Abstract
Background:
Arboviruses are RNA viruses and some have the potential to cause neuroinvasive disease and are a growing threat to global health.
Objectives:
Our objective is to identify and map all aspects of arbovirus neuroinvasive disease, clarify key concepts, and identify gaps within our knowledge with appropriate future directions related to the improvement of global health.
Methods:
Sources of Evidence: A scoping review of the literature was conducted using PubMed, Scopus, ScienceDirect, and Hinari. Eligibility Criteria: Original data including epidemiology, risk factors, neurological manifestations, neuro-diagnostics, management, and preventive measures related to neuroinvasive arbovirus infections was obtained. Sources of evidence not reporting on original data, non-English, and not in peer-reviewed journals were removed. Charting Methods: An initial pilot sample of 30 abstracts were reviewed by all authors and a Cohen’s kappa of κ = 0.81 (near-perfect agreement) was obtained. Records were manually reviewed by two authors using the Rayyan QCRI software.
Results:
A total of 171 records were included. A wide array of neurological manifestations can occur most frequently, including parkinsonism, encephalitis/encephalopathy, meningitis, flaccid myelitis, and Guillain-Barré syndrome. Magnetic resonance imaging of the brain often reveals subcortical lesions, sometimes with diffusion restriction consistent with acute ischemia. Vertical transmission of arbovirus is most often secondary to the Zika virus. Neurological manifestations of congenital Zika syndrome, include microcephaly, failure to thrive, intellectual disability, and seizures. Cerebrospinal fluid analysis often shows lymphocytic pleocytosis, elevated albumin, and protein consistent with blood-brain barrier dysfunction.
Conclusions:
Arbovirus infection with neurological manifestations leads to increased morbidity and mortality. Risk factors for disease include living and traveling in an arbovirus endemic zone, age, pregnancy, and immunosuppressed status. The management of neuroinvasive arbovirus disease is largely supportive and focuses on specific neurological complications. There is a need for therapeutics and currently, management is based on disease prevention and limiting zoonosis.
Keywords: Arboviruses, zoonosis, neuroinvasion, Zika virus, dengue virus, chikungunya virus, West Nile virus, Japanese encephalitis virus, St. Louis encephalitis virus, Powassan virus, Mayaro virus, tick-borne encephalitis virus
Introduction
Arboviruses are vector-borne RNA viruses that can infect the central and peripheral nervous system (PNS). 1 Arboviruses are arthropod-borne (i.e., ticks or mosquitos). During the past 75 years, numerous arbovirus epidemics have emerged around the world. Most epidemics have been from members of the Flaviviridae and the Togaviridae family of arboviruses. 1 Systemic symptoms are common and can include fever, myalgias, and arthralgias. Arbovirus neuroinvasion or neurotropism refers to the ability of the virus to cause neurological signs and symptoms by infecting the brain, meninges, and spinal cord. 2 Starting in the 21st century millions of cases of Zika virus and chikungunya virus have been reported. Two previously obscure viruses have threatened and tested our public health systems throughout the world. Newer strains of the Zika virus have emerged since 2016 with neuroinvasive capabilities. A feat not observed in prior strains. The ongoing zoonosis of arboviruses and their capability for neurovirulence is a continual global health and economic threat. 3 Numerous chemokines, receptors, and proteins have been implicated in arbovirus neuroinvasion including E-protein, toll-like receptor-3 (TLR-3), chemokine (C-C motif) ligand 5 (CCL5), and non-structural protein 5 (NS5) among others.4,5,190
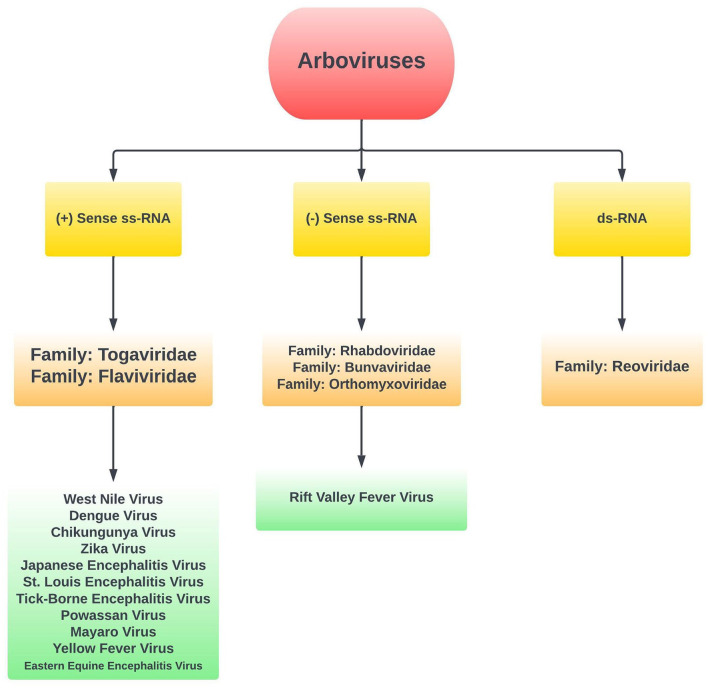
This review was conducted to inform clinicians on the varying neurological sequelae, neurodiagnostic measurements, outcomes, management, and preventative measures related to neuroinvasive emerging arbovirus infections. The scoping methodology of the literature review was planned to fully grasp and account for the breadth of material that can be reported on this important topic. We aim to identify and map out all aspects of arbovirus neuroinvasive disease, clarify key concepts, and identify gaps within our knowledge with appropriate future directions related to the improvement of global health. Furthermore, we aim to identify current management strategies, and those that are still in development that can help prevent disease progression and improve morbidity and mortality. We also provide a broad mechanistic overview by which neurotropic viruses gain access to the central nervous system. An overview of arbovirus classification is shown in Figure 1.
Figure 1.
Overview of arbovirus classification, and viruses included in review.
Methods
Registration and search strategy
Due to the broad topic and multiple related questions, a scoping review was planned. This scoping review was completed under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for scoping reviews (PRISMA-ScR) guidelines. The scoping review protocol was registered on Open Science Framework (Registration DOI: 10.17605/OSF.IO/GRKHA). The electronic databases PubMed/PubMedCentral/MEDLINE, Scopus, ScienceDirect, and Hinari were searched from the origin of their inception to 5th November 2022. Arbovirus selection was determined based on the experience of authors residing in endemic zones in addition to the recently established scientific literature on emerging arboviruses. 6 The following search terms were utilized: “Neuroinvasion arboviruses,” “neurotropic arboviruses,” “neurovirulent arboviruses,” “West Nile neuroinvasive disease,” “Zika virus,” “congenital Zika syndrome,” “Chikungunya virus,” “Dengue virus,” “Tick-borne encephalitis virus,” “St. Louis encephalitis virus,” “Japanese encephalitis virus,” “Powassan virus,” “Mayaro virus,” “Yellow fever virus,” and “Rift valley fever virus,” “Eastern Equine encephalitis virus.” When appropriate Boolean operators such as “AND”/“OR,” and the wildcard “*” were used within and between search terms. A combination of both medical subject headings as well as key words was utilized to take advantage of built-in database indexing. A gray literature search was conducted by reviewing the first 100 records obtained in Google Scholar. OpenGrey, a gray literature database was reviewed to obtain further bibliographic records. Backward and forward reverse citation was utilized to further increase the scope of the search. The search strategy and included viruses were determined based on prior and ongoing zoonosis with known neurological sequelae.
Inclusion and exclusion criteria
Studies were deemed eligible for inclusion if they provided original data on the primary outcomes: epidemiology, risk factors, neurological manifestations, neurodiagnostic measures, management strategy, and outcomes related to neuroinvasive arbovirus disease. Records that were not published in peer-reviewed journals, non-English, and without the full text available were excluded from the qualitative synthesis. During the article screening stage, records that were not associated with arboviruses were removed. Duplicated data provided by two or more articles were also excluded.
Data extraction and synthesis
All records obtained were exported to EndNote X9 3.3. Duplicate records were removed based on redundant bibliographic data. Records were then uploaded into the Rayyan QCRI software for systematic and scoping reviews to collaborate among authors. All records had their abstract, and title screened for relevancy by two independent assessors (B.S.S. and S.Z.). If any discrepancy arose between assessors, a third assessor (V.K.) was consulted. An initial pilot sample of records including 30 abstracts and titles was assessed for inclusion by all authors. We undertook the full screening of records only if the team had achieved a Cohen’s kappa of κ = 0.81 (near-perfect agreement).
After initial screening, full-text records were procured to check for relevancy to the topic. At this stage, all records not in the English language, and without the full-text available were excluded. Significant efforts were taken to obtain full records when not readily available. Full-text records with inappropriate data were assessed by two authors and then removed. Duplicated data was assessed when reporting on all records in this review. Thus, the original study was always referred to in the event of duplicated data from two or more records. Data extracted from each record included author(s), country of origin, study type, methodology/methods, population/sample size, and the primary outcomes (i.e., neurological manifestations, complications, neuro-diagnostics, epidemiological data, risk factors, treatment, and preventative strategies).
Data analysis
Simple descriptive methods were utilized to describe the data. When appropriate, data were summarized in a table for convenience. A critical appraisal of the study quality was determined to be overly complex given the vastness of the search, and heterogeneity of the procured records. This decision was made in support of all the authors and concordance with approved PRISMA-ScR guidelines.
Results
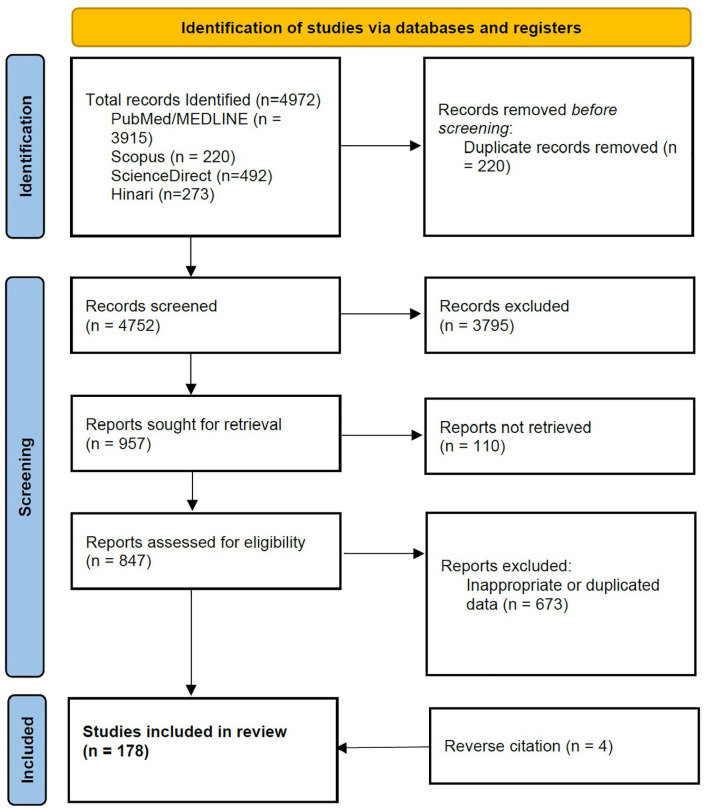
A total of 4972 records were obtained from which 178 records have been included in this review. A summary of the search results and record selection is visually depicted in the PRISMA flow chart (Figure 2). All identified records provided data related to some or all the following topics related to neuroinvasive arbovirus disease: epidemiology, risk factors, clinical presentation, neuro-diagnostics, management, and preventative strategies. The viruses presented in this review have been grouped by their respective families (Table 1).
Figure 2.
PRISMA-Scr flow diagram.
Table 1.
Viruses classified by family included within review.
