Abstract
Alcohol Use Disorders (AUD) is characterized by compulsion-like alcohol drinking (CLAD), where intake despite negative consequences can be a major clinical obstacle. With few treatment options available for AUD, there is a significant need for novel therapies. The noradrenergic system is an important hub for regulating stress responses and maladaptive drives for alcohol. Studies have shown that drugs targeting α1 adrenenergic receptors (ARs) may represent a pharmacological treatment for pathological drinking. However, the involvement of β ARs for treating human drinking has received scant investigation, and thus we sought to provide pre-clinical validation for possible AR utility for CLAD by analyzing whether β AR antagonists propranolol (β1/2), betaxolol (β1), and ICI, 118 551 (β2) impacted CLAD and alcohol-only drinking (AOD) in male Wistar rats. We found that the highest dose of propranolol tested systemically (10mg/kg) reduced alcohol drinking, while 5mg/kg propranolol reduced drinking with a trend to impact CLAD more than AOD, and with no effects of 2.5mg/kg. Betaxolol (2.5mg/kg) also decreased drinking, while ICI 118.551 had no effects. Also, while AR compounds might have utility for AUD, they can also lead to undesirable side effects. Here, a combination of ineffective doses of propranolol and prazosin reduced both CLAD and AOD. Finally, we investigated the effect of propranolol and betaxolol in two brain areas related to pathological drinking, the anterior insula (aINS) and medial prefrontal cortex (mPFC). Surprisingly, propranolol (1–10μg) in aINS or mPFC did not affect CLAD or AOD. Together, our findings provide new pharmacological insights into noradrenergic regulation of alcohol consumption, which may inform AUD therapy.
1. INTRODUCTION
Alcohol Use Disorder (AUD) ranks among the most prevalent mental disorders and is characterized by compulsive heavy alcohol use and loss of control over alcohol intake, with negative consequences on both physical and mental health (Larimer et al., 1999; Koob et al., 2010; Hopf et al., 2014; Carvalho et al., 2019; G.B.D. Collaborators, 2020; Epstein, 2020). Also, the compulsion for alcohol, where consumption persists despite negative consequences, can often relate to negative affect and activation of brain stress systems (Koob et al., 2014). In addition, currently approved medications for AUD show modest therapeutic efficacy, and there is a critical need for novel therapies, especially drugs that are already FDA approved and could be quickly repurposed (Spanagel, 2009; Kranzler et al., 2018; Downs et al., 2022). However, even with robust evidence for an important relation between AUD and stress activation, there are no approved medication targeting the brain stress system to modulate excessive alcohol drinking (Haass-Koffler et al., 2018; Downs et al., 2022; Varodayan et al., 2022).
The noradrenergic system is an important hub in the stress response system (Valentino et al., 2008; Downs et al., 2022, see Discussion), as well as maladaptive drives for alcohol (Vazey et al., 2018). Preclinical and clinical studies have shown that functional inhibition of α1 adrenergic receptors (AR) may represent a therapeutic target for AUD (Simpson et al., 2018; Sinha et al., 2021; Sinha et al., 2022). In humans, recent studies found that the α1 AR antagonist prazosin reduces drinking and craving in patients with more severe alcohol withdrawal symptoms (Sinha et al., 2021), and decreases alcohol withdrawal effects on the prefrontal-striatal activation (Sinha et al., 2022). In parallel, we used an animal model for compulsion-like alcohol drinking (CLAD), where animals will tolerate alcohol adulterated with quinine levels that are strongly avoided in water (Hopf et al., 2010, Lei et al., 2016, Sneddon et al., 2019; Katner et al., 2022, reviewed in Radke et al., 2021), including in our recent findings in Wistar rats where quinine 10mg/L decreased water intake >70% (De Oliveira Sergio et al., biorxiv) (similar to Domi et al., 2021, see Discussion). Also, our recent findings show that systemic administration of prazosin reduces both alcohol-only drinking (AOD) and CLAD in male rats (De Oliveira Sergio et al., 2021). We also examined the impact of prazosin in the anterior insula (aINS), a key regulator of emotional states and a strong contributor to many aspects of addiction and emotion in humans and rodents (Centanni et al., 2021; Sommer et al., 2022). Interestingly, prazosin injection into aINS also decreases both CLAD and AOD, while, in contrast, aINS projections to the Locus Coeruleus area mediate CLAD but not AOD (or sweet fluid intake) (De Oliveira Sergio et al., 2021). Thus, while α1 AR modulation can influence both CLAD and AOD, some aspects of noradrenergic signaling may be more selective for challenge-resistant action. Similarly, we previously found that CLAD but not AOD requires aINS inputs to striatal areas (Seif et al., 2013), as well as striatal inputs from mPFC (another area that can regulate drinking, e.g. Linsenbardt et al., 2019; Barbier et al., 2021; Dao et al., 2021), and compulsion-like responding for alcohol activates a similar insular circuit in heavy drinking humans (Grodin et al., 2018) (see also Arcurio et al., 2015), validating the importance of these circuits for at least some aspects of problem drinking in humans.
The involvement of β ARs for AUD has received limited study (Haass-Koffler et al., 2018; Downs et al., 2022). Early clinical studies found that non-selective β AR antagonists propranolol and atenolol can reduce symptoms related to alcohol withdrawal and cravings (Carlsson et al., 1971; Zilm et al., 1975; Horwitz et al., 1989; Bailly et al., 1992). In rodents, propranolol (but not nadolol) reduces alcohol consumption in alcohol-dependent rats, suggesting that central (but not peripheral) β ARs are involved in regulating alcohol consumption (Gilpin et al., 2010). Furthermore, a higher dose of propranolol decreases alcohol intake in dependent P rats during early withdrawal (Rasmussen et al., 2014).
Given that compulsion-like drives can be an important feature of AUD, the likely relation of CLAD with stress, and our previous work showing α1 AR importance for CLAD and AOD, we hypothesized here that β AR signaling would also regulate CLAD and AOD. We first investigated if systemic administration of propranolol (2.5, 5 and 10mg/kg), the β1 AR antagonist betaxolol (2.5 and 5mg/kg), or the β2 ARs antagonist ICI 118, 551 (1 mg/kg), would alter alcohol intake. Further, while clinical and preclinical studies show the beneficial effects of α1 and β antagonists on AUD, these compounds can also lead to undesirable side effects on blood pressure and cardiovascular system (Vazey et al., 2018; Downs et al., 2022). Thus, any strategy lowering the dose of these compounds to reduce drinking could have broad utility. Importantly, we found that co-administering ineffective doses of propranolol and prazosin reduced CLAD and AOD. Finally, we also investigated the potential role of β AR signaling in aINS and mPFC, two brain areas related to AUD and CLAD, through local injection of propranolol and betaxolol. Together, our results provide pharmacological and neurocircuit insights into a novel treatment strategy (prazosin+propranolol) that already is FDA-approved and could be used for AUD treatment.
2. MATERIALS AND METHODS
All experimental procedures were conducted in accordance with the Guide for Care and Use of Laboratory animals provided by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Indiana University. All efforts were undertaken to reduce the number of animals needed and to minimize pain and suffering.
2.1. Subjects and Alcohol Drinking Methods
Male Wistar rats (Envigo) 45–50 days old were singly housed with ad libitum food and water. After two weeks of acclimatization to the vivarium, rats had access to alcohol (20% v/v diluted in water) in the intermittent two-bottle choice paradigm (IA2BC) (with the second bottle containing water). Briefly, three times a week (starting Monday, Wednesday and Friday), rats had an 18–24hr period where alcohol was available concurrently with water. The alcohol and water bottle positions were alternated across days to prevent a position bias. Several studies showed that Wistar and other outbred rat strains need at least 3 months of IA2BC to develop CLAD (Hopf et al., 2010; Seif et al., 2013; Seif et al., 2015; Spoelder et al., 2015; Spoelder et al., 2017). Thus, we allowed rats ~3 months of IA2BC, at which point rats were shifted to limited daily access two-bottle choice (LDA), with 20 min access to 20% alcohol or water Monday through Friday. After at least 2–3wk LDA, rats had 2–3 alcohol-quinine sessions (with 10mg/L quinine) to habituate to the novelty of quinine in alcohol, and then were returned to AOD. These methods are as we previously published (Hopf et al., 2010; Seif et al., 2013; Seif et al., 2015; Darevsky et al., 2019; Darevsky et al., 2020; De Oliveira Sergio et al., 2021).
One week before starting the experimental sessions rats were gently handled by the experimenter, once a day, for ~5 min, to familiarize them with the experimental conditions and to reduce non-specific stress responses (and see Section 2.4). Experimental days were generally carried out twice per week, with at least one day of AOD between test sessions; all other alcohol drinking days in the week involved alcohol-only.
