Abstract
Importance:
Surgical site infections increase patient morbidity and healthcare costs. The Centers for Disease Control and Prevention emphasizes improved basic preventive measures to reduce bacterial transmission and infections for patients undergoing surgery.
Objective:
To determine whether improved basic preventive measures can reduce perioperative S. aureus transmission and surgical site infections.
Design:
A randomized clinical trial conducted over one year (9/20/2018–9/20/2019) with a 60-day follow-up period. Nineteen surgeons and their associated patients were randomized 1:1 via a random number generator to treatment or to usual care. Observers were blinded to patient groupings during assessment of outcome measures.
Setting:
Major academic medical center.
Participants:
A random sample of adult patients undergoing orthopedic total joint, orthopedic spine, oncologic gynecological, thoracic, general, colorectal, open vascular, plastic, or open urological surgery requiring general or regional anesthesia.
Intervention:
Sustained improvements in perioperative provider hand hygiene, vascular care, environmental cleaning, and patient decolonization efforts.
Main outcome measures:
Perioperative S. aureus transmission assessed by the number of isolates transmitted and the incidence of transmission among patient care units (primary) and the incidence of surgical site infections (secondary).
Results:
A total of 106 and 130 adult patients in the treatment and control groups, respectively, received the intended treatment and were analyzed for the primary outcome. Treatment reduced the mean (± standard deviation) number of transmitted perioperative S. aureus isolates (1.25 ± 2.11 control vs. 0.47 ± 1.13 treatment, Wilcoxon-Mann-Whitney test P=0.002). Treatment reduced the incidence of S. aureus transmission (incidence risk ratio 0.56; 95% CI 0.37–0.86, P=0.008; clustering by surgeon, 95% CI 0.42–0.76, P<0.001). Approximately 4% of patients (11/236) suffered from surgical site infections, 7.69% in the control group (10/130) and 0.94% (1/106) in the treatment group. Transmission was associated with an increased risk of surgical site infection (11.0% [8/73] with transmission vs. 1.8% [3/163] without, risk ratio 5.95, 95% CI 1.62–21.86, P=0.007). Treatment reduced the risk of surgical site infection (Cox regression hazard ratio 0.12; 95% CI 0.015–0.918, P=0.041; with clustering by surgeon, 95% CI 0.027–0.506, P=0.004).
Conclusions:
Improved basic preventive measures in the perioperative arena can reduce S. aureus transmission and surgical site infections.
Objective:
Surgical site infections (SSIs) increase patient morbidity and healthcare costs.1–8 The Centers for Disease Control and Prevention (CDC) emphasizes strategic improvements in basic preventive measures to prevent bacterial spread and associated infection development.6,7
Host defenses, pathogen virulence, the microenvironment of the wound, and the size of the inoculum contribute to the pathophysiology of SSI development.9 The Surgical Care Improvement Project (SCIP) focused on host defenses and inhibition of bacterial virulence with complete adherence predicting a decrease in postoperative infection rates.10 Elevated intraoperative inspired oxygen was found to be ineffective for SSI prevention in a meta-analysis involving 12 randomized trials.11
Single interventions targeting improvements in intraoperative hand hygiene, 12 vascular care, 13 and patient decolonization14 have reduced the incidence of SSIs. The bacterial inoculum has been linked by whole cell genome analysis to 50% of S. aureus SSIs.15Single interventions however are prone to failure,16 and evidence indicates the need for a multi-faceted approach to maximally control high-risk intraoperative bacterial transmission events.17
We hypothesized that sustained improvements in perioperative provider hand hygiene, intravascular catheter hub disinfection, environmental cleaning, and patient decolonization efforts would generate substantial reductions in perioperative reservoir S. aureus transmission that would result in SSI reduction.
Design and Methods:
Trial Design:
Randomized, prospective, clinical trial with parallel, 1:1 allocation of surgeons and their patients to treatment and usual care groups (Appendix A, trial protocol) following the CONSORT reporting guideline for randomized clinical trials (Appendix B).
Definitions:
Surveillance:
Bacterial cultures obtained for each patient allowed for identification of potential S. aureus transmission events occurring within and between patients. Serially collected samples included the enrolled patient at home (patient nose, axilla, and groin), baseline and case-end anesthesia environment (adjustable pressure-limiting valve and agent dial of the anesthesia machine), anesthesia provider hands (attending physician and assistant), patient skin sites (nasopharynx, axilla, and groin), and intravascular catheter samples for the enrolled patient and patient to follow when able, and patient nasopharynx and axilla, provider hands, bedrail, patient skin site proximal to the wound dressing, and injection port samples during recovery. 18
Transmission:
S. aureus transmission was defined as ≥ 2 S. aureus isolates obtained from ≥ 2 distinct, temporally-associated reservoirs and/or the isolation of ≥ 1 pathogen from a reservoir at case end that was not present at case start. 12,18
Participants:
Eligibility:
Adult patients scheduled to undergo orthopedic total joint, orthopedic spine, oncologic gynecological, thoracic, general, colorectal, open vascular, plastic, and open urological surgery requiring general/regional anesthesia and who provided written consent were eligible for enrollment. Patients <18 years of age, scheduled to undergo procedures outside of the surgical specialties listed above or not requiring general or regional anesthesia, or who had a documented allergy to iodine, shellfish, or chlorhexidine were excluded.
Setting:
This prospective, randomized clinical trial involved 236 patients (130 control, 106 treatment) and was performed over one year (9/20/2018 to 9/20/2019) at the University of Iowa. The study was IRB approved (201802843) and registered at clinicaltrials.gov (NCT03638947) prior to first patient enrollment. Each of nineteen surgeons prospectively identified and enrolled eligible patients after obtaining consent. Consents were placed by the surgeon and/or assistant into bins within the clinic and collected at the end of each day.
