Abstract
Oxysterol-binding protein (OSBP) and its related proteins (ORPs) are a family of lipid transfer proteins (LTPs) that mediate non-vesicular lipid transport. ORP9 and ORP10, members of the OSBP/ORPs family, are located at the endoplasmic reticulum (ER)-trans-Golgi network (TGN) membrane contact sites (MCSs). It remained unclear how they mediate lipid transport. In this work, we discovered that ORP9 and ORP10 form a binary complex through intermolecular coiled-coil (CC) domain-CC domain interaction. The PH domains of ORP9 and ORP10 specially interact with phosphatidylinositol 4-phosphate (PI4P), mediating the TGN targeting. The ORP9-ORP10 complex plays a critical role in regulating PI4P levels at the TGN. Using in vitro reconstitution assays, we observed that while full-length ORP9 efficiently transferred PI4P between two apposed membranes, the lipid transfer kinetics was further accelerated by ORP10. Interestingly, our data showed that the PH domains of ORP9 and ORP10 participate in membrane tethering simultaneously, whereas ORDs of both ORP9 and ORP10 are required for lipid transport. Furthermore, our data showed that the depletion of ORP9 and ORP10 led to increased vesicle transport to the plasma membrane (PM). These findings demonstrate that ORP9 and ORP10 form a binary complex through the CC domains, maintaining PI4P homeostasis at ER-TGN MCSs and regulating vesicle trafficking.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-023-04728-5.
Keywords: OSBP/ORPs, Membrane contact sites, Coiled-coil domain, Lipid transport, Lipid homeostasis, Vesicle trafficking
Introduction
In eukaryotic cells, the endoplasmic reticulum (ER) communicates with a variety of membrane-bound organelles directly through membrane contact sites (MCSs). These MCSs play critical roles in ion dynamics, lipid homeostasis, and cell signaling [1–3]. Both vesicle fusion and non-vesicular lipid transport contribute to the lipid exchange between membranous organelles. Unlike vesicle fusion, which supplies the organelles with a bulk of non-specific lipids, non-vesicular lipid transport can selectively transfer demanded lipids [4–7]. MCSs with a gap of short distances provide an ideal platform at which lipid transfer proteins (LTPs) can efficiently exchange lipids between apposed membranes via non-vesicular transport [8]. Increasing evidence suggests that LTPs play a key role in maintaining the architectures of MCSs, sensing the dynamic changes of lipids, and regulating the lipid homeostasis of membranous organelles [8–10].
One important class of LTPs is oxysterol-binding protein (OSBP) and its related proteins (ORPs), divided into six subfamilies according to the sequence homology [11]. An LTP of the OSBP/ORPs family is characterized by a conserved OSBP-related domain (ORD) that has a hydrophobic pocket harboring certain lipids, such as cholesterol, phosphatidylserine (PS), and phosphatidylinositol polyphosphates (PIPs) [12, 13]. Most OSBP/ORPs contain a pleckstrin homology (PH) domain and a two phenylalanines in an acidic tract (FFAT) motif. While the FFAT recognizes vesicle-associated membrane protein (VAMP)-associated proteins (VAPs) on the ER, the PH domain interacts with PIPs and contributes to the membrane tethering [14–17]. ORP5 and ORP8, two well-studied members of the family, are localized to the ER by their C-terminal transmembrane domains (TMDs) [18, 19]. The membrane-targeting characteristics of OSBP/ORPs facilitate the establishment of MCSs and mediate lipid transfer between the ER and other organelles.
OSBP, as the initially identified member of this family, has been well-studied in the last decade. OSBP contains the ER-targeting FFAT motif and recognizes PI4P through its PH domain, thus locating OSBP to MCSs, for example, the ER-Golgi contact sites [20–22]. Structure studies suggest OSBP contains a dimeric region with the T-geometry, facilitating the movement of ORDs between membranes [23]. The N-terminal intrinsically disordered region of OSBP limits protein density and facilitates protein mobility in the narrow and crowded MCSs environment [24]. The ORD domain is capable of countertransport PI4P/cholesterol to regulate lipid homeostasis at MCSs [20]. Moreover, OSBP-mediated cholesterol transport from the ER to lysosomes drives the recruitment of mTORC1 through the Rag GTPases [25]. A recent study found that OSBP can be recruited into damaged lysosomes to repair damaged lysosome membranes [26].
PIPs are essential lipid signal molecules that regulate numerous physiological activities in the cell [27, 28]. The most abundant PIPs at the trans-Golgi network (TGN) is PI4P, which serves as a precursor of other PIPs and modulates the functional integrity of TGN [29–32]. The level of PI4P at the TGN is primarily maintained by the phosphatidylinositol-4 kinase PI4KIIIβ and phosphatidylinositol-4 phosphatase Sac1. Nevertheless, Sac1 is a transmembrane protein that localizes at the ER rather than TGN [33]. Hence, LTPs at ER-TGN MCSs are essential to maintaining PI4P homeostasis [20, 34–38]. Multiple OSBP/ORPs, including OSBP, ORP9, and ORP10, have been reported to mediate lipid transport at ER-TGN MCSs. OSBP mediates the countertransport of cholesterol and PI4P in ER-TGN MCSs [20]. ORP9 has been reported to bind sterol and PI4P [35, 36]. Unlike OSBP and ORP9, ORP10 contains neither the FFAT motif nor TMD, indicating it can not directly target the ER similarly to other OSBP/ORPs [39]. Recent studies suggested that ORP10 plays a critical role in maintaining the integrity of ER-TGN MCSs by transferring PS from the ER to the TGN [38].
In this work, we revealed a unique lipid transport machinery composed of ORP9 and ORP10, which form a binary complex through their respective coiled-coil (CC) domains localized between the PH domain and ORD. They target TGN through the specific PH domain-PI4P interactions. In vitro lipid transfer assays showed that full-length (FL) ORP9 efficiently transferred PI4P, which was further improved by FL ORP10. Mechanically, the two PH domains are involved in the membrane targeting, whereas both ORDs in the ORP9-ORP10 complexes can transfer lipids simultaneously. Depletion of ORP9 and ORP10 significantly increases the PI4P levels at TGN and remarkably accumulates VSV-G on the plasma membrane (PM). Therefore, ORP9 and ORP10 are a pair of LTPs that coordinately maintain lipid homeostasis at ER-TGN MCSs and regulate vesicle trafficking.
Results
ORP9 determines the subcellular localization of ORP10 at ER-TGN MCSs
A previous study showed that ORP10 colocalizes with VAPs (VAPA and VAPB) [14]. However, this interaction appeared to be indirect as ORP10 does not contain the FFAT motif. We used co-immunoprecipitation (co-IP) and mass spectrometry (MS) to determine ORP10-associated proteins. The data revealed that ORP9, another member of the OSBP/ORPs family, is associated with ORP10 (Fig. S1a), consistent with a recent study [40]. Western blotting confirmed the interaction between ORP9 and ORP10 (Fig. S1b and S2).
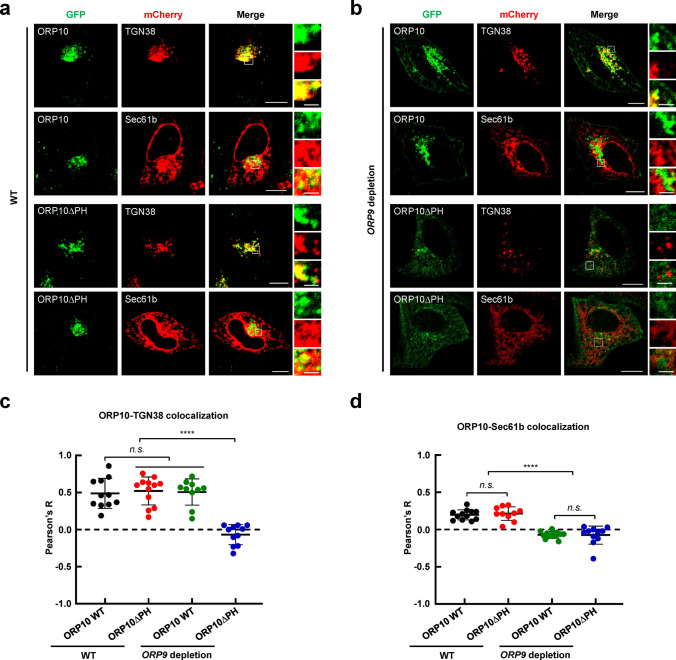
We co-expressed GFP-tagged ORP9, mCherry-tagged TGN38 (a TGN marker), and BFP-tagged VAPA (an ER marker) in HeLa cells, and observed that ORP9 localized at ER-TGN MCSs (Fig. S3), consistent with the previous studies [38]. Since ORP9 can bind to VAPs through the FFAT motif, we speculate that ORP10 may localize to the ER by bridging ORP9 and VAPs. To test this possibility, we generated recombinant GST-tagged VAPA protein and performed in vitro GST pull-down assay, which showed that ORP10 was associated with VAPA only in the presence of ORP9 (Fig. S4a) [40]. We then co-expressed mCherry-tagged ORP9, GFP-tagged ORP10 and BFP-tagged VAPA to investigate their colocalization. ORP9, ORP10 and VAPA showed perfect colocalization (Fig. S4b). We then examined whether the localization of ORP10 at ER-TGN MCSs depends on ORP9 (Fig. S4c). When GFP-tagged ORP10 was co-expressed with mCherry-tagged TGN38 or Sec61b (an ER marker), we observed that ORP10 partially colocalized with the ER and the TGN (Fig. 1a, c), consistent with previous studies [33, 38]. However, the localization of ORP10 at the ER was abolished in ORP9-depleted cells (Fig. 1b, d).
Fig. 1.
