Abstract
Mammalian oocytes are particularly susceptible to accumulating DNA damage. However, unlike mitotic cells in which DNA damage induces G2 arrest by activating the ATM-Chk1/2-Cdc25 pathway, oocytes readily enter M-phase immediately following DNA damage. This implies a lack of a robust canonical G2/M DNA damage checkpoint in oocytes. Here we show that MDC1 plays a non-canonical role in controlling G2/M transition by regulating APC/C-Cdh1-mediated cyclin B1 degradation in response to DNA damage in mouse oocytes. Depletion of MDC1 impaired M-phase entry by decreasing cyclin B1 levels via the APC/C-Cdh1 pathway. Notably, the APC/C-Cdh1 regulation mediated by MDC1 was achieved by a direct interaction between MDC1 and APC/C-Cdh1. This interaction was transiently disrupted after DNA damage with a concomitant increase in Cdh1 levels, which, in turn, decreased cyclin B1 levels and delayed M-phase entry. Moreover, MDC1 depletion impaired spindle assembly by decreasing the integrity of microtubule organizing centers (MTOCs). Therefore, our results demonstrate that MDC1 is an essential molecule in regulating G2/M transition in response to DNA damage and in regulating spindle assembly in mouse oocytes. These results provide new insights into the regulation of the G2/M DNA damage checkpoint and cell cycle control in oocytes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04241-1.
Keywords: MDC1, G2/M transition, DNA damage, APC/C-Cdh1, Spindle assembly, Oocytes
Introduction
To maintain genomic integrity, eukaryotic cells activate cell cycle checkpoints in response to DNA damage [1]. The G2/M DNA damage checkpoint serves to delay or prevent cells from entering M-phase with genomic DNA damage. This allows for the repair of DNA damage prior to M-phase. This checkpoint is primarily controlled by the activity of the Cdk1/cyclin B1 complex and is initiated by ATM/ATR kinases. ATM/ATR is a member of a phosphoinisitide-3-kinase-related protein kinase family that has a central role in coordinating the DNA damage response [2]. A key function of ATM/ATR in G2/M DNA checkpoint signaling is to phosphorylate and activate Chk1/2 kinases. Chk1/2 kinase activation inhibits Cdc25 phosphatases, thereby preventing Cdk1/cyclin B1 activation and M-phase entry [3].
MDC1 is a mediator protein that plays a vital role in the DNA damage response by regulating a cell cycle checkpoint and recruiting DNA repair proteins [4, 5]. In response to DNA damage, especially double-strand breaks (DSBs), ATM phosphorylates histone H2AX (γ-H2AX) in the chromatin surrounding the damaged sites. The phosphorylated tail of γ-H2AX creates a docking platform for MDC1 to amplify ATM signaling by recruiting additional ATM to the vicinity of the DSBs, allowing for the spread of γ-H2AX to adjacent chromatin [6–8]. This stepwise amplification of ATM and γ-H2AX signaling ensures the repair of damaged DNA by stabilizing the broken DNA ends and recruiting repair factors, including 53BP1 and BRCA1 [9]. The MDC1-dependent accumulation of ATM is also important for G2/M checkpoint arrest through Chk1/2 phosphorylation. Therefore, cells lacking MDC1 are sensitive to DNA damage and defective in damage-induced γ-H2AX foci formation. Moreover, MDC1-depleted cells fail to activate the G2/M checkpoint in response to DNA damage. This failure is associated with an inability to regulate Chk1 properly [5].
Mammalian oocytes remain arrested at G2/prophase of the first meiosis, also termed the germinal vesicle (GV) stage, within primordial follicles for a prolonged period. This arrest is maintained by suppressing the activity of Cdk1 via inhibitory phosphorylation at Thr14/Tyr15 residues. Wee1B/Myt1 kinases mediate these inhibitory phosphorylations that are counteracted by Cdc25 phosphatases [10–12]. In addition to inhibitory phosphorylation, Cdk1 inhibition is also achieved by cyclin B1 degradation, which is regulated by the anaphase-promoting complex/cyclosome (APC/C) in association with Cdh1 (APC/C-Cdh1) during G2/prophase arrest in fully grown oocytes [13, 14]. Following hormone stimulation, oocytes resume meiosis after Cdk1 activation by removing the inhibitory phosphorylation and increasing cyclin B1 levels, which results in GV breakdown (GVBD). Accompanying with chromosome condensation and spindle assembly, oocytes proceed through the meiosis I (MI), and then become arrested again at metaphase II (MII).
Because of prolonged arrest at the G2/prophase stage, oocytes are particularly susceptible to the accumulation of DNA damage [15]. Therefore, a robust surveillance mechanism is of paramount importance to ensure that oocytes repair DNA damage to maintain oocyte and embryo quality. Surprisingly, however, fully grown oocytes lack a robust G2/M DNA damage checkpoint [16–20]. Therefore, oocytes enter M-phase despite the presence of substantial levels of DNA damage. Only severe DNA damage partially activates the G2/M DNA damage checkpoint via the ATM-Chk1-Cdc25 pathway in mouse oocytes [16]. However, a recent study showed that oocytes are able to mount a G2/M DNA damage checkpoint response via an APC/C-Cdh1-mediated proteolytic pathway. However, this non-canonical pathway acts slowly and does not evoke an immediate response [21]. The mechanism underlying the fully grown oocyte response to DNA damage during G2/prophase arrest is unclear.
In the absence of a canonical G2/M DNA damage checkpoint, oocytes have the capacity to induce MI arrest in response to DNA damage. This arrest is associated with activation of the spindle assembly checkpoint (SAC), which normally prevents the onset of anaphase until all chromosomes are correctly attached to the spindle [18, 19]. Although the reason is unclear, DNA damage in MI oocytes is sensed at the kinetochores and increases the level of Mad2 and Bub1 at kinetochores. Moreover, MDC1 was found to be localized at kinetochores and required for kinetochore recruitment of Mad2 in response to DNA damage [22]. Given that centromeres are particularly fragile due to their repetitive sequences [23], the MDC1 recruitment at kinetochores may reflect that damage in centromeric DNA leads to unstable kinetochore-microtubule (kMT) attachment and subsequent SAC activation. However, the role of MDC1 in spindle assembly and SAC activation in response to DNA damage remains unclear.
