Abstract
Termites are a clade of eusocial wood-feeding roaches with > 3000 described species. Eusociality emerged ~ 150 million years ago in the ancestor of modern termites, which, since then, have acquired and sometimes lost a series of adaptive traits defining of their evolution. Termites primarily feed on wood, and digest cellulose in association with their obligatory nutritional mutualistic gut microbes. Recent advances in our understanding of termite phylogenetic relationships have served to provide a tentative timeline for the emergence of innovative traits and their consequences on the ecological success of termites. While all “lower” termites rely on cellulolytic protists to digest wood, “higher” termites (Termitidae), which comprise ~ 70% of termite species, do not rely on protists for digestion. The loss of protists in Termitidae was a critical evolutionary step that fostered the emergence of novel traits, resulting in a diversification of morphology, diets, and niches to an extent unattained by “lower” termites. However, the mechanisms that led to the initial loss of protists and the succession of events that took place in the termite gut remain speculative. In this review, we provide an overview of the key innovative traits acquired by termites during their evolution, which ultimately set the stage for the emergence of “higher” termites. We then discuss two hypotheses concerning the loss of protists in Termitidae, either through an externalization of the digestion or a dietary transition. Finally, we argue that many aspects of termite evolution remain speculative, as most termite biological diversity and evolutionary trajectories have yet to be explored.
Keywords: Sociality, Lower termites, Higher termites, Nutritional mutualism, Symbiosis, Protists, Fungi, Termitomyces, Bacteria
Introduction
The emergence of new symbiotic associations is a major source of novel evolutionary trajectories. In insect societies, such as those formed by termites, symbionts are vertically transmitted from parents to offspring and among nest mates during social interactions [1, 2]. Termites are a classic example of evolutionary innovation through the acquisition of obligate symbionts [3–6], and this symbiosis has been implicated as a key factor in the origin of termite eusociality [7, 8]. Although they are often compared to eusocial Hymenoptera owing to a convergence of their social traits [9], termites became eusocial independently, and through a different pathway than ants, bees, and wasps [8]. Termites form a clade of eusocial cockroaches (traditionally the order Isoptera, today a subgroup of Blattaria [10, 11]), and all termite species rely upon their nutritional symbionts to digest plant material [2, 12]. The most prominent gut symbiotic microbes are cellulolytic protists, present in all non-termitid termite families (i.e., “lower” termites: Fig. 1) as well as the extant sister group to termites, the wood roach Cryptocercus (Cryptocercidae). These symbionts allowed termites to become one of the few animal groups capable of digesting lignocellulose [13], resulting in termites attaining abundances and a global biomass comparable to ants [14, 15] and wielding a significant impact on ecosystem functioning, especially in the tropics [16].
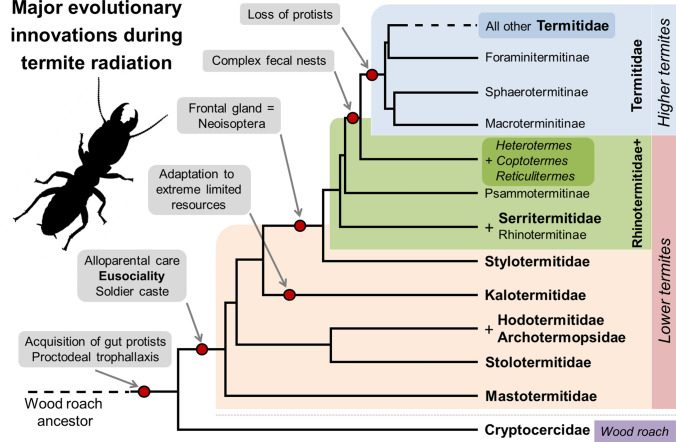
Fig. 1.
The appearance of certain innovative traits during the course of termite evolution. Phylogeny simplified from [28]
The cellulolytic protists found in the guts of all “lower” termites and Cryptocercus originate from two independent groups, Parabasalia and Oxymonadida (Preaxostyla) [17, 18]. Gut protists have evolved through varying degrees of horizontal transfer and episodes of co-speciation with their hosts [18–24]. In some species of Cryptocercus, there can be up to 25 species of protists. By contrast, many termite species host a limited number of protist species. This is especially true for more phylogenetically derived species of termites [23], such as certain Rhinotermitidae that are associated with a handful of protist species, down to a single species in Termitogeton [25]. This reduction in symbiont diversity portends the complete loss of protists in one termite lineage nested within the paraphyletic Rhinotermitidae—the Termitidae (i.e., “higher” termites: Fig. 1). The loss of protists in Termitidae was a major mutualistic shift that was compensated by the acquisition, or by the repurposing, of bacterial and fungal nutritional mutualists [26]. Paradoxically, while the acquisition of lignocellulolytic protists played a fundamental role in the emergence of termite eusociality and their remarkable evolutionary trajectories [8], the subsequent loss of protists in Termitidae and their alternative mutualisms allowed for an unprecedented diversification in diet and ecological success [27, 28]. Today, Termitidae are the most diverse termite lineage, making up more than 70% of all termite species [11], and representing one of the dominant groups of decomposers throughout the terrestrial tropics [14, 26].
Cellulolytic termite gut protists have been transmitted across generations since their acquisition in the common ancestor of termites and Cryptocercus, and Termitidae are the only lineage in which protists were lost [29]. Within this unique symbiotic context, we here review the main events of termite natural history. First, we highlight some of the key innovations that evolved in termites, from the advent of eusociality in their ancestors, to the diversification of diet and the rise to dominance of Termitidae. Second, we discuss the two principal evolutionary scenarios that have been proposed to explain the loss of protists among Termitidae. Finally, while our insight into many aspects of termite evolution has improved over the last two decades, we discuss some of the more relevant uncertainties that remain.
The main evolutionary transitions of termite evolution
“Among the living termites, Mastotermes darwiniensis Froggatt (Mastotermitidae) is universally admitted to be the most primitive morphologically. However, the descriptions of its nesting activities (Hill 1921, 1925) would seem to indicate an advance over the behavior of kalotermitids usually considered more advanced from a morphological standpoint. As Imms (1919) and Emerson (1926, p.92) have remarked, in certain morphological details Archotermopsis (Kalotermitidae) is more primitive than Mastotermes. One may either conclude that Mastotermes has undergone evolution toward more intricate behavior after its divergence from the ancestral isopteran stock, or else that degenerative evolution of the behavior patterns has occurred in the Termopsinae and other kalotermitids. With only meager evidence, I am inclined toward the former hypothesis”.
Alfred E. Emerson (1938) pertinently musing about the evolution of nesting behaviour in termites [30].
“The use of termites as models of the termite ancestor is untenable, because all termites, no matter how basal, are eusocial and thus defined and characterized by highly derived characters”.
Christine Nalepa (2011), noting the limitations of inferring the origin of complex termite societies using modern termite models [31].
Our understanding of termite evolution and diversification has improved owing to increasingly robust phylogenies [28, 32–36]. When used in combination with a solid theoretical framework [8, 11, 26, 34], modern phylogenetic estimates provide refined inferences of the timeline of key evolutionary transitions and their roles in termite success. Here, we present some of the key evolutionary innovations progressively acquired by termite ancestors prior to the emergence of Termitidae (Fig. 1). This timeline is largely based on several extant, species-poor, and early-diverging lineages of “lower” termites. These lineages are deemed “basal” and exhibit many traits considered ancestral; however, they have also evolved their own unique traits [31, 34, 35]. As a consequence, it can be challenging to distinguish between plesiomorphic (ancestral) and apomorphic (derived) traits, and there are still limitations to determine whether a trait evolved multiple times independently, or whether it evolved once and was then lost on repeated occasions [30, 37, 38]. These uncertainties can create confusion and be the subject of debate among proponents of contradicting hypotheses. One such debate is the evolution of workers, a permanently wingless caste incapable of developing into alate imagoes, which has important implications for models of termite social evolution. The true worker is the result of an irreversible deviation from the basic egg-to-imago developmental line, as opposed to a false worker (pseudergate sensu lato according to [39]), which is only a temporarily specialised aide retaining the capacity to develop wings and establish a new colony. The true worker caste is viewed by many as a derived trait, having evolved in several lineages independently after the origin of eusociality in termites [40–47], and by others as a plesiomorphic trait that evolved once in the ancestor of all modern termites and was lost independently in several lineages [37, 48–51]. Similar debates continue regarding other key innovations among termites, such as the role of trophallaxis and brood care, in the initial emergence of termite eusociality [26, 52–54]. A careful consideration of the biology and ecology of select taxa can provide valuable clues, and may allow for hypothesis-testing of the origin of certain innovations and their impact on termite diversification and ecological success [26, 55]. The following sections provide an overview of some major innovations in termites prior to the emergence of Termitidae.
Acquisition of gut cellulolytic protists and proctodeal trophallaxis
Termites and the wood-feeding roaches, Cryptocercus, inherited many gut bacteria and cellulolytic protists [56–58] from their common ancestor, indicating that symbiosis with intestinal microbes preceded the origin of eusociality in termites. The ancestor of termites and Cryptocercus was a gregarious wood-feeding roach [59, 60], living, confined and protected, within a single piece of decaying wood, presumably in contact with the soil. This ancestor progressively evolved from a primarily detritivorous diet to a xylophagous diet, supplemented by coprophagy [6, 26, 61]. The acquisition of a xylophagous diet was made possible by association with new gut symbionts, in particular with cellulolytic protozoa capable of decomposing lignocellulose, and with bacteria and archaea that provided nutritional and recycling functions [62–66].
The evolution of intricate mutualistic associations, such as that of termites and Cryptocercus with their beneficial gut microbes, is dependent upon an effective route for transmission across host generations [2]. In termites and Cryptocercus, transmission flows through proctodeal trophallaxis, during which a droplet of faecal food is provided to a nest mate together with the microbes it contains [8, 67]. Proctodeal trophallaxis presumably evolved from coprophagy [61, 68], which originally allowed the transfer of encysted protists surviving in faecal pellets [6]. The presumptive transition from coprophagy to proctodeal trophallaxis in the ancestor of Cryptocercus and termites was a critical behavioural shift that provided a dependable path for the transmission of gut microbes across generations of their hosts. This mechanism of gut symbiont acquisition is one potential factor that facilitated eusociality in the wood-roach ancestor of crown-group termites [8, 61].
Shift to alloparental care and the emergence of eusociality
Numerous factors have contributed to the evolution of eusociality in termites, and these have been the subject of various reviews (e.g., [8, 9, 43, 44, 46, 69]). We do not aim to provide an exhaustive summary and will focus on one catalyst of eusocial evolution in termites: symbiosis with gut microbes.
Parental care is widespread in insects, ranging from rudimentary behaviours to complex forms of brood care [70–72], and increases the survival rate of offspring and their chance of reaching adulthood. Cryptocercus and termites are altricial, as their offspring receive food and protists from their parents, and, in the case of termites, from their nest mates (older siblings) [7, 8, 56, 73]. Overlapping generations in the termite ancestor, possibly favoured by slow ontogenetic development, allowed older nutritionally-independent immatures to take over the food provisioning of younger individuals. The shift from biparental to alloparental brood care resulted in a reallocation of parental resources toward reproduction with a resulting increased brood size [8, 74]. The interdependency of individuals became fixed with the dependence of immatures on other nest mates for the reacquisition of intestinal symbionts lost during moulting. Therefore, in the “proto-termite”, a stable symbiosis with gut microbes necessitated obligate group-living conditions for all members of the family unit [6, 61].
Within the mutualistic constraints of protists, the switch to alloparental care precipitated the termite ancestor toward eusociality [8], as developmentally-arrested juvenile offspring, not engaged in reproduction, took over brood care duties [7]. This shift also resulted in a change from semelparity to iteroparity, with overlapping generations, as parents invested in multiple broods and increasing colony sizes. Ultimately, the “proto-termite” became fully eusocial with the emergence of the first sterile castes. The worker caste assumed various functions of extended care within the colony, while the soldier caste took on the role of defending colonies otherwise composed primarily of vulnerable individuals with juvenile morphologies [31, 39].
Lessons from termite fossils
Once eusociality was achieved in the ancestor of termites, it allowed for a series of novel innovations. Modern termites descend from a common ancestor that lived around the end of the Jurassic, some 150 million years ago [28, 34–36]. They are the earliest eusocial organisms known from the fossil record, with fossils extending back to the early stages of the Early Cretaceous, nearly 130 million years ago [35, 75]. Many of the first termite fossils maintained a relatively plesiomorphic morphology, retaining several cockroach-like features that have been lost by all, or most, modern termites [11, 75]. The faunas of termites from the Early Cretaceous through earliest Late Cretaceous reveal remarkable species diversity, with considerable morphological diversification [11, 34]. Most of these fossils belong to the “Meiatermes grade” and intercalate among modern termite families deemed “basal” (early diverging), but cannot be confidently placed within any of them, representing stem groups to either individual families or entire clades of families (e.g., some comprise the stem to the Icoisoptera and Neoisoptera, respectively) [11, 34]. Nonetheless, these species inform us greatly of the rich variety of early termite diversity, including the earliest examples of specialized workers and soldiers in the fossil record [35]. In fact, from the Early Cretaceous, we see evidence of a group that was perhaps already relict in its day, with Cratomastotermitidae representing the earliest-diverging termite group (diverging prior to all modern termites) and, therefore, coming closest to approximating that suite of features to be found in the ancestral termite [34].
The first fossils of extant termite families are more recent, with the exception of Mastotermitidae and Stolotermitidae whose fossil record dates back to the Early and Middle Cretaceous, respectively [11, 34, 76]. Although the lineages that gave rise to many of the more early-diverging extant families are ancient, their crown groups are comparatively young (e.g., Stolotermitidae, Archotermopsidae, Hodotermitidae, Kalotermitidae). Stem groups to these would have extended back into the Early Cretaceous, but fossil representatives of the crown groups are currently confined to the Eocene or younger. The reality of this pattern indicates that the reconstruction of the ancestral termite based on information from extant termite species is largely imperfect because of the paucity of species clearly allied to the earliest termite fossils. In addition, the early-diverging termite lineages intercalating among fossils of the Meiatermes grade have evolved some unique traits on their own, and lost other traits previously acquired by the termite ancestor [75]. Admittedly, all organisms, including those still living, are mosaics of plesiomorphic and apomorphic traits and it requires comparison across a diverse grade of species, ideally early-diverging species, to pull together the suite of plesiomorphies that may have characterized the taxon ancestral to them all. Disentangling plesiomorphic and apomorphic traits among basal termites has been the subject of intense debate and remains complicated, and perhaps impossible, based on observations of extant species alone [31]. Indeed, all modern termites are phylogenetically, temporally, and biologically divorced from the ancestral termite and the unique paleoecological and paleoclimatological factors that were integral to its appearance. Furthermore, no species today can approximate the varied paleobiotic influences from other Late Jurassic lineages whose unique species are similarly long extinct. We are left looking through a glass, darkly, with our clearest insights from those taxa (cratomastotermitids, basal Meiatermes grade members) closest to that auspicious first termite. In any case, the biology of early-branching termite lineages is indicative of the diversity of those evolutionary trajectories taken by the first termites.