| Family | Viruses |
|---|---|
| Flaviviridae | West Nile virus, Dengue virus, Zika virus, Japanese Encephalitis virus, St. Louis Encephalitis virus, Tick-borne encephalitis virus, Powassan virus, and Yellow fever virus |
| Togaviridae | Chikungunya virus, Eastern Equine encephalitis virus, and Mayaro virus |
| Bunyaviridae | Rift Valley fever virus |
Flaviviridae family
West Nile virus
West Nile virus epidemiology and pathogenesis
The West Nile virus (WNV) is an RNA virus that belongs to the Flaviviridae family transmitted by the Culex mosquito and is endemic to East Africa, Europe, Asia, and North America. 7 It emerged in the Americas in 1999, the virus has resulted in more than 48,000 reported cases, 24,000 reported neuroinvasive cases, >2300 deaths, and an estimated 7 million total human infections in the continental United States. 8 Following WNV infection, individual risk factors and comorbidities contribute to increased severity or death from neuroinvasive disease. The incidence of neuroinvasive disease increases approximately 1.5 times for each decade of life. 9 Individuals infected with WNV from infected donor organs are more likely to acquire severe neurologic disease and death compared to patients infected by mosquito bites. Elevated temperature also affects the rate of virus replication, causing increased infectivity. 10 Risk factors for WNV-associated meningoencephalitis include an age > 50 diabetes, and immunosuppression. 11
WNV clinical manifestations and neurodiagnostics
The neuroinvasive disease can present with fever, headaches, nuchal rigidity, confusion, seizures, and muscle weakness, among other focal neurological deficits. 12 Patients with West Nile encephalitis can develop tremors, especially in the upper extremities compared to the lower. Postural tremors are prevalent as well.13–16 Other neurological manifestations include myoclonus, bradykinesia, and other parkinsonian features. 16 A case of Anton syndrome and possible posterior reversible encephalopathy syndrome (PRES) secondary to WNV encephalitis has also been reported. 17 In some individuals, the involvement of respiratory muscles may lead to diaphragmatic and intercostal muscle paralysis and subsequent respiratory failure that requires emergent endotracheal intubation.18,19
A clinical presentation suspicious for WNV encephalitis should allow for cerebrospinal fluid (CSF) assessment for WNV Immunoglobulin M (IgM) antibodies.11,20 The presence of IgG antibodies indicates a chronic infection (weeks to months). The polymerase chain reaction test is often reserved for immunosuppressed patients who may lack sufficient antibody formation, and computed tomography of the brain is often non-contributory.21,22 Magnetic resonance imaging (MRI) abnormalities can appear within several days-to-weeks after the onset of the disease. Patients with meningitis secondary to WNV may have leptomeningeal enhancement.22,23 Lesions on MRI often include subcortical structures including the thalamus, basal ganglia, posterior limb of the internal capsule, midbrain, and pons.22–24
WNV management
Neuroinvasive West Nile disease has no specific cure. In general, some studies support the administration of intravenous immunoglobulin (IVIG) to patients with WNV encephalitis. 25 However, further research is still needed to evaluate the role of IVIG in WNV encephalitis. The antiviral agent ribavirin has shown in vitro activity against WNV infection, but its efficacy has not yet been demonstrated in animal models or humans.25,26 Supportive care is the main management strategy in West Nile encephalitis. Pain management is important as headaches are recorded in 69.7% of patients during hospital admission. 27 Convulsions and respiratory failure may warrant intensive care unit admission. 28 In some scenarios, mannitol and steroids have been administered in the management of inflammation, electrolyte derangements, and elevated intracranial pressure. 27
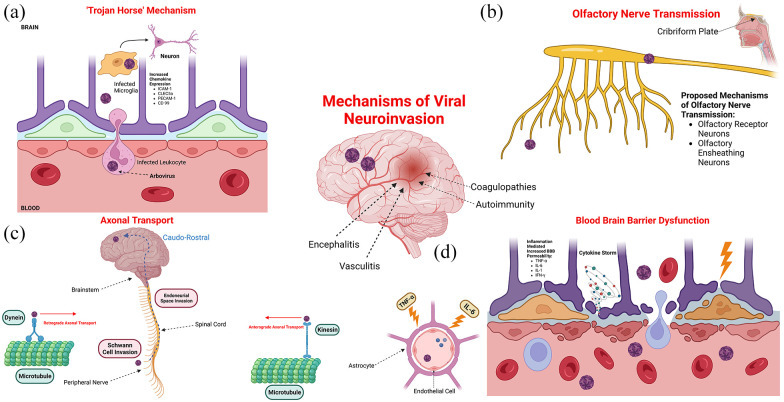
Long-term neurological sequelae include resting tremors, flaccidity, and cognitive disorders. 29 Cognitive dysfunction, including attention and lack of concentration, have also been reported long-term. 16 Hearing loss has also been documented in those who have recovered from WNV infection. These symptoms suggest vestibular involvement of the WNV, which may progress to chronic vestibulocochlear neuritis and loss of function. 30 Visual depiction of viral mechanisms of neurotropism is depicted in Figure 3.
Figure 3.
Mechanisms of viral neuroinvasion including entry via (a) the “trojan horse” mechanism, (b) olfactory nerve transmission, (c) axonal transport, and (d) blood-brain barrier dysfunction.
Dengue virus
Dengue epidemiology and pathogenesis
Dengue is a flavivirus-mediated disease first discovered in the 1950s and has had a devastating effect on human health and the global economy, with about 390 million people infected yearly.31,32 The burden of the high infectious rate translates into approximately 500,000 hospital admissions with 25,000 deaths yearly and billions spent on its prevention and management. 33 The dengue virus is an RNA virus within the family Flaviviridae and is transmitted by the bite of an infected mosquito of the Aedes species. Four antigenically distinct serotypes have been identified with their manifestations ranging from self-limiting symptoms to fatalities due to hemorrhagic fever and Dengue shock syndrome. 34 A meta-analysis conducted in 2021 analyzed 21 studies between 2007 and 2020 and demonstrated that age, lack of mosquito control measures, urban residence, climate change, and recent travel history are the leading risk factors for contracting dengue disease. 35
Old age is a significant risk factor for infection and severe disease, especially in the context of repeated exposure to flaviviruses. Although an individual obtains lifelong immunity after primary infection with one of the dengue serotypes, secondary infection with virulent strains or heterologous serotypes is known to be associated with severe disease.34,36 Dengue is spread primarily through the Aedes mosquito, and a key measure of prevention lies in the regulation of the presence and quantity of mosquitoes, as well as in the limitation of their contact with humans.37,38 There is a high prevalence of these mosquitoes in tires, water jars, and cans found near settlements. Therefore, overpopulated regions lead to a proportional increase in the number of susceptible hosts, and infected individuals, and an increase in the size of the mosquito population. 39 A challenge that slows progress in mosquito control is increasing resistance to insecticides, which are one of the most effective forms of vector control. This resistance occurs through mutations of target sites, detoxification, reduced penetration of insecticides via the cuticle, and mosquito behavior. 40 Socioeconomic risk factors that are also identified include occupation type, lower/lack of education, and poor income, with comorbidities such as diabetes mellitus being implicated in the severity of the disease. 35 The risk factors for Dengue are multifaceted, stemming from environmental sources, socioeconomic factors, and demographic factors.
For the past 7 decades, since its discovery in epidemics in the Philippines and Thailand, Dengue has spread rapidly throughout the world with an ever-increasing global burden of all arboviruses. 32 It is estimated that half of the world’s population lives in Dengue-infested areas, especially in urban centers within tropical and subtropical zones.33,41 The fast-paced spread of dengue is partially attributed to climate change which leads to amplified viral replication, vector survival, Aedes reproduction, and the bite rate. This trajectory ultimately leads to prolonged transmission, a higher number of infections, and spread beyond normal endemic boundaries.34,42
Dengue clinical manifestations and neurodiagnostics
Endothelial dysfunction is believed to be a key component in Dengue pathogenesis. 43 The dengue virus typically causes broken bone fever characterized by fever, muscle and joint pain, lymphadenopathy, and maculopapular rash. Hemorrhagic fever is a feared complication and is characterized by bleeding from the nose or gums, melena, and hematemesis. When not treated, Dengue shock syndrome may occur and is characterized by abdominal pain, vomiting, and eventual septic shock. 44 The dengue virus can also cause atypical neurological manifestations that fall under the expanded Dengue syndrome. 45 Neurological manifestations involving the CNS and PNS were observed in 2.64% of the participants in a study by Kulkarni et al. 46 Reported neurological manifestations include febrile seizures in young children, syncope, encephalopathy/encephalitis, meningitis, myositis, and intracranial bleeding. 47 Other reported complications include neuro-ophthalmic involvement, peripheral demyelinating disease (Guillain-Barré syndrome (GBS)) and myopathies. 47 These neurological manifestations can be broadly categorized into three categories: (1) direct neurotropic (such as encephalitis, meningitis, myelitis, and myositis), (2) systemic complications (including encephalopathy, stroke, and hypokalemic paralysis), and (3) post-infectious/immune-mediated (acute disseminated encephalomyelitis, GBS and optic neuritis).
Encephalopathy and encephalitis are the most common neurological complications of dengue fever.46,48 Encephalopathy is usually secondary to multisystemic derangements, such as shock, hepatic injury, coagulopathy, and concurrent bacterial infection with normal CSF findings. 49 Dengue encephalitis may occur due to direct neuronal infiltration by the dengue virus with signs of cerebral involvement. In such cases, the direct detection of dengue virus RNA aids in the diagnosis. Optic neuritis in dengue fever presents as impaired visual acuity and color vision due to inflammation of the optic disc, which can spontaneously resolve or lead to permanent visual deficits. 50 Radiographic findings often include T2/FLAIR hyperintensities involving the white matter, temporal lobes and basal ganglia. Cases of PRES have also been reported secondary to dengue. 51 Domingues et al. 52 conducted a retrospective review of 85 patients with confirmed dengue virus infection. Neurological manifestations involving the CNS were observed in 18/85 (21.2%). Chronic dengue virus panencephalitis with progressive dementia and extrapyramidal symptoms with antibodies in CSF measured using VirScan have been reported. 53 VirScan combines DNA micro-synthesis and bacteriophage providing a human virome of past and present viral antibodies. 54 The prevalence of neurological disease did not vary between dengue fever and dengue hemorrhagic fever. Dengue virus CSF RNA was only detected in 7/13 (53.8%) patients. 52 CSF findings of blood-brain barrier (BBB) dysfunction, including elevated albumin and protein may be present, indicative of neuroinflammation. However, the presence of IgM antibodies from the dengue virus or the direct detection of viral RNA through the polymerase chain reaction is of the highest diagnostic yield when considering the direct CNS involvement of the dengue virus. 55
Dengue management
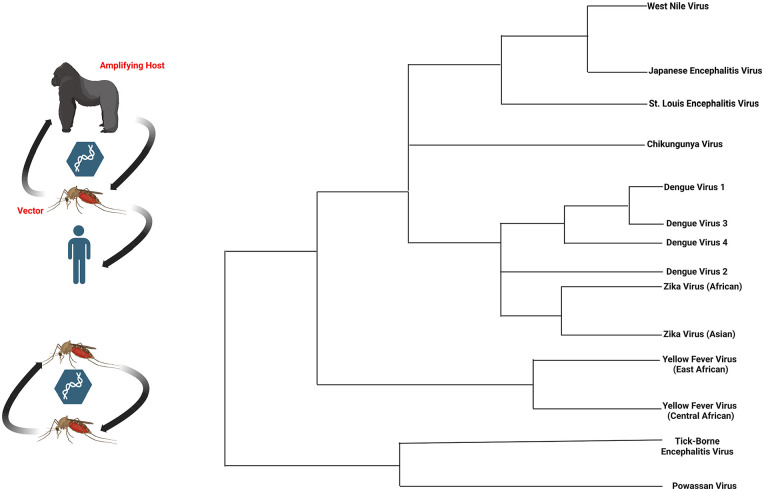
Currently, there are no approved antiviral agents for dengue; therefore, preventive measures are critically important. Dengvaxia is the only vaccine approved by the Food and Drug Administration for Dengue. 56 Ischemic strokes should be treated according to the standard of care. Optic neuropathy associated with dengue fever may resolve spontaneously or progress to severe and permanent visual loss. 50 Corticosteroids have shown some benefits in patients with Dengue-related myositis. 57 Standard treatment protocols (IVIG, plasmapheresis, etc.) for Dengue-related GBS and other peripheral demyelinating disorders have been effective. 58 Neurological involvement of the dengue virus may be fatal. A study of children inflicted with dengue in India reported a mortality rate of 7.5% in the group of patients who had neurological manifestations. Several vaccine preparations are under investigation and provide great hope for prevention and control. 59 Figure 4 provides a visual representation of the arbovirus transmission cycle and select flavivirus phylogeny.
Figure 4.
Arbovirus transmission cycle and flavivirus phylogeny. (BioRender Agreement: BN24TN2UWX).
Zika virus
Zika virus epidemiology and pathogenesis
The Zika virus (ZIKV) also belongs to the Flaviviridae family of viruses and is spread through the Aedes mosquito. There are two main ZIKV lineages: (1) African lineage and (2) Asian lineage. Since the virus was first discovered in the 1950s, it remained confined to equatorial regions in Asia and Africa. 60 However, in the mid-1900s reports of infections were observed outside of the prior endemic zone. The transmissibility factor (R0) for ZIKV has been estimated to be between 1.4 and 6.6. 61 Risk factors for ZIKV infection are similar to other arboviruses. Residence and travel to one of the endemic locations, especially during the summer months, sexual intercourse with an infected individual, blood transfusions, and vertical transmission through pregnancy are commonly seen. 62
ZIKV is replicated in the cells of the mosquito midgut and salivary glands. A 5–10-day period is needed for the virus to have seeded the mosquito saliva. When an infected mosquito’s saliva is introduced into human skin the virus begins to infect Langerhans cells and keratinocytes. Shortly afterward, the lymph nodes and blood stream are seeded with the virus. 63 Specific receptors targeted by ZIKV include phosphatidylserine, T-cell immunoglobulin mucin receptor, and C-type lectin receptors.64,65 The systemic symptoms of ZIKV include a series of symptoms known as Zika fever.
ZIKV clinical manifestations and neurodiagnostics
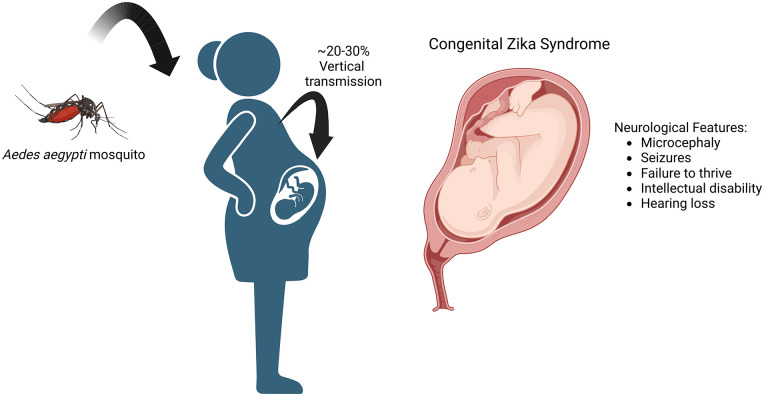
Symptoms of Zika fever include elevated body temperature, conjunctival injection, arthralgia, and a maculopapular rash. Zika fever may persist for an average of 7 days. Most cases are mild and may be similar in presentation to dengue fever. Neurological signs and symptoms secondary to ZIKV have also been reported. ZIKV was traditionally not associated with CNS involvement or disease until 2015. A new circulating strain is known as ZIKVBR with increased neurovirulence. In vitro studies revealed that this strain invaded neuroprogenitor cells and cortical neurons, allowing viral replication and apoptosis. Other strains exhibiting neurovirulence include Brazilian ZIKV, IbH30656, and FB-GWUH-2016. 66 These strains have also been shown to impair growth in fetal neural progenitor stem cells. 67 During the ZIKV outbreak in Brazil, numerous cases of microcephaly were reported in newborns. The Brazilian Ministry of health confirmed approximately 2000 cases of fetal neonate microcephaly with co-infection with ZIKV. Microcephaly is a sequalae of cephalic defects that include occipital protuberance, small brain, ventriculomegaly, asymmetric cerebral lobes, lissencephaly (loss of gyri), enlarged extra-axial spaces, and dysgenesis of the corpus callosum. 68 Other neuroradiographic findings include ventriculomegaly, cortical malformations, calcifications, cerebellar hypoplasia, and delayed myelination. 69 Microcephaly from ZIKV is typically identified after birth; however, the diagnosis can also be made intrauterine using ultrasound. 70 The World Health Organization (WHO) defines microcephaly as a newborn occipitofrontal measurement with 37 weeks of gestation as ⩽31.9 cm for boys and ⩽31.5 cm for girls. 71 Secondary complications from microcephaly include seizures, severe intellectual disability, and failure to thrive (Figure 5). Given the significant morbidity and mortality of neonatal microcephaly from vertical transmission of ZIKV, efforts to screen young women of reproductive age in the endemic zone are important.
