Abstract
Mechanosensitive hair cells (HCs) in the cochlear sensory epithelium are critical for sound detection and transduction. Mammalian HCs in the cochlea undergo cytogenesis during embryonic development, and irreversible damage to hair cells postnatally is a major cause of deafness. During the development of the organ of Corti, HCs and supporting cells (SCs) originate from the same precursors. In the neonatal cochlea, damage to HCs activates adjacent SCs to act as HC precursors and to differentiate into new HCs. However, the plasticity of SCs to produce new HCs is gradually lost with cochlear development. Here, we delineate an essential role for the guanine nucleotide exchange factor Net1 in SC trans-differentiation into HCs. Net1 overexpression mediated by AAV-ie in SCs promoted cochlear organoid formation and HC differentiation under two and three-dimensional culture conditions. Also, AAV-Net1 enhanced SC proliferation in Lgr5-EGFPCreERT2 mice and HC generation as indicated by lineage tracing of HCs in the cochleae of Lgr5-EGFPCreERT2/Rosa26-tdTomatoloxp/loxp mice. We further found that the up-regulation of Wnt/β-catenin and Notch signaling in AAV-Net1-transduced cochleae might be responsible for the SC proliferation and HC differentiation. Also, Net1 overexpression in SCs enhanced SC proliferation and HC regeneration and survival after HC damage by neomycin. Taken together, our study suggests that Net1 might serve as a potential target for HC regeneration and that AAV-mediated gene regulation may be a promising approach in stem cell-based therapy in hearing restoration.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-023-04743-6.
Keywords: Hair cell regeneration, Net1, AAV, Organoid, Cochlea
Introduction
Sensorineural hearing loss is mainly caused by hair cell (HC) damage and is one of the leading causes of hearing loss in humans [1]. In mammals, there are two types of mechanosensory HCs and surrounding non-sensory supporting cells (SCs), and these are arranged in rows extending from the basal to apical turns along the cochlear spiral. HCs, the main sensors for sound stimuli, include three rows of outer HCs (OHCs) and one row of inner HCs (IHCs). SCs consist of several types, including Hensen’s cells, Deiters’ cells (DCs), outer pillar cells, inner pillar cells (IPCs), inner phalangeal cells (IPhCs), and inner border cells (IBCs) [1].
HCs are differentiated during embryonic development in mammals, and mammalian HC damage is irreversible. HCs and SCs are derived from the same ancestral progenitor cells [2, 3], and studies have shown that SCs are able to serve as the source of HC regeneration in the mouse cochlea [4–7]. SCs in neonatal mice sorted by flow cytometry have the ability to divide and can differentiate into HCs in culture [8, 9], and this also occurs in the cochlear epithelia after HC damage or ablation [10–13] Multiple regulatory pathways have been shown to be involved in HC regeneration [1, 14–17]. Overexpression of Atoh1 positively promotes the trans-differentiation of SCs into HCs with high efficiency [18, 20], and upregulation of Wnt/β-catenin signaling induces the proliferation of SCs and HC differentiation in mice [5, 21, 22]. Also, Notch inhibition activates Wnt/β-catenin signaling to produce new HCs. However, the reprogrammed HCs induced by these signaling pathways do not acquire all of the features of mature cells [23, 24], and thus there is an urgent need to identify new genes involved in HC development and regeneration.
We previously sorted Lgr5+ SCs and Lgr6+ SCs from neonatal Lgr5-EGFPCreERT2 and Lgr6-EGFPCreERT2 transgenic mice by flow cytometry and then analyzed the transcriptomic differences between the two cell groups by RNA sequencing [9]. We found that the expression of neuroepithelial transforming gene 1 (Net1) was significantly different in Lgr5+ SCs and Lgr6+ SCs. Net1 is a guanine nucleotide exchange factor of RhoA GTPase that was originally isolated from neuroepithelial tumor cells and is considered a novel oncogene [25, 26], and Rho proteins belong to the Ras superfamily of small GTPases and are involved in the regulation of the actin cytoskeleton, signal transduction, and gene transcription [27]. Net1 is involved in diverse biological processes, including cell proliferation, apoptosis, differentiation, and cytoskeleton reorganization by regulating RhoA activity [28, 29]. Previous studies have shown that Net1 can enhance the proliferation of Nalm-6 B-cells in patients with acute lymphoblastic leukemia, and Net1 promotes carcinoma cell proliferation, invasion, and metastasis in gastric cancer tumorigenesis, which can be attenuated by the use of Net1 siRNA [30–32]. Net1 can also activate signaling pathways such as Jnk, NF-κB, and Wnt/β-catenin [33]. Combined with Dishevelled, Net1 activates RhoA to regulate gastrulation in Xenopus [34] In zebrafish, Net1 acts upstream of Pak1 to promote β-catenin phosphorylation at S675, thereby activating Wnt/β-catenin signaling during early embryonic development [34, 35]. In this study, we found that Net1 is expressed in the cochlea after birth and that its expression increases then decreases as the cochlea develops, indicating its role in SC and HC development. Thus, we hypothesize that Net1 is involved in SC plasticity and HC differentiation in the Wnt/β-catenin signaling pathway in the inner ear.
Previously, we designed and optimized a novel and safe adeno-associated virus (AAV-inner ear, AAV-ie) that has high transfection efficiency for SCs, which act as HC progenitors [36], thus establishing an AAV-mediated gene delivery system for cochlear progenitor cells. Here, we achieved Net1 overexpression in SCs using AAV-ie-Net1 (referred to as AAV-Net1 hereafter), and we provide evidence that AAV-Net1 reinforces SC proliferation and HC differentiation in cochlear organoid culture. Further, delivery of AAV-Net1 through the round window membrane into the postnatal mouse inner ear increased the proliferation activity of SCs along with activation of the Wnt/β-catenin signaling pathway. Meanwhile, forced Net1 expression in SCs by AAV-Net1 increased the trans-differentiate ability of SCs into HCs via a Wnt/β-catenin and Notch signal-dependent pathway. Finally, we demonstrated that AAV-Net1 could mediate SC reprogramming and HC survival after exposure to the ototoxic aminoglycoside neomycin. Our results suggest the potential application of AAV-mediated Net1 regulation in HC regeneration.
Methods
Experimental animals
FVB wildtype mice, Lgr5-EGFPCreERT2 mice (stock number: 008875, Jackson Laboratory), and Rosa26-CAG-LSL(Loxp-Stop-Loxp)-tdTomato mice (stock number: 007914, Jackson Laboratory) of both sexes were used in our experiments. The transgenic mice were genotyped using genomic DNA from tail tips after digestion in 180 μl of 50 mM NaOH (incubation for 1 h at 98 °C) and termination in 20 μl of 1 M Tris–HCl. The primers and sequences used for genotyping were as follows: Lgr5-wildtype-F: 5′-ATA CCC CAT CCC TTT TGA GC-3′ Lgr5-mutant-F: 5′-CTG CTC TCT GCT CCC AGT CT-3′, Lgr5-common: 5′-GAA CTT CAG GGT CAG CTT GC-3′; tdTomato-wildtype-F: 5′-AAG GGA GCT GCA GTG GAG TA-3′, tdTomato-wildtype-R: 5′-CCG AAA ATC TGT GGG AAG TC-3′, tdTomato-mutant-F: 5′-GGC ATT AAA GCA GCG TAT CC-3′, tdTomato-mutant-R: 5′-CTG TTC CTG TAC GGC ATG G-3′. For Cre activation, tamoxifen (Sigma, T5648, diluted with corn oil) was intraperitoneally injected at a dose of 0.075 mg/g body weight in P1-2 mice. For EdU labeling, EdU (Beyotime, ST067, diluted with PBS (Multicell, 311-010-CL)) was intraperitoneally injected at a dose of 0.05 mg/g body weight in P2-4 mice. All experiments were approved by the Institutional Animal Care and Use Committee of Southeast University, China, and all efforts were made to minimize the number of mice used.
