Abstract
Uveal melanoma (UM) is the most common intraocular malignant tumor in adults with an extremely high mortality rate. Genetic and epigenetic dysregulation contribute to the development of UM. Recent discoveries have revealed dysregulation of the expression levels of microRNAs (miRNAs) as one of the epigenetic mechanisms underlying UM tumorigenesis. Based on their roles, miRNAs are characterized as either oncogenic or tumor suppressive. This review focuses on the roles of miRNAs in UM tumorigenesis, diagnosis, and prognosis, as well as their therapeutic potentials. Particularly, the actions of collective miRNAs are summarized with respect to their involvement in major, aberrant signaling pathways that are implicated in the development and progression of UM. Elucidation of the underlying functional mechanisms and biological aspects of miRNA dysregulation in UM is invaluable in the development of miRNA-based therapeutics, which may be used in combination with conventional treatments to improve therapeutic outcomes. In addition, the expression levels of some miRNAs are correlated with UM initiation and progression and, therefore, may be used as biomarkers for diagnosis and prognosis.
Keywords: Ocular melanoma, microRNAs, Signaling pathway, Biomarkers, Therapeutic
Introduction
Uveal melanoma and differences between uveal and cutaneous melanoma
Uveal melanoma (UM) is the most common adult primary intraocular cancer with an annual incidence of six to seven cases per million [1, 2]. UM and cutaneous melanoma (CM) both derive from melanocytes of the same embryonic origin and cellular function [1]; however, they display remarkable differences in etiology, mutational profile, and clinical progression [2]. Although ultraviolet radiation (UVR) exposure is a proven environmental risk factor for CM, UVR is not involved in UM etiology. Additionally, UM exhibits relatively simple chromosomal alterations, unlike CM, which has very complex cytogenetic alterations. Due to the differences in biological, molecular, and genetic tumor features, the effective clinical therapies for metastatic CM patients are ineffective for metastatic UM patients. The differences between UM and CM have already been comprehensively reviewed by Pandiani et al. [3]. Therefore, the current review will focus on the genetic alterations and abnormal gene expression profiles and signaling pathways in UM. UM mainly originates from melanocytic cells of the choroid (~ 85%), whereas the rest of the cases are found in the iris and the ciliary body [4]. Posterior UM of the ciliary body and choroidal region are more likely to become metastatic due to their location and late detection [5]. Despite good control of the primary UM tumor by surgery or radiation therapy, more than 50% of UM patients will develop metastatic disease within 10–15 years of enucleation [6], and the median survival rate after diagnosis of metastatic UM is 2–9 months [7]. The liver is the most common organ prone to metastasis (89%), followed by the lungs (29%), bones (17%), and skin (12%) [6]. To date, there is not an approved treatment for metastatic UM that can significantly increase the overall survival (OS) rate of patients [1].
Specific gene mutations and dysregulated pathways in UM
Cytogenetic analyses have shown that loss of an entire chromosome 3 homolog (monosomy 3) and an amplification of chromosomal 6p and 8q are the major chromosomal aberrations in UM [8]. UM tumors with monosomy 3 (often associated with amplification of chromosome 8q) are prone to metastasis and have a poor prognosis [9, 10]. Chromosome 3 hosts the tumor suppressor gene BRCA-associated protein 1 (BAP1). The BAP1 gene is located on chromosome 3p21, which encodes an enzyme that is a tumor suppressor protein with deubiquitinase activity and that functions in DNA double-strand break repair [11, 12]. BAP1 mutations occur in approximately 40% of metastatic UM patients [13, 14]. Loss-of-function and/or inactivating mutations of the BAP1 gene are highly associated with metastatic risk [15]. Somatic mutations in the guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) and guanine nucleotide-binding protein subunit alpha-11 (GNA11) genes are oncogenic drivers in UM. Mutually exclusive mutations in GNAQ or GNA11 are present in over 85% of all examined UM cases [3, 13]. The proteins encoded by the GNAQ/GNA11 genes transduce signals from G protein-coupled receptors (GPCRs) to intracellular pathways [16]. Most of the GNAQ/GNA11 mutations are within the GTPase catalytic domains, resulting in the loss of the GTPase activity. These mutations lead to constitutive activation of downstream signaling pathways, such as PI3K/AKT and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), and influence melanocyte transformation. The GNAQ/GNA11 mutations activate phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2), indicating that both GNAQ/GNA11 mutants behave as dominant-acting oncogene proteins [14, 17].
Dysregulated pathways downstream of mutant GNAQ/GNA11
RHO/RAC pathway
The members of the RHO family of GTPases are proteins that are involved in a wide variety of cellular functions. Particularly relevant to UM, GNAQ/GNA11 stimulate RAS homolog family member A (RHOA) and RAS-related C3 botulinum toxin substrate 1 (RAC1) and their associated signaling networks through direct activation of triple function domain protein (TRIO) [18–20]. TRIO is a member of the RHO guanine nucleotide exchange factors, and it plays a pivotal role in mediating mitogenic signals in UM. Interestingly, TRIO selectively activates RHOA and RAC1. RAC1 is a small GTP-binding protein that regulates cell proliferation and migration through actin polymerization [21, 22]. The RHO/RAC pathway is activated by oncogenic GNAQ/GNA11 mutations, thus suggesting a new therapeutic target in UM.
MAPK/ERK and PI3K/AKT pathways
Activation of the MAPK/ERK pathway by mutant GNAQ/GNA11 is critical for the development of UM [14]. The incidence of ERK1/2 activation in UM is high and occurs in 86% of primary UM tumors [23]. Mitogen-activated protein kinase (MEK) phosphorylates and activates ERK1/2 and subsequently regulates cellular processes, including proliferation, differentiation, and apoptosis [19, 24]. Another dysregulated pathway in response to GNAQ/GNA11 mutation is the phosphatidylinositol (4,5)-bisphosphate 3-kinase (PI3K)/protein kinase B (AKT) pathway. PI3K induces the membrane translocation of AKT to promote cell proliferation and survival [24]. Phosphorylated AKT is observed in approximately 55% of UM cases and is associated with a high risk of metastatic disease [25].
UM and miRNAs
Roles of miRNAs in UM
Recent epigenetic research has elucidated the involvement of microRNAs (miRNAs) in UM [26, 27]. MiRNAs are small (18–22 nucleotides in length), endogenous, noncoding, single-stranded RNAs involved in the regulation of a variety of biological processes, including carcinogenesis. The production of miRNAs involves multiple processing steps that require a large number of molecular events for proper coordination [28]. Gene expression can be altered by miRNAs via binding to the 3′-untranslated region (3′-UTR) of their respective target genes or by destabilizing the messenger RNA (mRNA), the latter of which leads to altered gene expression at the post-transcriptional level [29]. Both oncogenes and tumor suppressor genes are regulated by miRNAs. Thus, a dysregulated miRNA network is a typical feature of many cancers [30]. Emerging evidence has highlighted the implication of miRNAs in UM. Many miRNAs are dysregulated in UM tissues and cell lines, and some of them have been validated as critical regulators in the development of UM [31]. Interestingly, studies have found a link between miRNAs and monosomy 3/BAP1 mutation. Although the major miRNA biogenesis factors are not encoded on chromosome 3, miRNA-processing factors are altered in monosomy 3, regulating the expression levels of certain miRNAs to facilitate metastasis in UM [32]. A miRNA cluster (miR-31-5p, miR-125 family, miR-140-3p, miR-200a-3p, and miR-423-5p) is embedded in the 3′-UTR region of the BAP1 gene. Therefore, BAP1 mutations may contribute to the dysregulated expression of these BAP1-associated miRNAs and their target genes [11]. Elucidation of miRNA target genes, whether oncogenes, tumor suppressor genes, or other cancer-related genes, will explicate how specific miRNAs regulate UM tumorigenesis and provide the basis for novel targeted therapies [33].
UM and OncomiRs
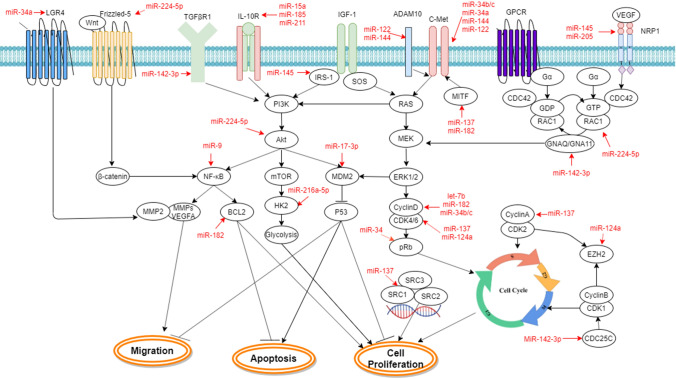
OncomiRs are miRNAs that play crucial roles in the initiation and progression of human cancer. They are generally upregulated in cancers and typically target tumor suppressors to promote tumorigenesis. When oncomiRs are inhibited, tumor cell proliferation, survival, and metastasis can be remarkably reduced. Based on these criteria, several specific oncomiRs have been identified in UM (Table 1), and their target genes and related signaling pathways are summarized in Fig. 1.