Figure 5.
Overview of congenital Zika syndrome, and common neurological features. (BioRender Agreement Number: FB24RNJ6LI).
Zika testing, particularly in areas where both dengue and Zika viruses are prevalent, primarily relies on nucleic acid amplification tests (NAATs) for diagnosis in patients showing symptoms of either disease. These tests are most effective when performed on serum samples taken within 7 days of symptom onset. IgM antibody testing is also used, especially when NAATs are negative or if the serum is collected more than 7 days after symptoms begin. However, interpreting IgM results is challenging due to potential cross-reactivity between the two viruses, and it is difficult to pinpoint the exact timing of infection. This issue is especially critical for pregnant women, to ascertain whether Zika infection occurred during or before pregnancy. For symptomatic pregnant women, it is recommended to collect serum and urine samples as early as possible, preferably within 12 weeks from symptom onset, for concurrent testing of both dengue and Zika viruses using NAATs and IgM antibody tests. If IgM tests are positive but NAATs are negative, additional tests like neutralizing antibody tests should be conducted for confirmation, especially in pregnant women. 72
Other neurological manifestations include GBS, meningoencephalitis, seizures, headaches (primarily frontal), and flaccid paralysis, among others. Neuroradiographic findings of ZIKV often include vasogenic edema of deep subcortical structures, and spinal imaging may show T2/FLAIR changes associated with transverse myelitis. 27 The first reported case of significant neurological involvement from a case of ZIKV occurred in 2016. An 81-year-old man required admission to the intensive care unit for ZIKV-associated meningoencephalitis. The patient had recently visited New Caledonia and MRI was significant for multiple subcortical T2/FLAIR hyperintensities and multiple punctuate areas of diffusion restriction consistent with ischemic foci. The diagnosis was confirmed with a positive viral culture from CSF. 73 A fatal case of meningoencephalitis was reported in an immunosuppressed patient after a heart transplant. During the 2015–2016 outbreak in South America, 1474 cases of GBS were reported. 74 Furthermore, another report from South America in 2016 revealed that 60% of patients with symptomatic GBS were serologically positive for ZIKV. 75 GBS may also be present in patients infected with dengue virus, as well as Chikungunya virus (CHIKV). Lebov et al. 76 studied the longitudinal neuropsychological manifestations of children infected with ZIKV in comparison to noninfected children. Neuropsychological manifestations were seen in both groups; however, no differences were observed between both groups (ZIKV infected vs non-infected). Romani et al. 77 conducted a cohort analysis of 152 children born to ZIKV-infected pregnant women. Only 11 women met the confirmed ZIKV infection standard. 2/11 (18.1%) newborns were diagnosed with congenital Zika syndrome.
ZIKV management
An accurate diagnosis of ZIKV should be done by measuring Zika viral RNA in a real-time polymerase chain reaction. Currently, there are no approved therapeutics against ZIKV in people with neurological disease. Clinical trials are currently underway to examine a possible vaccine. However, they may not be widely available until the mid-late 2020s. Vaccination may be an effective strategy to prevent ZIKV-mediated neonatal microcephaly by actively vaccinating pregnant women in the endemic zone and those traveling there. 78 The use of monoclonal antibodies against ZIKV is an area of further research. 79
Japanese encephalitis virus
Japanese encephalitis virus epidemiology and pathogenesis
Like other members of the Flaviviridae family, the Japanese encephalitis virus (JEV) is also a single-stranded positive-sense RNA virus, which results in the most commonly diagnosed endemic encephalitis. 80 It is horizontally transmitted between vertebrates such as pigs, humans, birds, and arthropods (i.e., Culex mosquito). 81 Pigs serve as the amplifying host, while humans are referred to as the dead-end host, given the inability of feeding mosquitoes to become infected because of inadequate viremia. Migratory water birds and bovine birds are natural reservoirs for JEV. 82 Transmission of JEV through non-vector mechanisms, particularly oral and nasal shedding has been experimentally demonstrated in pigs, macaques, guinea pigs, hamsters, squirrels, and mice. 83 However, viral RNA in throat swab samples from Japanese encephalitis (JE) patients suggests that oral shedding may also be possible in the human population. 83 Such vector-free transmission that could occur from direct blood, or sexual transmission has also been described for other flaviviruses such as Zika virus, WNV, Bagaza virus, St. Louis encephalitis virus, Tembusu virus, and Wesselsbron virus. 84 The epidemiological significance of this pattern of non-vector transmission of JEV remains unclear. Furthermore, persistence in the vaginal epithelium has brought to light important implications related to potential sexual and transplacental transmission of JEV. 85 Nosocomial transmission of JEV through transfusion of blood components, as well as post-transplantation, has also been reported to result in symptomatic encephalitis in an immunocompromised recipient.86,87
The annual incidence of JE estimated in the late 1980s was 2 per 100,000 while the incidence calculated almost a decade later revealed that 175,000 cases of JE would occur among unvaccinated children under 15 years of age, resulting in an estimated incidence of 25 per 100,000. 88 A systematic review by Campbell et al. 89 reported approximately 68,000 annual cases with a global incidence of 1.8 per 100,000 and an age-specific incidence of 5.4 per 100,000 in children under the age of 15. Interestingly, despite JE being a notifiable disease, only 10% of these cases are reported to the WHO. 89
JE was first documented as an epidemic in Japan in the year 1871 and has since been reported throughout the world after being isolated initially in 1935. It is a particularly important infection in the geographic regions of South Asia, Southeastern Asia, Northern Australia, and Eastern Asia. JEV has been reported in 27 countries in these regions and can be categorized into four groups based on various characteristics (Table 2). 90
Table 2.
Classification of Japanese encephalitis grouped by country and features.
| Classification | Countries | Features |
|---|---|---|
| Group 1 | Japan, South Korea, and Taiwan | These countries experience JE a epidemics and have established immunization and surveillance programs. Future programs should focus on the long-term prevention of adult JE cases following large-scale immunization |
| Group 2 | Cambodia, the People’s Republic of China, India, Malaysia, Nepal, Sri Lanka, Thailand, and Vietnam | These countries, India and the People’s Republic of China in particular contribute majorly to the worldwide incidence of JE and have national or sentinel surveillance programs. Immunization is carried out in high-risk areas |
| Group 3 | Bangladesh, Bhutan, Brunei Darussalam, Burma (Myanmar), Indonesia, Laos, North Korea, Pakistan, PNG, Philippines, and Timor-Leste | These countries report fewer than 100 cases annually and do not have any known surveillance programs. Immunization against JEV b is not carried out. Nationwide surveillance systems should be established to study the disease burden in these countries |
| Group 4 | Australia, Guam, Russia (Siberia), Singapore, and Saipan | These countries report fewer than 3 cases annually. Surveillance systems have been established. In the future, surveillance programs should be maintained, and immunization programs should be established for the target population |
Japanese encephalitis.
Japanese encephalitis virus.
The JEV is classified into five distinct genotypes that have originated from a common ancestor in the Southeast Asian regions of Indonesia and Malaysia. Genotypes IV, III, II, and I have evolved from the oldest common ancestor Genotype V. Genotype V, also known as the ‘Muar strain’, was regarded as the only isolate of Genotype V since 1952 and remained undetected for over 6 decades. 91 A recent national monitoring of the mosquito population and flavivirus epidemiology in South Korea revealed that the seven JEV-positive mosquitoes were Genotype V. 92 The geographic distribution of the five JEV genotypes is highlighted in Table 3. 93
Table 3.
Classification of Japanese encephalitis grouped by country and features.
| Genotype | Countries | Years |
|---|---|---|
| GI | Northern Australia, Northern Cambodia, China, India, Japan, Korea, Laos, Malaysia, Taiwan, Thailand, and Vietnam | 1967–2013 |
| GII | Sporadic cases in Northern Australia, Indonesia, Korea, Malaysia, Papua New Guinea, and Southern Thailand | 1951–1999 |
| GIII | Epidemics in China, India, Indonesia, Japan, Korea, Malaysia, Myanmar, Nepal, Philippines, Sri Lanka, the former Soviet Union, Taiwan, Thailand, and Vietnam | 1935–2013 |
| GIV | Seven isolates in Indonesia | 1980–1981 |
| GV | Malaysia, China, and South Korea | 1952–2020 |
JEV clinical manifestations and neurodiagnostics
JE is a severe infection with mortality ranging from 5% to 50%, and about 50% of patients who develop JE experience neurological symptoms. 94 Nonspecific prodromal symptoms, such as fever, coryza, diarrhea, headache, body aches, and vomiting, can precede acute encephalitis syndrome by 3–4 days while the primary course of the infection can last up to 3 weeks. Most JEV infections remain asymptomatic or present with a nonspecific febrile illness; however, only 1% of these infections progress to neuroinvasive disease. Studies with longer follow-up periods report the inability of children to meet their pre-infection level of educational performance even after making a full recovery. 82 Patients with meningeal or brain parenchymal involvement can develop seizures, headaches, and altered state of consciousness which may progress to coma and death. Neurological examination may show cognitive dysfunction, neck stiffness, reduced power in the limbs, cranial nerve palsies, and mute deep tendon reflexes. Parkinsonian features that include facial masking and resting tremor show a propensity for basal ganglia involvement in JE.82,95 Other reported movement disorders include lip-smacking, bruxism, hemiballismus, hypokinesia, choreoathetosis, and hypophonia due to incoordination of the vocal muscles. 82
Acute flaccid paralysis, particularly in the pediatric population, indicates damage to the anterior horn cells that can be histologically demonstrated on autopsy. Involvement of anterior horn cells may highlight a poorer prognosis which is often missed on clinical assessment. GBS, transverse myelitis (<3 segments), longitudinally extensive transverse myelitis (⩾3 segments), acute disseminated encephalomyelitis, dystonic storm, have all been reported neurological complications in patients with JE.95–99 Furthermore, a case of N-methyl d-aspartate receptor encephalitis secondary to JE has also been reported pointing toward the possibility of secondary autoimmune events. 100 A case of JE mimicking poliomyelitis and presenting with primary respiratory failure has also been reported. 101
Furiya et al. 102 reported a patient with multiple bouts of hyperthermia during the summer after a JEV infection. MRI revealed T2 hyperintensities in both thalamic paraventricular subcortical regions, which project to the hypothalamic paraventricular nucleus. However, involvement of the limbic system can present with hypothermia as reported in an 18-year-old patient with JE with bilateral lateral rectus palsy and asymmetric flaccid paresis of the four limbs. 98 A detailed ophthalmologic examination is necessary in patients with suspected JEV infection, highlighted by several cases of ischemic maculopathy, chorioretinitis, and retinal hemorrhages.103–105
Japanese encephalitis may appear clinically identical to some of the other causes of infectious meningoencephalitis and subsequently, it becomes imperative to correlate clinical and laboratory findings. Peripheral blood may reveal neutrophil-predominant leukocytosis, mild thrombocytopenia, and mildly elevated liver enzymes. The CSF analysis may appear normal or show mild pleocytosis, as seen during the 2005 JE epidemic in India. Anti-JEV IgM can be detected in one or two CSF collections according to WHO recommendations. Within the first week, IgM is detectable in CSF of 70% of JE cases, while on day 10 it is detectable in up to 95% of patients. Therefore, samples should ideally be collected after the fifth day of the disease course to detect IgM. Further confirmation of diagnosis may be done by detecting viral antigen, nucleic acid detection, or plaque reduction neutralization test which is the gold standard.104,106,107
MRI findings of JEV include T2/FLAIR and diffusion restriction in sub-cortical structures including the thalamus, substantia nigra, and basal ganglia. However, brain stem, cerebral cortex, cerebellum, and spinal cord lesions are possible. 108 CT images of the head often show subcortical hypodensities. In newborns, DWI can detect earlier lesions as compared to T2WI and FLAIR sequences due to the higher water content in the developing brain. 108 Dung et al. 109 conducted a cohort analysis of 75 patients with thalamic involvement on CT and/or MRI and had a 100% positive predictive value and specificity, as well as a sensitivity of 23%, and 42.1% negative predictive value.
