Abstract
Excessive activation of the ionotropic N-methyl-d-aspartate (NMDA) receptor has been shown to cause abnormally high levels of Ca2+ influx, thereby leading to excitotoxic neuronal death. In this study, exposure of mouse primary cortical neurons to NMDA resulted in the cleavage and activation of mammalian sterile 20-like kinase-1 (MST1), both of which were mediated by calpain 1. In vitro cleavage assay data indicated that calpain 1 cleaves out the autoinhibitory domain of MST1 to generate an active form of the kinase. Furthermore, calpain 1 mediated the cleavage and activation of wild-type MST1, but not of MST1 (G339A). Intriguingly, NMDA/calpain-induced MST1 activation promoted the nuclear translocation of the kinase and the phosphorylation of histone H2B in mouse cortical neurons, leading to excitotoxicity. Thus, we propose a previously unrecognized mechanism of MST1 activation associated with NMDA-induced excitotoxic neuronal death.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-021-04103-2.
Keywords: Calcium-dependent cleavage, Glutamate receptor, Histone H2B, Neurotoxicity, Protein kinase
Introduction
Glutamate, the major excitatory amino acid neurotransmitter in the mammalian central nerve system, exerts its crucial role in development of brain circuits, synaptic activity and plasticity, and learning and memory through binding to glutamate receptors, including the N-methyl-d-aspartate (NMDA) receptor (NMDAR), which is permeable to cations, such as Ca2+ and Na+ [1, 2]. Under normal conditions, the action of glutamate is quickly terminated by the reuptake of glutamate by astrocytes [3]. Under pathological conditions, however, glutamate causes neuronal cell death known as excitotoxicity [4]. In particular, when it is released and accumulated excessively, glutamate over-activates the NMDAR and consequently causes intracellular calcium overload, thereby activating calcium-dependent cytotoxic events, such as reactive oxygen species/reactive nitrogen species production [5], mitochondrial dysfunction [6], and DNA damage [7].
Many lines of evidence have shown that calpain, a calcium-activated cysteine protease, plays a pivotal role in excitotoxicity in cortical neurons [8–11]. There are at least 15 different types of calpains in mammals, among which calpain 1 (or μ-calpain) and calpain 2 (or m-calpain) are the classical calpains found ubiquitously in the brain [12]. At physiological calcium levels, calpains modulate important signaling pathways to control diverse behaviors of intracellular proteins and organelles [13, 14]. At higher calcium levels, however, they are aberrantly activated and promote excitotoxic processes in neurons with cleaving several death-regulating proteins, such as pro-caspase-3 [15], Src tyrosine kinase [8], debrin [9], p35 [10], and Bid [11]. It has been also reported that an endogenous calpain inhibitor reduces glutamate-induced excitotoxicity in murine primary neurons [16].
Mammalian STE20-like kinase 1 (MST1), a member of the class II germinal center kinase family [17, 18], has been shown to play a role in a variety of cellular processes, including cell growth, cellular stress, and tumor suppression [19, 20]. In particular, MST1 functions as a key mediator of various cell death, including IFN-γ-induced cell death in microglia [21], and oxidative stress-induced cell death in neurons [22–25]. Additionally, MST1 has been associated with the mechanism for ischemic stroke-induced microglial activation, which triggers the inflammatory reactions leading to neurotoxicity [26]. MST1 has been also shown to mediate the neurotoxicity of motor neurons in spinal cords in mouse models of spinal cord injury [27] and of amyotrophic lateral sclerosis [28].
MST1 consists of an amino-terminal catalytic domain, a central autoinhibitory domain, and a carboxy-terminal regulatory SARAH domain (Salvador/Rassf/Hippo) [18]. Several mechanisms have been proposed for MST1 activation in cellular response to various stimuli. In response to apoptotic stimuli, active caspases can mediate MST1 activation through cleaving MST1 into a 36-kDa active fragment [29]. MST1 can also be activated by homo-dimerization and subsequent autophosphorylation [30, 31]. MST1 has been shown to phosphorylate histone H2B at Ser14, thereby leading to histone modification that is uniquely associated with apoptotic chromatin formation [32]. MST1 can also mediate the phosphorylation of a transcription factor FOXO3 in neurons, thereby promoting neuronal cell death [22].
In order to better understand a molecular mechanism for excitotoxic neuronal death, we have investigated a role of MST1 in the NMDAR-mediated excitotoxicity in primary cultures of mouse cortical neurons. Here, we have found that calpain 1 mediates the NMDA-induced stimulation of MST1 in cortical neurons. Furthermore, the calpain-mediated activation of MST1 facilitates the nuclear translocation of the cleaved form of MST1 and the phosphorylation of histone H2B at serine 14. Taken together, our findings suggest a previously unrecognized mechanism of MST1 activation associated with NMDA-induced excitotoxic neuronal death.
Materials and methods
Animals
MST1−/− C57BL/6 mice were described previously [33]. Mice were maintained at the Korea University Laboratory Animal Center, and all animal procedures were approved by the Institutional Animal Care and Use Committee of Korea University. CrljOri:CD1 (ICR) TP13 mice were purchased locally from Orient Bio (Gyeonggi-do, South Korea) and were immediately sacrificed for preparation of primary cortical neuron culture.
