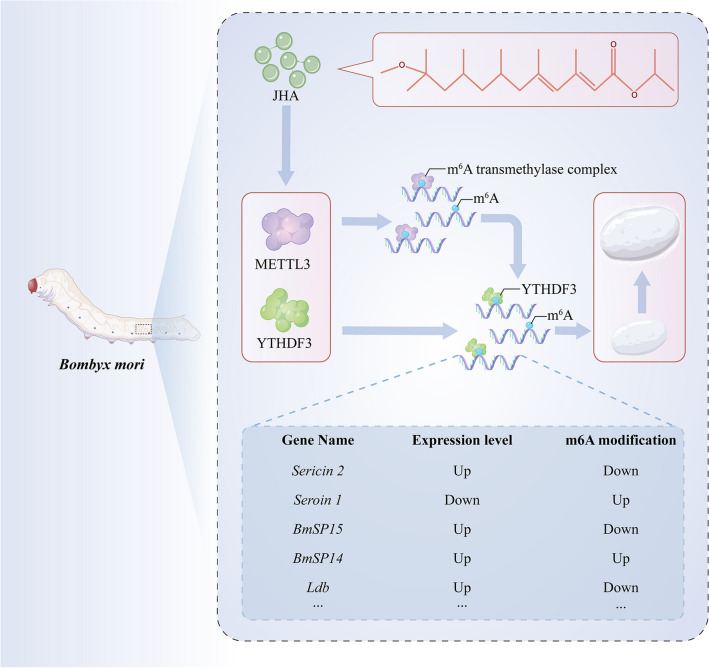
Abstract
Juvenile hormone (JH) is an indispensable insect hormone that is critical in regulating insect development and physiology. N6-methyladenosine (m6A) is the most abundant modification of RNA that regulates RNA fate in eukaryotic organisms. However, the relationship between m6A and JH remains largely unknown. Here, we found that the application of a Juvenile hormone analog (JHA) extended the larval period of Bombyx mori and increased the weight and thickness of the cocoon. Interestingly, global transcriptional patterns revealed that m6A-related genes are specifically regulated by JHA in the posterior silk gland (PSG) that synthesizes the major component of cocoon silk. By transcriptome and m6A sequencing data conjointly, we discovered that JHA significantly regulated the m6A modification in the PSG of B. mori and many m6A-containing genes are related to nucleic acid binding, nucleus, and nucleobase-containing compound metabolism. Notably, 547 genes were significantly regulated by JHA at both the m6A modification and expression levels, especially 16 silk-associated genes, including sericin2, seroin1, Serine protease inhibitors 4 (BmSPI4), Serine protease inhibitors 5 (BmSPI5), and LIM domain-binding protein 2 (Ldb). Among them, 11 silk associated genes were significantly affected by METTL3 knockdown, validating that these genes are targets of m6A modification. Furthermore, we confirm that JHA directly regulates the expression of BmSPI4 and BmSPI5 through m6A modification of CDS regions. These results demonstrate the essential role of m6A methylation regulated by JH in PSG, and elucidate a novel mechanism by which JH affects silk gland development via m6A methylation. This study uncovers that m6A modification is a critical factor mediating the effect of JH in insects.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-023-04996-1.
Keywords: Insect, Juvenile hormone, m6A, Silk gland, Bombyx mori
Introduction
Silk is a natural protein fiber known for its shine, strength, luster, and durability. In recent years, regenerated silk solutions were used to form a variety of biomaterials. Silk is usually made from cocoons that are spun by the silk gland of the domesticated silkworm, Bombyx mori. The increasing demand has driven notable efforts to improve silk production through hybrid breeding, but silk production has reached the long-expected plateau. The silk gland of B. mori is composed of three regions. The anterior silk gland is responsible for silk spinning, and the middle silk gland secretes sericin. The posterior silk gland synthesizes fibroin, the major component of cocoon silk. Juvenile hormone (JH) was used to improve the yield of silk in silk production. Juvenile hormone (JH) plays a crucial role in preserving larval characteristics and regulating various other biological processes in insects [1]. In B. mori, JH could induce the expression of Bmdimm, a transcription factor in the larval silk gland and contribute to the synthesis of fibroin H-chain protein [2–4]. JH restrains BmTINP1, which is involved in silk production, translocation from the nucleus to the cytoplasm [5]. The cross-talk of 20-hydroxyecdysone and JH regulates the transcription of FMBP-1, a novel transcription factor influencing fib-H transcription [6]. The idea that stimulating fibroin protein synthesis by JHA treatment could improve silk yield is very attractive; however, little is known about the molecular mechanisms underlying the effect of JHA in the silkworm posterior silk gland on silk yield increase [7].
The regulation of JH is extensively involved in numerous aspects of insect life. Previous studies on JH in insects have mainly concentrated on JH directly regulating the transcription, translation, and translocation of specific genes [8–11]. For example, JH induced the expression of Kruppel homolog 1, thereby repressing the metamorphic differentiation of Drosophila adult abdominal epidermis [12]. Epigenetic studies on JH have become widespread and are gradually gaining attention. For example, the potential targets of CoREST, an integral component of chromatin corepressor complexes, are involved in the JH signaling pathway and lead to a switch to minor-like caste foraging behavior in Camponotus floridanus [13]. In Tribolium castaneum, deacetylation and acetylation of histones mediated by acetylation proteins play an important role in JH action [14]. CREB-binding protein, a transcriptional coregulator with histone acetyltransferase activity, mediates acetylation of H3K27 for JH induction of target genes in T. castaneum [15]. N6-methyladenosine modification is the most abundant and widespread internal modification found in the RNA of eukaryotic organisms. This modification plays a crucial role in maintaining the stability and effectiveness of mRNA during the process of translation [16–20]. The regulation and recognition of m6A modification is a dynamic process that involves the concerted action of different proteins, including demethylases, methyltransferases, and m6A binding proteins. These proteins work together to ensure that the m6A modification is properly recognized and regulated within the RNA, allowing for the efficient translation of mRNA [21, 22]. The evolutionary relationship of m6A-related genes, including METTL3, METTL14, YTHDF3, YTHDC, and FL2D, is relatively conserved in insects [23]. m6A is involved in various biological processes of insects. For example, m6A plays a critical role in sex determination and neuronal function in Drosophila [24, 25]. The m6A site in the 5’ UTR of a P450 gene, CYP4C64, leads to thiamethoxam resistance in Bemisia tabaci [26]. METTL3, the indispensable component of the m6A demethylase complex, influences Bombyx mori nucleopolyhedrovirus infection, cell cycle progression, and chromosome alignment in BmN cells [27, 28]. In Apis mellifera, three genes in JH biosynthesis, JHAMT, Vg, and CYP314A1, have different m6A methylation levels between workers and queens in different larval stages. Treatment with the SAH hydrolysis inhibitor DAA, which has been confirmed in peripheral cells to mainly affect RNA processing through inhibition of m6A methylation, could alter the titer of JH in worker larvae [29]. The present study indicates the potential correlation between the JH signaling pathway and m6A modification. However, whether and how JH influences m6A modification in insects is still unclear. Considering the significant association of m6A modification with growth and development, this study aimed to investigate the potential role of m6A in the action of JH.
