Abstract
Objective
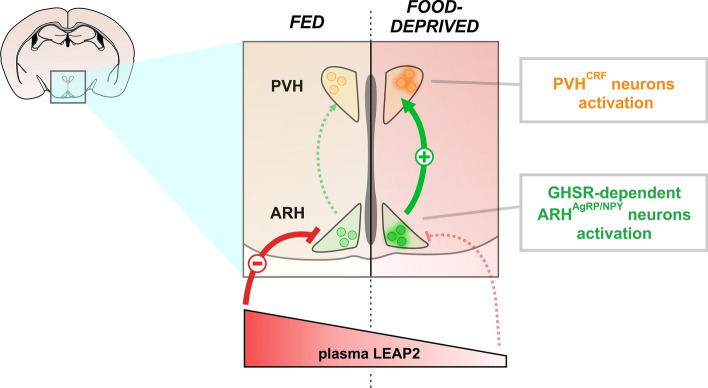
Prolonged fasting is a major challenge for living organisms. An appropriate metabolic response to food deprivation requires the activation of the corticotropin-releasing factor-producing neurons of the hypothalamic paraventricular nucleus (PVHCRF neurons), which are a part of the hypothalamic–pituitary–adrenal axis (HPA), as well as the growth hormone secretagogue receptor (GHSR) signaling, whose activity is up- or down-regulated, respectively, by the hormones ghrelin and the liver-expressed antimicrobial peptide 2 (LEAP2).
Since ghrelin treatment potently up-regulates the HPA axis, we studied the role of GHSR in mediating food deprivation-induced activation of the PVHCRF neurons in mice.
Methods
We estimated the activation of the PVHCRF neurons, using immuno-staining against CRF and the marker of neuronal activation c-Fos in brain sections, and assessed plasma levels of corticosterone and glucose in different pharmacologically or genetically manipulated mouse models exposed, or not, to a 2-day food deprivation protocol. In particular, we investigated ad libitum fed or food-deprived male mice that: (1) lacked GHSR gene expression, (2) had genetic deletion of the ghrelin gene, (3) displayed neurotoxic ablation of the hypothalamic arcuate nucleus, (4) were centrally treated with an anti-ghrelin antibody to block central ghrelin action, (5) were centrally treated with a GHSR ligand that blocks ghrelin-evoked and constitutive GHSR activities, or (6) received a continuous systemic infusion of LEAP2(1–12).
Results
We found that food deprivation results in the activation of the PVHCRF neurons and in a rise of the ghrelin/LEAP2 molar ratio. Food deprivation-induced activation of PVHCRF neurons required the presence and the signaling of GHSR at hypothalamic level, but not of ghrelin. Finally, we found that preventing the food deprivation-induced fall of LEAP2 reverses the activation of the PVHCRF neurons in food-deprived mice, although it has no effect on body weight or blood glucose.
Conclusion
Food deprivation-induced activation of the PVHCRF neurons involves ghrelin-independent actions of GHSR at hypothalamic level and requires a decrease of plasma LEAP2 levels. We propose that the up-regulation of the actions of GHSR associated to the fall of plasma LEAP2 level are physiologically relevant neuroendocrine signals during a prolonged fasting.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04302-5.
Keywords: Ghrelin, Constitutive GHSR activity, CRH neurons
Introduction
Fasting refers to the metabolic condition in which an organism is completely deprived of food but has ad libitum access to water. To cope with food deprivation, several neuroendocrine systems are engaged and coordinately act to assure animal survival. The neuroendocrine responses recruited during fasting include the activation of the hypothalamic–pituitary–adrenal (HPA) axis, which comprises the hypo-physiotropic neurons of the paraventricular nucleus of the hypothalamus (PVH) that produce corticotropin-releasing factor (CRF), hereafter named PVHCRF neurons [1]. Upon activation, PVHCRF neurons secrete CRF into the median eminence that, in turn, stimulates the secretion of adrenocorticotropic hormone (ACTH) from corticotrope cells of the pituitary. ACTH acts on the adrenal cortex and releases glucocorticoids, which display a myriad of important effects that include the upregulation of mechanisms that tend to increase blood glucose (for which they were named for). The activation of the HPA axis is essential to maintain blood glucose within life compatible limits under food deprivation conditions. Indeed, CRF-deficient mice fail to increase plasma glucocorticoid levels and show severe hypoglycemia in response to prolonged food deprivation [2]. Notably, the mechanisms controlling food deprivation-induced activation of PVHCRF neurons are poorly known.
An appropriate response to food deprivation also involves the growth hormone secretagogue receptor (GHSR). GHSR is a G protein-coupled receptor highly expressed in the hypothalamus and the pituitary, and that acts as the unique known receptor for the stomach-derived hormone ghrelin [3, 4]. Plasma ghrelin levels increase under fasting [1, 5], and ghrelin administration increases food intake, drives food-seeking behaviors and promotes a variety of hyperglycemic mechanisms, including secretion of growth hormone (GH) and release of glucocorticoids [4, 6, 7]. Importantly, GHSR also displays ghrelin-independent actions that include an unusually high constitutive activity and its capacity to modulate other G protein-coupled receptors [8]. Such ligand-independent actions of GHSR seem to gain more relevance under food deprivation, when ghsr gene expression increases in the hypothalamic arcuate nucleus (ARH) [5, 9, 10]. In the ARH, GHSR is enriched in neurons expressing agouti-related protein and neuropeptide Y (ARHAgRP/NPY neurons), which are key mediators of ghrelin’s actions [9, 11–15]. Notably, fasted GHSR-deficient mice exhibit lower blood glucose than fasted wild-type (WT) mice [16], whereas ghrelin-KO mice display similar fasting-induced hypoglycemia than WT mice [17]. Also, the compensatory hyperphagia that follows a period of fasting is reduced in GHSR-deficient mice and in mice centrally treated with a GHSR ligand that blocks both ghrelin-evoked and constitutive GHSR activities, but not in ghrelin-KO mice [5]. Altogether this evidence indicates that ligand-independent actions of GHSR activity play significant metabolic and behavioral roles under food deprivation in mice.
Recently, a novel GHSR ligand was identified: the liver-expressed antimicrobial peptide 2 (LEAP2) [18]. LEAP2 is mainly produced in liver and jejunum [18], and its plasma levels decrease upon food deprivation. LEAP2 acts as a GHSR antagonist blocking ghrelin-evoked GHSR activity [18] as well as an inverse agonist blocking the constitutive GHSR activity [19, 20]. In vivo, LEAP2 treatment dose-dependently blocks the acute orexigenic and GH secretagogue effects of ghrelin treatment [18]. The role and the physiological implications of endogenous LEAP2 are just beginning to be explored. Ad libitum fed mice with viral-mediated over expression of LEAP2 do not show evident alterations of food intake, body weight or blood glucose [18]. Similarly, LEAP2-KO mice show similar food intake, body weight or blood glucose than WT mice in ad libitum fed or overnight fasting conditions [21]. Under fasting, however, the immunoneutralization of LEAP2 does enhance GH release in mice [18], and viral-mediated over expression of LEAP2 in calorie-restricted mice leads to more dramatic weight loss and severe hypoglycemia [18]. Thus, the fall of plasma LEAP2 levels in energy deficit conditions seems to contribute to regulate some neuroendocrine responses.
Ghrelin-induced up-regulation of the GHSR signaling stimulates the HPA axis. Indeed, ghrelin treatment potently increases glucocorticoid levels in rodents and in healthy individuals [6, 22]. In rodents, ghrelin-induced activation of the HPA axis mainly occurs at hypothalamic level via an indirect activation of the PVNCRF neurons, which lack GHSR expression [23]. Since the hypothalamic GHSR mRNA levels as well as plasma ghrelin levels increased in mice food-deprived for longer periods of time [1], we hypothesized that GHSR signaling could control the food deprivation-induced activation of the HPA axis. To test our hypothesis, we used mouse models with pharmacological or genetic manipulation of the GHSR system, and found evidence indicating that food deprivation-induced activation of the PVHCRF neurons involves ghrelin-independent upregulation of the actions of GHSR. Next, we reasoned that the food deprivation-induced fall in LEAP2 levels in plasma could contribute to upregulate the actions of GHSR and, consequently, activate the PVHCRF neurons. Thus, we performed continuous infusion of LEAP2(1−12) in food-deprived mice to investigate whether preventing the fall in LEAP2 levels could reverse the food deprivation-induced activation of the PVHCRF neurons and HPA axis.
Materials and methods
Mice
All studies were performed using 10–14-week-old male mice generated in the animal facility of either the Multidisciplinary Institute of Cell Biology (IMBICE, La Plata, Argentina) or the Center of Psychiatry and Neurosciences (CPN, INSERM UMR-S 894, Paris, France). Mouse models included: (1) C57BL/6 WT mice, (2) GHSR-deficient mice, which fail to express the GHSR [24], (3) ghrelin-KO mice, which lack the preproghrelin gene [25], and 4) ARH-ablated mice. GHSR-deficient and ghrelin-KO mice and their respective WT littermates were derived from crosses between heterozygous animals backcrossed for > 10 generations onto a C57BL/6 genetic background. Mice with ablation of the ARH were generated as previously described [26]. Briefly, 4-day-old WT mice were subcutaneously (SC)-treated with saline alone (ARH-intact mice) or containing monosodium glutamate (2.5 mg/g body weight (BW), Sigma-Aldrich, Cat# G1626, ARH-ablated mice). As shown in the past [13, 26, 27], ARH-ablated mice had a reduced number of thionin-stained cells in the ARH but not in other brain areas (such as the PVH or the area postrema) and a dramatic reduction of the number of neurons immunoreactive for NPY (~ 88%), tyrosine hydroxylase (– 80%) and proopiomelanocortin (– 60%), as compared to ARH-intact mice (not shown). Mice were maintained under controlled temperature (22 ± 1 °C) and photoperiod (12 h light/dark cycle from 6:00 h to 18:00 h) with regular chow diet and water available ad libitum, unless otherwise specified. At IMBICE (La Plata), chow was provided by Gepsa and contains 2.5 kcal/g of metabolizable energy (weight composition: 28.8% carbohydrates, 25.5% proteins, 3.6% fat, 27.4% fibers, 8.1% minerals and water 6.7%). At CPN (Paris), chow was provided by Safe A04 and contains 2.79 kcal/g of metabolizable energy (weight composition: 60% carbohydrate, 16% protein, 3% fat, 4% fibers). All protocols received approval from the Institutional Animal Care and Use Committee of the IMBICE (N°10-0112) and the Animal Experimentation Committee CEEA.34 of Paris Descartes University (N°03,422.02).