One concern is that the 10mg/L dose of quinine might be too low, and that inability of this quinine level to reduce alcohol intake might reflect it’s failure to reduce consumption more generally. While we did not test quinine sensitivity in water in the present study, studies from our lab and others have confirmed that rodents strongly avoid quinine in water at quinine doses that do not reduce alcohol drinking (Hopf et al., 2010, Lei et al., 2016, Sneddon et al., 2019; Katner et al., 2022, reviewed in Radke et al., 2021). In addition, we (De Oliveira Sergio et al., biorxiv) and other recent work (Domi et al., 2021, Suppl.Fig.5E) found that quinine-water intake with 10mg/L is reduced 70–75% relative to water alone. These findings concur that this quinine dose greatly reduces water intake, suggesting that alcohol drinking rodents can sense and avoid quinine when in water, while the willingness to continue drinking alcohol adulterated with quinine suggests that they their responding is aversion-resistant (compulsion-like) for alcohol (reviewed in Radke et al., 2021).
2.2. Cannulas implantation and drugs infusion
After 3–4 weeks in LDA and 2–3 quinine sessions for habituation, surgery was performed to bilaterally implant guide cannulae (Plastics One; 26 gauge) aimed 1mm above the aINS (AP +2.8, ML ± 4.8, and DV −4.7 mm) or mPFC (AP +3.2, ML ± 1.2, and DV −3.0 mm with a 10° angle) (as in Seif et al., 2013; Seif et al., 2015; De Oliveira Sergio et al., 2021). After 7 days of recovery rats returned to LDA and had 2–3 quinine sessions again. Animals were then randomized to receive drug or vehicle during experimental sessions. As described in (De Oliveira Sergio et al., 2021), during a test session, the injection needle was connected to a 10μl microsyringe (Hamilton 701-RN, USA) through a polyethylene tube. The injection needle (Plastics One; 33 gauge) was lowered to reach 1 mm below the lower end of the cannulae. To control the volume and duration of injections, a digital syringe pump (KD Scientific Inc., USA) was programmed to inject a volume of 0.6 μl at 0.2 μl/min of drugs. To reduce reflux, needle was left in place for 1 min before removal.
2.3. Reagents.
Propranolol hydrochloride was obtained from Tocris. Prazosin hydrochloride, Betaxolol hydrochloride and ICI 118,551 hydrochloride were all from Sigma-Aldrich (USA). All drugs were dissolved in sterile saline (0.9%) except prazosin that was dissolved in sterile water. The references for the doses chosen for each compound are described in Supplemental Table 1. All drugs were prepared at the same day minutes before beginning of experiments.
2.4. Overview of experimental design during drinking sessions.
For all studies (systemic or central), animals were exposed to each drinking condition (alcohol-only and quinine-alcohol) and pharmacological treatments (drug vs vehicle) using a within-subject design. Experimental groups were randomized across animals before the beginning of the test sessions. Also, all conditions were balanced to assure that some rats in each experimental condition were tested on same test day. For all systemic administrations (i.p), rats were habituated to handling by experimenter, and with 1–3 sessions of habituation to i.p. vehicle injection before test sessions (rats received more than one i.p. vehicle habituation sessions until alcohol drinking was not reduced after such injections). This prior habituation with the vehicle is important to reduce stress during the injection procedure. Supplemental Table 2 summarizes the different cohorts of rats used. In study 1, propranolol or vehicle were injected 20 minutes before drinking test sessions at 2.5mg/kg (n=10; Fig. 1A), 5mg/kg (n=16; Fig. 1B) or 10mg/kg (n=10; Fig. 1C). Some animals were tested with the three doses. Only for propranolol 5mg/kg, a second cohort with 6 rats were added to the experiment. Supplemental materials show a schematic timeline for the propranolol experiments (Suppl.Fig.1A), to show how multiple doses of propranolol were administered within the same rats across time, and how a second cohort was added to test 5mg/kg propranolol.
Figure 1. Dose dependent effects of inhibiting β ARs and β1 ARs with propranolol and betaxolol on alcohol drinking.
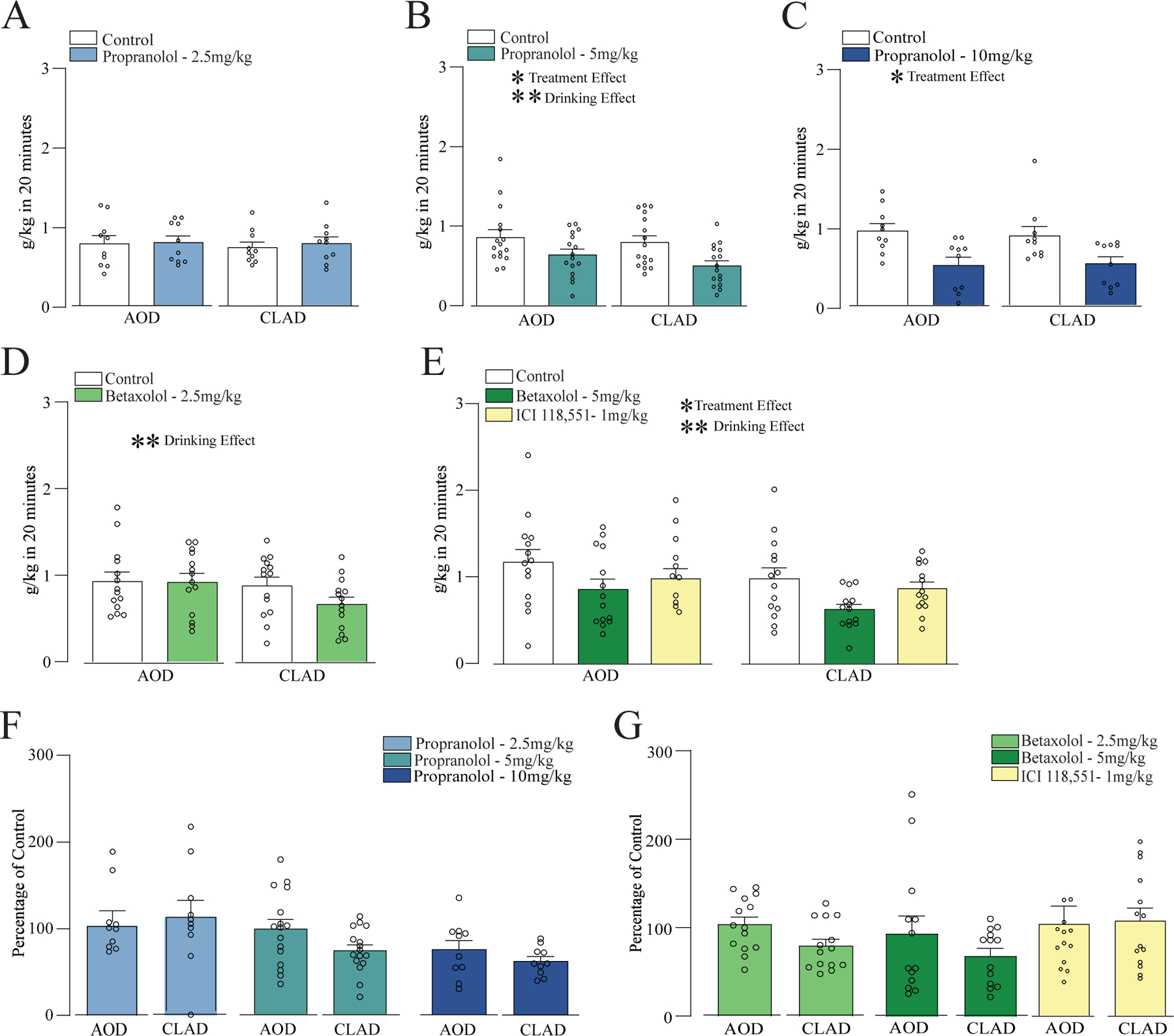
(A-C) Systemic administration of propranolol at (A) 2.5mg/kg, (B) 5mg/kg, and (C) 10mg/kg. (D) Systemic administration of β1 AR antagonist betaxolol at (D) 2.5mg/kg. (E) Systemic administration of betaxolol 5mg/kg or β2 ARs antagonist ICI 118,551. (F) Percentage change in drinking (relative to vehicle) for propranolol at 2.5, 5 and 10mg/kg. (G) Percentage of vehicle change for betaxolol 2.5, 5mg/kg and ICI 118,551. *,** p<0.05, p<0.01 for treatment or drinking condition effects, from two-way ANOVA.
Another cohort of animals were injected with betaxolol or vehicle at 2.5mg/kg (n=14; Fig. 1D), 5mg/kg (n=15; Fig. 1E) and ICI 118,551 or vehicle 1mg/kg (n=15; Fig. 1F) 30 minutes before the drinking sessions. Betaxolol 5mg/kg and ICI were tested in the same rat, with the order of vehicle, BTX (5 mg/kg) and ICI randomized across rats. For co-administration of an ineffective dose of prazosin (0.25mg/kg) and an ineffective dose of propranolol (2.5mg/kg) rats were first injected with prazosin or vehicle (water), and 10 minutes later to propranolol or vehicle (saline). 20 minutes after the last injection, rats went to drinking session (n=13; Fig. 3B).
