Abstract
Proliferative diabetic retinopathy (PDR), proliferative vitreoretinopathy (PVR), and neovascular age-related macular degeneration (nAMD) are among the leading causes of blindness. Due to the multifactorial nature of these vitreoretinal diseases, omics approaches are essential for a deeper understanding of the pathophysiologic processes underlying the evolution to a proliferative or neovascular etiology, in which patients suffer from an abrupt loss of vision. For many years, it was thought that the function of the vitreous was merely structural, supporting and protecting the surrounding ocular tissues. Proteomics studies proved that vitreous is more complex and biologically active than initially thought, and its changes reflect the physiological and pathological state of the eye. The vitreous is the scenario of a complex interplay between inflammation, fibrosis, oxidative stress, neurodegeneration, and extracellular matrix remodeling. Vitreous proteome not only reflects the pathological events that occur in the retina, but the changes in the vitreous itself play a central role in the onset and progression of vitreoretinal diseases. Therefore, this review offers an overview of the studies on the vitreous proteome that could help to elucidate some of the pathological mechanisms underlying proliferative and/or neovascular vitreoretinal diseases and to find new potential pharmaceutical targets.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04670-y.
Keywords: Age-related macular degeneration, Proliferative diabetic retinopathy, Proliferative vitreoretinopathy, Vitreous proteomics
Background: translational research in proliferative vitreoretinal diseases
Visual impairment and blindness severely affect the quality of life of the patients and are a significant burden for healthcare systems [1, 2]. Despite improvements achieved in the prevention and control of ocular diseases, the number of people with moderate-to-severe visual impairment is expected to increase due to the growth and aging of the world population [3, 4]. Diabetic retinopathy (DR) and age-related macular degeneration (AMD) are leading causes of visual impairment and blindness in middle-income and industrialized countries [5, 6]. Proliferative vitreoretinopathy (PVR) represents the most common cause of failure in retinal detachment (RD) surgery, accounting for 3.9–13.7% of all rhegmatogenous retinal detachment (RRD) cases [7, 8]. Despite the multiple treatments available, there is still progression to visual impairment and blindness in proliferative etiology [9], which reinforces the need for designing better strategies for the management of these diseases [10]. Considering their multifactorial nature, omics approaches provide a deeper understanding of the pathophysiological processes underlying these diseases and their evolution to a proliferative or neovascular etiology, in which patients experience significant vision loss. Ocular proteomics has emerged as a tool for discovering new biomarkers, which could help to unveil the pathophysiologic mechanisms underlying many ocular diseases, anticipate their progression, and predict the response to therapy [9, 11, 12].
Vitreous humor proteomics
Vitreous, also termed vitreous body or vitreous humor, is a translucent gel-like substance that fills the posterior cavity of the eye, surrounded by the retina, pars plana, and lens [13–15]. It is an avascular and virtually acellular connective tissue, composed mainly of water and a network of collagen fibrils surrounded by glycosaminoglycans, electrolytes, and soluble proteins [14, 16]. For many years, it was thought that vitreous function was merely structural, allowing to support and protect the surrounding ocular tissues from physical impact [14, 17, 18]. Nowadays, it is known that the vitreous has many other physiological functions. In addition to allowing the passage of light toward the retina, the vitreous contributes to eye growth and shape, serves as a barrier to biomolecules and cells, allows the repository and diffusion of the substances involved in the eye metabolism, and regulates oxygen levels within the eye [14, 18, 19]. In the last years, there has been a growing interest in the role of the vitreous proteome in different pathophysiological contexts, which is evidenced by the increase in the number of identified proteins from 545 to over 6538 in only 5 years [9, 20]. Vitreous proved to be extremely attractive from biological and analytical perspectives. The proteome and biochemical properties of the vitreous reflect the physiological and pathological conditions of the eye due to its close contact with the inner retina, lens, and ciliary body [11, 21]. Furthermore, aging-related changes in the vitreous, such as vitreous liquefaction and posterior vitreous detachment (PVD), are presumed to be underlying several retinal diseases [17, 19, 22]. Therefore, the outcome of vitreous proteome studies could help to reveal some of the pathological mechanisms underlying proliferative retinal diseases. This review aims to provide an overview of the reported changes in vitreous proteome in patients with PDR, neovascular AMD (nAMD), and PVR, and how these could be correlated with pathological events, such as inflammation, fibrosis, oxidative stress, neurodegeneration, and vitreous remodeling.
Characterization of the vitreous proteome in vitreoretinal diseases
Diabetic retinopathy
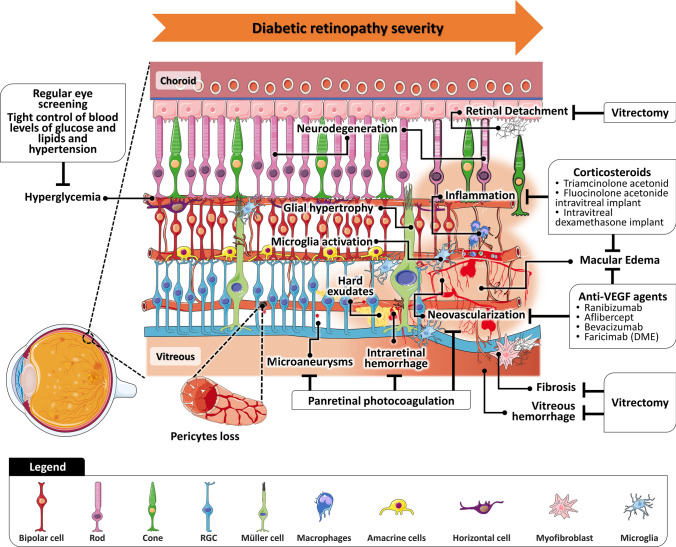
According to World Health Organization (WHO), about 146 million (34.6%) of 422 million adults with Diabetes Mellitus suffer from some form of DR [23], accounting for 1.1% of blindness among middle-aged adults [24]. The public health burden associated with DR will increase as a result of the substantial expansion of diabetes in low- and middle-income countries [25, 26]. The duration and type of diabetes, poor glycemic control, hypertension, and high levels of glycated hemoglobin A1c and triglycerides are the main risk factors for DR [27, 28]. The pathophysiology and treatment of DR, which have been reviewed by several authors [26, 29–32], are summarized in Fig. 1. In an early stage, microvascular changes occur in response to hyperglycemia, but increasing evidence suggests that neuroglial degeneration may precede microvascular changes [26, 29, 30, 33, 34]. As the severity of DR progresses, capillary nonperfusion and occlusion lead to retinal ischemia, which combined with an imbalance in the levels of inflammatory and pro-angiogenic cytokines can trigger intra-retinal and intravitreal neovascularization (NV) and, eventually, diabetic macular edema (DME) [26, 32, 33]. The association of NV, retinal gliosis, and fibrosis favors the formation and growth of fibrovascular proliferative tissue on the retinal surface. Tractional forces at the vitreoretinal interface due to the contraction of fibrovascular tissue can lead to tractional RD and, ultimately, vitreous hemorrhage [33, 35]. Furthermore, vitreoretinal structural and molecular changes are underlying the progression to PDR, since the production of advanced glycation-end products (AGEs) associated with hyperglycemia, among other processes, triggers abnormal crosslinks between collagen fibrils, causing the destabilization of vitreous structure [36–38].
Fig. 1.
Vitreous and retinal anatomy in pathophysiological events in diabetic retinopathy and proliferative diabetic retinopathy and current treatments for each clinical feature. RGC retinal ganglion cell
The characterization of the proteome of vitreous humor has contributed extensively to identify pathways involved in DR/PDR [37, 39–41] and DME [42], as well as potential disease biomarkers. Proteins that have been associated with these diseases using proteomic and multiplex ELISA approaches are listed in Supplementary Table 1.1. Overall, the first proteomic analyses, using two-dimensional electrophoresis (2DE) coupled with mass spectrometry, reported increased levels of plasma proteins, including apolipoproteins complement and coagulation proteins, and acute-phase proteins in DR [43–46] and PDR [44, 46–49] compared to non-diabetic controls, as well in DME compared to the non-DME group [50, 51]. Levels of pigment epithelium-derived factor (PEDF) and gamma-enolase (ENO2) were inconsistent among studies comparing PDR vitreous with non-diabetic controls [44, 46–49, 52]. In the case of DME, PEDF was consistently reported as augmented in proteomics studies [50, 53, 54], suggesting a protective effect on DME due to its role as an anti-angiogenic/neurotrophic factor [42]. Nevertheless, other authors reported lower levels of PEDF in DME [55, 56], implying that its protective effect depends on the pathological phases of DR [54]. Clusterin (CLU), inter-alpha-trypsin inhibitor heavy chain H2, and several retinol-binding proteins and crystallins were reported as underexpressed in PDR [48, 49, 52], but in DME, only CLU and crystallin S were found diminished [51]. Although these studies have provided some potential biomarkers of the progression to PDR, the complexity and wide dynamic range of human vitreous represent a challenge for quantitative analysis by 2DE [57, 58]. To overcome some of the limitations of gel-based techniques, proteomics techniques such as liquid chromatography coupled to mass spectrometry (LC–MS) and capillary electrophoresis coupled to mass spectrometry (CE–MS) have been used in recent years. In addition to the aforementioned proteins that were implicated in DR/PDR pathogenesis [53, 54, 59–66], these studies contributed to the identification of new potential protein biomarkers.
