Abstract
Heat shock proteins (HSPs) play oncogenic roles in human tumours. We reported a somatic inactivating mutation of HSP110 (HSP110DE9) in mismatch repair-deficient (dMMR) cancers displaying microsatellite instability (MSI) but did not assess its impact. We evaluated the impact of the Hsp110DE9 mutation on tumour development and the chemotherapy response in a dMMR knock-in mouse model (Hsp110DE9KIMsh2KO mice). The effect of the Hsp110DE9 mutation on tumorigenesis and survival was evaluated in Msh2KO mice that were null (Hsp110wt), heterozygous (Hsp110DE9KI/+), or homozygous (Hsp110DE9KI/KI) for the Hsp110DE9 mutation by assessing tumoral syndrome (organomegaly index, tumour staging) and survival (Kaplan–Meier curves). 5-Fluorouracil (5-FU), which is the backbone of chemotherapy regimens in gastrointestinal cancers and is commonly used in other tumour types but is not effective against dMMR cells in vivo, was administered to Hsp110DE9KI/KI, Hsp110DE9KI/+, and Hsp110wtMsh2KO mice. Hsp110, Ki67 (proliferation marker) and activated caspase-3 (apoptosis marker) expression were assessed in normal and tumour tissue samples by western blotting, immunophenotyping and cell sorting. Hsp110wt expression was drastically reduced or totally lost in tumours from Msh2KOHsp110DE9KI/+ and Msh2KOHsp110DE9KI/KI mice. The Hsp110DE9 mutation did not affect overall survival or tumoral syndrome in Msh2KOHsp110DE9KI/+ and Msh2KOHsp110DE9KI/KI mice but drastically improved the 5-FU response in all cohorts (Msh2KOHsp110DE9KI/KI: P5fu = 0.001; Msh2KOHsp110DE9KI/+: P5fu = 0.005; Msh2KOHsp110wt: P5fu = 0.335). Histopathological examination and cell sorting analyses confirmed major hypersensitization to 5-FU-induced death of both Hsp110DE9KI/KI and Hsp110DE9KI/+ dMMR cancer cells. This study highlights how dMMR tumour cells adapt to HSP110 inactivation but become hypersensitive to 5-FU, suggesting Hsp110DE9 as a predictive factor of 5-FU efficacy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04293-3.
Keywords: Microsatellite instability, MMR deficiency, Mouse model, Hsp110, 5-FU chemotherapy
Introduction
Cancer is a major cause of death worldwide. A relatively large subset of tumours has defective mismatch repair (MMR) systems, causing high nucleotide instability, especially microsatellite instability (MSI). The clinicopathological characteristics of MSI tumours (i.e., immunogenic profile and low metastatic potential) make the MSI phenotype a theranostic biomarker [1]. In a recent study, we reported an unexpectedly frequent somatic inactivating mutation in the Hsp110 chaperone (Hsp110DE9) [2]. In addition, other publications and unpublished results from our group (data not shown) have revealed that HSP110 is also mutated in a substantial number of other primary MSI tumours and that HSP110 is implicated in lymphoma [3, 4]. Heat shock proteins (HSPs) are a large family of chaperones that perform multiple roles in eukaryotic cells, but their primary functions involve maturation and protein folding. HSPs are overexpressed in multiple cancer types. Their elevated expression is implicated in the molecular orchestration of cancer development and in the suppression of spontaneous and treatment-induced apoptosis; this characteristic antiapoptotic role of HSPs is important for aiding tumour progression and resistance to treatment [5]. The chaperone Hsp110 is overexpressed in a variety of cancers, including melanoma, breast cancer, colorectal cancer (CRC) and pancreatic cancer [6]. In CRC and melanoma, its overexpression is associated with disease progression [7, 8]. Consistent with these data, Hsp110 overexpression is suggested to be a poor prognostic factor in CRC [8, 9]. Mutation of HSP110 occurs due to MSI-driven somatic deletions in the noncoding microsatellite T17 of the HSP110 gene located in the vicinity of the splicing acceptor site of intron 8. A large deletion (> 4 bp) of this cis-splicing HSP110 T17 microsatellite leads to aberrant exon 9 skipping and a frameshift HSP110DE9 mRNA mutant containing a premature termination codon (PTC) before the last exon, which is therefore sensitive to mRNA decay and mostly degraded by the nonsense-mediated decay (NMD) system in cancer cells. The consequence of this mutation is the loss of wild-type HSP110 expression [10, 11].
According to a retrospective study investigating patients with resected MSI colon cancer treated with 5-fluorouracil (5-FU) in combination with oxaliplatin, only patients with the T17 mutation inducing the suppression of Hsp110 function appeared to benefit from 5-FU adjuvant chemotherapy even when used alone [10]. In addition, we demonstrated that HSP110 is likely to underlie cell growth in colon cancer in vitro, ex vivo (xenografts) and in vivo (human biopsies), putatively through its ability to induce STAT3 activation [12].
With the aim of further investigating the consequences of the Hsp110DE9 mutation in terms of tumorigenesis, cancer growth and response to 5-FU chemotherapy in MSI cancer, we created a germinal knock-in (KI) mouse model reproducing the effect of the deletion of T17 found in human MSI cancer (i.e., the Hsp110DE9 mutation) [10, 11]. These mice were crossed with MMR-deficient (dMMR) Msh2-knockout (KO) mice, which are predisposed to develop MSI T-cell lymphoma and, to a lesser extent, MSI intestinal adenocarcinoma [13–15]. Our results show that loss of Hsp110 function due to the Hsp110DE9 mutation in a germline context has no influence on either the initiation or progression of MSI lymphoma or adenocarcinoma in mice. In contrast, this event enhances the responsiveness to 5-FU and is associated with increased survival in 5-FU-treated mice with tumours that arise from defective DNA MMR. These results provide valuable knowledge about the MSI tumorigenesis model and the target genes for MSI cancers. Moreover, they provide important insights that reinforce the notion that the HSP110 mutation frequently observed in MSI tumours is a biomarker of response to chemotherapy and allows the identification of tumours sensitive to treatment with 5-FU alone.
