Abstract
Genome-wide association studies across diverse populations may help validate and confirm genetic contributions to risk of disease. We estimated the extent of population stratification as well as the predictive accuracy of polygenic scores (PGS) derived from European samples to a data set from India. We analysed 2685 samples from two data sets, a population neurodevelopmental study (cVEDA) and a hospital-based sample of bipolar affective disorder (BD) and obsessive-compulsive disorder (OCD). Genotyping was conducted using Illumina′s Global Screening Array.
Population structure was examined with principal component analysis (PCA), uniform manifold approximation and projection (UMAP), support vector machine (SVM) ancestry predictions, and admixture analysis. PGS were calculated from the largest available European discovery GWAS summary statistics for BD, OCD, and externalizing traits using two Bayesian methods that incorporate local linkage disequilibrium structures (PGS-CS-auto) and functional genomic annotations (SBayesRC). Our analyses reveal global and continental PCA overlap with other South Asian populations. Admixture analysis revealed a north-south genetic axis within India (FST 1.6%). The UMAP partially reconstructed the contours of the Indian subcontinent.
The Bayesian PGS analyses indicates moderate-to-high predictive power for BD. This was despite the cross-ancestry bias of the discovery GWAS dataset, with the currently available data. However, accuracy for OCD and externalizing traits was much lower. The predictive accuracy was perhaps influenced by the sample size of the discovery GWAS and phenotypic heterogeneity across the syndromes and traits studied. Our study results highlight the accuracy and generalizability of newer PGS models across ancestries. Further research, across diverse populations, would help understand causal mechanisms that contribute to psychiatric syndromes and traits.
INTRODUCTION:
Psychiatric genomics has witnessed tremendous growth over the past two decades CITE. The advent of genome-wide association studies (GWAS), along with the formation of international consortia and biobanks, have led to a plethora of genetic associations for psychiatric disorders and brain traits. The success of GWAS has also led to the development of multiple analysis methods to elucidate biological and clinical relevance of these findings (1).
Polygenic score (PGS) is one such method that predicts an individual’s inherited risk for a trait, and is derived as a weighted sum of genomic risk alleles (2) based on GWAS findings. While GWAS requires large sample sizes to detect common variants of small effects, the PGS can be calculated at the level of individuals. Therefore, PGS can be useful for stratifying individuals, for risk of disease, risk based on their genomic variants (3).PGS has gained popularity as a predictive tool for various common disorders. For instance, in coronary artery disease, PGS can identify individuals at increased risk, and the predictive power could be increased even further by combining PGS with clinical risk factors (4–6). In psychiatry, although the discriminative power of PGS is not yet suitable for clinical application, the latest GWAS on schizophrenia has demonstrated promising outcomes, revealing a notably high odds ratio (OR) of 39 (CI 29–53) when comparing the case/control ratio between the highest and lowest deciles of the PGS (7).
However, the under-representation of several global populations represents a major limitation in psychiatric genomics and PGS research. Recent estimates from GWAS of schizophrenia, bipolar disorder (BD) and major depressive disorder suggest that individuals of South Asian ancestry make up less than 1% of these samples (8). The Eurocentric nature of GWAS may limit the optimal transferability of PGS, and may thus restrict the extent to which the genetic discoveries can be extrapolated across diverse populations. As a case in point, the largest meta-analysis from the PGC Schizophrenia Working Group included only 25% participants of non-European ancestry (7). Further, the use of PGS, derived mainly from European-based GWAS, may reinforce or even widen existing health disparities when applied in clinical settings (9). There is now accumulating evidence to suggest that the use of multi-ancestry PGS may perform better than single-ancestry PGS (10). Hence, addressing the lack of ancestral diversity is critical for finessing our findings; as also for an equitable distribution of its potential benefits.
Despite its large share in the global population, the contribution to psychiatry GWAS from India has been sparse. Given the complex population history (11–13), population substructures resulting from geography, linguistics and the historical practice of endogamy (14), there has been a reluctance to apply GWAS and PGS methods in India. However, the advent of large imputation panels and globally representative genotyping arrays, studies have demonstrated feasibility and potential utility of polygenic approaches in India (14). A GWAS on schizophrenia from southern India identified a genome-wide significant locus modifying the expression of NAPRT1 (15). GWAS in other complex diseases such as diabetes (16) and dyslipidemia (17) have implicated loci, which were previously identified in other continental populations.
