Abstract
The traditional functions of cytoskeletal-associated proteins (CAPs) in line with polymerization and stabilization of the cytoskeleton have evolved and are currently underrated in oncology. Although therapeutic drugs have been developed to target the cytoskeletal components directly in cancer treatment, several recently established therapeutic agents designed for new targets block the proliferation of cancer cells and suppress resistance to existing target agents. It would seem like these targets only work toward inhibiting the polymerization of cytoskeletal components or hindering mitotic spindle formation in cancer cells, but a large body of literature points to CAPs and their culpability in cell signaling, molecular conformation, organelle trafficking, cellular metabolism, and genomic modifications. Here, we review those underappreciated functions of CAPs, and we delineate the implications of cellular signaling instigated by evasive properties induced by aberrant expression of CAPs in response to stress or failure to exert normal functions. We present an analogy establishing CAPs as vulnerable targets for cancer systems and credible oncotargets. This review establishes a paradigm in which the cancer machinery may commandeer the conventional functions of CAPs for survival, drug resistance, and energy generation; an interesting feature overdue for attention.
Keywords: CAPs-Cytoskeltal-associated proteins, Metabolism, MAPs-Microtubule-associated proteins, IFAPs-Intermediate filament-associated proteins, Cell signaling
Introduction
We have come a long way in understanding the complexities and the control of the cytoskeleton in cellular dynamics and some roles of the cytoskeletal component (actin filaments, microtubule, and intermediate filaments) in metastasis. Nevertheless, important questions about the roles of the cytoskeleton and its components in major signaling underlying cancer progression remain exclusive. The cytoskeleton is a dynamic multiplex set of networks controlling cell shape, migration, stiffness, adhesion, and response to cell stress [1]. These functions and dynamics of the cytoskeleton are dependent on regulations by cytoskeletal-associated proteins (CAPs) and post-translational modifications [2]. CAPs are proteins involved in the regulation of cytoskeletal components individually or capable of synergistic regulation of two or all cytoskeletal components. These include microtubule (MT)-associated proteins (MAPs), actin-related proteins (ARPs), and intermediate filament (IF)-associated proteins (IFAPs) [3]. However, the traditional functions of CAPs in stabilizing and polymerizing the cytoskeleton may be regarded as a downplay of the absolute potentials these molecules harbor, which is currently unexplored or understudied.
Our growing knowledge of CAPs helped pave the way for new insights in cancer development. Several CAPs have multiple functions that emerged based on subcellular localization, such as modulation of growth factor receptors, regulation of cell signaling pathways, and mitotic spindle assembly [4, 5]. Their increased expression also possesses significant roles in tumorigenesis and can induce genomic instability and cancer progression, including aberrant proliferation and migration [6–11]. Hence, it is imperative to understand these molecules’ underlying functions and full potentials, and their mechanism of action. In context; How are CAPs related to cancer progression? Are they naturally pro-oncogenic in nature, or do cancer machinery easily take over their functions faster than other types of molecules? These questions remain unanswered and are begging to be understood.
It is important to state that even though we are not writing specifically about the cytoskeletal components (IF, MT, and Actin), our thoughts may sometimes shift toward those unusual functions of these components, a fraction of which are summarized herein to make specific points along with our understanding and hypothesis. Ergo, this review is not about the functions of the cytoskeleton in migration or cell shape. Rather, it details the implications of specific CAPs capable of regulating multiple cytoskeletal components in cancer progression, with regard to their traditional functions and their corresponding roles in drug resistance and cancer metabolism, which is presumed to be easily commandeered by those factors driving cancer initiation and progression.
CAPs are easy targets for cancer treatment
Upregulation of CAPs is related to the high invasiveness of cancer cells [12–14]. Despite the high level of conservation, the cytoskeleton adapts to different control mechanisms either by constant modification of their biophysical properties or by the dynamic association with associated proteins that convey specific signals responsible for cellular functions [12, 15]. CAPs are simultaneously engaged in survival, recurrence, or drug resistance. Hence, CAPs assume a more prominent role in malignancy. However, these proteins are not only subjective to the cytoskeletal components but are also involved in cell signaling processes that lead to cell survival, drug resistance, and recurrence [3, 16]. Indeed, drugs that target the cytoskeleton also effectively attenuate crucial signaling pathways directed at major cellular events. Consequently, it becomes inherently of note to understand the intricacies of these unpopular conjectures and draw a firm conclusion to give prominence to this interesting concern and to identify the limitations as certain trends are being revealed to project CAPs in part, a holy grail of oncology.
Targeting the MTs and MAPs in cancer
The MT is an established chemotherapeutic target in cancer therapy
Some MT-destabilizing drugs are current standard chemotherapeutics in some cancers. MT-active substances currently referred to as spindle poisons have recorded tremendous clinical success in the treatment of malignancies. As such, taxanes are termed “stabilizers” and vincas are called “destabilizers”. In fact, most, if not all MT-binding agents destabilize MT dynamics at low concentrations, and this potency also affects spindle development, hence, the arrest of cell division. There are currently several anticancer drugs designed to alter the mitotic spindle at different stages of development, while several others are now at developmental stages. A typical example is that exerted by taxol, the first MT-stabilizing drug described in literature, and an antitumor drug effective for cancer treatment (reviewed in [17]). However, cancer cells easily circumvent drug potency by reorganizing the cytoskeleton to adapt to the drug’s effect (Fig. 1). For instance, βIII-tubulin is one of seven isoforms of human β-tubulin isoforms (out of nine in mammals), and it is mainly involved in cellular response to oxidative stress [18]. It has recently been regarded as a marker of taxane resistance in cytoskeletal-targeted therapy in cancer cells as a consequence of unstable polymer assembly of heterodimers evading the stabilizing effects exerted by taxol on MTs [19, 20] (Fig. 1). In glioblastoma, βIII-tubulin is expressed across different morphologic phenotypes as well as in hypoxic neoplastic tumors bordering palisading necrosis. Expression of βIII-tubulin has also been reported in poorly differentiated cells and stem cells responsible for neurogenesis [21]. βIII-tubulin is also aberrantly expressed in prostate cancer and is associated with PTEN deletion [22]. However, there are competing opinions disputing the adoption of the above analogy (βIII-tubulin as a marker of taxane resistance), citing that these functions may have been exaggerated, and explaining that although βIII-tubulin may serve as a prognostic marker of certain neoplasms, current evidence are inadequate to adopt βIII-tubulin as a predictive marker of taxane resistance [23–25]. Therefore, elucidation of βIII-tubulin’s response to taxane treatments merits further studies.
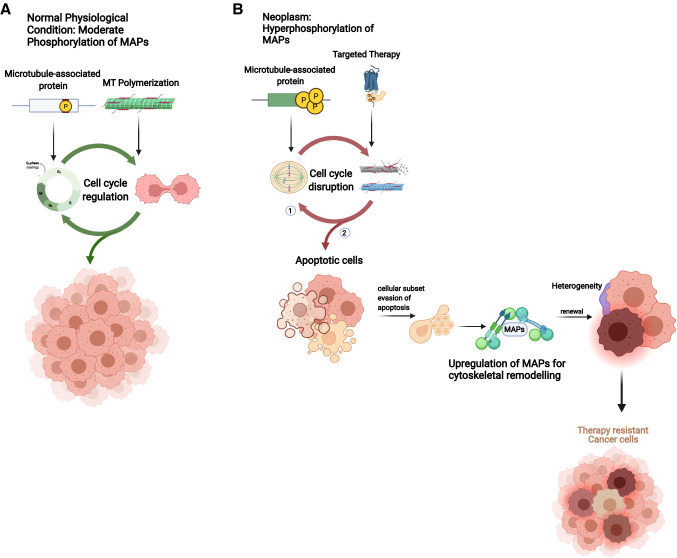
Fig. 1.
CAPs confer therapeutic resistance on cancer cells. A MAPs stabilize and polymerize MTs for effective and maximal functional capacity of cellular functions. In normal physiology, moderate phosphorylation of MAPs stabilizes and polymerizes the MT to regulate numerous cellular functions and positive control of the cell cycle. B MAPs clip αβ-heterodimers of tubulins together and failure to exert these functions due to hyperphosphorylation in cancer cells either leads to, (1) distortion of MT polymerization and stabilization leading to mitotic-slippage and misregulation of the cell cycle, or (2) evasion of apoptosis in response to targeted therapy by virtue of unstable MT polymers and high expression of MAPs in response to MT destabilization-rescue, and successful rescue leads to heterogeneity in the cell population, giving rise to more aggressive and drug-resistant clones
So far, there are varying reports of resistance to spindle poisons because of the evasive properties conferred on tumor cells via the actions of CAPs. This has led to discoveries of new drugs, such as IMB5046 and epothilones, which confer significant antitumor effects on cells resistant to colchicine, vincristine, and paclitaxel [26, 27]. IMB5046 is a newly developed MT-depolymerizing agent that exhibits potent cytotoxicity against multi-drug-resistant cell lines and in mice at well-tolerated doses. IMB5046 possesses a unique chemical structure and does not bind to P-glycoprotein (P-gp) unlike most MT-binding agents; hence, IMB5046 can circumvent P-gp-mediated multidrug resistance in cancers. Moreover, this feature distinguishes it in its mechanism of action as it functions by binding to the colchicine pockets on tubulins to inhibit MT polymerization in cancer cells [26].
Epothilones inhibit the cell cycle by binding to tubulins in cancer cells to promote polymerization and ultimately induce apoptosis. Compared with paclitaxel, epothilones have a simpler structure, higher water solubility, and can be effective in a lower dosage to curb drug resistance and have a better prospect of development [28]. However, it may be that the polymerization effect of these drugs competes with the binding and polymerization of MAPs, hence rendering tubulin monomers responsive to apoptotic signals (Fig. 1). Thus, the expression of CAPs and in part, other components of the cytoskeleton (and their isotypes) in malignancies and their involvement in those signaling downstream and along the maintenance of undefined functions adds another layer of functional complexities to the cytoskeleton and may be relevant to finding a cure to specific neoplasms. Hence, it is imperative to explore more on these findings as it may be of significance in the diagnosis of treatment resistance in some malignancies, while it may not be the same in other malignancies because of the unique properties and the relatively available binding proteins in some cancers that are absent or not as highly expressed in other cancers.
Exploitation of MAPs in cancer
In oncology, a migrating cell is deemed a proliferating cell, and the emergence of more cells sets off metastases. CAPs are involved in these processes underlying cytoskeletal remodeling, mislocalization, and aberrant expression. However, these functions are mostly classical features of CAPs that regulate normal functions of cellular physiology that can be hijacked to become targets of cancer machinery because of the susceptibilities in their roles in cytoskeletal regulation (Table 1).
Table 1.
