Abstract
The innate immune response constitutes the first line of defense against pathogens. It involves the recognition of pathogen-associated molecular patterns (PAMPs) by pathogen recognition receptors (PRRs), the production of inflammatory cytokines and the recruitment of immune cells to infection sites. Recently, ADP-heptose, a soluble intermediate of the lipopolysaccharide biosynthetic pathway in Gram-negative bacteria, has been identified by several research groups as a PAMP. Here, we recapitulate the evidence that led to this identification and discuss the controversy over the immunogenic properties of heptose 1,7-bisphosphate (HBP), another bacterial heptose previously defined as an activator of innate immunity. Then, we describe the mechanism of ADP-heptose sensing by alpha-protein kinase 1 (ALPK1) and its downstream signaling pathway that involves the proteins TIFA and TRAF6 and induces the activation of NF-κB and the secretion of inflammatory cytokines. Finally, we discuss possible delivery mechanisms of ADP-heptose in cells during infection, and propose new lines of thinking to further explore the roles of the ADP-heptose/ALPK1/TIFA axis in infections and its potential implication in the control of intestinal homeostasis.
Keywords: Innate immunity, PAMP, ADP-heptose, Bacterial infection, ALPK1, TIFA
Introduction
Throughout life, complex organisms are constantly exposed to pathogens present in the environment such as bacteria, viruses and fungi. To deal with this threat, they have developed molecular mechanisms to discriminate self and non-self and built integrated defense strategies. Innate immunity constitutes the first line of defense. It is based on the recognition of microbial-specific molecules referred to as pathogen-associated molecular patterns (PAMPs) by evolutionarily conserved, germline-encoded host sensors called pathogen recognition receptors (PRRs). The latter are expressed on innate immune cells such as macrophages, neutrophils and dendritic cells, but also on epithelial and endothelial cells that function as sentinels. Mechanisms of pathogen recognition elicit the secretion of a broad range of immune effectors such as cytokines, chemokines and antimicrobial peptides. They also induce the cell surface expression of adhesion receptors that facilitate the recruitment of immune cells to infection sites, and activate the inflammasomes involved in the proteolytic cleavage, maturation and secretion of pro-inflammatory cytokines interleukin-1β and interleukin-18, and in the induction of pyroptosis. In addition to launching the first line of defense against microbes, PAMP recognition contributes to the initiation of adaptive immunity, which provides long-lasting protection against pathogens and immunological memory.
To date, different families of PRRs and their cognate PAMPs have been identified and extensively reviewed [1–4]. For instance, toll-like receptors (TLRs) can sense a variety of microbial molecular traits including lipopolysaccharide (LPS) [5], flagellin [6], lipoproteins [7, 8], or bacterial [9] and viral DNA [10]. NOD1 and NOD2, two proteins of the NOD-like receptor (NLR) family, recognize the peptidoglycan motifs meso-diaminopimelic acid [11, 12] or muramyl-dipeptide [13, 14], respectively. RIG-I-like receptors (RLRs) sense the presence of viral RNA [15], whereas C-type-lectin receptors (CLRs) detect carbohydrate structures [16, 17].
PAMPs can thus be of different nature including proteins, lipids, nucleic acids and carbohydrates and are well conserved across species since they play essential roles in microbial physiology [18]. Recently, ADP-heptose, a soluble intermediate of the LPS biosynthetic pathway, was identified as a bacterial PAMP by three independent research groups [19–21]. The purpose of this review is to describe the strategies that led to the identification of ADP-heptose as a PAMP and to recapitulate current knowledge about the molecular mechanism of its cellular detection in bacterial infections.
The LPS biosynthesis intermediate ADP-heptose is a bacterial PAMP
LPS: structure and immunogenic properties
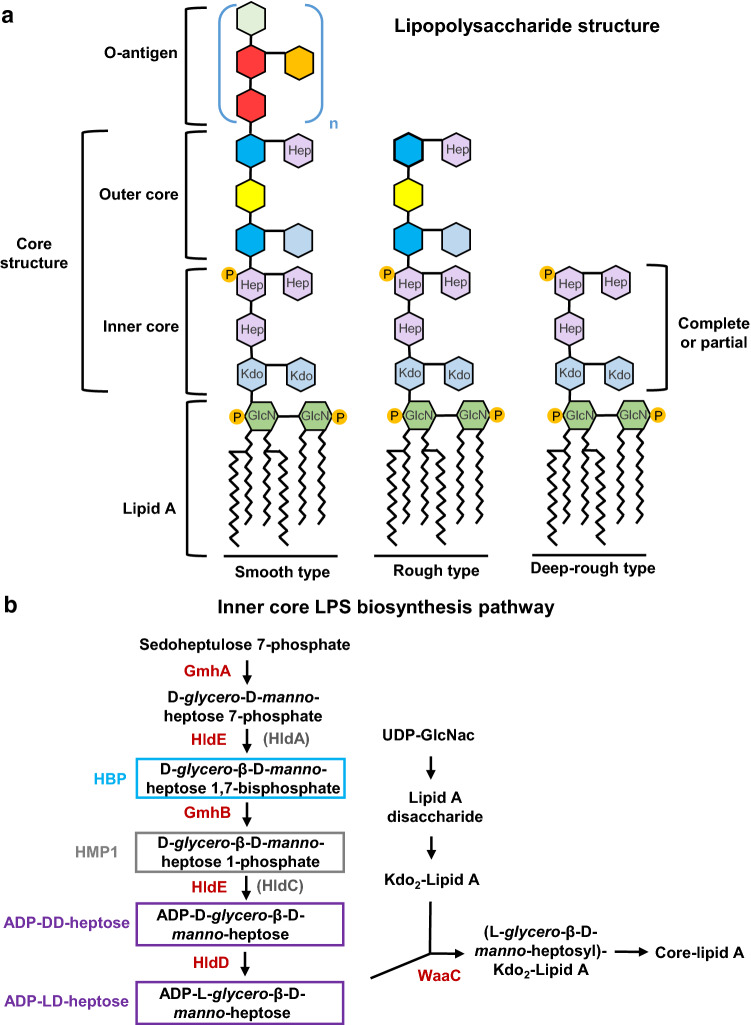
In most Gram-negative bacteria, LPS is a major constituent of the external leaflet of the outer membrane. It has been extensively studied for its immunogenic properties during bacterial infections. It is typically composed of three main regions: the lipid A, which is the hydrophobic part of the LPS inserted in the outer membrane; the O-antigen extending from the cell to the aqueous environment; and the core oligosaccharide, which links the O-antigen part to the lipid A (Fig. 1a) [22]. The latter is the main virulence factor of LPS. It consists of a phosphorylated glucosamine dimer with fatty acids attached. It elicits a strong innate immune response through activation of TLR4 at the cell surface and caspase 4/11 inflammasomes in the cytoplasm [23, 24]. The O-antigen, consisting of a repetitive glycan polymer, is highly variable across bacterial strains and possesses antigenic properties. During infection, it can be targeted by antibodies generated by the adaptive immune system [25]. In contrast, the core oligosaccharide of the LPS molecule, divided into the inner and outer cores, is not known to induce an immune response. The inner core starts with one to three residues of 3-deoxy-D-manno-oct-2-ulosonic acid or keto-deoxyoctulosonate (Kdo), followed by two to three residues of L-glycero-D-manno-heptose that can be phosphorylated [22, 26]. The outer core is made of hexose residues such as D-glucose, D-mannose and D-galactose or heptose. They are attached to the last heptose residue in the inner core. An LPS molecule containing only lipid A and a complete or partial inner core is referred to as “deep-rough LPS”, while a molecule containing lipid A and a complete core oligosaccharide is called “rough LPS” (Fig. 1a). Compared to “smooth LPS” bacteria that harbor a complete LPS, deep-rough and rough LPS bacteria exhibit enhanced cell surface hydrophobicity, higher sensitivity to detergents, certain antibiotics and bile salts [27]. They also display increased auto-aggregation and enhanced adhesion to eukaryotic cells [28]. Finally, they are more sensitive to serum [28] and acidity [29], which renders them therefore less virulent in in vivo infection models.
