in HCC therapy and 3p-GPC-3 down-regulation by siRNA improves the immunotherapy of HCC [128]. When expression level of Nrf2 is suppressed by siRNA, it causes inhibition of HIF-1α/HSP70 axis in impairing progression of HCC cells [129]. However, siRNA application in HCC therapy has been limited due to lack of specific accumulation at tumor cells. Hence, drug delivery systems have been developed for siRNA delivery in HCC. The tumor homing of siRNA in HCC cells can be improved using nanocarriers [130] and functionalization of nanostructures with materials including hyaluronic acid improves the ability and targetability of nanocarriers towards HCC cells and their efficiency in siRNA delivery [131]. The increased penetration into cancer cells [132] and more efficiency in gene expression down-regulation [133] are other features of using nanoparticles for siRNA delivery in HCC therapy. The current section focuses on the application of liposomes in gene delivery for HCC therapy. The pH-responsive liposomes co-delivery sorafenib and VEGF-siRNA in HCC therapy and the reason of focusing on the VEGF is the fact that VEGF shows overexpression in HCC cells and if its expression is suppressed, it causes significant reduction in tumorigenesis. The liposomes co-deliver siRNA and sorafenib in apoptosis induction and suppressing tumorigenesis in cells and in animal model [134]. The enhancement in biological behavior of HCC cells can result in their resistance to therapy. Vimentin upregulation is vital for progression and metastasis of HCC cells that can be mediated by ZEB1 [135]. The stability of vimentin increases by CMTM6 that is vital for EMT induction and increasing invasion of HCC [136]. Moreover, overexpression of vimentin by osteopontin stimulates EMT in HCC [137]. The galactosylated liposomes derived from cationic lipids, cholesterol and PEG2000-DSPE and others can be used for co-delivery of vimentin-siRNA and doxorubicin in HCC therapy that improve transfection and suppress tumorigenesis [138]. Another method for synthesis of liposomes is thin-film hydration method and their modification with PEI increases potential in siRNA delivery due to negative charge of siRNA and positive charge of PEI that such delivery enhances internalization in tumor cells and its co-delivery by sorafenib promotes capacity in HCC suppression [139]. In addition, reverse-phase evaporation can be utilized for liposome preparation. NF-κB is another pathway in HCC tumorigenesis and its upregulation by PIGU promotes tumorigenesis in HCC [140]. Suppression of NF-κB renders cell cycle arrest in HCC cells [141] and NF-κB down-regulation is responsible for PNU-74,654′s therapeutic indexes [142]. During the preparation of liposomes by reverse-phase evaporation, beta-Sitosterol is used to mediate liver-targeting feature in HCC cells and NF-κB-siRNA has been loaded in cationic liposomes to protect it against degradation and suppress carcinogenesis [143]. In addition to siRNA, shRNA can also be used for treatment of HCC and it is capable of reducing expression level of genes such as Gli2 [144] and HULC [145]. However, there is a similar problem for shRNA that is its targeted delivery to HCC cells that can be mediated by nanostructures [146,147]. Loading doxorubicin and Trx1-shRNA on liposomes can reduce cancer cell viability and increases chemosensitivity. Moreover, cationic liposomes can increase intracellular uptake that is because of folate functionalization [148]. Another type of delivery of microRNAs (miRNAs) as epigenetic factors with dysregulation in HCC cells. The function of miRNAs in HCC is different that can increase [149] or decrease [150] tumorigenesis and their expression can be regulated by upstream mediators such as circRNAs [151,152]. Liposomes can mediate co-delivery of miR-375 and doxorubicin that after release of cargo by micelles, miR-375 diminishes expression levels of AEG-1, YAP1, and ATG7 and then, doxorubicin induces apoptosis and cell cycle arrest through enhancing p53, Bax, caspase-3, JNK and p38 [153]. The liposomes can increase the internalization of miRNAs in HCC cells up to 15-fold. Anti-miR-221 delivery by anionic liposomes results in its down-regulation to enhance PTEN, p27 and TIMP3 levels in suppressing tumorigenesis in vitro and in vivo [154]. miR-101 is considered as a tumor-suppressor factor in HCC therapy and it suppresses Beclin-1-induced autophagy in increasing oxaliplatin sensitivity [155]. Autophagy inhibition by miR-101 increases CP-mediated apoptosis [156] and its down-regulation by SNHG6 increases tumorigenesis [157]. Co-delivery of miR-101 and doxorubicin by liposomes stimulates apoptosis and suppresses progression and growth of tumor cells [158]. Therefore, genes can be delivered by liposomes in cancer therapy (Fig. 4).
Fig. 4.
The delivery of genes by liposomes in HCC therapy. The delivery of genes alone or in combination with drugs can provide new insights in the treatment of HCC. The liposomes can increase the internalization of genes in HCC cells to enhance their potential in gene regulation. Liposomes have bene used to deliver siRNAs for targeting VEGF, vimentin and Trx-1, among others, that are responsible for the cancer progression. Moreover, epigenetic factors such as miR-101 and miR-375 can be loaded on liposomes for HCC therapy.
Stimuli-responsive liposomes
Cancer cells have a unique TME that can be utilized for the development of stimuli-responsive nanocarriers. The stimuli-responsive nanoparticles are ableto respond to the pH, redox and light to release drug in tumor site, improve the anti-cancer activity of drugs and decrease in the adverse impacts. The pH-sensitive nanoparticles respond to the endogenous stimulus. The TME has a lower pH and mild acidic compared to the normal cells resulting from the enhancement of metabolic activitiy and generation of lactic acid through anaerobic glycolysis. This acidic pH can be found in the extracellular matrix of tumor and within in the intracellular organelles including endosomes and lysosomes. The pH-responsive nanostructures respond to the acidic pH through induction of structural changes in the nanostructures to release drug in the tumor site. The application of polymers including poly(β-amino esters) and lipids enriched in acid-labile linkages (hydrazone, ortho esters, or acetal groups) can result in the development of pH-responsive nanostructures. These nanoparticles should be deisnged in a way to be stable at the netural pH, while undergo changes in the acidic pH to release the cargo. Another type of nanoparticle is redox-responsive nanostructures in which respond to the glutathione (GSH), since the GSH levels in the tumor cells are higher (4-time increase) compared to the normal cells. Upon exposure to the redox status of TME, the cleavage of disulfide bonds occurs to release the drug from the nanoparticles. The exogenous stimulus for the development of nanoparticles is the light-sensitive nanostructures that can be controlled by the exogenous light exposure. The o-nitrobenzyl derivatives or azobenzene-containing compounds can be utilized for the development of light-responsive nanoparticles. More information regarding the stimuli-responsive nanoparticles in cancer therapy can be found in the recent reviews [159], [160], [161], [162], [163], [164].
pH-responsive
The acidicity or alkalinity of a tissue or environment is determined by the pH level that can affect the cellular mechanisms, especially in cancer. The pH level is different among normal and cancer tissues due to the metabolic alterations occurring in cancer cells. The normal tissue possesses a pH level of 7.35–7.45 that is slightly alkaline and this pH level is vital for cellular functions and homeostasis. However, due to the Warburg effect in the tumor cells, they utilize glycolysis for energy generation even in the presence of oxygen, resulting in the production of lactic to reduce pH of TME. Therefore, the extracellular pH levels of tumors are lower than the normal tissues at the range of 6.5–7.2 and this acidic pH is required for increasing the progression of tumor through increasing metastasis and mediating immune evasion. Recently, the nanoparticles have been developed to respond to pH for cancer therapy and drug release. The pH-responsive tumor-tropism hybrid membrane-coated MnO2 nanostructures have been developed for the delivery of BPTES as a glutamine metabolism inhibitors to suppress cancer progression, increase the infiltration of T lymphocytes and promote M1 polarization of macrophages [165]. In another experiment, the pH-responsive CaCO3 nanostructures have been developed for the delivery of TCL and immune adjuvant CpG that can act as nanovaccine and promote M1 macrophage polarization in cancer therapy [166]. Therefore, the pH-sensitive nanoparticles can be promising carriers for tumor site delivery and efficient tumor eradication [167].
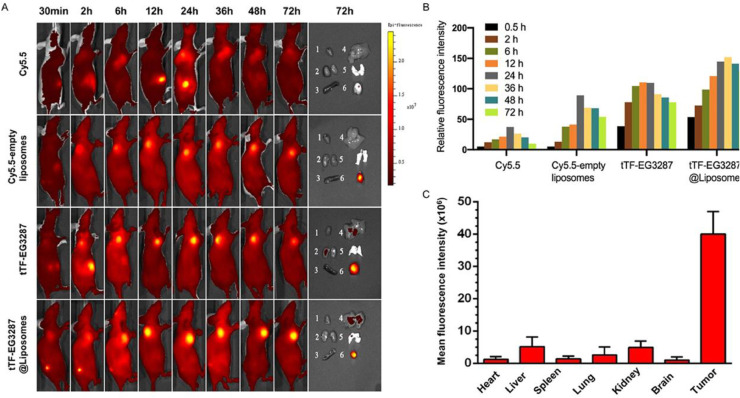
The development of stimuli-responsive nanocarriers has been shown to be promising in HCC therapy, since they can respond to stimulus in TME and this causes their stimulation for cargo release. The stimuli for inducing drug release from nanocarriers are different and overall, they are categorized into endogenous and exogenous stimuli that pH is one of the most well-known endogenous stimuli for drug release from nanostructures. The pH of TME is lower compared to normal cells and tissues, and therefore, using it as a stimulus is vital for development of smart liposomes. The pH-sensitive nanoparticles have been extensively applied for purpose of HCC therapy such as development of niosomes sensitive to pH and their functionalization with galactose in delivery of tanshinone IIA for apoptosis induction in HCC [168]. Triptolide is a natural product that can be loaded on pH-sensitive folate-targeted nanostructures to selectively target HCC cells with folate upregulation in cancer therapy [169]. Two essential hallmarks of HCC cells are their stemness and chemoresistance that by development of pH-sensitive nanoclusters and their application for HCC therapy, due to their important features such as long blood circulation time and DNA damage stimulation, there is possibility of suppressing tumorigenesis in HCC [170]. Pyrrolidinedithiocarbamate and doxorubicin can be co-loaded on pH-sensitive nanostructures for synergistic inhibition of HCC [154]. Hence, pH-sensitive nanostructures have opened their way in HCC therapy [171], [172], [173]. Because of the wide application of liposomes in HCC therapy, there has been trend and willing towards using pH-sensitive liposomes in HCC removal. In previous section, some of the experiments revealed that pH-sensitive liposomes can mediate targeted release of drugs and genes in HCC therapy, confirming development and potential of these nanocarriers. tTF-EG3287 is a procoagulant protein that can be loaded in liposomes with pH-sensitive feature and they release drug in pH 6.5 with 100 nm size. The show prolonged release of drug in 72 h that was 83 % and administration to an animal model of HCC led to preferential accumulation in cancer site and suppressing tumorigenesis(Fig. 5) [174]. Overall, the field of pH-sensitive liposomes has been ignored a little in HCC therapy that requires more attention.
Fig. 5.
In vivo tumor targeting and biodistribution of tTF-EG3287@Liposomes. (A) In vivo fluorescence images of HepG2 tumor-bearing mice injected with free Cy5.5, Cy5.5-empty liposomes, tTF-EG3287, and tTF-EG3287@Liposomes were acquired in real time up until 72 h after administration. Ex vivo imaging showing the distribution of particles in the excised tumors and major organs at 72 h after injection. 1, heart; 2, kidney; 3, spleen; 4, liver; 5, lung; 6, tumor. (B) Quantitative analysis of the fluorescence intensity in tumors of all experimental groups for 0.5, 2, 6, 12, 24, and 72 h after administration. (C) Quantitative analysis of the fluorescence intensity of Cy5.5-tTF-EG3287@Liposomes in tumors and major organs 72 h after administration.. Reprinted with permission from ACS [174].
Redox-responsive
There is relationship between glutathione (GSH) levels and cancer that is complicated. GSH participates in the cellular defense mechanism, redox homeostasis and regulation of cell death pathways. GSH is a tripeptide comprised of glutamine, cysteine and glycine and has the highest level as intracellular antioxidant. GSH is responsible for the protection of cancer cells against reactive oxygen species (ROS). The increase in antioxidant ability, resistance to therapy, inhibition of apoptosis and tumor microenvironment remodeling can be mediated by GSH. More information regarding the GSH and cancer can be found in the recent review [175]. The TME has a dysregulation of a redox environment that can be used for the development of redox-responsive nanoparticles in cancer therapy. Different kinds of polymers and materials can be utilized for the development of redox-responsive nanocarriers. The disulfide bond-containing polymers can be incorporated in the liposomes and other nanostructures to develop redox-responsive nanoparticles., When there is high levels of GSH, the cleavage of disulfide bonds occurs. Then, the encapsulated drugs are released from the nanoparticles into the tumor site. For instance, the modification of liposomes with PEG has disulfide bonds and can lead to release to drug. The thiol-responsive materials can be used for the development of redox-responsive nanoparticles and in the presence of thiols, the degradation or alterations in the structure occur to release drug at tumor site. Moreover, ferrocene-containing compounds undergo oxidation–reduction reactions in response to the redox conditions. The selenium-containing polymers and selenium such as sulfur can result in the development of bonds sensitive to redox. In the recent years, the redox-responsive nanoparticles have been extensively used in the treatment of cancer [176], [177], [178], [179], [180]. There are several advantageous regarding targeting GSH for cancer therapy. The targeted release of drug, increase in penetration and retention of nanoparticles, suppressing drug resistrancd, controlled drug release kinetics, selective activation of prodrugs and reduced systemic toxicity.
