Abstract
Glioblastoma is the most life-threatening tumor of the central nervous system. Despite recent therapeutic advancements, maximum survival of glioblastoma patients remains dismal. The mediator complex is a set of proteins, essential for eukaryotic gene expression. Abnormal expression/mutations of specific mediator genes have been associated with progression of various cancers, however, its role and status in glioblastoma remains largely unknown. Our work shows overexpression of a subunit of kinase assembly of mediator complex, MED12, in various glioblastoma patient cohorts including Indian glioblastoma patients and cell lines. Functional characterization of MED12 using both overexpression and knockdown approach revealed that it promotes glioblastoma cell proliferation, migration and inhibits apoptosis. Transcriptome analysis post MED12 knockdown revealed Vitamin D receptor (VDR) pathway to be one of the key pathways affected by MED12 in glioblastoma. We studied direct interaction of MED12 with VDR protein using docking studies and co-immunoprecipitation assay. We identify BCL6, a secondary regulator of VDR signaling, to be directly regulated by MED12 through a combination of chromatin immunoprecipitation, qRT-PCR and western analyses. We further show that MED12 brings about the inhibition of p53 levels and apoptosis partly through induction of BCL6 in glioblastoma. Overall, this stands as the first report of MED12 over-expression and involvement in glioblastoma pathogenesis and identifies MED12 as an important mediator of VDR signaling and an attractive molecule for development of new therapeutic interventions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-021-04056-6.
Keywords: Glioblastoma, Mediator complex, MED12, BCL6, p53
Introduction
Eukaryotic gene expression is a complex process with multifaceted regulatory networks. The mediator complex is a set of proteins that are essential controllers of RNA pol II-mediated transcription. It can be divided into the head, middle, tail (core mediator) and the kinase domain. While the core mediator function is imperative for basal transcription, the kinase domain of the mediator complex is present at the heart of transcription regulation and plays a central role in stimuli-controlled transcription programs. As such its alteration is a hallmark in many diseases including cancer [1, 2]. The kinase module consists of four subunits namely CDK8, CCNC, MED12 and MED13. Paralogs of CDK8, MED12 and MED13 namely CDK19, MED12L and MED13L, respectively exist and are present in the kinase module in a mutually exclusive manner [3, 4]. As a part of the whole mediator complex, the kinase assembly associates with transcription factors bound to enhancer regions to bring about DNA looping and aid in transcription initiation, however, kinase assembly has additional distinct functions in transcription regulation independent of its function as a part of the core mediator complex. For example, it aids in the phosphorylation of key transcription factors through CCNC-CDK8 kinase activity and it also has transcription repressive functions contrasting the core mediator which is exclusively associated with transcription activation [5–8]. Initial biochemical studies showed that kinase module when associated with core mediator blocks the interaction between Pol II and core mediator implicating transcription repression as its sole function [9, 10]. However, recent studies have shown that the kinase module is an essential requirement for stimuli-derived transcription implicating context-dependent transcription repressive and/or activating activity of this module. The kinase module as the name suggests is capable of phosphorylating targets via its CDK8-CCNC kinases. Phosphorylation of the carboxyl terminal domain (CTD) of Pol II by kinase module is a prerequisite for transcription of genes of the NFkB pathway. Similarly, CDK8 also phosphorylates various transcription factors to drive downstream transcription [11–14]. A vast array of transcription factors have been shown to physically target MED12 for activation of transcription of target genes and as such a loss of kinase functionality results in loss of expression of these genes [15, 16]. Therefore, it is safe to say that the kinase module has context dependent transcription repressive or transcription promoting functions, making it unique in context of mediator complex overall.
Given the diverse functions of the kinase module in transcription regulation, it is not surprising that individual subunits have been implicated across several malignancies. MED12 alterations, especially missense point mutations have been observed in uterine leiomyomas, fibroepithelial tumors of breast, ovarian cancer, thyroid cancer, prostate cancer, and chronic lymphocytic leukaemia. Oncogenic roles for MED12 have also been well established in, castration resistant prostate cancer, non-small cell lung cancer and ovarian cancer [15–25]. Similarly, CDK8 is a well identified oncogene in colon cancer, breast cancer and melanoma. It also has tumor suppressive roles in endometrial cancer [26–28]. CCNC have tumor suppressive roles in osteosarcoma and T-ALL [29, 30].
Glioblastoma is the most aggressive form of brain cancer with a uniformly poor prognosis. The treatment challenges include diffuse nature of the tumor which makes it almost impossible to remove the tumor completely by surgery. The presence of blood–brain barrier makes it difficult for most of the chemotherapy drugs to pass through to tumor location. The current chemo-drug that is used for treatment is temozolomide, marketed by the name temodar. But of late, we see a current trend in glioblastoma patients of developing resistance towards temozolomide, making the prognosis poorer [31, 32]. Furthermore, temozolomide has been shown to sometimes enrich CD133 + glioma stem cells population leading to a more aggressive and genetically diverse secondary tumor [33, 34]. The tightly integrated network of tumor cells connected by tumor microtubes further helps to evade therapy by using brain parenchyma to efflux chemo agents and decrease cytotoxic damage [35]. All these factors combined with the fact that the tumor is highly heterogeneous in nature draws attention towards the development of novel targeted therapies. It is widely agreed that a better understanding of the transcription landscape of such tumors will help target oncogenic signalling pathways associated with gliomagenesis.
Intriguingly, even though the mediator complex plays a major role in the regulation of gene expression, the status and role of mediator complex subunits remain largely unknown so far in glioblastoma. There is only a single report from our group that showed alterations in MED30 (a head module subunit) in glioblastoma and its role in glioblastoma pathogenesis [36]. Here, we analyzed the expression levels of kinase mediator complex subunits in glioblastoma patient cohorts and identified MED12 to be significantly overexpressed. We conclude that MED12 is a novel oncogene, promoting cancer hallmarks of proliferation and migration while inhibiting apoptosis in glioblastoma cells. We further show that MED12 physically interacts with Vitamin D receptor in glioblastoma cells, leading to induction in the expression of the downstream target genes of this pathway. We found BCL6, a vitamin D responsive gene and a known oncogene in glioblastoma, to be positively correlated with MED12 and established that MED12 aids VDR binding to BCL6 promoter thereby promoting its transcription. BCL6 is known to inhibit p53 pathway in glioblastoma we show that MED12 too negatively regulates p53 expression in glioblastoma. We finally conclude that MED12 exerts its oncogenic effects partly via a MED12-BCL6-p53 axis in glioblastoma.
Materials and methods
Cell culture
A172 and T98G (source—ATCC) cells were a kind gift from Dr. Ellora Sen, NBRC, Manesar. Cells were cultured in DMEM (GIBCO) supplemented with 10% Fetal Bovine Serum (GIBCO, USA), 100 U/mL penicillin/streptomycin (GIBCO) at 37 °C in a 5% CO2 atmosphere. The cells were routinely checked for mycoplasma infection.
Patient samples
Glioblastoma (GBM) samples collected during surgery at the All-India Institute of Medical Sciences (AIIMS) were used in this study. The study was ethically approved by the Institute Ethics Committee and has been performed in accordance with the ethical standards. Briefly, tumor samples were snap-frozen in liquid nitrogen and stored at − 80 °C until use. Hematoxylin and eosin slides of all cases were reviewed independently by three neuropathologists as per WHO 2016 classification. Samples containing significant regions of normal cell contamination (> 10%) and/or excessively large amounts of necrotic material were excluded. Based on such criteria, a total of 25 glioblastoma and 3 epileptic brain samples (representing non-cancerous brain tissues) were finally selected. RNA from these samples was isolated using RNeasy mini kit (M/s Qiagen) and was quantified using Nanodrop. 500 ng of RNA from each sample was used to synthesize cDNA using IScirpt kit (M/s Biorad). MED12 levels were quantified in samples by SyBr green using GAPDH as the internal control. The relative fold change was calculated by double detla CT method and data were plotted in comparison to control.
Glioblastoma patient data analysis
The GBM patients data were analyzed using various public datasets such as GlioVis (http://gliovis.bioinfo.cnio.es), The human protein Atlas (https://www.proteinatlas.org/) and Prognoscan (http://gibk21.bse.kyutech.ac.jp/PrognoScan/index.html).
Construction of recombinant plasmids
Plasmid having human MED12 cds cloned downstream of a polyhedrin promoter (Addgene # 49240) was purchased from Addgene. The MED12 cds was amplified using this plasmid as template and subcloned into PC DNA3.1(+) with a C-terminal 6X histidine tag. The tag sequence was introduced in the reverse primer. The human VDR cds sequence was retrieved from NCBI and cloned with an N-terminal Flag tag in PC DNA3.1(+) vector using specific primers. The tag sequence was introduced in the forward primer. The human BCL6 cds sequence was retrieved from NCBI and cloned in PC DNA3.1(+) vector using specific primers. All the primer sequences used can be found in Supplementary Table 1.
Transfections
Cells were seeded (1 × 10 5 cells per well) in 12-well plates. Different concentrations (10–100 nM) of MED12 siRNA (Sigma siRNA id: SASI_Hs01_00016177) or its respective controls (SIC001Sigma) and MED12 over-expressing plasmid (PC-MED12) or its control (PC DNA 3.1+) were transfected using lipofectamine 2000 (Invitrogen) as per manufacturer’s guidelines. Cells were processed after 48 h for further experiments. 100 nM of siRNA was standardized for knockdown experiments. For plasmid, a concentration of 1 μg per 1 × 105 cells was used.
RNA isolation and RT-qPCR
Total RNA was isolated from transfected cells using RNeasy mini kit (Qiagen) according to the manufacture’s protocol and reverse transcribed using Revertaid first strand cDNA synthesis kit (Biorad). cDNA formed was then further amplified for the specific genes with respective primers using Eva Green mastermix kit (Biorad). Glyceraldehyde 3-phosphate dehydrogenase or β-actin were used as controls for the normalization of the data. List of gene-specific primers is provided in the Supplementary Table 1.
Cellular assays
The cell lines were transfected with MED12 siRNA or MED12 overexpression plasmid along with their respective controls as described above. Post-transfection, cell proliferation was scored using MTT assay, Cyquant Cell proliferation assay and colony formation assay, cellular migration was scored using scratch assay and Boyden chamber assay, and apoptosis was scored using Caspase 3/7 Glo assay and 7-AAD, PE-Annexin V/PI, FITC-Annexin V, based FACS analyses as previously described [36]. The detailed methodology for these assays is also provided in electronic supplementary material 1.