Interventions:
Usual Care:
Wall-mounted and anesthesia cart-based 62% ethanol dispensers (B4 Brands Avant foaming hand sanitizer, Lisbon, IA 52243), 70% isopropyl alcohol pads (Covidien, Mansfield, MA 02048) for injection port disinfection, top down cleaning of the anesthesia machine and equipment with cotton cloths soaked in a quaternary ammonium compound (Virex, Diversey Inc, Sturtevant, Wisconsin 53177), and patient decolonization including nasal mupirocin ointment and chlorhexidine wipes for 5 days including the morning of surgery, no decolonization protocol, or chlorhexidine wipes the day before and morning of surgery.
Treatment:
Hand hygiene:
A bag of 70% isopropyl alcohol connected to a one-handed pump (Frantz Medical, Mentor, Ohio 44060) was attached to the IV pole prior to arrival to the OR and to the bedrail IV pole prior to transport to the recovery unit. 12
Organization of the anesthesia work area:
A wire basket (Clinton Industries, York PA 17403) lined with a plastic bag was placed on the IV pole prior to patient OR entry. This served as a receptacle and storage location for contaminated/used equipment. 19
Frequency and quality of environmental cleaning:
A microfiber cloth (16 in. x 16 in., The Rag Company, Boise, ID 83703) was soaked in a quaternary ammonium compound (Virex, Diversey Inc, Sturtevant, Wisconsin 53177) and used to clean the anesthesia machine and monitors before patient OR entry and before patient admission to the recovery unit. A top down cleaning approach was utilized. Use of surface disinfection wipes containing a quaternary ammonium compound and isopropyl alcohol (PDI healthcare, Woodcliff Lake, New Jersey 07677) were used to clean the anesthesia machine following induction of anesthesia and patient stabilization.19,20
Intravascular catheter and syringe tip disinfection:
Disinfection caps containing 70% isopropyl alcohol (Frantz Medical, Mentor, Ohio, 44060) were attached to the IV pole prior to arrival to the operating room and to the bedrail IV pole prior to departure to the recovery unit. These devices can disinfect in 10 seconds and with one turn.13
Patient decolonization with nasal povidone iodine (3M ST Paul, Minnesota 55144): 14
This product was used as directed on the morning of surgery in same day holding prior to OR entry and after induction of anesthesia and patient stabilization in the operating room.
Targeted UV-C light therapy (Helios, Surfacide, Waukesha, WI 53188):
UV-C therapy was directed to operating room environments that had been exposed to S. aureus transmission within the prior 2 weeks.21 Surveillance was utilized for the detection process (RDB Bioinformatics, Omaha, NE 68154).
Quarterly feedback via surveillance failure mode analysis (RDB Bioinformatics, Omaha, NE 68154):15, 18
Surveillance unit reservoirs contributing to temporally-associated S. aureus transmission events were identified and processed to generate typical transmission maps. Contributing reservoirs falling in the 90th percentile were highlighted and became improvement targets.
Outcomes:
Primary:
We observed the number of transmitted S. aureus isolates and surveillance unit S. aureus transmission exposure from the patient at home prior to surgery until 10:00 A.M. on the first postoperative day. We utilized previously described microbiological analyses.15,18
Secondary:
Patients were followed for 60 postoperative days. Research assistants screened all patient charts for evidence of elevated white blood cell count, fever, anti-microbial order, office note documentation of infection, and culture acquisition. Patients were called up to 3 times following study completion. Patients with ≥ 1 of the 5 criteria and/or positive on the phone call were flagged for review by the principal investigator to determine if possible infections met National Healthcare Safety Network (NHSN) definitions of SSI.22
Patient Demographics and Procedural Information:
Information pertaining to patient discharge location, age greater than 50 years,18 sex, American Society of Anesthesiologists (ASA) physical status classification > 2, dirty or infected surgery, duration > 2 hours, orthopedic, plastic, and general abdominal surgery, and decolonization strategy (nasal mupirocin and chlorhexidine, chlorhexidine only, or no protocol) was collected.
Sample Size:
We originally planned to screen 1000 patients from the 10 surgical specialties over a two-year study period with the surgeons randomized 1:1, yielding 500 patients in each treatment arm on which to evaluate the primary outcome. We calculated conservatively that this would provide 85% power to detect a 30% relative reduction in any S. aureus transmission event (40% down to 28%) (Appendix A trial protocol).23Our planned analysis of the study data exactly midway through the trial revealed significant effect for the primary outcome (P ≤ 0.0078) and resulted in study termination.
Randomization:
Hospital and orthopedic, general abdominal and plastic surgical specialties have been previously associated with intraoperative bacterial transmission.17,18 The association of bacterial transmission with surgical specialty is likely related to variation in preoperative decolonization protocols.18 Thus, this study involved randomization by surgeon via a random number generator (by statistician) to ensure that previous decolonization practices that might impact infections were balanced among the treatment and control groups. All patients enrolled by a given surgeon after obtaining informed, written consent were randomized to receive the treatment assigned to the surgeon. Approximately 44% (19/43) of the surgeons involved in the specialties meeting inclusion criteria participated.
Blinding:
Patients were not informed of their treatment assignment. All outcomes were measured by research personnel that were not aware of patient grouping assignments.
Statistical Methods:
Statistical analysis:
Multiple statistical methods were used. For simplification, we list the statistical methods in the precise sequence as reported in the results, and vice-versa.
Fisher’s exact test was used to compare patient and procedural demographic information for treatment vs. control groups and for cases involving S. aureus transmission. Similarly, ASA > 2 and sex for enrolled vs. eligible patients were compared by surgeon using the Mantel-Haenszel test.
Fisher’s exact test was used to examine the potential association of each of the covariates mentioned above with S. aureus transmission; none was associated (all P ≥ 0.120).
A two-sided Wilcoxon-Mann-Whitney test was used to examine the association between treatment assignment and the number of transmitted S. aureus isolates.
Poisson regression was used to estimate the incidence risk ratio (IRR) of S. aureus transmission for the independent variable of treatment. Poisson regression was used to estimate the incidence risk ratio (IRR) because the incidence of transmission (30.9% of N = 73 of 236 cases) was so large that the odds ratio estimated using logistic regression would be a biased estimator of the relative risk.24 We repeated the analysis using multi-level Poisson regression clustering to the surgeon or specialty levels.
