Abstract
Kindlin3 (K3), a FERM domain containing protein expressed in hematopoietic cells controls integrin activation and thus hemostatic and inflammatory responses. However, its role in the mechanics of plasma membrane remains unclear. Here, we show that genetic knockout of K3 in microglia and macrophages resulted in defective plasma membrane tension and membrane blebbing. Atomic force microscopy (AFM) of K3-deficient cells revealed a significant loss in membrane-to-cortex attachment (MCA), and consequently reduced membrane tension. This loss in MCA is amplified by the mislocalization of the cell cortex proteins—ezrin, radixin, and moesin (ERM)—to the plasma membrane of microglia and macrophages. Re-expression of K3 in K3-deficient macrophages rescued the defects and localization of ERMs implying a key role for K3 in MCA. Analysis of two K3 mutants, K3int affecting integrin binding and activation, and K3pxn/act disrupting binding to paxillin and actin but not integrin functions, demonstrated that the role of K3 in membrane mechanics is separate from integrin activation. The K3pxn/act mutant substantially diminished both membrane tension and Yes-associated protein (YAP) translocation to the nucleus, while preserving integrin activation, cell spreading, and migration. Together, our results show that K3 coordinates membrane mechanics, ERM protein recruitment to the membrane, and YAP translocation by linking integrin at the membrane to paxillin and actin of the cytoskeleton. This novel function of K3 is distinct from its role in integrin activation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-021-03817-7.
Keywords: Atomic force microscopy (AFM), Tether forces, Myeloid cells, Osmotic pressure, Young’s modulus, Membrane tension
Introduction
Myeloid cells, from monocytes/macrophages to microglia of the central nervous system, represent the most mobile cells in the body. Due to their roles in immunity, tissue development, homeostasis, and wound repair, myeloid cells require not only fast migration through tissues of various mechanical properties, but also well-controlled retention within inflamed regions [1]. Cell migration, and to a greater extent, cell immobilization within the extracellular matrix (ECM), are primarily mediated by the transmembrane extracellular matrix receptors, integrins [2–4]. On myeloid cells, integrins undergo activation or inside-out signaling, which requires a direct binding of two key adaptors—Kindlin and talin—to integrin’s cytoplasmic domain. Integrin activation is a prerequisite for ligand binding, cell adhesion, spreading, and migration on the ECM. Moreover, most myeloid cells are known as “shape-shifters” because they are capable of rapid transformation, such as formation of protrusions or processes (for microglia), polarization, spreading, and phagocytosis. All of these essential cellular responses are tightly controlled by the mechanics of the cell surface, which is an important cellular barrier [5]. The cell surface consists of plasma membrane, which is a heterogenous bilipid layer with glycocalyx on the surface and numerous embedded proteins, as well as a cortical cytoskeleton connected by a complex of proteins known as a membrane-to-cortex adhesion complex (MCA) [5].
A key factor controlling cell shape is membrane tension, generated from the in-plane tension within the lipid bilayers of the membrane, in response to external or internal forces on the membrane surface [6]. Membrane tension deforms the plasma membrane and the extracellular force is transmitted from surface integrins to the actin cytoskeleton [4] via focal adhesion (FA) complex. The FA complex is made up of a diverse array of proteins and their complexes, together with a dynamic set of receptors, regulators, and adapters, including Kindlins and talins [1, 7–10]. Talins have been shown to contribute to the MCA by forming a structural link between β-integrins and the actin cytoskeleton thereby regulating the dynamics of adhesion proteins [11, 12], while the specific role of kindlins in this process is unknown.
Kindlins are a family of membrane-bound cytoplasmic proteins consisting a C-terminal FERM (4.1, ezrin, radixin, and moesin) domain, a PH (pleckstrin homology) domain, and an N-terminal domain [13–15]. Kindlins directly bind integrins through highly-conserved QW614/615 residues on the F3 subdomain [1]. Mutations in QW614/615 (designated here as K3int) result in a lack of kindlin-integrin interactions and lead to diminished integrin-mediated cell adhesion, spreading, and migration [1]. The PH domain binds phospholipids such as PtdIns-3,4,5-P3 and other ligands in the membrane [16, 17]. The F0 domain interacts with actin and the cytoskeletal protein paxillin that share a common residue (mutation of paxillin/actin binding site is designated as K3pxn/act) [18–20]. The kindlin family constitutes three members that have differential tissue-specific expression [14, 17]. In humans, the importance of Kindlin3 (K3) is underscored by the lack of leukocyte adhesion and integrin activation, leading to life-threatening bleeding and immune disorder [14]. In microglia, K3 is required for cell adhesion, phagocytosis, response to injury [1], as well as mechanosensing [21].
Kindlins exhibit similarity to the FERM domain containing ERMs, major players within MCA complex. Similar to K3, they have two binding sites on the membrane, PIP2 and the cytosolic tail of the membrane receptors, usually CD44 or integrin β2 [22, 23]. The ERMs localize to adhesion sites, filopodia and membrane ruffles where the plasma membrane interacts with F-actin [24]. ERMs have been implicated in numerous pathologies including immune problems and cancer [25]. The current models of ERM activity and localization are conformationally regulated by C-terminal threonine phosphorylation and PIP2 binding on the plasma membrane [26].
Here, using K3 knockout and knockin primary microglia, myeloid cells either lacking K3 or expressing K3-mutants (K3int and K3pxn/act), and atomic force microscopy (AFM) probing, we establish the role of Kindlin and its binding partners in MCA and membrane mechanics.
Results
K3 in microglia is crucial for regulating the MCA
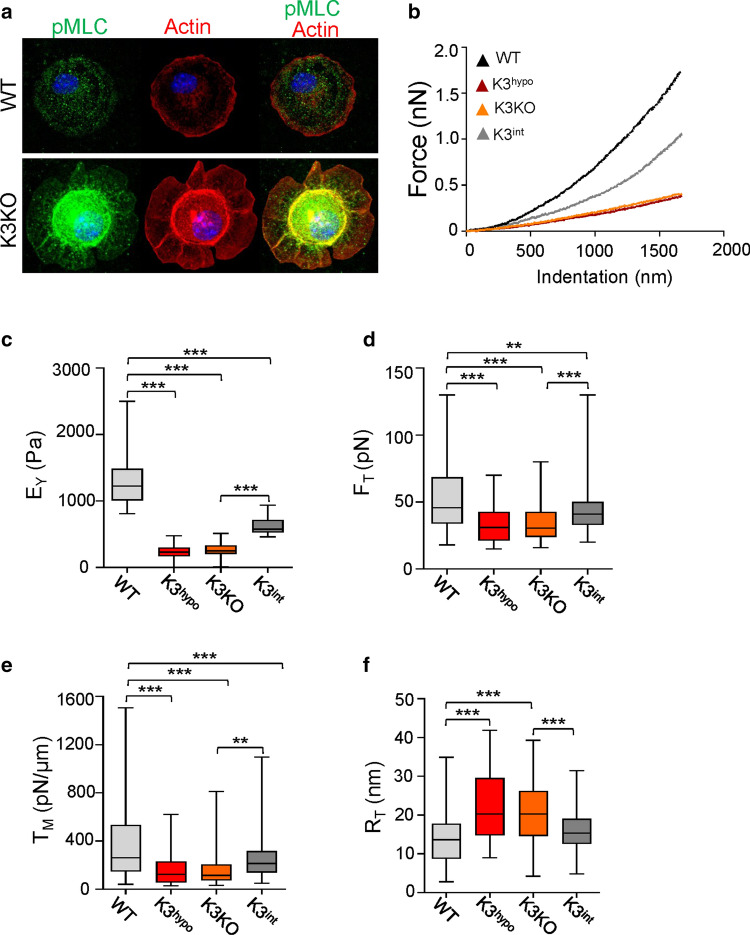
Absence of K3 in primary microglia isolated from wild-type (WT) and K3 knockout (K3KO) mice results in mislocalization of myosin light-chain (MLC) and actin from the membrane (Fig. 1a) indicating defective MCA. To test the role of K3 in regulating the MCA, we had analyzed the WT, K3KO, K3 hypomorph (K3hypo), and K3-integrin mutant knockin (K3int) primary microglia cells using AFM. Two K3 deficient microglia lines, a hypomorph with a low expression (K3hypo) and an inducible knockout of K3 (K3KO), were used to ascertain the importance of K3 levels. K3int microglia expressing K3 bearing the mutation QW-to-AA (in the integrin binding domain) at the level similar to WT (Fig. S1a) were used to determine the importance of K3-integrin binding. We approximated the cell surface as a two-dimensional sheet composed of a lipid bilayer and MCA complex (linkage to cytoskeletal proteins), which together contribute to the apparent membrane tension, TM, experienced during bending under strain [27]. Young’s modulus (EY) was derived from the initial part of the force–displacement curves (Figs. 1b, S1b–e) obtained from AFM analysis. K3 deficiency in K3hypo and K3KO, or K3 mutation in integrin-binding site (K3int), had significantly reduced the Young’s modulus (fivefold in K3hypo and K3KO, and twofold in K3int) compared to WT (Fig. 1c; Table 1), reflecting low stiffness. The EY values had wider distribution from 750 to 2500 Pa in WT cells, but a significant narrowing and shift to lower values in K3int (400–900 Pa), and between 25 and 500 Pa in K3hypo and K3KO cases (Fig. S1f). Similarly, non-specific membrane tethers (both single and multiple tethers) during the retraction phase of the AFM tip from the cell surface showed that the absence of K3 or lack of K3 interaction with integrins results in substantially lower tether forces compared to WT (Fig. 1d). The narrower distributions of tether forces and elastic moduli values (measured at multiple locations on numerous cells in each case) in K3KO cells (including K3hypo, K3int) stand in contrast to their WT counterparts (Fig. S1f, g), attesting to the homogeneity in the decoupling of membrane and cytoskeleton. Thus, the lack of K3 (in K3KO and K3hypo) or its altered interaction with integrin β-subunit in K3int substantially diminished MCA, indicating that the direct connection between K3 and integrin is crucial for regulating membrane mechanics.
