Abstract
Gremlin-1 is part of the TGF-β superfamily and is a BMP antagonist that blocks BMP signalling to precisely control BMP gradients. Gremlin-1 is primarily involved in organogenesis and limb patterning however, has recently been described as being involved in fibrotic diseases. Initially described as a key factor involved in diabetic kidney fibrosis due to being induced by high glucose, it has now been described as being associated with lung, liver, eye, and skin fibrosis. This suggests that it is a key conserved molecule mediating fibrotic events irrespective of organ. It appears that Gremlin-1 may have effects mediated by BMP-dependent and independent pathways. The aim of this review is to evaluate the role of Gremlin-1 in fibrosis, its mechanisms and if this can be targeted therapeutically in fibrotic diseases, which currently have very limited treatment options and are highly prevalent.
Keywords: Gremlin-1, BMP, VEGF, Fibrosis, Myofibroblasts
Introduction
Gremlin-1 is a bone morphogenetic protein (BMP) antagonist containing a cysteine knot, that is part of the cysteine knot superfamily [1]. Gremlin-1 regulates BMP signalling and is critical in organ formation and limb patterning. As well as its role in organ formation, it has been associated with specific cancers [2] and in recent years organ fibrosis including, kidney fibrosis [3], lung fibrosis [4], liver fibrosis [5] and recently we identified this involved in skin fibrosis in systemic sclerosis [6] (Fig. 1). It appears that the reactivation of Gremlin-1 in these ‘wound healing’ situations leads to organ fibrosis and may be a conserved response irrespective of organ system affected. Various different organs in fibrosis appear to be affected by Gremlin-1 and may all converge on gremlin-1 regardless of aetiology. The aim of this review is to give an overview of Gremlin-1 in fibrosis, its regulation and possible therapeutic targeting.
Fig. 1.
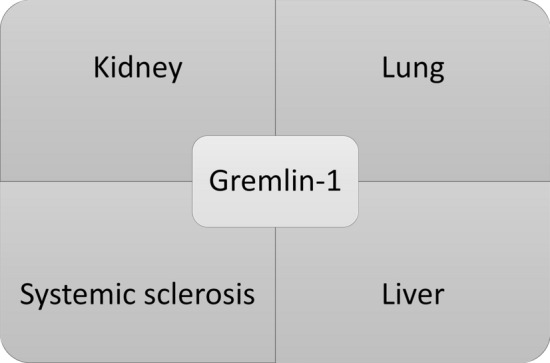
Main fibrotic diseases in which Gremlin-1 has been implicated as playing a cardinal role
Gremlin-1 protein
Gremlin-1 is part of the family of BMP antagonists that include noggin, chordin and follistatin and follistatin-like proteins [1]. These antagonists of BMPs are highly regulated in a temporospatial manner. Interestingly, when comparing amino acid sequences no significant similarities are found. Most BMP antagonists are classified based on their cysteine-knot size [1] and Gremlin-1 belongs to the Differential screening-selected gene Aberrative in Neuroblastoma (DAN) family. Human Gremlin-1 gene has been mapped to chromosome 15q13-15 [7].
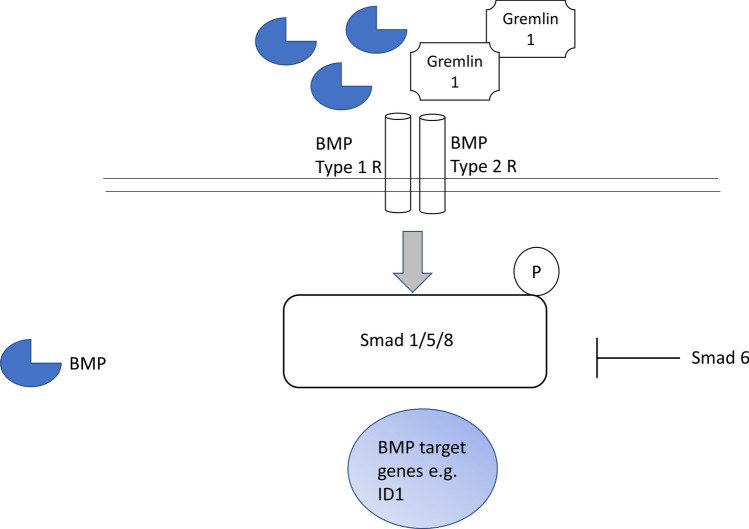
Predicted amino acid sequences have revealed sites for glycosylation and sites for phosphorylation. Gremlin-1 preferentially binds BMP 2 and BMP 4 [8], this leads to inhibition of canonical BMP signalling by preventing phosphorylation of SMAD1/5/8 and gene expression (Fig. 2). In common with the TGF-β cytokines, BMPs induce their effects through interaction with type I and type II cell surface receptors that activates the serine/threonine kinases. Type I receptors (ALK2, ALK3, ALK6) and Type II receptors (BMPR2, ActRIIa, ActR2b) when bound the BMP ligand form together Type I receptor-mediated phosphorylation [9].