Fig. 1.
a Smooth, rough and deep-rough lipopolysaccharide (LPS) structures of a prototypical Gram-negative bacterium. The lipid A, the inner and outer core, and the O-antigen structures are depicted. P phosphate, Hep heptose, GlcN N-acteyl-glucosamine, Kdo 3-deoxy-d-manno-oct-2-ulosonic acid. b Schematic representation of the enzymes (in red) and metabolites (in black) involved in the inner-core lipopolysaccharide biosynthesis pathway. The heptoses that possess immunogenic properties are highlighted in blue (HBP), gray (HMP1) and purple (ADP-DD-heptose and ADP-LD-heptose). The enzymes present in Neisseria species are shown in brackets
ADP-heptose is a precursor of core oligosaccharide synthesis
Several important genetic and biochemical studies led to the characterization of the LPS biosynthetic pathway in Gram-negative bacteria such as Escherichia coli and Salmonella Typhimurium [30–37]. In E. coli, the biosynthetic pathway of the inner core (Fig. 1b) starts with sedoheptulose 7-phosphate, which is transformed into D-glycero-D-manno-heptose 7-phosphate by the ketose-aldose isomerase GmhA. This product is then phosphorylated by the bifunctional enzyme HldE to form D-glycero-β-D-manno-heptose 1,7-bisphosphate, also known as HBP. A dephosphorylation step at position 7 is then carried out by GmhB, forming D-glycero-β-D-manno-heptose 1-monophosphate (HMP1). HldE subsequently exerts its second enzymatic activity and catalyzes the transfer of an adenylyl moiety to the phosphate group at position 1, making ADP-D-glycero-β-D-manno-heptose (ADP-D,D-heptose). HldD epimerase transforms it into ADP-L-glycero-β-D-manno-heptose (ADP-L,D-heptose), which is then transferred to Kdo2-lipidA by the heptosyltransferase WaaC. It should be noted that this pathway can vary slightly across species. In Neisseria species, the bifunctional protein HldE is replaced by two independent enzymes, HldA and HldC [38]. Since the presence of heptose residues in the inner core of LPS is well conserved across species, ADP-heptose is produced in most Gram-negative bacteria. Interestingly, few exceptions exist. For instance, Moraxella [39], Rhizobium [40], Francisella [41], Legionella [42] and Brucella [43] do not possess the ADP-heptose synthetic pathway. In these, the heptose residues of the inner core are replaced by other sugars such as mannose [44] or glucose phosphate [45]. For certain pathogenic bacteria, the absence of ADP-heptose may have been selected along host–pathogen co-evolution because it probably allows bacterial dissemination in the tissues sheltered from the immune system. In the case of Francisella, this may be part of a global camouflage strategy, since these bacteria have also an atypical lipid A molecule which very poorly stimulates the innate immunity of the host [46]. On the other hand, the Gram-positive bacteria Streptomyces fimbriatus and Streptomyces hygroscopicus can also synthesize ADP-heptose [47]. In these soil bacteria, ADP-heptose is a precursor of the biosynthesis of secondary metabolites such as septacidin and hygromycin B.
First evidence for a role of bacterial heptoses in innate immunity
In recent years, the ability of some bacterial heptoses to induce immune responses has been reported. A first study showed that Neisseria gonorroehae-derived heptose monophosphate (D-glycero-β-D-manno-heptose 1-monophosphate or HMP) induces a pro-inflammatory cytokine response and expression of HIV-1 in an NF-κB-dependent manner in human CD4 T lymphocytes [38]. In 2015, the same laboratory reported that HBP, and not HMP, was the molecule responsible for the effects observed in T cells [48], and that this intermediate of LPS biosynthesis was a Gram-negative bacterial PAMP. In Neisseria meningitidis infection, they demonstrated that the presence of HldA in bacteria was essential to activate NF-κB during infection, whereas the enzymes GmhB and HldD, acting downstream of HldA in the LPS biosynthetic pathway, were not. They also showed that enzymatically synthesized HBP was sufficient to activate NF-κB in a cellular luciferase assay and that it also induced the production of inflammatory cytokines in vitro and in vivo [48]. The role of HBP as a bacterial PAMP was further supported by studies showing that the activation of NF-κB and the production of inflammatory cytokines following Shigella flexneri, S. Typhimurium and Helicobacter pylori infections was HBP dependent [49–52]. In addition to HBP, HMP1 was shown to act as an immune agonist able to activate NF-κB and to induce cytokine production [53].
ADP-heptose, and not HBP, is a PAMP
Interestingly, all results showing that HBP and HMP1 caused inflammation were obtained from experiments in which the cells were stimulated for several hours [48, 52]. Since the oligomerization of TIFA proteins, known as the most upstream signaling event following HBP recognition [48, 49], was observed within minutes after infection with S. flexneri [49] and H. pylori [52], the dynamics of HBP sensing had to be investigated on a shorter timescale. Surprisingly, the formation of TIFA oligomers, called TIFAsomes, was not significant until 2 h of treatment of digitonin-permeabilized cells with chemically synthesized HBP, even for concentrations in the millimolar range [19]. This unexpected delay suggested that HBP is not directly recognized by a host sensor and that it has to be transformed within cells to trigger inflammation. In this sense, Zhou et al. showed that HBP might be converted into ADP-heptose 7-phosphate (ADP-heptose 7P) by host adenylyltransferase enzymes of the nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT) family to induce inflammatory signaling [20]. The observation of a delay also suggested that another bacterial molecule was responsible for triggering the early mechanism of TIFAsome formation observed during bacterial infection [49, 52]. Given that inflammation was completely abolished upon infection with an hldE deletion mutant (ΔhldE) of S. flexneri [49], we hypothesized that a more downstream intermediate of LPS biosynthesis was acting as a bacterial PAMP. A systematic analysis of the pathway using gene deletion and complementation experiments revealed that both the kinase and the adenylyltransferase activities of HldE are required to induce inflammation and that the PAMP is produced upstream of HldD and WaaC, pointing to the role of ADP-heptose. This result was directly confirmed by showing that chemically synthesized ADP-heptose was able to induce rapid TIFAsome formation and cytokine production down to the concentration of 10–10 M in digitonin-permeabilized cells [19].
Interestingly, Shao’s group discovered the PAMP activity of ADP-heptose from a different angle, as they were originally seeking for bacterial factors involved in Yersinia pseudotuberculosis-induced NF-κB mediated cytokine expression. By screening a large library of transposon mutants, they identified a strain that was severely impaired in its ability to activate NF-κB although having a functional type 3 secretion system (T3SS) [20]. In this mutant, the transposon is inserted into the hldE gene. Deleting gmhB or hldD did not affect Yersinia-induced NF-κB activation, whereas deleting gmhA phenocopied the ΔhldE mutant. These results could be explained by a role of HBP in Yersinia-induced NF-κB activation. However, they also found that, unlike the ΔhldE and ΔgmhA mutants, the ΔgmhB still supports ADP-heptose synthesis, as ADP-heptose-dependent autotransporter heptosylation is maintained in these bacteria. In addition, the role of ADP-heptose as an immune modulator was confirmed by showing that synthesized ADP-heptose leads to NF-κB activation and IL-8 production in a much more potent manner than HBP [20].