Table 1.
Individual upregulated oncomiRs and their target genes in UM
| OncomiRs | Expression in UM cell lines and/or UM tumor tissues | Function | Targets | Refs. |
|---|---|---|---|---|
| miR‑20a | MUM-2B and MUM-2C metastatic UM cells; UM patient tumor tissues | Promotes cell growth, migration, and invasion | N/A | [36] |
| miR-20a, miR-146a, miR-155, miR-181a, miR-223 | MUM-2B and OCM-1 metastatic UM cells | Suppress NK cells, promote metastasis | N/A | [38] |
| miR-21 | OCM-1, M619, and MUM-2B UM cells | Promotes cell proliferation, migration, and invasion | p53 | [48] |
| miR-27a | Genistein-treated human C918 UM cells | Promotes cell growth | ZBTB10 | [50] |
| miR-92a-3p | Decreased expression by histone deacetylase inhibitor MS-275 in OCM-1 UM cells | Decreases susceptibility of UM to TRAIL-mediated apoptosis | MYCBP2 | [39] |
| miR-155 | OCM-1A, MUM-2C, C918, and MUM-2B UM cells; UM patient tumor tissues | Promotes cell proliferation and invasion | NDFIP1 | [38, 44] |
| miR-181b | UM cell lines: SP6.5, VUP, OCM-1, 92–1, OCM-1a, and MUM-2b; UM patient tumor tissues | Promotes cell proliferation | CTDSPL | [49] |
| miR-367 | UM cell lines: M17, M23, MUM-2B, and C918; UM patient tumor tissues | Promotes cell proliferation and migration | PTEN | [42] |
| miR-454 | UM cell lines: OCM‐1A, MUM‐2B, MUM‐2C, and C918; UM patient tumor tissues | Promotes cell proliferation and invasion | PTEN | [43] |
| miR-652 | UM cell lines: MUM-2B and MEL270; UM patient tumor tissues | Promotes cell proliferation and migration | HOXA9 | [26] |
Fig. 1.
Involvement of oncomiRs in UM tumorigenesis via activation of aberrant signaling pathways
The first well-characterized oncomiR is the miR-17–92 polycistronic cluster, which includes miR-17-5p, miR-18a, miR-19a, miR-19b-1, miR-20a, and miR-92a-1 [34, 35]. As a member of the miR-17–92 cluster, miR-20a is significantly upregulated in UM tissues, cell lines, and the plasma of UM patients. Its oncogenic potential has been shown in UM via promotion of cancer cell proliferation and migration [36, 37]. Furthermore, suppression of natural killer (NK) cell activity by miR-20a is implicated in UM metastasis [38]. Another member of the miR-17–92 cluster, miR-92a-3p, is able to reduce the susceptibility of UM cells to TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis by directly downregulating MYC-binding protein 2 (MYCBP2) [39].
Aberrant miRNA expression in UM can change the expression of genes in diverse cancer-related signaling pathways. Phosphatase and tensin homolog (PTEN), a lipid phosphatase, functions as downregulator of the PI3K/AKT signaling pathway. PTEN expression is strongly associated with disease-free survival of UM patients [40, 41]. Interestingly, both miR-367 and miR-454 are able to directly downregulate tumor suppressor PTEN expression [42, 43]. In addition, miR-155 interferes with the PTEN and AKT signaling pathways by directly targeting NEDD4-family interacting protein 1 (NDFIP1) [44], which has a key role in the ubiquitination and nuclear translocation of PTEN [45]. Accordingly, high expression and secretion levels of miR-155 are detected in cancer stem cells and circulating CD271+ cells that have cancer stem cell features from metastatic UM patients [38], suggesting that the suppression of NK cells by miR-155 may contribute to metastatic progression. Mutation of the tumor suppressor p53 gene is rare in UM, but disruption of the p53 signaling pathway is common [46, 47]. The p53 gene is identified as a direct target of miR-21, which is highly expressed in invasive UM cells. Furthermore, inactivation of p53 and its downstream events by miR-21 lead to more aggressive phenotypes of UM cells [48].
The oncogenic potential of miR-652 has been shown by promotion of hypoxia-inducible factor 1-alpha (HIF-1α) signaling via repression of tumor suppressor homeobox A9 (HOXA9) in UM cells [26]. Overexpression of miR-181b in UM cells promotes cell proliferation through suppression of the target C-terminal domain small phosphatase-like (CTDSPL), which consequently induces the phosphorylation of retinoblastoma (RB, a prototype tumor suppressor) and increases the downstream cell cycle effector transcription factor E2F1 [49]. It is confirmed that miR-27a downregulates the zinc finger and BTB domain-containing 10 (ZBTB10) gene, resulting in inhibition of cell growth [50].
Tumor suppressor miRNAs
Tumor suppressor miRNAs are defined by their properties of downregulating oncogenes [51]. In general, they are often lost or under expressed in cancer cells. GNAQ/GNA11 mutations are the most common somatic mutations occurring in UM, and multiple signaling pathways are induced as a consequence of GNAQ/GNA11 activation [19]. GNAQ/GNA11 and some components of the downstream RHO/RAC, MAPK/ERK, and PI3K/AKT pathways are direct targets of a few tumor suppressor miRNAs. In addition, a number of tumor suppressor miRNAs have been identified by either suppressing cell growth through targeting the genes involved in cell survival signaling pathways or inducing UM cell death through modulating apoptotic signaling (Fig. 2). The expression, function, characterization, and direct gene targets of each individual miRNA are summarized in Table 2.
Fig. 2.
Interference of aberrant UM signaling pathways by tumor suppressor miRNAs
Table 2.
Tumor suppressor miRNAs expression and targets in UM
| Tumor suppressor miRNAs | Downregulated in | Functions | Targets | Refs. |
|---|---|---|---|---|
| let-7b | Radioresistant UM cell lines: OM431 and OCM1 | Increases radiosensitivity | cyclin D1 | [81] |
| miR-9 | Highly invasive cell lines: MUM-2B and C918 | Suppresses cell migration and invasion | NF-κB | [82] |
| miR-17-3p | UM patient tumor tissues; UM cell lines: OCM-1A, MUM-2C, C918, and MUM-2B | Suppresses tumorigenesis and metastasis | MDM2 | [83] |
| miR-34a | UM cell lines: M17, M21, M23, and SP6.5; UM patient tumor tissues | Inhibits cell proliferation and migration | C-MET | [74] |
| UM cell lines: M17, M23, and SP6.5 | Inhibits migration and invasion | LGR4 | [84] | |
| UM cell lines: SP6.5 and OM431 | Induces apoptosis | TRAIL | [85] | |
| miR-34b/c | UM cell line: SP6.5; UM patient tumor tissues (upregulated in doxorubicin- and epigenetic drug-treated SP6.5 cells) | Inhibits cell proliferation and migration | C-MET | [76] |
| miR-122 | UM cell lines: 92.1, MEL270, OMM2.5, UPMM2, and UPMM3; the TCGA UM dataset, and a cohort of 7 UM patient tumor tissues | Inhibits cell proliferation and migration | C-MET, ADAM10 | [77] |
| miR-124a | UM cell lines: M17, M21, M23, SP6.5; UM patient tumor tissues | Inhibits cell proliferation and invasion | CDK4, CDK6, cyclin D2, EZH2 | [86] |
| miR-137 | UM cell lines: OMM1.3, Mel202, 92.1, OMM1, OCM1, and OCM3 | Inhibits cell proliferation | p160SRC3 | [87] |
| UM cell lines: SP6.5 and OM431 | Induces apoptosis and | TRAIL | [85] | |
| UM cell lines: M17, M23, and SP6.5 | Inhibits cell proliferation | MITF, CDK6 | [88] | |
| miR-142-3p | UM cell lines: M17, SP6.5; UM patient tumor tissues | Suppresses cell proliferation and migration | CDC25C, TGFβR1, GNAQ, WASL, RAC1 | [52] |
| miR-144 | UM cell lines: MUM-2B, C918, MUM-2C, and OCM-1A; UM patient tumor tissues | Inhibits cell proliferation and invasion | C-MET | [75] |
| UM cell lines: 92.1, MEL270, OMM2.5, UPMM2, and UPMM3; The TCGA UM dataset and a cohort of 7 UM patient tumor tissues | Inhibits cell proliferation and migration | C-MET, ADAM10 | [77] | |
| miR-145 | UM cell lines: MUM-2B and OCM-1; UM patient tumor tissues | Inhibits cell growth and promotes cell apoptosis | IRS-1 | [65] |
| UM patient tumor tissues (both high- and low-invasive UM) | Inhibits proliferation and invasion | NPR1 | [60] | |
| miR-182 | UM cell lines: M23 and SP6.5; UM patient tumor tissues; (upregulated by p53 activation) | Decreases cell growth, migration, and invasion | MITF, BCL2, cyclin D2 | [80] |
| UM cell lines: SP6.5 and OM431 | Induce apoptosis | TRAIL | [85] | |
| miRNA-205 | UM patient tumor tissues (both high- and low-invasive UM) | Inhibits proliferation and invasion | NPR1 | [60] |
| miR-216a-5p | UM cell line: MUM-2B; UM patient tumor tissues | Inhibits cell growth through the Warburg effect | HK2 | [69] |
| miR-224-5p | UM cell line: OCM-1A; UM patient tumor tissues | Inhibits proliferation, migration, and invasion, | PIK3R3/AKT3 | [62] |
| UM cell lines: OCM-1A, MUM-2C, C918, and MUM-2B; UM patient tumor tissues | Suppresses cell proliferation and migration | FTH1P3, RAC1, Fizzled 5 | [58] | |
| miR-15a, miR-185, miR-211 | Cell line: OCM-1; UM patient tumor tissues | Inhibit cell proliferation | IL-10Rα | [67] |
RHO/RAC pathway-targeting miRNAs
Peng et al. showed that miR-142-3p regulates GNAQ/GNA11 and the downstream RHO/RAC signaling pathway as well as components of the MAPK/ERK and PI3K/AKT pathways. GNAQ, RAC1, transforming growth factor beta receptor 1 (TGFβR1), cell division cycle 25C (CDC25C), or Wiskott–Aldrich syndrome protein (WASL) are target genes of miR-142-3p [52, 53]. TGFβR1 functions as a transducer and acts as an oncogene in UM. CDC25C is a phosphatase that triggers entry into mitosis by dephosphorylating cyclin B-CDK1 [54–56]. WASL holds key roles in the regulation of endocytosis, actin polymerization, and cell junction formation [57]. Based on their functions, downregulation of the expression of these five genes accounts for decreased cell proliferation and migration [52]. RAC1 is also a direct target of miR-224-5p. Additionally, miR-224-5p targets frizzled-5, a receptor protein in the WNT/β-catenin pathway, which is activated in many cancers and is believed to contribute to tumor recurrence [58, 59]. A recent study suggested that miR-145 and miR-205 function as onco-suppressors in UM by directly targeting neuropilin 1 (NRP1) to reduce the expression of human cell division control protein 42 (CDC42), a small GTPase of the RHO family [60]. The homologous RHO GTPases, RAC, and CDC42, play key roles in regulating the occurrence and development of tumors by controlling diverse cellular functions [61]. NRP1 is a transmembrane coreceptor for both vascular endothelial growth factor (VEGF) and semaphorin family members. It also interacts with various membrane receptors, such as C-MET, integrins, and transforming growth factor receptors to promote angiogenesis, tumor growth, invasion, and metastasis.