In this study, we investigated whether JH regulates silk gene expression levels through RNA m6A methylation. To detect the effect of insect hormones on m6A-related genes, we performed transcriptome sequencing of multiple silkworm tissues after JHA and 20E treatment. Interestingly, many m6A-related genes were specifically regulated by JHA in silkworm PSGs. To further reveal the regulation of JH in numerous biological processes of silkworms through m6A modification, we conducted m6A methylation and RNA sequencing in the silkworm PSGs after JHA treatment. Furthermore, we knocked down METTL3 and performed transcriptome profiling analysis to identify the mechanism by which m6A modification mediates the regulatory effect of JH. Conjoint analysis of the transcriptome and m6A sequencing reveal that JH regulates many silk-associated genes in the silkworm PSG via m6A modification. Our findings provide new insights into the pivotal regulatory role of JH mediated by m6A.
Results
JH enhances the weight and thickness of silkworm cocoon
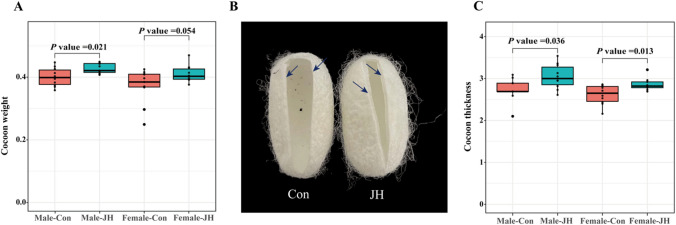
To identify the function of JHA in improving silk protein production in silkworms, we performed individual experiments on silkworm larvae. After JHA treatment for 24 h, we found that the application of JHA significantly extended the fifth larval instar of silkworm. JHA treatment extended the B. mori fifth instar from 8 to 11 days (Fig.S1). We further measured the effect of JHA on the weight and thickness of the cocoon. The results showed that JHA enhanced the weight of silkworm cocoons, especially those of male silkworm (Fig. 1A). In addition, we found that JHA treatment significantly increased the thickness of silkworm cocoons, both in males and females (Fig. 1B–C). Taken together, these results showed that the application of JH extended the larval period, increased the weight and thickness of the cocoon, and further enhanced silk production in silkworms.
Fig. 1.
JHA treatment enhanced the weight and thickness of silkworm cocoons. Comparison of cocoon weight between the control and JHA-treated groups (A). Comparison of cocoon thickness between the control and JHA-treated groups (B and C)
Characteristics of m6A modification in the transcriptome of silkworm posterior silk gland
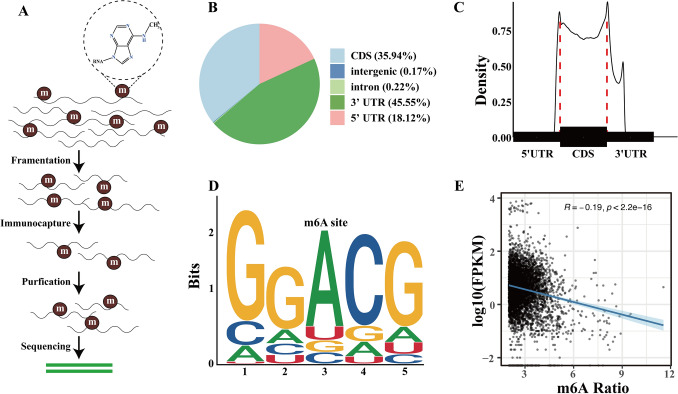
To investigate the characteristics of m6A modification in the PSG of silkworm, we performed m6A methylation sequencing to identify specific locations of m6A sites in the mRNA of PSG (Fig. 2A). Our results showed that m6A modification was mainly enriched in exons and 3’ UTRs, with particularly high levels of m6A peaks observed around stop codons (Fig. 2B–C). Through the analysis of consensus motifs, we observed that the GGAC motif was enriched within m6A sites (Fig. 2D). The motif has been frequently found in other studies on humans, mice, and fruit flies. To confirm the role of m6A in the expression of genes, we further analyzed the relationship between the expression level, represented by FPKM, and the m6A ratio of m6A-containing genes. The results showed that the expression levels of m6A-containing genes had a negative relationship with the m6A modification levels of m6A-containing genes (Fig. 2E). Collectively, the m6A sequencing data provided clear evidence of the distribution features and consensus motifs of m6A in the mRNA of silkworm PSG. These results suggested that m6A modification in the mRNA of silkworm PSG tends to down-regulate gene expression.
Fig. 2.
Analysis of m6A methylation sequencing in silkworm PSG: distribution, motif identification, and relationship with gene expression. Workflow of m6A methylation sequencing, including RNA fragmentation, immunoprecipitation with a m6A-specific antibody, mRNA purification, and sequencing (A). Pie chart showing the proportion of m6A peaks in the indicated regions, such as CDS, 3’ UTRs, and 5’ UTRs (B). The profile of m6A distribution across the transcriptome in silkworm PSG (C). The sequence motif of m6A modification identified in silkworm PSG mRNA (D). Relationship between the gene m6A modification ratio and expression levels (E)
m6A-containing genes involved in several important biological pathways
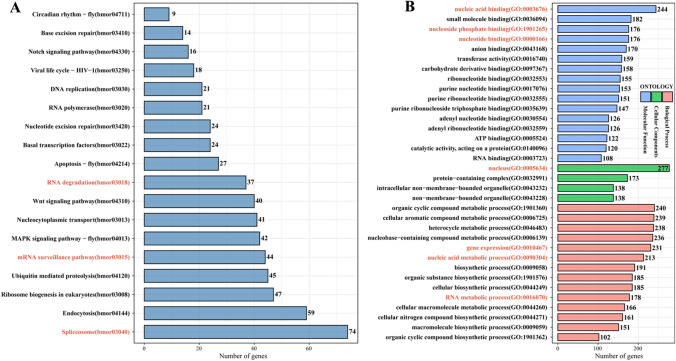
m6A modification is involved in various gene expression regulation and biological processes [30]. To investigate the role of m6A in silkworm PSG, we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analysis on high-confidence m6A-containing genes. From the m6A-seq data, we identified 4962 high-confidence m6A-containing genes. Seventy-four m6A-containing genes are involved in the spliceosome and are critical for mRNA alternative splicing. Forty-four and 37 m6A-containing genes were enriched in the mRNA surveillance pathway and RNA degradation, respectively (Fig. 3A). These pathways are important for the stability of mRNA. The results showed that m6A-containing genes are involved in regulating the stability, splicing and translation of mRNA.
Fig. 3.
KEGG and GO enrichment analysis of significant m6A-containing genes identified in silkworm PSG m6A-seq data. KEGG enrichment analysis of m6A-containing genes identified in the m6A-seq (A). GO enrichment analysis of biological process, cellular components, and molecular function for m6A-containing genes identified in the m6A-seq (B). Top 18 KEGG terms and GO terms containing more than 100 genes are listed to display the potential function of m6A modification in silkworm PSG. Enriched GO and KEGG terms with p < 0.05 were represent in red color
Furthermore, GO enrichment analysis revealed that 244 and 176 m6A-containing genes were enriched in nucleic acid binding and nucleotide binding, respectively. Compared to molecular function terms, biological process terms, including gene expression, nucleic acid metabolic process, and RNA metabolic process, were enriched. Additionally, 277 m6A-containing genes were enriched in the nucleus in the cellular component category (Fig. 3B). These results indicated that m6A is closely related to nucleic acid binding and metabolic processes. Taken together, our findings suggest that m6A plays a critical role in influencing RNA fate and regulating gene expression in the PSG of B. mori.