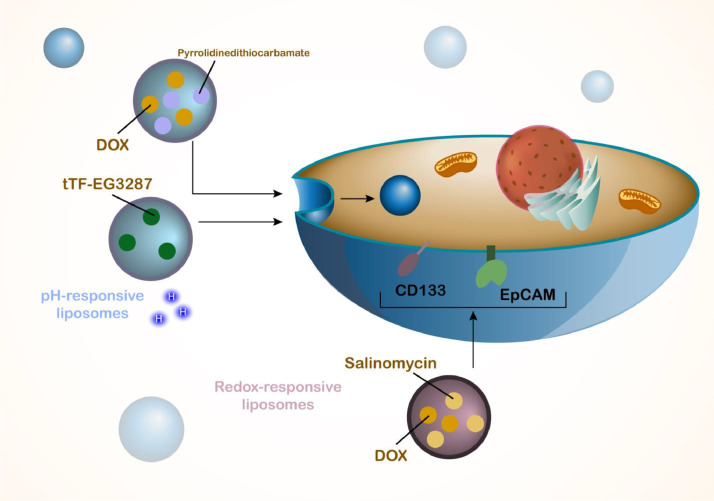
Another kind of stimulus for development of smart nanostructures is redox and nanoparticles can be developed in a way to respond to GSH levels in TME and then, cargo release occurs due to changes in their structures. Increasing evidence has evaluated application of redox-sensitive nanostructures in HCC therapy such as redox-responsive PLGA nanostructures for delivery of oridonin, spherical shape, high cellular uptake and apoptosis stimulation in tumor cells [181]. The nanoparticles can be developed in layers that each layer has different response to TME such as multifunctional nanostructures with albumin shell and a core that is responsive to redox for release of siRNA in HCC therapy [182]. Moreover, miR-34a can be loaded on nanostructures to be released in response to redox status in suppressing tumorigenesis in HCC [183]. The nanoparticles obtain response to redox due to presence of disulfide bond that undergoes cleavage [184] and dual-functionalized nanoparticles responsive to pH and redox can be developed in HCC therapy [185]. The presence of liver cancer stem cells can cause development of HCC and redox-responsive liposomes have been functionalized for targeting CD133 and EpCAM receptors on the surface of cells to co-deliver doxorubicin and salinomycin in suppressing cancer cells and impairing HCC tumorigenesis (Fig. 6) [186].
Fig. 6.
The pH- and redox-sensitive nanoparticles and liposomes in HCC therapy. The tumor microenvironment has a low pH level and also, due to the changes in the redox status, the nanoparticles can be developed to respond to pH and redox levels in the tumor microenvironment. In response to pH and redox, the liposomes release the cargo to specifically target tumor cells and enhance accumulation of cargo in tumor cells.
Phototherapy and light-responsive liposomes
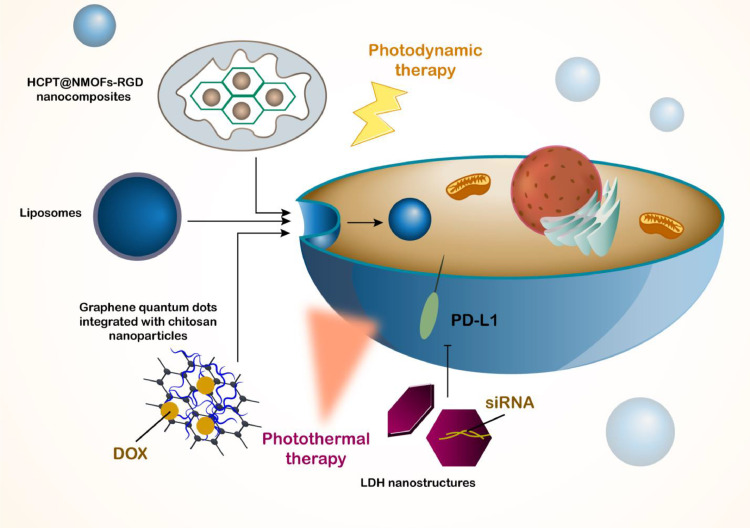
Phototherapy has been emerged as a new kind of therapy for HCC and in according to various difficulties in therapy of HCC including chemoresistance and relapse after surgery, phototherapy can be combined with other therapies to resolve their problems and by complete suppression of cancer cells, it can prevent development of recurrence after surgical resection in HCC. Different kinds of light sources can be utilized for the stimulation of phototherapy. The ultraviolet light is one of them that has wavelength range of 100–400 nm and can increase the generation of ROS to mediate DNA for induction of apoptosis in tumor cells. Visible light is another light source with wavelength range of 400–700 nm that can cause photodynamic and photothermal therapy. The structures such as gold nanorods or copper selenide can be utilized for photothermal therapy and through conversion of light into heat, they can suppress tumor cells. In photodynamic therapy, the photosensitizer-loaded nanostructures increase ROS levels. The wavelength of 600–700 nm is vital for deep tissue penetration and decreased damage to helath tissues and organs. The near-infrared (NIR) light is another method of phototherapy with wavelength of 700–1000 nm (NIR-I) and 1000–1700 nm (NIR-II) that has deep tissue penetration, increased photothermalk efficiency and decrease in photodamage. There are also a number of specialized light sources that can be used for phototherapy including lase light and LED light. In cancer therapy, phototherapy is vital for impairing progression of cancer cells and it can increase ROS generation or mediate hyperthermia to trigger tumor ablation. More information regaerding phototherapy-mediated tumor ablation can be found in recent reviews [187], [188], [189], [190], [191]. The first type of phototherapy is photothermal therapy (PDT) that aims in development of nano-scale delivery systems for photosensitizers and sometimes, chemotherapy drugs are also loaded on nanoparticles to mediate combination of phototherapy and chemotherapy. Graphene quantum dots have been integrated with chitosan nanoparticles for delivery of doxorubicin in HCC therapy and quantum dots can function as photosensitizers to induce PTT in tumor ablation and such combination of PTT and chemotherapy improves potential in HCC inhibition [192]. The UiO-66/Bi2S3 nanocomposite can also be utilized for delivery of doxorubicin in HCC therapy and in addition to pH-responsive release of drug, they can induce PTT for tumor suppression [193]. Interestingly, LDH nanostructures can be loaded with siRNAs and then, by mediating PTT impact, they can combine gene therapy and chemotherapy in down-regulation of PD-L1 and NR2F6 in suppressing tumorigenesis in HCC [194]. Importantly, nanoparticle-mediated PTT can increase infiltration of NK cells in tumor microenvironment in triggering immunotherapy in HCC [195]. Moreover, PTT can be used for overcoming drug resistance in HCC cells [196]. The aim of PTT is to increase heat in tumor ablation and another kind is photodynamic therapy (PDT) that increases ROS generation in cancer cell death. The theranostic nanoplatforms can be disassembled by light and this results in PDT and combination with chemotherapy in HCC inhibition [197]. The HCPT@NMOFs-RGD nanocomposites can be employed for PDT and they are ideal candidates in HCC therapy due to their modification with RGD that enhances selectivity towards HCC cells [198]. More importantly, PTT and PDT can be combined by nanostructures in HCC therapy [199] and it is a minimally-invasive tool in cancer therapy [200]. The nanostructures used for PDT can respond to wavelength of 532 nm to increase ROS production in HCC therapy [201]. Liposomes have been considered as novel nanostructures for purpose of phototherapy in HCC and although a few studies have evaluated this potential, the current experiments provide the idea that using liposomes for phototherapy is a novel strategy in HCC suppression and more studies should focus on this part, since liposomes have high biocompatibility and their clinical application is completely possible in near future. Interestingly, since the structure of liposomes has been comprised of bilayer membranes, exposure to light can cause disassembly and such property is used for development of stimulus-responsive liposomes. The method for synthesis of such nanoparticles is like that first, liposomes are prepared and fabricated through various methods such as thin-film hydration or reverse-phase evaporation and in the next step, they are loaded with agents responsive to light such as ICG [202] or IR-780 [203] that after irradiation, it causes changes in structure of liposomes for release of drug and suppressing tumorigenesis in HCC (Fig. 7).
Fig. 7.
An overview of phototherapy in HCC and role of light-responsive liposomes. Light is an exogenous stimuli for the regulation of nanoparticles in cancer therapy. Since liposomes lack photo-responsive feature, they should be conjugated to other nanoparticles with photo-resppnsive activity such as graphene quantum dots or LDH nanostructures. Moroever, the photosensitizers can be loaded in liposomes to respond to the light for cancer phototherapy. Liposomes can be utilized for the induction of photothermal and photodynamic therapy that finally stimulate cell death in HCC.
One of the major benefits of nanoparticles that are responsive to light is their ability to better deliver the cargo and drugs into the tumor site. Currently, the systemic administration of nanoparticles can release the drug in tumor site, but when they are developed in a way that they are light-responsive, the application of light can cause the release of drug from the nanoparticles by causing changes in their structures to specifically release drug at tumor site for the specific suppression of cancer cells. The benchmark experiments are vital to investigate the reactive behavior and impacts of nanostructures in animal models and evaluate the safety, biodistribution, efficacy and potential toxicity of nanostructures. Such impacts of nanostructures are vital for the clinical translation of findings. Moreover, the benchmark studies are essential for the interactions among the nanostructures and biological systems, the potential of certain targeting tissues or cells, stimulation of therapeutic impacts and mediating side impacts on immune system or healthy tissues.
Functionalized liposomes
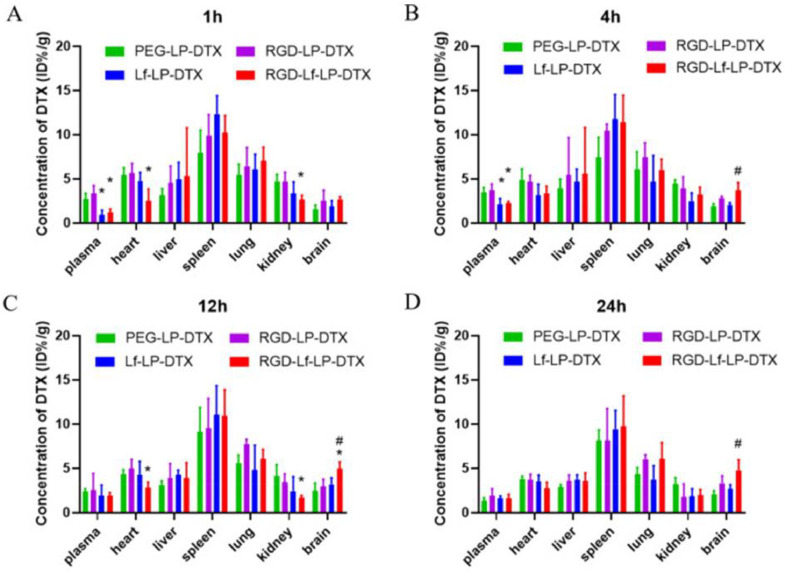
The functionalization of nanoparticles has been suggested as a promising strategy in increasing targeting ability. One of the reasons of focusing on liposomes is their ease of functionalized that has been confirmed in other cancers. Due to urgency in effective therapy of HCC, surface-functionalized liposomes have been also employed for HCC therapy. It is worth mentioning that surface modification of liposomes may increase their size such as modification of liposomes with lactobionic acid (LA) that enhanced size from 256 nm to 310 nm. Such surface modification increased internalization in HCC cells and promoted ability of these nanostructures in targeted delivery of drugs [204]. Asialoglycoprotein receptor (ASGPR) is a receptor with glycoprotein feature that shows presence on the surface of liver hepatocytes, and it also undergoes upregulation in HCC cells [205], [206], [207]. The reason of being important for drug delivery is ability of ASGPR for mediating endocytosis. The ASGPR ligands have been used for modification of nanoparticles and liposomes [208,209]. The doxorubicin-loaded liposomes have been modified with palmitoylated arabinogalactan (PAG) to selectively target HCC cells overexpressing ASGPR that have 200 nm size, negative zeta potential and ability of releasing more levels of doxorubicin in pH 5.5 compared to pH 7.4. The cytotoxicity was higher in HCC cells overexpressing ASGPR and improved pharmacokinetic feature of doxorubicin and anti-cancer activity were observed in surface-functionalized liposomes [210]. Hence, targeting ASGPR is of importance in HCC therapy [211]. Another agent for surface functionalization of liposomes is lactoferrin (Lf) that can mediate delivery of drugs to brain for suppressing glioblastoma [212]. Furthermore, Lf-functionalized liposomes can be utilized for docetaxel delivery in tumorigenesis suppression (Fig. 8) [213].