Figure 3. Co-administration of ineffective doses of prazosin and propranolol reduced AOD and CLAD.
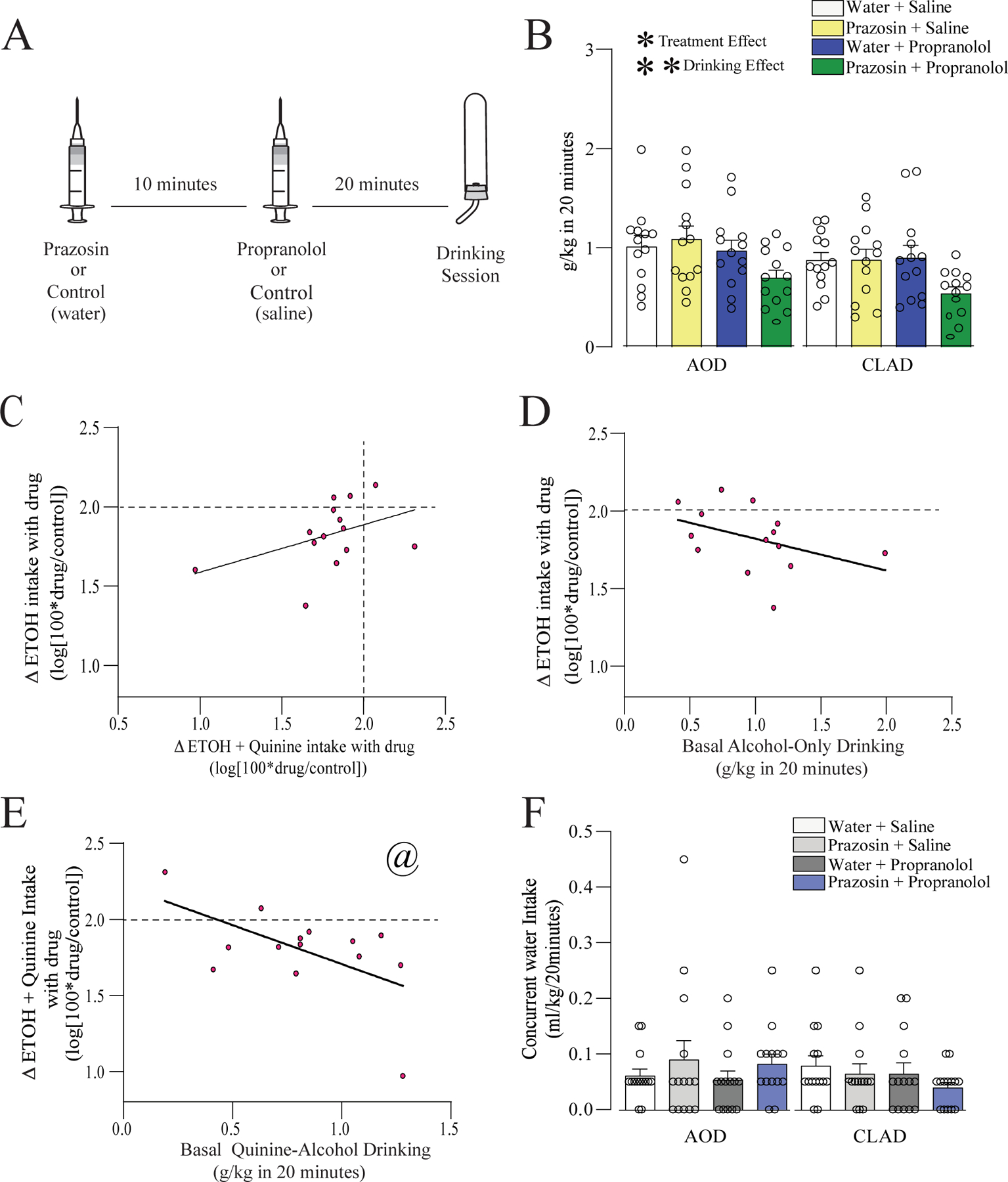
(A) Schematic experimental timeline for co-administration of ineffective doses of prazosin and propranolol. Prazosin (0.25mg/kg) or vehicle was first injected, and 10 minutes animals were injected with propranolol (2.5mg/kg) or vehicle and then 20 minutes later were exposed to AOD or CLAD drinking. (B) Systemic administration of prazosin or propranolol alone did not affect AOD or CLAD. However, co-administration of these drugs together decreased both drinking conditions. (C) Prazosin+propranolol changes in AOD and CLAD were not correlated. (D) Changes in AOD were not correlated with the basal AOD. (E) Changes on CLAD were negatively correlated with basal quinine-alcohol drinking. (F) Co-administration of prazosin and propranolol did not change the concurrent water intake. A dashed line in (C-E) indicates where drug intake was not different from vehicle, and values below the dashed line indicate that alcohol drinking under drug exposure was reduced relative to vehicle treatment. *,** p<0.05, p<0.01 for treatment or drinking condition effects, from two-way ANOVA. @ p<0.05 correlation.
For bilateral intra-aINS administration, propranolol or vehicle was tested at 0.5 and 2ug/0.6ul (n=7; Fig. 4A) or 5 and 10ug/0.6ul (n=9; Fig. 4B) 10 minutes before drinking session, and betaxolol or vehicle at 307ng/0.6ul 30 minutes before drinking sessions (n=9; Fig. 4C). For bilateral intra-mPFC administration, cannulae and injections were targeted to prelimbic cortex (following previous studies from our, Seif et al., 2013, 2015, and other labs), although we cannot rule out some impact in infralimbic. Propranolol or vehicle was tested at 0.5 and 5ug/0.6ul (n=7; Fig. 5A) 10 minutes before the drinking session. To test the effect of intra-mPFC betaxolol and prazosin, rats were bilaterally injected with 307ng/0.6ul of betaxolol or vehicle (n=6; Fig. 5B), or 0.3ug/0.6ul of prazosin or vehicle (n=7; Fig. 5C) 30 minutes before drinking sessions.
Figure 4. Inhibition of β ARs in aINS did not affect AOD or CLAD.
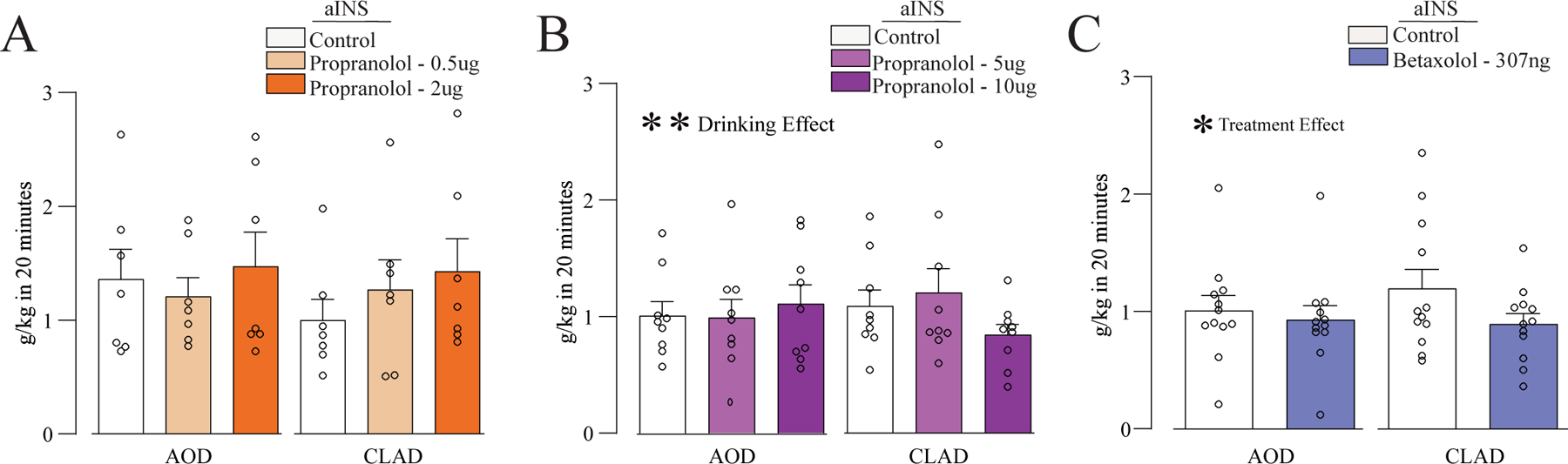
(A,B) Intra-aINS administration of propranolol (0.5, 2, 5, or 10μg) had no impact on alcohol intake. (C) Administration of β1 ARs antagonist betaxolol (307ng) into aINS had limited effects on CLAD (see Results). *,** p<0.05, p<0.01 for treatment or drinking condition effects, from two-way ANOVA.
Figure 5. Inhibition of β and α1 ARs in mPFC did not affect AOD or CLAD.
