Abstract
Background
Marathon running poses unique cardiovascular challenges, sometimes leading to syncopal episodes. We present a case series of athletes who experienced pre-/syncope during the Zurich Marathon 2023, accompanied by elevated cardiac biomarkers.
Case summary
Eight athletes (2 females, 6 males) aged 21–35 years, with pre-/syncope and various additional diverse symptoms such as dizziness and palpitations during the (half-)marathon, were admitted to two emergency departments in Zurich, Switzerland. Clinical evaluations included electrocardiogram, echocardiography, telemetry, coronary computed tomography (CT) scans, and cardiac biomarker assessments. High-sensitive troponin T (hs-cTnT) was elevated in all cases at initial assessment and returned to normal at follow-up. All athletes who received CT scans had normal coronary and brain CT results. None of the eight athletes had underlying cardiovascular disease. Renal function normalized post-admission, and neurological symptoms resolved within hours. Creatinine levels indicated transient acute kidney injury. A common feature was inexperience in running, inadequate race preparation, particularly regarding fluid, electrolyte, and carbohydrate intake, along with pacing issues and lack of coping strategies with heat.
Discussion
From a clinician perspective, the case series highlights the challenge in the management of patients with a pre-/syncopal event during strenuous exercise and elevated cardiac biomarkers. Diverse initial symptoms prompted tailored investigations. Adequate training, medical assessments, and awareness of syncope triggers are essential for marathon participants. Caution and pacing strategies are crucial, especially among novices in competitive running. This information is pertinent given the growing popularity of marathon events and prompts a standardized diagnostic approach after these events.
Keywords: Marathon, Collapse, Syncope, Cardiac biomarkers, Case series
Learning points.
The case series highlights syncope and elevated cardiac biomarkers in athletes after intense exercise, likely due to ‘exercise-induced elevation’ without known cardiac issues.
Athletes with pre-/syncope commonly share factors like inexperience, inadequate training, and poor race preparation.
Pre-participation screening is crucial for athletes’ health, safety, and performance in sports management.
The series stresses the need for immediate medical attention during (half-)marathons to investigate serious causes of pre-/syncope like spontaneous coronary artery dissection, acute coronary syndrome peri-/myocarditis, or channelopathies.
Introduction
Marathon running is a strenuous endurance activity that places substantial demands on the cardiovascular system. Athletes participating in marathons may occasionally experience cardiovascular events, including syncope or presyncope. These events can be alarming for both athletes and medical personnel, prompting further investigation to understand their underlying mechanisms and implications. Syncope in the emergency department requires a standardized approach to ideally categorize it as orthostatic hypotension, reflex syncope, or cardiac syncope.
Cardiac biomarker elevation during or after a marathon may indicate damage to the heart muscle.1 There are many factors that can contribute to such damage, including physical overexertion, dehydration, heat stroke, underlying cardiac conditions, and other medical conditions. Whether exercise-induced elevation of cardiac biomarkers is clinically relevant is still under debate.
Summary figure
Patient 1
A 26-year-old female marathon runner experienced back pain after 39 km and syncope at km 41. Initial vital signs included a blood pressure (BP) of 99/57 mmHg, heart rate of 85 b.p.m., and normal oxygen saturation. Her initial electrocardiogram (ECG) was unremarkable, and troponin levels were not measured. Creatinine was elevated with 109 µmol/L. A transthoracic echocardiogram (TTE) showed a left ventricular ejection fraction (LVEF) of 59% without valvular disease, and 48 h ECG monitoring showed continuous sinus rhythm without relevant arrhythmia. Cardiorespiratory fitness assessment revealed a VO2max of 53.92 mL/kg/min, a maximum exercise capacity of 210 W on cycle ergometry (175% of age-predicted maximum), and a VE/VCO2 Slope of 22.54. She took daily lisdexamfetamine (30 mg/day) for attention deficit hyperactivity disorder (ADHD), is a law student, an experienced runner with five marathons, and has trained for 14 years, including eight 20 km runs in preparation. During follow-up, there were no abnormalities noted in the laboratory analysis, ECG, 48 h ECG monitoring, cardiopulmonary exercise testing (CPET), or echocardiography. The use of supplements and doping was denied and there was no family history of sudden cardiac death (SCD).