Western blotting
Whole cell protein was isolated after 48 h of transfection by using RIPA protein extraction buffer and sonicated at 30% amplitude for 2 min followed by centrifugation at 15,000 rpm for 20 min at 4 °C. Protein quantification was performed by Bradford assay. 30 µg of protein was mixed with 4X Laemmli buffer followed by SDS-PAGE. The proteins were then transferred to a nitrocellulose membrane followed by 2 h of blocking in 5% BSA. The membrane was incubated with primary antibody overnight (1:1000 dilution of MED12 antibody cell signaling technology #4529, 1:1000 dilution of cleaved parp antibody cell signaling technology #9541S, 1:200 dilution of p53 antibody Santacruz biotechnology # sc-126, 1:250 dilution of BCL6 antibody Thermo scientific #MA5-11493, 1:1000 dilution of β-actin antibody cell signaling technology #4970) at 4 °C followed by incubation with secondary antibody for 2 h (Santa Cruz Biotechnology anti-mouse # SC-2005, anti-rabbit# SC-2357, Dilution 1:7000). Detection was done using a ECL chemiluminescent substrate (Biorad).
Co-immunoprecipitation
A172 and T98G cells were transfected with histidine-tagged MED12 over-expressing plasmid alone or along with Flag-tagged VDR over expressing plasmid. Post 48 h of transfection cells were washed with ice-cold PBS and lysed using RIPA buffer with 10 mM of PMSF. Cells were then sonicated at 30% amplitude for 5 min at 10 s on and 30 s off pulse using Branson Sonicator followed by centrifugation at 12,000 rpm for 20 min at 4 °C. The supernatant was collected and incubated with 50ul of anti-MED12 antibody (1:50, Cell Signaling Technology #4529) overnight. Pull down was performed using Protein A Sepharose beads (Thermo Fisher Scientific) followed by immunoblotting using anti-flag (1:1000 Abkine #A0210) antibody.
Chromatin immunoprecipitation
T98G cells over-expressing Flag-tagged VDR were divided into two sets, one set transfected with MED12 siRNA and the other with siRNA control. 48 h post transfection the cells were cross-linked using 0.75% of formaldehyde for 20 min at RT followed by treatment with 0.125 M glycine for 5 min. The cells were then lysed with RIPA buffer and sonicated for 8 min at 50% amplitude at 10 s on 30 s off cycle. Each sample was further divided into two sets one pulled down using anti-IgG antibody (1:50, Santacruz Biotechnology #SC2025) and the other using anti-FLAG antibody (1:50, Abkine #A0210) for one hour at 4 °C. The samples were then incubated with 50ul of Protein A Sepharose beads overnight at 4 °C. The samples were washed using high salt (0.1% SDS,1% Triton X-100, 2 mM EDTA, 20 mM Tris–HCl pH 8.0, 500 mM NaCl) and low salt buffers (0.1% SDS,1% Triton X-100, 2 mM EDTA, 20 mM Tris–HCl pH 8.0, 150 mM NaCl) followed by DNA elution and de-crosslinking overnight. DNA was isolated using phenol:chloroform extraction and analysed using q-RTPCR. Target gene enrichment was calculated as per standard methods using the formula:
Gene expression profiling
A172 cells were seeded in a six-well plate and transfected using MED12 siRNA (sigma) or its control and total RNA was isolated post 48 h of transfection. The RNA was sent for microarray analysis to Imperial Life Sciences Pvt. Ltd, India. Microarray-based gene expression analysis was performed using an Affymetrix platform. The differentially regulated genes (fold-change = or > ± 1.5) with p value < 0.05 was considered significant. The microarray data have been submitted to GEO repository. The accession number for the same is GSE176187. The list of differentially regulated genes can also be found in electronic supplementary material 2. The results of pathway analysis of de-regulated genes can be found in electronic supplementary material 3.
Docking studies
The amino acid sequence of human MED12 (accession no: Q93074) was retrieved from the UniProt KB database (http://www.uniprot.org/). A homology detection and structure prediction were performed using the I-TASSER (https://zhanglab.dcmb.med.umich.edu/I-TASSER/) and resulted in the best match electron microscopic structure of the Cdk8 kinase module (CKM) (PDB ID: 7KPX) with good similarity and making it the best template for fishing out the human MED12 3D structure. The 3D structure of MED12 was generated using I-TASSER server. The final model was validated using the Ramachandran plot. Similarly, the crystal structure of the human VDR structure (PDB ID: 3B0T) was chosen for the interaction analysis. The molecular docking was performed using the HDOCK server for the human MED12 against VDR. The pictures were rendered using PyMOL and LigPlot + software.
Statistical analysis
All in vitro experiments were performed in triplicates unless mentioned otherwise. A two-tailed Student’s t test was applied for the calculation of p values using Microsoft Excel. Data with a p value < 0.05 was considered significant.
Results
MED12 is overexpressed in glioblastoma patients and cell lines
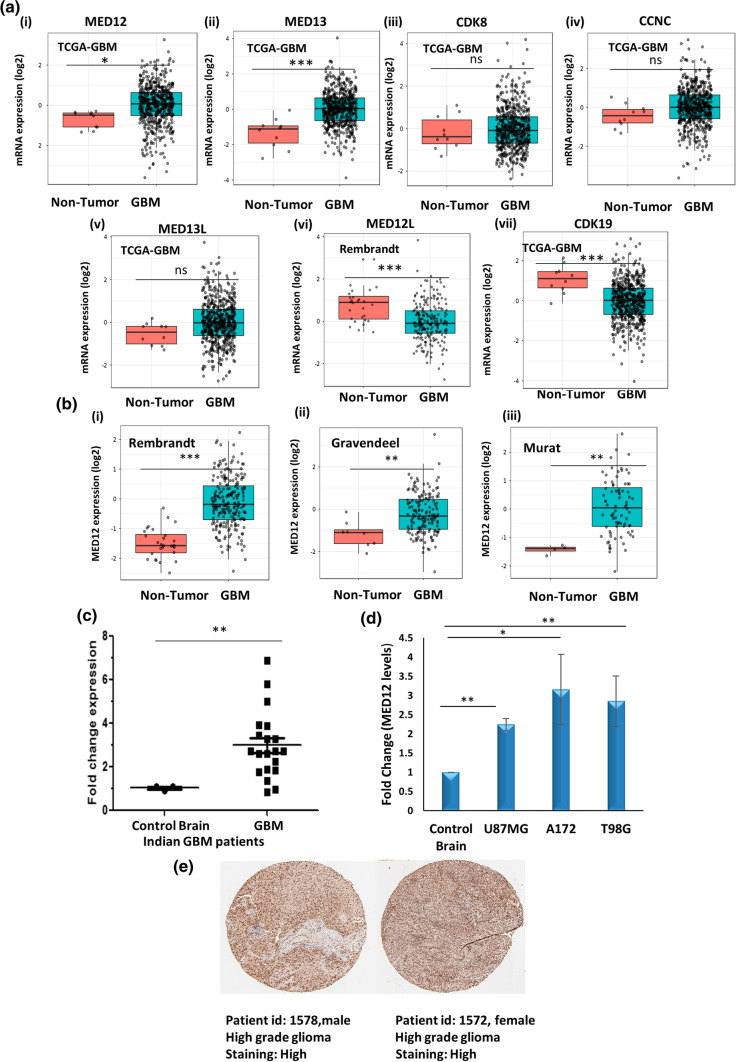
The role of certain kinase subunits such as CDK8 and MED12 in various cancers is very well-reported and extensively studied, however, no report of these subunits in glioblastoma is available so far. The first step in this direction was to check if these subunits had de-regulation of expression in glioblastoma patients. For this, we performed TCGA-GBM (HG-U133A; GBM (n) = 528, non-tumor (n) = 10) patient data analyses using GlioVis (a web application for data visualization and analysis to explore brain tumors expression datasets). We found MED12 and MED13 to be overexpressed in glioblastoma patients while CDK19 was downregulated in glioblastoma patients. Since MED12L gene expression was not available in TCGA data, we checked its expression in Rembrandt dataset and found it to be significantly downregulated. This is in line with the observation that MED12 and MED12L are expressed in a mutually exclusive manner (Fig. 1a).
Fig. 1.
MED12 is over-expressed in glioblastoma patients and cell lines. GlioVis (a web application for data visualization and analysis to explore brain tumors expression datasets) was used for expression analysis of kinase subunits in TCGA-GBM (GBM (n) = 528, non-tumor (n) = 10) glioblastoma patient dataset. a Graph showing expression pattern of kinase subunits (MED12, MED13, CDK8, CCNC, MED13L, MED12L, CDK19) in TCGA_GBM patients (HG-U133A) versus non-tumor control. MED12L levels are shown from Rembrandt dataset. b Analysis of MED12 over-expression in glioblastoma in (i) Rembrandt patient dataset (GBM n = 219 non-tumor n = 28) (ii) Gravendeel patient dataset (GBM n = 159 non-tumor n = 8) (iii) Murat patient dataset (GBM n = 80 non-tumor n = 4). c Graph showing fold change in expression of MED12 in a cohort of 25 Indian glioblastoma patients versus non-tumor control (n = 3). d Graph showing fold change in expression of MED12 across three glioblastoma cell lines (A172, U87MG and T98G) versus normal brain RNA (Agilent MVP total brain mRNA). GAPDH was used for normalization. e Representative images of IHC of two glioma patients (patient id: 1572, gender: female, age: 55, glioma: high grade, antibody used: HPA003184; patient id: 1578, gender: male, age: 56, glioma: high grade, antibody used: HPA003184) showing over-expression in MED12 protein. Image was retrieved from the Human Protein Atlas. The graphical data points represent mean ± S.D of at least three independent experiments (* represents p value < 0.05 and ** represents p value < 0.001). Error bars denote ± SD
The functional diversity of kinase module comes from its CCNC-CDK8 phosphorylation activity which is entirely dependent on MED12. The binding of MED12 to CCNC-CDK8 is imperative for kinase activation. Further, MED12 has been reported as a fine tuner of transcription driving carcinogenesis across several malignancies. We therefore focussed our attention on functionally characterizing MED12 in glioblastoma. We checked other datasets available on GlioVis for alterations in MED12 expression in glioblastoma and found significant over-expression of MED12 in glioblastoma patients as compared to non-tumor controls across three other patient datasets namely Rembrandt (GBM n = 219 non-tumor n = 28), Gravendeel (GBM n = 159 non-tumor n = 8) and Murat (GBM n = 80 non-tumor n = 4) (Fig. 1b). We checked for the expression of MED12 in Indian Patients as well. For this, tumor samples operated at All India Institute of Medical Sciences (AIIMS) were included in this study. We found significant upregulation in MED12 expression in the Indian patient cohort studied (Fig. 1c). We next checked for MED12 transcript status in the various glioblastoma cell lines and found MED12 to be overexpressed across three glioblastoma cell lines of varying genetic make-up (U87MG, A172 and T98G cell lines) (Fig. 1d). We also confirmed the enhanced expression of MED12 at protein levels in glioma patients by analyzing data available at the human protein atlas. We found that out of the 12 patients studied, 10 had significant overexpression in MED12 protein levels while three showed medium expression (Fig. 1e, Supplementary Fig. 1).