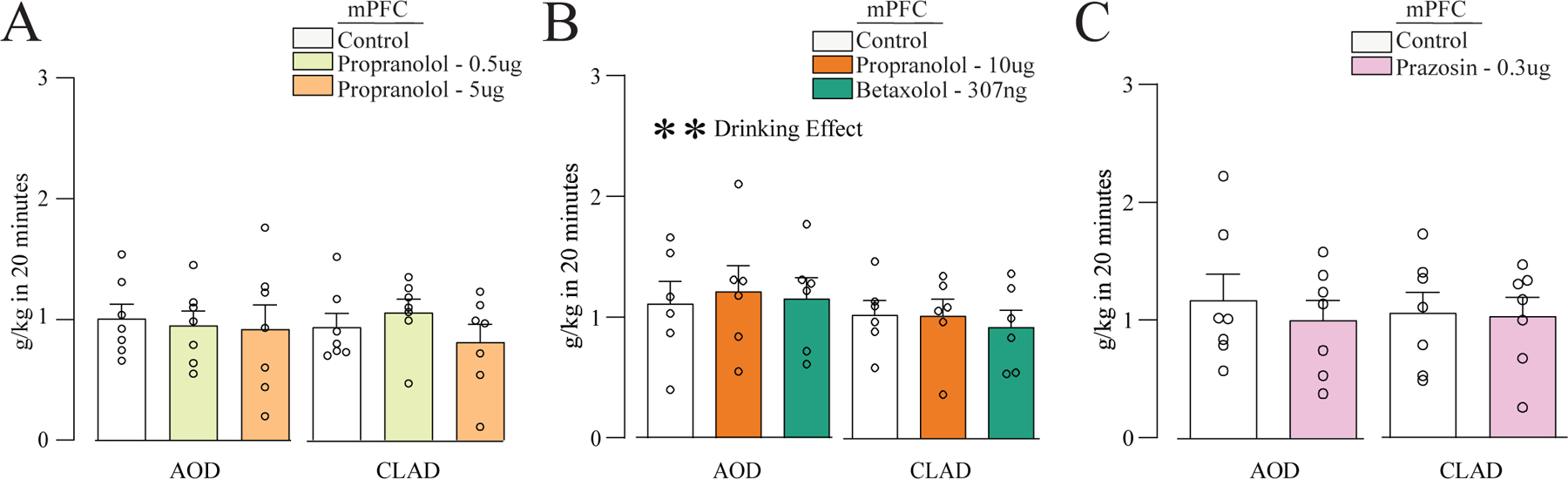
Intra-mPFC injection of propranolol 0.5 or 5μg (A), propranolol 10μg or betaxolol 307ng (B), or prazosin 0.3μg (C), had no impact on AOD or CLAD.
2.5. Statistics and Data Analysis
Alcohol consumption was determined through changes in bottle weight before and after a drinking session and converted to grams ethanol/kilograms body weight. Concurrent water intake was determined by changes in bottle weights before and after a drinking session and expressed in ml consumption/kilograms body weight. Statistical comparisons were primarily performed in a within-subject manner. Data were mostly analyzed by one- or two-way ANOVA with repeated-measures, while some comparisons used paired t-test or correlation. Statistical analysis was performed using GraphPad Prism or SPSS. All data are shown as mean ± SEM. Also, we describe some potential differences as trends, since such differences would not be considered significant with correction for multiple comparisons, but we include them as information for the reader.
We also examined the effects of drug treatment by normalizing drinking level during drug treatment to the corresponding vehicle session, yielding a percent change in drinking with drug treatment, as we have done before (Seif et al., 2013, 2015; Lei et al., 2019; De Oliveira Sergio et al., 2021). In particular, to further examine drinking changes, across groups and for correlations, we determined the impact of a given treatment using log[100 × (intake during drug treatment)/(intake during vehicle)], as we previously used (detailed in Lei et al., 2019). Log value of 2 (log[100]) indicates no effect of drug on alcohol drinking relative to it’s matched vehicle condition, while values lower than 2 indicate a decrease in drinking level with drug. One additional possible concern is that shifts in basal drinking level would impact the change in firing with drug. However, across our many studies, we have found that some treatments reduce drinking level while others don’t, irrespective of variation in basal intake (e.g. Seif et al., 2013, Wegner et al., 2019). In addition, we have correlated basal intake with the percent change in drinking with drug, and, e.g. in De Oliveira Sergio et al. (2021), systemic prazosin causes a greater percent reduction in CLAD drinking in higher-drinking individuals, while prazosin reduction in drinking level does not correlate with basal AOD intake level. Thus, having somewhat lower basal intake does not impede ability to meaningfully assess the change in drinking with treatment. For this reason, we have found that drug-related percent change in drinking (vs vehicle) to be a valuable measure that is robust to differences in basal intake across conditions that we examine.
Finally, we largely do not examine drug effects on concurrent water intake, since water intake in the 20min sessions is quite low, as we observed in previous studies (e.g. De Oliveira Sergio et al., 2021). Nonetheless, we did examine concurrent water data for Fig. 3F for comparison and information purposes only.
3. Results
3.1. Effect of β1/2 ARs antagonists on AOD and CLAD
We first tested if systemic administration of a nonselective β ARs antagonist propranolol, at the doses of 2.5, 5, and 10mg/kg, would affect CLAD and/or AOD. Our results showed that 2.5mg/kg of propranolol had no effect on CLAD or AOD [Fig. 1A, n=10; two-way ANOVA; F(treatment;1,9)=0.563, p=0.472; F(drinking-condition;1,9)=0.238, p=0.637; F(interaction;1,9)=0.066, p=0.802]. Results for propranolol 5mg/kg also reduced drinking levels, with a significant effect of treatment and drinking condition, although no interaction [Fig. 1B, n=16; F(treatment;1,15)=9.150, p=0.009; F(drinking-condition;1,15)=4.878, p=0.043; F(interaction;1,15)=0.469, p=0.504]. In addition, systemic administration of propranolol 10mg/kg had a significant effect of treatment [Fig. 1C, n=10; two-way ANOVA; F(treatment;1,9)=26.517, p<0.001; F(drinking-condition;1,9)=0.057, p=0.816; F(interaction;1,9)=0.250, p=0.629]. Together, these data suggest that β-ARs could regulate both AOD and CLAD.
Since propranolol acts through β1 and β2 ARs, we next evaluated if these dose-dependent effects of propranolol on CLAD and AUD could be related to a differential activation of these two ARs. Specifically, we systemically injected betaxolol, a β1 ARs antagonist, or ICI 118,551, a β2 ARs antagonist, and measured AOD and CLAD intake. Our results showed that betaxolol 2.5mg/kg decreased alcohol drinking, with a trend for greater impact on CLAD [Fig. 1D, n=14; two-way ANOVA; F(treatment;1,13)=3.578, p=0.081; F(drinking-condition;1,13)=6.341, p=0.025; F(interaction;1,13)=4.498, p=0.054]. Betaxolol 5mg/kg was tested in the same rats on a different day as 1 mg/kg of ICI 118,551, with the same vehicle (n=14), and these tests had a significant effect of treatment and drinking condition [Fig. 1E, two-way ANOVA; F(treatment;2,26)=5.258, p=0.012; F(drinking-condition;1,13)=7.046, p=0.020; F(interaction;2,26)=0.241, p=0.788]. In a preliminary attempt to examine the effects of betaxolol 5 mg/kg or ICI 118,551alone, we ran separate two-way ANOVAs, which showed a significant effect of treatment and drinking condition for betaxolol [F(treatment;1,13)=7.950, p=0.014; F(drinking-condition;1,13)=14.373, p=0.002; F(interaction;1,13)=0.057, p=0.815] but not ICI 118,551 [F(treatment;1,13)=2.557, p=0.134; F(drinking-condition;1,13)=2.591, p=0.131; F(interaction;1,13)=0.203, p=0.659]. Taken together, our findings suggest that the effects of propranolol on CLAD are through β2 rather than β1 ARs.
Studies above indicate that propranolol and BTX could reduce alcohol drinking. Thus, we next examined the results by normalizing drinking level during drug treatment to the corresponding vehicle session, as we have done before (Seif et al., 2013, 2015; Lei et al., 2019; Wegner et al., 2019; De Oliveira Sergio et al., 2021) (and see Methods). With this analysis, we found a difference in the impact of 5 mg/kg propranolol on CLAD vs AOD (Fig. 1G, paired t-test t(15)=2.455, p=0.027) but not propranolol 10mg/kg (Fig. 1G, t(9)=1.113, p=0.294); betaxolol 2.5mg/kg also showed a difference between CLAD and AOD percent decrease in drinking (Fig.1H, t(13) = 2.24, p=0.043). However, none of these patterns would survive multiple corrections, and should be considered trends.