Materials and methods
Generation of the Hsp110DE9KI/KI mouse strain
Hsp110DE9KI mice were generated by Clinique de la Souris Institut (ICS) and approved by the Ministère de la Recherche et de la Technologie (France). All information about the construction of the model and the protocol to introduce the mutation into C57BL/6 J ES cells are available on request. A schematic representation of the construction approach is shown in Supplementary Fig. S1. The study cohorts were generated by crossing Msh2± mice with Hsp110DE9KI/KI mice and intercrossing their F1 progeny to obtain the three cohort groups: Msh2KOHsp110wt, Msh2KOHsp110DE9KI/+, and Msh2KOHsp110DE9KI/KI mice (shown in Supplementary Fig. S2b).
Genotyping
Genotyping was performed on DNA isolated from tail snips. To identify Hsp110wt and Hsp110DE9 alleles, the following gene-specific primer sequences were used: forward: 5' GGAACTCATGCACATCAACAGAGTCAG 3' and 5' CCTTACGCTAATCACCAGCCACAAAG 3'; reverse: 5' CACGGCAGCACATGCCTTTAATAAGAG 3' (Sigma–Aldrich, France). The thermal cycling conditions used were as follows: 95 °C for 5 min followed by 95 °C for 1 min, 72 °C for 1 min, 72 °C for 1 min and a final extension for 10 min at 72 °C. To identify MSH2wt and MSH2KO alleles, the following gene-specific primer sequences were used: forward: 5' CGGCCTTGAGCTAAGTCTATTATAAGG 3'; reverse 5' GGTGGGATTAGATAATGCCTGCTCT 3' and 5' CCAAGATGACTGGTCGTACATAAG 3' (Sigma–Aldrich, France). The thermal cycling conditions used were as follows: 95 °C for 5 min followed by 95 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min and a final extension for 10 min at 72 °C. The PCR amplifications were performed in a total volume of 25 μl and cycled in the ABI Applied Biosystems 9700 thermal cycler. The PCR products were visualized on a 1% agarose gel containing 0.25 mg/ml ethidium bromide. The Hsp110wt allele has a corresponding 227 bp band, and the Hsp110DE9 allele has a corresponding 240 bp band. The MSH2wt allele has a corresponding 164 bp band, and the MSH2KO allele has a corresponding 194 bp band.
Animals
Animals were held in cages in the animal research facility at Centre de Recherche Saint Antoine (Paris). The air temperature and humidity were kept at 21–24 °C and 50–60%, respectively. A 12-h light/dark cycle was implemented. Food and water were given ad libitum. All animal experiments were conducted in accordance with the ethical regulations of the French Ministry of Research and Technology (Record number #9175–2,017,030,810,379,961 v3; Charles Darwin Ethics Committee). All efforts were made to minimize animal suffering. The mice were sacrificed by cervical dislocation at the first sign of suffering, at which time the tumours and organs were removed. This was considered the endpoint. Age, organomegaly, lymphoma staging and adenocarcinoma development were evaluated to classify mice and assess changes due to Hsp110DE9 mutation in untreated (or PBS-injected) and treated (5-FU-treated) mice. The spleen, liver, thymus and intestinal tract were systematically removed, a tail snip was obtained, and a portion of each type of tissue was stored at – 80 °C. The remaining portions of tissue were formalin fixed, embedded in paraffin, and sectioned for histological analysis and immunohistochemical staining or dissociated to obtain cell suspensions for cytometry analysis and/or immunoblotting analysis.
Lymphoma staging and organomegaly analysis
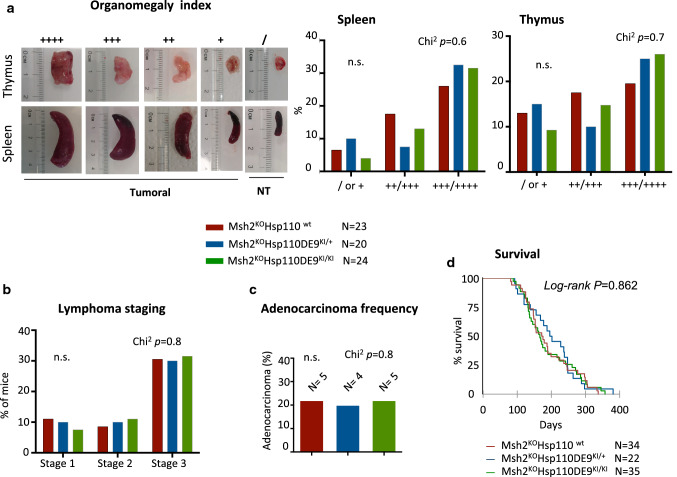
At the endpoint, mice were autopsied and assessed by macroanalysis. The COTSWOLD staging classification was adapted to classify the stage of lymphoma in mice. Stage 1 corresponds to the involvement of a single lymph node region (the same side of the diaphragm, i.e., the thymus or liver/spleen). Stage 2 corresponds to the involvement of two lymph node regions (both sides of the diaphragm, i.e., the thymus and liver/spleen). Stage 3 corresponds to the involvement of two lymph node regions and dissemination to other areas (i.e., the intestine or kidney). A schematic representation of staging is shown in Supplementary Fig. S2c. Macroanalysis made it possible to classify tumours into four classes of organomegaly according to their size: low organomegaly was noted if the spleen and thymus were 20 and 6 mm, respectively (indicated as + in Fig. 3); medium organomegaly was noted if the sizes were 25 and 8 mm (indicated as + + in Fig. 3); high organomegaly was noted if the sizes were 35 and 10 mm (indicated as + + + in Fig. 3); and very high organomegaly was noted if the sizes were 40 and 15 mm (indicated as + + + + in Fig. 3).
Fig. 3.
Hsp110 depletion does not suppress or change the tumour phenotype in the MSI mouse model (II). a Left panel: Representative pictures of organomegaly in the two localizations (spleen and thymus) and the associated index depending on the size of the organ at the macroanalysis after sacrifice. Right panel: Analysis of the organomegaly index of the three groups of mice. No significant differences were observed among the three mouse genotypes in terms of the development of splenomegaly (Chi2 P = 0.6) or thymomegaly (Chi2 P = 0.7). b Lymphoma staging analysis of the three study cohorts (for more details, see the Materials and Methods section and Supplementary Fig. S2c). c Adenocarcinoma formation rate in the three groups of this study. No significant differences were observed among the three mouse genotype groups in terms of adenocarcinoma development (Chi2 P = 0.8). d Kaplan–Meier survival analysis of the Msh2KOHsp110wt (red line), Msh2KOHsp110DE9KI/+ (blue line) and Msh2KOHsp110DE9KI/KI (green line) mouse groups. The log-rank test was used for statistical analysis. An unpaired t test was performed to determine significance
Experimental design and evaluation of the effects of Hsp110DE9 mutation on tumorigenesis and the response to 5-FU treatment
We developed an experimental protocol to evaluate the effect of Hsp110DE9 mutation on tumorigenesis and the 5-FU response. This experimental protocol is represented in Supplementary Fig. S3. Briefly, in the genetic context of the mouse model with dMMR and lymphoma, we defined a delay in the appearance of signs of suffering and reaching the endpoint as an effect of the Hsp110DE9 mutation; we compared the endpoints of untreated mice versus 5-FU-treated mice in the Hsp110wt group and the Hsp110 mutant group. This delay was calculated as the median survival (quartiles determined by Kaplan–Meier survival analysis) of the 5-FU group minus that of the PBS group.