Arahata et al. 110 reported a case of a 10-month-old boy with longitudinal brain MRI depicting a patchy unilateral thalamic injury that progressed to bilateral involvement. The lesions were consistent with cytotoxic edema and showed the possibility of progressive involvement of the inter-thalamic tract. The T2/FLAIR sequences may be important in the chronic phase of JE showing vasogenic edema and gliosis as a result of necrosis. Although radiological findings on MRI may overlap with herpes encephalitis and autoimmune encephalitis when the temporal lobe is majorly involved in patients with JE, MRI still has an important role in recording the extent and chronicity of lesions. Hemorrhagic lesions, particularly in the thalamus, are also rarely observed in JE patients. 108
JEV management
Currently, no definitive treatment has been shown to alter the course of JEV; therefore, an increasing emphasis is placed on primary prevention. However, an estimated 80% of cases occur in regions with established JEV vaccination programs; this is likely due to ineffective programs, vaccines, and coverage. 94 Supportive therapy is the mainstay treatment in patients with JE and should focus on adequate fluid therapy, maintenance of airways, breathing, circulation, electrolyte balance, fever management, management of immobility complications, and treatment as well as prevention of aspiration pneumonia. Antibiotics are routinely administered due to the similar presentation of bacterial meningitis; however, antibiotics may not be used if the bacterial infection is unlikely based on neuroimaging or CSF findings. 94
Only a few randomized controlled trials have been conducted to investigate potentially beneficial therapeutic agents; however, results remain inconclusive. A double-blind, randomized placebo control trial of high-dose dexamethasone failed to show clinical benefit and did not alter mortality rates between the placebo and control groups. 111 A comparative study by Rathi et al. 112 did not favor dexamethasone use in patients with JE and a retrospective study by Johnson et al., 113 demonstrated similar results. However, corticosteroids and mannitol are routinely used in patients with JE, particularly those with elevated intracranial pressure. 112
Two randomized controlled trials investigating the use have demonstrated conflicting evidence. Clinical trials have 114 demonstrated a significant reduction in the duration of fever, hospital stay, and improved level of consciousness by administering minocycline to patients with JE. However, long-term neurological sequelae and mortality at 1 year remained unchanged. Kumar et al. 115 did not show a significant mortality difference after minocycline use. Similarly, alpha-2a interferon did not show a significant improvement in a clinical trial. 116 However, one case has recorded an improvement in short-term symptoms following ribavirin use, but long-term outcomes remained uninfluenced. 117 A case series by Harinasuta et al. 118 has shown better clinical outcomes and improved symptoms following interferon use in two patients, while patients not receiving interferon met fatal outcomes.
St. Louis encephalitis virus
St. Louis encephalitis virus epidemiology and pathogenesis
The St. Louis Encephalitis (SLE) virus belongs to the Flaviviridae family and is a single-stranded RNA viral agent. SEV is transmitted to humans and animals (e.g., horses) as accidental dead-end hosts via the bite of female mosquitoes from the Culex species, for example C. nigripalpus, C. quinquefasciatus, C. pipiens, C. tarsalis, C. stigmatosoma, and C. erythrothorax. Humans and animals do not develop significant viremia to transmit the disease (to other humans or viral vectors); however, wild birds, for example, pigeons, house sparrows/finches, robins, and blue jays do develop sufficient viremia to infect mosquitoes that bite them and, thus, act as amplifying hosts in the enzootic cycle of SLE virus.119,120
Risk factors for SLE include travel to endemic areas, mosquito bites, outdoor activities during mosquito feeding times or mosquito breeding areas, blood transfusions, and old age (higher risk of encephalitis). The risk of neuroinvasive disease is elevated in subjects who have undergone solid organ transplantation and in other individuals who suffer from immunosuppression. 121 Most reports of SLE come from the United States of America, specifically from the Mississippi River and/or Gulf Coast regions, although cases have been reported in other geographical areas as well: Argentina, Brazil, Canada, Colombia, Cuba, The Caribbean, Mexico, Panama, Peru, Trinidad Tobago, Uruguay, Venezuela. The transmission of the SLE virus appears to occur primarily in the late summer and/or early fall in temperate regions and throughout the year in warmer climates. 122
SEV clinical manifestations and neurodiagnostics
Although most SLE infections do not affect the brain, when the BBB is crossed by the viral agent it can predominantly affect gray matter (with consequent lymphocytic meningitis), although cases of acute demyelinating encephalomyelitis due to white matter involvement have been described. The cerebellar/cerebral cortex, the hypothalamus, the brainstem, basal ganglia, and the spinal cord can all be severely affected by SLE. Encephalitis is generally seen in the elderly, whereas aseptic meningitis, which is the most frequent neurologic discovery, develops primarily in children or adults of younger ages. SLE can either be asymptomatic or present with non-specific/flu-like symptoms (e.g., headache, fever/rigors, arthralgias/myalgias, nausea, vomiting, rash, diarrhea) to those related to the development of meningitis (e.g., agitation/confusion, altered mental status, photophobia, neck pain, nuchal rigidity, seizure, or even coma) or acute flaccid paralysis.123–125 The neurodiagnostic findings of the SLE virus are described in Table 4.
Table 4.
Neurodiagnostic findings in St. Louis encephalitis virus.
| Neurodiagnostic modality | Findings |
|---|---|
| Computed tomography | Usually, normal |
| Magnetic resonance imaging | Usually normal; however, may have subcortical T2 hyperintensities |
| Cerebrospinal fluid | ↑opening pressure, cell count <500 /mmc, normal/↓glucose, ↑protein, lymphocytic pleocytosis |
| Electroencephalogram | ± diffuse slowing, ± delta-wave activity with isolated spikes |
| Enzyme-linked immunosorbent assay | Serum/CSF anti-SLE virus IgM antibodies (diagnostic) |
| Nucleic acid amplification testing | Can identify viral nucleic acid, usually performed to exclude other viral agents with similar presentations. Limited utility as clinical symptoms develops usually when viremia is no longer detectable |
| Metagenomic next-generation sequencing | Useful when other targeted approaches have failed to detect the SLE virus |
| Viral cultures | Usually, unsuccessful. The cytopathic effect usually occurs in ~5 days |
| Other laboratory findings | ± Leukocytosis, ± hyponatremia, fluid overload via SIADH |
SIADH: syndrome of inappropriate antidiuretic hormone.
SEV management
The management of SLE is based on supportive care (intravenous fluids, antipyretics) as no antiviral agents/vaccines have yet been approved. Fluid restriction is employed to manage the accompanying SIADH. Thus, the prevention of mosquito bites remains of uttermost importance in order not to contract SLE. Prevention measures include wearing protective clothing, the use of mosquito repellants (permethrin, lemon eucalyptus, DEET)/nets/screens, avoidance of outdoor activities between dawn and dusk when feeding of viral vectors occurs, and of areas with standing water/wading pools/tire swings where mosquitoes tend to breed. 126
Most subjects who contract the virus will recover fully; however, SLE can be fatal in 5%–20% of cases, particularly in the elderly. Other complications of neuroinvasive disease include SIADH-related hyponatremia, cerebral edema, central poutine myelinolysis, subacute thyroiditis, or post-infectious encephalomyelitis. Neurological sequelae including intellectual disability, (extra) pyramidal signs, and/or convulsions, have been observed primarily in children, while adults can exhibit prolonged convalescence characterized by anxiety, depression, emotional lability, irritability, attention/memory deficits, asthenia, dizziness, tremor, or gait unsteadiness. 124
Tick-borne encephalitis virus
TBEV epidemiology and pathogenesis
TBEV is a single-stranded RNA virus that is transmitted by the Ixodes Spp tick and has a varied presentation from asymptomatic to neuroinvasive disease. 127 There are three major subtypes of the TBEV: (1) the European virus (TBEV-Eu), (2) the Far Eastern virus (TBEV-Fe), and (3) the Siberian virus (TBEV-Sib). The Ixodes Ricinus and Ixodes Persulcatus ticks are the two main vectors implicated in transmission. I. Persulcatus is primarily responsible for spreading the transmission of TBEV of the Far Eastern and Siberian subtypes, and I. Ricinus is responsible for the transmission of the European subtype of TBEV. 128
Countries in Europe that are known to be endemic to TBEV include Austria, Denmark, Estonia, Finland, Germany, Hungary, Italy, Latvia, Norway, Poland, Romania, Switzerland, Sweden, Slovenia, and Slovakia. 129 Non-European endemic countries include Japan, China, and Russia. 130 An analysis of 128 patients with tick-borne encephalitis (TBE) demonstrated that a functional TLR-3 was associated with infection. 131 Lifestyle factors that lead to frequent and prolonged exposure to forested areas are associated with an increased risk of TBE. In a national population-based survey it was determined that spending <10 h/week in forests, and unemployment were associated with the highest risk of TBE. 132
TBEV clinical manifestations and neurodiagnostics
Following a bite from a tick, replication of TBEV will begin to start locally. Initially, it will start by replicating in dendritic skin cells and will be transported to local lymph nodes. Once local lymph nodes are infected the next affected area consists of the spleen, liver, bone marrow, and ultimately the brain; however, the exact mechanism of penetration of the blood-brain barrier is not clearly understood. 133 The spectrum of neurologic disease ranges from meningitis to severe encephalitis. Seizures, cranial neuropathies, and dysphagia have also been reported. TBE often follows a biphasic trajectory: (1) Phase I (2–10 days) systemic symptoms (i.e., fever, malaise, arthralgia, etc.) followed by (2) Phase II (7–21 days) leukopenia, thrombocytopenia, and neurologic symptoms. 134 A post-encephalitic syndrome is an umbrella term that describes various neurological complications seen in patients after the acute phase of the infection. This includes cognitive dysfunction, apathy, irritability, memory problems, and inability to concentrate. 135
Hematologic abnormalities (i.e., leukopenia, thrombocytopenia) are observed in approximately 70% of patients. 133 Serological examination of IgG and IgM antibodies with clinical symptoms is the gold standard for the diagnosis of TBE. CSF analysis may also reveal lymphocytic pleocytosis. 136 MRI is often not associated with the diagnosis; however, in cases of severe subcortical lesions including the basal ganglia and thalamus may be observed. 137
TBEV management
There are no approved antiviral agents for TBEV, and management is supportive. 138 Supprovtive management includes antiemetics, antipyretics, analgesics, and intravenous fluids.
Powassan virus
Powassan virus epidemiology and pathogenesis
The first case of the Powassan virus (POWV) was reported in the town of Powassan in 1958 in Canada. It is endemic to the United States and Canada. Risk factors related to POWV infection include exposure to wild animals and time spent outside in wooded areas where POWV is endemic. 139 Approximately 110 cases have been reported in the United States and 20 in Russia. Less than 300 cases of POWV have been reported worldwide. The incidence of infection has increased over the last few decades, likely related to increased detection. The Powassan virus has an incubation period of 7–34 days.69,139,140
POWV clinical manifestations and neurodiagnostics
POWV generally presents with systemic symptoms such as fever, malaise, and headache. Neurological manifestations are rarely reported; however, seizures and malignant fevers have been reported. 139 Several cases of nystagmus, facial palsy, and myelitis have been reported. The diagnosis of POWV is best supported by the presence of neutralizing IgM antibodies. Neuroimaging including CT and MRI is often non-contributory; however, may show subcortical T2/FLAIR hyperintensities and diffusion restriction. Positive magnetic resonance findings are typically observed in people with severe encephalitis.139,141,142 Of a study reporting 98 cases from which 88 reported neuroinvasive involvement, 11 deaths were recorded with an estimated mortality of 11.22%. 143
POWV management
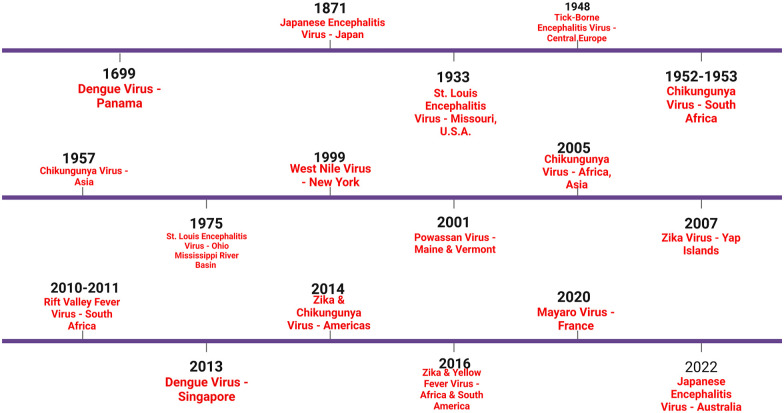
Like other arboviral diseases, the management of POWV is supportive. Few case reports have shown benefits in the use of corticosteroids or IVIG. Thus, primary prevention and surveillance are key strategies. Wearing protective clothing and utilization of insect repellent measures should be carried out during the summer season and in a wooded area where POWV is endemic. Additionally, patients presenting with encephalitis of POWV in the endemic zone can be considered. Efforts are currently underway to develop a vaccine against POWV.144–146 A timeline of major arbovirus epidemics is provided in Figure 6.
Figure 6.
A timeline of major arbovirus epidemics over the centuries. (BioRender Agreement Number: WF24UB7EXG).
Togaviridae family
Yellow Fever virus
Yellow Fever virus epidemiology and pathogenesis
The yellow fever virus (YFV) is another RNA virus that belongs to the Flavivirus family. Similar to other members of this family, the virus is transmitted to humans via the bite of the Aedes aegypti mosquito. In 2013 an outbreak of YFV led to more than 45,000 reported deaths, with 90% of reported deaths occurring in Africa. The endemic zone of YFV places nearly 1 billion people at risk of infection. Infections are most commonly reported in Africa and South America and rarely in the Asian continent (2014). 189 The pathogenesis of the virus involves infection of dendritic cells and the subsequent lymph node. YFV is known to infect Kupffer cells and other hepatocytes, often leading to acute liver injury and failure. Cytokine storm, organ failure, and septic shock are common causes of mortality. 147
YFV clinical manifestations, neurodiagnostics and management
Neurological involvement of the YFV is rare, however, and has been reported. Cases of seizures, cranial nerve palsies, seizures, and encephalitis have been reported. Marinho et al. 148 performed a postmortem CSF analysis of a 3-year-old girl who died from septic shock secondary to YFV. The CSF analysis was positive for the yellow fever virus RNA showing neuroinvasive potential. Similarly, to other neuroinvasive diseases, children are at increased risk. Ho et al. 149 completed a retrospective descriptive cohort study on 79 cases of YFV that required admission to the intensive care unit. They found 25% of patients with severe YFV had seizures. Seizures likely occurred due to severe systemic inflammation and lowering of the seizure threshold rather than direct viral neurotropism. Lewis Stevenson performed a brain pathological report on 20 individuals infected with YFV and died from the virus. He noted that perivascular hemorrhages more often affect subcortical structures, including the mamillary bodies, subthalamic area, and the periventricular region. 150 Pathological inflammatory changes were not observed. The presence of perivascular hemorrhages can lead to cerebral edema, occlusive microvasculopathy, and failure of cellular metabolism, rather than direct neuroinflammation. Liu and Chambers 151 inoculated mice deficient in interferon-gamma (IFN- γ) and knockout mice with a neuroadapted yellow fever 17D virus. The results indicated that Th1-specific CD4+ lymphocytes, as well as antibody production, are key in the survival of YFV encephalitis. Neurological manifestations secondary to the YFV vaccine have also been reported. McMahon et al. 152 reported on neurological disease sent to the National Vaccine Adverse Events Reporting System (VAERS). Six cases of GBS, one of encephalitis and two of acute disseminated encephalomyelitis, were classified as “suspect” vaccine-associated diseases. Other adverse events related to the YFV vaccine can include hyperthermia and jaundice.