Fig. 2.
BMP signalling pathway. BMP signalling is induced by binding to its receptors both type I and type II that leads to phosphorylation of R-Smads 1/5/8 which leads to translocation to the nucleus and eventually to BMP-dependent gene expression and protein products. Smad 6 is an inhibitory Smad that inhibits BMP signalling. Negative regulation can occur through extracellular Gremlin-1 which is a natural antagonist of this pathway by sequestering BMPs
Functionally Inhibition impairs BMP signalling maintaining the tightly balanced gradient of morphogens. Gremlin-1 has a molecular weight of 20.7KDa but runs at around 24-25KDa due to glycosylation. It is clear that Gremlin-1 is critical for organogenesis [10] and is clearly involved in kidney formation and kidney fibrosis, with gremlin-1 KO mice dying of renal aplasia [11–13]. Also siRNA knockdown of Gremlin-1 reduced fibrosis in a diabetic mouse model of kidney inflammation and fibrosis [14]. Although classically defined as a BMP antagonist that regulates BMP signalling through inhibition a few other studies have described Gremlin-1 as having BMP-independent signalling [15]. Mitola et al. have described Gremlin-1 as being a VEGFR2 agonist at least in endothelial cells [15]. Others have noted EGFR activation [16] in relation to cancer. Although an exhaustive study has recently not found activation of VEGFR2 by Gremlin-1 on endothelial cells or augmentation of VEGFR by Gremlin-1 [17]. Another study suggested that Gremlin-1 mediated upregulation of Nrf2 via VEGF activation [18]. Thus, inconsistencies in the literature currently exist. It has also been demonstrated that Gremlin-1 is an inhibitor of macrophage migration inhibitory factor [19] in specific contexts. The current accepted view is that primarily Gremlin-1 is a BMP antagonist.
Furthermore, it is now clear that Gremlin-1 plays a key role in maintaining the stem cell niche [20] and most recently was associated with multiple myeloma [21]. Thus Gremlin-1 is a multifunctional protein with a variety of functions that are likely context and cell-dependent.
Fibrosis
Fibrosis represents a final common end point in a variety of organs and depending on the organ affected can be fatal. It is the result of a wound healing response that is in response to repeated injury. The injury can take the form of multiple different types, e.g., in the liver it may be viral in the case of HCV or through toxins or in the skin it could be due to physical trauma and this sets in motion a wound healing response that fails to terminate. In a general sense if this termination does not cease the organ affected may cease to function appropriately. The process may be associated with an inflammatory response but the main cell type involved is the myofibroblast. This is a critical cell type that is generated from either a quiescent fibroblast or epithelial cells in response to injury or stress [22], 23. The myofibroblast is critically involved as its phenotype supports fibrosis through increased contractility altering tissue architecture and increased expression of α-smooth muscle actin. Myofibroblasts also secrete copious amounts of ECM protein and have reduced matrix proteases also. The net effect being ECM deposition. One of the key features also is that they are endowed with resistance to apoptosis [22]. TGF-β1 has been the archetypal cytokine that regulates transition to fibrosis but other factors including epigenetics could also be operative [24]. Currently limited therapies exist.
Gremlin and fibrosis
Gremlin-1 was first described associated with kidney development and the development of kidney fibrosis associated with diabetic kidney disease [3] and indeed is upregulation very robustly in response to high glucose [3]. Interestingly, in normal adult kidney it is not expressed and expressed only in kidney disease [25]. Moreover a disease Single Nucleotide Polymorphism (SNP) in the Grem1 gene is associated with diabetic kidney disease [26]. There appears to be a relationship between Gremlin-1 expression and NOTCH expression [27] in diabetic kidney fibrosis, suggesting that Gremlin-1 regulates NOTCH signalling to facilitate fibrosis.
