Abstract
Ependymal cells lining the central canal of the spinal cord play a crucial role in providing a physical barrier and in the circulation of cerebrospinal fluid. These cells express the FOXJ1 and SOX2 transcription factors in mice and are derived from various neural tube populations, including embryonic roof and floor plate cells. They exhibit a dorsal–ventral expression pattern of spinal cord developmental transcription factors (such as MSX1, PAX6, ARX, and FOXA2), resembling an embryonic-like organization. Although this ependymal region is present in young humans, it appears to be lost with age. To re-examine this issue, we collected 17 fresh spinal cords from organ donors aged 37–83 years and performed immunohistochemistry on lightly fixed tissues. We observed cells expressing FOXJ1 in the central region in all cases, which co-expressed SOX2 and PAX6 as well as RFX2 and ARL13B, two proteins involved in ciliogenesis and cilia-mediated sonic hedgehog signaling, respectively. Half of the cases exhibited a lumen and some presented portions of the spinal cord with closed and open central canals. Co-staining of FOXJ1 with other neurodevelopmental transcription factors (ARX, FOXA2, MSX1) and NESTIN revealed heterogeneity of the ependymal cells. Interestingly, three donors aged > 75 years exhibited a fetal-like regionalization of neurodevelopmental transcription factors, with dorsal and ventral ependymal cells expressing MSX1, ARX, and FOXA2. These results provide new evidence for the persistence of ependymal cells expressing neurodevelopmental genes throughout human life and highlight the importance of further investigation of these cells.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-023-04811-x.
Keywords: Niche, Ependyma, Regeneration, Injury, Neural tube, Stenosis, Neural stem cells
Introduction
The ependyma is a layer of cells that lines the ventricular system of the brain and central canal of the spinal cord. Spinal cord ependymal cells are a heterogeneous population derived from different subpopulations of the neural tube in mice [1–8]. They play an important role in the production and movement of cerebrospinal fluid [6, 9] and typically express FOXJ1 and SOX2 transcription factors [8]. Adult spinal cord ependymal cells also keep expressing several spinal cord developmental transcription factors such as ARX, FOXA2, MSX1, PAX6, and SOX2,4,6,11 in mice and young humans [10]. In particular, the ependyma has an embryonic-like dorsal–ventral regionalization and long radial NESTIN+ cells have been observed in the dorsal and ventral regions [6, 7, 11]. These radial cells are remnants of the embryonic spinal cord roof and floor plates [2, 8, 10] which are well-known developmental organizing centers [12]. Compared to the brain ependyma, the mouse spinal cord ependyma maintains proliferation [4, 6, 11, 13].
While much of our knowledge of the ependymal region is based on studies in rodents, it is important to study spinal cord ependymal cells in non-human primates and humans, which have a much longer lifespan and may exhibit significant differences. For example, ependymal cells in mice are mainly bi- and uniciliated, whereas macaques have uni-, bi-, and multiciliated cells [6, 14]. Human spinal cord ependymal cells remain largely understudied, with limited research on adult tissues [14–24]. Most of these studies were based on spinal cords removed from cadavers.
In contrast to rodents, the ependymal region in humans appears to disappear from early childhood or the second decade in the majority of individuals, resulting in extensive accumulation of disorganized ependymal cells, pronounced astrogliosis, and perivascular pseudo-rosettes that resemble ependymomas [14, 17–19, 24]. After the second decade, no expression of Sox2 or CD133 (PROM1), which are ependymal cell markers, is found [17]. Although a fraction of ependymal cells in mice can act as neural progenitors and replace lost cells in spinal cord injury [1, 7, 8, 25–28], it appears that the ependyma remnants in adult humans do not proliferate after injury, suggesting that these cells are unlikely to be involved in cell replacement after a lesion [29].
Contrary to the notion of disappearance of the ependymal region, our previous study on two freshly collected spinal cords from organ donors aged seventeen and forty-six, showed the presence of a distinctive ependymal region with an observable lumen, even in the older donor [10]. RNA profiling of these cells combined with immunofluorescence validation showed good conservation of this region in humans and mice. Therefore, this study aimed to address the issue of the persistence of an ependymal region during human aging using a new collection of 17 fresh spinal cords obtained from organ donors aged between 37 and 83 years. Immunohistochemistry (IHC) and immunofluorescence (IF) were performed on lightly-fixed cryosections to determine whether a typical ependymal region with a lumen surrounded by FOXJ1+ PAX6+ SOX2+ cells could be observed in aged spinal cord and whether these cells could still be detected when the central canal was obliterated. We also examined whether the embryonic-like organization and expression of spinal cord developmental transcription factors can be maintained during aging. Finally, we investigated whether different types of FOXJ1+ ependymal cells were detected in human spinal cord.
Our results show that a typical ependymal region with an embryonic-like organization can persist even in very old individuals, at least in some portions of the spinal cord. The high heterogeneity among samples, coupled with non-standardized procedures for both spinal cord collection and histology, provides a plausible explanation for the observed disparate results.
Materials and methods
Human spinal cord extraction
Human spinal cords were dissected from organ donors at Montpellier Hospital with the approval of the French Institution for Organ Transplantation (Agence de Biomédecine). None of the patients had a history of Chiari infection, hydrocephalus, or operated scoliosis. Informed consent was obtained from the families by the organ procurement organization for this study. Before vascular aortic clamping, blood was replaced by perfusion with 4 °C-chilled IGL1 (Institut Georges Lopez, Lissieu, France) or Custodiol® (Essential Pharmaceuticals, Durham, UK) organ preservation solutions for abdominal or thoracic organ collection, respectively. The average duration between vascular clamping and spinal cord extraction was four hours when the heart, lungs, liver, and kidneys were removed, and an average of 2 h when only the liver and kidneys were removed. The body cavities were then cooled using crushed ice. The surgery was performed as previously described [30] and the thoracic or lumbar segments were immediately placed on ice before processing for immunohistochemistry. The donor characteristics and elapsed time between brain death, clamping, and spinal cord extraction are presented in Table S1.
Histology
Spinal cord segments were flash frozen in a 40-mL flat-bottom plastic tube immersed in a pre-chilled at − 80 °C isopentane bath placed in a SnapFrost® apparatus (Excilone, Elancourt, France) and then stored in a − 80 °C freezer. The spinal cords were cryosectioned transversally (14 µm thick sections) using a NX70 cryostat (Microm Microtech, Brignais, France) and sections were placed on SuperFrost® Plus Menzel Gläser slides (Thermo Scientific, Illkirch, France). The slides were stored in a − 80 °C freezer until used. Before immunolabeling, sections were lightly fixed in a 4% paraformaldehyde-PBS (phosphate-buffered saline) solution for 20 min at 4 °C and then washed twice with PBS. The antibodies and dilutions used for staining are listed in Table S2. Immunohistochemistry was performed using Polink-2 HRP Plus rabbit, mouse and goat DAB detection kits (Diagomics, Blagnac, France). The sections were incubated with primary antibodies overnight. Images were taken with a Nikon Eclipse microscope. To evaluate the presence of a closed versus an open central canal, sections were stained with hematoxylin. The number of sections examined is listed in Table S3.
