Abstract
The blood–brain barrier (BBB) provides essential neuroprotection from environmental toxins and xenobiotics, through high expression of drug efflux transporters in endothelial cells of the cerebral capillaries. However, xenobiotic exposure, stress, and inflammatory stimuli have the potential to disrupt BBB permeability in fetal and post-natal life. Understanding the role and ability of the BBB in protecting the developing brain, particularly with respect to drug/toxin transport, is key to promoting long-term brain health. Drug transporters, particularly P-gp and BCRP are expressed in early gestation at the developing BBB and have a crucial role in developmental homeostasis and fetal brain protection. We have highlighted several factors that modulate drug transporters at the developing BBB, including synthetic glucocorticoid (sGC), cytokines, maternal infection, and growth factors. Some factors have the potential to increase expression and function of drug transporters and increase brain protection (e.g., sGC, transforming growth factor [TGF]-β). However, others inhibit drug transporters expression and function at the BBB, increasing brain exposure to xenobiotics (e.g., tumor necrosis factor [TNF], interleukin [IL]-6), negatively impacting brain development. This has implications for pregnant women and neonates, who represent a vulnerable population and may be exposed to drugs and environmental toxins, many of which are P-gp and BCRP substrates. Thus, alterations in regulated transport across the developing BBB may induce long-term changes in brain health and compromise pregnancy outcome. Furthermore, a large portion of neonatal adverse drug reactions are attributed to agents that target or access the nervous system, such as stimulants (e.g., caffeine), anesthetics (e.g., midazolam), analgesics (e.g., morphine) and antiretrovirals (e.g., Zidovudine); thus, understanding brain protection is key for the development of strategies to protect the fetal and neonatal brain.
Keywords: Blood–brain barrier, ABC transporter, Fetal, Prenatal glucocorticoids, Maternal infection
Introduction
The blood–brain barrier (BBB) provides essential neuroprotection from environmental toxins and xenobiotics that may be circulating in the peripheral blood, through high expression of drug efflux transporters in endothelial cells of the cerebral capillaries. The BBB plays a key role in maintaining an optimal microenvironment for neuronal cells through hormone, nutrient and ion transport, as well as removal of waste products [1]. However, changes in its surrounding microenvironment, such as xenobiotic exposure, stress and inflammatory stimuli, have the potential to disrupt BBB permeability and induce brain injury, neurodegeneration and disease [2].
The developing fetal brain is highly susceptible to the influences of environmental factors; thus understanding the role and ability of the BBB in protecting the developing brain, particularly with respect to drug/toxin transport, is key to long-term brain health. In this regard, a number of drugs prescribed during pregnancy are actively transported at the level of the placenta and the fetal BBB. This review focuses on the ontogeny and regulation of key ATP-binding cassette (ABC) drug efflux transporter systems at the BBB, P-glycoprotein (P-gp; encoded by ABCB1) and breast cancer resistance protein (BCRP/ABCG2); which among other solute carrier (SLC) transporters, such as organic anion transporter 2 (OAT2/SLC22A7) and monocarboxylate transporter 1 (MCT1/SLC16A1), are present at high levels at the human BBB [3].
The role of the placenta in fetal brain protection, and how the fetal BBB and the placenta coordinate to protect the developing fetal brain will also be considered. Finally, we outline potential modulators of BBB-efflux transporter function during development, including exposure to infection, corticosteroids, and other modulators, which will directly impact fetal brain permeability and protection. Knowledge as to how brain protection is acquired and disrupted during development may offer new opportunities to reduce obstetric complications and improve pediatric outcomes.
BBB structure and function
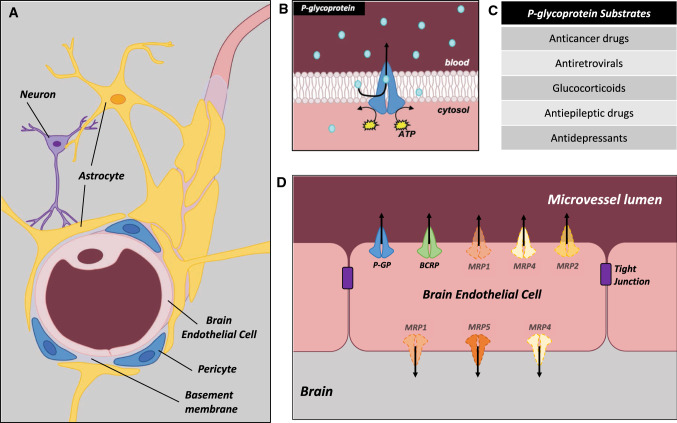
The BBB is a highly organized biological system providing a physical barrier between the brain and the systemic circulation. Histologically, the BBB is comprised of brain microvessel endothelial cells (BEC)s, connected by intricate tight junctions, ensheathed at the abluminal surface by astrocyte foot processes and resident pericytes, which aid in the regulation of endothelial cell permeability (Fig. 1A). At the level of cerebral capillaries, this barrier is essential, as in the adult human, it covers a large surface area (12–18 m2) with a diffusion distance of 25 µm between the lumen and nearby neurons (to maximize cerebral–blood exchange rates) [4]. Here, we summarize important features of the BBB components related to brain protection.
Fig. 1.
Cellular structure of the blood–brain barrier (BBB). A Cerebral capillaries are ensheathed by astrocyte foot processes, and share a continuous basement membrane where pericytes are located. Nearby neurons make contact with astrocytes. Together these cells form the BBB or “neurovascular unit”. B Mechanism of substrate efflux. P-glycoprotein (P-gp) is a transmembrane transporter that effluxes its substrates against their concentration gradients by utilizing ATP. Studies have shown that P-gp acts as a flippase, grabbing lipophilic substrates as they cross the plasma membrane, and excluding them. C Categories of P-gp substrates. Listed are some of the major categories of P-gp substrates. Most P-gp substrates are lipophilic and there is large substrate overlap with other drug transporters. D Localization of drug transporters in brain endothelial cells. P-gp and breast cancer resistance (BCRP) protein are primarily localized at the luminal side of BECs where they prevent entry of their substrates into the brain parenchyma. Multidrug-related proteins (Mrp) 1,3, 4, 5 have been detected in brain endothelial cells, and some studies have suggested their localization to the luminal or basolateral side of endothelial cells
Endothelial cells
At the BBB, endothelial cells have several unique characteristics compared to those in the systemic circulation. These include low vesicular transport, and high expression of nutrient and drug transporters, with the latter controlling transcellular passage of molecules into and from the brain. One study found BECs also have a higher mitochondrial volume compared to systemic endothelial cells [5], which many postulate supports diverse specialized active transport systems [1, 6]; however, more research is required to confirm this relationship.
Cerebral capillaries lack fenestrations and possess unique tight junction structures to efficiently restrict paracellular transport [7]. The high complexity and number of tight junctions are unique adaptive features of BECs compared to other endothelial cells [8, 9]. The primary proteins that make up tight junctions in BECs are occludins, claudins, and zonula occludens. Occludin, a 65kDA protein, with 4 transmembrane domains was the first integral membrane protein discovered at the tight junction. However, knockout of occludin did not affect tight junction strand formation, suggesting the necessity of other membrane proteins at the tight junction [10]. The claudin family of proteins has a similar structure to occludin but shares little sequence homology. In the brain, claudins-5 is specifically expressed in endothelial cells and not at tight junctions of other cell types [11]. Knock-out of Cldn5 in mice leads to size-selective leakiness at the BBB, and was therefore deemed the integral protein of the tight junction system [12]. Zonula Occludens (ZO) proteins connect the intercellular domains of the tight junction to the cytoskeleton and add structural support [4]. Together, tight junction proteins confer an important barrier by restricting the intercellular space and therefore limiting the entrance of molecules as small as 4.7 ˜A (sucrose) [13]. Additionally, the combination of a small intercellular space and charged extracellular loops of claudin proteins, results in limited paracellular ion transport. The restricted movement of charged molecules across the paracellular space creates high resistance across the cerebral endothelium and helps to establish concentration gradients [7, 8, 14]. However, many potentially toxic endogenous and exogenous compounds are lipophilic, and able to freely cross transcellularly via passive diffusion across the plasma membrane. In this case, protection is provided by active transporter systems in the BBB (discussed in detail in “ATP-Binding Cassette (ABC) Transporters”), which limit the entry of a myriad of lipophilic and non-lipophilic drugs and environmental toxins into brain [15].
Pericytes
Pericytes play a key role in maintaining BBB homeostasis and integrity through secreted factors and regulation of tight junction integrity and vesicular trafficking. Pericyte coverage is higher in brain vessels than in any other tissue vasculature. Pericytes together with astrocyte foot processes are present in the basement membrane of capillary endothelial cells (Fig. 1A), and make direct contact with endothelial cells via adheren (N-cadherin) and gap junctions (connexin 43) [16]. During BBB development in mice, pericytes are closely associated with BECs, and are present from initial migration of the vascular plexus into the neural tissue [17]. Pericytes are key cellular components modulating specificity of transport across the neurovascular unit since they reduce vesicular trafficking in endothelial cells [18]. In mice, decreased pericyte number is associated with increased BBB permeability resulting from structural abnormalities in endothelial cell contacts, despite normal tight junction formation [17, 19]. Regulation of BECs by pericytes appears region-specific since a uniform reduction in pericyte number throughout the brain only affects BBB permeability in some specific regions (e.g., cortex, hippocampus) [20]. Less is known about the role of pericytes in specifically regulating drug transporter expression at the BBB. One study identified increased P-gp function in mouse immortalized brain capillary endothelial cells after co-culture with rat brain pericytes; this effect was in part mediated by transforming growth factor beta (TGF-β) secreted by pericytes [21]. The specific intracellular signaling pathways involved in TGF-β mediated upregulation of P-gp and BCRP at the BBB are unknown. Recently, studies in hepatocellular carcinoma cells have implicated miRNAs and lnRNAs in mediating the effects of TGF-β induction of P-gp and BCRP expression. Specifically, a SMAD4/HOTAIR/miR-145 axis was identified that responds to TGF-β by up regulating P-gp and BCRP [22]. While, this signaling pathway has not been investigated in physiological/non-cancerous tissues, it could be hypothesized as one possible mechanism mediating pericyte regulation of P-gp and BCRP expression/function in BECs. Of note, TGF-β is also secreted by astrocytes, and plays a key role in maintaining BBB integrity via regulation of P-gp. However, another study found no change in Abcb1 mRNA expression after co-culture with pericytes [23]. The mechanisms through which pericytes regulate drug transporters in BECs warrants further investigation.
Astrocytes
Astrocyte foot processes surround the abluminal face of endothelial cells, and share a continuous basement membrane (Fig. 1A) [24]. Astrocytes regulate tight junction function between endothelial cells, decreasing the size of the intercellular cleft [25]. This has been demonstrated in in vitro co-cultures of astrocytes and BECs, where trans-endothelial electrical resistance (TEER), is increased [26, 27]. By inducing tight junction function, astrocytes also contribute to the polarization of transporters to the luminal membrane, including the GLUT1 glucose transporter and P-gp. Astrocyte-endothelial cell interactions lead to differentiation in both cell types. Agrin in the basal lamina, which is a heparin sulfate proteoglycan, secreted by endothelial cells at the time of BBB tightening, stimulates polarization of astrocytes, including sequestering of the water channel aquaporin 4 (AQP4) to astrocytic end feet [28]. In this connection, in a rat model of temporal lobe epilepsy, disruption of aquaporins expression by the drug acetazolamide, leads to disruption of P-gp expression in hippocampal tissue [29]. Furthermore, astrocyte secreted factors also increase expression and function of the efflux drug transporter P-gp in BECs from neonatal guinea pigs [27], further demonstrating an interplay between these two cell types regulating transport function at the developing BBB.