More recently, ADP-heptose was also described as a PAMP in H. pylori infection [21]. Analytical methods revealed that H. pylori lysates contain only residual amounts of HBP and that these are too low to elicit NF-κB activation as observed during infection. Pfannkuch et al. utilized ultraperformance liquid chromatography to fractionate bacterial lysates and found that a single fraction activates NF-κB in luciferase reporter cells. Mass spectrometry (MS) analysis combined with chemical and enzymatic treatments strongly suggested that the active substance in the fraction was ADP-heptose. This assumption was further confirmed by showing that enzymatically and chemically synthesized ADP-heptose induces NF-κB activation and IL-8 expression.
The phenotype of ΔgmhB in the center of the HBP/ADP-heptose controversy
In several studies, the phenotype of the ΔhldE and ΔgmhB mutants, respectively incapable and capable of inducing inflammation, was used as a rational basis for determining that the PAMP was produced downstream of HldE and upstream of GmhB and, therefore, that it was HBP [48–51]. However, accumulating evidence strongly suggests that the ΔgmhB mutant has only a partial phenotype in some bacterial species. Zhou et al. showed that ADP-heptose-dependent heptosylation of the AIDA-I autotransporter is partially maintained in the Y. pseudotuberculosis ΔgmhB mutant, indicating that significant amounts of ADP-heptose are still synthesized in these bacteria [20]. In E. coli, the ΔgmhB mutant exhibits a lack of complete heptoseless phenotype, strongly suggesting that ADP-heptose synthesis is also partially maintained [34]. Finally, the observation that S. flexneri ΔgmhB bacteria display only weak cell adhesion and invasiveness compared to the ΔhldE mutant indicates that they have a nearly smooth LPS phenotype and that a certain level of ADP-heptose is still likely produced [49]. A partial phenotype may result from functional redundancy to GmhB in ADP-heptose biosynthesis and the existence of a yet-unidentified phosphatase activity capable of dephosphorylating HBP. Such redundant mechanism does not seem to exist in N. meningitidis for which the ΔgmhB mutant displays a truncated, heptoseless lipooligosaccharide [33, 48]. In this case, the activation of NF-κB observed in cells transfected with the ΔgmhB lysate [48] may result from the conversion of HBP into ADP-heptose 7P by the host adenylyltransferases NMNAT1-3 [20]. As the cells were analyzed several hours post-transfection, the ADP-heptose 7P produced in the cytoplasm may have reached a concentration sufficient to activate the host sensor ALPK1 [20]. Finally, in H. pylori, the existence of a redundant mechanism is unclear. No ADP-heptose is found in ΔgmhB bacteria and this mutant is unable to induce inflammation [21]. However, these results contradict earlier studies showing that an H. pylori ΔgmhB mutant has a nearly complete LPS and induces IL-8 production [50, 51].
Mechanism of ADP-heptose sensing and inflammatory response
Identification of the ALPK1/TIFA/TRAF6 axis as a new pathway of innate immunity
The adaptor protein TIFA was the first host factor identified in the control of inflammation following bacterial heptose recognition [48]. TIFA is a 20-kDa protein, which was first identified as a TRAF2 and TRAF6-binding protein involved in NF-κB activation [54, 55]. TIFA contains a conserved threonine in position 9 (T9), a forkhead-associated domain (FHA) responsible for the recognition of phosphorylated threonine and serine residues and a glutamic acid at position 178 involved in TRAF6 binding [56, 57]. In resting conditions, TIFA is found as an intrinsic dimer (Fig. 2a). After TNFα stimulation, TIFA is phosphorylated at T9. Phosphorylated T9s are then recognized by FHA domains of adjacent dimers, leading to massive oligomerization of TIFA proteins (Fig. 2b,c). As TRAF6 is constitutively bound to TIFA, TIFA oligomerization leads to TRAF6 oligomerization, a process known to enhance its ubiquitin ligase activity toward proteins of the NF-κB pathway [58]. The crystal structure determination of TIFA suggests a model for TIFA oligomerization based on antiparallel pairs of head-to-tail binding between pT9 and the FHA domain, which allow the exposure of the C-terminal region for TRAF6 binding [57] (Fig. 2b). Gaudet et al. found that prolonged treatment of macrophages and epithelial cells with HBP induces the formation of TIFAsomes and that HBP-induced inflammation is TIFA and TRAF6 dependent [48]. The formation of TIFAsomes is also observed during S. flexneri and S. Typhimurium infections [49, 59]. It is dependent on the phosphorylation of T9 and the FHA domain of TIFA, but not on TIFA’s ability to bind TRAF6. In contrast, binding to TRAF6 is absolutely required to induce inflammation in response to infection. The implication of TIFA in the control of inflammation was also shown during H. pylori [50–52] and Citrobacter rodentium [60] infections. Our laboratory identified alpha-protein kinase 1 (ALPK1) as the kinase controlling the formation of TIFAsomes, linking for the first time this poorly characterized kinase to bacterial infection [49]. ALPK1 is a member of the alpha-kinase family, a class of atypical protein kinases that share little sequence similarity in their catalytic domain with conventional kinases. In humans, the alpk1 gene is located on chromosome 4q25 and is directly adjacent to that of tifa [61]. It is restricted to vertebrates, conferring them novel signaling capacities [62]. ALPK1 is a 139 kDa protein that contains an N-terminal alpha helical domain, an unstructured linker region and a C-terminal alpha-kinase domain [20]. The central role of ALPK1 in the induction of TIFAsomes during S. flexneri infection requires its kinase domain [49]. ALPK1 is also necessary in H. pylori-induced TIFAsomes [52].
Fig. 2.