Results
To probe the presence of ependymal cells during aging, we collected spinal cords from 17 organ donors (thoracic and/or lumbar levels) aged between 37 and 83 years (the donors are described in Table S1). In most cases, the spinal cord was removed within two to 4 h after aortic clamping and 6–27 h after death. To preserve antigens and avoid tissue shrinkage due to formaldehyde fixation [31], the spinal cords were frozen without fixation, and IHC was performed on lightly fixed sections to preserve antigens.
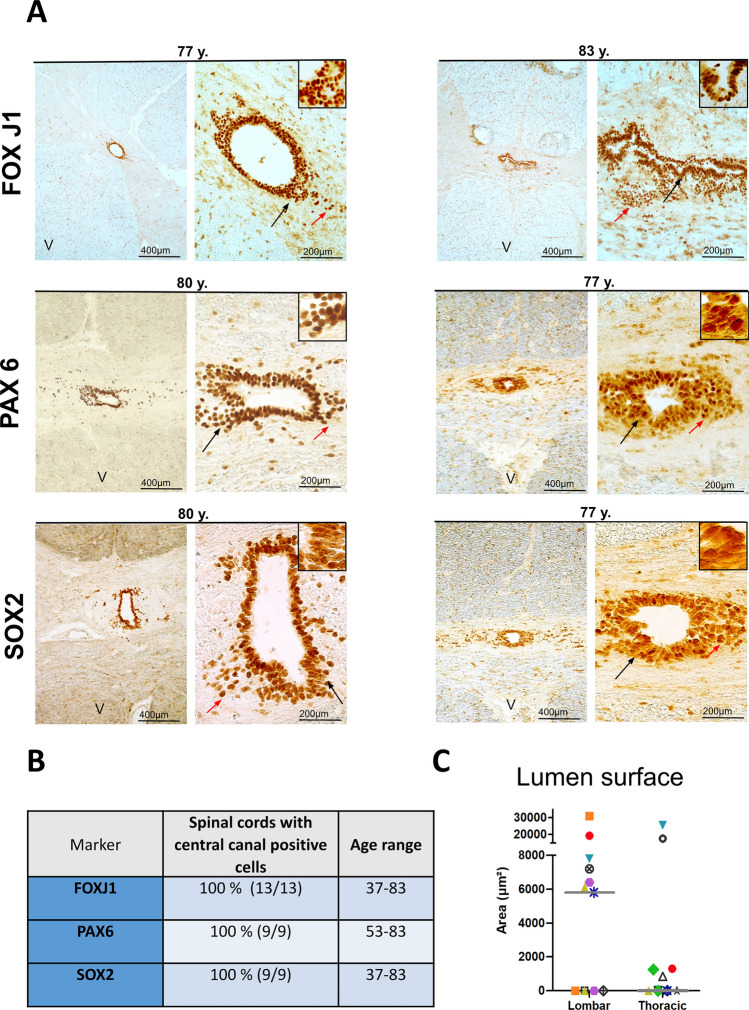
We began our analysis by examining the expression of the transcription factor FOXJ1 which is a very good marker of ependymal cells in mouse and human spinal cords [32]. Figure 1A illustrates the presence of FOXJ1+ cells around the central lumen of a 77-year-old and an 83-year-old donor. FOXJ1+ cells were also detected in all other examined cases (13/13) (Fig. 1B, all cases are presented in Fig. S1). We noted that in approximately 50% of spinal cords, a patent lumen was not observed and FOXJ1+ cells tended to appear as a mass of cells located in the central region (see for instance donor 12, upper thoracic, Fig. S1). When a central canal was present we also observed that in addition to FOXJ1+ cells surrounding the lumen, isolated and probably delaminating FOXJ1+ cells were often found away from the lumen (red arrows in Fig. 1 and ventral FOXJ1+ cells in Figs. 7, 8). In addition to FOXJ1, in mice and in young humans [10, 16], ependymal cells also express SOX2, which is a well-known neural stem cell transcription factor, together with PAX6, which is a homeodomain transcription factor that is highly expressed in spinal cord neuroepithelial cells during development [33]. As illustrated in Fig. 1A, both SOX2 and PAX6 patent staining were present in cells surrounding the lumen of the two 77- and 80-year-old donors, as well as in all other examined cases (9/9, Fig. 1B). We previously reported the specific expression of cilia-related genes in human and mouse ependymal cells [10]. To validate this expression in aged samples, we performed IHC for RFX2, which is an important transcription factor controlling ciliogenesis [34], and ARL13B, a regulatory GTPase involved in the sonic hedgehog pathway that is highly enriched in cilia [35, 36]. Strong nuclear expression of RFX2 and ARL13B at the border of ependymal cells was observed in all examined cases (Fig. 2A–C). Taken together these results demonstrate the persistence of ependymal cells expressing ARL13B, FOXJ1, PAX6, RFX2, and SOX2 in the human spinal cord during aging.
Fig. 1.
Detection of ependymal cells in aged spinal cords. A Immunohistochemistry of the indicated proteins. Left-hand images are low-magnification images (25×) showing the expression of specific markers in cells around the central canal. The black arrows indicate the magnified areas presented in the insets. Red arrows show stained cells away from the central canal. The letter V on the images indicates the ventral part of the spinal cord. B Summary of the staining results. C Scatter plot of the lumen surfaces measured in lumbar and thoracic spinal cords. Each donor is depicted by a different colored symbol. The gray bar shows the median value
Fig. 7.