In this study, we found that MDC1 plays a non-canonical function in regulating G2/M transition and spindle assembly in mouse oocytes. Unlike in mitotic cells, depletion of MDC1 in oocytes impairs M-phase entry by decreasing cyclin B1 levels via an APC/C-Cdh1 pathway. Importantly, MDC1 modulates APC/C-Cdh1 activity by directly interacting with APC/C-Cdh1 in a DNA damage-dependent manner during G2/prophase arrest in oocytes. Additionally, we found that MDC1 regulates spindle assembly during meiotic maturation.
Materials and methods
Animals and reagents
All mice were used in accordance with established guidelines, and experimental procedures were approved by the Institutional Animal Care and Use Committees of Sungkyunkwan University (approval ID: SKKUIACUC2020-11-06-1). Mice were purchased from a local company (Koatech, Korea). All chemicals and reagents were purchased from Sigma-Aldrich unless otherwise stated.
Oocyte collection, oocyte culture and chemical treatment
Ovaries were isolated from 3–4-week-old CD1 female mice 46–48 h after injection of 5 IU of pregnant mare’s serum gonadotropin (PMSG; HOR-272, Prospec). Oocytes at the GV stage were collected by puncturing ovaries with a fine needle in M2 medium (M7167) supplemented with 100 μM of 3-isobutyl-1-methylxanthine (IBMX; I5897). Follicular cells surrounding GV oocytes were mechanically removed using a pipette with an inner diameter of around 100 μm. For in vitro maturation, oocytes were cultured in IBMX-free M2 medium in a 5% CO2 atmosphere at 37 °C and imaged using a Nikon Eclipse Ti inverted microscope equipped with a CCD-cooled camera (Nikon). During in vitro maturation, dissolution of the nuclear membrane was defined as GVBD. The developmental stage of oocytes was determined by their morphology. GV oocytes were identified by the presence of a clearly defined GV. MI oocytes were defined by the absence of a GV and polar body, while oocytes with the first polar body were assigned as MII oocytes.
To induce DNA DSBs, oocytes were treated with 50 μg/ml etoposide (E1383) for 30 min. Control oocytes were treated with an equivalent volume of DMSO.
Microinjection, siRNAs, mRNA constructs and morpholinos
Microinjection was performed as previously described [24]. Briefly, fully grown GV oocytes were placed in M2 medium with 100 μM IBMX to maintain G2 arrest; microinjection into oocytes was performed on the stage of an inverted microscope (Leica DM IRB) using a microinjector (Eppendorf FemtoJet®) and micromanipulators (Narishige MN-4). Approximately 5–10 pl of 20 μM siRNA, 800 ng/μl mRNA or 1 mM morpholino (MO) were injected into the cytoplasm of oocytes. After microinjection, oocytes were kept in M2 medium containing IBMX for 24 h.
To deplete MDC1, MDC1 siRNA (5’-UCACCUCUAUAGAACAGCCUGUUAU-3; Invitrogen) was used with a scrambled siRNA as a negative control.
For the cyclin B1 rescue experiment, the pRN3-cyclin B1-GFP was prepared as described previously [24]. The fidelity of the constructs was confirmed by DNA sequencing. The mRNAs for microinjection were prepared with a T3 mMESSAGE mMACHINE (AM1348, Ambion) and purified using an RNA purification kit (#740,984.50, MACHEREY–NAGEL) according to the manufacturer’s protocol.
For Cdh1 knockdown, we used a morpholino (MO) designed to target Cdh1 as described previously [24, 25].
Immunofluorescence
Oocytes were fixed with 4% paraformaldehyde (158,127) in PBS for 10 min and permeabilized with 0.1% Triton X-100 (T8532) and 0.01% Tween-20 (P9416) for 20 min at room temperature. Then, oocytes were blocked with 3% BSA-supplemented PBS for 1 h and incubated with primary antibodies overnight at 4 °C. After washing three times, oocytes were incubated with Alexa Fluor 488‐conjugated (1:500, 115–545-146, Jackson ImmunoResearch) or Alex Fluor 594-conjugated secondary antibodies (1:500, 115–585-044, Jackson ImmunoResearch) at room temperature for 2 h. Oocytes were then mounted on glass slides after counterstaining with DAPI to visualize DNA and observed under an LSM 700 laser scanning confocal microscope (Zeiss) with a C‐Apochromat 63 × /1.2 water immersion objective. The primary antibodies used were acetylated-α-tubulin (1:1000, T7451), γ-H2AX (1:250, ab22551, Abcam), p-ATM (1:100, ab36810, Abcam), MDC1 (1:250, ab241048, Abcam), p-MDC1 (1:250, ab35967, Abcam), cyclin B1 (1:100, #4138, Cell Signaling), Cdh1 (1:200, PA5-85,303, Invitrogen), PCNT (1:100, ab4448, Abcam), p-Aurora A (1:250, #3079, Cell Signaling), Cep192 (1:500, AR07-PA0001, Young In Frontier) and anti-centromere antibody (ACA; 1:100, 15-234-0001, Antibodies Incorporated).
For measurement of immunofluorescence intensity, images were captured with the same laser power and the mean intensity of fluorescence signals was measured. Data were analyzed using ZEN 2012 Blue (Zeiss) and ImageJ software (National Institutes of Health, NIH) under the same processing parameters.
For analysis of kMT attachment, oocytes at the MI stage were incubated in ice-cold M2 medium for 10 min. After cold treatment, oocytes were fixed and subjected to the immunofluorescence analysis. Stable kinetochores were scored by quantifying the number of kinetochores identified by ACA signals that had bundles of microtubules.