Recruitment of ORP10 to ER-TGN MCSs depends on ORP9. a Representative confocal microscopy images showing the subcellular localization of ORP10 and ORP10∆PH with the ER (mCherry-Sec61b) or TGN (TGN38-mCherry) in WT HeLa cells. Scale bar: 5 μm (entire cell), or 1 μm (zoom). b Representative confocal microscopy images showing the subcellular localization of ORP10 and ORP10∆PH with the ER or TGN in ORP9-depleted HeLa cells. Scale bar: 5 μm (entire cell), or 1 μm (zoom). c Pearson’s correlation coefficient (R) of GFP-ORP10 or GFP-ORP10∆PH with TGN38-mCherry. Data are presented as means ± SD, three independent experiments, n = 10–12 cells/condition. p values were calculated using ordinary one-way ANOVA with Tukey’s multiple comparisons test. n.s., p > 0.05. ****p < 0.0001. d Pearson’s correlation coefficient (R) of GFP-ORP10 or GFP-ORP10∆PH with mCherry-Sec61b. Data are presented as means ± SD, three independent experiments, n = 10–14 cells/condition. p values were calculated using ordinary one-way ANOVA with Tukey’s multiple comparisons test. n.s., p > 0.05. ****p < 0.0001
The PH domain is a β-sandwich-like protein module commonly found in a variety of proteins [17, 41, 42]. Recent studies suggest that multiple OSBP/ORPs employ the PH domain to specifically recognize PIPs on the apposed membrane, contributing to its subcellular localization at MCSs [19, 20, 23]. We then performed experiments to explore whether the PH domain is required for targeting ORP10 to the TGN membrane. Unexpectedly, ORP10 was still localized to ER-TGN MCSs when its PH domain was removed in wild-type (WT) cells. However, the deletion of the PH domain from ORP10 abolished its TGN targeting in ORP9-depleted cells (Fig. 1). These results indicate that the PH domain of ORP10 is dispensable for its TGN localization in the presence of ORP9. Together, these findings demonstrate that ORP9 determines the recruitment of ORP10 to ER-TGN MCSs.
ORP9 and ORP10 interact through their CC domains
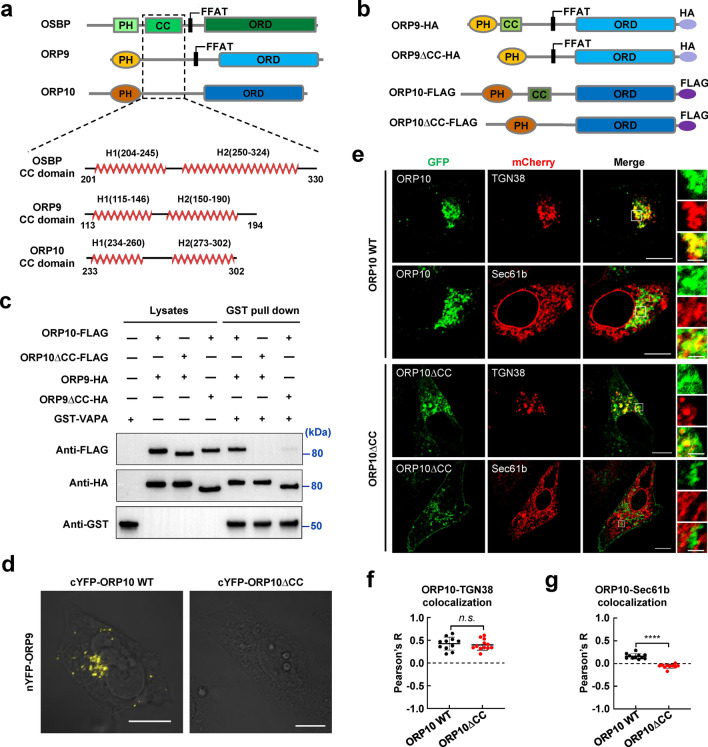
Next, we investigated how ORP10 is associated with ORP9 by co-IP, which was performed by transient expression of FLAG-tagged ORP9 with GFP-tagged WT or mutant ORP10 (Fig. S5a). We found that both the PH domain and ORD are dispensable, indicating ORP10 interacts with ORP9 through another region than these two known domains (Fig. S5b). Recent studies reported that OSBP dimerizes through the CC domain between the PH domain and the FFAT motif [20, 23, 24]. We speculated that CC domain interactions also mediate the association of ORP9 and ORP10. The structural prediction and secondary structural analysis were then performed using AlphaFold (AlphaFold Protein Structure Database (ebi.ac.uk)) and PSIPRED (PSIPRED Workbench (ucl.ac.uk)) [43–46]. We found a predicted α-helix region in the linker region between the PH domain and ORD in ORP9 and ORP10. The predicted secondary structure of these regions is similar to that of OSBP, albeit the primary sequences are not conserved (Fig. 2a).
Fig. 2.
The interaction of ORP9 and ORP10 was mediated by the CC domains. a The structural prediction and secondary structural analysis of OSBP, ORP9, and ORP10 using AlphaFold [AlphaFold Protein Structure Database (ebi.ac.uk)] and PSIPRED [PSIPRED Workbench (ucl.ac.uk)]. b Schematic diagrams of WT and mutant ORP9 or ORP10. c Immunoblots showing the binding of ORP9 to ORP10 through their CC domains. GST pull-down and immunoblotting were carried out as described in Fig. S4a. d BiFC images showing the physical interaction signals of ORP9 and ORP10 in HeLa cells. ORP9 fused with nYFP (a.a. 1–173) was transiently co-expressed with ORP10 or ORP10∆CC fused with cYFP (a.a. 174–238) in HeLa cells. Scale bar: 5 μm. e Representative confocal microscopy images showing the subcellular localization of ORP10 and ORP10∆CC with the ER or TGN in HeLa cells. Scale bar: 5 μm (entire cell), or 1 μm (zoom). f Pearson’s correlation coefficient (R) of GFP-ORP10 WT or GFP-ORP10∆CC with TGN38-mCherry. Data are presented as means ± SD, three independent experiments, n = 11–12 cells/condition. p values were calculated using unpaired t test. n.s., p > 0.05. g Pearson’s correlation coefficient (R) of GFP-ORP10 or GFP-ORP10∆CC with mCherry-Sec61b. Data are presented as means ± SD, three independent experiments, n = 10–11 cells/condition. p values were calculated using unpaired t test. ****p < 0.0001
We then carried out the GST pull-down assay to examine the role of the CC domains in the interaction of ORP9 and ORP10. Removal of either CC domain disrupted the association between ORP9 and ORP10 (Fig. 2b, c). Using confocal microscopy, we observed that ORP9 and ORP10 generated a strong bimolecular fluorescence complementation (BiFC) signal that consistent with a previous study [47], indicating the two proteins interact in the cell. Interestingly, the BiFC signal diminished when the CC domain was deleted, consistent with the GST pull-down data (Fig. 2d). Moreover, when we deleted the CC domain, ORP10 was no longer colocalized with the ER, similar to its distribution in ORP9-depleted cells (Fig. 2e–g). Immunofluorescence studies confirmed that ORP10ΔCC did not affect the morphology of endogenous ER but lost its ER localization (Fig. S6). These data demonstrated that the CC domains are essential for the interaction between ORP9 and ORP10, and are required for their colocalization at ER-TGN MCSs.
ORP9 and ORP10 form a heterocomplex but not homocomplexes
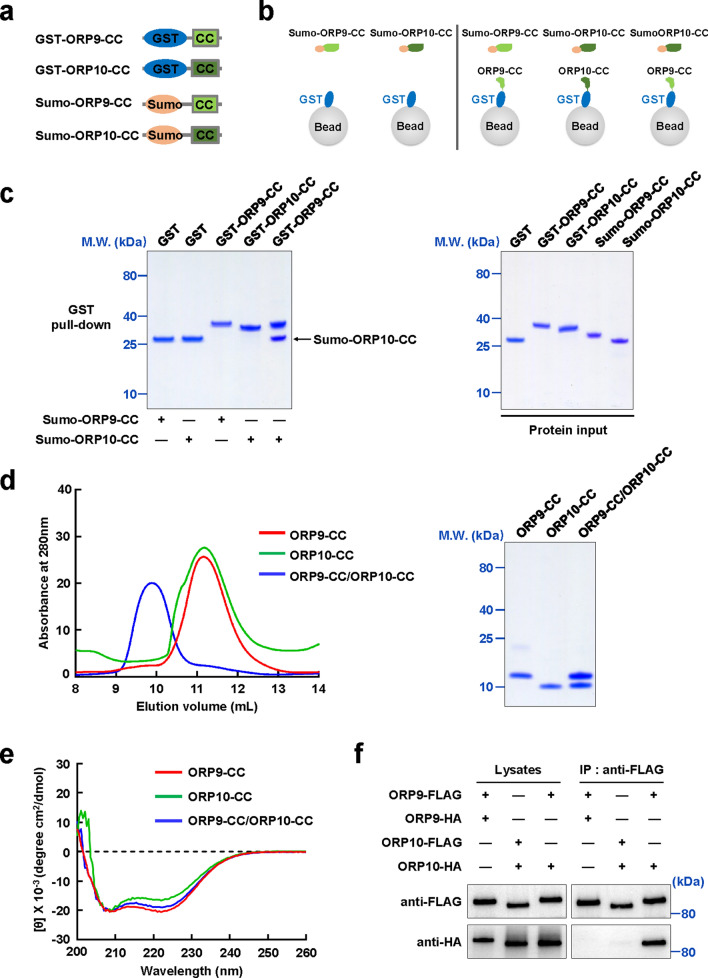
Next, we expressed recombinant CC domains of ORP9 and ORP10, and examined their interaction using in vitro GST pull-down assay (Fig. 3a, b). Unlike OSBP, neither the ORP9 CC domain nor the ORP10 CC domain can form a homocomplex. Interestingly, the CC domains of ORP9 and ORP10 interacted with each other to form a heterocomplex, which was further demonstrated by the gel filtration chromatography (GFC) analysis (Fig. 3c, d). The circular dichroism (CD) spectra of CC domains showed a typical α-helix structure for individual CC domain fragments and their binary complex, confirming the structural prediction of the CC domains in ORP9 and ORP10 (Fig. 3e). The co-IP data showed that FL ORP9 and FL ORP10 formed the heterologous rather than homologous complex, in agreement with the in vitro CC domain-CC domain binding data (Fig. 3f). Together, these data indicated that ORP9 and ORP10 form a heterocomplex through their CC domains.
Fig. 3.