ATP-binding cassette (ABC) transporters
The ABC transporters are critical for maintaining brain homeostasis and provide a route for xenobiotic clearance from the cerebral parenchyma into the circulation. The human ABC superfamily of efflux transporters consists of 48 proteins, divided into seven subfamilies from ABCA to ABCG [30]. In humans and mammals, the ABC superfamily is largely comprised of drug and lipid transporters, and generally possess a basic structure with four domains. Two transmembrane domains (TMD) hold the transporters in the lipid bilayers and are responsible for substrates’ permeation, and two nucleotide-binding domains (NBD), responsible for ATP-driven energy supply [31].
The ABC drug transporters P-gp (encoded by ABCB1 in humans and by Abcb1a and Abcb1b in mice and rats), BCRP (encoded by ABCG2), and the Multidrug Resistance Proteins, isoforms 4 and 5 (Mrp 4 and 5, encoded by ABCC4 and 5, respectively) are the most abundant ABC drug transporters at the BBB. Prior to determining their role in physiological barrier sites (i.e., placenta, kidney, intestine, BBB) P-gp and BCRP were first identified and studied as sources of multidrug resistance (MDR) in cancer cells [32, 33]. P-gp and BCRP are highly expressed in the luminal surface of the BECs (facing the systemic circulation) [3, 34, 35], although some studies have shown that they may also be weakly localized on the abluminal membrane (facing neuronal tissue) [36] (Fig. 1). Drug transporters have a wide spectrum of substrate specificities, with some overlapping substrates. Pharmaceutical agents that are substrates for ABC transporters include anti-cancer drugs, HIV inhibitors, opioids, antibiotics, antidepressants, antiepileptics, immunosuppressants and synthetic glucocorticoids (sGC) among others [37, 38]. Table 1 presents a summary of important ABC transporter substrates. Drug transporters exert an essential function in reducing the transfer of pesticides, toxins, and potentially harmful substances from the systemic circulation into the brain. However, they also represent a challenge to therapeutic treatment of brain tumors as they limit drug bioavailability, a major obstacle, for example, in the use of peripherally administered anti-cancer drugs. Additionally, drug transporters regulate the uptake of several endogenous substrates (e.g., steroids, lipids) that may be important for normal function and development.
Table 1.
Blood–brain barrier ABC transporters and their major substrates relevant in pregnancy and neonatal life
| Class | Substrate | ABC transporter(s) | Relevance in pregnancy and neonatal life | References |
|---|---|---|---|---|
| Anti-epileptic | Phenobarbital | P-gp | First-line treatment for most neonatal seizures | [39, 40] |
| Lamotrigine | P-gp | Safe for use during pregnancy (26,416,395) | [39, 41] | |
| Phenytoin |
P-gp Mrp1 |
Second line treatment for neonatal seizures | [39, 40, 42] | |
| Topiramate | P-gp | Second-generation AED, sometimes used to treat refractory neonatal seizures (Sandoval 2019) | [39, 40] | |
| Anti-HIV | Zidovudine |
P-gp BCRP Mrp4 |
Indicated for treatment of neonates with HIV | [43, 44] |
| Ritonavir |
P-gp Mrp1, Mrp2 |
Infants and children, combined with Lopinavir | [42, 45] | |
| AZT |
BCRP Mrp4 |
Safe for use in pregnant women and their infants in combination w/Zidovudine | [42, 46] | |
| Analgesics | Morphine | P-gp | Management for post-operative pain | [39, 47] |
| Methadone | P-gp | Neonatal abstinence syndrome; therapeutic treatment for opiate abuse during pregnancy | [47, 48] | |
| Anti-cancer | Doxorubicine |
P-gp BCRP Mrp1, Mrp2, Mrp6 |
[42] | |
| Mitoxantrone |
P-gp BCRP |
[42] | ||
| Daunorubicin |
P-gp BCRP Mrp1, Mrp6 |
[42] | ||
| Methotrexate |
P-gp Mrp1, Mrp2, Mrp3, Mrp4 |
[42] | ||
| Anti-depresssants | Amitryptiline | P-gp | Increased rate of cardiac abnormalities when taken during pregnancy | [42, 49] |
| Doxepin | P-gp | No increased incidence of neonatal cardiac abnormalities when used during pregnancy | [42, 49] | |
| Other substrates | Verapamil |
P-gp Mrp1 |
Calcium channel blocker; a competitive inhibitor of P-gp | [42] |
| Cyclosporin A |
P-gp Mrp1 |
Immunosuppressants; also a competitive inhibitor of P-gp | [42] |
P-gp is one of the major BBB drug transporters and is the most well-studied ABC transporter, based on its crucial role in neuroprotection and drug resistance. P-gp, is predominantly located on the luminal surface of BECs [50–52] (Fig. 1), where it functions as a drug transporter at the plasma membrane excluding a large spectrum of substrates (please see Table 1). P-gp is also localized intracellularly in the Golgi complex, endosomes/lysosomes/proteasome and in the endoplasmic reticulum (ER) which are important sites for P-gp post-translational modification, traffic/recycling/degradation and synthesis, respectively [53–56]. P-gp has a predicted molecular weight of 140 kDa, and the mature N-glycosylated protein migrates through SDS-PAGE at an apparent molecular weight of 170 kDa [57, 58]. P-gp protein and Abcb1 mRNA have also been identified in the primate brain parenchyma, neuronal and glial cells, though at lower levels than in the BEC [59–61]. In this context, while the expression of P-gp and BCRP is likely of importance, less is known about their functional role in neuronal and glial cells.
Functional P-gp deficiency dramatically increases transfer of many drugs and xenobiotics into the brain. In vivo studies in Abcb1 knockout mice revealed up to 100-fold increased brain accumulation of P-gp substrates [50, 62, 63]. Further, exposure to ivermectin during pregnancy in mice, lead to congenital head anomalies in 100% of homozygous Abcb1−/− offspring [64]. Recently, an epidemiological study supported the effect of P-gp in protecting the fetal human brain, by showing an increased risk for specific CNS congenital anomalies after exposure to clinical P-gp substrates (including cimetidine, ranitidine, risperidone, citalopram) along with the use of P-gp inhibitors (including omeprazole, pantoprazole, haloperidol) in women in the first-trimester of pregnancy [65].
Although P-gp was the first drug transporter described at the BBB [66, 67], BCRP is the most abundant ABC transporter in the human BBB at the level of protein [68] and mRNA [69]. BCRP is a “half-transporter”, with 70 kDa and only one TMD and one NBD, that requires homodimerization to become functionally active [70]. Similar to P-gp, BCRP is highly expressed in the luminal membrane of brain microvascular endothelium (Fig. 1) [71]. The absence of BCRP in Abcg2−/− mice results in increased accumulation of BCRP substrates in the brain [72, 73]. Intracellular localization of BCRP has also been demonstrated in many cell lines [74–76]; however, specific localization and function in organelles/vesicles in developing BBB require further investigation.
P-gp and BCRP have overlapping substrates, and a number of studies have suggested a synergistic/complimentary relationship between the two transporters [77–88]. There is evidence for a compensatory mechanism by which downregulation of one transporter results in increased expression and function of the other [89–91]. In coordinating the response to infection, this appears to be the case. In hCMEC/D3 cells, LPS and poly:ic exposure results in increased P-gp function but decreased BCRP activity. ssRNA-40 had the opposite effect where P-gp function was decreased, but BCRP activity increased [92]. This complementary relationship may be due to differences in the signaling pathways induced by different infective insults. Cross-talk between the regulatory pathways of P-gp and BCRP likely has important implications for drug delivery into the brain. While there is a considerable knowledge of P-gp regulation at the BBB relatively little is known concerning the mechanisms regulating BCRP expression and function. Further studies are required to better understand the potential cross-talk between these two transporter systems in the developing brain.
MRPs are a subfamily of ABC transporters with 13 members, that are generally larger than other ABC transporters; approximately 200 kDa [93]. MRP-1 to 6, 9 and 10 have been detected in human BECs, with MRP4 and 5 expressed at highest levels [34, 35, 94, 95]. MRPs are present either at the luminal or abluminal membranes or both in BECs, and contribute to efflux of substrates in both directions at the BBB [36, 96–99]. MRP substrates include antibiotics, antiretrovirals, immunosuppressive drugs, antiepileptic and anti-cancer agents [100–102] (Table 1). However, relatively little is known concerning the regulation of MRPs or the expression and regulation of other ABC transporters in the adult or developing BBB; many of which may have important transport functions.
Other ABC transporters have been detected at the rodent and human BBB, through mass spectrometry-based proteomics. These include the ABCA superfamily, which are involved in lipid transport (e.g., cholesterol) [3]. ABCA2 and ABCA8 are the two most abundant ABC transporters at the BBB after BCRP and P-gp. However, their functional role remains unclear. ABCA2 is expressed in several brain cell types including oligodendrocytes, some neurons, and brain endothelial cells. Studies in oligodendrocytes have determined that ABCA2 is localized to the lysosomal compartment, rather than the plasma membrane, and could therefore be involved in intracellular lipid transport [103, 104]. Importantly, there is increasing recognition for the role of specific ABC transporters proteins, such as ABCA1, ABCA2, BCRP, MRP-1 and P-gp clearing β-amyloid protein (the major amyloid plaque component) out of the brain. As such, dysregulation of ABC transporters has therefore been implicated in the pathogenesis of Alzheimer’s Disease [105, 106]. Since ABCA1 has been demonstrated to neutralize β-amyloid aggregation capacity via Apolipoprotein E (ApoE) [107], the expression of ABCA1 and ABCA2 at the BBB as a site of waste removal from the brain, may suggest a potential role in Alzheimer’s disease, which warrants further investigation. Little is known about the ontogeny of ABCA transporters or a potential role at the BBB during development. As Kim et al. postulated in their 2008 review, ABC transporters involved in lipid transport, that are expressed in the brain, likely play an important role in brain–lipid homeostasis.
In addition to the BBB, which is the focus of this review, ABC and other drug transporters are expressed elsewhere in the central nervous system (CNS), including the blood–cerebral spinal fluid barrier (choroid plexus), blood–retinal barrier and spinal chord. In this context, P-gp has been described in the epithelial cells of the (choroid plexus) of mouse, rat and human, where it may act in the regulation of the efflux transport function in the cerebrospinal fluid (CSF) [99]. In the porcine blood–retinal barrier, BCRP, P-gp and MCT1 transporters were detected, demonstrating a potential role of these transporters in regulating xenobiotic clearance in different types of CNS-related barriers. A detailed review of other brain barriers during development can be found here [108].
BBB development
The onset of the brain vasculogenesis is well documented in mice. It begins at gestational day (GD) 7.5 with the migration of mesenchyme-derived angioblasts into the outer part of the neural tube head region to form a perineural vascular plexus (PNVP) that sprouts and invades the surrounding inner neural tube, at around GD9.5 [109]. The newly formed vessels then lose their fenestrations, form tight junctions, and adopt a BBB phenotype [9, 110]. BEC tight junction complexes are observable as early as GD13.5 in mice, and 8 weeks gestation in humans [111]. While the process of brain vascularization, tight junction formation, and the factors that guide these events have been described [112], little is known about how and when drug transporter expression is induced and then regulated during development. This knowledge is key to our understanding of how maternal and external factors, isolated or combined, may affect BBB drug transporters during neurodevelopment, and in turn, uptake of substrates (i.e., toxins, xenobiotics, hormones) into the fetal brain.