Fig. 8.
Tissue distribution of DTX after intravenous administration of DTX loaded liposomes, data represented the mean ± SD (n = 3). *p < 0.05 compared with RGD-LP-DTX, #p < 0.05 compared with Lf-LP-DTX. Reprinted with permission from BMC [213].
In order to provide an anchor for functionalized of liposomes, they have been first modified with PEG and then, Lf is conjugated to the liposomes that compared to unmodified liposomes, they demonstrate preferential accumulation in HCC tissue [214]. In addition to improving ability of liposomes in drug delivery, modification with ligands increase potential in DNA delivery such as using a ligand targeting 5-HT receptors in HCC cells that increases cellular uptake [215]. As it was mentioned, liposomes can be employed for doxorubicin delivery in cancer therapy. Increasing evidence highlights function of liposomes as effective materials for purpose of doxorubicin delivery in cancer suppression and pH-sensitive liposomes can increase potential of doxorubicin in cancer suppression [216].
Another kind of compound used to modify the surface of liposomes is hyaluronic acid (HA). The rational for functionalization with HA is to specifically target the CD44 receptors upregulating on the surface of HCC cells. In this line, liposomes were modified with glycyrrhetinic acid (GA) and HA to co-deliver aprepitant and curcumin. Such drug-loaded liposomes showed uptake by HCC cells and cancer-associatd fibroblasts that suppressed tumorigenesis in vitro and in vivo, and disrupted the invasion [217]. In another effort, GA/HA-functionalized liposomes were utilized to co-deliver berberine and curcumin that were able to stimulate apoptosis in tumor cells and impair their proliferation [110]. The HCC cells demonstrate overexpression of folate receptor to bring up folate for their proliferation. Therefore, functionalization with folate is also of importance in HCC therapy that folate receptor-targeted liposomal nanostructures can deliver a diacid metabolite of norcantharidin to trigger apoptosis in HCC cells [218]. Noteworthy, folate-targeted liposomes have shown potential to deliver thioredoxin 1 shRNA and doxorubicin in synergistic drug and gene delivery with efficacy in nuclear delivery [148]. Moreover, antibodies have been applied to functionalize the liposomes. The modification of liposomes with carbonic anhydrase IX antibody and BR2 peprtide has been performed to deliver cantharidin to enhance cellular uptake and suppress tumor progression in vitro and in vivo [219]. The functionalization is of importance for increasing cellular uptake of cargo, since tumor cells demonstrate upregulation of specific receptors and therefore, the side effects of drugs would also diminish due to the increased accumulation in tumor cells. On the other hand, there are a number of limitations and drawbacks including increase in the particle size of nanoparticles and the inductin of immune reactions, especially in case of modification with peptides and antibodies.
Doxorubicin can be co-delivered with simvastatin in suppressing lung cancer progression [220]. Another type is thermosensitive magnetic liposomes for doxorubicin delivery [221] and adjusting protein corona component around liposomes improves their potential in doxorubicin delivery [222]. However, more progressed liposomes have been employed for doxorubicin delivery in HCC and they have been dually modified with glycyrrhetinic acid (GA) and peanut agglutinin (PNA) to selectively target liver tumor cells in inhibiting tumorigenesis in vitro and in vivo [223]. Since there is upregulation of transferrin (Tf) on the surface of HCC cells, functionalization of nanostructures with Tf has been conducted. The Tf-functionalized polymeric nanostructures deliver CP and doxorubicin in suppressing carcinogenesis in HCC [224]. Moreover, poly(vinyl alcohol) nanomaterials can be functionalized with Tf in co-delivery of doxorubicin and sorafenib in HCC suppression [225]. However, targeting Tf receptors on the surface of HCC cells can be performed in another way that is using peptides such as LT7 (L(HAIYPRH)) peptide for functionalization of liposomes and then, their targeting capacity towards HCC cells increases to deliver docetaxel in tumor suppression (Fig. 9) [226]. Hence, increasing evidence advocates the fact that functionalization of liposomes is vital for HCC therapy (Fig. 10, Table 1).
Fig. 9.
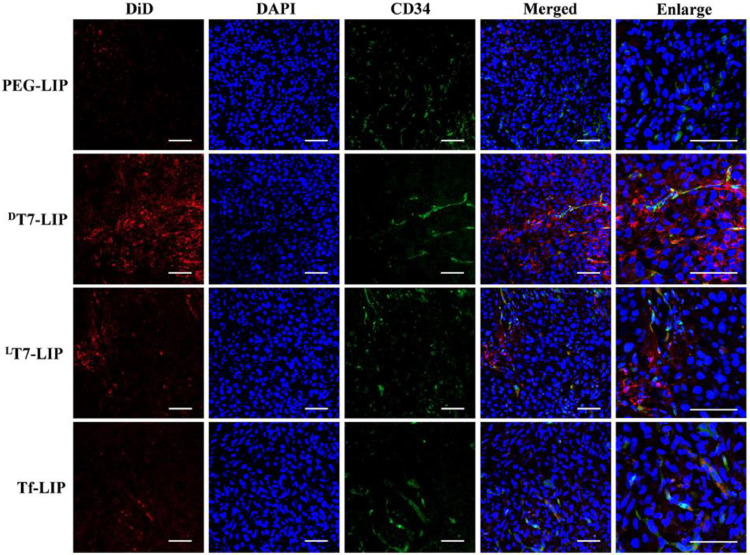
Distribution of DiD-loaded liposomes in subcutaneous HepG2 xenograft tumor tissues at 24 h after intravenous administration of DiD-loaded liposomes. Red: DiD-loaded liposomes; blue: DAPI-stained cell nucleus, green: anti-CD34-stained blood vessels. Bars represent 40 µm. Reprinted with permission from Elsevier [226].
Fig. 10.
The surface-functionalized liposomes in HCC therapy. The biological studies have highlighted the expression of receptors on the surface of HCC cells. Therefore, the functionalization of liposomes with lactobionic acid, lactoferrin, glycyrrhetinic acid, LT7 peptide and palmitoylated arabinogalactan has been performed to increase their selectivity towards tumor cells.
Table 1.
The application of liposomes in HCC therapy.
| Nanovehicle | Remark | Ref |
|---|---|---|
| CXCR4-guided liposomes | Co-delivery of sorafenib and PLX3397 to increase CD8+ T cell infiltration and suppressing tumorigenesis | [227] |
| 11-DGA-3- O-Gal-Modified Cantharidin Liposomes | Suppressing invasion and increasing cytotoxicity towards tumor cells | [228] |
| Glycyrrhetinic Acid and TAT Peptide Modified Dual-functional Liposomes | 135.55 nm size −4.57 mV zeta potential Apoptosis stimulation |
[229] |
| Mild Hyperthermia Responsive Liposomes | High internalization and cytotoxicity | [230] |
| Chemotherapeutic Nanoparticle-Based Liposomes | Mild microwave ablation along with doxorubicin chemotherapy to induce 80 % cell death | [231] |
| P94 Modified Liposomes Loaded with N-14NCTDA | Higher cellular uptake compared to conventional liposomes | [232] |
| ApoE-modified liposomes | Apoptosis induction and increasing survival of animal model | [233] |
| Dual-Functional Liposomes with Carbonic Anhydrase IX Antibody and BR2 Peptide Modification | Providing the targeted delivery of cantharidin to tumor cells | [219] |
| Folate receptor-targeted liposomes | Loading norcantharidin metabolite in suppressing carcinogenesis in vitro and in vivo | [218] |
| Hyaluronated and PEGylated Liposomes | High cellular uptake by tumor cells and stimulating M1 polarization of macrophages | [234] |
| Glycyrrhetinic acid and cell transmembrane peptides modified with liposomes | Stimulation of apoptosis and preferential accumulation in tumor cells and tissues | [235] |
| Polyinosinic-polycytidylic acid liposome | Triggering apoptosis due to increasing expression levels of RIG-I-like receptors | [236] |
| GA&HA-Modified Liposomes | Co-delivery of curcumin and aprepitant in impairing chemoresistance and invasion | [217] |
| Liposomes | Co-loading ursolic acid and ginsenoside Rg3 in apoptosis induction and reducing viability of tumor cells | [237] |
| Glycyrrhetinic acid-modified liposomes | Delivery of Murrayafoline A and high cytosolic absorption | [238] |
| Light-activatable liposomes | Co-delivery of triptolide and Ce6 to suppress tumorigenesis | [239] |
There are several methods for analyszing the functionalization of nanoparticles that can be utilized for liposomes. The flow cytometry and fluorescence-based techniques are commonly used for the functionalization efficacy of nanoparticles. The flow cytometry allows to evaluat the physical and chemical features of nanoparticles in a quantitative manner and this can be understood through the evaluiation of fluorescence intensity of labeled nanostructures. Moreover, the flow cytometery allows to evalaut the size and granulity of the nanoparticles. The fluorescent techniques can also be utilized to evaluate and visualize the binding to fluorescently labeled molecules into nanostructures to investigate the potential and uniformity of functionalization.
Liposomes in hepatocellular carcinoma diagnosis
The diagnosis of HCC is mainly based on the application of cytology or histology and during biopsy, the lesion tissue is achieved that are traditional ways for the diagnosis of HCC [240]. However, advances in the technology has resulted in the development of new methods such as computed tomography (CT) and magnetic resonance imaging (MRI) that do not depend on biopsy for the diagnosis [241]. Notably, liposomes can be utilized as novel nanostructures for the diagnosis of HCC [242]. In order to develop liposomal nanostructures for the HCC diagnosis, it is required to load a number of imaging agents such as indocyanine green [202] and IR780iodide [243] in liposomes. Since a number of receptors undergo upregulation on the surface of HCC cells including CD44, the modification of liposomes with CD44-antibody can increase potential in molecular imaging [244].
MRI
MRI has been widely used in the biomedicine as a diagnostic toll due to its non-invasive nature, spatial resolution, high anatomical contrast and sofr-tissue differentiation. However, the gadolinium-based contrast agents (CA) are required to enhance the differences in signals between diseased areas and normal tissue in many cases. Liposomes are considered as biocompatible nanostructures and two methods are used for binding CA into liposomes including encapsulation of water-soluble reagents of gadolinium chelates in liposome cavities or functionalization of lipid bilayers by gadolinium chelates [245]. In an experiment, the rare-earth-doped nanoparticle (Gd-REs@Lips) were used for imaging HCC as primary liver cancer and nanostructure was utilized as a T2-weighted imaging contrast agent to elevate the difference in the signal intensities among the HCC tissues and surrounding normal liver tissue on MRI, enhancing the accuracy in the diagnosis of HCC [246]. Moreover, compared to the traditional gadolinium-based contrast agents, the gadolinium-based liposomes display high biocompatibility, while Gadolinium deposition can impair intracellular lysosomal function and mediate oxudative stress and fibrous deposition [247]. In line with this, an experiment invetistated the gadolinium-based liposome complexes containing 1, 2-dimyristoyl-sn‑glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (PE-DTPA) chelated with Gd+3 and the findings of RT-PCR demonstrated that such complexes do not cause upregulation of stress-related factors including EGR1, ATF3, GDF15 and FGF21. Moreover, such complexes did not affect the inflammatory factors and demonstrated desirable biocompatibility [248]. In CT, the diagnostic agents should be utilized for the diagnosis purposes. It has been shown that iodomelanol-containing liposomes could significantly improve the liver contrast and enhance the diagnostic rate up to 60 % higher than the pre-push control values when detecting liver tumors in rats [249].
Multimodal imaging
The purpose of multimodal imaging is to combine two or more imaging strategies and it usually integrates the anatomical information with high-resolution molecular-level biological information. The multimodal imaging enables to provide high spatial resolution, soft tissue contrast and increased sensitivity to molecular level biological information. Moreover, it can overcome to a number of challenges in invidial imaging strategies for the full description of the diseases and generates accurate images. For instance, PET/CT complements the low resolution of the PET and inactivity of CT to generate functional imaging [250]. The diagnosis has been improved through application of radiolabeled liposomes to control the distribution of drugs in vivo and real time, optimizing the therapeutic potetnail of liposomal nanostructures. The [111In]-liposome nanoarchitecture has been shown as a promising deliver system to encapsulate 18F-labeled carboplatin drug derivative ([18F]-FCP) as a biomolecular imaging tool. Moreover, 18F positron emission tomography (PET) and 111In single-photon emission CT (SPECT)-mediated dual imaging can be performed when both the nanopartiucle and drug are labelled. The PET/SPECT imaging is utilized to significantly increase the movement of encapsulated drugs in vivo [251]. The gold nanotod@liposome core-shell nanostructures have been developed for the delivery of ICG as a NIR fluorescent dye, but it has a number of problems and limitation for being used as a bimocal contrast agent such as aggregation, rapid clearance, flouresence burst and poor potential in transformation of laser energy into heat. Moreover, the gold nanorods have been promising as photoacoustic probes owing to their poor biocompatibility and easy exudation in tumor site. The gold nanotod@liposome-ICG-induced photoacoustic imaging increases photoacoustic tomography (PAT) signals that can beacheived for preoperative diagnosis and safe surgical resection of tumors [252].