Food deprivation protocol and experimental groups
Mice were single-housed and ad libitum fed with chow diet. After 3 days, individually housed mice were maintained fed ad libitum or food-deprived for 2 days by removing the chow diet from the food hopper at 10:00 h; water was freely available. In all cases, body weight and food intake were daily monitored at 10:00 h. Experimental groups included: (1) fed (n = 6) or food-deprived (n = 6) WT mice, (2) fed (n = 7) or food-deprived (n = 9) GHSR-deficient mice and their fed (n = 7) or food-deprived (n = 9) WT littermates; (3) fed (n = 7) or food-deprived (n = 8) ARC-ablated mice and fed (n = 7) or food-deprived (n = 7) ARC-intact mice; and (4) fed (n = 4) or food-deprived (n = 5) ghrelin-KO mice and their fed (n = 4) or food-deprived (n = 5) WT littermates. Between 9:00 h and 11:00 h of the second day, mice were decapitated to collect their trunk blood and brains, which were placed overnight in formalin.
Central manipulations of the GHSR signaling in fed and food-deprived mice
Here, WT mice were first permanently implanted with a single indwelling intracerebroventricular (ICV) guide cannula (Plastics One) into the lateral ventricle using stereotaxic surgeries, as previously described [5]. Placement coordinates were anteroposterior: − 0.34 mm, mediolateral: + 1.00 mm and dorsoventral: − 2.30 mm. After surgery, mice were individually housed and allowed to recover for 5 days. During these days, mice were accustomed to handling by removal of the dummy cannula and connection to an empty cannula connector. Next, mice were either fed or food-deprived as indicated above. During the following two days, all mice received ICV injections every 8 h starting at 16:00 h of the first experimental day. Thus, each mouse received a total of six ICV injections. Between 9:00 h and 11:00 h of the second experimental day, mice were decapitated and their blood and brains were processed as described above. In all cases, the correct placement of the cannula was confirmed by histological observation at the end of the experiment.
In one set of experiments, fed and food-deprived WT mice were ICV-treated with 2 μL of artificial cerebrospinal fluid (CSF) alone (n = 4 and n = 6, respectively) or containing the GHSR inverse agonist K-(D-1-Nal)-FwLL-NH2 (1 nmol/mouse, n = 4 and n = 6 respectively). K-(D-1-Nal)-FwLL-NH2 was synthesized by automated solid-phase peptide synthesis as described elsewhere [28]. The dose was chosen based on our previous experience showing that it reduces ad libitum food intake in the early dark-phase period (from 18:00 h to 23:00 h), blocks the orexigenic effects of ICV-injected ghrelin and reduces the fasting‐induced compensatory hyperphagia in WT mice but not in GHSR-deficient mice [5].
In another set of experiments, fed and food-deprived WT mice were ICV-treated with 2 μL of artificial CSF alone (n = 4 and n = 6, respectively) or containing a chicken anti-ghrelin antibody (0.1 pmol/mouse, GeneTex, Cat# GTX-78202, n = 4 and n = 7, respectively). The dose of anti-ghrelin antibody was chosen based on previous studies which showed that it reduces food intake after a fasting event [29] and in response to systemically-injected ghrelin [30] in mice.
Continuous infusion of LEAP2(1−12) in fed and food-deprived mice
Here, mice were implanted with ALZET mini-osmotic pumps (Model# 1003D 3-day delivery at 1 µL/h rate, DURECT Corporation). The mini-pumps were weighed, aseptically filled with 100 µL of saline alone or containing LEAP2(1−12), and weighed again after filling to ensure adequate fill volume. LEAP2(1–12) was synthesized, purified and chemically characterized using mass spectrometry analyses, as described in detail in the past [19]. LEAP2(1–12) shows similar binding Ki in competition binding assays as well as similar EC50 and Emax in dose–response curves of inositol-1-phosphate production assays in GHSR-expressing HEK293T cells and similar capacity to abrogate ghrelin-induced food intake in mice as seen for full-length LEAP2 [19]. Mini-pumps were SC-implanted in the interscapular region of anesthetized WT mice 1 day before starting the food deprivation protocol. Experimental mice included: fed and food-deprived WT mice SC-infused with saline (n = 6 and n = 6, respectively) or containing LEAP2(1−12) (n = 6 and n = 6, respectively). LEAP2(1 − 12) concentration was 1.42 μg/μl, which corresponds to the dose of 1.36 μg/g/day (0.78 nmol/g/day) in mice weighing – 25 g. The dose was chosen to continuously reach – 16 ng/mL of LEAP2(1–12) in plasma, and was estimated using a constant-rate infusion model combined with a two-compartment model, which was fitted with the previously reported data for plasma LEAP2 clearance kinetics [18, 31, 32]. Between 9:00 h and 11:00 h of the second experimental day, mice were decapitated to collect their trunk blood and brains, which were placed overnight in formalin. After euthanasia, the implantation sites were checked to lack abnormalities, such as compound crystallization, local inflammation, or infection.
Assessments in blood samples
Blood samples were collected in tubes pre-treated with ethylenediaminetetraacetic acid (EDTA, 1 mg/mL final) and centrifuged at 5000 rpm for 10 min at 4 °C to obtain plasma. A fraction of plasma was separated immediately treated with the protease inhibitor p‐hydroxy‐mercuribenzoic acid (0.4 mM final) and acidified with HCl (0.1 M final) to preserve ghrelin acylation. All samples were stored frozen at − 80 °C until analysis. All untreated plasma samples were used to assess corticosterone levels using a specific radioimmunoassay as previously described [33] and glucose levels using a commercial enzymatic assay from Wiener Argentina. A sub-set of acidified plasma samples was used to assess ghrelin or LEAP2 using a specific sandwich enzyme-immunoassay from Bertin Pharma, (cat# A05117) or a competitive enzyme-immunoassay from Phoenix Pharmaceuticals (cat# EK-075-40), respectively, as previously reported [5, 30, 34]. Importantly, the commercial assay used to assess LEAP2 levels did not detect LEAP2(1–12) (unpublished observations).
Immunohistochemistry (IHC) in brain samples
After overnight fixation, brains were immersed overnight in 20% sucrose, frozen, and coronally cut at 40 µm into four equal series on a sliding cryostat. One series of brain sections was used for fluorescent IHC against CRF whereas another series was used for double chromogenic IHC against c-Fos and CRF. In the former case, brain sections were treated with blocking solution (3% normal donkey serum and 0.25% Triton X-100 in PBS) and then incubated with a rabbit anti-CRF antibody (1:1000) for 48 h at 4 °C. This antibody recognizes mature CRF(1–41) and full pre-proCRF prohormone, and consequently allows the visualization of the neuropeptide present in the cell body of the PVHCRF neurons [6]. Next, sections were incubated with a donkey anti-rabbit Alexa Fluor 594 antibody (1/1000; Thermo Fisher Cat# A-21207) for 2 h. Sections were washed, sequentially mounted on glass slides and cover-slipped with mounting media. Fluorescence images were acquired with 20x/0.80 and 40x/0.95 objectives using a Zeiss AxioObserver D1 equipped with an Apotome.2 structured illumination module and an AxioCam 506 monochrome camera. All image processing and analysis were performed in the ImageJ-based open-source image-processing package Fiji [35]. The average fluorescence CRF immunoreactive (denoted as CRF +) signal intensity was blindly and bilaterally quantified in the PVH in 20 × images between bregma − 0.58 and − 0.94 mm, using the neuroanatomical references of the mouse brain atlas [36]. The compact part of the PVH was identified using the atlas [36] and recognized beforehand by the distribution of cell nuclei labeled with Hoechst. For each image, the histogram of signal intensity was obtained, and the tissue background level was estimated by fitting a Gaussian curve, which coincided with the dominant peak. With these parameters, a specific signal detection threshold defined as the mean of the distribution plus five standard deviations was calculated. A region of interest was created according to this threshold. For each binarized image, the mean fluorescence CRF + intensity within the compact part of the PVH region, representing the average fluorescent signal per pixel, was obtained.
For double c-Fos and CRF IHC, brain sections were first treated with 0.5% H2O2, next treated with blocking solution and then incubated with a rabbit anti-c-Fos antibody (Oncogene, cat# PC38-100UL, 1:20.000) for 48 h at 4 °C. Next, sections were sequentially incubated with a biotinylated donkey anti-rabbit antibody (Jackson ImmunoResearch Laboratories, cat# 711-065-152, 1:4000), reagents of the Vectastain Elite ABC kit (Vector Laboratories Cat# PK-6200) and a DAB/nickel solution that generates a black nuclear precipitate in c-Fos + cells. Then, sections were incubated with the anti-CRF antibody (1:2000) for 48 h and sequentially incubated with the biotinylated donkey anti-rabbit antibody, reagents of the Vectastain Elite ABC kit, and a DAB solution without nickel, which generates a brown cytoplasmic precipitate in CRF + cells. Sections were sequentially mounted on glass slides and cover-slipped with mounting media. Bright-field color images were acquired with a Nikon Eclipse 50i and a DS-Ri1 Nikon digital camera with a 0.45 × adapter using a 10x /0.3 objective. All images were taken in comparable areas and under the same optical and light conditions. Here, all CRF + cells that were c-Fos + and c-Fos- were counted in the PVH. The results were expressed as a percentage, which represents the fraction of CRF + neurons that were also positive for c-Fos compared to the total number of CRF + neurons (% c-Fos + /CRF +). Data were corrected for double counting, according to the method of Abercrombie [37]. Blind quantitative analysis was performed independently by at least two observers in one series per animal.