Cell culture and DNA transfection
Primary cortical neuron cultures were prepared using the cortical region dissected from the E13 embryonic brain of ICR, or wild-type or MST1−/− C57BL/6 mice. Cortical neurons in culture dishes coated with poly-d-lysine (Sigma-Aldrich, St. Louis, Missouri) were cultivated in Neurobasal medium (Thermo Fisher Scientific, Waltham, Massachusetts) supplemented with 1% B-27 (Invitrogen, Carlsbad, California), 1% fetal bovine serum, 0.5 mM glutamine, and 25 μM β-mercaptoethanol, under a humidified atmosphere of 5% CO2 at 37 °C, and the cultured cells at DIV11 were used for experiments. For DNA transfection, primary cultures of mouse cortical neurons at DIV9 were transfected with indicated cDNA constructs with the use of NeuroMag (OZ biosciences, San Diego, California). HEK293T cells were transfected with indicated expression vectors with the use of polyethylenimine.
Plasmids, antibodies, and reagents
The pHM6/HA-MST1 was described previously [21]. The pHM6/HA-MST1 (G339A) and pEGFP-C1/GFP-MST1 (G339A) mutant constructs were prepared from pHM6/HA-MST1 and pEGFP-C1/GFP, respectively, by site-directed mutagenesis (Stratagene, La Jolla, California). Rabbit polyclonal antibodies for MST1 and for phospho-H2B (Ser14) were from Cell Signaling Technology (Danvers, Massachusetts). Mouse monoclonal antibodies for calpain 1, spectrin, and for GFP were from Santa Cruz Biotechnology (Dallas, Texas). Mouse monoclonal antibodies for tubulin and for Flag were from Sigma-Aldrich. MK-801 was purchased from Tocris (Bristol, United Kingdom). NMDA and ALLN were purchased from Sigma-Aldrich. BAPTA-AM was purchased from A.G. Scientific, Inc. (San Diego, California).
Immune complex kinase assay
Cells were lysed with buffer A containing 1% Triton X-100, 5 mM EGTA, 20 mM Tris–HCl (pH 7.4), 150 mM sodium chloride, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 10 mM sodium fluoride, 12 mM β-glycerophosphate, and 0.5% sodium deoxycholate. Cell lysates were subjected to immunoprecipitation with indicated antibodies, and the resulting precipitates were examined for immune complex kinase assay with myelin basic protein or histone 2B (New England Biolabs, Ipswich, MA, USA) as substrate [21, 34].
RNA interference
Primary cortical neuron cultures at DIV 9 were transfected with calpain 1 siRNA (Santa Cruz Biotechnology) using Lipofectamine RNAiMAX (Invitrogen).
In vitro calpain cleavage assay of MST1
In vitro calpain cleavage assays were performed by incubating HA-MST1 immunoprecipitates with calpain 1 (Sigma-Aldrich) under the indicated conditions in a reaction buffer containing 50 mM Tris–HCl (pH 7.5), 100 mM NaCl, 2 mM dithiothreitol, 1 mM EDTA, and 5 mM CaCl2. Then, the reaction mixtures were subjected to SDS-PAGE, followed by immunoblot analysis with antibody to HA.
Immunoblot analysis
Cultured cells were lysed with ice-cold buffer A. The lysates were subjected to centrifugation at 12,000×g at 4 °C for 20 min, and the resulting supernatants were subjected to immunoblot analysis with appropriate antibodies as described previously [34].
Immunostaining
Cells cultured on cover slips were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton-X-100, blocked with 10% horse serum, and incubated overnight at 4 °C with mouse anti-MAP2 (Santa Cruz Biotechnology) or rabbit anti-phospho-H2B (Ser14) antibodies (Cell Signaling Technology), as indicated. Then, the samples were incubated for 2 h at room temperature with Texas Red-conjugated anti-mouse or anti-rabbit secondary antibodies (Vector Laboratories, Burlingame, California), followed by nuclear staining with DAPI. The fluorescent images of the slides were examined by a Zeiss LSM 800 confocal microscope, or Olympus BX53 Olympus BX53 fluorescence microscope equipped with a DP72CCD camera.
Lactate dehydrogenase (LDH) release assay
After the indicated treatments, culture media from primary cortical neurons were collected and subjected to LDH assay with the use of a LDH-Cytotoxicity Colorimetric Assay Kit II (BioVision, Milpitas, California). Cytotoxicity values were calculated according to the manufacturer’s protocol.
Statistical analysis
Quantitative data are presented as mean ± SD. All statistical analyses were done using GraphPad Prism 9 (San Diego, California). Data were analyzed with one-way ANOVA followed by Tukey post hoc test. P values < 0.05 were considered statistically significant.