YFV management
Similar to other members of the Flaviviridae family, there are no approved therapeutic agents. Treatment is often supportive, including pain relief, anti-inflammatory agents, and hydration. Vaccination and vector control remain the mainstay methods in disease prevention and progression. Few studies mainly in animal models have shown potential with antiviral agents such as ribavirin. 147
Chikungunya virus
CHIKV epidemiology and pathogenesis
CHIKV is an alphavirus (genus Alphavirus, Togaviridae family) transmitted through the bite of the Aedes mosquito. CHIKV has an incubation period of between 1 and 12 days. The mechanism of pathogenicity is poorly understood; however, viral replication initially occurs in human epithelial and endothelial cells.153,154 CHIKV outbreaks have been reported as far back as the 18th century and the virus was first isolated in Tanzania, in 1952.155,156 CHIKV has traditionally been endemic to Sub-Saharan Africa and a few outbreaks of CHIKV were observed in Asia in the 20th century. Beginning in 2013 CHIKV has been spreading to the Americas, and one outbreak was observed in France, in 2014. 157 After the 2013 outbreak, a study in Saint Martin determined a seroprevalence of 16.9% with 39.0% of cases being asymptomatic. 158
Systemic symptoms often observed include headache, fever, myalgia, arthralgia, and rash that lasts 7–14 days on average. 159 Galatas et al. 160 conducted a field investigation in Trapeang, Roka, in 2012. They determined that an indoor occupation was associated with a lower probability of infection compared to people who remained at home (AOR: 0.32, 95% CI: 0.12–0.82).
CHIKV clinical manifestations and neurodiagnostics
In April 2005, Economopoulou et al. 161 collected data from 660 Chikungunya cases. They observed neurological manifestations in 147 patients. 222 patients had severe CHIKV infection and 65 died. Crosby et al. 162 conducted an observational study in 2013–2014 on 65 patients admitted to the ICU with CHIKV. Twenty-eight (18%) patients had neurological manifestations including GBS and encephalitis. Lemant et al. 163 reported on 33 patients admitted to the ICU for CHIKV, 14/33 (42.4%) patients had encephalopathy, and one was diagnosed with GBS. In a systematic review by Mehta et al., 164 93.0% of patients with neurological complications were directly infected by mosquitoes and 7.0% were vertically infected from mother to child. Patients admitted to hospitalization with CHIKV are older and at increased risk of developing neurological complications. Fetal infection is rare; however, neonates are at increased risk of developing neurological signs and symptoms. 165 Almost 50% of neonates born to viremic mothers develop long-term neurologic sequelae. 166 From the 2014 to 2015 outbreak in France, 66,000 cases were reported with an attack rate of 25%. Among the patients admitted for Chikungunya fever, nine cases of GBS were reported. Eight patients had IgG or IgM antibodies for CHIKV. MRI findings in seven patients showed cranial neuritis (CN V, CN VII), vasogenic edema of the deep cortical structures, and electromyography revealed motor conduction abnormalities consistent with GBS. 167 Although rare, the neurological manifestations reported by CHIKV are summarized in Table 5. Neurodiagnostic measures should be guided by the underlying clinical presentation and localization through the neural axis. Direct measurement of CHIKV RNA in CSF may be possible and should be sought after when clinical suspicion is high. 168 Similar to other neuroinvasive viruses, CSF studies often reveal lymphocytic pleocytosis. 169
Table 5.
Summary of neurological manifestations seen in Chikungunya virus.
| Common neurological manifestations of CHIKV | Less common neurological manifestations of CHIKV |
|---|---|
| Encephalopathy/encephalitis | Seizures |
| Guillain Barré syndrome | Sensorineural hearing loss |
| Acute disseminated encephalomyelitis | Cranial neuropathy |
| Myelopathy and myelitis | Carpal tunnel syndrome |
| Neonatal hypotonia | Cerebellitis |
CHIKV: Chikungunya virus.
CHIKV management
In patients with neurologic sequelae, treatment is guided by the underlying disease (i.e., IVIG for GBS). Currently, there are no approved antiviral medications or vaccines approved for the treatment of CHIKV. 170 However, a novel CHIKV vaccine (PXVX0317) is currently being studied. Currently, the PXVX0317 vaccine shows promise in generating neutralizing antibodies and has a favorable safety profile. 171 One clinical trial showed no benefit in the utilization of chloroquine in comparison to meloxicam for the treatment of musculoskeletal pain and arthritis secondary to CHIKV. Ravichandran et al. 172 reported improvement in CHIKV musculoskeletal pain and arthritis using Ribavirin 200 mg twice daily in ten patients. Larger randomized controlled trials are needed to further assess antiviral agents in acute infection.
Mayaro virus
Mayaro virus epidemiology and pathogenesis
The Mayaro virus (MAYV) is a mosquito-borne infection that belongs to the Togaviridae family of arboviruses. The natural reservoir of MAYV is the wild primates, and the Haemagogus species mosquito is the vector. The main risk factor for infection includes residence and long-term exposure to tropical forests. Reported cases have been restricted to South America. The clinical manifestations of MAYV infection are similar to those of dengue virus and other arboviruses. Symptoms include fever, myalgia, arthralgia, and maculopapular rash. 173 The ongoing zoonosis of MAYV is a continued global health threat. Within the past 15 years, multiple outbreaks of MAYV have been reported. In 2007, 12 people were infected in Bolivia. 174 In 2010, 69 people were diagnosed with MAYV in Venezuela. 175
MAYV clinical manifestations, neurodiagnostics, and management
Neurological disease secondary to MAYV has been studied in vitro. Bengue et al. 4 reported MAYV infection of pericytes, astrocytes, and neural progenitor cells. Elevated inflammatory markers such as IL-6, IL-12, IL-15, and the chemokines CXCL10 and CXCL11 have been observed. Thus far, a limited number of cases have shown neurological complications. However, MAYV neurotropism is possible and a threat to global public health. Currently, there are no approved treatment options for MAYV and the management is generally supportive. However, ongoing studies are underway to assess the role of macrophage migration inhibitory factor as a potential early-use therapeutic agent. 176
Eastern equine encephalitis virus
Eastern equine encephalitis virus epidemiology and pathogenesis
Eastern Equine Encephalitis (EEE) virus is a significant arbovirus primarily found in the eastern regions of the United States. It is transmitted through the bite of infected mosquitoes, mainly Culiseta melanura. The virus primarily circulates among wild birds in swampy areas, but can spill over into humans and horses, among other mammals. The incidence of EEE is relatively low, but it has been increasing in recent years, potentially due to changes in climate and land use patterns. Despite its low incidence EEE has >30% mortality and >60% long-term neurological damage. 177 The virus’s pathogenesis involves initial replication in regional lymph nodes post-mosquito bite, followed by viremia, which can cross the BBB leading to neuroinvasion. Once in the central nervous system, the virus causes intense inflammation, neuronal damage, and can lead to severe neurological sequelae or death. 178
EEEV clinical manifestations and neurodiagnostics
The majority of EEE virus infections are subclinical, but when symptoms do develop, they typically include a sudden onset of fever, headache, chills, and vomiting. The neuroinvasive form of EEE, although rare, is particularly severe, often progressing rapidly to include symptoms such as disorientation, seizures, or coma. Neurological damage is often severe, and survivors may experience long-term neurological deficits. 179 Diagnosis of EEE primarily relies on serological methods, such as the detection of virus-specific IgM antibodies in the CSF or serum. Polymerase chain reaction (PCR) assays can also detect viral RNA in the early stages of infection. Neuroimaging, such as MRI, may reveal characteristic abnormalities in the brain, particularly in the basal ganglia and thalami, but is not specific for EEE. 180
EEEV management
There is no specific treatment for EEE; management is mainly supportive, focusing on treating symptoms and complications. This may include hospitalization, respiratory support, IV fluids, and anticonvulsants for seizure control. Due to the high mortality rate associated with neuroinvasive EEE, early recognition and supportive care are crucial. Wilcox et al. 181 performed a retrospective analysis of 17 patients with EEE and found that delayed IVIG treatment correlated with worse long-term disability, while steroid use showed no significant impact on outcomes. Mortality stood at 12%, indicating a lower rate than previously reported, but with high morbidity. This suggests the potential benefit of early IVIG administration alongside standard care in treating EEE. However, in a case report by Cho et al. 182 a 5-day course of IVIG therapy did not lead to significant improvement. The case by Wendell et al. 183 of a 21-year-old man required intracranial pressure monitoring and osmolar therapy for the management of intracranial hypertension in addition to IVIG and steroids. Preventative measures are primarily focused on mosquito control and personal protective measures to avoid mosquito bites. Vaccines are available for horses, but not yet for humans. Proposed mechanisms for the benefit of IVIG and steroids include prevention of cerebral vasogenic edema, demyelination, and neuroinflammation. 183
Bunyaviridae family
Rift Valley fever virus
Rift Valley fever virus epidemiology and pathogenesis
Rift Valley fever virus (RVFV) is an arbovirus that belongs to the Bunyaviridae family. The primary mosquito vectors are Culex tritaeniorhynchus and Aedes vexans. 184 Although vector-borne transmission is possible, exposure to contaminated animal blood and fluids is the most common route of transmission. Numerous outbreaks of RVFV have occurred in the past century. This includes East Africa, 1931, and numerous outbreaks in Africa in the early 2000s. A recent outbreak of RVF occurred in France in 2018–2019. One hundred and forty-two cases were confirmed and 73% reported exposure to animals or their biologic fluids. 185
RVFV clinical manifestations, neurodiagnostics and management
Symptoms are generally mild and include fever, myalgia, and headaches that can last for weeks. Neurological signs and symptoms have also been reported in RVF. In a cohort analysis of 886 patients with RVF, approximately 17% of the patients had neurological manifestations and were associated with increased morbidity and mortality. 186 Encephalitis and retinitis are severe complications of RVF and can occur 1–2 weeks after initial symptoms. Other neurological complications reported in RVF include meningoencephalitis, hallucinations, locked-in syndrome, and choreiform movements.
In a patient with RVF, postmortem tissue pathological findings indicated perivascular cuffing and round cell infiltration. In one case report, MRI depicted subcortical hyperintense lesions involving the thalamus on T2WI and CSF findings were significant for elevated albumin, and leukocyte counts consistent with BBB dysfunction. 187 The diagnosis of RVFV is best supported by RT-PCR for viral RNA or ELISA for IgM antibodies. Management of RVF is largely supportive and there are no approved therapeutic agents. Ribavirin has been used inconclusively and newer antiviral treatments are being tested. 188 An inactivated human vaccine has been developed; however, it is not largely available.
Discussion
Summary of findings
The viral families Togaviridae, Flaviviridae, Rhabdoviridae, Bunyaviridae, Orthomyxoviridae, and Reoviridae encompass a diverse set of pathogens with various clinical presentations, risk factors, and pathogenic mechanisms. Common clinical manifestations across these families include generalized symptoms such as fever, malaise, and fatigue. Vector transmission is a shared risk factor among Togaviridae, Flaviviridae, and Bunyaviridae, all of which rely on arthropods such as mosquitoes and ticks for spread.
However, these families also display distinct characteristics. For instance, hemorrhagic fevers are more commonly associated with certain Bunyaviridae and Flaviviridae viruses, while musculoskeletal symptoms often accompany Togaviridae infections like Chikungunya. Seasonal variation in infection rates is notable in all families of arboviruses. Pathogenically, complex immune interactions are observed in Flaviviridae, such as antibody-dependent enhancement in Dengue virus, setting it apart from the other viral families under consideration.
Table 6 provides a summary of review findings related to arboviruses included in the review.
Table 6.