This study investigates the predictive accuracy of cross-ancestry PGS for psychiatric phenotypes.. We use state-of-the-art Bayesian methods and also incorporate functional genomic annotations to improve portability from European discovery GWAS. We evaluate the predictive accuracy for BD, OCD, and externalizing symptoms. Moreover, we assess population stratification and admixture levels within these Indian samples, to inform future sampling strategies for Indian ancestry GWAS.
METHODS:
Samples and phenotypes
Individuals diagnosed to have BD or OCD through the clinical services at NIMHANS were identified, and DNA samples were collected, after informed consent. The cVEDA sample consisted of community samples, identified at random, as part of an ongoing study (ref here)Control subjects were volunteers from the general population, with no lifetime history of psychiatric disease in themselves, or their first degree relatives. All subjects provided written informed consent, and the studies were approved by the ethics committee at NIMHANS.
We used genetic data of consenting individuals from the general population and also patients with OCD or BD. Individuals diagnosed with psychiatric disorders, specifically BD and OCD were identified through clinical services at NIMHANS, Bengaluru, India. All diagnoses were independently confirmed by two experts through detailed clinical interviews and review of medical records, as per the ICD-10 criteria for BD (codes F30 and F31) and OCD (code F42). Most BD patients met the criteria for BD1 syndrome as defined by the DSM5-TR; while those with OCD patients had a moderate to severe illness according to the Clinical Global Impressions Scale.
Individuals without a lifetime psychiatric diagnosis or first-degree family history of psychiatric illness were included as controls along with cVEDA participants. The cVEDA, is a longitudinal study investigating the effects of environmental factors and genomic variations on neurodevelopment and susceptibility to externalizing disorders. The study participants have undergone assessments that covered socio-demographics, temperament, environmental exposures, parenting styles, psychiatric morbidity, and cognitive functioning (18,19). For this study, we focused on the externalizing score from the Strengths and Difficulties Questionnaire (SDQ) CITE, which measures hyperactivity/inattention and conduct problems.
Genotyping, quality control and imputation
Genotyping was conducted in three batches using the Illumina Global Screening Array, followed by batch-specific quality control using a customized pipeline based on Plink 1.9 and KING version 2.3.2. Samples with genotyping rate < 0.95, F_heterozygosity > 0.2, and genotype-phenotype sex mismatches were removed from the analysis. SNPs were filtered out if these were monomorphic, or had a call rate < 0.95, or deviated from Hardy-Weinberg equilibrium p < 1e−6. Pairs of related individuals were identified using the --related function in KING and all first, second, and third degree relatives were excluded. After quality control, the genotypic data of each batch were as follows: Batch1-GSAv2+ had 996 subjects and 619,022 SNPs, Batch2-GSAv3 had 915 subjects and 545,877 SNPs, and Batch3-GSAv3+ included 774 subjects with 633,293 SNPs. Figure S2 illustrates a consistent ~81% overlap in SNP intersections across the three batches, maintained both pre- and post-QC. A detailed description of genotyping and quality control is provided in the supplementary material.
Following quality control, genotype data was phased using Eagle v2.4 and unobserved genotypes were imputed using Minimac4 with four reference panels: Trans-Omics for Precision Medicine (TOPMed) version r2, Haplotype Reference Consortium (HRC) Version r1.1 2016, 1000 Genomes Phase 3 (Version 5) and the Genome Asia Pilot v1 – (GAsP) on the Michigan Imputation Server. The imputation accuracy was evaluated using info scores (r2) across a range of allele frequencies for each reference panel. Following this, “hard call” genotypes from the top-performing panel were filtered for an imputation info score (r2) > 0.3 and a minor allele frequency > 0.01. The filtered genotypes were merged for further analysis, resulting in 7,325,802 markers.
Principal Component Analysis (PCA)
To examine population structure, we conducted PC analysis at three levels: global PCA in conjunction with 1000 Genomes (1000G), continental PCA with Human Genome Diversity Project (HGDP) and Genome Asia Pilot (GAsP), and within-sample PCA. Prior to conducting PCA, the genotype data were pruned for linkage disequilibrium (LD) using an r2 of 0.2 and window size of 50 kb and step size of 5 variants. In addition, 24 autosomal regions exhibiting long-range LD and spanning more than 2 Mb were excluded because long-range LD regions (e.g., the extended MHC region) can unduly influence PCA, which can lead to the masking of more subtle genome-wide variation patterns, particularly in admixed samples. This resulted in a final set of 759,439 markers.