ORP9 and ORP10 form a heterocomplex through the CC domain-CC domain interaction. a Schematic diagrams of ORP9 and ORP10 CC domains used in the pull-down assay. b Illustration of the GST pull-down assay measuring homologous or heterologous CC domain-CC domain interaction. c Left: Coomassie-blue-stained SDS-PAGE gel showing the binding of ORP10-CC to ORP9-CC from GST pull-down assay. Right: Coomassie-blue-stained SDS-PAGE gel showing input proteins used in the pull-down assay. d GFC analyses showing the formation of binary ORP9-CC/ORP10-CC complex using Superdex 75 increase 10/300 column. e CD spectra of ORP9-CC, ORP10-CC, and the ORP9-CC/ORP10-CC complex. f Immunoblots showing the heterologous but not the homologous interactions between the FL ORP9 and FL ORP10. The ORP9/ORP10-3 × FLAG proteins were immunoprecipitated from the cell lysates using the anti-FLAG antibody. The presence of ORP9/ORP10-3 × FLAG (top) and ORP9/ORP10-HA (bottom) in lysates and immunoprecipitates were detected using anti-FLAG and anti-HA antibodies, respectively
The PH domains of ORP9 and ORP10 specifically target TGN through PI4P
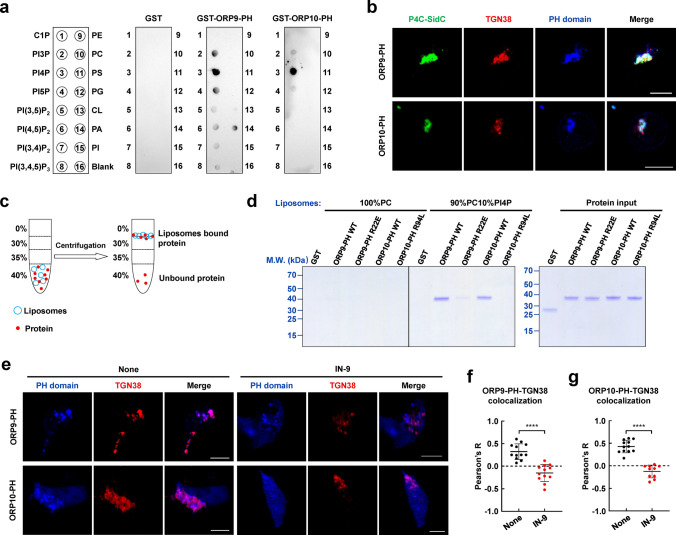
To investigate how ORP9 and ORP10 target the TGN, we expressed and purified the PH domains and examined their phospholipid binding preference through the PIPs binding assay. We observed that both PH domains of ORP9 and ORP10 selectively bind to PI4P (Fig. 4a), consistent with previous studies [35, 47]. Using confocal microscopy, we observed that the PH domains colocalized with TGN38 and P4C-SidC, a PI4P marker employed to monitor the distribution of cellular PI4P (Fig. 4b) [48]. In a liposome coflotation assay, we found that the PH domain did not bind to neutral liposomes prepared by phosphatidylcholine (PC). However, when PI4P was included, the PH domains interacted strongly with the PI4P-containing liposomes. The mutations, R22E in ORP9 and R94L in ORP10, markedly reduced the PH domains-membrane associations, confirming the specific interactions between the PH domains and PI4P (Fig. 4c, d). TGN PI4P is mainly generated by PI4KIIIβ. Therefore, PI4KIIIβ inhibitors can be used to control PI4P levels [49–51]. We then treated HeLa cells with IN-9, a PI4KIIIβ inhibitor, to determine whether PI4P mediates the recruitments of ORP9 and ORP10 to the TGN. After IN-9 treatment, the PH domains were no longer localized at the TGN (Fig. 4e–g). ORP9 and ORP10 localize at ER-endosome contact sites as well (Fig. S7) [40]. Our study suggested that ORP10 showed more colocalization with late endosomes after treatment with IN-9 (Fig. S8). These data demonstrated that the PH domains of ORP9 and ORP10 interact with PI4P on the TGN, contributing to their subcellular localization at MCSs.
Fig. 4.
The PH domains of ORP9 and ORP10 specifically bind PI4P on the TGN. a The PIPs binding assay showing the binding of the PH domains to the various phospholipids. b Representative confocal microscopy images showing the subcellular localization of PH domains, PI4P, and TGN in HeLa cells. Scale bar: 5 µm. c Diagram of the liposome coflotation assay used to study the interactions between the PH domains and liposomes. d Left, Coomassie blue-stained SDS-PAGE gel showing the binding of the PH domains to liposomes. Right, Coomassie-blue-stained SDS-PAGE gel showing input proteins used in liposome coflotation assay. The amount of protein was loaded as 25% of the total input. e Representative confocal microscopy images showing intracellular localizations of the PH domains in the absence or presence of IN-9. Scale bar: 5 µm. f Pearson’s correlation coefficient (R) of BFP-ORP9-PH and TGN38-mCherry. Data are presented as means ± SD, three independent experiments, n = 12 cells/condition. p values were calculated using unpaired t test. ****p < 0.0001. g Pearson’s correlation coefficient (R) of BFP-ORP10-PH and TGN38-mCherry. Data are presented as means ± SD, three independent experiments, n = 10–12 cells/condition. p values were calculated using unpaired t test. ****p < 0.0001
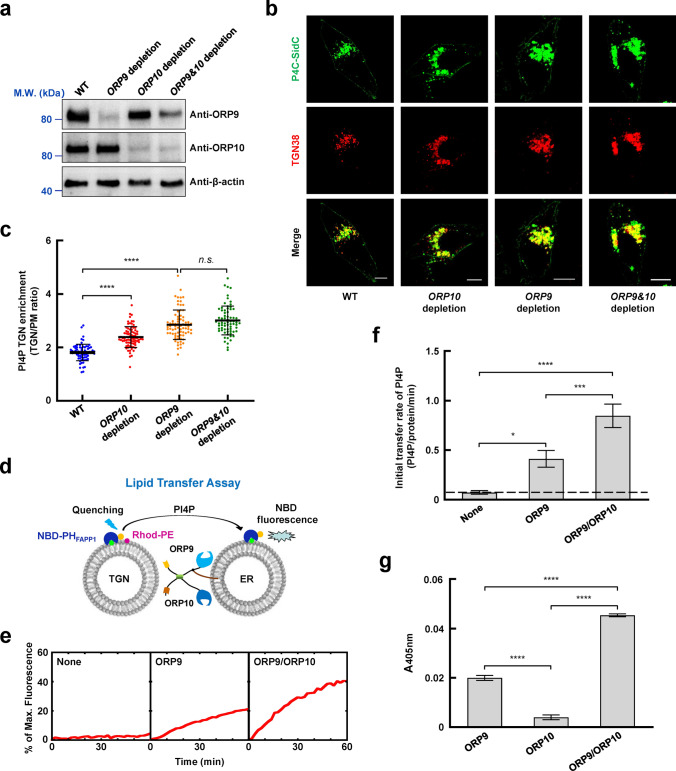
ORP9 and ORP10 regulate PI4P homeostasis at the TGN
PI4P is the most abundant PIPs species at the TGN generated by PI4KIIIβ. We speculated that the ORP9-ORP10 complex might be involved in PI4P homeostasis at the TGN. We then created ORP9 or ORP10-depleted cells to test this possibility (Fig. 5a). Based on the statistical analysis of GFP-P4C-SidC fluorescence, we observed that the level of TGN PI4P was significantly elevated when ORP9 or ORP10 was depleted. Notably, the depletion of ORP9 and ORP10 did not increase the TGN PI4P level more than ORP9-depleted cells (Fig. 5b, c), almost consistent with previous studies [33, 40]. Immunofluorescence measurements further confirmed the increase of PI4P on TGN (Fig. S9). Therefore, ORP9 and OPR10 are critical in maintaining PI4P homeostasis at the TGN.
Fig. 5.
ORP9 and ORP10 regulate PI4P homeostasis by mediating PI4P transport between the ER and the TGN. a Immunoblots showing the expression of the indicated proteins in WT, ORP9-depleted, ORP10-depleted, and ORP9&ORP10-depleted HeLa cells. b Representative confocal microscopy images showing the distribution and levels of PI4P in WT, ORP9-depleted, ORP10-depleted, and ORP9&ORP10-depleted HeLa cells. Scale bar: 5 µm. c The quantitative analysis of TGN PI4P levels in WT, ORP9-depleted, ORP10-depleted, and ORP9&ORP10-depleted HeLa cells. Error bars indicate standard deviation. Data are presented as means ± SD, three independent experiments, n = 72–80 cells/condition. p values were calculated using ordinary one-way ANOVA with Tukey’s multiple comparisons test. n.s., p > 0.05. ****p < 0.0001. d Schematic diagram of the FRET-based lipid transfer assay. Prior to the lipid transfer reaction, NBD emission from the TGN-like liposomes was quenched by neighboring rhodamine molecules through FRET. The transfer of fluorescence-labeled lipids results in the dequenching of the NBD fluorescence. e Lipid transfer of the liposomes in the absence or presence of LTPs. The data are presented as the percentage of maximum fluorescence change. f Initial lipid transfer rates of PI4P in the reactions shown in e. Error bars indicate standard deviation. Data are presented as mean ± SD (n = 3 independent replicates). p values were calculated using one-way ANOVA with Tukey’s multiple comparisons test. *p = 0.0140. ***p = 0.0004. ****p < 0.0001. g Effects of LTPs on membrane tethering in a liposome tethering assay. Turbidity was evaluated by measuring the absorbance at 405 nm, reflecting the clusters of liposomes induced by proteins. Error bars indicate standard deviation. Data are presented as mean ± SD (n = 3 independent replicates). p values were calculated using one-way ANOVA with Tukey’s multiple comparisons test. ****p < 0.0001
ORP9 and ORP10 coordinately mediate PI4P/PS transport in vitro
Next, we examined if ORP9-ORP10 heterocomplex directly transfers PI4P in vitro by a Förster resonance energy transfer (FRET)-based lipid transfer assay. The FL VAPA was reconstituted into ER-like liposomes, which were used to anchor FL ORP9 and ORP10. Protein-free liposomes containing PI4P (TGN-like liposomes) were used to simulate the TGN membrane. PI4P can be detected by the NBD-labeled PH domain of FAPP1 (NBD-PHFAPP1) [20]. Before the lipid transfer reaction, PI4P was reconstituted in the TGN-like liposomes, in which the NBD-PHFAPP1 fluorescence was quenched by neighboring Rhod-PE through FRET. The transfer of PI4P from the TGN-like liposomes to the ER-like liposomes results in the dequenching of the NBD fluorescence (Fig. 5d). We observed that ORP9 drove a comparable level of PI4P transfer in the lipid transfer assay. When ORP10 was added, however, lipid transfer efficiency was strongly elevated (Fig. 5e, f). Previous studies indicated that PS and/or cholesterol might be the countertransport lipids of PI4P [20, 40, 52]. PS and cholesterol transport experiments were then performed (Fig. S10a, d). The results showed that ORP9 and ORP10 could transport PS rapidly (Fig. S10b, c). However, the cholesterol transport efficiency of ORP9 and ORP10 is very slow (Fig. S10e, f). Therefore, PI4P and PS, rather than cholesterol, are effective lipids transferred by the ORP9-ORP10 complex.
