Abstract
The hypothalamus is a critical brain region for the regulation of energy homeostasis. Over the years, studies on energy metabolism primarily focused on the neuronal component of the hypothalamus. Studies have recently uncovered the vital role of glial cells as an additional player in energy balance regulation. However, their inflammatory activation under metabolic stress condition contributes to various metabolic diseases. The recruitment of monocytes and macrophages in the hypothalamus helps sustain such inflammation and worsens the disease state. Neurons were found to actively participate in hypothalamic inflammatory response by transmitting signals to the surrounding non-neuronal cells. This activation of different cell types in the hypothalamus leads to chronic, low-grade inflammation, impairing energy balance and contributing to defective feeding habits, thermogenesis, and insulin and leptin signaling, eventually leading to metabolic disorders (i.e., diabetes, obesity, and hypertension). The hypothalamus is also responsible for the causation of systemic aging under metabolic stress. A better understanding of the multiple factors contributing to hypothalamic inflammation, the role of the different hypothalamic cells, and their crosstalks may help identify new therapeutic targets. In this review, we focus on the role of glial cells in establishing a cause–effect relationship between hypothalamic inflammation and the development of metabolic diseases. We also cover the role of other cell types and discuss the possibilities and challenges of targeting hypothalamic inflammation as a valid therapeutic approach.
Keywords: Aging, Diabetes, Hypertension, Hypothalamus, Inflammation, Obesity
Introduction
The hypothalamus is an important brain structure that controls various basic physiological functions ranging from whole-body metabolism to aging [1]. An important function of the hypothalamus is linking the nervous system to the endocrine system via the pituitary gland [2]. Notably, hypothalamus receives several hormonal and cellular signals from the periphery, which act on distinct groups of hypothalamic neurons and non-neuronal cells. Additionally, it employs an integrated neuroendocrine network to direct peripheral metabolic activities. Through these mechanisms, the hypothalamus acts as a metabolic center that controls a variety of functions, such as glucose and fat metabolism [3, 4], nutrient sensing [5], food intake behavior [6], body temperature regulation [7], energy homeostasis [8], blood pressure (BP) regulation [9], and aging [10]. At the cellular level, these physiological functions are conducted by several groups of neurons located in the mediobasal hypothalamus (MBH), paraventricular nucleus (PVN), and lateral hypothalamus (LH), which regulate the controlled synthesis, release, and action of hypothalamic neuropeptides and neurotransmitters [11]. The arcuate nucleus (ARC) located next to the third ventricle in the MBH is the well-studied region concerning energy balance regulation, which comprises two distinct groups of interconnected neurons. One group releases orexigenic neuropeptide Y (NPY) and agouti-related peptide (AgRP), whereas another group releases anorexigenic peptides-pro-opiomelanocortin (POMC) and cocaine-amphetamine-related transcript (CART). These two groups of neurons reciprocally regulate each other and form a neuronal circuit that has projections to the PVN and LH nuclei, where they regulate the release of other neurohormones [11, 12]. Furthermore, POMC and AgRP/NPY neurons receive glutamatergic feedback signals from the PVN, whereas PVN neurons receive inhibitory innervation from LH neurons that promote feeding [13, 14]. Neurons are fundamental to various functions of hypothalamus, but they rely on the surrounding non-neuronal cells to function properly. Glia, epithelial cells, pericytes, and endothelia are examples of non-neuronal cell types that supply vital substances to neurons while also shielding them from harmful substances and circumstances [15]. Moreover, it is now clear that these non-neuronal cells play an important role in deciding the outcomes of neuronal signaling in the hypothalamus.
Hypothalamic inflammation has been identified as a critical factor that disrupts energy balance [16]; it is characterized by activation of various molecular mediators or pathways, such as cytokines [17, 18], chemokines [19], signaling molecules [20], oxidative and endoplasmic reticulum (ER) stress [20, 21], and autophagy [22]. Over the years, hypothalamic inflammation has been linked to a variety of metabolic disorders. It all began with the observation in preclinical studies that chronic high-fat diet (HFD) feeding induces hypothalamic inflammation [23]. Later research linked this hypothalamic inflammation to a broader spectrum of conditions, such as hypertension [18, 24], impaired glucose tolerance [25–27], and diabetes [28]. Recent studies further revealed that inflammatory markers are upregulated even in the offsprings and affect hypothalamic function and plasticity, resulting in alteration of energy homeostasis when pregnant rodents and non-human primates are exposed to a high-calorie diet [29–31]. Amid these findings, it was surprising that hypothalamic inflammation is found to programmatically regulate whole-body aging on a far larger scale [32, 33] (Fig. 1). Aging is associated with a systemic inflammatory state that has a negative association with longevity [34, 35] and a positive correlation with hypothalamic inflammation and related metabolic diseases [36–38]. These findings suggest that the hypothalamic dysregulation that occurs in metabolic disorders and aging share a similar paradigm, even though aging is more than just a metabolic disorder.
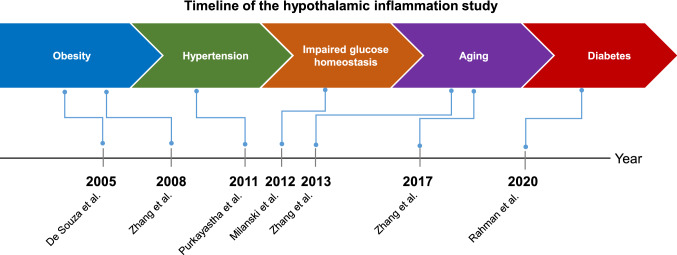
Fig. 1.
Timeline of hypothalamic inflammation in metabolic dysfunction and aging study. The importance of hypothalamic inflammation in metabolic dysfunction and aging is known for the last few decades. The concept began with the observation of increased inflammatory cytokines level in the hypothalamus of high-fat diet (HFD)-fed obese mice by De Souza et al. in 2005. Later studies by several other groups have linked hypothalamic inflammation to hypertension, glucose homeostasis, aging, and diabetes. The ongoing conceptual shifts about our knowledge of hypothalamic inflammation reflect an increasing appreciation that hypothalamic inflammation leads to several pathological and pathophysiological processes despite being a molecular event
Unlike metabolic inflammation in the peripheral tissues, hypothalamic inflammation starts early when animals are exposed to a hypercaloric environment; it is apparent even before the development of disease phenotypes, implying that hypothalamic inflammation is a key driver of metabolic disease [39]. Non-neuronal cells, including microglia [40, 41], astrocytes [24, 42], tanycytes [43], and macrophages [44, 45] are the key cells that have been attributed to hypothalamic inflammation associated with metabolic disorders and aging. Neurons are usually thought to be the victims of hypothalamic inflammation, but recent studies suggest that they can also contribute to inflammation by communicating quiescence or stress signals to surrounding non-neuronal cells [46, 47]. In this scenario, evaluating cellular sources of hypothalamic inflammation and targeting various components of the inflammatory processes can aid in the prevention or treatment of metabolic disorders and aging. This review focuses on recent conceptual advances in the hypothalamic inflammatory basis of metabolic diseases and aging, emphasizing the role of various cell types and mediators in the hypothalamus and their crosstalks. Finally, we discuss the implications, opportunities, and future perspectives of targeting hypothalamic inflammation as a therapy for metabolic disease and aging.
Factors causing hypothalamic inflammation
-
Fatty acids
A fatty acid is a carboxylic acid with a long aliphatic chain, which is either saturated or unsaturated [48]. The human body requires various types of fatty acids as they provide energy, form cell membranes, aid in the absorption of specific vitamins and minerals, and contain essential hormones [49]. Over time, the human diet has shifted to higher consumption of processed food rich in fat content which has led to obesity, diabetes, hypertension, and aging [50–52]. Specifically, long-chain saturated fatty acids (LC-SFAs) are increasingly being identified as the primary dietary triggers of hypothalamic inflammation [53]. LC-SFAs interact with different cell types through toll-like receptors (TLR) 2 and 4 in the hypothalamus. Further, these dietary fatty acids can induce an increased expression of the TLR gene on their own [54, 55]. Previously, researchers demonstrated that LC-SFAs activates predominantly TLR4, resulting in the induction of ER stress, which leads to the activation of inflammatory pathways in the hypothalamus via cytokine expression [56]. However, the direct binding of saturated fatty acids (SFAs) to TLR4 was questioned [57–60], suggesting that SFAs may indirectly interact with TLR4 through endogenous ligands or buildup of various derivatives of fatty acids [61, 62]. In fact, the HFD was found to increase the levels of hypothalamic ceramide, lysophosphatidylcholine, cholesterol esters, and diacylglycerol, and the accumulation of these derivatives increases inflammatory activity [53, 63].
Another concept regarding SFAs-induced inflammation is related to their metabolism. LC-SFAs are less susceptible to β-oxidation than medium-chain fatty acids (carbon chain length 12) and unsaturated fatty acids [64]. This causes elevation of SFAs in the hypothalamus, which is sufficient to induce lipotoxic stress and cause impairment in insulin and leptin signaling [65, 66]. This further helps to explain why a high intake of LC-SFAs causes metabolic inflammation. The condition is supported by the fact that enteric gavage of LC-SFAs causes hypothalamic inflammation, whereas a similar response is not observed with coconut oil, which is mainly composed of medium-chain fatty acids (rich in lauric acid), or olive oil, which is primarily composed of monounsaturated fatty acids (rich in the omega-9 oleic acid) [41]. Furthermore, the key ingredient of the experimental diet used to cause obesity in rodents is lard, and partial replacement of the fatty acids present in a standard HFD with either flax oil (rich in omega-3 fatty acid), fish oil (rich in omega-6 fatty acid, linolenic acid), or olive oil (rich in omega-9 fatty acid, oleic acid), all of which reversed the inflammatory response elicited by a lard-based HFD, improved hypothalamic and whole-body insulin sensitivity, decreased food intake and adiposity [67]. Other researchers confirmed these results upon direct intracerebroventricular (icv) infusion of linolenic acid or oleic acid into the hypothalamus or treatment of hypothalamic cells with these fatty acids, resulting in decreased inflammatory markers or increased expression of anti-inflammatory cytokines like interleukin (IL)-10 [56, 58, 67, 68]. In a recent study, palmitate, an SFA was found increased in cerebrospinal fluid (CSF) of overweight and obese patients with mild cognitive impairment. It was also found that palmitate-induced astrocyte and microglial activation, and its harmful impact was mediated by microglia-derived tumor necrosis factor (TNF)-α signaling [69]. As palmitate elicited proinflammatory responses, ER stress, and insulin resistance in the hypothalamus [66, 70, 71], its dysregulatory effect on hypothalamic functions in humans must be evaluated. Therefore, LC-SFAs-induced TLR activation, lipotoxicity, and ER stress, all of which coexist and reinforce each other by triggering parallel pathways, appear to be pivotal in hypothalamic inflammation in rodents and humans. More recently, it has been found that microbiota-depleted mice are protected from HFD-induced hypothalamic inflammation [72]. The mechanism behind this finding can be because of an increased percentage of Gram-negative bacteria in the gut microbiota following HFD, resulting in increased lipopolysaccharide (LPS) plasma concentration and systemic inflammation, which may eventually lead to hypothalamic inflammation [73]. These findings show that SFAs or HFD can be a potential trigger for hypothalamic inflammation. Amid these findings, one ray of hope is that not all dietary fatty acids cause hypothalamic inflammation as dietary fatty acids, such as omega-3 and omega-9, provide protective effects against hypothalamic inflammation and metabolic dysfunction.
-
Hyperglycemia
Our regular diet consists of both dietary fat and refined carbohydrates and sugar. It is also worth noting that HFD commonly used to induce obesity in rodents includes sucrose as the main carbohydrate source. Further, researchers have shown that overconsumption of simple carbohydrates, especially those found in soft drinks made with high fructose corn syrup, were linked to insulin resistance, obesity, type 2 diabetes mellitus (T2DM), and hypertension [74]. The consumption of a high-calorie diet leads to an undesirable increase in blood glucose. If blood glucose is not kept in check, it can lead to a condition called hyperglycemia [75]. Hyperglycemia is one of the key factors for developing glucotoxicity in the nervous system [76–81]. Previously, several glucotoxicity mechanisms were proposed, such as (1) the induction of oxidative stress through activation of the polyol and hexosamine pathway; (2) activation of protein kinase C (PKC), a serine/threonine-protein kinase that plays a central role in many intracellular signaling pathways; and (3) the formation of non-enzymatic glycated proteins, generating more reactive molecules from glucose [82]. Most studies about the effect of elevated glucose levels on neurotoxicity concentrated mainly on the peripheral nervous system [83]. The association between hyperglycemia and different brain pathologies is an underexplored but emerging research area. Lately, several researchers have tried to address the issue using a different animal model of hyperglycemia or diabetes. Diabetes is a syndrome defined by the presence of abnormally high blood glucose levels or hyperglycemia [84]. During poorly regulated diabetes, brain tissue is chronically exposed to markedly elevated glucose levels and may thus be subject to long-term adverse effects of hyperglycemia as found in peripheral tissues [79]. However, most of the studies of hypothalamic inflammation are performed using HFD-induced obesity and the T2DM model, where several factors confound the effect of hyperglycemia in brain pathology.
Recently, a study by Gao et al. showed the importance of sugar in a diet as it determines whether an HFD induces hypothalamic inflammation and consequent obesity or not [76]. The researchers found that a higher level of N (ε)-(Carboxymethyl)-Lysine (CML), an advanced glycation end product (AGE) is produced by hypothalamic neurons, which then binds to the receptor for AGE (RAGE) in microglia, causing microglial activation and inflammation following consumption of HFD rich in carbohydrates [76]. This idea was strengthened when studies using the type 1 diabetes model, which does not include an HFD, also showed increased microglial hypertrophy, astrocytic activation and proliferation, and increased proinflammatory gene expression in the hypothalamus [77, 85, 86]. Further, a recent study showed an increase in the microglial density in the hypothalamus of the binge sucrose rats [87]. Apart from the different hyperglycemic mice models, the direct administration of glucose into the mouse third ventricle has shown a significant increase in the nuclear factor kappa light chain enhancer of activated B cells (NF-κB) activity in the hypothalamus [20]. In line with this, other studies reported that direct intraperitoneal injection of glucose in rodents also increased hypothalamic Il-1α, Il-1β mRNA and prostaglandins (PG) level [3, 88]. Additionally, in vitro studies have shown that primary cultures of microglia, astrocytes, or Muller glial cells exposed to high glucose conditions exhibited increased production of inflammatory cytokines and reactive oxygen species (ROS) via activation of inflammatory signaling pathways [28, 89–91]. It is also found that incubation of hypothalamic explants at high glucose concentration also increased the levels of Il-1α, Il-1β, and Tnf-α mRNA [28, 88]. Further, hyperglycemia-induced activation of human astrocytes alters synaptic function and neurotoxicity [92]. Various mechanisms were proposed for how hyperglycemia induces an inflammatory response in glial cells. A study by Quan et al. showed that high glucose-induced TNF-α and chemokine C–C motif ligand-2 (CCL2), also called monocyte chemoattractant protein 1 (MCP-1) secretion by microglia is mediated by the ROS and NF-κB [91]. Another study found that the nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome signaling may alter microglia-related inflammation and pyroptosis under high-glucose conditions [93]. Similarly, recent research by Chen et al. demonstrated that chronic hyperglycemia might gradually induce microglia polarization into an increasingly proinflammatory subtype [94]. Apart from the direct effect of hyperglycemia, diabetes leads to the production of other metabolites, such as lactic acid and ketone bodies, which might play inflammatory roles in the hypothalamus [28]. A recent study by Rahman et al. shows that diabetes-induced hyperglycemia induces expression of pyruvate dehydrogenase kinase (PDK)-2, a mitochondrial enzyme that causes a glycolytic shift in astrocytes and increased accumulation of lactic acid in the hypothalamus [28]. The increased lactic acid induces an acidic extracellular microenvironment that may further enhance inflammatory activation of glial cells and the release of proinflammatory cytokines. Similarly, lactic acid also activates and recruits other immune cells, such as monocytes/macrophages or lymphocytes [95, 96], another potential mechanism for hypothalamic inflammation. Collectively, these findings indicate a link between hyperglycemia and neuroinflammation, constituting potential mechanisms for the pathogenesis of metabolic disorders, such as diabetes.
-
Aging
Aging is one of the most intricate and complex biological phenomena. Chronic inflammation and high levels of proinflammatory cytokines are common in the elderly, and they are powerful predictors of various age-related complications [97–99]. This aging-induced inflammatory response, also called inflammaging, is characterized by chronic, sterile (occurring in the absence of infection and primarily driven by endogenous signals), low-grade inflammation [100]. The root causes of hypothalamic inflammation with aging and its link to metabolic dysfunction remain poorly understood. Previously, researchers have reported that glial cell activity and cytokine production in the hypothalamus of mice are elevated with aging [33]. Further, there has been a great interest in the use of drugs that modulates several signaling pathways and delay the onset and progression of aging. However, there are no drugs approved by the Food and Drug Administration (FDA) for extension of lifespan in aging people. Several candidates like acarbose, nordihydroguaiaretic acid, and 17-α-estradiol have been reported to extend life span in animal studies [101]. Taking this into account, a study by Sadagurski et al. has shown that application of these drugs to aging mice blocks hypothalamic reactive gliosis and neuroinflammation indices [102], implying that altered glial activity in the hypothalamus with aging can be a potential mechanism of hypothalamic inflammation. Moreover, the reasons for inflammaging hypothalamus might be due to cellular senescence (a phenomenon characterized by irreversible cell cycle arrest) [103, 104]. Senescent cells accumulate with age in many tissues and are prominent at sites of many age-related pathologies [105]. Senescent cells, albeit not proliferating, are metabolically and transcriptionally active, thereby affecting their microenvironment, notably via the production of inflammatory mediators. These mediators maintain and propagate the senescence process to neighboring cells and recruit immune cells for clearing senescent cells [106, 107]. Importantly, senescent cells frequently secrete cytokines, growth factors, and matrix metalloproteinases, which form the so-called senescence-associated secretory phenotype, which might be a potential mediator of hypothalamic inflammation in aging. Further, strategic targeting of senescent cells in different tissues is associated with decreased age-associated gene signatures, attenuated low-grade chronic inflammation, and improved physical function [108]. Increased inflammation may also derive from defective autophagy in the hypothalamus. Autophagy is a highly controlled process regulating the turnover of cellular components and maintenance of cellular homeostasis. The hypothalamus of aged mice exhibited defective autophagy characterized by lowering of autophagy proteins, such as autophagy-related 7 (ATG7) and microtubule-associated protein light chain 3 (LC3)-II levels and autophagy flux rate [10]. Furthermore, damaged macromolecules and cells (self-debris) that accumulate with age due to increased buildup or incomplete removal may cause an inflammaging hypothalamus. This self-debris is recognized as a threat signal by a network of receptors, which trigger immune responses for physiological repair. However, as damage accumulates, the danger responses will become persistent and maladaptive [109]. These findings suggest that age-related changes in the hypothalamus might act as a substrate for neuroinflammation.
Metabolic disorders, aging, and hypothalamic inflammation
-
Obesity and hypothalamic inflammation
Obesity-induced hypothalamic inflammation in animals and humans: Obesity is associated with chronic low-grade inflammation in the hypothalamus. The first investigation that led to this revelation was based on a study that found changes in several proinflammatory cytokines and inflammatory responsive proteins in the hypothalamus of HFD-fed rats [23]. The finding was further corroborated by various subsequent studies in the coming years [20, 56, 65]. Later studies demonstrated that HFD-induced inflammatory response in the hypothalamus is paralleled by a glial cell accumulation (gliosis) specifically in the ARC of the hypothalamus [40, 41, 110–116]. This diet-induced inflammatory response in the hypothalamus occurs in two phases. The first phase is characterized by increased production of chemokines (and chemokine-related proteins) such as C-X3-C motif chemokine ligand 1 (CX3CL1) also called as fractalkine, leukemia inhibitory factor (LIF), MCP-1, and atypical chemokine receptor 2 (ACKR2); and cytokines, such as TNF-α and IL-6, ranging from few hours to days after exposure to dietary lipids [40, 47, 117, 118]. As the HFD is continued, the hypothalamic inflammatory response returns to near normal after 1 week, and then reappears as the second phase of inflammation, after 4 weeks [40, 119]. This obesity-mediated inflammatory responses have been reported to affect the synthesis and release of neuropeptides and neurotransmitters, which ultimately dysregulate the brain circuit involved in important physiological functions, such as food intake and energy expenditure, eventually leading to obesity and diabetes [120] (Fig. 2).
So far, only a few clinical investigations have been conducted to study the link between hypothalamic inflammation and obesity. When compared to the healthy control group, obese patients without chronic or systemic illness have significantly higher inflammatory markers in the hypothalamus [121]. In line with this, a retrospective analysis of magnetic resonance imaging (MRI) data of obese patients revealed gliosis in the MBH region [40]. Later studies using MRI techniques further supported the notion that hypothalamic gliosis is associated with obesity and insulin resistance in humans [122, 123]. Apart from the metabolic diseases, approximately half of the craniopharyngioma patients who develop tumor in the hypothalamus have severe obesity phenotypes [124]. Further, the use of anti-inflammatory treatment (statin or aspirin) was related to a 2-fold increase in the chance of weight reduction in patients with type 2 diabetes after 1 year of follow-up [125]. Overall, recent studies have enabled to obtain some information in humans and the changes observed in human studies are similar to those seen in rodent models, indicating the translational significance of hypothalamic pathology in obesity.
Signaling pathways involved in hypothalamic inflammation in obesity: Studies have demonstrated that dietary excess can activate various inflammatory signaling pathways in the hypothalamus. Zhang et al. have established an intriguing connection between metabolic inflammation and hypothalamic dysfunction via IκB kinase-β (IKKβ) activation [20]. In their study, chronic dietary or genetic obesity resulted in IKKβ/NF-κB activation in the neurons of MBH. Further, experimental activation of the IKK/NF-κB signaling pathway in the hypothalamus resulted in weight gain and an increase in food intake, as well as significantly impaired insulin and leptin signaling in the hypothalamus [20]. In addition, the authors show that inhibiting IKK/NF-κB signaling in the MBH protects against insulin and leptin resistance and subsequent development of obesity. Further studies by different groups have repeatedly established the participation of several components of the NF-κB signaling pathway including TLR4 and myeloid differentiation primary response 88 (MyD88) in hypothalamic inflammation [56, 126]. Valdearcos et al. later found that activation of NF-κB in microglia is required for HFD-induced microgliosis in the MBH that leads to metabolic dysfunction associated with obesity [113]. Accordingly, reducing the microglial inflammatory activity by targeting NF-κB signaling or microglia depletion protects HFD-fed mice from hyperphagia and obesity [113]. Similarly, Douglass et al. found that IKKβ/NF-κB signaling in astrocytes is required for HFD-induced hypothalamic inflammation and subsequent development of obesity in mice [42]. In this study, selective ablation of hypothalamic astrocyte IKKβ reduces weight gain and glucose intolerance following HFD feeding. Additionally, Zhang et al. reported that the HFD-induced activation of astrocytic IKKβ/NF-κB signaling impaired the astrocytic process plasticity. Such activation caused a reduction of gamma-aminobutyric acid (GABA) and brain-derived neurotrophic factor (BDNF) levels in the hypothalamus, resulting in impaired glucose homeostasis, increase in BP, and body weight gain in mice [24]. These findings suggest that NF-κB signaling remains the principal component of hypothalamic inflammation in obesity.