Conclusion and remarks
Liposomes brought the fact that therapy is HCC is no longer a kind of dream for physicians around the world and therefore, if development of smart liposomes is followed and it is mixed with conventional therapies, there is much hope to improv prognosis and survival of patients and maybe in near future, completely cure the patients. The HCC patients are asymptomatic in early stages and bioimaging tools for diagnosis of biomarkers should be provided. However, when this disease is diagnosed, all the efforts and powers should be integrated in a way to treat patients and prevent another death in patients. When discussion of nanoparticles for HCC therapy is brought, there is high demand that which kind of nanostructure is preferred to another in treatment of HCC and the first answer to this question is that the nanostructure that has high biocompatibility and its future application in patients is not forbidden. Liposomes have been already used in clinical trials and even Pfizer vaccine uses liposomes for delivery of mRNA. Hence, long-term biocompatibility of liposomes has been confirmed, they are well-tolerated and their potential for delivery is high. Since there is hope introduction of liposomes in clinic for treatment of HCC patients, the current paper was dedicated in understanding potential of liposomes in HCC therapy. The first problem in HCC therapy is low bioavailability and therapeutic index of chemotherapy drugs that can be followed by drug resistance that due to delivery by liposomes, increased blood circulation time and targeted accumulation in cancer site, their cytotoxicity increases and chemoresistance is prevented. Furthermore, when liposomes encapsulate genes, they protect them against degradation and this is interesting for their increased efficiency in gene expression regulation and also, their internalization in HCC cells is accelerated. Due to potential of liposomes in delivery of photosensitizers, they can be employed for purpose of PDT and PTT that its combination with chemotherapy provides combination tumor suppression. Moreover, functionalization and surface modification of liposomes promote their anti-cancer activity against HCC cells and their smart ones including pH-, redox- and light-responsive can provide targeted suppression of HCC. One of the limitations of current studies is that they have focused on light-responsive liposomes with adding IR-780 or ICG, but other kinds of agents can also be used. The experiments have only shown response of liposomes to light for release of drug at cancer site, but they have not focused on the synergistic HCC therapy by phototherapy and chemotherapy through liposomes. Moreover, since entrapment efficiency of liposomes is high, other nanostructures such as gold nanoparticles or quantum dots can be loaded in them to absorb light for phototherapy of HCC that can be focus of future studies. Another limitation is low attention to development of smart liposomes in HCC therapy, especially pH- and redox-responsive liposomes.
Although studies provided significant findings and results regarding application of liposomal nanocarriers for the treatment of hepatocellular carcinoma, there are a number of limitations that should be addressed. Although the studies have shown the application of liposomes for the delivery of genes and drugs in cancer therapy, the co-deliveyr of drugs and genes for the synergistic tumor suppression has been ignored. Moreover, the natural products have been recently introduced for the treatment of HCC that their targeted delivery by liposomes can suppress HCC progression through increasing therapeutic index and pharmacokinetic profile of these compounds. Various kinds of stimuli-responsive liposomes have been introduced for the treatment of HCC. However, it is suggested to also load liposomes into hydrogels to provide prolonged release of liposomes for better cancer suppression. The surface-functionalized liposomes have been utilized for the HCC therapy, but the studies have focused on the delivery of traditional chemotherapy drugs, while natural products should also come into attention. The diagnosis of HCC is also of importance, since the biomarkers for the diagnosis of HCC have been developed and liposomes can be used for the non-invasive diagnosis of HCC. Moreover, CRISPR/Cas9 has been widely applied in the treatment of HCC, but the off-targeting feature is a problem that future studies can focus on the delivery of this genetic tool by liposomes in cancer therapy.
CRediT authorship contribution statement
Seyedeh Setareh Samaei: Writing – original draft, Conceptualization. Mahshid Daryab: Writing – original draft, Conceptualization. Sarah Gholami: Writing – original draft, Conceptualization. Aryan Rezaee: Writing – original draft, Conceptualization. Navid Fatehi: Writing – original draft, Investigation. Romina Roshannia: Investigation, Data curation. Saeed Hashemi: Investigation, Data curation. Nazanin Javani: Visualization, Investigation. Parham Rahmanian: Visualization, Investigation. Reza Amani-Beni: Resources, Investigation. Mohammad Arad Zandieh: Visualization. Noushin Nabavi: Writing – review & editing. Mohsen Rashidi: Writing – review & editing, Supervision. Neda Malgard: Supervision. Mehrdad Hashemi: Supervision. Afshin Taheriazam: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.101975.
Contributor Information
Mohsen Rashidi, Email: mrashidi@mazums.ac.ir.
Neda Malgard, Email: neda.malgard@gmail.com.
Mehrdad Hashemi, Email: mhashemi@iautmu.ac.ir.
Afshin Taheriazam, Email: a.taheriazam@iautmu.ac.ir.
Appendix. Supplementary materials
References
- 1.Allahou, L.W., S.Y. Madani, and A.J.I.j.o.b. Seifalian, Investigating the application of liposomes as drug delivery systems for the diagnosis and treatment of cancer. 2021. 2021. [DOI] [PMC free article] [PubMed]
- 2.Holban A.M., Grumezescu A.M. William Andrew; 2016. Nanoarchitectonics for smart delivery and drug targeting. [Google Scholar]
- 3.Yau A., Lee J., Chen Y. Nanomaterials for protein delivery in anticancer applications. Pharmaceutics. 2021;13(2) doi: 10.3390/pharmaceutics13020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W., et al. Cell membrane-camouflaged bufalin targets NOD2 and overcomes multidrug resistance in pancreatic cancer. Drug Resist. Updates. 2023;71 doi: 10.1016/j.drup.2023.101005. [DOI] [PubMed] [Google Scholar]
- 5.Li T., et al. Chitosan-functionalized bioplatforms and hydrogels in breast cancer: immunotherapy, phototherapy and clinical perspectives. Drug Discov. Today. 2024;29(1) doi: 10.1016/j.drudis.2023.103851. [DOI] [PubMed] [Google Scholar]
- 6.Li B., Ashrafizadeh M., Jiao T. Biomedical application of metal-organic frameworks (MOFs) in cancer therapy: stimuli-responsive and biomimetic nanocomposites in targeted delivery, phototherapy and diagnosis. Int. J. Biol. Macromol. 2024;260 doi: 10.1016/j.ijbiomac.2024.129391. [DOI] [PubMed] [Google Scholar]
- 7.Ashrafizadeh M., et al. (Nano)platforms in breast cancer therapy: drug/gene delivery, advanced nanocarriers and immunotherapy. Med. Res. Rev. 2023;43(6):2115–2176. doi: 10.1002/med.21971. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y., et al. Autophagy-driven regulation of cisplatin response in human cancers: exploring molecular and cell death dynamics. Cancer Lett. 2024;587 doi: 10.1016/j.canlet.2024.216659. [DOI] [PubMed] [Google Scholar]
- 9.Ashrafizadeh M., et al. Molecular panorama of therapy resistance in prostate cancer: a pre-clinical and bioinformatics analysis for clinical translation. Cancer Metastasis Rev. 2024 doi: 10.1007/s10555-024-10168-9. [DOI] [PubMed] [Google Scholar]
- 10.Qin Y., et al. Autophagy and cancer drug resistance in dialogue: pre-clinical and clinical evidence. Cancer Lett. 2023;570 doi: 10.1016/j.canlet.2023.216307. [DOI] [PubMed] [Google Scholar]
- 11.Hu C.M.J., Zhang L. Therapeutic nanoparticles to combat cancer drug resistance. Curr. Drug Metab. 2009;10(8):836–841. doi: 10.2174/138920009790274540. [DOI] [PubMed] [Google Scholar]
- 12.Yao Y., et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020;7:193. doi: 10.3389/fmolb.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torchilin, V.P.J.N.r.D.d., Recent advances with liposomes as pharmaceutical carriers. 2005. 4(2): p. 145–160. [DOI] [PubMed]
- 14.Symon, Z., et al., Selective delivery of doxorubicin to patients with breast carcinoma metastases by stealth liposomes. 1999. 86(1): p. 72–78. [PubMed]
- 15.Perez, A.T., et al., Pegylated liposomal doxorubicin (Doxil®) for metastatic breast cancer: the Cancer research network, inc., experience. 2002. 20(sup2): p. 22–29. [DOI] [PubMed]
- 16.O'Shaughnessy, J.A.J.C.b.c., Pegylated liposomal doxorubicin in the treatment of breast cancer. 2003. 4(5): p. 318–328. [DOI] [PubMed]
- 17.Schwonzen, M., C. Kurbacher, and P.J.A.C.D. Mallmann, Liposomal doxorubicin and weekly paclitaxel in the treatment of metastatic breast cancer. 2000. 11(9): p. 681–685. [DOI] [PubMed]
- 18.Goncalves, A., et al., Phase I study of pegylated liposomal doxorubicin (Caelyx) in combination with carboplatin in patients with advanced solid tumors. 2003. 23(4): p. 3543–3548. [PubMed]
- 19.Harrington, K., et al., Phase II study of pegylated liposomal doxorubicin (Caelyx™) as induction chemotherapy for patients with squamous cell cancer of the head and neck. 2001. 37(16): p. 2015–2022. [DOI] [PubMed]
- 20.Schmidinger, M., et al., Pilot study with pegylated liposomal doxorubicin for advanced or unresectable hepatocellular carcinoma. 2001. 85(12): p. 1850–1852. [DOI] [PMC free article] [PubMed]
- 21.Wollina, U., et al., Multicenter study of pegylated liposomal doxorubicin in patients with cutaneous T-cell lymphoma. 2003. 98(5): p. 993–1001. [DOI] [PubMed]
- 22.Skubitz, K.M.J.C.i., Phase II trial of pegylated-liposomal doxorubicin (Doxil™) In sarcoma* ORIGINAL ARTICLE. 2003. 21(2): p. 167–176. [DOI] [PubMed]
- 23.Seiden, M.V., et al., A phase II study of liposomal lurtotecan (OSI-211) in patients with topotecan resistant ovarian cancer. 2004. 93(1): p. 229–232. [DOI] [PubMed]
- 24.Peng, P., et al., Polysaccharide-modified liposomes and their application in cancer research. 2023. [DOI] [PubMed]
- 25.Samad, A., Y. Sultana, and M. Aqil, Liposomal drug delivery systems: an update review. Curr. Drug Deliv., 2007. 4(4): p. 297–305. [DOI] [PubMed]
- 26.Almeida, B., et al., Recent progress in bioconjugation strategies for liposome-mediated drug delivery. 2020. 25(23): p. 5672. [DOI] [PMC free article] [PubMed]
- 27.Aghdam, M.A., et al., Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release. 2019. 315: p. 1–22. [DOI] [PubMed]
- 28.Allen, T.M. and P.R.J.A.d.d.r. Cullis, Liposomal drug delivery systems: from concept to clinical applications. 2013. 65(1): p. 36–48. [DOI] [PubMed]
- 29.Kozhikhova, K.V., et al., Preparation of chitosan-coated liposomes as a novel carrier system for the antiviral drug Triazavirin. 2018. 23(4): p. 334–342. [DOI] [PubMed]
- 30.Tan, C., J. Wang, and B.J.B.A. Sun, Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. 2021. 48: p. 107727. [DOI] [PubMed]
- 31.Zahednezhad, F., et al., Liposome and immune system interplay: Challenges and potentials. 2019. 305: p. 194–209. [DOI] [PubMed]
- 32.Kakudo, T., et al., Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: an artificial viral-like delivery system. 2004. 43(19): p. 5618–5628. [DOI] [PubMed]
- 33.Kumar, S., et al., A systematic study on chitosan-liposome based systems for biomedical applications. 2020. 160: p. 470–481. [DOI] [PubMed]
- 34.Nsairat, H., et al., Liposomes: structure, composition, types, and clinical applications. 2022. 8(5): p. e09394. [DOI] [PMC free article] [PubMed]
- 35.Ong, S.G.M., et al., Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. 2016. 8(3): p. 25. [DOI] [PMC free article] [PubMed]
- 36.Wu X., et al. Investigation on drug entrapment location in liposomes and transfersomes based on molecular dynamics simulation. J. Mol. Model. 2021;27(4):111. doi: 10.1007/s00894-021-04722-3. [DOI] [PubMed] [Google Scholar]