Immunoblotting
Proteins from 50 oocytes at the appropriate stage of maturation were collected in 5 μl of 2 × reducing SDS loading buffer and heated for 10 min at 95 °C. Proteins were separated by 8% SDS-PAGE and transferred to polyvinylidene difluoride membranes (IPVH0010, Immobilon). The membranes were blocked in TBST containing 3% BSA for 1 h at room temperature and then incubated with primary antibodies against β-actin (1:5000, #4967S, Cell Signaling), cyclin B1 (1:1000, #4138, Cell Signaling), Cdh1 (1:200, PA5-85,303, Invitrogen) or pY15-Cdk1 (1:1000, #9111, Cell Signaling) overnight at 4 °C. After washing three times, the membranes were incubated with the appropriate HRP-conjugated anti-rabbit (1:5000, 111-035-144, Jackson ImmunoResearch) or anti-mouse (1:5000, 111-585-144, Jackson ImmunoResearch) secondary antibodies for 1 h at room temperature. Detected targets on the membranes were visualized on X-ray films using the ECL Plus Western Blotting Detection kit (#32,132, Pierce) according to the manufacturer’s instructions. Band density was quantified using ImageJ software (National Institutes of Health, NIH).
Proximity ligation assay (PLA)
To detect protein–protein interaction between MDC1 and Cdh1 in mouse oocytes, a proximity ligation assay (PLA) was performed using the in situ detection reagents Orange kit Mouse/Rabbit (DUO92007). Mouse anti-MDC1 and rabbit anti-Cdh1 antibodies were conjugated with PLA PLUS and PLA MINUS probes, respectively. Oocytes injected with MDC1 siRNA were used as negative controls. The PLA signals were visualized using the LSM 700 laser scanning confocal microscope (Zeiss).
TUNEL assay
The TUNEL assay was performed using the In Situ Cell Death Detection kit (#12,156,792,910, Roche) according to the manufacturer’s instructions. In brief, paraformaldehyde-fixed oocytes were washed three times with PBS and permeabilized with 0.15% Triton-X100 and 0.1% sodium citrate for 1 h on ice. Oocytes were washed and incubated with fluorescent-conjugated terminal deoxynucleotide transferase dUTP for 2 h at 37 °C. After washing three times, oocytes were mounted on glass slides after counterstaining with DAPI, and the fluorescence signal was detected using the LSM 700 laser scanning confocal microscope (Zeiss).
Statistical analysis
All statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software). Data are representative of at least three independent experiments unless otherwise specified, and each experimental group included at least 15 oocytes. The numbers of oocyte for analyzed were indicated on each graph. Differences between two groups were analyzed by Student’s t-test, and comparisons between more than two groups were analyzed by one-way ANOVA with Tukey’s post hoc test. Percentages of maturation were analyzed using arcsine transformation. P < 0.05 was considered statistically significant.
Results
MDC1 depletion impairs DNA damage response in oocytes
To explore the function of MDC1 in mouse oocytes, we injected siRNAs targeting MDC1 (siMDC1). Control oocytes were injected with non-specific scrambled siRNAs (siCTL). Immunostaining analysis revealed that MDC1 localized at the nucleus was efficiently depleted by siMDC1 injection (Fig. 1A, B). Because MDC1 is a key protein in the DNA damage response, we first examined the effect of MDC1 depletion on the DNA damage response in oocytes. To induce DNA damage, oocytes were treated with etoposide for 30 min. TUNEL assay showed that the extent of DNA damage was comparable between control and MDC1-depleted oocytes (Fig. 1C, D). However, after MDC1 depletion, γ-H2AX and p-ATM signals decreased significantly in response to etoposide treatment (Fig. 1E–H). These results suggest that MDC1 depletion impairs γ-H2AX and p-ATM recruitment at DNA damage sites and thereby inhibits the activation of the DNA damage response in oocytes.
Fig. 1.
MDC1 is required for ATM-mediated DNA damage response in mouse oocytes. A GV oocytes were injected with either MDC1 siRNA (siMDC1) or control siRNA (siCTL) and cultured for 24 h in the presence of IBMX for knockdown. Depletion of MDC1 was confirmed by immunostaining with MDC1 antibodies. Scale bar, 10 μm. B Quantification of MDC1 levels after knockdown. Data are expressed as the mean ± SEM of three independent experiments. C–H After treating with etoposide (ETP) or DMSO for 30 min, oocytes were fixed and subjected to TUNEL assay or immunostaining with γ-H2AX and p-ATM antibodies. The fluorescence intensity of TUNEL, γ-H2AX and p-ATM were measured and shown with representative images. Scale bar, 10 μm. Data are expressed as the mean ± SEM of three independent experiments. ***P < 0.0001; ns, not significant
MDC1 depletion impairs GVBD by preventing cyclin B1 accumulation
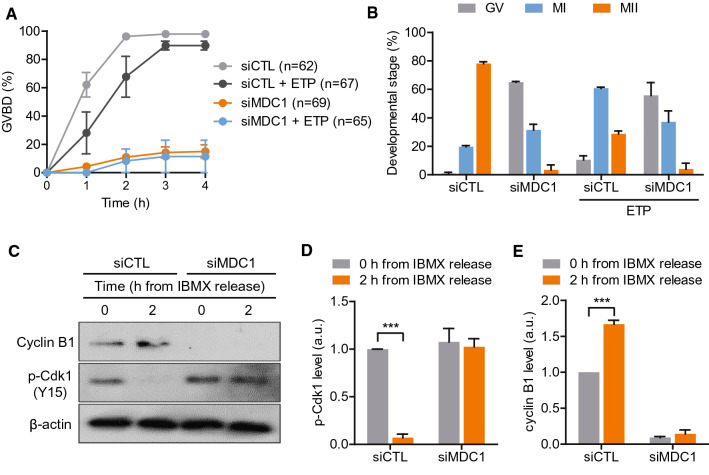
Despite a weak G2/M DNA damage checkpoint, oocytes are able to induce G2/prophase arrest by activating the ATM-Chk1-Cdc25B pathway when DNA damage is substantial [16]. Because MDC1 depletion impaired ATM recruitment to DNA damage sites, we sought to test whether MDC1-depleted oocytes could mount a G2/M DNA damage checkpoint response. To this end, oocytes were treated with etoposide and released from IBMX-mediated GV arrest. Consistent with previous reports, control oocytes showed delayed GVBD and then were arrested at metaphase I (MI) stage after etoposide treatment (Fig. 2A, B). Unexpectedly, however, MDC1-depleted oocytes were arrested at the GV stage. This arrest was irrespective of etoposide treatment (Fig. 2A). Moreover, the impaired GVBD was not rescued by prolonged culture of up to 24 h (Fig. 2B), implying that MDC1 plays a non-canonical role in the regulation of G2/M transition in mouse oocytes.