Insights from the basal groups
The earliest-diverging lineage among modern termites is the once cosmopolitan family Mastotermitidae, which includes a single extant species, the Australian Mastotermes darwiniensis [77]. Mastotermes darwiniensis displays several roach-like features, such as eggs laid in an ootheca-like structure [78] or the association with the intracellular endosymbiont Blattabacterium [79]. It also displays a bifurcated developmental pathway with a true worker caste [80], a partially subterranean lifestyle [30, 81], and is the only termite species known to explore its environment individually or in tandems instead of following trails with a large number of foragers [82]. Whether some of these unique characteristics represent the condition of the last common ancestor of termites (basal traits), or whether some of these emerged independently within the mastotermitid lineage (derived traits), remains unclear [37], as both mutually exclusive scenarios are equally parsimonious [51].
Stolotermitidae, Archotermopsidae, and Hodotermitidae form a clade (Teletisoptera) that is the extant sister group to the remaining termites, with the exclusion of Mastotermitidae [28, 36]. Their earliest-known fossils are from ~ 99-million-year-old Burmese amber [76], and time-calibrated phylogenetic trees indicate that the lineage as a whole diverged from other termites during the Early Cretaceous, about 130 million years ago [28, 36]. It is likely that the individual crown groups of each family in this clade are young in comparison to their individual and collective stem groups. Because of the clade’s overall antiquity, they could provide clues on the biology of the first termites, although it is equally likely that their biology are uniquely derived for their clade and divergent from that of the ancestral termite. Extant Stolotermitidae include two genera and ten species, and extant Archotermopsidae include three genera and six species [11]. Both families are characterised by a linear development and are thence devoid of a true worker caste [83]. With few exceptions (e.g., Porotermes), these termites build relatively small colonies that generally include less than ten thousand individuals, and feed on decaying wood logs that serve as both shelter and food source [84, 85], although they have the ability to relocate their nests [86–89]. Several species of Stolotermitidae and Archotermopsidae have fertile soldiers [90, 91]. Hodotermitidae, the third family of this lineage, include three extant genera and 21 extant species, and are phylogenetically nested within Archotermopsidae [36]. Hodotermitidae differ remarkably from Archotermopsidae, both morphologically and ecologically, as they build large, fully subterranean colonies; have bifurcated developmental pathways with a sterile worker caste [92], with functional eyes; and feed on dry grass that they actively forage in arid environments [83]. The diversity of basal termite lineages (both extant and extinct) suggests a rapid ecological diversification following the acquisition of eusociality in the ancestor of termites [34]. The discovery of new stem-termite fossils could provide supplementary or alternative sources of information from which to infer the biology of the common ancestor of termites, and potentially help refine the timeline of emergence of novel traits in the main termite lineages.
Adaptation to extremely limited resources in Kalotermitidae
The Kalotermitidae are the second most diverse family of termites [11], and form the sister group of Neoisoptera (collectively known as Icoisoptera = Kalotermitidae + Neoisoptera), to which Termitidae belong [28, 34–36, 93]. Time-calibrated phylogenetic trees estimate that Kalotermitidae diverged from Neoisoptera ~ 125 million years ago [28, 34, 36], and their current earliest fossils belong to the genus Proelectrotermes, from ~ 99-million-year-old Burmese amber [35, 94]. All modern Kalotermitidae reveal a linear development and thus lack a true worker caste [83]. They live in rather small colonies, only rarely exceeding 1000 individuals at maturity [85]. The degree of moisture each species can tolerate varies greatly among genera [95], and some species, such as those in the genus Cryptotermes, evolved a complete intolerance to water and must infest wood pieces that are never exposed to liquid water [96]. Kalotermitidae are relatively weak competitors but strong dispersers, and have colonized even the most remote islands. This has likely been a diagnostic aspect of their biology for considerable time as kalotermitids are the only fossil termites to have occupied Zealandia during the Miocene [97]. Their ability to sustain colonies in harsh environments, with limited resources has permitted them to occupy the broadest geographical distribution of any termite family, as their nesting habits may have allowed for repeated transoceanic dispersal events. Kalotermitids can thrive in even small dead branches of trees, without any contact to the soil, allowing them to dominate tree canopies in the tropics [98].
The life history of many species of Kalotermitidae—producing small colonies in finite wood pieces on which they feed, and presumably unable to colonise new wood pieces—has been hypothesized by some as resembling that of the common ancestor of termites [99]. However, Kalotermitidae are well separated phylogenetically from the base of the termite tree, and no more-basal lineages are known to have similar biology. Thus, attributing this life history to the ancestor of Isoptera would necessitate, quite unparsimoniously, repeated parallel and entirely ad hoc losses of this biology in the plethora of living and fossil groups leading up to Kalotermitidae as well as in Neoisoptera. A more rational conclusion is that this is instead an apomorphic feature found within Kalotermitidae. Moreover, this lifestyle is not universal to Kalotermitidae and therefore may not be part of the kalotermitid groundplan. Some species of Kalotermitidae feed on large rotting logs on the ground and may form remarkably large colonies numbering well over 10,000 individuals, which is especially true for those living in areas where Termitidae are uncommon (tropical mountains of Southeast Asia or Queensland; Bourguignon and Šobotník, personal observations). A special case is the subterranean Paraneotermes simplicicornis in the deserts of the southern USA that actively dig galleries in the soil [100–102]. In the absence of “higher” termites, Kalotermitidae possess a life history similar to that of Archotermopsidae and Stolotermitidae [86–88], these features likely representing the ancestral condition for kalotermitids. If this is the case, then canopy-living kalotermitids derived from ground-living ancestors, supposedly pushed into marginal niches secondarily by more advanced competitors [30, 37]. Currently, the most complete phylogenetic estimate for Kalotermitidae is almost exclusively focused on the Australian lineages and is therefore far from a comprehensive representation of the family as a whole [103]. Nevertheless, this tree leaves no doubt concerning the derived position of Cryptotermes, the best example of kalotermitids that produce diminutive colonies in small, dry, dead branches. Future studies, providing a more comprehensive phylogeny for Kalotermitidae, are needed to determine patterns of trait evolution within the family.
Emergence of the frontal gland
The emergence of the frontal gland likely facilitated the ecological dominance of Neoisoptera, sister clade to Kalotermitidae (Fig. 1). The frontal gland is a unique character defining the Neoisoptera and is an unpaired saccular defensive gland with no equivalent among other insects [104]. Although the gland was long considered to be a soldier-specific organ, it also occurs in presoldiers, imagoes, and workers of some lineages [105–110]. The opening, the fontanelle, is positioned on the forehead, and the secretory cell-lined reservoir is often confined to the head but can reach deep into the abdomen in soldiers of certain species and imagoes of Rhinotermitinae [105, 107, 108, 111–113]. The frontal gland secretion has several functions, including the production of contact poisons, irritants or repellents, glues and incapacitating agents, anti-healing compounds, or alarm pheromones coordinating defensive activities [105, 107, 114]. The secretion, generally released following mandibular action in soldiers, is accumulated in copious amounts in many termite species, rendering them unpalatable to larger predators [105, 107, 115].
The evolution of the frontal gland allowed for the development of new defensive strategies. Termite groups basal to Neoisoptera primarily rely upon static warfare, i.e., robust and heavily sclerotized soldiers with toothed crushing mandibles defending key junctions of the gallery system. By contrast, Neoisoptera often rely on soldiers with smooth, sharp, and elongate slashing mandibles, that are mobile and actively search for enemies to combat [107, 111, 116]. Slashing mandibles overlap to a greater degree compared to crushing mandibles, and can therefore inflict more serious injury with smaller force [111]. This trend led to a decrease in the volume of mandibular muscles, freeing space in the head capsule for specialization of the frontal gland, potentially allowing for novel adaptive strategies against emerging predation pressures [111, 117–119].
Setting the plot for the rise of Termitidae: the ecological and developmental diversity of non-termitid Neoisoptera
Similar to Kalotermitidae, the earliest fossil of Neoisoptera, Archeorhinotermes rossi, was recovered from ~ 99-million-year-old Burmese amber [120]. Other fossils of Neoisoptera are from the Cenozoic and universally belong to extant termite families (see [11]). Neoisoptera are composed of four families—Stylotermitidae, Serritermitidae, Rhinotermitidae, and Termitidae—which exhibit considerable variation in life history. When compared with the remaining “lower” termites, the relationship between protozoan gut communities and their termite host seems to have been altered in some non-termitid Neoisoptera, with a notable reduction in protistan species diversity [25]. Stylotermitidae were the first to diverge, while Rhinotermitidae, as currently constituted, are paraphyletic to both Serritermitidae and Termitidae [28, 36, 121]. Relationships among the main lineages of Rhinotermitidae are not fully resolved, and vary among studies (e.g., compare [28, 36, 93]), confounding the reconstruction of life-history patterns among the constituent groups. However, the Termitidae are unambiguously recovered as sister to a clade composed of Reticulitermes, Coptotermes, and Heterotermes (which could be reconstituted as Heterotermitidae), allowing us to speculate on those attributes that perhaps preceded the termitid divergence (Fig. 1).
Non-termitid Neoisoptera have diverse ecological and developmental strategies. Stylotermitidae, represented solely by the extant genus Stylotermes, diverged from other Neoisoptera ~ 85 million years ago [28, 121]. Stylotermitidae live inside large trunks, usually associated with wounds or hollows, and feed at the margin of living tissues [121]. They make small colonies, composed of a few hundred individuals [122], and seem to have a linear developmental pathway with no worker caste [121], although this remains to be confirmed.
Serritermitidae and Rhinotermitidae are represented by two and 12 genera, respectively. Serritermitidae and the three rhinotermitid genera Prorhinotermes, Termitogeton, and Psammotermes have linear developmental pathways without worker castes and make relatively small colonies composed of a few thousand individuals [123–127], with the exception of Psammotermes that lives in large colonies comprising hundreds of thousands of individuals [85, 126]. Prorhinotermes has limited foraging abilities, but can move out of their log and colonise new wood pieces; [128], while among “lower” termites, Serritermes is the sole true inquiline (i.e., species living inside the nest of another termite species) [11, 127].
In sharp contrast, the nine other “rhinotermitid” genera possess a true worker caste [83], and make large colonies that can include more than several million individuals [85, 129]. Many have extensive foraging abilities and are categorised as separate-piece nesters (sensu Abe [129]) or multiple-piece nesters (sensu Shellman-Reeve [43]), for their ability to extend their colony across many wood items, without the construction of a central nest physically separated from the food source [43, 130]. Several genera also build centralised nests using faecal material, and display elaborate soil-excavation behaviours [30, 131, 132]. These traits are particularly well characterised in the sister group of Termitidae (Fig. 1), the clade comprising the three most economically important pest genera of “subterranean termites”—Coptotermes, Heterotermes, and Reticulitermes [11, 27, 36, 133–136]. These genera extensively reuse faecal wastes for construction, ranging from simple faecal lining alongside galleries, to complex sponge-like structures (= carton nest, Fig. 2) filling the voids created from feeding damage in large pieces of wood. These structures are also used to replace the original wood’s mechanical properties and increase the surface-to-volume ratio within voids [137].
Fig. 2.

Nest centre, including royal chamber, of the carton nest of Coptotermes gestroi, resulting from the reuse of faecal material, organic matter from the soil, and soil microbes (photo: T. Chouvenc)
The success of the clade comprising Coptotermes, Heterotermes, and Reticulitermes is possibly a direct result of their ability to sustain populous colonies that actively manipulate their surrounding environment and maintain suitable homeostatic conditions in the nest and foraging sites [129, 138, 139]. In addition, species of this clade often supplement their nitrogen-poor diet (wood) with organic-rich and microbial-rich matter acquired from the soil [140, 141], and which may have opened the door for the soil microbes to their faecal nest [141, 142]. These peculiar biological traits may provide some clues about the life history of the common ancestor of Termitidae prior to the loss of gut protists.
The rise of Termitidae and diet diversification
The loss of protozoa marks the transition to Termitidae
The loss of protists and their associated bacterial endosymbionts in Termitidae arguably marked the most important evolutionary innovation in termites since the emergence of eusociality (Fig. 3). The nutritional mutualists that replaced protistan functions allowed for the specialization of Termitidae into new niches, promoting their diversification and ecological dominance [26]. These nutritional mutualisms include novel symbiotic partners in combination with pre-existing intestinal bacterial and archaeal symbionts [5]. The emergence and rise of Termitidae were consequently marked by a series of specializations unique to the family, physiologically and ecologically separating them from “lower” termites, and beyond the critical mutualistic and dietary shifts discussed hereafter. Among such innovative traits, all Termitidae have inherited and maintained a true worker caste, rooted within a rigid bifurcated developmental scheme [83, 143]. Worker gut morphology and physiology departed from the ancestral conformation found among “lower” termites [144, 145]. Termitidae have also evolved a staggering diversity of morphologies and functions in soldiers, characterised by extreme modification of the mandibles and the frontal gland. Some species have soldiers with vestigial mandibles, fully dependent on chemical secretion for defence [107]. On the opposite end of the spectrum, soldiers with symmetric or asymmetric snapping mandibles have evolved independently several times within Termitidae [146, 147]. Finally, some lineages of Termitidae lost the soldier caste entirely and are exclusively defended by workers [148].
Fig. 3.
