Abstract
Background
Breast cancer is a lethal malignancy affecting females worldwide. It has been reported that upregulated centromere protein A (CENPA) expression might indicate unfortunate prognosis and can function as a prognostic biomarker in breast cancer. This study aimed to investigate the accurate roles and downstream mechanisms of CENPA in breast cancer progression.
Methods
CENPA protein levels in breast cancer tissues and cell lines were analyzed by Western blot and immunohistochemistry assays. We used gain/loss-of-function experiments to determine the potential effects of CENPA and phospholipase A2 receptor (PLA2R1) on breast cancer cell proliferation, migration, and apoptosis. Co-IP assay was employed to validate the possible interaction between CENPA and DNA methyltransferase 1 (DNMT1), as well as PLA2R1 and hematopoietically expressed homeobox (HHEX). PLA2R1 promoter methylation was determined using methylation-specific PCR assay. The biological capabilities of CENPA/PLA2R1/HHEX axis in breast cancer cells was determined by rescue experiments. In addition, CENPA-silenced MCF-7 cells were injected into mice, followed by measurement of tumor growth.
Results
CENPA level was prominently elevated in breast cancer tissues and cell lines. Interestingly, CENPA knockdown and PLA2R1 overexpression both restrained breast cancer cell proliferation and migration, and enhanced apoptosis. On the contrary, CENPA overexpression displayed the opposite results. Moreover, CENPA reduced PLA2R1 expression through promoting DNMT1-mediated PLA2R1 promoter methylation. PLA2R1 overexpression could effectively abrogate CENPA overexpression-mediated augment of breast cancer cell progression. Furthermore, PLA2R1 interacted with HHEX and promoted HHEX expression. PLA2R1 knockdown increased the rate of breast cancer cell proliferation and migration but restrained apoptosis, which was abrogated by HHEX overexpression. In addition, CENPA silencing suppressed tumor growth in vivo.
Conclusion
CENPA knockdown restrained breast cancer cell proliferation and migration and attenuated tumor growth in vivo through reducing PLA2R1 promoter methylation and increasing PLA2R1 and HHEX expression. We may provide a promising prognostic biomarker and novel therapeutic target for breast cancer.
Keywords: Breast cancer, CENPA, Biomarker, Environmental factor, PLA2R1, HHEX
Introduction
Breast cancer is a lethal malignancy affecting females worldwide [1]. Its incidence and mortality rates are increasing rapidly in recent decades [2]. Breast cancer exhibits an insidious clinical manifestation in the beginning phase, so that numerous patients are diagnosed at the advanced stage [3]. Traditionally, breast cancer in females has been assumed to be driven by genetic, environmental, and lifestyle factors. Environmental factors are thought to be responsible for roughly 70% of malignant tumors, whereas 90–95% of breast cancer cases fall into this category. Genetic variation and aberrant expression induced by environmental pollutants are particularly helpful for tumorigenesis, tumor metastasis, and drug resistance [4–6]. Although rapid development in the clinical therapeutic approaches for breast cancer has been achieved [7, 8], the curative efficiency for breast cancer remains unsatisfactory because of high postoperative recurrence and inevitable drug side effects. Thus, it is essential to explore comprehensive mechanistic insights into its pathogenesis and explore more effective treatment strategies.
Centromere protein A (CENPA) functions as a critical centromere protein. Centromere dysfunction can lead to aneuploidy, which is a well-established feature of human cancers [9], thus implying that CENPA may be functioned in the development of human cancers. Indeed, extensive prior studies have illustrated that abnormal CENPA expression was prominently related to malignant cancer progression and it has been identified as a prognostic indicator in human cancers. For instance, CENPA mRNA was significantly overexpressed in the tumor tissues of hepatocellular carcinoma, and thus it might act as an oncogenic molecule as well as predictive factor in hepatocellular carcinoma development [10]. An integrated bioinformatics analysis also demonstrated that high CENPA expression exhibited a worse prognosis in chromophobe renal cell carcinoma [11]. CENPA overexpression was found to promote renal cancer cell proliferation and metastasis, which may serve as an independent marker [12]. Moreover, it was previously reported that upregulated CENPA expression might be intimately related to the shorter disease-free survival in breast cancer, thereby indicating its potential to be a prognostic biomarker [13]. However, the biological capabilities of CENPA in breast cancer have not been illuminated in detail.
Phospholipase A2 receptor (PLA2R1) belongs to the mannose receptor family [14] and plays essential roles in different inflammatory and autoimmune diseases, such as idiopathic membranous nephropathy [15] and asthma [16]. In addition, recent studies have uncovered that PLA2R1 functions as a tumor suppressor gene by facilitating certain tumor suppressive responses, such as apoptosis, senescence, and cell transformation inhibition [14]. PLA2R1 was also found to induce DNA damage and repress aging-induced spontaneous tumor formation [17]. PLA2R1 expression was reported to be markedly decreased in renal cell carcinoma tissues, and PLA2R1 knockdown promoted the in vivo tumor growth [18]. More importantly, PLA2R1 downregulation and promoter hypermethylation were observed in different breast cancer subtypes, which were related to aggressive tumor subtypes and considered as a promising therapeutic target [19]. However, the biological capabilities of PLA2R1 in breast cancer have not been completely understood.
Our study confirmed that the potential biomarker, CENPA, was conspicuously upregulated in both breast cancer. Thereafter, we investigated the potential biological effects and downstream regulatory mechanisms of CENPA in breast cancer cell progression, with an aim to furnish a promising treatment strategy.
Materials and methods
Clinical samples
We recruited breast cancer patients aged 34–66 years N old ( = 30) at the First Affiliated Hospital of Jinzhou Medical University. We harvested breast cancer tissues and matched paracancerous tissues during surgery. Informed consent was signed by all patients, and the experiments were performed after review and approval by the ethics committee of the First Affiliated Hospital of Jinzhou Medical University.
Cell culture and transfection
Breast cancer cell lines (MDA-MB-231, MCF-7, BT-474, and BT-579) and control cell line MCF-10A were acquired from ATCC. The cells were cultured in DMEM (Solarbio, Beijing, China) containing 10% fetal bovine serum (FBS) in 5% CO2 at 37 °C. Gene overexpression vectors pcDNA-CENPA, pcDNA-PLA2R1, and pcDNA-HHEX, and short hairpin RNAs (sh-CENPA and sh-PLA2R1) were purchased from RiboBio (Guangzhou, China). When cell confluence reached approximately 70–80%, breast cancer cells were transfected with expression vector or shRNA (50 nM) utilizing Lipofectamine 3000 reagent (Invitrogen, USA) referred to the reported method [20].
Western blot assay
The various intracellular proteins were isolated by lysing RIPA solution. After separating on 10% SDS-PAGE, proteins (30 µg) were transferred to PVDF membranes, blocked with 5% nonfat milk, and then incubated with the following primary antibodies (Abcam, USA) overnight at 4 °C and secondary antibody for 2 h. ECL solution was used to visualize the protein signal. The protein level was analyzed by ImageJ 1.8.0 software and normalized with GAPDH. The various primary antibodies used were as follows: CENPA (1:1000, ab45694), PLA2R1 (1:1000, ab211573), HHEX (1:1000, ab34222), and GAPDH (1:2500, ab9485).