5-FU experimental design
After grouping mice into homogenous groups based on their genotype, sex and age, mice were treated with 40 or 60 mg/kg 5-FU (FLUOROURACILE ACCORD, France: stock solution at 50 mg/ml) daily for 2 days every 2 weeks via intraperitoneal (IP) injection from approximately 80 days old until the endpoint (Supplementary Fig. S3). Twelve MSH2KO mice were sacrificed before 80 days of life and examined for the tumoral syndrome. None of them presented with the tumoral syndrome. Therefore, 5-FU was administered very early before the appearance of clinically detectable tumours in all animals (the liver, spleen, thymus and lymph nodes were examined). The doses applied were in line with the doses given to mice as high doses in other studies [16]. The human equivalent doses (HEDs) were calculated using guide conversion as described [17]: mouse 40 mg/kg and 60 mg/kg 5-FU doses were calculated to be equivalent to human doses of 118 mg/m2 and 180 mg/m2, respectively.
5-FU toxicity evaluation
To evaluate the toxicity of 5-FU at two doses (40 mg/kg and 60 mg/kg) in the wild-type and Hsp110DE9KI/KI mice, mice were observed every day to detect any pain. Survival analysis was performed by the Kaplan–Meier method. Weight loss was used as an objective measurement to reflect toxicity [18]. Every day for the two days of treatment every two weeks, the body weight change (BWC) was calculated using the following formula as described previously [19]: BWC (%) = [(bodyweight on the second day) − (bodyweight on the first day)]/(bodyweight on the first day) × 100 (%). The average BWC was calculated by adding all the BWC values for each mouse and dividing by the number of treatments for each mouse.
Evaluation of 5-FU impact according to tumour mass loss
In agreement with the observation that bodyweight stabilizes or increases with tumour size, particularly for lymphoma pathology [20], to evaluate the efficacy of 5-FU treatment according to tumour mass loss in mice after treatment, we calculated the % BWC as described above. The analysis excluded the four weeks prior to sacrifice to disregard weight loss due to suffering.
Flow cytometry
Flow cytometry was performed on cell suspensions from tumoral and nontumoral spleen and thymus samples. Cells were washed once in PBS supplemented with 1% BSA (Merck, France) and resuspended in PBS/1% BSA at a concentration of 106 cells/100 μl. Cells were stained with IgG-Alexa647 or FITC (Biolegend, France) and IgG-PE (BD Biosciences, France) to detect nonspecific binding of antibodies and autofluorescence. The primary antibodies used were CD3-Alexa 647 (1:200; clone 17A2, Biolegend, France), CD19-PE (1:200, clone 1D3, BD Biosciences) and CD45-FITC (1:200, clone 30-F11, Biolegend, France). Cells were incubated for 30 min at 4 °C in the dark and then washed in buffer (PBS, 1% BSA, and 0.5 mM EDTA). DAPI was added before analysis at a final concentration of 1 µg/ml. The expression of cell surface markers was detected with a Gallios flow cytometer (Beckman Coulter, France), and data were analysed using Kaluza software [20].
Immunohistopathology
For histological analysis, 4-µm sections of paraffin-embedded tissue samples were cut and placed onto silane-treated Super Frost slides (CML, Nemours, France) and left to dry at 37 °C overnight. The tumour sections were deparaffinized in xylene and rehydrated in pure ethanol. Before immunostaining, antigen retrieval was performed by immersing the sections in citrate buffer at pH 6.0 (Ki67) and pH 9.0 (caspase-3) (15 min at 95 °C); the sections were washed twice in PBS for 3 min and treated with 3% H2O2-PBS for 15 min to inhibit endogenous peroxidases. After washing in PBS, the slides were saturated for 25 min in 3% BSA PBS. The sections were then incubated for 1 h at room temperature with antibodies against Ki67 (dilution 1:50; clone NCL-Ki67-MM1, Leica Biosystems, France) and caspase-3 (dilution 1:150; clone ab52293, Abcam, Cambridge, UK). After washing in PBS, a secondary antibody (8114P, Cell Signaling Technology, The Netherlands) was added and incubated for 30 min at room temperature. The slides were washed twice for 5 min in PBS and assessed using a NovarRED kit (Vector, Burlingame, USA). The slides were washed twice in water for 5 min and counterstained with 10% Mayer's haematoxylin. After one wash in water, the slides were dehydrated in 100% ethanol and in xylene for 30 s each. Apoptosis and proliferation were quantified by counting the number of cells labelled with anti-caspase-3 or anti-Ki67 antibody per 2000 tumour cells in the most affected areas as described previously [21].
Masson’s trichrome staining
For direct visualization of collagen fibers and histological assessment of collagen deposition, trichrome staining was performed using the Masson Trichrome Staining Kit (Sigma-Aldrich, St Louis, MO, USA).
H&E Staining
Paraffin-embedded tissues Sects. (4 μm) were stained with hematoxylin (Sigma-Aldrich, France) for 40 s and with eosin (Sigma-Aldrich, France) for 30 s. The tissue sections were examined under a light microscope after mounting with Permount mounting medium (Thermo Fisher, France).
CRC cell lines
CRC cell lines were purchased from the American Type Culture Collection. All of the cells were cultured in DMEM GlutaMAX (Thermo Fisher, France) containing 10% foetal bovine serum and 1% antibiotics (penicillin/streptomycin; Thermo Fisher, France). The cell lines were free of mycoplasma.