Physiological roles of Cytoskeletal-associated proteins and their functions in cancer cells
| CAPs | Physiological Function | Neoplastic Function | |
|---|---|---|---|
| MAPs | DCX | Promotes neural progenitor cells proliferation and formation of new-born neurons during adult neurogenesis [6] | Promotes glioma cells proliferation and invasiveness [37] |
| Tau | Regulates cellular migration and axonal MT functions [32] | Regulates MTs and actin remodeling for glioblastoma migration [33] | |
| DAB1 | Regulates cellular migration during neurogenesis [39] | Promotes colorectal cancer metastasis [41] | |
| TPPP/p25 | Modulates MT dynamics and stability via tubulin acetylation and bundling [170] | Regulation of tumor growth via spindle orientation and tubulin acetylation [171–173] | |
| MAP4 | Regulates MT dynamics via interactions with Septin [174] | Promotes cancer cell invasion via MAP4-ERK-Jun-VEGF signaling [175] | |
| DCLK | Regulates MT dynamics and structure, and mediates pro-metaphase to metaphase transition in a dynein-dependent manner during mitosis [176] | Regulates mitotic spindle and promotes proliferation. Silencing DCLK results in alteration of genes involved in oxidative stress and oxidative phosphorylation and induction of caspase-dependent apoptosis in cancer cells [177] | |
|
Microtubule‐ Associated Tumor Suppressor Protein (MATSP) |
Interacts with mitochondria membrane proteins to regulate mitochondria morphology [178] | Acts as tumor suppressor in cancer cells and interrupts cell division by prolonging metaphase [179, 180] | |
| Stathmin | Regulates global dynamics of interphase microtubules [181] | Instigates cellular resistance to paclitaxel-induced apoptosis [182] | |
| ARPs | Gelsolin | Severs actin filament in a Ca(2 +)-dependent manner [183] | Mediates colorectal cancer invasion via the uPA/uPAR cascade modulation at metastatic sites [51] |
| Tropomyosin | Controls access to myosin’s interaction with actin in a Ca(2 +)-dependent manner [184] | Regulates cellular migration, glucose uptake, and can mediate the ERK signaling in cancers [53] | |
| ARP2/3 | Required for actin nucleation and crosslinks polymerizing actin filaments [185] | Downstream effector activated by mTORC1 to promote Akt/Rac1 signaling in PDAC [186] | |
| Formin-like Protein | Regulates cytokinesis, cell polarity, and morphogenesis [187] | Promotes cell proliferation by inhibiting P27 stability and nuclear translocation [188] | |
| DIAPH1 | Crucial roles in thrombosis and vascular remodeling [189] | And inhibits apoptosis via ATR/p53/Caspase-3 Signaling [190] | |
| CAPZA | Binds to actin filaments in a Ca(2 +)-independent manner to prevent exchange of subunits at fast growing ends of actin [191] | Promotes EMT in HCC cells via F-actin remodeling [141] | |
| Nck‐associated protein 1 | Involved in phagocytosis, lymphocyte development, and neutrophil migration [192] | Associates with heat shock protein and enhances MMP9 secretion for cancer cell migration [193] | |
| Abelson interactor 1 | Promote WAVE2 membrane translocation and links Rac activation | Regulates cell proliferation via Ras small G-protein to alter cellular migration in an Rac-dependent manner [194] | |
| IFAPs | Plakin | Provides anchorage sites for IFs. Involved in the differentiation of epidermal keratinocytes (also regarded as incomplete apoptosis) [195] | Mediates interaction with the extracellular matrix. Activates PKC and MAPK signaling in response to stress [71] |
| Mrj | Confers stability to Keratin filaments as a chaperone with Hsp/c70 [77] | Confers tumor resistance to TNF-induced apoptosis [79] | |
| Spectraplakin | Provides organizational support and cytoskeletal integrity. Simultaneous cross-linking of actin and microtubules during axonal growth [196] | Suppresses tumor growth by restricting YAP activation [197] | |
| Trichohyalin-like 1 | Promotes keratinocytes proliferation via ERK1/2) phosphorylation [198] | Promotes squamous cell carcinoma proliferation via the phosphorylation of ERK1/2, AKT, and STAT3 [198] |
Known neuronal MAPs, like Tau and MAP2, are also expressed in non-neuronal neoplasm. While the expression of MAP2 correlates with taxane resistance in metastatic melanoma, Tau protein is associated with poor prognosis and can indicate taxane sensitivity [29]. Tau is a MAP primarily localized in axons. It regulates the proper functioning of MTs in normal neuronal activities based on its phosphorylation state [30]. Tau stabilizes the MTs to maintain a straight protofilament conformation by binding to a hydrophobic pocket at the interface between tubulin heterodimers [31]. Under normal physiological conditions, tau regulates cellular migration and axonal MT functions via regular phosphorylation. Interestingly, recent findings have revealed a relationship between tau pathology and glioblastoma, as CD44 could be responsible for the phosphorylation and aggregation of tau in both pathologies [32]. Tau regulates MTs and actin remodeling in a RHO/ROCK-dependent manner for migrative purposes in glioblastoma cells [33]. Moreover, tau exerts nearly similar functions and structure in normal neurons with increased activity in several malignancies (including prostate, breast, pancreatic, and colorectal carcinomas) [34].
Doublecortin (DCX) is also a MAP first discovered with regard to its role in X-linked lissencephaly and subcortical band heterotopia; two neurodevelopmental disorders involved in the abnormal migration of the cerebral cortical neurons. In normal physiological conditions, cells originating from neurogenic zones, traveling through the rostral migratory stream (RMS) toward the olfactory bulb and functional sites express DCX. Hence, DCX is utilized as a gold standard marker of adult neurogenesis. Interestingly, it has recently been proposed as a prognostic marker of glioblastoma survival [6, 35, 36]. Like tau, DCX binds between protofilaments from which MTs are built, and its phosphorylation on different serine and threonine residues regulates its physiological functions in normal cells and promotes MT stabilization and migration [6, 36]. Although DCX is traditionally known as a cytoplasmic protein, recent findings in our laboratory have revealed that a significant amount of DCX translocate to the nucleus of glioblastoma cells in response to MARK’s phosphorylation on its ser 47 residue, and the cells expressing a higher level of nuclear DCX are located at the edge of the invasive front of glioma tissues [37]. More importantly, the traditional function of DCX in adult neurogenesis and migration of newborn neurons has been implicated in metastasis of brain tumors to the prostate region and prostate cancer initiation [38].
Reelin-Disabled-1 (Dab1) is another MAP that regulates neuronal migration during neurogenesis. Dab1 is involved in the inverted lamination of the neocortex. When phosphorylated on its tyrosine residues, Dab1 recruits SH2 domain-molecules to initiate a series of signaling cascades that results in cytoskeletal remodeling [39]. To exert its migrative functions during neurogenesis, Dab1 actively utilizes the Notch signaling synergistically. More importantly, Reelin-deficient mice are characterized by reduced Notch ICD (a cleaved form of its intracellular domain), and it protects Notch ICD degradation via Dab1 [40], an interesting event that can be adopted by cancer cells for metastatic progression. As such, genetic depletion of Dab1 in mice engineered for Notch signaling activation results in the suppression of invasive and metastatic capabilities of cancer cells. Phosphorylation of Dab1 on its tyrosine residues by ABL kinase reciprocally activates ABL. This chain of events subsequently initiates RAC/RHOGEF protein TRIO's phosphorylation at Tyr residue 2681 (pY2681) in favor of metastasis in colorectal cancer [41].
Consequently, the aforementioned reversible reactions underscore weaknesses in some CAPs and set precedence for cancer networks utilizing a predetermined function in cancer progression. Although direct inhibition of specific MAPs may be detrimental to signaling cascades responsible for cellular survival, the overexpression of those oncogenes related to the cytoskeleton are vulnerabilities through which targeting downstream effectors may render cancer cells susceptible to chemotherapy.
Targeting Actin and ARPs in Cancer
While MT-targeting drugs are well-established, drugs capable of targeting actin have been more elusive. Like spindle poisons, several promising actin-targeting chemotherapeutics either function as stabilizers, or as depolymerizing agents and inhibitors of actin cross-linking to abrogate polymerization. A large number of actin-targeting chemotherapies have been developed including latrunculins, jasplakinolide, miuraenamide, cytochalasin, chaetoglobosins, and MKT-077 [42–45]. However, advances in molecular biology have revealed that the complex nature of actin biology exceeds regulation for the polymerization and depolymerization of the actin polymers. Rather, actin-binding proteins regularly compete with each other for the interaction with actin or interactions on actin-binding sites (typical examples include MRTF-G-actin [46], and thymosin beta4-profilin [47]), hence regulating the “actin interactome” and corresponding cellular functions. As such latrunculins and cytochalasins are actin destabilizers. Latrunculin is the most widely used actin-depolymerizing agent, and it binds actin monomers near nucleotide-binding cleft. Latrunculin induces disassembly of actin filaments by sequestering monomeric G-actin and disrupting actin bundling and association with associated proteins [48]. Conversely, cytochalasin tends to bind to F-actin, and consequently blocks actin polymerization [49]. Alternatively, jasplakinolide and miuraenamide act as actin stabilizing compounds that nucleate and polymerize the F-actin. Unlike jasplakinolide which competes with gelsolin or Arp2/3 for F-actin-binding, miuraenamide competes with cofilin [50]. Due to its crucial roles in RhoA/ROCK-mediated migration of cancer cells during metastasis, actin employs ARPs to mediate response to stress induced by some of these drugs. For instance, high expression of the actin-modulating cytoskeletal protein, gelsolin correlates with aggressive phenotype and drug resistance in specific tumors, and it is highly expressed at tumor borders infiltrating adjacent organs. In addition, gelsolin mediates colorectal cancer invasion via the uPA/uPAR cascade modulation at metastatic sites [51]. Gelsolin can also interact with PI3K-Akt signaling to mediate HGF-mediated cell scattering and E-cadherin transcriptional repression through Snail, Twist, and Zeb2 in diffuse gastric cancers [52]. Unfortunately, targeting actin in tumor therapy has been marred with difficulties because of the universal effects of actin-targeting drugs on all actin filament systems, consequently rendering them highly toxic to humans, particularly cardiotoxicity. The complex nature of actin biology prompts investigation into whether ARPs or other actin-related proteins might be targeted to inhibit cancer cell proliferation.
Tropomyosin is also an ARP that regulates many key cellular processes. Tpm3.1 is a major isoform of tropomyosin in most cancer cells, and it regulates cellular migration, glucose uptake, and can mediate the ERK signaling. Isoforms like Tpm2.1 regulate anoikis, while others are involved in endoplasmic reticulum (ER) stress, actin polymerization, and focal adhesion formation [53]. Newer classes of drugs have been developed to target cancer cells based on their tropomyosin isoforms composition. Interestingly, TR100 effectively inhibits Tpm3.1, resulting in diminished neuroblastoma cell proliferation and melanoma progression both in vivo and in vitro without compromising cardiac structure and function [54], as opposed to the adverse effects of other drugs targeting actin filaments. This led to the development of more anti-tropomyosin analogs. Combination of newly developed anti-tropomyosin analog like ATM-3507 with spindle poisons presented enhanced inhibition of tumor growth and proliferation in mice models [55].
Hence, for subsequent drug designs to qualify for trials and approval, they must selectively target specific structures involved in cellular motility and division, while maintaining the contractility of the heart and the diaphragm. Hence, further studies on drug composition, side effects, and specificity could present advanced knowledge required for the initiation of trials which may subsequently lead to approval of anti-actin therapeutics.
Targeting IFs and IFAPs in cancers
Although not all forms of cases are associated with IF’s induction, numerous IF family proteins are involved in different cellular processes. Unlike MTs, there is currently no routinely utilized IF-targeting drug [56]. Hence, the scarcity of depolymerizing drugs targeting IFs has highly been decisive in the shortage of distinctive researches on the IF network. Therefore, the suppression of several IFAPs is often required for the IF network targeting for extensive studies on the IF. Alternatively, constructing truncated proteins that can act as dominant-negative constructs has proven effective in the complete disorganization of the IF network [57, 58]. Nonetheless, there is still a large gap in understanding IF-complex functions and the potentials in cellular activities. Thus, IF commands more attention not only because of its involvement in support and tissue integrity but also for its vast potential. Post-translational modifications like phosphorylation, glycosylation, sumoylation, farnesylation, and acetylation contribute to the heterogeneity of the IF. These factors endow IF with metabolic functions and signaling amplification [58, 59]. The expression of IFs like vimentin correlates with invasive and metastatic tendencies in cancer cells [60]. Its downregulation has been proven to decrease tumor invasion as it can promote migration and invasion of epithelial carcinoma cells independently of E-cadherin, forcing cells into hyperplastic forms without undergoing epithelial-mesenchymal transition (EMT) [58, 61]. Several new compounds have recently been developed to target vimentin via the TGF-β1 pathway to suppress EMT in cancer cells, including Dioscin, Ginsenoside 20(R)-Rg3, Resveratrol, and Volasertib [62–65]. While several others like Aojene, Simvastatin, and withaferin A induces vimentin destabilization [66–68]. However, only FiVe1, a vimenting binding small molecule inhibitor shows an actual prospect in targeting IFs in cancers. It induces vimentin collapse through the phosphorylation on ser56 during metaphase, leading to mitotic catastrophe and loss of stemness in cancer cells [69].