a Schematic representation of the structure of inactive TIFA as an intrinsic dimer. Note the threonine 9, the FHA domain with its pT9 recognizing domain and the E178 residue responsible for TRAF6 binding. b Schematic representation of TIFAsome formation upon threonine 9 phosphorylation. Note that TRAF6 oligomerization is responsible for NF-κB activation (Adapted from Huang et al. 2012). c Fluorescence microscopy images of HeLa cells transfected with a TIFA-GFP encoding plasmid stimulated or not with ADP-heptose. Cell nuclei are shown in blue, TIFA-GFP in green. Scale bar 20 µm. d Schematic representation of ALPK1 activation upon ADP-heptose recognition
ALPK1 is the receptor for ADP-heptose
In 2018, Zhou et al. elegantly demonstrated that ALPK1 is the receptor for ADP-heptose [20]. First, they found that a complex of N-terminal and kinase domains of ALPK1 purified from wild-type (wt) E. coli is sufficient to phosphorylate TIFA, whereas such complex purified from ΔhldE bacteria is unable to do so. These results constituted the first indication that a bacterial metabolite produced in wt bacteria, and not in the ΔhldE mutant, binds to the ALPK1 complex and induces the direct phosphorylation of TIFA by the kinase domain of ALPK1. This initial observation was further supported by electrospray ionization MS data, showing that the mass of native ALPK1 N-terminal domain (ALPK1-NTD) exceeds that of denatured ALPK1-NTD by 619.32 Da, which corresponds to the mass-to-charge ratio of ADP-heptose. The recognition of ADP-heptose by ALPK1 was then directly demonstrated by the crystal structure of ALPK1-NTD in complex with ADP-heptose. In brief, the N-terminal domain harbors 18 alpha-helices with 14 of them assembled into 7 antiparallel pairs that form a right-hand solenoid. ADP-heptose binds in a pocket present on the concave side of the solenoid. Although the pocket is spacious enough for ADP-D,D-heptose, ADP-L,D-heptose is more specifically recognized [20]. Several residues of ALPK1 interact with ADP-heptose. R116, R150, R153 and K233 amino acids interact with the two phosphates of ADP via a network of hydrogen bonds. The adenosine moiety of ADP-heptose interacts with F295, S236 and T237. The heptose C3 and C4 hydroxyls are anchored by Q67 and D231. C2 and C6 interact with K233 and C7 is fixed by R153. Finally, the heptose backbone interacts with F61 [20]. Interestingly, all ALPK1 residues interacting with ADP-heptose are conserved in other vertebrates, confirming that they are central to the recognition of ADP-heptose and that this newly identified pathway of innate immunity may broadly operate in vertebrates. Regarding the activation of ALPK1, it has been proposed that the binding of ADP-heptose to the N-terminal domain of ALPK1 interferes with the interactions that this domain has with K1140 and K1149, two residues of the kinase domain, which are predicted to be located near the kinase catalytic cleft. This mechanism may induce a conformational change, which would make the catalytic cleft more exposed, thereby promoting its kinase activity [20] (Fig. 2d). The authors confirmed that neither HBP, sedoheptulose 7-phosphate, nor H1MP is capable of activating ALPK1. The contribution of the residues contacting the adenosine part of the ADP-heptose molecule to the activation of ALPK1 may explain why these other bacterial metabolites are not efficiently recognized. As previously mentioned, Zhou et al. identified the role of the host adenylyltransferases NMNAT1-3 in the cellular response to HBP and showed that these enzymes can convert HBP into ADP-heptose 7P. Although less efficient than ADP-heptose, ADP-heptose 7P can bind and activate ALPK1 (Fig. 3a). The Q67 and Y68 residues of ALPK1 are important for ADP-heptose 7P recognition, but not for canonical ADP-heptose. The facts that HBP has to be transformed by cellular enzymes and that ADP-heptose 7P is less efficient in activating ALPK1 may explain the weak and delayed induction of TIFAsomes observed in HBP-treated cells [19]. Since HMP1 fails to activate ALPK1, the response observed in HMP1-treated cells by Adekoya et al. may also rely on the cellular synthesis of ADP-heptose 7P [53]. ALPK1 is not the only PRR harboring a kinase domain. In plants, various receptor-like kinases (RLKs) with serine/threonine kinase activity have been described for their roles in pathogen recognition [63]. However, unlike ALPK1, these receptors are cell surface localized. They have a ligand-binding ectodomain, a single-pass transmembrane domain, and an intracellular kinase domain. Furthermore, while they can phosphorylate substrates [64], other associated kinases are generally needed to initiate PAMP-triggered immunity [65]. In animals, none of the TLRs, NLRs, RLRs and CLRs has kinase activity [4]. Whereas some of them can be tyrosine phosphorylated after binding of their cognate ligand(s), this occurs through the recruitment of cytosolic kinases such as Src, Syk or Btk [66]. Interestingly, the mode of action of ALPK1 is reminiscent of that of double-stranded RNA-dependent protein kinase (PKR), which was originally characterized as a pathogen sensor regulating innate immune responses against viral infections [67]. Following binding of virus-derived dsRNA molecules to its N-terminal dsRNA binding motifs, the kinase domain of PKR is activated, and phosphorylates the alpha subunit of eukaryotic initiation factor 2, resulting in the inhibition of viral mRNA translation [68]. Note that the activation of PKR results in its dimerization and autophosphorylation [69]. Such molecular processes have not been described so far for ALPK1. The ligand, localization and kinase activity of ALPK1 make it a unique PRR that triggers immune signaling in Gram-negative bacterial infections. It would be of particular interest to explore if other atypical kinases like ALPK2 and ALPK3 [70] exhibit similar properties.
Fig. 3.
a Schematic representation of direct ADP-heptose-dependent (black bold arrows) and indirect HBP-dependent (gray dotted arrows) ALPK1 activation, leading to TIFA phosphorylation and oligomerization. b Schematic representation of the activation of the ADP-heptose/ALPK1/TIFA/TRAF6/NF-κB pathway resulting in pro-inflammatory cytokine release and neutrophil recruitment. c Schematic representation of the possible delivery mechanisms of ADP-heptose during infection by Shigella, Neisseria, Yersinia and Helicobacter bacteria
Mechanistic insights into the activation of TIFA
In response to ADP-heptose recognition, ALPK1 can directly phosphorylate TIFA in its T9 and induce the formation of TIFAsomes (Figs. 2c and 3b). Zhou et al. found that both proteins co-immunoprecipitate in response to ADP-heptose sensing in HEK cells co-transfected with ALPK1 and TIFA constructs [20]. However, they did not see any co-localization of both proteins in co-transfection experiments assessed by immunofluorescence. Moreover, Zimmermann et al. did not find ALPK1 in the composition of TIFAsomes by MS analysis [52]. These results suggest that ALPK1 and TIFA can physically interact following ADP-heptose recognition, but that the interaction is only transient and that ALPK1 does not remain stably associated with TIFAsomes. Interestingly, ALPK1 may not be the only kinase able to phosphorylate TIFA. Upon TNFα treatment, the kinase AKT can phosphorylate TIFA at T9, thereby promoting NLRP3 inflammasome assembly and activation [71]. Aurora A may also phosphorylate the T9 of TIFA, triggering the activation of an NF-κB-dependent survival pathway [72].
TIFAsomes are large multiprotein complexes. A total of 77 TIFAsome-associated proteins enriched after H. pylori infection were identified by MS [52]. They include classical NF-κB regulators, including TAB2 and TRAF2, TRIM21, a factor involved in ubiquitination and proteasomal degradation [73] as well as the microtubule- and actin-associated proteins KIF11 and myosin IIA. Interestingly, it has been reported that ALPK1 phosphorylates myosin IIA modulating TNF-α trafficking in gout flares [74]. Annexin A2, which can interact with the p50 subunit of NF-κB to regulate p50 complex translocation into the nucleus, was also found to be associated with TIFAsomes. Noteworthy, several proteins that act on TIFA have important implications in the resolution of inflammation. For instance, TIFAB can change TIFA’s conformation and inhibit TRAF6 activation, thus limiting the proliferation and maturation of immune cells [75, 76]. Moreover, zinc finger protein ZCCHC11 is able to suppress NF-κB signaling after LPS/TLR4 stimulation by directly interacting with TIFA [77]. Whether these proteins are also associated with TIFAsomes and/or regulate TIFA in response to ADP-heptose sensing remains to be elucidated.
