Abstract
Doxorubicin (DOX) is an anthracycline chemotherapy drug used in the treatment of various types of cancer. However, short-term and long-term cardiotoxicity limits the clinical application of DOX. Currently, dexrazoxane is the only approved treatment by the United States Food and Drug Administration to prevent DOX-induced cardiotoxicity. However, a recent study found that pre-treatment with dexrazoxane could not fully improve myocardial toxicity of DOX. Therefore, further targeted cardioprotective prophylaxis and treatment strategies are an urgent requirement for cancer patients receiving DOX treatment to reduce the occurrence of cardiotoxicity. Accumulating evidence manifested that Sirtuin 1 (SIRT1) could play a crucially protective role in heart diseases. Recently, numerous studies have concentrated on the role of SIRT1 in DOX-induced cardiotoxicity, which might be related to the activity and deacetylation of SIRT1 downstream targets. Therefore, the aim of this review was to summarize the recent advances related to the protective effects, mechanisms, and deficiencies in clinical application of SIRT1 in DOX-induced cardiotoxicity. Also, the pharmaceutical preparations that activate SIRT1 and affect DOX-induced cardiotoxicity have been listed in this review.
Keywords: Doxorubicin, Cardiotoxicity, SIRT1, Deacetylation, Mechanism, SIRT1 agonists
Introduction
Doxorubicin (DOX) is an anthracycline chemotherapeutic drug that is widely used to treat various neoplastic diseases, including lymphomas, breast cancer, prostatic cancer, osteosarcoma, lung cancer, and neuroblastoma [1]. DOX exerts an antitumor effect mainly by intercalation into DNA and inhibition of topoisomerase II. Regrettably, DOX also causes apoptosis or necrosis of healthy tissues, and hence, proving to be toxic to the brain, heart, liver, and kidney [2]. Clinical data have shown that the toxicities of this drug, especially cardiotoxicity, severely limit its application [3, 4]. The myocardial toxicity of DOX is closely associated with its dosage, which might ultimately result in progressive heart failure and irreversible cardiac dysfunction. The cumulative DOX doses of 400, 500, and 550 mg/m2 were estimated to correlate with the incidence of heart failure equal to 5%, 16%, and 26%, respectively [5]. The mechanisms of DOX-induced cardiotoxicity have been studied extensively, including oxidative stress, inflammatory response, mitochondrial injury, endoplasmic reticulum (ER) stress, calcium (Ca2+) dyshomeostasis, apoptosis, fibrosis, and dysregulation of autophagy [6–11].
However, to date, there is no effective targeted therapeutic strategy, neither prophylactic nor curative, to protect against DOX-induced cardiotoxicity. Dexrazoxane is the only drug currently approved for reducing DOX-induced cardiotoxicity. Recently, Li et al. investigated the effects of continuous DOX infusion or dexrazoxane pre-treatment on DOX-induced cardiotoxicity. The results exhibited that changes in global longitudinal strain and left ventricular ejection fraction were scarcely observed. However, the increased levels of high-sensitivity troponin T were observed in > 59% of the patients who received continuous DOX infusion or dexrazoxane pre-treatment, suggesting that pre-treatment with dexrazoxane could not entirely improve subclinical DOX-induced cardiotoxicity [12]. Therefore, it is highly vital to investigate the prevention and treatment strategies as well as potential molecular mechanism for DOX-induced cardiotoxicity.
Sirtuin 1 (SIRT1), as a nicotinamide adenosine dinucleotide (NAD+)-dependent deacetylase, has been verified to play a critical role in DOX-induced cardiotoxicity. A previous study summarized some mechanisms of SIRT1 regarding DOX-induced cardiotoxicity, while the involved regulatory pathways are not comprehensive, only including the regulation of transforming growth factor-beta (TGF-β), tumor protein p53 (p53), and forkhead box O (FOXO) proteins [13]. In addition, we identified several shortcomings with respect to the application of SIRT1 in DOX-induced cardiotoxicity. Hence, this review summarizes the recent advances related to protective effects and mechanisms, as well as deficiencies in clinical application of SIRT1 in DOX-induced cardiotoxicity. First, we outline the mechanisms of DOX-induced cardiotoxicity and then explain the significant protective role of SIRT1 in the heart. Subsequently, we highlight and summarize SIRT1-mediated signaling pathways in DOX-induced cardiotoxicity. Finally, we indicate several SIRT1 agonists and their protective roles in DOX-induced cardiotoxicity, as well as deficiencies of SIRT1 in clinical application.
Mechanisms of doxorubicin-induced cardiotoxicity
Oxidative stress and inflammatory response
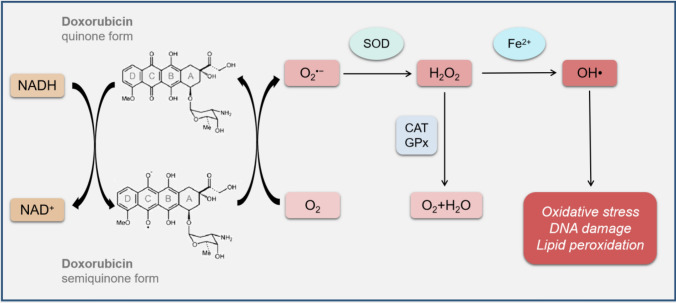
Oxidative stress refers to an imbalance between reactive oxygen species (ROS) production and antioxidant defense system, which is one of the main mechanisms of DOX-induced cardiotoxicity. Typically, mitochondria are the primary sites of ROS generation [14]. The enzymes that produce ROS in mitochondria can convert quinone moiety in ring C of DOX into semiquinone form through single-electron reduction. The semiquinone form of DOX is easy to react with oxygen molecules (O2) to form superoxide anion (), which is neutralized by superoxide dismutase (SOD) to become relatively stable and low-toxic hydrogen peroxide (H2O2). In the presence of iron (Fe2+), H2O2 may also generate highly reactive and toxic hydroxyl radical (OH∙). ROS reacts with DNA, proteins, and lipids, eventually leading to DNA damage and lipid peroxidation [15, 16] (Fig. 1). A previous study demonstrated that DOX could increase cardiac ROS generation and malondialdehyde (MDA, an end product of lipid hydroperoxide) level to result in oxidative stress. Nuclear factor erythroid 2-related factor 2 (NRF2) regulates numerous antioxidant proteins, including heme oxygenase-1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1), SOD, and catalase (CAT). Cardamonin increases the expression and reduces the degradation of NRF2 to improve DOX-induced oxidative stress through inhibiting ROS production and MDA level and upregulating the expression of antioxidant enzymes [17]. Reportedly, NAD phosphate (NADPH) oxidase 2 (NOX2) and NOX4 are involved in signal transduction during oxidative stress, which can be stimulated by DOX to increase ROS production and subsequent oxidative stress in cardiomyocytes [18]. Recently, Chen et al. found that metformin could inhibit the expression of mitogen-activated protein kinase (MAPK) and activate the expression of AMP-activated protein kinase (AMPK) to exert antioxidative and anti-apoptotic effects on cardiomyocytes during DOX treatment [19].
Fig. 1.
DOX-related redox cycling. The mitochondrial NADH-dependent enzymes can convert quinone moiety in ring C of DOX into semiquinone form through single-electron reduction. The semiquinone form of DOX reacts with O2 to form , which is neutralized by SOD to H2O2. In the presence of Fe2+, H2O2 might also generate highly reactive and toxic OH∙, which reacts with DNA, proteins and lipids, leading to DNA damage and lipid peroxidation. DOX doxorubicin, NADH nicotinamide adenine dinucleotide hydrate, O2 oxygen molecule, superoxide anion, SOD superoxide dismutase, H2O2 hydrogen peroxide, Fe2+ iron, OH∙, hydroxyl radical, CAT catalase, GPx glutathione-dependent peroxidase, H2O water
Inflammation is positively correlated with the oxidative stress, and thus, accelerates myocardial injury. Hwang et al. elucidated that oxidative stress could lead to inflammatory response through activating nuclear factor kappa B (NF-κB), a redox-sensitive transcription factor [20]. DOX has shown to upregulate the levels of several inflammatory factors, including interleukin-1β (IL-1β), IL-6, IL-17, and tumor necrosis factor-alpha (TNF-α) in the heart [21, 6]. In myeloid differentiation protein 1 (MD-1)-knockout mice, Toll-like receptor (TLR4)/MAPKs/NF-κB pathway was over-activated by DOX, accelerating myocardial inflammatory response and suggesting that MD-1 plays a pivotal role in DOX-induced cardiac inflammation [22]. NOD-like receptor family pyrin domain-containing protein 3 (NLRP3) inflammasome, as a critical regulator of the innate immune system, could be activated by DOX to result in myocardial inflammation [23]. Moreover, the transient receptor potential ankyrin 1 (TRPA1) channel is stimulated by DOX to cause cardiotoxicity through promoting oxidative stress and inflammation [6]. Based on the above-mentioned findings, oxidative stress and inflammation are regarded as significant risk factors for DOX-induced cardiotoxicity, while the specific correlation and mechanisms need to be further investigated.
Mitochondrial injury
As sites of cellular respiration and energy production, mitochondria play a central role in regulating pathophysiological activities, such as cell growth, proliferation, differentiation, damage, repair, and death [24]. Recent studies demonstrated that mitochondria are key targets of DOX-induced cardiotoxicity. The heart is more remarkably sensitive to toxicity of DOX than other organs owing to cardiolipin, which is localized in the mitochondrial inner membrane, has a high affinity to DOX and leads to the accumulation of DOX-cardiolipin complex in the heart [25]. When the accumulated dose of DOX is > 50–100 μM in the mitochondria, the electron transport chain is disrupted by inhibiting complexes I, and simultaneously, ROS production is increased [26]. Some studies have shown that the primary mechanism of DOX-induced mitochondrial dysfunction might be related to mitochondrial permeability transition (MPT). MPT is the phenomenon whereby the inner membrane suddenly allows free passage of solutes up to 1.5 kDa in size. The MPT pore (mPTP) could be opened by DOX-induced mitochondrial oxidative damage and Ca2+ overload, which might lead to the collapse of the inner membrane potential, halting of mitochondrial adenosine triphosphate (ATP) synthesis, and uncoupling of respiratory chain, promoting mitochondrial osmotic swelling and rupture, and cell death [27, 28]. While the biophysical properties of the mPTP are well-established, the exact molecular nature is yet an enigma. Cyclophilin D (CypD) is a genetically verified and undisputed regulator of mPTP function. Dhingra et al. reported that DOX could impair NF-κB signaling accompanied by mitochondrial injury and CypD-mediated mPTP opening through the activation of mitochondrial death protein Bcl-2/19 kDa interacting protein 3. However, the precise mechanism by which CypD controls mPTP opening has not been explored [29]. In addition, some studies suggested that adenine nucleotide translocator is not a requisite component of the mPTP but regulates mPTP activity in heart mitochondria from DOX-treated rats [27]. Recently, Catanzaro et al. pointed out that DOX could also enhance mitochondrial fragmentation and accelerate mitochondrial degradation by the lysosome, eventually leading to cardiotoxicity [30]. As an inhibitor of mitophagy, liensinine could decrease the phosphorylation of dynamin-related protein 1 (Drp1) at the Ser616 site to inhibit mitochondrial fragmentation, mitophagy, and cytochrome c (Cyt C) leakage, thereby ameliorating mitochondrial dysfunction and cardiac injury following DOX treatment [7]. Although the effects of DOX on mitochondrial injury in the heart have been widely studied, the specific mechanisms and pathways by which DOX induces mitochondrial mPTP opening and mitochondrial fragmentation need to be further explored.
Endoplasmic/sarcoplasmic reticulum stress and calcium dyshomeostasis in cardiac cells
The changes in the function of ER lead to the accumulation of unfolded or misfolded proteins, resulting in a cellular condition called ER stress [31]. ER stress is mediated by three ER-resident transmembrane sensors, including activating transcription factor 6 (ATF6), protein RNA-like ER kinase(PERK), and inositol-requiring enzyme 1 (IRE1) [32]. When ER stress is sustained or aggravated, the c-JUN NH2-terminal kinase (JNK), caspase-12, and C/EBP homologous protein (CHOP) signaling pathways-mediated cell apoptosis occurs [33]. DOX could increase the expression of ER stress-related proteins, such as p-PERK, ATF6, ATF4, 78-kDa glucose-regulated protein precursor (GRP78), and CHOP to result in apoptosis. However, CACNA1H-specific inhibitor ABT-639 could reverse the DOX-mediated increased levels of ER stress-related proteins and pro-apoptotic proteins in H9c2 cells, suggesting that CACNA1H is involved in DOX-induced cardiotoxicity through effecting ER stress [34]. Another study found that DOX could increase transcriptional intermediary factor 1 (TIF1) expression but inhibit GRP78 expression to simulate the expression of ATF6 and IRE1, resulting in ER stress [35]. Recently, Wang et al. found that the DOX-activated TRPA1 channel in cardiomyocytes also could cause cardiotoxicity by promoting ER stress [6].