Mice with allelic depletion of Gremlin-1 have reduced kidney fibrosis [11]. Homozygous KO mice are lethal due to developmental abnormalities. Church et al. developed a Tubular epithelia cell Gremlin-1 knockout mouse using the ksp-cadherin-Cre mouse and they showed that tubular epithelial cell KO of Gremlin-1 protected mice from kidney fibrosis [28]. This model circumvents the issue with global KO of Gremlin-1. Conversely tubular-specific overexpression of Gremlin-1 aggravates diabetic kidney fibrosis [29]. In cell culture of HK2 cells overexpression of Gremlin-1 led to an enhanced TGF-β response and in response to TGF-β1 there was a prolonged phosphorylation of Smad3 in the overexpressed cells suggesting an enhancement of Smad3 signalling [30].
Mechanistically it has been implied that kidney fibrosis mediated by Gremlin-1 protein is facilitated through the Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) leading to downstream gene activation [31]. However, an exhaustive study subsequently could find no activation (or inhibition) of the VEGFR in endothelial cells [17]. Thus further clarification of the role-if any- of VEGFR in Gremlin-1 effects is required. Other studies in kidney cells have implied that Gremlin-1-mediated fibrosis is through canonical Smad2/3 signalling [32], which is classic signalling downstream of TGF-β. Although other Smads, in other cells, can be phosphorylated via TGF-β. Furthermore, overexpression of Gremlin-1 in kidneys of mice led to fibrosis in these animals and activation of VEGFR2 and the inflammatory transcription factor nf-kb [33].
Lung fibrosis
The first study to demonstrate an association with Gremlin-1 and lung fibrosis was in 2006 and this study examined Gremlin-1 expression in Idiopathic pulmonary fibrosis (IPF) [4]. IPF is a progressive form of lung fibrosis that is associated with no known cause. Koli et al. demonstrated higher levels of Gremlin-1 in IPF and that it is induced itself by TGF-β [4]. Furthermore, overexpression by plasmids of Gremin-1 in A549 cells sensitized them to become mesenchymal [4]. Higher levels of Gremlin-1 could be found in IPF tissue compared to non-specific pneumonia [34]. Using a model of asbestos-induced fibrosis, it was found that Gremlin-1 was upregulated significantly coincident with reduced BMP signalling [35]. Moreover, in human bronchial epithelial cells Gremlin-1 was induced by incubation with asbestos. Transient overexpression of Gremlin-1 in rat lungs using adenoviral vectors resulted in reduced BMP signalling and a transient fibrosis associated with activation of epithelial cells [36]. A further study identified elevated Gremlin-1 in IPF tissue and the bleomycin model of lung fibrosis [37]. Gremlin-1 was also found to be regulated in IPF fibroblasts by IL-6 [38], an effect we had seen in skin also [6] suggesting, at least in some situations, that inflammation may regulate Gremlin-1.
Skin fibrosis/systemic sclerosis
We first identified that Gremln-1 was involved in skin fibrosis associated with a disease called systemic sclerosis (SSc). SSc is an autoimmune rheumatic skin disease in which there is inflammation and fibrosis of the skin, and in some individuals, the lung [23, 24]. Key to the fibrosis, like all fibrosis, is the myofibroblast cell type that is activated and secretes copious amounts of ECM. We demonstrated that Interleukin-6 trans signalling mediated increased collagen in skin fibroblasts via Gremlin-1 [6]. This appeared to be associated with Signal Transducer and Activator of Transcription 3 (STAT3) activation [6]. More recently using an overexpression system we could show that overexpression of Gremlin-1 led to elevated ECM and that this was associated with the TGF-β pathway and neutralisation of TGF-β or TGF-β receptors led to reduced collagen expression [39]. Interestingly, although Gremlin-1 appeared to be dependent on STAT3 via IL-6 signalling it was not upregulated by IL-11, another of the IL-6 family cytokines [39]. Furthermore, we recently demonstrated elevated levels of Gremlin-1 in the sera of SSc patients, particularly those with interstitial lung disease [40].
