Abstract
Abstract
Amphiregulin (AREG) is an epidermal growth factor (EGF)-like growth factor that binds exclusively to the EGF receptor (EGFR). Treatment with luteinizing hormone (LH) and/or human chorionic gonadotropin dramatically induces the expression of AREG in the granulosa cells of the preovulatory follicle. In addition, AREG is the most abundant EGFR ligand in human follicular fluid. Therefore, AREG is considered a predominant propagator that mediates LH surge-regulated ovarian functions in an autocrine and/or paracrine manner. In addition to the well-characterized stimulatory effect of LH on AREG expression, recent studies discovered that several local factors and epigenetic modifications participate in the regulation of ovarian AREG expression. Moreover, aberrant expression of AREG has recently been reported to contribute to the pathogenesis of several ovarian diseases, such as ovarian hyperstimulation syndrome, polycystic ovary syndrome, and epithelial ovarian cancer. Furthermore, increasing evidence has elucidated new applications of AREG in assisted reproductive technology. Collectively, these studies highlight the importance of AREG in female reproductive health and disease. Understanding the normal and pathological roles of AREG and elucidating the molecular and cellular mechanisms of AREG regulation of ovarian functions will inform innovative approaches for fertility regulation and the prevention and treatment of ovarian diseases. Therefore, this review summarizes the functional roles of AREG in ovarian function and disease.
Graphical abstract
Keywords: Amphiregulin, Ovary, Granulosa cells, Assisted reproductive technology
Introduction
Ovaries have two primary biological functions, the production of steroid hormones and the release of a mature oocyte for fertilization. The ovarian follicle is the functional unit of the ovary, and it is composed of germ cells and several types of somatic cells, including granulosa and theca cells. Ovarian function depends on endocrine regulators, such as pituitary gonadotropins, and a variety of locally produced hormonal factors that exert their effects via autocrine and paracrine manners. Given the increasing evidence of cross-talk between the different endocrine and intraovarian signaling systems, it is apparent that all aspects of ovarian function, from steroidogenesis to folliculogenesis, ovulation, corpus luteum formation and regression, are regulated by multi-factorial mechanisms [1]. Different lines of evidence indicate that amphiregulin (AREG), a member of the epidermal growth factor (EGF) family ligand, can be induced by the luteinizing hormone (LH) and plays an important role as a mediator of the pathway linking LH surge [2]. Recent studies demonstrated that AREG expression in the ovaries could also be regulated via other LH-independent mechanisms, which revealed the complex regulation of ovarian AREG expression. In addition, recent evidence supports new concepts that AREG plays important roles in the preovulatory follicle and the corpus luteum. The functions of AREG in ovarian diseases such as ovarian hyperstimulation syndrome (OHSS), polycystic ovary syndrome (PCOS), and epithelial ovarian cancer (EOC), as well as its applications in assisted reproductive technology (ART) were also reported recently. The objectives of this review are to provide an overview of the physiological function of AREG in the ovaries, which has been summarized in a previous excellent review [3], and to outline the more recent perspectives on the regulation of ovarian AREG expression and the role of AREG in regulating the ovarian function and disease. New applications of AREG to advances in ART are also examined. Overall, this review provides an overview of the literature on the expression and function of ovarian AREG and summarizes the functional role of AREG in ovarian function and disease.
The discovery and structure of AREG
AREG was first purified from the serum-free conditioned medium of phorbol 12-myristate 13-acetate (PMA)-treated MCF-7 human breast carcinoma cells [4]. The “amphiregulin” was named because AREG was initially defined as a bi-functional growth factor that inhibits the growth of several human cancer cell lines in culture but stimulates normal fibroblasts, keratinocytes, and certain human cancer cell growth [4]. Amino acid sequence analysis reveals that the C-terminal of human AREG had 38% and 32% of sequence homology to human EGF and transforming growth factor-α (TGF-α), respectively [5, 6]. In addition, AREG partially competes with 125I-EGF for binding to the epidermal growth factor receptor (EGFR), which suggests that AREG is an EGFR ligand [6]. Later on, the stimulatory effect of AREG on EGFR is confirmed by examining its ability to induction of EGFR phosphorylation in several human cancer cell lines [7].
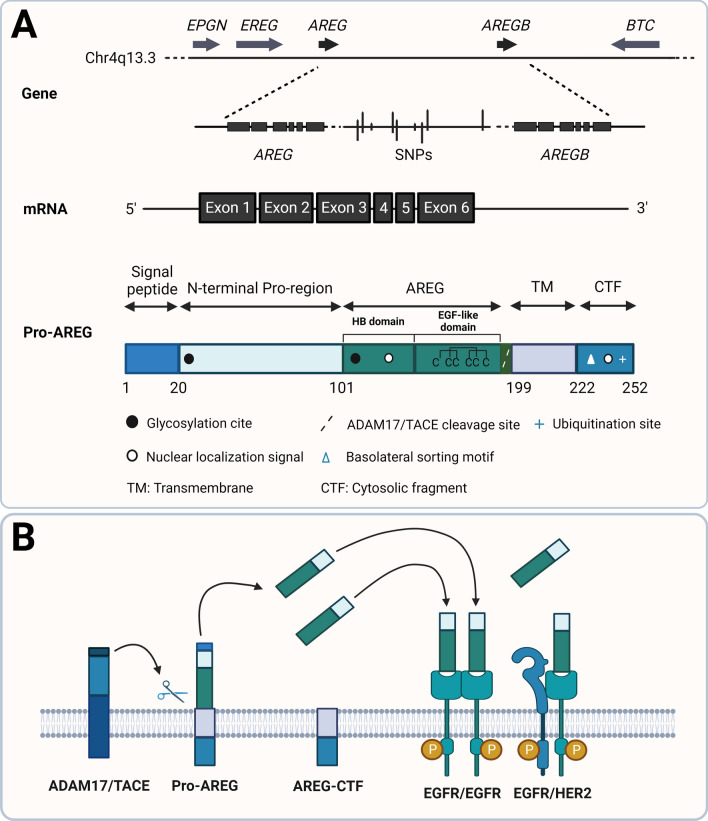
Human AREG is encoded by the AREG gene located in chromosomal region 4q13-4q21, which is flanked by the epiregulin (EREG) and epigen (EPGN) genes in the 5′ region and by the betacellulin (BTC) gene in the 3′ region [8]. The human AREG gene consists of six exons and spans 10 kb in the genomic DNA. The transcription of the human AREG gene produces a 1.4 kb mRNA [5]. There are two copies of the AREG gene (AREG and AREGB) located 160 kb apart in humans. Several single-nucleotide polymorphisms have been identified in the intergenic region between AREG and AREGB genes [9]. The relative contribution of each AREG gene copy to the overall levels of AREG expression is currently unknown. AREG is synthesized as a transmembrane glycoprotein precursor with 252 amino acids [5, 6]. The AREG precursor protein contains multiple domains that, from N- to C-terminal, are a hydrophobic signal peptide, a hydrophilic N-terminal pro-region, a heparin-binding (HB) domain, an EGF-like domain, a hydrophobic transmembrane domain, and a hydrophilic cytoplasmic C-terminal domain [8]. Two N-glycosylation sites are located in the N-terminal pro-region domain and HB domain, respectively. In the EGF-like domain, the presence of conserved spacing of six cysteines that form disulfide bridges and the three-looped structure represents the similarity of AREG to other EGF family members [5] (Fig. 1A).
Fig. 1.
Schematic diagram summarizing the features of AREG gene and protein structure and the AREG proteolytic processing. A Schematic representation of chromosomal localization, the structure of AREG and AREGB genes, mature AREG mRNA, and pro-AREG protein. B Schematic representation of the proteolytic processing of pro-AREG protein. BTC, betacellulin; CTF, cytosolic fragment; EPGN, epigen; EREG, epiregulin; HB domain, heparin-binding domain; TM, transmembrane domain. The figure is derived based on figures published by Berasain and Avila [8]
Like other ErbB ligands, AREG precursor protein undergoes a proteolytic cleavage, also known as ectodomain shedding, at the cell surface to release a mature ectodomain [10]. Ectodomain shedding produces a soluble AREG, a plasma membrane-anchored remnant C-terminal fragment, and a significant amount of un-shed AREG precursor. Lys187 located in the juxtamembrane stalk of AREG precursor is the cleavage site for the ectodomain shedding that mediates by a disintegrin and metalloprotease 17 (ADAM17), which is also known as tumor necrosis factor-α converting enzyme (TACE) [11–14]. In addition to the ectodomain shedding, the AREG precursor also undergoes sequential proteolytic processing, which results in multiple membrane-bound (16, 26, 28, and 50 kDa) and soluble isoforms (9, 19, 21, and 43 kDa). All soluble AREG forms are derived directly from proteolytic cleavage of membrane AREG precursor, and all isoforms contain the EGF-like domain [15]. The cytoplasmic domain of AREG contains a novel mono-leucine-based motif (EEXXXL) which is necessary for delivering AREG to the basolateral membrane in polarized epithelial cells [16]. Lysine240 on the cytoplasmic domain can be ubiquitinized, and this ubiquitination accelerates the half-life of AREG on the cell membrane and regulates AREG endocytosis and ectodomain shedding [17] (Fig. 1B).
AREG signaling pathways
Depending on the processing and trafficking of the protein, AREG exerts its biological functions through EGFR-dependent and independent manners. After proteolytic processing, soluble AREG proteins mainly engage in autocrine and paracrine signaling by binding to the EGFR. The transmembrane AREG precursor also activates EGFR in a juxtacrine manner and which can be enhanced by the binding with the CD9 tetraspanin protein localized on the plasma membrane [18, 19]. In addition to the plasma membrane, full-length AREG protein is also localized in the exosome with proper orientation, which allows AREG to bind to and activate the EGFR that is expressed in adjacent cells. A single exosome contains an average of 24 AREG molecules. AREG exosomes are rapidly internalized by recipient cells and exhibit fivefold effects on cell invasion over equivalent amounts of recombinant AREG [20]. The expressions of exosomes are detected in both male and female reproductive organs and play important roles in the regulation of physiological and pathological events [21]. However, thus far, the majority of research focuses on the functional role of exosomal small RNA in ovarian function and disease [22]. Whether AREG is expressed in ovarian exosomes is currently unknown and warrants further examination. The expression levels of circulating AREG protein in the serum of healthy women are either very low or undetectable [23]. Therefore, the endocrine role of AREG in physiological conditions remains to be defined. However, aberrant serum AREG levels are observed in several human diseases and serve as a biomarker and therapeutic target [24]. Collectively, AREG is now considered a multicrine signaling protein.
For the EGFR-independent action, AREG regulates cellular functions by its nuclear localization. The presence of the putative nuclear localization signal (NLS) in the HB domain distinguishes AREG from other EGF family members [5, 25]. After ectodomain shedding, under the mediation of endocytosis, the C-terminal fragment translocates to the lysosome. The un-shed AREG precursor translocates from the plasma membrane to the inner nuclear membrane through retrograde trafficking, where it interacts with A-type lamin and downregulates global transcription [26]. In response to DNA damage, p53 induces AREG expression, and the nuclear AREG stimulates apoptosis by binding with DDX5 and Drosha to regulate microRNA processing [27]. In female reproductive organs, the nuclear localization of AREG is also observed in the ovarian surface epithelial cells and the syncytiotrophoblast cells of the placental villi [28, 29]. However, the role of nuclear AREG remains largely unknown and needs further investigation.
Activation of EGFR by different ligands results in distinct downstream intracellular signaling pathways and biological functions. These observations could be attributed to the differences in ligand-receptor affinity, preferred ErbB dimer sets, and induced phosphorylation tyrosine residues on the receptor [30, 31]. Compared to EGF and TGF-α, AREG has a lower binding affinity to EGFR. Upon binding to the EGFR, AREG produces an equal portion of EGFR/EGFR and EGFR/HER2 dimer, whereas EGF and TGF-α prefer to produce EGFR/HER2 heterodimer. In vitro assays show that AREG at a low dose (3 and 10 nM) binds to an EGFR monomer and then induces receptor dimerization. High dose of AREG (30, 100, and 300 nM) mainly binds to pre-existing receptor dimers [32]. Like other EGFR ligands, AREG binds to EGFR and actives many intracellular signaling pathways with high complexity. The binding of AREG to the EGFR leads to the activation of the intrinsic tyrosine kinase and autophosphorylation of specific tyrosine residues at the C-terminal of EGFR. These phosphorylated tyrosine residues provide docking sites for adaptor proteins, kinases, and protein tyrosine phosphatases that contain Src homology 2 (SH2) or phosphotyrosine binding (PTB) domains. Simultaneous activation of diverse and complementary signaling pathways follows to produce a physiological outcome [33]. These intracellular pathways include RAS/RAF/MEK/ERK1/2, JNK, P38 MAPK, PI3K/AKT, and PLC-γ/PKC. In a cell type-dependent manner, activation of these signaling pathways leads to the activation of various transcription factors which regulate the expression of different AREG target genes [34].
