Abstract
Insect flight is a complex physiological process that involves sensory and neuroendocrinal control, efficient energy metabolism, rhythmic muscle contraction, and coordinated wing movement. As a classical study model for insect flight, locusts have attracted much attention from physiologists, behaviorists, and neuroendocrinologists over the past decades. In earlier research, scientists made extensive efforts to explore the hormone regulation of metabolism related to locust flight; however, this work was hindered by the absence of molecular and genetic tools. Recently, the rapid development of molecular and genetic tools as well as multi-omics has greatly advanced our understanding of the metabolic, molecular, and neuroendocrinal basis of long-term flight in locusts. Novel neural and molecular factors modulating locust flight and their regulatory mechanisms have been explored. Moreover, the molecular mechanisms underlying phase-dependent differences in locust flight have also been revealed. Here, we provide a systematic review of locust flight physiology, with emphasis on recent advances in the neuroendocrinal, genetic, and molecular basis. Future research directions and potential challenges are also addressed.
Keywords: Flight physiology, Energy metabolism, Hormone, Neuropeptide, Phase-related flight traits, Aging
Introduction
Flight is a fascinating biological trait that is possessed only by insects, birds, and some mammals. Insects have developed this capacity to the extreme [1]. Benefiting from their flight ability, insects are a highly successful class of animals, and they are capable of radiating into most types of terrestrial environments [2]. Locusts have attracted much attention from researchers over the past decades due to their extraordinary long-distance flight ability, easy mass rearing, and convenient handling. Beyond their flight mastery, locusts have been among the most destructive agricultural pests around the world since ancient times. Even today, locust plagues often cause serious threats to the economic and food security of over 60 countries. Locust swarms formed by millions of gregarious locusts can cover several hundred square kilometers and migrate up to 200 km per day (FAO 2020, https://www.fao.org/locusts/en/) [3]. The long-distance flight capacity of locusts greatly reduces the effects of pesticide application and further exacerbates the difficulty of prevention and control. Therefore, exploring the physiological, genetic, and molecular basis of flight is essential for locust plague control.
Flight is a complex behavioral event that involves precise sensory input and processing [4], rhythmic muscle contraction [5], efficient energy utilization [6], and coordinated wing movements [7]. Locusts have evolved physiological adaptations for each aspect of flight, especially high plasticity in metabolism and sustained energy supply for flight muscles. The plasticity of flight ability is also observed in locusts that display density-dependent polyphenism, particularly the migratory locust Locusta migratoria and the desert locust Schistocerca gregaria [8]. Gregarious locusts occurring in high population density can perform sustained flight for over ten hours, whereas solitarious locusts with low population density show weak flight ability [9]. Given these features, locusts constitute a long-standing favored model insect for flight-related physiological activities, such as energy metabolism and motor pattern generation [10, 11].
Many early studies on locust flight focused largely on the dynamics of energy consumption in flight muscles [12], mobilization of substrates in the fat body [13], and hormone regulation of flight-related metabolism [14]. Moreover, a few neurons and circuits involved in the control of flight motor patterns and muscle metabolism have also been demonstrated [15–17]. These findings have been discussed or reviewed in early separate reviews [18–21], which have laid an important foundation for understanding the regulatory mechanisms underlying locust flight. The subject of locust flight specifically focused on the hormonal regulation of lipid metabolism was reviewed over 10 years ago [11]. Since then, omics and molecular studies have significantly promoted research advances in locust biology. On this basis, novel genes or molecules involved in flight control and neuroendocrinal mechanisms underlying long-term flight in locusts have been identified. Here, we provide a comprehensive review of the regulation of locust flight by summarizing important findings, with an emphasis on recent advances in hormone control and the molecular basis for the plasticity of locust flight performance.
Physiological basis of locust flight
All types of movements are induced by muscle contractions that convert chemical energy to mechanical activity with different speeds, forces, and durations. Insect flight muscles are fundamental devices for flight performance and are particularly interesting because of their high rate of power output. In contrary to most insect species using indirect flight muscle that power wing beating via deformation of the thoracic exoskeleton, the locust uses direct flight muscle to drive wing movement directly [22]. The locust flight muscles consist of many fibers innerved by motoneurons located in the thoracic ganglion [23]. Muscular contraction is induced by action potentials in the motoneurons supplying the muscle. The release of neurotransmitter substances from motor nerve terminals induces excitation of the muscle cell membrane, which is followed by an intracellular contraction-elicitation process leading to muscle contraction and finally resulting in wing movement (Fig. 1). Unlike the patterns of muscle innervation in vertebrates in which one motoneuron supplies one muscle fiber [24], flight muscle fibers in locusts and other insects are innervated by multiple types of motoneurons, including excitatory, inhibitory, and neuromodulator motoneurons, and display graded electrical potentials that produce graded contraction forces [25].
Fig. 1.
Physiological and mechanical basis for wing movement driven by direct flight muscle in locusts. Successive steps initiated by motoneuron activation are required for the generation of wing movement. A single action potential of motoneuron induces depolarization of the muscle cell membrane, leading to muscle contraction, which causes wing movement in turn
The muscle metabolism rate rises by 50–200-fold during insect flight, which is much higher than that in skeletal muscle in mammals performing continuous exercise [6]. Thus, insect flight muscle has evolved a specialized ultrastructure and biochemistry to form an effective catabolic machine. Compared to vertebrate muscle, insect flight muscle comprises larger fibers, more glycogen stores, less sarcoplasmic reticulum, and more giant mitochondria that comprise 40% of the muscle mass [26]. As a key cellular organelle producing ATP by oxidative phosphorylation, the biogenesis of mitochondria exactly reflects the capacity of oxidative phosphorylation and is adapted to cell-specific energy demands [27]. Quantitative electron microscopy has demonstrated that mitochondria enlarge 30-fold in adult locusts with sustained flight capacity compared to the last instar of locust nymphs [28]. Two alternative mechanisms are thought to enhance mitochondrial mass: mitochondrial proliferation and mitochondrial differentiation. Developmental analysis of mitochondrial DNA copy number, mitochondrial transcripts, and the mitochondrial translation rate of flight muscle in L. moratoria indicates that enhanced mitochondrial differentiation accompanied by increased translation of mitochondrial proteins contributes significantly to functional improvement [29]. However, the genetic and molecular basis underlying mitochondrial differentiation in locusts has rarely been reported. With the availability of both nuclear and mitochondrial genome data as well as genetic tools, we believe that regulatory mechanisms underlying mitochondrial differentiation in the flight muscle of adult locusts will soon be determined. The results will shed light on the metabolic regulation related to mitochondrial differentiation that is commonly observed in mammals [30].
Neuroendocrinal regulation of energy metabolism during locust flight
As a representative migratory insect with long-distance flight ability, locusts consume both carbohydrates and lipids for energy supply during flight [6]. Within the first 15 min of flight, the concentrations of glycogen and glucose dramatically decrease, indicating that the glycolytic pathway mainly contributes to the energy supply for the initiation period of flight. In contrast, fatty acids (FAs) mobilized from adipose triacylglycerol (TAG) serve as exclusive substrates consumed to meet energy demands during prolonged flight. Dynamic changes in the contents of intermediates and products of the glycolytic pathway during flight have been described in detail in early studies [12]. Research progress on lipid mobilization, lipid transport, and the uptake and utilization of FAs has also been thoroughly reviewed previously. The readers are referred to these references for details on metabolism dynamics during locust flight [11, 20, 31, 32].
The neuroendocrinal control of insect flight occurs at several levels. First, neuromodulators could directly control the flight motor system by acting on the electronic response of motoneurons or flight muscles, thus having a releaser effect; second, endocrinal hormones exert fewer direct effects on general flight activity but modulate energy utilization, displaying a priming effect; and third, endocrine factors may affect flight by controlling the development of the flight system, such as wing polymorphism, flight muscle organization, motoneurons, or central pattern generation. Unlike the flight traits of wing-polymorphic insects (e.g., plant hoppers, crickets, and aphids) that are controlled by wing forms [33], locust flight is mainly indirectly controlled by hormones and neuromodulators through metabolic regulation, with octopamine (OA) exhibiting direct modulation of muscle glycolysis (see below). This review outlines the regulatory roles and action models of neuroendocrinal factors and their potential interactions in regulating locust flight, particularly substrate consumption.