Several other distinct signaling pathways are also involved in hypothalamic inflammation. c-Jun N-terminal kinases (JNKs), a member of the mitogen-activated protein kinase (MAPK) family is one of them. The JNKs are activated through phosphorylation by upstream MAPK kinases and increase the production of inflammatory cytokines [127]. Several factors, such as nutritional components, hyperglycemia, intracellular ER stress, and oxidative stress, are known to cause JNK activation. It has been reported that selective ablation of JNK1 from the mouse central nervous system (CNS) significantly reduces HFD-induced obesity [128, 129]. Similarly, other signaling pathways, such as the Janus kinase 2 (JAK2)/signal transducer and activator of transcription-3 (STAT3) [130, 131], forkhead box protein O1 (FOXO1) [132, 133], and mechanistic target of rapamycin (mTOR) [134–137] might get dysregulated by inflammatory insults in the hypothalamus during obesity. This leads to an increase in the protein–tyrosine phosphatase 1B (PTP1B) and suppressor of cytokine signaling 3 (SOCS3) levels through the activation of IKKβ/NF-κB, thereby eventually causing aberrant energy balance and obesity [20, 138]. This evidence provides the causal link between hypothalamic inflammation and obesity.
How does hypothalamic inflammation lead to obesity: involvement of leptin and insulin signaling?: Hypothalamic inflammation is involved in the dysregulation of leptin and insulin function. Various studies have revealed that leptin and insulin are the major hormones that convey “adiposity negative feedback” signals to the hypothalamus regarding the amount of body fuel stored as fat [139]. The adiposity negative feedback model of energy homeostasis is based on the idea that circulating signals alert the brain of changes in body fat mass, and that the brain adapts energy balance to stabilize fat storage in response to this information [140]. Taking this into account, various researchers have investigated if obesity-induced hypothalamic inflammation has anything to do with leptin and insulin signaling in the hypothalamus of obese animals. In fact, leptin transport to the brain is hindered by the saturation of leptin transporters, limiting further leptin accumulation in the brain during obesity [141]. This corresponds to the fact that leptin level in the CSF of obese people is significantly lower in comparison to lean individuals [142, 143]. In addition to leptin transport theory, impairment of leptin signaling in the hypothalamus has been the major concern of obesity-induced hypothalamic inflammation. It has been found that hypothalamic SOCS3 expression is enhanced in obese mice [144]. The elevated SOCS3 binds to Tyr985 of leptin receptor (LRb) to mediate the inhibition of LRb signaling in hypothalamus [145]. Interestingly, two putative NF-κB DNA binding motifs are found in the promoter region of the gene encoding SOCS3, and hypothalamic SOCS3 expression is markedly decreased in neuronal IKK-β-deficient mice [20]. Hence, increased SOCS3 has been shown to mediate hypothalamic leptin resistance induced by activated IKKβ/NF-κB signaling [20, 145, 146]. Further, leptin itself is known to induce the expression of SOCS3 in mouse hypothalamus and various in vitro conditions [147, 148] that ultimately cause phosphorylation of Tyr985 in LRb receptor [145], thereby attenuating leptin signaling and promoting leptin resistance in obesity. Similar to SOCS3, PTP1B is another important mediator expressed in the hypothalamus during obesity that inhibits leptin signaling via JAK2-STAT3 pathway [138, 149]. PTP1B also negatively regulates both hypothalamic leptin and insulin signaling via 5′ adenosine monophosphate-activated protein kinase (AMPK) [150]. Therefore, both SOCS3 and PTP1B along with other inflammatory mediators may provide a bridge between hypothalamic inflammation and leptin resistance.
Apart from leptin, insulin resistance is another common feature of obesity in the CNS. It was found that insulin receptors are widely distributed within the hypothalamus [151, 152] particularly the PVN [153] and ARC [154, 155]. The exogenous administration of insulin in the brain might act on these receptors and reduce food intake, body weight [156], and endogenous glucose production [157]. This is further supported by the fact that brain-specific insulin receptor (IR) knockout mice have been used to study obesity and insulin resistance [158]. On the other hand, various studies have found that the hypothalamus regulates insulin secretion and glucose metabolism presumably by the extensive neural connections that exist between the hypothalamus and pancreas [159–161]. However, inflammation in the hypothalamus has been found to affect this pathway too. In the study by Arruda et al., icv injection of recombinant TNF-α protein increases base-line plasma insulin and insulin secretion by pancreatic islets, which is accompanied by an impaired insulin signal transduction in the liver and skeletal muscle [162]. This is important as insulin released from the pancreas acts on different tissues and regulates peripheral glucose metabolism and maintains body weight. Similar to leptin resistance, HFD-induced hypothalamic upregulation of SOCS3, PTP1B, and PKC have been shown to impair hypothalamic insulin signaling pathways [163–165]. Interestingly, several studies have conclusively shown that persistent exposure to elevated leptin levels during obesity also induces insulin resistance via impairment of neuronal insulin signaling in the hypothalamus [166, 167]. Furthermore, HFD-induced activation of hypothalamic inflammatory pathways including IKKβ/NF-κB, TLR4/MyD88, and JNK, as well as the subsequent increase in proinflammatory cytokines results in hypothalamic insulin resistance [11, 20, 56, 126, 168, 169]. When inflammation disrupts insulin and leptin signaling in hypothalamic neurons, a state of positive energy balance develops, which causes accumulation of body fat and a rise in plasma leptin and insulin levels sufficient to overcome the resistance. This state of increased plasma leptin and insulin levels, coupled with an elevated level of body weight is characteristic of common obesity.
Controversies or limitations of the hypothalamic inflammation and obesity studies: Although several studies suggest that hypothalamic inflammation has an important role in the development of diet-induced obesity (DIO), several inconsistencies cast doubt over this hypothesis. A recent study found that defective regulation of hypothalamic POMC is the earliest marker of obesity that even precedes hypothalamic inflammation [170]. However, it was previously found that the acute HFD-induced gliosis in the hypothalamus is thought to limit or repair a neuronal injury, due to induction of neuroprotective substances like heat shock protein 72 (HSP72) mainly in POMC neurons in the hypothalamus [40]. It was assumed that neurons directly respond to SFAs by expressing HSP72 and producing factors that attract local microglia and trigger their activation [40]. However, a study by Valdearcos et al. showed that microglia depletion abolishes HSP72 expression in neurons, suggesting that neuronal stress is triggered by increased microglial activity responding to SFAs [41]. As of late, Dalvi et al. showed that the levels of HSP70, a member of the HSP72 family, and ciliary neurotrophic factor (CNTF) do not alter in the hypothalamic ARC following acute intralipid (an emulsion of fatty acids containing 7–14% palmitic acid together with a mixture of linoleic and oleic acids) infusion; however, they are significantly increased after the extended exposure to HFD feeding for 8 weeks [119]. Further, there has been a concern that an acute increase in TNF-α in the ARC following intralipid infusion or HFD feeding might likely limit or reverse the injury caused by high fatty acid exposure, despite TNF-α being a proinflammatory cytokine [119]. In line with this, it has recently been shown that HFD-induced inflammation in astrocytes may have a protective effect against the development of obesity [171]. Further, it was reported that hypothalamic gliosis caused by HFD is mostly reversible along with the reversal of the HFD-induced obesity phenotype [172]. In contrast to this finding, it is found that HFD-fed rats, when switched back to a regular chow diet for 8 weeks, lose all their excess weight despite sustained elevations in hypothalamic inflammatory gene expression [173]. Interestingly, profound weight loss has been found to induce reactive astrogliosis in the hypothalamus of obese mice, which was probably linked to increased non-essential fatty acids in the circulation [174].
Another aspect of hypothalamic inflammation is the infiltration of peripheral myeloid cells into the hypothalamus during obesity condition. Recent studies have revealed that myeloid cells leave the circulation and accumulate in the MBH of HFD-fed mice and have an important role in the progression of obesity [175, 176]. Researchers have faced difficulties for distinguishing macrophages from resident microglia for some time now. There are limited markers that can exclusively distinguish these cells. Further, the immunoreactivity of recently available microglia-specific markers like purinergic receptor P2RY12 or transmembrane (TMEM) 119 is found to be reduced by HFD consumption [175]. In addition, the reports that infiltrating myeloid cells display morphologies characteristic of resident microglia further complicate studies to determine the independent impact of microglia versus infiltrating myeloid cells on metabolic regulation [177]. Although researchers have tried to address these issues using bone marrow chimeras [47, 171], these studies have been inconsistent [115] and confounded by the effects of irradiation on blood–brain barrier (BBB) [178]. Future research should concentrate on understanding what causes chemotaxis of peripheral myeloid cells into the hypothalamus in the setting of nutritional excess, as well as the metabolic consequences of inhibiting this process. Similar to microglia, astrocytes in the brain also cannot be identified by a single common marker. In fact it has been found that the frequently used glial fibrillary acidic protein (GFAP, a reactive astrocyte marker) can only identify a subset of astrocytes [179]. In this condition, use of a combination of genetic manipulations targeting subpopulations of astrocytes that express aldehyde dehydrogenase 1 family member L1 (Aldh1l1) [180] or glutamate aspartate transporter (Glast) [181] may be required to fully comprehend astrocyte function in the context of obesity and metabolic disorders. On the other hand, one has to be careful as the strategies used to overexpress or downregulate specific proteins in the hypothalamus can itself damage the BBB and hence cause an increased influx of peripheral immune cells.
Knockout mice are widely used in studies to determine the involvement of certain genes or proteins in obesity. One of the studies showed that neuron-specific peroxisome proliferator-activated receptor-δ knockout mice are resistant to HFD-induced hypothalamic inflammation yet are more susceptible to DIO than wild-type control animals [182]. Similarly, various studies using proinflammatory cytokine and cytokine receptor knockout models demonstrated obesity phenotypes rather than being protected from DIO [183–186]. In the study by Schreyer et al., deletion of TNF-α receptors (TNFRs) in mice fed with HFD exhibited marked fasting hyperinsulinemia, glucose tolerance, and insulin sensitivity. Further, loss of both TNFRs and leptin resulted in more dramatic protection from obesity-induced insulin resistance [186]. Another study found that TNFRs knockout mice are heavier than wild-type controls from an early age, and their weight increases further after 14 weeks of high-fat high-sucrose (HFHS) diet feeding [183]. Similar results were reported with IL-1 receptor knockout mice, which showed decreased suppression of body weight and food intake in response to systemic leptin treatment. The decreased leptin responsiveness was even more pronounced in older obese animals [184]. Similarly, Wallenius et al. found that mice lacking the gene encoding IL-6 developed obesity, which was reversed by icv administration of IL-6 in these mice [187, 188]. Because these are primarily whole-body gene knockouts that affect numerous tissues and may induce developmental changes, interpreting these findings for hypothalamic inflammation in DIO is inherently challenging. Fewer other studies found improved insulin sensitivity under inflammatory conditions in peripheral tissues, suggesting that the relationship between hormone signaling and inflammation is situational [189, 190]. Apart from these findings, a new study has revealed that mice with DIO have a functional leptin transport system and have normal leptin level in the MBH or circumventricular organs, although they are leptin resistant [191], which contrasts with the previous findings in both animals and humans [142, 143]. Further, despite activating the same pathways, diet-induced chronic low-grade inflammation leads to obesity, but strong acute inflammation that occurs as part of the illness response leads to weight reduction [162, 192]. In the middle of these confusing results, future research must inquire into these facts and disagreements must be resolved, and a comprehensive perspective on the subject is necessary.
-
Diabetes and hypothalamic inflammation
Hypothalamic inflammation was primarily studied in animal models of obesity or obesity-associated T2DM (Fig. 2). However, very little is known about the role of hypothalamic inflammation in type 1 diabetes. The results from preclinical studies have shown higher levels of microglia and astrocyte activation and increased expressions of proinflammatory genes, such as Tnf-α, Il-1β, and inducible nitric oxide synthase (iNOS) in the hypothalamus of diabetic rodents [28, 85, 193, 194]. A study by Hu et al. further found that diabetes enhances the infiltration of bone marrow-derived macrophages (BMDMs) into the hypothalamus which in turn activates resident microglia [85]. In another study by the same group, it was reported that loss of a cluster of differentiation (CD) 39 in the vasculature of the hypothalamus results in facilitated adhesion and subsequent extravasation of inflammatory cells in these regions. They also believed that increase in activation of astrocytes observed in the brains of the diabetic mice would facilitate BBB leakage and monocyte extravasation [77]. Further, a study by Rana et al. has shown that microglial activation occurs specifically in the PVN and supraoptic nucleus (SON) of the hypothalamus of diabetic mice. They also found that neuronal activation in the hypothalamus precedes the onset of microglial changes suggesting the over-excitation of neurons as a mechanism of microglial activation in diabetic conditions [195]. Thinschmidt et al. used both streptozotocin (STZ) and genetic (Ins2Akita mouse) models of type 1 diabetes and found that nuclear release of proinflammatory mediator high-mobility group box-1 (HMBG1) in the hypothalamus was associated with depressed basal neuronal activity [196]. Similarly, hypothalamic neurons in diabetic mice are found to undergo degeneration [194], showing electron-dense cytoplasm, distension of rough endoplasmic reticulum, and swollen mitochondria [197, 198]. Clinically, diabetic patients develop abnormal autoimmunity or inflammation [199] with a variety of functional and structural brain alterations, such as swelling and edema [200], with discrete brain activation patterns [201]. A brain MRI study of diabetic patients had an enlarged hypothalamus with thickened and compressed third ventricle. These hypothalamic structural changes correlate with classical inflammatory features, including tissue swelling, redness, and aberrant tissue function [202]. In light of the limited knowledge, a recent study revealed the role of hypothalamic inflammation in diabetes [28]. It was found that PDK2-induced metabolic shift from oxidative phosphorylation to glycolysis in astrocytes resulted in an increment of diabetes-induced hypothalamic inflammation with increased gliosis (microglia and astrocytes) and production of proinflammatory cytokines and lactic acid in diabetic mice [28]. This process further led to the dysregulation of AMPK and protein kinase B (AKT) signaling and associated orexigenic neuropeptidergic circuit, which likely accounts for the increased food intake seen in diabetic mice. Further, the pharmacological inhibition of hypothalamic inflammation and site- and cell-specific genetic ablation of Pdk2 inhibited the diabetes-induced hypothalamic inflammation, which was associated with normalized feeding behavior [28]. These findings suggest that hypothalamic inflammation is crucial in diabetes.
-
Hypertension and hypothalamic inflammation
Similar to obesity and diabetes, inflammation has been linked to the development of hypertension in both animals and humans [203, 204]. The PVN in the hypothalamus is a crucial BP regulation center [205], controlling other important nuclei and their circuits in the CNS, especially the rostroventrolateral medulla and nucleus tractus solitarius in the hindbrain, which is necessary for BP control [206–208]. Several studies have reported elevated proinflammatory cytokines expression in the PVN of animal hypertension models [209–213]. Further, the microinjection of inflammatory cytokines, such as TNF-α or IL-1β, into the PVN results in increased sympathetic outflow and increased afferent cardiac sympathetic reflex associated with an elevated BP [214]. Alternatively, the overexpression of anti-inflammatory IL-10 in the PVN of rats attenuates BP induced by chronic infusion of angiotensin (Ang) II [215]. These inflammatory cytokines disrupt critical pathways, including the renal sympathetic nerve activity, PVN sympathetic pathway, and dorsomedial hypothalamic sympathetic pathway, in the hypothalamus that control BP and cause hypertension [216–218]. Alternatively, these cytokines can activate perivascular macrophages (PVMs) in the BBB, causing increased production of PGE2 leading to increased activity of the sympathetic nervous system in the PVN, subsequently increasing BP [216, 219]. The hypothalamic synthesis of Ang II is related to another possible mechanism attributed to inflammatory changes [216]. It is believed that increased levels of Ang II may lead to resetting of the barostat by induction of hypothalamic inflammation, causing higher BP levels [216]. On the other hand, Ang II administration increases the expression of the proinflammatory cytokines IL-1β, IL-6, and TNF-α in the PVN [220]. It was found that Ang II binds to angiotensin II receptor type 1 (AT1) receptors, promoting inflammation through a variety of mechanisms, including the generation of free radicals and TLR4 activation in immune cells, followed by increased secretion of proinflammatory cytokines [221, 222]. A recent study showed that TNFR activation was required for N-methyl-D-aspartate (NMDA)-mediated signaling in the PVN neurons following Ang II-infusion. Further, silencing of TNFR1 in the PVN prevented high BP in these animals [223]. These findings demonstrate that hypothalamic inflammation is closely related to hypertension regulation.
Obesity is one of the major causes of hypertension [203]. With this in mind, researchers are now focusing their attention on the study of hypertension using rodent obesity models with a high clinical translation potential [224, 225]. Obesity-related inflammation in endothelial cells, smooth muscles, and vasculature-resident macrophages in the periphery were linked to hypertension development [226–228]. In the hypothalamus, HFD feeding initiates an inflammatory cascade in both the PVN and subfornical organ (SFO), two brain regions critical for regulation of BP and energy balance [229]. This obesity‐related hypothalamic inflammation can trigger sympathetic BP upregulation [18, 21, 230]. Further, obesity induces increased glial reactivity and proinflammatory gene expression within the PVN of HFD-fed mice that depend on neuronal AT1a receptor signaling [229]. It was found that AT1a contributes to the microglial recruitment in the PVN during obesity [229], which is involved in neurogenic hypertension [215, 231]. Another pathway linked to obesity-induced hypertension was related to leptin, a cytokine originating from adipose tissue overproduced in obese people. This idea is based on the observation that mice lacking leptin (ob/ob) or leptin receptor (db/db) had a higher body weight but no elevated heart rate or BP. Furthermore, leptin therapy causes a rise in heart rate and BP in these mice, which fades as body weight decreases. These results demonstrate that obesity alone is insufficient to affect cardiovascular outcomes and that leptin mediates these effects [232]. However, this finding was connected to inflammation, as researchers discovered that cytokines like TNF-α could be essential in understanding the function of leptin in mediating a pathological BP increase [233, 234]. Further, a new study suggests that leptin signaling in the ARC may contribute to the exaggerated sympathoexcitation observed in the HFD obese rats. The study further reported that energy-sensing enzyme sirtuin 1 (Sirt1) and FOXO1 are the key modulators of leptin signaling in the hypothalamus, which contribute to sympathetic activation and inflammation contributing to increased BP [235]. Previously, Purkayastha et al. identified IKKβ/NF‐κB in hypothalamic POMC neurons as responsible for the central obesity‐related hypertension mechanism [18]. The intriguing finding of the study is that the impact on arterial pressure, arising from the modulation of hypothalamic IKKβ/NF-κB signaling, is unrelated to changes in body weight and adiposity. Such a result indicates that hypothalamic inflammation induces BP imbalance and insulin resistance in an obesity-independent manner [18]. In another study by the same group, brain ER stress was identified as the event upstream of hypothalamic NF-κB activation in the development of CNS inflammation-induced hypertension [21]. In the recent study, Zhang et al. identified the NF-κB pathway as a critical regulator of astrocytic process plasticity, with functional implications for acutely regulated daytime BP [24]. Further, studies using rodent hypertension models other than obesity have also shown the involvement of NF‐κB signaling in the brain [209, 220]. Lately, researchers have also found that mice that were fed with an HFHS diet developed microvascular remodeling in the hypothalamus, including the preautonomic centers. Interestingly, these changes began before the changes in systemic arterial BP, along with an increase in body weight and elevated serum leptin level [236]. They observed that both local astrocytes and the vasculature in the hypothalamus go through significant pathological changes not observed in other brain regions. Further, they identified that leptin acts as an upstream regulator of hypoxia-inducible factor-1α (HIF-1α)-vascular endothelial growth factor (VEGF) pathway in hypothalamic astrocytes associated with the pathological remodeling of the local blood vessels [236]. However, there is a lack of a more complete understanding of the underlying processes linking astrocytic VEGF with autonomic output and BP. Future research should include different pharmacological and transgenic animal models in conjunction with microendoscopy or fiber photometry to examine pathological alterations in astrocytic and neuronal activity within the MBH in vivo. Considered together, our understanding of the several potential mechanisms within the hypothalamus to control BP has vastly improved in recent years. The PVN remains a dominant site within the hypothalamus where neuroinflammation disrupts several signaling pathways, eliciting autonomic and endocrine dysfunction and driving elevations in both sympathetic outflow and BP.
-
Aging and hypothalamic inflammation
Aging is marked by a steady deterioration of multiple bodily functions and increased sensitivity to different age-related disorders, such as diabetes and cardiovascular diseases. Recently, Suda et al. identified exhausted and senescent microglia and activated astrocytes in the hypothalamus of normal aging mice, contributing to aging-related emotional and physical dyscoordination [237]. Another study found that microglia play a significant role in the induction of reactive astrocytes with aging [238]. Further, Zhang et al. reported that the rise in age-related inflammation is mediated by the hypothalamic microglial cells with activated NF-κB and its upstream IKK-β, and they produce inflammatory cytokines like TNF-α, further stimulating NF-κB activity and TNF-α production in neighboring neurons through a cytokine-directed positive feedback mechanism [33]. Another study using a Drosophila model also reported that NF-κB signaling regulates healthy aging and age-related neurodegenerative disease [239]. Zhang et al. further found that hypothalamic inflammation during aging is partially attributed to defective gonadotropin-releasing hormone (GnRH) neurons, which do not sufficiently produce GnRH, a neuropeptide that is essential for neurogenesis [33]. The result is a wide array of physiological, cognitive, and behavioral changes, such as bone degeneration, skin atrophy, muscle deterioration, declined ability to learn, and memory impairment, all of which are direct indicators of systemic aging [33]. The correlation between impairment in neurogenesis with aging and related disorders was reported previously [240]. In particular, adult neural stem cells (NSCs), similar to other stem cells, are self-renewing and can develop into many different cell types. These cells exist in the hypothalamic third-ventricle wall and MBH parenchyma [241]. Previously it was reported that the proliferation of hypothalamic NSCs (htNSCs) was reduced in HFD-induced inflammatory conditions [241, 242]. Considering the defective production of gonadotropin hormone essential for neurogenesis, Zhang et al. further extended their study and reported that NF-κB-mediated inflammatory activation of the hypothalamus in aging mice also leads to the loss of stem cells in the hypothalamus (Fig. 3). These stem cells are responsible for releasing exosomes rich in microRNAs, which help to regulate gene expression to exert endocrine control over the speed of systemic aging [32]. This finding was in line with the understanding that reduced neurogenesis is linked to aging and the onset of aging-related diseases [243–245]. However, Zhang et al. did not address whether microRNAs could mitigate age-related physiological decline on their own. It will be important to see if other exosomal components play a role in aging. Further, the proteins and pathways targeted by the exosomal microRNAs released from the htNSCs and exactly how these affect aging are areas of interest for future research. A recent study found that htNSCs express long noncoding RNAs (lncRNAs) that may play a key role in influencing the fate of these stem cells through regulation of cell senescence [246]. They found a lncRNA, Hnscr, which regulated the senescence of htNSCs and mice aging by binding to Y-box protein 1 (YB-1), protecting it from molecular level degradation. However, they did not employ the Hnscr overexpression strategy in htNSCs of older mice to levels observed in younger mice to see if this is enough to prevent senescence and enhance physiological aging. Another constraint is the relationship between Hnscr and YB-1. While YB-1 is a key target of Hnscr in affecting the senescence of these cells, it may not be the only one [246]. These findings show an emerging correlation between age-related hypothalamic inflammation and stem cell depletion, thus indicating the substantial utility of the development of drugs that target hypothalamic inflammation and neural stem cell maintenance for extending health and life span.
