Abstract
The success of investigations on the structure and function of the genome (genomics) has been paralleled by an equally awesome progress in the analysis of protein structure and function (proteomics). We propose that the investigation of carbohydrate structures that go beyond a cell’s metabolism is a rapidly developing frontier in our expanding knowledge on the structure and function of carbohydrates (glycomics). No other functional system appears to be suited as well as the nervous system to study the functions of glycans, which had been originally characterized outside the nervous system. In this review, we describe the multiple studies on the functions of LewisX, the human natural killer cell antigen-1 (HNK-1), as well as oligomannosidic and sialic (neuraminic) acids. We attempt to show the sophistication of these structures in ontogenetic development, synaptic function and plasticity, and recovery from trauma, with a view on neurodegeneration and possibilities to ameliorate deterioration. In view of clinical applications, we emphasize the need for glycomimetic small organic compounds which surpass the usefulness of natural glycans in that they are metabolically more stable, more parsimonious to synthesize or isolate, and more advantageous for therapy, since many of them pass the blood brain barrier and are drug-approved for treatments other than those in the nervous system, thus allowing a more ready access for application in neurological diseases. We describe the isolation of such mimetic compounds using not only Western NIH, but also traditional Chinese medical libraries. With this review, we hope to deepen the interests in this exciting field.
Keywords: Glycans, Neurons, Brain, Regeneration, Synaptic plasticity, Glycomimetics
Introduction
Interest in carbohydrates was historically founded on their importance in the physiology of cell metabolism. Research in this area led to important insights into the molecular mechanisms that allow cells to survive by generation of energy via, for instance, glycolysis used by cells to produce the energy source adenosine triphosphate (ATP). Later, the biological value of carbohydrates became apparent in their capacity to represent recognition motifs that allow a multitude of interactions between cells, cells and pathogens, and ligands and their receptors. Carbohydrates (hereafter called more briefly ‘glycans’) can display their motifs in a seemingly unlimited wealth of diverse structures. A multitude of monomers of sophisticated chemistry linked by diverse covalent linkages into short and long linear or branched polymers are attached to protein and lipid backbones. This structural diversity has opened a vast spectrum of individualities at the level of recognition between cells and organisms which has laid the basis of a rapidly expanding platform ushering a new era: glycomics. We expect this era not only to follow, but also to interdigitate with the highly successful eras of genomics and proteomics.
Glycans are biological molecules occurring as monosaccharides or polymers of various combinations of monosaccharides of different lengths. At least several hundred distinct monosaccharides and their derivatives are known with 800 monosaccharides described in the international glycan structure repository (https://glytoucan.org/ [1]). Monosaccharides are either aldehydes or ketones that have multiple hydroxyl groups attached to the asymmetric chiral carbon atoms forming the backbone chain of the molecule (e.g., five groups in the six-carbon monosaccharide glucose). Monosaccharide stereoisomers have the same chemical composition but differ in the position of the hydroxyl groups relative to the chiral carbon atoms and have distinct properties. l and d isomers of the monosaccharide are mirror images differing in the position of the hydroxyl group relative to the carbon before the last carbon atom in the carbon chain. Most of vertebrate monosaccharides are d isomers with the exception of fucose and iduronic acid (IdoA) which are l sugars [2]. Hydroxyl groups in the monosaccharides serve to form glycosidic linkages to different carbon atoms within another monosaccharide resulting in a large number of possible combinations. Further diversity is generated by differences in the glycosidic linkages at the anomeric carbon atoms that can be either in α or β configuration [3]. Formation of three or more glycosidic linkages to one monosaccharide results in the formation of branched glycans [4]. Complex glycans are found in all naturally occurring organisms. Glycosylation in bacteria and archaea is far more diverse, both in terms of diversity of monosaccharides and types of linkages and modifications, than in eukaryotic cells, but is less studied [5].
Glycans can be conjugated by glycosidic bonds to a wide variety of molecules including proteins and lipids, resulting in the formation of glycoproteins and glycolipids. Conjugation via an ether group, i.e., via an oxygen connecting the glycan to another molecule, is called O-glycosylation. This type of glycosylation plays a key role in conjugation of glycans to lipids [6]. In N-glycosidic bonds, the oxygen is replaced by nitrogen. In eukaryotic cells, N-glycosylation and O-glycosylation are the most abundant forms of attachment of glycans to proteins. N-glycosidic bonds between d-glucose (Glc) or N-acetyl-d-glucosamine (GlcNAc) and the amide side chain of asparagine result in N-glycosylation. O-glycosidic bonds between d-galactose (Gal), d-mannose (Man), d-fucose (Fuc), d-xylose (Xyl), d-arabinose (Ara), N-acetyl-d-galactosamine (GalNAc) or GlcNAc and the amino acids containing a hydroxyl group, i.e., serine, threonine, tyrosine, hydroxylysine or hydroxyproline, result in O-glycosylation [7]. C-type glycosylation is also present in many eukaryotic proteins and displays the attachment of a single α-mannopyranosyl residue to carbon atom 2 of the indole nucleus of tryptophan [8].
The central and peripheral nervous system of vertebrates is the predominantly rich source of glycans in comparison to other organs. Large-scale analysis of glycoproteins in the mouse brain shows over 4000 N-glycosylation sites on over 1500 N-glycoproteins [9]. Proteins that play essential roles in brain development, such as cell adhesion molecules of the immunoglobulin superfamily, which contain immunoglobulin and fibronectin-homologous domains, are enriched among the N-glycosylated carriers [9]. Not surprisingly, nearly all glycosylation disorders caused by genetic defects in the activity of enzymes involved in glycosylation pathways are characterized by neurological abnormalities [10], and high N-glycosylation site multiplicity is critical for neural adhesion underlying development [11].
In this review, we summarize current knowledge on glycans highly expressed in the nervous system and their role in neural development and function. We also describe changes in glycan expression in response to pathological conditions and discuss the efficiency of glycomimetic peptides and small organic compounds mimicking glycan structure and functions in improving regeneration of the injured nervous system.
Glycans that are highly expressed in the nervous system
LewisX
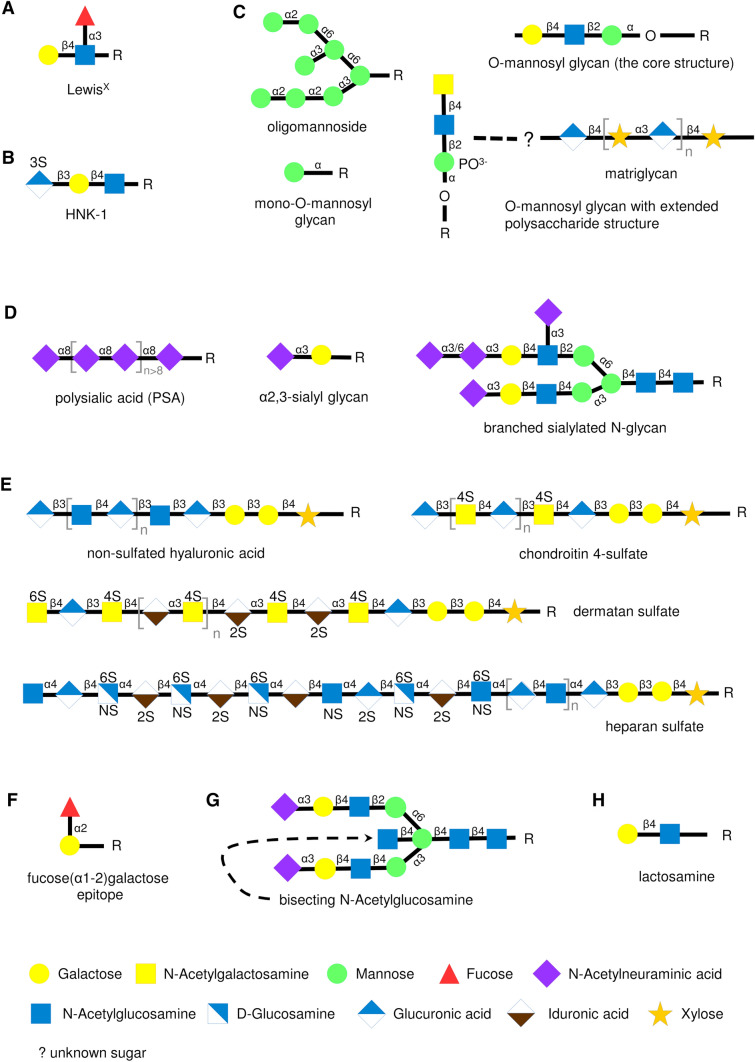
The LewisX structure (Fig. 1a) was identified as an epitope detected by a monoclonal antibody derived by fusion of mouse myeloma cells with spleen cells from a mouse immunized with F9 teratocarcinoma cells. It was originally termed stage-specific embryonic antigen-1 (SSEA-1) or cluster of differentiation 15 (CD15), because the antibody reacted with preimplantation stage mouse embryos [12]. The LewisX epitope is comprised of a trisaccharide (Galβ1-4 (Fucα1-3) GlcNAc). LewisX expression is lost in brains of mice with ablated expression of α1,3-fucosyltransferase 9 (FUT9), indicating that this enzyme is responsible for the biosynthesis of most LewisX structures in the nervous system [13]. LewisX can also be synthesized by α1,3-fucosyltransferase 10 (FUT10) in neural stem cells, where its activity is required for the maintenance of stem cells in an undifferentiated state [14].
Fig. 1.
The structures of the glycan epitopes described in the text. Monosaccharide compositions of the LewisX (a), HNK-1 (b), oligomannosidic glycans, mono-O-mannosyl glycans, the core structure of O-mannosyl glycans, and O-mannosyl glycan with matriglycan (c), PSA, α2,3-sialic acid and branched sialylated N-glycan (d), non-sulfated hyaluronic acid, chondroitin 4-sulfate, dermatan sulfate and heparan sulfate (e), fucose(α1-2)galactose epitope (f), bisecting N-acetylglucosamine (g), and lactosamine (h) are shown
LewisX is present on O- and N-linked glycans in the nervous system [15, 16]. O-mannosylated glycans play a key role in presenting the LewisX epitope: LewisX expression is reduced in mouse brains with ablated expression of O-linked-mannose β1,2-N-acetylglucosaminyl transferase 1 (POMGnT1), a N-acetylglucosaminyl transferase that is essential for the synthesis of O-mannosylated glycans [17].
LewisX carriers
Several proteins have been identified as LewisX carriers, including the astrocytic glycoforms of the cell adhesion molecule CD24 [18], synapsin I [19] and lipoprotein receptor-related protein 1 (LRP1) [20]. The carrier proteins of LewisX change from embryonic toward postnatal central nervous system development [20]. LewisX is detected on phosphacan in the postnatal but not embryonic mouse brain. Similarly, tenascin-C and the L1 cell adhesion molecule (L1) carry more LewisX postnatally than in the embryo [20]. The O-mannose-linked glycans on phosphacan/receptor protein tyrosine phosphatase β (RPTPβ) are the major glycan structures carrying the LewisX epitope in postnatal brains [17] (Fig. 2).
Fig. 2.
Examples of glycan carriers and glycan-mediated interactions in excitatory synapses. The LewisX epitope is carried by RPTPβ, NCAM and L1. LewisX epitopes interact homophilically, and these interactions can potentially strengthen homophilic interactions of NCAM (a), mediate heterophilic interactions of other proteins, such as RPTPβ and L1 (d), or promote clustering of the intracellular LewisX carrier, synapsin I, and synaptic vesicles that it binds to (f). The HNK1 epitope is carried by NCAM, L1, RPTPβ and the GluR2 subunit of the AMPA receptor (AMPAR). It mediates the heterophilic interaction of GluR2 with N-cadherin (c) and can also modulate the homophilic interactions of NCAM (a). L1 is a carrier of oligomannosidic glycans, which mediate its interactions with NCAM (e). Neurexin is a carrier of the heparan sulfate glycosaminoglycan, which binds neuroligins, and may potentially link neurexins to other heparan sulfate binding proteins, such as NCAM (b). NCAM is the major carrier of PSA, which interacts with and potentiates currents through AMPAR (g)
LewisX receptors
LewisX can interact with LewisX, thereby mediating homophilic adhesion [21] and, possibly, clustering of LewisX carriers, such as synaptic vesicle-associated synapsin I (Fig. 2). Contactin-1 and -2 are receptors for the LewisX carried by CD24 [18]. Interestingly, contactin-1 and -2 bind to CD24 and mediate CD24-induced and LewisX-dependent effects on neurite outgrowth of cerebellar and dorsal root ganglion neurons. Yet, only contactin-2 but not contactin-1 interacts with a synthetic LewisX glycan [18], suggesting that binding to the glycan depends on its presentation by the protein backbone that it is attached to. In the developing nervous system, LewisX-presenting molecules bind to and regulate activity of growth factors, including fibroblast growth factor 2 (FGF2) [22, 23] and wingless-type MMTV integration site family member 1 (Wnt-1) [24]. Myelin basic protein (MBP) binds to L1 in a LewisX-dependent manner, leading to the generation of a functionally important L1 fragment [25].
LewisX expression during brain development
During mouse embryonic development, LewisX is expressed already at embryonic day 9.5 (E9.5) by neural progenitor cells, including neural stem cells, neuroblasts and glioblasts, but not by their more differentiated descendants [24, 26]. The LewisX-carrying N-glycans in neural stem cells modulate the expression level of Musashi-1, an activator of the Notch signaling pathway, and thereby promote cell proliferation [27]. During postnatal mouse development, expression of the LewisX epitope increases after birth reaching the highest level at 14 days with high expression levels maintained also in adult brain [17]. Interestingly, different antibodies against the LewisX epitope label different cell populations at postnatal stages, indicating that cells express structurally distinct LewisX glycans [28].
LewisX expression in the adult brain
In the adult mouse nervous system, LewisX expression is particularly strong in glial cells [29] and in neurogenic zones [30].
LewisX in synaptic plasticity
Glycan analysis of chicken synaptic plasma membrane glycoproteins demonstrated that LewisX is one of the most abundant synaptic glycans [31]. Mice lacking FUT9 show reduced numbers of calbindin-positive neurons in the basolateral amygdala and exhibit increased anxiety-like responses in dark–light preference and in elevated plus maze tests [13] suggesting that LewisX plays a role in regulating some aspects of emotional behavior in mice.
LewisX mimicking compounds
Since glycans are metabolically labile, thus leading to their rapid degradation under physiological conditions, it appears important to find ways that allow stabilization of their structural and thus functional properties. This view led to efforts to find compounds that would mimic glycans. First attempts succeeded in identification of more stable compounds by screening phage display libraries that express peptides at the tip of their pili, thus presenting them to an antibody or lectin that recognizes the glycan of interest. In this approach, the glycan is substrate-coated in multiwell plates and detected by the relevant antibodies by enzyme-linked immunosorbent assay (ELISA, see for instance [32]). Mimicking peptides, or later on small organic compounds from libraries commercially available from the National Institutes of Health (NIH) or from traditional Chinese medicine collections, are screened in the so-called competition ELISA. Competition signifies the fact that the glycan-specific antibody is incubated with individual compounds such that the antibody is saturated with a reactive compound, prior to its addition to the glycan substrate-coated ELISA plate. If the compound interacts with the antibody, the antibody is inhibited in its binding to the glycan, representing an endpoint that can easily be measured in multiwell spectrophotometers. Examples for this approach are referenced here and will be referred to in subsequent chapters.
A glycomimetic peptide of LewisX was identified by its inhibition of a LewisX specific antibody to LewisX. Like the LewisX oligosaccharide, the identified LewisX glycomimetic peptide inhibited the CD24-induced neurite outgrowth of cultured cerebellar neurons [33]. Competitive ELISA also served to identify small organic compounds that mimic this glycan. Gossypol, orlistat, ursolic acid, folic acid and tosufloxacin were thereby identified as glycomimetics of LewisX. These enhanced neurite outgrowth and promoted survival of cultured mouse cerebellar neurons by activating distinct intracellular signal transduction pathways, in agreement with the findings on the LewisX mimicking peptide [32].
Human natural killer-1 (HNK-1) glycan
The HNK-1 epitope is recognized by a monoclonal antibody against a membrane antigen on a cultured T cell line and prominently expressed on natural killer cells [34]. The antigenic HNK-1 epitope consists of sulfated glucuronic acid attached to the non-reducing terminal of a N-acetyllactosamine residue (HSO3-3GlcAβ1-3Galβ1-4GlcNAc-) [35, 36] (Fig. 1b). The sulfate moiety is a required epitope for some HNK-1 antibodies, but not for others [35, 36]. HNK-1 biosynthesis depends on the activities of glucuronyl transferase P (GlcAT-P) encoded by the beta-1,3-glucuronyltransferase 1 (B3GAT1) gene [37] and HNK-1 sulfotransferase (HNK1ST) encoded by the carbohydrate sulfotransferase 14 (CHST14) gene [38, 39]. The Galβ1-4GlcNAc structure in HNK-1 is mainly synthesized by beta4-galactosyl transferase-II [40]. In mice deficient in GlcAT-P, the HNK-1 epitope is retained in perineuronal nets [41]. This GlcAT-P-independent HNK-1 epitope is carried by aggrecan and represents a sulfated linkage region comprising HSO3-GlcA-Gal-Gal-Xyl-R in glycosaminoglycans (GAGs) synthesized by glucuronyl transferase GlcAT-I [41].