Clinical association of MED12 expression across different grades of glioma and its correlation with patient prognosis
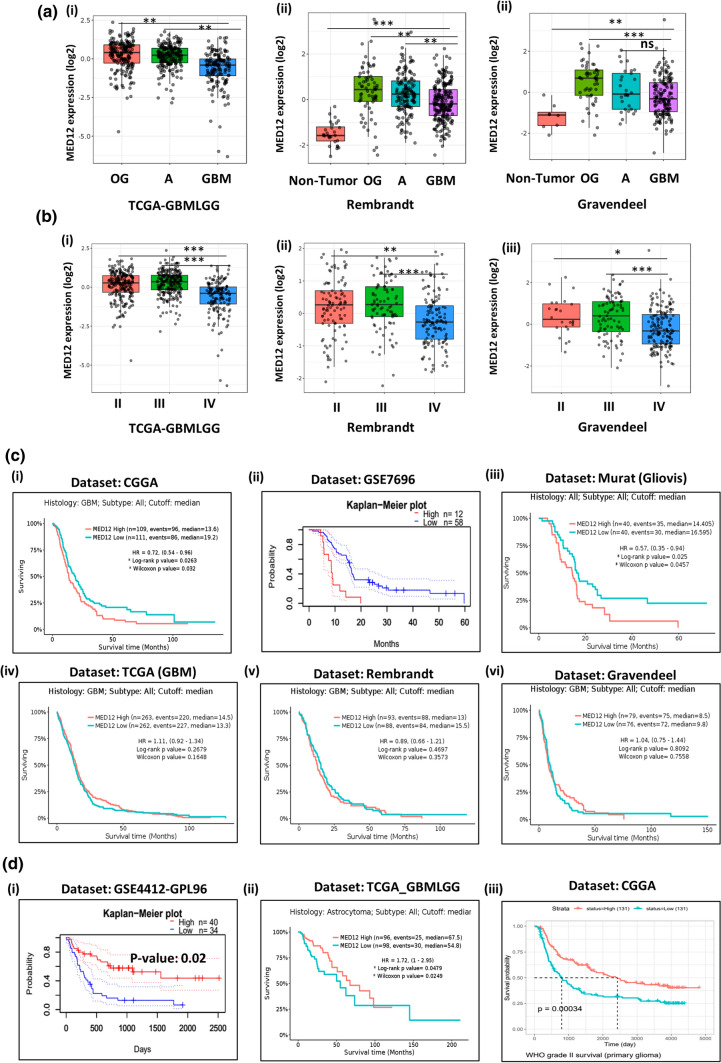
When we compared the expression of MED12 across different subtypes of glioma, we found that it is higher in oligodendroglioma, and astrocytoma as compared to glioblastoma (Fig. 2a). Astrocytoma and oligodendroglioma are considered low-grade glial tumors arising from astrocytes and oligodendrocyte, respectively. Grade wise expression analysis further revealed that its expression is higher in grade II and grade III tumors as compared to grade IV glioblastoma (Fig. 2b). These observations hint towards a possible involvement of MED12 in early events of gliomagenesis.
Fig. 2.
Clinical association of MED12 expression across different grades of glioma and its correlation with patient prognosis: a expression of MED12 across different subtypes of gliomas. Graph from GlioVis showing higher expression of MED12 in astrocytoma and oligodendroglioma as compared to glioblastoma in (i) TCGA_GBMLGG dataset (ii) Rembrandt dataset. (iii) Gravendeel dataset. b Expression of MED12 across low grade gliomas. Graph from GlioVis showing higher expression of MED12 in grade II and grade III tumors as compared to glioblastoma in (i) TCGA_GBMLGG dataset. (ii) Rembrandt dataset. (iii) Gravendeel dataset. c Prognosis of glioblastoma patients in relation to MED12 over-expression (i) Kaplan–Meier curve from GlioVis (CGGA dataset) showing significant correlation of MED12 high expression with poor patient prognosis in glioblastoma. (ii) Kaplan–Meier curve from Prognoscan database displaying significant correlation of MED12 high expression with poor patient prognosis in glioblastoma. (iii) Kaplan–Meier curve from GlioVis (Murat dataset) database displaying significant correlation of MED12 high expression with poor patient prognosis in glioblastoma. (iv, v, vi) Kaplan–Meier curve from GlioVis (TCGA_GBM, Rembrandt and Gravendeel dataset) database displaying no significant correlation of MED12 expression with patient prognosis in glioblastoma. d Prognosis of glioma patients in relation to MED12 over-expression. (i) Kaplan–Meier curve from Prognoscan analysing GSE4412-GPL96 dataset showing high expression of MED12 promotes patient prognosis in glioma. (ii) Kaplan–Meier curve from GlioVis analysing TCGA_GBMLGG dataset showing high expression of MED12 promotes patient prognosis in astrocytoma. (iii) Kaplan–Meier curve from CGGA showing high expression of MED12 promotes patient prognosis in grade II glioma
We next checked for the prognostic significance of MED12 expression alterations in glioblastoma and found varied results. In CGGA and MURAT GBM patient cohort, high expression of MED12 correlated with poor survival. The same correlation was observed across one more dataset of glioblastoma patients analysed using Prognoscan software (Fig. 2c). However, in TCGA-GBM, Rembrandt and Gravendeel GBM patients, we found no correlation between MED12 expression and GBM patient prognosis (Fig. 2c).
Interestingly, in the case of low-grade gliomas we found that high expression of MED12 improves survival outcomes in patients (Fig. 2d). We discuss these points in detail later in the manuscript.
MED12 promotes cellular proliferation in glioblastoma
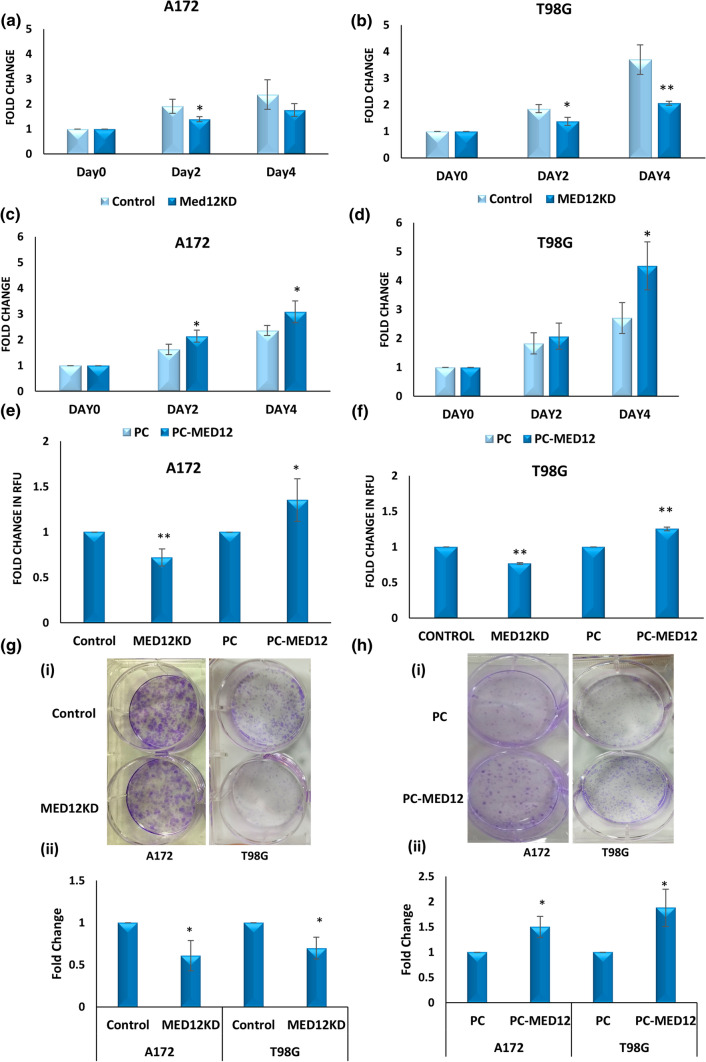
To elucidate the role of MED12 in glioblastoma, we checked for the effects of MED12 modulation on cell proliferation. For this, we performed MED12 overexpression and knockdown in T98G and A172 cell lines as described in the Methods section. The modulation of MED12 levels was confirmed by qRT-PCR (Supplementary Fig. 2).
MTT assay was performed in the transfected T98G and A172 cells. Results obtained showed an increase in proliferation upon MED12 over-expression while opposite results were obtained post MED12 knockdown (Fig. 3a–d). Next, we confirmed our results by performing cyquant cell proliferation assay in A172 and T98G cells having MED12 modulation. Like our observation with MTT, we found MED12 to promote cell proliferation (Fig. 3e, f). The results were replicated in the colony formation potential of MED12 modulated cells. The MED12 overexpressing T98G and A172 cells showed an increase in the colony-forming potential while opposite results were obtained upon knockdown (Fig. 3g, h). Thus, we show MED12 to enhance the proliferative potential of glioblastoma cells.
Fig. 3.