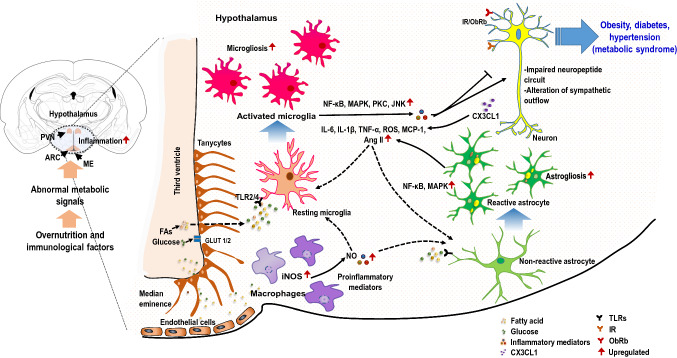
Fig. 2.
Hypothalamic inflammation in the pathophysiology of metabolic diseases. Abnormal metabolic signals associated with overnutrition, and immunological factors appear to induce hypothalamic inflammation. Aberrant nutritional elements, including fatty acids (FAs) and glucose transported into the hypothalamus primarily through the permeable blood–brain barrier (BBB), may induce glial activation, which leads to the release of various inflammatory mediators. Tanycytes located in the lateral walls and surface of the third ventricle allow the dynamic passage of nutrients from the circulation to the hypothalamus. Increased levels of FAs and glucose can activate both microglia and astrocytes through diverse inflammatory signaling pathways, leading to the release of proinflammatory mediators. In addition, abnormal metabolic states can cause the recruitment of macrophages in the hypothalamus, which help in sustaining such inflammation and thus worsening the disease state. Collectively, inflammatory conditions impair insulin and leptin signaling and negatively affect the proper functioning of hypothalamic neuronal circuits, which include neuropeptides and sympathetic outflow in the control of metabolism, leading to metabolic diseases, such as obesity, diabetes, or hypertension. Solid black arrows, reported links; dotted black arrows, possible links, solid red arrows, upregulated. FAs fatty acids, GLUT1/2 glucose transporter ½, IR insulin receptor, MAPK mitogen-activated protein kinase, NF-κB nuclear factor-kappa B, JNK c-Jun N-terminal kinases, PKC protein kinase C, MCP-1 monocyte chemoattractant protein 1, Ang II angiotensin II, ObRb leptin receptor b, ROS reactive oxygen species, TLR2/4 toll-like receptor 2/4
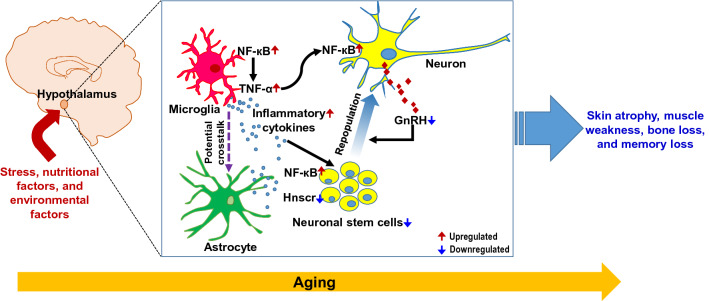
Fig. 3.
Inflammation in the hypothalamus and the regulation of systemic aging. Aging and associated factors, such as stress, nutritional factors, and environmental factors, induce activation of glial cells like microglia and astrocytes in the hypothalamus of the brain. Glial activation increases proinflammatory cytokine levels through NF-κB pathway, which acts on neurons and neuronal stem cells, causing various defects in the secretary function and neurogenesis in the hypothalamus. Finally, the hypothalamic control of whole-body physiology is compromised, leading to age-related changes, including bone loss, skin atrophy, muscle weakness, and memory loss, contributing profoundly to the development of systemic aging. See the text for detail. Solid black arrows, reported link; solid red arrows, upregulated; solid blue arrows, downregulated, dotted purple arrows, potential crosstalk. NF-κB nuclear factor kappa light chain enhancer of activated B cells, GnRH gonadotropin-releasing hormone
Cellular contribution to hypothalamic inflammation
-
Microglia
Microglia are first responder cells in a variety of CNS insults [247]. It has been found that hypothalamic microglia are readily activated in response to an HFD [41, 56, 110, 175], hyperglycemia [28], or a hypertensive state [248]. The presence of lipid-sensitive receptors in microglia, such as TLRs, triggering receptors expressed on myeloid cells 2 (TREM2), and the CD 36 makes them susceptible to incoming lipids during HFD-fed condition [249]. Microglia might utilize these receptors for directly picking up fatty acids within the hypothalamus in animals fed with SFAs-rich diet, leading to their activation [40, 41]. Further, in vitro studies using primary microglia confirmed the ability of SFAs to initiate an inflammatory response, characterized by the production of proinflammatory cytokines and oxidative stress response [41, 250–252]. A recent study found that HFD induces a rapid and transient increase in uncoupling protein 2 (Ucp2), a condition that is linked to mitochondrial dysfunction. In this study, the selective deletion of microglial Ucp2 inhibited HFD-induced impairment in mitochondrial dynamics and function, microglia activation, and the expression of proinflammatory cytokines [111]. It was noted that Ucp2 was deleted from whole brain microglia and not only from the hypothalamus. It is also unclear how UCP2 activation during HFD feeding controls mitochondrial dynamics and function. Further, UCP2 has been found to protect hypothalamic cells from oxidative and pro-apoptotic damage generated by inflammatory stimuli [253]. These issues need to be resolved which necessitates further study to elucidate the molecular mechanism of UCP2 in hypothalamic inflammation with respect to human conditions.
Apart from obesity or HFD, microglial cells are also stimulated when exposed to a hyperglycemic environment. iNOS expression is significantly increased in microglial cells following exposure to a high-glucose environment [254]. High glucose also mediates transition to M1 phenotype in BV-2 microglial cells. Furthermore, interleukin-1 receptor-associated kinase 1 (IRAK1), TNF receptor-associated factor 6 (TRAF6), and NF-κB expression are significantly increased in the high glucose-treated BV2-microglial cells [255]. A recent study in which mice were administered with STZ for 3 weeks (type 1 diabetes) showed increased microglial activation and proliferation in the hypothalamus [28]. Similarly, several studies also underline the essential role of microglia in neuroinflammation and BP regulation [231, 256–258]. Microglia are activated by hypertensive triggers, such as Ang II, and release proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 during hypertension [231, 259]. Contrarily, central Ang II signaling inhibition results in a decrement in neuroinflammation [260, 261], restoring BP [209]. These observations strongly support the idea that microglia participate in the regulation of neuroinflammation in a hypertensive state. Further, microglia appeared to be the initial players in aging. With normal aging, microglia developed an inflammatory phenotype [262], characterized by the increased number and overproduction of inflammatory cytokines [33]. A recent study found that microglia accumulate lipid droplets with aging in mouse and human brains. These microglia exhibit insufficient phagocytic activity, producing high ROS levels, and secreting proinflammatory cytokines [263]. Contrarily, liver X receptor target gene products in the lipid-laden microglia/macrophages facilitate the efflux of lipid and cholesterol to support remyelination by oligodendrocytes during multiple sclerosis [264]. As the latter study was conducted in adult mice, it is possible to speculate from these findings that age determines microglial phenotype toward either proinflammatory or anti-inflammatory. It would be interesting to see how these adult microglia respond to a hypercaloric state. More recently, Maya-Monteiro et al. revealed that both lipid-laden microglia and astrocytes are found to be concentrated mainly in the walls of the third ventricle in the normal hypothalamus of both mice and humans [265]. They also found that HFD-fed mice accumulate lipid droplets in the hypothalamus. However, the hypothalamus of the human brain with T2DM showed lower amounts of lipid droplet accumulation, thus bringing into question the translational significance of the study [265]. Although it is too early to conclusively resolve these discrepancies, it is possible to speculate that HFD in animal models does not fully mimic the human T2DM pathogenesis. This condition is a significant obstacle hampering translational studies on brain dysfunction in T2DM patients [266]. Amid these findings, understanding microglial activity in hypothalamic inflammation and its impact on metabolic diseases and aging will be beneficial in microglial research for designing successful treatment techniques.
-
Astrocytes
Astrocytes are another type of glial cell in the CNS that have an essential role in hypothalamic inflammation. Reactive astrocytosis is observed in the hypothalamus of animals with a metabolic disorder, such as obesity and diabetes [28, 42]. Astrocytes generally detect peripheral signals because of their physical proximity to blood vessels and play an important role in transporting/storing nutrients in the CNS. Mainly peripherally administered fatty acids accumulate in the astrocytes in the hypothalamus, and these lipid‐laden cells display higher expression of GFAP and inflammatory cytokines (e.g., TNF-α, IL‐1β, and IL‐6) and CCL2 or MCP-1 [267–269]. The lipid‐laden astrocytes released CCL2, which causes microglia migration and activation, augmenting hypothalamic inflammatory responses [267]. Further, astrocytes also express TLRs, which activate the NF-κB pathway in response to inflammatory triggers, regulating the production of proinflammatory cytokines during HFD feeding [24, 270–272]. Astrocytes also produce transforming growth factor (TGF)-β during obesity, triggering an RNA stress response in the hypothalamus and leading to atypical NF-κB activation in the hypothalamus [25]. Besides obesity, very little is known about the role of hypothalamic astrocytes in diabetic conditions. Preclinical studies have shown an increased astrocytic reactivity and proliferation along with increased proinflammatory gene expression in the hypothalamus of rodent models of type 1 diabetes [77, 85].
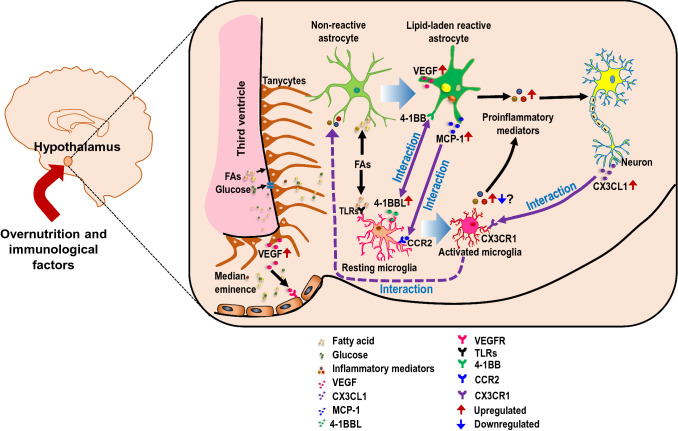
Furthermore, aging is another factor that primes astrocytes toward proinflammatory type [237, 238]. Previous research revealed that aged rat brain astrocytes overexpress genes associated with increased reactivity, inflammation, and oxidative stress, such as lipocalin-2 (Lcn2), C-X-C motif chemokine ligand 10 (Cxcl10), and complement component 3a (C3a), compared to astrocytes from younger individuals [273, 274]. Others reported similar changes in astrocytes isolated from aged mouse brains via fluorescence-activated cell sorting [275]. However, changes were also observed in microglia as they enter a reactive state during aging [276], which might ultimately affect the reactive phenotype of astrocytes during aging. It is to be noted that aging astrocytes can also cause changes in microglia, resulting in a negative feedback loop. Further, astrocytes secrete VEGF-A under the neuroinflammatory condition, which binds to VEGF receptor 2 (VEGFR2) on endothelial cells and enhances BBB permeability. As a result, the astrocyte-derived VEGF-A likely leads to hypothalamic inflammation by causing BBB hyperpermeability in the ARC of obese mice [277, 278] and contributes to hypothalamic inflammation by allowing the free entry of circulating SFAs, inflammatory mediators, and immune cells.
-
Macrophages
Macrophages are white blood cells that play an essential role in the immune system and the inflammatory response of the body [279]. The infiltration of macrophages into the hypothalamus has been observed in various metabolic diseases, such as obesity [44, 45, 175, 280], diabetes [85], and hypertension [281, 282]. Recent research indicates an increased permeability of the BBB following an HFD diet, which agrees with the fact that HFD exposure causes hypervascularization, angiopathy, and endothelial damage in the hypothalamus [283–285]. Compromised BBB integrity during HFD feeding may induce an increase in macrophage recruitment from the general circulation to help the resident glial cells induce an immune response [116]. This idea was confirmed by Buckman et al., who used bone marrow chimeras and found that prolonged HFD feeding promotes BMDM infiltration into different regions of CNS, including the hypothalamus [176]. This finding differed from that by several scientists, as mice received irradiation of the whole body, including the head that can disrupt the integrity of BBB and allow the artificial invasion of peripheral immune cells into the brain. To protect the BBB from irradiation-induced disruption, Valdearcos et al. employed a head and neck shielding approach, which eliminated such bone-marrow transplant monocyte infiltration; however, they did not check this phenotype in HFD-fed conditions [41]. Additionally, Baufeld et al. observed no significant influx of BMDMs into the hypothalamus of green fluorescent protein (GFP+)-harboring bone marrow chimeric mice in HFD-fed conditions, although head and neck shielding strategy was not followed [115]. To resolve the issue, Valdearcos et al. later performed another study using chimeric mice and recently available microglia-specific markers and found that BMDMs infiltration has a significant contribution to HFD-induced microgliosis in the hypothalamus [113]. They also identified that some of the infiltrated cells in the hypothalamus were morphologically similar to local perivascular and meningeal macrophages. The number of cells increased somewhat in response to the HFD, indicating that perivascular or meningeal macrophages also play a role in the diet-induced inflammatory response in the brain [113]. In line with this, researchers have identified the presence of monocyte-derived macrophages in the median eminence (ME) and ARC of the hypothalamus [116, 286]. These PVMs have a vital role in sensing metabolic signals from circulation and controlling BBB functions. For example, they secrete VEGF in response to HFD-induced reduction in brain glucose uptake and maintain brain glucose uptake by increasing BBB glucose transporter 1 (GLUT1) expression [287]. Recently, Lee et al. demonstrated that PVMs activation and aggregation are visible after 2 weeks of HFD feeding, indicating that these cells are stimulated later than microglia during HFD feeding [44]. These activated PVMs migrate from the perivascular space to the ARC parenchyma changing their morphology from linear to reactive microglia-like cells [44]. These activated PVMs in the ARC express higher amounts of iNOS and can release a significant amount of nitric oxide (NO) after being exposed to an HFD [44]. Further, iNOS inhibition in ARC macrophages significantly reduces proinflammatory cytokine overproduction, microgliosis, astrogliosis, macrophage activation/accumulation, and vascular hyperpermeability in HFD-induced hypothalamic inflammation [44]. However, there was some confusion regarding the source of macrophage in the hypothalamus in the HFD-fed condition. Indeed, numerous debates have discussed whether monocytes and macrophages contribute to CNS inflammation [288–290]. Lee et al. extended their study and found that circulating lysozyme-M (LysM) myeloid cells are not actively recruited to the hypothalamic ARC, even under the conditions of chronic HFD feeding. In contrast, a significant LysM-GFP-positive cell infiltration was observed within CNS sites, such as the choroid plexus and meninges adjacent to the hypothalamus. This lack of hypothalamic infiltration of circulating LysM-GFP-positive cells in mice with DIO was unexpected and surprising as the ARC vasculature becomes permeable from the early course of HFD feeding [45]. This study provides evidence that macrophages primarily infiltrate the hypothalamus during the early postnatal cycle before the BBB closure, maintaining their pool through local proliferation, even under chronic HFD feeding conditions.
-
Neurons
Neurons were traditionally thought of as victims of activated glial cells. However, several observations have cast doubts on this assumption. Research have shown previously that both neurons and glia could express cytokines in response to their activation [291, 292]. Neurons express TLRs, which may mediate the pro-inflammatory effects in the hypothalamus. Specifically, TLR4 expression is found in PVN neurons [293] and the immortalized hypothalamic neuronal cell lines, such as rHypoE-7 [294], and N43/5 [295]. Further, TLR2 expression has been demonstrated in mouse ARC neurons in vivo and the mHypoE-42 (N42) hypothalamic neuronal cell line [296]. In line with this, Dwarkasing et al. found that the two hypothalamic neuronal cell lines, HypoE-46 and HypoA-2/12, can produce inflammatory mediators when exposed to LPS, TNF-α, or IL-6 [297]. This finding necessitated the considerable attention of future research to confirm whether similar changes may be observed in terms of primary neurons or not. However, it was found that SFAs or high glucose exposure does not induce inflammatory signaling in cultured hypothalamic neurons [28, 295]. Recently, Sergi et al. showed that LC-SFAs and palmitic acid, but not the medium-chain fatty acid or lauric acid, induce the upregulation of proinflammatory gene expression in cultured N42 hypothalamic neurons [58]. The role of neuronal cells in hypothalamic inflammation has also been studied in several in vivo conditions. Within the first week of HFD exposure, hypothalamic neurons initiated a stress response (as determined by upregulation of the chaperone protein hsp72), close to the initiation of hypothalamic inflammation [40]. Further, hypothalamic neurons are known to express glycated proteins and chemokines following HFD feeding that regulates the activity of nearby microglia and neuroinflammation [47, 76]. Other researchers suggested that inflammation within specific hypothalamic neurons (i.e., AgRP neurons) is critical for disrupting leptin and insulin signaling, favoring weight gain [20]. These findings show that hypothalamic neurons can detect nutrients, such as fatty acids and glucose, via receptor or non-receptor mechanisms. When these neurons detect diverse stimuli, they can initiate a stress response, produce ROS or inflammatory cytokines, which culminate into induction hypothalamic neuroinflammation.
-
Tanycytes
Tanycytes are radial glia-like cells located in the walls of the third ventricle. They are mainly classified into two main subtypes, α- and β-tanycytes, based upon their location and morphological distribution, which are further subdivided into α1-, α2-, β1-, and β2-tanycytes. Specifically, α-tanycytes live dorsally in the ME, whereas β-tanycytes occupy the ventral sidewall and surface of the third ventricle in the ME [298]. Tanycytes serve as a switchboard between the periphery and hypothalamus, given their interface between blood and CSF or brain parenchyma [298]. Recent studies suggest that tanycytes sense glucose [299] and ghrelin [300] and also regulate BBB plasticity and transport leptin into the CNS depending upon nutrient signaling by releasing VEGF-A [301, 302]. Apart from nutrient signaling, hypothalamic tanycytes accumulate lipid droplets in obesity [303]. Further, HFD-fed mice were observed to have an early loss of β1-tanycytes structural organization in the ME [304]. Interestingly, β2-tanycytes in the ME region showed increased neurogenesis upon HFD exposure. Selective inhibition of this mechanism using targeted radiation reduced weight gain in HFD-fed mice, implying that tanycytes proliferation plays a role in obesity [242]. However, unlike microglia and astrocytes, the inflammatory role of tanycytes has not been well studied in different metabolic conditions. Recently, Bottcher et al. found that intravenous administration of IL-1β increases the activity of the transcription factor NF-κB in tanycytes, which lead to increased expression of cyclooxygenase-2 (Cox-2) and the release of the anorexigenic PGE2 from tanycytes [43]. The selective ablation of the IKK subunit NF-κB essential modulator (NEMO) in tanycytes alleviates the IL-1β-induced anorexia. These findings suggest that tanycytes may contribute to hypothalamic inflammation in response to selective stimuli [43]. More studies are required to determine factors involved in the modulation of tanycytes activity that play a causal role in hypothalamic inflammation and the consequent metabolic dysregulation.
-
Neuron-glial antigen 2-positive glial cells
Neuron-glial antigen 2 (NG2) cells are one of the glial cell types found in the CNS, which can be identified by the expression of the NG2 proteoglycan. They are known as oligodendrocyte precursor cells (OPCs) because of their ability to produce and regenerate oligodendrocytes during development and adulthood [305]. However, a growing body of evidence suggests that NG2 cells play much broader and more complex roles in the nervous system function and disease, prompting them to be classified as the fourth major glial cell type [306–308]. After years of study, researchers have reported that NG2 glia are not only precursors to myelinating oligodendrocytes but also play a role in other physiological processes, including synaptic transmission in neurons, body weight control, cognition, and immune response [306, 309–312]. Researchers in recent studies showed an essential role for NG2 glia in various neuroinflammatory conditions. Liu et al. showed that NG2 glia exerts a crucial influence on microglia cellular states relevant to brain aging and neurodegenerative diseases [313]. The NG2 cells were also observed to suppress neuroinflammation and microglial activation through TGF-β signaling [314]. The deficiency of NG2 glia contributes to neuroinflammation and nigral dopaminergic neuron loss in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse Parkinson’s disease model [314]. In results from another study, the ablation of NG2 glial cells produced defects in hippocampal neurons due to excessive neuroinflammation via activation of the IL-1β proinflammatory pathway, resulting in hippocampal atrophy [306]. In the hypothalamus, NG2 glia physically engaged with the dendritic processes of ARC neurons and regulated their responsiveness to leptin signaling [311]. It was also found that genetic and pharmacological ablation of adult NG2 glia resulted in primary leptin resistance and obesity in mice [311]. Further studies are required to determine whether NG2 glia play a causal role in metabolic disorders.