HNK-1 carriers
HNK-1 glycan is carried by a number of cell adhesion molecules, including neural cell adhesion molecule (NCAM) [42], neural cell adhesion molecule 2 (NCAM2) [43], cell adhesion molecule L1 [42], myelin protein zero (P0) [44] and myelin-associated glycoprotein (MAG) [42]. HNK-1 was therefore suggested to be a marker for adhesion molecules. In agreement with this view was the observation that adhesion molecules contactin-1 and -2 carry HNK-1 [45]. HNK-1 is also carried by components of the extracellular matrix, including chondroitin sulfate proteoglycan [46], tenascin-C and -R [45], aggrecan [41] and phosphacan [47]. Interestingly, also neurotransmitter receptors, such as the glutamate ionotropic receptor alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) type subunit 2 (GluR2) [48], and receptor-associated glycolipids are HNK-1 positive [35]. Of note also, early studies demonstrated that HNK-1 is present on some but not all NCAM molecules, suggesting that this specialization results in molecular and thus functional heterogeneity [42].
HNK-1 receptors
Laminin was the first receptor discovered for HNK-1 [49]. The binding domain of 21 amino acids [50] was thereafter discovered in the adhesion molecule N-cadherin, the receptor of advanced glycation end products (RAGE) and high mobility group box 1 (HMGB1) (also called amphoterin) which also function as HNK-1 receptors [51, 52]. In developing neurons, contactin-1 at the neuronal surface is a receptor for HNK-1 carried by tenascin-C [45]. Interestingly, the neurite-promoting activity of HNK-1 attached to tenascin-C requires neuronal HNK-1 expression [45]. In excitatory synapses of mature neurons, N-cadherin is a receptor for HNK-1 carried by GluR2 [48]. In inhibitory perisomatic synapses, HNK-1 binds to gamma aminobutyric acid B (GABAB) receptors [53]. Similar to the homophilic feature of LewisX, HNK-1 may also engage in homophilic adhesion and in mediating homophilic interaction of the immunoglobulin superfamily peripheral nervous system myelin glycoprotein P0 [54]. HNK-1 may also be involved in regulation of NCAM-mediated homophilic adhesion [55], indicating that NCAM and P0 are not only carriers but also receptors for HNK-1. This feature will have to be verified using the glycans without their protein backbones to which they are attached.
HNK-1 expression during brain development
Antibodies against HNK-1, HNK-1 carrying glycolipids and the HNK-1 glycan itself inhibit neuron-to-neuron, neuron-to-astrocyte, astrocyte-to-astrocyte adhesion and neurite outgrowth [56, 57], indicating that HNK-1 is required for interactions between different neural cell types and may therefore be essential for brain development. In mice, HNK-1 is expressed starting from embryonic day 11 in most regions of the developing nervous system, with high levels in postmigratory cells located around proliferative zones [58]. HNK-1 expression peaks at postnatal day 0 and gradually decreases in adulthood [40, 58]. Mouse embryonic neural stem cells express HNK-1, which is mostly carried by tenascin-C, and HNK-1 promotes neural stem cell proliferation by enhancing expression of the epidermal growth factor receptor and inducing rat sarcoma viral oncogene homolog (Ras)—mitogen-activated protein kinase (MAPK) activation [59]. In the developing sciatic nerve, HNK-1 is expressed before myelination by both motor and sensory fibers. However, starting from 8 weeks after birth, HNK-1 is mostly associated with compact myelin associated with motor fibers and is largely excluded from sensory non-myelinated axons [60].
HNK-1 expression in the adult brain
HNK-1 accumulates in synapses at early postnatal ages, thereafter declining in adult mouse brains [48]. In the adult hippocampus, HNK-1 is highly expressed at the cell surface of parvalbumin-positive somata of interneurons and at the terminal fields of their axons surrounding somata of neurons in the pyramidal cell layer [61]. In the adult cerebellum, HNK-1 is expressed in stripes of Purkinje cells in the molecular layer and by Golgi cells [62] possibly indicating topographic specificity in function. In NCAM knock-out mice, HNK-1 expression in Purkinje cells is lost, indicating that NCAM is the major carrier of HNK-1 in these cells [62].
The role of HNK-1 in synaptic plasticity and Alzheimer’s disease
Acute antibody-mediated perturbation of HNK-1 functions in mice with antibodies against the HNK-1 epitope inhibits GABAA-dependent perisomatic inhibitory postsynaptic currents and enhances long-term potentiation (LTP), which is reduced by a HNK-1 mimetic peptide [61]. HNK-1 antibodies do not affect inhibitory currents in tenascin-R knock-out mice, suggesting that covalent attachment of HNK-1 to tenascin-R is important for synaptic plasticity. Further, a HNK-1 antibody constitutively activates GABAB receptors, whereas synthetic HNK-1 and HNK-1 mimetic peptide inhibit activity of GABAB receptors, suggesting that HNK-1 regulates the homeostasis of GABAA receptor-mediated perisomatic inhibition by suppressing postsynaptic GABAB receptor activity [53]. Intra-hippocampal administration of an antibody against HNK-1 impairs memory consolidation in a learning task in mice [63]. Similarly, antibodies against HNK-1 inhibit memory consolidation after active avoidance conditioning in zebrafish [64].
In mice deficient in HNK-1 sulfotransferase, basal synaptic transmission in pyramidal cells of hippocampal CA1 is increased, LTP is reduced, and long-term memory and spatial learning are impaired [65]. Similarly, LTP at Schaffer collateral-CA1 synapses is reduced in GlcAT-P-deficient mice, leading to defective spatial memory formation [66]. β4-galactosyltransferase II-deficient mice also show impaired spatial learning and memory as well as abnormal motor coordination [40]. In GlcAT-P-deficient mice, pyramidal neurons are characterized by higher densities of filopodium-like immature spines, suggesting that HNK-1 promotes memory formation by regulating spine morphogenesis [67]. Also, HNK-1 is attached to GluR2 and promotes interaction with N-cadherin and cell surface expression of GluR2 [48].
Levels of HNK-1 are decreased in the brain of individuals with Alzheimer’s disease, in amyloid precursor protein (APP)-overexpressing transgenic mice and in cultured human neuroblastoma cells and cortical neurons treated with Aβ [68, 69], suggesting that HNK-1 biosynthesis is disrupted in this disease. Alzheimer’s disease is also associated with increased proteolysis of several adhesion molecules, including HNK-1 carriers NCAM, NCAM2 and L1 [70, 71]. How the loss of HNK-1 contributes to synapse dysfunction and loss of spines and synapses in Alzheimer’s disease remains to be investigated.
The role of HNK-1 in regeneration after trauma
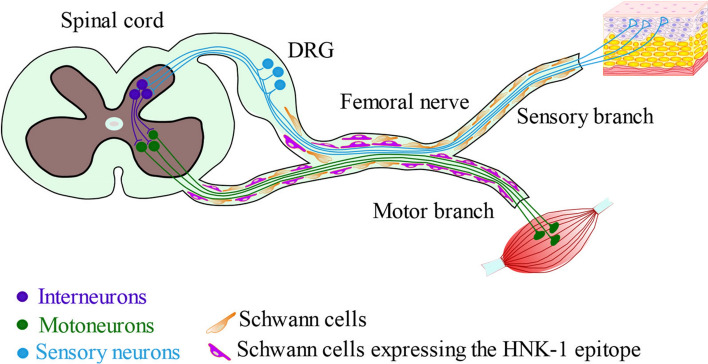
HNK-1 is carried by MAG in Schwann cells associated with motor, but remarkably not sensory axons in the femoral nerves of adult mice [72] (Fig. 3). After transection, motor axons preferentially reinnervate distal motor branches, a process termed “preferential motor reinnervation”. Regrowing axons from the muscle branch induce HNK-1 expression by Schwann cells of the muscle branch, which promotes preferential regrowth of motor axons into the muscle rather than sensory branch, but does not affect the growth of sensory neurons [73, 74]. After nerve transection, the density of HNK-1 positive structures in the distal nerve branches declines, and this decline can be prevented by short-term low-frequency electrical stimulation of the lesioned and surgically repaired femoral nerve. Preferential motor reinnervation and functional recovery are reduced in heterozygous brain-derived neurotrophic factor (BDNF) or tropomyosin receptor kinase B (TrkB)-deficient mice indicating that BDNF/TrkB signalling is involved in the control of HNK-1 expression [75].
Fig. 3.
Expression of the HNK-1 epitope in the femoral nerve. HNK-1 is expressed by Schwann cells in the motor branch of the femoral nerve innervating the skeletal muscle, but not in the sensory branch innervating the sensory organs. DRG, dorsal root ganglion
In the mouse sciatic nerve, HNK-1 is expressed starting at the end of the second post-natal week and is localized on the outer profiles of thick myelin sheaths. Transection of the sciatic nerve results in the loss of HNK-1 expression, which recovers only after the nerve fascicles return to the normal state of myelination at up to 1 year after transection [76].
In zebrafish, levels of glucuronyl transferase and HNK-1 sulfotransferase are increased in neurons that are intrinsically capable of regeneration. Reducing levels of the glucuronyl transferase but not HNK-1 sulfotransferase by application of anti-sense morpholinos results in reduction of locomotor recovery, indicating that HNK-1 contributes to regeneration after spinal cord injury in adult zebrafish in the absence of its sulfate moiety [77].
HNK-1 glycomimetics
The HNK-1 mimetic peptide was isolated using the phage display method using a HNK-1 antibody [78]. The peptide binds to motor neurons, preferentially promotes outgrowth of motor rather than sensory axons in vitro [78] and promotes functional recovery after transection of the femoral nerve in mice [79] and monkeys [80].
Although the HNK-1 mimetic peptide does not improve locomotor recovery after mouse spinal cord injury, it improves myelination in the proximal part of the lesion site [81]. Yet, the HNK-1 mimetic small compound ursolic acid promotes functional recovery in spinal cord injured mice [82].
Oligomannosidic glycans
Research on oligomannosidic glycans (Fig. 1c) was facilitated by the development of monoclonal antibodies recognizing N-linked oligomannosidic glycans which were raised by immunizing rats with membrane glycoproteins from the mouse brain [83]. The findings that oligomannosides are present at the cell surface were unexpected, since this glycan was considered mainly to be a transient structure initiating glycan synthesis in the Golgi apparatus, where N-glycosidic synthesis on glycoproteins and glycolipids is kicked off [84].
Carriers of oligomannosidic glycans
Synapsin 1 was identified as a major carrier of oligomannosidic glycans in the mouse brain [19]. Oligomannosidic glycans are also carried by some adhesion molecules including L1, ATPase Na+/K+ transporting subunit beta 2 [also called adhesion molecule on glia (AMOG)] and MAG, but not by NCAM and tenascin-C [85, 86]. Oligomannosidic glycans are also detected on other molecules at the cell surface, such as the dopamine D1 receptor [87].
Receptors for oligomannosidic glycans
The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans, which mediate its interaction with L1 [85] and synapsin 1 [19]. A similar domain is present in the first immunoglobulin-like domain of basigin, which binds to oligomannosidic glycans carried by L1, MAG and AMOG [88]. Synapsin 1 not only carries oligomannosidic glycans, but also is an oligomannose-binding lectin [19]. The high-molecular weight complexes with proteins carrying oligomannosidic glycans contain the cellular prion protein (PrPc) adhesion molecule [89]. Whether PrPc binds to oligomannosidic glycans is unknown.
Expression of oligomannosidic glycans during brain development
In cultures of mouse cerebellar cells, oligomannosidic glycans are expressed by neurons and a subpopulation of oligodendrocytes, but not by astrocytes. These glycans are not detectable on cultured dorsal root ganglia neurons and Schwann cells from the peripheral nervous system [86]. The levels of oligomannosidic glycans in mouse brain are developmentally regulated and decline during development [90].
Expression of oligomannosidic glycans in the adult brain
Oligomannosidic glycans constitute approximately 15% of the total pool of N-glycans in the adult rat brain [15]. Oligomannosidic glycans with 9, 8, 7, 6 and 5 mannose residues are the major glycans accumulating in synapses of chicken [31]. Glycans containing five mannoses are the predominant species in adult synapses whereas synaptic levels of glycans with eight mannoses decrease during development [91].
The role of oligomannosidic glycans in synaptic plasticity
Soluble oligomannosides inhibit theta-burst stimulation-induced LTP in rat CA1 hippocampal neurons [92]. Peptides derived from the fourth immunoglobulin-like domain of NCAM containing the lectin binding domain and mediating the interaction with oligomannosides also partially inhibit LTP induction and maintenance [92]. Oligomannosidic glycans are highly expressed in the brain regions involved in the motor control of song production in zebra finches and their expression is enhanced after subcutaneous injection of testosterone [93].
The role of oligomannosidic glycans in regeneration after trauma.
Peptides carrying oligomannosidic glycans infused into the tectum of goldfish interfere with the sharpening of retinotectal projections that regenerate after optic nerve crush, indicating that the regrowth of axons depends on oligomannosidic glycans for accuracy in regeneration [94].
Oligomannose mimicking compounds
The oligomannose-recognizing antibodies were used to identify the oligomannose-mimicking peptide TISWWHLWPSPA in a phage display screen [19]. This peptide stimulates neurite outgrowth and disturbs adhesion between neurons and astrocytes [19].
Polysialic acid
The observation that the apparent molecular weight of the embryonic form of NCAM is higher than the molecular weight of the adult form led to the discovery of polysialic acid (PSA), a unique carbohydrate predominantly carried by NCAM [95]. PSA constitutes approximately 30% of embryonic NCAM’s apparent molecular weight, whereas in the adult brain only 10% of NCAM molecules carry this carbohydrate [95, 96]. PSA is an N-linked polymer of α2-8 linked neuraminic acid units [96, 97] (Fig. 1d). The core structures of PSA comprise predominantly fucosylated, partially sulfated 2,6-branched isomers of triantennary and tetraantennary complex-type glycans [98, 99]. PSA is synthesized in the Golgi apparatus and takes as much as several days to reach the cell surface [100]. Polysialylation of NCAM is catalyzed by two polysialyltransferases, ST8Sia II [also called sialyltransferase X (STX)] and ST8Sia IV [also called polysialyltransferase-1 (PST)] [101, 102], which belong to the family of α2,-sialyltranferases [103]. While STX is primarily expressed in embryonic tissues, PST is expressed in the adult brain [104]. Polysialylation depends on the highly specific recognition of NCAM by polysialyltransferases. Polysialyltransferase ST8Sia-IV binds to the first fibronectin type III repeat and fifth immunoglobulin-like domain of NCAM [105], and NCAM binds to the polybasic region of ST8Sia-IV [106]. Thus, complex interactions determine PSA functions.
Carriers of the polysialic acid
A comparison of NCAM and PSA concentrations in the brain led to the conclusion that NCAM is the major carrier of PSA in the brain [96]. In agreement with this idea, nearly all PSA expression is lost in NCAM knock-out mice [107]. In lower amounts, PSA is also found on voltage-sensitive Na channels [108, 109], on N-glycans of the first immunoglobulin-like domain of the synaptic cell adhesion molecule SynCAM1 [110] and neuropilin 2 [111].
Binding partners of PSA
Multiple functions of PSA have become evident in many studies: aggregation of lipid vesicles containing the embryonic form of NCAM is strongly reduced when compared to the aggregation of vesicles with the adult form of NCAM. This effect is lost after removal of PSA by treating vesicles with neuraminidase resulting in a fourfold increase in vesicle aggregation, indicating that PSA reduces homophilic interactions of NCAM [112, 113]. PSA also inhibits homophilic interactions of SynCAM1 [110]. Injection of endoneuraminidase into the eye of 3.5-day-old chicken embryos results in abnormalities consistent with abnormally increased adhesion between cells, such as thickening of the neural epithelium in the posterior eye, showing a failure of cells to elongate radially and form an ectopic optic fiber layer [113] thereby indicating that lack of PSA results in enhanced adhesion (see also below). Interestingly, PSA can also promote adhesion. Adhesion of PSA-expressing mouse F11 cells and chick embryonic brain cells to the heparan sulfate proteoglycans agrin and 6C4 is inhibited by PSA and antibodies against PSA, indicating that PSA can either bind to these proteoglycans or promote binding between these proteoglycans and the NCAM heparin-binding domain [114].
Cell surface-exposed PSA binds to the neurotrophins brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin (NT)-3, and NT-4 [115]. PSA-bound BDNF can interact with and activate BDNF receptors, trkB and p75NTR [115]. Enzymatic removal of PSA results in a decreased activity of trkB and addition of BDNF restores trkB activation [116]. PSA potentiates synaptic AMPA receptor currents [117] (Fig. 2) and reduces the glutamate-elicited extrasynaptic N-methyl-d-aspartate (NMDA) receptor currents [118, 119] via mechanisms probably involving direct PSA-AMPA receptor and PSA-NMDA receptor interactions. PSA also binds to the neurotransmitter dopamine [120].
PSA can bind to the antennapedia homeodomain peptide, a synthetic peptide that corresponds to the homeodomain sequence of antennapedia protein (pAntp), which is captured by neurons, delivered to the nucleus, where it induces morphological differentiation of neurons [121]. Structural analysis suggests that a sequence of eight sialic acid residues mimics one large groove of the deoxyribonucleic acid (DNA) double helix allowing binding of the homeodomain peptide [121]. Cellular secretion of homeodomain proteins may play an important role in cell–cell interactions [122]. Yet, interactions of PSA with homeodomain proteins and other transcription factors remain to be characterized. Recent studies show that PSA is delivered from the cell surface via importins into the cell nucleus, where PSA regulates circadian rhythm activities [123, 124]. PSA can bind to DNA and DNA-binding proteins involved in gene expression regulation, including histones and lactoferrin [125]. These findings suggest that PSA may function in the nucleus by regulating transcription. Another intracellular binding partner of PSA is myristoylated alanine-rich C kinase substrate (MARCKS), which is required for PSA-dependent neurite outgrowth [126]. That a cell surface glycan can interact with an intracellular binding partner is a noteworthy feature of glycan biology: It shows that PSA penetrates the surface membrane, possibly by exhibiting hydrophobic characteristics in a helical conformation, allowing interaction with the hydrophobic cell surface plasma membrane.
