Abstract
Chemokines are pivotal players in instigation and perpetuation of synovitis through leukocytes egress from the blood circulation into the inflamed articulation. Multitudinous literature addressing the involvement of the dual-function interferon (IFN)-inducible chemokines CXCL9, CXCL10 and CXCL11 in diseases characterized by chronic inflammatory arthritis emphasizes the need for detangling their etiopathological relevance. Through interaction with their mutual receptor CXC chemokine receptor 3 (CXCR3), the chemokines CXCL9, CXCL10 and CXCL11 exert their hallmark function of coordinating directional trafficking of CD4+ TH1 cells, CD8+ T cells, NK cells and NKT cells towards inflammatory niches. Among other (patho)physiological processes including infection, cancer, and angiostasis, IFN-inducible CXCR3 ligands have been implicated in autoinflammatory and autoimmune diseases. This review presents a comprehensive overview of the abundant presence of IFN-induced CXCR3 ligands in bodily fluids of patients with inflammatory arthritis, the outcomes of their selective depletion in rodent models, and the attempts at developing candidate drugs targeting the CXCR3 chemokine system. We further propose that the involvement of the CXCR3 binding chemokines in synovitis and joint remodeling encompasses more than solely the directional ingress of CXCR3-expressing leukocytes. The pleotropic actions of the IFN-inducible CXCR3 ligands in the synovial niche reiteratively illustrate the extensive complexity of the CXCR3 chemokine network, which is based on the intercommunion of IFN-inducible CXCR3 ligands with distinct CXCR3 isoforms, enzymes, cytokines, and infiltrated and resident cells present in the inflamed joints.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-023-04715-w.
Keywords: Adult-onset Still’s disease, Chemokine, CXCR3, Juvenile idiopathic arthritis, Rheumatoid arthritis, Spondyloarthritis
Introduction
The chemokine superfamily constitutes a group of low molecular mass (± 8–12 kDa) chemotactic cytokines that orchestrate directional leukocyte trafficking in a spatially and temporally specific manner [1–3]. As such, chemokines are protagonists in homeostatic and pathophysiological settings, thereby fulfilling frontline actions in embryogenesis, leukocyte homing, (neo)angiogenesis, inflammation, autoimmunity and cancer [4–13]. From a biological perspective, chemokines may be classified into functional subgroups, which are referred to as inflammatory, homeostatic and dual-function chemokines [14]. Inflammatory chemokines entail prior stimuli-mediated induction and navigate effector leukocytes to inflammatory niches, whereas homeostatic chemokines exhibit constitutive expression and govern basal migration and homing of leukocytes [13, 14]. In addition, certain chemokines display both inflammatory and homeostatic actions and are, therefore, termed ‘dual-function’ chemokines, e.g., CXCL12 [12, 14, 15]. Furthermore, the relative position and number of conserved cysteine (Cys) residues in the amino (NH2)-terminal region of the sequence of a chemokine define its structural classification in one of the four subfamilies, i.e., C, CC, CXC or CX3C chemokines [1, 16–18]. Conventional chemokine signaling occurs through seven transmembrane spanning G protein-coupled receptors (GPCRs), which are complementary categorized according to the subfamily of chemokines predominantly recognized by the respective receptor [17]. In addition, multiple regulatory mechanisms fine-tune chemokine activity and receptor specificity. These mechanisms include transcriptional and translational events (e.g., alternative splicing), mRNA stability (e.g., miRNA dependent), chemokine interaction with glycosaminoglycans (GAGs), binding to atypical chemokine receptors (ACKRs), interindividual antagonism and synergism between chemokines, and posttranslational modifications (PTMs) [19–21].
A chemokine is allocated to the aforementioned CXC chemokine subfamily based on the occurrence of one random amino acid (“X”) positioned in between the two most NH2-terminal cysteines [17]. Based on the presence or absence of a Glu–Leu–Arg (“ELR”) amino acid motif preceding the “CXC” sequence, CXC chemokines may be further categorized as ELR+ and ELR− CXC chemokines, respectively [1, 17]. ELR+ CXC chemokines are neutrophil chemo-attractants with angiogenic actions and encompass CXCL1–3 and CXCL5–8 in humans [1, 18]. The majority of CXC chemokines that lack the ELR motif interact with CXC chemokine receptor 3 (CXCR3) [1], thereby exerting angiostatic activities and mediating chemotaxis of natural killer (NK) cells and activated T cells [1, 22]. The CXCR3 ligands include three interferon-γ (IFN-γ)-induced proteins [23], i.e., monokine induced by interferon- (Mig/CXCL9), interferon- inducible protein of 10 kDa (IP-10/CXCL10), and interferon-inducible T-cell α chemoattractant (I-TAC/CXCL11) [24–28]. In addition, platelet factor-4 (PF-4/CXCL4) and the product of the non-allelic platelet factor-4 gene variant (PF-4var1/CXCL4L1) also bind to CXCR3 [29, 30]. CXCL9, CXCL10 and CXCL11 are generally acknowledged to orchestrate chemotaxis of activated CD4+ TH1 cells, CD8+ T cells, NK cells and NKT cells towards inflammatory and immunoprivileged sites [31–40]. Additionally, IFN-inducible CXCR3 ligands exhibit angiostatic properties [9, 41–43] and coordinate homing of mature thymocytes during T lymphopoiesis in the human postnatal thymus [44]. As such, CXCL9, CXCL10 and CXCL11 are considered dual-function chemokines [14]. IFN-inducible CXCR3 ligands are produced by a variety of cells including fibroblasts, keratinocytes and human microvascular endothelial cells [27, 45–50] and exhibit pronounced homology in their amino acid sequence [46], i.e., mature secreted CXCL10 shares 37.7% and 34.2% amino acid identity with mature CXCL9 and CXCL11, respectively. Moreover, their genes are located in close vicinity on the chromosomal chemokine cluster in the q21.1 region of chromosome 4 [25, 26, 51]. Despite these similarities conferring apparent analogous properties in vitro, non-redundant biological roles of the IFN-inducible CXCR3 ligands have been evidenced in vivo [22, 46]. The non-redundancy of the IFN-dependent CXCR3 chemokine network plausibly relies on the complex outcome of ligand-specific features including distinct GAG and receptor specificity, differential stimuli and cell types responsible for chemokine production, intracellular signaling cascades, and differential susceptibility to enzymatic processing resulting in PTMs [46].
Regarding chemotactic actions of the IFN-inducible CXCR3 ligands, the originally discovered canonical human CXCR3 [32]—later renamed CXCR3A upon discovery of alternative splicing of the CXCR3 gene [52]—is the receptor responsible for mediating cellular migratory responses and cell proliferation [25, 32, 34, 53]. CXCR3A couples to the inhibitory type of Gα proteins (Gαi) and to phospholipase C (PLC)-coupling Gα proteins (Gαq) [54, 55], thereby eliciting a downstream pathway that includes mobilization of intracellular calcium (Ca2+i), phosphorylation of extracellular signal-regulated kinase (ERK), and intracellular reduction of cyclic adenosine monophosphate (cAMP) [25, 32, 34, 53, 55–60]. The other identified CXCR3 variants that emerge from alternative splicing of the CXCR3 gene were named CXCR3B and CXCR3-alt, respectively [52, 61]. Distinctive downstream signaling and functions were designated to the three CXCR3 isoforms [57, 62]. CXCR3B differs from CXCR3A in its amino (NH2)-terminal tail of 51 amino acids. CXCR3 ligand-mediated CXCR3B stimulation was reported to evoke opposite cellular responses compared to CXCR3A [52]. In particular, CXCR3B-induced signaling was reported to engender apoptosis, inhibition of proliferation and chemotaxis of endothelial cells, and is considered to exert anti-angiogenic actions [52]. Furthermore, CXCR3-alt is a receptor with four-or-five transmembrane regions that lacks 101 amino acid compared to CXCR3A and arises as a result of posttranscriptional exon skipping [61]. The CXCL11-CXCR3-alt axis elicits Ca2+i mobilization and chemotaxis of CXCR3-alt-transfected human embryonal kidney 293 (HEK) cells [61]. Moreover, CXCL9, CXCL10- and CXCL11-induced ERK1/2 phosphorylation and receptor internalization on CXCR3-alt-transfected HEK cells [57]. In addition to the alternatively spliced forms of CXCR3, other receptors and interaction partners have been identified (Fig. 1). Given the inflammatory nature of the IFN-inducible CXCR3 ligands, immobilization on GAGs is crucial for their in vivo functioning. Sequestration of CXCL10 on endothelial GAGs was shown to be critical for CXCL10-mediated transendothelial migration of T cells and recruitment of T cells and plasmacytoid dendritic cells (pDC) [63, 64]. Furthermore, the anti-proliferative, anti-viral and anti-fibrotic properties of CXCL10 were also attributed to the interaction between CXCL10 and GAG [65–69]. In addition, binding to heparin, heparan sulfate and chondroitin sulfate was found to systemically shield these CXCR3 ligands from proteolytic inactivation by dipeptidyl peptidase IV (DPPIV/CD26) [70]. In terms of ACKRs, CXCL9 and CXCL10 displayed weak affinity for the Duffy antigen receptor for chemokines (DARC/ACKR1) whereas CXCL11 exhibited potent binding to this receptor [71]. Since ACKR1 was reported to transport pro-inflammatory chemokines CXCL8 and CCL2 across cell monolayers [72], this receptor may also mediate chemokine transcytosis of the pro-inflammatory CXCR3 ligands [72, 73]. Furthermore, CXCL10 and CXCL11 also interact with ACKR2/D6 or ACKR3/CXCR7, respectively [74, 75]. ACKR2 was shown to efficiently internalize CXCL10 and thereby exerts a prominent CXCL10 scavenging function [75]. ACKR3 conferred a growth and survival advantage to cells [74], but whether the CXCL11–ACKR3 axis could also instigate this response was not investigated. Concerning CC chemokine receptors, at high chemokine concentrations (1 µM), all three CXCR3 ligands exhibit natural antagonism for CCR3 [76, 77], whereas solely CXCL11 also antagonizes CCL4- and CCL5-mediated CCR5 signaling [78]. Hence, these various interaction partners of the IFN-inducible CXCR3 ligands provide an additional dimension to the versatility of the actions of the CXCR3 chemokine network (Table 1). Detailed overviews on the interaction features and downstream processes of receptor- and GAG-CXCR3 ligand interaction were recently published [46, 79]. Noteworthy, the intercommunion of these chemokines and the respective responder cells becomes even more complicated in the framework of the synovial microenvironment as multiple joint-resident cells are altered by the CXCL9/10/11–CXCR3 axis (vide infra).
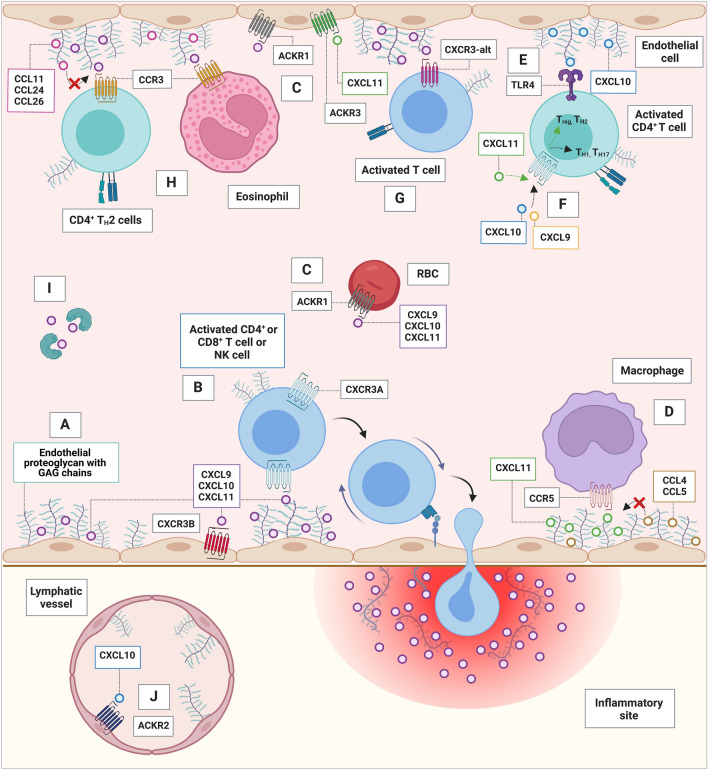
Fig. 1.
Receptors and interaction partners of the IFN-inducible CXCR3 ligands in blood and lymph vessels. For ACKRs, chemokine–receptor interaction as displayed has not been confirmed for the particularly displayed cell type (rather on transfected cells). Key mechanisms of the IFN-inducible CXCR3 ligands are depicted and include A immobilization on GAGs of proteoglycans located on endothelial cells and in tissues, B chemotaxis of activated T cells and NK cells through CXCR3A, C binding to ACKR1, D antagonistic activity on CCR5, E pro-inflammatory cytokine release through interaction of CXCL10 with TLR4 on CD4+ T cells, (F) CD4+ T cell polarization into TH1 or TH17 cells (by CXCL9 and CXCL10) or Treg or TH2 cells (by CXCL11), G binding to CXCR3-alt on activated T cells, H antagonistic actions on CCR3, I posttranslational modifications, and J CXCL10 scavenging through ACKR2. ACKR atypical chemokine receptor, CCL CC chemokine receptor ligand, CCR CC chemokine receptor, CXCR CXC chemokine receptor, CXCL CXC chemokine receptor ligand, GAG glycosaminoglycan, RBC red blood cell, TLR4 Toll-like receptor 4
Table 1.
Receptors and interaction partners of the IFN-inducible CXCR3 ligands
| IFN-inducible CXCR3 ligand | Receptor/interaction partner | Responder cell | Process affected by the respective IFN-inducible CXCR3 liganda | References |
|---|---|---|---|---|
| CXCL9 | CXCR3A |
Hu activated CD4+ TH1 cells, Hu-activated CD8+ T cells, Hu NK cells, Hu NKT cells, Hu pDC, Hu CXCR3+ neutrophils Hu B cells |
Chemotaxis | [31–39, 64, 80–83] |
| Mu CD4+ T cellsb | Polarization towards TH1 and TH17 cells | [84] | ||
| CXCR3B |
CXCR3B-transfected HMEC Primary HMEC |
Inhibition of proliferation Inhibition of cell migration Inhibition of vessel formation (angiostasis) |
[52] | |
| CXCR3-alt | CXCR3-alt-transfected HEK cells | Weak signaling | [57] | |
| CCR3 |
Hu primary eosinophils CCR3-B300-19 cells 4DE4-CCR3 cells |
Antagonism of CCL11-, CCL24- and CCL26-mediated chemotaxis at high concentrations of CXCL9 (1 µM) | [76, 77] | |
|
ACKR1/DARC (weak binding) |
Hu erythrocytes | N.D. for CXCL9 (presumably mediating scavenging and/or endothelial transcytosis) | [71, 72] | |
| Heparin | N.A. (soluble GAGs) | Protection against proteolytic inactivation by CD26 | [70] | |
| Heparan sulfate | ||||
| Chondroitin sulfate | ||||
| CXCL10 | CXCR3A |
Hu activated CD4+ TH1 cells, Hu-activated CD8+ T cells, Hu NK cells, Hu NKT cells, Hu pDC, Hu CXCR3+ neutrophils Hu B cells |
Chemotaxis | [31–39, 64, 80–83] |
| Mu CD4+ T cellsb | Polarization towards TH1 and TH17 cells | [84] | ||
| CXCR3B |
CXCR3B-transfected HMEC Primary HMEC |
Inhibition of proliferation Inhibition of cell migration Inhibition of vessel formation (angiostasis) |
[52] | |
| CXCR3-alt | CXCR3-alt-transfected HEK cells | Weak signaling | [57] | |
| CCR3 |
Primary hu eosinophils CCR3-B300-19 cells 4DE4-CCR3 cells |
Antagonism of CCL11-, CCL24- and CCL26-mediated chemotaxis at high concentrations of CXCL10 (1 µM) | [76, 77] | |
| TLR4 | Pancreatic β cells |
Apoptosis ↓ insulin expression and secretion |
[85] | |
| CD4+ T cells | Production of osteoclastogenic cytokines RANKL, IL-6 and TNF-α | [86] | ||
| ACKR1/DARC (weak binding) | Hu erythrocytes | N.D. for CXCL10 (presumably mediating scavenging and/or endothelial transcytosis) | [71–73] | |
| ACKR2/D6 | ACKR2-transfected HEK cells | CXCL10 internalization and scavenging/depletion | [75] | |
| Unidentified receptor | Cultured non-small cell lung cancer cells | Chemotaxis | [87] | |
| Heparin | N.A. (soluble GAGs) | Protection against proteolytic inactivation by CD26 | [70] | |
| Heparan sulfate | Primary hu pDC | Hapto-repulsion and transendothelial migration | [64] | |
| Endothelial cells | Inhibition of proliferation | [65] | ||
|
Mu cardiac fibroblasts Primary human lung fibroblasts |
Inhibition of cell migration | [66, 67] | ||
| Hepatocytes (Hepa1-6 cells) | Inhibition of Dengue virus binding to cell-surface heparan sulfate | [68] | ||
| N.A. (soluble GAGs) | Protection against proteolytic inactivation by CD26 | [70] | ||
| Primary HMEC | Oligomerization | [88] | ||
| Chondroitin sulfate | Mu cardiac fibroblasts | Inhibition of fibroblast migration | [66] | |
| N.A. (soluble GAGs) | Protection against proteolytic inactivation by CD26 | [70] | ||
| Endothelial GAG | Primary hu CD4+ T cells | Transendothelial migration | [63] | |
| Mu memory CD45RO+ T cells | Arterial recruitment and accumulation within the intima of the vessel wall in vivo | [63] | ||
| Primary hu endothelial cells (HMEC and HUVEC) | Inhibition of proliferation | [69] | ||
| CXCL11 | CXCR3A | Hu activated CD4+ TH1 cells, Hu-activated CD8+ T cells, Hu NK cells, Hu NKT cells, Hu pDC, Hu CXCR3+ neutrophils Hu B cells | Chemotaxis | [31–39, 64, 80–83] |
| Mu CD4+ T cellsb | Polarization towards Tr1 and TH2 cells | [84] | ||
| CXCR3B |
CXCR3B-transfected HMEC Primary HMEC |
Inhibition of proliferation Inhibition of cell migration Inhibition of vessel formation (angiostasis) |
[52] | |
| CXCR3-alt | CXCR3-alt transfectant HEK cells | Chemotaxis | [59, 61] | |
| CCR3 |
Primary hu eosinophils CCR3-B300-19 cells 4DE4-CCR3 cells |
Antagonism of CCL11-, CCL24- and CCL26-mediated chemotaxis at high concentrations of CXCL11 (1 µM) | [76, 77] | |
| CCR5 |
Primary hu monocytes CCR5-transfected mu B300.19 cells |
Antagonism of CCL4- and CCL5-induced chemotaxis at high concentrations of CXCL11 (1 µM) | [78] | |
| ACKR1/DARC (strong binding) | Hu erythrocytes | N.D. for CXCL11 (presumably mediating scavenging and/or endothelial transcytosis) | [71, 72] | |
| ACKR3/CXCR7 | Hu epithelial breast cancer cells (MCF-7) | N.D. for CXCL11 (may increase growth, survival and adhesion) | [74] | |
| Heparin | N.A. (soluble GAGs) | Protection against proteolytic inactivation by CD26 | [70] | |
| Heparan sulfate | N.A. (soluble GAGs) | Protection against proteolytic inactivation by CD26 | [70] | |
| N.A. (soluble GAGs) | Oligomerization (dimer formation) | [89, 90] | ||
| Chondroitin sulfate | N.A. (soluble GAGs) | Protection against proteolytic inactivation by CD26 | [70] | |
| Endothelial GAGs | Mu leukocytes | Chemotaxis in vivo towards peritoneal cavity | [91] |
ACKR atypical chemokine receptor, CCR CC chemokine receptor, CCL CC chemokine ligand, CD26/DPPIV dipeptidyl peptidase 4, CXCL CXC chemokine ligand, CXCR CXC chemokine receptor, DARC Duffy antigen receptor for chemokines, GAG glycosaminoglycan, HEK cells human embryonal kidney, HMEC human microvascular endothelial cells, hu human, HUVEC human umbilical cord endothelial cells, IL-6 interleukin 6, mu murine, N.A. not applicable, N.D. not determined, NK natural killer, pDC plasmacytoid dendritic cells, RANKL Receptor activator of nuclear factor kappa-Β ligand, TNF-α tumor necrosis factor α
aAffected signaling pathways were not mentioned
bPolarization of murine T cells, whether polarization of human CD4+ T cells happens through CXCR3A or another CXCR3 isoform has not been evidenced yet. All findings were obtained in vitro, except when indicated otherwise
The IFN-dependent CXCR3 chemokine network in diseases characterized by chronic inflammatory arthritis
A myriad of chemokines has been implicated in diseases characterized by chronic joint inflammation. Numerous manuscripts have been published in the last three decades on CXCR3 and its ligands in the context of inflammatory arthropathies in humans and mice models. This progressively generated knowledge coincided with research enabling our understanding on the general biology of CXCR3 to mature, thereby sparking interest and generating opportunities for drug development. In the present review, we provide an overview of IFN-inducible CXCR3 ligands in inflammatory diseases characterized by chronic inflammatory arthritis and focus on their pro-inflammatory actions in the articular environment, whereby rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA) serve as paradigmatic pathologies (Fig. 2; Suppl. tables 1–2).
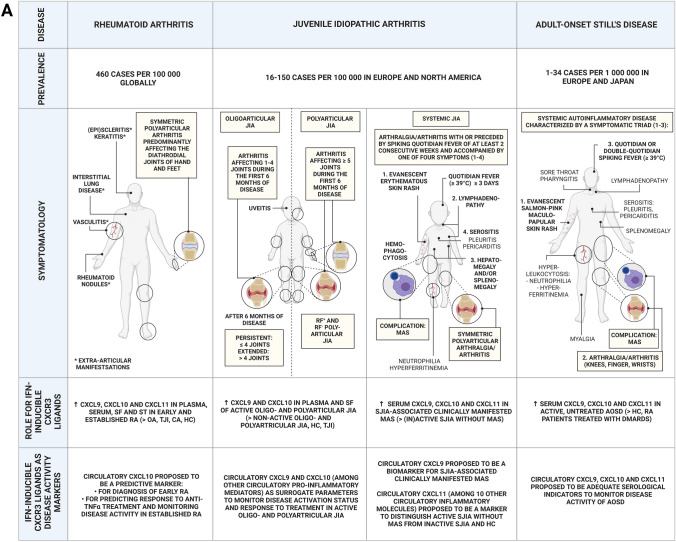
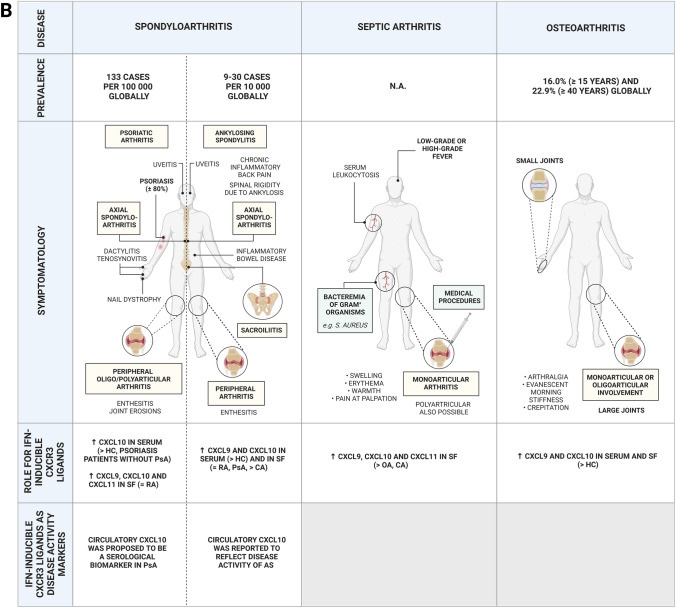
Fig. 2.
Chronic inflammatory arthropathies in which IFN-inducible CXCR3 ligands have been implicated. Prevalence, symptomatology, role and disease marker potential of the IFN-inducible CXCR3 ligands in A RA, JIA, AOSD and B in spondyloarthritis, septic arthritis and osteoarthritis. Prevalence numbers were based on [114–119]. Global prevalence of septic arthritis has not been described recently, whereas incidence of septic arthritis was recently reported to be 21/100,000 person-years in New Zealand with pronounced interethnic variation [120]. AOSD adult-onset Still’s disease, CA crystal-induced arthritis, CXCL CXCL chemokine receptor ligand, DMARDs disease-modifying anti-rheumatic drugs, HC healthy control, JIA juvenile idiopathic arthritis, MAS macrophage activation syndrome, N.A. not available in literature, OA osteoarthritis, PsA psoriatic arthritis, RA rheumatoid arthritis, RF rheumatoid factor, S. aureus Staphylococcus aureus, SF synovial fluid, sJIA systemic juvenile idiopathic arthritis, ST synovial tissue, TNF-α tumor necrosis factor α, TJI traumatic joint injury
Rheumatoid arthritis
RA is a systemic, inflammatory, and disabling disease characterized by progressive polyarticular arthritis, which can elicit substantial cartilage and bone damage [92, 93]. The etiopathology of RA has not been completely elucidated but a wide spectrum of pro-inflammatory chemokines and cytokines have been implicated in directional trafficking of leukocyte subsets towards the inflamed joints [94–96]. Despite the fact that the etiology of RA has remained partially elusive, a pivotal role of T cells, in particular type-1 T-helper (TH1), type-17 T-helper (TH17) cells and regulatory T (Treg) cells, has been evidenced in RA pathogenesis [97]. Evidently, when addressing T cell (patho)physiology, one cannot overlook CXCR3 since this chemokine receptor enables T cell trafficking towards and entry into inflammatory niches in vivo [98–101]. Studies from 1997 onwards already reported that approximately 90% of synovial CD4+ T cells in established RA expressed CXCR3 [102–104]. This abundant presence of CXCR3+ T cells in the rheumatoid synovium was corroborated by others [105, 106]. CXCR3 expression is conventionally allocated to the TH1 phenotype [107]. However, extensive immunophenotyping of synovial T cells in RA recently showed that other T cell subsets also express CXCR3 including type-2 T-helper (TH2) cells, TH17 cells, peripheral T helper cells and follicular T helper cells expressing high levels of programmed cell death protein 1 (PD-1) [107]. Intriguingly, successful treatment of RA patients with TNF-α inhibitors resulted in a marked increase of CXCR3+ CD4+ T cells in the peripheral blood of patients, indicating peripheral pooling of these inflammatory cells upon disease amelioration [108]. In addition, CXCR3 expression was shown on various heterogenous leukocyte subsets in the synovial fluid including NK cells, plasma cells, memory B cells, neutrophils, pDC, monocytes and mast cells [80, 81, 83, 106, 109–113].
IFN-inducible CXCR3 ligands in bodily fluids of patients with rheumatoid arthritis
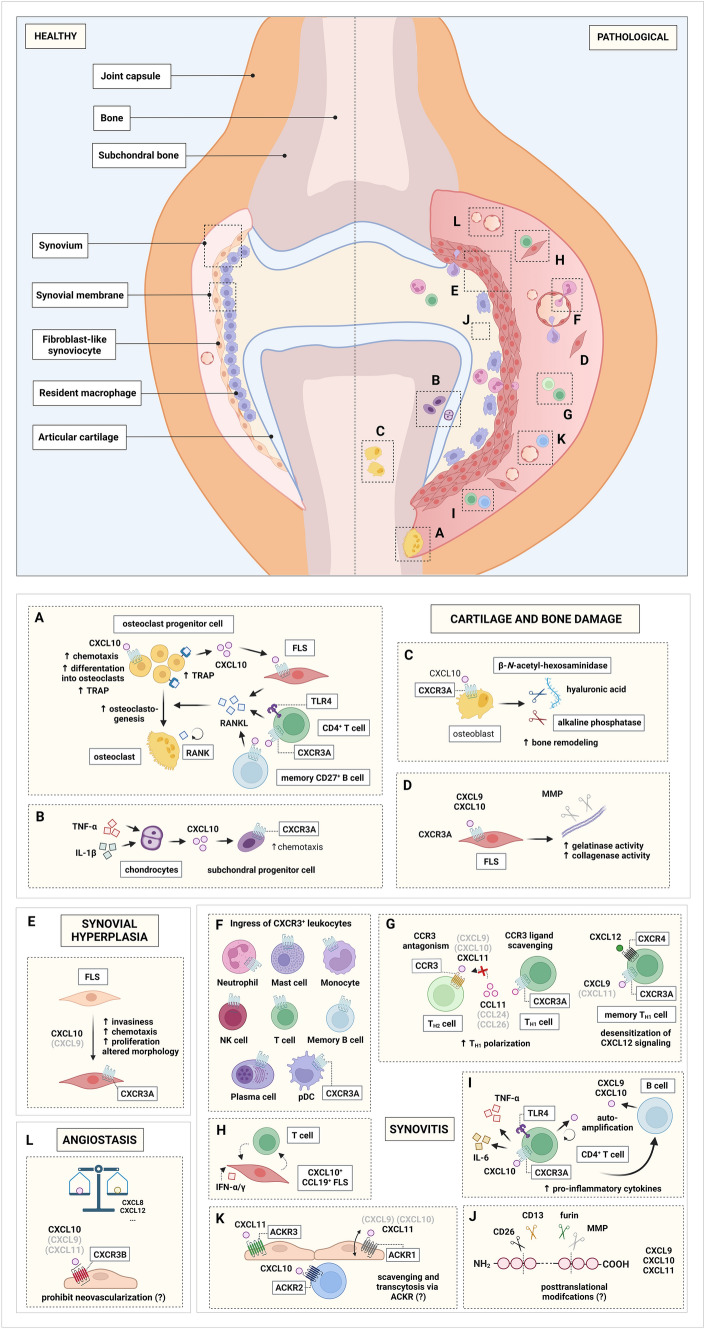
The anatomy of the synovial joints enables reciprocal exchange of inflammatory mediators between the articulation and blood circulation. In synovial joints, the joint capsule consists of an external articular capsule and an internal envelope of highly vascularized connective tissue that seals the joint cavity, referred to as the synovium. The internal surface layer of the synovium is outlined by a synovial membrane [121]. Under physiological circumstances, fibroblast-like synoviocytes (FLS) embedded in the synovial membrane/lining secrete nutrients, regulatory cytokines and extracellular matrix (ECM) components (e.g., hyaluronic acid) into the synovial cavity, thereby fueling and regulating the herein-present synovial fluid (SF) [121, 122]. The hallmark function of SF is biological lubrication of synovial joints [121]. As such, SF equips the articular cartilage surface with low-friction and load-bearing properties, which facilitates joint movement. In addition, SF is an ultrafiltrate of blood plasma filtered through the synovial membrane and thereby also functions as biochemical reservoir [121]. This signature architecture of synovial joints—in which synovial tissue, SF and the blood circulation are in continuous intercommunion—underscores the relevance to investigate these anatomical locations to clarify the role of inflammatory molecules in arthropathies at the inflammatory site. All three IFN-inducible CXCR3 ligands have been detected in plasma sera and SF of patients suffering from RA [45, 48, 80, 102, 107, 123–135] (Fig. 2A, Suppl. table 1).
Circulatory IFN-inducible CXCR3 ligands
Circulatory CXCL9 and CXCL10 levels were significantly higher in early and long-standing RA compared to healthy controls (HC) [125, 127, 129, 130, 132–134, 136] and gradually attenuated upon clinical improvement after treatment [126, 132, 135]. Furthermore, serum CXCL10 levels were increased in patients with early RA in comparison with patients with long-standing RA [125] or patients suffering from other arthropathies including osteoarthritis [137], psoriatic arthritis [128] or ankylosing spondylitis [128]. In addition, CXCL9 and CXCL10 concentrations were significantly augmented in plasma of patients with early RA compared to those of matched pre-patients, whereby plasma levels of these chemokines increased in pre-patients when they were closer to onset of symptoms [123]. These findings underscore the potential relevance of CXCL9 and CXCL10 as diagnostic markers for the detection of early RA. Indeed, ROC analysis confirmed that serum CXCL10 had an adequate diagnostic sensitivity and specificity to predict early RA [125, 134]. Intriguingly, serum CXCL10 levels were significantly higher in RA patients with anti-cyclic citrullinated peptide (anti-CCP) antibodies—a marker routinely used to diagnose RA—compared to anti-CPP negative patients [126, 138]. In addition, the observation that baseline serum CXCL10 levels were elevated in RA patients who responded adequately to tumor necrosis factor α (TNF-α) inhibitor treatment as opposed to non-responders [126] suggests that increased serum CXCL10 at baseline may also be a valuable tool to predict a favorable response to anti-TNF-α therapy. Hence, the T cell chemoattractant CXCL10—among other chemokines such as the major human neutrophil attractant CXCL8 and the B cell chemotactic protein CXCL13—is believed to be a promising candidate biomarker in RA [94–96, 125, 126, 132, 139–142]. Pandya et al. recently proposed that solely CXCL10—and not other chemokines—may serve as a suitable disease activity marker in untreated, early RA [133]. A blood chemokine signature comprising of CXCL9, CXCL10, CXCL13, CCL4 and CCL22 was defined through multivariate discriminant analysis that enabled to discriminate patients with untreated, early RA from HC [133]. Among these chemokines, only plasma CXCL10 levels correlated with all clinical disease activity parameters including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), swollen joint counts (SJC) in 66 joints, Clinical Disease Activity Index (CDAI), and disease activity score in 28 joints (DAS-28) based on ESR and CRP. Accordingly, another study revealed that serum CXCL10 has a higher diagnostic sensitivity and specificity for prediction of RA compared to serum CXCL8 [134]. As such, these relatively novel studies point towards a potential key positioning of blood CXCL10 in the framework of monitoring tools for RA disease activity.
Noteworthy, conflicting data exist in literature on the correlation of serum CXCL10 levels with disease activity parameters in RA. On the one hand, serum CXCL10 levels did not correlate with systemic disease parameters including C-reactive protein (CRP) and ESR [124–126, 135]. For example, Ueno et al. reported that serum levels of all three CXCR3 ligands failed to correlate with disease parameters including ESR, CRP, presence of the rheumatoid factor (RF) or treatment modality [124]. On the other hand, multiple studies—including the one of Pandya et al.—showed that blood CXCL10 levels in RA correlated with joint pathology-related disease indexes including SJC, tender joint count (TJC), and DAS-28 based on CRP or ESR [125, 133, 135]. However, contrasting findings were reported in other studies, whereby serum CXCL10 did not correlate with either SJC and TJC or DAS-28, respectively [126, 135]. Thus, these seemingly inconsistent findings may be caused by general diversity in patient cohorts, but also heterogeneity in individual characteristics of recruited patients (e.g., systemic and joint-related disease status and therapy at sampling). In addition, CXCL10 protein detection may be biased as CXCL10 is highly susceptible to proteolytic processing, which is especially relevant given the massive protease release during clothing processes at the moment of serum collection.