Results
Calpain 1 mediates the NMDA-induced cleavage and stimulation of MST1 in cortical neurons
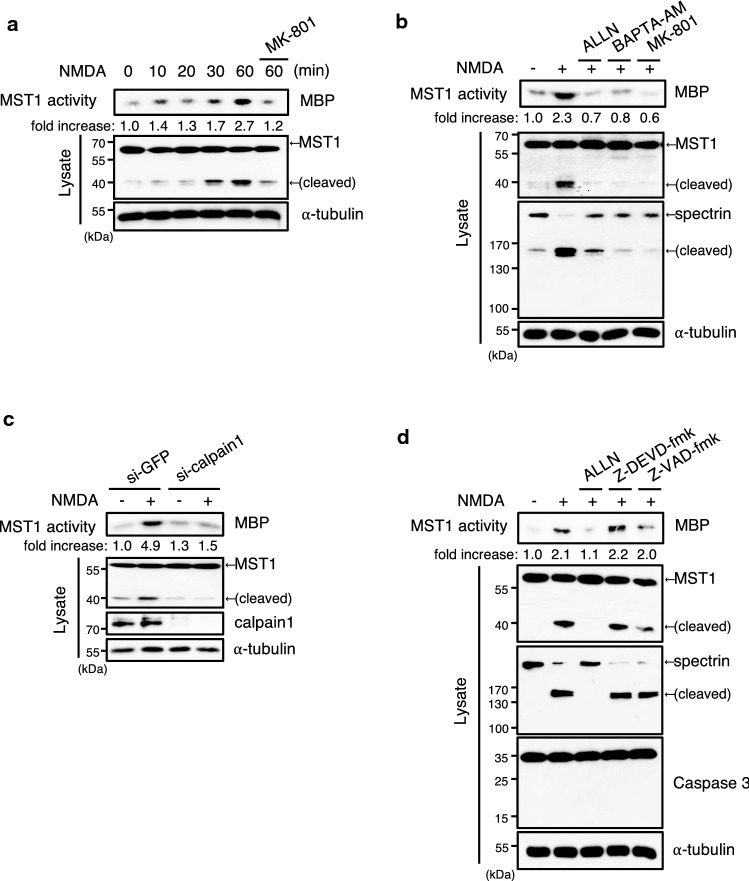
In order to clarify a role of MST1 in NMDA-induced excitotoxicity, we initially examined the kinase activity of MST1 in primary cultures of mouse cortical neurons after NMDA treatment. Our results revealed that NMDA induced the stimulation of MST1 activity in the cortical neurons (Fig. 1a). Intriguingly, NMDA treatment also resulted in the cleavage of MST1, and this cleavage coincided with the stimulation of MST1 activity. Furthermore, MK-801, an antagonist of the NMDAR, mitigated the effect of NMDA on both the stimulation and cleavage of MST1 in the cortical neurons.
Fig. 1.
NMDA induces the activation and cleavage of MST1 in primary cultures of mouse cortical neurons. a, b, d Primary cultures (DIV11) of cortical neurons were treated with 100 μM NMDA for the indicated times (a) or 60 min (b, d) in the absence or presence of indicated chemicals (1 μM MK-801, 10 μM ALLN, 100 μM BAPTA-AM, 20 μM Z-DEVD-fmk, and 20 μM Z-VAD-fmk). Cell lysates were immunoprecipitated with anti-MST1 antibody, and the resulting precipitates were examined for MST1 activity by immune complex kinase assay with myelin basic protein (MBP) as substrate. The lysates were also immunoblotted directly with antibodies to MST1, spectrin, or to tubulin. c Primary cultures (DIV9) of cortical neurons were transfected with GFP (control) or calpain 1 siRNA. After 48 h of transfection, the cells were treated with 100 μM NMDA for 1 h, and then immunoprecipitated with anti-MST1 antibody. The resulting precipitates were examined for MST1 activity by immune complex kinase assay
Calpain, a calcium-dependent protease, has been shown to be a key mediator of NMDA-induced excitotoxicity [8–11]. We, therefore, examined whether calpain would be involved in the NMDA-induced cleavage of MST1 in primary cortical neurons. As expected, NMDA induced the cleavage of spectrin, a typical natural substrate of calpain, in primary cortical neurons, and this effect of NMDA was blocked by a calpain inhibitor N-acetyl-Leu-Leu-Nle-aldehyde (ALLN) and a calcium chelator BAPTA-AM as well as MK-801 (Fig. 1b). Moreover, ALLN and BAPTA-AM abolished the NMDA-induced cleavage and stimulation of MST1 in the cells. Additionally, siRNA-mediated depletion of calpain 1 expression mitigated the NMDA-induced cleavage and stimulation of MST1 in the cells, compared with those of control siRNA-transfected cells (Fig. 1c). Taken together, these results suggested that calpain 1 mediates the NMDA-induced cleavage and stimulation of MST1 in primary cultures of mouse cortical neurons. Of note, Z-DEVD-fmk, a caspase-3 inhibitor, and Z-VAD-fmk, a pan-caspase inhibitor, did not affect the NMDA-induced cleavage and stimulation of MST1 in the primary cortical neurons (Fig. 1d). These caspase inhibitors did not prevent the NMDA-induced cleavage of spectrin, either.