Liver fibrosis
The first study to identify Gremlin-1 upregulation in liver fibrosis was in 2006 [5]. Boers et al. used transcriptomics of activated hepatic stellate cells and found transcripts belonging to Gremlin-1 to be upregulated compared to healthy controls [5]. This was also recapitulated in mice models of liver fibrosis [5], suggesting that gremlin-1 is critical in liver fibrosis. This was further validated and in hepatic stellate cells it appears that Gremlin-1 activated these cells through and upregulation of TGF-β. This indicates that Gremlin-1 mediated liver fibrosis may, at least in part, be mediated by TGF-β.
Furthermore activation in vitro of hepatocytes to stellate cells, the cells responsible for ECM deposition and cell contraction, could be induced by Gremlin-1 [41]. Gremlin-1 was found to be regulated epigenetically by microRNA27a/b that will bind to the 3’ Untranslated Region (UTR) of Gremlin-1 [41] which would lead to its degradation as microRNAs are negative regulators of gene expression [42]. In a further animal study of experimental liver fibrosis, Gremlin-1 was elevated [43].
Pancreatic fibrosis
Gremlin-1 has been found to be associated with pancreatic fibrosis in a similar way to liver fibrosis. In mouse models of pancreatic fibrosis, Gremlin-1 was found to be hugely upregulated in these animal models [44]. In a heterozygous Grem1 KO mouse, experimental pancreatic fibrosis was reduced by over 30% [44]. Suggesting that Gremlin-1 is very important in pancreatic fibrosis. In vitro, in isolated cells they found that TGF-β induced Gremlin-1 expression in pancreatic cells [15, 44].
Intestinal fibrosis
Most recently a role form Gremlin-1 has been described in intestinal fibrosis- at least in an pre clinical animal model of intestinal fibrosis. It was demonstrated in the Dextran Sodium Sulfate (DSS) model of fibrosis—a standard inflammatory colitis model that can lead to fibrosis—that the levels of Gremlin-1 mRNA and protein where significantly elevated [45]. Furthermore, in isolated cell lines human-derived Gremlin-1 mediated increased fibrosis and deposition of ECM. This increased ECM was mediated via activation of MAPK pathway that ultimately led to increased metabolic rerouting to fatty acid oxidation through metabolic repurposing. Finally, they used a specific VEGFR inhibitor in vivo in the animal model to demonstrate that VEGFR blockade reduced fibrosis in this model of disease [45]. This suggest that Gremlin-1 was mediating its effects through the VEGR2 receptor in a BMP-independent manner. The authors suggest that targeting gremlin-1 directly with an antibody would have been a preferable route but none was commercially available.
Retinopathy
Gremlin-1 has been found to be associated with increased fibrosis and epithelial to mesenchymal transition in retinal pigment epithelial cells (RPE) that are associated with retinal disease, such as aged related macular degeneration [46]. Further research demonstrated increased Gremlin-1 in these RPE cells and that reduction in Gremlin-1 by siRNA reduced collagen expression mediated by incubation of the cells with both TGF-β1 and β2 [47]. Gremlin-1 was also found to be associated with RPE mesenchymal transition which was reduced on siRNA-mediated knockdown of gremlin-1 protein [48].
Multiple organ fibrosis differing mechanisms
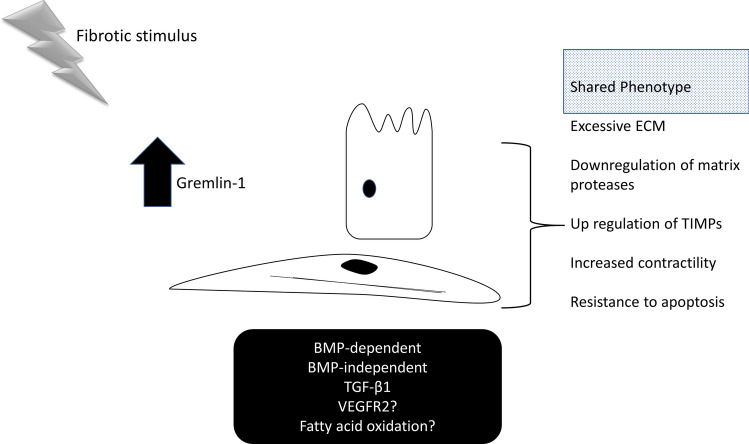
It is clear that Gremlin-1 is elevated in multiple organ-specific fibrotic conditions (Fig. 1). Whilst Gremlin-1 is clearly present in elevated amounts the precise mechanism appears to depend on the specific organ and cell type, but may lead to common end point myofibroblast formation (Fig. 3). For instance, it appears in systemic sclerosis that it is due to an upregulation of TGF-β1, as mitigation of TGF-β1 [39] reduced ECM deposition. In kidney for instance, it appears BMP inhibition is important but VEGFR2 activation has also been demonstrated [31], but a recent study could not reproduce this finding [17]. Indeed a more recent study utilising pulmonary endothelial cells demonstrated that Gremlin1 acts as a antagonist to VEGFR2, and not an agonist [49].