Fisher’s exact test was used to compare (i) the proportion treatment cases with transmission events before vs. after surveillance feedback and (ii) the proportion of patients with SSI with and without documented S. aureus transmission. Poisson regression was used to compare the proportion of patients with SSI with and without documented S. aureus transmission clustering to the surgeon.
The proportion of patients with surgical site infections (SSIs) was compared by the Fisher’s exact test. Time to infection was evaluated by Cox proportional hazard modeling. We repeated the analysis using multi-level Poisson regression clustering to the surgeon or specialty levels.
There was no missing data for the primary and secondary outcomes. Calculations were performed using Stata version 16.1. All P-values and confidence intervals (CI) were 2-sided. P < 0.05 was taken to indicate statistical significance.
Results:
A total of 106 and 130 adult patients in the treatment and control groups, respectively, received the intended treatment and were analyzed for the primary outcome.
Participant flow:
The overall patient enrollment schematic is shown in Fig. 1. Approximately 2,372 patients among the 19 participating surgeons were eligible for enrollment. A total of 236 patients were enrolled (130 control, 106 treatment) with no systematic differences between enrolled and eligible patients among randomized surgeons according to ASA status > 2 and sex (Mantel-Haenszel test P = 0.66 and P = 0.18, respectively).
Figure 1.
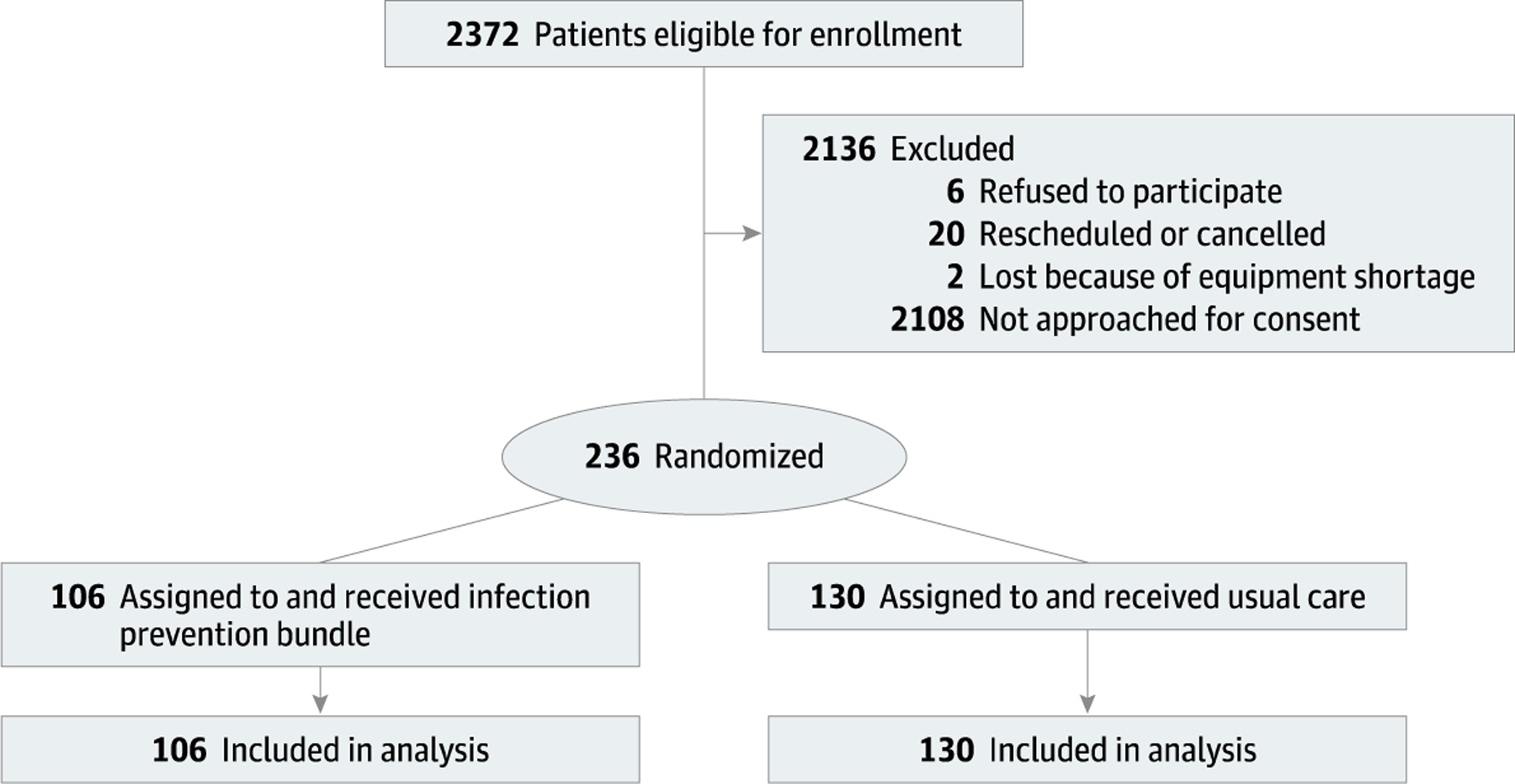
Patient Enrollment Schematic
Recruitment:
Patients were recruited from 9/20/2018 to 9/20/2019.
Baseline data:
Baseline patient and procedural demographics stratified by treatment and transmission are shown (Table 1). Floor or intensive care unit (ICU) discharge, age greater than 50 years,18 male sex, ASA > 2, and plastic surgery were associated with the treatment group. None of those covariates associated with the treatment assignment were associated with S. aureus transmission.