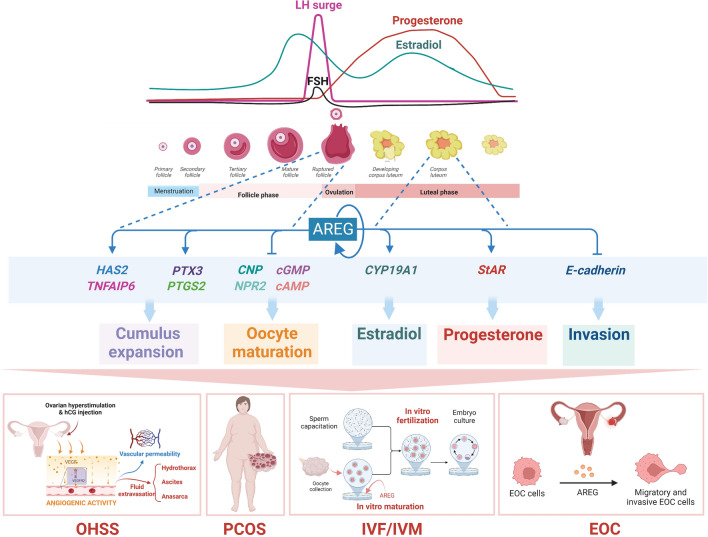
AREG is the predominant EGFR ligand in the ovaries that mediates the function of LH
EGF, AREG, TGF-α, and EPGN bind specifically to the EGFR [31]. Depending on the mouse strain, global knockout of Egfr results in embryonic or postnatal lethality, which limits further assessment of the role of EGFR in reproductive function [35, 36]. Ovarian intrabursal injection of the EGFR kinase inhibitor AG1478 inhibits oocyte maturation and ovulation [37]. These studies indicate that EGFR plays a pivotal role in the regulation of ovarian function. Early in vitro studies have shown that EGF induces oocyte maturation in several mammalian species, including humans [38–41]. Therefore, for many years, EGF has been considered a critical EGFR ligand that regulates ovarian function. However, later studies have demonstrated that the expression of EGF is not immunolocalized in human preovulatory follicles and the expression levels of EGF in human follicular fluid (FF) are not detectable or are very low, depending on the methods of measurement [42, 43]. These findings suggest that other EGFR ligands regulate oocyte maturation and ovulation via an autocrine and/or paracrine manner. A previous study demonstrated that the expression of three ErbB ligands, Areg, Btc, and Ereg, were rapidly and transiently upregulated by LH and/or human chorionic gonadotropin (hCG) stimulation in the mouse ovary [44]. LH/hCG treatment did not affect the expression of other ErbB ligands, such as Egf, Tgfa, and heparin-binding EGF-like growth factor (Hbegf) [45]. Many studies in the last two decades showed that AREG, BTC, EREG, and EPGN were critical propagators of the LH/hCG signal and mediated its effects on oocyte maturation, cumulus expansion, ovulation, and luteinization [46]. Notably, the expression of BTC and EREG is not detected in human FF or conditioned media of mouse cumulus-oocyte complexes (COCs) which suggests that BTC and EREG exert their signals in a juxtacrine manner within the ovarian follicle [47]. Although the expression levels of EPGN are not clear, AREG is the most abundant EGFR ligand in human FF [42]. Based on the specific binding of AREG to EGFR, AREG is now believed to act as a predominant EGFR ligand that mediates LH function in the ovaries.
Regulation of AREG expression in the ovaries
AREG is expressed in several normal human tissues. The ovary and placenta express the highest levels of AREG, and the breast, testis, pancreas, heart, colon, lungs, spleen, and kidneys express lower but measurable levels of AREG [5]. Due to the possible functional redundancy or compensation of the EGF family, Areg knockout mice are fertile. However, Areg deficiency led to abnormal mammary gland development and impaired ovarian functions [48–50] (Table 1). The findings on the induction of AREG expression by LH/hCG in rodent granulosa cells launch a new research area in the autocrine/paracrine role of EGF-like factors in regulating ovarian function. Injection of an ovulatory dose of hCG induced a rapid and transient expression of Areg in mouse ovaries. In situ hybridization analysis showed that the expression of Areg was restricted to mural granulosa cells of preovulatory follicles [44]. Injection of pregnant mare serum gonadotropin (PMSG) in immature rat ovaries induced the expression of Areg mRNA levels. Subsequent hCG injections also rapidly induced Areg mRNA levels. PMSG- and hCG-induced Areg mRNA expression was localized in the granulosa cells of large antral follicles. In addition, Areg mRNA is only highly expressed in the granulosa cells of preovulatory follicles obtained in the afternoon of proestrus, which coincides with the preovulatory LH surge [51]. After these initial studies, many subsequent studies also confirmed the stimulatory effect of LH/hCG on AREG expression in the ovaries of different mammalian species [3]. In addition to LH, treatment of follicle-stimulating hormone (FSH) also stimulates AREG expression in the granulosa cells of different species, although the induction level of AREG by FSH is much lower than that by LH/hCG[47, 52–54].
Table 1.
Abnormal reproductive phenotypes in Areg null mice
| Genotype | Strain | Type of knockout | Phenotypes examined | References |
|---|---|---|---|---|
| Areg−/− | C57BL/6J x 129Sv | Global knockout | Survival and growth rate of Areg−/− pups born to the Areg−/− parents were similar to wild-type | [50] |
| Impaired mammary gland development and function | ||||
| Partial disrupted cumulus expansion | [49] | |||
| Delayed resumption of meiosis | ||||
| Normal ovulation | ||||
| Decreased litter size | [48] | |||
| Decreased percentage of two-cell embryos | ||||
| Increased spindle morphology detected in MII oocytes |
Areg, amphiregulin; MII, metaphase II
In addition to the endocrine regulations, several local factors also regulate AREG expression in ovaries. The LH surge induces the expression of prostaglandin-endoperoxide synthase-2 (PTGS2), which is commonly known as cyclooxygenase-2 (COX-2), in granulosa cells, which increases the production of prostaglandin E2 (PGE2). PGE2 is an essential paracrine mediator of the LH surge that mediates several LH surge-regulated ovarian functions [55]. Early evidence reported that the hCG-induced Areg mRNA levels in granulosa cells and COCs of Ptgs2 null mice were significantly lower than in wild-type mice. Reduced induction of AREG protein levels was also observed in whole ovaries of Ptgs2 null mice [56]. Treatment with the COX-2 inhibitor nimesulide blocked LH-induced AREG expression in human granulosa cells [57]. The siRNA-mediated knockdown of COX-2 significantly inhibited forskolin plus PMA-induced AREG expression in bovine granulosa cells [58]. These studies document that LH/hCG-induced AREG expression is dependent on the function and expression of COX-2. Direct treatment of human granulosa or cumulus cells with PGE2 upregulated the expression of AREG [57]. These studies provide evidence that LH/hCG-induced COX-2 expression and subsequent PGE2 production relayed LH/hCG-induced AREG expression in the ovaries.
AREG treatment upregulated its own expression in granulosa cells. The EGFR tyrosine kinase inhibitor AG1478 blocked AREG-induced AREG expression in granulosa cells. This result further confirms the autocrine loop or auto-stimulatory feedback mechanism of AREG [59]. Activation of the ERK1/2 and p38 MAPK signaling pathways is required for AREG-induced AREG expression [59, 60]. AREG, EREG, and EGF also upregulate Areg mRNA levels in mouse cumulus cells [47, 61]. Collectively, these studies provide evidence that the positive AREG autocrine feedback loop is a critical mechanism that controls AREG expression in granulosa cells. In addition, the expression of AREG can be induced by other ligands of the EGF family.
Estradiol (E2) exerts its biological functions via genomic and non-genomic signaling pathways. Two nuclear estrogen receptors (ER), ERα and ERβ, primarily mediate the genomic signaling of E2 [62]. The G protein-coupled estrogen receptor (GPER), which is also known as GPR30, mediates non-genomic E2 signaling [63]. GPER is expressed in mouse theca cells, mural granulosa cells, cumulus cells, and oocytes. Activation of GPER by E2 or GPER agonist resulted in rapid and transient induction of AREG expression in mouse cumulus cells. A GPER antagonist blocked E2-induced AREG expression. E2 activated the cAMP/PKA/CREB signaling pathway via GPER but not ERs, and it was required for the activation of GPER-induced AREG expression in mouse cumulus cells [64]. A recent study using RNA-sequencing identified that treatment of cultured mouse COCs with a physiological concentration of lysophosphatidic acid (LPA) stimulated the expression of several cumulus expansion-related genes, including Areg [65]. A previous study showed that treatment of mouse COCs with full-length recombinant human versican, a large matricellular proteoglycan, induced the expression of genes related to cumulus expansion and oocyte maturation, including Areg. These results indicate that versican has EGF-like effects and may mediate the regulatory functions of LH in cumulus expansion and oocyte maturation [61]. The hypothalamus-secreted neuropeptide kisspeptin (KISS) acts as an important regulator for the female reproductive function [66]. The expressions of KISS and its receptor have been identified in granulosa cells and the corpus luteum of various animals and humans, which indicates the local physiological function of KISS in the ovaries [67]. Treatment of human granulosa-lutein (hGL) cells obtained from patients undergoing in vitro fertilization (IVF) with KISS inhibited the expression of AREG. In addition, KISS suppressed the stimulatory effect of FSH on AREG expression in hGL cells [67].
The expression of ovarian AREG is impaired in several gene-knockout mouse models (Table 2). The hCG-induced Areg mRNA and protein levels were significantly reduced in the ovaries of Ptgs2 and progesterone receptor (Pgr) null mice [56]. Treatment with PGR antagonists blocked the FSH plus hCG-induced AREG mRNA levels in the granulosa cells of rhesus macaques, which supports the involvement of PGR signaling in the regulation of AREG [68]. The reduction in hCG-induced Areg mRNA levels in granulosa cell-specific Erk1/2 knockout (Erk1/2gc−/−) mice further confirmed the requirement of the ERK1/2 signaling pathway in mediating AREG expression in granulosa cells [69]. In vivo studies showed that hCG-induced Areg mRNA levels were reduced in COCs obtained from granulosa cell-specific Mapk14 knockout (Mapk14gc−/−) mice. In contrast, the hCG-induced Areg mRNA levels in granulosa cells of Mapk14gc−/− mice were higher than in control mice. Although the molecular mechanisms that mediate these differences are not clear, MAPK14 acts differently in cumulus and granulosa cells [60]. Oocyte-derived growth and differentiation factor-9 (GDF-9) and bone morphogenic protein 15 (BMP-15) promote cumulus expansion and ovulation via the SMAD signaling pathway. hCG-induced Areg mRNA levels were reduced in granulosa cells of Smad4gc−/− mice. The impaired hCG-induced cumulus expansion and follicle rupture in Smad4gc−/− mice may be attributed to the reduction in AREG expression [70]. The natural LH surge-induced and hCG-induced Areg mRNA levels were significantly reduced in the ovaries of Ereg null mice, but hCG-induced Btc mRNA levels were not affected. The reduction in hCG-induced AREG expression suggests that EREG is an autocrine and/or paracrine factor mediating AREG expression in the ovaries [71]. Receptor-interacting protein 140 (RIP140) is a co-regulator of nuclear receptors, and it is required for cumulus expansion and ovulation. Rip140 null mice are infertile. The AREG- and forskolin-induced Areg mRNA levels were lower in granulosa cells and COCs of Rip140 null mice compared to wild-type mice. These observations are attributed to the recruitment of RIP140 to the CRE of the Areg promoter and its subsequent action with CREB and c-Jun [72]. Nuclear factor IL-3 (NFIL3) belongs to the basic leucine zipper transcription factor superfamily, which includes CREB. The NFIL3-binding site sequence is homologous to the CRE sequence. NFIL3 binds to the CRE in rat granulosa cells, and overexpression of NFIL3 blocked forskolin plus PMA-induced AREG expression [73]. These results strengthen the critical role of CREB in the transcriptional regulation of AREG expression. NR5A2, also called liver receptor homologue-1, is expressed in granulosa cells. Cumulus expansion, ovulation, and luteinization were impaired in granulosa cell-specific Nr5a2 knockout mice [74, 75]. hCG-induced Areg expression was also impaired in the mural and cumulus cells of granulosa cell-specific Nr5a2 knockout mice [76]. A recent study showed that Cre-loxP-mediated knockout of Yap and Taz, which are the major downstream transcriptional co-activators of the Hippo pathway, blunted LH-induced Areg expression in mouse granulosa cells [77].
Table 2.