Fig. 2.
MDC1 regulates G2/M transition by stabilizing cyclin B1 in mouse oocytes. A, B Control (siCTL) and siMDC1-injected oocytes were treated with etoposide (ETP). The incidence of GVBD for 4 h and the developmental stage of oocytes after 24-h culture were quantified. C Immunoblotting analysis of cyclin B1 and pY15-Cdk1 at 0 and 2 h after IBMX release in siCTL- or siMDC1-injected oocytes. D, E Levels of pY15-Cdk1 and cyclin B1 were normalized to β-actin, quantified, and expressed in arbitrary unit (a.u.). Data are expressed as the mean ± SEM from three independent experiments. *** P < 0.0001
To dissect mechanisms underlying GV arrest mediated by MDC1 depletion, we examined the Tyr15 phosphorylation of Cdk1 (pY15-Cdk1) and cyclin B1 levels, critical regulators of M-phase entry in oocytes. Immunoblot analysis showed that pY15-Cdk1 levels were not different between control and MDC1-depleted oocytes in the GV stage. However, by 2 h following IBMX release, pY15-Cdk1 levels significantly decreased in control oocytes but did not change in MDC1-depleted oocytes (Fig. 2C, D). In contrast to pY15-Cdk1 levels, cyclin B1 levels at the GV stage significantly decreased after MDC1 depletion. Following IBMX release, cyclin B1 levels increased in control oocytes but remained undetectable in MDC1-depleted oocytes (Fig. 2C, E). The decrease in cyclin B1 levels after MDC1 depletion was confirmed by immunostaining analysis (Fig. S1). Collectively, our results suggest that MDC1 is required to stabilize cyclin B1 in mouse oocytes.
Cyclin B1 destabilization by MDC1 depletion is caused by an increased Cdh1 level
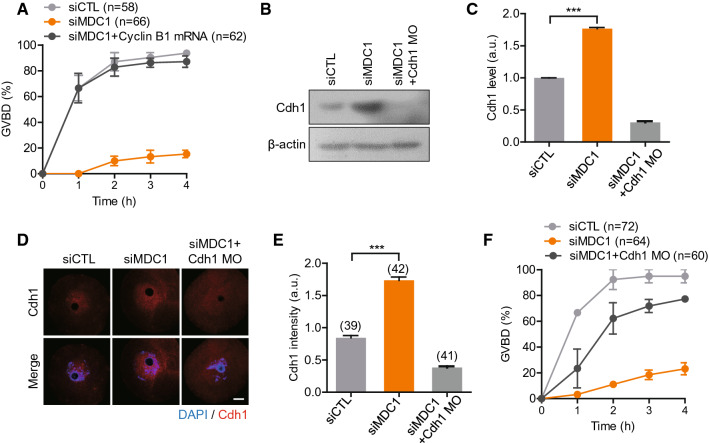
To further investigate whether a failure to stabilize cyclin B1 is a main cause of GV arrest in MDC1-depleted oocytes, we injected cyclin B1 mRNA and monitored GVBD. Notably, the impaired GVBD by MDC1 depletion was fully rescued by cyclin B1 overexpression, suggesting that MDC1 is required for cyclin B1 stabilization in oocytes (Fig. 3A). Since cyclin B1 levels are regulated by APC/C-Cdh1 activity during G2/M transition in mouse oocytes [13, 14], we next examined Cdh1 levels in MDC1-depleted oocytes. Interestingly, Cdh1 levels increased in MDC1-depleted oocytes compared to those in the control oocytes (Fig. 3B–E). This suggests that the GV arrest mediated by MDC1 depletion is associated with cyclin B1 destruction by increased APC/C-Cdh1 activity. To confirm the role of Cdh1 in MDC1-depleted oocytes, we injected Cdh1 morpholino (MO) and monitored GVBD. Indeed, the impaired GVBD was rescued by Cdh1 depletion (Fig. 3B–F), indicating that the GV arrest after MDC1 depletion is caused by cyclin B1 destruction as the result of increased APC/C-Cdh1 activity.
Fig. 3.