Fig. 3.
Gremlin-1 mediates myofibroblast transition. Schematic demonstrating that fibrotic stimuli drives Gremlin-1-mediated fibrosis with a shared phenotype from divergent cells. Epithelial and fibroblast stromal cells shown. Lower box gives examples of signalling pathways mediated by Gremlin-1 in different cell types. Cellular phenotype characteristics are given on the right hand side. TIMPs; Tissue Inhibitors of Matrix Metalloproteinases
Therefore, what is the precise role of VEGR2 in mediating fibrosis? Are there other, unknown, pathways that mediate Gremlin-1 effects? It was shown that Gremlin-1 can bind to fibrilin microfibrils in mesothelioma [50]. Mesothelioma is a cancer that often affects the pleura of the lungs. The biding of Gremlin-1 in this study to fibrillin microfibrils is interesting [50], because microfibrils are used for the integration of TGF-β signals and thus Gremlin-1-targeted fibrillin microfibrils may serve to suppress BMP signalling and as a consequence enhance pro-fibrotic TGF-β signalling in a disease scenario.
In intestinal fibrosis, it appears that VEGFR2 does indeed play a role with induction of fatty acid oxidation being important in fibrosis. Furthermore, because it appears that there are BMP-independent pathways of Gremlin-1-mediated fibrosis one could speculate that Gremlin-1 has a specific interaction with an unidentified receptor? For instance, Gremlin-1 has been identified to interact with SLIT proteins [51], at least in immune cells. Furthermore, it has also been found to be an antagonists of macrophage migration inhibitory factor [19] and a recent study suggested that Gremlin-1 activated a STAT3 pathway in breast cancer cells mediating metastasis [52]. So how does the context determine the response? This is a key question that remains unanswered. Regulation of BMPs by Gremlin-1 is well characterised but the precise upstream regulators of Gremlin-1 in the context of fibrosis are not that clear. A few studies have demonstrated that TGF-β1 can induce Gremlin-1 expression [44] suggesting that TGF-β lies upstream of Gremlin-1 in fibrosis. However, in other studies including ours we did not see that effect [39], more that Gremlin-1 enhanced TGF-β signalling [39]. Factors outside of classic cytokines including mechanical stretch have also been found to upregulate Gremlin-1 levels [53]. Identifying regulators of Gremlin-1 expression requires more research.
Targeting Gremlin-1 would also be an attractive option due to the fact that postnatally there is barely any expression of Gremlin-1, thus inhibition would be specific and with no side effects. Currently no specific chemical inhibitor exists thus hampering clinical development as a possible therapeutic. In vivo in an animal model of chronic hypoxia-induced pulmonary hypertension—which is associated with systemic sclerosis—treatment with a specific antibody to Gremlin-1-retarded pathology [54]. Also, in vivo in an animal model of myeloma antiGremlin-1 antibody treatment compared to IgG control reduced tumour burden [21]. Thus, therapeutic targeting with monoclonal antibodies seems a facile approach. A different approach could be with the reduction of Gremlin-1 mediated by PRoteolysis TArgeting Chimeras (PROTACs). PROTACs are used to target proteins for specific protein degradation by hijacking the ubiquitin proteosome system [55]. PROTACs are hetero bifunctional molecules that connect your protein of interest ligand to an E3 ubiquitin ligase recruiting ligand with a linker molecule. Subsequently protein of interest degradation occurs when the ubiquitination machinery is brought into close proximity and the ubiquitinated protein is recognized and degraded by the 26S proteosome, which is part of the ubiquitin-proteosome system in cells [56]. This targeted degradation making use of the hosts own recycling machinery is highly specific and could be used to degrade Gremlin-1. Such PROTACs have been used to degrade proteins such as STAT3 [57].