PI3K/AKT and MAPK/ERK pathway-targeting miRNAs
The PI3K/AKT signaling pathway is activated in a setting of GNAQ/GNA11 mutation and is involved in promotion of cell survival and cell migration in UM [24]. Tumor suppressor miR-224-5p directly targets PI3K/AKT pathway-related proteins, such as phosphatidylinositol 3-kinase regulatory subunit gamma (PIK3R3) and AKT3 (protein kinase B gamma), to exert its inhibitory effects on UM cell growth and invasion [62]. Insulin receptor substrate-1 (IRS-1) is an oncogene that is implicated in the upregulation of the PI3K/AKT pathway [63, 64]. Cell proliferation is inhibited and UM cell death is promoted by miR-145 through targeting IRS-1 [65]. IL-10, an immunoregulatory cytokine, can induce phosphorylation of AKT to active PI3K/AKT signaling [66]. The expression of IL-10 receptor (IL-10Rα) in UM is downregulated by miR-15a, miR-185, and miR-211, individually and in combination [67]. Single or combined ectopic expression of these three miRNAs leads to a significant reduction in UM cell proliferation [67]. The expression of hexokinase-2 (HK2) is induced by activated PI3K/AKT signaling [68], and it is the primary rate-determining enzyme involved in aerobic glycolysis, which preferentially occurs in cancer cells and is referred to as the Warburg effect. UM growth is reduced by miR-216a-5p through suppression of the Warburg effect by directly targeting HK2 [69].
The C-MET tyrosine-kinase receptor is implicated in the upstream pathway of PI3K/AKT [70, 71]. In UM, C-MET is overexpressed in over 60% of tumors, and it is associated with tumor aggression and metastasis [72, 73]. To date, several miRNAs have been identified that regulate C-MET expression, such as miR-34, miR-122, miR-144, and miR-182 [74–77]. The members of the miR-34 family are miR-34a, miR-34b, and miR-34c. Although three of them are encoded by two different transcriptional units, their sequences are very similar [78]. They act as tumor suppressors by abrogating several signaling pathways involved in cell proliferation and migration [74, 76]. They downregulate C-MET expression, which in turn interferes with the downstream AKT signaling pathway in an hepatocyte growth factor (HGF)-dependent manner [70]. The expression of C-MET can be directly and indirectly downregulated in UM by miR-122 and miR-144 [75, 77] because they both directly target disintegrin metalloproteinase 10 (ADAM10), a zinc‐dependent transmembrane protein with pro-invasive roles in UM cells. ADAM1 is associated with UM progression by cleaving c-Met into soluble c-Met [79]. Nevertheless, miR-182 interferes the C-MET signaling pathway by targeting melanogenesis associated transcription factor (MITF) [80].
Microphthalmia-associated transcription factor (MITF) encodes a basic helix-loop-helix leucine zipper transcriptional factor and is an oncogene that is associated with the AKT and ERK1/2 pathways in UM. C-MET is a direct transcriptional target of MITF [89], and the latter is targeted by miR-182 in UM cells. Furthermore, UM cell growth is suppressed by miR-182 via the binding of MITF, cyclin D2, and B-cell lymphoma 2 (BCL2) [80]. BCL-2 is a mitochondrial protein that promotes cellular survival and inhibits the activity of pro-apoptotic proteins [80]. Additionally, miR-182 participates in the tumor suppression network of p53 in UM because miR-182 expression depends on p53 activation [80]. Similar to the function of miR-182, miR-137 suppresses the expression of MITF and cell cycle-related genes. The expression of miR-137 is epigenetically downregulated during UM tumorigenesis [85, 88]. Functional analysis has indicated that miR-137 significantly increases G1 cell cycle arrest of UM cells by suppressing the expression of MITF, cyclin-dependent kinases CDK2 and CDK6, and three p160 steroid receptor coactivators (SRCs) [87]. Of special note, SRCs are transcriptional coactivators and “master regulators” of genes involved in tumorigenesis.
NF-κB signaling pathway interference by miRNAs
The aggression of UM is believed to be associated with the nuclear factor kappa B (NF-κB) pathway. NF-κB1 is known to play key roles in cell proliferation, tumoral angiogenesis, and metastasis. Liu et al. reported that miR-9 downregulates the expression of NF-κB1 and its downstream targets, such as matrix metalloproteinases-2 and -9 (MMP-2, -9) and VEGFA [82]. MMP-2- and MMP-9-mediated extracellular matrix degradation is essential for cellular invasion and cancer cell metastasis [90]. MMP-2 is a downstream effector for leucine-rich repeat-containing G protein-coupled receptor 4 (LGR4). LGR4 is involved in tumor metastasis, and miR-34a targets LGR4 to regulate the migration and invasion of UM cells [84, 91].
P53 and other cell cycle-related pathway-targeting mRNAs
The p53 pathway is central among the molecular mechanisms that influence cell cycle arrest and apoptosis [92]. Nonetheless, the retinoblastoma tumor suppressor protein (pRB) is a key player in cell cycle progression, and one of its major functions is to prevent excessive cell growth. However, the RB pathway is frequently dysregulated in many cancers [93]. The canonical RB pathway consists of RB1, cyclin D1, cyclin-dependent kinase 4/6 (CDK4/6), p16, and the E2F family. The principal function of CDK4/6 is to phosphorylate pRB and consequently control G1/S progression. Cancer cells often restrain the inhibitory function of pRB on cell cycle progression via CDK4/6 [94]. It has been reported that miR-17-3p increases the transcriptional activity of p53 by downregulating the expression of oncoprotein murine double minute clone 2 (MDM2) [83], which mediates the proteasomal degradation of p53 through its E3 ligase activity [95]. UM cell proliferation is reduced by miR-34a and miR-34b/c through indirect modulation of the cell cycle proteins RB, CDC2, E2F3, and CDK4/6 [74, 76]. It has been shown that miR-124a suppresses the functions of CDK4/6, cyclin D2, and EZH2, and it also exhibits strong inhibitory effects on UM cell migration and invasion as well as tumor growth in vivo [86].The expression of miR-124a is epigenetically silenced in UM development, and its expression can be restored by treatment with a DNA-hypomethylating agent or histone deacetylase inhibitor [86]. Let-7b is a well-known tumor suppressor miRNA and is downregulated in radioresistant UM cells [96]. Direct targeting of cyclin D1 leads to increased radiosensitivity of UM cells by let-7b [81].