AAV virus preparation
The target transgene plasmids with Net1 tagged with HA or the fluorescent protein mNeonGreen were cloned into the AAV plasmid containing the CAG promoter and the WPRE cassette flanked by AAV2 inverted terminal repeats. All AAV viruses were produced by a triple plasmid co-transfection system including the rep-cap-fused plasmid AAV-ie, the helper plasmid, and the transgene plasmid in the HEK 293T cells. Purification of AAVs was performed as described previously [36]. The concentration of AAVs was determined by SYBR (Vazyme, Q311) analysis, and the qPCR primers for the WPRE region were same as the previous report [36]: Forward, 5′-GTC AGG CAA CGT GGC GTG GTG TG-3′; Reverse, 5′-GGC GAT GAG TTC CGC CGT GGC-3′.
Immunofluorescence staining
The dissected cochleae were placed in PBS and fixed in 4% paraformaldehyde (Biosharp, BL539A) for 2–3 h at room temperature. The cochleae of mice over 7 days old were decalcified in 0.5 M EDTA for at least 2–3 h at room temperature. The cochleae were incubated in blocking medium (10% donkey serum, Solarbio, SL050) and 0.5% Triton-X100 (Life Science, TB200) in PBS at (pH 7.2) for 1 h at room temperature and then incubated with the primary antibodies overnight at 4 °C. The primary antibodies were Myosin7a (Proteus Bioscience, 1:1000 dilution), Sox2 (R&D systems, 1:200 dilution), Net1 (Santa Cruz, sc-271947, 1:200 dilution), Ctbp2 (BD Biosciences, 612044.0, 1:400 dilution), and HA (Roche, 11867431001, 1:200 dilution). The cochlear samples were washed with PBS three times and then incubated with the secondary antibody for 1 h at room temperature. The cochlear samples were washed with PBS three times again and mounted with DAKO anti-fluorescence quenching mounting medium (S3023). Immunofluorescence images were captured by a laser confocal microscope (Zeiss LSM 900).
Lgr5+ SC isolation
P1-3 Lgr5-EGFP-IRES-creERT2 mice were used. The basilar membrane was isolated in cold HBSS (Gibco, 8118227) and digested in 80 µl 0.25% trypsin–EDTA (Invitrogen, 25200-056) for 8 min at 37 °C. The digestion was stopped by addition of 80 µl trypsin inhibitor (Worthington Biochem, LS003570), and the cochlear tissue was dissociated into a single cell suspension. The cells were filtered through a 40 μl strainer (BD Biosciences, 21008-949) to eliminate clumps.
Cochlear organoid culture
For two-dimensional culture, single cells were obtained from 0.25% trypsin–EDTA treatment as described above. Cell suspensions were seeded in low-adhesion 96-well plates (Coring, 3474) at 5000 cells per well and cultured for 5 days in expansion medium. The cells were then transferred to laminin-coated glass slides for 10 days of culture in differentiation medium, and EdU (3 μM, Thermo Fisher, C10337) was added at day 3–5. The differentiation medium included DMEM/F12 (Thermo Fisher, C11995500BT), N2 (1%, Thermo Fisher, 175020-48), B-27 (2%, Thermo Fisher, 17504-044), IGF (50 ng/ml, Sigma, I8779), EGF (20 ng/mL, Sigma, E9644), b-FGF (10 ng/ml, Sigma, F0291), heparan sulfate (20 ng/ml, Sigma, H4777), and ampicillin (1%, Sigma, A9518).
For three-dimensional culture, the single cell suspension was centrifuged and the supernatant was removed. The single cells were resuspended in a 1:3 mix of Matrigel (Corning, 356234) and DMEM/F12 (Gibco, C11995500BT) and seeded into 24-well plates (Griner Bio-one, 662160) at 5000 cells per well. Expansion medium was added and cultured for 10 days, and EdU (3 μM, Thermo Fisher, C10337) was added 1 h before the end of the expansion culture. The expansion medium contained DMEM/F12 (Thermo Fisher, C11995500BT), N2 (1%, Thermo Fisher, 175020-48), B-27 (2%, Thermo Fisher, 17504-044), IGF (50 ng/ml, Sigma, I8779), EGF (20 ng/mL, Sigma, E9644), b-FGF (10 ng/ml, Sigma, F0291), CHIR99021 (3 μM, Sigma, SML1046), VPA (1 mM, Sigma, P4543), 616452 (2 μM, Sigma, 446859-33-2), and ampicillin (1%, Sigma, A9518). For differentiation, cells were cultured for 10 days in expansion medium and then cultured in differentiation medium for 10 days. EdU (3 μM, Invitrogen, C10337) was added during day 3–5 of culture. The differentiation medium consisted of DMEM/F12 (Thermo Fisher, C11995500BT), N2 (1%, Thermo Fisher, 175020-48), B-27 (2%, Thermo Fisher, 17504-044), CHIR99021 (3 μM, Sigma, SML1046), LY411575 (5 μM, Sigma, SML0506), and ampicillin (1%, Sigma, A9518).
Round window membrane injection
We followed the injection protocol as published previously [36]. P1-2 mice were chosen for AAV injection through the round window membrane. The AAV dose was 7.5e10 GCs, and the maximal volume of virus was 1.5 µL for each ear. After injection, the pups were sent back to their mother for nursing.
Cochlear explant culture
We followed a previously published protocol [51]. The P2 FVB wildtype mouse basilar membrane was isolated in cold HBSS (Thermo Fisher, 8118227), and the lower apical turn and the upper basal turn from each ear were attached to a Cell-Tak-coated slide (Corning, 354240). A total of 3 × 1010 GCs of AAVs was added to the culture medium for 24 h, and neomycin (0.5 µM, Sigma, N6386) was added to the culture medium for 12 h. The AAVs and neomycin were removed, and EdU (3 μM, Thermo Fisher, C10337) was added to the culture medium for 3 days.
RNA extraction and quantitative real-time PCR
The cochleae or the organoids were harvested in cold PBS and homogenized by grinding with the addition of 1 ml of Trizol (Thermo Fisher, 15596-018). RNA was dissolved in DEPC water and was reverse transcribed to cDNA with an RNA reverse transcription kit (Thermo Fisher, 01134583). The SYBR system (Vazyme, Q311) was used on a qPCR instrument (Thermo Fisher Scientific, QuantStudio3). RNA expression levels of Lgr5, Net1, and other genes were normalized to Gapdh in the same samples. The qPCR primers are listed in Table S1. For RNA sequencing, the qualified RNAs ware analyzed and digitalized by NovoGene Inc.
Auditory Brainstem Response (ABR) measurement
Pentobarbital sodium (10 mg/ml in PBS) was intraperitoneally injected into the mice at a dose of 0.1 mg/1 g body weight to achieve deep anesthesia. Then we according the previous protocol to achieve the closed-field ABR thresholds for AAV injected mice by using a TDT system III workstation (Tucker-Davis Technologies). There are three electrodes, the positive one on the top of the head, the negative one under the ear, and the ground wire to the thigh. The 4, 8, 12, 16, 24, and 32 kHz tone pips were performed in the ABR test, and the sound intensities were decreased from 90 dB in 5 dB steps.
Statistical analysis
Raw images were export by the LSM 700 or 900 (Zeiss) as.tif images, and these were then adjusted and analyzed in Fiji software (Fiji, Inc.). All of the data are shown as the mean ± SEM, and statistical analyses were conducted in GraphPad Prism 7 software. Two-tailed, unpaired Student’s t-tests or one-way ANOVA was used to determine the statistical difference. A value of p < 0.05 was considered to be statistically significant.
Results
Net1 is expressed in the cochlear epithelium
We first used biochemical methods to observe the spatiotemporal expression of Net1 in the sensory epithelium of the postnatal mouse cochlea. Cochlear RNA was extracted from postnatal day (P)1, P7, P14, and P21 wild-type mice, and quantitative real-time PCR (qPCR) results showed that the expression level of Net1 in the cochlea increased and then decreased (Fig. S1A). Similarly, we used a commercially available antibody against Net1, and western blotting (Fig. S1B, C) confirmed that the expression level of Net1 in the cochlea showed an increasing and then decreasing trend. The high expression level of Net1 in the immature cochlea suggests its potential function during cochlear maturation.