Sarcoplasmic reticulum (SR) has been considered as a specialized form of the ER in cardiac cells, which is primarily responsible for the regulation of Ca2+ fluxes and consequently, the control of excitation–contraction coupling [36]. Ca2+ homeostasis disequilibrium is involved in the development of DOX-induced cardiotoxicity because of its pivotal role in cardiac electrical activity and excitation–contraction coupling. Llach et al. showed that low-dose of DOX could disrupt the cardiac function with a reduction in ejection fraction and slight dilation at 15 weeks after the end of the treatment, which could be associated with a decline in the intracellular free Ca2+ concentration [8]. Early DOX treatment could induce SR Ca2+ leak by the interaction between DOX and ryanodine receptor and the sarco/ER Ca2+-ATPase; subsequently, Ca2+/calmodulin-dependent protein kinase II (CaMKII) was activated to result in DOX-induced cardiotoxicity. CaMKII is associated with caspase-dependent apoptosis pathways. SR Ca2+ homeostasis and ER stress are closely interlinked. GRP78 is not only a central mediator of the unfolded protein response during ER stress but also a regulator of Ca2+ homeostasis in the ER. Low levels of GRP78 overexpression could protect cardiomyocytes from DOX-induced cell apoptosis by reducing CaMKII activation and p53 accumulation. Mechanism study further demonstrated above CaMKII activation and p53 accumulation was occurred in a Ca2+-dependent manner, thereby explaining the beneficial action of GRP78 was to normalize Ca2+ handling [37]. Taken together, ER stress and Ca2+ dyshomeostasis are crucial in DOX-induced cardiotoxicity. However, the functional correlation between the ER and SR in cardiomyocytes is yet confusing; therefore, the interaction between ER stress and Ca2+ dyshomeostasis in DOX-induced cardiotoxicity need further investigation.
Apoptosis
Apoptosis is a form of programmed cell death in multicellular organisms. It plays a substantial role in the evolution of organisms, stability of internal environment, and normal cell renewal [38]. A study reported that DOX-induced cardiotoxicity is related to nuclear translocation of p53 and p53-dependent apoptosis. Extracellular signal-regulated kinases (ERKs) could be activated by DOX to phosphorylate p53 at Ser15. The phosphorylation of p53 could lead to cardiomyocyte apoptosis via downregulation of anti-apoptotic B-cell lymphoma 2 (Bcl-2), upregulation of pro-apoptotic Bcl-2-associated X protein (Bax), and activation of caspase-3, caspase-9, and poly-ADP-ribose polymerase [9, 39]. Moreover, DOX-induced oxidative stress opens the voltage-dependent anion channel (VDAC) to result in MPT and subsequently release Cyt C from the mitochondria [40]. In the presence of ATP, Cyt C modulates the allosteric activation and hepta-oligomerization of the adaptor molecule apoptosis-protease activating factor 1 to produce apoptosome, which recruits the dimers of caspase-9 to facilitate the activation of caspase-3, eventually initiating the apoptotic degradation phase [41]. Mitofusin 2 (Mfn2) is a mitochondrial GTPase fusion protein that plays a crucial role in mitochondrial fusion and fission. DOX treatment decreased the expression of Mfn2 accompanied by an increase in mitochondrial fragmentation and ROS generation, further causing cardiomyocyte apoptosis [42]. However, detailed mechanisms underlying the Mfn2 inhibition by DOX and ROS production regulation by Mfn2 are not yet clarified. Furthermore, Leboucher et al. found that Mfn2 is a substrate for JNK, which phosphorylates Mfn2 to result in its ubiquitin-dependent proteasomal degradation, leading to mitochondrial fragmentation and apoptosis in DOX-treated sarcoma U2OS cells [43]. Hitherto, the exact mechanism by which inhibition of Mfn2 leads to cardiomyocyte apoptosis in DOX-induced cardiotoxicity is unknown and needs to be further investigated. Intriguingly, death receptor-mediated apoptosis in cardiomyocytes could be a major mechanism of DOX-induced cardiotoxicity [44]. Recently, a study explored the role of nuclear factor of activated T cells (NFAT)/Fas/Fas ligand (FasL) axis in cardiomyocyte apoptosis during DOX treatment in rats and found that DOX could increase the levels of Fas, FasL and NFAT to induce caspase-8-mediated intrinsic cell death, resulting in elevated expression of Bax or caspase-3. Further mechanistic investigation unveiled that the activation of NFAT/Fas/FasL axis was mediated by upregulated expression of calcineurin and p38 MAPK and downregulated expression of mammalian target of rapamycin (mTOR) [45]. The activation of E2F transcription factor 1 (E2F1)/AMPKα2 signaling pathway was also found to be involved in DOX-induced cardiomyocyte apoptosis [46]. Although the effects and mechanisms of DOX on cardiomyocyte apoptosis have been studied extensively, the specific signaling pathways involved in the DOX-induced apoptosis are deemed complicated.
Autophagy
Autophagy is a catabolic process involving the elimination of cellular misfolded proteins and damaged or old organelles to maintain cellular homeostasis by the regulation of autophagy-related (Atg) genes and lysosomal proteolysis [47, 48]. Some studies have demonstrated the role of autophagy in DOX-induced cardiotoxicity (Table 1). On the one hand, a number of studies pointed out that DOX could stimulate cardiac autophagy and conduce to the pathogenic mechanism of DOX-induced cardiotoxicity [35, 49–53]. Xu et al. demonstrated that DOX could stimulate autophagy through increased ratio of microtubule-associated proteins 1A/1B light chain 3-II (LC3-II)/LC3-I and upregulated expression of p62, Beclin-1, and Atg5 by stimulating the expression of JNK1 and p70S6 kinase (p70S6K) [35]. Moreover, mTOR has been proved to be one of the major negative regulators of autophagy. The inhibition of mTOR by DOX promotes autophagy by increased expression of LC3-II, Atg5, Atg6, Atg8 and Atg12 via AMPK activation and p38 MAPK inhibition [49]. Rutin is a polyphenolic flavonoid that suppresses DOX-induced autophagy by increased phosphorylation of protein kinase B (Akt) [50]. However, the effect of DOX on autophagy regulation in cardiomyocytes is controversial. Gu et al. found that DOX could cause cardiotoxicity via inhibition of autophagy rather than stimulation of autophagic flux [10, 46, 54, 55–57]. DOX inhibits autophagy via a sequence of pathways, such as AMPK/Unc-51 like autophagy activating kinase 1 (ULK1) [56] and E2F1/mTOR complex 1 (mTORC1) [46]. Recently, Pan et al. reported that autophagy in human AC16 cells was upregulated by the protective mechanism of cytotoxicity, and autophagy-related proteins (for example, the ratio of LC3-II/LC3-I and Beclin-1) showed a short-term increase in the early stage of DOX intervention. As the concentration and time of DOX intervention increased, the autophagy flux would be decompensated and reduced to lose the ability of self-cleaning and gradually move towards apoptosis [58]. The above-mentioned controversial findings about the effects of DOX on cardiac autophagy might be associated with the specific species, dosage of DOX, experimental conditions, and observation of different and intertwined pathways.
Table 1.
The effect of autophagy in DOX-induced cardiotoxicity
| Animal or cellular model | DOX dose | Treatment period | Autophagy markers | Effect on autophagy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| C57BL/6 mice | 24 mg/kg (cumulative dose) | 12 days | LC3; p62; Beclin-1; Atg5 | Induction | DOX stimulates autophagy through increased ratio of LC3-II/LC3-I, and increased expression of p62, Beclin-1, and Atg5 by upregulated expression of JNK1 and p70S6K | [35] |
| C57BL/6 mice and NRCs | 21 mg/kg (cumulative dose) and 1 μM | 2 weeks and 24 h | LC3; p62; Atg5 | Induction | DOX stimulates autophagy through increased expression of LC3-II, p62, and Atg5 by reduced phosphorylation of Akt | [50] |
| C57BL/6 mice and H9c2 cells | 32 mg/kg (cumulative dose) and 10 μM | 4 weeks and 24 h | LC3; Atg5; Atg6; Atg8; Atg12 | Induction | DOX stimulates autophagy through increased expression of LC3-II, Atg5, Atg6, Atg8, and Atg12 by AMPK activation and p38 MAPK inhibition and subsequent mTOR inhibition | [49] |
| H9c2 cells | 5 μg/ml | 24 h | LC3; p62; Beclin-1 | Induction | DOX stimulates autophagy through increased expression of LC3-II and Beclin-1 and decreased expression of p62 | [51] |
| NRCs | 1 μM | 18 h | LC3; p62; Atg5; Atg12-Atg5 complex | Induction | DOX stimulates autophagy through increased expression of Atg5 and Atg12-Atg5 complex and decreased expression of p62 by upregulation of p70S6K expression | [52] |
| NRCs | 1 μM | 18 h | LC3; p62; Beclin-1; Atg5; Atg7; Atg12 | Induction | DOX stimulates autophagy through increased expression of LC3-II, Beclin-1, Atg5, Atg7, and Atg12 and decreased expression of p62 by depleting GATA4 protein levels | [53] |
| C57BL/6 mice and H9c2 cells | 20 mg/kg (cumulative dose) and 1 μM | 4 weeks and 24 h | LC3; p62; Beclin-1; LAMP1 | Inhibition | DOX inhibits autophagy through suppression of Beclin-1/LAMP1 pathway | [10] |
| C57BL/6 mice | 15 mg/kg (single dose) | 3 days | Beclin-1 | Inhibition | DOX inhibits autophagy through decreased Beclin-1 by inhibition of AMPK and activation of mTOR | [54] |
| C57BL/6 mice and H9c2 cells | 20 mg/kg (cumulative dose) and 1 μM | 4 weeks and 24 h | LC3 | Inhibition | DOX inhibits autophagy through decreased ratio of LC3-II/LC3-I by activating E2F1/mTORC1 pathway | [46] |
| GFP-LC3 transgenic mice and H9c2 cells | 20 mg/kg (cumulative dose) and 3 μM | 15 days and 24 h | LC3; p62 | Inhibition | DOX inhibits autophagy as shown in the accumulation of LC3-I and p62 | [55] |
| GFP-LC3 transgenic mice and neonatal mice cardiomyocytes | 20 mg/kg (cumulative dose) and 0.1 μM | 5 days and 6 h | LC3; p62 | Inhibition | DOX inhibits autophagy through decreased expression of AMPK and ULK1 | [56] |
DOX doxorubicin, H9c2 cells rat cardiomyocyte cell line, NRCs neonatal rat ventricular cardiomyocytes, LC3 microtubule-associated proteins 1A/1B light chain 3, Atg autophagy-related, LAMP1 lysosomal-associated membrane proteins 1, JNK1 c-JUN NH2-terminal kinase 1, p70S6K p70S6 kinase, Akt protein kinase B, AMPK AMP-activated protein kinase, MAPK mitogen-activated protein kinase, mTORC1 mammalian target of rapamycin complex 1, E2F1 E2F transcription factor 1, ULK1 Unc-51 like autophagy activating kinase 1
Fibrosis
Cardiac fibrosis is a vital mechanism of DOX-induced cardiotoxicity [59, 60]. It has been proved that matrix metalloproteinases (MMPs) regulate cardiac fibrosis via degradation of extracellular matrix (ECM). Narikawa et al. demonstrated that DOX could increase the expression of MMP1, TGF-β, and collagen in human cardiac fibroblasts and speculated that DOX-induced upregulation of MMP1 could impair the balance of ECM via activating phosphoinositide 3-kinase (PI3K)/Akt signaling pathway to result in cardiac dysfunction and fibrosis [61]. Moreover, MMP2 activation is involved in DOX-induced cardiotoxicity, causing intracellular matrix and ECM remodeling through myofilament lysis by proteolyzing cardiac titin [11]. Another study found that DOX treatment could induce cardiac fibrosis via the neurokinin 1 receptor (NK-1R) and collagen production by cardiac fibroblasts [62]. Currently, only limited information is available about the effect of cardiac fibrosis on DOX-induced cardiotoxicity, and the specific mechanisms and pathways underlying DOX-induced cardiac fibrosis need further exploration. A schematic diagram of mechanisms involved in DOX-induced cardiotoxicity is illustrated in Fig. 2.
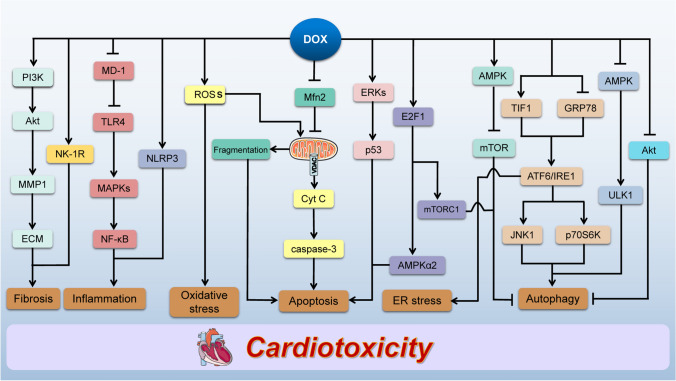
Fig. 2.