In summary, some miRNAs can influence UM tumorigenesis and metastasis and are potential targets for the development of novel therapeutic strategies. A specific miRNA may have diverse roles in different subtypes of UM because a single miRNA can target multiple genes to interact with several signaling pathways. Therefore, interpretation of the effects of specific miRNAs in UM should be relevant to the biological backgrounds.
Biomarker miRNAs for diagnosis and prognosis of UM
Efforts to understand the molecular biology of UM have revealed some miRNAs that correlate with patient prognoses [97]. In UM, miRNAs can be detected and quantified in tumor tissues [frozen and formalin-fixed, paraffin-embedded tissues (FFPE)] and blood. Particular miRNA signatures appear different from healthy cells [5, 98]. The data summarized here may help guide UM biomarker development to identify metastatic UM (Table 3).
Table 3.
Summary of miRNAs involved in human UM detection
| Samples from UM patients | Techniques for miRNA detection | Overexpressed underexpressed in primary UM |
Overexpressed underexpressed in metastasis/high metastatic risk UM |
Refs. |
|---|---|---|---|---|
| Tumor tissues | miRNA microarray chips; qPCR | miR-17, miR-20a, miR-21, miR-34a, miR-106a, miR-145, miR-204 | [99] | |
| Tumor tissues | miRNA sequencing | miR-16-5p, miR-17-5p, miR-21-5p miR-99a-5p, miR-99a-3p, miR-101-3p, miR-132-5p, miR-151a-3p, miR-181a-2-3p, miR-181b-5p, miR-378d, miR-1537-3p, let-7c-5p | [102] | |
| Tumor tissues | Agilent miRNA microarray | let-7b, miR-143, miR-193b, miR-199a, miR-199a*, miR-652 | [105] | |
| FFPE tumor tissues | miRNA Agilent arrays- hybridization | miR-1, miR-10a, miR-18a, miR-19b-1*, miR-26a-2, miR-34c-5p, miR-129*, miR-133a, miR-154, miR-181a*, miR-218, miR-369-3p, miR-377, miR-376c, miR-493*, miR-495, miR-586, let-7e | miR-33a, miR-99a*, miR-135b, miR-196a*, miR-325, miR-497*, miR-512-5p, miR-549, miR-556-5p miR-585, miR-640, miR-885-5p | [100] |
| FFPE tumor tissues | miRNA Agilent microarray | miR-134, miR-143, miR146b, miR-199a, miR-214 | miR-134, miR-149* | [104] |
| FFPE tumor tissues | Tissue microarrays (TMA); qPCR | miR-592, miR-346, miR-1247; miR-506, miR-513c | [106] | |
| TCGA UM database | large-scale genome sequencing | miR-195, miR-224, miR-365a, miR-365b, miR-452, miR-513c, miR-873, miR-4709, miR-7702 | [108] | |
| TCGA UM database | Illumina Hiseq | miR‑592, miR‑199a‑5p; miR-211-5p, miR-514a-3p, miR-508-3p, miR-509–3-5p, miR-513a-5p, miR-513c-5p | [107] | |
| Serum | MELmiR-17 panel qPCR | miR-16, miR-145, miR-146a, miR-204, miR-211, miR-363-3p | miR-211 | [5] |
| Plasma, tumor tissues | Illumina microRNA profiling BeadChip, quantitative nuclease protection assay, qPCR | miR-199-5p, miR-223, miR-92b | [32] | |
| Serum | qPCR | miR-20a, 125b, 146a, 155 and 223, miR-181a | miR-20a, 125b, 146a, 155 and 223, miR-181a | [37] |
| Vitreous humor, serum, vitreal and serum exosomes | TaqMan low density array | Vitreous humor and vitreal exosomes: miR-21, miR-34a, miR-146a, serum and serum exosomes: miR-146a | [110] | |
| Serum, FFPE tumor samples | TaqMan low density array, TaqMan miRNA assay | Serum: miR-146a, miR-523; miR-19a, miR-30d, miR-127, miR-451, miR-518f, miR-1274B FFPE tumor samples: miR-146a | [111] |
Detection of miRNAs in UM tissues and cells
Comparison of the miRNA-expression profiles of UM and normal uveal tissues revealed that miR-17, miR-20a, miR-21, miR-34a, and miR-106a are upregulated, whereas miR-145 and miR-204 are downregulated in UM. Thus, it is suggested that differentially expressed miRNAs may help in UM diagnosis [99]. Radhakrishnan et al. performed miRNA-expression profiling on FFPE sections of UM tumors and found that 18 miRNAs are differentially expressed in non-metastatic UM, whereas 12 miRNAs are differentially expressed in metastatic UM [100]. Londin et al. also reported that miRNA isoforms are associated with UM metastasis and patient survival [101]. After analyzing the expression of miRNAs in 26 human UM samples, Smit et al. identified 13 differentially expressed miRNAs and three known oncomiRs (miR-17-5p, miR-21-5p, and miR-151a-3p) that are overexpressed in high-risk patients (with an average disease-free survival rate of 28 months, a BAP1 mutation, and BAP1-negative immunoreactivity). This study suggests that some miRNAs play key roles in the development of UM metastasis [102]. Thus, further validation of these findings is warranted to explore the potential role of miRNAs as biomarkers in UM tumor progression and metastasis In UM, alterations on chromosome 3 (a predictor of metastasis) have been linked to metastatic death [103], and links between miRNAs and clinicopathological features in UM with monosomy 3 or disomy 3 have been studied by several research groups. Using univariate and multivariate analyses of miRNA expression in FFPE UM samples [104], Venkatesan et al. discovered that five miRNAs (miR-134, miR-143, miR-146b, miR-199a, and miR-214) have different expression patterns in monosomy 3/disomy 3 UM tumors in a South Asian Indian cohort. The expression of miR-134 and miR-149-3p are strongly correlated with liver metastasis [104]. Worley et al. screened differentially expressed miRNAs using the Agilent miRNA microarray platform to predict metastatic risk and revealed that expression of a group of miRNAs (let-7b, miR-143, miR-193b, miR-199a, miR-199a-3p, and miR-652) is upregulated in metastatic UM tumors and is associated with chromosome 3 status. Two miRNAs (let-7b and miR-199a) are validated as the most significant discriminators for metastatic UM [105]. Wróblewska et al. reported significant upregulation of miR-346, miR-592, and miR-1247, but downregulation of miR-506 and miR-513c, in tumors of metastatic UM patients. Moreover, miR-592 expression is correlated with monosomy 3 tumors [106]. Overall, these independent studies strongly suggest that the expression pattern of miRNAs can be used to stratify UM and to predict metastasis and overall survival of UM patients.
In recent years, different computational approaches have been used to analyze the miRNA-expression data in the TCGA (The Cancer Genome Atlas) UM database to identify miRNAs as prognostic biomarkers. Using this approach, Falzone et al. found that five miRNAs of the miR‑506‑514 cluster (including miR-508-3p, miR-509-3-5p, miR-513c-5p, miR-513a-5p, and miR-514a-3p) are downregulated, whereas miR‑592 and miR‑199a‑5p are upregulated in UM patients with high grade or high risk of metastatic disease. The downregulation of miR-211-5p plus the five miRNAs of the miR‑506‑514 cluster are significantly associated with a negative prognosis in UM patients [107]. Nonetheless, Xin et al. analyzed the miRNA-expression profiles of 80 UM patients from the TCGA database and identified a signature of nine miRNAs (miR-195, miR-224, miR-365a, miR-365b, miR-452, miR-513c, miR-873, miR-4709, miR-7702) for the prognosis of UM. This set of miRNAs can be used to distinguish high-risk UM patients with significantly shorter overall survival rates from those in the low-risk group [108]. Taken together, these preliminary findings need to be further validated by in vitro and translational approaches to develop specific miRNAs as diagnostic/prognostic biomarkers for the management of UM.
Contrary to the aforementioned findings, Larsen et al. performed multiple analyses to determine the association of miRNA expression and chromosomal changes with metastasis and patient survival in UM. They analyzed the miRNA expression of 13 metastatic UM patients compared with that of 13 age‐ and gender‐matched non-metastatic UM control patients, but they did not find miRNAs related to metastasis or overall survival. The prognostic value of miRNA expression has not been confirmed in this study [109].
Circulating biomarker miRNA
Circulating miRNAs are determined to be eminently specific and sensitive for identifying UM malignancy. They are expected to become a new type of blood biomarkers for the diagnosis and prognosis of UM [5, 33, 98, 110, 111]. A group of six miRNAs (miR-16, miR-145, miR-146a, miR-204, miR-211, and miR-363-3p) are differentially expressed in the sera of UM patients and patients with benign melanocytic lesions of the posterior uvea (known as choroidal nevi). Notably, miR-211 is differently expressed in both metastatic and localized UM [5]. Achberger et al. observed high levels of miR-20a, miR-125b, miR-146a, miR-155, miR-181a, and miR-223 in the sera of UM patients. When metastasis occurs in these patients, levels of these miRNAs increase, but miR-181a levels decrease [37]. Russo et al. analyzed the serum levels of miRNAs and found eight miRNAs that are differentially expressed in UM patients compared to normal controls; miR-146a and miR-523 are upregulated, whereas miR-19a, miR-30d, miR-127, miR-451, miR-518f, and miR-1274b) are downregulated. Importantly, the level of miR-146a is increased in both serum and FFPE UM samples [111]. Ragusa et al. also reported the upregulation of miR-146a in the sera, serum exosomes, and FFPE samples of UM patients [110]. Remarkably, miR-146a is upregulated in the four aforementioned investigations, which strongly suggests that miR-146a is a potential circulating marker of UM. Triozzi et al. investigated the plasma levels of miRNAs in UM patients with either tumor monosomy 3 or disomy 3 compared with normal controls. They noticed that miR-92b, miR-199a-5p, and miR-223 are specifically overexpressed in patients with monosomy 3 [32].