Regulatory role of AKH in locust flight
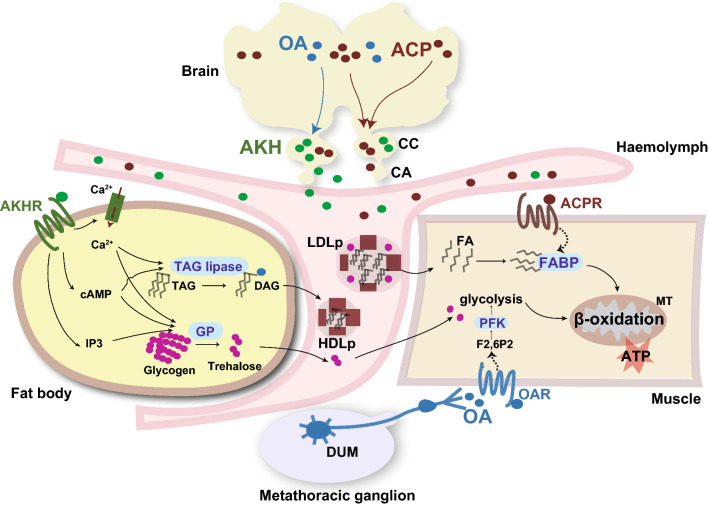
During the prolonged flight of locusts, the mobilization of stored fuels in the fat body is controlled by the neuropeptide adipokinetic hormone (AKH), which is synthesized and stored in neurosecretory cells in the glandular lobe of the corpora cardiaca (CC) [20, 34]. In general, AKH peptides consist of 8–11 amino acids and are characterized by a pyroglutamic acid on the N-terminus and an amidated C-terminus [35]. Accumulating evidence has revealed the significant role of AKH in integrating energy metabolism during flight. Contrary to its name, AKH not only participates in lipid mobilization and carbohydrate (trehalose) release from fat bodies but also affects the anabolism of nucleic acids, proteins, and lipids [36, 37]. Although the regulatory roles of AKH in the mobilization of either carbohydrates, lipids, or amino acids (especially proline) have been extensively reported in different insect species, such as cockroaches [38, 39], moths [40], and fruit beetles [41], the biosynthesis, release, and signal transduction of the hormone are best studied in locusts [20, 34, 35, 42]. The action of AKH starts with its specific membrane receptor (AKHR), which couples with GTP-binding protein and then activates glycogen phosphorylase and TAG lipase in the fat body in a time- and dose-dependent manner, with activation of the former occurring earlier but the latter being activated later. However, signal transduction messengers activating the two enzymes vary in their response to AKH release, in which cyclic AMP and Ca2+ are competent for the activation of both TAG lipase and glycogen phosphorylase, while IP3 is only required for glycogen phosphorylase activation [43] (shown in Fig. 2). The mode of action of AKH-induced energy mobilization and lipophorin conversion has been described in detail, and the readers are referred to previous reviews [20, 44].
Fig. 2.
Neuronal and endocrinal regulatory mechanisms underlying energy mobilization and utilization during flight. Energy metabolism associated with locust flight is integrated by three neuroendocrinal factors, AKH, ACP, and OA. AKH produced by corpora cardiaca (CC) is responsible for the mobilization of lipids and carbohydrates in the fat body by promoting the enzyme activities of TAG lipase and glycogen phosphatase [11, 20]. Different second messengers have been implicated in AKH receptor-mediated activation of lipase and glycogen phosphatase [21]. OA in the brain has been revealed to promote AKH release from CC and subsequent mobilization of lipids in the fat body [93]. The ACP peptide generated in the brain acts as a hormone to facilitate FA transport and oxidation in flight muscle by acting on its membrane receptor. The expression level of FABP changes in response to variation in ACP levels to fine-tune the beta-oxidation rate in mitochondria [54]. Moreover, OA released from DUM neurons in the metathoracic ganglion tightly controls glycolysis by modulating PFK activity through adjusting F2,6P2 levels in the flight muscle. However, DUM neuron activity and OA level in the flight muscle rapidly decrease after flight, indicating the distinct role of OA in takeoff [17]. Dotted lines indicate unknown molecular mechanisms. TAG triacylglycerol, DAG diacylglycerol, GP glycogen phosphatase, LDLp low-density lipophorin, HDLp high-density lipophorin, PFK phosphofructokinase, DUM neuron, dorsal unpaired median neuron, MT mitochondrion, F2,6P2 fructose-2, 6-diphosphate
To date, more than 50 AKH peptides have been identified from representative insects [45]. The numbers of AKH peptides vary from 1 to 3 depending on the distinct insect species [46], and different AKH peptides that exist in certain insect species usually show overlapping functions [47, 48]. Four AKH precursor genes have been identified based on the locust genome [49]. However, only three of them (AKH-I, AKH-II, and AKH-III) have been biochemically validated through peptide characterization [47]. The mRNA of all three AKH precursor genes is colocalized in the cell bodies of the glandular lobe of the corpora cardiaca (CC) [50]. Upon flight, all three AKH peptides could be released from the CC proportionally to the hemolymphs for lipid mobilization of the fat body [51]. In locusts, compared to AKH-II and AKH-III, AKH-I has the highest content in the CC (AKH-I:AKH-II: AKH-III = 14:2:1) and the greatest potential to stimulate lipid mobilization [52]. Comparative studies on the degradation rates of the three AKHs revealed that AKH-I and AKH-II degraded much more slowly than AKH-III under both flight and resting conditions. Moreover, AKH-I and AKH-III degraded faster during flight compared to the resting state, whereas AKH-II displayed stable half-lives under flight and resting conditions [53]. Therefore, the abundance ratio of the three AKH peptides changed drastically after release. The highest initial amounts together with their relatively slow degradation suggest that AKH-I is the most important hormone for sustained flight. AKH-II, which is less abundant and displays identical degradation rates at rest and flight, is supposed to play a distinct role at the onset of flight when carbohydrates are consumed as a major resource [53]. Notably, knockdown of AKH-II significantly suppressed the total duration and total distance within 1 h of tethered flight, supporting the alternative function of this peptide in fine-tuning prolonged flight [54]. As AKH-III had the lowest abundance, it is assumed to be essential for metabolic modulation at rest [42]. In both L. migratoria and S. gregaria, prolonged flight could increase the expression levels and released contents of three AKHs to varying degrees [55, 56]. Whether different AKH peptides in locusts act separately or synergistically in regulating flight-related energy metabolism remains elusive. The AKH peptides have been shown to play distinct roles in reproduction, another energy-intensive physiological process in locusts, by either inhibiting or promoting the synthesis of proteins or lipids [57–59], implying that three AKHs may undertake distinct tasks in the metabolic trade-off between flight and reproduction. Using 3-D structure modeling, three locust AKHs were individually docked to AKHR in the desert locust. All three endogenous AKHs are predicted to bind with AKHR with similar binding constants, despite their different amino acid sequences (Table 1) [60]. Intriguingly, these two locust species share a common peptide sequence for AKH-I and a very similar sequence for AKH-II, whereas the Schgr-AKH-III peptide is identical to the predicted Locmi-AKH-IV peptide. Whether the AKH IV peptide is present in migratory locusts needs further biochemical validation. Comparative studies on the different roles of the three AKHs in physiology and behavior are warranted, including those that take advantage of specific gene knockdown or knockout together with dynamic metabolic analysis. Several neuropeptides, such as locustatachykinin, SchistoFLRFamide, and FMRFamide, which are detected in the axon terminals contacting the glandular cells of the CC, have been shown to either induce or inhibit the release of AKH in locusts [61]. Further exploration of the regulatory mechanisms underlying the release of distinct AKH peptides in response to flight will shed light on the action model of the AKH peptide system during locust flight.
Table 1.
Comparison of the biochemical properties of AKH and ACP peptides in locusts
| Peptide name | Sequence | Abundance | mRNA Fold change upon 1 h flight | Binding constants for Sg AKHR | Half-lives (rest/flight) |
|---|---|---|---|---|---|
| LmAKH-I/Sg-AKH-I | pELNFTPNWGTamide | 14 | 2 | − 98 kcal/mol | 51/35 min |
| LmAKH-II | pELNFSAGWamide | 2 | 2 | – | 40/37 min |
| LmAKH-III | pELNFTPWWamide | 1 | 4.2 | – | 5/3 min |
| LmAKH IV/Sg-AKH-III | pELTFTPSWamide | – | – 88 kcal/mol | – | |
| Sg-AKH-II | pELNFSTGWamide | – | – 94 to – 116 kcal/mol | – | |
| LmACP | pEVTFSRDWSPamide | – | 2 | – | – |
Regulatory role of ACP in locust flight
The so-called AKH/corazonin-related peptide (ACP) is named based on its high structural similarity with both AKH and corazonin, which belong to the gonadotropin releasing hormone (GnRh) family peptides. Phylogenetic tree analyses show that AKH, ACP, and corazonin peptides and their corresponding receptors present a typical receptor/ligand coevolution case [62]. The ACP/ACPR peptide system is supposed to originate by gene duplication from the same ancestor as the AKH/AKHR system and thus displays a much closer evolutionary relationship. Unlike AKH, which is produced in the CC, the ACP peptide is generated in the brains of most insects, such as Locusta and Tribolium [54]. The ACP peptide initially isolated from the CC of the migratory locust does not affect hemolymph trehalose levels in locusts; however, it has a hypertrehalosemic effect in cockroaches and is thus named locust hypertrehalosemic hormone (Lom-HrTH) [63]. As a newly discovered peptide signaling pathway, the ACP system has been lost several times during evolution and is absent in several insect species, including the model insect Drosophila [64]. The injection of ACP peptide has no significant effects on either hemolymph lipid levels or heartbeat rates in Rhodnius prolixus [65]. The biological functions of the ACP peptide and its receptor have not been clearly unraveled in insects until a recent study reported a novel role in facilitating the sustained flight of locusts [54].