Schematic representation of mechanisms involved in DOX-induced cardiotoxicity. DOX increases ROS production that results in cardiac oxidative stress. Moreover, DOX accelerates myocardial inflammation by MD-1/TLR4/MAPKs/NF-κB and NLRP3 pathways. DOX increases TIF1 expression and inhibits GRP78 expression to simulate the expression of ATF6 and IRE1, resulting in ER stress. DOX-induced oxidative stress causes the release of Cyt C from mitochondria via the opening of VDAC to activate caspase-3, leading to the cardiomyocyte apoptosis. Furthermore, DOX inhibits Mfn2 expression to cause mitochondrial fragmentation, resulting in cardiomyocyte apoptosis, and induces cardiomyocyte apoptosis by ERKs/p53 and E2F1/AMPKα2 pathways. DOX stimulates cardiac autophagy via upregulating the expression of JNK1 and p70S6K, as well as activating AMPK/mTOR pathway and inhibiting Akt expression. Conversely, DOX inhibits cardiac autophagy through inhibiting AMPK/ULK1 pathway and activating E2F1/mTORC1 pathway. DOX-induced upregulation of MMP1 impairs the balance of ECM via activating PI3K/Akt pathway, resulting in cardiac fibrosis. DOX also induces cardiac fibrosis through NK-1R. DOX doxorubicin, ROS reactive oxygen species, MD-1 myeloid differentiation protein 1, TLR4 toll-like receptor, MAPK mitogen-activated protein kinase, NF-κB nuclear factor kappa B, NLRP3 NOD-like receptor family pyrin domain-containing protein 3, TIF1 transcriptional intermediary factor 1, GRP78 78-kDa glucose-regulated protein precursor, ATF6 activating transcription factor 6, IRE1 inositol-requiring enzyme 1, ER endoplasmic reticulum, Cyt C cytochrome c, VDAC voltage-dependent anion channel, Mfn2 mitofusin 2, ERKs extracellular signal-regulated kinases, p53 tumor protein p53, E2F1 E2F transcription factor 1, AMPK AMP-activated protein kinase, JNK1 c-JUN NH2-terminal kinase 1, p70S6K p70S6 kinase, mTORC1 mammalian target of rapamycin complex 1, Akt protein kinase B, ULK1 Unc-51 like autophagy activating kinase 1, MMP1 matrix metalloproteinase 1, ECM extracellular matrix, PI3K phosphoinositide 3-kinase, NK-1R neurokinin 1 receptor
The role of SIRT1 in the heart
In rodents, the terminal differentiation of cardiomyocytes occurs over the first 2 weeks of postnatal life, characterized by the exit from the cell cycle, binucleation and loss of ability to proliferate. The expression of SIRT1 was low in embryonic hearts and notably increased in rats’ hearts at postnatal day 7. In contrast to the expression trend of SIRT1, that of cardiac-specific micorRNA-133a (miR-133a) was remarkable in embryonic hearts while that decreased markedly in the hearts of rats at postnatal day 7. Notably, SIRT1 suppression could reduce binucleated cardiomyocytes after birth, suggesting that endogenous SIRT1 and its upstream miR-133a may play substantial roles in the terminal differentiation and maturation of cardiomyocytes during cardiac development [63]. Furthermore, SIRT1-deficient mice indicated cardiac developmental defects and rarely postnatal survival [64]. Therefore, SIRT1 is a favorable factor for cardiac development and may be a potential therapeutic strategy for heart injury or diseases. Accumulating evidence suggested a protective role of SIRT1 in myocardial ischemia/reperfusion (I/R) injury, diabetic cardiomyopathy (DCM), and cardiac aging (Fig. 3).
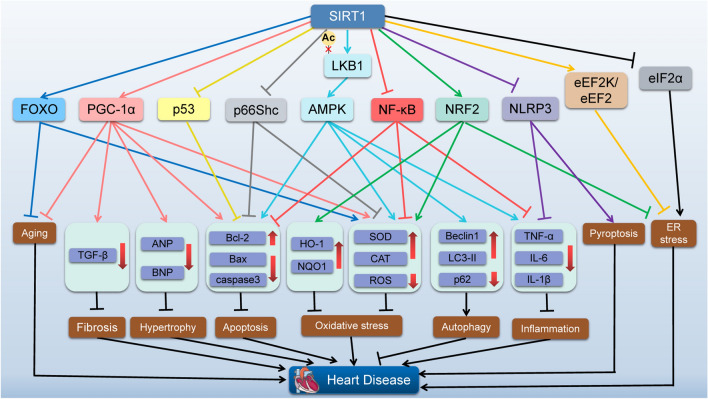
Fig. 3.
The role of SIRT1 in the heart. Diagram showing proposed downstream targets of SIRT1 and their related effects implicated in myocardial protection. SIRT1 can activate or inhibit some signaling pathways to exert a protective effect on the heart. SIRT1 Sirtuin 1, FOXO forkhead box O, PGC-1α peroxisome proliferator-activated receptor gamma coactivator-1 alpha, p53 tumor protein p53, LKB1 liver kinase B1, AMPK AMP-activated protein kinase, NF-κB nuclear factor kappa B, NRF2 nuclear factor erythroid 2-related factor 2, NLRP3 NOD-like receptor family pyrin domain-containing protein 3, eEF2K eukaryotic elongation factor-2 kinase, eIF2α eukaryotic initiation factor 2 alpha, TGF-β transforming growth factor-beta, ANP atrial natriuretic peptide, BNP brain natriuretic peptide, Bcl-2 B-cell lymphoma 2, Bax Bcl-2-associated X protein, HO-1 heme oxygenase-1, NQO1 NAD(P)H quinone dehydrogenase 1, SOD superoxide dismutase, CAT catalase, ROS reactive oxygen species, LC3-II microtubule-associated proteins 1A/1B light chain 3-II, TNF-α tumor necrosis factor-alpha, IL interleukin, ER endoplasmic reticulum
The role of SIRT1 in myocardial ischemia/reperfusion injury
I/R injury is a major cause of myocardial injury that results in cardiac remodeling and heart failure. Accumulating evidence suggested that changes in SIRT1 levels affect myocardial injury and cardiac function. Cardiomyocyte-specific knockout of Sirt1 gene sensitized myocardium to I/R injury via metabolic shift, oxidative stress, apoptosis, and impaired autophagic influx. On the contrary, SIRT1 overexpression could improve cardiac function and decrease the myocardial infarction size caused by I/R injury via liver kinase B1 (LKB1) deacetylation and subsequent AMPK activation [65]. Guan et al. demonstrated that oxidative stress and Ca2+ overload were major mechanisms in the processes of I/R injury in cardiomyocytes, which could be improved by CD38 deficiency via activation of SIRT1/FOXOs pathway [66]. Moreover, the suppression of apoptosis is one of the major mechanisms of SIRT1 to protect the heart from injury. A study reported that SIRT1 activation by quercetin could stimulate peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) to upregulate Bcl-2 expression and downregulate Bax expression, thereby playing a pivotal anti-apoptosis role in improving I/R-induced myocardial damage [67]. DJ-1, a member of the eponymous DJ-1 superfamily, can suppress oxidative stress, regulate transcription, and promote cell survival. The increase in DJ-1 expression by resveratrol could stimulate SIRT1 activity via directly binding to SIRT1, which inhibited p53 acetylation, thereby attenuating hypoxia/reoxygenation-induced cardiomyocyte apoptosis [68]. Furthermore, the role of SIRT1-mediated anti-ER stress is an essential mechanism in the treatment of myocardial I/R injury. Wang et al. demonstrated that crocin could stimulate SIRT1/NRF2 signaling pathway to increase the expression of anti-apoptotic Bcl-2 and decrease the expression of ER stress makers (GRP78 and CHOP) and pro-apoptotic Bax and caspase-3, thereby improving the cardiac damage caused by I/R injury [69]. In addition, SIRT1 could alleviate ER stressor tunicamycin-induced ER stress in cardiomyocytes via regulating eukaryotic initiation factor 2 alpha (eIF2α) [70] and eukaryotic elongation factor-2 kinase (eEF2K)/eEF2 pathways [71]. However, whether SIRT1/eIF2α or SIRT1/eEF2K/eEF2 pathway plays an anti-ER stress role in the treatment of myocardial I/R injury needs to be further explored.
The role of SIRT1 in diabetic cardiomyopathy
DCM is one of the major complications in people with diabetes, characterized by cardiac fibrosis, myocardial hypertrophy, and ventricular systolic and/or diastolic dysfunction. Numerous studies have demonstrated that increased expression of SIRT1 is a crucial therapeutic target for the prevention and treatment of DCM. SIRT1 activation could exercise antioxidant function by reducing ROS production and MDA level, as well as activating SOD. Concurrently, it effectuated the anti-fibrotic effect by inhibiting the expression of fibrotic markers (α-smooth muscle actin, type-I collagen, and type-III collagen) via suppressing TGF-β1/mothers against decapentaplegic homolog 3 (SMAD3) pathway in DCM [72]. NF-κB is translocated into the nucleus as a response to oxidative stress, where it induces the transcription of several proinflammatory cytokines, serving as a negatively regulated downstream target of SIRT1. Bagul et al. found that activation of SIRT1 by resveratrol could deacetylate p65 subunit of NF-κB at Lys310 and histone 3 at Lys9 to reduce the expression of NOX1, NOX4, TNF-α, and IL-6, thereby inhibiting DCM-mediated cardiac oxidative stress and inflammation [73]. In addition, SIRT1 also exerts a cardioprotective role through attenuating myocardial hypertrophy in DCM. Waldman et al. reported that caloric restriction could protect the heart from DCM by inhibiting the expression of TNF-α, TGF-β, atrial natriuretic peptide (ANP), and brain natriuretic peptide (BNP) in diabetic mice, and the molecular mechanism of protective effects might be associated with the increase in cardiac SIRT1 and PGC-1α levels [74]. Furthermore, emerging evidence indicated that mitochondrial dysfunction might be one of the vital factors underlying the pathology in DCM. A study on cardiac-specific Sirt1 knockout mice demonstrated that SIRT1 could promote the expression of mitochondrion-related genes, such as nuclear respiratory factor 1 (Nrf-1), Nrf-2, mitochondrial transcription factor A (Tfam), and estrogen-related receptor-α to improve DCM via PGC-1α deacetylation [75]. Additionally, SIRT1/PGC-1α pathway is also involved in the inhibition of mitochondrial fission and apoptosis in diabetic hearts [76]. As described above, SIRT1 plays a pivotal cardioprotective role in DCM through inhibiting cardiac oxidative stress, fibrosis, inflammation, apoptosis, and mitochondrial fission, as well as improving myocardial hypertrophy and mitochondrial dysfunction by targeting diverse cellular factors and signaling pathways.
The role of SIRT1 in cardiac aging
Cardiac aging is characterized by increased apoptosis and necrosis, myocyte nuclei proliferation, cardiac hypertrophy, interstitial fibrosis, and cardiac dysfunction. A previous study demonstrated that SIRT1 exerts a protective role in cardiovascular aging [77]. Ralph et al. reported that 2.5–7.5-fold cardiac-specific overexpression of SIRT1 could inhibit the cellular senescence through regulating the INK4/ARF family proteins and p53 protein. In addition, 7.5-fold cardiac-specific overexpression of SIRT1 could upregulate the levels of some molecule markers such as heat shock protein 40 (HSP40), HSP70, HSP90, telomere reverse transcriptase, telomere repeat-binding factor 2, and Klotho, and subsequently improve age-dependent cardiac hypertrophy, fibrosis, apoptosis, and cardiac dysfunction. Conversely, the excessive elevation of SIRT1 might not always be advantageous. Transgenic mice with 12.5-fold overexpression of SIRT1 showed significantly increased cardiac β-myosin heavy chain, atrial natriuretic factor, α-skeletal actin, and TUNEL-positive cell, which eventually leaded to cardiac dysfunction [78]. In addition, exercise training could improve aging-induced inflammation, which might be associated with the upregulation of SIRT1 and PGC-1α in the hearts of aging rats [79]. Another study found that exercise training could also attenuate aging-induced cardiomyocyte apoptosis via stimulating SIRT1 expression [80]. SIRT1 also plays a cardioprotective role by regulating autophagy and mitochondrial integrity in aging hearts. Ren et al. demonstrated that Akt2 ablation could improve cardiac aging through restoring FOXO1-related mitochondrial integrity, which might be associated with SIRT1-mediated autophagy regulation [81]. Another study focused on the role of SIRT1 in aging human hearts and found that the expression of SIRT1 could be associated with gender. Lower expression of antioxidative protein SOD2 and higher expression of macrophages and proinflammatory cytokines were more obviously observed in old than young female hearts, which could be related to the diverse expression of cardiac SIRT1 and SIRT3 [82]. Although several studies have confirmed the anti-aging role of SIRT1 in myocardium, the specific mechanisms need to be further assessed.
Pathway regulation of SIRT1 in doxorubicin-induce cardiotoxicity
SIRT1 is involved in the regulation of oxidative stress [83–85], mitochondrial function [83], apoptosis [85, 86], inflammatory response [86], ER stress [87], fibrosis [88], and other processes by mediating various signaling pathways to play a significant role in DOX-induced cardiotoxicity. Thus, we summarize and introduce the signaling pathways regulated by SIRT1 in DOX-induced cardiotoxicity, including SIRT1/PGC-1α, SIRT1/AMPK, SIRT1/p53, SIRT1/p66Shc, SIRT1/NF-κB, SIRT1/p38 MAPK, SIRT1/FOXOs, SIRT1/TGF-β, and SIRT1/NLRP3 (Table 2; Figs. 3, 4).
Table 2.