We next examined the relationship between the impact of propranolol changes AOD and CLAD versus basal drinking levels. We previously showed that the α1 ARs antagonist (prazosin) reduces drinking in both AOD and CLAD, but the changes in AOD with prazosin are unrelated to changes in CLAD, suggesting different α1 ARs-regulated pathways for AOD and CLAD (De Oliveira Sergio et., 2021). In addition, prazosin changes in drinking were related to basal drinking level for CLAD (bigger change in higher basal drinkers) but not AOD. For these analyses, we determined the drinking change with drug versus it’s matched vehicle session using log[100 × (intake during drug treatment)/(intake during vehicle)], as we have done previously (De Oliveira Sergio et al., 2021; detailed in Lei et al., 2019). A dashed line indicates where drug intake was not different from vehicle, and values below the dashed line indicate that alcohol drinking under drug exposure was reduced relative to vehicle treatment.
Here, we found a related pattern for propranolol and betaxolol as we previously observed for prazosin. In particular, there was no correlation between 5mg/kg propranolol change in AOD versus change in CLAD (Fig. 2A, R2=0.078, F(1,14)=1.197, p=0.292). Similarly, there were no relations between CLAD changes and AOD changes for 10 mg/kg propranolol (Fig. 2B, R2=0.372, F(1,8)=0.892, p=0.373) or 2.5 mg/kg betaxolol (Fig. 2C, R2=0.009, F(1,12)=0.113, p=0.743). We then examined whether drug-related changes in drinking correlated with basal intake levels. For propranolol 5mg/kg, the drug-related change in firing had a trend relationship with basal AOD intake (Fig. 2D, R2=0.245, F(1,14)=4.559, p=0.051), and the propranol change in CLAD was significant negatively correlated with basal CLAD drinking levels (Fig. 2E, R2=0.247, F(1,14)=4.614, p=0.049), similar to what we reported for prazosin (De Oliveira Sergio et al., 2021), and suggesting a greater propranolol 5mg/kg effect in higher alcohol drinkers. For 10 mg/kg, there was no relation between basal intake and change in AOD (Fig. 2F, R2=0.000, F(1,8)=0.007, p=0.935) or CLAD (Fig. 2G, R2=0.003, F(1,8)=0.026, p=0.875). Similarly, changes in drinking with 2.5 mg/kg betaxolol were not related to basal AOD (Fig. 2H, R2=0.084, F(1,12)=1.121, p=0.311) or basal CLAD (Fig. 2I, R2=0.003, F(1,8)=0.039, p=0.846) drinking. Finally, we found that the AOD change and CLAD change with 5 mg/kg betaxolol were not correlated (Fig. 2J, R2=0.006, F(1,12)=0.075, p=0.789), while changes in drinking with 5 mg/kg betaxolol were significantly related to basal AOD (Fig. 2K, R2=0.400, F(1,12)=8.004, p=0.015) or basal CLAD (Fig. 2L, R2=0.616, F(1,12)=19.26, p<0.001) drinking.
Figure 2. Correlations related to propranolol and betaxolol effects on drinking.
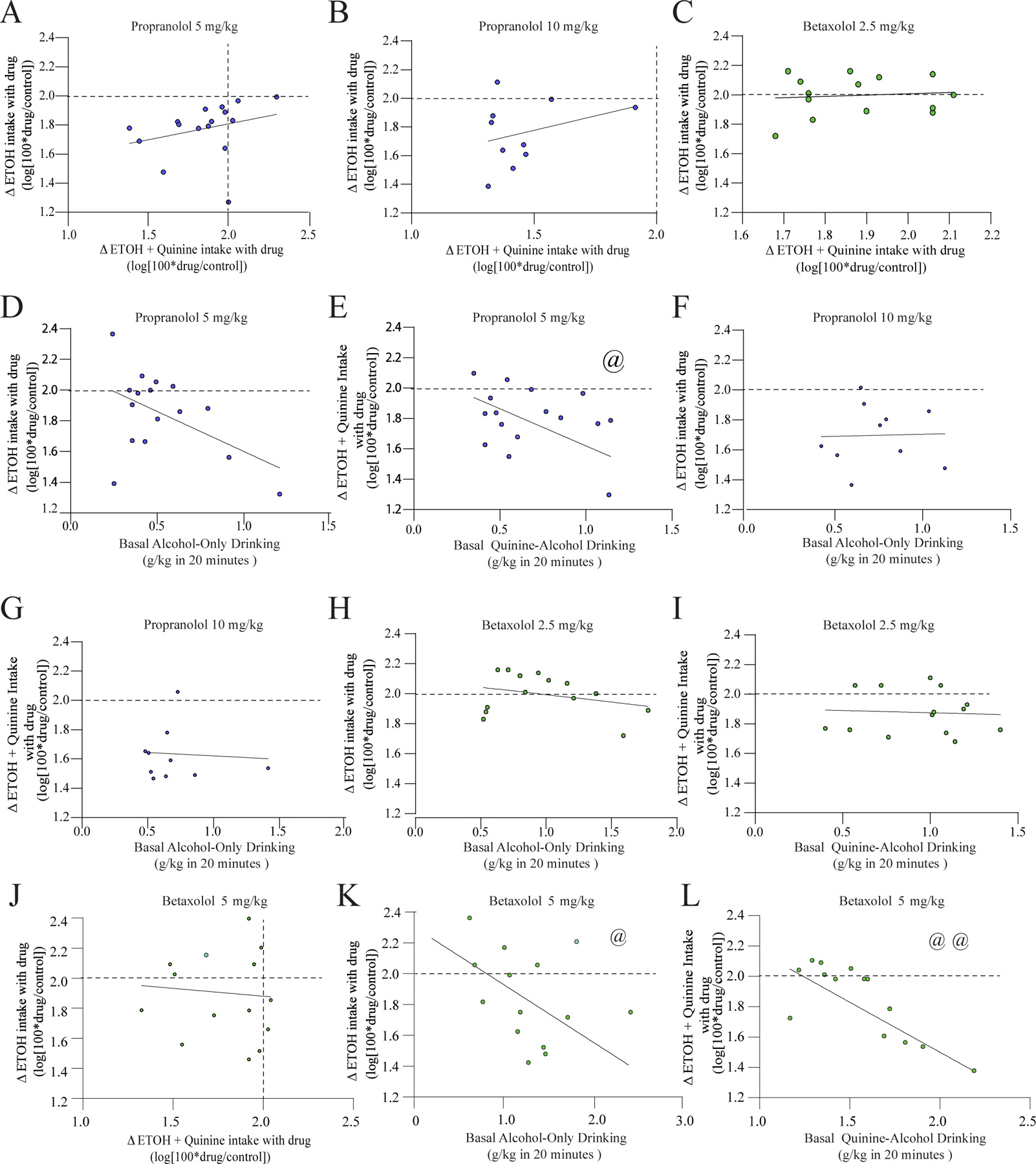
(A-C) Drug-related changes in AOD and changes in CLAD were not correlated for 5 mg/kg propranolol (A), 10 mg/kg propranolol (B), or 2.5 mg/kg betaxolol (C). (D,E) Relation between changes in drinking with 5 mg/kg propranolol (y-axis) and basal intake levels (x-axis) for AOD (D) and CLAD (E). (F,G) Relation between change in drinking with 10 mg/kg propranolol and basal intake levels for AOD (F) and CLAD (G). (H,I) Relation between change in drinking with 2.5 mg/kg betaxolol and basal intake levels for AOD (H) and CLAD (I). (J) Drug-related changes in AOD and changes in CLAD were not correlated for 5 mg/kg betaxolol. (K,L) Relation between change in drinking with 5 mg/kg betaxolol and basal intake levels for AOD (K) and CLAD (L). A dashed line indicates where drug intake was not different from vehicle, and values below the dashed line indicate that alcohol drinking under drug exposure was reduced relative to vehicle treatment. @, @@ p<0.05, p<0.01 correlation.
3.2. Targeting α1 and β ARs together to regulate CLAD and AOD
Despite our findings showing the effects of α1 and β antagonists on CLAD and AOD, these compounds can also provoke a series of undesirable side effects (see Introduction). Thus, any strategy allowing lower the dose of these compounds to reduce drinking could have broad utility. We next investigated if combining a sub-therapeutic doses of these both compounds could affect CLAD and AOD intake. The results showing that combining 0.25mg/kg of prazosin with 2.5mg/kg of propranolol had main effects of treatment and drinking condition, [Fig. 3B, n=14; two-way ANOVA; F(treatment;3,39)=7.753, p<0.001; F(drinking-condition;1,13)=10.722, p=0.006; F(interaction;3,39)=0.227, p=0.877]. Moreover, for prazosin+propranolol AOD changes were not correlated with CLAD changes (Fig. 3C, R2=0.181, F(1,12)=2.663, p=0.128). Also, basal intake was not correlated with change in drinking for AOD (Fig. 3D, R2=0.166, F(1,12)=2.405, p=0.146) however, changes in CLAD were significantly negatively correlated for basal CLAD intake (Fig. 3E, R2=0.328, F(1,12)=5.859, p=0.033). Further, the administration of prazosin+propranolol did not affect the concurrent water intake [Fig. 3F, n=14; two-way ANOVA; F(treatment;3,39)=0.386, p=0.763; F(drinking-condition;1,13)=1.018, p=0.331; F(interaction;3,39)=1.586, p= 0.208]. Water intake in the 20min sessions was quite low, as we observed in previous studies (De Oliveira Sergio et al., 2021). Nonetheless, we did this concurrent water data for this particular comparison for information purposes only. Suppl.Fig.2 compares percent change in firing versus vehicle for prazosin+propranolol experiments with other experimental groups. Thus, our data suggest that the ineffective doses of prazosin+propranolol can be used for reducing CLAD and AOD intake.