AAV-Net1 activation in cochlear organoids increased the proliferation of SCs and the generation of HCs
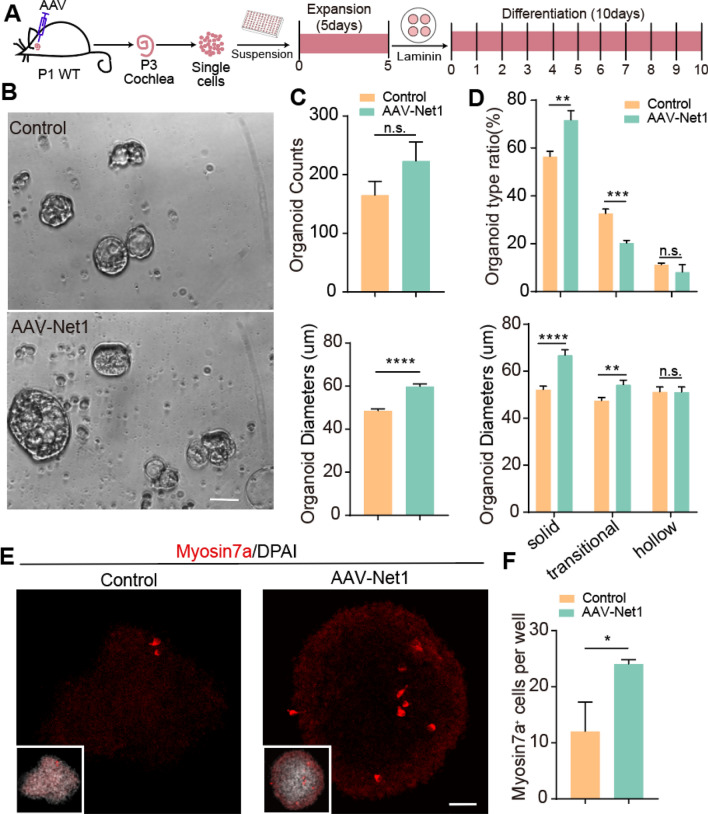
We next delivered exogenous Net1 into the SCs of P1-2 mouse cochleae using AAV-ie. The transduction efficiency of AAV-ie in SCs can be more that 90%, and we verified the transduction efficiency of AAV-Net1 in SCs using immunofluorescence imaging of an HA tag conjugated to the C terminus of NET1 (Fig. S2). After 2 days of AAV delivery, single cochlear cells were obtained by an enzymatic dissociation method, and these were used for organoid culture in both expansion and differentiation conditions. Organoids were expanded in suspension in ultra-low-attachment 96-well plates and then transferred onto laminin-coated cover glasses for differentiation culture (Fig. 1A). The numbers and diameters of the organoids were measured after 5 days of expansion (Fig. 1B). AAV-Net1 increased the average diameter of the cochlear organoids compared with those transduced with control AAVs (Fig. 1C), and organoid morphologies in in vitro culture varied from solid to hollow subtypes. Solid organoids contain more stem cells that are actively going through cell cycle progression compared to hollow or transitional organoids [37]. The proportion of solid organoids was higher in Net1-overexpressing organoids compared to controls and the proportion of transitional organoids was decreased, while the proportion of hollow organoids was similar between the two groups (Fig. 1D). The average diameter of the solid and transitional organoids was also increased after AAV-Net1 transduction (Fig. 1D). We next investigated HC differentiation in cochlear organoids mediated by AAV-Net1 (Fig. 1A). After 10 days of differentiation, immunostaining images of organoids (Fig. 1E) showed that the proportion of Myosin7a+-positive cells (a marker of HCs) was significantly increased in cultured organoids after Net1 overexpression (Fig. 1F).
Fig. 1.
AAV-Net1 promotes cochlear organoid formation and HC regeneration in two-dimensional culture assay. A Illustration of the experimental design. All mice were injected with the indicated AAVs at a dose of 7.5E10 GCs in the left ears. B Representative bright-field images of cochlear organoids overexpressing AAV-control and AAV-Net1 after expansion. Scale bar, 50 μm. C Cochlear organoid counts per cell and average organoid diameters. D The proportions and average diameters of three organoid morphologies after 5 days of expansion. E Representative confocal images of cochlear organoids overexpressing AAV-control or AAV-Net1 showing newly formed HCs in cochlear organoids after 10 days of differentiation. Scale bar, 40 μm. F The proportion of Myosin7a+ organoids after 10 days of differentiation. The controls here refer to AAV-NLS-mNeonGreen. NLS, nuclear localization sequence. Data are presented as the mean ± SEM. The p-value was calculated by two-tailed unpair Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n.s., no significance
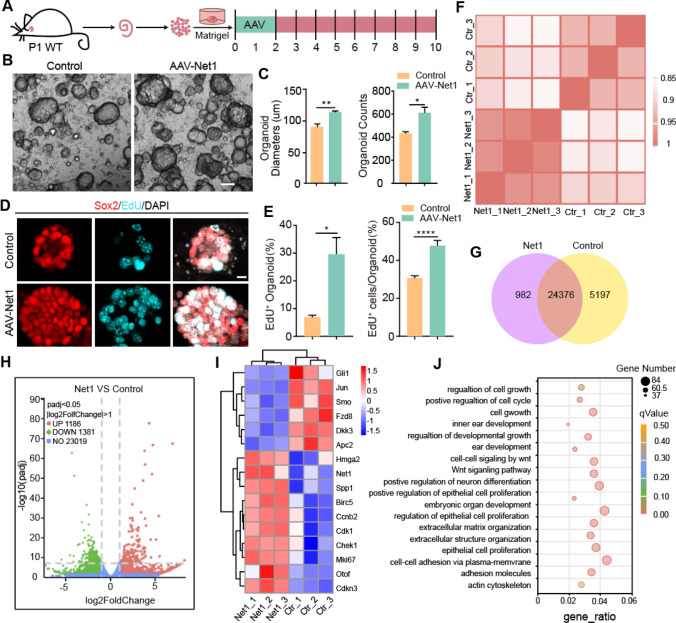
We further explored the effect of Net1 upregulation on organoid expansion and differentiation using Matrigel-based hydrogel scaffolds to simulate the in vivo environment. Organoids were derived from the P1 mouse cochlea. Net1 and control AAV viruses with the same number of genome-containing particles (GCs) (2 × 1010 GCs) were added on days 1–2 of expansion, and the analyses were conducted 8 days later (Fig. 2A). Similarly, AAV-Net1 promoted organoid expansion with significantly increased diameters and numbers compared to the control group (Fig. 2B, C). At the end of the expansion culture on day 10, a single EdU pulse was administered for 1 h (Fig. 2A) to label the proliferating cells (Fig. 2D). Compared with controls, the percentage of EdU+ organoids increased by nearly 4-fold after Net1 overexpression, and the ratio of EdU+ cells in each organoid also increased by about 1.5-fold (Fig. 2E).
Fig. 2.
AAV-Net1 administration promotes cochlear SC expansion in optimized three-dimensional culture assay. A Experimental design for cochlear organoid expansion. Corresponding AAVs were introduced at a dose of 2E10 GCs into every well. B Representative bright-field images of cochlear organoids overexpressing AAV-control or AAV-Net1 after expansion. Scale bar, 100 μm. C Average organoid diameters and cochlear organoid counts per cell. D Representative confocal images of cochlear organoids immunostained with Sox2 after 10 days of expansion. Sox2 (red) marked SCs. EdU incorporation (cyan) was analyzed after 1 h EdU treatment on day 10 of expansion. Scale bar, 10 μm. E The proportion of EdU+ organoids and the proportion of EdU+ cells in the organoids at day 10 of expansion. F The cluster analysis of all replicated samples of AAV-control and AAV-Net1. G The amount of gene expression between AAV-control and AAV-Net1 in the venn diagram. H The number of differential genes between AAV-control and AAV-Net1 in the volcano Plot. I The changes of AAV-control and AAV-Net1 differential genes in heatmap. J The GO analysis of differential genes in AAV-control and AAV-Net1. The controls here refer to AAV-NLS-mNeonGreen. Data are presented as the mean ± SEM. The p-value was calculated by two-tailed unpaired Student’s t-test. *p < 0.05, **p < 0.01, ****p < 0.0001
In order to further explore the mechanism of Net1 in organoids expansion, we used the RNA-sequencing to analyze the transcriptome difference between AAV-control and AAV-Net1-transduced organoids after 10 days of expansion. Each group of samples had 3 replicates with high repeatability (Pearson’s r = 0.964–0.98 for AAV-Net1 groups and 0.928–0.931 for AAV-control groups) (Fig. 2F). There are 25,358 and 29,573 genes expressed in the AAV-Net1 group and AAV-control group respectively, and 24,376 genes expressed in both groups (Fig. 2G). Compared with the AAV-control group, there are 1186 up-regulated differential genes, 1381 down-regulated differential genes and 23,019 no differential genes in the Net1 overexpression group (Fig. 2H). In the Net1 overexpressed organoids the cell cycle genes Cdkn3, Cdk1, birc5, ccnb2 and Chek1 were significantly increased, Mki67, the marker of cell proliferation, was significantly increased. And the inhibitory factors Dkk3 and Apc2 of Wnt/β-catenin signaling were significantly down-regulated.