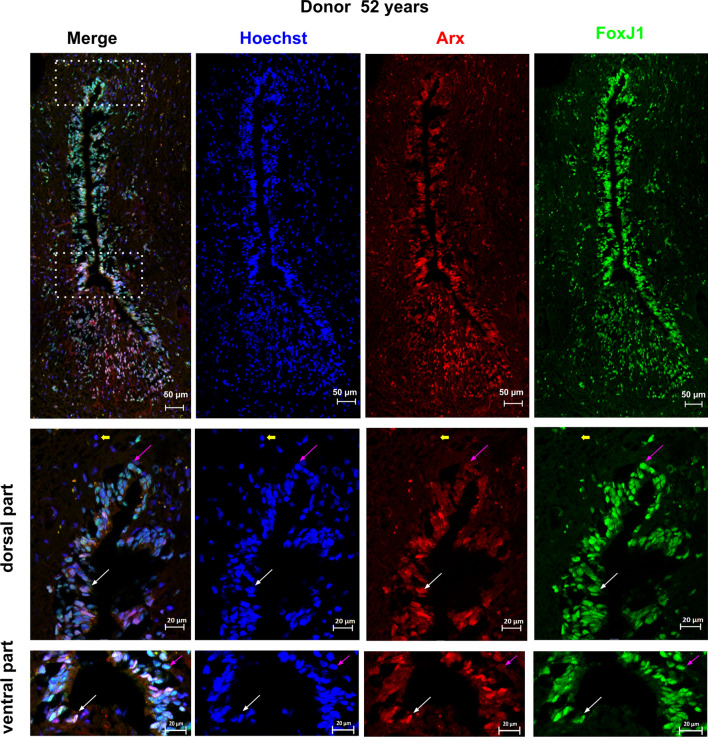
Expression of ARX by FOXJ1+ cells. The expression of ARX in FOXJ1+ cells within the spinal cord was analyzed using co-stainings for FOXJ1 and ARX in one donor aged 52 years. Double-labeled cells, indicating co-expression of FOXJ1 and ARX, are indicated by white arrows. To show labeling specificity, double-negative cells are indicated by yellow arrows. Pink arrows show FOXJ1+ cells negative for ARX. The dotted boxes in the image indicate areas that were used for high magnification images. Notably, in this donor, ARX + cells appear to have delaminated from the ventral part of the central canal. These findings suggest that ARX is expressed in some, but not all, FOXJ1+ cells within the spinal cord and that delamination of ARX+ cells from the central canal may occur in some cases
Fig. 8.
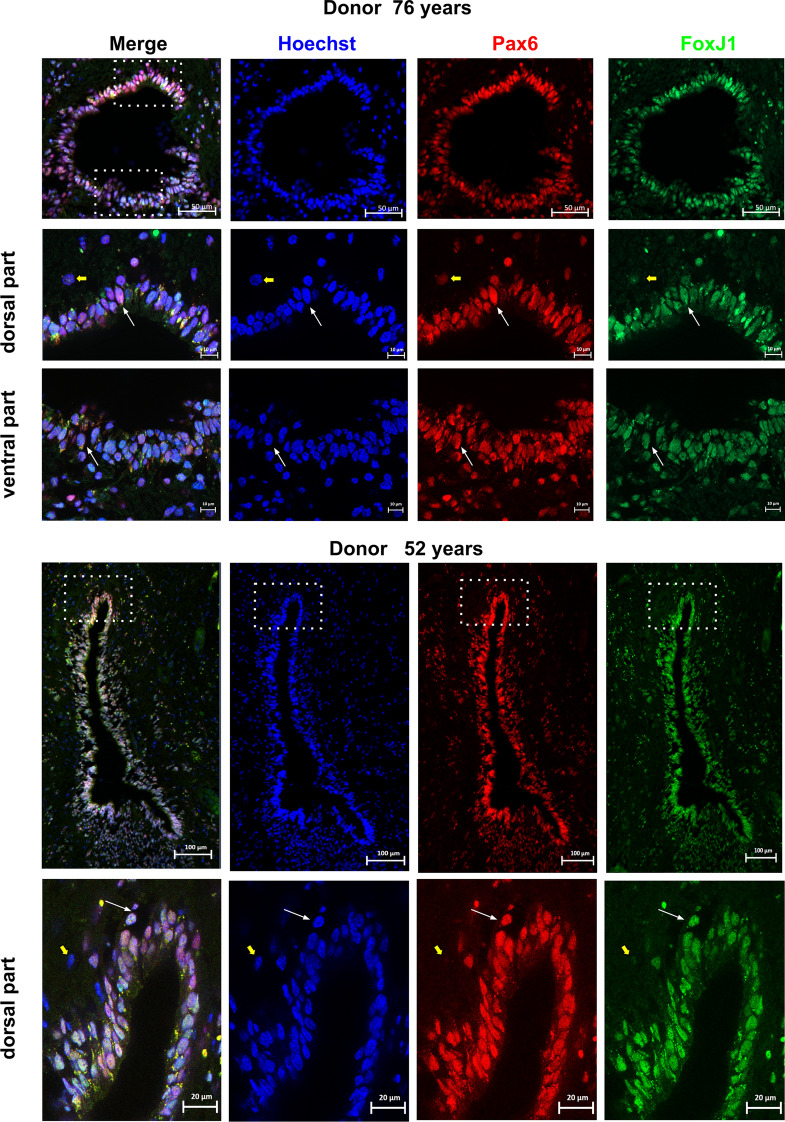
Expression of ARX by FOXJ1+ cells. The expression of ARX in FOXJ1+ cells within the spinal cord was analyzed using co-stainings for FOXJ1 and ARX in a second donor aged 76 years. Double-labeled cells, indicating co-expression of FOXJ1 and ARX, are indicated by white arrows. To show labeling specificity, double-negative cells are indicated by yellow arrows. Pink arrows show FOXJ1+ cells negative for ARX. Notably, in this donor, ARX+ cells appear to have delaminated from the ventral part of the central canal. For this panel, the presented ventral and dorsal high magnification images were taken from a section adjacent to the low magnification image. These findings suggest that ARX is expressed in some, but not all, FOXJ1+ cells within the spinal cord and that delamination of ARX+ cells from the central canal may occur in some cases
Fig. 2.
Detection of cilia-associated proteins in aged spinal cords. A, B Immunohistochemistry of the indicated proteins. A Staining for RFX2. Left-hand images are low magnification images (25×) showing specific expression of RFX2 in cells around the central canal. Black arrows indicate the magnified area presented in the inset. The letter V on the images indicates the ventral part of the spinal cord. B Staining for ARL13B. Arrows show accumulation of staining at the apical side of ependymal cells. C Summary of the staining results
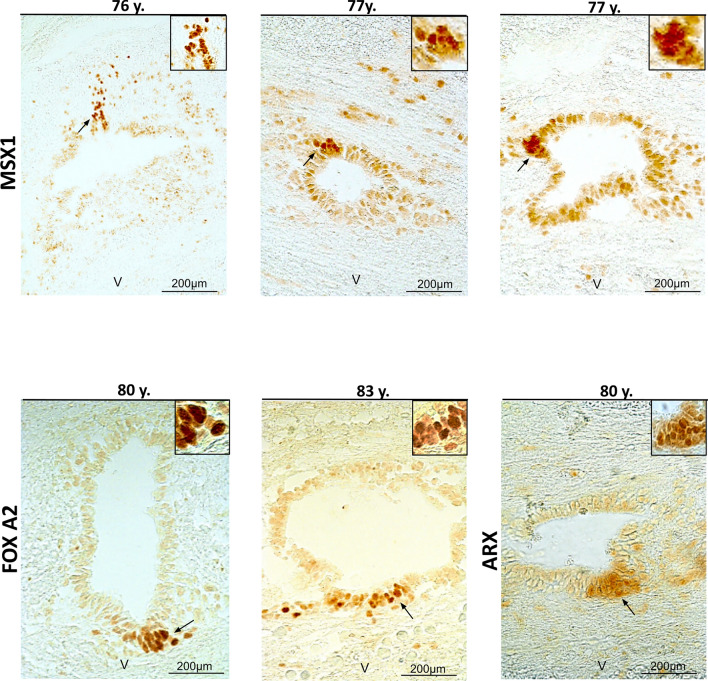
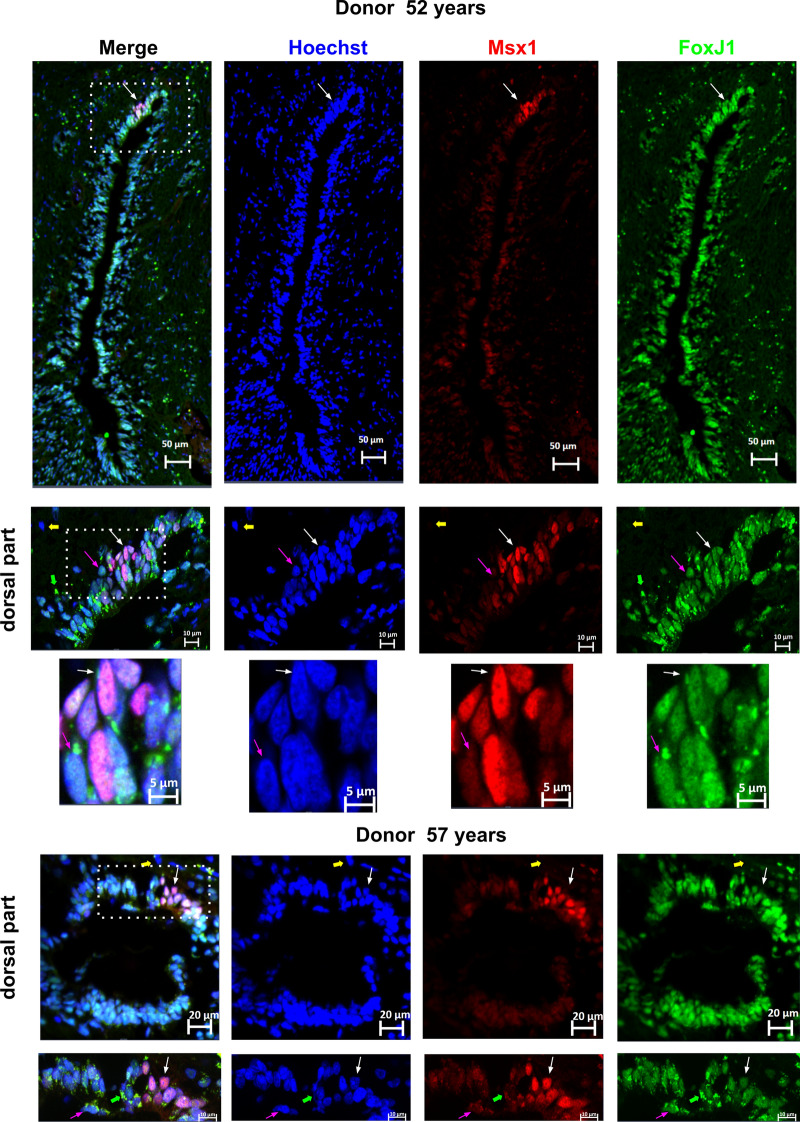
We previously reported the regionalized expression of the spinal cord developmental transcription factors ARX-FOXA1/2-MSX1, in mouse and human ependymal [10]. This pattern of expression is reminiscent of the situation during spinal cord development, where MSX1 is expressed by cells in the dorsal neuroepithelium, whereas ARX and FOXA1/2 expression are restricted to the ventral neural tube [12, 37]. We performed IHC of these three factors to determine whether the fetal-like regionalization could be maintained with aging. As presented in Fig. 3, for the three studied cases, we observed weak expression of MSX1 in most ependymal cells, however, a group of dorsal cells clearly exhibited higher MSX1 staining. In contrast to MSX1, ARX and FOXA2 staining were confined to a group of cells located in the ventral ependyma (Fig. 3).
Fig. 3.
Maintenance of a dorsal–ventral regionalization of the central canal in aged spinal cords. Immunohistochemistry of the indicated proteins in three aged donors. Black arrows indicate the magnified area presented in the inset. The letter V on the images indicates the ventral part of the spinal cord
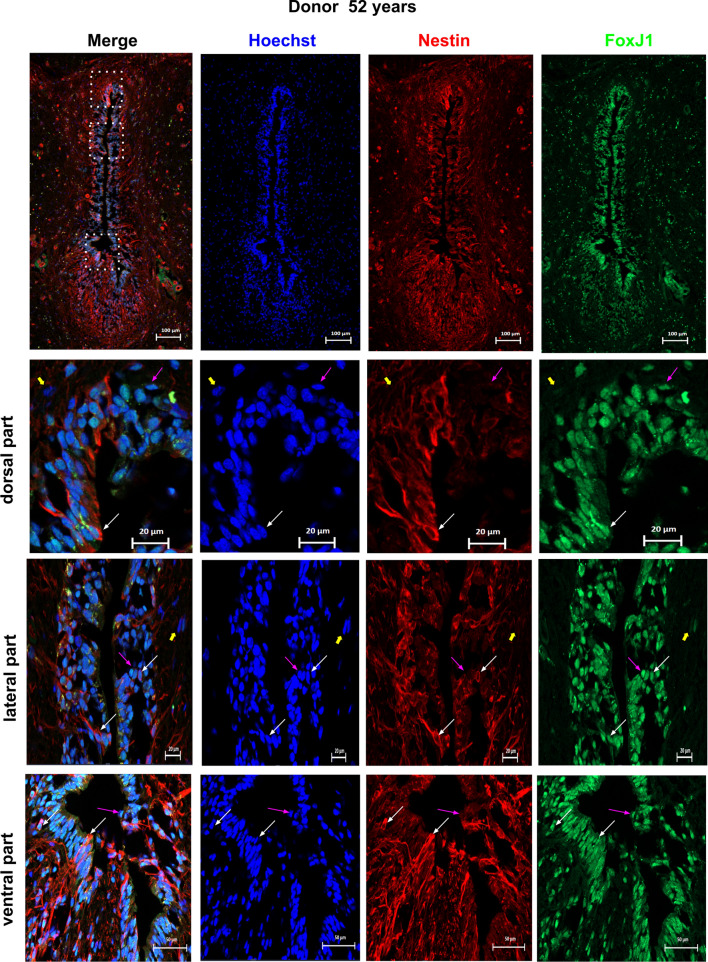
To ascertain whether ARX, FOXA2, MSX1, PAX6 and SOX2 are expressed in adult ependymal cells, we performed double stainings with FOXJ1. As presented in Figs. 4, 5, 6, 7, 8, 9, we observed that these developmental transcription factors were clearly expressed by all ependymal cells or subpopulations of FOXJ1+ cells. In addition to transcription factors, in a 52-year-old spinal cord, we also examined the expression of the well-known neural stem cell marker NESTIN (NES) in FOXJ1+ cells. Figure 10 shows that this cytoskeletal protein was expressed heterogeneously in ependymal FOXJ1+ cells.
Fig. 4.
Expression of PAX6 by FOXJ1+ cells. Expression of PAX6 by FOXJ1+ cells in the spinal cord was analyzed using co-stainings for FOXJ1 and PAX6 in two donors aged 52 and 76 years. Double-labeled cells are indicated by white arrows, demonstrating co-expression of FOXJ1 and PAX6. To show labeling specificity, double-negative cells are indicated by yellow arrows. The dotted boxes in the image indicate areas that were used for high magnification images. These findings indicate that PAX6 expression is present in FOXJ1+ cells within the spinal cord
Fig. 5.
Expression of SOX2 by FOXJ1+ cells. Expression of SOX2 by FOXJ1+ cells within the spinal cord was analyzed using co-stainings for FOXJ1 and SOX2 in one donor aged 57 years. Double-labeled cells, indicating co-expression of FOXJ1 and SOX2, are indicated by white arrows. To show labeling specificity, double-negative cells are indicated by yellow arrows. The dotted boxes in the image indicate areas that were used for high magnification images. These findings suggest that SOX2 is expressed in FOXJ1+ cells within the spinal cord
Fig. 6.
Expression of MSX1 by FOXJ1+ cells. The expression of MSX1 in FOXJ1+ cells within the spinal cord was analyzed using co-stainings for FOXJ1 and MSX1 in two donors aged 52 and 57 years. Double-labeled cells, indicating co-expression of FOXJ1 and MSX1, are indicated by white arrows. To show labeling specificity, double-negative cells are indicated by yellow arrows. Pink arrows show FOXJ1+ cells negative for MSX1. Green arrows indicate artifactual stainings, such as lipofuscin, commonly observed in old tissues. The dotted boxes in the image indicate areas that were used for high magnification images. These findings suggest that MSX1 expression is present in some, but not all, FOXJ1+ cells within the spinal cord
Fig. 9.
Expression of FOXA2 by FOXJ1+ cells. The expression of FOXA2 in FOXJ1+ cells within the spinal cord was analyzed using co-stainings for FOXJ1 and FOXA2 in one donor aged 57 years. Double-labeled cells, indicating co-expression of FOXJ1 and FOXA2, are indicated by white arrows. To show labeling specificity, double-negative cells are indicated by yellow arrows. FOXJ1+ cells negative for FOXA2 are shown by pink arrows, while FOXA2+ cells negative for FOXJ1 are shown by sky blue arrows. The dotted box in the image indicates the area used for the ventral high magnification image. These findings suggest that while some FOXJ1+ cells within the spinal cord co-express FOXA2, there are also distinct populations of FOXJ1+ cells and FOXA2 + cells that do not co-express these markers
Fig. 10.
Expression of NESTIN by FOXJ1+ cells. The expression of NESTIN in FOXJ1+ cells within the spinal cord was analyzed using co-stainings for FOXJ1 and NESTIN in one donor aged 52 years. Double-labeled cells, indicating co-expression of FOXJ1 and NESTIN, are indicated by white arrows. To show labeling specificity, double-negative cells are indicated by yellow arrows. FOXJ1+ cells negative for NESTIN are shown by pink arrows. Dotted boxes in the image indicate the areas used for high magnification images. These findings suggest that while some FOXJ1+ cells within the spinal cord co-express NESTIN, there are also distinct populations of FOXJ1+ cells that do not express NESTIN
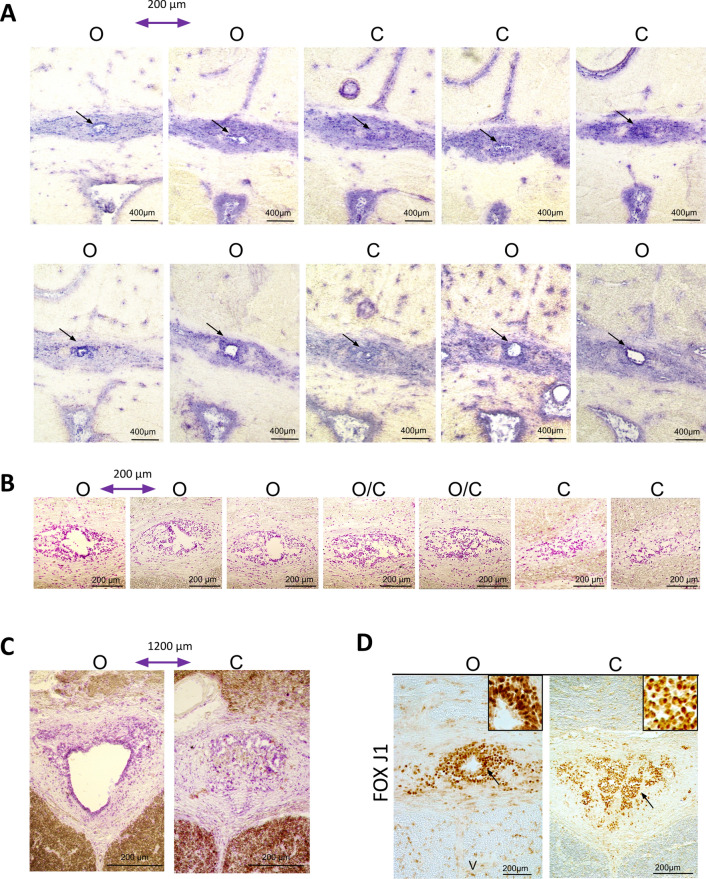
Finally, loss of the central canal and stenosis have been previously reported during aging of the human spinal cord [17–19, 24]. We found three types of situations in our cohort (Table S3–S4): (1) cases in which all examined sections had a lumen (45% of explored segments). It is worth noting that this lumen was not always circular and could be distorted (see, for instance, Fig. 1A, top right-hand image of an 83-year-old donor). However, a wide and round lumen was observed even in old donors (Fig. 1A left-hand image). The surface of the lumen was generally larger in the lumbar spinal cord than in the thoracic segment (Fig. 1C) where the lumen can be very small (see, for instance, Fig. 1A PAX6 staining, 77-year-old donor). (2) Cases in which no patent lumen was found (40% of explored segments). Examples of this type of pattern are shown in Fig. S1. However it is important to note that even in this situation, ependymal cells expressing FOXJ1, PAX6 and SOX2 were still detected (Figs. 11D and Fig. S2). (3) Cases in which, depending on sections in the same segment, we noted the absence or the presence of a central canal (15% of explored segments; Fig. 11). To further explore this pattern, we performed serial sectioning of four samples. We observed the coexistence of open and closed central canals over a distance of 1000–2000 µm for three donors (Fig. 11A–C), whereas all sections over 5000 µm were closed for the fourth donor (not shown).
Fig. 11.
Alternation of closed-open central canals. A, B: The series of images show hematoxylin-stained sections, spaced at 200 µm each, from a 77 year old (A) and a 55 year old (B) upper thoracic spinal cord. C Two images from the ependymal region of a 66 year old spinal cord taken 1200 µm apart. D Immunohistochemistry of FOXJ1 in one 77 year old spinal cord donor showing stained cells in sections with an open (left) or a closed (right) central canal. The letters O and C indicate open and closed central canals, respectively (dark arrows)
Discussion
This article reports that ependymal cells expressing FOXJ1 and spinal cord neurodevelopmental and stem cell proteins (i.e., ARX, FOXA2, MSX1, NESTIN, PAX6, and SOX2) are maintained during aging of the human spinal cord. These findings contrast with those of previous studies, suggesting that these cells may be lost with age [17–19, 24].
These varying conclusions are likely due to differences in the origin and heterogeneity of human samples and the technical approaches employed, which are typical in human tissue research [see, for instance, [38]. First, we used organ donors as sources of the spinal cord. A critical factor in accurate detection of marker proteins is the post-mortem delay, i.e., the time between the death of a person and fixation of the spinal cord [38, 39]. Unlike autopsy, which is generally used in most studies of human spinal cord ependyma, the organ donors were perfused with an organ preservation solution and the spinal cords were removed only a few hours after clamping, typically 2–4 h. This procedure is likely to preserve the ependymal cells as close as possible to their original state. Second, after collection, the spinal cords were directly frozen at a low temperature without fixation and IHC was performed on frozen sections that were only lightly fixed. In contrast, most studies on the human spinal cord ependyma are based on an extended paraformaldehyde fixation followed by paraffin embedding which has been shown to remove lipids and induce substantial tissue shrinkage (up to 60% for mouse brain [40]). This histological procedure may affect the structure of the ependymal region and the reactivity of ependymal cells to antibodies.
Although the conditions for collecting the spinal cord after death were more favorable for histological analysis, we still noted stenosis or a disorganized central canal in approximately 50% of cases. This finding is consistent with previous histological studies that have reported partial or complete stenosis of the ependyma with aging [17–19, 24]. This underscores the significant heterogeneity of human spinal cord samples and emphasizes the necessity of studying large cohorts. Even in the case of central canal stenosis, we detected cells expressing FOXJ1, SOX2 and PAX6 indicating that the ependymal cells did not disappear. We observed cases with both open and closed central canal regions, indicating that the disorganization of the central canal was not uniform throughout the spinal cord. Indeed, by examining 232 cases, Milhorat et al. found only four complete occlusions (1.7%) of the entire central canal across the seven levels that were analyzed [19]. Combined with our observations of closed and open ependymal regions in the same donors, these data suggest that the obliteration of the central canal is not continuous.
We previously reported that in mice and in humans, aged 17 and 46 years, the ependyma exhibits an embryonic-like organization, with differential dorsal–ventral expression of spinal cord neurodevelopmental transcription factors such as ARX-FOXA1/2-MSX1 [10]. This organization very likely arises from the persistence of cells originating from the neural tube roof and floor plates [2, 10, 12]. Strikingly, we found that such an organization could be observed even in donors over 75 years of age. During development, these dorsal and ventral cells secrete important morphogens such as BMP6 and SHH that regulate the growth and fate of developing spinal cord stem cells [12]. The role and contribution of these dorsal and ventral cells in the maintenance of ependymal cells during aging of the human spinal cord remain to be elucidated.
Our double-staining experiments with FOXJ1 revealed heterogeneity of ependymal cells in humans, with subpopulations of FOXJ1+ ependymal cells that differentially express NESTIN and neurodevelopmental transcription factors. This is consistent with studies conducted in mice and macaques, notably using single-cell RNA sequencing, which revealed the heterogeneity of these cells [5, 6, 10, 11, 14, 21]. These findings raise questions about the different functions of these cells.
While this work was in progress, single-cell RNA-seq analysis was performed on seven lumbar spinal cords from some of the cases included in the present article and aged 50–80 years [41]. A database for gene expression in the human spinal cord is available on a website (vmenon.shinyapps.io/humanspinalcord). By interrogating this resource, we found that 6/7 of the genes we explored in our study by IHC; namely, Arx, FoxJ1, Msx1, Pax6, Rfx2 and Sox2 were well expressed in ependymal cells at the single-cell RNA level (Fig. S3). FoxA2 expression was not detected in ependymal cells or in the entire spinal cord through scRNA sequencing, which may be due to its expression being restricted to only a few cells located in the ventral central canal, as detected by IHC (Fig. 3). These independent data further support the notion of maintenance of ependymal cells during human spinal cord aging.
In conclusion, our results based on IHC and IF, combined with those of three other studies, provide strong evidence for the maintenance of human ependymal cells with immature traits throughout life. These studies included (1) PCR analysis demonstrating enriched expression of Pax6, FoxJ1, and Msi1 (Musashi) genes in ependyma from patients aged 32–76 years [17], (2) single-cell RNA sequencing identifying ependymal cells in donors aged 50–80 years [41], and (3) recent IF for FOXJ1 and RNA in situ hybridization for Arx demonstrating their expression in up to 52-year-old human spinal cord ependymas [21]. The degree of stenosis and disorganized ependyma displayed significant variations across studies, which may be partly attributed to differences in current protocols. This highlights the need for standardization of collection and histological analysis of human spinal cords. Additionally, the origins of spinal cord stenosis, which vary with the spinal cord level, may be influenced by a multitude of factors, including but not limited to the local-lifestyle, ethnicity, and unknown parameters.
Limitations
Technical variability among samples represents a potential limitation of our study on ependymal cells in the human spinal cord, stemming from factors such as non-standardized sample collection, variation in postmortem intervals until sample collection, and diverse clinical histories.
Our study was also limited by our inability to demonstrate maintenance of proliferation and differentiation capacities in aged ependymal cells, as we were unable to detect proliferative ependymal cells using the proliferation marker mKI67 (data not shown). Human ependymal cells likely undergo mitosis at a low rate, rendering this approach unsuitable. Alternatively, analysis of cell proliferation via the incorporation of 14C derived from nuclear bomb testing would be more suitable [42]
Future directions
In mice, a fraction of the spinal cord ependymal cells behaves as stem cells and can form multipotent and passageable neurospheres in culture [26, 28]. After spinal cord injury, ependymal cells rapidly proliferate via Ras signaling activation [43] and then migrate to the lesion site. This was notably observed when the lesion compromised the integrity of the ependymal region [44]. Unfortunately, these cells do not produce neurons, generate only a few oligodendrocytes, and mainly form astrocytes [45]. However these astrocytes contribute to the core of the glial scar and are beneficial for recovery by reducing axonal loss and secondary lesion [43]. In addition, recent studies in mice, have revealed that ependymal cells are in a permissive chromatin state that enables unfolding of a normally latent gene expression program for oligodendrogenesis which could be used to replace a large number of lost oligodendrocytes in the injured mouse spinal cord [46]. Unlike in rodents, it has been observed that ependymal cells in humans do not proliferate after injury, regardless of the time or distance from the lesion [29]. Nonetheless, there is an increase in NESTIN+ ependymal cells in human spinal cord following traumatic injury implying the presence of a certain degree of plasticity among these cells to revert to an immature state [15].
Considering the heterogeneity of human ependymal cells, it is necessary to continue studying these cells to gain clarity and determine their potential for regeneration in spinal cord injuries or degenerative diseases such as multiple sclerosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figures S1–3 (PDF 5879 KB)
Acknowledgements
We thank the French Agency of biomedicine and all Montpellier Biocampus facilities for help (RHEM, MRI) and excellent technical work. We also warmly thank all organ donors and their family as well as nurses and all medical staff from the Unit of Donation and Transplantation (Montpellier hospital). We thank Dr Ariel Levine (NIH, USA) for permission to use screen capture from the Human Spinal Cord snRNAseq Viewer (https://vmenon.shinyapps.io/humanspinalcord/)
Author contributions
All authors contributed to the study conception and design. Spinal cords were mainly extracted and collected by LB, RC, NL and GP. Chantal Ripoll designed and performed histology, quantifications and figure preparation. RC participated in histology, performed data analysis and quantifications. FV-L coordinated informed consents and spinal cord collections. The first draft of the manuscript was written by JPH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.”
Funding
This work was supported by grants from IRP (Switzerland, 2013), IRME (France, 2020), AFM (France, 2019), ANR ERANET Neuroniche (JP Hugnot, 2019), ARSEP (France, 2020). T Chevreau was supported by an AFM PhD fellowship.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted to Dr Luc Bauchet by the French “Agence de la biomédecine”, n° SPGED19-3-4692, April, 2nd, 2019.
Consent to participate
Informed consent was obtained from relatives of organ donors included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luc Bauchet and Jean-Philippe Hugnot contributed equally.
Change history
7/16/2023
The ESM file has been updated.
Change history
7/15/2023
A Correction to this paper has been published: 10.1007/s00018-023-04856-y
References
- 1.Stenudd M, Sabelström H, Llorens-Bobadilla E, et al. Identification of a discrete subpopulation of spinal cord ependymal cells with neural stem cell properties. Cell Rep. 2022;38:110440. doi: 10.1016/j.celrep.2022.110440. [DOI] [PubMed] [Google Scholar]
- 2.Xing L, Anbarchian T, Tsai JM, et al. Wnt/β-catenin signaling regulates ependymal cell development and adult homeostasis. Proc Natl Acad Sci USA. 2018;115:E5954–E5962. doi: 10.1073/pnas.1803297115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu H, Qi Y, Tan M, et al. Molecular mapping of the origin of postnatal spinal cord ependymal cells: evidence that adult ependymal cells are derived from Nkx6.1+ ventral neural progenitor cells. J Comp Neurol. 2003;456:237–244. doi: 10.1002/cne.10481. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton LK, Truong MKV, Bednarczyk MR, et al. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience. 2009;164:1044–1056. doi: 10.1016/j.neuroscience.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Milich LM, Choi JS, Ryan C, et al. Single-cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord. J Exp Med. 2021;218:e20210040. doi: 10.1084/jem.20210040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfaro-Cervello C, Soriano-Navarro M, Mirzadeh Z, et al. Biciliated ependymal cell proliferation contributes to spinal cord growth. J Comp Neurol. 2012;520:3528–3552. doi: 10.1002/cne.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marichal N, Reali C, Rehermann MI, et al. Progenitors in the ependyma of the spinal cord: a potential resource for self-repair after injury. Adv Exp Med Biol. 2017;1015:241–264. doi: 10.1007/978-3-319-62817-2_13. [DOI] [PubMed] [Google Scholar]
- 8.Becker CG, Becker T, Hugnot J-P. The spinal ependymal zone as a source of endogenous repair cells across vertebrates. Prog Neurobiol. 2018;170:67–80. doi: 10.1016/j.pneurobio.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Deng S, Gan L, Liu C, et al. Roles of ependymal cells in the physiology and pathology of the central nervous system. Aging Dis. 2023;14:468–483. doi: 10.14336/AD.2022.0826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghazale H, Ripoll C, Leventoux N, et al. RNA profiling of the human and mouse spinal cord stem cell niches reveals an embryonic-like regionalization with MSX1+ roof-plate-derived cells. Stem Cell Rep. 2019;12:1159–1177. doi: 10.1016/j.stemcr.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabourin J-C, Ackema KB, Ohayon D, et al. A mesenchymal-like ZEB1(+) niche harbors dorsal radial glial fibrillary acidic protein-positive stem cells in the spinal cord. Stem Cells (Dayt Ohio) 2009;27:2722–2733. doi: 10.1002/stem.226. [DOI] [PubMed] [Google Scholar]
- 12.Wilson L, Maden M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Dev Biol. 2005;282:1–13. doi: 10.1016/j.ydbio.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 13.Pfenninger CV, Steinhoff C, Hertwig F, Nuber UA. Prospectively isolated CD133/CD24-positive ependymal cells from the adult spinal cord and lateral ventricle wall differ in their long-term in vitro self-renewal and in vivo gene expression. Glia. 2011;59:68–81. doi: 10.1002/glia.21077. [DOI] [PubMed] [Google Scholar]
- 14.Alfaro-Cervello C, Cebrian-Silla A, Soriano-Navarro M, et al. The adult macaque spinal cord central canal zone contains proliferative cells and closely resembles the human. J Comp Neurol. 2014;522:1800–1817. doi: 10.1002/cne.23501. [DOI] [PubMed] [Google Scholar]
- 15.Cawsey T, Duflou J, Weickert CS, Gorrie CA. Nestin-positive ependymal cells are increased in the human spinal cord after traumatic central nervous system injury. J Neurotrauma. 2015;32:1393–1402. doi: 10.1089/neu.2014.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dromard C, Guillon H, Rigau V, et al. Adult human spinal cord harbors neural precursor cells that generate neurons and glial cells in vitro. J Neurosci Res. 2008;86:1916–1926. doi: 10.1002/jnr.21646. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Ovejero D, Arevalo-Martin A, Paniagua-Torija B, et al. The ependymal region of the adult human spinal cord differs from other species and shows ependymoma-like features. Brain J Neurol. 2015;138:1583–1597. doi: 10.1093/brain/awv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasantikul V, Netsky MG, James AE. Relation of age and cerebral ventricle size to central canal in man. Morphological analysis. J Neurosurg. 1979;51:85–93. doi: 10.3171/jns.1979.51.1.0085. [DOI] [PubMed] [Google Scholar]
- 19.Milhorat TH, Kotzen RM, Anzil AP. Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J Neurosurg. 1994;80:716–722. doi: 10.3171/jns.1994.80.4.0716. [DOI] [PubMed] [Google Scholar]
- 20.Paniagua-Torija B, Arevalo-Martin A, Ferrer I, et al. CB1 cannabinoid receptor enrichment in the ependymal region of the adult human spinal cord. Sci Rep. 2015;5:17745. doi: 10.1038/srep17745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigo Albors A, Singer GA, Llorens-Bobadilla E, et al. An ependymal cell census identifies heterogeneous and ongoing cell maturation in the adult mouse spinal cord that changes dynamically on injury. Dev Cell. 2023;58:239–255.e10. doi: 10.1016/j.devcel.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Saker E, Henry BM, Tomaszewski KA, et al. The human central canal of the spinal cord: a comprehensive review of its anatomy, embryology, molecular development, variants, and pathology. Cureus. 2016;8:e927. doi: 10.7759/cureus.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torrillas de la Cal A, Paniagua-Torija B, Arevalo-Martin A, et al. The structure of the spinal cord ependymal region in adult humans is a distinctive trait among mammals. Cells. 2021;10:2235. doi: 10.3390/cells10092235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasui K, Hashizume Y, Yoshida M, et al. Age-related morphologic changes of the central canal of the human spinal cord. Acta Neuropathol (Berl) 1999;97:253–259. doi: 10.1007/s004010050982. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert EAB, Lakshman N, Lau KSK, Morshead CM. Regulating endogenous neural stem cell activation to promote spinal cord injury repair. Cells. 2022;11:846. doi: 10.3390/cells11050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno-Manzano V. Ependymal cells in the spinal cord as neuronal progenitors. Curr Opin Pharmacol. 2020;50:82–87. doi: 10.1016/j.coph.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Sabelström H, Stenudd M, Frisén J. Neural stem cells in the adult spinal cord. Exp Neurol. 2014;260:44–49. doi: 10.1016/j.expneurol.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Weiss S, Dunne C, Hewson J, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paniagua-Torija B, Norenberg M, Arevalo-Martin A, et al. Cells in the adult human spinal cord ependymal region do not proliferate after injury. J Pathol. 2018;246:415–421. doi: 10.1002/path.5151. [DOI] [PubMed] [Google Scholar]
- 30.Bauchet L, Poulen G, Lonjon N, et al. Isolation and culture of precursor cells from the adult human spinal cord. Methods Mol Biol Clifton NJ. 2022;2389:103–110. doi: 10.1007/978-1-0716-1783-0_10. [DOI] [PubMed] [Google Scholar]
- 31.Mouritzen Dam A. Shrinkage of the brain during histological procedures with fixation in formaldehyde solutions of different concentrations. J Hirnforsch. 1979;20:115–119. [PubMed] [Google Scholar]
- 32.Jacquet BV, Salinas-Mondragon R, Liang H, et al. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Dev Camb Engl. 2009;136:4021–4031. doi: 10.1242/dev.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgala PA, Carr CB, Price DJ. The role of Pax6 in forebrain development. Dev Neurobiol. 2011;71:690–709. doi: 10.1002/dneu.20895. [DOI] [PubMed] [Google Scholar]
- 34.Chung M-I, Peyrot SM, LeBoeuf S, et al. RFX2 is broadly required for ciliogenesis during vertebrate development. Dev Biol. 2012;363:155–165. doi: 10.1016/j.ydbio.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim YS, Chua CEL, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell. 2011;103:209–221. doi: 10.1042/BC20100150. [DOI] [PubMed] [Google Scholar]
- 36.Bay SN, Long AB, Caspary T. Disruption of the ciliary GTPase Arl13b suppresses Sonic hedgehog overactivation and inhibits medulloblastoma formation. Proc Natl Acad Sci USA. 2018;115:1570–1575. doi: 10.1073/pnas.1706977115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho G, Lim Y, Cho I-T, et al. Arx together with FoxA2, regulates Shh floor plate expression. Dev Biol. 2014;393:137–148. doi: 10.1016/j.ydbio.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee H, Thuret S. Adult human hippocampal neurogenesis: controversy and evidence. Trends Mol Med. 2018;24:521–522. doi: 10.1016/j.molmed.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Kempermann G, Gage FH, Aigner L, et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23:25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wehrl HF, Bezrukov I, Wiehr S, et al. Assessment of murine brain tissue shrinkage caused by different histological fixatives using magnetic resonance and computed tomography imaging. Histol Histopathol. 2015;30:601–613. doi: 10.14670/HH-30.601. [DOI] [PubMed] [Google Scholar]
- 41.Yadav A, Matson KJE, Li L, et al. A cellular taxonomy of the adult human spinal cord. Neuron. 2023;111:328–344.e7. doi: 10.1016/j.neuron.2023.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spalding KL, Bhardwaj RD, Buchholz BA, et al. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 43.Sabelström H, Stenudd M, Réu P, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342:637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- 44.Ren Y, Ao Y, O’Shea TM, et al. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Sci Rep. 2017;7:41122. doi: 10.1038/srep41122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meletis K, Barnabé-Heider F, Carlén M, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llorens-Bobadilla E, Chell JM, Le Merre P, et al. A latent lineage potential in resident neural stem cells enables spinal cord repair. Science. 2020 doi: 10.1126/science.abb8795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1–3 (PDF 5879 KB)
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).