Fig. 1.
K3 deficiency diminishes microglia plasma membrane tension. a Representative confocal images of WT, and K3KO primary microglia in their initial stages of spreading on fibronectin in vitro. The cells were stained with pMLC (green) and phallodin for actin (red). The K3KO cells show significant levels of pMLC and actin localized to the cell center instead of the cell membrane as compared to WT. b Representative force-indentation curves showing significantly less resistance to indentation in K3-deficient cells. c Cell stiffness represented as Young’s modulus (EY, Pa) for WT, K3hypo, K3KO, and K3int cells. n = 68 (WT), 69 (K3hypo), 69 (K3KO) and 45 (K3int) for the analyzed force-indentation curves. d Membrane tether forces (FT) of primary microglia measured by retraction of the AFM tip from the cell surface (i.e., tether). e The apparent plasma membrane tension of K3hypo, K3KO, and K3int microglia is significantly lowered compared to WT. f The K3hypo and K3KO cells have greater tether radius compared to WT and were obtained using , where KB is the bending modulus. n = 106 (WT), 109 (K3hypo), 93 (K3KO) and 119 (K3int) for the analyzed membrane tether forces, plasma membrane tension, and tether radius. All box and whisker plots show median (line within box), upper and lower quartiles (bounds of box), and minimum and maximum values (bars) analyzed by one-way ANOVA with Tukey’s post hoc; **p < 0.01, ***p < 0.001
Table 1.
Average values of membrane mechanics parameters of microglia measured by AFM
| WT | K3hypo | K3KO | K3int | |
|---|---|---|---|---|
| EY (Pa) | 1308 ± 47.7 | 236.3 ± 14.6 | 249.3 ± 14.1 | 619.4 ± 17.4 |
| FT (pN) | 57.9 ± 3.5 | 33.5 ± 1.3 | 34.6 ± 1.5 | 44.3 ± 1.4 |
| TM (pN/µm) | 592.4 ± 91.1 | 164.4 ± 12.2 | 206.5 ± 33.2 | 279.3 ± 23.1 |
| RT (nm) | 14.5 ± 0.74 | 21.7 ± 0.8 | 20.8 ± 0.8 | 15.6 ± 0.4 |
| ΔP (kPa) | 81.8 ± 12.1 | 15.1 ± 1.2 | 19.8 ± 3.7 | 35.7 ± 3.4 |
Mean ± St. error of all the parameters (rounded to the nearest tenth) calculated as described in Methods. The effects of K3 depletion in K3hypo and K3KO, or K3 mutation in K3int primary microglial cells, was shown in comparison to WT. Here EY is the Young’s modulus, FT the tether force, TM the apparent membrane tension, RT the radius of tether, and ΔP is the osmotic pressure difference across the plasma membrane
K3 in microglia regulates membrane mechanics
Considering typical bending modulus (KB ≈ 0.1–0.2 pN μm) values for lipid bilayers [28], the average apparent membrane tension (TM) was threefold and twofold lower in K3hypo/ K3KO and K3int respectively, compared to WT (Fig. 1e). In these cells, TM is generated by a combination of the lipid bilayer tension (Tb) and MCA (γ), i.e., TM = Tb + γ. Here, MCA is regulated by K3 (γK3) that could include contribution from other linker proteins (such as ERMs) between membrane and cytoskeleton (γlink), i.e., γ = γK3 + γlink. Since K3KO cells lack K3-mediated MCA (γK3 = 0), the membrane tension in K3KO cells is contributed by Tb and γlink, i.e., TM (K3KO) = Tb + γlink. Assuming changes in Tb are relatively insignificant between the various cell types considered here, γK3 = TM (WT) − TM (K3KO) ≈ 385 pN/µm in WT cells, suggesting that it contributes to > 65% of the overall MCA due to K3. The radius of curvature of tethers (RT) from these cells, calculated from the respective tether forces, showed significantly higher values for K3hypo, K3KO and K3int compared to WT cells (Fig. 1f), indicating lower resistance to deformation and a weaker MCA in the absence of K3 (parameter values shown in Table 1).
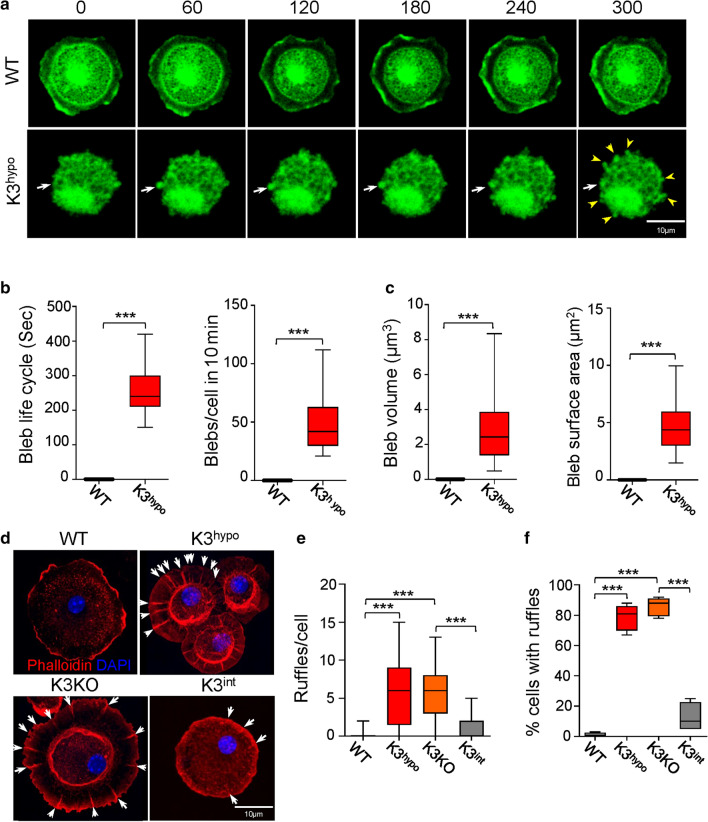
A characteristic of the disruption in membrane–actin cortex interactions is the formation of membrane blebbing [29, 30]. The K3 deficient K3hypo cells show a significant increase in the levels of phosphorylated myosin light chain (Fig. 1a), indicative of increased contractile activity of cytoskeleton required for the generation of membrane blebs in these cells. We noted that K3hypo cells plated on fibronectin-coated dishes showed significant membrane blebbing (Fig. 2a–c), especially during the early phases of attachment, and cell spreading. The K3hypo cells show constant expansion and retraction of multiple blebs per cell, covering a significant portion of the cell membrane surface, with an average bleb life span of 250 s (Fig. 2b, c). Another manifestation of weak MCA and loose membrane is the appearance of membrane folds/ruffles. Once the cells adhered on fibronectin, almost 80% of the K3-deficient cells exhibited significant number of membrane ruffles per cell, while the K3int cells exhibited significantly less ruffles compared to WT microglia (Fig. 2d–f). The phenotype of these cells one day after seeding is shown in Fig. S1h–j.
Fig. 2.
