Abstract
Recent studies have illustrated that psoriatic lesions are innervated by dense sensory nerve fibers. Psoriatic plaques appeared to improve after central or peripheral nerve injury. Therefore, the nervous system may play a vital role in psoriasis. We aimed to clarify the expression of nerve fibers in psoriasis and their relationship with immune cells and keratinocytes, and to explore the effect of skin nerve impairment. Our results illustrated that nerve fibers in psoriatic lesions increased and were closely innervated around immune cells and keratinocytes. RNA-seq analysis showed that peripheral sensory nerve-related genes were disrupted in psoriasis. In spinal cord hemi-section mice, sensory impairment improved psoriasiform dermatitis and inhibited the abnormal proliferation of keratinocytes. Botulinum toxin A alleviated psoriasiform dermatitis by inhibiting the secretion of calcitonin gene-related peptide. Collectively, cutaneous nerve fibers participate in the progression of psoriasis by linking epidermal keratinocytes and immunocytes. Neurological intervention may be a new treatment strategy for psoriasis.
Keywords: Psoriasis, Sensory nerve, Spinal cord hemi-section model, Botulinum toxin A
Introduction
Psoriasis is a chronic immune-mediated inflammatory disease mediated by T cells and dendritic cells [1]. The pathogenesis of psoriasis has not been completely clear. Most researches focus on the immune-related mechanism [2], few researchers on the role of neurons and neurotransmitters as mediators. However, psychosis or mental abnormal can induce onset of psoriasis [3]. Nerve damage can play an important role in skin diseases. For example, negative emotions contribute to skin diseases such as alopecia areata and vitiligo [4]. However, there are limited researches reporting the role of neural factors in the occurrence and development of psoriasis.
As early as 1965, histopathological study had shown increased number of nerve fibers, Schwann cells and other nerve cells in the epiderma of psoriasis plaques [5, 6]. Till 2016, 12 cases reported that after central or peripheral nerve injury (such as cerebrovascular accident, trauma, polio, etc.), the psoriasis plaques in neurological dysfunction skin were improved or got completely remission [7]. These case reports provide clinical evidence for the involvement of the nervous system in the pathogenesis of psoriasis. Taken together, these factors shed a light on the role of nerve endings in psoriasis.
The peripheral nervous system can induce protective nociceptive nerve reflexes and release neurotransmitters to resist external dangers. More evidence supports the hypothesis of the two-way neuroimmune communication in body homeostasis [8, 9]. Immune cells express multiple receptors that respond to neuropeptides, regulating local antibacterial responses [10]. For example, after C. albicans infection, calcitonin gene-related peptide (CGRP) released by skin neurons stimulates the production of IL-23 by dermal conventional type-2 dendritic cells (cDC2) resulting in Type-17 immunity [11]. Skin peripheral nerves in the skin can also release neuropeptides leading to inflammatory skin diseases. In atopic dermatitis, the initiation of allergic response activates substance p (SP)-producing TRPV1+ nociceptors and then triggers mast cell degranulation via MRGPRB2 [12].
Therefore, exploring the role of peripheral nervous system will help us better understand the pathogenesis of psoriasis. Herein, we defined the distribution pattern of nerve fibers in the lesional skin of psoriasis by immunohistochemistry, combined with the analysis of the results of psoriasis bulk skin RNA-seq in the GEO database. Furthermore, in the mouse model of psoriatic-like dermatitis, hemisection of the spinal cord and intervention of type A botulinum toxin were used to further confirm the role of the nervous system in the occurrence and development of psoriasis.
Materials and methods
Patients
The skin biopsies used in this project were from 4 psoriasis patients and 4 healthy volunteers in the dermatology clinic. After local anesthesia using 2% lidocaine, an incisional biopsy with 6 mm diameter was performed with a scalpel. Biopsies of the lesional skin from psoriasis patients and normal skin from healthy volunteers were taken. The collection and processing experiments were approved by the Institutional Review Board of the Second Affiliated Hospital, Zhejiang University School of Medicine. Written and informed consent was obtained from all psoriasis patients and healthy controls.
Mice
Female C57BL/6 wild-type mice at 6 weeks old were purchased from Shanghai SLAC Laboratory Animal Co., Ltd., and bred in the Animal Experiment Center of the Second Affiliated Hospital, Zhejiang University School of Medicine. All animal experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine.
Imiquimod (IMQ) induced psoriasis-like mouse model
Imiquimod cream (Sichuan Mingxin Pharmaceutical Co., Ltd.) was used to induce psoriasis-like dermatitis in mice. The mice were depilated on the dorsal skin and then 62.5 mg 5% imiquimod cream was topically applied for 6 consecutive days. The sham controls were treated with the same dose of base ointment.
Co-culture of mouse dorsal root ganglion (DRG) neurons and keratinocytes
The method of culturing keratinocytes is the same as described before [13]. Skin samples from mouse were incubated with 0.5% dispase (Gibco) at 37 °C for 2 h. Then the epidermis was peeled off and incubated with 0.25% trypsin (Gibco) for 10 min, neutralize by 10% FBS (Gibco), centrifuged at 1000 rpm/min for 10 min and then cultured in keratinocyte medium (Millipore). Mouse L4-L6 DRG was taken, digested with 0.1% collagenase (Sigma) and 0.25% trypsin for 30 min, neutralized with 10% FBS and centrifuged at 1000 rpm for 10 min. The precipitate was mixed with keratinocyte culture medium, and added to the keratinocyte culture flask. Nerve growth factor (Sigma) and B-27 supplement (Gibco) were added for neuron incubation. After 4–5 days, cells were used for immunofluorescence detection.
Spinal cord hemisection model
The mouse was anesthetized with 1% sodium pentobarbital. The T10 thoracic spinous process was used as surface landmark. Under microscope observation and with the spinal cord anterior and posterior veins as the boundary, microsurgery scissors were used to cut the left half of the spinal cord. Then the muscle and skin were sutured layer by layer. The effectiveness of the mouse model of spinal cord injury was evaluated by trunk imbalance and paralysis of the ipsilesional hind limb and pain and temperature sensory disturbance on the contralesional side of the injury.