3.3. Impacts of inhibiting β receptors in aINS on CLAD and AOD
In order to investigate the β ARs signaling in a brain area that regulates CLAD,we next evaluated if the effects of injection into aINS of propranolol or betaxolol. We used a widely utilized doses of intracranial propranolol or betaxolol (see Suppl. Table 2). Intra-aINS propranolol (0.5 and 2 μg/side) did not affect CLAD or AOD [Fig. 4A, n=7; two-way ANOVA; F(treatment;2,12)=3.574, p=0.061; F(drinking-condition;1,6)=0.565, p=0.481; F(interaction;2,12) =2.679, p=0.109; post hoc veh-vs- propranolol 0.5ug for AOD: p=0.836; CLAD p=0.508; post hoc veh-vs- propranolol 2ug for AOD: p = 0.940; CLAD: p=0.103]. In a separate cohort, propranolol (5 and 10 μg/side) did not impact CLAD or AOD [Fig. 4B, n=9; two-way ANOVA; F(treatment;2,16)=1.785, p=0.200; F(drinking-condition;1,8)=0.011, p=0.917; F(interaction;2,16)=1.102, p=0.356]. Also, betaxolol (307ng/side) injection into aINS did not affect CLAD or AOD [Fig. 4C, n=12; F(treatment;1,11)=11.798, p=0.006; F(drinkingcondition;1,11)=0.446, p=0.518; F(interaction;1,11) =1.922, p=0.193; post hoc veh-vs- betaxolol for AOD: p=0.469; CLAD: p=0.136]. Moreover, when we normalized drinking level to vehicle for each animal that received aINS injections of betaxolol we found a trend for greater change in CLAD versus AOD (t (8)=2.229, p=0.074). Suppl. Fig.3 shows aINS histology, and Suppl. Fig. 4 shows the percent change in drinking with drug vs vehicle for intra-aINS studies. Together, our data suggests that aINS β AR signaling did not regulate CLAD or AOD.
3.4. Impacts of inhibiting β receptors in mPFC on CLAD and AOD
Since our data showed no effect of intra-aINS propranolol or betaxolol on alcohol drinking levels, we next examine the potential involvement of β ARs within mPFC (another region that can regulate CLAD, see Discussion). Our results showed that propranolol (0.5 or 5μg/side) did not impact CLAD or AOD [Fig. 5A, n=7; two-way ANOVA; F(treatment;2,12)=0.487, p=0.626; F(drinking-condition;1,6)=0.180, p=0.687; F(interaction;2,12)=0.590, p=0.569]. The higher dose of propranolol (10μg) or betaxolol (307ng) also did not affect CLAD or AOD [Fig. 5B, n=6; two-way ANOVA; F(treatment;2,10)=0.122, p=0.887; F(drinking-condition;1,5)=12.944, p=0.016; F(interaction;2,10)=0.489, p=0.627]. Based on the lack of effects of the β ARs antagonists into mPFC, we next investigated if the prazosin, an α1 adrenergic antagonist could affect CLAD or AOD in this structure. For this we used the same dose (0.3μg/side) used in our previous results showing that prazosin decreases AOD and CLAD when administrated into aINS (De Oliveira Sergio et al., 2021). However, prazosin into mPFC did not affect CLAD or AOD [Fig. 5C, n=7; two-way ANOVA; F(treatment;1,6)=2.002, p=0.207; F(drinking-condition;1,6)=0.051, p=0.828; F(interaction;1,6)=0.586, p=0.473]. Suppl. Fig.3 shows mPFC histology, and Suppl. Fig. 5 shows the percent change in drinking with drug vs vehicle for intra-aINS studies. Thus, these data suggest that mPFC β and α1 AR signaling did not regulate CLAD or AOD.
4. Discussion
Compulsion-like alcohol drinking, where consumption continues despite negative consequences, is a major obstacle to treating AUD, and is likely related to activation of the stress response system (Koob et al., 2014; Carvalho et al., 2019). Here we investigated the effect of β1/2 antagonists on CLAD and AOD in male rats through systemic administration and intracranial injections into aINS and mPFC, two brain areas related to the compulsion for alcohol in animals and humans. Based on our findings with systemic injections, we also investigated if co-administration of ineffective doses of β ARs antagonist propranolol and α1 ARs antagonist prazosin could affect CLAD and AOD intake.
We found that there was a dose dependent effect of propranolol on CLAD and AOD intake. While 2.5mg/kg of propranolol had no effects, the intermediate dose (5mg/kg) and higher dose (10mg/kg) significantly reduced alcohol drinking, with the intermediate dose having a trend for greater effect on CLAD. Interestingly, these same doses of propranolol decrease operant responding for alcohol reinforced in dependent rats, with only propranolol 10mg/kg affecting the consumption in non-dependent rats (Gilpin et al., 2010). Further, in alcohol-dependent P rats, 10 but not 5 mg/kg propranolol decrease alcohol intake during early withdrawal (Rasmussen et al., 2014). It is possible that discrepancies in these results, relative to our findings here, is related to the alcohol model used and breed of the animals (P rats versus Wistar rats). Our findings also showed that CLAD drinking changes and AOD drinking changes with the highest dose of propranolol were not correlated, suggesting the presence of different β AR mechanisms that regulate CLAD and AOD. Similarly, our recent study (De Oliveira Sergio et al., 2021) found that the systemic effects of the α1 ARs antagonist prazosin on CLAD versus AOD were not correlated. We speculate that noradrenergic modulation of CLAD and AOD could be regulated through different mechanisms, and future studies will be necessary to better understand these processes.
We next investigated if the dose dependent effect of propranolol on CLAD and AOD intake could be regulated by differential inhibition of β1 ARs and/or β2 ARs, through injection of the β1 antagonist betaxolol and the β2 antagonist ICI 118,551. To our knowledge this is the first study to investigate the effects of betaxolol and ICI 118,551 in animal models of AUD. Our results showed that ICI 118,551 had no effects. However, betaxolol (2.5mg/kg) did significantly reduce drinking, with a trend for a preferential effect on CLAD. Taken together, our findings suggest that propranolol regulation of alcohol intake could be related to an inhibition of β1 rather than β2 ARs. Betaxolol is considered a highly selective antagonist for β1 ARs (Tondo et al., 1985; Satoh et al., 1993) and ICI 118,551 a selective antagonist for β2 ARs (Nathanson, 1988; Willette et al., 1999), and it is known that both ICI 118, 551 and betaxolol promptly cross the blood brain barrier (Swartz, 1998; Moresco et al., 2000; Hare et al., 2006). Prior findings investigating the effects of betaxolol and ICI 118, 551 in animal addiction models have produced mixed results. Systemic administration of betaxolol at 5mg/kg during early cocaine withdrawal decreases cocaine withdrawal-related anxiety-like behavior in rats, without affecting the locomotor activity (Rudoy et al., 2007). However, betaxolol has no effect on cocaine self-administration (Wee et al., 2008), and ICI 118,551 but not betaxolol (10mg/kg) blocks stress-induced reinstatement of cocaine in mice (Mantsch et al., 2010). Furthermore, 20mg/kg betaxolol (but not lower doses) block reinstatement of cocaine induced by stress (Vranjkovic et al., 2012). Interestingly, betaxolol and ICI alone have no effect on the compulsion-like behavior assessed by the nestlet shredding model, but the combination of both drugs decreases this compulsion-like behavior (as does propranolol at 10mg/kg) (Lustberg et al., 2020), suggesting β2 importance for some compulsion-related conditions.