K3 is a crucial regulator of microglia membrane mechanics. a Time-lapse video confocal microscopy of membrane dynamics in WT and K3hypo primary microglia during their initial stages of spreading in vitro. A sequence of snapshots depicts membrane blebbing of K3hypo microglia but absent in WT over a period of 300 s. The white arrowheads indicate the three phases of a bleb life cycle that spans 300 s: bleb initiation, expansion and retraction. The yellow arrow heads in the last frame indicate all the blebs visible in that plane. Data shown were from one of four representative experiments. b Graphs quantifying average time for membrane formation and retraction, and number of blebs/ cells in ten minutes. c Average volume and surface area of a single bleb derived from calculations using measured bleb diameters and assuming the bleb shape as spheroid. d Representative confocal images of WT, K3hypo, K3KO, and K3int primary microglia in their initial stages of spreading on fibronectin in vitro. The K3hypo and K3KO cells display significant membrane ruffling (indicated by white arrow heads). e, f Quantification of the number of ruffles per cell and the percentage (%) of cells showing ruffles in each case. n = 30 (WT, K3hypo and K3KO), and 17 (K3int). All box and whisker plots show median (line within box), upper and lower quartiles (bounds of box), and minimum and maximum values (bars) analyzed by one-way ANOVA with Tukey’s post hoc; ***p < 0.001
We evaluated whether changes in osmotic pressure differences across the membrane (ΔP) might be contributing to the effective stiffness of these tethers. The Young–LaPlace equation ( relates the membrane tension on the cell, and thereby tether forces, to osmotic pressure difference across the plasma membrane [31]. For the range of forces imparted on the cells from indentation, ΔP is significantly lower in K3 deficient (K3hypo, K3KO) compared to WT microglia (Table 1). This implicates a mechanistic role of lowered ΔP across the membrane on the formation of ruffles and blebs in K3-deficient cells. For comparison, typical ΔP values across bacterial cell wall are typically of the order 100 kPa.
Rescue of MCA and membrane tension by re-expression of K3
We investigated whether K3 plays a similar role in macrophages by generating two independent K3 knockout clones of RAW 264.7 cells (K3KO1 and K3KO2) using the CRISPR/Cas9 system. Absence of K3 protein in these cell lines was confirmed by western blot analysis (Fig. S2a). Similar to microglia, both K3KO1 and K3KO2 cells exhibited substantial changes in membrane mechanics. The average Young’s modulus and tether forces reduced by ~ 70 and ~ 35% in the K3KO1 and K3KO2 cell lines, respectively, compared to control cells (Fig. 3a, b), indicating the loss of MCA. This translated to a decrease in membrane tension by more than twofold in both the K3KO cell lines compared to controls (Fig. 3c). The tether radius, calculated from their respective tether forces, was ~ 35% higher in K3KO cell lines (Fig. 3d). To confirm the role of K3, we re-expressed DsRed-tagged human Kindlin-3 (hK3) in both the K3KO lines (K3KO1-hK3 and K3KO2-hK3) at protein levels similar to those in WT controls (Fig. S2b). As anticipated, re-expression of hK3 in K3KO1 and K3KO2 cells rescued the cell phenotype (Fig. S2c–e) including the membrane characteristics, membrane stiffness, tether forces (MCA), apparent membrane tension, and tether radius, establishing K3 as a main regulator of membrane tension and MCA (Fig. 3). Representative force-indentation curves for control, K3KO1, and K3KO1-hK3 are shown in Fig. S2f–h. These patterns were also evident from the EY and FT distributions, wherein controls and K3KO cells were on the ends of the spectrum with no overlap, while hK3-rescued cells showed values in between (Fig. S3a–d).
Fig. 3.
Re-expression of K3 restores membrane tension in macrophages. Two clones of K3 knockout RAW 264.7 cells (K3KO1 and K3KO2) generated using CRISPR/Cas9 were transfected with human K3 (K3KO1-hK3 & K3KO2-hK3). a Cell stiffness represented as Young’s modulus (Pa) was calculated from force-indentation curves using a Hertz model. Control (n = 54), K3KO1 (n = 44), K3KO1-hK3 (n = 50), K3KO2 (n = 30), and K3KO2-hK3 (n = 30). b Tether forces (FT) were calculated from the retraction part of force-indentation curves. c The apparent membrane tension was calculated from the tether forces assuming KB, bending modulus, as 0.1 pN µm. d Membrane tether force was used to calculate the tether radius (RT). For b–d, Control (n = 81), K3KO1 (n = 59), K3KO1-hK3 (n = 77), K3KO2 (n = 40), and K3KO2-hK3 (n = 39). All box and whisker plots show median (line within box), upper and lower quartiles (bounds of box), and minimum and maximum values (bars) analyzed by one-way ANOVA with Tukey’s post hoc; *p < 0.05, **p < 0.01, ***p < 0.001
The role of K3 in membrane mechanics is distinct from that in integrin activation
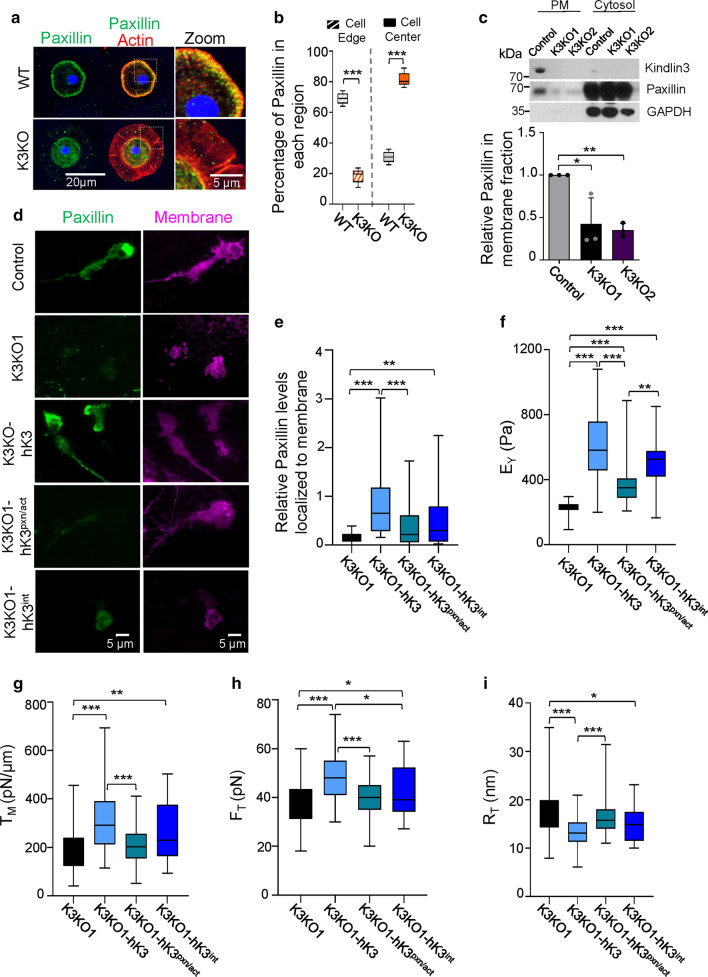
We and others have demonstrated kindlin association with actin and paxillin [20, 32], thereby establishing the connection between K3 and the cytoskeleton. While in WT microglia, paxillin staining overlaps with actin cortex and is associated with the plasma membrane, there was virtually no localization of paxillin and actin in K3KO cells to the cell membrane (Figs. 1a, 4a). Quantitative analysis of paxillin distribution in microglia shows that > 70% of paxillin is near the cell membrane edge while < 30% associates with the cell membrane edge in the K3KO cells (Fig. 4b), which is in stark contrast to WT cells. Paxillin distribution was also tested in K3 knockout RAW cells (K3KO1 and K3KO2) by western blot analysis of membrane and cytosolic fractions (Fig. 4c). While paxillin is detected in the plasma membrane fraction of control cells, it is absent in the membrane fraction of K3KO null clones. Likewise, TIRF imaging revealed paxillin localization to the membrane of control cells but not in K3KO cells (Fig. 4d). However, re-expression of human Kindlin-3 in the K3KO cells (K3KO1-hK3) rescued paxillin localization to the membrane (Fig. 4d, e) suggesting that K3-mediated recruitment of paxillin might be eventually involved in MCA. An increased variance in paxillin localization to cell membrane in the K3KO1-hK3 cells is indicative of different stages of cell spreading that is absent in K3KO cells (Fig. 4e).
Fig. 4.
