Abstract
Fatty acid synthase (FASN) participates in many fundamental biological processes, including energy storage and signal transduction, and is overexpressed in many cancer cells. We previously showed in a context of lipogenesis that FASN is protected from degradation by its interaction with O-GlcNAc transferase (OGT) in a nutrient-dependent manner. We and others also reported that OGT and O-GlcNAcylation up-regulate the PI3K/AKT/mTOR pathway that senses mitogenic signals and nutrient availability to drive cell cycle. Using biochemical and microscopy approaches, we show here that FASN co-localizes with OGT in the cytoplasm and, to a lesser extent, in the membrane fraction. This interaction occurs in a cell cycle-dependent manner, following the pattern of FASN expression. Moreover, we show that FASN expression depends on OGT upon serum stimulation. The level of FASN also correlates with the activation of the PI3K/AKT/mTOR pathway in hepatic cell lines, and in livers of obese mice and in a chronically activated insulin and mTOR signaling mouse model (PTEN-null mice). These results indicate that FASN is under a dual control of O-GlcNAcylation and mTOR pathways. In turn, blocking FASN with the small-molecule inhibitor C75 reduces both OGT and O-GlcNAcylation levels, and mTOR activation, highlighting a novel reciprocal regulation between these actors. In addition to the role of O-GlcNAcylation in tumorigenesis, our findings shed new light on how aberrant activity of FASN and mTOR signaling may promote the emergence of hepatic tumors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-021-03857-z.
Keywords: Cell proliferation, Protein interactions, Proximity ligation assay, siRNA, Ob/ob mice
Introduction
Fatty acid synthase (FASN) is the major enzyme responsible for the synthesis of fatty acids. It is a very large protein comprising six different catalytic domains. The best studied role played by FASN is energy storage via lipogenesis, a metabolic process in which de novo fatty acids are synthesized from carbohydrates. Fatty acids can then be condensed with glycerol to form triglycerides to be stored into lipid droplets in adipocytes or secreted in the form of VLDL (very low density lipoproteins) by the liver. But, the activity of FASN is not confined solely to “fat accumulation”. FASN also produces palmitoyl–CoA that serves to covalently modify cell signaling proteins such as the small G-protein N-Ras [1] and the secreted factor Wnt1 whose S-palmitoylation reduces degradation of β-catenin in immortalized prostate cell lines [2]. In addition, with microsomal elongases (Elovl, elongation of very long chain fatty acids proteins), FASN takes part in the production of long-chain saturated fatty acids which are crucial for the building of lipid microdomains that ensure the correct localization and activation of receptors, hence potentiating mitogenic signal transduction [3, 4]. Finally, FASN participates in the production of secondary messengers like PIP3 (phosphatidylinositol–3,4,5–triphosphate) and LPA (lysophosphatidic acid) that, respectively, activate the mitogenic PI3K/AKT pathway [5] and GPCR (G protein coupled-receptors) [6]. Many of these functions are particularly valued by cancer cells in which FASN activity is important for membrane growth, sustaining the high proliferation rate of these cells [7]. To enhance tumor cell division, FASN is upregulated in many cancers among which breast [8], colon [9], prostate [10], stomach [11], esophageal [12], melanoma [13], lung [14], pancreatic [15], and ovarian [16] cancers. Blocking FASN activity with the small-molecule inhibitor C75 [17] or more recently with G28UCM in two ovarian cell lines [18] proved to have anticancer properties in vitro, making this enzyme a promising target for cancer therapy. Recently, FASN has been defined as a poor prognosis factor in acute lymphoblastic leukemia (ALL) [19]. The authors proposed that phytochemicals extracted from ginger may be used as inhibitors of FASN to oppose resistance to glucocorticoids in childhood ALL.
O-GlcNAc transferase (OGT) that modifies a large subset of cytoplasmic, nuclear and mitochondrial proteins by O-GlcNAcylation is also more heavily expressed and active in cancers [20]. Silencing or inhibiting OGT impairs breast cancer cell growth and invasion [21]. O-GlcNAcase (OGA) antagonizes the activity of OGT by removing the sugar moiety from proteins, making O-GlcNAcylation a dynamic post-translational modification (PTM) akin to phosphorylation with which it can competes on the same or on neighboring serine or threonine residues [22, 23]. O-GlcNAcylation regulates fundamental cellular functions among which signaling, transcription, subcellular localization or protein fate [24, 25]. Concerning the latter role played by O-GlcNAcylation, we previously reported that FASN was a substrate of OGT and that its modification by O-GlcNAc moieties reduced its proteasomal degradation in a context of lipogenesis [26]. Similarly, Hsieh and collaborators showed that the silencing or inhibition of GFAT-1 (glutamine:fructose–6–phosphate amidotransferase–1), the rate-limiting enzyme of HBP (hexosamine biosynthetic pathway) providing UDP-GlcNAc necessary for OGT catalytic activity, reduced the expression of FASN and other lipogenic actors and inhibited adipogenesis [27]. Of crucial importance, increased activity of HBP is a general hallmark of highly proliferating cancer cells [28]. Besides, inactivation of Emeg32—the glucosamine–6–phosphate acetyltransferase belonging to HBP—decreases MEF (mouse embryonic fibroblast) proliferative rate, underlying the pivotal function of UDP-GlcNAc in cell proliferation [29].
Importantly, O-GlcNAcylation levels fluctuate all along the cell cycle. In a general way, an increase in O-GlcNAcylation tends to activate cell cycle, as shown in pre-B cells for which the treatment with the OGT inhibitor OSMI-1 leads to a cell cycle arrest [30]. On the other hand, blocking HBP flux with azaserine or DON treatment dramatically reduces MCF7 cells proliferation while inhibition of OGA by NButGT increases proliferation [31]. Interfering with the expression of either OGT or OGA is also deleterious for cell cycle progression as demonstrated especially in HeLa and 3T3-L1 cells [32]. Moreover, we reported that stimulation of starved cells by growth factors [33] or insulin [34] elevates the expression of OGT and O-GlcNAc levels when cells enter G1 phase. The O-GlcNAc content decreases in G1/S transition [35], and then re-increases at the G2/M transition [36, 37]. Thus, available literature suggests that FASN and OGT are both critical for proliferation, but how FASN is regulated by O-GlcNAcylation during this process has never been explored.
The mTOR (mechanistic target of rapamycin) pathway is another signaling pathway that senses nutrient availability and growth factor or hormones to enable cell growth [38]. O-GlcNAc cycling and mTOR pathway are closely linked, a reciprocal control of each other being demonstrated [39, 40]. In this study, we provided additional evidence reinforcing previous findings that O-GlcNAcylation takes part in the regulation of FASN expression and activation of mTOR pathway. This suggests that FASN, OGT and mTOR pathway have a synergistic effect on cell cycle and proliferation. Using several cellular and mouse models we show a correlation between the steady-state level of FASN and mTOR activation. Moreover, we provide the evidence that FASN is cyclically expressed along the cell cycle and its level is not only dependent upon O-GlcNAcylation but also mTOR activation during G1 phase. In turn, blocking FASN activity decreases OGT activity and reduces mTOR signaling pathway, defining a novel feedback loop between O-GlcNAc cycling, the growth factors/nutrient-dependent pathway and the lipogenic enzyme. Overall, these findings provide evidence of a complex cross-regulation between O-GlcNAcylation processes, FASN and mTOR pathway, and that a deregulation in any of them could impact the two others leading to loss of control of cell cycle and ultimately to carcinogenesis.
Materials and methods
Reagents, siRNA and antibodies
Ac5SGlcNAc (kind gift from Pr. David Vocadlo’s laboratory), Rapamycin (Santa Cruz, sc-3504), and C75 (CliniSciences, sc-1547-1) were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 50 mM, 1 mM, and 50 mM as stock solutions, respectively. Control siRNA (siCtrl) (MISSION siRNA universal negative control) and siRNA targeting human OGT (siOGT) were from Sigma-Aldrich. The antibodies used are listed in Supp. table 1.
Cell culture
All cell lines were provided from the ATCC except the IHH cells that is from the EGID (European Genomic Institute For Diabetes) in Lille. The human hepatocarcinoma HepG2 and the human colon carcinoma HCT116 cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Lonza) supplemented with 25 mM glucose. The human hepatocarcinoma Hep3B cell line was cultured in Minimal Essential Medium (MEM, Biowest) supplemented with 5 mM glucose. The immortalized human hepatocyte IHH cell line was cultured in William’s E Medium (Lonza) supplemented with 10 mM glucose, and human fetal colon CCD841CoN cells were cultured in Eagle’s Minimum Essential Medium (EMEM, Lonza) supplemented with 5 mM glucose. All cells were maintained in medium supplemented with 10% (v/v) fetal calf serum (FCS) (Dominique Dutscher) and 2 mM l-glutamine and incubated at 37 °C, in a 5% (v/v) CO2-enriched, humidified atmosphere.
Cell cycle synchronization, cell treatment and cell transfection
The cells were synchronized by preventing them from serum. Twenty-four hours after seeding, cells were washed twice with Phosphate-Buffered Saline (PBS) and kept in serum-free medium for 24 h or 48 h (time 0 h). Then they were stimulated with 10% (v/v) FCS for the indicated times. The cell cycle progression was monitored by propidium iodide (PI) staining and analysis by flow cytometry, as previously described [41].
Serum-starved HepG2 cells were treated, respectively, with the OGT inhibitor Ac5SGlcNAc (50 µM) or the mTOR inhibitor rapamycin (50 nM) for the indicated time periods. For time-course experiments, cells were pre-treated 1 h prior stimulation with serum. Regarding the FASN inhibitor C75, HepG2 cells were treated for 72 h at concentrations ranging from 40 µM to 100 µM. For each inhibitor, DMSO was used as negative control (vehicle).