- 37.Akbarzadeh A., et al. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakhaei P., et al. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.705886. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Pavelić Z., Skalko-Basnet N., Jalsenjak I. Characterisation and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitis. Int. J. Pharm. 2005;301(1–2):140–148. doi: 10.1016/j.ijpharm.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Sahoo S.K., Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today. 2003;8(24):1112–1120. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- 41.Calvagno M.G., et al. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes. Curr. Drug Deliv. 2007;4(1):89–101. doi: 10.2174/156720107779314749. [DOI] [PubMed] [Google Scholar]
- 42.Large D.E., et al. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113851. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Y., et al. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J. Adv. Res. 2023;49:159–173. doi: 10.1016/j.jare.2022.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou J. Site-specific delivery of cisplatin and paclitaxel mediated by liposomes: a promising approach in cancer chemotherapy. Environ. Res. 2023;238(Pt 1) doi: 10.1016/j.envres.2023.117111. [DOI] [PubMed] [Google Scholar]
- 45.Wang C., et al. Ultrasound-responsive low-dose doxorubicin liposomes trigger mitochondrial DNA release and activate cGAS-STING-mediated antitumour immunity. Nat. Commun. 2023;14(1):3877. doi: 10.1038/s41467-023-39607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng P., et al. Polysaccharide-modified liposomes and their application in Cancer research. Chem. Biol. Drug Des. 2023;101(4):998–1011. doi: 10.1111/cbdd.14201. [DOI] [PubMed] [Google Scholar]
- 47.Qu R., et al. Afterglow/Photothermal bifunctional polymeric nanoparticles for precise postbreast-conserving surgery adjuvant therapy and early recurrence theranostic. Nano Lett. 2023;23(10):4216–4225. doi: 10.1021/acs.nanolett.3c00191. [DOI] [PubMed] [Google Scholar]
- 48.Su W., et al. Targeted degradation of PD-L1 and activation of the STING pathway by Carbon-Dot-based PROTACs for Cancer immunotherapy. Angew. Chem. Int. Ed. Engl. 2023;62(11) doi: 10.1002/anie.202218128. [DOI] [PubMed] [Google Scholar]
- 49.Li X., et al. Ultrasmall graphene oxide for combination of enhanced chemotherapy and photothermal therapy of breast cancer. Colloids. Surf. B Biointerfaces. 2023;225 doi: 10.1016/j.colsurfb.2023.113288. [DOI] [PubMed] [Google Scholar]
- 50.Wang G., et al. Eudragit S100 prepared pH-responsive liposomes-loaded betulinic acid against colorectal cancer in vitro and in vivo. J. Liposome Res. 2022;32(3):250–264. doi: 10.1080/08982104.2021.1999974. [DOI] [PubMed] [Google Scholar]
- 51.Tang C., et al. Maleimide-functionalized liposomes: prolonged retention and enhanced efficacy of doxorubicin in breast cancer with low systemic toxicity. Molecules. 2022;(14):27. doi: 10.3390/molecules27144632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye X., et al. Enhanced anti-breast cancer efficacy of co-delivery liposomes of docetaxel and curcumin. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.969611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tefas L.R., et al. Co-delivery of gemcitabine and salinomycin in PEGylated liposomes for enhanced anticancer efficacy against colorectal cancer. J. Liposome Res. 2022:1–17. doi: 10.1080/08982104.2022.2153139. [DOI] [PubMed] [Google Scholar]
- 54.Chen T., et al. Co-delivery of 5-fluorouracil and paclitaxel in mitochondria-targeted KLA-modified liposomes to improve triple-negative breast Cancer treatment. Pharmaceuticals. (Basel) 2022;15(7) doi: 10.3390/ph15070881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li M., et al. Cationic liposomes co-deliver chemotherapeutics and siRNA for the treatment of breast cancer. Eur. J. Med. Chem. 2022;233 doi: 10.1016/j.ejmech.2022.114198. [DOI] [PubMed] [Google Scholar]
- 56.Ayoub A.M., et al. Photodynamic and antiangiogenic activities of parietin liposomes in triple negative breast cancer. Biomater. Adv. 2022;134 doi: 10.1016/j.msec.2021.112543. [DOI] [PubMed] [Google Scholar]
- 57.Pivetta T.P., et al. Liposomes encapsulating methylene blue and acridine orange: an approach for phototherapy of skin cancer. Colloids. Surf. B Biointerfaces. 2022;220 doi: 10.1016/j.colsurfb.2022.112901. [DOI] [PubMed] [Google Scholar]
- 58.Cai X., et al. Glutamine metabolism targeting liposomes for synergistic chemosensitization and starvation therapy in ovarian cancer. Acta Biomater. 2022 doi: 10.1016/j.actbio.2022.12.052. [DOI] [PubMed] [Google Scholar]
- 59.Liu X.M., et al. Rapamycin liposomes combined with 5-fluorouracil inhibits angiogenesis and tumor growth of APC ((Min/+)) Mice and AOM/DSS-induced colorectal Cancer Mice. Int. J. Nanomedicine. 2022;17:5049–5061. doi: 10.2147/IJN.S373777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang H., et al. Synergistic strategy with hyperthermia therapy based immunotherapy and engineered exosomes-liposomes targeted chemotherapy prevents tumor recurrence and metastasis in advanced breast cancer. Bioeng. Transl. Med. 2022;7(2):e10284. doi: 10.1002/btm2.10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song J.W., et al. Nano-liposomes double loaded with curcumin and Tetrandrine: preparation, characterization, hepatotoxicity and anti-tumor effects. Int. J. Mol. Sci. 2022;23(12) doi: 10.3390/ijms23126858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Llovet J.M., et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer. 2022;3(4):386–401. doi: 10.1038/s43018-022-00357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Llovet J.M., et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 64.Villanueva A. Hepatocellular carcinoma. N. Engl. J. Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 65.Schulze K., et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015;47(5):505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zucman-Rossi J., et al. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149(5):1226–1239.e4. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 67.Llovet J.M., et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018;15(10):599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PubMed] [Google Scholar]
- 68.Llovet J.M., et al. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022;19(3):151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 69.Rizzo A., Ricci A.D., Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Fut. Med. 2020:2587–2589. doi: 10.2217/fon-2020-0669. [DOI] [PubMed] [Google Scholar]
- 70.Rizzo A., Ricci A.D., Brandi G. Trans-arterial chemoembolization plus systemic treatments for hepatocellular carcinoma: an update. J. Pers. Med. 2022;12(11) doi: 10.3390/jpm12111788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ding X., et al. Genomic and epigenomic features of primary and recurrent hepatocellular carcinomas. Gastroenterology. 2019;157(6):1630–1645.e6. doi: 10.1053/j.gastro.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Sia D., et al. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152(4):745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 73.Xue R., et al. Variable intra-tumor genomic heterogeneity of multiple lesions in patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):998–1008. doi: 10.1053/j.gastro.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 74.Losic B., et al. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat. Commun. 2020;11(1):291. doi: 10.1038/s41467-019-14050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pfister D., et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cucarull B., et al. Hepatocellular carcinoma: molecular pathogenesis and therapeutic advances. Cancers. (Basel) 2022;14(3) doi: 10.3390/cancers14030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73 Suppl 1(Suppl 1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mittal S., El-Serag H.B. Epidemiology of hepatocellular carcinoma: consider the population. J. Clin. Gastroenterol. 2013;47 Suppl(0):S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Llovet J.M., et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 80.Arvanitakis K., et al. Tumor-associated macrophages in hepatocellular carcinoma pathogenesis, prognosis and therapy. Cancers. (Basel) 2022;14(1) doi: 10.3390/cancers14010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruix J., et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 82.Abou-Alfa G.K., et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruix J., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 84.Kudo M., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 85.Llovet J.M., et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 86.Parikh N.D., Singal A.G., Hutton D.W. Cost effectiveness of regorafenib as second-line therapy for patients with advanced hepatocellular carcinoma. Cancer. 2017;123(19):3725–3731. doi: 10.1002/cncr.30863. [DOI] [PubMed] [Google Scholar]
- 87.Santoni M., et al. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: the MOUSEION-01 study. Crit. Rev. Oncol. Hematol. 2022;170 doi: 10.1016/j.critrevonc.2022.103596. [DOI] [PubMed] [Google Scholar]
- 88.Mollica V., et al. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin. Exp. Med. 2023;23(8):5039–5049. doi: 10.1007/s10238-023-01159-1. [DOI] [PubMed] [Google Scholar]
- 89.Zhang D., et al. Mitochondrial TSPO promotes hepatocellular carcinoma progression through ferroptosis inhibition and immune evasion. Adv. Sci. (Weinh) 2023;10(15) doi: 10.1002/advs.202206669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen C., et al. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1133308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang F., et al. Oxaliplatin-resistant hepatocellular carcinoma drives immune evasion through PD-L1 Up-regulation and PMN-singular recruitment. Cell Mol. Gastroenterol. Hepatol. 2023;15(3):573–591. doi: 10.1016/j.jcmgh.2022.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao R., et al. Robust radiosensitization of hemoglobin-curcumin nanoparticles suppresses hypoxic hepatocellular carcinoma. J. Nanobiotechnology. 2022;20(1):115. doi: 10.1186/s12951-022-01316-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fu C., et al. Galactosed and reduction-responsive nanoparticles assembled from trimethylchitosan-camptothecin conjugates for enhanced hepatocellular carcinoma therapy. Pharmaceutics. 2022;14(7) doi: 10.3390/pharmaceutics14071315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y., et al. Repeated trans-arterial treatments of LDL-DHA nanoparticles induce multiple pathways of tumor cell death in hepatocellular carcinoma bearing rats. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1052221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song C., et al. Improved anti-hepatocellular carcinoma effect by enhanced Co-delivery of Tim-3 siRNA and sorafenib via multiple pH triggered drug-eluting nanoparticles. Mater. Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao X., et al. Targeting delivery of synergistic dual drugs with elastic PEG-modified multi-functional nanoparticles for hepatocellular carcinoma therapy. Int. J. Pharm. 2022;616 doi: 10.1016/j.ijpharm.2022.121567. [DOI] [PubMed] [Google Scholar]
- 97.Ding J., et al. Facile synthesis of NaYF4:yb up-conversion nanoparticles modified with photosensitizer and targeting antibody for in vitro photodynamic therapy of hepatocellular carcinoma. J. Healthc. Eng. 2022;2022 doi: 10.1155/2022/4470510. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Geinguenaud F., et al. Iron oxide nanoparticles functionalized with Fucoidan: a potential Theranostic nanotool for hepatocellular carcinoma. Chembiochem. 2022;23(16) doi: 10.1002/cbic.202200265. [DOI] [PubMed] [Google Scholar]
- 99.Kumar P., et al. Mechanistic exploration of the activities of poly(lactic-co-glycolic acid)-loaded nanoparticles of betulinic acid against hepatocellular carcinoma at cellular and molecular levels. Arch. Physiol. Biochem. 2022;128(3):836–848. doi: 10.1080/13813455.2020.1733024. [DOI] [PubMed] [Google Scholar]
- 100.Li P., et al. circMRPS35 promotes malignant progression and cisplatin resistance in hepatocellular carcinoma. Mol. Ther. 2022;30(1):431–447. doi: 10.1016/j.ymthe.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li X., et al. Mitochondrial fission factor promotes cisplatin resistancein hepatocellular carcinoma. Acta Biochim. Biophys. Sin. (Shanghai) 2022;54(3):301–310. doi: 10.3724/abbs.2022007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu X.F., et al. Dysregulation of non-coding RNAs mediates cisplatin resistance in hepatocellular carcinoma and therapeutic strategies. Pharmacol. Res. 2022;176 doi: 10.1016/j.phrs.2021.105906. [DOI] [PubMed] [Google Scholar]