-
Endothelial cells
Endothelial cells form the part of microvessel walls that assist in transferring metabolites through the BBB in the CNS [315]. Exposure of endothelial cells to inflammatory cytokines like IL-1β leads to a BBB dysfunction by repressing the transcription of claudin-5, a tight junction protein essential for maintaining BBB integrity [316, 317]. Surprisingly, during systemic inflammation, vessel-associated microglia can phagocytize astrocytic end-feet, impairing the BBB function [318]. Similarly, endothelial cells are also responsive to various dietary insults. HFD feeding induces CML secretion by neuronal cells in the hypothalamus that binds to both RAGE and activated leukocyte cell-adhesion molecule in endothelial cells and assist neovasculature formation in the hypothalamic ARC [76]. Researchers found that a 3-day HFD reduces the glucose transporter GLUT1 expression in endothelial cells in the CNS, resulting in lower glucose uptake [287]. Further, pathologic changes in the hypothalamic vasculature were observed in response to HFHS diet in rodents and patients with T2DM [285]. Pillon et al. showed that saturated fats increase adhesion molecule expression in microvascular endothelial cells, promoting monocyte adhesion and transmigration [319]. In addition, Freeman et al. showed that the long-term intake of a hypercaloric diet decreased the expression of tight junction proteins on brain endothelial cells and increased microgliosis [320]. Apart from the role of endothelial cells as a physical barrier or regulator of nutrient transport between the CNS and systemic circulation, they were reported to have an important role in neuroinflammation. Like other immune cells in the CNS, endothelial cells can produce cytokines and other mediators that have either neurotrophic or neuroprotective functions [321]. Studies have shown that endothelial cells produce COX-2 and release PGE2 in response to an inflammatory stimulus like IL-1 and LPS [322, 323]. Given the paucity of information about the function of endothelial cells in hypothalamic inflammation, more research is required to establish their significance in metabolic disorders and aging.
Cellular crosstalk in hypothalamic inflammation
The hypothalamus comprises astrocytes, oligodendrocytes, microglia, endothelial cells, ependymal cells, and various neuronal subgroups [15, 324]. Identifying which cells initiate an inflammatory response as a result of metabolic or age-related signals may provide crucial information. Accumulating evidence suggests that hypothalamic inflammation is not limited to the activity of a single-cell type but includes interaction among multiple cell types, which play important roles in the modulation of immune and inflammatory responses in the CNS [325–327]. In general, the communication between glial cells is a critical contributor to hypothalamic inflammation and neurometabolic diseases. Microglia are usually the first to respond to pathological insults in the CNS, accompanied by an astrocytic response. Microglia and astrocytes often release various signaling molecules to provide autoregulatory feedback or create a mutual conversation [328, 329]. Specifically, microglia–astrocyte interaction potentiates their activation states, mainly through the cell surface receptors and subsequent activation of various intracellular signaling pathways [175, 328, 330]. It was recently shown that mitochondria in the microglia of rats fed a HFD are smaller in size and higher in number [111]. This shift in size is mediated by a protein called UCP2 that ultimately regulates the activation of dynamin-related protein 1 (DRP 1), a well-known pro-fission protein [111]. This mitochondrial fragmentation has been attributed to the activation of microglial cells in various studies. A study by Joshi et al. has revealed an increased mitochondrial fragmentation and cytokine production in a mouse model of amyotrophic lateral sclerosis (ALS) [331]. In an extension of their study, many defective mitochondria were observed in activated microglial-conditioned medium and treating primary astrocytes with this conditioned media led to mitochondrial fragmentation and an activation state in astrocytes [331]. From these findings, it can be assumed that there can occur similar intercellular crosstalk through fragmented mitochondria from microglia to astrocyte in the hypothalamus of obese and diabetic mice triggering A1 astrocytic response as shown in the recent studies [111, 331]. However, it is important to consider the reciprocal regulation of microglia by astrocytes as they establish bidirectional conversation by releasing diverse signaling molecules [328]. In line with this, a recent study by Jin et al. showed that astrocyte-specific Myd88 deletion blocked HFD-induced increase in the number, intensity, and size of microglial cells in the hypothalamic ARC [332]. Kwon et al. further found that hypothalamic astrocytes accumulate lipid droplets under SFA-rich environments and get activated to release inflammatory cytokines like TNF-α, IL-1β, IL-6, and MCP-1 [267]. Further, medium conditioned by these astrocytes stimulates microglial chemotactic activity and increases transcripts of the microglial activation marker Iba-1 and inflammatory cytokines [267]. In another study by the same group, mice fed with HFD showed an increased expression of 4-1BB receptor and 4-1BB ligand (4-1BBL) transcripts in the hypothalamus [329]. The 4-1BB, also known as CD137, is a costimulatory and inflammatory receptor found in activated T cells and specific non-immune cells, such as endothelial cells and adipocytes. The 4-1BBL, also known as CD137L, is strongly expressed in macrophages and antigen-presenting cells and can transfer signals into other cells by binding to the 4-1BB receptor [329, 330]. In the CNS, astrocytes express 4-1BB, whereas microglia express 4-1BBL [333, 334]. Such expression suggests that these molecules may have the ability to mediate astrocyte–microglia crosstalk. Taking this into account, Kim and colleagues observed the expression of 4-1BB in astrocytes and 4-1BBL in microglia when these cells were treated with obesity-related factors, such as FFA, glucose, and adipose tissue-conditioned medium [330]. The 4-1BB stimulation on astrocytes produced an inflammatory signal, which increased astrocyte reactivity, increasing the production and release of proinflammatory mediators, such as TNF-α, IL-6, and MCP-1. Concurrently, the 4-1BBL stimulation in microglial cells increases their activity, causing the release of proinflammatory mediators, such as MCP-1 and IL-6, which were mediated through the MAPK pathway [330]. They found that inhibition of 4-1BB/4-1BBL crosstalk with a neutralizing antibody decreases the inflammatory responses in the astrocyte–microglia co-culture [330]. They further confirmed their finding as 4-1BB-deficient astrocytes displayed a reduction in inflammatory responses compared to astrocytes/microglia isolated from wild-type animals when co-cultured with microglia. These results imply that 4-1BBL/4-1BB signaling promotes glial cell-mediated inflammatory crosstalk and plays a role in obesity-induced hypothalamic inflammation.
Most of the researches have found that neurons in the hypothalamus are typically on the receiving end of various metabolic conditions. Recent research, on the other hand, suggests that it may be the other way around. A study has revealed that neurons and astrocytes combine to promote microglial homeostatic signature genes and also repressed inflammatory responses [335]. Another study reported that neuron–astrocyte metabolic coupling protects neurons from fatty acid toxicity during excitatory conditions [336]. In the hypothalamus, overnutrition activates the IKKβ/NF-κB pathway in neurons [20]. Further, neurons release various chemokines, such as CXCL1 [337], CCL21 [338], and CXCL10 [339], under the endangered condition, which might facilitate the migration of glial cells and promote hypothalamic inflammation. Morari et al. revealed that the chemokine CX3CL1 is rapidly induced in hypothalamic neurons of mice after the introduction of HFD [47]; inhibition of CX3CL1 decreases diet-induced hypothalamic inflammation and recruitment of bone marrow-derived monocytes to the hypothalamus. The CX3CL1 is a transmembrane chemokine that signals through its unique receptor CX3CR1 which is primarily expressed in the microglial cells [340, 341]. It is reasonable to infer that inhibiting neuronal CX3CL1 prevents its potential interaction with microglial receptor CX3CR1 in the hypothalamus, resulting in less inflammation after HFD [47]. In another study, inhibition of microglial CX3CR1 attenuates fructose-induced hypertension and chronic brain inflammation [256]. Contrarily, CX3CL1 is known to interact with the CX3CR1 receptor in the microglia and relay “off” signals, keeping them in a resting state [47, 342, 343]. In line with this, Dorfman et al. found that HFD-induced enhanced CX3CR1 signaling renders female mice less susceptible to DIO via reduced microglial activation in the hypothalamus, while male mice lack this adaptive mechanism owing to CX3CL1 and CX3CR1 downregulation after HFD feeding and hence obese phenotype [19]. This might provide a potential insight as to why male rodents are more susceptible to obesity and insulin resistance induced by HFD compared to female counterparts [19, 344–347]. Further analyses are needed to determine whether the membrane-bound or soluble CX3CL1 is responsible for the regulation of microglial function. These pieces of evidence are increasingly evolving, and it is becoming apparent that proper functioning of the neuron–microglia–astrocyte “triad” is critical for brain function [348].
Astrocytes interact with surrounding capillary endothelial cells by extending their endfeet cellular processes that ensheath blood arteries to regulate BBB-endothelial cell functions by cytokine production, such as TGF, glial cell-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), and angiopoietin 1. In parallel, endothelial cells have also been demonstrated to affect astrocyte development by secreting leukemia inhibitory factor and astrocyte activity by supplying key signaling molecules such as lactate and thyroid hormones [349–351]. In the hypothalamus, astrocytes and tanycytes are VEGF sources that facilitate microvessel permeability and help in the reorganization of close junction complexes in the ME and ARC [302, 349–352]. As a result, coordination between circulating metabolites and brain parenchyma can be facilitated. Overall, it is suggested that coordination between tanycytes, astrocytes, and endothelial cells could have a potential role in overnutrition-induced hypothalamic inflammation by increasing the permeability of BBB. Further, bidirectional communication between astrocytes and oligodendrocytes exists, as they express a wide array of receptors that are responsive to inflammatory stimuli secreted by astrocytes and vice versa [353–356]. This relationship is to be looked at in metabolic disorders in future (Fig. 4). In recent years, studying the molecular conversation between microglia and astrocytes has provided novel insights into our understanding of the CNS in health and disease. The interaction between different cell types through secreted molecules is evident in normal brain physiology, and changes in their molecular communication can underpin or facilitate pathological conditions. Future research is needed to broaden our understanding of the functional importance of crosstalk among neurons, astrocytes, and resident and infiltrating immune cells in the hypothalamus. With such knowledge in hands, it will be possible to understand better the pathophysiology of metabolic disorders and aging.
Fig. 4.
Schematic representation of potential intercellular interaction in hypothalamic inflammation associated with metabolic diseases. Hypercaloric environments, such as increased FAs and glucose or immunological factors, induce glial activation and hypothalamic inflammation. Higher circulating FAs and glucose are transported into the hypothalamus through the permeable blood capillaries. Tanycytes, a specialized ependymal cell type located in the lateral walls and surface of the third ventricle, allow the dynamic passage of nutrients, including FAs and glucose, from the circulation to the hypothalamus. Tanycytes express VEGF, which targets microvessel loops and increases the permeability of the vessels and may also contribute to chronic inflammation. In metabolic diseases like obesity and diabetes, increased level of FAs activates both microglia and astrocytes, primarily by binding to TLRs in microglia. Similarly, FAs cause accumulation of lipid droplets in astrocytes, leading to inflammatory activation of astrocytes and releasing proinflammatory mediators, including chemokine MCP-1, which may further activate microglia by molecular interaction, causing sustainable and enhanced inflammation. The lipid-laden reactive astrocytes can also interact with the microglia through the membrane-bound costimulatory receptor/ligand, such as 4-1BB/4-1BBL, enhancing the inflammatory activity of both microglia and astrocytes. Neurons release CX3CL1 following hypercaloric insult and are thought to bind with a chemokine receptor CX3CR1 expressed in the microglia, leading to inflammatory activation of the microglia. Solid purple arrows, reported cellular interaction; dotted purple arrows, possible interaction; solid red arrows, upregulated; solid blue arrows, downregulated. FAs fatty acids, TLR toll-like receptor, VEGF vascular endothelial growth factor, VEGFR vascular endothelial growth factor receptor, MCP-1 monocyte chemoattractant protein 1, CCR2 C–C chemokine receptor type 2, CX3CL1 chemokine (C-X-C motif) ligand 1, CXCL1R CX3C chemokine receptor 1
Hypothalamic inflammation: cause or consequence of the metabolic dysfunction and aging
Over the years, research has focused on elucidating mechanisms linked to the progression of metabolic disorders and aging. When exposed to high fat or high glucose, hypothalamic glial cells are activated and incite increased expression and secretion of diverse inflammatory molecules thought to cause leptin and insulin resistance within central and peripheral tissues. Leptin and insulin resistance is one of the hallmarks of obesity and accounts for many of its comorbidities, including diabetes and hypertension [357, 358]. However, experimental evidence demonstrates that, under defined conditions, inflammatory mediators alone can trigger insulin resistance in cells in the absence of other triggering factors, such as obesity. This observation suggests that inflammation is proximal to the metabolic deterioration [359]. Youn et al. demonstrated that excessive vascular ROS production by a smooth muscle cell in HFD-fed mice had a causal role in obesity by promoting inflammation rather than the other way around [360]. In the hypothalamus, the proliferation and activation of microglia were linked to a rise in proinflammatory factors, such as IL-6 and TNF-α, in rodents with different metabolic disorders, such as obesity [110, 361], hypertension [231, 362], and diabetes [86, 122]. However, researchers showed the upregulation of hypothalamic proinflammatory markers within few hours to days after starting HFD feeding, a period too brief to indicate substantial weight gain, refuting the idea that obesity causes hypothalamic inflammation [40, 118]. Further, direct icv treatment with a low dose of TNF-α inhibits leptin action, thermogenesis, and causes impairment of insulin release and its transduction pathways in peripheral tissues, similar to the effect produced by SFAs-induced hypothalamic inflammation [162, 363]. Numerous reports demonstrated that the induction and pathological actions of ER stress are associated with inflammation [364, 365]. Purkayastha et al. showed that induction of ER stress in the mice brain induced glucose intolerance, insulin resistance, and increased BP, which were accompanied by elevated sympathetic tone [21]. Further, direct microinjection of inflammatory cytokines in the PVN showed a linear correlation between the doses of cytokines with sympathetic activity and BP in animals [214]. These findings indicate that neuroinflammation alone is sufficient to cause changes in parameters usually affected by obesity, T2DM, or hypertension.
Numerous epidemiologic studies characterized inflammation as a feature of aging. The level of inflammatory mediators generally increases with age, even in the absence of acute infection or other physiological stress [366, 367]. In the brain, glial reactivity usually increases with aging, priming the brain for neuroinflammation [368]. While levels are still in the sub-acute range, this chronic inflammation underlies many aging-related metabolic conditions. There is ample evidence to suggest that obesity causes the upregulation of different pathways considered as aging accelerators [369]. Since most metabolic diseases develop with growing age, it is difficult to assess the causal relationship between inflammatory states observed in the hypothalamus of animals with metabolic diseases. Therefore, whether the feedback or feedforward mechanism prevails and consequently whether neuroinflammation is a cause or rather the effect of metabolic disorders or aging remains to be unveiled. Until now, the “chicken or egg” question has no answer. This situation raises a variety of follow-up questions, all of which are essential in addressing the first: is hypothalamic inflammation seen in rodents or humans because of metabolic disease, or is it the opposite case? If both the cases are observed, when will these facts become apparent during disease development? Thus, the evidence supporting a direct role of hypothalamic inflammation in promoting metabolic disease is evidently mixed.
Targeting hypothalamic inflammation for the treatment of metabolic diseases and aging
Evidence from both rodent models and clinical trials supports the idea that targeting metabolic inflammation can be a promising way of treating or preventing various metabolic disorders. In recent years, several researchers have focused on multiple cell types, inflammatory cytokines, and signaling pathways in the hypothalamus to find a new drug for the treatment of metabolic disorders and aging (Table 1). Andre et al. inhibited HFD-induced microglial proliferation in the hypothalamus by delivering arabinofuranosyl cytidine (AraC, an antimitotic drug), resulting in a reduced body weight gain and adiposity [110]. AraC therapy also reduced microglial activation, expression of the proinflammatory cytokine TNF-α, and activation of the NF-κB pathway in the hypothalamus [110]. Similarly, depletion of hypothalamic microglia using PLX5622, a selective inhibitor of colony-stimulating factor 1 receptor (CSF1R) reduced weight gain, overall body fat and food intake in mice with DIO [113]. Further, microglial depletion has been known to disrupt the gustatory circuit that connects the hypothalamus with the paraventricular nucleus of the thalamus, resulting in considerable weight loss largely due to the reduced food intake [370]. Similarly, aerobic training-induced improvement in glucose tolerance and normalization of autonomic dysfunction was associated with decreased microgliosis in the hypothalamus [371, 372]. Minocycline is another drug that was used to inhibit microglial activation and proliferation in the hypothalamus. Minocycline treatment reversed the effect of the neonatal overfeeding-induced increase in microglial numbers [373]. Further, minocycline treatment prevented the overall neuroinflammatory response in the PVN by attenuating microglial activation exerting antihypertensive effects [215, 231, 257]. Moreover, using microglial depletion and adoptive transfer strategies, researchers found that TGF-β modulated BP by suppressing microglial activation [258, 362]. These findings suggest that targeting microglial cells in the hypothalamus can be one strategy for treating metabolic disorders. One limitation of targeting hypothalamic microglia is being unable to distinguish the central microglia and peripheral macrophage. PLX5622 is frequently cited as a CSF1R inhibitor that specifically depletes microglia from the CNS without affecting peripheral monocyte and macrophage populations [374]. Recent research suggests that in response to metabolic derangements, peripheral immune cells may be mobilized to the CNS, contributing to the inflammatory response [176]. While the idea that bone marrow-derived cells play a role in preserving microglial numbers in adult mice under normal physiological conditions is contentious [289], there is growing interest in investigating this possibility to prevent neurodegenerative diseases. If peripheral monocytes or progenitor cells may achieve microglia-like functionality in the CNS, they could be designed as therapeutic resources and delivered to the CNS with incredible therapeutic potential.
Table 1.
Targeting hypothalamic inflammation in preclinical disease models
| Experimental models (animal/tissue) | Treatment/strategy | Effects | References |
|---|---|---|---|
| C57BL/6, HFD |
PLX5622 (a CSF1R inhibitor) Oral route |
Depletes microglia in MBH, reduces weight gain, total body fat, and food intake | [113] |
| Hypothalamic slice culture | Liposomal clodronate treatment |
Depletes microglia, abolishes palmitic acid-induced M1 cytokine secretion |
[41] |
| DTRBMT mice (C57BL/6, SFA gavage with clarified milk fat, coconut oil) |
Ki20227, PLX5622 (CSF1R inhibitors) Oral route |
Depletes microglia in the hypothalamus, enhances leptin-induced STAT3 activation by ARC neurons, decreases food intake | [41] |
| C57BL/6J, HFD |
Arabinofuranosyl cytidine (a mitotic inhibitor) Infusion into the lateral ventricle |
Inhibits microglial proliferation in ARC, inhibits excessive calorie intake and body weight gain, restores hypothalamic leptin sensitivity, and decreases peripheral inflammation | [110] |
| C57BL/6J, HFD |
Butein icv route |
Inhibits hypothalamic IKKβ/NF-κB signaling, improves glucose homeostasis | [375] |
| C57BL/6J, HFD | ARC-directed injection of AAV2-IκBα-mt | Overexpresses IκBα-mt in the ARC, attenuates diet-induced obesity, improves glucose homeostasis | [375] |
| IKKβMGKO mice, HFD | Microglia-specific deletion of Ikbkb | Reduces food intake, decreases weight gain | [113] |
| Male Wistar rats, HFD |
Antibody against TLR4 Infliximab (antibody against TNF-α) icv route |
Reduces hypothalamic resistance to leptin, improves insulin signal transduction in the liver, reduces liver steatosis | [27] |
| C57BL/6 J, HFD | LKB1 overexpression in hypothalamus | Inhibits hypothalamic inflammation, inhibits food intake and body weight gain, improves glucose tolerance, and insulin sensitivity | [387] |
| 4‐1BB‐deficient mice (C57BL/6 J background), HFD; glial cell culture | TKS‐1 treatment (a neutralizing antibody that interrupts interaction between 4-1BBL and 4-1BB) | Reduces hypothalamic inflammation | [330] |
| C57BL/6 J mice, HFD |
Epigallocatechin-3-gallate Oral route |
Attenuates hypothalamic inflammation and microglial overactivation, inhibits diet-induced obesity, and enhances BAT thermogenesis | [383] |
| Wistar rats |
Minocycline Intraperitoneal (ip) route |
Reverses the effect of a neonatal overfeeding-induced increase in microglial numbers | [373] |
| Swiss mice, HFD |
Flax seed oil, olive oil Oral route |
Reduces diet-induced hypothalamic inflammation, reduces food intake and body-mass gain, and improves insulin action and glucose homeostasis | [67] |
| Wistar rats, HFD |
α-Linolenic, oleic acid icv route |
Reduces hypothalamic inflammation and expression of the apoptotic protein | [67] |
| C57B/L6 mice, HFD |
Beta-aminoisobutyric acid Oral route |
Reduces microglial activation and hypothalamic inflammation and may affect obesity pathogenesis | [382] |
| B6/SJL mice, HFD |
Quercetin Oral route |
Reduces microglial activation and inflammatory cytokines in the hypothalamus, attenuates neuronal damage markers | [384] |
| C57B/L6 mice, HFD |
Filbertone Oral route |
Reduces HFD-induced microglial activation, inhibits MAPK and NF-κB pathways, and protects from obesity-induced hypothalamic inflammation | [381] |
| C57B/L6 mice, STZ |
AZD7545 (a PDK2 inhibitor) Oxamate (an LDH inhibitor) GSK2837808 (an LDH inhibitor) Ki20227 (a CSF1R inhibitor) Bay 11–7085 (an inhibitor of I-κB phosphorylation) icv route |
Attenuates diabetes-induced gliosis, inflammatory cytokines and lactic acid production in the hypothalamus, attenuates diabetes-induced increase in food intake | [28] |
| SHR rats (Spontaneously hypertensive rats) |
Tetracycline-3 (CMT3) icv route |
Reduces the microglial number, activation, and proinflammatory cytokine production in the PVN, decreases mean arterial pressure, normalizes sympathetic activity, and left ventricular hypertrophy | [257] |
| Sprague–Dawley rats (s.c. infusion of Ang II) |
Minocycline icv route |
Reduces the microglial number and inflammatory cytokines in PVN, attenuates Ang II-induced hypertension | [215] |
| SHR rats (Spontaneously hypertensive rats) | Aerobic training | Decreases microglia activation, inhibits proinflammatory cytokines expression in PVN, and improves autonomic dysfunction associated with hypertension | [372] |
| SHR rats (Spontaneously hypertensive rats) |
L-798106 [E3-class prostanoid (EP3) receptor inhibitor] infusion into PVN |
Attenuates ROS and proinflammatory cytokines in the PVN, ameliorates hypertension and cardiac hypertrophy | [212] |
| Dahl salt-sensitive (S) rats |
Pyrrolidine dithiocarbamate (a selective NF-κB inhibitor) Bilateral infusion into PVN |
Inhibits NF-κB activity in PVN, attenuates NLRP3, IL-1β, and oxidative stress in the PVN, decreases hypertension | [378] |
| SHR rats (Spontaneously hypertensive rats) |
Pentoxifylline, Etanercept (TNF-α blockers) Bilateral infusion into PVN |
Attenuates PVN NF-κB p65 activity and oxidative stress, attenuates hypertension-induced sympathetic hyperactivity and cardiac hypertrophy | [380] |
| Dahl salt-sensitive (S) rats (High-salt diet) |
PD98059 (a p44/42 MAPK inhibitor) Bilateral infusion into PVN |
Attenuates oxidative stress, modulates neurotransmitters, and decreases hypertension | [388] |
| C57BL/6 mice (HFD) | MBH-directed viral delivery of dominant-negative IκBα (IκBαDN) | Suppresses NF-κB in the hypothalamus and reduces obesity-related hypertension | [18] |
| shRNA (VEGF)GFAP/+ mice, HFHS diet (C57BL/6 J background) | shRNA-mediated knockdown of Vegfa mRNA in astrocytes | Inhibits astrocyte VEGF, reduces sympathetic hyperactivity, and prevents obesity-induced hypertension | [236] |
| MBH-IκBα mice (C57BL/6 mice background) | MBH-directed lentiviral gene delivery of dominant-negative IκBα (IκBαDN) | Improves cognition, attenuates aging-related muscle weakness, and decreases aging-related biomarkers | [236] |
| Ames dwarf mice, Snell dwarf, and GHR−/− mice | Low growth hormone (GH) action | Lowers hypothalamic inflammation, extension of lifespan | [385] |
| UM-HET3 mice | Calorie restriction | Reduces reactive gliosis associated with aging, reduces TNF-α production in microglial cells | [386] |
| UM-HET3 mice |
Acarbose, 17-α-estradiol, nordihydroguaiaretic acid Oral route |
Reduces microglial activation and inflammatory response in the hypothalamus of old mice | [101, 102] |
HFD high-fat diet, HFHS high-fat high sucrose, SFAs saturated fatty acid, icv intracerebroventricular, sc subcutaneous, PVN paraventricular nucleus, MBH mediobasal hypothalamus, ARC arcuate nucleus, GH growth hormone, GHR growth hormone receptor, VEGF vascular endothelial growth factor, BAT brown adipose tissue, STZ streptozotocin, CSF1R colony-stimulating factor 1 receptor, LDH lactate dehydrogenase, PDK2 pyruvate dehydrogenase kinase, LKB1 liver kinase B1, TLR4 toll-like receptor 4
Apart from the depletion of glial cells, blockade of synthesis or release of inflammatory cytokines from hypothalamic cells is another strategy for targeting inflammation. As discussed above, the NF-κB cascade is a central proinflammatory signaling pathway in the hypothalamus that is activated in metabolic diseases and aging. Further, NF-κB is also a key target in receptor-independent hypothalamic microinflammation through intracellular organelle stress, including the RNA stress response, ER stress [20], and defective autophagy [22]. Many independent experiments have now demonstrated that inhibition of hypothalamic NF-κB activation reverses various aspects of metabolic disorders and aging [18, 20, 24, 42, 126, 175, 375–378]. Recent research further reported that the interaction between circulating SFAs and TLR4 is responsible for obesity-associated activation of inflammatory pathways and that mice bearing a CNS-specific deletion of the Myd88 gene are protected against HFD-induced obesity through the alleviation of hypothalamic inflammation, leptin resistance, and weight gain [126]. Use of different neutralizing antibodies to block the activity of inflammatory cytokines and their receptors can be another strategy for targeting hypothalamic inflammation. It was found that icv administration of neutralizing antibodies against TLR4 or TNF-α reduces hypothalamic inflammation, normalize the expression of neurotransmitters involved in the control of feeding, thermogenesis and BP, improve leptin signaling, and restore defective glucose production in the liver [27, 379, 380]. Similarly, Rahman et al. showed that PDK2 plays a crucial role in hypothalamic inflammation and its sequelae in diabetes mouse models. Cell type-specific genetic ablation and pharmacological inhibition of Pdk2 in hypothalamic astrocytes attenuated diabetes-induced local neuroinflammation and increased food intake [28]. In line with this, icv administration of Bay11-7085 (an inhibitor of I-κB phosphorylation), Ki20227 (an inhibitor of CSF1R), AZD7545 (a PDK2 inhibitor), GSK2837808A (an LDHA inhibitor), and oxamate (an LDH inhibitor) are effective attenuating diabetes-induced hypothalamic inflammation and increasing food intake in mice [28]. They further emphasized that lactic acid produced by cells in the hypothalamus of mice with metabolic disorders is a mediator of an increased level of inflammatory cytokines, indicating that lactic acid can be a new therapeutic target in metabolic disorder.