Patient 2
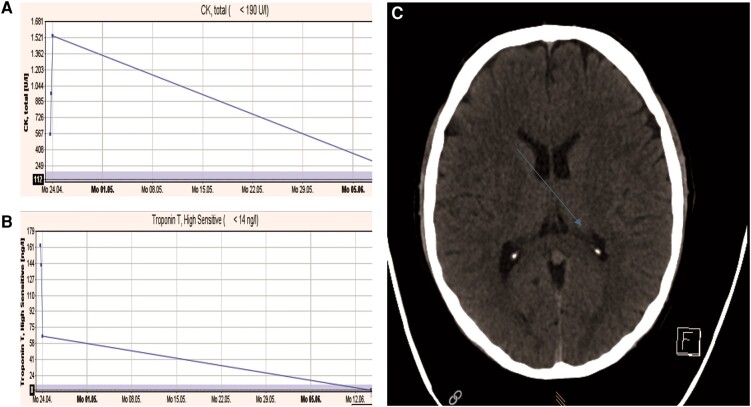
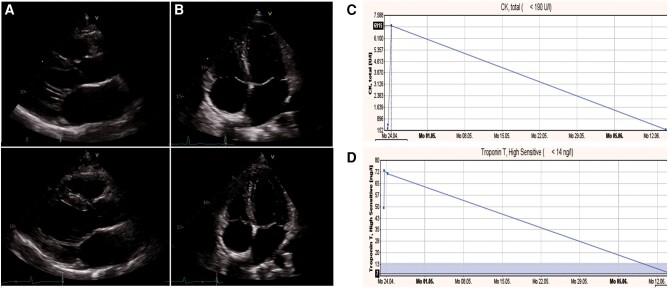
A 35-year-old male half-marathon runner experienced dizziness and disorientation after 20.5 km, nearly fainting. Initial vital signs showed a BP of 115/56 mmHg, heart rate of 67 b.p.m., SpO2 at 98%, and body temperature of 37.5°C. The initial ECG was normal. Lab results showed elevated high-sensitivity troponin T (163 ng/mL), creatine kinase (1538 U/L), and creatinine (139 µmol/L) levels (Figure 1). Follow-up assessments with TTE revealed an LVEF of 53%, no valvular disease, and sinus rhythm on a 48 h Holter ECG. Cardiorespiratory fitness showed a VO2max of 43.26 mL/min/kg, a maximum exercise capacity of 284 W (135% of age-predicted), and a VE/VCO2 Slope of 27.53. The patient had slightly elevated BP and participated in 10 km races yearly. He works as an engineer. He typically trains two to three times a week, including interval training and 8–10 km runs. This has been his second half-marathon, with a personal best of 1 h and 40 min. During follow-up, there were no abnormalities noted in the laboratory analysis, ECG, 48 h ECG monitoring, CPET, or echocardiography. The use of supplements and doping was denied and there was no family history of SCD.
Figure 1.
Patient 2: (A) creatine kinase levels on admission and at follow-up, (B) troponin levels on admission and at follow-up, and (C) computed tomography scan of the brain after the marathon: blue arrow shows non-specific small calcification in the 4th ventricle, no bleeding, no acute territorial infarction, and normal external and internal cerebrospinal fluid spaces.
Patient 3
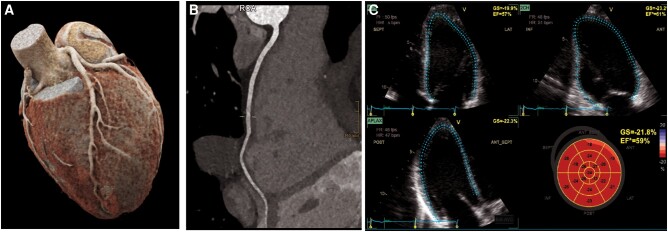
A 26-year-old female half-marathon runner experienced tachycardia and dizziness at 17.5 km, followed by syncope. Initial vital signs showed a BP of 102/55 mmHg, heart rate of 57 bpm, SpO2 at 99%, and a body temperature of 36.2°C. Her initial ECG was normal, and thorax and brain CT scans revealed no abnormalities. Laboratory findings showed an elevated peak troponin T (80 ng/mL), creatine kinase level (174 U/L), and creatinine level (125 µmol/L) (Figure 2). Follow-up assessments included a TTE reporting an LVEF of 59% with no valvular disease and both the ECG and a 48 h ECG monitoring showed sinus rhythm without rhythm disorders. Cardiopulmonary exercise testing revealed a VO2max of 39.3 mL/min/kg, a maximum threshold of 239 W (191% of expected), and a VE/VCO2 Slope of 24.9. The patient had experienced a viral infection 5 days before the race and was not on any medication. She is a medical student who engages in strength training twice a week and jogging three times a week, covering a total of 30 km weekly. She has completed 10 half-marathons since 2016, with a personal best time of 1 h and 52 min. The use of supplements and doping was denied and there was no family history of SCD.