Extracellular vesicles and miRNAs
Extracellular vesicles (EVs) include exosomes (size ranges from 30 to 100 nm), microparticles (size ranges from 100 to 1000 nm), and other cell-derived membranous structures. EVs contain various proteins, lipids, metabolites, and nucleic acids, and they transfer bioactive molecules from their parental cells to recipient cells to facilitate intercellular communication. It has been shown that EVs transfer miRNAs into target organs or cells and play a key role in tumorigenesis, metastasis, and therapeutic responses [33]. EVs are also present in many human bodily fluids and have been tested as markers for the diagnosis and prognosis of different diseases [112, 113]. Eldh et al. showed that patients with liver metastases have significantly more exosomes in their peripheral blood compared with healthy controls, and the exosomes from liver perfusates contain different miRNA clusters compared with the exosomes released from other types of cancer cells [114]. Ragusa et al. analyzed the miRNA profiles of exosomes from vitreous humor (VH) and serum exosomes of UM patients. They found high levels of miR-21, miR-34a, and miR-146a in the exosomes from VH and high levels of miR-146a in the serum exosomes, which suggests that these miRNAs may be released by the eyes affected by UM and could be considered as potential circulating biomarkers of UM [110].
Among these studies, only a few miRNAs (e.g., miR-146, miR-199a) are common, and some miRNAs, such as miR-181a, exhibit discordant expression patterns. Possible reasons for these discrepancies are discussed in a very recent review article [33]. Bande Rodriguez et al. suggest the following possible causes: UM sample quality, classification and inclusion criteria, clinical treatments, sample processing, tumor heterogeneity, different racial groups, miRNA quantification methods, and other patient clinical features. All these differences should be considered during interpretation of the data. Although using blood miRNA biomarkers is a less invasive diagnostic method, varied results have been generated from different studies. In the future, unification of the criteria for inclusion and exclusion of specimen collection and standardization of analysis methods is needed to produce more reliable data.
Therapeutic use of miRNAs for UM treatment
Current therapeutic approaches of chemotherapies or targeted therapies yield very low response rates for metastatic UM [115]. For example, selumetinib selectively targets the MAPK pathway, and AZD 8055 interferes with the mammalian target of rapamycin (mTOR) signaling pathway in UM cells; however, they both target these same signaling pathways in healthy cells as well. Emerging studies suggest a key role of a number of miRNAs in UM progression and that some specific miRNAs are involved in chemotherapeutic resistance [116]. Despite having potential to be developed as therapeutic tools, some disadvantages of miRNAs reduce their therapeutic activity. For example, miRNAs have very low stability because they can be degraded by enzymes circulating in the blood [117]. They also have a low endocytosis rate due to their negative charge [118]. Finally, they non-selectively target both healthy and cancer cells [117]. To overcome these limitations, nanoparticle delivery systems have been developed. Nanocarriers include liposomes, viral vectors, polymeric or peptide nanoparticles, lipid nanoparticles, etc. [119–124]. Rois et al. successfully used gold nanoparticles (AuNPs) as carriers to improve the stability and internalization of four miRNAs and chemotherapeutic SN38. Furthermore, the four miRNAs (miR-34a, miR-137 miR-144, and miR-182) were chosen based on their downregulation in UM, and their combined application exhibited a synergistic effect [125]. Moreover, SN38 (7-ethyl-10-hidroxycamptothecin) is a topoisomerase I inhibitor with poor aqueous solubility [126], and the combination of these four miRNAs increases UM cell sensitivity to chemotherapeutic SN38 [116]. Remarkably, these studies demonstrate that the conjugated effect of the four miRNAs and SN38 with AuNP delivery overcomes the innate constraints of these individual molecules and proves to be a highly effective therapeutic approach against UM [116].
Perspectives
Future studies are expected to explicate the involvement of miRNAs in UM tumorigenesis and resistance to chemotherapy and radiation. Proper interpretation of the effects of specific miRNAs in UM progression requires consideration of the appropriate biological backgrounds and further verification with larger sample sizes. The use of well-developed nanotechnology drug-delivery systems may overcome the limitations of therapeutic miRNAs (miRNA mimics and inhibitors of miRNA) and chemotherapy. Combination of conventional therapies with miRNA-based strategies may allow more effective targeting of UM cancer cells and minimize the risk of patient relapse. Besides their therapeutic potential, miRNAs can be developed as biomarkers for diagnosis and prognosis because unique miRNA profiles are associated with specific tissues and cancer stages of UM.
Funding Statement
This work was supported by the Canadian Institutes of Health Research under an operating grant [362383].
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Basile MS, Mazzon E, Fagone P, Longo A, Russo A, Fallico M, et al. Immunobiology of uveal melanoma: state of the art and therapeutic targets. Front Oncol. 2019;9:1145. doi: 10.3389/fonc.2019.01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Bosch T, Kilic E, Paridaens D, de Klein A. Genetics of uveal melanoma and cutaneous melanoma: two of a kind? Dermatol Res Pract. 2010;2010:360136. doi: 10.1155/2010/360136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandiani C, Beranger GE, Leclerc J, Ballotti R, Bertolotto C. Focus on cutaneous and uveal melanoma specificities. Genes Dev. 2017;31(8):724–743. doi: 10.1101/gad.296962.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berus T, Halon A, Markiewicz A, Orlowska-Heitzman J, Romanowska-Dixon B, Clinical DP. Histopathological and cytogenetic prognosticators in . Anticancer Res. 2017;37(12):6541–6549. doi: 10.21873/anticanres.12110. [DOI] [PubMed] [Google Scholar]
- 5.Stark MS, Gray ES, Isaacs T, Chen FK, Millward M, McEvoy A, et al. A panel of circulating microRNAs detects uveal melanoma with high precision. Transl Vis Sci Technol. 2019;8(6):12. doi: 10.1167/tvst.8.6.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messineo D, Barile G, Morrone S, La Torre G, Turchetti P, Accetta L, et al. Meta-analysis on the utility of radiotherapy for the treatment of ocular melanoma. Clin Ter. 2020;170(1):e89–e98. doi: 10.7417/ct.2020.2195. [DOI] [PubMed] [Google Scholar]
- 7.Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: collaborative ocular melanoma study group report no. 26. Arch Ophthalmol. 2005;123(12):1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 8.Sandinha MT, Farquharson MA, Roberts F. Identification of monosomy 3 in choroidal melanoma by chromosome in situ hybridisation. The Br J Ophthalmol. 2004;88(12):1527–1532. doi: 10.1136/bjo.2004.044768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lange MJ, Razzaq L, Versluis M, Verlinde S, Dogrusoz M, Bohringer S, et al. Distribution of GNAQ and GNA11 mutation signatures in uveal melanoma points to a light dependent mutation mechanism. PLoS ONE. 2015;10(9):e0138002. doi: 10.1371/journal.pone.0138002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Versluis M, de Lange MJ, van Pelt SI, Ruivenkamp CA, Kroes WG, Cao J, et al. Digital PCR validates 8q dosage as prognostic tool in uveal melanoma. PLoS ONE. 2015;10(3):e0116371. doi: 10.1371/journal.pone.0116371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Biswas A, Liu H, Sen S, Paruchuri A, Katsonis P, et al. Mutational landscape of the BAP1 locus reveals an intrinsic control to regulate the miRNA network and the binding of protein complexes in uveal melanoma. Cancers. 2019 doi: 10.3390/cancers11101600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismail IH, Davidson R, Gagné JP, Xu ZZ, Poirier GG, Hendzel MJ. Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res. 2014;74(16):4282–4294. doi: 10.1158/0008-5472.Can-13-3109. [DOI] [PubMed] [Google Scholar]