Furthermore, the use of natural compounds like butein, epigallocatechin-3-gallate, quercetin, filbertone, and olive oil showed therapeutic potential against hypothalamic inflammation and metabolic disorders [67, 381–384]. Butein, a dietary flavonoid that inhibits IKKβ/NF-κB signaling, showed dose-dependent glucose-lowering activity, regardless of whether obesity is caused by leptin deficiency or HFD [375]. Yang et al. reported that quercetin supplementation reduced the levels of IL-6, IL-1β, nitric oxide, and microglial activation markers via induction of heme oxygenase (HO)-1 in the hypothalamus of HFD-fed obese mice [384]. Researchers recently found that filbertone supplementation significantly reduced the expression of inflammatory cytokines and a microglial activation marker in the hypothalamus of obese mice [381]. Mechanistically, filbertone decreased HFD-induced microglial activation by inhibiting the MAPK and NF-κB pathways, thus protecting against obesity-induced hypothalamic inflammation [381]. In addition to the treatment of metabolic diseases, these strategies have also been shown to be useful in extending the lifespan of animals [33, 101, 102, 385, 386] (Table 1). Although several inhibitors or receptor antagonists have been widely used in several experimental conditions, their clinical relevance is yet to be established.
Concluding remarks and perspectives
In the last decade, chronic, low-grade inflammation has been identified as a crucial link in the pathogenesis of various diseases. While this low-grade atypical inflammation takes a long time to manifest, it can have adverse consequences for metabolic and physiological processes if left unchecked. In line with this, hypothalamic inflammation has been linked to the pathogenesis of various metabolic diseases and early aging process. Further, metabolism and aging are inextricably linked, metabolism influences aging and vice versa. At times when the medical field is struggling to provide adequate therapy for metabolic disorders, the aging process further imposes a significant burden on patients and their caregivers. Hence, targeting hypothalamic inflammation can be a potential strategy in the treatment of various metabolic sequelae.
The crucial role of hypothalamic inflammation in the pathogenesis of obesity, diabetes, hypertension, and aging is more transparent than ever, thanks to a large body of evidence collected in recent years. Recent research showed that the hypothalamic basis for metabolic syndrome and aging is mediated by NF-κB-dependent inflammatory activation in neurons, neural stem/progenitor cells, and glial cells. It would be interesting to see how NF-κB interacts with other aging-regulating molecular events and whether some NF-κB-unrelated molecular pathways are held accountable. There are still many aspects that are yet to be understood. For example, (1) the connections between hypothalamic inflammation and peripheral inflammation triggered by excessive overeating and aging, (2) the cause–effect relationship of hypothalamic inflammation vs. metabolic diseases and aging, (3) if hypothalamic inflammation is the direct effect of diet (high-fat and carbohydrate diet) or are their effects exerted via an endogenous ligand?, (4) how does hypothalamic microinflammation affect the functions of other brain areas that can lead to the development of a metabolic syndrome and aging?, and (5) are there any differences between natural aging and aging-related chronic diseases in terms of hypothalamic microinflammation?
Glial cells are essential for regulating energy metabolism in both physiological and pathological conditions. Specifically, they have been the subject of research to understand the inflammatory aspect of metabolic diseases. Although the findings are intriguing, further research is needed to determine whether and how hypothalamic glial cells detect diet-induced metabolic stress and communicate with one another and how heterogeneous hypothalamic neurons interact with glial cells. Further, these glial cells attain an inflammatory state with aging, which might affect the progression of metabolic diseases. It is now high time to check the effect of aging glial cells as one of the confounding factors in different animal models, as the study time point for metabolic diseases extends from a few months to years. Another aspect would be the evaluation of sexual diversity in research. As the effect of gender differences in energy homeostasis and other physiological processes are becoming more apparent, essential links might be missing, as female animals are purposefully excluded from most trials to minimize costs and time. Recently, viral vectors with specific promoters were widely used in vivo to selectively modulate gene expression in specific cell populations in the hypothalamus. While these viral techniques are useful in laboratory models, their clinical translation effectiveness is constrained because even modified viruses can cause nonspecific inflammation and toxicity when transmitted to different brain regions.
Furthermore, unlike viral vectors, which are incapable of delivering chemical compounds, nanoparticles may effectively deliver therapeutic agents to specific cells. In addition, recent technological advancements, such as CLARITY-based neuroimaging, nanotechnology, optogenetics, chemogenetics, induced pluripotent stem cells, organoids, and single-cell multiomics, may make preclinical research easier. As a result, current and future studies could improve glia-based clinical treatments for metabolic disorders and aging. Since animal models showed the causal role in hypothalamic microinflammation-associated metabolic syndrome and aging, profiling these inflammatory modifications in human conditions and addressing the relevance of this hypothalamic microinflammatory disorder in terms of human aging and related diseases should be highly important.
Author contributions
All authors have made a substantial intellectual contribution to this work and approved submission of the manuscript. AB and MHR wrote the manuscript. KS edited the manuscript and was involved in all aspects of manuscript preparation.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2017R1A5A2015391, 2020M3E5D9079764).
Declarations
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu T, Xu Y, Yi CX, Tong Q, Cai D. The hypothalamus for whole-body physiology: from metabolism to aging. Protein Cell. 2021 doi: 10.1007/s13238-021-00834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morita-Takemura S, Wanaka A. Blood-to-brain communication in the hypothalamus for energy intake regulation. Neurochem Int. 2019;128:135–142. doi: 10.1016/j.neuint.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Lee ML, Matsunaga H, Sugiura Y, Hayasaka T, Yamamoto I, Ishimoto T, Imoto D, Suematsu M, Iijima N, Kimura K, Diano S, Toda C. Prostaglandin in the ventromedial hypothalamus regulates peripheral glucose metabolism. Nat Commun. 2021;12:2330. doi: 10.1038/s41467-021-22431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 5.Tonon MC, Lanfray D, Castel H, Vaudry H, Morin F. Hypothalamic glucose-sensing: role of glia-to-neuron signaling. Horm Metab Res. 2013;45:955–959. doi: 10.1055/s-0033-1355357. [DOI] [PubMed] [Google Scholar]
- 6.Palkovits M. Hypothalamic regulation of food intake. Ideggyogy Sz. 2003;56:288–302. [PubMed] [Google Scholar]
- 7.Jolicoeur FB, Bouali SM, Fournier A, St-Pierre S. Mapping of hypothalamic sites involved in the effects of NPY on body temperature and food intake. Brain Res Bull. 1995;36:125–129. doi: 10.1016/0361-9230(94)00176-2. [DOI] [PubMed] [Google Scholar]
- 8.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 9.Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med. 2018;119:108–114. doi: 10.1016/j.freeradbiomed.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K, Choe HK. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech Ageing Dev. 2019;177:74–79. doi: 10.1016/j.mad.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Valdearcos M, Xu AW, Koliwad SK. Hypothalamic inflammation in the control of metabolic function. Annu Rev Physiol. 2015;77:131–160. doi: 10.1146/annurev-physiol-021014-071656. [DOI] [PubMed] [Google Scholar]
- 12.Kumar RB, Aronne LJ. Hypothalamic inflammation: is there evidence for human obesity? Curr Obes Rep. 2014;3:242–247. doi: 10.1007/s13679-014-0104-0. [DOI] [PubMed] [Google Scholar]
- 13.Timper K, Bruning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. 2017;10:679–689. doi: 10.1242/dmm.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waterson MJ, Horvath TL. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 2015;22:962–970. doi: 10.1016/j.cmet.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Freire-Regatillo A, Argente-Arizon P, Argente J, Garcia-Segura LM, Chowen JA. Non-neuronal cells in the hypothalamic adaptation to metabolic signals. Front Endocrinol (Lausanne) 2017;8:51. doi: 10.3389/fendo.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaler JP, Choi SJ, Schwartz MW, Wisse BE. Hypothalamic inflammation and energy homeostasis: resolving the paradox. Front Neuroendocrinol. 2010;31:79–84. doi: 10.1016/j.yfrne.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Mendes NF, Gaspar JM, Lima-Junior JC, Donato J, Jr, Velloso LA, Araujo EP. TGF-beta1 down-regulation in the mediobasal hypothalamus attenuates hypothalamic inflammation and protects against diet-induced obesity. Metabolism. 2018;85:171–182. doi: 10.1016/j.metabol.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorfman MD, Krull JE, Douglass JD, Fasnacht R, Lara-Lince F, Meek TH, Shi X, Damian V, Nguyen HT, Matsen ME, Morton GJ, Thaler JP. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat Commun. 2017;8:14556. doi: 10.1038/ncomms14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purkayastha S, Zhang H, Zhang G, Ahmed Z, Wang Y, Cai D. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci USA. 2011;108:2939–2944. doi: 10.1073/pnas.1006875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Q, Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. J Biol Chem. 2011;286:32324–32332. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Reichel JM, Han C, Zuniga-Hertz JP, Cai D. Astrocytic process plasticity and IKKbeta/NF-kappaB in central control of blood glucose, blood pressure, and body weight. Cell Metab. 2017;25(1091–1102):e1094. doi: 10.1016/j.cmet.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan J, Zhang H, Yin Y, Li J, Tang Y, Purkayastha S, Li L, Cai D. Obesity- and aging-induced excess of central transforming growth factor-beta potentiates diabetic development via an RNA stress response. Nat Med. 2014;20:1001–1008. doi: 10.1038/nm.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benzler J, Ganjam GK, Legler K, Stohr S, Kruger M, Steger J, Tups A. Acute inhibition of central c-Jun N-terminal kinase restores hypothalamic insulin signalling and alleviates glucose intolerance in diabetic mice. J Neuroendocrinol. 2013;25:446–454. doi: 10.1111/jne.12018. [DOI] [PubMed] [Google Scholar]
- 27.Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, Roman EA, Romanatto T, Pascoal LB, Caricilli AM, Torsoni MA, Prada PO, Saad MJ, Velloso LA. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes. 2012;61:1455–1462. doi: 10.2337/db11-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman MH, Bhusal A, Kim JH, Jha MK, Song GJ, Go Y, Jang IS, Lee IK, Suk K. Astrocytic pyruvate dehydrogenase kinase-2 is involved in hypothalamic inflammation in mouse models of diabetes. Nat Commun. 2020;11:5906. doi: 10.1038/s41467-020-19576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schellong K, Melchior K, Ziska T, Ott R, Henrich W, Rancourt RC, Plagemann A. Hypothalamic insulin receptor expression and DNA promoter methylation are sex-specifically altered in adult offspring of high-fat diet (HFD)-overfed mother rats. J Nutr Biochem. 2019;67:28–35. doi: 10.1016/j.jnutbio.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Barrand S, Crowley TM, Wood-Bradley RJ, De Jong KA, Armitage JA. Impact of maternal high fat diet on hypothalamic transcriptome in neonatal Sprague Dawley rats. PLoS ONE. 2017;12:e0189492. doi: 10.1371/journal.pone.0189492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noble EE, Kanoski SE. Early life exposure to obesogenic diets and learning and memory dysfunction. Curr Opin Behav Sci. 2016;9:7–14. doi: 10.1016/j.cobeha.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52–57. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monti D, Ostan R, Borelli V, Castellani G, Franceschi C. Inflammaging and human longevity in the omics era. Mech Ageing Dev. 2017;165:129–138. doi: 10.1016/j.mad.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and ‘garb-aging’. Trends Endocrinol Metab. 2017;28:199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Cai D, Khor S. “Hypothalamic microinflammation” paradigm in aging and metabolic diseases. Cell Metab. 2019;30:19–35. doi: 10.1016/j.cmet.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age-related diseases. Front Immunol. 2017;8:1745. doi: 10.3389/fimmu.2017.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mobbs CV, Moreno CL, Poplawski M. Metabolic mystery: aging, obesity, diabetes, and the ventromedial hypothalamus. Trends Endocrinol Metab. 2013;24:488–494. doi: 10.1016/j.tem.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jais A, Bruning JC. Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest. 2017;127:24–32. doi: 10.1172/JCI88878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP. Astrocyte IKKbeta/NF-kappaB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab. 2017;6:366–373. doi: 10.1016/j.molmet.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bottcher M, Muller-Fielitz H, Sundaram SM, Gallet S, Neve V, Shionoya K, Zager A, Quan N, Liu X, Schmidt-Ullrich R, Haenold R, Wenzel J, Blomqvist A, Engblom D, Prevot V, Schwaninger M. NF-kappaB signaling in tanycytes mediates inflammation-induced anorexia. Mol Metab. 2020;39:101022. doi: 10.1016/j.molmet.2020.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee CH, Kim HJ, Lee YS, Kang GM, Lim HS, Lee SH, Song DK, Kwon O, Hwang I, Son M, Byun K, Sung YH, Kim S, Kim JB, Choi EY, Kim YB, Kim K, Kweon MN, Sohn JW, Kim MS. Hypothalamic macrophage inducible nitric oxide synthase mediates obesity-associated hypothalamic inflammation. Cell Rep. 2018;25(934–946):e935. doi: 10.1016/j.celrep.2018.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee CH, Shin SH, Kang GM, Kim S, Kim J, Yu R, Kim MS. Cellular source of hypothalamic macrophage accumulation in diet-induced obesity. J Neuroinflammation. 2019;16:221. doi: 10.1186/s12974-019-1607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee CH, Suk K, Yu R, Kim MS. Cellular contributors to hypothalamic inflammation in obesity. Mol Cells. 2020;43:431–437. doi: 10.14348/molcells.2020.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morari J, Anhe GF, Nascimento LF, de Moura RF, Razolli D, Solon C, Guadagnini D, Souza G, Mattos AH, Tobar N, Ramos CD, Pascoal VD, Saad MJ, Lopes-Cendes I, Moraes JC, Velloso LA. Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes. 2014;63:3770–3784. doi: 10.2337/db13-1495. [DOI] [PubMed] [Google Scholar]
- 48.Di Pasquale MG. The essentials of essential fatty acids. J Diet Suppl. 2009;6:143–161. doi: 10.1080/19390210902861841. [DOI] [PubMed] [Google Scholar]
- 49.Calder PC. Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enter Nutr. 2015;39:18S–32S. doi: 10.1177/0148607115595980. [DOI] [PubMed] [Google Scholar]
- 50.Kearney J. Food consumption trends and drivers. Philos Trans R Soc Lond B Biol Sci. 2010;365:2793–2807. doi: 10.1098/rstb.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alonso-Pedrero L, Ojeda-Rodriguez A, Martinez-Gonzalez MA, Zalba G, Bes-Rastrollo M, Marti A. Ultra-processed food consumption and the risk of short telomeres in an elderly population of the Seguimiento Universidad de Navarra (SUN) Project. Am J Clin Nutr. 2020;111:1259–1266. doi: 10.1093/ajcn/nqaa075. [DOI] [PubMed] [Google Scholar]
- 52.Nardocci M, Polsky JY, Moubarac JC. Consumption of ultra-processed foods is associated with obesity, diabetes and hypertension in Canadian adults. Can J Public Health. 2021;112:421–429. doi: 10.17269/s41997-020-00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sergi D, Williams LM. Potential relationship between dietary long-chain saturated fatty acids and hypothalamic dysfunction in obesity. Nutr Rev. 2020;78:261–277. doi: 10.1093/nutrit/nuz056. [DOI] [PubMed] [Google Scholar]
- 54.Nam KN, Mounier A, Wolfe CM, Fitz NF, Carter AY, Castranio EL, Kamboh HI, Reeves VL, Wang J, Han X, Schug J, Lefterov I, Koldamova R. Effect of high fat diet on phenotype, brain transcriptome and lipidome in Alzheimer’s model mice. Sci Rep. 2017;7:4307. doi: 10.1038/s41598-017-04412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu YJ, Wang C, Song G, Zang SS, Liu YX, Li L. Toll-like receptor-2 and -4 are associated with hyperlipidemia. Mol Med Rep. 2015;12:8241–8246. doi: 10.3892/mmr.2015.4465. [DOI] [PubMed] [Google Scholar]
- 56.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29:1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 58.Sergi D, Morris AC, Kahn DE, McLean FH, Hay EA, Kubitz P, MacKenzie A, Martinoli MG, Drew JE, Williams LM. Palmitic acid triggers inflammatory responses in N42 cultured hypothalamic cells partially via ceramide synthesis but not via TLR4. Nutr Neurosci. 2020;23:321–334. doi: 10.1080/1028415X.2018.1501533. [DOI] [PubMed] [Google Scholar]
- 59.Murumalla RK, Gunasekaran MK, Padhan JK, Bencharif K, Gence L, Festy F, Cesari M, Roche R, Hoareau L. Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis. 2012;11:175. doi: 10.1186/1476-511X-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lancaster GI, Langley KG, Berglund NA, Kammoun HL, Reibe S, Estevez E, Weir J, Mellett NA, Pernes G, Conway JRW, Lee MKS, Timpson P, Murphy AJ, Masters SL, Gerondakis S, Bartonicek N, Kaczorowski DC, Dinger ME, Meikle PJ, Bond PJ, Febbraio MA. Evidence that TLR4 Is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 2018;27(1096–1110):e1095. doi: 10.1016/j.cmet.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 61.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 62.Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borg ML, Omran SF, Weir J, Meikle PJ, Watt MJ. Consumption of a high-fat diet, but not regular endurance exercise training, regulates hypothalamic lipid accumulation in mice. J Physiol. 2012;590:4377–4389. doi: 10.1113/jphysiol.2012.233288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr. 2000;72:905–911. doi: 10.1093/ajcn/72.4.905. [DOI] [PubMed] [Google Scholar]
- 65.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng L, Yu Y, Szabo A, Wu Y, Wang H, Camer D, Huang XF. Palmitic acid induces central leptin resistance and impairs hepatic glucose and lipid metabolism in male mice. J Nutr Biochem. 2015;26:541–548. doi: 10.1016/j.jnutbio.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 67.Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, Velloso LA. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS ONE. 2012;7:e30571. doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dragano NRV, Solon C, Ramalho AF, de Moura RF, Razolli DS, Christiansen E, Azevedo C, Ulven T, Velloso LA. Polyunsaturated fatty acid receptors, GPR40 and GPR120, are expressed in the hypothalamus and control energy homeostasis and inflammation. J Neuroinflammation. 2017;14:91. doi: 10.1186/s12974-017-0869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melo HM, Seixas da Silva GDS, Sant'Ana MR, Teixeira CVL, Clarke JR, Miya Coreixas VS, de Melo BC, Fortuna JTS, Forny-Germano L, Ledo JH, Oliveira MS, Figueiredo CP, Pardossi-Piquard R, Checler F, Delgado-Garcia JM, Gruart A, Velloso LA, Balthazar MLF, Cintra DE, Ferreira ST, De Felice FG. Palmitate is increased in the cerebrospinal fluid of humans with obesity and induces memory impairment in mice via pro-inflammatory TNF-alpha. Cell Rep. 2020;30(2180–2194):e2188. doi: 10.1016/j.celrep.2020.01.072. [DOI] [PubMed] [Google Scholar]
- 70.Marwarha G, Claycombe K, Schommer J, Collins D, Ghribi O. Palmitate-induced endoplasmic reticulum stress and subsequent C/EBPalpha homologous protein activation attenuates leptin and Insulin-like growth factor 1 expression in the brain. Cell Signal. 2016;28:1789–1805. doi: 10.1016/j.cellsig.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mayer CM, Belsham DD. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5' monophosphate-activated protein kinase activation. Endocrinology. 2010;151:576–585. doi: 10.1210/en.2009-1122. [DOI] [PubMed] [Google Scholar]
- 72.Heiss CN, Manneras-Holm L, Lee YS, Serrano-Lobo J, Hakansson Gladh A, Seeley RJ, Drucker DJ, Backhed F, Olofsson LE. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021;35:109163. doi: 10.1016/j.celrep.2021.109163. [DOI] [PubMed] [Google Scholar]
- 73.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 74.Ferder L, Ferder MD, Inserra F. The role of high-fructose corn syrup in metabolic syndrome and hypertension. Curr Hypertens Rep. 2010;12:105–112. doi: 10.1007/s11906-010-0097-3. [DOI] [PubMed] [Google Scholar]
- 75.Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci. 2016;53:52–67. doi: 10.3109/10408363.2015.1084990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, Bernhard W, Guzman-Ruiz M, Layritz C, Legutko B, Zinser E, Garcia-Caceres C, Buijs RM, Woods SC, Kalsbeek A, Seeley RJ, Nawroth PP, Bidlingmaier M, Tschop MH, Yi CX. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab. 2017;6:897–908. doi: 10.1016/j.molmet.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu P, Thinschmidt JS, Caballero S, Adamson S, Cole L, Chan-Ling T, Grant MB. Loss of survival factors and activation of inflammatory cascades in brain sympathetic centers in type 1 diabetic mice. Am J Physiol Endocrinol Metab. 2015;308:E688–698. doi: 10.1152/ajpendo.00504.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharma R, Buras E, Terashima T, Serrano F, Massaad CA, Hu L, Bitner B, Inoue T, Chan L, Pautler RG. Hyperglycemia induces oxidative stress and impairs axonal transport rates in mice. PLoS ONE. 2010;5:e13463. doi: 10.1371/journal.pone.0013463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jacob RJ, Fan X, Evans ML, Dziura J, Sherwin RS. Brain glucose levels are elevated in chronically hyperglycemic diabetic rats: no evidence for protective adaptation by the blood brain barrier. Metabolism. 2002;51:1522–1524. doi: 10.1053/meta.2002.36347. [DOI] [PubMed] [Google Scholar]
