Abstract
Esophageal squamous cell carcinoma (ESCC) is a common malignancy worldwide with a low survival rate due to a lack of therapeutic targets. Here, our results showed that nuclear mitotic apparatus protein 1 (NUMA1) transcript and protein levels are significantly upregulated in ESCC patient samples and its high expression predicated poor prognosis. Knock-down of NUMA1 promoted cell apoptosis and suppressed cell proliferation and colony formation. By using cell-derived xenograft (CDX) and patient-derived xenograft (PDX) mice models, we found silencing the NUMA1 expression suppressed tumor progression. In addition, conditional knocking-out of NUMA1 reduced 4NQO-induced carcinogenesis in mice esophagus, which further confirmed the oncogenic role of NUMA1 in ESCC. Mechanistically, from the immunoprecipitation assay we revealed that NUMA1 interacted with GSTP1 and TRAF2, promoted the association of TRAF2 with GSTP1 while inhibited the interaction of TRAF2 and ASK1, thus to regulate sustained activation of JNK. In summary, our findings suggest that NUMA1 plays an important role during ESCC progression and it functions through regulating ASK1-MKK4-SAPK/JNK signaling pathway.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-023-04854-0.
Keywords: Nuclear mitotic apparatus protein 1 (NUMA1), NUMA1 conditional knock out mice, Esophageal squamous cell carcinoma (ESCC), ASK1-MKK4-SAPK/JNK signaling pathway
Introduction
Esophageal cancer (EC) is one of the most lethal cancers worldwide according to the global cancer statistics 2020 and it is the sixth leading cause of cancer death, and seventh in terms of incidence [1]. There are two histological types of esophageal cancer, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) [2]. ESCC is the major subtype, accounting for 85% of esophageal cancer, with the highest incidence rates in Asia and South Africa [3]. For the treatment of ESCC, surgical resection, chemotherapy and chemoradiotherapy are the primary methods; however, the outcomes are not satisfied [4–6]. Despite improvements have made during the past few years, the five-year survival rates are still very low, ranging from 10 to 30%, due to recurrence, inadequate therapeutic strategies, and limited effective therapeutic targets [3, 4, 7, 8]. Therefore, the identification of molecular targets regulating ESCC progression and the investigation of their pathogenic mechanisms are urgently needed.
NUMA1, also known as nuclear matrix protein-22, is a nuclear protein with high molecular weight (238 kDa). During mitosis in cell cycle, NUMA1 drives tethering the bulk of spindle microtubules to the spindle poles, helps spindle orientation and anchors dynein at the cell cortex [9–11]. In addition, NUMA1 interacts with 53BP1 and prevents 53BP1 accumulation at DNA breaks [12]. Moreover, NUMA1 interacts with different oncogenic partners, such as cyclin-dependent kinase 1 and aurora kinase A, which bind with NUMA1 and phosphorylate NUMA1, thus to regulate cell division [10, 13–15]. During oxidative DNA damage repair, NUMA1 is enriched at the promoters and can promote oxidative damage repair [16]. NUMA1 also interacts with p53 and modulates p53-mediated gene transcription in cancer cells [17, 18]. Although there are some evidences about the function of NUMA1 during cell mitosis and cancer progression, it still needed more explore about the function of NUMA1 during cancer progression, and there is no study of NUMA1 in ESCC.
In the present study, we report that NUMA1 is upregulated in ESCC patient tissues and associated with poor clinical outcome in patients and promotes ESCC progression in vitro and in vivo. NUMA1 knock-down in ESCC cells induces cell apoptosis and cell cycle arrest, and NUMA1 conditional knock-out suppresses 4NQO-induced esophageal carcinogenesis in mice. Mechanistically, we show the non-canonical oncogenic function of NUMA1, and it can regulate cell apoptosis through binding with GSTP1 and TRAF2. NUMA1 promotes the interaction between GSTP1 and TRAF2 and inhibits the interaction between ASK1 and TRAF2, thereby interfering the formation of the active ASK1 complex and inhibiting the sustained activation of the downstream JNK signaling pathway. Finally, silencing NUMA1 expression using lentivirus intratumor injection significantly retards tumor growth in ESCC patient-derived xenograft (PDX) models. Taken together, our findings demonstrate that NUMA1 promotes ESCC progression by regulating the complex of GSTP1, TRAF2 and ASK1. It might be considered as a potential target for the therapy of ESCC.
Materials and methods
Materials
The antibody conjugated agarose such as anti-Flag magnetic agarose and anti-HA magnetic agarose used in this study was obtained from Thermo Fisher scientific (A36797, MA, USA). Anti-c-Myc Magnetic agarose was purchased from MCE (Junction, NJ, USA). Anti-Flag antibody was obtained from Sigma-Aldrich (St Louis, MO, USA). Antibodies to detect HA-tag, NUMA1, phospho-SAPK/JNK (Thr183/Tyr185), SAPK/JNK, His-tag, Bcl-2, cleaved PARP (Asp214), caspase-3, cleaved caspase-3 (Asp175), Bim, cyclin D1, cyclin D3, GSTP1, TRAF2, SEK1/MKK4, phospho-SEK1/MKK4 (Ser257), phospho-c-Jun (Ser73) and c-Jun were all bought from Cell Signaling Technology (Beverly, MA, USA). GAPDH and GSTP1 were obtained from Proteintech (Chicago, Illinois, USA). His-tag antibody used to do co-immunoprecipitation was obtained from MBL (Beijing, China), NUMA1 and Ki67 antibodies used to do immunohistochemistry were obtained from Abcam (Cambridge, MA, USA).
Cell culture and transfection
Human esophageal squamous cell carcinoma (ESCC) cell line KYSE30, KYSE70, KYSE140, KYSE150, KYSE410, KYSE450 and KYSE510 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI-1640 medium. HEK293T cells were bought from ATCC and maintained in Dulbecco’s modified Eagle’s medium. All cells were mycoplasma-free and authenticated by STR analysis. Culture medium was supplemented with 10% fetal bovine serum (Biological Industries, Kibbutz Beit Haemek, Israel) and 100 units/mL Penicillin/streptomycin. Cells were cultured in a humidified incubator under 37 °C with 5% CO2. Transfection was performed using Simple-Fect reagent (Signaling Dawn Biotech, Wuhan, Hubei, China) for HEK293T cells and jetPRIME reagent (Polyplus, Illkirch, France) for ESCC cells, following the manufacturer’s instructions.
Cell proliferation and colony formation assay
MTT assay was performed to detect cell proliferation. Cells (1 ~ 2 × 103 cells/well) were seeded into 96-well plates and detected the absorbance at the time point of 0, 24, 48 and 72 h. For the absorbance determining, 5 mg/mL MTT (20 μl/well) was added to the wells and incubated at 37 °C incubator for 3 h. After removing the medium, 100 μl DMSO was added to every well and measured the absorbance at 570 nm. Foci formation and soft agar experiments were used to test the colony formation. For the foci formation assay, cells (0.8 ~ 1 × 103 cells/well) were seeded in six-well plate and cultured for 1 to 2 weeks. After then, cells were washed with phosphate buffered saline (PBS) and stained with 0.2% crystal violet. Foci numbers were counted using Image Pro Plus software (v.6.0) program (Media Cybernetics, Rockville, MD, USA). For the soft agar colony formation assay (anchorage-independent cell growth), cells (8 × 103) were suspended in 1 mL of RPMI-1640 medium containing 2 mM glutamine, 5 μg/mL gentamycin and 0.3% soft agar as top layer. About the base layer, 3 mL 0.5% soft agar was added to the six-well plate. After 1–3 weeks, colonies were photographed under a microscope and counted using Image Pro Plus software.
Lentivirus construction and infection
To generate the NUMA1, TRAF2 and GSTP1 knock-down cells, shNUMA1, shTRAF2 and shGSTP1 plasmids were constructed and the shRNA sequences were designed as described in Supplementary Table 3. The virus plasmid pLKO.1-scramble, shNUMA1, shTRAF2 or shGSTP1 were co-transfected with the package plasmid (pSPAX2 and pMD2.G) into HEK293T cells together with Simple-Fect reagent. Medium was changed after transfection for 4 h, and virus particles were collected 48 h later using a 0.45 µm filter. After infecting with virus particles media mixed with 8 μg/mL polybrene (Millipore, Billerica, MA) for 24 h, ESCC cells were selected with puromycin (Solarbio, Beijing, China) and used to do subsequent experiments.
Western blot analysis
Cell pellets were resuspended using RIPA lysis buffer (50 mM Tris–HCl pH 7.4, 1% NP40, 1 mM EDTA, 150 mM NaCl, 0.25% deoxycholic acid disodium salt, 0.1% SDS, 10% protease inhibitor and phosphatase inhibitor), and incubated on ice for 1 h to fully lysis the cells. After centrifuged at 12,000 rpm for 15 min, collected the supernatant as whole cell extracts. The concentration of the extracts was examined using BCA Quantification kit (Solarbio, Beijing, China, Cat#PC0020). Proteins were separated by SDS/PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were then blocked using 5% milk in PBS for 1 h and incubated with the appropriate primary antibody overnight at 4 °C cold room. Subsequently, the membranes were washed three times using PBS containing 0.5% tween-20 and incubated with a secondary antibody conjugated with horseradish peroxidase for 2 h at room temperature (RT). Finally, the specific protein bands were visualized using enhanced chemiluminescence (ECL) reagent.
Co-immunoprecipitation
Cell pellets were resuspended with IP lysis buffer (25 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40 and 5% Glycerin) containing 10% protease inhibitor and phosphatase inhibitor and incubated on ice for 1 h. The whole cell extracts were collected by centrifuge (12,000 rpm 15 min), and protein concentration was measured using BCA Quantification kit. Appropriate cell extracts were mixed with specific antibodies and rotated at 4 °C for 12 h. Protein A/G agarose (#sc-2003, Santa Cruz) was added and rotated for another 3 h. Finally, the agarose was washed three times using lysis buffer and added loading buffer then eluted proteins at 95 °C for 8 min. Specific target proteins were examined by Western blot analysis.
Protein digestion for LC–MS/MS analysis
Excise the protein gel bands of interest and cut into pieces (1 mm2), wash with 200 μL water. Then, use 100 mM ammonium bicarbonate/acetonitrile (1:1, vol/vol) treat and incubated at 37 °C for 30 min. After neat acetonitrile treat and dry the gel use a speed Vac concentrator, reduce the gel and treat with acetonitrile. Then, use IAA treat the gel and keep in the dark for 45 min. Next, wash and dry the gel, then treat with 25 ng/μL trypsin to digest gel overnight. At last, use 2% formic acid/60% acetonitrile extracted the peptides, then dry down in speed Vac and dissolve the peptide with 20 ~ 30 μL 0.1% formic acid for LC–MS/MS analysis.