Immunohistochemistry (IHC) assay
Immunohistochemistry (IHC) staining was conducted according to the standard method previously [21]. The 4-μm tissue sections underwent microwave heating in 10 mM sodium citrate solution, and inactivated by 3% H2O2 for 10 min. Thereafter, the goat serum-blocked sections were incubated with the primary antibodies overnight at 4 °C and secondary antibody for 1 h at 37 °C. Finally, the section signals were stained with a diaminobenzidine chromogen (Dako North America, Carpinteria, USA). Images were obtained under a light microscope (Olympus, Tokyo, Japan) and analyzed by ImageJ 1.8.0 software.
Cell counting kit-8 (CCK-8) assay
CCK-8 proliferation assay was carried out using the standard method [22]. The cells (5 × 103, 200 µL/well) were first inoculated in 96-well plates at 37 °C in 5% CO2. 10 µL/well of CCK-8 solution (Dojindo, Japan) was added for 2 h incubation after 0, 24, 48, and 72 h of culture. The absorbance at 450 nm was measured using a microplate reader (Thermo Fisher, USA).
EdU assay
EdU proliferation assay was conducted as formerly reported [22]. Cancer cells (2 × 105 cells/well) were incubated with 50 μM EdU solution (Beyotime, China) for 2 h at 37 °C. Next, cells were fixed with 4% paraformaldehyde for 15 min and then incubated with click reaction solution (100 μL) for 30 min in the dark environment. Thereafter, cells were observed under a fluorescence microscopy (Olympus, Tokyo, Japan).
Transwell migration assay
A Transwell chamber (8-μm; Millipore Corporation, USA) was used for analysis of migration ability. 200 µL of breast cancer cells (5 × 104) suspended in DMEM were added to upper chamber. DMEM containing 10% FBS (600 µL) was placed at lower chamber. Thereafter, the migrating cells were stained after 48 h of culture, and subsequently imaged under a microscope. The images were analyzed using ImageJ 1.8.0.
Flow cytometry analysis
Flow cytometry was employed for cell apoptosis detection [23]. After indicated treatment, the cell suspension (4 × 105 cells/mL) was treated with 10 μL Annexin V-FITC and 5 μL PI for 15 min of staining in dark condition. Flow cytometry (BD Biosciences, NJ, USA) was employed for apoptosis evaluation.
RT-qPCR
Total RNA was extracted using Trizol reagent. The iScriptcDNA synthesis kit (Bio-Rad, USA) was used to obtain cDNAs. RT-qPCR was conducted using SYBR green SuperReal Premix Plus (Tiangen Biotech, Beijing, China) and ABI 7500 Fast Real-Time PCR System (Applied Biosystems, USA). PLA2R1 mRNA expression was calculated by 2−ΔΔCT method and normalized to GAPDH.
Co-immunoprecipitation (Co-IP) assay
Co-IP assay was utilized to assess protein interactions in reference to the reported approach [24]. MCF-7 cell lysates were incubated with various indicated antibodies for 30 min at 4 °C, and subsequently incubated with protein A/G beads (100 μL; Takara, Dalian, China) overnight at 4 °C. Finally, immunoprecipitated products were analyzed using the Western blot assay.
Methylation-specific PCR (MSP)
MSP assay was employed to assess gene methylation [25]. The genomic DNAs were isolated and bisulfite modified with an EZ DNA Methylation kit (Zymo Research, Beijing, China). MSP assay was performed using the EpiTect Methyl II DNA Restriction Kit (Qiagen, USA) and analyzed via RT-qPCR using SYBR green SuperReal Premix Plus.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was employed with reference to a reported method [26]. MCF-7 cells were fixed with 1% formaldehyde for 15 min to generate DNA–protein crosslink, followed by 0.125 M glycine inactivation. Thereafter, the cell lysates were sonicated with several pulses for 3 min to obtain 200–1000 bp chromatin fragments, which were then immunoprecipitated with anti-DNMT1 antibody and ChIP-grade protein A/G magnetic beads. The precipitated products were eluted and then determined by RT-qPCR assay.
In vivo studies
Female BALB/c nude mice (4–5 weeks old, 16–20 g) were obtained from the experimental animal center of Jinzhou Medical University. Mice were maintained in a standard SPF-grade experiment environment. Xenograft tumor model was established as previously reported [27]. MCF-7 cells transfected with sh-CENPA or sh-NC (1 × 106, 200 μL) were subcutaneously injected into the right axilla of mice (n = 8 per group). Tumor volume was recorded every 7 days. The mice were euthanized 28 days later, and we excised the complete tumors and recorded the weights. The Ethics Committee of the First Affiliated Hospital of Jinzhou Medical University gave its approval to all animal experiments.
Statistical analysis
SPSS 22.0 was utilized to perform the data analysis. Data from independent triplicate experiments were presented as mean ± standard error of mean (SEM). The sample size was n = 6 for cell experiments and n = 8 for animal experiments. Student’s t test was employed to compare differences between the two groups, and one-way analysis of variance (ANOVA) was for comparisons among groups. P < 0.05 was defined statistically significant.
Results
CENPA was substantially upregulated in breast cancer
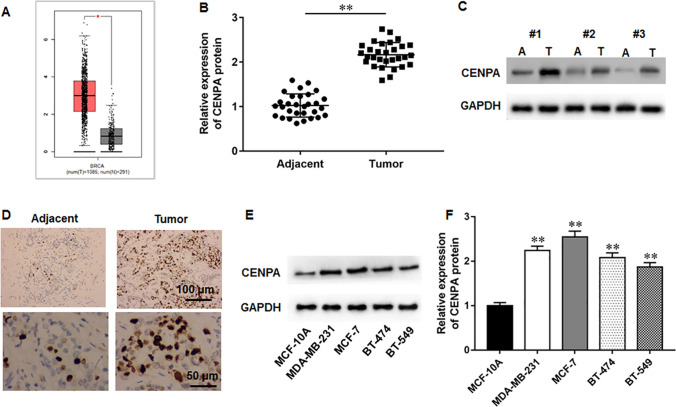
We found that CENPA was substantially upregulated in breast cancer via the GEPIA database (Fig. 1A). Subsequently, we evaluated CENPA protein level in the tumor tissues from the recruited breast cancer patients, and findings demonstrated that CENPA protein level was markedly elevated in tumor tissues (Fig. 1B–D). Besides, CENPA was also upregulated in breast cancer cell lines (Fig. 1E, F). Thus, we hypothesized that CENPA might participate in breast cancer progression.
Fig. 1.