- 103.Jegannathan S.D., Arul S., Dayalan H. Zerumbone sensitizes the anti-cancer efficacy of cisplatin in hepatocellular Carcinoma cells. AntiCancer Agents Med. Chem. 2022;22(16):2885–2895. doi: 10.2174/1871520622666220324090801. [DOI] [PubMed] [Google Scholar]
- 104.Cheng Y., et al. Cisplatin and curcumin co-loaded nano-liposomes for the treatment of hepatocellular carcinoma. Int. J. Pharm. 2018;545(1–2):261–273. doi: 10.1016/j.ijpharm.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y., et al. Curcumin-loaded liposomes with the hepatic and lysosomal dual-targeted effects for therapy of hepatocellular carcinoma. Int. J. Pharm. 2021;602 doi: 10.1016/j.ijpharm.2021.120628. [DOI] [PubMed] [Google Scholar]
- 106.Luo Y., et al. Berberine prevents non-alcoholic steatohepatitis-derived hepatocellular carcinoma by inhibiting inflammation and angiogenesis in mice. Am. J. Transl. Res. 2019;11(5):2668–2682. [PMC free article] [PubMed] [Google Scholar]
- 107.Chen J., et al. Berberine upregulates miR-22-3p to suppress hepatocellular carcinoma cell proliferation by targeting Sp1. Am. J. Transl. Res. 2016;8(11):4932–4941. [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang F., et al. Janus nanocarrier-based co-delivery of doxorubicin and berberine weakens chemotherapy-exacerbated hepatocellular carcinoma recurrence. Acta Biomater. 2019;100:352–364. doi: 10.1016/j.actbio.2019.09.034. [DOI] [PubMed] [Google Scholar]
- 109.Wang Z., et al. Berberine-loaded Janus nanocarriers for magnetic field-enhanced therapy against hepatocellular carcinoma. Chem. Biol. Drug Des. 2017;89(3):464–469. doi: 10.1111/cbdd.12866. [DOI] [PubMed] [Google Scholar]
- 110.Wu J., et al. Curcumin and berberine co-loaded liposomes for anti-hepatocellular carcinoma therapy by blocking the cross-talk between hepatic stellate cells and tumor cells. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.961788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gao F., et al. Resveratrol suppresses human hepatocellular carcinoma via targeting HGF-c-Met signaling pathway. Oncol. Rep. 2017;37(2):1203–1211. doi: 10.3892/or.2017.5347. [DOI] [PubMed] [Google Scholar]
- 112.Dai H., et al. Resveratrol inhibits the malignant progression of hepatocellular carcinoma via MARCH1-induced regulation of PTEN/AKT signaling. Aging (Albany. NY) 2020;12(12):11717–11731. doi: 10.18632/aging.103338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Q., et al. Resveratrol exerts antitumor effects by downregulating CD8(+)CD122(+) Tregs in murine hepatocellular carcinoma. Oncoimmunology. 2020;9(1) doi: 10.1080/2162402X.2020.1829346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jagwani S., et al. Pharmacokinetic and pharmacodynamic evaluation of resveratrol loaded cationic liposomes for targeting hepatocellular carcinoma. ACS. Biomater. Sci. Eng. 2020;6(9):4969–4984. doi: 10.1021/acsbiomaterials.0c00429. [DOI] [PubMed] [Google Scholar]
- 115.Lin H., et al. Disulfiram enhances chemotherapeutic effects of doxorubicin liposomes against human hepatocellular carcinoma via activating ROS-induced cell stress response pathways. Cancer Chemother Pharmacol. 2022;90(6):455–465. doi: 10.1007/s00280-022-04481-9. [DOI] [PubMed] [Google Scholar]
- 116.Yu Z., et al. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS. Nano. 2020;14(4):4816–4828. doi: 10.1021/acsnano.0c00708. [DOI] [PubMed] [Google Scholar]
- 117.Ma Z., et al. Lgr5-mediated p53 Repression through PDCD5 leads to doxorubicin resistance in Hepatocellular Carcinoma. Theranostics. 2019;9(10):2967–2983. doi: 10.7150/thno.30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cao Y., et al. LncRNA MALAT1 mediates doxorubicin resistance of hepatocellular carcinoma by regulating miR-3129-5p/Nova1 axis. Mol. Cell Biochem. 2021;476(1):279–292. doi: 10.1007/s11010-020-03904-6. [DOI] [PubMed] [Google Scholar]
- 119.Zhu F., et al. Effectiveness of localized ultrasound-targeted microbubble destruction with doxorubicin liposomes in H22 mouse hepatocellular carcinoma model. J. Drug Target. 2015;23(4):323–334. doi: 10.3109/1061186X.2014.996759. [DOI] [PubMed] [Google Scholar]
- 120.Zhou X., et al. Lactosylated liposomes for targeted delivery of doxorubicin to hepatocellular carcinoma. Int. J. Nanomedicine. 2012;7:5465–5474. doi: 10.2147/IJN.S33965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wei M., et al. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int. J. Nanomedicine. 2015;10:5123–5137. doi: 10.2147/IJN.S87011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luo Y., et al. Effect of a controlled-release drug delivery system made of oleanolic acid formulated into multivesicular liposomes on hepatocellular carcinoma in vitro and in vivo. Int. J. Nanomedicine. 2016;11:3111–3129. doi: 10.2147/IJN.S108445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lv Y.F., et al. Gene delivery to breast cancer by incorporated EpCAM targeted DARPins into AAV2. BMC. Cancer. 2023;23(1):1220. doi: 10.1186/s12885-023-11705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu M., et al. A dual-functional buformin-mimicking poly(amido amine) for efficient and safe gene delivery. J. Drug Target. 2020;28(9):923–932. doi: 10.1080/1061186X.2020.1729770. [DOI] [PubMed] [Google Scholar]
- 125.Zhao J., et al. Calcium phosphate nanoneedle based gene delivery system for cancer genetic immunotherapy. Biomaterials. 2020;250 doi: 10.1016/j.biomaterials.2020.120072. [DOI] [PubMed] [Google Scholar]
- 126.Chen G., et al. Engineering bifunctional calcium alendronate gene-delivery nanoneedle for synergistic chemo/immuno-therapy against HER2 positive ovarian Cancer. Adv. Sci. (Weinh) 2023;10(14) doi: 10.1002/advs.202204654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mekuria S.L., et al. Facile formation of PAMAM Dendrimer nanoclusters for enhanced gene delivery and cancer gene therapy. ACS. Appl. Bio Mater. 2021;4(9):7168–7175. doi: 10.1021/acsabm.1c00743. [DOI] [PubMed] [Google Scholar]
- 128.Shao L., et al. Enhancing anti-tumor efficacy and immune memory by combining 3p-GPC-3 siRNA treatment with PD-1 blockade in hepatocellular carcinoma. Oncoimmunology. 2022;11(1) doi: 10.1080/2162402X.2021.2010894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Duan X., et al. Nrf2-siRNA Enhanced the anti-tumor effects of As(2)O(3) in 5-fluorouracil-resistant hepatocellular carcinoma by inhibiting HIF-1α/HSP70 signaling. J. HepatoCell Carcinoma. 2022;9:1341–1352. doi: 10.2147/JHC.S388077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou X., et al. Tumour-derived extracellular vesicle membrane hybrid lipid nanovesicles enhance siRNA delivery by tumour-homing and intracellular freeway transportation. J. ExtraCell Vesicles. 2022;11(3):e12198. doi: 10.1002/jev2.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xia Y., et al. siRNA-loaded selenium nanoparticle modified with hyaluronic acid for enhanced hepatocellular carcinoma therapy. Int. J. Nanomed. 2018;13:1539–1552. doi: 10.2147/IJN.S157519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu J., et al. Enhanced penetrative siRNA delivery by a nanodiamond drug delivery platform against hepatocellular carcinoma 3D models. Nanoscale. 2021;13(38):16131–16145. doi: 10.1039/d1nr03502a. [DOI] [PubMed] [Google Scholar]
- 133.Xia Y., et al. Folate-targeted selenium nanoparticles deliver therapeutic siRNA to improve hepatocellular carcinoma therapy. RSC. Adv. 2018;8(46):25932–25940. doi: 10.1039/c8ra04204g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yao Y., et al. Co-delivery of sorafenib and VEGF-siRNA via pH-sensitive liposomes for the synergistic treatment of hepatocellular carcinoma. Artif. Cells Nanomed. Biotechnol. 2019;47(1):1374–1383. doi: 10.1080/21691401.2019.1596943. [DOI] [PubMed] [Google Scholar]
- 135.Qin Y., et al. ZEB1 promotes tumorigenesis and metastasis in hepatocellular carcinoma by regulating the expression of vimentin. Mol. Med. Rep. 2019;19(3):2297–2306. doi: 10.3892/mmr.2019.9866. [DOI] [PubMed] [Google Scholar]
- 136.Huang X., et al. CMTM6 promotes migration, invasion, and EMT by interacting with and stabilizing vimentin in hepatocellular carcinoma cells. J. Transl. Med. 2021;19(1):120. doi: 10.1186/s12967-021-02787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dong Q., et al. Osteopontin promotes epithelial-mesenchymal transition of hepatocellular carcinoma through regulating vimentin. Oncotarget. 2016;7(11):12997–13012. doi: 10.18632/oncotarget.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oh H.R., et al. Galactosylated liposomes for targeted co-delivery of doxorubicin/vimentin siRNA to hepatocellular carcinoma. Nanomaterials. (Basel) 2016;6(8) doi: 10.3390/nano6080141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun W., et al. Codelivery of sorafenib and GPC3 siRNA with PEI-modified liposomes for hepatoma therapy. Biomater. Sci. 2017;5(12):2468–2479. doi: 10.1039/c7bm00866j. [DOI] [PubMed] [Google Scholar]
- 140.Wei X., et al. PIGU promotes hepatocellular carcinoma progression through activating NF-κB pathway and increasing immune escape. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118476. [DOI] [PubMed] [Google Scholar]
- 141.Wang W.S., et al. DNA damage and NF-κB inactivation implicate glycyrrhizic acid-induced G(1) phase arrest in hepatocellular carcinoma cells. J. Food Biochem. 2022;46(7):e14128. doi: 10.1111/jfbc.14128. [DOI] [PubMed] [Google Scholar]
- 142.Wu M.Y., et al. The therapeutic role of PNU-74654 in Hepatocellular carcinoma may involve suppression of NF-κB signaling. Medicina (Kaunas) 2022;58(6) doi: 10.3390/medicina58060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cho H.A., et al. Suppression of hepatitis B virus-derived human hepatocellular carcinoma by NF-kappaB-inducing kinase-specific siRNA using liver-targeting liposomes. Arch. Pharm. Res. 2009;32(7):1077–1086. doi: 10.1007/s12272-009-1714-z. [DOI] [PubMed] [Google Scholar]
- 144.Zhang D., et al. shRNA-mediated silencing of Gli2 gene inhibits proliferation and sensitizes human hepatocellular carcinoma cells towards TRAIL-induced apoptosis. J. Cell Biochem. 2011;112(11):3140–3150. doi: 10.1002/jcb.23240. [DOI] [PubMed] [Google Scholar]
- 145.Yan C., et al. LncRNA HULC shRNA disinhibits miR-377-5p to suppress the growth and invasion of hepatocellular carcinoma in vitro and hepatocarcinogenesis in vivo. Ann. Transl. Med. 2020;8(20):1294. doi: 10.21037/atm-20-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu S., et al. Self-activated cascade-responsive sorafenib and USP22 shRNA Co-delivery system for synergetic hepatocellular carcinoma therapy. Adv. Sci. (Weinh) 2021;8(5) doi: 10.1002/advs.202003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen X., et al. Stat3 shRNA delivery with folate receptor-modified multi-functionalized graphene oxide particles for combined infrared radiation and gene therapy in hepatocellular carcinoma. Anticancer Drugs. 2022 doi: 10.1097/CAD.0000000000001461. [DOI] [PubMed] [Google Scholar]
- 148.Li W., et al. Co-delivery of thioredoxin 1 shRNA and doxorubicin by folate-targeted gemini surfactant-based cationic liposomes to sensitize hepatocellular carcinoma cells. J. Mater. Chem. B. 2014;2(30):4901–4910. doi: 10.1039/c4tb00502c. [DOI] [PubMed] [Google Scholar]
- 149.Wei Y., et al. miR-3154 promotes hepatocellular carcinoma progression via suppressing HNF4α. Carcinogenesis. 2022;43(10):1002–1014. doi: 10.1093/carcin/bgac067. [DOI] [PubMed] [Google Scholar]
- 150.Chen M., et al. miR-6071 inhibits hepatocellular carcinoma progression via targeting PTPN11. Arch. Biochem. Biophys. 2022;727 doi: 10.1016/j.abb.2022.109345. [DOI] [PubMed] [Google Scholar]
- 151.Liu L., et al. CircGPR137B/miR-4739/FTO feedback loop suppresses tumorigenesis and metastasis of hepatocellular carcinoma. Mol. Cancer. 2022;21(1):149. doi: 10.1186/s12943-022-01619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang L., et al. CircLIFR suppresses hepatocellular carcinoma progression by sponging miR-624-5p and inactivating the GSK-3β/β-catenin signaling pathway. Cell Death. Dis. 2022;13(5):464. doi: 10.1038/s41419-022-04887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fan Y.P., et al. MiR-375 and doxorubicin co-delivered by liposomes for combination therapy of hepatocellular carcinoma. Mol. Ther. Nucleic. Acids. 2017;7:181–189. doi: 10.1016/j.omtn.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li H., et al. pH-Sensitive pullulan-DOX conjugate nanoparticles for co-loading PDTC to suppress growth and chemoresistance of hepatocellular carcinoma. J. Mater. Chem. B. 2015;3(41):8070–8078. doi: 10.1039/c5tb01210d. [DOI] [PubMed] [Google Scholar]