Cell cycle analysis
KYSE450 and KYSE510 cells (2 × 105) were seeded into 60-mm plates and incubated for 18 h in 10% serum-containing RPMI-1640 media. Then, cells were cultured for 48 h in 10% serum-containing RPMI-1640 after starvation for 24 h. Cells were collected by trypsinization and washed using PBS, then fixed with 70% ethanol and stored at − 20 °C refrigerator for more than 24 h. After rehydration, cells were digested with RNase for 1 h and stained with propidium iodide for 15 min. Finally, the cell cycle distribution was analyzed using a BD FACS Calibur Flow Cytometer (BD Biosciences, San Jose, CA, USA).
Apoptosis assay
For cell apoptosis assay, we conducted TUNEL staining and flow cytometry analysis using annexin-V and propidium iodide (PI) double staining. For TUNEL staining assay, ESCC cells (3 × 104) were seeded on glass slides in 24 well plates and incubated for 36 h in 10% FBS containing RPMI-1640 media. Then, cells were fixed using 4% paraformaldehyde for 30 min and stained using TUNEL staining kit (#MA0224, Meilunbio, Dalian, China) as manufacture’s protocol. For Annexin V apoptosis assay, cells (9 × 104) were seeded into 60-mm plates. After cultured for 48 h, the cells were collected by trypsinization and washed using ice cold PBS, and then, stained cells with annexin-V and propidium iodide at RT for 15 min. The apoptotic cells were analyzed using a BD FACS Calibur Flow Cytometer.
Plasmid construction
Eukaryotic expression constructs, including pcDNA3.1-Flag-NUMA1, pcDNA3.1-HA-GSTP1 and pcDNA3.1-His-TRAF2, were purchased from Youbao Biotechnology Company (Changsha, China). Through enzyme digestion and DNA ligase ligation, pcDNA3.1-Flag-TRAF2 (BamHI + XbaI), pcDNA3.1-His-GSTP1(BamHI + XhoI), and pcDNA3.1-Myc-MKK4 (BamHI + XhoI) plasmids were constructed. The domain deletion mutations of NUMA1 were amplified by PCR with the designed specific primers (Table S3). The domain deletion mutations for TRAF2 were purchased from Youbao Biotechnology. The lentivirus package plasmids (PLKO.1, pMD2.0 G and psPAX2) were obtained from Addgene Inc (Cambridge, MA, USA).
Immunohistochemistry
An human ESCC tissue microarray (Shanghai Xinchao Biotech Company, Shanghai, China) and tumor tissues of CDX and PDX mice were used to do immunohistochemistry. The tumor tissues of the CDX and PDX mice were embedded in paraffin before doing immunohistochemistry. After deparaffinized and rehydrated, antigen retrieval was performed using sodium citrate buffer (10 mmol/L, pH 6.0) by boiling for 90 s. Then, used 3% H2O2 treated the slides for 10 min, and blocked the slides using 10% goat serum for 1 h at RT. The slides were then incubated with primary antibodies (Ki-67, NUMA1, p-SAPK/JNK (Thr183/Tyr185) or p–c-Jun (Ser73) at 4 °C overnight. Subsequently, washed the slides three times using PBS and incubated with secondary antibody for 30 min at RT. Then, stained the slides using 3,3′-diaminobenzidine and finally, counterstained with hematoxylin. Photographed and analyzed the slides by Image Scope software program and quantified the percentage of positive cells.
Cell-derived xenograft (CDX) mouse model
Animal studies were approved by the Ethics Committee of China-US(Henan) Hormel Cancer Institute (Zhengzhou, Henan, China). KYSE450 cells and 6–8 weeks old NU/NU mice (Vital River Labs, Beijing, China) were used to prepare the CDX mice model. The mice were randomly divided into three groups and seeded with the corresponding KYSE450 (Scramble, shNUMA1#3 and shNUMA1#4) cells. Each group contains eight mice, and 1 × 107 corresponding cells were injected subcutaneously into the right flank of the mouse. After 3 weeks, the tumor volume was measured twice a week using a Vernier caliper. Tumor volume was calculated as follows: length × width × height × 0.52. Finally, the mice were euthanized and tumors were extracted for further Western blot and immunohistochemical analysis.
Patient-derived xenograft (PDX) mouse model
Seven- to eight-week-old female nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice were used for the PDX mice experiments. Tumor tissues were cut to pieces with the same weight and then, transplanted into the right back of the mice. When the tumor volume reached about 150 mm3, mice were randomly divided into three groups (scramble, shNUMA1#3, shNUMA1#4) based on the tumor volume. The lentivirus used to do intratumor injection were produced using HEK293T cells as previous mentioned. After collected the lentivirus with centrifugated at 30,000 rpm, 4 °C for 4 h, mice tumors were injected with the corresponding lentivirus at a dose of 1 × 108 pfu/100 μL per mouse, twice a week for 3 weeks. Mice weight and tumor volume were monitored once a week. When tumor volume reached about 1200 mm3, the mice were euthanized and tumors were extracted for further Western blot and immunohistochemical analysis.
4-nitroquinoline-1-oxide (4NQO)-induced esophageal tumors in mice
NUMA1 flox heterozygous recombinant C57BL/6N mice were purchased from the Cyagen biosciences (CKOCMS190429JW1), and esophagus Cre mice (ED-L2-Cre) were purchased from Beijing Vitalstar Biotechnology, China. After breeding the mice, obtained wild type control mice (WT, flox homozygous and Cre gene negative) and NUMA1 conditional knock out mice (CKO, flox homozygous and Cre gene positive). Mice were genotyped by PCR analysis using the specific primers (Table S3) according to the manufactures protocol. When the mice, 10 weeks old, were randomly divided to four groups: (1) Wild type vehicle (n = 16, 8 male and 8 female), (2) Wild type 4NQO (n = 52, 26 male and 26 female), (3) NUMA1 CKO vehicle (n = 16, 8 male and 8 female), (4) NUMA1 CKO 4NQO (n = 52, 26 male and 26 female). 4NQO (Cas#56-57-5, 100 μg/ml) was administered in the drinking water for 16 weeks, followed by 9 weeks of regular water. Mice body weight was continuously monitored once a week until the mice euthanized. Mice esophagus weight and length were measured, and microscopically visible tumors were counted. Finally, photographed the esophagus and fixed in formalin for further histological analysis.
Statistical analysis
In this study, GraphPad Prism 7.0. and SPSS software were used for statistical analysis. All results data were showed as mean value ± SD. Statistically significant difference was determined by two-tailed Student’s t test and Chi-square test. p values less than 0.05 were considered to be statistically significant, *p < 0.05, ** p < 0.01 and *** p < 0.001.
Results
NUMA1 is overexpressed in human ESCC and is associated with patient survival viability
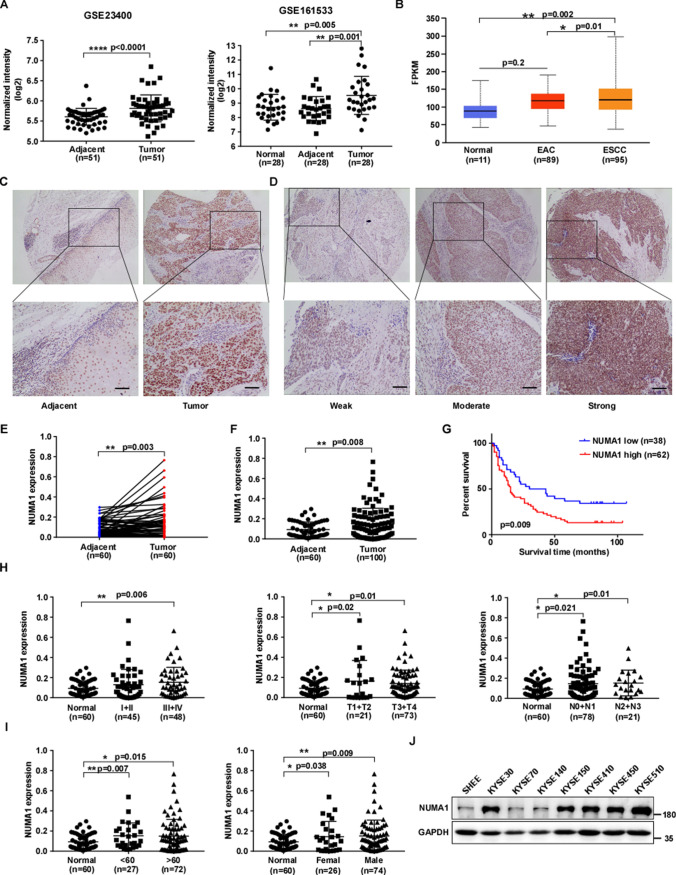
To explore the potential target for therapy of ESCC, we analyzed high-level gene expression in ESCC by using two ESCC microarray datasets (GSE23400, GSE161533), and the datasets were downloaded from the Gene Expression Omnibus. From the datasets, we found the mRNA level of NUMA1 was significantly upregulated in the tissues of patient-derived ESCC tumors compared with paired adjacent tissues and normal tissues (Fig. 1A). Next, we used the UALCAN to assess NUMA1 expression information in the TCGA database [19], and bioinformatical analysis results showed that the NUMA1 mRNA level was significantly up-regulated in ESCC, but not in EAC (Fig. 1B). To further explore the clinical significance of NUMA1 in ESCC, we measured NUMA1 expression in an ESCC tissue microarray by immunohistochemical staining. The expression of NUMA1 was significantly elevated in cancer tissues compared with paired (Fig. 1C–E) and unpaired (Fig. 1F) adjacent tissues. Survival analysis revealed that patients with high NUMA1 expression had worse overall survival and an adverse prognosis (Fig. 1G). Furthermore, clinical association analysis results showed that NUMA1 expression was not significantly correlated with clinical stage, lymph node metastasis, age and gender (Fig. 1H, I and Table S1). We further analyzed the expression of NUMA1 in seven paired ESCC and adjacent tissues and found higher expression of NUMA1 in ESCC tissues (Fig. S1). Furthermore, we examined the expression of NUMA1 in different ESCC cell lines and normal human immortalized esophageal epithelial cell line (Shantou human embryonic esophageal cell line, SHEE). The results showed NUMA1 expression was increased in ESCC cell lines than that of normal SHEE cells (Fig. 1J). These results indicate that NUMA1 is upregulated in ESCC tissues and predicts poor prognosis in ESCC patients.