Botulinum toxin A (BTX-A) injection
Normal saline was applied to formulate BTX-A (Allergen) dry powder into 0.005 U/μl solution. The back of the mice was depilated. Four evenly distributed injection points were selected on the back, and 50 μl of BTX-A of working concentration was injected subcutaneously, and each mouse was injected with 1 unit in total. The control group was injected with 50 μl of normal saline.
Immunofluorescence (IF) and Immunohistochemistry (IHC)
Skin biopsy was embedded in OCT (Sakura Finetek), sectioned by freezing microtome (Leica), fixed in ice acetone for 15 min and blocked with 10% BSA (Sigma) for 1 h. DRG was also put in OCT immediately after isolation and then blocked with 10% BSA without fixation. Tissues were stained with rabbit monoclonal anti-keratin 14 antibody (1:500, Abcam), mouse monoclonal anti-βIII-tubulin antibody (1:200, Abcam), rabbit monoclonal anti-CGRP antibody (1:200, CST), rabbit monoclonal anti-TRPV1 antibody (1:200, Alomone), rabbit monoclonal anti PGP9.5 antibody (1:200, Abcam), rabbit monoclonal anti-CD103 antibody (1:400, Abcam), rabbit monoclonal anti-CD11b antibody (1:200, Abcam) or rabbit monoclonal anti-Nav1.8 antibody (1:200, Abcam) in PBS with 5% FBS for 1 h, and then incubated with Alexa Fluro 488 or 555-conjugated secondary antibody (1:2000, Invitrogen) for 1 h. Nuclei were counterstained with DAPI (1:5000, Roche). Images were pictured by Leica DM5500B. In skin section IF, cells surrounded by nerve fibers were counted. In IF of DRG, ImageJ was used for semi-quantitative statistics.
For IHC of Ki67 and PGP9.5, skin sections from mice were blocked with 10% BSA 1 h, incubated with anti-Ki67 antibody (1:500, Abcam) or anti-PGP9.5 antibody (1:500, Abcam), washed, then incubated with secondary HRP conjugated antibody and stained with DAB (Vector Laboratories). Images were scanned by Nano Zoomer (Hamamatsu) and captured by NDP.view software.
Western blotting
Skin biopsies were grinded to powder in mortar and lysed with RIPA buffer. Lysates were boiled with 5 × Loading buffer for 10 min. Protein samples were separated with SDS-PAGE gel and immunoblotted with mouse monoclonal anti-βIII-tubulin antibody (1:1000, Abcam), rabbit monoclonal anti-CGRP antibody (1:1000, CST), rabbit monoclonal anti-TRPV1 antibody (1:1000, Alomone), rabbit monoclonal anti-Nav1.8 antibody (1:1000, Abcam), followed by an incubation with a secondary antibody. The immunoreactive bands were detected using ECL Substrate (Thermo Scientific).
Immunoelectron microscopy
The psoriatic lesional skin from psoriasis patients was placed in 4% paraformaldehyde–0.5% glutaraldehyde fixative (PG) for fixation overnight and was cut into 50 μm sections. Then the tissue pieces were fixed in 4% paraformaldehyde (Sangon Biotech). Skin slices were treated with 1% hydrogen peroxide for 15 min and then incubated with goat serum (Beyotime) at room temperature for 1 h. Then the sample slice was incubated with the anti-βIII-tubulin antibody (1:200, Abcam) for 24 h at 4 °C. After washed with PBS for 3 times, the sample was incubated with colloidal gold-labeled antibody solution for 1 h at room temperature and washed with ddH2O. Picture was taken with Tecnai G2 Spirit 120 kV cryo-electron microscope.
qRT-PCR
RNA was isolated from DRG with Trizol (Thermo). Nanodrop2000 (Thermo) was used for RNA concentration measurement. SuperScript II reverse transcriptase (Invitrogen) was used for cDNA synthesis. RT-PCR was conducted using SYBR Green Master Mix (Applied Biosystems) in QuantStudio 5 real-time system (Applied Biosystems). Data were presented as relative expression of the target gene to β-actin as housekeeping gene using the 2−ΔΔCT method, analyzed by qBase Plus 2 software.
The following primer sets were used: β-actin, forward 5′-GTCATTCCAAATATGAGATGCGT-3′ and reverse 5′-GCTATCACCTCCCCTGTGTG-3′, ifn-αr1 forward 5′-TCCACATGGTATGAGGTTGA-3′ and reverse 5′-AGCTTGAACGATCCATAGCC-3′, ifn-αr2, forward 5′-GTCTTGACACCCTACAAACC-3′ and reverse 5′-TCAGGCCACTTTGACTGCAA-3′, il-17rc, forward 5′-ATGCCTGTGTCCTGGTTCCT-3′ and reverse 5′-TTCTAGTGTAGTGCAGGGTC-3′.
ELISA
A 6 mm skin punch was taken from each mouse and quickly transferred to a 12-well cell culture plate containing 500 μl DMEM (Gibco). The skin was incubated on a 32 °C shaker for 30 min. Supernatant was used for CGRP ELISA. The protein expression levels of CGRP secreted into the supernatants of cultured skin sheets from mice were quantified via ELISA kits (Cusabio) following the manufacturer’s instructions.
RNA sequence reanalysis from GEO
The transcriptome data were obtained from the GSE121212 and GSE53552 in Gene Expression Omnibus (GEO) database [14, 15]. Read counts were normalized by TMM, and the VOOM transformation was used to model the mean–variance relationship. Bayes linear model in the limma package was used to analyze DEGs.
Statistical analysis
Statistical analyses were performed using GraphPad Prism6. Analyses were carried out using One-way ANOVA. P value < 0.05 was considered statistically significant.
Results
Increased sensory nerve fibers surround epidermal keratinocytes and immune cells in psoriatic lesional skin
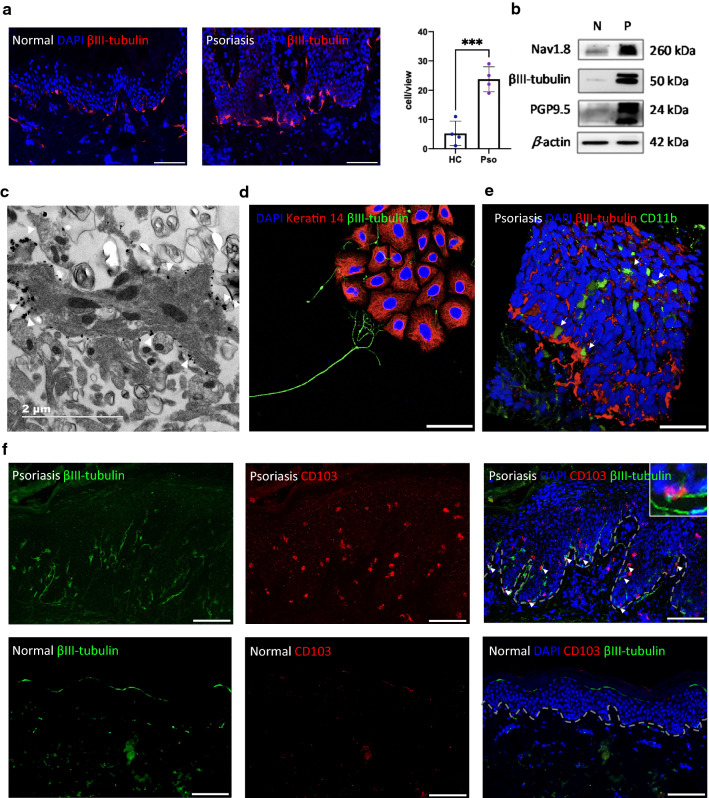
To clarify the distribution of nerve fibers, we determined the expression of βIII-tubulin, a cytoskeletal protein that is often used as a neuron marker, by IF in healthy and psoriatic lesional skin. Numerous nerve fibers were detected in the skin and were densest along the basement membrane zone (Fig. 1a, b). Compared with healthy skin, the density of nerve fibers is increased in psoriatic lesional skin. Some cells in the basal layer were closely surrounded by nerve fibers. The number of these cells was statistically different between psoriatic and normal skin (P < 0.001, Fig. 1a). Furthermore, three specific markers of sensory nerve fibers, protein levels of βIII-tubulin, Nav1.8, and PGP9.5, were all significantly upregulated in the psoriatic epidermis as defined by Western blotting (Fig. 1b).
Fig. 1.
Location and expression of nerves in psoriasis lesions. a IF of βIII-tubulin in healthy and psoriatic lesional skin (left). Qualification of innervated cells (right), scale bar = 100 μm. b Western blotting of βIII-tubulin, Nav1.8, PGP9.5 and β-actin in the epidermis of normal skin (N) and psoriatic lesion (P). c Immunoelectron microscopy to detect βIII-tubulin in psoriatic epidermis, scale bar = 2 μm. d IF to detect the localization of mouse keratinocytes (K14, red) and DRG neurons (βIII-tubulin, green), scale bar = 50 μm. e IF of CD11b (green) and βIII-tubulin (red) in psoriatic lesion, white arrow marked CD11b+ cells, scale bar = 50 μm. f IF of CD103 (red) and βIII-tubulin (green) in psoriatic lesion, white arrow marked CD103+ cells co-localized with nerve fibers, scale bar = 100 μm
To further establish the relationship between keratinocytes and nerve fibers, immunoelectron microscopy was performed to localize nerve fibers in the psoriatic epidermis. A keratinocyte containing a nuclear structure and transparent keratinous particles is well recognized in Fig. 1c. The βIII-tubulin-positive particles (arrowhead) were distributed around the cell membrane of keratinocytes. Next, we built a co-culture system of keratinocytes and DRG neurons. It is very clear that the DRG neurons extended their neurites toward the keratinocytes like a hand to catch things (Fig. 1d).
CD11b+ DCs and CD103+ tissue-resident memory T (Trm) cells are barely present in normal skin, but in psoriatic epidermis, especially in the basal layer, their number increased, and almost all CD11b+ DCs and CD103+ Trm cells were innervated by dense nerve fibers (Fig. 1e, f, white arrow). These results suggest that in addition to epidermal keratinocytes, increased peripheral nerves in psoriatic lesional epidermis were also in close contact with immune cells.
Disordered sensory nerve-related genes in psoriatic lesions
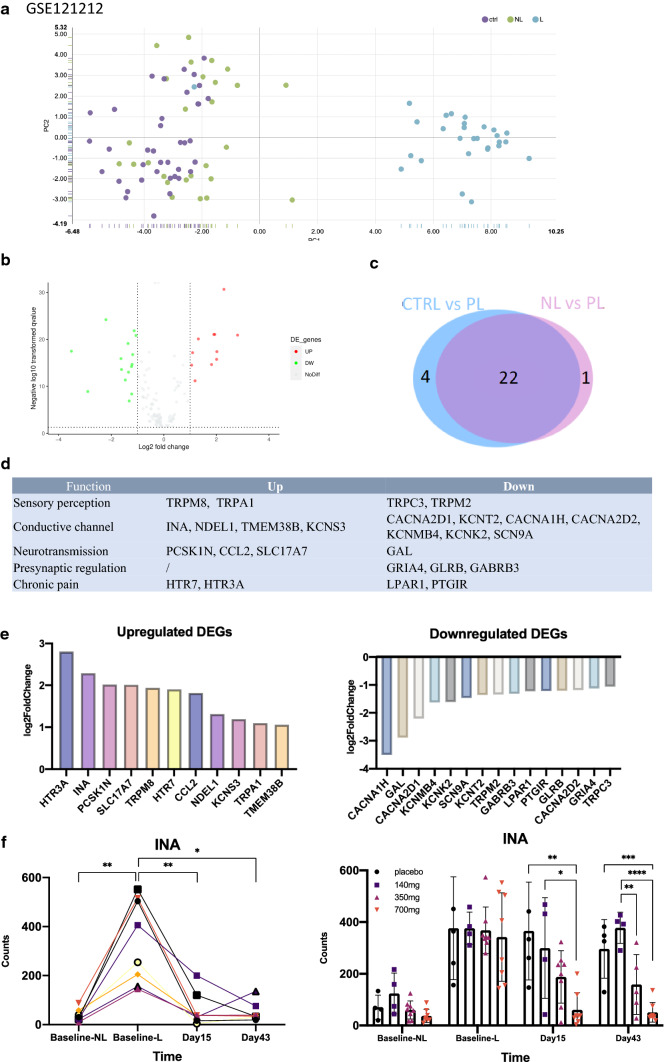
To further clarify the role of nerves in psoriasis, we conducted an in-depth analysis of related genes in neural pathways from transcriptome data (GSE121212) [14], which includes normal skin, non-lesional, and lesional psoriatic skin. According to single-cell RNA-seq of the DRG [16], 175 genes enriched in the sensory nerve were identified. These genes are involved in various operational components of sensory neurons, including perception, conduction, signal transmission, synaptic regulation, and chronic pain perception. There were 26 differentially expressed genes (DEGs) between normal and psoriatic lesional skin (Fig. 2b, c). DEGs were scattered in every component, as shown in Fig. 2d. Among the upregulated differentially expressed genes, 5-hydroxytryptamine receptor 3A (HTR3A) showed the largest fold change (Fig. 2d). Internexin neuronal intermediate filament protein alpha (INA) encodes α-internexin, a neurofilament protein that promotes the growth of neuronal processes and regulates the expression of other neurofilament proteins.
Fig. 2.
Gene expression of sensory nerves in skin. a The top two principal components for the samples in the cohort. b Volcano plot detecting proteins by FC and P value. c Venn diagram showing the overlap between DEGs. d List of DEGs in different functions of sensory nerve. e Up-regulated DEGs and down-regulated DEGs. f INA gene expression at baseline, 15 days and 43 days after treatment with control substance, 140 mg, 350 mg and 700 mg doses of Brodalumab. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, one-way ANOVA. (Dataset from GSE121212)
To explore whether these DEGs will change with the improvement of psoriatic lesions, we analyzed another transcriptome dataset, GSE53552, in the GEO database [15]. This dataset includes 99 paired psoriatic RNA-seq data of non-lesional and lesional full-thickness skin before and after (baseline, 15 days, 43 days) an IL-17 receptor monoclonal antibody, brodalumab, treatment at different doses (control, 140 mg, 350 mg, 700 mg). The RNA level of INA in the lesional skin was higher than that in the non-lesional area, but decreased after brodalumab treatment, especially in the high-dose (700 mg) group. On day 43, the lesional INA was close to the non-lesion level (Fig. 2f).
Sensory neurons and cytokine receptors IFN-αR1, IFN-αR2, and IL-17RC are increased in the DRG of IMQ-treated mice
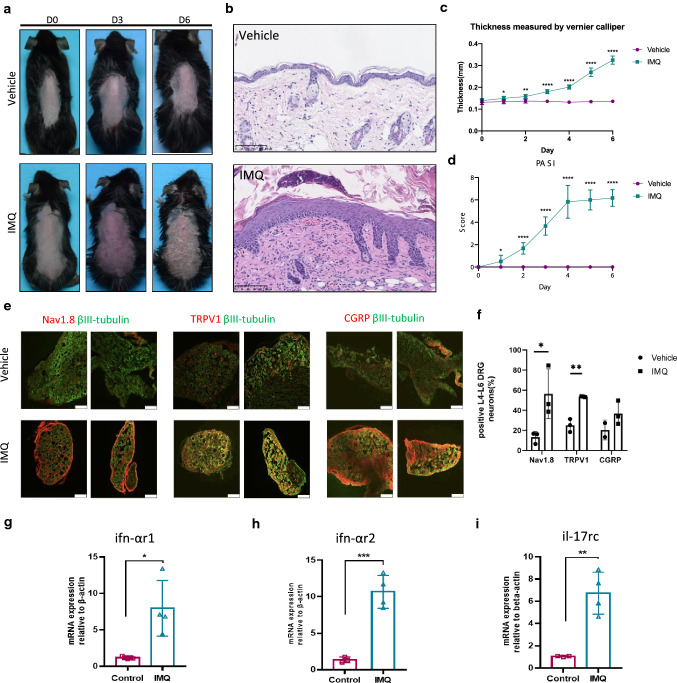
IMQ-induced psoriasis-like skin inflammation [17] was included to explore whether the peripheral nervous system was abnormally activated in psoriasis (Fig. 3a–d) [18]. First, we used DRG to detect the expression of neuron subtypes. Transient receptor potential V1 (TRPV1) is a transient receptor potential cation channel that is expressed in the central and peripheral nervous systems and is responsible for the conduction of pain and itching [19]. IF showed that the fluorescence intensity of Nav1.8, TRPV1, and neuropeptide CGRP in the DRG of psoriasis-like dermatitis was markedly increased compared with that in control mice (Fig. 3e, f). Immune cells communicate with neurons via cytokine receptors. Thus, we next used qRT-PCR to detect the expression of cytokine receptors ifn-αr1, ifn-αr2, and il-17rc at the gene level (Fig. 3g–j). IFN-α plays an indispensable role in the initial stage of psoriasis after skin injury [20], and ifn-α receptors r1 and r2 mRNA were significantly upregulated after IMQ induction (Fig. 3g, h). The mRNA level of il-17rc, a core factor in the pathogenesis of psoriasis, was also significantly upregulated (Fig. 3j). We suggest that the neuro-immune interaction might be enhanced by the increased expression of these cytokine receptors in neurons.
Fig. 3.
Expression of nerve markers and cytokine receptors on mouse DRG. a–d The phenotype, Hematoxylin–eosin (HE) staining, hole back skin thickness and PASI of mice induced by IMQ and vehicle, scale bar = 100 μm. e IF to detect the expression of nerve-related marker proteins βIII-tubulin, Nav1.8, TRPV1 and neuropeptide CGRP in frozen sections of DRG 6 days after modeling, scale bar = 100 μm. f Semi-quantitative statistics using ImageJ. g–i qRT-PCR analysis of IFN-αR1, IFN-αR2 and IL-17RC in DRG, β-actin as internal reference gene. *P < 0.05, **P < 0.01, ****P < 0.0001, one-way ANOVA
IMQ-induced psoriatic dermatitis was attenuated on the sensory impairment side of spinal cord hemisection mice
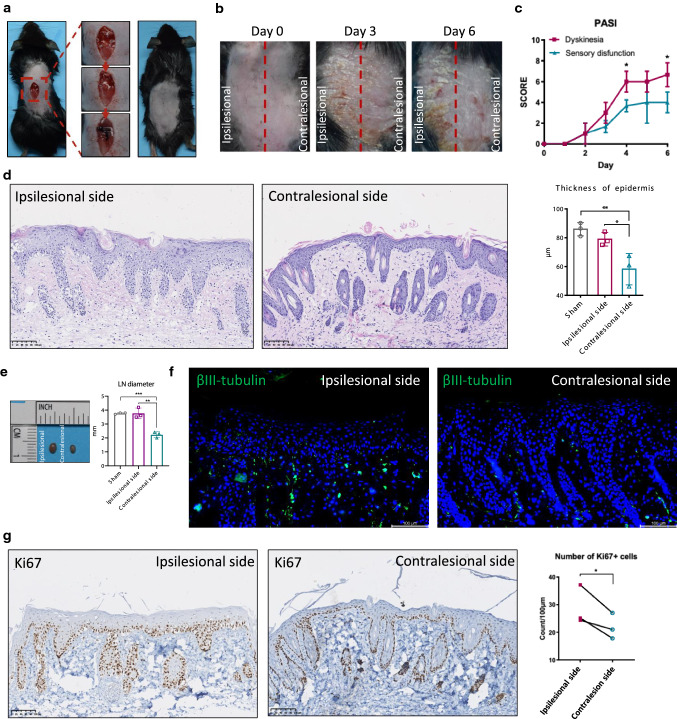
In recent years, a number of cases have reported that after central nervous system injury, such as cerebrovascular accident, spinal cord injury, polio, etc., psoriatic plaques in the neurological dysfunction area were improved or completely relieved [21]. We established a mouse model of spinal cord hemi-section (Fig. 4a) to simulate Brown–Séquard syndrome, a common type of spinal cord injury caused by a lesion on half of the spinal cord, the clinical manifestations of which were pain and temperature sensory disturbance on the contralesional side, and dyskinesia (paralysis of hind limbs) on the ipsilesional side [22]. After IMQ induction, the ipsilesional side showed a phenotype similar to that of wild-type mice, while psoriasis-like dermatitis was not obvious on the contralesional side (Fig. 4b), including mild erythema, slight induration, and fine scales. Taking the midline of the back as the boundary, there is a clear contrast between the skin on the contralesional and ipsilesional sides (Fig. 4b). On the 4th and 6th day of modeling, the PASI score of the contralesional side was significantly lower than that of the ipsilesional side (Fig. 4c).
Fig. 4.
IMQ-induced psoriasiform dermatitis was attenuated on the sensory impairment side of spinal cord hemi-section mouse. a Schematic diagram of spinal cord hemi-section operation. b Mouse phenotype on days 0, 3, and 6 after IMQ induction. Red dashed line marked midline of the ipsilesional side and contralesional side. c PASI score of each side. d HE staining and the thickness of the epidermis (μm), scale bar = 100 μm. e Gross map and diameter of inguinal lymph nodes. f IF of βIII-tubulin (green), scale bar = 100 μm. g IHC staining of Ki67 (left) and Ki67-positive keratinocytes counts (right). *P < 0.05, **P < 0.01, one-way ANOVA
In addition, the diameter of the inguinal lymph nodes on the ipsilesional side was larger than that on the contralesional side, suggesting that the skin inflammation on the sensory impairment side was relatively reduced (Fig. 4e). A large part of the nerves in the skin are sensory neurons [23]. IF showed that the number of βIII-tubulin-positive neurons in the dermis and epidermis of the contralesional side was reduced (Fig. 4f). Abnormal proliferation of keratinocytes is one of the typical pathological features of psoriasis [24]. We used immunohistochemistry (IHC) to detect the proliferation marker Ki67 in spinal cord hemisectioned mice after IMQ induction. The number of Ki67-positive keratinocytes on the contralesional side was significantly reduced (Fig. 4g). Our results illustrated that IMQ-induced psoriatic dermatitis was attenuated on the sensory impairment side of spinal cord hemisection, possibly by inhibiting inflammation and proliferation of keratinocytes.
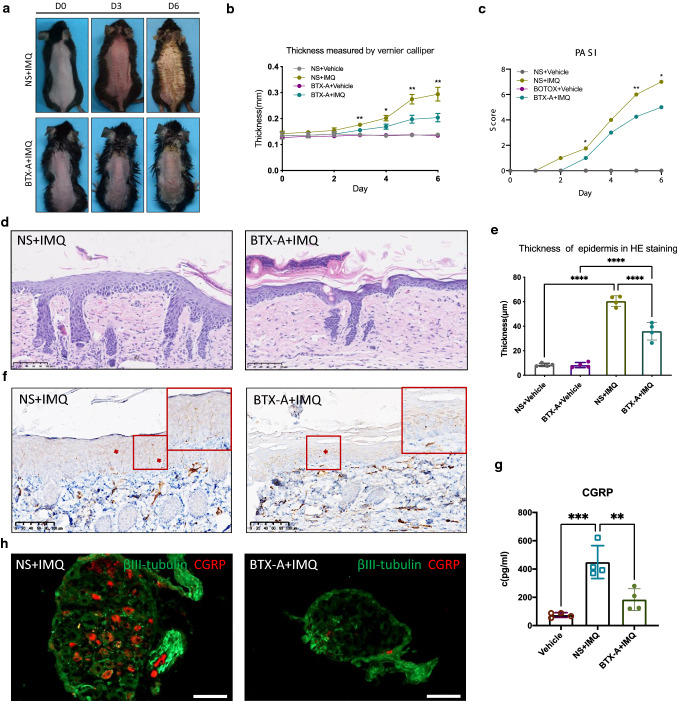
Subcutaneous injection of BTX-A alleviated imiquimod-induced psoriasis-like dermatitis
BTX-A is a neurotoxin that can inhibit the release of acetylcholine and other neurotransmitters and block nerve conduction [25]. Therefore, BTX-A was applied to pretreat mice to determine whether the inhibition of neurotransmitters can alleviate or block psoriasis inflammation. We administered a single subcutaneous injection of BTX-A to the backs of these mice and started to induce psoriasis-like dermatitis in mice with imiquimod for 5 consecutive days. Compared with the vehicle group, the mice treated with BTX-A showed a dramatically alleviated phenotype, which was manifested by a reduction in scales and skin thickening (Fig. 5a, b). The PASI score of BTX-A-treated mice was lower than that of the control group (P < 0.05, Fig. 5c). The epidermal thickness measured by H&E staining also decreased after BTX-A injection (Fig. 5d, e).
Fig. 5.
BTX-A reduced IMQ-induced psoriasiform dermatitis. a After NS or BTX-A injection, the phenotype of mice on the 0th, 3rd, and 6th days of IMQ application. b Skin thickness on the back skin. c PASI score from day 0 to day 6. P value compared NS + IMQ group with BTX-A + IMQ group. d HE staining of back skin, scale bar = 100 μm. e The statistics of epidermal thickness (μm) in HE staining, measured by NDP.view software. f IF of PGP9.5, scale bar = 100 μm. g The secretion concentration of CGRP in the skin tissue of mice on the 3rd day of modeling. h IF of βIII-tubulin and CGRP in DRG, scale bar = 100 μm. **P < 0.01, ***P < 0.0001, one-way ANOVA
A previous report showed that the number of PGP9.5-positive sensory nerve fibers and neuropeptides secreted by psoriasis skin lesions was increased, especially CGRP, which acts as a bridge between the nervous and immune systems in various skin infections and atopic dermatitis [26]. PGP9.5-positive nerve fibers in the skin of mice after BTX-A injection was reduced compared with the control group (Fig. 5f). Subsequently, we measured the concentration of CGRP in the supernatant from tissue-cultured skin by ELISA. The results showed that the CGRP secreted by the skin of mice induced with IMQ was much higher than that in the vehicle group (Fig. 5g). After BTX-A pre-treatment, the CGRP concentration was markedly lower than that of the IMQ group, and there was no statistical difference between the two groups (Fig. 5g).
Next, immunofluorescence of DRG was performed to further define the effect of BTX-A. CGRP-positive neurons in the imiquimod model showed that approximately 40% of the DRG neurons were CGRP-positive. However, after BTX-A pre-treatment, there were less than 10% CGRP-positive neurons in the DRG (Fig. 5h). These data showed that subcutaneous injection of BTX-A can reduce cutaneous nerve fibers and thus alleviate IMQ-induced psoriasiform dermatitis.
Discussion
The important role of the neuroimmune microenvironment in skin diseases has recently attracted attention [27]. Communication and cooperation between the nervous and immune systems is an indispensable part of the body's homeostasis [8, 9]. Abnormal neuroimmune communication may not only concern psoriasis but also diseases with different immune responses spectrum, such as atopic dermatitis [28].
Whether nerve fibers increase or decrease in psoriasis remains controversial. One of the reasons for this is the difference in the detection methods [29, 30]. Our results showed that the distribution of nerve fibers in the epidermis of psoriasis was denser, as determined by IF and Western blotting. Peripheral sensory nerve endings can participate in immune regulation by communicating with a variety of immune cells [31, 32]. We found that in psoriasis, nerve endings were in close contact with CD11c+ DC and CD103+ Trm cells. CD103 in dermis was reported to play a pivotal role in the development of psoriasis by controling the function of cDCs [33]. Whereas, in epidermis, CD103+ T cells were mostly CD8+ memory T cells that are associated with a progressive clinical course of psoriasis [34]. In addition, nerve endings in the epidermis are distributed around and innervated keratinocytes. Thus, the peripheral nervous system in the skin may link both cutaneous immune cells and keratinocytes and participate in the disease process of psoriasis.
Sensory neurons in the skin are mainly responsible for identifying pathogens, uploading danger signals, and mobilizing immune responses. Under these conditions, sensory neurons can activate the type 17 immune responses [35]. The density of PGP 9.5-positive nerve was found engaged in itch occurring in psoriasis patients. Moreover, PGP9.5 expressed by keratinocytes was increased in itchy skin lesions [36]. Similarly, our results also showed that the expression of PGP9.5 increased in psoriatic lesions. And in IMQ-induced psoriasis-like mouse model, the expression of PGP9.5 decreased after BTX pre-treatment. TRPV1+ sensory neurons, often co-expressing Nav1.8, have been confirmed to play a role in regulating barrier immunity in local skin infections [11]. TRPV1+ neurons can also mediate psoriasiform dermatitis in mice through IL-23 [32]. The increased number of TRPV1 and CGRP-positive neurons in the DRG might account for IMQ-induced skin inflammation.
Sensory nerve fibers can secrete neuropeptides in the skin to regulate the local immune responses. Among them, CGRP has a key regulatory function in skin immunity and inflammation. CGRP-containing nerves are intimately associated with epidermal Langerhans cells (LCs) by regulating the antigen-presenting capability of Langerhans cells [37]. In addition, the immune system can transmit signals to neurons through cytokines, activate neurons to cause itching, pain, and other sensations, and transmit signals to the central nervous system [38]. Our data showed that in the mouse DRG, receptors of IFN-α and IL-17 increased, suggesting that the strengthened neuro-immune communication may aggravate the local inflammatory response.