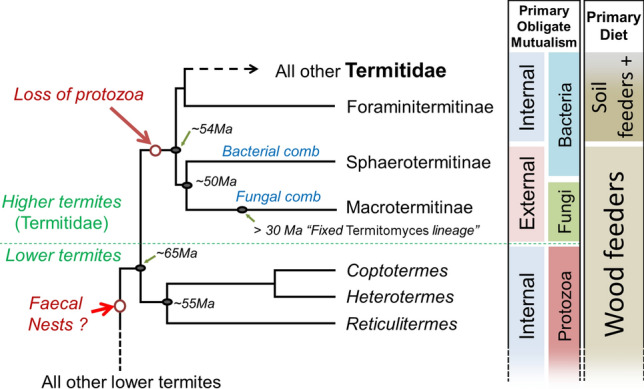
Simplified diversification of derived Neoisoptera from [28]. The loss of nutritional mutualistic protozoa marked the emergence of Termitidae. While all termites possess various degrees of mutualistic relationships with intestinal Bacteria and Archaea, this figure highlights the characteristic partners involved in their obligate mutualistic relationship for nutritional purposes, and their respective locations relative to the termite hindgut. Excluding the subfamilies Sphaerotermitinae and Macrotermitinae, most other Termitidae are soil feeders, with many instances of independent reversal to a wood-feeding diet
As Termitidae have a wide diversity of diets, morphologies, and behaviours, not seen in any “lower” termite, it is hypothesized that the initial loss of protozoa and the concomitant nutritional changes intrinsically played a critical role in permitting such a breadth of specializations to appear. However, the processes that allowed for the transition from a mutualism with protists toward alternative (and arguably more efficient) mutualistic associations in Termitidae remain speculative [4, 19, 28, 149]. The fundamental diets of termites are coarsely based on either wood (poorly decayed plant organic matter) or soil (highly decayed matter) [150]. All Termitidae descend from a wood-feeding ancestor, but up to 85% of termitid species have evolved to feed on soil in association with their symbiotic gut bacteria and archaea [58, 151]. Many species within some derived termitid clades reverted to the plesiomorphic condition of digesting wood, which they digest with the help of symbiotic intestinal bacteria [28, 152, 153]. In addition, two lineages of wood-feeding Termitidae, Macrotermitinae and Sphaerotermitinae, evolved an external pre-stomach [154], sometimes incorrectly referred as an “external rumen” [26, 149]. The external digestion of Macrotermitinae is composed of a fungal comb, while that of Sphaerotermitinae is a bacterial comb [155–157]. Both an early externalization of the digestion or an early switch to soil-feeding during termitid evolution are potential explanations for the loss of protists and the subsequent emergence of Termitidae (Fig. 3). The following two sections provides an overview of the biology and evolution of the two primary feeding strategies used in Termitidae.
The external digestion of Macrotermitinae–Sphaerotermitinae
Two sister lineages of extant Termitidae use an external digestion (Fig. 4): Sphaerotermitinae and Macrotermitinae [28]. The only described species of Sphaerotermitinae, Sphaerotermes sphaerothorax, builds bacterial combs with undetermined functions, but that presumably participate in their nutrition [155]. Macrotermitinae comprise 12 genera and 373 species and have an obligate mutualism with 34 known species of Termitomyces (Agaricales: Basidiomycota) fungi [158] that participate in the nutrition of their host, including the digestion of the lignocellulose matrix, provision of essential amino acids, and production of metabolic water [159–161]. Therefore, the nutritional mutualistic functions largely moved from the termite gut to the fungal comb in Macrotermitinae [19, 162]. A similar shift has been hypothesized for Sphaerotermitinae and their bacterial combs, but this remains to be confirmed. While Macrotermitinae and Sphaerotermitinae most likely inherited their externalized digestion from a common ancestor [28], it is unknown whether the initial nutritional mutualist was fungal or bacterial. However, it is noteworthy that the diversification of extant Termitomyces and macrotermitine lineages in Africa both started some 31–37 Ma [27, 163, 164], which roughly coincides with the Eocene–Oligocene extinction event at ~ 34 Ma [165]. This raises the possibility that either termite fungiculture could have already been established and that all Macrotermitinae are descendants of a single fungus-growing ancestral lineage that survived the extinction event, or that termite fungiculture emerged in context with the Eocene–Oligocene extinctions, which would imply that the ancestral nutritional comb could have been other than fungal.
Fig. 4.

Fungal comb of Macrotermes (photo: J. Šobotník)
External digestive systems have evolved independently multiple times in insects, and fungiculture specifically may have evolved several times in beetles, once in ants (Attini), and once in the termite subfamily Macrotermitinae [166–168]. The mechanisms of coevolution between Macrotermitinae and their Termitomyces symbiont has received extensive attention and revealed a dynamic association with frequent switches between partners [167, 169–172]. Species of Termitomyces are not specific to one termite host species, and host switches at lower taxonomic levels of their termite hosts are frequent, but exhibit a lineage-specific pattern of associations at higher taxonomic levels of their host termites [163, 167, 173–175]. These frequent switches are linked to the mode of transmission of Termitomyces. In most species of Macrotermitinae, the sexual spores of Termitomyces that serve as the inoculum of fungus gardens are acquired from the environment by the first foraging workers [169, 176], implying an open system in which various strains of fungi compete and are selected during termite colony foundation [177]. This horizontal mode of Termitomyces transmission, relying on the acquisition of spores from the surrounding soil, presumably represents the ancestral condition of fungus acquisition and transmission. Vertical transmission of Termitomyces is also known to have evolved secondarily in at least two independent lineages of Macrotermitinae, once in a single Macrotermes species, Macrotermes bellicosus, and once in Microtermes [178], allowing for oversea colonization of Madagascar from continental Africa in the latter [177].
All modern Macrotermitinae and Termitomyces have paired to establish an intricate mutualistic system, which has similarities with other “insect farmers” [179]. The ecological conditions that allowed for this symbiosis to emerge remain speculative. One hypothesis for the origin of fungal gardening in social insects is that the nest structure, built using colony wastes, provided a nutritional substrate for potential mutualists [166]. Sands [180] suggested that the mutualistic association between fungi and termites emerged as the ancestor of fungus-growing termites stored, or incorporated in their nest structure, unprocessed or partially digested wood material that inadvertently promoted the growth of saprophytic microorganisms. The opportunistic Agaricales fungi, which may have gradually invaded the termite nest structure [181, 182] may have then been progressively used and subsequently farmed by the ancestor of Macrotermitinae [162, 168, 171]. Following this scenario, the emerging nest structures fostered the colonization of the nest by opportunistic soil fungi that later became obligate nutritional symbionts (Leucocoprinus in attine ants, Termitomyces in Macrotermitinae) [162, 166]. A similar scenario can be posited for the origin of bacterial combs in Sphaerotermitinae.
Soil-feeding termite biology
While the “lower” termites primarily feed on wood, and occasionally on grass, most termitid species feed on dead plant matter at an advanced stage of decomposition, from rotten wood to bare soil [150, 152]. Species feeding on highly rotten wood that has become soil-like, or on soil below rotten logs, are referred to as wood/soil interface feeders or feeding-group III, while those feeding on the fully humified organic matter present in apparently mineral soil are referred to as true soil-feeders or feeding-group IV [152]. As these groups overlap broadly in the state of decomposition of their diet [150], we here use the term soil-feeder indistinctively for both feeding-group III and IV.
Soil-feeding has been adopted by about half of all known termite species, and by a majority of the species of Termitidae, in which about ~ 85% of the species are soil-feeders [11, 183]. All subfamilies of Termitidae include soil-feeding species, with the exception of the two subfamilies endowed with externalized digestive systems, Macrotermitinae and Sphaerotermitinae, which only include species relying on wood as their primary food source. The prevalence of soil-feeding among Termitidae is likely underestimated as soil-feeding lineages, such as Apicotermitinae, are particularly in need of taxonomic revision and are known to include many undescribed species [148, 183, 184]. This is best illustrated by faunistic surveys of African and South American tropical rainforests, which reveal that soil-feeding termites are diverse, and generally make up > 50%, and sometimes up to 80%, of the termite fauna (e.g., [148, 150, 185–187]. Soil-feeding termites are also extremely abundant in tropical rainforests, and their biomass often outweighs that of termites with different feeding habits [187–189]. The acquisition of a soil-based diet therefore contributed significantly to the ecological success of Termitidae.
Both wood-feeding and soil-feeding Termitidae are associated with stable communities of intestinal prokaryotes, the composition of which differs substantially among the two feeding types [58, 151, 190]. Soil-feeders have been reported to have an increased abundance of Ruminococcaceae and Lachnospiraceae (Firmicutes) compared to wood-feeders, which have increased abundance of Treponema (Spirochaetes) and Fibrobacteres often associated with wood-fiber particles [151, 190–193]. These patterns remain to be confirmed by studies with more comprehensive termite sampling. In both wood-feeders and soil-feeders, the gut bacterial communities actively participate in the nutrition of their termite host. Soil-feeders are able to digest cellulose [194, 195], despite the relative scarcity of cellulose in their diet. A large part of their diet appears to be the microbial biomass of the soil, and the nitrogen-rich organic residues associated with clay particles [195–198]. In consequence, the ammonia concentration in the intestines of soil-feeding termites reaches levels similar to that of carnivorous organisms [199], confirming that soil-feeding Termitidae feed on a nitrogen-rich diet, unlike wood-feeding Termitidae and all “lower” termites that feed on nitrogen-poor wood [200].
External digestion and soil-feeding as potential proximal causes for the loss of protozoa
The proximal causes for the loss of protozoa remain speculative. Both the external nutritional comb and soil-feeding habits evolved early in Termitidae (~ 65–54 Ma), either of which might have been the driving factor in the loss of mutualistic protozoa. The externalization of the digestion hypothesis has been most discussed [4, 19, 139, 149, 162, 180], although based on an erroneous placement of Macrotermitinae as sister to all other Termitidae [28, 36]. Such a phylogenetic placement is no longer supported as the most recent estimate of relationships, based on thousands of nuclear genes, recovered all termites with an external digestion (Macrotermitinae and Sphaerotermitinae) as reciprocally monophyletic sister groups and together sister to all remaining Termitidae, allowing for the possibility of an alternative narrative for the loss of protozoa [28].
In the case of the early externalization of the digestion, external symbionts may have acted as a new source of enzymes and nutrients, making symbiotic gut protozoa redundant and ultimately obsolete. Following this scenario, the loss of protozoa was achieved while conserving a primary wood diet, and soil-feeders appeared subsequently during a re-internalization of the digestion event. The alternative explanation for the loss of protozoa is an early shift from wood-feeding to soil-feeding in the ancestor of all modern Termitidae, depriving the cellulolytic gut protozoa from a cellulose-rich diet and starving them to extinction [28]. This shift might have been eased by the association with new gut microbes trophically acquired from the soil. In this scenario, the externalization of the digestion in the nutritional comb of Macroterminae and Sphaerotermitinae occurred secondarily, following the rapid return to a wood-feeding diet from a soil-feeding ancestor [28]. However, data regarding diet and nesting structures are almost exclusively available for extant termites only owing to the incomplete fossil record [34, 164], rendering it challenging to accurately reconstruct the pattern of events [201, 202]. Molecular phylogenetic trees fail to resolve the matter as the branching pattern among early termitid lineages leaves both scenarios equally probable. Therefore, the actual sequence of events that eventually led to the loss of gut protozoa and the emergence of Termitidae remains unclear. We outline the two possible scenarios here.
Loss of protozoa in Termitidae scenario A: the externalization of the digestion in a nutritional comb
In this scenario, the externalization of the primary functions of lignocellulose digestion outside the termite hindgut was the proximal cause for the loss of protists in a subterranean ancestor (Fig. 5). The common ancestor to the Heterotermes-Coptotermes-Reticulitermes lineage and Termitidae was a wood-feeder and most likely lived in populous colonies, with the ability to forage through a system of underground galleries connecting many wood items. We here argue that these three primary traits (wood-feeding, large colonies, soil foragers) were likely necessary requirements for the externalization of the digestion in the Macrotermitinae + Sphaerotermitinae ancestor, as the nutritional comb may have emerged only under a narrow range of conditions [168]. Given that termites evolved from a wood roach ancestor with a nitrogen-limited but carbon-rich diet, all “lower termites” retain hardwired nitrogen-conservation mechanisms [8, 203–205] and a remarkable absence of a carbon-conservation strategy [206]. Therefore, such a subterranean ancestor with large colonies and a relatively fast, wood-based metabolism, owing to easy access to resources through subterranean foraging (water, space, wood, organic-rich soil layers) resulted in the excretion and accumulation of excess carbon within their faecal matter.
Fig. 5.
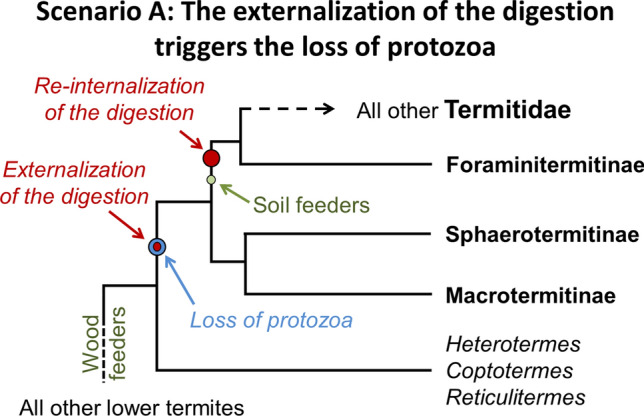
Scenario A: the externalization of the digestion hypothesis
Species that make large colonies in many of the derived Rhinotermitidae often reuse such faecal material as part of their nest structure, which is taken to an extreme in Coptotermes, with elaborate carton nests [19]. Such faecal nests could be interpreted as analogous to the excretion of excess carbon by aphids via honeydew from similar physiological constraints. Aphid honeydew is a potential nutritional resource for ants, which in return protect the aphids in a loose mutualistic relationship [207]. Similarly, in subterranean termites, the faecal nest serves as a nutritional niche for a wide range of opportunistic symbiotic soil microorganisms, primarily bacteria [208]. Although such microbes are unlikely to provide a nutritional benefit to the termites in Rhinotermitidae, microbial communities have attained secondary functions in providing homeostatic conditions within the termite nest, and levels of protection against the invasion of the colony by soil entomopathogens [139]. Therefore, carton nests, such as in Coptotermes, are essentially bacterial combs with no putative nutritional benefits for the termites.
We here suggest that such faecal nests and associated microbes eventually attained an alternative secondary function in the ancestor of Termitidae, with an exaptation involving external nutritional symbionts. The recurrent incorporation of soil microbes into the faecal nest [141] eventually included microbial communities with lignocellulolytic capabilities and other functions complementary to termite metabolism. One argument for such reuse of faecal material is that in Macrotermitinae the nutritional comb is the result of a primary passage of masticated wood through the gut as “faecal pellets” which are then inoculated with fungus [171, 172]. The required passage of the wood through the termite gut may, therefore, reflect the ancestral mechanism of how nutritional external symbionts initially took advantage of the feacal nest. An added argument is that carton nests in dying Coptotermes colonies or abandoned sections of the nest, can be invaded by a series of saprophytic microorganisms, including Basidiomycetes soil fungi, such as Leucocoprinus [209] (Fig. 6). Such observations demonstrate that opportunistic microorganisms may be suppressed from the carton by Coptotermes or its allied microorganisms, but it remains a niche for potential decomposers.
Fig. 6.