ADP-heptose-induced inflammatory response
As for other PRRs, TRAF6 is involved in the signaling pathway activated by ALPK1 in response to ADP-heptose recognition. TRAF6 is constitutively bound to TIFA [55, 78]. After activation, TIFA oligomerization subsequently induces the oligomerization of TRAF6 [49], a mechanism known to increase its E3 ubiquitin ligase activity [79] (Fig. 2b). As a consequence, it promotes TAK1, NEMO and IKK activation through ubiquitination and phosphorylation events [58]. This results in the ubiquitination and proteasome-dependent degradation of IκB, allowing the translocation of p65 to the nucleus and the activation of an NF-κB-dependent transcriptional program regulating the expression of multiple cytokines. In response to ADP-heptose sensing, IL-8, IL-6, and IL-4 are produced by human epithelial cells in vitro [19]. In addition, the injection of ADP-heptose in the dorsal air pouches of mice induces the expression of IL-6, IP-10, MCP-1, TNFα, IFN-γ, MCP-3, GM-CSF, MIP-1α, MIP-1β and RANTES and leads to the massive recruitment of neutrophils [20] (Fig. 3b). This immune response is strictly ALPK1 dependent, since no cytokine is produced in alpk1 knock-out mice (alpk1−/−). In line with this result, the production of cytokines is increased in the lungs of mice infected with Burkholderia cenocepacia in an ALPK1-dependent manner [20]. The production of cytokines is impaired and a higher bacterial load is observed in the lungs of alpk1−/− mice compared to wt animals, confirming the role of the ADP-heptose sensor ALPK1 in the control of inflammation in vivo and in the clearance of bacteria during infection.
Mechanisms of ADP-heptose delivery during infection
How a bacterial cytosolic metabolite like ADP-heptose is delivered during infection in the cytoplasm of host cells where it can be sensed by ALPK1, remains an open question (Fig. 3c). In the case of the extracellular pathogen N. meningitidis, ADP-heptose may be locally secreted by bacteria adhering at the cell surface or released upon bacterial lysis. It may then be internalized into cells. In contrast to HBP, ADP-heptose can enter cells in the absence of transfection reagents or permeabilization [20]. The mechanism by which cells are permeable to ADP-heptose is unknown and the search for a potential cellular transporter is of particular interest. For the pathogen Y. pseudotuberculosis that harbors a T3SS, ADP-heptose-induced NF-κB activation requires functional secretion [20]. ADP-heptose may thus be directly delivered into the cytoplasm of host cells by passing through the needle of the T3SS. In a non-mutually exclusive manner, it may also be residually secreted via T3SS in the extracellular medium before being internalized inside cells. The fact that ADP-heptose delivery is T3SS dependent suggests that this sophisticated nanomachine, well known for the translocation of bacterial proteins, may also secrete bacterial metabolites. Interestingly, a similar mechanism has been proposed for the type 4 secretion system (T4SS) of H. pylori. Indeed, functional T4 secretion is also required to activate the ALPK1/TIFA axis in response to ADP-heptose sensing [21]. During infection by intracellular bacteria, several non-mutually exclusive mechanisms of ADP-heptose delivery may also be at work. Like extracellular pathogens, they may use T3SS or T4SS systems to inject ADP-heptose in the early process of infection while they are still outside host cells. Depending on the intracellular lifestyle of the bacteria, they may also inject or release ADP-heptose into the cytoplasm while they are residing inside internalization vacuoles or in the cytoplasm of host cells. S. flexneri is a well-suited model to study the mechanisms of ADP-heptose delivery during infection with an intracellular bacterium. It harbors a T3SS used by bacteria to inject a series of effector proteins that promote the formation of actin-dependent cell protrusions and bacterial engulfment in an internalization vacuole [80]. After rupture of the vacuolar membrane, bacteria are released into the cytoplasm of host cells where they can move from cell to cell via actin-based motility [81]. During S. flexneri infection, TIFAsomes are visible within minutes of infection [49]. This early response is compatible with a mechanism of ADP-heptose delivery mediated by the T3SS of extracellular bacteria and/or a release of ADP-heptose into the cytoplasm after vacuolar rupture, but this remains to be shown. Since TIFAsomes are visible for several hours, ADP-heptose may also be released by cytosolic replicating or lysed bacteria as previously shown for HBP [59]. Interestingly, TIFAsomes are also present in uninfected cells surrounding the infected ones [49]. Such process of bystander cell activation results from a mechanism of gap junction-mediated cell–cell communication that amplifies the production of inflammatory cytokines during infection [19, 82]. It must be determined whether ADP-heptose is the molecule diffusing through gap junctions that activates the ALPK1/TIFA axis in bystander cells.
Concluding remarks and perspectives
The identification of ADP-heptose as a new bacterial PAMP brings new insights into our understanding of the diversity of pathogen recognition mechanisms and reveals the complexity of the regulation of innate immunity. During infection, ADP-heptose is likely detected together with other bacterial PAMPs including LPS, peptidoglycan-derived peptides, flagellin or bacterial DNA. Whether the recognition of ADP-heptose only modulates the expression of target genes shared by other PAMP/PRR pathways, or whether it can induce a specific profile of immune effectors and thereby trigger a specific immune response, remains to be established. This question could be addressed by a comparative transcriptome analysis of cells stimulated by a panel of different PAMPs used individually or in combinations. A thorough analysis of immune responses in wt and alpk1−/− animal models infected by Gram-negative bacteria producing ADP-heptose, or not, would also be very valuable. At the molecular level, the ADP-heptose sensing mechanism could directly interfere with other known pro-inflammatory signaling pathways. For instance, the fact that the ADP-heptose sensing pathway activates NF-κB via TRAF6 suggests that it may alter the activation of TLR1-2,4–9 that are localized at the cell membrane or in endosomes and that also rely on this E3 ubiquitin ligase to trigger inflammation [83]. Indeed, part of the TRAF6 proteins are constitutively bound to TIFA, but the TIFA/TRAF6 interaction is further enhanced upon TIFA oligomerization [56]. TRAF6, massively associated with TIFAsomes, could then be sequestrated away from the TLRs. In contrast, inflammasomes or cytosolic PRRs such as NLRs do not rely on TRAF6 to elicit pro-inflammatory signaling [84]. In this sense, the novel cytosolic PRR ALPK1 exploits a signaling cascade distinct from those of other cytosolic sensors.
Beyond a role in innate immunity, the mechanism of ADP-heptose sensing may also have implications in the control of intestinal homeostasis. A first hint was provided by two studies using different mouse models of colitis showing that the genomic loci, Cdcs1 (C3Bir-derived cytokine deficiency-induced colitis susceptibility 1) and Hiccs (Helicobacter hepaticus-induced colitis and associated cancer susceptibility), which both contain alpk1, are involved in the maintenance of intestinal homeostasis upon microbial challenge [85, 86]. These initial observations were further supported by directly identifying alpk1 as the key player within the Hiccs locus [87]. In response to infection with the commensal pathobiont H. hepaticus, alpk1−/− mice display enhanced IL-12/IL-23-dependent colitis characterized by an augmented Th1/IFN-γ response at the expense of Treg and Th17 cells, indicating that ALPK1 promotes gut immune homeostasis by regulating the balance of type 1/type 17 immunity. The question of whether such a role of ALPK1 is linked to its PRR activity for ADP-heptose is an important question that remains to be elucidated at this stage. To note, a connection between PAMP recognition and intestinal inflammation has already been established. For instance, in certain Crohn’s disease patients, intestinal inflammation is associated with defects in the cytosolic PRRs NOD1/2 pathways [88]. Alternatively, ALPK1 may regulate intestinal homeostasis independently of ADP-heptose. Interestingly, mutations in alpk1 are associated with several diseases, including nephropathies [89], breast cancer [90], acute lymphoblastic leukemia [91, 92] and oral squamous carcinoma [93], suggesting that ALPK1 may be involved in many biological processes beyond its central role in ADP-heptose sensing.