MED12 promotes cellular proliferation in glioblastoma. a, b Graph showing relative proliferation using MTT assay in cells transfected with MED12 siRNA or universal negative control siRNA in a A172 cells b T98G cells. c, d Graph showing relative proliferation using MTT assay in cells transfected with MED12 over-expressing plasmid or PC DNA 3.1(+) in c A172 cells d T98G cells. e Graph showing relative proliferation using cyquant cell proliferation assay in A172 cells having modulation in MED12 levels using MED12 siRNA and over-expressing plasmids as well as their respective controls. f Graph showing relative proliferation using cyquant cell proliferation assay in T98G cells having modulation in MED12 levels using MED12 siRNA and over-expressing plasmids as well as their respective controls. g, h Colony formation assay results in T98G and A172 cells upon g MED12 knockdown or h MED12 over-expression [(i) images of colonies (ii) graphical representation of fold change in number of colonies]. The graphical data points represent mean ± SD of at least three independent experiments (* represents p value < 0.05 and ** represents p value < 0.001). Error bars denote ± SD
MED12 promotes cellular migration in glioblastoma
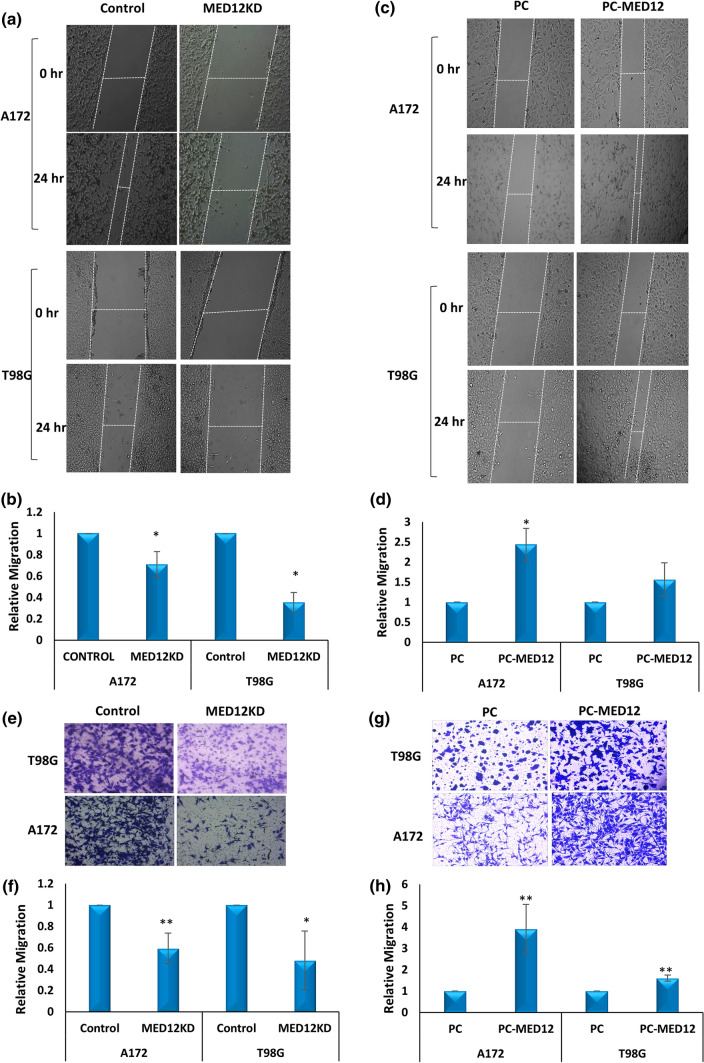
We checked effects of MED12 alteration on cell migration using a scratch assay in both MED12 overexpressing and MED12 silenced A172 and T98G cells. MED12 was shown to enhance the migratory potential of glioblastoma cells (Fig. 4a–d). To validate the results further, a trans-well migration assay (Boyden chamber Assay) was performed in both the sets of A172 and T88G cells. We found a similar result with a decrease in migration rate with MED12 knockdown and increased migration with MED12 over-expression (Fig. 4e–h). Thus, MED12 was shown to promote cellular migration in glioblastoma.
Fig. 4.
MED12 promotes cellular migration in glioblastoma: a images of T98G and A172 cells showing wound healing assay post MED12 knockdown b graphical representation of the data showing relative migration. c Microscopic images of T98G and A172 cells showing wound healing assay post MED12 over-expression. d Graphical representation of the data showing relative migration. e Microscopic Images showing transwell migration assay post MED12 knockdown in T98G and A172 cells. f Graphical representation of the data. g Microscopic Images showing transwell migration assay post MED12 over-expression in T98G and A172 cells. h Graphical representation of the quantification of transwell migration assay using ImageJ. The graphical data points represent mean ± SD of at least three independent experiments (*represents p value < 0.05 and ** represents p value < 0.001). Error bars denote ± SD
MED12 inhibits apoptosis in glioblastoma
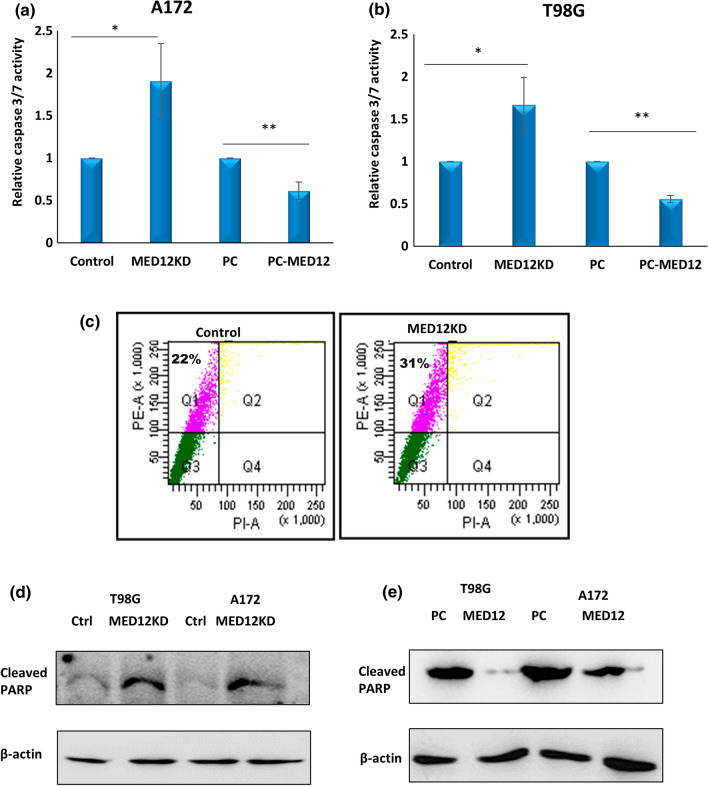
We checked if MED12 had any effects on cellular apoptosis in glioblastoma. To do this, we performed caspase 3/7 glo assay in A172 and T98G cells, having MED12 over-expression and knockdown. Caspase 3/7 glo assay works by enzymatic breakdown of the luminogenic substrate by activated caspases. The amount of substrate breakdown can be quantified by measuring the luminescent signal. We found that MED12 over-expression inhibits apoptosis in A172 and T98G cells while knockdown gave opposite results (Fig. 5a, b). We next performed flow cytometric-based analysis to analyse the percentage of cells having phosphatidylserine externalization, which is considered a hallmark of apoptosis induction, in cells having MED12 knockdown. FACS analysis again confirmed that MED12 knockdown lead to apoptosis induction (Fig. 5c). We finally confirmed these results by performing western blotting for cleaved PARP, in A172 and T98G cells having MED12 over-expression and knockdown. The results obtained, further confirmed the apoptosis inhibitory effects of MED12 in glioblastoma (Fig. 5d, e).
Fig. 5.
MED12 inhibits apoptosis in glioblastoma: substrate based caspase 3/7 glo assay was performed to check for apoptosis. a, b Graph showing fold change in relative caspase 3/7 activity in cells having MED12 over-expression and knockdown in a A172 cells b T98G cells. c FACS based detection of annexin-V externalisation was checked in A172 cells post MED12 knockdown. d, e Western blotting was performed to detect cleaved PARP protein levels in A172 and T98G cells having d MED12 knockdown and e having MED12 over-expression. The actin blot was run on a different gel. The western blotting experiment was performed in duplicates. The graphical data points represent mean ± SD of at least three independent experiments (*represents p value < 0.05 and ** represents p value < 0.001). Error bars denote ± SD
Vitamin D receptor pathway is one of the key pathways regulated by MED12 in glioblastoma
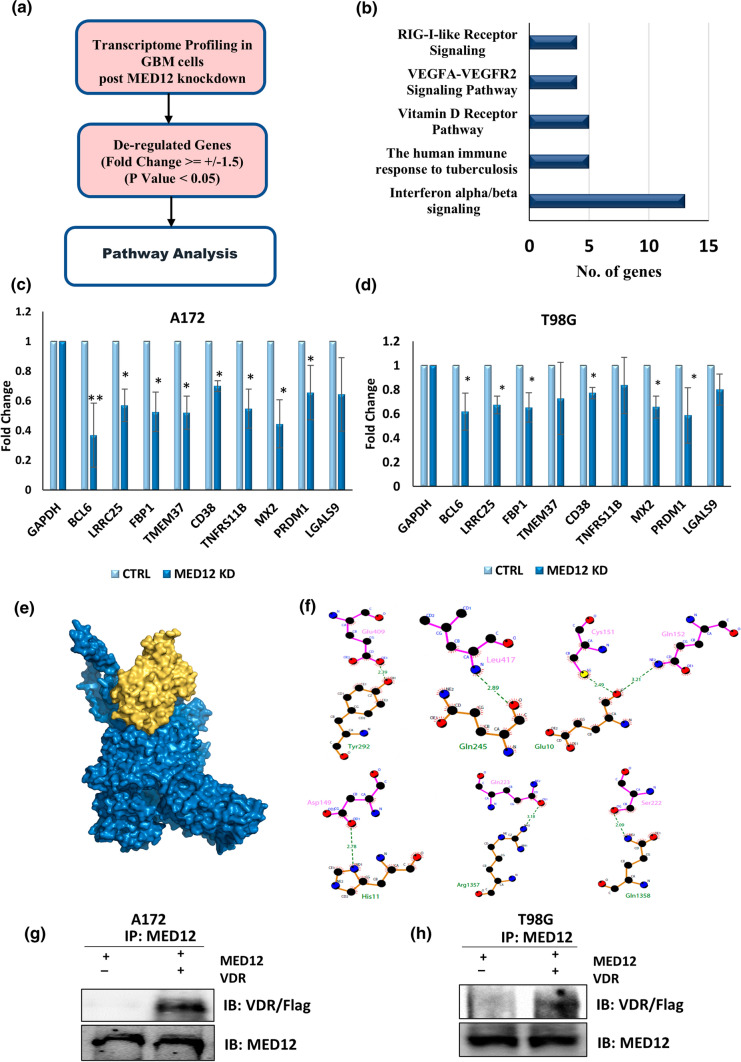
Having established the oncogenic role of MED12 in glioblastoma, we were interested to know the mechanistic consequences of its de-regulation. For this we performed whole transcriptome analysis upon MED12 knockdown in A172 cells and did pathway analysis of genes that were significantly de-regulated (fold change > or = ± 1.5, p value < 0.05) using wiki-pathways. We found interferon-alpha/beta signalling, the human immune response to tuberculosis, Vitamin D receptor pathway, VEGFA-VEGFR2 signalling pathway and RIG I like receptor signalling as the topmost pathways affected by MED12 alteration in glioblastoma cells (Fig. 6a, b). The N-terminal region of the MED12 protein contains two overlapping LxxLL motifs which are the standard nuclear hormone receptor recognition signature. Incidentally, we found vitamin D receptor pathway which is a nuclear receptor pathway as one of the pathways affected by MED12. Since MED12 has been known to control gene expression via physical interaction with several transcription factors, we focussed our attention on elucidating the role of MED12 in VDR signalling. We checked the expression of some of the genes commonly associated with VDR pathway in response to MED12 modulation in glioblastoma cells. For this, we performed over-expression and knockdown of MED12 in two glioblastoma cell lines (A172, T98G) and checked the transcript levels of some of the common VDR responsive genes by qRT-PCR. We found a positive correlation in the expression of VDR responsive genes and MED12 (Fig. 6c, d, Supplementary Fig. 3). We then used the in-silico approach, to evaluate the ability of VDR to interact with MED12 and found that VDR has a high binding affinity for MED12 with a docking score of -303.55 kcal/mol. These two proteins interact with 16 hydrophobic and 7 strong hydrogen bonds (Supplementary Tables 2, 3). The binding site of MED12 contains both hydrophobic residues (Leu126, Val158, Val288, Ser1398, and Ser1405) and aromatic residues (Tyr9, Phe237, Phe242, Phe244, His252 and Trp1354) which form weak interactions. This suggests a possible interaction between VDR and MED12 stabilized by weak interactions. From the molecular docking study, we concluded that VDR has a good binding affinity with MED12 (Fig. 6e, f). We finally confirmed this interaction by co-immunoprecipitation experiment. We cloned the cds region of VDR with a N-terminal fusion FLAG tag and the cds region of MED12 with a C-terminal fusion histidine tag. We over-expressed both these proteins by transiently transfecting them in A172 and T98G glioblastoma cells. 48 h post transfection we prepared whole cell lysates and precipitated the lysates using MED12 antibody followed by immunoblotting with anti-Flag antibody. The results obtained confirmed physical interaction between MED12 and VDR (Fig. 6g, h). Hence, we identify vitamin D receptor pathway to be a key pathway affected by MED12 in glioblastoma, and VDR to be a physical binding partner of MED12.