Downstream targets of SIRT1 that are regulated during DOX-induced cardiotoxicity
| Substrate | Animal or cell model | DOX dose | Treatment period | Functions of SIRT1 | Mechanism | Reference |
|---|---|---|---|---|---|---|
| PGC-1α | C57BL/6 mice and H9c2 cells | 20 mg/kg (cumulative dose) and 1 μM | 6 days and 24 h | Antioxidation and regulate mitochondrial function | Deacetylate PGC-1α | [97] |
| Human cardiomyocyte AC16 cells | 125 nM | 24 h | Regulate mitochondrial function and biogenesis | Deacetylate PGC-1α | [95] | |
| AMPK | 129S1/SvlmJ mice and H9c2 cells | 20 mg/kg (cumulative dose) and 5 μg/ml | 4 weeks and 22 h | Anti-inflammation, antioxidation and anti-apoptosis | Deacetylate LKB1 and activate AMPK | [105] |
| p53 | Balb/c mice | 24 mg/kg (cumulative dose) | 3 weeks | Anti-apoptosis | Deacetylate p53 | [110] |
| H9c2 cells | 1 μM | 24 h | Anti-apoptosis | Deacetylate p53 | [111] | |
| p66Shc | Sprague–Dawley rats | 16 mg/kg or 32 mg/kg (cumulative dose) | 4 or 8 weeks | Anti-apoptosis | Suppress p66Shc expression | [116] |
| Sprague–Dawley rats | 20 mg/kg (cumulative dose) | 9 days | Regulate mitochondrial function, antioxidation and anti-apoptosis | Suppress p66Shc expression | [117] | |
| NF-κB | C57BL/6 mice | 15 mg/kg (single dose) or 15 mg/kg (cumulative dose) | 8 days or 6 weeks | Anti-inflammation | Suppress nuclear translocation of NF-κB | [86] |
|
p38 MAPK |
C57BL/6 mice | 20 mg/kg (single dose) | 5 days | Antioxidation and anti-apoptosis | Suppress p38 MAPK expression | [129] |
| FOXOs | H9c2 cells | 5 μM | 24 h | Anti-apoptosis | Suppress FOXO1 expression | [136] |
| TGF-β | Fischer 344 rats | 15 mg/kg (cumulative dose) | 2 weeks | Anti-fibrosis | Suppress TGF-β/SMAD3 pathway | [88] |
| NLRP3 | Sprague–Dawley rats and H9c2 cells | 15 mg/kg (cumulative dose) and 5 μM | 6 weeks and 24 h | Anti-inflammation | Suppress the NLRP3 inflammasome | [23] |
| Kunming mice and H9c2 cells | 15 mg/kg (single dose) and 5 μM | 7 days and 24 h | Anti-inflammation | Suppress the NLRP3 inflammasome | [151] |
SIRT1 Sirtuin 1, DOX doxorubicin, PGC-1α peroxisome proliferator-activated receptor gamma coactivator-1 alpha, LKB1 liver kinase B1, AMPK AMP-activated protein kinase, p53 tumor protein p53, NF-κB nuclear factor kappa B, MAPK mitogen-activated protein kinase, FOXO forkhead box O, TGF-β transforming growth factor-beta, NLRP3 NOD-like receptor family pyrin domain-containing protein 3, H9c2 cells rat cardiomyocyte cell line, SMAD3 mothers against decapentaplegic homolog 3
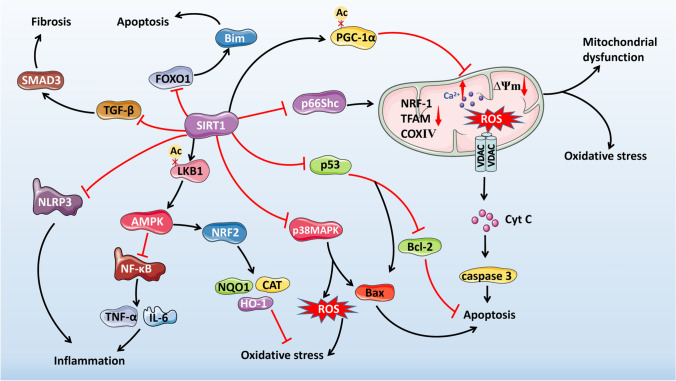
Fig. 4.
Mechanisms of SIRT1-mediated signaling pathways involved in DOX-induced cardiotoxicity. Diagram showing proposed downstream targets of SIRT1 and their related effects implicated in DOX-induced cardiotoxicity. SIRT1 Sirtuin 1, DOX doxorubicin, PGC-1α peroxisome proliferator-activated receptor gamma coactivator-1 alpha, NRF-1 nuclear respiratory factor 1, TFAM mitochondrial transcription factor A, COXIV cytochrome c oxidase IV, ∆Ψm mitochondrial membrane potential, ROS reactive oxygen species, VDAC voltage-dependent anion channel, Cyt C cytochrome c, LKB1 liver kinase B1, AMPK AMP-activated protein kinase, NF-κB nuclear factor kappa B, TNF-α tumor necrosis factor-alpha, IL interleukin, NRF2 nuclear factor erythroid 2-related factor 2, NQO1 NAD(P)H quinone dehydrogenase 1, CAT catalase, HO-1 heme oxygenase-1, p53 tumor protein p53, Bcl-2 B-cell lymphoma 2, Bax Bcl-2-associated X protein, MAPK mitogen-activated protein kinase, FOXO1 forkhead box O 1, TGF-β transforming growth factor-beta, SMAD3 mothers against decapentaplegic homolog 3, NLRP3 NOD-like receptor family pyrin domain-containing protein 3
SIRT1/PGC-1α pathway
PGC-1α is a transcriptional coactivator that regulates mitochondrial biogenesis and function, antioxidant defense systems, and cell metabolism by interacting with specific co-activated genes [89, 90]. Previous studies have reported that SIRT1 is a significant upstream regulator of PGC-1α [91, 92]. When the energy storage of the cell is reduced, the increasing levels of NAD+ can activate SIRT1 expression, and then SIRT1 is combined with PGC-1α at 200–400 amino acids to deacetylate PGC-1α, thereby facilitating mitochondrial ATP production by mitochondrial substrate oxidation [93]. Fang et al. pointed out that SIRT1 could strengthen cellular antioxidant capacity and alleviate mitochondrial dysfunction to improve DCM by PGC-1α deacetylation [94]. Moreover, the activation of SIRT1/PGC-1α pathway could also protect the heart from I/R injury via inhibition of apoptosis [67]. A study concentrated on the DOX-treated human cardiomyocyte AC16 cells and demonstrated that DOX could increase PGC-1α acetylation and suppress the downstream genes related to mitochondrial function and biogenesis, such as SOD2, NRF-1, Cyt C oxidase IV and TFAM to result in oxidative stress, loss of mitochondrial membrane potential (∆Ψm) and disruption of mitochondrial energy, which could be reversed through SIRT1 activation by erythropoietin pre-treatment [95]. Moreover, Govender et al. found that melatonin could improve mitochondrial morphology, increase mitochondrial fusion, repair ATP generation and decrease myocardial apoptosis to ameliorate DOX-induced cardiotoxicity. The study on the underlying mechanism further demonstrated that the protective role of melatonin was associated with upregulation of cardiac SIRT1 and PGC-1α [96]. Recently, another study indicated that pterostilbene treatment could also upregulate the expression and deacetylation of PGC-1α via AMPK and SIRT1 cascades to improve DOX-induced mitochondrial injury and oxidative stress in cardiomyocytes [97]. Therefore, the efficient regulation of SIRT1/PGC-1α pathway plays a crucial role in the prevention and treatment of DOX-induced cardiotoxicity by maintaining mitochondrial homeostasis, as well as inhibiting oxidative stress and apoptosis.
SIRT1/AMPK pathway
AMPK is a conserved energy sensor that regulates cellular metabolism. The activation of AMPK increases the ATP-producing catabolism, including glucose metabolism and fatty acid oxidation, and decreases ATP-consuming anabolism, such as protein and lipid synthesis, aiming to maintain cellular energy storage [98]. The activation of AMPK also results in advanced mitochondrial biogenesis by increasing PGC-1α expression [99]. Kobashigawa et al. reported that AMPK is a sensitive target of DOX-induced cardiotoxicity [100]. DOX could inhibit AMPK phosphorylation and activate downstream mTOR to cause pathological cardiac phenotype [101]. SIRT1 was considered to be implicated in the metabolic regulation of AMPK in the heart. A study on SIRT1 in the treatment of I/R injury discovered that SIRT1 could improve cardiac function, reduce myocardial infarction size, and attenuate the oleate oxidation to rescue the tolerance of aged hearts to I/R injury via LKB1 deacetylation and subsequent AMPK activation [65]. In addition, the activation of SIRT1/AMPK pathway could improve cardiac metabolic remodeling in ventricular cardiomyocytes induced by aldosterone stimulation [102]. Alternatively, other studies revealed that AMPK could also activate SIRT1 by modulating intracellular NAD+ metabolism [103, 104]. Recently, we found that fibroblast growth factor 21 (FGF21) could facilitate the interaction of SIRT1 with LKB1 to enhance LKB1 deacetylation and subsequently activate AMPK to prevent DOX-induced oxidative stress, inflammation, and apoptosis, as well as ameliorate cardiac dysfunction partly through elevating nuclear NRF2 expression and inhibiting NF-κB expression both in vivo and in vitro[105]. In addition to the regulation of oxidative stress, inflammation, and apoptosis, AMPK is also a crucial regulator of autophagy in DOX-induced cardiotoxicity [49]; thus, whether this protective effect is dependent on the expression of SIRT1 remains obscure and needs to be further investigated.
SIRT1/p53 pathway
p53 is a primary tumor suppressor that mainly acts as a transcription factor in response to numerous stressors to control cell proliferation, senescence, apoptosis, and DNA repair [106]. Under normal circumstances, murine double minute-2 (Mdm2), an E3 ubiquitin ligase, targets p53 for proteasomal degradation and maintains the expression of p53 at a low-level. In response to acute stress, Mdm2 is inactivated, following which, it elevates p53 level that prohibits cell division and induces apoptosis [107]. A study on angiotensin II-induced cardiomyocyte apoptosis found that SIRT1 could inhibit p53 acetylation and its binding to the mitochondrial fission protein Drp1 promoter to suppress mitochondrial fission and cardiomyocyte apoptosis [108]. In addition, SIRT1/p53 pathway was also reported to be involved in the treatment of myocardial hypoxia/reoxygenation injury [68]. Generally, SIRT1 could regulate cardiac apoptosis through both p53 transcription-dependent and p53 transcription-independent pathways. The former is effectuated on the expression of the apoptosis-related target genes, such as Bax, PUMA, and NOXA [109], whereas the latter is initiated through Cyt C release from the mitochondria intramembrane [41]. For mouse models with DOX treatment, DOX-induced cardiomyocyte apoptosis was amplified through promoting Cyt C release from mitochondria and increasing p53 acetylation and p53-dependent transcription of Bax. These effects were inhibited by resveratrol. Moreover, resveratrol could also upregulate SIRT1 expression, suggesting that the cardioprotective effect of resveratrol was associated with SIRT1-mediated deacetylation of p53 and subsequent inhibition of p53 transcription-dependent and transcription-independent apoptosis [110]. Furthermore, Zhang et al. demonstrated that HSP25 knockdown could increase p53 acetylation on K379 by attenuating the correlation between SIRT1 and p53 to aggravate DOX-induced H9c2 cell apoptosis [111]. Therefore, pharmacological interventions that regulate SIRT1/p53 pathway might provide an effective pathway for the treatment of DOX-induced cardiotoxicity.
SIRT1/p66Shc pathway
p66Shc is a member of the spontaneous human combustion (shc) family that regulates the redox balance in the cells via elevation of mitochondrial ROS production [112]. p66Shc deletion could improve oxidative stress and cardiac dysfunction in pressure overload-induced heart failure [113]. Consistent with this study, Liu et al. reported that miR-124 could decrease ROS generation and MDA level, as well as increase activity of SOD to alleviate DOX-induced oxidative stress in the heart through inhibiting p66Shc expression [114], suggesting that inhibition of p66Shc might be a potential therapeutic strategy for DOX-induced cardiotoxicity. SIRT1 has emerged as a critical upstream regulator of p66Shc, while deletion of cardiomyocyte-specific Sirt1 gene could increase oxidative stress of aged hearts in response to ischemic insults by enhancing p66Shc phosphorylation [65]. Moreover, overexpression of SIRT1 could decrease the level of p66Shc by binding to the p66Shc promoter to prevent hyperglycemia-induced endothelial dysfunction through reducing oxidative stress and the expression of endothelial dysfunction marker plasminogen activator inhibitor-1 [115]. On the other hand, p66Shc was reported to be positively correlated with pro-apoptotic Bax expression and caspase-3 activation, while that is negatively associated with the expression of anti-apoptotic Bcl-2. Zhu et al. demonstrated that miR-34a-5p could inhibit SIRT1 expression and increase p66shc level to promote cardiomyocyte apoptosis after DOX treatment, highlighting that miR-34a-5p/SIRT1/p66Shc is a significant axis that is conducive to DOX-induced cardiotoxicity [116]. Moreover, another study showed that berberine could upregulate SIRT1 expression and subsequently downregulate p66Shc expression to exert antioxidative and anti-apoptotic effects, and improve mitochondrial dysfunction, thereby ameliorating DOX-induced cardiotoxicity [117]. The above-mentioned findings indicate a potential therapeutic target of SIRT1/p66Shc pathway in DOX-induced cardiotoxicity.
SIRT1/NF-κB pathway
NF-κB, known as a critical regulator of inflammatory and immunological responses, plays a pivotal role in the development of DOX-induced cardiotoxicity. A previous study pointed out that DOX could upregulate NF-κB expression to cause cardiac inflammation [118]. SIRT1 has been exhibited to inhibit NF-κB expression through deacetylation of RelA/p65 subunit of NF-κB on Lys310 to regulate inflammatory phases and energy supply for metabolic processes. Concurrently, the deacetylation of RelA/p65 protein on Lys310 makes its own methylation on Lys314 and Lys315 to enhance its ubiquitination and degradation [119, 120]. Bagul et al. found that SIRT1 activation by resveratrol pre-treatment could inhibit NF-κB expression to improve cardiac oxidative stress and inflammation in diabetic rats [73]. SIRT1/NF-κB pathway also participates in the inhibition of myocardial apoptosis to protect H9c2 cells against hypoxia-induced injury [121]. A recent study by Yuan et al. indicated that overexpression of SIRT1 by C1q/tumor necrosis factor-related protein-3 (CTRP3) could exert anti-inflammatory effect to improve DOX-induced cardiotoxicity through reduced levels of TNF-α and nuclear translocation of NF-κB [86]. Based on these findings, the anti-inflammatory effect of SIRT1 on DOX-induced cardiotoxicity might be partially due to the inhibition of NF-κB, while further investigation is required to elucidate the regulatory mechanism of NF-κB by SIRT1 in DOX-induced cardiotoxicity.