Carton material from a dead Coptotermes colony invaded by Leucocoprinus (as observed in [209])
The opportunistic saprophytes are indeed inhibited by termites and their associated microbes [139, 141, 209], however, the ancestor of Termitidae may have let certain saprophytes use parts of the fecal nest or abandoned foraging sites. Once termites started reusing such processed faecal nests, it would have allowed access to novel metabolites and enhanced wood-digestion processes. The new microbial association with such a termitid ancestor ultimately resulted in the protozoa being redundant, allowing for the potential of their loss. Once such a loss occurred it would have opened an ecological vacuum within termite guts, providing a newly available niche in the termite intestine for facultative gut-inhabiting bacteria [5]. In addition, once termites started feeding on such decayed materials, it might have easily opened the door for feeding on similar materials already present in the soil, making the nutritional comb dispensable. While the physiological constraints of the termite gut may have rendered the direct and immediate substitution of protists to bacteria within the hindgut unlikely, the process of first externalizing the digestion would have eased the reinternalization in the termitid ancestor where the nutritional comb was not yet engaged in a fully obligate relationship. Accordingly, in this scenario, three major consecutive events were necessary for the emergence of extant Termitidae: (1) the externalization of the digestion to a nutritional comb that led to the loss of protozoa, (2) a shift to a soil-like diet in one of the termitid lineages, which in turn led to (3) the reinternalization of the digestion in soil-feeders, with steps 2 and 3 potentially being interdependent.
Loss of protozoa in Termitidae scenario B: The transition to soil-feeders
As an alternative scenario, the loss of intestinal protists in Termitidae takes its origin in the early switch to a soil-feeding habit in the common ancestor of modern Termitidae (Fig. 7). Such a switch potentially triggered the extinction of gut protists because of their inability to feed on substrates other than lignocellulose [28]. Soil is impoverished in organic compounds that are efficiently decomposed, such as cellulose, and is enriched in recalcitrant materials, such as lignin, tannins, and other aromatic compounds, that aggregate with carbohydrates and proteins to form humic and fulvic acids [210, 211]. Studies on soil-feeding termites suggest that they feed on the microbial biomass present in the soil, and are able to mobilize the nitrogen-rich organic residues associated with clay particles [195–198]. Soil-feeding termites have also retained prokaryotic communities that encode for many glycoside hydrolase families, suggesting that they retained, to a certain extent, a carbohydrate metabolism [212], which may have allowed for subsequent reversals to wood-feeding habits. However, the high ammonia concentration in the intestines of soil-feeding termites indicates that their diet is nitrogen-rich [199], and therefore that the proportion of cellulose in their diet is relatively small, possibly to the extent that it led to the extinction of microbial clades unable to use feeding substrates other than cellulose, including protists. Alternatively, the gut microbial communities of wood-feeding termites may have experienced significant changes following the host switching to a diet based on soil, and the extinction of gut protists would have been triggered by drastic changes in gut physiochemical conditions, such as extreme alkalinity, with pH > 12 [213, 214], which might have taken place in a soil-feeding ancestor to mobilize recalcitrant humic compounds.
Fig. 7.
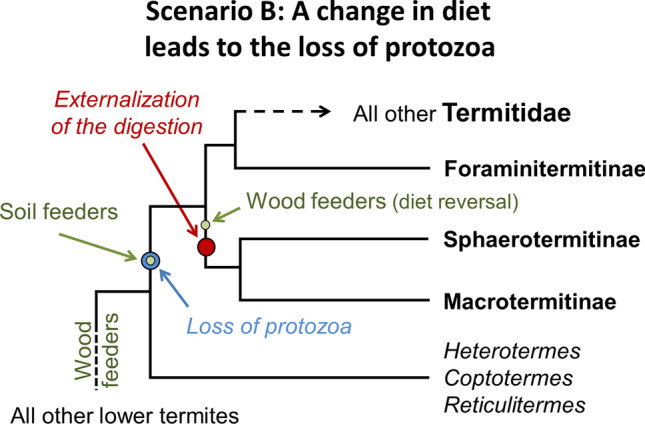
Scenario B: the soil-feeder (edaphophagy) hypothesis
The exploitation of a new dietary niche may have been the primary driver for protistan loss in the ancestor of Termitidae. The sister clade to Termitidae, comprised of Heterotermes, Coptotermes, and Reticulitermes, has the ability to forage in soil layers, with potential trophic interactions for nitrogen acquisition [129, 140, 141], suggesting that their common ancestor already possessed such behavioural and foraging traits. However, in the termitid lineage, the fundamental shift towards soil-feeding associated with a relatively nitrogen-rich diet, would have improved the acquisition of the building blocks necessary for colony growth, while maintaining the minimal cellulolytic functions required for the energy metabolism of the colony with diverse endogenous and exogenous cellulase sources [215]. In such an optimized diet, protists may not only have become obsolete in their functions to their host and been passively lost over time; they may have been actively suppressed by the inherent biochemical changes within the termite gut and/or starved to extinction. In addition, in a positive feedback, the loss of protists freed up space within termite guts and may have allowed for a reconfiguration of gut morphology [145], with new layers of competition and mutualism among new putative microbial partners, as a novel ecological niche to exploit.
This scenario, whereby the switch to soil-feeding triggered the loss of protists, therefore implies that the emergence of the external nutritional comb in Macrotermitinae and Sphaerotermitinae was secondary, and derived from a soil-feeding termitid ancestor. However, such digestion externalization could only have been possible in the presence of a nest structure nutritionally suitable for the growth of external symbionts, which may only have emerged from the excess of carbon-rich faeces of a wood feeder (as explained in scenario A). Therefore, the externalization of the digestion mandates that the soil-feeding ancestor of Macrotermitinae and Sphaerotermitinae likely reverted to a wood-feeding diet prior to the colonization of microorganisms of such faecal nests. Thus, in this scenario, three major consecutive events were necessary for the emergence of an external nutritional comb: (1) the switch to a soil diet and loss of protozoa, (2) a reversal to wood-feeding in the ancestral stem of the Macrotermitinae + Sphaerotermitinae lineage, and (3) the acquisition of external nutritional mutualists, with steps 2 and 3 potentially being interdependent. Alternatively, the ancestral termitid was only facultatively a soil-feeder, potentially sufficiently so as to lead to protistan loss (as outlined above), but with enough flexibility that termitids began to switch back to principle wood-feeding in the ancestor of Macrotermitinae + Sphaerotermitinae. This scenario might have been precipitated by environmental changes taking place after the Eocene–Oligocene transition, such as global cooling and aridification. Such a modification of the scenario outlined would have eased step 2.
Discussion
Termites: 150 Ma of evolutionary uncertainty since eusociality
Extant termites display a broad spectrum of traits that emerged from innovations, gains and losses of characters and functions, which propelled their evolutionary trajectories away from their ancient blattodean roots. Undoubtedly, the transition to eusociality in the termite ancestor allowed, and ultimately precipitated, the evolution of unique characteristics that fostered their ecological success. As a result, the initial conditions and traits that have led to the emergence of eusociality in termites ~ 150 Ma ago have received ample scrutiny, resulting in a plethora of hypotheses and tentative explanations over the decades and revealing routes to eusociality distinct from those proposed for social Hymenoptera [4, 8, 9, 30, 69, 90, 216, 217]. The evolutionary processes that led various traits to emerge after eusociality was attained often remain highly speculative owing to the many convergences and repeated losses during termite diversification, but these ultimately led to diverse physiological, ecological, and morphological adaptations and exaptations [30, 37, 51]. As highlighted in this review, many evolutionary scenarios pertaining to the emergence of traits and their timelines possess various degrees of uncertainty. However, we here support the hypothesis that two distinct mutualistic shifts had a critical role within the history of termite evolution: (1) the initial acquisition of intestinal protists within the context of alloparental care as one of the key events that enabled or facilitated the emergence of eusociality in termites, and (2) the much later loss of these protists, associated with the gain of alternative mutualists, that ultimately triggered the emergence of the most successful termite group, the Termitidae. Interestingly, there is a progressive loss of protozoan diversity in more derived “lower” termites [23, 25], which culminates in their complete loss in the “higher” termites. Regardless of the factors that led to this protistan disappearance from the guts, they presumably cascaded a series of changes that fundamentally altered the inherent physiology and ecological performance of Termitidae.
Toward the resolution of the loss-of-protists scenario
The recent clarification of the phylogenetic position of Sphaerotermes within Termitidae has revealed a need to reassess the putative events that led to the loss of protists [28]. While the general hypothesis that the initial externalization of the digestion resulted in the loss of protists remains plausible, an alternative explanation, namely a switch to soil-feeding as the proximal cause for protistan loss, is in fact equally parsimonious. In this review, we argue that these two scenarios are equally probable, and that it remains unclear which came first—the external nutritional comb or soil-feeding. There is also a certain possibility that both scenarios occurred independently on two distinct branches of the termitid tree, in which case protists were lost twice independently, once through the externalization of the digestion to a nutritional comb in the ancestor of Macrotermitinae and Sphaerotermitinae, and once in the ancestor of all other Termitidae through the acquisition of soil-feeding habits. Regardless of which scenario triggered the initial loss of protists, the origin of Termitidae took place ~ 65–54 Ma, following the end-Cretaceous mass extinction and leading into the Paleocene-Eocene Thermal Maximum, raising the possibility that changing global conditions and niche openings played a critical role in the initial dietary switch in the progenitors of the termitids. The discovery of new termite fossils spanning either side of the Mesozoic–Cenozoic boundary, as well as from the Paleocene and Early Eocene, may provide morphological clues from early termitids (such as mandibular structures associated with wood- versus soil-feeding), allowing inference of their diets. Alternatively, the potential discovery of trace fossils, particularly nest structures, from early termitids would provide evidence, or absence thereof, of a primitive nutritional comb [164].
Although insect paleontology is experiencing a revival, as illustrated by the many new fossils that have been described during the past decade, only one fossil, that of Nanotermes isaacae [218], provides relevant information on early termitid lineages. Nanotermes isaacae is known from ~ 50-million-year-old Cambay amber, and is upward of 20 million years older than all other known fossils of Termitidae, such as those found in Dominican amber [219], which are exclusively comprised of crown-Termitidae. The only available alate imago of N. isaacae was smaller than that of any known extant termites, and its actual affiliation with modern lineages of Termitidae is unclear. Because no other castes of N. isaacae are yet known, the shape of the alate imago mandible is the only character informative of the diet of this minute, early termitid species [220]. Unfortunately, the mandibles of the only known specimen of N. isaacae are not exposed, and the Cambay amber in which the fossil is preserved is inadequate for micro-CT scanning owing to minimal differential density between the matrix and comparatively soft-bodied arthropods, such as this specimen. It is possible that N. isaacae is representative of the termitid stem group, or could be a stem group to one of the constituent lineages within the family. While the diet of early termitid lineages cannot be inferred from currently available fossils, future discoveries of stem-Termitidae might help determine the feeding ecology of early termitid lineages.
Both a robust termite phylogeny and more fossil occurrences have the potential to provide important clues to resolve such questions. The phylogenetic relationships among the main termite lineages are now well-resolved, and often with high support, providing the opportunity to reconstruct the evolution of various traits (e.g., [221]), including diet. Because “lower” termites are all wood-feeders, there is no doubt that Termitidae descend from an initially wood-feeding ancestor. However, ancestral diet reconstructions indicate that soil-feeding habits evolved early in the evolution of Termitidae. This transition was either directly after termitids diverged from their sister lineage (Coptotermes + Heterotermes + Reticulitermes), in which case the switch to soil-feeding coincides with the loss of gut protists (unless there are as-of-yet undiscovered fossil taxa that intercalate between these two events), or in the common ancestor of the sister group to Macrotermitinae + Sphaerotermitinae, in which case the loss of gut protists has another cause, putatively an externalization of the digestion. The present molecular phylogenies are inconclusive and neither support nor reject any of the two scenarios, although they do support that the most recent common ancestor of the termitid sister group to Macrotermitinae + Sphaerotermitinae was a soil-feeder, and that wood-feeding habits were secondarily reacquired in some lineages in this clade [28]. Future phylogenetic works that resolve the position of Foraminitermitinae, or include new key taxa, such as the foraminitermitine Pseudomicrotermes alboniger, have the potential to shed brighter light on the precise timing of the initial acquisition of soil-feeding in Termitidae.
Epilogue: our comprehension of termite evolution remains fragmentary
In this review, we provide an overview of crucial steps of termite evolutionary scenarios and their consequences to the recent global fauna. From the initial emergence of eusociality to the remarkable diversification it generated, major strides in our understanding of termite evolution have been achieved within the last two decades. Nonetheless, these clarifications and new discoveries only scratch the surface of the complexity of an often-misunderstood group of eusocial roaches. All major representative groups of termites invariably possess species that display traits that differ from what often defines their genera, or even sometimes their own family, reflective of the mosaic nature of taxa. Arguably, termites often rule by the exception. Unfortunately, aside from a limited number of studied termite species (most of them owing to their pest status), the vast majority of termite diversity and their inherent biology remains to be investigated, revealing a large void of biological knowledge, ultimately limiting our ability to decipher and interpret such social complexity and its evolution. Accordingly, our extrapolations and explanations are assuredly over-simplifications for the time being, and this review highlights the still-limited state of our knowledge. Ultimately, there is a need for engaged research in this fascinating and important group of eusocial animals whose abundance is comparable to that of ants and humans [14, 26]. Regardless of shortfalls, recent breakthroughs in our understanding of phylogenetic relationships among most major termite groups and in their inherent nutritional requirements have opened new avenues of research, expanding possible evolutionary trajectories termites may have undertaken and that are in need of critical investigation.
Acknowledgements
TC thanks Nan-Yao Su, Paul Bardunias, Aaron Mullins for the many discussions over the years about the various aspects of evolutionary trajectories in termites. MSE is grateful to the late Kumar Krishna for his many stimulating discussions regarding termites and their evolution. All authors are thankful to all the participants of the ‘2019 termite course’ (Ft Lauderdale, FL), the event that nurtured this collaboration.
Author contributions
TC and TB jointly prepared the initial draft, and JŠ and MSE contributed additional information. All authors were actively involved with the development of the main narrative of this review.
Funding
This study was supported in part by, a grant from USDA National Institute of Food and Agriculture Hatch projects number FLA-FLT 005660 (TC), by a NSF-DEB grant, under the agreement no. 1754083 (TC), by the project IGA 20205014 realized at Faculty of Tropical AgriSciences, Czech University of Life Sciences Prague (JŠ and TB), and by the subsidiary funding to OIST (TB).
Compliance with ethical standards
Conflict of interest
The author(s) declare no competing interests
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas Chouvenc, Email: tomchouv@ufl.edu.
Thomas Bourguignon, Email: thomas.bourguignon@oist.jp.