For the global and continental PCAs, we merged our samples with publicly available reference datasets. While merging, SNPs with unambiguous allele labels were auto-flipped, while those with ambiguous (A/T, C/G) or inconsistent allele labels were excluded. We also removed any first-, second- and third-degree relatives present in the reference data. The global PCA used 1000G data, including 631 African, 330 Amerindian, 494 East Asian, 500 European, and 454 South Asian samples. Two subsequent Asian sub-continental PCAs (HGDP and GAsP) were conducted to examine how our sample clusters within these sub-continental population structures.
Ancestry Prediction
We implemented a SVM-based method to identify the most likely ancestral groups for each individual in our sample based on the first 10 global PCs and known ancestry from the 1000 Genomes Project data. The R package ‘e1071’ was used for SVM. An initial parameter grid for the radial kernel SVM was defined in terms of cost and gamma parameters. We used 5-fold cross-validation, repeating the process twice to identify the optimal hyperparameters. Using the best parameters, an SVM model was trained and employed to predict the most probable ancestral group of each individual. The predicted ancestral groups were assigned along with associated probabilities.
Uniform Manifold Approximation and Projection (UMAP)
The UMAP algorithm was implemented in Python, utilizing the UMAP-learn library, configured with Euclidean distance metric, a neighborhood size of 15, and a minimum distance of 0.1. The first 15 PCs from within-sample PCA were selected for UMAP analysis. The resulting low-dimensional embeddings were plotted with the SVM predicted ancestry labels from the previous step to visualize the within-sample population structure.
Admixture
Admixture proportions were assessed using ADMIXTURE (v1.3.0). The analysis was performed on genotype data that had been pruned for LD (see above). In alignment with prior literature CITATION, we employed a larger number of markers (272,963) for analysis to achieve effective population resolution, given that the required marker count is inversely proportional to the genetic distance (FST) between the populations under study. The optimal number of ancestral components (K) was determined based on the lowest 10-fold cross-validation error. The algorithm for ADMIXTURE employs maximum likelihood estimation, block relaxation for iterative parameter updates, and a quasi-Newton method for accelerated convergence. A subset of samples with high-confidence self-reported geocodes were plotted on the map of India to examine the geographical distribution of ancestry proportions.
Polygenic scores (PGS):
Cross-ancestry PGS were constructed for BD, OCD, and externalizing symptoms using public GWAS summary statistics from European ancestry populations. The discovery cohorts, of individuals of confirmed European ancestry, included 41,917 BD cases and 371,549 controls from the 2021 PGC3 analysis (20); and another 6,848 OCD cases and 18,812 controls from the OCD PGC GWAS (21). For the broad externalizing phenotype, we utilized summary statistics from the genomic structural equation modeling (Genomic SEM) with 1,045,957 participants in the Externalizing Consortium (excluding 23andMe samples as required by this company) (22). For the PGS calculation, we employed three methods: classical clumping and thresholding (C+T) via PRSice-2 (23) and the Bayesian methods PGS-CS-auto (24) and SBayesRC (25). For PRSice-2, the best performing PGS was calculated at a range of P-value thresholds (0.001,0.05,0.1,0.2,0.3,0.4,0.5,1) with default parameters for clumping with a window of 250 kb and r2 of 0.1. PGS-CS-auto utilizes a Bayesian regression model with continuous shrinkage priors for posterior SNP effect inference and is robust to different genetic architectures. It models local LD structures using an external reference panel of around 1 million HapMap3 markers. SBayesRC incorporates an LD panel with about 7 million imputed common SNPs along with 96 functional genomic annotations. It employs a hierarchical multi-component mixture prior to adjusting annotation information, thereby influencing the probability and effect size of a SNP being causal. PGS-CS-auto and SBayesRC estimate all parameters jointly within a Bayesian framework, eliminating the need for parameter tuning.
PGS Predictive performance:
To evaluate the predictive performance of the PGS, we employed a comprehensive set of metrics, with adjustments for covariates including sex, SNP batch, and the first seven principal components of the genotype data. These metrics included: 1) Nagelkerke’s R2 (for BD and OCD) and adjusted R2 (for externalizing symptoms); 2) Area under the receiver operating characteristic curve (AUC) was calculated for BD and OCD to evaluate the discriminatory power of the PGS; and 3) ORs were estimated to compare the risk between the top 10% of individuals for a given PGS, to the bottom and middle 10% of the sample.