PSA expression in brain development
The essential role of PSA in brain development is underscored by studies showing that high levels of sialic acid are present in human milk [127] and that sialic acid as a milk supplement promotes expression of PST in piglets [128]. Expression of PST and STX in mouse brain starts at embryonic day 9, and, accordingly, PSA is not expressed during the early phases of neurogenesis in the developing brain [129–132], while levels of PSA increase starting at embryonic day 9 in neuronal and glial membranes in areas which are enriched in moving cells [129, 133–135]. Levels of STX decline within 10 days after birth, whereas PST expression persists in the adult brain [136].
At the subcellular level, PSA expression is higher in growth cones and filopodia of developing neurons than, for instance, immobile somata [137] in agreement with the view that PSA is important for cell migration, neuritogenesis and guidance of neurites. In agreement, removal of PSA from NCAM results in accumulation of olfactory precursors in the subependymal germinal cell layer and reduces formation of growth cone-like processes oriented along the cell migration route [138]. PSA also increases collateral sprouting and defasciculation of mossy fibers and leads to aberrant innervation of the hippocampal pyramidal cell layer by mossy fibers [139]. PSA-NCAM also promotes survival of neuronal progenitors, and lack of PSA reduces the survival of newly generated neurons [140].
Negative charge and bulky hydration of PSA can impede molecular interactions between apposing membranes and, specifically, interactions of NCAM with other proteins at the cell surface in cis- or trans-interactions [141]. Simultaneous deletion of murine PST and STX results in a severely abnormal phenotype showing neuronal network wiring defects, progressive hydrocephalus, postnatal growth retardation and precocious death [142]. In the forebrain of doubly deficient mutants, the following abnormal phenotypes are observed: apoptotic cell death, defects in tangential and radial migration of neural precursors during development, aberrant positioning of neuronal and glial cells, and increased glial cell differentiation [143]. These abnormalities are rescued by deletion of NCAM, demonstrating that they are due to aberrant interactions of NCAM with presently unknown binding partners that depend on PSA [142].
PSA expression in the adult brain
PSA expression is retained in the adult brain, but its distribution is restricted to cell populations and cellular domains undergoing remodeling. For example, while PSA is expressed broadly in hippocampus and substantia nigra in the brains of 3-day-old rats, punctate PSA immunoreactivity is found in 2- to 3-month-old rats, probably representing synapses [133]. PSA expression is retained in the functionally active adult brain structures: olfactory bulb and piriform cortex in neurons of layer II, which receive input from the olfactory bulb, where neurogenesis and formation of neural circuits take place [129, 135]; in the supraoptic and paraventricular nuclei of the hypothalamus and in the neurohypophysis, which undergo neuronal-glial and synaptic rearrangements in response to physiological stimuli [144]; in the hippocampal dentate gyrus, where postnatal neurogenesis continues into adulthood in the deep part of the granule cell layer [145]. At the subcellular level, PSA is often found in synapses, for example in some, but not all spine synapses in the outer third of the molecular layer of the dentate gyrus [146], suggesting that PSA contributes to synaptic activity and plasticity.
PSA in synaptic plasticity
PSA is selectively expressed in immature synaptic boutons of hippocampal mossy fibers, whereas mature synaptic boutons are negative for PSA, indicating that PSA expression is required for remodeling of synapses [147]. Polysialylation of NCAM transiently increases during learning in multiple brain regions suggesting that PSA is involved in learning and memory. Polysialylation of the largest postsynaptic isoform of NCAM, NCAM180, transiently increases in a subpopulation of granule-like cells in the dentate gyrus of adult rats during acquisition and consolidation of a passive avoidance response [148, 149]. Spatial learning also induces polysialylation of NCAM in the hippocampal CA1-CA3 regions and in the enthorinal cortex [150].
Expression of PSA-NCAM is increased in response to NMDA receptor activation in oligodendrocyte precursor cells [151]. However, PSA is also synthesized in a NMDA receptor-independent manner in the dentate gyrus of rats following LTP induction [152]. Polysialylation of NCAM is negatively regulated by protein kinase C delta, which phosphorylates polysialyltransferases [153]. PSA expression can therefore also be regulated by downregulation of this signal transduction pathway. STX and PST mRNA levels increase after induction of LTP [154] suggesting that polysialylation is also regulated at the transcriptional level via the relevant glucoronyl synthesizing enzymes and sulfunoryl transferases.
Acquisition and retention of the spatial memory in the Morris water maze are impaired by localized enzymatic removal of PSA in the adult rat hippocampus, whereas visual and motor functions are not affected [155]. These changes correlate with defects in synaptic plasticity induced by removal of PSA. Tetanic stimulation-induced LTP in Schaffer collaterals is blocked in hippocampal slices of adult rats after enzymatic removal of PSA [155]. Similarly, enzymatic removal of PSA results in inhibition of LTP and long-term depression (LTD) in hippocampal organotypic cultures [156].
NCAM knock-out mice are impaired in spatial learning in the Morris water maze, whereas activity and motor abilities appear normal [107]. Similarly, ST8SialV/PST-1 knockout mice are reduced in spatial and reversal learning [157]. LTP and LTD in the synapses of Schaffer collaterals, which express PSA in the wild types, are impaired in adult PST knock-out mice, whereas these forms of synaptic plasticity are not affected in young PST knock-out animals [158]. In contrast, loss of STX does not impair hippocampal synaptic plasticity, but instead results in misguidance of infrapyramidal mossy fibers and abnormal formation of ectopic hippocampal synapses [159].
Enzymatic removal of PSA with endoneuraminidase-N abolishes preferential formation of synapses on NCAM-expressing cells in heterogenotypic cocultures of wild-type and NCAM-deficient hippocampal neurons and blocks the increase in numbers of perforated spine synapses associated with NMDA receptor-dependent LTP in CA1 of organotypic hippocampal cultures, indicating that PSA-NCAM promotes synaptogenesis and activity-dependent remodeling of synapses [160]. PSA carried by NCAM also regulates plasticity and learning by inhibiting the GluN2B-Ras-GRF1-p38 MAPK signaling pathway [119]. In the cortex, NCAM and ephrinAs/EphA3 constrain GABAergic interneuronal arborization and perisomatic innervation, and PSA is required for ephrinA5-induced axon remodeling of basket interneurons [161]. Enzymatic removal of PSA results in increased spine density on spiny interneurons in the hippocampus, indicating that PSA is also involved in regulating inhibitory connectivity [162].
PSA is also involved in other types of neural plasticity. Suprachiasmatic nuclei express high levels of PSA-NCAM, and enzymatic removal of PSA disrupts circadian rhythmicity [163] and reduces photic induction of FBJ murine osteosarcoma viral oncogene homolog (Fos) and light-induced phase-resetting of the circadian locomotor activity rhythm [164]. Levels of PSA-NCAM decrease during lactation in supraoptic nuclei and neurohypophysis and return to initial levels after weaning, whereas levels of total NCAM protein are not affected [165]. Enzymatic removal of PSA by microinjection of endoneuraminidase close to the hypothalamic magnocellular nuclei prevents withdrawal of astrocytic processes and increase in synaptic contacts normally induced by lactation and dehydration [166].
PSA in regeneration after trauma and in brain disorders
Following injury, PSA is re-expressed in neurons in organotypic hippocampal cultures, where CA3-CA1 synapses express PSA, being required for axonal regrowth and formation of new synapses [167]. Similarly, lesions in the sensorimotor cortex of adult rats induce an increase in the levels of NCAM-PSA in the subventricular zone [168]. After femoral nerve injury, axons of the motor neurons and, to a lesser extent, sensory neurons re-express high levels of PSA. Preferential motor reinnervation is inhibited in NCAM-deficient mice and wild-type mice after enzymatic removal of PSA from the regrowing axons, indicating that PSA promotes correct regeneration [169].
These observations led to the idea that regeneration can be improved by increasing levels of PSA at the injury site. Indeed, virus-mediated overexpression of polysialyltransferase in glial scar astrocytes results in sustained expression of high levels of PSA and promotes regrowth of severed corticospinal tract axons through the injury site [170]. Regeneration of cortical lesions is also improved when PSA is overexpressed in a pathway extending from subventricular zone to the lesion site, thereby allowing better migration of precursor cells [170]. While Purkinje cells are generally unable to survive after axotomy, transplanted Schwann cells overexpressing PST promote axonal regrowth from Purkinje cells after a cerebellar stab wound [171]. It should be noted, however, that persistent overexpression of PSA may be detrimental, because down-regulation of polysialic acid is required for efficient myelin formation, and continuous PSA overexpression causes axonal degeneration in transgenic mice expressing the polysialyltransferase ST8SiaIV under control of the myelin-specific proteolipid protein promoter [172].
PSA expression is increased in the hippocampus and entorhinal cortex of humans with temporal lobe epilepsy, a condition associated with neuronal loss and axonal sprouting [173]. Enzymatic removal of PSA counteracts the status epilepticus-induced increase in neurogenesis in rats. Although absence of PSA does not impact the development of spontaneous seizures in this animal model, it reduces cognition deficits in the Morris water maze paradigm, suggesting that transient modulation of NCAM polysialylation may be beneficial for ameliorating long-term deficits [174]. Levels of PSA are increased in the hippocampus of Alzheimer’s disease patients [175] suggesting attempts for remodeling by surviving neurons; however the disease is also associated with loss of PSA due to loss of PSA-positive interneurons in the entorhinal cortex [176]. PSA expression is increased in the substantia nigra pars reticulata of some patients with Parkinson’s disease [177], i.e., in the brain region characterized by extensive loss of dopaminergic neurons. This increase could be explained by an attempt to counteract cell death by enhancing synaptic plasticity in surviving neurons. Changes in PSA levels also occur in schizophrenia and other mental disorders (extensively reviewed in [178]).
PSA mimicking compounds
A PSA mimicking peptide was identified by screening of a linear 12-mer phage display peptide library with a PSA-specific monoclonal antibody. This PSA mimetic peptide, applied in a polyethylene cuff used to surgically reconnect the severed stumps of the femoral nerve before it bifurcates into the motor and sensory branches, improves motor recovery by enhancing Schwann cell proliferation and remyelination of regenerated axons distal to the injury site [179]. Interestingly, this peptide acts via NCAM and fibroblast growth factor receptor (FGFR) since it cannot enhance proliferation of NCAM-deficient Schwann cells, and its effects are reduced by FGFR inhibitors [179]. Further research identified several small organic compounds (Fig. 4), which mimic the neurostimulatory functions of PSA, promote nervous system repair and stimulate neuronal survival and neurite outgrowth by signaling via protein kinase C [180–182].
Fig. 4.
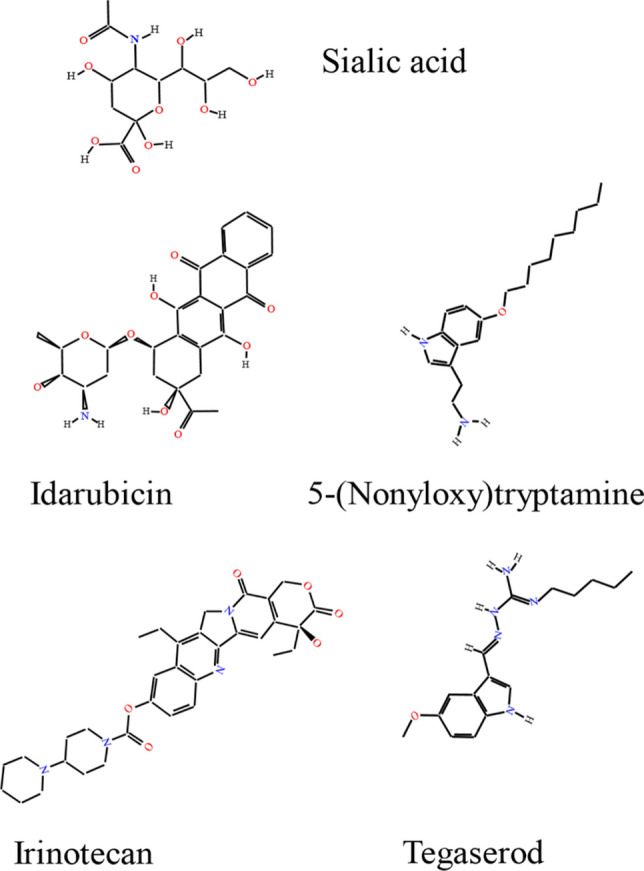
The structure of the sialic acid and small organic compounds mimicking the neurostimulatory functions of PSA
Glycosaminoglycans
Glycosaminoglycans (GAGs) or mucopolysaccharides were first detected biochemically and immunocytochemically in nervous tissue in the 1950s and 1960s [183, 184]. These early studies showed that non-sulfated hyaluronic acid and chondroitin 4-sulfate are the most abundant GAGs, while chondroitin 6-sulphate, dermatan sulfate and heparan sulfate are present at lower levels [185, 186]. GAGs are long chains of repeating disaccharide units with disaccharides of glucuronic acid and N-acetylgalactosamine found in chondroitin sulfate and dermatan sulfate, while disaccharides of glucuronic acid and N-acetylglucosamine are found in heparan sulfates and hyaluronic acid (Fig. 1e). In dermatan sulfate, disaccharides containing iduronic acid instead of glucuronic acid are also present at varying proportions.
Carriers of GAGs and GAG sulfation
GAG biosynthesis is initiated via the addition of a linker tetrasaccharide consisting of xylose, two galactose units and a glucuronic acid to a serine residue in the core protein, followed by addition of disaccharide units and resulting in formation of proteoglycans [187]. GAG chains are then sulfated in the Golgi apparatus by sulfotransferases, which transfer sulfate groups to specific positions on the sugar moieties in some but not all monosaccharides in GAG chains, resulting in different sulfation patterns which are the basis of appropriate function [188]. Mature proteoglycans can either be cell membrane-bound (e.g., syndecans, receptor-type protein tyrosine phosphatase zeta (PTPRZ)) or form part of the extracellular matrix (e.g., neurocan, agrin, phosphacan) (reviewed in [189]). Sulfation of heparan sulfates in the extracellular space is further regulated by extracellular sulfatases, which remove sulfate from GAGs [190]. Interestingly, a recent study reported that heparan sulfate can also be carried by the synaptic adhesion molecules neurexins [191]. Whether other adhesion molecules carry GAGs is an intriguing question for future research.
GAG expression in brain development
Early studies noted already that levels of mucopolysaccharides and rates of their synthesis measured by estimating incorporation of [3H]glucosamine into GAGs increase transiently within several days after birth and then decline with age until reaching a plateau in the adult brain [185, 192], suggesting that GAGs are important for brain development. Further studies on changes in GAG levels induced by enzymes degrading specific GAGs or via application of purified GAGs reported that these manipulations cause defects in axonal growth, guidance and fasciculation verifying that GAGs are important for brain development (reviewed in [189]). Early studies also reported that an enzyme incorporating 35S into rat brain mucopolysaccharides is more active at birth and decreasingly in age, suggesting that sulfation of mucopolysacchirides is developmentally regulated [193]. The importance of GAG sulfation during development has been extensively reviewed [194]. Not only is the GAG sulfation rate during biosynthesis important, but also crucial is sulfation pattern remodeling by extracellular sulfatases Sulf1 and Sulf2, which specifically remove 6-O-sulfate groups from heparan sulfate chains [195]. Neurite outgrowth in cultures of hippocampal and cerebellar neurons from Sulf1 and Sulf2 knock-out mice is decreased, and Sulf2 deficiency causes higher embryonic lethality and congenital hydrocephalus in mice [196].
GAG expression in adult brain, synaptic plasticity and brain disorders
In the adult brain, GAG-containing proteoglycans show diffuse and condensed forms of extracellular matrix deposition. Condensed extracellular matrix is observed in perineuronal nets and at the nodes of Ranvier of myelinated axons, whereas the diffuse form is seen in the neuropil (reviewed in [197]). Early studies showed that GAGs are present at high levels in isolated synaptosomes and synaptic vesicles [198]. Severe neurological impairment in cats caused by removal of GAGs by chronic administration of testicular hyaluronidase to the brain was described in probably the first study, demonstrating the importance of GAGs for the normal brain function [199]. Following work showed that GAGs are important modulators of the structural and functional synaptic plasticity [197]. For example, enzymatic digestion of chondroitin sulfates promotes motility and functional plasticity of dendritic spines in the visual cortex of adult mice indicating that chondroitin sulfate proteoglycans restrain structural changes [200]. A developmental increase in the 4-sulfation/6-sulfation ratio of chondroitin sulfate proteoglycans leads to the termination of the critical period for ocular dominance plasticity in the mouse visual cortex [201]. Spine density and LTP are impaired in the CA1 region of Sulf1-deficient mice, and Sulf1 and Sulf2-deficient mice are characterized by behavioral alterations, further indicating that remodeling of sulfation is involved in regulation of synaptic plasticity [196]. Mice lacking heparan sulfate chains on neurexin-1 also show structural and functional deficits at central nervous system synapses [191]. The importance of GAG remodeling in normal brain function is highlighted by the fact that impaired GAG degradation, caused by deficiencies in lysosomal enzymes, results in mucopolysaccharidosis characterized by short life span, various multiple anatomical changes, and severe cognitive and behavioral disturbances (reviewed in [202, 203]). Altered abnormal expression of GAGs has also been found in mental and neurodegenerative disorders, including schizophrenia and Alzheimer’s disease (reviewed in [204]).