Fig. 6.
MED12 physically interacts with Vitamin D Receptor and regulates the Vitamin D receptor pathway in glioblastoma: a schematic of work-flow for analysis of pathways affected by MED12 in glioblastoma. b Bar graph showing five topmost pathways affected by MED12 in glioblastoma cells. The data from whole transcriptome analysis post MED12 knockdown was analysed and significantly de-regulated genes (fold change ≥ ± 1.5, p value < 0.05) were used for pathway analysis using wiki-pathways. c, d Graph showing fold change in expression of genes of VDR pathway analysed by qRT-PCR post MED12 knockdown in c A172 and d T98G cells. e Surface representation of MED12 complex (blue colour) and VDR (yellow colour). f Interaction analysis of MED12 with VDR, LigPlot + image representing the hydrogen bond interactions between the MED12 (labelled in green colour) and VDR (labelled in pink colour). g, h Co-Immunoprecipitation results establishing physical interaction between MED12 and VDR. A172 (g), T98G (h) cells over-expressing MED12 and a flag tagged VDR were subjected to immunoprecipitation using MED12 antibody. Post precipitation western blotting with anti-flag antibody was performed to check for physical interaction between MED12 and VDR. Western Blotting with anti-MED12 was performed to confirm successful precipitation. The graphical data points represent mean ± SD of at least three independent experiments (*represents p value < 0.05 and ** represents p value < 0.001). Error bars denote ± SD
MED12 enhances VDR mediated transcription induction of BCL6 and inhibits p53 expression in glioblastoma
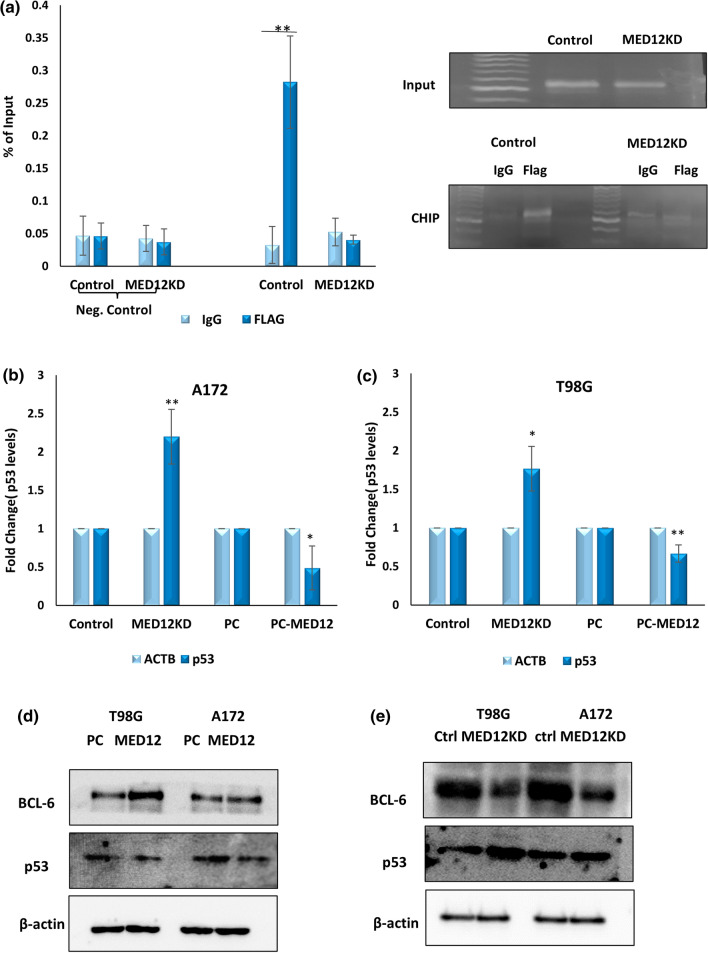
We previously performed qRT-PCR, post MED12 modulation in glioblastoma cells and found BCL6 to be one of the VDR responsive genes affected by MED12 (Fig. 6c, d). BCL6 is a very well characterised oncogene in glioblastoma. There have been several reports citing BCL6 over-expression and its association with poor survival outcome in glioblastoma. A recent study showed that BCL6 negatively regulates p53 signalling in glioblastoma, thereby inhibiting apoptosis, and promoting tumor progression [37, 38]. Since MED12 also showed strong oncogenic effects in glioblastoma cells, we were interested to check if BCL6 up-regulation might be one of the contributing factors through which MED12 exerts its oncogenic effects. For this, we first confirmed BCL6 up-regulation by MED12 in glioblastoma. We checked if MED12 modulation affected VDR binding on BCL6 promoter. For this chromatin immunoprecipitation studies were performed in T98G cells. Cells overexpressing VDR tagged with FLAG peptide were subjected to siRNA mediated knockdown of MED12 and respective control. CHIP was performed using anti-flag antibody and an IgG control. Chromatin extract without any antibody treatment was used as input. The binding of VDR to BCL6 promoter was confirmed by qRT-PCR using sequence-specific primers. We confirm that post MED12 knockdown the enrichment of BCL6 promoter with VDR is reduced significantly as compared to control indicating that MED12 regulates BCL6 expression at transcript levels by modulating VDR binding on BCL6 promoter. We used primers for hypoxia response elements in the promoter of hsa-mir-196a as negative control (Fig. 7a). Since BCL6 is known to inhibit apoptosis in glioblastoma via the suppression of p53 pathway, we checked if MED12 had any effects on p53 levels in glioblastoma. For this, we performed qRT-PCR post MED12 knockdown and over-expression in A172 and T98G cells and found that MED12 also suppresses the expression of p53 in glioblastoma cells (Fig. 7b, c). We also confirmed BCL6 mediated inhibition of p53 expression in glioblastoma cells (Supplementary Fig. 5a, b). We further confirmed effects of MED12 on p53 and BCL6 on protein levels as well. We found that MED12 overexpression brings about an increase in BCL6 levels while decreasing p53 levels in both the cell lines while MED12 knockdown showed opposite results (Fig. 7d, e). Overall, MED12 promotes VDR mediated transcriptional induction of BCL6 leading to inhibition of p53 levels.
Fig. 7.
MED12 enhances VDR mediated transcription induction of BCL6 and inhibits p53 expression in glioblastoma: a graph and gel image showing Chromatin Immunoprecipitation results indicating that enrichment of BCL6 promoter by VDR decreases significantly post MED12 knockdown. The experiment was performed in duplicates. b Graph showing fold change in p53 expression analysed by qRT-PCR post MED12 knockdown and over-expression in A172 cells. c Graph showing fold change in p53 expression analysed by qRT-PCR post MED12 knockdown and over-expression in T98G cells. d, e Western blotting results showing effects of MED12 modulation on BCL6 and p53 protein levels in A172 and T98G cells in d MED12 over-expressing cells e cells having MED12 knockdown. The western blotting experiment was performed in duplicates. The actin blot was run on a different gel. The graphical data points represent mean ± SD of at least three independent experiments (*represents p value < 0.05 and ** represents p value < 0.001). Error bars denote ± SD
p53 and apoptosis inhibition by MED12 in glioblastoma is BCL6 mediated
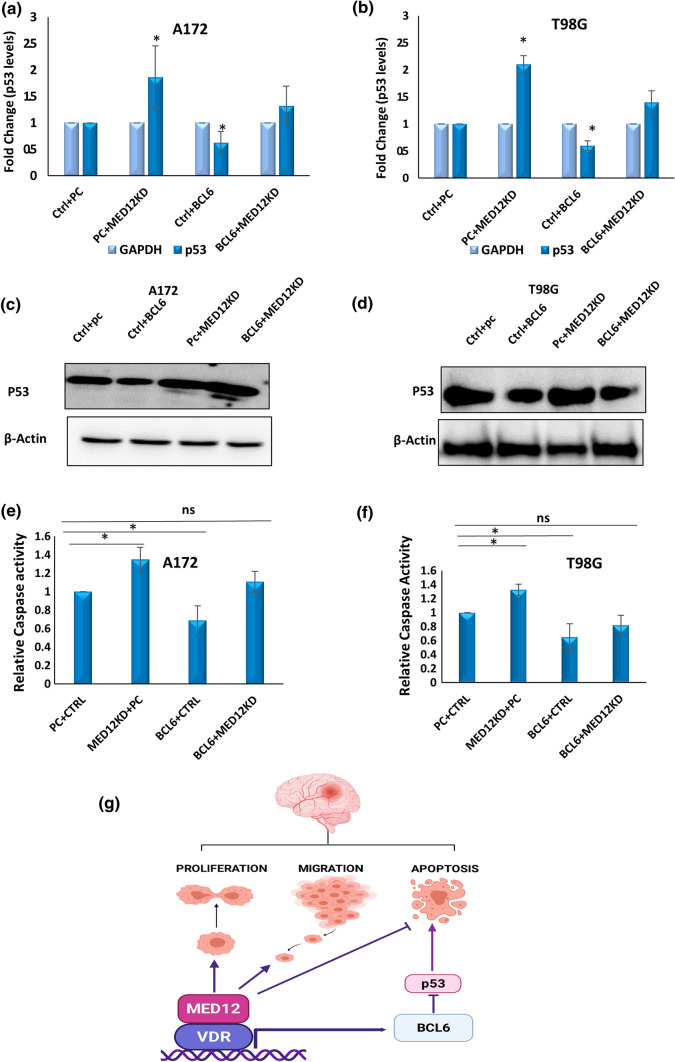
To further explore the link between the MED12-BCL6-p53 axis and its possible pro-oncogenic, anti-apoptotic role in glioblastoma, we checked if the anti-apoptotic and p53 inhibitory activity of MED12 is affected by BCL6 modulation. For this, we performed simultaneous overexpression of BCL6 and MED12 knockdown in A172 and T98G cells and checked if BCL6 over-expression could rescue the induction in apoptosis and p53 expression due to MED12 knockdown. Our results show that p53 expression on the transcript as well as protein levels was enhanced upon MED12 knockdown, however, the enhancement in p53 expression by MED12 knockdown was reduced upon BCL6 over-expression (Fig. 8a–d). Consequently, we found that apoptosis induction by MED12 knockdown was somewhat rescued by BCL6 over-expression (Fig. 8e, f, Supplementary Fig. 6a–c). Thus, we conclude that the oncogenic effects of MED12 are partially mediated by BCL6/p53 axis in glioblastoma.