Areg expression in the ovaries of gene knockout mouse models.
| Genotype | Gene name | Type of knockout | Effects | References |
|---|---|---|---|---|
| Ereg−/− | Epiregulin | Global knockout |
hCG-induced Areg mRNA in ovaries (−) LH surge-induced Areg mRNA in ovaries (−) |
[71] |
| Mapk14gc−/− | Mitogen-Activated Protein Kinase 14/p38 MAPKα | GC-specific |
hCG-induced Areg mRNA in COCs (−) hCG-induced Areg mRNA in GCs (+) |
[60] |
| Nr5a2Cyp19−/− |
Nuclear receptor Subfamily 5 Group A |
GC-specific (antral follicles) | hCG-induced Areg mRNA in GCs (−) | [76] |
| Nr5a2Amhr2−/− | Member 2 | GC-specific (primary follicles) | hCG-induced Areg mRNA in GCs (−) | |
| Pgr−/− | Progesterone receptor | Global knockout |
hCG-induced Areg mRNA in GCs (−) hCG-induced AREG protein in GCs (−) |
[56] |
| Ptgs2−/− | Prostaglandinendoperoxide synthase 2/cyclooxygenase-2 | Global knockout |
hCG-induced Areg mRNA in COCs (−) hCG-induced Areg mRNA in GCs (−) hCG-induced AREG protein in ovaries (−) |
[56] |
| Rip140−/− | Nuclear receptor interacting protein 1 | Global knockout |
AREG-induced Areg mRNA in GCs and COCs (−) FSK-induced Areg mRNA in GCs and COCs (−) PGE2-induced Areg mRNA in GCs and COCs (x) |
[72] |
| Smad4gc−/− | SMAD Family Member 4 | GC-specific | hCG-induced Areg in GC (−) | [70] |
| Yapf/f;Tazf/f |
Yes-associated protein; transcriptional coactivator with PDZ-binding motif |
Global knockout | LH-induced Areg in GC (−) | [77] |
Areg, amphiregulin; COC, cumulus-oocyte complex; FSK, forskolin; GC, granulosa cells; hCG, human chorionic gonadotropin; LH, luteinizing hormone; PGE2, prostaglandin E2
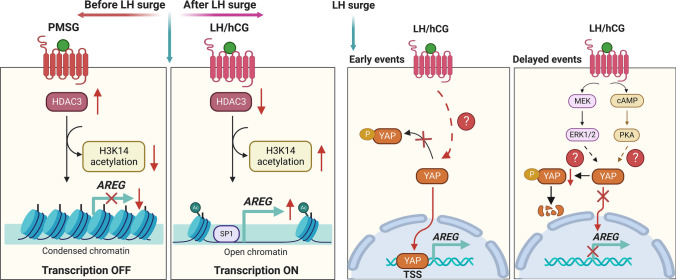
A previous study used a microarray to examine the temporal changes in the expression of EGF-like growth factor mRNAs in immature wild-type mouse ovaries primed with PMSG and 48 h later by hCG injection. The Areg mRNA levels were very low before the hCG injection. Injection of an ovulatory dose of hCG resulted in rapid and transient induction of Areg mRNA [44, 45]. Analysis of the mRNA levels of LH receptor (Lhr) collected from follicles in different developmental stages showed that the highest level of Lhr mRNA was detected in granulosa cells from preovulatory follicles just before ovulation induction. Granulosa cells from small antral follicles and immature follicles express similar levels of Lhr mRNA, which is approximately tenfold lower than preovulatory follicles. Cumulus cells express very low Lhr mRNA levels relative to granulosa cells of the same stage of ovarian follicles [78]. This spatiotemporal expression pattern of Lhr may explain why the expression level of AREG is very low before the LH surge and LH/hCG-induced Areg is restricted to mural granulosa cells of preovulatory follicles [44]. In addition to the impact of the differential expression of LHR, the other underlying mechanisms that affect the temporal and LH-induced AREG expression during the ovarian cycle remain largely unknown. An early study provided additional evidence that the epigenetic modification mechanism in mouse granulosa cells was involved in LH/hCG-induced AREG expression [79]. This study showed that PMSG treatment upregulated the expression of histone deacetylase 3 (HDAC3), which was then downregulated by subsequent hCG treatment in a time-dependent manner. Before the LH surge, PMSG-stimulated HDAC3 suppressed the transcription of Areg. The LH surge decreased HDAC3 expression, which enabled histone H3 lysine 14 (H3K14) acetylation and binding of the SP1 transcription factor to the Areg promoter to stimulate the transcription of Areg in granulosa cells. Notably, the LH/hCG-stimulated induction of Areg is fast and transient. A recent study used ChIP-qPCR and demonstrated that YAP was rapidly recruited to the transcription start site of the Areg promoter after hCG treatment in mouse granulosa cells. Although the detailed mechanisms that mediate the recruitment and binding of YAP to the Areg promoter are not clear, this machinery contributes to the rapid upregulation of Areg mRNA in response to LH/hCG. However, LH/hCG increases the phosphorylation of YAP via PKA signaling upon binding to the receptor, which inactivates YAP [77]. Treatment with hCG for 2 h downregulated YAP expression in granulosa cells. The inhibitory effect of hCG on YAP expression requires the activation of ERK1/2 signaling [80]. The LH/hCG-downregulated YAP activity and expression may contribute to the transient induction of LH/hCG-induced Areg mRNA expression. Taken together, these findings provide novel mechanisms for how AREG expression is controlled before and after the LH surge (Fig. 2).
Fig. 2.
Schematic diagram summarizing mechanisms for how AREG expression is controlled before and after the LH surge in the ovaries. Before the LH surge, PMSG-stimulated HDAC3 suppresses the transcription of AREG. The LH surge decreases HDAC3 expression, which enables H3K14 acetylation and binding of the SP1 transcription factor to the AREG promoter to stimulate the transcription of AREG (left panel). YAP protein is rapidly recruited to the TSS of the AREG promoter after LH/hCG treatment which contributes to the rapid upregulation of AREG expression in response to LH/hCG. Later, LH/hCG inactivates and downregulates YAP by activating PKA and ERK1/2 signaling pathways, which contributes to the transient expression of AREG in response to LH/hCG (right panel). H3K14, histone H3 lysine 14; hCG, human chorionic gonadotropin; HDAC3, histone deacetylase 3; LH, luteinizing hormone; PMSG, pregnant mare serum gonadotropin; TSS, transcription start site
The physiological functions of AREG in the ovaries
Steroidogenesis
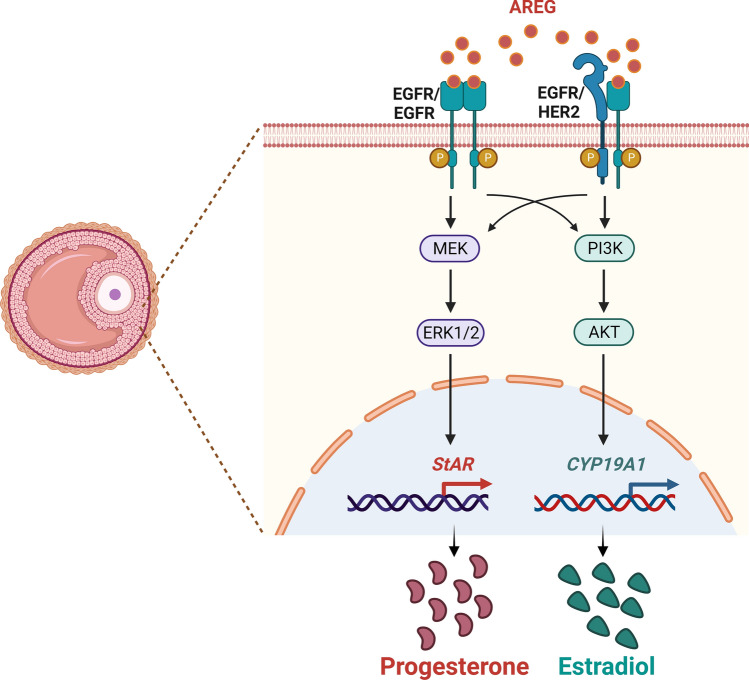
Ovarian steroidogenesis is a complex process that involves multiple subcellular compartments, several enzymes, substrates, and products, and it converts cholesterol to two biologically active steroid hormones, progesterone (P4) and E2. The key regulatory step involved in ovarian steroidogenesis is the transport of cholesterol from the outer to the inner membrane of the mitochondria. The transfer of cholesterol from the outer to the inner membrane of the mitochondria is mediated by steroidogenic acute regulatory protein (StAR) [81]. Blockade of LH-induced P4 production by EGFR tyrosine kinase inhibitor revealed that EGFR signaling was required for ovarian steroidogenesis [82]. Treatment of AREG alone induced steroidogenesis-related genes, such as Star, Cyp11a1, and Pgr [56]. AREG also stimulated the expression of Star and Cyp11a1 and the production of P4 in cultured mouse and rat granulosa cells. The AREG-induced upregulation of Star and Cyp11a1 required activation of the ERK1/2 signaling pathway [59, 83]. A study showed that AREG induced the expression of another steroidogenesis-related gene, Hsd3b, when mouse granulosa cells were cultured on fibronectin-coated plates [84]. In contrast, AREG did not affect the expression of CYP11A1 or P4 production in cultured porcine COCs [85]. In humans, treatment of hGL cells with AREG stimulated P4 production [86, 87]. Our previous work demonstrated that AREG upregulated the mRNA and protein levels of StAR and P4 production in hGL cells. AREG activated the ERK1/2 and PI3K/AKT signaling pathways in hGL cells. However, only the activation of ERK1/2 was required for the induction of StAR expression and P4 production by AREG. In addition, the AREG levels in human FF positively correlated with P4 levels in FF and serum [88]. The CYP19A1 gene encodes aromatase, which mediates the final step in the biosynthesis of estrogens from androgens. Treatment with AREG alone induced E2 production in hGL cells. The induction of E2 production was attributed to the AREG-induced upregulation of CYP19A1 expression. The PI3K/AKT signaling pathway is involved in AREG-induced CYP19A1 expression in hGL cells. The AREG levels in human FF also positively correlated with E2 levels in the human FF and serum. However, the mRNA levels of CYP11A1, HSD3B, and HSD17B1 were not affected by the AREG treatment in hGL cells [89]. Notably, treatment with AREG alone did not affect the expression of Cyp19a1 in rat granulosa cells [59]. A recent study also shows that AREG administration exhibits a significant increase in P4 and E2 production in hGL cells [67]. Interestingly, although the underlying mechanism remains unknown, AREG at a low dose (1 ng/mL) promotes testosterone release but reduces its release at a high dose (100 ng/mL) [67]. Collectively, these results indicate that AREG promotes P4 and E2 production in granulosa cells by regulating the expression of steroidogenesis-related enzymes (Fig. 3). However, these regulatory effects seem to occur in a species-dependent manner.
Fig. 3.
