Abstract
The neurotransmitter serotonin has been implicated in a range of complex neurological disorders linked to alterations of neuronal circuitry. Serotonin is synthesized in the developing brain before most neuronal circuits become fully functional, suggesting that serotonin might play a distinct regulatory role in shaping circuits prior to its function as a classical neurotransmitter. In this study, we asked if serotonin acts as a guidance cue by examining how serotonin alters growth cone motility of rodent sensory neurons in vitro. Using a growth cone motility assay, we found that serotonin acted as both an attractive and repulsive guidance cue through a narrow concentration range. Extracellular gradients of 50 µM serotonin elicited attraction, mediated by the serotonin 5-HT2a receptor while 100 µM serotonin elicited repulsion mediated by the 5-HT1b receptor. Importantly, high resolution imaging of growth cones indicated that these receptors signalled through their canonical pathways of endoplasmic reticulum-mediated calcium release and cAMP depletion, respectively. This novel characterisation of growth cone motility in response to serotonin gradients provides compelling evidence that secreted serotonin acts at the molecular level as an axon guidance cue to shape neuronal circuit formation during development.
Electronic supplementary material
The online version of this article (10.1007/s00018-020-03628-2) contains supplementary material, which is available to authorized users.
Keywords: Serotonin, Growth cone, Axon guidance, Guidance cue, Circuit development, Axon pathfinding
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is a crucial neurotransmitter with a range of nervous system functions including modulation of cognition, sleep, decision-making and attention [1, 2] in the adult brain. Serotonin also regulates early central nervous system (CNS) development including neuronal migration, proliferation, differentiation and synaptogenesis [3–5]. In neuronal circuit development, extracellular application of serotonin promotes outgrowth of embryonic mouse [6] and postnatal rat [7] thalamic axons, while inhibiting the outgrowth of snail [8, 9] and chick [10] axons in culture. While these studies have suggested that serotonin alters growth of developing axons, they do not provide any insight into how serotonin might act to directly coordinate axon guidance events during the formation of neuronal circuits.
There are numerous lines of evidence to support the notion that in addition to the classical neurotransmitter and neuromodulator functions of serotonin, it may also act as an axon guidance cue. Serotonin is released into the CNS from sources such as synapses, axons and the systemic circulation (reviewed in [11]). At the cellular level, focal application of serotonin generates cytosolic calcium signals [12] that regulate cytoskeletal rearrangements in growth cones [13, 14]. At the circuit level, serotonin is well known as a key player in fine-tuning development of CNS circuits, such as the somatosensory system where elevation of serotonin alters thalamocortical axon targeting and cortical barrel field formation (reviewed in [15] [16]). Alterations of extracellular serotonin during brain development have been implicated in the onset of many neuropsychiatric and neurodevelopmental disorders (as reviewed by [17]), such as schizophrenia [18] and autism [19]. Therefore, a better understanding of how serotonin regulates axon guidance during development will inform our understanding of the aetiology of such neurological disorders.
Seven serotonin receptor sub-types are present in the embryonic brain and their activation has been shown to influence brain development [20]. For example, activation of the serotonin htr2a gene, encoding the 5-HT2a receptor, abolished ephrin-mediated midline axon crossing in zebrafish. This suggests that serotonin signaling, through the 5-HT2a receptor, is necessary for telencephalic axons to correctly respond to environmental guidance signals [21]. Embryonic thalamocortical axons are also tuned during their growth and guidance by 5-HT1b/1d receptor activation which can switch axon responses to netrin-1 from attraction to repulsion during cortical network formation [22], suggesting that serotonin interacts with other key guidance cues during early stages of neural circuit formation. While these studies are consistent with a crucial role for serotonin in modulating other guidance cues to shape the connectome, the question of whether serotonin is capable of playing an instructive role in its own right in regulating growth cone guidance, remains unclear.
In this study, we hypothesized that serotonin acts as a guidance molecule that alters growth cone motility through the regulation of specific second messenger signalling mechanisms. Using a combination of an established growth cone motility assay, pharmacology and high-resolution imaging, we provide compelling evidence that serotonin functions, in a dose-dependent manner, as an instructional guidance cue to regulate attraction and repulsion of neuronal growth cones in vitro and illustrates a potential mechanism whereby serotonin regulates the fine tuning of circuit formation in the developing brain.
Materials and methods
Animals
Female Sprague–Dawley rats were maintained under standard conditions and embryonic (E17.5-18.5) tissue was used for all experiments. Animal procedures were approved by the Animal Ethics Committee of the University of Tasmania and performed in accordance with the National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Scientific Purposes.
Primary embryonic dorsal root ganglia cell culture
Sensory neuron cultures from dorsal root ganglia (DRG) were prepared as previously described [23]. Briefly, thoracic and lumbar DRGs were excised from E17.5-18.5 embryonic rats and mechanically dissociated prior to culturing. Neurons were plated on glass coverslips, previously coated with poly-l-ornithine hydrochloride (1 mg/mL; Sigma-Aldrich) and laminin (50 µg/mL; Invitrogen), in sensory neuron medium (SNM) Dulbecco’s Modified Eagle’s Medium (DMEM/Ham’s:F12 (Invitrogen), comprising foetal calf serum (5%v/v; Invitrogen), penicillin–streptomycin (100 μg/ml; Invitrogen), N2 neural medium supplement (1%v/v; Gibco), nerve growth factor (50 ng/mL; Sigma-Aldrich). Cultures were maintained in a humified incubator at the following conditions: 37 °C and 5% CO2 for 4–6 h prior to use.
In vitro growth cone turning assay
Growth cone turning assays were performed as previously described [23, 24]. Micropipettes were loaded with the following guidance cues: brain-derived neurotrophic factor human (BDNF; 10 μg/ml; Sigma-Aldrich) and semaphorin-3A (sema-3a; 20 μg/ml; R&D Systems), serotonin (0.5–100 mM; Sigma-Aldrich) and vehicle (DMEM, 20% HCl in DMEM, 10% DMSO in DMEM). Images were acquired at 0.15 Hz for 30 min using custom software (Matlab, MathWorks) and videos exported in avi format. Axon extension and growth cone turning angles were quantitated using ImageJ.
Pharmacological agents
Pharmacological agents were bath-applied to cultures 20 min prior to growth cone turning assay as listed: chlorpromazine (5 μM,Sigma-Aldrich), ritanserin (1 nM, Tocris), GR-55562 dihydrochloride (500 nM, R&D Systems), Sp-cAMPs (20 μM, BioLog Life Science), U-731222 (20 nM, Sigma-Aldrich), forskolin (5 μM, Sigma-Aldrich), nifedipine (5 μM, Alomone Labs), thapsigargin (50 nM, Alomone Labs), ondansentron (10 nM, Tocris), TCB-2 ((4-Bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide, 2 nM, Tocris), CP 94253 hydrochloride (5-Propoxy-3-(1,2,3,6-tetrahydro-4-pyridinyl)-1H-pyrrolo[3,2-b]pyridine hydrochloride, 5 nM, Tocris).
Immunocytochemistry
DRG sensory neurons were fixed with paraformaldehyde (PFA) (4%, Sigma-Aldrich) in phosphate buffered saline (PBS) overnight at 4 °C, washed in PBS (3 × 10 min), for some experiments, cells were permeabilised for 1 h with blocking solution (PBS containing 0.4% Triton X-100; 5% goat serum, Sigma-Aldrich & Gibco) and immunostained with a rabbit antibody to serotonin receptors 5-HT1b (1:500; Abcam) or 5-HT2a (1:500; Alomone Labs) in blocking solution overnight at 4 °C. Following washes in PBS (3 × 10 min), Alexa Fluor goat anti-rabbit 488/568/647 secondary antibodies (1:1000, Invitrogen) were incubated for 1 h and rinsed in PBS (3x10 min) prior to 4% PFA incubation overnight at 4 °C. Following washes in PBS (3x10 min) and 1 h incubation of blocking solution, primary polyclonal rabbit antibodies 5-HT1b or 5-HT2a were added to the blocking solution for 5 h and rinsed in PBS (3 × 10 min). Alexa Fluor goat anti-rabbit 488/568/647 secondary antibodies (1:1000, Invitrogen) were added for 1 h then rinsed in PBS (3 × 10 min) prior to mounting.
For direct immunocytochemistry experiments, a Zenon Rabbit IgG Labelling Kit (Molecular Probes, Alexa Fluor 488) was used. Fixed DRGs were rinsed in PBS and incubated for 30 min with non-permeabilising blocking solution (PBS containing 10% goat serum). Primary antibodies for receptors 5-HT1b and 5-HT2a were conjugated for 10 min at 20–24 °C and applied to cell cultures for 1 h (1:200). Following washes in PBS, cells were fixed with PFA for 10 min at RT and stained with Alexa Fluor 568 Phalloidin for 1 h at RT prior to mounting.
Confocal Imaging
Confocal stacks were acquired in 0.2 μm increments covering total growth cone thickness of 1.5 μm, using a spinning disk confocal microscope (UltraView, PerkinElmer, USA) equipped with a × 100 oil objective and Volocity software (Perkin–Elmer).