During fetal brain development, the specialized phenotype of BECs is driven by factors secreted by pericytes and neural progenitor cells. Canonical Wnt-signaling is involved in driving vascularization of the developing BBB, by guiding angiogenesis in concert with vascular endothelial growth factor (VEGF) [112, 113]. Wnt is also involved in the induction of P-gp gene expression in the BBB. Differentiation of stem cells into BECs in vitro has also helped elucidate key factors in driving the maturation of the BBB. Sequential Wnt and retinoic acid (RA) pathway activation in human pluripotent stem cells (hPSCs) lead to their differentiation [114]. Direct and indirect effects of Wnt/β-catenin on P-gp expression have been demonstrated in models of adult and fetal BBB, where activation with Wnt ligands leads to increased P-gp expression and activity [115, 116]. Wnt/β-catenin signaling interacts with other pathways (e.g., TGF-β, Notch) that are activated by pericytes and neural progenitors during early vascularization [113]. TGF-β is secreted from pericytes and binds to the TGF-βII and I receptors on the abluminal face of endothelial cells to increase P-gp expression and activity. This has been demonstrated in vitro, in developing guinea pig BECs and in adult rat and murine endothelial cells [21, 117, 118]. TGF-β can also induce developing BBB tight junction function and be secreted by astrocytes [118]. In this context, we have shown that astrocytes in co-culture with BECs derived from the fetal and neonatal brain increase TEER, and reduce dextran movement across the monolayer [27]. Pericytes and not astrocytes are present at the neurovascular unit from early BBB formation, and as such, secreted factors from pericytes are likely most important in initial BBB phenotype acquisition. The fact that P-gp and BCRP are regulated by key signaling pathways in embryonic and fetal development highlights their functional importance in the establishment and maturation of the developing BBB.
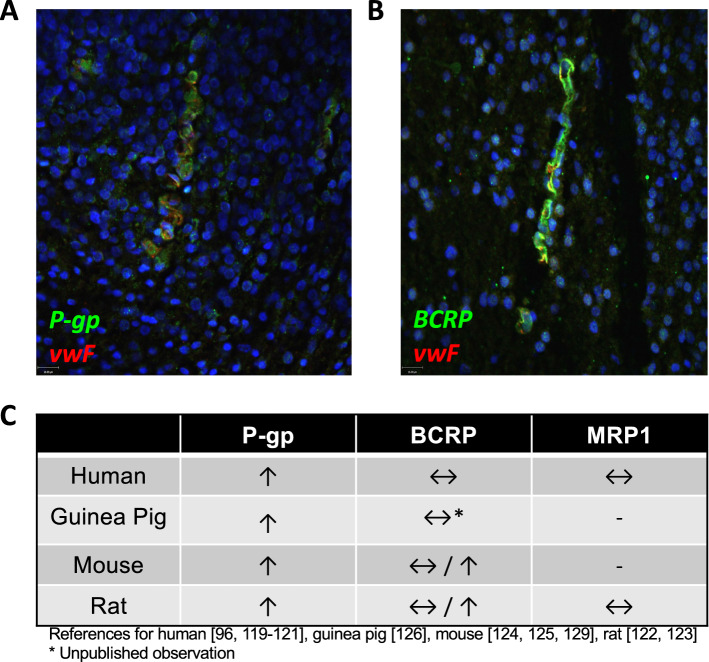
The ontogeny of drug transporters at the developing BBB is summarized in Table 2. Appearance of drug transporters is sequential, and different ABC transporters exhibit different developmental patterns of expression across gestation. P-gp is considered one the earliest markers of brain microvasculature, detectable at GD10.5 in mice, and has been detected as early as 6–8 weeks post conception in humans [111, 119, 120]. In humans, guinea pigs, mice and rats, levels of ABCB1 (Abcb1) mRNA and P-gp protein increase with development [96, 116, 121] (Fig. 2). Studies in mice and rats have also shown these increases in gene and protein expression correspond with increased P-gp function [122–124]. While limited studies have been undertaken, P-gp levels have been shown to be low in microvessels in early human BBB development, and primarily cytosolic. Toward 18 weeks of gestation, P-gp expression becomes diffuse along the length of capillaries, and by 22 weeks, P-gp is expressed evenly along cerebral capillaries [125]. See Table 2 and Fig. 2 for a summary of timing and pattern of P-gp expression at the developing BBB.
Table 2.
Expression of ABC transporters at the developing BBB of human, guinea pig, mouse and rat
| ABC transporter | Earliest expression at BBB during development | |||
|---|---|---|---|---|
| Human | Guinea pig | Mouse | Rat | |
| ABCB1 (P-gp) |
GW 7–8 |
GD 40* [126] |
GD10.5 [127] |
GD13* [122] |
| ABCG2 (BCRP) |
GW 5 GW [119] |
GD 40* [126] |
GD12.5* [128] |
GD13* [122] |
| ABCC1 (MRP-1) |
GW 5 GW [119] |
N/A | N/A |
GD13* [122] |
| ABCC4 (MRP4) | N/A | N/A | N/A |
GD13* [122] |
GW gestation week, GD gestation day, N/A not available
*Earliest studied
Fig. 2.
Developmental expression of drug transporters at the blood–brain barrier. P-glycoprotein (P-gp) (A) and breast cancer resistance protein (BCRP) (B) are expressed in second trimester human cerebral microvessels. Pattern of drug transporter expression throughout development in different species (C). P-gp expression increases with advancing gestation in all species, while BCRP expression stays fairly constant. Little is known concerning the developmental regulation of multidrug resistance proteins (MRPs)
BCRP has been identified as one of the first ABC transporters expressed in the neurovasculature. Studies in humans and guinea pigs have identified expression of BCRP in cerebral capillaries from mid-gestation (22 weeks in human; GD 40 in guinea pigs), with little change in expression levels through fetal development [96, 129, 130]. However, studies in rats have reported increasing expression of Abcg2/BCRP during fetal BBB maturation, corresponding with an increase in BCRP activity [116, 123, 131], suggesting potential species differences in BCRP expression during BBB development. Recently, BCRP has been detected as early as 5 weeks post conception in humans, in the early stages of brain vascularization, before even P-gp is detectable by immunostaining [119]. BCRP is present in microvessels throughout gestation [96]; and of note, we have shown microvessels in 2nd trimester (~ 17 weeks) fetal brains to express both P-gp and BCRP (Fig. 2).
The role for P-gp and BCRP may not be limited to BBB protection during fetal brain development but may comprise other functions including modulation of migration of BBB cell precursors and angiogenesis, whereas in neural progenitor cells (NPCs) they may function as markers of immaturity/stemness. In this regard, our group has recently demonstrated that silencing of ABCB1 (encoding P-gp) in human extravillous trophoblast cells (EVT; HTR8/SVneo) impaired invasion and migration and induced tube formation [132]. Similarly, silencing of ABCG2 (encoding BCRP) inhibited EVT cell migration while it did not affect cell proliferation [133]. EVTs are endovascular invading cells with relatively high expression of P-gp and BCRP in capillaries early on in development. It is possible that P-gp and BCRP being present in BECs during phases of intense angiogenesis and lateral branching formation, may play a role in these processes; a hypothesis currently being investigated. With respect to NPCs, expression of ABCB1 is high in early development when these cells are immature (Nestin positive) [134]. Upon differentiation, ABCB1 expression is reduced, suggesting that beyond protecting an important stem cell pool, P-gp may have a role in stemness itself in this cell type. Taken together, the high expression of P-gp and BCRP in proliferative and invasive cell types, including side population cells and cancer cells, should be considered in discussing alternate roles for these transporters in the BBB.
MRPs are detectable in the fetal brain, however at lower levels compared to P-gp and BCRP. MRPs are present at higher levels in ependymal cells of the choroid plexus in humans and rats, compared to BECs [96, 98]. In humans, MRP-1 is present at high levels in the choroid plexus and cerebellum by 22 weeks gestation, but was undetectable in cerebral capillaries, suggesting a less important role for MRPs at the developing BBB [96]. However, another study detected Mrp1 in the human cerebrovasculature at 5 weeks post conception [119]. Further studies are required to elucidate the presence and role of MRPs at the developing BBB. Figure 2C provides a summary of developmental changes in drug transporter expression for the different species that have been studied, to date.
Drugs, environmental factors, and the developing BBB
Drugs are frequently used during pregnancy, particularly in cases of chronic diseases (depression, diabetes, epilepsy), as well as to treat pregnancy-associated symptoms or infections [135]. Approximately 70% of women take at least one medication (excluding vitamins & supplements) during pregnancy [136, 137]. Many drugs prescribed during pregnancy are P-gp, BCRP and MRP substrates or modulate their activity. Given that increased drug exposure can negatively impact fetal brain development, it is critical to understand the levels and regulation of drug transporters in the developing BBB. Table 1 summarizes major classes of P-gp and BCRP substrates, with a focus on those relevant in pregnancy and neonatal life.
During pregnancy, the fetus and the developing brain may be exposed to factors present in the maternal environment. These include agrochemicals, toxins or their residues, that may enter the mother through water [138, 139], dietary [140, 141] or airborne routes [142, 143]. Exposure of the fetus to a number of these factors have been associated with altered pregnancy outcomes including preterm birth [144, 145] and reduced birthweight. Longer-term effects associated with prenatal and post-natal pesticide exposure have also been identified, such as increased blood pressure [146], altered glucose metabolism [147], poor respiratory outcomes [148, 149], and neurobehavioural deficits in children [150, 151]. For example, one study in this systematic review [150] found cord blood levels of chlorpyrifos were associated with attention deficits in toddlers; chlorpyrifos is known to interact with BCRP [152] and P-gp. Chlorpyrifos has been shown to regulate human BCRP expression in placental trophoblast cell lines [152] and placental explants [153, 154]. In addition to chlorpyrifos, several organochlorine and pyrethroid pesticides have been shown to regulate the expression and function of human P-gp and BCRP in vitro [155, 156]. Most studies investigating the effects of organochlorine and pyrethroid pesticides on human drug transporters must make use of in vitro cell culture or membrane vesicle preparations; studies identifying pesticides as P-gp or BCRP substrates face similar challenges. While knowledge of the in vivo role for drug transporter efflux of pesticides is limited, studies utilizing mosquitos have identified permethrin and temefos as potential drug transporter substrates [157, 158]. Epidemiological data on the effects of pesticides on human drug transporters in vivo, in particular at the BBB, are limited. Therefore, the possibility that drug transporters may play a crucial role in protecting the developing fetus from maternal pesticide exposure and subsequent short-term and long-term effects is an assumption that requires investigation. In this case, alterations in drug transporter function due to the presence of pharmacological or physiological (e.g., inflammation, infection) inhibitors, could increase accumulation of their substrates into the CNS. The impacts of infection and pro-inflammatory cytokines on BBB drug transporter function are discussed in detail below. Increasing access of such pesticides and toxins to the fetus is likely to have consequences for development. A retrospective study found that when pregnant women were exposed in their first-trimester to both clinical P-gp substrates (including cimetidine, ranitidine, risperidone, citalopram) concurrently with P-gp inhibitors (including omeprazole, pantoprazole, haloperidol), this was associated with increased risk for specific congenital anomalies of the CNS [65]. Ultimately, more research is required to fully understand the role of fetal and placental drug transporters in preventing teratogenesis.