References
- 1.Cleveland LR. Symbiosis among animals with special reference to termites and their intestinal flagellates. Q Rev Biol. 1926;1(1):51–60. doi: 10.1086/394236. [DOI] [Google Scholar]
- 2.Engel P, Moran NA. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol Rev. 2013;37(5):699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 3.Higashi M, Abe T. Global diversification of termites driven by the evolution of symbiosis and sociality. In: Abe T, Levin SA, Higashi M, editors. Biodiversity: an ecological perspective. New York: Springer; 1997. pp. 83–112. [Google Scholar]
- 4.Aanen DK, Eggleton P. Symbiogenesis: beyond the endosymbiosis theory? J Theor Biol. 2017;434:99–103. doi: 10.1016/j.jtbi.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Bourguignon T, Lo N, Dietrich C, Šobotník J, Sidek S, Roisin Y, Brune A, Evans TA. Rampant host switching shaped the termite gut microbiome. Curr Biol. 2018;28(4):649–654. doi: 10.1016/j.cub.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Nalepa CA. Origin of mutualism between termites and flagellated gut protists: transition from horizontal to vertical transmission. Front Ecol Evol. 2020;8:14. doi: 10.3389/fevo.2020.00014. [DOI] [Google Scholar]
- 7.Nalepa CA. Altricial development in wood-feeding cockroaches: the key antecedent of termite eusociality. In: Bignell DE, Roisin Y, Lo N, editors. Biology of termites: a modern synthesis. Dordrecht: Springer; 2010. pp. 69–95. [Google Scholar]
- 8.Nalepa CA. Origin of termite eusociality: trophallaxis integrates the social, nutritional, and microbial environments. Ecol Entomol. 2015;40(4):323–335. doi: 10.1111/een.12197. [DOI] [Google Scholar]
- 9.Howard KJ, Thorne BL. Eusocial evolution in termites and Hymenoptera. In: Bignell DE, Roisin Y, Lo N, editors. Biology of termites: a modern synthesis. Dordrecht: Springer; 2010. pp. 97–132. [Google Scholar]
- 10.Lo N, Engel MS, Cameron S, Nalepa CA, Tokuda G, Grimaldi D, Kitade O, Krishna K, Klass K-D, Maekawa K, Miura T, Thompson GJ, et al. Save Isoptera: a comment on Inward et al. Biol Lett. 2007;3(5):562–563. doi: 10.1098/rsbl.2007.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishna K, Grimaldi DA, Krishna V, Engel MS. Treatise on the Isoptera of the world. Bull Am Mus Nat Hist. 2013;377:1–2704. doi: 10.1206/377.1. [DOI] [Google Scholar]
- 12.Bignell DE. Introduction to symbiosis. In: Abe T, Bignell DE, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Dordrecht: Kluwer Academic Publishers; 2000. pp. 189–208. [Google Scholar]
- 13.Cragg SM, Beckham GT, Bruce NC, Bugg TDH, Distel DL, Dupree P, Etxabe AG, Goodell BS, Jellison J, McGeehan JE, McQueen-Mason SJ, Schnorr K, Walton PH, Watts JEM, Zimmer M. Lignocellulose degradation mechanisms across the tree of life. Curr Opin Chem Biol. 2015;29:108–119. doi: 10.1016/j.cbpa.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. Proc Natl Acad Sci USA. 2016;115(25):6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggleton P. The state of the world’s insects. Annu Rev Environ Res. 2020 doi: 10.1146/annurev-environ-012420-050035. [DOI] [Google Scholar]
- 16.Jouquet P, Bottinelli N, Shanbhag RR, Bourguignon T, Traoré S, Abbasi SA. Termites: the neglected soil engineers of tropical soils. Soil Sci. 2016;181(3–4):157–165. doi: 10.1097/SS.0000000000000119. [DOI] [Google Scholar]
- 17.Brugerolle G, Radek R. Symbiotic protozoa of termites. In: König H, Varma A, editors. Intestinal microorganisms of termites and other invertebrates. Cham: Springer; 2006. pp. 243–269. [Google Scholar]
- 18.Ohkuma M, Brune A. Diversity, structure, and evolution of the termite gut microbial community. In: Bignell DE, Roisin Y, Lo N, editors. Biology of termites: a modern synthesis. Dordrecht: Springer; 2010. pp. 413–438. [Google Scholar]
- 19.Eggleton P. The termite gut habitat: its evolution and co-evolution. In: König H, Varma A, editors. Intestinal microorganisms of termites and other invertebrates. Berlin: Springer; 2006. pp. 373–404. [Google Scholar]
- 20.Lo N, Eggleton P. Termite phylogenetics and co-cladogenesis with symbionts. In: Bignell DE, Roisin Y, Lo N, editors. Biology of termites: a modern synthesis. Dordrecht: Springer; 2010. pp. 27–50. [Google Scholar]
- 21.Tai V, James ER, Nalepa CA, Scheffrahn RH, Perlman SJ, Keeling PJ. The role of host phylogeny varies in shaping microbial diversity in the hindguts of lower termites. Appl Environ Microbiol. 2015;81(3):1059–1070. doi: 10.1128/AEM.02945-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taerum SJ, De Martini F, Liebig J, Gile GH. Incomplete co-cladogenesis between Zootermopsis termites and their associated protists. Environ Entomol. 2018;47(1):184–195. doi: 10.1093/ee/nvx193. [DOI] [PubMed] [Google Scholar]
- 23.Radek R, Meuser K, Strassert JFH, Arslana O, Teßmer A, Šobotník J, Sillam-Dussès D, Nink RA, Brune A. Exclusive gut flagellates of Serritermitidae suggest a major transfaunation Eevent in lower termites: description of Heliconympha glossotermitis gen. nov. spec. nov. J Eucaryot Microbiol. 2018;65(1):77–92. doi: 10.1111/jeu.12441. [DOI] [PubMed] [Google Scholar]
- 24.Mee ED, Gaylor MG, Jasso-Selles DE, Mizumoto N, Gile GH. Molecular phylogenetic position of Hoplonympha natator (Trichonymphea, Parabasalia): horizontal symbiont transfer or differential loss? J Eukaryotic Microbiol. 2020;67(2):268–272. doi: 10.1111/jeu.12765. [DOI] [PubMed] [Google Scholar]
- 25.Kitade O, Matsumoto T. Characteristics of the symbiotic flagellate composition within the termite family Rhinotermitidae (Isoptera) Symbiosis. 1998;25:271–278. [Google Scholar]
- 26.Bignell DE. The role of symbionts in the evolution of termites and their rise to ecological dominance in the tropics. In: Hurst CJ, editor. The mechanistic benefits of microbial symbionts. Cham: Springer; 2016. pp. 121–172. [Google Scholar]
- 27.Bourguignon T, Lo N, Šobotník J, Ho SYW, Iqbal N, Coissac E, Lee M, Jendryka M, Sillam-Dussès D, Křížková B, Roisin Y, Evans TA. Mitochondrial phylogenomics resolves the global spread of higher termites, ecosystem engineers of the tropics. Mol Biol Evol. 2017;34(3):589–597. doi: 10.1093/molbev/msw253. [DOI] [PubMed] [Google Scholar]
- 28.Buček A, Šobotník J, He S, Shi M, McMahon DP, Holmes EC, Roisin Y, Lo N, Bourguignon T. Evolution of termite symbiosis informed by transcriptome-based phylogenies. Curr Biol. 2019;29(21):3728–3734. doi: 10.1016/j.cub.2019.08.076. [DOI] [PubMed] [Google Scholar]
- 29.Brune A, Dietrich C. The gut microbiota of termites: digesting the diversity in the light of ecology and evolution. Annu Rev Microbiol. 2015;69:145–166. doi: 10.1146/annurev-micro-092412-155715. [DOI] [PubMed] [Google Scholar]
- 30.Emerson AE. Termite nests—a study of the phylogeny of behavior. Ecol Monogr. 1938;8(2):247–284. doi: 10.2307/1943251. [DOI] [Google Scholar]
- 31.Nalepa CA. Body size and termite evolution. Evol Biol. 2011;38(3):243–257. doi: 10.1007/s11692-011-9121-z. [DOI] [Google Scholar]
- 32.Lo N, Tokuda G, Watanabe H, Rose H, Slaytor M, Maekawa K, Bandi C, Noda H. Evidence from multiple gene sequences indicates that termites evolved from wood-feeding cockroaches. Curr Biol. 2000;10(13):801–804. doi: 10.1016/S0960-9822(00)00561-3. [DOI] [PubMed] [Google Scholar]
- 33.Inward D, Beccaloni G, Eggleton P. Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol lett. 2007;3(3):331–335. doi: 10.1098/rsbl.2007.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engel MS, Grimaldi DA, Krishna K. Termites (Isoptera): their phylogeny, classification, and rise to ecological dominance. Am Mus Novitates. 2009;3650:1–27. doi: 10.1206/651.1. [DOI] [Google Scholar]
- 35.Engel MS, Barden P, Riccio ML, Grimaldi DA. Morphologically specialized termite castes and advanced sociality in the Early Cretaceous. Curr Biol. 2016;26(4):522–530. doi: 10.1016/j.cub.2015.12.061. [DOI] [PubMed] [Google Scholar]
- 36.Bourguignon T, Lo N, Cameron SL, Šobotník J, Hayashi Y, Shigenobu S, Watanabe D, Roisin Y, Miura T, Evans TA. The evolutionary history of termites as inferred from 66 mitochondrial genomes. Mol Biol Evol. 2015;32(2):406–421. doi: 10.1093/molbev/msu308. [DOI] [PubMed] [Google Scholar]
- 37.Watson JAL, Sewell JJ. Caste development in Mastotermes and Kalotermes: which is primitive? In: Watson JAL, Okot-Kotber BM, Noirot C, editors. Current themes in tropical science, caste differentiation in social insects. Oxford: Pergamon Press; 1985. pp. 27–40. [Google Scholar]
- 38.Scheffrahn RH, Bourguignon T, Akama PD, Sillam-Dussès D, Šobotník J. Roisinitermes ebogoensis gen. & sp. n., an outstanding drywood termite with snapping soldiers from Cameroon (Isoptera, Kalotermitidae) ZooKeys. 2018;787:91–105. doi: 10.3897/zookeys.787.28195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roisin Y, Korb J. Social organization and the status of workers in termites. In: Bignell DE, Roisin Y, Lo N, editors. Biology of termites: a modern synthesis. Dordrecht: Springer; 2010. pp. 133–164. [Google Scholar]
- 40.Noirot C, Pasteels JM. Ontogenetic development and evolution of the worker caste in termites. Experientia. 1987;43(8):851–860. doi: 10.1007/BF01951642. [DOI] [Google Scholar]
- 41.Noirot C, Pasteels JM. The worker caste is polyphyletic in termites. Sociobiology. 1988;14(1):15–20. [Google Scholar]
- 42.Roisin Y. Intragroup conflicts and the evolution of sterile castes in termites. Am Nat. 1994;143(5):751–765. doi: 10.1086/285631. [DOI] [Google Scholar]
- 43.Shellman-Reeve JS. The spectrum of eusociality in termites. In: Choe JC, Crespi BJ, editors. The evolution of social behavior in insects and arachnids. Cambridge: Cambridge University Press; 1997. pp. 52–93. [Google Scholar]
- 44.Thorne BL. Evolution of eusociality in termites. Annu Rev Ecol Syst. 1997;28(1):27–54. doi: 10.1146/annurev.ecolsys.28.1.27. [DOI] [Google Scholar]
- 45.Thorne BL, Traniello JFA. Comparative social biology of basal taxa of ants and termites. Annu Rev Entomol. 2003;48(1):283–306. doi: 10.1146/annurev.ento.48.091801.112611. [DOI] [PubMed] [Google Scholar]
- 46.Korb J. The ecology of social evolution in termites. In: Korb J, Heinze J, editors. Ecology of social evolution. Berlin: Springer; 2008. pp. 151–174. [Google Scholar]
- 47.Legendre F, Whiting MF, Bordereau C, Cancello EM, Evans TA, Grandcolas P. The phylogeny of termites (Dictyoptera: Isoptera) based on mitochondrial and nuclear markers: implications for the evolution of the worker and pseudergate castes, and foraging behaviors. Mol Phylogenet Evol. 2008;48(2):615–627. doi: 10.1016/j.ympev.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 48.Watson JAL, Sewell JJ. The origin and evolution of caste systems in termites. Sociobiology. 1981;6(1):101–118. [Google Scholar]
- 49.Thompson GJ, Kitade O, Lo N, Crozier RH. Phylogenetic evidence for a single, ancestral origin of a ‘true’ worker caste in termites. J Evol Biol. 2000;13(6):869–881. doi: 10.1046/j.1420-9101.2000.00237.x. [DOI] [Google Scholar]
- 50.Thompson GJ, Kitade O, Lo N, Crozier RH. On the origin of termite workers: weighing up the phylogenetic evidence. J Evol Biol. 2004;17(1):217–220. doi: 10.1046/j.1420-9101.2003.00645.x. [DOI] [PubMed] [Google Scholar]
- 51.Bourguignon T, Chisholm RA, Evans TA. The termite worker phenotype evolved as a dispersal strategy for fertile wingless individuals before eusociality. Am Nat. 2016;187(3):372–387. doi: 10.1086/684838. [DOI] [PubMed] [Google Scholar]
- 52.Korb J, Buschmann M, Schafberg S, Liebig J, Bagnères A-G. Brood care and social evolution in termites. Proc R Soc B Lond. 2012;279(1738):2662–2671. doi: 10.1098/rspb.2011.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nalepa CA. ‘Cost’ of proctodeal trophallaxis in extant termite individuals has no relevance in analysing the origins of eusociality. Ecol Entomol. 2016;41(1):27–30. doi: 10.1111/een.12276. [DOI] [Google Scholar]
- 54.Roisin Y. What makes the cost of brood care important for the evolution of termite sociality? Its insignificance. Ecol Entomol. 2016;41(1):31–33. doi: 10.1111/een.12278. [DOI] [Google Scholar]
- 55.Bignell DE, Roisin Y, Lo N. Biology of termites: a modern synthesis. Dordrecht: Springer; 2010. [Google Scholar]
- 56.Cleveland LR, Hall SK, Sanders EP, Collier J. The wood-feeding roach Cryptocercus, its protozoa, and the symbiosis between protozoa and roach. Mem Am Acad Arts Sci. 1934;17(2):185–342. [Google Scholar]
- 57.Nalepa CA. Ancestral transfer of symbionts between cockroaches and termites: an unlikely scenario. Proc R Soc B Lond. 1991;246(1316):185–189. doi: 10.1098/rspb.1991.0143. [DOI] [PubMed] [Google Scholar]