Table 1:
Baseline Patient and Procedural Demographics Stratified by Treatment and Transmission
| Baseline Patient and Procedural Demographics (N=236 patients enrolled) | Treatment | Control |
|---|---|---|
| Treatment assignment N (%) | 106 (44.92) | 130 (55.08) |
| Age > 50 N (%) | 88 (83.02) | 81 (62.31) |
| Female N (%) | 43 (40.57) | 113 (86.92) |
| ASA > 2 N (%) | 62 (58.49) | 44 (33.85) |
| Dirty or Infected Site N (%) | 3 (2.83) | 6 (4.62) |
| Duration > 2 Hours N (%) | 97 (91.51) | 119 (91.54) |
| Plastic surgery N (%) | 1 (0.94) | 27 (20.77) |
| Orthopedic surgery N (%) | 27 (25.47) | 20 (15.38) |
| General abdominal surgery N (%) | 5 (4.72) | 6 (4.62) |
| Preoperative decolonization strategy | ||
| Nasal mupirocin and chlorhexidine for 5 days N (%) | 27 (25.47) | 20 (15.38) |
| Chlorhexidine for day before and morning of surgery N (%) | 55 (51.89) | 80 (61.54) |
| No protocol N (%) | 24 (22.64) | 30 (23.08) |
| Discharge location | ||
| Same day | 27 (25.47) | 54 (41.54) |
| Floor | 69 (65.09) | 73 (56.15) |
| Intensive care unit | 10 (9.43) | 3 (2.31) |
| Baseline Patient and Procedural Demographics by S. aureus Transmission N (%), Total N=236 (106 treatment, 130 control) | Yes | No | P-Value |
|---|---|---|---|
| S. aureus Transmission N (%) | 73 (30.93) | 163 (69.07) | |
| Age > 50 N (%) | 48 (65.75) | 121 (74.23) | 0.21 |
| Female N (%) | 53 (72.60) | 103 (63.19) | 0.18 |
| ASA > 2 N (%) | 27 (36.99) | 79 (48.47) | 0.12 |
| Dirty or Infected Site N (%) | 5 (6.85) | 4 (2.45) | 0.14 |
| Duration > 2 Hours N (%) | 68 (93.15) | 148 (90.80) | 0.62 |
| Plastic surgery N (%) | 11 (15.07) | 17 (10.43) | 0.38 |
| Orthopedic surgery N (%) | 12 (16.44) | 35 (21.47) | 0.39 |
| General abdominal surgery N (%) | 4 (5.48) | 7 (4.29) | 0.74 |
| Preoperative decolonization strategy | |||
| Nasal mupirocin and chlorhexidine for 5 days N (%) | 12 (16.44) | 35 (21.47) | 0.39 |
| Chlorhexidine for day before and morning of surgery N (%) | 45 (61.64) | 90 (55.21) | 0.39 |
| No protocol N (%) | 16 (21.92) | 38 (23.31) | 0.87 |
| Discharge location | |||
| Same day | 25 (34.25) | 56 (34.36) | 1.0 |
| Floor | 43 (58.90) | 99 (60.74) | 0.89 |
| Intensive care unit | 5 (6.85) | 8 (4.91) | 0.76 |
ASA=American Society of Anesthesiologists (ASA) heath classification status.
Outcomes and estimation:
Treatment reduced the number of transmitted perioperative S. aureus isolates (1.25 ± 2.11 control vs. 0.47 ± 1.13 treatment, Wilcoxon-Mann-Whitney test P=0.002; Fig. 2). S. aureus transmission and SSI development by surgeon is shown in Table 2.
Figure 2.
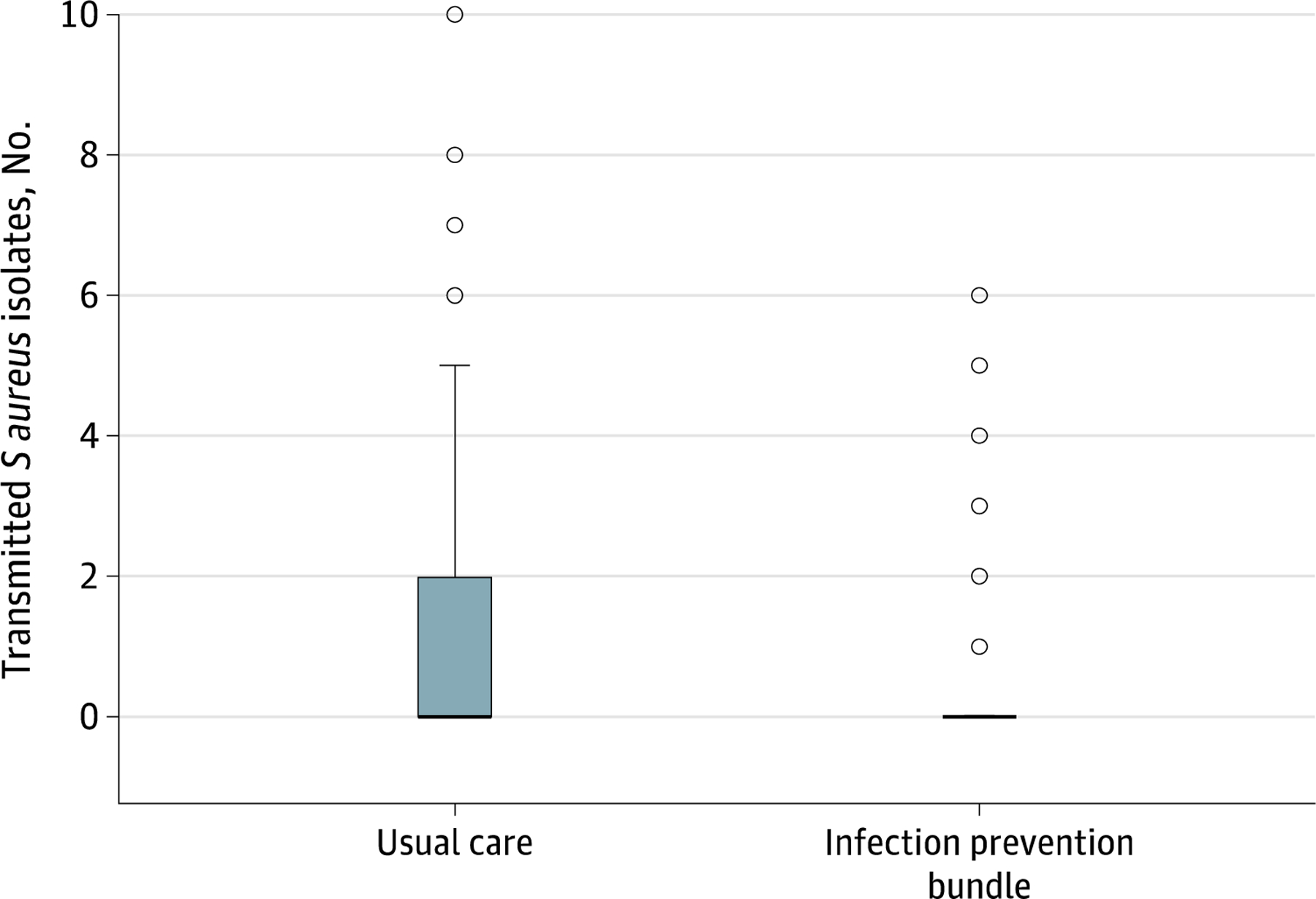
Effect of the multifaceted approach on perioperative staphylococcus aureus reservoir transmission.