IFAPs are just as important in driving cancer progression. Emerging evidence shows that proteins interacting with the elaborate network of IFs (extension connecting the actomyosin cortex to intracellular organelles) not only contribute to the biophysical properties of cancer cells for diagnosis but also serve as drivers of cancer progression and are sometimes regarded as markers of cancer survival or recurrence [58]. Newer evidence indicates that knockdown of IFAP molecules either reduced cancer invasion, increased proliferation, or promote survival [56]. In contrast, opposite phenotypes have been recorded in studies overexpressing some of these IF proteins. Furthermore, IFAPs like plectin and desmoplakin possess caspase cleavage sites and the activation of corresponding caspases can destabilize the entire IF network [70]. Plectin is a cytolinker that binds all three cytoskeletal components, and it has been touted as a pro-tumorigenic regulator of cancer progression [71]. Targeting plectin may be promising in cancer therapy thanks to its localization on the cell surface in some cancers [72]. As an IF regulator, plectin interacts with RACK1 to regulate PKC and MAPK/ERK signaling pathways for the proliferation of some cancer cells [73]. Hence, some studies have utilized plectin-targeted drug delivery to enhance targeted therapy. Plectin’s unique mislocalization to the cell surface and subsequent proliferating effects renders it an ideal therapeutic target. As such plectin-targeted peptides (KTLLPTPC) have been utilized to guide the delivery of Quinazolinedione-based compounds such as QD242-encapsulated polymeric nanoparticles in PDAC cells [74]. Furthermore, in ovarian cancer cells, plectin-targeted liposome selectively binds to neoplastic cells in vitro and in vivo [75], and this was effective in increasing the drug payload of PARP inhibitor, AZ7379 both in vitro and in vivo [76].
Mrj is a DnaJ/Hsp40 family protein recognized as an IFAP because of its binding affinity for K18. It binds to K18 through its C-terminal and can simultaneously bind to Hsp/c70 which is an established K8/K18 binding protein at its N-terminal region. Via this interaction, Mrj may confer stability to K8/K18 filaments as a chaperone with Hsp/c70, an interaction which may explain the protective roles conferred on cells by interactions between IFs and heat shock proteins (HSPs) [77]. Through its central rod domain, K18 can also bind with tumor necrosis factor (TNF) receptor (TNFR) 1-associated death domain protein (TRADD), a crucial TNFR signaling adaptor molecule and an adaptor IFAP [78]. High activity of this interaction or aberrant expression of the TRADD-bound K18 fragment confers tumor resistance to TNF-induced apoptosis, and direct targeting of this interaction may initiate apoptosis in a TNFR-dependent manner [79].
Although there is a large gap in the development of high-specificity ligand targeting IF functions in cancers, more promising candidate peptides are in development. However, further research is required to fully identify and develop better therapeutics to regulate more vulnerable targets in IF-targeted therapy in cancers.
Role of keratins in oncogenic signaling
Keratins are IF-forming proteins of epithelial cells and are reliable diagnostic markers of malignancies and cancer cell signaling regulators. Keratins are involved in tumorigenesis and metastasis of numerous cancers including lungs [80] and colorectal cancers [81]. Moreover, some keratins participate in cell survival and apoptosis in cancer cells. Depleting the Keratin isoform Keratin 8 and 18 (K8/18) increases cisplatin-induced apoptosis via the extrinsic pathway involving Fas receptor membrane targeting regulated by the tight junction protein, claudin1 [82]. Furthermore, loss of K8/18 induces nuclear translocation of claudin1, and knockdown of K8/18 stimulates transcriptional activity and expression of NF-ΚBp65 and MMP2/MMP9, respectively, albeit also moderating TNF-α mediated NF-ΚB activity to aid or provide resistance to the apoptotic effects of TNF-α [82, 83]. K8/18 also interacts/binds with TNF receptor-2 (a receptor IFAP), highlighting keratins' interactive abilities with other signaling molecules [83]. This particular interaction alters TNF-dependent activation of downstream effectors, such as c-jun-NH2-kinase (an enzymatic IFAP) and NF-ΚB, to modify apoptotic and cell survival processes [84]. It is important to note that most type-I keratins possess caspase cleavage sites directly responsible for their involvement in apoptotic signaling. In contrast, type-II keratins do not possess cleavage sites and are, therefore, resistant to proteolysis by caspase cleavage [70, 85]. However, these keratins are not necessarily redundant during apoptosis because they are frequently associated with other keratins [86]. For example, cleaving K18 cleavage by caspase 9 sets up a series of apoptotic events that leads to DNA fragmentation and the activation of other caspases [87]. In a slightly similar process, Desmin, a type III IF, can also be cleaved by caspase6 in cells undergoing apoptosis [88].
K17 indicates susceptibility to IF-targeted therapy
Escobar-Hoyos et al. explored an exciting feature for K17 in tumor biology. They explained that K17 functions specially among keratins as an oncoprotein by controlling the ability of tumor suppressor p27KIP1 to influence cervical cancer pathogenesis [89]; their report revealed the promotion of p27KIP1’s nuclear export by K17, and the resulting degradation of p27KIP1, thus, stimulating tumor proliferation by regulating G1–S checkpoint in cancer cells [89]. It is important to note that p27KIP1 regulates effective G1 timing due to improved nuclear export of p27KIP1 and degradation, which triggers required tumor growth, increased mutation, and defective DNA replication [90]. In the case of K17 deficiency, cancer cells undergoing early G1 arrest complemented with p27KIP1 nuclear accumulation and stabilization results in decreased phosphorylation of pRb, consistent with inhibition of G1 cyclin-dependent kinases by elevated p27KIP1 [89]. In cervical cancer specimens, K17 expression inversely correlates with p27KIP1 nuclear levels and reduced cell proliferation in basal cell carcinoma. However, this reduction is not linked to cell cycle events, suggesting that K17 deregulates critical tumor suppressor programs in G1, promoting p27KIP1 nuclear export, sustained proliferation, and tumor growth [89]. K17 acts as a linker between CRM1 and p27KIP1, as point mutations in the nuclear export signals (NES) of K17, and CRM1’s inhibition with CRM1 specific inhibitor (LMB) instigated nuclear accumulation of both p27KIP1 and K17. Hence, K17-NES is the primary mediator of p27KIP1 nuclear export in K17-expressing cancer cells [91]. Although p27KIP1 nuclear export may be mediated via various mechanisms, it is now popular knowledge that K17 promotes p27KIP1 nuclear export in different malignancies. K17-positive cancer cells undergo rapid proliferation and are relatively chemoresistant, and diminished cell death by sequestering the death adaptor TRADD [92] or minimizing apoptotic effects [93]. Furthermore, K17 expression is associated with poor prognosis, and silencing K17 results in a twofold increase in cisplatin sensitivity [89]. In fact, within both low- and high-grade cervical cancers, high K17 status identifies patients at the greatest risk of mortality who could be most likely to benefit from the more aggressive therapeutic intervention in tumors characterized with high K17 expression [89, 94–97]. Besides, K17 status is understood to be a better negative prognostic marker than p27KIP1 and tumor stage. Notably, K17 expression provides information beyond classical clinicopathologic parameters currently used to guide patient management decisions.
Keratin fusion in cancer progression
Technological advances in gene sequencing have improved our abilities to identify oncogenes for specific druggable targets. Hence, gene fusion which was previously attributed to hematologic malignancies has now been recognized in heterogeneous solid tumors [98]. Keratins as members of the IF protein family has recently been attributed extended roles in cell growth, apoptosis, as well as stress sensing and response [99, 100]. Keratins have also been identified as a critical player in stem cell differentiation since this process is highly mechanosensitive [101, 102]. In fact, keratin expression changes influence EMT and cancer stem cell development, which forms a major step in tumor progression and metastasis [103–107]. Furthermore, K6 [108], K14 [103, 104], K15 [109], and K19 overexpressions are implicated in cancer progression [110, 111], while K8/K18 downregulation are faulted for cancer progression [82]. Tsai et al. have also recently reported novel keratin fusions while studying the global keratin fusion arrangement, among which K6-K14/V3, V5, and V7 were shown to have significant clinical relevance, which can potentially be considered as prognostic markers in the prediction of clinical outcomes. Higher expression of this particular fusion was found to improve oral squamous cell carcinoma stemness by promoting TGF-β and G-CSF activities, then subsequently activated downstream EMT transcription factors and rendered the cells drug-resistant and more aggressive [112]. Similar results were reported by some researchers and the TCGA group in their cancer genomics project in head and neck cancer (HNSCC), where they characterized several novel gene fusions, including those involving Keratins [113, 114]. Furthermore, Guo et al. also reported distinct gene fusion profiles specific to HPV-positive HNSCC, including certain KRT fusions like APAF1-K14 and EFTUD2-K24 [115]. Their findings may support the notion that keratin fusion functions as one possible early event during cancer initiation and development. These keratin fusions take up active roles as driver of oncogenesis, but not as passengers. However, Keratin does not get enough research focus it deserves and the potentials of several IFAPs in these processes are not fully understood. Like gene fusion and miRNAs, keratin fusion is a significant study that can unbox the complex intricacies underlying known mechanisms evading successful targeting in cancer therapy. The IF has somewhat been underrated in its functional capacity in cancer progression. In fact, drugs targeted at MAPs for therapeutic intervention against cancer cells have been in use, and more drugs are currently in the production stage. However, because the IFAPs are understudied, their potential in cancer progression is fairly known. This underscores the massive potential therapeutic intervention that can be developed to facilitate drug delivery or weaken resistance to those conventional anticancer drugs currently being used in cancer therapeutics.
Traditional functions of CAPs as stress proteins have evolved into metabolic stress regulation
The cytoskeleton orchestrates cellular signaling via CAPs in response to stress. Cellular stress induced by exposure to toxic microenvironment may be characterized by hypoxia, nutrient deprivation, and increased metabolic switch [116]. The cytoskeleton responds to many of these stress signals and actively participates in specific metabolic processes as a pro-survival reaction from aggressive cancers [24, 117]. These cancers possess abundant regions creating niches for stem cells resistant to conventional therapies and are capable of tumor recurrence and re-initiation [118].