For double c-Fos and AgRP IHC, brain sections were processed similarly as described for double c-Fos and CRF IHC. For fluorescent against AgRP, brain sections immuno-labeled for c-Fos were incubated with a rabbit anti-AgRP antibody (1/1000; Phoenix Pharmaceuticals Cat# H-003-57) for 48 h at 4 °C. Next, sections were incubated with a donkey anti-rabbit Alexa Fluor 594 antibody (1/1000; Thermo Fisher Cat# A-21207) for 2 h. Sections were processed as described above, and fluorescence images were acquired and analyzed as also described above. The average fluorescence AgRP immunoreactive (denoted as AgRP +) signal intensity as well as the fraction of AgRP + neurons that were also positive for c-Fos compared to the total number of AgRP + neurons (% of c-Fos + /AgRP +) were blindly and bilaterally quantified in the ARH in 20 × images between bregma − 1.58 and − 1.94 mm.
Quantification of GHSR mRNA levels
An independent set of fed (n = 5) and food-deprived (n = 6) WT mice were used to collect the ARH and PVH brain punches to assess the levels of GHSR mRNA using qRT-PCR, as we have done in the past [5]. Briefly, brains were removed after decapitation, placed in cold diethylpyrocarbonate-treated phosphate-buffered saline, and sectioned into 1 mm coronal slices by the use of a mouse brain matrix. Punches of tissue corresponding to the location of the ARH and the PVH, identified by comparing the coronal slices with a mouse brain atlas [36], were excised with a 1 mm micropuncher. Punches were collected in TRIzol reagent (Thermo Scientific, cat# 15,596,018), total RNA was isolated and reverse-transcribed into cDNA using random hexamer primers and MMLV reverse transcriptase (Thermo Scientific, cat# 28,025,021). Quantitative PCR for GHSR was performed in duplicate with HOT FIREPol EvaGreen qPCR Mix Plus (Solis BioDyne, cat# 08-24-00,001) using a StepOne Cycler (Applied Biosystems). Fold change from fed values was determined using the relative standard curve method, normalizing the expression to the ribosomal protein L19 (reference gene). Primers sequences for GHSR were sense: 5′-GCTCTGCAAACTCTTCCA-3′, antisense: 5′-AAGCAGATGGCGAAGTAG-3′ [GenBank Accession No. NM_177330.4], product size 99 bp. Primers sequences for ribosomal protein L19 (housekeeping) were sense: 5′-AGCCTGTGACTGTCCATTCC-3′, antisense: 5′-TGGCAGTACCCTTCCTCTTC-3′ [GenBank Accession No. NM_009078.2], product size 99 bp.
Statistical analyses
Data are expressed as the mean ± standard error of the mean (SEM). Normality was tested using the D’Agostino and Pearson’s test, and homogeneity of variances, using Bartlett’s test. When a normal distribution was found, data were compared with Student’s unpaired t tests or regular two-way ANOVA followed by Tukey’s post test, depending on the number of groups or variables in the experimental design. When a normal distribution was not found, data were logarithmic transformed prior to analysis. The statistical tests and post tests of each dataset are reported in the figure captions, as well as the statistical outcomes obtained in each case. Differences were considered significant when p < 0.05. Analyses were performed using GraphPad Prism, version 9.0 (GraphPad Software).
Results
PVHCRF neurons are activated in food-deprived mice
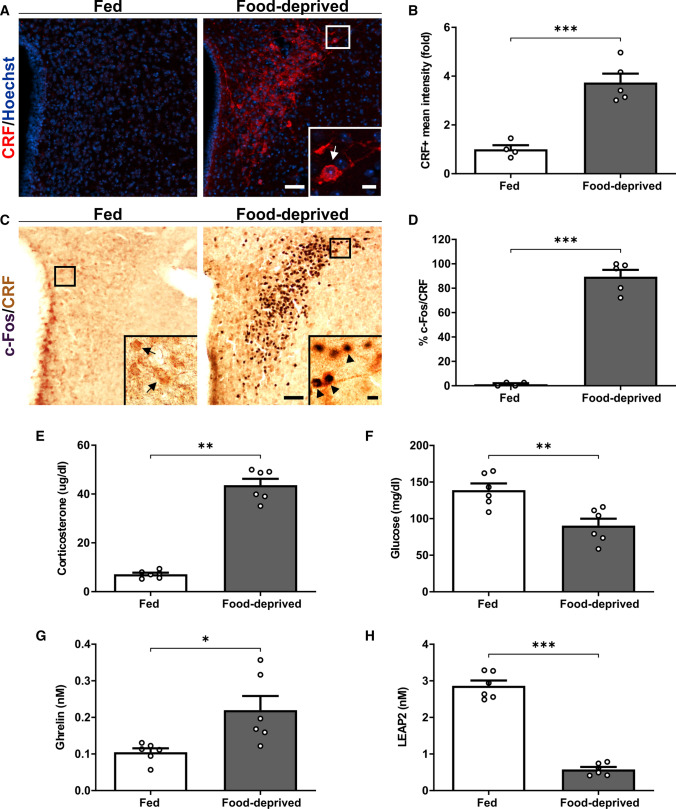
First, we performed an IHC against CRF to estimate the levels of this neuropeptide in the PVH of fed and food-deprived WT mice, and found that CRF + signal increased in the PVH of food-deprived mice, as compared to fed mice (Fig. 1A–B). To estimate if PVHCRF neurons are activated under food deprivation, we performed a double IHC against CRF and the marker of neuronal activation c-Fos. Since fluorescent IHC against CRF difficulted the obvious identification of cell bodies, we labeled PVHCRF neurons using chromogenic IHC that includes an enzymatic amplification step that enhances the IHC signal and consequently improves the visualization of the cell body of the PVHCRF neurons. We found that the vast majority of PVHCRF neurons was activated upon food deprivation (Fig. 1C–D). As previously reported [1], we confirmed that corticosterone levels and glucose levels in plasma increased and decreased, respectively, in food-deprived mice, as compared to fed mice (Fig. 1E–F). Also, ghrelin levels increased 2.1-fold and LEAP2 levels decreased 5.0-fold in the plasma of food-deprived mice, as compared to fed mice (Fig. 1G-H). Thus, the ghrelin/LEAP2 molar ratio increased from – 0.04 to – 0.41 for fed and food-deprived mice, respectively.
Fig. 1.
Food deprivation-induced activation of PVHCRF neurons is associated with an increase of ghrelin and a decrease of LEAP2 levels in plasma. A Representative photomicrographs of the PVH in coronal brain sections of fed and food-deprived WT mice subjected to immunofluorescence against CRF (red). Cell nuclei were labeled with Hoechst (blue). Scale bars: 50 µm (low magnification) and 10 µm (high magnification). B Quantitative analysis of the mean intensity of the CRF + signal in the PVH of each experimental group. C Representative photomicrographs of the PVH in coronal brain sections of fed and food-deprived WT mice subjected to double IHC against c-Fos (black) and CRF (brown). Insets depict high magnification images of the areas marked in low magnification images. Arrows point to CRF + cells while arrowheads point to c-Fos + /CRF + cells. Scale bars: 50 µm (low magnification) and 10 µm (high magnification). D Percentage of CRF + cells positive for c-Fos in the PVH in each experimental condition. E Plasma corticosterone, F glucose, G ghrelin and H LEAP2 levels of mice in each experimental group. Bars indicate mean ± SEM. Circles represent individual values. P values for unpaired comparisons were calculated by two-tailed unpaired Student’s t test. *p < 0.05, **p < 0.01 and ***p < 0.001
Food deprivation-induced activation of PVHCRF neurons is impaired in GHSR-deficient mice
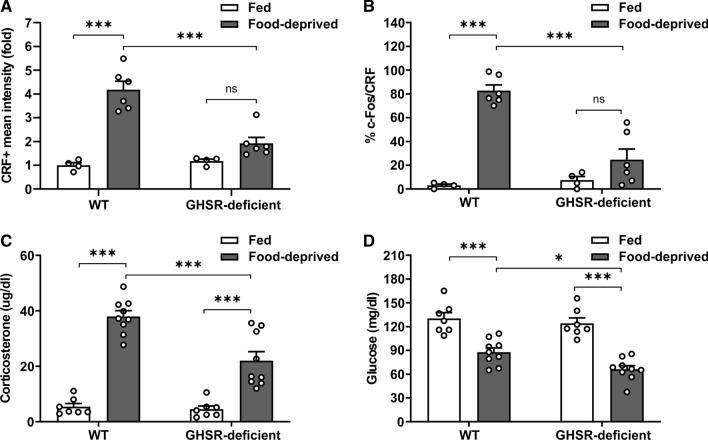
Since GHSR signaling is up-regulated under fasting, we hypothesized that food deprivation-induced activation of PVHCRF neurons depends on GHSR. Thus, we tested if PVHCRF neurons were activated in GHSR-deficient mice exposed, or not, to food deprivation. As shown in the past [5], food deprivation-induced increase of the number of c-Fos + cells in the ARH was impaired in GHSR-deficient mice, as compared to WT mice (Fig. S1). Also, food deprivation-induced increase of CRF + signal and the fraction of c-Fos + /CRF + cells in the PVH depended on the genotype. In particular, the CRF + signal and the fraction of c-Fos + /CRF + cells increased in the PVH of food-deprived WT mice whereas it was unaffected in GHSR-deficient mice (Fig. 2A–B). To estimate the systemic implications of the impaired activation of the PVHCRF neurons in food-deprived GHSR-deficient mice, we assessed corticosterone and glucose levels in the plasma of GHSR-deficient mice exposed, or not, to food deprivation. Here, corticosterone levels increased and plasma glucose decreased in food-deprived GHSR-deficient, as compared to fed GHSR-deficient mice, but both parameters were smaller than in food-deprived WT mice (Fig. 2C–D).
Fig. 2.