Table 2:
S. aureus Reservoir Exposure, Transmission and SSI Development by Surgeon and Treatment
| Surgeon | Treatment group | No. Cases | Isolates Transmission N (% group total) | Any Transmission N (% group total) | Between Transmission N (% group total) | Surgical Site Infection N (% group total) |
|---|---|---|---|---|---|---|
| 7 | 1 | 41 | 23 (46) | 10 (43.5) | 2 (66.7) | 1 (100) |
| 8 | 1 | 27 | 15 (30) | 7 (30.4) | 0 | 0 |
| 9 | 1 | 4 | 7 (14) | 2 (8.7) | 0 | 0 |
| 10 | 1 | 16 | 4 (8) | 3 (13) | 1 (33.3) | 0 |
| 11 | 1 | 6 | 0 (0) | 0 (0) | 0 | 0 |
| 12 | 1 | 6 | 0 (0) | 0 (0) | 0 | 0 |
| 14 | 1 | 3 | 0 (0) | 0 (0) | 0 | 0 |
| 16 | 1 | 1 | 0 (0) | 0 (0) | 0 | 0 |
| 18 | 1 | 1 | 1 (2) | 1(4.3) | 0 | 0 |
| 19 | 1 | 1 | 0 (0) | 0 (0) | 0 | 0 |
| Group Total | 106 | 50 | 23 | 3 | 1 | |
| 1 | 0 | 45 | 41 (25) | 19 (38) | 3 (16) | 5 (50) |
| 2 | 0 | 26 | 42 (26) | 12 (24) | 7 (37) | 2 (20) |
| 3 | 0 | 19 | 35 (22) | 7 (14) | 6 (32) | 2 (20) |
| 4 | 0 | 6 | 4 (3) | 2 (4) | 0 | 0 |
| 5 | 0 | 20 | 12 (7) | 5 (10) | 1 (5) | 0 |
| 6 | 0 | 8 | 13 (8) | 3 (6) | 1 (5) | 1 (10) |
| 15 | 0 | 1 | 0 (0) | 0 (0) | 0 | 0 |
| 17 | 0 | 2 | 7 (4) | 0 (0) | 0 | 0 |
| 13 | 0 | 3 | 8 (5) | 2 (4) | 1 (5) | 0 |
| Group Total | 130 | 162 | 50 | 19 | 10 |
Treatment 0=usual practice, 1= program, isolate transmission is the number of transmitted isolates recovered among all cases by surgeon; any transmission is the number of cases by surgeon where at least one transmission event was detected with or between cases; and between transmission is that which occurred between patient care environments.
Treatment reduced the number of surveillance units exposed to S. aureus transmission (incidence risk ratio [IRR] 0.56; 95% CI 0.37–0.86, P = 0.008; with robust variance clustering by surgeon, 95% CI 0.42–0.76, P<0.001; and with robust variance clustering by specialty, 95% CI 0.39–0.81, P=0.002).
Transmission was associated with an increased risk of SSI (11.0% [8/73] with S. aureus transmission detection had infection vs. 1.8% [3/163] without S. aureus transmission detection had infection, risk ratio 5.95, 95% CI 1.62–21.86, P=0.007; with clustering by surgeon, 95% CI 2.47–14.38, P < 0.001; and with clustering by specialty, 95% CI 3.04–11.67, P < 0.001.
Approximately 4% of patients (11/236) suffered from SSIs, 7.69% in the control group (10/130) and 0.94% (1/106) in the treatment group. One patient in the treatment group suffered from a deep organ space infection involving MSSA that was complicated by bacteremia. Ten patients in the control group suffered from wound infections with 2 complicated by bacteremia and one complicated by septic shock and death.
Treatment reduced risk of SSI (Cox regression hazard ratio [HR] 0.12; 95% CI 0.015–0.918, P=0.041; with clustering by surgeon, 95% CI 0.027–0.506, P=0.004; and with clustering by specialty, 95% CI 0.025–0.546, P=0.006; Fig.3).
Figure 3.