MAPs regulate microtubule function in cellular metabolism
Metabolic stress is a product of aberrant cell proliferation in nutrient-deficient conditions. In response to specific stressors, the MT stimulates metabolic processes via MAPs to regulate and maintain cellular energy levels, and may serve as a sensor of energy levels in the cells [119]. The measure of energy levels can be translated by the stability in detyrosination of the MT plus end, which may be affected by ATP levels in the cell [120]. Modulation of MT dynamics and post-translational modifications of tubulins in response to stress allows metabolic programs in cells. A typical example is the epigenetic modification of tubulins via hypermethylation in nutrient-deficient conditions for the initiation of autophagy as a part of AMPK signaling triggered by the loss of ATP [121]. The MT via tubulins and MAPs actively modulates mitochondria metabolism by virtue of the reversible interactions between tubulins and VDAC to regulate ATP production and energy generation in hypoxic conditions via the Warburg effects in proliferating cells [122, 123]. In particular, βIII-tubulin associates with the TCA cycle and glycolytic intermediates [20]. However, specific mechanisms of modulation are still not clearly understood. The interaction between tubulins and specific MAPs with GAPDH promotes cellular trafficking and ATP generation, which may fuel the energy consumption of motor proteins in favor of MT dynamics [124]. This interaction further extends to membrane fusion for the regulation of vesicular trafficking through the membrane during glycolytic stress [125], and the recruitment of Rab2 protein to maintain and regulate the shuttle between ER and the Golgi apparatus [126], which may be crucial to protein synthesis during metabolism. It should also be noted that GAPDH also binds to the neuronal MAP1B; however, subsequent translocation occurs in response to oxidative stress [127]. These interactions between metabolic intermediates and CAPs are mostly crucial for cancer progression and evasion of apoptotic signals. Interactions with pyruvate kinase stimulate the depolymerization of the MTs [128]. While interactions between βIII-tubulin and PKM2 in the mitochondria favorably regulates the metabolic flux and influences the Warburg effect [129].
MAPs orchestrate MT’s response to hypoxia
The MT undergoes conformational changes in response to oxygen deprivation, which may arise from uncontrolled proliferation. Furthermore, there is a sudden drop in polymerization in anoxic conditions, while hypoxia positively regulates MT polymerization [130, 131]. In hypoxic conditions, polymerization is accompanied by detyrosination of tubulins and modification of specific metabolic reactions via GSK3β inhibition [130]. Simultaneously, rapid phosphorylation of MAPs like MAP4, tau, and DCX depolymerizes the MT due to hypoxia-induced p38/MAPK pathway [6, 130]. Thus, remodeling of the MT in response to hypoxic conditions may influence different signaling in the cell, including all implicating pathways associated with hypoxic response in cancer cells, which subsequently influences the metastatic progression of invasive cancer cells (Fig. 2).
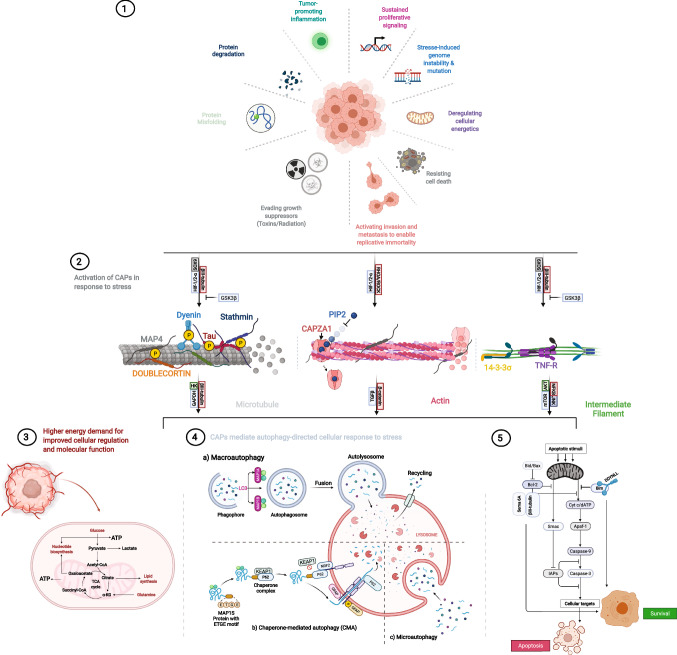
Fig. 2.
CAPs orchestrate the modification of the cytoskeletal components in response to stress to regulate homeostasis and cancer progression. 1 Cancer cells respond to different types of stress, in the form of genetic, oxidative, mechanical, protein misfolding, and radiation, stemming from the environment or targeted therapy. 2 Cells respond to these stressors via cytoskeletal remodeling and upregulating CAPs with respective response signals that initiate a rapid post-translational modification of CAPs, and subsequent activation of several downstream signaling and interactions with specific metabolic intermediates, which trigger secondary functions for CAPs leading to relative metabolic processes. 3 Aberrant energy generation leads to more uncontrolled proliferation and an unstable microtubule results in abnormal cell division. 4 The aberrant energy generation in cancer cells prompts hyperactivity, and maintenance of each process requires homeostasis, another secondary function of specific CAPs; here, MAP1 binds to tubulin to promote autophagosome formation, while its homolog MAP1S is involved in chaperone-mediated autophagy. 5. Failure to effectively regulate the constant demand for energy or cellular response to therapy initiates apoptotic signals which are intercepted by other CAPs via interactions with anti-apoptotic genes leading to survival
Moreover, dynein light chain tctex-type1 (DYNLT1) is also a phosphorylation target that results in MT's depolymerization in response to hypoxia [130]. When unphosphorylated, DYNLT1 protects against the permeabilization of the mitochondria by stabilizing the MT to maintain ATP production in hypoxic conditions [132]. In addition, βIII-tubulin directly binds to HIF-1 and HIF-2 via the E-box(50-RCGTG-3) located at its flanking region [116]. Also, Sox9 and HIF-2α mediate regulation of βIII-tubulin expression as a pro-survival reaction in ovarian cancer. Similarly, invasive glioblastoma also responds to hypoxia with the overexpression of βIII-tubulin stimulated by HIF-2α [118]. Interestingly, a possible mode of regulation can be explained with the stabilization of HIF-1α by HuR because this molecule also regulates the expression of βIII-tubulin [133]. This may explain a complex mechanism in the hypoxia-induced cancer cell proliferation via MAPs. Given the relative correlation between increased expression of CAPs and HIF stimulation initiated by hypoxia, silencing CAPs with targeted genetic screening or specific pharmacologic inhibition of their downstream effectors can amount to an effective strategy in the treatment of specific cancers.
CAPs regulate actin remodeling in response to stress
Hypoxia renders detrimental effects in several pathological conditions involving the dysregulation of the actin cytoskeleton [134]. For example, MAP-1 light chain 3 (LC3) expression can be induced by hypoxic stress, and this is characteristic of epithelial ovarian cancer migration and invasion. Disruption in LC3 expression affects the actin cytoskeleton organization and increases RhoA expression, and it has been hyped as a marker of hypoxia-induced metastasis in ovarian cancers [135]. Also, disruption in cell to cell contact under hypoxic conditions may rely on the formation of polarized actin mediating paracellular gaps in the monolayer [136]. Reported increase in parallel gap in chick vascular bed, instigated by hypoxia revealed induction of F-actin polymerization, which indicated possible causes of leakiness induced by hypoxia in epithelial cells [137].
Conversely, ARPs are also susceptible to oxidative stress. ARPs like gelsolin impact VDAC and the redox balance in cancer cells. Gelsolin’s interaction with Cu/ZnSOD limits the catalytic activities of Cu/ZnSOD and consequently elevates superoxide levels, and this disequilibrium can contribute to invasiveness and survival in cancer cells [138]. Studies have reported that hypoxia can induce cytoskeletal injury or remodeling via HIF-1α stimulation of the RhoA/ROCK signaling pathway [139]. Hence, it is safe to assume that since the essence of EMT is mainly for cytoskeletal remodeling, as hypoxia is capable of inducing EMT via RhoA/ROCK signaling pathway [140]. Moreover, the capping actin protein of muscle Z-line (CapZ) is an actin-binding protein capable of binding to the barbed end of F-actin. The α subunit of CapZ, CapZA1 promotes EMT in HCC cells as it regulates F-actin's remodeling [141]. Through the RhoA/ROCK signaling pathway in response to stress, specific undefined mechanisms are associated with the accumulation of thin filaments in support of hypertrophy attributed to phosphatidylinositol (4,5) bisphosphate (PIP2) expression [142]. Under hypoxic conditions, PIP2 combines with CapZA1 (via HIF-1α/RhoA/ROCK1 pathway) to facilitate its release from the F-actin barbed ends to mediate actin cytoskeleton remodeling (Fig. 2), which propagates EMT in HCC cells [140]. Also, F-actin-capping/severing proteins including CAPZ and other ARPs like cofilin and gelsolin can influence gene transcription and cell proliferation in response to mechanical stress regulated by YAP/TAZ activity and localization in cancer cells [143]. In essence, CAPZA1 is a potential therapeutic target to inhibit the invasion and migration driven by hypoxia in HCC and a potential clinical biomarker to predict the outcome of HCC patients. Regulating the level of PIP2 in HCC cells may be a new method to inhibit HCC invasion and metastasis, and this can be replicated in specific tumor types sharing similar heterogeneity.
IFs regulate cytoskeletal in response to hypoxia
Regulation of the cytoskeleton in response to hypoxia is not limited to ARPs and MAPs. In endothelial cells, hypoxic stress triggers several IF-mediated signals via IFAPs as cancer cells aggressively remodel the cytoskeleton for excessive energy requirements. As such, vimentin is regulated by hypoxia. Exposure of endothelial cells to hypoxic conditions initially redistributes vimentin perinuclearly, and this is followed by subsequent reformation and prolonged appearance than in normoxic conditions. Vimentin phosphorylation is also affected under hypoxic conditions by PAK1 activation [144]. This may promote adhesive properties and endothelial barrier stabilization. Hypoxia also induces keratin IF alterations in the alveolar epithelial-type cells of rats. Na et al. reported a time-dependent degradation of keratin 8 and 18 proteins under hypoxic conditions accompanied by elevated ROS generated from the mitochondria, rendering the keratin disassembly and subsequent migration, thus, progressing to the invasion of neoplastic cells [145].
IFAPs modulate stress response by regulating cellular metabolism
Aberrant protein synthesis is also the hallmark of cancer survival and an escape route for cancer cells in response to stress. During cellular development, cells undergo continuous modifications of the intracellular milieu, resulting in injury or damages to the DNA or RNA synthesis products. Although the consequential damages are manifold, ranging from biological stressors, chemical, or mechanically induced stress, the response is however conserved and may either be in the form of stress response–gene activation or alteration of housekeeping genes [146]. Conversely, CAPs are implicated in cellular metabolism [147, 148]. With respect to the universal nature and pattern of stress response and the protective effects that ensue, exposure to a specific form of stress renders a cross-protective response to other forms of stress [149]. It is safe to assume that CAPs may be involved in processes like these since key fundamental and emerging concepts indicate that modulation of post-translational modifications is a potential target or mechanism that can be targeted at metabolic dysfunctions in cancer cells driven by defective IF proteins [59]. Similar features exhibited by IF proteins suggest that they should be considered as stress proteins.
IFAPs have been associated with metabolic activities in response to cellular stress, and this is a major ammunition for cancer progression. Some cytoskeletal protein’s expression triples in response to specific stress conditions, while some CAPs are responsive to both intrinsic and extrinsic stresses, which may either be of genetic and endoplasmic reticulum stress, mechanical, wound, or metabolic stress [150]. IFAPs are implicated in chemokine stimulation, oxidative stress, infection, ischemia, electroporation, and regeneration of astrocytes, muscle, and skin fibroblasts [151, 152]. Failure to successfully aid astrocytes regeneration is a current research interest expected to explain astrocytes' transformation to glioma cells, as aberrant expression of those protective proteins and stress response molecules become concentrated and instigates malignancy. For instance, KRT17 upregulates protein synthesis and cell growth through its binding to adaptor IFAP 14-3-3σ by activating the Akt/mTOR pathway incisively without affecting other kinases’ activity [153], a process which is crucial for glutamine-dependent survival of cancer cells [154, 155]. KRT17 also utilizes two amino acid residues located at its amino-terminal head domain for serum-dependent nucleocytoplasmic translocation of 14-3-3σ, which concomitantly stimulates mTOR activity (Fig. 2). Thus, cell growth and proliferation ensue [153]. Interestingly, phosphorylation of K18 on ser33 also stimulates its binding to 14-3-3σ protein, whereas a mutation of this ser residue distorts the nucleocytoplasmic distribution of the 14-3-3σ, which results in aberrant cytokinesis due to imbalanced G2/M phase (a dais for aberrant protein synthesis ensuing from metabolic activities) [156, 157]. It is also important to note that the loss of K8/18 leads to a reduction in protein synthesis and affects cell size in hepatic cells [158].