Conclusions
Gremlin-1 appears to play a critical role in multiple fibrotic diseases including kidney [28], systemic sclerosis [39], lung [4], eye [46] and liver [5]. The downstream signalling pathways appear different among different organs and cells and likely represent differential signalling nodes. Gremlin-1 may be a conserved response to injury and that is why multiple organs are expressing this. To date no small molecule inhibitors exist, although antibodies to Gremlin-1 do exist, they are not commercially available. Neutralisation of Gremlin-1 with specific monoclonal antibodies maybe a therapeutic option in fibrotic diseases and should be investigated further. Furthermore, PROTACs that can degrade proteins using the proteosomal system should be investigated. Given the importance of fibrotic diseases and the estimation that over 50% of deaths are associated with fibrosis in the western world [58] further investigation is critical into the role of Gremlin-1.
Author contributions
SOR designed, conceived and wrote this work solely.
Funding
No funding was provided for this work.
Availability of data and material
NA.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
NA.
Consent for publication
NA.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brazil DP, et al. BMP signalling: agony and antagony in the family. Trends Cell Biol. 2015;25(5):249–264. doi: 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Sneddon JB, et al. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci U S A. 2006;103(40):14842–14847. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMahon R, et al. IHG-2, a Mesangial Cell Gene induced by high glucose, is human gremlin: regulation by extracellular glucose concentration, cyclic mechanical strain, and transforming growth factor-β1. J Biol Chem. 2000;275(14):9901–9904. doi: 10.1074/jbc.275.14.9901. [DOI] [PubMed] [Google Scholar]
- 4.Koli K, et al. Bone morphogenetic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol. 2006;169(1):61–71. doi: 10.2353/ajpath.2006.051263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boers W, et al. Transcriptional profiling reveals novel markers of liver fibrogenesis: gremlin and insulin-like growth factor-binding proteins. J Biol Chem. 2006;281(24):16289–16295. doi: 10.1074/jbc.M600711200. [DOI] [PubMed] [Google Scholar]
- 6.O'Reilly S, et al. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J Biol Chem. 2014;289(14):9952–9960. doi: 10.1074/jbc.M113.545822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topol LZ, et al. Identification of drm, a novel gene whose expression is suppressed in transformed cells and which can inhibit growth of normal but not transformed cells in culture. Mol Cell Biol. 1997;17(8):4801–4810. doi: 10.1128/mcb.17.8.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church RH, et al. Gremlin1 preferentially binds to bone morphogenetic protein-2 (BMP-2) and BMP-4 over BMP-7. Biochem J. 2015;466(1):55–68. doi: 10.1042/BJ20140771. [DOI] [PubMed] [Google Scholar]
- 9.Allendorph GP, Vale WW, Choe S. Structure of the ternary signaling complex of a TGF-beta superfamily member. Proc Natl Acad Sci U S A. 2006;103(20):7643–7648. doi: 10.1073/pnas.0602558103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeller R, López-Ríos J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10(12):845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- 11.Roxburgh SA, et al. Allelic depletion of grem1 attenuates diabetic kidney disease. Diabetes. 2009;58(7):1641–1650. doi: 10.2337/db08-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khokha MK, et al. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet. 2003;34(3):303–307. doi: 10.1038/ng1178. [DOI] [PubMed] [Google Scholar]
- 13.Michos O, et al. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131(14):3401–3410. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, et al. In vivo delivery of Gremlin siRNA plasmid reveals therapeutic potential against diabetic nephropathy by recovering bone morphogenetic protein-7. PLoS ONE. 2010;5(7):e11709. doi: 10.1371/journal.pone.0011709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitola S, et al. Gremlin is a novel agonist of the major proangiogenic receptor VEGFR2. Blood. 2010;116(18):3677–3680. doi: 10.1182/blood-2010-06-291930. [DOI] [PubMed] [Google Scholar]