Fig. 1.
NUMA1 is upregulated in human ESCC and predicts poor prognosis in ESCC patients. A Plots illustrating NUMA1 transcript expression levels in paired adjacent-tumor and normal-adjacent-tumor GEO ESCC datasets. B The mRNA level of NUMA1 in the TCGA esophageal cancer patient cohort. C Representative immunohistochemical staining images of human ESCC tissue microarray using NUMA1 antibody (upper panel, 40 × ; lower panel, 100 × ; scale bar, 50 μm). D Representative immunohistochemical staining images indicate increasing expression levels of NUMA1 (40 × and 100 × ; Scale bar: 50 μm). E–F Statistical analysis of NUMA1 expression is shown in paired (E) and unpaired (F) ESCC tissues. G Relationship between NUMA1 expression and the overall survival of patients from the ESCC tissue microarray. H The expression profile of NUMA1 in different clinical stages for tissue microarray. I Expression of NUMA1 in different age and gender. J NUMA1 expression levels in human immortalized esophageal epithelial cell line SHEE and ESCC cell lines. The data statistical analysis was performed using Student’s unpaired t test in B, F, H, I; Student’s paired t test in A and E; Kaplan–Meier analysis in G. Error bars represent the mean ± SD
The silence of NUMA1 decreased cell proliferation in vitro and retarded tumor growth in vivo
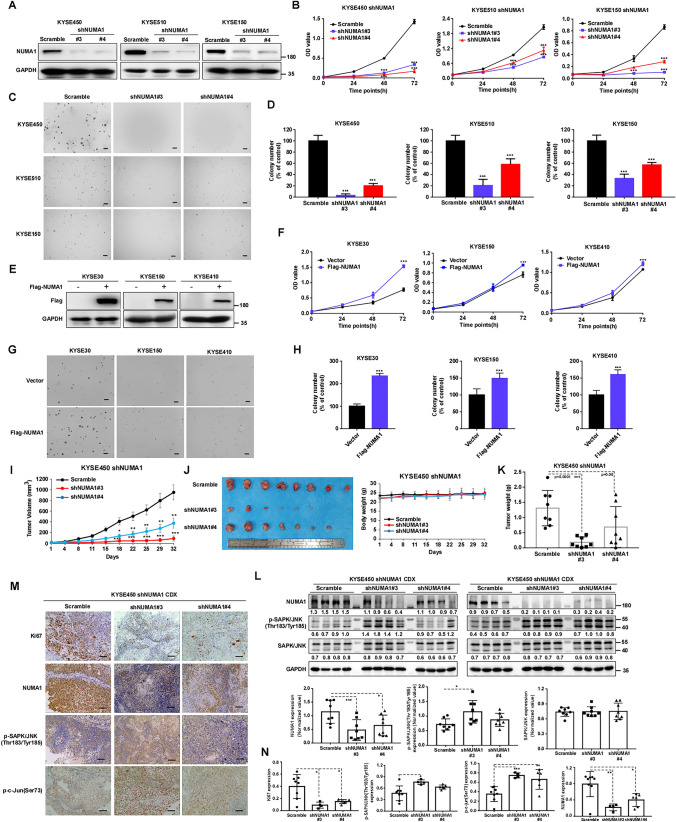
To investigate the roles of NUMA1 in ESCC cells, we constructed specific lentiviral shRNA targeting NUMA1 and scramble control, and infected into ESCC cells. Western blot results showed that NUMA1 expression was markedly decreased (Figs. 2A and S2A). Furthermore, we use qPCR confirmed the knockdown of NUMA1 in each cell (Fig. S2B). After knocking-down NUMA1 in KYSE450, KYSE510 and KYSE150 cells, cell proliferation and colony formation were significantly inhibited (Figs. 2B–D and S2C). Moreover, we overexpressed NUMA1 in KYSE30, KYSE150 and KYSE410 cell lines by transfection of flag-NUMA1 plasmid and confirmed the expression by Western blot and qPCR (Figs. 2E and S2D–E). The overexpression of NUMA1 markedly increased cell proliferation and colony formation (Fig. 2F–H). To further explore whether NUMA1 could promote tumor growth in vivo, we used KYSE450 cells silencing NUMA1 expression or scramble controls subcutaneously injected into nude mice. The results showed that silencing the NUMA1 expression significantly suppressed tumor growth in vivo (Fig. 2I–K). Compared with scramble control group, the tumor growth rate, tumor volume and tumor weight were markedly decreased without significant difference of body weight (Fig. 2I–K). Moreover, in shNUMA1 group, the expressions of NUMA1 were reduced significantly, and the phosphorylation levels of SAPK/JNK (Thr183/Tyr185) were upregulated (Fig. 2L). Immunohistochemical staining results of tumors excised from the CDX mice also showed that Ki67 and NUMA1 expression reduced in the NUMA1 knocking-down group, while the phosphorylation level of SAPK/JNK and c-Jun was upregulated significantly in tumors of NUMA1 silencing group (Fig. 2M–N). Overall, these results show that NUMA has a crucial role during ESCC progression and relates with SAPK/JNK (Thr183/Tyr185) activation.
Fig. 2.
Knocking-down of NUMA1 inhibits ESCC cells proliferation and colony formation in vitro and repressed tumor growth in vivo. A, E Verification of NUMA1 knock-down cells (KYSE450, KYSE510, KYSE150) and overexpression cells (KYSE30, KYSE150, KYSE410) using Western blot. B, F Cell viability was measured by MTT assay (n = 3, independent experiments). C, G Representative images of soft agar colony formation, scale bars, 200 μm. D, H Quantified colony numbers which calculated using Image Pro Plus software (n = 3, independent experiments). I Cell-derived xenograft (CDX) model was established in nude mice; KYSE450 cells stably infected with scramble and NUMA1 knock-down shRNA was subcutaneously injected into the nude mice (n = 8 every group), and tumor volume was recorded twice a week. J Photographs of excised tumor and mice body weight. K Tumor weight. L Western blot evaluated the expression of NUMA1, p-SAPK/JNK (Thr183/Tyr185), SAPK/JNK and GAPDH, and quantified the expression using ImageJ software (down panel) (n = 8). M Representative immunohistochemistry staining images of Ki67, NUMA1, p-SAPK/JNK(Thr183/Tyr185) and p–c-Jun (Ser73). N Statistical analysis of Ki67, NUMA1, p-SAPK/JNK(Thr183/Tyr185) and p–c-Jun (Ser73) expression (Scramble, n = 8; shNUMA1#3, n = 4; shNUMA1#4, n = 6). Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
Silencing NUMA1 expression enhanced ESCC cell apoptosis and cell cycle arrest
To assess the contribution of NUMA1 to cell apoptosis, after silencing NUMA1 expression, we examined cell apoptosis through TUNEL assay and annexin-V staining. The TUNEL assay results showed that apoptosis was significantly induced after knock-down of NUMA1 expression (Fig. 3A). In addition, flow cytometry analysis results also showed increased apoptotic cell population in NUMA1 knock-down cells group (Fig. 3B). Then, we measured the expression of pro-apoptotic and anti-apoptotic markers by Western blot. Knock-down of NUMA1 upregulated cleaved-PARP, cleaved caspase-3 and short isoform of Bim expression, and downregulated Bcl-2 expression (Figs. 3C and S3A). We next measured cell cycle distribution after knocking-down NUMA1 in KYSE450 and KYSE510 cells and flow cytometry analysis results showed that significantly induced G1 phase arrest (Fig. 3D). Furthermore, we examined the expression of proteins associated with the G1 phase of the cell cycle, NUMA1 silencing reduced the expression of cyclin D1 and cyclin D3 as compared with the scramble control (Figs. 3E and S3B). These results show that deficient of NUMA1 in ESCC induces apoptosis and G1 cell cycle arrest with regulation of related biomarkers.
Fig. 3.
Knocking-down NUMA1 expression in ESCC cells induces cell apoptosis and cell cycle arrest. A TUNEL staining in NUMA1 deficient cells; representative images of TUNEL (TRITC) in left panel and the corresponding quantification analysis in right panel (n = 3, independent experiments). B Using annexin V and the PI double staining method to measure the cell apoptosis after knock-down NUMA1 expression by flow cytometry (n = 3, independent experiments). C The effects of NUMA1 knock-down on the expression of apoptosis biomarkers using Western blot. D Cell cycle distribution after knocking-down NUMA1 expression by Flow cytometry analysis (n = 3, independent experiments). E The expression of Cyclin D1 and Cyclin D3 after silencing NUMA1 expression. *p < 0.05, **p < 0.01, ***p < 0.001. Error bars represent the mean ± SD
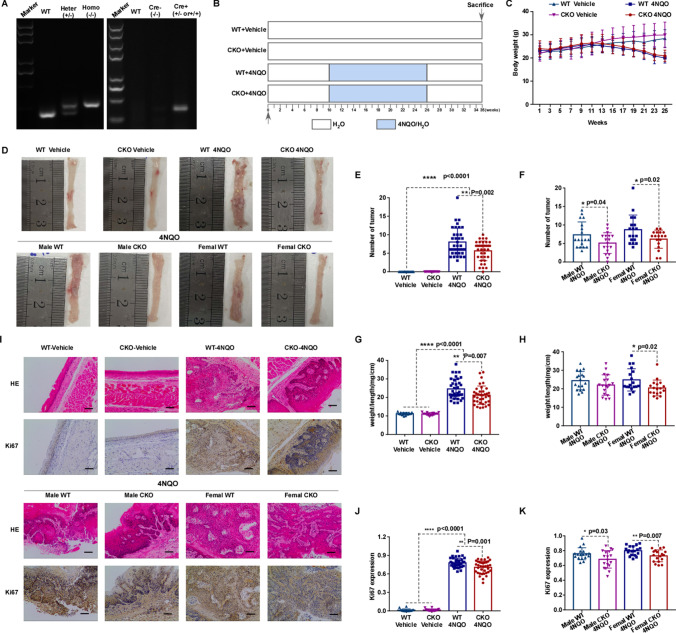
NUMA1 conditional knock out suppresses 4NQO-induced carcinogenesis in mice esophagus
To determine whether the absence of NUMA1 could retard esophageal carcinogenesis or tumor progression in 4NQO-induced esophageal carcinogenesis model, we prepared Wild type (WT, flox homozygous and Cre gene negative) and NUMA1 conditional knock-out (CKO, flox homozygous and Cre gene positive) mice. First, PCR genotyping of NUMA1 flox heterozygous (Heter), flox homozygous (Homo), Cre gene positive (Cre +), Cre gene negative (Cre −) was performed (Fig. 4A). We also confirmed the conditional knockout of NUMA1 use Western blot and qPCR (Fig. S4A and C). Then, we grouped and treated the mice with water vehicle and 4NQO according to schematic diagram (Fig. 4B). The body weight loss was significantly in 4NQO-treated mice compared with Vehicle group (Fig. 4C). Especially, WT male mice in 4NQO treated group suffered severer body weight loss than the NUMA1 CKO male group at the thirteenth and fifteenth week (Fig. S4E). Almost, all of 4NQO treated mice developed esophageal tumors, and no tumor formed in vehicle control group. Furthermore, tumorigenesis in NUMA1 CKO mice was lower compared to WT control (Fig. 4D–F). The expression of NUMA1 has no significant change with or without 4NQO treatment (Fig. S4B and D). Additionally, esophageal weight of WT group was significantly increased compared to NUMA1 CKO group, and female mice showed more significant difference (Fig. S4F–G). The ratio of weight to length was significantly higher in WT group compared to NUMA1 CKO group, and especially in the female NUMA1 CKO group (Fig. 4G–H). After 4NQO treatment, we found more severe dysplasia in esophageal sections of the WT mice compared to CKO group, and some mice already developed carcinoma in situ and invasive carcinoma. Consistently, Ki67 levels were highly expressed in esophagus tissues with higher tumor burden (Fig. 4I–K). These data reveal that NUMA1 acts as an oncogene, accelerates ESCC carcinogenesis and promotes 4NQO-induced tumor development.