Immunoblot analysis
Cells were washed in PBS and lysed on ice in cold lysis buffer (RIPA buffer, Thermo Scientific, France) containing protease and phosphatase inhibitors (Halt Protease and Phosphatase Inhibitor Cocktail, Pierce). Tissues were washed in PBS and homogenized mechanically. For 5 mm × 5 mm tissues, 200 µl of cold lysis buffer (T-Per: Tissue Protein Extraction Reagent, Thermo Scientific, France) was added in the presence of protease and phosphatase inhibitors (Halt Protease and Phosphatase Inhibitor Cocktail, Pierce). Proteins were separated by PAGE and transferred following standard protocols before analysis with an Odyssey Infrared Imaging System. Primary antibodies against the following molecules were used: Hsp110 (ab24503 dilution 1:100; Abcam, Cambridge, UK) and actin (926–42,210 dilution 1:5000; Licor, The Netherlands). IRDye680 and IRDye800 secondary antibodies were used (926–68,021 and 926–32,211, respectively, dilution 1:15,000, Licor). Protein quantification was performed using ImageStudio software.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Mouse survival rates were calculated using the Kaplan–Meier method, and statistical significance was evaluated using the log-rank (Mantel–Cox) test with XLstat software. Statistical comparisons were made using unpaired t tests with Prism v8.0 software (GraphPad, San Diego, CA, USA); differences with p values ≤ 0.05 were considered statistically significant.
Results
Expression of Hsp110wt and Hsp110DE9 mutant proteins in mice with Hsp110KI germline mutations
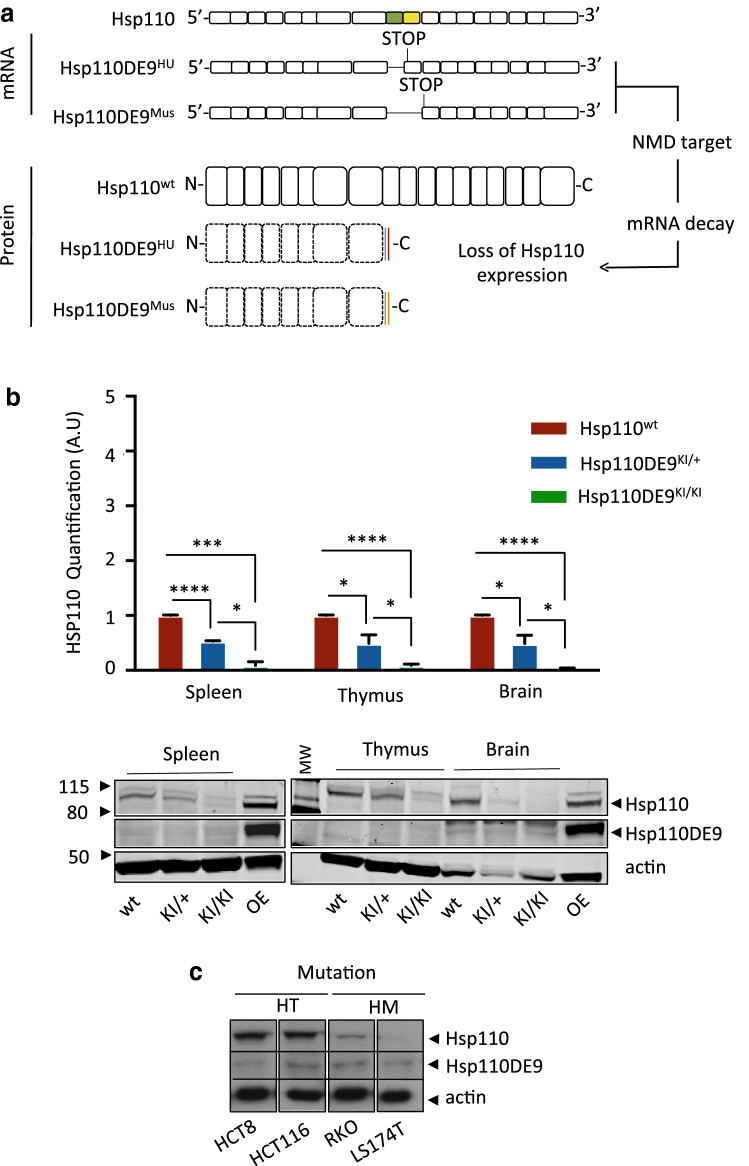
We generated KI mice with the Hsp110DE9 mutation to recreate the effect of homologous Hsp110DE9 mutation in human MSI CRC (see Fig. 1a and Supplementary Fig. S1 for further details concerning genetic model construction). No abnormal health phenotypes were observed in either Hsp110DE9KI/+ or Hsp110DE9KI/KI mice compared to Hsp110wt animals (mice were observed over a 2-year period, 15 mice per group, Supplementary Fig. S2a).
Fig. 1.
Hsp110DE9 KI model induces the loss of wild-type HSP110 expression in mouse tissues. a Schematic drawing of Hsp110 mRNA and the consequence of the Hsp110DE9 mutation (exon 9 skipping and stop codon formation in humans (HU) (Hsp110DE9HU)). In the Hsp110DE9 KI mouse allele, the endogenous HSP110 locus was modified to produce the same effect as the human Hsp110DE9 mutation and the putative Hsp110DE9 mutant protein. The mutated pre-mRNA is a target of the NMD system, as we described previously [11], with consecutive loss of the expression of the putative mutant Hsp110DE9 protein. b Upper panel: Hsp110 protein quantification analysis in non-tumoral tissues (spleen, thymus and brain) from wild-type, Hsp110DE9KI/+ and Hsp110DE9KI/KI mice. Bottom panel: The western blot shown is representative of 3 independent experiments. c Western blot analysis of 4 MSI CRC cell lines: HCT8 and HCT116 cells heterozygous for the Hsp110DE9 mutation; RKO and LS174T cells homozygous for the Hsp110DE9 mutation. Actin was used as a loading control. An unpaired t test was performed to assess significance. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
As expected, protein analysis of whole-cell extracts from normal tissues of animals (spleen, thymus and brain) revealed the complete or partial loss of Hsp110wt protein expression in Hsp110DE9KI/KI and/or Hsp110DE9KI/+ mice, respectively, compared to Hsp110wt mice (Fig. 1b). No increase in Hsp110DE9 mutant protein expression was observed in either Hsp110DE9KI/KI or Hsp110DE9KI/+ strains, probably due to the decay of Hsp110DE9 mutant mRNA through NMD, as previously reported [10, 11]. We concluded that the generated KI mouse model reproduced the functional effect of the human HSP110 microsatellite T17 somatic mutation, which we observed either heterozygously or homozygously in MSI cancer cell lines (Fig. 1c) and primary tumours [10].