Schematic diagram summarizing mechanisms for the regulatory roles of AREG in steroidogenesis. AREG activates the ERK1/2 and PI3K/AKT signaling pathways in human granulosa-lutein cells. The activation of ERK1/2 is required for the induction of StAR expression and progesterone production by AREG. The activation of the PI3K/AKT signaling pathway is required for the induction of CYP19A1 expression and estradiol production by AREG
Oocyte maturation
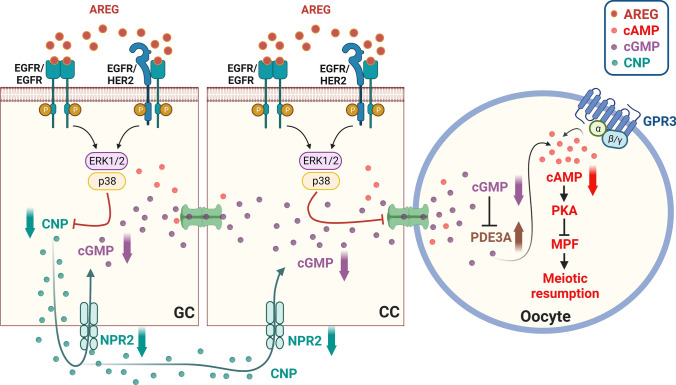
Oocyte meiotic maturation begins with the mid-cycle LH surge, which involves various intercellular, intracellular, and molecular processes. During this process, the LH surge activates LHR, which leads to a diverse signal cascade within the ovarian follicle that induces the resumption of oocyte meiosis and the completion of the first meiotic division. Because LHR is primarily expressed in the mural granulosa cells of the ovarian follicle, and almost no LHR expression is detected in cumulus cells or the oocyte, the LH signal must be transmitted from the mural granulosa cells to the oocyte. It is well established that high levels of cAMP within the oocyte are required for maintaining meiotic arrest [90]. High levels of intra-oocyte cAMP activate PKA, which consequently inhibits the activity of maturation promoting factor (MPF) and maintains the meiotic arrest. In contrast, the reduced cAMP level activates MPF, which initiates germinal vesicle breakdown (GVBD) and chromosome segregation by phosphorylating its downstream targets, including anaphase-promoting complex/cyclosome and spindle assembly checkpoint proteins [91]. The cAMP generated by granulosa cells or cumulus cells may diffuse from cumulus cells to oocytes via gap junctions, and it has been considered the major source of intra-oocyte cAMP. Interestingly, later studies have provided evidence that cAMP can also be produced by the oocyte itself. The G-protein coupled receptor 3 (GPR3), located in the oocyte plasma membrane, is a constitutive activator of adenylate cyclase and thus can activate adenylate cyclase in the absence of a ligand. GPR3-mediated cAMP production maintains the high intra-oocyte cAMP levels. Cyclic GMP (cGMP) is another major factor in the ovarian follicle that mediates oocyte meiotic arrest. The cGMP produced by granulosa cells and cumulus cells diffuses to oocytes, and there keeps a sustained high level of intra-oocyte cAMP after inhibition of the activity of oocyte phosphodiesterase 3A (PDE3A) [92]. The binding of C-natriuretic peptide (CNP), also known as natriuretic peptide precursor C (NPPC), to its receptor guanylyl cyclase natriuretic peptide receptor 2 (NPR2) produces cGMP in the ovarian follicle. Areg knockout mice have delayed oocyte meiotic resumption and impaired oocyte developmental competence [48, 49]. AREG-induced oocyte meiotic resumption required the activation of the ERK1/2 and p38 MAPK signaling pathways [93]. Activation of EGFR by treatment with AREG or EREG decreased cGMP levels in cultured mouse ovarian follicles [94–96]. The activation of EGFR-induced decrease in cGMP levels may be attributed to its suppressive effects on CNP and NPR2 expression [97]. The direct communication channel provided by the gap junction plays an important role in the transmission of molecules, such as cAMP and cGMP, between follicular somatic cells and between cumulus cells and oocytes. LH decreases the permeability of gap junctions by phosphorylating the major component protein of gap junctions, connexin-43 (Cx43). This effect establishes a barrier to molecular diffusion between somatic cells and oocytes. LH reduced gap junction communication in mouse ovarian follicles, and this inhibitory effect was blocked by an EGFR inhibitor. Similar to the effect of LH, AREG or EREG decreased gap junction communication in mouse ovarian follicles [95]. Activation of the ERK1/2 signaling pathway was required for AREG-induced Cx43 phosphorylation and oocyte maturation in mouse preovulatory follicles [98]. Taken together, it is clear that AREG induces oocyte meiotic resumption by decreasing CNP/NPR2-mediated cGMP production and gap junction communication between somatic cells and the oocyte (Fig. 4).
Fig. 4.
Schematic diagram summarizing mechanisms for the effects of AREG on oocyte maturation. High levels of cAMP within the oocyte are required for maintaining meiotic arrest by activating PKA, which consequently inhibits the activity of MPF and maintains the meiotic arrest. The cAMP generated by granulosa cells or cumulus cells may be diffused from cumulus cells to oocytes via gap junctions. Oocyte itself also produces cAMP by GPR3, which is a constitutive activator of adenylate cyclase, and it activates adenylate cyclase in the absence of a ligand. The cGMP produced by granulosa cells and cumulus cells diffuses to oocytes, and there keeps a sustained high level of intra-oocyte cAMP after inhibition of the activity of oocyte PDE3A. The binding of CNP to its receptor guanylyl cyclase NPR2 produces cGMP. Diffusion of cGMP from mural granulosa cells and cumulus cells to oocytes through gap junction inhibits meiotic resumption. AREG induces oocyte meiotic resumption by decreasing CNP/NPR2-mediated cGMP production and gap junction communication between somatic cells and the oocyte. CC, cumulus cell; CNP, C-natriuretic peptide; GC, granulosa cell, GPR3, G-protein-coupled receptor 3; MPF, maturation promoting factor; NPR2, natriuretic peptide receptor 2; PDE3A, phosphodiesterase-3; PKA, protein kinase A. The figure is derived based on figures published by Conti et al. [92]
Cumulus expansion
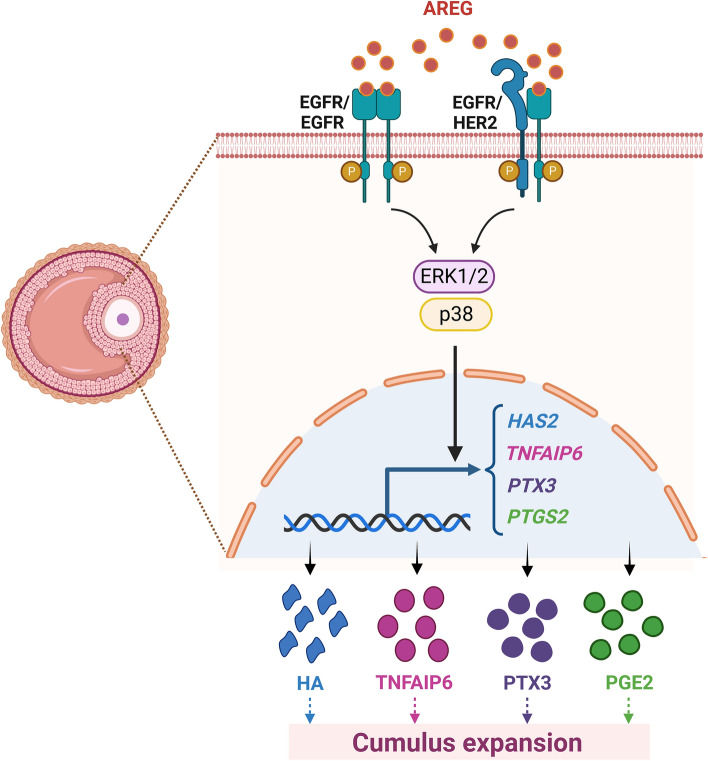
Cumulus expansion enables the detachment of COCs from the follicle wall, and it is a necessary process for subsequent ovulation [99]. The LH surge promotes the synthesis of a large amount of hyaluronic acid (HA)-enriched extracellular matrix in cumulus cells, which increases the deposition of extracellular spaces and allows the outward migration and dissociation of cumulus cells. The synthesis of HA is mediated by hyaluronan synthase 2 (HAS2) in cumulus cells, which is upregulated by hCG [100]. HA synthesis is not sufficient for organizing an extracellular matrix. HA-binding proteins are a group of proteins that have highly homologous sequences for HA binding and are needed for the retention and organization of the HA-rich matrix. Three HA-binding proteins, tumor necrosis factor alpha-induced protein 6 (TNFAIP6), pentraxin 3 (PTX3), and inter-α-trypsin inhibitor (IαI), are expressed in COCs and required for the proper formation and stability of the HA matrix of COCs [101]. A matricellular proteoglycan, versican, is another HA-binding factor that regulates the stability of the COC matrix [101]. The expression of versican was detected in ovulated COCs collected from the oviducts [102]. Treatment with recombinant versican induced mouse COC maturation through its EGF-like activity that activated the EGFR [61]. In vitro experiments with an EGFR inhibitor showed that LH-induced cumulus expansion required transactivation of the EGFR [103]. Reduced cumulus expansion was observed in Areg null mice [49]. Interestingly, the released numbers of oocytes into the oviducts were similar in wild-type and Areg null mice, and the disruption of fertility in Areg null mice was moderate. These results are likely attributed to the redundancy of other EGF-like growth factors that compensate for the loss of AREG production [49]. Reduced expression levels of Has2, Tnfaip6, and Ptgs2 were detected in the COCs of Areg null mice [49]. Treatment of cultured mouse COCs with AREG upregulated the expression of Has2, Tnfaip6, and Ptx3 [44, 56]. The stimulatory effects of AREG on HAS2, TNFAIP6, and PTGS2 were also observed in pig COCs. AREG-induced HAS2 and TNFAIP6 expression required the activation of the ERK1/2 and p38 MAPK signaling pathways in pig COCs [85, 93]. A study from our group demonstrated that treatment with LH or 8-Br-cAMP stimulated the mRNA and protein levels of COX-2, but not COX-1, in an immortalized hGL cell line, SVOG. EGFR knockdown attenuated LH- or 8-Br-cAMP-induced COX-2 mRNA and protein expression. Treatment of SVOG cells with AREG upregulated COX-2 expression and PGE2 production, and these effects were mediated by the ERK1/2 but not the PI3K/AKT signaling pathway [104]. Collectively, these findings indicate that AREG plays important roles in promoting cumulus expansion (Fig. 5).
Fig. 5.
Schematic diagram summarizing mechanisms for the regulatory roles of AREG in cumulus expansion. AREG stimulates HAS2, TNFAIP6, PTX3, and PTGS2 in granulosa cells. These genes mediate the AREG-induced cumulus expansion and ovulation. HA, hyaluronic acid; HAS2, hyaluronan synthase 2; PGE2, prostaglandin E2; PTGS2, prostaglandin-endoperoxide synthase 2; PTX3, pentraxin 3; TNFAIP6, tumor necrosis factor-induced protein 6
Luteal function
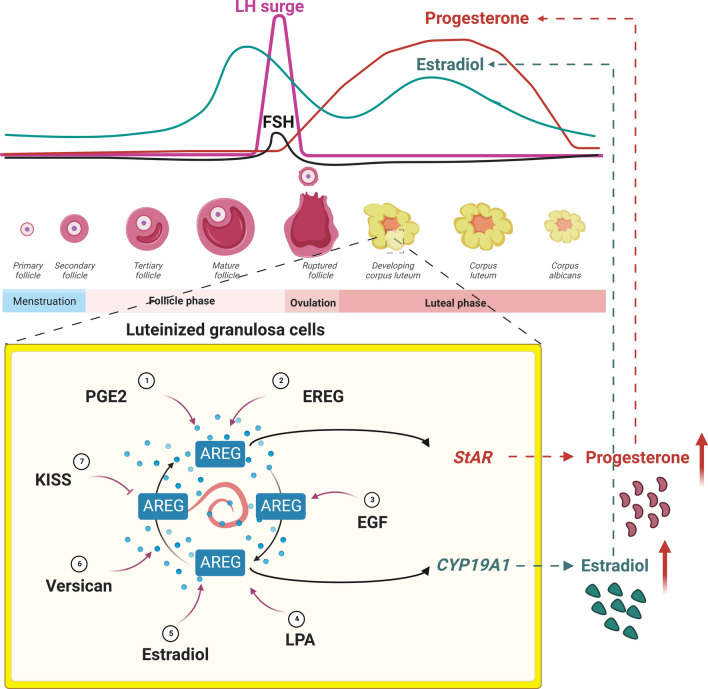
EGFR is not expressed in the primordial follicle in humans, but it is detected in the preovulatory follicles and corpus luteum [105]. In addition to its roles in mediating LH-induced cumulus expansion and oocyte maturation, the expression of EGFR in the corpus luteum suggests that EGFR ligands also affect the function of the corpus luteum. P4 is a well-known steroid hormone that protects the corpus luteum from cell death [106]. Treatment of hGL cells with AREG reduced serum starvation-induced apoptosis. The pro-survival effect of AREG in hGL cells is attributed to its stimulatory effect on P4 production [86]. Similarly, a recent study reported that treatment of hGL cells with AREG increased cell viability and proliferation and inhibited apoptosis [67]. Because LH/hCG-induced AREG expression is transient, the expression of AREG after the LH surge may be maintained by the AREG autocrine loop or regulated by factors that are expressed in the corpus luteum, such as PGE2, EREG, EGF, LPA, E2, versican, and KISS (Fig. 6). E2 also participates in the regulation of luteal cell functions [107]. CYP19A1 aromatase is the critical enzyme that mediates E2 synthesis. FSH is the best-known factor in granulosa cells that induces the expression of CYP19A1. However, the levels of FSH decrease after the LH surge. Therefore, there should be an undiscovered FSH-independent mechanism that regulates CYP19A1 expression and E2 production in the luteal phase. Our study showed that AREG stimulated CYP19A1 expression and E2 production in hGL cells. The stimulatory effects of AREG on CYP19A1 expression and E2 production were mediated by the PI3K/AKT signaling pathway. AREG also mediated hCG-induced upregulation of CYP19A1 expression and E2 production in hGL cells. In addition, AREG protein levels in the human FF positively correlated with E2 levels in human serum and FF [89]. Collectively, these studies indicate that AREG controls luteal function by regulating luteal cell survival and steroid hormone production.
Fig. 6.