Neuroinvasive arboviruses included in the review and summary of findings.
| Virus | Incubation period | Neurological symptoms | Location of MRI findings | Management | Route(s) of neuroinvasion | Pathomechanism of neurovirulence | Global events |
|---|---|---|---|---|---|---|---|
| WNV | 3–15 days | Seizures, altered mental status, Anton syndrome, meningitis, myelitis | Subcortical structures (thalamus, basal ganglia), bilateral occipital lobes | Supportive (intravenous fluids, vasopressors) | Hematogenous, axonal transport | E-protein, TLR3, CCR5 | New York: 1999 |
| CHIKV | 2–12 days | High fever, altered mental status, GBS, ADEM, encephalomyelitis | Thalamus, brainstem, spinal cord, and cerebral cortex | Supportive (intravenous fluids, vasopressors) | Hematogenous, direct invasion (high viral burden) | E-protein, IL-8 | East Africa: 1950s, South America: 2013 |
| DV | 3–14 days | Meningitis, encephalitis, GBS, dengue hemorrhagic fever, cranial neuropathy | Subcortical structures | Supportive (intravenous fluids, vasopressors), blood products, monitor for late sequelae | Hematogenous, direct invasion, post-infectious autoimmunity | E-protein, NS1, IL-8, CCL-2 | Southeast Asia: 1950s, Caribbean: 1970s, Africa, Asia, Americas: 2000s |
| ZIKV | 3–14 days | Congenital Zika syndrome (vertical transmission), altered mental status, GBS, conjunctivitis | Brainstem, cerebellum, thalamus, basal ganglia | Supportive (hydration, pain relief, anti-pyrectics) | Axonal transport, trans-paracellular route, olfactory nerve transmission | E-protein, NS5, IL-8, CCL5, AXL receptor tyrosine kinase, Tyk2 | Yap Islands: 2007, French Polynesia: 2013–2014, India: 2018, Singapore: 2019, Gabon: 2020 |
| JEV | 5–15 days | Encephalitis, confusion, paralysis | Brainstem, cerebral cortex, cerebellum, hippocampus | Supportive, ribavirin, interferon-alpha | Direct transcytosis, passive diffusion, receptor-mediated transcytosis | E-protein, IL-8 | India: 2019, Thailand: 2018 |
| SEV | 3–15 days | Seizures, encephalitis, long-term (paralysis, parkinsonism, amnesia) | Brainstem, cerebellum, spinal cord, hippocampus | Supportive, ASMs | Hematogenous, BBB dysfunction | E-protein, IL-8 | Missouri: 1933, Mississippi: 1963, Midwest USA: 2007, South & Mid-west USA: 2019 |
| TBEV | 3–28 days | Encephalitis, meningismus, seizures, paralysis, coma | Meningoencephalitis, spinal cord | Active immunization (prevention). Immunoglobulins (early), supportive (late) | Trans-synaptic, peripheral nerve axonal transport, hematogenous | C, NS, and E proteins | Central Europe: 1948 |
| PWV | 3–30 days | Encephalitis, meningitis, seizures, amnesia. Long-term (mood changes, ataxia) | The spinal cord, cerebral cortex | Supportive, physical therapy and rehabilitation (long-term) | ? | ? | New England: 2017, Minnesota: 2018 |
| MAYV | 2–14 days | Encephalitis, meningitis | ? | Supportive | ? | ? | Brazil: 2018 |
| YFV | 3–10 days | Seizures, altered mental status | ? | Supportive | Olfactory nerve transmission, BBB dysfunction | CXCL10, CCL2, DC-SIGN, AXL, Tyro3, NS5, RhoGDI | Massachusetts: 1649, Peru: 1928, Brazil: 2017 |
| RVFV | 1–14 days | Encephalitis, Meningitis, optic neuritis | Cerebral cortex, cerebellum, and brainstem | Supportive | Peripheral and olfactory nerve axonal transport, hematogenous | CCL2, CCL3, CCL5, integrin receptor, VP7, phosphatidylserine receptor | Tanzania: 1998, Egypt: 2009, Yemen: 2019 |
| EEEV | 4–10 days | Encephalitis | Cerebral cortex, basal ganglia, thalamus | IVIG, steroids, supportive | ? | VLDLR | Michigan: 2019 |
MRI: magnetic resonance imaging; WNV: West Nile virus; CHIKV: Chikungunya virus; DV: Dengue virus; ZIKV: Zika virus; JEV: Japanese Encephalitis virus; SEV: St. Louis encephalitis virus; TBEV: Tick-borne encephalitis virus; POWV: Powassan virus; MAYV: Mayaro virus; YFV: Yellow fever virus; RVFV: Rift valley fever virus; GBS: Guillain-Barre syndrome; ADEM: acute disseminated encephalomyelitis; ASM: antiseizure medications; BBB: blood-brain barrier; TLR3: toll-like receptor 3; CCL2/3/5: chemokine (C-C motif) ligand 2/3/5; CCR5: C-C chemokine receptor type 5; Tyk2: tyrosine kinase 2; NS1/5: non-structural protein 1/5; AXL: tyrosine-protein kinase receptor UFO; DC-SIGN: dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; RhoGDL: Rho GTPase-activating protein with a death domain-like; VP7: viral envelope protein.
Limitations and future directions
This scoping review mapped out many of the neuroinvasive viruses that belong to both the Flaviviridae and Togaviridae family of arboviruses. However, neuroinvasive disease secondary to other arboviruses including members of the Bunyaviridae family (i.e., Crimean-Congo Hemorrhagic Fever) and Orthomyxviridae has also been observed. Additionally, other viruses that can be considered in future reviews include Eastern Equine encephalitis virus, La Crosse encephalitis virus, and Cache Valley virus among others. The treatment of neuroinvasive arbovirus infections, such as those caused by the Zika virus, remains a significant challenge. While current treatments are primarily supportive and aim to alleviate symptoms, there is a need for more effective and specific treatments. Antiviral agents (e.g., interferon, favipiravir, remdesivir) could potentially halt the replication of the virus and prevent it from causing damage to the brain and nervous system. Vaccines are a key tool in preventing arbovirus infections and are an ongoing field of research for the prevention and management of arbovirus infections. There is a need for therapies that can protect the brain and nervous system from the damage caused by the virus. Corticosteroids, monoclonal antibodies, and other anti-inflammatory agents may be able to reduce inflammation and neurodegeneration caused by the virus. There is also a growing interest in targeting the host’s immune response to the virus as a potential treatment strategy. This may involve modulating the immune response to reduce inflammation and prevent the virus from replicating in the brain.
Conclusions
Neuroinvasion occurs primarily through transneural transmission, BBB dysfunction/breakdown, and infected immune cells (“trojan horse” mechanism). ZIKV is the most common arbovirus exhibiting vertical transmission. The most common neurological symptoms include encephalitis/encephalopathy, headache, GBS, and parkinsonism. Long-term neurological sequelae can include cognitive dysfunction and parkinsonism. CSF findings in neuroinvasive diseases often include signs of BBB dysfunction such as elevated protein, albumin, and IgG index. Lymphocytic pleocytosis, antibody detection, and positive viral RNA cultures may also be present. MRI findings show a predominance of subcortical lesions most often as T2/FLAIR hyperintensities. The continued zoonosis of arboviruses is a significant threat to global health. Furthermore, newer strains of arboviruses (i.e., ZIKV) have emerged with significant neurological sequelae. Older strains of these viruses never showed neuroinvasive potential. Therefore, significant efforts must be made toward primary screening and prevention to anticipate the genesis of neurovirulent arbovirus strains.
Acknowledgments
Figures were generated using BioRender.
Footnotes
Author contributions: B.S.S.: Intellectual conceptualization, writing- original draft preparation, writing- review and editing, and visualization. S.Z.: writing- original draft preparation, writing-review, and editing. M.R.M., T.S., V.K., A.D., S.D., D.C.A., R.C.S. K.N., M.A.G.: writing- original draft preparation.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: OSF Registration: DOI:10.17605/OSF.IO/GRKHA.
Informed consent: Not applicable.
ORCID iD: Vincent Kipkorir  https://orcid.org/0009-0008-5978-7211
https://orcid.org/0009-0008-5978-7211
References
- 1. Barrows NJ, Campos RK, Liao KC, et al. Biochemistry and molecular biology of flaviviruses. Chem Rev 2018; 118(8): 4448–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pielnaa P, Al-Saadawe M, Saro A, et al. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology 2020; 543: 34–42. [DOI] [PubMed] [Google Scholar]
- 3. Mordecai EA, Ryan SJ, Caldwell JM, et al. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet Health 2020; 4(9): e416–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bengue M, Ferraris P, Barthelemy J, et al. Mayaro virus infects human brain cells and induces a potent antiviral response in human astrocytes. Viruses 2021; 13(3): 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pingen M, Bryden SR, Pondeville E, et al. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity 2016; 44(6): 1455–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chauhan L, Matthews E, Piquet AL, et al. Nervous system manifestations of arboviral infections. Curr Trop Med Rep 2022; 9(4): 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Debiasi RL. West nile virus neuroinvasive disease. Curr Infect Dis Rep 2011; 13(4): 350–359. [DOI] [PubMed] [Google Scholar]
- 8. Hadfield J, Brito AF, Swetnam DM, et al. Twenty years of West Nile virus spread and evolution in the Americas visualized by Nextstrain. PLoS Pathog 2019; 15(10): e1008042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sejvar JJ. West Nile virus infection. Microbiol Spectr 2016; 4(3): 1–19. [DOI] [PubMed] [Google Scholar]
- 10. Kilpatrick AM, Meola MA, Moudy RM, et al. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog 2008; 4(6): e1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Debiasi RL, Tyler KL. West nile virus meningoencephalitis. Nat Clin Pract Neurol 2006; 2(5): 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai F, Thompson EA, Vig PJS, et al. Current understanding of west nile virus clinical manifestations, immune responses, neuroinvasion, and immunotherapeutic implications. Pathogens 2019; 8(4): 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burton JM, Kern RZ, Halliday W, et al. Neurological manifestations of West Nile virus infection. Can J Neurol Sci 2004; 31(2): 185–193. [DOI] [PubMed] [Google Scholar]
- 14. Emig M, Apple DJ. Severe West nile virus disease in healthy adults. Clin Infect Dis 2004; 38(2): 289–292. [DOI] [PubMed] [Google Scholar]
- 15. Sayao AL, Suchowersky O, Al-Khathaami A, et al. Calgary experience with West Nile virus neurological syndrome during the late summer of 2003. Can J Neurol Sci 2004; 31(2): 194–203. [DOI] [PubMed] [Google Scholar]
- 16. Sejvar JJ, Haddad MB, Tierney BC, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA 2003; 290(4): 511–515. [DOI] [PubMed] [Google Scholar]
- 17. Srichawla BS. Neuroinvasive West Nile Virus (WNV) encephalitis with anton syndrome: epidemiology and pathophysiology review. Cureus 2022; 14(6): e26264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan E, Needham DM, Brunton J, et al. West Nile virus infection in the intensive care unit: a case series and literature review. Can Respir J 2004; 11(5): 354–358. [DOI] [PubMed] [Google Scholar]
- 19. Sejvar JJ, Bode AV, Marfin AA, et al. West Nile virus-associated flaccid paralysis. Emerg Infect Dis 2005; 11(7): 1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Long MT, Jeter W, Hernandez J, et al. Diagnostic performance of the equine IgM capture ELISA for serodiagnosis of West Nile virus infection. J Vet Intern Med 2006; 20(3): 608–613. [DOI] [PubMed] [Google Scholar]
- 21. Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA 2013; 310(3): 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zak IT, Altinok D, Merline JR, et al. West Nile virus infection. AJR Am J Roentgenol 2005; 184(3): 957–961. [DOI] [PubMed] [Google Scholar]
- 23. Ali M, Safriel Y, Sohi J, et al. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol 2005; 26(2): 289–297. [PMC free article] [PubMed] [Google Scholar]
- 24. Petropoulou KA, Gordon SM, Prayson RA, et al. West Nile virus meningoencephalitis: MR imaging findings. AJNR Am J Neuroradiol 2005; 26(8): 1986–1995. [PMC free article] [PubMed] [Google Scholar]
- 25. Haley M, Retter AS, Fowler D, et al. The role for intravenous immunoglobulin in the treatment of West Nile virus encephalitis. Clin Infect Dis 2003; 37(6): e88–e90. [DOI] [PubMed] [Google Scholar]
- 26. Jordan I, Briese T, Fischer N, et al. Ribavirin inhibits West Nile virus replication and cytopathic effect in neural cells. J Infect Dis 2000; 182(4): 1214–1217. [DOI] [PubMed] [Google Scholar]
- 27. Koch M, Pozsgai E, Soos V, et al. Identifying risks for severity of neurological symptoms in Hungarian West Nile virus patients. BMC Infect Dis 2021; 21(1): 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hawkes MA, Carabenciov ID, Wijdicks EFM, et al. Critical West Nile neuroinvasive disease. Neurocrit Care 2018; 29(1): 47–53. [DOI] [PubMed] [Google Scholar]
- 29. Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis 2007; 44(12): 1617–1624. [DOI] [PubMed] [Google Scholar]
- 30. Weatherhead JE, Miller VE, Garcia MN, et al. Long-term neurological outcomes in West Nile virus-infected patients: an observational study. Am J Trop Med Hyg 2015; 92(5): 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis 2010; 4(5): e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Messina JP, Brady OJ, Golding N, et al. The current and future global distribution and population at risk of dengue. Nat Microbiol 2019; 4(9): 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496(7446): 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol 2013; 5: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mwanyika GO, Mboera LEG, Rugarabamu S, et al. Dengue virus infection and associated risk factors in Africa: a systematic review and meta-analysis. Viruses 2021; 13(4): 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eldigail MH, Abubaker HA, Khalid FA, et al. Recent transmission of dengue virus and associated risk Facors among residents of Kassala state, eastern Sudan. BMC Public Health 2020; 20(1): 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Higa Y, Abilio AP, Futami K, et al. Abundant Aedes (Stegomyia) aegypti aegypti mosquitoes in the 2014 dengue outbreak area of Mozambique. Trop Med Health 2015; 43(2): 107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Msellemu D, Gavana T, Ngonyani H, et al. Knowledge, attitudes and bite prevention practices and estimation of productivity of vector breeding sites using a Habitat Suitability Score (HSS) among households with confirmed dengue in the 2014 outbreak in Dar es Salaam, Tanzania. PLoS Negl Trop Dis 2020; 14(7): e0007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lifson AR. Mosquitoes, models, and dengue. Lancet 1996; 347(9010): 1201–1202. [DOI] [PubMed] [Google Scholar]