CENPA level was substantially upregulated in breast cancer tissues and cell lines. A GEPIA database illustrated that CENPA was upregulated in breast cancer tissues. B, C CENPA protein levels in the tumor tissues and matched paracancerous tissues were analyzed by Western blot assay (N = 30). D CENPA protein levels in tumor tissues and matched paracancerous tissues were examined by immunohistochemistry assay. E, F CENPA protein levels in breast cancer cell lines were determined by Western blot assay (N = 6). The data have been presented as mean ± SEM. **P < 0.01
CENPA silencing restrained proliferation and migration and enhanced apoptosis in breast cancer cells
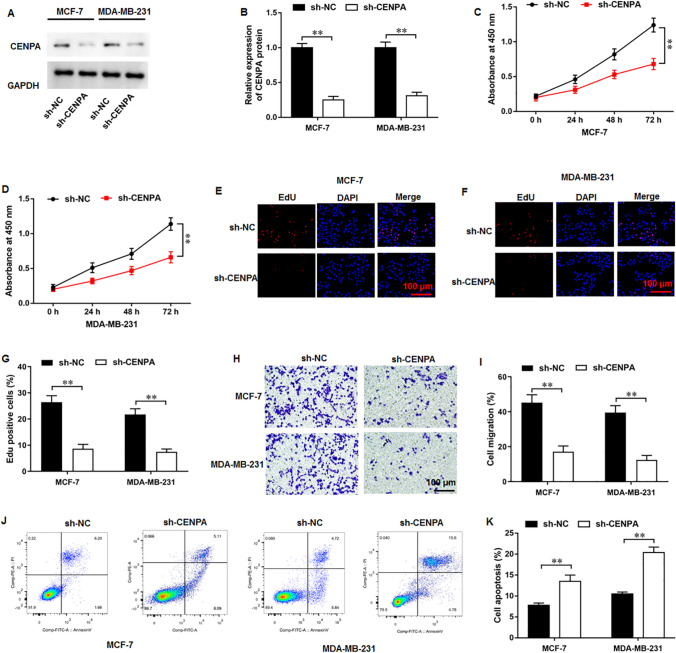
First, the biological functions of shRNA-mediated CEPNA silencing in breast cancer cells were evaluated. It was implicated that transfection of sh-CENPA reduced CENPA protein level in MCF-7 and MDA-MB-231 cells (Fig. 2A, B). Moreover, CENPA silencing remarkably restrained breast cancer cell proliferation (Fig. 2C, D). In addition, EdU assay showed the similar results (Fig. 2E–G). CENPA silencing inhibited breast cancer cell migration (Fig. 2H, I). Besides, it was illustrated that CENPA silencing could significantly augment cell apoptosis (Fig. 2J, K).
Fig. 2.
CENPA silencing inhibited proliferation and migration and augmented apoptosis in breast cancer cells. A, B CENPA protein level was analyzed by Western blot assay. C, D CCK-8 and E–G EdU assay were employed to test the cell proliferation. H, I Cell migration was measured using Transwell assay. J, K Cell apoptosis was examined with flow cytometry. N = 6. The data have been presented as mean ± SEM. **P < 0.01
CENPA overexpression augmented proliferation and migration and diminished apoptosis in breast cancer cells
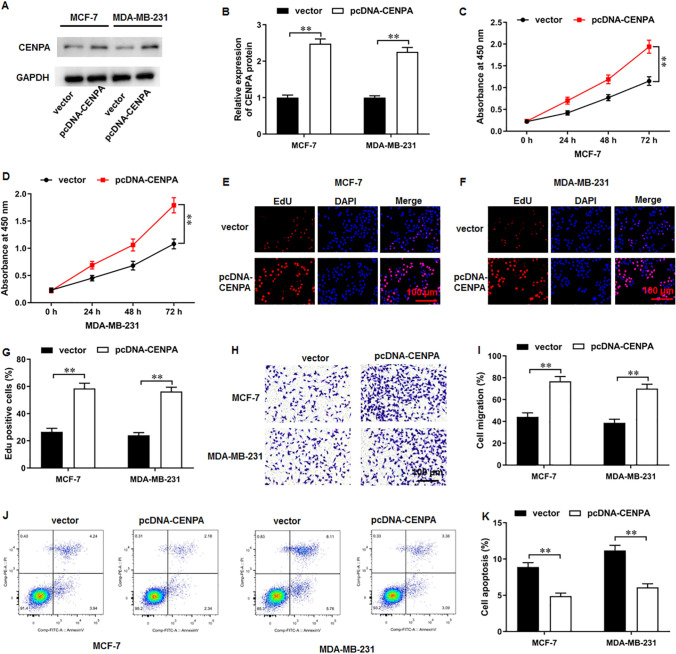
We then examined CEPNA overexpression-mediated possible effects on breast cancer cell behaviors. Western blot assay implicated that pcDNA-CENPA transfection substantially increased CENPA protein level (Fig. 3A, B). Thereafter, results of CCK-8 assay illustrated that CENPA overexpression obviously intensified breast cancer cell proliferation (Fig. 3C, D). EdU assay data also showed that CENPA overexpression facilitated cell proliferation (Fig. 3E–G). Furthermore, CENPA overexpression promoted cell migration (Fig. 3H, I). Moreover, we observed that CENPA overexpression diminished cell apoptosis (Fig. 3J, K).
Fig. 3.
CENPA overexpression intensified breast cancer cell proliferation and migration and diminished apoptosis. A, B CENPA protein level was examined by Western blot assay. C, D CCK-8 and E–G EdU assay were employed to test the cell proliferation. H, I Cell migration was measured using Transwell assay. J, K Cell apoptosis was examined with flow cytometry. N = 6. The data have been presented as mean ± SEM. **P < 0.01
CENPA inhibited PLA2R1 expression through facilitating PLA2R1 promoter methylation
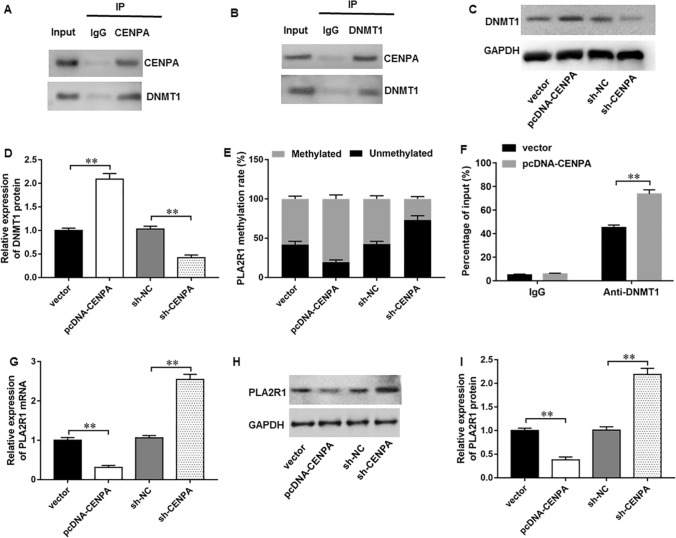
The BioGRID database (http://thebiogrid.org/) indicated that CENPA could potentially interact with DNA methyltransferase 1 (DNMT1). We found that DNMT1 protein could be immunoprecipitated by CENPA antibody (Fig. 4A). Meanwhile, CENPA protein could be immunoprecipitated by DNMT1 antibody (Fig. 4B), thus indicating that CENPA could directly interact with DNMT1 in MCF-7 cells. Moreover, CENPA overexpression facilitated DNMT1 expression, whereas CENPA silencing reduced DNMT1 expression in MCF-7 cells (Fig. 4C, D). PLA2R1 hypermethylation has been previously reported in breast cancer tissues [19]. MSP assay suggested that CENPA overexpression increased PLA2R1 methylation level, and CENPA silencing decreased PLA2R1 methylation level (Fig. 4E). ChIP assay verified that CENPA overexpression augmented DNMT1 enrichment in PLA2R1 promoter (Fig. 4F). In addition, CENPA overexpression could substantially decrease PLA2R1 expression (Fig. 4G–I) in breast cancer cells, whereas CENPA silencing displayed the opposite results.
Fig. 4.