- 155.Sun W., et al. miR-101-3p sensitizes hepatocellular carcinoma cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int. J. Clin. Exp. Pathol. 2019;12(6):2056–2065. [PMC free article] [PubMed] [Google Scholar]
- 156.Xu Y., et al. miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 2013;29(5):2019–2024. doi: 10.3892/or.2013.2338. [DOI] [PubMed] [Google Scholar]
- 157.Chang L., et al. Upregulation of SNHG6 regulates ZEB1 expression by competitively binding miR-101-3p and interacting with UPF1 in hepatocellular carcinoma. Cancer Lett. 2016;383(2):183–194. doi: 10.1016/j.canlet.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 158.Xu F., et al. MiR-101 and doxorubicin codelivered by liposomes suppressing malignant properties of hepatocellular carcinoma. Cancer Med. 2017;6(3):651–661. doi: 10.1002/cam4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xie A., et al. Stimuli-responsive prodrug-based cancer nanomedicine. EBioMedicine. 2020;56 doi: 10.1016/j.ebiom.2020.102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chang D., et al. Stimuli-responsive polymeric nanoplatforms for cancer therapy. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.707319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Song Y., Ding Y., Dong C.M. Stimuli-responsive polypeptide nanoassemblies: recent progress and applications in cancer nanomedicine. Wiley. Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022;14(2):e1742. doi: 10.1002/wnan.1742. [DOI] [PubMed] [Google Scholar]
- 162.Li C., et al. Stimuli-responsive gold nanocages for cancer diagnosis and treatment. Pharmaceutics. 2022;14(7) doi: 10.3390/pharmaceutics14071321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jain A., Jain S.K. Stimuli-responsive smart liposomes in cancer targeting. Curr. Drug Targets. 2018;19(3):259–270. doi: 10.2174/1389450117666160208144143. [DOI] [PubMed] [Google Scholar]
- 164.Wei D., et al. Stimuli-responsive polymer-based nanosystems for cancer theranostics. ACS. Nano. 2023;17(23):23223–23261. doi: 10.1021/acsnano.3c06019. [DOI] [PubMed] [Google Scholar]
- 165.Zhang J., et al. pH-sensitive tumor-tropism hybrid membrane-coated nanoparticles for reprogramming the tumor microenvironment and boosting the antitumor immunity. Acta Biomater. 2023;166:470–484. doi: 10.1016/j.actbio.2023.05.040. [DOI] [PubMed] [Google Scholar]
- 166.Ding Y., et al. Construction of ph-sensitive nanovaccines encapsulating tumor cell lysates and immune adjuvants for breast cancer therapy. Small. 2023;19(37) doi: 10.1002/smll.202301420. [DOI] [PubMed] [Google Scholar]
- 167.Chen B., et al. B7H3 targeting gold nanocage pH-sensitive conjugates for precise and synergistic chemo-photothermal therapy against NSCLC. J. Nanobiotechnol.. 2023;21(1):378. doi: 10.1186/s12951-023-02078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hu X., et al. Galactose-modified ph-sensitive niosomes for controlled release and hepatocellular carcinoma target delivery of Tanshinone IIA. AAPS. PharmSciTech. 2021;22(3):96. doi: 10.1208/s12249-021-01973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ling D., et al. pH-sensitive nanoformulated triptolide as a targeted therapeutic strategy for hepatocellular carcinoma. ACS. Nano. 2014;8(8):8027–8039. doi: 10.1021/nn502074x. [DOI] [PubMed] [Google Scholar]
- 170.Xia H., et al. pH-sensitive pt nanocluster assembly overcomes cisplatin resistance and heterogeneous stemness of hepatocellular carcinoma. ACS. Cent. Sci. 2016;2(11):802–811. doi: 10.1021/acscentsci.6b00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Y., et al. pH-sensitive pullulan-based nanoparticle carrier of methotrexate and combretastatin A4 for the combination therapy against hepatocellular carcinoma. Biomaterials. 2013;34(29):7181–7190. doi: 10.1016/j.biomaterials.2013.05.081. [DOI] [PubMed] [Google Scholar]
- 172.Zou Y., et al. Galactose-installed photo-crosslinked pH-sensitive degradable micelles for active targeting chemotherapy of hepatocellular carcinoma in mice. J. Control Release. 2014;193:154–161. doi: 10.1016/j.jconrel.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 173.Zhao R., et al. Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials. 2017;143:1–16. doi: 10.1016/j.biomaterials.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 174.Wang L., et al. pH-Responsive liposomes loaded with targeting procoagulant proteins as potential embolic agents for solid tumor-targeted therapy. Mol. Pharm. 2022;19(5):1356–1367. doi: 10.1021/acs.molpharmaceut.1c00912. [DOI] [PubMed] [Google Scholar]
- 175.van Zandwijk N. N-acetylcysteine (NAC) and glutathione (GSH): antioxidant and chemopreventive properties, with special reference to lung cancer. J. Cell Biochem. Suppl. 1995;22:24–32. doi: 10.1002/jcb.240590805. [DOI] [PubMed] [Google Scholar]
- 176.Xu L., et al. Redox-responsive polymeric nanoparticle for nucleic acid delivery and cancer therapy: progress, opportunities, and challenges. Macromol. Biosci. 2023 doi: 10.1002/mabi.202300238. [DOI] [PubMed] [Google Scholar]
- 177.Xu X., et al. Redox-responsive nanoparticle-mediated systemic RNAi for effective Cancer therapy. Small. 2018;14(41) doi: 10.1002/smll.201802565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen H., et al. Redox responsive nanoparticle encapsulating black phosphorus quantum dots for cancer theranostics. Bioact. Mater. 2021;6(3):655–665. doi: 10.1016/j.bioactmat.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu Z., et al. Redox chemistry-enabled stepwise surface dual nanoparticle engineering of 2D MXenes for tumor-sensitive T(1) and T(2) MRI-guided photonic breast-cancer hyperthermia in the NIR-II biowindow. Biomater. Sci. 2022;10(6):1562–1574. doi: 10.1039/d1bm01957k. [DOI] [PubMed] [Google Scholar]
- 180.Wu J., et al. Hydrophobic cysteine poly(disulfide)-based redox-hypersensitive nanoparticle platform for cancer theranostics. Angew. Chem. Int. Ed. Engl. 2015;54(32):9218–9223. doi: 10.1002/anie.201503863. [DOI] [PubMed] [Google Scholar]
- 181.Fang X.B., et al. A redox-sensitive and RAGE-targeting nanocarrier for hepatocellular carcinoma therapy. Mol. Pharm. 2016;13(11):3613–3625. doi: 10.1021/acs.molpharmaceut.6b00116. [DOI] [PubMed] [Google Scholar]
- 182.Xu B., et al. A multifunctional nanoparticle constructed with a detachable albumin outer shell and a redox-sensitive inner core for efficient siRNA delivery to hepatocellular carcinoma cells. J. Drug Target. 2018;26(10):941–954. doi: 10.1080/1061186X.2018.1455840. [DOI] [PubMed] [Google Scholar]
- 183.Hu Q., et al. A redox-sensitive, oligopeptide-guided, self-assembling, and efficiency-enhanced (ROSE) system for functional delivery of microRNA therapeutics for treatment of hepatocellular carcinoma. Biomaterials. 2016;104:192–200. doi: 10.1016/j.biomaterials.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 184.Yang H., et al. Redox-responsive nanoparticles from disulfide bond-linked poly-(N-ε-carbobenzyloxy-l-lysine)-grafted hyaluronan copolymers as theranostic nanoparticles for tumor-targeted MRI and chemotherapy. Int. J. Biol. Macromol. 2020;148:483–492. doi: 10.1016/j.ijbiomac.2020.01.071. [DOI] [PubMed] [Google Scholar]
- 185.Chen F., et al. Tumor pH(e)-triggered charge-reversal and redox-responsive nanoparticles for docetaxel delivery in hepatocellular carcinoma treatment. Nanoscale. 2015;7(38):15763–15779. doi: 10.1039/c5nr04612b. [DOI] [PubMed] [Google Scholar]
- 186.Wang Z., et al. A novel CD133- and EpCAM-Targeted liposome with redox-responsive properties capable of synergistically eliminating liver cancer stem cells. Front. Chem. 2020;8:649. doi: 10.3389/fchem.2020.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sadeghi M.S., et al. Graphene oxide nanoarchitectures in cancer therapy: drug and gene delivery, phototherapy, immunotherapy, and vaccine development. Environ. Res. 2023;237(Pt 2) doi: 10.1016/j.envres.2023.117027. [DOI] [PubMed] [Google Scholar]
- 188.Saadh M.J., et al. The bioengineered and multifunctional nanoparticles in pancreatic cancer therapy: bioresponisive nanostructures, phototherapy and targeted drug delivery. Environ. Res. 2023;233 doi: 10.1016/j.envres.2023.116490. [DOI] [PubMed] [Google Scholar]
- 189.Li S., et al. Cell membrane-based biomimetic technology for cancer phototherapy: mechanisms, recent advances and perspectives. Acta Biomater. 2024;174:26–48. doi: 10.1016/j.actbio.2023.11.029. [DOI] [PubMed] [Google Scholar]
- 190.Lu H., et al. Cancer phototherapy with nano-bacteria biohybrids. J. Control Release. 2023;360:133–148. doi: 10.1016/j.jconrel.2023.06.009. [DOI] [PubMed] [Google Scholar]
- 191.Zhou J., et al. Gas-assisted phototherapy for cancer treatment. J. Control Release. 2023;360:564–577. doi: 10.1016/j.jconrel.2023.07.015. [DOI] [PubMed] [Google Scholar]
- 192.Chen L., et al. Graphene quantum dots mediated magnetic chitosan drug delivery nanosystems for targeting synergistic photothermal-chemotherapy of hepatocellular carcinoma. Cancer Biol. Ther. 2022;23(1):281–293. doi: 10.1080/15384047.2022.2054249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu L., et al. Doxorubicin-loaded UiO-66/Bi(2)S(3) nanocomposite-enhanced synergistic transarterial chemoembolization and photothermal therapy against hepatocellular carcinoma. ACS. Appl. Mater. Interfaces. 2022;14(6):7579–7591. doi: 10.1021/acsami.1c19121. [DOI] [PubMed] [Google Scholar]
- 194.Lu Y.F., et al. Ultra-thin layered double hydroxide-mediated photothermal therapy combine with asynchronous blockade of PD-L1 and NR2F6 inhibit hepatocellular carcinoma. J. Nanobiotechnology. 2022;20(1):351. doi: 10.1186/s12951-022-01565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jia C., et al. Black phosphorus-Au-thiosugar nanosheets mediated photothermal induced anti-tumor effect enhancement by promoting infiltration of NK cells in hepatocellular carcinoma. J. Nanobiotechnology. 2022;20(1):90. doi: 10.1186/s12951-022-01286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Du Z., et al. TPGS-galactose-modified Polydopamine co-delivery nanoparticles of nitric oxide donor and doxorubicin for targeted chemo-Photothermal therapy against drug-resistant hepatocellular carcinoma. ACS. Appl. Mater. Interfaces. 2021;13(30):35518–35532. doi: 10.1021/acsami.1c09610. [DOI] [PubMed] [Google Scholar]
- 197.Xu J., et al. Chemo-photodynamic therapy with light-triggered disassembly of Theranostic Nanoplatform in combination with checkpoint blockade for immunotherapy of hepatocellular carcinoma. J. Nanobiotechnology. 2021;19(1):355. doi: 10.1186/s12951-021-01101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shang Y., et al. Fluorescent imaging-guided chemo- and photodynamic therapy of hepatocellular carcinoma with HCPT@NMOFs-RGD nanocomposites. Int. J. Nanomedicine. 2022;17:1381–1395. doi: 10.2147/IJN.S353803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Qi S., et al. Targeted multifunctional nanoplatform for imaging-guided precision diagnosis and photothermal/photodynamic therapy of orthotopic hepatocellular carcinoma. Int. J. Nanomedicine. 2022;17:3777–3792. doi: 10.2147/IJN.S377080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hu J., et al. Bimodal treatment of hepatocellular carcinoma by targeted minimally interventional photodynamic/chemotherapy using glyco-covalent-organic frameworks-guided porphyrin/sorafenib. Acta Biomater. 2022;148:206–217. doi: 10.1016/j.actbio.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 201.Abuduwaili W., et al. Iridium complex-loaded sorafenib nanocomposites for synergistic chemo-photodynamic therapy of hepatocellular carcinoma. ACS. Appl. Mater. Interfaces. 2022;14(33):37356–37368. doi: 10.1021/acsami.2c07247. [DOI] [PubMed] [Google Scholar]
- 202.He Q., et al. Sorafenib and indocyanine green co-loaded in photothermally sensitive liposomes for diagnosis and treatment of advanced hepatocellular carcinoma. J. Mater. Chem. B. 2018;6(36):5823–5834. doi: 10.1039/c8tb01641k. [DOI] [PubMed] [Google Scholar]