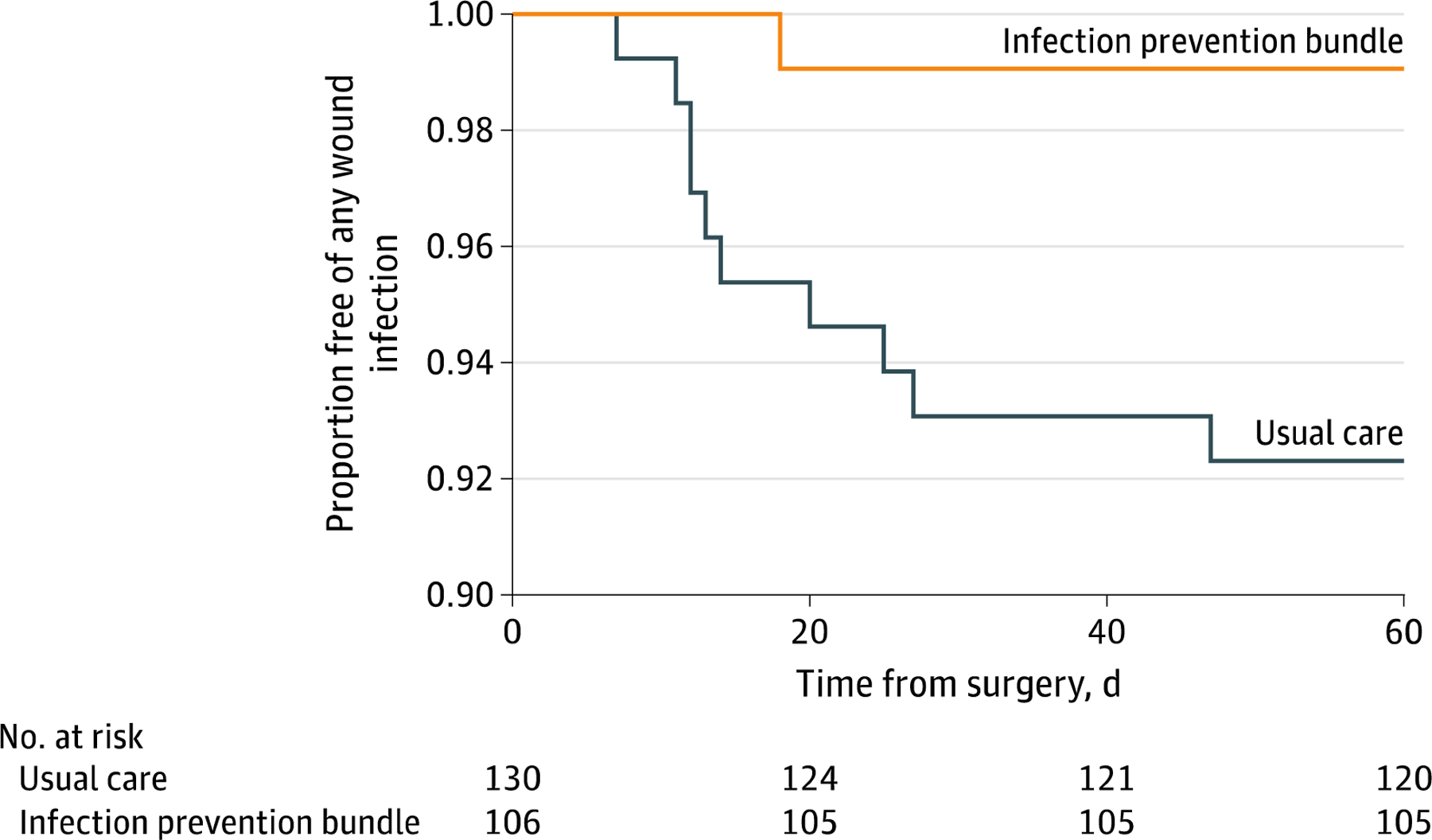
Effect of the multifaceted program on surgical site infections
Ancillary analyses:
There were fewer treatment surveillance units with any S. aureus transmission detection with feedback as compared to surveillance units before feedback, (3/38 cases study months 5–8 (after feedback) vs. 11/38 cases study months 1–4 (before feedback), risk ratio 0.27, 95% CI 0.08–0.90, P = 0.04). The impact of failure mode analysis in achieving a sustained reduction in S. aureus transmission during the study period and a description of specific feedback utilized and lessons learned can be found in appendix C.
Discussion
The CDC has emphasized the importance of basic preventive measures for infection prevention.6,7 We have shown that improvements in basic, perioperative preventive measures can reduce S. aureus transmission and SSIs.
While most SSI prevention efforts have focused on host optimization and inhibition of bacterial virulence,9–11 a substantial body of evidence indicates the need for improved basic preventive measures. 12–15 A multimodal program targeting parallel improvements in provider hand hygiene, intravascular catheter disinfection, environmental cleaning, and patient decolonization is needed to address the complex interplay of intraoperative bacterial reservoirs. 17 Surveillance is needed to mitigate component failure and/or fatigue.15,18 The individual efficacy of several interventions in addressing these reservoirs has been demonstrated,12–14 and a surveillance system for monitoring perioperative bacterial transmission has been developed as a feedback mechanism.15,18 We combined these individual components to create an evidence-based, multifaceted approach for perioperative infection prevention that we hypothesized would generate substantial reductions in perioperative S. aureus transmission and SSIs.