Acknowledgements
We thank Anne-Sophie Dangeard and Veronica Teixeira for critically reviewing the manuscript.
Abbreviations
- ADP-heptose
ADP-L-glycero-β-D-manno-heptose
- ADP-heptose 7P
ADP-heptose 7-phosphate
- AIDA
Adhesin involved in diffuse adherence
- AKT
AK strain-transforming protein/protein kinase B
- ALPK1
Alpha-protein kinase 1
- CD
Cluster of differentiation
- CLR
C-type lectin receptor
- dsRNA
Double-stranded RNA
- FHA
Forkhead-associated domain
- GM-CSF
Granulocyte–macrophage colony stimulating factor
- HBP
D-glycero-β-D-manno-heptose 1,7-bisphosphate
- HEK
Human embryonic kidney
- HIV
Human immunodeficiency virus
- HMP1
D-glycero-β-D-manno-heptose 1-monophosphate
- IFN
Interferon
- IKK
IκB kinase
- IL
Interleukin
- IP
Interferon-γ induced protein
- IκB
Inhibitor of κB
- Kdo
3-Deoxy-D-manno-oct-2-ulosonic acid
- KIF
Kinesin superfamily protein
- LPS
Lipopolysaccharide
- MCP
Monocyte chemoattractant protein
- MIP
Macrophage inflammatory protein
- MS
Mass spectrometry
- NEMO
NF-κB essential modulator
- NF-κB
Nuclear-factor kappa B
- NLR
NOD-like receptor
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- NMNAT
Nicotinamide mononucleotide adenylyltransferase
- NOD
Nucleotide-binding and oligomerization domain
- NTD
N-terminal domain
- PAMP
Pathogen-associated molecular pattern
- PRR
Pathogen recognition receptor
- pT9
Phospho-threonine 9
- RANTES
Regulated on activation, normal T-cell expressed and secreted
- RIG
Retinoid acid-inducible gene
- RLK
Receptor-like kinase
- RLR
RIG-like receptor
- T3/4SS
Type 3/4 secretion system
- T9
Threonine 9
- TAB
TAK-1-binding protein
- TAK
Transforming growth factor β-activated kinase 1
- Th1
Type 1 helper T cell
- Th17
Type 17 helper T cell
- TIFA
TRAF-interacting protein with FHA domain-containing protein A
- TLR
Toll-like receptor
- TNFα
Tumor necrosis factor alpha
- TRAF
Tumor necrosis factor receptor-associated factor
- Treg
Regulatory T cell
- TRIM
Tripartite motif
- Wt
Wild type
- ZCCHC
Zinc finger CCHC-type containing protein
Funding
We gratefully acknowledge financial support from the Agence Nationale de la Recherche (Grants no: ANR‐14‐ACHN‐0029‐01 and ANR‐17‐CE15‐0006, including postdoctoral fellowships to DGW) and from Fondation ARC pour la Recherche sur le Cancer (Grant No: ARC—PJA20171206187).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Creagh EM, O’Neill LAJ. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Baccala R, Gonzalez-Quintial R, Lawson BR, et al. Sensors of the innate immune system: their mode of action. Nat Rev Rheumatol. 2009;5:448–456. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz-Wolf N, Lavelle EC. Innate immune receptors. Methods Mol Biol Clifton NJ. 2016;1417:1–43. doi: 10.1007/978-1-4939-3566-6_1. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Gewirtz AT, Navas TA, Lyons S, et al. (2001) Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol Baltim Md. 1950;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 7.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 8.Hirschfeld M, Kirschning CJ, Schwandner R, et al. (1999) Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol Baltim Md. 1950;163:2382–2386. [PubMed] [Google Scholar]
- 9.Bauer S, Kirschning CJ, Häcker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii KJ, Coban C, Kato H, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 11.Chamaillard M, Hashimoto M, Horie Y, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 12.Girardin SE, Boneca IG, Carneiro LAM, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 13.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 14.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 15.Yoneyama M, Kikuchi M, Natsukawa T, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 16.Sato K, Yang X, Yudate T, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–38866. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 17.Wells CA, Salvage-Jones JA, Li X, et al. (2008) The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol Baltim Md. 1950;180:7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 18.Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 19.García-Weber D, Dangeard AS, Cornil J, et al (2018) ADP-heptose is a newly identified pathogen-associated molecular pattern of Shigella flexneri. EMBO Rep 10.15252/embr.201846943 [DOI] [PMC free article] [PubMed]
- 20.Zhou P, She Y, Dong N, et al. Alpha-kinase 1 is a cytosolic innate immune receptor for bacterial ADP-heptose. Nature. 2018;561:122–126. doi: 10.1038/s41586-018-0433-3. [DOI] [PubMed] [Google Scholar]
- 21.Pfannkuch L, Hurwitz R, Traulsen J, et al. ADP heptose, a novel pathogen-associated molecular pattern identified in Helicobacter pylori. FASEB J Off Publ Fed Am Soc Exp Biol. 2019 doi: 10.1096/fj.201802555R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raetz CRH. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 23.Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 24.Park BS, Lee J-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunin CM, Beard MV. Serological studies of o antigens of Escherichia Coli by means of the hemagglutination test. J Bacteriol. 1963;85:541–548. doi: 10.1128/JB.85.3.541-548.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endotoxin in Health and Disease. In: CRC Press. https://www.routledge.com/Endotoxin-in-Health-and-Disease/Brade/p/book/9780824719449. Accessed 11 June 2020
- 27.Møller AK, Leatham MP, Conway T, et al. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infect Immun. 2003;71:2142–2152. doi: 10.1128/iai.71.4.2142-2152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu D, Zhang W, Zhang B, et al. Characterization of a biofilm-forming Shigella flexneri phenotype due to deficiency in Hep biosynthesis. PeerJ. 2016;4:e2178. doi: 10.7717/peerj.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinić M, Hoare A, Contreras I, Álvarez SA. Contribution of the lipopolysaccharide to resistance of Shigella flexneri 2a to extreme acidity. PLoS ONE. 2011 doi: 10.1371/journal.pone.0025557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coleman WG, Deshpande KS. New cysE-pyrE-linked rfa mutation in Escherichia coli K-12 that results in a heptoseless lipopolysaccharide. J Bacteriol. 1985;161:1209–1214. doi: 10.1128/JB.161.3.1209-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valvano MA, Marolda CL, Bittner M, et al. The rfaE gene from Escherichia coli encodes a bifunctional protein involved in biosynthesis of the lipopolysaccharide core precursor ADP-L-glycero-d-manno-heptose. J Bacteriol. 2000;182:488–497. doi: 10.1128/jb.182.2.488-497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gronow S, Brade H. Lipopolysaccharide biosynthesis: which steps do bacteria need to survive? J Endotoxin Res. 2001;7:3–23. [PubMed] [Google Scholar]
- 33.Shih GC, Kahler CM, Carlson RW, et al. gmhX, a novel gene required for the incorporation of L-glycero-D-manno-heptose into lipooligosaccharide in Neisseria meningitidis. Microbiol Read Engl. 2001;147:2367–2377. doi: 10.1099/00221287-147-8-2367. [DOI] [PubMed] [Google Scholar]