In summary, further research is warranted to uniformly conclude whether blood CXCL10 adequately reflects disease activity in RA. Circulatory levels of CXCL10 may serve as a predictive marker for diagnosis of early RA [123, 125, 134], for predicting the response to treatment in established RA [126] and for monitoring disease activity/remission in RA [125, 132, 133]. Nevertheless, it is important to realize that chemokines are in essence local inflammatory actors and their presence in the bloodstream merely reflects chemokine egress from inflammatory sites. Therefore, correlation of serum CXCL10 levels with systemic disease measures may be less relevant from a clinico-biological perspective, whereas the evidenced correlation of serum or synovial CXCL10 levels to joint-specific pathology parameters (e.g., DAS-28, SJC and TJC) could be more informative.
Synovial IFN-inducible CXCR3 ligands
Immunoreactivity for CXCR3 in synovial tissues of RA patients was detected on CD3+ (T) lymphocytes [104, 105], peri-vascularly located lymphocytes [102, 103] and in particular perivascular CD2+ T cells [102], synovial fibroblasts [106], mast cells in the perivascular, interstitial and sublining region [106] and CD183+ plasma cells in the sublining layer of the synovial membrane [110]. In addition, CXCR3 was also described on vascular endothelial cells and on infiltrating mononuclear cells in lymphoid aggregates in the rheumatoid synovium, for which staining became more pronounced in severely inflamed areas [143]. Immunohistochemistry further demonstrated substantial interindividual variability of RA patients in the proportion of CXCR3-expressing synovial cells (ranging from 20 to 60%) [106]. Moreover, significantly increased RNA levels of CXCR3 were detected in synovia of RA patients compared to those of patients with osteoarthritis [106]. Accordingly, upregulated CXCR3 protein expression in rheumatoid synovia relative to synovia of patients with osteoarthritis was observed [106].
In terms of the CXCR3 ligands, CXCL9 and CXCL10 protein levels were significantly increased in SF of RA patients, compared to patients suffering from traumatic joint injury, osteoarthritis, and crystal-induced arthritis (Fig. 2A, Suppl. table 1) [45, 48, 80, 102, 124, 131, 137, 144]. Synovial CXCL11 levels were enhanced in RA relative to osteoarthritis [80, 124] and ankylosing spondylitis [48], but not in comparison with crystal-induced arthritis [48]. In rheumatoid synovial tissue biopsies, pronounced protein and mRNA expression of CXCR3, CXCL9 and CXCL10 was recurrently reported [102, 104–106, 110, 124, 131, 143, 145, 146]. Enhanced mRNA levels of CXCL9 and CXCL10 were detected in synovial tissue biopsies of RA patients compared to those of osteoarthritis patients [106, 124, 146] and of patients who underwent synovial tissue biopsy due to suspected articular damage [145]. Remarkably, synovial mRNA levels of CXCL9 and CXCL10 were upregulated in RA compared to osteoarthritis by 135- and 340-fold, respectively [106]. Recently, CXCL9 and CXCL10 genes were identified as differentially expressed genes that encoded biomarkers in a meta-analysis of two gene expression microarray datasets of synovial tissues from patients with RA [147]. Taken together, CXCL9 and CXCL10 levels were uniformly found to be upregulated in the circulation and even to a greater extend in the SF and tissue [102, 107, 124, 136, 137], thereby establishing a chemotactic gradient from blood towards the synovium along which leukocyte can migrate. Indeed, CD8+ T cells isolated from the peripheral blood of RA patients migrated to recombinant CXCL10 in an in vitro transwell assay [148]. The chemotactic response of RA patient-derived CD8+ T cells could be abolished by the addition of an anti-human CXCL10 monoclonal antibody [148]. Likewise, CXCR3-transfected HEK cells—expressing solely CXCR3 and no other chemokine receptor—migrated towards SF of RA patients in a Transwell filter assay, which was abrogated by administration of a CXCR3-neutralizing antibody [149]. Thus, CXCL10 appears to be an important synovial T cells chemoattractant. Furthermore, RT-PCR and immunohistochemistry performed on synovial tissue revealed coinciding expression of CXCL9 and CXCL10 in the synovial lining and sublining [110, 146]. In situ hybridization revealed marked CXCL9 mRNA expression in the synovial lining and in cellular infiltrates in synovial tissues of patients with RA [150]. Moreover, CXCL9 immunoreactivity was found on synovial fibroblasts in synovial sublining regions, perivascular fibroblast-like cells and endothelial cells in vascular regions [110]. In addition, cytological analysis of rheumatoid synovial tissue showed that CXCL9 expression was primarily present in macrophages (i.e., monocytic cells expressing macrophage antigen Ki-M6 [151]) and in some vessel-associated lymphocytes [150]. Similar to CXCL9, immunohistochemistry revealed CXCL10 expression in rheumatoid synovium on fibroblast-like cells and macrophage-like cells [131]. Synovial B cells were also proposed to be a source of CXCL9 and CXCL10, since B cells isolated from SF of RA patients prominently expressed CXCL9 and CXCL10 mRNA, to an even higher extent as synovial CD4+ T cells and CD8+ T cells [152]. Hence, FLS, synovial macrophages, and (perivascular) lymphocytes probably constitute important cellular sources for CXCL9 and CXCL10 in the joints of RA patients.
In contrast to the consistent findings corroborating CXCL9 and CXCL10 upregulation in joints and circulation of RA patients, data on the expression profile of CXCL11 in clinical samples of RA patients is less congruent. In terms of CXCL11 expression in the rheumatoid synovia, one study unveiled increased mRNA levels of CXCL11 in RA compared to osteoarthritis [124] whereas other researchers reported that CXCL11 could not be detected in rheumatoid synovia via RT-PCR [110]. In addition, the established blood-to-SF gradient of CXCL9 and CXCL10, could not be confirmed for CXCL11. Ueno et al. showed that serum and synovial levels of CXCL11 did not significantly differ from each other [124], whereas others found that plasma CXCL11 was significantly increased relative to synovial CXCL11 in paired samples of RA patients [107]. Notable, these findings regarding blood levels, SF concentrations, mRNA expression in the synovial tissue and immunolocalization of CXCL11 in RA are rather ambiguous and often only partially accord with the data on CXCL9 and CXCL10. Indeed, a definitive conclusion concerning the involvement of CXCL11 in RA remains challenging as multiple confounders may hamper a straightforward interpretation. Firstly, concentrations of naturally secreted CXCL11 are often low compared to CXCL9 and CXCL10, both in human cell culture supernatant [47, 124, 153, 154] and in bodily fluids of RA patients [80, 107, 124]. Secondly, CXCL11 concentrations have been explored to a lesser degree in vivo relative to the other IFN-inducible CXCR3 ligands [46], especially in the context of RA. Nevertheless, CXCL11 is the most potent ligand for CXCR3A, characterized by the highest affinity for the receptor [25, 32] and the most pronounced ability to induce Ca2+i mobilization, chemotaxis and receptor internalization [25, 154]. In addition, CXCL11, in contrast to CXCL9 and CXCL10, is also a strong ligand for ACKR3, a chemokine receptor that fails to signal through G proteins [74]. Intriguingly, CXCL11 also affects CD4+ T cell polarization in an antithetical manner compared to CXCL9 and CXCL11 [84, 155]. CXCL9 and CXCL10 polarizes CD4+ T cells towards a TH1 and TH17 effector phenotype, whereas CXCL11 skews CD4+ T cells towards TH2 or IL-10high T regulatory 1 subset (Tr1) cells [46, 84, 155]. This type of biased signaling is established via CXCL10-induced CXCR3 activation, resulting in phosphorylation of signal transducer and activator (STAT) 1, STAT4 and STAT5, thereby activating T-box transcription factor (T-bet) [46, 84, 155]. CXCL11-CXCR3 interactions activate STAT3- and STAT6-dependent pathways and thereby GATA-binding protein 3 (GATA3). Conceivably, these CXCL11-induced Tr1 cells may restrain inflammation. Despite its restricted concentrations in vivo, CXCL11 may be highly relevant in RA and other inflammatory arthropathies. Given the prioritized binding of CXCL11 to CXCR3, CXCL11 may supersede the actions of CXCL9/10 through receptor internalization making it unavailable for CXCL9/10 [155] and through polarization towards TH2 or Tr1 cells, thereby resulting in an anti-inflammatory outcome.
In vitro production of IFN-inducible CXCR3 ligands by rheumatoid synovial cells
The major chemokine-secreting cell lineages of the rheumatoid synovia include synovial fibroblasts [110, 124, 156] or FLS [157, 158], macrophage-like synoviocytes [158], synovial follicular dendritic cells in ectopic lymphoid like structures (ELS) [159] and synovial endothelial cells [160]. FLS are mesenchymal cells that represent the most abundant tissue-resident cell type in the human synovial membrane [161]. On the one hand, these cells exhibit hallmark characteristics of fibroblasts including expression of vimentin, and type IV and V collagens. On the other hand, FLS display distinctive joint-specific features that distinguishes them from other fibroblast lineages including the secretion of nutrients and ECM components [162]. In contrast to these nurturing physiological functions, the aggressive phenotype of FLS in RA perpetuates synovial inflammation [161]. In addition to their loss of contact inhibition, increased invasiveness and proliferation, FLS secrete various pro-inflammatory and osteoclastogenic cytokines, matrix metalloproteinases (MMPs) and chemokines [157, 163, 164] including the IFN-inducible CXCR3 chemokines (Table 2) [110, 124, 135, 165].
Table 2.
Expression and secretion of IFN-inducible CXCR3 ligands by human synovial cells isolated from SF and synovial tissues of RA patients
| Cell type (status of cells) | Stimuli | Secreted IFN-inducible CXCR3 ligand in cell culture supernatant | mRNA expression CXCR3 ligand by the cells | References |
|---|---|---|---|---|
| Synovial tissue cells (freshly isolated cells) | DMEM + 10% FCS | CXCL9, CXCL10, CXCL11 | N.D | [124] |
| TNF-α | CXCL10 | CXCL9, CXCL10 | [137, 165] | |
| IL-1β | N.D | CXCL10 | [156] | |
| Synovial tissue cells (cultured for 2 weeks[135] or third to ninth passage[166]) | DMEM + 10% FCS | CXCL10 | N.D | [166] |
| IFN-γ | CXCL10 | N.D | [135] | |
| TNF-α | CXCL10 | N.D | [135, 166] | |
| hTWEAK | CXCL10 | CXCL10 | [166] | |
| hTWEAK + TNF-α | CXCL10 | N.D | ||
| Synovial fibroblasts (fourth or fifth passage[124] or third to sixth passage[110]) | IFN-γ | CXCL9, CXCL10 | CXCL9, CXCL10, CXCL11 | [110, 124] |
| TNF-α | CXCL10 | CXCL9, CXCL10, CXCL11 | [110, 124] | |
| IL-1β | CXCL10 | CXCL9, CXCL10, CXCL11 | [124] | |
| IFN-γ + TNF-α | CXCL9, CXCL10, CXCL11 | N.D | [124] | |
| IFN-γ + IL-1β | CXCL9, CXCL10, CXCL11 | N.D | [124] | |
| SF monocytes (freshly isolated) | Fibroblast-like synoviocytes (cultured after isolation) | CXCL10 | CXCL10 in synovial monocytes | [131] |
| SF PMNs (freshly isolated) | CXCL10 | CXCL10 in synovial PMNs | ||
| SF CD1c+ mDC | RPMI medium + 10% human AB serum | CXCL9, CXCL10 | N.D | [167] |
DMEM Dulbecco's Modified Eagle Medium, FCS fetal calf serum, hTWEAK human tumor necrosis factor (TNF)-like weak inducer of apoptosis, IFN interferon, IL interleukin, mDC myeloid dendritic cells, N.D. not determined, PMNs polymorphonuclear neutrophils, RPMI Roswell Park Memorial Institute, SF synovial fluid, TNF-α tumor necrosis factor α
In 1993, mRNA expression of CXCL10 by synovial tissue cells of RA patients was described for the first time [156]. Intriguingly, all three IFN-inducible CXCR3 ligands were secreted by freshly isolated synovial tissue cells upon incubation in medium containing 10% fetal calf serum (FCS) [124, 166] and their concentrations were significantly higher in supernatant of synovial cells of RA patients compared to those of osteoarthritis patients [124]. Also, CD1c+ myeloid DC (mDC)—isolated via magnetic-activated cell sorting (MACS) from SF of RA patients and cultured for 20 h in medium supplemented with 10% human serum—secreted substantial amounts of CXCL9 and CXCL10 [167]. Furthermore, cultured synovial fibroblasts after several passages did not spontaneously express CXCR3 ligands but these chemokines were profusely secreted after stimulation with both IFN-γ and TNF-α [124], which are cytokines known to be present in SF of RA patients [168]. Upon IFN-γ stimulation, CXCL9 and CXCL10 were secreted, whereas stimulation with TNF-α or interleukin 1β (IL-1β) resulted in the presence of CXCL10 in the cell culture supernatant [124]. Moreover, mRNA expression of all three IFN-inducible CXCR3 ligands was detected via RT-PCR after stimulation with either IFN-γ or TNF-α or IL-1β [110, 124]. Thus, IFN-induced CXCR3 chemokines seemed to be constitutively expressed to some extent, but these chemokines are also robustly induced upon cytokine stimulation in rheumatoid synovial cells. More specifically, IFN-γ and TNF-α may orchestrate a cooperative, synergistic induction of IFN-inducible CXCR3 ligands in synovial cells of patients with RA. Synergism between IFN-γ and TNF-α enabling increased production of all three CXCR3 ligands has been previously described for human skin/muscle-derived fibroblasts [45, 48] and human microvascular endothelial cells [45, 48]. In addition, CXCL10 production was also synergically induced by IFN-γ and TNF-α in synovial fibroblast of patients with temporomandibular joint disorders (TMD) [169] and in THP-1 monocytes [170]. Etanercept treatment was shown to neutralize the synergic IFN-γ and TNF-α-mediated CXCL9 production in human microvascular endothelial cells [48]. Hence, this obliteration of synergic induction of IFN-inducible CXCR3 ligands could, at least in part, explain the reduced serum protein CXCL10 levels in RA patients after receiving anti-TNF-α therapy [126]. Consequently, CXCR3+ inflammatory cells may be less “trapped” in the synovium, which would explain the enhanced peripheral pooling of CXCR3+ cells after anti-TNF therapy [108]. Mechanistically, CXCL10 production by leukocytes appeared to be dependent, at least in part, on physical interaction between leukocytes and FLS [131]. Co-culturing of FLS with monocytes or polymorphonuclear neutrophils (PMNs) isolated from SF induced substantial mRNA expression and protein secretion of CXCL10 by synovial monocytes and PMNs, which could be abrogated by blocking ICAM-1/integrin interaction or by physical separation in a transwell system [131]. Hence, in addition to constitutive expression and cytokine-mediated chemokine induction, the intercommunion between tissue-resident cells (e.g., FLS) and infiltrating leukocytes in the synovium may further contribute to CXCR3 ligand production and consequently joint inflammation.
Interference with cytokine-mediated chemokine production by synovial cells may be interesting in the framework of developing novel therapies for RA. For example, Kuranobu et al. proposed that the anti-inflammatory and joint-protective actions of activin A—which is a homodimeric glycoprotein that is abundantly present in the inflamed synovium—are rooted partially in its regulation of CXCL10 production [165]. Activin A significantly attenuated TNF-α-induced mRNA expression and protein secretion of CXCL10 by rheumatoid synovial cells [165]. Similarly, others evaluated the in vitro anti-inflammatory potential of blockage of human tumor necrosis factor (TNF)-like weak inducer of apoptosis (hTWEAK) [166]. hTWEAK is a widely tissue-distributed member of the TNF superfamily that has pro-inflammatory activity and is pronouncedly expressed in rheumatoid synovia [166, 171]. In rheumatoid synovial cells, hTWEAK potently stimulated secretion of various chemokines including CXCL10 [166]. This CXCL10 secretion was abrogated by an anti-hTWEAK antibody. As such, these strategies targeting cytokine-induced chemokine expression may be interesting tools to reduce the localized inflammatory response in the synovium.
Disease models of rheumatoid arthritis
CXCR3 in experimentally induced arthritis
In general, disease-contributing roles of CXCL10 and CXCR3 in the context of experimentally induced arthritis have been widely explored (Fig. 3, Suppl. tables 3–5). Targeting CXCR3 alleviated arthritis disease symptoms in animal models of arthritis including type II collagen-induced arthritis (CIA) [172], type II collagen antibody-induced arthritis (CAIA) and rat adjuvant arthritis (AA) [100] (Fig. 3A, Suppl. tables 3–4). First, genetic ablation of CXCR3 (CXCR3−/−) in collagen type II antibodies-challenged C57BL/6 mice resulted in a mitigated CAIA phenotype, marked by attenuated clinical arthritis and histopathological scores, less proteoglycan loss and reduced osteoclast activation in the articular cartilage compared to WT mice [86]. Relative to WT mice, CXCR3−/− mice with CAIA also had reduced infiltration of CD4+ T cells and F4/80+ macrophages in the joints and diminished serum levels of receptor activator of nuclear factor kappa-Β ligand (RANKL), TNF-α and IL-6 [86]. Second, the severity of adoptively transferred rat AA was ameliorated by blockage of CXCR3 via treatment with a neutralizing anti-CXCR3 monoclonal antibody, i.e., XR3.2 [100]. In particular, naïve Lewis rats treated with XR3.2—receiving adoptively transferred T cells of Lewis rats with AA—had delayed onset of arthritis, decreased clinical joint scores, less cartilage proteoglycan loss and more than 50% reduction in synovial neutrophil accumulation compared to untreated animals [100]. In addition, CXCR3 expression on T cells turned out to be crucial for T cell ingress in the inflamed joints in rat AA [100]. Intravenous (IV) injection of Cr51-labeled CXCR3+ and CXCR3− T cells—isolated from the spleen of healthy animals—in Lewis rats with AA demonstrated that nearly 2.5-fold more CXCR3+ T cells than CXCR3− T cells accumulated in the inflamed joints [100]. Another finding that prompted the CXCR3 dependence of synovial T cell infiltration was the XR3.2-mediated diminished synovial recruitment of IV injected radiolabeled nodal T cells, that were exogenously activated [100]. Hence, these data point towards a pivotal role of CXCR3 in T cell trafficking from the circulation towards the inflamed articulation in rat AA. Third, a decreased incidence of CIA development and reduced clinical score was described in CXCR3−/− C57BL/6 mice [172]. In contrast to rat AA and CAIA, Al-Banna et al. reported that intravenously injected Cr51-labeled TH1 cells from CXCR3−/− C57BL/6 mice and WT mice migrated to a similar extend into inflamed paws of WT C57BL/6 mice with CIA [172]. As such, these authors speculated that CXCR3-dependent T cell migration towards the synovial compartment plays a more limited role in CIA, in which joint inflammation may be rather antibody dependent. Noteworthy, these intravenously injected TH1 cells of WT mice were not specifically pre-selected for being CXCR3+. Hence, CXCR3-mediated T cell homing in the inflamed joints and its relative contribution to synovitis in CIA may follow the same trend as observed in other animal models.
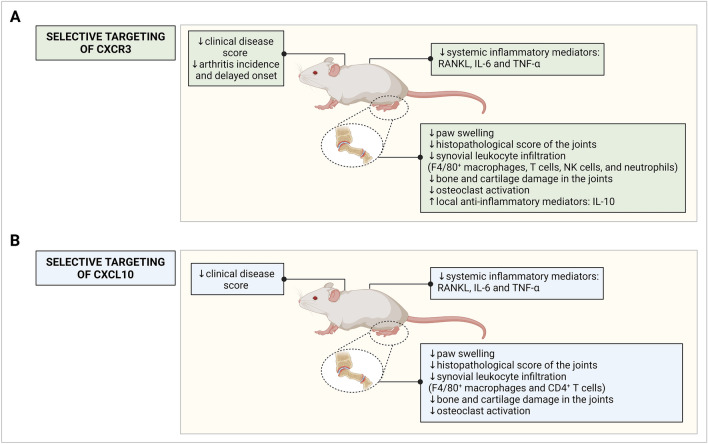
Fig. 3.
Schematic representation of the effects of targeting the IFN-inducible CXCR3 chemokine network on arthritis symptoms in rodent models. Symptomatology, cellular and molecular outcomes are depicted for rodent models undergoing therapies or depletions targeting A CXCR3 and B CXCL10. Selective targeting of CXCR3 was established through genetic ablation, CXCR3 antagonists (AMG 487, TAK-779, SCH 546,738, or JN-2) or CXCR3-targeting monoclonal antibodies. Selective targeting of CXCL10 was realized through genetic ablation, a CXCL10-encoding DNA vaccine, CXCL10-targeting monoclonal antibodies, a CXCL10-encoding retrovirus or a bispecific antibody targeting CXCL10 and TNF-α. CXCL, CXC chemokine receptor ligand, CXCR CXC chemokine receptor, IFN interferon, IL interleukin, NK natural killer, RANKL Receptor activator of nuclear factor kappa-Β ligand, TNF-α tumor necrosis factor α
In disagreement with the aforementioned data supporting a disease-contributing role of CXCR3, similar ankle swelling was observed in WT mice as in CXCR3−/− C57BL/6 mice during the development of serum-transferred arthritis upon serum transfer of K/BxN mice [173]. This prompted the assumption that CXCR3 is not critical for this type of K/BxN serum-transferred arthritis [173]. The discrepancy between the findings in other rodent arthritis models was explained by the fact that solely the transferred antibodies are sufficient to induce disease in the K/BxN serum-transferred arthritis model [173], whereas T cell trafficking to the joints is required for other forms of experimentally induced arthritis. However, beside ankle swelling, other clinical disease parameters or histopathological features were not examined in these mice, rendering conclusions in terms of the involvement of CXCR3 in serum-transferred arthritis rather premature. In general, depletion of CXCR3 offered arthritis-restraining outcomes in multiple rodent models.
In line with our conclusions concerning the role of CXCR3 in CIA (vide supra), recent studies that examined small-molecule CXCR3 antagonists in CIA further corroborated the involvement of CXCR3 in CIA-associated synovitis and CIA disease development (Fig. 4, Suppl. table 4). CIA-developing mice treated with CXCR3 antagonists displayed reduced synovial inflammation [174–179] and showed mitigated clinical disease development [175, 176, 178, 179]. First, the small-molecule CXCR3 antagonist AMG 487 (Fig. 4A) improved the clinical arthritis score and synovial histopathological manifestations, and re-directed T cell polarization towards a Foxp3+ IL-10-producing Treg cell phenotype in CIA-developing DBA/1 J mice [178]. Moreover, AMG 487 suppressed production of inflammatory mediators (e.g., IFN-γ, TNF-α, NFκB p65, IL-6) in knee tissue [174, 177] and shifted the inflammatory B cell phenotype towards IL-4 and IL-27-producing B cells in the spleen in CIA [174]. In addition, AMG 487 upregulated the production of TH2 cytokine IL-4, anti-inflammatory cytokine IL-10 and ‘anti-arthritic’ cytokine IL-27 in the inflamed joints in CIA [174, 178, 180]. Second, the small-molecule CCR5/CXCR3/CCR2 antagonist TAK-779 (Fig. 4B) was found to partially inhibit CIA development and reduced severity of CIA [179]. TAK-779 treatment decreased the incidence of CIA development and significantly reduced the arthritic index [179]. Since TAK-779 did not affect anti-collagen T cell responses, nor CCR5 induction on T cells in CIA, this effect is likely mediated by suppression of T cell ingress in the inflamed paws. Indeed, TAK-779 strongly inhibited leukocyte infiltration into joint lesions in CIA [179]. Whether the anti-inflammatory action of TAK-779 was based on its CCR5 or CXCR3 antagonism was not investigated in detail. Third, B10.RIII mice with CIA were treated with a selective, high affinity and non-competitive CXCR3 antagonist SCH 546738 (Fig. 4C) [175]. This compound ameliorated disease development at a dosing regimen of 40 mg/kg bodyweight, as evidenced by the attenuated disease score, reduced synovial leukocyte infiltration and less structural damage to bone and cartilage [175]. Recently, another small-molecule CXCR3 antagonist JN-2, which structurally resembles AMG 487, was developed (Fig. 4D) [181]. Similar to SCH 546738, JN-2 significantly reduced clinical disease scores, paw swelling, bone erosion and histopathological scores in CIA-developing DBA/1 mice [176]. JN-2 also attenuated serum protein and splenic mRNA levels of inflammatory cytokines including IL-6 and TNF-α [176]. Altogether, CXCR3 antagonism was overall substantially successful in restraining CIA [174–179].
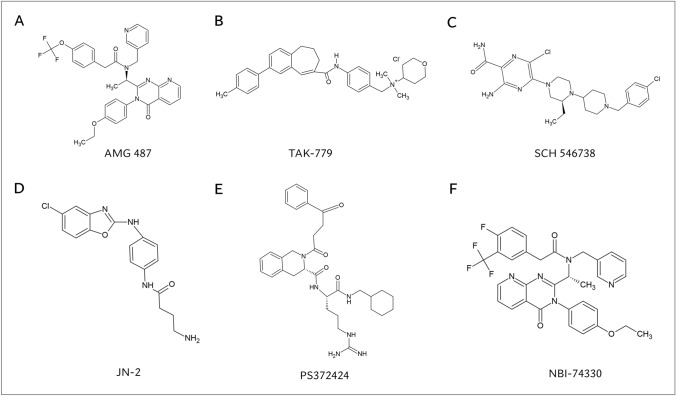
Fig. 4.
Chemical structures of the small-molecule CXCR3 antagonists and CXCR3 agonist that were evaluated in rodent models of arthritis. Chemical structures of A small-molecule CXCR3 antagonist AMG 487, B small-molecule CCR5/CXCR3/CCR2 antagonist TAK-779, C small-molecule CXCR3 antagonist SCH 546,738, D small-molecule CXCR3 antagonist JN-2, E small-molecule CXCR3 agonist PS372424, and F small-molecule CXCR3 antagonist NBI-74330. AMG 487, N-1-[(3-4(-Ethoxyphenyl)-3,4-dihydro-4-oxopyrido[2,3-d]pyrimidin-2-yl]ethyl]-N-(3-pyridinylmethyl)-4-(trifluoromethoxy)benzeneacetamide; JN-2, N-(4-(5-chlorobenzo[d]oxazol-2-ylamino)phenyl)-4-aminobutanamide; NBI-74330, N-1-[(3–4(-Ethoxyphenyl)-3,4-dihydro-4-oxopyrido[2,3-d]pyrimidin-2-yl]ethyl]-4-fluoro-N-(3-pyridinylmethyl)-3-(trifluoromethyl)benzene-acetamide; PS372424, (S)-N-((S)-1-((cyclohexylmethyl)amino)-5-guanidino-1-oxopentan-2-yl)-2-(4-oxo-4-phenylbutanoyl)-1,2,3,4-tetrahydro-isoquinoline-3-carboxamide; SCH 546738, 3-Amino-6-chloro-5-[(3S)-4-[1-[(4-chlorophenyl)methyl]-4-piperidinyl]-3-ethyl-1-piperazinyl]-2-pyrazinecarboxamide; TAK-779; N, N-dimethyl-N-(4-[[[2-(4-methylphenyl)-6, 7-dihydro-5H-benzocyclohepten-8-yl]carbon-yl]amino]benzyl)-tetrahydro-2H-pyran-4-aminium chloride
In addition to the abundant evidence supporting CXCR3 antagonism as a strategy to restrain experimental arthritis, promising results were obtained with the small-molecule CXCR3 agonist PS372424 in a humanized mouse air-pouch arthritis model (Fig. 4E, Suppl. table 4) [149]. As a result of an E196Q amino acid difference in human and mice CXCR3 (Glu196 Gln196), PS372424 is solely a CXCR3 antagonist in humans [182], thereby hampering the research on this compound in conventional rodent arthritis models. In the humanized mouse air-pouch arthritis model, severely immunodeficient NOD scid gamma (NSG) mice receive human peripheral blood mononuclear cells (PBMCs) by intraperitoneal injection and subsequently an air pouch is created through subcutaneous injection of sterile air into the back of the animal [149]. After 28 days, these immunodeficient mice have a peripheral T cell population that is nearly completely human. Intriguingly, intravenous administration of CXCR3 agonist PS372424 significantly reduced CD45+ leukocyte trafficking towards the air pouch, either filled with PBS containing CXCL11, CCL5, CXCL12 or SF of RA patients [149]. The small-molecule CXCR3 antagonist NBI-74330 (Fig. 4F) and CXCR3-neutralizing antibodies did not affect leukocyte infiltration in the pouch. Hence, chemotaxis antagonism of PS372424 was attributed to PS372424-mediated receptor cross-phosphorylation of CCR5 in CXCR3–CCR5 heterodimer complexes (vide infra) [149].
CXCL10 in experimentally induced arthritis
In general, CXCL10−/− mice have impaired T cell responses in response to allogeneic or antigenic stimulation in vivo, characterized by severely reduced T cell trafficking towards inflammatory sites and compromised T cell priming marked by decreased proliferation and IFN-γ secretion [183]. Intriguingly, selective depletion of CXCL10 provided consistent protective effects in various arthritis models including AA [184], CIA [185], CAIA [86], human TNF-α transgenic Tg197 mice [148], LPS-induced bone erosion [148] and K/BxN serum transfer-induced arthritis [148] (Fig. 3B, Suppl. table 5). First, IV administration of CXCL10 neutralizing antibodies ameliorated severity of CIA, as evidenced by reduced serum levels of RANKL and TNF-α, decreased infiltration of CD4+ T cells and F4/80+ macrophages, and less bone erosion [185]. In addition, serum levels and synovial expression of CXCL10 in untreated CIA-developing mice were significantly increased compared to control mice without CIA [185]. These data suggest that the induction of CXCL10 occurs mainly in inflamed joints and is important for leukocyte ingress into and bone erosion in the inflammatory articulation in CIA [185]. Second, CXCL10−/− C57BL/6 mice developed an attenuated CAIA phenotype, characterized by a reduced arthritis score, less synovial infiltration of CD4+ T cells and F4/80+ macrophages, diminished serum levels of IL-6, RANKL and TNF-α, and decreased bone and cartilage damage compared to WT mice [86]. These observations in CXCL10−/− mice with CAIA perfectly accord to the data of CIA-developing mice treated with anti-CXCL10 neutralizing antibodies [86, 185]. Third, Salomon et al. administered a naked DNA vaccine encoding for rat CXCL10 before and during the onset of AA in Lewis rats, thereby breaking immunological self-tolerance marked by high titers of self-specific CXCL10-targeting antibodies [184]. Lewis rats that received the vaccine before or during the onset of rat AA, exhibited ameliorated arthritis characterized by a decreased clinical disease score and improved joint histological parameters compared to the untreated littermates [184]. In addition, adoptive transfer of self-specific anti-CXCL10 antibodies purified from sera of these vaccinated rats into rats, in which clinical AA was initiated two days earlier, largely protected the AA-developing rats against development of full-blown clinical AA [184]. Rats receiving CXCL10-targeting antibodies exhibited significantly reduced clinical disease scores and polarization of lymph node CD4+ T cells to cells producing high levels of IL-4 and low levels of IFN-γ and TNF-α [184]. As such, CXCL10 may not solely orchestrate chemo-attraction of TH1 cells towards the inflamed joints but also skews the polarization of naïve infiltrating T cells into TH1 cells. In addition, the collective production of IFN-γ by these accumulating TH1 cells further propagates CXCL10 production by other cell types in the synovial niche. Fourth, the anti-arthritic efficacy of a bispecific antibody targeting both TNF-α and CXCL10 (BsAb) was demonstrated in three distinct arthritis models [148]. To evaluate the additional beneficial value of neutralization of downstream actions of CXCL10 aside from TNF-α blockage, mice with experimental arthritis treated with BsAb were compared to adalimumab-treated mice [148]. Treatment with BsAb ameliorated K/BxN serum transfer-induced arthritis to a similar extent as adalimumab. In human TNF-α transgenic Tg197 mice, which spontaneously develop arthritis due to constitutive overexpression of human TNF-α, BsAb-mediated ameliorated arthritis was characterized by reduced serum levels of human TNF-α and mouse IL-1β compared to adalimumab-treated mice. In mice with LPS-induced bone erosion, treatment with BsAb—but not adalimumab—instigated reduced bone resorption. Mechanistically, the reduced systemic inflammation and decreased bone damage after treatment with BsAb in Tg197 mice and mice with LPS-induced bone erosion, respectively, probably emerge from inhibition of synergic effects of CXCL10 and TNF-α on the production of inflammatory and osteoclastogenic cytokines. Noteworthy, CXCL10-mediated potentiation of bone destruction and cartilage damage was further confirmed in healthy ICR mice [185]. An intra-articular injection of CXCL10-encoding retrovirus caused more extended bone erosion in ICR mice compared to injection of a control retrovirus [185]. In addition to the observation described in the aforementioned models of arthritis, CXCL10 was found to be upregulated in the synovium following trauma in rodents with post-traumatic arthritis [186, 187], a feature that was also observed in the articular cartilage of patients after articular fractions [186]. However, whether synovial CXCL10 has deleterious or protective actions following articular fractions was not investigated [186]. To conclude, the CXCL10-CXCR3 axis plays a pivotal role in the progression of experimental arthritis. The downstream in vivo actions of CXCL10 affected the physiology in rodent arthritis models on a systemic level (e.g., increased circulatory cytokines) and on localized levels in the joints (e.g., leukocyte homing, reduced bone integrity, articular cartilage damage and TH1 cell polarization), thereby propagating inflammation and progressive destruction of the joints.
Altogether, these findings may also potentially point towards a redundant functioning of the CXCR3 chemokine network in rodent models of arthritis [86]. First, genetic ablation of CXCR3 in CAIA-developing mice pronouncedly mitigated clinical arthritis symptoms, whereas targeted knock-out of CXCL10 alleviated clinical arthritis score to a lesser extent compared to CXCR3−/− mice [86]. Second, adoptively transferred self-specific CXCL10-targeting antibodies provided only partial protection against development of full-blown clinical rat AA [184]. These results indicate that the absence of CXCL10 may be partially overcome by other IFN-inducible CXCR3 ligands in experimentally induced arthritis. However, findings obtained in rodent models should be interpreted with considerable caution, especially those related to the murine IFN-inducible CXCR3 ligands. This notion is anchored on multiple levels. First, the widely used C57BL/6 mouse strain were reported to not endogenously express CXCL11 [84, 155, 188], as a result of an insertion of two base pairs located closely to the start codon that cause a frame shift leading to an early stop codon [188]. Therefore, the potential anti-inflammatory and autoimmune-restraining actions of CXCL11 related to CD4+ T cell polarization towards TH2 or IL-10high Tr1 cells may be overlooked in CAIA-developing C57BL/6 mice. Second, the absence of a CXCR3B splice variant in mice marks another important discrepancy [69]. Accordingly, the high failure rate of effective translational application of CXCR3 antagonists further confirms that extrapolation of CXCR3 ligand-related observations from rodents to humans is not straightforward [189, 190]. Third, targeted knock-out of CXCL9 and CXCL11 has not been explored yet in the framework of experimentally induced arthritis, despite marked upregulation of these chemokines in rheumatoid synovia [80, 102, 106, 124, 145]. Fourth, rodent arthritis models were considered to mimic human RA to a rather limited extent, given the predominance of neutrophils in human RA for which the chemokine receptor functioning profoundly differs between these species [191]. Nevertheless, some similarities between RA and mice models of arthritis with regard to the IFN-inducible CXCR3 ligands may be present, since comparison of single-cell RNA-sequencing (scRNA-seq) data of whole joints of 129/Sv mice with antigen-induced arthritis and gene microarray data of human joints revealed that the TH1 pathway was a mutual upregulated pathway with IFN-γ as the most significant shared upstream regulator [147]. Finally, mounting evidence points towards in vivo non-redundancy of the three IFN-inducible CXCR3 ligands [46], in particular also in rodent models mimicking autoimmune disease [192, 193]. In summary, complementary data from clinical settings is warranted to validate potential protective actions of blockage of the CXCR3 chemokine network in human inflammatory arthritides.