Multi-PGS Association Analysis
To assess the specificity of associations for BD, OCD and externalizing symptoms, we also attempted an exploratory multi-PGS association analysis with over 3,800 PGSs spanning 18 trait categories, defined according to the Experimental Factor Ontology (EFO) in the PGS Catalog (26). These scores were calculated with ‘pgs-calc’ using default parameters without adjusting for ancestral differences in linkage disequilibrium (LD) or minor allele frequency (MAF). A Bonferroni-corrected significance threshold was set to α = 1.3 × 10−5 for this analysis, conservatively correcting for total number of PGS tested.
RESULTS:
Following quality control and imputation, the final sample available for subsequent analyses consisted of 2,685 unrelated individuals. These included those with BD (N=463; 47% female), OCD (N=554; 40% female), other psychiatric diagnoses (N=154; 32% female), and neurotypical community controls (N=1514; 48.5% female). The SDQ data on 963 individuals showed a mean Externalizing score of 5.9 (SD=3.6.
As expected, imputation accuracy improved with increasing allele frequency for Indian samples across all four imputation reference panels: HRC, TOPMed, GAsP1, and 1000G (see Supplementary Fig 1). HRC demonstrated the highest accuracy, followed by TOPMed and GAsP1, while 1000G showed the lowest accuracy.
Population Structure
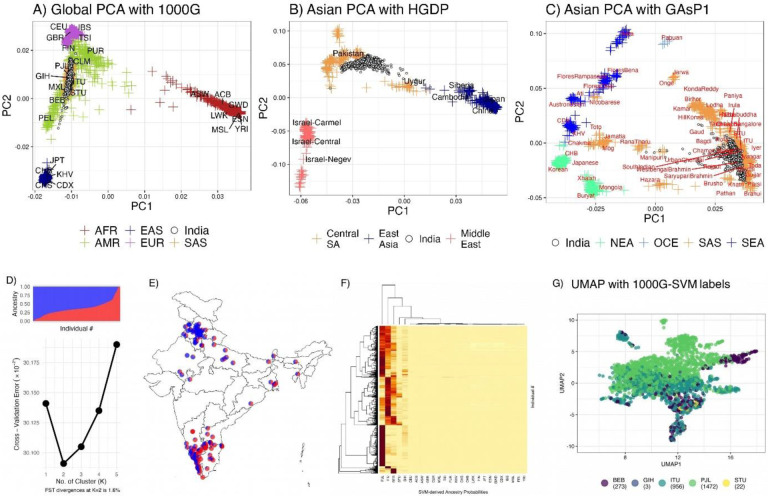
We employed PCA, UMAP, and ADMIXTURE for assessment of population structure in our study. Global PCA with 1000G data showed that the Indian samples positioned between European and East Asian clusters, with overlap on all the South Asian population labels (Fig 1A). We also observed intra-regional variability, extending along the PC1 axis but condensed along PC2.
Figure 1:
Population structure and genetic diversity in Indian samples
Global PCA (1A) illustrates the placement of Indian samples between European and East Asian clusters, highlighting notable regional variability. Asian PCA with HGDP samples (1B) reveals distinct clustering of Indian samples, absent in HGDP, situated between Pakistani and Uyghur populations. Asian PCA with Genome Asia samples (1C) demonstrates significant overlap with urban South Asians, indicating urban genetic diversity. ADMIXTURE analysis (1D) reveals a two-cluster genetic structure with a 1.6% F_ST, reflecting modest diversity. Geographic mapping of a subset of samples (1E) presents a north-south ancestral gradient, correlated with latitude. SVM predictions (F) show clustering around key South Asian populations, while UMAP visualization (G) delineates distinct ancestral clusters corresponding to geographical regions, highlighting spatial genetic patterns in India
The Asian PCA plots reveal two contrasting patterns. In the first plot that incorporates HGDP populations, Indian samples do not directly overlap with any HGDP groups. This may well be due to lack of specific Indian ancestry data in HGDP (Fig 1B). Our samples clustered between the Pakistani and Uyghur (China) populations. PCA with Genome Asia Phase 1 data shows that most of our samples form a cluster, substantially overlapping with urban samples from the Genome Asia Phase 1 data (Fig 1C). Considering Genome Asia’s strategic sampling to capture diversity, this overlap suggests that our samples predominantly reflect the genomic spread among urban South Asian populations.