JH regulates the m6A modification and expression level of genes in silkworm PSGs
To investigate the relationship between JH and m6A modification, we performed transcriptome sequencing of multiple silkworm tissues after JHA treatment. Our findings indicated that the expression of m6A-related genes was significantly changed by JHA treatment in PSG compared to other tissues (Fig.S2A). Western blotting also showed that JHA treatment significantly induced the protein expression of METTL3 and YTHDF3 in silkworm PSG (Fig.S2B). Additionally, we further found that JHA treatment significantly increased the m6A abundance of RNA in the PSG (Fig.S2C). The results indicated that JHA treatment affects the expression of METTL3 and YTHDF3, thereby regulating the m6A abundance of RNA in the PSG of silkworms.
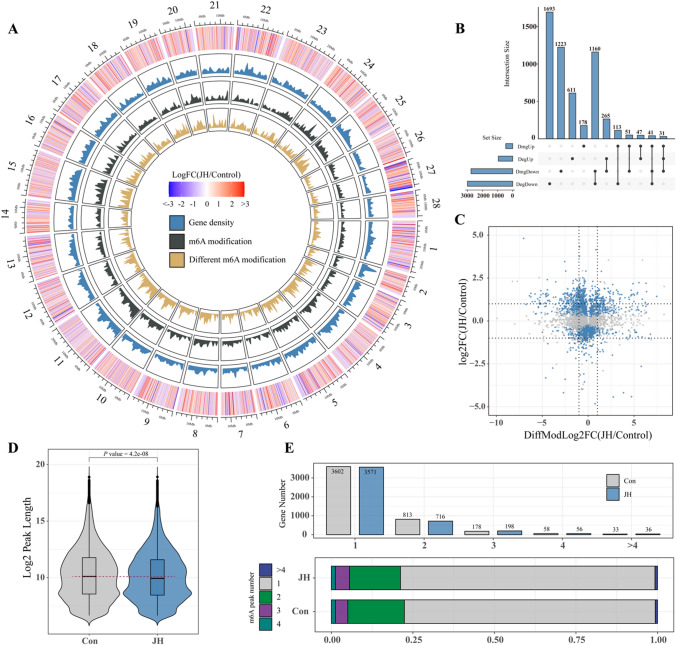
We further performed m6A sequencing of PSGs after JHA treatment. By analyzing transcriptome and m6A sequencing data conjointly, we found 3795 differentially m6A-modified genes (DMGs) and 3961 differentially expressed genes (DEGs) in m6A-seq and RNA-seq of silkworm PSG, respectively. DMGs and DEGs were mapped on B. mori chromosomes, and we found that the density of m6A modifications, different m6A modifications, and gene density on chromosomes were highly consistent with each other. In most B. mori chromosomes, downregulated and upregulated DEGs were distributed uniformly. However, the downregulated DEGs were enriched significantly at the telomeres of chromosomes 27 (Fig. 4A). Moreover, 261 downregulated and 222 upregulated genes had upregulated m6A modification levels. A total of 438 and 614 genes with downregulated m6A modification levels were downregulated and upregulated, respectively. These results revealed that JHA widely regulates gene expression and mainly downregulates gene expression by regulating m6A modification of mRNA. Notably, 2304 DEGs were not DMGs, and 1455 DMGs were not DEGs (Fig. 4B–C). Comparing m6A peak lengths between the control and JHA treatment groups revealed that JHA treatment significantly decreased the length of mRNA, which was modified by m6A (Fig. 4D). Through statistical analysis of the number of m6A peaks in each gene, we found that most genes had one or two m6A peaks. JHA treatment decreased the proportion of genes with one or two m6A peaks and increased the number of genes with three m6A peaks (Fig. 4E). These results show that JH affects the density and distribution of m6A modifications, thereby regulating m6A-containing gene expression.
Fig. 4.
JHA regulates m6A modification of mRNA and gene expression in the silkworm PSG. Circos plot of the log2FC of DEGs, gene density, m6A modification density, and DMGs on B. mori chromosomes, from the outside to the inside (A). Upset plot displayed the DEGs and DMGs identified in RNA-seq and m6A-seq data (B). In the volcano plot, genes with significantly increased and decreased expression levels are highlighted in blue. The dashed line indicates y/x = ± 1 (C). Comparison of peak lengths of m6A in silkworm PSGs in the control and JHA treatment groups (D). The number and proportion of genes with different m6A peaks in the control and JHA treatment groups. The proportion of genes with 1, 2, 3, 4, and over 4 m6A peaks were mapped in different color respectively (E)
Enrichment analysis of differentially m6A-modified DEGs after JHA treatment
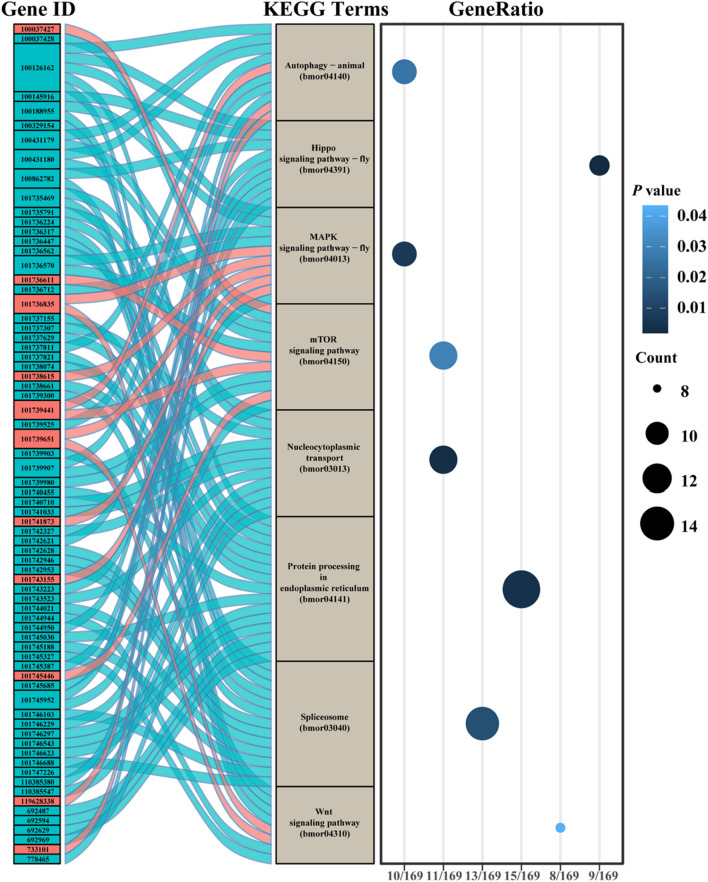
To investigate the effects of JHA treatment on silkworm PSGs, we performed GO and KEGG enrichment analyses of genes with different expression levels and m6A peaks in RNA-seq and m6A-seq data. We found DEGs with significantly different m6A modifications after JHA treatment. To uncover the function of these genes, we performed GO enrichment analysis. Most of the genes were enriched in molecular function terms related to binding activity, especially nucleic acid binding activity. Two cellular component terms, nucleus and protein-containing complex, as well as five biological process terms, gene expression and biological regulation, were enriched in the GO analysis (Fig. S3). The results further confirmed that m6A modification affected the fate of nucleic acids by regulating nucleic acid binding and nuclear components, thereby regulating gene expression and biological processes. KEGG enrichment analysis of these 547 genes found that 8 pathways, including autophagy, Hippo signaling pathway, MAPK signaling pathway, mTOR signaling pathway, nucleocytoplasmic transport, protein processing in endoplasmic reticulum, spliceosome, and Wnt signaling pathway, were highly enriched (Fig. 5). In total, 61 of 72 DEGs in these 8 pathways were downregulated after JHA treatment. All DEGs were downregulated in 4 pathways, including the Hippo signaling pathway, nucleocytoplasmic transport, spliceosome, and protein processing in the endoplasmic reticulum. The results suggested JHA affects multiple biological processes in PSGs through m6A modification.