Receptors for GAGs
GAG functions in the developing and adult nervous system depend on a large number of proteins that GAGs interact with at the neuronal cell surface and in the extracellular matrix. The binding partners include cell adhesion molecules, receptor protein tyrosine phosphatases, growth factors and their receptors, and other proteins (summarized in [194, 205, 206]). In addition to limiting structural changes, GAGs of the extracellular matrix limit the mobility of proteins at cell surface membranes and regulate their functions. For example, GAGs limit the mobility of AMPA receptors at synapses [207]. The chondroitin sulfate proteoglycan neurocan inhibits spine remodeling by binding to synaptic adhesion molecule NrCAM and blocking the semaphorin3F-induced spine elimination, which is mediated through NrCAM and other subunits of the semaphorin3F holoreceptor, neuropilin-2, and plexinA3 [208]. In addition, heparan sulfate chains on neurexins enhance their interactions with postsynaptic adhesion molecules neuroligins and leucine-rich repeat transmembrane neuronal (LRRTM) glycoproteins [191]. The proteins that GAGs bind to also regulate the distribution of the GAG-containing proteoglycans in neural tissues: For example, neuronal pentraxin 2 binds to GAGs in perineuronal nets via both chondroitin sulfate and hyaluronic acid, and enhances perineuronal net formation [209].
GAGs in regeneration after trauma
Early studies demonstrated that GAGs are present at high levels in spinal cord and peripheral nerves [210]. Expression of chondroitin sulfate proteoglycans increases at the spinal cord lesion site and around denervated synaptic targets in the spinal dorsal column nuclei [211]. These GAGs are considered to be most abundant and potent inhibitors of axonal regrowth and regeneration. Removal of chondroitin sulfates with chondroitinase ABC promotes regeneration after the spinal cord injury [212]. A similar effect is observed in transgenic mice with inhibited synthesis of chondrotin sulfates, for example in mice deficient in chondroitin sulfate N-acetylgalactosaminyl transferase 1 (CSGALNACT1), an enzyme responsible for transferring the first GalNAc to the protein linker region during chondroitin sulfate synthesis [213]. Interestingly, regeneration after severe compression injury of the spinal cord is reduced in dermatan-4O-sulfotransferase1-deficient mice, which express chondroitin sulfates, but not dermatan sulfates [214] suggesting that dermatan sulfates may promote regeneration. It should be noted, however, that peripheral nerve regeneration is accelerated in dermatan-4O-sulfotransferase1-deficient mice [215].
GAG function inhibiting compounds
A screen of a phage display library for peptides binding to chondroitin-4-sulfate led to the discovery of three peptides that bind to chondroitin-4-sulfate, but not chondroitin-6-sulfate, heparin sulfate or dermatan sulfate. The peptides neutralized the inhibitory functions of chondroitin sulfate proteoglycans on cell adhesion, neuronal migration and neurite outgrowth in cultured mouse cerebellar neurons [216]. Peptides binding to chondroitin-6-sulfate have been identified through peptide array screening [217] and shown to block the inhibitory activity of chondroitin-6-sulfate on neurite outgrowth of cultured cortical neurons [218].
Other glycans
Fucose(α1-2)galactose
Terminal galactose residues in brain glycoproteins can be fucosylated in the α(1–2) position (Fig. 1f). Fucα(1–2)Galβ moieties are synthesized by the GDP-l-fucose:β(1 → 4)-d-galactosyl-R2-α-l-fucosyltransferases (FUT1 and FUT2) [219]. Analysis of immunoprecipitates with antibodies against the fucα(1–2)Galβ epitope showed the presence of this linkage in a subfraction of cadherin, neuroplastin (also called gp65) and NMDA receptor subunit 1 (NR1), but not in NCAM [220]. Fucα(1–2)Galβ is highly expressed by pyramidal and granule neurons and glial cells of the hippocampus [220].
Fucα(1–2)Galβ recognized by the Ulex europeaus agglutinin I lectin is expressed in developing hippocampal neurons and treatment of neurons with either the lectin or synthetic fucα(1–2)Galβ glycan promotes neurite outgrowth [221]. A fucose analog, 2-deoxygalactose, disrupts the synthesis of fucα(1–2)Galβ on glycoproteins and inhibits neurite outgrowth and synapse formation [221]. 2-Deoxygalactose suppresses the maintenance of hippocampal LTP [222] and blocks long-term memory formation in several learning tasks [223, 224].
α2,3-Sialyl containing glycans
CD24 is a carrier of α2,3-sialic acid (Fig. 1d) specifically recognized by the lectin M. amurensis agglutinin. The glycan mediates the interaction of CD24 with L1, which function as a receptor for α2,3-sialic acid [225]. α2,3-Sialyl residues of CD24 bind to L1 within a structural motif in the first fibronectin type III domain [18]. This glycan is also recognized by sialoadhesins including sialoadhesin, CD22, MAG and CD33 [226]. Inhibition of neurite outgrowth by MAG depends on its binding to α2,3-sialic acid presented by the ganglioside GT1b at the neuronal cell surface [227].
Mono-O-mannosyl glycans
Rabbit monoclonal antibody recognizing mono-O-mannosyl glycans (Fig. 1c) showed that these glycans are present in all regions of the adult mouse brain. Mono-O-mannosyl containing glycans are particularly present in inhibitory neurons, such as Purkinje cells in the cerebellum [228]. Mono-O-mannosyl glycans are carried by the components of the extracellular matrix and perineuronal nets, such as neurocan, and cell adhesion molecules, including neurexin 3, cadherins and protocadherins [228].
O-mannosidic glycans
O-mannose containing glycans predominantly represent different types of the tetrasaccharide NeuAcα2-3Galβ1-4GlcNAcβ1-2Manα1-Ser/Thr, which can differ in length (e.g., with or without sialic acid) and content of fucose α1,3-linked to GlcNAc, and can also display the HNK-1 epitope in the form of HSO4-3GlcAβ1-3Galβ1-4GlcNacβ1-2Man-Ser/Thr glycans (reviewed in [229]). An additional β1-6 linkage to mannose results in formation of the branched forms of O-mannose glycans. The major carriers of O-mannosidic glycans are cadherins and protocadherins [230]. The characterized carrier of O-mannosdic glycans in the brain is α-dystroglycan, a cell surface glycoprotein anchored to the plasma membrane by binding to the transmembrane glycoprotein β-dystroglycan. A small subset of O-mannosidic sites in α-dystroglycan extend into the GalNAcβ1-3GlcNAcβ1-4Man-Ser/Thr trisaccharide, which serves as a carrier for matriglycan with its repeating Xyl-GlcA disaccharide [231] (Fig. 1c). O-mannose containing glycans are also found on RPTPβ [232], CD24 [233], neurofascin [234], proteins of the perineural net, lecticans [235], plexins and other glycoproteins [230]. O-mannosidic glycans mediate the interaction of α-dystroglycan with laminin G-like domain containing proteins, including laminin, agrin and neurexin [236, 237]. Defects in the O-mannosylation pathway result in reduced glycosylation of α-dystroglycan and cause several human disorders, including muscle-eye-brain disease (MEB), Walker-Warburg syndrome (WWS), Fukuyama congenital muscular dystrophy (FCMD), and congenital muscular dystrophy 1C and 1D (MDC1C and MDC1D) (reviewed in [238]).
Bisecting GlcNAc
N-glycan structures are characterized by several GlcNAc branches, which are synthesized by specific glycosyltransferases of the Golgi apparatus. N-glycans can contain a central GlcNAc branch designated as “bisecting GlcNAc”, which is not elongated or capped by sialic acid (Fig. 1g). Synthesis of α-Gal and sialyl N-glycans and other branches is reduced in N-glycans with the bisecting GlcNAc, suggesting that it suppresses synthesis of other epitopes in N-glycans (reviewed in [239]). The bisecting GlcNAc modification is carried by NCAM [240] and beta1 integrin [241] and plays a role in regulating neural development. Glycosyltransferase GnT-III responsible for bisecting GlcNAc modifications is expressed in the brain starting at mouse embryonic day 10.5 and deficiency in GnT-III activity leads to neurological problems [242]. GnT-III overexpression promotes serum deprivation-induced beta1 integrin-dependent neurite outgrowth in mouse neuroblastoma cells [241] but inhibits neurite outgrowth in rat pheochromocytoma PC12 cells in response to co-stimulation of epidermal growth factor and integrins [243]. Bisecting GlcNAc is also carried by the Beta-Site APP-Cleaving Enzyme 1 (BACE1) [244]. GnT-III is upregulated in Alzheimer disease affected brains [245], which have higher levels of bisecting GlcNAc on BACE1 [244]. Bisecting GlcNAc on BACE1 promotes BACE1 localization to early endosomes, stabilizes this protein and reduces its targeting to lysosomes for degradation [246], thereby promoting generation of cytotoxic Aβ [244]. Levels of N-glycans containing bisecting GlcNAc are also increased in the cerebrospinal fluid from patients with Alzheimer’s disease [247]. Other carriers of bisecting GlcNAc have been described outside of the nervous system and in cancers, including basigin, L1, Thy-1 and others [248]. The functions of bisecting GlcNAc on these carriers in neurons remain to be characterized.
Branched sialylated N-glycans
Branched sialylated N-glycans (Fig. 1d) on the immunoglobulin superfamily adhesion molecules neurotrimin and limbic system-associated membrane protein (LAMP) accumulate in synapses, where they mediate the interactions of dendritic spines with microglia via interactions with the microglia-specific sialic acid-binding immunoglobulin-like lectin H [249].
Lactosamine disaccharide
Olfactory sensory neurons are unique in their expression of a terminal lactosamine [250] that is recognized by monoclonal antibody 1B2 [251]. The lactosamine disaccharide (Fig. 1h) occurs widely on N-glycans and is typically capped by sialic acid or other glycans, and β1,3-N-acetylglucosaminyl transferase 1 (β3GnT1) plays a key role in its synthesis. Lactosamines can be extended by β3Gn transferase and β4GalT transferase to form polylactosamines, which frequently occur on alpha-1,6-mannosylglycoprotein 6-beta-N-acetylglucosaminyl transferase (MGAT5)-generated branched glycans and are preferentially recognized by the 1B2 antibody. Deficiency in β3GnT1 in mice results in the loss of a subset of odorant receptor-expressing neurons and in a severely disorganized olfactory bulb, indicating that terminal lactosamine is important for axonal pathfinding [250].
Concluding remarks
In this review, we have tried to describe the sophistication of glycans with regard to their overwhelming functional impact as structurally diverse molecules. We focused on the most well-known glycans and are aware that we may have not covered them all in their complexity. Also, it may well be that we have neglected mentioning glycans and their protein carriers other than those here described, considering that a focus on these glycans would deem sufficient to expose the vast range of functions that glycans display in the nervous system. The functional versatility of glycans is of tremendous impact not only in ontogenetic development, but also in the adult, where they influence the most challenging features of the nervous system: synaptic plasticity and regeneration not only in the acutely injured nervous system, but also in mental and neurodegenerative diseases. It is noteworthy that the glycans of the nervous system were discovered outside the nervous system, before they attracted attention for understanding neural functions. Their structural and functional versatility would seem well placed in the nervous system, since it is a most complex organ. Finally, we would like to point out that glycans are metabolically very demanding in their biosynthesis, more so than proteins or nucleic acids on a molar basis. It seems that this expense in energy has been invested well, since it has led to what we would like to call a most beautiful embellishment bestowed onto neural glycoproteins.
Author contributions
All authors have made substantial contributions to the conception and design of the review, analyzed the literature, and drafted the manuscript.
Funding
This work was supported by a grant from the National Health and Medical Research Council (APP1129869 to V.S.). The Li Kashing Foundation is gratefully acknowledged for support (LD030302 to M.S.).