- 203.Peng Y., et al. Near-infrared light laser-triggered release of doxorubicin and sorafenib from temperaturesensitive liposomes for synergistic therapy of hepatocellular carcinoma. J. Biomed. Nanotechnol. 2020;16(9):1381–1393. doi: 10.1166/jbn.2020.2975. [DOI] [PubMed] [Google Scholar]
- 204.Bansal D., et al. Lactobionic acid coupled liposomes: an innovative strategy for targeting hepatocellular carcinoma. Drug Deliv. 2016;23(1):140–146. doi: 10.3109/10717544.2014.907373. [DOI] [PubMed] [Google Scholar]
- 205.Schwartz A.L., et al. Identification and quantification of the rat hepatocyte asialoglycoprotein receptor. Proceed. Nat. Acad. Sci. 1981;78(6):3348–3352. doi: 10.1073/pnas.78.6.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Weigel P.H. Characterization of the asialoglycoprotein receptor on isolated rat hepatocytes. J. Biolog. Chem. 1980;255(13):6111–6120. [PubMed] [Google Scholar]
- 207.Trere D., et al. The asialoglycoprotein receptor in human hepatocellular carcinomas: its expression on proliferating cells. Br. J. Cancer. 1999;81(3):404–408. doi: 10.1038/sj.bjc.6690708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Franssen E.J., et al. Hepatic and intrahepatic targeting of an anti-inflammatory agent with human serum albumin and neoglycoproteins as carrier molecules. Biochem. Pharmacol. 1993;45(6):1215–1226. doi: 10.1016/0006-2952(93)90273-y. [DOI] [PubMed] [Google Scholar]
- 209.Rensen P.C., et al. Recombinant lipoproteins: lipoprotein-like lipid particles for drug targeting. Adv. Drug Deliv. Rev. 2001;47(2–3):251–276. doi: 10.1016/s0169-409x(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 210.Shah S.M., et al. Liposomes for targeting hepatocellular carcinoma: use of conjugated arabinogalactan as targeting ligand. Int. J. Pharm. 2014;477(1–2):128–139. doi: 10.1016/j.ijpharm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 211.Pathak P., et al. Design of cholesterol arabinogalactan anchored liposomes for asialoglycoprotein receptor mediated targeting to hepatocellular carcinoma: in silico modeling, in vitro and in vivo evaluation. Int. J. Pharm. 2016;509(1–2):149–158. doi: 10.1016/j.ijpharm.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 212.Kuo Y.C., Lee Y.J., Rajesh R. Enhanced activity of AZD5582 and SM-164 in rabies virus glycoprotein-lactoferrin-liposomes to downregulate inhibitors of apoptosis proteins in glioblastoma. Biomater. Adv. 2022;133 doi: 10.1016/j.msec.2021.112615. [DOI] [PubMed] [Google Scholar]
- 213.Qi N., et al. Combined integrin α(v)β(3) and lactoferrin receptor targeted docetaxel liposomes enhance the brain targeting effect and anti-glioma effect. J. Nanobiotechnol. 2021;19(1):446. doi: 10.1186/s12951-021-01180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wei M., et al. Hepatocellular carcinoma targeting effect of PEGylated liposomes modified with lactoferrin. Eur. J. Pharm. Sci. 2012;46(3):131–141. doi: 10.1016/j.ejps.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 215.Gopal V., et al. Targeted liposomes to deliver DNA to cells expressing 5-HT receptors. Int. J. Pharm. 2011;419(1–2):347–354. doi: 10.1016/j.ijpharm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 216.Gomes E.R., et al. Fusion of tumor-derived exosomes with long-circulating and ph-sensitive liposomes loaded with doxorubicin for the treatment of breast Cancer. AAPS. PharmSciTech. 2022;23(7):255. doi: 10.1208/s12249-022-02349-y. [DOI] [PubMed] [Google Scholar]
- 217.Li Y., et al. GA&HA-modified liposomes for Co-delivery of aprepitant and curcumin to inhibit drug-resistance and metastasis of hepatocellular carcinoma. Int. J. Nanomedicine. 2022;17:2559–2575. doi: 10.2147/IJN.S366180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liu M.C., et al. Folate receptor-targeted liposomes loaded with a diacid metabolite of norcantharidin enhance antitumor potency for H22 hepatocellular carcinoma both in vitro and in vivo. Int. J. Nanomedicine. 2016;11:1395–1412. doi: 10.2147/IJN.S96862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang X., et al. Dual-functional liposomes with carbonic anhydrase IX antibody and BR2 peptide modification effectively improve intracellular delivery of cantharidin to treat orthotopic hepatocellular Carcinoma Mice. Molecules. 2019;(18):24. doi: 10.3390/molecules24183332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Barbalata C.I., et al. The use of the QbD approach to optimize the Co-loading of simvastatin and doxorubicin in liposomes for a synergistic Anticancer effect. Pharmaceuticals. (Basel) 2022;15(10) doi: 10.3390/ph15101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nitica S., et al. Doxorubicin loaded thermosensitive magneto-liposomes obtained by a gel hydration technique: characterization and in vitro magneto-chemotherapeutic effect assessment. Pharmaceutics. 2022;14(11) doi: 10.3390/pharmaceutics14112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li H., et al. Regulation of protein corona on liposomes using albumin-binding peptide for targeted tumor therapy. J. Control Release. 2023;355:593–603. doi: 10.1016/j.jconrel.2023.02.004. [DOI] [PubMed] [Google Scholar]
- 223.Li X., et al. Improved efficacy of doxorubicin delivery by a novel dual-ligand-modified liposome in hepatocellular carcinoma. Cancer Lett. 2020;489:163–173. doi: 10.1016/j.canlet.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 224.Zhang X., Li J., Yan M. Targeted hepatocellular carcinoma therapy: transferrin modified, self-assembled polymeric nanomedicine for co-delivery of cisplatin and doxorubicin. Drug Dev. Ind. Pharm. 2016;42(10):1590–1599. doi: 10.3109/03639045.2016.1160103. [DOI] [PubMed] [Google Scholar]
- 225.Malarvizhi G.L., et al. Transferrin targeted core-shell nanomedicine for combinatorial delivery of doxorubicin and sorafenib against hepatocellular carcinoma. Nanomedicine. 2014;10(8):1649–1659. doi: 10.1016/j.nano.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 226.Tang J., et al. A stabilized retro-inverso peptide ligand of transferrin receptor for enhanced liposome-based hepatocellular carcinoma-targeted drug delivery. Acta Biomater. 2019;83:379–389. doi: 10.1016/j.actbio.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 227.Wang Y., et al. CXCR4-guided liposomes regulating hypoxic and immunosuppressive microenvironment for sorafenib-resistant tumor treatment. Bioact. Mater. 2022;17:147–161. doi: 10.1016/j.bioactmat.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhou L., et al. Development of 11-DGA-3-O-gal-modified cantharidin liposomes for treatment of hepatocellular carcinoma. Molecules. 2019;24(17) doi: 10.3390/molecules24173080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Huang S., et al. Glycyrrhetinic acid and TAT Peptide modified dual-functional liposomes for treatment of hepatocellular cancer. Curr. Top. Med. Chem. 2020;20(27):2493–2505. doi: 10.2174/1568026620666200722110244. [DOI] [PubMed] [Google Scholar]
- 230.Rahim M.A., et al. Mild hyperthermia responsive liposomes for enhanced in vitro and in vivo anticancer efficacy of doxorubicin against hepatocellular carcinoma. Pharmaceutics. 2021;13(8) doi: 10.3390/pharmaceutics13081310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wu S., et al. Chemotherapeutic nanoparticle-based liposomes enhance the efficiency of mild microwave ablation in hepatocellular carcinoma therapy. Front. Pharmacol. 2020;11:85. doi: 10.3389/fphar.2020.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jiang Y., et al. In vitro and in vivo evaluation of SP94 modified liposomes loaded with N-14NCTDA, a norcantharimide derivative for hepatocellular carcinoma-targeting. AAPS. PharmSciTech. 2020;21(7):277. doi: 10.1208/s12249-020-01829-3. [DOI] [PubMed] [Google Scholar]
- 233.Mu X., et al. ApoE-modified liposomes mediate the antitumour effect of survivin promoter-driven HSVtk in hepatocellular carcinoma. Cancer Gene Ther. 2020;27(10–11):754–767. doi: 10.1038/s41417-019-0145-3. [DOI] [PubMed] [Google Scholar]
- 234.Cannito S., et al. Hyaluronated and PEGylated Liposomes as a potential drug-delivery strategy to specifically target liver cancer and inflammatory cells. Molecules. 2022;27(3) doi: 10.3390/molecules27031062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li L., et al. Preparation and pharmacokinetics of glycyrrhetinic acid and cell transmembrane peptides modified with liposomes for liver targeted-delivery. Biomed. Mater. 2022;17(4) doi: 10.1088/1748-605X/ac6b73. [DOI] [PubMed] [Google Scholar]
- 236.Peng S., et al. Polyinosinic-polycytidylic acid liposome induces human hepatoma cells apoptosis which correlates to the up-regulation of RIG-I like receptors. Cancer Sci. 2009;100(3):529–536. doi: 10.1111/j.1349-7006.2008.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang B., et al. Liposomes co-loaded with ursolic acid and ginsenoside Rg3 in the treatment of hepatocellular carcinoma. Acta Biochim. Pol. 2021;68(4):711–715. doi: 10.18388/abp.2020_5608. [DOI] [PubMed] [Google Scholar]
- 238.Dinh C.T., et al. Synthesis of glycyrrhetinic acid-modified liposomes to deliver Murrayafoline A for treatment of hepatocellular carcinoma. J. Mater. Sci. Mater. Med. 2022;33(10):72. doi: 10.1007/s10856-022-06692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yu L., et al. Synergetic delivery of triptolide and Ce6 with light-activatable liposomes for efficient hepatocellular carcinoma therapy. Acta Pharm. Sin. B. 2021;11(7):2004–2015. doi: 10.1016/j.apsb.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li Y., et al. Advances in nanoliposomes for the diagnosis and treatment of liver cancer. Int. J. Nanomed. 2022:909–925. doi: 10.2147/IJN.S349426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yang J.D., et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li H., et al. iRGD peptide-mediated liposomal nanoparticles with photoacoustic/ultrasound dual-modality imaging for precision theranostics against hepatocellular carcinoma. Int. J. Nanomed. 2021:6455–6475. doi: 10.2147/IJN.S325891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mu W., et al. Promoting early diagnosis and precise therapy of hepatocellular carcinoma by glypican-3-targeted synergistic chemo-photothermal theranostics. ACS. Appl. Mater. Interfaces. 2019;11(26):23591–23604. doi: 10.1021/acsami.9b05526. [DOI] [PubMed] [Google Scholar]
- 244.Wang L., et al. CD44 antibody-targeted liposomal nanoparticles for molecular imaging and therapy of hepatocellular carcinoma. Biomaterials. 2012;33(20):5107–5114. doi: 10.1016/j.biomaterials.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 245.Cao Y., et al. Gadolinium-based nanoscale MRI contrast agents for tumor imaging. J. Mater. Chem. B. 2017;5(19):3431–3461. doi: 10.1039/c7tb00382j. [DOI] [PubMed] [Google Scholar]
- 246.Ren Y., et al. An NIR-II/MR dual modal nanoprobe for liver cancer imaging. Nanoscale. 2020;12(21):11510–11517. doi: 10.1039/d0nr00075b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wallnöfer E.A., et al. Albumin-based nanoparticles as contrast medium for MRI: vascular imaging, tissue and cell interactions, and pharmacokinetics of second-generation nanoparticles. Histochem. Cell Biol. 2021;155(1):19–73. doi: 10.1007/s00418-020-01919-0. [DOI] [PubMed] [Google Scholar]
- 248.Šimečková P., et al. Gadolinium labelled nanoliposomes as the platform for MRI theranostics: in vitro safety study in liver cells and macrophages. Sci. Rep. 2020;10(1):4780. doi: 10.1038/s41598-020-60284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Fouillet X., et al. Enhancement of computed tomography liver contrast using iomeprol-containing liposomes and detection of small liver tumors in rats. Acad. Radiol. 1995;2(7):576–583. doi: 10.1016/s1076-6332(05)80118-7. [DOI] [PubMed] [Google Scholar]
- 250.Lee S.Y., et al. Targeted multimodal imaging modalities. Adv. Drug Deliv. Rev. 2014;76:60–78. doi: 10.1016/j.addr.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 251.Lamichhane N., et al. [(18)F]-Fluorinated carboplatin and [(111)In]-liposome for image-guided drug delivery. Int. J. Mol. Sci. 2017;18(5) doi: 10.3390/ijms18051079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Guan T., et al. From detection to resection: photoacoustic tomography and surgery guidance with indocyanine green loaded gold nanorod@liposome core-shell nanoparticles in liver cancer. Bioconjug. Chem. 2017;28(4):1221–1228. doi: 10.1021/acs.bioconjchem.7b00065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.