More importantly, Notch pathway activators Gli1 and Spp1 were significantly decreased in the Net1 overexpressed organoids after 10 days of expansion (Fig. 2I). Notch inhibition is sufficient to reprogram SCs into inner ear HCs [23], suggesting that the activation of Net1 may prime SCs for HC fate induction. To further identify the functional network of gene involved in Net1 overexpression organoids, GO function analysis based on differential genes was performed (Fig. 2J). The scatter plots showed the significantly enriched GO terms, including cell cycle regulation, cell proliferation, cell growth, and Wnt/β-catenin signaling, all of which indicated that Net1 overexpression could promote the proliferation of SCs. Also, 45 and 37 genes in ear development and inner ear development were detected.
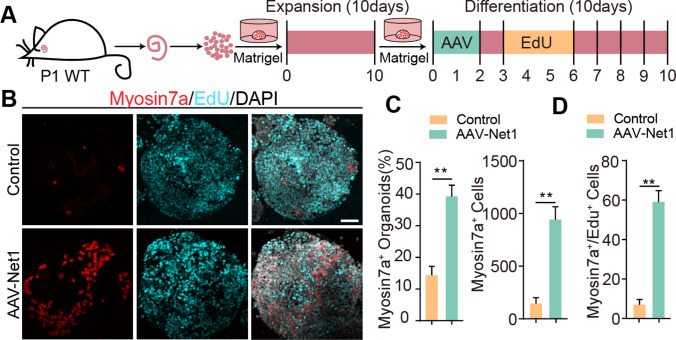
Next, we further explored the effect of AAV-Net1 on HC production in the organoids. After 10 days of expansion, the organoids were transferred into differentiation medium and transduced with AAV-control or AAV-Net1 on day 1–2 of culture. HC production was investigated at day 10, and EdU was added on day 4–6 of differentiation culture to label mitotic HCs (Fig. 3F). HC formation in cultured organoids was determined using the HC marker myosin7a (Fig. 3G). There was a nearly 3-fold increase in the proportion of Myosin7a+ organoids after Net1 overexpression compared with the controls (Fig. 3H). Also, a significant difference in the total number of HCs induced by Net1 overexpression was seen at the end of the differentiation culture (Fig. 2H). In addition, we observed a dramatic increase in the number of mitotic HCs (Myosin7a+/EdU+) (Fig. 3I). Taken together, these results indicated that Net1 overexpression in SCs mediated by AAV-ie could effectively promote the proliferation of inner ear progenitors and the formation of HCs in organoid culture.
Fig. 3.
AAV-Net1 administration promotes HC production in optimized three-dimensional culture assay. A Experimental design for cochlear organoid differentiation after expansion. Corresponding AAVs were introduced at a dose of 2E10 GCs into every well. B Representative confocal images of cochlear organoids immunostained with Myosin7a after 10 days of differentiation. Myo7a (red) marked HCs. EdU incorporation (cyan) was also analyzed. Scale bar, 50 μm. C The proportions of Myosin7a+ organoids and Myosin7a+ cells after 10 days of differentiation. D Myosin7a+/ EdU+ cell counts. The controls here refer to AAV-NLS-mNeonGreen. Data are presented as the mean ± SEM. The p-value was calculated by two-tailed unpaired Student’s t-test. **p < 0.01
AAV-Net1 induced SC division by activating the Wnt/β-catenin signaling pathway in the neonatal cochlea
To determine whether higher than normal Net1 levels will boost the regenerative capacity of SCs in situ, we delivered AAV-Net1 into the neonatal cochlea through the round window membrane. EdU (0.05 mg/g) was injected intraperitoneally from day 2 to day 4 after AAV administration locally, and the cochleae were analyzed at 2 weeks (Fig. 4A). Normally, SCs are quiescent after birth and have no ability to divide [2, 19, 22]. After AAV-Net1 injection, EdU+/Sox2+ double-positive SCs were detected in the IPhC/IBC region of the cochlear epithelium (Fig. 4B) with a significantly increased cell number count (Fig. 4C), indicating a positive effect of Net1 on SC proliferation. Wnt/β-catenin signaling is a classical signaling pathway that regulates SC plasticity, and upregulation of the Wnt/β-catenin pathway contributes to SC proliferation and HC differentiation in both healthy and injured cochleae [5, 22, 23]. Lgr5 expression decreases with postnatal development and is barely detected in the cochlea at P16 [5]. Here, we delivered AAV-Net1 into the cochlea from P1 Lgr5CerERT2-EGFP mice and observed a stronger EGFP signal, which represented Lgr5 expression in the ipsilateral AAV-injected cochlea and its contralateral side (Fig. 4D, E). The real-time qPCR results showed that the transcriptome level of Lgr5 in the cochlea with overexpression of Net1 was increased more than 2-fold compared to control cochleae (Fig. 4F). Lgr5+ SCs are considered to be HC progenitors [5], so we next directly investigated the effect of Net1 on the plasticity of Lgr5+ SCs. Lgr5+ SCs were sorted and collected by fluorescence activated cell sorting from P1 Lgr5-EGFPCreERT2 mice for organoid culture. AAV-Net1 was added during the 1st to 2nd day of culture (Fig. 4G). After 5 days of expansion, we found that the number of organoids formed from AAV-Net1-injected cochleae was significantly increased, with more solid organoids (Fig. 4H–J). These results suggested that Net1 overexpression promotes SC proliferation through a Wnt/β-catenin-dependent pathway.
Fig. 4.