Figure 2.
Patient 3: (A and B) coronary computed tomography: normal coronary anatomy, no coronary atherosclerosis. (C) Echocardiography including strain imaging reveiled normal left ventricular function.
Patient 4
A 27-year-old male first-time half-marathon runner experienced vomiting followed by syncope at km 18. Initial vital signs included a BP of 113/53 mmHg, heart rate of 75 b.p.m., SpO2 at 94%, and a body temperature of 36.5°C. The initial ECG was normal. Laboratory findings included a creatine kinase of 151 IU/L and creatinine levels of 133 µmol/L, but troponin T was not measured. Follow-up assessments with TTE revealed an LVEF of 52% without valvular disease. Both the ECG and 48 h ECG monitoring showed sinus rhythm without rhythm disorders. Cardiopulmonary exercise testing showed a VO2max of 35.2 mL/min/kg, a maximum threshold of 216 W (94% of expected), and a VE/VCO2 Slope of 20.30. The patient had no significant past medical history and was not on medication. Professionally, he works as a depositary. He initiated targeted running training with coaching in December 2022, losing ∼16 kg. His regimen consisted of six sessions per week with a goal of a 2 h, 10 min half-marathon, completing several 20 km runs in preparation. The use of supplements and doping was denied and there was no family history of SCD.
Patient 5
A 32-year-old male, a former professional football player, experienced syncope at km 41 during his run, followed by shivering and amnesia upon regaining consciousness. Initial vital signs included a BP of 107/55 mmHg, heart rate of 67 b.p.m., SpO2 at 96%, and a body temperature of 37.1°C. The initial ECG revealed non-significant ST-segment elevation in the inferior leads. Laboratory findings showed elevated peak troponin T (117 ng/L), creatine kinase (1047 IU/L), and creatinine (178 µmol/L) levels. Follow-up assessments included a TTE with an LVEF of 53% without valvular disease. Both the ECG and a 48 h ECG monitoring showed sinus rhythm with no rhythm disorders. The patient was not on regular medication. Cardiopulmonary exercise testing showed a VO2max of 43.38 mL/min/kg, a maximum threshold of 243 W (115% of expected), and a VE/VCO2 Slope of 18.3. He works as a consultant in finance and had been a professional football goalkeeper until age 20 and engaged in regular running training for the past 10 years, covering around 60 km/week, often with multiple runs weekly. In preparation for the marathon, he completed five long jogs, each exceeding 40 km. The use of supplements and doping was denied and there was no family history of SCD.
Patient 6
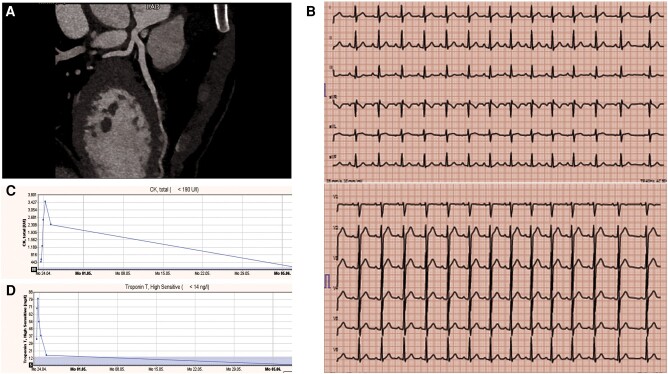
A 36-year-old male participating in his first marathon experienced exhaustion around km 21, followed by syncope at km 32, preceded by dizziness and imbalance. Initial vital signs showed a BP of 118/59 mmHg, a heart rate of 77 b.p.m., SpO2 at 99%, and a body temperature of 37.7°C. Laboratory results revealed elevated troponin T (146 ng/L), a high creatine kinase (3463 U/L), and elevated peak creatinine (147 µmol/L) (Figure 3). Subsequent evaluations with TTE showed an LVEF of 59% with no valvular heart disease. Both the ECG and 48 h ECG monitoring showed sinus rhythm with no rhythm disorders. Cardiopulmonary exercise testing showed a VO2max of 37.3 mL/kg/min, a maximum threshold of 223 W (133% of expected), and a VE/VCO2 Slope of 24.99. The patient had a history of catching a cold 2 weeks before the race but was not on medications. He worked as a consultant in finance and resumed regular training 6 years ago, running over 10 km every other day. In preparation for the marathon, he completed five long jogs, each covering 30 km. The use of supplements and doping was denied and there was no family history of SCD.