ORP9 and ORP10 constitutively colocalize at ER-TGN MCSs, where they span the two apposed membranes. We then performed a liposome tethering assay to study how ORP9 and ORP10 mediate membrane tethering. By measuring liposome clustering-induced turbidity, we observed that ORP9, but not ORP10, mediated liposome tethering. However, the addition of ORP10 markedly increased ORP9-mediated membrane tethering capacity (Fig. 5g). We postulate that ORP9 provides a fulcrum for ORP10, facilitating membrane tethering. While the ORP9-ORP10 complex is anchored to the ER through ORP9, both proteins are involved in TGN membrane targeting. Together, these data suggested that ORP9 and ORP10 form a binary complex that efficiently mediates membrane tethering and inter-membrane PI4P transport.
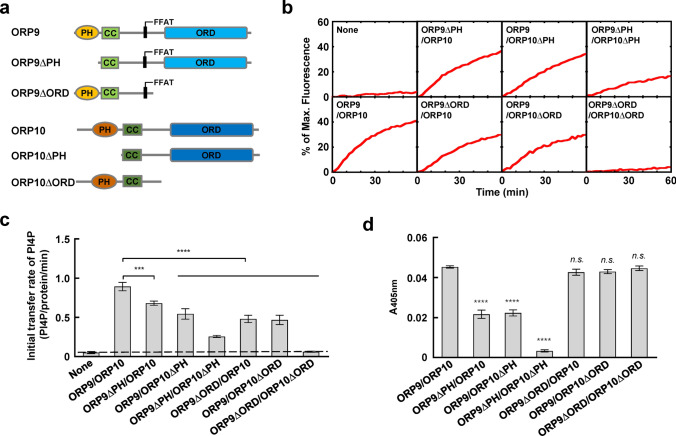
Both ORP9 and ORP10 contribute to TGN targeting and PI4P transport
Both ORP9 and ORP10 contain a PH domain and an ORD. We then examined the functional importance of the PH domains and ORDs in lipid transport by individual domain deletions (Fig. 6a). Interestingly, removing either one PH domain or ORD from the ORP9-ORP10 complex reduced the lipid transfer efficiency. The omission of two PH domains from ORP9 and ORP10 significantly reduced the lipid transfer activity of the ORP9-ORP10 complex, whereas the double deletion of ORDs completely abolished the lipid transfer activity (Fig. 6b, c).
Fig. 6.
ORP9 and ORP10 contribute to ORP9-ORP10 complex-mediated membrane tethering and PI4P transport. a Schematic diagrams of WT and mutant ORP9 and ORP10 proteins. b Effect of the individual PH domain and ORD on the lipid transfer activity. Lipid transfer assays were carried out as described in Fig. 5e. c Initial lipid transfer rates of PI4P in the reactions shown in b. Error bars indicate standard deviation. Data are presented as mean ± SD (n = 3 independent replicates). p values were calculated using one-way ANOVA with Tukey’s multiple comparisons test. ***p = 0.0005. ****p < 0.0001. d Effect of the individual PH domain and ORD on membrane tethering in a liposome tethering assay. Error bars indicate standard deviation. Data are presented as mean ± SD (n = 3 independent replicates). p values were calculated using one-way ANOVA with Tukey’s multiple comparisons test. n.s., p > 0.05. ****p < 0.0001
In the liposome tethering assay, we observed that removing either the PH domain of ORP9 or ORP10 reduced the tethering activity. Removal of both PH domains completely abolished membrane tethering, suggesting that the ORP9-ORP10 complex tethers membranes mainly through their PH domains. In contrast, the deletion of ORDs had little effect on membrane tethering (Fig. 6d), confirming our conclusion that the PH domains and ORDs carry out distinct functions in the ORP9-ORP10 complex. While ORP9 and ORP10 transfer lipids fundamentally through the ORDs, efficient lipid transport requires the PH domains-mediated membrane tethering. These data demonstrated that while the two PH domains of ORP9 and ORP10 contribute to TGN targeting, both ORDs are involved in PI4P transport.
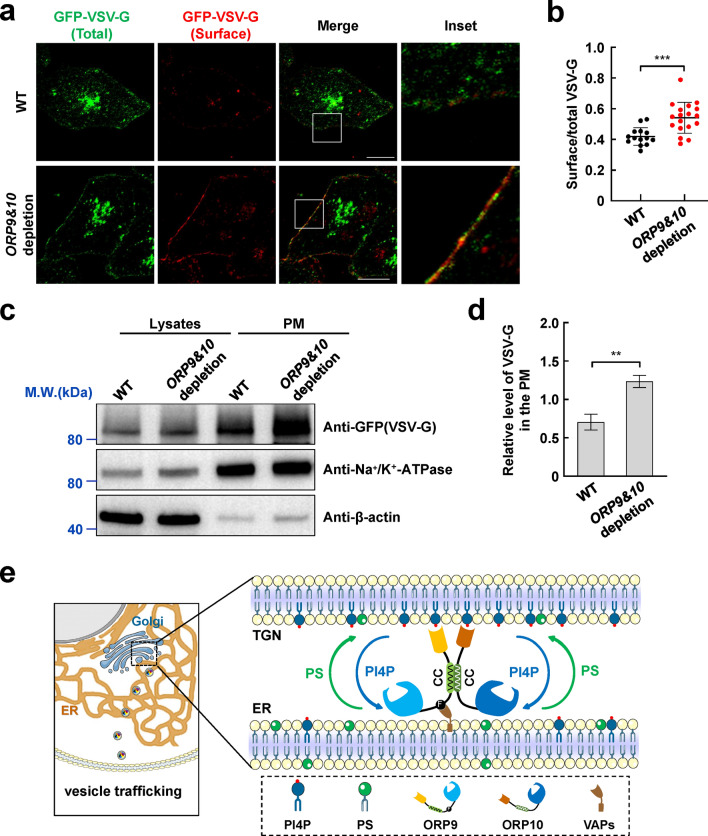
ORP9 and ORP10 regulate vesicle transport to the PM
Multiple key regulators, including OSBP/ORPs, PITPNC1, Arfs, and GOLPH3, that target TGN through PI4P are involved in vesicle biogenesis [29]. A previous report suggested PI4KIIIβ-derived PI4P synthesis could activate vesicular release from the Golgi to enhance secretion [53]. We then performed temperature-sensitive mutant vesicular stomatitis virus-G (VSV-G) assays to examine whether ORP9 and ORP10 regulate the vesicle release and transport to the PM. At restrictive temperatures, VSV-G accumulates in the TGN due to impaired secretory function and is released at permissive temperatures. Compared with the WT cells, ORP9 and ORP10-depleted cells accumulated more VSV-G on the PM (Fig. 7a, b). The increase of VSV-G in the PM components of mutant cells was further confirmed by western blotting (Fig. 7c, d). These data suggested that ORP9 and ORP10 regulate vesicle trafficking by maintaining the PI4P homeostasis at TGN.
Fig. 7.
ORP9 and ORP10 regulate vesicle transport to the PM. a Representative confocal microscopy images showing the distribution of GFP-VSV-G in WT and ORP9&ORP10-depleted HeLa cells. Scale bar: 5 µm. b The quantitative analysis of surface VSV-G to total VSV-G in WT and ORP9&ORP10-depleted cells. Error bars indicate standard deviation. Data are presented as means ± SD, n = 14–18 cells/condition. p values were calculated using unpaired t test. ***p = 0.0004. c Immunoblots showing the accumulation of the VSV-G in the PM components of WT and ORP9&ORP10-depleted cells. The presence of GFP-VSV-G (top), Na+/K+-ATPase (middle, PM marker), and β-actin (bottom) in the immunoblots were detected using anti-GFP, anti-Na+/K+-ATPase, and anti-β-actin antibodies, respectively. d The quantitative analysis of VSV-G to Na+/K+-ATPase in the PM components of WT and ORP9&ORP10-depleted cells. Error bars indicate standard deviation. Data are presented as means ± SD (n = 3 independent replicates). p values were calculated using unpaired t test. **p = 0.0021. e Working model for ORP9-ORP10 heterocomplex-mediated lipid transport at ER-TGN MCSs. ORP9 and ORP10 form heterocomplex through the CC domains. The ORP9 FFAT motif and two PH domains act as membrane tethers that localize the ORP9-ORP10 complex at ER-TGN MCSs, whereas the ORDs transport PI4P/PS to maintain lipid homeostasis and further regulate vesicle trafficking
Discussion
PI4P is an essential regulatory molecule in many physiological processes, including organelle biogenesis, membrane trafficking, lipid metabolism, and multiple signaling pathways [27, 30, 31]. The levels of PI4P are precisely controlled by phosphatases and phosphoinositide kinases [54–56]. As the most abundant PIPs in the TGN, PI4P is generated by the Golgi-resident PI4KIIIβ and hydrolyzed by ER-resident Sac1 [55, 57]. The different localization of PI4KIIIβ and Sac1 requires an efficient lipid exchange between the two organelles. There are multiple LTPs that mediate non-vesicular lipid transport at the ER-TGN interface. However, the functions and molecular mechanisms of these LTPs remain largely unclear.