Fig. 4.
NUMA1 conditional knock-out suppresses tumorigenesis in 4NQO-induced esophageal carcinogenesis model. A Example of PCR genotyping for NUMA1 conditional knock out mice, Wild type (WT), flox heterozygous (Heter, +/–), and flox homozygous (Homo, −/−), Cre gene positive (Cre +) and Cre gene negative (Cre−). B Illustration of the experimental procedures used to induce esophageal carcinogenesis in NUMA1 conditional knock out mice (CKO, flox homozygous and Cre gene positive) and Wild type control mice (WT, flox homozygous and Cre gene negative). Vehicle (H2O) and 4NQO (100 μg/ml) was administered in the drinking water for 16 weeks, followed by 9 weeks of regular water. C Body weight of mice, mice weighed once every week. D Representative images of esophageal at necropsy. E Number of tumors (WT + Vehicle, n = 16; WT + 4NQO, n = 36; NUMA1-CKO + Vehicle, n = 16; NUMA1-CKO + 4NQO, n = 36). F Number of tumors (Male WT + 4NQO, n = 18; Male NUMA1-CKO + 4NQO, n = 18; Female WT + 4NQO, n = 18; Female NUMA1-CKO + 4NQO, n = 18). G weight to length ratio (WT + Vehicle, n = 16; WT + 4NQO, n = 36; NUMA1-CKO + Vehicle, n = 16; NUMA1-CKO + 4NQO, n = 36). H weight to length ratio (Male WT + 4NQO, n = 18; Male NUMA1-CKO + 4NQO, n = 18; Female WT + 4NQO, n = 18; Female NUMA1-CKO + 4NQO, n = 18). I H&E staining of tumor morphology and Ki67 staining in esophageal tissue (100 × ; Scale bar: 50 μm). J The expression level of Ki67 (WT + Vehicle, n = 16; WT + 4NQO, n = 36; NUMA1-CKO + Vehicle, n = 16; NUMA1-CKO + 4NQO, n = 36). K Ki67 expression (Male WT + 4NQO, n = 18; Male NUMA1-CKO + 4NQO, n = 18; Female WT + 4NQO, n = 18; Female NUMA1-CKO + 4NQO, n = 18). Results are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
NUMA1 interacts with GSTP1 and promotes the interaction between GSTP1 and TRAF2
To identify the oncogenic potential role of NUMA1 and the molecular mechanisms, we continued to explore the potential substrate of NUMA1 in ESCC by using co-IP (co-immunoprecipitation) assay and following LC–MS/MS analysis (Fig. S5A). We found GSTP1 was the top protein according to the MS score (Table S2). We further performed co-IP assays to confirm whether GSTP1 interacts with NUMA1. The results showed that endogenous NUMA1 can precipitate endogenous GSTP1 in KYSE510 cells (Fig. 5A). Furthermore, we ectopically expressed NUMA1 in KYSE450 and KYSE510, and the co-IP results showed that Flag-NUMA1 precipitated endogenous GSTP1 (Fig. 5B). We also performed co-IP assay with ectopically expressed NUMA1 and GSTP1 in KYSE150 cells, and Flag-NUMA1 was found to precipitate HA-GSTP and vice versa (Fig. 5C). To identify the domain of NUMA1 involved in its association with GSTP1, we constructed a series of Flag-tagged NUMA1 domain deletion mutants, and the schematic diagram is shown (Fig. 5D). The results of co-IP assay using Flag magnetic agarose revealed that C-terminal domain of NUMA1 was responsible for its interaction with GSTP1 (Fig. 5E). It was reported that GSTP1 regulated JNK downstream signaling and apoptosis through interacted with TRAF2 [20]. Therefore, we confirmed the interaction between GSTP1 and TRAF2 through exogenous immunoprecipitation, and indeed, GSTP1 interacted with TRAF2 (Fig. 5F). Then, we investigated whether NUMA1 could bind with TRAF2 through exogenous immunoprecipitation. Results showed that NUMA1 interacted with TRAF2 (Fig. 5G). Furthermore, we explored whether NUMA1 binding with GSTP1 could affect the association of GSTP1 and TRAF2. HA-GSTP1 and His-TRAF2 were co-transfected with increasing plasmid concentrations of Flag-NUMA1 (0–5 µg) into KYSE150 cells, following immunoprecipitation of His-TRAF2, HA-GSTP1 and Flag-NUMA1 was precipitated, and quantified by Western blot. Results showed an increasing NUMA1 expression promoted the interaction between TRAF2 and GSTP1 (Fig. 5H). We also got the same results using HEK293T cells (Fig. S5B). On the contrary, reduced NUMA1 expression decreased the endogenous association between GSTP1 and TARF2 (Fig. 5I). We then further investigated which domain of TRAF2 could bind with NUMA1, a series of His-tagged TRAF2 domain deletion mutants were constructed, and the schematic diagram is shown (Fig. 5J). The results of co-IP assay using Flag-tagged NUMA1 revealed that the Zinc finger motif of TRAF2 directly bound with NUMA1 (Fig. 5K). Collectively, these findings reveal that NUMA1 interacts with GSTP1 and TRAF2, and promotes the interaction between TRAF2 and GSTP1.
Fig. 5.
NUMA1 interacts with GSTP1 and promotes the interaction between GSTP1 and TRAF2. A Lysates from KYSE510 cells were immunoprecipitated with anti-NUMA1 antibody and then, immunoblotted with GSTP1 antibody. B KYSE450 and KYSE510 were transiently transfected Flag-tagged NUMA1 plasmid, 48 h later, collected cell pellet and got the cell lysates, immunoprecipitated Flag-tagged NUMA1 by anti-Flag tag antibody, and Western blot checked the expression of GSTP1. C Flag-tagged NUMA1 and HA tagged GSTP1 were transiently transfected into KYSE150 cells, and the cell lysates were then subjected to co-immunoprecipitation using anti-Flag tag and anti-HA tag antibody, and analysis the expression with the indicated antibodies by Western blotting. D Schematic diagram of wild type NUMA1 and its deletion mutants. E HEK293T cells were transfected with wild type NUMA1 and its different deletion mutants together with HA tagged GSTP1, cell lysates were immunoprecipitated with anti-Flag magnetic agarose, and co-precipitated HA-GSTP1 was detected by Western blotting. F HA tagged GSTP1 was transiently co-transfected with Flag-tagged TRAF2 into HEK293T cells, Flag-tagged TRAF2 was immunoprecipitated by anti-Flag magnetic agarose, co-precipitated HA tagged GSTP1 was detected by immunoblot. G Flag-tagged NUMA1 and His tagged TRAF2 were co-transfected into HEK293T cells, cell lysates were subjected to immunoprecipitation using anti-Flag magnetic agarose, then analysis the indicated antibodies. H KYSE150 cells were transfected with the indicated amounts of HA tagged GSTP1 (5 μg), Flag-tagged NUMA1 (2.5 μg or 5 μg) and His tagged TRAF2 (5 μg), then immunoprecipitated with anti-His antibody, and analyzed by Western blotting used the indicated antibodies. I KYSE150 cells were infected with shNUMA1 lentivirus and scramble control lentivirus, collected the cell pellet and used the cell lysates do immunoprecipitation using anti-GSTP1 antibody, then checked the expression of GSTP1 and TRAF2. J Illustration of wild type TRAF2 and different TRAF2 domain deletion constructs. K Flag-tagged NUMA1 was transiently co-transfected into HEK293T cells with expression plasmids for wild type and deletion mutate constructs of TRAF2, then immunoprecipitated with anti-Flag magnetic agarose, and examined the expression of indicated antibodies
NUMA1 regulates ASK1-MKK4-SAPK/JNK signaling through binding with TRAF2 and GSTP1
The above results prompted us to examine the function of NUMA1 on regulating of ASK1-MKK4-SAPK/JNK signaling. Apoptosis signal-regulating kinase 1 (ASK1) can activate MKK4 and then, continuously phosphorylates and activates JNK, leading to cell apoptosis [21–24]. It has been reported that JNK activated by ASK1 promotes cancer cell apoptosis in different types of cancer, including lung adenocarcinoma [25, 26], hepatocellular carcinoma [27], and breast cancer [21, 28, 29]. We already confirmed that C-terminal domain of NUMA1 is important for its interaction with GSTP1, and Zinc finger motif of TRAF2 is important for its interaction with NUMA1. Then, we investigated which domain of NUMA1 is responsible for its binding with TRAF2. Co-IP results using Flag-tagged NUMA1 domain deletion mutants revealed that Globular domain, C-terminal domain and part of the coiled domain interacted with TRAF2 (Fig. 6A). In the next, we examined the domain of TRAF2 responsible for its interaction with GSTP1. Results showed that both RING finger motif and TRAF domain of TRAF2 interacted with GSTP1 (Fig. 6B). We further found that NUMA1 can significantly downregulated the amount of TRAF2 immunoprecipitated by ASK1 (Fig. 6C). What is more, increasing NUMA1 expression decreased the association between TRAF2 and ASK1 more significantly (Fig. 6D). In summary, NUMA1 can promote the interaction between TRAF2 and GSTP1, and inhibit the interaction between TRAF2 and ASK1, through regulating protein–protein interaction. To further determine the effect of NUMA1 on ASK1-MKK4-SAPK/JNK signaling, we measured the phosphorylation status of MKK4 and JNK in ESCC cells after knock-down or overexpression NUMA1. The phosphorylation of MKK4, JNK and c-Jun was increased after knocking-down NUMA1, and the expression level of GSTP1 and TRAF2 was not changed (Fig. 6E). On the contrary, overexpression of NUMA1 in KYSE150 cells inhibited the phosphorylation of SAPK/JNK and c-Jun (Fig. 6F). Furthermore, we do co-knockdown of TRAF2, NUMA1 and GSTP1 in KYSE150 and KYSE450 cells, and results showed that cell proliferation was significantly downregulated (Fig. S6A, B). On the contrary, overexpression of TRAF2, NUMA1 and GSTP1 significantly upregulated cell proliferation in ESCC cells (Fig. S6C, D). To further confirm that NUMA1 inhibited the activation of JNK signaling through the regulation of interaction between GSTP1, TRAF2 and ASK1, we co-transfected the overexpression plasmid of Flag-NUMA1, His-GSTP1, His-TRAF2, Myc-MKK4 and HA-ASK1 into HEK293T and KYSE150 cells and co-immunoprecipitated using anti-c-Myc magnetic agarose and then, immunoblotted with p-SEK1/MKK4 antibody. We found a significant downregulation of MKK4 and JNK phosphorylation level (Figs. 6G and S6E). Next, the endogenous p-SAPK/JNK and p-MKK4 levels in HEK293T cells after transiently overexpression GSTP1, TRAF2 and NUMA1 were evaluated. The results showed that the overexpression of NUMA1 together with TRAF2 and GSTP1 significantly inhibited the activation of MKK4 and JNK (Fig. 6H). Taken together, these results indicate that NUMA1 regulates ASK1-MKK4-SAPK/JNK signaling through regulate the interaction of TRAF2 and GSTP1.