Figure 3.
Patient 6: (A) coronary computed tomography after the marathon showing normal coronary anatomy and no coronary atherosclerosis of the left anterior descending artery, (B) electrocardiogram after the marathon, (C) creatine kinase levels after marathon and at follow-up, and (D) troponin levels after on admission and at the follow-up.
Patient 7
A 22-year-old experienced male runner, suffered syncope, tachycardia, limited vision, and imbalance during a half-marathon at km 22. Initial assessments revealed a BP of 125/59 mmHg, heart rate of 113 b.p.m., SpO2 of 96%, and body temperature of 38.7°C. Electrocardiogram showed sinus tachycardia and non-significant ST-segment depression in V3–V6. Troponin T (71 ng/L) and creatine kinase (6915 U/L) were substantially elevated while creatinine was normal (Figure 4). Initial TTE showed a mildly dilated left atrium immediately after the marathon and syncope, which normalized in the follow-up. In TTE, LVEF was 52% without valvular disease. Initial CT scan of the brain and heart were normal. Electrocardiogram and 48 h ECG monitoring showed normal sinus rhythm. Cardiopulmonary exercise testing showed a VO2max of 44.0 mL/min/kg, max threshold of 266 W (114% of expected), and a VE/VCO2 Slope of 22.26. There was no significant medical history or medication. He studied business management. Regarding training, he had not specifically prepared for the half-marathon. He played professional football as a winger for 7 years until 2020. Subsequently, he trained 3–4 times weekly, focusing on both strength and endurance. He also participated in university sports and jogged thrice weekly for distances up to 10 km. The use of supplements and doping was denied and there was no family history of SCD.
Figure 4.
Patient 7: (A) mildly dilated left atrium left atrial volume index 35 mL/m² immediately after marathon/syncope, (B) normalized atrium at the follow-up after 8 weeks left atrial volume index 20 mL/m², (C) creatine kinase levels initial and at follow-up, and (D) troponin levels on admission and at follow-up.
Patient 8
A 21-year-old male with a decade of athletic experience noted an elevated heart rate (200/min) from the start, followed by imbalance, dizziness at km 18, and syncope at km 21. Two weeks prior, he had a viral infection with fever (up to 38.5°C). He was not on medication and occasionally used tetrahydrocannabinol. He studied architecture and had no significant medical history. He participated in track and field since childhood with professional coaching. His routine involved interval runs, 3–4 track sessions weekly, and 1–2 runs spanning 10 to 20 km/week. The use of supplements and doping was denied and there was no family history of SCD.
Case series
We present a case series involving eight individuals (2 females/6 males) who received treatment after pre-/syncope during the Zurich Marathon and Half-Marathon that took place on 23 April 2023. The cases were managed in two emergency departments, namely, the City Hospital Zurich and the University Hospital Zurich. In total, 3561 people participated in the Zurich Marathon and 6388 participated in the Half-Marathon bringing the total participation to 9949 individuals. The sunny weather conditions in the morning were quite usual for Switzerland during spring with a temperature around 8°C, but during the course of the day, it became quite warm for the season, reaching up to 18°C. All cases shared common clinical features following the pre-/syncope episodes, as outlined in Table 1. The sports cardiological follow-up at the outpatient department of the University Clinic Zurich took place 3 months after the emergency treatment in the hospital.
Table 1.