CENPA inhibited PLA2R1 expression through facilitating PLA2R1 promoter methylation. A, B The potential interaction between CENPA and DNMT1 was validated by Co-IP assay. C, D PLA2R1 protein level was determined with Western blot assay. E MSP assay was employed to examine PLA2R1 methylation in MCF-7 cells. F ChIP assay was used to measure DNMT1 enrichment on PLA2R1 promoter. G–I PLA2R1 mRNA and protein levels were examined by RT-qPCR and Western blot analysis. N = 6. The data have been presented as mean ± SEM. **P < 0.01
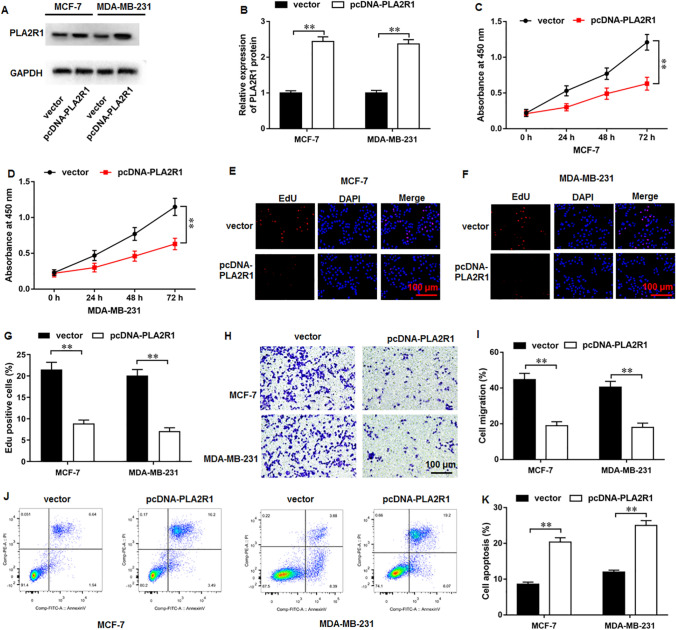
PLA2R1 overexpression restrained proliferation and migration and augmented apoptosis in breast cancer cells
Thereafter, the potential capabilities of PLA2R1 were analyzed. Western blot results indicated that pcDNA-PLA2R1 transfection facilitated PLA2R1 protein expression (Fig. 5A, B). Subsequently, results of CCK-8 (Fig. 5C, D) and EdU (Fig. 5E–G) assays demonstrated that PLA2R1 overexpression conspicuously suppressed breast cancer cell proliferation. Furthermore, PLA2R1 overexpression remarkably restrained cell migration (Fig. 5H, I). Besides, PLA2R1 overexpression could markedly augment cell apoptosis (Fig. 5J, K).
Fig. 5.
PLA2R1 overexpression inhibited proliferation and migration and augmented apoptosis in breast cancer cells. A, B PLA2R1 protein level was examined with Western blot assay. C, D CCK-8 assay and E–G EdU assay were employed to test MCF-7 cell proliferation. H, I MCF-7 cell migration was measured using Transwell assay. J, K MCF-7 cell apoptosis was determined with flow cytometry. N = 6. The data have been presented as mean ± SEM. **P < 0.01
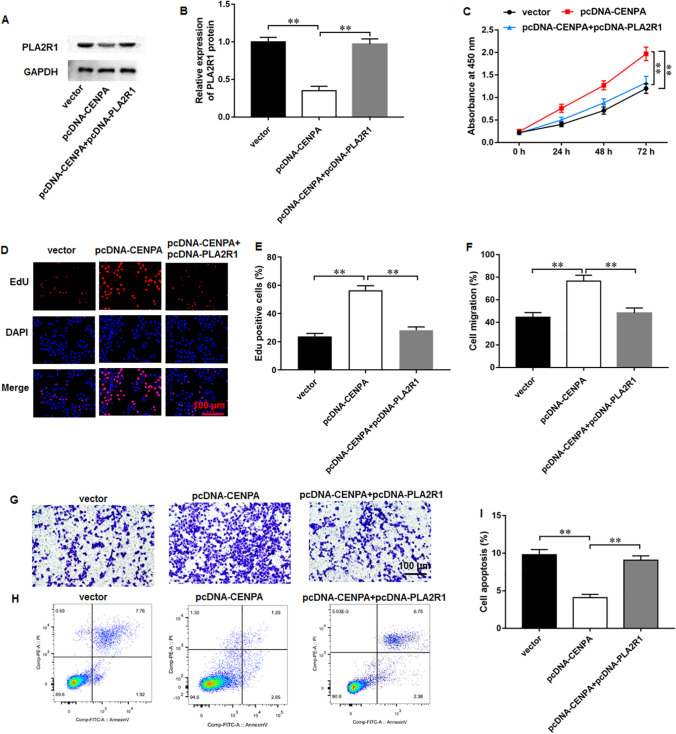
CENPA facilitated breast cancer development through inhibiting PLA2R1 expression
We performed rescue experiments to investigate the possible biological effects of CENPA and PLA2R1. Western blot results illustrated that the transfection of pcDNA-CENPA prominently downregulated PLA2R1 expression, whereas transfection of pcDNA-PLA2R1 increased PLA2R1 expression in MCF-7 cells (Fig. 6A, B). As reflected by CCK-8 (Fig. 6C) and EdU (Fig. 6D, E) results, CENPA overexpression enhanced proliferation in MCF-7 cells, and PLA2R1 overexpression abrogated these effects. Moreover, CENPA overexpression promoted migration, which was abolished by PLA2R1 overexpression (Fig. 6F, G). Besides, CENPA overexpression repressed cell apoptosis in MCF-7 cells, which was abrogated by PLA2R1 overexpression (Fig. 6H, I). We revealed that CENPA facilitated breast cancer progression by regulating PLA2R1 expression.
Fig. 6.
CENPA inhibited breast cancer progression by suppressing PLA2R1 expression. A, B Western blot assay was used to examine PLA2R1 protein level. C CCK-8 and D, E EdU assay were employed to test MCF-7 cell proliferation. F, G MCF-7 cell migration was measured using Transwell assay. H, I MCF-7 cell apoptosis was examined with flow cytometry. N = 6. The data have been presented as mean ± SEM. **P < 0.01
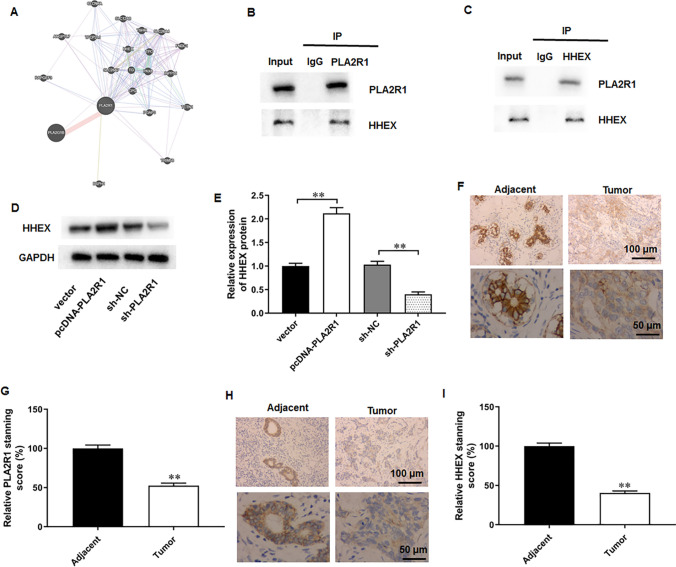
PLA2R1 interacted with HHEX in breast cancer cells
It was implicated that hematopoietically expressed homeobox (HHEX) was a potential breast cancer suppressor [28]. It was observed that HHEX was a potential interacting protein of PLA2R1 though the Genemania website (Fig. 7A). Thereafter, we performed Co-IP assay to explore their relationship in breast cancer cells. The results suggested that HHEX could be immunoprecipitated by PLA2R1 antibody (Fig. 7B), and PLA2R1 also could be immunoprecipitated by PLA2R1 antibody (Fig. 7C). The results indicated that PLA2R1 could directly interact with HHEX in breast cancer cells. Thereafter, it was observed that PLA2R1 overexpression remarkably promoted HHEX expression in MCF-7 cells, and PLA2R1 silencing restrained HHEX expression (Fig. 7D, E). In addition, we also found that PLA2R1 (Fig. 7F, G) and HHEX (Fig. 7H, I) levels were also downregulated in tumor tissues obtained from breast cancer patients (Fig. 7F–I).