Fig. 6.
NUMA1 regulates ASK1-MKK4-SAPK/JNK signaling through binding with TRAF2 and GSTP1. A HEK293T cells were transfected with His tagged TRAF2 and Flag-tagged wild type NUMA1 and different NUMA1 domain deletion mutants, cell lysates were immunoprecipitated with anti-Flag magnetic agarose, performed Western blot. B His tagged wild type and deletion mutants of TRAF2 were co-transfected with HA tagged GSTP1, proteins were extracted and immunoprecipitated with anti-HA magnetic agarose, and checked the expression of co-precipitated TRAF2 domain by Western blotting. C His tagged TRAF2(2.5 μg), Flag-tagged NUMA1(5 μg) and HA tagged ASK1(5 μg) were co-transfected into HEK293T cells, cell lysates were extracted and co-immunoprecipitation were performed use anti-HA magnetic agarose, checked the indicated antibodies by Western blot. D HEK293T cells were transfected with the indicated amounts of HA tagged ASK1 (5 μg), Flag-tagged NUMA1 (5 μg or 10 μg) and His tagged TRAF2 (2.5 μg), then immunoprecipitated with anti-HA magnetic agarose, and analyzed by Western blotting used the indicated antibodies. E Cell lysates from KYSE150, KYSE450 and KYSE510 scramble control cells and NUMA1 knock down cells were collected, p-SEK1/MKK4 (Ser257), p-SAPK/JNK (Thr183/Tyr185), p–c-Jun (Ser73), GSTP1 and TRAF2 were detected by Western blot. F Cell lysates from KYSE150 control cells and NUMA1 overexpression cells were immunoblotted with the indicated antibodies. G HEK293T cells were transfected with the indicated plasmids, 48 h later, collected cell pellet, protein extracted and immunoprecipitated with anti-c-Myc magnetic agarose, immunoblotted with the indicated antibodies and quantified the expression using ImageJ software (n = 3, representative Western blots). H HEK293T cells were transfected with the indicated plasmids, the expression of the indicated proteins was detected by Western blot and quantified by ImageJ software (n = 3, representative Western blots)
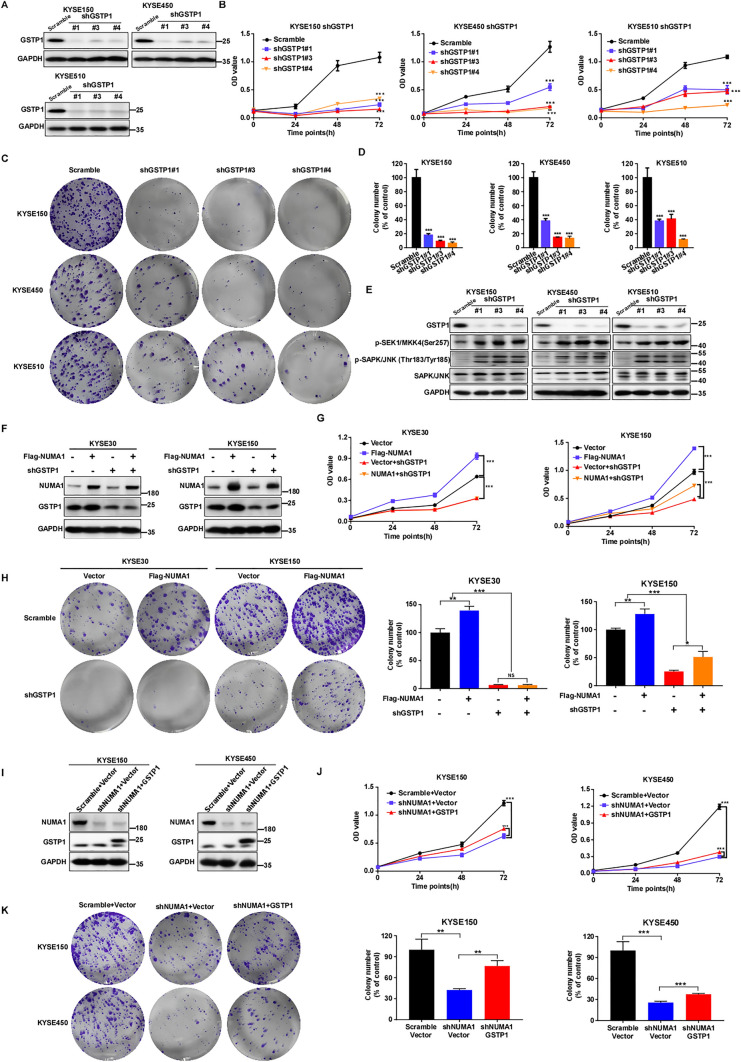
GSTP1 mediates the oncogenic effect of NUMA1 in ESCC
To further verify the function of GSTP1 in human ESCC, we generated GSTP1 knock-down cells (Fig. 7A) and evaluated cell proliferation and colony formation. Silencing the GSTP1 expression significantly inhibited ESCC cell proliferation and colony formation (Figs. 7B–D, S7A). Furthermore, we examined the signaling cascade after knocking-down GSTP1 and found MKK4 and JNK phosphorylation levels were upregulated (Fig. 7E). To investigate whether GSTP1 mediated the oncogenic effect of NUMA1 in ESCC, rescue experiments were performed. KYSE30 and KYSE150 cells transfected with vector or flag-tagged NUMA1 were infected with scramble and shGSTP1 lentivirus, respectively (Fig. 7F). Compared with control cells, overexpression of NUMA1 significantly increased cell proliferation and colony formation, while GSTP1 knock-down rescued this phenomenon partially (Figs. 7G, H and S7B). In addition, after scramble or shNUMA1- KYSE150 and -KYSE450 cells were overexpressed with control or HA tagged GSTP1 (Fig. 7I), cell proliferation and colony formation after silencing NUMA1 expression were significantly inhibited, while GSTP1 overexpression partially rescued this phenotype (Figs. 7J, K and S7C). Taken together, these results demonstrate that GSTP1 mediates oncogenic function of NUMA1 in ESCC cells.
Fig. 7.
GSTP1 mediates the oncogenic effect of NUMA1 in ESCC. A KYSE150, KYSE450 and KYSE510 cells with GSTP1 knock-down were established, GSTP1 expression was detected by Western blot. B Cell proliferation was tested by MTT in different GSTP1 knock down cells (n = 3, independent experiments). C Colony formation was measured after silencing GSTP1 in ESCC cells, the represent images were shown. D Number of colonies determined by Image Pro Plus software (Scale bar: 200 μm, n = 3, independent experiments). E p-SEK1/MKK4(Ser257) and p-SAPK/JNK (Thr183/Tyr185) were detected after knockdown GSTP1 in KYSE150, KYSE450 and KYSE510 by Western blot. F Cells with NUMA1 overexpression and GSTP1 knock-down was established, and the expression of NUMA1 and GSTP1 was detected by Western blot. G Cell proliferation was measured by MTT assay in KYSE30 and KYSE150 cells with NUMA1 overexpression and GSTP1 knock-down (n = 3, independent experiments). H Colony formation was detected for the indicated cells. Left panels: representative images (Scale bar: 200 μm). Right panels: colony number was counted use Image Pro Plus software (n = 3, independent experiments), and statistical analysis were shown. I Cells with NUMA1 knock-down and GSTP1 overexpression was established and examined the expression of NUMA1 and GSTP1 by Western blot. J Cell proliferation was measured by MTT assay for the indicated cells (n = 3, independent experiments). K Colony formation was tested in KYSE150 and KYSE450 after NUMA1 knock-down and GSTP1 overexpression. Left panels: representative images (Scale bar: 200 μm). Right panels: Image Pro Plus software counted the colony number and statistical analysis were shown (n = 3, independent experiments). Data were presented as mean ± SD. Statistical analysis using Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001
Silencing NUMA1 expression delays tumor growth in ESCC patient-derived xenograft mice model
To further asses the effect of NUMA1 on ESCC progression in vivo, we established a patient-derived xenograft (PDX) mice model and performed lentivirus-mediated NUMA1 knock-down. The expression of NUMA1 in patient samples was evaluated by Western blot, and we selected high expressed cases including LEG32 and LEG104 to establish PDX model and conduct lentivirus infection (Fig. S8A). The patient characteristics of LEG32 and LEG104 were shown (Fig. 8A). Knock-down of NUMA1 by lentivirus infection significantly retarded tumor growth, the tumor volume and tumor weight were downregulated significantly compared to the control group (Fig. 8B, C). While, for the mice average body weight, there is no significant change (Fig. S8B). Next, to further confirm whether the shNUMA1 lentivirus infection antitumor effect was associated with silencing NUMA1 expression, we measured the expression of NUMA1 using tumor tissue extracts from each group and quantified the expression. The results showed a significantly downregulated expression of NUMA1 compared to scrambled control (Fig. 8D, E). Moreover, immunohistochemical analysis results also showed a reduced expression of NUMA1 and Ki67, and a significantly induced expression of p-SAPK/JNK (Thr183/Tyr185) and p-c-Jun (Ser73) compared to scrambled control group (Fig. 8F, G). These findings reveal that inhibition of NUMA1 expression retards ESCC PDX tumor growth in vivo.