AAV-Net1 promotes the proliferation of Sox2+ SCs and Lgr5+ progenitors via Wnt/β-catenin signaling. A Illustration of the experimental design. All mice were injected with corresponding AAVs at a dose of 7.5E10 GCs in the left ears. B EdU was stained (cyan) in cochleae transduced by AAV-control and AAV-Net1, respectively. Sox2 (red) marked SCs. Magnified images and orthogonal views are shown to the right. Arrows indicate the Sox2+/EdU+ SCs. Scale bars, 40 µm and 20 μm, respectively. C Sox2+/EdU+ SC counts. D Representative images of Lgr5-EGFP (green) signals in AAV-injected cochleae and the contralateral cochleae. Myosin7a (magenta) marked HCs. Scale bar, 50 μm. E Immunosignal intensity of Lgr5-EGFP in cochleae transduced with AAV-control and AAV-Net1 in the DC region and IPC region. F Relative Lgr5 mRNA expression in cochleae transduced with AAV-control and AAV-Net1. A, M, and B refer to the apical, middle, and basal turns of the cochlea. G Illustration of the experimental design. Corresponding AAVs were introduced at a dose of 2E10 GCs into every well. H Representative bright-field images of cochlear organoids overexpressing AAV-control and AAV-Net1 after expansion. Scale bar, 40 μm. I Cochlear organoid counts per cell. J The proportions of the three organoid morphologies after 5 days of expansion. The controls in D refer to Lgr5-EGFPCreERT2 mice, and the controls in the other panels refer to AAV-NLS-mNeonGreen. Data are presented as the mean ± SEM. The p-value was calculated by two-tailed unpair Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n.s., no significance
AAV-Net1 promoted SC to HC conversion in the postnatal cochlea
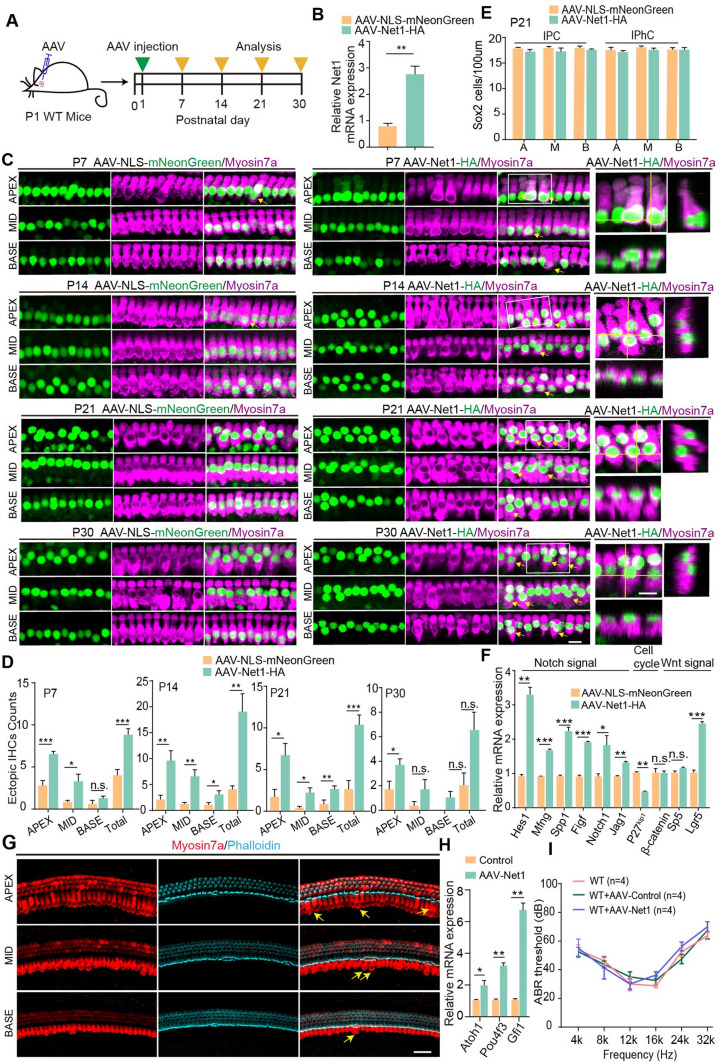
To investigate the role of Net1 in HC differentiation in the cochlea, AAV-ie-Net1 was injected into P1 mice to induce exogenous Net1 overexpression in SCs. AAV-Net1-injected mice were sacrificed at P7, and cochlear samples were collected (Fig. 5A). The real-time qPCR results showed that the transcriptome level of Net1 in cochleae overexpressing Net1 was increased more than 3-fold higher compared to control cochleae (Fig. 5B). Ectopic IHCs were observed in the apical, middle, and basal turns of the cochlea (Fig. 5C), and no ectopic OHCs were detected (Fig. S3). Although ectopic IHCs were also seen in the control cochleae, statistical analyses showed a significantly increased number of IHCs after AAV-Net1 transduction compared with controls (Fig. 5D). The number of ectopic IHCs in the cochlea transduced by AAV-Net1 decreased from the apical to basal turns (Fig. 5D). The numbers of various SC types near the IHCs, including IPCs and IPhCs, were quantified, and while these were slightly reduced the differences were not significantly different (Fig. 5E). Also, no mitotic HCs (Myosin7a+/EdU+) were detected in any of the AAV-Net1-transduced cochleae (Fig. S4), suggesting that the ectopic HCs were derived from direct trans-differentiation of SCs. Previous reports have shown that newly regenerated ectopic HCs gradually degenerate as the mouse ages [10], so we also analyzed the survival of ectopic IHCs in the cochlea. A significantly different number of ectopic IHCs was found in the cochleae injected with AAV-Net1 in P14, P21, and P30 mice (Fig. 5C, D), suggesting that some ectopic IHCs can survive at least to P30. And the phalloidin dye was used to analyze the stereocilia of the ectopic IHCs in the AAV-Net1-transduced cochleae, and the staining results showed that phalloidin was expressed in ectopic IHCs at P14 (Fig. 5G), suggesting that some ectopic IHCs had the stereocilia morphology. Atoh1, Pou4f3 and Gfi1 were the HCs marker, we examined those gene expression by qPCR, the result showed the expression of Atoh1, Pou4f3 and Gfi1 were increased in AAV-Net1 transduced cochleae compared with control at P14 (Fig. 5H). Those results showed the Net1 overexpression could promote HCs regeneration in postnatal mice.
Fig. 5.
AAV-Net1 transduction in SCs results in increased HC numbers in postnatal cochleae. A Illustration of the experimental design. All mice were injected with corresponding AAVs at a dose of 7.5E10 GCs in the left ears. B Relative Net1 mRNA expression in cochleae transduced with AAV-NLS-mNeonGreen and AAV-Net1-HA. C Extra IHCs (arrows) were seen in the apical (APEX), middle (MID), and basal (BASE) turns of cochleae transduced with AAV-NLS-mNeonGreen and AAV-Net1-HA. Myosin7a (magenta) marked the HCs. Magnified images and orthogonal views are shown to the right. Scale bar, 10 μm. D Ectopic IHC counts in P7, P14, P21, and P30 cochleae transduced with AAV-NLS-mNeonGreen (control) and AAV-Net1-HA. E Sox2 counts in P21 cochleae transduced with AAV-NLS-mNeonGreen and AAV-Net1-HA. A, M, B refer to the apical, middle, and basal turns of the cochlea. F Relative mRNA expression in cochleae transduced with AAV-NLS-mNeonGreen and AAV-Net1-HA, respectively. G Representative confocal images of Mosin7a staining (red) and phalloidin (cyan) in cochleae transduced at P14. Myosin7a (red) marked HCs. phalloidin (cyan) marked HC stereocilia. The yellow arrow indicated the ectopic IHCs in the cochleae. All mice were injected with corresponding AAVs at a dose of 7.5E10 GCs in the left ears. Scale bar, 40 μm. H Relative Atoh1, Pou4f3 and Gfi1 mRNA expression in the AAV-Net1 transduced cochleae at P14. All P1 mice were injected with corresponding AAVs at a dose of 7.5E10 GCs in the left ears. I The P1 wildtype mice were injected with AAV-control and AAV-Net1 and the ABR measurement was performed at P30. Corresponding AAVs were introduced at a dose of 2E10 GCs into every left ear. AAV-NLS-mNeonGreen refers to the control. Data are presented as the mean ± SEM. The p-value was calculated by two-tailed unpaired Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., no significance
To determine the mechanism of HC regeneration mediated by Net1 overexpression, mRNA from AAV-Net1-transduced cochleae was extracted for real-time qPCR to quantify the classic signaling pathways involved in HC formation, including the Wnt/β-catenin and Notch signaling pathways. As expected, the activation of Wnt/β-catenin and inhibition of Notch could be detected in AAV-Net1-transduced cochleae. We also found that the cell cycle repressor p27kip1 was significantly down-regulated (Fig. 5F). These results indicated that the up-regulated Net1 in SCs leads to new HC generation mainly through the Wnt/β-catenin and Notch signaling pathways.
To evaluate the safety of AAV mediated Net1 overexpression in mice, we delivered AAV-control and AAV- Net1 to P1 mice respectively, and the Auditory Brainstem Response (ABR) test result showed that AAV-Net1 had no effect on the mice's hearing compared to wildtype mice at P30 (Fig. 5I). This suggested that delivering Net1 via AAV was a safe approach in mice and AAV vectors could be applied in HCs regeneration in mice. To further investigate the role of Net1 in adult mice cochleae, AAV- Net1 was injected into P30 mice. And, an unchanged HCs counts were detected in the AAV-control and AAV-Net1 transduced cochleae at P40 (Fig. S5), indicating that Net1 had no effect on the SCs plasticity in the adult mice.