Besides contributing to the understanding of the role of carbonic anhydrase (CA) in the activation of the kallikrein–kinin system (KKS), Gao et al. reported lower levels of extracellular superoxide dismutase, neuroserpin, cell adhesion molecules (e.g., calsyntenin-1), and amyloid-forming proteins (e.g., amyloid-beta A4 protein [APP]) in PDR compared with diabetic patients [53, 54]. Kita et al. also investigated the role of the KKS in DME. Since no correlation was found between the levels of plasma kallikrein and vascular endothelial growth factor (VEGF), they demonstrated that KKS induces retinal thickening and vascular permeability in DM through a VEGF-independent mechanism. However, it was observed a positive correlation between kallikrein levels and several plasma proteins (e.g., complement components and acute-phase proteins) in DME, suggesting that KKS activation in the vitreous may result from plasma proteins influx after blood–retinal barrier (BRB) dysfunction [67]. Balaiya et al. also associated the levels of complement components and coagulation systems with KKS in PDR, whereas anti-angiogenic proteins, immune modulators, acute-phase proteins, proteases, and extracellular matrix (ECM) components were only detected in patients with epiretinal membranes (ERM) or macular hole [62].
Wang et al. identified 96 differentially expressed proteins in PDR vitreous compared to healthy donor samples, many of them related to the glycolytic process, visual perception, and hypoxia-inducible factor (HIF-1) pathways [60]. In another study, Gardner and Sundstrom proposed a new personalized medicine method for the prevention and treatment of early DR based on proteomics approaches. Proteins related to NRF2-mediated oxidative stress response and cell proliferation were found up-regulated in PDR vitreous, which suggests the activation of these processes. On the other hand, HIPPO signaling pathway and central nervous system development were predicted as inhibited [65]. In a more recent study, Schori et al. described increased levels of proteins involved in the regulation of cell adhesion, and ECM organization, including metalloproteinases (MMPs) and inhibitors, and proteoglycans in PDR vitreous. In turn, molecules associated with lysosomal activity/autophagy and biological processes in the nervous system, including developmental processes, ECM organization, and cell adhesion, were found downregulated. Interestingly, the reduction in the levels of some of these proteins, including APP, neuroserpin, acid ceramidase, and ceroid-lipofuscinosis neuronal protein 5, has been related to neurodegeneration [63].
Using a label-free quantitative method, Loukovaara et al. compared vitreous from patients with PDR, including those treated with bevacizumab. Overall, this study reported 1351 quantified vitreous proteins, describing higher levels of inflammatory mediators, cell adhesion molecules, oxidative stress markers, and ECM proteoglycans. In addition, the downregulation of 72 proteins was found after the administration of bevacizumab, including apolipoproteins, crystallins, immunoglobulins, insulin-like growth factor-binding proteins (IGFBPs), and proteins involved in cell adhesion and apoptosis [64]. More recently, Zou et al. studied the changes induced by ranibizumab in the vitreous proteome of patients with PDR and found that platelet degranulation and integrin cell surface interaction pathways are severely affected. Many complement and coagulation proteins, crystallins, and immunoglobulins were not detected in the vitreous after treatment. By contrast, metalloproteinase inhibitor 2 (TIMP2) was found up-regulated in response to ranibizumab, as well as several glycolytic proteins and antioxidant proteins (catalase, peroxiredoxin) [68]. Alternatively to proteomic assays, multiplex ELISA assays were used for the quantification of proteins like VEGF, whose intravitreal concentration varies in the range of picograms [69–71]. VEGF has been established as one of the key molecules in DR pathogenesis, and its levels in vitreous and plasma are correlated with the progression of PDR [72] and clinical features such as macular volume and central retinal thickness [73, 74]. Therefore, it is not surprising that anti-VEGF therapy was established as the first-line therapy for DME and PDR [75, 76]. Although anti-VEGF therapy displays some contraindications and requires further optimization to improve its long-term effectiveness, drugs, such as aflibercept, ranibizumab, and bevacizumab, have been shown to promote the regression of NV in DR patients [77–79] (reviewed in Refs. [75, 76]). Several studies found a positive correlation between VEGF intravitreal levels in PDR and levels of vascular cell adhesion protein-1 (VCAM-1) [80], alpha-crystallin B chain [81], MMPs [82, 83], oxidative stress markers [84–86], and proteins related to the renin-angiotensin system [82, 87]. Angiopoietins 1 and 2 (Ang-1 and Ang-2) were also implicated in the modulation of later stages of angiogenesis in DR/PDR [88]. They exert their effect by binding to a specific tyrosine kinase receptor (Tie-2), with Ang-1 acting as an activator, maintaining cell survival and vascular stability, and Ang-2 as an antagonist, promoting abnormal angiogenesis (reviewed in Refs. [88, 89]). Considering that, faricimab, a new drug that targets both Tie-2/Ang-2 and VEGF pathways, was recently approved for the treatment of DME, showing gains in visual acuity statistically superior to ranibizumab in treatment-naïve patients [90, 91]. Several authors reported higher levels of Ang-2 in the vitreous of patients with PDR [70, 92, 93]. Klaassen et al. found that the levels of Ang-2 and other pro-angiogenic mediators, such as PDGF and PlGF, were strongly correlated to the degree of fibrosis in PDR, whereas Ang-1 shows a strong correlation with NV [70]. The concentration of Ang-2 was twice that of Ang-1 in non-proliferative DR with DME, whereas only Ang-1 was increased in PDR [94]. Loukovaara et al. observed a significant correlation between Ang-2 levels and matrix metalloproteinase-9 (MMP9), VEGF, erythropoietin, and transforming growth factor (TGF-β) levels in vitreous from DR patients. Although Ang-1 were also increased, the Ang-1/Ang-2 ratio was found to be decreased, which is considered a critical control for a switch in inflammatory processes [95]. Apart from VEGF and Ang, other regulators of angiogenesis were found up-regulated in PDR vitreous compared to non-diabetic controls, including IGFBPs [70, 96], placental growth factor (PlGF) [70, 97], platelet-derived growth factor (PDGF) [70, 98–100], hepatocyte growth factor [70, 99, 101], and soluble VEGF receptor 1 (VEGFR-1) [93].
Other studies pointed to the role of chronic neuroinflammation in PDR through the quantitation of inflammatory mediators, adhesion molecules, and neurotrophic factors. The measurement of pro-inflammatory mediators could be relevant to assessing the use of alternatives (e.g., corticosteroids) to the standard treatment, especially in refractory DME and unresponsive cases to anti-VEGF therapy [102–104]. On the other hand, the measurement of neurotrophins opens new doors for the design of new therapies based on neuroprotection (see “Mechanisms of neurodegeneration and neuroprotection” section). Klaassen et al. found higher levels of intercellular adhesion molecule-1 (ICAM-1), tumor necrosis factor (TNF-α) receptors, and neurotrophic factor neuregulin-1 in PDR compared with non-diabetic patients with vitreous debris [70]. Other studies reported the up-regulation of cytokines in PDR vitreous, including interferon-gamma (INF-γ) [71], TNF-α [71, 99], granulocyte Colony-Stimulating Factor (CSF3) [71], interleukins 1 beta (IL1-β), 6 (IL-6), and 8 (IL-8) [69, 71], chemokines [e.g., C-X-C motif chemokine 10 (CXCL10)] [71], and adhesion molecules (e.g. ICAM-1, VCAM-1) [69, 80]. Downregulation of neurotrophins/anti-angiogenic molecules such as PEDF [93, 101] or nerve growth factor (NGF) [105] in PDR has been described, whereas levels of TNF-α, IL-8, neurotrophin-3 (NT-3), NGF, glial cell-derived neurotrophic factor (GDNF), and ciliary neurotrophic factor (CNTF) were higher in eyes with non-proliferative DR compared with active PDR [106]. By measuring the levels of inflammatory mediators at distinct stages of DR, Kovacs et al. found that IL-8 levels gradually increase with the progression of the disease to PDR [99]. Koskela et al. observed higher levels of IL-6 and IL-8, but not of adhesion molecules, in the vitreous compared to the plasma of patients with PDR, which suggests an active local production of these interleukins at the retinal level. Furthermore, they found higher levels of interleukin-10 (IL-10) and several adhesion molecules (ICAM-1, platelet endothelial cell adhesion molecule [PECAM-1], E-selectin, and VCAM-1) in PDR compared with controls [69]. Yoshida et al. detected significantly higher levels of monocyte chemoattractant protein-1 (MCP-1) and IL-6, but not IL-8, in patients with PDR after vitrectomy, suggesting a possible association between inflammation and post-operative macular edema. Also, levels of MCP-1 were higher in the vitreous of patients with DME compared to those without DME [107]. Deuchler et al. found a linear correlation between DR grade and the expression of ICAM-1, IL-8, and PlGF and between the average retinal thickness and the levels of ICAM-1 and IL-8. PDR has been associated with higher levels of ICAM-1, IL-8, and PlGF, whereas the levels of ICAM-1, interferon-gamma-inducible protein, PlGF, VEGF, and interleukins (IL-6 and IL-8) were higher in DME patients with PDR than those with non-proliferative DR [108]. More recently, Kuo et al. performed a systematic literature review to find reliable inflammatory biomarkers in DR and relate them to the disease progression. The activation of the inflammasome pathway as DR progresses is suggested by the increase of IL-1β and IL-18 [109]. Other studies also assessed several cytokines in vitreous during DME, but they have demonstrated inconsistent findings, as recently reviewed [110]. Higher levels of VEGF [55, 56, 71, 111–113], IL-6 [56, 71, 113, 114], IL-8 [71, 112, 114], and ICAM-1 [56, 111] MCP-1 [56, 107, 114], and acute-phase factors [115] have been associated to DME. Although these studies were included in the systematic review of Minaker et al., only VEGF levels were considered significantly higher in DME compared to controls, according to meta-analysis, while IL-8 failed the sensitivity analysis due to the lack of studies [110].