Fig. 8.
Lentivirus-mediated NUMA1 knock-down inhibits ESCC tumor growth in PDX mice model. A Patient clinical information for tumor tissues used in the PDX mice models. B, C LEG32 and LEG104 tumor bearing mice received lentivirus (scramble, shNUMA1#3, shNUMA1#4) intratumor injection twice one week for three weeks, tumor volume was measured twice one week, tumor photographs were shown and tumor weight was checked after mice sacrifice (LEG32, n = 9/group; LEG104, n = 11/group). D Western blot evaluated the expression of NUMA1 in tumor tissues of PDX mice. E Quantification of NUMA1 expression (LEG32, n = 8/group; LEG104, n = 11/group). F Immunohistochemical analysis showed the expression of Ki67, NUMA1, p-SAPK/JNK (Thr183/Tyr185) and p-c-Jun (Ser73) in harvested tumor tissues, representative images are shown. G Statistical analysis of Ki67, NUMA1, p-SAPK/JNK (Thr183/Tyr185) and p–c-Jun (Ser73) expression levels (LEG32 scramble n = 9; LEG32 shNUMA1#3 n = 6; LEG32 shNUMA1#4 n = 9; LEG104 n = 11/group). All data are presented as mean ± SD, results were analyzed with the unpaired student’s t-test, *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
We reported here for the first time that the expression of NUMA1 is upregulated in ESCC tissues compared with corresponding adjacent tissues and increased NUMA1 expression predicated poor patient survival (Fig. 1A–G). To further determine the impact of NUMA1 knock-down on ESCC cells, we established NUMA1 knock-down cells using lentivirus-based shRNA delivery. Results showed that silencing NUMA1 expression significantly reduced ESCC cell proliferation and colony formation, induced cell apoptosis and cell cycle arrest (Figs. 2A–H and 3). Moreover, in silencing NUMA1 CDX mice model, the tumor growth was significantly decreased in vivo (Fig. 2I–N). It means that NUMA1 might play an important role in the progression of ESCC.
4NQO-induced ESCC mice model is based on its function to induce DNA damage and mutations [30, 31]. Specifically, esophageal tumors developed in this model showed the pathophysiological and molecular features of human ESCC [30, 32]. In this study, we used 4NQO-induced ESCC model confirmed the oncogenic functions of NUMA1 during ESCC progression and esophageal conditional knockout of NUMA1 significantly inhibited the esophageal tumorigenesis induced by 4NQO (Fig. 4). The PDX model accurately recapitulates the tumor behavior observed in patients and is a preferred mice model for clinical translation research [33–35]. Therefore, to investigate the potential function of NUMA1 in ESCC patient-derived tumors, we used lentivirus intratumor injection silencing NUMA1 expression in ESCC PDX mice and found NUMA1 silencing significantly suppressed ESCC tumor growth (Fig. 8). Thus, NUMA1 plays an important role during ESCC progression.
In order to elucidate the molecular mechanisms of NUMA1, we successfully identified GSTP1 as a novel NUMA1 interaction protein (Fig. 5A–C). GSTP1 is a phase II detoxifying enzyme, and it participates in various physiological processes including antioxidant, detoxification and regulate signal transduction through protein–protein interaction [36–38]. GSTP1 can regulate the activation of JNK through directly interact with JNK, or regulate the interaction between TRAF2 and ASK1 [20, 36, 39–41]. GSTP1 is ubiquitously expressed in mammalian cells and evaluated in many different cancers, such as colorectal cancer [42], triple-negative breast cancer [43] and glioblastoma [44]. Our results show that NUMA1 interacts with GSTP1 and TRAF2, and promote the interaction between GSTP1 and TRAF2 (Fig. 5G–I). Then, we investigated whether NUMA1 can modulate the interaction of GSTP1, TRAF2 and ASK1. We confirmed the interaction domain of NUMA1, TRAF2 and GSTP1 through domain truncation. The C-terminal domain of NUMA1 is important for its interaction with GSTP1 and TRAF2, and TRAF2 can also interact with the globular domain and part of coiled domain of NUMA1. TRAF2 interacts with NUMA1 through its Zinc finger motif and interacts with GSTP1 through its RING finger motif and TRAF domain. Co-IP results also confirmed that NUMA1 can inhibit the interaction between TRAF2 and ASK1 (Fig. 6C, D). What is more, knock-down of NUMA1 upregulated the phosphorylation of MKK4 and JNK. In order to further confirm whether NUMA1 can inhibit the activation of JNK signaling through promote the interaction between GSTP1 and TRAF2, we overexpressed NUMA1 together with TRAF2 and GSTP1 into HEK293T cells, and NUMA1 can significantly inhibit the activation of MKK4 and JNK when co-overexpression with GSTP1 (Fig. 6H). No matter Knockdown GSTP1 after overexpression NUMA1 or overexpression GSTP1 after knockdown NUMA1, GSTP1 all can partially reverse the function of NUMA1(Fig. 7F–K). Therefore, NUMA1 modulates ASK1-MKK4-SAPK/JNK signaling pathway through promote the interaction between GSTP1 and TRAF2, thus to inhibit the association of TRAF2 and ASK1, and finally, inhibit TRAF2-activated ASK1-MKK4-SAPK/JNK signaling cascade (Fig. 9).
Fig. 9.
A schematic model for the findings of this paper. NUMA1 can interact with GSTP1 and TRAF2, promote the interaction of GSTP1 and TRAF2, and inhibit the interaction between TRAF2 and ASK1, thus to promote the inhibition of ASK1 activation induced by interaction with TRAF2. Aberrant NUMA1 in ESCC inhibited TRAF2-ASK1-induced cell apoptosis by suppressing the sustained activation of JNK
Our results indicate that NUMA1 is a critical oncogenic factor during ESCC progression, and its high expression predicts poor patient survival in ESCC patient. Moreover, we report here that NUMA1 exerts a strong accelerative effect on ESCC tumorigenesis. Mechanically, NUMA1 regulates cell apoptosis through inhibition of sustained JNK activation via interaction with GSTP1 and TRAF2. This novel mechanism might be used for design new therapeutic approaches for ESCC and worthy more investigation.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
SY, M-HL and ZD designed the study, revised the article and drafted the manuscript. SY, ZZ, RW and JT performed the experiments and obtained the data. SY, SZ, FL, YS, RY and MS performed mouse experiments and interpretation of data. SY, JL, XM, KL and RZ supported materials. SY, M-HL and ZD wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grant funding from the National Natural Science Foundation of China (NSFC 82103195, NSFC81972839) and Major Science and Technology Projects in Henan Province (Grant number: 221100310100).
Data availability
All data generated or analyzed during this study are available from the corresponding authors on reasonable request.
Declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
Animal studies were approved by the Ethics Committee of China-US(Henan) Hormel Cancer Institute (CUHCI2019032).
Consent to participate
Not applicable.
Consent to publish
All authors have given consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mee-Hyun Lee, Email: mhyun_lee@hanmail.net.