CAPs can regulate apoptosis independently of mitotic spindle modulation
Interactions between tubulins and apoptotic genes either directly or via MAPs further validate the involvement of CAPs in apoptosis. These interactions facilitate the final stages of cell death in cancer cells. Most drugs designed to target the cytoskeleton are mostly attenuators of apoptotic signaling. Some cancer cells upregulate the expression of bcl2 without the G2/M arrest in resistance to apoptotic signals transmitted by MT-binding agents targeting the apoptotic networks in cancer cells [159, 160]. In response, the TBAs can phosphorylate bcl2 to render its upregulation useless to its effects on apoptosis [161]. Interactions of TBAs with CAPs are also implicated in pro-apoptotic signaling. Semaphorin 6A is downregulated in several cancer cells, but its interaction with βIII-tubulin in ovarian cancer cells initiates resistance to different chemotherapeutics [162]. Bim (Bcl-2 interacting mediator of cell death) also prevents apoptosis by tacit interaction with MTs via the dynein light chain interactions [163]. Its translocation may follow bim’s release from this interaction into the mitochondria, where it interacts with bcl family members to initiate apoptosis [164].
Cellular stress often results in apoptosis induced by caspase-mediated degradation of proteins, including IF proteins which are substrates for caspases [165, 166]. K8/K18 are anti-apoptotic in action. Their binding capabilities with TNF-receptor-associated death domain (TRADD) are deemed the rationale for the vast multifactorial effects in blocking apoptosis [83]. Moreover, post-translational modifications like the phosphorylation of Keratins are crucial for liver injury management and prevention of apoptosis [150], which is a prerequisite for oncogenesis. Keratin 8 has also been linked to important signaling effects in the immune system. Dong et al. recently reported that downregulating keratin 8 in mice results in uncontrollable innate inflammatory response rendering them susceptible to bacterial infection, tissue damage, increased death rate, and reduced survival [167]. The ability to limit inflammatory response is synonymous with cancer cells’ ability to co-opt some of the relevant signaling molecules of the innate immune system, which triggers the fostering of novel anti-inflammatory therapeutic intervention. However, translating these effects on inflammation goes both ways. On the one hand, reduced expression of Keratin 8 may be toxic for a normal body, while on the other hand, we could deduce that the same effect can fuel oncogenesis and/or maintain cancer cells development; this is feasible because of the resulting uncontrollable inflammatory immune response (cancer nature) resulting from Keratin’s downregulation.
Conclusion
Cellular dynamics is a multistep process that relies on cytoskeletal network coordination in space and time [58]. Studies directed toward the cytoskeletal components and interacting molecules unraveled key signaling pathway effectors activated via CAPs to mediate cancer cell migration, invasion, and subsequent metastasis [168]. Similarly, there is existing evidence progressively pointing toward the cytoskeleton and the changes in the composition of its components and associated proteins as important parameters regulating intricate signaling during cell development and tumor invasion. This has also led to vast discoveries on the crosstalk between these cytoskeletal components in some cancer cells to initiate cancer invasion and division [169]. The cancer machinery hijacks several typical signaling cascades in normal tissues and subverts these functions to their advantage, first for oncogenesis and then for proliferation and survival. Recent works have revealed that several CAPs involved in intricate signaling in non-neoplastic tissues are implicated in cancer cells and are easily subvertible by the cancer machinery to promote oncogenesis, invasion, apoptosis, metastasis, or drug resistance. Further studies also revealed that the normal processes coordinated by CAPs are implicated in these malignancies either as a result of aberrant expression of these molecules or by failure to exert their protective or traditional functions, leading to uncontrollable signaling boost that stimulates those critical processes promoting proliferation, migration, and relative metabolic events that trigger multiple adaptive responses (like glycolysis, hypoxia, glutaminolysis, and ATP generation) (Fig. 2).
Tissues and cells are constantly subjected to stressful conditions, meaning that cell components, including proteins, organelles, and DNA, can be damaged. These stresses are both intrinsic (e.g., genetic and endoplasmic reticulum stress) and extrinsic/environmental [e.g., heat, toxin or radiation, mechanical, wound and regenerative, infection, metabolic (e.g., hypoxia and autophagy), osmotic and oxidative] [77], resulting in abnormal cell growth. These malignancies require abundant energy to maintain those uncontrollable events. Hence, cancer cells' constant demand for energy prompts extended metabolic activities toward unusual energy sources, including those controlled by CAPs. In some cases, these metabolic functions are natural, and in other cases, aberrant expression of those signaling products of stress response associated with the cytoskeleton casts additional roles for CAPs. Hence, the initiation of more comprehensive signaling events linked with different subcellular compartments ensues. Most especially those factors that are implicated in evasive abilities and drug-resistant features of cancer cells. Therefore, our collection of extensive studies projects secondary roles for CAPs beyond the cytoskeleton's polymerization and stabilization.
Author contributions
AAA developed and wrote the manuscript, all other authors contributed with manuscript data, and GDS approved the manuscript for submission.
Funding
Researches in our laboratories are funded by the National Natural Science Foundation of China, Natural Science Foundation of Jiangsu, Qing Lan Project, and a project Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors approve the submission of the manuscript.
Footnotes
Abiola Abdulrahman Ayanlaja: Primary author.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benoit B, Baillet A, Pous C. Cytoskeleton and associated proteins: pleiotropic JNK substrates and regulators. Int J Mol Sci. 2021;22(16):8375. doi: 10.3390/ijms22168375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacTaggart B, Kashina A. Posttranslational modifications of the cytoskeleton. Cytoskeleton (Hoboken) 2021;78(4):142–173. doi: 10.1002/cm.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiewek J, et al. Clinical relevance of cytoskeleton associated proteins for ovarian cancer. J Cancer Res Clin Oncol. 2018;144(11):2195–2205. doi: 10.1007/s00432-018-2710-9. [DOI] [PubMed] [Google Scholar]
- 4.van Helvert S, Storm C, Friedl P. Mechanoreciprocity in cell migration. Nat Cell Biol. 2018;20(1):8–20. doi: 10.1038/s41556-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGregor AL, Hsia CR, Lammerding J. Squish and squeeze-the nucleus as a physical barrier during migration in confined environments. Curr Opin Cell Biol. 2016;40:32–40. doi: 10.1016/j.ceb.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayanlaja AA, et al. Distinct features of doublecortin as a marker of neuronal migration and its implications in cancer cell mobility. Front Mol Neurosci. 2017;10:199. doi: 10.3389/fnmol.2017.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laks DR, et al. A molecular cascade modulates MAP1B and confers resistance to mTOR inhibition in human glioblastoma. Neuro Oncol. 2018;20(6):764–775. doi: 10.1093/neuonc/nox215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu W, et al. The role of katanin p60 in breast cancer bone metastasis. Oncol Lett. 2018;15(4):4963–4969. doi: 10.3892/ol.2018.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nehlig A, et al. Regulation of end-binding protein EB1 in the control of microtubule dynamics. Cell Mol Life Sci. 2017;74(13):2381–2393. doi: 10.1007/s00018-017-2476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pohl M, et al. Keratin 34betaE12/keratin7 expression is a prognostic factor of cancer-specific and overall survival in patients with early stage non-small cell lung cancer. Acta Oncol. 2016;55(2):167–177. doi: 10.3109/0284186X.2015.1049291. [DOI] [PubMed] [Google Scholar]
- 11.Afghani N, et al. Microtubule actin cross-linking factor 1, a novel target in glioblastoma. Int J Oncol. 2017;50(1):310–316. doi: 10.3892/ijo.2016.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otani Y, et al. Dynamic reorganization of microtubule and glioma invasion. Acta Med Okayama. 2019;73(4):285–297. doi: 10.18926/AMO/56930. [DOI] [PubMed] [Google Scholar]
- 13.Lin YN, et al. Drosophila homologue of Diaphanous 1 (DIAPH1) controls the metastatic potential of colon cancer cells by regulating microtubule-dependent adhesion. Oncotarget. 2015;6(21):18577–18589. doi: 10.18632/oncotarget.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rath O, Kozielski F. Kinesins and cancer. Nat Rev Cancer. 2012;12(8):527–539. doi: 10.1038/nrc3310. [DOI] [PubMed] [Google Scholar]
- 15.Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206(4):461–472. doi: 10.1083/jcb.201406055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W, et al. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE. 2012;7(10):e46609. doi: 10.1371/journal.pone.0046609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang CH, Horwitz SB. Taxol((R)): the first microtubule stabilizing agent. Int J Mol Sci. 2017;18(8):1733. doi: 10.3390/ijms18081733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng R, et al. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet. 2016;53(10):662–671. doi: 10.1136/jmedgenet-2016-103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10(3):194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 20.Parker AL, Kavallaris M, McCarroll JA. Microtubules and their role in cellular stress in cancer. Front Oncol. 2014;4:153. doi: 10.3389/fonc.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan T, et al. Neuronal markers are expressed in human gliomas and NSE knockdown sensitizes glioblastoma cells to radiotherapy and temozolomide. BMC Cancer. 2011;11:524. doi: 10.1186/1471-2407-11-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsourlakis MC, et al. betaIII-tubulin overexpression is an independent predictor of prostate cancer progression tightly linked to ERG fusion status and PTEN deletion. Am J Pathol. 2014;184(3):609–617. doi: 10.1016/j.ajpath.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Katsetos CD, et al. Emerging microtubule targets in glioma therapy. Semin Pediatr Neurol. 2015;22(1):49–72. doi: 10.1016/j.spen.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Mariani M, et al. Class III beta-tubulin in normal and cancer tissues. Gene. 2015;563(2):109–114. doi: 10.1016/j.gene.2015.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karki R, et al. betaIII-Tubulin: biomarker of taxane resistance or drug target? Expert Opin Ther Targets. 2013;17(4):461–472. doi: 10.1517/14728222.2013.766170. [DOI] [PubMed] [Google Scholar]
- 26.Zheng YB, et al. A novel nitrobenzoate microtubule inhibitor that overcomes multidrug resistance exhibits antitumor activity. Sci Rep. 2016;6:31472. doi: 10.1038/srep31472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larkin JM, Kaye SB. Epothilones in the treatment of cancer. Expert Opin Investig Drugs. 2006;15(6):691–702. doi: 10.1517/13543784.15.6.691. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H, Huang G. Synthesis and antitumor activity of epothilones B and D and their analogs. Fut Med Chem. 2018;10(12):1483–1496. doi: 10.4155/fmc-2017-0320. [DOI] [PubMed] [Google Scholar]