Age-related macular degeneration
AMD is a multifactor ocular disease that affects central vision and is characterized by a degeneration of photoreceptors and retinal pigment epithelium (RPE) in the macula [116, 117]. AMD represents the most common cause of blindness among the elderly in developed countries [118, 119]. According to WHO, about 195.6 million people suffered from AMD in 2020, of which about 10.4 million had moderate-to-severe vision impairment or blindness [23, 118]. Risk factors, such as age, smoking, high body mass index, and genetic factors, are strongly associated with the development of AMD [119–121].
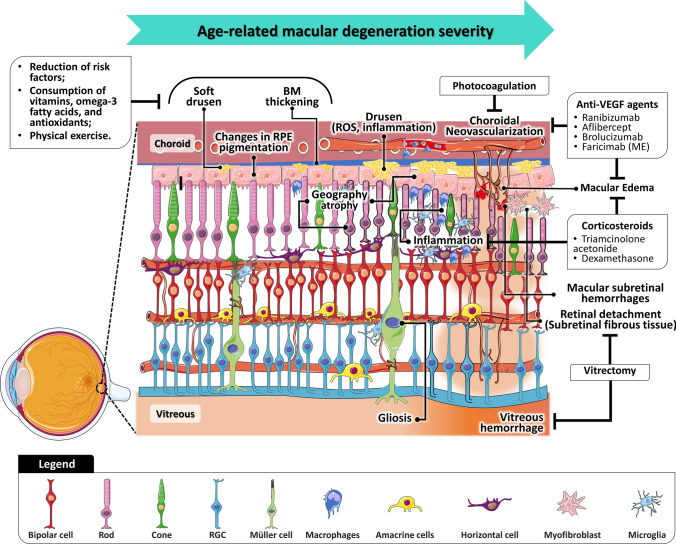
In the early phase of AMD, yellowish deposits (drusen) are accumulated underneath the retina, accompanied by changes in RPE pigmentation, and thickening of Bruch’s membrane (Fig. 2) [116, 122–124]. AMD can be further classified as early or intermediate based on the area and size of the drusen in the macula, with the presence of larger soft drusen being associated with an increased risk of disease progression [123, 125, 126]. At more advanced stages, patients may develop geographic atrophy, which is characterized by progressive atrophy of RPE and overlying neurosensory retina and choriocapillaris [122, 127, 128]. Currently, the management of dry AMD is based on a regular follow-up evaluation, to detect signals of visual function deterioration and early NV. Changes in lifestyle and antioxidant micronutrient supplementation are also recommended to reduce the risk of developing AMD and the progression of early-to-late AMD [129–134]. Furthermore, several therapeutic strategies show the potential to prevent and delay the progression of dry AMD and restore vision [135, 136], including cell-based therapies [137, 138] and several therapeutic agents [139–143].
Fig. 2.
Vitreous and retinal anatomy in pathophysiological events in the dry age-related macular degeneration and neovascular age-related macular degeneration and current treatments for each clinical feature. BM Bruch’s membrane, RGC retinal ganglion cell, ROS reactive oxygen species, oxidative stress, RPE retinal pigment epithelium
Approximately 10–15% of all AMD patients develop nAMD, the main responsible for severe vision loss [124]. Until recently, the neovascular component was referred to as choroidal NV (CNV), but this nomenclature was replaced by macular NV (MNV), since NV does not always originate from the choroid [144, 145]. According to the Consensus on Neovascular Age-Related Macular Degeneration Nomenclature (CONAN) Study Group, nAMD includes type 1 MNV (occult CNV), type 2MNV (classic CNV), and type 3 MNV [145]. While MNV in type 1 arises from the choriocapillaris and proliferates into the sub-RPE space, contributing to the detachment of the fibrovascular pigment epithelium, type 2 refers to the proliferating of vessels above the RPE into the sub-neurosensory retinal space, where exudation or hemorrhage may occur [145, 146]. Type 3 refers to retinal angiomatous proliferation and is characterized by NV within the retinal vasculature, that extends posteriorly into the subretinal space, and eventually anastomose with the CNV [147, 148]. Laser treatments were the first treatments approved for nAMD, but currently, the first-line treatment has been the regular intravitreal injection of anti-VEGF agents (e.g., ranibizumab, aflibercept, and brolucizumab) [125, 149, 150]. Recently, the FDA-approved faricimab, a bispecific antibody that targets both VEGF and Ang-2 growth factors, showed a comparable but more durable therapeutic effect than aflibercept, which reduces a treatment burden in patients with nAMD [151]. The efficiency of biosimilar anti-angiogenic therapies based on sustained-release implants, encapsulated cell technology, or gene therapy has been also explored in several clinical trials [136, 152, 153].
Although complement cascade, inflammation, angiogenesis, and oxidative stress have been proposed as key pathways for the onset and progression of AMD [154, 155], a better understanding of pathophysiologic processes is crucial for the implementation of more effective treatments [117, 146]. Despite a few studies have focused on the characterization of vitreous proteomics in AMD, some potential disease biomarkers have been proposed (Supplementary Table 1.2) [63, 156, 157]. Using CE–MS, Koss et al. identified 19 putative biomarkers in the nAMD vitreous compared to idiopathic floaters. Changes in vitreous proteome reflected the up-regulation in nAMD of several proteins related to transport, immune responses, complement cascades, and protection against oxidative stress (e.g., glutathione peroxidase 3) [156]. Nobl et al. identified four biomarkers (opticin, CLU, PEDF, prostaglandin-H2 D-isomerase) by CE–MS and LC–MS by comparing vitreous collected from nAMD eyes with different degrees of CNV [157]. Using a label-free LC–MS/MS, Schori et al. found increased levels of lysosomal proteins (cathepsin Z and prosaposin) and inflammatory mediators (chitinase-3-like protein-1) in nAMD compared to ERM controls. Nonetheless, proteins associated with the same processes were found downregulated in dry AMD, suggesting that some of these pathways may be activated later in nAMD. Other proteins involved in the glycolytic process (phosphoglycerate mutase 2), oxidative stress (superoxide dismutase, glutathione reductase), and APP fibril formation (beta-2-microglobulin) were also found up-regulated in nAMD [63]. The role of VEGF in CNV, a hallmark of nAMD, has been strongly supported by its expression in choroidal neovascular membranes and by the clinical efficacy of anti-VEGF treatment [158, 159]. Our research group found lower levels of both VEGF-A and VEGF-B in nAMD compared to patients with retinal vein occlusion and PDR.[73]. Huber et al. observed increased intravitreal levels of VEGFR-1 and decreased levels of PEDF in nAMD vitreous, but the concentration of angiogenic proteins, such as VEGF and Ang-2, remained unchanged [93]. The levels of PEDF are in agreement with the other studies [160, 161], which can indicate that the reduced anti-angiogenic activity also contributes to CNV during AMD. Using a cytometric bead assay, Koss et al. found higher levels of MCP-1 but lower levels of IL-6 and VEGF in nAMD compared to DME vitreous [162]. An increase of inactive (pro-IL-1β) and active forms of IL-1β [163] and TGF-β [164] were also detected in the vitreous of nAMD patients. They also found that semaphorin 3A inhibits the CNV mediated by TGF-β1, as well as the VEGF and TGF-β responses [164]. Elevated levels of MMP9, interleukins (IL-8), PDGF receptor, and BCL-2 associated death promoter, among other molecules, were related to subretinal fluid (SRF) accumulation in AMD patients [165]. To determine the role of the complement activation in the progression of AMD, complement C3 (C3), and factors B and D were quantified in vitreous at distinct severity levels. Although there is no increase in these factors in the vitreous in parallel with the progression of AMD, the activation of the alternative complement pathway is suggested due to the higher levels of factor B fragments observed in more advanced stages of AMD [166].
Proliferative vitreoretinopathy
PVR is a major complication of RRD characterized by the growth and contraction of cellular membranes on the detached retina and within the vitreous cavity [8, 167]. Although it can occur in untreated eyes with RD, the incidence of PVR increases after surgery [7]. Extension and duration of RD, retinal tear size, presence of choroidal detachment and vitreous hemorrhage, chronic intraocular inflammation, and unsuccessful RRD repair are predisposing factors to the development of PVR [168–170]. The pathogenesis of PVR is summarized in Fig. 3. PVR progresses into three main wound-healing phases: inflammation, proliferation, and scar modulation [171, 172]. BRB breakdown leads to the influx of growth factors and inflammatory mediators, which increases the chemotactic and mitogenic activity in the vitreous and foments’ inflammatory recruitment and cellular proliferation [7, 167, 172]. The physical separation of the neurosensory retina and the underlying RPE creates an ischemic environment causing the death of the photoreceptors and neurons [8, 167]. Altogether, these events incite structural and cellular changes in surrounding RPE and glial cells by mechanisms not fully understood [173, 174]. One of these mechanisms is the epithelial–mesenchymal transition (EMT) of the RPE cells, in which cells lose their epithelial characteristics and gain the capacity of migrating and proliferating [8, 173]. In this early phase of PVR, vitreous haze, protein flare, and the presence of pigment clumps are manifested in the eye due to the multiplication of RPE in the vitreous [175]. The migration of proliferating cells, such as RPE and glial cells, into the vitreous cavity, their adhesion to the detached retina, and the uncontrolled production of ECM components lead to the formation of fibroproliferative membranes. The contraction of these membranes and the resulting tractional forces pull the retina into fixed folds [168, 171]. If not addressed promptly, these forces progressively create retinal wrinkles, tears, and tractional RD, which hinders surgical reattachment [7, 171, 172]. Scleral buckling, pars plana vitrectomy, and pneumatic retinopexy are commonly used for the management of RRD and early PVR (grade A) [176], whereas other surgical procedures (Fig. 3) are recommended at more advanced stages [8, 167]. Numerous drugs have been proposed as adjunctive in the treatment of PVR, including anti-inflammatory molecules [177, 178], anti-neoplastic/anti-proliferative agents [179, 180], anti-growth factor pathway inhibitors [181, 182], and anti-oxidants [183]. Several clinical trials (e.g., GUARD trial) [184, 185] have tested the efficiency of intravitreal injections of methotrexate during the post-operative period and an improvement in the retinal reattachment rate has been observed, suggesting a promising alternative for the management of advanced PVR [186].
Fig. 3.
Vitreous and retinal anatomy in pathophysiological events related to the progression from retinal detachment to proliferative vitreoretinopathy and current treatments for each clinical feature. The experimental treatments are marked with *. ECM extracellular matrix, iBRB inner blood-retinal barrier, oBRB outer blood-retinal barrier, RGC retinal ganglion cell, RPE retinal pigment epithelium
In recent years, different studies of vitreous proteome have been carried out to elucidate the mechanisms underlying the PVR pathogenesis. Shitama conducted one of the first proteomic approaches in PVR by studying the vitreous proteome in several vitreoretinal diseases. Increased levels of alpha-1-antitrypsin (AAT) and apolipoprotein A4 were found in PVR, while higher levels of PEDF were reported in RRD compared to PVR. Cathepsin D, transthyretin (TTR), and CLU were also found up-regulated in RRD and PVR compared to DR and PDR samples [46]. The increase of TTR levels in PVR vitreous, as well as complement C4, was confirmed by other authors using 2DE and ELISA [187]. By applying distinct proteomics approaches, kininogen 1 and insulin-like growth factor-binding protein 6 (IGFBP-6) were proposed as new biomarkers of PVR, and p53 and transcription factor E2F1 as potential therapeutic targets [188–190]. These studies showed an increase of alpha-2-HS-glycoprotein, alpha-1B-glycoprotein, serpin family members, and complement factors, suggesting that plasma proteins accumulate in vitreous as the PVR progresses [188, 189]. Although the increase of these components in the vitreous is not PVR specific, it can provide information on the integrity of BRB, and the degree of inflammation and wound healing [11, 170]. These studies also suggest that the complement and coagulation cascade plays an important role in PVR pathogenesis [188, 189]. Furthermore, ECM remodeling during PVR is suggested by the downregulation of several actin family members, tubulin, and opticin [188, 189]. Deregulation in the glycolysis/gluconeogenesis process was also implicated in PVR, since many proteins, some associated with the HIF-1 signaling pathway (e.g., glyceraldehyde-3-phosphate dehydrogenase, ENO1 or 2), were found downregulated in moderate and severe PVR [188]. Interestingly, several glycolytic enzymes were reported as up-regulated in RRD vitreous by our research group and Öhman et al. [191, 192], suggesting that in an early phase, retinal cells increase their metabolism to obtain more energy through glycolysis to compensate for the metabolic “stress” state of the retina [191].
Comprehensive multiplex and ELISA assays have analyzed the vitreous from patients with PVR. Among the factors found differently expressed in PVR are IFN-γ [193, 194], interleukins (e.g., IL-6, IL-8) [193–197], chemokines (e.g., CXCL10) [194, 195], colony-stimulating factors [193, 195], growth factors (e.g., VEGF, PDGF, TGF-β) [193, 194, 198, 199], and MMPs [196] (Supplementary Table 1.3). The results link these factors with PVR events, such as exacerbated wound healing, activation and proliferation of RPE and glial cells, ECM production, and even potential survival mechanisms [200, 201]. Banerjee et al. found a complex pattern of inflammatory mediators in PVR vitreous compared to other vitreoretinal disorders (e.g., PDR). The detection of IL-6, IL-10, TNF-α, IFN-γ, and CSF3, among others, in PVR vitreous reinforces the crucial role of inflammation in PVR. Curiously, VEGF was found at higher levels in PVR than in PDR, whereas fibroblast growth factor (FGF) was only detected in PVR [193]. The increase of VEGF levels in the vitreous and SRF from patients with PVR was confirmed by other authors [194, 198, 199, 202], suggesting a role in the pathogenesis of PVR. In another study, the increase in the levels of CSF3, interleukins (e.g., IL-6), and inflammatory chemokines (e.g., CXCL10) were more evident in PVR compared with ERM controls than in primary RD, showing an increase in inflammation with the progression to PVR [195]. Through the screening of 200 cytokines in the vitreous samples from patients at distinct stages of PVR, Roybal et al. found that 25 cytokines significantly up-regulated in PVR and 20 associated with advanced PVR [194]. The levels of cell adhesion molecules (ICAM-1, PECAM-1), growth factors (VEGF), and chemotactic factors (CXCL10, CCL15) increased gradually from the early to more severe stages of PVR. The up-regulation of cytokines associated with T-cell recruitment, fibrosis (e.g., PDGF), and mTOR activation (e.g., IL-6) was more evident in the early stages of PVR, whereas fibroblast markers (e.g., CXCL12), macrophage inflammatory proteins (e.g., CCL3), and stromal cell-derived factor 1 were more predominant in PVR-C [194].
Molecular mechanisms common to proliferative and neovascular vitreoretinal diseases
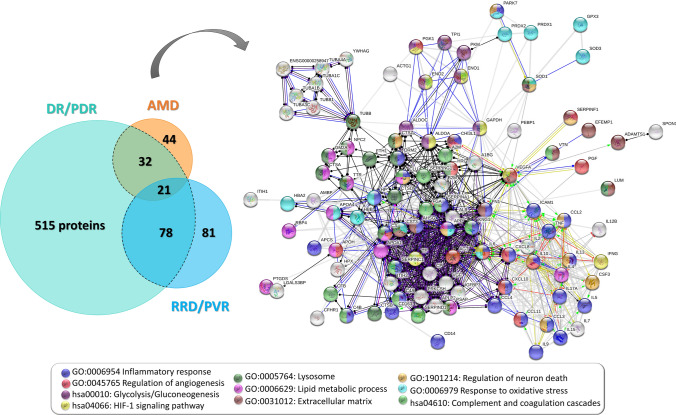
The proteins associated with DR/PDR, AMD, and RRD/PVR in diverse proteomics and multiplex studies were individually analyzed by STRING v11.5 [203], based on their protein interactions and functional enrichment, according to gene ontology for biological processes and pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome (Supplementary Table 2). These proteins and the related functional enrichment data were compared, as illustrated in Fig. 4, to set relationships and overlapping biological processes/pathways between their pathological mechanisms. Of these, biological processes, such as inflammation, fibrosis, oxidative stress, neurodegeneration, and vitreous remodeling, stand out in the functional analysis and, therefore, will be discussed below.
Fig. 4.
Proteins detected in proteomics and multiplex studies as differentially expressed in the vitreous of patients with non- and proliferative diabetic retinopathy (DR/PDR), age-related macular degeneration (AMD), and reghmatogeneous retinal detachment (RRD)/proliferative vitreoretinopathy are depicted in the Venn diagram. Protein–protein interaction network of the proteins found to be differentially expressed in more than one disease (black dashed line in Venn diagram) obtained with high confidence in STRING v11.5. Nodes corresponding to the top enriched terms of Gene Ontology and KEGG pathways are highlighted in different colors, as shown in the caption of the figure. Disconnected nodes were removed from the protein–protein interaction network
Mechanisms of inflammation
Nowadays, inflammation is known to play a central role in the development of proliferative pathologies, such as PDR [204–207], AMD [122, 208], and PVR [200, 209, 210]. Inflammatory events in PVR/PDR have been more strongly associated with the ischemic retinal environment resulting from BRB disruption [200, 206, 207, 209], whereas chronic inflammation in AMD may be linked to dysfunction of lipid homeostasis, in particular drusen formation [211]. Although the exact mechanisms have not been fully elucidated, pro-inflammatory cytokines, growth factors, ECM components, and proteolytic enzymes provide crosstalk between inflammation and other processes, including angiogenesis [212, 213], fibrosis [114, 201], oxidative stress [211], and retinal degeneration [214, 215].
The presence of these mediators in the vitreous can provide information on the integrity of BRB and the state of inflammation [11, 170]. As a matter of fact, the breakdown of the BRB and the massive influx of plasma proteins into the retina and vitreous cavity is a suitable predictor of the progression to a proliferative etiology [216, 217]. Increased intravitreal chemotactic and mitogenic activity stimulates the migration of macrophages and the proliferation of RPE and/or glial cells [217, 218]. Nevertheless, growing evidence indicates that changes in the immunosuppressive ocular microenvironment can precede changes in vascular permeability and BRB dysfunction [219]. Hyperglycemia-induced pathways [36, 220], hypoxic–ischemic conditions [221], and the activation of retinal glial cells, macrophages, and retinal neurons [222, 223] promote a pro-inflammatory environment that may disrupt the delicate balance of BRB. VEGF [224, 225], TNF-α [226], IL-1β [227], IGFBPs [228], ICAM-1 [111, 229], MMPs [230, 231], and the KKS [53, 67] induce the BRB breakdown through increased vascular and endothelial cells permeability, changes in tight junction proteins and cadherins, leukocyte recruitment, among other mechanisms. While most of these results are relevant to inner BRB dysfunction in hypoxic–ischemic and diabetic conditions, the outer BRB disruption in nAMD is more related to oxidative stress [221].
IL-1β was reported as the initiator of ocular inflammation [232] and, in combination with TNF-α, amplifies the inflammatory cascade through the modulation of VEGF, colony-stimulating Factors, MMPs, and adhesion molecules [232, 233]. Several studies suggest that IL-1β release is mediated by the activation of the NLRP3 inflammasome complex in response to the accumulation of drusen components and necrosis of adjacent RPE cells [234–236]. In turn, active IL-1β, TNF-α, and the induced pro-inflammatory mediators, such as IL-6 or IL-17, promote the recruitment of inflammatory cells, dysfunction, and proliferation of RPE, microglia, and Müller cells, activation of the complement cascade, and the production of ECM components. This leads to an inflammatory and post-fibrotic environment in the retina, which contributes to the degeneration of retinal neurons, RPE, and vascular endothelial cells [232, 237, 238].
IL-6 regulates the synthesis of acute-phase proteins and pro-inflammatory mediators, in addition to its role in hematopoiesis and the proliferation of fibroblast and glial cells [233, 239]. Acute-phase proteins are expressed by Müller cells in response to an inflammatory stimulus to restore homeostasis, but they can exert a protective effect or exacerbate tissue damage [240]. While AAT can have a protective effect [241], the presence of c-reactive protein, AAT, and serum amyloid in drusen has been associated with the activation of the complement cascade and immune cells, and neurodegeneration [121, 239, 242]. In its turn, molecules, such as ICAM-1 and VCAM-1, contribute to inflammation by mediating the adhesion of leukocytes to activated endothelial cells [225, 243], whereas other (e.g., PECAM1) mediates leukocyte transmigration [244]. Adhesion molecules are constitutively expressed by human retinal endothelial cells and are further increased in pathological conditions, which can predispose the retina to inflammation, and also lead to the death of endothelial cells and pericytes [245]. Vascular dysfunction favors a retinal ischemic environment, inducing the expression of VEGF and triggering the NV [204, 225]. Augmented levels of ICAM-1 in vitreous, vascular endothelium, and ERM have also been correlated with an increased risk of developing PVR [246, 247]. These correlations suggest that adhesion molecules could be common mediators between inflammation, fibrosis, and angiogenesis.
Activation of complement and coagulation cascades and fibrosis
Low levels of activation of complement and coagulation cascades are characteristics of the immune-privileged status that contributes to retinal homeostasis and integrity. Therefore, chronic activation of both cascades has been implicated in a variety of pathophysiological processes in the eye [248, 249]. Genetic variants in complement genes, such as C3, complement Factor H (CFH), and complement factor B, are considered as risk factors for AMD [166, 250]. CFH polymorphisms have been associated with sub-RPE accumulation of drusen and the extensive activation of the alternative pathway [250]. The activation of complement components like C3a and C5a, and their deposition in drusen, RPE, and choriocapillaris layers stimulate inflammatory responses, the recruitment of mononuclear phagocytes, and inhibit NV. On the other hand, the membrane attack complex has been related to the destruction of choroidal endothelium and the loss of pericytes [250–252]. Furthermore, the removal of retinal debris by the mononuclear phagocyte system could be compromised with aging, resulting in a sustained pro-inflammatory environment [253].
Although genetic studies have strongly supported the association of complement factors with AMD [250], a few studies have reported the quantification of these proteins in nAMD vitreous [63]. Changes in complement pathways also have a genetic component in PDR [254], but unlike nAMD, up-regulation of these components in PDR vitreous, was reported (see “Diabetic retinopathy” section). Increased levels of both full-length C3 and its activated fragment and CFH were found in vitreous and serum samples of patients with PDR, suggesting the activation of the alternative pathway of complement [255]. Although their role in PDR is still unclear [255], activation of complement and coagulation cascades could be associated with increased leukostasis and vascular permeability, BRB breakdown, and microglial activation [53, 54, 60, 64]. Likewise, the complement activation was reported in RD/PVR [187–189, 256], as well as its involvement in pathological processes, such as increased vascular permeability, endothelial cell proliferation, migration, RPE atrophy, reactive gliosis, and loss of photoreceptor outer segments [189, 257]. Sweigard et al. demonstrated that the activation of the alternative complement pathway promotes early photoreceptor cell death during RD. By contrast, deficient levels of complement components were related to impaired signaling function in retinal layers, highlighting the relevance of this system to retinal homeostasis [257].
The role of the coagulation system in the development of PVR is well established and is evidenced by intraocular fibrin deposition [258]. The exposition of RPE cells to serum components, such as thrombin, fibrin, plasmin, or fibronectin upon BRB breakdown, triggers EMT in these cells [35, 201]. Increased levels of kininogen 1, Factor Xa, and thrombin activity, which were detected in the vitreous in PVR, can contribute to its pathogenesis through the increase of levels of PDGF, TGF, and pro-inflammatory cytokines [189, 190, 258, 259]. It has been suggested that these effects are exerted in RPE cells via NF-κB, by the activation of mediators of pro-inflammatory signaling, angiogenesis, and fibrosis [190, 258, 259]. In addition to coagulation components, intraocular levels of IL-6 [260–262], osteopontin [263], connective tissue growth factor (CCN2) [6, 264], insulin-like growth factors (IGFs) [262, 265], and other growth factors (e.g., TGF-β, PDGF) [70, 262, 266], were correlated to fibrosis. The role of inflammatory and fibrogenic factors in PVR has been recently reviewed [209, 210]. Although its role is less clear in DR and AMD, retinal fibrosis is also a unifying feature of neovascular retinal diseases, particularly at late stages [267]. The elucidation of these mechanisms has recently paved the way for the development of new antifibrotic therapies (e.g., TGF-β signaling antagonists) that can benefit patients by reducing ocular morbidity associated with ocular fibrosis [268]. Some of these potential therapeutics are described below.
TGF-β is a key molecule in the activation of the EMT and fibrotic process. In addition to its role in ECM remodeling, it is capable of inducing itself and other pro-fibrotic factors, interleukins, and MMPs [209, 269, 270]. Several therapeutic strategies have been devised to target TGF-β in fibrotic diseases, including the administration of neutralizing antibodies [266], TGF-β agonists [182, 269], or inhibitors of TGF-β-induced pathways, such as Smad (e.g., pirfenidone) [271, 272], Notch (e.g., RO4929097) [273], and RhoA/Rho-kinase (e.g., Y27632) [274], and TAK1 signaling (e.g., 5Z-7 oxozeaenol) [275]. Anti-fibrotic drugs, such as pirfenidone [276] or OM-101 [277], showed both efficacy and safety for the treatment of PVR in animal models by reducing significantly the fibrotic response [276], and the incidence of RD and PVR [277].
PDGF is a potent chemoattractant and mitogen for fibroblasts, glial cells, and RPE cells, and promotes EMT, cellular contraction, and collagen synthesis [278, 279]. The co-expression of PDGF and its receptor (PDGFR) in ERMs suggests that RPE cells gain the capacity of autocrine stimulation upon the loss of cell–cell contact [280, 281], which was also verified for IGFs [265, 282]. As with TGF-β, several therapeutic agents targeting PDGF and its receptor have been tested in animal models, including aptamers [283], RNA interference [284], and antibodies [262, 285]. Remarkably, the neutralization of intravitreal PDGFs in a rabbit experimental model did not reduce PVR, suggesting that PDGF could be a poor therapeutic target [285]. Nevertheless, non-PDGF growth factors are capable of activating the PDGFR, whereas PDGFs could act as a protective agent [262, 285, 286]. The mechanism of activation of PDGFR by non-PDGF growth factors is mediated by increased cellular levels of reactive oxygen species (ROS), leading to the activation of Src family kinases that promote phosphorylation and activation of PDGFR [287]. In light of these findings, targeting the activation of PDGFR with anti-oxidants (e.g., n-acetylcysteine) [183] or kinases inhibitors (e.g., rapamycin that targets mTOR pathway) [194, 288], could prevent PVR in patients undergoing retinal reattachment surgery or help to ameliorate the symptoms.
The role of VEGF in fibrosis is also intermediated by the binding to PDGFR [286, 289] and by its up-regulation in vitreous, SRF, and ERMs in vitreoretinal diseases associated with fibrosis [198, 202]. However, the contribution of VEGF to angio-fibrotic switch mechanisms in angiogenic diseases, such as PDR or nAMD, is still unclear [290–292]. The ratio between CCN2 and VEGF levels was found to be the strongest predictor of fibrosis [290, 291], which means that the angio-fibrotic switch may be accomplished by a reduction of intravitreal VEGF levels and a progressive increase of the CCN2 [291]. This fact could explain the increase in intraocular fibrosis observed after treatment with anti-VEGF drugs [290, 291, 293]. Therefore, controlling the fibrosis after the administration of anti-VEGF drugs in patients with nAMD and PDR could be beneficial to explore the feasibility of combining this treatment with anti-fibrotic therapy [293].
Oxidative stress
The high demand for energy and oxygen, the presence of high levels of polyunsaturated fatty acids, and constant exposure to light make the retina particularly susceptible to oxidative stress and lipid peroxidation [294, 295]. Impaired redox balance has been associated with several ophthalmologic disorders, including DR, AMD, and PVR, as recently reviewed by our research group [296]. The rate of production and accumulation of ROS increases with age, where chronic low-grade inflammation and hypoxia in the aged retina can be potential sources [297]. Also, the ability of the eye to counteract the effect of ROS declines with age [294, 297]. One of the protective mechanisms against oxidative stress of the retina and surrounding tissues is provided by the high antioxidant capacity of the vitreous. However, it also decreases with age, contributing to oxidative damage [295, 298, 299]. The activation of protective mechanisms is suggested by the increase of intravitreal antioxidant enzymes, such as glutathione peroxidase, catalase, and peroxiredoxins [296, 298].
Hyperglycemia stimulates oxidative stress in the ocular tissues, which culminates in changes in vitreous structure, Bruch’s membrane thickening, and loss of retinal and capillary cells [300, 301]. Multiple mechanisms in diabetes are responsible for mitochondrial dysfunction and subsequent ROS production, including the activation of protein kinase C, hexosamine, and polyol pathways and the increased production of AGEs and their receptors (RAGE), [302, 303]. On the other hand, polymorphisms in antioxidant enzyme genes, cigarette smoke, and exposure to sunlight, among other environmental factors, are considered risk factors for AMD [211, 299, 304]. A combination of light exposition, dysfunction in lipid homeostasis, and oxidative stress promotes lipid peroxidation, which is one of the pathological events in AMD [211]. It has been suggested that these mediators could cause geographic atrophy by promoting inflammatory responses and oxidative damage to cellular proteins, lipids, and DNA (particularly within mitochondria) [211, 305]. Lipid peroxidation and the consequent accumulation of lipofuscin contribute to lysosomal dysfunction in RPE cells, which are essential for removing waste products and maintaining neural retina nutrition and homeostasis [306–308]. Impaired autophagy contributes to the accumulation of intracellular lipofuscin and drusen in Bruch's membrane [309, 310], creating a physical barrier to the influx of oxygen and nutrients to the photoreceptors and the waste removal between RPE and choroid [311]. Metabolic dysregulation and hypoxia stimulate the production of growth factors and cytokines that compromises the integrity of BRB [221, 312], while lipofuscin accumulation mediates light-induced damages in RPE cells and adjacent photoreceptors [306, 313]. Furthermore, the increase of free radicals, oxidative stress byproducts, and drusen can generate chronic inflammation through the production of pro-inflammatory interleukins and adhesion molecules [304, 314].
It was also reported that oxidative stress plays a role in EMT, which contributes to the pathogenesis of PVR and AMD [304, 315]. A synergistic effect between TGF, macrophage migration inhibitory factor, and hydrogen peroxide induces EMT through the up-regulation of α-smooth muscle actin, vimentin, and fibronectin and downregulation of cadherins [315, 316]. Interestingly, RPE cells increase autophagic activity and secretion of exosomes in response to oxidative stress, aging, and in the presence of drusen [309, 317, 318], probably to remove damaged and toxic materials [319]. Although, initially, these mechanisms may contribute to retinal protection and survival, the dysfunction of lysosomal degradation contributes to drusen formation, whereas exosomes could carry proteins and miRNAs that promote NV and EMT in neighboring cells [309, 317–319].
Mechanisms of neurodegeneration and neuroprotection
The complex architecture and functionality of the retina and its high energy and oxygen requirements make it very susceptible to stress [320, 321]. As neurodegeneration depends on a balance between the levels of neurotoxic and neuroprotective factors, it has been suggested that neurotrophic factors, anti-oxidants, and anti-apoptotic therapy could mitigate the effects of secondary degenerative events and reduce the loss of neurons in the retina [322]. Therefore, neuroprotection is gaining interest to design therapies for preserving the photoreceptors and ganglion cells after retinal damage in DR [323–325], AMD [326, 327], and RD/PVR [8, 328, 329]. In this context, it is relevant to understand the endogenous protective mechanisms triggered in the eye, with RPE and Müller cells being the main ones responsible for the secretion of neurotrophic factors [330, 331].
In response to acute stress signals, microglia produce neurotrophic mediators, such as GDNF and NT3, that act directly in neurons. In turn, brain-derived neurotrophic factor (BDNF) and CNTF induce Müller cells secretion of secondary mediators (e.g., FGF, GDNF) that mediate the survival of photoreceptors [215, 332]. RPE cells also contribute to cell survival signaling by secreting a wide range of neurotrophic factors (e.g., PEDF, IGFs, and NGF), among others, including cytokines, angiogenic, and anti-angiogenic factors [333]. Neurotrophic factors are also expressed in the retina in response to mechanical injury, suggesting an important role in photoreceptor rescue. Several of these factors diminished the death of photoreceptors in experimental models of RD [328, 334]. However, the chronic activation of these mechanisms can initiate severe alterations in retinal integrity, and exacerbate neuronal death [215, 335]. Complement factors [255, 336], IL-6 [337, 338], TGF-β1 [339, 340], and VEGF [202, 341] can act as neurotrophic factors and have an important role in neuronal, glial, and vascular homeostasis. Nevertheless, due to their role in other pathological events (see “Mechanisms of inflammation” and “Activation of complement and coagulation cascades and fibrosis” sections), they can eventually contribute to neurodegeneration.
Glial and RPE dysfunction, activation of Müller cells, and the loss of neurotrophic support are critical for the survival of photoreceptors and retinal ganglion cells [330, 331]. Activated Müller cells suffer morphological, biochemical, and physiological changes as a result of the dramatic increase in the expression of glial fibrillary acidic protein (GFAP) and vimentin [342]. The increase in the levels of these proteins in the RD vitreous was correlated with the severity of PVR, suggesting that reactive gliosis may play a key role in PVR formation [343]. Recently, Eastlake et al. described that proteins related to cytoskeleton, ECM, and ribosomes are up-regulated in gliosis compared to the normal retina, with Müller glia appearing to be the main source of vimentin, GFAP, polyubiquitin, and HSP90a [344]. Photoreceptor degeneration is greatly reduced in the absence of GFAP, vimentin, and other intermediate filaments, indicating that reactive gliosis impacts retinal damage [345, 346]. Taking this into account, the regulation of their expression in Müller cells might promote retinal regeneration after disease or injury [344].
Activated microglia also express retinal damage biomarkers such as ENO2 [347], and phagocytize toxic proteins like APP [330, 348]. ENO2 can act either as a neurotoxic or neuroprotector factor (e.g., against amyloid-β peptide toxicity), but upon neuron injury, it is expressed in M1-type microglia, promoting neurodegeneration [347, 349]. APP is a key component of drusen and its accumulation in the eye has been associated with neurodegeneration in AMD and glaucoma [350–352]. The increased phagocytic capacity of microglia and the expression of APP degrading enzymes could initially contribute to Aβ clearance. However, it eventually promotes neuroinflammation by the induction of pro-inflammatory cytokines and NLRP3 inflammasome [330, 348]. Likewise, it has been suggested that APP toxicity in neurons is mediated by its intralysosomal accumulation through macroautophagy, and consequent lysosomal membrane permeabilization [353]. On the other hand, the presence of αβ-crystallins in the drusen may reduce the aggregation of toxic proteins and augment autophagy-mediated clearance [354]. Crystallin mutant and knockout animal models exhibit inefficient lysosomal clearance, evidenced by the accumulation of lipofuscin-like material and increased susceptibility of RPE to oxidative stress and apoptosis [307, 355]. Moreover, several studies in animal models indicated that overexpression of heat shock proteins supports the survival of injured RPE, retinal ganglion cells, and photoreceptors [355–357].
AKT/mTOR pathway in RPE has also been associated with the impairment of autophagic flux and increased oxidative stress, glycolysis, and glycogen storage [358–360]. mTOR is activated in response to RPE stress, impairing autophagy and inducing dedifferentiation, hypertrophy, and metabolic reprogramming in RPE cells to promote its survival. However, the return to basal levels of mTOR activity is necessary to diminish the RPE glycolytic metabolism and to increase glucose supply to photoreceptors, indicating that chronic mTOR activation could lead to glucose deprivation and degeneration of photoreceptors [360, 361]. In turn, degenerative photoreceptors display low mTOR activity and high HIF-1 levels, as well as the activation of chaperone-mediated autophagy, suggesting that nutrient deprivation and prolonged starvation might underlie neurodegeneration.
Vitreous aging and ECM remodeling
With aging, the human vitreous suffers a progressive remodeling characterized by the loss of type IX collagen and short-range interactions with other ECM components, as well as the new synthesis and aggregation of collagen fibrils, which eventually leads to vitreous liquefaction and PVR [17, 362]. Although age-related ECM changes are frequent, they are intensified by pathological events, such as hyperglycemia, ocular inflammation, and oxidative stress [17, 22, 298, 362]. Therefore, vitreous liquefaction and PVD have been associated with various retinal pathologies [17, 19, 22]. ECM dynamics are tightly regulated by a balance between proteinases and their neutralizing substances [22, 363, 364], which suggests that the deregulation of these mechanisms might be underlying degenerative changes in vitreous and vitreoretinal diseases [365].
MMPs are the main proteases involved in the degradation of collagen and other ECM components [363, 366], although other proteases are also involved, including ADAMTS, cathepsins, and plasminogen [363, 364]. Under physiological conditions, MMPs are synthesized and secreted into the vitreous in their inactive form [22], but their expression and activity increase in tissue repair, inflammation, oxidative stress, or under-remodeling processes [366]. MMPs levels correlate with pathological features, such as SRF accumulation in nAMD [165], the duration and extent of RD [367], the grade of post-operative PVR [368], and PVD [369], and NV in DR [264]. ECM dynamics modulate a series of pathological features of these vitreoretinal diseases, like vascular permeability, NV [366, 370], inflammation [244, 371], and fibrosis [209, 372]. The degradation of ECM components by MMPs modulates these processes by providing a scaffolding via ECM–integrin binding that facilitates the cell adhesion and migration of immune cells and vascular endothelial cells [244, 363, 370]. Proteolysis via MMPs also changes the bioavailability of factors sequestered in ECM, including growth factors, chemoattractants, and other signaling molecules such as matricellular proteins or bioactive ECM fragments [363, 364, 370]. Besides that, MMPs process other bioactive molecules, including growth factors and other cytokines and cell surface molecules, which may promote their inactivation or potentiate their effects [244, 363, 371].
On the other hand, increased levels of metalloproteinase inhibitor 1 (TIMP1) were found in RRD/PVR [196, 367, 368] and PDR [63, 264], whereas TIMP2 only slightly increased in PDR [64]. The up-regulation of TIMP1 by pro-fibrotic factors (factor Xa, TGF-β) was associated with fibrosis, since their combined effect potentiates ECM production and prevents the destruction of the newly synthesized matrix [259, 368]. Nevertheless, it has been suggested that MMPs/TIMPs levels suffer concomitant changes during RRD/PVR, and that secretion of MMPs by RPE cells might assist in its migration into vitreous [209, 368]. In accordance, ERM removed from patients with PVR and PDR are particularly rich in structural (e.g., collagens) and adhesive ECM components (e.g., fibronectin) and matricellular proteins (e.g., tenascin, thrombospondin 1), but also contain MMPs/TIMPs [70, 373]. Decreased 72 kDa Type IV Collagenase (MMP2) and MMP9 activity were also associated with Bruch’s membrane thickening and accumulation of lipid-rich debris in AMD [374]. Conversely, higher levels of MMPs were associated with the progression of CNV, which was confirmed using an MMP2 and MMP9 knockout mouse model [375]. TIMP1 levels also correlated with the degree of NV, but its correlation with activated TGF-β2 levels was independent of the degree of DR, suggesting that it might have a more relevant role in angiogenesis than in fibrosis [264]. Furthermore, TIMP1 could result from the breakdown of BRB, in contrast with TIMP2 that is specifically secreted by intraocular tissues, which could explain its correlation with NV degree in PDR [376]. More recently, it was verified that TIMP2 and other protease inhibitors (e.g., SERPINA5) are up-regulated after treatment with ranibizumab [68]. TIMP2 seems to be more efficient at inhibiting angiogenesis than TIMP1, and it may mediate the effects of treatment with ranibizumab [68]. This can be related to the fact that TIMP2 inhibits growth and angiogenesis, not only by inhibiting MMPs but also via α3β1 integrin-mediated binding of TIMP2’s N-terminal domain to endothelial cells [377]. Although the role of ECM remodeling in proliferative diseases is not clear, it might be a unifying mechanism between vitreous degeneration and pathological events, such as fibrosis, inflammation, and NV. The structural integrity of the vitreous seems to be one of the decisive factors for the progression to a proliferating and/or neovascular etiology and, therefore, ECM molecules may be a potential therapeutic target.
Conclusions and future perspectives
During the progression of vitreoretinal diseases, the vitreous acts as a repository of the mediators involved in inflammation, fibrosis, oxidative stress, neurodegeneration, and remodeling of the ECM. Therefore, the study of vitreous proteome has shed light to elucidate some of the pathological mechanisms underlying these diseases and discover potential pharmaceutical targets. The characterization of the vitreous humor proteome has largely contributed to the recognition of pathways involved in PDR and to the identification of new therapeutic targets, in addition to VEGF. The lack of validation in a larger cohort of patients might explain the absence of reliable vitreous biomarkers. Proteomics studies focusing on nAMD and PVR are scarce, although they provided interesting evidence on the pathogenesis of these diseases. In light of proteomic studies, it would be beneficial to incorporate other therapeutic agents targeting fibrosis, neurodegeneration, oxidative stress, and vitreous degeneration in PVR, nAMD, and PDR.
However, the interpretation of proteomics results in the context of vitreoretinal pathologies could be challenging due to the high complexity of vitreous, as illustrated by the presence of several proteoforms, including isoforms and post-translational modified proteins, which have distinct or dual functions. In this context, proteomics studies should be complemented with functional proteomics studies, other -omics analyses (e.g., genomics and metabolomics), and in vitro and in vivo experiments. Genomics analysis could be relevant in nAMD, since this disorder has a significant genetic component. Cooperation between clinicians and basic researchers is essential to fill the gap between medical practice and basic science, contributing to discovering reliable pharmaceutical targets and finding correlations between biomarkers and disease progression. Unfortunately, vitreous is collected at later stages of vitreoretinal pathologies (e.g., ERM and vitreous hemorrhage), reducing dramatically the probability of finding reliable therapeutic targets for the earliest stages of the disease. Finally, it must be considered that invasive sampling hampers the use of the vitreous for diagnosis but, when obtained as part of clinical treatment, these biomarkers may be used for prognosis and/or to predict the response to treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Fátima Millhano Santos acknowledges a doctoral fellowship [SFRH/BD/112526/2015] from the Portuguese Foundation for Science and Technology (FCT). This work was developed within the scope of the CICS-UBI projects UIDB/00709/2020 and UIDP/00709/2020, financed by national funds through the FCT/MCTES. It was also funded by the European Regional Development Fund through the “Programa Operacional Regional do Centro (Centro 2020)—Sistema de Apoio à Investigação Científica e Tecnológica—Programas Integrados de IC&DT”, Centro-01-0145-FEDER-000019-C4-Centro de Competências em Cloud Computing. CNB-CSIC proteomics lab is a member of Proteored, PRB3-ISCIII, supported by grant PT17/0019/0001. CNB-CSIC has been designated Centre of Excellence “Severo Ochoa” and is funded by the Spanish Government through Grant SEV2017-0712. This work was also supported by national funds from FCT in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences-UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB. The authors acknowledge the COMBINE project “CombinatiOn aptaMer-drug Based therapeutics for ocular neovascular dIsease: wheN two are bettEr than one”, funded by BInov 2021, Innovation Grant of the Southern Regional Section and Autonomous Regions (SRSRA) of Portuguese Pharmacists Order.