Zigang Dong, Email: dongzg@zzu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, Cunningham D. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. doi: 10.1038/nrdp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J, Arnold M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163(3):649–658. doi: 10.1053/j.gastro.2022.05.054. [DOI] [PubMed] [Google Scholar]
- 4.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo e Silva G, Chen W-Q, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP, Bouzbid S, Hamdi-Chérif M, Zaidi Z, Meguenni K, Regagba D, Bayo S, Cheick Bougadari T, Manraj SS, Bendahhou K, Fabowale A, Bradshaw D, Somdyala NIM, Kumcher I, Moreno F, Calabrano GH, Espinola SB, Carballo Quintero B, Fita R, Diumenjo MC, Laspada WD, Ibañez SG, Lima CA, De Souza PCF, Del Pino K, Laporte C, Curado MP, de Oliveira JC, Veneziano CLA, Veneziano DB, Latorre MRDO, Tanaka LF, Rebelo MS, Santos MO, Galaz JC, Aparicio Aravena M, Sanhueza Monsalve J, Herrmann DA, Vargas S, Herrera VM, Uribe CJ, Bravo LE, Garcia LS, Arias-Ortiz NE, Morantes D, Jurado DM, Yépez Chamorro MC, Delgado S, Ramirez M, Galán Alvarez YH, Torres P, Martínez-Reyes F, Jaramillo L, Quinto R, Castillo J, Mendoza M, Cueva P, Yépez JG, Bhakkan B, Deloumeaux J, Joachim C, Macni J, Carrillo R, Shalkow Klincovstein J, Rivera Gomez R, Poquioma E, Tortolero-Luna G, Zavala D, Alonso R, Barrios E, Eckstrand A, Nikiforuk C, Noonan G, Turner D, Kumar E, Zhang B, McCrate FR, Ryan S, MacIntyre M, Saint-Jacques N, Nishri DE, McClure CA, Vriends KA, Kozie S, Stuart-Panko H, Freeman T, George JT, Brockhouse JT, O'Brien DK, Holt A, Almon L, Kwong S, Morris C, Rycroft R, Mueller L, Phillips CE, Brown H, Cromartie B, Schwartz AG, Vigneau F, Levin GM, Wohler B, Bayakly R, Ward KC, Gomez SL, McKinley M, Cress R, Green MD, Miyagi K, Ruppert LP, Lynch CF, Huang B, Tucker TC, Deapen D, Liu L, Hsieh MC, Wu XC, Schwenn M, Gershman ST, Knowlton RC, Alverson G, Copeland GE, Bushhouse S, Rogers DB, Jackson-Thompson J, Lemons D, Zimmerman HJ, Hood M, Roberts-Johnson J, Rees JR, Riddle B, Pawlish KS, Stroup A, Key C, Wiggins C, Kahn AR, Schymura MJ, Radhakrishnan S, Rao C, Giljahn LK, Slocumb RM, Espinoza RE, Khan F, Aird KG, Beran T, Rubertone JJ, Slack SJ, Garcia L, Rousseau DL, Janes TA, Schwartz SM, Bolick SW, Hurley DM, Whiteside MA, Miller-Gianturco P, Williams MA, Herget K, Sweeney C, Johnson AT, Keitheri Cheteri MB, Migliore Santiago P, Blankenship SE, Farley S, Borchers R, Malicki R, Espinoza JR, Grandpre J, Wilson R, Edwards BK, Mariotto A, Lei Y, Wang N, Chen JS, Zhou Y, He YT, Song GH, Gu XP, Mei D, Mu HJ, Ge HM, Wu TH, Li YY, Zhao DL, Jin F, Zhang JH, Zhu FD, Junhua Q, Yang YL, Jiang CX, Biao W, Wang J, Li QL, Yi H, Zhou X, Dong J, Li W, Fu FX, Liu SZ, Chen JG, Zhu J, Li YH, Lu YQ, Fan M, Huang SQ, Guo GP, Zhaolai H, Wei K, Zeng H, Demetriou AV, Mang WK, Ngan KC, Kataki AC, Krishnatreya M, Jayalekshmi PA, Sebastian P, Nandakumar A, Malekzadeh R, Roshandel G, Keinan-Boker L, Silverman BG, Ito H, Nakagawa H, Sato M, Tobori F, Nakata I, Teramoto N, Hattori M, Kaizaki Y, Moki F, Sugiyama H, Utada M, Nishimura M, Yoshida K, Kurosawa K, Nemoto Y, Narimatsu H, Sakaguchi M, Kanemura S, Naito M, Narisawa R, Miyashiro I, Nakata K, Sato S, Yoshii M, Oki I, Fukushima N, Shibata A, Iwasa K, Ono C, Nimri O, Jung KW, Won YJ, Alawadhi E, Elbasmi A, Ab Manan A, Adam F, Sanjaajmats E, Tudev U, Ochir C, Al Khater AM, El Mistiri MM, Teo YY, Chiang CJ, Lee WC, Buasom R, Sangrajrang S, Kamsa-ard S, Wiangnon S, Daoprasert K, Pongnikorn D, Leklob A, Sangkitipaiboon S, Geater SL, Sriplung H, Ceylan O, Kög I, Dirican O, Köse T, Gurbuz T, Karaşahin FE, Turhan D, Aktaş U, Halat Y, Yakut CI, Altinisik M, Cavusoglu Y, Türkköylü A, Üçüncü N, Hackl M, Zborovskaya AA, Aleinikova OV, Henau K, Van Eycken L, Valerianova Z, Yordanova MR, Šekerija M, Dušek L, Zvolský M, Storm H, Innos K, Mägi M, Malila N, Seppä K, Jégu J, Velten M, Cornet E, Troussard X, Bouvier AM, Guizard AV, Bouvier V, Launoy G, Arveux P, Maynadié M, Mounier M, Woronoff AS, Daoulas M, Robaszkiewicz M, Clavel J, Goujon S, Lacour B, Baldi I, Pouchieu C, Amadeo B, Coureau G, Orazio S, Preux PM, Rharbaoui F, Marrer E, Trétarre B, Colonna M, Delafosse P, Ligier K, Plouvier S, Cowppli-Bony A, Molinié F, Bara S, Ganry O, Lapôtre-Ledoux B, Grosclaude P, Bossard N, Uhry Z, Bray F, Piñeros M, Stabenow R, Wilsdorf-Köhler H, Eberle A, Luttmann S, Löhden I, Nennecke AL, Kieschke J, Sirri E, Emrich K, Zeissig SR, Holleczek B, Eisemann N, Katalinic A, Asquez RA, Kumar V, Petridou E, Ólafsdóttir EJ, Tryggvadóttir L, Clough-Gorr K, Walsh PM, Sundseth H, Mazzoleni G, Vittadello F, Coviello E, Cuccaro F, Galasso R, Sampietro G, Giacomin A, Magoni M, Ardizzone A, D'Argenzio A, Castaing M, Grosso G, Lavecchia AM, Sutera Sardo A, Gola G, Gatti L, Ricci P, Ferretti S, Serraino D, Zucchetto A, Celesia MV, Filiberti RA, Pannozzo F, Melcarne A, Quarta F, Russo AG, Carrozzi G, Cirilli C, Cavalieri d'Oro L, Rognoni M, Fusco M, Vitale MF, Usala M, Cusimano R, Mazzucco W, Michiara M, Sgargi P, Boschetti L, Borciani E, Seghini P, Maule MM, Merletti F, Tumino R, Mancuso P, Vicentini M, Cassetti T, Sassatelli R, Falcini F, Giorgetti S, Caiazzo AL, Cavallo R, Cesaraccio R, Pirino DR, Contrino ML, Tisano F, Fanetti AC, Maspero S, Carone S, Mincuzzi A, Candela G, Scuderi T, Gentilini MA, Piffer S, Rosso S, Barchielli A, Caldarella A, Bianconi F, Stracci F, Contiero P, Tagliabue G, Rugge M, Zorzi M, Beggiato S, Brustolin A, Berrino F, Gatta G, Sant M, Buzzoni C, Mangone L, Capocaccia R, De Angelis R, Zanetti R, Maurina A, Pildava S, Lipunova N, Vincerževskiené I, Agius D, Calleja N, Siesling S, Larønningen S, Møller B, Dyzmann-Sroka A, Trojanowski M, Góźdź S, Mężyk R, Mierzwa T, Molong L, Rachtan J, Szewczyk S, Błaszczyk J, Kępska K, Kościańska B, Tarocińska K, Zwierko M, Drosik K, Maksimowicz KM, Purwin-Porowska E, Reca E, Wójcik-Tomaszewska J, Tukiendorf A, Grądalska-Lampart M, Radziszewska AU, Gos A, Talerczyk M, Wyborska M, Didkowska JA, Wojciechowska U, Bielska-Lasota M, Forjaz de Lacerda G, Rego RA, Bastos J, Silva MA, Antunes L, Laranja Pontes J, Mayer-da-Silva A, Miranda A, Blaga LM, Coza D, Gusenkova L, Lazarevich O, Prudnikova O, Vjushkov DM, Egorova AG, Orlov AE, Kudyakov LA, Pikalova LV, Adamcik J, Safaei Diba C, Primic-Žakelj M, Zadnik V, Larrañaga N, Lopez de Munain A, Herrera AA, Redondas R, Marcos-Gragera R, Vilardell Gil ML, Molina E, Sánchez Perez MJ, Franch Sureda P, Ramos Montserrat M, Chirlaque MD, Navarro C, Ardanaz EE, Guevara MM, Fernández-Delgado R, Peris-Bonet R, Carulla M, Galceran J, Alberich C, Vicente-Raneda M, Khan S, Pettersson D, Dickman P, Avelina I, Staehelin K, Camey B, Bouchardy C, Schaffar R, Frick H, Herrmann C, Bulliard JL, Maspoli-Conconi M, Kuehni CE, Redmond SM, Bordoni A, Ortelli L, Chiolero A, Konzelmann I, Matthes KL, Rohrmann S, Broggio J, Rashbass J, Fitzpatrick D, Gavin A, Clark DI, Deas AJ, Huws DW, White C, Montel L, Rachet B, Turculet AD, Stephens R, Chalker E, Phung H, Walton R, You H, Guthridge S, Johnson F, Gordon P, D'Onise K, Priest K, Stokes BC, Venn A, Farrugia H, Thursfield V, Dowling J, Currow D, Hendrix J, Lewis C (2018) Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet 391 (10125):1023–1075. 10.1016/s0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed]
- 5.van Rossum PSN, Mohammad NH, Vleggaar FP, van Hillegersberg R. Treatment for unresectable or metastatic oesophageal cancer: current evidence and trends. Nat Rev Gastroenterol Hepatol. 2018;15(4):235–249. doi: 10.1038/nrgastro.2017.162. [DOI] [PubMed] [Google Scholar]
- 6.Yang YM, Hong P, Xu WW, He QY, Li B. Advances in targeted therapy for esophageal cancer. Signal Transduct Target Ther. 2020;5(1):229. doi: 10.1038/s41392-020-00323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan W, Liu Z, Wang Y, Liu M, Pan Y, Lei W, Yang H, Xu R, Zhang L, Cai H, Li J, Ke Y. Clonal evolution of esophageal squamous cell carcinoma from normal mucosa to primary tumor and metastases. Carcinogenesis. 2019;40(12):1445–1451. doi: 10.1093/carcin/bgz162. [DOI] [PubMed] [Google Scholar]
- 8.Lin DC, Wang MR, Koeffler HP. Genomic and epigenomic aberrations in esophageal squamous cell carcinoma and implications for patients. Gastroenterology. 2018;154(2):374–389. doi: 10.1053/j.gastro.2017.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radulescu AE, Cleveland DW. NuMA after 30 years: the matrix revisited. Trends Cell Biol. 2010;20(4):214–222. doi: 10.1016/j.tcb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polverino F, Naso FD, Asteriti IA, Palmerini V, Singh D, Valente D, Bird AW, Rosa A, Mapelli M, Guarguaglini G. The Aurora-A/TPX2 axis directs spindle orientation in adherent human cells by regulating NuMA and microtubule stability. Curr Biol. 2021;31(3):658–667. doi: 10.1016/j.cub.2020.10.096. [DOI] [PubMed] [Google Scholar]