- 29.Song Z, et al. Increased expression of MAP2 inhibits melanoma cell proliferation, invasion and tumor growth in vitro and in vivo. Exp Dermatol. 2010;19(11):958–964. doi: 10.1111/j.1600-0625.2009.01020.x. [DOI] [PubMed] [Google Scholar]
- 30.Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15(3):112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Kadavath H, et al. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc Natl Acad Sci USA. 2015;112(24):7501–7506. doi: 10.1073/pnas.1504081112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim S, et al. Glioblastoma-secreted soluble CD44 activates tau pathology in the brain. Exp Mol Med. 2018;50(4):5. doi: 10.1038/s12276-017-0008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breuzard G, Pagano A, Bastonero S, Malesinski S, Parat F, Barbier P, Peyrot V, Kovacic H. Tau regulates the microtubule-dependent migration of glioblastoma cells via the Rho-ROCK signaling pathway. J Cell Sci. 2019;32(3):jcs222851. doi: 10.1242/jcs.222851. [DOI] [PubMed] [Google Scholar]
- 34.Huda MN, Erdene-Ochir E, Pan CH. Assay for phosphorylation and microtubule binding along with localization of tau protein in colorectal cancer cells. J Vis Exp. 2017 doi: 10.3791/55932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich JN, et al. Gene expression profiling and genetic markers in glioblastoma survival. Cancer Res. 2005;65(10):4051–4058. doi: 10.1158/0008-5472.CAN-04-3936. [DOI] [PubMed] [Google Scholar]
- 36.Ortensi B, et al. Rai is a new regulator of neural progenitor migration and glioblastoma invasion. Stem Cells. 2012;30(5):817–832. doi: 10.1002/stem.1056. [DOI] [PubMed] [Google Scholar]
- 37.Ayanlaja AA, et al. Doublecortin undergo nucleocytoplasmic transport via the RanGTPase signaling to promote glioma progression. Cell Commun Signal. 2020;18(1):24. doi: 10.1186/s12964-019-0485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauffrey P, et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature. 2019;569(7758):672–678. doi: 10.1038/s41586-019-1219-y. [DOI] [PubMed] [Google Scholar]
- 39.Gao Z, Godbout R. Reelin-Disabled-1 signaling in neuronal migration: splicing takes the stage. Cell Mol Life Sci. 2013;70(13):2319–2329. doi: 10.1007/s00018-012-1171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto-Torii K, et al. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron. 2008;60(2):273–284. doi: 10.1016/j.neuron.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonoshita M, et al. Promotion of colorectal cancer invasion and metastasis through activation of NOTCH-DAB1-ABL-RHOGEF protein TRIO. Cancer Discov. 2015;5(2):198–211. doi: 10.1158/2159-8290.CD-14-0595. [DOI] [PubMed] [Google Scholar]
- 42.Trendowski M. Exploiting the cytoskeletal filaments of neoplastic cells to potentiate a novel therapeutic approach. Biochim Biophys Acta. 2014;1846(2):599–616. doi: 10.1016/j.bbcan.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Luo H, et al. Chaetoglobosin K inhibits tumor angiogenesis through downregulation of vascular epithelial growth factor-binding hypoxia-inducible factor 1alpha. Anticancer Drugs. 2013;24(7):715–724. doi: 10.1097/CAD.0b013e3283627a0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schweikart K, et al. The effects of jaspamide on human cardiomyocyte function and cardiac ion channel activity. Toxicol In Vitro. 2013;27(2):745–751. doi: 10.1016/j.tiv.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knudsen PB, et al. Chaetoglobosin A preferentially induces apoptosis in chronic lymphocytic leukemia cells by targeting the cytoskeleton. Leukemia. 2014;28(6):1289–1298. doi: 10.1038/leu.2013.360. [DOI] [PubMed] [Google Scholar]
- 46.Miralles F, et al. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113(3):329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 47.Goldschmidt-Clermont PJ, et al. The control of actin nucleotide exchange by thymosin beta 4 and profiling. A potential regulatory mechanism for actin polymerization in cells. Mol Biol Cell. 1992;3(9):1015–1024. doi: 10.1091/mbc.3.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujiwara I, et al. Latrunculin A accelerates actin filament depolymerization in addition to sequestering actin monomers. Curr Biol. 2018;28(19):3183–3192 e2. doi: 10.1016/j.cub.2018.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trendowski M. Using cytochalasins to improve current chemotherapeutic approaches. Anticancer Agents Med Chem. 2015;15(3):327–335. doi: 10.2174/1871520614666141016164335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S, et al. Actin stabilizing compounds show specific biological effects due to their binding mode. Sci Rep. 2019;9(1):9731. doi: 10.1038/s41598-019-46282-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhuo J, et al. Gelsolin induces colorectal tumor cell invasion via modulation of the urokinase-type plasminogen activator cascade. PLoS ONE. 2012;7(8):e43594. doi: 10.1371/journal.pone.0043594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang B, et al. Gelsolin-mediated activation of PI3K/Akt pathway is crucial for hepatocyte growth factor-induced cell scattering in gastric carcinoma. Oncotarget. 2016;7(18):25391–25407. doi: 10.18632/oncotarget.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gunning PW, Hardeman EC. Tropomyosins. Curr Biol. 2017;27(1):R8–R13. doi: 10.1016/j.cub.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 54.Stehn JR, et al. A novel class of anticancer compounds targets the actin cytoskeleton in tumor cells. Cancer Res. 2013;73(16):5169–5182. doi: 10.1158/0008-5472.CAN-12-4501. [DOI] [PubMed] [Google Scholar]
- 55.Currier MA, et al. Identification of cancer-targeted tropomyosin inhibitors and their synergy with microtubule drugs. Mol Cancer Ther. 2017;16(8):1555–1565. doi: 10.1158/1535-7163.MCT-16-0873. [DOI] [PubMed] [Google Scholar]
- 56.Strouhalova K, et al. Vimentin intermediate filaments as potential target for cancer treatment. Cancers (Basel) 2020;12(1):184. doi: 10.3390/cancers12010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Helfand BT, et al. Vimentin organization modulates the formation of lamellipodia. Mol Biol Cell. 2011;22(8):1274–1289. doi: 10.1091/mbc.E10-08-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leduc C, Etienne-Manneville S. Intermediate filaments in cell migration and invasion: the unusual suspects. Curr Opin Cell Biol. 2015;32:102–112. doi: 10.1016/j.ceb.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Snider NT, Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol. 2014;15(3):163–177. doi: 10.1038/nrm3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68(18):3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol. 2013;25(5):600–612. doi: 10.1016/j.ceb.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh R et al (2019) Non-canonical cMet regulation by vimentin mediates Plk1 inhibitor-induced apoptosis. EMBO Mol Med 11(5) [DOI] [PMC free article] [PubMed]
- 63.Ji Q, et al. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015;15:97. doi: 10.1186/s12885-015-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim YJ, et al. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-beta1-induced epithelial-mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology. 2014;322:23–33. doi: 10.1016/j.tox.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Chen B, et al. Dioscin inhibits the invasion and migration of hepatocellular carcinoma HepG2 cells by reversing TGF-beta1-induced epithelial-mesenchymal transition. Molecules. 2019;24(12):2222. doi: 10.3390/molecules24122222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaschula CH, et al. The garlic compound ajoene covalently binds vimentin, disrupts the vimentin network and exerts anti-metastatic activity in cancer cells. BMC Cancer. 2019;19(1):248. doi: 10.1186/s12885-019-5388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trogden KP, et al. An image-based small-molecule screen identifies vimentin as a pharmacologically relevant target of simvastatin in cancer cells. FASEB J. 2018;32(5):2841–2854. doi: 10.1096/fj.201700663R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee DH, et al. Withaferin A inhibits matrix metalloproteinase-9 activity by suppressing the Akt signaling pathway. Oncol Rep. 2013;30(2):933–938. doi: 10.3892/or.2013.2487. [DOI] [PubMed] [Google Scholar]
- 69.Bollong MJ, et al. A vimentin binding small molecule leads to mitotic disruption in mesenchymal cancers. Proc Natl Acad Sci USA. 2017;114(46):E9903–E9912. doi: 10.1073/pnas.1716009114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marceau N, et al. Dual roles of intermediate filaments in apoptosis. Exp Cell Res. 2007;313(10):2265–2281. doi: 10.1016/j.yexcr.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 71.Buckup M, et al. Plectin is a regulator of prostate cancer growth and metastasis. Oncogene. 2021;40(3):663–676. doi: 10.1038/s41388-020-01557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raymond AC, et al. Unbiased peptoid combinatorial cell screen identifies plectin protein as a potential biomarker for lung cancer stem cells. Sci Rep. 2019;9(1):14954. doi: 10.1038/s41598-019-51004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Osmanagic-Myers S, et al. Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration. J Cell Biol. 2006;174(4):557–568. doi: 10.1083/jcb.200605172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanna V, et al. Targeted nanoparticles for the delivery of novel bioactive molecules to pancreatic cancer cells. J Med Chem. 2016;59(11):5209–5220. doi: 10.1021/acs.jmedchem.5b01571. [DOI] [PubMed] [Google Scholar]
- 75.Yuan Y, et al. Chaperonin-GroEL as a smart hydrophobic drug delivery and tumor targeting molecular machine for tumor therapy. Nano Lett. 2018;18(2):921–928. doi: 10.1021/acs.nanolett.7b04307. [DOI] [PubMed] [Google Scholar]
- 76.Dasa SSK, et al. Plectin-targeted liposomes enhance the therapeutic efficacy of a PARP inhibitor in the treatment of ovarian cancer. Theranostics. 2018;8(10):2782–2798. doi: 10.7150/thno.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toivola DM, et al. Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 2010;20(2):79–91. doi: 10.1016/j.tcb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Inada H, et al. Keratin attenuates tumor necrosis factor-induced cytotoxicity through association with TRADD. J Cell Biol. 2001;155(3):415–426. doi: 10.1083/jcb.200103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nishimura Y, Kasahara K, Inagaki M. Intermediate filaments and IF-associated proteins: from cell architecture to cell proliferation. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95(8):479–493. doi: 10.2183/pjab.95.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujioka M, et al. Dimethylarsinic acid (DMA) enhanced lung carcinogenesis via histone H3K9 modification in a transplacental mouse model. Arch Toxicol. 2020;94(3):927–937. doi: 10.1007/s00204-020-02665-x. [DOI] [PubMed] [Google Scholar]
- 81.Misiorek JO, et al. Keratin 8-deletion induced colitis predisposes to murine colorectal cancer enforced by the inflammasome and IL-22 pathway. Carcinogenesis. 2016;37(8):777–786. doi: 10.1093/carcin/bgw063. [DOI] [PubMed] [Google Scholar]
- 82.Fortier AM, Asselin E, Cadrin M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J Biol Chem. 2013;288(16):11555–11571. doi: 10.1074/jbc.M112.428920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caulin C, et al. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J Cell Biol. 2000;149(1):17–22. doi: 10.1083/jcb.149.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ju JH, et al. Regulation of cell proliferation and migration by keratin19-induced nuclear import of early growth response-1 in breast cancer cells. Clin Cancer Res. 2013;19(16):4335–4346. doi: 10.1158/1078-0432.CCR-12-3295. [DOI] [PubMed] [Google Scholar]
- 85.Marceau N, Gilbert S, Loranger A. Uncovering the roles of intermediate filaments in apoptosis. Methods Cell Biol. 2004;78:95–129. doi: 10.1016/s0091-679x(04)78005-x. [DOI] [PubMed] [Google Scholar]