- 80.Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 81.Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002;946:221–231. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]
- 82.Rahman MH, Jha MK, Suk K. Evolving insights into the pathophysiology of diabetic neuropathy: implications of malfunctioning glia and discovery of novel therapeutic targets. Curr Pharm Des. 2016;22:738–757. doi: 10.2174/1381612822666151204001234. [DOI] [PubMed] [Google Scholar]
- 83.Bhusal A, Lee WH, Suk K. Lipocalin-2 in diabetic complications of the nervous system: physiology, pathology, and beyond. Front Physiol. 2021;12:638112. doi: 10.3389/fphys.2021.638112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khan RMM, Chua ZJY, Tan JC, Yang Y, Liao Z, Zhao Y. From pre-diabetes to diabetes: diagnosis, treatments and translational research. Medicina (Kaunas) 2019 doi: 10.3390/medicina55090546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu P, Thinschmidt JS, Yan Y, Hazra S, Bhatwadekar A, Caballero S, Salazar T, Miyan JA, Li W, Derbenev A, Zsombok A, Tikhonenko M, Dominguez JM, 2nd, McGorray SP, Saban DR, Boulton ME, Busik JV, Raizada MK, Chan-Ling T, Grant MB. CNS inflammation and bone marrow neuropathy in type 1 diabetes. Am J Pathol. 2013;183:1608–1620. doi: 10.1016/j.ajpath.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klein JP, Hains BC, Craner MJ, Black JA, Waxman SG. Apoptosis of vasopressinergic hypothalamic neurons in chronic diabetes mellitus. Neurobiol Dis. 2004;15:221–228. doi: 10.1016/j.nbd.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 87.Patkar OL, Mohamed AZ, Narayanan A, Mardon K, Cowin G, Bhalla R, Stimson DHR, Kassiou M, Beecher K, Belmer A, Alvarez Cooper I, Morgan M, Hume DA, Irvine KM, Bartlett SE, Nasrallah F, Cumming P. A binge high sucrose diet provokes systemic and cerebral inflammation in rats without inducing obesity. Sci Rep. 2021;11:11252. doi: 10.1038/s41598-021-90817-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mizuno TM, Lew PS, Spirkina A, Xu Y. Mediation of glucose-induced anorexia by central nervous system interleukin 1 signaling. Behav Brain Res. 2013;256:512–519. doi: 10.1016/j.bbr.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 89.Li W, Liu X, Tu Y, Ding D, Yi Q, Sun X, Wang Y, Wang K, Zhu M, Mao J. Dysfunctional Nurr1 promotes high glucose-induced Muller cell activation by up-regulating the NF-kappaB/NLRP3 inflammasome axis. Neuropeptides. 2020;82:102057. doi: 10.1016/j.npep.2020.102057. [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Li G, Wang Z, Zhang X, Yao L, Wang F, Liu S, Yin J, Ling EA, Wang L, Hao A. High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience. 2012;202:58–68. doi: 10.1016/j.neuroscience.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 91.Quan Y, Jiang CT, Xue B, Zhu SG, Wang X. High glucose stimulates TNFalpha and MCP-1 expression in rat microglia via ROS and NF-kappaB pathways. Acta Pharmacol Sin. 2011;32:188–193. doi: 10.1038/aps.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bahniwal M, Little JP, Klegeris A. High glucose enhances neurotoxicity and inflammatory cytokine secretion by stimulated human astrocytes. Curr Alzheimer Res. 2017;14:731–741. doi: 10.2174/1567205014666170117104053. [DOI] [PubMed] [Google Scholar]
- 93.Huang L, You J, Yao Y, Xie M. High glucose induces pyroptosis of retinal microglia through NLPR3 inflammasome signaling. Arq Bras Oftalmol. 2021;84:67–73. doi: 10.5935/0004-2749.20210010. [DOI] [PubMed] [Google Scholar]
- 94.Chen C, Wu S, Hong Z, Chen X, Shan X, Fischbach S, Xiao X. Chronic hyperglycemia regulates microglia polarization through ERK5. Aging (Albany NY) 2019;11:697–706. doi: 10.18632/aging.101770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yabu M, Shime H, Hara H, Saito T, Matsumoto M, Seya T, Akazawa T, Inoue N. IL-23-dependent and -independent enhancement pathways of IL-17A production by lactic acid. Int Immunol. 2011;23:29–41. doi: 10.1093/intimm/dxq455. [DOI] [PubMed] [Google Scholar]
- 96.Shime H, Yabu M, Akazawa T, Kodama K, Matsumoto M, Seya T, Inoue N. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 97.Newman AB, Sanders JL, Kizer JR, Boudreau RM, Odden MC, Zeki Al Hazzouri A, Arnold AM. Trajectories of function and biomarkers with age: the CHS All Stars Study. Int J Epidemiol. 2016;45:1135–1145. doi: 10.1093/ije/dyw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52:M201–208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 100.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 101.Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sadagurski M, Cady G, Miller RA. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell. 2017;16:652–660. doi: 10.1111/acel.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Di Micco R, Krizhanovsky V, Baker D, d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22:75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018;128:1238–1246. doi: 10.1172/JCI95148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020;16:263–275. doi: 10.1038/s41574-020-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lasry A, Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36:217–228. doi: 10.1016/j.it.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 108.Cai Y, Zhou H, Zhu Y, Sun Q, Ji Y, Xue A, Wang Y, Chen W, Yu X, Wang L, Chen H, Li C, Luo T, Deng H. Elimination of senescent cells by beta-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020;30:574–589. doi: 10.1038/s41422-020-0314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Franceschi C, Valensin S, Bonafe M, Paolisso G, Yashin AI, Monti D, De Benedictis G. The network and the remodeling theories of aging: historical background and new perspectives. Exp Gerontol. 2000;35:879–896. doi: 10.1016/s0531-5565(00)00172-8. [DOI] [PubMed] [Google Scholar]
- 110.Andre C, Guzman-Quevedo O, Rey C, Remus-Borel J, Clark S, Castellanos-Jankiewicz A, Ladeveze E, Leste-Lasserre T, Nadjar A, Abrous DN, Laye S, Cota D. Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral inflammation. Diabetes. 2017;66:908–919. doi: 10.2337/db16-0586. [DOI] [PubMed] [Google Scholar]
- 111.Kim JD, Yoon NA, Jin S, Diano S. Microglial UCP2 mediates inflammation and obesity induced by high-fat feeding. Cell Metab. 2019;30(952–962):e955. doi: 10.1016/j.cmet.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Balland E, Cowley MA. Short-term high-fat diet increases the presence of astrocytes in the hypothalamus of C57BL6 mice without altering leptin sensitivity. J Neuroendocrinol. 2017 doi: 10.1111/jne.12504. [DOI] [PubMed] [Google Scholar]
- 113.Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, Gerritse I, Fasnacht R, Barres BA, Thaler JP, Koliwad SK. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017;26(185–197):e183. doi: 10.1016/j.cmet.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guillemot-Legris O, Masquelier J, Everard A, Cani PD, Alhouayek M, Muccioli GG. High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J Neuroinflammation. 2016;13:206. doi: 10.1186/s12974-016-0666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016;132:361–375. doi: 10.1007/s00401-016-1595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gao Y, Ottaway N, Schriever SC, Legutko B, Garcia-Caceres C, de la Fuente E, Mergen C, Bour S, Thaler JP, Seeley RJ, Filosa J, Stern JE, Perez-Tilve D, Schwartz MW, Tschop MH, Yi CX. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia. 2014;62:17–25. doi: 10.1002/glia.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fioravante M, Bombassaro B, Ramalho AF, de Moura RF, Haddad-Tovolli R, Solon C, Dragano NR, Vettorazzi JF, Gaspar RS, Ropelle ER, Carneiro EM, Morari J, Velloso LA. Hypothalamic expression of the atypical chemokine receptor ACKR2 is involved in the systemic regulation of glucose tolerance. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1126–1137. doi: 10.1016/j.bbadis.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 118.Cansell C, Stobbe K, Sanchez C, Le Thuc O, Mosser CA, Ben-Fradj S, Leredde J, Lebeaupin C, Debayle D, Fleuriot L, Brau F, Devaux N, Benani A, Audinat E, Blondeau N, Nahon JL, Rovere C. Dietary fat exacerbates postprandial hypothalamic inflammation involving glial fibrillary acidic protein-positive cells and microglia in male mice. Glia. 2021;69:42–60. doi: 10.1002/glia.23882. [DOI] [PubMed] [Google Scholar]
- 119.Dalvi PS, Chalmers JA, Luo V, Han DY, Wellhauser L, Liu Y, Tran DQ, Castel J, Luquet S, Wheeler MB, Belsham DD. High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-alpha on appetite-regulating NPY neurons. Int J Obes (Lond) 2017;41:149–158. doi: 10.1038/ijo.2016.183. [DOI] [PubMed] [Google Scholar]
- 120.Cazettes F, Cohen JI, Yau PL, Talbot H, Convit A. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Res. 2011;1373:101–109. doi: 10.1016/j.brainres.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Puig J, Blasco G, Daunis IEJ, Molina X, Xifra G, Ricart W, Pedraza S, Fernandez-Aranda F, Fernandez-Real JM. Hypothalamic damage is associated with inflammatory markers and worse cognitive performance in obese subjects. J Clin Endocrinol Metab. 2015;100:E276–281. doi: 10.1210/jc.2014-2682. [DOI] [PubMed] [Google Scholar]
- 122.van de Sande-Lee S, Melhorn SJ, Rachid B, Rodovalho S, De-Lima-Junior JC, Campos BM, Pedro T, Beltramini GC, Chaim EA, Pareja JC, Cendes F, Maravilla KR, Schur EA, Velloso LA. Radiologic evidence that hypothalamic gliosis is improved after bariatric surgery in obese women with type 2 diabetes. Int J Obes (Lond) 2020;44:178–185. doi: 10.1038/s41366-019-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schur EA, Melhorn SJ, Oh SK, Lacy JM, Berkseth KE, Guyenet SJ, Sonnen JA, Tyagi V, Rosalynn M, De Leon B, Webb MF, Gonsalves ZT, Fligner CL, Schwartz MW, Maravilla KR. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity (Silver Spring) 2015;23:2142–2148. doi: 10.1002/oby.21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roth CL, Eslamy H, Werny D, Elfers C, Shaffer ML, Pihoker C, Ojemann J, Dobyns WB. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity (Silver Spring) 2015;23:1226–1233. doi: 10.1002/oby.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boaz M, Lisy L, Zandman-Goddard G, Wainstein J. The effect of anti-inflammatory (aspirin and/or statin) therapy on body weight in Type 2 diabetic individuals: EAT, a retrospective study. Diabet Med. 2009;26:708–713. doi: 10.1111/j.1464-5491.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 126.Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Bruning JC. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 128.Sabio G, Cavanagh-Kyros J, Barrett T, Jung DY, Ko HJ, Ong H, Morel C, Mora A, Reilly J, Kim JK, Davis RJ. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Belgardt BF, Mauer J, Wunderlich FT, Ernst MB, Pal M, Spohn G, Bronneke HS, Brodesser S, Hampel B, Schauss AC, Bruning JC. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci USA. 2010;107:6028–6033. doi: 10.1073/pnas.1001796107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu H, Du T, Li C, Yang G. STAT3 phosphorylation in central leptin resistance. Nutr Metab (Lond) 2021;18:39. doi: 10.1186/s12986-021-00569-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsunekawa T, Banno R, Mizoguchi A, Sugiyama M, Tominaga T, Onoue T, Hagiwara D, Ito Y, Iwama S, Goto M, Suga H, Sugimura Y, Arima H. Deficiency of PTP1B attenuates hypothalamic inflammation via activation of the JAK2-STAT3 pathway in microglia. EBioMedicine. 2017;16:172–183. doi: 10.1016/j.ebiom.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, DePinho RA, Wardlaw SL, Accili D. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- 134.Mori H, Inoki K, Munzberg H, Opland D, Faouzi M, Villanueva EC, Ikenoue T, Kwiatkowski D, MacDougald OA, Myers MG, Jr, Guan KL. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009;9:362–374. doi: 10.1016/j.cmet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andre C, Catania C, Remus-Borel J, Ladeveze E, Leste-Lasserre T, Mazier W, Binder E, Gonzales D, Clark S, Guzman-Quevedo O, Abrous DN, Laye S, Cota D. mTORC1 pathway disruption abrogates the effects of the ciliary neurotrophic factor on energy balance and hypothalamic neuroinflammation. Brain Behav Immun. 2018;70:325–334. doi: 10.1016/j.bbi.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 136.Cota D. Mammalian target of rapamycin complex 1 (mTORC1) signaling in energy balance and obesity. Physiol Behav. 2009;97:520–524. doi: 10.1016/j.physbeh.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 137.Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012;75:425–436. doi: 10.1016/j.neuron.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P, Kroeger PE, White DW, Jirousek MR, Trevillyan JM. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol. 2002;195:109–118. doi: 10.1016/s0303-7207(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 139.Wisse BE, Schwartz MW. Does hypothalamic inflammation cause obesity? Cell Metab. 2009;10:241–242. doi: 10.1016/j.cmet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 140.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 141.Burguera B, Couce ME. Leptin access into the brain: a saturated transport mechanism in obesity. Physiol Behav. 2001;74:717–720. doi: 10.1016/s0031-9384(01)00615-1. [DOI] [PubMed] [Google Scholar]
- 142.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 143.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 144.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 145.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 146.Velloso LA, Schwartz MW. Altered hypothalamic function in diet-induced obesity. Int J Obes (Lond) 2011;35:1455–1465. doi: 10.1038/ijo.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 148.Emilsson V, Arch JR, de Groot RP, Lister CA, Cawthorne MA. Leptin treatment increases suppressors of cytokine signaling in central and peripheral tissues. FEBS Lett. 1999;455:170–174. doi: 10.1016/s0014-5793(99)00874-1. [DOI] [PubMed] [Google Scholar]
- 149.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 150.Xue B, Pulinilkunnil T, Murano I, Bence KK, He H, Minokoshi Y, Asakura K, Lee A, Haj F, Furukawa N, Catalano KJ, Delibegovic M, Balschi JA, Cinti S, Neel BG, Kahn BB. Neuronal protein tyrosine phosphatase 1B deficiency results in inhibition of hypothalamic AMPK and isoform-specific activation of AMPK in peripheral tissues. Mol Cell Biol. 2009;29:4563–4573. doi: 10.1128/MCB.01914-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 152.Hopkins DF, Williams G. Insulin receptors are widely distributed in human brain and bind human and porcine insulin with equal affinity. Diabet Med. 1997;14:1044–1050. doi: 10.1002/(SICI)1096-9136(199712)14:12<1044::AID-DIA508>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 153.Young WS., 3rd Periventricular hypothalamic cells in the rat brain contain insulin mRNA. Neuropeptides. 1986;8:93–97. doi: 10.1016/0143-4179(86)90035-1. [DOI] [PubMed] [Google Scholar]
- 154.Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford ML. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–1965. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- 156.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93:1339–1344. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 157.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 158.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 159.Chan O, Sherwin RS. Hypothalamic regulation of glucose-stimulated insulin secretion. Diabetes. 2012;61:564–565. doi: 10.2337/db11-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Osundiji MA, Lam DD, Shaw J, Yueh CY, Markkula SP, Hurst P, Colliva C, Roda A, Heisler LK, Evans ML. Brain glucose sensors play a significant role in the regulation of pancreatic glucose-stimulated insulin secretion. Diabetes. 2012;61:321–328. doi: 10.2337/db11-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pozo M, Claret M. Hypothalamic control of systemic glucose homeostasis: the pancreas connection. Trends Endocrinol Metab. 2018;29:581–594. doi: 10.1016/j.tem.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 162.Arruda AP, Milanski M, Coope A, Torsoni AS, Ropelle E, Carvalho DP, Carvalheira JB, Velloso LA. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology. 2011;152:1314–1326. doi: 10.1210/en.2010-0659. [DOI] [PubMed] [Google Scholar]
- 163.Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, Migrenne S, Lefevre AL, Cruciani-Guglielmacci C, Magnan C, Yu F, Niswender K, Irani BG, Holland WL, Clegg DJ. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J Clin Invest. 2009;119:2577–2589. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283:14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 166.Burgos-Ramos E, Chowen JA, Arilla-Ferreiro E, Canelles S, Argente J, Barrios V. Chronic central leptin infusion modifies the response to acute central insulin injection by reducing the interaction of the insulin receptor with IRS2 and increasing its association with SOCS3. J Neurochem. 2011;117:175–185. doi: 10.1111/j.1471-4159.2011.07191.x. [DOI] [PubMed] [Google Scholar]
- 167.Benomar Y, Roy AF, Aubourg A, Djiane J, Taouis M. Cross down-regulation of leptin and insulin receptor expression and signalling in a human neuronal cell line. Biochem J. 2005;388:929–939. doi: 10.1042/BJ20041621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 169.Kwon O, Kim KW, Kim MS. Leptin signalling pathways in hypothalamic neurons. Cell Mol Life Sci. 2016;73:1457–1477. doi: 10.1007/s00018-016-2133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Souza GF, Solon C, Nascimento LF, De-Lima-Junior JC, Nogueira G, Moura R, Rocha GZ, Fioravante M, Bobbo V, Morari J, Razolli D, Araujo EP, Velloso LA. Defective regulation of POMC precedes hypothalamic inflammation in diet-induced obesity. Sci Rep. 2016;6:29290. doi: 10.1038/srep29290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Buckman LB, Thompson MM, Lippert RN, Blackwell TS, Yull FE, Ellacott KL. Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice. Mol Metab. 2015;4:58–63. doi: 10.1016/j.molmet.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Berkseth KE, Guyenet SJ, Melhorn SJ, Lee D, Thaler JP, Schur EA, Schwartz MW. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology. 2014;155:2858–2867. doi: 10.1210/en.2014-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang X, Ge A, Cheng M, Guo F, Zhao M, Zhou X, Liu L, Yang N. Increased hypothalamic inflammation associated with the susceptibility to obesity in rats exposed to high-fat diet. Exp Diabetes Res. 2012;2012:847246. doi: 10.1155/2012/847246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Harrison L, Pfuhlmann K, Schriever SC, Pfluger PT. Profound weight loss induces reactive astrogliosis in the arcuate nucleus of obese mice. Mol Metab. 2019;24:149–155. doi: 10.1016/j.molmet.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, Gerritse I, Fasnacht R, Barres BA, Thaler JP, Koliwad SK. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2018;27:1356. doi: 10.1016/j.cmet.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 176.Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, Weller K, Ellacott KL. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, Thal DR, Charo IF, Heppner FL, Aguzzi A, Garaschuk O, Ransohoff RM, Jucker M. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci USA. 2012;109:18150–18155. doi: 10.1073/pnas.1210150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Diserbo M, Agin A, Lamproglou I, Mauris J, Staali F, Multon E, Amourette C. Blood-brain barrier permeability after gamma whole-body irradiation: an in vivo microdialysis study. Can J Physiol Pharmacol. 2002;80:670–678. doi: 10.1139/y02-070. [DOI] [PubMed] [Google Scholar]
- 179.Tatsumi K, Isonishi A, Yamasaki M, Kawabe Y, Morita-Takemura S, Nakahara K, Terada Y, Shinjo T, Okuda H, Tanaka T, Wanaka A. Olig2-lineage astrocytes: a distinct subtype of astrocytes that differs from GFAP astrocytes. Front Neuroanat. 2018;12:8. doi: 10.3389/fnana.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Srinivasan R, Lu TY, Chai H, Xu J, Huang BS, Golshani P, Coppola G, Khakh BS. New transgenic mouse lines for selectively targeting astrocytes and studying calcium signals in astrocyte processes in situ and in vivo. Neuron. 2016;92:1181–1195. doi: 10.1016/j.neuron.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M. Inducible gene deletion in astroglia and radial glia-a valuable tool for functional and lineage analysis. Glia. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- 182.Kocalis HE, Turney MK, Printz RL, Laryea GN, Muglia LJ, Davies SS, Stanwood GD, McGuinness OP, Niswender KD. Neuron-specific deletion of peroxisome proliferator-activated receptor delta (PPARdelta) in mice leads to increased susceptibility to diet-induced obesity. PLoS ONE. 2012;7:e42981. doi: 10.1371/journal.pone.0042981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pamir N, McMillen TS, Kaiyala KJ, Schwartz MW, LeBoeuf RC. Receptors for tumor necrosis factor-alpha play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology. 2009;150:4124–4134. doi: 10.1210/en.2009-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Garcia MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahren B, Enerback S, Ohlsson C, Wallenius V, Jansson JO. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 185.Chida D, Osaka T, Hashimoto O, Iwakura Y. Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes. 2006;55:971–977. doi: 10.2337/diabetes.55.04.06.db05-1250. [DOI] [PubMed] [Google Scholar]