Food deprivation-induced activation of PVHCRFneurons is impaired in GHSR-deficient mice. A Quantitative analysis of the mean intensity of CRF + signal in the PVH of WT and GHSR-deficient mice in each experimental group. Two-way ANOVA detected condition x genotype interaction: P = 0.0004, F (1,16) = 19.44. B Percentage of CRF + cells positive for c-Fos in the PVH of WT and GHSR-deficient mice in each experimental group. Two-way ANOVA detected significant condition x genotype interaction: P = 0.0002, F (1,16) = 22.97. C Plasma corticosterone levels of WT and GHSR-deficient mice in each experimental group. Two-way ANOVA detected significant condition x genotype interaction: P = 0.0030, F (1,28) = 10.60. D Plasma glucose levels of WT and GHSR-deficient mice in each experimental group. Two-way ANOVA revealed no condition x genotype interaction, but a main effect of both condition: P < 0.0001, F (1,28) = 71.22 and genotype: P = 0.0276, F (1,28) = 5.398. Bars indicate mean ± SEM. Circles represent individual values. Tukey's multiple comparisons test shows significant differences between ‘condition’ and ‘genotype’. *p < 0.05, ***p < 0.001 and ns not significant
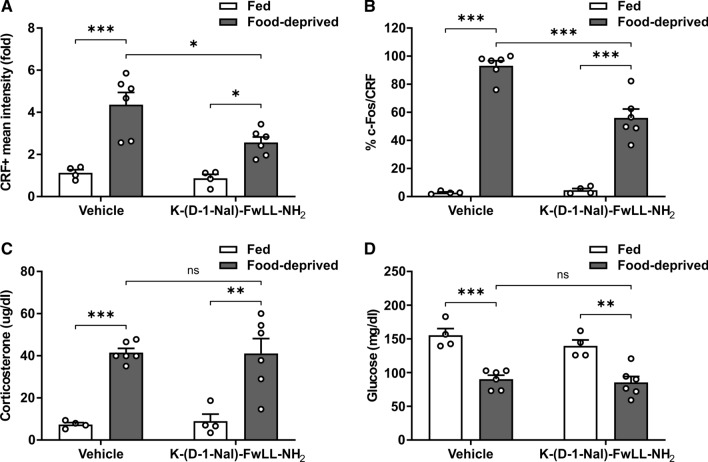
As an additional maneuver to test whether fasting-induced activation of PVHCRF neurons involves GHSR actions, we used a pharmacological approach. In particular, we investigated if the PVHCRF neurons were activated in food-deprived mice ICV-treated with K-(D-1-Nal)-FwLL-NH2, a GHSR ligand that blocks ghrelin-dependent and ghrelin-independent actions of GHSR. As recently reported [5], the food deprivation-induced increase in the number of c-Fos + cells in the ARH was impaired in food-deprived WT mice ICV-treated with K-(D-1-Nal)-FwLL-NH2, as compared to food-deprived WT mice ICV-treated with vehicle (Fig. S2). Also, we found that the CRF + signal and the fraction of c-Fos + /CRF + cells increased in the PVH of food-deprived mice ICV-treated with K-(D-1-Nal)-FwLL-NH2, but such increase was smaller than the one detected in food-deprived WT mice ICV-treated with vehicle (Fig. 3A–B). Plasma levels of corticosterone and glucose in food-deprived mice ICV-treated with K-(D-1-Nal)-FwLL-NH2 did not differ from the values found in food-deprived mice ICV-treated with vehicle (Fig. 3C–D).
Fig. 3.
Full food deprivation-induced activation of PVHCRF neurons involves ghrelin-independent actions of GHSR. A Quantitative analysis of the mean intensity of the CRF + signal in the PVH of WT mice ICV-treated with vehicle or with the GHSR blocker K-(D-1-Nal)-FwLL-NH2 in each experimental group. Two-way ANOVA revealed no condition x treatment interaction, but a main effect of both condition: P < 0.0001, F (1,16) = 36.40, and treatment: P = 0.0228, F (1,16) = 6.346. B Percentage of CRF + cells positive for c-Fos in the PVH of WT mice ICV-treated with vehicle or with the GHSR blocker K-(D-1-Nal)-FwLL-NH2 in each experimental group. Two-way ANOVA detected significant condition x treatment interaction: P = 0.0007, F (1,16) = 17.81. C Plasma corticosterone levels of WT mice ICV-treated with vehicle or with the GHSR blocker K-(D-1-Nal)-FwLL-NH2 in each experimental group. Two-way ANOVA revealed no condition x treatment interaction, but a main effect of condition: P < 0.0001, F (1,16) = 48.29. D Plasma glucose levels of WT mice ICV-treated with vehicle or with the GHSR blocker K-(D-1-Nal)-FwLL-NH2 in each experimental group. Two-way ANOVA revealed no condition x treatment interaction, but a main effect of condition: P < 0.0001, F (1,16) = 50.95. Bars indicate mean ± SEM. Circles represent individual values. Tukey's multiple comparisons test shows significant differences between ‘condition’ and ‘treatment’. *p < 0.05, **p < 0.001, ***p < 0.001 and ns not significant
Food deprivation-induced activation of PVHCRF neurons requires the integrity of the ARH
To clarify the putative hypothalamic targets of GHSR mediating the fasting-induced activation of PVHCRF neurons, we assessed the mRNA levels of GHSR in the PVH and the ARH, which strongly innervates the PVH and mediates PVHCRF neurons activation [26], of fed and food-deprived mice. Here, GHSR mRNA levels were increased not only in the ARH of food-deprived mice (3.1 ± 0.6-fold, p = 0.0018, Student’s t test), as we previously shown [5], but also in the PVH of food-deprived mice (1.8 ± 0.2-fold, p = 0.0105, Student’s t test).
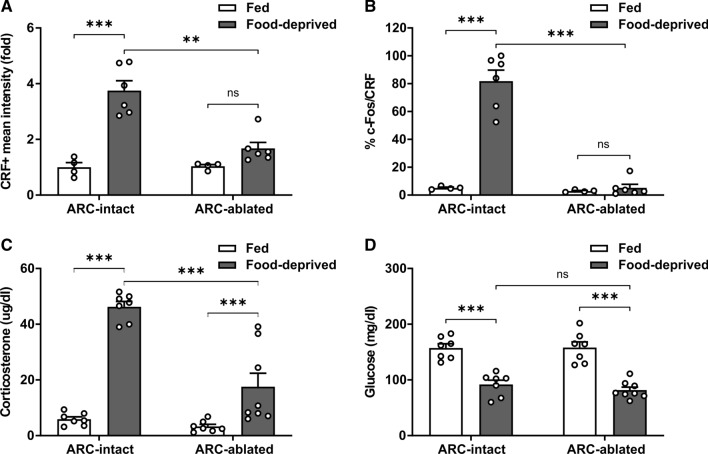
Since the ARH not only innervates the PVH but also plays a key role sensing circulating signals, we investigated if the PVHCRF neurons were activated in ARH-ablated mice exposed to food deprivation. ARH-ablated mice were tested at 9–11 weeks of age, when their body weights (24.2 ± 0.7 and 23.3 ± 0.6 g, respectively) and overnight food intake (3.4 ± 0.2 and 3.3 ± 0.2 g/day/mouse, respectively) did not differ from ARH-intact mice. As shown in the past [26], we found a similar food deprivation-induced decrease in body weight in ARH-ablated and ARH-intact mice, as compared to their fed groups; however, fasting-induced increase of the number of c-Fos + cells in the ARH was impaired in food-deprived ARH-ablated mice, as compared to food-deprived ARH-intact mice (Fig. S3). Also, the CRF + signal and the fraction of c-Fos + /CRF + cells increased in the PVH of food-deprived ARH-intact mice whereas it was unaffected in ARH-ablated mice (Fig. 4A–B). To estimate the systemic implications of the impaired activation of the PVHCRF neurons in food-deprived ARH-ablated mice, we assessed corticosterone and glucose levels in the plasma. We found that the fasting-induced increase of plasma corticosterone levels was attenuated in food-deprived ARH-ablated mice, as compared to ARH-intact mice, whereas the fasting-induced decrease of plasma glucose levels did not differ between food-deprived ARH-ablated mice and food-deprived ARH-intact mice (Fig. 4C–D).
Fig. 4.
Food deprivation-induced activation of PVHCRF neurons requires the integrity of the ARH. A Quantitative analysis of the mean intensity of the CRF + signal in the PVH of ARH-intact and ARH-ablated mice in each experimental group. Two-way ANOVA detected significant condition x group interaction: P = 0.0012, F (1,16) = 15.39. B Percentage of CRF + cells positive for c-Fos in the PVH of ARH-intact and ARH-ablated mice in each experimental group. Two-way ANOVA detected significant condition x group interaction: P = 0.0002, F (1,16) = 23.22. C Plasma corticosterone levels of ARH-intact and ARH-ablated mice in each experimental group. Two-way ANOVA revealed no condition x group interaction, but a main effect of both condition: P < 0.0001, F (1,25) = 83.65 and group: P < 0.0001, F (1,25) = 22.26. D Plasma glucose levels of ARH-intact and ARH-ablated mice in each experimental group. Two-way ANOVA revealed no condition x group interaction, but a main effect of condition: P < 0.0001, F (1, 25) = 82.00. Bars indicate mean ± SEM. Circles represent individual values. Tukey’s multiple comparisons test shows significant differences between ‘condition’ and ‘group’. **p < 0.001, ***p < 0.001 and ns not significant
Food deprivation-induced activation of PVHCRF neurons involves ghrelin-independent actions of GHSR
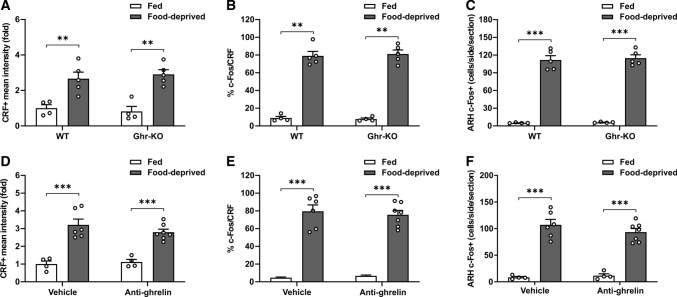
To test if fasting-induced activation of PVHCRF neurons involves an increase of plasma ghrelin levels, we investigated if PVHCRF neurons were activated in food-deprived ghrelin-KO mice. We found that the number of c-Fos + cells in the ARH, the CRF + signal in the PVH and the fraction of c-Fos + /CRF + cells in the PVH increased in food-deprived ghrelin-KO mice, as compared to fed ghrelin-KO mice, in a similar extent as seen in the WT littermates exposed to similar experimental conditions (Fig. 5A–C). As an additional strategy to test if fasting-induced activation of PVHCRF neurons involves ghrelin, we centrally administered food-deprived mice with an anti-ghrelin antibody, to immuno-neutralize ghrelin present in the CSF, and assessed if the PVHCRF neurons were activated. We found that the number of c-Fos + cells in the ARH, the CRF + signal in the PVH and the fraction of c-Fos + /CRF + cells in the PVH were not different between food-deprived WT mice ICV-treated with an anti-ghrelin antibody or with vehicle (Fig. 5D–F).