In this work, we initially identified the localization of ORP9 and ORP10 at ER-TGN MCSs. Although lacking the ER targeting sequences, ORP10 can still be recruited to the ER through binding to ORP9. We showed that the PH domains of ORP9 and ORP10 could interact with PI4P on the TGN, providing the membrane tethering to target TGN. Our data suggested that ORP9 and ORP10 are functional partners at ER-TGN MCSs, appearing as integral binary complexes, consistent with a recent study showing the recruitment of ORP10 to the ER-endosome MCSs by ORP9 [40]. It seems that the ORP9 and ORP10 may target the MCSs formed between ER and another PI4P-enriched organelle. Further studies with immuno-electron microscopy will be helpful in explicitly dissecting their distributions and PI4P dependence. Similarly, ORP9 and ORP11 could also form complexes, serving as functional units of intracellular lipid sensors or transporters [26, 58]. ORP5 may contribute to the recruitment of ORP8 to the ER-PM MCSs, where PI4P/PS were effectively countertransported to maintain lipid homeostasis [52]. Recent studies suggested that ORP5 and ORP8 form a complex localized at ER-mitochondria contact sites and ER subdomains in contact with mitochondria and nascent lipid droplets [59, 60]. These studies revealed that the functional coupling between LTPs might be a common characteristic of the OSBP/ORPs family.
We demonstrate that the interaction of ORP9 and ORP10 is not from the PH domains or ORDs, but mediated by the CC domains in the linker region connected to the PH domain and ORD. Interestingly, previous reports showed that OSBP formed homologous dimers through the CC domains in a similar linker region [23]. However, our studies suggested that ORP9 and ORP10 could not form homologous complexes individually like OSBP. Instead, the CC domains of ORP9 and ORP10 mediate heterologous interactions of these two ORPs. Interestingly, a recent study showed ORP5 recruits ORP8 to MCSs through its CC domain [60]. It might be conserved that OSBP/ORPs interact with each other through the CC domains. However, the binding modes of their CC domain-CC domain interactions could be divergent.
It is challenging to delineate an individual LTP in the complex ER-TGN interfaces. We take advantage of the FL LTP reconstitution assay to address the mechanisms of the ORP9-ORP10 complex in a defined system without the complications of other factors naturally present in cells [20, 23, 34]. This reconstitution system demonstrated that the ORP9-ORP10 complex efficiently transfers PI4P/PS between the apposed membranes. Previous studies showed that ORP10 maintains the ER-TGN MCSs integrity with the requirement of PS transfer from the ER to the TGN [38]. Early evidence indicated that ORP9 could extract and transfer cholesterol between the ER and the TGN to maintain the integrity of the early secretory pathway [35, 36]. Our study showed that ORP9 and ORP10 efficiently transport PS rather than cholesterol. Thus, ORP9 and ORP10 are LTPs that mediate PI4P/PS transport at ER-TGN MCSs.
By the application of the reconstitution assay, we observed that both the PH domains of ORP9 and ORP10 are involved in membrane targeting. Therefore, while the ORP9-ORP10 complexes are anchored to the ER through the ORP9-VAP interactions, they target the TGN membrane through the two PH domains, thereby tethering the ER and the TGN. Correspondingly, the two ORDs coordinately mediate PI4P transport from the TGN to the ER. (Fig. 7e).
In summary, our findings unravel a mechanism of ORP9 and ORP10 at ER-TGN MCSs. The two LTPs form a heterocomplex through the CC domain-CC domain interaction, which may represent a conserved mechanism for intermolecular assemblies of OSBP/ORPs. ORP9 and ORP10 mediate membrane tethering and simultaneously transport PI4P, further regulating lipid homeostasis and vesicle trafficking.
Materials and methods
Plasmids
The human FL VAPA gene was cloned into a pET-SUMO vector. The sequence of truncated VAPA (a.a.8–212) was subcloned into a pGEX4T-3 vector. The human ORP9 sequences [FL (a.a.1–736), ΔPH (a.a.113–736), ΔORD (a.a.1–306)] and human ORP10 sequences [FL (a.a.1–764), ΔORD (a.a.1–392)] were subcloned into a pFast-Bac vector. ORP10ΔPH (a.a.233–764) was subcloned into a pFast-Bac-MBP vector. The fragments of ORP9-CC (a.a.113–194) and ORP10-CC (a.a.223–302) were subcloned into the pET-SUMO and pGEX6P-1 vector, respectively. The PH domain of FAPP1 (a.a.1–100) was cloned into the pET-SUMO vector and was modified by C37S/C94S/T13C mutagenesis. The C2 domain of lactadherin (a.a.270–427) was cloned into pET-SUMO vector and was modified by C270A/C427A/H352C mutagenesis.
The human ORP9 gene was cloned into the pmCherry-C1, pcDNA3.1, SHC003BSD (Addgene, #133300), and pnYFP-C3 vector. The human ORP10 gene was cloned into the pEGFP-C3, pcDNA3.1, SHC003BSD, and pcYFP-C3 vector. The ORP9 and ORP10 mutants were generated by site-directed mutagenesis. The P4C domain of SidC (a.a.614–744) was cloned into the pEGFP-C3 vector. TGN38, a biomarker of TGN, was cloned into the pmCherry-N1 vector. The pmCherry-Sec61b plasmid was kindly provided by Dr. Zonghong Li (Guangzhou Laboratory).
Cell culture
HeLa cells and 293 T cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS and penicillin/streptomycin. Sf9 insect cells were cultured in SF-900™ II SFM Medium.
CRISPR-Cas9 genome editing
For single gene depletion, the guide sequence targeting ORP9 or ORP10 was individually subcloned into the pLenti-CRISPR-V2 vector (Addgene, #52961). For double depletion of ORP9 and ORP10, the gRNA targeting the ORP10 gene was subcloned into the BsmBI site of the pLenti-CRISPR-V2 vector. Meanwhile, the gRNA targeting the ORP9 gene was subcloned into the BbsI site of the pmU6-gRNA vector (Addgene, #53187). Next, the expression cassette of the ORP9 gRNA, including the mU6 promoter, the gRNA and the gRNA scaffold, was amplified by PCR with KpnI sites introduced at both ends. The PCR product was subcloned into the KpnI site of the pLenti-CRISPR-V2 containing the ORP10 gRNA [61]. The plasmids were transfected into 293 T cells along with pAdVAntage (Promega, #E1711), pCMV-VSVG (Addgene, #8454), and psPax2 (Addgene, #12260) [62, 63]. The media containing lentiviral particles were harvested and centrifuged at 25,000 rpm for 1.5 h in a Beckman SW28 rotor. Viral pellets were resuspended in media and used to infect HeLa cells. Infected cells were selected using 1 µg/mL puromycin. The sequence of gRNA targeting the human ORP9 gene was CAATTGGGGTGCACAACATA, and the sequence of gRNA targeting the human ORP10 gene was GCCCTTGCGGCCCTCGTGAA.
Confocal imaging and analysis
HeLa cells were transiently transfected with the indicated plasmids using Lipofectamine 2000 (Thermo Fisher Scientific, #11668-019) and harvested 24 h after transfection for analysis. Cells grown on coverslips were fixed using 4% paraformaldehyde and mounted to adhesion microscope slides using the anti-fade mounting solution. Confocal images were visualized under a Nikon A1 microscope (Nikon Corporation, Japan) and analyzed using FIJI software. To evaluate the degrees of colocalization, Coloc2 in ImageJ was used to calculate the Person’s coefficient, which measures the strength of the association between two continuous variables.
For analysis of PI4P levels on TGN, indicated cells were transfected with GFP-P4C-SidC and mCherry-TGN38. The fluorescent regions of GFP-P4C-SidC colocalized with mCherry-TGN38 were selected as the region of interest (ROI). The mean fluorescence intensity of ROI and PM was analyzed and normalized with the mean fluorescence intensity of the background to adjust the expression differences. The normalized PI4P levels on TGN were indicated by the ratios between the mean GFP fluorescence intensity of ROI and PM.
Immunofluorescence microscopy
Cells grown on coverslips were fixed using 4% paraformaldehyde, and then permeabilized with 0.2% Triton X-100 for 10 min. The samples were blocked with 1% BSA for 1 h, incubated with primary antibodies at 4 ℃ overnight, immunolabeled fluorescence-conjugated secondary antibodies for 1 h at room temperature, and mounted to adhesion microscope slides using the anti-fade mounting solution. Confocal images were visualized under a Nikon A1 microscope (Nikon Corporation, Japan).
To detect PI4P levels at TGN, the cells were fixed with 2% paraformaldehyde for 15 min and washed three times with PBS containing 50 mM NH4Cl. Cells were permeabilized with immunofluorescence buffer [ 20 mM PIPES (pH 6.8), 137 mM NaCl, 2.7 mM KCl] supplemented with 20 μM digitonin for 5 min and were blocked for 45 min in immunofluorescence buffer containing 5% normal goat serum and 50 mM NH4Cl. Incubate with anti-PI4P antibodies (echelon biosciences, Z-P004) and immunolabeled 488-conjugated secondary antibodies (ABclonal, AS037) for 1 h and 45 min at room temperature, respectively. Cells were postfixed with 2% paraformaldehyde for 10 min and then were mounted to adhesion microscope slides using the anti-fade mounting solution. Confocal images were visualized under a Nikon A1 microscope (Nikon Corporation, Japan).
IP and immunoblotting
HeLa cells were transiently transfected with the indicated plasmids using Lipofectamine 2000. The cells were harvested 24 h after transfection for analysis. In IP experiments, cells were lysed in a lysis buffer [25 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 5% glycerol, and a protease inhibitor cocktail]. Proteins were precipitated from cell lysates using anti-FLAG M2 antibody (Millipore-Sigma, #F1804) and protein A agarose beads. For endogenous IP experiments, cells were directly harvested without transfection. Endogenous ORP9 was precipitated from cell lysates using an anti-ORP9 antibody (Proteintech, 11879-1-AP#F1804) and protein A agarose beads. Immunoprecipitates and whole cells were lysed in 1 × SDS loading buffer and resolved by SDS-PAGE. Proteins were detected using primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies.