Admixture analysis identified a two-cluster solution for the dataset, supported by an inflection point at K=2 in the cross-validation error curve (Fig 1D). The FST between these inferred ancestries was 1.6%, surpassing the standard 1% threshold, indicating a modest level of genetic diversity within the sample. Geographic mapping of a subset of samples with available geocodes indicated that ancestral proportions across India are distributed along a north-south gradient (Fig 1E, correlation with latitude r=0.7), thereby suggesting a spatial alignment of genetic ancestry within the country.
The SVM model robustly predicted ancestral groups, assigning probabilities that revealed significant clustering around the PJL, ITU, and BEB populations, with notable representation in STU and GIH, while other groups were less represented (Fig F). Using ancestry predicted by SVM with 1000G global principal components, the UMAP visualization (Fig 1G) approximates the geographical distribution of ancestries in India. Notably, the plot delineates distinct clusters corresponding to Eastern (BEB), Northern (PJL), and Southern Indian (ITU, STU) ancestry groups, which align closely with their respective geographical origins.
Polygenic Prediction of Psychiatry Phenotypes
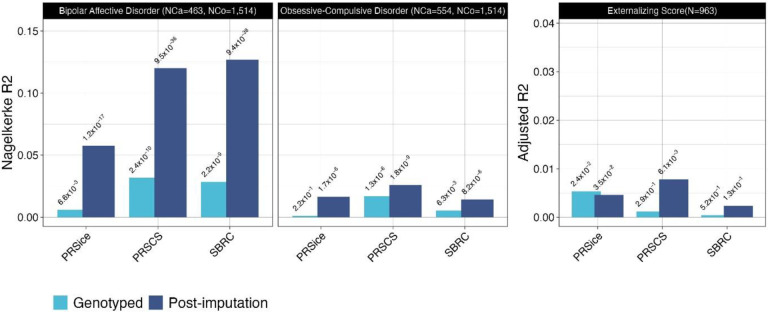
We assessed the predictive accuracy of PGS models for BD, OCD, and externalizing traits using PRSice-2, PGS-CS-auto, and SBayesRC. The patterns across the PGS models for BD, OCD, and externalizing traits were consistent (as expected) with imputed data yielding higher predictive accuracy than genotyped data (Figure 2). Notably, Bayesian methods (PGS-CS-auto and SBayesRC) outperformed the classical C+T approach (PRSice-2).
Figure 2:
Cross-ancestry polygenic score model comparison for psychiatric phenotypes
Comparison of European-GWAS derived PGS predictive accuracy for BD, OCD, and externalizing traits in an Indian cohort using PRSice-2, PGS-CS-auto, and SBayesRC. Imputed data shows enhanced prediction, with Bayesian methods outperforming PRSice-2 in cross-ancestry application.
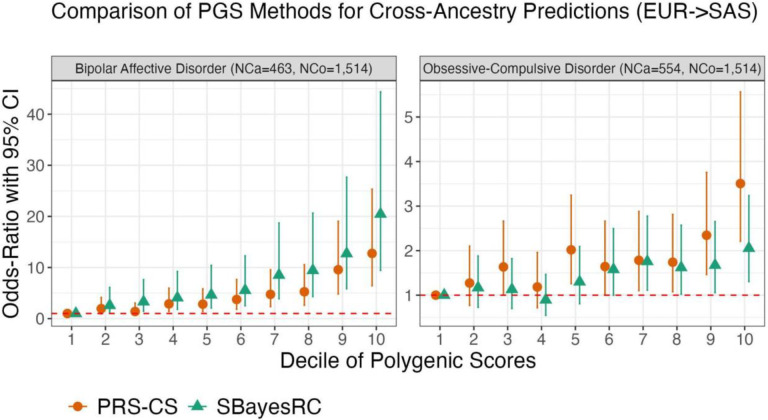
For BD, the application of PGS yielded statistically significant results using both Bayesian methodologies. The p-values were highly significant at 9.49×10−36 for PGS-CS-auto and 9.38×10−38 for SBayesRC, accompanied by Nagelkerke R2 values of 0.120 and 0.127, respectively. Furthermore, when assuming a 1% population prevalence, these PGS models explain approximately 6.5% (using PGS-CS-auto) and 6.9% (using SBayesRC) of the phenotypic variance on the liability scale. The discriminatory ability of these models is substantiated by an AUC of around 71% for both methods. In terms of clinical risk stratification, the PRS-CS-auto method yields an ORs of 3.5 (95% CI 2.2–5.6) and 12.8 (95% CI 6.4–25.3) for the individuals in the top decile of being affected with the disorder, when compared to individuals in the middle and lowest deciles, respectively (Figure 3). In contrast, SBayesRC yields higher corresponding ORs of 3.8 (95% CI 2.4–5.9) and 20.5 (95% CI 9.4–44.4).