Fig. 5.
KEGG enrichment analysis of genes with differential m6A peaks and expression levels in silkworm PSG m6A-seq data after JHA treatment. Genes with different m6A peaks and expression levels were analyzed for KEGG enrichment. Eight KEGG terms with the highest gene counts were mapped. Seventy-two genes involved in these 8 KEGG terms are listed on the left-hand side of the Sankey plot. Blue and red blocks represent down- and up-regulated genes, respectively. The dot plot on the right-hand side of the analysis shows the gene counts, gene ratio, and P value of 8 KEGG terms
JHA treatment regulated the m6A modification and expression level of silk-associated genes
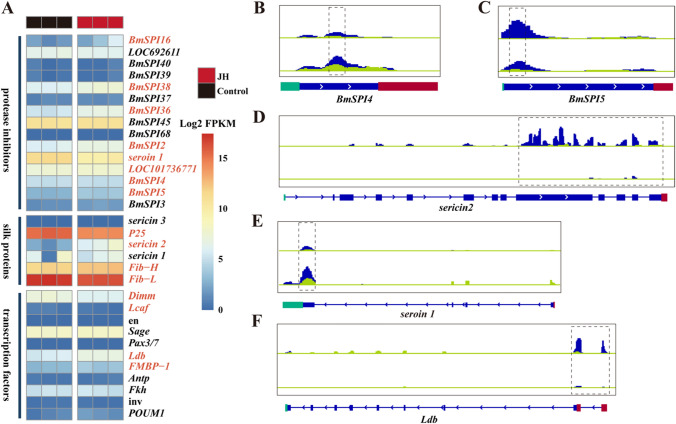
To better understand the effect of JHA on silk protein synthesis in PSGs, we conducted an analysis of the expression changes in silk-associated genes, which are critical for silk protein synthesis, transport, and silk fiber formation (Table S1) [31]. These silk-associated genes were grouped into three functional categories: protease inhibitors, silk proteins, and transcription factors. Among these silk-associated genes, 32 genes were identified in transcriptome sequencing data (Fig. 6A), and 16 genes were significantly differentially expressed (Fig.S4). We further performed qPCR of 6 silk-associated genes to identify the reliability of RNA-sequencing. The result of qPCR confirmed the transcriptome data (Fig.S5). In addition, 6 of the 35 silk-associated genes showed significant changes in m6A modification (Fig. 6B–F), suggesting that these genes are targets of m6A modification. Notably, m6A modification was mainly concentrated in the 3’ UTRs and CDS regions of these 6 silk-associated genes. Furthermore, we observed that JHA resulted in different m6A modification changes for different gene transcripts. JHA significantly increased the m6A modification levels of seroin 1, proteasome inhibitor PI31, and BmSPI4. However, the m6A modification levels of sericin 2, BmSPI5, and Ldb were downregulated by JHA. Taken together, the results suggest that the expression of BmSPI4, BmSPI5, sericin2, and seroin1 is regulated by m6A modification.
Fig. 6.
Analysis of silk-associated genes in control and JHA-treated groups using RNA-seq and m6A-seq data. Heatmap representing the Log2 FPKM of 32 silk-associated genes in RNA-seq data. Significantly regulated genes (P value < 0.05) were represented in red color (A). Silk-associated genes were grouped by their function in silk gland biological activities. IGV tracks in the blue panels display m6A-seq reads, while the light green panels display RNA-seq reads, for 5 silk-associated genes along the locus in both control and JH-treated groups (B–F)
Knocking down METTL3 regulated the expression level of silk-associated genes
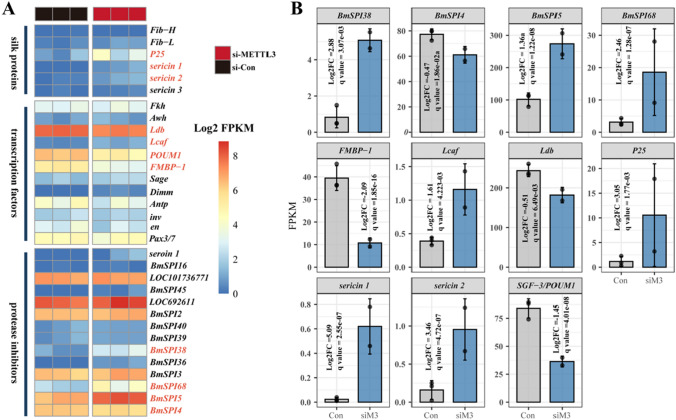
To investigate the role of m6A in the expression of silk-associated genes, we further knocked down the expression of METTL3 in embryonic of silkworm. Through transcriptome sequencing data analysis, we identified 3561 DEGs after METTL3 knockdown, and 1359 DEGs were m6A-containing genes. The m6A sequencing data in this study revealed that multiple genes in the JH signaling pathway are also m6A-methylated (Table S2). Knocking down METTL3 widely affected the expression of many genes in the JH signaling pathway, including Juvenile hormone esterase 1 (JHE1), Juvenile hormone epoxide hydrolase 2 (JHEH2), Juvenile hormone epoxide hydrolase 3 (JHEH3), Farnesyl pyrophosphate syntase (FPS), etc. (Table S3). We further identified 32 silk-associated genes in the RNA-seq of silkworm embryos (Fig. 7A). In total, 11 silk-associated genes were DEGs (Fig. 7B). Interestingly, 5 DEGs, including BmSPI4, BmSPI5, sericin2, Ldb, and seroin1, had significantly different m6A modification levels after JHA treatment in the PSG (Table S4). Taken together, the results suggested that the expression of BmSPI4, BmSPI5, sericin2, and seroin1 is regulated by m6A modification.
Fig. 7.