SIRT1/p38 MAPK pathway
p38 MAPK, a stress-activated kinase, can be induced by inflammatory response and osmotic stress. The activity of p38 MAPK is mediated by the upstream kinases, known as MAPK kinases (MKK), such as MKK3 and MKK6 [122]. Previous studies pointed out that p38 MAPK activation was involved in several cardiac pathological changes, and p38 MAPK inhibition could improve cardiac fibrosis [123], pressure-loaded right ventricular hypertrophy [124], cardiac oxidative stress, and myocardial I/R injury [125]. Guo et al. found that the inhibition of p38 MAPK/NF-κB pathway could decrease the levels of inflammatory factors IL-1β, IL-6, and TNF-α, which in turn, protected H9c2 cells from DOX toxicity [126]. SIRT1 has been reported to be a negative regulator of p38 MAPK. Previously, it was shown that the overexpression of SIRT1 could attenuate mitochondrial dysfunction, oxidative stress, and apoptosis to improve I/R injury of cardiomyocytes. Further mechanistic investigation revealed that the cardioprotective role of SIRT1 is involved in reduction of p38 MAPK and JNK phosphorylation as well as enhancement of ERK phosphorylation [127]. In addition, SIRT1/MAPK pathway is known to participate in the treatment of DCM [128]. Another study focused on SIRT1 in the treatment of DOX-induced cardiotoxicity and demonstrated that SIRT1 could inhibit oxidative stress and cardiomyocyte apoptosis partially via the inhibition of p38 MAPK expression [129]. Currently, only a limited number of studies are available on the role of SIRT1/p38 MAPK pathway in DOX-induced cardiotoxicity. Therefore, further studies are required to clarify the exact functions and mechanisms of SIRT1/p38 MAPK pathway.
SIRT1/FOXOs pathway
FOXO transcription factors, including four members, FOXO1, FOXO3, FOXO4, and FOXO6 in mammals, are widely involved in stress response, cellular homeostasis, and longevity. To carry out these functions, FOXOs regulate various cellular processes, such as oxidative stress, metabolic homeostasis, protein homeostasis, cell apoptosis, and repair of DNA damage [130]. In normal physiological conditions, FOXO1 are primarily localized in the cytoplasm. Under various stress stimulations, SIRT1 could deacetylate FOXO1 at K242, K245, and K262 and enhance the nuclear localization of FOXO1 in the heart [131–133]. Moreover, SIRT1 could enhance the abilities of oxidative stress resistance and DNA damage repair in the heart through the deacetylation and activation of FOXOs [132, 134]. On the contrary, other studies reported that SIRT1 negatively regulates the expression of FOXOs; for instance, Wang et al. found that the ability of FOXO3a could be inhibited by SIRT1 to improve oxidative stress-induced endothelial progenitor cell apoptosis through ubiquitination and degradation of FOXO3a [135]. Consistent with this study, resveratrol could protect the heart from DOX-induced apoptosis accompanied by increased expression of SIRT1, and reduced expression of FOXO1 and its target cell death gene Bim. Therefore, the anti-apoptotic function of SIRT1 on DOX-induced cardiotoxicity might be associated with the negative regulation of FOXO1 expression [136]. The above controversial reports about the regulation of FOXOs by SIRT1 might be related to the different post-translational modifications of FOXOs. FOXOs deacetylation by SIRT1 might increase its transcription and activity, whereas deacetylation of FOXOs promotes its own poly-ubiquitination and degradation [137]. Therefore, the specific effects and mechanisms related to the SIRT1/FOXOs pathway in DOX-induced cardiotoxicity need further investigation.
SIRT1/TGF-β pathway
TGF-β family is a multipotent cytokine involved in numerous cellular functions, such as modulating cell growth, proliferation, differentiation, and apoptosis. It can also regulate the production of ECM, including collagen and fibronectin. TGF-β family has three structurally similar isoforms: TGF-β1, TGF-β2, and TGF-β3, encoded by three different genes [138, 139]. A previous study demonstrated that TGF-β could directly stimulate its downstream targets SMADs to induce the overexpression of pro-fibrotic genes [140]. SIRT1 plays a potential regulatory role in abolishing TGF-β-induced collagen synthesis and myofibroblast differentiation. Li et al. found that SIRT1 activation by tetrahydrocurcumin could inhibit TGF-β1/SMAD3 pathway to exercise anti-fibrotic function and ameliorate DCM [72]. Another study on isoproterenol-induced cardiac fibrosis demonstrated that SIRT1 exerts a protective role by endothelial-to-mesenchymal transition via downregulation of TGF-β/SMAD2/3 pathway [141]. In addition, TGF-β is known to be involved in the occurrence of DOX-induced cardiotoxicity[142]. In a rat model of DOX-induced cardiotoxicity, activation of SIRT1 by resveratrol could decrease the levels of TGF-β and pSMAD3/SMAD3 ratio, as well as the expression of fibronectin and type-I collagen, which subsequently inhibited cardiac fibrosis and fibroblast activation to improve diastolic dysfunction and myocardial remodeling [88]. Therefore, the anti-fibrotic function mediated by SIRT1/TGF-β pathway may play an indispensable function in the treatment of DOX-induced cardiotoxicity.
SIRT1/NLRP3 pathway
NLRP3, a critical regulator of the innate immune system, is activated in response to the stimulation of endogenous and exogenous factors [143]. The activation of NLRP3 inflammasome results in the activation of caspase-1 and transforms pro-IL-1β and pro-IL-18 into their bioactive forms (IL-1β and IL-18), leading to inflammatory response and tissue injury [144]. Moreover, NLRP3-induced innate immune response could lead to pyroptosis, an inflammatory form of programmed cell death [145]. Previous studies showed that NLRP3 inflammasome plays a critical role in the pathogenic mechanism of cardiovascular diseases, including myocardial I/R injury [146], atherosclerosis [147], and DOX-induced cardiotoxicity [148]. Furthermore, it was previously found that SIRT1 could downregulate the NLRP3 inflammasome to modulate cellular inflammation [149]. A recent study on the protective role of SIRT1 in myocardial I/R injury demonstrated that SIRT1 could protect cardiomyocytes from I/R injury through inhibiting NLRP3-dependent inflammation and pyroptosis via Akt-dependent metabolic regulation [150]. Recently, Zhai et al. pointed out that SIRT1 activation by calycosin could not only upregulate the cardiac activity of glutathione peroxidase, CAT, and SOD but also decrease the levels of ROS, MDA, IL-1β, and NLRP3 in DOX-treated mice, suggesting that the protective effects of calycosin on DOX-induced cardiac dysfunction are involved in SIRT1/NLRP3 pathway [151]. In addition, dihydromyricetin could also protect against DOX-induced inflammation by SIRT1/NLRP3 pathway [23]. Hence, it could be concluded that SIRT1/NLRP3 signaling pathway is a potential therapeutic target for DOX-induced cardiotoxicity; however, the specific mechanisms related to the inhibition of NLRP3 by SIRT1 in DOX-induced cardiotoxicity remain elusive, highlighting the necessity of conducting further research.
SIRT1 agonists and their roles in DOX-induced cardiotoxicity
As discussed earlier, the activation of SIRT1 is a major cardioprotective therapy for DOX-induced cardiotoxicity. A number of substances, such as resveratrol, pterostilbene, melatonin, erythropoietin, berberine, sesamin, CTRP3, FGF21, dihydromyricetin, and calycosin, stimulate SIRT1 to exert protective functions on DOX-induced cardiotoxicity (summarized in Table 3). Resveratrol is the earliest and most studied SIRT1 agonist that can stimulate SIRT1 to antagonize DOX-induced cardiotoxicity. It is a natural phytoalexin that belongs to polyphenols and is mainly found in the skin of grapes and red wines [152]. Numerous studies indicated that resveratrol increased SIRT1 expression to alleviate DOX-induced mitochondrial dysfunction, ER stress, cardiac fibrosis, and apoptosis [83, 87, 88, 110]. Pterostilbene is a natural resveratrol analogue and acknowledged as an antioxidant found in grapes and blueberries [153]. Relevant studies have shown that pterostilbene is an effective myocardial protective agent to prevent various heart diseases, such as I/R injury, myocardial infarction, and DCM through free radical elimination and anti-inflammatory roles [154–156]. It also upregulates SIRT1 and AMPK cascades and subsequently activates PGC-1α to resist DOX-induced mitochondrial injury and oxidative stress [97]. Notably, a clinical study demonstrated that pterostilbene is generally safe for the human body and can attenuate adult blood pressure [157].
Table 3.
The main roles of each SIRT1 agonist in DOX-induced cardiotoxicity
| SIRT1 agonist | Roles in DOX-induced cardiotoxicity |
|---|---|
| Resveratrol | Regulate mitochondrial function, inhibit ER stress, anti-fibrosis and anti-apoptosis |
| Pterostilbene | Antioxidation and regulate mitochondrial function |
| Melatonin | Regulate mitochondrial function and anti-apoptosis |
| Erythropoietin | Regulate mitochondrial function and biogenesis |
| Berberine | Regulate mitochondrial function, antioxidation and anti-apoptosis |
| Sesamin | Antioxidation |
| CTRP3 | Anti-inflammation and anti-apoptosis |
| FGF21 | Antioxidation, anti-inflammation and anti-apoptosis |
| Dihydromyricetin | Anti-inflammation |
| Calycosin | Anti-inflammation |
SIRT1 Sirtuin 1, DOX doxorubicin, ER endoplasmic reticulum, CTRP3 C1q/tumor necrosis factor-related protein-3, FGF21 fibroblast growth factor 21
Melatonin is another drug that enhances the expression of SIRT1 and PGC-1α and improves DOX-mediated mitochondrial dysfunction and cardiomyocyte apoptosis [96]. Reportedly, melatonin, as a mitochondria-targeted antioxidant, exerts an effect on mitochondrial homeostasis and functions [158]. Erythropoietin and its receptor on hearts have been manifested to play pivotal protective roles in myocardial I/R injury and cyanotic congenital heart disease by modulating mitochondrial morphology and biogenesis [159, 160]. The beneficial functions of erythropoietin mentioned above are also effective in antagonizing DOX-induced cardiotoxicity, which is implicated in the regulation of SIRT1/PGC-1α pathway [95]. In addition, berberine, a broad-spectrum antibiotic with antineoplastic and cardioprotective effects, also inhibits DOX-induced mitochondrial dysfunction by adjusting ∆Ψm, mitochondrial Ca2+ concentration, and mitochondrial biogenesis. Importantly, pre-treatment with berberine upregulates SIRT1 expression and downregulates p66Shc expression to exercise antioxidative and anti-apoptotic functions in the heart following DOX exposure [117, 161]. Sesamin, as another SIRT1 agonist with antioxidative and anti-hypertensive roles, ameliorates DOX-induced cardiotoxicity through the regulation of manganese SOD (MnSOD) expression [84]. Moreover, CTRP3, an endogenous lipopolysaccharide antagonist, activates SIRT1 and subsequently reduces DOX-induced inflammation and apoptosis [86]. As an effective adjuster of glucose and lipid metabolism in adipose and liver tissue, FGF21 is also verified to express in the myocardium. In a previous study, we found that it could restore the DOX-disrupted interaction of SIRT1 and LKB1 and then decrease LKB1 acetylation, thereby inducing AMPK activation to improve cardiac oxidative stress, inflammation, and apoptosis [105]. Recently, Sun et al. pointed out that dihydromyricetin, a flavonoid compound with cardioprotective effect extracted from the Japanese raisin tree (Hovenia dulcis), could inhibit DOX-induced activation of NLRP3 inflammasome to protect against cardiac inflammation through stimulating SIRT1 signaling [23]. Furthermore, as the primary active constituent in astragalus membranaceus with anti-tumor, anti-inflammatory, and cardioprotective roles, calycosin also activates SIRT1/NLRP3 pathway to ameliorate DOX-induced inflammation [151]. Although the effects of numerous SIRT1 agonists on DOX-induced cardiotoxicity have been reported, various animal or cellular models and different doses and timescales of DOX make it difficult to reach consensus on protective molecular mechanisms. Moreover, some SIRT1 agonists might not be specific, and further assessment is required to identify specific SIRT1 agonists for clinical application.
Conclusion
SIRT1 plays a protective role in DOX-induced cardiotoxicity by modulating various signaling pathways. Although previous studies have reported the protective effects of SIRT1 on DOX-induced cardiotoxicity in rodent models, no systematic study has yet evaluated the effects of clinical combination therapy and the optimum time to take precaution. In addition, several studies ignored many aspects of human cancer management in clinical practice; for example, various studies used a single dose or multiple high doses and intraperitoneal injection of DOX to treat animals, while human cancer management was typically implemented with continuous cycles of DOX and intravenous administration. Therefore, a recent editorial emphasized the importance of horizontally integrating research on DOX-induced cardiotoxicity, such as using intravenous administration and small repeated doses in animal models [162]. Notably, the experimental results in animals need to be observed and confirmed in humans. Another study on mouse model has verified that an appropriate amount of SIRT1 (2.5- to 7.5-fold) expression is beneficial for the heart, whereas higher levels of SIRT1 (12.5-fold or greater) can trigger cardiomyopathy [78]. Thus, appropriate doses of SIRT1 agonists should be carefully evaluated for the optimal use of the therapeutic potential of SIRT1 in clinical application.
Notably, although numerous studies have shown that resveratrol, as an effective agonist of SIRT1, plays a critical role in DOX-induced cardiotoxicity, its application in clinical practice is limited due to low solubility in water and poor systemic bioavailability [163]. In order to improve the bioavailability, newer methods of delivery need to be developed so that it can be adequately absorbed from the gut. Recently, Quagliariello et al. reported that nanoemulsions loaded with anti-inflammatory nutraceuticals and nano-encapsulation of coenzyme Q10 in nano-emulsions acted against DOX-induced cardiotoxicity, thereby providing an effective method of delivery [21, 164]. Resveratrol solid lipid nanoparticles and PLGA nanoparticles have been found to be submicron drug delivery systems for delivering resveratrol, enabling its gradual release and better distribution in the body [165, 166]. Furthermore, SIRT1, as a redox-sensitive deacetylase, can be downregulated under oxidative/inflammatory conditions through post-translational modification [167]. Thus, reversing the post-translational modification of SIRT1 might be an ideal approach to treat chronic diseases associated with oxidative stress and inflammation. In conclusion, further in-depth studies on the effects and mechanisms of SIRT1 would provide novel ideas for the prevention and treatment of DOX-induced cardiotoxicity.