- 186.Schreyer SA, Chua SC, Jr, LeBoeuf RC. Obesity and diabetes in TNF-alpha receptor- deficient mice. J Clin Invest. 1998;102:402–411. doi: 10.1172/JCI2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wallenius K, Wallenius V, Sunter D, Dickson SL, Jansson JO. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun. 2002;293:560–565. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 188.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 189.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, Jung DY, Ko HJ, Ong H, Kim JK, Mynatt R, Martin RJ, Keenan M, Gao Z, Ye J. Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J Biol Chem. 2010;285:4637–4644. doi: 10.1074/jbc.M109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Harrison L, Schriever SC, Feuchtinger A, Kyriakou E, Baumann P, Pfuhlmann K, Messias AC, Walch A, Tschop MH, Pfluger PT. Fluorescent blood-brain barrier tracing shows intact leptin transport in obese mice. Int J Obes (Lond) 2019;43:1305–1318. doi: 10.1038/s41366-018-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Arruda AP, Milanski M, Romanatto T, Solon C, Coope A, Alberici LC, Festuccia WT, Hirabara SM, Ropelle E, Curi R, Carvalheira JB, Vercesi AE, Velloso LA. Hypothalamic actions of tumor necrosis factor alpha provide the thermogenic core for the wastage syndrome in cachexia. Endocrinology. 2010;151:683–694. doi: 10.1210/en.2009-0865. [DOI] [PubMed] [Google Scholar]
- 193.Serino R, Ueta Y, Tokunaga M, Hara Y, Nomura M, Kabashima N, Shibuya I, Hattori Y, Yamashita H. Upregulation of hypothalamic nitric oxide synthase gene expression in streptozotocin-induced diabetic rats. Diabetologia. 1998;41:640–648. doi: 10.1007/s001250050962. [DOI] [PubMed] [Google Scholar]
- 194.Luo Y, Kaur C, Ling EA. Neuronal and glial response in the rat hypothalamus-neurohypophysis complex with streptozotocin-induced diabetes. Brain Res. 2002;925:42–54. doi: 10.1016/s0006-8993(01)03258-9. [DOI] [PubMed] [Google Scholar]
- 195.Rana I, Badoer E, Alahmadi E, Leo CH, Woodman OL, Stebbing MJ. Microglia are selectively activated in endocrine and cardiovascular control centres in streptozotocin-induced diabetic rats. J Neuroendocrinol. 2014;26:413–425. doi: 10.1111/jne.12161. [DOI] [PubMed] [Google Scholar]
- 196.Thinschmidt JS, Colon-Perez LM, Febo M, Caballero S, King MA, White FA, Grant MB. Depressed basal hypothalamic neuronal activity in type-1 diabetic mice is correlated with proinflammatory secretion of HMBG1. Neurosci Lett. 2016;615:21–27. doi: 10.1016/j.neulet.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dheen ST, Tay SS, Wong WC. Arginine vasopressin- and oxytocin-like immunoreactive neurons in the hypothalamic paraventricular and supraoptic nuclei of streptozotocin-induced diabetic rats. Arch Histol Cytol. 1994;57:461–472. doi: 10.1679/aohc.57.461. [DOI] [PubMed] [Google Scholar]
- 198.Dheen ST, Tay SS, Wong WC. Ultrastructural changes in the hypothalamic paraventricular nucleus of the streptozotocin-induced diabetic rat. Acta Anat (Basel) 1994;149:291–299. doi: 10.1159/000147590. [DOI] [PubMed] [Google Scholar]
- 199.Cabrera SM, Henschel AM, Hessner MJ. Innate inflammation in type 1 diabetes. Transl Res. 2016;167:214–227. doi: 10.1016/j.trsl.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Warncke K, Dressel P, Ziegler AG, Steinborn M, Bonfig W, Burdach S, Engelsberger I. Severe pretreatment cerebral edema in newly diagnosed type 1 diabetes. Horm Res Paediatr. 2014;81:285–288. doi: 10.1159/000357140. [DOI] [PubMed] [Google Scholar]
- 201.Gallardo-Moreno GB, Gonzalez-Garrido AA, Gudayol-Ferre E, Guardia-Olmos J. Type 1 diabetes modifies brain activation in young patients while performing visuospatial working memory tasks. J Diabetes Res. 2015;2015:703512. doi: 10.1155/2015/703512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 203.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–376. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li JJ, Fang CH, Hui RT. Is hypertension an inflammatory disease? Med Hypotheses. 2005;64:236–240. doi: 10.1016/j.mehy.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 205.Basting T, Xu J, Mukerjee S, Epling J, Fuchs R, Sriramula S, Lazartigues E. Glutamatergic neurons of the paraventricular nucleus are critical contributors to the development of neurogenic hypertension. J Physiol. 2018;596:6235–6248. doi: 10.1113/JP276229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 207.Dampney RA, Michelini LC, Li DP, Pan HL. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol. 2018;315:H1200–H1214. doi: 10.1152/ajpheart.00216.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- 209.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gao HL, Yu XJ, Liu KL, Zuo YY, Fu LY, Chen YM, Zhang DD, Shi XL, Qi J, Li Y, Yi QY, Tian H, Wang XM, Yu JY, Zhu GQ, Liu JJ, Kang KB, Kang YM. Chronic infusion of Astaxanthin into hypothalamic paraventricular nucleus modulates cytokines and attenuates the renin-angiotensin system in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2021;77:170–181. doi: 10.1097/FJC.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 212.Wang FF, Ba J, Yu XJ, Shi XL, Liu JJ, Liu KL, Fu LY, Su Q, Li HB, Kang KB, Yi QY, Wang SQ, Gao HL, Qi J, Li Y, Zhu GQ, Kang YM. Central blockade of E-prostanoid 3 receptor ameliorated hypertension partially by attenuating oxidative stress and inflammation in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Cardiovasc Toxicol. 2021;21:286–300. doi: 10.1007/s12012-020-09619-w. [DOI] [PubMed] [Google Scholar]
- 213.Wu Q, Chen Y, Zhang W, Song S, Xu Z, Zhang H, Liu L, Sun J. Upregulation of chemokines in the paraventricular nucleus of the hypothalamus in rats with stress-induced hypertension. Med Sci Monit. 2020;26:e926807. doi: 10.12659/MSM.926807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 2011;203:289–297. doi: 10.1111/j.1748-1716.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 215.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Khor S, Cai D. Hypothalamic and inflammatory basis of hypertension. Clin Sci (Lond) 2017;131:211–223. doi: 10.1042/CS20160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Carmichael CY, Wainford RD. Hypothalamic signaling mechanisms in hypertension. Curr Hypertens Rep. 2015;17:39. doi: 10.1007/s11906-015-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 219.Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–659. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaBin the paraventricular nucleus. Hypertension. 2012;59:113–121. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Biancardi VC, Stranahan AM, Krause EG, de Kloet AD, Stern JE. Cross talk between AT1 receptors and Toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol. 2016;310:H404–415. doi: 10.1152/ajpheart.00247.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Woods C, Marques-Lopes J, Contoreggi NH, Milner TA, Pickel VM, Wang G, Glass MJ. Tumor necrosis factor alpha receptor type 1 activation in the hypothalamic paraventricular nucleus contributes to glutamate signaling and angiotensin II-dependent hypertension. J Neurosci. 2021;41:1349–1362. doi: 10.1523/JNEUROSCI.2360-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension. 2000;35:1009–1015. doi: 10.1161/01.hyp.35.4.1009. [DOI] [PubMed] [Google Scholar]
- 225.Mills E, Kuhn CM, Feinglos MN, Surwit R. Hypertension in CB57BL/6J mouse model of non-insulin-dependent diabetes mellitus. Am J Physiol. 1993;264:R73–78. doi: 10.1152/ajpregu.1993.264.1.R73. [DOI] [PubMed] [Google Scholar]
- 226.Duan SZ, Usher MG, Mortensen RM. PPARs: the vasculature, inflammation and hypertension. Curr Opin Nephrol Hypertens. 2009;18:128–133. doi: 10.1097/MNH.0b013e328325803b. [DOI] [PubMed] [Google Scholar]
- 227.Harrison DG, Guzik TJ, Goronzy J, Weyand C. Is hypertension an immunologic disease? Curr Cardiol Rep. 2008;10:464–469. doi: 10.1007/s11886-008-0073-6. [DOI] [PubMed] [Google Scholar]
- 228.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- 229.de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, Sumners C. Obesity induces neuroinflammation mediated by altered expression of the renin-angiotensin system in mouse forebrain nuclei. Physiol Behav. 2014;136:31–38. doi: 10.1016/j.physbeh.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rahmouni K, Davisson RL, Sigmund CD. Inflaming hypothalamic neurons raises blood pressure. Cell Metab. 2011;14:3–4. doi: 10.1016/j.cmet.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, Shi P. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66:309–316. doi: 10.1161/HYPERTENSIONAHA.115.05333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG, Jr, Licinio J, Brown RD, Enriori PJ, O'Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–1416. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Han C, Wu W, Ale A, Kim MS, Cai D. Central leptin and tumor necrosis factor-alpha (TNFalpha) in diurnal control of blood pressure and hypertension. J Biol Chem. 2016;291:15131–15142. doi: 10.1074/jbc.M116.730408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Liu X, Zheng H. Modulation of Sirt1 and FoxO1 on hypothalamic leptin-mediated sympathetic activation and inflammation in diet-induced obese rats. J Am Heart Assoc. 2021;10:e020667. doi: 10.1161/JAHA.120.020667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gruber T, Pan C, Contreras RE, Wiedemann T, Morgan DA, Skowronski AA, Lefort S, De Bernardis MC, Le Thuc O, Legutko B, Ruiz-Ojeda FJ, Fuente-Fernandez M, Garcia-Villalon AL, Gonzalez-Hedstrom D, Huber M, Szigeti-Buck K, Muller TD, Ussar S, Pfluger P, Woods SC, Erturk A, LeDuc CA, Rahmouni K, Granado M, Horvath TL, Tschop MH, Garcia-Caceres C. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metab. 2021;33:1155–1170.e1110. doi: 10.1016/j.cmet.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Suda Y, Nakashima T, Matsumoto H, Sato D, Nagano S, Mikata H, Yoshida S, Tanaka K, Hamada Y, Kuzumaki N, Narita M. Normal aging induces PD-1-enriched exhausted microglia and A1-like reactive astrocytes in the hypothalamus. Biochem Biophys Res Commun. 2021;541:22–29. doi: 10.1016/j.bbrc.2020.12.086. [DOI] [PubMed] [Google Scholar]
- 238.Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA. 2018;115:E1896–E1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kounatidis I, Chtarbanova S, Cao Y, Hayne M, Jayanth D, Ganetzky B, Ligoxygakis P. NF-kappaB immunity in the brain determines fly lifespan in healthy aging and age-related neurodegeneration. Cell Rep. 2017;19:836–848. doi: 10.1016/j.celrep.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, Aja S, Ford E, Fishell G, Blackshaw S. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15:700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014;346:89–93. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sun F, Mao X, Xie L, Ding M, Shao B, Jin K. Notch1 signaling modulates neuronal progenitor activity in the subventricular zone in response to aging and focal ischemia. Aging Cell. 2013;12:978–987. doi: 10.1111/acel.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xiao YZ, Yang M, Xiao Y, Guo Q, Huang Y, Li CJ, Cai D, Luo XH. Reducing hypothalamic stem cell senescence protects against aging-associated physiological decline. Cell Metab. 2020;31(534–548):e535. doi: 10.1016/j.cmet.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 247.Catalin B, Cupido A, Iancau M, Albu CV, Kirchhoff F. Microglia: first responders in the central nervous system. Rom J Morphol Embryol. 2013;54:467–472. [PubMed] [Google Scholar]
- 248.Takesue K, Kishi T, Hirooka Y, Sunagawa K. Activation of microglia within paraventricular nucleus of hypothalamus is NOT involved in maintenance of established hypertension. J Cardiol. 2017;69:84–88. doi: 10.1016/j.jjcc.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 249.Mauerer R, Walczak Y, Langmann T. Comprehensive mRNA profiling of lipid-related genes in microglia and macrophages using taqman arrays. Methods Mol Biol. 2009;580:187–201. doi: 10.1007/978-1-60761-325-1_10. [DOI] [PubMed] [Google Scholar]
- 250.Button EB, Mitchell AS, Domingos MM, Chung JH, Bradley RM, Hashemi A, Marvyn PM, Patterson AC, Stark KD, Quadrilatero J, Duncan RE. Microglial cell activation increases saturated and decreases monounsaturated fatty acid content, but both lipid species are proinflammatory. Lipids. 2014;49:305–316. doi: 10.1007/s11745-014-3882-y. [DOI] [PubMed] [Google Scholar]
- 251.Tracy LM, Bergqvist F, Ivanova EV, Jacobsen KT, Iverfeldt K. Exposure to the saturated free fatty acid palmitate alters BV-2 microglia inflammatory response. J Mol Neurosci. 2013;51:805–812. doi: 10.1007/s12031-013-0068-7. [DOI] [PubMed] [Google Scholar]
- 252.Wang Z, Liu D, Wang F, Liu S, Zhao S, Ling EA, Hao A. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-kappaB signalling. Br J Nutr. 2012;107:229–241. doi: 10.1017/S0007114511002868. [DOI] [PubMed] [Google Scholar]
- 253.Degasperi GR, Romanatto T, Denis RG, Araujo EP, Moraes JC, Inada NM, Vercesi AE, Velloso LA. UCP2 protects hypothalamic cells from TNF-alpha-induced damage. FEBS Lett. 2008;582:3103–3110. doi: 10.1016/j.febslet.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 254.Hsieh CF, Liu CK, Lee CT, Yu LE, Wang JY. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci Rep. 2019;9:840. doi: 10.1038/s41598-018-37215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Huang Y, Liao Z, Lin X, Wu X, Chen X, Bai X, Zhuang Y, Yang Y, Zhang J. Overexpression of miR-146a might regulate polarization transitions of BV-2 cells induced by high glucose and glucose fluctuations. Front Endocrinol (Lausanne) 2019;10:719. doi: 10.3389/fendo.2019.00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ho CY, Lin YT, Chen HH, Ho WY, Sun GC, Hsiao M, Lu PJ, Cheng PW, Tseng CJ. CX3CR1-microglia mediates neuroinflammation and blood pressure regulation in the nucleus tractus solitarii of fructose-induced hypertensive rats. J Neuroinflammation. 2020;17:185. doi: 10.1186/s12974-020-01857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sharma RK, Yang T, Oliveira AC, Lobaton GO, Aquino V, Kim S, Richards EM, Pepine CJ, Sumners C, Raizada MK. Microglial cells impact gut microbiota and gut pathology in angiotensin II-induced hypertension. Circ Res. 2019;124:727–736. doi: 10.1161/CIRCRESAHA.118.313882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li Y, Shen XZ, Li L, Zhao TV, Bernstein KE, Johnson AK, Lyden P, Fang J, Shi P. Brain transforming growth factor-beta resists hypertension via regulating microglial activation. Stroke. 2017;48:2557–2564. doi: 10.1161/STROKEAHA.117.017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR. IL-1beta and IL-18: inflammatory markers or mediators of hypertension? Br J Pharmacol. 2014;171:5589–5602. doi: 10.1111/bph.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yu Y, Wei SG, Weiss RM, Felder RB. Angiotensin II type 1a receptors in the subfornical organ modulate neuroinflammation in the hypothalamic paraventricular nucleus in heart failure rats. Neuroscience. 2018;381:46–58. doi: 10.1016/j.neuroscience.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Liu M, Shi P, Sumners C. Direct anti-inflammatory effects of angiotensin-(1–7) on microglia. J Neurochem. 2016;136:163–171. doi: 10.1111/jnc.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Spittau B. Aging microglia-phenotypes, functions and implications for age-related neurodegenerative diseases. Front Aging Neurosci. 2017;9:194. doi: 10.3389/fnagi.2017.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens DW, Kim J, Tevini J, Felder TK, Wolinski H, Bertozzi CR, Bassik MC, Aigner L, Wyss-Coray T. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci. 2020;23:194–208. doi: 10.1038/s41593-019-0566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Berghoff SA, Spieth L, Sun T, Hosang L, Schlaphoff L, Depp C, Duking T, Winchenbach J, Neuber J, Ewers D, Scholz P, van der Meer F, Cantuti-Castelvetri L, Sasmita AO, Meschkat M, Ruhwedel T, Mobius W, Sankowski R, Prinz M, Huitinga I, Sereda MW, Odoardi F, Ischebeck T, Simons M, Stadelmann-Nessler C, Edgar JM, Nave KA, Saher G. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat Neurosci. 2021;24:47–60. doi: 10.1038/s41593-020-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Maya-Monteiro CM, Correa-da-Silva F, Hofmann SS, Hesselink MKC, la Fleur SE, Yi CX. Lipid droplets accumulate in the hypothalamus of mice and humans with and without metabolic diseases. Neuroendocrinology. 2021;111:263–272. doi: 10.1159/000508735. [DOI] [PubMed] [Google Scholar]
- 266.Stott NL, Marino JS. High fat rodent models of type 2 diabetes: from rodent to human. Nutrients. 2020 doi: 10.3390/nu12123650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kwon YH, Kim J, Kim CS, Tu TH, Kim MS, Suk K, Kim DH, Lee BJ, Choi HS, Park T, Choi MS, Goto T, Kawada T, Ha TY, Yu R. Hypothalamic lipid-laden astrocytes induce microglia migration and activation. FEBS Lett. 2017;591:1742–1751. doi: 10.1002/1873-3468.12691. [DOI] [PubMed] [Google Scholar]
- 268.Bernoud N, Fenart L, Benistant C, Pageaux JF, Dehouck MP, Moliere P, Lagarde M, Cecchelli R, Lecerf J. Astrocytes are mainly responsible for the polyunsaturated fatty acid enrichment in blood-brain barrier endothelial cells in vitro. J Lipid Res. 1998;39:1816–1824. doi: 10.1016/S0022-2275(20)32169-6. [DOI] [PubMed] [Google Scholar]
- 269.Morand O, Baumann N, Bourre JM. In vivo incorporation of exogenous [1-14C]stearic acid into neurons and astrocytes. Neurosci Lett. 1979;13:177–181. doi: 10.1016/0304-3940(79)90038-7. [DOI] [PubMed] [Google Scholar]
- 270.Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59:242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- 271.Krasovska V, Doering LC. Regulation of IL-6 secretion by astrocytes via TLR4 in the fragile X mouse model. Front Mol Neurosci. 2018;11:272. doi: 10.3389/fnmol.2018.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shen Y, Qin H, Chen J, Mou L, He Y, Yan Y, Zhou H, Lv Y, Chen Z, Wang J, Zhou YD. Postnatal activation of TLR4 in astrocytes promotes excitatory synaptogenesis in hippocampal neurons. J Cell Biol. 2016;215:719–734. doi: 10.1083/jcb.201605046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bellaver B, Souza DG, Souza DO, Quincozes-Santos A. Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol. 2017;54:2969–2985. doi: 10.1007/s12035-016-9880-8. [DOI] [PubMed] [Google Scholar]
- 274.Jiang T, Cadenas E. Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell. 2014;13:1059–1067. doi: 10.1111/acel.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I, Klooster J, Bossers K, Hol EM. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging. 2014;35:1–14. doi: 10.1016/j.neurobiolaging.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 276.Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gruber T, Pan C, Contreras RE, Wiedemann T, Morgan DA, Skowronski AA, Lefort S, De Bernardis MC, Le Thuc O, Legutko B, Ruiz-Ojeda FJ, Fuente-Fernandez M, Garcia-Villalon AL, Gonzalez-Hedstrom D, Huber M, Szigeti-Buck K, Muller TD, Ussar S, Pfluger P, Woods SC, Erturk A, LeDuc CA, Rahmouni K, Granado M, Horvath TL, Tschop MH, Garcia-Caceres C. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metab. 2021;33(1155–1170):e1110. doi: 10.1016/j.cmet.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lainez NM, Jonak CR, Nair MG, Ethell IM, Wilson EH, Carson MJ, Coss D. Diet-induced obesity elicits macrophage infiltration and reduction in spine density in the hypothalami of male but not female mice. Front Immunol. 2018;9:1992. doi: 10.3389/fimmu.2018.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kerkhofs D, van Hagen BT, Milanova IV, Schell KJ, van Essen H, Wijnands E, Goossens P, Blankesteijn WM, Unger T, Prickaerts J, Biessen EA, van Oostenbrugge RJ, Foulquier S. Pharmacological depletion of microglia and perivascular macrophages prevents vascular cognitive impairment in Ang II-induced hypertension. Theranostics. 2020;10:9512–9527. doi: 10.7150/thno.44394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Iyonaga T, Shinohara K, Mastuura T, Hirooka Y, Tsutsui H. Brain perivascular macrophages contribute to the development of hypertension in stroke-prone spontaneously hypertensive rats via sympathetic activation. Hypertens Res. 2020;43:99–110. doi: 10.1038/s41440-019-0333-4. [DOI] [PubMed] [Google Scholar]
- 283.Yi CX, Tschop MH, Woods SC, Hofmann SM. High-fat-diet exposure induces IgG accumulation in hypothalamic microglia. Dis Model Mech. 2012;5:686–690. doi: 10.1242/dmm.009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Salameh TS, Mortell WG, Logsdon AF, Butterfield DA, Banks WA. Disruption of the hippocampal and hypothalamic blood-brain barrier in a diet-induced obese model of type II diabetes: prevention and treatment by the mitochondrial carbonic anhydrase inhibitor, topiramate. Fluids Barriers CNS. 2019;16:1. doi: 10.1186/s12987-018-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Yi CX, Gericke M, Kruger M, Alkemade A, Kabra DG, Hanske S, Filosa J, Pfluger P, Bingham N, Woods SC, Herman J, Kalsbeek A, Baumann M, Lang R, Stern JE, Bechmann I, Tschop MH. High calorie diet triggers hypothalamic angiopathy. Mol Metab. 2012;1:95–100. doi: 10.1016/j.molmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kalin S, Heppner FL, Bechmann I, Prinz M, Tschop MH, Yi CX. Hypothalamic innate immune reaction in obesity. Nat Rev Endocrinol. 2015;11:339–351. doi: 10.1038/nrendo.2015.48. [DOI] [PubMed] [Google Scholar]
- 287.Jais A, Solas M, Backes H, Chaurasia B, Kleinridders A, Theurich S, Mauer J, Steculorum SM, Hampel B, Goldau J, Alber J, Forster CY, Eming SA, Schwaninger M, Ferrara N, Karsenty G, Bruning JC. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell. 2016;166:1338–1340. doi: 10.1016/j.cell.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 288.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 289.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 290.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 291.Acarin L, Gonzalez B, Castellano B. Neuronal, astroglial and microglial cytokine expression after an excitotoxic lesion in the immature rat brain. Eur J Neurosci. 2000;12:3505–3520. doi: 10.1046/j.1460-9568.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- 292.Shibata M. Hypothalamic neuronal responses to cytokines. Yale J Biol Med. 1990;63:147–156. [PMC free article] [PubMed] [Google Scholar]