Despite the effects of propranolol and betaxolol on alcohol drinking, one significant challenge with ARs compounds is that they also regulate cardiovascular function, with potential for significant side effects (also, betaxolol is only FDA approved to treat an ophthalmic condition). Thus, the development of strategies that could decrease the doses of these drugs used could be an advantage for AUD pharmacotherapy. We thus investigated how combined inhibition of β ARs and α1 ARs, using ineffective doses of propranolol (2.5mg/kg) and prazosin (0.25mg/kg) in combination, could impact alcohol consumption. We found that combining the lower doses of propranolol and prazosin significantly reduced alcohol intake. Interestingly, changes on AOD and CLAD were not correlated, and the ability of this combined treatment to reduce CLAD was correlated with basal intake, with a greater impact in higher drinkers, as we observed here with 5mg/kg of propranolol and BTX, and we showed in our previous study with 0.75mg/kg prazosin (De Oliveira Sergio et al., 2021). In addition, the ineffective dose of prazosin used here (0.25mg/kg) also did not show effects in a model of self-administration of nicotine (Forget et al., 2010). Further, one study combined lower, ineffective doses of propranolol and prazosin (10mg/kg and 1mg/kg, respectively), which decreased alcohol intake in dependent P rats during early withdrawal without affecting locomotion (Rasmussen et al., 2014). Co-administration of prazosin and propranolol also decreases compulsion-like behavior in mice in the marble burying model, and the combination of each drug was more effective than each drug alone (Lustberg et al., 2020). We found that combining prazosin and propranolol impacted both CLAD and AOD (Fig. 3, Suppl. Fig.1), supporting the possible use of combined therapy to reduce alcohol use disorder.
We then examined whether the systemic effects of propranolol and betaxolol could be mediated by two brain areas relate to CLAD, aINS and mPFC. However, all doses tested of intra-aINS propranolol and betaxolol did not affect CLAD or AOD. Furthermore, our findings showed no effect of propranolol or betaxolol into mPFC on CLAD or AUD. Similarly, 0.3μg of prazosin within mPFC did not affect CLAD or AOD intake, while a similar dose of prazosin within aINS decreases both CLAD and AOD (De Oliveira Sergio et al., 2021). The aINS and mPFC receive noradrenergic projections from the Locus Coeruleus (Robertson et al., 2013). Although we found no effects of intra-aINS propranolol, a prior study showed that propranolol at 2.5μg into aINS decreases incidental taste learning, but not aversive taste learning (Miranda et al., 2008). Also, 5μg of propranolol in aINS decreases arousal-induced neophobia although the doses of 1 and 10μg have no effect (Rojas et al., 2015). The same has been shown for mPFC, where propranolol control different responses. 5μg propranolol in mPFC affects aversive taste association as well as aversive retrieval, but not incidental taste memory formation (Reyes-Lopez et al., 2010). Also, systemic administration of propranolol (10mg/kg) regulates the stress-induced changes, mediated by mPFC, that contribute to impaired fear extinction (Fitzgerald et al., 2015).
Although, our data found no effects of propranolol on CLAD and AOD when injected direct into aINS or mPFC, a recent study showed that propranolol injection into central amygdala decreases alcohol intake only in dependent rats, while the prazosin injection in this area decreases alcohol intake only in non-dependent rats (Varodayan et al., 2022). Also, propranolol in basolateral amygdala prevents reinstatement of alcohol seeking in rats (Chesworth et al., 2018). Thus, we speculate that systemic effects of propranolol on alcohol intake could be mediated through amygdala regions, while the effects of prazosin, based on our recent study, could be mediated through aINS (De Oliveira Sergio et al., 2021).
This study has some limitations. First, we only used male rats, and another recent study from our laboratory showed that males and females have different strategies on CLAD intake, with females being more persistent than males for alcohol (De Oliveira Sergio et al., biorxiv). Thus, it would be of value to investigate the effect of these compounds in females. However, despite these limitations, the present findings provide new insights about the effect of the β ARs modulators on alcohol drinking, and, especially, the advantage of using lower doses of propranolol and prazosin in combination as a pharmacological strategy for AUD treatment.
Supplementary Material
Highlights.
Systemic beta-adrenergic receptor inhibition reduces drinking through beta-1 receptors.
Beta-receptor inhibition of drinking is larger in rats with higher basal drinking levels.
Combining subthreshold doses of beta-receptor blocker (propranolol) and alpha1-receptor blocker (prazosin) decreases alcohol drinking.
Effectiveness of combined lower doses may represent a novel therapeutic intervention with fewer side effects.
Beta-receptor inhibition in the anterior insula surprisingly did not reduce alcohol intake.
Beta- and alpha1-receptor inhibition in the medial prefrontal cortex surprisingly did not reduce alcohol intake.
6. Acknowledgements
The authors thank Sydney Hoffman, Heidi Cult, and Rebecca J. Smith for technical assistance with animal drinking and histology.
7. Funding and disclosure
This work was supported by AA024109 (FWH) from the National Institute on Alcohol and Alcoholism. Raw data are available from communicating author.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
All authors declare no competing interests.
8. References
- Arcurio LR, Finn PR, James TW, 2015. Neural mechanisms of high-risk decisions-to-drink in alcohol-dependent women. Addict Biol 20, 390–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly D, Servant D, Blandin N, Beuscart R, Parquet PJ, 1992. Effects of beta-blocking drugs in alcohol withdrawal: a double-blind comparative study with propranolol and diazepam. Biomed Pharmacother 46, 419–424. [DOI] [PubMed] [Google Scholar]
- Barbier E, Barchiesi R, Domi A, Chanthongdee K, Domi E, Augier G, Augier E, Xu L, Adermark L, Heilig M, 2021. Downregulation of Synaptotagmin 1 in the Prelimbic Cortex Drives Alcohol-Associated Behaviors in Rats. Biol Psychiatry 89, 398–406. [DOI] [PubMed] [Google Scholar]
- Carlsson C, Johansson T, 1971. The psychological effects of propranolol in the abstinence phase of chronic alcoholics. Br J Psychiatry 119, 605–606. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Heilig M, Perez A, Probst C, Rehm J, 2019. Alcohol use disorders. Lancet 394, 781–792. [DOI] [PubMed] [Google Scholar]
- Centanni SW, Janes AC, Haggerty DL, Atwood B, Hopf FW, 2021. Better living through understanding the insula: Why subregions can make all the difference. Neuropharmacology, 108765. [DOI] [PubMed]
- Chesworth R, Corbit LH, 2018. Noradrenergic beta-receptor antagonism in the basolateral amygdala impairs reconsolidation, but not extinction, of alcohol self-administration: Intra-BLA propranolol impairs reconsolidation of alcohol self-administration. Neurobiol Learn Mem 151, 59–70. [DOI] [PubMed] [Google Scholar]
- Dao NC, Brockway DF, Suresh Nair M, Sicher AR, Crowley NA, 2021. Somatostatin neurons control an alcohol binge drinking prelimbic microcircuit in mice. Neuropsychopharmacology 46, 1906–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darevsky D, Gill TM, Vitale KR, Hu B, Wegner SA, Hopf FW, 2019. Drinking despite adversity: behavioral evidence for a head down and push strategy of conflict-resistant alcohol drinking in rats. Addict Biol 24, 426–437. [DOI] [PubMed] [Google Scholar]
- Darevsky D, Hopf FW, 2020. Behavioral indicators of succeeding and failing under higher-challenge compulsion-like alcohol drinking in rat. Behav Brain Res 393, 112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira Sergio T, Darevsky D, de Paula Soares V, de Cassia Albino M, Maulucci D, Wean S, Hopf FW, Evidence for different greater-persistence strategies under lower and higher challenge for alcohol in female rats. Biorxiv, 10.1101/2022.1105.1118.492488v492481. [DOI]
- De Oliveira Sergio T, Lei K, Kwok C, Ghotra S, Wegner SA, Walsh M, Waal J, Darevsky D, Hopf FW, 2021. The role of Anterior Insula-brainstem projections and alpha-1 noradrenergic receptors for compulsion-like and alcohol-only drinking. Neuropsychopharmacology, 46, 1918–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domi E, Xu L, Toivainen S, Nordeman A, Gobbo F, Venniro M, Shaham Y, Messing RO, Visser E, van den Oever MC, Holm L, Barbier E, Augier E, Heilig M, 2021. A neural substrate of compulsive alcohol use. Scie Adv, 7, eabg9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs AM, McElligott ZA, 2022. Noradrenergic circuits and signaling in substance use disorders. Neuropharmacology 208, 108997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, 2020. Let’s agree to agree: a comment on Hogarth (2020), with a plea for not-so-competing theories of addiction. Neuropsychopharmacology 45, 715–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Giustino TF, Seemann JR, Maren S, 2015. Noradrenergic blockade stabilizes prefrontal activity and enables fear extinction under stress. Proc Natl Acad Sci U S A 112, E3729–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B, 2010. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35, 1751–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators GBD, 2020. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF, 2010. Effects of beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology 212, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Sussman L, Sundby K, Brennan GM, Diazgranados N, Heilig M, Momenan R, 2018. Neural Correlates of Compulsive Alcohol Seeking in Heavy Drinkers. Biol Psych Cogn Neuro Neuroimag 2, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Haass-Koffler CL, Swift RM, Leggio L, 2018. Noradrenergic targets for the treatment of alcohol use disorder. Psychopharmacology (Berl) 235, 1625–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare GM, Worrall JM, Baker AJ, Liu E, Sikich N, Mazer CD, 2006. Beta2 adrenergic antagonist inhibits cerebral cortical oxygen delivery after severe haemodilution in rats. Br J Anaesth 97, 617–623. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A, 2010. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res 34, 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HM, 2014. Rodent models for compulsive alcohol intake. Alcohol 48, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz RI, Gottlieb LD, Kraus ML, 1989. The efficacy of atenolol in the outpatient management of the alcohol withdrawal syndrome. Results of a randomized clinical trial. Arch Intern Med 149, 1089–1093. [PubMed] [Google Scholar]