Fig. 7.
PLA2R1 interacted with HHEX in breast cancer cells. A The potential interacting proteins of PLA2R1 were predicted from the Genemania website. B, C Co-IP assay was employed to validate the interaction between PLA2R1 and HHEX. D, E HHEX expression was tested by Western blot assay. F–I PLA2R1 and HHEX levels in the tumor tissues and matched paracancerous tissues were accessed with immunohistochemistry assay. N = 6. The data have been presented as mean ± SEM. **P < 0.01
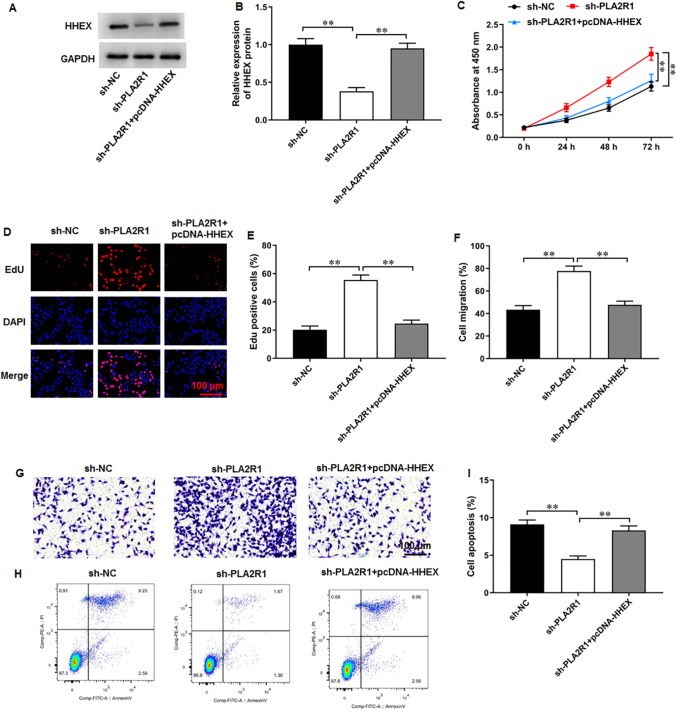
PLA2R1 knockdown facilitated breast cancer progression by inhibiting HHEX expression
MCF-7 cells were transfected with sh-PLA2R1 and pcDNA-HHEX. Western blot results demonstrated that sh-PLA2R1 transfection markedly inhibited HHEX expression, whereas pcDNA-HHEX transfection augmented HHEX expression (Fig. 8A, B). PLA2R1 knockdown augmented MCF-7 cell proliferation, and HHEX overexpression reversed this effect (Fig. 8C–E). Thereafter, PLA2R1 knockdown facilitated migration in MCF-7 cells (Fig. 8F, G), while HHEX overexpression abrogated this effect. Moreover, PLA2R1 knockdown decreased MCF-7 cell apoptosis, which was abolished by HHEX overexpression (Fig. 8H, I). Thus, it was concluded that PLA2R1 knockdown facilitated breast cancer progression by inhibiting HHEX expression.
Fig. 8.
PLA2R1 knockdown facilitated breast cancer progression by inhibiting HHEX expression. A, B HHEX protein level was evaluated by Western blot assay. C CCK-8 and D, E EdU assay were employed to test MCF-7 cell proliferation. F, G MCF-7 cell migration was measured using Transwell assay. H, I MCF-7 cell apoptosis was examined with flow cytometry. N = 6. The data have been presented as mean ± SEM. **P < 0.01
CENPA silencing suppressed tumor growth of breast cancer
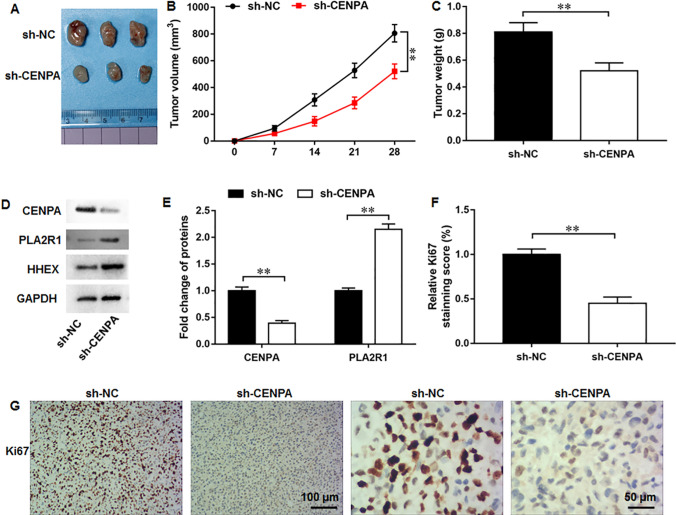
We further established a xenograft tumor mouse model to determine the in vivo function of CENPA. It was observed that CENPA silencing could markedly decrease the both tumor volume and weight (Fig. 9A–C). Moreover, compared with sh-NC group, CENPA protein level was downregulated and PLA2R1 and HHEX protein levels were upregulated in tumor tissues of sh-CENPA group (Fig. 9D, E). IHC staining demonstrated that CENPA silencing markedly decreased the protein level of proliferation marker Ki67 (Fig. 9F, G). Collectively, these results revealed that CENPA silencing could significantly suppress breast cancer tumor growth under in vivo settings. We summed our results in a graphical abstract (Fig. 10).
Fig. 9.
CENPA silencing suppressed tumor growth under in vivo settings. A Tumor images in sh-NC and sh-CENPA groups. B Tumor volume and C tumor weight were analyzed. D, E CENPA and PLA2R1 protein levels were examined by Western blot assay. F, G Ki67 protein level was determined using IHC staining. N = 8. The data have been presented as mean ± SEM. **P < 0.01
Fig. 10.