Analysis of silk-associated genes in silkworm embryos of the control and siMETTL3 group using RNA-seq data. Heatmap representing the Log2 FPKM of 32 silk-associated genes in RNA-seq data after knocking down METTL3 in the embryonic stage of silkworm. Significantly regulated genes (P value < 0.05) were represented in red color (A). Silk-associated genes were grouped by their function in silk gland biological activity. Bar charts representing 11 silk-associated genes that have significantly different expression in transcriptome data after knocking down METTL3 (B)
JHA regulated the expression of BmSPI4 and BmSPI5 via m6A modification
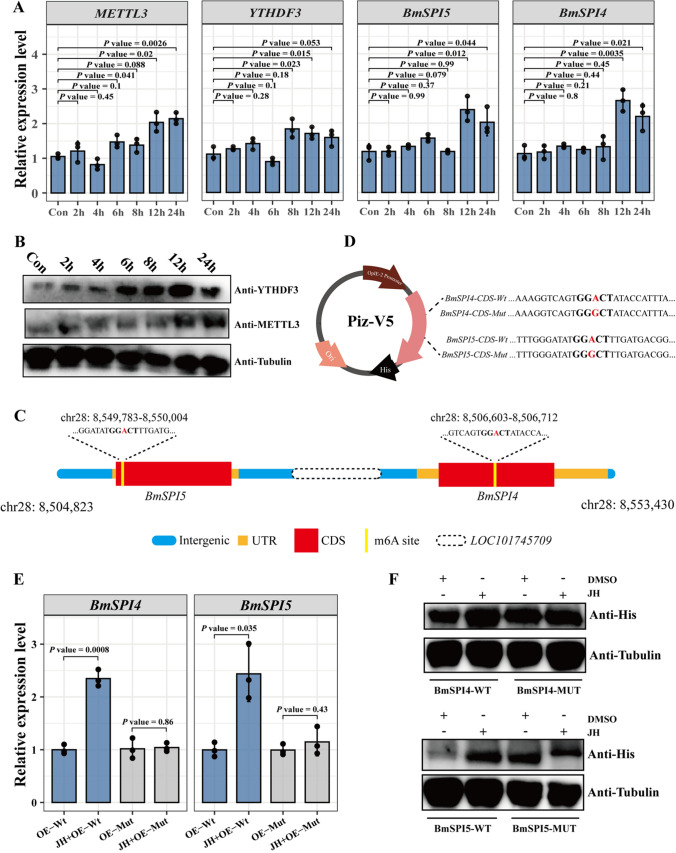
To investigate whether JH directly regulates silk-associated genes, we treated BmN cells with JHA for 2 h, 4 h, 6 h, 8 h, 12 h, and 24 h (Fig. 8A, Fig S6). The qPCR results suggested that JHA significantly increased the expression of METTL3 and YTHDF3 (Fig. 8A), and the results were confirmed by western blotting analysis (Fig. 8B). We also found that BmSPI4 and BmSPI5 were induced by JHA in BmN cells at 12 h. Given that JHA altered the m6A modification levels of BmSPI4 and BmSPI5, we therefore investigated whether JHA directly regulates the expression of BmSPI4 and BmSPI5 through m6A in BmN cells. According to the results of m6A sequencing, we identified that m6A peaks in the BmSPI4 CDS region ranged from NC_051385.1: 8,549,783–8,550,004, and the BmSPI5 CDS region ranged from NC_051385.1: 8,506,603–8,506,712 (Fig. 8C). We further generated reporter plasmids containing His-tag following the wild-type BmSPI4, BmSPI5, or mutant BmSPI4, BmSPI5 CDS. To avoid the influence of background expression of BmSPI4 and BmSPI5 in BmN cells, we designed specific qPCR primers located in the pIZ/V5-His vector, and BmSPI4 or BmSPI5 CDS regions (Fig. 8D). We performed qPCR and western blotting assays to detect the expression of PizV5-BmSPI4 and PizV5-BmSPI5 with or without JHA treatment. The results suggested that JHA treatment significantly enhanced the expression levels of pIZ/V5-BmSPI4-WT and PizV5-BmSPI5-WT (Fig. 8E–F). However, JHA did not improve the expression of pIZ/V5-BmSPI4-Mut or pIZ/V5-BmSPI5-Mut (Fig. 8E–F). Taken together, the results reveal that JHA directly regulates BmSPI4 and BmSPI5 through m6A modification of CDS regions.
Fig. 8.
Expression of BmSPI4 and BmSPI5 was directly regulated by JHA through m6A modification in BmN cells. qPCR results of METTL3, YTHDF3, BmSPI4, and BmSPI5 after JHA treatment for 2 h, 4 h, 6 h, 8 h, 12 h, and 24 h in BmN cells (A). Protein expression of METTL3 and YTHDF3 in BmN cells after JHA treatment for 2 h, 4 h, 6 h, 8 h, 12 h, 24 h, and 24 h was measured by western blotting analysis (B). Schematic representation of the positions of m6A motifs within BmSPI4 and BmSPI5. The elements of genes were presented in different color. Intergenic region, UTR, CDS and m6A sites were mapped in blue, brown, red and yellow, respectively (C). Schematic representation of mutated CDS regions of BmSPI4 and BmSPI5, which were cloned into the pIZ/V5 vector (D). qPCR and western blotting of pIZ/V5-BmSPI4-WT, pIZ/V5-BmSPI4-Mut, pIZ/V5-BmSPI5-WT, and pIZ/V5-BmSPI5-Mut in the control and JHA treatment groups (E and F)
Discussion
In this study, we identified that JHA enhanced the weight and thickness of silkworm cocoons. Through conjoint analysis of RNA-seq and m6A-seq, we uncovered the regulatory relationship of JH to silk-associated gene expression levels by changing m6A modification in silkworm PSG. Knockdown of METTL3 further validated that many silk genes are targets of m6A modification. Furthermore, we confirmed that JHA directly regulated expression of BmSPI4 and BmSPI5 through m6A modification of the CDS regions in BmN cells. This study provided evidence in vivo and in vitro illustrating that m6A modification plays a novel epigenetic mechanism in mediating the effects of JH. Our findings advance the understanding of the epitranscriptomic regulation of JH and provide new insights into the role of m6A modification in insects.
Recent studies have focused on the relationship between JH and epigenetic modification [14]. Epigenetic modifications, acetylation and deacetylation, play critical roles in JH action. However, the correlation between JH and m6A, the most prevalent chemical modification of eukaryotic RNA, is still unclear. An increasing number of studies have suggested that m6A modification participates in numerous biological functions in mammals and insects [24, 25, 32]. In this study, we found that JHA treatment significantly induced the protein expression of METTL3 and YTHDF3 in silkworm PSG. We further performed m6A methylation sequencing to characterize the profile of m6A modifications in silkworm PSG. m6A peaks are enriched in the CDS, especially around the stop codon region. The results showed that the m6A distribution is conserved compared to that in other species [33, 34]. We also found m6A peaks enriched in the start codon in silkworm PSG. Surprisingly, the peak in the start codon region did not appear in the mRNA of silkworm midgut and BmN cells [26, 27], suggesting that the feature of the m6A modification in different tissues of silkworm has tiny differences. Further investigation is needed to fully understand the mechanism by which JHA regulates the expression of METTL3 and YTHDF3.
m6A modification has been shown to play a critical role in RNA-mediated regulation of numerous biological processes. In esophageal squamous cell carcinoma, m6A modification regulates Notch1 expression and further promotes the activation of the Notch signaling pathway [35]. In this study, 16 m6A-containing genes involved in the Notch signaling pathway were also enriched. In gastric cancer research, m6A suppression enhanced GC cell proliferation and invasiveness by activating the Wnt signaling pathway [36]. The Wnt signaling pathway was enriched after knocking down METTL3 in the embryonic stage of silkworm [23]. Here, 40 m6A-containing genes involved in the Wnt signaling pathway were also found in silkworm PSG. Collectively, these results showed that the correlation between m6A and the Notch and Wnt signaling pathways might be conserved and widespread in eukaryotes. Knocking down METTL3 also affected the expression of many genes in the JH signaling pathway including JHE1, JHEH2, JHEH3, FPS, Hmg-r, Mk, Mpk, etc. Compared to a study of A. mellifera [29], the m6A sequencing data in this study revealed that multiple genes in the JH signaling pathway are also m6A-methylated. Further experiments are required to determine the specific mechanisms by which METTL3 regulates the expression of many genes in the JH signaling pathway.