Protein expression and purification
Recombinant FL VAPA was expressed and purified by nickel affinity chromatography, using a previously established procedure [64]. Briefly, the recombinant plasmid was transformed into BL21 (DE3) competent cell. The protein expression was induced by isopropyl β-d-galactopyranoside (IPTG) when the OD600 of culture reached 0.6–0.8. The culture continued to grow for 3.5 h at 37 °C and then harvested by centrifugation at 6000g for 30 min. The cell pellets were resuspended in a lysis buffer [25 mM HEPES (pH 7.4), 400 mM KCl, 10% glycerol, 20 mM imidazole, 1% Triton X-100, 2 mM b-mercaptoethanol, and EDTA-free protease inhibitor cocktail]. After lysis, the protein was purified by nickel affinity chromatography. The purified VAPA was stored in membrane protein storage buffer containing 25 mM HEPES (pH 7.4), 400 mM KCl, 1% n-octyl-β-d-glucoside, 10% (vol/vol) glycerol, and 1 mM DTT. ORP9-CC, ORP10-CC, FAPP1-PH and Lact-C2 were produced and purified similarly to VAPA. The His6-SUMO tags were removed by SUMO proteases. Recombinant FL ORP9, ORP9ΔPH, ORP9ΔORD, FL ORP10, ORP10ΔPH, and ORP10ΔORD were produced in Sf9 insect cells using baculovirus infection as previously described [65]. Protein was expressed in Sf9 cells according to the manufacturer’s instruction (Bac-to-Bac Baculovirus Expression System, Life Technologies). The cells were lysed in the lysis buffer. The cell extract was centrifuged at 18,500 rpm for 30 min at 4 ℃. Proteins were purified by nickel affinity chromatography and were subsequently dialyzed overnight against a storage buffer [25 mM HEPES (pH 7.4), 150 mM KCl, 10% glycerol, and 1 mM DTT].
Protein labeling
FAPP1-PH and Lact-C2 were fluorescence-labeled by the NBD labeling kit following the manufacturer’s instructions. Briefly, the protein solution was dialyzed against the labeling buffer [25 mM HEPES (pH 7.4), 150 mM KCl, and 10% glycerol] and then mixed with tenfold molar excess of N,N′-dimethyl-N-(iodoacetyl)-N′-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) ethylenediamine (IANBD-amide, # D2004) for 2 h on ice. The reaction was stopped by adding a tenfold molar excess of l-cysteine. The free dye was removed by overnight dialysis, and the labeled protein was analyzed by SDS-PAGE and absorption spectroscopy.
Pull-down assay
Pull-down assay was performed as previously described [62, 66]. The indicated recombinant proteins were expressed in BL21 (DE3) E. coli. When OD600 of E. coli cultured in 2 × YT media reached ~ 0.6–0.8, 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to induce protein expression. After two hours of incubation at 37 °C, cells were harvested and lysed. After centrifugation, proteins were isolated using glutathione beads (Thermo Fisher Scientific, #16101), resolved by SDS-PAGE, and stained with Coomassie Blue.
HeLa cells were transfected with the indicated plasmids using Lipofectamine 2000 for recombinant proteins that need to be expressed in mammalian cells. 24 h after transfection, the cells were washed and lysed in the lysis buffer. The obtained lysates were incubated with GST-VAPA (8-212) and glutathione beads for 1 h at 4℃. The beads were then washed three times with lysis buffer, resuspended in 1 × SDS loading buffer, and resolved by SDS-PAGE. Proteins were detected using primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies.
CD spectroscopy
Near UV CD spectra were measured using a Chirascan spectropolarimeter at 20 ℃ with a 1-mm quartz cell. The readings were made at 0.5-nm intervals, and each data point represented the average of six scans at a speed of 50 nm/min over the wavelength range of 195–260 nm. The data were converted into mean residue weighted molar ellipticity using the following equation: [θ]MRW = (100 × θ)/Cnl, where C is the protein concentration (mM), θ is the measured ellipticity (milli-degree), n is the number of residues, and l is the path length (cm).
PIPs binding assay
Lipids were dissolved in chloroform and then spotted onto the nitrocellulose membrane (PALL, 66485) strips. All the lipids used in this study were purchased from Avanti Polar Lipids, including N-oleoyl-ceramide-1-phosphate(C1P, #860599), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol-3′-phosphate) (PI3P, #850150P), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol-4′-phosphate) (PI4P, #850151P), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol-5′-phosphate) (PI5P, #850152P), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol-3′,5′-bisphosphate) (PI(3,5)P2, #850154P), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol-4′,5′-bisphosphate) (PtdIns(4,5) P2, #850155P), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol-3′,4′-bisphosphate) (PI(3,4)P2, #850153P), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol-3′,4′,5′-trisphosphate) (PI(3,4,5)P3, #850156P), l-α-phosphatidylethanolamine (PE, #840024P),
l-α-phosphatidylcholine (PC, #840054P), 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (PS, #840035), l-α-phosphatidylglycerol (PG, #841148P), 1′,3′-bis[1,2-dimyristoyl-sn-glycero-3-phospho]-glycerol (CL, #710332), l-α-phosphatidic acid (PA, #840074P) and l-α-phosphatidylinositol (PI, #840044P). The PIP strips were blocked with 3% fatty acid-free bovine serum albumin in 1 × TBS-T [20 mM Tris (pH 7.4), 150 mM NaCl, 0.1%(v/v) Tween20] for 1 h and then incubated with purified GST-tagged proteins at 4℃ overnight. After washing, the strips were incubated with the rabbit polyclonal anti-GST antibody (Proteintech, #10000-0-AP) for 1 h, followed by incubating with goat anti-rabbit IgG-HRP antibody for 1 h at room temperature. Then, the strips were analyzed by a chemiluminescence gel imaging system.
Liposome preparation
All lipids used in this work were acquired from Avanti Polar Lipids. To prepare TGN-like liposomes in PI4P transport assays, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC, #850457P), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE, #850757P), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine (POPS, #840034P), l-α-phosphatidylinositol-4-phosphate (PI4P, # 840045P) and N-(Lissamine rhodamine B sulfonyl)-1,2-dipalmitoyl phosphatidylethanolamine (Rhod-PE, #810158P) were mixed at a molar ratio of 66:18:10:4:2. Lipid mixture was dried to form a film under a stream of N2 gas, and further dried in a vacuum desiccator for 1 h. The lipid film was rehydrated in HKM buffer [50 mM HEPES (pH 7.4), 120 mM potassium acetate, 1 mM MgCl2]. The multilamellar liposome suspension was processed by seven freeze–thaw cycles and extruded through polycarbonate filters of 200 nm pore size using an Avanti Mini-Extruder (Avanti Polar Lipids). For ER-like liposomes in PI4P transport assays, POPC, POPE, and POPS were mixed in a molar ratio of 70:20:10. Lipid mixture was dried and dissolved in membrane protein storage buffer. VAPA proteoliposomes were formed by detergent dilution and isolated on a Nycodenz density gradient flotation [67, 68]. Complete detergent removal was achieved by overnight dialysis of the samples in Novagen dialysis tubes against the reconstitution buffer [25 mM HEPES (pH 7.4), 100 mM KCl, 10% glycerol, and 1 mM DTT]. Liposomes used in other assays were generated by the same procedure but with different lipid components.
Liposome coflotation assay
The binding of soluble factors with membranes was examined using a liposome coflotation assay, as we previously described [65, 69]. Protein was incubated with liposomes at 4 ℃ with gentle agitation. An equal volume (150 μL) of 80% Nycodenz (w/v) in the reconstitution buffer was added after 1 h, and the mixture was transferred to 5-mm by 41-mm centrifuge tubes. The samples were overlaid with 200 μL each of 35% and 30% Nycodenz, and then with 20 μL reconstitution buffer on the top. The gradients were centrifuged for 4 h at 48,000 rpm in a Beckman SW55 rotor. Liposome samples were collected from the 0/30% Nycodenz interface (2 × 20 μL) and analyzed by SDS-PAGE.
FRET-based lipid transfer assay
The lipid transfer reaction was conducted in a 96-well Nunc plate at 37 °C through a FRET-based assay. The donor and acceptor liposomes (200 μM total lipids for each) were mixed in the presence or absence of ORPs to initiate the reactions. Increase in NBD-fluorescence at 538 nm (excitation 460 nm) was measured every two minutes in a BioTek Synergy HT microplate reader [70–72]. At the end of the reaction, 10 μL of 10% CHAPS (Sangon Biotech, # A600110) was added to the liposomes. The data were presented as the percentage of maximum fluorescence change.
For PI4P transport, The TGN-like liposomes were incubated with NBD-FAPP1-PH at 37 °C for 5 min. Then, the ER-like liposomes were added to initiate the reactions in the presence or absence of ORPs. For PS transport, The ER-like liposomes (POPC:POPE:POPS: Rhod-PE = 75:18:5:2) were incubated with NBD-Lact-C2 at 37 °C for 5 min. Then, the TGN-like liposomes (POPC:POPE:PI4P = 76:20:4) were added to initiate the reactions in the presence or absence of ORPs. For cholesterol transport, The ER-like liposomes (POPC:POPE:cholesterol:NBD-cholesterol:Rhod-PE = 75:18.5:3.5:1.5:1.5) and TGN-like liposomes (POPC:POPE:PI4P = 76:20:4) were added to initiate the reactions in the presence or absence of ORPs.
The initial lipid transfer rates of ORPs were calculated using the formula: Vtransfer = [(Nlipid × Plipid)/NORPs]/treaction. N represents the total amount of lipid or protein concentration in each reaction. P represents the percentage of lipid transferred during the initial 10 min reaction. Full accounting of statistical significance was included for each figure based on at least three independent experiments.
Liposome tethering assay
The turbidity, which reflects liposome clustering, was employed to evaluate liposome tethering [73, 74]. The reactions were performed similar to those in FRET-based lipid transfer assays, except the absorbance at 405 nm was measured at 30 min using a BioTek Synergy HT microplate reader [70, 71]. Full accounting of statistical significance was included for each figure based on at least three independent experiments.
VSV-G transport
HeLa cells were transiently transfected with GFP-VSV-G (ts045) (Addgene, #11912). After 6 h, the cells were transferred to the restrictive temperature of 39.5 °C for 20 h, then moved to the permissive temperature of 32 °C for 2 h in the presence of 100 μg/mL of cycloheximide. The cells were fixed with 4% paraformaldehyde and incubated with 2% bovine serum albumin. Surface VSV-G was stained with anti-GFP antibodies and Alexa Fluor 555-conjugated secondary antibodies (abcam, #ab150078). Images were captured using a 100 × oil immersion objective on a Nikon A1 Laser Scanning confocal microscope.
Statistical analysis
All data were presented as the mean ± SD and were analyzed using GraphPad Prism 8.0.2 software for Windows. Statistical significance was calculated using one-way ANOVA or unpaired t test, and p value < 0.05 was considered statistically significant.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Dr. Jingshi Shen and Dr. Chun Wan for helpful discussions and advice. We thank Dr. Zonghong Li for providing the pmCherry-Sec61b plasmid. We thank Dr. Xiaojun Wang for technical assistance.
Author contributions
HY, CZ, and YL conceived the project. RH, FL, HW, and SH performed the experiments. RH, FL, KX, and YL analyzed the data. RH, KX, YL, CZ, and HY wrote the manuscript with input from all authors.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) Grants (nos. 32270735, 91854117, 31871425, and 32100546), Natural Science Foundation of Jiangsu Province (BK20200036), Jiangsu Province’s Innovation Program (JSSCTD202142), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Availability of data and material
All data and materials are available in the manuscript, the supplementary information, or from the corresponding authors upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yinghui Liu, Email: yinghuiliu@njnu.edu.cn.
Haijia Yu, Email: yuhaijia@njnu.edu.cn.
References
- 1.Masone MC, Morra V, Venditti R. Illuminating the membrane contact sites between the endoplasmic reticulum and the trans-Golgi network. FEBS Lett. 2019;593:3135–3148. doi: 10.1002/1873-3468.13639. [DOI] [PubMed] [Google Scholar]
- 2.Prinz WA, Toulmay A, Balla T. The functional universe of membrane contact sites. Nat Rev Mol Cell Biol. 2020;21:7–24. doi: 10.1038/s41580-019-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siehler J, Blochinger AK, Meier M, Lickert H. Engineering islets from stem cells for advanced therapies of diabetes. Nat Rev Drug Discov. 2021;20:920–940. doi: 10.1038/s41573-021-00262-w. [DOI] [PubMed] [Google Scholar]
- 4.Vance JE. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16:1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- 5.Stefan CJ, Trimble WS, Grinstein S, Drin G, Reinisch K, De Camilli P, Cohen S, Valm AM, Lippincott-Schwartz J, Levine TP, et al. Membrane dynamics and organelle biogenesis-lipid pipelines and vesicular carriers. BMC Biol. 2017;15:102. doi: 10.1186/s12915-017-0432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lev S. Nonvesicular lipid transfer from the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2012;4:a013300. doi: 10.1101/cshperspect.a013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinisch KM, Prinz WA. Mechanisms of nonvesicular lipid transport. J Cell Biol. 2021;220:e202012058. doi: 10.1083/jcb.202012058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong LH, Gatta AT, Levine TP. Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes. Nat Rev Mol Cell Biol. 2019;20:85–101. doi: 10.1038/s41580-018-0071-5. [DOI] [PubMed] [Google Scholar]
- 9.Lipp NF, Ikhlef S, Milanini J, Drin G. Lipid exchangers: cellular functions and mechanistic links with phosphoinositide metabolism. Front Cell Dev Biol. 2020;8:663. doi: 10.3389/fcell.2020.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiapparino A, Maeda K, Turei D, Saez-Rodriguez J, Gavin AC. The orchestra of lipid-transfer proteins at the crossroads between metabolism and signaling. Prog Lipid Res. 2016;61:30–39. doi: 10.1016/j.plipres.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Kentala H, Weber-Boyvat M, Olkkonen VM. OSBP-related protein family: mediators of lipid transport and signaling at membrane contact sites. Int Rev Cell Mol Biol. 2016;321:299–340. doi: 10.1016/bs.ircmb.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Raychaudhuri S, Prinz WA. The diverse functions of oxysterol-binding proteins. Annu Rev Cell Dev Biol. 2010;26:157–177. doi: 10.1146/annurev.cellbio.042308.113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olkkonen VM. OSBP-related proteins: liganding by glycerophospholipids opens new insight into their function. Molecules. 2013;18:13666–13679. doi: 10.3390/molecules181113666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber-Boyvat M, Kentala H, Peranen J, Olkkonen VM. Ligand-dependent localization and function of ORP-VAP complexes at membrane contact sites. Cell Mol Life Sci. 2015;72:1967–1987. doi: 10.1007/s00018-014-1786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiser SE, Brickner JH, Reilein AR, Fenn TD, Walter P, Brunger AT. Structural basis of FFAT motif-mediated ER targeting. Structure. 2005;13:1035–1045. doi: 10.1016/j.str.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Murphy SE, Levine TP. VAP, a versatile access point for the endoplasmic reticulum: review and analysis of FFAT-like motifs in the VAPome. Biochim Biophys Acta. 2016;1861:952–961. doi: 10.1016/j.bbalip.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Lemmon MA. Pleckstrin homology (PH) domains and phosphoinositides. Biochem Soc Symp. 2007;1:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galmes R, Houcine A, Vliet AR, Agostinis P, Jackson CL, Giordano F. ORP5/ORP8 localize to endoplasmic reticulum–mitochondria contacts and are involved in mitochondrial function. EMBO Rep. 2016;17:800–810. doi: 10.15252/embr.201541108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghai R, Du X, Wang H, Dong J, Ferguson C, Brown AJ, Parton RG, Wu JW, Yang H. ORP5 and ORP8 bind phosphatidylinositol-4, 5-biphosphate (PtdIns(4,5)P 2) and regulate its level at the plasma membrane. Nat Commun. 2017;8:757. doi: 10.1038/s41467-017-00861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesmin B, Bigay J, Moser von Filseck J, Lacas-Gervais S, Drin G, Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 21.Levine TP, Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr Biol CB. 2002;12:695–704. doi: 10.1016/s0960-9822(02)00779-0. [DOI] [PubMed] [Google Scholar]
- 22.Peretti D, Dahan N, Shimoni E, Hirschberg K, Lev S. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol Biol Cell. 2008;19:3871–3884. doi: 10.1091/mbc.e08-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de la Mora E, Dezi M, Di Cicco A, Bigay J, Gautier R, Manzi J, Polidori J, Castano-Diez D, Mesmin B, Antonny B, et al. Nanoscale architecture of a VAP-A-OSBP tethering complex at membrane contact sites. Nat Commun. 2021;12:3459. doi: 10.1038/s41467-021-23799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamecna D, Polidori J, Mesmin B, Dezi M, Levy D, Bigay J, Antonny B. An intrinsically disordered region in OSBP acts as an entropic barrier to control protein dynamics and orientation at membrane contact sites. Dev Cell. 2019;49:220–234. doi: 10.1016/j.devcel.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Lim CY, Davis OB, Shin HR, Zhang J, Berdan CA, Jiang X, Counihan JL, Ory DS, Nomura DK, Zoncu R. ER-lysosome contacts enable cholesterol sensing by mTORC1 and drive aberrant growth signalling in Niemann-Pick type C. Nat Cell Biol. 2019;21:1206–1218. doi: 10.1038/s41556-019-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan JX, Finkel T. A phosphoinositide signalling pathway mediates rapid lysosomal repair. Nature. 2022;609:815–821. doi: 10.1038/s41586-022-05164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond GRV, Burke JE. Novel roles of phosphoinositides in signaling, lipid transport, and disease. Curr Opin Cell Biol. 2020;63:57–67. doi: 10.1016/j.ceb.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santiago-Tirado FH, Bretscher A. Membrane-trafficking sorting hubs: cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol. 2011;21:515–525. doi: 10.1016/j.tcb.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Matteis MA, Wilson C, D'Angelo G. Phosphatidylinositol-4-phosphate: the Golgi and beyond. BioEssays. 2013;35:612–622. doi: 10.1002/bies.201200180. [DOI] [PubMed] [Google Scholar]
- 31.Tan J, Brill JA. Cinderella story: PI4P goes from precursor to key signaling molecule. Crit Rev Biochem Mol Biol. 2014;49:33–58. doi: 10.3109/10409238.2013.853024. [DOI] [PubMed] [Google Scholar]
- 32.Waugh MG. The Great Escape: how phosphatidylinositol 4-kinases and PI4P promote vesicle exit from the Golgi (and drive cancer) Biochem J. 2019;476:2321–2346. doi: 10.1042/BCJ20180622. [DOI] [PubMed] [Google Scholar]
- 33.Venditti R, Masone MC, Rega LR, Di Tullio G, Santoro M, Polishchuk E, Serrano IC, Olkkonen VM, Harada A, Medina DL, et al. The activity of Sac1 across ER-TGN contact sites requires the four-phosphate-adaptor-protein-1. J Cell Biol. 2019;218:783–797. doi: 10.1083/jcb.201812021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesmin B, Bigay J, Polidori J, Jamecna D, Lacas-Gervais S, Antonny B. Sterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OSBP. EMBO J. 2017;36:3156–3174. doi: 10.15252/embj.201796687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngo M, Ridgway ND. Oxysterol binding protein-related Protein 9 (ORP9) is a cholesterol transfer protein that regulates Golgi structure and function. Mol Biol Cell. 2009;20:1388–1399. doi: 10.1091/mbc.E08-09-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Ridgway ND. Characterization of the sterol and phosphatidylinositol 4-phosphate binding properties of Golgi-associated OSBP-related protein 9 (ORP9) PLoS ONE. 2014;9:e108368. doi: 10.1371/journal.pone.0108368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda K, Anand K, Chiapparino A, Kumar A, Poletto M, Kaksonen M, Gavin AC. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature. 2013;501:257–261. doi: 10.1038/nature12430. [DOI] [PubMed] [Google Scholar]
- 38.Venditti R, Rega LR, Masone MC, Santoro M, Polishchuk E, Sarnataro D, Paladino S, D'Auria S, Varriale A, Olkkonen VM, et al. Molecular determinants of ER-Golgi contacts identified through a new FRET-FLIM system. J Cell Biol. 2019;218:1055–1065. doi: 10.1083/jcb.201812020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakatsu F, Kawasaki A. Functions of oxysterol-binding proteins at membrane contact sites and their control by phosphoinositide metabolism. Front Cell Dev Biol. 2021;9:664788. doi: 10.3389/fcell.2021.664788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawasaki A, Sakai A, Nakanishi H, Hasegawa J, Taguchi T, Sasaki J, Arai H, Sasaki T, Igarashi M, Nakatsu F. PI4P/PS countertransport by ORP10 at ER-endosome membrane contact sites regulates endosome fission. J Cell Biol. 2022;221:e202103141. doi: 10.1083/jcb.202103141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saraste M, Hyvönen M. Pleckstrin homology domains: a fact file. Curr Opin Struct Biol. 1995;5:403–408. doi: 10.1016/0959-440x(95)80104-9. [DOI] [PubMed] [Google Scholar]
- 42.Singh N, Reyes-Ordonez A, Compagnone MA, Moreno JF, Leslie BJ, Ha T, Chen J. Redefining the specificity of phosphoinositide-binding by human PH domain-containing proteins. Nat Commun. 2021;12:4339. doi: 10.1038/s41467-021-24639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- 46.Buchan DWA, Jones DT. The PSIPRED Protein Analysis Workbench: 20 years on. Nucleic Acids Res. 2019;47:W402–W407. doi: 10.1093/nar/gkz297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nissila E, Ohsaki Y, Weber-Boyvat M, Perttila J, Ikonen E, Olkkonen VM. ORP10, a cholesterol binding protein associated with microtubules, regulates apolipoprotein B-100 secretion. Biochim Biophys Acta. 2012;1821:1472–1484. doi: 10.1016/j.bbalip.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Luo X, Wasilko DJ, Liu Y, Sun J, Wu X, Luo ZQ, Mao Y. Structure of the legionella virulence factor, SidC reveals a unique PI(4)P-specific binding domain essential for its targeting to the bacterial phagosome. PLoS Pathog. 2015;11:e1004965. doi: 10.1371/journal.ppat.1004965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boura E, Nencka R. Phosphatidylinositol 4-kinases: function, structure, and inhibition. Exp Cell Res. 2015;337:136–145. doi: 10.1016/j.yexcr.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 50.van der Schaar HM, Leyssen P, Thibaut HJ, de Palma A, van der Linden L, Lanke KH, Lacroix C, Verbeken E, Conrath K, Macleod AM, et al. A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIbeta. Antimicrob Agents Chemother. 2013;57:4971–4981. doi: 10.1128/AAC.01175-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Mikrani R, Hu Y, Faran Ashraf Baig MM, Abbas M, Akhtar F, Xu M. Research progress of phosphatidylinositol 4-kinase and its inhibitors in inflammatory diseases. Eur J Pharmacol. 2021;907:174300. doi: 10.1016/j.ejphar.2021.174300. [DOI] [PubMed] [Google Scholar]
- 52.Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349:428–432. doi: 10.1126/science.aab1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan X, Banerjee P, Pham EA, Rutaganira FUN, Basu K, Bota-Rabassedas N, Guo HF, Grzeskowiak CL, Liu X, Yu J, et al. PI4KIIIβ is a therapeutic target in chromosome 1q-amplified lung adenocarcinoma. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.aax3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balla A, Balla T. Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 2006;16:351–361. doi: 10.1016/j.tcb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Del Bel LM, Brill JA. Sac1, a lipid phosphatase at the interface of vesicular and nonvesicular transport. Traffic. 2018;19:301–318. doi: 10.1111/tra.12554. [DOI] [PubMed] [Google Scholar]
- 56.Burke JE. Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol Cell. 2018;71:653–673. doi: 10.1016/j.molcel.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Dornan GL, McPhail JA, Burke JE. Type III phosphatidylinositol 4 kinases: structure, function, regulation, signalling and involvement in disease. Biochem Soc Trans. 2016;44:260–266. doi: 10.1042/BST20150219. [DOI] [PubMed] [Google Scholar]
- 58.Zhou Y, Li S, Mayranpaa MI, Zhong W, Back N, Yan D, Olkkonen VM. OSBP-related protein 11 (ORP11) dimerizes with ORP9 and localizes at the Golgi-late endosome interface. Exp Cell Res. 2010;316:3304–3316. doi: 10.1016/j.yexcr.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Monteiro-Cardoso VF, Rochin L, Arora A, Houcine A, Jaaskelainen E, Kivela AM, Sauvanet C, Le Bars R, Marien E, Dehairs J, et al. ORP5/8 and MIB/MICOS link ER-mitochondria and intra-mitochondrial contacts for non-vesicular transport of phosphatidylserine. Cell Rep. 2022;40:111364. doi: 10.1016/j.celrep.2022.111364. [DOI] [PubMed] [Google Scholar]
- 60.Guyard V, Monteiro-Cardoso VF, Omrane M, Sauvanet C, Houcine A, Boulogne C, Ben Mbarek K, Vitale N, Faklaris O, El Khallouki N, et al. ORP5 and ORP8 orchestrate lipid droplet biogenesis and maintenance at ER-mitochondria contact sites. J Cell Biol. 2022 doi: 10.1083/jcb.202112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S, Crisman L, Miller J, Datta I, Gulbranson DR, Tian Y, Yin Q, Yu H, Shen J. Inducible Exoc7/Exo70 knockout reveals a critical role of the exocyst in insulin-regulated GLUT4 exocytosis. J Biol Chem. 2019;294:19988–19996. doi: 10.1074/jbc.RA119.010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gulbranson DR, Crisman L, Lee M, Ouyang Y, Menasche BL, Demmitt BA, Wan C, Nomura T, Ye Y, Yu H, et al. AAGAB controls AP2 adaptor assembly in clathrin-mediated endocytosis. Dev Cell. 2019;50:436–446. doi: 10.1016/j.devcel.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gulbranson DR, Davis EM, Demmitt BA, Ouyang Y, Ye Y, Yu H, Shen J. RABIF/MSS4 is a Rab-stabilizing holdase chaperone required for GLUT4 exocytosis. Proc Natl Acad Sci USA. 2017;114:E8224–E8233. doi: 10.1073/pnas.1712176114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muretta JM, Romenskaia I, Mastick CC. Insulin releases Glut4 from static storage compartments into cycling endosomes and increases the rate constant for Glut4 exocytosis. J Biol Chem. 2008;283:311–323. doi: 10.1074/jbc.M705756200. [DOI] [PubMed] [Google Scholar]
- 65.Yu H, Rathore SS, Lopez JA, Davis EM, James DE, Martin JL, Shen J. Comparative studies of Munc18c and Munc18-1 reveal conserved and divergent mechanisms of Sec1/Munc18 proteins. Proc Natl Acad Sci USA. 2013;110:E3271–E3280. doi: 10.1073/pnas.1311232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wan C, Crisman L, Wang B, Tian Y, Wang S, Yang R, Datta I, Nomura T, Li S, Yu H, et al. AAGAB is an assembly chaperone regulating AP1 and AP2 clathrin adaptors. J Cell Sci. 2021;134:jcs258587. doi: 10.1242/jcs.258587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rathore SS, Liu Y, Yu H, Wan C, Lee M, Yin Q, Stowell MHB, Shen J. Intracellular vesicle fusion requires a membrane-destabilizing peptide located at the juxtamembrane region of the v-SNARE. Cell Rep. 2019;29:4583–4592. doi: 10.1016/j.celrep.2019.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Wan C, Rathore SS, Stowell MHB, Yu H, Shen J. SNARE zippering is suppressed by a conformational constraint that is removed by v-SNARE splitting. Cell Rep. 2021;34:108611. doi: 10.1016/j.celrep.2020.108611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu H, Shen C, Liu Y, Menasche BL, Ouyang Y, Stowell MHB, Shen J. SNARE zippering requires activation by SNARE-like peptides in Sec1/Munc18 proteins. Proc Natl Acad Sci USA. 2018;115:E8421–E8429. doi: 10.1073/pnas.1802645115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qian T, Li C, He R, Wan C, Liu Y, Yu H. Calcium-dependent and -independent lipid transfer mediated by tricalbins in yeast. J Biol Chem. 2021;296:100729. doi: 10.1016/j.jbc.2021.100729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qian T, Li C, Liu F, Xu K, Wan C, Liu Y, Yu H. Arabidopsis synaptotagmin 1 mediates lipid transport in a lipid composition-dependent manner. Traffic. 2022;23:346–356. doi: 10.1111/tra.12844. [DOI] [PubMed] [Google Scholar]
- 72.Yu H, Liu Y, Gulbranson DR, Paine A, Rathore SS, Shen J. Extended synaptotagmins are Ca2+-dependent lipid transfer proteins at membrane contact sites. Proc Natl Acad Sci USA. 2016;113:4362–4367. doi: 10.1073/pnas.1517259113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bian X, Saheki Y, De Camilli P. Ca(2+) releases E-Syt1 autoinhibition to couple ER-plasma membrane tethering with lipid transport. EMBO J. 2018;37:219–234. doi: 10.15252/embj.201797359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saheki Y, Bian X, Schauder CM, Sawaki Y, Surma MA, Klose C, Pincet F, Reinisch KM, De Camilli P. Control of plasma membrane lipid homeostasis by the extended synaptotagmins. Nat Cell Biol. 2016;18:504–515. doi: 10.1038/ncb3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials are available in the manuscript, the supplementary information, or from the corresponding authors upon reasonable request.