Figure 3:
Decile-based risk stratification in BD and OCD
Both PRS-CS-auto and SBayesRC methods stratify risk across deciles for BD (3A) and OCD (3B), with BD showing greater discriminative power.
For OCD, the application of PGS also showed significant findings using both PRS-CS-auto and SBayesRC methods. The p-values were significant at 1.84×10−9 for PRS-CS-auto and 8.23×10−6 for SBayesRC, with corresponding Nagelkerke R2 values of 0.026 and 0.014, respectively. When assuming a 1% population prevalence, these models account for approximately 1.3% (PRS-CS-auto) and 0.7% (SBayesRC) of the phenotypic variance on the liability scale. The models’ discriminatory ability was lower compared to BD, with an AUC of approximately 59% for both methods. In terms of clinical risk stratification for OCD, PRS-CS-auto yields odds ratios of 2.2 (95% CI 1.4–3.4) and 3.5 (95% CI 2.2–5.6) individuals in the top and lowest deciles, respectively, as compared to those in the middle decile. SBayesRC demonstrates lower odds ratios of 1.3 (95% CI 0.9–2.1) and 2.1 (95% CI 1.3–3.2).
For externalizing symptoms, the PRS-CS-auto model accounted for approximately 0.78% of the variance in the trait, with a coefficient estimate of 0.094 (95% CI: 0.027–0.160, p-value = 0.006). In contrast, the SBayesRC method explained a smaller variance of 0.23% and a coefficient estimate of 0.057 (95% CI: −0.017–0.130, p-value = 0.133).
Discussion
In this study, we evaluated the predictive accuracy of PGS derived from European ancestry populations as applied to an Indian sample. We also evaluated the genetic structure of several samples ascertained in India with the intent of assessing its genetic diversity, both globally and regionally.
We identified a robust cross-ancestry overlap in BD PGS. Our sample included only those with well-characterized BD1 and high heritability. This hints at considerable overlap in underlying biological mechanisms across ancestries. Interestingly, OCD PGS also showed a significant cross-ancestry prediction, albeit with smaller effect size compared to BD likely due to the far smaller number of cases in the European OCD GWAS. Overall, our analyses demonstrate that PGS models, developed from European cohorts and applied to Indian samples using Bayesian methodologies that adjust for local LD patterns and incorporate functional genomic annotations, maintain statistical significance for psychiatric diagnoses. These findings suggest a shared polygenic liability across populations, indicating that the genetic foundations of psychiatric disorders remain consistent, and are detectable, across diverse ethnic backgrounds (27).
The PGS for externalizing symptoms exhibited lesser overlap than that found for BD and OCD. This difference could be attributed to the methods of phenotypic ascertainment. Individuals with BD and OCD were identified through the clinical services, evaluated using structured face-to-face interviews, and diagnosed using well established and reliable diagnostic guidelines. Those with externalizing traits are typically gauged through standardized questionnaires in varied settings, resulting ina lower diagnostic accuracy. Moreover, the inherent genetic attributes of each disorder may impact the effectiveness of PGS. For example, BD may present more consistent genetic signals, on account of its greater heritability, when compared to externalizing traits, which are likely influenced by a wider array of genetic and environmental factors.
The genetic diversity observed in our sample is broadly comparable to that seen in European populations, which exhibit variation along both East-West and North-South axes (28,29). The investigation of genetic risks for common disorders, prevalent across all populations, underscores the necessity of including large, continental samples. Despite these encouraging findings, it may still be useful to assess the impact of specific patterns of diversity, within sub-populations, on these risks. Such approaches promise to enhance the generalizability of genetic models in research, targeting the genetic underpinnings of common medical conditions
The lack of global diversity in genetic studies impacts the generalizability of findings for underrepresented populations, and constrains the understanding of genetic bases of traits across varied groups. This is relevant in psychiatric disorder research, which has predominantly included individuals of European ancestry to identify associated genetic loci. The epidemiology, and semiology, of psychiatric syndromes such as BD1 and OCD are quite similar all over the world. This suggests that there is likely a similar genetic liability, with a shared set of genomic loci influencing these phenotypes across populations (30). Nonetheless, ancestry-related variations in LD patterns, with the true causal variant, as well as diverse environmental interactions, may affect the size of the genetic effect.