- 13.Onken MD, Worley LA, Long MD, Duan S, Council ML, Bowcock AM, et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49(12):5230–5234. doi: 10.1167/iovs.08-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(6009):1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubbard KB, Hepler JR. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal. 2006;18(2):135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363(23):2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz S, Freichel-Blomquist A, Yang Y, Rumenapp U, Jakobs KH, Schmidt M, et al. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem. 2005;280(12):11134–11139. doi: 10.1074/jbc.M411322200. [DOI] [PubMed] [Google Scholar]
- 19.Chua V, Lapadula D, Randolph C, Benovic JL, Wedegaertner PB, Aplin AE. Dysregulated GPCR signaling and therapeutic options in uveal melanoma. Mol Cancer Res. 2017;15(5):501–506. doi: 10.1158/1541-7786.MCR-17-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaque JP, Dorsam RT, Feng X, Iglesias-Bartolome R, Forsthoefel DJ, Chen Q, et al. A genome-wide RNAi screen reveals a trio-regulated Rho GTPase circuitry transducing mitogenic signals initiated by G protein-coupled receptors. Mol Cell. 2013;49(1):94–108. doi: 10.1016/j.molcel.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su J, Li H. RAC1 overexpression promotes the proliferation, migration and epithelial-mesenchymal transition of lens epithelial cells. Int J Clin Exp Pathol. 2015;8(9):10760–10767. [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida T, Zhang Y, Rivera Rosado LA, Chen J, Khan T, Moon SY, et al. Blockade of rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2010;9(6):1657–1668. doi: 10.1158/1535-7163. [DOI] [PubMed] [Google Scholar]
- 23.Weber A, Hengge UR, Urbanik D, Markwart A, Mirmohammadsaegh A, Reichel MB, et al. Absence of mutations of the BRAF gene and constitutive activation of extracellular-regulated kinase in malignant melanomas of the uvea. Lab Invest. 2003;83(12):1771–1776. doi: 10.1097/01.lab.0000101732.89463.29. [DOI] [PubMed] [Google Scholar]
- 24.Patel M, Smyth E, Chapman PB, Wolchok JD, Schwartz GK, Abramson DH, et al. Therapeutic implications of the emerging molecular biology of uveal melanoma. Clin Cancer Res. 2011;17(8):2087–2100. doi: 10.1158/1078-0432.Ccr-10-3169. [DOI] [PubMed] [Google Scholar]
- 25.Saraiva VS, Caissie AL, Segal L, Edelstein C, Burnier MN., Jr Immunohistochemical expression of phospho-Akt in uveal melanoma. Melanoma Res. 2005;15(4):245–250. doi: 10.1097/00008390-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Xia Z, Yang C, Yang X, Wu S, Feng Z, Qu L, et al. miR-652 promotes proliferation and migration of uveal melanoma cells by targeting HOXA9. Med Sci Monit. 2019;25:8722–8732. doi: 10.12659/msm.917099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Jia R, Ge S. Role of epigenetics in uveal melanoma. Int J Biol Sci. 2017;13(4):426–433. doi: 10.7150/ijbs.18331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartel DP. microRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomase VS, Parundekar AN. microRNA: human disease and development. Int J Bioinform Res Appl. 2009;5(5):479–500. doi: 10.1504/ijbra.2009.028678. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Yu X, Shen J, Jiang Y. microRNA dysregulation in uveal melanoma: a new player enters the game. Oncotarget. 2015;6(7):4562–4568. doi: 10.18632/oncotarget.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Triozzi PL, Achberger S, Aldrich W, Crabb JW, Saunthararajah Y, Singh AD. Association of tumor and plasma microRNA expression with tumor monosomy-3 in patients with uveal melanoma. Clin Epigenetics. 2016;8:80. doi: 10.1186/s13148-016-0243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bande Rodriguez MF, Fernandez Marta B, Lago Baameiro N, Santiago-Varela M, Silva-Rodriguez P, Blanco-Teijeiro MJ, et al. Blood biomarkers of uveal melanoma: current perspectives. Clin Ophthalmol. 2020;14:157–169. doi: 10.2147/opth.s199064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grillari J, Hackl M, Grillari-Voglauer R. miR-17-92 cluster: ups and downs in cancer and aging. Biogerontology. 2010;11(4):501–506. doi: 10.1007/s10522-010-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20(12):1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, Jiang J, Wang S, Xia X. Oncogenic role of microRNA20a in human uveal melanoma. Mol Med Rep. 2016;14(2):1560–1566. doi: 10.3892/mmr.2016.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Achberger S, Aldrich W, Tubbs R, Crabb JW, Singh AD, Triozzi PL. Circulating immune cell and microRNA in patients with uveal melanoma developing metastatic disease. Mol Immunol. 2014;58(2):182–186. doi: 10.1016/j.molimm.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi P, Kooshki M, Aldrich W, Varghai D, Zborowski M, Singh AD, et al. Expression of natural killer cell regulatory microRNA by uveal melanoma cancer stem cells. Clin Exp Metastasis. 2016;33(8):829–838. doi: 10.1007/s10585-016-9815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venza M, Visalli M, Beninati C, Benfatto S, Teti D, Venza I. miR-92a-3p and MYCBP2 are involved in MS-275-induced and c-myc-mediated TRAIL-sensitivity in melanoma cells. Int Immunopharmacol. 2016;40:235–243. doi: 10.1016/j.intimp.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Dupont J, Renou JP, Shani M, Hennighausen L, LeRoith D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J Clin Invest. 2002;110(6):815–825. doi: 10.1172/jci13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdel-Rahman MH, Yang Y, Zhou XP, Craig EL, Davidorf FH, Eng C. High frequency of submicroscopic hemizygous deletion is a major mechanism of loss of expression of PTEN in uveal melanoma. J Clin Oncol. 2006;24(2):288–295. doi: 10.1200/jco.2005.02.2418. [DOI] [PubMed] [Google Scholar]
- 42.Ling JW, Lu PR, Zhang YB, Jiang S, Zhang ZC. miR-367 promotes uveal melanoma cell proliferation and migration by regulating PTEN. Genet Mol Res. 2017 doi: 10.4238/gmr16039067. [DOI] [PubMed] [Google Scholar]
- 43.Sun L, Wang Q, Gao X, Shi D, Mi S, Han Q. MicroRNA-454 functions as an oncogene by regulating PTEN in uveal melanoma. FEBS Lett. 2015;589(19 Pt B):2791–2796. doi: 10.1016/j.febslet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Peng J, Liu H, Liu C. MiR-155 promotes uveal melanoma cell proliferation and invasion by regulating NDFIP1 expression. Technol Cancer Res Treat. 2017;16(6):1160–1167. doi: 10.1177/1533034617737923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howitt J, Low LH, Putz U, Doan A, Lackovic J, Goh CP, et al. Ndfip1 represses cell proliferation by controlling Pten localization and signaling specificity. J Mol Cell Biol. 2015;7(2):119–131. doi: 10.1093/jmcb/mjv020. [DOI] [PubMed] [Google Scholar]
- 46.Chana JS, Wilson GD, Cree IA, Alexander RA, Myatt N, Neale M, et al. c-myc, p53, and Bcl-2 expression and clinical outcome in uveal melanoma. Br J Ophthalmol. 1999;83(1):110–114. doi: 10.1136/bjo.83.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brantley MA, Jr, Harbour JW. Deregulation of the Rb and p53 pathways in uveal melanoma. Am J Pathol. 2000;157(6):1795–1801. doi: 10.1016/s0002-9440(10)64817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang YC, Yang X, Wei WB, Xu XL. Role of microRNA-21 in uveal melanoma cell invasion and metastasis by regulating p53 and its downstream protein. Int J Ophthalmol. 2018;11(8):1258–1268. doi: 10.18240/ijo.2018.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, He X, Li F, Pan H, Huang X, Wen X, et al. The miR-181 family promotes cell cycle by targeting CTDSPL, a phosphatase-like tumor suppressor in uveal melanoma. J Exp Clin Cancer Res. 2018;37(1):15. doi: 10.1186/s13046-018-0679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Q, Cong R, Yan H, Gu H, Zeng Y, Liu N, et al. Genistein inhibits growth of human uveal melanoma cells and affects microRNA-27a and target gene expression. Oncol Rep. 2009;22(3):563–567. doi: 10.3892/or_00000472. [DOI] [PubMed] [Google Scholar]
- 51.Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? the duplicity of microRNAs in cancer. Cancer Res. 2016;76(13):3666–3670. doi: 10.1158/0008-5472.Can-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng D, Dong J, Zhao Y, Peng X, Tang J, Chen X, et al. miR-142-3p suppresses uveal melanoma by targeting CDC25C, TGFbetaR1, GNAQ, WASL, and RAC1. Cancer Manag Res. 2019;11:4729–4742. doi: 10.2147/cmar.s206461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levovitz C, Chen D, Ivansson E, Gyllensten U, Finnigan JP, Alshawish S, et al. TGFbeta receptor 1: an immune susceptibility gene in HPV-associated cancer. Cancer Res. 2014;74(23):6833–6844. doi: 10.1158/0008-5472.Can-14-0602-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993;12(1):53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darbon JM, Penary M, Escalas N, Casagrande F, Goubin-Gramatica F, Baudouin C, et al. Distinct Chk2 activation pathways are triggered by genistein and DNA-damaging agents in human melanoma cells. J Biol Chem. 2000;275(20):15363–15369. doi: 10.1074/jbc.275.20.15363. [DOI] [PubMed] [Google Scholar]