Compliance with ethical standards
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent for publication
All authors consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aoki-Kinoshita K, Agravat S, Aoki NP, Arpinar S, Cummings RD, Fujita A, Fujita N, Hart GM, Haslam SM, Kawasaki T, Matsubara M, Moreman KW, Okuda S, Pierce M, Ranzinger R, Shikanai T, Shinmachi D, Solovieva E, Suzuki Y, Tsuchiya S, Yamada I, York WS, Zaia J, Narimatsu H. GlyTouCan 1.0–the international glycan structure repository. Nucleic Acids Res. 2016;44(D1):D1237–1242. doi: 10.1093/nar/gkv1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeberger PH, et al. Monosaccharide diversity. In: Varki A, Cummings RD, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor; 2015. pp. 19–30. [Google Scholar]
- 3.Prestegard JH, Liu J, Widmalm G, et al. Oligosaccharides and polysaccharides. In: Varki A, Cummings RD, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor; 2015. pp. 31–40. [Google Scholar]
- 4.Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, Stanley P, Hart G, Darvill A, Kinoshita T, Prestegard JJ, Schnaar RL, Freeze HH, Marth JD, Bertozzi CR, Etzler ME, Frank M, Vliegenthart JF, Lutteke T, Perez S, Bolton E, Rudd P, Paulson J, Kanehisa M, Toukach P, Aoki-Kinoshita KF, Dell A, Narimatsu H, York W, Taniguchi N, Kornfeld S. Symbol nomenclature for graphical representations of glycans. Glycobiology. 2015;25(12):1323–1324. doi: 10.1093/glycob/cwv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagneux P, Aebi M, Varki A, et al. Evolution of glycan diversity. In: Varki A, Cummings RD, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor; 2015. pp. 253–264. [Google Scholar]
- 6.Kopitz J. Lipid glycosylation: a primer for histochemists and cell biologists. Histochem Cell Biol. 2017;147(2):175–198. doi: 10.1007/s00418-016-1518-4. [DOI] [PubMed] [Google Scholar]
- 7.Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12(4):43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- 8.de Beer T, Vliegenthart JF, Loffler A, Hofsteenge J. The hexopyranosyl residue that is C-glycosidically linked to the side chain of tryptophan-7 in human RNase Us is alpha-mannopyranose. Biochemistry. 1995;34(37):11785–11789. doi: 10.1021/bi00037a016. [DOI] [PubMed] [Google Scholar]
- 9.Xiao H, Chen W, Smeekens JM, Wu R. An enrichment method based on synergistic and reversible covalent interactions for large-scale analysis of glycoproteins. Nat Commun. 2018;9(1):1692. doi: 10.1038/s41467-018-04081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeze HH, Eklund EA, Ng BG, Patterson MC. Neurology of inherited glycosylation disorders. Lancet Neurol. 2012;11(5):453–466. doi: 10.1016/S1474-4422(12)70040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medina-Cano D, Ucuncu E, Nguyen LS, Nicouleau M, Lipecka J, Bizot JC, Thiel C, Foulquier F, Lefort N, Faivre-Sarrailh C, Colleaux L, Guerrera IC, Cantagrel V. High N-glycan multiplicity is critical for neuronal adhesion and sensitizes the developing cerebellum to N-glycosylation defect. eLife. 2018 doi: 10.7554/eLife.38309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proc Natl Acad Sci USA. 1978;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo T, Fujii T, Ikegami S, Inokuchi K, Takayama Y, Ikehara Y, Nishihara S, Togayachi A, Takahashi S, Tachibana K, Yuasa S, Narimatsu H. Mice lacking alpha1,3-fucosyltransferase IX demonstrate disappearance of Lewis x structure in brain and increased anxiety-like behaviors. Glycobiology. 2007;17(1):1–9. doi: 10.1093/glycob/cwl047. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Torii T, Ishino Y, Muraoka D, Yoshimura T, Togayachi A, Narimatsu H, Ikenaka K, Hitoshi S. The Lewis X-related alpha1,3-fucosyltransferase, Fut10, is required for the maintenance of stem cell populations. J Biol Chem. 2013;288(40):28859–28868. doi: 10.1074/jbc.M113.469403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YJ, Wing DR, Guile GR, Dwek RA, Harvey DJ, Zamze S. Neutral N-glycans in adult rat brain tissue–complete characterisation reveals fucosylated hybrid and complex structures. Eur J Biochem. 1998;251(3):691–703. doi: 10.1046/j.1432-1327.1998.2510691.x. [DOI] [PubMed] [Google Scholar]
- 16.Chai W, Yuen CT, Kogelberg H, Carruthers RA, Margolis RU, Feizi T, Lawson AM. High prevalence of 2-mono- and 2,6-di-substituted manol-terminating sequences among O-glycans released from brain glycopeptides by reductive alkaline hydrolysis. Eur J Biochem. 1999;263(3):879–888. doi: 10.1046/j.1432-1327.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 17.Yaji S, Manya H, Nakagawa N, Takematsu H, Endo T, Kannagi R, Yoshihara T, Asano M, Oka S. Major glycan structure underlying expression of the Lewis X epitope in the developing brain is O-mannose-linked glycans on phosphacan/RPTPbeta. Glycobiology. 2015;25(4):376–385. doi: 10.1093/glycob/cwu118. [DOI] [PubMed] [Google Scholar]
- 18.Lieberoth A, Splittstoesser F, Katagihallimath N, Jakovcevski I, Loers G, Ranscht B, Karagogeos D, Schachner M, Kleene R. Lewis(x) and alpha2,3-sialyl glycans and their receptors TAG-1, Contactin, and L1 mediate CD24-dependent neurite outgrowth. J Neurosci. 2009;29(20):6677–6690. doi: 10.1523/JNEUROSCI.4361-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Cesca F, Loers G, Schweizer M, Buck F, Benfenati F, Schachner M, Kleene R. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci. 2011;31(20):7275–7290. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennen E, Safina D, Haussmann U, Worsdorfer P, Edenhofer F, Poetsch A, Faissner A. A LewisX glycoprotein screen identifies the low density lipoprotein receptor-related protein 1 (LRP1) as a modulator of oligodendrogenesis in mice. J Biol Chem. 2013;288(23):16538–16545. doi: 10.1074/jbc.M112.419812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakomori S. Le(X) and related structures as adhesion molecules. Histochem J. 1992;24(11):771–776. doi: 10.1007/bf01046348. [DOI] [PubMed] [Google Scholar]
- 22.Dvorak P, Hampl A, Jirmanova L, Pacholikova J, Kusakabe M. Embryoglycan ectodomains regulate biological activity of FGF-2 to embryonic stem cells. J Cell Sci. 1998;111(Pt 19):2945–2952. doi: 10.1242/jcs.111.19.2945. [DOI] [PubMed] [Google Scholar]
- 23.Jirmanova L, Pacholikova J, Krejci P, Hampl A, Dvorak P. O-linked carbohydrates are required for FGF-2-mediated proliferation of mouse embryonic cells. Int J Dev Biol. 1999;43(6):555–562. [PubMed] [Google Scholar]
- 24.Capela A, Temple S. LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Dev Biol. 2006;291(2):300–313. doi: 10.1016/j.ydbio.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Lutz D, Loers G, Kleene R, Oezen I, Kataria H, Katagihallimath N, Braren I, Harauz G, Schachner M. Myelin basic protein cleaves cell adhesion molecule L1 and promotes neuritogenesis and cell survival. J Biol Chem. 2014;289(19):13503–13518. doi: 10.1074/jbc.M113.530238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karus M, Hennen E, Safina D, Klausmeyer A, Wiese S, Faissner A. Differential expression of micro-heterogeneous LewisX-type glycans in the stem cell compartment of the developing mouse spinal cord. Neurochem Res. 2013;38(6):1285–1294. doi: 10.1007/s11064-013-1048-6. [DOI] [PubMed] [Google Scholar]
- 27.Yagi H, Saito T, Yanagisawa M, Yu RK, Kato K. Lewis X-carrying N-glycans regulate the proliferation of mouse embryonic neural stem cells via the Notch signaling pathway. J Biol Chem. 2012;287(29):24356–24364. doi: 10.1074/jbc.M112.365643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennen E, Czopka T, Faissner A. Structurally distinct LewisX glycans distinguish subpopulations of neural stem/progenitor cells. J Biol Chem. 2011;286(18):16321–16331. doi: 10.1074/jbc.M110.201095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gocht A, Struckhoff G, Lhler J. CD15-containing glycoconjugates in the central nervous system. Histol Histopathol. 1996;11(4):1007–1028. [PubMed] [Google Scholar]
- 30.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35(5):865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 31.Koles K, McDowell W, Mileusnic R, Rose SP. Glycan analysis of the chicken synaptic plasma membrane glycoproteins–a major synaptic N-glycan carries the LewisX determinant. Int J Biol Sci. 2005;1(4):126–134. doi: 10.7150/ijbs.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theis T, Johal AS, Kabat M, Basak S, Schachner M. Enhanced neuronal survival and neurite outgrowth triggered by novel small organic compounds mimicking the LewisX glycan. Mol Neurobiol. 2018;55(10):8203–8215. doi: 10.1007/s12035-018-0953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katagihallimath N, Mehanna A, Guseva D, Kleene R, Schachner M. Identification and validation of a Lewis x glycomimetic peptide. Eur J Cell Biol. 2010;89(1):77–86. doi: 10.1016/j.ejcb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Abo T, Balch CM. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1) J Immunol. 1981;127(3):1024–1029. [PubMed] [Google Scholar]
- 35.Chou DK, Ilyas AA, Evans JE, Costello C, Quarles RH, Jungalwala FB. Structure of sulfated glucuronyl glycolipids in the nervous system reacting with HNK-1 antibody and some IgM paraproteins in neuropathy. J Biol Chem. 1986;261(25):11717–11725. doi: 10.1016/S0021-9258(18)67303-X. [DOI] [PubMed] [Google Scholar]
- 36.Chou KH, Ilyas AA, Evans JE, Quarles RH, Jungalwala FB. Structure of a glycolipid reacting with monoclonal IgM in neuropathy and with HNK-1. Biochem Biophys Res Commun. 1985;128(1):383–388. doi: 10.1016/0006-291x(85)91690-0. [DOI] [PubMed] [Google Scholar]
- 37.Terayama K, Oka S, Seiki T, Miki Y, Nakamura A, Kozutsumi Y, Takio K, Kawasaki T. Cloning and functional expression of a novel glucuronyltransferase involved in the biosynthesis of the carbohydrate epitope HNK-1. Proc Natl Acad Sci USA. 1997;94(12):6093–6098. doi: 10.1073/pnas.94.12.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakker H, Friedmann I, Oka S, Kawasaki T, Nifant'ev N, Schachner M, Mantei N. Expression cloning of a cDNA encoding a sulfotransferase involved in the biosynthesis of the HNK-1 carbohydrate epitope. J Biol Chem. 1997;272(47):29942–29946. doi: 10.1074/jbc.272.47.29942. [DOI] [PubMed] [Google Scholar]
- 39.Ong E, Yeh JC, Ding Y, Hindsgaul O, Fukuda M. Expression cloning of a human sulfotransferase that directs the synthesis of the HNK-1 glycan on the neural cell adhesion molecule and glycolipids. J Biol Chem. 1998;273(9):5190–5195. doi: 10.1074/jbc.273.9.5190. [DOI] [PubMed] [Google Scholar]
- 40.Yoshihara T, Sugihara K, Kizuka Y, Oka S, Asano M. Learning/memory impairment and reduced expression of the HNK-1 carbohydrate in beta4-galactosyltransferase-II-deficient mice. J Biol Chem. 2009;284(18):12550–12561. doi: 10.1074/jbc.M809188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yabuno K, Morise J, Kizuka Y, Hashii N, Kawasaki N, Takahashi S, Miyata S, Izumikawa T, Kitagawa H, Takematsu H, Oka S. A sulfated glycosaminoglycan linkage region is a novel type of human natural killer-1 (HNK-1) epitope expressed on aggrecan in perineuronal nets. PLoS ONE. 2015;10(12):e0144560. doi: 10.1371/journal.pone.0144560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kruse J, Mailhammer R, Wernecke H, Faissner A, Sommer I, Goridis C, Schachner M. Neural cell adhesion molecules and myelin-associated glycoprotein share a common carbohydrate moiety recognized by monoclonal antibodies L2 and HNK-1. Nature. 1984;311(5982):153–155. doi: 10.1038/311153a0. [DOI] [PubMed] [Google Scholar]
- 43.Yoshihara Y, Kawasaki M, Tamada A, Fujita H, Hayashi H, Kagamiyama H, Mori K. OCAM: a new member of the neural cell adhesion molecule family related to zone-to-zone projection of olfactory and vomeronasal axons. J Neurosci. 1997;17(15):5830–5842. doi: 10.1523/JNEUROSCI.17-15-05830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voshol H, van Zuylen CW, Orberger G, Vliegenthart JF, Schachner M. Structure of the HNK-1 carbohydrate epitope on bovine peripheral myelin glycoprotein P0. J Biol Chem. 1996;271(38):22957–22960. doi: 10.1074/jbc.271.38.22957. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura A, Morise J, Yabuno-Nakagawa K, Hashimoto Y, Takematsu H, Oka S. Site-specific HNK-1 epitope on alternatively spliced fibronectin type-III repeats in tenascin-C promotes neurite outgrowth of hippocampal neurons through contactin-1. PLoS ONE. 2019;14(1):e0210193. doi: 10.1371/journal.pone.0210193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margolis RK, Ripellino JA, Goossen B, Steinbrich R, Margolis RU. Occurrence of the HNK-1 epitope (3-sulfoglucuronic acid) in PC12 pheochromocytoma cells, chromaffin granule membranes, and chondroitin sulfate proteoglycans. Biochem Biophys Res Commun. 1987;145(3):1142–1148. doi: 10.1016/0006-291x(87)91556-7. [DOI] [PubMed] [Google Scholar]
- 47.Morise J, Kizuka Y, Yabuno K, Tonoyama Y, Hashii N, Kawasaki N, Manya H, Miyagoe-Suzuki Y, Takeda S, Endo T, Maeda N, Takematsu H, Oka S. Structural and biochemical characterization of O-mannose-linked human natural killer-1 glycan expressed on phosphacan in developing mouse brains. Glycobiology. 2014;24(3):314–324. doi: 10.1093/glycob/cwt116. [DOI] [PubMed] [Google Scholar]
- 48.Morita I, Kakuda S, Takeuchi Y, Itoh S, Kawasaki N, Kizuka Y, Kawasaki T, Oka S. HNK-1 glyco-epitope regulates the stability of the glutamate receptor subunit GluR2 on the neuronal cell surface. J Biol Chem. 2009;284(44):30209–30217. doi: 10.1074/jbc.M109.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall H, Deutzmann R, Timpl R, Vaughan L, Schmitz B, Schachner M. HNK-1 carbohydrate-mediated cell adhesion to laminin-1 is different from heparin-mediated and sulfatide-mediated cell adhesion. Eur J Biochem. 1997;246(1):233–242. doi: 10.1111/j.1432-1033.1997.t01-1-00233.x. [DOI] [PubMed] [Google Scholar]
- 50.Hall H, Vorherr T, Schachner M. Characterization of a 21 amino acid peptide sequence of the laminin G2 domain that is involved in HNK-1 carbohydrate binding and cell adhesion. Glycobiology. 1995;5(4):435–441. doi: 10.1093/glycob/5.4.435. [DOI] [PubMed] [Google Scholar]
- 51.Chou DK, Evans JE, Jungalwala FB. Identity of nuclear high-mobility-group protein, HMG-1, and sulfoglucuronyl carbohydrate-binding protein, SBP-1, in brain. J Neurochem. 2001;77(1):120–131. doi: 10.1046/j.1471-4159.2001.t01-1-00209.x. [DOI] [PubMed] [Google Scholar]
- 52.Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Mol Neurobiol. 2012;45(3):499–506. doi: 10.1007/s12035-012-8264-y. [DOI] [PubMed] [Google Scholar]
- 53.Saghatelyan AK, Snapyan M, Gorissen S, Meigel I, Mosbacher J, Kaupmann K, Bettler B, Kornilov AV, Nifantiev NE, Sakanyan V, Schachner M, Dityatev A. Recognition molecule associated carbohydrate inhibits postsynaptic GABA(B) receptors: a mechanism for homeostatic regulation of GABA release in perisomatic synapses. Mol Cell Neurosci. 2003;24(2):271–282. doi: 10.1016/S1044-7431(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 54.Griffith LS, Schmitz B, Schachner M. L2/HNK-1 carbohydrate and protein-protein interactions mediate the homophilic binding of the neural adhesion molecule P0. J Neurosci Res. 1992;33(4):639–648. doi: 10.1002/jnr.490330417. [DOI] [PubMed] [Google Scholar]
- 55.Cole GJ, Schachner M. Localization of the L2 monoclonal antibody binding site on chicken neural cell adhesion molecule (NCAM) and evidence for its role in NCAM-mediated cell adhesion. Neurosci Lett. 1987;78(2):227–232. doi: 10.1016/0304-3940(87)90638-0. [DOI] [PubMed] [Google Scholar]
- 56.Keilhauer G, Faissner A, Schachner M. Differential inhibition of neurone-neurone, neurone-astrocyte and astrocyte-astrocyte adhesion by L1, L2 and N-CAM antibodies. Nature. 1985;316(6030):728–730. doi: 10.1038/316728a0. [DOI] [PubMed] [Google Scholar]
- 57.Kunemund V, Jungalwala FB, Fischer G, Chou DK, Keilhauer G, Schachner M. The L2/HNK-1 carbohydrate of neural cell adhesion molecules is involved in cell interactions. J Cell Biol. 1988;106(1):213–223. doi: 10.1083/jcb.106.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarting GA, Jungalwala FB, Chou DK, Boyer AM, Yamamoto M. Sulfated glucuronic acid-containing glycoconjugates are temporally and spatially regulated antigens in the developing mammalian nervous system. Dev Biol. 1987;120(1):65–76. doi: 10.1016/0012-1606(87)90104-7. [DOI] [PubMed] [Google Scholar]
- 59.Yagi H, Yanagisawa M, Suzuki Y, Nakatani Y, Ariga T, Kato K, Yu RK. HNK-1 epitope-carrying tenascin-C spliced variant regulates the proliferation of mouse embryonic neural stem cells. J Biol Chem. 2010;285(48):37293–37301. doi: 10.1074/jbc.M110.157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martini R, Bollensen E, Schachner M. Immunocytological localization of the major peripheral nervous system glycoprotein P0 and the L2/HNK-1 and L3 carbohydrate structures in developing and adult mouse sciatic nerve. Dev Biol. 1988;129(2):330–338. doi: 10.1016/0012-1606(88)90380-6. [DOI] [PubMed] [Google Scholar]