Cross-talk between the placenta and fetal BBB in protection of the developing brain
The human fetal brain is protected from xenobiotics by two key barriers: the placenta and the fetal BBB. The placenta represents the primary protective barrier separating maternal and fetal circulations, and it is important to note that the a greater proportion of the placenta is of fetal origin. The placenta delivers nutrients and oxygen to the fetus, while simultaneously functioning as a route for fetal metabolic waste elimination. In parallel, it limits the entrance of several factors present in the maternal circulation that could be harmful to the developing fetus. The placental barrier in humans is formed by syncytiotriophoblasts, which are in close contact with the maternal blood bathing the intervillous space [159]. A number of members of the ABC transporter family have been identified in the placenta, including P-gp, BCRP, MDR3 (encoded by Abcb4), MRPs 1,2,3,5 and the lipid transporters ABCA1, ABCA6 and ABCG1 [37, 160, 161]. Transport proteins may be present in the apical and/or the basolateral surfaces of the syncytiotrophoblast layer, facing both the maternal blood and the placental core in a transporter specific manner. This allows them to control the entrance and accumulation of substrates, depending on their placental location. A number of transporters are also be expressed in the luminal membrane of the fetal capillaries, controlling the efflux of substrates into/or out from the fetal circulation.
There is a critical interplay of the placenta and fetal BBB in protecting the developing fetal brain. Placental protection through the expression of efflux drug transporters is critical in early stages of pregnancy when fetal brain capillaries and the BBB are still being formed. The primary drug transporters, P-gp and BCRP are expressed at high levels in the placental syncitiotrophoblast in the first trimester human placenta [162–164]. P-gp and BCRP are expressed at the apical membrane, and efflux their substrates from the placenta to the maternal circulation [165]. Levels of placental P-gp decline in late gestation, while BCRP levels remain relatively constant [162–164]. Similar patterns have been identified in animal models [166]. In the mouse, the reduction in placental P-gp results in increased accumulation of P-gp substrates in the fetus and amniotic fluid [167]. In parallel to the decrease in protection provided by the placenta, there are increases in P-gp and BCRP in the fetal BBB [121, 124]. As such, it appears that the fetal BBB becomes critically important for brain protection in late gestation. This increase in protection also represents an important transition from fetal to post-natal life. In this context, in most mammalian species there is a major surge of endogenous glucocorticoid that occurs in late gestation, and emerging evidence would suggest this may drive the transition (discussed in detail below). The rising glucocorticoid levels correspond with up-regulation of P-gp, which also suggests developmental regulation of P-gp and BCRP is coordinated at least in part through separate signaling pathways (as BCRP does not increase). Additionally, emphasis has been placed on understanding the role of P-gp and BCRP in drug resistance/barrier function, when these transporters may have other physiological functions. The BBB also plays an important role in sequestering neurotransmitters such as serotonin, which cannot cross the BBB and thus must be synthesized locally in the CNS [25, 168]. This is important for neurotransmitter balance and signal specificity. It is likely that drug transporters play a role in controlling movement of key hormones/signals during development, an important area of research that requires further focus.”
Glucocorticoids and the developing BBB
Glucocorticoids are key in the transition from fetal to neonatal life. The late gestation surge in fetal glucocorticoid is essential for maturing several organs, including the lungs, liver, kidney and brain. Glucocorticoids exert their actions via glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) signaling. As discussed above (see cross-talk), P-gp expression and activity at the BBB increases with advancing gestation. This maturation could be mediated by glucocorticoids, as both endogenous (cortisol), and sGC (betamethasone, dexamethasone) are potent modulators of drug transporter expression and activity, as well as tight junction function in the BBB [126, 129, 130, 169–171]. In the context of preterm birth (~ 12% of all pregnancies), antenatal sGC are administered to mature the fetal lung and reduce the risk of respiratory distress syndrome [172, 173] Unlike endogenous cortisol which binds both MR and GR, sGC are not inactivated by placental 11 beta HSD-2 and activate only GR [174]. We have shown in the guinea pig that maternal treatment of cortisol and sGC increase expression and function of BBB P-gp/Abcb1 in late gestation/early postnatal life, but not in mid-gestation [126]. The role for glucocorticoids in inducing BCRP at the developing BBB is less clear. Administration of sGCs to pregnant mice led to altered mRNA levels of Abcg2 at the fetal BBB but not BCRP protein expression and function [124, 129], demonstrating a disconnect between mRNA and protein levels for this transporter. sGC increase trans-endothelial electrical resistance (TEER) in brain endothelial cells in vitro, and this is associated with increased tight junction function [175, 176]. In development, maternal antenatal dexamethasone exposure increases expression of tight junction proteins in the BBB of fetal sheep, and this was associated with decreased BBB permeability (increased function) [170, 171], thus showing strong evidence for modulation of fetal BBB transporter activity and permeability by sGC.
In addition to GR signaling, sGC also activate drug-sensing pathways via pregnane-x-receptor (PXR) and constitutive androstane receptor (CAR) [177–179]. PXR and CAR are nuclear receptors, which upon binding xenobiotics, translocate to the nucleus and activate transcription through binding “xenobiotic response elements” in genes, namely cytochrome p450 enzymes, and drug transporters in liver [180]. PXR and CAR are also expressed in brain endothelial cells [181]; it is likely that PXR and CAR play a role in drug-sensing and metabolism at the BBB. In porcine BECs, PXR activators, rifampicin and hyperforin. lead to increased expression of Abcb1 and Abcg2 mRNA, BCRP and P-gp protein, and P-gp function [182, 183]. These studies provide evidence that activation of PXR and CAR by drugs leads to increased expression and/or function of P-gp at the BBB. With respect to sGC mediated up-regulation of drug transporters, this is likely a coordinated action of GR and PXR/CAR. The sGC dexamethasone is a PXR ligand, and dexamethasone exposure in adult rat brain capillaries leads to increased P-gp protein expression and function [181]. A study conducted in retinal pigment epithelium, which is another protective brain barrier, found that dexamethasone interacts only with GR to up-regulate P-gp, and PXR played a supporting role, potentially through a GR-mediated PXR expression mechanism [178]. Other studies examining the dynamics of GR-PXR activation of P-gp at the BBB and liver do report direct interaction between dexamethasone and PXR, suggesting tissue-specific glucocorticoid responses [177, 184].
Infection/inflammation and the developing BBB
Prenatal exposure to infection and inflammation can disrupt brain development and lead to life-long neurological and behavioral changes [185–191]. Studies in rodents demonstrated that offspring from dams prenatally exposed to infection mimics, including polyinosinic:polycytidylic acid (PolyI:C, a TLR-3 ligand), lipopolysaccharide (LPS, a TLR-4 agonist) and Imiquimod (a TLR-7 ligand) exhibited higher risk of brain injury and adverse neurologic outcomes, including autism, cerebral palsy and schizophrenia [192–199]. Further, viral infection, such as Zika virus and cytomegalovirus [200, 201], also induce adverse neurological outcomes including congenital viral syndrome and microcephaly [202–204]. Recently, it was shown that prenatal maternal exposure to the viral-mimic (PolyI:C) in mice leads to increased accumulation of the P-gp selective substrate [3H]digoxin in the fetal brain at GD15.5, indicating a reduction of P-gp functional activity at the fetal BBB [205]. In this connection, maternal PolyI:C exposure in mid-pregnancy led to long-term offspring motor and cognitive dysfunction [206]. At the placenta, LPS-mediated inflammation impaired P-gp activity and led to greater fetal accumulation of [3H]digoxin at GD15.5 but not at GD17.5 [207], without eliciting changes in placental Abcb1a/Abcb1b mRNA [208] and P-gp labyrinthine expression (the exchange site in the mouse placenta). Moreover, maternal infection with Plasmodium berghei ANKA (a mouse model of malaria in pregnancy) and Zika virus (ZIKV) in mice, decreased the placental expression of P-gp [209, 210]. Whereas, in the mouse yolk sac, Plasmodium berghei ANKA increased Abcb1a and P-gp expression[211]. Interestingly, LPS challenge had no effect[212]. Together, these studies indicate that tissue barrier P-gp expression and activity respond differently to specific infective-stimuli and that this response also depends on gestational age, suggesting specific gestational windows of fetal vulnerability to infection and resultant exposure to xenobiotics and environmental toxins.
Previous in vitro studies using porcine and human (hCMEC/D3) BECs derived from adult brains have shown infection and inflammation to inhibit P-gp function [92, 100, 213, 214]. However, little is known as to how infection and inflammation impact P-gp in the developing BBB, and how BCRP and MRPs are affected. In primary cultures of BECs derived from fetal and neonatal guinea pigs at various stages of development, pro-inflammatory cytokines including interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α induced a dose-dependent inhibition of P-gp function and decreased Abcb1 gene expression [130]. The magnitude of the effects was highly dependent on the developmental stage at which the BECs were derived. Effects were greatest in cells derived from neonatal animals, were less pronounced in BECs derived from fetuses near term (gestational day (GD) 65; Term ~ GD67), while BECS derived at GD50 (75% gestation) did not respond to pro-inflammatory cytokines [130]. However, a key interaction exists between glucocorticoids and pro-inflammatory cytokines at the level of the developing BBB. In guinea pigs, maternal antenatal treatment with glucocorticoids (single-course dexamethasone at GD50) in vivo, increased P-gp expression and function in the fetal BBB. BECs derived from glucocorticoid exposed fetuses at GD50 demonstrated robust inhibition of P-gp function following cytokine exposure, an effect that was mediated, at least in part by increased cytokine receptor levels [130]. These are important observations because infection is present in 40% of all cases of preterm birth[90], and many of these women will have received prenatal sGC treatment. Given the findings in animal studies, it might be anticipated that sGC treatment of pregnant women might sensitize the fetal brain to the effects of infection, potentially decreasing P-gp-mediated protection of the fetal brain. Clearly, further research is required to investigate this possibility further.
Implications of altered BBB function for neonates
It has been established that fetal BBB function can be modulated by maternal exposure to factors, such as stress, sGC, infection and inflammation. Infants and neonates, particularly those in the neonatal intensive care unit (NICU), represent a vulnerable population who are exposed to a number of drugs, many of which are P-gp and BCRP substrates [215]. Evidently, there are limitations in optimizing drugs for the neonatal population, and its estimated only 35% are FDA approved in neonates. In fact, a large portion of neonatal adverse drug reactions are attributed to agents that target or access the nervous system, such as stimulants (e.g., caffeine), anesthetics (e.g., midazolam), analgesics (e.g., morphine) and antiretrovirals (e.g., Zidovudine). These are some of many agents that are regulated by drug transporters at the BBB, or impact their function [216–218]. In this context, prenatal exposures that lead to long-term changes in BBB function may have consequences for drug efficacy in the post-natal period. There is growing evidence that early life activation of drug-sensing pathways lead to long-term changes in drug metabolism in the liver, effects which are mediated by altered activity and expression of nuclear receptors, such as GR, CAR, and PXR [219]; these same xenobiotic receptors and systems are present at the BBB. If expression and function of drug transporters at the fetal BBB is altered by a prenatal exposure (to glucocorticoids or infection, for example), and these effects persist into post-natal life, this could alter drug uptake in neonates potentially leading to adverse longer-term health outcomes. Understanding how prenatal/post-natal environmental exposures may lead to subsequent post-natal drug interactions is essential to informing the use of these drugs in perinatal care.