- 16.Park SA, et al. Gremlin-1 augments the oestrogen-related receptor α signalling through EGFR activation: implications for the progression of breast cancer. Br J Cancer. 2020;123(6):988–999. doi: 10.1038/s41416-020-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutton LR, et al. No evidence of Gremlin1-mediated activation of VEGFR2 signaling in endothelial cells. J Biol Chem. 2019;294(48):18041–18045. doi: 10.1074/jbc.AC119.010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji C, et al. Gremlin inhibits UV-induced skin cell damages via activating VEGFR2-Nrf2 signaling. Oncotarget. 2016;7(51):84748–84757. doi: 10.18632/oncotarget.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller I, et al. Gremlin-1 is an inhibitor of macrophage migration inhibitory factor and attenuates atherosclerotic plaque growth in ApoE-/- Mice. J Biol Chem. 2013;288(44):31635–31645. doi: 10.1074/jbc.M113.477745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worthley DL, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1–2):269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark KC, et al. Targeted disruption of bone marrow stromal cell-derived Gremlin1 limits multiple myeloma disease progression in vivo. Cancers. 2020;12(8):2149. doi: 10.3390/cancers12082149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinz B, Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16(1):11–31. doi: 10.1038/s41584-019-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinchcliff M, O'Reilly S. Current and potential new targets in systemic sclerosis therapy: a new hope. Curr Rheumatol Rep. 2020;22(8):42. doi: 10.1007/s11926-020-00918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson J, Distler J, O’Reilly S. The role of epigenetic modifications in systemic sclerosis: a druggable target. Trends Mol Med. 2019;25(5):395–411. doi: 10.1016/j.molmed.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Dolan V, et al. Expression of gremlin, a bone morphogenetic protein antagonist, in human diabetic nephropathy. Am J Kidney Dis. 2005;45(6):1034–1039. doi: 10.1053/j.ajkd.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 26.McKnight AJ, et al. A GREM1 gene variant associates with diabetic nephropathy. J Am Soc Nephrol. 2010;21(5):773–781. doi: 10.1681/ASN.2009070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh DW, et al. Co-regulation of Gremlin and Notch signalling in diabetic nephropathy. Biochimica et Biophysica Acta Mol Basis Dis. 2008;1782(1):10–21. doi: 10.1016/j.bbadis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Church RH, et al. Gremlin1 plays a key role in kidney development and renal fibrosis. Am J Physiol Renal Physiol. 2017;312(6):F1141–F1157. doi: 10.1152/ajprenal.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchant V, et al. Tubular overexpression of Gremlin in transgenic mice aggravates renal damage in diabetic nephropathy. Am J Physiol Renal Physiol. 2015;309(6):F559–F568. doi: 10.1152/ajprenal.00023.2015. [DOI] [PubMed] [Google Scholar]
- 30.Murphy M, et al. IHG-1 amplifies TGF-beta1 signaling and is increased in renal fibrosis. J Am Soc Nephrol. 2008;19(9):1672–1680. doi: 10.1681/ASN.2007101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquez-Exposito L, et al. Gremlin regulates tubular epithelial to mesenchymal transition via VEGFR2: potential role in renal fibrosis. Front Pharmacol. 2018;9:1195. doi: 10.3389/fphar.2018.01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues-Diez R, et al. Gremlin activates the Smad pathway linked to Epithelial Mesenchymal Transdifferentiation in cultured Tubular Epithelial cells. BioMed Res Int. 2014;2014:802841. doi: 10.1155/2014/802841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavoz C, et al. Gremlin regulates renal inflammation via the vascular endothelial growth factor receptor 2 pathway. J Pathol. 2015;236(4):407–420. doi: 10.1002/path.4537. [DOI] [PubMed] [Google Scholar]
- 34.Myllärniemi M, et al. Gremlin localization and expression levels partially differentiate idiopathic interstitial pneumonia severity and subtype. J Pathol. 2008;214(4):456–463. doi: 10.1002/path.2300. [DOI] [PubMed] [Google Scholar]
- 35.Myllärniemi M, et al. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(3):321–329. doi: 10.1164/rccm.200706-945OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farkas L, et al. Transient overexpression of Gremlin results in Epithelial activation and reversible fibrosis in rat lungs. Am J Respir Cell Mol Biol. 2011;44(6):870–878. doi: 10.1165/rcmb.2010-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy N, et al. Altered expression of bone morphogenetic protein accessory proteins in murine and human pulmonary fibrosis. Am J Pathol. 2016;186(3):600–615. doi: 10.1016/j.ajpath.