Schematic diagram summarizing the local factors that regulate AREG expression in the corpus luteum. The LH levels are reduced in the luteal phase. The expression of AREG in the corpus luteum can be regulated by the AREG autocrine loop or by factors that are expressed in the corpus luteum, such as PGE2, EREG, EGF, LPA, E2, and versican. AREG stimulates the expression of StAR and CYP19A1, which subsequently contributes to the production of progesterone and estradiol. E2, estradiol; EGF, epidermal growth factor; EREG, epiregulin; LPA, lysophosphatidic acid; PGE2, prostaglandin E2
The roles of AREG in ovarian diseases
Ovarian hyperstimulation syndrome
Ovarian hyperstimulation syndrome (OHSS) is a serious complication induced by gonadotropin stimulation for ovarian follicle growth and the subsequent hCG administration for oocyte maturation during controlled ovarian hyperstimulation treatment. Although OHSS is an iatrogenic complication, it may sometimes lead to maternal death [108]. The pathophysiology of OHSS remains incompletely understood. However, the upregulation of vascular endothelial growth factor (VEGF) expression and COX-2-induced increases in capillary permeability are major causes of OHSS [109]. The KGN cell line was derived from human ovarian granulosa cell tumors, but it maintains many of the physiological characteristics of normal granulosa cells [110]. AREG treatment stimulated VEGF expression and secretion in KGN cells by activating ERK1/2 signaling [111]. Our study showed that the mRNA levels of AREG, EGFR, and HER2 in hGL cells and FF AREG protein levels were upregulated in OHSS patients. The FF AREG protein levels positively correlated with several clinical OHSS indices, including the levels of FF VEGF. Treatment with AREG stimulated VEGF expression and secretion more rapidly and profoundly in hGL cells from OHSS patients than in non-OHSS patients. These results provide direct evidence that AREG/EGFR signaling plays an important role in the development of OHSS and may be a potential therapeutic target for OHSS [112]. Sprouty (SPRY) proteins are important intracellular regulators of receptor tyrosine kinase-mediated ERK1/2 signaling [113]. SPRY2 is expressed in hGL cells but not in theca lutein cells or the ovarian stroma. Immunohistochemistry showed that only granulosa cells of ruptured follicles expressed SPRY2 in rat ovaries, but unruptured follicles did not, which suggests a role for SPRY2 during follicle maturation and corpus luteum formation [114]. We showed that the mRNA and protein levels of SPRY2 were upregulated in hGL cells from OHSS patients. SPRY2 knockdown attenuated AREG-activated ERK1/2 signaling and AREG-induced COX-2 expression and PGE2 production. These results indicate that the higher expression levels of SPRY2 in hGL cells of OHSS patients prolong AREG/EGFR signaling and enhance AREG-induced COX-2 expression, which contributes to the development of OHSS [115]. The soluble receptor for advanced glycation end products (sRAGE) is expressed in human FF, and its levels positively correlated with the number of oocytes retrieved in patients undergoing IVF treatments [116]. A recent study reported that serum and FF levels of sRAGE were lower in OHSS patients. Treatment with sRAGE decreased VEGF and AREG expression in hGL cells obtained from OHSS patients [117]. Although only a handful of studies investigated the role of AREG in the pathogenesis of OHSS, these results suggest that the aberrant expression or activity of AREG/EGFR signaling contributes to the development of OHSS.
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is a hormonal and metabolic disorder that affects reproductive-age women. PCOS is the most common cause of infertility, which is characterized by polycystic ovaries as verified using ultrasonography, the hypersecretion of LH, hyperandrogenism, hyperinsulinemia, and ovulatory dysfunction. The tonic hypersecretion of LH during the follicular phase is commonly observed in women with PCOS and suppresses the function of FSH, which results in premature luteinization and premature oocyte maturation [118]. The results from microarray analyses revealed that AREG and EGFR levels were downregulated in the cumulus cells of PCOS patients [119, 120]. However, the expression of EGFR was similar in cumulus cells from PCOS patients and controls reported by another study [121]. Proteomics and ELISA also revealed the downregulation of AREG protein levels in the FF of PCOS patients [122]. Notably, the TaqMan low-density qPCR array showed that AREG levels were downregulated in the central stroma of PCOS ovaries, and AREG levels in granulosa cells of PCOS patients were similar to controls [123]. Taken together, these studies indicate that AREG expression is downregulated in cumulus cells, stromal cells, and the FF of PCOS ovaries. However, the factors and underlying mechanisms that cause the reduced AREG in ovaries of PCOS and the pathological function of AREG in PCOS are not known.
Epithelial ovarian cancer
Many studies have highlighted the functional role of AREG in several aspects of tumorigenesis in various types of human cancers [34]. Ovarian cancer is the most lethal gynecological cancer. It represents the 7th most commonly diagnosed cancer among women in the world [124]. Both benign and malignant ovarian tumors can arise from different ovarian cell types. Approximately 3% of ovarian cancers are germ cell tumors. Sex cord-stromal tumors, such as granulosa cell tumors and thecomas, are derived from the connective tissue of the ovary and account for 7% of ovarian cancers [125, 126]. Epithelial ovarian cancer (EOC) is the most common type; it comprises approximately 90% of all ovarian cancers [127]. In human EOC, EGFR gene amplification, mutation, and overexpression of EGFR protein levels are frequently observed and are associated with more aggressive clinical behavior and poor prognosis [128]. These results indicate the critical role of EGFR in regulating the development and progression of human EOC. It has been shown that the concentration of AREG in the peritoneal fluid of EOC patients was significantly higher than that of TGF-α [129]. In addition, a previous study measuring the concentrations of EGF-like ligands in ascites fluid from 43 ovarian cancer patients showed that AREG is the most abundant and generalized ligand secreted by tumors compared with EGF, TGF-α, BTC, and heparin-binding EGF-like growth factor (HB-EGF) [130]. Moreover, in EOC tissues and cell lines, AREG expression levels were higher than the other two EGFR ligands, EGF and TGF-α [130–132]. Importantly, higher AREG protein levels correlated with the advanced stages of EOC, and patients with high AREG expression in tumors had significantly shorter survival than those with low AREG expression [133]. Treatment of AREG stimulated cell proliferation, migration, and invasion [134, 135]. In addition, the stemness and drug resistance of EOC cells were enhanced in response to the AREG treatment [133]. The pro-invasive effect of AREG on the EOC cells can be attributed to its inhibitory role on the E-cadherin expression by inducing the expression of E-cadherin transcriptional repressors, Snail and Slug [135–138]. YAP-dependent AREG secretion mediated the EGFR-dependent LPA-induced EOC cell migration [139]. Notably, the autocrine effect or self-reinforcing loop of AREG played critical roles in mediating the function of AREG in EOC tumorigenesis [134, 140, 141]. The siRNA-mediated knockdown of AREG or treating anti-AREG monoclonal antibodies inhibited the tumorigenic growth of human EOC cells [130, 142]. Taken together, these studies indicate that AREG plays a dominant role in the regulation of human EOC progression, and targeting AREG might be an effective therapeutic approach.
Roles and applications of AREG in assisted reproductive technology
In vitro fertilization
Since the birth of Louise Brown in 1978, over 8 million children have been born using ART worldwide, and over 2.5 million cycles are performed annually and result in over 500,000 deliveries annually [143]. A prior study reported that the FF AREG concentration negatively correlated with the fertilization rate. Although no significant difference was observed, FF AREG levels were associated with poor embryo quality. However, the use of pooled follicular fluid samples prevented correlation analyses of FF AREG with IVF outcomes of the individual [42]. Using individual follicle aspirates, ovarian follicles yielding GV oocytes or abnormally fertilized oocytes had significantly lower AREG levels than normally producing ovarian follicles or unfertilized oocytes [144]. Another study comparing AREG concentrations in FF between four controlled ovary hyperstimulation (COH) protocols showed that AREG levels were highest in the FF of the GnRH-agonist (GnRH-a) ultra-long protocol, followed by the GnRH-a long protocol, GnRH antagonist protocol, and GnRH-a short protocol. FF EGF levels and serum levels of AREG and EGF were similar between the four COH protocols. FF AREG levels also positively correlate with the number of available embryos [145]. A later study from the same group reported that the mRNA levels of AREG were much higher in pregnant patients than in non-pregnant patients. The AREG mRNA levels in mural granulosa and cumulus cells positively correlated with oocyte retrieval numbers, MII oocyte numbers, and good-quality embryo numbers [146]. AREG mRNA levels in cumulus granulosa cells positively correlated with the number of 2PN zygotes [146]. A GnRH-agonist (GnRH-a) trigger, instead of an hCG trigger, for the final maturation of oocytes, has been applied to patients undergoing COH GnRH antagonist cycles to prevent or reduce the risk of OHSS. Unlike the hCG trigger, the GnRH-a trigger stimulates an LH and FSH surge. The FSH surge positively impacts oocyte maturation, the induction of LH receptor expression in granulosa cells, and cumulus expansion [147]. A comparison of the effects of an hCG trigger and GnRH-a trigger on FF AREG protein levels showed that AREG concentrations in FF of natural menstrual were significantly higher than GnRH-a triggering but considerably lower than hCG triggering. Compared to the hCG trigger, the GnRH-a trigger resulted in substantially more MII oocytes and transferable embryos [148]. Notably, another study did not find a difference in FF AREG levels between patients with GnRH-a and hCG triggers. However, AREG mRNA levels were significantly higher in the granulosa cells of patients with the GnRH-a trigger compared to patients triggered with hCG [149]. Using the combination of GnRH-a and hCG, also known as a dual trigger, for final follicular maturation improves IVF outcomes [150]. The AREG mRNA levels were significantly higher in the granulosa cells of patients with dual trigger than patients with hCG trigger alone [151]. Collectively, these studies indicate that different protocols or treatments during IVF treatment affect the expression of AREG in the ovaries. The AREG levels in the ovaries may be the decisive factor for IVF outcome.
In vitro maturation
In vitro maturation (IVM) of the oocyte is an assisted reproductive technology in which a meiotically immature oocyte is recovered from an antral follicle and matured in vitro prior to fertilization. IVM is no longer considered an experimental treatment by the American Society of Reproductive Medicine, which suggests the potential for wider clinical application of this technology [152]. IVM has been widely practiced in domestic animal breeding. Due to the variable success rates, IVM is less commonly practiced in humans, but it is applied to patients with PCOS and patients at risk of OHSS. IVM may be used for fertility preservation in women with estrogen-sensitive cancers or before gonadotoxic cancer treatments [153, 154]. Many factors, particularly culture medium and supplementation, influence the outcome of IVM [155]. Microarray analysis of mRNA from cumulus cells following in vivo maturation (IVO) or IVM of mouse COCs showed greatly reduced Areg levels in IVM-derived cumulus cells compared to IVO-derived cumulus cells [156]. Microarray analysis revealed similar results that AREG mRNA levels were lower in cumulus cells obtained from PCOS patients with IVM than IVO [120]. There was a qualitative difference in AREG mRNA levels between IVM and IVO in rhesus macaque cumulus cells, with IVO samples consistently expressing AREG mRNA. AREG mRNA levels in granulosa cells were lower in the IVM group than in the IVO group [157]. AREG mRNA levels at the end of FSH-induced IVM were also significantly lower than after IVM in cumulus cells obtained from ≤ 6 mm antral follicles of non-PCOS and PCOS patients [158]. IVM medium supplemented with AREG enhanced oocyte developmental competence in domestic animals [85]. Compared to FSH alone, AREG supplementation improved oocyte developmental competence in mouse COCs [47]. This beneficial effect of AREG on IVM outcomes is attributed to the greater oocyte mitochondrial and glucose metabolism induced by AREG [159]. A culture medium supplemented with AREG in the presence of gonadotrophins exhibited an increased percentage of MII oocytes and a decreased percentage of MI oocytes relative to controls in COCs of rhesus macaques. However, AREG supplementation does not affect fertilization and first cleavage rates [160]. Incubation of human GV-stage oocytes in a standard medium supplemented with AREG plus EREG resulted in a significantly higher rate of MII oocytes [161]. Fertilization and cleavage outcomes after intracytoplasmic sperm injection (ICSI) were not affected by supplementation of the maturation medium with AREG plus EREG [161]. The results of these studies suggest that the addition of exogenous AREG, instead of the commonly used additive FSH, to the culture medium improves the outcomes of IVM.
Conclusions and future directions
Ovarian function is regulated by endocrine factors, such as the pituitary gonadotropin hormones FSH and LH. However, the normal ovarian function also depends on a number of locally produced hormonal factors that exert their effects in an autocrine and paracrine fashion. EGF plays a role in regulating ovarian functions. However, the presence of EGF in the preovulatory follicle and follicular fluid remains inconclusive. Since the first study reported in 2004 by Park et al., subsequent in vitro and animal studies in multiple species have delineated that the expression of three EGF-like growth factors, Areg, Btc, and Ereg, is not detectable before the LH surge but is rapidly and transiently induced in the somatic cells of the preovulatory follicle by LH/hCG. Unexpectedly, Egf expression was not affected by LH/hCG. Notably, compared to EGF and TGF-α, AREG is the most abundant EGFR in human FF, and the levels of AREG are much higher in FF than in serum. BTC and EREG proteins have not been detected in mouse COC-conditioned media or human FF. Therefore, AREG is now considered the essential local factor that mediates the functions of the LH surge because it transmits the LH signal from the periphery of the follicle to the oocyte. Studies in the past two decades demonstrated that several intra-follicular factors also regulated AREG expression in the ovarian follicle. Direct effects of AREG on steroidogenesis, oocyte maturation, cumulus expansion, and luteal function in different species have been described. The aberrant expression of AREG has been detected in the ovaries of patients diagnosed with OHSS, PCOS, and EOC, and it contributes to the pathogenesis of these diseases. These observations suggest that AREG may be used as a biomarker or therapeutic target for OHSS, PCOS, and EOC. Therefore, AREG plays an important role in regulating ovarian physiology and the pathogenesis of ovarian diseases. Different ART treatment protocols affect ovarian AREG levels, and clinical use of recombinant AREG protein may improve IVM outcomes. However, despite the obvious physiological and clinical implications, the underlying molecular mechanisms that mediate the physiological and pathological function of AREG in the ovaries remain poorly understood and need to be further explored. Future mechanistic investigations toward the effect physiological and pathological function of AREG will be required. In addition, most published results regarding the physiological and pathological function of AREG in the ovaries were obtained from animal studies and some roles of AREG in the regulation of ovarian function are in a species-dependent manner. Therefore, future studies are required to delineate the species-dependent effects of AREG on ovarian function and disease. A comprehensive understanding of the regulation, actions, and underlying molecular mechanisms of AREG signaling in the ovaries could inform innovative approaches to fertility regulation and the prevention and treatment of ovarian disorders.