- 40. Gan SJ, Leong YQ, Bin Barhanuddin MFH, et al. Dengue fever and insecticide resistance in Aedes mosquitoes in Southeast Asia: a review. Parasit Vectors 2021; 14(1): 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kraemer MU, Sinka ME, Duda KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. Elife 2015; 4: e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21(st) century. Trop Med Health 2011; 39(4 Suppl): 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calderon-Pelaez MA, Coronel-Ruiz C, Castellanos JE, et al. Endothelial dysfunction, HMGB1, and dengue: an enigma to solve. Viruses 2022; 14(8): 1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alejandria. Dengue haemorrhagic fever or dengue shock syndrome in children. BMJ Clin Evid 2015; 2015: 0917. [PMC free article] [PubMed] [Google Scholar]
- 45. Arif A, Abdul Razzaque MR, Kogut LM, et al. Expanded dengue syndrome presented with rhabdomyolysis, compartment syndrome, and acute kidney injury: a case report. Medicine (Baltimore) 2022; 101(7): e28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kulkarni R, Pujari S, Gupta D. Neurological manifestations of dengue fever. Ann Indian Acad Neurol 2021; 24(5): 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carod-Artal FJ, Wichmann O, Farrar J, et al. Neurological complications of dengue virus infection. Lancet Neurol 2013; 12(9): 906–919. [DOI] [PubMed] [Google Scholar]
- 48. Jackson CA, Sudlow CLM, Mishra GD. Education, sex and risk of stroke: a prospective cohort study in New South Wales, Australia. BMJ Open 2018; 8(9): e024070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koley TK, Jain S, Sharma H, et al. Dengue encephalitis. J Assoc Phys India 2003; 51: 422–423. [PubMed] [Google Scholar]
- 50. Sanjay S, Wagle AM, Au Eong KG. Optic neuropathy associated with dengue fever. Eye (Lond) 2008; 22(5): 722–724. [DOI] [PubMed] [Google Scholar]
- 51. Mai NTH, Phu NH, Nghia HDT, et al. Dengue-associated posterior reversible encephalopathy syndrome, Vietnam. Emerg Infect Dis 2018; 24(2): 402–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Domingues RB, Kuster GW, Onuki-Castro FL, et al. Involvement of the central nervous system in patients with dengue virus infection. J Neurol Sci 2008; 267(1–2): 36–40. [DOI] [PubMed] [Google Scholar]
- 53. Johnson TP, Larman HB, Lee MH, et al. Chronic dengue virus panencephalitis in a patient with progressive dementia with extrapyramidal features. Ann Neurol 2019; 86(5): 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shrock EL, Shrock CL, Elledge SJ. VirScan: high-throughput profiling of antiviral antibody epitopes. Bio Protoc 2022; 12(13): e4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cristiane S, Marzia PS. Diagnosis criteria of dengue encephalitis. Arq Neuropsiquiatr 2014; 72(3): 263. [DOI] [PubMed] [Google Scholar]
- 56. Norshidah H, Vignesh R, Lai NS. Updates on dengue vaccine and antiviral: where are we heading? Molecules 2021; 26(22): 6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Finsterer J, Kongchan K. Severe, persisting, steroid-responsive dengue myositis. J Clin Virol 2006; 35(4): 426–428. [DOI] [PubMed] [Google Scholar]
- 58. van Alfen N. The neuralgic amyotrophy consultation. J Neurol 2017; 254(6): 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Simmons CP, Farrar JJ, Nguyen V, et al. Dengue. N Engl J Med 2012; 366(15): 1423–1432. [DOI] [PubMed] [Google Scholar]
- 60. Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46(5): 521–534. [DOI] [PubMed] [Google Scholar]
- 61. Lessler J, Chaisson LH, Kucirka LM, et al. Assessing the global threat from Zika virus. Science 2016; 353(6300): aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mehrjardi MZ. Is Zika virus an emerging TORCH agent? An invited commentary. Virology (Auckl) 2017; 8: 1178122X17708993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Buckley A, Gould EA. Detection of virus-specific antigen in the nuclei or nucleoli of cells infected with Zika or Langat virus. J Gen Virol 1988; 69(Pt 8): 1913–1920. [DOI] [PubMed] [Google Scholar]
- 64. Hamel R, Dejarnac O, Wichit S, et al. Biology of Zika virus infection in human skin cells. J Virol 2015; 89(17): 8880–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Perera-Lecoin M, Meertens L, Carnec X, et al. Flavivirus entry receptors: an update. Viruses 2013; 6(1): 69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cugola FR, Fernandes IR, Russo FB, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016; 534(7606): 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gabriel E, Ramani A, Karow U, et al. Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 2017; 20(3): 397–406.e5. [DOI] [PubMed] [Google Scholar]
- 68. Hazin AN, Poretti A, Di Cavalcanti Souza Cruz D, et al. Computed tomographic findings in microcephaly associated with Zika virus. N Engl J Med 2016; 374(22): 2193–2195. [DOI] [PubMed] [Google Scholar]
- 69. Kemenesi G, Banyai K. Tick-borne flaviviruses, with a focus on Powassan virus. Clin Microbiol Rev 2019; 32(1): e00106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Soares de Oliveira-Szejnfeld P, Levine D, Melo AS, et al. Congenital brain abnormalities and Zika virus: what the radiologist can expect to see prenatally and postnatally. Radiology 2016; 281(1): 203–218. [DOI] [PubMed] [Google Scholar]
- 71. Faizan MI, Abdullah M, Ali S, et al. Zika virus-induced microcephaly and its possible molecular mechanism. Intervirology 2016; 59(3): 152–158. [DOI] [PubMed] [Google Scholar]
- 72. Sharp TM, Fischer M, Munoz-Jordan JL, et al. Dengue and zika virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recomm Rep 2019; 68(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med 2016; 374(16): 1595–1596. [DOI] [PubMed] [Google Scholar]
- 74. Dos Santos T, Rodriguez A, Almiron M, et al. Zika virus and the Guillain-Barre syndrome—case series from seven countries. N Engl J Med 2016; 375(16): 1598–1601. [DOI] [PubMed] [Google Scholar]
- 75. Dirlikov E, Major CG, Mayshack M, et al. Guillain-Barre syndrome during ongoing Zika virus transmission—Puerto Rico, January 1–July 31, 2016. MMWR Morb Mortal Wkly Rep 2016; 65(34): 910–914. [DOI] [PubMed] [Google Scholar]
- 76. Lebov JF, Hooper SR, Pugh N, et al. Neurological and neuropsychological sequelae of Zika virus infection in children in Leon, Nicaragua. Rev Panam Salud Publica 2022; 46: e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Romani N, Pieras M, Frick MA, et al. Neurological short-term outcomes of a cohort of children born to Zika virus-infected mothers in Barcelona. Children (Basel) 2022; 9(10): 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chang C, Ortiz K, Ansari A, et al. The Zika outbreak of the 21st century. J Autoimmun 2016; 68: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sapparapu G, Fernandez E, Kose N, et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 2016; 540(7633): 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yun SI, Lee YM. Early events in Japanese encephalitis virus infection: viral entry. Pathogens 2018; 7(3): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yap G, Mailepessov D, Lim XF, et al. Detection of Japanese encephalitis virus in culex mosquitoes in Singapore. Am J Trop Med Hyg 2020; 103(3): 1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sharma KB, Vrati S, Kalia M. Pathobiology of Japanese encephalitis virus infection. Mol Aspects Med 2021; 81: 100994. [DOI] [PubMed] [Google Scholar]
- 83. Bharucha T, Sengvilaipaseuth O, Seephonelee M, et al. Detection of Japanese encephalitis virus RNA in human throat samples in Laos - a pilot study. Sci Rep 2018; 8(1): 8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Garcia-Nicolas O, Braun RO, Milona P, et al. Targeting of the nasal mucosa by Japanese encephalitis virus for non-vector-borne transmission. J Virol 2018; 92(24): e01091–e01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chapagain S, Pal Singh P, Le K, et al. Japanese encephalitis virus persists in the human reproductive epithelium and porcine reproductive tissues. PLoS Negl Trop Dis 2022; 16(7): e0010656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cheng VCC, Sridhar S, Wong SC, et al. Japanese encephalitis virus transmitted via blood transfusion, Hong Kong, China. Emerg Infect Dis 2018; 24(1): 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Qi ZL, Sun LY, Bai J, et al. Japanese encephalitis following liver transplantation: a rare case report. World J Clin Cases 2020; 8(2): 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tsai TF. New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine 2000; 18(Suppl 2): 1–25. [DOI] [PubMed] [Google Scholar]
- 89. Campbell GL, Hills SL, Fischer M, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 2011; 89(10): 766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang H, Liang G. Epidemiology of Japanese encephalitis: past, present, and future prospects. Ther Clin Risk Manag 2015; 11: 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li MH, Fu SH, Chen WX, et al. Genotype v Japanese encephalitis virus is emerging. PLoS Negl Trop Dis 2011; 5(7): e1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Seo MG, Lee HS, Yang SC, et al. National monitoring of mosquito populations and molecular analysis of flavivirus in the Republic of Korea in 2020. Microorganisms 2021; 9(10): 2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schuh AJ, Ward MJ, Brown AJ, et al. Phylogeography of Japanese encephalitis virus: genotype is associated with climate. PLoS Negl Trop Dis 2013; 7(8): e2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Turtle L, Solomon T. Japanese encephalitis—the prospects for new treatments. Nat Rev Neurol 2018; 14(5): 298–313. [DOI] [PubMed] [Google Scholar]
- 95. Verma R, Praharaj HN. Bilateral facial palsy as a manifestation of Japanese encephalitis. BMJ Case Rep 2012; 2012: bcr2012006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen WL, Liao MF, Chiang HL, et al. A possible case of acute disseminated encephalomyelitis after Japanese encephalitis. Acta Neurol Taiwan 2013; 22(4): 169–173. [PubMed] [Google Scholar]
- 97. Ghosh R, Dubey S, Das S, et al. Case report: dystonic storm following Japanese encephalitis virus infection. Am J Trop Med Hyg 2022; 107(3): 557–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Narayanan S, Thulaseedharan NK, Subramaniam G, et al. Hypothermia due to limbic system involvement and longitudinal myelitis in a case of Japanese encephalitis: a case report from India. Int J Gen Med 2017; 10: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu S, Wang J, Yang J, et al. The underlying mechanism of Guillain-Barre syndrome in a young patient suffered from Japanese encephalitis virus infection: a case report. Virol J 2022; 19(1): 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ma J, Zhang T, Jiang L. Japanese encephalitis can trigger anti-N-methyl-D-aspartate receptor encephalitis. J Neurol 2017; 264(6): 1127–1131. [DOI] [PubMed] [Google Scholar]
- 101. Tzeng SS. Respiratory paralysis as a presenting symptom in Japanese encephalitis—a case report. Zhonghua Yi Xue Za Zhi (Taipei) 1989; 43(3): 208–212. [PubMed] [Google Scholar]
- 102. Fang ST, Chu SY, Lee YC. Ischaemic maculopathy in Japanese encephalitis. Eye (Lond) 2006; 20(12): 1439–1441. [DOI] [PubMed] [Google Scholar]
- 103. Furiya Y, Hirano M, Nakamuro T, et al. A case of post-Japanese encephalitis with partial hypothalamic dysfunction showing repetitive hyperthermia in summertime. J Infect 2006; 52(5): e143–e146. [DOI] [PubMed] [Google Scholar]
- 104. Singh S, Kumar A. Ocular manifestations of emerging flaviviruses and the blood-retinal barrier. Viruses 2018; 10(10): 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Van K, Korman TM, Nicholson S, et al. Case report: Japanese encephalitis associated with chorioretinitis after short-term travel to Bali, Indonesia. Am J Trop Med Hyg 2020; 103(4): 1691–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kumar R. Prevention, diagnosis, and management of Japanese encephalitis in children. Pediatr Health Med Therap 2014; 5: 99–110. [Google Scholar]
- 107. Pichl T, Wedderburn CJ, Hoskote C, et al. A systematic review of brain imaging findings in neurological infection with Japanese encephalitis virus compared with dengue virus. Int J Infect Dis 2022; 119: 102–110. [DOI] [PubMed] [Google Scholar]
- 108. Phukan P, Sarma K, Sharma BK, et al. MRI spectrum of Japanese encephalitis in Northeast India: a cross-sectional study. J Neurosci Rural Pract 2021; 12(2): 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dung NM, Turtle L, Chong WK, et al. An evaluation of the usefulness of neuroimaging for the diagnosis of Japanese encephalitis. J Neurol 2009; 256(12): 2052–2060. [DOI] [PubMed] [Google Scholar]
- 110. Arahata Y, Fujii K, Nishimura T, et al. Longitudinal magnetic resonance imaging changes in Japanese encephalitis. Brain Dev 2019; 41(8): 731–734. [DOI] [PubMed] [Google Scholar]
- 111. Hoke CH, Vaughn DW, Nisalak A, et al. Effect of high-dose dexamethasone on the outcome of acute encephalitis due to Japanese encephalitis virus. J Infect Dis 1992; 165(4): 631–637. [DOI] [PubMed] [Google Scholar]
- 112. Rathi AK, Kushwaha KP, Singh YD, et al. JE virus encephalitis: 1988 epidemic at Gorakhpur. Indian Pediatr 1993; 30(3): 325–333. [PubMed] [Google Scholar]
- 113. Johnson RT, Intralawan P, Puapanwatton S. Japanese encephalitis: identification of inflammatory cells in cerebrospinal fluid. Ann Neurol 1986; 20(6): 691–695. [DOI] [PubMed] [Google Scholar]
- 114. Kumar Singh A, Mehta A, Kushwaha KP, et al. Minocycline trial in Japanese encephalitis: a double blind, randomized placebo study. Pediatr Rev Int J Pediatr Res 2016; 3(5): 371–377. [Google Scholar]
- 115. Kumar R, Basu A, Sinha S, et al. Role of oral minocycline in acute encephalitis syndrome in India—a randomized controlled trial. BMC Infect Dis 2016; 16: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Solomon T, Dung NM, Wills B, et al. Interferon alfa-2a in Japanese encephalitis: a randomised double-blind placebo-controlled trial. Lancet 2003; 361(9360): 821–826. [DOI] [PubMed] [Google Scholar]