Patients’ characteristics and investigations as well as follow-up of all the eight athletes in the case series
| Patient 1, marathon | Patient 2, marathon | Patient 3, half-marathon | Patient 4, half-marathon | Patient 5, marathon | Patient 6, marathon | Patient 7, half-marathon | Patient 8, half-marathon | |
|---|---|---|---|---|---|---|---|---|
| Pre-/syncope | S | P | S | S | S | S | S | S |
| Age, years | 26 | 35 | 26 | 27 | 32 | 36 | 22 | 21 |
| Sex | f | m | f | m | m | m | m | M |
| Presenting complaint | Back pain and syncope | Dizziness and disorientation | Tachycardia, dizziness, syncope | Vomiting and syncope | Syncope, shivering, amnesia | Exhaustion, syncope, dizziness, imbalance | Syncope, tachycardia, limited vision, imbalance | Elevated heart rate, imbalance, dizziness, syncope |
| Distance at symptoms, km | 41 | 20.5 | 17.5 | 18 | 41 | 32 | 16.5 | 19 |
| Initial blood pressure, mmHg | 99/57 | 115/56 | 102/55 | 113/53 | 107/55 | 118/94 | 125/59 | 117/63 |
| Initial heart rate, b.p.m. | 85 | 67 | 57 | 75 | 67 | 77 | 113 | 79 |
| Initial oxygen saturation, SpO2 | 98 | 98 | 99 | 94 | 96 | 99 | 96 | 98 |
| Initial body temperature (tympanic), °C | 36.5 | 37.5 | 36.2 | 36.5 | 37.1 | 37.7 | 38.7 | 37 |
| Initial ECG | ECG normal | ECG normal | ECG normal | ECG normal | Sinus tachycardia, non-significant ST-segment depression in inferior leads | ECG normal | Sinus tachycardia, non-significant ST-segment depression in V4–V6 | ECG normal |
| Initial laboratory findings | ||||||||
| hsTroponinT, ng/L | — | 163 | 80 | — | 117 | 146 | 71 | 247 |
| Creatine kinase, U/L | — | 1538 | 174 | 151 | 1047 | 3463 | 6915 | 4901 |
| Creatinine, µmol/L | 109 | 139 | 125 | 133 | 178 | 147 | 99 | 131 |
| Initial investigations | Lab test, ECG, medical assessment | Lab test, ECG, medical assessment, CT heart and brain, echocardiography | Lab test, ECG, medical assessment, CT heart and brain, echocardiography | Lab test, ECG, medical assessment | Lab test, ECG, medical assessment | Lab test, ECG, medical assessment, CT heart, echocardiography | Lab test, ECG, medical assessment, Echo, CT heart and brain, echocardiography | Lab test, ECG, medical assessment |
| Follow-up echocardiogram LVEF, % | 59 | 53 | 59 | 52 | 53 | 59 | 52 | 58 |
| 48 h ECG monitoring | Sinus rhythm, no relevant arrhythmia | Sinus rhythm, no relevant arrhythmia | Sinus rhythm, no relevant arrhythmia | Sinus rhythm, no relevant arrhythmia | Sinus rhythm, no relevant arrhythmia | Sinus rhythm, no relevant arrhythmia | Sinus rhythm, no relevant arrhythmia | Sinus rhythm, no relevant arrhythmia |
| Cardiorespiratory fitness assessment | ||||||||
| VO2max, mL/kg/min | 53.92 | 43.26 | 39.30 | 35.26 | 43.38 | 37.38 | 44.01 | 77.91 |
| Maximum exercise capacity, W | 210 (175% of predicted) | 284 (135% of predicted) | 239 (191% of predicted) | 216 (94% of predicted) | 243 (115% of predicted) | 296 (133% of predicted) | 266 (114% of predicted) | 321 (136% of predicted) |
| VE/VCO2 Slope | 22.54 | 27.53 | 24.90 | 20.30 | 18.23 | 24.99 | 22.26 | 16.42 |
| medication | Daily lisdexamfetamine (30 mg/day) for ADHD | — | — | — | — | — | — | — |
| Doping | — | — | — | — | — | — | — | — |
| follow-up blood pressure, mmHg | 124/92 | 130/74 | 103/68 | 129/76 | 133/79 | 130/78 | 133/76 | 135/71 |
| Running history | Experienced runner (14 years of training) | Yearly 10-kilometre races | Experienced runner | Novice | Former professional football player | Novice | Played professional football as a winger for 7 years until 2020 | Participation in track and field since childhood with professional coaching |
| Training frequency | 4–6 training sessions per week, eight 20 km runs in preparation | 2–3 training sessions per week (1× interval training, 2× 8–10 km runs) | 3 training sessions a week/weekly distance 30 km | 6 training sessions per week, starting 6 months before | Approximately 60 km/week, with long-distance sessions and intervals | Resumed six years ago part of a consistent running routine, trains every other day | Trained 3–4 times weekly, focusing on strength and endurance | Interval runs and 3–4 track sessions weekly |
| Half/marathon experience | Experienced runner with 5 completed M | Finished one HM | 10 finished HM since 2016 | — | — | — | — | Finished one HM |
| Personal best | around 3 h M | 1 h 40 min HM | 1 h 52 min HM | — | — | — | 1 h 20 min HM | |