Acknowledgements
This study was supported by Qilu Young Scholar's Program of Shandong University (21330089963007), National Natural Science Foundation of China (81700329, 81770375) and Jilin Science and Technology Department (20200801061GH).
Funding
This study was supported by Qilu Young Scholar’s Program of Shandong University (21330089963007), National Natural Science Foundation of China (81700329, 81770375) and Jilin Science and Technology Department (20200801061GH).
Compliance with ethical standards
Conflict of interest
There are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gergely S, Hegedus C, Lakatos P, Kovacs K, Gaspar R, Csont T, Virag L. High throughput screening identifies a novel compound protecting cardiomyocytes from doxorubicin-induced damage. Oxid Med Cell Longev. 2015;2015:178513. doi: 10.1155/2015/178513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 3.Baech J, Hansen SM, et al. Cumulative anthracycline exposure and risk of cardiotoxicity; a Danish nationwide cohort study of 2440 lymphoma patients treated with or without anthracyclines. Br J Haematol. 2018;183(5):717–726. doi: 10.1111/bjh.15603. [DOI] [PubMed] [Google Scholar]
- 4.Narayan HK, Finkelman B, French B, Plappert T, Hyman D, Smith AM, Margulies KB, Ky B. Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation. 2017;135(15):1397–1412. doi: 10.1161/CIRCULATIONAHA.116.023463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64(9):938–945. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Wang M, Liu J, Ye J, Jiang H, Xu Y, Ye D, Wan J. Inhibition of TRPA1 attenuates doxorubicin-induced acute cardiotoxicity by suppressing oxidative stress, the inflammatory response, and endoplasmic reticulum stress. Oxid Med Cell Longev. 2018;2018:5179468. doi: 10.1155/2018/5179468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang X, Wang S, Wang L, Ceylan AF, Ren J, Zhang Y. Mitophagy inhibitor liensinine suppresses doxorubicin-induced cardiotoxicity through inhibition of Drp1-mediated maladaptive mitochondrial fission. Pharmacol Res. 2020;157:104846. doi: 10.1016/j.phrs.2020.104846. [DOI] [PubMed] [Google Scholar]
- 8.Llach A, Mazevet M, et al. Progression of excitation-contraction coupling defects in doxorubicin cardiotoxicity. J Mol Cell Cardiol. 2019;126:129–139. doi: 10.1016/j.yjmcc.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Cunha-Oliveira T, Ferreira LL, Coelho AR, Deus CM, Oliveira PJ. Doxorubicin triggers bioenergetic failure and p53 activation in mouse stem cell-derived cardiomyocytes. Toxicol Appl Pharmacol. 2018;348:1–13. doi: 10.1016/j.taap.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Li C, et al. Tanshinone IIA restores dynamic balance of autophagosome/autolysosome in doxorubicin-induced cardiotoxicity via targeting beclin1/LAMP1. Cancers (Basel) 2019;11(7):190. doi: 10.3390/cancers11070910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan BYH, Roczkowsky A, Cho WJ, Poirier M, Sergi C, Keschrumrus V, Churko JM, Granzier H, Schulz R. MMP inhibitors attenuate doxorubicin cardiotoxicity by preventing intracellular and extracellular matrix remodeling. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Chang HM, Banchs J, Araujo DM, Hassan SA, Wagar EA, Yeh ETH, Meng QH. Detection of subclinical cardiotoxicity in sarcoma patients receiving continuous doxorubicin infusion or pre-treatment with dexrazoxane before bolus doxorubicin. Cardiooncology. 2020;6:1. doi: 10.1186/s40959-019-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolinsky VW. The role of sirtuins in mitochondrial function and doxorubicin-induced cardiac dysfunction. Biol Chem. 2017;398(9):955–974. doi: 10.1515/hsz-2016-0316. [DOI] [PubMed] [Google Scholar]
- 14.Skulachev VP. Mitochondria-targeted antioxidants as promising drugs for treatment of age-related brain diseases. J Alzheimers Dis. 2012;28(2):283–289. doi: 10.3233/jad-2011-111391. [DOI] [PubMed] [Google Scholar]
- 15.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261(7):3060–3067. doi: 10.1016/S0021-9258(17)35746-0. [DOI] [PubMed] [Google Scholar]
- 16.Cappetta D, De Angelis A, et al. Oxidative stress and cellular response to doxorubicin: a common factor in the complex milieu of anthracycline cardiotoxicity. Oxid Med Cell Longev. 2017;2017:1521020. doi: 10.1155/2017/1521020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi W, Boliang W, Xiaoxi T, Guoqiang F, Jianbo X, Gang W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed Pharmacother. 2020;122:109547. doi: 10.1016/j.biopha.2019.109547. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Fang L, Li H, Li Z, Lyu L, Wang H, Xiao J. Astragaloside IV alleviates doxorubicin induced cardiomyopathy by inhibiting NADPH oxidase derived oxidative stress. Eur J Pharmacol. 2019;859:172490. doi: 10.1016/j.ejphar.2019.172490. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Zhang S, et al. Modulatory effect of metformin on cardiotoxicity induced by doxorubicin via the MAPK and AMPK pathways. Life Sci. 2020;249:117498. doi: 10.1016/j.lfs.2020.117498. [DOI] [PubMed] [Google Scholar]
- 20.Hwang J-w, Yao H, Caito S, Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radical Biol Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quagliariello V, Vecchione R, et al. Cardioprotective effects of nanoemulsions loaded with anti-inflammatory nutraceuticals against doxorubicin-induced cardiotoxicity. Nutrients. 2018;10(9):1304. doi: 10.3390/nu10091304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YJ, Huang H, Liu Y, Kong B, Wang G. MD-1 deficiency accelerates myocardial inflammation and apoptosis in doxorubicin-induced cardiotoxicity by activating the TLR4/MAPKs/nuclear factor kappa B (NF-kappaB) signaling pathway. Med Sci Monit. 2019;25:7898–7907. doi: 10.12659/MSM.919861. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Sun Z, Lu W, Lin N, Lin H, Zhang J, Ni T, Meng L, Zhang C, Guo H. Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem Pharmacol. 2020;175:113888. doi: 10.1016/j.bcp.2020.113888. [DOI] [PubMed] [Google Scholar]
- 24.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung KG, Cole LK, Xiang B, Chen K, Ma X, Myal Y, Hatch GM, Tong Q, Dolinsky VW. Sirtuin-3 (SIRT3) protein attenuates doxorubicin-induced oxidative stress and improves mitochondrial respiration in H9c2 cardiomyocytes. J Biol Chem. 2015;290(17):10981–10993. doi: 10.1074/jbc.M114.607960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Songbo M, Lang H, Xinyong C, Bin X, Ping Z, Liang S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett. 2019;307:41–48. doi: 10.1016/j.toxlet.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira PJ, Wallace KB. Depletion of adenine nucleotide translocator protein in heart mitochondria from doxorubicin-treated rats—relevance for mitochondrial dysfunction. Toxicology. 2006;220(2–3):160–168. doi: 10.1016/j.tox.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Kwong JQ, Molkentin JD. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. 2015;21(2):206–214. doi: 10.1016/j.cmet.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhingra R, Guberman M, Rabinovich-Nikitin I, Gerstein J, Margulets V, Gang H, Madden N, Thliveris J, Kirshenbaum LA. Impaired NF-κB signalling underlies cyclophilin D-mediated mitochondrial permeability transition pore opening in doxorubicin cardiomyopathy. Cardiovasc Res. 2020;116(6):1161–1174. doi: 10.1093/cvr/cvz240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catanzaro MP, Weiner A, et al. Doxorubicin-induced cardiomyocyte death is mediated by unchecked mitochondrial fission and mitophagy. FASEB J. 2019;33(10):11096–11108. doi: 10.1096/fj.201802663R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 32.Fu HY, Sanada S, et al. Chemical endoplasmic reticulum chaperone alleviates doxorubicin-induced cardiac dysfunction. Circ Res. 2016;118(5):798–809. doi: 10.1161/circresaha.115.307604. [DOI] [PubMed] [Google Scholar]
- 33.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J, Wu Q, Wang Z, Hong J, Chen R, Li B, Hu Z, Hu X, Zhang M. Inhibition of CACNA1H attenuates doxorubicin-induced acute cardiotoxicity by affecting endoplasmic reticulum stress. Biomed Pharmacother. 2019;120:109475. doi: 10.1016/j.biopha.2019.109475. [DOI] [PubMed] [Google Scholar]
- 35.Xu ZM, Li CB, Liu QL, Li P, Yang H. Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through the inhibition of autophagy and endoplasmic reticulum stress in mice. Int J Mol Sci. 2018;19(11):3658. doi: 10.3390/ijms19113658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalak M, Opas M. Endoplasmic and sarcoplasmic reticulum in the heart. Trends Cell Biol. 2009;19(6):253–259. doi: 10.1016/j.tcb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Tscheschner H, Meinhardt E, Schlegel P, Jungmann A, Lehmann LH, Muller OJ, Most P, Katus HA, Raake PW. CaMKII activation participates in doxorubicin cardiotoxicity and is attenuated by moderate GRP78 overexpression. PLoS ONE. 2019;14(4):e0215992. doi: 10.1371/journal.pone.0215992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49(5):330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Mao W, Ding B, Liang C-s. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295(5):H1956–H1965. doi: 10.1152/ajpheart.00407.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res. 2002;62(16):4592–4598. [PubMed] [Google Scholar]
- 41.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13(9):1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 42.Tang H, Tao A, Song J, Liu Q, Wang H, Rui T. Doxorubicin-induced cardiomyocyte apoptosis: role of mitofusin 2. Int J Biochem Cell Biol. 2017;88:55–59. doi: 10.1016/j.biocel.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Leboucher GP, Tsai YC, Yang M, Shaw KC, Zhou M, Veenstra TD, Glickman MH, Weissman AM. Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol Cell. 2012;47(4):547–557. doi: 10.1016/j.molcel.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L, Zhang B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci Rep. 2017;7(1):44735. doi: 10.1038/srep44735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shati AA. Doxorubicin-induces NFAT/Fas/FasL cardiac apoptosis in rats through activation of calcineurin and P38 MAPK and inhibition of mTOR signalling pathways. Clin Exp Pharmacol Physiol. 2020;47(4):660–676. doi: 10.1111/1440-1681.13225. [DOI] [PubMed] [Google Scholar]
- 46.Gu J, Fan YQ, Zhang HL, Pan JA, Yu JY, Zhang JF, Wang CQ. Resveratrol suppresses doxorubicin-induced cardiotoxicity by disrupting E2F1 mediated autophagy inhibition and apoptosis promotion. Biochem Pharmacol. 2018;150:202–213. doi: 10.1016/j.bcp.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 47.Shabalala S, Muller CJF, Louw J, Johnson R. Polyphenols, autophagy and doxorubicin-induced cardiotoxicity. Life Sci. 2017;180:160–170. doi: 10.1016/j.lfs.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Wang XL, et al. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem Pharmacol. 2014;88(3):334–350. doi: 10.1016/j.bcp.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 50.Ma Y, Yang L, Ma J, Lu L, Wang X, Ren J, Yang J. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):1904–1911. doi: 10.1016/j.bbadis.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Wang H, Liang EY, Zhou LX, Dong ZL, Liang P, Weng QF, Yang M. Thrombopoietin protects H9C2 cells from excessive autophagy and apoptosis in doxorubicin-induced cardiotoxicity. Oncol Lett. 2018;15(1):839–848. doi: 10.3892/ol.2017.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X, Chen K, Kobayashi S, Timm D, Liang Q. Resveratrol attenuates doxorubicin-induced cardiomyocyte death via inhibition of p70 S6 kinase 1-mediated autophagy. J Pharmacol Exp Ther. 2012;341(1):183–195. doi: 10.1124/jpet.111.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J Biol Chem. 2010;285(1):793–804. doi: 10.1074/jbc.M109.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nazari Soltan Ahmad S, Sanajou D, Kalantary-Charvadeh A, Hosseini V, Roshangar L, Khojastehfard M, Haiaty S, Mesgari-Abbasi M. β-LAPachone ameliorates doxorubicin-induced cardiotoxicity via regulating autophagy and Nrf2 signalling pathways in mice. Basic Clin Pharmacol Toxicol. 2020;126(4):364–373. doi: 10.1111/bcpt.13340. [DOI] [PubMed] [Google Scholar]
- 55.Sishi BJ, Loos B, van Rooyen J, Engelbrecht AM. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem Pharmacol. 2013;85(1):124–134. doi: 10.1016/j.bcp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Kawaguchi T, Takemura G, et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc Res. 2012;96(3):456–465. doi: 10.1093/cvr/cvs282. [DOI] [PubMed] [Google Scholar]
- 57.Chen C, Jiang L, Zhang M, Pan X, Peng C, Huang W, Jiang Q. Isodunnianol alleviates doxorubicin-induced myocardial injury by activating protective autophagy. Food Funct. 2019;10(5):2651–2657. doi: 10.1039/c9fo00063a. [DOI] [PubMed] [Google Scholar]