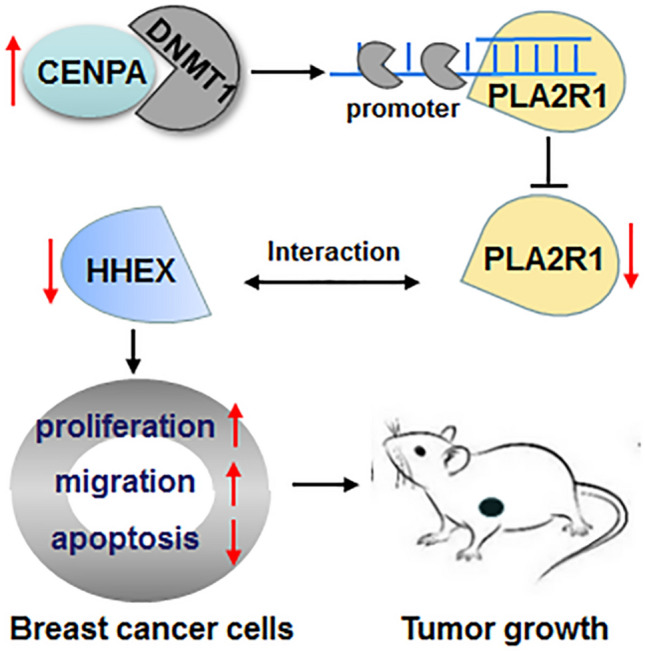
Graphical abstract. CENPA inhibited PLA2R1 expression by promoting DNMT1-mediated promoter methylation. PLA2R1 interacted with HHEX in breast cancer cells. The upregulated CENPA promoted breast cancer progression through inhibiting the PLA2R1/HHEX axis
Discussion
It has been generally recognized that breast cancer, specifically metastatic breast, is a common malignant cancer among women characterized by increasing morbidity and mortality, as well as poor prognosis. The vast majority of breast cancer patients continue to exhibit high risk of recurrence, metastasis, drug resistance, and serious side effects, thereby hampering therapeutic efficacy and prognosis [29]. Especially, genetic variation and aberrant expression induced by environmental factors contribute to tumor metastasis and drug resistance. Environmental factors include ionizing radiation, chemical pollutants, hormonal therapy, alcohol, as well as other dietary factors [30]. It was reported that the industrial organic pollutants, polychlorinated biphenyls, enhanced breast cancer migration and the development of bone, lung, and liver metastasis in mice by activating Rho-associated kinase [31]. Moreover, chemical phthalates were found to reduce the sensitivity to tamoxifen, which suggests a role in chemotherapeutic drug resistance [31]. A plethora of genetic alterations and multitude of molecular events are involved in breast cancer initiation and progression. Thus, in-depth studies to clarify the pathological mechanisms in breast cancer are still needed.
Accumulating studies have suggested that CENPA might act as a potential oncogenic molecule as well as prognostic factor in several human malignant cancers and abnormal CENPA expression could significantly promote cancer development [10–12]. Silico analysis found that CENPA was upregulated in 20 types of solid cancer, and increased CENPA expression is involved in cancer progression and indicated poor outcome for patients [32]. More notably, current evidence has demonstrated that IHC expression of CENPA is upregulated in malignant breast tissues compared with normal tissues, and upregulated CENPA expression was conspicuously correlated with shorter disease-free survival in breast cancer, thus indicating its potential as a potential prognostic biomarker [13]. Consistently, our Western blot and IHC assays illustrated that CENPA was substantially upregulated in breast cancer tissues. Moreover, it was previously reported that elevated CENPA level could impact chemotherapy response and lead to poor prognosis in breast cancer patients [33]. An integrated expression profiling analysis identified that high CENPA level in triple-negative breast cancer was correlated with poor prognosis, thereby suggesting its potential clinical therapeutic applications [34]. In addition, a recent study revealed the upstream regulatory genes of CENPA, which suggested that lncRNA MBNL1-AS1/ MBNL1-AS1/ZFP36 axis suppressed proliferation and cancer stem-like properties of breast cancer cells through reducing CENPA mRNA stability [35]. Given the momentous roles of CENPA in breast cancer, our study investigated the accurate functions and downstream pathways of CENPA in breast cancer progression. We found that CENPA silencing restrained proliferation and migration, and enhanced apoptosis in breast cancer cells. Interestingly, CENPA silencing retarded the tumor growth of breast cancer in xenograft mouse model. Mechanistically, CENPA inhibited the PLA2R1/HHEX axis in breast cancer cells.
Low PLA2R1 expression has been identified in some malignant tumors and it was found to suppress tumor growth by facilitating cancer cell apoptosis and inhibiting transformation. It was suggested that PLA2R1 expression was downregulated in the thyroid cancer samples, and PLA2R1 overexpression repressed proliferation and migration in thyroid cancer cells [36]. Moreover, PLA2R1 expression was decreased in tissues of renal cell carcinoma, and PLA2R1 knockdown increased in vivo renal cancer cell growth [18]. In addition, recent discoveries have revealed that PLA2R1 promoter hypermethylation-induced gene expression suppression could be observed in several cancer cells, including thyroid cancer [37], leukemia [38], and renal cell carcinoma [18]. Similarly, PLA2R1 downregulation and promoter hypermethylation were observed in breast cancer, and it was related to aggressive subtypes and might be a diagnostic target in breast cancer [19]. Moreover, it was also reported that high DNA-methylation-induced PLA2R1 expression reduction were found in several breast cancer cells [39]. Importantly, PLA2R1 promoter hypermethylation was coupled to the upregulation of DNMT1 [39]. Consistently, our study confirmed the PLA2R1 promoter hypermethylation in breast cancer cells. Furthermore, our results illustrated that CENPA inhibited PLA2R1 expression through directly facilitating DNMT1-mediated PLA2R1 promoter methylation. Besides, PLA2R1 overexpression inhibited proliferation and migration, and enhanced cell apoptosis in breast cancer cells. PLA2R1 overexpression reversed the oncogenic effects of CENPA overexpression on breast cancer cell progression.
HHEX, also known as PRH, has been reported to play crucial roles in cancer progression. For example, Li et al. reported that HHEX could significantly suppress cell migration and cell protrusion formation in human lung cancer cells [40]. Moreover, HHEX was identified to be downregulated in thyroid cancer. HHEX knockdown could effectively enhance cell proliferation, migration, and invasion, whereas HHEX overexpression exhibited opposite effects [41]. More notably, it was recently proposed that HHEX levels were downregulated in breast cancer patients, and decreased HHEX expression indicated poor survival in breast cancer patients [42]. Moreover, the author revealed that HHEX overexpression substantially decreased proliferation, migration, and invasion in breast cancer cells [42]. A mechanism research demonstrated that HHEX inhibited cancer cell migration and invasion partly by directly transcriptional regulating Endoglin [43]. Besides, an in vivo study suggested that HHEX overexpression inhibited the breast tumor formation in mice [28]. Similarly, our study confirmed that HHEX was downregulated in the tumor tissues of breast cancers. Mechanistically, HHEX could directly interact with PLA2R1 and its expression was primarily regulated by PLA2R1. HHEX overexpression abrogated PLA2R1 knockdown-mediated promotion effects on breast cancer progression. Thus, our results revealed that CENPA knockdown markedly modulated breast cancer progression through the PLA2R1/HHEX axis.
This study still has some important potential limitations. We accessed to a limited sample size for breast cancer patients, which may have affected the generalizability of our results. Moreover, due to time and funding constraints, we have insufficient experimental sample size for statistical measurements, which may impact the accuracy of our results. We only explored the potential mechanism of CENPA regulating the PLA2R1/HHEX axis, and there may be other CENPA-related molecular mechanisms in breast cancer progression. Therefore, in our future work, we will expand the sample size of experiments and strive to eliminate potential bias factors to get valid research results. Moreover, we intend to investigate other potential molecular mechanisms of CENPA-mediated breast cancer progression.