| Supplements, drugs, or doping | — | — | — | — | — | — | — | Occasionally used THC |
| Medical history | ADHS | Caught a cold 5 days before the race | — | — | Caught a cold two weeks before the race | — | Caught a cold 2 weeks before the race, with fever up to 38.5°C | |
| Occupation | Law student | Engineer | Medical student | Depositary | Finance consultant | Finance consultant | Business management student | Student of architecture |
M, marathon; HM, half-marathon; S, syncope; P, presyncope; hsTroponinT, high-sensitivity troponin T; LVEF, left ventricular ejection fraction.
Discussion
We here report a case series of eight athletes with pre-/syncope during the Zurich (Half-)Marathon 2023 that agreed to have a structured follow-up examination and also agreed to be included in this cases series.
According to guidelines for syncope of the European Society of Cardiology, our athletes displayed low-risk features, including classic prodromal symptoms preceding syncope. However, their syncope occurring during exertion categorized them as high-risk individuals.2 None of the athletes reported chest discomfort upon reaching the emergency department.
Initial symptoms upon presentation varied, leading to tailored investigations. In practice, we observed diverse approaches to subsequent clinical investigations, with some athletes undergoing immediate coronary CT scans and echocardiography following elevated cardiac biomarkers.
All eight athletes underwent comprehensive screening for underlying medical conditions associated with their pre-/syncope episodes during half-/marathons. None of them had underlying cardiovascular disease. Patient 7 exhibited mild left atrial dilatation during the initial emergency department echocardiogram, which had completely resolved upon follow-up.
Endurance exercises like long-distance triathlons, marathons, and half-marathons are known to induce specific adaptations in the heart muscle. These adaptations can manifest as reduced left and/or right ventricular systolic and/or diastolic function.3 The elevated cardiac output during these events places significant strain on the heart’s structures, especially in cavities with thin walls such as the right ventricle, left atrium, and right atrium. This can lead to temporary decreases in function or myocardial remodelling.3,4
In a low-risk population with elevated cardiac biomarkers, performing a coronary CT scan supports risk stratification by detecting coronary artery disease, coronary anomalies, or spontaneous coronary artery dissection (SCAD) and analysing plaque composition and the tissue surrounding the vessel to pinpoint patients who are at risk of coronary events.5 Recent advances in CT technology have significantly improved coronary CT angiography, although its resolution remains limited for assessing small distal coronary arteries.6
All CT scans conducted on the athletes revealed normal results, reinforcing the concept of exercise-induced elevation in cardiac biomarkers. In the context of exercise-induced high-sensitivity troponin elevation, most studies have found no association between post-exercise troponin elevations and severity of coronary artery disease.5 Patients presenting post-exercise with any clinical concern for an acute coronary syndrome should undergo immediate evaluation including non-invasive and/or invasive risk stratification—irrespective of cardiac biomarker levels. However, due to its low diagnostic yield, assessment of CAD is not indicated solely on the basis of post-exercise troponin elevation.
Interestingly, we noted a concurrent elevation in creatinine levels in some athletes indicating acute kidney injury. It can be assumed that syncope had different, and potentially mixed, underlying mechanisms, which calls for categorizing as precisely as possible. Marathon running subjects the body to various stressors, including environmental heat exposure, exercise-induced dehydration, elevated core body temperature, reduced renal blood flow, and systemic inflammation.7 However, during follow-up assessments, all athletes exhibited normalized renal function. Diagnosing a potential heat stroke, only the core temperature (tympanic or rectal) should be considered, as in our patients. Peripheral temperatures should not be used to define heat stroke during sports practice. Heat stroke can occur not only due to the weather but mainly due to the athlete’s acclimatization conditions.
Neurological symptoms experienced by the athletes completely resolved within hours of admission to the hospital. Two athletes underwent brain CT scans that revealed no underlying pathologies. One patient had a follow-up appointment with an outpatient neurologist (Patient 7), which was also normal.
The elevated cardiac biomarkers observed in these athletes were interpreted in the context of ‘exercise-induced elevation’, a well-documented phenomenon in the literature. The precise mechanisms driving this phenomenon remain incompletely understood, but it is likely linked to physical stress during endurance exercise, leading to increased membrane permeability of cardiomyocytes and the passive diffusion of troponins.8
Three of the athletes (Patients 3, 6, and 8) had fallen ill with a viral infection. Patients 3 and 6 measured subfebrile temperatures and complained of bronchitis and rhinitis. Patient 8 also had a fever 2 weeks before the race. All patients did not feel fully recovered from the infection at the time of competition but did not want to miss the event. Therefore, further diagnostic steps were taken to exclude peri-/myocarditis and consecutive arrhythmogenic syncope. Patient 8 had a sinus tachycardia right from the start; there was no evidence of an underlying supraventricular tachycardia in the analysis of the heart rate monitor from the race, during follow-up exercise test or in the 48 h ECG monitoring. The elevated heart rate could be interpreted as sinus tachycardia.
An important differential diagnosis of syncope is also represented by channelopathies. None of our athletes showed signs of a channelopathy in both the initial and follow-up ECGs, either at rest or under exercise testing and there was no family history of sudden cardiac arrest in the medical history of each athlete. Therefore, high-lead position ECGs were not routinely performed. However, in cases of recurrent syncope, further investigations including high-lead position ECGs are warranted.
The pre-/syncope episodes had diverse causes among the athletes. A common feature was inadequate race preparation, particularly regarding fluid, electrolyte, and carbohydrate intake, along with pacing issues. Most of these athletes were inexperienced in competitive running or attempting a (half-)marathon for the first time. None of the investigated athletes had undergone a sports medical examination before participating in the competition. The correct pacing strategy in the marathon is difficult to achieve and requires thorough preparation, recreational runners should try to maintaining a consistent speed increases the likelihood of reaching the finish line compared with inconsistent pacing. The more experienced the athlete, the better they can maintain their speed.9 All of our examined athletes ran the first quarter of the competition at a pace significantly faster than the rest of the race, most likely in an anaerobic phase, which lead to significantly faster exhaustion and an unintentional accumulation of lactate. Competitive racing, especially among novices, demands a solid understanding of pacing strategies and proper nutritional intake for a safe and successful race.10
Patient’s perspective (Patient 3)
Around km 15, it got really rough. I could not run anymore and felt dizzy, like I had no control over my legs. I was staggering as if I were drunk, and the sensory overload from the crowds was overwhelming. I felt nauseous and had strange tingling sensations in my legs that went away quickly.
I felt extremely nauseous, unsure if I was going to vomit. When the ambulance arrived, my neurological symptoms gradually subsided. In the hospital, they gave me medication to relieve the nausea and pain. As I lay on the hospital bed, the symptoms disappeared entirely. Even my low BP did not bother me much.
When I was discharged, I felt weak and unsteady on my feet. Three days after the race, I developed a fever, but 2 days after that, I was back at work with no symptoms. A week later, I was able to start light training again. It was a scary experience, but I’m grateful for the help I received during the race.
Conclusion
This case series highlights the phenomenon of syncope and elevated biomarkers after intense and prolonged exercise and underscores the importance of proper marathon preparation and medical attention for participants. Even though findings suggested mainly orthostatic hypotension as mechanism for (pre-/)syncope, elevated cardiac biomarkers prompted sequential testing and additional tests to rule out acute coronary syndrome, SCAD, or peri-/myocarditis. Adequate training, medical examinations, and awareness of syncope causes are crucial for marathon participants. Caution and proper pacing are essential, particularly given the growing trend in marathon running.