Acknowledgements
This work was supported by operating grants from the National Natural Science Foundation of China to Jung-Chien Cheng (32170868) and Lanlan Fang (32070848). This work was also supported by the International (Regional) Cooperation and Exchange Projects (81820108016) from the National Natural Science Foundation of China to Ying-Pu Sun. All figures were created with BioRender.com. We have been granted a license to use all figures in journal publications.
Author contributions
JCC and LF wrote the manuscript and prepared the figures and tables. YPS reviewed and provided suggestions for the manuscript. All authors read and approved the final manuscript.
Funding
Funding was provided by National Natural Science Foundation of China (Grant nos. 32170868, 32070848, and 81820108016).
Availability of data and material
This is a narrative review based on published data.
Declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update. 2016;23:1–18. doi: 10.1093/humupd/dmw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol. 2006;20:715–723. doi: 10.1210/me.2005-0185. [DOI] [PubMed] [Google Scholar]
- 3.Richani D, Gilchrist RB. The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum Reprod Update. 2018;24:1–14. doi: 10.1093/humupd/dmx029. [DOI] [PubMed] [Google Scholar]
- 4.Shoyab M, McDonald VL, Bradley JG, Todaro GJ. Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc Natl Acad Sci USA. 1988;85:6528–6532. doi: 10.1073/pnas.85.17.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plowman GD, Green JM, McDonald VL, Neubauer MG, Disteche CM, Todaro GJ, Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol. 1990;10:1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- 7.Johnson GR, Kannan B, Shoyab M, Stromberg K. Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. J Biol Chem. 1993;268:2924–2931. doi: 10.1016/S0021-9258(18)53862-X. [DOI] [PubMed] [Google Scholar]
- 8.Berasain C, Avila MA. Amphiregulin. Semin Cell Dev Biol. 2014;28:31–41. doi: 10.1016/j.semcdb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Sebio A, et al. Intergenic polymorphisms in the amphiregulin gene region as biomarkers in metastatic colorectal cancer patients treated with anti-EGFR plus irinotecan. Pharmacogenom J. 2014;14:256–262. doi: 10.1038/tpj.2013.29. [DOI] [PubMed] [Google Scholar]
- 10.Sanderson MP, Dempsey PJ, Dunbar AJ. Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors. 2006;24:121–136. doi: 10.1080/08977190600634373. [DOI] [PubMed] [Google Scholar]
- 11.Levano KS, Kenny PA. Clarification of the C-terminal proteolytic processing site of human amphiregulin. FEBS Lett. 2012;586:3500–3502. doi: 10.1016/j.febslet.2012.07.078. [DOI] [PubMed] [Google Scholar]
- 12.Hinkle CL, Sunnarborg SW, Loiselle D, Parker CE, Stevenson M, Russell WE, Lee DC. Selective roles for tumor necrosis factor alpha-converting enzyme/ADAM17 in the shedding of the epidermal growth factor receptor ligand family: the juxtamembrane stalk determines cleavage efficiency. J Biol Chem. 2004;279:24179–24188. doi: 10.1074/jbc.M312141200. [DOI] [PubMed] [Google Scholar]
- 13.Sahin U, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunnarborg SW, et al. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem. 2002;277:12838–12845. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- 15.Brown CL, Meise KS, Plowman GD, Coffey RJ, Dempsey PJ. Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor. Release of a predominant N-glycosylated 43-kDa soluble form. J Biol Chem. 1998;273:17258–17268. doi: 10.1074/jbc.273.27.17258. [DOI] [PubMed] [Google Scholar]
- 16.Gephart JD, Singh B, Higginbotham JN, Franklin JL, Gonzalez A, Folsch H, Coffey RJ. Identification of a novel mono-leucine basolateral sorting motif within the cytoplasmic domain of amphiregulin. Traffic. 2011;12:1793–1804. doi: 10.1111/j.1600-0854.2011.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda S, Nishida-Fukuda H, Nakayama H, Inoue H, Higashiyama S. Monoubiquitination of pro-amphiregulin regulates its endocytosis and ectodomain shedding. Biochem Biophys Res Commun. 2012;420:315–320. doi: 10.1016/j.bbrc.2012.02.156. [DOI] [PubMed] [Google Scholar]
- 18.Inui S, Higashiyama S, Hashimoto K, Higashiyama M, Yoshikawa K, Taniguchi N. Possible role of coexpression of CD9 with membrane-anchored heparin-binding EGF-like growth factor and amphiregulin in cultured human keratinocyte growth. J Cell Physiol. 1997;171:291–298. doi: 10.1002/(SICI)1097-4652(199706)171:3<291::AID-JCP7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Willmarth NE, Ethier SP. Autocrine and juxtacrine effects of amphiregulin on the proliferative, invasive, and migratory properties of normal and neoplastic human mammary epithelial cells. J Biol Chem. 2006;281:37728–37737. doi: 10.1074/jbc.M606532200. [DOI] [PubMed] [Google Scholar]
- 20.Higginbotham JN, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowalczyk A, Wrzecinska M, Czerniawska-Piatkowska E, Kupczynski R. Exosomes—spectacular role in reproduction. Biomed Pharmacother. 2022;148:112752. doi: 10.1016/j.biopha.2022.112752. [DOI] [PubMed] [Google Scholar]
- 22.Di Pietro C. Exosome-mediated communication in the ovarian follicle. J Assist Reprod Genet. 2016;33:303–311. doi: 10.1007/s10815-016-0657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson EA, Shabbeer S, Kenny PA. Normal range of serum Amphiregulin in healthy adult human females. Clin Biochem. 2012;45:460–463. doi: 10.1016/j.clinbiochem.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Singh SS, Chauhan SB, Kumar A, Kumar S, Engwerda CR, Sundar S, Kumar R. Amphiregulin in cellular physiology, health, and disease: potential use as a biomarker and therapeutic target. J Cell Physiol. 2022;237:1143–1156. doi: 10.1002/jcp.30615. [DOI] [PubMed] [Google Scholar]
- 25.Modrell B, McDonald VL, Shoyab M. The interaction of amphiregulin with nuclei and putative nuclear localization sequence binding proteins. Growth Factors. 1992;7:305–314. doi: 10.3109/08977199209046413. [DOI] [PubMed] [Google Scholar]
- 26.Isokane M, Hieda M, Hirakawa S, Shudou M, Nakashiro K, Hashimoto K, Hamakawa H, Higashiyama S. Plasma-membrane-anchored growth factor pro-amphiregulin binds A-type lamin and regulates global transcription. J Cell Sci. 2008;121:3608–3618. doi: 10.1242/jcs.031443. [DOI] [PubMed] [Google Scholar]
- 27.Taira N, Yamaguchi T, Kimura J, Lu ZG, Fukuda S, Higashiyama S, Ono M, Yoshida K. Induction of amphiregulin by p53 promotes apoptosis via control of microRNA biogenesis in response to DNA damage. Proc Natl Acad Sci USA. 2014;111:717–722. doi: 10.1073/pnas.1313675111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson GR, Saeki T, Auersperg N, Gordon AW, Shoyab M, Salomon DS, Stromberg K. Response to and expression of amphiregulin by ovarian carcinoma and normal ovarian surface epithelial cells: nuclear localization of endogenous amphiregulin. Biochem Biophys Res Commun. 1991;180:481–488. doi: 10.1016/S0006-291X(05)81090-3. [DOI] [PubMed] [Google Scholar]
- 29.Lysiak JJ, Johnson GR, Lala PK. Localization of amphiregulin in the human placenta and decidua throughout gestation: role in trophoblast growth. Placenta. 1995;16:359–366. doi: 10.1016/0143-4004(95)90093-4. [DOI] [PubMed] [Google Scholar]
- 30.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh B, Carpenter G, Coffey RJ. EGF receptor ligands: recent advances. F1000Res. 2016;5:2270. doi: 10.12688/f1000research.9025.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macdonald-Obermann JL, Pike LJ. Different epidermal growth factor (EGF) receptor ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation. J Biol Chem. 2014;289:26178–26188. doi: 10.1074/jbc.M114.586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 34.Busser B, Sancey L, Brambilla E, Coll JL, Hurbin A. The multiple roles of amphiregulin in human cancer. Biochim Biophys Acta. 2011;1816:119–131. doi: 10.1016/j.bbcan.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Threadgill DW, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 36.Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 37.Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- 38.Prochazka R, Srsen V, Nagyova E, Miyano T, Flechon JE. Developmental regulation of effect of epidermal growth factor on porcine oocyte-cumulus cell complexes: nuclear maturation, expansion, and F-actin remodeling. Mol Reprod Dev. 2000;56:63–73. doi: 10.1002/(SICI)1098-2795(200005)56:1<63::AID-MRD8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 39.Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, Dhont M. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13:1638–1644. doi: 10.1093/humrep/13.6.1638. [DOI] [PubMed] [Google Scholar]
- 40.Das K, Phipps WR, Hensleigh HC, Tagatz GE. Epidermal growth factor in human follicular fluid stimulates mouse oocyte maturation in vitro. Fertil Steril. 1992;57:895–901. doi: 10.1016/S0015-0282(16)54977-2. [DOI] [PubMed] [Google Scholar]
- 41.Dekel N, Sherizly I. Epidermal growth factor induces maturation of rat follicle-enclosed oocytes. Endocrinology. 1985;116:406–409. doi: 10.1210/endo-116-1-406. [DOI] [PubMed] [Google Scholar]
- 42.Inoue Y, Miyamoto S, Fukami T, Shirota K, Yotsumoto F, Kawarabayashi T. Amphiregulin is much more abundantly expressed than transforming growth factor-alpha and epidermal growth factor in human follicular fluid obtained from patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2009;91:1035–1041. doi: 10.1016/j.fertnstert.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Reeka N, Berg FD, Brucker C. Presence of transforming growth factor alpha and epidermal growth factor in human ovarian tissue and follicular fluid. Hum Reprod. 1998;13:2199–2205. doi: 10.1093/humrep/13.8.2199. [DOI] [PubMed] [Google Scholar]
- 44.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh M, Zamah AM, Conti M. Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med. 2009;27:52–61. doi: 10.1055/s-0028-1108010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arroyo A, Kim B, Yeh J. Luteinizing hormone action in human oocyte maturation and quality: signaling pathways, regulation, and clinical impact. Reprod Sci. 2020;27:1223–1252. doi: 10.1007/s43032-019-00137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richani D, Ritter LJ, Thompson JG, Gilchrist RB. Mode of oocyte maturation affects EGF-like peptide function and oocyte competence. Mol Hum Reprod. 2013;19:500–509. doi: 10.1093/molehr/gat028. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, et al. Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat Cell Biol. 2013;15:1415–1423. doi: 10.1038/ncb2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsieh M, et al. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 51.Sekiguchi T, Mizutani T, Yamada K, Kajitani T, Yazawa T, Yoshino M, Miyamoto K. Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol. 2004;33:281–291. doi: 10.1677/jme.0.0330281. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita Y, Kawashima I, Yanai Y, Nishibori M, Richards JS, Shimada M. Hormone-induced expression of tumor necrosis factor alpha-converting enzyme/A disintegrin and metalloprotease-17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor. Endocrinology. 2007;148:6164–6175. doi: 10.1210/en.2007-0195. [DOI] [PubMed] [Google Scholar]
- 53.Puri P, Little-Ihrig L, Chandran U, Law NC, Hunzicker-Dunn M, Zeleznik AJ. Protein kinase A: a master kinase of granulosa cell differentiation. Sci Rep. 2016;6:28132. doi: 10.1038/srep28132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freimann S, Ben-Ami I, Dantes A, Armon L, Ben Ya'cov-Klein A, Ron-El R, Amsterdam A. Differential expression of genes coding for EGF-like factors and ADAMTS1 following gonadotropin stimulation in normal and transformed human granulosa cells. Biochem Biophys Res Commun. 2005;333:935–943. doi: 10.1016/j.bbrc.2005.04.177. [DOI] [PubMed] [Google Scholar]
- 55.Duffy DM. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Hum Reprod Update. 2015;21:652–670. doi: 10.1093/humupd/dmv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 57.Ben-Ami I, et al. PGE2 up-regulates EGF-like growth factor biosynthesis in human granulosa cells: new insights into the coordination between PGE2 and LH in ovulation. Mol Hum Reprod. 2006;12:593–599. doi: 10.1093/molehr/gal068. [DOI] [PubMed] [Google Scholar]
- 58.Shrestha K, Lukasik K, Baufeld A, Vanselow J, Moallem U, Meidan R. Regulation of ovulatory genes in bovine granulosa cells: lessons from siRNA silencing of PTGS2. Reproduction. 2015;149:21–29. doi: 10.1530/REP-14-0337. [DOI] [PubMed] [Google Scholar]
- 59.Wayne CM, Fan HY, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21:1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- 60.Liu Z, Fan HY, Wang Y, Richards JS. Targeted disruption of Mapk14 (p38MAPKalpha) in granulosa cells and cumulus cells causes cell-specific changes in gene expression profiles that rescue COC expansion and maintain fertility. Mol Endocrinol. 2010;24:1794–1804. doi: 10.1210/me.2010-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dunning KR, Watson LN, Zhang VJ, Brown HM, Kaczmarek AK, Robker RL, Russell DL. Activation of mouse cumulus-oocyte complex maturation in vitro through EGF-like activity of versican. Biol Reprod. 2015;92:116. doi: 10.1095/biolreprod.114.127274. [DOI] [PubMed] [Google Scholar]
- 62.Heldring N, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 63.Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab. 2009;20:409–416. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, et al. Mechanisms of estradiol-induced EGF-like factor expression and oocyte maturation via G protein-coupled estrogen receptor. Endocrinology. 2020;161:bqaa190. doi: 10.1210/endocr/bqaa190. [DOI] [PubMed] [Google Scholar]
- 65.Ma Y, et al. Lysophosphatidic acid improves oocyte quality during IVM by activating the ERK1/2 pathway in cumulus cells and oocytes. Mol Hum Reprod. 2021;27:gaab032. doi: 10.1093/molehr/gaab032. [DOI] [PubMed] [Google Scholar]
- 66.Ruohonen ST, Poutanen M, Tena-Sempere M. Role of kisspeptins in the control of the hypothalamic-pituitary-ovarian axis: old dogmas and new challenges. Fertil Steril. 2020;114:465–474. doi: 10.1016/j.fertnstert.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 67.Fabova Z, Loncova B, Mlyn Ek M, Sirotkin AV. Interrelationships between amphiregulin, kisspeptin, FSH and FSH receptor in promotion of human ovarian cell functions. Reprod Fertil Dev. 2022;34:362–377. doi: 10.1071/RD21230. [DOI] [PubMed] [Google Scholar]
- 68.Puttabyatappa M, Brogan RS, Vandevoort CA, Chaffin CL. EGF-like ligands mediate progesterone's anti-apoptotic action on macaque granulosa cells. Biol Reprod. 2013;88:18. doi: 10.1095/biolreprod.112.103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu C, Zhang YL, Fan HY. Selective Smad4 knockout in ovarian preovulatory follicles results in multiple defects in ovulation. Mol Endocrinol. 2013;27:966–978. doi: 10.1210/me.2012-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim K, Lee H, Threadgill DW, Lee D. Epiregulin-dependent amphiregulin expression and ERBB2 signaling are involved in luteinizing hormone-induced paracrine signaling pathways in mouse ovary. Biochem Biophys Res Commun. 2011;405:319–324. doi: 10.1016/j.bbrc.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 72.Nautiyal J, et al. The nuclear receptor cofactor receptor-interacting protein 140 is a positive regulator of amphiregulin expression and cumulus cell-oocyte complex expansion in the mouse ovary. Endocrinology. 2010;151:2923–2932. doi: 10.1210/en.2010-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li F, Liu J, Jo M, Curry TE., Jr A role for nuclear factor interleukin-3 (NFIL3), a critical transcriptional repressor, in down-regulation of periovulatory gene expression. Mol Endocrinol. 2011;25:445–459. doi: 10.1210/me.2010-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bertolin K, Gossen J, Schoonjans K, Murphy BD. The orphan nuclear receptor Nr5a2 is essential for luteinization in the female mouse ovary. Endocrinology. 2014;155:1931–1943. doi: 10.1210/en.2013-1765. [DOI] [PubMed] [Google Scholar]
- 75.Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22:1871–1876. doi: 10.1101/gad.472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bertolin K, Meinsohn MC, Suzuki J, Gossen J, Schoonjans K, Duggavathi R, Murphy BD. Ovary-specific depletion of the nuclear receptor Nr5a2 compromises expansion of the cumulus oophorus but not fertilization by intracytoplasmic sperm injection. Biol Reprod. 2017;96:1231–1243. doi: 10.1093/biolre/iox045. [DOI] [PubMed] [Google Scholar]
- 77.Godin P, Tsoi MF, Morin M, Gevry N, Boerboom D. The granulosa cell response to luteinizing hormone is partly mediated by YAP1-dependent induction of amphiregulin. Cell Commun Signal. 2022;20:72. doi: 10.1186/s12964-022-00843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeppesen JV, et al. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97:E1524–E1531. doi: 10.1210/jc.2012-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H, et al. HDAC3 maintains oocyte meiosis arrest by repressing amphiregulin expression before the LH surge. Nat Commun. 2019;10:5719. doi: 10.1038/s41467-019-13671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ji SY, et al. The polycystic ovary syndrome-associated gene Yap1 is regulated by gonadotropins and sex steroid hormones in hyperandrogenism-induced oligo-ovulation in mouse. Mol Hum Reprod. 2017;23:698–707. doi: 10.1093/molehr/gax046. [DOI] [PubMed] [Google Scholar]
- 81.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 82.Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA. 2005;102:16257–16262. doi: 10.1073/pnas.0508521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noma N, et al. LH-induced neuregulin 1 (NRG1) type III transcripts control granulosa cell differentiation and oocyte maturation. Mol Endocrinol. 2011;25:104–116. doi: 10.1210/me.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitasaka H, Kawai T, Hoque SAM, Umehara T, Fujita Y, Shimada M. Inductions of granulosa cell luteinization and cumulus expansion are dependent on the fibronectin-integrin pathway during ovulation process in mice. PLoS ONE. 2018;13:e0192458. doi: 10.1371/journal.pone.0192458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prochazka R, Petlach M, Nagyova E, Nemcova L. Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: comparison with gonadotropins. Reproduction. 2011;141:425–435. doi: 10.1530/REP-10-0418. [DOI] [PubMed] [Google Scholar]
- 86.Ben-Ami I, Armon L, Freimann S, Strassburger D, Ron-El R, Amsterdam A. EGF-like growth factors as LH mediators in the human corpus luteum. Hum Reprod. 2009;24:176–184. doi: 10.1093/humrep/den359. [DOI] [PubMed] [Google Scholar]
- 87.Negishi H, Ikeda C, Nagai Y, Satoh A, Kumasako Y, Makinoda S, Ustunomiya T. Regulation of amphiregulin, EGFR-like factor expression by hCG in cultured human granulosa cells. Acta Obstet Gynecol Scand. 2007;86:706–710. doi: 10.1080/00016340701314959. [DOI] [PubMed] [Google Scholar]
- 88.Fang L, Yu Y, Zhang R, He J, Sun YP. Amphiregulin mediates hCG-induced StAR expression and progesterone production in human granulosa cells. Sci Rep. 2016;6:24917. doi: 10.1038/srep24917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fang L, et al. Human chorionic gonadotropin-induced amphiregulin stimulates aromatase expression in human granulosa-lutein cells: a mechanism for estradiol production in the luteal phase. Hum Reprod. 2019;34:2018–2026. doi: 10.1093/humrep/dez171. [DOI] [PubMed] [Google Scholar]
- 90.Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187:153–159. doi: 10.1016/S0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 91.Jones KT. Turning it on and off: M-phase promoting factor during meiotic maturation and fertilization. Mol Hum Reprod. 2004;10:1–5. doi: 10.1093/molehr/gah009. [DOI] [PubMed] [Google Scholar]
- 92.Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356:65–73. doi: 10.1016/j.mce.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prochazka R, Blaha M, Nemcova L. Signaling pathways regulating FSH- and amphiregulin-induced meiotic resumption and cumulus cell expansion in the pig. Reproduction. 2012;144:535–546. doi: 10.1530/REP-12-0191. [DOI] [PubMed] [Google Scholar]
- 94.Vaccari S, Weeks JL, 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Norris RP, Freudzon M, Nikolaev VO, Jaffe LA. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140:655–662. doi: 10.1530/REP-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X, Xie F, Zamah AM, Cao B, Conti M. Multiple pathways mediate luteinizing hormone regulation of cGMP signaling in the mouse ovarian follicle. Biol Reprod. 2014;91:9. doi: 10.1095/biolreprod.113.116814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsuji T, Kiyosu C, Akiyama K, Kunieda T. CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol Reprod Dev. 2012;79:795–802. doi: 10.1002/mrd.22114. [DOI] [PubMed] [Google Scholar]
- 98.Hsieh M, Thao K, Conti M. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS ONE. 2011;6:e21574. doi: 10.1371/journal.pone.0021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richards JS, Ascoli M. Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol Metab. 2018;29:313–325. doi: 10.1016/j.tem.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 100.Fulop C, Salustri A, Hascall VC. Coding sequence of a hyaluronan synthase homologue expressed during expansion of the mouse cumulus-oocyte complex. Arch Biochem Biophys. 1997;337:261–266. doi: 10.1006/abbi.1996.9793. [DOI] [PubMed] [Google Scholar]
- 101.Richards JS. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234:75–79. doi: 10.1016/j.mce.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 102.Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–42339. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- 103.Reizel Y, Elbaz J, Dekel N. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol. 2010;24:402–411. doi: 10.1210/me.2009-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fang L, Cheng JC, Chang HM, Sun YP, Leung PC. EGF-like growth factors induce COX-2-derived PGE2 production through ERK1/2 in human granulosa cells. J Clin Endocrinol Metab. 2013;98:4932–4941. doi: 10.1210/jc.2013-2662. [DOI] [PubMed] [Google Scholar]
- 105.Tamura M, Sasano H, Suzuki T, Fukaya T, Funayama Y, Takayama K, Takaya R, Yajima A. Expression of epidermal growth factors and epidermal growth factor receptor in normal cycling human ovaries. Hum Reprod. 1995;10:1891–1896. doi: 10.1093/oxfordjournals.humrep.a136203. [DOI] [PubMed] [Google Scholar]
- 106.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 107.Devoto L, Henriquez S, Kohen P, Strauss JF., 3rd The significance of estradiol metabolites in human corpus luteum physiology. Steroids. 2017;123:50–54. doi: 10.1016/j.steroids.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 108.Practice Committee of the American Society for Reproductive Medicine. Electronic address, A.a.o., Practice Committee of the American Society for Reproductive, M Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106:1634–1647. doi: 10.1016/j.fertnstert.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 109.McClure N, Healy DL, Rogers PA, Sullivan J, Beaton L, Haning RV, Jr, Connolly DT, Robertson DM. Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. Lancet. 1994;344:235–236. doi: 10.1016/S0140-6736(94)93001-5. [DOI] [PubMed] [Google Scholar]
- 110.Nishi Y, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142:437–445. doi: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- 111.Karakida S, Kawano Y, Utsunomiya Y, Furukawa Y, Sasaki T, Narahara H. Effect of heparin-binding EGF-like growth factor and amphiregulin on the MAP kinase-induced production of vascular endothelial growth factor by human granulosa cells. Growth Factors. 2011;29:271–277. doi: 10.3109/08977194.2011.607136. [DOI] [PubMed] [Google Scholar]
- 112.Fang L, Yu Y, Li Y, Wang S, He J, Zhang R, Sun YP. Upregulation of AREG, EGFR, and HER2 contributes to increased VEGF expression in granulosa cells of patients with OHSSdagger. Biol Reprod. 2019;101:426–432. doi: 10.1093/biolre/ioz091. [DOI] [PubMed] [Google Scholar]
- 113.Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- 114.Haimov-Kochman R, et al. Expression and regulation of Sprouty-2 in the granulosa-lutein cells of the corpus luteum. Mol Hum Reprod. 2005;11:537–542. doi: 10.1093/molehr/gah203. [DOI] [PubMed] [Google Scholar]
- 115.Cheng JC, Fang L, Chang HM, Sun YP, Leung PC. hCG-induced Sprouty2 mediates amphiregulin-stimulated COX-2/PGE2 up-regulation in human granulosa cells: a potential mechanism for the OHSS. Sci Rep. 2016;6:31675. doi: 10.1038/srep31675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang B, Li J, Yang Q, Zhang F, Hao M, Guo Y. Decreased levels of sRAGE in follicular fluid from patients with PCOS. Reproduction. 2017;153:285–292. doi: 10.1530/REP-16-0359. [DOI] [PubMed] [Google Scholar]
- 117.Wang B, Wang J, Liu Y, Wang L, Du M, Zhang Z, Guan Y. sRAGE downregulates the VEGF expression in OHSS ovarian granulosa cells. Gynecol Endocrinol. 2021;37:836–840. doi: 10.1080/09513590.2021.1942453. [DOI] [PubMed] [Google Scholar]
- 118.McCartney CR, Marshall JC. CLINICAL PRACTICE. polycystic ovary syndrome. N Engl J Med. 2016;375:54–64. doi: 10.1056/NEJMcp1514916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haouzi D, Assou S, Monzo C, Vincens C, Dechaud H, Hamamah S. Altered gene expression profile in cumulus cells of mature MII oocytes from patients with polycystic ovary syndrome. Hum Reprod. 2012;27:3523–3530. doi: 10.1093/humrep/des325. [DOI] [PubMed] [Google Scholar]
- 120.Ouandaogo ZG, Frydman N, Hesters L, Assou S, Haouzi D, Dechaud H, Frydman R, Hamamah S. Differences in transcriptomic profiles of human cumulus cells isolated from oocytes at GV, MI and MII stages after in vivo and in vitro oocyte maturation. Hum Reprod. 2012;27:2438–2447. doi: 10.1093/humrep/des172. [DOI] [PubMed] [Google Scholar]
- 121.Patil K, Shinde G, Hinduja I, Mukherjee S. Compromised cumulus-oocyte complex matrix organization and expansion in women with PCOS. Reprod Sci. 2022;29:836–848. doi: 10.1007/s43032-021-00775-0. [DOI] [PubMed] [Google Scholar]
- 122.Ambekar AS, et al. Proteomics of follicular fluid from women with polycystic ovary syndrome suggests molecular defects in follicular development. J Clin Endocrinol Metab. 2015;100:744–753. doi: 10.1210/jc.2014-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmidt J, Weijdegard B, Mikkelsen AL, Lindenberg S, Nilsson L, Brannstrom M. Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Mol Hum Reprod. 2014;20:49–58. doi: 10.1093/molehr/gat051. [DOI] [PubMed] [Google Scholar]
- 124.Doherty JA, Peres LC, Wang C, Way GP, Greene CS, Schildkraut JM. Challenges and opportunities in studying the epidemiology of ovarian cancer subtypes. Curr Epidemiol Rep. 2017;4:211–220. doi: 10.1007/s40471-017-0115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen VW, Ruiz B, Killeen JL, Cote TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97:2631–2642. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 126.Romero I, Bast RC., Jr Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology. 2012;153:1593–1602. doi: 10.1210/en.2011-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gurung A, Hung T, Morin J, Gilks CB. Molecular abnormalities in ovarian carcinoma: clinical, morphological and therapeutic correlates. Histopathology. 2013;62:59–70. doi: 10.1111/his.12033. [DOI] [PubMed] [Google Scholar]
- 128.Lassus H, Sihto H, Leminen A, Joensuu H, Isola J, Nupponen NN, Butzow R. Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. J Mol Med (Berl) 2006;84:671–681. doi: 10.1007/s00109-006-0054-4. [DOI] [PubMed] [Google Scholar]
- 129.Yagi H, et al. Clinical significance of heparin-binding epidermal growth factor-like growth factor in peritoneal fluid of ovarian cancer. Br J Cancer. 2005;92:1737–1745. doi: 10.1038/sj.bjc.6602536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carvalho S, et al. An antibody to amphiregulin, an abundant growth factor in patients' fluids, inhibits ovarian tumors. Oncogene. 2016;35:438–447. doi: 10.1038/onc.2015.93. [DOI] [PubMed] [Google Scholar]
- 131.Yotsumoto F, et al. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun. 2008;365:555–561. doi: 10.1016/j.bbrc.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 132.Tanaka Y, et al. Clinical significance of heparin-binding epidermal growth factor-like growth factor and a disintegrin and metalloprotease 17 expression in human ovarian cancer. Clin Cancer Res. 2005;11:4783–4792. doi: 10.1158/1078-0432.CCR-04-1426. [DOI] [PubMed] [Google Scholar]
- 133.Tung SL, et al. miRNA-34c-5p inhibits amphiregulin-induced ovarian cancer stemness and drug resistance via downregulation of the AREG-EGFR-ERK pathway. Oncogenesis. 2017;6:e326. doi: 10.1038/oncsis.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bolitho C, Moscova M, Baxter RC, Marsh DJ. Amphiregulin increases migration and proliferation of epithelial ovarian cancer cells by inducing its own expression via PI3-kinase signaling. Mol Cell Endocrinol. 2021;533:111338. doi: 10.1016/j.mce.2021.111338. [DOI] [PubMed] [Google Scholar]
- 135.So WK, Fan Q, Lau MT, Qiu X, Cheng JC, Leung PC. Amphiregulin induces human ovarian cancer cell invasion by down-regulating E-cadherin expression. FEBS Lett. 2014;588:3998–4007. doi: 10.1016/j.febslet.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 136.Cheng JC, Chang HM, Xiong S, So WK, Leung PC. Sprouty2 inhibits amphiregulin-induced down-regulation of E-cadherin and cell invasion in human ovarian cancer cells. Oncotarget. 2016;7:81645–81660. doi: 10.18632/oncotarget.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.So WK, Cheng JC, Liu Y, Xu C, Zhao J, Chang VT, Leung PC. Sprouty4 mediates amphiregulin-induced down-regulation of E-cadherin and cell invasion in human ovarian cancer cells. Tumour Biol. 2016;37:9197–9207. doi: 10.1007/s13277-016-4790-y. [DOI] [PubMed] [Google Scholar]
- 138.Qiu X, Cheng JC, Klausen C, Fan Q, Chang HM, So WK, Leung PC. Transforming growth factor-alpha induces human ovarian cancer cell invasion by down-regulating E-cadherin in a Snail-independent manner. Biochem Biophys Res Commun. 2015;461:128–135. doi: 10.1016/j.bbrc.2015.03.180. [DOI] [PubMed] [Google Scholar]
- 139.Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal. 2013;11:31. doi: 10.1186/1478-811X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Panupinthu N, et al. Self-reinforcing loop of amphiregulin and Y-box binding protein-1 contributes to poor outcomes in ovarian cancer. Oncogene. 2014;33:2846–2856. doi: 10.1038/onc.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lindzen M, et al. Targeting autocrine amphiregulin robustly and reproducibly inhibits ovarian cancer in a syngeneic model: roles for wildtype p53. Oncogene. 2021;40:3665–3679. doi: 10.1038/s41388-021-01784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Casamassimi A, De Luca A, Agrawal S, Stromberg K, Salomon DS, Normanno N. EGF-related antisense oligonucleotides inhibit the proliferation of human ovarian carcinoma cells. Ann Oncol. 2000;11:319–325. doi: 10.1023/A:1008350811639. [DOI] [PubMed] [Google Scholar]
- 143.Fauser BC. Towards the global coverage of a unified registry of IVF outcomes. Reprod Biomed Online. 2019;38:133–137. doi: 10.1016/j.rbmo.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 144.Zamah AM, Hsieh M, Chen J, Vigne JL, Rosen MP, Cedars MI, Conti M. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod. 2010;25:2569–2578. doi: 10.1093/humrep/deq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu N, Ma Y, Li R, Jin H, Li M, Huang X, Feng HL, Qiao J. Comparison of follicular fluid amphiregulin and EGF concentrations in patients undergoing IVF with different stimulation protocols. Endocrine. 2012;42:708–716. doi: 10.1007/s12020-012-9706-z. [DOI] [PubMed] [Google Scholar]
- 146.Huang Y, Zhao Y, Yu Y, Li R, Lin S, Zhang C, Liu P, Qiao J. Altered amphiregulin expression induced by diverse luteinizing hormone receptor reactivity in granulosa cells affects IVF outcomes. Reprod Biomed Online. 2015;30:593–601. doi: 10.1016/j.rbmo.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 147.Alyasin A, Mehdinejadiani S, Ghasemi M. GnRH agonist trigger versus hCG trigger in GnRH antagonist in IVF/ICSI cycles: a review article. Int J Reprod Biomed. 2016;14:557–566. [PMC free article] [PubMed] [Google Scholar]
- 148.Humaidan P, Westergaard LG, Mikkelsen AL, Fukuda M, Yding Andersen C. Levels of the epidermal growth factor-like peptide amphiregulin in follicular fluid reflect the mode of triggering ovulation: a comparison between gonadotrophin-releasing hormone agonist and urinary human chorionic gonadotrophin. Fertil Steril. 2011;95:2034–2038. doi: 10.1016/j.fertnstert.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 149.Haas J, Ophir L, Barzilay E, Yerushalmi GM, Yung Y, Kedem A, Maman E, Hourvitz A. GnRH agonist vs. hCG for triggering of ovulation–differential effects on gene expression in human granulosa cells. PLoS ONE. 2014;9:e90359. doi: 10.1371/journal.pone.0090359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin MH, Wu FS, Lee RK, Li SH, Lin SY, Hwu YM. Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril. 2013;100:1296–1302. doi: 10.1016/j.fertnstert.2013.07.1976. [DOI] [PubMed] [Google Scholar]
- 151.Haas J, Ophir L, Barzilay E, Machtinger R, Yung Y, Orvieto R, Hourvitz A. Standard human chorionic gonadotropin versus double trigger for final oocyte maturation results in different granulosa cells gene expressions: a pilot study. Fertil Steril. 2016;106:653–659 e1. doi: 10.1016/j.fertnstert.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 152.Practice Committees of the American Society for Reproductive Medicine, t.S.o.R.B., Technologists and the Society for Assisted Reproductive Technology. Electronic address, j.a.o In vitro maturation: a committee opinion. Fertil Steril. 2021;115:298–304. doi: 10.1016/j.fertnstert.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 153.Krisher RL. Present state and future outlook for the application of in vitro oocyte maturation in human infertility treatment. Biol Reprod. 2022;106:235–242. doi: 10.1093/biolre/ioac010. [DOI] [PubMed] [Google Scholar]
- 154.Vuong LN, et al. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: a randomized non-inferiority controlled trial. Hum Reprod. 2020;35:2537–2547. doi: 10.1093/humrep/deaa240. [DOI] [PubMed] [Google Scholar]
- 155.Yang H, et al. Factors influencing the in vitro maturation (IVM) of human oocyte. Biomedicines. 2021;9:1904. doi: 10.3390/biomedicines9121904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kind KL, Banwell KM, Gebhardt KM, Macpherson A, Gauld A, Russell DL, Thompson JG. Microarray analysis of mRNA from cumulus cells following in vivo or in vitro maturation of mouse cumulus-oocyte complexes. Reprod Fertil Dev. 2013;25:426–438. doi: 10.1071/RD11305. [DOI] [PubMed] [Google Scholar]
- 157.Nyholt de Prada JK, Lee YS, Latham KE, Chaffin CL, VandeVoort CA. Role for cumulus cell-produced EGF-like ligands during primate oocyte maturation in vitro. Am J Physiol Endocrinol Metab. 2009;296:E1049–E1058. doi: 10.1152/ajpendo.90930.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guzman L, Adriaenssens T, Ortega-Hrepich C, Albuz FK, Mateizel I, Devroey P, De Vos M, Smitz J. Human antral follicles <6 mm: a comparison between in vivo maturation and in vitro maturation in non-hCG primed cycles using cumulus cell gene expression. Mol Hum Reprod. 2013;19:7–16. doi: 10.1093/molehr/gas038. [DOI] [PubMed] [Google Scholar]
- 159.Richani D, Sutton-McDowall ML, Frank LA, Gilchrist RB, Thompson JG. Effect of epidermal growth factor-like peptides on the metabolism of in vitro- matured mouse oocytes and cumulus cells. Biol Reprod. 2014;90:49. doi: 10.1095/biolreprod.113.115311. [DOI] [PubMed] [Google Scholar]
- 160.Peluffo MC, Ting AY, Zamah AM, Conti M, Stouffer RL, Zelinski MB, Hennebold JD. Amphiregulin promotes the maturation of oocytes isolated from the small antral follicles of the rhesus macaque. Hum Reprod. 2012;27:2430–2437. doi: 10.1093/humrep/des158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ben-Ami I, Komsky A, Bern O, Kasterstein E, Komarovsky D, Ron-El R. In vitro maturation of human germinal vesicle-stage oocytes: role of epidermal growth factor-like growth factors in the culture medium. Hum Reprod. 2011;26:76–81. doi: 10.1093/humrep/deq290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a narrative review based on published data.