HepG2 cells were cultured in DMEM-10% (v/v) FCS on coverslips and when 70% of confluence was reached, cells were transiently co-transfected for 24 h with GFP-OGT and Cherry-FAS (1 µg/well in 6-wells plate) using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen).
For small interfering RNA (siRNA) reverse transfection, siCtrl or siRNA targeting OGT (siOGT, 10 nM) were administered to HepG2 cells (1 × 106 cells/100-mm dish) with Lipofectamine RNAiMAX (Invitrogen) as described in [41]. Forty-eight hours later, cells were serum-starved for 24 h and then stimulated with serum for the indicated time periods.
Cell viability assay
HepG2 cells were seeded in 96-well plates treated with C75 at various concentrations (40–100 µM) for 72 h. Efficiency of C75 was evaluated by measuring cell viability using the MTS reagent (Promega) according to the manufacturer’s instructions.
Fluorescence microscopy and proximity ligation assay (PLA)
For cells transfected with GFP-OGT and Cherry-FAS, cells were washed three times in ice-cold PBS before fixation in 4% (w/v) paraformaldehyde (PAF) in PBS at room temperature for 20 min. After three washes with PBS, the nuclei were labeled with a DAPI solution at 50 µg/mL for 10 min. The coverslips were rinsed with ultrapure water and mounted on slides using Mowiol medium (Calbiochem, Merck chemicals). The fluorescence was detected on a Leica AF 6000LX microscope and the images were analyzed with Fiji software (inter-Schindelin).
For indirect immunofluorescence and PLA, the fixation with PAF was followed by quenching with 100 mM glycine (pH 7.4) in PBS for 20 min and three washes in PBS (5 min per wash, at RT). Then cells were permeabilized with 0.5% (v/v) Triton X-100 in PBS for 20 min. After three washes, coverslips were incubated with blocking buffer (2% (v/v) FCS, 2% (w/v) bovine serum albumin and 0.2% (w/v) gelatin in PBS) for 1 h at RT and subsequently incubated with the primary antibodies (1:100 for OGT (DM17) and 1:50 for FAS) (Supp. Table 1) diluted in the blocking buffer, overnight at 4 °C. For immunofluorescence, coverslips were washed tree times with PBS and incubated with Alexa Fluor-conjugated secondary antibodies (1:600 in blocking buffer) (Supp. Table 1) for 1 h in the dark, at RT (Alexa 488 (rabbit) and Alexa 568 (mouse). For PLA (Duolink in situ kit, Sigma-Aldrich), after primary antibodies incubation and two washes in PBS, the coverslips were incubated with PLA PLUS and MINUS probes for mouse and rabbit, respectively, for 1 h, followed by 30 min incubation with the ligase (ligation step), and followed by incubation with the polymerase for 3 h (amplification step, Duolink in situ detection reagents Green, λex = 495 nm and λem = 527 nm) in a humidifier chamber at 37 °C. Two washes with buffer A (Tris–HCl, NaCl, Tween-20, pH 7.4) were performed between each step as described in [41]. Finally, coverslips were washed twice with buffer B (Tris–HCl, NaCl, pH 7.5) for 10 min before mounting slides in mounting solution which also allows to stain the nucleus (ProLong Diamond Antifade Mountant with DAPI, Molecular Probes). Negative controls were done using only the secondary antibodies for immunofluorescence and using only one primary antibody for PLA. The fluorescence was detected with an inverted Zeiss LSM700 confocal microscope with a 40 × oil immersion lens at room temperature and data were collected with the ZEN 2010 software (Zeiss, Oberkochen, Germany). The images were analyzed with ImageJ.
Cell fractionation
HepG2 cells were fractionated using a commercial kit (Subcellular Proteome Extraction Kit, Calbiochem) according to the manufacturer’s instructions.
Co-immunoprecipitation
Co-immunoprecipitation (Co-IP) experiments were conducted as described in [42]. Briefly, cell lysis was performed in a buffer containing 10 mM Tris–HCl, 150 mM NaCl, 1% (v/v) Triton-X100, 0.5% (m/v) sodium deoxycholate (NaDOC), pH 7.5 (Co-IP buffer). The cell lysates were centrifuged at 20,000×g for 15 min at 4 °C. Thirty µg of proteins were set aside for control (input) and 1 mg was precleared with a mix of protein A and protein G-coupled Sepharose beads (50:50 ratio) (GE Healthcare) in Co-IP buffer for 1 h at 4 °C (20 µL per mg of proteins). After centrifugation at 1200×g for 5 min, the supernatant was incubated with anti-OGT (Ti14) antibodies overnight at 4 °C (5 µg antibodies per mg of proteins). Then, protein A-coupled Sepharose beads were added and incubated for 1 h (30 µL per mg of proteins). After centrifugation at 200×g for 5 min, beads were washed three times with Co-IP buffer (5 min), once in high-salt containing Co-IP buffer (450 mM NaCl), and a last one with Co-IP buffer, and finally boiled in Laemmli buffer before resolution by SDS-PAGE and analysis by Western blotting.
Western blotting
Cells were first washed twice with ice-cold PBS and then incubated for 20 min with lysis buffer (10 mM Tris–HCl, 150 mM NaCl, 0.1% (m/v) sodium dodecyl sulfate (SDS), 1% (v/v) Triton-X100, 0.5% (m/v) sodium deoxycholate (NaDOC), pH 7.5) containing protease inhibitors (protease cocktail inhibitors, Sigma-Aldrich), 50 mM sodium fluoride (NaF, Sigma-Aldrich) and 1 mM sodium orthovanadate (Sigma-Aldrich). The cell lysates were then centrifuged at 20,000×g for 15 min at 4 °C. The supernatants were collected and protein concentrations were measured using the micro BCA protein assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Proteins (20 µg per lane) were resolved by 6 or 8% SDS-PAGE in electrophoresis buffer (25 mM Tris–HCl, 192 mM glycine, 0.1% (m/v) SDS, pH 8.8) and transferred onto nitrocellulose membranes (HybondTM-C EXTRA, GE Healthcare) in transfer buffer (25 mM Tris–HCl, 192 mM glycine, 20% (v/v) methanol, pH 8.8). Membranes were stained with Ponceau red (5% (v/v) acetic acid and 0.1% (w/v) Ponceau red) to assess equal loading. Membranes were destained with TBS (Tris-Buffered Saline)—Tween20 (20 mM Tris–HCl, 150 mM NaCl, 0.05% (v/v) Tween20 (Sigma-Aldrich), pH 7.5) (TBS-T), subsequently blocked in 5% (w/v) nonfat dry milk or BSA in TBS-T, and probed overnight at 4 °C with primary antibodies (Supp. Table 1). After three TBS-T washes, membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (anti-mouse or anti-rabbit IgG-HRP linked, Supp. Table 1) for 1 h at room temperature. After three TBS-T washes, blots were developed using enhanced chemiluminescence (West Pico Plus, ThermoScientific). The images were acquired using a CCD camera (Fusion Solo, Vilbert Lourmat). For additional probing, the membranes were stripped in the Antibody Stripping Buffer (Gene Bio-Application L.T.D.) for 15 min at RT, washed in TBS-T and re-probed with antibodies. Densitometry analyses of Western blot images were done using Image J software.
mRNA extraction and RT-qPCR analysis
Extraction of mRNA was performed using the Nucleospin “DNA, RNA and protein purification” kit (Macherey-Nagel) according to the manufacturer’s instructions. Assay of RNA was carried out using a Nanodrop type system. Reverse transcription was carried out on 1 µg of RNA with the Maxima First Strand cDNA Synthesis kit for RT-qPCR (Thermo Scientific) and the following incubation step: 10 min at 25 °C, 30 min at 50 °C and 5 min at 85 °C. The FASN, OGT, SREBP, ChREBP and RPLP0 transcripts were analyzed by RT-qPCR using Mx4000 Multiplex Quantitative PCR system (Stratagene). Each PCR reaction contains 12.5 µL of SyberGreen, 300 nM of each primer (Supp. Table 2) and 2 µL of cDNA for a total volume of 25 µL.
In vivo experiments
All the procedures were carried out according to the French guidelines for the care of experimental animals and the experimental procedure was approved by the Animal Care Committee of the French Research Ministry (Autor. APAFiS #1879-2018121918307521 and A75-14-08). Eight-weeks-old C57BL6 and ob/ob obese male mice were purchased from Charles River Elevage (Saint-Germain sur l’Arbresle, France) and adapted to the environment for 1 week prior to experiments. The mice were maintained in a 12 h light/12 h dark cycle with free access to water and food. The body weight and blood glucose levels were determined. Before being sacrificed, mice were fasted by preventing access to food for 18 h. Livers were collected and lysed in 1 mL of lysis buffer for proteins or 1 mL of RA1 buffer (containing chaotropic salt) for mRNA extraction. Gentle MACS Dissociator (MACS Miltengi Biotec) and gentle MACS M Tubes were used for tissue disruption. The soluble fractions were obtained after centrifugation at 1000×g for 3 min (mRNA) and 20,000×g for 15 min (proteins).
Hepatic mouse mutant of PTEN has been previously described [43]. Pre-tumoral male mice between 5–6 months of age were used in the experiments. Animals were maintained in grouped cages in a temperature-controlled pathogen-free facility on 12 h/12 h (8 am–8 pm) light/dark cycle and had free access to water and standard chow (Teklad global protein diet; 20% protein, 75% carbohydrate, 5% fat). Animals were sacrificed in random fed state between 2 and 4 pm (ZT6-ZT8).
Histological and morphometric analyses
For immunohistochemical analysis, liver tissue was fixed overnight in phosphate-buffered 10% (w/v) formalin and embedded in paraffin. Four μm sections were cut and processed for immunohistochemical analyses. Immunohistochemistry of liver tissue sections was performed using anti-phospho-rpS6, anti-FASN or anti-O-GlcNAc antibodies (see Supp. Table 1 for references) following manufacturer guidelines and procedure described before [44]. The signal was quantified using Fiji as previously detailed in [45]. Results were expressed as optical densities (OD) calculated from intensity values as follows: OD = log (max intensity/mean intensity) in a total area of at least ten sequential fields of 33,500 μm2 tissue analyzed.
Results
OGT and FASN functionally interact.
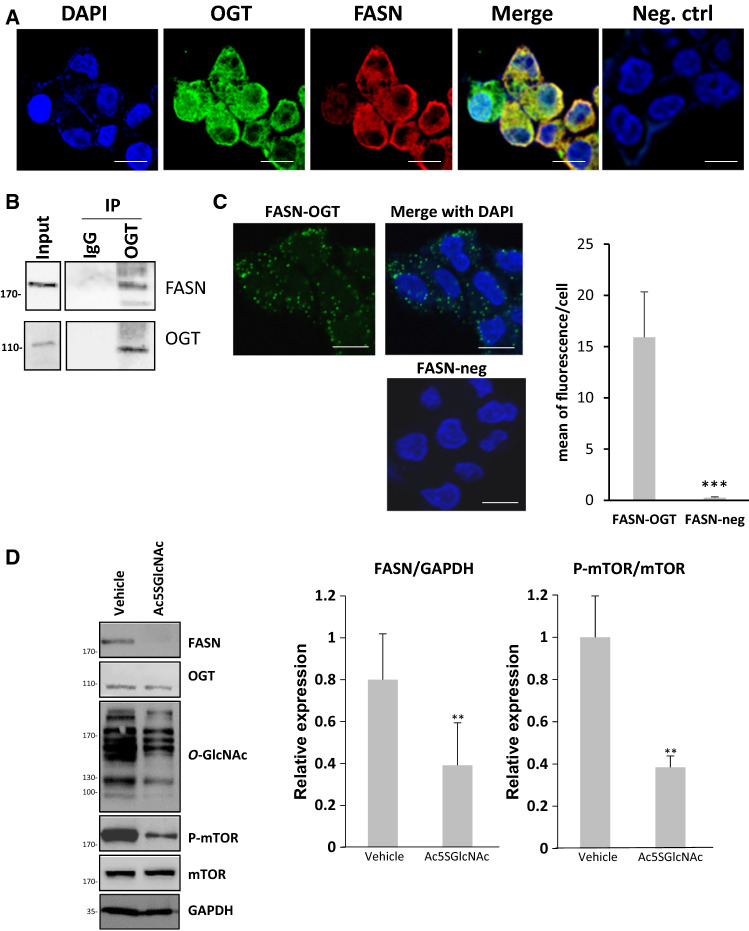
OGT is mainly expressed in the cytoplasm and the nucleus [46], while the mOGT mitochondrial isoform of OGT (mOGT) localizes to the mitochondria [47]. Cytoplasmic localization of FASN has been extensively reported albeit Madigan and collaborators have detected the enzyme in the nucleus of prostate cancer cells and in prostate cancer tissues of patients [48]. By immunofluorescence (Fig. 1A) and using fusion proteins (Supp. Fig. 1A) we observed that both OGT and FASN are distributed in the cytoplasm of the hepatocellular carcinoma HepG2 cell line. A similar observation was made in the hepatocellular carcinoma Hep3B cell line (Supp. Fig. 1B). Subcellular fractionation of cell cycle phase synchronized HepG2 cells depleted from serum (G0 phase) or serum-depleted and then stimulated with serum to re-enter the G1 phase strengthened that OGT and FASN co-localize in the cytoplasmic fraction (Supp. Fig. 1C). OGT was also found in the nucleus, as expected, while we did not detect FASN in the nuclear fraction. Both enzymes are also detected in the membrane fraction, as previously reported for the glycosyltransferase [34, 49]. Interestingly, the cytoplasmic levels of FASN and OGT increase following serum stimulation of starved cells (attested by the presence of phosphorylated-AKT in the membrane fraction) (Supp. Fig. 1C). Co-IP experiments (Fig. 1B) and in situ proximity ligation assays (PLA) (Fig. 1C) corroborated the interaction between FASN and OGT in HepG2 cells, as we previously showed [26]. As shown by PLA, OGT and FASN are found in close proximity mainly in the cytoplasm (Fig. 1C). Since O-GlcNAcylation levels are dependent upon glucose concentration [50, 51], we evaluated the interaction between FASN and OGT in a physiological concentration of glucose (5 mM) versus in high glucose conditions (25 mM) (Supp. Fig. 2A). Interestingly, this interaction seems to be dependent upon glucose concentration since a higher PLA signal was observed in the high glucose condition compared to the low glucose one. Western blot analysis showed that FASN and O-GlcNAcylation levels are much more elevated in high glucose concentration (Supp. Fig. 2B) indicating that an increased interaction between the two enzymes would result in a greater part from an increased expression of FASN.
Fig. 1.
OGT interacts with FASN and inhibition of its catalytic activity reduces FASN expression. a Confocal microscopy analysis of HepG2 cells indicate that OGT and FASN are mainly co-expressed in the cytoplasm (bar size: 15 µm). b Co-IP and c in situ PLA approach (bar size: 15 µm) reveal that OGT and FASN are interacting partners (negative control was performed by omitting one of the two primary antibodies). Nuclei were counterstained with DAPI. Quantification of PLA is presented at the right. d Asynchronous HepG2 cells were treated with Ac5SGlcNAc, an inhibitor of OGT, for 18 h or with vehicle (DMSO). Efficiency of OGT inhibition was checked by staining proteins with an anti-O-GlcNAc antibody, and mTOR inactivation was evaluated with an anti-phospho-mTOR (Ser2448) antibody. Analysis of protein lysates by Western blot shows that OGT inhibition reduces FASN expression and mTOR activation. Optical densities corresponding to FASN, phospho-mTOR and mTOR were measured and accordingly FASN/GAPDH (n = 6) and phospho-mTOR/mTOR (n = 3) were calculated and reported. For Western blots, molecular mass markers are indicated on the left (kDa). Bar size: 15 µm. **P < 0.01, ***P < 0.001
Owing to that (i) FASN expression is dependent on O-GlcNAcylation in a context of lipogenesis [26], and (ii) that activation of mTOR pathway is under the control of O-GlcNAcylation status and reciprocally [40], we further investigate whether both the level of FASN and activation of mTOR are dependent on OGT activity. Treatment of HepG2 cells with Ac5SGlcNAc, a potent OGT's inhibitor, for 24 h revealed that both the steady-state level of FASN and activation of mTOR, as evaluated by phosphorylation at Ser2448, are significantly reduced when OGT is inhibited (Fig. 1D).
FASN protein expression positively correlates with mTOR activation
We then hypothesized that, in addition to be regulated by O-GlcNAcylation, FASN expression depends on mTOR status. To test this hypothesis, we explored GEPIA (Gene Expression Profiling Interactive Analysis) database and performed correlation analyses to evaluate the pair-wise co-expression of the gene encoding FASN versus a PI3K/AKT/mTOR signaling signature (PI3KCA, AKT, MTOR, RPTOR, RPS6KB1, EIF4E) (Supp. Fig. 3A). Data showed a significant positive correlation between the levels of these transcripts in healthy liver or colon/rectum and corresponding cancer tissues, with a correlation coefficient values of R = 0.41 (n = 50; p value, 0.0037) for healthy liver, R = 0.29 (n = 369; p value, 1.3e−8) for LIHC (liver hepatocarcinoma), R = 0.56 (n = 51; p value, 3.1e−05) for healthy colon and rectum, and R = 0.48 (n = 367, p value, 3.2e−22) for colorectal cancer (COAD/READ). Next, we analyzed the expression levels of FASN and OGT, and the activation status of mTOR in four different liver or colon asynchronous cell lines (Supp. Fig. 3B). As shown by Western blot, expression of FASN is higher in HT29 and HCT116 colon cancer cells compared with the non-cancerous CCD841CoN colon cell line. Moreover, FASN protein is more strongly expressed in hepatocarcinoma HepG2 cells compared to colon cancer cells. Interestingly, the expression of FASN positively correlated with the O-GlcNAcylation profile and the phosphorylation of mTOR in the four cell lines (Supp. Fig. 3B).
Next we used two distinct mouse models in which upregulation of mTOR was described: the ob/ob (obese) mice [52] and mice with liver-specific deletion of PTEN (phosphatase and tensin homolog) [43]. To further characterize the relationship between FASN, O-GlcNAcylation and mTOR activation, we first compared livers from C57Bl6 mice to those from ob/ob mice (Fig. 2). Prior animal sacrifice, mice were starved for 18 h, and blood glucose levels and body weights were measured (Supp. Fig. 4). While no significant difference between the two groups was found for the blood glucose concentrations, ob/ob mice had a double weight compared to the control group as expected (Supp. Fig. 4). As shown by Western blotting from liver lysates, a dramatic increase in the FASN expression level was observed in ob/ob mice compared to control mice, concomitantly to higher O-GlcNAc levels and mTOR activation which was assessed by the phosphorylation of the downstream target p70S6K (p70 Ribosomal protein S6 Kinase) at Thr389 (Fig. 2A, B). The protein and mRNA levels of OGT were steady between the two groups suggesting that the increase O-GlcNAcylation levels observed in livers of obese mice may be due to changes in OGT activity (Fig. 2A, B). RT-qPCR analysis showed an increase in mRNA expression of FASN and SREBP (Sterol Responsive Element Binding Protein), a driver of FASN transcription (Fig. 2C), indicating that FASN expression is upregulated at the transcriptional level in obese mouse liver. However, the increase in FASN protein levels (Fig. 2A, B) cannot be only explained by the increase in FASN transcripts (80-fold increase for the protein versus 2.5-fold increase for mRNA), suggesting that FASN protein expression is likely also upregulated in a post-translational manner in ob/ob mice. Overall, in obese mice, increased FASN protein expression positively correlates with mTOR activation and levels of O-GlcNAc.
Fig. 2.
Obese mice exhibit high FASN and O-GlcNAcylation contents, and mTOR activation in liver. a Livers from ob/ob mice versus C57Bl6 mice were analyzed according to their FASN, OGT and O-GlcNAc levels by Western blot. Activation of the mTOR pathway was evaluated using an anti-phospho-p70S6K antibody. Molecular mass markers are indicated on the left (kDa). b Optical densities corresponding to FASN, OGT, O-GlcNAcylation, P-p70S6K, and p70S6K were measured and accordingly FASN/total proteins, OGT/total proteins, O-GlcNAc/total proteins, and P-p70S6K/p70S6K ratios were calculated and reported. c Levels of mRNA encoding FASN, OGT and SREBP were measured by RT-qPCR. Values were normalized to RPLP0. NS non-significant; *P < 0.05; **P < 0.01; ***P < 0.001 (n = 5)
Then, we used a mouse model of chronically activated insulin and mTOR signaling due to hepatocyte specific inactivation of lipid phosphatase PTEN (AlbCre+:PTENf/f versus PTENf/f mice) [43]. The hepatic specific PTEN KO develop severe liver hypertrophy and steatosis due to increased de novo lipid synthesis dependent on transcriptional activity of PPARγ [44, 53]. As PTEN-null mice develop hepatic adenomas, followed by hepatocellular carcinoma and cholangiocellular carcinoma with 100% penetrance, they represent a pertinent liver cancer model recapitulating major manifestations of human disease associated with fatty liver [43]. Thus, we asked whether marked liver steatosis in pre-tumoral PTEN mutants correlated with changes in O-GlcNAc pathway. For this, livers from 5–6-months-old pre-tumoral PTEN-null mice were analyzed by Western blot (Fig. 3A, B) and IHC (Fig. 3C, D). As expected, in line with activated insulin signaling, Western blot analyses revealed an activation of AKT as well as a significant increase in phosphorylation of p70S6K (Fig. 3A, B). The activation of mTOR was also evidenced by the phosphorylation of AKT substrate PRAS40 (proline-rich AKT substrate of 40 kDa) protein, an inhibitor of mTOR of which phosphorylation at Thr246 relieves its repressive function on mTORC1. Furthermore, the immunohistological analyses demonstrated increased phospho-rpS6 (ribosomal protein S6, effector of p70S6K) levels in the liver tissue of PTEN mutants compared to controls (Fig. 3C, D). Consistent with previous reports and in line with increased steatosis of PTEN mutants, the expression of FASN protein was significantly upregulated and most evidently observed in steatosis regions of liver tissue (Fig. 3C, D). Notably, both increased FASN and activated mTOR signaling were positively correlated with increased O-GlcNAc levels (Fig. 3C, D). Surprisingly, we found that OGT protein levels were decreased in livers of PTEN mutants compared to age-matched controls (Fig. 3A, B). No difference in protein levels of unprocessed and mature form of SREBP (nuclear form ~ 50 kDa) was observed between the two groups of mice (Fig. 3A, B). Altogether, the observations in these two distinct animal models of hepatic steatosis indicate that FASN-driven activated de novo lipogenesis and mTOR signaling are positively correlated with increased O-GlcNAcylation.
Fig. 3.
PTEN-null mice show hepatic FASN and O-GlcNAcylation increase levels and enhanced mTOR activation. a Livers from hepatic PTEN mutants versus wild type mice were analyzed by Western blot with indicated antibodies. Molecular mass markers are indicated on the left (kDa). b Optical densities corresponding to FASN, OGT, O-GlcNAcylation, SREBP (precursor and nuclear form), P-p70S6K, and p70S6K were measured and accordingly FASN/actin, OGT/actin, O-GlcNAc/actin, SREBP (precursor or nuclear form)/actin, and P-p70S6K/p70S6K ratios were calculated and reported. c The same samples were also analyzed by immunohistochemistry for expression of FASN, activation of mTOR pathway (phospho-rpS6) and evaluation of O-GlcNAcylation levels. d Quantification was done with Fiji software and values were accordingly represented as histograms. NS non-significant; *P < 0.05; **P < 0.01; ***P < 0.001 (n = 5)
Expression of FASN depends on OGT activity in serum stimulated-cells
FASN is not only necessary for energy storage, it is also crucial for cell proliferation, a process in which the enzyme plays diverse functions including the supply in fatty acids that contribute to the building of membranes in dividing cells. To analyze the expression profile of FASN along the cell cycle, we synchronized HepG2 cells at G0/G1 using the serum-starvation method followed by serum addition [33]. Serum-induced cells were collected at the indicating times for expression analyses during cell cycle progression (Fig. 4A). The efficiency of cell synchronization and cell cycle progression was monitored by FACS analysis and expression of cyclin D1 (G1 phase progression) and cyclin B1 (G2/M transition) (Fig. 4A and Supp. Fig. 5). FASN expression is rapidly induced upon serum stimulation and its level follows cyclin D1 profile and activation of the mTOR pathway as attested by phosphorylation of mTOR at Ser2448 and phosphorylation of its downstream target p70S6K at Thr389 (Fig. 4A). Then, the enzyme content decreased to be minimal fourteen hours after cell cycle release (cyclin D1 is degraded, both phosphorylation of mTOR and p70S6K decrease, and S phase is maximal) and slightly re-increased later when cyclin B1 was expressed (Fig. 4A). Similar fluctuation of FASN expression during cell cycle was also observed in the immortalized human hepatocyte cell line IHH (Supp. Fig. 6). As we previously reported [33], upon mitogenic stimulation, the OGT level is increased in a constant manner (Fig. 4A and Supp. Fig. 6). Notably, the effect on FASN protein levels was post-transcriptional since its transcript levels were unmodified throughout our serum stimulation experiments (Fig. 4B). At the same time, the transcripts of OGT, SREBP and ChREBP (Carbohydrate Responsive Element Binding Protein), another transcriptional factor driving FASN mRNA transcription, varied along cell cycle (Fig. 4B). Thus, the cell cycle phase dependent fluctuation of FASN protein rather results from proteostasis and PTMs than from transcriptional processes. This experiment shows that the expression of FASN progressively increases during G1 phase, decreases in S phase and then re-augments in G2 phase. Moreover, OGT regularly increased upon stimulation all along the cell cycle as previously observed for serum [33] or insulin [34] stimulation.
Fig. 4.
FASN expression is induced by serum, and FASN and OGT interact differentially along cell cycle progression. a HepG2 cells were serum-starved for 24 h and then cell cycle was released by addition of serum. Serum-induced cells were collected at the indicated time periods after serum addition and analyzed by FACS: the percentage of cells in G0/G1, S and G2/M phases are indicated. Cell lysates were analyzed by Western blot according to their FASN, OGT and O-GlcNAc contents. Phosphorylation of mTOR and p70S6K attests of activation of the mTOR pathway. Progression of the cells along cell cycle is visualized by expression of cyclins D1 and B1. GAPDH serves as equal loading control. Molecular mass markers are indicated on the left (kDa). Optical densities corresponding to FASN were measured (four independent experiments) and accordingly FASN/GAPDH ratios were calculated and reported. b Levels of mRNA encoding FASN, OGT, ChREBP and SREBP were measured by RT-qPCR. Values were normalized to RPLP0. HepG2 cells were serum-starved for 24 h and then cell cycle was released by addition of serum. c Cells were analyzed by in situ PLA to visualize the interaction between the two partners at different time periods (bar: 15 µm). Negative controls were performed by omitting one of the two primary antibodies. Nuclei were counterstained with DAPI. Quantification of PLA is presented at the right. Molecular mass markers are indicated on the left (kDa). NS non-significant; *P < 0.05; **P < 0.01; ***P < 0.001 (n = 4 for proteins, n = 3 for mRNA)
Next, we wondered whether the complex between OGT and FASN changes along the cell cycle progression. For this the synchronized HepG2 cells were analyzed using PLA (Fig. 4C). The basal interaction between the partners observed in starved cells (t = 0) increased after 8 h of serum stimulation when cells were mostly in G1 phase. Then the interaction decreased in S phase (t = 14 h) and re-increased 24 h after stimulation, when the cell population was in G2/M. The changes in the interaction between OGT and FASN are likely to follow the cyclic expression of FASN during cell cycle progression. Finally, we performed the same time-course experiment in HepG2 cells transfected with siRNA targeting OGT (Fig. 5). As shown by Western blot, the strong decrease in O-GlcNAcylation in siOGT cells compared to siCtrl cells disturbed the serum-induced time-course expression of FASN, reduced the phosphorylation of both AKT and p70S6K, and disturbed the distribution of the cells in the cell cycle. OGT expression is thus fundamental for the correct induction of FASN expression and activation of mTOR pathway during cell cycle entry.
Fig. 5.
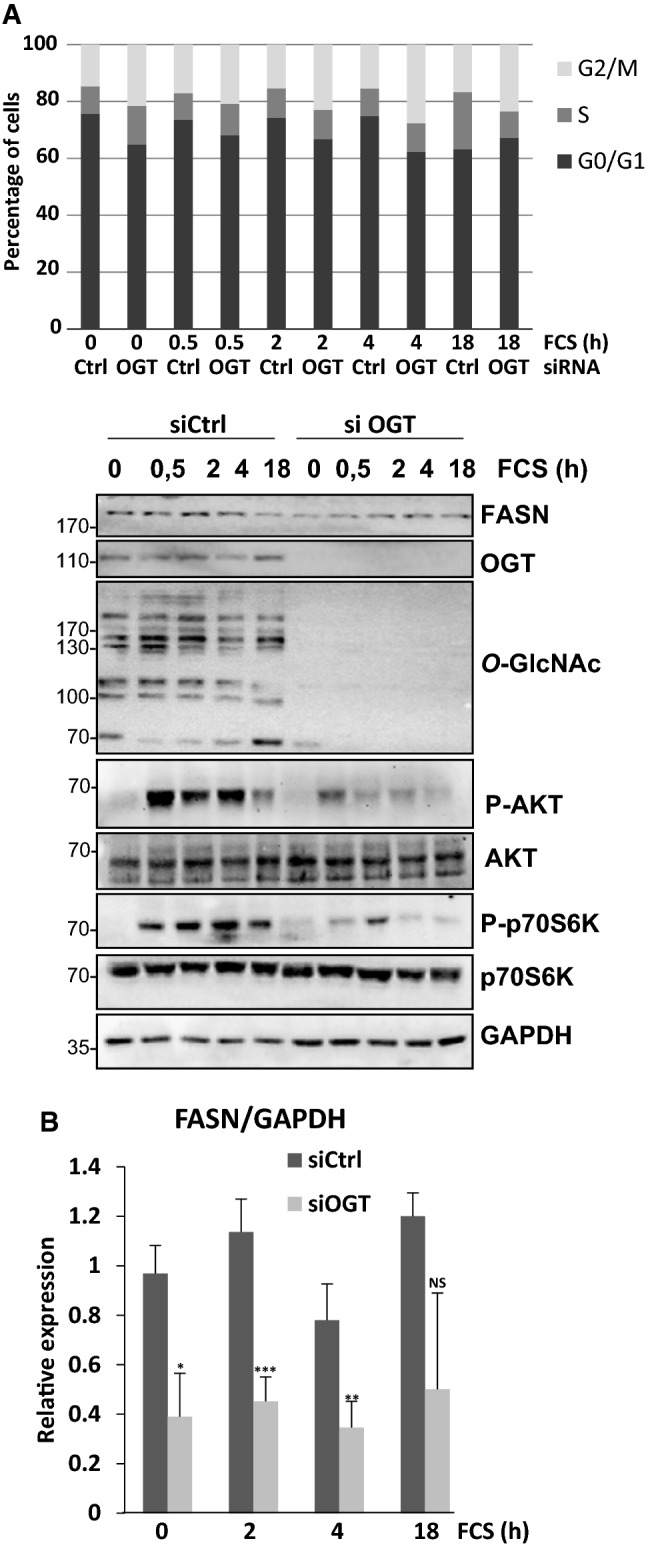
Silencing OGT reduces FASN expression concomitantly to the inactivation of the mTOR pathway during cell cycle progression. a HepG2 cells were transfected with siRNA targeting OGT mRNA prior to serum starvation. Then cell cycle was released by addition of serum and cells were collected at the indicated time periods after serum addition and analyzed by FACS: the percentage of cells in G0/G1, S and G2/M phases are indicated. Cell lysates were analyzed by Western blot according to their FASN, OGT and O-GlcNAc contents. Phosphorylation of AKT was evaluated, and activation of the mTOR pathway attested by monitoring the phosphorylation of p70S6K. Molecular mass markers are indicated on the left (kDa). b Optical densities corresponding to FASN were measured and accordingly FAS/GAPDH ratios were calculated and reported. NS non-significant; *P < 0.05; **P < 0.01; ***P < 0.001 (n = 4)
Expression of FASN during G1 phase is dependent upon mTOR activation
We reported previously that expression of FASN was dependent upon O-GlcNAcylation status during lipogenesis [26] and that OGT and mTOR regulate each other in a reciprocal manner [40]. Here, we showed that induction of FASN expression follows the serum-induced activation of mTOR (Fig. 4), while inhibition or silencing OGT reduced FASN expression and mTOR activation concomitantly (Figs. 1D, 5). Altogether, these observations suggested that synthesis of FASN may also depend on mTOR pathway. We therefore investigated the role of mTOR pathway on FASN expression during G1 phase. Starved HepG2 cells were synchronized by serum starvation for 48 h and then re-stimulated with serum for 3 h, with or without the mTOR inhibitor rapamycin (Fig. 6). As shown by Western blotting, FASN levels significantly decreased upon mTOR inhibition which was ascertained by the lack of phosphorylation of p70S6K. At the same time mTOR inhibition by rapamycin did not affect neither expression nor maturation of SREBP (Fig. 6A). Moreover, qRT-PCR analysis revealed that blockade of mTOR did not impact the mRNA levels of FASN, SREBP or ChREBP (Fig. 6B).
Fig. 6.
Inhibition of mTOR drastically reduces FASN expression. a Serum-starved HepG2 cells were released from G0 to G1 by incubation with serum for 3 h with or without the potent mTOR inhibitor rapamycin. FASN and SREBP expression, and activation of mTOR were analyzed by Western blot. Molecular mass markers are indicated on the left (kDa). b Optical densities corresponding to FASN and SREBP were measured and accordingly FASN/GAPDH and SREBP/GAPDH ratios were calculated and reported. Levels of mRNA encoding FASN, ChREBP, SREBP, GK and ACC were measured by RT-qPCR. Values were normalized to RPLP0. NS non-significant; **P < 0.01; ***P < 0.001 (n = 3)
Next, we tested the effect of Ac5SGlcNAc and rapamycin on IHH cells for 24 or 48 h (Supp. Fig. 7). Both inhibitors decreased the expression of FASN but the combined action of these two molecules resulted in only a very moderate synergistic effect. This indicates that FASN expression is not only under the control of O-GlcNAcylation and mTOR pathway in cancer-derived cells but also in immortalized human hepatocytes.
Blockade of FASN activity impacts OGT expression and mTOR activation
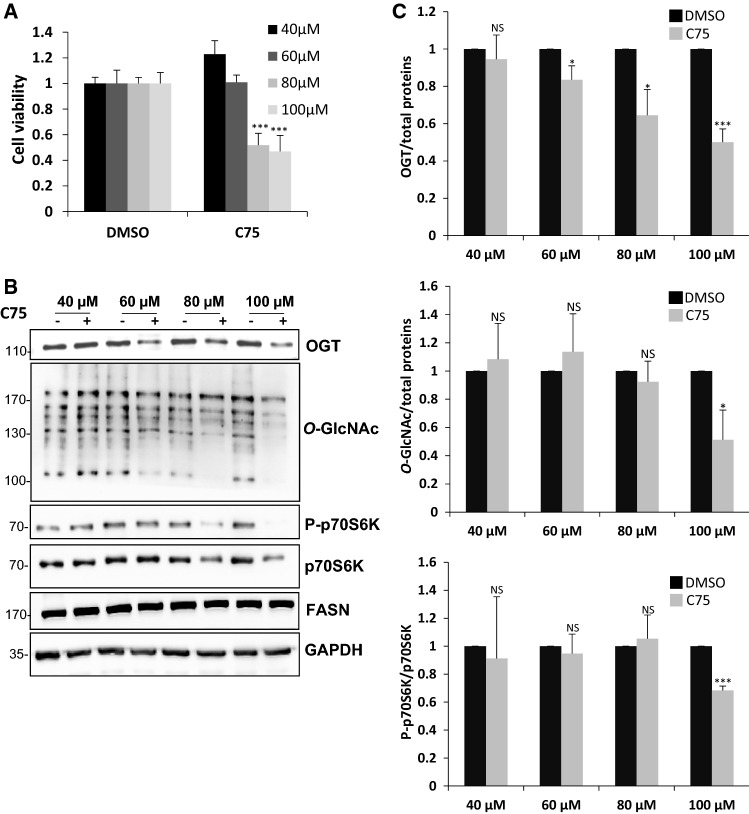
Since we showed that FASN expression correlates with the activity of both OGT and mTOR, we wondered whether FASN could in turn control OGT, as well as activation of the mTOR pathway. To answer this question, we treated asynchronous HepG2 cells with increasing concentrations of the small-molecule inhibitor C75, a synthetic analog of the natural inhibitor of FASN cerulenin [54]. Efficiency of C75 was first evaluated by the MTS method [55]: we observed a 50% decrease in cellular viability at 80 µM and 100 µM of C75 (Fig. 7A). In treated cells, we observed a significant decrease in OGT expression level in a dose-dependent manner, from 60 to 100 µM C75; O-GlcNAcylation levels and activation of mTOR pathway were significantly reduced at 100 µM C75 (Fig. 7B, C). These results show that FASN inhibition affects both O-GlcNAc cycling and mTOR activation, highlighting a reciprocal regulation between FASN, OGT and mTOR pathway.
Fig. 7.
Inhibition of FASN reduces OGT and O-GlcNAc levels, and inactivates mTOR. HepG2 cells were treated with increasing concentrations of the FASN inhibitor C75. a Cell viability was monitored to attest of the efficiency of C75. b Cell lysates were prepared and analyzed by Western blot according to their OGT, FASN and O-GlcNAc levels, and activation of mTOR pathway. Molecular mass markers are indicated on the left (kDa). c Optical densities corresponding to OGT, O-GlcNAc, P-p70S6K and p70S6K were measured and accordingly OGT/total proteins, O-GlcNAc/total proteins and P-p70S6K/p70S6K ratios were calculated and reported below their representative Western blot. NS non-significant; *P < 0.05; ***P < 0.001 (n = 3)
Discussion
FASN is a fundamental pillar of all living beings, its knock-down preventing implantation of embryos [56]. Using acetyl-CoA, malonyl-CoA and NADPH,H+, FASN builds acyl-CoA which is widely used in cells, especially for sustaining membrane formation, this process being particularly high in cancer cells. Here, we show that serum mitogenic stimulation of starved cells induces FASN protein expression in an OGT- and PI3K/mTOR-dependent manner.
Induction of OGT expression is also dependent upon mitogenic pathways [33, 34]; in turn OGT activity is necessary for MAPK and PI3K pathways activation [34, 57–59]. Moreover, FASN expression is partly controlled by OGT as we previously reported [26] and observed herein. Inhibition of OGT impairs FASN expression in asynchronous cells, but also reduces mTOR activation as already described in colon cell lines [40]. Since the expression of both OGT and FASN responds to serum stimulation and is closely related to mTOR pathway in HepG2 cells, we therefore, asked whether OGT activity was a prerequisite for FASN expression upon G0/G1 stimulation. We show that silencing of OGT delays the expression of FASN and decreases mTOR activation in synchronized HepG2 cells. Moreover, inhibition of mTORC1 by rapamycin prior to serum stimulation also prevented the expression of FASN, as reported in hepatocarcinoma mouse models [60]. Our findings are in accordance with reported observations of mTOR acting upstream of FASN expression at the protein level but not mRNA level in breast cancer cells [61]. It was reported that the PI3K/AKT/mTOR pathway regulates FASN by inducing the transcription factor SREBP under hypoxia conditions in human breast carcinoma cell lines [62]. But, in our hands, we did not observe any difference in the expression of SREBP in response to mTOR activation in HepG2 cells. To further understand the cross-regulation of FASN and O-GlcNAcylation by the mTOR pathway in a more physiological context, we moved on two mice models in which mTOR is aberrantly activated, namely the (ob/ob) obese mice [52] and the liver-specific PTEN mutant [43]. Our in vivo observations show that mTOR activation positively correlates with FASN accumulation and a slight increase in global O-GlcNAcylation in liver of both animal models. However, upregulation of O-GlcNAcylation was not related to an increase in OGT protein levels, indicating that O-GlcNAcylation contents depend also on the fine-control of the catalytic activity of OGT and likely of the antagonist enzyme OGA. In addition, our results suggest a reciprocal regulation of mTOR by FASN. Inhibition of the lipogenic enzyme with C75 led to a decrease in phosphorylation of the mTOR downstream target, p70S6K in liver cancer cells. Interestingly, in ovarian cancer cells, blockade of FASN interferes with mTORC1 activation at multiple levels [63]. In another work, Bruning and co-workers demonstrated that downregulation of endothelial FASN inhibits mTOR by accumulation of malonyl-CoA, the main substrate of FASN [64]. Indeed malonyl-CoA covalently links mTOR at Lys1218, hence impairing the kinase activity and consequently angiogenesis.
This cross-regulation between OGT, FASN and mTOR is of particular interest since each of these actors are dependent on the nutritional status. The highly conserved kinase mTOR is part of two complexes, namely mTORC1 and mTORC2. mTORC1, for which the bacterial macrolide rapamycin is an inhibitor [65], is involved in many key cellular processes that integrate various signals like growth factors, energy, oxygen, and nutrients levels to activate protein, lipid and nucleotide synthesis, glutamine metabolism and glycolysis [38]. O-GlcNAc homeostasis is directly link to nutrients availability through HBP that is highly dependent among other on glutamine metabolism [51]. Therefore, our data and others strongly suggest that activation of mTORC1 and OGT may converge towards the induction of FASN in a growth factor- and nutrient-dependent manner to coordinate cell cycle progression, especially G1 phase characterized by a sustained protein synthesis, and cellular proliferation. As suggested by our results, this may occur through direct interaction of FASN with OGT on one hand, and, probably, FASN and mTOR on the other hand. In addition, we show in the present study that a small fraction of FASN and OGT is localized at the plasma membrane, as reported in various cell lines [34, 49, 66, 67]. Indeed, at the membrane level, both FASN and OGT are more specifically found in lipid rafts [34, 66] in which the former controls the production of phospholipids [3]. Lipid rafts concentrate modulators of signal transduction and trafficking, thus playing a fundamental role in cell adhesion, migration and proliferation in physiological conditions and cancers [68]. Interestingly, FASN and OGT have been shown to be phosphorylated by tyrosine-kinase receptors [69, 70]. To further elucidate the functional reciprocal regulation of OGT and FASN, it could be interesting to determine whether their tyrosine-phosphorylation status may facilitate their recruitment to the plasma membrane, increases their activity and potentiates the activation of signaling pathways to drive cell proliferation.
While it is well-known that OGT and FASN play both a leading role in cell proliferation, our work sheds additional light on the dual regulation and concerted action of these two enzymes in cell cycle entry and progression in different cell lines and mice models. In addition to this duo, we bring new evidence supporting the crosstalk between FASN, OGT and the nutrient-dependent mTOR pathway, and consequently identifying a new enzymatic triad involved in cell cycle progression.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Dr. Julien Thévenet (INSERM, CHU Lille, UMR1190 Translational Research for Diabetes, European Genomic Institute for Diabetes, Lille, France) for helping in harvesting livers form C57Bl6 and ob/ob mice. We also thank Pr. David Vocadlo and Dr. Matthew Alteen from the Simon Fraser University for providing Ac5SGlcNAc and the SFR-Necker small animal histology and morphology platform for histological slide preparation.
Abbreviations
- ALL
Acute lymphoblastic leukemia
- ChREBP
Carbohydrate Responsive Element Binding Protein
- Co-IP
Co-immunoprecipitation
- DMEM
Dulbecco’s Modified Eagle’s Medium
- DMSO
Dimethyl sulfoxide
- Elovl
Elongation of very long chain fatty acids proteins
- FASN
Fatty acid synthase
- FCS
Fetal calf serum
- GEPIA
Gene Expression Profiling Interactive Analysis
- GFAT-1
Glutamine:fructose–6–phosphate amidotransferase–1
- GPCR
G protein coupled-receptors
- HBP
Hexosamine biosynthetic pathway
- HRP
Horseradish peroxidase
- IHH
Immortalized human hepatocytes
- LPA
Lysophosphatidic acid
- MEF
Mouse embryonic fibroblasts
- MEM
Minimal Essential Medium
- mTOR
Mechanistic target of rapamycin
- NaDOC
Sodium deoxycholate
- OD
Optical density
- OGA
O-GlcNAcase
- OGT
O-GlcNAc transferase
- p70S6K
p70 Ribosomal protein S6 Kinase
- PAF
Paraformaldehyde
- PBS
Phosphate-Buffered Saline
- PIP3
Phosphatidylinositol–3,4,5–triphosphate
- PLA
Proximity ligation assay
- PRAS40
Proline-rich AKT substrate of 40 kDa
- PTEN
Phosphatase and tensin homolog
- PTM
Post-translational modification
- rpS6
Ribosomal protein S6
- RT
Room temperature
- RT-qPCR
Reverse transcription-quantitative polymerase chain reaction
- SDS
Sodium dodecyl sulfate
- SDS-PAGE
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- siRNA
Small interfering RNA
- SREBP
Sterol Responsive Element Binding Protein
- TBS
Tris-buffered saline
- VLDL
Very low density lipoproteins
- WB
Western blot
Funding
This research was supported by the University of Lille, the “Centre National de la Recherche Scientifique (CNRS)” and from the “Agence Nationale de la Recherche” (ANR-JCJC-NUTRISENSPIK-16-CE14-0029) to G.P. SR is a recipient of a fellow from the “Ministère de l’Enseignement Supérieur et de la Recherche” and from the “Région Hauts-de-France”.
Declarations
Conflict of interest
The authors declare neither conflict of interest nor competing interests.