Newly formed HCs induced by AAV-Net1 originated from Lgr5+ SCs
SCs subtypes have a variety of differentiation potentials, and newly regenerated HCs in the OHC region are derived from DCs while newly regenerated HCs in the IHC region are derived from IPCs, IPhCs, and IBCs [38]. We only observed ectopic HCs along the modiolus side of the IHCs in cochleae with Net1 overexpression, so we next used Lgr5-EGFPCreERT2/Rosa26-tdTomatoloxp/loxp mice to trace the new HC lineage induced by AAV-Net1. Tomato expression driven by the Lgr5 promoter could label all lineages of HCs from Lgr5+ progenitors (including the third row of DCs, IPCs, IPhCs, and IBCs) after Cre recombinase activation. Mice were injected intraperitoneally with tamoxifen at P1 and with AAV virus at P2 and were sacrificed at P14 for cochlear sample collection for comprehensive tracing of all HCs from Lgr5+ SCs (Fig. 6A). Immunofluorescence results showed a 3-fold increase in the number of Myosin7a+/tdTomato+ IHCs, and this was statistically different in both the AAV-Net1-injected cochleae and the contralateral cochleae compared to controls, mainly in the apical turns of the cochlea (Fig. 6B–D). Meanwhile, we confirmed that the newly formed ectopic IHCs made a comparable number of synapses to native IHCs (Fig. S6). In addition, although ectopic OHCs were barely detected, we found a significant increase in the total number of tdTomato+ OHCs co-labeled with Myosin7a, demonstrating that most regenerated OHCs induced by Net1 overexpression were of SC origin (Fig. 6B–D). Together, these results indicate that AAV-Net1 activation of SCs significantly enhanced SC-to-HC trans-differentiation, and these reprogramed HCs were derived from Lgr5+ SCs.
Fig. 6.
AAV-Net1 facilities SC-to-HC conversion in postnatal cochleae. A Illustration of the experimental design. All mice were injected with the indicated AAVs at a dose of 7.5E10 GCs in the left ears. B Lineage tracing images of cochlear Lgr5+ SCs in Lgr5CreER/+-Rosa26-tdTomatoloxp/loxp mice injected with AAV-control or AAV-Net1. Arrows and stars indicated tdTomato + OHCs and IHCs, respectively. Scale bar, 10 μm. C The magnified images and orthogonal views from the (B). Scale bar, 10 μm. D Myosin7a+/tdTomato+ hair cells count in Lgr5CreER/+-Rosa26-tdTomatoloxp/loxp mice cochleae transduced with AAV-NLS-mNeonGreen (control) and AAV-Net1-HA. The proportions of Myosin7a+ organoids and Myosin7a+ cells after 10 days of differentiation. AAV-NLS-mNeonGreen refers to control AAV. Data are presented as the mean ± SEM. The p-value was calculated by one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001
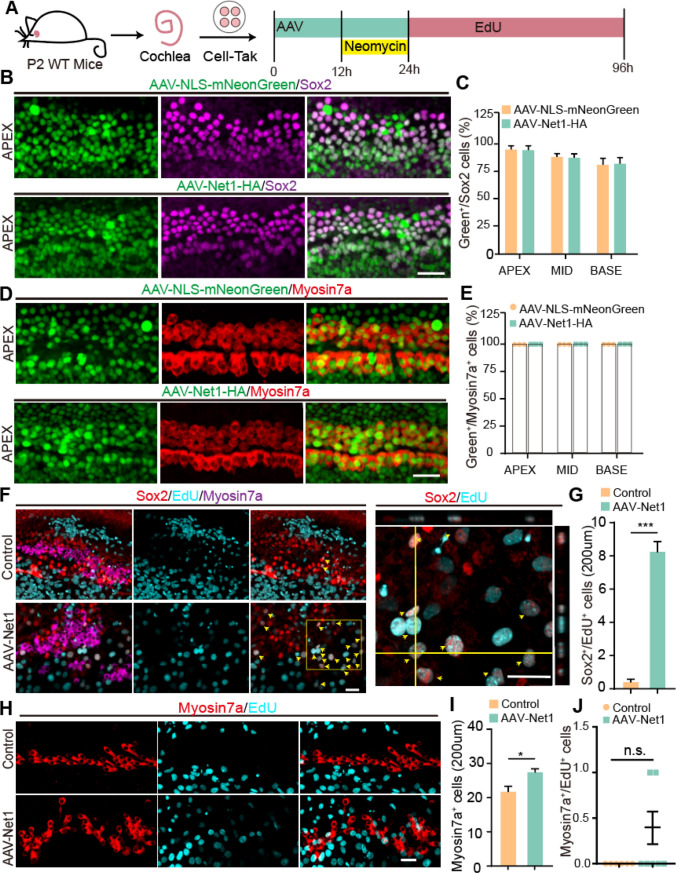
AAV-Net1 promoted SC reprogramming after neomycin injury
Exposure to noise or ototoxic drugs can cause damage to the cochlear HCs, and HC regeneration may be a potential treatment approach for deafness caused by noise or ototoxic drugs. To determine how SCs respond to HC damage after Net1 overexpression, we explored HC fate determination from SCs in detail in a model of HC damage induced by the aminoglycoside antibiotic neomycin. AAV-Net1 was used to genetically regulate the SCs in cultured cochlear explants. Here, neomycin was used to injure HCs in the cochlear epithelium, and EdU was administrated to label dividing SCs and mitotic HCs during damage repair. Cultures were then continued for another 3 days for further imaging analysis (Fig. 7A). AAV-Net1 had a clear effect and transduced > 75% of SCs and almost 100% of HCs (Fig. 7B–E). Next, we investigated the proliferation of cochlear SCs in the control and AAV-Net1 groups. More dividing SCs (EdU+/Sox2+) were observed in the cochlear epithelia, while very few were seen in the control cochleae (Fig. 7F, G). In addition, AAV-Net1-transduced cochleae had a significantly greater number of HCs compared to the control groups (Fig. 7H, I) but no Myo7a + /EdU + cells (mitotic HCs) were detected in either the AAV-control or AAV-Net1-treated cochleae (Fig. 7J). These results indicated that the activation of Net1 induces the proliferation of SCs and an increased number of HCs in the injured cochlea but fails to induce the proliferated SCs to differentiate into HCs. Taken together, this suggested that Net1 plays an important role in regulating the plasticity of cochlear SCs after neomycin-induced injury.
Fig. 7.
AAV-Net1 facilities SC reprogramming after neomycin exposure in postnatal cochleae. A Illustration of the experimental design. Corresponding AAVs were introduced at a dose of 3E10 GCs into every well. B Representative confocal images of NLS-mNeonGreen/Net1-HA fluorescence (green) and Sox2 staining (magenta) in the apical turns (APEX) of cochlea transduced at P2 with the indicated AAV at a dose of 3E10 GCs per well. Scale bar, 30 μm. C The ratio of AAV-positive SCs (green+/Sox2+) in the apical (APEX), middle (MID), and basal (BASE) turns of the cochlea. D Representative confocal images of NLS-mNeonGreen/Net1-HA fluorescence (green) and Myosin7a (red) staining in the apical turns (APEX) of cochleae transduced at P2 with the corresponding AAV at a dose of 3E10 GCs per well. Scale bar, 30 μm. E The ratio of AAV-positive HCs (green+/Myosin7a+) in the apical (APEX), middle (MID), and basal (BASE) turns of the cochlea. F EdU was stained (cyan) in cochleae transduced by AAV-NLS-mNeonGreen and AAV-Net1. Sox2 (red) marked SCs, and Myosin7a (magenta) marked HCs. Magnified images and their orthogonal views are shown to the right. Arrows indicate the Sox2+/EdU+ SCs. Scale bars, 30 μm. G The number of Sox2+/EdU+ SCs per 200 µm corresponding to (F). H Representative confocal images of NLS-mNeonGreen/Net1-HA fluorescence (green) and Sox2 Myosin7a (red) staining in cochleae transduced at P2 with the indicated AAVs at a dose of 3E10 GCs per well. Myosin7a (red) marked HCs. Scale bar, 30 μm. I The number of Myosin7a+ cells per 200 µm corresponding to (H). J The Myosin7a+/EdU+ cell counts in cochleae transduced with AAV-NLS-mNeonGreen and AAV-Net1-HA, respectively. AAV-NLS-mNeonGreen refers to control AAVs. Data are presented as the mean ± SEM. The p-value was calculated by two-tailed unpaired Student’s t-test. *p < 0.05, ***p < 0.001, n.s., no significance
Discussion
In the mammalian cochlea, HCs cannot regenerate spontaneously once damaged. Currently, clinical treatments rely mainly on hearing aids and cochlear implants, which depend on the quality and quantity of the remaining HCs and spiral ganglion neurons. Thus, damage to HCs will cause permanent hearing loss, and the use of stem cell regenerative medicine to obtain new HCs is a potential way to treat sensorineural hearing loss. Because HCs and SCs are derived from the same progenitor cells, SC regeneration of HCs from SCs has become a key research direction, and our study suggests that Net1 is involved in the mechanism of SC to HC conversion.