Fig. 8.
Inhibition of apoptosis and p53 levels by MED12 is BCL6 mediated: simultaneous over-expression of BCL6 and MED12 knockdown was performed in A172 and T98G cells. a, b Graph showing fold change in p53 expression analysed by qRT-PCR upon simultaneous over-expression of BCL6 and MED12 knockdown in a A172 cells, b T98G cells. c, d Western blotting results showing effects of simultaneous knockdown of MED12 and BCL6 over-expression in c A172 cells, d T98G cells. The western blotting experiment was performed in duplicates. The actin blot was run on a different gel. e, f Graph showing fold change in relative caspase 3/7 activity in cells having BCL6 over-expression and MED12 knockdown in e A172 cells f T98G cells. g Cartoon summarizing the role of MED12 in glioblastoma. The illustration was created with BioRender.com. The graphical data points represent mean ± SD of at least three independent experiments (*represents p value < 0.05 and ** represents p value < 0.001). Error bars denote ± SD
Discussion
Our work here delves into the status and role of a member of the mediator complex (kinase domain), in glioblastoma. There is a vast body of literature on alterations in mediator subunits across several cancers. CDK8 is a well-characterized oncogene in colon cancer, prostate cancer and breast cancer. CCNC has been shown to be a tumor suppressor in osteosarcoma and T-ALL. Oncogenic amplifications in MED13 gene locus have been reported in breast cancer [15–29, 39]. However, the status of mediators in glioblastoma remains mostly unknown. Recent work from our lab showed the involvement of MED30, a subunit of core mediator complex, in glioblastoma pathogenesis [36]. We show that MED30 is overexpressed in glioblastoma and promotes cell proliferation making glioblastoma cells more sensitive to chemotherapy. Other than this, to our knowledge, there are no reports of alterations of mediator complex subunits in glioblastoma.
Based on patient data analyses, we found overexpression of two subunits of kinase domain namely, MED12 and MED13 in glioblastoma, while CDK19 and MED12L showed significant downregulation. The kinase activity of the complex that makes it functionally independent of the core mediator relies on activation by MED12. In addition to this, MED12 alterations have been widely reported across several other malignancies. Exon1 and exon2 mutations in MED12 affecting its interaction with CCNC-CDK8 kinase have been reported in uterine leiomyomas, fibroepithelial tumours of breast, ovarian leiomyomas, chronic lymphocytic leukaemia, and non-anaplastic thyroid cancer. MED12 alterations especially in hormone-dependent tumors of the female reproductive system is gaining importance in therapeutics as well as diagnosis. MED12 is also linked to therapy resistance in lung cancer and breast cancer [15–25]. Thus, in this study we focussed our attention on studying the role of MED12 in glioblastoma, though there is a possibility that other altered MED subunits of the kinase domain too play an important role in glioblastoma pathogenesis which needs functional validation.
In glioblastoma, we found that MED12 was upregulated in tissues as well as cell lines. The data was further replicated in a cohort of 25 Indian patients. Although MED12 mutations are widespread in several cancers, we found that in glioblastoma, the frequency of somatic mutations in MED12 is just 1% (cBioportal). The majority of these mutations are missense mutations, with few frameshift deletions. One of these mutations, A1383T has the status of being probably damaging for MED12 function by polyphen analysis, however, the frequency of its incidence in patients is very less.
When we compared grade-wise expression patterns, we found that as the tumor progresses the expression of MED12 decreases being more in grade II and grade III tumors as compared to grade IV glioblastoma indicating that it might be associated with the early events of gliomagenesis. The expression of MED12 was also found to be more in astrocytoma and oligodendroglioma as compared to glioblastoma. This observation also coincides with the fact that in low-grade gliomas its high expression is associated with either better overall survival outcomes or else it is not significantly correlated. Further studies are needed to establish its role, if at all in the progression of gliomas vis-a-vis secondary lesions, however, one thing that we noticed is that its expression in IDH mutant tumors is more as compared to IDH wild-type tumors (Supplementary Fig. 4). IDH mutations are majorly present in secondary glioblastomas, along with the fact that they are well-established genetic alterations in low-grade glioma with IDH mutant tumors having a better prognosis [40]. Thus, it is fair to anticipate that a detailed study of this axis might shed some light on the mechanistic insights of such an expression pattern of this gene across several grades in glioblastoma.
TCGA data analysis revealed a negligible frequency of copy number alterations in MED12, nullifying genomic alterations as a possible cause for MED12 over-expression in glioblastoma (cBioportal). Thus, the underlying cause for expression alterations in MED12 in glioblastoma may be attributed to post-transcriptional mechanisms. It will be interesting to explore the network of transcription factors, miRNAs, and other non-coding RNAs that target MED12 in glioblastoma to decipher the cause of its over-expression.
Functional characterisation of MED12 in glioblastoma revealed that it promotes cellular proliferation and migration while inhibiting apoptosis. We confirmed these results across two glioblastoma cell lines (A172 and T98G). Thus, we conclude that the effect of MED12 over-expression in glioblastoma is oncogenic in nature. MED12 is known to promote cancer cell growth and proliferation in other cancers such as castration-resistant prostate cancer, uterine leiomyomas, and non-small lung cancer as well indicating its diverse oncogenic role in several cancers [17, 23, 25]. Apoptosis induction is generally believed to be an irreversible commitment to cell death and is often the most targeted process for anti-cancer drug development. Therefore, its role in apoptosis regulation sheds light on the importance of MED12 alterations in glioblastoma.
Understanding the oncogenic effects of MED12 modulation in glioblastoma, we next wanted to check the possible interactions of MED12 with transcription regulators to decipher the mechanistic landscape of its de-regulation in glioblastoma. Mediators are often referred to as relay molecules passing transcription on/off signals from stimuli-induced transcription factors to the downstream cellular machinery. MED12 drives downstream expression of target genes in Wnt pathway by physically interacting with β-catenin in uterine leiomyomas while it modulates TGFBR2 cell surface expression in lung cancer and therefore controls multi-drug response. In ovarian cancer, it drives expression through EGFR promoter and its loss causes tumor dormancy [17, 20, 24]. The presence of unique structural motifs in the MED12 protein makes it a suitable interaction partner with effectors of these pathways. One feature of MED12 protein is the presence of two overlapping LxxLL motifs at its N terminal. LxxLL motif is the standard nuclear hormone receptor recognition signature. Other than MED12, LxxLL motif is present in MED1. TRα and VDR as well as many other nuclear receptors that include TRβ, ERα/β, PPARα/γ, GR, AR, RARα and RXRα physically interact with MED1.Work from our lab reported that MED1 promotes ER-dependent oncogenic programs in breast cancer [41, 42]. Incidentally, whole transcriptome analysis upon MED12 knockdown revealed vitamin D receptor pathway, which is a nuclear receptor, to be one of the key pathways affected by MED12 in glioblastoma cells. Since, MED12 has been shown to regulate transcription by physically interacting with transcription factors, we used publicly available protein interaction databases like The Biological General Repository for Interaction Datasets (BioGRID) to check for a possible link between VDR and MED12 and found that vitamin D receptor has been reported to have physical interaction with MED12 by three independent studies. Thus, we focussed our attention on studying the VDR-MED12 axis. We performed docking studies and found the potential points of physical interaction between MED12 and VDR. VDR has a high binding affinity for MED12 with a docking score of − 303.55 kcal/mol. These two proteins interact with 16 hydrophobic and 7 strong hydrogen bonds suggesting that that VDR has a good binding affinity to MED12. Subsequently, we confirmed a physical interaction between the two by co-immunoprecipitation experiment. Thus, we identify vitamin D receptor pathway to be one of the key pathways regulated by MED12 in glioblastoma and physical protein–protein interaction between the two as a possible means of regulation. Other than VDR, nuclear receptors like ESR1, ESR2 and THRA are also predicted interactors of MED12 (BioGRID), however, gene expression analysis post MED12 knockdown did not reveal these receptor pathways to be among the top regulated pathways by MED12 in glioblastoma.
To understand, how VDR and MED12 interaction affects glioblastoma, we looked at the downstream genes of the pathway that MED12 positively regulates. One of these genes is BCL6 which is a very well characterised oncogene in glioblastoma and in several other cancers. It is over-expressed and correlated with poor survival in glioblastoma patients. BCL6 is known for its apoptosis inhibitory effects. In glioblastoma too, it promotes tumorigenesis by inhibiting apoptosis and promoting cell proliferation [37, 38, 43–45]. Since MED12 also showed strong oncogenic effects in glioblastoma cells, we were interested to check if BCL6 up-regulation might be one of the contributing factors through which MED12 exerts its oncogenic effects. We first checked if MED12 modulation could affect the transcription of BCL6 through VDR. We found that MED12 regulates BCL6 expression by modulating VDR binding on BCL6 promoter. BCL6, being a strong transcription repressor, is reported to suppress p53 pathway in glioblastoma, thereby inhibiting apoptosis. We found that MED12 too inhibits p53 expression in glioblastoma. The P53 pathway is de-regulated in 84% of glioblastoma patients [46], highlighting its importance in tumor maintenance and dissemination. Restoration of p53 to induce cell cycle arrest, senescence and apoptosis is a promising approach in precision medicine. Therefore, the involvement of MED12 in the BCL6-p53 axis opens a new understanding of the molecular landscape of the disease. To further study the MED12-BCL6-p53 axis in glioblastoma we performed simultaneous knockdown of MED12 and over-expression of BCL6 in glioblastoma cells to check if the effects of MED12 knockdown in p53 expression and apoptosis could be rescued by BCL6 over-expression and found that p53 and apoptosis inhibition by MED12 is BCL6 mediated. Thus, we conclude that the oncogenic effects of MED12 in glioblastoma might be partially mediated by BCL6. Having said that, it should be mentioned that the MED12-VDR axis is one of the several pathways by which MED12 regulates cancer hallmarks in glioblastoma. Transcriptome studies post MED12 knockdown in glioblastoma cells revealed other pathways such as the VEGF-VEGFR2, Rig like receptor pathway, Interferon alpha/beta signaling pathway affected as well (Electronic supplementary material 3), hinting towards the possibility that MED12 would exert its oncogenic effects through a network of various signaling cascades in glioblastoma. Similarly, we have focussed our attention on exploring MED12-BCL6 axis, however, we cannot deny the possibility that other genes of the VDR pathway might also be involved in mediating the oncogenic effects of MED12.
These observations give a proof of concept for novel molecular targets in glioblastoma. Although cancer is a consequence of multiple molecular aberrations, years of research point out that inactivation of even a single oncogene could lead to significant tumor regression, which highlights the importance of molecular characterization of de-regulated genes in cancer. Our work is a step in this direction: we identify a new oncogene, MED12, in glioblastoma as a promising target for future studies in the context of glioblastoma pathogenesis.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Detailed methods employed to perform cellular assays (DOCX 14 KB)
Supplementary file2 List of differentially regulated genes (p-value < 0.05, fold change > or = + -1.5) obtained by gene expression profiling in A172 cells upon MED12 knockdown (XLSX 31 KB)
Supplementary file3 Results of pathway analysis of differentially regulated genes (p-value < 0.05, fold change > or = + -1.5) obtained by gene expression profiling in A172 cells upon MED12 knockdown (XLSX 15 KB)
Supplementary file4 Supplementary tables with list of primers, list of amino acids in MED12 with VDR having hydrogen bond interactions and list of amino acids in MED12 with VDR having hydrophobic interactions. (DOCX 18 KB)
Supplementary file5 Supplementary Fig. 1: (1–10) IHC Images of glioma patients (antibody used: HPA003184) showing medium (n = 2) to high (n = 8) expression in MED12 protein. Images were retrieved from the Human Protein Atlas (TIF 21424 KB)
Supplementary file6 Supplementary Fig. 2: MED12 transcript levels were measured 48 h post-transfection in GBM cells (a) qRT-PCR data showing knockdown of MED12 transcript levels in A172 cell line upon transfection with MED12 specific siRNA (Sigma). (b) qRT-PCR data show induction in MED12 transcript levels upon transfection with MED12 over-expression construct in A172 cell line. (c) qRT-PCR data showing knockdown of MED12 transcript levels in T98G cell line upon transfection with MED12 specific siRNA (Sigma). (d) qRT-PCR data shows induction in MED12 transcript levels upon transfection with MED12 over-expression construct in T98G cell line (TIF 21424 KB)
Supplementary file7 Supplementary Fig. 3: (a, b) Graph showing fold change in expression of genes of VDR pathway analysed by qRT-PCR post MED12 over-expression in (a) A172 and (b) T98G cells (TIF 21424 KB)
Supplementary file8 Supplementary Fig. 4: MED12 is enhanced in IDH mutant tumors. Data analysis from GlioVis showed enhanced expression of MED12 in IDH mutant tumors as compared to IDH wild type tumors across various datasets (a) analysis of CGGA patient dataset (b) analysis of TCGA_GBMLGG patient dataset (c) analysis of Gravendeel patient dataset (d) analysis of Bao patient dataset (TIF 21424 KB)
Supplementary file9 Supplementary Fig. 5: BCL6 inhibits p53 expression in GBM cells. Western Blotting data to show inhibition in p53 levels post BCL6 overexpression in (a) A172 cells (b) T98G cells. The western blotting experiment was performed in duplicates. The actin blot was run on a different gel (TIF 7142 KB)
Supplementary file10 Supplementary Fig. 6: Inhibition of apoptosis by MED12 is BCL6 mediated: Simultaneous over-expression of BCL6 and MED12 knockdown was performed in A172 and T98G cells. (a, b) Western blotting results showing effects of MED12 knockdown and BCL6 over-expression alone or in combination on cleaved PARP levels in (a) A172 cells (b) T98G cells. The western blotting experiment was performed in duplicates. (c) FACS based detection of phosphatidylserine externalisation was checked in A172 cells post simultaneous over-expression of BCL6 and MED12 (TIF 21424 KB)
Acknowledgements
SS thanks Ministry of Human Resource and Development (MHRD), Govt. of India for senior research fellowship. HM thanks Department of Science and Technology, Govt. of India for post-doctoral fellowship. VS thanks Department of Biotechnology, Govt. of India for the post-doctoral fellowship. CS thanks Science and Engineering Research Board (SERB), Govt. of India for the JC Bose fellowship.
Abbreviations
- CDS
Coding sequence
- VDR
Vitamin D receptor
- qRT-PCR
Quantitative real time polymerase chain reaction
- T-ALL
T-cell acute lymphoblastic leukemia
- ESR1
Estrogen receptor alpha
- ESR2
Estrogen receptor beta
- THRA
Thyroid hormone receptor alpha
- siRNA
Small interfering RNA
- MED
Mediator
- MTT
3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide
- TCGA
The Cancer Genome Atlas
- CGGA
Chinese Glioma Genome Atlas
- GBM
Glioblastoma
- TGFBR2
Transforming growth factor β receptor type 2
Author contributions
RK conceptualized and coordinated the whole study. SS performed all the cell line experiments and data analyses of patient data. HM and SS performed co-immunoprecipitation experiments. HM performed docking studies.VS1, VS2 and CS performed analyses in the Indian GBM Patient samples. SS, HM and RK wrote the manuscript.
Funding
RK and CS thank Department of Biotechnology (DBT), Government of India for financial support (BT/PR16851/MED/122/45/2016). CS also thanks the Science and Engineering Research Board, Govt. of India for the JC Bose fellowship.
Data availability
Upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing financial or other interest in relation to this work.
Ethics approval
The study was approved by the Ethics committee (Ref. No. IEC-130/07.04.2017, RP-24/2017) of All India Institute of Medical Sciences, New Delhi, and informed consent was obtained from the patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Donner AJ, Szostek S, Hoover JM, Espinosa JM. CDK8 is a stimulus-specific positive coregulator of p53 target genes. Mol Cell. 2007;27(1):121–133. doi: 10.1016/j.molcel.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galbraith MD, Donner AJ, Espinosa JM. CDK8: a positive regulator of transcription. Transcription. 2010;1(1):4–12. doi: 10.4161/trns.1.1.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fj A, Yw J, Lc M, Cm G, Rd K. Conserved structures of mediator and RNA polymerase II holoenzyme. Science (New York, NY) 1999;283:5404. doi: 10.1126/science.283.5404.985. [DOI] [PubMed] [Google Scholar]
- 4.Daniels L, D. Mutual exclusivity of MED12/MED12L, MED13/13L, and CDK8/19 paralogs revealed within the CDK-mediator kinase module. J Proteom Bioinform. 2013;01:S2. doi: 10.4172/jpb.S2-004. [DOI] [Google Scholar]
- 5.Allen BL, Taatjes DJ. The mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol. 2015;16(3):155. doi: 10.1038/nrm3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fant CB, Taatjes DJ. Regulatory functions of the mediator kinases CDK8 and CDK19. Transcription. 2018;10(2):76–90. doi: 10.1080/21541264.2018.1556915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirst M, Kobor MS, Kuriakose N, Greenblatt J, Sadowski I. GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8. Mol Cell. 1999;3(5):673–678. doi: 10.1016/S1097-2765(00)80360-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, Feng D, Wang Q, Abdulla A, Xie X-J, Zhou J, Sun Y, Yang ES, Liu L-P, Vaitheesvaran B, Bridges L, Kurland IJ, Strich R, Ni J-Q, Wang C, Ericsson J, Pessin JE, Ji J-Y, Yang F. Regulation of lipogenesis by cyclin-dependent kinase 8-mediated control of SREBP-1. J Clin Investig. 2012;122(7):2417. doi: 10.1172/JCI61462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cj H, Ve M, Sm L, Cj W, Ss K, Ra Y. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol Cell. 1998;2:1. doi: 10.1016/s1097-2765(00)80112-4. [DOI] [PubMed] [Google Scholar]
- 10.Akoulitchev S, Chuikov S, Reinberg D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature. 2000;407(6800):102–106. doi: 10.1038/35024111. [DOI] [PubMed] [Google Scholar]
- 11.Liang J, Chen M, Hughes D, Chumanevich AA, Altilia S, Kaza V, Lim C-U, Kiaris H, Mythreye K, Pena MM, Broude EV, Roninson IB. CDK8 selectively promotes the growth of colon cancer metastases in the liver by regulating gene expression of TIMP3 and matrix metalloproteinases. Can Res. 2018;78(23):6594–6606. doi: 10.1158/0008-5472.CAN-18-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bancerek J, Poss ZC, Steinparzer I, Sedlyarov V, Pfaffenwimmer T, Mikulic I, Dölken L, Strobl B, Müller M, Taatjes DJ, Kovarik P. CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity. 2013;38(2):250. doi: 10.1016/j.immuni.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alarcón C, Zaromytidou A-I, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, Sapkota G, Pan D, Massagué J. Nuclear CDKs drive smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell. 2009;139(4):757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poss ZC, Ebmeier CC, Taatjes DJ. The mediator complex and transcription regulation. Crit Rev Biochem Mol Biol. 2013 doi: 10.3109/10409238.2013.840259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava S, Kulshreshtha R. Insights into the regulatory role and clinical relevance of mediator subunit, MED12, in human diseases. J Cell Physiol. 2020;236(5):3163–3177. doi: 10.1002/jcp.30099. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, O’Regan R, Xu W. The emerging role of mediator complex subunit 12 in tumorigenesis and response to chemotherapeutics. Cancer. 2020;126(5):939–948. doi: 10.1002/cncr.32672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Andaloussi A, Al-Hendy A, Ismail N, Boyer TG, Halder SK. Introduction of somatic mutation in MED12 induces Wnt4/β-catenin and disrupts autophagy in human uterine myometrial cell. Reprod Sci. 2020;27:823–832. doi: 10.1007/s43032-019-00084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu B, Słabicki M, Sellner L, Dietrich S, Liu X, Jethwa A, Hüllein J, Walther T, Wagner L, Huang Z, Zapatka M, Zenz T. MED12 mutations and NOTCH signalling in chronic lymphocytic leukaemia. Br J Haematol. 2017;179:421–429. doi: 10.1111/bjh.14869. [DOI] [PubMed] [Google Scholar]
- 19.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat J-P, White TA, Stojanov P, Van Allen E, Stransky N, Nickerson E, Chae S-S, Boysen G, Auclair D, Onofrio RC, Park K, Kitabayashi N, MacDonald TY, Sheikh K, Garraway LA. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo X-L, Deng C, Su X, Wang F, Chen Z, Wu X-P, Liang S-B, Liu J, Fu L. Loss of MED12 induces tumour dormancy in human epithelial ovarian cancer via downregulation of EGFR. Cancer Res. 2018 doi: 10.1158/0008-5472.CAN-18-0134. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Zeng H, Wang Q, Zhao Z, Boyer TG, Bian X, Xu W. MED12 methylation by CARM1 sensitizes human breast cancer cells to chemotherapy drugs. Sci Adv. 2015;1:e1500463. doi: 10.1126/sciadv.1500463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciebiera M, Ali M, Zgliczyńska M, Skrzypczak M, Al-Hendy A. Vitamins and uterine fibroids: current data on pathophysiology and possible clinical relevance. Int J Mol Sci. 2020;21(15):5528. doi: 10.3390/ijms21155528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu M, Wang F, Li G, Wang X, Fang X, Jin H, Chen Z, Zhang J, Fu L. MED12 exerts an emerging role in actin-mediated cytokinesis via LIMK2/cofilin pathway in NSCLC. Mol Cancer. 2019;18:93. doi: 10.1186/s12943-019-1020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang S, Hölzel M, Knijnenburg T, Schlicker A, Roepman P, McDermott U, Garnett M, Grernrum W, Sun C, Prahallad A, Groenendijk FH, Mittempergher L, Nijkamp W, Neefjes J, Salazar R, ten Dijke P, Uramoto H, Tanaka F, Beijersbergen RL, Wessels LFA, Bernards R. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signalling. Cell. 2012;151:937–950. doi: 10.1016/j.cell.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaikhibrahim Z, Offermann A, Braun M, Menon R, Syring I, Nowak M, Halbach R, Vogel W, Ruiz C, Zellweger T, Rentsch CA, Svensson M, Andren O, Bubendorf L, Biskup S, Duensing S, Kirfel J, Perner S. MED12 overexpression is a frequent event in castration-resistant prostate cancer. Endocr Relat Cancer. 2014;21:663–675. doi: 10.1530/ERC-14-0171. [DOI] [PubMed] [Google Scholar]
- 26.Crown J. CDK8: A new breast cancer target. Oncotarget. 2017;8(9):14269. doi: 10.18632/oncotarget.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu W, Wang C, Li W, Hsu F-N, Tian L, Zhou J, Yuan C, Xie X-J, Jiang T, Addya S, Tai Y, Kong B, Ji J-Y. Tumor-suppressive effects of CDK8 in endometrial cancer cells. Cell Cycle. 2013;12(6):987–999. doi: 10.4161/cc.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapoor A, Goldberg MS, Cumberland LK, Ratnakumar K, Segura MF, Emanuel PO, Menendez S, Vardabasso C, LeRoy G, Vidal CI, Polsky D, Osman I, Garcia BA, Hernando E, Bernstein E. The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature. 2010;468(7327):1105. doi: 10.1038/nature09590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li N, Fassl A, Chick J, Inuzuka H, Li X, Mansour MR, Liu L, Wang H, King B, Shaik S, Gutierrez A, Ordureau A, Otto T, Kreslavsky T, Baitsch L, Bury L, Meyer CA, Ke N, Mulry KA, Sicinski P. Cyclin C is a haploinsufficient tumour suppressor. Nat Cell Biol. 2014;16(11):1080–1091. doi: 10.1038/ncb3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohata N, Ito S, Yoshida A, Kunisada T, Numoto K, Jitsumori Y, Kanzaki H, Ozaki T, Shimizu K, Ouchida M (2006) Highly frequent allelic loss of chromosome 6q16–23 in osteosarcoma: Involvement of cyclin C in osteosarcoma. Int J Mol Med 18(6). https://pubmed.ncbi.nlm.nih.gov/17089020/ [PubMed]
- 31.Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res. 2014;23:10. doi: 10.1158/1055-9965.EPI-14-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stupp R, Mason WP, van Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. Mass Med Soc. 2009 doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:7412. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee G, Auffinger B, Guo D, Hasan T, Deheeger M, Tobias AL, Kim JY, Atashi F, Zhang L, Lesniak MS, James CD. Dedifferentiation of glioma cells to glioma stem-like cells by therapeutic stress-induced HIF signaling in the recurrent GBM model. Mol Cancer Therap. 2016;15:12. doi: 10.1158/1535-7163.MCT-15-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noch EK, Ramakrishna R, Magge R. Challenges in the treatment of glioblastoma: multisystem mechanisms of therapeutic resistance. World Neurosurg. 2018;116:505–517. doi: 10.1016/j.wneu.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Shukla A, Srivastava S, Darokar J, Kulshreshtha R. HIF1α and p53 regulated MED30, a mediator complex subunit, is involved in regulation of glioblastoma pathogenesis and temozolomide resistance. Cell Mol Neurobiol. 2020 doi: 10.1007/s10571-020-00920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu L, Chen Y, Dutra-Clarke M, Mayakonda A, Hazawa M, Savinoff SE, Doan N, Said JW, Yong WH, Watkins A, Yang H, Ding L-W, Jiang Y-Y, Tyner JW, Ching J, Kovalik J-P, Madan V, Chan S-L, Müschen M, Koeffler HP. BCL6 promotes glioma and serves as a therapeutic target. Proc Natl Acad Sci USA. 2017;114(15):3981. doi: 10.1073/pnas.1609758114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabre M-S, Stanton NM, Slatter TL, Lee S, Senanayake D, Gordon RMA, Castro ML, Rowe MR, Taha A, Royds JA, Hung N, Melnick AM, McConnell MJ. The oncogene BCL6 is up-regulated in glioblastoma in response to DNA damage, and drives survival after therapy. PLoS One. 2020;15(4):e0231470. doi: 10.1371/journal.pone.0231470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark AD, Oldenbroek M, Boyer TG. Mediator kinase module and human tumorigenesis. Crit Rev Biochem Mol Biol. 2015;50(5):393. doi: 10.3109/10409238.2015.1064854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J-R, Yao Y, Xu H-Z, Qin Z-Y. Isocitrate dehydrogenase (IDH)1/2 mutations as prognostic markers in patients with glioblastomas. Medicine. 2016;95(9):e2583. doi: 10.1097/MD.0000000000002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, Roeder RG. Mediator-dependent nuclear receptor function. Semin Cell Dev Biol. 2011;22(7):749–758. doi: 10.1016/j.semcdb.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagpal N, Sharma S, Maji S, Durante G, Ferracin M, Thakur JK, Kulshreshtha R. Essential role of MED1 in the transcriptional regulation of ER-dependent oncogenic miRNAs in breast cancer. Sci Rep. 2018;8(1):11805. doi: 10.1038/s41598-018-29546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nurminen V, Neme A, Ryynanen J, Heikkinen S, Seuter S, Carlberg C. The transcriptional regulator BCL6 participates in the secondary gene regulatory response to vitamin D. Biochim Biophys Acta. 2015;1849:3. doi: 10.1016/j.bbagrm.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Vukić M, Neme A, Seuter S, Saksa N, de Mello VDF, Nurmi T, Uusitupa M, Tuomainen T-P, Virtanen JK, Carlberg C. Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS One. 2015;10(4):e0124339. doi: 10.1371/journal.pone.0124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warwick T, Schulz MH, Günther S, Gilsbach R, Neme A, Carlberg C, Brandes RP, Seuter S. A hierarchical regulatory network analysis of the vitamin D induced transcriptome reveals novel regulators and complete VDR dependency in monocytes. Sci Rep. 2021;11(1):1–16. doi: 10.1038/s41598-021-86032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Dube C, Gibert M, Cruickshanks N, Wang B, Coughlan M, Yang Y, Setiady I, Deveau C, Saoud K, Grello C. The p53 pathway in glioblastoma. Cancers. 2018;10:9. doi: 10.3390/cancers10090297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowman RL, Wang Q, Carro A, Verhaak RGW, Squatrito M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro Oncol. 2017;19(1):139. doi: 10.1093/neuonc/now247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizuno H, Kitada K, Nakai K, Sarai A. PrognoScan: a new database for meta-analysis of the prognostic value of genes. BMC Med Genom. 2009;2(1):1–11. doi: 10.1186/1755-8794-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thul PJ, Lindskog C. The human protein atlas: a spatial map of the human proteome. Prot Sci Publ Prot Soc. 2018;27(1):233. doi: 10.1002/pro.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stark C, Breitkreutz B-J, Reguly T, Boucher L, Breitkreutz A, Tyers M. BioGRID: a general repository for interaction datasets. Nucleic Acids Res. 2006;34(Database issue):D535. doi: 10.1093/nar/gkj109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Detailed methods employed to perform cellular assays (DOCX 14 KB)
Supplementary file2 List of differentially regulated genes (p-value < 0.05, fold change > or = + -1.5) obtained by gene expression profiling in A172 cells upon MED12 knockdown (XLSX 31 KB)
Supplementary file3 Results of pathway analysis of differentially regulated genes (p-value < 0.05, fold change > or = + -1.5) obtained by gene expression profiling in A172 cells upon MED12 knockdown (XLSX 15 KB)
Supplementary file4 Supplementary tables with list of primers, list of amino acids in MED12 with VDR having hydrogen bond interactions and list of amino acids in MED12 with VDR having hydrophobic interactions. (DOCX 18 KB)
Supplementary file5 Supplementary Fig. 1: (1–10) IHC Images of glioma patients (antibody used: HPA003184) showing medium (n = 2) to high (n = 8) expression in MED12 protein. Images were retrieved from the Human Protein Atlas (TIF 21424 KB)
Supplementary file6 Supplementary Fig. 2: MED12 transcript levels were measured 48 h post-transfection in GBM cells (a) qRT-PCR data showing knockdown of MED12 transcript levels in A172 cell line upon transfection with MED12 specific siRNA (Sigma). (b) qRT-PCR data show induction in MED12 transcript levels upon transfection with MED12 over-expression construct in A172 cell line. (c) qRT-PCR data showing knockdown of MED12 transcript levels in T98G cell line upon transfection with MED12 specific siRNA (Sigma). (d) qRT-PCR data shows induction in MED12 transcript levels upon transfection with MED12 over-expression construct in T98G cell line (TIF 21424 KB)
Supplementary file7 Supplementary Fig. 3: (a, b) Graph showing fold change in expression of genes of VDR pathway analysed by qRT-PCR post MED12 over-expression in (a) A172 and (b) T98G cells (TIF 21424 KB)
Supplementary file8 Supplementary Fig. 4: MED12 is enhanced in IDH mutant tumors. Data analysis from GlioVis showed enhanced expression of MED12 in IDH mutant tumors as compared to IDH wild type tumors across various datasets (a) analysis of CGGA patient dataset (b) analysis of TCGA_GBMLGG patient dataset (c) analysis of Gravendeel patient dataset (d) analysis of Bao patient dataset (TIF 21424 KB)
Supplementary file9 Supplementary Fig. 5: BCL6 inhibits p53 expression in GBM cells. Western Blotting data to show inhibition in p53 levels post BCL6 overexpression in (a) A172 cells (b) T98G cells. The western blotting experiment was performed in duplicates. The actin blot was run on a different gel (TIF 7142 KB)
Supplementary file10 Supplementary Fig. 6: Inhibition of apoptosis by MED12 is BCL6 mediated: Simultaneous over-expression of BCL6 and MED12 knockdown was performed in A172 and T98G cells. (a, b) Western blotting results showing effects of MED12 knockdown and BCL6 over-expression alone or in combination on cleaved PARP levels in (a) A172 cells (b) T98G cells. The western blotting experiment was performed in duplicates. (c) FACS based detection of phosphatidylserine externalisation was checked in A172 cells post simultaneous over-expression of BCL6 and MED12 (TIF 21424 KB)
Data Availability Statement
Upon reasonable request.