The important regulatory role of the ACP peptide system in flight-related lipid oxidation is revealed by combined multi-omics and CRISPR/Cas9 technology [54]. In this study, Hou et al. performed comparative neuropeptidome analysis and found that the ACP peptide was abundantly detected in the adult retrocerebral complex (mainly including the CC-CA). The ACP precursor gene is specifically expressed in the brain and is significantly induced after 1 h of sustained flight. Loss of function of ACP or its receptor gene (ACPR) dramatically attenuates the prolonged flight ability of locusts of both sexes. Integrated transcriptome and metabolome analyses demonstrate obvious reductions in fatty acid transport and oxidation in the flight muscle of ACP mutant locusts. The key cellular FA transporter in flight muscle, fatty acid binding protein (FABP) [66], mediates the regulatory effects of ACP on lipid oxidation and prolonged flight (Fig. 2). This study provides a previously undetermined molecular basis for effective lipid utilization in flight muscle, indicating that brain-derived neuropeptides act as neuromodulators to directly facilitate substrate consumption in flight muscle to maintain flight [54]. The regulatory role of the ACP peptide system in flight-related metabolism provides a novel case for the involvement of neuropeptides in flight control. Whether ACP plays conserved roles requires validation in other insects engaged in long-term flight.
As a downstream molecule of ACP for flight control, FABP has been shown to determine FA utilization capacity in the flight muscle of locusts, since its protein accounts for as much as 18% of total cytosolic muscle proteins, a value more than three times that found in mammalian muscle [67, 68]. In both S. gregaria and L. migratoria, RNA interference (RNAi) of the FABP gene significantly attenuated prolonged flight ability in both locust species [54, 69], indicating the conserved role of FABP in long-term flight in locusts. FABP displays a truly adult-specific expression pattern, and its concentration is concomitant with the flight ability of locusts [70]. Moreover, injection of either ACP or AKH peptide or low-density lipoprotein (LDLp) induces increased mRNA level of FABP in the flight muscle [54, 71], suggesting that the temporally and spatially dependent expression of the gene is controlled at multiple levels. To date, the molecular mechanism underlying multilevel-controlled FABP transcription remains unknown. It has been suggested that the inaccessibility of RNA polymerase to the FABP promoter may result in complete silencing of the FABP gene in nymphs. Considering the key function of epigenetic factors in temporal and spatial gene expression [72], epigenetic changes occurring in the FABP promoter region, such as DNA methylation or histone modifications, may be involved in the developmental regulation of FABP gene transcription. FA-induced upregulation of FABP expression is common throughout the animal kingdom. A conserved fatty acid response element (FARE) has been identified in the promoter regions of FABP from desert locusts, flies, chickens, rats, and humans [73, 74]. Thus, FA-activated transcription factors may be involved in the regulation of FABP expression by ACP or AKH manipulation. Further studies are required to identify distinct transcription factors mediating the effects of ACP and AKH on FABP transcription activation in locusts.
Despite being expressed in the muscle, the ACP receptor is also detected in the fat body at a relatively high level, implying that the peptide system plays a role in fat body biology [54]. In the two-spotted cricket Gryllus bimaculatus, the ACP precursor gene is only detected in the CA, and ACP peptide injection promotes increases in hemolymph carbohydrate and lipid levels, which is similar to the effects caused by AKH treatment [75]. However, the hypertrehalosemic effect of ACP has been excluded in locusts, and the metabolic changes in the fat body in response to ACP manipulations remain unclear. Interestingly, ACP knockout locusts display obviously larger body sizes, which is probably attributed to the energy trade-off between flight and body growth that is commonly observed in organisms [76]. Similarly, the loss of function of the AKH gene also leads to adult obesity in Drosophila [77]. Therefore, ACP and AKH may play common roles in modulating energy homeostasis, yet the relative action modes may differ. Clearly, both AKH and ACP are required for integrating substrate utilization with nonredundant functions to maintain sustained flight activity in the locust (Fig. 2). The former peptide governs energy mobilization from the fat body, whereas the latter peptide directly facilitates lipid utilization in flight muscle. It will be very interesting to further explore whether and how the two neuropeptide systems interact with each other to affect metabolic changes in the fat body, where their receptors are expressed. A comprehensive understanding of the independent or interactive roles of the two related peptide systems will be achieved in future work, especially comparative studies of signal transduction and physiological and behavioral changes induced by separate or joint manipulations of the two peptide systems.
Regulatory roles of OA in locust flight
The biogenic monoamine OA is a crucial modulator of invertebrate physiology and behavior. OA can act as a neurotransmitter or a neurohormone and is found in a variety of biological contexts in insects, including feeding, sleep, locomotion, learning, aggregation, reproduction, and stress response [78, 79]. A series of studies has revealed that OA participates in the modulation of insect flight by regulating energy metabolism as well as flight motor patterns [80, 81]. Here, the multilevel regulation of OA in locust flight is summarized, with an emphasis on its direct control of muscle glycolysis at the early stage of flight.
Glycogen or glucose breakdown by glycolysis supplies the primary energy resource at the initial phase of flight. The primary control element of glycolysis is phosphofructokinase (PFK), which is activated by the synergistic action of AMP and fructose-2, 6-diphosphate (F2,6P2) [82, 83]. Injection of high doses of OA neutralizes the flight-induced reduction in F2,6P2 levels in the flight muscle [84]. It has been demonstrated that the only source of OA in the flight muscle is released by central dorsal unpaired median (DUM) neurons with bilaterally symmetrical efferent axons on both sides of muscles [10, 85]. Upon antidromic stimulation of DUM neurons, OA is only released to a single side of the wing elevator muscle, and the content of F2,6P2 in nervated muscle increases sevenfold compared with the contralateral unstimulated control muscle, clearly demonstrating a direct and specific regulation of glycolysis by OA-containing central modulatory neurons during insect flight (Fig. 2) [17]. Pharmacological experiments demonstrate that protein kinase A (PKA) is required, but not sufficient, for OA-containing motoneuron increased F2,6P2 level, implying that another pathway is involved in the signal transduction from OA to F2,6P2. The receptor subtype and subsequent pathway that transduces the effects of OA remain to be determined [86]. During flight, all DUM neuron activity is strongly suppressed, followed by a reduction in OA levels in flight muscles [10]. Flight-induced sequential inhibition of DUM motor neuron activities and OA levels was in line with the decrease in F2,6P2 content in flight muscle, highlighting the tight control of DUM motor neuron-released OA on muscle glycolysis during flight. However, the accumulation of F2,6P2 alone in the resting state cannot trigger glycolytic activity in the absence of AMP. The dramatic turnover of ATP after flight will lead to rapid AMP generation, which then ensures high glycolytic rates for energy demand in the flight muscle after flight initiation [10, 17]. Mentel et al. (2003) provided the first study that revealed the direct control of muscle metabolism during flight by the central nervous system (for review, see [25]). It is reasonable to ask whether the metabolic transition from glycolytic flux to lipid oxidation during prolonged flight is due to the decreased activity of OA-containing DUM neurons or the integrated effects of dynamic activity changes in multiple neuroendocrinal factors involved in flight control, e.g., AKH and ACP, as described above.
OA is also supposed to have adipokinetic activity in locusts because the transmitter is shown to be released to the hemolymph after a few minutes of motor activity and induces cAMP production and lipid mobilization from the fat body both in vivo and in vitro [87–89]. Based on the biphasic pattern of increases in hemolymph lipid level during flight (a rapid increase at 10 min and a later steady increase at 20 min after flight) and the sequential release of OA and two AKH peptides, a regulatory model incorporating lipid mobilization during flight has been proposed [85]. The initial increase in hemolymph lipids is caused by the short-lived elevation of OA [90], but the second phase of hemolymph lipid increase is attributed to AKH release [91]. However, the involvement of OA in the mobilization of lipids from the fat body was challenged by another study, in which neither lipase activity nor the hemolymph lipid level was changed upon OA injection [43]. Anatomical and physiological evidence suggests that OA exists in the synapses between axons of nervi corporis cardiaci II (NCCII) and neurosecretory cells in the CC [92]. Pharmacological treatment with aminergic agonists (exogenous application of OA) and antagonists (adrenergic receptor antagonists) suggests that synaptically released OA stimulates AKH release at least in part through the elevation of intracellular cAMP levels in the glandular lobe [93]. Obviously, the control of energy mobilization during flight is complex and involves the integration of different hormones. It is difficult but very meaningful to elucidate the hormone network coordinating metabolic demands for sustained flight in the fat body where both storage and output of substrate occur.