- 117. Zhao J, Chen F, Lu L, et al. Japanese encephalitis (JE) mimicking acute ischemic stroke: a case report. Medicine (Baltimore) 2020; 99(45): e23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Harinasuta C, Nimmanitya S, Titsyakorn U. The effect of interferon-alpha A on two cases of Japanese encephalitis in Thailand. Southeast Asian J Trop Med Public Health 1985; 16(2): 332–336. [PubMed] [Google Scholar]
- 119. Barbosa Costa G, Marinho PES, Vilela APP, et al. Silent circulation of the Saint Louis encephalitis virus among humans and equids, Southeast Brazil. Viruses 2019; 11(11): 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gimenez-Richarte A, Ortiz de Salazar MI, Gimenez-Richarte MP, et al. Transfusion-transmitted arboviruses: update and systematic review. PLoS Negl Trop Dis 2022; 16(10): e0010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Curren EJ, Lindsey NP, Fischer M, et al. St. Louis encephalitis virus disease in the United States, 2003–2017. Am J Trop Med Hyg 2018; 99(4): 1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ortiz-Martinez Y, Vega-Useche L, Villamil-Gomez WE, et al. Saint Louis encephalitis virus, another re-emerging arbovirus: a literature review of worldwide research. Infez Med 2017; 25(1): 77–79. [PubMed] [Google Scholar]
- 123. Hartmann CA, Vikram HR, Seville MT, et al. Neuroinvasive St. Louis encephalitis virus infection in solid organ transplant recipients. Am J Transplant 2017; 17(8): 2200–2206. [DOI] [PubMed] [Google Scholar]
- 124. Venkat H, Krow-Lucal E, Kretschmer M, et al. Comparison of characteristics of patients with West Nile virus or St. Louis encephalitis virus neuroinvasive disease during concurrent outbreaks, Maricopa County, Arizona, 2015. Vector Borne Zoonotic Dis 2020; 20(8): 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ridenour CL, Cocking J, Poidmore S, et al. St. Louis encephalitis virus in the Southwestern United States: a phylogeographic case for a multi-variant introduction event. Front Genet 2021; 12: 667895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Smith DR. Waiting in the wings: the potential of mosquito transmitted flaviviruses to emerge. Crit Rev Microbiol 2017; 43(4): 405–422. [DOI] [PubMed] [Google Scholar]
- 127. Kaiser R. Tick-borne encephalitis. Infect Dis Clin North Am 2008; 22(3): 561–575. [DOI] [PubMed] [Google Scholar]
- 128. Blomqvist G, Naslund K., Svensson L, et al. Mapping geographical areas at risk for tick-borne encephalitis (TBE) by analysing bulk tank milk from Swedish dairy cattle herds for the presence of TBE virus-specific antibodies. Acta Vet Scand 2021; 63(1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ponomareva EP, Mikryukova TP, Gori AV, et al. Detection of Far-Eastern subtype of tick-borne encephalitis viral RNA in ticks collected in the Republic of Moldova. J Vector Borne Dis 2015; 52(4): 334–336. [PubMed] [Google Scholar]
- 130. Im JH, Baek JH, Durey A, et al. Geographic distribution of Tick-borne encephalitis virus complex. J Vector Borne Dis 2020; 57(1): 14–22. [DOI] [PubMed] [Google Scholar]
- 131. Kindberg E, Vene S, Mickiene A, et al. A functional Toll-like receptor 3 gene (TLR3) may be a risk factor for tick-borne encephalitis virus (TBEV) infection. J Infect Dis 2011; 203(4): 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Stefanoff P, Rosinska M, Samuels S, et al. A national case-control study identifies human socio-economic status and activities as risk factors for tick-borne encephalitis in Poland. PLoS One 2012; 7(9): e45511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Dorrbecker B, Dobler G, Spiegel M, et al. Tick-borne encephalitis virus and the immune response of the mammalian host. Travel Med Infect Dis 2010; 8(4): 213–222. [DOI] [PubMed] [Google Scholar]
- 134. Lasala PR, Holbrook M. Tick-borne flaviviruses. Clin Lab Med 2010; 30(1): 221–235. [DOI] [PubMed] [Google Scholar]
- 135. Bogovic P, Strle F. Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World J Clin Cases 2015; 3(5): 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Taba P, Schmutzhard E, Forsberg P, et al. EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis. Eur J Neurol 2017; 24(10): 1214–e1261. [DOI] [PubMed] [Google Scholar]
- 137. Alkadhi H, Kollias SS. MRI in tick-borne encephalitis. Neuroradiology 2000; 42(10): 753–755. [DOI] [PubMed] [Google Scholar]
- 138. Kaiser R. The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994–1998: a prospective study of 656 patients. Brain 1999; 122(Pt 11): 2067–2078. [DOI] [PubMed] [Google Scholar]
- 139. Romero JR, Simonsen KA. Powassan encephalitis and Colorado tick fever. Infect Dis Clin North Am 2008; 22(3): 545–559. [DOI] [PubMed] [Google Scholar]
- 140. Leonova GN, Isachkova LM, Baranov NI, et al. [Role of Powassan virus in the etiological structure of tick-borne encephalitis in the Primorsky Kray]. Vopr Virusol 1980; 2: 173–176. [PubMed] [Google Scholar]
- 141. Embil JA, Camfield P, Artsob H, et al. Powassan virus encephalitis resembling herpes simplex encephalitis. Arch Intern Med 1983; 143(2): 341–343. [PubMed] [Google Scholar]
- 142. Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 2016; 62(6): 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Madison-Antenucci S, Kramer LD, Gebhardt LL, et al. Emerging Tick-borne diseases. Clin Microbiol Rev 2020; 33(2): e00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bogaty C, Drebot M. Powassan virus—an emerging public health concern. CMAJ 2018; 190(15): E472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Choi H, Kudchodkar SB, Ho M, et al. A novel synthetic DNA vaccine elicits protective immune responses against Powassan virus. PLoS Negl Trop Dis 2020; 14(10): e0008788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Pach JJ, Zubair AS, Traner C, et al. Powassan meningoencephalitis: a case report highlighting diagnosis and management. Cureus 2021; 13(7): e16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Monath TP. Treatment of yellow fever. Antiviral Res 2008; 78(1): 116–124. [DOI] [PubMed] [Google Scholar]
- 148. Marinho PES, Alvarenga PPM, Crispim APC, et al. Wild-type yellow fever virus RNA in cerebrospinal fluid of child. Emerg Infect Dis 2019; 25(8): 1567–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ho YL, Joelsons D, Leite GFC, et al. Severe yellow fever in Brazil: clinical characteristics and management. J Travel Med 2019; 26(5): taz040. [DOI] [PubMed] [Google Scholar]
- 150. Stevenson LD. Pathologic changes in the nervous system in yellow fever. Arch Pathol 1939; 27(2): 249–266. [Google Scholar]
- 151. Liu T, Chambers TJ. Yellow fever virus encephalitis: properties of the brain-associated T-cell response during virus clearance in normal and gamma interferon-deficient mice and requirement for CD4+ lymphocytes. J Virol 2001; 75(5): 2107–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. McMahon AW, Eidex RB, Marfin AA, et al. Neurologic disease associated with 17D-204 yellow fever vaccination: a report of 15 cases. Vaccine 2007; 25(10): 1727–1734. [DOI] [PubMed] [Google Scholar]
- 153. Burt FJ, Rolph MS, Rulli NE, et al. Chikungunya: a re-emerging virus. Lancet 2012; 379(9816): 662–671. [DOI] [PubMed] [Google Scholar]
- 154. Ozden S, Huerre M, Riviere JP, et al. Human muscle satellite cells as targets of chikungunya virus infection. PLoS One 2007; 2(6): e527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Carey DE. Chikungunya and dengue: a case of mistaken identity? J Hist Med Allied Sci 1971; 26(3): 243–262. [DOI] [PubMed] [Google Scholar]
- 156. Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond) 1956; 54(2): 177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Weaver SC, Forrester NL. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res 2015; 120: 32–39. [DOI] [PubMed] [Google Scholar]
- 158. Gay N, Rousset D, Huc P, et al. Seroprevalence of Asian Lineage chikungunya virus infection on Saint Martin Island, 7 months after the 2013 emergence. Am J Trop Med Hyg 2016; 94(2): 393–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rudolph KE, Lessler J, Moloney RM, et al. Incubation periods of mosquito-borne viral infections: a systematic review. Am J Trop Med Hyg 2014; 90(5): 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Galatas B, Ly S, Duong V, et al. Long-lasting immune protection and other epidemiological findings after chikungunya emergence in a Cambodian Rural Community, April 2012. PLoS Negl Trop Dis 2016; 10(1): e0004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Economopoulou A, Dominguez M, Helynck B, et al. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on reunion. Epidemiol Infect 2009; 137(4): 534–541. [DOI] [PubMed] [Google Scholar]
- 162. Crosby L, Perreau C, Madeux B, et al. Severe manifestations of chikungunya virus in critically ill patients during the 2013–2014 Caribbean outbreak. Int J Infect Dis 2016; 48: 78–80. [DOI] [PubMed] [Google Scholar]
- 163. Lemant J, Boisson V, Winer A, et al. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit Care Med 2008; 36(9): 2536–2541. [DOI] [PubMed] [Google Scholar]
- 164. Mehta R, Gerardin P, de Brito CAA, et al. The neurological complications of chikungunya virus: a systematic review. Rev Med Virol 2018; 28(3): e1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 2015; 372(13): 1231–1239. [DOI] [PubMed] [Google Scholar]
- 166. Gerardin P, Barau G, Michault A, et al. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Reunion. PLoS Med 2008; 5(3): e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Oehler E, Fournier E, Leparc-Goffart I, et al. Increase in cases of Guillain-Barre syndrome during a Chikungunya outbreak, French Polynesia, 2014 to 2015. Euro Surveill 2015; 20(48): 30079. [DOI] [PubMed] [Google Scholar]
- 168. Acevedo N, Waggoner J, Rodriguez M, et al. Zika virus, chikungunya virus, and dengue virus in cerebrospinal fluid from adults with neurological manifestations, Guayaquil, Ecuador. Front Microbiol 2017; 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ortiz-Quezada J, Rodriguez EE, Hesse H, et al. Chikungunya encephalitis, a case series from an endemic country. J Neurol Sci 2021; 420: 117279. [DOI] [PubMed] [Google Scholar]
- 170. Silva LA, Dermody TS. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest 2017; 127(3): 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Stephenson KE. Novel chikungunya vaccine shows promise for durable protection. Lancet Infect Dis 2022; 22(9): 1259–1261. [DOI] [PubMed] [Google Scholar]
- 172. Ravichandran R, Manian M. Ribavirin therapy for chikungunya arthritis. J Infect Dev Ctries 2008; 2(2): 140–142. [PubMed] [Google Scholar]
- 173. Acosta-Ampudia Y, Monsalve DM, Rodriguez Y, et al. Mayaro: an emerging viral threat? Emerg Microbes Infect 2018; 7(1): 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Coimbra TL, Santos CL, Suzuki A, et al. Mayaro virus: imported cases of human infection in Sao Paulo State, Brazil. Rev Inst Med Trop Sao Paulo 2007; 49(4): 221–224. [DOI] [PubMed] [Google Scholar]
- 175. Receveur MC, Grandadam M, Pistone T. Infection with Mayaro virus in a French traveller returning from the Amazon region, Brazil, January, 2010. Euro Surveill 2010; 15(18): 19563. [PubMed] [Google Scholar]
- 176. Herrero LJ, Nelson M, Srikiatkhachorn A, et al. Critical role for macrophage migration inhibitory factor (MIF) in Ross River virus-induced arthritis and myositis. Proc Natl Acad Sci U S A 2011; 108(29): 12048–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ruiz C, Gibson G, Rojas S. Eastern equine encephalitis virus: a case report and brief literature review of current therapeutic and preventative strategies. Vector Borne Zoonotic Dis. Online ahead of print 2023. DOI: 10.1089/vbz.2023.0011. [DOI] [PubMed] [Google Scholar]
- 178. Armstrong PM, Andreadis TG. Ecology and epidemiology of eastern equine encephalitis virus in the Northeastern United States: an historical perspective. J Med Entomol 2022; 59(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. de Heus P, Bago Z, Weidinger P, et al. Severe neurologic disease in a horse caused by tick-borne encephalitis virus, Austria, 2021. Viruses 2023; 15(10): 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Sun JA, Hallowell TC. Magnetic resonance imaging, clinicopathologic findings, and clinical progression of a puppy with confirmed Eastern equine encephalitis virus. Can Vet J 2021; 62(12): 1298–1303. [PMC free article] [PubMed] [Google Scholar]
- 181. Wilcox DR, Collens SI, Solomon IH, et al. Eastern equine encephalitis and use of IV immunoglobulin therapy and high-dose steroids. Neurol Neuroimmunol Neuroinflamm 2021; 8(1): e917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Cho JJ, Wong JK, Henkel J, et al. Acute seroconversion of Eastern equine encephalitis coinfection with California Serogroup encephalitis virus. Front Neurol 2019; 10: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wendell LC, Potter NS, Roth JL, et al. Successful management of severe neuroinvasive eastern equine encephalitis. Neurocrit Care 2013; 19(1): 111–115. [DOI] [PubMed] [Google Scholar]
- 184. Turell MJ, Presley SM, Gad AM, et al. Vector competence of Egyptian mosquitoes for Rift Valley fever virus. Am J Trop Med Hyg 1996; 54(2): 136–139. [DOI] [PubMed] [Google Scholar]
- 185. Youssouf H, Subiros M, Dennetiere G, et al. Rift valley fever outbreak, Mayotte, France, 2018–2019. Emerg Infect Dis 2020; 26(4): 769–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Madani TA, Al-Mazrou YY, Al-Jeffri MH, et al. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis 2003; 37(8): 1084–1092. [DOI] [PubMed] [Google Scholar]
- 187. Alrajhi AA, Al-Semari A, Al-Watban J. Rift valley fever encephalitis. Emerg Infect Dis J 2004; 10(3): 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hartman A. Rift Valley fever. Clin Lab Med 2017; 37(2): 285–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Yellow fever in Africa and South America, 2013. Wkly Epidemiol Rec 2014; 89(27): 297–306. [PubMed] [Google Scholar]
- 190. Beasley DW, Whiteman MC, Zhang S, et al. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J Virol 2005; 79(13): 8339–8347. [DOI] [PMC free article] [PubMed] [Google Scholar]