- 58.Dietrich C, Köhler T, Brune A. The cockroach origin of the termite gut microbiota: patterns in bacterial community structure reflect major evolutionary events. App Environ Microbiol. 2014;80(7):2261–2269. doi: 10.1128/AEM.04206-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klass K-D, Nalepa C, Lo N. Wood-feeding cockroaches as models for termite evolution (Insecta: Dictyoptera): Cryptocercus vs Parasphaeria boleiriana. Mol Phylogenet Evol. 2008;46(3):809–817. doi: 10.1016/j.ympev.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 60.Ohkuma M, Noda S, Hongoh Y, Nalepa CA, Inoue T. Inheritance and diversification of symbiotic trichonymphid flagellates from a common ancestor of termites and the cockroach Cryptocercus. Proc R Soc B Lond. 2009;276(1655):239–245. doi: 10.1098/rspb.2008.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nalepa CA, Bignell DE, Bandi C. Detritivory, coprophagy, and the evolution of digestive mutualisms in Dictyoptera. Insect Soc. 2001;48(3):194–201. doi: 10.1007/PL00001767. [DOI] [Google Scholar]
- 62.Warnecke F, Luginbühl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, Cayouette M, McHardy AC, Djordjevic G, Aboushadi N, Sorek R, Tringe SG, Podar M, Garcia Martin H, Kunin V, Dalevi D, Madejska J, Kirton E, Platt D, Szeto E, Salamov A, Barry K, Mikhailova N, Kyrpides NC, Matson EG, Ottesen EA, Zhang X, Hernández M, Murillo C, Acosta LG, Rigoutsos I, Tamayo G, Green BD, Chang C, Rubin EM, Mathur EJ, Robertson DE, Hugenholtz P, Leadbetter JR. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450(169):560–565. doi: 10.1038/nature06269. [DOI] [PubMed] [Google Scholar]
- 63.Yamada A, Inoue T, Noda S, Hongoh Y, Ohkuma M. Evolutionary trend of phylogenetic diversity of nitrogen fixation genes in the gut community of wood-feeding termites. Mol Ecol. 2007;16(18):3768–3777. doi: 10.1111/j.1365-294X.2007.03326.x. [DOI] [PubMed] [Google Scholar]
- 64.Hongoh Y, Sharma VK, Prakash T, Noda S, Toh H, Taylor TD, Kudo T, Sakaki Y, Toyoda A, Hattori M, Ohkuma M. Genome of an endosymbiont coupling N2 fixation to cellulolysis within protist cells in termite gut. Science. 2008;322(5904):1108–1109. doi: 10.1126/science.1165578. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe H, Tokuda G. Cellulolytic systems in insects. Annu Rev Entomol. 2010;55:609–632. doi: 10.1146/annurev-ento-112408-085319. [DOI] [PubMed] [Google Scholar]
- 66.Ohkuma M, Noda S, Hattori S, Iida T, Yuki M, Starns D, Inoue J-I, Darby AC, Hongoh Y. Acetogenesis from H2 plus CO2 and nitrogen fixation by an endosymbiotic spirochete of a termite-gut cellulolytic protist. Proc Acad Natl Sci USA. 2015;112(33):10224–10230. doi: 10.1073/pnas.1423979112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nalepa CA. Colony composition, protozoan transfer and some life history characteristics of the woodroach Cryptocercus punctulatus Scudder (Dictyoptera: Cryptocercidae) Behav Ecol Sociobiol. 1984;14:273–279. doi: 10.1007/BF00299498. [DOI] [Google Scholar]
- 68.Nalepa CA. Nourishment and the origin of termite eusociality. In: Hunt JH, Nalepa CA, editors. Nourishment and evolution in insect societies. Boulder: Westview Press; 1994. pp. 57–104. [Google Scholar]
- 69.Korb J, Thorne B. Sociality in termites. In: Rubenstein D, Abbot P, editors. Comparative social evolution. Cambridge: Cambridge University Press; 2017. pp. 124–153. [Google Scholar]
- 70.Costa JT. The other insect societies. Cambridge: Harvard University Press; 2006. p. 767. [Google Scholar]
- 71.Trumbo ST. Patterns of parental care in invertebrates. In: Royle NJ, Smiseth PT, Kölliker M, editors. The evolution of parental care. Oxford: Oxford University Press; 2012. pp. 81–100. [Google Scholar]
- 72.Wong JWY, Meunier J, Kölliker M. The evolution of parental care in insects: the roles of ecology, life history and the social environment. Ecol Entomol. 2013;38(2):123–137. doi: 10.1111/een.12000. [DOI] [Google Scholar]
- 73.Nalepa CA, Maekawa K, Shimada K, Saito Y, Arellano C, Matsumoto T. Altricial development in subsocial wood-feeding cockroaches. Zool Sci. 2008;25(12):1190–1198. doi: 10.2108/zsj.25.1190. [DOI] [PubMed] [Google Scholar]
- 74.Chouvenc T, Su N-Y. Irreversible transfer of brood care duties and insights into the burden of caregiving in incipient subterranean termite colonies. Ecol Entomol. 2017;42(6):777–784. doi: 10.1111/een.12443. [DOI] [Google Scholar]
- 75.Barden P, Engel MS. Fossil social insects. In: Starr CK, editor. Encyclopedia of social insects. Cham: Springer International; 2020. pp. 1–21. [Google Scholar]
- 76.Zhao Z, Yin X, Shih C, Gao T, Ren D. Termite colonies from mid-Cretaceous Myanmar demonstrate their early eusocial lifestyle in damp wood. Natl Sci Rev. 2020;7(2):381–390. doi: 10.1093/nsr/nwz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barden P, Ware JL. Relevant relicts: the impact of fossil distributions on biogeographic reconstruction in ants and dragonflies. Insect Syst Div. 2017;1(1):73–80. doi: 10.1093/isd/ixx005. [DOI] [Google Scholar]
- 78.Nalepa CA, Lenz M. The ootheca of Mastotermes darwiniensis Froggatt (Isoptera: Mastotermitidae): homology with cockroach oothecae. Proc R Soc B Lond. 2000;267(1454):1809–1813. doi: 10.1098/rspb.2000.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sacchi L, Nalepa CA, Lenz M, Bandi C, Corona S, Grigolo A, Bigliardi E. Transovarial transmission of symbiotic bacteria in Mastotermes darwiniensis (Isoptera: Mastotermitidae): ultrastructural aspects and phylogenetic implications. Ann Entomol Soc Am. 2000;93(6):1308–1313. doi: 10.1603/0013-8746(2000)093[1308:TTOSBI]2.0.CO;2. [DOI] [Google Scholar]
- 80.Watson JAL, Metcalf EC, Sewell JJ. A re-examination of the development of castes in Mastotermes darwiniensis Froggatt (Isoptera) Aust J Zool. 1977;25(1):25–42. doi: 10.1071/ZO9770025. [DOI] [Google Scholar]
- 81.Hill GF. Notes on Mastotermes darwiniensis Froggatt (Isoptera) Proc R Soc Vic. 1925;37:119–124. [Google Scholar]
- 82.Sillam-Dussès D, Sémon E, Lacey MJ, Robert A, Lenz M, Bordereau C. Trail-following pheromones in basal termites, with special reference to Mastotermes darwiniensis. J Chem Ecol. 2007;33(10):1960–1977. doi: 10.1007/s10886-007-9363-5. [DOI] [PubMed] [Google Scholar]
- 83.Roisin Y. Diversity and evolution of caste patterns. In: Abe T, Bignell DE, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Dordrecht: Kluwer Academic Publishers; 2000. pp. 95–119. [Google Scholar]
- 84.Imms AD. On the structure and biology of Archotermopsis, together with descriptions of new species of intestinal Protozoa, and general observations on the Isoptera. Philos Trans R Soc Lond. 1919;209:75–180. [Google Scholar]
- 85.Lepage M, Darlington JPEC. Population dynamics of termites. In: Abe T, Bignell DE, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Dordrecht: Kluwer Academic Publishers; 2000. pp. 333–361. [Google Scholar]
- 86.Castle GB. The damp-wood termites of western United States, genus Zootermopsis (formerly Termopsis) In: Kofoid CA, editor. Termites and termite control. 2. Berkeley: University of California Press; 1934. pp. 273–310. [Google Scholar]
- 87.Morgan FD. The ecology and external morphology of Stolotermes ruficeps Brauer (Isoptera: Hodotermitidae) Trans R Soc N Zeal. 1959;86:155–195. [Google Scholar]
- 88.Nkunika POY. Field composition and size of the populations of the primitive damp wood termite, Porotermes adamsoni (Isoptera: Termopsidae) in South Australia. Sociobiology. 1990;16:251–258. [Google Scholar]
- 89.Bordereau C, Pasteels JM. Pheromones and chemical ecology of dispersal and foraging in termites. In: Bignell DE, Roisin Y, Lo N, editors. Biology of termites: a modern synthesis. Dordrecht: Springer; 2010. pp. 279–320. [Google Scholar]
- 90.Myles TG. Reproductive soldiers in the Termopsidae (Isoptera) Pan-Pac Entomol. 1986;62(4):293–299. [Google Scholar]
- 91.Thorne BL, Breisch NL, Muscedere ML. Evolution of eusociality and the soldier caste in termites: influence of intraspecific competition and accelerated inheritance. Proc Acad Natl Sci USA. 2003;100(22):12808–12813. doi: 10.1073/pnas.2133530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watson JAL. The worker caste of the hodotermitid harvester termites. Insect Soc. 1973;20(1):1–20. doi: 10.1007/BF02223558. [DOI] [Google Scholar]
- 93.Inward DJG, Vogler AP, Eggleton P. A comprehensive phylogenetic analysis of termites (Isoptera) illuminates key aspects of their evolutionary biology. Mol Phylogenet Evol. 2007;44(3):953–967. doi: 10.1016/j.ympev.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 94.Engel MS, Grimaldi DA, Krishna K. Primitive termites from the Early Cretaceous of Asia (Isoptera) Stuttgarter Beiträge zur Naturkunde, Serie B, Geologie und Paläontologie. 2007;371:1–32. [Google Scholar]
- 95.Scheffrahn RH. Expanded new world distributions of genera in the termite family Kalotermitidae. Sociobiology. 2019;66(1):136–153. doi: 10.13102/sociobiology.v66i1.3492. [DOI] [Google Scholar]
- 96.Scheffrahn RH, Křeček J, Ripa R, Luppichini P. Endemic origin and vast anthropogeneic dispersal of the West Indian drywood termite. Biol Invas. 2009;11(4):787–799. doi: 10.1007/s10530-008-9293-3. [DOI] [Google Scholar]
- 97.Engel MS, Kaulfuss U. Diverse, primitive termites (Isoptera: Kalotermitidae, incertae sedis) from the early Miocene of New Zealand. Aust Entomol. 2017;56(1):94–103. doi: 10.1111/aen.12216. [DOI] [Google Scholar]
- 98.Roisin Y, Dejean A, Corbora B, Orivel J, Samaniego M, Leponce M. Vertical stratification of the termite assemblage in a neotropical rainforest. Oecologia. 2006;149(2):301–311. doi: 10.1007/s00442-006-0449-5. [DOI] [PubMed] [Google Scholar]
- 99.Korb J. Workers of a drywood termite do not work. Front Zool. 2007;4:7. doi: 10.1186/1742-9994-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nutting WL. Colonizing flights and associated activities of termites. I. The desert damp-wood termite Paraneotermes simplicicornis (Kalotermitidae) Psyche. 1966;73(2):131–149. doi: 10.1155/1966/59080. [DOI] [Google Scholar]
- 101.Mizumoto N, Bourguignon T. Modern termites inherited the potential of collective construction from their common ancestor. Ecol Evol. 2020;10:6775–6784. doi: 10.1002/ece3.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mizumoto N, Bardunias PM, Pratt SC. Complex relationship between tunneling patterns and individual behaviors in termites. Am Nat. 2020;196(5):555–565. doi: 10.1086/711020. [DOI] [PubMed] [Google Scholar]
- 103.Thompson GJ, Miller LR, Lenz M, Crozier RH. Phylogenetic analysis and trait evolution in Australian lineages of drywood termites (Isoptera, Kalotermitidae) Mol Phylogenet Evol. 2000;17(3):419–429. doi: 10.1006/mpev.2000.0852. [DOI] [PubMed] [Google Scholar]
- 104.Noirot C. Glands and secretions. In: Krishna K, Weesner FM, editors. Biology of termites. New York: Academic Press; 1969. pp. 89–123. [Google Scholar]
- 105.Prestwich GD. Defense mechanisms of termites. Annu Rev Entomol. 1984;29(1):201–232. doi: 10.1146/annurev.en.29.010184.001221. [DOI] [Google Scholar]
- 106.Šobotník J, Weyda F, Hanus R, Kyjaková P, Doubský J. Ultrastructure of the frontal gland in Prorhinotermes simplex (Isoptera: Rhinotermitidae) and quantity of the defensive substance. Euro J Entomol. 2004;101(1):153–163. doi: 10.14411/eje.2004.020. [DOI] [Google Scholar]
- 107.Šobotník J, Jirošová A, Hanus R. Chemical warfare in termites. J Insect Physiol. 2010;56(9):1012–1021. doi: 10.1016/j.jinsphys.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 108.Šobotník J, Sillam-Dussès D, Weyda F, Dejean A, Roisin Y, Hanus R, Bourguignon T. The frontal gland in workers of Neotropical soldierless termites. Naturwissenschaften. 2010;97(5):95–503. doi: 10.1007/s00114-010-0664-0. [DOI] [PubMed] [Google Scholar]
- 109.Piskorski R, Hanus R, Kalinová B, Valterová I, Křeček J, Bourguignon T, Roisin Y, Šobotník J. Temporal and geographic variations in the morphology and chemical composition of the frontal gland in imagoes of Prorhinotermes species (Isoptera: Rhinotermitidae) Biol J Linnean Soc. 2009;98(2):384–392. doi: 10.1111/j.1095-8312.2009.01286.x. [DOI] [Google Scholar]
- 110.Kutalová K, Hanus R, Bourguignon T, Roisin Y, Šobotník J. Armed reproductives: evolution of the frontal gland in imagoes of Termitidae. Arthrop Struct Develop. 2013;42(4):339–348. doi: 10.1016/j.asd.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 111.Deligne J, Quennedey A, Blum MS. The enemies and defense mechanisms of termites. In: Hermann HR, editor. Social insects. New York: Academic Press; 1981. pp. 1–76. [Google Scholar]
- 112.Quennedey A. Morphology and ultrastructure of termite defense glands. In: Hermann HR, editor. Defensive mechanisms in social insects. New York: Praeger; 1984. pp. 151–200. [Google Scholar]
- 113.Šobotník J, Bourguignon T, Hanus R, Weyda F, Roisin Y. Structure and function of defensive glands in soldiers of Glossotermes oculatus (Isoptera: Serritermitidae) Biol J Linnean Soc. 2010;99(4):839–848. doi: 10.1111/j.1095-8312.2010.01392.x. [DOI] [Google Scholar]
- 114.Šobotník J, Hanus R, Kalinová B, Piskorski R, Cvačka J, Bourguignon T, Roisin Y. E, E)-α-farnesene, the alarm pheromone of Prorhinotermes canalifrons (Isoptera: Rhinotermitidae. J Chem Ecol. 2008;34(4):478–486. doi: 10.1007/s10886-008-9450-2. [DOI] [PubMed] [Google Scholar]