- 56.Turowski P, Franckhauser C, Morris MC, Vaglio P, Fernandez A, Lamb NJ. Functional cdc25C dual-specificity phosphatase is required for S-phase entry in human cells. Mol Biol Cell. 2003;14(7):2984–2998. doi: 10.1091/mbc.e02-08-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alekhina O, Burstein E, Billadeau DD. Cellular functions of WASP family proteins at a glance. J Cell Sci. 2017;130(14):2235–2241. doi: 10.1242/jcs.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng X, Tang H, Zhao X, Sun Y, Jiang Y, Liu Y. Long non-coding RNA FTH1P3 facilitates uveal melanoma cell growth and invasion through miR-224-5p. PLoS ONE. 2017;12(11):e0184746. doi: 10.1371/journal.pone.0184746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Luo JT, Liu YM, Wei WB. miRNA-145/miRNA-205 inhibits proliferation and invasion of uveal melanoma cells by targeting NPR1/CDC42. Int J Ophthalmol. 2020;13(5):718–724. doi: 10.18240/ijo.2020.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maldonado MDM, Dharmawardhane S. Targeting Rac and Cdc42 GTPases in cancer. Cancer Res. 2018;78(12):3101–3111. doi: 10.1158/0008-5472.Can-18-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, Liu X, Li C, Wang W. miR-224-5p inhibits proliferation, migration, and invasion by targeting PIK3R3/AKT3 in uveal melanoma. J Cell Biochem. 2019;120(8):12412–12421. doi: 10.1002/jcb.28507. [DOI] [PubMed] [Google Scholar]
- 63.Vigneri PG, Tirro E, Pennisi MS, Massimino M, Stella S, Romano C, et al. The insulin/IGF system in colorectal cancer development and resistance to therapy. Front Oncol. 2015;5:230. doi: 10.3389/fonc.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dearth RK, Cui X, Kim HJ, Kuiatse I, Lawrence NA, Zhang X, et al. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol. 2006;26(24):9302–9314. doi: 10.1128/mcb.00260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Huang Q, Shi X, Jin X, Shen L, Xu X, et al. MicroRNA 145 may play an important role in uveal melanoma cell growth by potentially targeting insulin receptor substrate-1. Chin Med J (Engl) 2014;127(8):1410–1416. [PubMed] [Google Scholar]
- 66.Sharma S, Yang B, Xi X, Grotta JC, Aronowski J, Savitz SI. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. 2011;1373:189–194. doi: 10.1016/j.brainres.2010.11.096. [DOI] [PubMed] [Google Scholar]
- 67.Venza I, Visalli M, Beninati C, Benfatto S, Teti D, Venza M. IL-10Ralpha expression is post-transcriptionally regulated by miR-15a, miR-185, and miR-211 in melanoma. BMC Med Genomics. 2015;8:81. doi: 10.1186/s12920-015-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhuo B, Li Y, Li Z, Qin H, Sun Q, Zhang F, et al. PI3K/Akt signaling mediated Hexokinase-2 expression inhibits cell apoptosis and promotes tumor growth in pediatric osteosarcoma. Biochem Biophys Res Commun. 2015;464(2):401–406. doi: 10.1016/j.bbrc.2015.06.092. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, Huo Y, Wang D, Tai Y, Li J, Pang D, et al. MiR-216a-5p/Hexokinase 2 axis regulates uveal melanoma growth through modulation of warburg effect. Biochem Biophys Res Commun. 2018;501(4):885–892. doi: 10.1016/j.bbrc.2018.05.069. [DOI] [PubMed] [Google Scholar]
- 70.Granito A, Guidetti E, Gramantieri L. c-MET receptor tyrosine kinase as a molecular target in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2015;2:29–38. doi: 10.2147/jhc.S77038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park M, Dean M, Cooper CS, Schmidt M, O'Brien SJ, Blair DG, et al. Mechanism of met oncogene activation. Cell. 1986;45(6):895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- 72.Mallikarjuna K, Pushparaj V, Biswas J, Krishnakumar S. Expression of epidermal growth factor receptor, ezrin, hepatocyte growth factor, and c-Met in uveal melanoma: an immunohistochemical study. Curr Eye Res. 2007;32(3):281–290. doi: 10.1080/02713680601161220. [DOI] [PubMed] [Google Scholar]
- 73.Bakalian S, Marshall JC, Logan P, Faingold D, Maloney S, Di Cesare S, et al. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin Cancer Res. 2008;14(4):951–956. doi: 10.1158/1078-0432.Ccr-06-2630. [DOI] [PubMed] [Google Scholar]
- 74.Yan D, Zhou X, Chen X, Hu DN, Dong XD, Wang J, et al. MicroRNA-34a inhibits uveal melanoma cell proliferation and migration through downregulation of c-Met. Invest Ophthalmol Vis Sci. 2009;50(4):1559–1565. doi: 10.1167/iovs.08-2681. [DOI] [PubMed] [Google Scholar]
- 75.Sun L, Bian G, Meng Z, Dang G, Shi D, Mi S. MiR-144 inhibits uveal melanoma cell proliferation and invasion by regulating c-Met expression. PLoS ONE. 2015;10(5):e0124428. doi: 10.1371/journal.pone.0124428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Dong F, Lou D. MicroRNA-34b/c suppresses uveal melanoma cell proliferation and migration through multiple targets. Mol Vis. 2012;18:537–546. [PMC free article] [PubMed] [Google Scholar]
- 77.Amaro A, Croce M, Ferrini S, Barisione G, Gualco M, Perri P, et al. Potential onco-suppressive role of miR122 and miR144 in uveal melanoma through ADAM10 and C-Met inhibition. Cancers. 2020 doi: 10.3390/cancers12061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L, Liao Y, Tang L. MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res. 2019;38(1):53. doi: 10.1186/s13046-019-1059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gangemi R, Amaro A, Gino A, Barisione G, Fabbi M, Pfeffer U, et al. ADAM10 correlates with uveal melanoma metastasis and promotes in vitro invasion. Pigment Cell Melanoma Res. 2014;27(6):1138–1148. doi: 10.1111/pcmr.12306. [DOI] [PubMed] [Google Scholar]
- 80.Yan D, Dong XD, Chen X, Yao S, Wang L, Wang J, et al. Role of microRNA-182 in posterior uveal melanoma: regulation of tumor development through MITF, BCL2 and cyclin D2. PLoS ONE. 2012;7(7):e40967. doi: 10.1371/journal.pone.0040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Y, Zhang L, Fan J, Jia R, Song X, Xu X, et al. Let-7b overexpression leads to increased radiosensitivity of uveal melanoma cells. Melanoma Res. 2015;25(2):119–126. doi: 10.1097/cmr.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 82.Liu N, Sun Q, Chen J, Li J, Zeng Y, Zhai S, et al. MicroRNA-9 suppresses uveal melanoma cell migration and invasion through the NF-kappaB1 pathway. Oncol Rep. 2012;28(3):961–968. doi: 10.3892/or.2012.1905. [DOI] [PubMed] [Google Scholar]
- 83.Wu S, Chen H, Han N, Zhang C, Yan H. Long noncoding RNA PVT1 silencing prevents the development of uveal melanoma by impairing microRNA-17-3p-dependent MDM2 upregulation. Invest Ophthalmol Vis Sci. 2019;60(14):4904–4914. doi: 10.1167/iovs.19-27704. [DOI] [PubMed] [Google Scholar]
- 84.Hou Q, Han S, Yang L, Chen S, Chen J, Ma N, et al. The interplay of microRNA-34a, LGR4, EMT-associated factors, and MMP2 in regulating uveal melanoma cells. Invest Ophthalmol Vis Sci. 2019;60(13):4503–4510. doi: 10.1167/iovs.18-26477. [DOI] [PubMed] [Google Scholar]
- 85.Liu J, Ma L, Li C, Zhang Z, Yang G, Zhang W. Tumor-targeting TRAIL expression mediated by miRNA response elements suppressed growth of uveal melanoma cells. Mol Oncol. 2013;7(6):1043–1055. doi: 10.1016/j.molonc.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen X, He D, Dong XD, Dong F, Wang J, Wang L, et al. MicroRNA-124a is epigenetically regulated and acts as a tumor suppressor by controlling multiple targets in uveal melanoma. Invest Ophthalmol Vis Sci. 2013;54(3):2248–2256. doi: 10.1167/iovs.12-10977. [DOI] [PubMed] [Google Scholar]
- 87.Eedunuri VK, Rajapakshe K, Fiskus W, Geng C, Chew SA, Foley C, et al. miR-137 targets p160 steroid receptor coactivators SRC1, SRC2, and SRC3 and inhibits cell proliferation. Mol Endocrinol. 2015;29(8):1170–1183. doi: 10.1210/me.2015-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen X, Wang J, Shen H, Lu J, Li C, Hu DN, et al. Epigenetics, microRNAs, and carcinogenesis: functional role of microRNA-137 in uveal melanoma. Invest Ophthalmol Vis Sci. 2011;52(3):1193–11929. doi: 10.1167/iovs.10-5272. [DOI] [PubMed] [Google Scholar]
- 89.McGill GG, Haq R, Nishimura EK, Fisher DE. c-Met expression is regulated by Mitf in the melanocyte lineage. J Biol Chem. 2006;281(15):10365–10373. doi: 10.1074/jbc.M513094200. [DOI] [PubMed] [Google Scholar]