Our results demonstrate the promising portability of PGS based on European GWAS for South Asian samples. The genetic variation between populations, manifested in LD structure and allele frequency differences, provides opportunities to discover new loci linked to psychiatric disorders. Such differences in LD structure are instrumental in fine-mapping associated regions, helping to close in on causal variants responsible for the observed associations. Our findings underline the need for further refinement of these models through more diverse and inclusive multi-ancestral research efforts. Initiatives like the Ancestral Population Network studies (https://www.nimh.nih.gov/about/organization/dnbbs/genomics-research-branch/ancestral-populations-network-apn), including the Asian Bipolar Genetics Network (A-BIG-NET) (31), are pivotal in promoting genetic diversity within psychiatric genetics research. Such efforts are expected to deepen our understanding of the genetics underlying psychiatric conditions, highlighting the shared and unique aspects of psychiatric phenotypes across different populations.
Supplementary Material
Funding:
DBT/Wellcome Trust India Alliance: Intermediate Clinical Fellowship (IA/CPHI/20/1/505266), Scientific Knowledge for Ageing and Neurological Ailments (SKAN) trust : (SKAN/002/208/2021/014), Indian Council of Medical Research for CVEDA and OCD genetics, ADBS program funded by the Department of Biotechnology and the Pratiksha Trust ((BT/PR17316/MED/31/326/2015), Center for Brain and Mind (CBM) funded by the Rohini Nilekani Philanthropies, NIMH grants, NIMH R01 MH130675-01 (Asian Bipolar Genetics Network), NIMH R01 MH121545 (Genetics at an extreme: an efficient genomic study of individuals with clinically severe major depression receiving ECT).
Funding Statement
DBT/Wellcome Trust India Alliance: Intermediate Clinical Fellowship (IA/CPHI/20/1/505266), Scientific Knowledge for Ageing and Neurological Ailments (SKAN) trust : (SKAN/002/208/2021/014), Indian Council of Medical Research for CVEDA and OCD genetics, ADBS program funded by the Department of Biotechnology and the Pratiksha Trust ((BT/PR17316/MED/31/326/2015), Center for Brain and Mind (CBM) funded by the Rohini Nilekani Philanthropies, NIMH grants, NIMH R01 MH130675-01 (Asian Bipolar Genetics Network), NIMH R01 MH121545 (Genetics at an extreme: an efficient genomic study of individuals with clinically severe major depression receiving ECT).
Footnotes
- Supplementary Material (see below)
- Figures
- Tables
- Data Availability Statement
REFERENCES:
- 1.Abdellaoui A, Yengo L, Verweij KJH, Visscher PM. 15 years of GWAS discovery: Realizing the promise. Am J Hum Genet. 2023. Feb 2;110(2):179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wray NR, Lin T, Austin J, McGrath JJ, Hickie IB, Murray GK, et al. From Basic Science to Clinical Application of Polygenic Risk Scores: A Primer. JAMA Psychiatry. 2021. Jan 1;78(1):101–9. [DOI] [PubMed] [Google Scholar]
- 3.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018. Sep;50(9):1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marston NA, Pirruccello JP, Melloni GEM, Koyama S, Kamanu FK, Weng LC, et al. Predictive Utility of a Coronary Artery Disease Polygenic Risk Score in Primary Prevention. JAMA Cardiology. 2023. Feb 1;8(2):130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel AP, Wang M, Ruan Y, Koyama S, Clarke SL, Yang X, et al. A multi-ancestry polygenic risk score improves risk prediction for coronary artery disease. Nat Med. 2023. Jul;29(7):1793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klarin D, Natarajan P. Clinical utility of polygenic risk scores for coronary artery disease. Nat Rev Cardiol. 2022. May;19(5):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022. Apr;604(7906):502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson RE, Kuchenbaecker K, Walters RK, Chen CY, Popejoy AB, Periyasamy S, et al. Genome-wide Association Studies in Ancestrally Diverse Populations: Opportunities, Methods, Pitfalls, and Recommendations. Cell. 2019. Oct 17;179(3):589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019. Apr;51(4):584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Kember RL, Deak JD, Xu H, Toikumo S, Yuan K, et al. Multi-ancestry study of the genetics of problematic alcohol use in over 1 million individuals. Nat Med. 2023. Dec;29(12):3184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu A, Sarkar-Roy N, Majumder PP. Genomic reconstruction of the history of extant populations of India reveals five distinct ancestral components and a complex structure. Proceedings of the National Academy of Sciences. 