Serotonin receptor distribution and image analysis
To assess 5-HT1b and 5-HT2a receptor translocation during turning, cells were rapidly fixed and processed for immunocytochemistry (as described above). Growth cone images were divided into “near” vs “far” with respect to the micropipette, by drawing a line from the distal 10 μm of the axon through the final growth cone orientation. Integrated density of pixel intensity was measured within the two regions of the central zone and normalised to the background fluorescence using ImageJ software, as previously described [25, 26]. Serotonin receptor density in filopodia was plotted as receptor puncta per micron of filopodia. Filopodia shorter than 1 μm were excluded from analysis. Colocalized 5-HT1b and 5-HT2a puncta were measured on background subtracted images of growth cones and expressed as percentages compared to the total amount of colocalized puncta. Phalloidin staining was used to define organelle-rich central-domain and the actin-rich peripheral-domain. Percentage of filopodia containing serotonin receptors was quantitated using ImageJ software and plotted as near/far ratio and total. Quantitation of f-actin was measured as integrated density of pixel and plotted as a near/far ratio.
Calcium imaging
To measure cytosolic calcium levels during turning, cells were loaded with Fluo-4AM (5 μM, Molecular Probes) in SNM for 10 min at RT. Cultures were rinsed with SNM and incubated at 37 °C for a further 30–40 min prior to imaging. Growth cones were exposed to serotonin microgradients for 15 min and images were acquired every 5 s at 488 nm using an EMCCD digital camera (Evolve, Photometrics) and inverted microscope (Eclipse TiE; Nikon Instruments). Intracellular calcium changes were quantitated using a region of interest (ROI), covering the entire growth cone.
Cyclic nucleotide imaging
To measure changes in cAMP levels, neurons were transfected with Flamindo2 (Flamindo2 was a gift from Tetsuya Kitaguchi (Addgene plasmid # 73938; http://n2t.net/addgene:73938; RRID:Addgene_73938) using a Rat Neuron Nucleofection kit (Lonza). Growth cones were exposed to serotonin microgradients for 15 min and images acquired every 10 s at 514 nm using an EMCCD camera (Evolve, Photometrics) and inverted microscope (Eclipse TiE; Nikon Instruments). Intracellular cAMP changes were quantitated using a region of interest (ROI), covering the entire growth cone.
Experimental design and statistical analysis
All growth cone turning experiments were performed using 90–100 independent imaging sessions, using dorsal root ganglia from 45 to 50 rats. Each imaging session contained at least one negative (vehicle) and one positive (BDNF, sema3a) control. No more than one growth cone was imaged from each dish. Operators were blinded to pharmacological treatments throughout imaging and analysis. Statistical analysis of turning angles and receptor distributions were performed using Prism 6 (GraphPad Software, USA). Normality of all experimental data was assessed using D’Agostino-Pearson omnibus or the Shapiro–Wilk test for normality. Normally distributed data were analysed using unpaired t tests with Welch’s correction and one-way ANOVA followed by Tukey’s multiple comparison post hoc test. Non-parametric data were analysed using Mann–Whitney u-test or Kruskal–Wallis followed by Dunn’s multiple comparison post hoc test. See figure legends for specific details. All scatter plots denote mean and standard deviation.
Results
Extracellular serotonin regulates growth cone motility in a dose-dependent manner
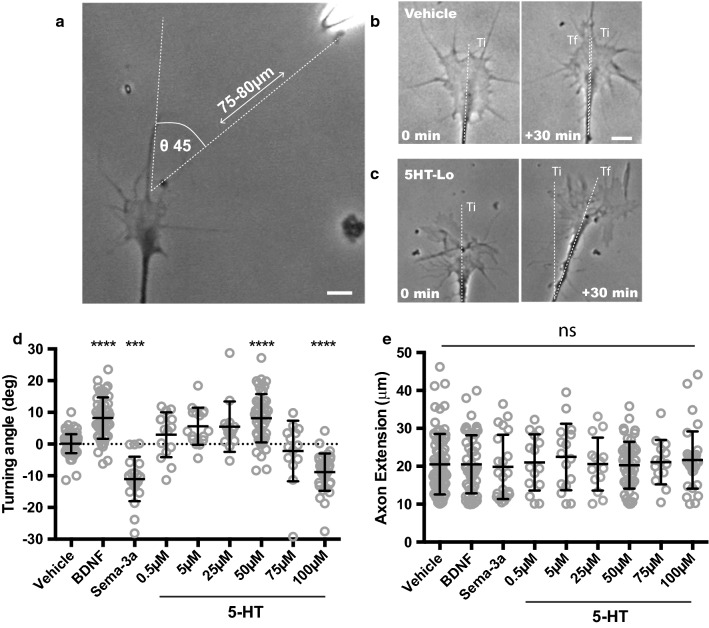
Serotonin is a neurotransmitter known to be released during early circuit development [27, 28] and we hypothesized that serotonin functions as a guidance cue during axon pathfinding. Using a well described method (Fig. 1a–c) to measure growth cone motility [23–26], we exposed embryonic growth cones from dorsal root ganglion sensory neurons to micro-gradients of serotonin and compared turning responses to the “gold-standard” guidance cues, BDNF and sema-3a (Fig. 1d). To determine a dose response curve for serotonin responses, we used a range of serotonin concentrations. Interestingly, growth cone responses to doses in the range of 0.5 µM to 25 µM or 75 µM were not statistically different from vehicle (Fig. 1d). However, 50 µM and 100 µM (described herein as 5HT-Lo and 5HT-Hi) elicited growth cone attraction and repulsion, respectively (Fig. 1d). Importantly, the growth cone response to 5HT-Lo was not significantly different (p = 0.9668) to the turning angle elicited by the established chemoattractant, BDNF, and growth cone responses to 5HT-Hi, were not significantly different (p = 0.2244) to the turning angle elicited by the gold standard repulsive cue, sema3a. To exclude the possibility that these guidance effects were caused by non-specific changes in axon outgrowth, we measured axon extension and showed that none of the serotonin doses tested altered axon extension (Fig. 1e), confirming that observed responses to serotonin were specific to growth cone motility.
Fig. 1.
Gradients of serotonin elicit distinct growth cone behaviors in a dose-dependent manner. a Isolated growth cone exposed to pulsatile ejections of serotonin gradient. The pipette is located in the upper right corner. b–c Phase contrast images of growth cones trajectories at the start (0 min) and at the end (30 min). Ti = initial trajectory; Tf = final trajectory. Dotted lines denote the change in axon trajectory in respect to the micropipette that can be measured as angle of turning. b is an example of vehicle and c 5HT-Lo turning. d Growth cones turned predictably towards BDNF (n = 51, p = <0.0001) and away from sema-3A (n = 21, p = 0.0009) compared to vehicle (n = 77). Serotonin concentrations (0.5, 5, 25 and 75 μM) were not significantly different (n = 15, p =>0.999), (n = 16, p =>0.1614), (n = 14, p = 0.573), (n = 14, p =>0.999) to vehicle turning angles. Only growth cones exposed to 50 μM (n = 51, p = <0.001) and 100 μM serotonin (n = 34, p = 0.0002) showed significant motile responses. Positive angles denote attractive turning and negative angles denote repulsive turning, when compared to random growth (vehicle) (Kruskal–Wallis with Dunn’s multiple comparison post hoc test). (e) Axon extension was not significantly different in any experimental treatment (ns, p > 0.05). Scale bars are 5 μm
Sensory neuron growth cones express 5-HT1b and 5-HT2a receptors
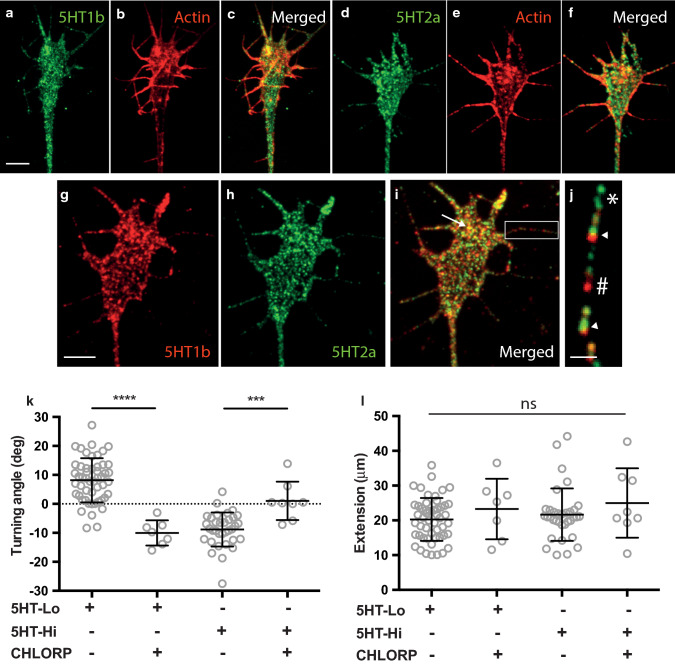
The biphasic nature of growth cone responses to high and low concentrations of serotonin suggests that multiple serotonin receptors might act to mediate attraction and repulsion. Serotonin receptors have been well characterised in rodent dorsal root ganglia sensory neurons with 5-HT1b and 5-HT2a being the most abundant receptor subtypes [29]. We used immunocytochemistry to confirm the expression of 5-HT1b (Fig. 2a–c) and 5-HT2a receptors (Fig. 2d–f) in growth cones in vitro. Dual label experiments using antibodies directed against 5-HT1b and 5-HT2a receptor subtypes revealed colocalization of 5HT receptors across the entire surface of growth cones (Fig. 2 g–i, arrow) and filopodia (Fig. 2j, arrowheads). Significantly, 80% of colocalized puncta were seen in peripheral areas including filopodia (Fig. S1). Filopodia are the key signalling structures of growth cones, necessary for initiating signal transduction pathways that regulate growth cone responses to guidance cues [26, 30]. Hence, the localisation of serotonin receptors on distal filopodial tips supports our hypothesis that 5-HT1b and 5-HT2a receptors could regulate growth cone turning to serotonin gradients. To explore this hypothesis, we tested whether chlorpromazine (a non-selective 5-HT1 and 5-HT2 antagonist) could perturb growth cone motility seen in response to serotonin. Bath application of chlorpromazine (5 µM) abolished both attractive and repulsive turning in response to 5HT-Lo and 5HT-Hi (Fig. 2k), respectively, confirming that growth cone responses to extracellular serotonin are regulated by serotonin receptors, possibly 5-HT1 and 5HT2. Consistent with the serotonin turning data (Fig. 1e), treatment with chlorpromazine had no effect on axon extension when growth cones were exposed to gradients of serotonin (Fig. 2l).
Fig. 2.
5-HT1b and 5-HT2a receptors are expressed in growth cones and filopodia. a–j Sensory neuron growth cones immunostained for serotonin receptors, 5-HT1b (green in a, red in g), 5-HT2a (green, d and h) as well as filamentous actin to highlight the extent of the growth cone (Phalloidin, red; b and e). The receptors 5-HT1b and 5HT2a were observed as a widespread punctate distribution throughout the growth cone (g–h). Punctate expression was evident in filopodia (j) with isolated 5-HT1b (green, *), 5-HT2a (red, #) as well as colocalised (arrowheads) puncta on filopodial shafts. There was prominent colocalization of 5-HT2a and 5-HT1b receptors in peripheral areas (i, arrow). Scale bars are 5 μm (a–i) and 1 μm (j). k Turning responses to serotonin were sensitive to chlorpromazine. Attractive turning of growth cones to 5HT-Lo (n = 7, p = <0.0001) and repulsion to 5HT-Hi (n = 8, p = 0.003) were significantly different when cultures were treated with chlorpromazine. l Axon extension was not significantly different in any experimental treatments (ns, p > 0.05). (Mann–Whitney u-test)
Growth cone attraction to serotonin is regulated by the 5-HT2a receptor
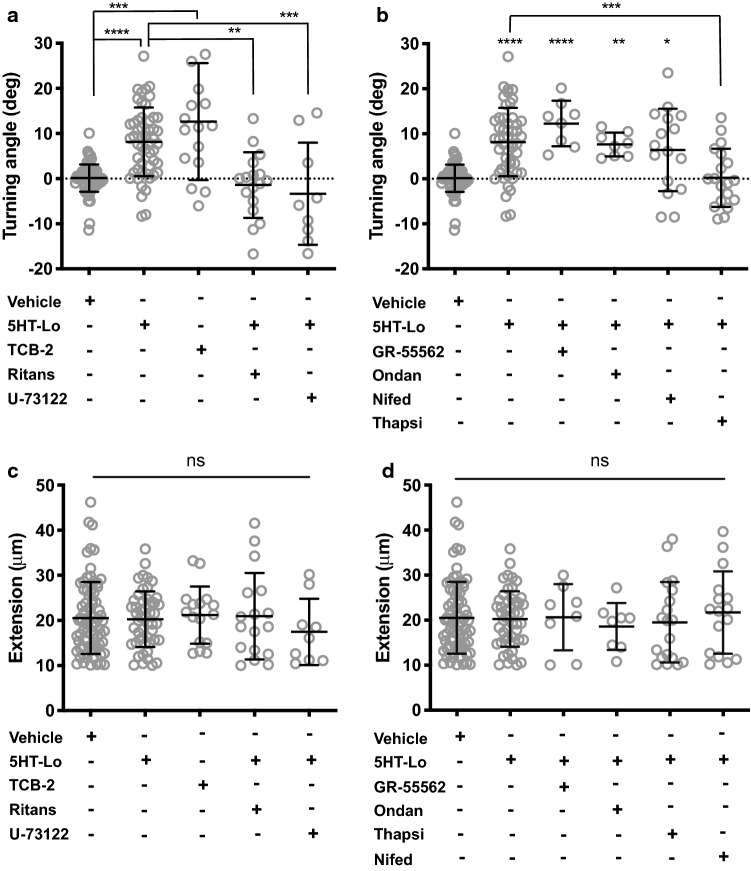
Activation of the 5-HT2a receptor signals through phospholipase-C (PLC)-mediated calcium release from the endoplasmic reticulum (ER) and inositol triphosphate (IP3) signalling [20]. Given that ER-mediated calcium release sustains attractive turning of growth cones by BDNF [25, 26], we hypothesized that 5-HT2a signaling mediates growth cone attraction in response to 5HT-Lo. We exposed growth cones to asymmetric gradients of TCB-2 (2 nM), a potent and highly selective 5-HT2a agonist, which induced robust attraction (Fig. 3a), suggesting that activation of 5-HT2a receptor mediates attraction. To confirm that 5-HT2a receptor regulates growth cone attraction towards 5HT-Lo, we bath applied a specific 5-HT2a receptor antagonist, ritanserin (1 nM) or the PLC inhibitor U-73122 (20 nM) to sensory neurons and exposed them to a gradient of 5HT-Lo. Both drugs abolished attraction towards 5HT-Lo (Fig. 3a; Fig S2). To exclude the possibility that other serotonin receptors could regulate growth cone attraction towards 5HT-Lo, we bath applied antagonists to the 5-HT1b and 5-HT3 receptors (5-HT3 is also commonly expressed on neurons from dorsal root ganglia [29]). Neither the 5-HT1b receptor antagonist GR-55562 (500 nM) or the 5-HT3 receptor antagonist ondansetron (10 nM) altered attractive turning towards 5HT-Lo (Fig. 3b). Given the importance of calcium signals in initiating growth cone motility [31, 32], and that release of calcium from ER via IP3 receptor activation is a hallmark feature of 5-HT2a signalling, we asked whether calcium signals from the ER can sustain attractive turning towards serotonin. We bath applied the sarco-endoplasmic reticulum ATPase (SERCA) inhibitor, thapsigargin (50 nM), to deplete ER calcium prior to serotonin exposure. We observed that thapsigargin abolished attractive turning to 5HT-Lo (Fig. 3b). Conversely, extracellular calcium influx through voltage-gated calcium channels (VGCCs) was not required for turning towards 5HT-Lo. Bath application of the L-type VGCC inhibitor nifedipine (5 µM) failed to perturb serotonin-mediated attraction (Fig. 3b). The application of these pharmacological agents did not alter axon extension during the imaging period, confirming these treatments affected only growth cone turning responses (Fig. 3c, d). These data demonstrate that 5-HT2a regulates growth cone attraction towards serotonin in an ER calcium-dependent manner.
Fig. 3.
5HT-Lo elicits growth cone attraction through the 5-HT2a receptor. a Growth cone attraction was measured in the presence of 5HT-Lo gradients (p = <0.0001) and TCB-2 gradients (p = 0.0002). Growth cone attraction to 5HT-Lo was abolished by pharmacological application of the selective 5-HT2a receptor antagonist, ritanserin (n = 17, p = 0.0001) and the PLC inhibitor, U-73122 (n = 9, p = 0.0018). b Growth cone attraction to 5HT-Lo was not perturbed when growth cones treated with a specific 5-HT1b antagonist, GR55562 (n = 8, p = <0.0001), or the 5-HT3 antagonist ondansetron (n = 8, p = 0.0082). Depletion of ER calcium with thapsigargin (n = 19, p = 0.0003) abolished attractive turning to 5HT-Lo, while inhibition of VGCCs with nifedipine (n = 15, p = 0.0330) had no significant effect. Turning angles were compared to vehicle and 5HT-Lo and only significant differences shown in (a) and (b). Kruskal–Wallis, Dunn’s multiple comparison test. c, d Axon extension was not significantly different in any experimental treatments (ns, p > 0.05). Kruskal–Wallis, Dunn’s multiple comparison test
Growth cone repulsion from serotonin is regulated by the 5-HT1b receptor
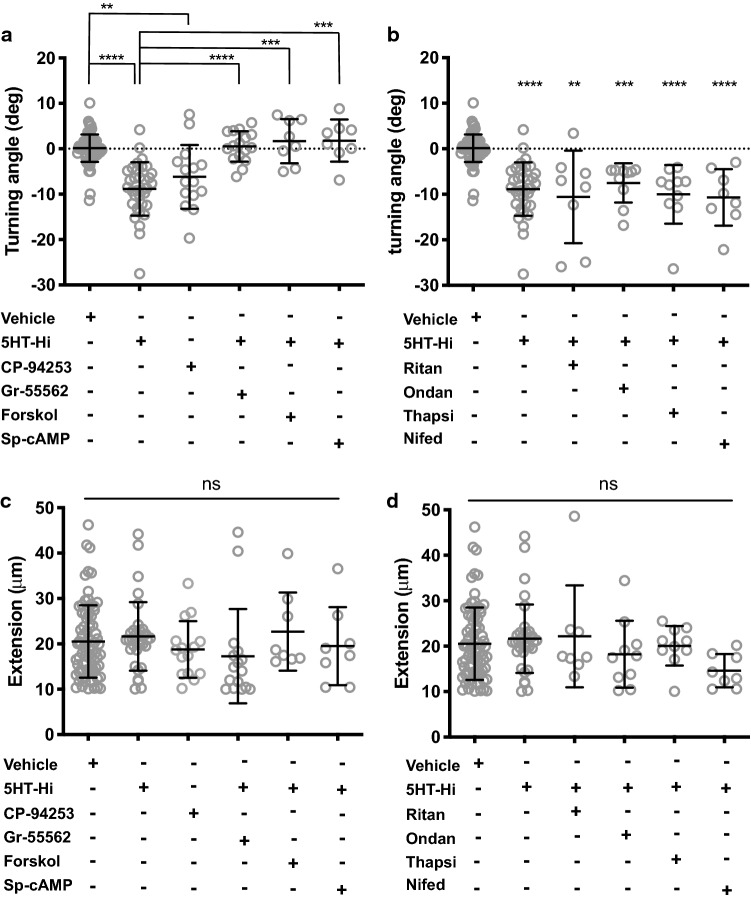
Early in vitro studies have demonstrated significant dose-dependent and cell-specific responses of neurons to serotonin [6–9]. The observation that exposure of growth cones to 5HT-Hi resulted in repulsion rather than attraction, as seen with 5HT-Lo, suggests the activation of a different serotonergic receptor. The 5-HT1b receptor is known to be coupled to an inhibitory G-protein [20], and we hypothesized that it could mediate growth cone repulsion. Asymmetric gradients of CP-94253 hydrochloride (5 nM), a potent and highly selective 5-HT1b agonist, induced robust growth cone repulsion (Fig. 4a). Bath application of the selective 5-HT1b receptor antagonist GR-55562 (500 nM) abolished repulsive turning to 5HT-Hi (Fig. 4a). Since 5-HT1b receptors are known to act through inhibition of adenylate cyclase [20], we sought to determine whether activating cAMP would perturb growth cone repulsion to 5HT-Hi. Application of the adenylate cyclase activator, forskolin (5 µM) or the activator of cAMP signalling, Sp-cAMPs (20 µM) both abolished repulsive turning (Fig. 4a). To exclude the involvement of 5-HT2a or 5-HT3 in growth cone repulsion from 5HT-Hi, we applied ritanserin (1 nM) or ondansetron (10 nM), respectively. Neither drug altered growth cone repulsion from 5HT-Hi, suggesting that 5-HT1b, and not 5-HT2a or 5-HT3, mediated growth cone repulsion from 5HT-Hi (Fig. 4b). To determine whether intracellular or extracellular calcium sources contributed to 5HT-Hi-induced growth cone repulsion, we applied thapsigargin (50 nM) and nifedipine (5 µM) to growth cones exposed to 5HT-Hi gradients. Both drugs failed to impair growth cone responses from 5HT-Hi (Fig. 4b), suggesting a mechanism of growth cone repulsion, that does not rely on relatively large calcium signals, potentially similar to the mechanisms that govern sema3a-mediated repulsion [33]. Bath application of these pharmacological agents did not affect the normal axonal growth (Fig. 4c, d). Together, these data demonstrate that 5-HT1b signalling mediates growth cone repulsion in response to 5HT-Hi in sensory growth cones.
Fig. 4.
5HT-Hi promotes growth cone repulsion through the 5-HT1b receptor. a Growth cone repulsion was measured in the presence of 5HT-Hi gradients (p = <0.0001) and CP-94253 gradients (p = 0.0018). Growth cone repulsion to 5HT-Hi was altered to levels indistinguishable from random growth (vehicle) with application of the selective antagonist to 5-HT1b, GR55562 (n = 16, p = <0.0001), by activating adenylate cyclase with forskolin (n = 8, p = 0.0003) and restoration of cAMP signals with Sp-cAMPs (n = 8, p = 0.0001). b Repulsion to 5HT-Hi was not perturbed when growth cones were treated with the 5-HT2a receptor antagonist ritanserin (n = 8, p = 0.002) or the 5-HT3 antagonist ondansetron (n = 10, p = 0.0003). Inhibition of ER calcium release with thapsigargin (n = 10, p = <0.0001) or calcium influx with nifedipine (n = 8, p = <0.0001) did not alter growth cone repulsion from 5HT-Hi. Turning angles were compared to vehicle and 5HT-Hi. c, d Axon extension was not perturbed by any pharmacological application (ns, p > 0.05). Turning angles were compared to vehicle and 5HT-Hi. (Kruskal–Wallis, Dunn’s multiple comparison test)
Growth cone attraction to 5HT-Lo is regulated by mobilisation of ER calcium
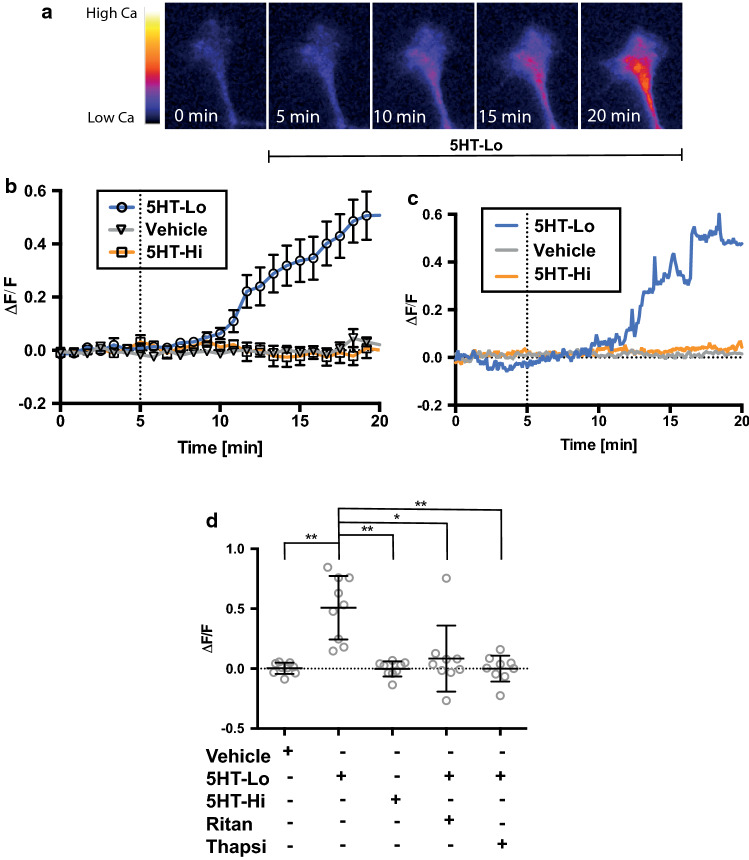
Coordinated cytosolic calcium signalling is crucial for growth cone motility [34] and our findings suggest that serotonin-mediated attraction, but not repulsion, requires the activation of cytosolic calcium signals. To demonstrate the calcium dependency of growth cone turning to serotonin, we performed live cell calcium imaging in growth cones exposed to gradients of serotonin (Fig. 5a). We observed a rise in intracellular calcium in growth cones exposed to 5HT-Lo, while microgradients of 5HT-Hi and vehicle did not cause any detectable changes in cytosolic calcium levels within growth cones (Fig. 5a–c). As predicted from the growth cone turning data (Fig. 3), bath application of the 5-HT2a inhibitor ritanserin (1 nM) or depletion of ER calcium with thapsigargin (50 nM), abolished calcium elevations induced by 5-HT-Lo microgradients (Fig. 5d). These results confirm our pharmacological experiments, demonstrating that activation of 5-HT2a signalling and subsequent calcium mobilisation from the ER regulates attractive turning towards 5HT-Lo, while growth cone repulsion from 5HT-Hi is consistent with a calcium-independent mechanism.
Fig. 5.
Growth cone attraction to 5HT-Lo is regulated by specific calcium signals. a Representative time-lapse images of a growth cone exposed to 5HT-Lo while recording cytosolic calcium. b The average (n = 9 ± sem) change in calcium levels in growth cones exposed to vehicle, 5HT-Lo and 5HT-Hi gradients. c Representative growth cone responses in cytosolic calcium to vehicle, 5HT-Lo and 5HT-Hi exposure. d Quantitation of average change in growth cones after 15 min of exposure to serotonin gradients. While growth cones exposed to 5HT-Lo showed significant (n = 9, p = 0.0027) increase in calcium influx, vehicle (n = 9) and 5HT-Hi (n = 9, p = 0.0018) exposure did not elicit any calcium influx. Significant reduction in cytosolic calcium occurred with ritanserin (n = 9, p = 0.0344) and thapsigargin (n = 9, p = 0.0033) in response to 5HT-Lo exposure. All conditions were compared to vehicle and 5HT-Lo. (Kruskal–Wallis, Tukey’s multiple comparison test)
Growth cone repulsion from 5HT-Hi is transduced through inhibition of cAMP signaling
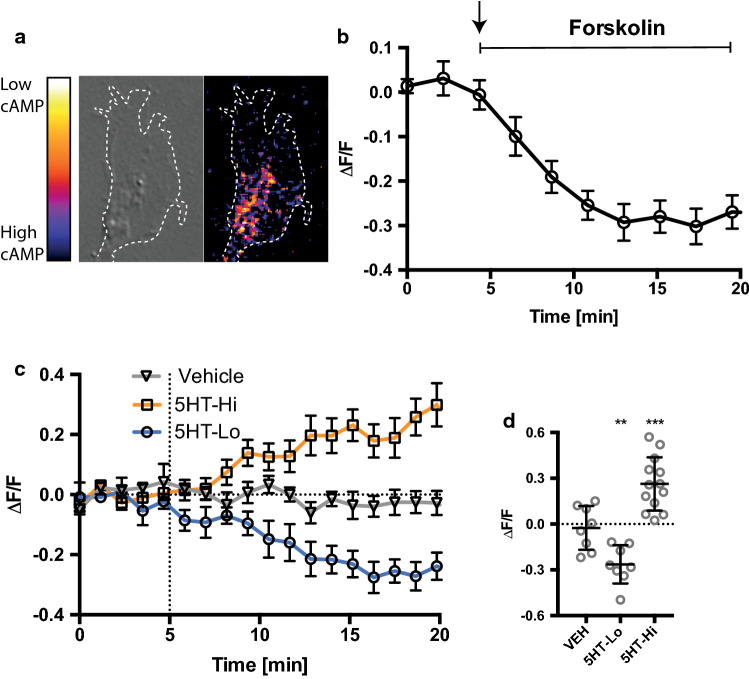
The ratio of cyclic nucleotides, cAMP and cGMP, has been previously shown to regulate growth cone motility [35], with low ratios regulating repulsive turning [36] and high ratios regulating attraction [37]. Spatially restricted changes in cAMP or cGMP alter the nucleotide ratio in a reciprocal manner in neurons to promote axon protrusion and dendrite differentiation [38]. Furthermore, crosstalk between cyclic nucleotides and calcium signals refines receptor signaling [39]. Signaling through the 5-HT1b receptor is known to inhibit adenylate cyclase and hence decrease cAMP activation. We therefore asked if we could detect 5-HT1b-mediated decreases in cAMP levels in growth cones exposed to 5HT-Hi during growth cone repulsion. We performed time-lapse imaging of cAMP levels in growth cones transfected with flamindo-2 [40], a biosensor for cAMP and exposed to serotonin gradients (Fig. 6a, c). We first confirmed the specificity and ability of flamindo-2 to detect changes in cAMP levels in growth cones. We bath applied forskolin (20μM) and measured a significant increase in cAMP levels, observed as a decrease in the fluorescence emission of the sensor (Fig. 6b). There was no significant change in cAMP levels in growth cones exposed to vehicle (Fig. 6c, d) and in support of our pharmacological data (Figs. 3,4), 5HT-Hi exposure caused a significant decrease in cAMP (Fig. 6c, d), further confirming the role of the 5-HT1b receptor in serotonin-mediated growth cone repulsion. Conversely, microgradients of 5HT-Lo resulted in activation of cAMP signalling (Fig. 6c, d), suggesting that cAMP is activated downstream of store-released calcium in response to 5-HT2a receptor activation, highlighting the crosstalk mechanism between calcium and cAMP signals.
Fig. 6.
5HT-Hi repulsion is regulated by cAMP signalling. Flamindo-2 accurately reports cAMP signaling in growth cones. a Representative images showing localization of cAMP. b Bath application of forskolin (n = 7, ± sem) to growth cones elicited a decrease in fluorescence indicating the activation of cAMP signaling. c Average change in cAMP in growth cones exposed to gradients of vehicle (open triangles, n = 8, ± sem), 5HT-Lo (open circles, n = 8, ± sem) and 5HT-Hi (open squares, n = 13, ± sem). d Quantitation of average changes in cAMP levels within growth cones after 15 min of serotonin exposure. 5HT-Hi significantly decreased (n = 12, p = 0.0003) cAMP levels and 5HT-Lo significantly increased (n = 8, p = 0.0076) cAMP signalling. (One-way ANOVA followed by Tukey’s multiple comparison post hoc test)
Serotonin receptors are translocated within growth cones during asymmetric serotonin exposure
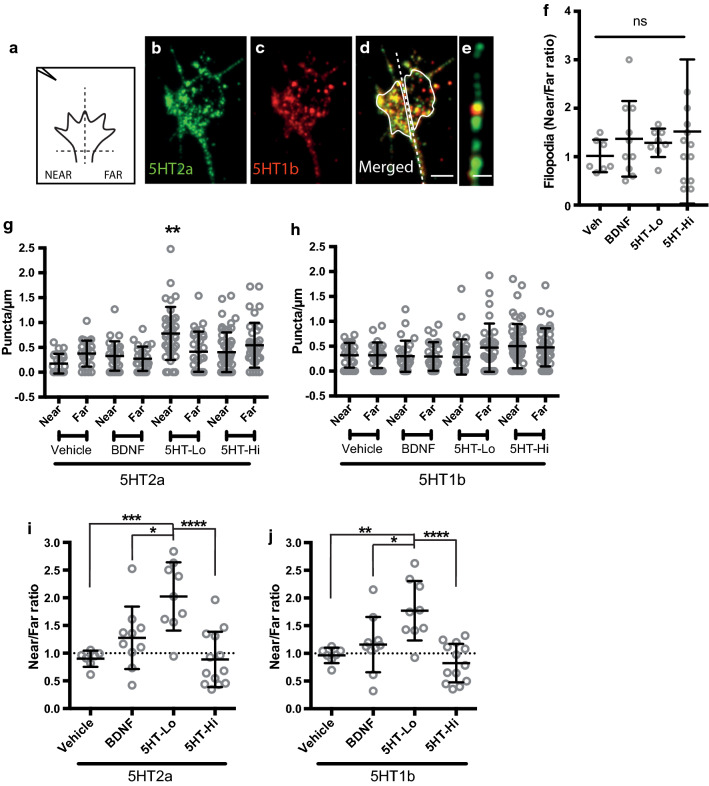
Growth cone turning is underpinned by the asymmetric reorganisation of signaling proteins and receptors required for motility [41, 42]. It is well established that molecules required for transduction of external guidance cues are actively translocated by the cytoskeleton to the active or turning side of growth cones, including filopodia [26]. Our observation of a biphasic growth cone turning response to serotonin led us to hypothesise that spatial reorganisation of serotonin receptors might explain the opposing motile responses of growth cones to low and high serotonin gradients. We assessed the spatial distribution of receptor puncta in growth cones and filopodia as previously described [26] (Fig. 7a). Briefly, growth cones were rapidly fixed during turning and processed for serotonin receptor 5-HT2a (Fig. 7b) and 5-HT1b (Fig. 7c) localisation using immunocytochemistry. Serotonin receptor expression was measured in growth cones (Fig. 7d, i–j) and filopodia (Fig. 7e, g–h) in response to gradients of vehicle, BDNF (used here as a control), 5HT-Lo, and 5HT-Hi. Given the importance of filipodia in the initiation of growth cone motility [26], we sought to determine whether receptor distribution along filopodia was biased with respect to gradient orientation. Individual filopodial lengths were measured and categorised as “near” or “far” with respect to the pipette. There was no significant bias in overall number of filopodia detected across growth cones in all experimental conditions (Fig. 7f). Serotonin receptor puncta were counted on filopodia longer than 1 μm. Immunocytochemistry revealed prominent serotonin receptor localisation on many filopodia, and only filopodia oriented towards a source of 5HT-Lo showed a small, but significant increase in 5-HT2a receptor density (Fig. 7g, h) when compared to filopodia oriented towards a 5HT-Hi, vehicle or BDNF gradient. There was no detectable bias or asymmetry in receptor localisation on filopodia exposed to 5HT-Hi, vehicle or BDNF (Fig. 7g, h). These data suggest that growth cone responses to 5HT-Lo require the translocation of 5-HT2a receptors to the motile or turning side of growth cones, including the sensory structures, the filopodia.
Fig. 7.
5HT-Lo exposure causes asymmetric distribution of 5-HT receptors to the turning or motile side of growth cones. Schematic a showing growth cone divided into “near” and “far” regions with respect to micropipet position (located on the upper left side). b–d Growth cones immunostained after turning in response to 5HT-Lo and stained for 5-HT2a (green, b), 5-HT1b (red, c) and merged in (d). Dotted line separates the near and far regions of growth cone (near vs far). (e) Representative image of filopodia with 5-HT2a (green), 5-HT1b (red) receptor puncta. (f) There was no significant near/far bias in the number of filopodia following exposure to 5HT-Lo (n = 8, p = 0.9004), 5HT-Hi (n = 13, p = 0.5450) or BDNF (n = 10, p = 0.7907) compared to vehicle. (Data not normally distributed: Kruskal–Wallis, Dunn’s multiple comparison test). g There were significantly more (n = 36, p = 0.0033) 5-HT2a puncta in filopodia on the near side of growth cones exposed to 5HT-Lo compared to all other treatments. h There was no bias in 5-HT1b puncta in filopodia of growth cones. (Mann–Whitney U-test). (i-j) When entire growth cones were analysed, i 5HT-2a (n = 9, p = 0.0006) and j 5HT-1b (n = 9, p = 0.0028) receptor translocation was significantly biased in growth cones exposed to 5HT-Lo. All ratios were compared to vehicle (Data normally distributed: Shapiro–Wilk. One-way ANOVA, Tukey’s multiple comparison test). Scale bars are 5 μm (Fig. 7b–d) and 1 μm (Fig. 7e)
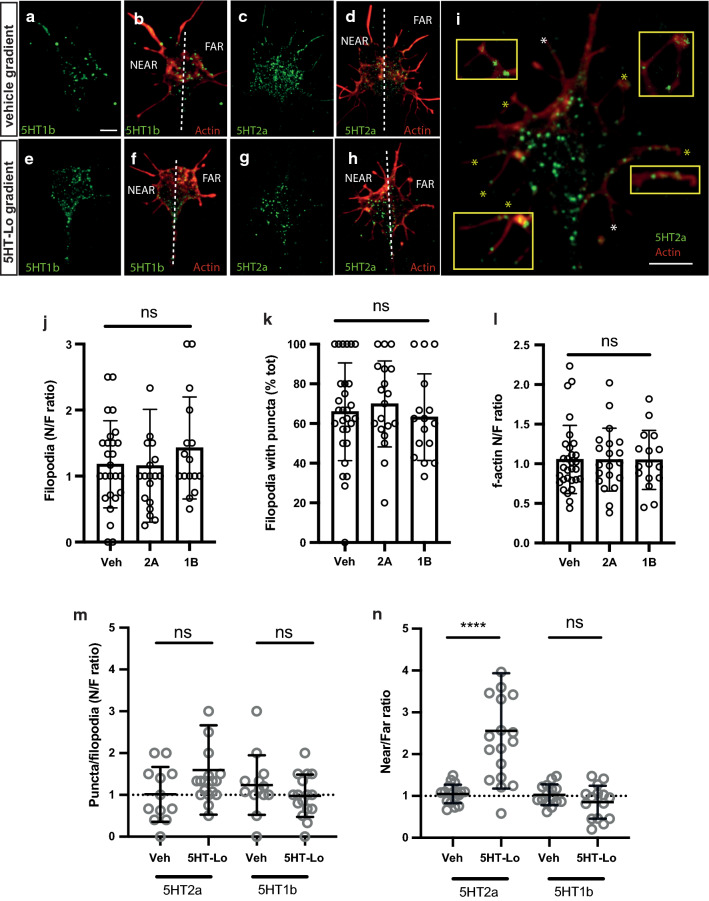
When growth cones were exposed to gradients of vehicle or BDNF there was no spatial reorganisation of 5-HT2a or 5-HT1b receptor puncta across the growth cone (Fig. 7i, j). However, there was a significant bias of receptor expression in response to 5-HTLo, with both receptors, 5-HT2a and 5-HT1b puncta expressed at higher levels on the “near” or turning side of growth cones (Fig. 7i, j). Interestingly, in response to 5HT-Hi, no expression bias was detected for either receptor on the near or far side of growth cones turning away from 5HT-Hi (Fig. 7i, j). These data suggest that there is an active translocation of both 5-HT2a and 5-HT1b receptors in response to 5HT-Lo exposure. However, these experiments were conducted using permeabilising reagents (Triton-X) in the staining protocol, and so reflect receptors within the cytosol, as well as any receptors at the remaining membrane. To focus on membrane localisation of serotonin receptors, we performed a second set of experiments using the same primary antibodies conjugated directly to a fluorescent probe. Growth cones were fixed during turning to 5HT-Lo or vehicle and processed for immunocytochemistry without permeabilization (Fig. 8a, i). Although non-permeabilised ICC showed less immunoreactivity, serotonin receptor puncta were present throughout growth cones and filopodia (asterisks, insets, Fig. 8i). The number of filopodia detected across growth cones was not significantly different between near vs far regions (Fig. 8j) and among all experimental conditions, 60-70% of filopodia were enriched with 5-HT2a and 5-HT1b receptor puncta (Fig. 8k). The amount of f-actin was not significantly different between near vs far regions (Fig. 8l). We then assessed the near:far distribution of 5-HT2a and 5-HT1b receptor puncta in growth cones and filopodia (Figs. 8m, n). As expected, growth cones and filopodia exposed to gradients of vehicle showed an equal, or random “near/far” distribution of both 5-HT2a and 5-HT1b receptors with no bias of puncta to the motile, or “near” side (Fig. 8m, n). Similarly, there was no near:far bias in the amount of 5-HT2a and 5-HT1b puncta detected in filopodia exposed to 5HT-Lo (Fig. 8m). Importantly, we detected a significant translocation of 5-HT2a in growth cones exposed to 5HT-Lo, with higher 5-HT2a receptor density on the near side (Fig. 8n), with no detectable translocation of 5-HT1b receptors in the membrane of growth cones exposed to 5HT-Lo (Fig. 8n). These data confirm that 5-HT2a receptor distribution is sensitive to the extracellular serotonin concentration, mobilising asymmetrically to the membrane on the near side of growth cones exposed to 5HT-Lo.
Fig. 8.
5HT-Lo exposure causes asymmetric distribution of membrane 5-HT2a receptors to the motile side of growth cones. a–h Representative growth cones exposed to vehicle and 5HT-Lo gradients (micropipette on upper left side) and stained for membrane localization of receptors 5-HT1b (green, a, e), 5-HT2a (green, c, g) and merged with actin, (red, b, d, f, h). Dotted line separates the near and far regions of the growth cone. i Increase magnification of representative image h to show the presence of 5-HT2a receptor membrane on most filopodia (*) of the growth cone (yellow *, insets). Scale bars are 5 μm. (j-k)There was no significant near/far bias in the amount of f-actin (/area) (j) and the number of filopodia (k) in growth cones exposed to vehicle and 5HT-Lo. Kruskal–Wallis, Dunn’s multiple comparison post hoc test. (l) There was no significant bias in the total percentage of filopodia containing 5HT2a and 5HT1b puncta (60%). One-way ANOVA followed by Tukey’s multiple comparison post hoc test. (m) There was no significant near/far bias in the amount of 5-HT2a and 5-HT1b puncta per filopodia. (n) Analysis of 5-HT receptor membrane localization showed translocation of 5-HT2a (n = 18, p = <0.0001) to the near side of growth cones exposed to 5HT-Lo while no significant translocation of 5-HT1b (n = 15, p = 0.346) was observed. (Mann–Whitney U-test)
Discussion
Serotonin, a vital neuromodulator of the mature nervous system, is also prominently expressed during development of the CNS [27, 28]. In this study, we asked whether serotonin acts as a bona fide guidance cue during axon pathfinding, capable of modulating growth cone motility. Our experiments have revealed that serotonin can indeed function as an instructional guidance molecule by regulating the bidirectional turning of rodent sensory neuronal growth cones, in vitro, without altering the rate of axon growth. We found that serotonin initiates and sustains growth cone attraction and repulsion in a dose-dependent manner through 5-HT2a and 5-HT1b receptors, coupled to canonical second messenger signalling pathways. To our knowledge, this is the first direct demonstration of serotonin acting in a dose-dependent manner to activate growth cone chemoattraction and chemorepulsion. Our data support a novel mechanism where extracellular serotonin might regulate axon guidance in a binary manner, to attract and subsequently stop axons during development of circuits in the embryonic brain.
The role of serotonin in shaping CNS circuits has been studied intensively over past decades, primarily by studying in vivo models that manipulate the extracellular serotonin milieu through altered serotonin synthesis or reuptake. This body of work illustrates a spectrum of subtle guidance and targeting defects and suggests normal circuit development requires optimum extracellular levels of serotonin (extensively reviewed in [2, 43, 44]). Similarly, previous in vitro work has demonstrated that serotonin levels could alter both axon extension and branching in vertebrate and invertebrate neurons [7–9, 45], yet how developing neurons, during axon pathfinding, are able to discern and respond appropriately to extracellular serotonin was unclear. Our study provides a model where extra-synaptic gradients of serotonin can function in chemoattraction and repulsion in the “guidance landscape” of the developing CNS.
Neurotransmitters are well known to regulate neuronal migration and axon growth (reviewed in [46]), with neurotransmitter receptors expressed on progenitor cells [47] and coupled to second messengers [5] are able to regulate developmental processes such as neuronal growth cone motility and guidance. For example, acetylcholine induces attractive turning through the activation of nicotinic receptors and a significant rise in cytosolic calcium, without significantly affecting the rate of extension [48]. Similarly, glutamate induces growth cone attraction mediated by NMDA receptors and calcium signaling [49]. Interestingly, dopamine is also capable of activating attraction and repulsion. Dopamine acts as a chemoattractant for target cell growth cones but at the same concentration, also acts as a chemorepellent for non-target cell growth cones [50].
Serotonin has been shown to have disparate effects in vitro, both inhibiting and enhancing neurite outgrowth [6–10]. These seemingly contradictory effects of serotonin on developing neurons in vitro suggest that serotonin action is defined by (a) pleiotropic expression of serotonin receptor subtypes, (b) downstream effectors of receptor signaling and (c) heterogeneity of serotonin concentration in the developing CNS. The presence of diverse serotonin receptor subtypes in embryonic neurons [29] and our demonstration of serotonin receptors in growth cones, together with the coupling of selected receptors to distinct second messenger signaling cascades [20], supports the notion that serotonin could regulate opposing growth cone turning responses at specific concentrations. We have shown that serotonin elicits attraction through specific activation of 5-HT2a receptors and PLC-mediated ER calcium release. Significantly, inhibition of the 5-HT2a receptor completely abolished attraction, suggesting little or no involvement by other receptor subtypes. Surprisingly, the exposure of growth cones to a higher serotonin concentration (100 µM) induced significant growth cone repulsion which was abolished by specific antagonism of 5-HT1b receptors and restoration of cAMP signalling with the adenylate cyclase activator, forskolin. These results are consistent with previous research demonstrating the second messengers, cytosolic calcium and cAMP, regulate turning and guidance of growth cones in response to molecular cues such as serotonin (as reviewed in [51]).
It is well established that growth cone attraction to guidance cues such as BDNF is characterised by generation of spatially restricted calcium gradients that are sustained temporally and spatially by store operated calcium entry (SOCE) [25, 26]. We found that attraction of growth cones to 5HT-Lo was dependent on replete ER stores and activation of PLC- and IP3 mediated signal transduction. The sustained calcium response supports the hypothesis that 5HT-Lo activation of 5HT-2a signals via IP3-mediated calcium release/influx and is consistent with our previous work [23, 25, 26] demonstrating that other calcium-dependent guidance cues such as BDNF activate ER-medicated calcium dynamics that drive cytoskeletal reorganisation during growth cone turning. The exact spatiotemporal nature of calcium release in the case of serotonergic stimulation seen in this study has yet to be determined. Exposure of growth cones to 5HT-Hi, however, did not alter cytosolic calcium levels, but instead relied on 5-HT1b-mediated inhibition of cAMP signalling. Coincidently, 5HT-Lo stimulation elicited a small but significant increase in cAMP levels confirming downstream crosstalk between cAMP and calcium signalling during attractive turning [39]. Exactly how the biphasic responses, from attraction to repulsion, are initiated over a relatively narrow concentration gradient are not completely understood. A similar concentration-dependent bimodal turning response has been observed in embryonic cortical axons, upon exposure to netrin-1 gradients [52], where low concentrations of netrin-1 promoted attraction and higher concentrations of netrin-1 caused repulsion, without perturbing axon growth [52].
Immunolocalization experiments designed to track receptors after exposure to serotonin revealed significant translocation of the 5-HT2a receptor in response to low serotonin exposure. Exactly how this translocation might be regulated in highly motile structures such as growth cones is unclear. Specific binding motifs between serotonin receptors and the cytoskeleton are unknown at this time. However, it has been shown that heterotrimeric G proteins (G ), which are the components of G protein-coupled receptors, interact with microtubules [53], [54]. These studies demonstrate that G subunits inhibit microtubule polymerization and G promotes microtubule assembly. Our experiments provide compelling functional data that suggest a mechanism for growth cone turning towards 5-HTLo whereby the G subunit could initiate the PLC-mediated ER calcium release, while the G subunits could interact with the tubulin cytoskeleton to promote microtubule assembly in the direction of attractive turning. A more detailed biophysical analysis of receptor localization and/or translocation would be necessary to understand these mechanisms but is beyond the scope of the current study. Notably, desensitization of G-protein coupled receptors by prolonged agonist or antagonist exposure has been well documented in the case of the 5-HT2a receptor. The 5-HT2a receptor exhibits a time-dependent desensitization at serotonin concentrations of 10-100 µM in rodent cortical neurons [55]. Prolonged exposure of 5-HT2a receptors to both serotonin and ritanserin in a glioma cell line results in significant receptor internalisation through a clathrin and dynamin-dependent process [56]. Signalling in this case was reduced through the inability of 5-HT2a to couple downstream G-proteins resulting in attenuation of PLC-mediated calcium release. Our results suggest that once desensitized, serotonin signaling might “spill over” to 5-HT1b receptors, mediating growth cone repulsion.
Our data demonstrate that neuronal growth cones are exquisitely tuned in their responses to extracellular concentrations of serotonin. Given the variable secretion of serotonin from a variety of extra-synaptic sites, including soma and axons [11] of serotonergic neurons, our results would be consistent with a mechanism for subsequent waves of extending axons guided through a process of growth cone attraction and repulsion, to target serotonergic neurons in early developmental landscape of the CNS. Our data support a novel hypothesis for early neuronal circuit development whereby serotonin might contribute to guiding growth cones towards synaptic targets, acting as a chemoattractant by activating 5HT-2a receptors and IP3 mediated calcium signals. The very narrow tuning responses of growth cones in the micromolar range seen in this study would suggest that as growth cones approach the domains of higher serotonin concentration, desensitisation of 5HT-2a receptors occurs and higher serotonin levels subsequently activate the 5HT-1b receptors, possibly acting as molecular “stop” signals. In conclusion, these data suggest a novel guidance mechanism for serotonin, where rather than simply modulating existing guidance cues it also acts as “stop” or “go” instructional signal during circuit formation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors sincerely thank Dr. James Crane and colleagues from the Foa, Young and Lin lab groups for valuable comments and suggestions during preparation of this manuscript. This work was supported by an Australian Government Postgraduate Award (SV), the National Health and Medical Research Council, Australia (LF and RG: #1165616).
Abbreviations
- 5-HT
5-hydroxytryptamine; serotonin
- CNS
Central nervous system
- DRG
Dorsal root ganglia
- SNM
Sensory neuron medium
- DMEM
Dulbecco’s Modified Eagle’s Medium
- BDNF
Brain-derived neurotrophic factor
- sema-3a
Semaphorin-3A
- ROI
Region of interest
- PFA
Paraformaldehyde
- PBS
Phosphate buffered saline
- PLC
Phospholipase-C
- ER
Endoplasmic reticulum
- IP3
Inositol triphosphate
- SERCA
Sarco-endoplasmic reticulum ATPase
- VGCCs
Voltage-gated calcium channels
- SOCE
Store operated calcium entry
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Azmitia EC. Serotonin and brain: evolution. Neuroplast Homeost. 2007;77:31–56. doi: 10.1016/S0074-7742(06)77002-7. [DOI] [PubMed] [Google Scholar]
- 2.Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TF. Developmental changes in serotonin signaling: implications for early brain function, behavior and adaptation. Neuroscience. 2017;342:212–231. doi: 10.1016/j.neuroscience.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- 4.Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 5.Lipton SA, Kater SB. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989;12:265–270. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- 6.Lotto B, Upton L, Price DJ, Gaspar P. Serotonin receptor activation enhances neurite outgrowth of thalamic neurones in rodents. Neurosci Lett. 1999;269:87–90. doi: 10.1016/S0304-3940(99)00422-X. [DOI] [PubMed] [Google Scholar]
- 7.Lieske V, Bennett-Clarke CA, Rhoades RW. Effects of serotonin on neurite outgrowth from thalamic neurons in vitro. NSC. 1999;90:967–974. doi: 10.1016/s0306-4522(98)00501-6. [DOI] [PubMed] [Google Scholar]
- 8.Haydon P, McCobb D, Kater S. Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons. Science. 1984;226:561–564. doi: 10.1126/science.6093252. [DOI] [PubMed] [Google Scholar]
- 9.Koert CE, Spencer GE, van Minnen J, et al. Functional implications of neurotransmitter expression during axonal regeneration: serotonin, but not peptides, auto-regulate axon growth of an identified central neuron. J Neurosci. 2001;21:5597–5606. doi: 10.1523/JNEUROSCI.21-15-05597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Igarashi M, Li WW, Sudo Y, Fishman MC. Ligand-induced growth cone collapse: amplification and blockade by variant GAP-43 peptides. J Neurosci. 1995;15:5660–5667. doi: 10.1523/JNEUROSCI.15-08-05660.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De-Miguel FF, Trueta C. Synaptic and extrasynaptic secretion of serotonin. Cell Mol Neurobiol. 2005;25:297–312. doi: 10.1007/s10571-005-3061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohan CS, Connor JA, Kater SB. Electrically and chemically mediated increases in intracellular calcium in neuronal growth cones. J Neurosci. 1987;7:3588–3599. doi: 10.1523/JNEUROSCI.07-11-03588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X-F, Hyland C, Van Goor D, Forscher P. Calcineurin-dependent cofilin activation and increased retrograde actin flow drive 5-HT-dependent neurite outgrowth in Aplysia bag cell neurons. Mol Biol Cell. 2012;23:4833–4848. doi: 10.1091/mbc.E12-10-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X-F, Ajeti V, Tsai N, et al. Regulation of axon growth by myosin II–dependent mechanocatalysis of cofilin activity. J Cell Biol. 2019;218:2329–2349. doi: 10.1083/jcb.201810054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 16.van Kleef ESB, Gaspar P, Bonnin A. Insights into the complex influence of 5-HT signaling on thalamocortical axonal system development. Eur J Neurosci. 2012;35:1563–1572. doi: 10.1111/j.1460-9568.2012.8096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56:479–485. doi: 10.1016/S0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 18.Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295:853–861. [PubMed] [Google Scholar]
- 19.Maloney SE, Rieger MA, Dougherty JD. Identifying essential cell types and circuits in autism spectrum disorders. Int Rev Neurobiol. 2013;113:61–96. doi: 10.1016/B978-0-12-418700-9.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirth A, Holst K, Ponimaskin E. How serotonin receptors regulate morphogenic signalling in neurons. Progr Neurobiol. 2017;151:35–56. doi: 10.1016/j.pneurobio.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Xing L, Son JH, Stevenson TJ, et al. A serotonin circuit acts as an environmental sensor to mediate midline axon crossing through EphrinB2. J Neurosci. 2015;35:14794–14808. doi: 10.1523/JNEUROSCI.1295-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnin A, Torii M, Wang L, et al. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- 23.Gasperini R, Choi-Lundberg D, Thompson MJ, et al. Homer regulates calcium signalling in growth cone turning. Neural Dev. 2009;4:29. doi: 10.1186/1749-8104-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell CB, Gasperini RJ, Small DH, Foa L. STIM1 is necessary for store-operated calcium entry in turning growth cones. J Neurochem. 2012;122:1155–1166. doi: 10.1111/j.1471-4159.2012.07840.x. [DOI] [PubMed] [Google Scholar]
- 26.Pavez M, Thompson AC, Arnott HJ, et al. STIM1 is required for remodelling of the endoplasmic reticulum and microtubule cytoskeleton in steering growth cones. J Neurosci. 2019 doi: 10.1523/jneurosci.2496-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi H, Nakashima S, Ohama E, Takeda S, Ikuta F, et al. Distribution of serotonin-containing cell bodies in the brainstem of the human fetus determined with immunohistochemistry using antiserotonin serum. Brain and Development. 1986;8:355–365. doi: 10.1016/S0387-7604(86)80055-9. [DOI] [PubMed] [Google Scholar]
- 28.Sundström E, Kölare S, Souverbie F, et al. Neurochemical differentiation of human bulbospinal monoaminergic neurons during the first trimester. Brain Res Dev Brain Res. 1993;75:1–12. doi: 10.1016/0165-3806(93)90059-J. [DOI] [PubMed] [Google Scholar]
- 29.Chen JJ, Vasko MR, Wu X, et al. Multiple subtypes of serotonin receptors are expressed in rat sensory neurons in culture. J Pharmacol Exp Ther. 1998;287:1119–1127. [PubMed] [Google Scholar]
- 30.Davenport RW, Dou P, Mills LR, Kater SB. Distinct calcium signaling within neuronal growth cones and filopodia. J Neurobiol. 1996;31:1–15. doi: 10.1002/(SICI)1097-4695(199609)31:1<1::AID-NEU1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Gomez TM, Snow DM, Letourneau PC. Characterization of spontaneous calcium transients in nerve growth cones and their effect on growth cone migration. Neuron. 1995;14:1233–1246. doi: 10.1016/0896-6273(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 32.Spitzer NC. Calcium: first messenger. Nat Neurosci. 2008;11:243–244. doi: 10.1038/nn0308-243. [DOI] [PubMed] [Google Scholar]
- 33.Togashi K, von Schimmelmann MJ, Nishiyama M, et al. Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron. 2008;58:694–707. doi: 10.1016/j.neuron.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Gasperini RJ, Pavez M, Thompson AC, et al. How does calcium interact with the cytoskeleton to regulate growth cone motility during axon pathfinding? Mol Cell Neurosci. 2017 doi: 10.1016/j.mcn.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 36.Nishiyama M, Hoshino A, Tsai L, et al. Cyclic AMP/GMP-dependent modulation of Ca2 + channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- 37.Ming GL, Song HJ, Berninger B, et al. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/S0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 38.Shelly M, Lim BK, Cancedda L, et al. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 39.Forbes EM, Thompson AW, Yuan J, Goodhill GJ. Calcium and cAMP levels interact to determine attraction versus repulsion in axon guidance. Neuron. 2012;74:490–503. doi: 10.1016/j.neuron.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 40.Odaka H, Arai S, Inoue T, Kitaguchi T. Genetically-encoded yellow fluorescent camp indicator with an expanded dynamic range for dual-color imaging. PLoS ONE. 2014;9:e100252–e100257. doi: 10.1371/journal.pone.0100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mortimer D, Feldner J, Vaughan T, et al. Bayesian model predicts the response of axons to molecular gradients. Proc Natl Acad Sci USA. 2009;106:10296–10301. doi: 10.1073/pnas.0900715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tojima T, Hines JH, Henley JR, Kamiguchi H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat Rev Neurosci. 2011;12:191–203. doi: 10.1038/nrn2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deneris E, Gaspar P. Serotonin neuron development: shaping molecular and structural identities. WIREs Dev Biol. 2017;7:e301–e326. doi: 10.1002/wdev.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sykes PA, Condron BG. Development and sensitivity to serotonin of Drosophila serotonergic varicosities in the central nervous system. Dev Biol. 2005;286:207–216. doi: 10.1016/j.ydbio.2005.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruediger T, Bolz J. Neurotransmitters and the development of neuronal circuits. Adv Exp Med Biol. 2007;621:104–115. doi: 10.1007/978-0-387-76715-4_8. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen L, Rigo J-M, Rocher V, et al. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 47.Zheng JQ, Felder M, Connor JA, Poo M-M. Turning of nerve growth cones induced by neurotrasmitters. Nature. 1994;368:1–5. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 48.Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996;16:1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spencer GE, Lukowiak K, Syed NI. Transmitter-receptor interactions between growth cones of identified Lymnaea neurons determine target cell selection in vitro. J Neurosci. 2000;20:8077–8086. doi: 10.1523/JNEUROSCI.20-21-08077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song H, Poo M. The cell biology of neuronal navigation. Nat Cell Biol. 2001;3:E81–E88. doi: 10.1038/35060164. [DOI] [PubMed] [Google Scholar]
- 51.Taylor AM, Menon S, Gupton SL. Passive microfluidic chamber for long-term imaging of axon guidance in response to soluble gradients. Lab Chip. 2015;15:2781–2789. doi: 10.1039/C5LC00503E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roychowdhury S, Martinez L, Salgado L, et al. G protein activation is prerequisite for functional coupling between Gα/Gβγ and tubulin/microtubules. Biochem Biophys Res Commun. 2006;340:441–448. doi: 10.1016/j.bbrc.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 53.Montoya V, Gutierrez C, Najera O, et al. G protein βγ subunits interact with αβ- and γ-tubulin and play a role in microtubule assembly in PC12 cells. Cell Motil Cytoskeleton. 2007;64:936–950. doi: 10.1002/cm.20234. [DOI] [PubMed] [Google Scholar]
- 54.Rahman S, Neuman RS. Multiple mechanisms of serotonin 5-HT2 receptor desensitization. Eur J Pharmacol. 1993;238:173–180. doi: 10.1016/0014-2999(93)90845-9. [DOI] [PubMed] [Google Scholar]
- 55.Hanley NRS, Hensler JG. Mechanisms of ligand-induced desensitization of the 5-hydroxytryptamine(2A) receptor. J Pharmacol Exp Ther. 2002;300:468–477. doi: 10.1124/jpet.300.2.468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.