K3-Paxillin/actin interactions are crucial for regulating MCA. a Immunostaining of primary mouse microglia from WT and K3KO mice for paxillin (green), actin (phalloidin in red), nuclei (DAPI in blue), and WGA for membrane (not shown). Cell images were acquired using a confocal microscope and the z-planes were stacked to produce a superimposed image. An enlarged view shows co-localization of paxillin and actin to the cell membrane edge of WT microglia but not in K3KO. Representative cells from four independent experiments are shown. b Proteins localization to the membrane (outer 1/3rd of the area) or center (inner 2/3rd) of the cell is expressed in % from the total value (n = 10 cells of each genotype). c Western blot analysis shows the presence of K3 within the plasma membrane (PM) fraction of the control (WT) RAW cells but not in K3KO1 and K3KO2 clones. Note the presence of paxillin in membrane fraction of control but not K3KO cells. GAPDH was used as loading control for the cytosolic fraction. Quantification of paxillin in the membrane fraction is shown in the lower panel (n = 3; mean ± SEM analyzed by one-way ANOVA with Tukey’s post hoc; *p < 0.05, **p < 0.01). d TIRF microscopy images of control, K3KO1 clone, and K3KO1 cells re-expressing DsRed tagged-human K3 (K3KO1-hK3), K3-integrin mutant (hK3int), and K3-paxillin mutant (hK3pxn/act) immunostained for paxillin (green) and membrane (magenta). e Quantification of TIRF images showing the relative levels of paxillin localized to the membrane of control, K3KO1, and rescue cells. (n = 44 cells). f AFM analysis of cell stiffness represented as Young’s modulus (Pa) was calculated from force-indentation curves using the Hertz model. K3KO1 (n = 44), expressing hK3 (n = 51), hK3pxn/act (n = 50), and hK3int (n = 26). g Apparent membrane tension was calculated from the tether forces (FT) that were obtained from the retraction part of force-indentation curves (h). For f–i, K3KO1 (n = 59), K3KO1 expressing hK3 (n = 77), hK3int (n = 32), and hK3pxn/act (n = 67). i Membrane tether force was used to calculate the tether radius (RT). All box and whisker plots show median (line within box), upper and lower quartiles (bounds of box), and minimum and maximum values (bars) analyzed by one-way ANOVA with Tukey’s post hoc; *p < 0.05, **p < 0.01, ***p < 0.001
Kindlins, via their F0 domains, bind actin and paxillin that share a highly conserved, common binding region (L46 in K2 and L47 in K3). Mutation of the conserved residues G43 and L47 in the F0 domain of K3 (corresponding to G42 and L46 in K2) into Lysine (K) and Glutamate (E) respectively, were shown to disrupt the K3-paxillin interaction [32, 33]. The mutation in the L47 (corresponding to L46 in K2) should simultaneously disrupt the K3 interaction with actin [20]. This combination of mutations would completely disrupt the K3-cytoskeleton link. We re-expressed DsRed tagged-human Kindlin-3 (hK3) and DsRed tagged-mutant human Kindlin-3 (hK3pxn/act bearing the mutations GL to KE in the F0 domain) in K3KO cell lines at similar K3 and paxillin protein levels (Figs. S2b, S4a). Cells expressing hK3 rescued paxillin to the cell membrane however, hK3pxn/act cannot localize paxillin to the cell membrane (Fig. 4d, e). While hK3 rescued mechanical defects of K3KO cells, hK3pxn/act was largely ineffective. The average EY reduced by ~ 38% in hK3pxn/act cells compared to hK3 cells (Figs. 4f, S4b). Moreover, the defect in membrane tension of hK3pxn/act cells was comparable to that of K3KO cells (Figs. 3c, 4g, S4c). This was mirrored in the measurements of tether forces and tether radius (Figs. 4h–i, S4d, e). K3pxn/act mutant cells expressed β1, αm and β2 integrin subunits at levels comparable to that on hK3 cells (Fig. S5a, c, d, respectively).
Previously, we have shown that a mutation in the integrin binding site of the F3 domain of K3 results in the lack of K3-integrin interactions leading to diminished integrin functions [1]. To confirm the role of K3-integrin interactions in MCA, we re-expressed the DsRed tagged-F3 domain mutant human Kindlin-3 (hK3int bearing the QW/AA mutation in the F3 domain) in both the K3KO macrophage lines at similar protein levels (Fig. S2b, c). Re-expression of hK3int in K3KO cells only partly rescued mechanical defects of K3KO cells (Fig. S2c–e, S3). The average EY was lower by ~ 18 and ~ 13%, respectively, in K3KO1 and K3KO2 cells re-expressed with hK3int, compared to those re-expressed with hK3 (Figs. 4f, S4b). The loss in membrane tension in hK3int cells was comparable to the effect of total loss of K3 in K3KO cells (Figs. 3c, 4g, S4c), which mirrored in the measurements of tether forces and tether radius (Figs. 4h–i, S4d, e). The distributions of EY and FT values in control, K3KO1 and K3KO2 RAW cells, and in their respective hK3, hK3pxn/act and hK3int genotypes were shown in Fig. S3.
In contrast to impaired integrin function in K3KO and K3int mutants, K3pxn/act mutant fully supported integrin activation as evidenced by the binding of activation-dependent antibody 9EG7 (Fig. S5b) and soluble ligand, fibrinogen (Fig. S5e). Consistent with normal integrin function, hK3pxn/act cells adhered and spread on integrin substrate similar to or better than hK3-expressing cells (Fig. S2c). Thus, K3pxn/act mutant supports integrin functions and subsequent cell spreading but not membrane tension, thereby separating the role of K3 in integrin activation from that in membrane mechanics and MCA.
Differential effects of paxillin and actin binding to K2 and K3 on membrane tension
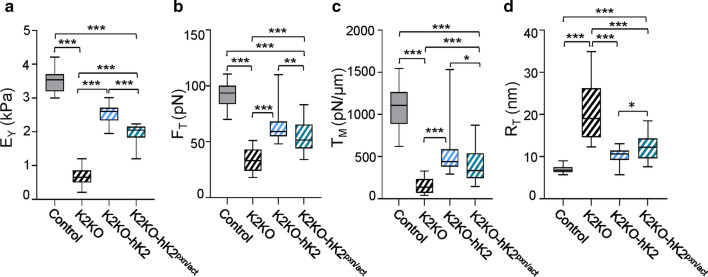
To assess whether MCA function is restricted to K3, we generated CRISPR-based K2 knockouts in mouse embryonic fibroblasts (MEF) (K2KO). Absence of K2 was confirmed by western blot analysis (Fig. S6a). Similar to K3KO, K2KO exhibited substantial changes in membrane mechanics. Lack of K2 in MEFs reduced average EY by ~ 81% (Fig. 5a; Table 2) and the average tether force by ~ 63% (Fig. 5b). The apparent membrane tension reduced by ~ 85% in the K2KO cells (Fig. 5c), consistent with the corresponding increases in the tether radii (Fig. 5d). Importantly, re-expression of HA tagged-human Kindlin2 (hK2) in K2KO cells (Fig. S6a) substantially rescued all the phenotypic and membrane associated mechanical characteristics (Figs. 5, S6b–d), similar to K3. Taken together, these results establish that the absence of kindlins causes destabilization of MCA resulting in lower membrane tension.
Fig. 5.
K2 but not its interaction with paxillin is crucial for MCA in MEFs. a-d AFM analysis of CRISPR K2 knockout MEF cells (K2KO), K2KO expressing HA tagged-human K2 (hK2), and K2KO expressing K2-paxillin mutant (hK2pxn/act). a Cell stiffness represented as Young’s modulus (kPa) were derived from force-indentation curves using the Hertz model. b Tether forces (FT) were calculated from the retraction part of force-indentation curves and used to calculate the apparent membrane tension (c) and tether radius (d) of MEF cells. Control (n = 49), K2KO (n = 31), K2KO re-expressed hK2 (n = 41), and hK2pxn/act (n = 41). All box and whisker plots show median (line within box), upper and lower quartiles (bounds of box), and minimum and maximum values (bars) analyzed by one-way ANOVA with Tukey’s post hoc; *p < 0.05, **p < 0.01, ***p < 0.001
Table 2.