Genetic and molecular basis underlying long-term flight
Before the availability of genome information, studies on locust flight were limited to physiological and biochemical levels. Insect biology is set to enter the omics era with the increase in omics technologies being applied in this area. In recent years, studies using genomics, transcriptomics, metabolomics, and effective genetic tools have greatly advanced our understanding of flight-related physiological adaptation in locusts.
Molecular mechanisms underlying long-term flight
The assembly of the L. migratoria genome lays a foundation for understanding the genetic and molecular basis for long-term flight [94]. Wang et al. have annotated a high number of genes involved in wing and muscle morphology, neuroendocrine regulation, and energy metabolism. Based on comparative gene expression analysis of flying and nonflying adults, 472 flight-related genes were enriched, and 80% of these genes were multicopy genes. A comparative genome analysis of six insect species revealed that the copy numbers of genes associated with lipid metabolism and antioxidant protection significantly increased in the locust genome (Fig. 3a). Given the extensive requirement of energy and redox homeostasis during sustained flight [95], the significant increase in the copy numbers of lipid metabolism genes and antioxidant genes properly reflects a genetic adaptation for effective energy consumption as well as a powerful antioxidative protection system to counter extensive energy costs during long-term flight in the locust [94]. Future genetic and molecular experiments are needed to validate the distinct roles of these genes.
Fig. 3.
Mechanisms underlying long-term flight in locusts. A Genetic and molecular basis of long-term flight in locusts. Increased copy number of genes related to energy metabolism and antioxidation may contribute to excellent sustained flight capacity in locusts [94]. The alternative splicing form of hypoxia inducible factor Hif-1α2 can facilitate sustained flight by maintaining redox homeostasis through activating the expression level of DJ-1 gene in the flight muscle [96]. B Molecular mechanism underlying flight plasticity in the migratory locust. Flight ability in locusts displays a physiological state and phase-dependent changing patterns controlled by nongenetic mechanisms. During the aging process, downregulation of metabolism-related genes and abnormal mitochondria result in deteriorated flight performance [107]. Locusts display distinct flight traits in response to population density variation. Moderate metabolism, stable redox homeostasis, and higher energy storage facilitate long-term flight capacity with relatively lower speed in gregarious locusts, whereas robust muscle metabolism and overloaded ROS lead to short-term flight with higher initial flight speed in solitarious locusts [9]. PLIN perilipin, Prdx6 peroxiredoxin 6, GST glutathione S-transferase, LIPT1 lipoyltransferase 1, OXPHOS oxidative phosphorylation, ROS reactive oxygen species
In addition to the genome-based study on long-term flight, functional molecules involved in the modulation of long-term flight have also been demonstrated through molecular validation in locusts. Ding et al. recently revealed a modulatory role of hypoxia inducible factor (Hif) during prolonged flight in the migratory locust [96]. They identified two splice isoforms of locust Hif-1α, namely, Hif-1α1 and Hif-1α2. The ubiquitously expressed isoform Hif-1α1 was strictly controlled by hypoxic conditions and retained classic regulatory roles in hypoxia adaptation, as extensively reported in many animals [97]. By contrast, the isoform Hif-1α2, which is abundantly expressed in flight muscle, was less sensitive to hypoxia but significantly upregulated upon sustained flight. Prolonged flight activity was substantially reduced by isoform-specific knockdown of Hif-1α2, rather than Hif-1α1 (Fig. 3a). Further transcriptome and experimental analyses revealed that Hif-1α2 facilitated prolonged flight performance by maintaining redox homeostasis during locust flight by activating the expression of the DJ-1 gene [96], which is an evolutionarily conserved ROS quencher [98, 99]. Intense physical activities usually lead to overloaded reactive oxidative species (ROS) that may cause damage to the cell membrane and biochemical molecules [100, 101]. The findings raised by Ding et al. (2021) thus expand the functional scope of Hif genes and provide clear experimental evidence for the importance of redox balancing in the maintenance of long-term flight in flying insects.
Molecular mechanisms underlying aging-related flight activity
The essential role of muscle ultrastructure and organization in flight performance has been revealed in many flying insects [102–105]. The swelling, degradation, and encapsulation of mitochondria, together with the apoptosis of muscle cells, have been attributed to decreased muscle function and the resulting reduction in flight performance associated with aging [106]. Guo et al. carried out time-course analyses of aging-related features of the migratory locust at the physiological, cellular, and transcriptional levels [107]. All four flight parameters, namely, flight distance, flight duration, average flight velocity, and maximum flight velocity, significantly declined at 28 days post-adult eclosion (PAE), indicating dramatic flight deterioration at the late stage of locust life history. Significant transcriptional alterations occur in aged flight muscle and fat body, whereas the brain and testis show little transcriptional changes during the aging process in the migratory locust [107]. Aging-related transcriptional changes in muscle and fat body are mainly related to the mitochondrion, carbon metabolism, and detoxification, indicating strong disruptions to metabolism homeostasis in the flight muscle and fat body of aged locusts. Homologs of 1426 aging-related genes reported in model species, including humans, mice, fruit flies, worms, and yeast [108], have been identified in the locust. During the aging process, 313 of these homologs displayed variable expression levels. Furthermore, a coexpression network analysis revealed four genes that displayed significant expression changes in all tested tissues during aging, including lipoyltransferase 1 (LIPT1), death-associated inhibitor of apoptosis 1 (IAP1), transcription factor JUN, and peptidoglycan recognition protein SA (PGRP-SA). LIPT1, which modulates mitochondrial metabolism by catalyzing lipoylation of mitochondrial proteins [109], was validated to be involved in aging-related flight decline in locusts through RNAi-mediated gene knockdown experiments (Fig. 3b) [107]. Therefore, these results indicate that the deterioration of muscle metabolism and flight activity during aging might be driven by mitochondrial metabolism suppression.
Molecular basis of phase-dependent differences in locust flight
Locust displays strong density-dependent plasticity in fly capacity. Gregarious locusts can fly continuously for long distances in a swarming form, whereas solitarious locusts rarely perform sustained flight [8]. Unlike the flight polymorphisms of wing-dimorphic insects that develop into long wing (LW) or short wing (SW)/wingless morph adults [33], flight plasticity in locusts occurs in response to changes in population density without wing size variation. Compared with the widely studied wing polymorphisms, the molecular mechanisms underlying locust phase-related flight plasticity are rarely reported. Through integrated analyses of flight traits, transcriptomes, and metabolomes in solitarious and gregarious locusts, Du et al. provided the first study to explore the molecular regulatory mechanisms on phase-dependent flight plasticity in the migratory locust [9]. They revealed that solitarious locusts displayed higher flight speed than gregarious locusts at the initial phase of flight (within 15 min), whereas gregarious locusts exhibited much longer flight distances and durations during prolonged flight. This divergence in flight traits perfectly matched the ecological adaptation of the two phases of locusts [19].
Gregarious locusts have more lipid reserves, as revealed by higher TAG contents in the fat body [110]. However, the shorter flight duration of solitarious locusts may not be due to poor energy storage because forced flying for 1 h did not induce any change in the TAG content of solitarious locusts [9]. By monitoring metabolic changes across both phases of locusts during sustained flight, Du et al. (2022) demonstrated that the contents of important antioxidants, including uric acid and glutathione (GSH), displayed rapidly decreased, whereas ROS production significantly increased in solitarious locusts but not in gregarious locusts after the same flight treatment [9]. The injection of paraquat resulted in increased ROS production but decreased GSH/GSSG level (an index representing the antioxidation activity [111]) and subsequently repressed flight distance and duration, indicating that reduced sustained flight is attributed to the accumulation of oxidative stress-related molecules [9]. This study thus hypothesized that the long-term flight ability of gregarious locusts is attributed to the balance of metabolic rate and redox homeostasis in flight muscle.
Transcriptome analyses of flight muscle showed that energy metabolism-related gene expressions were much higher in solitarious locusts than in gregarious locusts under both resting and flight conditions [9]. Knockdown of the citrate synthase (CS) gene, a key molecule for metabolic activity regulation, significantly repressed respiratory metabolism and initial flight speed, suggesting that active energy metabolism is necessary for high flight speed at the beginning of locust flight in the solitarious phase [9]. The oxidation of carbohydrates in flight muscle gives rise to rapid ATP production at the initial phase of locust flight [112]. Glycogen phosphorylase-dependent mobilization of glycogen stored in the fat body is thus essential for the energy supplied to flight muscle during early flight [113]. Glycogen phosphorylase is immediately activated after a 5-min flight and promoted by all three AKH peptides in a dose-dependent manner [43]. Interestingly, higher contents of AKH I and AKH II were observed in the CC of resting solitary migratory locusts than in that of gregarious locusts [114]. Thus, the abundant AKH levels probably serve as a hormone basis that is ready for rapid carbohydrate mobilization by activating glycogen phosphorylase at the early stage of locust flight. Moreover, solitarious locusts contain lower hemolymph lipid levels and display a less intense hyperlipemia response to AKH, reflecting a coarse adipokinetic strategy [110, 115]. Further work on the phase-dependent regulation of AKH-induced activation of glycogen phosphorylase and lipase is required to understand the endocrine mechanism underlying distinct metabolic patterns during the flight of both phases of locusts.