Ethics approval
All procedures were carried out according to the French guidelines for the care of experimental animals. Experimental procedure was approved by the Animal Care Committee of the French Research Ministry (Autor. APAFiS #1879-2018121918307521 and A75-14-08).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zambetti NA, Firestone AJ, Remsberg JR, Huang BJ, Wong JC, Long AM, Predovic M, Suciu RM, Inguva A, Kogan SC, Haigis KM, Cravatt BF, Shannon K. Genetic disruption of N-RasG12D palmitoylation perturbs hematopoiesis and prevents myeloid transformation in mice. Blood. 2020;135(20):1772–1782. doi: 10.1182/blood.2019003530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorentino M, Zadra G, Palescandolo E, Fedele G, Bailey D, Fiore C, Nguyen PL, Migita T, Zamponi R, Di Vizio D, Priolo C, Sharma C, Xie W, Hemler ME, Mucci L, Giovannucci E, Finn S, Loda M. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab Investig. 2008;88(12):1340–1348. doi: 10.1038/labinvest.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swinnen JV, Van Veldhoven PP, Timmermans L, De Schrijver E, Brusselmans K, Vanderhoydonc F, Van de Sande T, Heemers H, Heyns W, Verhoeven G. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003;302(4):898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 4.Helms JB, Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 2004;5(4):247–254. doi: 10.1111/j.1600-0854.2004.0181.x. [DOI] [PubMed] [Google Scholar]
- 5.Faes S, Dormond O. PI3K and AKT: unfaithful partners in cancer. Int J Mol Sci. 2015;16(9):21138–21152. doi: 10.3390/ijms160921138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, An S, Ward R, Yang Y, Guo XX, Li W, Xu TR. G protein-coupled receptors as promising cancer targets. Cancer Lett. 2016;376(2):226–239. doi: 10.1016/j.canlet.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 7.Baldini SF, Lefebvre T. O-GlcNAcylation and the metabolic shift in high-proliferating cells: all the evidence suggests that sugars dictate the flux of lipid biogenesis in tumor processes. Front Oncol. 2016;22(6):6. doi: 10.3389/fonc.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3(11):2115–2120. [PubMed] [Google Scholar]
- 9.Rashid A, Pizer ES, Moga M, Milgraum LZ, Zahurak M, Pasternack GR, Kuhajda FP, Hamilton SR. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol. 1997;150(1):201–208. [PMC free article] [PubMed] [Google Scholar]
- 10.Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, Baert L, Heyns W, Verhoeven G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98(1):19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- 11.Kusakabe T, Nashimoto A, Honma K, Suzuki T. Fatty acid synthase is highly expressed in carcinoma, adenoma and in regenerative epithelium and intestinal metaplasia of the stomach. Histopathology. 2002;40(1):71–79. doi: 10.1046/j.1365-2559.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- 12.Orita H, Coulter J, Tully E, Abe M, Montgomery E, Alvarez H, Sato K, Hino O, Kajiyama Y, Tsurumaru M, Gabrielson E. High levels of fatty acid synthase expression in esophageal cancers represent a potential target for therapy. Cancer Biol Ther. 2010;10(6):549–554. doi: 10.4161/cbt.10.6.12727. [DOI] [PubMed] [Google Scholar]
- 13.Innocenzi D, Alò PL, Balzani A, Sebastiani V, Silipo V, La Torre G, Ricciardi G, Bosman C, Calvieri S. Fatty acid synthase expression in melanoma. J Cutan Pathol. 2003;30(1):23–28. doi: 10.1034/j.1600-0560.2003.300104.x. [DOI] [PubMed] [Google Scholar]
- 14.Visca P, Sebastiani V, Botti C, Diodoro MG, Lasagni RP, Romagnoli F, Brenna A, De Joannon BC, Donnorso RP, Lombardi G, Alo PL. Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer Res. 2004;24(6):4169–4173. [PubMed] [Google Scholar]
- 15.Alo PL, Amini M, Piro F, Pizzuti L, Sebastiani V, Botti C, Murari R, Zotti G, Di Tondo U. Immunohistochemical expression and prognostic significance of fatty acid synthase in pancreatic carcinoma. Anticancer Res. 2007;27(4B):2523–2527. [PubMed] [Google Scholar]
- 16.Veigel D, Wagner R, Stübiger G, Wuczkowski M, Filipits M, Horvat R, Benhamú B, López-Rodríguez ML, Leisser A, Valent P, Grusch M, Hegardt FG, García J, Serra D, Auersperg N, Colomer R, Grunt TW. Fatty acid synthase is a metabolic marker of cell proliferation rather than malignancy in ovarian cancer and its precursor cells. Int J Cancer. 2015;136(9):2078–2090. doi: 10.1002/ijc.29261. [DOI] [PubMed] [Google Scholar]
- 17.Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci USA. 2000;97(7):3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunt TW, Slany A, Semkova M, Colomer R, López-Rodríguez ML, Wuczkowski M, Wagner R, Gerner C, Stübiger G. Membrane disruption, but not metabolic rewiring, is the key mechanism of anticancer-action of FASN-inhibitors: a multi-omics analysis in ovarian cancer. Sci Rep. 2020;10(1):14877. doi: 10.1038/s41598-020-71491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaeidamini Harouni M, Rahgozar S, Rahimi Babasheikhali S, Safavi A, Ghodousi ES. Fatty acid synthase, a novel poor prognostic factor for acute lymphoblastic leukemia which can be targeted by ginger extract. Sci Rep. 2020;10(1):14072. doi: 10.1038/s41598-020-70839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fardini Y, Dehennaut V, Lefebvre T, Issad T (2013) O-GlcNAcylation: a new cancer hallmark? Front Endocrinol (Lausanne) 4:99. 10.3389/fendo.2013.00099(eCollection 2013) [DOI] [PMC free article] [PubMed]
- 21.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29(19):2831–2842. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre T, Alonso C, Mahboub S, Dupire MJ, Zanetta JP, Caillet-Boudin ML, Michalski JC. Effect of okadaic acid on O-linked N-acetylglucosamine levels in a neuroblastoma cell line. Biochim Biophys Acta. 1999;1472(1–2):71–81. doi: 10.1016/s0304-4165(99)00105-1. [DOI] [PubMed] [Google Scholar]
- 23.Olivier-Van Stichelen S, Dehennaut V, Buzy A, Zachayus JL, Guinez C, Mir AM, El Yazidi-Belkoura I, Copin MC, Boureme D, Loyaux D, Ferrara P, Lefebvre T. O-GlcNAcylation stabilizes β-catenin through direct competition with phosphorylation at threonine 41. FASEB J. 2014;28(8):3325–3338. doi: 10.1096/fj.13-243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardivillé S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 2014;20(2):208–213. doi: 10.1016/j.cmet.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18(7):452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldini SF, Wavelet C, Hainault I, Guinez C, Lefebvre T. The nutrient-dependent O-GlcNAc modification controls the expression of liver fatty acid synthase. J Mol Biol. 2016;428(16):3295–3304. doi: 10.1016/j.jmb.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh TJ, Lin T, Hsieh PC, Liao MC, Shin SJ. Suppression of glutamine:fructose-6-phosphate amidotransferase-1 inhibits adipogenesis in 3T3-L1 adipocytes. J Cell Physiol. 2012;227(1):108–115. doi: 10.1002/jcp.22707. [DOI] [PubMed] [Google Scholar]
- 28.Vasconcelos-Dos-Santos A, de Queiroz RM, da Costa RB, Todeschini AR, Dias WB. Hyperglycemia and aberrant O-GlcNAcylation: contributions to tumor progression. J Bioenerg Biomembr. 2018;50(3):175–187. doi: 10.1007/s10863-017-9740-x. [DOI] [PubMed] [Google Scholar]
- 29.Boehmelt G, Wakeham A, Elia A, Sasaki T, Plyte S, Potter J, Yang Y, Tsang E, Ruland J, Iscove NN, Dennis JW, Mak TW. Decreased UDP-GlcNAc levels abrogate proliferation control in EMeg32-deficient cells. EMBO J. 2000;19(19):5092–5104. doi: 10.1093/emboj/19.19.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DH, Kwon NE, Lee WJ, Lee MS, Kim DJ, Kim JH, Park SK. Increased O-GlcNAcylation of c-Myc promotes pre-B cell proliferation. Cells. 2020;9(1):158. doi: 10.3390/cells9010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivier-Van Stichelen S, Guinez C, Mir AM, Perez-Cervera Y, Liu C, Michalski JC, Lefebvre T. The hexosamine biosynthetic pathway and O-GlcNAcylation drive the expression of β-catenin and cell proliferation. Am J Physiol Endocrinol Metab. 2012;302(4):E417–E424. doi: 10.1152/ajpendo.00390.2011. [DOI] [PubMed] [Google Scholar]
- 32.Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem. 2005;280(38):32944–32956. doi: 10.1074/jbc.M503396200. [DOI] [PubMed] [Google Scholar]
- 33.Olivier-Van Stichelen S, Drougat L, Dehennaut V, El Yazidi-Belkoura I, Guinez C, Mir AM, Michalski JC, Vercoutter-Edouart AS, Lefebvre T. Serum-stimulated cell cycle entry promotes ncOGT synthesis required for cyclin D expression. Oncogenesis. 2012;1(12):e36. doi: 10.1038/oncsis.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Cervera Y, Dehennaut V, Aquino Gil M, Guedri K, Solórzano Mata CJ, Olivier-Van Stichelen S, Michalski JC, Foulquier F, Lefebvre T. Insulin signaling controls the expression of O-GlcNAc transferase and its interaction with lipid microdomains. FASEB J. 2013;27(9):3478–3486. doi: 10.1096/fj.12-217984. [DOI] [PubMed] [Google Scholar]
- 35.Drougat L, Olivier-Van Stichelen S, Mortuaire M, Foulquier F, Lacoste AS, Michalski JC, Lefebvre T, Vercoutter-Edouart AS. Characterization of O-GlcNAc cycling and proteomic identification of differentially O-GlcNAcylated proteins during G1/S transition. Biochim Biophys Acta. 2012;1820(12):1839–1848. doi: 10.1016/j.bbagen.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Lefebvre T, Baert F, Bodart JF, Flament S, Michalski JC, Vilain JP. Modulation of O-GlcNAc glycosylation during Xenopus oocyte maturation. J Cell Biochem. 2004;93(5):999–1010. doi: 10.1002/jcb.20242. [DOI] [PubMed] [Google Scholar]