- 115.Waller DA, La Fage JP. Unpalatability as a defense of Coptotermes formosanus Shiraki soldiers against ant predation. J Appl Entomol. 1987;103(15):148–153. doi: 10.1111/j.1439-0418.1987.tb00973.x. [DOI] [Google Scholar]
- 116.Haverty MI. The proportion of soldiers in termite colonies: a list and a bibliography (Isoptera) Sociobiology. 1977;2(3):199–216. [Google Scholar]
- 117.Labandeira CC, Johnson KR, Wilf P. Impact of the terminal Cretaceous event on plant-insect associations. Proc Natl Acad Sci USA. 2002;99(4):2061–2066. doi: 10.1073/pnas.042492999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barden P. Fossil ants (Hymenoptera: Formicidae): ancient diversity and the rise of modern lineages. Myrmecol News. 2017;14:1–30. doi: 10.25849/myrmecol.news_024:001. [DOI] [Google Scholar]
- 119.Tuma J, Eggleton P, Fayle TM. Ant-termite interactions: an important but under-explored ecological linkage. Biol Rev. 2020;95(3):555–572. doi: 10.1111/brv.12577. [DOI] [PubMed] [Google Scholar]
- 120.Krishna K, Grimaldi DA. The first Cretaceous Rhinotermitidae (Isoptera): a new species, genus, and subfamily in Burmese amber. Am Mus Novitates. 2003;3390:1–10. doi: 10.1206/0003-0082(2003)390<0001:TFCRIA>2.0.CO;2. [DOI] [Google Scholar]
- 121.Wu L-W, Bourguignon T, Šobotník J, Wen P, Liang W-R, Li H-F. Phylogenetic position of the enigmatic termite family Stylotermitidae. Invertebr Syst. 2018;32(5):1111–1117. doi: 10.1071/IS17093. [DOI] [Google Scholar]
- 122.Tsai P-H, Ping C-K, Li G-X. Four new species of the genus Stylotermes Holmgren, K. et N. (Isoptera: Rhinotermitidae, Stylotermitinae) from Kwangsi. Acta Entomol Sinica. 1978;21:429–436. [Google Scholar]
- 123.Roisin Y. Morphology, development and evolutionary significance of the working stages in the caste system of Prorhinotermes (Insecta, Isoptera) Zoomorphol. 1988;107(6):339–347. doi: 10.1007/BF00312217. [DOI] [Google Scholar]
- 124.Parmentier D, Roisin Y. Caste morphology and development in Termitogeton nr. planus (Insecta, Isoptera, Rhinotermitidae) J Morphol. 2003;255(1):69–79. doi: 10.1002/jmor.10047. [DOI] [PubMed] [Google Scholar]
- 125.Bourguignon T, Šobotník J, Hanus R, Roisin Y. Developmental pathways of Glossotermes oculatus (Isoptera, Serritermitidae): at the cross-roads of worker caste evolution in termites. Evol Dev. 2009;11(6):659–668. doi: 10.1111/j.1525-142X.2009.00373.x. [DOI] [PubMed] [Google Scholar]
- 126.Bourguignon T, Šobotník J, Sillam-Dussès D, Jiroš P, Hanus R, Roisin Y, Miura T. Developmental pathways of Psammotermes hybostoma (Isoptera: Rhinotermitidae): old pseudergates make up a new sterile caste. PLoS ONE. 2012;7:e44527. doi: 10.1371/journal.pone.0044527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barbosa JRC, Constantino R. Polymorphism in the neotropical termite Serritermes serrifer. Entomol Exp Appl. 2017;163(1):43–50. doi: 10.1111/eea.12532. [DOI] [Google Scholar]
- 128.Rupf T, Roisin Y. Coming out of the woods: do termites need a specialized worker caste to search for new food sources? Naturwissenschaften. 2008;95(9):811–819. doi: 10.1007/s00114-008-0387-7. [DOI] [PubMed] [Google Scholar]
- 129.Su N-Y, Scheffrahn RH. Foraging population and territory of the Formosan subterranean termite (Isoptera: Rhinotermitidae) in an urban environment. Sociobiology. 1988;14(2):353–359. [Google Scholar]
- 130.Abe T. Evolution of life types in termites. In: Kawano S, Connell JH, Hidaka T, editors. Evolution and coadaptation in biotic communities. Tokyo: University of Tokyo Press; 1987. pp. 125–148. [Google Scholar]
- 131.Li H-F, Su N-Y. Sand displacement during tunnel excavation by the Formosan subterranean termite (Isoptera: Rhinotermitidae) Ann Entomol Soc Am. 2008;101(2):456–462. doi: 10.1603/0013-8746(2008)101[456:SDDTEB]2.0.CO;2. [DOI] [Google Scholar]
- 132.Bardunias P, Su N-Y. Opposing headings of excavating and depositing termites facilitate branch formation in the Formosan subterranean termite. Anim Behav. 2009;78(3):755–759. doi: 10.1016/j.anbehav.2009.06.024. [DOI] [Google Scholar]
- 133.Rust MK, Su N-Y. Managing social insects of urban importance. Annu Rev Entomol. 2012;57:355–375. doi: 10.1146/annurev-ento-120710-100634. [DOI] [PubMed] [Google Scholar]
- 134.Evans TA, Forschler BT, Grace JK. Biology of invasive termites: a worldwide review. Annu Rev Entomol. 2013;58:455–474. doi: 10.1146/annurev-ento-120811-153554. [DOI] [PubMed] [Google Scholar]
- 135.Bourguignon T, Lo N, Šobotník J, Sillam-Dussès D, Roisin Y, Evans TA. Oceanic dispersal, vicariance, and human introduction shaped the modern distribution of the termites Reticulitermes, Heterotermes and Coptotermes. Proc R Soc B Lond. 2016;283(1827):1827–1835. doi: 10.1098/rspb.2016.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chouvenc T, Li H-F, Austin J, Bordereau C, Bourguignon T, Cameron S, Cancello E, Constantino R, Costa-Leonardo A-M, Eggleton P, Evans T, Forschler B, Grace JK, Husseneder C, Křeček J, Lee C-Y, Lee T, Lo N, Messenger M, Mullins A, Robert A, Roisin Y, Scheffrahn RH, Sillam-Dussès D, Šobotník J, Szalanski A, Takematsu Y, Vargo EL, Yamada A, Yoshimura T, Su N-Y. Revisiting Coptotermes (Isoptera: Rhinotermitidae): a global taxonomic roadmap for species validity and distribution of the economically important subterranean termite genus. Syst Entomol. 2016;41(2):299–306. doi: 10.1111/syen.12157. [DOI] [Google Scholar]
- 137.Oberst S, Lai JCS, Evans TA. Termites utilise clay to build structural supports and so increase foraging resources. Scie Rep. 2016;6(1):20990. doi: 10.1038/srep20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wood TG. Termites and the soil environment. Biol Fertile Soils. 1988;6(3):228–236. doi: 10.1007/BF00260819. [DOI] [Google Scholar]
- 139.Chouvenc T, Efstathion CA, Elliott ML, Su N-Y. Extended disease resistance emerging from the faecal nest of a subterranean termite. Proc R Soc B Lond. 2013;280(1770):20131885. doi: 10.1098/rspb.2013.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mullins A, Su N-Y. Parental nitrogen transfer and apparent absence of N2 fixation during colony foundation in Coptotermes formosanus Shiraki. Insects. 2018;9(2):37. doi: 10.3390/insects9020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chouvenc T, Elliott ML, Šobotník J, Efstathion CA, Su N-Y. The termite fecal nest: a framework for the opportunistic acquisition of beneficial soil Streptomyces (Actinomycetales: Streptomycetaceae) Environ Entomol. 2018;47(6):1431–1439. doi: 10.1093/ee/nvy152. [DOI] [PubMed] [Google Scholar]
- 142.Arango RA, Carlson CM, Currie CR, McDonald BR, Book AJ, Green F, III, Lebow NK, Raffa KF. Antimicrobial activity of actinobacteria isolated from the guts of subterranean termites. Environ Entomol. 2016;45(6):1415–1423. doi: 10.1093/ee/nvw126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Legendre F, Condamine FL. When Darwin’s special difficulty promotes diversification in insects. Syst Biol. 2018;67(5):873–887. doi: 10.1093/sysbio/syy014. [DOI] [PubMed] [Google Scholar]
- 144.Noirot C. The gut of termites (Isoptera). Comparative anatomy, systematics, phylogeny. I. Lower termites. Ann Soc Entomol France. 1995;31(3):197–226. [Google Scholar]
- 145.Noirot C. The gut of termites (Isoptera). Comparative anatomy, systematics, phylogeny. II. Higher termites (Termitidae) Ann Soc Entomol France. 2001;37(4):431–471. [Google Scholar]
- 146.Scholtz OI, MacLeod N, Eggleton P. Termite soldier defence strategies: a reassessment of Prestwich’s classification and an examination of the evolution of defence morphology using extended eigenshape analyses of head morphology. Zool J Linn Soc Lond. 2008;153(4):631–650. doi: 10.1111/j.1096-3642.2008.00396.x. [DOI] [Google Scholar]
- 147.Kuan K-C, Chiu C-I, Shih M-C, Chi K-J, Li H-F. Termite’s twisted mandible presents fast, powerful, and precise strikes. Sci Rep. 2020;10(9462):1–12. doi: 10.1038/s41598-020-66294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bourguignon T, Šobotník J, Dahlsjö C, Roisin Y. The soldierless Apicotermitinae: insights into a poorly known and ecologically dominant tropical taxon. Insect Soc. 2016;63(1):39–50. doi: 10.1007/s00040-015-0446-y. [DOI] [Google Scholar]
- 149.Bignell DE. Termites as soil engineers and soil processors. In: König H, Varma A, editors. Intestinal microorganisms of termites and other invertebrates. Cham: Springer; 2006. pp. 183–220. [Google Scholar]
- 150.Bourguignon T, Šobotník J, Lepoint G, Martin J-M, Hardy OJ, Dejean A, Roisin Y. Feeding ecology and phylogenetic structure of a complex neotropical termite assemblage, revealed by nitrogen stable isotope ratios. Ecol Entomol. 2011;36(2):261–269. doi: 10.1111/j.1365-2311.2011.01265.x. [DOI] [Google Scholar]
- 151.Mikaelyan A, Dietrich C, Köhler T, Poulsen M, Sillam-Dussès D, Brune A. Diet is the primary determinant of bacterial community structure in the guts of higher termites. Mol Ecol. 2015;24(20):5284–5285. doi: 10.1111/mec.13376. [DOI] [PubMed] [Google Scholar]
- 152.Donovan SE, Eggleton P, Bignell DE. Gut content analysis and a new feeding group classification of termites. Ecol Entomol. 2001;26(4):356–366. doi: 10.1046/j.1365-2311.2001.00342.x. [DOI] [Google Scholar]
- 153.Tokuda G, Watanabe H. Hidden cellulases in termites: revision of an old hypothesis. Biol Lett. 2007;3(3):336–339. doi: 10.1098/rsbl.2007.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chiu C-I (2020) Termite fungal cultivation as a ruminant-like digestive system? In: Proceedings of the 13th conference of the Pacific Rim termite research group, 12 Feb 2020
- 155.Garnier-Sillam E, Toutain F, Villemin G, Renoux J. Études préliminaires des meules originales du termite xylophage Sphaerotermes sphaerothorax (Sjöstedt) Insect Soc. 1989;36:293–312. doi: 10.1007/BF02224882. [DOI] [Google Scholar]
- 156.Rouland-Lefèvre C, Bignell DE. Cultivation of symbiotic fungi by termites of the subfamily Macrotermitinae. In: Seckbach J, editor. Symbiosis, mechanisms and model systems. Cham: Springer; 2001. pp. 731–756. [Google Scholar]
- 157.Hyodo F, Tayasu I, Inoue T, Azuma J-I, Kudo T. Differential role of symbiotic fungi in lignin degradation and food provision for fungus-growing termites (Macrotermitinae: Isoptera) Funct Ecol. 2003;17(2):186–193. doi: 10.1046/j.1365-2435.2003.00718.x. [DOI] [Google Scholar]
- 158.Mossebo DC, Essouman EPF, Machouart MC, Gueidan C. Phylogenetic relationships, taxonomic revision and new taxa of Termitomyces (Lyophyllaceae, Basidiomycota) inferred from combined nLSU- and mtSSU-rDNA sequences. Phytotaxa. 2017;321(1):71–102. doi: 10.11646/phytotaxa.321.1.3. [DOI] [Google Scholar]
- 159.Grassé P-P. Termitologia. Anatomie–physiologie–biologie–systématique des termites, fondation des societes, construction. Paris: Masson; 1984. [Google Scholar]
- 160.Rouland-Lefèvre C. Symbiosis with fungi. In: Abe T, Bignell DE, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Dordrecht: Kluwer Academic Publishers; 2000. pp. 289–306. [Google Scholar]
- 161.Chiu C-I, Ou J-H, Chen C-Y, Li H-F. Fungal nutrition allocation enhances mutualism with fungus-growing termite. Fungal Ecol. 2019;41:92–100. doi: 10.1016/j.funeco.2019.04.001. [DOI] [Google Scholar]
- 162.Aanen DK, Boomsma JJ. Evolutionary dynamics of the mutualistic symbiosis between fungus-growing termites and Termitomyces fungi. In: Vega FE, Blackwell M, editors. Insect-fungal associations: ecology and evolution. Oxford: Oxford University Press; 2005. pp. 191–210. [Google Scholar]
- 163.Nobre T, Koné NA, Konaté S, Linsenmair KE, Aanen DK. Dating the fungus-growing termites’ mutualism shows a mixture between ancient codiversification and recent symbiont dispersal across divergent hosts. Mol Ecol. 2011;20(12):2619–2627. doi: 10.1111/j.1365-294X.2011.05090.x. [DOI] [PubMed] [Google Scholar]
- 164.Roberts EM, Todd CN, Aanen DK, Nobre T, Hilbert-Wolf HL, O’Connor PM, Tapanila L, Mtelela C, Stevens NJ. Oligocene termite nests with in situ fungus gardens from the Rukwa Rift basin, Tanzania, support a Paleogene African origin for insect agriculture. PLoS ONE. 2016;11(6):e0156847. doi: 10.1371/journal.pone.0156847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ivany LC, Patterson WP, Lohmann KC. Cooler winters as a possible cause of mass extinctions at the Eocene/Oligocene boundary. Nature. 2000;407(6806):887–890. doi: 10.1038/35038044. [DOI] [PubMed] [Google Scholar]
- 166.Mueller UG, Schultz TR, Currie CR, Adams RMM, Malloch D. The origin of the attine ant-fungus mutualism. Q Rev Biol. 2001;76(2):169–197. doi: 10.1086/393867. [DOI] [PubMed] [Google Scholar]
- 167.Aanen DK, Eggleton P, Rouland-Lefèvre C, Guldberg-Frøslev T, Rosendahl S, Boomsma JJ. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci USA. 2002;99(23):14887–14892. doi: 10.1073/pnas.222313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. The evolution of agriculture in insects. Annu Rev Ecol Evol Syst. 2005;36:563–595. doi: 10.1146/annurev.ecolsys.36.102003.152626. [DOI] [Google Scholar]