Fig. 5.
Food deprivation-induced activation of PVHCRF neurons does not involve ghrelin-dependent actions of GHSR. A Quantitative analysis of the mean intensity of the CRF + signal in the PVH of WT and ghrelin-KO mice in each experimental group. Two-way ANOVA revealed no condition x genotype interaction, but a main effect of condition: P < 0.0001, F (1,14) = 39.77. B Percentage of CRF + cells positive for c-Fos in the PVH of WT and ghrelin-KO mice in each experimental group. Two-way ANOVA revealed no condition x genotype interaction, but a main effect of condition: P < 0.0001, F (1,14) = 338.2. C Bar graphs displaying the quantitative analysis of the number of c-Fos + cells in the ARH of WT and ghrelin-KO mice in each experimental group. Two-way ANOVA revealed no condition x genotype interaction, but a main effect of condition: P < 0.0001, F (1,14) = 416.7. D Quantitative analysis of the mean intensity of the CRF + signal in the PVH of WT mice ICV-treated with vehicle or with an anti-ghrelin antibody in each experimental group. Two-way ANOVA revealed no condition x treatment interaction, but a main effect of condition: P < 0.0001, F (1,17) = 64.55. E Percentage of CRF + cells positive for c-Fos in the PVH of WT mice ICV-treated with vehicle or with an anti-ghrelin antibody in each experimental group. Two-way ANOVA revealed no condition x treatment interaction, but a main effect of condition: P < 0.0001, F (1,17) = 166.8. F Bar graphs displaying the quantitative analysis of the number of c-Fos + cells in the ARH of WT mice ICV-treated with vehicle or an anti-ghrelin antibody in each experimental group. Two-way ANOVA revealed no condition x treatment interaction, but a main effect of condition: P < 0.0001, F (1,17) = 133.7. Bars indicate mean ± SEM. Circles represent individual values. Tukey’s multiple comparisons test shows significant differences between ‘condition’ and ‘genotype’ or ‘condition’ and ‘treatment’. **p < 0.01 and ***p < 0.001
Food deprivation-induced activation of PVHCRF neurons requires a fall in plasma LEAP2 levels
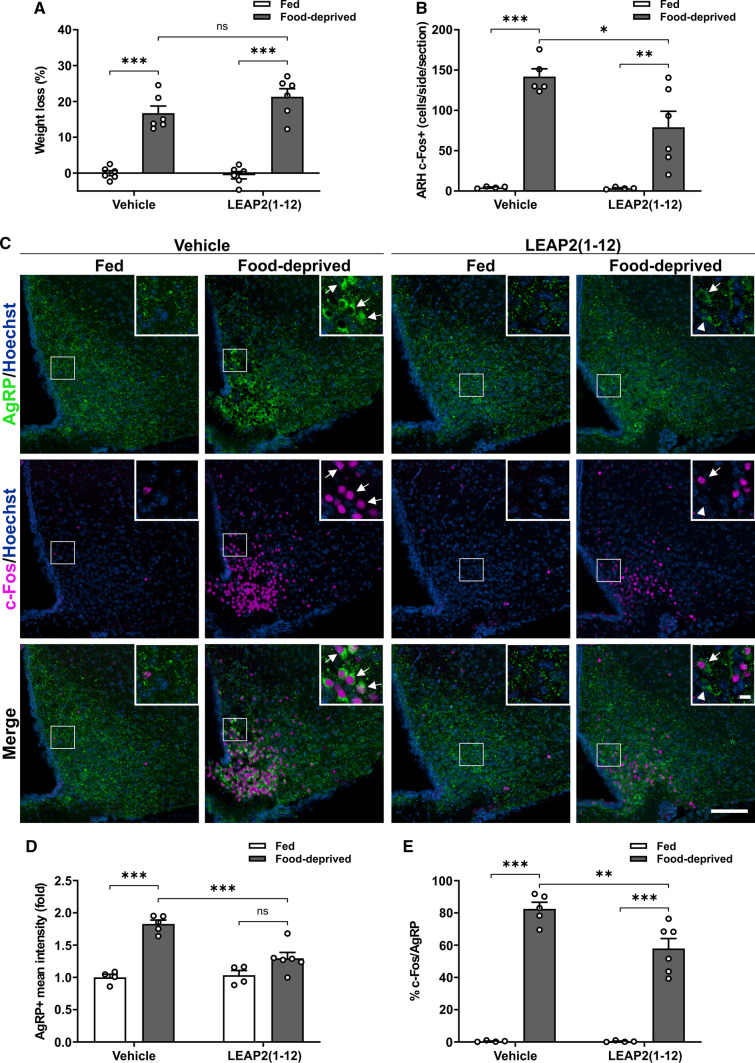
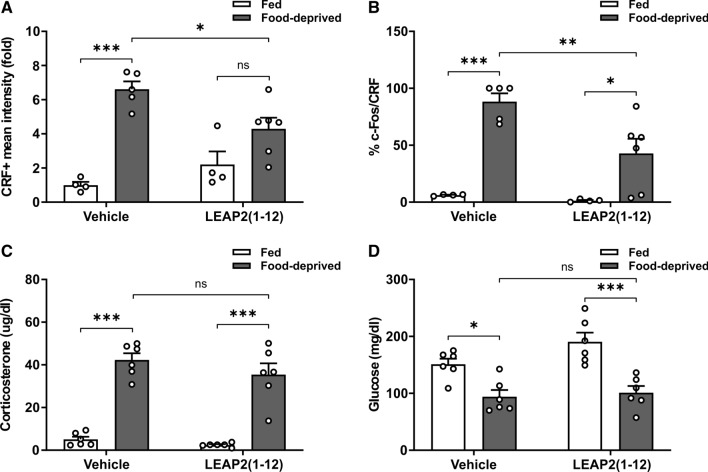
Since plasma levels of LEAP2 decrease under fasting, we investigated if the food deprivation-induced activation of PVHCRF neurons is affected in WT mice receiving a continuous infusion of LEAP2(1−12). Body weight of LEAP2(1−12)-treated food-deprived mice did not differ from body weight of vehicle-treated food-deprived mice (Fig. 6A), but the fasting-induced increase in the number of c-Fos + cells in the ARH was larger in vehicle-treated food-deprived WT mice than in LEAP2(1−12)-treated food-deprived WT mice (Fig. 6B). Since ARHAgrR/NPY neurons are activated in fasting conditions in a GHSR-dependent manner [5, 9, 11, 26], we next used double IHC against AgRP and c-Fos to assess if the response of this neuronal set is affected by LEAP2(1–12) treatment. As shown in the past [26], AgRP + cell bodies are only evident in the ARH of food-deprived mice. Interestingly, the AgRP + signal and the fraction of c-Fos + /AgRP + neurons were higher in the ARH of vehicle-treated food-deprived mice than in LEAP2(1−12)-treated food-deprived mice (Fig. 6C–E). In the PVH, the CRF + signal did not increase in LEAP2(1–12)-treated food-deprived mice, as compared to LEAP2(1–12)-treated fed mice, (Fig. 7A) whereas the fraction of c-Fos + /CRF + cells in the PVH (Fig. 7B) increased in LEAP2(1–12)-treated food-deprived mice, as compared to LEAP2(1–12)-treated fed mice, but such increase was smaller than the fasting-induced increase detected in vehicle-treated WT mice. In contrast, plasma levels of corticosterone and glucose in LEAP2(1–12)-treated food-deprived mice did not differ from the values found in vehicle-treated food-deprived mice (Fig. 7C–D).
Fig. 6.
Full food deprivation-induced activation of ARHAgRP/NPY neurons requires a decrease of the plasma LEAP2 levels. A Percentage of body weight change of WT mice SC-treated with vehicle- or LEAP2(1−12) in each experimental group. Two-way ANOVA revealed no condition x treatment interaction, but a main effect of condition: P < 0.0001, F (1,20) = 140.8. B Bar graphs displaying the quantitative analysis of the number of c-Fos + cells in the ARH of WT mice SC-treated with vehicle- or LEAP2(1−12) in each experimental group. Two-way ANOVA detected significant condition x treatment interaction: P = 0.0435, F (1,15) = 4.860. C Representative photomicrographs of the ARH in coronal brain sections subjected to double IHC against c-Fos (Pseudo-colored to magenta) and AgRP (green). Insets depict high magnification images of the areas marked in low magnification images. Arrowheads point to AgRP + cells while arrows point to c-Fos + /AgRP + cells. Scale bars: 100 µm (low magnification) and 10 µm (high magnification). D Quantitative analysis of the mean intensity of the AgRP + signal in the ARH of WT mice SC-treated with vehicle- or LEAP2(1−12) in each experimental group. Two-way ANOVA detected significant condition x treatment interaction: P = 0.0021, F (1,15) = 13.69. E Percentage of AgRP + cells positive for c-Fos in the ARH of WT mice SC-treated with vehicle- or LEAP2(1−12) in each experimental group. Two-way ANOVA detected significant condition x treatment interaction: P = 0.0181, F (1,15) = 7.030. Bars indicate mean ± SEM. Circles represent individual values. Tukey's multiple comparisons test shows significant differences between ‘condition’ and ‘treatment’. *p < 0.05, **p < 0.001, ***p < 0.001 and ns not significant
Fig. 7.