- 61.Saghatelyan AK, Gorissen S, Albert M, Hertlein B, Schachner M, Dityatev A. The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate perisomatic inhibition and long-term potentiation in the CA1 region of the hippocampus. Eur J Neurosci. 2000;12(9):3331–3342. doi: 10.1046/j.1460-9568.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- 62.Marzban H, Sillitoe RV, Hoy M, Chung SH, Rafuse VF, Hawkes R. Abnormal HNK-1 expression in the cerebellum of an N-CAM null mouse. J Neurocytol. 2004;33(1):117–130. doi: 10.1023/B:NEUR.0000029652.96456.0d. [DOI] [PubMed] [Google Scholar]
- 63.Strekalova T, Wotjak CT, Schachner M. Intrahippocampal administration of an antibody against the HNK-1 carbohydrate impairs memory consolidation in an inhibitory learning task in mice. Mol Cell Neurosci. 2001;17(6):1102–1113. doi: 10.1006/mcne.2001.0991. [DOI] [PubMed] [Google Scholar]
- 64.Pradel G, Schachner M, Schmidt R. Inhibition of memory consolidation by antibodies against cell adhesion molecules after active avoidance conditioning in zebrafish. J Neurobiol. 1999;39(2):197–206. doi: 10.1002/(SICI)1097-4695(199905)39:2<197::AID-NEU4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 65.Senn C, Kutsche M, Saghatelyan A, Bosl MR, Lohler J, Bartsch U, Morellini F, Schachner M. Mice deficient for the HNK-1 sulfotransferase show alterations in synaptic efficacy and spatial learning and memory. Mol Cell Neurosci. 2002;20(4):712–729. doi: 10.1006/mcne.2002.1142. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto S, Oka S, Inoue M, Shimuta M, Manabe T, Takahashi H, Miyamoto M, Asano M, Sakagami J, Sudo K, Iwakura Y, Ono K, Kawasaki T. Mice deficient in nervous system-specific carbohydrate epitope HNK-1 exhibit impaired synaptic plasticity and spatial learning. J Biol Chem. 2002;277(30):27227–27231. doi: 10.1074/jbc.C200296200. [DOI] [PubMed] [Google Scholar]
- 67.Morita I, Kakuda S, Takeuchi Y, Kawasaki T, Oka S. HNK-1 (human natural killer-1) glyco-epitope is essential for normal spine morphogenesis in developing hippocampal neurons. Neuroscience. 2009;164(4):1685–1694. doi: 10.1016/j.neuroscience.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Ayllon MS, Botella-Lopez A, Cuchillo-Ibanez I, Rabano A, Andreasen N, Blennow K, Avila J, Saez-Valero J. HNK-1 carrier glycoproteins are decreased in the Alzheimer's disease brain. Mol Neurobiol. 2017;54(1):188–199. doi: 10.1007/s12035-015-9644-x. [DOI] [PubMed] [Google Scholar]
- 69.Thomas SN, Soreghan BA, Nistor M, Sarsoza F, Head E, Yang AJ. Reduced neuronal expression of synaptic transmission modulator HNK-1/neural cell adhesion molecule as a potential consequence of amyloid beta-mediated oxidative stress: a proteomic approach. J Neurochem. 2005;92(4):705–717. doi: 10.1111/j.1471-4159.2004.02892.x. [DOI] [PubMed] [Google Scholar]
- 70.Leshchyns'ka I, Liew HT, Shepherd C, Halliday GM, Stevens CH, Ke YD, Ittner LM, Sytnyk V. Abeta-dependent reduction of NCAM2-mediated synaptic adhesion contributes to synapse loss in Alzheimer's disease. Nat Commun. 2015;6:8836. doi: 10.1038/ncomms9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leshchyns'ka I, Sytnyk V. Synaptic cell adhesion molecules in Alzheimer's disease. Neural Plast. 2016;2016:6427537. doi: 10.1155/2016/6427537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Low K, Orberger G, Schmitz B, Martini R, Schachner M. The L2/HNK-1 carbohydrate is carried by the myelin associated glycoprotein and sulphated glucuronyl glycolipids in muscle but not cutaneous nerves of adult mice. Eur J Neurosci. 1994;6(12):1773–1781. doi: 10.1111/j.1460-9568.1994.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 73.Martini R, Schachner M, Brushart TM. The L2/HNK-1 carbohydrate is preferentially expressed by previously motor axon-associated Schwann cells in reinnervated peripheral nerves. J Neurosci. 1994;14(11 Pt 2):7180–7191. doi: 10.1523/JNEUROSCI.14-11-07180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martini R, Xin Y, Schmitz B, Schachner M. The L2/HNK-1 carbohydrate epitope is involved in the preferential outgrowth of motor neurons on ventral roots and motor nerves. Eur J Neurosci. 1992;4(7):628–639. doi: 10.1111/j.1460-9568.1992.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 75.Eberhardt KA, Irintchev A, Al-Majed AA, Simova O, Brushart TM, Gordon T, Schachner M. BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery after peripheral nerve repair. Exp Neurol. 2006;198(2):500–510. doi: 10.1016/j.expneurol.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 76.Nieke J, Schachner M. Expression of the neural cell adhesion molecules L1 and N-CAM and their common carbohydrate epitope L2/HNK-1 during development and after transection of the mouse sciatic nerve. Differentiation. 1985;30(2):141–151. doi: 10.1111/j.1432-0436.1985.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 77.Ma L, Shen HF, Shen YQ, Schachner M. The adhesion molecule-characteristic HNK-1 carbohydrate contributes to functional recovery after spinal cord injury in adult zebrafish. Mol Neurobiol. 2017;54(5):3253–3263. doi: 10.1007/s12035-016-9876-4. [DOI] [PubMed] [Google Scholar]
- 78.Simon-Haldi M, Mantei N, Franke J, Voshol H, Schachner M. Identification of a peptide mimic of the L2/HNK-1 carbohydrate epitope. J Neurochem. 2002;83(6):1380–1388. doi: 10.1046/j.1471-4159.2002.01247.x. [DOI] [PubMed] [Google Scholar]
- 79.Simova O, Irintchev A, Mehanna A, Liu J, Dihne M, Bachle D, Sewald N, Loers G, Schachner M. Carbohydrate mimics promote functional recovery after peripheral nerve repair. Ann Neurol. 2006;60(4):430–437. doi: 10.1002/ana.20948. [DOI] [PubMed] [Google Scholar]
- 80.Irintchev A, Wu MM, Lee HJ, Zhu H, Feng YP, Liu YS, Bernreuther C, Loers G, You SW, Schachner M. Glycomimetic improves recovery after femoral injury in a non-human primate. J Neurotrauma. 2011;28(7):1295–1306. doi: 10.1089/neu.2011.1775. [DOI] [PubMed] [Google Scholar]
- 81.Mehanna A, Jakovcevski I, Acar A, Xiao M, Loers G, Rougon G, Irintchev A, Schachner M. Polysialic acid glycomimetic promotes functional recovery and plasticity after spinal cord injury in mice. Mol Ther. 2010;18(1):34–43. doi: 10.1038/mt.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahu S, Li R, Kadeyala PK, Liu S, Schachner M. The human natural killer-1 (HNK-1) glycan mimetic ursolic acid promotes functional recovery after spinal cord injury in mouse. J Nutr Biochem. 2018;55:219–228. doi: 10.1016/j.jnutbio.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 83.Schmitz B, Peter-Katalinic J, Egge H, Schachner M. Monoclonal antibodies raised against membrane glycoproteins from mouse brain recognize N-linked oligomannosidic glycans. Glycobiology. 1993;3(6):609–617. doi: 10.1093/glycob/3.6.609. [DOI] [PubMed] [Google Scholar]
- 84.Verbert A, Cacan R. Trafficking of oligomannosides released during N-glycosylation: a clearing mechanism of the rough endoplasmic reticulum. Biochim Biophys Acta. 1999;1473(1):137–146. doi: 10.1016/s0304-4165(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 85.Horstkorte R, Schachner M, Magyar JP, Vorherr T, Schmitz B. The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans implicated in association with L1 and neurite outgrowth. J Cell Biol. 1993;121(6):1409–1421. doi: 10.1083/jcb.121.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kucherer A, Faissner A, Schachner M. The novel carbohydrate epitope L3 is shared by some neural cell adhesion molecules. J Cell Biol. 1987;104(6):1597–1602. doi: 10.1083/jcb.104.6.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jarvie KR, Booth G, Brown EM, Niznik HB. Glycoprotein nature of dopamine D1 receptors in the brain and parathyroid gland. Mol Pharmacol. 1989;36(4):566–574. [PubMed] [Google Scholar]
- 88.Heller M, von der Ohe M, Kleene R, Mohajeri MH, Schachner M. The immunoglobulin-superfamily molecule basigin is a binding protein for oligomannosidic carbohydrates: an anti-idiotypic approach. J Neurochem. 2003;84(3):557–565. doi: 10.1046/j.1471-4159.2003.01537.x. [DOI] [PubMed] [Google Scholar]
- 89.Watts JC, Huo H, Bai Y, Ehsani S, Jeon AH, Shi T, Daude N, Lau A, Young R, Xu L, Carlson GA, Williams D, Westaway D, Schmitt-Ulms G. Interactome analyses identify ties of PrP and its mammalian paralogs to oligomannosidic N-glycans and endoplasmic reticulum-derived chaperones. PLoS Pathog. 2009;5(10):e1000608. doi: 10.1371/journal.ppat.1000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshimi Y, Yamazaki S, Ikekita M. Developmental changes in Asn-linked neutral oligosaccharides in murine cerebrum. Biochim Biophys Acta. 1999;1426(1):69–79. doi: 10.1016/s0304-4165(98)00123-8. [DOI] [PubMed] [Google Scholar]
- 91.Fu SC, Gurd JW. Developmental changes in the oligosaccharide composition of synaptic junctional glycoproteins. J Neurochem. 1983;41(6):1726–1734. doi: 10.1111/j.1471-4159.1983.tb00886.x. [DOI] [PubMed] [Google Scholar]
- 92.Luthl A, Laurent JP, Figurov A, Muller D, Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372(6508):777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- 93.Burkhardt-Holm P, Kafitz KW, Guttinger HR, Schachner M. Testosterone elevates expression of tenascin-R and oligomannosidic carbohydrates in developing male zebra finches. J Neurosci Res. 1996;46(3):385–392. doi: 10.1002/(SICI)1097-4547(19961101)46:3<385::AID-JNR12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt JT, Schachner M. Role for cell adhesion and glycosyl (HNK-1 and oligomannoside) recognition in the sharpening of the regenerating retinotectal projection in goldfish. J Neurobiol. 1998;37(4):659–671. doi: 10.1002/(SICI)1097-4695(199812)37:4<659::AID-NEU13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 95.Rothbard JB, Brackenbury R, Cunningham BA, Edelman GM. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J Biol Chem. 1982;257(18):11064–11069. doi: 10.1016/S0021-9258(18)33933-4. [DOI] [PubMed] [Google Scholar]
- 96.Finne J, Finne U, Deagostini-Bazin H, Goridis C. Occurrence of alpha 2–8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983;112(2):482–487. doi: 10.1016/0006-291x(83)91490-0. [DOI] [PubMed] [Google Scholar]
- 97.McCoy RD, Vimr ER, Troy FA. CMP-NeuNAc:poly-alpha-2,8-sialosyl sialyltransferase and the biosynthesis of polysialosyl units in neural cell adhesion molecules. J Biol Chem. 1985;260(23):12695–12699. doi: 10.1016/S0021-9258(17)38929-9. [DOI] [PubMed] [Google Scholar]
- 98.Liedtke S, Geyer H, Wuhrer M, Geyer R, Frank G, Gerardy-Schahn R, Zahringer U, Schachner M. Characterization of N-glycans from mouse brain neural cell adhesion molecule. Glycobiology. 2001;11(5):373–384. doi: 10.1093/glycob/11.5.373. [DOI] [PubMed] [Google Scholar]
- 99.Geyer H, Bahr U, Liedtke S, Schachner M, Geyer R. Core structures of polysialylated glycans present in neural cell adhesion molecule from newborn mouse brain. Eur J Biochem. 2001;268(24):6587–6599. doi: 10.1046/j.0014-2956.2001.02613.x. [DOI] [PubMed] [Google Scholar]
- 100.Scheidegger P, Papay J, Zuber C, Lackie PM, Roth J. Cellular site of synthesis and dynamics of cell surface re-expression of polysialic acid of the neural cell adhesion molecule. Eur J Biochem. 1994;225(3):1097–1103. doi: 10.1111/j.1432-1033.1994.1097b.x. [DOI] [PubMed] [Google Scholar]
- 101.Nakayama J, Fukuda MN, Fredette B, Ranscht B, Fukuda M. Expression cloning of a human polysialyltransferase that forms the polysialylated neural cell adhesion molecule present in embryonic brain. Proc Natl Acad Sci USA. 1995;92(15):7031–7035. doi: 10.1073/pnas.92.15.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scheidegger EP, Sternberg LR, Roth J, Lowe JB. A human STX cDNA confers polysialic acid expression in mammalian cells. J Biol Chem. 1995;270(39):22685–22688. doi: 10.1074/jbc.270.39.22685. [DOI] [PubMed] [Google Scholar]
- 103.Angata K, Fukuda M. Polysialyltransferases: major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie. 2003;85(1–2):195–206. doi: 10.1016/s0300-9084(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 104.Angata K, Nakayama J, Fredette B, Chong K, Ranscht B, Fukuda M. Human STX polysialyltransferase forms the embryonic form of the neural cell adhesion molecule. Tissue-specific expression, neurite outgrowth, and chromosomal localization in comparison with another polysialyltransferase, PST. J Biol Chem. 1997;272(11):7182–7190. doi: 10.1074/jbc.272.11.7182. [DOI] [PubMed] [Google Scholar]
- 105.Thompson MG, Foley DA, Colley KJ. The polysialyltransferases interact with sequences in two domains of the neural cell adhesion molecule to allow its polysialylation. J Biol Chem. 2013;288(10):7282–7293. doi: 10.1074/jbc.M112.438374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bhide GP, Prehna G, Ramirez BE, Colley KJ. The polybasic region of the polysialyltransferase ST8Sia-IV binds directly to the neural cell adhesion molecule. NCAM Biochem. 2017;56(10):1504–1517. doi: 10.1021/acs.biochem.6b01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367(6462):455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- 108.James WM, Agnew WS. Multiple oligosaccharide chains in the voltage-sensitive Na channel from electrophorus electricus: evidence for alpha-2,8-linked polysialic acid. Biochem Biophys Res Commun. 1987;148(2):817–826. doi: 10.1016/0006-291x(87)90949-1. [DOI] [PubMed] [Google Scholar]
- 109.Zuber C, Lackie PM, Catterall WA, Roth J. Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. J Biol Chem. 1992;267(14):9965–9971. doi: 10.1016/S0021-9258(19)50186-7. [DOI] [PubMed] [Google Scholar]
- 110.Galuska SP, Rollenhagen M, Kaup M, Eggers K, Oltmann-Norden I, Schiff M, Hartmann M, Weinhold B, Hildebrandt H, Geyer R, Muhlenhoff M, Geyer H. Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc Natl Acad Sci USA. 2010;107(22):10250–10255. doi: 10.1073/pnas.0912103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Curreli S, Arany Z, Gerardy-Schahn R, Mann D, Stamatos NM. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem. 2007;282(42):30346–30356. doi: 10.1074/jbc.M702965200. [DOI] [PubMed] [Google Scholar]
- 112.Hoffman S, Edelman GM. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc Natl Acad Sci USA. 1983;80(18):5762–5766. doi: 10.1073/pnas.80.18.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rutishauser U, Watanabe M, Silver J, Troy FA, Vimr ER. Specific alteration of NCAM-mediated cell adhesion by an endoneuraminidase. J Cell Biol. 1985;101(5 Pt 1):1842–1849. doi: 10.1083/jcb.101.5.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Storms SD, Rutishauser U. A role for polysialic acid in neural cell adhesion molecule heterophilic binding to proteoglycans. J Biol Chem. 1998;273(42):27124–27129. doi: 10.1074/jbc.273.42.27124. [DOI] [PubMed] [Google Scholar]
- 115.Kanato Y, Kitajima K, Sato C. Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology. 2008;18(12):1044–1053. doi: 10.1093/glycob/cwn084. [DOI] [PubMed] [Google Scholar]
- 116.Muller D, Djebbara-Hannas Z, Jourdain P, Vutskits L, Durbec P, Rougon G, Kiss JZ. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid-neural cell adhesion molecule-deficient hippocampus. Proc Natl Acad Sci USA. 2000;97(8):4315–4320. doi: 10.1073/pnas.070022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vaithianathan T, Matthias K, Bahr B, Schachner M, Suppiramaniam V, Dityatev A, Steinhauser C. Neural cell adhesion molecule-associated polysialic acid potentiates alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor currents. J Biol Chem. 2004;279(46):47975–47984. doi: 10.1074/jbc.M407138200. [DOI] [PubMed] [Google Scholar]
- 118.Hammond MS, Sims C, Parameshwaran K, Suppiramaniam V, Schachner M, Dityatev A. Neural cell adhesion molecule-associated polysialic acid inhibits NR2B-containing N-methyl-d-aspartate receptors and prevents glutamate-induced cell death. J Biol Chem. 2006;281(46):34859–34869. doi: 10.1074/jbc.M602568200. [DOI] [PubMed] [Google Scholar]
- 119.Kochlamazashvili G, Senkov O, Grebenyuk S, Robinson C, Xiao MF, Stummeyer K, Gerardy-Schahn R, Engel AK, Feig L, Semyanov A, Suppiramaniam V, Schachner M, Dityatev A. Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors. J Neurosci. 2010;30(11):4171–4183. doi: 10.1523/JNEUROSCI.5806-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Isomura R, Kitajima K, Sato C. Structural and functional impairments of polysialic acid by a mutated polysialyltransferase found in schizophrenia. J Biol Chem. 2011;286(24):21535–21545. doi: 10.1074/jbc.M111.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Joliot AH, Triller A, Volovitch M, Pernelle C, Prochiantz A. alpha-2,8-Polysialic acid is the neuronal surface receptor of antennapedia homeobox peptide. New Biol. 1991;3(11):1121–1134. [PubMed] [Google Scholar]
- 122.Prochiantz A, Joliot A. Can transcription factors function as cell-cell signalling molecules? Nat Rev Mol Cell Biol. 2003;4(10):814–819. doi: 10.1038/nrm1227. [DOI] [PubMed] [Google Scholar]