Silk secretion is one of the most mysterious and desirable biological phenomena in nature and has been studied for a long time, especially in B. mori and Nephila clavipes [37]. Transcriptome analysis of the B. mori silk gland, including the PSG and middle silk gland (MSG), showed that the PSG and MSG differ greatly in energy metabolism, silk protein synthesis and secretion [31]. Insect hormones are the critical factor controlling silk protein biosynthesis [38]. JH plays a crucial role in regulating silk gland development and silk fiber production in silkworm [3, 39, 40]. Moreover, previous reports highlighted that a JHA called methoprene could increase silk yield in silkworm [41, 42]. Here, we found that m6A-related genes are regulated by JHA in the PSG.
Silk-associated genes are divided into three parts according to the function in the process of silk fiber formation. BmSPI4, BmSPI5, BmSPI36, BmSPI38, seroin2, etc. are protease inhibitors which are critical for protecting the silk protein from degrading by protease. Fib-L, Fib-H, seroin2, etc. are the components of silk fiber. Ldb, Dimm, sage etc. are important transcription factors for regulating the process of silk production. Here, we found that 16 silk-associated genes enriched in the PSG of silkworms had significantly different expression after JHA treatment. In this study, we further investigated the role of m6A in mediating the effect of JH on PSG, especially in silk-associated genes. Five silk-associated genes, BmSPI4, BmSPI5, seroin2, sericin2, and Ldb were differentially m6A methylated after JHA treatment. The results indicated that these genes are potential targets of m6A modification. In the mammal animals, the m6A reader protein YTHDF1 and YTHDF2 play essential roles in enhancing mRNA translation and degradation, respectively. So far we did not find YTHDF1 and YTHDF2 in the silkworm. In addition, the function of YTHDF3 in B. mori is still unclear. Further research about the mechanism of YTHDF3 regulating expression of silk-associated genes via recognizing m6A modification is needed. In another aspect, silk-associated genes undergo regulating by complex system which is not limited in m6A system [2, 3]. This also interpreted why the expression level of some genes is consistent with the m6A modification level, while the other part is opposite.
To further identify the targets of m6A, we altered the m6A abundance by knocking down METTL3. METTL3, as the most important component of the m6A transmethylase complex, plays a critical role in RNA m6A modification [43]. Here, we found that knocking down METTL3 led to changes in the expression level of many silk-associated genes. In addition, the results also suggested that the alternation of m6A modification in different tendencies led to inverse expression changes in silk-associated genes. The results suggest that there is a direct regulatory mechanism of m6A and silk-associated genes. Point mutagenesis of m6A sites in the regions of the BmSPI4 and BmSPI5 CDSs further identified that m6A plays a key role in enhancing the expression of BmSPI4 and BmSPI5 after JHA treatment. These results reveal that m6A modification regulated by JH plays a significant role in regulating the expression of silk-associated genes in silkworm PSGs.
Concluding remarks
In summary, we characterized the detailed profile of m6A modification in mRNA of silkworm PSG and revealed that many m6A-related genes were regulated in the PSG of silkworm. m6A marks in the PSG were differentially altered by JHA. Transcriptome and m6A sequencing data show that JHA regulates numerous biological processes of PSG through m6A modification. In addition, the data suggest that JHA regulates the expression of many silk associated genes in the PSG by m6A methylation. Furthermore, we explored the mechanism by which JHA regulates the expression of silk associated genes through m6A modification by knocking down METTL3 and point mutant genesis. These results reveal the essential role of m6A methylation regulated by JH in silk gland development and silk production. These results may help to overcome sericultural bottleneck. More importantly, this study demonstrates that m6A modification is a critical factor mediating the effect of JH in insects.
Experimental procedures
Methylated RNA immunoprecipitation and sequencing
Total RNA of silkworm larvae PSG at the 3rd day of the 5th instar was isolated with TRIzol. Poly RNA was purified from 50 µg of total RNA using Dynabeads Oligo 25–61,005. The poly RNA was fragmented into small pieces using the Magnesium RNA Fragmentation Module and then incubated with m6A-specific antibody in IP buffer at 4 °C for 2 h. After reverse transcription of the IP RNA to cDNA using SuperScript™ II Reverse Transcriptase, the cDNA was used to synthesize U-labeled second-stranded DNA with E. coli DNA polymerase I, RNase H and dUTP Solution. Following ligation with indexed adapters, size selection was performed with AMPureXP beads. After U-labeled second-stranded DNA was treated with the heat-labile UDG enzyme, the ligated products were amplified by PCR. Finally, paired-end sequencing on an Illumina Novaseq™ 6000 platform was performed according to the vendor’s recommended protocol.
Sequencing data analysis
Trim-galore was used to remove the reads that contained adaptor contamination, low-quality bases and undetermined bases with default parameters. FastQC was used to verify the sequence quality of the IP and input samples. STAR was used to map reads to the reference genome of B. mori. The peaks were called and analyzed using MACS2 and subsequently annotated using the ChIPseeker R package. After peak calling of m6A peaks with MACS2, the bed file with the sites and annotation of m6A peaks on B. mori genome was imported in R, and further made the Txdb object which having the information of starting and ending of transcripts with R package Guitar. And then, the density of m6A peaks on transcripts was calculated and plotted with the function GuitarPlot from R package Guitar. De novo and known motif finding were performed using the MEME suite and HOMER. RSEM was used to perform expression level estimation for all transcripts and genes from input libraries by calculating FPKM. Furthermore, DEGs were identified using the edgeR R package with thresholds of |log2FC|≥ 1 and p value < 0.05. GO and KEGG enrichment analyses of genes were performed using the ClusterProfiler R package. The data are presented as the mean ± SD from at least three repeated biological and technical experiments. The p value was calculated using two-tailed unpaired Student’s t test between two groups in R with a p value < 0.05 considered statistically significant.
Measurement of cocoon parameters
The cocoons were cut open carefully, and the pupae were separated before calculating the shell weight and cocoon thickness. The shell weight was measured in grams with an electronic digital balance. The thickness of each cocoon shell was measured in millimeters using a fabric thickness meter with a presser foot area of 50 mm2 and pressure of 0.2 ± 0.0005 kPa.
Sample preparation
The domesticated silkworm strain (Qiufeng × Baiyu) was reared in 25 °C incubator with fresh mulberry. For juvenile hormone (JH), juvenile hormone analog (JHA) (Yuanye Bio-Technology, China) was used instead of JH in larvae. Acetone was used for dissolving and applying JHA. The tissues of silkworm were collected and extracted for total RNA after JHA treating 24 h at L5D3.
Cell culture, treatments, and transfection
BmN cells were maintained in Sf-900 II SFM medium (Thermo Fisher Scientific, USA) with 3% fetal bovine serum (Gibco, USA). JHA and DMSO in final concentration 3 µM was used to treat BmN cells for 2 h, 4 h, 6 h, 8 h, 12 h, and 24 h. For overexpression of BmSPI4, BmSPI5I, BmN cells were transfected with plasmids by Lipo8000 (Beyotime, China) according to the manufacturers’ instructions. After transfected with pIZ/V5-BmSPI4-CDS-WT, pIZ/V5-BmSPI4-CDS-Mut, pIZ/V5-BmSPI5-CDS-WT or pIZ/V5-BmSPI5-CDS-Mut for 24 h, BmN cells were further treated with or without JHA (3 µM) for 12 h.