Conclusion
Drug transporters, particularly P-gp and BCRP at the developing BBB are crucial for developmental homeostasis and fetal brain protection. Despite their importance, relatively little is known regarding drug transporter regulation in the developing brain, particularly when exposed to environmental factors (e.g., stress, toxins, xenobiotics). There is a key interplay of P-gp and BCRP in fetal brain protection as well as interplay between the placenta and the fetal BBB. In this review, we have highlighted several factors that modulate drug transporters at the developing BBB, including sGC, pro-inflammatory cytokines, maternal infection, and growth factors (TGF-β, Wnt). While some have the potential to increase brain protection (e.g., sGC, TGF-β), others may have profound consequences in increasing xenobiotic exposure (e.g., maternal infection). It is also unknown whether changes at the developing BBB induced by environmental factors will persist into post-natal life. This could have implications for post-natal brain protection, as well as with respect to drug exposure/interactions. The mechanisms underlying these regulatory networks demand further investigation, as well as the potential role of other transporters, including MRPs. Knowledge of the underlying mechanisms of drug transporter regulation at the developing BBB will help to identify potential therapeutic approaches for increasing fetal brain protection.
Author contributions
MEE: conceptualization, visualization—figures and tables, writing—original draft. GEI: conceptualization, writing—original draft. EB: conceptualization, writing—review and editing, supervision. SGM: conceptualization, writing—review and editing, supervision, project administration, funding acquisition.
Funding and Acknowledgments
This work was funded by a Foundation grant from Canadian Institutes of Health Research (CIHR: FDN-148368) to S.G.M. M.E.E. was supported in part by a Queen Elizabeth II Graduate Scholarship in Science and Technology (QEII-GSST). E.B. is supported by the Higher Education Personnel Improvement Coordination (Coordenação de Aperfeiçoamento Pessoal de Nível Superior [CAPES]; finance code 001, CAPES-Print fellowship: 88887.370196/2019-00), The National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq]: 10578/2020-5) and the Research Support Foundation of Minas Gerais State (Fundação de Amparo à Pesquisa do Estado de Minas Gerais [FAPEMIG]: APQ-00338-18). We thank Ms Phetcharawan Lye for the H & E images displayed on Fig. 2 A & B. This work was undertaken in compliance with REB policies at Sinai Health System (protocol n# 18-0057-E).
Data availability
Enquiries about data availability should be directed to the authors.
Declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
N ot applicable
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 3.Al-Majdoub ZM, Al Feteisi H, Achour B, et al. Proteomic quantification of human blood-brain barrier SLC and ABC transporters in healthy individuals and dementia patients. Mol Pharm. 2019;16:1220–1233. doi: 10.1021/acs.molpharmaceut.8b01189. [DOI] [PubMed] [Google Scholar]
- 4.Abbott NJ, Patabendige AAK, Dolman DEM, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 6.Malinovskaya NA, Komleva YK, Salmin VV, et al. Endothelial progenitor cells physiology and metabolic plasticity in brain angiogenesis and blood-brain barrier modeling. Front Physiol. 2016;7:599. doi: 10.3389/fphys.2016.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolburg H, Lippoldt A, Ebnet K (2007) Tight junctions in the blood-brain barrier. In: Handbook of neurochemistry and molecular neurobiology: neural membranes and transport. pp 1–27
- 8.Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/a:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebner S, Czupalla CJ, Wolburg H. Current concepts of blood-brain barrier development. Int J Dev Biol. 2011;55:467–476. doi: 10.1387/ijdb.103224sl. [DOI] [PubMed] [Google Scholar]
- 10.Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krause G, Winkler L, Mueller SL, et al. Structure and function of claudins. Biochim Biophys Acta Biomembr. 2008;1778:631–645. doi: 10.1016/J.BBAMEM.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5–deficient mice. J Cell Biol. 2001;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohno K, Pettigrew KD, Rapoport SI. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. Am J Physiol. 1978;235:H299–307. doi: 10.1152/ajpheart.1978.235.3.H299. [DOI] [PubMed] [Google Scholar]
- 14.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 16.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 20.Villaseñor R, Kuennecke B, Ozmen L, et al. Region-specific permeability of the blood–brain barrier upon pericyte loss. J Cereb Blood Flow Metab. 2017;37:3683–3694. doi: 10.1177/0271678X17697340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohgu S, Takata F, Yamauchi A, et al. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-β production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Kong J, Qiu Y, Li Y, et al. TGF-β1 elevates P-gp and BCRP in hepatocellular carcinoma through HOTAIR/miR-145 axis. Biopharm Drug Dispos. 2019;40:70–80. doi: 10.1002/bdd.2172. [DOI] [PubMed] [Google Scholar]
- 23.Berezowski V, Landry C, Dehouck MP, et al. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain Res. 2004;1018:1–9. doi: 10.1016/j.brainres.2004.05.092. [DOI] [PubMed] [Google Scholar]
- 24.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 26.Dehouck M-P, Méresse S, Delorme P, et al. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J Neurochem. 1990;54:1798–1801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 27.Baello S, Iqbal M, Gibb W, Matthews SG. Astrocyte-mediated regulation of multidrug resistance P-glycoprotein in fetal and neonatal brain endothelial cells: age-dependent effects. Physiol Rep. 2016;4:1–17. doi: 10.14814/phy2.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 29.Duan L, Di Q. Acetazolamide suppresses multi-drug resistance-related protein 1 and P-Glycoprotein expression by inhibiting aquaporins expression in a mesial temporal epilepsy rat model. Med Sci Monit. 2017;23:5818–5825. doi: 10.12659/MSM.903855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locher KP. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol. 2016;23:487–493. doi: 10.1038/nsmb.3216. [DOI] [PubMed] [Google Scholar]
- 31.Linton KJ. Structure and function of ABC transporters. Physiol. 2007;22:122–130. doi: 10.1152/physiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- 32.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta - Biomembr. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 33.Austin Doyle L, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shawahna R, Uchida Y, Decleves X, et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8:1332–1341. doi: 10.1021/mp200129p. [DOI] [PubMed] [Google Scholar]
- 35.Uchida Y, Ohtsuki S, Katsukura Y, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117:333–345. doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- 36.Saidijam M, Karimi Dermani F, Sohrabi S, Patching SG. Efflux proteins at the blood-brain barrier: review and bioinformatics analysis. Xenobiotica. 2018;48:506–532. doi: 10.1080/00498254.2017.1328148. [DOI] [PubMed] [Google Scholar]
- 37.Bloise E, Ortiga-Carvalho TM, Reis FM, et al. ATP-binding cassette transporters in reproduction: a new frontier. Hum Reprod Updat. 2016;22:164–181. doi: 10.1093/humupd/dmv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–127. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 39.Löscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandoval Karamian AG, Wusthoff CJ. Antiepileptic drug therapy in neonates. Amsterdam: Elsevier Inc; 2019. [Google Scholar]
- 41.Voinescu PE, Pennell PB. Management of epilepsy during pregnancy. Expert Rev Neurother. 2015;15:1171–1187. doi: 10.1586/14737175.2015.1083422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Löscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 43.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 44.Filia MF, Marchini T, Minoia JM, et al. Induction of ABCG2/BCRP restricts the distribution of zidovudine to the fetal brain in rats. Toxicol Appl Pharmacol. 2017;330:74–83. doi: 10.1016/j.taap.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. https://clinicalinfo.hiv.gov/en/guidelines/pediatric-arv
- 46.Lambert JS, Nogueira SA, Abreu T, et al. A pilot study to evaluate the safety and feasibility of the administration of AZT/3TC fixed dose combination to HIV infected pregnant women and their infants in Rio de Janeiro, Brazil. Sex Transm Infect. 2003;79:448–452. doi: 10.1136/sti.79.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allegaert K, van den Anker JN. Neonatal pain management: still in search for the Holy Grail. Int J Clin Pharmacol Ther. 2016;54:514–523. doi: 10.5414/CP202561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.L. Mercer S, Coop A, Opioid analgesics and P-glycoprotein efflux transporters: a potential systems-level contribution to analgesic tolerance. Curr Top Med Chem. 2011;11:1157–1164. doi: 10.2174/156802611795371288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ornoy A, Weinstein-Fudim L, Ergaz Z. Antidepressants, antipsychotics, and mood stabilizers in pregnancy: what do we know and how should we treat pregnant women with depression. Birth Defects Res. 2017;109:933–956. doi: 10.1002/bdr2.1079. [DOI] [PubMed] [Google Scholar]
- 50.Schinkel AH, Wagenaar E, Mol CAAM, Van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller DS, Nobmann SN, Gutmann H, et al. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol. 2000;58:1357–1367. doi: 10.1124/MOL.58.6.1357. [DOI] [PubMed] [Google Scholar]
- 52.Beaulieu E, Demeule M, Ghitescu L, Béliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326(Pt 2):539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu D, Bebawy M, Kable EP, Roufogalis BD. Dynamic and intracellular trafficking of P-glycoprotein-EGFP fusion protein: implications in multidrug resistance in cancer. Int J Cancer. 2004;109:174–181. doi: 10.1002/ijc.11659. [DOI] [PubMed] [Google Scholar]
- 54.Fu D, Roufogalis BD. Actin disruption inhibits endosomal traffic of P-glycoprotein-EGFP and resistance to daunorubicin accumulation. Am J Physiol Cell Physiol. 2007;292:C1543–C1552. doi: 10.1152/ajpcell.00068.2006. [DOI] [PubMed] [Google Scholar]
- 55.Lavie Y, Fiucci G, Liscovitch M. Up-regulation of caveolae and caveolar constituents in multidrug-resistant cancer cells. J Biol Chem. 1998;273:32380–32383. doi: 10.1074/jbc.273.49.32380. [DOI] [PubMed] [Google Scholar]
- 56.Yamori T, Ota DM, Cleary KR, Irimura T. Increased content of chondroitin sulfate proteoglycan in human colorectal carcinoma metastases compared with the primary tumor as determined by an anti-chondroitin-sulfate monoclonal antibody. J Cell Biochem. 1988;36:405–416. doi: 10.1002/jcb.240360409. [DOI] [PubMed] [Google Scholar]
- 57.Greer DA, Ivey S. Distinct N-glycan glycosylation of P-glycoprotein isolated from the human uterine sarcoma cell line MES-SA/Dx5. Biochim Biophys Acta - Gen Subj. 2007;1770:1275–1282. doi: 10.1016/j.bbagen.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loo TW, Bartlett MC, Clarke DM. Thapsigargin or curcumin does not promote maturation of processing mutants of the ABC transporters, CFTR, and P-glycoprotein. Biochem Biophys Res Commun. 2004;325:580–585. doi: 10.1016/j.bbrc.2004.10.070. [DOI] [PubMed] [Google Scholar]
- 59.Decleves X, Regina A, Laplanche JL, et al. Functional expression of P-glycoprotein and multidrug resistance-associated protein (Mrp1) in primary cultures of rat astrocytes. J Neurosci Res. 2000;60:594–601. doi: 10.1002/(SICI)1097-4547(20000601)60:5<594::AID-JNR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 60.Ronaldson PT, Bendayan M, Gingras D, et al. Cellular localization and functional expression of P-glycoprotein in rat astrocyte cultures. J Neurochem. 2004;89:788–800. doi: 10.1111/j.1471-4159.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- 61.Schlachetzki F, Pardridge WM. P-glycoprotein and caveolin-1α in endothelium and astrocytes of primate brain. NeuroReport. 2003;14:2041–2046. doi: 10.1097/00001756-200311140-00007. [DOI] [PubMed] [Google Scholar]