- 34.Kneidinger B, Marolda C, Graninger M, et al. Biosynthesis pathway of ADP-L-glycero-beta-D-manno-heptose in Escherichia coli. J Bacteriol. 2002;184:363–369. doi: 10.1128/jb.184.2.363-369.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valvano MA, Messner P, Kosma P. Novel pathways for biosynthesis of nucleotide-activated glycero-manno-heptose precursors of bacterial glycoproteins and cell surface polysaccharides. Microbiol Read Engl. 2002;148:1979–1989. doi: 10.1099/00221287-148-7-1979. [DOI] [PubMed] [Google Scholar]
- 36.Kim C-H. A Salmonella typhimurium rfaE mutant recovers invasiveness for human epithelial cells when complemented by wild type rfaE (controlling biosynthesis of ADP-L-glycero-D-mannoheptose-containing lipopolysaccharide) Mol Cells. 2003;15:226–232. [PubMed] [Google Scholar]
- 37.McArthur F, Andersson CE, Loutet S, et al. Functional analysis of the glycero-manno-heptose 7-phosphate kinase domain from the bifunctional HldE protein, which is involved in ADP-L-glycero-D-manno-heptose biosynthesis. J Bacteriol. 2005;187:5292–5300. doi: 10.1128/JB.187.15.5292-5300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malott RJ, Keller BO, Gaudet RG, et al. Neisseria gonorrhoeae-derived heptose elicits an innate immune response and drives HIV-1 expression. Proc Natl Acad Sci U S A. 2013;110:10234–10239. doi: 10.1073/pnas.1303738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pokorny B, Kosma P. Synthesis of 5-O-oligoglucosyl extended α-(2→4)-Kdo disaccharides corresponding to inner core fragments of Moraxellaceae lipopolysaccharides. Carbohydr Res. 2016;422:5–12. doi: 10.1016/j.carres.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadrmas JL, Brozek KA, Raetz CR. Lipopolysaccharide core glycosylation in Rhizobium leguminosarum. An unusual mannosyl transferase resembling the heptosyl transferase I of Escherichia coli. J Biol Chem. 1996;271:32119–32125. doi: 10.1074/jbc.271.50.32119. [DOI] [PubMed] [Google Scholar]
- 41.Kay W, Petersen BO, Duus JØ, et al. Characterization of the lipopolysaccharide and beta-glucan of the fish pathogen Francisella victoria. FEBS J. 2006;273:3002–3013. doi: 10.1111/j.1742-4658.2006.05311.x. [DOI] [PubMed] [Google Scholar]
- 42.Knirel YA, Moll H, Zähringer U. Structural study of a highly O-acetylated core of Legionella pneumophila serogroup 1 lipopolysaccharide. Carbohydr Res. 1996;293:223–234. doi: 10.1016/0008-6215(96)00194-2. [DOI] [PubMed] [Google Scholar]
- 43.Moreno E, Pitt MW, Jones LM, et al. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979;138:361–369. doi: 10.1128/JB.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okan NA, Kasper DL. The atypical lipopolysaccharide of Francisella. Carbohydr Res. 2013;378:79–83. doi: 10.1016/j.carres.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vries SPW, Bootsma HJ, Hays JP, Hermans PWM. Molecular aspects of Moraxella catarrhalis pathogenesis. Microbiol Mol Biol Rev MMBR. 2009;73:389–406. doi: 10.1128/MMBR.00007-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajjar AM, Harvey MD, Shaffer SA, et al. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun. 2006;74:6730–6738. doi: 10.1128/IAI.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang W, Guo Z, Cao Z, et al. d-Sedoheptulose-7-phosphate is a common precursor for the heptoses of septacidin and hygromycin B. Proc Natl Acad Sci U S A. 2018;115:2818–2823. doi: 10.1073/pnas.1711665115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaudet RG, Sintsova A, Buckwalter CM, et al. Innate immunity. Cytosolic detection of the bacterial metabolite HBP activates TIFA-dependent innate immunity. Science. 2015;348:1251–1255. doi: 10.1126/science.aaa4921. [DOI] [PubMed] [Google Scholar]
- 49.Milivojevic M, Dangeard A-S, Kasper CA, et al. ALPK1 controls TIFA/TRAF6-dependent innate immunity against heptose-1,7-bisphosphate of gram-negative bacteria. PLoS Pathog. 2017;13:e1006224. doi: 10.1371/journal.ppat.1006224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein SC, Faber E, Bats SH, et al. Helicobacter pylori modulates host cell responses by CagT4SS-dependent translocation of an intermediate metabolite of LPS inner core heptose biosynthesis. PLoS Pathog. 2017;13:e1006514. doi: 10.1371/journal.ppat.1006514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gall A, Gaudet RG, Gray-Owen SD, Salama NR (2017) TIFA Signaling in Gastric Epithelial Cells Initiates the cag Type 4 Secretion System-Dependent Innate Immune Response to Helicobacter pylori Infection. mBio 10.1128/mBio.01168-17 [DOI] [PMC free article] [PubMed]
- 52.Zimmermann S, Pfannkuch L, Al-Zeer MA, et al. ALPK1- and TIFA-dependent innate immune response triggered by the Helicobacter pylori Type IV Secretion System. Cell Rep. 2017;20:2384–2395. doi: 10.1016/j.celrep.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 53.Adekoya IA, Guo CX, Gray-Owen SD, et al. (2018) d-Glycero-β-d-Manno-heptose 1-phosphate and d-glycero-β-d-manno-heptose 1,7-biphosphate are both innate immune agonists. J Immunol Baltim Md. 1950;201:2385–2391. doi: 10.4049/jimmunol.1801012. [DOI] [PubMed] [Google Scholar]
- 54.Kanamori M, Suzuki H, Saito R, et al. T2BP, a novel TRAF2 binding protein, can activate NF-kappaB and AP-1 without TNF stimulation. Biochem Biophys Res Commun. 2002;290:1108–1113. doi: 10.1006/bbrc.2001.6315. [DOI] [PubMed] [Google Scholar]
- 55.Takatsuna H, Kato H, Gohda J, et al. Identification of TIFA as an adapter protein that links tumor necrosis factor receptor-associated factor 6 (TRAF6) to interleukin-1 (IL-1) receptor-associated kinase-1 (IRAK-1) in IL-1 receptor signaling. J Biol Chem. 2003;278:12144–12150. doi: 10.1074/jbc.M300720200. [DOI] [PubMed] [Google Scholar]
- 56.Huang C-CF, Weng J-H, Wei T-YW, et al. Intermolecular binding between TIFA-FHA and TIFA-pT mediates tumor necrosis factor alpha stimulation and NF-κB activation. Mol Cell Biol. 2012;32:2664–2673. doi: 10.1128/MCB.00438-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weng J-H, Hsieh Y-C, Huang C-CF, et al. Uncovering the mechanism of forkhead-associated domain-mediated TIFA oligomerization that plays a central role in immune responses. Biochemistry. 2015;54:6219–6229. doi: 10.1021/acs.biochem.5b00500. [DOI] [PubMed] [Google Scholar]
- 58.Ea C-K, Sun L, Inoue J-I, Chen ZJ. TIFA activates IkappaB kinase (IKK) by promoting oligomerization and ubiquitination of TRAF6. Proc Natl Acad Sci U S A. 2004;101:15318–15323. doi: 10.1073/pnas.0404132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaudet RG, Guo CX, Molinaro R, et al. Innate recognition of intracellular bacterial growth is driven by the TIFA-dependent cytosolic surveillance pathway. Cell Rep. 2017;19:1418–1430. doi: 10.1016/j.celrep.2017.04.063. [DOI] [PubMed] [Google Scholar]
- 60.Carson D, Barry R, Hopkins EGD, et al. Citrobacter rodentium induces rapid and unique metabolic and inflammatory responses in mice suffering from severe disease. Cell Microbiol. 2020;22:e13126. doi: 10.1111/cmi.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hillier LW, Graves TA, Fulton RS, et al. Generation and annotation of the DNA sequences of human chromosomes 2 and 4. Nature. 2005;434:724–731. doi: 10.1038/nature03466. [DOI] [PubMed] [Google Scholar]