Full food deprivation-induced activation of PVHCRF neurons requires a decrease of the plasma LEAP2 levels. A Quantitative analysis of the mean intensity of the CRF + signal in the PVH of WT mice SC-treated with vehicle- or LEAP2(1−12) in each experimental group. Two-way ANOVA detected significant condition x treatment interaction: P = 0.0094, F (1,15) = 8.874. B Percentage of CRF + cells positive for c-Fos in the PVH of WT mice SC-treated with vehicle- or LEAP2(1−12) in each experimental group. Two-way ANOVA detected significant condition x treatment interaction: P = 0.0463, F (1,15) = 4.716. C Plasma corticosterone levels of WT mice SC-treated with vehicle- or LEAP2(1−12) in each experimental group. Two-way ANOVA revealed no condition x treatment interaction, but a main effect of both condition: P < 0.0001, F (1,20) = 179.8, and treatment: P = 0.0254, F (1,20) = 5.831. D Plasma glucose levels of WT mice SC-treated with vehicle- or LEAP2(1−12) in each experimental group. Two-way ANOVA revealed no condition x treatment interaction, but a main effect of condition: P < 0.0001, F (1,20) = 34.57. Bars indicate mean ± SEM. Circles represent individual values. Tukey’s multiple comparisons test shows significant differences between ‘condition’ and ‘treatment’. *p < 0.05, **p < 0.001, ***p < 0.001 and ns not significant
Discussion
Here, we reveal a role of GHSR in mediating food deprivation-induced activation of the PVHCRF neurons in male mice. The results of the current study are summarized in Table 1. In brief, we found that the food deprivation-induced activation of PVHCRF neurons requires both the presence of GHSR and the integrity of the ARH. We also found that food deprivation-induced activation of PVHCRF neurons does not require the presence of ghrelin but is impaired by a GHSR ligand that reduces the constitutive GHSR activity. Finally, we found that preventing the fall of plasma LEAP2 levels in food-deprived mice impairs the activation of PVHCRF neurons. Thus, current observations support the notions that GHSR controls fasting-induced activation of the PVHCRF neurons and that food deprivation-induced fall of plasma LEAP2 levels contributes to such adaptation.
Table 1.
Summary of findings
| Does the food deprivation induced activation of the PVHCRF neurons require | Observations | ||
|---|---|---|---|
| Key experimental groups in food-deprived conditions | PVHCRF neuron activation | Plasma corticosterone | |
| ARC integrity? | ARH-ablated mice as compared to ARH-intact mice | ↓ | ⇊ |
| GHSR signaling? |
Genetic approach GHSR-deficient mice as compared to WT mice |
⇊ | ⇊ |
|
Pharmacological approach K-(D-1-Nal)-FwLL-NH2-treated mice as compared to Vehicle-treated mice |
↓ | NC | |
| ghrelin-evoked GHSR signaling? |
Genetic approach Ghrelin-KO mice as compared to WT mice |
NC | - |
|
Pharmacological approach Anti-ghrelin-treated mice as compared to Vehicle-treated mice |
NC | - | |
| A fall of LEAP2 plasma levels? |
Pharmacological approach LEAP2(1-12)-treated mice as compared to Vehicle-treated mice |
↓ | NC |
↓, decrease; ⇊, strong decrease;NC No change
Here, we gained insights into the neurobiological basis by which GHSR signaling becomes essential to cope against severe energy deficit conditions. Indeed, mice lacking ghrelin exposed to a chronic starvation protocol, in contrast to WT mice, cannot appropriately maintain glycemia within limits compatible with life and become moribund [38]. Rather than resorting to a chronic calorie-restriction, we studied the role GHSR on the activation of the PVHCRF neurons in mice subjected to a 2-day fasting because it is a less severe condition in which mice show normal overall health status and locomotor activity [5]. In our experience, the use of a 2-day food deprivation protocol allows to unmask some roles of endogenous GHSR that are not observed after a single day of food deprivation, presumably because the hypothalamic GHSR mRNA levels are higher in 2-day food-deprived mice than in 1-day food-deprived mice, despite plasma ghrelin levels do not differ between these two conditions [1]. To the best of our knowledge, we report here for the first time that plasma LEAP2 levels in 2-day food-deprived mice are – fivefold lower than in ad libitum fed mice. Previous studies reported that plasma LEAP2 levels decrease – 1.5–threefold in 1-day fasted mice [18, 39]. Thus, the more drastic fall of plasma LEAP2 levels in 2-day food-deprived mice is likely another factor that enhances the relevance GHSR signaling under this experimental condition.
We reveal here a previously unknown role of the GHSR signaling mediating food deprivation-induced activation of the PVHCRF neurons, which constitute the primary driver of the food deprivation-induced activation of the HPA axis [40]. As stated before, the genetic ablation of CRF in mice abrogates fasting-induced increase of plasma glucocorticoid levels and results in severe hypoglycemia [2]. Here, we found that both the CRF + signal, which estimates the levels of CRF and its precursors, and the fraction of c-Fos + /CRF + cells increase in the PVH of food-deprived mice, indicating that the neuropeptide biosynthesis and the transcriptional activity of the PVHCRF neurons are enhanced during prolonged food deprivation. Of note, the activation of PVHCRF neurons co-exist with high plasma corticosterone levels in food-deprived mice indicating that the negative feedback of glucocorticoids at hypothalamic level was reset under fasting, presumably as a way to sustain its systemic hyperglycemic actions [40]. The observation that PVHCRF neurons were not activated in food-deprived GHSR-deficient mice indicates that GHSR is essential for its fasting-induced activation. Still, plasma corticosterone levels increased in food-deprived GHSR-deficient mice, although in a lower extent than in WT mice, suggesting that GHSR-independent mechanisms contribute to activate the HPA axis under fasting. In this regard, it is likely that the hypoleptinemia, another typical feature of fasting, contributes to increase corticosterone release since leptin supplementation in fasted mice leads to a partial reduction of plasma corticosterone levels [41]. Leptin mainly inhibits the HPA axis by acting on the adrenal gland; thus, the reduction of plasma leptin levels may favor the release of glucocorticoid without a concomitant activation of the PVHCRF neurons [42]. Also, we report here that food-deprived GHSR-deficient mice display a more severe hypoglycemia than food-deprived WT mice. It is likely that the incomplete activation of the HPA axis in food-deprived GHSR-deficient mice, together with other alterations such as an impaired fasting-induced elevation of plasma glucagon levels [43], is another reason why the absence of GHSR reduces the capability of mice to properly maintain glycemia during a prolonged food deprivation.
Here, we explored the putative pathways that could mediate GHSR-dependent PVHCRF neurons activation and found that the ARH is essential for food deprivation-induced activation of the PVHCRF neurons. The PVH is located adjacent to the third ventricle, and reached by systemically-injected ghrelin, which crosses the blood–CSF barrier, access to the CSF and then diffuses to the periventricular hypothalamic nuclei [30]. We have shown that PVHCRF neurons lack GHSR, but intra-PVH or systemic administration of ghrelin can activate PVHCRF neurons in an ARH-independent manner [6, 23]. Thus, we tested here if immuno-neutralization of ghrelin in the CSF of food-deprived mice impaired fasting-induced activation of PVHCRF neurons, and we found no evidence supporting such possibility. Since systemically injected ghrelin gains access to the ventral region of the ARH [27, 44, 45], we tested if food deprivation-induced activation of the PVHCRF neurons occurs in ARH-ablated mice. We found that PVHCRF neurons were not activated and that plasma corticosterone levels showed a smaller increment in food-deprived ARH-ablated mice, as compared to food-deprived ARH-intact mice, suggesting that the ARH is essential for fasting-induced activation of the PVHCRF neurons. It is worth noting that we and others have shown that monosodium glutamate treatment in neonatal mice ablates almost all ARHAgRP/NPY neurons but only a fraction of other ARH neurons [46, 47]. Thus, the impaired fasting-induced activation of the PVHCRF neurons in mice with monosodium glutamate-induced ablation of the ARH may indicate that ARHAgRP/NPY play a preferential role mediating GHSR actions in food deprivation. Still, mice with neonatal ablation of the ARH also develop some neuroendocrine abnormalities that result in obesity later in life. Despite we careful design experiments to minimize the impact of such abnormalities (e.g., experimental mice were young, when body weight and food intake are still unaltered), we cannot rule out that the impaired response of the PVHCRF neurons observed in food-deprived ARH-ablated mice is an indirect consequence of the hypothalamic lesion.
Since plasma ghrelin levels as well as the GHSR mRNA levels in the ARH increase during fasting, we wondered if ghrelin-dependent or ghrelin-independent modes of GHSR action mediates food deprivation-induced activation of the PVHCRF neurons. We found that PVHCRF neurons were activated in food-deprived ghrelin-KO mice and in food-deprived WT mice in which endogenous ghrelin was immuno-neutralized suggesting that ghrelin is not required for fasting-induced activation of the HPA axis. Also, we found that activation of PVHCRF neurons is partially abrogated in food-deprived mice treated with K-(D-1-Nal)-FwLL-NH2. Altogether, these data suggest that food deprivation-induced activation PVHCRF neurons requires ghrelin-independent actions of GHSR. The reason why fasting-induced activation PVHCRF was partially affected in mice treated with the GHSR blocker but fully abrogated in GHSR-deficient mice is uncertain. It is possible that K-(D-1-Nal)-FwLL-NH2 did not fully block GHSR actions in vivo. In support of this possibility, it was shown that K-(D-1-Nal)-FwLL-NH2 is less potent than the substance P analog, another well-characterized GHSR inverse agonist, to reduce the constitutive activity of the receptor [48, 49]. However, we cannot rule out that a higher dose of the GHSR blocker or its continuous infusion may fully abrogate the activation of the PVHCRF neurons. Of note, plasma levels of corticosterone and glucose changed in food-deprived mice treated with the GHSR blocker in a similar extent as that detected in vehicle-treated food-deprived mice. In this regard, it is likely that the partial activation of the PVHCRF neurons detected in K-(D-1-Nal)-FwLL-NH2-treated food-deprived mice was sufficient to fully activate the HPA axis at systemic level. Interesting, we have shown that the compensatory hyperphagic response observed after a prolonged fasting event relies on, in part, on ghrelin-independent actions of GHSR in the ARH [5]. Similarly, fasting-induced remodeling of ARHAgRP/NPY projections to the PVH involved ghrelin-independent actions of GHSR [26]. Now, we show that food deprivation-induced activation of the PVHCRF neurons implicates ghrelin-independent actions of GHSR. Thus, ghrelin-independent actions of GHSR seem to play diverse and relevant roles in prolonged food deprivation conditions.