- Katner SN, Sentir AM, Steagall KB, Ding Z-M, Wetherill L, Hopf FW, Engelmann EA, 2022. Modeling Aversion Resistant Alcohol Intake in Indiana Alcohol-Preferring (P) Rats. Brain Sci 12, 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW Jr., George O, 2014. Addiction as a stress surfeit disorder. Neuropharmacology 76 Pt B, 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharm 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Soyka M, 2018. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA 320, 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer ME, Palmer RS, Marlatt GA, 1999. Relapse prevention. An overview of Marlatt’s cognitive-behavioral model. Alcohol Res Health 23, 151–160. [PMC free article] [PubMed] [Google Scholar]
- Lei K, Kwok C, Darevsky D, Wegner SA, Yu J, Nakayama L, Pedrozo V, Anderson L, Ghotra S, Fouad M, Hopf FW, 2019. Nucleus Accumbens Shell Orexin-1 Receptors Are Critical Mediators of Binge Intake in Excessive-Drinking Individuals. Front Neurosci 13, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Simms JA, Hopf FW, 2016. A single alcohol drinking session is sufficient to enable subsequent aversion-resistant consumption in mice. Alcohol 55, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Timme NM, Lapish CC, 2019. Encoding of the Intent to Drink Alcohol by the Prefrontal Cortex Is Blunted in Rats with a Family History of Excessive Drinking. eNeuro 6, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustberg D, Iannitelli AF, Tillage RP, Pruitt M, Liles LC, Weinshenker D, 2020. Central norepinephrine transmission is required for stress-induced repetitive behavior in two rodent models of obsessive-compulsive disorder. Psychopharmacology (Berl) 237, 1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H, 2010. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology 35, 2165–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, Rodriguez-Garcia G, Reyes-Lopez JV, Ferry B, Ferreira G, 2008. Differential effects of beta-adrenergic receptor blockade in basolateral amygdala or insular cortex on incidental and associative taste learning. Neurobiol Learn Mem 90, 54–61. [DOI] [PubMed] [Google Scholar]
- Moresco RM, Matarrese M, Soloviev D, Simonelli P, Rigamonti M, Gobbo C, Todde S, Carpinelli A, Kienle MG, Fazio F, 2000. Synthesis and in vivo evaluation of [11C]ICI 118551 as a putative subtype selective beta2-adrenergic radioligand. Int J Pharm 204, 101–109. [DOI] [PubMed] [Google Scholar]
- Nathanson JA, 1988. Stereospecificity of beta adrenergic antagonists: R-enantiomers show increased selectivity for beta-2 receptors in ciliary process. J Pharmacol Exp Ther 245, 94–101. [PubMed] [Google Scholar]
- Radke AK, Sneddon EA, Frasier RM, Hopf FW, 2021. Recent Perspectives on Sex Differences in Compulsion-Like and Binge Alcohol Drinking. Int J Mol Sci 22, 3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Beckwith LE, Kincaid CL, Froehlich JC, 2014. Combining the alpha1 - adrenergic receptor antagonist, prazosin, with the beta-adrenergic receptor antagonist, propranolol, reduces alcohol drinking more effectively than either drug alone. Alcohol Clin Exp Res 38, 1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Lopez J, Nunez-Jaramillo L, Moran-Guel E, Miranda MI, 2010. Differential effects of beta-adrenergic receptor blockade in the medial prefrontal cortex during aversive and incidental taste memory formation. Neuroscience 169, 195–202. [DOI] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, de Marchena J, Jensen P, 2013. Developmental origins of central norepinephrine neuron diversity. Nat Neurosci 16, 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas S, Diaz-Galarce R, Jerez-Baraona JM, Quintana-Donoso D, Moraga-Amaro R, Stehberg J, 2015. The insula modulates arousal-induced reluctance to try novel tastes through adrenergic transmission in the rat. Front Behav Neurosci 9, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy CA, Van Bockstaele EJ, 2007. Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Prog Neuropsychopharmacol Biol Psychiatry 31, 1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh E, Narimatsu A, Hosohata Y, Tsuchihashi H, Nagatomo T, 1993. The affinity of betaxolol, a beta 1-adrenoceptor-selective blocking agent, for beta-adrenoceptors in the bovine trachea and heart. Br J Pharmacol 108, 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW, 2013. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 16, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, Hopf FW, 2015. D-Serine and D-Cycloserine Reduce Compulsive Alcohol Intake in Rats. Neuropsychopharm 40, 2357–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Stappenbeck C, Malte CA, Lyons R, Tell D, Millard SP, Raskind M, 2018. Double-Blind Randomized Clinical Trial of Prazosin for Alcohol Use Disorder. Am J Psychiatry 175, 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fogelman N, Wemm S, Angarita G, Seo D, Hermes G, 2022. Alcohol withdrawal symptoms predict corticostriatal dysfunction that is reversed by prazosin treatment in alcohol use disorder. Addict Biol 27, e13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Wemm S, Fogelman N, Milivojevic V, Morgan PM, Angarita GA, Hermes G, Fox HC, 2021. Moderation of Prazosin’s Efficacy by Alcohol Withdrawal Symptoms. Am J Psychiatry 178, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon EA, White RD, Radke AK, 2019. Sex Differences in Binge-Like and Aversion-Resistant Alcohol Drinking in C57BL/6J Mice. Alcohol Clin Exp Res 43, 243–249. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Canals S, Bifone A, Heilig M, Hyytia P, 2022. From a systems view to spotting a hidden island: A narrative review implicating insula function in alcoholism. Neuropharmacology 209, 108989. [DOI] [PubMed] [Google Scholar]
- Spanagel R, 2009. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev 89, 649–705. [DOI] [PubMed] [Google Scholar]
- Spoelder M, Hesseling P, Baars AM, Lozeman-van ‘t Klooster JG, Rotte MD, Vanderschuren LJ, Lesscher HM, 2015. Individual Variation in Alcohol Intake Predicts Reinforcement, Motivation, and Compulsive Alcohol Use in Rats. Alcohol Clin Exp Res 39, 2427–2437. [DOI] [PubMed] [Google Scholar]
- Spoelder M, Pol S, Janssen BSG, Baars AM, Vanderschuren L, Lesscher HMB, 2017. Loss of control over alcohol seeking in rats depends on individual vulnerability and duration of alcohol consumption experience. Behav Pharmacol 28, 334–344. [DOI] [PubMed] [Google Scholar]
- Swartz CM, 1998. Betaxolol in anxiety disorders. Ann Clin Psychiatry 10, 9–14. [DOI] [PubMed] [Google Scholar]
- Tondo L, Conway PG, Brunswick DJ, 1985. Labeling in vivo of beta adrenergic receptors in the central nervous system of the rat after administration of [125I] iodopindolol. J Pharmacol Exp Ther 235, 1–9. [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, 2008. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 583, 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Patel RR, Matzeu A, Wolfe SA, Curley DE, Khom S, Gandhi PJ, Rodriguez L, Bajo M, D’Ambrosio S, Sun H, Kerr TM, Gonzales RA, Leggio L, Natividad LA, Haass-Koffler CL, Martin-Fardon R, Roberto M, 2022. The Amygdala Noradrenergic System Is Compromised With Alcohol Use Disorder. Biol Psychiatry 91, 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazey EM, den Hartog CR, Moorman DE, 2018. Central Noradrenergic Interactions with Alcohol and Regulation of Alcohol-Related Behaviors. Handb Exp Pharmacol 248, 239–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O, Hang S, Baker DA, Mantsch JR, 2012. beta-adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for beta1 and beta2 adrenergic receptors. J Pharmacol Exp Ther 342, 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF, 2008. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol 18, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner SA, Hu B, De Oliveira Sergio T, Darevsky D, Kwok CC, Lei K, Hopf FW, 2019. A novel NMDA receptor-based intervention to suppress compulsion-like alcohol drinking. Neuropharmacology 157, 107681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Carroll RJ, Harden KK, Wu G, 2012. Comparison of treatment means when factors do not interact in two-factorial studies. Amino Acids 42, 2031–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette RN, Aiyar N, Yue TL, Mitchell MP, Disa J, Storer BL, Naselsky DP, Stadel JM, Ohlstein EH, Ruffolo RR Jr., 1999. In vitro and in vivo characterization of intrinsic sympathomimetic activity in normal and heart failure rats. J Pharmacol Exp Ther 289, 48–53. [PubMed] [Google Scholar]
- Zilm DH, Sellers EM, MacLeod SM, Degani N, 1975. Letter: Propranolol effect on tremor in alcoholic withdrawal. Ann Intern Med 83, 234–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