- 62.Scheeff ED, Bourne PE. Structural evolution of the protein kinase-like superfamily. PLoS Comput Biol. 2005;1:e49. doi: 10.1371/journal.pcbi.0010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zipfel C. Plant pattern-recognition receptors. Trends Immunol. 2014;35:345–351. doi: 10.1016/j.it.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Greeff C, Roux M, Mundy J, Petersen M. Receptor-like kinase complexes in plant innate immunity. Front Plant Sci. 2012;3:209. doi: 10.3389/fpls.2012.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dardick C, Ronald P. Plant and animal pathogen recognition receptors signal through non-RD kinases. PLoS Pathog. 2006;2:e2. doi: 10.1371/journal.ppat.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chattopadhyay S, Sen GC. Tyrosine phosphorylation in toll-like receptor signaling. Cytokine Growth Factor Rev. 2014;25:533–541. doi: 10.1016/j.cytogfr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samuel CE. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993;268:7603–7606. doi: 10.1016/S0021-9258(18)52994-X. [DOI] [PubMed] [Google Scholar]
- 68.Balachandran S, Roberts PC, Brown LE, et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13:129–141. doi: 10.1016/s1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- 69.Dey M, Mann BR, Anshu A, Mannan MA. Activation of protein kinase PKR requires dimerization-induced cis-phosphorylation within the activation loop. J Biol Chem. 2014;289:5747–5757. doi: 10.1074/jbc.M113.527796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Middelbeek J, Clark K, Venselaar H, et al. The alpha-kinase family: an exceptional branch on the protein kinase tree. Cell Mol Life Sci CMLS. 2010;67:875–890. doi: 10.1007/s00018-009-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin T-Y, Wei T-YW, Li S, et al. TIFA as a crucial mediator for NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2016;113:15078–15083. doi: 10.1073/pnas.1618773114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei T-YW, Wu P-Y, Wu T-J, et al. Aurora A and NF-κB survival pathway drive chemoresistance in acute myeloid leukemia via the TRAF-interacting protein TIFA. Cancer Res. 2017;77:494–508. doi: 10.1158/0008-5472.CAN-16-1004. [DOI] [PubMed] [Google Scholar]
- 73.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 74.Lee C-P, Chiang S-L, Ko AM-S, et al. ALPK1 phosphorylates myosin IIA modulating TNF-α trafficking in gout flares. Sci Rep. 2016;6:25740. doi: 10.1038/srep25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsumura T, Semba K, Azuma S, et al. TIFAB inhibits TIFA, TRAF-interacting protein with a forkhead-associated domain. Biochem Biophys Res Commun. 2004;317:230–234. doi: 10.1016/j.bbrc.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 76.Matsumura T, Kawamura-Tsuzuku J, Yamamoto T, et al. TRAF-interacting protein with a forkhead-associated domain B (TIFAB) is a negative regulator of the TRAF6-induced cellular functions. J Biochem (Tokyo) 2009;146:375–381. doi: 10.1093/jb/mvp080. [DOI] [PubMed] [Google Scholar]
- 77.Minoda Y, Saeki K, Aki D, et al. A novel Zinc finger protein, ZCCHC11, interacts with TIFA and modulates TLR signaling. Biochem Biophys Res Commun. 2006;344:1023–1030. doi: 10.1016/j.bbrc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 78.Huang W-C, Liao J-H, Hsiao T-C, et al. Binding and enhanced binding between key immunity proteins TRAF6 and TIFA. Chembiochem Eur J Chem Biol. 2019;20:140–146. doi: 10.1002/cbic.201800436. [DOI] [PubMed] [Google Scholar]
- 79.Yin Q, Lin S-C, Lamothe B, et al. E2 interaction and dimerization in the crystal structure of TRAF6. Nat Struct Mol Biol. 2009;16:658–666. doi: 10.1038/nsmb.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parsot C. Shigella type III secretion effectors: how, where, when, for what purposes? Curr Opin Microbiol. 2009;12:110–116. doi: 10.1016/j.mib.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Kuehl CJ, Dragoi A-M, Talman A, Agaisse H. Bacterial spread from cell to cell: beyond actin-based motility. Trends Microbiol. 2015;23:558–566. doi: 10.1016/j.tim.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kasper CA, Sorg I, Schmutz C, et al. Cell-cell propagation of NF-κB transcription factor and MAP kinase activation amplifies innate immunity against bacterial infection. Immunity. 2010;33:804–816. doi: 10.1016/j.immuni.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 83.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Platnich JM, Muruve DA. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch Biochem Biophys. 2019;670:4–14. doi: 10.1016/j.abb.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 85.Bleich A, Büchler G, Beckwith J, et al. Cdcs1 a major colitis susceptibility locus in mice; subcongenic analysis reveals genetic complexity. Inflamm Bowel Dis. 2010;16:765–775. doi: 10.1002/ibd.21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boulard O, Kirchberger S, Royston DJ, et al. Identification of a genetic locus controlling bacteria-driven colitis and associated cancer through effects on innate inflammation. J Exp Med. 2012;209:1309–1324. doi: 10.1084/jem.20120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ryzhakov G, West NR, Franchini F, et al. Alpha kinase 1 controls intestinal inflammation by suppressing the IL-12/Th1 axis. Nat Commun. 2018;9:3797. doi: 10.1038/s41467-018-06085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caruso R, Warner N, Inohara N, Núñez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41:898–908. doi: 10.1016/j.immuni.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuo T-M, Hsu H-T, Chung C-M, et al. Enhanced alpha-kinase 1 accelerates multiple early nephropathies in streptozotocin-induced hyperglycemic mice. Biochim Biophys Acta. 2016;1862:2034–2042. doi: 10.1016/j.bbadis.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 90.Strietz J, Stepputtis SS, Preca B-T, et al (2016) ERN1 and ALPK1 inhibit differentiation of bi-potential tumor-initiating cells in human breast cancer. Oncotarget 7:83278–83293. 10.18632/oncotarget.13086 [DOI] [PMC free article] [PubMed]
- 91.Li C, Kuang L, Zhu B, et al. Identification of prognostic risk factors of acute lymphoblastic leukemia based on mRNA expression profiling. Neoplasma. 2017;64:494–501. doi: 10.4149/neo_2017_402. [DOI] [PubMed] [Google Scholar]
- 92.Ji C, Lin S, Yao D, et al. Identification of promising prognostic genes for relapsed acute lymphoblastic leukemia. Blood Cells Mol Dis. 2019;77:113–119. doi: 10.1016/j.bcmd.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 93.Chen P-K, Hua C-H, Hsu H-T, et al. ALPK1 expression is associated with lymph node metastasis and tumor growth in oral squamous cell carcinoma patients. Am J Pathol. 2019;189:190–199. doi: 10.1016/j.ajpath.2018.09.003. [DOI] [PubMed] [Google Scholar]