- 123.Westphal N, Kleene R, Lutz D, Theis T, Schachner M. Polysialic acid enters the cell nucleus attached to a fragment of the neural cell adhesion molecule NCAM to regulate the circadian rhythm in mouse brain. Mol Cell Neurosci. 2016;74:114–127. doi: 10.1016/j.mcn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 124.Westphal N, Loers G, Lutz D, Theis T, Kleene R, Schachner M. Generation and intracellular trafficking of a polysialic acid-carrying fragment of the neural cell adhesion molecule NCAM to the cell nucleus. Sci Rep. 2017;7(1):8622. doi: 10.1038/s41598-017-09468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuhnle A, Lutteke T, Bornhofft KF, Galuska SP. Polysialic acid modulates the binding of external lactoferrin in neutrophil extracellular traps. Biology. 2019 doi: 10.3390/biology8020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Theis T, Mishra B, von der Ohe M, Loers G, Prondzynski M, Pless O, Blackshear PJ, Schachner M, Kleene R. Functional role of the interaction between polysialic acid and myristoylated alanine-rich C kinase substrate at the plasma membrane. J Biol Chem. 2013;288(9):6726–6742. doi: 10.1074/jbc.M112.444034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakano T, Sugawara M, Kawakami H. Sialic acid in human milk: composition and functions. Acta Paediatr Taiwan. 2001;42(1):11–17. [PubMed] [Google Scholar]
- 128.Wang B, Hu H, Yu B. Molecular characterization of pig ST8Sia IV–a critical gene for the formation of neural cell adhesion molecule and its response to sialic acid supplement in piglets. Nutr Neurosci. 2006;9(3–4):147–154. doi: 10.1080/10284150600903594. [DOI] [PubMed] [Google Scholar]
- 129.Seki T, Arai Y. Expression of highly polysialylated NCAM in the neocortex and piriform cortex of the developing and the adult rat. Anat Embryol. 1991;184(4):395–401. doi: 10.1007/bf00957900. [DOI] [PubMed] [Google Scholar]
- 130.Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80(3):129–164. doi: 10.1016/j.pneurobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 131.Prodromidou K, Papastefanaki F, Sklaviadis T, Matsas R. Functional cross-talk between the cellular prion protein and the neural cell adhesion molecule is critical for neuronal differentiation of neural stem/precursor cells. Stem Cells. 2014;32(6):1674–1687. doi: 10.1002/stem.1663. [DOI] [PubMed] [Google Scholar]
- 132.Huang R, Yuan DJ, Li S, Liang XS, Gao Y, Lan XY, Qin HM, Ma YF, Xu GY, Schachner M, Sytnyk V, Boltze J, Ma QH, Li S. NCAM regulates temporal specification of neural progenitor cells via profilin2 during corticogenesis. J Cell Biol. 2020 doi: 10.1083/jcb.201902164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aaron LI, Chesselet MF. Heterogeneous distribution of polysialylated neuronal-cell adhesion molecule during post-natal development and in the adult: an immunohistochemical study in the rat brain. Neuroscience. 1989;28(3):701–710. doi: 10.1016/0306-4522(89)90015-8. [DOI] [PubMed] [Google Scholar]
- 134.Hekmat A, Bitter-Suermann D, Schachner M. Immunocytological localization of the highly polysialylated form of the neural cell adhesion molecule during development of the murine cerebellar cortex. J Comp Neurol. 1990;291(3):457–467. doi: 10.1002/cne.902910311. [DOI] [PubMed] [Google Scholar]
- 135.Chung WW, Lagenaur CF, Yan YM, Lund JS. Developmental expression of neural cell adhesion molecules in the mouse neocortex and olfactory bulb. J Comp Neurol. 1991;314(2):290–305. doi: 10.1002/cne.903140207. [DOI] [PubMed] [Google Scholar]
- 136.Ong E, Nakayama J, Angata K, Reyes L, Katsuyama T, Arai Y, Fukuda M. Developmental regulation of polysialic acid synthesis in mouse directed by two polysialyltransferases, PST and STX. Glycobiology. 1998;8(4):415–424. doi: 10.1093/glycob/8.4.415. [DOI] [PubMed] [Google Scholar]
- 137.van den Pol AN, Kim WT. NILE/L1 and NCAM-polysialic acid expression on growing axons of isolated neurons. J Comp Neurol. 1993;332(2):237–257. doi: 10.1002/cne.903320208. [DOI] [PubMed] [Google Scholar]
- 138.Ono K, Tomasiewicz H, Magnuson T, Rutishauser U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron. 1994;13(3):595–609. doi: 10.1016/0896-6273(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 139.Seki T, Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci. 1998;18(10):3757–3766. doi: 10.1523/JNEUROSCI.18-10-03757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vutskits L, Gascon E, Zgraggen E, Kiss JZ. The polysialylated neural cell adhesion molecule promotes neurogenesis in vitro. Neurochem Res. 2006;31(2):215–225. doi: 10.1007/s11064-005-9021-7. [DOI] [PubMed] [Google Scholar]
- 141.Yang P, Major D, Rutishauser U. Role of charge and hydration in effects of polysialic acid on molecular interactions on and between cell membranes. J Biol Chem. 1994;269(37):23039–23044. doi: 10.1016/S0021-9258(17)31616-2. [DOI] [PubMed] [Google Scholar]
- 142.Weinhold B, Seidenfaden R, Rockle I, Muhlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280(52):42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- 143.Angata K, Huckaby V, Ranscht B, Terskikh A, Marth JD, Fukuda M. Polysialic acid-directed migration and differentiation of neural precursors are essential for mouse brain development. Mol Cell Biol. 2007;27(19):6659–6668. doi: 10.1128/MCB.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Theodosis DT, Rougon G, Poulain DA. Retention of embryonic features by an adult neuronal system capable of plasticity: polysialylated neural cell adhesion molecule in the hypothalamo-neurohypophysial system. Proc Natl Acad Sci USA. 1991;88(13):5494–5498. doi: 10.1073/pnas.88.13.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Seki T, Arai Y. The persistent expression of a highly polysialylated NCAM in the dentate gyrus of the adult rat. Neurosci Res. 1991;12(4):503–513. doi: 10.1016/s0168-0102(09)80003-5. [DOI] [PubMed] [Google Scholar]
- 146.Schuster T, Krug M, Stalder M, Hackel N, Gerardy-Schahn R, Schachner M. Immunoelectron microscopic localization of the neural recognition molecules L1, NCAM, and its isoform NCAM180, the NCAM-associated polysialic acid, beta1 integrin and the extracellular matrix molecule tenascin-R in synapses of the adult rat hippocampus. J Neurobiol. 2001;49(2):142–158. doi: 10.1002/neu.1071. [DOI] [PubMed] [Google Scholar]
- 147.Seki T, Arai Y. Different polysialic acid-neural cell adhesion molecule expression patterns in distinct types of mossy fiber boutons in the adult hippocampus. J Comp Neurol. 1999;410(1):115–125. doi: 10.1002/(sici)1096-9861(19990719)410:1<115::aid-cne10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 148.Doyle E, Nolan PM, Bell R, Regan CM. Hippocampal NCAM180 transiently increases sialylation during the acquisition and consolidation of a passive avoidance response in the adult rat. J Neurosci Res. 1992;31(3):513–523. doi: 10.1002/jnr.490310315. [DOI] [PubMed] [Google Scholar]
- 149.Fox GB, O'Connell AW, Murphy KJ, Regan CM. Memory consolidation induces a transient and time-dependent increase in the frequency of neural cell adhesion molecule polysialylated cells in the adult rat hippocampus. J Neurochem. 1995;65(6):2796–2799. doi: 10.1046/j.1471-4159.1995.65062796.x. [DOI] [PubMed] [Google Scholar]
- 150.O'Connell AW, Fox GB, Barry T, Murphy KJ, Fichera G, Foley AG, Kelly J, Regan CM. Spatial learning activates neural cell adhesion molecule polysialylation in a corticohippocampal pathway within the medial temporal lobe. J Neurochem. 1997;68(6):2538–2546. doi: 10.1046/j.1471-4159.1997.68062538.x. [DOI] [PubMed] [Google Scholar]
- 151.Wang C, Pralong WF, Schulz MF, Rougon G, Aubry JM, Pagliusi S, Robert A, Kiss JZ. Functional N-methyl-d-aspartate receptors in O-2A glial precursor cells: a critical role in regulating polysialic acid-neural cell adhesion molecule expression and cell migration. J Cell Biol. 1996;135(6 Pt 1):1565–1581. doi: 10.1083/jcb.135.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rodriguez JJ, Dallerac GM, Tabuchi M, Davies HA, Colyer FM, Stewart MG, Doyere V. N-methyl-d-aspartate receptor independent changes in expression of polysialic acid-neural cell adhesion molecule despite blockade of homosynaptic long-term potentiation and heterosynaptic long-term depression in the awake freely behaving rat dentate gyrus. Neuron Glia Biol. 2008;4(3):169–178. doi: 10.1017/S1740925X09990159. [DOI] [PubMed] [Google Scholar]
- 153.Gallagher HC, Murphy KJ, Foley AG, Regan CM. Protein kinase C delta regulates neural cell adhesion molecule polysialylation state in the rat brain. J Neurochem. 2001;77(2):425–434. doi: 10.1046/j.1471-4159.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 154.Guiraudie-Capraz G, Chaillan FA, Truchet B, Franc JL, Mourre C, Roman FS. Increase in polysialyltransferase gene expression following LTP in adult rat dentate gyrus. Hippocampus. 2011;21(11):1180–1189. doi: 10.1002/hipo.20835. [DOI] [PubMed] [Google Scholar]
- 155.Becker CG, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res. 1996;45(2):143–152. doi: 10.1002/(SICI)1097-4547(19960715)45:2<143::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 156.Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17(3):413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- 157.Markram K, Gerardy-Schahn R, Sandi C. Selective learning and memory impairments in mice deficient for polysialylated NCAM in adulthood. Neuroscience. 2007;144(3):788–796. doi: 10.1016/j.neuroscience.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 158.Eckhardt M, Bukalo O, Chazal G, Wang L, Goridis C, Schachner M, Gerardy-Schahn R, Cremer H, Dityatev A. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci. 2000;20(14):5234–5244. doi: 10.1523/JNEUROSCI.20-14-05234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Angata K, Long JM, Bukalo O, Lee W, Dityatev A, Wynshaw-Boris A, Schachner M, Fukuda M, Marth JD. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J Biol Chem. 2004;279(31):32603–32613. doi: 10.1074/jbc.M403429200. [DOI] [PubMed] [Google Scholar]
- 160.Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, Muller D, Schachner M. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J Neurosci. 2004;24(42):9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brennaman LH, Zhang X, Guan H, Triplett JW, Brown A, Demyanenko GP, Manis PB, Landmesser L, Maness PF. Polysialylated NCAM and ephrinA/EphA regulate synaptic development of GABAergic interneurons in prefrontal cortex. Cereb Cortex. 2013;23(1):162–177. doi: 10.1093/cercor/bhr392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guirado R, Perez-Rando M, Sanchez-Matarredona D, Castillo-Gomez E, Liberia T, Rovira-Esteban L, Varea E, Crespo C, Blasco-Ibanez JM, Nacher J. The dendritic spines of interneurons are dynamic structures influenced by PSA-NCAM expression. Cereb Cortex. 2014;24(11):3014–3024. doi: 10.1093/cercor/bht156. [DOI] [PubMed] [Google Scholar]
- 163.Shen H, Watanabe M, Tomasiewicz H, Rutishauser U, Magnuson T, Glass JD. Role of neural cell adhesion molecule and polysialic acid in mouse circadian clock function. J Neurosci. 1997;17(13):5221–5229. doi: 10.1523/JNEUROSCI.17-13-05221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Glass JD, Shen H, Fedorkova L, Chen L, Tomasiewicz H, Watanabe M. Polysialylated neural cell adhesion molecule modulates photic signaling in the mouse suprachiasmatic nucleus. Neurosci Lett. 2000;280(3):207–210. doi: 10.1016/s0304-3940(00)00786-2. [DOI] [PubMed] [Google Scholar]
- 165.Nothias F, Vernier P, von Boxberg Y, Mirman S, Vincent JD. Modulation of NCAM polysialylation is associated with morphofunctional modifications in the hypothalamo-neurohypophysial system during lactation. Eur J Neurosci. 1997;9(8):1553–1565. doi: 10.1111/j.1460-9568.1997.tb01513.x. [DOI] [PubMed] [Google Scholar]
- 166.Theodosis DT, Bonhomme R, Vitiello S, Rougon G, Poulain DA. Cell surface expression of polysialic acid on NCAM is a prerequisite for activity-dependent morphological neuronal and glial plasticity. J Neurosci. 1999;19(23):10228–10236. doi: 10.1523/JNEUROSCI.19-23-10228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Muller D, Stoppini L, Wang C, Kiss JZ. A role for polysialylated neural cell adhesion molecule in lesion-induced sprouting in hippocampal organotypic cultures. Neuroscience. 1994;61(3):441–445. doi: 10.1016/0306-4522(94)90424-3. [DOI] [PubMed] [Google Scholar]
- 168.Szele FG, Chesselet MF. Cortical lesions induce an increase in cell number and PSA-NCAM expression in the subventricular zone of adult rats. J Comp Neurol. 1996;368(3):439–454. doi: 10.1002/(SICI)1096-9861(19960506)368:3<439::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 169.Franz CK, Rutishauser U, Rafuse VF. Polysialylated neural cell adhesion molecule is necessary for selective targeting of regenerating motor neurons. J Neurosci. 2005;25(8):2081–2091. doi: 10.1523/JNEUROSCI.4880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.El Maarouf A, Petridis AK, Rutishauser U. Use of polysialic acid in repair of the central nervous system. Proc Natl Acad Sci USA. 2006;103(45):16989–16994. doi: 10.1073/pnas.0608036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang Y, Zhang X, Yeh J, Richardson P, Bo X. Engineered expression of polysialic acid enhances Purkinje cell axonal regeneration in L1/GAP-43 double transgenic mice. Eur J Neurosci. 2007;25(2):351–361. doi: 10.1111/j.1460-9568.2007.05311.x. [DOI] [PubMed] [Google Scholar]
- 172.Fewou SN, Ramakrishnan H, Bussow H, Gieselmann V, Eckhardt M. Down-regulation of polysialic acid is required for efficient myelin formation. J Biol Chem. 2007;282(22):16700–16711. doi: 10.1074/jbc.M610797200. [DOI] [PubMed] [Google Scholar]
- 173.Mikkonen M, Soininen H, Kalvianen R, Tapiola T, Ylinen A, Vapalahti M, Paljarvi L, Pitkanen A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann Neurol. 1998;44(6):923–934. doi: 10.1002/ana.410440611. [DOI] [PubMed] [Google Scholar]
- 174.Pekcec A, Fuest C, Muhlenhoff M, Gerardy-Schahn R, Potschka H. Targeting epileptogenesis-associated induction of neurogenesis by enzymatic depolysialylation of NCAM counteracts spatial learning dysfunction but fails to impact epilepsy development. J Neurochem. 2008;105(2):389–400. doi: 10.1111/j.1471-4159.2007.05172.x. [DOI] [PubMed] [Google Scholar]
- 175.Mikkonen M, Soininen H, Tapiola T, Alafuzoff I, Miettinen R. Hippocampal plasticity in Alzheimer's disease: changes in highly polysialylated NCAM immunoreactivity in the hippocampal formation. Eur J Neurosci. 1999;11(5):1754–1764. doi: 10.1046/j.1460-9568.1999.00593.x. [DOI] [PubMed] [Google Scholar]
- 176.Murray HC, Swanson MEV, Dieriks BV, Turner C, Faull RLM, Curtis MA. Neurochemical characterization of PSA-NCAM(+) cells in the human brain and phenotypic quantification in Alzheimer's disease entorhinal cortex. Neuroscience. 2018;372:289–303. doi: 10.1016/j.neuroscience.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 177.Yoshimi K, Ren YR, Seki T, Yamada M, Ooizumi H, Onodera M, Saito Y, Murayama S, Okano H, Mizuno Y, Mochizuki H. Possibility for neurogenesis in substantia nigra of parkinsonian brain. Ann Neurol. 2005;58(1):31–40. doi: 10.1002/ana.20506. [DOI] [PubMed] [Google Scholar]
- 178.Sato C, Hane M. Mental disorders and an acidic glycan-from the perspective of polysialic acid (PSA/polySia) and the synthesizing enzyme, ST8SIA2. Glycoconj J. 2018;35(4):353–373. doi: 10.1007/s10719-018-9832-9. [DOI] [PubMed] [Google Scholar]
- 179.Mehanna A, Mishra B, Kurschat N, Schulze C, Bian S, Loers G, Irintchev A, Schachner M. Polysialic acid glycomimetics promote myelination and functional recovery after peripheral nerve injury in mice. Brain. 2009;132(Pt 6):1449–1462. doi: 10.1093/brain/awp128. [DOI] [PubMed] [Google Scholar]
- 180.Loers G, Astafiev S, Hapiak Y, Saini V, Mishra B, Gul S, Kaur G, Schachner M, Theis T. The polysialic acid mimetics idarubicin and irinotecan stimulate neuronal survival and neurite outgrowth and signal via protein kinase C. J Neurochem. 2017;142(3):392–406. doi: 10.1111/jnc.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Saini V, Lutz D, Kataria H, Kaur G, Schachner M, Loers G. The polysialic acid mimetics 5-nonyloxytryptamine and vinorelbine facilitate nervous system repair. Sci Rep. 2016;6:26927. doi: 10.1038/srep26927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bushman J, Mishra B, Ezra M, Gul S, Schulze C, Chaudhury S, Ripoll D, Wallqvist A, Kohn J, Schachner M, Loers G. Tegaserod mimics the neurostimulatory glycan polysialic acid and promotes nervous system repair. Neuropharmacology. 2014;79:456–466. doi: 10.1016/j.neuropharm.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Clausen J, Rosenkast P. Isolation of acid mucopolysaccharides of human brain. J Neurochem. 1962;9:393–398. doi: 10.1111/j.1471-4159.1962.tb09465.x. [DOI] [PubMed] [Google Scholar]
- 184.Abood LG, Abul-Haj SK. Histochemistry and characterization of hyaluronic acid in axons of peripheral nerve. J Neurochem. 1956;1(2):119–125. doi: 10.1111/j.1471-4159.1956.tb12062.x. [DOI] [PubMed] [Google Scholar]
- 185.Singh M, Bachhawat BK. The distribution and variation with age of different uronic acid-containing mucopolysaccharides in brain. J Neurochem. 1965;12(6):519–525. doi: 10.1111/j.1471-4159.1965.tb06779.x. [DOI] [PubMed] [Google Scholar]