- 86.Schutte B, et al. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res. 2004;297(1):11–26. doi: 10.1016/j.yexcr.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 87.Leers MP, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187(5):567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 88.Chen F, et al. Caspase proteolysis of desmin produces a dominant-negative inhibitor of intermediate filaments and promotes apoptosis. J Biol Chem. 2003;278(9):6848–6853. doi: 10.1074/jbc.M212021200. [DOI] [PubMed] [Google Scholar]
- 89.Escobar-Hoyos LF, et al. Keratin-17 promotes p27KIP1 nuclear export and degradation and offers potential prognostic utility. Cancer Res. 2015;75(17):3650–3662. doi: 10.1158/0008-5472.CAN-15-0293. [DOI] [PubMed] [Google Scholar]
- 90.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 91.Tomoda K, et al. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem. 2002;277(3):2302–2310. doi: 10.1074/jbc.M104431200. [DOI] [PubMed] [Google Scholar]
- 92.Tong X, Coulombe PA. Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion. Genes Dev. 2006;20(10):1353–1364. doi: 10.1101/gad.1387406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dinsdale D, et al. Intermediate filaments control the intracellular distribution of caspases during apoptosis. Am J Pathol. 2004;164(2):395–407. doi: 10.1016/S0002-9440(10)63130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang HS, et al. Clinical significance of MUC1, MUC2 and CK17 expression patterns for diagnosis of pancreatobiliary arcinoma. Biotech Histochem. 2012;87(2):126–132. doi: 10.3109/10520295.2011.570276. [DOI] [PubMed] [Google Scholar]
- 95.He X, et al. Differentiation of a highly tumorigenic basal cell compartment in urothelial carcinoma. Stem Cells. 2009;27(7):1487–1495. doi: 10.1002/stem.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim CY, et al. Proteomic analysis reveals overexpression of moesin and cytokeratin 17 proteins in colorectal carcinoma. Oncol Rep. 2012;27(3):608–620. doi: 10.3892/or.2011.1545. [DOI] [PubMed] [Google Scholar]
- 97.Toyoshima T, et al. Cytokeratin 17 mRNA expression has potential for diagnostic marker of oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2008;134(4):515–521. doi: 10.1007/s00432-007-0308-8. [DOI] [PubMed] [Google Scholar]
- 98.Maher CA, et al. Transcriptome sequencing to detect gene fusions in cancer. Nature. 2009;458(7234):97–101. doi: 10.1038/nature07638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene. 2011;30(2):127–138. doi: 10.1038/onc.2010.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Loschke F, et al. Regulation of keratin network organization. Curr Opin Cell Biol. 2015;32:56–64. doi: 10.1016/j.ceb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 101.Engler AJ, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 102.Swift J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149):1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheung KJ, et al. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155(7):1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheung KJ, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci USA. 2016;113(7):E854–E863. doi: 10.1073/pnas.1508541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Willipinski-Stapelfeldt B, et al. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11(22):8006–8014. doi: 10.1158/1078-0432.CCR-05-0632. [DOI] [PubMed] [Google Scholar]
- 106.Joosse SA, et al. Changes in keratin expression during metastatic progression of breast cancer: impact on the detection of circulating tumor cells. Clin Cancer Res. 2012;18(4):993–1003. doi: 10.1158/1078-0432.CCR-11-2100. [DOI] [PubMed] [Google Scholar]
- 107.Seltmann K, et al. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc Natl Acad Sci USA. 2013;110(46):18507–18512. doi: 10.1073/pnas.1310493110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bu W, et al. Keratin 6a marks mammary bipotential progenitor cells that can give rise to a unique tumor model resembling human normal-like breast cancer. Oncogene. 2011;30(43):4399–4409. doi: 10.1038/onc.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang GY, et al. Basal cell carcinomas arise from hair follicle stem cells in Ptch1(+/-) mice. Cancer Cell. 2011;19(1):114–124. doi: 10.1016/j.ccr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kawai T, et al. Keratin 19, a cancer stem cell marker in human hepatocellular carcinoma. Clin Cancer Res. 2015;21(13):3081–3091. doi: 10.1158/1078-0432.CCR-14-1936. [DOI] [PubMed] [Google Scholar]
- 111.Kawai T, et al. Identification of keratin 19-positive cancer stem cells associating human hepatocellular carcinoma using (18)F-fluorodeoxyglucose positron emission tomography. Clin Cancer Res. 2017;23(6):1450–1460. doi: 10.1158/1078-0432.CCR-16-0871. [DOI] [PubMed] [Google Scholar]
- 112.Tsai FJ, et al. Novel K6–K14 keratin fusion enhances cancer stemness and aggressiveness in oral squamous cell carcinoma. Oncogene. 2019;38(26):5113–5126. doi: 10.1038/s41388-019-0781-y. [DOI] [PubMed] [Google Scholar]
- 113.Cancer Genome Atlas N (2015) Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517(7536):576–82 [DOI] [PMC free article] [PubMed]
- 114.Yoshihara K, et al. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene. 2015;34(37):4845–4854. doi: 10.1038/onc.2014.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo T, et al. Characterization of functionally active gene fusions in human papillomavirus related oropharyngeal squamous cell carcinoma. Int J Cancer. 2016;139(2):373–382. doi: 10.1002/ijc.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raspaglio G, et al. Sox9 and Hif-2alpha regulate TUBB3 gene expression and affect ovarian cancer aggressiveness. Gene. 2014;542(2):173–181. doi: 10.1016/j.gene.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 117.McCarroll JA, et al. TUBB3/betaIII-tubulin acts through the PTEN/AKT signaling axis to promote tumorigenesis and anoikis resistance in non-small cell lung cancer. Cancer Res. 2015;75(2):415–425. doi: 10.1158/0008-5472.CAN-14-2740. [DOI] [PubMed] [Google Scholar]
- 118.Bordji K, et al. Hypoxia-inducible factor-2alpha (HIF-2alpha), but not HIF-1alpha, is essential for hypoxic induction of class III beta-tubulin expression in human glioblastoma cells. FEBS J. 2014;281(23):5220–5236. doi: 10.1111/febs.13062. [DOI] [PubMed] [Google Scholar]
- 119.Saks VA, et al. Control of cellular respiration in vivo by mitochondrial outer membrane and by creatine kinase. A new speculative hypothesis: possible involvement of mitochondrial-cytoskeleton interactions. J Mol Cell Cardiol. 1995;27(1):625–645. doi: 10.1016/s0022-2828(08)80056-9. [DOI] [PubMed] [Google Scholar]
- 120.Infante AS, et al. Detyrosinated (Glu) microtubules are stabilized by an ATP-sensitive plus-end cap. J Cell Sci. 2000;113(Pt 22):3907–3919. doi: 10.1242/jcs.113.22.3907. [DOI] [PubMed] [Google Scholar]
- 121.Geeraert C, et al. Starvation-induced hyperacetylation of tubulin is required for the stimulation of autophagy by nutrient deprivation. J Biol Chem. 2010;285(31):24184–24194. doi: 10.1074/jbc.M109.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sheldon KL, et al. Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS ONE. 2011;6(10):25539. doi: 10.1371/journal.pone.0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maldonado EN, et al. Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J Biol Chem. 2013;288(17):11920–11929. doi: 10.1074/jbc.M112.433847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zala D, et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152(3):479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 125.Glaser PE, Han X, Gross RW. Tubulin is the endogenous inhibitor of the glyceraldehyde 3-phosphate dehydrogenase isoform that catalyzes membrane fusion: Implications for the coordinated regulation of glycolysis and membrane fusion. Proc Natl Acad Sci USA. 2002;99(22):14104–14109. doi: 10.1073/pnas.222542999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tisdale EJ, Azizi F, Artalejo CR. Rab2 utilizes glyceraldehyde-3-phosphate dehydrogenase and protein kinase C{iota} to associate with microtubules and to recruit dynein. J Biol Chem. 2009;284(9):5876–5884. doi: 10.1074/jbc.M807756200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cueille N, et al. Microtubule-associated protein 1B binds glyceraldehyde-3-phosphate dehydrogenase. J Proteome Res. 2007;6(7):2640–2647. doi: 10.1021/pr070081z. [DOI] [PubMed] [Google Scholar]
- 128.Kovacs J, et al. Phosphoenolpyruvate-dependent tubulin-pyruvate kinase interaction at different organizational levels. J Biol Chem. 2003;278(9):7126–7130. doi: 10.1074/jbc.M210244200. [DOI] [PubMed] [Google Scholar]
- 129.Cicchillitti L, et al. Proteomic characterization of cytoskeletal and mitochondrial class III beta-tubulin. Mol Cancer Ther. 2008;7(7):2070–2079. doi: 10.1158/1535-7163.MCT-07-2370. [DOI] [PubMed] [Google Scholar]
- 130.Hu JY, et al. The p38/MAPK pathway regulates microtubule polymerization through phosphorylation of MAP4 and Op18 in hypoxic cells. Cell Mol Life Sci. 2010;67(2):321–333. doi: 10.1007/s00018-009-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fang YD, et al. MAP4 mechanism that stabilizes mitochondrial permeability transition in hypoxia: microtubule enhancement and DYNLT1 interaction with VDAC1. PLoS ONE. 2011;6(12):e28052. doi: 10.1371/journal.pone.0028052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu X, et al. Phosphorylation of DYNLT1 at serine 82 regulates microtubule stability and mitochondrial permeabilization in hypoxia. Mol Cells. 2013;36(4):322–332. doi: 10.1007/s10059-013-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raspaglio G, et al. HuR regulates beta-tubulin isotype expression in ovarian cancer. Cancer Res. 2010;70(14):5891–5900. doi: 10.1158/0008-5472.CAN-09-4656. [DOI] [PubMed] [Google Scholar]
- 134.Zieseniss A. Hypoxia and the modulation of the actin cytoskeleton—emerging interrelations. Hypoxia (Auckl) 2014;2:11–21. doi: 10.2147/HP.S53575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang Z, et al. Inhibition of microtubule-associated protein 1 light chain 3B via small-interfering RNA or 3-methyladenine impairs hypoxia-induced HO8910PM and HO8910 epithelial ovarian cancer cell migration and invasion and is associated with RhoA and alterations of the actin cytoskeleton. Oncol Rep. 2015;33(3):1411–1417. doi: 10.3892/or.2015.3742. [DOI] [PubMed] [Google Scholar]
- 136.Kolluru GK, et al. Nitric oxide/cGMP protects endothelial cells from hypoxia-mediated leakiness. Eur J Cell Biol. 2008;87(3):147–161. doi: 10.1016/j.ejcb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 137.Swaminathan A, et al. Hypoxia perturbs endothelium by re-organizing cellular actin architecture: Nitric oxide offers limited protection. Tissue Cell. 2018;50:114–124. doi: 10.1016/j.tice.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 138.Tochhawng L, et al. Gelsolin-Cu/ZnSOD interaction alters intracellular reactive oxygen species levels to promote cancer cell invasion. Oncotarget. 2016;7(33):52832–52848. doi: 10.18632/oncotarget.10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang L, et al. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor -1alpha in hepatocellular carcinoma. BMC Cancer. 2013;13:108. doi: 10.1186/1471-2407-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang D, et al. Hypoxia induces actin cytoskeleton remodeling by regulating the binding of CAPZA1 to F-actin via PIP2 to drive EMT in hepatocellular carcinoma. Cancer Lett. 2019;448:117–127. doi: 10.1016/j.canlet.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 141.Huang D, Cao L, Zheng S. CAPZA1 modulates EMT by regulating actin cytoskeleton remodelling in hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36(1):13. doi: 10.1186/s13046-016-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li J, Russell B. Phosphatidylinositol 4,5-bisphosphate regulates CapZbeta1 and actin dynamics in response to mechanical strain. Am J Physiol Heart Circ Physiol. 2013;305(11):H1614–H1623. doi: 10.1152/ajpheart.00477.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aragona M, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 144.Liu T, et al. Regulation of vimentin intermediate filaments in endothelial cells by hypoxia. Am J Physiol Cell Physiol. 2010;299(2):C363–C373. doi: 10.1152/ajpcell.00057.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Na N, et al. Mitochondrial reactive oxygen species are required for hypoxia-induced degradation of keratin intermediate filaments. FASEB J. 2010;24(3):799–809. doi: 10.1096/fj.08-128967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kampinga HH, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Snider NT, et al. Glucose and SIRT2 reciprocally mediate the regulation of keratin 8 by lysine acetylation. J Cell Biol. 2013;200(3):241–247. doi: 10.1083/jcb.201209028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang RC, et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012;338(6109):956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Akerfelt M, et al. Heat shock factors at a crossroad between stress and development. Ann N Y Acad Sci. 2007;1113:15–27. doi: 10.1196/annals.1391.005. [DOI] [PubMed] [Google Scholar]