In conclusion, our findings illustrated that CENPA level was upregulated in breast cancer. CENPA silencing substantially restrained proliferation and migration, and enhanced apoptosis in breast cancer cells, and retarded tumor growth in breast cancer xenograft mouse model. Mechanistically, CENPA inhibited PLA2R1 expression by promoting DNMT1-mediated promoter methylation. PLA2R1 interacted with HHEX and augmented HHEX expression in breast cancer cells. We revealed that upregulated CENPA promoted breast cancer progression through inhibiting the PLA2R1/HHEX axis. We may provide a promising prognostic marker and novel therapeutic target for breast cancer. Targeting the potential prognostic marker, reagents and methods for early diagnosis of tumors can be developed to improve the early detection rate of tumors. In addition, our research provides theoretical basis for clinical breast cancer treatment and new drug development.
Abbreviations
- CENPA
Centromere protein A
- PLA2R1
Phospholipase A2 receptor
- DNMT1
DNA methyltransferase 1
- HHEX
Hematopoietically expressed homeobox
- Co-IP
Co-immunoprecipitation
- MSP
Methylation-specific PCR
Author contributions
GW and XL were responsible for research design. ZF and XL were responsible for conducting the experiments. GW and ZF were responsible for data acquisition and data analysis. The first draft of the manuscript was written by XL and all authors commented on previous versions of the manuscript.
Funding
This work was supported by Liaoning Provincial Department of Science and Technology (No.2021-MS-331), Liaoning Province College Students Innovation, and Entrepreneurship Training Program (No. S202110160024).
Data availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This study was approved by the Ethnic Committee of the First Affiliated Hospital of Jinzhou Medical University, and was conducted in accordance to the tenets of the Declaration of Helsinki. All animal care and experimental procedures were approved by the Ethics Committee of Jinzhou Medical University.
Consent to participate
All patients had read and signed the informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
$ Zhongkai Fan and Xin Li contributed equally to this work.
Contributor Information
Zhongkai Fan, Email: FZK0506@163.com.
Xin Li, Email: lix@jzmu.edu.cn.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Guo L, Jing Y. Construction and identification of a novel 5-gene signature for predicting the prognosis in breast cancer. Front Med (Lausanne) 2021;8:669931. doi: 10.3389/fmed.2021.669931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Z, Chen Z, Tan M, Elingarami S, Liu Y, Li T, Deng Y, He N, Li S, Fu J, Li W. A review on methods for diagnosis of breast cancer cells and tissues. Cell Prolif. 2020;53:e12822. doi: 10.1111/cpr.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Li L, Wang S, Wang Z, Qu L, Wang C, Xu K. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine. 2023;118:154940. doi: 10.1016/j.phymed.2023.154940. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Van der Jeught K, Zhou Z, Zhang L, Yu T, Sun Y, Li Y, Wan C, So KM, Liu D, Frieden M, Fang Y, Mosley AL, He X, Zhang X, Sandusky GE, Liu Y, Meroueh SO, Zhang C, Wijeratne AB, Huang C, Ji G, Lu X. Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J Clin Invest. 2021 doi: 10.1172/jci146832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan L, Feng F, Wu J, Fan S, Han J, Wang S, Yang L, Liu W, Wang C, Xu K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol Res. 2022;181:106270. doi: 10.1016/j.phrs.2022.106270. [DOI] [PubMed] [Google Scholar]
- 7.Lou JS, Zhao LP, Huang ZH, Chen XY, Xu JT, Tai WC, Tsim KWK, Chen YT, Xie T. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine. 2021;80:153370. doi: 10.1016/j.phymed.2020.153370. [DOI] [PubMed] [Google Scholar]
- 8.Sun Q, Tao Q, Ming T, Tang S, Zhao H, Liu M, Yang H, Ren S, Lei J, Liang Y, Peng Y, Wang M, Xu H. Berberine is a suppressor of Hedgehog signaling cascade in colorectal cancer. Phytomedicine. 2023;114:154792. doi: 10.1016/j.phymed.2023.154792. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Lai M, Wang Y, Chai R, Li N, Ou L, Zheng K, Li J, Xu G, Wang S, Dong Y, Wang S. Upregulation of centromere proteins as potential biomarkers for esophageal squamous cell carcinoma diagnosis and prognosis. Biomed Res Int. 2022;2022:3758731. doi: 10.1155/2022/3758731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Yang L, Shi J, Lu Y, Chen X, Yang Z. The oncogenic role of CENPA in hepatocellular carcinoma development: evidence from bioinformatic analysis. Biomed Res Int. 2020;2020:3040839. doi: 10.1155/2020/3040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Xu Y, Zhang J, Wu J. Identification and analysis of novel biomarkers involved in chromophobe renal cell carcinoma by integrated bioinformatics analyses. Biomed Res Int. 2020;2020:2671281. doi: 10.1155/2020/2671281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Xu J, Xiong Z, Xu T, Liu J, Liu Y, Chen J, Shi J, Shou Y, Yue C, Liu D, Liang H, Yang H, Yang X, Zhang X. CENPA promotes clear cell renal cell carcinoma progression and metastasis via Wnt/β-catenin signaling pathway. J Transl Med. 2021;19:417. doi: 10.1186/s12967-021-03087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajput AB, Hu N, Varma S, Chen CH, Ding K, Park PC, Chapman JA, Sengupta SK, Madarnas Y, Elliott BE, Feilotter HE. Immunohistochemical assessment of expression of centromere protein-A (CENPA) in human invasive breast cancer. Cancers (Basel) 2011;3:4212–4227. doi: 10.3390/cancers3044212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard D, Vindrieux D. PLA2R1: expression and function in cancer. Biochim Biophys Acta. 2014;1846:40–44. doi: 10.1016/j.bbcan.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Gao C, Liu Z, Dai H, Feng Z, Dong Z, Zheng Y, Gao Y, Tian X, Liu B. Idiopathic membranous nephropathy: glomerular pathological pattern caused by extrarenal immunity activity. Front Immunol. 2020;11:1846. doi: 10.3389/fimmu.2020.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolin JD, Ogden HL, Lai Y, Altemeier WA, Frevert CW, Bollinger JG, Naika GS, Kicic A, Stick SM, Lambeau G, Henderson WR, Jr, Gelb MH, Hallstrand TS. Identification of epithelial phospholipase A(2) receptor 1 as a potential target in asthma. Am J Respir Cell Mol Biol. 2016;55:825–836. doi: 10.1165/rcmb.2015-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huna A, Griveau A, Vindrieux D, Jaber S, Flaman JM, Goehrig D, Azzi L, Médard JJ, Djebali S, Hernandez-Vargas H, Dante R, Payen L, Marvel J, Bertolino P, Aubert S, Dubus P, Bernard D. PLA2R1 promotes DNA damage and inhibits spontaneous tumor formation during aging. Cell Death Dis. 2021;12:190. doi: 10.1038/s41419-021-03468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vindrieux D, Devailly G, Augert A, Le Calvé B, Ferrand M, Pigny P, Payen L, Lambeau G, Perrais M, Aubert S, Simonnet H, Dante R, Bernard D. Repression of PLA2R1 by c-MYC and HIF-2alpha promotes cancer growth. Oncotarget. 