Impact of the Hsp110DE9 mutation on the tumour phenotype and survival of Msh2KO mice
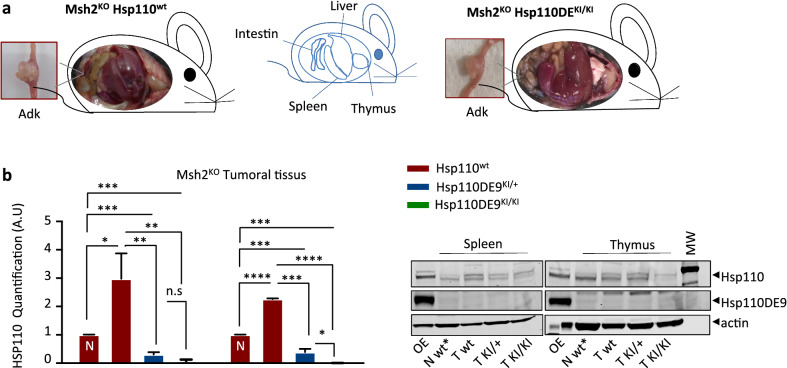
We investigated whether the Hsp110DE9 mutation reduces MSI-driven tumorigenesis in Msh2KO mice. As expected, 100% of Msh2KO mice developed MSI T-cell lymphoma with consequent organomegaly of primary (thymus) and secondary (spleen) lymphoid organs and/or intestinal adenocarcinoma (10–30% of mice) (Fig. 2a) as described previously [15]. Protein extracts from tumour tissues (spleen and thymus) infiltrated by lymphoma cells from Msh2KOHsp110wt mice showed overexpression of the Hsp110 protein compared to those from normal tissues (Fig. 2b), in line with the previously reported oncogenic role of Hsp110 during lymphoma development [4, 22].
Fig. 2.
Hsp110 depletion does not suppress or change the tumour phenotype in the MSI mouse model (I). a The pictures show representative images of the two mouse genotypes evaluated in this study (i.e., Msh2KOHsp110wt, left panel, and Msh2KOHsp110DE9KI/KI, right panel). Each mouse exhibited signs suggestive of organomegaly in the three organs (spleen, thymus and liver), consistent with lymphoma development. The boxed images represent an enlargement of the intestinal tract with representative adenocarcinoma. b Left panel: Hsp110 protein quantification analysis of T cells infiltrating spleen and thymus tissues from Msh2KOHsp110wt, Msh2KOHsp110DE9KI/+ and Msh2KOHsp110DE9KI/KI mice; (N) indicates extracts from nontumoral tissues. Right panel: Representative western blot membranes from 3 independent experiments. Actin was used as a loading control. An unpaired t test was performed to determine significance. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
We evaluated lymphoma development in mice by assessing organomegaly in the thymus and spleen (Fig. 3a), lymphoma stage (Fig. 3b), adenocarcinoma development (Fig. 3c) and survival (Fig. 3d). We did not observe any impact of Hsp110DE9 mutation on lymphoma development or the frequency of adenocarcinoma development in MSH2KO mice. Overall, no impact of this mutation on the survival of Msh2KOHsp110DE9KI/+ or Msh2KOHsp110DE9KI/KI mice compared to Msh2KOHsp110wt mice was observed (P = 0.862, log-rank test, Fig. 3d and Supplementary Fig. S4). The partial or total loss of the Hsp110wt protein in Msh2KOHsp110DE9KI/+ and Msh2KOHsp110DE9KI/KI mice did not reduce tumour syndrome characteristics or extend the survival of the mice to the endpoint (for more details, see the experimental protocol design and working hypothesis in Supplementary Fig. S3), which led us to believe that the Hsp110DE9 mutation has no relevant impact on the development of MSI tumours.
Impact of the Hsp110DE9 mutation on the response to 5-fluorouracil chemotherapy in Msh2-deficient mice
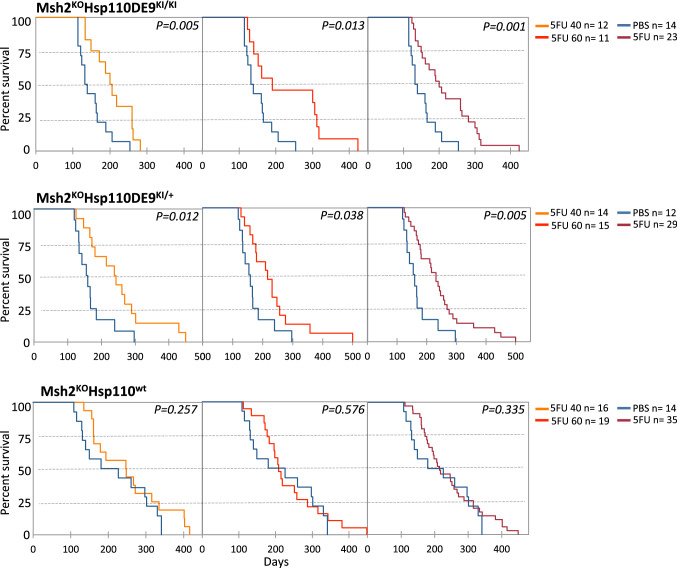
We next examined whether the Hsp110DE9 mutation affects the responsiveness of Msh2KO mice to 5-FU. Tolerance to 5-FU was previously examined with survival analysis (5-FU 60 mg/kg; Supplementary Fig. S5a) and BWC (5-FU 40 and 60 mg/kg, see Supplementary Fig. S5b and the Materials and Methods section for more details). As expected, the survival of Msh2KOHsp110wt (n = 35) mice did not increase following treatment with 5-FU, confirming the resistance of MMR-deficient tumours from these animals to this anticancer agent as previously described [23, 24] (Fig. 4 bottom panel). In contrast, the survival of both Msh2KOHsp110DE9KI/KI (n = 23) and Msh2KOHsp110DE9KI/+ (n = 29) mice (compared to Msh2KOHSsp110wt control mice) drastically increased following treatment with 5-FU (Kaplan–Meier analysis; 5-FU 40 mg/kg: log-rank P = 0.005 and P = 0.012, respectively; 5-FU 60 mg/kg: log-rank P = 0.013 and P = 0.038, respectively; Fig. 4 upper and middle panels). The effects of the two concentrations of 5-FU were not significantly different in terms of survival (Supplementary Fig. S6). Similarly, the BWC of Msh2KOHsp110DE9KI/+ and Msh2KOHsh110DE9KI/KI mice was increased by 5-FU compared to that of Msh2KOHsp110wt mice, probably because there was a reduction in tumour mass only in mice carrying the Hsp110DE9 mutation; the BWC was more pronounced in males than females (Supplementary Fig. S7).
Fig. 4.
Hsp110 depletion improved the response to 5-FU chemotherapy in the MSI mouse model. Kaplan–Meier survival analysis of Msh2KOHsp110DE9KI/KI (upper panel), Msh2KOHsp110DE9KI/+ (middle panel), Msh2KOHsp110wt (bottom panel) mice treated with 5-FU (5-FU 40 mg/kg orange line; 5-FU 60 mg/kg red line; 5-FU all doses burgundy line) and/or placebo (PBS; blue line). The log-rank test was used for statistical analysis
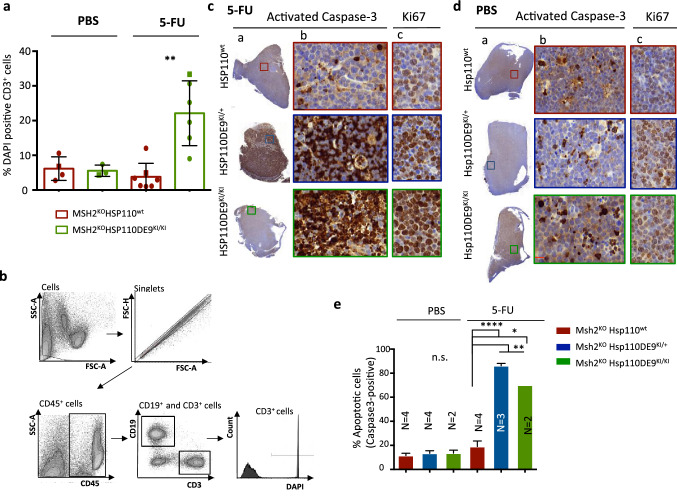
Loss of Hsp110 protein sensitizes tumour cells to death, inducing apoptosis after 5-FU treatment
We next examined the effect of Hsp110 on tumour cell death and apoptosis associated with the response to 5-FU treatment in mice. Compared to lymphoma cells from Msh2KOHsp110wt mice under the same conditions, Msh2KOHsp110DE9KI/KI lymphoma cells not only showed increased cell death, as measured by DAPI staining and cytometry analysis (t test P = 0.002; Fig. 5a and b) but also showed an increased apoptotic rate, as measured by caspase-3 staining, following exposure to 5-FU without any morphological change (P = 0.002; Fig. 5c–e, Supplementary Figs. S8 and S9). In contrast, Msh2KOHsh110DE9KI/KI and Msh2KOHsp110wt lymphoma cells displayed similar proliferation rates (Supplementary Fig. S10) according to our results described in Fig. 3, indicating that the Hsp110DE9 mutation does not affect cell proliferation or disease progression.
Fig. 5.
Hsp110 protein depletion increases the susceptibility of MSI T-lymphoma cells to apoptosis after 5-FU treatment. a Quantification of cell viability based on a comparison of the percentage of DAPI-stained T-lymphoma cells (CD3+) in tumoral organs (spleen and thymus) determined by cytometry analysis with the organomegaly status of Msh2KOHsh110wt and Msh2KOHsp110DE9KI/KI mice treated with 5-FU or with placebo. The square points correspond to representative flow cytometry data shown in Supplementary Fig. S8 b Representative FACS dot plots showing the gating of cells used to detect T-lymphoma cells positive for DAPI staining. An unpaired t test was performed to determine significance. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (c and d) Representative activated caspase-3 immunostaining of the entire field of view (a) or a magnified region (b) corresponding to the coloured boxed area after 5-FU (c) and/or placebo (PBS) (d) treatment for the three groups (Msh2KOHsp110wt, Msh2KOHsp110DE9KI/+ and Msh2KO Hsp110DE9KI/KI). The image in (c) is an enlargement of the coloured boxes for Ki67 immunostaining. e Apoptotic index calculated as the percentage of activated caspase-3-positive cells in at least 2000 cells of the area of the slide with the most marked staining
Discussion
Cancer cells undergo extensive rewiring of their metabolic and signal transduction pathways, thereby becoming dependent on proteins such as HSPs that are dispensable for the survival of normal cells outside stressful conditions. HSP110DE9 is a unique example of an HSP loss-of-function mutation in human cancer. The HSP110DE9 mutation is a surprising event since it is deleterious for tumour cells and has a proapoptotic role and a chemosensitizing effect [2, 10, 25]. In this study, we wanted to investigate the impact of the loss of Hsp110 protein expression on tumour development and progression as well as on the response to 5-FU-based chemotherapy. Our results first showed that even though the Hsp110 protein was overexpressed in MSI lymphoma cells from Msh2KO mice, impairment of Hsp110 function due to the Hsp110DE9 mutation in these tumour cells had no significant deleterious impact on tumour initiation or progression. These results are consistent with the high frequency of this mutation in MSI CRC, which occurs somatically in approximately one-third of MSI CRC tumours and to a lesser extent in gastric, endometrial and other MSI carcinomas at all tumour stages (data not shown) without being associated with disease prognosis in patients who are not treated with chemotherapy, as published previously [10]. Our unpublished data show that there is no association between this mutation and tumour stage in human CRC (data not shown). These results interestingly suggest that MSI tumour cells can adapt to Hsp110 inactivation via mechanisms that will be interesting to highlight in future studies.
Of more immediate clinical interest, our data reinforce the idea that the addiction of tumour cells to Hsp110 is observed only in the context of chemotherapy, making the HSP110DE9 mutation an “Achilles heel” in MSI cancer under specific treatment conditions, as suggested previously [2, 10]. The concept of oncogene addiction has been introduced to highlight the apparent dependence of certain tumours on one or a few genes for the maintenance of malignant phenotype, growth and/or resistance to chemotherapy. In this context, our results confirm that there is a conditional oncogenic addiction of MSI tumour cells to HSP110 (Fig. 3), i.e., in the context of chemotherapy treatment, in which case, the mutation results in induction of sensitization to 5-FU alone (Fig. 4). In this study, our data establish that the chemosensitivity and clinical survival of Msh2KOHsp110KI/+ and Msh2KOHsp110KI/KI mice treated with 5-FU were similar, while those in Msh2KOHsp110wt mice were different. It seems therefore that Hsp110 haploinsufficiency is sufficient to chemosensitize MMR-deficient lymphoma cells to 5-FU. This is probably also the case in human tumours, although this hypothesis could not be confirmed previously because it is difficult to determine the exact number of mutated copies of HSP110 per tumour due to intratumoral heterogeneity. It should be noted that patients with MMR-deficient tumours usually do not appear to derive any benefit from 5-FU adjuvant or neoadjuvant chemotherapy. There is evidence to suggest that this drug may even be detrimental to patients with MSI CRC and gastric cancer, for instance [26–28]. This is because the MMR protein recognizes 5-FU incorporation in DNA, and this is a critical step in activating signalling pathways related to cell cycle arrest and cell death [29]. In a previous study, we demonstrated that the HSP110DE9 mutation was associated with an excellent response to chemotherapy in MSI cancer patients treated with 5-FU-based chemotherapy [10]. This mutation is therefore definitively a functionally important molecular event unique to MSI tumours with the potential to serve as a biomarker for chemotherapy response in this class of tumours. Since 2004, different studies have revealed that adding oxaliplatin to a regimen of 5-FU combined with leucovorin (FOLFOX) produces a significant improvement in overall survival for patients with MSI and microsatellite stable (MSS) colon cancers [30, 31]. Despite its efficacy, FOLFOX has a `significantly higher cost and toxicity and is less inconvenient. In particular, oxaliplatin-induced peripheral neuropathy occurs in 92.1% of patients receiving treatments, 12% of MOSAIC trial patients showed grade 3 neurotoxicity 1 year after completion of chemotherapy, and approximately 50% of patients suffered from grade 1 or 2 neurotoxicity in the second posttreatment year [30, 31]. This work suggests that HSP110 could be used as a biomarker as part of a precision medicine approach to contribute to enhanced therapeutic efficacy and minimize treatment-related toxicity in patients with MSI cancer. We think our findings are important for successfully employing HSP110 status as a marker to select MSI cancer patients to be treated with 5-FU monotherapy with the objective of avoiding the toxicity of other drugs whenever possible. This is even more important since MSI has emerged as a tissue-agnostic, major predictive biomarker for the efficacy of immune checkpoint inhibitors [30, 31]. Strategies with immunotherapy alone or combined with 5-FU-based chemotherapy are under investigation, and HSP10 status might provide valuable predictive information in this context [32].
The limitation of this study is that we investigated the effects of the Hsp110DE9 mutation on the 5-FU response mainly in a lymphoma model. Adenocarcinoma is the most common type of MSI cancer, but MSI lymphoma is at least as frequent as MSI gastrointestinal tumours in patients with constitutional mismatch repair deficiency (CMMRD) [33]. To decipher the role of the Hsp110DE9 mutation in intestinal MSI cancer, a conditional and/or inducible mouse model of intestine-specific MSH2 somatic mutation may be used.
In conclusion, in this study, we confirmed that the Hsp110DE9 mutation and the consequent loss of Hsp110 expression sensitize tumour cells to 5-FU-based chemotherapy treatment. Together with our previous publications, this work further highlights that the HSP110DE9 mutation can be used as a biomarker and predictive factor for 5-FU treatment. Hopefully, HSP110DE9 mutation information could become a significant tool in predicting a positive response to adjuvant 5-FU chemotherapy in MSI cancer to allow more personalized treatment for patients with this drug, especially when the toxicity of other chemotherapeutic agents, such as oxaliplatin, must be avoided.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The present work has benefited from the animal facility of Saint-Antoine Research Center (PHEA, Mrs Tatiana Ledent) and the core facility of UMS30-LUMIC, CISA (Cytometry and Imagery Saint Antoine, Mrs Annie Munier), Sorbonne University, UPMC Univ Paris 06, INSERM, Saint-Antoine (CRSA), F-75012 Paris, France.
Abbreviations
- CRC
Colorectal cancer
- MMR
Mismatch repair
- dMMR
MMR deficiency
- MSI
Microsatellite instability
- 5-FU
5-Fluorouracil
Author contributions
KN, AB, RB, FR, PB, MS, and ACJ (acquisition of data; analysis and interpretation of data; approval of the final version of the manuscript); AD and AC (conception of the study; analysis and interpretation of data; drafting of the manuscript; revision of the manuscript; approval of the final version of the manuscript).
Funding
This work was supported by the Institut National de la santé et la recherche Medicale (INSERM) and by a grant from the Ligue Nationale Contre le Cancer (LNCC) « Labeled teams».