- 186.Singh M, Chandrasekaran EV, Cherian R, Bachhawat BK. Isolation and characterization of glycosaminoglycans in brain of different species. J Neurochem. 1969;16(7):1157–1162. doi: 10.1111/j.1471-4159.1969.tb05961.x. [DOI] [PubMed] [Google Scholar]
- 187.Brandt AE, Distler J, Jourdian GW. Biosynthesis of the chondroitin sulfate-protein linkage region: purification and properties of a glucuronosyltransferase from embryonic chick brain. Proc Natl Acad Sci USA. 1969;64(1):374–380. doi: 10.1073/pnas.64.1.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kusche-Gullberg M, Kjellen L. Sulfotransferases in glycosaminoglycan biosynthesis. Curr Opin Struct Biol. 2003;13(5):605–611. doi: 10.1016/j.sbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 189.Masu M. Proteoglycans and axon guidance: a new relationship between old partners. J Neurochem. 2016;139(Suppl 2):58–75. doi: 10.1111/jnc.13508. [DOI] [PubMed] [Google Scholar]
- 190.Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277(51):49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang P, Lu H, Peixoto RT, Pines MK, Ge Y, Oku S, Siddiqui TJ, Xie Y, Wu W, Archer-Hartmann S, Yoshida K, Tanaka KF, Aricescu AR, Azadi P, Gordon MD, Sabatini BL, Wong ROL, Craig AM. Heparan sulfate organizes neuronal synapses through neurexin partnerships. Cell. 2018;174(6):1450–1464. doi: 10.1016/j.cell.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Werz W, Fischer G, Schachner M. Glycosaminoglycans of rat cerebellum: II. A developmental study. J Neurochem. 1985;44(3):907–910. doi: 10.1111/j.1471-4159.1985.tb12902.x. [DOI] [PubMed] [Google Scholar]
- 193.Balasubramanian AS, Bachhawat BK. Enzymic transfer of sulphate from 3'-phosphoadenosine 5'-phosphosulphate to mucopolysaccharides in rat brain. J Neurochem. 1964;11:877–885. doi: 10.1111/j.1471-4159.1964.tb06739.x. [DOI] [PubMed] [Google Scholar]
- 194.Yu P, Pearson CS, Geller HM. Flexible roles for proteoglycan sulfation and receptor signaling. Trends Neurosci. 2018;41(1):47–61. doi: 10.1016/j.tins.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kalus I, Rohn S, Puvirajesinghe TM, Guimond SE, Eyckerman-Kolln PJ, Ten Dam G, van Kuppevelt TH, Turnbull JE, Dierks T. Sulf1 and Sulf2 differentially modulate heparan sulfate proteoglycan sulfation during postnatal cerebellum development: evidence for neuroprotective and neurite outgrowth promoting functions. PLoS ONE. 2015;10(10):e0139853. doi: 10.1371/journal.pone.0139853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kalus I, Salmen B, Viebahn C, von Figura K, Schmitz D, D'Hooge R, Dierks T. Differential involvement of the extracellular 6-O-endosulfatases Sulf1 and Sulf2 in brain development and neuronal and behavioural plasticity. J Cell Mol Med. 2009;13(11–12):4505–4521. doi: 10.1111/j.1582-4934.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Miyata S, Kitagawa H. Formation and remodeling of the brain extracellular matrix in neural plasticity: roles of chondroitin sulfate and hyaluronan. Biochim Biophys Acta Gen Subj. 2017;10:2420–2434. doi: 10.1016/j.bbagen.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 198.Vos J, Kuriyama K, Roberts E. Distribution of acid mucopolysaccharides in subcellular fractions of mouse brain. Brain Res. 1969;12(1):172–179. doi: 10.1016/0006-8993(69)90064-x. [DOI] [PubMed] [Google Scholar]
- 199.Custod JT, Young IJ. Cat brain mucopolysaccharides and their in vivo hyaluronidase digestion. J Neurochem. 1968;15(8):809–813. doi: 10.1111/j.1471-4159.1968.tb10326.x. [DOI] [PubMed] [Google Scholar]
- 200.de Vivo L, Landi S, Panniello M, Baroncelli L, Chierzi S, Mariotti L, Spolidoro M, Pizzorusso T, Maffei L, Ratto GM. Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nat Commun. 2013;4:1484. doi: 10.1038/ncomms2491. [DOI] [PubMed] [Google Scholar]
- 201.Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15(3):414–422. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- 202.Wegrzyn G, Jakobkiewicz-Banecka J, Narajczyk M, Wisniewski A, Piotrowska E, Gabig-Ciminska M, Kloska A, Slominska-Wojewodzka M, Korzon-Burakowska A, Wegrzyn A. Why are behaviors of children suffering from various neuronopathic types of mucopolysaccharidoses different? Med Hypotheses. 2010;75(6):605–609. doi: 10.1016/j.mehy.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 203.Bigger BW, Begley DJ, Virgintino D, Pshezhetsky AV. Anatomical changes and pathophysiology of the brain in mucopolysaccharidosis disorders. Mol Genet Metab. 2018;125(4):322–331. doi: 10.1016/j.ymgme.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 204.Sethi MK, Zaia J. Extracellular matrix proteomics in schizophrenia and Alzheimer's disease. Anal Bioanal Chem. 2017;409(2):379–394. doi: 10.1007/s00216-016-9900-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 2008;72(6):455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 206.Dyck SM, Karimi-Abdolrezaee S. Chondroitin sulfate proteoglycans: key modulators in the developing and pathologic central nervous system. Exp Neurol. 2015;269:169–187. doi: 10.1016/j.expneurol.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 207.Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12(7):897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 208.Mohan V, Wyatt EV, Gotthard I, Phend KD, Diestel S, Duncan BW, Weinberg RJ, Tripathy A, Maness PF. Neurocan inhibits semaphorin 3F induced dendritic spine remodeling through NrCAM in cortical neurons. Front Cell Neurosci. 2018;12:346. doi: 10.3389/fncel.2018.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Van't Spijker HM, Rowlands D, Rossier J, Haenzi B, Fawcett JW, Kwok JCF. Neuronal pentraxin 2 binds PNNs and enhances PNN formation. Neural Plast. 2019;2019:6804575. doi: 10.1155/2019/6804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chandrasekaran EV, Bachhawat BK. Isolation and characterization of glycosaminoglycans in peripheral nerve and spinal cord of monkey. J Neurochem. 1969;16(11):1529–1532. doi: 10.1111/j.1471-4159.1969.tb09908.x. [DOI] [PubMed] [Google Scholar]
- 211.Massey JM, Amps J, Viapiano MS, Matthews RT, Wagoner MR, Whitaker CM, Alilain W, Yonkof AL, Khalyfa A, Cooper NG, Silver J, Onifer SM. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol. 2008;209(2):426–445. doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bradbury EJ, Carter LM. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. 2011;84(4–5):306–316. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 213.Igarashi M, Takeuchi K, Sugiyama S. Roles of CSGalNAcT1, a key enzyme in regulation of CS synthesis, in neuronal regeneration and plasticity. Neurochem Int. 2018;119:77–83. doi: 10.1016/j.neuint.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 214.Rost S, Akyuz N, Martinovic T, Huckhagel T, Jakovcevski I, Schachner M. Germline ablation of dermatan-4O-sulfotransferase1 reduces regeneration after mouse spinal cord injury. Neuroscience. 2016;312:74–85. doi: 10.1016/j.neuroscience.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 215.Akyuz N, Rost S, Mehanna A, Bian S, Loers G, Oezen I, Mishra B, Hoffmann K, Guseva D, Laczynska E, Irintchev A, Jakovcevski I, Schachner M. Dermatan 4-O-sulfotransferase1 ablation accelerates peripheral nerve regeneration. Exp Neurol. 2013;247:517–530. doi: 10.1016/j.expneurol.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 216.Loers G, Liao Y, Hu C, Xue W, Shen H, Zhao W, Schachner M. Identification and characterization of synthetic chondroitin-4-sulfate binding peptides in neuronal functions. Sci Rep. 2019;9(1):1064. doi: 10.1038/s41598-018-37685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Butterfield KC, Caplan M, Panitch A. Identification and sequence composition characterization of chondroitin sulfate-binding peptides through peptide array screening. Biochemistry. 2010;49(7):1549–1555. doi: 10.1021/bi9021044. [DOI] [PubMed] [Google Scholar]
- 218.Butterfield KC, Conovaloff A, Caplan M, Panitch A. Chondroitin sulfate-binding peptides block chondroitin 6-sulfate inhibition of cortical neurite growth. Neurosci Lett. 2010;478(2):82–87. doi: 10.1016/j.neulet.2010.04.070. [DOI] [PubMed] [Google Scholar]
- 219.Domino SE, Zhang L, Gillespie PJ, Saunders TL, Lowe JB. Deficiency of reproductive tract alpha(1,2)fucosylated glycans and normal fertility in mice with targeted deletions of the FUT1 or FUT2 alpha(1,2)fucosyltransferase locus. Mol Cell Biol. 2001;21(24):8336–8345. doi: 10.1128/MCB.21.24.8336-8345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Smalla KH, Angenstein F, Richter K, Gundelfinger ED, Staak S. Identification of fucose alpha(1–2) galactose epitope-containing glycoproteins from rat hippocampus. NeuroReport. 1998;9(5):813–817. doi: 10.1097/00001756-199803300-00009. [DOI] [PubMed] [Google Scholar]
- 221.Kalovidouris SA, Gama CI, Lee LW, Hsieh-Wilson LC. A role for fucose alpha(1–2) galactose carbohydrates in neuronal growth. J Am Chem Soc. 2005;127(5):1340–1341. doi: 10.1021/ja044631v. [DOI] [PubMed] [Google Scholar]
- 222.Krug M, Jork R, Reymann K, Wagner M, Matthies H. The amnesic substance 2-deoxy-d-galactose suppresses the maintenance of hippocampal LTP. Brain Res. 1991;540(1–2):237–242. doi: 10.1016/0006-8993(91)90513-u. [DOI] [PubMed] [Google Scholar]
- 223.Rose SP, Jork R. Long-term memory formation in chicks is blocked by 2-deoxygalactose, a fucose analog. Behav Neural Biol. 1987;48(2):246–258. doi: 10.1016/S0163-1047(87)90808-9. [DOI] [PubMed] [Google Scholar]
- 224.Tiunova AA, Anokhin KV, Rose SP. Two critical periods of protein and glycoprotein synthesis in memory consolidation for visual categorization learning in chicks. Learn Mem. 1998;4(5):401–410. doi: 10.1101/lm.4.5.401. [DOI] [PubMed] [Google Scholar]
- 225.Kleene R, Yang H, Kutsche M, Schachner M. The neural recognition molecule L1 is a sialic acid-binding lectin for CD24, which induces promotion and inhibition of neurite outgrowth. J Biol Chem. 2001;276(24):21656–21663. doi: 10.1074/jbc.M101790200. [DOI] [PubMed] [Google Scholar]
- 226.Vinson M, van der Merwe PA, Kelm S, May A, Jones EY, Crocker PR. Characterization of the sialic acid-binding site in sialoadhesin by site-directed mutagenesis. J Biol Chem. 1996;271(16):9267–9272. doi: 10.1074/jbc.271.16.9267. [DOI] [PubMed] [Google Scholar]
- 227.Vinson M, Strijbos PJ, Rowles A, Facci L, Moore SE, Simmons DL, Walsh FS. Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition. J Biol Chem. 2001;276(23):20280–20285. doi: 10.1074/jbc.M100345200. [DOI] [PubMed] [Google Scholar]
- 228.Bartels MF, Winterhalter PR, Yu J, Liu Y, Lommel M, Mohrlen F, Hu H, Feizi T, Westerlind U, Ruppert T, Strahl S. Protein O-mannosylation in the murine brain: occurrence of mono-O-mannosyl glycans and identification of new substrates. PLoS ONE. 2016;11(11):e0166119. doi: 10.1371/journal.pone.0166119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lommel M, Strahl S. Protein O-mannosylation: conserved from bacteria to humans. Glycobiology. 2009;19(8):816–828. doi: 10.1093/glycob/cwp066. [DOI] [PubMed] [Google Scholar]
- 230.Vester-Christensen MB, Halim A, Joshi HJ, Steentoft C, Bennett EP, Levery SB, Vakhrushev SY, Clausen H. Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc Natl Acad Sci USA. 2013;110(52):21018–21023. doi: 10.1073/pnas.1313446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Praissman JL, Willer T, Sheikh MO, Toi A, Chitayat D, Lin YY, Lee H, Stalnaker SH, Wang S, Prabhakar PK, Nelson SF, Stemple DL, Moore SA, Moremen KW, Campbell KP, Wells L. The functional O-mannose glycan on alpha-dystroglycan contains a phospho-ribitol primed for matriglycan addition. eLife. 2016 doi: 10.7554/eLife.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dwyer CA, Baker E, Hu H, Matthews RT. RPTPzeta/phosphacan is abnormally glycosylated in a model of muscle-eye-brain disease lacking functional POMGnT1. Neuroscience. 2012;220:47–61. doi: 10.1016/j.neuroscience.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bleckmann C, Geyer H, Lieberoth A, Splittstoesser F, Liu Y, Feizi T, Schachner M, Kleene R, Reinhold V, Geyer R. O-glycosylation pattern of CD24 from mouse brain. Biol Chem. 2009;390(7):627–645. doi: 10.1515/BC.2009.044. [DOI] [PubMed] [Google Scholar]
- 234.Pacharra S, Hanisch FG, Breloy I. Neurofascin 186 is O-mannosylated within and outside of the mucin domain. J Proteome Res. 2012;11(8):3955–3964. doi: 10.1021/pr200996y. [DOI] [PubMed] [Google Scholar]
- 235.Pacharra S, Hanisch FG, Muhlenhoff M, Faissner A, Rauch U, Breloy I. The lecticans of mammalian brain perineural net are O-mannosylated. J Proteome Res. 2013;12(4):1764–1771. doi: 10.1021/pr3011028. [DOI] [PubMed] [Google Scholar]
- 236.Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan.. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J Biol Chem. 1997;272(4):2156–2162. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- 237.Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418(6896):417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 238.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119(Pt 2):199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 239.Kizuka Y, Taniguchi N. Neural functions of bisecting GlcNAc. Glycoconj J. 2018;35(4):345–351. doi: 10.1007/s10719-018-9829-4. [DOI] [PubMed] [Google Scholar]
- 240.Wuhrer M, Geyer H, von der Ohe M, Gerardy-Schahn R, Schachner M, Geyer R. Localization of defined carbohydrate epitopes in bovine polysialylated NCAM. Biochimie. 2003;85(1–2):207–218. doi: 10.1016/s0300-9084(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 241.Shigeta M, Shibukawa Y, Ihara H, Miyoshi E, Taniguchi N, Gu J. Beta1,4-N-Acetylglucosaminyltransferase III potentiates beta1 integrin-mediated neuritogenesis induced by serum deprivation in Neuro2a cells. Glycobiology. 2006;16(6):564–571. doi: 10.1093/glycob/cwj100. [DOI] [PubMed] [Google Scholar]
- 242.Bhattacharyya R, Bhaumik M, Raju TS, Stanley P. Truncated, inactive N-acetylglucosaminyltransferase III (GlcNAc-TIII) induces neurological and other traits absent in mice that lack GlcNAc-TIII. J Biol Chem. 2002;277(29):26300–26309. doi: 10.1074/jbc.M202276200. [DOI] [PubMed] [Google Scholar]
- 243.Gu J, Zhao Y, Isaji T, Shibukawa Y, Ihara H, Takahashi M, Ikeda Y, Miyoshi E, Honke K, Taniguchi N. Beta 1,4-N-acetylglucosaminyltransferase III down-regulates neurite outgrowth induced by costimulation of epidermal growth factor and integrins through the Ras/ERK signaling pathway in PC12 cells. Glycobiology. 2004;14(2):177–186. doi: 10.1093/glycob/cwh016. [DOI] [PubMed] [Google Scholar]
- 244.Kizuka Y, Kitazume S, Fujinawa R, Saito T, Iwata N, Saido TC, Nakano M, Yamaguchi Y, Hashimoto Y, Staufenbiel M, Hatsuta H, Murayama S, Manya H, Endo T, Taniguchi N. An aberrant sugar modification of BACE1 blocks its lysosomal targeting in Alzheimer's disease. EMBO Mol Med. 2015;7(2):175–189. doi: 10.15252/emmm.201404438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Akasaka-Manya K, Manya H, Sakurai Y, Wojczyk BS, Kozutsumi Y, Saito Y, Taniguchi N, Murayama S, Spitalnik SL, Endo T. Protective effect of N-glycan bisecting GlcNAc residues on beta-amyloid production in Alzheimer's disease. Glycobiology. 2010;20(1):99–106. doi: 10.1093/glycob/cwp152. [DOI] [PubMed] [Google Scholar]
- 246.Kizuka Y, Nakano M, Kitazume S, Saito T, Saido TC, Taniguchi N. Bisecting GlcNAc modification stabilizes BACE1 protein under oxidative stress conditions. Biochem J. 2016;473(1):21–30. doi: 10.1042/BJ20150607. [DOI] [PubMed] [Google Scholar]
- 247.Schedin-Weiss S, Gaunitz S, Sui P, Chen Q, Haslam SM, Blennow K, Winblad B, Dell A, Tjernberg LO. Glycan biomarkers for Alzheimer disease correlate with T-tau and P-tau in cerebrospinal fluid in subjective cognitive impairment. FEBS J. 2019 doi: 10.1111/febs.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Allam H, Aoki K, Benigno BB, McDonald JF, Mackintosh SG, Tiemeyer M, Abbott KL. Glycomic analysis of membrane glycoproteins with bisecting glycosylation from ovarian cancer tissues reveals novel structures and functions. J Proteome Res. 2015;14(1):434–446. doi: 10.1021/pr501174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Handa-Narumi M, Yoshimura T, Konishi H, Fukata Y, Manabe Y, Tanaka K, Bao GM, Kiyama H, Fukase K, Ikenaka K. Branched sialylated N-glycans are accumulated in brain synaptosomes and interact with siglec-H. Cell Struct Funct. 2018;43(2):141–152. doi: 10.1247/csf.18009. [DOI] [PubMed] [Google Scholar]
- 250.Henion TR, Raitcheva D, Grosholz R, Biellmann F, Skarnes WC, Hennet T, Schwarting GA. Beta 1,3-N-acetylglucosaminyltransferase 1 glycosylation is required for axon pathfinding by olfactory sensory neurons. J Neurosci. 2005;25(8):1894–1903. doi: 10.1523/JNEUROSCI.4654-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Young WW, Jr, Portoukalian J, Hakomori S. Two monoclonal anticarbohydrate antibodies directed to glycosphingolipids with a lacto-N-glycosyl type II chain. J Biol Chem. 1981;256(21):10967–10972. doi: 10.1016/S0021-9258(19)68541-8. [DOI] [PubMed] [Google Scholar]