Since LEAP2 was recently recognized as an endogenous blocker of GHSR and plasma levels of LEAP2 decrease under fasting [8], we investigated if the ghrelin-independent actions of GHSR mediating the food deprivation-induced activation of the PVHCRF neurons depend on a decrease of plasma LEAP2 levels. Rather than administration of full-length LEAP2, we used here LEAP2(1–12) since we have shown that the N-terminal residues of LEAP2 are required and sufficient for GHSR binding and bioactivity [19]. LEAP2(1–12) fully resembles the actions of full-length native LEAP2, but it presumably displays a shorter half-life in vivo because the C-terminal end includes two disulfide bridges that protect the peptide from degradation in plasma [50]. It is noteworthy that we synthetized the LEAP2(1–12) used in the current studies allowing not only produce the large amounts required for the experiments but also make us confident about its chemical identity. For the supplementation, we choose to implant mice with osmotic mini-pumps to allow the delivery of LEAP2(1–12) at continuous and controlled rates during the entire food deprivation period. The previous referred study inducing a long-lasting increase of plasma LEAP2 levels used a virally-mediated overexpression system, but we considered that such strategy was not appropriated to our study due to several reasons including that it requires several weeks to increase plasma LEAP2 levels making it inconvenient for a 2-days treatment [18], and that it may trigger immune responses that could secondarily impact on the neuroendocrine system [51].
Our studies supplementing food-deprived mice with LEAP2(1–12) provide evidence here indicating that the fall of plasma LEAP2 levels drives some hypothalamic adaptations that take place during a prolonged food deprivation. Of note, we confirmed here the molar concentration of LEAP2 is higher than the molar concentration of ghrelin in fed mice suggesting that LEAP2 affects GHSR more significantly than ghrelin in satiated conditions, since LEAP2 and ghrelin display similar affinity for GHSR [8]. In ad libitum fed mice, we found that continuous infusion of LEAP2(1–12) does not affect c-Fos in the ARH, the HPA axis or glycemia, in line with previous observations that a single bolus injection of intact LEAP2 or its N-terminal end does not affect food intake or glycemic control in fed mice [18, 19]. Thus, it is likely that the relatively high plasma LEAP2 levels and the presumably low level of GHSR activity preclude observing effects of exogenously administered LEAP2 in ad libitum fed conditions. In contrast, plasma LEAP2 levels decreased – fivefold in food-deprived mice, and LEAP2(1–12) administration partially abrogated food deprivation-induced activation of the ARHAgRP/NPY neurons. Notably, food deprivation-induced increase in GHSR gene expression in the ARH was previously shown to specifically take place in ARHAgRP/NPY neurons [9, 10, 52], and an increase of GHSR levels was shown to result in higher constitutive intracellular signaling in vitro [53]. In addition, electrophysiological recording in mouse brain sections showed that LEAP2 potently hyperpolarizes ARHAgRP/NPY neurons [39]. Thus, we propose that the food deprivation-induced fall of plasma LEAP2 levels contributes to enhance GHSR actions in ARHAgRP/NPY neurons and, by doing so, helps to cope against energy deficit conditions.
Here, we also show that food deprivation-induced fall of LEAP2 contributes to activate the PVHCRF neurons since LEAP2(1–12) treatment reduced the activation of the PVHCRF neurons in food-deprived mice. Although it was not directly tested, it is likely that the impaired activation PVHCRF neurons in LEAP2(1–12)-treated food-deprived mice arises from an impaired activation the ARHAgRP/NPY neurons. Indeed, PVHCRF neurons are densely innervated by ARHAgRP/NPY neurons [54–56], and activated by NPY [57, 58]. Of note, projections from the ARHAgRP/NPY neurons to the PVH increase in the food-deprived WT mice, but not in food-deprived GHSR-deficient mice, and such morphological remodeling also contributes to activate the PVH neurons [26]. Thus, NPY released from GHSR-expressing ARHAgRP/NPY neurons could mediate food deprivation-induced activation of PVHCRF neurons. Alternatively, ARHAgRP/NPY neurons may act on other neuronal populations that subsequently act on PVHCRF neurons [59]. The reason why LEAP2(1–12) treatment in food-deprived mice only partially reduces the food deprivation-induced activation of ARHAgRP/NPY and PVHCRF neurons is uncertain, but it seems likely that other neuroendocrine systems contribute to activate ARHAgRP/NPY and PVHCRF neurons. Notably, LEAP2(1–12) treatment in food-deprived mice did not affect plasma levels of corticosterone and glucose, similarly as found for K-(D-1-Nal)-FwLL-NH2 treatment in food-deprived mice. Thus, current results seem to indicate that a partial fasting-induced activation of the PVHCRF neurons is sufficient to fully activate the HPA axis at systemic level. Although the neurobiological bases of our observations are uncertain, it is possible that the GHSR ligands do not completely block GHSR signaling in our experimental conditions as it is the case in mice lacking GHSR expression. Of note, we expected to find a lower plasma glucose levels in LEAP2(1–12)-treated food-deprived mice since the previously referred study using virally-mediated overexpression of LEAP2 found that LEAP2 supplementation leads to a more severe hypoglycemia in calorie-restricted mice [18]. The reason why the treatment with LEAP2(1–12) (or with K-(D-1-Nal)-FwLL-NH2) in food-deprived mice did not affect glycemia is uncertain. Unfortunately, the short version of LEAP2 was not detected by the commercial immunoassay, and, consequently, we were unable to confirm if LEAP2(1–12) infusion induced the predicted plasma level increase. Interestingly, a recent study reported that LEAP2(1–10) increases glucose-stimulated insulin release from cultured human pancreatic pseudo-islets but does not affect glucose homeostasis in mice or in healthy humans fasted for 10 h [60]. Thus, the extent to which shorter versions LEAP2 display the capacity to fully mimic the effects of intact LEAP2 in vivo needs to be further investigated.
Overall, the current study shows that the food deprivation-induced activation of the PVHCRF neurons involves hypothalamic actions of GHSR that depend on LEAP2, but not on ghrelin. Thus, we propose that food deprivation-induced decrease of plasma LEAP2 levels plays a key role as a neuroendocrine signal during a prolonged fasting. Since the current work made use of different type of mouse models (e.g., mice with genetic- or pharmacological manipulations of the GHSR signaling), our overall conclusion should not be strongly conditioned by putative caveats associated to each experimental model (e.g., compensatory developmental mechanisms in genetically-modified mice; off-target effects in pharmacologically-modified mice, etc.). Still, further investigations using different and complementary experimental strategies may help, or not, to further refine the proposed model to describe how GHSR regulates food deprivation-induced activation of the PVHCRF.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank MinCyT-ECOS-Sud (A13B01) and Bec.Ar for support covering exchanges, Dr. Zigman (University of Texas Southwestern Medical Center, Dallas, TX) for providing the GHSR-deficient mice, and Dr. Tomasetto (Institut de Genetique et de Biologie Moleculaire et Cellulaire, Strasbourg, France) for providing the ghrelin-KO mice. The technical assistance of Dr. Tolosa, Dr. García-Romero, DVM Lacunza and Mr. Aguilar is also acknowledged. This work was supported by grants from the Fondo para la Investigación Científica y Tecnológica (FONCyT, PICT2016-1084, PICT2017-3196 and PICT2019-3054 to MP), from CONICET (PUE105 to MP) and from The National Qatar Research Foundation (NPRP13S-0209-200315).
Author contributions
GF, AC, PNDeF, MU, MR, DC, GZ and SC performed experiments; GF, AC and GZ analyzed data; GF, AG, SD, JAF, VT, HBS and M. Perello designed the experiments and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Fondo para la Investigación Científica y Tecnológica (FONCyT, PICT2016-1084, PICT2017-3196 and PICT2019-3054 to MP), from CONICET (PUE105 to MP) and from The National Qatar Research Foundation (NPRP13S-0209-200315).
Data availability
The datasets generated and/or analyzed during the current study are included in this published article and are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the US National Research Council and the European Communities Council 447 Directive (86/609/EEC). All protocols received approval from the Institutional Animal Care and Use Committee of the IMBICE (N°10-0112) and the Animal Experimentation Committee CEEA.34 of Paris Descartes University (N°03422.02).