- 62.Schinkel AH, Smit JJM, van Tellingen O, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 63.Schinkel AH, Wagenaar E, van Deemter L, et al. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lankas GR, Wise LD, Cartwright ME, et al. Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reprod Toxicol. 1998;12:457–463. doi: 10.1016/S0890-6238(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 65.Daud ANA, Bergman JEH, Bakker MK, et al. P-glycoprotein-mediated drug interactions in pregnancy and changes in the risk of congenital anomalies: a case-reference study. Drug Saf. 2015;38:651–659. doi: 10.1007/s40264-015-0299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cordon-Cardo C, O’Brien JP, Casals D, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thiebaut F, Tsuruo T, Hamada H, et al. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989;37:159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- 68.Aday S, Cecchelli R, Hallier-Vanuxeem D, et al. Stem cell-based human blood-brain barrier models for drug discovery and delivery. Trends Biotechnol. 2016;34:382–393. doi: 10.1016/J.TIBTECH.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Dauchy S, Dutheil F, Weaver RJ, et al. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem. 2008;107:1518–1528. doi: 10.1111/j.1471-4159.2008.05720.x. [DOI] [PubMed] [Google Scholar]
- 70.Ni Z, Bikadi Z, Rosenberg MF, Mao Q. Structure and function of the human breast cancer resistance protein (BCRP/ABCG2) Curr Drug Metab. 2010;11:603–617. doi: 10.2174/138920010792927325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han B, Zhang JT. Multidrug resistance in cancer chemotherapy and xenobiotic protection mediated by the half ATP-binding cassette transporter ABCG2. Curr Med Chem Anticancer Agents. 2004;4:31–42. doi: 10.2174/1568011043482205. [DOI] [PubMed] [Google Scholar]
- 72.Marchetti S, de Vries NA, Buckle T, et al. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type mice. Mol Cancer Ther. 2008;7:2280–2287. doi: 10.1158/1535-7163.MCT-07-2250. [DOI] [PubMed] [Google Scholar]
- 73.Marchetti S, Pluim D, van Eijndhoven M, et al. Effect of the drug transporters ABCG2, Abcg2, ABCB1 and ABCC2 on the disposition, brain accumulation and myelotoxicity of the aurora kinase B inhibitor barasertib and its more active form barasertib-hydroxy-QPA. Invest New Drugs. 2013;31:1125–1135. doi: 10.1007/s10637-013-9923-1. [DOI] [PubMed] [Google Scholar]
- 74.Bhatia P, Bernier M, Sanghvi M, et al. Breast cancer resistance protein (BCRP/ABCG2) localises to the nucleus in glioblastoma multiforme cells. Xenobiotica. 2012;42:748–755. doi: 10.3109/00498254.2012.662726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lemos C, Kathmann I, Giovannetti E, et al. Impact of cellular folate status and epidermal growth factor receptor expression on BCRP/ABCG2-mediated resistance to gefitinib and erlotinib. Br J Cancer. 2009;100:1120–1127. doi: 10.1038/sj.bjc.6604980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solazzo M, Fantappie O, D’Amico M, et al. Mitochondrial expression and functional activity of breast cancer resistance protein in different multiple drug-resistant cell lines. Cancer Res. 2009;69:7235–7242. doi: 10.1158/0008-5472.CAN-08-4315. [DOI] [PubMed] [Google Scholar]
- 77.Kodaira H, Kusuhara H, Ushiki J, et al. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther. 2010;333:788–796. doi: 10.1124/jpet.109.162321. [DOI] [PubMed] [Google Scholar]
- 78.Oberoi RK, Mittapalli RK, Elmquist WF. Pharmacokinetic assessment of efflux transport in sunitinib distribution to the brain. J Pharmacol Exp Ther. 2013;347:755–764. doi: 10.1124/jpet.113.208959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agarwal S, Hartz AMS, Elmquist WF, Bauer B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des. 2011;17:2793–2802. doi: 10.2174/138161211797440186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bauer M, Römermann K, Karch R, et al. Pilot PET study to assess the functional interplay between ABCB1 and ABCG2 at the human blood-brain barrier. Clin Pharmacol Ther. 2016;100:131–141. doi: 10.1002/cpt.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poller B, Wagenaar E, Tang SC, Schinkel AH. Double-transduced MDCKII cells to study human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) interplay in drug transport across the blood-brain barrier. Mol Pharm. 2011;8:571–582. doi: 10.1021/mp1003898. [DOI] [PubMed] [Google Scholar]
- 82.Sisodiya SM, Martinian L, Scheffer GL, et al. Vascular colocalization of P-glycoprotein, multidrug-resistance associated protein 1, breast cancer resistance protein and major vault protein in human epileptogenic pathologies. Neuropathol Appl Neurobiol. 2006;32:51–63. doi: 10.1111/j.1365-2990.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 83.de Vries NA, Zhao J, Kroon E, et al. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- 84.Polli JW, Olson KL, Chism JP, et al. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino }met. Drug Metab Dispos. 2009;37:439–442. doi: 10.1124/dmd.108.024646. [DOI] [PubMed] [Google Scholar]
- 85.Lagas JS, van Waterschoot RA, van Tilburg VA, et al. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res. 2009;15:2344–2351. doi: 10.1158/1078-0432.CCR-08-2253. [DOI] [PubMed] [Google Scholar]
- 86.Lagas JS, van Waterschoot RA, Sparidans RW, et al. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther. 2010;9:319–326. doi: 10.1158/1535-7163.MCT-09-0663. [DOI] [PubMed] [Google Scholar]
- 87.Tang SC, de Vries N, Sparidans RW, et al. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) gene dosage on plasma pharmacokinetics and brain accumulation of dasatinib, sorafenib, and sunitinib. J Pharmacol Exp Ther. 2013;346:486–494. doi: 10.1124/jpet.113.205583. [DOI] [PubMed] [Google Scholar]
- 88.Agarwal S, Elmquist WF. Insight into the cooperation of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) at the blood-brain barrier: a case study examining sorafenib efflux clearance. Mol Pharm. 2012;9:678–684. doi: 10.1021/mp200465c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cisternino S, Mercier C, Bourasset F, et al. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res. 2004;64:3296–3301. doi: 10.1158/0008-5472.CAN-03-2033. [DOI] [PubMed] [Google Scholar]
- 90.do Imperio GE, Bloise E, Javam M,, et al. Chorioamnionitis induces a specific signature of placental ABC transporters associated with an increase of miR-331-5p in the human preterm placenta. Cell Physiol Biochem. 2018;45:591–604. doi: 10.1159/000487100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiong H, Callaghan D, Jones A, et al. ABCG2 is upregulated in Alzheimer’s brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Abeta(1–40) peptides. J Neurosci. 2009;29:5463–5475. doi: 10.1523/JNEUROSCI.5103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eustaquio Do Imperio G, Lye P, Bloise E, Matthews SG. Function of multidrug resistance transporters is disrupted by infection mimics in human brain endothelial cells. Tissue barriers. 2021;9:1860616. doi: 10.1080/21688370.2020.1860616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hipfner DR, Deeley RG, Cole SP. Structural, mechanistic and clinical aspects of MRP1. Biochim Biophys Acta. 1999;1461:359–376. doi: 10.1016/s0005-2736(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 94.Nies AT, Jedlitschky G, Konig J, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129:349–360. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 95.Suhy AM, Webb A, Papp AC, et al. Expression and splicing of ABC and SLC transporters in the human blood-brain barrier measured with RNAseq. Eur J Pharm Sci. 2017;103:47–51. doi: 10.1016/J.EJPS.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 96.Daood M, Tsai C, Ahdab-Barmada M, Watchko JF. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics. 2008;39:211–218. doi: 10.1055/s-0028-1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gazzin S, Berengeno AL, Strazielle N, et al. Modulation of Mrp1 (ABCc1) and Pgp (ABCb1) by bilirubin at the blood-CSF and blood-brain barriers in the Gunn rat. PLoS ONE. 2011;6:e16165. doi: 10.1371/journal.pone.0016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gazzin S, Strazielle N, Schmitt C, et al. Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood-brain interfaces. J Comp Neurol. 2008;510:497–507. doi: 10.1002/cne.21808. [DOI] [PubMed] [Google Scholar]
- 99.Rao VV, Dahlheimer JL, Bardgett ME, et al. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA. 1999;96:3900–3905. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hartz AM, Bauer B. Regulation of ABC transporters at the blood-brain barrier: new targets for CNS therapy. Mol Interv. 2010;10:293–304. doi: 10.1124/mi.10.5.6. [DOI] [PubMed] [Google Scholar]
- 101.Hayashi K, Pu H, Andras IE, et al. HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood-brain barrier. J Cereb Blood Flow Metab. 2006;26:1052–1065. doi: 10.1038/sj.jcbfm.9600254. [DOI] [PubMed] [Google Scholar]
- 102.Potschka H, Fedrowitz M, Loscher W. Multidrug resistance protein MRP2 contributes to blood-brain barrier function and restricts antiepileptic drug activity. J Pharmacol Exp Ther. 2003;306:124–131. doi: 10.1124/jpet.103.049858. [DOI] [PubMed] [Google Scholar]
- 103.Mack J, Townsend D, Beljanski V, Tew K. The ABCA2 transporter: intracellular roles in trafficking and metabolism of LDL-derived cholesterol and sterol-related compounds. Curr Drug Metab. 2006;8:47–57. doi: 10.2174/138920007779315044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim WS, Weickert CS, Garner B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J Neurochem. 2008;104:1145–1166. doi: 10.1111/j.1471-4159.2007.05099.x. [DOI] [PubMed] [Google Scholar]
- 105.Wolf A, Bauer B, Hartz AM. ABC transporters and the Alzheimer’s disease enigma. Front Psychiatry. 2012;3:54. doi: 10.3389/fpsyt.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elali A, Rivest S. The role of ABCB1 and ABCA1 in beta-amyloid clearance at the neurovascular unit in Alzheimer’s disease. Front Physiol. 2013 doi: 10.3389/fphys.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saunders NR, Dziegielewska KM, Møllgård K, Habgood MD. Recent developments in understanding barrier mechanisms in the developing brain: drugs and drug transporters in pregnancy, susceptibility or protection in the fetal brain? Annu Rev Pharmacol Toxicol. 2019;59:487–505. doi: 10.1146/annurev-pharmtox-010818-021430. [DOI] [PubMed] [Google Scholar]
- 109.Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 2014;355:687–699. doi: 10.1007/s00441-014-1811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bauer HC, Bauer H, Lametschwandtner A, et al. Neovascularization and the appearance of morphological characteristics of the blood-brain barrier in the embryonic mouse central nervous system. Dev Brain Res. 1993;75:269–278. doi: 10.1016/0165-3806(93)90031-5. [DOI] [PubMed] [Google Scholar]
- 111.Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR. Barriers in the developing brain and neurotoxicology. Neurotoxicology. 2012;33:586–604. doi: 10.1016/j.neuro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 112.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liebner S, Plate KH. Differentiation of the brain vasculature: the answer came blowing by the Wnt. J Angiogenes Res. 2010;2:1. doi: 10.1186/2040-2384-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qian T, Maguire SE, Canfield SG, et al. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci Adv. 2017;3:e1701679. doi: 10.1126/sciadv.1701679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lim JC, Kania KD, Wijesuriya H, et al. Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J Neurochem. 2008;106:1855–1865. doi: 10.1111/j.1471-4159.2008.05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harati R, Benech H, Villégier AS, Mabondzo A. P-glycoprotein, breast cancer resistance protein, organic anion transporter 3, and transporting peptide 1a4 during blood-brain barrier maturation: involvement of Wnt/β-catenin and endothelin-1 signaling. Mol Pharm. 2013;10:1566–1580. doi: 10.1021/mp300334r. [DOI] [PubMed] [Google Scholar]