Our randomized study design accounted for patient decolonization strategies that would otherwise potentially confound the primary endpoint, and enrolled patients represented typical surgical populations. While treatment patients were older and sicker overall, these variables were not associated with the primary or secondary study outcomes which is consistent with prior work. 17,18
Treatment reduced perioperative S. aureus transmission, an outcome associated with increased risk of SSI development. In turn, a reduction in S. aureus transmission correlated with a reduction in SSIs. Two prior trials have shown a correlation between attenuation of perioperative bacterial transmission and infection reduction.12,13 A controlled before and after study involving 111 ORs randomized to a hand hygiene improvement strategy leveraging provider proximity was conducted at Dartmouth. The authors found that a several-fold improvement in provider hand hygiene above baseline significantly reduced stopcock and environmental transmission along with postoperative healthcare-associated infections (HAIs). 12 In another study at Dartmouth, a randomized clinical trial involving 572 patients demonstrated the efficacy of a catheter care station incorporating improved disinfection of injection ports and syringe tips in reducing high-risk stopcock transmission events and postoperative HAIs. HAI reductions included SSIs for both trials. Trial limitations included single site implementation, a single intervention approach, an intraoperative focus, and failure to demonstrate sustainability during the intervention period or to account for seasonal variation.12,13,25
The current study addressed these prior limitations. Intervention12,13 efficacy was confirmed at the University of Iowa, thereby providing evidence of intervention efficacy beyond Dartmouth. We utilized a multimodal approach to address all perioperative reservoirs with proven contributions to transmission and infection including patient skin sites, environmental sites, provider hands, intravascular catheter injection ports, and syringe tips.13,17 The interventions were applied perioperatively, and surveillance was employed to monitor the interventions to achieve sustainability during the study period.15,18, 25 The study duration accounted for seasonal variation.26 As expected, this comprehensive, evidence-based approach generated an effect that exceeded prior studies utilizing a single intervention approach12,13 and those addressing a single contributing reservoir (i.e. patient decolonization).14, 30 Thus, we have confirmed greater efficacy of a set of evidence-based interventions in preventing S. aureus transmission and infection development as compared to usual perioperative infection control practice. The measures that we utilized are simple to implement, widely available, and serve to facilitate better patient care.12,13,14,15,18,19–21
A solid body of published evidence utilized whole cell genome and single nucleotide variant analysis to confirm the contributions of perioperative patient, provider hand, environmental, and stopcock reservoirs to postoperative infections and increased patient mortality across multiple academic medical centers. 13,15, 17, 28, 29 The current study supports these prior findings by showing an association of S. aureus transmission with increased risk of SSI development. Based on past and current evidence of causality and demonstrative ability to attenuate known risk factors for SSI development by addressing proven reservoirs, it is ill-advised to ignore CDC recommendations to improve basic preventive measures to prevent bacterial spread and associated infection development.6,7
Study Limitations:
Bacterial strain characteristics at the University of Iowa may not represent those at other hospitals. However, the studied interventions have proven efficacy at multiple hospital sites,12–14 and surveillance feedback will allow hospital sites to optimize bundle implementation and address pathogens beyond S. aureus.18,27 Most epidemiologically-related transmission links identified via our model for study of bacterial cross contamination are confirmed with single nucleotide variant analysis. 17,18,28,29 We have demonstrated the utility of this approach in group level feedback, an important contribution to the substantial and sustained reductions in S. aureus transmission and SSIs achieved in this study.
In conclusion, improved perioperative basic preventive measures can reduce perioperative S. aureus transmission and SSIs. Widespread adherence to CDC recommendations for improved basic preventive measures to reduce the spread of bacteria and associated infections is indicated to improve perioperative patient safety.
Supplementary Material
Key Points:
Questions:
What is the impact of Centers for Disease Control and Prevention recommendations emphasizing improved basic preventive measures for prevention of bacterial transmission and infection development on perioperative S. aureus transmission and surgical site infections?
Findings:
In this randomized clinical trial involving 236 adult patients, sustained improvements in basic, perioperative preventive measures resulted in a substantial reduction in S. aureus transmission that correlated with a significant reduction in surgical site infections.
Meaning:
Perioperative adherence to CDC recommendations for improved basic preventive measures to prevent bacterial spread and infection development is indicated to improve perioperative patient safety.
Acknowledgements:
This study was funded by the Anesthesia Patient Safety Foundation (APSF) with provided funds utilized for collection, management, analysis, and interpretation of the data and by the Department of Anesthesia, University of Iowa, with funds utilized for data collection and management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Randy W. Loftus reported research funding from Sage Medical Inc., BBraun, Draeger, and Kenall, has one or more patents pending, and is a partner of RDB Bioinformatics, LLC, and 1055 N 115th St #301, Omaha, NE 68154, a company that owns OR PathTrac, and has spoken at educational meetings sponsored by Kenall (AORN) and BBraun (APIC). Other authors reported no conflicts of interest. Surfacide, Waukesha, WI 53188 provided the Helios equipment for use during the study period at no charge. Surfacide had no access to the study data and provided no contributions to the manuscript. The authors acknowledge Hogan J, Rosenberg R, Glass L, Kass G, Greene S, Clark B, and Lyons Y for their assistance with patient enrollment. The authors also acknowledge MacKenzie T for assistance with trial design. Written permission was received for acknowledgement. Drs. Loftus and Brown had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Randy W. Loftus, Department of Anesthesia, University of Iowa.
Franklin Dexter, Department of Anesthesia, University of Iowa.
Michael J. Goodheart, Department of Gynecology/Oncology, University of Iowa.
Megan McDonald, Department of Gynecology/Oncology, University of Iowa.
John Keech, Department of Thoracic Surgery, University of Iowa.
Nicolas Noiseux, Department of Orthopaedic Surgery and Rehabilitation, University of Iowa.
Andrew Pugely, Department of Orthopaedic Surgery and Rehabilitation, University of Iowa.
William Sharp, Department of Vascular Surgery, University of Iowa.
Mel Sharafuddin, Department of Vascular Surgery, University of Iowa.
W. Thomas Lawrence, Department of Surgery, Plastic and Reconstructive Surgery, University of Iowa.
Mark Fisher, Department of Plastic and Reconstructive Surgery, University of Iowa.
Patrick McGonagill, Department of Acute Care Surgery, University of Iowa.
Jennifer Shanklin, Department of Acute Care Surgery, University of Iowa, now at Allina Health Surgical Specialists, Abbott Northwestern.
Dionne Skeete, Department of Acute Care Surgery, University of Iowa.
Chad Tracy, Department of Urology, University of Iowa.
Bradley Erickson, Department of Urology, University of Iowa.
Thomas Granchi, Department of Acute Care Surgery, University of Iowa.
Lance Evans, Department of Anesthesia, University of Iowa.
Eli Schmidt, Department of Anesthesia, University of Iowa.
Joshua Godding, Department of Anesthesia, University of Iowa.
Raven Brenneke, Department of Anesthesia, University of Iowa.
Deanna Persons, Department of Anesthesia, University of Iowa.
Alexia Herber, Department of Anesthesia, University of Iowa.
Mark Yeager, The Department of Anesthesia, Geisel School of Medicine at Dartmouth.
Brent Hadder, Department of Anesthesia, University of Iowa.
Jeremiah Brown, The Department of Epidemiology, Geisel School of Medicine at Dartmouth.