- 37.Dehennaut V, Slomianny MC, Page A, Vercoutter-Edouart AS, Jessus C, Michalski JC, Vilain JP, Bodart JF, Lefebvre T. Identification of structural and functional O-linked N-acetylglucosamine-bearing proteins in Xenopus laevis oocyte. Mol Cell Proteomics. 2008;7(11):2229–2245. doi: 10.1074/mcp.M700494-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24(7):400–406. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sodi VL, Khaku S, Krutilina R, Schwab LP, Vocadlo DJ, Seagroves TN, Reginato MJ. mTOR/MYC axis regulates O-GlcNAc transferase expression and O-GlcNAcylation in breast cancer. Mol Cancer Res. 2015;13(5):923–933. doi: 10.1158/1541-7786.MCR-14-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Very N, Steenackers A, Dubuquoy C, Vermuse J, Dubuquoy L, Lefebvre T, El Yazidi-Belkoura I. Cross regulation between mTOR signaling and O-GlcNAcylation. J Bioenerg Biomembr. 2018;50(3):213–222. doi: 10.1007/s10863-018-9747-y. [DOI] [PubMed] [Google Scholar]
- 41.Leturcq M, Mortuaire M, Hardivillé S, Schulz C, Lefebvre T, Vercoutter-Edouart AS. O-GlcNAc transferase associates with the MCM2-7 complex and its silencing destabilizes MCM-MCM interactions. Cell Mol Life Sci. 2018;75(23):4321–4339. doi: 10.1007/s00018-018-2874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masclef L, Dehennaut V, Mortuaire M, Schulz C, Leturcq M, Lefebvre T, Vercoutter-Edouart AS. Cyclin D1 stability is partly controlled by O-GlcNAcylation. Front Endocrinol (Lausanne) 2019;22(10):106. doi: 10.3389/fendo.2019.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, Naito M, Enomoto K, Watanabe S, Mak TW, Nakano T. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Investig. 2004;113(12):1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patitucci C, Couchy G, Bagattin A, Cañeque T, de Reyniès A, Scoazec JY, Rodriguez R, Pontoglio M, Zucman-Rossi J, Pende M, Panasyuk G. Hepatocyte nuclear factor 1α suppresses steatosis-associated liver cancer by inhibiting PPARγ transcription. J Clin Investig. 2017;127(5):1873–1888. doi: 10.1172/JCI90327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crowe AR, Yue W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. 2019;9(24):e3465. doi: 10.21769/BioProtoc.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272(14):9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 47.Lazarus BD, Love DC, Hanover JA. Recombinant O-GlcNAc transferase isoforms: identification of O-GlcNAcase, yes tyrosine kinase, and tau as isoform-specific substrates. Glycobiology. 2006;16(5):415–421. doi: 10.1093/glycob/cwj078. [DOI] [PubMed] [Google Scholar]
- 48.Madigan AA, Rycyna KJ, Parwani AV, Datiri YJ, Basudan AM, Sobek KM, Cummings JL, Basse PH, Bacich DJ, O'Keefe DS. Novel nuclear localization of fatty acid synthase correlates with prostate cancer aggressiveness. Am J Pathol. 2014;184(8):2156–2162. doi: 10.1016/j.ajpath.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451(7181):964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 50.Steenackers A, Olivier-Van Stichelen S, Baldini SF, Dehennaut V, Toillon RA, Le Bourhis X, El Yazidi-Belkoura I, Lefebvre T. Silencing the nucleocytoplasmic O-GlcNAc transferase reduces proliferation, adhesion, and migration of cancer and fetal human colon cell lines. Front Endocrinol (Lausanne) 2016;25(7):46. doi: 10.3389/fendo.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biwi J, Biot C, Guerardel Y, Vercoutter-Edouart AS, Lefebvre T. The many ways by which O-GlcNAcylation may orchestrate the diversity of complex glycosylations. Molecules. 2018;23(11):2858. doi: 10.3390/molecules23112858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng D, Youn DY, Zhao X, Gao Y, Quinn WJ, 3rd, Xiaoli AM, Sun Y, Birnbaum MJ, Pessin JE, Yang F. mTORC1 down-regulates cyclin-dependent kinase 8 (CDK8) and cyclin C (CycC) PLoS One. 2015;10(6):e0126240. doi: 10.1371/journal.pone.0126240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panasyuk G, Espeillac C, Chauvin C, Pradelli LA, Horie Y, Suzuki A, Annicotte JS, Fajas L, Foretz M, Verdeguer F, Pontoglio M, Ferré P, Scoazec JY, Birnbaum MJ, Ricci JE, Pende M. PPARγ contributes to PKM2 and HK2 expression in fatty liver. Nat Commun. 2012;3:672. doi: 10.1038/ncomms1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pizer ES, Chrest FJ, DiGiuseppe JA, Han WF. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 1998;58(20):4611–4615. [PubMed] [Google Scholar]
- 55.Rae C, Fragkoulis GI, Chalmers AJ. Cytotoxicity and radiosensitizing activity of the fatty acid synthase inhibitor C75 is enhanced by blocking fatty acid uptake in prostate cancer cells. Adv Radiat Oncol. 2020;5(5):994–1005. doi: 10.1016/j.adro.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chirala SS, Chang H, Matzuk M, Abu-Elheiga L, Mao J, Mahon K, Finegold M, Wakil SJ. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci USA. 2003;100(11):6358–6363. doi: 10.1073/pnas.0931394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dehennaut V, Hanoulle X, Bodart JF, Vilain JP, Michalski JC, Landrieu I, Lippens G, Lefebvre T. Microinjection of recombinant O-GlcNAc transferase potentiates Xenopus oocytes M-phase entry. Biochem Biophys Res Commun. 2008;369(2):539–546. doi: 10.1016/j.bbrc.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 58.Jiang M, Qiu Z, Zhang S, Fan X, Cai X, Xu B, Li X, Zhou J, Zhang X, Chu Y, Wang W, Liang J, Horvath T, Yang X, Wu K, Nie Y, Fan D. Elevated O-GlcNAcylation promotes gastric cancer cells proliferation by modulating cell cycle related proteins and ERK 1/2 signaling. Oncotarget. 2016;7(38):61390–61402. doi: 10.18632/oncotarget.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang P, Wang C, Ma T, You S. O-GlcNAcylation enhances the invasion of thyroid anaplastic cancer cells partially by PI3K/Akt1 pathway. Onco Targets Ther. 2015;9(8):3305–3313. doi: 10.2147/OTT.S82845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li T, Weng J, Zhang Y, Liang K, Fu G, Li Y, Bai X, Gao Y. mTOR direct crosstalk with STAT5 promotes de novo lipid synthesis and induces hepatocellular carcinoma. Cell Death Dis. 2019;10(8):619. doi: 10.1038/s41419-019-1828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon S, Lee MY, Park SW, Moon JS, Koh YK, Ahn YH, Park BW, Kim KS. Up-regulation of acetyl-CoA carboxylase a and fatty acid synthase by human epidermal growth factor 2 at the translational level in breast cancer cells. J Biol Chem. 2007;282:26122–26131. doi: 10.1074/jbc.M702854200. [DOI] [PubMed] [Google Scholar]
- 62.Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, Kamada S, Saito K, Iiizumi M, Liu W, Ericsson J, Watabe K. Fatty acid synthase gene is up-regulated by hypoxia via activation of AKT and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- 63.Wagner R, Stübiger G, Veigel D, Wuczkowski M, Lanzerstorfer P, Weghuber J, Karteris E, Nowikovsky K, Wilfinger-Lutz N, Singer CF, Colomer R, Benhamú B, López-Rodríguez ML, Valent P, Grunt TW. Multi-level suppression of receptor-PI3K-mTORC1 by fatty acid synthase inhibitors is crucial for their efficacy against ovarian cancer cells. Oncotarget. 2017;8(7):11600–11613. doi: 10.18632/oncotarget.14591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bruning U, Morales-Rodriguez F, Kalucka J, Goveia J, Taverna F, Queiroz KCS, Dubois C, Cantelmo AR, Chen R, Loroch S, Timmerman E, Caixeta V, Bloch K, Conradi LC, Treps L, Staes A, Gevaert K, Tee A, Dewerchin M, Semenkovich CF, Impens F, Schilling B, Verdin E, Swinnen JV, Meier JL, Kulkarni RA, Sickmann A, Ghesquière B, Schoonjans L, Li X, Mazzone M, Carmeliet P. Impairment of angiogenesis by fatty acid synthase inhibition involves mTOR malonylation. Cell Metab. 2018;28(6):866–880.e15. doi: 10.1016/j.cmet.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 66.Di Vizio D, Adam RM, Kim J, Kim R, Sotgia F, Williams T, Demichelis F, Solomon KR, Loda M, Rubin MA, Lisanti MP, Freeman MR. Caveolin-1 interacts with a lipid raft-associated population of fatty acid synthase. Cell Cycle. 2008;7(14):2257–2267. doi: 10.4161/cc.7.14.6475. [DOI] [PubMed] [Google Scholar]
- 67.Xue T, Zhang Y, Zhang L, Yao L, Hu X, Xu LX. Proteomic analysis of two metabolic proteins with potential to translocate to plasma membrane associated with tumor metastasis development and drug targets. J Proteome Res. 2013;12(4):1754–1763. doi: 10.1021/pr301100r. [DOI] [PubMed] [Google Scholar]
- 68.Mollinedo F, Gajate C. Lipid rafts as major platforms for signaling regulation in cancer. Adv Biol Regul. 2015;57:130–146. doi: 10.1016/j.jbior.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Jin Q, Yuan LX, Boulbes D, Baek JM, Wang YN, Gomez-Cabello D, Hawke DH, Yeung SC, Lee MH, Hortobagyi GN, Hung MC, Esteva FJ. Fatty acid synthase phosphorylation: a novel therapeutic target in HER2-overexpressing breast cancer cells. Breast Cancer Res. 2010;12(6):R96. doi: 10.1186/bcr2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whelan SA, Lane MD, Hart GW. Regulation of the O-linked beta-N-acetylglucosamine transferase by insulin signaling. J Biol Chem. 2008;283(31):21411–21417. doi: 10.1074/jbc.M800677200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.