Many signaling pathways are involved in the process of regenerating cochlear HCs from SCs, and our previous studies have shown that the canonical Wnt/β-catenin signaling pathway regulates the fate of HC proliferation and differentiation during early cochlear development [39, 40]. In embryonic cochlear explant cultures, inhibition of the Wnt/β-catenin signaling pathway using small molecules can reduce the proliferation of precursor cells. In contrast, activating the Wnt/β-catenin signaling pathway can promote the formation of the presensory domain that expresses Sox2 and thus boosts the regeneration of HCs. Crucially, similar results have been obtained in vivo in β-catenin transgenic mice, and HC differentiation from progenitor cells is prevented when β-catenin is downregulated, whereas HC regeneration is promoted when β-catenin is overexpressed [39, 40]. Net1 has been shown to be involved in the Wnt/β-catenin signaling pathway in zebrafish [35]. In the inner ear, Lgr5 marks inner ear stem cells as a downstream target gene of Wnt/β-catenin [41], and in Lgr5-EGFPCreERT2 mice the fluorescence intensity of EGFP gradually decreases with age as does the stemness of SCs [5]. We injected AAV-Net1 into the cochlea at the neonatal stage in Lgr5-EGFPCreERT2 mice, and compared with the control group at P16 the cochleae after injection of Net1 maintained the green fluorescence of EGFP, indicating that the overexpression of Net1 could increase the expression of Lgr5 in vivo and activate Wnt/β-catenin signaling. Also, Lgr5+ SCs showed a positive response to Net1 overexpression, manifested as a significant increase in cell proliferation capacity. These results suggested that Net1 could affect the proliferation of SCs by regulating Wnt/β-catenin signaling.
Inhibition of the Notch signaling pathway can promote the differentiation of HCs, and we indeed observed an inhibitory effect of Net1 on Notch signaling. Mfng, a negative regulator of Notch signaling [42, 43], was up-regulated when Net1 was overexpressed in vivo. It has been reported that Mfng is also critical for the boundary setting of the organ of Corti and for determination of cochlear sensory cell fate by regulating Notch signaling [44]. The inhibition of Notch triggered downstream Hes1 activation, and thus HC differentiation, which was in response to the forced expression of Net1 in this study. These results further confirmed that re-activation of Net1 in the cochlear SCs induces HC differentiation via Notch inhibition.p27kip1 is a member of the cyclin-dependent kinase (Cdk) family of inhibitors, which inhibit a variety of Cdk protein complexes, including cyclin D, E, and A, thereby inhibiting re-entry into the cell cycle [45]. Inner ear HC production is also regulated by Cip/Kip and Ink4 Cdk inhibitors. p27kip1 is a quiescent regulator of HCs and SCs [46], and mice carrying the defective form of p27kip1 produce ectopic HCs and SCs in the organ of Corti [46, 47]. Combining the depletion of p27kip1 with ectopic Atoh1 expression overcomes the age-related decline in SC plasticity and induces SC to HC transformation in adult mice [19]. In the present study, Net1 overexpression in the cochlea significantly decreased the expression of p27kip1, thus possibly promoting SC proliferation and indicating a potential role for Net1 in the conversion of SCs to HCs in the mature mouse cochlea with Wnt/β-catenin-activation and Notch inhibition.
Aminoglycosides are commonly used in the clinic against microbial infections, but adverse reactions in the use of aminoglycosides include ototoxicity, nephrotoxicity, and neuromuscular blockade [48, 49]. The ototoxicity of aminoglycosides can lead to hearing loss [50]. Here, we found that overexpression of Net1 in the cochlea results in the re-entry of SCs into the cell cycle and supports HC regeneration and survival. Activation of Wnt/β-catenin signaling in HCs is capable of protecting HCs against neomycin-induced HC loss. Due to the high efficiency of AAV-ie in HCs, the increased numbers of HCs in neomycin-treated cochleae transduced by AAV-Net1 were mainly derived from SC-to-HC transformation or the enhanced survival of HCs in response to Net1 overexpression. And, this needs to be further investigated in the future.
In summary, we report here that the exogenous activation of Net1 by AAV in neonatal mouse cochleae results in the reprogramming of HC progenitors. Considering the promising clinical applications of recombinational AAV, AAV-mediated gene regulation in HC production will be a good approach for future studies of HC regeneration in mammals. By combining Net1 regulation with modulation of signaling pathways that induce HC regeneration, like Notch signaling, AAV-mediated reprograming of SCs has great potential as a therapeutic strategy for the effective restoration of HCs.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
JQ and RC conceived and designed the experiments. LZ, YF, and FT performed most of the experiments. LZ contributed to the data analysis. FG completed the ABR test and data analyses of RNA sequencing. ZZ, NL, and QS helped with the experiments and the data analysis. JQ, LZ, YF, and FT discussed the data analysis, interpretation, and presentation and wrote the manuscript with contributions from all authors.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFA1101300, 2020YFA0113600, 2021YFA1101800 and 2020YFA0112503), the Strategic Priority Research Program of the Chinese Academy of Science (XDA16010303), the National Natural Science Foundation of China (82000984 , 92149304, 82030029 and 81970882), the China National Postdoctoral Program for Innovative Talents (BX20200082), the China Postdoctoral Science Foundation (2020M681468), the Science and Technology Department of Sichuan Province (2021YFS0371), the Shenzhen Fundamental Research Program (JCYJ20190814093401920 and JCYJ20210324125608022), and the Open Research Fund of State Key Laboratory of Genetic Engineering, Fudan University (SKLGE-2104).
Data and materials availability
All data associated with this study are present in the paper or the Supplementary Materials.
Declarations
Conflict of interest
Jieyu Qi had filed a patent on the use of AAV-ie for gene therapy in the inner ear. The authors declare no other competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liyan Zhang, Yuan Fang, Fangzhi Tan and Fangfang Guo contributed equally to this work.
Contributor Information
Jieyu Qi, Email: jieyuqi@seu.edu.cn.
Renjie Chai, Email: renjiec@seu.edu.cn.