MDC1 regulates APC/C-Cdh1-mediated cyclin B1 degradation during G2/M transition. A The incidence of GVBD was quantified for 4 h after IBMX release in oocytes injected with siCTL, siMDC1 or siMDC1 + cyclin B1 mRNA. B–F Oocytes were injected with siCTL, siMDC1 and siMDC1 + Cdh1 MO. After 24-h culture, Cdh1 levels were determined using immunoblotting and immunostaining analyses. B, C Immunoblotting analysis of Cdh1. Levels of Cdh1 were normalized to β-actin, quantified, and expressed in arbitrary unit (a.u.). Data are expressed as the mean ± SEM from three independent experiments. D, E Immunostaining of Cdh1. The fluorescence intensity of Cdh1 was measured and shown with representative images. Scale bar, 10 μm. *** P < 0.0001. F The incidence of GVBD was quantified for 4 h after IBMX release
MDC1 directly interacts with Cdh1 in a DNA damage-dependent manner in oocytes
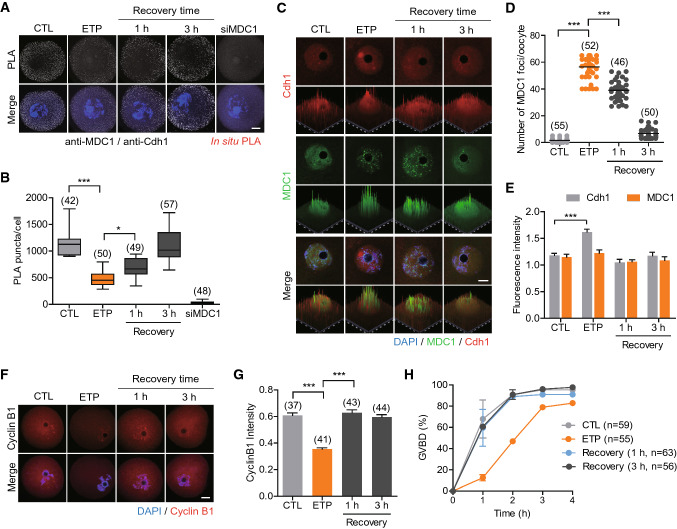
Both MDC1 and Cdh1 localize in the nucleus in mouse oocytes [20, 26]. Thus, we raised an interesting question whether MDC1 directly interacts with Cdh1 at the nucleus of GV oocytes. To test this, we performed an in situ proximity ligation assay (PLA), which allows for visualization of weak or transient in vivo interactions between two proteins [27]. Although a negligible signal was detected in the negative controls (siMDC1), strong positive signals were observed in the presence of anti-MDC1 and anti-Cdh1 antibodies (Fig. 4A, B), implying a direct interaction between MDC1 and Cdh1. It is well established that MDC1 forms foci at DNA damage sites by directly binding to γ-H2AX [5, 8]. We therefore asked whether the interaction between MDC1 and Cdh1 might be affected by DNA damage. Indeed, MDC1 clustered and formed foci with γ-H2AX after etoposide treatment and then gradually returned to normally distributed patterns during recovery (Fig. 4C, D, Fig. S2). However, overall MDC1 levels did not change (Fig. 4C, E). In contrast, Cdh1 did not change its nuclear distribution, but total Cdh1 levels increased after etoposide treatment. These returned to normal levels after recovery (Fig. 4C, E). Strikingly, the number of PLA foci significantly decreased after etoposide treatment but gradually returned to normal levels during recovery (Fig. 4A, B). Therefore, our results suggest that MDC1 regulates Cdh1 levels by modulating its interaction with Cdh1 in a DNA damage-dependent manner.
Fig. 4.
MDC1 interacts with APC/C-Cdh1 in a DNA damage dependent manner. Oocytes were exposed to etoposide (ETP) and cultured in fresh medium for recovery for 1 h and 3 h. Oocytes injected with siMDC1 were used as a negative control. A In situ PLA of oocytes. B The number of PLA puncta per oocyte was quantified. C–E Immunostaining analysis of Cdh1 and MDC1. Scale bar, 10 μm. D The number of MDC1 foci was quantified. E The fluorescence intensity of Cdh1 and MDC1 were quantified. F Immunostaining analysis of cyclin B1. Scale bar, 10 μm. G The fluorescence intensity of cyclin B1 was quantified. * P < 0.05; *** P < 0.0001. H The incidence of GVBD was quantified for 4 h after IBMX release
To further investigate the impact of MDC1 dissociation from Cdh1 on APC/C-Cdh1 activity, we measured cyclin B1 levels after etoposide treatment. Importantly, cyclin B1 levels decreased after etoposide treatment and were restored during recovery (Figs. 4F, G and S3). Consistent with cyclin B1 levels, timing of GVBD was delayed after etoposide treatment but was restored after recovery (Fig. 4H). Together, our results suggest that MDC1 negatively regulates APC/C-Cdh1 activity through direct interaction with Cdh1 during G2 arrest in oocytes. However, in the presence of DNA damage, MDC1 is dissociated from Cdh1 and is recruited to DNA damage sites forming distinct foci with γ-H2AX. This activity increases APC/C-Cdh1 activity, degrades cyclin B1 and delays M-phase entry.
MDC1 depletion impairs chromosome alignment and spindle organization
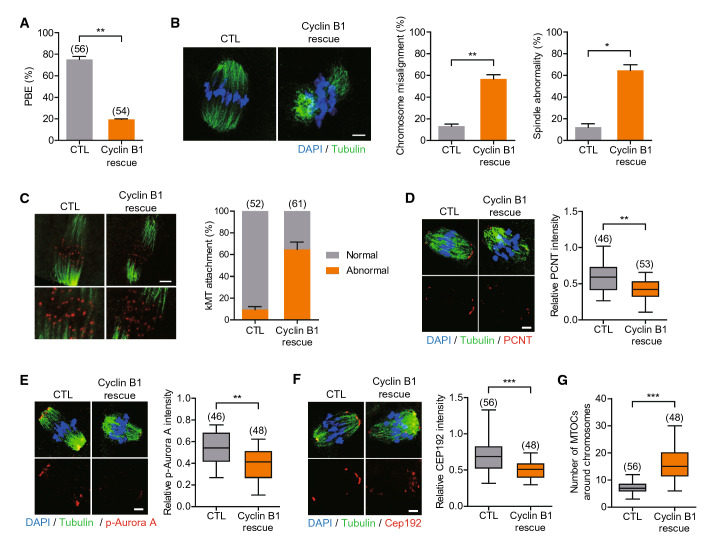
Because the GV arrest mediated by MDC1 depletion was fully rescued by cyclin B1 overexpression, we next investigated the impact of MDC1 depletion on meiotic progression after GVBD using MDC1-depleted oocytes overexpressing cyclin B1 (cyclin B1-rescued oocytes). We confirmed that control oocytes injected with cyclin B1 mRNA showed no discernible defects in spindle and chromosome organization and polar body extrusion, excluding side effects of cyclin B1 overexpression in meiotic maturation (Fig. 5A, B). In striking contrast, the rate of polar body extrusion (PBE) significantly decreased in MDC1-depleted oocytes overexpressing cyclin B1. Moreover, spindle organization, chromosome alignment and kMT attachment were severely impaired in these oocytes (Fig. 5A-C). Therefore, our results suggest that MDC1 is required for spindle assembly and chromosome alignment after GVBD.
Fig. 5.
MDC1 is essential for MTOC organization during meiotic maturation. Oocytes were injected with cyclin B1 mRNA alone (CTL) or siMDC1 + cycin B1 mRNA (Cyclin B1 rescue). After 24-h culture, oocytes were released from IBMX. A Polar body extrusion (PBE) rate was scored after 16-h culture post-IBMX release. B–G After 8 h post-IBMX release, oocytes were fixed and immunostained with α-tubulin, ACA, PCNT, p-Aurora A or CEP192 antibodies. For ACA staining, oocytes were briefly placed on ice before fixation. Scale bar, 10 μm. Chromosome misalignment; spindle abnormality; kMT attachment; and intensity of PCNT, p-Aurora A, and CEP192 were quantified. (G) The number of MTOCs around chromosomes was quantified using CEP192 foci. * P < 0.05; ** P < 0.001; *** P < 0.0001
MDC1 regulates CEP192-mediated MTOC organization
To investigate how MDC1 regulates spindle organization and chromosome alignment in oocytes, we examined localization of MDC1 during meiotic maturation. In agreement with the role of MDC1 in the DNA damage response, MDC1 localized to the nucleus at the GV stage and associated with chromosomes after GVBD (Fig. S4A). However, the p-MDC1 signal appeared as discernible foci at the GV stage and was distributed in the vicinity of chromosomes and spindle microtubules at GVBD. Importantly, the p-MDC1 signal was enriched at the spindle pole at MI and MII stages (Fig. S4B). This result prompted us to investigate whether spindle defects and chromosome misalignment in MDC1-depleted oocytes were associated with spindle pole integrity. In oocytes, multiple microtubule organizing centers (MTOCs) cluster together and form spindle poles without canonical centrosomes [28]. Thus, we examined MTOC organization after MDC1 depletion. Intriguingly, levels of PCNT, p-Aurora A and CEP192 at MTOCs were markedly reduced after MDC1 depletion (Fig. 5D-F). Also, the number of CEP192 foci decreased in MDC1-depleted oocytes (Fig. 5G), indicating a decrease in MTOC number. Therefore, our results suggest that MDC1 regulates CEP192-mediated MTOC organization in mouse oocytes.
Discussion
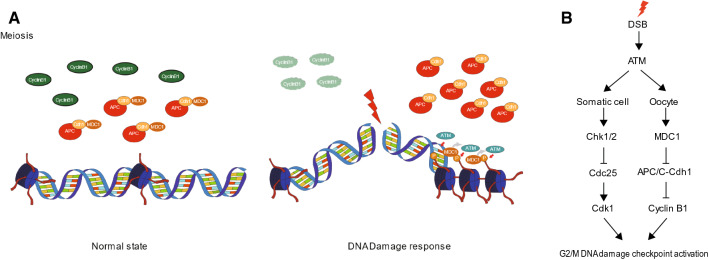
In this study, we showed that MDC1 regulates APC/C-Cdh1-mediated G2/M transition and spindle assembly in mouse oocytes. Importantly, the APC/C-Cdh1 activity associated with MDC1 is regulated by a direct interaction between MDC1 and APC/C-Cdh1. Moreover, this interaction is temporarily disrupted by DNA damage suggesting the existence of a MDC1-dependent non-canonical G2/M DNA damage checkpoint in mouse oocytes. In the normal state, MDC1 regulates cyclin B1 levels by modulating APC/C-Cdh1 activity via direct interaction with APC/C-Cdh1. After DNA damage, however, MDC1 is recruited to DNA damage sites and dissociates from APC/C-Cdh1. MDC1 and APC/C-Cdh1 enhance DNA damage repair by promoting the recruitment of DNA repair factors to DNA damage sites and delay M-phase entry by increasing APC/C-Cdh1-mediated cyclin B1 degradation, respectively (Fig. 6A).
Fig. 6.
Model of MDC1-mediated regulation of G2/M transition in response to DNA damage. A Working model for the functions of MDC1 during G2/M transition. In the normal state, MDC1 is associated with APC/C-Cdh1 and regulates cyclin B1 to maintain threshold levels. However, in the presence of DNA damage, MDC1 is dissociated from APC/C-Cdh1 and recruited to DNA damage sites. This activity increases APC/C-Cdh1-medidated cyclin B1 degradation. B Comparison of the G2/M DNA damage checkpoint between somatic cells and oocytes
We demonstrate here that MDC1 regulates G2/M transition via APC/C-mediated proteolytic degradation of cyclin B1 in mouse oocytes. Notably, MDC1-mediated regulation of APC/C-Cdh1 activity is likely to occur through a direct interaction between MDC1 and Cdh1. However, given that positive PLA signals might be produced when two molecules are in close proximity without a physical interaction [27], we could not exclude the possibility that MDC1 interacts with other APC/C subunits; and MDC1 has been demonstrated to bind to Cdc27, an APC/C subunit, in vitro [29]. However, in contrast to our data showing that the MDC1-Cdh1 interaction is disrupted by DNA damage, the MDC1-Cdc27 interaction is enhanced by DNA damage [29]. Also, mitotic cells lacking MDC1 exhibit markedly reduced levels of APC/C activity [30]. This is in contrast to our results that APC/C-Cdh1 activity increased in MDC1-depleted oocytes. Therefore, MDC1-mediated regulation of APC/C activity in mitotic cells and oocytes is probably different. Further studies are required to clarify the interaction between MDC1 and APC/C-Cdh1.
MDC1 depletion in somatic cells results in failure to maintain the DNA damage-induced G2/M checkpoint, thereby leading to early M-phase entry [5]. However, our data show that MDC1-depleted oocytes fail to enter M-phase suggesting that MDC1 differentially regulates the G2/M checkpoint in mouse oocytes. In somatic cells, the G2/M checkpoint is mainly controlled by the ATM/ATR-Chk1/2-Cdc25 pathway [3]. Although APC/C-Cdh1-mediated proteolysis of cyclin B1 has been reported in some somatic cells after DNA damage, this response induces irreversible withdrawal from the cell cycle [31–33]. Therefore, this response is likely to be distinct from the G2/M checkpoint activation that prevents or delays M-phase entry. Indeed, the checkpoint failure induced by MDC1 depletion is associated with an impaired activation of the ATM/ATR-mediated Chk1/2 pathway, not the APC/C-Cdh1-mediated proteolytic pathway [5]. In contrast to somatic cells, the canonical ATM-mediated G2/M checkpoint is dampened in oocytes [16, 20]. Thus, oocytes are unable to respond immediately to DNA damage. However, our finding that cyclin B1 decreased temporarily after DNA damage suggests that oocytes mount an immediate G2/M DNA damage checkpoint response. However, this response might not be sufficient to induce G2 arrest because of the time required to destroy the quantity of cyclin B1 required to prevent M-phase entry. MDC1 recruitment to DNA damage sites may be delayed due to its association with APC/C-Cdh1 in oocytes, which eventually disturbs an immediate activation of the ATM-mediated canonical G2/M checkpoint. Taken together, unlike somatic cells in which the canonical ATM/ATR-Chk1/2-Cdc25 pathway is the predominant G2/M DNA damage checkpoint, oocytes possess a MDC1-mediated proteolytic pathway via APC/C-Cdh1 regulation as the G2/M DNA damage checkpoint (Fig. 6B).
In addition to revealing a non-canonical function of MDC1 in the regulation of the G2/M transition, our study also demonstrates a novel function of MDC1 in spindle assembly during meiotic maturation in mouse oocytes. Unlike somatic cells, oocytes assemble a bipolar spindle by fragmenting and merging their non-centriolar MTOCs without canonical centrosomes [28, 34]. Our data suggest that p-MDC1 is a MTOC component that is indispensable for spindle assembly in mouse oocytes. Consistent with our results, MDC1 phosphorylated at the T4 residue has been shown to colocalize with γ-tubulin and Plk1 at spindle poles during mitosis [35]. Although many proteins including pericentriolar materials are involved in this process, Aurora A and Plk1 are undoubtedly key kinases in the coordination of MTOC assembly into spindle poles [36, 37]. In this respect, we assumed that p-MDC1 detected at spindle poles in oocytes is likely to be associated with Plk1 instead of ATM. Indeed, MDC1 has been shown to be phosphorylated by Plk1 at the T4 residue during mitosis, independently of DNA damage and ATM [38]. Also, either depleting MDC1 or abrogating MDC1 T4 phosphorylation causes a delay in mitotic progression [38]. Therefore, the role of MDC1 in spindle assembly might be conserved between mitosis and meiosis.
In summary, we demonstrate that MDC1 is a key molecule in the regulation of APC/C-Cdh1-mediated G2/M transition and that this novel regulatory pathway functions as a G2/M DNA damage checkpoint in mouse oocytes. Additionally, MDC1 is indispensable for spindle assembly by regulating the maintenance of MTOC integrity in oocytes. Our data provide new insights into MDC1’s role in the regulation of the DNA damage response and spindle assembly in mouse oocytes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
JL and JSO conceived and designed the experiments. JL performed all experiments. JL and JSO analyzed and interpreted the data. JSO supervised the study. JL and JSO wrote the manuscript.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1A6A1A03015642 and NRF-2019R1I1A2A01041413).