Contributor Information
Greta Hametner, Department of Cardiology, University Hospital Zurich, University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Doris Eis, Department of Emergency Medicine, University Hospital Zurich, University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Muriel Kruijver, Department of Cardiology, University Hospital Zurich, University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Michael Stiefel, Department of Cardiology, University Hospital Zurich, University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Jan Gerrit van der Stouwe, Department of Cardiology, University Hospital Zurich, University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Department of Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Melina Stüssi-Helbling, Department of Internal Medicine, Clinic for Internal Medicine, City Hospital Zurich, Triemli, Birmensdorferstrasse 497, 8063 Zürich, Switzerland.
Anja Forrer, Department of Internal Medicine, Clinic for Internal Medicine, City Hospital Zurich, Triemli, Birmensdorferstrasse 497, 8063 Zürich, Switzerland.
David Niederseer, Department of Cardiology, University Hospital Zurich, University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Hochgebirgsklinik, Medicine Campus Davos, Herman-Burchard-Strasse 1, 7265 Davos Wolfgang, Switzerland; Christine Kühne Center for Allergy Research and Education (CK-CARE), Medicine Campus Davos, Herman-Burchard-Strasse 1, 7265 Davos Wolfgang, Switzerland.
Lead author biography

Dr Greta Hametner completed her training in internal medicine and cardiology at the University Hospital Zurich in Switzerland in 2023. She served as a Resident Physician in Cardiology and Intensive Care Medicine at Cantonal Hospital St. Gallen from 2017 to 2022. During an interim period, she held the position of Senior Physician in Internal Medicine at Cantonal Hospital Münsterlingen from 2020 to 2021. Dr Hametner’s medical journey began with her role as a Resident Physician in Internal Medicine in 2015 in Salzburg, Austria. She completed her medical education at the Medical University Graz, Austria, from 2009 to 2015. Her professional interests centre on sports cardiology, exercise testing, and advanced echocardiography, reflecting her dedication to enhancing cardiovascular care.
Consent: The authors confirm that written consent for submission and publication of the case series has been obtained from all patients in line with COPE guidance.
Funding: This case series was not supported by any funding.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. Aengevaeren VL, Froeling M, Hooijmans MT, Monte JR, van den Berg-Faay S, Maria TE, et al. Myocardial injury and compromised cardiomyocyte integrity following a marathon run. JACC Cardiovasc Imaging 2020;13:1445–1447. [DOI] [PubMed] [Google Scholar]
- 2. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–1948. [DOI] [PubMed] [Google Scholar]
- 3. Vitiello D, Palacin F, Poinsard L, Kirsch M, Jouini S, Billat V, et al. Marathon-induced cardiac fatigue: a review over the last decade for the preservation of the athletes’ health. Int J Environ Res Public Health 2021;18:8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanz-de la Garza M, Grazioli G, Bijnens BH, Sarvari SI, Guasch E, Pajuelo C, et al. Acute, exercise dose-dependent impairment in atrial performance during an endurance race: 2D ultrasound speckle-tracking strain analysis. JACC Cardiovasc Imaging 2016;9:1380–1388. [DOI] [PubMed] [Google Scholar]
- 5. Antonopoulos AS, Angelopoulos A, Tsioufis K, Antoniades C, Tousoulis D. Cardiovascular risk stratification by coronary computed tomography angiography imaging: current state-of-the-art. Eur J Prev Cardiol 2022;29:608–624. [DOI] [PubMed] [Google Scholar]
- 6. Gupta S, Meyersohn NM, Wood MJ, Steigner ML, Blankstein R, Ghoshhajra BB, et al. Role of coronary CT angiography in spontaneous coronary artery dissection. Radiol Cardiothorac Imaging 2020;2:e200364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atkins WC, Butts CL, Kelly MR, Troyanos C, Laursen RM, Duckett A, et al. Acute kidney injury biomarkers and hydration outcomes at the Boston marathon. Front Physiol 2022;12:813554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aengevaeren VL, Baggish AL, Chung EH, George K, Kleiven Ø, Mingels AMA, et al. Exercise-induced cardiac troponin elevations: from underlying mechanisms to clinical relevance. Circulation 2021;144:1955–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ristanović L, Cuk I, Villiger E, Stojiljković S, Nikolaidis PT, Weiss K, et al. The pacing differences in performance levels of marathon and half-marathon runners. Front Psychol 2023;14:1273451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smyth B. How recreational marathon runners hit the wall: a large-scale data analysis of late-race pacing collapse in the marathon. PLoS One 2021;16:e0251513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.