Skin nerve denervation prior to the application of IMQ could attenuate the induction of psoriasiform dermatitis [39]. Using the spinal cord hemisection model, we found that the performance of psoriasiform dermatitis in mice on the sensory impairment side was also significantly improved. Recent studies have found that in the pathogenesis of psoriasis, neuropeptides may be involved in promoting the proliferation of keratinocytes [40, 41]. On the sensory impairment side, the proliferation marker Ki67 in epidermal keratinocytes was significantly reduced. Combined with the dense innervation of keratinocytes by nerve endings, it may directly inhibit the abnormal proliferation of keratinocytes. After pre-treatment with BTX-A, IMQ-induced psoriasiform dermatitis in mice was attenuated. Previous studies have found that botulinum toxin type B (BTX-B) can ameliorate psoriasiform dermatitis in mice [42]. The dose used here was half that of BTX-B (1 unit), and the results showed a more significant difference. It has been reported that the number of CGRP-positive nerves in the skin of psoriasis lesions has increased significantly, which is also confirmed by our aforementioned results. Nevertheless, CGRP-positive neurons and the secretion of CGRP in skin tissue culture both decreased after BTX-A pre-treatment. Furthermore, our results showed that BTX-A improved psoriasiform dermatitis by inhibiting CGRP secretion.
In summary, our study suggests that cutaneous nerve fibers communicate with immune cells and keratinocytes and are abnormally activated in psoriasis. Neurological intervention, especially blocking the secretion and transmission of neurotransmitters, may be a new treatment strategy for psoriasis.
Abbreviations
- BTX-A
Botulinum toxin A
- CGRP
Calcitonin gene-related peptide
- DEG
Differential expression genes
- DRG
Dorsal root ganglion
- ELISA
Enzyme linked immunosorbent assay
- FC
Fold change
- GEO
Gene Expression Omnibus
- HE
Hematoxylin–eosin staining
- IF
Immunofluorescence
- IFN
Interferon
- IHC
Immunohistochemistry
- IL
Interleukin
- IMQ
Imiquimod
- NS
Normal saline
- PASI
Psoriasis Area Severity Index
- PGP9.5
Protein gene product 9.5
- qRT-PCR
Real-Time Quantitative Reverse Transcription PCR
Author contributions
Conceptualization was performed by X-YM and S-QC. Data curation and formal analysis and writing were performed by S-QC, X-YC and Y-ZC. Supervision and validation were performed by YZ, B-XY and Z-YW. Review and editing were performed by FX, Y-ZH and Y-XZ.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81930089).
Data availability
The datasets analyzed during the current study are available in the GEO repository. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse121212 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse53552.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study was approved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine. All animal experiments were performed in accordance with protocols approved by the Animal Care and Use Committee.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
No individual information, image or video was included in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Si-Qi Chen, Xue-Yan Chen and Ying-Zhe Cui contributed equally to this work.
References
- 1.Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, Armstrong AW, Connor C, Cordoro KM, Davis DMR, Elewski BE, Gelfand JM, Gordon KB, Gottlieb AB, Kavanaugh A, Kiselica M, Korman NJ, Kroshinsky D, Lebwohl M, Leonardi CL, Lichten J, Lim HW, Mehta NN, Paller AS, Parra SL, Pathy AL, Rupani RN, Siegel M, Wong EB, Wu JJ, Hariharan V, Elmets CA. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 2.Liang Y, Sarkar MK, Tsoi LC, Gudjonsson JE. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr Opin Immunol. 2017;49:1–8. doi: 10.1016/j.coi.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evers AWM, van Beugen S. How stress affects the skin: from designs to mechanisms. Br J Dermatol. 2021;185:12–13. doi: 10.1111/bjd.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon LJ, Witcraft SM, McCowan NK, Brodell RT. Stress and skin disease quality of life: the moderating role of anxiety sensitivity social concerns. Br J Dermatol. 2018;178:951–957. doi: 10.1111/bjd.16082. [DOI] [PubMed] [Google Scholar]
- 5.Weddell G, Cowan MA, Palmer E, Ramaswamy S. Psoriatic skin. Arch Dermatol. 1965;91:252–266. doi: 10.1001/archderm.1965.01600090060012. [DOI] [PubMed] [Google Scholar]
- 6.Zhu TH, Nakamura M, Farahnik B, Abrouk M, Lee K, Singh R, Gevorgyan A, Koo J, Bhutani T. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257–263. doi: 10.1007/s40257-016-0183-7. [DOI] [PubMed] [Google Scholar]
- 7.Lee EB, Reynolds KA, Pithadia DJ, Thiyanaratnam J, Wu JJ. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74–76. [PubMed] [Google Scholar]
- 8.Limjunyawong N, Dong X. Spicy immunity: pain to gain. Immunity. 2019;51:426–428. doi: 10.1016/j.immuni.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JA, Wu J, Kaplan DH. Neuronal regulation of cutaneous immunity. J Immunol. 2020;204:264–270. doi: 10.4049/jimmunol.1901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagomarsino VN, Kostic AD, Chiu IM. Mechanisms of microbial-neuronal interactions in pain and nociception. Neurobiol Pain. 2021;9:100056. doi: 10.1016/j.ynpai.2020.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen JA, Edwards TN, Liu AW, Hirai T, Jones MR, Wu J, Li Y, Zhang S, Ho J, Davis BM, Albers KM, Kaplan DH. Cutaneous TRPV1 neurons trigger protective innate type 17 anticipatory immunity. Cell. 2019 doi: 10.1016/j.cell.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, Reber LL, Marichal T, Starkl P, Cenac N, Dong X, Tsai M, Galli SJ, Gaudenzio N. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat Immunol. 2019;20:1435–1443. doi: 10.1038/s41590-019-0493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Wang P, Yan B-X, Chen X-Y, Landeck L, Wang Z-Y, Li X-X, Zhang J, Zheng M, Man X-Y. Quantitative proteomic profile of psoriatic epidermis identifies OAS2 as a novel biomarker for disease activity. Front Immunol. 2020;11:1432–1532. doi: 10.3389/fimmu.2020.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, Szymczak S, Swindell WR, Sarkar MK, Raja K, Shao S, Patrick M, Gao Y, Uppala R, Perez White BE, Getsios S, Harms PW, Maverakis E, Elder JT, Franke A, Gudjonsson JE, Weidinger S. Atopic dermatitis is an il-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Investig Dermatol. 2019;139:1480–1489. doi: 10.1016/j.jid.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell CB, Rand H, Bigler J, Kerkof K, Timour M, Bautista E, Krueger JG, Salinger DH, Welcher AA, Martin DA. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192:3828–3836. doi: 10.4049/jimmunol.1301737. [DOI] [PubMed] [Google Scholar]
- 16.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 17.van der Fits L, Mourits S, Voerman JSA, Kant M, Boon L, Laman JD, Cornelissen F, Mus A-M, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 18.Schön MP, Manzke V, Erpenbeck L. Animal models of psoriasis-highlights and drawbacks. J Allergy Clin Immunol. 2021;147:439–455. doi: 10.1016/j.jaci.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 20.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu Y-J, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph T, Kurian J, Warwick DJ, Friedmann PS. Unilateral remission of psoriasis following traumatic nerve palsy. Br J Dermatol. 2005;152:185–186. doi: 10.1111/j.1365-2133.2005.06330.x. [DOI] [PubMed] [Google Scholar]
- 22.Filli L, Zörner B, Weinmann O, Schwab ME. Motor deficits and recovery in rats with unilateral spinal cord hemisection mimic the Brown–Sequard syndrome. Brain. 2011;134:2261–2273. doi: 10.1093/brain/awr167. [DOI] [PubMed] [Google Scholar]
- 23.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43:249–259. doi: 10.1067/mjd.2000.105567. [DOI] [PubMed] [Google Scholar]
- 26.Tillmaand EG, Anapindi KDB, De La Toba EA, Guo CJ, Krebs J, Lenhart AE, Liu Q, Sweedler JV. Quantitative characterization of the neuropeptide level changes in dorsal horn and dorsal root ganglia regions of the murine itch models. J Proteome Res. 2020;19:1248–1257. doi: 10.1021/acs.jproteome.9b00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu C, Artis D, Chiu IM. Neuro-immune interactions in the tissues. Immunity. 2020;52:464–474. doi: 10.1016/j.immuni.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blake KJ, Jiang XR, Chiu IM. Neuronal regulation of immunity in the skin and lungs. Trends Neurosci. 2019;42:537–551. doi: 10.1016/j.tins.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taneda K, Tominaga M, Negi O, Tengara S, Kamo A, Ogawa H, Takamori K. Evaluation of epidermal nerve density and opioid receptor levels in psoriatic itch. Br J Dermatol. 2011;165:277–284. doi: 10.1111/j.1365-2133.2011.10347.x. [DOI] [PubMed] [Google Scholar]
- 30.Tan Y, Ng WJ, Lee SZX, Lee BTK, Nattkemper LA, Yosipovitch G, Ng LG, Tey HL. Dimensional optical clearing and imaging of pruritic atopic dermatitis and psoriasis skin reveals downregulation of epidermal innervation. J Investig Dermatol. 2019;139:1201–1204. doi: 10.1016/j.jid.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Chen O, Donnelly CR, Ji R-R. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol. 2020;62:17–25. doi: 10.1016/j.conb.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity. 2015;43:515–526. doi: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukui T, Fukaya T, Uto T, Takagi H, Nasu J, Miyanaga N, Nishikawa Y, Koseki H, Choijookhuu N, Hishikawa Y, Yamashita Y, Sato K. Pivotal role of CD103 in the development of psoriasiform dermatitis. Sci Rep. 2020;10:8371. doi: 10.1038/s41598-020-65355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurihara K, Fujiyama T, Phadungsaksawasdi P, Ito T, Tokura Y. Significance of IL-17A-producing CD8+CD103+ skin resident memory T cells in psoriasis lesion and their possible relationship to clinical course. J Dermatol Sci. 2019;95:21–27. doi: 10.1016/j.jdermsci.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Fattori V, Ferraz CR, Rasquel-Oliveira FS, Verri WA., Jr Neuroimmune communication in infection and pain: friends or foes? Immunol Lett. 2021;229:32–43. doi: 10.1016/j.imlet.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Kupczyk P, Reich A, Gajda M, Hołysz M, Wysokińska E, Paprocka M, Nevozhay D, Chodaczek G, Jagodziński PP, Ziółkowski P, Szepietowski JC. UCHL1/PGP 9.5 dynamic in neuro-immune-cutaneous milieu: focusing on axonal nerve terminals and epidermal keratinocytes in psoriatic itch. Biomed Res Int. 2018;2018:7489316. doi: 10.1155/2018/7489316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granstein RD, Wagner JA, Stohl LL, Ding W. Calcitonin gene-related peptide: key regulator of cutaneous immunity. Acta Physiol (Oxf) 2015;213:586–594. doi: 10.1111/apha.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu JA, Yu J, Cheung CW. Immune actions on the peripheral nervous system in pain. Int J Mol Sci. 2021 doi: 10.3390/ijms22031448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin B, Sun C, Chen L, Wang S, Yang J, Xie Z, Shen Z. The nerve injuries attenuate the persistence of psoriatic lesions. J Dermatol Sci. 2021;102:85–93. doi: 10.1016/j.jdermsci.2021.02.0066. [DOI] [PubMed] [Google Scholar]
- 40.Ostrowski SM, Belkadi A, Loyd CM, Diaconu D, Ward NL. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Investig Dermatol. 2011;131:1530–1538. doi: 10.1038/jid.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding W, Stohl LL, Xu L, Zhou XK, Manni M, Wagner JA, Granstein RD. Calcitonin gene-related peptide-exposed endothelial cells bias antigen presentation to CD4+ T cells toward a Th17 response. J Immunol. 2016;196:2181–2194. doi: 10.4049/jimmunol.1500303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amalia SN, Uchiyama A, Baral H, Inoue Y, Yamazaki S, Fujiwara C, Sekiguchi A, Yokoyama Y, Ogino S, Torii R, Hosoi M, Ishikawa O, Motegi S-I. Suppression of neuropeptide by botulinum toxin improves imiquimod-induced psoriasis-like dermatitis via the regulation of neuroimmune system. J Dermatol Sci. 2021;101:58–68. doi: 10.1016/j.jdermsci.2020.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available in the GEO repository. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse121212 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse53552.