Data availability
The authors confirmed that all data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Declarations
Conflict of interests
The authors have no conflict of interests to declare.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19(2):238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell. 2017;66(6):801–817. doi: 10.1016/j.molcel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3(5):421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 4.Lou Z, Minter-Dykhouse K, Wu X, Chen J. MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature. 2003;421(6926):957–961. doi: 10.1038/nature01447. [DOI] [PubMed] [Google Scholar]
- 5.Stewart GS, Wang B, Bignell CR, Taylor AM, Elledge SJ. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature. 2003;421(6926):961–966. doi: 10.1038/nature01446. [DOI] [PubMed] [Google Scholar]
- 6.Panier S, Boulton SJ. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol. 2014;15(1):7–18. doi: 10.1038/nrm3719. [DOI] [PubMed] [Google Scholar]
- 7.Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25(5):409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123(7):1213–1226. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol. 2003;5(7):675–679. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 10.Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol. 2005;15(18):1670–1676. doi: 10.1016/j.cub.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Morgan DO. Principles of CDK regulation. Nature. 1995;374(6518):131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 12.Adhikari D, Busayavalasa K, Zhang J, Hu M, Risal S, Bayazit MB, Singh M, Diril MK, Kaldis P, Liu K. Inhibitory phosphorylation of Cdk1 mediates prolonged prophase I arrest in female germ cells and is essential for female reproductive lifespan. Cell Res. 2016;26(11):1212–1225. doi: 10.1038/cr.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt JE, Tran SM, Stewart JL, Minahan K, Garcia-Higuera I, Moreno S, Jones KT. The APC/C activator FZR1 coordinates the timing of meiotic resumption during prophase I arrest in mammalian oocytes. Development. 2011;138(5):905–913. doi: 10.1242/dev.059022. [DOI] [PubMed] [Google Scholar]
- 14.Reis A, Chang HY, Levasseur M, Jones KT. APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat Cell Biol. 2006;8(5):539–540. doi: 10.1038/ncb1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll J, Marangos P. The DNA damage response in mammalian oocytes. Front Genet. 2013;4:117. doi: 10.3389/fgene.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol. 2012;22(11):989–994. doi: 10.1016/j.cub.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 17.Collins JK, Jones KT. DNA damage responses in mammalian oocytes. Reproduction. 2016;152(1):R15–22. doi: 10.1530/REP-16-0069. [DOI] [PubMed] [Google Scholar]
- 18.Collins JK, Lane SIR, Merriman JA, Jones KT. DNA damage induces a meiotic arrest in mouse oocytes mediated by the spindle assembly checkpoint. Nat Commun. 2015;6:8553. doi: 10.1038/ncomms9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marangos P, Stevense M, Niaka K, Lagoudaki M, Nabti I, Jessberger R, Carroll J. DNA damage-induced metaphase I arrest is mediated by the spindle assembly checkpoint and maternal age. Nat Commun. 2015;6:8706. doi: 10.1038/ncomms9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer A, Baran V, Sakakibara Y, Brzakova A, Ferencova I, Motlik J, Kitajima TS, Schultz RM, Solc P. DNA damage response during mouse oocyte maturation. Cell Cycle. 2016;15(4):546–558. doi: 10.1080/15384101.2015.1128592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian GN, Greaney J, Wei Z, Becherel O, Lavin M, Homer HA (2020) Oocytes mount a noncanonical DNA damage response involving APC-Cdh1-mediated proteolysis. J Cell Biol 219 (4). doi:10.1083/jcb.201907213 [DOI] [PMC free article] [PubMed]
- 22.Eliezer Y, Argaman L, Kornowski M, Roniger M, Goldberg M. Interplay between the DNA damage proteins MDC1 and ATM in the regulation of the spindle assembly checkpoint. J Biol Chem. 2014;289(12):8182–8193. doi: 10.1074/jbc.M113.532739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barra V, Fachinetti D. The dark side of centromeres: types, causes and consequences of structural abnormalities implicating centromeric DNA. Nat Commun. 2018;9(1):4340. doi: 10.1038/s41467-018-06545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai GY, Choe MH, Kim JS, Oh JS (2020) Mis12 controls cyclin B1 stabilization via Cdc14B-mediated APC/C(Cdh1) regulation during meiotic G2/M transition in mouse oocytes. Development 147 (8). doi:10.1242/dev.185322 [DOI] [PubMed]
- 25.Homer H, Gui L, Carroll J. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science. 2009;326(5955):991–994. doi: 10.1126/science.1175326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holt JE, Weaver J, Jones KT. Spatial regulation of APCCdh1-induced cyclin B1 degradation maintains G2 arrest in mouse oocytes. Development. 2010;137(8):1297–1304. doi: 10.1242/dev.047555. [DOI] [PubMed] [Google Scholar]
- 27.Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3(12):995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 28.Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130(3):484–498. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Coster G, Hayouka Z, Argaman L, Strauss C, Friedler A, Brandeis M, Goldberg M. The DNA damage response mediator MDC1 directly interacts with the anaphase-promoting complex/cyclosome. J Biol Chem. 2007;282(44):32053–32064. doi: 10.1074/jbc.M705890200. [DOI] [PubMed] [Google Scholar]
- 30.Townsend K, Mason H, Blackford AN, Miller ES, Chapman JR, Sedgwick GG, Barone G, Turnell AS, Stewart GS. Mediator of DNA damage checkpoint 1 (MDC1) regulates mitotic progression. J Biol Chem. 2009;284(49):33939–33948. doi: 10.1074/jbc.M109.009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krenning L, Feringa FM, Shaltiel IA, van den Berg J, Medema RH. Transient activation of p53 in G2 phase is sufficient to induce senescence. Mol Cell. 2014;55(1):59–72. doi: 10.1016/j.molcel.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Mullers E, Silva Cascales H, Jaiswal H, Saurin AT, Lindqvist A. Nuclear translocation of Cyclin B1 marks the restriction point for terminal cell cycle exit in G2 phase. Cell Cycle. 2014;13(17):2733–2743. doi: 10.4161/15384101.2015.945831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiebusch L, Hagemeier C. p53- and p21-dependent premature APC/C-Cdh1 activation in G2 is part of the long-term response to genotoxic stress. Oncogene. 2010;29(24):3477–3489. doi: 10.1038/onc.2010.99. [DOI] [PubMed] [Google Scholar]
- 34.Clift D, Schuh M. A three-step MTOC fragmentation mechanism facilitates bipolar spindle assembly in mouse oocytes. Nat Commun. 2015;6:7217. doi: 10.1038/ncomms8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rai R, Phadnis A, Haralkar S, Badwe RA, Dai H, Li K, Lin SY. Differential regulation of centrosome integrity by DNA damage response proteins. Cell Cycle. 2008;7(14):2225–2233. doi: 10.4161/cc.7.14.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Namgoong S, Kim NH. Meiotic spindle formation in mammalian oocytes: implications for human infertility. Biol Reprod. 2018;98(2):153–161. doi: 10.1093/biolre/iox145. [DOI] [PubMed] [Google Scholar]
- 37.Joukov V, Walter JC, De Nicolo A. The Cep192-organized aurora A-Plk1 cascade is essential for centrosome cycle and bipolar spindle assembly. Mol Cell. 2014;55(4):578–591. doi: 10.1016/j.molcel.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Shao C, Kong Y, Carlock C, Ahmad N, Liu X (2017) DNA Damage Response-Independent Role for MDC1 in Maintaining Genomic Stability. Molecular and cellular biology 37 (9). 10.1128/MCB.00595-16 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirmed that all data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.