References:
- 1.Magill SS, O’Leary E, Janelle SJ et al. ; Emerging Infections Program Hospital Prevalence Survey Team. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N Engl J Med 2018; 379:1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magill SS, Hellinger W, Cohen J, et al. Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol 2012; 33: 283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher HW, Talbot GH, Bradley JS, et al. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clinical Infectious Diseases 2009; 48:1–12. [DOI] [PubMed] [Google Scholar]
- 4.Vogel TR, Dombrovskiy VY, Lowry SF. Impact of Infectious Complications after Elective Surgery on Hospital Re-Admission and late Deaths in the U.S. Medicare Population. Surg Infect (Larchmt) 2012; 13: 307–1. [DOI] [PubMed] [Google Scholar]
- 5.Awad SS, “Adherence to surgical care improvement project measures and postoperative surgical site infections”. Surgical Infection (Larchmt) 2012, 13: 234–7. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Updated guidelines for evaluating public health surveillance systems: recommendations from the guidelines working group. MMWR 2001; 50:1–35. [PubMed] [Google Scholar]
- 7.http://www.whitehouse.gov/the-press-office/2014/09/18/fact-sheet-obama-administration-takes-actions-combat-antibiotic-resistan. FACT SHEET: Obama Administration Takes Actions to Combat Antibiotic-Resistant Bacteria. The White House, Office of the Press Secretary. Accessed September 1st, 2018.
- 8.https://grants.nih.gov/grants/guide/pa-files/PA-16-423.html. Accessed March 5th, 2019.
- 9.Rubin RH. Surgical wound infection: epidemiology, pathogenesis, diagnosis and management. BMC Infectious Disease 2006; 6: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stulberg JJ, Delaney CP, Neuhauser DV, et al. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA 2010; 303: 2479–85. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Hong S, Liu Y, Duan Y et al. High inspired oxygen versus low inspired oxygen for reducing surgical site infection: a meta-analysis. Int Wound J 2017; 14: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koff MD, Loftus RW, Burchman CC, et al. Reduction in intraoperative bacterial contamination of peripheral intravenous tubing through the use of a novel device. Anesthesiology 2009; 110:978–85. [DOI] [PubMed] [Google Scholar]
- 13.Loftus RW, Brindeiro BS, Kispert DP, et al. Reduction in intraoperative bacterial contamination of peripheral intravenous tubing through the use of a passive catheter care system. Anesth Analg 2012; 115:1315–23. [DOI] [PubMed] [Google Scholar]
- 14.Phillips M, Rosenberg A, Shopsin B, et al. Preventing Surgical Site Infections: A Randomized, Open-label Trial of Nasal Mupirocin Ointment and Nasal Povidone Iodine Solution. Infect Control Hosp Epidemiol 2014; 35: 826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loftus RW, Dexter F, Robinson ADM. High-risk Staphylococcus aureus transmission in the operating room: A call for widespread improvements in perioperative hand hygiene and patient decolonization practices. Am J Infect Control 2018; 46:1134–1141. [DOI] [PubMed] [Google Scholar]
- 16.Kretzer EK, Larson EL. Behavioral interventions to improve infection control practices. Am J Infect Control 1998; 26: 245–53. [DOI] [PubMed] [Google Scholar]
- 17.Loftus RW, Brown JR, Koff MD, et al. Multiple reservoirs contribute to intraoperative bacterial transmission. Anesth Analg 2012; 114: 1236–48. [DOI] [PubMed] [Google Scholar]
- 18.Robinson ADM, Dexter F, Renkor V, et al. Operating room PathTrac analysis of current intraoperative Staphylococcus aureus transmission dynamics. Am J Infect Control 2019; 47:1240–1247. [DOI] [PubMed] [Google Scholar]
- 19.Clark C, Taenzer A, Charette K, et al. Decreasing contamination of the anesthesia environment. Am J Infect Control 2014; 42:1223–5. [DOI] [PubMed] [Google Scholar]
- 20.Wilson AP, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011; 39:651–8. [DOI] [PubMed] [Google Scholar]
- 21.Anderson DJ, Chen LF, Weber DJ, et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): A cluster-randomized, multicenter crossover study. Lancet 2017; 389: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC. National Healthcare Safety Network (NHSN) Patient Safety Component Manual https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed November 15th, 2018.
- 23.Loftus RW, Koff MD, Brown JR, et al. The epidemiology of Staphylococcus aureus transmission in the anesthesia work area. Anesth Analg 2015; 120:807–18. [DOI] [PubMed] [Google Scholar]
- 24.Prasad K, Jaeschke P, Wyer S, et al. Tips for teachers of evidence-based medicine: understanding odds ratios and their relationship to risk ratios. J Gen Intern Med 2008; 23: 635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durkin MJ, Dicks KV, Baker AW, et al. Seasonal Variation of Common Surgical Site Infections: Does Season Matter? Infect Control Hosp Epidemiol 2015; 36: 1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area. Infect Control Hosp Epidemiol 2018:1–17. doi: 10.1017/ice.2018.303. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Hadder B, Patel HM, Loftus RW. Dynamics of intraoperative Klebsiella, Acinetobacter, Pseudomonas, and Enterobacter transmission. Am J Infect Control 2018; 46:526–532. [DOI] [PubMed] [Google Scholar]
- 28.Loftus RW, Dexter F, Robinson ADM. Methicillin-resistant Staphylococcus aureus has greater risk of transmission in the operating room than methicillin-sensitive S aureus. Am J Infect Control 2018; 46: 520–525. [DOI] [PubMed] [Google Scholar]
- 29.Loftus RW, Dexter F, Robinson ADM, et al. Desiccation Tolerance is Associated with Staphylococcus aureus Hyper Transmissibility, Resistance, and Infection Development in the Operating Room. J Hosp Infect 2018;100: 299–308. [DOI] [PubMed] [Google Scholar]
- 30.Schweizer ML, Chiang HY, Septimus E, Moody J, Braun B, Hafner J, Ward MA, Hickok J, Perencevich EN, Diekema DJ, Richards CL, Cavanaugh JE, Perlin JB, Herwaldt LA. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA 2015; 313:2162–71. PMID: 26034956 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