- 58.Pan JA, Tang Y, Yu JY, Zhang H, Zhang JF, Wang CQ, Gu J. miR-146a attenuates apoptosis and modulates autophagy by targeting TAF9b/P53 pathway in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2019;10(9):668. doi: 10.1038/s41419-019-1901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pei XM, Yung BY, Yip SP, Ying M, Benzie IF, Siu PM. Desacyl ghrelin prevents doxorubicin-induced myocardial fibrosis and apoptosis via the GHSR-independent pathway. Am J Physiol Endocrinol Metab. 2014;306(3):E311–E323. doi: 10.1152/ajpendo.00123.2013. [DOI] [PubMed] [Google Scholar]
- 60.Rodrigues PG, Miranda-Silva D, et al. Early myocardial changes induced by doxorubicin in the nonfailing dilated ventricle. Am J Physiol Heart Circ Physiol. 2019;316(3):H459–h475. doi: 10.1152/ajpheart.00401.2018. [DOI] [PubMed] [Google Scholar]
- 61.Narikawa M, Umemura M, et al. Doxorubicin induces trans-differentiation and MMP1 expression in cardiac fibroblasts via cell death-independent pathways. PLoS ONE. 2019;14(9):e0221940. doi: 10.1371/journal.pone.0221940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levick SP, Soto-Pantoja DR, Bi J, Hundley WG, Widiapradja A, Manteufel EJ, Bradshaw TW, Melendez GC. Doxorubicin-induced myocardial fibrosis involves the neurokinin-1 receptor and direct effects on cardiac fibroblasts. Heart Lung Circ. 2019;28(10):1598–1605. doi: 10.1016/j.hlc.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin AN, Han L, Dasgupta C, Huang L, Yang S, Zhang L. SIRT1 increases cardiomyocyte binucleation in the heart development. Oncotarget. 2018;9(8):7996–8010. doi: 10.18632/oncotarget.23847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng HL, Mostoslavsky R, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA. 2003;100(19):10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Quan N, Sun W, Chen X, Cates C, Rousselle T, Zhou X, Zhao X, Li J. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc Res. 2018;114(6):805–821. doi: 10.1093/cvr/cvy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guan XH, Liu XH, et al. CD38 deficiency protects the heart from ischemia/reperfusion injury through activating SIRT1/FOXOs-mediated antioxidative stress pathway. Oxid Med Cell Longev. 2016;2016:7410257. doi: 10.1155/2016/7410257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang J, Lu L, et al. Quercetin improve ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1alpha signaling. J Cell Biochem. 2019;120(6):9747–9757. doi: 10.1002/jcb.28255. [DOI] [PubMed] [Google Scholar]
- 68.Xu RY, Xu XW, Deng YZ, Ma ZX, Li XR, Zhao L, Qiu LJ, Liu HY, Chen HP. Resveratrol attenuates myocardial hypoxia/reoxygenation-induced cell apoptosis through DJ-1-mediated SIRT1-p53 pathway. Biochem Biophys Res Commun. 2019;514(2):401–406. doi: 10.1016/j.bbrc.2019.04.165. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Yuan B, Cheng B, Liu Y, Zhang B, Wang X, Lin X, Yang B, Gong G. Crocin alleviates myocardial ischemia/reperfusion-induced endoplasmic reticulum stress via regulation of miR-34a/Sirt1/Nrf2 pathway. Shock. 2019;51(1):123–130. doi: 10.1097/shk.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 70.Prola A, Pires Da Silva J, et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2alpha deacetylation. Cell Death Differ. 2017;24(2):343–356. doi: 10.1038/cdd.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pires Da Silva J, Monceaux K, Guilbert A, Gressette M, Piquereau J, Novotova M, Ventura-Clapier R, Garnier A, Lemaire C. SIRT1 protects the heart from ER stress-induced injury by promoting eEF2K/eEF2-dependent autophagy. Cells. 2020;9(2):426. doi: 10.3390/cells9020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li K, Zhai M, et al. Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxid Med Cell Longev. 2019;2019:6746907. doi: 10.1155/2019/6746907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bagul PK, Deepthi N, Sultana R, Banerjee SK. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J Nutr Biochem. 2015;26(11):1298–1307. doi: 10.1016/j.jnutbio.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Waldman M, Cohen K, et al. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1alpha’. Cardiovasc Diabetol. 2018;17(1):111. doi: 10.1186/s12933-018-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma S, Feng J, et al. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid Med Cell Longev. 2017;2017:4602715. doi: 10.1155/2017/4602715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding M, Feng N, et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1alpha pathway. J Pineal Res. 2018;65(2):e12491. doi: 10.1111/jpi.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.D'Onofrio N, Servillo L, Balestrieri ML. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal. 2018;28(8):711–732. doi: 10.1089/ars.2017.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alcendor RR, Gao S, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100(10):1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 79.Chen WK, Tsai YL, et al. Exercise training augments Sirt1-signaling and attenuates cardiac inflammation in d-galactose induced-aging rats. Aging (Albany NY) 2018;10(12):4166–4174. doi: 10.18632/aging.101714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lai CH, Ho TJ, et al. Exercise training enhanced SIRT1 longevity signaling replaces the IGF1 survival pathway to attenuate aging-induced rat heart apoptosis. Age (Dordr) 2014;36(5):9706. doi: 10.1007/s11357-014-9706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ren J, Yang L, Zhu L, Xu X, Ceylan AF, Guo W, Yang J, Zhang Y. Akt2 ablation prolongs life span and improves myocardial contractile function with adaptive cardiac remodeling: role of Sirt1-mediated autophagy regulation. Aging Cell. 2017;16(5):976–987. doi: 10.1111/acel.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barcena de Arellano ML, Pozdniakova S, Kuhl AA, Baczko I, Ladilov Y, Regitz-Zagrosek V. Sex differences in the aging human heart: decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense. Aging (Albany NY) 2019;11(7):1918–1933. doi: 10.18632/aging.101881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med. 2009;46(12):1589–1597. doi: 10.1016/j.freeradbiomed.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Su S, Li Q, et al. Sesamin ameliorates doxorubicin-induced cardiotoxicity: involvement of Sirt1 and Mn-SOD pathway. Toxicol Lett. 2014;224(2):257–263. doi: 10.1016/j.toxlet.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 85.Xu C, Liu C-H, Zhang D-L. MicroRNA-22 inhibition prevents doxorubicin-induced cardiotoxicity via upregulating SIRT1. Biochem Biophys Res Commun. 2020;521(2):485–491. doi: 10.1016/j.bbrc.2019.10.140. [DOI] [PubMed] [Google Scholar]
- 86.Yuan YP, Ma ZG, Zhang X, Xu SC, Zeng XF, Yang Z, Deng W, Tang QZ. CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. J Mol Cell Cardiol. 2018;114:38–47. doi: 10.1016/j.yjmcc.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Lou YU, Wang Z, Xu YI, Zhou P, Cao J, Li Y, Chen Y, Sun J, Fu LU. Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. Int J Mol Med. 2015;36(3):873–880. doi: 10.3892/ijmm.2015.2291. [DOI] [PubMed] [Google Scholar]
- 88.Cappetta D, Esposito G, et al. SIRT1 activation attenuates diastolic dysfunction by reducing cardiac fibrosis in a model of anthracycline cardiomyopathy. Int J Cardiol. 2016;205:99–110. doi: 10.1016/j.ijcard.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 89.Jones AW, Yao Z, Vicencio JM, Karkucinska-Wieckowska A, Szabadkai G. PGC-1 family coactivators and cell fate: roles in cancer, neurodegeneration, cardiovascular disease and retrograde mitochondria-nucleus signalling. Mitochondrion. 2012;12(1):86–99. doi: 10.1016/j.mito.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Tan Z, Luo X, Xiao L, Tang M, Bode AM, Dong Z, Cao Y. The role of PGC1alpha in cancer metabolism and its therapeutic implications. Mol Cancer Ther. 2016;15(5):774–782. doi: 10.1158/1535-7163.MCT-15-0621. [DOI] [PubMed] [Google Scholar]
- 91.Guo P, Pi H, et al. Melatonin improves mitochondrial function by promoting MT1/SIRT1/PGC-1 alpha-dependent mitochondrial biogenesis in cadmium-induced hepatotoxicity in vitro. Toxicol Sci. 2014;142(1):182–195. doi: 10.1093/toxsci/kfu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bu X, Wu LuX, Yang L, Xu X, Wang J, Tang J. Role of SIRT1/PGC-1alpha in mitochondrial oxidative stress in autistic spectrum disorder. Neuropsychiatr Dis Treat. 2017;13:1633–1645. doi: 10.2147/NDT.S129081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Di W, Lv J, Jiang S, Lu C, Yang Z, Ma Z, Hu W, Yang Y, Xu B. PGC-1: the energetic regulator in cardiac metabolism. Curr Issues Mol Biol. 2018;28:29–46. doi: 10.21775/cimb.028.029. [DOI] [PubMed] [Google Scholar]
- 94.Fang W-j, Wang C-j, He Y, Zhou Y-l, Peng X-d, Liu S-k. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol Sin. 2017;39(1):59–73. doi: 10.1038/aps.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cui L, Guo J, et al. Erythropoietin activates SIRT1 to protect human cardiomyocytes against doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol Lett. 2017;275:28–38. doi: 10.1016/j.toxlet.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 96.Govender J, Loos B, Marais E, Engelbrecht AM. Melatonin improves cardiac and mitochondrial function during doxorubicin-induced cardiotoxicity: a possible role for peroxisome proliferator-activated receptor gamma coactivator 1-alpha and sirtuin activity? Toxicol Appl Pharmacol. 2018;358:86–101. doi: 10.1016/j.taap.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 97.Liu D, Ma Z, Xu L, Zhang X, Qiao S, Yuan J. PGC1alpha activation by pterostilbene ameliorates acute doxorubicin cardiotoxicity by reducing oxidative stress via enhancing AMPK and SIRT1 cascades. Aging (Albany NY) 2019;11(22):10061–10073. doi: 10.18632/aging.102418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA. 2002;99(25):15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kobashigawa LC, Xu YC, Padbury JF, Tseng YT, Yano N. Metformin protects cardiomyocyte from doxorubicin induced cytotoxicity through an AMP-activated protein kinase dependent signaling pathway: an in vitro study. PLoS ONE. 2014;9(8):e104888. doi: 10.1371/journal.pone.0104888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gratia S, Kay L, Potenza L, Seffouh A, Novel-Chate V, Schnebelen C, Sestili P, Schlattner U, Tokarska-Schlattner M. Inhibition of AMPK signalling by doxorubicin: at the crossroads of the cardiac responses to energetic, oxidative, and genotoxic stress. Cardiovasc Res. 2012;95(3):290–299. doi: 10.1093/cvr/cvs134. [DOI] [PubMed] [Google Scholar]
- 102.Liu GZ, Zhang S, et al. Aldosterone stimulation mediates cardiac metabolism remodeling via Sirt1/AMPK signaling in canine model. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(7):851–863. doi: 10.1007/s00210-019-01641-2. [DOI] [PubMed] [Google Scholar]
- 103.Dziewulska A, Dobosz AM, Dobrzyn A, Smolinska A, Kolczynska K, Ntambi JM, Dobrzyn P. SCD1 regulates the AMPK/SIRT1 pathway and histone acetylation through changes in adenine nucleotide metabolism in skeletal muscle. J Cell Physiol. 2020;235(2):1129–1140. doi: 10.1002/jcp.29026. [DOI] [PubMed] [Google Scholar]
- 104.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang S, Wang Y, Zhang Z, Liu Q, Gu J. Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis. 2017;8(8):e3018. doi: 10.1038/cddis.2017.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lacroix M, Riscal R, Arena G, Linares LK, Le Cam L. Metabolic functions of the tumor suppressor p53: implications in normal physiology, metabolic disorders, and cancer. Mol Metab. 2020;33:2–22. doi: 10.1016/j.molmet.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mak TW, Hauck L, Grothe D, Billia F. p53 regulates the cardiac transcriptome. Proc Natl Acad Sci USA. 2017;114(9):2331–2336. doi: 10.1073/pnas.1621436114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qi J, Wang F, Yang P, Wang X, Xu R, Chen J, Yuan Y, Lu Z, Duan J. Mitochondrial fission is required for angiotensin II-induced cardiomyocyte apoptosis mediated by a Sirt1-p53 signaling pathway. Front Pharmacol. 2018;9:176. doi: 10.3389/fphar.2018.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu Z, Wang H, Zhang L, Tang A, Zhai Q, Wen J, Yao L, Li P. Both p53-PUMA/NOXA-Bax-mitochondrion and p53–p21cip1 pathways are involved in the CDglyTK-mediated tumor cell suppression. Biochem Biophys Res Commun. 2009;386(4):607–611. doi: 10.1016/j.bbrc.2009.06.083. [DOI] [PubMed] [Google Scholar]
- 110.Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo Q, Liu M, Chen G, Xiao X. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res. 2011;90(3):538–545. doi: 10.1093/cvr/cvr022. [DOI] [PubMed] [Google Scholar]