References
- 1.Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7(11):837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- 2.Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18(19):7811–7821. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driver EC, Sillers L, Coate TM, et al. The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev Biol. 2013;376(1):86–98. doi: 10.1016/j.ydbio.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White PM, Doetzlhofer A, Lee YS, et al. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 5.Chai R, Kuo B, Wang T, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci USA. 2012;109(21):8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinkkonen ST, Chai R, Jan TA, et al. Intrinsic regenerative potential of murine cochlear supporting cells. Sci Rep. 2011;1:26. doi: 10.1038/srep00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson PJ, Dong Y, Gu S, et al. Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea. J Clin Invest. 2018;128(4):1641–1656. doi: 10.1172/JCI97248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng C, Guo L, Lu L, et al. Characterization of the transcriptomes of Lgr5+ hair cell progenitors and Lgr5– supporting cells in the mouse cochlea. Front Mol Neurosci. 2017;10:122. doi: 10.3389/fnmol.2017.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Guo L, Lu X, et al. Characterization of Lgr6+ cells as an enriched population of hair cell progenitors compared to Lgr5+ cells for hair cell generation in the neonatal mouse cochlea. Front Mol Neurosci. 2018;11:147. doi: 10.3389/fnmol.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox BC, Chai R, Lenoir A, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014;141(4):816–829. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Chai R, Kim GS, et al. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat Commun. 2015;6:6613. doi: 10.1038/ncomms7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Zhang Y, Yu P, et al. Characterization of Lgr5+ progenitor cell transcriptomes after neomycin injury in the neonatal mouse cochlea. Front Mol Neurosci. 2017;10:213. doi: 10.3389/fnmol.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bramhall NF, Shi F, Arnold K, et al. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports. 2014;2(3):311–322. doi: 10.1016/j.stemcr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schimmang T. Expression and functions of FGF ligands during early otic development. Int J Dev Biol. 2007;51(6–7):473–481. doi: 10.1387/ijdb.072334ts. [DOI] [PubMed] [Google Scholar]
- 15.Groves AK, Fekete DM. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139(2):245–257. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansson L, Kim GS, Cheng AG. Making sense of Wnt signaling-linking hair cell regeneration to development. Front Cell Neurosci. 2015;9:66. doi: 10.3389/fncel.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Żak M, Klis SF, Grolman W. The Wnt and Notch signalling pathways in the developing cochlea: formation of hair cells and induction of regenerative potential. Int J Dev Neurosci. 2015;47(Pt B):247–258. doi: 10.1016/j.ijdevneu.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Costa A, Powell LM, Lowell S, et al. Atoh1 in sensory hair cell development: constraints and cofactors. Semin Cell Dev Biol. 2017;65:60–68. doi: 10.1016/j.semcdb.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Walters BJ, Coak E, Dearman J, et al. In vivo interplay between p27(Kip1), GATA3, ATOH1, and POU4F3 converts non-sensory cells to hair cells in adult mice. Cell Rep. 2017;19(2):307–320. doi: 10.1016/j.celrep.2017.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo BR, Baldwin EM, Layman WS, et al. In vivo cochlear hair cell generation and survival by coactivation of β-Catenin and Atoh1. J Neurosci. 2015;35(30):10786–10798. doi: 10.1523/JNEUROSCI.0967-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi F, Hu L, Edge AS. Generation of hair cells in neonatal mice by β-catenin overexpression in Lgr5-positive cochlear progenitors. Proc Natl Acad Sci USA. 2013;110(34):13851–13856. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni W, Zeng S, Li W, et al. Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea. Oncotarget. 2016;7(41):66754–66768. doi: 10.18632/oncotarget.11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Wu J, Yang J, et al. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc Natl Acad Sci USA. 2015;112(1):166–171. doi: 10.1073/pnas.1415901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Li W, Lin C, et al. Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles. Sci Rep. 2016;6:29418. doi: 10.1038/srep29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan AM, Takai S, Yamada K, et al. Isolation of a novel oncogene, NET1, from neuroepithelioma cells by expression cDNA cloning. Oncogene. 1996;12(6):1259–1266. [PubMed] [Google Scholar]
- 26.Murray D, Horgan G, Macmathuna P, et al. NET1-mediated RhoA activation facilitates lysophosphatidic acid-induced cell migration and invasion in gastric cancer. Br J Cancer. 2008;99(8):1322–1329. doi: 10.1038/sj.bjc.6604688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. doi: 10.1042/bj3480241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 29.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6(2):167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 30.Zong W, Feng W, Jiang Y, et al. LncRNA CTC-497E21.4 promotes the progression of gastric cancer via modulating miR-22/NET1 axis through RhoA signaling pathway. Gastric Cancer. 2020;23(2):228–240. doi: 10.1007/s10120-019-00998-w. [DOI] [PubMed] [Google Scholar]
- 31.Leyden J, Murray D, Moss A, et al. Net1 and Myeov: computationally identified mediators of gastric cancer. Br J Cancer. 2006;94(8):1204–1212. doi: 10.1038/sj.bjc.6603054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider EH, Hofmeister O, Kälble S, et al. Apoptotic and anti-proliferative effect of guanosine and guanosine derivatives in HuT-78 T lymphoma cells. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(7):1251–1267. doi: 10.1007/s00210-020-01864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Xia P, Zhang W, et al. Short interfering RNA targeting Net1 reduces the angiogenesis and tumor growth of in vivo cervical squamous cell carcinoma through VEGF down-regulation. Hum Pathol. 2017;65:113–122. doi: 10.1016/j.humpath.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Miyakoshi A, Ueno N, Kinoshita N. Rho guanine nucleotide exchange factor xNET1 implicated in gastrulation movements during Xenopus development. Differentiation. 2004;72(1):48–55. doi: 10.1111/j.1432-0436.2004.07201004.x. [DOI] [PubMed] [Google Scholar]
- 35.Wei S, Dai M, Liu Z, et al. The guanine nucleotide exchange factor Net1 facilitates the specification of dorsal cell fates in zebrafish embryos by promoting maternal β-catenin activation. Cell Res. 2017;27(2):202–225. doi: 10.1038/cr.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan F, Chu C, Qi J, et al. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat Commun. 2019;10(1):3733. doi: 10.1038/s41467-019-11687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li XJ, Doetzlhofer A. LIN28B/let-7 control the ability of neonatal murine auditory supporting cells to generate hair cells through mTOR signaling. Proc Natl Acad Sci USA. 2020;117(36):22225–22236. doi: 10.1073/pnas.2000417117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Gu Y, Li Y, et al. Generation of mature and functional hair cells by co-expression of Gfi1, Pou4f3, and Atoh1 in the postnatal mouse cochlea. Cell Rep. 2021;35(3):109016. doi: 10.1016/j.celrep.2021.109016. [DOI] [PubMed] [Google Scholar]
- 39.Jacques BE, Puligilla C, Weichert RM, et al. A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development. 2012;139(23):4395–4404. doi: 10.1242/dev.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi F, Hu L, Jacques BE, et al. β-Catenin is required for hair-cell differentiation in the cochlea. J Neurosci. 2014;34(19):6470–6479. doi: 10.1523/JNEUROSCI.4305-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chai R, Xia A, Wang T, et al. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J Assoc Res Otolaryngol. 2011;12(4):455–469. doi: 10.1007/s10162-011-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veeraraghavalu K, Pett M, Kumar RV, et al. Papillomavirus-mediated neoplastic progression is associated with reciprocal changes in JAGGED1 and manic fringe expression linked to notch activation. J Virol. 2004;78(16):8687–8700. doi: 10.1128/JVI.78.16.8687-8700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golson ML, Le Lay J, Gao N, et al. Jagged1 is a competitive inhibitor of Notch signaling in the embryonic pancreas. Mech Dev. 2009;126(8–9):687–699. doi: 10.1016/j.mod.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basch ML, Brown 2nd RM, Jen HI et al (2016) Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. Elife 5 [DOI] [PMC free article] [PubMed]
- 45.Oesterle EC, Chien WM, Campbell S, et al. p27(Kip1) is required to maintain proliferative quiescence in the adult cochlea and pituitary. Cell Cycle. 2011;10(8):1237–1248. doi: 10.4161/cc.10.8.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126(8):1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 47.Löwenheim H, Furness DN, Kil J, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci USA. 1999;96(7):4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen TC, Albenberg L, Bittinger K, et al. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015;125(7):2841–2850. doi: 10.1172/JCI79214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldmann T, Overlack N, Möller F, et al. A comparative evaluation of NB30, NB54 and PTC124 in translational read-through efficacy for treatment of an USH1C nonsense mutation. EMBO Mol Med. 2012;4(11):1186–1199. doi: 10.1002/emmm.201201438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huth ME, Han KH, Sotoudeh K, et al. Designer aminoglycosides prevent cochlear hair cell loss and hearing loss. J Clin Invest. 2015;125(2):583–592. doi: 10.1172/JCI77424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landegger LD, Pan B, Askew C, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol. 2017;35(3):280–284. doi: 10.1038/nbt.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials.