Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer-related death due to its late diagnosis that removes the opportunity for surgery and metabolic plasticity that leads to resistance to chemotherapy. Metabolic reprogramming related to glucose, lipid, and amino acid metabolism in PDAC not only enables the cancer to thrive and survive under hypovascular, nutrient-poor and hypoxic microenvironments, but also confers chemoresistance, which contributes to the poor prognosis of PDAC. In this review, we systematically elucidate the mechanism of chemotherapy resistance and the relationship of metabolic programming features with resistance to anticancer drugs in PDAC. Targeting the critical enzymes and/or transporters involved in glucose, lipid, and amino acid metabolism may be a promising approach to overcome chemoresistance in PDAC. Consequently, regulating metabolism could be used as a strategy against PDAC and could improve the prognosis of PDAC.
Keywords: Pancreatic cancer, Glycolysis, Glutamine, Lipogenesis, Chemotherapy
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains an intractable malignancy and ranks as the seventh leading cause of cancer-related death worldwide [1, 2]. According to the data, only approximately 10%–20% of patients are eligible for surgical resection at the time of diagnosis; because PDAC is characteristically hard to detect at the early stage and invades adjacent tissue early in the disease process and there is a lack of effective early detection strategies, the 5-year survival rate of PDAC is merely 10% [3–5]. In addition, surgery remains the first option for PDAC if specific tumor and patient criteria are present. Radical resection of tumors can improve the 5-year survival rate to 20%–25%, and surgery combined with adjuvant chemotherapy dramatically enhances the 5-year survival rate to 30% [6–9]. However, the abovementioned data clearly show that even comprehensive treatment for PDAC does not guarantee a good prognosis, and chemoresistance is one of the challenges that contributes to dismal outcomes.
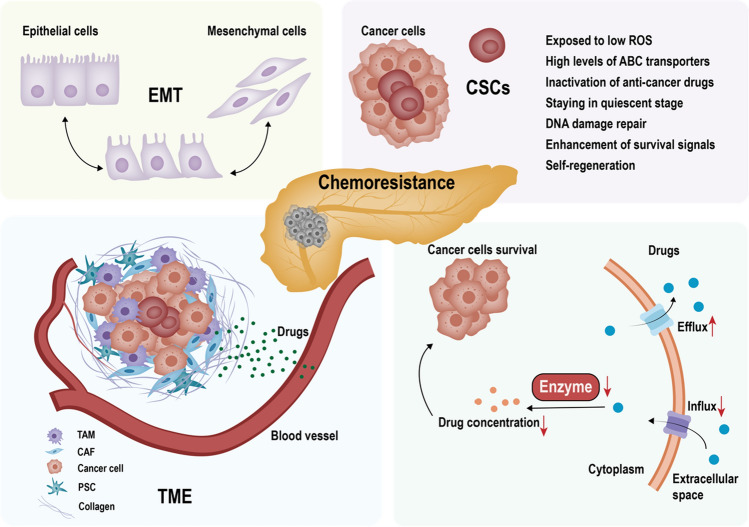
Surgery combined with adjuvant chemotherapy is currently the standard treatment for resectable PDAC [10]. Chemotherapy also plays a crucial role in nonresectable PDAC. In general, chemotherapy regimens primarily consist of gemcitabine [11], ABRAXANE (gemcitabine with albumin-bound paclitaxel) [12], and FOLFIRINOX (oxaliplatin, irinotecan, leucovorin, and 5-fluorouracil) [13]. However, chemoresistance is a frustrating and challenging reality for clinical practitioners. The underlying mechanism of drug resistance in PDAC is very complicated, and a variety of factors, such as the origin of the tumor, tumor microvascularity, and the tumor microenvironment, contribute to drug resistance (Fig. 1).
Fig. 1.
The mechanism of chemoresistance in PDAC. The epithelial–mesenchymal transition phenotype, cancer stem cells, the tumor microenvironment and the deregulation of metabolic pathways induced by chemotherapeutic agents, including changes in drug influx and efflux transporters and changes in enzymes that participate in drug effects, contribute to chemoresistance in PDAC. The arrows in black indicate shifts or bioconversion, and the upward and downward arrows in red indicate upregulation and downregulation, respectively. EMT epithelial-mesenchymal transition, CSCs cancer stem cells, TME tumor microenvironment, CAFs cancer-associated fibroblasts, TAMs tissue-associated macrophages, ROS reactive oxygen species, PSCs pancreatic stellate cells
Metabolism is indispensable for cell proliferation and differentiation and produces adenosine 5′-triphosphate (ATP) to satisfy the energetic demands of cells. Cancer cells, unlike normal cells, need to grow rapidly with abnormal morphology and even preferentially invade adjacent tissues or metastasize, which undoubtedly requires large amounts of energy. Therefore, metabolic reprogramming is crucial for cancer cells to meet the demands for survival and growth. Moreover, as a hallmark of malignant tumors, metabolic reprogramming is regulated by mutations in oncogenes and tumor suppressor genes, the tissue of origin, and the tumor microenvironment. [14, 15] In 1927, Otto Warburg discovered that cancer cells prefer glycolysis over mitochondrial oxidative phosphorylation (OXPHOS) to acquire ATP even under aerobic circumstances. This phenomenon, termed the Warburg effect, [16, 17], is a hallmark of cancer and a prime example of metabolic reprogramming. Cancer cells in PDAC upregulate glycolysis, which produces ATP far less efficiently than OXPHOS. However, intermediates and precursors accumulated in aerobic glycolysis serve as critical building blocks of fast proliferation and tumor progression [18]. Cancer cells upregulate glucose influx and promote biosynthetic pathways, such as the pentose phosphate pathway, which produces ribose-5-phosphate and nicotinamide adenine dinucleotide phosphate (NADPH), which are critical substrates of lipid and nucleic acid biosynthesis [18]. Moreover, NADPH plays an essential role in maintaining redox balance. Therefore, it can be inferred that there is a balance between biosynthesis and ATP production. A typical example in PDAC is the overexpressed major glycolytic enzyme PKM2, which exists in tetrameric and dimeric forms [19]. The tetrameric form of PKM2 possesses high catalytic activity and thereby generates more ATP, similar to PKM1, while the low-activity dimeric form of PKM2 causes the accumulation of intermediates, which subsequently flow into anabolic pathways to produce intermediates to maintain redox balance and support the proliferation of cancer cells at the expense of ATP production [19]. Additionally, there is a shift between the tetrameric and dimeric forms of PKM2 according to the biosynthetic or bioenergetic needs of cells [19].
Although cancer cells upregulate glycolysis for their own benefit, it consequently results in the accumulation of lactate in the ECM. However, cancer cells are not defeated by acidosis of the ECM. In contrast, an acidic extracellular microenvironment promotes the activity of matrix metalloproteinases (MMPs) via cathepsin B action [20]. Subsequently, upregulated MMPs affect growth signals such as the transforming growth factor-β (TGF-β) pathway in the TME, favor cancer cells to resist apoptosis, be involved in tumor angiogenesis and degradation of ECM, mediate vascular stability and assist in forming the metastatic niche [21]; thereby, inducing cancer cells to escape immune surveillance and facilitating the invasiveness and migration capability of cancer cells.
PDAC features dense fibrosis, hypovascularization, and increased interstitial pressure, which subsequently contribute to hypoxia and nutrient limitation [22], and these harsh conditions force PDAC tumors to adapt and alter their metabolism not only to survive but also to achieve invasion of adjacent and remote tissues. An in vitro study showed that there were differences in the metabolome between gemcitabine-resistant pancreatic cancer cells and gemcitabine-sensitive cells [23], suggesting that there is a strong correlation between chemoresistance and cancer metabolism.
Mechanism of chemoresistance in PDAC
Chemotherapy resistance is generally classified into two categories: intrinsic (innate or de novo) and acquired drug resistance [24]. Intrinsic resistance entails a failure of drug effectiveness from the initiation of therapy owing to genetic factors of patients, while acquired resistance includes situations in which the patient first shows decent sensitivity to chemotherapy at the start of therapy but gradually becomes resistant after a certain period of exposure to anticancer drugs. Therefore, continued usage of anticancer drugs when acquired resistance occurs will eventually result in recurrence and further invasion of tumors [24]. In this paper, we take gemcitabine resistance as an example because relatively less research has been conducted on other drugs.
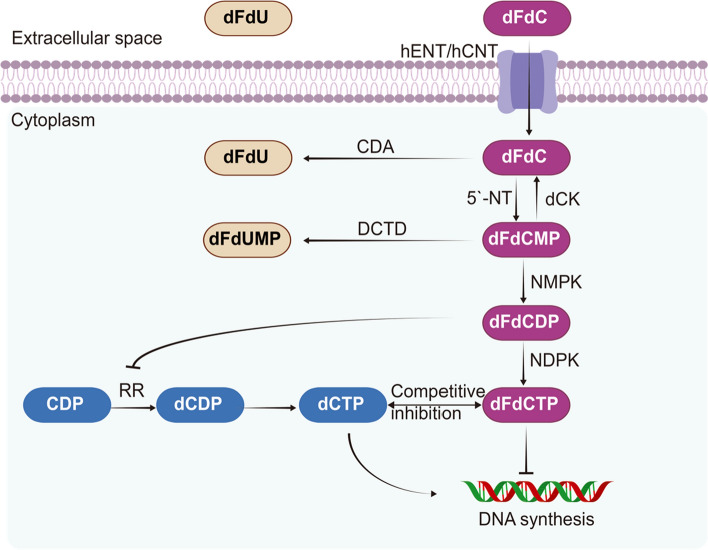
Gemcitabine (2′,2′-difluorodeoxycytidine, dFdC), the most typically used drug in the treatment of PDAC, has been considered the first-line drug for locally advanced or metastatic pancreatic cancer since 1997 [11]. It is a nucleoside cytidine analog that competes with pyrimidine and is incorporated into replicating DNA, resulting in the blockage of DNA synthesis. As a prodrug, gemcitabine must be recognized and transported into the cytoplasm by nucleoside transporters (hNTs) and then phosphorylated by deoxycytidine kinase (dCK), which converts it into the active forms gemcitabine diphosphate (dFdCDP) and triphosphate (dFdCTP) to exert cytotoxic effects [25]. dFdCDP and dFdCTP inhibit ribonucleotide reductase and DNA polymerase, respectively, to terminate DNA synthesis, giving rise to cell death [26] (Fig. 2). Overall, the underlying mechanism of gemcitabine resistance can be broadly attributed to gemcitabine metabolism, the tumor microenvironment, the epithelial–mesenchymal transition (EMT) phenotype, and cancer stem cells (CSCs).
Fig. 2.
The pharmacological mechanism and metabolism of gemcitabine in cancer cells. Gemcitabine (dFdC) is transported into the cytoplasm by hENT/hCNT and phosphorylated by dCK, NMPK and NDPK into active forms to terminate DNA synthesis in cancer cells. The arrows in black indicate shifts or bioconversion, and the T-ended stop bar indicates negative regulation. dFdC 2′,2′-difluorodeoxycytidine, gemcitabine, dFdCMP gemcitabine monophosphate, dFdCDP gemcitabine diphosphate, dFdCTP gemcitabine triphosphate, dFdU 2′,2′-difluorodeoxyuridine, dFdUMP 2′,2′-difluorodeoxyuridine monophosphate, CDP cytidine diphosphate, dCDP deoxycytidine diphosphate, dCTP deoxycytidine triphosphate, DCK deoxycytidine kinase, DCTD deoxycytidine monophosphate deaminase, CDA cytidine deaminase, 5′-NT 5′-nucleotidase, RR ribonucleotide reductase, hCNTs concentrative nucleoside transporters, hENTs equilibrative nucleoside transporters
The influx and efflux of gemcitabine
As described previously, gemcitabine is a prodrug and must be delivered into the cytoplasm by hNTs. hNTs are classified into two types: concentrative nucleoside transporters (hCNTs) and equilibrative nucleoside transporters (hENTs) [27]. To a large extent, the intracellular uptake of gemcitabine is mediated by hENT1, and the remainder of gemcitabine transport is mediated by hENT2, hCNT1, and hCNT3 [28]. Therefore, hNTs play a crucial role in determining sensitivity and resistance to gemcitabine.
Moreover, the export processes for gemcitabine and 5-fluorouracil (5-FU) are mediated by multidrug resistance protein 5 (MRP-5), which belongs to the ATP-binding cassette transporter (ABC transporter) family [29]. Therefore, inhibition of efflux transporters has been regarded as a strategy to overcome chemoresistance.
Enzymes involved in gemcitabine metabolism
In addition to the factors involved in the influx and efflux of gemcitabine, other factors, such as dCK, cytidine deaminase (CDA), and ribonucleotide reductase (RR), also influence the effectiveness of gemcitabine, thus having an impact on gemcitabine chemoresistance. Gemcitabine phosphorylation is mediated intracellularly by dCK in the rate-limiting step of the conversion of gemcitabine to the active metabolite form [30]. Therefore, inactivation of dCK contributes to gemcitabine resistance in some pancreatic cancer cell lines [31, 32], whereas upregulation of dCK leads to enhanced chemosensitivity [10]. Moreover, an RNA-binding protein termed Hu antigen R (HuR) regulates dCK and sensitizes cancer cells to gemcitabine by promoting the level of dCK [33]. In addition, inactivation of gemcitabine to 2',2'-difluoro-2'-deoxyuridine (dFdU) is catalyzed by CDA [34]. Accordingly, low activity of CDA leads to enhanced gemcitabine effectiveness [35]. In addition, RR catalyzes the processing of ribonucleotides into deoxyribonucleotides, which is a crucial step in the production of dCTP (deoxycytidine triphosphate) [36]. Therefore, enhanced levels of RR lead to an increase in dCTP, which competes with dFdCTP in DNA synthesis, thus resulting in gemcitabine chemoresistance.
Epithelial–mesenchymal transition
EMT refers to the phenotypic conversion of cancer epithelial cells to mesenchymal cells [37]. It plays crucial roles in mediating embryonic development and other physiological and pathological processes in adults, such as wound healing, regeneration of tissues, and the progression and metastasis of cancers, including PDAC [37, 38]. The process is characterized by a reduction in cell–cell adhesion in epithelial cells and the acquisition of motile and invasive capabilities, which enables tumor cells to disseminate and metastasize [37]. Therefore, the EMT phenotype features a decrease in E-cadherin, which is responsible for adhesion, and an increase in mesenchymal markers, such as vimentin and fibronectin [39]. Moreover, tumor cells undergoing EMT become resistant to chemotherapy and apoptosis [38]. Several transcription factors, such as Snail, Slug, Twist, zinc-finger E-box-bonding homeobox1 (ZEB1), and ZEB2, play a pivotal role in modulating the process of EMT [40]. Therefore, downregulation of these transcription factors has shown enhancement of chemosensitivity in pancreatic cell lines [37, 41, 42]. In addition, EMT can lead to the enhancement of CSCs, and intrinsic changes, such as decreased proliferation, increased DNA repair ability, and resistance to apoptosis, can result in EMT-induced chemoresistance [43].
Cancer stem cells
CSCs are present in very small numbers in tumors and are characterized by self-renewal and the ability to differentiate into cancer cells. CSCs are heterogeneous and tumorigenic and correlate with tumor metastasis, relapse, strong resistance to chemotherapy, and poor prognosis [44]. Pancreatic CSCs exclusively possess the ability to generate tumors and display great resistance to chemotherapy [45]. CSCs contribute to chemoresistance by settling in hypoxic niches that provide low levels of reactive oxygen species (ROS) [46, 47], express high levels of ABC transporters [48], possess abundant enzymes that can inactivate anticancer drugs [46], largely remain quiescent [46, 49], maintain competent DNA repair [50], and upregulate prosurvival mechanisms [46, 51, 52].
Tumor microenvironment
The tumor microenvironment (TME) serves as a nest for tumors; as such, it plays a pivotal role in tumor growth, progression, metastasis, and anticancer drug effects. The TME comprises cellular components such as cancer-associated fibroblasts (CAFs), vascular cells, and immune cells, as well as extracellular matrix, which contains collagen, cytokines, and growth factors [53]. Furthermore, the dense fibrosis present in the pancreatic cancer stroma acts as a barrier that influences drug delivery and leads to chemoresistance [53]. CAFs, as the major cellular component, are activated under pathological conditions and take on a myofibroblast phenotype; this phenotypic change enables them to secrete various cytokines, which creates a favorable environment for tumor growth. In addition, according to multiple studies, CAFs assist in chemoresistance by promoting EMT and preventing apoptosis [54, 55]. Pancreatic stellate cells (PSCs) are classified as quiescent PSCs and activated PSCs, and the former express several nonspecific protein markers and play an important role in sustaining the normal physiological state of the pancreas. Under pathological conditions, resident quiescent PSCs are activated and transform into activated PSCs, which are the primary precursors of CAFs [56]. Furthermore, both activated PSCs and CAFs contribute to dense fibrosis of the stroma in PDAC by secreting laminins, fibronectins, and collagens [57]. In addition, PSCs play a critical role in PDAC metabolism by secreting nonessential amino acids, which act as alternative carbon sources to fuel the tricarboxylic acid (TCA) cycle, thus enabling PDAC survival [58].
Immune cells, such as tissue-associated macrophages (TAMs), as the dominant immune component in the TME, are correlated with tumor progression and chemoresistance [54]. Moreover, TAMs have a prominent M2 phenotype and an increase in fatty acid uptake and L-arginine metabolism, thereby promoting tumor progression [59, 60]. In addition, TAMs showed a preference for glycolysis in the early stage of cancer but oxidative phosphorylation in the late stage [61]. Similarly, TAMs also rewire lipid metabolism in cancer progression, such as increased lipid accumulation and preference for fatty acid oxidation [62]. TAMs have been reported to secrete CDA and avoid apoptosis to decrease the efficacy of gemcitabine [63]. In addition, the acellular components collagen, hyaluronan, and laminin impact chemoresistance to some extent. Pancreatic cancer is characterized by dense fibrosis that mainly contains type I collagen [64], which induces chemoresistance to gemcitabine by modifying genes related to gemcitabine metabolism and activating signaling pathways that prevent apoptosis [64]. The dense fibrosis in PDAC leaves little room for blood vessels, and consequently, there is impaired blood perfusion and hypoxia, which ultimately give rise to decreased hNTs and enhanced EMT [54, 65]. Hyaluronan has been reported to be an independent prognostic factor in patients with PDAC, and its binding to CD44 eventually contributes to antiapoptotic mechanisms and drug resistance [66].
Glucose metabolism reprogramming contributes to chemoresistance
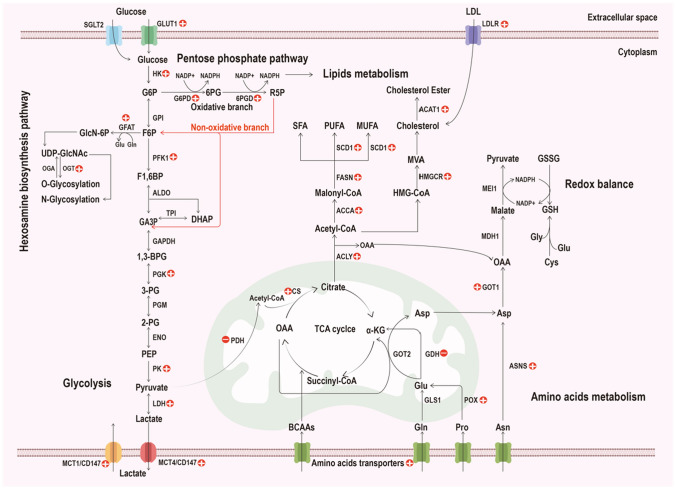
Glucose metabolism includes glycolysis, the pentose phosphate pathway (PPP), the hexosamine biosynthesis pathway (HBP) and gluconeogenesis. PDAC cells rewire their glucose metabolism pathway to acquire energy, carbon and many intermediates to thrive under nutrient-poor conditions. More importantly, the reprogramming of glucose metabolism enables PDAC to resist anticancer drugs, resulting in chemoresistance (Fig. 3).
Fig. 3.
Overview of reprogrammed glucose, amino acid and lipid metabolism in PDAC. Cancer cells regulate biochemical transporters and enzymes involved in metabolic pathways to survive harsh conditions and highly toxic anticancer drugs, and this regulation can confer chemoresistance in PDAC. The arrows indicate shifts or bioconversion, and the plus and minus symbols in the red circle indicate upregulation and downregulation, respectively. GLUTs glucose transporters, SGLTs sodium-dependent glucose transporters, HK hexokinase, G6P glucose 6-phosphate, GPI phosphohexose isomerase, F6P fructose 6 phosphate, PFK1 phosphofructokinase-1; F1,6BP, fructose 1,6-bisphosphate, ALDO aldolase, TPI triose phosphate isomerase, GA3P glyceraldehyde 3-phosphate, DHAP dihydroxyacetone phosphate, GAPDH glyceraldehyde 3-phosphate dehydrogenase, 1,3-BPG 1,3-bisphosphoglycerate, PGK phosphoglycerate kinase, 3-PG 3-phosphoglycerate, PGM phosphoglycerate mutase, 2-PG 2-phosphoglycerate, ENO enolase, PEP phosphoenolpyruvate, PK pyruvate kinase, LDH lactate dehydrogenase, MCT monocarboxylate transporter, G6PD glucose-6-phosphate dehydrogenase, 6PG 6-phosphogluconate, 6PGD 6-phosphogluconate dehydrogenase, R5P ribose-5-phosphate, NADPH nicotinamide adenine dinucleotide phosphate hydrogen, GFAT glutamine fructose-6-phosphate amidotransferase, GlcN-6P glucosamine 6-phosphate, UDP-GlcNAc uridine 5′-diphospho-N-acetylglucosamine, OGT O-linked N-acetylglucosamine transferase, OGA O-GlcNAcase, PDH pyruvate dehydrogenase, CS citrate synthase, OAA oxaloacetate, TCA tricarboxylic acid, α-KG α-ketoglutarate, ACLY ATP citrate lyase, ACCA acetyl-CoA carboxylase, FASN fatty acid synthase, SCD1, stearoyl-CoA desaturase, SFA saturated fatty acids, PUFA polyunsaturated fatty acids, MUFA monounsaturated fatty acids, HMG-CoA 3-hydroxy-3-methylglutaryl coenzyme A, HMGCR 3-hydroxy-3-methylglutaryl coenzyme A reductase, MVA mevalonate, ACAT1 acyl-CoA cholesterol acyltransferase, LDL low-density lipoprotein, LDLR low-density lipoprotein receptor, Gln glutamine, Glu glutamate, Pro proline, Asn asparagine, Asp aspartate, Cys cysteine, Gly glycerine, BCAAs branched chain amino acids, GLS1 glutaminase, GDH glutamate dehydrogenase, GOT glutamic oxaloacetic transaminase, POX proline oxidase, ASNS asparagine synthetase, MDH1 malate dehydrogenase, MEI1 malic enzyme, GSH reduced glutathione, GSSG oxidized glutathione
Glycolysis
Unlike normal differentiated cells that rely on OXPHOS, the majority of cancer cells favor glycolysis to produce energy. Cancer cells are highly proliferative and obviously require much energy for growth and survival, but why vigorously growing cancer cells choose the less efficient metabolic pathway and how glycolysis meets the demands of highly active cancer cells are not yet fully understood [67, 68]. Warburg hypothesized that malfunction of mitochondria leads to impaired OXPHOS in cancer cells, which consequently brings about alterations in the metabolic pathway from OXPHOS to glycolysis [16]. Nevertheless, mitochondrial damage does not occur in the majority of cancers. Instead, glycolysis and OXPHOS can be carried out simultaneously in cancer cells [68, 69]. Glycolysis benefits cancer cells by producing ATP at a faster rate, providing a plethora of intermediates for vigorous biosynthesis, maintaining redox balance, and creating a microenvironment with low immunity [67, 68].
The increase in glycolysis promotes the demand for glucose in PDAC, which consequently leads to upregulation of glucose transporters (GLUTs) [70]. According to the recent research, GLUT1 expression is related to the grade and size of tumors, therapeutic efficacy and the prognosis of patients with PDAC [71, 72]. Increased expression of GLUT1 in PDAC results in poor prognosis and resistance to chemoradiotherapy [72]. Inhibition of GLUT1 at the transcriptional level by a peroxisome proliferator-activated receptor α (PPARα) agonist in colorectal cancer cell lines resulted in reduced tumor growth and enhanced chemotherapeutic efficacy by affecting the mTOR pathway [73]. It has also been reported that the knockdown of Glut1 improves chemosensitivity to cisplatin in neck and head cancers [74]. However, the underlying mechanism underlying the specific relationship between GLUT1 and chemoresistance needs further study. In addition, another type of GLUT, termed sodium-dependent glucose transporters (SGLTs), also plays a critical role in PDAC [75]. Scafoglio et al. reported that treatment with SGLT2 inhibitors in pancreatic cancer xenografts led to cancer cell death. Surprisingly, research has also indicated that SGLT2 inhibitors enhance sensitivity to gemcitabine in a pancreatic cancer model [75]. These findings shed light on chemoresistance in PDAC treatment and indicate that combination treatments containing GLUT inhibitors and chemotherapy will increase efficacy.
Moreover, after entering the cell, glucose is processed through several steps in the process of glycolysis, which involves a number of enzymes, such as hexokinase-1 (HK1) and HK2, phosphofructokinase-1 (PFK1), pyruvate kinase (PK), lactate dehydrogenase A (LDHA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). HKs catalyze the first step of glycolysis, transforming glucose to glucose-6-phosphate; there are four HK isoforms, HK1-HK4, and they each exhibit different affinities for glucose and different cellular distributions [76]. HK2 has been found to be actively expressed in several cancers [77–79], and Ahn K.J. et al. reported an increase in HK2 expression and activity in hepatocellular cancer cells, which exhibited enhanced survival and resistance to cisplatin treatment [77]. Our previous work demonstrated that HK2 was overexpressed in PDAC [78], and overexpression of HK2 was clinically related to cancer recurrence and poor prognosis [78, 80]. In our previous study [78], we found that high expression of HK2 was correlated with gemcitabine resistance in pancreatic cancer cells, whereas gemcitabine efficacy was enhanced through HK2 knockdown in pancreatic cancer cells both in vitro and in vivo. In addition, gemcitabine resistance was attributed to interactions of the HK2 dimer with voltage-dependent anion channels. In addition, 2-deoxy-d-glucose (2DG), a glucose analog, can be catalyzed by HK to 2-deoxyglucose-6-phosphate, leading to blockade of glycolysis [79]. Recent studies have indicated that 2DG not only sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine but also inhibits EMT and CSC phenotypes [81, 82]. However, scientists are still trying to determine the appropriate dose of 2DG in terms of clinical efficacy and patient tolerance [79]. Another inhibitor targeting HK, termed 3-bromopyruvate (3-BP), has been found to exhibit toxicity in cancer cells [79], and its inhibition of glycolysis implies that it can be a potential chemotherapeutic agent. Studies have indicated that 3-BP enhances the chemosensitivity of cancer cells to oxaliplatin and 5-FU by inactivating ABC transporters, and strategies combining chemotherapeutics with 3-BP show an increase in cytotoxicity and slow tumor growth [83, 84]. Since 3-BP impairs mitochondria and induces necrosis in pancreatic cancer cells and remains effective in harsh hypoxic environments [85, 86], we believe that it may help overcome chemoresistance in pancreatic cancer, especially when combined with chemotherapeutic agents.
PFK-1 catalyzes the conversion of fructose-6-phosphate (F6P) to fructose-1,6-bisphosphate, which is the rate-limiting step in glycolysis. PFK1 activity can be increased by an allosteric activator termed fructose-2,6-bisphosphate that drives glycolysis. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphates (PFKFBs) are responsible for phosphorylating F6P to produce F26BP. However, among the four isoforms of PFKFBs, PFKFB3 possesses the highest activity and consequently plays a pivotal role in driving glycolysis [87]. Furthermore, the activity of PFKFB3 can be dramatically enhanced under hypoxic conditions, and overexpression of PFKFB3 has been reported in many cancers, including PDAC, which is characterized by hypoxia [88, 89]. Recent research has indicated that PFKFB3 plays a significant role in PDAC cells by mediating plasma membrane calcium ATPases (PMCAs), and inhibition of PFKFB3 gives rise to calcium overload and subsequent apoptosis in PDAC [89]. In addition, PFKFB2 has also been reported to be highly expressed in PDAC; in addition, it is required for fructose-2,6-bisphosphate synthesis, maintaining the glycolytic phenotype and proliferation of PDAC cells [90]. However, the roles of PFKFBs in chemoresistance in PDAC have scarcely been investigated. Given that the evidence mentioned above emphasizes the importance of PFKFBs in PDAC cell metabolism and cell fate, the study of their impact on chemotherapeutic agents should be performed.
Phosphoglycerate kinase 1 (PGK1) is another essential enzyme and the first enzyme to produce ATP in glycolysis [91]. It is responsible for catalyzing the conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate, which produces ATP. Significantly, PGK1 mediates cancer cell proliferation and progression by maintaining its activity under hypoxic conditions [92]. More noticeably, multiple lines of evidence have demonstrated that PGK1 is related to drug resistance [93, 94]. Chemotherapy-resistant cancer cells are the strongest and most versatile cells, and they survive not only the toxic effects of chemotherapeutic agents but also the harsh conditions induced by nutritional deficiency and hypoxia, which subsequently activate PGK1 to sustain glycolysis [91]. Furthermore, PGK1 has been reported to induce chemoresistance by upregulating autophagy, which contributes to drug resistance in cancer cells, and another way of inducing resistance is scavenging ROS [91, 93, 95, 96]. In addition, in an in vitro study, inhibition of PGK1 not only prevented the proliferation of cancer cells but also enhanced the sensitivity of endometrial cancer cells to cisplatin, and further experiments suggested that PGK1 promoted chemoresistance by mediating the repair and methylation of DNA [94]. Another in vitro study indicated that the treatment with a combination of PGK1 inhibitors and chemotherapeutic agents, such as 5-FU and mitomycin in human gastric cancer cells showed better anticancer effects than PGK1 inhibitors or chemotherapy alone [97]. A recent study from our team revealed that PGK1 expression in the nucleus and cytoplasm determines the phenotype of SMAD4-negative PDAC cells, and PGK1 in the nucleus can mediate gene transcription; thereby, promoting metastasis; in addition, high expression of PGK1 in the cytoplasm supports the proliferation of PDAC [98], suggesting that targeting PGK1 in combination with chemotherapy may be a promising strategy to combat drug resistance in PDAC.
PKM2, an isoform of PK, catalyzes the last rate-limiting step of glycolysis, which produces pyruvate and ATP. More importantly, it plays a crucial role in aerobic glycolysis and cancer cell growth [99]. Two genes (PKLR and PKM) encode four PK isoforms (L, R, M1 and M2) in mammals. PKLR encodes the PKL isoform in the liver, kidney and intestine, and it encodes the PKR isoform in red blood cells. Moreover, PKM encodes the PKM1 and PKM2 isoforms; the former is expressed in adult differentiated tissues that highly demand energy, such as brain and muscle, while the latter is expressed in embryonic cells and cancer cells [99]. In addition, numerous cancers, including PDAC, overexpress PKM2, which determines the fate of glycolysis towards biosynthesis or bioenergetics [100]. PKM2 exists as either a dimeric or tetrameric form. The tetrameric form is highly active and leads to rapid and increased production of ATP and pyruvate, similar to PKM1, which constantly exists in the form of high-activity tetrameric. Nevertheless, the dimeric form is nearly inactive and thereby favors the generation of glycolytic intermediates, which then enter the biosynthetic pathway to meet the demand of cancer cells to proliferate and metastasize [101]. Therefore, the dimeric form of PKM2 is regarded as an oncogenic enzyme that is primarily expressed in cancer cells. The dimeric form of PKM2 and tetrameric form convert mutually according to different metabolic demands of cells. For example, the dimeric form of PKM2 takes effect at the phase of G1/S during the cell cycle, which are highly in need of biosynthesis, while the tetrameric form is activated at the phase of G2/M or the phase of tumor initiation that contains nonproliferating cancer cells that highly demand energy [19, 102]. In addition, posttranslational modifications of PKM2, such as phosphorylation by tyrosine kinases, acetylation, and oxidation of cysteine, steer glycolysis to the biosynthetic pathway to fulfill the needs of cancer cells by disrupting the tetrameric form [103].
In addition, PKM2 inhibition in PDAC leads to the inhibition of aerobic glycolysis and plasma membrane calcium pumps, which gives rise to subsequent calcium overload, decreased cell proliferation, and cell death [104]. Moreover, the impact of PKM2 on cancer chemoresistance has drawn interest and has been studied intensely. In bladder cancer, downregulation of PKM2 results in improved efficacy of pirarubicin by inducing activation of AMPK and inhibition of STAT3 [105]. Another study reported that PKM2 inhibition enhanced cisplatin-induced toxicity in the treatment of advanced bladder cancer, but even better drug efficacy occurred when cisplatin was combined with a PKM2 inhibitor [106]. Furthermore, PKM2 also plays an important role in the chemoresistance of PDAC, and studies indicate that PKM2 upregulation leads to gemcitabine resistance in PDAC [107], while knockdown of PKM2 significantly increases gemcitabine efficacy by inducing effects such as gemcitabine-induced cell apoptosis [103]. However, the function of PKM2 in cell proliferation remains controversial. Yu, L and his colleagues reported that PKM1/2 knockdown in pancreatic cancer cells did not induce any decrease in cancer cell proliferation; however, the cancer cells rewired their metabolism to acquire pyruvate from other metabolic pathways, such as the serine synthesis and cysteine metabolism pathways [108], suggesting that PDAC cells are extremely resilient and adaptable. Nevertheless, according to contradictory results of PKM2 knockdown or knockout in cancer cells concerning tumor growth, the function of PKM2 in cancer cells remains controversial. A possible explanation is that the relative activity of PKM2 plays a crucial role in conferring a cancer phenotype instead of the total amount of PKM2 [19].
Although the concurrent treatment of PDAC with chemotherapy and PKM2 inhibitors in some studies has shown positive outcomes, conflicting results have raised concerns about the utility of PKM2 as a target for overcoming chemoresistance. Therefore, further convincing and validated results are needed.
LDH is the last step in aerobic glycolysis; it catalyzes the interconversion of pyruvate and nicotinamide adenine dinucleotide (NADH) to lactate and NAD+. As a form of LDH, LDHA is expressed in various cancers, including PDAC, while the majority of noncancerous tissues scarcely express LDHA [109, 110]. Therefore, inhibition of LDHA leads to impairment in cancer cell proliferation and reduces the rate of tumor growth [111, 112]. In PDAC, the expression of LDHA acts as a poor prognostic factor [109]. Moreover, LDHA has been found to be correlated with chemoresistance, and targeting LDHA is one of the approaches to overcome chemoresistance [113]. Liu et al. reported that inhibition of LDHA enhances the sensitivity of Taxol-resistant cancer cells to Taxol by promoting apoptosis [113]. LDHA expression is increased significantly by hypoxia, which influences gemcitabine activity by downregulating the enzyme dCK and decreasing the synthesis of an active form of gemcitabine [114]. Interestingly, NHI compounds, which are LDHA inhibitors, increase the level of dCK and decrease the number of CSCs stimulated by gemcitabine treatment, thus improving gemcitabine toxicity in PDAC cells, and synergistic efficacy was observed when NHI compounds and gemcitabine were administered together [114]. Similarly, another study of malignant pleural mesothelioma cells indicated that NHI-1 is related to the recovery of hENT1 expression, which is downregulated under hypoxia, thereby enhancing the efficacy of gemcitabine under hypoxic conditions [115] and suggesting that targeting LDHA is a promising way to improve chemosensitivity and anticancer effects.
Monocarboxylate transporters (MCTs) play a critical role in regulating pH and the levels of intracellular lactate produced from pyruvate, and an increase in MCT1 and/or MCT4 is a characteristic of some malignant tumors [116]. Furthermore, inhibition of MCT1 in aerobic cancer cells leads to termination of lactate transport, which subsequently results in enhanced uptake of glucose. This enhanced uptake of glucose gives rise to a glucose shortage in hypoxic cancer cells; thereby, inducing apoptosis of hypoxic cancer cells, which predominantly exhibit resistance to chemotherapy. The remaining aerobic cancer cells are responsive to chemotherapeutic agents; thus, inhibition of MCT1 enhances drug efficacy [117, 118]. Moreover, inhibition of MCT4 contributes to increases in lactate and H+ in cells; thus, cytosolic acidification causes cell death [117]. An in vitro study reported stable expression of MCT1 and MCT4 in PDAC cells, and knockdown of either of them resulted in a decline in invasiveness [119], suggesting that MCT1 and MCT4 are potential anticancer targets. However, there is still a lack of research demonstrating a direct relationship between MCTs and chemoresistance, and more studies are needed to illuminate the underlying mechanism.
In addition, the function of MCT1 and MCT4 is dependent on the assistance of the chaperone CD147 [120], which is overexpressed in many tumors and has been reported to mediate chemoresistance in tumor cells [121–123]. Silencing of CD147 by RNA interference (RNAi) leads to decreased expression of MCT1 and MCT4 and an increase in the efficacy of cisplatin [121]. Similarly, CD147 silencing in PDAC cells results in inhibition of lactate transport by suppressing the expression of MCT1 and MCT4 and a reduction in invasiveness and tumorigenicity both in vivo and in vitro [124]. Furthermore, CD147 mediates chemoresistance in breast cancers by regulating vacuolar H+-ATPase, which plays a key role in balancing intracellular pH and chemoresistance in cancer cells [122]. In addition, silencing CD147 improves the efficacy of 5-FU by inducing apoptosis of cancer cells [125]. Another study indicated that inhibition of CD147 not only impairs CSC growth but also restores the efficacy of 5-FU in CSC-like cells [126]. In addition, CD147 regulates ABCG2 by increasing its expression and dimerization and influencing its cellular localization, thus mediating its function in transporting drugs in breast cancer [123]. Interestingly, CD147 confers chemoresistance by improving the expression and localization of ABCG2 on the cell surface, which is responsible for the efflux of chemotherapeutic agents. A very recent study demonstrated that CD147 contributes to gemcitabine resistance in PDAC by targeting the ATM/ATR/p53 axis and improving the DNA damage response [127]. Therefore, CD147 acts as a promising therapeutic target for overcoming chemoresistance in PDAC.
Pentose phosphate pathway (PPP)
The PPP, as a pathway of glycolysis, consists of two biomedical branches and plays a pivotal role in synthesizing nucleic acids and NADPH, thus maintaining redox homeostasis and satisfying the biosynthetic demand of cancer cells [128]. The oxidative branch starts with G6P, which is produced by glucose-6-phosphate dehydrogenase (G6PD) and is eventually processed into ribulose-5-phosphate (R5P), NADPH and CO2. Furthermore, the nonoxidative branch produces F6P and glyceraldehyde-3-phosphate, which re-enter the glycolysis pathway [128]. More importantly, the PPP has been reported to be hyperactive in cancer cells and to participate in cancer cell proliferation and chemoresistance [129–132]. Knocking down regulators involved in the PPP in pancreatic cancer with small RNAi decreased the resistance of pancreatic cancer cells to gemcitabine and doxorubicin [129].
Furthermore, G6PD, a key enzyme, catalyzes the first reaction of the PPP, which is irreversible. Moreover, overexpression of G6PD has been found in various cancers, and inhibition of G6PD expression or activity causes decreased cancer cell growth [132–134]. In addition, G6PD is also involved in chemotherapy resistance, and its inhibition in cancer cells contributes to an enhancement in chemotherapy sensitivity in cancer cells [135, 136]. Yin et al. reported that a decrease in G6PD not only reduces cell proliferation but also restores the sensitivity of hepatocellular cancer cells to oxaliplatin [136]. Similarly, Sharma et al. demonstrated that upregulation of G6PD conferred erlotinib resistance in pancreatic cancer [135]. In addition, another enzyme, 6-phosphogluconate dehydrogenase (6PGD), is involved in the third step of the PPP, catalyzing the conversion of 6-phosphogluconate into R5P [130]. Overexpression and high activity of 6PGD have been observed in cisplatin-resistant ovarian and lung cancers [133]. Hu et al. reported upregulation of 6PGD in hepatocellular carcinoma tissues compared to adjacent normal tissues, and both genetic and pharmacological inhibition of 6PGD increased the efficacies of paclitaxel, doxorubicin and cisplatin [130]. In addition, an in vitro and in vivo study demonstrated that enhancement of paclitaxel and doxorubicin toxicity was observed in 6PGD-depleted breast cancer cells [137]. Recently, 6PGD has been reported to play a crucial role in the tumorigenesis of pancreatic cancer [138].
R5P is a substrate for synthesizing nucleic acids, and its upregulation improves the synthesis of nucleic acids; this increased nucleic acid synthesis subsequently promotes DNA repair after exposure to chemotherapeutics that cause DNA damage, thereby inducing chemoresistance and supporting the rapid proliferation of cancer cells [131]. NADPH plays a crucial role in reductive biosynthesis and maintaining reduced conditions to counteract ROS [79]. Furthermore, high ROS production leads to oxidative stress in cells, and glycolysis-dominant cancer cells are exposed to fewer ROS generated by OXPHOS, which subsequently provides cancer cells with protection from the oxidative stress caused by chemotherapy; thus, reducing ROS production is also an effective approach for overcoming chemoresistance [133, 139]. Moreover, NADPH is critical for maintaining reduced glutathione (GSH), which acts as a significant antioxidative agent and controls redox balance in cells. Furthermore, GSH also determines chemotherapy-induced apoptosis, and high levels of GSH lead to superior detoxification in KRAS mutant cancers such as PDAC, which enables cancer cell proliferation and chemoresistance [140, 141]. However, the role of ROS in chemoresistance is still under debate. Of note, higher levels of ROS decrease the expression of P-glycoprotein, which subsequently decreases the efflux of chemotherapeutic agents, thus enhancing the chemosensitivity of cancer [142].
In light of the aforementioned evidence, targeting critical enzymes in the PPP seems to enhance the chemosensitivity of cancer cells by decreasing NADPH and R5P. However, few studies have investigated the roles of these promising enzymes and NADPH in chemoresistance in PDAC, and targeting critical enzymes involved in the PPP and NADPH might be a promising strategy for overcoming drug resistance in PDAC.
Hexosamine biosynthesis pathway (HBP)
HBP is another branch of glycolysis that induces increased glucose influx in PDAC in response to oncogenic KRAS signaling [70]. HBP starts with F6P, which is converted into glucosamine-6-phosphate through catalysis of the rate-limiting enzyme glutamine fructose-6-phosphate amidotransferase (GFAT), and GFAT simultaneously catalyzes the conversion of glutamine into glutamate. Glucosamine-6-phosphate then undergoes several steps of catalysis and is ultimately converted into uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc) [143]. UDP-GlcNAc is a substrate for glycosylation and O-GlcNAc modification of proteins [144], which is catalyzed by O-GlcNAc transferase (OGT); O-GlcNAcase (OGA) catalyzes the removal of O-GlcNAc from proteins. O-GlcNAcylation has been reported to regulate the repair of DNA damage, thus playing a significant role in inducing resistance to drugs targeting DNA [145]. Ma et al. investigated O-GlcNAcylation in PDAC and reported high activity of the PPP and high levels of O-GlcNAcylation; these results support the idea that PDAC growth occurs through NF-kB oncogenic activation and antiapoptotic mechanisms [146]. In addition, inhibition of GFAT, the rate-limiting enzyme in the HBP, contributes to enhancement of the pharmacologic efficacy of cisplatin by downregulating binding immunoglobulin protein (BiP), which subsequently induces apoptosis [147]. Mechanistically, a recent study indicated that increased levels of O-GlcNAcylation in cancer cells confer cisplatin resistance by regulating p53 and c-Myc. [148] Liu et al. reported that high activity of HBP and a subsequent increase in O-GlcNAcylation were observed in cancer cells after exposure to chemotherapy [145]. In addition, the study also demonstrated that O-GlcNAcylation induced chemoresistance in cancer cells by inhibiting apoptosis and inducing the transcription factors NF-kB and AKT, which act as survival-promoting factors. In contrast, chemoresistance in cancer cells can be reduced through inhibition of O-GlcNAcylation or knockdown of OGT, suggesting that O-GlcNAcylation affecting the HBP is a potential key target for strategies to overcome drug resistance in PDAC.
Mitochondrial metabolism
The majority of metabolic pathways, including pathways related to the synthesis of lipids, amino acids, and nucleic acids, converge on mitochondria; thus, mitochondria play essential roles in cancer metabolic reprogramming [149, 150]. Even though glycolysis is the dominant metabolism in most PDAC cells, mitochondrial OXPHOS should not be ignored, and it is of great significance in PDAC relapse and progression [151–153]. It has been reported that the ferlin family member myoferlin, which is abundant in PDAC, plays a crucial role in tumor progression in PDAC by enhancing OXPHOS, and inhibition of myoferlin induces cell lines to switch to a glycolytic phenotype but results in reduced cell proliferation and ATP production [154]. Pancreatic cancer cells that survived KRAS ablation showed strong dependency on OXPHOS and highly expressed genes related to mitochondrial function, but these cells could not shift their metabolism to glycolysis when OXPHOS was inhibited [153], suggesting a potential therapeutic target in the treatment of PDAC. In an in vitro study, forcing pancreatic cancer cells to utilize OXPHOS resulted in the enrichment of pancreatic CSCs, which are highly plastic and able to alter their metabolism; this alteration in metabolism contributes to the enhancement of chemoresistance [152]. CSCs exert chemoresistance primarily through overexpression of ABC transporters (such as ABCG2) and correlate with increased autophagy [48, 51, 52]. Pancreatic cancer cells utilizing OXPHOS express more ABCG2 and less CNT1 than those utilizing other forms of metabolism, which subsequently leads to reduced intracellular concentrations of drugs and increased survival under treatment with different chemotherapeutics, including gemcitabine [152].
Gluconeogenesis
Gluconeogenesis is less investigated than other glucose metabolism pathways, such as aerobic glycolysis, OXPHOS and the PPP. Cells generate glucose using substrates such as glucogenic amino acids, glycerol and lactate in gluconeogenesis. However, gluconeogenesis also antagonizes the Warburg effect in cancer cells [155]. Therefore, gluconeogenesis is suppressed in cancer cells in favor of aerobic glycolysis.
Furthermore, fructose-bisphosphatase 1 (FBP1) is one of the rate-limiting enzymes of gluconeogenesis that catalyzes the conversion of fructose-1,6-biphosphate into F6P. The expression of FBP1 is downregulated in multiple cancers, including PDAC [156]. As FBP1 is a tumor suppressor, its downregulation is related to tumor progression and indicates a poor prognosis in PDAC [156]. Moreover, FBP1 inhibits cell proliferation and the progression of PDAC by inhibiting downstream genes of BRD4 [157]. Additionally, it suppresses tumor progression primarily by inhibiting aerobic glycolysis and the transcription factor hypoxia-inducible factor 1α (HIF1α) [158]. Notably, FBP1 contributes to gemcitabine resistance by suppressing the IQGAP1-MAPK interaction [156], suggesting that targeting gluconeogenesis may be a promising strategy for overcoming chemoresistance.
Amino acid metabolism reprogramming contributes to chemoresistance
Amino acids play indispensable roles in cancer metabolism, such as maintaining redox balance, regulating energy and providing nitrogen and carbon for biosynthesis [159]. In addition to modifying glucose metabolism, PDAC cells also rewire amino acid metabolism to satisfy their demand for rapid proliferation, which facilitates their survival under harsh conditions and resistance to chemotherapeutic agents. For example, PDAC cells upregulate transporters of amino acids, express more asparagine synthetase and even activate a noncanonical glutamine metabolism pathway [160, 161] (Fig. 3).
Glutamine metabolism
Among all essential and nonessential amino acids, glutamine is the most abundant in the blood [162]. Glutamine uptake into the cytoplasm is mediated by the glutamine transporter ASCT2 or via macropinocytosis [162, 163]. In general, after entering mitochondria, glutamine is converted into glutamate by glutaminase (GLS). Glutamate dehydrogenase (GDH) catalyzes the conversion of glutamate into α-ketoglutarate (α-KG) to fuel the TCA cycle, which produces NADPH. Nevertheless, oncogenic KRAS promotes the metabolic reprogramming of PDAC cells, including activation of a noncanonical pathway of glutamine metabolism [164]. The production of NADPH and NH4+ regulates the synthesis of pyrimidines and purines. In PDAC, KRAS mutation induces downregulation of glutamate dehydrogenase and upregulation of cytosolic glutamic oxaloacetic transaminase or aspartate aminotransferase (GOT1). Accordingly, mitochondrial aspartate aminotransferase (GOT2) catalyzes the conversion of glutamine-derived glutamine and oxaloacetate (OAA) into α-KG and aspartate. When aspartate enters the cytoplasm, GOT1 catalyzes its conversion into OAA, which is then processed by malate dehydrogenase 1 (MDH1) and malic enzyme into pyruvate. As a result, noncanonical glutamine metabolism increases the production of NADPH and contributes to redox balance in PDAC. Of note, either glutamine deprivation or inhibition of enzymes involved in this process results in an increased amount of ROS and a reduction in GSH [164].
One study used the glutamine analog 6-diazo-5-oxo-l-norleucine (DON) to investigate the specific mechanism underlying the chemoresistance induced by disruption of glutamine metabolism. Researchers elucidated that disruption of glutamine metabolism enhanced gemcitabine sensitivity in gemcitabine-resistant PDAC cells in three ways. First, downregulation of the epidermal growth factor receptor (EGFR)-dependent pathway has been correlated with chemoresistance in cancer. Second, HBP is impaired by interfering with GFAT, which leads to downregulation of UDP-GlcNAc and subsequent inhibition of protein glycosylation, including glycosylation of EGFR-related proteins and MRP1 or P-glycoprotein1. MRP1 and P-glycoprotein 1 without glycosylation are discharged from the cell. Third, redox imbalance promotes apoptosis and cell death [165].
Branched chain amino acid (BCAA) metabolism
BCAAs include leucine, isoleucine, and valine. All of them are essential amino acids and play a significant role in providing carbon for other metabolic syntheses, thus fueling the TCA cycle and providing cells with energy. Furthermore, BCAAs also provide nitrogen for nucleotide synthesis and impact protein synthesis [166]. A large prospective study with nearly 16 years of follow-up in Japan indicated that elevated levels of plasma BCAAs were strongly correlated with a high risk of pancreatic cancer occurrence [167]. Of note, BCAAs are elevated in pancreatic cancer at an early stage [168]. Because elevation of circulating BCAAs can subsequently enhance the synthesis of nucleic acids, metabolic reprogramming affecting BCAAs may confer chemoresistance by improving DNA repair, which is damaged by DNA-targeting chemotherapeutic agents.
Asparagine metabolism
Recently, a study from Krall et al. demonstrated that asparagine acts as an exchanger that controls the intracellular amounts of serine, arginine and histidine, thus indirectly mediating protein synthesis. Significantly, asparagine mediates serine uptake into cells and consequently becomes a determinant in nucleotide synthesis [169]. Asparagine synthetase (ASNS) catalyzes the conversion of glutamine and aspartate into asparagine. Additionally, ASNS has been reported to suppress apoptosis as a result of cell stress caused by altered cancer metabolism [170]. Moreover, Cui H and colleagues found overexpression of ASNS in glucose-deprived PDAC cells, which showed resistance to cisplatin- and carboplatin-induced apoptosis but not to gemcitabine, 5-FU and paclitaxel, which are commonly used in PDAC chemotherapy [161]. Furthermore, ASNS induced resistance to apoptosis partly by suppressing JNK/SAPK activation. Additionally, ASNS inhibition in gastric cancer not only reduces tumor growth but also synergizes with cisplatin toxicity [171]. In an in vivo study, asparagine depletion in brain tumors significantly enhanced the pharmacological efficacy of gemcitabine and etoposide [172]. Therefore, it can be deduced that overexpression of ASNS in PDAC confers chemoresistance through suppression of apoptosis and a subsequent increase in asparagine, which indirectly enhance nucleotide synthesis.
Proline metabolism
Proline metabolism is activated under hypoxic conditions, and as a subsequent metabolite of proline, hydroxyproline promotes cancer growth and confers sorafenib resistance in hepatocellular carcinoma (HCC) by regulating HIF1α [173]. Moreover, proline has been reported to promote cancer proliferation under nutrient-poor circumstances in PDAC both in vitro and in vivo; specifically, overexpressed proline oxidase (POX) produces glutamate [174], suggesting that POX might contribute to chemoresistance in PDAC by enhancing survival and regulating HIF1α, although this possibility has never been investigated.
Lipid metabolism reprogramming contributes to chemoresistance
The role of lipid metabolism reprogramming in PDAC chemoresistance is less studied than that of glucose metabolism. However, lipid metabolism is indispensable for building the cell membrane because it provides backbone structures for the formation of lipid rafts to recruit signaling proteins, which transduce signals and produce signaling molecules [175]. Excessive demand for lipids enables cancer cells to acquire lipids either through exogenous uptake or endogenous synthesis. Furthermore, lipid metabolism also satisfies the energy demands of cancer cells by generating lipid droplets, which store excess energy and can be utilized to supply energy [176]. Of note, lipid droplets have been reported to be involved in chemoresistance, and cancer cells rich in lipid droplets show more resistance to chemotherapy [177]. Lipid droplet accumulation mediated by lysophosphatidylcholine acyltransferase 2 impairs activation of the caspase cascade and endoplasmic reticulum stress (ER stress) responses, thus inducing resistance to 5-FU and oxaliplatin in colorectal cancer [178]. In PDAC, decreased levels of low-density lipoprotein (LDL) render cancer cells more sensitive to chemotherapy. Therefore, cancer cells mobilize cholesteryl ester, a component of lipid droplets, under such conditions [179] (Fig. 3).
Lipogenesis
Various enzymes involved in the de novo synthesis of fatty acids and cholesterol are upregulated in PDAC. For example, fatty acid synthase (FASN), ATP citrate lyase (ACLY), citrate synthase (CS), and 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase [179, 180]. FASN plays a crucial role in de novo lipogenesis and is regulated by transcriptional regulators, such as c-Myc [181]. Its overexpression has been observed in some chemotherapy-resistant cancer cells, including PDAC [182, 183]. Moreover, the expression of FASN in cancer has been reported to predict poor prognosis, and inhibition of FASN significantly reduces the proliferation of chemoresistant PDAC cells [183]. An in vitro study reported that a FASN inhibitor induced apoptosis and restored the sensitivity of ovarian cancer cells to platinum [184]. Tadros S et al. found overexpression of FASN in PDAC, and the inhibition of FASN profoundly enhanced the chemosensitivity of gemcitabine by mediating ER stress and promoting stemness in cancer cells [183]. Therefore, de novo lipid synthesis in PDAC plays a significant role in the development of chemoresistance.
The lipogenic gene transcription factor SREBP1 has been reported to mediate de novo lipogenesis and correlate with tumorigenesis. In PDAC, silencing SREBP1 impairs lipid metabolism and induces apoptosis, thereby inhibiting tumor progression. Moreover, a study also demonstrated the prognostic value of SREBP1 in PDAC; in the study, high expression of SREBP1 predicted poor survival [185]. PDAC shows high dependence on cholesterol uptake, thus exhibiting overexpression of low-density lipoprotein receptor (LDLR), which has been reported to correlate with a high risk of cancer recurrence. Of note, blocking cholesterol uptake by silencing LDLR sensitizes cancer cells to chemotherapy by suppressing the ERK1/2 survival pathway [179]. Suppressing the cholesterol pathway using melittin not only inhibited tumor growth but also restored gemcitabine efficacy in PDAC [186]. J Li et al. reported that aberrant cholesterol metabolism supported tumor progression and metastasis in PDAC. Researchers have found that cancer cells exhibit high accumulation of cholesterol ester and that inhibiting cholesterol esterification by targeting the acyl-CoA cholesterol acyltransferase-1 (ACAT-1) enzyme results in suppression of tumor growth and metastasis as a result of the increased ER stress and subsequent apoptosis induced by elevated free cholesterol [187]; these results suggest that the proportions of free cholesterol and cholesterol ester are dysregulated and thus that increasing the amount of free cholesterol will induce toxic effects [179]. Increasing the amount of cholesterol ester by increasing ACAT-1 gave rise to gemcitabine resistance in PDAC by downregulating Act, while inhibition of ACAT-1 significantly restored sensitivity to gemcitabine [188]. Lowering cholesterol via simvastatin in cancer cells enhances sensitivity to paclitaxel. Mechanistically, simvastatin impairs the cholesterol-rich domains of lipid rafts, inhibits the FAK signaling pathway, regulates TAMs, subsequently remodels the TME and suppresses EMT, thereby promoting drug efficacy [189].
Fatty acid oxidation (FAO)
Adipocytes confer chemoresistance by secreting arachidonic acid, which subsequently activates AKT and blocks the apoptosis induced by cisplatin [190]. In breast CSCs, inhibiting JAK/STAT3 blocks CSC self-renewal and the expression of diverse lipid metabolism genes, including carnitine palmitoyltransferase 1B (CPT1B), which encodes an enzyme for FAO. Adipocyte-derived leptin upregulates the aforementioned processes, and inhibiting FAO or leptin restores chemosensitivity and inhibits tumor growth [191]. FAO is a prominent energy production pathway in mitochondria and is highly related to NADPH production [192]. Furthermore, FAO was reported to support cancer cell invasion in PDAC in an in vitro study [193]. Additionally, adipocytes support the growth and survival of PDAC cells under glutamine shortage by secreting glutamine [194]. In addition, adipocytes in PDAC secrete the cytokine interleukin-1 (IL-1) to activate pancreatic stellate cells, which subsequently creates a microenvironment with high fibrosis and poor vascularization, thus conferring chemoresistance [195]. However, whether adipocytes or FAO directly influence anticancer drug toxicity in PDAC needs to be verified.
The plethora of evidence mentioned above indicates that reprogramming lipid metabolism to some extent contributes to chemoresistance in PDAC. Significantly, the PDAC phenotype involving proliferation and growth changes induced by altered lipid metabolism is classified as the lipid-dependent phenotype, and the lipid-dependent phenotype of PDAC shows sensitivity to inhibitors of lipogenesis [196], suggesting that targeting lipid metabolism in PDAC with this phenotype will probably enhance the effect of chemotherapy.
Conclusion
PDAC remains an intractable malignancy, and surgical resection is still the only opportunity to cure PDAC. However, the lack of overt clinical manifestations combined with the lack of effective measurements to detect early-stage PDAC remove the chance for surgery in the majority of patients with PDAC. Improvements in chemotherapy will not only enable some patients to be eligible for surgical resection but will also promote long-term outcomes in patients who undergo surgery and have advanced disease. Unfortunately, resistance to anticancer drugs impedes the effect of chemotherapy in PDAC. Reprogramming glucose, amino acid and lipid metabolism provides PDAC cells with energy and metabolites, which supports tumor growth, progression, metastasis and even resistance to chemotherapy. Recently, an increasing number of studies have focused on the role of metabolic reprogramming in chemoresistance. Upregulation or downregulation of critical enzymes or transporters involved in glucose, amino acid and lipid metabolism has been shown to confer chemoresistance in PDAC. There are some clinical trials concerning metabolic inhibitors in PDAC (Table 1). A plethora of in vivo and in vitro studies have demonstrated that targeting critical steps in metabolic pathways overcomes the chemoresistance of PDAC. However, more specific mechanisms involved in the promotion of chemoresistance should be verified in the future, and more convincing evidence from clinical trials of metabolic interventions to overcome chemoresistance is needed to move treatment strategies from bench to bedside. In summary, a better understanding of the anticancer drug resistance mechanism and metabolic reprogramming in PDAC and their relationship will aid the development of strategies that aim to overcome chemoresistance by targeting the Achilles' heel of PDAC: reprogrammed metabolism.
Table 1.
Clinical trials concerning metabolic inhibitors in pancreatic cancer
| Identifier | Recruitment status | Intervention | Study phase | Cancer stage |
|---|---|---|---|---|
| Target: Glucose metabolism | ||||
| NCT00096707 | Completed | 2-deoxy-d-glucose (2DG) | I | Locally advanced or metastatic solid malignancy |
| NCT04542291 | Recruiting | Dapagliflozin | I | Metastatic or locally advanced pancreatic cancer |
| NCT01835041 | Active, not recruiting | CPI-613/Modified FOLFIRINOX | I | Metastatic pancreatic adenocarcinoma |
| NCT03435289 | Unknown | CPI-613/Gemcitabine/Nab-paclitaxel | I | Locally advanced or metastatic pancreatic cancer |
| NCT03699319 | Recruiting | CPI-613 /Modified FOLFIRINOX | I/II | Locally advanced (including unresectable or borderline resectable) pancreatic cancer |
| NCT03504423 | Active, not recruiting | CPI-613 /mFolfirinox/Folfirinox | III | Metastatic stage IV adeno-carcinoma of the pancreas |
| NCT01839981 | Completed | CPI-613 | I | Locally advanced or metastatic pancreatic adenocarcinoma |
| NCT03854110 | Recruiting | GP-2250 | I/II | Advanced unresectable or metastatic pancreatic adenocarcinoma |
| Target: Lipid metabolism | ||||
| NCT02201381 | Not yet recruiting | Metformin/Atorva-statin/Doxycycline/Mebendazole | III | Any cancer type and stage |
| NCT00944463 | Completed | Gemcitabine/Simvastatin | II | Metastatic or unresectable pancreatic adenocarcinoma |
| NCT03889795 | Recruiting | Simvastatin/Metformin/Digoxin | I | Advanced pancreatic cancer/Advanced solid tumor |
| NCT00944463 | Completed | Gemcitabine/Simvastatin | II | Metastatic or unresectable pancreatic adenocarcinoma |
| NCT03889795 | Recruiting | Metformin/Simvastatin/Digoxin | I | Advanced pancreatic cancer/Advanced solid tumor |
| NCT01488513 | Completed | ABC294640 | I | Pancreatic cancer/Unspecified adult solid tumor |
| Target: Amino acid metabolism | ||||
| NCT01523808 | Completed | GRASPA | I | Locally advanced and non-resectable or metastatic pancreatic adenocarcinoma |
| NCT02195180 | Completed | ERY001/Gemcitabine/Folfox | I | Advanced or metastatic exocrine pancreatic adenocarcinoma |
| NCT02077881 | Completed | Nab-Paclitaxel/Gemcitabine/Indoximod | I/II | Metastatic pancreatic cancer |
| NCT03006302 | Recruiting | Epacadostat/Pembrolizumab/Cyclophosphamide/GVAX | II | Metastatic pancreatic adenocarcinoma |
Acknowledgements
We are grateful to the editors of AJE (American Journal Experts) for their help in editing the manuscript. This study was supported by the National Natural Science Foundation of China (No. 81902428, 81802352 and 81772555), the Shanghai Sailing Program (No. 19YF1409400), the National Science Foundation for Distinguished Young Scholars of China (No. 81625016), Clinical and Scientific Innovation Project of Shanghai Hospital Development Center (SHDC12018109) and Scientific Innovation Project of Shanghai Education Committee (2019-01-07-00-07-E00057).
Author contributions
CL, WW and XJY conceived the review, and AT, SS and JX undertook the initial research and writing. ZT, QCM, JH, JL, BZ, and WW were involved in writing, reviewing and graphing, and all authors contributed to the final version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81902428, 81802352 and 81772555), the Shanghai Sailing Program (No. 19YF1409400), the National Science Foundation for Distinguished Young Scholars of China (No. 81625016), Clinical and Scientific Innovation Project of Shanghai Hospital Development Center (SHDC12018109) and Scientific Innovation Project of Shanghai Education Committee (2019–01-07–00-07-E00057).
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
All authors consent to publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abudureyimu Tuerhong, Jin Xu and Si Shi contributed equally to this work.
Contributor Information
Xianjun Yu, Email: yuxianjun@fudanpci.org.
Chen Liang, Email: liangchen@fudanpci.org.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD. Jemal A (2019) Cancer statistics. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 4.Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, Hong TS, Kwak EL, Lauwers GY, Ryan DP, Wargo JA, Lillemoe KD, Ferrone CR. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a "true" R0 resection? Ann Surg. 2013;257(4):731–736. doi: 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- 5.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. The Lancet. 2020;395(10242):2008–2020. doi: 10.1016/s0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 6.Kunovsky L, Tesarikova P, Kala Z, Kroupa R, Kysela P, Dolina J, Trna J. The Use of Biomarkers in Early Diagnostics of Pancreatic Cancer. Can J Gastroenterol Hepatol. 2018;2018:5389820. doi: 10.1155/2018/5389820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):333–348. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 8.Seppanen H, Juuti A, Mustonen H, Haapamaki C, Nordling S, Carpelan-Holmstrom M, Siren J, Luettges J, Haglund C, Kiviluoto T. The Results of Pancreatic Resections and Long-Term Survival for Pancreatic Ductal Adenocarcinoma: A Single-Institution Experience. Scand J Surg. 2017;106(1):54–61. doi: 10.1177/1457496916645963. [DOI] [PubMed] [Google Scholar]
- 9.Valle S, Martin-Hijano L, Alcala S, Alonso-Nocelo M, Sainz B., Jr The Ever-Evolving Concept of the Cancer Stem Cell in Pancreatic Cancer. Cancers (Basel) 2018 doi: 10.3390/cancers10020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng S, Pottler M, Lan B, Grutzmann R, Pilarsky C, Yang H. Chemoresistance in pancreatic cancer. Int J Mol Sci. 2019 doi: 10.3390/ijms20184504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 12.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of U, Intergroup P FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 14.Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020 doi: 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayers JR, Torrence ME, Danai LV, Papagiannakopoulos T, Davidson SM, Bauer MR, Lau AN, Ji BW, Dixit PD, Hosios AM, Muir A, Chin CR, Freinkman E, Jacks T, Wolpin BM, Vitkup D, Vander Heiden MG. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science. 2016;353(6304):1161–1165. doi: 10.1126/science.aaf5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 17.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 19.Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW, Burga LN, Xie J, Jurczak MJ, DePinho RA, Clish CB, Jacks T, Kibbey RG, Wulf GM, Di Vizio D, Mills GB, Cantley LC, Vander Heiden MG. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155(2):397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kryczka J, Papiewska-Pajak I, Kowalska MA, Boncela J. Cathepsin B Is Upregulated and Mediates ECM Degradation in Colon Adenocarcinoma HT29 Cells Overexpressing Snail. Cells. 2019 doi: 10.3390/cells8030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, Thompson CB, Rabinowitz JD. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75(3):544–553. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujimura Y, Ikenaga N, Ohuchida K, Setoyama D, Irie M, Miura D, Wariishi H, Murata M, Mizumoto K, Hashizume M, Tanaka M. Mass spectrometry-based metabolic profiling of gemcitabine-sensitive and gemcitabine-resistant pancreatic cancer cells. Pancreas. 2014;43(2):311–318. doi: 10.1097/MPA.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, Sarkar FH. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8(1):27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 25.Ohhashi S, Ohuchida K, Mizumoto K, Fujita H, Egami T, Yu J, Toma H, Sadatomi S, Nagai E, Tanaka M. Down-regulation of deoxycytidine kinase enhances acquired resistance to gemcitabine in pancreatic cancer. Anticancer Res. 2008;28(4B):2205–2212. [PubMed] [Google Scholar]
- 26.Adamska A, Elaskalani O, Emmanouilidi A, Kim M, Abdol Razak NB, Metharom P, Falasca M. Molecular and cellular mechanisms of chemoresistance in pancreatic cancer. Adv Biol Regul. 2018;68:77–87. doi: 10.1016/j.jbior.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17(Suppl 5):v7–12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 28.Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, Hyde RJ, Karpinski E, Cass CE, Baldwin SA, Young JD. Mol Membr Biol. 2001;18(1):65–72. doi: 10.1080/09687680010026313. [DOI] [PubMed] [Google Scholar]
- 29.Hagmann W, Faissner R, Schnolzer M, Lohr M, Jesnowski R. Membrane drug transporters and chemoresistance in human pancreatic carcinoma. Cancers (Basel) 2010;3(1):106–125. doi: 10.3390/cancers3010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabini E, Ort S, Monnerjahn C, Konrad M, Lavie A. Structure of human dCK suggests strategies to improve anticancer and antiviral therapy. Nat Struct Biol. 2003;10(7):513–519. doi: 10.1038/nsb942. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Hu G, Jiang Z, Guo J, Wang K, Ouyang K, Wen D, Zhu M, Liang J, Qin X, Zhang L. Identification of NME5 as a contributor to innate resistance to gemcitabine in pancreatic cancer cells. FEBS J. 2012;279(7):1261–1273. doi: 10.1111/j.1742-4658.2012.08521.x. [DOI] [PubMed] [Google Scholar]
- 32.Ohmine K, Kawaguchi K, Ohtsuki S, Motoi F, Egawa S, Unno M, Terasaki T. Attenuation of phosphorylation by deoxycytidine kinase is key to acquired gemcitabine resistance in a pancreatic cancer cell line: targeted proteomic and metabolomic analyses in PK9 cells. Pharm Res. 2012;29(7):2006–2016. doi: 10.1007/s11095-012-0728-2. [DOI] [PubMed] [Google Scholar]
- 33.Tatarian T, Jiang W, Leiby BE, Grigoli A, Jimbo M, Dabbish N, Neoptolemos JP, Greenhalf W, Costello E, Ghaneh P, Halloran C, Palmer D, Buchler M, Yeo CJ, Winter JM, Brody JR. Cytoplasmic HuR Status Predicts Disease-free Survival in Resected Pancreatic Cancer: A Post-hoc Analysis From the International Phase III ESPAC-3 Clinical Trial. Ann Surg. 2018;267(2):364–369. doi: 10.1097/SLA.0000000000002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen R, Preta LH, Joste V, Curis E, Huillard O, Jouinot A, Narjoz C, Thomas-Schoemann A, Bellesoeur A, Tiako Meyo M, Quilichini J, Desaulle D, Nicolis I, Cessot A, Vidal M, Goldwasser F, Alexandre J, Blanchet B. Determinants of the interindividual variability in serum cytidine deaminase activity of patients with solid tumours. Br J Clin Pharmacol. 2019;85(6):1227–1238. doi: 10.1111/bcp.13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciccolini J, Serdjebi C, Peters GJ, Giovannetti E. Pharmacokinetics and pharmacogenetics of Gemcitabine as a mainstay in adult and pediatric oncology: an EORTC-PAMM perspective. Cancer Chemother Pharmacol. 2016;78(1):1–12. doi: 10.1007/s00280-016-3003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordheim LP, Seve P, Tredan O, Dumontet C. The ribonucleotide reductase large subunit (RRM1) as a predictive factor in patients with cancer. Lancet Oncol. 2011;12(7):693–702. doi: 10.1016/S1470-2045(10)70244-8. [DOI] [PubMed] [Google Scholar]
- 37.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcucci F, Stassi G, De Maria R. Epithelial–mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov. 2016;15(5):311–325. doi: 10.1038/nrd.2015.13. [DOI] [PubMed] [Google Scholar]
- 39.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 40.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 41.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69(14):5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsukasa K, Ding Q, Yoshimitsu M, Miyazaki Y, Matsubara S, Takao S. Slug contributes to gemcitabine resistance through epithelial–mesenchymal transition in CD133(+) pancreatic cancer cells. Hum Cell. 2015;28(4):167–174. doi: 10.1007/s13577-015-0117-3. [DOI] [PubMed] [Google Scholar]
- 43.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5(9):744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 44.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol Ther. 2016;160:145–158. doi: 10.1016/j.pharmthera.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashimoto O, Shimizu K, Semba S, Chiba S, Ku Y, Yokozaki H, Hori Y. Hypoxia induces tumor aggressiveness and the expansion of CD133-positive cells in a hypoxia-inducible factor-1alpha-dependent manner in pancreatic cancer cells. Pathobiology. 2011;78(4):181–192. doi: 10.1159/000325538. [DOI] [PubMed] [Google Scholar]
- 48.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 49.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140(1):62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 51.Hwang DW, So KS, Kim SC, Park KM, Lee YJ, Kim SW, Choi CM, Rho JK, Choi YJ, Lee JC. Autophagy Induced by CX-4945, a Casein Kinase 2 Inhibitor, Enhances Apoptosis in Pancreatic Cancer Cell Lines. Pancreas. 2017;46(4):575–581. doi: 10.1097/MPA.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 52.Yang MC, Wang HC, Hou YC, Tung HL, Chiu TJ, Shan YS. Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine. Mol Cancer. 2015;14:179. doi: 10.1186/s12943-015-0449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18(16):4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang C, Shi S, Meng Q, Liang D, Ji S, Zhang B, Qin Y, Xu J, Ni Q, Yu X. Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: where we are and where we are going. Exp Mol Med. 2017;49(12):e406. doi: 10.1038/emm.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oettle H. Progress in the knowledge and treatment of advanced pancreatic cancer: from benchside to bedside. Cancer Treat Rev. 2014;40(9):1039–1047. doi: 10.1016/j.ctrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Fu Y, Liu S, Zeng S, Shen H. The critical roles of activated stellate cells-mediated paracrine signaling, metabolism and onco-immunology in pancreatic ductal adenocarcinoma. Mol Cancer. 2018;17(1):62. doi: 10.1186/s12943-018-0815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sunami Y, Haussler J, Kleeff J. Cellular Heterogeneity of Pancreatic Stellate Cells, Mesenchymal Stem Cells, and Cancer-Associated Fibroblasts in Pancreatic Cancer. Cancers (Basel) 2020 doi: 10.3390/cancers12123770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, Kimmelman AC. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536(7617):479–483. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang CI, Liao JC, Kuo L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res. 2001;61(3):1100–1106. [PubMed] [Google Scholar]
- 60.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang Y, Miao H. Lipid Metabolism in Tumor-Associated Macrophages. Adv Exp Med Biol. 2021;1316:87–101. doi: 10.1007/978-981-33-6785-2_6. [DOI] [PubMed] [Google Scholar]
- 63.Amit M, Gil Z. Macrophages increase the resistance of pancreatic adenocarcinoma cells to gemcitabine by upregulating cytidine deaminase. Oncoimmunology. 2013;2(12):e27231. doi: 10.4161/onci.27231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dangi-Garimella S, Krantz SB, Barron MR, Shields MA, Heiferman MJ, Grippo PJ, Bentrem DJ, Munshi HG. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 2011;71(3):1019–1028. doi: 10.1158/0008-5472.CAN-10-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kikuta K, Masamune A, Watanabe T, Ariga H, Itoh H, Hamada S, Satoh K, Egawa S, Unno M, Shimosegawa T. Pancreatic stellate cells promote epithelial–mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403(3–4):380–384. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 66.Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009;284(39):26533–26546. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41(3):211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 70.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, DePinho RA. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149(3):656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basturk O, Singh R, Kaygusuz E, Balci S, Dursun N, Culhaci N, Adsay NV. GLUT-1 expression in pancreatic neoplasia: implications in pathogenesis, diagnosis, and prognosis. Pancreas. 2011;40(2):187–192. doi: 10.1097/MPA.0b013e318201c935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurahara H, Maemura K, Mataki Y, Sakoda M, Iino S, Kawasaki Y, Arigami T, Mori S, Kijima Y, Ueno S, Shinchi H, Natsugoe S. Significance of glucose transporter Type 1 (GLUT-1) expression in the therapeutic strategy for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2018;25(5):1432–1439. doi: 10.1245/s10434-018-6357-1. [DOI] [PubMed] [Google Scholar]
- 73.Gou Q, Dong C, Jin J, Liu Q, Lu W, Shi J, Hou Y. PPARalpha agonist alleviates tumor growth and chemo-resistance associated with the inhibition of glucose metabolic pathway. Eur J Pharmacol. 2019;863:172664. doi: 10.1016/j.ejphar.2019.172664. [DOI] [PubMed] [Google Scholar]
- 74.Wang YD, Li SJ, Liao JX. Inhibition of glucose transporter 1 (GLUT1) chemosensitized head and neck cancer cells to cisplatin. Technol Cancer Res Treat. 2013;12(6):525–535. doi: 10.7785/tcrt.2012.500343. [DOI] [PubMed] [Google Scholar]
- 75.Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, Moatamed NA, Huang J, Koepsell H, Barrio JR, Wright EM. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci USA. 2015;112(30):E4111–4119. doi: 10.1073/pnas.1511698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varghese E, Samuel SM, Liskova A, Samec M, Kubatka P, Busselberg D. Targeting glucose metabolism to overcome resistance to anticancer chemotherapy in breast cancer. Cancers (Basel) 2020 doi: 10.3390/cancers12082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahn KJ, Hwang HS, Park JH, Bang SH, Kang WJ, Yun M, Lee JD. Evaluation of the role of hexokinase type II in cellular proliferation and apoptosis using human hepatocellular carcinoma cell lines. J Nucl Med. 2009;50(9):1525–1532. doi: 10.2967/jnumed.108.060780. [DOI] [PubMed] [Google Scholar]
- 78.Fan K, Fan Z, Cheng H, Huang Q, Yang C, Jin K, Luo G, Yu X, Liu C. Hexokinase 2 dimerization and interaction with voltage-dependent anion channel promoted resistance to cell apoptosis induced by gemcitabine in pancreatic cancer. Cancer Med. 2019;8(13):5903–5915. doi: 10.1002/cam4.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10(9):671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 80.Sato-Tadano A, Suzuki T, Amari M, Takagi K, Miki Y, Tamaki K, Watanabe M, Ishida T, Sasano H, Ohuchi N. Hexokinase II in breast carcinoma: a potent prognostic factor associated with hypoxia-inducible factor-1alpha and Ki-67. Cancer Sci. 2013;104(10):1380–1388. doi: 10.1111/cas.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai S, Peng Y, Zhu Y, Xu D, Zhu F, Xu W, Chen Q, Zhu X, Liu T, Hou C, Wu J, Miao Y. Glycolysis promotes the progression of pancreatic cancer and reduces cancer cell sensitivity to gemcitabine. Biomed Pharmacother. 2020;121:109521. doi: 10.1016/j.biopha.2019.109521. [DOI] [PubMed] [Google Scholar]
- 82.Zhao H, Duan Q, Zhang Z, Li H, Wu H, Shen Q, Wang C, Yin T. Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. J Cell Mol Med. 2017;21(9):2055–2067. doi: 10.1111/jcmm.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakano A, Tsuji D, Miki H, Cui Q, El Sayed SM, Ikegame A, Oda A, Amou H, Nakamura S, Harada T, Fujii S, Kagawa K, Takeuchi K, Sakai A, Ozaki S, Okano K, Nakamura T, Itoh K, Matsumoto T, Abe M. Glycolysis inhibition inactivates ABC transporters to restore drug sensitivity in malignant cells. PLoS ONE. 2011;6(11):e27222. doi: 10.1371/journal.pone.0027222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, Gao G, Zhang A, Xia X, Brasher H, Widger W, Ellis LM, Weihua Z. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72(1):304–314. doi: 10.1158/0008-5472.CAN-11-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chapiro J, Sur S, Savic LJ, Ganapathy-Kanniappan S, Reyes J, Duran R, Thiruganasambandam SC, Moats CR, Lin M, Luo W, Tran PT, Herman JM, Semenza GL, Ewald AJ, Vogelstein B, Geschwind JF. Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer. Clin Cancer Res. 2014;20(24):6406–6417. doi: 10.1158/1078-0432.CCR-14-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao H, Li S, Zhang D, Liu T, Yu M, Wang F. Separate and concurrent use of 2-deoxy-D-glucose and 3-bromopyruvate in pancreatic cancer cells. Oncol Rep. 2013;29(1):329–334. doi: 10.3892/or.2012.2085. [DOI] [PubMed] [Google Scholar]
- 87.Clem BF, O'Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA, 2nd, Klarer AC, Redman R, Miller DM, Trent JO, Telang S, Chesney J. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther. 2013;12(8):1461–1470. doi: 10.1158/1535-7163.MCT-13-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kotiah SD, Caro J. Elevation of PFKFB3 and TIGAR levels in pancreatic cancer. J Clin Oncol. 2010;28(15_suppl):e14679–e14679. doi: 10.1200/jco.2010.28.15_suppl.e14679. [DOI] [Google Scholar]
- 89.Richardson DA, Sritangos P, James AD, Sultan A, Bruce JIE. Metabolic regulation of calcium pumps in pancreatic cancer: role of phosphofructokinase-fructose-bisphosphatase-3 (PFKFB3) Cancer Metab. 2020;8:2. doi: 10.1186/s40170-020-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ozcan SC, Sarioglu A, Altunok TH, Akkoc A, Guzel S, Guler S, Imbert-Fernandez Y, Muchut RJ, Iglesias AA, Gurpinar Y, Clem AL, Chesney JA, Yalcin A. PFKFB2 regulates glycolysis and proliferation in pancreatic cancer cells. Mol Cell Biochem. 2020;470(1–2):115–129. doi: 10.1007/s11010-020-03751-5. [DOI] [PubMed] [Google Scholar]
- 91.He Y, Luo Y, Zhang D, Wang X, Zhang P, Li H, Ejaz S, Liang S. PGK1-mediated cancer progression and drug resistance. Am J Cancer Res. 2019;9(11):2280–2302. [PMC free article] [PubMed] [Google Scholar]
- 92.Daly EB, Wind T, Jiang XM, Sun L, Hogg PJ. Secretion of phosphoglycerate kinase from tumour cells is controlled by oxygen-sensing hydroxylases. Biochim Biophys Acta. 2004;1691(1):17–22. doi: 10.1016/j.bbamcr.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Ariosa AR, Klionsky DJ. A novel role for a glycolytic pathway kinase in regulating autophagy has implications in cancer therapy. Autophagy. 2017;13(7):1091–1092. doi: 10.1080/15548627.2017.1321723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou JW, Tang JJ, Sun W, Wang H. PGK1 facilities cisplatin chemoresistance by triggering HSP90/ERK pathway mediated DNA repair and methylation in endometrial endometrioid adenocarcinoma. Mol Med. 2019;25(1):11. doi: 10.1186/s10020-019-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang Z, Zhou L, Chen Z, Nice EC, Huang C. Stress management by autophagy: implications for chemoresistance. Int J Cancer. 2016;139(1):23–32. doi: 10.1002/ijc.29990. [DOI] [PubMed] [Google Scholar]
- 96.Sun R, Meng X, Pu Y, Sun F, Man Z, Zhang J, Yin L, Pu Y. Overexpression of HIF-1a could partially protect K562 cells from 1,4-benzoquinone induced toxicity by inhibiting ROS, apoptosis and enhancing glycolysis. Toxicol In Vitro. 2019;55:18–23. doi: 10.1016/j.tiv.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 97.Schneider CC, Archid R, Fischer N, Buhler S, Venturelli S, Berger A, Burkard M, Kirschniak A, Bachmann R, Konigsrainer A, Glatzle J, Zieker D. Metabolic alteration–overcoming therapy resistance in gastric cancer via PGK-1 inhibition in a combined therapy with standard chemotherapeutics. Int J Surg. 2015;22:92–98. doi: 10.1016/j.ijsu.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 98.Liang C, Shi S, Qin Y, Meng Q, Hua J, Hu Q, Ji S, Zhang B, Xu J, Yu X. Localisation of PGK1 determines metabolic phenotype to balance metastasis and proliferation in patients with SMAD4-negative pancreatic cancer. Gut. 2020;69(5):888–900. doi: 10.1136/gutjnl-2018-317163. [DOI] [PubMed] [Google Scholar]
- 99.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 100.Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, Simmet T, Seufferlein T. PKM2 promotes tumor angiogenesis by regulating HIF-1alpha through NF-kappaB activation. Mol Cancer. 2016;15:3. doi: 10.1186/s12943-015-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tamada M, Suematsu M, Saya H. Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res. 2012;18(20):5554–5561. doi: 10.1158/1078-0432.CCR-12-0859. [DOI] [PubMed] [Google Scholar]
- 102.Icard P, Fournel L, Wu Z, Alifano M, Lincet H. Interconnection between metabolism and cell cycle in cancer. Trends Biochem Sci. 2019;44(6):490–501. doi: 10.1016/j.tibs.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Kim DJ, Park YS, Kang MG, You YM, Jung Y, Koo H, Kim JA, Kim MJ, Hong SM, Lee KB, Jang JJ, Park KC, Yeom YI. Pyruvate kinase isoenzyme M2 is a therapeutic target of gemcitabine-resistant pancreatic cancer cells. Exp Cell Res. 2015;336(1):119–129. doi: 10.1016/j.yexcr.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 104.James AD, Richardson DA, Oh IW, Sritangos P, Attard T, Barrett L, Bruce JIE. Cutting off the fuel supply to calcium pumps in pancreatic cancer cells: role of pyruvate kinase-M2 (PKM2) Br J Cancer. 2020;122(2):266–278. doi: 10.1038/s41416-019-0675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su Q, Tao T, Tang L, Deng J, Darko KO, Zhou S, Peng M, He S, Zeng Q, Chen AF, Yang X. Down-regulation of PKM2 enhances anticancer efficiency of THP on bladder cancer. J Cell Mol Med. 2018;22(5):2774–2790. doi: 10.1111/jcmm.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang X, Zhang F, Wu XR. Inhibition of pyruvate kinase M2 markedly reduces chemoresistance of advanced bladder cancer to cisplatin. Sci Rep. 2017;7:45983. doi: 10.1038/srep45983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tian S, Li P, Sheng S, Jin X. Upregulation of pyruvate kinase M2 expression by fatty acid synthase contributes to gemcitabine resistance in pancreatic cancer. Oncol Lett. 2018;15(2):2211–2217. doi: 10.3892/ol.2017.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu L, Teoh ST, Ensink E, Ogrodzinski MP, Yang C, Vazquez AI, Lunt SY. Cysteine catabolism and the serine biosynthesis pathway support pyruvate production during pyruvate kinase knockdown in pancreatic cancer cells. Cancer Metab. 2019;7:13. doi: 10.1186/s40170-019-0205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mohammad GH, Olde Damink SW, Malago M, Dhar DK, Pereira SP. Pyruvate kinase M2 and lactate dehydrogenase A are overexpressed in pancreatic cancer and correlate with poor outcome. PLoS ONE. 2016;11(3):e0151635. doi: 10.1371/journal.pone.0151635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu Y, Deck JA, Hunsaker LA, Deck LM, Royer RE, Goldberg E, Vander Jagt DL. Selective active site inhibitors of human lactate dehydrogenases A4, B4, and C4. Biochem Pharmacol. 2001;62(1):81–89. doi: 10.1016/s0006-2952(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 111.Li S, Gao J, Zhuang X, Zhao C, Hou X, Xing X, Chen C, Liu Q, Liu S, Luo Y. Cyclin G2 inhibits the warburg effect and tumour progression by suppressing LDHA phosphorylation in glioma. Int J Biol Sci. 2019;15(3):544–555. doi: 10.7150/ijbs.30297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohammad GH, Vassileva V, Acedo P, Olde Damink SWM, Malago M, Dhar DK, Pereira SP. Targeting pyruvate kinase M2 and lactate dehydrogenase A is an effective combination strategy for the treatment of pancreatic cancer. Cancers (Basel) 2019 doi: 10.3390/cancers11091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Z, Zhou M, Zhao Y, Yan D, Liu H, Tan M. Overcoming chemotherapy resistance by targeting LDHA mediated glycolysis. Cancer Res. 2011 doi: 10.1158/1538-7445.Am2011-2130. [DOI] [Google Scholar]
- 114.Maftouh M, Avan A, Sciarrillo R, Granchi C, Leon LG, Rani R, Funel N, Smid K, Honeywell R, Boggi U, Minutolo F, Peters GJ, Giovannetti E. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110(1):172–182. doi: 10.1038/bjc.2013.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Giovannetti E, Leon LG, Gomez VE, Zucali PA, Minutolo F, Peters GJ. A specific inhibitor of lactate dehydrogenase overcame the resistance toward gemcitabine in hypoxic mesothelioma cells, and modulated the expression of the human equilibrative transporter-1. Nucleosides Nucleotides Nucleic Acids. 2016;35(10–12):643–651. doi: 10.1080/15257770.2016.1149193. [DOI] [PubMed] [Google Scholar]
- 116.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McDonald PC, Chafe SC, Dedhar S. Overcoming hypoxia-mediated tumor progression: combinatorial approaches targeting pH regulation, angiogenesis and immune dysfunction. Front Cell Dev Biol. 2016;4:27. doi: 10.3389/fcell.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Puri S, Juvale K. Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: A review with structure–activity relationship insights. Eur J Med Chem. 2020;199:112393. doi: 10.1016/j.ejmech.2020.112393. [DOI] [PubMed] [Google Scholar]
- 119.Kong SC, Nohr-Nielsen A, Zeeberg K, Reshkin SJ, Hoffmann EK, Novak I, Pedersen SF. Monocarboxylate Transporters MCT1 and MCT4 regulate migration and invasion of pancreatic ductal adenocarcinoma cells. Pancreas. 2016;45(7):1036–1047. doi: 10.1097/MPA.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 120.Le Floch R, Chiche J, Marchiq I, Naiken T, Ilc K, Murray CM, Critchlow SE, Roux D, Simon MP, Pouyssegur J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci USA. 2011;108(40):16663–16668. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Afonso J, Santos LL, Miranda-Goncalves V, Morais A, Amaro T, Longatto-Filho A, Baltazar F. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol Carcinog. 2015;54(11):1451–1466. doi: 10.1002/mc.22222. [DOI] [PubMed] [Google Scholar]
- 122.Kuang Y, Wang S, Tang L, Hai J, Yan G, Liao L. Cluster of differentiation 147 mediates chemoresistance in breast cancer by affecting vacuolar H(+)-ATPase expression and activity. Oncol Lett. 2018;15(5):7279–7290. doi: 10.3892/ol.2018.8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou S, Liao L, Chen C, Zeng W, Liu S, Su J, Zhao S, Chen M, Kuang Y, Chen X, Li J. CD147 mediates chemoresistance in breast cancer via ABCG2 by affecting its cellular localization and dimerization. Cancer Lett. 2013;337(2):285–292. doi: 10.1016/j.canlet.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 124.Schneiderhan W, Scheler M, Holzmann KH, Marx M, Gschwend JE, Bucholz M, Gress TM, Seufferlein T, Adler G, Oswald F. CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut. 2009;58(10):1391–1398. doi: 10.1136/gut.2009.181412. [DOI] [PubMed] [Google Scholar]
- 125.Kuang YH, Chen X, Su J, Wu LS, Liao LQ, Li D, Chen ZS, Kanekura T. RNA interference targeting the CD147 induces apoptosis of multi-drug resistant cancer cells related to XIAP depletion. Cancer Lett. 2009;276(2):189–195. doi: 10.1016/j.canlet.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 126.Kang MJ, Kim HP, Lee KS, Yoo YD, Kwon YT, Kim KM, Kim TY, Yi EC. Proteomic analysis reveals that CD147/EMMPRIN confers chemoresistance in cancer stem cell-like cells. Proteomics. 2013;13(10–11):1714–1725. doi: 10.1002/pmic.201200511. [DOI] [PubMed] [Google Scholar]
- 127.Zhou Y, Zheng M, Liu Z, Yang H, Zhu P, Jiang JL, Tang J, Chen ZN. CD147 promotes DNA damage response and gemcitabine resistance via targeting ATM/ATR/p53 and affects prognosis in pancreatic cancer. Biochem Biophys Res Commun. 2020;528(1):62–70. doi: 10.1016/j.bbrc.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 128.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39(8):347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Al Hanjori AS, Alshaer W, Anati B, Wehaibi S, Zihlif M. Studying Antitumor effects of sirna gene silencing of some metabolic genes in pancreatic ductal adenocarcinoma. Curr Mol Pharmacol. 2020 doi: 10.2174/1874467213666201012162250. [DOI] [PubMed] [Google Scholar]
- 130.Chen H, Wu D, Bao L, Yin T, Lei D, Yu J, Tong X. 6PGD inhibition sensitizes hepatocellular carcinoma to chemotherapy via AMPK activation and metabolic reprogramming. Biomed Pharmacother. 2019;111:1353–1358. doi: 10.1016/j.biopha.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 131.Li Q, Qin T, Bi Z, Hong H, Ding L, Chen J, Wu W, Lin X, Fu W, Zheng F, Yao Y, Luo ML, Saw PE, Wulf GM, Xu X, Song E, Yao H, Hu H. Rac1 activates non-oxidative pentose phosphate pathway to induce chemoresistance of breast cancer. Nat Commun. 2020;11(1):1456. doi: 10.1038/s41467-020-15308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin R, Elf S, Shan C, Kang HB, Ji Q, Zhou L, Hitosugi T, Zhang L, Zhang S, Seo JH, Xie J, Tucker M, Gu TL, Sudderth J, Jiang L, Mitsche M, DeBerardinis RJ, Wu S, Li Y, Mao H, Chen PR, Wang D, Chen GZ, Hurwitz SJ, Lonial S, Arellano ML, Khoury HJ, Khuri FR, Lee BH, Lei Q, Brat DJ, Ye K, Boggon TJ, He C, Kang S, Fan J, Chen J. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nat Cell Biol. 2015;17(11):1484–1496. doi: 10.1038/ncb3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giacomini I, Ragazzi E, Pasut G, Montopoli M. The Pentose Phosphate Pathway and Its Involvement in Cisplatin Resistance. Int J Mol Sci. 2020 doi: 10.3390/ijms21030937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Preuss J, Richardson AD, Pinkerton A, Hedrick M, Sergienko E, Rahlfs S, Becker K, Bode L. Identification and characterization of novel human glucose-6-phosphate dehydrogenase inhibitors. J Biomol Screen. 2013;18(3):286–297. doi: 10.1177/1087057112462131. [DOI] [PubMed] [Google Scholar]
- 135.Sharma N, Bhushan A, He J, Kaushal G, Bhardwaj V. Metabolic plasticity imparts erlotinib-resistance in pancreatic cancer by upregulating glucose-6-phosphate dehydrogenase. Cancer Metab. 2020;8:19. doi: 10.1186/s40170-020-00226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yin X, Tang B, Li JH, Wang Y, Zhang L, Xie XY, Zhang BH, Qiu SJ, Wu WZ, Ren ZG. ID1 promotes hepatocellular carcinoma proliferation and confers chemoresistance to oxaliplatin by activating pentose phosphate pathway. J Exp Clin Cancer Res. 2017;36(1):166. doi: 10.1186/s13046-017-0637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang X, Peng X, Huang J. Inhibiting 6-phosphogluconate dehydrogenase selectively targets breast cancer through AMPK activation. Clin Transl Oncol. 2018;20(9):1145–1152. doi: 10.1007/s12094-018-1833-4. [DOI] [PubMed] [Google Scholar]
- 138.Bechard ME, Word AE, Tran AV, Liu X, Locasale JW, McDonald OG. Pentose conversions support the tumorigenesis of pancreatic cancer distant metastases. Oncogene. 2018;37(38):5248–5256. doi: 10.1038/s41388-018-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53(3):421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 140.Friesen C, Kiess Y, Debatin KM. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S73–85. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- 141.Recktenwald CV, Kellner R, Lichtenfels R, Seliger B. Altered detoxification status and increased resistance to oxidative stress by K-ras transformation. Cancer Res. 2008;68(24):10086–10093. doi: 10.1158/0008-5472.CAN-08-0360. [DOI] [PubMed] [Google Scholar]
- 142.Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. Reactive oxygen species and cancer paradox: to promote or to suppress? Free Radic Biol Med. 2017;104:144–164. doi: 10.1016/j.freeradbiomed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 143.Akella NM, Ciraku L, Reginato MJ. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019;17(1):52. doi: 10.1186/s12915-019-0671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 145.Liu Y, Cao Y, Pan X, Shi M, Wu Q, Huang T, Jiang H, Li W, Zhang J. O-GlcNAc elevation through activation of the hexosamine biosynthetic pathway enhances cancer cell chemoresistance. Cell Death Dis. 2018;9(5):485. doi: 10.1038/s41419-018-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem. 2013;288(21):15121–15130. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen W, Do KC, Saxton B, Leng S, Filipczak P, Tessema M, Belinsky SA, Lin Y. Inhibition of the hexosamine biosynthesis pathway potentiates cisplatin cytotoxicity by decreasing BiP expression in non-small-cell lung cancer cells. Mol Carcinog. 2019;58(6):1046–1055. doi: 10.1002/mc.22992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Luanpitpong S, Angsutararux P, Samart P, Chanthra N, Chanvorachote P, Issaragrisil S. Hyper-O-GlcNAcylation induces cisplatin resistance via regulation of p53 and c-Myc in human lung carcinoma. Sci Rep. 2017;7(1):10607. doi: 10.1038/s41598-017-10886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guerra F, Arbini AA, Moro L. Mitochondria and cancer chemoresistance. Biochim Biophys Acta Bioenerg. 2017;1858(8):686–699. doi: 10.1016/j.bbabio.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 150.Vyas S, Zaganjor E, Haigis MC. Mitochondria and cancer. Cell. 2016;166(3):555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Falasca M, Kim M, Casari I. Pancreatic cancer: current research and future directions. Biochim Biophys Acta. 2016;1865(2):123–132. doi: 10.1016/j.bbcan.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 152.Valle S, Alcala S, Martin-Hijano L, Cabezas-Sainz P, Navarro D, Munoz ER, Yuste L, Tiwary K, Walter K, Ruiz-Canas L, Alonso-Nocelo M, Rubiolo JA, Gonzalez-Arnay E, Heeschen C, Garcia-Bermejo L, Hermann PC, Sanchez L, Sancho P, Fernandez-Moreno MA, Sainz B., Jr Exploiting oxidative phosphorylation to promote the stem and immunoevasive properties of pancreatic cancer stem cells. Nat Commun. 2020;11(1):5265. doi: 10.1038/s41467-020-18954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, DePinho RA, Draetta GF. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rademaker G, Hennequiere V, Brohee L, Nokin MJ, Lovinfosse P, Durieux F, Gofflot S, Bellier J, Costanza B, Herfs M, Peiffer R, Bettendorff L, Deroanne C, Thiry M, Delvenne P, Hustinx R, Bellahcene A, Castronovo V, Peulen O. Myoferlin controls mitochondrial structure and activity in pancreatic ductal adenocarcinoma, and affects tumor aggressiveness. Oncogene. 2018;37(32):4398–4412. doi: 10.1038/s41388-018-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Z, Dong C. Gluconeogenesis in cancer: function and regulation of PEPCK, FBPase, and G6Pase. Trends Cancer. 2019;5(1):30–45. doi: 10.1016/j.trecan.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 156.Jin X, Pan Y, Wang L, Ma T, Zhang L, Tang AH, Billadeau DD, Wu H, Huang H. Fructose-1,6-bisphosphatase Inhibits ERK activation and bypasses gemcitabine resistance in pancreatic cancer by blocking IQGAP1-MAPK interaction. Cancer Res. 2017;77(16):4328–4341. doi: 10.1158/0008-5472.CAN-16-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang C, Zhu S, Yang H, Fan P, Meng Z, Zhao J, Zhang K, Jin X. FBP1 binds to the bromodomain of BRD4 to inhibit pancreatic cancer progression. Am J Cancer Res. 2020;10(2):523–535. [PMC free article] [PubMed] [Google Scholar]
- 158.Li B, Qiu B, Lee DS, Walton ZE, Ochocki JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I, Simon MC. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513(7517):251–255. doi: 10.1038/nature13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52(1):15–30. doi: 10.1038/s12276-020-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Coothankandaswamy V, Cao S, Xu Y, Prasad PD, Singh PK, Reynolds CP, Yang S, Ogura J, Ganapathy V, Bhutia YD. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br J Pharmacol. 2016;173(23):3292–3306. doi: 10.1111/bph.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cui H, Darmanin S, Natsuisaka M, Kondo T, Asaka M, Shindoh M, Higashino F, Hamuro J, Okada F, Kobayashi M, Nakagawa K, Koide H, Kobayashi M. Enhanced expression of asparagine synthetase under glucose-deprived conditions protects pancreatic cancer cells from apoptosis induced by glucose deprivation and cisplatin. Cancer Res. 2007;67(7):3345–3355. doi: 10.1158/0008-5472.CAN-06-2519. [DOI] [PubMed] [Google Scholar]
- 162.Biancur D, Kimmelman A. The plasticity of pancreatic cancer metabolism in tumor progression and therapeutic resistance. Biochim Biophys Acta Rev Cancer. 2018;1870(1):67–75. doi: 10.1016/j.bbcan.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497(7451):633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, Bardeesy N, Asara JM, Haigis MC, DePinho RA, Cantley LC, Kimmelman AC. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen R, Lai LA, Sullivan Y, Wong M, Wang L, Riddell J, Jung L, Pillarisetty VG, Brentnall TA, Pan S. Disrupting glutamine metabolic pathways to sensitize gemcitabine-resistant pancreatic cancer. Sci Rep. 2017;7(1):7950. doi: 10.1038/s41598-017-08436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sivanand S, Vander Heiden MG. Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell. 2020;37(2):147–156. doi: 10.1016/j.ccell.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Katagiri R, Goto A, Nakagawa T, Nishiumi S, Kobayashi T, Hidaka A, Budhathoki S, Yamaji T, Sawada N, Shimazu T, Inoue M, Iwasaki M, Yoshida M, Tsugane S. Increased levels of branched-chain amino acid associated with increased risk of pancreatic cancer in a prospective case-control study of a large cohort. Gastroenterology. 2018;155(5):1474–1482 e1471. doi: 10.1053/j.gastro.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 168.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS, Davidson SM, Papagiannakopoulos T, Yang A, Dayton TL, Ogino S, Stampfer MJ, Giovannucci EL, Qian ZR, Rubinson DA, Ma J, Sesso HD, Gaziano JM, Cochrane BB, Liu S, Wactawski-Wende J, Manson JE, Pollak MN, Kimmelman AC, Souza A, Pierce K, Wang TJ, Gerszten RE, Fuchs CS, Vander Heiden MG, Wolpin BM. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20(10):1193–1198. doi: 10.1038/nm.3686s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Krall AS, Xu S, Graeber TG, Braas D, Christofk HR. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun. 2016;7:11457. doi: 10.1038/ncomms11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gwinn DM, Lee AG, Briones-Martin-Del-Campo M, Conn CS, Simpson DR, Scott AI, Le A, Cowan TM, Ruggero D, Sweet-Cordero EA. Oncogenic KRAS regulates amino acid homeostasis and asparagine biosynthesis via ATF4 and alters sensitivity to l-asparaginase. Cancer Cell. 2018;33(1):91–107 e106. doi: 10.1016/j.ccell.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yu Q, Wang X, Wang L, Zheng J, Wang J, Wang B. Knockdown of asparagine synthetase (ASNS) suppresses cell proliferation and inhibits tumor growth in gastric cancer cells. Scand J Gastroenterol. 2016;51(10):1220–1226. doi: 10.1080/00365521.2016.1190399. [DOI] [PubMed] [Google Scholar]
- 172.Panosyan EH, Wang Y, Xia P, Lee WN, Pak Y, Laks DR, Lin HJ, Moore TB, Cloughesy TF, Kornblum HI, Lasky JL., 3rd Asparagine depletion potentiates the cytotoxic effect of chemotherapy against brain tumors. Mol Cancer Res. 2014;12(5):694–702. doi: 10.1158/1541-7786.MCR-13-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tang L, Zeng J, Geng P, Fang C, Wang Y, Sun M, Wang C, Wang J, Yin P, Hu C, Guo L, Yu J, Gao P, Li E, Zhuang Z, Xu G, Liu Y. Global metabolic profiling identifies a pivotal role of proline and hydroxyproline metabolism in supporting hypoxic response in hepatocellular carcinoma. Clin Cancer Res. 2018;24(2):474–485. doi: 10.1158/1078-0432.CCR-17-1707. [DOI] [PubMed] [Google Scholar]
- 174.Olivares O, Mayers JR, Gouirand V, Torrence ME, Gicquel T, Borge L, Lac S, Roques J, Lavaut MN, Berthezene P, Rubis M, Secq V, Garcia S, Moutardier V, Lombardo D, Iovanna JL, Tomasini R, Guillaumond F, Vander Heiden MG, Vasseur S. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun. 2017;8:16031. doi: 10.1038/ncomms16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Petan T, Jarc E, Jusovic M. Lipid droplets in cancer: guardians of fat in a stressful world. Mol. 2018 doi: 10.3390/molecules23081941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qiu B, Ackerman D, Sanchez DJ, Li B, Ochocki JD, Grazioli A, Bobrovnikova-Marjon E, Diehl JA, Keith B, Simon MC. HIF2alpha-dependent lipid storage promotes endoplasmic reticulum homeostasis in clear-cell renal cell carcinoma. Cancer Discov. 2015;5(6):652–667. doi: 10.1158/2159-8290.CD-14-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cotte AK, Aires V, Fredon M, Limagne E, Derangere V, Thibaudin M, Humblin E, Scagliarini A, de Barros JP, Hillon P, Ghiringhelli F, Delmas D. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat Commun. 2018;9(1):322. doi: 10.1038/s41467-017-02732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet O, Pinault M, Guimaraes C, Nigri J, Loncle C, Lavaut MN, Garcia S, Tailleux A, Staels B, Calvo E, Tomasini R, Iovanna JL, Vasseur S. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2015;112(8):2473–2478. doi: 10.1073/pnas.1421601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Swierczynski J, Hebanowska A, Sledzinski T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J Gastroenterol. 2014;20(9):2279–2303. doi: 10.3748/wjg.v20.i9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Edmunds LR, Sharma L, Kang A, Lu J, Vockley J, Basu S, Uppala R, Goetzman ES, Beck ME, Scott D, Prochownik EV. c-Myc programs fatty acid metabolism and dictates acetyl-CoA abundance and fate. J Biol Chem. 2014;289(36):25382–25392. doi: 10.1074/jbc.M114.580662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alo PL, Amini M, Piro F, Pizzuti L, Sebastiani V, Botti C, Murari R, Zotti G, Di Tondo U. Immunohistochemical expression and prognostic significance of fatty acid synthase in pancreatic carcinoma. Anticancer Res. 2007;27(4B):2523–2527. [PubMed] [Google Scholar]
- 183.Tadros S, Shukla SK, King RJ, Gunda V, Vernucci E, Abrego J, Chaika NV, Yu F, Lazenby AJ, Berim L, Grem J, Sasson AR, Singh PK. De novo lipid synthesis facilitates gemcitabine resistance through endoplasmic reticulum stress in pancreatic cancer. Cancer Res. 2017;77(20):5503–5517. doi: 10.1158/0008-5472.CAN-16-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bauerschlag DO, Maass N, Leonhardt P, Verburg FA, Pecks U, Zeppernick F, Morgenroth A, Mottaghy FM, Tolba R, Meinhold-Heerlein I, Brautigam K. Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer. J Transl Med. 2015;13:146. doi: 10.1186/s12967-015-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sun Y, He W, Luo M, Zhou Y, Chang G, Ren W, Wu K, Li X, Shen J, Zhao X, Hu Y. SREBP1 regulates tumorigenesis and prognosis of pancreatic cancer through targeting lipid metabolism. Tumour Biol. 2015;36(6):4133–4141. doi: 10.1007/s13277-015-3047-5. [DOI] [PubMed] [Google Scholar]
- 186.Wang X, Xie J, Lu X, Li H, Wen C, Huo Z, Xie J, Shi M, Tang X, Chen H, Peng C, Fang Y, Deng X, Shen B. Melittin inhibits tumor growth and decreases resistance to gemcitabine by downregulating cholesterol pathway gene CLU in pancreatic ductal adenocarcinoma. Cancer Lett. 2017;399:1–9. doi: 10.1016/j.canlet.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 187.Li J, Gu D, Lee SS, Song B, Bandyopadhyay S, Chen S, Konieczny SF, Ratliff TL, Liu X, Xie J, Cheng JX. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene. 2016;35(50):6378–6388. doi: 10.1038/onc.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li J, Qu X, Tian J, Zhang JT, Cheng JX. Cholesterol esterification inhibition and gemcitabine synergistically suppress pancreatic ductal adenocarcinoma proliferation. PLoS ONE. 2018;13(2):e0193318. doi: 10.1371/journal.pone.0193318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jin H, He Y, Zhao P, Hu Y, Tao J, Chen J, Huang Y. Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin beta3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics. 2019;9(1):265–278. doi: 10.7150/thno.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang J, Zaman MM, Vlasakov I, Roy R, Huang L, Martin CR, Freedman SD, Serhan CN, Moses MA. Adipocytes promote ovarian cancer chemoresistance. Sci Rep. 2019;9(1):13316. doi: 10.1038/s41598-019-49649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, Somlo G, Jandial R, Ann D, Hanash S, Jove R, Yu H. JAK/STAT3-Regulated Fatty Acid beta-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018;27(1):136–150 e135. doi: 10.1016/j.cmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13(4):227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shin SC, Thomas D, Radhakrishnan P, Hollingsworth MA. Invasive phenotype induced by low extracellular pH requires mitochondria dependent metabolic flexibility. Biochem Biophys Res Commun. 2020 doi: 10.1016/j.bbrc.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Meyer KA, Neeley CK, Baker NA, Washabaugh AR, Flesher CG, Nelson BS, Frankel TL, Lumeng CN, Lyssiotis CA, Wynn ML, Rhim AD, O'Rourke RW. Adipocytes promote pancreatic cancer cell proliferation via glutamine transfer. Biochem Biophys Rep. 2016;7:144–149. doi: 10.1016/j.bbrep.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bronte V, Tortora G. Adipocytes and neutrophils give a helping hand to pancreatic cancers. Cancer Discov. 2016;6(8):821–823. doi: 10.1158/2159-8290.CD-16-0682. [DOI] [PubMed] [Google Scholar]
- 196.Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu W, Liu J, Xiang J, Liang D, Hu Q, Liu L, Liu C, Luo G, Ni Q, Xu J, Yu X. Energy sources identify metabolic phenotypes in pancreatic cancer. Acta Biochim Biophys Sin (Shanghai) 2016;48(11):969–979. doi: 10.1093/abbs/gmw097. [DOI] [PubMed] [Google Scholar]