MST1 is a natural substrate of calpain 1
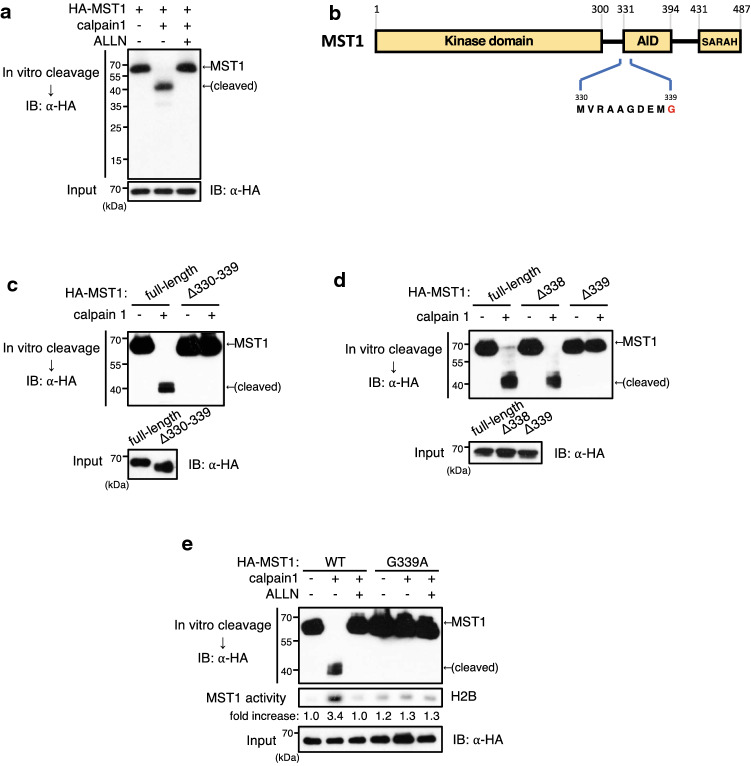
Given that calpain 1 mediates the NMDA-induced cleavage of MST1 in primary cortical neurons, we examined whether MST1 might be a substrate of calpain 1. In vitro calpain protease assay data revealed that calpain 1 cleaved MST1, thereby producing a 40-kDa fragment, and this cleavage was blocked by ALLN (Fig. 2a). Intriguingly, calpain 1 failed to cleave MST1 (Δ330–339), a MST1 mutant deleted for amino acid residues 330–339 in the front part of the autoinhibitory domain (Fig. 2b, c). Additionally, calpain 1 was found to cleave MST1 (Δ335–337) but not MST1 (Δ335–339) (Fig. S1), implicating that the amino acid residue 338 or 339 might be essential for the calpain 1-mediated cleavage. Moreover, calpain 1 failed to cleave MST1Δ339, while it was able to cleave MST1Δ338, in in vitro cleavage assays (Fig. 2d). Taken together, these results suggested that the Gly339 is a target site of calpain 1. We further investigated whether the Gly339 is a calpain 1 cleavage site by using a G339A substitution mutant of MST1, MST1 (G339A), in which Gly339 was replaced with alanine. Both wild-type and G339A mutant of MST1 were immunoprecipitated from 293 T cells overexpressing each of the MST1 variants, and the resulting immunoprecipitates were examined for calpain 1-mediated cleavage in vitro. Calpain 1 cleaved wild-type MST1, but not MST1 (G339A) (Fig. 2e). Furthermore, the calpain 1-catalyzed cleavage enhanced the kinase activity of wild-type MST1, but not of MST1 (G339A). It is noteworthy that the catalytic function of MST1 (G339A) is intact, because hydrogen peroxide, a typical stimuli of MST1, induced the activation of MST1 (G339A) as well as wild-type MST1 in transfection experiments using 293 T cells (Fig. S2). Together, these results suggest that Gly339 present in the autoinhibitory domain is critical for calpain 1-mediated cleavage and subsequent activation of MST1.
Fig. 2.
MST1 is a substrate of calpain 1. a In vitro calpain 1-catalyzed cleavage assay was performed in vitro by incubating calpain 1 (0.5 U) for 10 min at 10 °C with the HA immunoprecipitates of HA-MST1, which had been prepared from HEK293 cells transfected with a vector encoding HA-MST1, in the absence or presence of 10 μM ALLN. Then, the reaction mixture was subjected to immunoblot analysis with antibody to HA. b A schematic diagram of MST1 with the kinase domain, autoinhibitory domain (AID), and SARAH domain. The location of amino acid residues spanning 330–339 is shown. c, d Calpain 1-catalyzed cleavage assay was performed as in a with the HA immunoprecipitates of MST1 variants [full-length MST1 and MST1Δ330–339 (c) or full-length MST1, MST1Δ338, and MST1Δ339 (d)]. e Calpain 1-catalyzed cleavage assay was performed as in a with the HA immunoprecipitates of MST1 or MST1 (G339A) in the absence or presence of 10 μM ALLN. After the cleavage reactions, the HA immunoprecipitates were pulled down and examined for MST1 activity by immune complex kinase assay with histone 2B (H2B) as substrate
Calpain mediates the NMDA-induced nuclear translocation of MST1 in primary cortical neurons
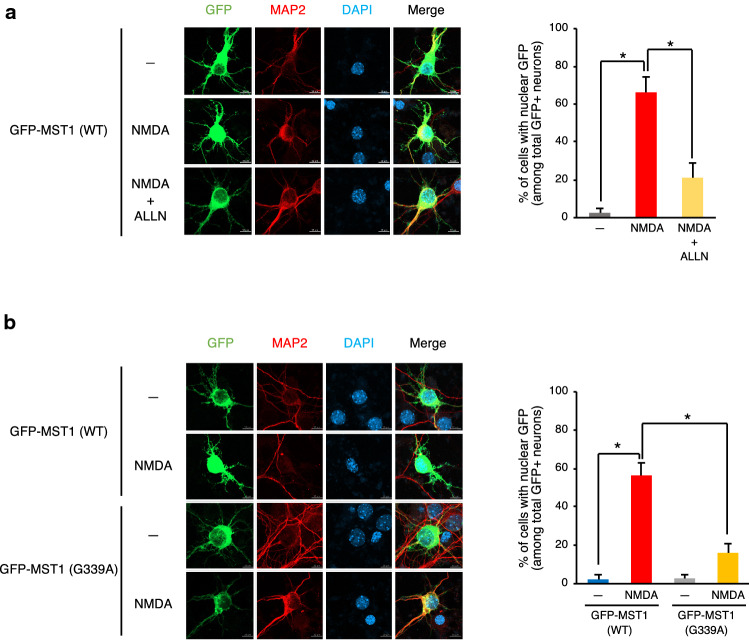
Given the presence of two nuclear export signal (NES) consensus sequences in the 361–370 and 441–451 amino acid residues of MST1 [35], calpain 1-mediated cleavage of MST1 at Gly339 is expected to generate a MST1 variant that lacks the NES sequences. Therefore, we decided to examine whether NMDA might promote the nuclear translocation of MST1 in primary mouse cortical neurons by transfecting the neurons with a vector encoding GFP-tagged MST1. While GFP-MST1 was mostly present in the cytoplasm in the neurons under basal conditions, NMDA increased the abundance of nuclear GFP-MST1 in the cells and this effect of NMDA was mitigated by a calpain inhibitor ALLN (Fig. 3a). Noticeably, a GFP-fused form of MST1 (G339A), which was resistant to a calpain-mediated cleavage (Fig. 2e), remained mostly in the cytoplasm in the neurons even after NMDA treatment (Fig. 3b). Together, these results suggested that the NMDA-induced calpain cleavage of MST1 enhances the nuclear translocation of the kinase in mouse cortical neurons.