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 38.Epstein Shochet G, et al. TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir Res. 2020;21(1):56. doi: 10.1186/s12931-020-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duffy L, et al. Bone Morphogenetic Protein Antagonist Gremlin-1 Increases Myofibroblast Transition in Dermal Fibroblasts: implications for systemic sclerosis. Front Cell Dev Biol. 2021;9:1451. doi: 10.3389/fcell.2021.681061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oreilly S. Circulating Gremlin-1 is elevated in systemic sclerosis patients. J Scleroderma Relat Disord. 2021 doi: 10.1177/23971983211036571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng X-Y, et al. Suppression of hepatic stellate cell activation through downregulation of gremlin1 expression by the miR-23b/27b cluster. Oncotarget. 2016;7(52):86198–86210. doi: 10.18632/oncotarget.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Reilly S. MicroRNAs in fibrosis: opportunities and challenges. Arthritis Res Ther. 2016;18(1):11. doi: 10.1186/s13075-016-0929-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang T, et al. Bone morphogenetic protein 7 suppresses the progression of hepatic fibrosis and regulates the expression of gremlin and transforming growth factor β1. Mol Med Rep. 2012;6(1):246–252. doi: 10.3892/mmr.2012.892. [DOI] [PubMed] [Google Scholar]
- 44.Staloch D, et al. Gremlin is a key pro-fibrogenic factor in chronic pancreatitis. J Mol Med. 2015;93(10):1085–1093. doi: 10.1007/s00109-015-1308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y, et al. Targeting Gremlin 1 prevents Intestinal Fibrosis Progression by inhibiting the fatty acid oxidation of fibroblast cells. Front Pharmacol. 2021;12:663774. doi: 10.3389/fphar.2021.663774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee H, et al. The role of gremlin, a BMP antagonist, and epithelial-to-mesenchymal transition in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2007;48(9):4291–4299. doi: 10.1167/iovs.07-0086. [DOI] [PubMed] [Google Scholar]
- 47.Qin D, Jin X, Jiang Y. Gremlin mediates the TGF-β-induced induction of profibrogenic genes in human retinal pigment epithelial cells. Exp Ther Med. 2020;19(3):2353–2359. doi: 10.3892/etm.2020.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D, et al. Gremlin-1: An endogenous BMP antagonist induces epithelial-mesenchymal transition and interferes with redifferentiation in fetal RPE cells with repeated wounds. Mol Vis. 2019;25:625–635. [PMC free article] [PubMed] [Google Scholar]
- 49.Rowan SC, et al. EXPRESS: Gremlin1 blocks vascular endothelial growth factor signalling in the pulmonary microvascular endothelium. Pulm Circ. 2018;10(1):2045894018807205. doi: 10.1177/2045894018807205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamminen JA, et al. Gremlin-1 associates with fibrillin microfibrils in vivo and regulates mesothelioma cell survival through transcription factor slug. Oncogenesis. 2013;2(8):e66–e66. doi: 10.1038/oncsis.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen B, et al. Cutting edge: bone morphogenetic protein Antagonists Drm/Gremlin and Dan interact with Slits and act as negative regulators of Monocyte Chemotaxis. J Immunol. 2004;173(10):5914. doi: 10.4049/jimmunol.173.10.5914. [DOI] [PubMed] [Google Scholar]
- 52.Sung NJ, et al. Gremlin-1 promotes metastasis of breast cancer cells by activating STAT3-MMP13 signaling pathway. Int J Mol Sci. 2020;21(23):9227. doi: 10.3390/ijms21239227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng L, et al. Vital roles of Gremlin-1 in pulmonary arterial hypertension induced by systemic-to-pulmonary shunts. J Am Heart Assoc. 2020;9(15):e016586. doi: 10.1161/JAHA.120.016586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciuclan L, et al. Treatment with anti-gremlin 1 antibody ameliorates chronic hypoxia/SU5416-induced pulmonary arterial hypertension in mice. Am J Pathol. 2013;183(5):1461–1473. doi: 10.1016/j.ajpath.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bond MJ, Crews CM. Proteolysis targeting chimeras (PROTACs) come of age: entering the third decade of targeted protein degradation. RSC Chem Biol. 2021;2(3):725–742. doi: 10.1039/d1cb00011j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schapira M, et al. Targeted protein degradation: expanding the toolbox. Nat Rev Drug Discovery. 2019;18(12):949–963. doi: 10.1038/s41573-019-0047-y. [DOI] [PubMed] [Google Scholar]
- 57.Bai L, et al. A potent and selective small-molecule degrader of STAT3 achieves complete tumor regression in vivo. Cancer Cell. 2019;36(5):498–511.e17. doi: 10.1016/j.ccell.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4(8):583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NA.