Collectively, flight trait differentiation between solitarious and gregarious locusts may be associated with the divergence of energy metabolism and antioxidant capacity. In solitarious locusts, robust muscle metabolism confers high flight speed for short-term flight, whereas overloaded ROS limits prolonged flight performance. In gregarious locusts, moderate aerobic metabolism, stable redox homeostasis, and higher energy storage facilitate a relatively lower speed but long-term flight (Fig. 3b). Moreover, flight traits, metabolic patterns, and antioxidant capacity can reversibly transform between gregarious and solitarious phases in response to population density variations [9]. Determining the mechanisms underlying density-dependent plasticity of energy metabolism and antioxidant capacity is essential for understanding phase-related divergence of locust flight between the two phases.
Conclusion and prospects
Since locusts have been adopted as study models for flight activity, much research progress has been made in understanding the physiological basis of flight and hormone-regulated metabolism. Recent omics-based analyses and molecular biological studies have further significantly deepened our comprehension of the genetic and molecular mechanisms underlying long-term flight. An obvious increase in copy number of genes related to lipid metabolism and antioxidant protection might be involved in the sustained flight capacity of the locust genome. An alternative splicing isoform of Hif-1α is involved in antioxidant capacity associated with sustained flight. A complex neuroendocrine network involving multiple hormones or neuropeptides is essential for the precise and effective control of tissue-dependent metabolism during flight. Phase-related flight plasticity is attributed to antioxidant ability and metabolic homeostasis. Moreover, a decline in flight capacity and the expression level of energy metabolism-related genes occurs during the aging process. These findings further highlight the significance of locusts as a model for exploring the regulatory mechanism underlying insect flight.
Regardless of these advances, we are still far from fully understanding how the sustained flight of locusts is coordinated at physiological, neuroendocrinal, and behavioral levels. Given the temporally and spatially dependent roles of multiple neuromodulators in the modulation of carbohydrate or lipid metabolism during flight, it is very interesting to address whether these modulators act separately or interactively to orchestrate flight-related metabolism. In addition, the extent to which distinct neuromodulators contribute to the metabolic transition from early carbohydrate consumption to later lipid utilization in flight muscle must be determined. Moreover, the neuroendocrinal mechanisms underlying significant divergence in flight-related metabolism between gregarious and solitarious locusts remain largely unknown. Resolving these questions is essential for understanding the hormone basis for metabolic adaptation in response to distinct physiological and environmental conditions. Another meaningful research direction is to explore the distinct roles and action modes of three AKH peptides in balancing energy allocation between flight muscles and ovaries, which will be helpful for understanding the trade-off between flight and reproduction in locusts. In addition, as a gene with adult-specific expression, muscle-specific FABP has been shown to play a central role in mediating lipid utilization during flight in both desert locusts and migratory locusts and thus may act as a conserved molecular target for locust control. The temporally and spatially dependent expression of FABP gene requires further investigation. Moreover, locust flight muscles have evolved highly efficient metabolism compared to that of other flying insects and vertebrates. The most striking feature is the 30-fold increase in mitochondrial volume in the flight muscle during adult development. Unraveling the mechanism of mitochondrial differentiation is essential for better understanding the cellular and molecular basis for rapid metabolic adaptation in response to extensive energy demand.
Acknowledgements
We thank Prof. Le Kang for helpful suggestions on the writing of this review. Figures were produced using Adobe Illustrator.
Author contributions
LH and SYG wrote the manuscript and drew the figures, DD and BZD revised the manuscript, XHW revised and finalized the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant NO. 31930012, 32070497, 32100388) and Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDPB16) and Youth Innovation Promotion Association CAS (No. 2021079).
Availability of data and material
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
The manuscript was reviewed and approved by all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Hou, Siyuan Guo have contributed equally to this paper.
References
- 1.Wegener G. Flying insects: Model systems in exercise physiology. Experientia. 1996;52(5):404–412. doi: 10.1007/Bf01919307. [DOI] [PubMed] [Google Scholar]
- 2.Dudley R, Pass G. Wings and powered flight: Core novelties in insect evolution. Arthropod Struct Dev. 2018;47(4):319–321. doi: 10.1016/j.asd.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Verlinden H, Sterck L, Li J, Li Z, Yssel A, Gansemans Y, Verdonck R, Holtof M, Song H, Behmer ST, Sword GA, Matheson T, Ott SR, Deforce D, Van Nieuwerburgh F, Van de Peer Y, Vanden Broeck J. First draft genome assembly of the desert locust, Schistocerca gregaria. F1000Rres. 2020;9:775. doi: 10.12688/f1000research.25148.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor GK, Krapp HG. Sensory systems and flight stability: What do insects measure and why? Adv Insect Physiol. 2007;34:231–316. doi: 10.1016/S0065-2806(07)34005-8. [DOI] [Google Scholar]
- 5.Robertson RM. Neuronal circuits controlling flight in the locust - central generation of the rhythm. Trends Neurosci. 1986;9(6):278–280. doi: 10.1016/0166-2236(86)90078-0. [DOI] [Google Scholar]
- 6.Candy DJ, Becker A, Wegener G. Coordination and integration of metabolism in insect flight. Comp Biochem Phys B. 1997;117(4):497–512. doi: 10.1016/S0305-0491(97)00212-5. [DOI] [Google Scholar]
- 7.Sadaf S, Reddy OV, Sane SP, Hasan G. Neural control of wing coordination in flies. Curr Biol. 2015;25(1):80–86. doi: 10.1016/j.cub.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 8.Pener MP, Simpson SJ. Locust phase polyphenism: an update. Adv Insect Physiol. 2009;36:1–272. doi: 10.1016/S0065-2806(08)36001-9. [DOI] [Google Scholar]
- 9.Du BZ, Ding D, Ma C, Guo W, Kang L. Locust density shapes energy metabolism and oxidative stress resulting in divergence of flight traits. Proc Natl Acad Sci U S A. 2022;119(1):e2115753118. doi: 10.1073/pnas.2115753118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duch C, Pfluger HJ. DUM neurons in locust flight: a model system for amine-mediated peripheral adjustments to the requirements of a central motor program. J Comp Physiol A. 1999;184(5):489–499. doi: 10.1007/s003590050349. [DOI] [Google Scholar]
- 11.Van der Horst DJ, Rodenburg KW. Locust flight activity as a model for hormonal regulation of lipid mobilization and transport. J Insect Physiol. 2010;56(8):844–853. doi: 10.1016/j.jinsphys.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Worm RAA, Beenakkers AMT. Regulation of substrate utilization in the flight-muscle of the locust, Locusta migratoria, during Flight. Insect Biochem. 1980;10(1):53–59. doi: 10.1016/0020-1790(80)90038-4. [DOI] [Google Scholar]
- 13.Vanmarrewijk WJA, Beenakkers AMT. Role of adipokinetic hormone in the control of glycogen mobilization for locust flight. B Soc Zool Fr. 1986;111(1–2):52–52. [Google Scholar]
- 14.Goldsworthy GJ, Jutsum AR, Robinson NL. Adipokinetic hormone and flight metabolism in locusts. J Endocrinol. 1975;64(3):P66–P67. [PubMed] [Google Scholar]
- 15.Wilson DM, Waldron I. Models for generation of motor output pattern in flying locusts. Pr Inst Electr Elect. 1968;56(6):1058. doi: 10.1109/Proc.1968.6457. [DOI] [Google Scholar]