- 11.Pirovano L, Culurgioni S, Carminati M, Alfieri A, Monzani S, Cecatiello V, Gaddoni C, Rizzelli F, Foadi J, Pasqualato S, Mapelli M. Hexameric NuMA:LGN structures promote multivalent interactions required for planar epithelial divisions. Nat Commun. 2019;10(1):2208. doi: 10.1038/s41467-019-09999-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvador Moreno N, Liu J, Haas KM, Parker LL, Chakraborty C, Kron SJ, Hodges K, Miller LD, Langefeld C, Robinson PJ, Lelievre SA, Vidi PA. The nuclear structural protein NuMA is a negative regulator of 53BP1 in DNA double-strand break repair. Nucleic Acids Res. 2019;47(6):2703–2715. doi: 10.1093/nar/gkz138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotak S, Busso C, Gonczy P. NuMA phosphorylation by CDK1 couples mitotic progression with cortical dynein function. EMBO J. 2013;32(18):2517–2529. doi: 10.1038/emboj.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ban KH, Torres JZ, Miller JJ, Mikhailov A, Nachury MV, Tung JJ, Rieder CL, Jackson PK. The END network couples spindle pole assembly to inhibition of the anaphase-promoting complex/cyclosome in early mitosis. Dev Cell. 2007;13(1):29–42. doi: 10.1016/j.devcel.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Gallini S, Carminati M, De Mattia F, Pirovano L, Martini E, Oldani A, Asteriti IA, Guarguaglini G, Mapelli M. NuMA phosphorylation by Aurora—a orchestrates spindle orientation. Curr Biol. 2016;26(4):458–469. doi: 10.1016/j.cub.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Ray S, Abugable AA, Parker J, Liversidge K, Palminha NM, Liao C, Acosta-Martin AE, Souza CDS, Jurga M, Sudbery I, El-Khamisy SF. A mechanism for oxidative damage repair at gene regulatory elements. Nature. 2022;609(7929):1038–1047. doi: 10.1038/s41586-022-05217-8. [DOI] [PubMed] [Google Scholar]
- 17.Endo A, Moyori A, Kobayashi A, Wong RW. Nuclear mitotic apparatus protein, NuMA, modulates p53-mediated transcription in cancer cells. Cell Death Dis. 2013;4:e713. doi: 10.1038/cddis.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohata H, Miyazaki M, Otomo R, Matsushima-Hibiya Y, Otsubo C, Nagase T, Arakawa H, Yokota J, Nakagama H, Taya Y, Enari M. NuMA is required for the selective induction of p53 target genes. Mol Cell Biol. 2013;33(12):2447–2457. doi: 10.1128/MCB.01221-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B, Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Fan Y, Xue B, Luo L, Shen J, Zhang S, Jiang Y, Yin Z. Human glutathione S-transferase P1–1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene. 2006;25(42):5787–5800. doi: 10.1038/sj.onc.1209576. [DOI] [PubMed] [Google Scholar]
- 21.Davila-Gonzalez D, Choi DS, Rosato RR, Granados-Principal SM, Kuhn JG, Li WF, Qian W, Chen W, Kozielski AJ, Wong H, Dave B, Chang JC. Pharmacological inhibition of NOS activates ASK1/JNK pathway augmenting docetaxel-mediated apoptosis in triple-negative breast cancer. Clin Cancer Res. 2018;24(5):1152–1162. doi: 10.1158/1078-0432.CCR-17-1437. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Lee EK, Kang DH, Lee J, Hong SH, Jeong W, Kang SW. Glutathione peroxidase-1 regulates ASK1-dependent apoptosis via interaction with TRAF2 in RIPK3-negative cancer cells. Exp Mol Med. 2021;53(6):1080–1091. doi: 10.1038/s12276-021-00642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichijo H, Nishida E, Fau-Irie K, Irie K, Fau-ten Dijke P, ten Dijke P, Fau-Saitoh M, Saitoh M, Fau-Moriguchi T, Moriguchi T Fau-Takagi M, Takagi M, Fau-Matsumoto K, Matsumoto K, Fau-Miyazono K, Miyazono K, Fau-Gotoh Y, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. (0036–8075 (Print)) [DOI] [PubMed]
- 24.Rusnak L, Tang C, Qi Q, Mo X, Fu H. Large tumor suppressor 2, LATS2, activates JNK in a kinase-independent mechanism through ASK1. J Mol Cell Biol. 2018;10(6):549–558. doi: 10.1093/jmcb/mjy061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mhone TG, Chen MC, Kuo CH, Shih TC, Yeh CM, Wang TF, Chen RJ, Chang YC, Kuo WW, Huang CY. Daidzein synergizes with Gefitinib to Induce ROS/JNK/c-Jun activation and inhibit EGFR-STAT/AKT/ERK pathways to enhance lung adenocarcinoma cells chemosensitivity. Int J Biol Sci. 2022;18(9):3636–3652. doi: 10.7150/ijbs.71870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang T, Liu Z, She Y, Deng J, Zhong Y, Zhao M, Li S, Xie D, Sun X, Hu X, Chen C. A novel protein encoded by circASK1 ameliorates gefitinib resistance in lung adenocarcinoma by competitively activating ASK1-dependent apoptosis. Cancer Lett. 2021;520:321–331. doi: 10.1016/j.canlet.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Ma W, Yao Y, Zhang Q, Li J, Wu X, Mei C, Jiang X, Chen Y, Wang G, Wang K, Liu Y, Guo Y, Liu Z, Yuan Y. Serum deprivation-response protein induces apoptosis in hepatocellular carcinoma through ASK1-JNK/p38 MAPK pathways. Cell Death Dis. 2021;12(5):425. doi: 10.1038/s41419-021-03711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Q, Liu Y, Zhong J, Bi Y, Liu Y, Ren Z, Li X, Jia J, Yu M, Yu X. Pristimerin induces apoptosis and autophagy via activation of ROS/ASK1/JNK pathway in human breast cancer in vitro and in vivo. Cell Death Discov. 2019;5:125. doi: 10.1038/s41420-019-0208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyari HRE, Rawling T, Chen Y, Sudarmana W, Bourget K, Dwyer JM, Allison SE, Murray M. A novel synthetic analogue of omega-3 17,18-epoxyeicosatetraenoic acid activates TNF receptor-1/ASK1/JNK signaling to promote apoptosis in human breast cancer cells. FASEB J. 2017;31(12):5246–5257. doi: 10.1096/fj.201700033R. [DOI] [PubMed] [Google Scholar]
- 30.Tang XH, Knudsen B, Fau-Bemis D, Bemis D, Fau-Tickoo S, Tickoo S, Fau-Gudas LJ, Gudas LJ Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. (1078–0432 (Print)) [DOI] [PubMed]
- 31.Li X, Ding F, Wang L, Chen H, Liu Z. Disruption of enhancer-driven S100A14 expression promotes esophageal carcinogenesis. Cancer Lett. 2022;545:215833. doi: 10.1016/j.canlet.2022.215833. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L, Ma M, Wu H, Zhang C, Dai S, Dong P, Huo B, Shan B. p-Hydroxylcinnamaldehyde slows the progression of 4NQO-induced oesophageal tumourigenesis via the RhoA-MAPK signaling pathway. Mol Carcinog. 2018;57(10):1319–1331. doi: 10.1002/mc.22847. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida GJ. Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 2020;13(1):4. doi: 10.1186/s13045-019-0829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Du R, Jia X, Liu K, Qiao Y, Wu Q, Yao N, Yang L, Zhou L, Liu X, Xiang P, Xin M, Wang Y, Chen X, Kim DJ, Dong Z, Li X. CDK15 promotes colorectal cancer progression via phosphorylating PAK4 and regulating β-catenin/MEK-ERK signaling pathway. Cell Death Differ. 2022;29(1):14–27. doi: 10.1038/s41418-021-00828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du R, Huang C, Chen H, Liu K, Xiang P, Yao N, Yang L, Zhou L, Wu Q, Zheng Y, Xin M, Dong Z, Li X. SDCBP/MDA-9/syntenin phosphorylation by AURKA promotes esophageal squamous cell carcinoma progression through the EGFR-PI3K-Akt signaling pathway. Oncogene. 2020;39(31):5405–5419. doi: 10.1038/s41388-020-1369-2. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee A, Gupta S. The multifaceted role of glutathione S-transferases in cancer. Cancer Lett. 2018;433:33–42. doi: 10.1016/j.canlet.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 37.Lei K, Xia Y, Wang XC, Ahn EH, Jin L, Ye K. C/EBPbeta mediates NQO1 and GSTP1 anti-oxidative reductases expression in glioblastoma, promoting brain tumor proliferation. Redox Biol. 2020;34:101578. doi: 10.1016/j.redox.2020.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Ye ZW, Chen W, Culpepper J, Jiang H, Ball LE, Mehrotra S, Blumental-Perry A, Tew KD, Townsend DM. Altered redox regulation and S-glutathionylation of BiP contribute to bortezomib resistance in multiple myeloma. Free Radic Biol Med. 2020;160:755–767. doi: 10.1016/j.freeradbiomed.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamura T, Antoun G, Keir ST, Friedman H, Bigner DD, Ali-Osman F. Phosphorylation of Glutathione S-Transferase P1 (GSTP1) by Epidermal Growth Factor Receptor (EGFR) Promotes Formation of the GSTP1-c-Jun N-terminal kinase (JNK) Complex and Suppresses JNK downstream signaling and apoptosis in brain tumor cells. J Biol Chem. 2015;290(52):30866–30878. doi: 10.1074/jbc.M115.656140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Arifoglu P, Ronai Z, Tew KD. Glutathione S-transferase P1–1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem. 2001;276(24):20999–21003. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- 41.Sau A, Filomeni G, Pezzola S, D'Aguanno S, Tregno FP, Urbani A, Serra M, Pasello M, Picci P, Federici G, Caccuri AM. Targeting GSTP1-1 induces JNK activation and leads to apoptosis in cisplatin-sensitive and -resistant human osteosarcoma cell lines. Mol Biosyst. 2012;8(4):994–1006. doi: 10.1039/c1mb05295k. [DOI] [PubMed] [Google Scholar]
- 42.FeiFei W, HongHai X, YongRong Y, PingXiang W, JianHua W, XiaoHui Z, JiaoYing L, JingBo S, Kun Z, XiaoLi R, Lu Q, XiaoLiang L, ZhiQiang C, Na T, WenTing L, YanQing D, Li L. FBX8 degrades GSTP1 through ubiquitination to suppress colorectal cancer progression. Cell Death Dis. 2019;10(5):351. doi: 10.1038/s41419-019-1588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louie SM, Grossman EA, Crawford LA, Ding L, Camarda R, Huffman TR, Miyamoto DK, Goga A, Weerapana E, Nomura DK. GSTP1 is a driver of triple-negative breast cancer cell metabolism and pathogenicity. Cell Chem Biol. 2016;23(5):567–578. doi: 10.1016/j.chembiol.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei K, Gu X, Alvarado AG, Du Y, Luo S, Ahn EH, Kang SS, Ji B, Liu X, Mao H, Fu H, Kornblum HI, Jin L, Li H, Ye K. Discovery of a dual inhibitor of NQO1 and GSTP1 for treating glioblastoma. J Hematol Oncol. 2020;13(1):141. doi: 10.1186/s13045-020-00979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding authors on reasonable request.