- 169.Korb J, Aanen DK. The evolution of uniparental transmission of fungal symbionts in fungus-growing termites (Macrotermitinae) Behav Ecol Sociobiol. 2003;53(2):65–71. doi: 10.1007/s00265-002-0559-y. [DOI] [Google Scholar]
- 170.Aanen DK. As you reap, so shall you sow: coupling of harvesting and inoculating stabilizes the mutualism between termites and fungi. Biol Lett. 2006;2(2):209–212. doi: 10.1098/rsbl.2005.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nobre T, Aanen DK. Fungiculture or termite husbandry? The ruminant hypothesis. Insects. 2012;3(1):307–323. doi: 10.3390/insects3010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Poulsen M. Towards an integrated understanding of the consequences of fungus domestication on the fungus-growing termite gut microbiota. Environ Microbiol. 2015;17(8):2562–2572. doi: 10.1111/1462-2920.12765. [DOI] [PubMed] [Google Scholar]
- 173.Nobre T, Fernandes C, Boomsma JJ, Korb J, Aanen DK. Farming termites determine the genetic population structure of Termitomyces fungal symbionts. Mol Ecol. 2011;20(9):2023–2033. doi: 10.1111/j.1365-294X.2011.05064.x. [DOI] [PubMed] [Google Scholar]
- 174.da Costa RR, Vreeburg SME, Shik JZ, Aanen DK, Poulsen M. Can interaction specificity in the fungus-farming termite symbiosis be explained by nutritional requirements of the fungal crop? Fungal Ecol. 2019;38:54–61. doi: 10.1016/j.funeco.2018.08.009. [DOI] [Google Scholar]
- 175.van de Peppel LJJ, Aanen DK. High diversity and low host-specificity of Termitomyces symbionts cultivated by Microtermes spp. indicate frequent symbiont exchange. Fungal Ecol. 2020;45:100917. doi: 10.1016/j.funeco.2020.100917. [DOI] [Google Scholar]
- 176.Aanen DK, Ros VID, de Fine Licht HH, Mitchell J, de Beer ZW, Slippers B, Rouland-LeFèvre C, Boomsma JJ. Patterns of interaction specificity of fungus-growing termites and Termitomyces symbionts in South Africa. BMC Evol Biol. 2007;7(115):1–11. doi: 10.1186/1471-2148-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nobre T, Eggleton P, Aanen DK. Vertical transmission as the key to the colonization of Madagascar by fungus-growing termites? Proc R Soc B Lond. 2010;277(1680):359–365. doi: 10.1098/rspb.2009.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aanen DK, de Fine Licht HH, Debets AJM, Kerstes NAG, Hoekstra RF, Boomsma JJ. High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science. 2009;326(5956):1103–1106. doi: 10.1126/science.1173462. [DOI] [PubMed] [Google Scholar]
- 179.Mueller UG, Gerardo N. Fungus farming insects: multiple origins and diverse evolutionary histories. Proc Natl Acad Sci USA. 2002;99(24):15247–15249. doi: 10.1073/pnas.242594799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sands WA. The association of termites and fungi. In: Krishna K, Weesner FM, editors. Biology of termites. New York: Academic Press; 1969. pp. 495–524. [Google Scholar]
- 181.Wood TG, Thomas RJ. The mutualistic association between Macrotermitinae and Termitomyces. In: Wilding N, Collins NM, Hammond PM, Webber JF, editors. Insect-fungus interactions: 14th symposium of the Royal Entomological Society of London in collaboration with the British Mycological Socety. New York: Academic Press; 1989. pp. 69–92. [Google Scholar]
- 182.Darlington JPEC. Nutrition and evolution in fungus-growing termites. In: Hunt JH, Nalepa CA, editors. Nourishment and evolution in insect societies. Boulder: Westview Press; 1994. pp. 105–130. [Google Scholar]
- 183.Brauman A, Bignell DE, Tayasu I. Soil-feeding termites: biology, microbial associations and digestive mechanisms. In: Abe T, Bignell DE, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Dordrecht: Kluwer Academic Publishers; 2000. pp. 233–259. [Google Scholar]
- 184.Eggleton P. Termite species description rates and the state of termite taxonomy. Insect Soc. 1999;46(1):1–5. doi: 10.1007/s000400050105. [DOI] [Google Scholar]
- 185.Davies RG, Eggleton P, Jones DT, Gathorne-Hardy FJ, Hernández LM. Evolution of termite functional diversity: analysis and synthesis of local ecological and regional influences on local species richness. J Biogeogr. 2003;30(6):847–877. doi: 10.1046/j.1365-2699.2003.00883.x. [DOI] [Google Scholar]
- 186.Ackerman IL, Constantino R, Gauch HG, Lehmann J, Riha SJ, Fernandes ECM. Termite (Insecta: Isoptera) species composition in a primary rain forest and agroforests in Central Amazonia. Biotropica. 2009;41(2):226–233. doi: 10.1111/j.1744-7429.2008.00479.x. [DOI] [Google Scholar]
- 187.Dahlsjö CAL, Parr CL, Malhi Y, Rahman H, Meir P, Jones DT, Eggleton P. First comparison of quantitative estimates of termite biomass and abundance reveals strong intercontinental differences. J Trop Ecol. 2014;30(2):143–152. doi: 10.1017/S0266467413000898. [DOI] [Google Scholar]
- 188.Abe T, Masumoto T. Studies on the distribution and ecological role of termites in a lowland rain forest of West Malaysia. Food and feeding habits of termites in Pasoh Forest Reserve. Jpn J Ecol. 1979;29(4):121–135. doi: 10.18960/seitai.29.4_337. [DOI] [Google Scholar]
- 189.Eggleton P, Bignell DE, Sands WA, Mawdsley NA, Lawton JH, Wood TG, Bignell NC. The diversity, abundance, and biomass of termites under differing levels of disturbance in the Mbalmayo Forest Reserve, southern Cameroon. Philos Trans R Soc Lond B. 1996;351(1335):51–68. doi: 10.1098/rstb.1996.0004. [DOI] [Google Scholar]
- 190.Mikaelyan A, Meuser K, Brune A. Microenvironmental heterogeneity of gut compartments drives bacterial community structure in wood-and humus-feeding higher termites. FEMS Microbiol Ecol. 2017;93(1):fiw10. doi: 10.1093/femsec/fiw210. [DOI] [PubMed] [Google Scholar]
- 191.Mikaelyan A, Strassert JFH, Tokuda G, Brune A. The fibre-associated cellulolytic bacterial community in the hindgut of wood-feeding higher termites (Nasutitermes spp.) Environ Microbiol. 2014;16(9):2711–2722. doi: 10.1111/1462-2920.12425. [DOI] [Google Scholar]
- 192.Tokuda G, Mikaelyan A, Fukui C, Matsuura Y, Watanabe H, Fujishima M, Brune A. Fiber-associated spirochetes are major agents of hemicellulose degradation in the hindgut of wood-feeding higher termites. Proc Natl Acad Sci. 2018;115(51):E11996–E12004. doi: 10.1073/pnas.1810550115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hongoh Y, Deevong P, Inoue T, Moriya S, Trakulnaleamsai S, Ohkuma M, Vongkaluang C, Noparatnaraporn N, Kudol T. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl Environ Microbiol. 2005;71(11):6590–6599. doi: 10.1128/AEM.71.11.6590-6599.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ji R, Kappler A, Brune A. Transformation and mineralization of synthetic 14C-labeled humic model compounds by soil-feeding termites. Soil Biol Biochem. 2000;32(8–9):1281–1291. doi: 10.1016/S0038-0717(00)00046-8. [DOI] [Google Scholar]
- 195.Ji R, Brune A. Transformation and mineralization of 14C-labeled cellulose, peptidoglycan, and protein by the soil-feeding termite Cubitermes orthognathus. Biol Fertil Soils. 2001;33(2):166–174. doi: 10.1007/s003740000310. [DOI] [Google Scholar]
- 196.Ji R, Brune A. Digestion of peptidic residues in humic substances by an alkali-stable and humic-acid-tolerant proteolytic activity in the gut of soil-feeding termites. Soil Biol Biochem. 2005;37(9):1648–1655. doi: 10.1016/j.soilbio.2005.01.026. [DOI] [Google Scholar]
- 197.Ngugi DK, Ji R, Brune A. Nitrogen mineralization, denitrification, and nitrate ammonification by soil-feeding termites: a 15N-based approach. Biogeochemistry. 2011;103(1–3):355–369. doi: 10.1007/s10533-010-9478-6. [DOI] [Google Scholar]
- 198.Ngugi DK, Brune A. Nitrate reduction, nitrous oxide formation, and anaerobic ammonia oxidation to nitrite in the gut of soil-feeding termites (Cubitermes and Ophiotermes spp.) Environ Microbiol. 2012;14(4):860–871. doi: 10.1111/j.1462-2920.2011.02648.x. [DOI] [PubMed] [Google Scholar]
- 199.Ji R, Brune A. Nitrogen mineralization, ammonia accumulation, and emission of gaseous NH3 by soil-feeding termites. Biogeochemistry. 2006;78(3):267–283. doi: 10.1007/s10533-00. [DOI] [Google Scholar]
- 200.Breznak JA. Ecology of prokaryotic microbes in the guts of wood- and litter-feeding termites. In: Abe T, Bignell DE, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Dordrecht: Kluwer Academic Publishers; 2000. pp. 209–231. [Google Scholar]
- 201.Baerends GP. Comparative methods and the concept of homology in the study of behaviour. Arch Neerl Zool. 1958;13(Suppl 1):401–417. [Google Scholar]
- 202.Wenzel JW. Behavioral homology and phylogeny. Annu Rev Ecol Syst. 1992;23(1):361–381. doi: 10.1146/annurev.es.23.110192.002045. [DOI] [Google Scholar]
- 203.Potrikus CJ, Breznak JA. Gut bacteria recycle uric acid nitrogen in termites: a strategy for nutrient conservation. Proc Natl Acad Sci USA. 1981;78(7):4601–4605. doi: 10.1073/pnas.78.7.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Higashi M, Abe T, Burns TP. Carbon—nitrogen balance and termite ecology. Proc R Soc B Lond. 1992;249(1326):303–308. doi: 10.1098/rspb.1992.0119. [DOI] [Google Scholar]
- 205.Machida M, Kitade O, Miura T, Matsumoto T. Nitrogen recycling through proctodeal trophallaxis in the Japanese damp-wood termite Hodotermopsis japonica (Isoptera, Termopsidae) Insect Soc. 2001;48(1):52–56. doi: 10.1007/PL00001745. [DOI] [Google Scholar]
- 206.Chouvenc T. Limited survival strategy in starving subterranean termite colonies. Insect Soc. 2020;67(1):71–82. doi: 10.1007/s00040-019-00729-5. [DOI] [Google Scholar]
- 207.Stadler B, Dixon AFG. Ant attendance in aphids: why different degrees of myrmecophily? Ecol Entomol. 1999;24(3):363–369. doi: 10.1046/j.1365-2311.1999.00195.x. [DOI] [Google Scholar]
- 208.Chouvenc T, Elliott ML, Su N-Y. Rich microbial community associated with the nest material of Reticulitermes flavipes (Isoptera: Rhinotermitidae) Florida Entomol. 2011;94(1):115–116. doi: 10.1653/024.094.0117. [DOI] [Google Scholar]
- 209.Chouvenc T, Bardunias P, Efstathion CA, Chakrabarti S, Elliott ML, Giblin-Davis R, Su N-Y. Resource opportunities from the nest of dying subterranean termite (Isoptera: Rhinotermitidae) colonies: a laboratory case of ecological succession. Ann Entomol Soc Am. 2013;106(6):771–778. doi: 10.1603/AN13104. [DOI] [Google Scholar]
- 210.Schulten HR, Schnitzer M. The chemistry of soil organic nitrogen: a review. Biol Fertil Soils. 1997;26(1):1–15. doi: 10.1007/s003740050335. [DOI] [Google Scholar]
- 211.Lavelle P, Spain AV. Soil ecology. Dordrecht: Kluwer Academic; 2001. [Google Scholar]
- 212.Marynowska M, Goux X, Sillam-Dussès D, Rouland-Lefevre C, Halder R, Wilmes P, Gawron P, Roisin Y, Delfosse P, Calusinska M. Compositional and functional characterisation of biomass-degrading microbial communities in guts of plant fibre-and soil-feeding higher termites. Microbiome. 2020;8(96):1–18. doi: 10.1186/s40168-020-00872-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Brune A, Kühl M. pH profiles of the extremely alkaline hindguts of soil-feeding termites (Isoptera: Termitidae) determined with microelectrodes. J Insect Physiol. 1996;42(11–12):1121–1127. doi: 10.1016/S0022-1910(96)00036-4. [DOI] [Google Scholar]
- 214.Brune A. Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol. 2014;12(3):168–180. doi: 10.1038/nrmicro3182. [DOI] [PubMed] [Google Scholar]
- 215.Lo N, Tokuda G, Watanabe H. Evolution and function of endogenous termite cellulases. In: Bignell DE, Roisin Y, Lo N, editors. Biology of termites: a modern synthesis. Dordrecht: Springer; 2010. pp. 51–67. [Google Scholar]
- 216.Krishna K, Weesner FM. Biology of termites. New York: Academic Press; 1969. [Google Scholar]
- 217.Grassé P-P. Termitologia. Anatomie–physiologie–biologie–systématique des termites, comportement, socialité, écologie, evolution, systematique. Paris: Masson; 1985. [Google Scholar]
- 218.Engel MS, Grimaldi DA, Nascimbine PC, Singh H. The termites of Early Eocene Cambay amber, with the earliest record of the Termitidae (Isoptera) ZooKeys. 2011;148:105–123. doi: 10.3897/zookeys.148.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Krishna K, Grimaldi DA. Diverse Rhinotermitidae and Termitidae (Isoptera) in Dominican Amber. Am Mus Novitates. 2009;3640:1–48. doi: 10.1206/633.1. [DOI] [Google Scholar]
- 220.Eggleton P. An introduction to termites: biology, taxonomy and functional morphology. In: Bignell DE, Roisin Y, Lo N, editors. Biology of termites: a modern synthesis. Dordrecht: Springer; 2010. pp. 1–26. [Google Scholar]
- 221.Scheffrahn RH, Bourguignon T, Bordereau C, Hernandez-Aguilar RA, Oelze VM, Dieguez P, Šobotník J, Pascual-Garrido A. White-gutted soldiers: simplification of the digestive tube for a non-particulate diet in higher old world termites (Isoptera: Termitidae) Insect Soc. 2017;64(4):525–533. doi: 10.1007/s00040-017-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]