- 111.Zhang C, Qu S, et al. HSP25 down-regulation enhanced p53 acetylation by dissociation of SIRT1 from p53 in doxorubicin-induced H9c2 cell apoptosis. Cell Stress Chaperones. 2016;21(2):251–260. doi: 10.1007/s12192-015-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boengler K, Bornbaum J, Schluter KD, Schulz R. P66shc and its role in ischemic cardiovascular diseases. Basic Res Cardiol. 2019;114(4):29. doi: 10.1007/s00395-019-0738-x. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y, Qu H, Liu J. P66Shc deletion ameliorates oxidative stress and cardiac dysfunction in pressure overload-induced heart failure. J Card Fail. 2020;26(3):243–253. doi: 10.1016/j.cardfail.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 114.Liu Y, Li Y, Ni J, Shu Y, Wang H, Hu T. MiR-124 attenuates doxorubicin-induced cardiac injury via inhibiting p66Shc-mediated oxidative stress. Biochem Biophys Res Commun. 2020;521(2):420–426. doi: 10.1016/j.bbrc.2019.10.157. [DOI] [PubMed] [Google Scholar]
- 115.Zhou S, Chen HZ, et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109(6):639–648. doi: 10.1161/CIRCRESAHA.111.243592. [DOI] [PubMed] [Google Scholar]
- 116.Zhu JN, Fu YH, et al. Activation of miR-34a-5p/Sirt1/p66shc pathway contributes to doxorubicin-induced cardiotoxicity. Sci Rep. 2017;7(1):11879. doi: 10.1038/s41598-017-12192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu YZ, Zhang L, Wu ZX, Shan TT, Xiong C. Berberine ameliorates doxorubicin-induced cardiotoxicity via a SIRT1/p66Shc-mediated pathway. Oxid Med Cell Longev. 2019;2019:2150394. doi: 10.1155/2019/2150394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bin Jardan YA, Ansari MA, Raish M, Alkharfy KM, Ahad A, Al-Jenoobi FI, Haq N, Khan MR, Ahmad A. Sinapic acid ameliorates oxidative stress, inflammation, and apoptosis in acute doxorubicin-induced cardiotoxicity via the NF-κB-mediated pathway. Biomed Res Int. 2020;2020:3921796. doi: 10.1155/2020/3921796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 120.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(8):861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han X, Zhang L, Liu Y, Wu M, Li X, Zhang ZT, Li T. Resveratrol protects H9c2 cells against hypoxia-induced apoptosis through miR-30d-5p/SIRT1/NF-κB axis. J Biosci. 2020;45(1):42. doi: 10.1007/s12038-020-9997-9. [DOI] [PubMed] [Google Scholar]
- 122.Martin ED, Bassi R, Marber MS. p38 MAPK in cardioprotection—are we there yet? Br J Pharmacol. 2015;172(8):2101–2113. doi: 10.1111/bph.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao L, Chen T, Hang P, Li W, Guo J, Pan Y, Du J, Zheng Y, Du Z. Choline attenuates cardiac fibrosis by inhibiting p38MAPK signaling possibly by acting on M3 muscarinic acetylcholine receptor. Front Pharmacol. 2019;10:1386. doi: 10.3389/fphar.2019.01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kojonazarov B, Novoyatleva T, et al. p38 MAPK inhibition improves heart function in pressure-loaded right ventricular hypertrophy. Am J Respir Cell Mol Biol. 2017;57(5):603–614. doi: 10.1165/rcmb.2016-0374OC. [DOI] [PubMed] [Google Scholar]
- 125.Wang X, Cui L, Joseph J, Jiang B, Pimental D, Handy DE, Liao R, Loscalzo J. Homocysteine induces cardiomyocyte dysfunction and apoptosis through p38 MAPK-mediated increase in oxidant stress. J Mol Cell Cardiol. 2012;52(3):753–760. doi: 10.1016/j.yjmcc.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo R, Wu K, et al. Exogenous hydrogen sulfide protects against doxorubicin-induced inflammation and cytotoxicity by inhibiting p38MAPK/NFkappaB pathway in H9c2 cardiac cells. Cell Physiol Biochem. 2013;32(6):1668–1680. doi: 10.1159/000356602. [DOI] [PubMed] [Google Scholar]
- 127.Becatti M, Taddei N, Cecchi C, Nassi N, Nassi PA, Fiorillo C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol Life Sci. 2012;69(13):2245–2260. doi: 10.1007/s00018-012-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luo W, Jin Y, Wu G, Zhu W, Qian Y, Zhang Y, Li J, Zhu A, Liang G. Blockage of ROS and MAPKs-mediated inflammation via restoring SIRT1 by a new compound LF10 prevents type 1 diabetic cardiomyopathy. Toxicol Appl Pharmacol. 2019;370:24–35. doi: 10.1016/j.taap.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 129.Ruan Y, Dong C, et al. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell Physiol Biochem. 2015;35(3):1116–1124. doi: 10.1159/000373937. [DOI] [PubMed] [Google Scholar]
- 130.Brown AK, Webb AE. Regulation of FOXO factors in mammalian cells. Curr Top Dev Biol. 2018;127:165–192. doi: 10.1016/bs.ctdb.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res Commun. 2009;378(3):389–393. doi: 10.1016/j.bbrc.2008.11.110. [DOI] [PubMed] [Google Scholar]
- 132.Hsu C-P, Zhai P, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122(21):2170–2182. doi: 10.1161/circulationaha.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol. 2015;309(9):H1375–H1389. doi: 10.1152/ajpheart.00053.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brunet A, Sweeney LB, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 135.Wang YQ, Cao Q, Wang F, Huang LY, Sang TT, Liu F, Chen SY. SIRT1 protects against oxidative stress-induced endothelial progenitor cells apoptosis by inhibiting FOXO3a via FOXO3a ubiquitination and degradation. J Cell Physiol. 2015;230(9):2098–2107. doi: 10.1002/jcp.24938. [DOI] [PubMed] [Google Scholar]
- 136.Liu MH, Shan J, Li J, Zhang Y, Lin XL. Resveratrol inhibits doxorubicin-induced cardiotoxicity via sirtuin 1 activation in H9c2 cardiomyocytes. Exp Ther Med. 2016;12(2):1113–1118. doi: 10.3892/etm.2016.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang F, Chan CH, Chen K, Guan X, Lin HK, Tong Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene. 2011;31(12):1546–1557. doi: 10.1038/onc.2011.347. [DOI] [PubMed] [Google Scholar]
- 138.Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35(2):83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 139.Ignotz RA, Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261(9):4337–4345. doi: 10.1016/S0021-9258(17)35666-1. [DOI] [PubMed] [Google Scholar]
- 140.Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 141.Liu ZH, Zhang Y, Wang X, Fan XF, Zhang Y, Li X, Gong YS, Han LP. SIRT1 activation attenuates cardiac fibrosis by endothelial-to-mesenchymal transition. Biomed Pharmacother. 2019;118:109227. doi: 10.1016/j.biopha.2019.109227. [DOI] [PubMed] [Google Scholar]
- 142.Chen L, Yan KP, Liu XC, Wang W, Li C, Li M, Qiu CG. Valsartan regulates TGF-beta/Smads and TGF-beta/p38 pathways through lncRNA CHRF to improve doxorubicin-induced heart failure. Arch Pharm Res. 2018;41(1):101–109. doi: 10.1007/s12272-017-0980-4. [DOI] [PubMed] [Google Scholar]
- 143.Bakker PJ, Butter LM, Kors L, Teske GJ, Aten J, Sutterwala FS, Florquin S, Leemans JC. Nlrp3 is a key modulator of diet-induced nephropathy and renal cholesterol accumulation. Kidney Int. 2014;85(5):1112–1122. doi: 10.1038/ki.2013.503. [DOI] [PubMed] [Google Scholar]
- 144.He Q, Li Z, Wang Y, Hou Y, Li L, Zhao J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int Immunopharmacol. 2017;50:208–215. doi: 10.1016/j.intimp.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 145.Ding S, Liu D, Wang L, Wang G, Zhu Y. Inhibiting microrna-29a protects myocardial ischemia-reperfusion injury by targeting SIRT1 and suppressing oxidative stress and NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther. 2020;372(1):128–135. doi: 10.1124/jpet.119.256982. [DOI] [PubMed] [Google Scholar]
- 146.Qiu Z, Lei S, et al. NLRP3 inflammasome activation-mediated pyroptosis aggravates myocardial ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev. 2017;2017:9743280. doi: 10.1155/2017/9743280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peng K, Liu L, et al. P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int J Mol Med. 2015;35(5):1179–1188. doi: 10.3892/ijmm.2015.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meng L, Lin H, et al. Doxorubicin induces cardiomyocyte pyroptosis via the TINCR-mediated posttranscriptional stabilization of NLR family pyrin domain containing 3. J Mol Cell Cardiol. 2019;136:15–26. doi: 10.1016/j.yjmcc.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 149.Park S, Shin J, Bae J, Han D, Park SR, Shin J, Lee SK, Park HW. SIRT1 alleviates LPS-induced IL-1beta production by suppressing NLRP3 inflammasome activation and ROS production in trophoblasts. Cells. 2020;9(3):728. doi: 10.3390/cells9030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Han Y, Sun W, Ren D, Zhang J, He Z, Fedorova J, Sun X, Han F, Li J. SIRT1 agonism modulates cardiac NLRP3 inflammasome through pyruvate dehydrogenase during ischemia and reperfusion. Redox Biol. 2020;34:101538. doi: 10.1016/j.redox.2020.101538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhai J, Tao L, Zhang S, Gao H, Zhang Y, Sun J, Song Y, Qu X. Calycosin ameliorates doxorubicin-induced cardiotoxicity by suppressing oxidative stress and inflammation via the sirtuin 1-NOD-like receptor protein 3 pathway. Phytother Res. 2020;34(3):649–659. doi: 10.1002/ptr.6557. [DOI] [PubMed] [Google Scholar]
- 152.Kwon HS, Ott M. The ups and downs of SIRT1. Trends Biochem Sci. 2008;33(11):517–525. doi: 10.1016/j.tibs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 153.Yu CL, Yang SF, Hung TW, Lin CL, Hsieh YH, Chiou HL. Inhibition of eIF2alpha dephosphorylation accelerates pterostilbene-induced cell death in human hepatocellular carcinoma cells in an ER stress and autophagy-dependent manner. Cell Death Dis. 2019;10(6):418. doi: 10.1038/s41419-019-1639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu Z, Wang S, Zhang X, Li Y, Zhao Q, Liu T. Pterostilbene protects against myocardial ischemia/reperfusion injury via suppressing oxidative/nitrative stress and inflammatory response. Int Immunopharmacol. 2017;43:7–15. doi: 10.1016/j.intimp.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 155.Kosuru R, Kandula V, Rai U, Prakash S, Xia Z, Singh S. Pterostilbene decreases cardiac oxidative stress and inflammation via activation of AMPK/Nrf2/HO-1 pathway in fructose-fed diabetic rats. Cardiovasc Drugs Ther. 2018;32(2):147–163. doi: 10.1007/s10557-018-6780-3. [DOI] [PubMed] [Google Scholar]
- 156.Lacerda D, Ortiz V, et al. Stilbenoid pterostilbene complexed with cyclodextrin preserves left ventricular function after myocardial infarction in rats: possible involvement of thiol proteins and modulation of phosphorylated GSK-3beta. Free Radic Res. 2018;52(9):988–999. doi: 10.1080/10715762.2018.1506115. [DOI] [PubMed] [Google Scholar]
- 157.Riche DM, Riche KD, Blackshear CT, McEwen CL, Sherman JJ, Wofford MR, Griswold ME. Pterostilbene on metabolic parameters: a randomized, double-blind, and placebo-controlled trial. Evid Based Complement Alternat Med. 2014;2014:459165. doi: 10.1155/2014/459165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61(3):253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 159.Qin C, Zhou S, Xiao Y, Chen L. Erythropoietin enhances mitochondrial biogenesis in cardiomyocytes exposed to chronic hypoxia through Akt/eNOS signalling pathway. Cell Biol Int. 2014;38(3):335–342. doi: 10.1002/cbin.10205. [DOI] [PubMed] [Google Scholar]
- 160.Ong SB, Hall AR, Dongworth RK, Kalkhoran S, Pyakurel A, Scorrano L, Hausenloy DJ. Akt protects the heart against ischaemia-reperfusion injury by modulating mitochondrial morphology. Thromb Haemost. 2015;113(3):513–521. doi: 10.1160/th14-07-0592. [DOI] [PubMed] [Google Scholar]
- 161.Coelho AR, Martins TR, et al. (2017) Berberine-induced cardioprotection and Sirt3 modulation in doxorubicin-treated H9c2 cardiomyoblasts. Biochim Biophys Acta Mol Basis Dis. 1863;11:2904–2923. doi: 10.1016/j.bbadis.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 162.Lipshultz SE, Herman EH. Anthracycline cardiotoxicity: the importance of horizontally integrating pre-clinical and clinical research. Cardiovasc Res. 2018;114(2):205–209. doi: 10.1093/cvr/cvx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Berretta M, Bignucolo A, Di Francia R, Comello F, Facchini G, Ceccarelli M, Iaffaioli RV, Quagliariello V, Maurea N. Resveratrol in cancer patients: from bench to bedside. Int J Mol Sci. 2020;21(8):2945. doi: 10.3390/ijms21082945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Quagliariello V, Vecchione R, De Capua A, Lagreca E, Iaffaioli RV, Botti G, Netti PA, Maurea N. Nano-encapsulation of coenzyme Q10 in secondary and tertiary nano-emulsions for enhanced cardioprotection and hepatoprotection in human cardiomyocytes and hepatocytes during exposure to anthracyclines and trastuzumab. Int J Nanomed. 2020;15:4859–4876. doi: 10.2147/IJN.S245170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang L, Zhu K, Zeng H, Zhang J, Pu Y, Wang Z, Zhang T, Wang B. Resveratrol solid lipid nanoparticles to trigger credible inhibition of doxorubicin cardiotoxicity. Int J Nanomedicine. 2019;14:6061–6071. doi: 10.2147/IJN.S211130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao Y, Huan ML, Liu M, Cheng Y, Sun Y, Cui H, Liu DZ, Mei QB, Zhou SY. Doxorubicin and resveratrol co-delivery nanoparticle to overcome doxorubicin resistance. Sci Rep. 2016;6:35267. doi: 10.1038/srep35267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE, Brookes PS, Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. 2010;24(9):3145–3159. doi: 10.1096/fj.09-151308. [DOI] [PMC free article] [PubMed] [Google Scholar]