- 293.Masson GS, Nair AR, Dange RB, Silva-Soares PP, Michelini LC, Francis J. Toll-like receptor 4 promotes autonomic dysfunction, inflammation and microglia activation in the hypothalamic paraventricular nucleus: role of endoplasmic reticulum stress. PLoS ONE. 2015;10:e0122850. doi: 10.1371/journal.pone.0122850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wellhauser L, Belsham DD. Activation of the omega-3 fatty acid receptor GPR120 mediates anti-inflammatory actions in immortalized hypothalamic neurons. J Neuroinflammation. 2014;11:60. doi: 10.1186/1742-2094-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Choi SJ, Kim F, Schwartz MW, Wisse BE. Cultured hypothalamic neurons are resistant to inflammation and insulin resistance induced by saturated fatty acids. Am J Physiol Endocrinol Metab. 2010;298:E1122–1130. doi: 10.1152/ajpendo.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Shechter R, London A, Kuperman Y, Ronen A, Rolls A, Chen A, Schwartz M. Hypothalamic neuronal toll-like receptor 2 protects against age-induced obesity. Sci Rep. 2013;3:1254. doi: 10.1038/srep01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Dwarkasing JT, Witkamp RF, Boekschoten MV, Ter Laak MC, Heins MS, van Norren K. Increased hypothalamic serotonin turnover in inflammation-induced anorexia. BMC Neurosci. 2016;17:26. doi: 10.1186/s12868-016-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Rizzoti K, Lovell-Badge R. Pivotal role of median eminence tanycytes for hypothalamic function and neurogenesis. Mol Cell Endocrinol. 2017;445:7–13. doi: 10.1016/j.mce.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 299.Barahona MJ, Llanos P, Recabal A, Escobar-Acuna K, Elizondo-Vega R, Salgado M, Ordenes P, Uribe E, Sepulveda FJ, Araneda RC, Garcia-Robles MA. Glial hypothalamic inhibition of GLUT2 expression alters satiety, impacting eating behavior. Glia. 2018;66:592–605. doi: 10.1002/glia.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Collden G, Balland E, Parkash J, Caron E, Langlet F, Prevot V, Bouret SG. Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Mol Metab. 2015;4:15–24. doi: 10.1016/j.molmet.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A, Anouar Y, Dehouck B, Trinquet E, Jockers R, Bouret SG, Prevot V. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19:293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, Balland E, Lacombe A, Mazur D, Carmeliet P, Bouret SG, Prevot V, Dehouck B. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013;17:607–617. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hofmann K, Lamberz C, Piotrowitz K, Offermann N, But D, Scheller A, Al-Amoudi A, Kuerschner L. Tanycytes and a differential fatty acid metabolism in the hypothalamus. Glia. 2017;65:231–249. doi: 10.1002/glia.23088. [DOI] [PubMed] [Google Scholar]
- 304.Ramalho AF, Bombassaro B, Dragano NR, Solon C, Morari J, Fioravante M, Barbizan R, Velloso LA, Araujo EP. Dietary fats promote functional and structural changes in the median eminence blood/spinal fluid interface-the protective role for BDNF. J Neuroinflammation. 2018;15:10. doi: 10.1186/s12974-017-1046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63:1429–1451. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Nakano M, Tamura Y, Yamato M, Kume S, Eguchi A, Takata K, Watanabe Y, Kataoka Y. NG2 glial cells regulate neuroimmunological responses to maintain neuronal function and survival. Sci Rep. 2017;7:42041. doi: 10.1038/srep42041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nishiyama A, Boshans L, Goncalves CM, Wegrzyn J, Patel KD. Lineage, fate, and fate potential of NG2-glia. Brain Res. 2016;1638:116–128. doi: 10.1016/j.brainres.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Nishiyama A, Suzuki R, Zhu X. NG2 cells (polydendrocytes) in brain physiology and repair. Front Neurosci. 2014;8:133. doi: 10.3389/fnins.2014.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kirby L, Jin J, Cardona JG, Smith MD, Martin KA, Wang J, Strasburger H, Herbst L, Alexis M, Karnell J, Davidson T, Dutta R, Goverman J, Bergles D, Calabresi PA. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun. 2019;10:3887. doi: 10.1038/s41467-019-11638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Falcao AM, van Bruggen D, Marques S, Meijer M, Jakel S, Agirre E, Samudyata FEM, Vanichkina DP, Ffrench-Constant C, Williams A, Guerreiro-Cacais AO, Castelo-Branco G. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat Med. 2018;24:1837–1844. doi: 10.1038/s41591-018-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Djogo T, Robins SC, Schneider S, Kryzskaya D, Liu X, Mingay A, Gillon CJ, Kim JH, Storch KF, Boehm U, Bourque CW, Stroh T, Dimou L, Kokoeva MV. Adult NG2-glia are required for median eminence-mediated leptin sensing and body weight control. Cell Metab. 2016;23:797–810. doi: 10.1016/j.cmet.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 312.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Liu Y, Aguzzi A. NG2 glia are required for maintaining microglia homeostatic state. Glia. 2020;68:345–355. doi: 10.1002/glia.23721. [DOI] [PubMed] [Google Scholar]
- 314.Zhang SZ, Wang QQ, Yang QQ, Gu HY, Yin YQ, Li YD, Hou JC, Chen R, Sun QQ, Sun YF, Hu G, Zhou JW. NG2 glia regulate brain innate immunity via TGF-beta2/TGFBR2 axis. BMC Med. 2019;17:204. doi: 10.1186/s12916-019-1439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 316.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Beard RS, Jr, Haines RJ, Wu KY, Reynolds JJ, Davis SM, Elliott JE, Malinin NL, Chatterjee V, Cha BJ, Wu MH, Yuan SY. Non-muscle Mlck is required for beta-catenin- and FoxO1-dependent downregulation of Cldn5 in IL-1beta-mediated barrier dysfunction in brain endothelial cells. J Cell Sci. 2014;127:1840–1853. doi: 10.1242/jcs.144550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Haruwaka K, Ikegami A, Tachibana Y, Ohno N, Konishi H, Hashimoto A, Matsumoto M, Kato D, Ono R, Kiyama H, Moorhouse AJ, Nabekura J, Wake H. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. 2019;10:5816. doi: 10.1038/s41467-019-13812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Pillon NJ, Azizi PM, Li YE, Liu J, Wang C, Chan KL, Hopperton KE, Bazinet RP, Heit B, Bilan PJ, Lee WL, Klip A. Palmitate-induced inflammatory pathways in human adipose microvascular endothelial cells promote monocyte adhesion and impair insulin transcytosis. Am J Physiol Endocrinol Metab. 2015;309:E35–44. doi: 10.1152/ajpendo.00611.2014. [DOI] [PubMed] [Google Scholar]
- 320.Freeman LR, Granholm AC. Vascular changes in rat hippocampus following a high saturated fat and cholesterol diet. J Cereb Blood Flow Metab. 2012;32:643–653. doi: 10.1038/jcbfm.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Reyes TM, Fabry Z, Coe CL. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain Res. 1999;851:215–220. doi: 10.1016/s0006-8993(99)02189-7. [DOI] [PubMed] [Google Scholar]
- 322.Ridder DA, Lang MF, Salinin S, Roderer JP, Struss M, Maser-Gluth C, Schwaninger M. TAK1 in brain endothelial cells mediates fever and lethargy. J Exp Med. 2011;208:2615–2623. doi: 10.1084/jem.20110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Quan N, He L, Lai W. Endothelial activation is an intermediate step for peripheral lipopolysaccharide induced activation of paraventricular nucleus. Brain Res Bull. 2003;59:447–452. doi: 10.1016/s0361-9230(02)00951-6. [DOI] [PubMed] [Google Scholar]
- 324.Prevot V, Dehouck B, Poulain P, Beauvillain JC, Buee-Scherrer V, Bouret S. Neuronal-glial-endothelial interactions and cell plasticity in the postnatal hypothalamus: implications for the neuroendocrine control of reproduction. Psychoneuroendocrinology. 2007;32(Suppl 1):S46–51. doi: 10.1016/j.psyneuen.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 325.Liu LR, Liu JC, Bao JS, Bai QQ, Wang GQ. Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol. 2020;11:1024. doi: 10.3389/fimmu.2020.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Chen T, Lennon VA, Liu YU, Bosco DB, Li Y, Yi MH, Zhu J, Wei S, Wu LJ. Astrocyte-microglia interaction drives evolving neuromyelitis optica lesion. J Clin Invest. 2020;130:4025–4038. doi: 10.1172/JCI134816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kirkley KS, Popichak KA, Afzali MF, Legare ME, Tjalkens RB. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J Neuroinflammation. 2017;14:99. doi: 10.1186/s12974-017-0871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Jha MK, Jo M, Kim JH, Suk K. Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist. 2019;25:227–240. doi: 10.1177/1073858418783959. [DOI] [PubMed] [Google Scholar]
- 329.Rahman MH, Kim MS, Lee IK, Yu R, Suk K. Interglial crosstalk in obesity-induced hypothalamic inflammation. Front Neurosci. 2018;12:939. doi: 10.3389/fnins.2018.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Kim J, Kwon YH, Kim CS, Tu TH, Kim BS, Joe Y, Chung HT, Goto T, Kawada T, Park T, Choi MS, Kim MS, Yu R. The involvement of 4–1BB/4-1BBL signaling in glial cell-mediated hypothalamic inflammation in obesity. FEBS Open Bio. 2018;8:843–853. doi: 10.1002/2211-5463.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Joshi AU, Minhas PS, Liddelow SA, Haileselassie B, Andreasson KI, Dorn GW, 2nd, Mochly-Rosen D. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat Neurosci. 2019;22:1635–1648. doi: 10.1038/s41593-019-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Jin S, Kim KK, Park BS, Kim DH, Jeong B, Kang D, Lee TH, Park JW, Kim JG, Lee BJ. Function of astrocyte MyD88 in high-fat-diet-induced hypothalamic inflammation. J Neuroinflammation. 2020;17:195. doi: 10.1186/s12974-020-01846-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Yeo YA, Martinez Gomez JM, Croxford JL, Gasser S, Ling EA, Schwarz H. CD137 ligand activated microglia induces oligodendrocyte apoptosis via reactive oxygen species. J Neuroinflammation. 2012;9:173. doi: 10.1186/1742-2094-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Reali C, Curto M, Sogos V, Scintu F, Pauly S, Schwarz H, Gremo F. Expression of CD137 and its ligand in human neurons, astrocytes, and microglia: modulation by FGF-2. J Neurosci Res. 2003;74:67–73. doi: 10.1002/jnr.10727. [DOI] [PubMed] [Google Scholar]
- 335.Baxter PS, Dando O, Emelianova K, He X, McKay S, Hardingham GE, Qiu J. Microglial identity and inflammatory responses are controlled by the combined effects of neurons and astrocytes. Cell Rep. 2021;34:108882. doi: 10.1016/j.celrep.2021.108882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, Pasolli HA, Xu CS, Pang S, Matthies D, Hess HF, Lippincott-Schwartz J, Liu Z. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177(1522–1535):e1514. doi: 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 337.Erichsen D, Lopez AL, Peng H, Niemann D, Williams C, Bauer M, Morgello S, Cotter RL, Ryan LA, Ghorpade A, Gendelman HE, Zheng J. Neuronal injury regulates fractalkine: relevance for HIV-1 associated dementia. J Neuroimmunol. 2003;138:144–155. doi: 10.1016/s0165-5728(03)00117-6. [DOI] [PubMed] [Google Scholar]
- 338.de Jong EK, Dijkstra IM, Hensens M, Brouwer N, van Amerongen M, Liem RS, Boddeke HW, Biber K. Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci. 2005;25:7548–7557. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Pawelec P, Ziemka-Nalecz M, Sypecka J, Zalewska T. The impact of the CX3CL1/CX3CR1 axis in neurological disorders. Cells. 2020 doi: 10.3390/cells9102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 342.Zujovic V, Benavides J, Vige X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. doi: 10.1002/(SICI)1098-1136(20000215)29:4<305::AID-GLIA2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 343.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 344.Morselli E, Frank AP, Palmer BF, Rodriguez-Navas C, Criollo A, Clegg DJ. A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int J Obes (Lond) 2016;40:206–209. doi: 10.1038/ijo.2015.114. [DOI] [PubMed] [Google Scholar]
- 345.Yang Y, Smith DL, Jr, Keating KD, Allison DB, Nagy TR. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity (Silver Spring) 2014;22:2147–2155. doi: 10.1002/oby.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Stubbins RE, Holcomb VB, Hong J, Nunez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51:861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 347.Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 2010;34:989–1000. doi: 10.1038/ijo.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Cerbai F, Lana D, Nosi D, Petkova-Kirova P, Zecchi S, Brothers HM, Wenk GL, Giovannini MG. The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PLoS ONE. 2012;7:e45250. doi: 10.1371/journal.pone.0045250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.da Silva SM, Campos GD, Gomes FCA, Stipursky J. Radial glia-endothelial cells' bidirectional interactions control vascular maturation and astrocyte differentiation: impact for blood-brain barrier formation. Curr Neurovascular Res. 2019;16:291–300. doi: 10.2174/1567202616666191014120156. [DOI] [PubMed] [Google Scholar]
- 350.Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc Res. 2005;65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 351.Garcia CM, Darland DC, Massingham LJ, D'Amore PA. Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties. Brain Res Dev Brain Res. 2004;152:25–38. doi: 10.1016/j.devbrainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 352.Garcia-Caceres C, Balland E, Prevot V, Luquet S, Woods SC, Koch M, Horvath TL, Yi CX, Chowen JA, Verkhratsky A, Araque A, Bechmann I, Tschop MH. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019;22:7–14. doi: 10.1038/s41593-018-0286-y. [DOI] [PubMed] [Google Scholar]
- 353.Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A, Kivisakk P, Kallas K, Ellezam B, Bakshi R, Prat A, Antel JP, Weiner HL, Quintana FJ. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. 2014;20:1147–1156. doi: 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Omari KM, John GR, Sealfon SC, Raine CS. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain. 2005;128:1003–1015. doi: 10.1093/brain/awh479. [DOI] [PubMed] [Google Scholar]
- 355.Ramesh G, Benge S, Pahar B, Philipp MT. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. J Neuroinflammation. 2012;9:72. doi: 10.1186/1742-2094-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Linnerbauer M, Wheeler MA, Quintana FJ. Astrocyte crosstalk in CNS inflammation. Neuron. 2020;108:608–622. doi: 10.1016/j.neuron.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Malone JI, Hansen BC. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr Diabetes. 2019;20:5–9. doi: 10.1111/pedi.12787. [DOI] [PubMed] [Google Scholar]
- 358.Sowers JR, Whaley-Connell A, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 360.Youn JY, Siu KL, Lob HE, Itani H, Harrison DG, Cai H. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes. 2014;63:2344–2355. doi: 10.2337/db13-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Mendes NF, Kim YB, Velloso LA, Araujo EP. Hypothalamic microglial activation in obesity: a mini-review. Front Neurosci. 2018;12:846. doi: 10.3389/fnins.2018.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Li Y, Wei B, Liu X, Shen XZ, Shi P. Microglia, autonomic nervous system, immunity and hypertension: is there a link? Pharmacol Res. 2020;155:104451. doi: 10.1016/j.phrs.2019.104451. [DOI] [PubMed] [Google Scholar]
- 363.Romanatto T, Cesquini M, Amaral ME, Roman EA, Moraes JC, Torsoni MA, Cruz-Neto AP, Velloso LA. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient–effects on leptin and insulin signaling pathways. Peptides. 2007;28:1050–1058. doi: 10.1016/j.peptides.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 364.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Matias I, Morgado J, Gomes FCA. Astrocyte heterogeneity: impact to brain aging and disease. Front Aging Neurosci. 2019;11:59. doi: 10.3389/fnagi.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Salvestrini V, Sell C, Lorenzini A. Obesity may accelerate the aging process. Front Endocrinol (Lausanne) 2019;10:266. doi: 10.3389/fendo.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.De Luca SN, Sominsky L, Soch A, Wang H, Ziko I, Rank MM, Spencer SJ. Conditional microglial depletion in rats leads to reversible anorexia and weight loss by disrupting gustatory circuitry. Brain Behav Immun. 2019;77:77–91. doi: 10.1016/j.bbi.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 371.Yi CX, Al-Massadi O, Donelan E, Lehti M, Weber J, Ress C, Trivedi C, Muller TD, Woods SC, Hofmann SM. Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol Behav. 2012;106:485–490. doi: 10.1016/j.physbeh.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 372.Masson GS, Nair AR, Silva Soares PP, Michelini LC, Francis J. Aerobic training normalizes autonomic dysfunction, HMGB1 content, microglia activation and inflammation in hypothalamic paraventricular nucleus of SHR. Am J Physiol Heart Circ Physiol. 2015;309:H1115–1122. doi: 10.1152/ajpheart.00349.2015. [DOI] [PubMed] [Google Scholar]
- 373.Soch A, Sominsky L, De Luca SN, Spencer SJ. Obesity after neonatal overfeeding is independent of hypothalamic microgliosis. J Neuroendocrinol. 2019;31:e12757. doi: 10.1111/jne.12757. [DOI] [PubMed] [Google Scholar]
- 374.Ali S, Mansour AG, Huang W, Queen NJ, Mo X, Anderson JM, Hassan QN, 2nd, Patel RS, Wilkins RK, Caligiuri MA, Cao L. CSF1R inhibitor PLX5622 and environmental enrichment additively improve metabolic outcomes in middle-aged female mice. Aging (Albany NY) 2020;12:2101–2122. doi: 10.18632/aging.102724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Benzler J, Ganjam GK, Pretz D, Oelkrug R, Koch CE, Legler K, Stohr S, Culmsee C, Williams LM, Tups A. Central inhibition of IKKbeta/NF-kappaB signaling attenuates high-fat diet-induced obesity and glucose intolerance. Diabetes. 2015;64:2015–2027. doi: 10.2337/db14-0093. [DOI] [PubMed] [Google Scholar]
- 376.Tsaousidou E, Paeger L, Belgardt BF, Pal M, Wunderlich CM, Bronneke H, Collienne U, Hampel B, Wunderlich FT, Schmidt-Supprian M, Kloppenburg P, Bruning JC. Distinct roles for JNK and IKK activation in agouti-related peptide neurons in the development of obesity and insulin resistance. Cell Rep. 2014;9:1495–1506. doi: 10.1016/j.celrep.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 377.Weissmann L, Quaresma PG, Santos AC, de Matos AH, Pascoal VD, Zanotto TM, Castro G, Guadagnini D, da Silva JM, Velloso LA, Bittencourt JC, Lopes-Cendes I, Saad MJ, Prada PO. IKKepsilon is key to induction of insulin resistance in the hypothalamus, and its inhibition reverses obesity. Diabetes. 2014;63:3334–3345. doi: 10.2337/db13-1817. [DOI] [PubMed] [Google Scholar]
- 378.Qi J, Yu XJ, Shi XL, Gao HL, Yi QY, Tan H, Fan XY, Zhang Y, Song XA, Cui W, Liu JJ, Kang YM. NF-kappaB blockade in hypothalamic paraventricular nucleus inhibits high-salt-induced hypertension through NLRP3 and caspase-1. Cardiovasc Toxicol. 2016;16:345–354. doi: 10.1007/s12012-015-9344-9. [DOI] [PubMed] [Google Scholar]
- 379.Amaral ME, Barbuio R, Milanski M, Romanatto T, Barbosa HC, Nadruz W, Bertolo MB, Boschero AC, Saad MJ, Franchini KG, Velloso LA. Tumor necrosis factor-alpha activates signal transduction in hypothalamus and modulates the expression of pro-inflammatory proteins and orexigenic/anorexigenic neurotransmitters. J Neurochem. 2006;98:203–212. doi: 10.1111/j.1471-4159.2006.03857.x. [DOI] [PubMed] [Google Scholar]
- 380.Song XA, Jia LL, Cui W, Zhang M, Chen W, Yuan ZY, Guo J, Li HH, Zhu GQ, Liu H, Kang YM. Inhibition of TNF-alpha in hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by inhibiting neurohormonal excitation in spontaneously hypertensive rats. Toxicol Appl Pharmacol. 2014;281:101–108. doi: 10.1016/j.taap.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 381.Mutsnaini L, Yang J, Kim J, Kim C-S, Lee C-H, Kim M-S, Park T, Goto T, Yu R. Filbertone protects obesity-induced hypothalamic inflammation by reduction of microglia-mediated inflammatory responses. Biotechnol Bioprocess Eng. 2021;26:86–92. doi: 10.1007/s12257-020-0220-5. [DOI] [Google Scholar]
- 382.Park BS, Tu TH, Lee H, Jeong DY, Yang S, Lee BJ, Kim JG. Beta-aminoisobutyric acid inhibits hypothalamic inflammation by reversing microglia activation. Cells. 2019 doi: 10.3390/cells8121609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Zhou J, Mao L, Xu P, Wang Y. Effects of (-)-epigallocatechin gallate (EGCG) on energy expenditure and microglia-mediated hypothalamic inflammation in mice fed a high-fat diet. Nutrients. 2018 doi: 10.3390/nu10111681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Yang J, Kim CS, Tu TH, Kim MS, Goto T, Kawada T, Choi MS, Park T, Sung MK, Yun JW, Choe SY, Lee JH, Joe Y, Choi HS, Back SH, Chung HT, Yu R. Quercetin protects obesity-induced hypothalamic inflammation by reducing microglia-mediated inflammatory responses via HO-1 induction. Nutrients. 2017 doi: 10.3390/nu9070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Sadagurski M, Landeryou T, Cady G, Kopchick JJ, List EO, Berryman DE, Bartke A, Miller RA. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging Cell. 2015;14:1045–1054. doi: 10.1111/acel.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Sadagurski M, Landeryou T, Cady G, Bartke A, Bernal-Mizrachi E, Miller RA. Transient early food restriction leads to hypothalamic changes in the long-lived crowded litter female mice. Physiol Rep. 2015 doi: 10.14814/phy2.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Wu Z, Xi P, Zhang Y, Wang H, Xue J, Sun X, Tian D. LKB1 up-regulation inhibits hypothalamic inflammation and attenuates diet-induced obesity in mice. Metabolism. 2021;116:154694. doi: 10.1016/j.metabol.2020.154694. [DOI] [PubMed] [Google Scholar]
- 388.Gao HL, Yu XJ, Liu KL, Shi XL, Qi J, Chen YM, Zhang Y, Bai J, Yi QY, Feng ZP, Chen WS, Cui W, Liu JJ, Zhu GQ, Kang YM. PVN blockade of p44/42 MAPK pathway attenuates salt-induced hypertension through modulating neurotransmitters and attenuating oxidative stress. Sci Rep. 2017;7:43038. doi: 10.1038/srep43038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.