Consent for publication
N/A.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luque RM, Park S, Kineman RD. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotropin-releasing hormone. Endocrinology. 2007;148(1):300–309. doi: 10.1210/en.2006-0592. [DOI] [PubMed] [Google Scholar]
- 2.Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373(6513):427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 3.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 4.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez G, Cabral A, Andreoli MF, Labarthe A, M'Kadmi C, Ramos JG, et al. Evidence supporting a role for constitutive ghrelin receptor signaling in fasting-induced hyperphagia in male mice. Endocrinology. 2018;159(2):1021–1034. doi: 10.1210/en.2017-03101. [DOI] [PubMed] [Google Scholar]
- 6.Cabral A, Suescun O, Zigman JM, Perello M. Ghrelin indirectly activates hypophysiotropic CRF neurons in rodents. PLoS ONE. 2012;7(2):e31462. doi: 10.1371/journal.pone.0031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolle V, Zizzari P, Tomasetto C, Rio MC, Epelbaum J, Bluet-Pajot MT. In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat. Neuroendocrinology. 2001;73(1):54–61. doi: 10.1159/000054620. [DOI] [PubMed] [Google Scholar]
- 8.Cornejo MP, Mustafa ER, Cassano D, Baneres JL, Raingo J, Perello M. The ups and downs of growth hormone secretagogue receptor signaling. FEBS J. 2021 doi: 10.1111/febs.15718. [DOI] [PubMed] [Google Scholar]
- 9.Henry FE, Sugino K, Tozer A, Branco T, Sternson SM. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. Elife. 2015 doi: 10.7554/eLife.09800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasrebi A, Hsieh A, Mamounis KJ, Krumm EA, Yang JA, Magby J, et al. Differential gene regulation of GHSR signaling pathway in the arcuate nucleus and NPY neurons by fasting, diet-induced obesity, and 17beta-estradiol. Mol Cell Endocrinol. 2016;422:42–56. doi: 10.1016/j.mce.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484–496. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornejo MP, Denis RGP, Garcia Romero G, Fernandez G, Reynaldo M, Luquet S, et al. Ghrelin treatment induces rapid and delayed increments of food intake: a heuristic model to explain ghrelin’s orexigenic effects. Cell Mol Life Sci. 2021;78(19–20):6689–6708. doi: 10.1007/s00018-021-03937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2014;3(1):64–72. doi: 10.1016/j.molmet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70(5):306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149(2):843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA. 2004;101(21):8227–8232. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge X, Yang H, Bednarek MA, Galon-Tilleman H, Chen P, Chen M, et al. LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metab. 2018;27(2):461–469. doi: 10.1016/j.cmet.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 19.M'Kadmi C, Cabral A, Barrile F, Giribaldi J, Cantel S, Damian M, et al. N-terminal liver-expressed antimicrobial peptide 2 (LEAP2) region exhibits inverse agonist activity toward the ghrelin receptor. J Med Chem. 2019;62(2):965–973. doi: 10.1021/acs.jmedchem.8b01644. [DOI] [PubMed] [Google Scholar]
- 20.Wang JH, Li HZ, Shao XX, Nie WH, Liu YL, Xu ZG, et al. Identifying the binding mechanism of LEAP2 to receptor GHSR1a. FEBS J. 2019;286(7):1332–1345. doi: 10.1111/febs.14763. [DOI] [PubMed] [Google Scholar]
- 21.Shankar K, Metzger NP, Singh O, Mani BK, Osborne-Lawrence S, Varshney S, et al. LEAP2 deletion in mice enhances ghrelin's actions as an orexigen and growth hormone secretagogue. Mol Metab. 2021;53:101327. doi: 10.1016/j.molmet.2021.101327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, et al. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001;86(3):1169–1174. doi: 10.1210/jcem.86.3.7314. [DOI] [PubMed] [Google Scholar]
- 23.Cabral A, Portiansky E, Sanchez-Jaramillo E, Zigman JM, Perello M. Ghrelin activates hypophysiotropic corticotropin-releasing factor neurons independently of the arcuate nucleus. Psychoneuroendocrinology. 2016;67:27–39. doi: 10.1016/j.psyneuen.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115(12):3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassouna R, Zizzari P, Tomasetto C, Veldhuis JD, Fiquet O, Labarthe A, et al. An early reduction in GH peak amplitude in preproghrelin-deficient male mice has a minor impact on linear growth. Endocrinology. 2014;155(9):3561–3571. doi: 10.1210/en.2014-1126. [DOI] [PubMed] [Google Scholar]
- 26.Cabral A, Fernandez G, Tolosa MJ, Rey Moggia A, Calfa G, De Francesco PN, et al. Fasting induces remodeling of the orexigenic projections from the arcuate nucleus to the hypothalamic paraventricular nucleus, in a growth hormone secretagogue receptor-dependent manner. Mol Metab. 2020;32:69–84. doi: 10.1016/j.molmet.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabral A, Valdivia S, Fernandez G, Reynaldo M, Perello M. Divergent neuronal circuitries underlying acute orexigenic effects of peripheral or central ghrelin: critical role of brain accessibility. J Neuroendocrinol. 2014;26(8):542–554. doi: 10.1111/jne.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Els S, Schild E, Petersen PS, Kilian TM, Mokrosinski J, Frimurer TM, et al. An aromatic region to induce a switch between agonism and inverse agonism at the ghrelin receptor. J Med Chem. 2012;55(17):7437–7449. doi: 10.1021/jm300414b. [DOI] [PubMed] [Google Scholar]
- 29.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 30.Uriarte M, De Francesco PN, Fernandez G, Cabral A, Castrogiovanni D, Lalonde T, et al. Evidence supporting a role for the blood-cerebrospinal fluid barrier transporting circulating ghrelin into the brain. Mol Neurobiol. 2019;56(6):4120–4134. doi: 10.1007/s12035-018-1362-8. [DOI] [PubMed] [Google Scholar]
- 31.Greenblatt DJ, Koch-Weser J. Clinical pharmacokinetics (second of two parts) N Engl J Med. 1975;293(19):964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- 32.Greenblatt DJ, Kock-Weser J. Drug therapy clinical pharmacokinetics (first of two parts) N Engl J Med. 1975;293(14):702–705. doi: 10.1056/NEJM197510022931406. [DOI] [PubMed] [Google Scholar]
- 33.Perello M, Chacon F, Cardinali DP, Esquifino AI, Spinedi E. Effect of social isolation on 24-h pattern of stress hormones and leptin in rats. Life Sci. 2006;78(16):1857–1862. doi: 10.1016/j.lfs.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Cornejo MP, Castrogiovanni D, Schioth HB, Reynaldo M, Marie J, Fehrentz JA, et al. Growth hormone secretagogue receptor signalling affects high-fat intake independently of plasma levels of ghrelin and LEAP2, in a 4-day binge eating model. J Neuroendocrinol. 2019;31(10):e12785. doi: 10.1111/jne.12785. [DOI] [PubMed] [Google Scholar]
- 35.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paxinos G, Franklin KBJ, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- 37.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 38.Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107(16):7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mani BK, Puzziferri N, He Z, Rodriguez JA, Osborne-Lawrence S, Metzger NP, et al. LEAP2 changes with body mass and food intake in humans and mice. J Clin Invest. 2019;129(9):3909–3923. doi: 10.1172/JCI125332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 42.Perello M, Gaillard RC, Chisari A, Spinedi E. Adrenal enucleation in MSG-damaged hyperleptinemic male rats transiently restores adrenal sensitivity to leptin. Neuroendocrinology. 2003;78(3):176–184. doi: 10.1159/000072799. [DOI] [PubMed] [Google Scholar]
- 43.Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, et al. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121(7):2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciofi P, Garret M, Lapirot O, Lafon P, Loyens A, Prevot V, et al. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology. 2009;150(12):5509–5519. doi: 10.1210/en.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, Roux T, et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci USA. 2013;110(4):1512–1517. doi: 10.1073/pnas.1212137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95(25):15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yulyaningsih E, Rudenko IA, Valdearcos M, Dahlen E, Vagena E, Chan A, et al. Acute lesioning and rapid repair of hypothalamic neurons outside the blood-brain barrier. Cell Rep. 2017;19(11):2257–2271. doi: 10.1016/j.celrep.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.M'Kadmi C, Leyris JP, Onfroy L, Gales C, Sauliere A, Gagne D, et al. Agonism, antagonism, and inverse agonism bias at the ghrelin receptor signaling. J Biol Chem. 2015;290(45):27021–27039. doi: 10.1074/jbc.M115.659250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen PS, Woldbye DP, Madsen AN, Egerod KL, Jin C, Lang M, et al. In vivo characterization of high Basal signaling from the ghrelin receptor. Endocrinology. 2009;150(11):4920–4930. doi: 10.1210/en.2008-1638. [DOI] [PubMed] [Google Scholar]
- 50.Henriques ST, Tan CC, Craik DJ, Clark RJ. Structural and functional analysis of human liver-expressed antimicrobial peptide 2. ChemBioChem. 2010;11(15):2148–2157. doi: 10.1002/cbic.201000400. [DOI] [PubMed] [Google Scholar]
- 51.Shirley JL, de Jong YP, Terhorst C, Herzog RW. Immune responses to viral gene therapy vectors. Mol Ther. 2020;28(3):709–722. doi: 10.1016/j.ymthe.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luckman SM, Rosenzweig I, Dickson SL. Activation of arcuate nucleus neurons by systemic administration of leptin and growth hormone-releasing peptide-6 in normal and fasted rats. Neuroendocrinology. 1999;70(2):93–100. doi: 10.1159/000054463. [DOI] [PubMed] [Google Scholar]
- 53.Lopez Soto EJ, Agosti F, Cabral A, Mustafa ER, Damonte VM, Gandini MA, et al. Constitutive and ghrelin-dependent GHSR1a activation impairs CaV2.1 and CaV2.2 currents in hypothalamic neurons. J Gen Physiol. 2015;146(3):205–219. doi: 10.1085/jgp.201511383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuzesi T, Wittmann G, Liposits Z, Lechan RM, Fekete C. Contribution of noradrenergic and adrenergic cell groups of the brainstem and agouti-related protein-synthesizing neurons of the arcuate nucleus to neuropeptide-y innervation of corticotropin-releasing hormone neurons in hypothalamic paraventricular nucleus of the rat. Endocrinology. 2007;148(11):5442–5450. doi: 10.1210/en.2007-0732. [DOI] [PubMed] [Google Scholar]
- 55.Li C, Chen P, Smith MS. Corticotropin releasing hormone neurons in the paraventricular nucleus are direct targets for neuropeptide Y neurons in the arcuate nucleus: an anterograde tracing study. Brain Res. 2000;854(1–2):122–129. doi: 10.1016/s0006-8993(99)02324-0. [DOI] [PubMed] [Google Scholar]
- 56.Liposits Z, Sievers L, Paull WK. Neuropeptide-Y and ACTH-immunoreactive innervation of corticotropin releasing factor (CRF)-synthesizing neurons in the hypothalamus of the rat an immunocytochemical analysis at the light and electron microscopic levels. Histochemistry. 1988;88(3–6):227–234. doi: 10.1007/BF00570278. [DOI] [PubMed] [Google Scholar]
- 57.Dimitrov EL, DeJoseph MR, Brownfield MS, Urban JH. Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity. Endocrinology. 2007;148(8):3666–3673. doi: 10.1210/en.2006-1730. [DOI] [PubMed] [Google Scholar]
- 58.Sarkar S, Lechan RM. Central administration of neuropeptide Y reduces alpha-melanocyte-stimulating hormone-induced cyclic adenosine 5'-monophosphate response element binding protein (CREB) phosphorylation in pro-thyrotropin-releasing hormone neurons and increases CREB phosphorylation in corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology. 2003;144(1):281–291. doi: 10.1210/en.2002-220675. [DOI] [PubMed] [Google Scholar]
- 59.Spencer SJ, Emmerzaal TL, Kozicz T, Andrews ZB. Ghrelin’s role in the hypothalamic-pituitary-adrenal axis stress response: implications for mood disorders. Biol Psychiatry. 2015;78(1):19–27. doi: 10.1016/j.biopsych.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 60.Hagemann CA, Zhang C, Hansen HH, Jorsal T, Rigbolt KTG, Madsen MR, et al. Identification and metabolic profiling of a novel human gut-derived LEAP2 fragment. J Clin Endocrinol Metab. 2021;106(2):e966–e981. doi: 10.1210/clinem/dgaa803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are included in this published article and are available from the corresponding author on reasonable request.