2016. Feb 9;113(6):1594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moorjani P, Thangaraj K, Patterson N, Lipson M, Loh PR, Govindaraj P, et al. Genetic evidence for recent population mixture in India. Am J Hum Genet. 2013. Sep 5;93(3):422–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narasimhan VM, Patterson N, Moorjani P, Rohland N, Bernardos R, Mallick S, et al. The formation of human populations in South and Central Asia. Science. 2019. Sep 6;365(6457):eaat7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall JD, Sathirapongsasuti JF, Gupta R, Rasheed A, Venkatesan R, Belsare S, et al. South Asian medical cohorts reveal strong founder effects and high rates of homozygosity. Nat Commun. 2023. Jun 8;14(1):3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Periyasamy S, John S, Padmavati R, Rajendren P, Thirunavukkarasu P, Gratten J, et al. Association of Schizophrenia Risk With Disordered Niacin Metabolism in an Indian Genome-wide Association Study. JAMA Psychiatry. 2019. Oct 1;76(10):1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabassum R, Chauhan G, Dwivedi OP, Mahajan A, Jaiswal A, Kaur I, et al. Genome-wide association study for type 2 diabetes in Indians identifies a new susceptibility locus at 2q21. Diabetes. 2013. Mar;62(3):977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandesh K, Prasad G, Giri AK, Kauser Y, Upadhyay M, INDICO, et al. Genome-wide association study of blood lipids in Indians confirms universality of established variants. J Hum Genet. 2019. Jun;64(6):573–87. [DOI] [PubMed] [Google Scholar]
- 18.Sharma E, Vaidya N, Iyengar U, Zhang Y, Holla B, Purushottam M, et al. Consortium on Vulnerability to Externalizing Disorders and Addictions (cVEDA): A developmental cohort study protocol. BMC Psychiatry. 2020. Dec;20(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Vaidya N, Iyengar U, Sharma E, Holla B, Ahuja CK, et al. The Consortium on Vulnerability to Externalizing Disorders and Addictions (c-VEDA): an accelerated longitudinal cohort of children and adolescents in India. Mol Psychiatry. 2020. Aug;25(8):1618–30. [DOI] [PubMed] [Google Scholar]
- 20.Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021. Jun;53(6):817–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strom NI, Yu D, Gerring ZF, Halvorsen MW, Abdellaoui A, Rodriguez-Fontenla C, et al. Genome-wide association study identifies new locus associated with OCD. medRxiv; 2021. p. 2021.10.13.21261078. [Google Scholar]
- 22.Karlsson Linnér R, Mallard TT, Barr PB, Sanchez-Roige S, Madole JW, Driver MN, et al. Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self-regulation and addiction. Nat Neurosci. 2021. Oct;24(10):1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi SW, O’Reilly PF. PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience. 2019. Jul 1;8(7):giz082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge T, Chen CY, Ni Y, Feng YCA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019. Dec;10(1):1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Z, Liu S, Sidorenko J, Yengo L, Turley P, Ani A, et al. Leveraging functional genomic annotations and genome coverage to improve polygenic prediction of complex traits within and between ancestries. bioRxiv; 2022. p. 2022.10.12.510418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert SA, Gil L, Jupp S, Ritchie SC, Xu Y, Buniello A, et al. The Polygenic Score Catalog as an open database for reproducibility and systematic evaluation. Nat Genet. 2021. Apr;53(4):420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam M, Chen CY, Li Z, Martin AR, Bryois J, Ma X, et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat Genet. 2019. Dec;51(12):1670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavalli-Sforza LL, Piazza A. Human Genomic Diversity in Europe: A Summary of Recent Research and Prospects for the Future. Eur J Hum Genet. 1993. Jan;1(1):3–18. [DOI] [PubMed] [Google Scholar]
- 29.Tian C, Kosoy R, Nassir R, Lee A, Villoslada P, Klareskog L, et al. European population genetic substructure: further definition of ancestry informative markers for distinguishing among diverse European ethnic groups. Mol Med. 2009;15(11–12):371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClellan JM, Zoghbi AW, Buxbaum JD, Cappi C, Crowley JJ, Flint J, et al. An evolutionary perspective on complex neuropsychiatric disease. Neuron. 2024. Jan 3;112(1):7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo PH, Huang H, Kendler K, Zandi P. Enhancing Diversity and Health Equity through Asian Bipolar Genetics Network (A-BIG-NET). European Neuropsychopharmacology. 2023. Oct 1;75:S17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.