Fig. 3.
Calpain mediates the NMDA-induced nuclear translocation of MST1 in primary mouse cortical neurons. Primary cultures of mouse cortical neurons were transfected with a vector for GFP-MST1 (a) or for either GFP-MST1 or GFP-MST1 (G339A) (b). After 48 h of transfection, the cells were left untreated or treated with 100 μM NMDA for 1 h in the absence or presence of 10 μM ALLN. Then, the cells were fixed and subjected to immunostaining with rabbit anti-MAP2 primary antibody and Texas Red-conjugated anti-rabbit secondary antibody, followed by DAPI staining. GFP-positive cells were analyzed for the nuclear localization of GFP-tagged proteins by fluorescence microscopy. Scale bar, 10 μm. Data are mean ± SD of the GFP-positive neurons expressing nuclear GFP-MST1 from three independent experiments. *P < 0.05
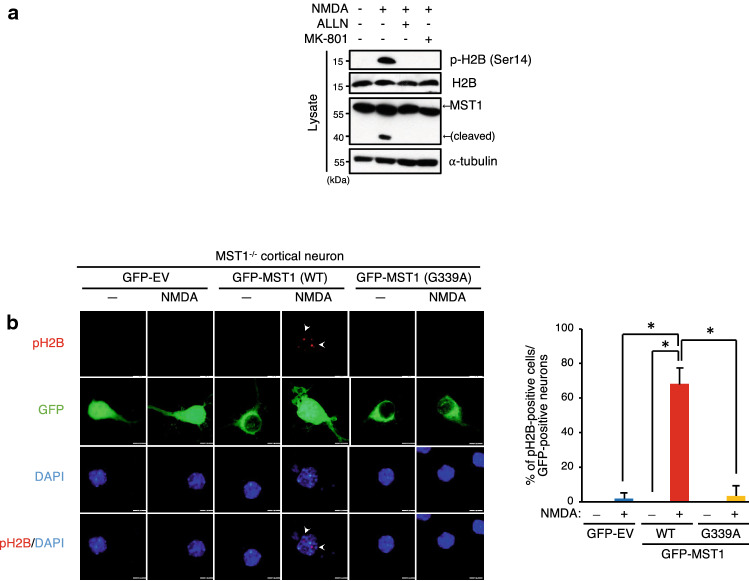
Given the NMDA-induced nuclear translocation of MST1 in cortical neurons, we next examined whether NMDA treatment could increase the phosphorylation of histone 2B, a well-studied nuclear substrate of MST1. MST1 has been shown to mediate the phosphorylation of histone 2B at Ser14, and this phosphorylation promotes chromatin condensation associated with apoptotic cell death [30, 32, 35]. We observed that NMDA treatment resulted in the cleavage of MST1 as well as the phosphorylation of histone 2B at Ser14 in primary mouse cortical neurons, and these effects of NMDA were inhibited by either ALLN or MK-801 (Fig. 4a). Furthermore, genetic ablation of MST1 abolished the NMDA-induced phosphorylation of histone 2B in MST1−/− primary mouse cortical neurons, while the phosphorylation of histone 2B in the MST1−/− neurons was restored by reconstitution of the cells with wild-type MST1, but not with MST1 (G339A) (Fig. 4b). Together, our results suggested that calpain mediates the NMDA-induced nuclear translocation of MST1 and the Ser14 phosphorylation of histone 2B in mouse cortical neurons.
Fig. 4.
The G339A mutation of MST1 abolishes the MST1-mediated phosphorylation of histone 2B under NMDA excitotoxic stress. a Mouse primary cortical neurons (DIV11) were left untreated or treated with 100 μM NMDA for 1 h in the absence or presence of 10 μM ALLN or 1 μM MK-801, and lysed. Cell lysates were immunoblotted with antibodies to phospho-histone 2B (Ser14), histone 2B, MST1, or to α-tubulin. b MST1−/− primary cortical neurons were transfected with either a GFP empty vector (GFP-EV) or a vector encoding GFP-tagged MST1 or MST1 (G339A). After 48 h of transfection, the cells were left untreated or treated with 100 μM NMDA for 1 h, then were fixed and subjected to immunostaining using rabbit anti-phospho-H2B (Ser14) primary antibody and Texas Red-conjugated anti-rabbit secondary antibody, followed by nuclear staining with DAPI. The fluorescence images were analyzed for phospho-H2B-positive cells among GFP-expressing neurons by fluorescence microscopy. Scale bar, 10 μm. Data are mean ± SD from three independent experiments. *P < 0.05
MST1 mediates NMDA-induced excitotoxicity
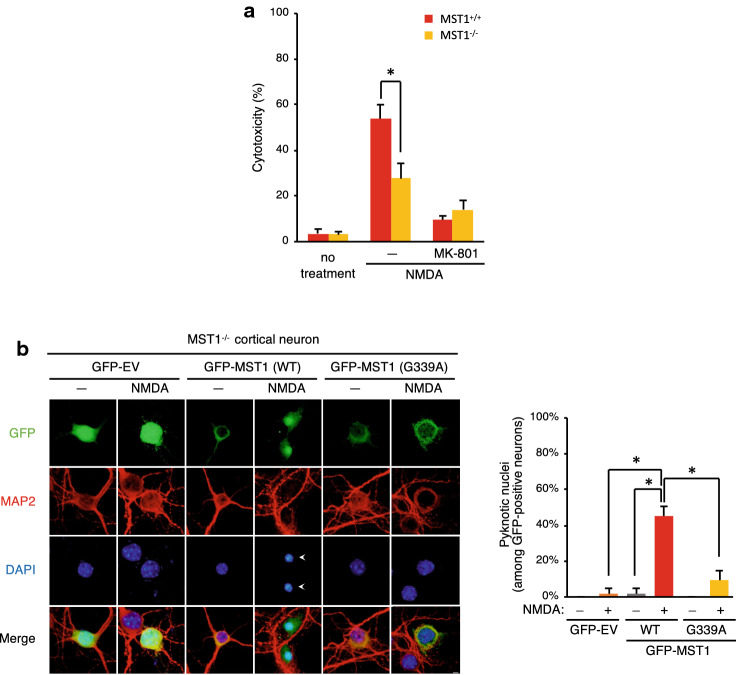
Next, we examined a role of MST1 in NMDA-induced excitotoxic neuronal death in primary cultures of mouse cortical neurons. We initially examined the effect of RNAi-mediated depletion of MST1 expression on NMDA-induced excitotoxicity in the cortical cells. NMDA treatment increased cytotoxicity of the cortical neurons in LDH release assays (Fig. S3a, b) and pyknotic nucleus formation assays (Fig. S3c, d), and this effect of NMDA was mitigated by RNAi-mediated depletion of MST1. We also found that NMDA-induced neurotoxicity was inhibited by ALLN, Z-VAD-fmk, or MK-801 (Fig. S4). Next, we examined the NMDA-induced excitotoxicity in primary cultures of cortical neurons obtained from either wild-type or MST1−/− mice. Noticeably, genetic ablation of MST1 reduced the NMDA-induced cytotoxicity in MST1−/− cortical neurons, compared to that of wild-type cells (Fig. 5a). Collectively, our data thus suggested that MST1 mediates excitotoxic neuronal injury induced by NMDA.
Fig. 5.
MST1 mediates NMDA-induced cell death in primary mouse cortical neurons. a Primary cultures (DIV11) of cortical neurons prepared from wild-type or MST1−/− mice were left untreated or treated with 30 μM NMDA for 24 h in the absence or presence of 1 μM MK-801. Then, culture media were collected and subjected to LDH release assay in triplicates. Data are mean ± SD from three independent experiments. b Primary cultures (DIV9) of cortical neurons prepared from MST1−/− mice were transfected with a vector expressing either wild-type or G339A mutant of GFP-tagged MST1. After 48 h of transfection, the cells were left untreated or treated with 30 μM NMDA for 24 h. Then, the cells were fixed and stained with rabbit anti-MAP2 primary antibody and Texas Red-conjugated anti-rabbit secondary antibody, followed by nuclear staining with DAPI. Scale bar, 5 μm. GFP-positive neurons were analyzed for pyknotic nuclei by fluorescence microscopy. Data are mean ± SD of the percentages of the cells with pyknotic nuclei among GFP-positive neurons from three independent experiments. *P < 0.05
Next, we examined the effect of the G339A mutation of MST1 on NMDA-induced excitotoxicity. We reconstituted MST1−/− cortical neurons with a vector expressing recombinant GFP-tagged MST1 or MST1 (G339A), and then examined for NMDA-induced excitotoxicity by quantifying the formation of pyknotic nuclei. Excitotoxic treatment of NMDA has been shown to cause the formation of condensed pyknotic nuclei in the damaged neurons [36–39]. NMDA markedly increased nuclear condensation in MST1−/− neurons reconstituted with wild-type MST1, but not in the cells reconstituted with an empty vector (Fig. 5b), indicating that MST1 mediates NMDA-induced excitotoxicity. Moreover, the NMDA-induced nuclear toxicity was dramatically reduced in the cells reconstituted with MST1 (G339A), compared with that of cells reconstituted with wild-type MST1. We also observed that NMDA-induced nuclear toxicity was lower in MST1 (G339A)-expressing MST1−/− neurons, compared with MST1+/+ neurons (Fig. S5). Taken together, these results suggest that the G339A mutation of MST1, which renders it resistant to calpain 1 cleavage, mitigates NMDA-induced excitotoxicity in primary cortical neurons.
Discussion
We have here shown that NMDA induces the stimulation of MST1 in the cortical neurons and that this action of NMDA is mediated by calpain. The calpain-mediated stimulation of MST1 promotes the nuclear translocation of this protein kinase, and thereby increases the phosphorylation of histone H2B as well as damaged nuclei in the neurons.
Intriguingly, NMDA treatment induces not only the stimulation but also a cleavage of MST1 in mouse cortical neurons. Moreover, the NMDA-induced cleavage and stimulation of the kinase was abolished by a calpain inhibitor ALLN or by siRNA-mediated depletion of calpain 1, suggesting that calpain 1 is involved in the NMDA-induced cleavage and stimulation of MST1 in the cortical neurons. Indeed, our in vitro data have indicated that calpain 1 directly cleaves MST1 and this cleavage increases its kinase activity. The Gly339 residue appears to be a critical site for the calpain 1-mediated cleavage. Collectively, these findings strongly suggest that MST1 is a novel substrate of calpain 1 and that the calpain 1-mediated cleavage is an important mechanism by which NMDA induces the stimulation of MST1 in the cortical neurons. Given that it is located at the front part of the autoinhibitory domain of MST1, one may propose that calpain 1 mediates the cleavage of MST1 at Gly339, thereby producing a catalytical active fragment of the kinase. Further studies are needed to verify an exact cleavage site of MST1. Additionally, we also tested whether a caspase, such as caspase-3, might mediate the NMDA-induced cleavage of MST1 in the cortical cells, because it has been reported that MST1 is a substrate of caspase-3 under apoptotic conditions [29]. In our study, however, we found that caspase inhibitors do not affect the NMDA-induced cleavage and stimulation of MST1 in the cortical neurons.
Calpain 1 is a known mediator of glutamate-induced neuronal cell death. Under excitotoxic stress, calpain 1 has been shown to mediate cleavage of proteins, such as Src tyrosine kinase [8], debrin [9], p53 [10], and Bid [11], leading to neuronal cell death. In this study, our data indicate that MST1 is a novel substrate of calpain 1, and that calpain 1-activated MST1 mediates the nuclear translocation of MST1 and histone 2B phosphorylation at Ser14 in cortical neurons after NMDA treatment. Given that histone 2B phosphorylation at Ser14 contributes to chromatin condensation leading to cell death [32], the MST1-mediated phosphorylation of histone 2B may be an integral part of the NMDA-induced neurotoxicity in cortical neurons. It is also worthwhile to mention that Src tyrosine kinase, which is another substrate of calpain 1 [8], has been shown to be indirectly involved in the mechanism for MST1 activation in microglial cells under cerebral ischemic conditions [26]. Thus, further studies are needed to clarify possible additional mechanisms by which calpain 1 mediates MST1 activation. In any event, our findings in this study have established that the MST1 functions as a key mediator of NMDA-induced excitotoxicity in mouse cortical neurons.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank D. S. Lim for MST1−/− mice.
Funding
This work was supported by a NRF Grant (NRF-2020R1A2C2011392) funded by the Ministry of Science and ICT of Korea as well as by a Korea University grant (E.-J.C.).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McEntee WJ, Crook TH. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology. 1993;111:391–401. doi: 10.1007/bf02253527. [DOI] [PubMed] [Google Scholar]
- 2.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–62. [PubMed] [Google Scholar]
- 3.Rothman DL, Behar KL, Hyder F, Shulman RG. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annu Rev of Physiol. 2003;65:401–427. doi: 10.1146/annurev.physiol.65.092101.142131. [DOI] [PubMed] [Google Scholar]
- 4.Sims NR, Zaidan E. Biochemical changes associated with selective neuronal death following short-term cerebral ischaemia. Int J Biochem Cell Biol. 1995;27:531–550. doi: 10.1016/1357-2725(95)00026-l. [DOI] [PubMed] [Google Scholar]
- 5.Gunasekar PG, Kanthasamy AG, Borowitz JL, Isom GE. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implications for cell death. J Neurochem. 1995;65:2016–2021. doi: 10.1046/j.1471-4159.1995.65052016.x. [DOI] [PubMed] [Google Scholar]
- 6.Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/jneurosci.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didier M, Bursztajn S, Adamec E, Passani L, Nixon RA, Coyle JT, Wei JY, Berman SA. DNA strand breaks induced by sustained glutamate excitotoxicity in primary neuronal cultures. J Neurosci. 1996;16:2238–2250. doi: 10.1523/jneurosci.16-07-02238.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hossain MI, Roulston CL, Kamaruddin MA, Chu PWY, Ng DCH, Dusting GJ, Bjorge JD, Williamson NA, Fujita DJ, Cheung SN, Chan TO, Hill AF, Cheng HC. A Truncated fragment of Src protein kinase generated by calpain-mediated cleavage is a mediator of neuronal death in excitotoxicity. J Biol Chem. 2013;288:9696–9709. doi: 10.1074/jbc.m112.419713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chimura T, Launey T, Yoshida N. Calpain-mediated degradation of debrin by excitotoxicity in vitro and in vivo. PLoS One. 2015 doi: 10.1371/journal.pone.0125119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276:30724–30728. doi: 10.1074/jbc.m103701200. [DOI] [PubMed] [Google Scholar]
- 12.Saez ME, Ramirez-Lorca R, Moron FJ, Ruiz A. The therapeutic potential of the calpain family: new aspects. Drug Discov Today. 2006;11:917–923. doi: 10.1016/j.drudis.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Mills LR, Kater SB. Neuron-specific and state-specific differences in calcium homeostasis regulate the generation and degeneration of neuronal architecture. Neuron. 1990;4:149–163. doi: 10.1016/0896-6273(90)90451-k. [DOI] [PubMed] [Google Scholar]
- 14.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGinnis KM, Gnegy ME, Park YH, Mukerjee N, Wang KK. Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun. 1999;263:94–99. doi: 10.1006/bbrc.1999.1315. [DOI] [PubMed] [Google Scholar]