RNA extraction and qPCR
RNA isolation and reverse transcription-quantitative PCR were performed as described before [23]. Briefly, total RNA extracted from B. mori tissues and BmN cells using Trizol Plus (TaKaRa, Japan) were used for cDNA synthesis. All the primers used in qPCR assays were list in Table S4 and the amplification efficiencies of the qPCR primers were between 90 and 110% (R2 > 0.98). qPCR assays were performed using SYBR green detection method. Gene expression was analyzed using three biological replicates and three technical replicates and normalized to the control gene BmRpl49.
Western blotting analysis
Western blot analysis was conducted as described in our previous study [23]. Briefly, silkworm PSG and BmN cells were collected and washed with pre-cool phosphate-buffered saline (PBS) as well as mechanically homogenized in ice-cold RIPA (FDbio Science, China) lysate (RIPA: PMSF = 100: 1) for 30 min. The protein content of the supernatant was quantified using bicinchoninic acid (BCA) assay. Equal quality of protein (15 µg) was separated by SDS-PAGE, and then transferred into PVDF membranes (Millipore, USA). The membranes were blocked with no-fat milk and treated overnight with polyclonal rabbit/mouse anti-METTL3, anti-YTHDF3, anti-Tubulin, or anti-His. After incubated with a secondary antibody (DingGuo ChangSheng Biotechnology, China) for 2 h, the ECL Plus Kit (FDbio Science, China) were used to detected signal.
Construction of plasmids for overexpression of BmSPI4 and BmSPI5, and purification of recombinant YTHDF3 (1-152aa)
The ORFs encoding the BmSPI4 and BmSPI5 were cloned into pIZ/V5-His vector respectively. 1–456 bp of YTHDF3 CDS was cloned into pCold-His vector (TaKaRa, Japan). All the primers were listed in Table S4.
Protein purification of recombinant YTHDF3 (1-152aa) and preparation of antiserum
YTHDF3 CDS (1-456bp) was subcloned into Pcold-His vector (TaKaRa, Japan) with 6 × His-tag at the C-terminus, and then transfected to Escheerichia coli (BL21, Tsingke, China). E. coli was raised in Luria–Bertani medium at 37˚C for 6 h and 18˚C for 18 h. The E. coli was collected and dissolved with ice-cold lysis solution and sonication for 30 min. The supernatant was collected after centrifuged for 30 min at 13,000 rpm and 4 °C, and purified with Ni-nitrilotriacetic acid (NTA) column (GE Healthcare, USA) as described in our previous study[23]. Western blotting and CBB staining were used to identify the purity of recombinant YTHDF3 (1-152aa) (Fig. S6). Purified protein was used to generate rabbit polyclonal antibody according to previous study [44].
Detection of m6A abundance in total RNA
m6A modification of total RNA was determined in four duplicates using the EpiQuick RNA Methylation Quantification Kit. Two hundred nanograms of total RNA was utilized per assay well and incubated with binding solutions at 37 °C for 90 min. After washing three times, capture antibody was added to each assay well, incubated for 60 min at room temperature and washed. Then, detection antibody was added and incubated at room temperature for 30 min. After washing three times with washing buffer, the enhancer solution for 30 min and the determined solution for 10 min away from the light were used. The signal was detected at 530/590 nm after the addition of fluorescence developer using a MultiSkan FC microplate reader.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 1382 KB) All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Acknowledgements
We thank Yinghui Li and Jianguo Tao for directions and assistance of cocoon thickness and weight measurement. We also thank Shiyu Zou and Zhihua Hao for silkworm rearing and tissues dissection.
Author contributions
S.L., H.T., and H.W. conceived the study, and designed the experiments. S.L., H.T., and H.W. performed experiments and most data analyses. Y.X., and H.W. contributed reagents or analytical tools. S.L., and H.W. wrote the manuscript draft. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (Grant No. LR22C040001), the National Natural Science Foundation of China (Grant No. 319704603; 32170483), the Zhejiang Provincial Science and Technology Plans (2021C02072-6), and the Zhejiang Provincial Key Laboratory Construction Plans (2020E10025).
Availability of data and material
All data are available in the manuscript and in the Supplementary Information. m6A sequencing and transcriptome sequencing data of JHA treatment in silkworm PSG have been uploaded in the National Center for Biotechnology Information Sequence Read Archive with accession number: PRJNA948114. The RNA-seq of knocking down METTL3 in the embryonic of silkworm in our previous study were also uploaded with accession number: PRJNA882243. All the codes generated in the study have been uploaded in Github (https://github.com/Shuaiqi-Liu/JH-m6A).
Declarations
Conflict of interest
The authors declare no conflict of interest with the contents of this article.
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 2.Zhao X-M, Liu C, Jiang L-J, Li Q-Y, Zhou M-T, Cheng T-C, et al. A juvenile hormone transcription factor Bmdimm-fibroin H chain pathway is involved in the synthesis of silk protein in silkworm, Bombyx mori. J Biol Chem. 2015;290:972–986. doi: 10.1074/jbc.M114.606921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G, Lan H, Lu Q, He C, Wei Y, Mo D, et al. The JH-Met2-Kr-h1 pathway is involved in pyriproxyfen-induced defects of metamorphosis and silk protein synthesis in silkworms, Bombys mori. Pesticide Biochem Physiol. 2021;179:104980. doi: 10.1016/j.pestbp.2021.104980. [DOI] [PubMed] [Google Scholar]
- 4.Cao J, Zheng H, Zhang R, Xu Y, Pan H, Li S, et al. Dimmed gene knockout shortens larval growth and reduces silk yield in the silkworm, Bombys mori. Insect Mol Biol. 2023;32:26–35. doi: 10.1111/imb.12810. [DOI] [PubMed] [Google Scholar]
- 5.Wang J-L, Zhang Y-P, Gu Y-Y, Wang J-X, Zhao X-F. Function of a TGF-β inducible nuclear protein in the silk gland in Bombyx mori. Insect Mol Biol. 2009;18:243–251. doi: 10.1111/j.1365-2583.2008.00861.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Wang Y, Li Y, Ding C, Zhao P, Xia Q, et al. Cross-talk between juvenile hormone and ecdysone regulates transcription of fibroin modulator binding protein-1 in Bombyx mori. Int J Biol Macromol. 2019;128:28–39. doi: 10.1016/j.ijbiomac.2019.01.092. [DOI] [PubMed] [Google Scholar]
- 7.Brindha S, Maragathavalli S, Gangwar SK, Annadurai B. Effect of Juvenile hormone on enhance silk production in Bombyx mori. IJABR. 2012;2:396–402. [Google Scholar]
- 8.Bownes M, Dübendorfer A, Smith T. Ecdysteroids in adult males and females of Drosophila melanogaster. J Insect Physiol. 1984;30:823–830. doi: 10.1016/0022-1910(84)90019-2. [DOI] [Google Scholar]
- 9.Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. 2002;47:883–916. doi: 10.1146/annurev.ento.47.091201.145302. [DOI] [PubMed] [Google Scholar]
- 10.Kozlova T, Thummel CS. Steroid regulation of postembryonic development and reproduction in Drosophila. Trends Endocrinol Metab. 2000;11:276–280. doi: 10.1016/S1043-2760(00)00282-4. [DOI] [PubMed] [Google Scholar]