- 150.Omary MB, et al. “Heads and tails” of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci. 2006;31(7):383–394. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 151.Perng MD, et al. Glial fibrillary acidic protein filaments can tolerate the incorporation of assembly-compromised GFAP-delta, but with consequences for filament organization and alphaB-crystallin association. Mol Biol Cell. 2008;19(10):4521–4533. doi: 10.1091/mbc.E08-03-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hagemann TL, et al. Suppression of GFAP toxicity by alphaB-crystallin in mouse models of Alexander disease. Hum Mol Genet. 2009;18(7):1190–1199. doi: 10.1093/hmg/ddp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441(7091):362–365. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 154.Wu SH, et al. Emerging function of mTORC2 as a core regulator in glioblastoma: metabolic reprogramming and drug resistance. Cancer Biol Med. 2014;11(4):255–263. doi: 10.7497/j.issn.2095-3941.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ku NO, Liao J, Omary MB. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. EMBO J. 1998;17(7):1892–1906. doi: 10.1093/emboj/17.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Toivola DM, et al. Disturbances in hepatic cell-cycle regulation in mice with assembly-deficient keratins 8/18. Hepatology. 2001;34(6):1174–1183. doi: 10.1053/jhep.2001.29374. [DOI] [PubMed] [Google Scholar]
- 158.Galarneau L, et al. Keratins modulate hepatic cell adhesion, size and G1/S transition. Exp Cell Res. 2007;313(1):179–194. doi: 10.1016/j.yexcr.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 159.Gajate C, et al. Induction of apoptosis in leukemic cells by the reversible microtubule-disrupting agent 2-methoxy-5-(2',3',4'-trimethoxyphenyl)-2,4,6-cycloheptatrien-1 -one: protection by Bcl-2 and Bcl-X(L) and cell cycle arrest. Cancer Res. 2000;60(10):2651–2659. [PubMed] [Google Scholar]
- 160.Esteve MA, et al. Bcl-2 down-regulation and tubulin subtype composition are involved in resistance of ovarian cancer cells to vinflunine. Mol Cancer Ther. 2006;5(11):2824–2833. doi: 10.1158/1535-7163.MCT-06-0277. [DOI] [PubMed] [Google Scholar]
- 161.Srivastava RK, et al. Involvement of microtubules in the regulation of Bcl2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol Cell Biol. 1998;18(6):3509–3517. doi: 10.1128/mcb.18.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Prislei S, et al. From plasma membrane to cytoskeleton: a novel function for semaphorin 6A. Mol Cancer Ther. 2008;7(1):233–241. doi: 10.1158/1535-7163.MCT-07-0390. [DOI] [PubMed] [Google Scholar]
- 163.Knipling L, Wolff J. Direct interaction of Bcl-2 proteins with tubulin. Biochem Biophys Res Commun. 2006;341(2):433–439. doi: 10.1016/j.bbrc.2005.12.201. [DOI] [PubMed] [Google Scholar]
- 164.Puthalakath H, et al. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3(3):287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 165.Franco R, et al. Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mutat Res. 2009;674(1–2):3–22. doi: 10.1016/j.mrgentox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 166.Capetanaki Y, et al. Muscle intermediate filaments and their links to membranes and membranous organelles. Exp Cell Res. 2007;313(10):2063–2076. doi: 10.1016/j.yexcr.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 167.Dong XM, et al. Keratin 8 limits TLR-triggered inflammatory responses through inhibiting TRAF6 polyubiquitination. Sci Rep. 2016;6:32710. doi: 10.1038/srep32710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fife CM, McCarroll JA, Kavallaris M. Movers and shakers: cell cytoskeleton in cancer metastasis. Br J Pharmacol. 2014;171(24):5507–5523. doi: 10.1111/bph.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Akhshi TK, Wernike D, Piekny A. Microtubules and actin crosstalk in cell migration and division. Cytoskeleton (Hoboken) 2014;71(1):1–23. doi: 10.1002/cm.21150. [DOI] [PubMed] [Google Scholar]
- 170.Tokesi N, et al. TPPP/p25 promotes tubulin acetylation by inhibiting histone deacetylase 6. J Biol Chem. 2010;285(23):17896–17906. doi: 10.1074/jbc.M109.096578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schofield AV, Steel R, Bernard O. Rho-associated coiled-coil kinase (ROCK) protein controls microtubule dynamics in a novel signaling pathway that regulates cell migration. J Biol Chem. 2012;287(52):43620–43629. doi: 10.1074/jbc.M112.394965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou W, et al. Depletion of tubulin polymerization promoting protein family member 3 suppresses HeLa cell proliferation. Mol Cell Biochem. 2010;333(1–2):91–98. doi: 10.1007/s11010-009-0208-0. [DOI] [PubMed] [Google Scholar]
- 173.Hu D, et al. Proteomic analyses identify prognostic biomarkers for pancreatic ductal adenocarcinoma. Oncotarget. 2018;9(11):9789–9807. doi: 10.18632/oncotarget.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xia X, et al. Microtubule-associated protein 4 is a prognostic factor and promotes tumor progression in lung adenocarcinoma. Dis Markers. 2018;2018:8956072. doi: 10.1155/2018/8956072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jiang YY, et al. Microtubule-associated protein 4 is an important regulator of cell invasion/migration and a potential therapeutic target in esophageal squamous cell carcinoma. Oncogene. 2016;35(37):4846–4856. doi: 10.1038/onc.2016.17. [DOI] [PubMed] [Google Scholar]
- 176.Shu T, et al. Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron. 2006;49(1):25–39. doi: 10.1016/j.neuron.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 177.Verissimo CS, et al. Combining doublecortin-like kinase silencing and vinca alkaloids results in a synergistic apoptotic effect in neuroblastoma cells. J Pharmacol Exp Ther. 2012;342(1):119–130. doi: 10.1124/jpet.111.188813. [DOI] [PubMed] [Google Scholar]
- 178.Wang Y, et al. Microtubule associated tumor suppressor 1 interacts with mitofusins to regulate mitochondrial morphology in endothelial cells. FASEB J. 2018;32(8):4504–4518. doi: 10.1096/fj.201701143RR. [DOI] [PubMed] [Google Scholar]
- 179.Parbin S, et al. DNA methylation regulates Microtubule-associated tumor suppressor 1 in human non-small cell lung carcinoma. Exp Cell Res. 2019;374(2):323–332. doi: 10.1016/j.yexcr.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 180.Zhao T, et al. MTUS1/ATIP3a down-regulation is associated with enhanced migration, invasion and poor prognosis in salivary adenoid cystic carcinoma. BMC Cancer. 2015;15:203. doi: 10.1186/s12885-015-1209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Belletti B, Baldassarre G. Stathmin: a protein with many tasks New biomarker and potential target in cancer. Expert Opin Ther Targets. 2011;15(11):1249–1266. doi: 10.1517/14728222.2011.620951. [DOI] [PubMed] [Google Scholar]
- 182.Balachandran R, Welsh MJ, Day BW. Altered levels and regulation of stathmin in paclitaxel-resistant ovarian cancer cells. Oncogene. 2003;22(55):8924–8930. doi: 10.1038/sj.onc.1207060. [DOI] [PubMed] [Google Scholar]
- 183.Takeda S, et al. Novel inter-domain Ca(2+)-binding site in the gelsolin superfamily protein fragmin. J Muscle Res Cell Motil. 2020;41(1):153–162. doi: 10.1007/s10974-019-09571-5. [DOI] [PubMed] [Google Scholar]
- 184.Racca AW, et al. M8R tropomyosin mutation disrupts actin binding and filament regulation: The beginning affects the middle and end. J Biol Chem. 2020;295(50):17128–17137. doi: 10.1074/jbc.RA120.014713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Molinie N, Gautreau A. The Arp2/3 regulatory system and its deregulation in cancer. Physiol Rev. 2018;98(1):215–238. doi: 10.1152/physrev.00006.2017. [DOI] [PubMed] [Google Scholar]
- 186.Zhao Y, et al. mTORC1 and mTORC2 converge on the Arp2/3 complex to promote kras(G12D)-induced acinar-to-ductal metaplasia and early pancreatic carcinogenesis. Gastroenterology. 2021;160(5):1755–177017. doi: 10.1053/j.gastro.2020.12.061. [DOI] [PubMed] [Google Scholar]
- 187.Faix J, Grosse R. Staying in shape with formins. Dev Cell. 2006;10(6):693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 188.Jiao X, et al. Formin-like protein 2 promotes cell proliferation by a p27-related mechanism in human breast cancer cells. BMC Cancer. 2021;21(1):760. doi: 10.1186/s12885-021-08533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ren Z, et al. Association of DIAPH1 gene polymorphisms with ischemic stroke. Aging (Albany NY) 2020;12(1):416–435. doi: 10.18632/aging.102631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang J, et al. DIAPH1 is upregulated and inhibits cell apoptosis through ATR/p53/Caspase-3 signaling pathway in laryngeal squamous cell carcinoma. Dis Markers. 2019;2019:6716472. doi: 10.1155/2019/6716472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Almenar-Queralt A, Gregorio CC, Fowler VM. Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils. J Cell Sci. 1999;112(Pt 8):1111–1123. doi: 10.1242/jcs.112.8.1111. [DOI] [PubMed] [Google Scholar]
- 192.Castro CN et al (2020) NCKAP1L defects lead to a novel syndrome combining immunodeficiency, lymphoproliferation, and hyperinflammation. J Exp Med 217(12) [DOI] [PMC free article] [PubMed]
- 193.Xiong Y, et al. Nck-associated protein 1 associates with HSP90 to drive metastasis in human non-small-cell lung cancer. J Exp Clin Cancer Res. 2019;38(1):122. doi: 10.1186/s13046-019-1124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang JL, et al. Oncogenic function and prognostic significance of Abelson interactor 1 in hepatocellular carcinoma. Int J Oncol. 2017;50(5):1889–1898. doi: 10.3892/ijo.2017.3920. [DOI] [PubMed] [Google Scholar]
- 195.Aho S. Plakin proteins are coordinately cleaved during apoptosis but preferentially through the action of different caspases. Exp Dermatol. 2004;13(11):700–707. doi: 10.1111/j.0906-6705.2004.00217.x. [DOI] [PubMed] [Google Scholar]
- 196.Wesley T, et al. The attributes of plakins in cancer and disease: perspectives on ovarian cancer progression, chemoresistance and recurrence. Cell Commun Signal. 2021;19(1):55. doi: 10.1186/s12964-021-00726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jain PB, et al. The spectraplakin Dystonin antagonizes YAP activity and suppresses tumourigenesis. Sci Rep. 2019;9(1):19843. doi: 10.1038/s41598-019-56296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Makino T, et al. Ultraviolet B irradiation increases the expression of trichohyalin-like 1 protein in human skin xenotransplants. Clin Exp Dermatol. 2019;44(7):773–776. doi: 10.1111/ced.13904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.