- 16.Gold M, Koczulla AR, Mengel D, Koepke J, Dodel R, Dontcheva G, Habib P, Bach JP. Reduction of glutamate-induced excitotoxicity in murine primary neurons involving calpain inhibition. J Neurol Sci. 2015;359:356–362. doi: 10.1016/j.jns.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Creasy CL, Chernoff J. Characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995;270:21695–21700. doi: 10.1074/jbc.270.37.21695. [DOI] [PubMed] [Google Scholar]
- 18.Creasy CL, Ambrose DM, Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J Biol Chem. 1996;271:21049–21053. doi: 10.1074/jbc.271.35.21049. [DOI] [PubMed] [Google Scholar]
- 19.Chan SW, Lim CJ, Chen L, Chong YF, Huang C, Song H, Hong W. The hippo pathway in biological control and cancer development. J Cell Physiol. 2011;226:928–939. doi: 10.1002/jcp.22435. [DOI] [PubMed] [Google Scholar]
- 20.Avruch J, Zhou D, Fitamant J, Bardeesy N. Mst1/2 signalling to Yap: gatekeeper for liver size and tumour development. Br J Cancer. 2011;104:24–32. doi: 10.1038/sj.bjc.6606011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yun HJ, Yoon JH, Lee JK, Noh KT, Yoon KW, Oh SP, Oh HJ, Chae JS, Hwang SG, Kim EH, Maul GG, Lim DS, Choi EJ. Daxx mediates activation-induced cell death in microglia by triggering MST1 signalling. EMBO J. 2011;30:2465–2476. doi: 10.1038/emboj.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Z, Lehtinen MK, Merlo P, Villen J, Gygi S, Bonni A. Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem. 2009;284:11285–11292. doi: 10.1074/jbc.M900461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao L, Chen D, Hu P, Wu J, Liu W, Zhao Y, Cao M, Fang Y, Bi W, Zheng Z, Ren J, Ji G, Wang Y, Yuan Z. The c-Abl-MST1 signaling pathway mediates oxidative stress-induced neuronal cell death. J Neurosci. 2011;31:9611–9619. doi: 10.1523/JNEUROSCI.0035-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan M, Rutten BPF, Kim MO. MST1 regulates neuronal cell death via JNK/Casp3 signaling pathway in HFD mouse brain and HT22 cells. Int J Mol Sci. 2019;20:2504. doi: 10.3390/ijms20102504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao S, Yin J, Zhou L, Yan F, He Q, Huang L, Peng S, Jia J, Cheng J, Chen H, Tao W, Ji X, Xu Y, Yuan Z. Hippo/MST1 signaling mediates microglial activation following acute cerebral ischemia-reperfusion injury. Brain Behav Immun. 2016;55:236–248. doi: 10.1016/j.bbi.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Tao W, Yuan Z, Liu Y. Mst-1 deficiency promotes post-traumatic spinal motor neuron survival via enhancement of autophagy flux. J Neurochem. 2017;143:244–256. doi: 10.1111/jnc.14154. [DOI] [PubMed] [Google Scholar]
- 28.Lee JK, Shin JH, Hwang SG, Gwag BJ, McKee AC, Lee J, Kowall NW, Ryu H, Lim DS, Choi EJ. MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS. PNAS. 2013;110:12066–12071. doi: 10.1073/pnas.1300894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graves JD, Gotoh Y, Draves KE, Ambrose D, Han DK, Wright M, Chernoff J, Clark EA, Krebs EG. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 1998;17:2224–2234. doi: 10.1093/emboj/17.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graves JD, Draves KE, Gotoh Y, Krebs EG, Clark EA. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J Biol Chem. 2001;276:14909–14915. doi: 10.1074/jbc.m010905200. [DOI] [PubMed] [Google Scholar]
- 31.Roh KH, Choi EJ. TRAF2 functions as an activator switch in the reactive oxygen species-induced stimulation of MST1. Free Radic Biol Med. 2016;91:105–113. doi: 10.1016/j.freeradbiomed.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, Beeser A, Etkin LD, Chernoff J, Earnshaw WC, Allis CD. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell. 2003;113:507–517. doi: 10.1016/s0092-8674(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 33.Oh S, Lee D, Kim T, Kim TS, Oh HJ, Hwang CY, Kong YY, Kwon KS, Lim DS. Crucial role for Mst1 and Mst2 kinases in early embryonic development of the mouse. Mol Cell Biol. 2009;29:6309–6320. doi: 10.1128/mcb.00551-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee IY, Lim JM, Cho H, Kim E, Kim Y, Oh HK, Yang WS, Roh KH, Park HW, Mo JS, Yoon JH, Song HK, Choi EJ. MST1 negatively regulates TNFα-induced NF-κB signaling through modulating LUBAC activity. Mol Cell. 2019;73:1138–1149. doi: 10.1016/j.molcel.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y, Khokhlatchev A, Figeys D, Avruch J. Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis. J Biol Chem. 2002;277:47991–48001. doi: 10.1074/jbc.M202630200. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Capetillo O, Allis CD, Nussenzweig A. Phosphorylation of histone H2B at DNA double-strand breaks. J Exp Med. 2004;199:1671–1677. doi: 10.1084/jem.20032247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam TT, Abler AS, Kwong JM, Tso MO. N-methyl-d-aspartate (NMDA)-induced apoptosis in rat retina. Investig Ophthalmol Vis Sci. 1999;40:2391–2397. [PubMed] [Google Scholar]
- 38.Gasull T, DeGregorio-Rocasolano N, Zapata A, Trullas R. Choline release and inhibition of phosphatidylcholine synthesis precede excitotoxic neuronal death but not neurotoxicity induced by serum deprivation. J Biol Chem. 2000;275:18350–18357. doi: 10.1074/jbc.m910468199. [DOI] [PubMed] [Google Scholar]
- 39.McKay S, Bengtson CP, Bading H, Wyllie DJA, Hardingham GE. Recovery of NMDA receptor currents from MK-801 blockade is accelerated by Mg2+ and memantine under conditions of agonist exposure. Neuropharmacology. 2013;74:119–125. doi: 10.1016/j.neuropharm.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.