- 90.Foda HD, Zucker S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov Today. 2001;6(9):478–482. doi: 10.1016/s1359-6446(01)01752-4. [DOI] [PubMed] [Google Scholar]
- 91.Wu J, Xie N, Xie K, Zeng J, Cheng L, Lei Y, et al. GPR48, a poor prognostic factor, promotes tumor metastasis and activates beta-catenin/TCF signaling in colorectal cancer. Carcinogenesis. 2013;34(12):2861–2869. doi: 10.1093/carcin/bgt229. [DOI] [PubMed] [Google Scholar]
- 92.Levine AJ, Hu W, Feng Z. The P53 pathway: what questions remain to be explored? Cell Death Differ. 2006;13(6):1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 93.Knudsen ES, Wang JY. Targeting the RB-pathway in cancer therapy. Clin Cancer Res. 2010;16(4):1094–1099. doi: 10.1158/1078-0432.Ccr-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.VanArsdale T, Boshoff C, Arndt KT, Abraham RT. Molecular pathways: targeting the cyclin D-CDK4/6 axis for cancer treatment. Clin Cancer Res. 2015;21(13):2905–2910. doi: 10.1158/1078-0432.Ccr-14-0816. [DOI] [PubMed] [Google Scholar]
- 95.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24(17):2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 96.Venza M, Dell’Aversana C, Visalli M, Altucci L, Teti D, Venza I. Identification of microRNA expression patterns in cutaneous and uveal melanoma cell lines. Tumori. 2014;100(1):e4–7. doi: 10.1700/1430.15828. [DOI] [PubMed] [Google Scholar]
- 97.Zhao G, Yin Y, Zhao B. miR-140-5p is negatively correlated with proliferation, invasion, and tumorigenesis in malignant melanoma by targeting SOX4 via the Wnt/beta-catenin and NF-kappaB cascades. J Cell Physiol. 2020;235(3):2161–2170. doi: 10.1002/jcp.29122. [DOI] [PubMed] [Google Scholar]
- 98.Ferracin M, Veronese A, Negrini M. Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Rev Mol Diagn. 2010;10(3):297–308. doi: 10.1586/erm.10.11. [DOI] [PubMed] [Google Scholar]
- 99.Yang C, Wei W. The miRNA expression profile of the uveal melanoma. Sci China Life Sci. 2011;54(4):351–358. doi: 10.1007/s11427-011-4149-y. [DOI] [PubMed] [Google Scholar]
- 100.Radhakrishnan A, Badhrinarayanan N, Biswas J, Krishnakumar S. Analysis of chromosomal aberration (1, 3, and 8) and association of microRNAs in uveal melanoma. Mol Vis. 2009;15:2146–2154. [PMC free article] [PubMed] [Google Scholar]
- 101.Londin E, Magee R, Shields CL, Lally SE, Sato T, Rigoutsos I. IsomiRs and tRNA-derived fragments are associated with metastasis and patient survival in uveal melanoma. Pigment Cell Melanoma Res. 2020;33(1):52–62. doi: 10.1111/pcmr.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smit KN, Chang J, Derks K, Vaarwater J, Brands T, Verdijk RM, et al. Aberrant microRNA expression and its implications for uveal melanoma metastasis. Cancers. 2019 doi: 10.3390/cancers11060815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sisley K, Rennie IG, Parsons MA, Jacques R, Hammond DW, Bell SM, et al. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes Chromosomes Cancer. 1997;19(1):22–28. doi: 10.1002/(sici)1098-2264(199705)19:1<22::aid-gcc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 104.Venkatesan N, Kanwar J, Deepa PR, Khetan V, Crowley TM, Raguraman R, et al. Clinico-pathological association of delineated miRNAs in uveal melanoma with monosomy 3/disomy 3 chromosomal aberrations. PLoS ONE. 2016;11(1):e0146128. doi: 10.1371/journal.pone.0146128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Worley LA, Long MD, Onken MD, Harbour JW. Micro-RNAs associated with metastasis in uveal melanoma identified by multiplexed microarray profiling. Melanoma Res. 2008;18(3):184–190. doi: 10.1097/CMR.0b013e3282feeac6. [DOI] [PubMed] [Google Scholar]
- 106.Wroblewska JP, Lach MS, Ustaszewski A, Kulcenty K, Ibbs M, Jagiello I, et al. The potential role of selected miRNA in uveal melanoma primary tumors as early biomarkers of disease progression. Genes (Basel) 2020 doi: 10.3390/genes11030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Falzone L, Romano GL, Salemi R, Bucolo C, Tomasello B, Lupo G, et al. Prognostic significance of deregulated microRNAs in uveal melanomas. Mol Med Rep. 2019;19(4):2599–2610. doi: 10.3892/mmr.2019.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xin X, Zhang Y, Ling F, Wang L, Sheng X, Qin L, et al. Identification of a nine-miRNA signature for the prognosis of uveal melanoma. Exp Eye Res. 2019;180:242–249. doi: 10.1016/j.exer.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 109.Larsen AC, Holst L, Kaczkowski B, Andersen MT, Manfe V, Siersma VD, et al. MicroRNA expression analysis and Multiplex ligation-dependent probe amplification in metastatic and non-metastatic uveal melanoma. Acta Ophthalmol. 2014;92(6):541–549. doi: 10.1111/aos.12322. [DOI] [PubMed] [Google Scholar]
- 110.Ragusa M, Barbagallo C, Statello L, Caltabiano R, Russo A, Puzzo L, et al. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: Pathological and diagnostic implications. Cancer Biol Ther. 2015;16(9):1387–1396. doi: 10.1080/15384047.2015.1046021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Russo A, Caltabiano R, Longo A, Avitabile T, Franco LM, Bonfiglio V, et al. Increased levels of miRNA-146a in serum and histologic samples of patients with uveal melanoma. Front Pharmacol. 2016;7:424. doi: 10.3389/fphar.2016.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 113.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101(36):13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eldh M, Olofsson Bagge R, Lasser C, Svanvik J, Sjostrand M, Mattsson J, et al. MicroRNA in exosomes isolated directly from the liver circulation in patients with metastatic uveal melanoma. BMC Cancer. 2014;14:962. doi: 10.1186/1471-2407-14-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vivet-Noguer R, Tarin M, Roman-Roman S, Alsafadi S. Emerging therapeutic opportunities based on current knowledge of uveal melanoma biology. Cancers. 2019 doi: 10.3390/cancers11071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Milan Rois P, Latorre A, Rodriguez Diaz C, Del Moral A, Somoza A. Reprogramming cells for synergistic combination therapy with nanotherapeutics against uveal melanoma. Biomimetics (Basel) 2018 doi: 10.3390/biomimetics3040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guzman-Villanueva D, El-Sherbiny IM, Herrera-Ruiz D, Vlassov AV, Smyth HD. Formulation approaches to short interfering RNA and MicroRNA: challenges and implications. J Pharm Sci. 2012;101(11):4046–4066. doi: 10.1002/jps.23300. [DOI] [PubMed] [Google Scholar]
- 118.Peng B, Chen Y, Leong KW. MicroRNA delivery for regenerative medicine. Adv Drug Deliv Rev. 2015;88:108–122. doi: 10.1016/j.addr.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bregoli L, Movia D, Gavigan-Imedio JD, Lysaght J, Reynolds J, Prina-Mello A. Nanomedicine applied to translational oncology: a future perspective on cancer treatment. Nanomedicine. 2016;12(1):81–103. doi: 10.1016/j.nano.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 120.Gou Y, Miao D, Zhou M, Wang L, Zhou H, Su G. Bio-inspired protein-based nanoformulations for cancer theranostics. Front Pharmacol. 2018;9:421. doi: 10.3389/fphar.2018.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mahajan S, Patharkar A, Kuche K, Maheshwari R, Deb PK, Kalia K, et al. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int J Pharm. 2018;548(1):540–558. doi: 10.1016/j.ijpharm.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 122.Tabatabaei SN, Derbali RM, Yang C, Superstein R, Hamel P, Chain JL, et al. Co-delivery of miR-181a and melphalan by lipid nanoparticles for treatment of seeded retinoblastoma. J Control Release. 2019 doi: 10.1016/j.jconrel.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 123.Manzano M, Vallet-Regi M. Mesoporous silica nanoparticles in nanomedicine applications. J Mater Sci Mater Med. 2018;29(5):65. doi: 10.1007/s10856-018-6069-x. [DOI] [PubMed] [Google Scholar]
- 124.Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115(19):10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 125.Dai X, Tan C. Combination of microRNA therapeutics with small-molecule anticancer drugs: mechanism of action and co-delivery nanocarriers. Adv Drug Deliv Rev. 2015;81:184–197. doi: 10.1016/j.addr.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 126.Palakurthi S. Challenges in SN38 drug delivery: current success and future directions. Expert Opin Drug Deliv. 2015;12(12):1911–1921. doi: 10.1517/17425247.2015.1070142. [DOI] [PubMed] [Google Scholar]