Average values of membrane parameters of RAW 264.7 and MEF cells measured by AFM
| Genotype | EY, Pa | FT, pN | TM, pN/μm | RT, nm | ΔP, kPa | |
|---|---|---|---|---|---|---|
|
Macrophages (RAW 264.7 cells) |
Control | 813.8 ± 26.9 | 61.7 ± 1.9 | 518.4 ± 31.1 | 11.7 ± 0.4 | 88.6 ± 5.2 |
| K3KO1 | 235.0 ± 6.1 | 39.7 ± 1.5 | 215.8 ± 18.3 | 17.0 ± 0.6 | 25.4 ± 2.1 | |
| K3KO2 | 245.2 ± 6.2 | 40.8 ± 1.4 | 220.0 ± 16.1 | 16.0 ± 0.5 | 27.5 ± 13.6 | |
| K3KO1hK3 | 615.6 ± 33.5 | 48.6 ± 1.3 | 317.1 ± 19.7 | 13.6 ± 0.3 | 46.6 ± 3.2 | |
| K3KO1hK3pxn/act | 380.8 ± 19.6 | 40.0 ± 1.2 | 209.0 ± 10.1 | 16.3 ± 0.5 | 25.6 ± 1.1 | |
| K3KO1hK3int | 504.8 ± 22.7 | 44.8 ± 1.6 | 269.1 ± 18.2 | 14.9 ± 0.5 | 36.1 ± 2.8 | |
| K3KO2hK3 | 576.9 ± 3.6 | 50.8 ± 1.3 | 334.6 ± 17.7 | 12.7 ± 0.3 | 52.7 ± 3.4 | |
| K3KO2hK3pxn/act | 402.4 ± 10.1 | 41.8 ± 1.5 | 234.8 ± 20.8 | 15.7 ± 0.4 | 29.9 ± 3.3 | |
| K3KO2hK3int | 504 ± 17.8 | 43.97 ± 1.5 | 258.5 ± 17.9 | 15.1 ± 0.5 | 34.3 ± 2.4 | |
| MEF cells | Control | 3499 ± 43 | 92.3 ± 1.22 | 1093.9 ± 28.3 | 6.9 ± 0.1 | 317 ± 8.6 |
| K2KO | 683 ± 51 | 33.9 ± 1.82 | 158.5 ± 15.9 | 20.4 ± 1.2 | 15.5 ± 1.2 | |
| K2KOhK2 | 2549 ± 41 | 62.6 ± 1.64 | 513.8 ± 30.1 | 10.3 ± 0.2 | 99.8 ± 6.7 | |
| K2KOhK2pxn/act | 1964 ± 38 | 54.8 ± 1.9 | 403.4 ± 28.6 | 12.1 ± 0.4 | 65 ± 5.5 |
Mean ± St. error of all the parameters measured by AFM for RAW and MEF cells, calculated as described in supplementary methods. The top panel shows effects of K3 deletions and re-expression of hK3 and hK3pxn/act or hK3int in RAW cells. Bottom panel shows similar analyses for K2 in MEF cells. The strong positive linear correlation between effective cell stiffness and ΔP across the membrane suggests that depletion of K2 or K3 in hematopoietic cells led to decreased internal osmotic pressure, thereby compromising the effective cell stiffness and encouraging higher deformation, with possible implications for hematopoietic cellular functions such as migration and adhesion
To test the role of K2-paxillin/actin link in non-hematopoietic cells, we re-expressed HA tagged-human Kindlin2 (hK2) and HA tagged-mutant human Kindlin2 (hK2pxn/act) bearing the mutations GL to KE in the F0 domain in the K2KO cells. Surprisingly, unlike hK3pxn/act, re-expression of hK2pxn/act rescued the mechanical defects of K2KO cells showing values closer to hK2 and not K2KO (Fig. 5). The average EY of MEF cells re-expressing hK2pxn/act mutant was almost threefold higher than K2KO cells, while only 23% lower than in cells re-expressing hK2 (Fig. 5a). Similarly, the average tether forces of hK2pxn/act cells were around 1.6-fold higher than K2KO cells while only 12% lower than cells re-expressing hK2 (Fig. 5b). Similar trends were noted for tether radius and apparent membrane tension (Fig. 5c, d). More importantly, the average TM with hK2pxn/act mutant is lowered by only 20% in comparison to hK2 but significantly higher than the membrane tension observed with K2KO. These results indicate that K2-paxillin/actin binding contributes only partially to K2-mediated membrane tension control.
Our results suggest that K3-integrin binding contributes partially to the K3-mediated MCA at the K3-membrane interface in RAW cells. However, at the K3-cytoskeleton interface, the K3-paxillin/actin binding has a significant role in K3-mediated MCA (Table 2). The connections between K3 and paxillin/actin seem to be primarily responsible for the K3-mediated support of membrane tension, suggesting that K3 is an important component of the MCA complex. On the other hand, the K2-paxillin/actin axis accounts for only a small fraction of K2-mediated membrane tension (Table 2), indicating a possible role for other K2 binding molecules as well.
The osmotic pressure difference across the membrane (ΔP) was remarkably high for WT macrophages and MEF cells (Table 2), while K3 and K2 knockouts in respective cell types significantly dropped ΔP to at least one order of magnitude lower than atmospheric pressure. Interestingly, hK3 and hk3int, but not hk3pxn/act, in K3KO RAW cells, and both hk2 and hk2pxn/act in K2KO MEF cells, promoted modest elevation in ΔP in respective cell types, but not to the levels noted in WT cells. Nevertheless, these results establish the roles of K2 and K3 in regulating the membrane tension, osmotic pressure difference across the membrane, and membrane mechanics in respective cell types.
K3 coordinates ERM proteins localization
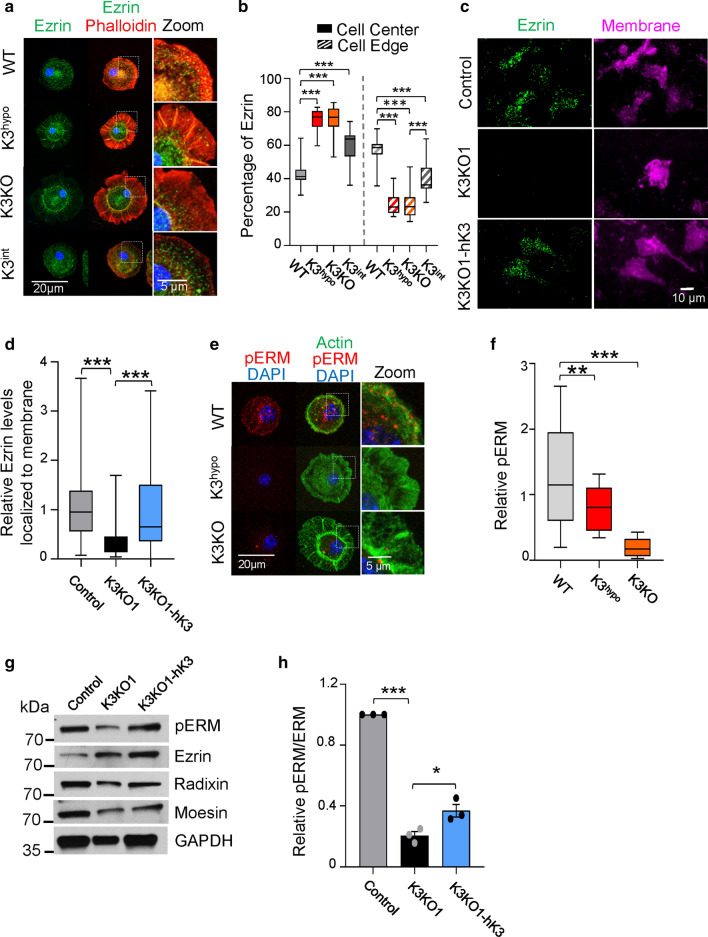
The main mechanical links between membrane and cytoskeleton are ERM (Ezrin, Radixin, Moesin) proteins, which, similar to K3, are characterized by the presence of FERM domains [34] and are localized directly below the plasma membrane [35]. In WT cells, ezrin is primarily associated with both the plasma membrane at the edge of the cell and actin cortex (Fig. 6a). In contrast, in K3KO and K3hypo cells, ezrin is primarily localized to the center of the cell lacking an association with the cell membrane edge. Similar patterns were observed for radixin and moesin (Fig. S7). Thus, the lack of K3 results in dissociation of ERM proteins from the plasma membrane and actin cortex. This finding was further confirmed in myeloid RAW cells. TIRF microscopy analysis of RAW cells demonstrated the absence of ERM proteins within the membrane footprint of K3KO cells in contrast to control cells (Figs. 6c, d, S7e–h). Again, this pattern was rescued by hK3 re-expression, indicating that K3 might serve as a main regulator of MCA complex. The increased variance in ezrin localization to cell membrane in the K3 expressing cells (control and K3KO1-hK3) is due to the different stages of cell spreading that is lacking in K3KO cells (Fig. 6d).
Fig. 6.