- 117.Baello S, Iqbal M, Bloise E, et al. TGF-β1 regulation of multidrug resistance P-glycoprotein in the developing male blood-brain barrier. Endocrinology. 2014;155:475–484. doi: 10.1210/en.2013-1472. [DOI] [PubMed] [Google Scholar]
- 118.Hawkins BT, Davis TP, Avemary J, et al. Glucocorticoids modify effects of TGF-beta1 on multidrug resistance in the fetal blood-brain barrier. Placenta. 2016;17:475–484. doi: 10.1111/j.1471-4159.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- 119.Møllgård K, Dziegielewska KM, Holst CB, et al. Brain barriers and functional interfaces with sequential appearance of ABC efflux transporters during human development. Sci Rep. 2017;7:11603. doi: 10.1038/s41598-017-11596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schumacher U, Mollgård K. The multidrug-resistance P-glycoprotein (Pgp, MDR1) is an early marker of blood-brain barrier development in the microvessels of the developing human brain. Histochem Cell Biol. 1997;108:179–182. doi: 10.1007/s004180050159. [DOI] [PubMed] [Google Scholar]
- 121.Lam J, Baello S, Iqbal M, et al. The ontogeny of P-glycoprotein in the developing human blood–brain barrier: implication for opioid toxicity in neonates. Pediatr Res. 2015 doi: 10.1038/pr.2015.119. [DOI] [PubMed] [Google Scholar]
- 122.Ek CJ, Wong A, Liddelow SA, et al. Efflux mechanisms at the developing brain barriers: ABC-transporters in the fetal and postnatal rat. Toxicol Lett. 2010;197:51–59. doi: 10.1016/j.toxlet.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 123.Soares RV, Do TM, Mabondzo A, et al. Ontogeny of ABC and SLC transporters in the microvessels of developing rat brain. Fundam Clin Pharmacol. 2016;30:107–116. doi: 10.1111/fcp.12175. [DOI] [PubMed] [Google Scholar]
- 124.Petropoulos S, Gibb W, Matthews SG. Developmental expression of multidrug resistance phosphoglycoprotein (P-gp) in the mouse fetal brain and glucocorticoid regulation. Brain Res. 2010;1357:9–18. doi: 10.1016/j.brainres.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 125.Virgintino D, Errede M, Girolamo F, et al. Fetal blood-brain barrier P-glycoprotein contributes to brain protection during human development. J Neuropathol Exp Neurol. 2008;67:50–61. doi: 10.1097/nen.0b013e31815f65d9. [DOI] [PubMed] [Google Scholar]
- 126.Iqbal M, Gibb W, Matthews SG. Corticosteroid regulation of P-glycoprotein in the developing blood-brain barrier. Endocrinology. 2011;152:1067–1079. doi: 10.1210/en.2010-1227. [DOI] [PubMed] [Google Scholar]
- 127.Qin Y, Sato TN. Mouse multidrug resistance 1a/3 gene is the earliest known endothelial cell differentiation marker during blood-brain barrier development. Dev Dyn. 1995;202:172–180. doi: 10.1002/aja.1002020209. [DOI] [PubMed] [Google Scholar]
- 128.Orford M, Mean R, Lapathitis G, et al. Generation of an ABCG2GFPn-puro transgenic line—a tool to study ABCG2 expression in mice. Biochem Biophys Res Commun. 2009;384:199–203. doi: 10.1016/j.bbrc.2009.04.089. [DOI] [PubMed] [Google Scholar]
- 129.Petropoulos S, Gibb W, Matthews SG. Breast cancer-resistance protein (BCRP1) in the fetal mouse brain: development and glucocorticoid regulation. Biol Reprod. 2011;84:783–789. doi: 10.1095/biolreprod.110.088468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iqbal M, Baello S, Javam M, et al. Regulation of multidrug resistance P-glycoprotein in the developing blood-brain barrier: interplay between glucocorticoids and cytokines. J Neuroendocrinol. 2016;28:12360. doi: 10.1111/jne.12360. [DOI] [PubMed] [Google Scholar]
- 131.Cygalova L, Ceckova M, Pavek P, Staud F. Role of breast cancer resistance protein (Bcrp/Abcg2) in fetal protection during gestation in rat. Toxicol Lett. 2008;178:176–180. doi: 10.1016/j.toxlet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 132.Dunk CE, Pappas JJ, Lye P, et al. P-Glycoprotein (P-gp)/ABCB1 plays a functional role in extravillous trophoblast (EVT) invasion and is decreased in the pre-eclamptic placenta. J Cell Mol Med. 2018;22:5378–5393. doi: 10.1111/jcmm.13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lye P, Bloise E, Nadeem L, et al. Breast cancer resistance protein (BCRP/ABCG2) inhibits extra villous trophoblast migration: the impact of bacterial and viral infection. Cells. 2019 doi: 10.3390/cells8101150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yamamoto A, Shofuda T, Islam MO, et al. ABCB1 is predominantly expressed in human fetal neural stem/progenitor cells at an early development stage. J Neurosci Res. 2009;87:2615–2623. doi: 10.1002/jnr.22094. [DOI] [PubMed] [Google Scholar]
- 135.Bakker M, Jentink J, Vroom F, et al. Maternal medicine: drug prescription patterns before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the Netherlands. BJOG An Int J Obstet Gynaecol. 2006;113:559–568. doi: 10.1111/j.1471-0528.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 136.Haas DM, Marsh DJ, Dang DT, et al. Prescription and other medication use in pregnancy. Obs Gynecol. 2018;131:789–798. doi: 10.1097/AOG.0000000000002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lupattelli A, Spigset O, Twigg MJ, et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open. 2014;4:e004365. doi: 10.1136/bmjopen-2013-004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sousa JCG, Ribeiro AR, Barbosa MO, et al. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J Hazard Mater. 2018;344:146–162. doi: 10.1016/j.jhazmat.2017.09.058. [DOI] [PubMed] [Google Scholar]
- 139.Shalat SL, Walker DB, Finnell RH. Role of arsenic as a reproductive toxin with particular attention to neural tube defects. J Toxicol Environ Health. 1996;48:253–272. doi: 10.1080/009841096161320. [DOI] [PubMed] [Google Scholar]
- 140.Banerjee K, Utture S, Dasgupta S, et al. Multiresidue determination of 375 organic contaminants including pesticides, polychlorinated biphenyls and polyaromatic hydrocarbons in fruits and vegetables by gas chromatography-triple quadrupole mass spectrometry with introduction of semi-quantificatio. J Chromatogr A. 2012;1270:283–295. doi: 10.1016/j.chroma.2012.10.066. [DOI] [PubMed] [Google Scholar]
- 141.Modgil S, Lahiri DK, Sharma VL, Anand A. Role of early life exposure and environment on neurodegeneration: implications on brain disorders. Transl Neurodegener. 2014;3:1–14. doi: 10.1186/2047-9158-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gibbs JL, Yost MG, Negrete M, Fenske RA. Passive sampling for indoor and outdoor exposures to chlorpyrifos, azinphos-methyl, and oxygen analogs in a rural agricultural community. Environ Health Perspect. 2017;125:333–341. doi: 10.1289/EHP425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mercier F, Glorennec P, Thomas O, Le BB. Organic contamination of settled house dust, a review for exposure assessment purposes. Environ Sci Technol. 2011;45:6716–6727. doi: 10.1021/es200925h. [DOI] [PubMed] [Google Scholar]
- 144.Parvez S, Gerona RR, Proctor C, et al. Glyphosate exposure in pregnancy and shortened gestational length: a prospective Indiana birth cohort study. Environ Heal. 2018;17:23. doi: 10.1186/s12940-018-0367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eskenazi B, Harley K, Bradman A, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112:1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harari R, Julvez J, Murata K, et al. Neurobehavioral deficits and increased blood pressure in school-age children prenatally exposed to pesticides. Environ Health Perspect. 2010;118:890–896. doi: 10.1289/ehp.0901582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Debost-Legrand A, Warembourg C, Massart C, et al. Prenatal exposure to persistent organic pollutants and organophosphate pesticides, and markers of glucose metabolism at birth. Environ Res. 2016;146:207–217. doi: 10.1016/j.envres.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 148.Ye M, Beach J, Martin JW, Senthilselvan A. Pesticide exposures and respiratory health in general populations. J Environ Sci (China) 2017;51:361–370. doi: 10.1016/j.jes.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 149.Reardon A, Perzanowski MS, Whyatt RM, et al. Associations between prenatal pesticide exposure and cough, wheeze, and IgE in early childhood. J Allergy Clin Immunol. 2009;123:S21. doi: 10.1016/j.jaci.2010.12.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sapbamrer R, Hongsibsong S. Effects of prenatal and postnatal exposure to organophosphate pesticides on child neurodevelopment in different age groups: a systematic review. Environ Sci Pollut Res Int. 2019;26:18267–18290. doi: 10.1007/s11356-019-05126-w. [DOI] [PubMed] [Google Scholar]
- 151.Rohlman DS, Arcury TA, Quandt SA, et al. Neurobehavioral performance in preschool children from agricultural and non-agricultural communities in Oregon and North Carolina. Neurotoxicology. 2005;26:589–598. doi: 10.1016/j.neuro.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 152.Halwachs S, Schäfer I, Kneuer C, et al. Assessment of ABCG2-mediated transport of pesticides across the rabbit placenta barrier using a novel MDCKII in vitro model. Toxicol Appl Pharmacol. 2016;305:66–74. doi: 10.1016/j.taap.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 153.Ridano ME, Racca AC, Flores-Martin JB, et al. Impact of chlorpyrifos on human villous trophoblasts and chorionic villi. Toxicol Appl Pharmacol. 2017;329:26–39. doi: 10.1016/j.taap.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 154.Ridano ME, Racca AC, Flores-Martín J, et al. Chlorpyrifos modifies the expression of genes involved in human placental function. Reprod Toxicol. 2012;33:331–338. doi: 10.1016/j.reprotox.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 155.Marchitti SA, Mazur CS, Dillingham CM, et al. Inhibition of the human ABC efflux transporters P-gp and BCRP by the BDE-47 hydroxylated metabolite 6-OH-BDE-47: Considerations for human exposure. Toxicol Sci. 2017;155:270–282. doi: 10.1093/toxsci/kfw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bircsak KM, Richardson JR, Aleksunes LM. Inhibition of human MDR1 and BCRP transporter ATPase activity by organochlorine and pyrethroid insecticides. J Biochem Mol Toxicol. 2013;27:157–164. doi: 10.1002/jbt.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Epis S, Porretta D, Mastrantonio V, et al. ABC transporters are involved in defense against permethrin insecticide in the malaria vector Anopheles stephensi. Parasit Vectors. 2014;7:1–7. doi: 10.1186/1756-3305-7-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Figueira-Mansur J, Ferreira-Pereira A, Mansur JF, et al. Silencing of P-glycoprotein increases mortality in temephos-treated Aedes aegypti larvae. Insect Mol Biol. 2013;22:648–658. doi: 10.1111/imb.12052. [DOI] [PubMed] [Google Scholar]