2014;5:1004–1013. doi: 10.18632/oncotarget.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitwally N, Yousef E, Abd Al Aziz A, Taha M. Clinical significance of expression changes and promoter methylation of PLA2R1 in tissues of breast cancer patients. Int J Mol Sci. 2020 doi: 10.3390/ijms21155453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Yuan D, Zhu D, Xu T, Huang A, Jiang L, Liu C, Qian H, Bu X. LncRNA-MSC-AS1 inhibits the ovarian cancer progression by targeting miR-425-5p. J Ovarian Res. 2021;14:109. doi: 10.1186/s13048-021-00857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Hu Z, Li J, Hu T. EZH2 exacerbates breast cancer by methylating and activating STAT3 directly. J Cancer. 2021;12:5220–5230. doi: 10.7150/jca.50675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Zheng F, Zhang L, Huang Z, Huang X, Pan Z, Chen S, Xu C, Jiang Y, Gu S, Zhao C, Zhang Q, Shi G. LncRNA HOTAIR-mediated MTHFR methylation inhibits 5-fluorouracil sensitivity in esophageal cancer cells. J Exp Clin Cancer Res. 2020;39:131. doi: 10.1186/s13046-020-01610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao B, Chen S, Li Y, Yang Z, Yang Y, Deng X, Ke S. LncRNA BLACAT1 promotes proliferation, migration and invasion of prostate cancer cells via regulating miR-29a-3p/DVL3 axis. Technol Cancer Res Treat. 2021;20:1533033820972342. doi: 10.1177/1533033820972342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang K, Zhang H, Wang F, Gao S, Sun C. HSPA8 Is identified as a novel regulator of hypertensive disorders in pregnancy by modulating the β-arrestin1/A1AR Axis. Reprod Sci. 2022;29:564–577. doi: 10.1007/s43032-021-00719-8. [DOI] [PubMed] [Google Scholar]
- 25.Lu C, Wei Y, Wang X, Zhang Z, Yin J, Li W, Chen L, Lyu X, Shi Z, Yan W, You Y. DNA-methylation-mediated activating of lncRNA SNHG12 promotes temozolomide resistance in glioblastoma. Mol Cancer. 2020;19:28. doi: 10.1186/s12943-020-1137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Li R, Wang C, Zhou S, Fan Y, Ma S, Gao D, Gai N, Yang J. Pinocembrin inhibits the proliferation and metastasis of breast cancer via suppression of the PI3K/AKT signaling pathway. Front Oncol. 2021;11:661184. doi: 10.3389/fonc.2021.661184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao S, Chen Q, Lin C, Dong H. Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway. J Exp Clin Cancer Res. 2020;39:191. doi: 10.1186/s13046-020-01676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kershaw RM, Roberts D, Wragg J, Shaaban AM, Humphreys E, Halsall J, Price L, Bicknell R, Gaston K, Jayaraman PS. Proline-rich homeodomain protein (PRH/HHEX) is a suppressor of breast tumour growth. Oncogenesis. 2017;6:e346. doi: 10.1038/oncsis.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gote V, Nookala AR, Bolla PK, Pal D. Drug resistance in metastatic breast cancer: tumor targeted nanomedicine to the rescue. Int J Mol Sci. 2021 doi: 10.3390/ijms22094673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolak A, Kamińska M, Sygit K, Budny A, Surdyka D, Kukiełka-Budny B, Burdan F. Primary and secondary prevention of breast cancer. Ann Agric Environ Med. 2017;24:549–553. doi: 10.26444/aaem/75943. [DOI] [PubMed] [Google Scholar]
- 31.Koual M, Tomkiewicz C, Cano-Sancho G, Antignac JP, Bats AS, Coumoul X. Environmental chemicals, breast cancer progression and drug resistance. Environ Health. 2020;19:117. doi: 10.1186/s12940-020-00670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X, Clermont PL, Jiao W, Helgason CD, Gout PW, Wang Y, Qu S. Elevated expression of the centromere protein-A(CENP-A)-encoding gene as a prognostic and predictive biomarker in human cancers. Int J Cancer. 2016;139:899–907. doi: 10.1002/ijc.30133. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Xie Y, Tian T, Yang Q, Zhou Y, Qiu J, Xu L, Wen N, Lv Q, Du Z. High expression levels of centromere protein A plus upregulation of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling pathway affect chemotherapy response and prognosis in patients with breast cancer. Oncol Lett. 2021;21:410. doi: 10.3892/ol.2021.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C, Han Y, Huang H, Min L, Qu L, Shou C. Integrated analysis of expression profiling data identifies three genes in correlation with poor prognosis of triple-negative breast cancer. Int J Oncol. 2014;44:2025–2033. doi: 10.3892/ijo.2014.2352. [DOI] [PubMed] [Google Scholar]
- 35.Ding Y, Li Y, Duan Y, Wang W, Zheng W, Cheng W, Qi Y, Feng J, Chen Z, Yu T, Hu A, Wang T, Li M, Zhang H, Li Y, Ma F, Guo B. LncRNA MBNL1-AS1 represses proliferation and cancer stem-like properties of breast cancer through MBNL1-AS1/ZFP36/CENPA axis. J Oncol. 2022;2022:9999343. doi: 10.1155/2022/9999343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng H, Zhang M, Gao D, Zhang X, Cai H, Cui Z, Gao Y, Lv Z. PLA2R1 inhibits differentiated thyroid cancer proliferation and migration via the FN1-mediated ITGB1/FAK axis. Cancers (Basel) 2023 doi: 10.3390/cancers15102720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Q, Yang H, Fan G, Zhang B, Yu J, Zhang Z, Jia G. Clinical importance of PLA2R1 and RASSF9 in thyroid cancer and their inhibitory roles on the Wnt/β-catenin pathway and thyroid cancer cell malignant behaviors. Pathol Res Pract. 2022;238:154092. doi: 10.1016/j.prp.2022.154092. [DOI] [PubMed] [Google Scholar]
- 38.Friedemann M, Gutewort K, Thiem D, Nacke B, Jandeck C, Lange BS, Sukocheva O, Suttorp M, Menschikowski M. Methylation of the phospholipase A2 receptor 1 promoter region in childhood B cell acute lymphoblastic leukaemia. Sci Rep. 2020;10:9058. doi: 10.1038/s41598-020-65825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menschikowski M, Hagelgans A, Nacke B, Jandeck C, Sukocheva O, Siegert G. Epigenetic control of phospholipase A2 receptor expression in mammary cancer cells. BMC Cancer. 2015;15:971. doi: 10.1186/s12885-015-1937-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Ma G, Guo W, Mu N, Wang Y, Liu X, Su L. Hhex inhibits cell migration via regulating RHOA/CDC42-CFL1 axis in human lung cancer cells. Cell Commun Signal. 2021;19:80. doi: 10.1186/s12964-021-00763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Z, Feng Y, Yan Y, Jin H, Chen Y, Han Y, Huang S, Feng F, Fu H, Yin Y, Huang Y, Wang H, Cheng W. HHEX suppresses advanced thyroid cancer by interacting with TLE3. Mol Cell Endocrinol. 2023;574:111988. doi: 10.1016/j.mce.2023.111988. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K, Zhao Q, Li Z, Fu F, Zhang H, Fu J, Zheng M, Zhang S. Clinicopathological significances of cancer stem cell-associated HHEX expression in breast cancer. Front Cell Dev Biol. 2020;8:605744. doi: 10.3389/fcell.2020.605744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kershaw RM, Siddiqui YH, Roberts D, Jayaraman PS, Gaston K. PRH/HHex inhibits the migration of breast and prostate epithelial cells through direct transcriptional regulation of endoglin. Oncogene. 2014;33:5592–5600. doi: 10.1038/onc.2013.496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.