Drugs targeting the CXCR3 chemokine network in experimentally induced arthritis and rheumatoid arthritis
Development and patent claiming of CXCR3 antagonists reached its prime between 2001 and 2009 [189, 194–202]. Initially, the pronounced degree of homology between rodent and human CXCR3 prompted researchers to evaluate CXCR3-targeting compounds in various models of inflammatory and autoimmune diseases. Detailed overviews on the discovery and exploration of small-molecule CXCR3 antagonists have been published elsewhere [189, 190, 203, 204]. Herein, we discuss compounds targeting CXCR3 and its IFN-inducible ligands that were evaluated in the context of experimentally induced arthritis and human arthropathies (Table 3).
Table 3.
Drugs targeting the CXCR3 chemokine system evaluated in the context of experimentally induced arthritis and rheumatoid arthritis
| Target | Drug type | Drug name | Type of study | Demonstrated (pre)clinical efficacy | Arthritis-related findings | References |
|---|---|---|---|---|---|---|
| CXCR3 | Small-molecule CXCR3 antagonist | AMG 487 | Preclinical | N.D |
↓ invasion of FLS of RA patients in Matrigel by 60% ↓ invasion of FLS of Dark Agouti rats with pristane-induced arthritis in Matrigel by 77% ↓ production of active MMP-1 by FLS of Dark Agouti rats with pristane-induced arthritis ↓ CXCL10-induced Ca2+i mobilization in FLS of Dark Agouti rats and RA patients ↓ number of thick actin filaments, ↓ number of elongated cells, ↓ formation of polarized lamellipodia, ↓ co-localization of phospho-FAK with lamellipodia in FLS of Dark Agouti rats and RA patients |
[214] |
| Preclinical | Yes | Ameliorated severity of CIA | [174, 177, 178] | |||
| Preclinical | N.D. | ↓ invasion of RA patient-derived B cells towards synovial biopsy suspensions of RA patients in Matrigel-filled microchamber | [83] | |||
| Phase IIa | Unknown | Status of the trial for use of AMG 487 in patients with moderate to severe RA is unknown | [212] | |||
| JN-2 | Preclinical | Yes |
Ameliorated systemic inflammation and severity of CIA in CIA-developing mice ↓ CXCL10 mRNA expression, CXCL10 secretion and CXCL10-induced chemotaxis of mouse breast cancer 4T1 cells ↓ CXCR3 ligand-induced cell migration and CXCL10-mediated pro-inflammatory cytokine expression of CD4 + T cells and BMMs |
[176, 181] | ||
| SCH 546738 | Preclinical | Yes | Ameliorated severity of CIA | [175] | ||
| TAK-779 | Preclinical | Yes |
↓ incidence of CIA and ameliorated severity of CIA = IL-12 production and proliferation rate in presence of collagen by co-cultures of LN T cells and LN APC (isolated from of CIA-mice treated with TAK-799 or with vehicle) |
[179] | ||
| Small-molecule CXCR3 agonist | PS372424 | Preclinical | N.D. |
↓ migration of activated CXCR3+ human T cells towards CCL5, CXCL12, CXCL11 or RA SF in vitro ↓ migration of CD45+ human leukocytes towards air pouch filled with RA SF, CCL5, CXCL11 or CXCL12 in humanized mice |
[149] | |
| CXCL10 | Neutralizing mAb | MDX-1100 | Preclinical | Unknown |
Prevented in vitro actions of CXCL10: Inhibits CXCL10-induced cell migration Blocks CXCL10-induced Ca2+i mobilization Inhibits induction of CXCL10-responsive genes |
[230, 231] |
| Phase I | Unknown | Properly tolerated at different dose levels (0.1–10 mg/kg) and favorable half-life (10 days) in HC and patients with ulcerative colitis | ||||
| Phase II | Yes |
ACR20 response at day 85 in MDX-1100- and MTX-treated group (54%) > placebo and MTX-treated group (17%) = ACR-50 response, ACR-70 response, and DAS28 in MDX-1100- and MTX-treated group compared to placebo- and MTX-treated group |
ACR American College of Rheumatology improvement criteria, APC antigen-presenting cells, BMMs bone marrow-derived macrophages, CIA type II collagen-induced arthritis, CCL CC chemokine ligand, CXCL CXC chemokine ligand, CXCR CXC chemokine receptor, DAS28 Disease Activity Score in 28 joints, DMSO dimethyl sulfoxide, ERK extracellular-signal-regulated kinases, FLS fibroblast-like synoviocytes, HC healthy controls, IL interleukin, LN lymph nodes, mAb monoclonal antibody, MMP matrix metalloproteinase, MTX methotrexate, N.D. not determined, phospho-FAK phosphorylated Focal Adhesion Kinase (FAK), PKC Protein Kinase C, RA rheumatoid arthritis, SF synovial fluid
CXCR3-based therapies for arthritis
In the early 2000s, optimization of CXCR3 binding potency and pharmacokinetic characteristics of a quinazolinone-derived compound led to the identification of an 8-azaquinazolinone derivative AMG 487 [202, 205, 206]. This non-competitive CXCR3 antagonist was properly absorbed after oral administration and showed low-to-moderate clearance after IV administration in rats and dogs [202]. In addition, AMG 487 significantly suppressed CXCR3-dependent in vivo leukocyte trafficking in bleomycin-mediated lung inflammation [202]. However, AMG 487-derived metabolites had cytochrome P450 3A4 (Cyp3A4)-inhibitory activity [207]. Despite this drawback, AMG 487 progressed to a Phase IIa clinical trial to treat patients with severe psoriasis in 2003, but was withdrawn since treated patients did not exhibit significant improvement in physician global assessment scores or psoriasis severity index compared to placebo-treated patients [208]. The high variability in drug exposure was speculated to underlie the lack of clinical efficacy observed in this study [203, 208]. In addition, CXCR3 may not be an optimal drug target to combat psoriasis in hindsight [190], given the incompletely understood role of CXCR3 and its ligands in psoriasis and the potentially overlooked relevance of other upregulated chemokine receptors in this disease (e.g., CCR6 and CCR4) [209–211]. AMG 487 remains the only small-molecule CXCR3 antagonist to have entered clinical trials so far [189]. Moreover, a Phase II clinical trial to evaluate AMG 487 in patients with moderate to severe RA was intended to commence in 2004 [212]. However, it is unknown whether this trial was eventually started or what its contemporary status is [203]. Hence, the nature of the failure of AMG 487 in clinical trials for psoriasis—probably due to drug-intrinsic pharmacokinetic properties—may have discouraged further implementation of this compound and other CXCR3 antagonists in clinical trials [190]. Nevertheless, preclinical research on AMG 487 progressively continued in the framework of experimentally induced arthritis and RA. AMG 487 was found to be successful in combatting several arthritis-related aberrant processes in which the IFN-inducible CXCR3 ligands are involved. First, the CXCR3–CXCL10 axis plays an important role in mediating chemotaxis and invasion of FLS [213–215]. In this context, AMG 487 was shown to significantly diminish in vitro matrigel invasion of FLS originating from Dark Agouti rats with pristane-induced arthritis or from RA patients [214]. Furthermore, AMG 487 reduced active MMP-1 production by rat FLS in vitro, attenuated CXCL10-induced Ca2+i mobilization, and partially rehabilitated elongated into round morphology of Dark Agouti rat-derived and RA FLS [214]. Second, CXCR3 was found to majorly contribute to infiltration of memory B cells towards rheumatoid synovial tissue [83]. AMG 487 potently reduced in vitro invasion of RA patient-derived B cells towards medium containing an ex vivo synovial biopsy suspension of RA patients [83]. Moreover, recently published manuscripts have reported on the in vivo potential of AMG 487 to suppress synovitis and improve clinical arthritis scores in CIA-developing DBA/1J mice [174, 177, 178] (vide supra). In addition, AMG 487 showed disease-restraining effects in other murine disease models of steatohepatitis, metastatic breast cancer and traumatic optic neuropathy [216–218].
A second relevant small-molecule CXCR3 antagonist TAK-779 was found to exert potent antagonistic actions on multiple chemokine receptors including CCR5, CCR2, and CXCR3 [219, 220]. In 1999, TAK-779 was initially discovered as a CCR5 antagonist with powerful anti-HIV-1 activity [221]. The IC50-values representing TAK-779-mediated inhibition of chemotaxis induced by the respective ligand–receptor pairs CCL3–CCR5, CCL2–CCR2 and CXCL11–CXCR3 are 1.86 nM [219], 5.78 nM [219] and 15.8 µM [220], respectively. In the context of CIA, TAK-779 partially suppressed CIA development and assuaged severity of the CIA arthritis phenotype [179]. The protective effects of TAK-779 in experimentally induced arthritis were attributed to its chemotaxis antagonistic actions since leukocyte ingress in joint lesions was drastically reduced (vide supra) [179]. The drug did not affect in vitro IL-12 production, proliferation of T cells or antigen-presenting cells (APC) isolated from lymph nodes of TAK-779-treated CIA-developing mice, nor CCR5 induction or anti-collagen response of their T cells [179]. In addition, TAK-779 also displayed substantial efficacy in other disease models, including preclinical models of experimental autoimmune encephalomyelitis [222], colitis [223], ischemia reperfusion injury [224] and cardiac allograft vasculopathy [225]. Despite its promising potential, TAK-779 was poorly absorbed after oral intake and attempts to modify TAK-779 into a compound with improved oral bioavailability resulted in loss of CXCR3 antagonistic activity [190, 220, 226]. Due to these pharmacokinetic inadequacies, exploration of TAK-779 was not pursued in the clinical setting.
Furthermore, the non-competitive small-molecule CXCR3 antagonist SCH 546738 is a oxadiazole-5-aminopyrazine derivate [227, 228] with remarkably high affinity for CXCR3 (0.4 nM) [175]. SCH 546738 potently inhibited CXCR3 binding of human CXCL10 and CXCL11 (IC50 of SCH 546738 ~ 1–2 nM) with additional cross-species activity [175]. In addition, SCH 546738 effectively suppressed CXCR3 ligand-mediated chemotaxis and had an adequate pharmacokinetic profile in rodents. Also, SCH 546738 induced an ameliorated phenotype in CIA, marked by attenuated synovitis and decreased cartilage and bone destruction (vide supra) [175]. In other rodent autoimmune models including mouse and rat experimental autoimmune encephalomyelitis, SCH 546738 also exhibited clinical efficacy [175]. Similar to AMG 487, SCH 546738 also reversed steatohepatitis in a preclinical study [218]. However, translation of SCH 546738 towards clinical trials has not been reported. A potential reason for this halt may be that SCH 546738 exhibited undesirable inhibitory activity on Human Ether-a-go-go Related Gene (hERG) kalium (K+) channel [228]. Recently, another promising small-molecule CXCR3 antagonist JN-2 was developed [181]. JN-2 emerged as a result of incorporation of an amide side chain into a benzoxazole-derived lead compound of Abbot Laboratories with CXCR3 antagonistic activity, in order to resemble AMG 487 [181]. Intriguingly, JN-2 suppressed CXCL10 mRNA expression, CXCL10 secretion and CXCL10-induced chemotaxis of 4T1 cells, which is a mouse breast cancer cell line [181]. Moreover, JN-2 was speculated to indirectly inhibit osteoclast formation in co-cultures in vitro and prevented 4T1 cell-induced bone destruction in BALB/c mice [181]. Accordingly, JN-2 induced less bone erosion and cartilage damage in CIA-developing DBA/1 mice, in addition to alleviation of clinical disease and reduction of CIA-induced pro-inflammatory cytokines (vide supra) [176]. Also, JN-2 counteracted CXCR3 ligand-induced cell migration and CXCL10-mediated pro-inflammatory cytokine expression of CD4+ T cells and bone marrow-derived macrophages (BMMs) in vitro [176]. No further information was published on whether JN-2 was explored in terms of clinical efficacy.
Counterintuitively, another route that was explored is the implementation of CXCR3 agonists. During a high-throughput screening for CXCR3 antagonists, a small-molecule CXCR3 agonist PS372424 was identified [229]. PS372424 contains a tetrahydroisoquinoline–arginine motif, on which its CXCR3 agonistic activity relies on [229]. This motif closely resembles the Pro-Arg dipeptide in the 30s loop of CXCL10, which is a key interaction site enabling CXCL10-mediated CXCR3 activation [182]. Intriguingly, PS372424 significantly reduced in vitro chemotaxis of activated human CXCR3+ T cells towards CCL5, CXCL11 or CXCL12, and also towards SF of RA patients. PS372424 also attenuated in vivo human leukocyte ingress in an air pouch filled with multiple chemokines or SF of RA patients in a humanized mouse air-pouch arthritis model (vide supra) [149]. In search for the underlying mechanisms of its activity, PS372424 was found to elicit cross-phosphorylation in a dose-dependent manner of CCR5 on CXCR3+—but not CXCR3−—activated T cells in vitro. Hence, the broad antagonistic activity of PS372424 in chemotaxis experiments was attributed to CXCR3-mediated cross-phosphorylation of CCR5 in CCR5–CXCR3 heterodimers [149]. In addition, the compound also decreased in vivo cell-surface expression of CXCR3 and CCR5 on splenic human T cells in immunodeficient mice with human PBMCs [149]. Thus, PS372424 plausibly desensitizes downstream CCR5 signaling and induces internalization of CXCR3, thereby reducing directional trafficking of CCR5+ CXCR3+ T cells.
CXCL10-based therapies for arthritis
In 2010, a phase II randomized, placebo-controlled study was performed to asses safety and clinical efficacy of eldelumab/MDX-1100—a fully human, neutralizing monoclonal antibody against CXCL10—in RA patients that responded insufficiently to methotrexate (Table 3; ClinicalTrials.gov, Identifier: NCT01017367) [230]. MDX-1100 exhibited selective and high-affinity binding for CXCL10, but not for CXCL9 or CXCL11 [230, 231]. Thereby, the drug blocked in vitro actions of CXCL10, including Ca2+i mobilization, induction of CXCL10-responsive genes and leukocyte chemotaxis in preclinical studies [230, 231]. Moreover, phase I single-dose studies proved that MDX-1100 was properly tolerated by healthy volunteers and patients with ulcerative colitis at various dose levels [230, 231]. In the phase II trial, MDX-1100-treated RA patients had significantly improved response according to the American College of Rheumatology 20% improvement criteria (ACR-20) on day 85 compared to the placebo-treated cohort [230, 232]. Each of the ACR core components, except for ESR, was improved compared to baseline in MDX-1100-treated patients compared to placebo-receiving patients [230]. However, ACR-50, ACR-70 and change of DAS28 over a period of 85 days were not ameliorated in the MDX-1100-receiving group compared to placebo-treated patients [230]. As such, the well-tolerated MDX-1100 was speculated to have favorable effects in RA, irrespective of alleviating systemic inflammation [6]. Despite moderate clinical benefit and adequate safety profile, final data on MDX-1100 or details on continuation towards phase III studies was not reported [233]. In addition, MDX-1100 also showed modest efficacy in patients with moderate to severe ulcerative colitis in Phase II clinical trials (NCT00656890) [234], but again no further data were released.
Targeting the CXCR3 chemokine network in arthritis: challenges and future perspectives
Defining new curative therapeutic targets marks a critical unmet need in refractory RA with suboptimal response to disease-modifying anti-rheumatic drugs (DMARDs) [235]. Despite the promising potential of many small-molecule CXCR3 antagonists developed over the last two decennia and their preclinical evaluation, only one compound progressed to the clinical setting [189]. This omnipresent reluctance towards clinical translation of CXCR3 antagonists has multiple reasons. First, the highly expensive failure of AMG 487 deterred others to continue in the same direction, consequently grounding clinical trials on CXCR3 antagonist to a halt [190]. Second, pharmacokinetic inadequacies often caused discontinuation of propitious CXCR3-targeting molecules (e.g., TAK-799). Third, CXCR3 targeting in autoimmunity and inflammation is speculated to be controversial to some extent, given the plausibility of concomitant inactivation of immunosuppressive effects mediated by CXCL11 [236]. These notions underscore the complicated pharmacology of the CXCR3 chemokine network and sparked the idea for another relevant strategy to tackle CXCR3, i.e., to disentangle biased signaling downstream of CXCR3 [237–239]. For example, boronic-acid based compounds that selectively inhibit either activation of G proteins or β-arrestin 2 recruitment may be relevant to combat autoimmune diseases [238, 239]. Thus, novel studies investigating CXCR3-targeting compounds including AMG 487, JN-2 and boronic-acid derivatives corroborate that CXCR3 antagonism remains a potential avenue to therapeutically restrain and relieve joint inflammation.
Noteworthy, high failure rates of drugs targeting the chemokine system, especially in RA, have demonstrated the importance of complete comprehension of chemokine functioning in a specific pathogenesis, thereby enabling precise selection of a valid target in that particular disease [191]. This concept is further illustrated by the lack of efficacy of CCR5 antagonist maraviroc in phase IIa clinical trials for patients with RA (NCT00427934), despite its successful application for HIV-1 treatment [240]. Especially, also in the context of MDX-1100, the absence of in-depth insight in the in vivo actions and reciprocal intercommunion of the three IFN-inducible CXCR3 ligands in arthritis marks an important knowledge hiatus. In addition, processing of chemokine ligands leading to posttranslationally modified forms (proteoforms) of IFN-inducible CXCR3 ligands and the implication of their structural heterogeneity on chemokine functioning—generating weak agonists or even antagonists—have remained incompletely understood in vitro and in vivo. Moreover, the unidentified predilection of MDX-1100 for distinct CXCL10 proteoforms further hampers a correct understanding of the mechanism of action of MDX-1100 in the pathogenesis of RA. Therefore, identification of CXCR3 ligand and receptor forms in synovial tissue/fluid and correlation to their biological activity is vital to fully grasp the functioning of the CXCR3 chemokine network in arthritis. Localized enzyme targeting may be a promising therapeutic strategy to prevent or promote selective processing of CXCR3 ligands into disease-contributing or inflammation-restraining proteoforms, respectively. For example, CD26 inhibitors sitagliptin and linagliptin are tested in combination with immune checkpoint inhibitors in Phase Ib/II clinical trials for gastric, esophageal and non-small cell lung cancer (NCT03281369; NCT03337698). These drugs would prevent CXCL10-mediated processing into the chemotaxis antagonist CXCL10(3–77), thereby facilitating CXCR3+ T cell tumoral ingress and potentiating the actions of immune checkpoint inhibitors [241].
Juvenile idiopathic arthritis
Juvenile idiopathic arthritis (JIA) encompasses a heterogenous group of chronic arthritides of unknown origin that commence before the age of 16 years [114, 242]. Seven distinct subsets of JIA were specified based on the number of affected joints and associated extra-articular manifestations (international classification criteria described elsewhere [243]). Far-reaching consequences that emerge as a result of this chronic disease—if not recognized timely—include reduced physical functioning, decreased societal participation and long-term psychological burdening [244–247]. As major players in the inflammatory response, a plethora of chemokines have been implicated in JIA [134, 248–253]. In particular, the IFN-inducible CXCR3 ligands-—especially CXCL10—were recurrently reported to contribute to the inflammatory synovial milieu in JIA by, inter alia, mediating inflammatory cell homing (Suppl. table 2) [248, 249, 251–254].
Oligo- and polyarticular JIA
Oligoarticular JIA is overall the most common JIA subtype, representing 50–80% of JIA cases in the Caucasian population (Fig. 2A) [114, 255]. Oligoarthritis is defined as arthritis affecting one to four joints during the first six months of disease, whereby persistent and extended forms of oligoarticular JIA are distinguished based on either less or more than four joints affected after the first 6 months of disease, respectively [243]. Furthermore, polyarticular JIA is identified as arthritis affecting five or more joints during the first 6 months of disease, in which the presence of rheumatoid factor (RF) enables to discriminate between RF-positive and RF-negative polyarthritis [243]. Although oligo- and polyarticular JIA have thus limited systemic involvement, the interchange of inflammatory mediators between SF and blood makes the circulation a valuable anatomic depot to explore.
Circulatory IFN-inducible CXCR3 ligands in oligo- and polyarticular JIA
In general, plasma CXCL9 and CXCL10 levels were found to be elevated during active disease in oligo- and polyarticular JIA in comparison to plasma of HC [134, 248, 249] and children with non-active JIA (Suppl. table 2) [248]. Cluster analysis based on plasma cytokine levels demonstrated a predominant pro-inflammatory cytokine cluster during active JIA, which included CXCL9 and CXCL10 [248]. Moreover, discrimination analysis revealed a panel of plasma cytokines including CXCL9 and CXCL10 to stratify and distinguish three different JIA subtypes during active disease, i.e., systemic JIA (sJIA), oligoarticular JIA and polyarticular JIA [248]. Accordingly, serum CXCL10 also had an improved diagnostic sensitivity and specificity for the prediction of JIA compared to serum CXCL8 in 79 Iraqi patients with oligo- or polyarticular JIA [134]. Noteworthy, protein fingerprinting revealed that CXCL9 and CXCL10 plasma levels showed a relatively limited increase in oligo- and polyarticular compared to the most prominently upregulated circulatory chemokines CCL5 and CCL18 [248]. Taken together, plasma CXCL9 and CXCL10 among other chemokines were speculated to reflect clinical disease activity in JIA and were proposed to be useful surrogate parameters to monitor disease activation status and response to treatment [248].
Synovial IFN-inducible CXCR3 ligands in oligo- and polyarticular jia
In SF of patients with oligoarticular and polyarticular JIA, CXCL9 and CXCL10 concentrations were found to be significantly augmented either compared to paired plasma of these patients [248, 249] or to SF of patients with traumatic joint injury or hip/skeletal dysplasia [252], respectively (Suppl. table 2). Protein fingerprinting illustrated that CXCL10 concentrations in SF of oligo- and polyarticular JIA were profoundly elevated compared to other upregulated chemokines including CCL2, CCL3, CCL5, CXCL8 and CXCL9 [248]. Also, CXCL10 mRNA expression was increased in T cells from SF compared to peripheral blood T cells [249]. These data signify that CXCL9 and CXCL10 may also establish a chemotactic gradient from the blood towards the inflamed joints in JIA. Indeed, similar to RA, enhanced proportions of CXCR3+ T cells were detected in SF compared to peripheral blood of patients with oligo- and polyarticular JIA [251, 253, 254, 256, 257]. In addition, CXCR3 expression levels were increased on synovial T cells compared to peripheral blood T cells [253]. These findings implicate selective trafficking of CXCR3+ T cells into the inflamed articulation in oligo- and polyarticular JIA [251, 253, 256]. The latter process is suggested to be coordinated, at least in part, by synovial IFN-inducible CXCR3 ligands [251, 252] and in particular by CXCL10 [249, 253]. This concept is supported by the notion that cell-free SF isolated from four patients with oligoarticular JIA exhibited chemotactic activity on 300-19 pre-B cells transfected with CXCR3, [253]. The SF-mediated chemotaxis of CXCR3+ 300-19 lymphocytes was inhibited upon addition of a neutralizing antibody against CXCL10 [253]. In addition to corroborating the presence of biologically active CXCL10 in SF of JIA patients, this finding further underscores that CXCL10 is involved in CXCR3+ cell chemo-attraction towards the inflamed joints [253]. Moreover, purified synovial T cells of two oligoarticular JIA patients displayed significantly enhanced ex vivo chemotaxis in response to CXCL10 in a Boyden Chamber [253]. Accordingly, CXCL11 also engendered directional trafficking of synovial T cells of JIA patients [251, 254]. First, CD4+ CD45RO+ memory T cells—which were freshly purified from SF of eight oligoarticular JIA patients—exhibited significant migration to CXCL11 in a transwell plate [251]. Second, synovial CD4+ T cells of oligo- and polyarticular JIA patients transmigrated upon CXCL11 stimulation in a transwell filter assay ex vivo [254]. In summary, CXCL9 and CXCL10 are upregulated in the circulation and, to an even greater extent, in SF of patients with oligo- and polyarticular JIA [248, 249, 252]. Thereby, CXCL9, CXCL10 and potentially CXCL11 constitute a gradient from the blood towards the synovial compartment [249], along which CXCR3+ inflammatory cells migrate from the circulation to the inflamed joints [251, 253, 256].
In terms of CXCR3-expressing cells, immunohistochemistry revealed that CXCR3+ cells were predominantly localized in the perivascular area and organized in a follicular pattern close to the luminal surface in synovium of JIA patients [253]. Similarly, others have described CXCR3 immunolocalization predominantly in lymphocyte-infiltrated area’s including lymphoid aggregates and perivascular infiltrates of the sublining layer of the synovial membrane [251]. Also, CXCR3 mRNA expression in synovial tissues of oligoarticular JIA patients were significantly higher relative to those of pediatric patients with bone dysplasia, bone fracture or hexadactylism [253]. A potential mechanism underlying this prominent CXCR3 expression on synovial T cells is that persistent antigen stimulation in the synovial niche causes in vivo T cell hyperreactivity and consequently, this aberrantly high CXCR3 expression profile is instigated and maintained through triggering of specific TCRs [253, 256]. In addition, previous studies have demonstrated that activated T cells assemble around activated DC in the JIA synovium [258, 259], which may explain the organization of CXCR3+ activated T cells in clustered lymphoid aggregates. An additional plausible reason for this characteristic synovial tissue distribution of CXCR3+ cells is ectopic germinal center formation [260]. Moreover, CXCR3 and CCR7 exhibited a similar pattern of synovial tissue distribution, in particular in these lymphocyte aggregates [251]. This finding combined with the established upregulation of their respective ligands (i.e., CXCL9, CXCL10 and CCL21[251]) in the inflamed joints suggests potential synergism of these chemokines in synovial recruitment of CCR7+ CXCR3+ memory T cells [251]. Similarly, CXCR3 expression was also enhanced on synovial CCR7+ memory B cells compared to those in the peripheral blood in oligo- and polyarticular JIA [261]. Conceivably, CXCR3 may be involved in synovial homing of memory T and B lymphocytes.
Furthermore, synovial tissue specimens of oligoarticular JIA patients exhibited strong immunoreactive staining for CXCL10 [253], whereas limited to absent expression was observed in synovial tissue biopsies of children with noninflammatory arthropathies [253]. CXCL10 was mostly expressed on the surface of CD68+ macrophages, but also on endothelial cells and epithelial-like cells in synovial tissues [253]. Accordingly, RT-PCR revealed that CXCL10 was primarily transcribed by myeloid cells, and not the lymphoid cells, within the peripheral blood and SF of patients with JIA [249]. Thus, the prevailing paradigm that macrophages are a major source of CXCL10 [262] seems to be also applicable to the synovial microenvironment in JIA. In addition, neutrophils are the most abundant immune cell present in SF of oligoarticular JIA [263, 264]. Isolated neutrophils of HC are also described to produce CXCL9 and CXCL10 in response to pro-inflammatory cytokines TNF-α and IFN-γ [265, 266], which are cytokines known to be upregulated in JIA SF [267]. Also, CXCL10 and CXCL11 mRNA expression in synovial monocytes of patients with oligoarticular JIA was more than twofold increased relative to paired peripheral blood monocytes [268]. Hence, neutrophils and monocytes may also constitute important cellular sources for IFN-inducible CXCR3 ligand secretion in JIA synovium. Intriguingly, ex vivo cultures of FLS isolated from synovial tissue of JIA patients secreted higher levels of various chemokines—including CXCL9 and CXCL11—compared to FLS obtained from patients with non-arthritic joint disease undergoing orthopedic procedures [252]. In conformity with RA, this finding indicates a role for FLS as perpetuators of joint inflammation in JIA via the release of large numbers of chemokines including CXCL9 and CXCL11 [252]. Moreover, in an attempt to discover biomarkers that enable to discriminate children with oligoarticular JIA that will proceed to develop the extended phenotype, CXCL9 was identified as a differentially expressed and highly upregulated gene in SF mononuclear cells (SFMCs) of children with oligoarticular JIA, which later on developed the extended phenotype [250]. Altogether, multiple cell types—including macrophages, monocytes, SFMCs, FLS and potentially neutrophils—presumably act in concert to enable expression of IFN-inducible CXCR3 ligands in the inflamed articulation of JIA patients.
Systemic juvenile idiopathic arthritis
Systemic JIA (sJIA) is a rare, pediatric autoinflammatory disorder that is characterized by quotidian spiking fever, evanescent erythematous skin rash, and chronic arthritis/arthralgia [243, 269], which is frequently symmetrical and polyarticular (Fig. 2A) [114]. Being initially defined as Still’s disease [270], sJIA was found to be clinically distinct from other JIA subtypes due to the presence of systemic extra-articular manifestations [243, 269, 271]. As a result of these prominent systemic clinical features combined with genetic associations of innate immune pathways and the absence of auto-antibodies, this intriguing childhood arthropathy is classified as an autoinflammatory disease [242, 269], which further underscores its distinctive character compared to other JIA subtypes. Despite accounting for approximately 10% of the JIA cases in North America and Europe [272], sJIA contributes disproportionately high to JIA-associated mortality. This striking feature primarily relies on a life-threating hyperinflammatory complication that emerges as a clinically overt disease in ~ 10% of sJIA cases and is termed macrophage activation syndrome (MAS) [273, 274]. However, subclinical or occult MAS is reported to occur in 30–40% of patients with sJIA [275, 276]. MAS arises as a result of uncontrolled and widespread activation and expansion of T cells and macrophages, provoking a profound hypersecretion of pro-inflammatory cytokines [277]. Although MAS can occur secondary of multiple autoimmune conditions, most cases of MAS are associated with sJIA or its adult counterpart, adult-onset Still’s disease (AOSD) [278] (vide infra). In addition to the immunologic resemblance, the shared clinical and laboratory aberrations of MAS and hemophagocytic lymphohistiocytosis (HLH) provides the basis for classification of MAS among secondary or acquired HLH [278, 279]. HLH is defined as a clinical syndrome of uncontrolled and pathogenic immune activation characterized by hyperinflammation and accumulation of mononuclear cells with a macrophage phenotype (i.e., histiocytes) exerting hemophagocytosis [277, 279–281]. The primary involvement of IFN- in the etiopathogenesis of HLH has been extensively substantiated [280–285]. However, despite similarities in terms of systemic inflammation between HLH and sJIA [269], the role of IFN- has remained incompletely understood in sJIA (Suppl. table 2) [286]. On the one hand, a limited role for IFN- and the three IFN-induced chemokines has been speculated in active sJIA in the absence of clinical MAS [287–289]. On the other hand, mounting evidence points towards a key pathogenic role of IFN- in MAS secondary to sJIA [280, 288, 290, 291].
IFN-inducible CXCR3 ligands in active sJIA in the absence of clinical MAS
The prevailing hypothesis of a minimal involvement of IFN- and the IFN-inducible CXCR3 ligands in active sJIA without clinical MAS is supported by several findings [287–289], but is also questioned to some extent [292, 293]. First, mRNA levels of CXCL9, CXCL10 and CXCL11 were drastically lower in synovial tissue of patients with active sJIA compared to extended oligoarticular JIA by 15.8-, 4.5- and 3.6-fold, respectively [287]. In addition, synovial CXCL9 and CXCL10 concentrations were decreased in active sJIA compared to oligoarticular JIA, although significance was not reported [248]. Second, the absence of an IFN- gene signature in PBMCs of patients with active sJIA without clinical MAS was recurrently corroborated [287, 289, 290, 294]. Gene expression of CXCL9 and STAT1 were significantly reduced in isolated and unstimulated CD14+ PBMCs of patients with active sJIA without MAS compared those of HC [287]. Additionally, mRNA levels of CXCL10, CXCL11, and interferon regulatory factor 1 (IRF1) tended to decrease in CD14+ peripheral blood monocytes of patients with active sJIA relative to HC, although not significantly [287]. Of note, IRF1 induces secondary IFN-γ-responsive genes and thereby normally perpetuates the IFN-γ signal [295]. In addition, gene expression profiling of PBMCs purified from patients with untreated new-onset active sJIA revealed that IFN--responsive genes were not upregulated compared to healthy pediatric controls [289]. Similarly, these genes were not upregulated in PBMCs of patients with active sJIA compared to inactive sJIA [294]. Also, incubation of PBMCs of healthy adult and pediatric controls with serum of four patients with active sJIA did not increase gene expression of IFN- or IFN-induced CXCR3 ligands as compared to incubation with autologous serum isolated from HC [296]. Noteworthy, some patients (29%) had subclinical MAS in one gene expression study [289], whereas in the other two gene expression studies the presence of clinical or subclinical MAS was not reported [294, 296]. Hence, despite these ambiguities in terms of the presence of subclinical MAS, PBMCs of patients with active JIA in the absence of clinical MAS probably display a lack of IFN--induced gene expression. Furthermore, downstream responses upon IFN stimulation in PBMCs of patients with active sJIA without MAS appeared to be unaffected [287, 290, 297]. For example, when purified peripheral blood monocytes of active sJIA and HC were pre-stimulated with IFN-, these cells displayed comparable mRNA levels of CXCL9, CXCL10, IRF1 and STAT1 in active sJIA compared to those of HC [287]. In addition, isolated populations of CD14− PBMCs (i.e., lymphocytes, NK cells and low density granulocytes) of patients with active sJIA also had only a minimal IFN-induced chemokine gene expression profile in the absence of stimuli and responded similarly to IFN- stimulation compared to those cells of HC [287]. Accordingly, others confirmed that incubation of PBMCs of patients with inactive and active sJIA with IFN- significantly increased CXCL10 mRNA expression and augmented CXCL10 and CXCL9 protein secretion compared to the respective unstimulated cells [290]. In addition, anakinra treatment of patients with active sJIA instigated upregulation of type I IFN-inducible genes in PBMCs, which was irrespective of the clinical response to anakinra [297]. More specially, CXCL10 gene expression in PBMCs and serum levels of CXCL10 were clearly elevated in patients with active sJIA after anakinra treatment compared to baseline [297]. Thus, PBMCs of active sJIA patients seem to be equally responsive to ex vivo IFN- stimulation compared to those of HC and are capable of upregulating downstream IFN-inducible genes in vivo. Hence, the lack of IFN- signature detected in PBMCs of these patients was speculated to be rather due to limited exposure to IFN- in vivo, lack of responsiveness to IL-18 due to altered phosphorylation of the IL-18 receptor, or the presence of IFN- inhibiting factors [287, 289, 298, 299].
Third, Bracaglia et al. reported similar serum levels of IFN- and all three IFN-inducible CXCR3 ligands in patients with active sJIA without MAS and clinically inactive sJIA [288]. However, whether circulatory levels of IFN- and IFN--upregulated chemokines are augmented in active sJIA in the absence of clinical MAS remains a disputable notion [248, 288, 290–293]. On the one hand, the absence of IFN-γ signature in PBMCs of patients with active sJIA without clinically overt MAS (vide supra) and the findings of Bracaglia and colleagues indicate a lack of significant upregulation of circulatory IFN- and IFN-induced CXCR3 ligands in active sJIA without clinical MAS. Also, de Jager et al. found reduced plasma IFN-, CXCL9 and CXCL10 concentrations in active sJIA compared to active oligo- and polyarticular JIA subtypes, whereas only plasma CXCL10 levels were significantly lower in active sJIA compared to active oligoarticular JIA [248]. Noteworthy, whether patients with sJIA had MAS at the time of sampling was not specifically defined in this study [248]. On the other hand, increased circulatory levels of IFN- and CXCL10 were observed in serum of patients with active sJIA without clinical MAS compared to HC [248, 290, 293] and inactive sJIA [248, 290], although these differences were only significant in one study [293]. Noteworthy, in the study of Bracaglia et al., comparison of serum levels of these chemokines in active sJIA patients in the absence of MAS to those of HC was not performed [288]. Moreover, a recent study reported that plasma CXCL11 was significantly upregulated in active sJIA without MAS compared to inactive sJIA and HC [292]. Plasma levels of CXCL9 and CXCL10 were also moderately—although not significantly—increased in active sJIA compared to HC [292]. Random forest analysis identified plasma levels of CXCL11, CXCL9 and CXCL10 as, respectively the 15th, 27th and 66th most important proteins out of 69 pre-selected immune-related proteins, to differentiate active sJIA in the absence of MAS, inactive sJIA and HC [292]. Moreover, paired analysis revealed that CXCL11 was the 5th most important protein out of 11 significantly upregulated plasma proteins in active sJIA without MAS compared to inactive sJIA that enabled to distinguish active sJIA in the absence of MAS from inactive sJIA [292]. Therefore, CXCL11, among these ten other inflammatory molecules, was proposed to be a novel biomarker to discriminate patients with active sJIA without MAS from inactive sJIA or HC [292]. In addition, dysregulation of IL-18-IFN-γ-CXCL9 axis was observed in patients with active sJIA without MAS [298, 300]. Serum levels of CXCL9 and CXCL10 were significantly increased in active sJIA without MAS at baseline visit compared to HC [298]. High ratios of serum IL-18: CXCL9 and IFN-γ: CXCL9 were found to correlate with persistent response to canakinumab therapy in active sJIA [298, 300].
Fourth, in an established mouse model of sJIA, targeted knock-out of IFN- aggravated clinical and pathological symptoms reminiscent of sJIA in Complete Freund’s adjuvant (CFA)-challenged BALB/c mice [301]. Moreover, IFN-−/− BALB/c mice displayed arthritis, hemophagocytosis and increased serum levels of IL-6 upon injection of CFA, whereas WT mice did not exhibit these features [301]. These data suggests that IFN- may have a protective role in this rodent model of sJIA [286]. Conversely, targeted knock-out of IFN-γ or treatment with IFN-γ neutralizing antibodies largely abolished symptoms of MAS-like syndrome in WT C57BL/6 mice repeatedly stimulated with Toll-like receptor (TLR) 9 ligand CpG DNA [302]. Accordingly, a treatment with an IFN- neutralizing antibody increased survival of IL6-transgenic mice challenged with TLR4 ligand LPS [303], which is another murine model of infection-triggered MAS [304]. These findings signify that IFN-γ functions as a driver in these rodent models of MAS-like disease. Consequently in animal models, IFN- is considered the double-edged sword mediating dichotomy dependent on the genetic background of the animal [286]. On the one hand, IFN- may facilitate immunopathology in mice strains mounting intrinsically potent TH1 cell responses, i.e., mice with C57BL/6 background (e.g., IL6-transgenic mice). On the other hand, IFN- may adequately inhibit IL-17-dominant inflammation in mice strains that are less genetically primed to develop potent TH1 responses (e.g., BALB/c mice). This provides a plausible explanation why IFN- has its respective sJIA-restraining actions and MAS-promoting effects in these distinct mice models.
Taken together, accumulating evidence implies a limited involvement of IFN- and its downstream-induced chemokines in active sJIA in the absence of clinical MAS. However, a definite conclusion is still lacking, since contravening data suggesting upregulated IFN- and IFN-induced CXCR3 ligands in both sJIA without MAS and AOSD in the absence of MAS (vide infra) fuel this ambiguity. In addition, the fact that the occurrence of clinical MAS in sJIA is occasionally not reported and that the presence of subclinical MAS is often not examined in patients with sJIA in these studies further complicates correct interpretation of these data and may hamper to draw accurate conclusions.
IFN-inducible CXCR3 ligands in sJIA-associated MAS
Emerging evidence hints towards a pivotal role of IFN- and IFN-inducible CXCR3 chemokines in MAS secondary to sJIA [280, 288, 290, 291]. First, serum levels of IFN- and the three IFN-inducible CXCR3 ligands were significantly augmented in patients with sJIA with clinical MAS compared to patients with active sJIA without clinical MAS [288]. Remarkably, serum concentrations of CXCL9 were elevated by approximately 15-fold in sJIA patients with clinical MAS relative to those without clinical MAS. Elevated CXCL9 levels in the serum were found to predispose MAS in sJIA, whereby also trends towards increased CXCL10 levels in serum of future MAS patients compared to patients with sJIA that did not proceed to develop MAS were present [298]. Furthermore, circulatory levels of IFN-, CXCL9, CXCL10 and CXCL11 were relatively comparable in sJIA patients with MAS and patients with secondary HLH [288]. Accordingly, others reported significantly elevated plasma concentrations of IFN- and CXCL10 in five patients with HLH—of which three patients had sJIA-associated MAS, one patient had primary HLH and one patient had Epstein-Barr virus (EBV)-associated HLH—compared to inactive sJIA and HC [290]. In addition, plasma levels of IFN- and CXCL10 were higher in these five patients with HLH relative to active sJIA, although not significant [290]. Taken together, IFN- and the IFN-inducible CXCR3 ligands are markedly enhanced in patients with sJIA complicated by clinical MAS. Individual cases of patients with overt MAS further corroborated this [288]. One patient was sampled at three clinical episodes of MAS and during intervals of clinical remission [288]. Serum levels of IFN-γ and all three IFN-inducible CXCR3 ligands were prominently increased during clinical MAS episodes compared to periods of remission, although significance was not evaluated. Similarly, a patient with severe MAS-associated central nervous system involvement was reported to have extremely elevated serum levels of CXCL9 and CXCL10 of 549.4 ng/ml and 35.1 ng/ml, respectively [288]. Second, analysis of paired samples of patients with active sJIA—that were obtained during clinically overt MAS and after clinical MAS had subsided—revealed that circulatory levels of IFN-, CXCL9, CXCL10 and CXCL11 diminished upon resolution of MAS [288]. Third, serum concentrations of CXCL9, CXCL10 and IFN- in sJIA patients suffering from MAS correlated with laboratory measures that are typically elevated in MAS, including serum levels of ferritin, lactate dehydrogenase (LDH), and alanine aminotransferase (ALT) [288, 305]. Circulatory CXCL11 levels also correlated with serum ferritin and LDH levels in sJIA patients with MAS [288, 305]. Accordingly, splenic and hepatic CXCL9 gene expression positively correlated with serum ferritin levels in MAS-like syndrome-developing IL6-transgenic mice stimulated with TLR4 ligand LPS [288].
Furthermore, fibroblasts, endothelial cells and PBMCs were identified as potential sources of IFN-inducible chemokines in sJIA-associated MAS [290]. In situ CXCL10 immunoreactivity in a lymph node was profoundly increased during an overt MAS episode compared active sJIA without MAS, whereby endothelial cells, fibroblasts and histiocytes stained for CXCL10 [290]. Moreover, IFN- stimulation of PBMCs of sJIA patients with MAS failed to substantially elevate mRNA levels of CXCL10 and protein secretion of CXCL9 and CXCL10 relative to unstimulated cells, suggesting ex vivo cellular hyporesponsiveness due to functional exhaustion [290]. In addition, IFN--producing lymphocytes were detected in a liver biopsy of a patient with sJIA-associated MAS [280].
In summary, markedly increased circulatory levels of IFN-γ and all three IFN-inducible chemokines is a hallmark feature of clinically overt MAS during disease course of active sJIA. Given the correlation of serum CXCL9 (and CXCL10) with various MAS-associated manifestations, circulatory levels of CXCL9—and presumably CXCL10—may reflect localized IFN-γ upregulation in the inflamed tissues and downstream induction of IFN-inducible CXCR3 ligands that subsequently leak in the circulation [288]. Hence, this biomarker potential of serological CXCL9 in MAS has been reported to be explored in a large multicenter study [288]. Moreover, hyperproduction of CXCL9 and IFN-γ has been observed in patients with other forms of HLH [306], thereby suggesting that this profound upregulation of CXCL9 and IFN-γ is a uniform feature of HLH, irrespective of the underlying etiology [288]. Accordingly, an IFN-γ neutralizing antibody (NI-0501/emapalumab) exhibited an acceptable safety profile and efficacy in a phase II/III clinical trial for children and adults with primary HLH [307] (NCT01818492, NCT03985423 and NCT02069899) and was approved in 2018 in the US for pediatric and adult HLH refractory to conventional treatment [308]. In 2022, a phase II clinical trial evaluating emapalumab in sJIA-associated MAS was completed (NCT03311854) and showed that emapalumab was effective in controlling MAS [309].
Mechanistically, augmented circulatory levels of IFN-γ may result from high levels of IL-18 [288], which is a cytokine that is primarily important for the induction of IFN-γ production in T cells and NK cells [310]. Indeed, patients with active sJIA that had increased levels of circulating IL-18 at disease onset were reported to be significantly more prone to develop MAS [311], suggesting that high levels of serum IL-18 predispose MAS development in active sJIA [311]. Furthermore, predominant upregulation of serum CXCL9 relative to the other IFN-inducible CXCR3 ligands during MAS episodes most plausibly resides on the presence of the γ-interferon response element (γIRE) in the CXCL9 promotor [312–314], thereby rendering CXCL9 protein expression exclusively dependent on IFN-γ [46]. In contrast, CXCL10 and CXCL11 promotors contain an interferon response element (IRSE) [312, 315–317] and consequently, their expression is mediated by type I and type II interferons [318, 319]. Indeed, increased circulatory levels of IFN-γ in sJIA patients suffering from MAS strongly and positively correlated with serum concentrations of CXCL9, whereas weaker and no correlation were observed for serum CXCL10 and CXCL11 with serum IFN-γ, respectively [288].
Adult-onset Still’s disease
AOSD is a multisystemic inflammatory disease of unknown etiology, which is generally acknowledged to be an auto-inflammatory disorder (Fig. 2A) [116, 320, 321]. In 1971, this rare disorder was initially described by Bywaters in 14 adults, who exhibited symptoms reminiscent to pediatric sJIA—at the time defined as Still’s disease—and thus was consequently termed “adult-onset” Still’s disease [322]. To date, sJIA and AOSD are still considered to represent one disease continuum with distinct ages of onset [323, 324] due to their superimposable clinical picture [325, 326], gene expression studies and successful outcome upon treatment with IL-1 antagonists [323, 324, 327]. Given the relative confined number of studies exploring the pathology of AOSD [321], the involvement of chemokines—and more specifically IFN-inducible CXCR3 ligands—in the immunopathogenesis of this afflicting disorder has only recently been brought to the fore (Suppl. table 2) [129, 130, 328–331].
First, circulatory CXCL9, CXCL10 and CXCL11 may be useful markers of disease activity in active AOSD [129, 130, 331]. In patients with active, untreated AOSD, serum IFN-, CXCL9, CXCL10 and CXCL11 levels were significantly increased compared to those of HC [129, 130, 328] and RA patients treated with DMARDs [129, 130]. Serum levels of all IFN-inducible CXCR3 ligands, but not IFN-, correlated with disease activity markers including ferritin [129, 130], CRP [130] and systemic disease scores [129, 130]. In addition, serum levels of these chemokines—but again not IFN- levels—significantly attenuated upon clinical remission of AOSD during follow-up after treatment with corticosteroids and immunosuppressive agents [129, 130]. During this follow-up period, changes in serum levels of all three IFN-inducible CXCR3 ligands also correlated with changes in systemic disease score, ferritin levels and LDH levels [129, 130]. Additionally, serum CXCL9 concentrations also correlated with serum levels of CXCL10 and CXCL11 [130]. At disease onset of AOSD, serum levels of CXCL10 were also significantly increased compared to patients with AOSD experiencing an episode of disease flare [129]. Thus, circulatory levels of CXCL9, CXCL10 and CXCL11 may be adequate serological indicators to monitor disease activity in patients with active AOSD [129, 130, 331]. At disease onset, serum levels of these chemokines probably reach their peak concentration, and subsequently decrease upon clinical remission to again augment during disease flares.
Second, immunohistochemistry revealed that inflammatory cells in skin lesions and lymph node tissues of patients with active AOSD even more profoundly expressed CXCR3 and CXCL10 compared to other inflammatory diseases affecting these organs [129, 130, 329, 330]. Expression levels of CXCL10 and CXCR3 were significantly increased in lymph node specimen of patients with AOSD compared to those of patients with lymphadenopathic pathologies including reactive hyperplasia, tuberculous lymphadenitis, histiocytic necrotizing lymphadenitis and T cell lymphoma [329]. Given their histopathological similarities, the aforementioned diseases marked by lymphadenopathy are often considered in the differential diagnosis of AOSD [332, 333]. Therefore, prominent immunoreactivity of CXCL10 and CXCR3 in lymph node tissues of patients with AOSD may help to efficiently identify this disease [329]. Especially, since examination of nodal histology is routinely required for diagnosis of AOSD to exclude infection or hematological malignancy [334]. Intriguingly, patients with arthralgia had higher nodal immunoreactivity of CXCR3 compared to those without arthralgia, thereby instigating the assumption that augmented nodal CXCR3 staining may be indicative for arthralgia/arthritis in AOSD [329]. Hence, after an initial priming and activation process in the lymph nodes, these CXCR3+ inflammatory leukocytes may voyage towards peripheral inflammatory sites where CXCR3 ligands are upregulated, e.g., the dermis and synovium. Interestingly, immunoreactivity of CXCL10—but not CXCR3—was elevated in lymph nodes of patients with AOSD, in which nodal cells exhibited necrosis and karyorrhexis [329]. Hence, nodal infiltration of these CXCL10-secreting inflammatory cells may worsen lymph node inflammation in AOSD-associated lymphadenopathy.
Furthermore, in skin lesions of patients with active AOSD, inflammatory cells expressed CXCR3 and its three respective ligands [129, 130, 330]. The proportion of CXCL10-expressing inflammatory cells was significantly greater in skin biopsies of patients with AOSD compared to those of HC and those of patients with either psoriasis or eczematous dermatitis [130]. In addition, serum CXCL10 levels correlated with the percentage of inflammatory cells expressing CXCL10 in skin lesions [130]. Moreover, serum concentrations of CXCL9 and CXCL10 in patients with AOSD suffering from skin rash were elevated compared to those without cutaneous manifestations [130]. Taken together, these results implicate a role for the CXCR3 chemokine network in AOSD-associated cutaneous inflammation [129, 130]. Actually, ample evidence from distinct pathologies affecting the skin supports this concept of CXCR3-orchestrated ingress of inflammatory cells in the dermis (reviewed elsewhere [8]). Mechanistically, the locally and systemically elevated concentrations of IFN-inducible CXCR3 ligands observed in patients with active AOSD probably contribute to the maintenance of the marked TH1 cell phenotype in skin lesions, lymph nodes and peripheral blood through chemotaxis of TH1 cells and TH1 cytokine induction [335]. Noteworthy, macrophages may be a potential source of IFN-inducible CXCR3 chemokines in skin lesion in AOSD and sJIA. Immunoreactivity of CXCL9 correlated with CD68 expression in cutaneous lesions of patients with active AOSD [130]. In addition, proportions of CXCL9-stained inflammatory cells were increased in skin specimen of patients with AOSD exhibiting dermal macrophage ingress compared to those who did not [130]. Moreover, injury-induced myeloid-related protein (MRP) 8 (S100A8) and MRP14 (S100A9)—which are upregulated in serum of patients with active AOSD or active sJIA and correlate with disease activity parameters in both diseases [336, 337]—induced CXCL10 production in macrophages and monocytes in vitro [338]. As such, this alternative S100A8/A9-dependent regulation of CXCL10, and potentially CXCL9 and CXCL11, may, at least in part, explain their upregulation in active AOSD and sJIA in the absence of MAS. Thus, the production of these chemokines may also potentially occur partially independent of IFN-γ, other IFNs or TNF-α. Indeed, circulatory levels of IFN-γ, IFN-β, IFN-α or TNF-α did not correlate with those of CXCL9, CXCL10 or CXCL11 in patients with active AOSD [130].
Similar to sJIA, the role of IFN-γ also remains enigmatic in the pathogenesis of AOSD and AOSD-associated MAS (reviewed elsewhere [286, 339]). Although inconsistent, some findings pointed towards upregulation of the three IFN-induced CXCR3-binding chemokines in AOSD complicated by MAS compared to active AOSD in the absence of clinical MAS. First, serum levels of all three IFN-inducible CXCR3 ligands—but not IFN-γ—were significantly higher in patients with AOSD complicated by MAS than in patients with active AOSD without MAS [130]. However, the same authors also reported comparable CXCL10 serum concentrations in patients with AOSD-associated MAS and in patients with AOSD in the absence of MAS in a former publication [129]. Second, the percentage of CXCR3+ inflammatory cells in cutaneous lesions were proportionally augmented in patients with AOSD with MAS compared to those without MAS [130]. Third, serum concentrations of other relevant chemokines with reported serological biomarker potential in active AOSD—i.e., CX3CL1 and CXCL13—were also significantly increased in AOSD with MAS compared to AOSD without MAS [129, 328]. Importantly, treatment, differential procedure of serum collection and distinct systemic inflammatory status (reflected by, inter alia, serum levels of ferritin, CRP, ESR and ALT) at the moment of sampling may drastically influence the chemokine blood profile and therefore also explain the differential outcomes of these studies involving patients with AOSD and sJIA. Further complicating this issue is the fact that patients with AOSD suffering from MAS were often included in the group of active AOSD when comparing serum levels of these three CXC chemokines to HC or RA patients [129, 130]. Therefore, we cannot fully affirm that the significantly increased serum levels were exclusively related to the active AOSD disease course irrespective of MAS. To summarize, these data merit further investigation to fully evaluate the potential of IFN-inducible CXCR3 ligands as indicators of systemic and cutaneous inflammation in AOSD and their potential exclusive association with AOSD-associated MAS.
Spondyloarthritis
Spondyloarthritis is a clinically heterogenous inflammatory disease that mainly affects the spine, sacroiliac and peripheral joints, and entheses (i.e., the insertions of tendons and ligaments to the bone) (Fig. 2B) [340, 341]. Based on the most predominant clinical involvement, two sub-entities were distinguished, i.e., axial and peripheral spondyloarthritis [341, 342]. Formally, axial spondyloarthritis solely encompassed ankylosing spondylitis [343], which is a historically developed term for a disease diagnosed based on advanced erosions and damage of sacroiliac joints observed after radiological examination [344, 345]. Axial spondyloarthritis is nowadays considered as an overarching term that describes a broad disease spectrum comprising of ankylosing spondylitis (or radiographic axial spondyloarthritis) and non-radiographic axial spondyloarthritis [343]. Peripheral spondyloarthritis, on the other hand, has enthesitis, dactylitis and/or arthritis as predominant symptomatology [346] and includes psoriatic arthritis [342, 346, 347]. In terms of the CXCR3 chemokine network, upregulation of CXCL9 and CXCL10 was recurrently described in SF of spondyloarthritic joints (Suppl. table 1) [45, 48, 80, 144, 150, 348, 349].
Psoriatic arthritis
Psoriatic arthritis is a common subtype of peripheral spondyloarthritis characterized by oligo/polyarthritis and/or enthesitis, affecting both peripheral and axial skeleton (Fig. 2B) [350–352]. Within 10 years after disease onset, the preponderance of patients with psoriatic arthritis (~ 88%) develops joint erosions [353], leading to functional disability and reduced quality of life. Moreover, given the importance of early diagnosis to prohibit joint damage and the common issue of under- and misdiagnosis, a circulatory biomarker—that enables to predict the susceptibility to develop psoriatic arthritis in patients with psoriasis and in the overall population—would be an indispensable tool in the clinical setting [348, 349, 354]. As such, CXCL10 was speculated to be a potential candidate to fulfill this biomarker role given its evidenced involvement in pathogenesis of psoriatic arthritis [45, 80, 348, 349, 355–358]. First, serum CXCL10 levels of patients suffering from psoriatic arthritis were significantly higher compared to HC [349, 355, 357, 358] and psoriasis patients that do not have psoriatic arthritis [348]. In addition, whole-blood gene expression profiling clearly showed in two independent cohorts that CXCL10 gene expression was significantly upregulated in patients with psoriatic arthritis compared to patients with psoriasis in the absence of psoriatic arthritis [356]. Moreover, serum levels of CXCL10 in patients with psoriasis were positively associated with the conversion state to develop psoriatic arthritis [348]. Thus, increased whole-blood gene expression and circulatory protein concentrations of CXCL10 in patients with psoriasis appear to precede the onset of psoriatic arthritis. However, this concept is not underpinned by all studies. First, other researchers have reported that serum levels of CXCL10 were unchanged [128]—or even lower [359]—in patients with psoriatic arthritis compared to controls [128, 359] and compared to patients with psoriasis in the absence of arthritis [359]. Similarly, circulatory levels of CXCL9 were reported to be comparable in psoriatic arthritis, psoriasis without arthritis and controls [357, 359]. Second, serum CXCL10 levels were not associated with any of the clinical indexes of psoriatic arthritis [357]. In addition, serum CXCL10 was reported to be inversely correlated with duration of psoriatic arthritis [355, 358], but again this was contradicted by another study [357]. These conflicting results may be attributed to discrepancies in methodology for chemokine detection (e.g., multiplex and ELISA) and in patient characteristics (e.g., treatment modalities and clinico-epidemiological features) [349, 355, 357]. For example, CXCL10 production is downregulated by distinct therapies, which are currently approved to treat psoriasis and psoriatic arthritis, including the phosphodiesterase-4 inhibitor apremilast and the TNF-α blocking agent etanercept [360, 361]. Although these treatments may induce intracohort variability, their effectiveness coinciding with reduction of CXCL9 and CXCL10 levels also underscores the relevance of these chemokines in psoriatic arthritis [349, 360, 362].
Synovial IFN-γ, CXCL9, CXCL10 and CXCL11 levels were comparably upregulated in patients with psoriatic arthritis relative to patients with RA [45, 48, 80, 349]. Concentrations of CXCL9 and CXCL10 reached similar levels in SF of patients with psoriatic arthritis as those with ankylosing spondylitis [45, 48], and were augmented compared to crystal-induced arthritis [45, 48, 349]. Also, synovial CXCL10 and IFN-γ levels were significantly elevated in patients with psoriatic arthritis compared to those of patients with osteoarthritis [80, 349]. In addition, mRNA levels of CXCL10 in synovial cells of patients with psoriatic arthritis were significantly increased relative to those of patients with gout and OA [348, 349]. Moreover, prominent in situ CXCL9 expression was detected in the synovial lining and cellular infiltrates of synovial tissue of patients with psoriatic arthritis exhibiting active synovitis [150]. Thus, IFN-inducible CXCR3 ligands are abundantly present in the inflamed joints of patients with psoriatic arthritis. Additionally, CXCL10 was augmented by more than 20-fold in SF compared to paired serum of patients with psoriatic arthritis [349]. Also, mRNA expression of CXCL10 was substantially increased in synovial cells compared to whole-blood cells of patients with psoriatic arthritis [348, 349]. Thus, CXCL10 establishes a chemotactic gradient from the circulation towards the synovium in psoriatic arthritis.
Intriguingly, parallels can be drawn between dermal and joint inflammation in patients with psoriatic arthritis, equipollently to those of AOSD (vide supra). Within psoriatic skin lesions, CXCL9 and CXCL10 were found to be prominently expressed [209, 363] and exactly colocalized with dermal CXCR3+ CD3+ lymphocytes [209]. In addition, CXCL10 stimulated in vitro migration of T cells isolated from psoriatic skin lesions [364], signifying that CXCL9 and CXCL10 are involved in T cell trafficking towards the dermis in psoriasis [209]. Accordingly, CXCL9 and CXCL10 probably contribute to CXCR3+ TH1 cell chemotaxis towards the joints, thereby inciting joint inflammation in patients with psoriasis. Indeed, mRNA levels of CXCL10 and CXCR3 in synovial cells of patients suffering from psoriatic arthritis positively correlated with each other [349]. Moreover, gene expression of CXCR3 was significantly increased in synovial cells of patients with psoriatic arthritis compared to osteoarthritis, gout and even compared to those of patients with RA [349]. Also, significantly decreased proportions of peripheral blood CXCR3+ CD4+ T cells were observed in psoriatic arthritis compared to controls, suggesting their recruitment towards peripheral inflammatory sites [359]. Moreover, CXCL9 mRNA expression was clearly attenuated in rheumatoid and psoriatic synovia exhibiting a thinner synovial lining and less pronounced cellular infiltrates, thereby suggesting that expression level of CXCL9 may be indicative for synovial cellular infiltration [150]. These findings imply a CXCR3 ligand-dependent polarization towards a CXCR3+ TH1 cell profile in the synovial niche in psoriatic arthritis. Moreover, sustained production of IFN-inducible CXCR3 ligands in the inflamed joints probably also contributes to synovial infiltration of pDC [80, 358]. Blood-derived and synovial pDC of patients with RA and psoriatic arthritis express CXCR3 and CXCR4 [80, 358] and these blood-derived pDC also exhibited chemotaxis towards all three IFN-inducible CXCR3 ligands in vitro [358].
Altogether, psoriatic arthritis shares most of its synovial expression profile of the CXCR3 ligands with RA. CXCL9 and CXCL10 seem to fulfill an equivalent role in joint inflammation in psoriatic arthritis as in RA, i.e., CXCR3-dependent orchestration of ingress of TH1 cells and pDCs into the synovium. In addition, cellular sources of CXCL9 were found to be similar in psoriatic arthritis compared to RA, i.e., synovial macrophages and perivascular infiltrating lymphocytes [150]. Nevertheless, further research is required to elucidate how CXCL9 and CXCL10 contribute to psoriatic arthritis-associated synovitis and whether their circulatory levels have clinical validity as marker of psoriatic arthritis susceptibility.
Ankylosing spondylitis
Ankylosing spondylitis is the prototype of axial spondyloarthritis of unascertained etiology [341], in which spinal inflammation and sacroiliitis may result in inflammatory back pain and ankylosis with advancing spinal rigidity (Fig. 2B) [365]. From the perspective that ankylosing spondylitis has an autoimmune component [341, 366], TH1 cytokines have been investigated in serum [367, 368] and SF [45, 48] of patients with ankylosing spondylitis. Serum levels of CXCL9 and CXCL10 were found to be significantly elevated in patients with untreated ankylosing spondylitis compared to HC [367, 368]. Importantly, circulatory levels of CXCL10 correlated with serum TNF-α levels, ESR, CRP and Ankylosing Spondylitis Disease activity score (ASDAS) in patients with untreated ankylosing spondylitis [367]. Upon anti-TNF-α treatment, serum CXCL10 levels were significantly diminished [367, 368] and retained their correlation with CRP and ASDAS [367]. These findings implicate that circulatory levels of CXCL10—probably synergically induced by TNF-α and IFN-γ—reflect disease activity in ankylosing spondylitis [367]. Conversely, other studies have reported that serum CXCL10 levels were comparable in patients with spondyloarthritis (ankylosing spondylitis and psoriatic arthritis) or untreated ankylosing spondylitis compared to HC [128, 368]. Noteworthy, only a very limited number of subjects with ankylosing spondylitis was included in these studies [128, 368]. Furthermore, synovial levels of CXCL9 and CXCL10 were comparable in patients with ankylosing spondylitis compared to those of patients with RA and psoriatic arthritis [45, 48]. Also, concentrations of these chemokines were significantly higher in SF of patients with ankylosing spondylitis compared to crystal-induced arthritis [45, 48]. Intriguingly, JIA patients with enthesitis-related arthritis—which is a common subtype of JIA in India that resembles adult spondyloarthritis—also had increased serum CXCL10 levels compared to HC with a gradient from the blood towards the SF [369]. Hence, although the number of studies reporting on IFN-inducible CXCR3 ligands in ankylosing spondylitis is particularly sparse in comparison to RA and psoriatic arthritis, these data suggest that CXCL9 and CXCL10 may exert comparable functions in the inflamed articulation of patients with ankylosing spondylitis.
Septic arthritis
Acute septic arthritis emerges due to bacteremic seeding in the vascular synovium of the joint [370] and typically manifests as monoarticular arthritis—marked by swelling, erythema, warmth and pain on palpation of the joint—and low- or high-grade fever (Fig. 2B) [371, 372]. The absence of a basement membrane within the joint lining renders the synovium susceptible to bacterial invasion from the circulation [373]. Importantly, all three IFN-inducible CXCR3 ligands are implicated in leukocyte accumulation in the synovium of patients with septic arthritis [47, 153]. Elevated levels of synovial CXCL9, CXCL10 and CXCL11 were detected in patients with septic arthritis compared to osteoarthritis [47, 153]. Moreover, synovial concentrations of CXCL9 and CXCL11 were also significantly augmented in patients with septic arthritis relative to crystal-induced arthritis [47]. In addition, in SF of patients with septic arthritis, CXCL10 levels were even twofold higher compared to those of CXCL8 [153], a chemokine which is also known to be significantly enhanced in septic arthritis compared to osteoarthritis [374]. High synovial concentrations of the IFN-inducible CXCR3 ligands may be explained by synergic induction in fibroblasts and human microvascular endothelial cells by IFN-γ combined with bacterial TLR ligands (e.g., LPS and peptidoglycans) [47, 48, 153]. Mechanistically, hypersecretion of the IFN-inducible CXCR3 ligands may contribute to bacterial clearance, given the direct defensin-like antimicrobial actions of these chemokines at high concentrations (i.e., µM range) [375]. This antibacterial activity of CXCL9, CXCL10 and CXCL11 presumably resides on their C-terminal segment, that is highly enriched with positively charged amino acids, thereby conferring interaction with anionic residues on the bacterial surface which leads to insertion and pore formation in the bacterial membrane [375]. Also, the positively charged α-helix of all three IFN-inducible CXCR3 ligands can be selectively cleaved off by virulence factor glycoprotein-63 (GP63) of Leishmania Major [376]. This mechanism was speculated to be an immune evasion strategy to counteract the antimicrobial actions of CXCL9, CXCL10 and CXCL11 [376].
Osteoarthritis
Osteoarthritis is the most prevalent degenerative joint disease and presents as low-grade arthritis of one or multiple diarthrodial joints characterized by arthralgia with a mechanical pain pattern, evanescent morning stiffness and crepitation (i.e., grinding that occurs when moving a joint) (Fig. 2B) [377, 378]. Of the various pro-inflammatory factors that have been identified in SF of patients with osteoarthritis, CXCL9 and CXCL10 were found to be present at relatively high levels [378]. However, patients with osteoarthritis are often included as disease controls in studies for autoimmune arthritides (vide supra), thereby serving comparative—rather than disease-uncovering—purposes. A confined number of studies addresses the involvement of IFN-inducible CXCR3 ligands in the specific framework of investigating osteoarthritis-associated synovitis [379–383].
In serum of patients with osteoarthritis, CXCL9 and CXCL10 levels were significantly increased compared to serum of HC [379] with a clear concentration gradient from the serum towards the osteoarthritic synovium [379]. Synovial concentrations of CXCL9 and CXCL10 were even relatively elevated compared to other inflammatory mediators (e.g., CXCL12a, CCL3, and CCL4) in patients with osteoarthritis [379], but evidently still decreased compared to those in SF of patients with RA [80, 102, 124, 131, 137, 144] and septic arthritis [47, 153]. Two studies reported that CXCL10 levels were significantly augmented in SF of osteoarthritis patients compared those in SF obtained from cadavers of HC [380, 381]. Conversely, another study identified none of the IFN-inducible CXCR3 ligands as being significantly upregulated proteins in osteoarthritic SF, when comparing the SF proteome of patients with osteoarthritis relative to those of HC [384]. In the latter study, proteins from SF were separated by one-dimensional gel electrophoresis and in-gel tryptic digest, followed by detection of via tandem mass spectrometry [384]. However, through this bottom-up methodology, chemokines may be easily overlooked, given their low relative molecular mass and high percentages of positively charged amino acids that lead to short peptides upon tryptic digest, which are easily lost during chromatographic separation. In addition, their broad structural heterogeneity—as a result of undergoing PTMs that are likely to occur in the enzyme-enriched environment of the SF [385]—renders mass spectrometry-based identification challenging. Taken together, CXCL9 and CXCL10 are probably also highly abundant proteins in osteoarthritic SF, although to a more limited extent compared to auto-immune arthropathies or septic arthritis. In addition, synovial CXCL10 concentrations were found to be elevated—although not significantly—in osteoarthritis patients with advanced arthritis compared to those with mild arthritis [382]. Moreover, principal component analysis identified six clusters of interrelated molecules in pooled data of SF of osteoarthritis and post-mortem HC donors [381]. Intriguingly, CXCL10 did not cluster together with other chemokines (e.g., CCL2 and CCL11), but was rather found in a cluster containing IFN-α and growth factors, implying that CXCL10 may be involved in homeostatic processes in the joint, aside from inflammation [381]. Indeed, this potential alternate and unknown functioning of CXCL10 in homeostatic processes may also explain its expression—although to a very limited extent—in SF of healthy controls [380, 381, 386].
In osteoarthritis-associated synovitis, macrophages, NK cells and neutrophils are prominent players [383, 387, 388]. Antibody-mediated depletion experiments showed that both NK cells and neutrophils deleteriously affect joint integrity during experimental osteoarthritis [389]. Moreover, sequential immunophenotyping of synovial cells in experimental osteoarthritis revealed that neutrophils are recruited first towards the joint and locally produce CXCL10, which, in turn, attracts macrophages and subsequently NK cells [389]. Infiltrating NK cells in the synovial tissue of patients with osteoarthritis represent 30% of the lymphocytic population [383]. Given that synovial tissue NK cells of these patients uniformly express CXCR3 [383], synovial ingress of NK cells in patients with osteoarthritis is considered to be orchestrated by CXCL9 and CXCL10 [383].
Pleiotropic actions of the IFN-inducible CXCR3 ligands that may contribute to an aberrant synovial niche
In addition to their widely acknowledged chemotactic actions enabling leukocyte homing in the synovial microenvironment, CXCL9 and CXCL10 also act as potent pro-inflammatory and osteoclastogenic mediators, thereby (in)directly contributing to the altered architecture of the arthritic joint (Fig. 5).
Fig. 5.
Pleiotropic actions of the IFN-inducible CXCR3 ligands that may contribute to joint inflammation. Pathogenic processes related to CXCL9 and CXCL10 that were established in vitro and that may contribute to an aberrant synovial microenvironment, include articular cartilage and bone damage through A induction of osteoclastogenesis (directly through their effect on osteoclast progenitor cells and indirectly through induction of RANKL secretion by FLS and CD4+ T cells), B stimulation of migration of CXCR3+ subchondral progenitor cells, C induction of enzyme secretion that affect bone remodeling by osteoblasts, and D augmenting the activity of MMPs secreted by FLS. In addition, IFN-inducible CXCR3 ligands may facilitate synovial hyperplasia through E alteration of morphology of FLS, stimulation of invasiveness, proliferation and chemotaxis and synovitis through F infiltration of CXCR3+ leukocytes, G TH1 cell polarization and infiltration via CCR3 antagonism, CCL11 scavenging, and desensitization of CXCL12 signaling, H interaction of CXCL10+ CCL19+ fibroblasts with T cells, I pro-inflammatory cytokine secretion and auto-amplification of CXCL10 production by CD4+ T cells, J actions mediated by posttranslationally modified IFN-inducible CXCR3 ligands generated after processing by synovial enzymes, K interaction with ACKR, and L angiostasis. How CXCL11 operates in the synovial microenvironment is less comprehended, as it may also reduce inflammation by outcompeting CXCL9/10 for CXCR3 and polarizing TH cells towards Tr1 and TH2 cells. ACKR atypical chemokine receptor, CCL CC chemokine receptor ligand, CCR CC chemokine receptor, CD13/APN metalloprotease aminopeptidase N, CD26/DPPIV dipeptidyl peptidase IV, CXCL CXC chemokine receptor ligand, CXCR CXC chemokine receptor, FLS fibroblast-like synoviocytes, GAG glycosaminoglycan, IL interleukin, MMP matrix metalloproteinase, pDC plasmacytoid DC, RANK Receptor activator of nuclear factor kappa-Β, RANKL RANKL Receptor activator of nuclear factor kappa-Β ligand, TLR4 Toll-like receptor 4, TNF-α tumor necrosis factor α, TRAP tartrate-resistant acid phosphatase
Upregulation of osteoclastogenic and pro-inflammatory cytokines
CXCL10-stimulated production of osteoclastogenic cytokines was demonstrated in various cell lineages including rheumatoid FLS [137, 148], human CD4+ T cells and memory CD27+ B cells isolated from HC blood [113, 137], Jurkat T cells [137], Hut 78 T cells [137], murine CD4+ T cells isolated from the spleen [86, 185] (Fig. 5A,I). First, combined stimulation of rheumatoid FLS with TNF-α and CXCL10 induced IL-6 secretion and mRNA expression of tumor necrosis factor ligand superfamily member 11 (TNFSF11) [148], which encodes for a crucial protein enabling osteoclast differentiation, i.e., receptor activator of nuclear factor kappa-Β ligand (RANKL) [390]. These actions could be prevented by pretreatment of FLS with a bispecific antibody targeting both TNF-α and CXCL10 [148]. Furthermore, another study confirmed that CXCL10 augmented RANKL expression in synoviocytes isolated from synovial specimen of RA patients [137]. CXCL10 engendered RANKL expression in Hut 78T cells, Jurkat T cells, CD4+ T cells and memory CD27+ B cells of healthy donors, but not in CD14+ monocytes [113, 137]. In addition, CXCL10 promoted mRNA expression and secretion of RANKL and TNF-α in CD4+ T cells isolated from mouse spleens [86, 185] and, in turn, RANKL also dose-dependently induced expression and secretion of CXCL10 in mouse osteoclast precursors [185]. Accordingly, co-culturing of murine CD4+ T cells and osteoclast precursors revealed that CXCL10 dose-dependently stimulated expression of osteoclast-activity marker tartrate-resistant acid phosphatase (TRAP) in osteoclast precursors, which could be abolished by addition of the decoy receptor for RANKL, i.e., osteoprotegerin [185, 391]. Thus, these data implicate that CXCL10 promotes osteoclast differentiation indirectly by mediating RANKL expression in CD4+ T cells and FLS.
Furthermore, CXCR3 and TLR4 were found to be indispensable receptors for CXCL10-mediated cytokine production by CD4+ T cells [86, 137]. RANKL expression in CXCL10-treated Jurkat T cells was suppressed by Gαi inhibition [137]. These results demonstrate that Gαi proteins are involved in CXCL10-dependent RANKL expression. Moreover, CXCL10 elicited mRNA expression and secretion of RANKL, TNF-α and IL-6 by CD4+ T cells isolated from spleens of WT C57BL/6 mice, but not in splenic CD4+ T cells purified from CXCR3−/− and TLR4−/− mice [86], showing the importance of both CXCR3 and TLR4 in CXCL10-stimulated cytokine production by T cells. Mechanistically, CXCL10-dependent RANKL expression was found to be molecularly established via the calcineurin/NFATc1 pathway [86]. To summarize, CXCL10 stimulates expression of pro-inflammatory cytokines (e.g., IL-6) and osteoclastogenic mediators (e.g., RANKL) in rheumatoid FLS and T cells in vitro. Consequently, CXCL10 may promote synovitis and may contribute to cartilage and bone degradation through osteoclast differentiation and activation in the synovial niche. Moreover, the reciprocal intercommunion between CXCL10, RANKL and TNF-α constitutes an amplification feedback loop and thereby may further propagate progressive inflammation and osteoclastogenesis in the inflamed joints [86, 137, 185]. In addition, some indirect evidence exits that CXCL10 gene expression may be associated with synovitis [146]. In this study, RA patients were clinically divided into two subgroups exhibiting ‘high’ and ‘low’ inflammation, respectively. This classification was based on clinical data of CRP and Krenn’s synovitis score and clustering patterns of genes. Although significance was not tested, gene expression of CXCL9 and CXCL10 was increased in RA patients with ‘high’ inflammation compared to those with ‘low’ inflammation [146]. This may indicate that elevated expression of CXCL9 and CXCL10 in the synovium is associated with a more pronounced inflammatory phenotype of RA marked by high-grade synovitis.
2.8.2. Osteoimmunology: intercommunion of CXCL10 with osteoblasts, osteoclast progenitor cells, subchondral progenitor cells and chondrocytes
CXCL10 clearly influences bone metabolism through its actions on osteoblasts, osteoclast progenitor cells and subchondral progenitor cells and its production by chondrocytes (Fig. 5A–C) [136, 386, 392, 393]. First, CXCL10 is an important regulator of human osteoblast activity through CXCR3-dependent selective upregulation of enzymes involved in bone remodeling (Fig. 5C) [392]. Human osteoblasts abundantly express functionally active CXCR3 [392]. Indeed, CXCL10 significantly and dose-dependently induced exocytosis of β-N-acetyl-hexosaminidase by human osteoblast in vitro, an effect that was abrogated by Gαi inhibitor pertussis toxin [392]. β-N-acetyl-hexosaminidase is an exoglycosidase that degrades extracellular matrix proteins, more specially GAGs such as hyaluronic acid [392]. Moreover, CXCL10 augmented alkaline phosphatase activity of human osteoblasts in vitro [392]. Bone-specific alkaline phosphatase is a primary regulator of bone mineralization, as it hydrolyzes inorganic pyrophosphate, which is a natural inhibitor of bone mineralization [394]. Hence, these findings demonstrate that CXCL10 participates in fine-tuning the activity of bone remodeling enzymes produced by human osteoblasts, a feature that may contribute to bone erosion in the joints of patients with arthritis.
Second, CXCL10 exhibits osteoclastogenic and chemotactic effects on osteoclast progenitor cells (Fig. 5A) [136]. Given the limited lifespan of individual osteoclasts [395] and the chronic osteoclast-driven periarticular bone resorption during RA, continuous replenishment of osteoclasts to the arthritic synovium is necessary [396, 397]. Osteoclast progenitors have the ability to mature into functional osteoclasts upon in vitro stimulation with RANKL and macrophage colony-stimulating factor (M-CSF) [396, 398]. Indeed, proportions of RANK+ osteoclast progenitors were found to be enlarged in SF compared to peripheral blood and correlated positively with tender joint counts in patients with RA [136]. Importantly, proportions of CXCR3+ osteoclast progenitors in the peripheral blood of patients with RA were significantly increased compared to those of HC [136]. Furthermore, CXCL10 stimulated maturation of in vitro-generated osteoclast progenitors derived from PBMCs of RA patients into mature TRAP+ multinuclear osteoclasts [136]. Also, CXCL10 promoted transwell migration of in vitro-generated osteoclast progenitors derived from PBMCs of RA patients [136]. Thus, synovial homing of CXCR3+ osteoclast progenitors and differentiation into multinucleated bone-resorbing osteoclasts are, at least partially, CXCL10-mediated mechanisms that may contribute to periarticular bone loss in arthritis.
Third, human chondrocytes isolated from healthy and osteoarthritic joints spontaneously secreted high levels of CXCL10 ex vivo, whereby stimulation with IL-1β or TNF-α potently increased CXCL10 secretion (Fig. 5B) [393]. Thus, articular cartilage may also actively contribute to CXCL10 hypersecretion in the synovial niche. Fourth CXCL10 efficiently stimulated in vitro migration of human subchondral mesenchymal progenitors derived from subchondral cortico-spongious bone isolated during osteotomy [386]. This suggests that synovial CXCL10 is involved in trafficking of subchondral progenitor cells from the subchondral bone marrow towards the cartilage (Fig. 5B) [386]. Accordingly, CXCR3 was the most pronouncedly expressed chemokine receptor on subchondral progenitor cells [386]. In contrast to the bone degenerative actions ascribed to the CXCL10-CXCR3 axis, CXCL10-mediated chemotaxis of subchondral progenitor cells to cartilage defects may contribute to formation of cartilage repair tissue.
Migration, invasiveness, morphology, proliferation and immune interaction of FLS
The CXCL10-CXCR3 axis is implicated in the regulation of FLS invasiveness [213–215]. Microarray analysis of gene expression was performed on FLS derived from Dark Agouti rats with pristane-induced arthritis and pristane-induced arthritis-resistant Dark Agouti.F344 (Cia5d) rats [213]. Cia5d rats have a Dark Agouti genetic background but carry alleles of the arthritis-resistant F344 strain at the arthritis severity locus Cia5d, in which consequently FLS exhibit attenuated invasiveness [399]. Among 66 significantly upregulated genes, the Cxcl10 gene was one of the most pronouncedly upregulated genes in highly invasive FLS of Dark Agouti rat-derived FLS compared to Cia5d rat-derived FLS [213]. Accordingly, Dark Agouti rat-derived FLS secreted increased levels of CXCL10 compared to Cia5d rat-derived FLS upon culturing in serum-containing medium in matrigels [214]. The distinct CXCL10 production by these FLS was speculated to partially underlie their difference in invasiveness. Indeed, exogenously administered CXCL10 induced a twofold increase in the number of matrigel-invading FLS isolated from Cia5d rat, which was inhibited by administration of anti-CXCR3 monoclonal antibody [214]. Similarly, migration of Dark Agouti rat-derived FLS to medium supplemented with serum was reduced by incubation with CXCR3 antagonist AMG 487 or a CXCR3 blocking antibody. Thus, CXCL10 stimulates invasion of rat FLS and this mechanism appears to be a CXCR3-dependent process.
Accordingly, similar findings were obtained in FLS isolated from synovial biopsies of RA patients. Cultured and unstimulated RA FLS were shown to constitutively express and bear functionally active chemokine receptors, including CXCR3, CCR5 and CXCR4 [215]. In addition, CXCL10 treatment induced Ca2+i mobilization in Dark Agouti rat-derived [214] and RA FLS [214, 215], which was attenuated by AMG 487 [214] and by the Gαi inhibitor pertussis toxin [215]. Moreover, RA FLS also migrated in matrigels towards serum-containing medium, which was significantly diminished by the CXCR3 antagonist AMG 487 [214]. A tenfold higher dose of AMG 487 was required to inhibit cell invasion of RA FLS compared to Dark Agouti rat-derived FLS [214]. This notion probably relies on the higher CXCR3 expression levels on RA FLS [214]. Also, CXCL10 triggered chemotaxis and transmigration of RA FLS in a transwell system and this chemotactic response was inhibited by pertussis toxin [215]. Hence, CXCL10 elicits chemo-attraction, transmigration and invasion of FLS derived from inflamed joints of RA patients. Consequently, in order to elucidate the underlying mechanism of CXCL10-stimulated FLS chemotaxis, morphology of FLS was examined. Unstimulated Dark Agouti rat-derived and RA FLS exhibited an elongated morphology, marked by parallel-organized, linearized and thick actin filaments and lamellipodia at the leading edge [214]. Interestingly, CXCR3 blockage with AMG 487 diminished formation of thick actin filaments and polarized lamellipodia of these FLS [214], indicating a role of CXCR3 signaling in actine cytoskeleton reorganization and consequently morphologic alteration enabling FLS migration.
Another crucial property of the aberrant synovial niche in RA is the multi-layered thickening of the synovial intimal lining, which is defined as synovial hyperplasia [400]. Importantly, synovial hyperplasia in RA is established by, inter alia, local proliferation of FLS in the synovial lining [401]. In this context, CXCL9 and CXCL10 induced significant cell proliferation of RA FLS after 72 h, thereby reaching similar proportions of proliferating cells as observed upon incubation with IL-1β [215]. A blocking monoclonal antibody against CXCL10 inhibited the proliferative response mediated by its ligand [215]. Thus, in addition to chemo-attraction, transmigration and invasion, CXCL10 also mediates proliferation of FLS derived from RA patients (Fig. 5E). A recent study characterized pro-inflammatory fibroblasts from distinct inflamed tissues—i.e., synovium of RA and osteoarthritis patients, salivary gland biopsy tissue from patients with Sjögren’s syndrome, intestinal biopsies from patients with ulcerative colitis, and lung biopsies from patients with interstitial lung disease—using single-cell RNA-sequencing [402]. A shared cluster identified as CXCL10+ CCL19+ fibroblasts was expanded in all these inflamed specimens and also in the synovium of CIA-developing DBA/1 mice [402]. These CXCL10+ CCL19+ fibroblasts had enriched marker genes for pathways implicated in direct interaction with immune cells, such as lymphocyte chemotaxis, T cell proliferation, and antigen presentation [402]. IFN-γ and IFN-α, the main inducers of the CXCR3 ligands, were identified as key pro-inflammatory cytokines to which these immune-interacting fibroblasts responded. Another group also described CXCL9 upregulation in synovial fibroblasts of RA patients [403]. Given the expression of HLA genes [402, 403], CXCL10+ CCL19+ fibroblasts were speculated to interact with T cells (Fig. 5H), as these cells functionally resemble to CCL19+ podoplanin+ immunofibroblasts of the salivary glands [402]. Since CXCL10 is clearly implicated in the development of the altered phenotype of FLS, CXCL10 may contribute to the emergence of key pathogenic manifestations in the synovial niche including synovial hyperplasia, synovitis, pannus formation and pannus invasion in cartilage.
Regulation and secretion of MMP by FLS
To further uncover the effector mechanisms of CXCL10–CXCR3-induced FLS invasion, secretion of active and pro-forms of MMPs by FLS was explored. MMPs are highly abundant proteolytic enzymes in rheumatoid synovia and are considered crucial orchestrators of cartilage degradation in RA [404]. The highly invasive and strongly CXCL10-producing Dark Agouti rat-derived FLS spontaneously secreted the active form of MMP-1 [214]. Furthermore, cultured and unstimulated RA FLS also spontaneously secreted collagenases MMP-1, proMMP-13 and MMP-13 [215, 405], and gelatinases proMMP-2, proMMP-9, MMP-2 and MMP-9 [215, 406]. Therefore, the effects of CXCR3 ligands on FLS-mediated MMP secretion and MMP activity were assessed. In the supernatant of RA FLS, CXCL9 and CXCL10 dose-dependently augmented gelatinase and collagenase activity, an effect that was abolished by pertussis toxin [215]. Hence, CXCL9 and CXCL10 upregulate MMP activity by RA FLS. This process was found to be G protein-dependent [215], and therefore likely mediated through CXCR3. Indeed, AMG 487 or anti-CXCR3 antibodies both substantially decreased spontaneous MMP-1 production by CXCL10-producing Dark Agouti rat-derived FLS [214]. Interestingly, chemokine-stimulated MMP activity by RA FLS is highly dependent on the presence of endogenous IL-1β, since blocking monoclonal antibodies against IL-1β markedly reduced CXCL9-mediated collagenase and gelatinase activity in RA FLS supernatant [215]. This finding raised the possibility of an indirect effect of chemokines on MMP activity of FLS by affecting their cytokine production [215], since IL-1β is a powerful inducer of MMP expression by RA FLS [407]. As such, CXCL9 and CXCL10 may ultimately contribute to matrix degradation via CXCR3-dependent cytokine- and MMP-release by FLS (Fig. 5D).
CCR3 antagonism
CXCR3 and its IFN-inducible ligands may subvert the development of TH2 cell responses in the synovium, which is a concept that is supported by two distinct mechanisms reported in vitro (Fig. 5G) [76, 77]. First, all three IFN-inducible CXCR3 ligands are natural antagonists for CCR3 at high concentrations (1 µM) [77]. These chemokines significantly inhibited CCR3-mediated chemotaxis induced by the three exotaxins (CCL11, CCL24, CCL26) on CCR3 transfectants and human eosinophils in vitro [76, 77]. CXCL11 exhibited the most pronounced chemotaxis antagonistic properties [76]. Hence, the CXCR3 chemokine network mediates CXCR3+ TH1 cell recruitment, while concomitantly reducing TH2 cell migration in response to CCR3 ligands [76, 77]. Second, CCL11 binds to CXCR3 with a high affinity (IC50 = 3.12 nM)—which is comparable to the affinity of CXCL11 for CXCR3—but does neither act as an agonist or antagonist on CXCR3 [76]. Thus, CXCR3 may function as a decoy receptor sequestering locally secreted CCL11 and thereby preventing its actions in re-directing polarization of TH1 cell to TH2 cell phenotype [76]. Hence, these two mechanisms may enhance the perpetuation of TH1 inflammatory responses at the expense of TH2 cell polarization in the inflamed synovium.
Heterologous desensitization of CXCR4 signaling by CXCR3 ligands
Intriguingly, co-stimulation of memory CXCR3+ CXCR4+ TH1 cells with CXCL9 was reported to result in desensitization of CXCL12 signaling (Fig. 5G) [408]. Pre-incubation of murine EL-4T cells, human IF12 memory TH1 cells, and human Jurkat T cells with CXCL9 or CXCL11—but not CXCL10—followed by treatment with CXCL12 resulted in reduced CXCL12-mediated chemotaxis [408]. In addition, TH1 memory clones, that were pre-treated with CXCL9, exhibited diminished CXCL12-driven crawling and transendothelial migration in vitro [408]. Importantly, this heterologous desensitization of CXCL12 signaling by CXCL9 is a CXCR3-dependent process since treatment with the highly specific CXCR3 antagonist NIBR2130 abolished CXCL9-mediated inhibition of CXCL12-induced chemotaxis [408]. Hence, a two-step mechanism of extravasation and chemotaxis of leukocytes in the synovium was proposed based on the distinct synovial localization of CXCL12 and IFN-inducible CXCR3 ligands at the synovial endothelial cells or more centrally in the synovial tissue, respectively [408]. First, CXCL12 instigates endothelial transmigration of memory CXCR3+ CXCR4+ TH1 cells from the blood into the synovial tissue. Subsequently, CXCL9 would orchestrate deeper tissue ingress of TH1 cells towards the epicenter of inflammation, irrespective of the opposing CXCL12 gradient. This coordinated process enables the IFN-inducible CXCR3 ligands to propagate inflammation by augmenting infiltration of immune cells and maintaining their localized presence at the inflammatory site.
Auto-amplification loop
Positive feedback through the CXCL10-CXCR3 axis was reported to amplify CXCL10 expression in vitro in an NFκB-dependent manner (Fig. 5I) [176, 181]. Exogenously added CXCL10 increased endogenous CXCL10 mRNA expression in murine breast cancer cells 4T1 cells [181], isolated murine bone marrow-derived macrophages (BMMs) [176] and purified murine splenic CD4+ T cells [176]. In addition, endogenous CXCL10 secretion was also stimulated by exogenous CXCL10 in BMMs and CD4+ T cells [176]. Interestingly, exogenous CXCL10-mediated CXCL10 mRNA expression was inhibited by the CXCR3 antagonist JN-2 in all these cell lineages [176, 181], signifying that the exogenous CXCL10 could contribute to endogenous CXCL10 expression in a CXCR3-dependent manner. To determine the downstream pathway of this auto-amplification loop, CXCL10 was found to induce transcriptional activity of NFκB and upregulate NFκB subunit P65 in 4T1 cells [181]. Importantly, the anti-inflammatory and protective actions of JN-2 in CIA were speculated to be, at least partially, dependent on the inhibition of CXCL10 auto-amplification, in addition to CXCR3 antagonism [176]. Synovial T cell–B cell interactions may also create positive feedback loop [152]. Synovial B cells of RA patients exhibit high mRNA expression of CXCL9 and CXCL10 (vide supra). This B cell-mediated chemokine expression was speculated to be dependent on stimulation by activated TH1 cells, since co-culturing of human B cells isolated from HCs with human active CXCR3+ CCR6− TH1 cells significantly induced mRNA expression and secretion of CXCL9 and CXCL10 by these B cells [152]. Hence, synovial B cells presumably become potent CXCR3 ligand producers in the TH1 cell milieu of the inflamed joint [152]. In turn, the secreted CXCL9 and CXCL10 could recruit additional CXCR3+ T cells, which consecutively will further stimulate B cells.
Posttranslational modifications
The co-localization of IFN-inducible CXCR3-binding chemokines and their respective processing enzymes (e.g., CD26 [409, 410], CD13 [411], MMPs [412, 413], furin [414, 415], etcetera) in the SF and the synovium provides an optimal environment to enable PTMs (Fig. 5J) [416]. For example, IFN-inducible CXCR3 ligand-mediated ingress of CXCR3+ CD26+ T cells into the synovium may result in a negative feedback loop, leading to auto-inactivation [47]. CD26 efficiently truncates CXCL9, CXCL10 and CXCL11 aminoterminally, thereby abrogating their chemotactic potential and converting these chemokines into chemotaxis antagonists [53, 417–420]. However, data on posttranslationally modified chemokines in the synovial environment is momentarily very scarce [385], since current methodology for detection of chemokine protein concentrations fail to discriminate between chemokine proteoforms.
Atypical chemokine receptors
ACKR1/DARC and ACKR3/CXCR7 have been identified on synovial endothelial cells in rheumatoid synovial biopsies [421, 422], whereas ACKR2/D6 was detected on stromal cells and CD45+ leukocytes located in aggregates in the synovial tissue of RA patients [423]. Although the role of ACKRs in arthritis remains to be unveiled, ACKR1 and ACKR3 have been examined in animal models of arthritis [94, 422, 424]. ACKR1 mediated neutrophil adhesion to endothelial cells in a co-culture of RA patient-derived FLS and endothelial cells, resembling the rheumatoid synovium [425]. In addition, ACKR1 was found to transport CXCL1 and CXCL2 through the joint endothelium in mice with K/BxN serum-transferred arthritis, thereby mediating transendothelial migration of neutrophils and their subsequent entry in the joints [424]. It remains to be determined whether IFN-inducible CXCR3 ligands—in particular CXCL11 given its high affinity for ACKR1 [71]—are also transcytosed by ACKR1 from the circulation into the synovium and vice versa. In addition, scavenging of CXCL10 in the inflamed synovia by ACKR2/D6 expressed on stromal cells and circulating leukocytes is another potential mechanism that remains to be explored. Furthermore, ACKR3 exerted angiogenic actions in the joints during CIA [422]. Treatment of CIA-developing DBA/1 J mice with an ACKR3 antagonist attenuated clinical arthritis score, coinciding with a reduced number of blood vessels present in the synovial tissue [422]. Since the utilized ACKR3 antagonist abrogates CXCL12-ACKR3 interaction [422], the angiogenic potential of ACKR3 in CIA may be mediated through CXCL12 signaling. Again, whether synovial CXCL11 may be involved in ACKR3-mediated actions in the inflamed joints is uncharted territory. Given the co-localization of ACKR and IFN-inducible CXCR3 ligands in the inflamed synovia, their interaction is likely to occur (Fig. 5K).
Angiostasis
Pronounced upregulation of the angiostatic IFN-inducible CXCR3 ligands in SF of patients with RA and JIA—diseases typically characterized by significant angiogenesis in the synovial tissue [93, 426]—seems counterintuitive. Erdem et al. speculated that augmented synovial levels of angiostatic CXCR3 ligands may be a compensating mechanism to cope with increased synovial levels of angiogenic chemokines (Fig. 5L) [144]. Thus, extensive neovascularization in the synovium may be partially prohibited by the IFN-inducible CXCR3 ligands, thereby possibly acting in an anti-inflammatory manner. Moreover, immunohistochemistry showed that synovial endothelial cells were positive for CXCL10 expression in JIA [253]. Hence, additional research is needed to elucidate whether the CXCR3 ligands may play a role in the regulation of angiostasis in synovia of patients with inflammatory arthritides.
Final remarks
In general, chemokines are considered protagonists in initiation and maintenance of synovitis through leukocytes diapedesis from the vasculature into the synovium. Eminent upregulation of CXCL9 and CXCL10 in the SF—and consequently also in the circulation due to chemokine egress—is evident in distinct rheumatic disease entities with a clear autoimmune component including RA, oligo- and polyarticular JIA, ankylosing spondylitis, and psoriatic arthritis. Given the superimposable clinical manifestations and symptomatology of autoimmune arthritides and inflammatory arthropathies without a clearly defined autoimmune component (e.g., osteoarthritis) [427], pronouncedly elevated circulatory and synovial levels of CXCL9 and CXCL10 may facilitate disease identification during differential diagnosis. In the search for suitable biomarkers, utility of serum levels of CXCL10 as biomarker may prevail over those of CXCL9 for monitoring of rheumatic disease activity, since repeated measurements of circulatory CXCL10—but not CXCL9—had acceptable longitudinal reliability [428]. The notion that the upregulation of IFN-inducible CXCR3 ligands is a mere “bystander” epiphenomenon of synovitis seems implausible, since CXCL9 and CXCL10 are equipped with an arsenal of pro-inflammatory actions that may deleteriously affect the articular environment (Fig. 5A–L), whereas actions of CXCL11 in the joints are less well understood and may even conversely restrain inflammation. In addition to their generally acknowledged function of mediating synovial ingress of distinct CXCR3+ leukocyte lineages, CXCL9 and CXCL10 exert a multitude of molecular activities including amplifying pro-inflammatory cytokine secretion, altering FLS morphology towards a proliferative, aggressive and invasive phenotype with augmented MMP secretion, promoting secretion of osteoblast-derived bone remodeling enzymes and invigorating osteoclastogenesis. When acting in concert, these localized actions of CXCL9 and CXCL10 presumably facilitate a plethora of pathogenic processes in the synovial niche including synovitis, synovial hyperplasia, pannus formation and invasion, bone remodeling, cartilage and bone erosion.
Surprisingly, the pivotal aspect of PTMs has remained unexplored for the IFN-inducible CXCR3 ligands in inflammatory arthritides, despite being an apparent major regulatory mechanism of these chemokines [46, 429]. First, the IFN-inducible CXCR3 ligands are extremely susceptible to PTMs, which drastically fine-tunes their receptor affinity and specificity, GAG binding avidity and chemokine potency [19, 46]. Second, experimental evidence exists that PTMs of CXCL10 have in vivo biological importance [430]. For example, in the regulation of tumor immunity, CD26 inhibition prevented processing of bioactive murine CXCL10 (mCXCL10) into mCXCL10(3–77) in C57BL/6 mice and thereby increased migration of adoptively transferred tumor-specific CXCR3+ T cells into B16F10 melanoma upon intra-tumoral injection of mCXCL10 [430]. Third, in clinical pathophysiological settings including chronic hepatis C virus (HCV), bladder carcinoma and active tuberculosis, antibody-based methodology to quantify CXCL10 proteoforms in plasma—i.e., CXCL10(1–77) and CD26-truncated antagonistic CXCL10(3–77)—has demonstrated that natural truncated CXCL10(3–77) is present in plasma and urine [431–433]. In addition, plasma CXCL10(3–77) was found to be the predominant CXCL10 proteoform of patients with chronic HCV that failed to respond to pegylated IFN-α2/ribavirin therapy [434] and was found to be positively correlated with viral replication in HCV [435]. However, antibody-based methods for chemokine proteoform identification are challenging given that C-terminally and/or N-terminally processed proteoforms should be simultaneously distinguished, requiring multiple epitopes present in confined levels and with only minor structural alterations to be recognized concurrently. This urges the development of sensitive biochemical techniques that permit simultaneous discrimination of full spectra of chemokine isoforms. Fourth, recent evidence revealing extensive processing of the pro-inflammatory chemokine CXCL8 into CXCL8 proteoforms with increased potency in SF of patients with RA and JIA, reaffirms the relevance of PTMs in the synovial niche [385]. Altogether, the identification of proteoforms of IFN-inducible CXCR3 ligands, offer a definitive substantiation that PTM are indeed crucial for modulating biological activity of chemokines and profoundly underscore the importance of grasping this facet in any clinicopathological context. As such, in addition to the CXCL11-mediated anti-inflammatory actions, enzymatic processing affecting the stability and modifying the activity of CXCR3 ligands into potential antagonists, may underlie the failure of the CXCR3-targeting interventions. Moreover, although currently incompletely understood in vivo, GAGs, ACKRs, and CXCR3 forms may also be important to fine-tune the molecular interactions and cellular outcomes of the CXCR3 ligands in the joints. Consequently, improved understanding of the in vivo actions of the posttranslational modified CXCR3 ligands, their interactions with distinct CXCR3 isoforms and ACKRs, their reciprocal intercommunion with each other, cytokines, GAGs, effector and resident cells in the synovial niche is needed to further comprehend their complex actions in the inflamed joints and to guarantee successful application of CXCR3-targeting drugs in diseases characterized by chronic inflammatory arthritis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Figures were created with Biorender.com and ACD/ChemSketch.
Abbreviations
- AA
Rat adjuvant arthritis
- ACKR
Atypical chemokine receptor
- ACR
American College of Rheumatology
- AOSD
Adult-onset Still’s disease
- AS
Ankylosing spondylitis
- BMMs
Bone marrow-derived macrophages
- CAIA
Type II collagen antibody-induced arthritis
- cAMP
Cyclic adenosine monophosphate
- CDAI
Clinical disease activity index
- CFA
Complete Freund’s adjuvant
- CIA
Type II collagen-induced arthritis
- CRP
C-reactive proteins
- DARC
Duffy antigen receptor for chemokines
- DAS28
Disease activity score in 28 joints
- DC
Dendritic cell
- DMARDs
Disease-modifying anti-rheumatic drugs
- DPP
Dipeptidyl peptidase
- ERK
Extracellular signal-regulated kinase
- ESR
Erythrocyte sedimentation rate
- FLS
Fibroblast-like synoviocytes
- GAG
Glycosaminoglycan
- GATA3
GATA-binding protein 3
- GPCR
G protein-coupled receptor
- HC
Healthy control
- HEK
Human embryonal kidney
- HLH
Hemophagocytic lymphohistiocytosis
- IFN
Interferon
- IL-
Interleukin
- IP-10/CXCL10
Interferon- inducible protein of 10 kDa
- IRSE
Interferon response element
- I-TAC/CXCL11
Interferon-inducible T-cell α chemoattractant
- JIA
Juvenile idiopathic arthritis
- MAS
Macrophage activation syndrome
- Mig/CXCL9
Monokine induced by interferon-
- MMPs
Matrix metalloproteinases
- NK
Natural killer
- NSAIDs
Non-steroidal anti-inflammatory drugs
- PBMC
Peripheral blood mononuclear cells
- pDC
Plasmacytoid dendritic cells
- PF-4/CXCL4
Platelet factor-4
- PF-4var1/CXCL4L1
Platelet factor-4 gene variant
- PLC
Phospholipase C
- RA
Rheumatoid arthritis
- RANKL
Receptor activator of nuclear factor kappa-Β ligand
- RF
Rheumatoid factor
- SF
Synovial fluid
- sJIA
Systemic JIA
- SJC
Swollen joint counts
- STAT
Signal transducer and activator
- TJC
Tender joint counts
- TJI
Traumatic joint injury
- TJR
Total joint replacement
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor α
- TRAP
Tartrate-resistant acid phosphatase
Author contributions
LD wrote the initial manuscript. All authors contributed to the study conception and design, provided their comments on former versions of the manuscript and approved the final version of the manuscript.
Funding
This work was funded by a C1 grant (C16/17/010) from KU Leuven, project G067123N of FWO Flanders, and by the Rega Foundation. LD obtained a predoctoral fellowship Fundamental Research of FWO Flanders (11L3122N).
Data availability
Information supporting the manuscript can be found in the Supplementary Material.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338(7):436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 3.Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9(9):953–959. doi: 10.1038/ni.f.207/1. [DOI] [PubMed] [Google Scholar]
- 4.Proost P, Struyf S, Van Damme J, Fiten P, Ugarte-Berzal E, Opdenakker G. Chemokine isoforms and processing in inflammation and immunity. J Autoimmun. 2017;85:45–57. doi: 10.1016/j.jaut.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Opdenakker G, Proost P, Van Damme J. Microbiomic and posttranslational modifications as preludes to autoimmune diseases. Trends Mol Med. 2016;22(9):746–757. doi: 10.1016/j.molmed.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Lee EY, Lee ZH, Song YW. The interaction between CXCL10 and cytokines in chronic inflammatory arthritis. Autoimmun Rev. 2013;12(5):554–557. doi: 10.1016/j.autrev.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Abron JD, Singh NP, Murphy AE, Mishra MK, Price RL, Nagarkatti M, et al. Differential role of CXCR3 in inflammation and colorectal cancer. Oncotarget. 2018;9(25):17928–17936. doi: 10.18632/oncotarget.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo PT, Zeng Z, Salim N, Mattarollo S, Wells JW, Leggatt GR. The role of CXCR3 and its chemokine ligands in skin disease and cancer. Front Med. 2018;5:271. doi: 10.3389/fmed.2018.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16(6):593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp Cell Res. 2011;317(5):685–690. doi: 10.1016/j.yexcr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Knaut H. Chemokine signaling in development and disease. Dev. 2014;141(22):4199–4205. doi: 10.1242/dev.101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116(1):1–12. doi: 10.1111/j.1365-2567.2005.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen K, Bao Z, Tang P, Gong W, Yoshimura T, Wang JM. Chemokines in homeostasis and diseases. Cell Mol Immunol. 2018;15(4):324–334. doi: 10.1038/cmi.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25(2):75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Gouwy M, Struyf S, Proost P, Van Damme J. Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine Growth Factor Rev. 2005;16(6):561–580. doi: 10.1016/j.cytogfr.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. J Cult Herit. 2000;1(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 17.Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. International union of pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66(1):1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacon K, Baggiolini M, Broxmeyer H, Horuk R, Lindley I, Mantovani A, et al. Chemokine/chemokine receptor nomenclature. Cytokine. 2003;21(1):48–49. doi: 10.1016/S1043-4666(02)00493-3. [DOI] [PubMed] [Google Scholar]
- 19.Moelants EAV, Mortier A, Van Damme J, Proost P. In vivo regulation of chemokine activity by post-translational modification. Immunol Cell Biol. 2013;91(6):402–407. doi: 10.1038/icb.2013.16. [DOI] [PubMed] [Google Scholar]
- 20.Mortier A, Gouwy M, Van Damme J, Proost P. Effect of posttranslational processing on the in vitro and in vivo activity of chemokines. Exp Cell Res. 2011;317(5):642–654. doi: 10.1016/j.yexcr.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Mortier A, Van DJ, Proost P. Regulation of chemokine activity by posttranslational modification. Pharmacol Ther. 2008;120(2):197–217. doi: 10.1016/j.pharmthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89(2):1–8. doi: 10.1038/icb.2010.158.CXCR3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015;26(3):311–327. doi: 10.1016/j.cytogfr.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315(6021):672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 25.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187(12):2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HH, Farber JM. Localization of the gene for the human MIG cytokine on chromosome 4q21 adjacent to IP10 reveals a chemokine “mini-cluster”. Cytogenet Genome Res. 1996;74(4):255–258. doi: 10.1159/000134428. [DOI] [PubMed] [Google Scholar]
- 27.Tensen CP, Flier J, Van Der Raaij-Helmer EMH, Sampat-Sardjoepersad S, Van Der Schors RC, Leurs R, et al. Human IP-9: a keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3) J Invest Dermatol. 1999;112(5):716–722. doi: 10.1046/j.1523-1747.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 28.Farber JM. A macrophage mRNA selectively induced by γ-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci USA. 1990;87(14):5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Struyf S, Salogni L, Burdick MD, Vandercappellen J, Gouwy M, Noppen S, et al. Angiostatic and chemotactic activities of the CXC chemokine CXCL4L1 (platelet factor-4 variant) are mediated by CXCR3. Blood. 2011;117(2):480–488. doi: 10.1182/blood-2009-11-253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller A, Meiser A, McDonagh EM, Fox JM, Petit SJ, Xanthou G, et al. CXCL4-induced migration of activated T lymphocytes is mediated by the chemokine receptor CXCR3. J Leukoc Biol. 2008;83(4):875–882. doi: 10.1189/jlb.1006645. [DOI] [PubMed] [Google Scholar]
- 31.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61(3):246–257. doi: 10.1002/jlb.61.3.246. [DOI] [PubMed] [Google Scholar]
- 32.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, et al. Chemokine Receptor Specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184(3):963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166(11):6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 34.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71(2):173–183. doi: 10.1189/jlb.71.2.173. [DOI] [PubMed] [Google Scholar]
- 35.Taub DD, Sayers TJ, Carter CRD, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–3888. doi: 10.4049/jimmunol.155.8.3877. [DOI] [PubMed] [Google Scholar]
- 36.Inngjerdingen M, Damaj B, Maghazachi AA. Expression and regulation of chemokine receptors in human natural killer cells. Blood. 2001;97(2):367–375. doi: 10.1182/blood.v97.2.367. [DOI] [PubMed] [Google Scholar]
- 37.Maghazaciu AA, Skålhegg BS, Rolstad B, Al-Aoukaty A. Interferon-inducible protein-10 and lymphotactin induce the chemotaxis and mobilization of intracellular calcium in natural killer cells through pertussis toxin-sensitive and -insensitive heterotrimeric G-proteins. FASEB J. 1997;11(10):765–774. doi: 10.1096/fasebj.11.10.9271361. [DOI] [PubMed] [Google Scholar]
- 38.Thapa M, Welner RS, Pelayo R, Carr D. CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J Immunol. 2008;180(2):1098–1106. doi: 10.4049/jimmunol.180.2.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wennerberg E, Kremer V, Childs R, Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother. 2015;64(2):225–235. doi: 10.1007/s00262-014-1629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y, et al. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol. 2003;73(6):771–780. doi: 10.1189/jlb.1102573. [DOI] [PubMed] [Google Scholar]
- 41.Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ. Interferon γ-inducible protein-10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun. 1995;210(1):51–57. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- 42.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182(1):155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang B, Wang W, Li Q, Wang Z, Yan B, Zhang Z, et al. Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone. Nat Commun. 2016;7:13885. doi: 10.1038/ncomms13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romagnani P, Annunziato F, Lazzeri E, Cosmi L, Beltrame C, Lasagni L, et al. Interferon-inducible protein 10, monokine induced by interferon gamma, and interferon-inducible T-cell alpha chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) αβ+ CD8+ single-positive T cells, TCRγδ+ T cells. Blood. 2001;97(3):601–607. doi: 10.1182/blood.V97.3.601. [DOI] [PubMed] [Google Scholar]
- 45.Proost P, Struyf S, Loos T, Gouwy M, Schutyser E, Conings R, et al. Coexpression and interaction of CXCL10 and CD26 in mesenchymal cells by synergising inflammatory cytokines: CXCL8 and CXCL10 are discriminative markers for autoimmune arthropathies. Arthritis Res Ther. 2006;8(4):R107. doi: 10.1186/ar1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metzemaekers M, Vanheule V, Janssens R, Struyf S, Proost P. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Front Immunol. 2018;8:1970. doi: 10.3389/fimmu.2017.01970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proost P, Verpoest S, De Borne K, Van SE, Struyf S, Put W, et al. Synergistic induction of CXCL9 and CXCL11 by Toll-like receptor ligands and interferon-γ in fibroblasts correlates with elevated levels of CXCR3 ligands in septic arthritis synovial fluids. J Leukoc Biol. 2004;75(5):777–784. doi: 10.1189/jlb.1003524. [DOI] [PubMed] [Google Scholar]
- 48.Loos T, Dekeyzer L, Struyf S, Schutyser E, Gijsbers K, Gouwy M, et al. TLR ligands and cytokines induce CXCR3 ligands in endothelial cells: enhanced CXCL9 in autoimmune arthritis. Lab Investig. 2006;86(9):902–916. doi: 10.1038/labinvest.3700453. [DOI] [PubMed] [Google Scholar]
- 49.Hensbergen PJ, Verzijl D, Balog CIA, Dijkman R, Van Der Schors RC, Van Der Raaij-Helmer EMH, et al. Furin is a chemokine-modifying enzyme: In vitro and in vivo processing of CXCL10 generates a C-terminally truncated chemokine retaining full activity. J Biol Chem. 2004;279(14):13402–13411. doi: 10.1074/jbc.M312814200. [DOI] [PubMed] [Google Scholar]
- 50.Hensbergen PJ, Van Der Raaij-Helmer EMH, Dijkman R, Van Der Schors RC, Werner-Felmayer G, Boorsma DM, et al. Processing of natural and recombinant CXCR3-targeting chemokines and implications for biological activity. Eur J Biochem. 2001;268(18):4992–4999. doi: 10.1046/j.0014-2956.2001.02433.x. [DOI] [PubMed] [Google Scholar]
- 51.Erdel M, Theurl M, Meyer M, Duba HC, Utermann G, Gabriele WF. High-resolution mapping of the human 4q21 and the mouse 5E3 SCYB chemokine cluster by fiber-fluorescence in situ hybridization. Immunogenetics. 2001;53(7):611–615. doi: 10.1007/s002510100363. [DOI] [PubMed] [Google Scholar]
- 52.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197(11):1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Proost P, Schutyser E, Menten P, Struyf S, Wuyts A, Opdenakker G, et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98(13):3554–3561. doi: 10.1182/blood.V98.13.3554. [DOI] [PubMed] [Google Scholar]
- 54.Thompzson BD, Jin Y, Wu KH, Colvin RA, Luster AD, Birnbaumer L, et al. Inhibition of Gai2 activation by Gai3 in CXCR3-mediated signaling. J Biol Chem. 2007;282(13):9547–9555. doi: 10.1074/jbc.M610931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smit MJ, Verdijk P, Van der Raaij-Helmer EMH, Navis M, Hensbergen PJ, Leurs R, et al. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi-and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood. 2003;102(6):1959–1965. doi: 10.1182/blood-2002-12-3945. [DOI] [PubMed] [Google Scholar]
- 56.Loos T, Mortier A, Gouwy M, Ronsse I, Put W, Lenaerts JP, et al. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: a naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood. 2008;112(7):2648–2656. doi: 10.1182/blood-2008-04-149039. [DOI] [PubMed] [Google Scholar]
- 57.Berchiche YA, Sakmar TP. CXC chemokine receptor 3 alternative splice variants selectively activate different signaling pathways. Mol Pharmacol. 2016;90(4):483–495. doi: 10.1124/mol.116.105502. [DOI] [PubMed] [Google Scholar]
- 58.Smith JS, Alagesan P, Desai NK, Pack TF, Wu JH, Inoue A, et al. C-X-C motif chemokine receptor 3 splice variants differentially activate beta-arrestins to regulate downstream signaling pathways. Mol Pharmacol. 2017;92(2):136–150. doi: 10.1124/mol.117.108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korniejewska A, Mcknight AJ, Johnson Z, Watson ML, Ward SG. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology. 2011;132(4):503–515. doi: 10.1111/j.1365-2567.2010.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonacchi A, Romagnani P, Romanelli RG, Efsen E, Annunziato F, Lasagni L, et al. Signal transduction by the chemokine receptor CXCR3: Activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem. 2001;276(13):9945–9954. doi: 10.1074/jbc.M010303200. [DOI] [PubMed] [Google Scholar]
- 61.Ehlert JE, Addison CA, Burdick MD, Kunkel SL, Strieter RM. Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol. 2004;173(10):6234–6240. doi: 10.4049/jimmunol.173.10.6234. [DOI] [PubMed] [Google Scholar]
- 62.Reynders N, Abboud D, Baragli A, Noman MZ, Rogister B, Niclou SP, et al. The distinct roles of CXCR3 variants and their ligands in the tumor microenvironment. Cells. 2019;8(6):613. doi: 10.3390/cells8060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ranjbaran H, Wang Y, Manes TD, Yakimov AO, Akhtar S, Kluger MS, et al. Heparin displaces interferon-γ–inducible chemokines (IP-10, I-TAC, and Mig) sequestered in the vasculature and and inhibits the transendothelial migration and arterial recruitment of T cells. Circulation. 2006;114:1293–1300. doi: 10.1161/CIRCULATIONAHA.106.631457. [DOI] [PubMed] [Google Scholar]
- 64.Kohrgruber N, Gröger M, Meraner P, Petzelbauer P, Brandt S, Stingl G, et al. Plasmacytoid dendritic cell recruitment by immobilized CXCR3 ligands. J Immunol. 2004;173(11):6592–6602. doi: 10.4049/jimmunol.173.11.6592. [DOI] [PubMed] [Google Scholar]
- 65.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182(1):219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saxena A, Bujak M, Frunza O, Dobaczewski M, Gonzalez-quesada C, Lu B, et al. CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res. 2014;103(2):217–227. doi: 10.1093/cvr/cvu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120(6):2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen JP, Lu HL, Lai SL, Gabriele S, Sung JM, Lu MY, et al. Dengue virus induces expression of CXC chemokine Ligand 10/IFN-γ-Inducible Protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J Immunol. 2006;177(5):3185–3195. doi: 10.4049/jimmunol.177.5.3185. [DOI] [PubMed] [Google Scholar]
- 69.Campanella GSV, Colvin RA, Luster AD. CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS ONE. 2010;5(9):e12700. doi: 10.1371/journal.pone.0012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Metzemaekers M, Mortier A, Janssens R, Boff D, Vanbrabant L, Lamoen N, et al. Glycosaminoglycans regulate CXCR3 ligands at distinct levels: protection against processing by dipeptidyl peptidase IV/CD26 and interference with receptor signaling. Int J Mol Sci. 2017;18(7):1513. doi: 10.3390/ijms18071513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gardner L, Patterson AM, Ashton BA, Stone MA, Middleton J. The human Duffy antigen binds selected inflammatory but not homeostatic chemokines. Biochem Biophys Res Commun. 2004;321(2):306–312. doi: 10.1016/j.bbrc.2004.06.146. [DOI] [PubMed] [Google Scholar]
- 72.Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol. 2009;10(1):101–108. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kashiwazaki M, Tanaka T, Kanda H, Ebisuno Y, Izawa D, Fukuma N, et al. A high endothelial venule-expressing promiscuous chemokine receptor DARC can bind inflammatory, but not lymphoid, chemokines and is dispensable for lymphocyte homing under physiological conditions. Int Immunol. 2003;15(10):1219–1227. doi: 10.1093/intimm/dxg121. [DOI] [PubMed] [Google Scholar]
- 74.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chevigné A, Janji B, Meyrath M, Reynders N, D’uonnolo G, Uchański T, et al. CXCL10 is an agonist of the CC family chemokine scavenger receptor ACKR2/D6. Cancers (Basel). 2021;13(5):1054. doi: 10.3390/cancers13051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xanthou G, Duchesnes CE, Williams TJ, Pease JE. CCR3 functional responses are regulated by both CXCR3 and its ligands CXCL9, CXCL10 and CXCL11. Eur J Immunol. 2003;33(8):2241–2250. doi: 10.1002/eji.200323787. [DOI] [PubMed] [Google Scholar]
- 77.Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, Bardi G, et al. The ligands of CXC chemokine receptor 3, I-TAC, mig, and IP10, are natural antagonists for CCR3. J Biol Chem. 2001;276(5):2986–2991. doi: 10.1074/jbc.M005652200. [DOI] [PubMed] [Google Scholar]
- 78.Petkovic V, Moghini C, Paoletti S, Uguccioni M, Gerber B. I-TAC/CXCL11 is a natural antagonist for CCR5. J Leukoc Biol. 2004;76(3):701–708. doi: 10.1189/jlb.1103570. [DOI] [PubMed] [Google Scholar]
- 79.Crijns H, Vanheule V, Proost P. Targeting chemokine—glycosaminoglycan interactions to inhibit inflammation. Front Immunol. 2020;11:483. doi: 10.3389/fimmu.2020.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lande R, Giacomini E, Serafini B, Rosicarelli B, Sebastiani GD, Minisola G, et al. Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J Immunol. 2004;173(4):2815–2824. doi: 10.4049/jimmunol.173.4.2815. [DOI] [PubMed] [Google Scholar]
- 81.Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F, et al. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol. 2008;181(11):8053–8067. doi: 10.4049/jimmunol.181.11.8053. [DOI] [PubMed] [Google Scholar]
- 82.Ichikawa A, Kuba K, Morita M, Chida S, Tezuka H, Hara H, et al. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am J Respir Crit Care Med. 2013;187(1):65–77. doi: 10.1164/rccm.201203-0508OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Floudas A, Neto N, Marzaioli V, Murray K, Moran B, Monaghan MG, et al. Pathogenic, glycolytic PD-1+ B cells accumulate in the hypoxic RA joint. JCI Insight. 2020 doi: 10.1172/jci.insight.139032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zohar Y, Wildbaum G, Novak R, Salzman AL, Thelen M, Alon R, et al. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest. 2014;124(5):2009–2022. doi: 10.1172/JCI71951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schulthess FT, Paroni F, Sauter NS, Shu L, Ribaux P, Haataja L, et al. CXCL10 impairs β cell function and viability in diabetes through TLR4 signaling. Cell Metab. 2009;9(2):125–139. doi: 10.1016/j.cmet.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 86.Lee JH, Kim B, Jin WJ, Kim HH, Ha H, Lee ZH. Pathogenic roles of CXCL10 signaling through CXCR3 and TLR4 in macrophages and T cells: relevance for arthritis. Arthritis Res Ther. 2017;19(1):163. doi: 10.1186/s13075-017-1353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soejima K, Rollins BJ. A functional IFN-γ-inducible protein-10/CXCL10-specific receptor expressed by epithelial and endothelial cells that is neither CXCR3 nor glycosaminoglycan. J Immunol. 2001;167(11):6576–6582. doi: 10.4049/jimmunol.167.11.6576. [DOI] [PubMed] [Google Scholar]
- 88.Campanella GSV, Grimm J, Manice LA, Colvin RA, Medoff BD, Gregory R, et al. Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol. 2006;177(10):6991–6998. doi: 10.4049/jimmunol.177.10.6991. [DOI] [PubMed] [Google Scholar]
- 89.Dyer DP, Migliorini E, Salanga CL, Thakar D, Handel TM, Richter RP. Differential structural remodelling of heparan sulfate by chemokines: the role of chemokine oligomerization. Open Biol. 2017;7(1):160286. doi: 10.1098/rsob.160286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dyer DP, Salanga CL, Volkman BF, Kawamura T, Handel TM. The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization. Glycobiology. 2015;26(3):312–326. doi: 10.1093/glycob/cwv100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Severin IC, Gaudry JP, Johnson Z, Kungl A, Jansma A, Gesslbauer B, et al. Characterization of the chemokine CXCL11-heparin interaction suggests two different affinities for glycosaminoglycans. J Biol Chem. 2010;285(23):17713–17724. doi: 10.1074/jbc.M109.082552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 93.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 94.Miyabe Y, Miyabe C, Iwai Y, Luster AD. Targeting the chemokine system in rheumatoid arthritis and vasculitis. JMA J. 2020;3(3):182–192. doi: 10.31662/jmaj.2020-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szekanecz Z, Koch AE. Successes and failures of chemokine-pathway targeting in rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(1):5–13. doi: 10.1038/nrrheum.2015.157. [DOI] [PubMed] [Google Scholar]
- 96.Elemam NM, Hannawi S, Maghazachi AA. Role of chemokines and chemokine receptors in Rheumatoid arthritis. ImmunoTarg Ther. 2020;9:43–56. doi: 10.2147/ITT.S243636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yap HY, Tee S, Wong M, Chow SK, Peh SC, Teow SY. Pathogenic role of immune cells in rheumatoid arthritis: implications in clinical treatment and biomarker development. Cells. 2018;7(10):161. doi: 10.3390/cells7100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–631. doi: 10.1016/j.yexcr.2010.12.017.CXCR3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Campanella GSV, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, et al. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci U S A. 2008;105(12):4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohan K, Issekutz TB. Blockade of chemokine receptor CXCR3 inhibits T Cell recruitment to inflamed joints and decreases the severity of adjuvant arthritis. J Immunol. 2007;179(12):8463–8469. doi: 10.4049/jimmunol.179.12.8463. [DOI] [PubMed] [Google Scholar]
- 101.Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K, et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol. 2009;183(7):4693–4704. doi: 10.4049/jimmunol.0802626. [DOI] [PubMed] [Google Scholar]
- 102.Patel DD, Zachariah JP, Whichard LP. CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clin Immunol. 2001;98(1):39–45. doi: 10.1006/clim.2000.4957. [DOI] [PubMed] [Google Scholar]
- 103.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101(4):746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loetscher P, Uguccioni M, Lorenza B, Baggiolini M, Moser B. CCR5 is characteristic of Th1 lymphocytes. Nature. 1997;391(6665):344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 105.Ruth JH, Rottman JB, Katschke KJ, Qin S, Wu L, Larosa G, et al. Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum. 2001;44(12):2750–2760. doi: 10.1002/1529-0131(200112)44:12<2750::AID-ART462>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 106.Ruschpler P, Lorenz P, Eichler W, Koczan D, Hänel C, Scholz R, et al. High CXCR3 expression in synovial mast cells associated with CXCL9 and CXCL10 expression in inflammatory synovial tissues of patients with rheumatoid arthritis. Arthritis Res Ther. 2003;5(5):241–252. doi: 10.1186/ar783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aldridge J, Ekwall AKH, Mark L, Bergström B, Andersson K, Gjertsson I, et al. T helper cells in synovial fluid of patients with rheumatoid arthritis primarily have a Th1 and a CXCR3+ Th2 phenotype. Arthritis Res Ther. 2020;22(1):245. doi: 10.1186/s13075-020-02349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aeberli D, Seitz M, Jüni P, Villiger PM. Increase of peripheral CXCR3 positive T lymphocytes upon treatment of RA patients with TNF-α inhibitors. Rheumatology. 2005;44(2):172–175. doi: 10.1093/rheumatology/keh437. [DOI] [PubMed] [Google Scholar]
- 109.Dalbeth N, Callan MFC. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum. 2002;46(7):1763–1772. doi: 10.1002/art.10410. [DOI] [PubMed] [Google Scholar]
- 110.Tsubaki T, Takegawa S, Hanamoto H, Arita N, Kamogawa J, Yamamoto H, et al. Accumulation of plasma cells expressing CXCR3 in the synovial sublining regions of early rheumatoid arthritis in association with production of Mig/CXCL9 by synovial fibroblasts. Clin Exp Immunol. 2005;141(2):363–371. doi: 10.1111/j.1365-2249.2005.02850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katschke KJ, Rottman JB, Ruth JH, Qin S, Wu L, LaRosa G, et al. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001;44(5):1022–1032. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 112.Nanki T, Takada K, Komano Y, Morio T, Kanegane H, Nakajima A, et al. Chemokine receptor expression and functional effects of chemokines on B cells: implication in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2009;11(5):R149. doi: 10.1186/ar2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ota Y, Niiro H, Ota S, Ueki N, Tsuzuki H, Nakayama T, et al. Generation mechanism of RANKL(+) effector memory B cells: relevance to the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2016;18:67. doi: 10.1186/s13075-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ravelli A, Schiappapietra B, Verazza S, Martini A (2017) Chapter 7—Juvenile idiopathic arthritis. In: The heart in rheumatic, autoimmune and inflammatory diseases, pp 167–87. 10.1016/B978-0-12-803267-1.00007-7
- 115.Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41(5):863–877. doi: 10.1007/s00296-020-04731-0. [DOI] [PubMed] [Google Scholar]
- 116.Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Sève P. Adult-onset Still’s disease. Autoimmun Rev. 2014;13(7):708–722. doi: 10.1016/j.autrev.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 117.Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29–30:100587. doi: 10.1016/j.eclinm.2020.100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Runsheng W, Ward MM. Epidemiology of axial spondyloarthritis: an update. Curr Opin Pharmacol. 2018;30(2):137–143. doi: 10.1097/BOR.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scotti L, Franchi M, Marchesoni A, Corrao G. Prevalence and incidence of psoriatic arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2018;48(1):28–34. doi: 10.1016/j.semarthrit.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 120.McBride S, Mowbray J, Caughey W, Wong E, Luey C, Siddiqui A, et al. Epidemiology, management, and outcomes of large and small native joint septic arthritis in adults. Clin Infect Dis. 2020;70(2):271–279. doi: 10.1093/cid/ciz265. [DOI] [PubMed] [Google Scholar]
- 121.Tamer TM. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip Toxicol. 2013;6(3):111–125. doi: 10.2478/intox-2013-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Balazs EA (1974) The physical properties of synovial fluid and the special role of hyaluronic acid. In: Disorders of the knee. Philadelphia, pp 63–75
- 123.Kokkonen H, Söderström I, Rocklöv J, Hallmans G, Lejon K, Dahlqvist SR. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62(2):383–391. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- 124.Ueno A, Yamamura M, Iwahashi M, Okamoto A, Aita T, Ogawa N, et al. The production of CXCR3-agonistic chemokines by synovial fibroblasts from patients with rheumatoid arthritis. Rheumatol Int. 2005;25(5):361–367. doi: 10.1007/s00296-004-0449-x. [DOI] [PubMed] [Google Scholar]
- 125.Imam AM, Hamed AM, Nasef SI, Hassan AM, Omar HH. Biochemical analysis of C-X-C motif chemokine ligand 10 (CXCL10) as a biomarker in patients with rheumatoid arthritis. Egypt J Immunol. 2019;26(2):79–86. [PubMed] [Google Scholar]
- 126.Han BK, Kuzin I, Gaughan JP, Olsen NJ, Bottaro A. Baseline CXCL10 and CXCL13 levels are predictive biomarkers for tumor necrosis factor inhibitor therapy in patients with moderate to severe rheumatoid arthritis: a pilot, prospective study. Arthritis Res Ther. 2016 doi: 10.1186/s13075-016-0995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jude C, Dejica D, Samasca G, Balacescu L, Balacescu O. Soluble CD163 serum levels are elevated and correlated with IL-12 and CXCL10 in patients with long-standing rheumatoid arthritis. Rheumatol Int. 2013;33(4):1031–1037. doi: 10.1007/s00296-012-2459-4. [DOI] [PubMed] [Google Scholar]
- 128.Hueber W, Tomooka BH, Zhao X, Kidd BA, Drijfhout JW, Fries JF, et al. Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with upregulation of proinflammatory cytokines. Ann Rheum Dis. 2007;66(6):712–719. doi: 10.1136/ard.2006.054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Han JH, Suh C, Jung J, Nam J, Kwon JE, Yim H, et al. Association of CXCL10 and CXCL13 levels with disease activity and cutaneous manifestation in active adult-onset Still’s disease. Arthritis Res Ther. 2015;17(1):260. doi: 10.1186/s13075-015-0773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han JH, Suh C, Jung J, Ahn M, Han MH, Kwon JE. Elevated circulating levels of the interferon-γ-induced chemokines are associated with disease activity and cutaneous manifestations in adult-onset Still’s disease. Sci Rep. 2017 doi: 10.1038/srep46652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hanaoka R, Kasama T, Muramatsu M, Yajima N, Shiozawa F, Miwa Y, et al. A novel mechanism for the regulation of IFN- γ inducible protein-10 expression in rheumatoid arthritis. Arthritis Res Ther. 2003;5(2):74–81. doi: 10.1186/ar616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuan WP, Tam L, Wong C, Ko FWS, Li T, Zhu T, et al. CXCL9 and CXCL10 as sensitive markers of disease activity in patients with rheumatoid arthritis. J Rheumatol. 2010;37(2):257–264. doi: 10.3899/jrheum.090769. [DOI] [PubMed] [Google Scholar]
- 133.Pandya JM, Lundell AC, Andersson K, Nordström I, Theander E, Rudin A. Blood chemokine profile in untreated early rheumatoid arthritis: CXCL10 as a disease activity marker. Arthritis Res Ther. 2017;19(1):20. doi: 10.1186/s13075-017-1224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muhsin HY, Kadri ZHM, Ad’hiah AH, Mayouf KZ. Predictive significance of CXCL8, CXCL10 and CXCL16 in juvenile idiopathic and rheumatoid arthritis Iraqi patients. Egypt Rheumatol. 2020;42(2):153–157. doi: 10.1016/j.ejr.2019.06.002. [DOI] [Google Scholar]
- 135.Ichikawa T, Kageyama Y, Kobayashi H, Kato N, Tsujimura K, Koide Y. Etanercept treatment reduces the serum levels of interleukin-15 and interferon-gamma inducible protein-10 in patients with rheumatoid arthritis. Rheumatol Int. 2010;30(6):725–730. doi: 10.1007/s00296-009-1356-y. [DOI] [PubMed] [Google Scholar]
- 136.Sucur A, Jajic Z, Artukovic M, Matijasevic MI, Anic B, Flegar D, et al. Chemokine signals are crucial for enhanced homing and differentiation of circulating osteoclast progenitor cells. Arthritis Res Ther. 2017;19(1):142. doi: 10.1186/s13075-017-1337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee EY, Seo MR, Juhnn YS, Kim JY, Hong YJ, Lee YJ, et al. Potential role and mechanism of IFN-gamma inducible protein-10 on receptor activator of nuclear factor kappa-B ligand (RANKL) expression in rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):R104. doi: 10.1186/ar3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yu K, Proost P. Insights into peptidylarginine deiminase expression and citrullination pathways. Trends Cell Biol. 2022 doi: 10.1016/j.tcb.2022.01.014. [DOI] [PubMed] [Google Scholar]
- 139.Meeuwisse CM, Van Der LMP, Rullmann TA, Allaart CF, Nelissen R, Huizinga TW, et al. Identification of CXCL13 as a marker for rheumatoid arthritis outcome using an in silico model of the rheumatic joint. Arthritis Rheumatol. 2011;63(5):1265–1273. doi: 10.1002/art.30273. [DOI] [PubMed] [Google Scholar]
- 140.Dennis Jr G, Holweg CTJ, Kummerfeld SK, Choy DF, Setiadi AF, Hackney JA, et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther. 2014;16(2):R90. doi: 10.1186/ar4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Greisen SR, Schelde KK, Rasmussen TK, Kragstrup TW, Stengaard-pedersen K, Hetland ML, et al. CXCL13 predicts disease activity in early rheumatoid arthritis and could be an indicator of the therapeutic ‘window of opportunity’. Arthritis Res Ther. 2014;16(5):434. doi: 10.1186/s13075-014-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bechman K, Dalrymple A, Southey-bassols C, Cope AP, Galloway JB. A systematic review of CXCL13 as a biomarker of disease and treatment response in rheumatoid arthritis. BMC Rheumatol. 2020;4(1):70. doi: 10.1186/s41927-020-00154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.García-López MÁ, Sánchez-Madrid F, Rodríguez-Frade JM, Mellado M, Acevedo A, García MI, et al. CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab Investig. 2001;81(3):409–418. doi: 10.1038/labinvest.3780248. [DOI] [PubMed] [Google Scholar]
- 144.Erdem H, Pay S, Musabak U, Simsek I, Dinc A, Pekel A, et al. Synovial angiostatic non-ELR CXC chemokines in inflammatory arthritides: does CXCL4 designate chronicity of synovitis? Rheumatol Int. 2007;27(10):969–973. doi: 10.1007/s00296-007-0317-6. [DOI] [PubMed] [Google Scholar]
- 145.Schmutz C, Hulme A, Burman A, Salmon M, Ashton B, Buckley C, et al. Chemokine receptors in the rheumatoid synovium: upregulation of CXCR5. Arthritis Res Ther. 2005;7(2):217–229. doi: 10.1186/ar1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yoshida S, Arakawa F, Higuchi F, Ishibashi Y, Goto M, Sugita Y, et al. Gene expression analysis of rheumatoid arthritis synovial lining regions by cDNA microarray combined with laser microdissection: Up-regulation of inflammation-associated STAT1, IRF1, CXCL9, CXCL10, and CCL5. Scand J Rheumatol. 2012;41(3):170–179. doi: 10.3109/03009742.2011.623137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee EJ, Lilja S, Li X, Schäfer S, Zhang H, Benson M. Bulk and single cell transcriptomic data indicate that a dichotomy between inflammatory pathways in peripheral blood and arthritic joints complicates biomarker discovery. Cytokine. 2020;127:154960. doi: 10.1016/j.cyto.2019.154960. [DOI] [PubMed] [Google Scholar]
- 148.Kang SE, Park JK, Yoo HJ, Kang H, Park YW, Park BC, et al. Efficacy of novel bispecific antibody targeting TNF-α/CXCL10 in the treatment of experimental arthritis. Transl Res. 2021;232:75–87. doi: 10.1016/j.trsl.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 149.O’Boyle G, Fox CRJ, Walden HR, Willet JDP, Mavin ER, Hine DW, et al. Chemokine receptor CXCR3 agonist prevents human T-cell migration in a humanized model of arthritic inflammation. Proc Natl Acad Sci USA. 2012;109(12):4598–4603. doi: 10.1073/pnas.1118104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.König A, Krenn V, Toksoy A, Gerhard N, Gillitzer R. Mig, GROα and RANTES messenger RNA expression in lining layer, infiltrates and different leucocyte populations of synovial tissue from patients with rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Virchows Arch. 2000;436(5):449–458. doi: 10.1007/s004280050472. [DOI] [PubMed] [Google Scholar]
- 151.Gloghini A, Volpe R, Carbone A. Ki-M6 immunostaining in routinely processed sections of reactive and neoplastic human lymphoid tissue. Am J Clin Pathol. 1990;94(6):734–741. doi: 10.1093/ajcp/94.6.734. [DOI] [PubMed] [Google Scholar]
- 152.Nakayama T, Yoshimura M, Higashioka K, Miyawaki K, Ota Y, Ayano M, et al. Type 1 helper T cells generate CXCL9/10-producing T-bet+ effector B cells potentially involved in the pathogenesis of rheumatoid arthritis. Cell Immunol. 2021;360:104263. doi: 10.1016/j.cellimm.2020.104263. [DOI] [PubMed] [Google Scholar]
- 153.Proost P, Vynckier AK, Mahieu F, Put W, Grillet B, Struyf S, et al. Microbial Toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-γ and provide a mechanism for enhanced synovial chemokine levels in septic arthritis. Eur J Immunol. 2003;33(11):3146–3153. doi: 10.1002/eji.200324136. [DOI] [PubMed] [Google Scholar]
- 154.Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD. CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell α chemoattractant (CXCL11) J Immunol. 2001;167(12):7084–7093. doi: 10.4049/jimmunol.167.12.7084. [DOI] [PubMed] [Google Scholar]
- 155.Karin N, Wildbaum G, Thelen M. Biased signaling pathways via CXCR3 control the development and function of CD4+ T cell subsets. J Leukoc Biol. 2016;99(6):857–862. doi: 10.1189/jlb.2MR0915-441R. [DOI] [PubMed] [Google Scholar]
- 156.Bédard PA, Golds EE. Cytokine-induced expression of mRNAs for chemotactic factors in human synovial cells and fibroblasts. J Cell Physiol. 1993;154(2):433–441. doi: 10.1002/jcp.1041540227. [DOI] [PubMed] [Google Scholar]
- 157.Yoshitomi H. Regulation of immune responses and chronic inflammation by fibroblast-like synoviocytes. Front Immunol. 2019;10:1395. doi: 10.3389/fimmu.2019.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nanki T, Shimaoka T, Hayashida K, Taniguchi K, Yonehara S, Miyasaka N. Pathogenic role of the CXCL16-CXCR6 pathway in rheumatoid arthritis. Arthritis Rheum. 2005;52(10):3004–3014. doi: 10.1002/art.21301. [DOI] [PubMed] [Google Scholar]
- 159.Shi K, Hayashida K, Kaneko M, Hashimoto J, Tomita T, Lipsky PE, et al. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001;166(1):650–655. doi: 10.4049/jimmunol.166.1.650. [DOI] [PubMed] [Google Scholar]
- 160.Rump L, Mattey DL, Kehoe O, Middleton J. An initial investigation into endothelial CC chemokine expression in the human rheumatoid synovium. Cytokine. 2017;97:133–140. doi: 10.1016/j.cyto.2017.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27(3):594–600. [PubMed] [Google Scholar]
- 163.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nygaard G, Firestein GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol. 2020;16(6):316–333. doi: 10.1038/s41584-020-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kuranobu T, Mokuda S, Oi K, Tokunaga T, Yukawa K, Kohno H, et al. Activin A expressed in rheumatoid synovial cells downregulates TNFα-induced CXCL10 expression and osteoclastogenesis. Pathobiology. 2020;87(3):198–207. doi: 10.1159/000506260. [DOI] [PubMed] [Google Scholar]
- 166.Chicheportiche Y, Chicheportiche R, Sizing I, Thompson J, Benjamin CB, Ambrose C, et al. Proinflammatory activity of TWEAK on human dermal fibroblasts and synoviocytes: Blocking and enhancing effects of anti-TWEAK monoclonal antibodies. Arthritis Res. 2002;4(2):126–133. doi: 10.1186/ar388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moret FM, Hack CE, Van Der Wurff-Jacobs KMG, De Jager W, Radstake TRDJ, Lafeber FPJG, et al. Intra-articular CD1c-expressing myeloid dendritic cells from rheumatoid arthritis patients express a unique set of T cell-attracting chemokines and spontaneously induce Th1, Th17 and Th2 cell activity. Arthritis Res Ther. 2013;15(5):R155. doi: 10.1186/ar4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fong KY, Boey ML, Koh WH, Feng PH. Cytokine concentrations in the synovial fluid and plasma of rheumatoid arthritis patients: correlation with bony erosions. Clin Exp Rheumatol. 1994;12(1):55–58. [PubMed] [Google Scholar]
- 169.Ohta K, Naruse T, Kato H, Ishida Y, Nakagawa T, Ono S, et al. Differential regulation by IFN-γ on TNF-α-induced chemokine expression in synovial fibroblasts from temporomandibular joint. Mol Med Rep. 2017;16(5):6850–6857. doi: 10.3892/mmr.2017.7432. [DOI] [PubMed] [Google Scholar]
- 170.Qi XF, Kim DH, Yoon YS, Jin D, Huang XZ, Li JH, et al. Essential involvement of cross-talk between IFN-γ and TNF-α in CXCL10 production in human THP-1 monocytes. J Cell Physiol. 2009;220(3):690–697. doi: 10.1002/jcp.21815. [DOI] [PubMed] [Google Scholar]
- 171.Van Kuijk AWR, Wijbrandts CA, Vinkenoog M, Zheng TS, Reedquist KA, Tak PP. TWEAK and its receptor Fn14 in the synovium of patients with rheumatoid arthritis compared to psoriatic arthritis and its response to tumour necrosis factor blockade. Ann Rheum Dis. 2010;69(1):301–304. doi: 10.1136/ard.2008.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Al-Banna NA, Vaci M, Slauenwhite D, Johnston B, Issekutz TB. CCR4 and CXCR3 play different roles in the migration of T cells to inflammation in skin, arthritic joints, and lymph nodes. Eur J Immunol. 2014;44(6):1633–1643. doi: 10.1002/eji.201343995. [DOI] [PubMed] [Google Scholar]
- 173.Jacobs JP, Ortiz-Lopez A, Campbell JJ, Gerard CJ, Mathis D, Christophe B. Deficiency of CXCR2, but not of other chemokine receptors attenuates a murine model of autoantibody-mediated arthritis. Arthritis Rheum. 2010;62(7):1921–1932. doi: 10.1002/art.27470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bakheet SA, Alrwashied BS, Ansari MA, Nadeem A, Attia SM, Alanazi MM, et al. CXC chemokine receptor 3 antagonist AMG487 shows potent anti-arthritic effects on collagen-induced arthritis by modifying B cell inflammatory profile. Immunol Lett. 2020;225:74–81. doi: 10.1016/j.imlet.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 175.Jenh CH, Cox MA, Cui L, Reich EP, Sullivan L, Chen SC, et al. A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection. BMC Immunol. 2012;13(1):2. doi: 10.1186/1471-2172-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim B, Lee JH, Jin WJ, Kim HH, Ha H, Lee ZH. JN-2, a C-X-C motif chemokine receptor 3 antagonist, ameliorates arthritis progression in an animal model. Eur J Pharmacol. 2018;823:1–10. doi: 10.1016/j.ejphar.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 177.Bakheet SA, Alrwashied BS, Ansari MA, Nadeem A, Attia SM, Assiri MA, et al. CXCR3 antagonist AMG487 inhibits glucocorticoid-induced tumor necrosis factor-receptor-related protein and inflammatory mediators in CD45 expressing cells in collagen-induced arthritis mouse model. Int Immunopharmacol. 2020;84:106494. doi: 10.1016/j.intimp.2020.106494. [DOI] [PubMed] [Google Scholar]
- 178.Bakheet SA, Ansari MA, Nadeem A, Attia SM, Alhoshani AR, Gul G, et al. CXCR3 antagonist AMG487 suppresses rheumatoid arthritis pathogenesis and progression by shifting the Th17/Treg cell balance. Cell Signal. 2019;64:109395. doi: 10.1016/j.cellsig.2019.109395. [DOI] [PubMed] [Google Scholar]
- 179.Yang YF, Mukai T, Gao P, Yamaguchi N, Ono S, Iwaki H, et al. A non-peptide CCR5 antagonist inhibits collagen-induced arthritis by modulating T cell migration without affecting anti-collagen T cell responses. Eur J Immunol. 2002;32(8):2124–2132. doi: 10.1002/1521-4141(200208)32:8<2124::AID-IMMU2124>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 180.Pickens SR, Chamberlain ND, Volin MV, Mandelin AM, Agrawal H, Matsui M, et al. Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum. 2011;63(8):2289–2298. doi: 10.1002/art.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jin WJ, Kim B, Kim D, Choo HYP, Kim HH, Ha H, et al. NF-κB signaling regulates cell-autonomous regulation of CXCL10 in breast cancer 4T1 cells. Exp Mol Med. 2017;49(2):e295. doi: 10.1038/emm.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nedjai B, Li H, Stroke IL, Wise EL, Webb ML, Merritt JR, et al. Small molecule chemokine mimetics suggest a molecular basis for the observation that CXCL10 and CXCL11 are allosteric ligands of CXCR3. Br J Pharmacol. 2012;166(3):912–923. doi: 10.1111/j.1476-5381.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168(7):3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 184.Salomon I, Netzer N, Wildbaum G, Schif-Zuck S, Maor G, Karin N. Targeting the function of IFN-γ-inducible protein 10 suppresses ongoing adjuvant arthritis. J Immunol. 2002;169(5):2685–2693. doi: 10.4049/jimmunol.169.5.2685. [DOI] [PubMed] [Google Scholar]
- 185.Kwak HB, Ha H, Kim HN, Lee JH, Hun SK, Lee S, et al. Reciprocal cross-talk between RANKL and interferon-γ-inducible protein 10 is responsible for bone-erosive experimental arthritis. Arthritis Rheum. 2008;58(5):1332–1342. doi: 10.1002/art.23372. [DOI] [PubMed] [Google Scholar]
- 186.Furman BD, Kent CL, Huebner JL, Kraus VB, McNulty AL, Farshid G, et al. CXCL10 is upregulated in synovium and cartilage following articular fracture. J orthop. 2018;36(4):1220–1227. doi: 10.1002/jor.23735.CXCL10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Collins KH, Reimer RA, Seerattan RA, Leonard TR, Herzog W. Using diet-induced obesity to understand a metabolic subtype of osteoarthritis in rats. Osteoarthr Cartil. 2015;23(6):957–965. doi: 10.1016/j.joca.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 188.Sierro F, Biben C, Martínez-Muñoz L, Mellado M, Ransohoff RM, Li M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA. 2007;104(37):14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Andrews SP, Cox RJ. Small molecule CXCR3 antagonists. J Med Chem. 2016;59(7):2894–2917. doi: 10.1021/acs.jmedchem.5b01337. [DOI] [PubMed] [Google Scholar]
- 190.Pease JE. Designing small molecule CXCR3 antagonists. Expert Opin Drug Discov. 2017;12(2):159–168. doi: 10.1080/17460441.2017.1268597. [DOI] [PubMed] [Google Scholar]
- 191.Schall TJ, Proudfoot AEI. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat Rev Immunol. 2011;11(5):355–363. doi: 10.1038/nri2972. [DOI] [PubMed] [Google Scholar]
- 192.Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MBA. Among CXCR3 chemokines, IFN-γ-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-γ (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol. 2003;171(12):6838–6845. doi: 10.4049/jimmunol.171.12.6838. [DOI] [PubMed] [Google Scholar]
- 193.Narumi S, Kaburaki T, Yoneyama H, Iwamura H, Kobayashi Y, Matsushima K. Neutralization of IFN-inducible protein 10/CXCL10 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32(6):1784–1791. doi: 10.1002/1521-4141(200206)32:6<1784::AID-IMMU1784>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 194.Crosignani S, Missotten M, Cleva C, Dondi R, Ratinaud Y, Humbert Y, et al. Discovery of a novel series of CXCR3 antagonists. Bioorgan Med Chem Lett. 2010;20(12):3614–3617. doi: 10.1016/j.bmcl.2010.04.113. [DOI] [PubMed] [Google Scholar]
- 195.Liu J, Fu Z, Li AR, Johnson M, Zhu L, Marcus A, et al. Optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorgan Med Chem Lett. 2009;19(17):5114–5118. doi: 10.1016/j.bmcl.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 196.Du X, Gustin DJ, Chen X, Duquette J, McGee LR, Wang Z, et al. Imidazo-pyrazine derivatives as potent CXCR3 antagonists. Bioorgan Med Chem Lett. 2009;19(17):5200–5204. doi: 10.1016/j.bmcl.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 197.Hayes ME, Wallace GA, Grongsaard P, Bischoff A, George DM, Miao W, et al. Discovery of small molecule benzimidazole antagonists of the chemokine receptor CXCR3. Bioorgan Med Chem Lett. 2008;18(5):1573–1576. doi: 10.1016/j.bmcl.2008.01.074. [DOI] [PubMed] [Google Scholar]
- 198.Watson RJ, Allen DR, Birch HL, Chapman GA, Hannah DR, Knight RL, et al. Development of CXCR3 antagonists. Part 2: Identification of 2-amino(4-piperidinyl)azoles as potent CXCR3 antagonists. Bioorgan Med Chem Lett. 2007;17(24):6806–6810. doi: 10.1016/j.bmcl.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 199.Allen DR, Bolt A, Chapman GA, Knight RL, Meissner JWG, Owen DA, et al. Identification and structure-activity relationships of 1-aryl-3-piperidin-4-yl-urea derivatives as CXCR3 receptor antagonists. Bioorgan Med Chem Lett. 2007;17(3):697–701. doi: 10.1016/j.bmcl.2006.10.088. [DOI] [PubMed] [Google Scholar]
- 200.Storelli S, Verdijk P, Verzijl D, Timmerman H, Van De Stolpe AC, Tensen CP, et al. Synthesis and structure-activity relationship of 3-phenyl-3H-quinazolin-4-one derivatives as CXCR3 chemokine receptor antagonists. Bioorgan Med Chem Lett. 2005;15(11):2910–2913. doi: 10.1016/j.bmcl.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 201.Heise CE, Pahuja A, Hudson SC, Mistry MS, Putnam AL, Gross MM, et al. Pharmacological characterization of CXC chemokine receptor 3 ligands and a small molecule antagonist. J Pharmacol Exp Ther. 2005;313(3):1263–1271. doi: 10.1124/jpet.105.083683. [DOI] [PubMed] [Google Scholar]
- 202.Johnson M, Li AR, Liu J, Fu Z, Zhu L, Miao S, et al. Discovery and optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorgan Med Chem Lett. 2007;17(12):3339–3343. doi: 10.1016/j.bmcl.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 203.Wijtmans M, Verzijl D, Leurs R, De Esch IJP, Smit MJ. Towards small-molecule CXCR3 ligands with clinical potential. ChemMedChem. 2008;3(6):861–872. doi: 10.1002/cmdc.200700365. [DOI] [PubMed] [Google Scholar]
- 204.Wijtmans M, Scholten D, Mooij W, Smit MJ, de Esch IJP, de Graaf C, et al. Exploring the CXCR3 chemokine receptor with small-molecule antagonists and agonist. Top Med Chem. 2014;10(October):169–244. doi: 10.1007/7355. [DOI] [Google Scholar]
- 205.Medina J, Johnson M, Li A, Liu Z, Huang A, Zhu L et al (2002) CXCR3 antagonists. WO02083143
- 206.Schall T, Dairaghi D, McMaster B (2001) Compounds and methods for modulating CXCR3 function. WO0116114
- 207.Henne KR, Tran TB, VandenBrink BM, Rock DA, Aidasani DK, Subramanian R, et al. Sequential metabolism of AMG 487, a novel CXCR3 antagonist, results in formation of quinone reactive metabolites that covalently modify CYP3A4 Cys239 and cause time-dependent inhibition of the enzyme. Drug Metab Dispos. 2012;40(7):1429–1440. doi: 10.1124/dmd.112.045708. [DOI] [PubMed] [Google Scholar]
- 208.Berry K, Friedrich M, Kersey K, Stempien MJ, Wagner F, van Lier JJ, et al. Evaluation of T0906487, a CXCR3 antagonist, in a phase 2a psoriasis trial. Inflamm Res. 2004;53(Suppl. 3):S222. [Google Scholar]
- 209.Rottman JB, Smith TL, Ganley KG, Kikuchi T, Krueger JG. Potential role of the chemokine receptors CXCR3, CCR4, and the integrin αEβ7 in the pathogenesis of psoriasis vulgaris. Lab Investig. 2001;81(3):335–347. doi: 10.1038/labinvest.3780242. [DOI] [PubMed] [Google Scholar]
- 210.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400(6746):776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 211.Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, et al. Up-regulation of macrophage inflammatory protein-3α/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164(12):6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 212.Goeddel DV (2004) Tularik expects to begin a Phase 2 study of T487 in patients with rheumatoid arthritis in the first quarter of this year. In: Presented at the 22nd annual JP Morgan healthcare conference, San Fransisco
- 213.Laragione T, Brenner M, Li W, Gulko PS. Cia5d regulates a new fibroblast-like synoviocyte invasion-associated gene expression signature. Arthritis Res Ther. 2008;10(4):R92. doi: 10.1186/ar2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Laragione T, Brenner M, Sherry B, Gulko PS. CXCL10 and its receptor CXCR3 regulate synovial fibroblast invasion. Arthritis Rheum. 2011;63(11):3274–3283. doi: 10.1002/art.30573.CXCL10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.García-Vicuña R, Gómez-Gaviro MV, Domínguez-Luis MJ, Pec MK, González-Alvaro I, Alvaro-Gracia JM, et al. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 2004;50(12):3866–3877. doi: 10.1002/art.20615. [DOI] [PubMed] [Google Scholar]
- 216.Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, et al. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66(15):7701–7707. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- 217.Ha Y, Liu H, Zhu S, Yi P, Liu W, Nathanson J, et al. Critical role of the CXCL10/C-X-C chemokine receptor 3 axis in promoting leukocyte recruitment and neuronal injury during traumatic optic neuropathy induced by optic nerve crush. Am J Pathol. 2017;187(2):352–365. doi: 10.1016/j.ajpath.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang X, Han J, Man K, Li X, Du J, Chu ESH, et al. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J Hepatol. 2016;64(1):160–170. doi: 10.1016/j.jhep.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 219.Hall SE, Mao A, Nicolaidou V, Finelli M, Wise EL, Nedjai B, et al. Elucidation of binding sites of dual antagonists in the human chemokine receptors CCR2 and CCR5. Mol Pharmacol. 2009;75(6):1325–1336. doi: 10.1124/mol.108.053470. [DOI] [PubMed] [Google Scholar]
- 220.Nedjai B, Viney JM, Li H, Hull C, Anderson CA, Horie T, et al. CXCR3 antagonist VUF10085 binds to an intrahelical site distinct from that of the broad spectrum antagonist TAK-779. Br J Pharmacol. 2015;172(7):1822–1833. doi: 10.1111/bph.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, et al. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A. 1999;96(10):5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ni J, Zhu YN, Zhong XG, Ding Y, Hou LF, Tong XK, et al. The chemokine receptor antagonist, TAK-779, decreased experimental autoimmune encephalomyelitis by reducing inflammatory cell migration into the central nervous system, without affecting T cell function. Br J Pharmacol. 2009;158(8):2046–2056. doi: 10.1111/j.1476-5381.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tokuyama H, Ueha S, Kurachi M, Matsushima K, Moriyasu F, Blumberg RS, et al. The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int Immunol. 2005;17(8):1023–1034. doi: 10.1093/intimm/dxh284. [DOI] [PubMed] [Google Scholar]
- 224.Akahori T, Sho M, Kashizuka H, Nomi T, Kanehiro H, Nakajima Y. A novel CCR5/CXCR3 antagonist protects intestinal ischemia/reperfusion injury. Transplant Proc. 2006;38(10):3366–3368. doi: 10.1016/j.transproceed.2006.10.115. [DOI] [PubMed] [Google Scholar]
- 225.Bastani S, Sherman W, Schnickel GT, Hsieh GR, Bhatia R, Fishbein MC, et al. Chemokine receptor blockade with a synthetic nonpeptide compound attenuates cardiac allograft vasculopathy. Transplantation. 2009;88(8):995–1001. doi: 10.1097/TP.0b013e3181b9ccd5. [DOI] [PubMed] [Google Scholar]
- 226.Baba M, Takashima K, Miyake H, Kanzaki N, Teshima K, Wang X, et al. TAK-652 inhibits CCR5-mediated human immunodeficiency virus type 1 infection in vitro and has favorable pharmacokinetics in humans. Antimicrob Agents Chemother. 2005;49(11):4584–4591. doi: 10.1128/AAC.49.11.4584-4591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nair AG, Wong MKC, Shu Y, Jiang Y, Jenh CH, Kim SH, et al. IV. Discovery of CXCR3 antagonists substituted with heterocycles as amide surrogates: Improved PK, hERG and metabolic profiles. Bioorgan Med Chem Lett. 2014;24(4):1085–1088. doi: 10.1016/j.bmcl.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 228.Kim SH, Anilkumar GN, Zawacki LG, Zeng Q, Yang DY, Shao Y, et al. III. Identification of novel CXCR3 chemokine receptor antagonists with a pyrazinyl-piperazinyl-piperidine scaffold. Bioorgan Med Chem Lett. 2011;21(23):6982–6986. doi: 10.1016/j.bmcl.2011.09.120. [DOI] [PubMed] [Google Scholar]
- 229.Stroke IL, Cole AG, Simhadri S, Brescia MR, Desai M, Zhang JJ, et al. Identification of CXCR3 receptor agonists in combinatorial small-molecule libraries. Biochem Biophys Res Commun. 2006;349(1):221–228. doi: 10.1016/j.bbrc.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 230.Yellin M, Paliienko I, Balanescu A, Ter-vartanian S, Tseluyko V, Xu L, et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64(6):1730–1739. doi: 10.1002/art.34330. [DOI] [PubMed] [Google Scholar]
- 231.Kuhne M, Preston B, Wallace S, Chen S, Geetha Vasudevan AW, Cardarelli P. MDX-1100, a fully human anti-CXCL10 (IP-10) antibody, is a high affinity, neutralizing antibody that has entered Phase I clinical trials for the treatment of Ulcerative Colitis. J Immunol. 2007;178:S241. doi: 10.4049/jimmunol.178.Supp.131.20. [DOI] [Google Scholar]
- 232.Medarex Inc (2009) Medarex announces primary endpoint achieved in MDX-1100 anti-IP-10 antibody phase 2 trial for rheumatoid arthritis. https://www.fiercebiotech.com/biotech/medarex-announces-primary-endpoint-achieved-mdx-1100-anti-ip-10-antibody-phase, p 3
- 233.Senolt L. Emerging therapies in rheumatoid arthritis: focus on monoclonal antibodies. F1000Res. 2019;8:F1000 Faculty Rev-1549. doi: 10.1268/f1000research.18688.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mayer L, Sandborn WJ, Stepanov Y, Maccarone J, Tao X, Lu LA, et al. A randomized, placebo-controlled trial of MDX-1100, an anti-IP-10 antibody, for moderately to severely active ulcerative colitis. Gastroenterology. 2010;139(1):e17–e18. doi: 10.1053/j.gastro.2010.05.065. [DOI] [Google Scholar]
- 235.Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):276–289. doi: 10.1038/nrrheum.2015.8. [DOI] [PubMed] [Google Scholar]
- 236.Nastase MV, Zeng-Brouwers J, Beckmann J, Tredup C, Christen U, Radeke HH, et al. Biglycan, a novel trigger of Th1 and Th17 cell recruitment into the kidney. Matrix Biol. 2018;68–69:293–317. doi: 10.1016/j.matbio.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 237.Smith JS, Nicholson LT, Suwanpradid J, Glenn RA, Knape NM, Alagesan P, et al. Biased agonists of the chemokine receptor CXCR3 differentially control chemotaxis and inflammation. Sci Signal. 2019;11(555):eaaq1075. doi: 10.1126/scisignal.aaq1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bernat V, Admas TH, Brox R, Heinemann FW, Tschammer N. Boronic acids as probes for investigation of allosteric modulation of the chemokine receptor CXCR3. ACS Chem Biol. 2014;9(11):2664–2677. doi: 10.1021/cb500678c. [DOI] [PubMed] [Google Scholar]
- 239.Bernat V, Brox R, Heinrich MR, Auberson YP, Tschammer N. Ligand-biased and probe-dependent modulation of chemokine receptor CXCR3 signaling by negative allosteric modulators. ChemMedChem. 2015;10(3):566–574. doi: 10.1002/cmdc.201402507. [DOI] [PubMed] [Google Scholar]
- 240.Fleishaker DL, Garcia Meijide JA, Petrov A, Kohen MD, Wang X, Menon S, et al. Maraviroc, a chemokine receptor-5 antagonist, fails to demonstrate efficacy in the treatment of patients with rheumatoid arthritis in a randomized, double-blind placebo-controlled trial. Arthritis Res Ther. 2012;14(1):R11. doi: 10.1186/ar3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee J, Ajani JA, Chung HC, Kang YK, Iqbal S, Allen S et a1 (2021) Phase Ib/II open-label, randomised evaluation of second-line atezolizumab + linagliptin vs ramucirumab + paclitaxel in MORPHEUS-Gastric cancer. In: 2021 EMSO congress
- 242.Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377(9783):2138–2149. doi: 10.1016/S0140-6736(11)60244-4. [DOI] [PubMed] [Google Scholar]
- 243.Petty RE, Taunton R, Southwood PM, Baum J, Glass DN, Goldenberg J, He X, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. [PubMed] [Google Scholar]
- 244.Minden K, Niewerth M, Listing J, Biedermann T, Bollow M, Schöntube M, et al. Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46(9):2392–2401. doi: 10.1002/art.10444. [DOI] [PubMed] [Google Scholar]
- 245.Packham JC, Hall MA. Long-term follow-up of 246 adults with juvenile idiopathic arthritis: education and employement. Rheumatology. 2002;41(12):1428–1435. doi: 10.1093/rheumatology/41.12.1428. [DOI] [PubMed] [Google Scholar]
- 246.Zak M, Pedersen FK. Juvenile chronic arthritis into adulthood: a long-term follow-up study. Rheumatology. 2000;39(2):198–204. doi: 10.1093/rheumatology/39.2.198. [DOI] [PubMed] [Google Scholar]
- 247.Peterson LS, Mason T, Nelson AM, O’Fallon WM, Gabriel SE. Psychosocial outcomes and health status of adults who have had juvenile rheumatoid arthritis: a controlled population-based study. Arthritis Rheum. 1997;40(12):2235–2240. doi: 10.1002/art.1780401219. [DOI] [PubMed] [Google Scholar]
- 248.De Jager W, Hoppenreijs EPAH, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. 2007;66(5):589–598. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pharoah DS, Varsani H, Tatham RW, Newton KR, De JW, Prakken BJ, et al. Expression of the inflammatory chemokines CCL5, CCL3 and CXCL10 in juvenile idiopathic arthritis, and demonstration of CCL5 production by an atypical subset of CD8+ T cells. Arthritis Res Ther. 2006;8(2):R50. doi: 10.1186/ar1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hunter PJ, Nistala K, Jina N, Eddaoudi A, Thomson W, Hubank M, et al. Biologic predictors of extension of oligoarticular juvenile idiopathic arthritis as determined from synovial fluid cellular composition and gene expression. Arthritis Rheum. 2010;62(3):896–907. doi: 10.1002/art.27284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gattorno M, Prigione I, Morandi F, Gregorio A, Chiesa S, Ferlito F, et al. Phenotypic and functional characterisation of CCR7+ and CCR7- CD4+ memory T cells homing to the joints in juvenile idiopathic arthritis. Arthritis Res Ther. 2004;7(2):256–267. doi: 10.1186/ar1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Brescia AMC, Simonds MM, Sullivan KE, Rose CD. Secretion of pro-inflammatory cytokines and chemokines and loss of regulatory signals by fibroblast-like synoviocytes in juvenile idiopathic arthritis. Proteom Clin Appl. 2017;11(5–6):1600088. doi: 10.1002/prca.201600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Martini G, Zulian F, Calabrese F, Bortoli M, Facco M, Cabrelle A, et al. CXCR3/CXCL10 expression in the synovium of children with juvenile idiopathic arthritis. Arthritis Res Ther. 2005;7(2):241–249. doi: 10.1186/ar1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Issekutz AC, Quinn PJ, Lang B, Ramsey S, Huber AM, Rowter D, et al. Coexpression of chemokine receptors CCR5, CXCR3, and CCR4 and ligands for P- and E-selectin on T lymphocytes of patients with juvenile idiopathic arthritis. Arthritis Rheum. 2011;63(11):3467–3476. doi: 10.1002/art.30521. [DOI] [PubMed] [Google Scholar]
- 255.Petty P, Cassidy J. Textbook for pediatric rheumatology. 6. Oxford: Elsevier; 2011. Oligoarthritis; pp. 262–271. [Google Scholar]
- 256.Wedderburn LR, Robinson N, Patel A, Varsani H, Woo P. Selective recruitment of polarized T cells expressing CCR5 and CXCR3 to the inflamed joints of children with juvenile idiopathic arthritis. Arthritis Rheum. 2000;43(4):765–774. doi: 10.1002/1529-0131(200004)43:4<765::AID-ANR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 257.Black APB, Bhayani H, Ryder CAJ, Pugh MT, Gardner-Medwin JMM, Southwood TR. An association between the acute phase response and patterns of antigen induced T cell proliferation in juvenile idiopathic arthritis. Arthritis Res Ther. 2003;5(5):277–284. doi: 10.1186/ar791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Silverman ED, Isacovics B, Petsche D, Laxer RM. Synovial fluid cells in juvenile arthritis: Evidence of selective T cell migration to inflamed tissue. Clin Exp Immunol. 1993;91(1):90–95. doi: 10.1111/j.1365-2249.1993.tb03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Murray KJ, Luyrink L, Grom AA, Passo MH, Emery H, Witte D, et al. Immunohistological characteristics of T cell infiltrates in different forms of childhood onset chronic arthritis. J Rheumatol. 1996;23(12):2116–2124. [PubMed] [Google Scholar]
- 260.Gregorio A, Gambini C, Gerloni V, Parafioriti A, Sormani MP, Gregorio S, et al. Lymphoid neogenesis in juvenile idiopathic arthritis correlates with ANA positivity and plasma cells infiltration. Rheumatology. 2007;46(2):308–313. doi: 10.1093/rheumatology/kel225. [DOI] [PubMed] [Google Scholar]
- 261.Corcione A, Ferlito F, Gattorno M, Gregorio A, Pistorio A, Gastaldi R, et al. Phenotypic and functional characterization of switch memory B cells from patients with oligoarticular juvenile idiopathic arthritis. Arthritis Res Ther. 2009;11(5):R150. doi: 10.1186/ar2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hardison JL, Wrightsman RA, Carpenter PM, Lane TE, Manning JE. The chemokines CXCL9 and CXCL10 promote a protective immune response but do not contribute to cardiac inflammation following infection with Trypanosoma cruzi. Infect Immun. 2006;74(1):125–134. doi: 10.1128/IAI.74.1.125-134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jarvis JN, Jiang K, Petty HR, Centola M. Neutrophils: the forgotten cell in JIA disease pathogenesis. Pediatr Rheumatol. 2007;5:13. doi: 10.1186/1546-0096-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Arve-Butler S, Schmidt T, Mossberg A, Berthold E, Gullstrand B, Bengtsson AA, et al. Synovial fluid neutrophils in oligoarticular juvenile idiopathic arthritis have an altered phenotype and impaired effector functions. Arthritis Res Ther. 2021;23(1):109. doi: 10.1186/s13075-021-02483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cassatella MA, Gasperini S, Calzetti F, Bertagnin A, Luster AD, McDonald PP. Regulated production of the interferon-γ-inducible protein-10 (IP-10) chemokine by human neutrophils. Eur J Immunol. 1997;27(1):111–115. doi: 10.1002/eji.1830270117. [DOI] [PubMed] [Google Scholar]
- 266.Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, et al. gene expression and production of the monokine induced by IFN-γ (MIG), IFN-inducible T cell α chemoattractant (I-TAC), and IFN-γ-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol. 1999;162(8):4928–4937. doi: 10.4049/jimmunol.162.8.4928. [DOI] [PubMed] [Google Scholar]
- 267.Metzemaekers M, Malengier-Devlies B, Yu K, Vandendriessche S, Yserbyt J, Matthys P, et al. Synovial fluid neutrophils from patients with juvenile idiopathic arthritis display a hyperactivated phenotype. Arthritis Rheumatol. 2021;73(5):875–884. doi: 10.1002/art.41605. [DOI] [PubMed] [Google Scholar]
- 268.Schmidt T, Berthold E, Arve-Butler S, Gullstrand B, Mossberg A, Kahn F, et al. Children with oligoarticular juvenile idiopathic arthritis have skewed synovial monocyte polarization pattern with functional impairment - a distinct inflammatory pattern for oligoarticular juvenile arthritis. Arthritis Res Ther. 2020;22(1):186. doi: 10.1186/s13075-020-02279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mellins ED, MacAubas C, Grom AA. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol. 2011;7(7):416–426. doi: 10.1038/nrrheum.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Still GF. On a form of chronic joint disease in children. Med-Chir Trans. 1897;80:47–60.9. doi: 10.1177/095952879708000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Vastert SJ, Kuis W, Grom AA. Systemic JIA: new developments in the understanding of the pathophysiology and therapy. Best Pract Res Clin Rheumatol. 2009;23(5):655–664. doi: 10.1016/j.berh.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sawhney S, Magalhães CS. Paediatric rheumatology—a global perspective. Best Pract Res Clin Rheumatol. 2006;20(2):201–221. doi: 10.1016/j.berh.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 273.Grom AA, Passo M. Macrophage activation syndrome in systemic juvenile idiopathic arthritis. J Pediatr. 1996;129(5):630–632. doi: 10.1016/s0022-3476(96)70140-3. [DOI] [PubMed] [Google Scholar]
- 274.Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child. 2001;85(5):421–426. doi: 10.1136/adc.85.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34(5):1133–1138. [PubMed] [Google Scholar]
- 276.Bleesing J, Prada A, Siegel DM, Villanueva J, Olson J, Ilowite NT, et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor α-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56(3):965–971. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 277.Ravelli A, Grom AA, Behrens EM, Cron RQ. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment. Genes Immun. 2012;13(4):289–298. doi: 10.1038/gene.2012.3. [DOI] [PubMed] [Google Scholar]
- 278.Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med. 2012;63:233–246. doi: 10.1146/annurev-med-041610-134208. [DOI] [PubMed] [Google Scholar]
- 279.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Billiau AD, Roskams T, Van Damme-Lombaerts R, Matthys P, Wouters C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-γ-producing lymphocytes and IL-6- and TNF-α-producing macrophages. Blood. 2005;105(4):1648–1651. doi: 10.1182/blood-2004-08-2997. [DOI] [PubMed] [Google Scholar]
- 281.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 282.Osugi Y, Hara J, Tagawa S, Takai K, Hosoi G, Matsuda Y, et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89(11):4100–4103. doi: 10.1182/blood.v89.11.4100. [DOI] [PubMed] [Google Scholar]
- 283.Henter J, Elinder G, Soder O, Hansson M, Andersson B, Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78(11):2918–2922. doi: 10.1182/blood.v78.11.2918.2918. [DOI] [PubMed] [Google Scholar]
- 284.Xu XJ, Tang YM, Song H, Yang SL, Xu WQ, Zhao N, et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr. 2012;160(6):984–990.e1. doi: 10.1016/j.jpeds.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 285.Schmid JP, Ho CH, Chrétien F, Lefebure JM, Pivert G, Kosco-Vilbois M, et al. Neutralization of IFNγ defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol Med. 2009;1(2):112–124. doi: 10.1002/emmm.200900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Canna SW. Interferon-γ: friend or foe in systemic juvenile idiopathic arthritis and adult still’s disease. Arthritis Rheumatol. 2014;66(5):1072–1076. doi: 10.1002/art.38362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sikora KA, Fall N, Thornton S, Grom AA. The limited role of interferon-γ in systemic juvenile idiopathic arthritis cannot be explained by cellular hyporesponsiveness. Arthritis Rheum. 2012;64(11):3799–3808. doi: 10.1002/art.34604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bracaglia C, De GK, Marafon DP, Guilhot F, Ferlin W, Prencipe G, et al. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2017;76(1):166–172. doi: 10.1136/annrheumdis-2015-209020. [DOI] [PubMed] [Google Scholar]
- 289.Fall N, Barnes M, Thornton S, Luyrink L, Olson J, Ilowite NT, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56(11):3793–3804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 290.Put K, Avau A, Brisse E, Mitera T, Proost P, Bader-meunier B, et al. Cytokines in systemic juvenile idiopathic arthritis and haemophagocytic lymphohistiocytosis: tipping the balance between interleukin-18 and interferon-γ. Rheumatology. 2015;54(8):1507–1517. doi: 10.1093/rheumatology/keu524. [DOI] [PubMed] [Google Scholar]
- 291.De Benedetti F, Prencipe G, Bracaglia C, Marasco E, Grom AA. Targeting interferon-γ in hyperinflammation: opportunities and challenges. Nat Rev Rheumatol. 2021;17(11):678–691. doi: 10.1038/s41584-021-00694-z. [DOI] [PubMed] [Google Scholar]
- 292.Qu H, Sundberg E, Aulin C, Neog M, Palmblad K, Horne AC, et al. Immunoprofiling of active and inactive systemic juvenile idiopathic arthritis reveals distinct biomarkers: a single-center study. Pediatr Rheumatol. 2021;19(1):173. doi: 10.1186/s12969-021-00660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gattorno M, Piccini A, Lasigliè D, Tassi S, Brisca G, Carta S, et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58(5):1505–1515. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- 294.Ogilvie EM, Khan A, Hubank M, Kellam P, Woo P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56(6):1954–1965. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 295.Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of Interferon gamma. Cytokine. 2010;50(1):1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 296.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201(9):1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Ann Rheum Dis. 2011;70(5):747–754. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hinze T, Kessel C, Hinze CH, Seibert J, Gram H, Foell D. A dysregulated interleukin-18-interferon-γ-CXCL9 axis impacts treatment response to canakinumab in systemic juvenile idiopathic arthritis. Rheumatol. 2021;60(11):5165–5174. doi: 10.1093/rheumatology/keab113. [DOI] [PubMed] [Google Scholar]
- 299.De Jager W, Vastert SJ, Beekman JM, Wulffraat NM, Kuis W, Coffer PJ, et al. Defective phosphorylation of interleukin-18 receptor β causes impaired natural killer cell function in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60(9):2782–2793. doi: 10.1002/art.24750. [DOI] [PubMed] [Google Scholar]
- 300.Weiss ES, Girard-Guyonvarc’h C, Holzinger D, De Jesus AA, Tariq Z, Picarsic J, et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood. 2018;131(13):1442–1455. doi: 10.1182/blood-2017-12-820852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Avau A, Mitera T, Put S, Put K, Brisse E, Filtjens J, et al. Systemic juvenile idiopathic arthritis-like syndrome in mice following stimulation of the immune system with freund’s complete adjuvant: regulation by interferon-γ. Arthritis Rheumatol. 2014;66(5):1340–1351. doi: 10.1002/art.38359. [DOI] [PubMed] [Google Scholar]
- 302.Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121(6):2264–2277. doi: 10.1172/JCI43157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Prencipe G, Caiello I, Pascarella A, Grom AA, Bracaglia C, Chatel L, et al. Neutralization of IFN-γ reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J Allergy Clin Immunol. 2018;141(4):1439–1449.. doi: 10.1016/j.jaci.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 304.Strippoli R, Carvello F, Scianaro R, De Pasquale L, Vivarelli M, Petrini S, et al. Amplification of the response to toll-like receptor ligands by prolonged exposure to interleukin-6 in mice: implication for the pathogenesis of macrophage activation syndrome. Arthritis Rheum. 2012;64(5):1680–1688. doi: 10.1002/art.33496. [DOI] [PubMed] [Google Scholar]
- 305.Bracaglia C, Marafon DP, Caiello I, de Graaf K, Guilhot F, Ferlin W et al (2015) High levels of interferon-gamma (IFNγ) in macrophage activation syndrome (MAS) and CXCL9 levels as a biomarker for IFNγ production in MAS. In: Pediatric rheumatology, p O84. 10.1186/1546-0096-13-S1-O84
- 306.Takada H, Takahata Y, Nomura A, Ohga S, Mizuno Y, Hara T. Increased serum levels of interferon-γ-inducible protein 10 and monokine induced by gamma interferon in patients with haemophagocytic lymphohistiocytosis. Clin Exp Immunol. 2003;133(3):448–453. doi: 10.1046/j.1365-2249.2003.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Jordan M, Prof FL, Allen C, De Benedetti F, Grom AA, Ballabio M, et al. A novel targeted approach to the treatment of hemophagocytic lymphohistiocytosis (HLH) with an anti-interferon gamma (IFNγ) monoclonal antibody (mAb), NI-0501: first results from a pilot phase 2 study in children with primary HLH. Blood. 2015;126(23):3. doi: 10.1182/blood.V126.23.LBA-3.LBA-3. [DOI] [Google Scholar]
- 308.Alfaleh MA, Alsaab HO, Mahmoud AB, Alkayyal AA, Jones ML, Mahler SM, et al. Phage display derived monoclonal antibodies: from bench to bedside. Front Immunol. 2020;11:1986. doi: 10.3389/fimmu.2020.01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.De Benedetti F, Grom AA, Brogan P, Bracaglia C, Pardeo M, Marucci G, et al. Macrophage Activation Syndrome (MAS) in Systemic Juvenile Idiopathic Arthritis (sJIA): treatment with emapalumab, an Anti-Interferon Gamma (IFNγ) monoclonal antibody. Blood. 2021;138(Supplement 1):2058–2058. doi: 10.1182/blood-2021-147596. [DOI] [Google Scholar]
- 310.Novick D, Kim S, Kaplanski G, Dinarello CA. Interleukin-18, more than a Th1 cytokine. Semin Immunol. 2013;25(6):439–448. doi: 10.1016/j.smim.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 311.Shimizu M, Nakagishi Y, Inoue N, Mizuta M, Ko G, Saikawa Y, et al. Interleukin-18 for predicting the development of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Clin Immunol. 2015;160(2):277–281. doi: 10.1016/j.clim.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 312.Ohmori Y, Hamilton TA. IL-4-induced STAT6 suppresses IFN-gamma-stimulated STAT1-dependent transcription in mouse macrophages. J Immunol. 1997;159(11):5474–5482. doi: 10.4049/jimmunol.159.11.5474. [DOI] [PubMed] [Google Scholar]
- 313.Wong P, Severns CW, Guyer NB, Wright TM. A unique palindromic element mediates gamma interferon induction of mig gene expression. Mol Cell Biol. 1994;14(2):914–922. doi: 10.1128/mcb.14.2.914-922.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wright TM, Farber JM. 5’ Regulatory region of a novel cytokine gene mediates selective activation by interferon γ. J Exp Med. 1991;173(2):417–422. doi: 10.1084/jem.173.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ohmori Y, Hamilton TA. Cooperative interaction between interferon (IFN) stimulus response element and κB sequence motifs controls IFNγ- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem. 1993;268(9):6677–6688. doi: 10.1016/s0021-9258(18)53303-2. [DOI] [PubMed] [Google Scholar]
- 316.Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J Immunol. 1998;161(9):4736–4744. doi: 10.4049/jimmunol.161.9.4736. [DOI] [PubMed] [Google Scholar]
- 317.Tensen CP, Flier J, Rampersad SS, Sampat-Sardjoepersad S, Scheper RJ, Boorsma DM, et al. Genomic organization, sequence and transcriptional regulation of the human CXCL 11 gene. Biochim Biophys Acta Gene Struct Expr. 1999;1446(1–2):167–172. doi: 10.1016/S0167-4781(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 318.Sandhya Rani MR, Foster GR, Leung S, Leaman D, Stark GR, Ransohoff RM. Characterization of β-R1, a gene that is selectively induced by interferon β (IFN-β) compared with IFN-α. J Biol Chem. 1996;271(37):22878–22884. doi: 10.1074/jbc.271.37.22878. [DOI] [PubMed] [Google Scholar]
- 319.Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183(11):6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Kadavath S, Efthimiou P. Adult-onset Still’s disease-pathogenesis, clinical manifestations, and new treatment options. Ann Med. 2015;47(1):6–14. doi: 10.3109/07853890.2014.971052. [DOI] [PubMed] [Google Scholar]
- 321.Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still’s disease. J Autoimmun. 2018;93:24–36. doi: 10.1016/j.jaut.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 322.Bywaters EG. Still’s disease in the adult. Ann Rheum Dis Rheum Dis. 1971;30(2):121–133. doi: 10.1136/ard.30.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Nirmala N, Brachat A, Feist E, Blank N, Specker C, Witt M, et al. Gene-expression analysis of adult-onset Still’s disease and systemic juvenile idiopathic arthritis is consistent with a continuum of a single disease entity. Pediatr Rheumatol. 2015 doi: 10.1186/s12969-015-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Vastert SJ, Jamilloux Y, Quartier P, Ohlman S, Osterling Koskinen L, Kullenberg T, et al. Anakinra in children and adults with Still’s disease. Rheumatol (United Kingdom) 2019;58(Suppl 6):vi9–vi22. doi: 10.1093/rheumatology/kez350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Pay S, Türkçapar N, Kalyoncu M, Şimşek I, Beyan E, Ertenli I, et al. A multicenter study of patients with adult-onset Still’s disease compared with systemic juvenile idiopathic arthritis. Clin Rheumatol. 2006;25(5):639–644. doi: 10.1007/s10067-005-0138-5. [DOI] [PubMed] [Google Scholar]
- 326.Ruscitti P, Cipriani P, Di Benedetto P, Liakouli V, Carubbi F, Berardicurti O, et al. Advances in immunopathogenesis of macrophage activation syndrome during rheumatic inflammatory diseases: toward new therapeutic targets? Expert Rev Clin Immunol. 2017;13(11):1041–1047. doi: 10.1080/1744666X.2017.1372194. [DOI] [PubMed] [Google Scholar]
- 327.Sfriso P, Bindoli S, Doria A, Feist E, Galozzi P. Canakinumab for the treatment of adult-onset Still’s disease. Expert Rev Clin Immunol. 2020;16(2):129–138. doi: 10.1080/1744666X.2019.1707664. [DOI] [PubMed] [Google Scholar]
- 328.Kasama T, Furuya H, Yanai R. Correlation of serum CX3CL1 level with disease activity in adult-onset Still’s disease and significant involvement in hemophagocytic syndrome. Clin Rheumatol. 2012;31(5):853–860. doi: 10.1007/s10067-012-1952-1. [DOI] [PubMed] [Google Scholar]
- 329.Kim HA, Kim YH, Jeon YK, Yang WI, Kwon JE, Han JH. Histopathology and expression of the chemokines CXCL10, CXCL13, and CXCR3 and the endogenous TLR-4 ligand S100A8/A9 in lymph nodes of patients with adult-onset Still’s disease. Sci Rep. 2019;9(1):7517. doi: 10.1038/s41598-019-44032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Han JH, Ahn MH, Jung JY, Suh CH, Kwon JE, Yim H, et al. The levels of CXCL12 and its receptor, CXCR4, as a biomarker of disease activity and cutaneous manifestation in adult-onset Still’s disease. Clin Exp Rheumatol. 2019;37(6):67–73. [PubMed] [Google Scholar]
- 331.Mitrovic S, Fautrel B. New markers for adult-onset still’s disease. Jt Bone Spine. 2018;85(3):285–293. doi: 10.1016/j.jbspin.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 332.Soy M, Ergin M, Paydas S. Lymphadenopathy in adult-onset still’s disease mimicking peripheral T-cell lymphoma. Clin Rheumatol. 2004;23(1):81–82. doi: 10.1007/s10067-003-0826-y. [DOI] [PubMed] [Google Scholar]
- 333.Jeon YK, Paik JH, Park SS, Park SO, Kim YA, Kim JE, et al. Spectrum of lymph node pathology in adult onset Still’s disease; analysis of 12 patients with one follow up biopsy. J Clin Pathol. 2004;57(10):1052–1056. doi: 10.1136/jcp.2004.018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Valente RM, Banks PM, Conn DL. Characterization of lymph node histology in adult onset Still’s disease. J Rheumatol. 1989;16(3):349–354. [PubMed] [Google Scholar]
- 335.Chen DY, Lan JL, Lin FJ, Hsieh TY, Wen MC. Predominance of Th1 cytokine in peripheral blood and pathological tissues of patients with active untreated adult onset Still’s disease. Ann Rheum Dis. 2004;63(10):1300–1306. doi: 10.1136/ard.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kim HA, An JM, Nam JY, Jeon JAY, Suh CH. Serum S100A8/A9, but not follistatin-like protein 1 and interleukin 18, may be a useful biomarker of disease activity in adult-onset still’s disease. J Rheumatol. 2012;39(7):1399–1406. doi: 10.3899/jrheum.120079. [DOI] [PubMed] [Google Scholar]
- 337.Holzinger D, Frosch M, Kastrup A, Prince FHM, Otten MH, Van Suijlekom-Smit LWA, et al. The toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann Rheum Dis. 2012;71(6):974–980. doi: 10.1136/annrheumdis-2011-200598. [DOI] [PubMed] [Google Scholar]
- 338.Wang J, Vodovotz Y, Fan L, Li Y, Liu Z, Namas R, et al. Injury-induced MRP8/MRP14 stimulates IP-10/CXCL10 in monocytes/macrophages. FASEB J. 2015;29(1):250–262. doi: 10.1096/fj.14-255992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Maranini B, Ciancio G, Govoni M. Adult-onset still’s disease: novel biomarkers of specific subsets, disease activity, and relapsing forms. Int J Mol Sci. 2021;22(24):13320. doi: 10.3390/ijms222413320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Walsh JA, Magrey M. Clinical manifestations and diagnosis of axial spondyloarthritis. JCR J Clin Rheumatol. 2021;27(8):e547–e560. doi: 10.1097/rhu.0000000000001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Mauro D, Thomas R, Guggino G, Lories R, Brown MA, Ciccia F. Ankylosing spondylitis: an autoimmune or autoinflammatory disease? Nat Rev Rheumatol. 2021;17(7):387–404. doi: 10.1038/s41584-021-00625-y. [DOI] [PubMed] [Google Scholar]
- 342.Rudwaleit M, Van Der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70(1):25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 343.Robinson PC, van der Linden S, Khan MA, Taylor WJ. Axial spondyloarthritis: concept, construct, classification and implications for therapy. Nat Rev Rheumatol. 2021;17(2):109–118. doi: 10.1038/s41584-020-00552-4. [DOI] [PubMed] [Google Scholar]
- 344.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 345.Goie The HS, Steven MM, van der Linden SM, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a comparison of the Rome, New York and modified New York criteria in patients with a positive clinical history screening test for ankylosing spondylitis. Rheumatology. 1985;24(3):242–249. doi: 10.1093/rheumatology/24.3.242. [DOI] [PubMed] [Google Scholar]
- 346.Carron P, De Craemer AS, Van Den Bosch F. Peripheral spondyloarthritis: a neglected entity—state of the art. RMD Open. 2020;6(1):e001136. doi: 10.1136/rmdopen-2019-001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Parida JR, Kumar S, Ahmed S, Chaurasia S, Mukherjee R, Singh R, et al. Reactive arthritis and undifferentiated peripheral spondyloarthritis share human leucocyte antigen B27 subtypes and serum and synovial fluid cytokine profiles. Rheumatol (United Kingdom) 2021;60(6):3004–3011. doi: 10.1093/rheumatology/keaa746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Abji F, Pollock RA, Liang K, Chandran V, Gladman DD. Brief report: CXCL10 is a possible biomarker for the development of psoriatic arthritis among patients with psoriasis. Arthritis Rheumatol. 2016;68(12):2911–2916. doi: 10.1002/art.39800. [DOI] [PubMed] [Google Scholar]
- 349.Muntyanu A, Abji F, Liang K, Pollock RA, Chandran V, Gladman DD. Differential gene and protein expression of chemokines and cytokines in synovial fluid of patients with arthritis. Arthritis Res Ther. 2016;18(1):296. doi: 10.1186/s13075-016-1196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391(10136):2273–2284. doi: 10.1016/S0140-6736(18)30830-4. [DOI] [PubMed] [Google Scholar]
- 351.Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med. 2017;17(1):65–70. doi: 10.7861/clinmedicine.17-1-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Olivieri I, D’Angelo S, Palazzi C, Padula A. Advances in the management of psoriatic arthritis. Nat Rev Rheumatol. 2014;10(9):531–542. doi: 10.1038/nrrheum.2014.106. [DOI] [PubMed] [Google Scholar]
- 353.Touma Z, Thavaneswaran A, Chandran V, Pellett F, Cook RJ, Gladman DD. Clinical and demographic characteristics of erosion-free and erosion-present status in psoriatic arthritis in a cohort study. J Rheumatol. 2016;43(6):1057–1062. doi: 10.3899/jrheum.150466. [DOI] [PubMed] [Google Scholar]
- 354.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74(6):1045–1050. doi: 10.1136/annrheumdis-2013-204858. [DOI] [PubMed] [Google Scholar]
- 355.Antonelli A, Fallahi P, Sedie AD, Ferrari SM, Maccheroni M, Bombardieri S, et al. High values of Th1 (CXCL10) and Th2 (CCL2) chemokines in patients with psoriatic arthtritis. Clin Exp Rheumatol. 2009;27(1):22–27. [PubMed] [Google Scholar]
- 356.Pollock RA, Abji F, Liang K, Chandran V, Pellett FJ, Virtanen C, et al. Gene expression differences between psoriasis patients with and without inflammatory arthritis. J Invest Dermatol. 2015;135(2):620–623. doi: 10.1038/jid.2014.414. [DOI] [PubMed] [Google Scholar]
- 357.Antonelli A, Fallahi P, Sedie AD, Ferrari SM, Maccheroni M, Bombardieri S, et al. High values of alpha (CXCL10) and beta (CCL2) circulating chemokines in patients with psoriatic arthritis, in presence or absence of autoimmune thyroiditis. Autoimmunity. 2008;41(7):537–542. doi: 10.1080/08916930802170401. [DOI] [PubMed] [Google Scholar]
- 358.Devito A. Interferon γ-induced chemokines in psoriatic arthritis. Clin Ter. 2014;165(6):e442–e446. doi: 10.7417/CT.2014.1790. [DOI] [PubMed] [Google Scholar]
- 359.Lima XT, Oliveira RTD, Braga FG, Magalhães RF, Mamoni RL, Blotta MHSL. Circulating levels of chemokines in psoriasis. Autoimmunity. 2015;48(1):57–60. doi: 10.3109/08916934.2014.947476. [DOI] [PubMed] [Google Scholar]
- 360.Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159(4):842–855. doi: 10.1111/j.1476-5381.2009.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175(4):2721–2729. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
- 362.Elia G. MIG in psoriatic arthritis. Clin Ter. 2018;169(6):E297–302. doi: 10.7417/CT.2018.2097. [DOI] [PubMed] [Google Scholar]
- 363.Gottlieb AB, Luster AD, Posnett DN, Martin CD. Detection of a γ interferon-induced protein IP-10 in psoriatic plaques. J Exp Med. 1988;168(3):941–948. doi: 10.1084/jem.168.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ottaviani C, Nasorri F, Bedini C, de Pità O, Girolomoni G, Cavani A. CD56brightCD16(-) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36(1):118–128. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 365.Zhu W, He X, Cheng K, Zhang L, Chen D, Wang X, et al. Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res. 2019;7:22. doi: 10.1038/s41413-019-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Generali E, Bose T, Selmi C, Voncken JW, Damoiseaux JGMC. Nature versus nurture in the spectrum of rheumatic diseases: Classification of spondyloarthritis as autoimmune or autoinflammatory. Autoimmun Rev. 2018;17(9):935–941. doi: 10.1016/j.autrev.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 367.Wang J, Zhao Q, Wang G, Yang C, Xu Y, Li Y, et al. Circulating levels of Th1 and Th2 chemokines in patients with ankylosing spondylitis. Cytokine. 2016;81:10–14. doi: 10.1016/j.cyto.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 368.Duftner C, Dejaco C, Kullich W, Klauser A, Goldberger C, Falkenbach A, et al. Preferential type 1 chemokine receptors and cytokine production of CD28-T cells in ankylosing spondylitis. Ann Rheum Dis. 2006;65(5):647–653. doi: 10.1136/ard.2005.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Aggarwal A, Agarwal S, Misra R. Chemokine and chemokine receptor analysis reveals elevated interferon-inducible protein-10 (IP)-10/CXCL10 levels and increased number of CCR5+ and CXCR3+ CD4 T cells in synovial fluid of patients with enthesitis-related arthritis (ERA) Clin Exp Immunol. 2007;148(3):515–519. doi: 10.1111/j.1365-2249.2007.03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002;15(4):527–544. doi: 10.1128/CMR.15.4.527-544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ross JJ. Septic arthritis of native joints. Infect Dis Clin North Am. 2017;31(2):203–218. doi: 10.1016/j.idc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 372.Long B, Koyfman A, Gottlieb M. Evaluation and management of septic arthritis and its mimics in the emergency department. West J Emerg Med. 2019;20(2):331–341. doi: 10.5811/westjem.2018.10.40974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Goldenberg DL, Reed JI. Bacterial arthritis. N Engl J Med. 1985;312(12):764–771. doi: 10.1016/S0140-6736(86)90235-7. [DOI] [PubMed] [Google Scholar]
- 374.Schutyser E, Struyf S, Wuyts A, Put W, Geboes K, Grillet B et al (2001) Selective induction of CCL18/PARC by staphylococcal enterotoxins in mononuclear cells and enhanced levels in septic and rheumatoid arthritis. Eur J Immunol 31(12):3755–62. 10.1002/1521-4141(200112)31:12<3755::aid-immu3755>3.0.co;2-o [DOI] [PubMed]
- 375.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167(2):623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 376.Antonia AL, Gibbs KD, Trahair ED, Pittman KJ, Martin AT, Schott BH, et al. Pathogen evasion of chemokine response through suppression of CXCL10. Front Cell Infect Microbiol. 2019;9:280. doi: 10.3389/fcimb.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat Rev Dis Prim. 2016;2:16072. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 378.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA J Am Med Assoc. 2021;325(6):568–578. doi: 10.1001/jama.2020.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14(1):R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Manferdini C, Paolella F, Gabusi E, Cattini L, Rojewski M, Schrezenmeier H, et al. Osteoarthritic milieu affects adipose-derived mesenchymal stromal cells. J Orthop Res. 2020;38(2):336–347. doi: 10.1002/jor.24446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Beekhuizen M, Gierman LM, van Spil WE, Van Osch GJVM, Huizinga TWJ, Saris DBF, et al. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthr Cartil. 2013;21(7):918–922. doi: 10.1016/j.joca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 382.Thomas Vangsness J, Burke WS, Narvy SJ, MacPhee RD, Fedenko AN. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis—a pilot study. Bull NYU Hosp Jt Dis. 2011;69(2):122–127. [PubMed] [Google Scholar]
- 383.Huss RS, Huddleston JI, Goodman SB, Butcher EC, Zabel BA. Synovial tissue-infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation. Arthritis Rheum. 2010;62(12):3799–3805. doi: 10.1002/art.27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Gobezie R, Kho A, Krastins B, Sarracino DA, Thornhill TS, Chase M, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther. 2007;9(2):R36. doi: 10.1186/ar2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Metzemaekers M, Abouelasrar Salama S, Vandooren J, Mortier A, Janssens R, Vandendriessche S, et al. From ELISA to immunosorbent tandem mass spectrometry proteoform analysis: the example of CXCL8/interleukin-8. Front Immunol. 2021;12:644725. doi: 10.3389/fimmu.2021.644725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Endres M, Andreas K, Kalwitz G, Freymann U, Neumann K, Ringe J, et al. Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthr Cartil. 2010;18(11):1458–1466. doi: 10.1016/j.joca.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 387.Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, Van Den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62(3):647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 388.Hsueh MF, Zhang X, Wellman SS, Bolognesi MP, Kraus VB. Synergistic roles of macrophages and neutrophils in osteoarthritis progression. Arthritis Rheumatol. 2021;73(1):89–99. doi: 10.1002/art.41486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Benigni G, Dimitrova P, Antonangeli F, Sanseviero E, Milanova V, Blom A, et al. CXCR3/CXCL10 axis regulates neutrophil–NK cell cross-talk determining the severity of experimental osteoarthritis. J Immunol. 2017;198(5):2115–2124. doi: 10.4049/jimmunol.1601359. [DOI] [PubMed] [Google Scholar]
- 390.Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells. 2017;40(10):706–713. doi: 10.14348/molcells.2017.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Clem K, Galdzicka M, Faryna DM, Soto E, Ostroff GR, Ginns EI. Development of a novel non-viral osteoprotegerin gene therapy for treating low bone density. Mol Ther. 2008;16(Supplement 1):S49–50. doi: 10.1016/s1525-0016(16)39531-4. [DOI] [Google Scholar]
- 392.Lisignoli G, Toneguzzi S, Piacentini A, Cattini L, Lenti A, Tschon M, et al. Human osteoblasts express functional CXC chemokine receptors 3 and 5: activation by their ligands, CXCL10 and CXCL13, significantly induces alkaline phosphatase and β-N-acetylhexosaminidase release. J Cell Physiol. 2003;194(1):71–79. doi: 10.1002/jcp.10188. [DOI] [PubMed] [Google Scholar]
- 393.De Ceuninck F, Dassencourt L, Anract P. The inflammatory side of human chondrocytes unveiled by antibody microarrays. Biochem Biophys Res Commun. 2004;323(3):960–969. doi: 10.1016/j.bbrc.2004.08.184. [DOI] [PubMed] [Google Scholar]
- 394.Szulc P, Bauer DC (2013) Biochemical markers of bone turnover in osteoporosis. In: Osteoporosis, 4th edn, pp 1573–1610. 10.1016/B978-0-12-415853-5.00067-4
- 395.Marks SC, Seifert MF. The lifespan of osteoclasts: experimental studies using the giant granule cytoplasmic marker characteristic of beige mice. Bone. 1985;6(6):451–455. doi: 10.1016/8756-3282(85)90223-6. [DOI] [PubMed] [Google Scholar]
- 396.Šućur A, Katavić V, Kelava T, Jajić Z, Kovačić N, Grčević D. Induction of osteoclast progenitors in inflammatory conditions: key to bone destruction in arthritis. Int Orthop. 2014;38(9):1893–1903. doi: 10.1007/s00264-014-2386-y. [DOI] [PubMed] [Google Scholar]
- 397.Deal C. Bone loss in rheumatoid arthritis: systemic, periarticular, and focal. Curr Rheumatol Rep. 2012;14(3):231–237. doi: 10.1007/s11926-012-0253-7. [DOI] [PubMed] [Google Scholar]
- 398.Ikić M, Jajić Z, Lazić E, Ivčević S, Grubišić F, Marušić A, et al. Association of systemic and intra-articular osteoclastogenic potential, pro-inflammatory mediators and disease activity with the form of inflammatory arthritis. Int Orthop. 2014;38(1):183–192. doi: 10.1007/s00264-013-2121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Laragione T, Brenner M, Mello A, Symons M, Gulko PS. The arthritis severity locus Cia5d is a novel genetic regulator of the invasive properties of synovial fibroblasts. Arthritis Rheum. 2008;58(8):2296–2306. doi: 10.1002/art.23610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Izquierdo E, Cañete JD, Celis R, Del Rey MJ, Usategui A, Marsal S, et al. Synovial fibroblast hyperplasia in rheumatoid arthritis: clinicopathologic correlations and partial reversal by anti-tumor necrosis factor therapy. Arthritis Rheum. 2011;63(9):2575–2583. doi: 10.1002/art.30433. [DOI] [PubMed] [Google Scholar]
- 401.Qu Z, Garcia CH, Orourke LM, Planck SR, Kohli M, Rosenbaum JT. Local proliferation of fibroblast-like synoviocytes contributes to synovial hyperplasia. Results of proliferating cell nuclear antigen/cyclin, c-myc, and nucleolar organizer region staining. Arthritis Rheum. 1994;37(2):212–220. doi: 10.1002/art.1780370210. [DOI] [PubMed] [Google Scholar]
- 402.Korsunsky I, Wei K, Pohin M, Kim EY, Barone F, Major T, et al. Cross-tissue, single-cell stromal atlas identifies shared pathological fibroblast phenotypes in four chronic inflammatory diseases. Med. 2022;3(7):481–518.e14. doi: 10.1016/j.medj.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Zhang F, Kevin W, Slowikowski K, Fonseka CY, Rao DA, Kelly S, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. 2019;20(7):928–942. doi: 10.1038/s41590-019-0378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Burrage PS, Mix KS, Constance E. Brinckerhoff. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 405.Bin SH, Yokota H. Reduction of cytokine-induced expression and activity of MMP-1 and MMP-13 by mechanical strain in MH7A rheumatoid synovial cells. Matrix Biol. 2002;21(3):263–270. doi: 10.1016/S0945-053X(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 406.Smolian H, Aurer A, Sittinger M, Zacher J, Bernimoulin JP, Burmester GR, et al. Secretion of gelatinases and activation of gelatinase A (MMP-2) by human rheumatoid synovial fibroblasts. Biol Chem. 2001;382(10):1491–1499. doi: 10.1515/BC.2001.183. [DOI] [PubMed] [Google Scholar]
- 407.Kumkumian GK, Lafyatis R, Remmers EF, Case JP, Kim SJ, Wilder RL. Platelet-derived growth factor and IL-1 interactions in rheumatoid arthritis. Regulation of synoviocyte proliferation, prostaglandin production, and collagenase transcription. J Immunol. 1989;143(3):833–837. doi: 10.4049/jimmunol.143.3.833. [DOI] [PubMed] [Google Scholar]
- 408.Giegold O, Ogrissek N, Richter C, Schröder M, Herrero San Juan M, Pfeilschifter JM, et al. CXCL9 causes heterologous desensitization of CXCL12-mediated memory T lymphocyte activation. J Immunol. 2013;190(7):3696–3705. doi: 10.4049/jimmunol.1101293. [DOI] [PubMed] [Google Scholar]
- 409.Buljevic S, Detel D, Pucar LB, Mihelic R, Madarevic T, Sestan B, et al. Levels of dipeptidyl peptidase IV/CD26 substrates neuropeptide y and vasoactive intestinal peptide in rheumatoid arthritis patients. Rheumatol Int. 2013;33(11):2867–2874. doi: 10.1007/s00296-013-2823-z. [DOI] [PubMed] [Google Scholar]
- 410.Cordero OJ, Varela-Calviño R, López-González T, Calviño-Sampedro C, Viñuela JE, Mouriño C, et al. CD26 Expression on T helper populations and sCD26 serum levels in patients with rheumatoid arthritis. PLoS ONE. 2015;10(7):e0131992. doi: 10.1371/journal.pone.0131992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Morgan R, Endres J, Behbahani-Nejad N, Phillips K, Ruth JH, Friday SC, et al. Expression and function of aminopeptidase N/CD13 produced by fibroblast-like synoviocytes in rheumatoid arthritis: role of CD13 in chemotaxis of cytokine-activated t cells independent of enzymatic activity. Arthritis Rheumatol. 2015;67(1):74–85. doi: 10.1002/art.38878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TWJ, TeKoppele JM, Hanemaaijer R, et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64(5):694–698. doi: 10.1136/ard.2004.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Kazantseva MG, Hung NA, Highton J, Hessian PA. MMP expression in rheumatoid inflammation: the rs11568818 polymorphism is associated with MMP-7 expression at an extra-articular site. Genes Immun. 2013;14(3):162–169. doi: 10.1038/gene.2012.65. [DOI] [PubMed] [Google Scholar]
- 414.Cao R, Zhang Y, Du J, Chen S, Wang N, Ying H, et al. Increased FURIN expression in rheumatoid arthritis patients and its anti-inflammatory effect. J Clin Lab Anal. 2020;34(12):e23530. doi: 10.1002/jcla.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Valli A, Ranta N, Grönholm A, Silvennoinen O, Pesu M, Isomäki P. Increased expression of the proprotein convertase enzyme FURIN in rheumatoid arthritis. Scand J Rheumatol. 2019;48(3):173–177. doi: 10.1080/03009742.2018.1520294. [DOI] [PubMed] [Google Scholar]
- 416.Repnik U, Starr AE, Overall CM, Turk B. Cysteine cathepsins activate ELR chemokines and inactivate non-ELR chemokines. J Biol Chem. 2015;290(22):13800–13811. doi: 10.1074/jbc.M115.638395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K, et al. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276(32):29839–29845. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- 418.Ludwig A, Schiemann F, Mentlein R, Lindner B, Brandt E. Dipeptidyl peptidase IV (CD26) on T cells cleaves the CXC chemokine CXCL11 (I-TAC) and abolishes the stimulating but not the desensitizing potential of the chemokine. J Leukoc Biol. 2002;72:183–191. doi: 10.1189/jlb.72.1.183. [DOI] [PubMed] [Google Scholar]
- 419.Ajami K, Pitman MR, Wilson CH, Park J, Menz RI, Starr AE, et al. Stromal cell-derived factors 1α and 1β, inflammatory protein-10 and interferon-inducible T cell chemo-attractant are novel substrates of dipeptidyl peptidase 8. FEBS Lett. 2008;582(5):819–825. doi: 10.1016/j.febslet.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 420.Proost P, Mortier A, Loos T, Vandercappellen J, Gouwy M, Ronsse I, et al. Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood. 2007;110(1):37–44. doi: 10.1182/blood-2006-10-049072. [DOI] [PubMed] [Google Scholar]
- 421.Patterson AM, Siddall H, Chamberlain G, Gardner L, Middleton J. Expression of the Duffy antigen/receptor for chemokines (DARC) by the inflamed synovial endothelium. J Pathol. 2002;197(1):108–116. doi: 10.1002/path.1100. [DOI] [PubMed] [Google Scholar]
- 422.Watanabe K, Penfold MET, Matsuda A, Ohyanagi N, Kaneko K, Miyabe Y, et al. Pathogenic role of CXCR7 in rheumatoid arthritis. Arthritis Rheum. 2010;62(11):3211–3220. doi: 10.1002/art.27650. [DOI] [PubMed] [Google Scholar]
- 423.Baldwin HM, Singh MD, Codullo V, King V, Wilson H, McInnes I, et al. Elevated ACKR2 expression is a common feature of inflammatory arthropathies. Rheumatol (United Kingdom) 2017;56(9):1607–1617. doi: 10.1093/rheumatology/kex176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Miyabe Y, Miyabe C, Mani V, Mempel TR, Luster AD. Atypical complement receptor C5aR2 transports C5a to initiate neutrophil adhesion and inflammation. Sci Immunol. 2019;4(35):eaav5951. doi: 10.1126/sciimmunol.aav5951. [DOI] [PubMed] [Google Scholar]
- 425.Smith E, McGettrick HM, Stone MA, Shaw JS, Middleton J, Nash GB, et al. Duffy antigen receptor for chemokines and CXCL5 are essential for the recruitment of neutrophils in a multicellular model of rheumatoid arthritis synovium. Arthritis Rheum. 2008;58(7):1968–1973. doi: 10.1002/art.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Świdrowska-Jaros J, Smolewska E. A fresh look at angiogenesis in juvenile idiopathic arthritis. Cent Eur J Immunol. 2018;43(3):325–330. doi: 10.5114/ceji.2018.80052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Mease PJ. Inflammatory musculoskeletal disease: Identification and assessment. J Rheumatol. 2011;38(3):557–561. doi: 10.3899/jrheum.101121. [DOI] [PubMed] [Google Scholar]
- 428.Linkov F, Gu Y, Arslan AA, Liu M, Shore RE, Koenig KL, et al. Reliability of tumor markers, chemokines, and metastasis-related molecules in serum. Eur Cytokine Netw. 2009;20(1):21–26. doi: 10.1684/ecn.2009.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Vanheule V, Metzemaekers M, Janssens R, Struyf S, Proost P. How post-translational modifications influence the biological activity of chemokines. Cytokine. 2018;109:29–51. doi: 10.1016/j.cyto.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 430.Barreira R, Laird ME, Yatim N, Fiette L, Ingersoll MA, Albert ML. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat Immunol. 2015;16(8):850–858. doi: 10.1038/ni.3201. [DOI] [PubMed] [Google Scholar]
- 431.Casrouge A, Bisiaux A, Stephen L, Schmolz M, Mapes J, Pfister C, et al. Discrimination of agonist and antagonist forms of CXCL10 in biological samples. Clin Exp Immunol. 2011;167(1):137–148. doi: 10.1111/j.1365-2249.2011.04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Petrone L, Bondet V, Vanini V, Cuzzi G, Palmieri F, Palucci I, et al. First description of agonist and antagonist IP-10 in urine of patients with active TB. Int J Infect Dis. 2019;78:15–21. doi: 10.1016/j.ijid.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 433.Meissner EG, Decalf J, Casrouge A, Masur H. Dynamic changes of post-translationally modified forms of CXCL10 and soluble DPP4 in HCV subjects receiving interferon-free therapy. PLoS ONE. 2015;10(7):0133236. doi: 10.1371/journal.pone.0133236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Casrouge A, Decalf J, Ahloulay M, Lababidi C, Mansour H, Vallet-Pichard A, et al. Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J Clin Invest. 2011;121(1):308–317. doi: 10.1172/JCI40594DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Riva A, Laird M, Casrouge A, Ambrozaitis A, Williams R, Naoumov NV, et al. Truncated CXCL10 is associated with failure to achieve spontaneous clearance of acute hepatitis C infection. Hepatology. 2014;60(2):487–496. doi: 10.1002/hep.27139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Information supporting the manuscript can be found in the Supplementary Material.