- 159.Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev. 2016;96:1509–1565. doi: 10.1152/physrev.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Imperio GE, Javam M, Lye P, et al. Gestational age-dependent gene expression profiling of ATP-binding cassette transporters in the healthy human placenta. J Cell Mol Med. 2019;23:610–618. doi: 10.1111/jcmm.13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Iqbal M, Audette MC, Petropoulos S, et al. Placental drug transporters and their role in fetal protection. Placenta. 2012;33:137–142. doi: 10.1016/j.placenta.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 162.Sun M, Kingdom J, Baczyk D, et al. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta. 2006;27:602–609. doi: 10.1016/j.placenta.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 163.Gil S, Saura R, Forestier F, Farinotti R. P-glycoprotein expression of the human placenta during pregnancy. Placenta. 2005;26:268–270. doi: 10.1016/j.placenta.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 164.Lye P, Bloise E, Dunk C, et al. Effect of oxygen on multidrug resistance in the first trimester human placenta. Placenta. 2013;34:817–823. doi: 10.1016/j.placenta.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 165.Walker N, Filis P, Soffientini U, et al. Placental transporter localization and expression in the Human: the importance of species, sex, and gestational age differences†. Biol Reprod. 2017;96:733–742. doi: 10.1093/biolre/iox012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kalabis GM, Kostaki A, Andrews MH, et al. Multidrug resistance phosphoglycoprotein (ABCB1) in the mouse placenta: fetal protection. Biol Reprod. 2005;73:591–597. doi: 10.1095/biolreprod.105.042242. [DOI] [PubMed] [Google Scholar]
- 167.Petropoulos S, Kalabis GM, Gibb W, Matthews SG. Functional changes of mouse placental multidrug resistance phosphoglycoprotein (ABCB1) with advancing gestation and regulation by progesterone. Reprod Sci. 2015;14:321–328. doi: 10.1177/1933719107303856. [DOI] [PubMed] [Google Scholar]
- 168.El-Merahbi R, Löffler M, Mayer A, Sumara G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015;589:1728–1734. doi: 10.1016/j.febslet.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 169.Iqbal M, Ho HL, Petropoulos S, et al. Pro-inflammatory cytokine regulation of P-glycoprotein in the developing blood-brain barrier. PLoS ONE. 2012;7:e43022. doi: 10.1371/journal.pone.0043022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sadowska GB, Malaeb SN, Stonestreet BS. Maternal glucocorticoid exposure alters tight junction protein expression in the brain of fetal sheep. Am J Physiol Heart Circ Physiol. 2010;298:H179–H188. doi: 10.1152/ajpheart.00828.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stonestreet BS, Sadowska GB, McKnight AJ, et al. Exogenous and endogenous corticosteroids modulate blood-brain barrier development in the ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2000;279:R468–477. doi: 10.1152/ajpregu.2000.279.2.R468. [DOI] [PubMed] [Google Scholar]
- 172.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017 doi: 10.1002/14651858.CD004454.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 2: Mechanisms. Nat Rev Endocrinol. 2014;10:403–411. doi: 10.1038/nrendo.2014.74. [DOI] [PubMed] [Google Scholar]
- 175.Romero IA, Radewicz K, Jubin E, et al. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett. 2003;344:112–116. doi: 10.1016/S0304-3940(03)00348-3. [DOI] [PubMed] [Google Scholar]
- 176.Calabria AR, Weidenfeller C, Jones AR, et al. Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J Neurochem. 2006;97:922–933. doi: 10.1111/j.1471-4159.2006.03793.x. [DOI] [PubMed] [Google Scholar]
- 177.Narang VS, Fraga C, Kumar N, et al. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood-brain barrier. Am J Physiol Cell Physiol. 2008;295:C440–C450. doi: 10.1152/ajpcell.00491.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang Y, Lu M, Sun X, et al. Expression and activity of p-glycoprotein elevated by dexamethasone in cultured retinal pigment epithelium involve glucocorticoid receptor and pregnane X receptor. Investig Ophthalmol Vis Sci. 2012;53:3508–3515. doi: 10.1167/iovs.11-9337. [DOI] [PubMed] [Google Scholar]
- 179.Savas Ü, Griffin KJ, Johnson EF, et al. Molecular mechanisms of cytochrome P-450 induction by xenobiotics: an expanded role for nuclear hormone receptors. Mol Pharmacol. 1999;56:851–857. doi: 10.1124/mol.56.5.851. [DOI] [PubMed] [Google Scholar]
- 180.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 181.Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–419. doi: 10.1124/mol.66.3. [DOI] [PubMed] [Google Scholar]
- 182.Ott M, Fricker G, Bauer B. Pregnane X receptor (PXR) regulates P-glycoprotein at the blood-brain barrier: functional similarities between pig and human PXR. J Pharmacol Exp Ther. 2009;329:141–149. doi: 10.1124/jpet.108.149690. [DOI] [PubMed] [Google Scholar]
- 183.Lemmen J, Tozakidis IEP, Galla HJ. Pregnane X receptor upregulates ABC-transporter Abcg2 and Abcb1 at the blood-brain barrier. Brain Res. 2013;1491:1–13. doi: 10.1016/j.brainres.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 184.Pascussi J-M, Drocourt L, Gerbal-Chaloin S, et al. Dual effect of dexamethasone on CYP3A4 gene expression in human hepatocytes. Eur J Biochem. 2001;268:6346–6358. doi: 10.1046/j.0014-2956.2001.02540.x. [DOI] [PubMed] [Google Scholar]
- 185.Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocr. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science (80-) 2016;353:772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Feng SYS, Hollis JH, Samarasinghe T, et al. Endotoxin-induced cerebral pathophysiology: differences between fetus and newborn. Physiol Rep. 2019;7:e13973. doi: 10.14814/phy2.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kwon J-Y, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387–390. doi: 10.1111/aji.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Murphy SK, Fineberg AM, Maxwell SD, et al. Maternal infection and stress during pregnancy and depressive symptoms in adolescent offspring. Psychiatry Res. 2017;257:102–110. doi: 10.1016/J.PSYCHRES.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Amodeo DA, Lai C-Y, Hassan O, et al. Maternal immune activation impairs cognitive flexibility and alters transcription in frontal cortex. Neurobiol Dis. 2019;125:211–218. doi: 10.1016/J.NBD.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Peloggia A, Ali M, Nanda K, Bahamondes L. Zika virus exposure in pregnancy and its association with newborn visual anomalies and hearing loss. Int J Gynecol Obstet. 2018;143:277–281. doi: 10.1002/ijgo.12663. [DOI] [PubMed] [Google Scholar]
- 192.Wang X, Rousset CI, Hagberg H, Mallard C. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonatal Med. 2006;11:343–353. doi: 10.1016/j.siny.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 193.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22:219–225. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- 195.Patterson PH. Maternal infection and autism. Brain Behav Immun. 2012;26:393. doi: 10.1016/j.bbi.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 196.Pakula AT, Van Naarden BK, Yeargin-Allsopp M. Cerebral palsy: classification and epidemiology. Phys Med Rehabil Clin N Am. 2009;20:425–452. doi: 10.1016/j.pmr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 197.Kentner AC, Bilbo SD, Brown AS, et al. Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology. 2019;44:245–258. doi: 10.1038/s41386-018-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Careaga M, Murai T, Bauman MD. Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biol Psychiatry. 2017;81:391–401. doi: 10.1016/j.biopsych.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Missig G, Robbins JO, Mokler EL, et al. Sex-dependent neurobiological features of prenatal immune activation via TLR7. Mol Psychiatry. 2019 doi: 10.1038/s41380-018-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dang J, Tiwari SK, Lichinchi G, et al. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell. 2016;19:258–265. doi: 10.1016/J.STEM.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yew KH, Harrison CJ. Blockade of Lyn kinase upregulates both canonical and non-canonical TLR-3 pathways in THP-1 monocytes exposed to human cytomegalovirus. Acta Virol. 2011;55:243–253. doi: 10.4149/av_2011_03_243. [DOI] [PubMed] [Google Scholar]
- 202.Lucey D, Cummins H, Sholts S. Congenital zika syndrome in 2017. JAMA. 2017;317:1368. doi: 10.1001/jama.2017.1553. [DOI] [PubMed] [Google Scholar]
- 203.de Miranda-Filho DB, Martelli CMT, de Ximenes RAA, et al. Initial description of the presumed congenital zika syndrome. Am J Public Health. 2016;106:598–600. doi: 10.2105/AJPH.2016.303115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 205.Bloise E, Petropoulos S, Iqbal M, et al. Acute effects of viral exposure on P-glycoprotein function in the mouse fetal blood-brain barrier. Cell Physiol Biochem. 2017;41:1044–1050. doi: 10.1159/000461569. [DOI] [PubMed] [Google Scholar]
- 206.Monteiro VRS, Andrade CBV, Gomes HR, Reginatto MW, Império GE, Fontes KN, Spiess DA, Rangel-Junior WS, Nascimento VMO, Lima COS, Sousa RPC, Bloise FF, Matthews SG, Bloise E, Pimentel-Coelho PM, Ortiga-Carvalho TM. Mid-pregnancy poly(I:C) viral mimic disrupts placental ABC transporter expression and leads to long-term offspring motor and cognitive dysfunction. Sci Rep. 2022 doi: 10.1038/s41598-022-14248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bloise E, Bhuiyan M, Audette MC, et al. Prenatal endotoxemia and placental drug transport in the mouse: placental size-specific effects. PLoS ONE. 2013;8:e65728. doi: 10.1371/journal.pone.0065728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Reginatto MW, Fontes KN, Monteiro VRS, et al. Effect of sublethal prenatal endotoxaemia on murine placental transport systems and lipid homeostasis. Front Microbiol. 2021;12:706499. doi: 10.3389/fmicb.2021.706499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fontes KN, Reginatto MW, Silva NL, et al. Dysregulation of placental ABC transporters in a murine model of malaria-induced preterm labor. Sci Rep. 2019;9:1–13. doi: 10.1038/s41598-019-47865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Andrade CBV, Monteiro VRS, Coelho SVA, Gomes HR, Sousa RPC, Nascimento VMO, Bloise FF, Matthews SG, Bloise E, Arruda LB, Ortiga-Carvalho TM. ZIKV disrupts placental ultrastructure and drug transporter expression in mice. Front Immunol. https:// 2021 doi: 10.3389/fimmu.2021.680246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Martinelli LM, Fontes KN, Reginatto MW, et al. Malaria in pregnancy regulates P-glycoprotein (P-gp/Abcb1a) and ABCA1 efflux transporters in the Mouse Visceral Yolk Sac. J Cell Mol Med. 2020;24:10636–10647. doi: 10.1111/jcmm.15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Martinelli LM, Reginatto MW, Fontes KN, et al. Breast cancer resistance protein (Bcrp/Abcg2) is selectively modulated by lipopolysaccharide (LPS) in the mouse yolk sac. Reprod Toxicol. 2020;98:82–91. doi: 10.1016/j.reprotox.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.von Wedel-Parlow M, Wolte P, Galla HJ. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem. 2009;111:111–118. doi: 10.1111/j.1471-4159.2009.06305.x. [DOI] [PubMed] [Google Scholar]
- 214.Qosa H, Miller DS, Pasinelli P, Trotti D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015 doi: 10.1016/j.brainres.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Krzyzaniak N, Bajorek B. Medication safety in neonatal care: a review of medication errors among neonates. Ther Adv Drug Saf. 2016;7:102–119. doi: 10.1177/2042098616642231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102:1677–1690. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- 217.Slosky LM, Thompson BJ, Sanchez-Covarrubias L, et al. Acetaminophen modulates P-glycoprotein functional expression at the blood-brain barrier by a constitutive androstane receptor-dependent mechanism. Mol Pharmacol. 2013;84:774–786. doi: 10.1124/mol.113.086298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Potschka H, Fedrowitz M, Löscher W. P-Glycoprotein-mediated efflux of phenobarbital, lamotrigine, and felbamate at the blood-brain barrier: evidence from microdialysis experiments in rats. Neurosci Lett. 2002;327:173–176. doi: 10.1016/S0304-3940(02)00423-8. [DOI] [PubMed] [Google Scholar]
- 219.Chen WD, Fu X, Dong B, et al. Neonatal activation of the nuclear receptor CAR results in epigenetic memory and permanent change of drug metabolism in mouse liver. Hepatology. 2012;56:1499–1508. doi: 10.1002/hep.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Enquiries about data availability should be directed to the authors.