- 16.Kutsch W, Neubauer K, Kramer H. Flight motor pattern in abdominal segments of locusts. J Exp Biol. 1991;156:629–635. [Google Scholar]
- 17.Mentel T, Duch C, Stypa H, Wegener G, Muller U, Pfluger HJ. Central modulatory neurons control fuel selection in flight muscle of migratory locust. J Neurosci. 2003;23(4):1109–1113. doi: 10.1523/JNEUROSCI.23-04-01109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gade G. The hormonal integration of insect flight metabolism. Zool Jahrb Allg Zool. 1992;96(2):211–225. [Google Scholar]
- 19.Lorenz MW, Gade G. Hormonal regulation of energy metabolism in insects as a driving force for performance. Integr Comp Biol. 2009;49(4):380–392. doi: 10.1093/icb/icp019. [DOI] [PubMed] [Google Scholar]
- 20.Van der Horst DJ. Insect adipokinetic hormones: release and integration of flight energy metabolism. Comp Biochem Phys B. 2003;136(2):217–226. doi: 10.1016/S1096-4959(03)00151-9. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Horst DJ, Van Marrewijk WJA, Vullings HGB, Diederen JHB. Metabolic neurohormones: release, signal transduction and physiological responses of adipokinetic hormones in insects. Eur J Entomol. 1999;96(3):299–308. [Google Scholar]
- 22.Cao T, Jin JP. Evolution of flight muscle contractility and energetic efficiency. Front Physiol. 2020;11:1038. doi: 10.3389/fphys.2020.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutsch W, Usherwood P. Studies of the innervation and electrical activity of flight muscles in the locust, Schistocerca gregaria. J Exp Biol. 1970;52(2):299–312. doi: 10.1242/jeb.52.2.299. [DOI] [Google Scholar]
- 24.Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J Neurosci. 1999;19(8):3007–3022. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfluger HJ, Duch C. Dynamic neural control of insect muscle metabolism related to motor behavior. Physiology (Bethesda) 2011;26(4):293–303. doi: 10.1152/physiol.00002.2011. [DOI] [PubMed] [Google Scholar]
- 26.Sacktor B. Biochemical adaptations for flight in the insect. Biochem Soc Symp. 1976;41:111–131. [PubMed] [Google Scholar]
- 27.Gagnon J, Kurowski TT, Wiesner RJ, Zak R. Correlations between a nuclear and a mitochondrial messenger-RNA of cytochrome-c-oxidase subunits, enzymatic-activity and total cessenger-RNA content, in rat-tissues. Mol Cell Biochem. 1991;107(1):21–29. doi: 10.1007/Bf02424572. [DOI] [PubMed] [Google Scholar]
- 28.Brosemer RW, Vogell W, Buecher T. Morphological and enzymatic patterns in the development of the indirect flight muscle of the Locust migratoria. Biochem Z. 1963;338:854–910. [PubMed] [Google Scholar]
- 29.Sogl B, Gellissen G, Wiesner RJ. Biogenesis of giant mitochondria during insect flight muscle development in the locust, Locusta migratoria (L.)—transcription, translation and copy number of mitochondrial DNA. Eur J Biochem. 2000;267(1):11–17. doi: 10.1046/j.1432-1327.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- 30.Bahat A, Gross A. Mitochondrial plasticity in cell fate regulation. J Biol Chem. 2019;294(38):13852–13863. doi: 10.1074/jbc.REV118.000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haunerland NH. Transport and utilization in insect flight muscles. Comp Biochem Phys B. 1997;117(4):475–482. doi: 10.1016/S0305-0491(97)00185-5. [DOI] [Google Scholar]
- 32.Van der Horst DJ, Roosendaal SD, Rodenburg KW. Circulatory lipid transport: lipoprotein assembly and function from an evolutionary perspective. Mol Cell Biochem. 2009;326(1–2):105–119. doi: 10.1007/s11010-008-0011-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhang CX, Brisson JA, Xu HJ. Molecular mechanisms of wing polymorphism in insects. Annu Rev Entomol. 2019;64:297–314. doi: 10.1146/annurev-ento-011118-112448. [DOI] [PubMed] [Google Scholar]
- 34.Oudejans RCHM, Harthoorn LF, Diederen JHB, Van der Horst DJ. Adipokinetic hormones - coupling between biosynthesis and release. Ann Ny Acad Sci. 1999;897:291–299. doi: 10.1111/j.1749-6632.1999.tb07900.x. [DOI] [PubMed] [Google Scholar]
- 35.Van der Horst DJ, Van Marrewijk WJ, Diederen JH. Adipokinetic hormones of insect: release, signal transduction, and responses. Int Rev Cytol. 2001;211:179–240. doi: 10.1016/s0074-7696(01)11019-3. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz MW. Synthesis of lipids in the fat body of Gryllus bimaculatus: age-dependency and regulation by adipokinetic hormone. Arch Insect Biochem Physiol. 2001;47(4):198–214. doi: 10.1002/arch.1052. [DOI] [PubMed] [Google Scholar]
- 37.Kodrik D, Goldsworthy GJ. Inhibition of RNA-synthesis by adipokinetic hormones and brain factor (S) in adult fat-body of Locusta migratoria. J Insect Physiol. 1995;41(2):127–133. doi: 10.1016/0022-1910(94)00096-Y. [DOI] [Google Scholar]
- 38.Steele JE, Ireland R. Hormonal activation of phosphorylase in cockroach fat body trophocytes: A correlation with trans-membrane calcium flux. Arch Insect Biochem Physiol. 1999;42(4):233–244. doi: 10.1002/(SICI)1520-6327(199912)42:4<233::AID-ARCH2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Keeley LL, Park JH, Lu KH, Bradfield JY. Neurohormone signal transduction for dual regulation of metabolism and gene expression in insects: hypertrehalosemic hormone as a model. Arch Insect Biochem. 1996;33(3–4):283–301. doi: 10.1002/(SICI)1520-6327(1996)33:3/4<283::AID-ARCH8>3.0.CO;2-T. [DOI] [Google Scholar]
- 40.Arrese EL, Canavoso LE, Jouni ZE, Pennington JE, Tsuchida K, Wells MA. Lipid storage and mobilization in insects: current status and future directions. Insect Biochem Mol Biol. 2001;31(1):7–17. doi: 10.1016/s0965-1748(00)00102-8. [DOI] [PubMed] [Google Scholar]
- 41.Gade G, Auerswald L. Beetles' choice–proline for energy output: control by AKHs. Comp Biochem Physiol B Biochem Mol Biol. 2002;132(1):117–129. doi: 10.1016/s1096-4959(01)00541-3. [DOI] [PubMed] [Google Scholar]
- 42.Oudejans RC, Vroemen SF, Jansen RF, Van der Horst DJ. Locust adipokinetic hormones: carrier-independent transport and differential inactivation at physiological concentrations during rest and flight. Proc Natl Acad Sci U S A. 1996;93(16):8654–8659. doi: 10.1073/pnas.93.16.8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auerswald L, Gade G. Endocrine control of TAG lipase in the fat body of the migratory locust, Locusta migratoria. Insect Biochem Mol Biol. 2006;36(10):759–768. doi: 10.1016/j.ibmb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Gade G, Auerswald L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen Comp Endocrinol. 2003;132(1):10–20. doi: 10.1016/s0016-6480(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 45.Gade G. Peptides of the adipokinetic hormone/red pigment-concentrating hormone family: a new take on biodiversity. Ann N Y Acad Sci. 2009;1163:125–136. doi: 10.1111/j.1749-6632.2008.03625.x. [DOI] [PubMed] [Google Scholar]
- 46.Siegert KJ, Kellner R, Gade G. A third active AKH is present in the pyrgomorphid grasshoppers Phymateus morbillosus and Dictyophorus spumans. Insect Biochem Mol. 2000;30(11):1061–1067. doi: 10.1016/S0965-1748(00)00081-3. [DOI] [PubMed] [Google Scholar]
- 47.Oudejans RC, Kooiman FP, Heerma W, Versluis C, Slotboom AJ, Beenakkers MT. Isolation and structure elucidation of a novel adipokinetic hormone (Lom-AKH-III) from the glandular lobes of the corpus cardiacum of the migratory locust, Locusta migratoria. Eur J Biochem. 1991;195(2):351–359. doi: 10.1111/j.1432-1033.1991.tb15713.x. [DOI] [PubMed] [Google Scholar]
- 48.Gade G. The revolution in insect neuropeptides illustrated by the adipokinetic hormone/red pigment-concentrating hormone family of peptides. Z Naturforsch C. 1996;51(9–10):607–617. doi: 10.1515/znc-1996-9-1001. [DOI] [PubMed] [Google Scholar]
- 49.Hou L, Jiang F, Yang P, Wang X, Kang L. Molecular characterization and expression profiles of neuropeptide precursors in the migratory locust. Insect Biochem Mol Biol. 2015;63:63–71. doi: 10.1016/j.ibmb.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Harthoorn LF, Diederen JH, Oudejans RC, Van der Horst DJ. Differential location of peptide hormones in the secretory pathway of insect adipokinetic cells. Cell Tissue Res. 1999;298(2):361–369. doi: 10.1007/s004419900094. [DOI] [PubMed] [Google Scholar]
- 51.Harthoorn LF, Oudejans RCHM, Diederen JHB, Van de Wijngaart DJ, Van der Horst DJ. Absence of coupling between release and biosynthesis of peptide hormones in insect neuroendocrine cells. Eur J Cell Biol. 2001;80(7):451–457. doi: 10.1078/0171-9335-00183. [DOI] [PubMed] [Google Scholar]
- 52.Oudejans RC, Mes TH, Kooiman FP, van der Horst DJ. Adipokinetic peptide hormone content and biosynthesis during locust development. Peptides. 1993;14(5):877–881. doi: 10.1016/0196-9781(93)90062-l. [DOI] [PubMed] [Google Scholar]
- 53.Diederen JH, Oudejans RC, Harthoorn LF, Van der Horst DJ. Cell biology of the adipokinetic hormone-producing neurosecretory cells in the locust corpus cardiacum. Microsc Res Tech. 2002;56(3):227–236. doi: 10.1002/jemt.10026. [DOI] [PubMed] [Google Scholar]
- 54.Hou L, Guo S, Wang Y, Nie X, Yang P, Ding D, Li B, Kang L, Wang X. Neuropeptide ACP facilitates lipid oxidation and utilization during long-term flight in locusts. Elife. 2021;10:e65279. doi: 10.7554/eLife.65279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Candy DJ. Adipokinetic hormones concentrations in the haemolymph of Schistocerca gregaria, measured by radioimmunoassay. Insect Biochem Mol Biol. 2002;32(11):1361–1367. doi: 10.1016/s0965-1748(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 56.Bogerd J, Kooiman FP, Pijnenburg MA, Hekking LH, Oudejans RC, Van der Horst DJ. Molecular cloning of three distinct cDNAs, each encoding a different adipokinetic hormone precursor, of the migratory locust, Locusta migratoria. Differential expression of the distinct adipokinetic hormone precursor genes during flight activity. J Biol Chem. 1995;270(39):23038–23043. doi: 10.1074/jbc.270.39.23038. [DOI] [PubMed] [Google Scholar]
- 57.Glinka AV, Kleiman AM, Wyatt GR. Roles of juvenile hormone, a brain factor and adipokinetic hormone in regulation of vitellogenin biosynthesis in Locusta migratoria. Biochem Mol Biol Int. 1995;35(2):323–328. [PubMed] [Google Scholar]
- 58.Moshitzky P, Applebaum SW. The role of adipokinetic hormone in the control of vitellogenesis in locusts. Insect Biochem. 1990;20(3):319–323. doi: 10.1016/0020-1790(90)90050-5. [DOI] [Google Scholar]
- 59.Zheng H, Chen C, Liu C, Song Q, Zhou S. Rhythmic change of adipokinetic hormones diurnally regulates locust vitellogenesis and egg development. Insect Mol Biol. 2020;29(3):283–292. doi: 10.1111/imb.12633. [DOI] [PubMed] [Google Scholar]
- 60.Jackson GE, Pavadai E, Gade G, Andersen NH. The adipokinetic hormones and their cognate receptor from the desert locust, Schistocerca gregaria: solution structure of endogenous peptides and models of their binding to the receptor. PeerJ. 2019;7:e7514. doi: 10.7717/peerj.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nassel DR, Passier PCCM, Elekes K, Dircksen H, Vullings HGB, Cantera R. Evidence that Locustatachykinin-I is involved in release of adipokinetic hormone from locust Corpora-Cardiaca. Regul Peptides. 1995;57(3):297–310. doi: 10.1016/0167-0115(95)00043-B. [DOI] [PubMed] [Google Scholar]
- 62.Hansen KK, Stafflinger E, Schneider M, Hauser F, Cazzamali G, Williamson M, Kollmann M, Schachtner J, Grimmelikhuijzen CJ. Discovery of a novel insect neuropeptide signaling system closely related to the insect adipokinetic hormone and corazonin hormonal systems. J Biol Chem. 2010;285(14):10736–10747. doi: 10.1074/jbc.M109.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siegert KJ. Locust corpora cardiaca contain an inactive adipokinetic hormone. Febs Lett. 1999;447(2–3):237–240. doi: 10.1016/s0014-5793(99)00299-9. [DOI] [PubMed] [Google Scholar]
- 64.Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, Kaufman TC, Kellis M, Gelbart W, Iyer VN, Pollard DA, Sackton TB, Larracuente AM, Singh ND, Abad JP, Abt DN, Adryan B, Aguade M, Akashi H, Anderson WW, Aquadro CF, Ardell DH, Arguello R, Artieri CG, Barbash DA, et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450(7167):203–218. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- 65.Patel H, Orchard I, Veenstra JA, Lange AB. The distribution and physiological effects of three evolutionarily and sequence-related neuropeptides in Rhodnius prolixus: Adipokinetic hormone, corazonin and adipokinetic hormone/corazonin-related peptide. Gen Comp Endocr. 2014;203:307–314. doi: 10.1016/j.ygcen.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Lucke C, Qiao Y, van Moerkerk HT, Veerkamp JH, Hamilton JA. Fatty-acid-binding protein from the flight muscle of Locusta migratoria: evolutionary variations in fatty acid binding. Biochemistry. 2006;45(20):6296–6305. doi: 10.1021/bi060224f. [DOI] [PubMed] [Google Scholar]
- 67.Haunerland NH, Andolfatto P, Chisholm JM, Wang Z, Chen X. Fatty-acid-binding protein in locust flight muscle. Developmental changes of expression, concentration and intracellular distribution. Eur J Biochem. 1992;210(3):1045–1051. doi: 10.1111/j.1432-1033.1992.tb17510.x. [DOI] [PubMed] [Google Scholar]
- 68.Haunerland NH, Chisholm JM. Fatty-acid binding-protein in flight-muscle of the locust Schistocerca gregaria. Biochim Biophys Acta. 1990;1047(3):233–238. doi: 10.1016/0005-2760(90)90521-X. [DOI] [PubMed] [Google Scholar]
- 69.Rajapakse S, Qu D, Ahmed AS, Rickers-Haunerland J, Haunerland NH. Effects of FABP knockdown on flight performance of the desert locust Schistocerca gregaria. J Exp Biol. 2019;222(Pt:21):jeb203455. doi: 10.1242/jeb.203455. [DOI] [PubMed] [Google Scholar]
- 70.Chen XM, Wang ZX, Haunerland NH. Flight-muscle fatty-acid binding-protein synthesis in juvenile and adult forms of the desert locust Schistocerca gregaria. Insect Biochem Mol. 1993;23(3):337–343. doi: 10.1016/0965-1748(93)90017-M. [DOI] [Google Scholar]
- 71.Chen XM, Haunerland NH. Fatty-acid-binding protein expression in locust flight-muscle-induction by flight, adipokinetic hormone, and low-density lipophorin. Insect Biochem Molec. 1994;24(6):573–579. doi: 10.1016/0965-1748(94)90093-0. [DOI] [Google Scholar]
- 72.Lim PS, Li J, Holloway AF, Rao S. Epigenetic regulation of inducible gene expression in the immune system. Immunology. 2013;139(3):285–293. doi: 10.1111/imm.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qu H, Cui L, Rickers-Haunerland J, Haunerland NH. Fatty acid-dependent expression of the muscle FABP gene—comparative analysis of gene control in functionally related, but evolutionary distant animal systems. Mol Cell Biochem. 2007;299(1–2):45–53. doi: 10.1007/s11010-005-9036-z. [DOI] [PubMed] [Google Scholar]
- 74.Wu QW, Haunerland NH. A novel fatty acid response element controls the expression of the flight muscle FABP gene of the desert locust, Schistocerca gregaria. Eur J Biochem. 2001;268(22):5894–5900. doi: 10.1046/j.0014-2956.2001.02538.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhou YJ, Fukumura K, Nagata S. Effects of adipokinetic hormone and its related peptide on maintaining hemolymph carbohydrate and lipid levels in the two-spotted cricket, Gryllus bimaculatus. Biosci Biotechnol Biochem. 2018;82(2):274–284. doi: 10.1080/09168451.2017.1422106. [DOI] [PubMed] [Google Scholar]
- 76.Lee WS, Monaghan P, Metcalfe NB. The trade-off between growth rate and locomotor performance varies with perceived time until breeding. J Exp Biol. 2010;213(19):3289–3298. doi: 10.1242/jeb.043083. [DOI] [PubMed] [Google Scholar]
- 77.Galikova M, Diesner M, Klepsatel P, Hehlert P, Xu YJ, Bickmeyer I, Predel R, Kuhnlein RP. Energy homeostasis control in Drosophila adipokinetic hormone mutants. Genetics. 2015;201(2):665. doi: 10.1534/genetics.115.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.White MA, Chen DS, Wolfner MF. She's got nerve: roles of octopamine in insect female reproduction. J Neurogenet. 2021 doi: 10.1080/01677063.2020.1868457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Novikova ES, Zhukovskaya MI. Octopamine, the insect stress hormone, alters grooming pattern in the cockroach Periplaneta americana. J Evol Biochem Phys. 2015;51(2):160–162. doi: 10.1134/S0022093015020118. [DOI] [PubMed] [Google Scholar]
- 80.Wolf H, Pearson KG. Comparison of motor patterns in the intact and deafferented flight system of the locust. 3. Patterns of interneuronal activity. J Comp Physiol A-Sens Neural and Behav Physiol. 1989;165(1):61–74. doi: 10.1007/Bf00613800. [DOI] [Google Scholar]
- 81.Ramirez JM, Pearson KG. Octopaminergic modulation of interneurons in the flight system of the locust. J Neurophysiol. 1991;66(5):1522–1537. doi: 10.1152/jn.1991.66.5.1522. [DOI] [PubMed] [Google Scholar]
- 82.Wegener G, Beinhauer I, Klee A, Newsholme EA. Properties of locust muscle 6-phosphofructokinase and their importance in the regulation of glycolytic flux during prolonged flight. J Comp Physiol B-Biochem Syst Environ Physiol. 1987;157(3):315–326. doi: 10.1007/Bf00693358. [DOI] [Google Scholar]
- 83.Wegener G, Michel R, Newsholme EA. Fructose 2,6-bisphosphate as a signal for changing from sugar to lipid oxidation during flight in locusts. Febs Lett. 1986;201(1):129–132. doi: 10.1016/0014-5793(86)80584-1. [DOI] [Google Scholar]
- 84.Blau C, Wegener G. Metabolic integration in locust flight - the effect of octopamine on fructose 2,6-bisphosphate content of flight-muscle in-vivo. J Comp Physiol B-Biochem Syst Environ Physiol. 1994;164(1):11–15. doi: 10.1007/Bf00714565. [DOI] [Google Scholar]
- 85.Orchard I, Ramirez JM, Lange AB. A multifunctional role for octopamine in locust flight. Annu Rev Entomol. 1993;38:227–249. doi: 10.1146/annurev.en.38.010193.001303. [DOI] [Google Scholar]
- 86.Robb S, Cheek TR, Hannan FL, Hall LM, Midgley JM, Evans PD. Agonist-specific coupling of a cloned Drosophila octopamine/tyramine receptor to multiple second messenger systems. Embo J. 1994;13(6):1325–1330. doi: 10.1002/j.1460-2075.1994.tb06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goosey MW, Candy DJ. The D-octopamine content of the hemolymph of the locust, Schistocerca-americana-gregaria and its elevation during flight. Insect Biochem. 1980;10(4):393–397. doi: 10.1016/0020-1790(80)90009-8. [DOI] [Google Scholar]
- 88.Orchard I, Carlisle JA, Loughton BG, Gole JW, Downer RG. In vitro studies on the effects of octopamine on locust fat body. Gen Comp Endocrinol. 1982;48(1):7–13. doi: 10.1016/0016-6480(82)90031-4. [DOI] [PubMed] [Google Scholar]
- 89.Wang ZW, Hayakawa Y, Downer RGH. Factors influencing cyclic-AMP and diacylglycerol levels in fat-body of Locusta migratoria. Insect Biochem. 1990;20(4):325–330. doi: 10.1016/0020-1790(90)90051-U. [DOI] [Google Scholar]
- 90.Orchard I, Loughton BG, Webb RA. Octopamine and short-term hyperlipaemia in the locust. Gen Comp Endocrinol. 1981;45(2):175–180. doi: 10.1016/0016-6480(81)90102-7. [DOI] [PubMed] [Google Scholar]
- 91.Orchard I, Lange AB. Cyclic-AMP in Locust Fat-Body - Correlation with octopamine and adipokinetic hormones during flight. J Insect Physiol. 1984;30(12):901–904. doi: 10.1016/0022-1910(84)90066-0. [DOI] [Google Scholar]
- 92.Orchard I, Martin RJ, Sloley BD, Downer RGH. The sssociation of 5-hydroxytryptamine, octopamine, and dopamine with the intrinsic (Glandular) lobe of the Corpus Cardiacum of Locusta migratoria. Can J Zool. 1986;64(1):271–274. doi: 10.1139/z86-045. [DOI] [Google Scholar]
- 93.Pannabecker T, Orchard I. Octopamine and cyclic-Amp mediate release of adipokinetic hormone-I and hormone-II from isolated locust neuroendocrine tissue. Mol Cell Endocrinol. 1986;48(2–3):153–159. doi: 10.1016/0303-7207(86)90037-7. [DOI] [PubMed] [Google Scholar]
- 94.Wang X, Fang X, Yang P, Jiang X, Jiang F, Zhao D, Li B, Cui F, Wei J, Ma C, Wang Y, He J, Luo Y, Wang Z, Guo X, Guo W, Wang X, Zhang Y, Yang M, Hao S, Chen B, Ma Z, Yu D, Xiong Z, Zhu Y, et al. The locust genome provides insight into swarm formation and long-distance flight. Nat Commun. 2014;5:2957. doi: 10.1038/ncomms3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cooper-Mullin C, McWilliams SR. The role of the antioxidant system during intense endurance exercise: lessons from migrating birds. J Exp Biol. 2016;219(Pt 23):3684–3695. doi: 10.1242/jeb.123992. [DOI] [PubMed] [Google Scholar]
- 96.Ding D, Zhang J, Du BZ, Wang XZ, Hou L, Guo SY, Chen B, Kang L. Non-canonical function of a Hif-1α splice variant contributes to the sustained flight of locusts. bioRxiv. 2021;76:11. doi: 10.7554/eLife.74554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rytkonen KT, Storz JF. Evolutionary origins of oxygen sensing in animals. Embo Rep. 2011;12(1):3–4. doi: 10.1038/embor.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Menzies FM, Yenisetti SC, Min KT. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15(17):1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 99.Taira T, Saito Y, Niki T, Iguchi-Ariga SMM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. Embo Rep. 2004;5(2):213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med. 2009;8:1. doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482(3):419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bulos BA, Shukla SP, Sacktor B. Bioenergetics of mitochondria from flight muscle of aging blowflies. Gerontologist. 1973;13(3):35–35. [Google Scholar]
- 103.Sohal RS. Mitochondrial changes with age in flight muscle of adult Drosophila melanogaster. J Cell Biol. 1973;59(2):A328–A328. [Google Scholar]
- 104.Miller MS, Lekkas P, Braddock JM, Farman GP, Ballif BA, Irving TC, Maughan DW, Vigoreaux JO. Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys J. 2008;95(5):2391–2401. doi: 10.1529/biophysj.108.130005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wone BWM, Pathak J, Davidowitz G. Flight duration and flight muscle ultrastructure of unfed hawk moths. Arthropod Struct Dev. 2018;47(5):457–464. doi: 10.1016/j.asd.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 106.Zheng J, Edelman SW, Tharmarajah G, Walker DW, Pletcher SD, Seroude L. Differential patterns of apoptosis in response to aging in Drosophila. Proc Natl Acad Sci U S A. 2005;102(34):12083–12088. doi: 10.1073/pnas.0503374102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo SY, Yang PC, Liang B, Zhou F, Hou L, Kang L, Wang XH. Aging features of the migratory locust at physiological and transcriptional levels. BMC Genom. 2021 doi: 10.1186/s12864-021-07585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Magalhaes JP, Toussaint O. GenAge: a genomic and proteomic network map of human ageing. Febs Lett. 2004;571(1–3):243–247. doi: 10.1016/j.febslet.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 109.Tort F, Ferrer-Cortes X, Thio M, Navarro-Sastre A, Matalonga L, Quintana E, Bujan N, Arias A, Garcia-Villoria J, Acquaviva C, Vianey-Saban C, Artuch R, Garcia-Cazorla A, Briones P, Ribes A. Mutations in the lipoyltransferase LIPT1 gene cause a fatal disease associated with a specific lipoylation defect of the 2-ketoacid dehydrogenase complexes. Hum Mol Genet. 2014;23(7):1907–1915. doi: 10.1093/hmg/ddt585. [DOI] [PubMed] [Google Scholar]
- 110.Ayali A, Golenser E, Pener MP. Flight fuel related differences between solitary and gregarious locusts (Locusts migratoria migratorioides) Physiol Entomol. 1996;21(1):1–6. doi: 10.1111/j.1365-3032.1996.tb00828.x. [DOI] [Google Scholar]
- 111.Levin E, Lopez-Martinez G, Fane B, Davidowitz G. Hawkmoths use nectar sugar to reduce oxidative damage from flight. Science. 2017;355(6326):733–734. doi: 10.1126/science.aah4634. [DOI] [PubMed] [Google Scholar]
- 112.Weisfogh T. Fat combustion and metabolic rate of flying locusts (Schistocerca gregaria Forskal) Philos T Roy Soc B. 1952;237(640):1–36. doi: 10.1098/rstb.1952.0011. [DOI] [Google Scholar]
- 113.Vanmarrewijk WJA, Vandenbroek ATM, Beenakkers AMT. Adipokinetic hormone is dependent on extracellular Ca2+ for its stimulatory action on the glycogenolytic pathway in locust fat-body in vitro. Insect Biochem. 1991;21(4):375–380. doi: 10.1016/0020-1790(91)90003-W. [DOI] [Google Scholar]
- 114.Ayali A, Pener MP, Sowa SM, Keeley LL. Adipokinetic hormone content of the corpora cardiaca in gregarious and solitary migratory locusts. Physiol Entomol. 1996;21(3):167–172. doi: 10.1111/j.1365-3032.1996.tb00851.x. [DOI] [Google Scholar]
- 115.Ayali A, Pener MP. Density-dependent phase polymorphism affects response to adipokinetic hormone in locusta. Comp Biochem Physiol a-Mol Integr Physiol. 1992;101(3):549–552. doi: 10.1016/0300-9629(92)90507-M. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.