- 11.Truman JW, Riddiford LM. Endocrine insights into the evolution of metamorphosis in insects. Annu Rev Entomol. 2002;47:467–500. doi: 10.1146/annurev.ento.47.091201.145230. [DOI] [PubMed] [Google Scholar]
- 12.Minakuchi C, Zhou X, Riddiford LM. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech Dev. 2008;125:91–105. doi: 10.1016/j.mod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pray-Grant MG, Grant PA. Ant-icipating change: an epigenetic switch in reprogramming the social lives of ants. Mol Cell. 2020;77:205–206. doi: 10.1016/j.molcel.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Roy A, Palli SR. Epigenetic modifications acetylation and deacetylation play important roles in juvenile hormone action. BMC Genom. 2018 doi: 10.1186/s12864-018-5323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Roy A, Palli SR. CREB-binding protein plays key roles in juvenile hormone action in the red flour beetle, Triboloum Castaneum. Sci Rep. 2018;8:14–26. doi: 10.1038/s41598-018-19667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holoch D, Moazed D. RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet. 2015;16:71–84. doi: 10.1038/nrg3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandola U, Das R, Panda B. Role of the N6-methyladenosine RNA mark in gene regulation and its implications on development and disease. Brief Funct Genom. 2015;14:169–179. doi: 10.1093/bfgp/elu039. [DOI] [PubMed] [Google Scholar]
- 19.Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;5:482–519. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell. 2015;162:299–308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frye M, Harada BT, Behm M, He C. RNA modifications modulate gene expression during development. Science. 2018;361:1346–1349. doi: 10.1126/science.aau1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Hsu PJ, Chen Y-S, Yang Y-G. Dynamic transcriptomic m6A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–624. doi: 10.1038/s41422-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Jia S, Tian H, Li Y, Hu K, Tao J, et al. Evolution of m6A-related genes in insects and the function of METTL3 in silkworm embryonic development. Insect Mol Biol. 2023;32:316–327. doi: 10.1111/imb.12832. [DOI] [PubMed] [Google Scholar]
- 24.Kan L, Ott S, Joseph B, Park ES, Dai W, Kleiner RE, et al. A neural m6A/Ythdf pathway is required for learning and memory in Drosophila. Nat Commun. 2021;12:1458. doi: 10.1038/s41467-021-21537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haussmann IU, Bodi Z, Sanchez-Moran E, Mongan NP, Archer N, Fray RG, et al. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Wei X, Yang J, Du T, Yin C, Fu B, et al. Epitranscriptomic regulation of insecticide resistance. Sci Adv. 2021 doi: 10.1126/sciadv.abe5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Zhang Y, Dai K, Liang Z, Zhu M, Pan J, et al. N6-methyladenosine level in silkworm midgut/ovary cell line is associated with Bombyx mori nucleopolyhedrovirus infection. Front Microbiol. 2020;10:2988. doi: 10.3389/fmicb.2019.02988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B, Wang X, Li Z, Lu C, Zhang Q, Chang L, et al. Transcriptome-wide analysis of N6-methyladenosine uncovers its regulatory role in gene expression in the lepidopteran Bombyx mori. Insect Mol Biol. 2019;28:703–715. doi: 10.1111/imb.12584. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Xiao Y, Li Y, Wang X, Qi S, Wang Y, et al. RNA m6A modification functions in larval development and caste differentiation in honeybee (Apis mellifera) Cell Rep. 2021;34:108–580. doi: 10.1016/j.celrep.2020.108580. [DOI] [PubMed] [Google Scholar]
- 30.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:187–200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi R, Ma S, He T, Peng J, Zhang T, Chen X, et al. Deep insight into the transcriptome of the single silk gland of Bombyx mori. IJMS. 2019;20:2491. doi: 10.3390/ijms20102491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang Y, Han H, Xiong Q, Yang C, Wang L, Ma J, et al. METTL3-mediated m6A methylation regulates muscle stem cells and muscle regeneration by Notch signaling pathway. Stem Cells Int. 2021 doi: 10.1155/2021/9955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540:242–247. doi: 10.1038/nature20568. [DOI] [PubMed] [Google Scholar]
- 35.Han H, Yang C, Zhang S, Cheng M, Guo S, Zhu Y, et al. METTL3-mediated m6A mRNA modification promotes esophageal cancer initiation and progression via Notch signaling pathway. Mol Ther Nucleic Acids. 2021;26:33–46. doi: 10.1016/j.omtn.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang C, Zhang M, Ge S, Huang W, Lin X, Gao J, et al. Reduced m6A modification predicts malignant phenotypes and augmented Wnt/PI3K-Akt signaling in gastric cancer. Cancer Med. 2019;8:4766–4781. doi: 10.1002/cam4.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland TD, Young JH, Weisman S, Hayashi CY, Merritt DJ. Insect silk: one name, many materials. Annu Rev Entomol. 2010;55:171–188. doi: 10.1146/annurev-ento-112408-085401. [DOI] [PubMed] [Google Scholar]
- 38.Maekawa H, Suzuki Y. Repeated turn-off and turn-on of fibroin gene transcription during silk gland development of Bombyx mori. Dev Biol. 1980;78:394–406. doi: 10.1016/0012-1606(80)90343-7. [DOI] [PubMed] [Google Scholar]
- 39.Hirayama C, Nakamura M. Regulation of glutamine metabolism during the development of Bombyx mori larvae. Biochimica et Biophysica Acta (BBA) 2002;1571:131–137. doi: 10.1016/S0304-4165(02)00207-6. [DOI] [PubMed] [Google Scholar]
- 40.Daillie J. Juvenile hormone modifies larvae and silk gland development in Bombyx mori. Biochimie. 1979;61:275–281. doi: 10.1016/S0300-9084(79)80072-3. [DOI] [PubMed] [Google Scholar]
- 41.Miranda JE, de Bortoli SA, Takahashi R. Development and silk production by silkworm larvae after topical application of methoprene. Sci Agric (Piracicaba, Braz) 2002;59:585–588. doi: 10.1590/S0103-90162002000300026. [DOI] [Google Scholar]
- 42.Chaitanya RK, Sridevi P, Senthilkumaran B, Dutta GA. Effect of juvenile hormone analog, methoprene on H-fibroin regulation during the last instar larval development of Corcyra cephalonica. Gen Comp Endocrinol. 2013;181:10–17. doi: 10.1016/j.ygcen.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee B-S, Huang J-S, Jayathilaka LP, Lee J, Gupta S (2016) Antibody production with synthetic peptides. In: Schwartzbach SD, Skalli O, Schikorski T, (Eds). High-resolution imaging of cellular proteins. Springer, New York. pp. 25–47. 10.1007/978-1-4939-6352-2_2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (PDF 1382 KB) All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Data Availability Statement
All data are available in the manuscript and in the Supplementary Information. m6A sequencing and transcriptome sequencing data of JHA treatment in silkworm PSG have been uploaded in the National Center for Biotechnology Information Sequence Read Archive with accession number: PRJNA948114. The RNA-seq of knocking down METTL3 in the embryonic of silkworm in our previous study were also uploaded with accession number: PRJNA882243. All the codes generated in the study have been uploaded in Github (https://github.com/Shuaiqi-Liu/JH-m6A).