K3 is important for recruitment and function of ERM proteins. a Immunostaining of primary microglia from WT, K3hypo, K3KO, and K3int mice for ezrin (green) and actin (phalloidin, red). Images were acquired using confocal microscopy and the z-planes were stacked to produce a superimposed image. An enlarged view of the cell edge shows co-localization of ERM proteins and actin to the membrane. Membrane was identified using Alexa Fluor-WGA (not shown). Representative cells from three independent experiments are shown. b Plot showing ERM proteins localization either to the membrane (outer 1/3rd area) or to the center (inner 2/3rd area) of the cell expressed as percentage of total amount. c K3 mediates ezrin localization to the membrane. TIRF microscopy images of WT, K3KO1 and K3KO cells expressing DsRed tagged-human K3 (K3KO1-hK3) immunostained for ezrin (green) and membrane (magenta). d Quantification of TIRF images showing relative levels of ezrin localized to the membrane of WT, K3KO1 and K3KO1-hK3 RAW cells. e Immunostaining of microglia from WT, K3hypo and K3KO mice for phosphorylated-Ezrin/Radixin/Moesin (pERM) in red co-stained with actin (phalloidin in green). Images were acquired using confocal microscopy and the z-planes were stacked to produce a superimposed image. Representative cells from three independent experiments are shown. f Graph showing the relative intensity of pERM in cells (from e; n = 3). g Western blot analysis of whole-cell lysates from control, K3KO1 and K3KO1-hK3 cells for pERM in comparison to ezrin, radixin, and moesin levels. GAPDH was used as a loading control. h Quantification of relative pERM levels normalized to total amount of ERM proteins from the western blots (n = 3, mean ± St. error analyzed by one-way ANOVA with Tukey’s post hoc; *p < 0.05, ***p < 0.001). All box and whisker plots show median (line within box), upper and lower quartiles (bounds of box), and minimum and maximum values (bars) analyzed by one-way ANOVA with Tukey’s post hoc; **p < 0.01, ***p < 0.001
Since the activity and localization of ERMs is dependent on their C-terminal threonine phosphorylation [24], we sought to show how K3 deficiency affects the levels of phospho-ezrin/radixin/moesin. In primary microglia, immunostaining with the antibody recognizing the phosphorylated forms of ezrin, radixin and moesin (pERM) revealed that substantial amount of pERM is associated with the plasma membrane and actin cortex in WT cells, whereas K3hypo cells exhibited substantially lower levels of pERM and K3KO microglia essentially lacked the staining (Fig. 6e, f). Similarly, in the RAW cell lines, knockout of K3 significantly diminished pERM levels as evidenced by western blotting (Fig. 6g, h). Similar to membrane tension experiments, low pERM levels in K3KO cells were at least partially rescued upon re-expression of hK3 (Fig. 6h). Thus, K3 controls recruitment of ERMs to the plasma membrane and their activity, consistent with its prominent role in membrane mechanics and MCA.
YAP translocation is dependent on K3 regulation of MCA
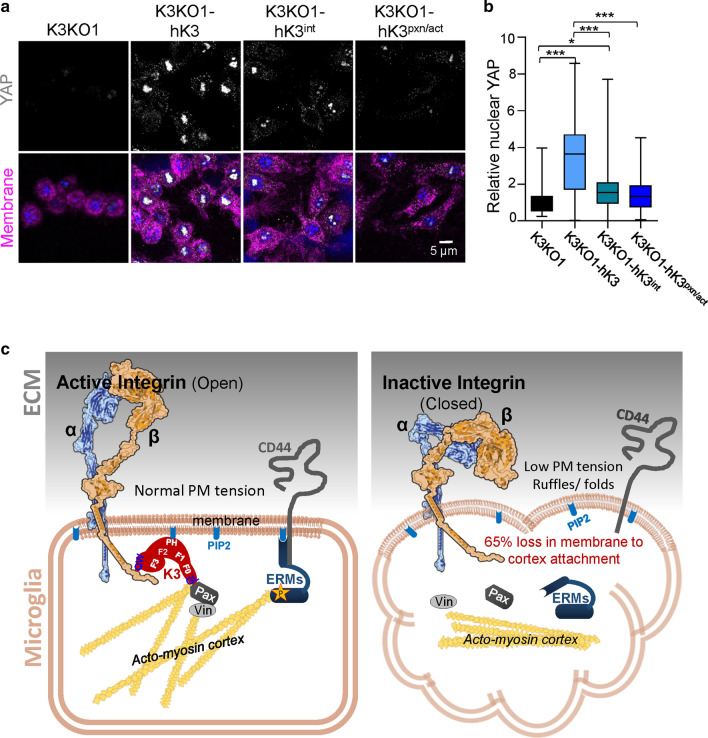
Yes-associated protein (YAP) is a mechanosensitive transcriptional regulator that is sensitive to cell tension [36], and is translocated as a downstream response to substrate stiffness sensing. Analysis of YAP in WT, K3KO and mutant Raw cells by immunostaining (Fig. 7a, b) revealed that YAP was translocated to the nucleus of hK3 cells seeded on fibronectin-coated substrates. Nuclear YAP was nearly absent in K3KO cells (Fig. 7a–d), thereby confirming the critical role of K3 in YAP translocation. To demonstrate whether translocation of YAP was dependent upon integrin-dependent functions rather than on the K3 role in MCA, comparison of hK3int and hK3pxn/act mutants with hK3 expressing cells was performed. As seen from Fig. 7a and quantification in Fig. 7b, nuclear localization of YAP was lowest in K3KO, hK3int and hK3pxn/act cells. The hK3int cells to a certain extent exhibited an intermediate phenotype with a large variability that is likely due to uneven adhesion of these mutant cells. Together, K3 deficiency or disruption of K3-integrin and K3-paxillin/actin binding leads to diminished membrane tension thereby preventing YAP localization to the nucleus.
Fig. 7.
K3 regulates YAP localization to the nucleus. a Confocal images of RAW cells (K3KO, K3KO-hK3. K3KO-hK3int and K3KO-hK3pxn/act) spread on fibronectin for 24 h and stained for YAP (gray), nuclei (DAPI, blue), and membrane with WGA-647 (magenta). b Quantification of YAP localized to the nucleus of RAW cells (n = 60 cells from three experiments). c Proposed role for K3 in regulation of MCA: K3 localizes to the membrane by binding to cytoplasmic domain of β-integrin (via QW residues in F3 domain) and PIP2 (via PH domain). Upon binding integrin, K3 recruits actin and paxillin (Pax) to its F0 domain (via the highly conserved GL residues) leading to integrin activation (open conformation). Paxillin, possibly via vinculin (Vin), further reinforces K3′s connection with the acto-myosin cortex. These events lead to the phosphorylation and activation of ERMs via effector kinase. Upon phosphorylation, ERMs undergo a conformational change, bind to actin, and localize to the membrane by binding PIP2 and transmembrane receptors such as CD44. The absence of K3 results in loss of MCA, low apparent membrane tension, ERMs mislocalization and membrane ruffling
Discussion
A key characteristic of myeloid cells, microglia in the central nervous system (CNS) and macrophages is their ability to migrate through various tissues, sensing and responding to the changes within their environment [21]. Cell motility, shape, and many other key functions of myeloid cells are regulated by the mechanical properties at the cell surface, foremost by membrane tension [37]. Here we show mechanistically that the functionality of myeloid cells depends upon K3, which coordinates membrane mechanics and regulates MCA, independent from its role in integrin activation. As the counterpart for K3 in adherent cells, K2 seems to play a similar role.
Kindlins serve as prime candidates for this function in membrane mechanics, as they contain three FERM domains that are similar to ERM proteins, a PH domain anchoring Kindlin to PIP2, and they interact with cytoskeletal proteins and adaptors [38]. Besides the docking sites characteristic for MCA proteins, Kindlins bind to the integrin cytoplasmic domain, which is an additional anchoring point enabling integration of ECM signaling mediated by integrins. Indeed, our results show that Kindlins (K3/K2) deficiency result in a loss of 65% of the apparent membrane tension. Interestingly, Kindlin deficiency diminished membrane tether force to a greater extent than the actin polymerization inhibitor, latrunculin A [39]. This defect was rescued by re-expression of human Kindlin, emphasizing the importance of Kindlins and the cytoskeleton in membrane tension control. The K3int mutant, which does not interact with integrins, hinders MCA, resulting in ~ 50% loss (out of 65% total loss seen with K3/K2 knockout) of apparent membrane tension. This implies that the Kindlin-integrin interactions serve as the major anchors for membrane binding with only a minimal role for K3-PIP2 interaction (~ 15%).
K2 is known to interact with integrin-linked kinase (ILK) via the F2PH domain [40], and with F-actin [20] and the cytoskeleton protein paxillin [18, 41] via the F0 domain [33]. NMR structural analysis revealed that both K2 and K3 use the same conserved residues (G and L) of the F0 domain to bind LIM4 domains of paxillin with each residue having an equal role [32, 33]. Interestingly, the residue L (46/47 in K2/K3, respectively) is also a binding site for actin [20]. While K2-paxillin interactions promote migration and survival of adherent cells, the same binding for K3 impairs adhesion, polarization, migration, and phagocytosis [32]. This difference between K3 and K2 could be explained by the different roles of these mutants (K3pxn/act and K2pxn/act) in membrane mechanics. The K3 interactions with paxillin/actin account for almost all of the K3-mediated membrane tension, while K2-paxillin/actin interactions have a smaller role. This is likely due to alternative connections between K2 and the cytoskeleton, including ILK [20].
Comparison of two K3 mutants, K3int and K3pxn/act, disrupting interactions with integrin cytoplasmic domain and paxillin/actin respectively, reveals that K3′s function in membrane mechanics is separate from its role in integrin activation. While K3-deficient and K3int mutant cells are characterized by impaired integrin activation, diminished adhesion and spreading, the K3pxn/act mutant fully supports integrin-dependent functions while diminishing apparent membrane tension. Recent structural insights into K3 show a role for the PH and F3 domains in K3 oligomerization, in addition to their known roles in membrane binding and integrin activation, respectively [14, 15]. This supports our functional conclusion that the main role of F0 domain in K3 is to bind paxillin/actin. Hence, the paxillin/actin, but not the integrin binding site that is hidden as a result of K3 oligomerization, is more crucial for the maintenance of K3 mediated MCA and membrane tension. Further, the mutations in the integrin binding site are unlikely to impair K3 oligomerization since these mutations were conclusively shown to impair integrin binding and integrin activation without affecting K3 oligomerization [14, 42]. Thus, the role of K3 in membrane tension control is not linked to integrin activation, cell adhesion and spreading. Numerous studies show that low membrane tension favors events resulting in “normalization” of this tension, including phagocytosis and endocytosis [5]. Indeed, our recent results show that phagocytosis of K3pxn/act mutant cells is substantially increased compared to control cells expressing WT-K3 [32]. Likewise, K3pxn/act mutant cells exhibit increased cell blebbing and motility in 3D, which also might be explained by lower membrane tension [5, 43]. Thus, this unique mutant not only permits analysis of K3 functions that are independent from integrin activation and ligation, but also demonstrates a link between membrane mechanics and essential myeloid cell functions.
Numerous cellular activities such as migration, adhesion, polarization, spreading and ruffle formation are critically dictated by membrane tension [44]. Transcriptional regulators (e.g., YAP) sense how cells perceive themselves and their tissue environment and communicate with it. YAP activity in a cell is directly controlled by its shape and polarity, which is dictated by both the cortical structure and plasma membrane [45, 46]. Our results show that the membrane tension (TM) was two to threefold greater in control macrophages and ~ sevenfold higher in control MEF cells, when plasma membrane was connected to the cytoskeleton via K3 and K2, respectively. This not only suggests the strong contribution of the kindlin-actin and kindlin-paxillin (probably via vinculin) nexus in FA-mediated overall TM, but also that it is cell-type- and kindlin-type-dependent. Similar reductions in TM were evident when membrane-cytoskeleton attachment proteins (e.g., ezrin, class I myosins), cytoskeleton, or membrane composition were perturbed [47].
Building on prior incremental models [39, 48], we propose that the total tether force (FT) would be the summation of forces arising from the cell membrane itself (Fm), between glycocalyx and membrane (Fg-m), between membrane and MCA complex (Fm-M), and between MCA complex and cytoskeleton (FM-c), i.e., . Considering the mean FT ≈ 58 pN for fully intact WT microglia and ≈ 62 pN for control macrophages in our study, the mean steady-state Fm ≈ 16 pN for plasma membrane vesicles on microglia surface [49], and Fg-m ≈ 8 pN in mammalian cells [39], the combined contribution from Fm-M and FM-c would thus be approximately 34 pN in microglia and 38 pN in macrophages, which is in close range to the mean FT values in K3KO cells observed experimentally in our current study.
The loss of apparent membrane tension resulting from K3 deficiency or impaired K3-paxillin/actin binding is profound and comparable to the effects of actin inhibitors or defects in ERM proteins known to mediate membrane-to-cytoskeleton attachment [43, 50]. Based on our results, K3 in hematopoietic cells could indeed serve as a regulator of ERM activation and localization to the cell membrane (Fig. 7c). Taken together, kindlins and their binding to integrins at the membrane side, or more importantly, binding paxillin and actin axis at the cytoskeleton side, serve as key regulators of membrane mechanics, ERM localization, and YAP translocation, although this function is more crucial for K3 in myeloid cells. Microglial morphology known to be dependent upon anatomical location and activation status [51, 52] is likely to be controlled by the role of K3 not only in integrin activation, but also in cell mechanics. The pathways regulating the shape of microglia and macrophages reciprocally affect a variety of physiological and pathological functions.
Methods (see supplementary methods for details)
The K3 hypomorph (K3hypo) mice with the mutation CAATGG to GCCGCC in exon-14 of the K3 gene express very low levels of mutant K3 expression, i.e., K3 deficient (Fig. S1a). The K3hypo mice were crossed with the FLP1 expressing mice to get K3int (K3 mutant Knockin) mice that match the endogenous K3 levels of WT mice, i.e., 100% K3 levels but mutant in integrin binding (Fig. S1a). The K3KO mice were generated by crossing K3 floxed mice with CX3CR1-cre (tamoxifen inducible) mice. Primary microglia were isolated from the brains of postnatal day 1 (P1) mice. The microglia were harvested by collecting the conditioned media and centrifugation. For the excision of K3 gene, the culture from tamoxifen inducible K3KO was treated with 10 nm 4-hydroxytamoxifen (4-HT) every 48 h. Cell lysates were prepared in osmolytic buffer (1 M TRIS at pH 7.4 and 0.5 M EDTA at pH 8.0) and centrifuged at 100,000×g for membrane and cytosolic fractions. The lysates were heated with Laemmli buffer, run on 12% polyacrylamide slab, and transferred to PVDF membrane. For immunocytochemistry, primary microglia were plated onto fibronectin-coated glass coverslips for 5 min at 37 °C and fixed with 4% paraformaldehyde. They were then stained with a membrane stain WGA-647, permeabilized with 0.5% Triton, blocked and incubated with primary antibody, and treated with fluorescently conjugated secondary antibody. For TIRF, RAW cells were plated onto fibronectin coated glass bottom dishes for 24 h, fixed with 4% paraformaldehyde, stained with WGA-647, permeabilized with 0.1% Triton, blocked and incubated with primary antibodies, and then treated with fluorescently conjugated secondary antibody and imaged at a depth of 110 nm. For AFM analysis, cells were plated onto fibronectin-coated cover glass adhered on a 50-mm petri dish maintained at 37 °C overnight. All measurements were made using an MFP-3D-Bio atomic force microscope (Oxford Instruments) mounted on an inverted epifluorescence microscope (Nikon Eclipse Ti) using TR400PSA cantilevers (nominal spring constant ~ 0.02 N/m). CRISPR-Cas9 technology was used to generate Kindlin-2 and Kindlin-3 knockouts in MEF and RAW 264.7 cells, respectively. For rescue, the human K3/K2 and its mutant CDS were sub-cloned into plvx-Dsred-monomer by PCR and transfected into Phoenix Packaging cells. Then the K2 and K3 knockout cells were infected by lentivirus from the culture medium with Lenti-X™ Packaging Single Shots. The cells were sorted for Ds-red expression followed by western blotting and functional experiments.
Statistics
All bar graphs are expressed as mean ± SEM. All box and whisker plots show median (line within box), upper and lower quartiles (bounds of box), and minimum and maximum values (bars). Unpaired Student’s t tests were performed to compare two groups and, when relevant, a between-groups analysis of variance (ANOVA) was performed followed by appropriate post hoc analysis. A p value < 0.05 was considered significant; *p < 0.05, **p < 0.01, ***p < 0.001, and ns is non-significant.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
TD designed and performed experiments, performed data analysis, and wrote the manuscript. GM, HL, IZ, CB, and DN performed experiments and data analysis. CRK and TVB conceived experiments, analyzed data, wrote the manuscript, and secured funding.
Funding
This work was supported by grants from NIH; R01 HL071625 to T.V.B. and funds from National Science Foundation (CBET, Award # 1337859) to C.R.K. We acknowledge the use of the Cleveland Clinic Imaging Core equipment and services supported by NIH SIG grant 1S10RR026820-01.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Conflict of interests
The authors declare that they have no conflict of interests.
Declarations
Ethics approval
Animal experimental procedures were performed in accordance with National Institutes of Health (NIH) guidelines on animal care and all protocols were approved by the Institutional Animal Care and Use Committee at Cleveland Clinic. The lentivirus infection was performed in accordance with the Cleveland Clinic IBC protocol.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chandrasekhar R. Kothapalli, Email: c.kothapalli@csuohio.edu
Tatiana V. Byzova, Email: byzovat@ccf.org
References
- 1.Meller J, Chen Z, Dudiki T, Cull RM, Murtazina R, et al. Integrin-kindlin3 requirements for microglial motility in vivo are distinct from those for macrophages. JCI Insight. 2017;2:e93002. doi: 10.1172/jci.insight.93002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fereol S, Fodil R, Labat B, Galiacy S, Laurent VM, et al. Sensitivity of alveolar macrophages to substrate mechanical and adhesive properties. Cell Motil Cytoskeleton. 2006;63:321–340. doi: 10.1002/cm.20130. [DOI] [PubMed] [Google Scholar]
- 3.Fereol S, Fodil R, Laurent VM, Planus E, Louis B, et al. Mechanical and structural assessment of cortical and deep cytoskeleton reveals substrate-dependent alveolar macrophage remodeling. Biomed Mater Eng. 2008;18:S105–118. [PubMed] [Google Scholar]
- 4.Svitkina TM. Ultrastructure of the actin cytoskeleton. Curr Opin Cell Biol. 2018;54:1–8. doi: 10.1016/j.ceb.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diz-Munoz A, Weiner OD, Fletcher DA. In pursuit of the mechanics that shape cell surfaces. Nat Phys. 2018;14:648–652. doi: 10.1038/s41567-018-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheetz MP, Dai J. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 1996;6:85–89. doi: 10.1016/0962-8924(96)80993-7. [DOI] [PubMed] [Google Scholar]
- 7.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case LB, Waterman CM. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat Cell Biol. 2015;17:955–963. doi: 10.1038/ncb3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton ER, Byron A, Askari JA, Ng DHJ, Millon-Fremillon A, et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol. 2015;17:1577–1587. doi: 10.1038/ncb3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–337. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- 12.Stutchbury B, Atherton P, Tsang R, Wang DY, Ballestrem C. Distinct focal adhesion protein modules control different aspects of mechanotransduction. J Cell Sci. 2017;130:1612–1624. doi: 10.1242/jcs.195362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Deng Y, Sun K, Yang H, Liu J, et al. Structural basis of kindlin-mediated integrin recognition and activation. Proc Natl Acad Sci USA. 2017;114:9349–9354. doi: 10.1073/pnas.1703064114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malinin NL, Zhang L, Choi J, Ciocea A, Razorenova O, et al. A point mutation in kindlin3 ablates activation of three integrin subfamilies in humans. Nat Med. 2009;15:313–318. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bu W, Levitskaya Z, Loh ZY, Jin S, Basu S, et al. Structural basis of human full-length kindlin-3 homotrimer in an auto-inhibited state. PLoS Biol. 2020;18:e3000755. doi: 10.1371/journal.pbio.3000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Fukuda K, Xu Z, Ma YQ, Hirbawi J, et al. Structural basis of phosphoinositide binding to kindlin-2 protein pleckstrin homology domain in regulating integrin activation. J Biol Chem. 2011;286:43334–43342. doi: 10.1074/jbc.M111.295352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malinin NL, Plow EF, Byzova TV. Kindlins in FERM adhesion. Blood. 2010;115:4011–4017. doi: 10.1182/blood-2009-10-239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottcher RT, Veelders M, Rombaut P, Faix J, Theodosiou M, et al. Kindlin-2 recruits paxillin and Arp2/3 to promote membrane protrusions during initial cell spreading. J Cell Biol. 2017;216:3785–3798. doi: 10.1083/jcb.201701176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao J, Huang M, Lai J, Mao K, Sun P, et al. Kindlin supports platelet integrin alphaIIbbeta3 activation by interacting with paxillin. J Cell Sci. 2017;130:3764–3775. doi: 10.1242/jcs.205641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bledzka K, Bialkowska K, Sossey-Alaoui K, Vaynberg J, Pluskota E, et al. Kindlin-2 directly binds actin and regulates integrin outside-in signaling. J Cell Biol. 2016;213:97–108. doi: 10.1083/jcb.201501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudiki T, Meller J, Mahajan G, Liu H, Zhevlakova I, et al. Microglia control vascular architecture via a TGFbeta1 dependent paracrine mechanism linked to tissue mechanics. Nat Commun. 2020;11:986. doi: 10.1038/s41467-020-14787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirao M, Sato N, Kondo T, Yonemura S, Monden M, et al. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang P, Cao C, Xu M, Zhang L. Cytoskeletal protein radixin activates integrin alpha(M)beta(2) by binding to its cytoplasmic tail. FEBS Lett. 2007;581:1103–1108. doi: 10.1016/j.febslet.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Ponuwei GA. A glimpse of the ERM proteins. J Biomed Sci. 2016;23:35. doi: 10.1186/s12929-016-0246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shabardina V, Kashima Y, Suzuki Y, Makalowski W. Emergence and evolution of ERM proteins and merlin in metazoans. Genome Biol Evol. 2020;12:3710–3724. doi: 10.1093/gbe/evz265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charrin S, Alcover A. Role of ERM (ezrin-radixin-moesin) proteins in T lymphocyte polarization, immune synapse formation and in T cell receptor-mediated signaling. Front Biosci. 2006;11:1987–1997. doi: 10.2741/1940. [DOI] [PubMed] [Google Scholar]
- 27.Shi Z, Graber ZT, Baumgart T, Stone HA, Cohen AE. Cell membranes resist flow. Cell. 2018;175:1769–1779.e13. doi: 10.1016/j.cell.2018.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochmuth FM, Shao JY, Dai J, Sheetz MP. Deformation and flow ofmembrane into tethers extracted from neuronal growth cones. Biophys J. 1996;70:358–369. doi: 10.1016/S0006-3495(96)79577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Litvinov RI, Chen X, Bach TL, Lian L, et al. Loss of PIP5KIgamma, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. J Clin Invest. 2008;118:812–819. doi: 10.1172/JCI34239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raucher D, Stauffer T, Chen W, Shen K, Guo S, et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/S0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 31.Bryant G, Wolfe J. Electromechanical stresses produced in the plasma membranes of suspended cells by applied electric fields. J Membr Biol. 1987;96:129–139. doi: 10.1007/BF01869239. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Zhu L, Dudiki T, Gabanic B, Good L, et al. Macrophage migration and phagocytosis are controlled by kindlin-3’s link to the cytoskeleton. J Immunol. 2020;204:1954–1967. doi: 10.4049/jimmunol.1901134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu L, Liu H, Lu F, Yang J, Byzova TV, et al. Structural basis of paxillin recruitment by kindlin-2 in regulating cell adhesion. Structure. 2019;27:1686–1697.e5. doi: 10.1016/j.str.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruckner BR, Pietuch A, Nehls S, Rother J, Janshoff A. Ezrin is a major regulator of membrane tension in epithelial cells. Sci Rep. 2015;5:14700. doi: 10.1038/srep14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2:392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- 36.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 37.Sitarska E, Diz-Munoz A. Pay attention to membrane tension: mechanobiology of the cell surface. Curr Opin Cell Biol. 2020;66:11–18. doi: 10.1016/j.ceb.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malinin NL, Pluskota E, Byzova TV. Integrin signaling in vascular function. Curr Opin Hematol. 2012;19:206–211. doi: 10.1097/MOH.0b013e3283523df0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun M, Graham JS, Hegedus B, Marga F, Zhang Y, et al. Multiple membrane tethers probed by atomic force microscopy. Biophys J. 2005;89:4320–4329. doi: 10.1529/biophysj.104.058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadry YA, Huet-Calderwood C, Simon B, Calderwood DA. Kindlin-2 interacts with a highly conserved surface of ILK to regulate focal adhesion localization and cell spreading. J Cell Sci. 2018;131:jcs221184. doi: 10.1242/jcs.221184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theodosiou M, Widmaier M, Bottcher RT, Rognoni E, Veelders M, et al. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Elife. 2016;5:e10130. doi: 10.7554/eLife.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadry YA, Maisuria EM, Huet-Calderwood C, Calderwood DA. Differences in self-association between kindlin-2 and kindlin-3 are associated with differential integrin binding. J Biol Chem. 2020;295:11161–11173. doi: 10.1074/jbc.RA120.013618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diz-Munoz A, Krieg M, Bergert M, Ibarlucea-Benitez I, Muller DJ, et al. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 2010;8:e1000544. doi: 10.1371/journal.pbio.1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pontes B, Monzo P, Gole L, Le Roux AL, Kosmalska AJ, et al. Membrane tension controls adhesion positioning at the leading edge of cells. J Cell Biol. 2017;216:2959–2977. doi: 10.1083/jcb.201611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rausch V, Hansen CG. The Hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol. 2020;30:32–48. doi: 10.1016/j.tcb.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Pontes B, Monzo P, Gauthier NC. Membrane tension: a challenging but universal physical parameter in cell biology. Semin Cell Dev Biol. 2017;71:30–41. doi: 10.1016/j.semcdb.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 48.Dai J, Sheetz MP. Membrane tether formation from blebbing cells. Biophys J. 1999;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pontes B, Ayala Y, Fonseca AC, Romao LF, Amaral RF, et al. Membrane elastic properties and cell function. PLoS ONE. 2013;8:e67708. doi: 10.1371/journal.pone.0067708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 51.Amadio S, De Ninno A, Montilli C, Businaro L, Gerardino A, et al. Plasticity of primary microglia on micropatterned geometries and spontaneous long-distance migration in microfluidic channels. BMC Neurosci. 2013;14:121. doi: 10.1186/1471-2202-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Not applicable.
Conflict of interests
The authors declare that they have no conflict of interests.