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Ethics approval
All animal experiments were conducted in accordance with the ethical regulations of the French Ministry of Research and Technology (Record number #9175–2017030810379961 v3; Charles Darwin Ethics Committee).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Svrcek M, Lascols O, Cohen R, Collura A, Jonchere V, Flejou JF, Buhard O, Duval A. MSI/MMR-deficient tumor diagnosis: Which standard for screening and for diagnosis? Diagnostic modalities for the colon and other sites: Differences between tumors. Bull Cancer. 2019;106:119–128. doi: 10.1016/j.bulcan.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Dorard C, de Thonel A, Collura A, et al. Expression of a mutant HSP110 sensitizes colorectal cancer cells to chemotherapy and improves disease prognosis. Nat Med. 2011;17:1283–1289. doi: 10.1038/nm.2457. [DOI] [PubMed] [Google Scholar]
- 3.Boudesco C, Verhoeyen E, Martin L, et al. HSP110 sustains chronic NF-κB signaling in activated B-cell diffuse large B-cell lymphoma through MyD88 stabilization. Blood J Am Soc Hematol. 2018;132(5):510–520. doi: 10.1182/blood-2017-12-819706. [DOI] [PubMed] [Google Scholar]
- 4.Zappasodi R, Bongarzone I, Ghedini GC, et al. Serological identification of HSP105 as a novel non-Hodgkin lymphoma therapeutic target. Blood. 2011;118:4421–4430. doi: 10.1182/blood-2011-06-364570. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee S, Burns TF. Targeting heat shock proteins in cancer: a promising therapeutic approach. Int J Mol Sci. 2017;18:1978. doi: 10.3390/ijms18091978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kai M, Nakatsura T, Egami H, Senju S, Nishimura Y, Ogawa M. Heat shock protein 105 is overexpressed in a variety of human tumors. Oncol Rep. 2003;10:1777–1782. [PubMed] [Google Scholar]
- 7.Park HS, Park CH, Choi BR, Lim MS, Heo SH, Kim CH, Kang SG, Whang KU, Cho MK. Expression of heat shock protein 105 and 70 in malignant melanoma and benign melanocytic nevi. J Cutan Pathol. 2009;36:511–516. doi: 10.1111/j.1600-0560.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- 8.Slaby O, Sobkova K, Svoboda M, et al. Significant overexpression of Hsp110 gene during colorectal cancer progression. Oncol Rep. 2009;21:1235–1241. doi: 10.3892/or_00000346. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kim KJ, Rhee YY, Oh S, Cho NY, Lee HS, Kang GH. Expression status of wild-type HSP110 correlates with HSP110 T17 deletion size and patient prognosis in microsatellite-unstable colorectal cancer. Mod Pathol. 2014;27:443–453. doi: 10.1038/modpathol.2013.160. [DOI] [PubMed] [Google Scholar]
- 10.Collura A, Lagrange A, Svrcek M, et al. Patients with colorectal tumors with microsatellite instability and large deletions in HSP110 T17 have improved response to 5-fluorouracil-based chemotherapy. Gastroenterology. 2014;146(401–411):e401. doi: 10.1053/j.gastro.2013.10.054. [DOI] [PubMed] [Google Scholar]
- 11.Bokhari A, Jonchere V, Lagrange A, et al. Targeting nonsense-mediated mRNA decay in colorectal cancers with microsatellite instability. Oncogenesis. 2018;7:70. doi: 10.1038/s41389-018-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berthenet K, Bokhari A, Lagrange A, et al. HSP110 promotes colorectal cancer growth through STAT3 activation. Oncogene. 2016;36:2328–2336. doi: 10.1038/onc.2016.403. [DOI] [PubMed] [Google Scholar]
- 13.de Wind N, Dekker M, Berns A, Radman M, te Riele H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell. 1995;82:321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]
- 14.Reitmair AH, Schmits R, Ewel A, et al. MSH2 deficient mice are viable and susceptible to lymphoid tumours. Nat Genet. 1995;11:64–70. doi: 10.1038/ng0995-64. [DOI] [PubMed] [Google Scholar]
- 15.Reitmair AH, Redston M, Cai JC, Chuang TC, Bjerknes M, Cheng H, Hay K, Gallinger S, Bapat B, Mak TW. Spontaneous intestinal carcinomas and skin neoplasms in Msh2-deficient mice. Cancer Res. 1996;56:3842–3849. [PubMed] [Google Scholar]
- 16.Orecchioni S, Talarico G, Labanca V, Calleri A, Mancuso P, Bertolini F. Vinorelbine, cyclophosphamide and 5-FU effects on the circulating and intratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br J Cancer. 2018;118:1329–1336. doi: 10.1038/s41416-018-0076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman K, Sewell F, Allais L, et al. A global pharmaceutical company initiative: an evidence-based approach to define the upper limit of body weight loss in short term toxicity studies. Regul Toxicol Pharmacol. 2013;67:27–38. doi: 10.1016/j.yrtph.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Bascuas T, Moreno M, Monaco A, Reyes L, Paolino A, Oliver P, Kramer MG, Engler H, Pacheco JP, Grille S, Chabalgoity JA. A novel non-Hodgkin lymphoma murine model closer to the standard clinical scenario. J Transl Med. 2016;14:323. doi: 10.1186/s12967-016-1073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paster EV, Villines KA, Hickman DL. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med. 2009;59:234–241. [PMC free article] [PubMed] [Google Scholar]
- 21.Bressenot A, Zimmer O, Fau-Marchal S, Marchal S, Fau-Gauchotte G, Gauchotte G, Fau-Montagne K, Montagne K, Fau-Plénat F, Plénat F. Detection of apoptosis in vivo: comparison of different methods in histological sections of subcutaneous xenografts of HT29 human colon adenocarcinoma. Ann Pathol. 2009;29:370–375. doi: 10.1016/j.annpat.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Boudesco C, Verhoeyen E, Martin L, et al. HSP110 sustains chronic NF-κB signaling in activated B-cell diffuse large B-cell lymphoma through MyD88 stabilization. Blood. 2018;132:510–520. doi: 10.1182/blood-2017-12-819706. [DOI] [PubMed] [Google Scholar]
- 23.Tajima A, Hess MT, Cabrera BL, Kolodner RD, Carethers JM. The mismatch repair complex hMutS alpha recognizes 5-fluorouracil-modified DNA: implications for chemosensitivity and resistance. Gastroenterology. 2004;127:1678–1684. doi: 10.1053/j.gastro.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Carethers JM, Smith EJ, Behling CA, Nguyen L, Tajima A, Doctolero RT, Cabrera BL, Goel A, Arnold CA, Miyai K, Boland CR. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology. 2004;126:394–401. doi: 10.1053/j.gastro.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Duval A, Collura A, Berthenet K, Lagrange A, Garrido C. Microsatellite instability in colorectal cancer: time to stop hiding! Oncotarget. 2011;2:826–827. doi: 10.18632/oncotarget.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietrantonio F, Miceli R, Raimondi A, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37:3392–3400. doi: 10.1200/jco.19.01124. [DOI] [PubMed] [Google Scholar]
- 28.Morton D. 523O FOxTROT: an international randomised controlled trial in 1053 patients evaluating neoadjuvant chemotherapy (NAC) for colon cancer. On behalf of the FOxTROT collaborative group. Ann Oncol. 2019;30:198. doi: 10.1093/annonc/mdz246.001. [DOI] [Google Scholar]
- 29.Jo WS, Carethers JM. Chemotherapeutic implications in microsatellite unstable colorectal cancer. Cancer Biomark. 2006;2:51–60. doi: 10.3233/cbm-2006-21-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 31.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 32.Pietrantonio F, Miceli R, Raimondi A, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37:3392–3400. doi: 10.1200/JCO.19.01124. [DOI] [PubMed] [Google Scholar]
- 33.Bodo S, Colas C, Buhard O, et al. Diagnosis of constitutional mismatch repair-deficiency syndrome based on microsatellite instability and lymphocyte tolerance to methylating agents. Gastroenterology. 2015;149(1017–1029):e1013. doi: 10.1053/j.gastro.2015.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Not applicable.