Abbreviations
- 2DE
Two-dimensional electrophoresis
- AAT
Alpha-1-antitrypsin
- AGEs
Advanced glycation end products
- AMD
Age-related macular degeneration
- Ang-1
Angiopoietin 1
- Ang-2
Angiopoietin-2
- APP
Amyloid-beta A4 protein
- BDNF
Brain-derived neurotrophic factor
- BRB
Blood-retinal barrier
- C3
Complement C3
- CA
Carbonic anhydrase
- CCN2
Connective tissue growth factor
- CE–MS
Capillary electrophoresis coupled to mass spectrometry
- CFH
Complement factor H
- CLU
Clusterin
- CNTF
Ciliary neurotrophic factor
- CNV
Choroidal neovascularization
- CSF3
Granulocyte colony-stimulating factor
- CXCL10
C-X-C motif chemokine 10
- DME
Diabetic macular edema
- DR
Diabetic retinopathy
- ECM
Extracellular matrix
- EMT
Epithelial-mesenchymal transition
- ENO2
Gamma-enolase
- ERM
Epiretinal membranes
- FGF
Fibroblast growth factor
- GDNF
Glial cell-derived neurotrophic factor
- GFAP
Glial fibrillary acidic protein
- HIF-1
Hypoxia-inducible factor
- ICAM-1
Intercellular adhesion molecule 1
- IGFBPs
Insulin-like growth factor-binding proteins
- IGFs
Insulin-like growth factors
- IL1-β
Interleukin-1 beta
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- IL-10
Nterleukin-10
- INF-γ
Interferon-gamma
- KKS
Kallikrein-Kinin system
- LC–MS
Liquid chromatography coupled to mass spectrometry
- MCP-1
Monocyte chemoattractant protein-1
- MMP9
Matrix metalloproteinase 9
- MMP2
72 KDa type IV collagenase
- MMPs
Metalloproteinases
- MNV
Macular neovascularization
- nAMD
“Wet” or neovascular age-related macular degeneration
- NGF
Nerve growth factor
- NT3
Neurotrophin-3
- NV
Neovascularization
- PDGF
Platelet-derived growth factor
- PDR
Proliferative diabetic retinopathy
- PECAM-1
Platelet endothelial cell adhesion molecule
- PEDF
Pigment epithelium-derived factor
- PlGF
Placental growth factor
- PVD
Posterior vitreous detachment
- PVR
Proliferative vitreoretinopathy
- RD
Retinal detachment
- ROS
Reactive oxygen species
- RPE
Retinal pigment epithelium
- RRD
Rhegmatogenous retinal detachment
- SRF
Subretinal fluid
- TGF-β
Transforming growth factor
- Tie-2
Tyrosine kinase receptor
- TIMP-1
Metalloproteinase inhibitor 1
- TIMP2
Metalloproteinase inhibitor 2
- TNF-α
Tumor necrosis factor
- TTR
Transthyretin
- VCAM-1
Vascular cell adhesion protein-1
- VEGF
Vascular endothelial growth factor
- VEGFR-1
Vascular endothelial growth factor receptor 1
Author contributions
FMS: conceptualization, formal analysis, writing—original draft, and writing—review and editing. SC: conceptualization; writing—review and editing. JM: conceptualization; writing—review and editing. JPCS: conceptualization; writing—review and editing. AP: supervision; writing—review and editing. CTT: supervision, conceptualization, and writing—review and editing. LAPP: supervision, conceptualization, and writing—review and editing.
Funding
Fundação para a Ciência e a Tecnologia (Grant no. SFRH/BD/112526/2015, UIDB/00709/2020, UIDP/04378/2020, UIDP/00709/2020, UIDB/04378/2020); European Regional Development Fund (Grant no. Centro-01-0145-FEDER-000019-C4); Fondo Europeo de Desarrollo Regional (Grant no. PT17/0019/0001); Spanish Government (Grant no. SEV2017-0712); Portuguese Pharmacists Order (Grant no. BInov 2021)
Data availability
The data reviewed in this paper is available in the original papers from proteomics and multiplex studies. These revised papers include the references [43–48, 52, 54, 59, 60, 62–64, 68–71, 98] for DR/PDR, [63, 93, 156, 157] for AMD, and [46, 188–195, 256] for RRD/PVR. The reviewed data is displayed in the supplementary table.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alberto Paradela, Cândida T. Tomaz and Luís António Paulino Passarinha are co-senior authors.
Contributor Information
Fátima Milhano dos Santos, Email: ftxsantos@gmail.com, Email: fr.milhano@cnb.csic.es.
Luís António Paulino Passarinha, Email: lpassarinha@fcsaude.ubi.pt, Email: lapp@ubi.pt.
References
- 1.Sabanayagam C, Cheng C-Y. Global causes of vision loss in 2015: are we on track to achieve the Vision 2020 target? Lancet Glob Heal. 2017;5:e1164–e1165. doi: 10.1016/S2214-109X(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 2.Bourne RRA, Flaxman SR, Braithwaite T, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Heal. 2017;5:e888–e897. doi: 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- 3.Ackland P, Resnikoff S, Bourne R. World blindness and visual impairment: despite many successes, the problem is growing. Community Eye Heal. 2018;30:71–73. [PMC free article] [PubMed] [Google Scholar]
- 4.Swenor BK, Ehrlich JR. Comment Ageing and vision loss: looking to the future. Lancet. 2021 doi: 10.1016/S2214-109X(21)00031-0. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida S, Nakama T, Ishikawa K, et al. Periostin in vitreoretinal diseases. Cell Mol Life Sci. 2017;74:4329–4337. doi: 10.1007/s00018-017-2651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuiper EJ, De Smet MD, Van Meurs JC, et al. Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch Ophthalmol. 2006;124:1457–1462. doi: 10.1001/archopht.124.10.1457. [DOI] [PubMed] [Google Scholar]
- 7.Constable IJ, Nagpal M (2013) Proliferative vitreoretinopathy. In: Retina, 5th edn. Elsevier, pp 1806–1825
- 8.Pastor JC, Rojas J, Pastor-Idoate S, et al. Proliferative vitreoretinopathy: a new concept of disease pathogenesis and practical consequences. Prog Retin Eye Res. 2016;51:125–155. doi: 10.1016/j.preteyeres.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Semba RD, Enghild JJ, Venkatraman V, et al. The Human Eye Proteome Project: perspectives on an emerging proteome. Proteomics. 2013;13:2500–2511. doi: 10.1002/pmic.201300075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramke J, Gilbert CE. Universal eye health: are we getting closer? Lancet Glob Heal. 2017;5:e843–e844. doi: 10.1016/S2214-109X(17)30302-9. [DOI] [PubMed] [Google Scholar]
- 11.Monteiro JP, Santos FM, Rocha AS, et al. Vitreous humor in the pathologic scope: Insights from proteomic approaches. PROTEOMICS Clin Appl. 2015;9:187–202. doi: 10.1002/prca.201400133. [DOI] [PubMed] [Google Scholar]
- 12.Velez G, Tang PH, Cabral T, et al. Personalized proteomics for precision health: identifying biomarkers of vitreoretinal disease. Transl Vis Sci Technol. 2018;7:12. doi: 10.1167/tvst.7.5.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodama M, Matsuura T, Hara Y. Structure of vitreous body and its relationship with liquefaction. J Biomed Sci Eng. 2013;06:739–745. doi: 10.4236/jbise.2013.67091. [DOI] [Google Scholar]
- 14.Chirila TV, Hong Y. Handbook of biomaterial properties. New York: Springer New York; 2016. Chapter C2 the vitreous humor; pp. 125–134. [Google Scholar]
- 15.Sebag J (2010) Vitreous Anatomy, Aging, and Anomalous Posterior Vitreous Detachment. In: Encyclopedia of the Eye. Elsevier, pp 307–315
- 16.Le Goff MM, Bishop PN. Adult vitreous structure and postnatal changes. Eye. 2008;22:1214–1222. doi: 10.1038/eye.2008.21. [DOI] [PubMed] [Google Scholar]
- 17.De Smet MD, Gad Elkareem AM, Zwinderman AH. The vitreous, the retinal interface in ocular health and disease. Ophthalmologica. 2013;230:165–178. doi: 10.1159/000353447. [DOI] [PubMed] [Google Scholar]
- 18.Alovisi C, Panico C, De Sanctis U, Eandi CM. Vitreous substitutes: old and new materials in vitreoretinal surgery. Journal of ophthalmology. 2017 doi: 10.1155/2017/3172138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holekamp NM. The vitreous gel: more than meets the eye. Am J Ophthalmol. 2010;149:32–36.e1. doi: 10.1016/j.ajo.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad MT, Zhang P, Dufresne C, et al. The human eye proteome project: updates on an emerging proteome. Proteomics. 2018;18:1–31. doi: 10.1002/pmic.201700394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahajan VB, Skeie JM. Translational vitreous proteomics. Proteomics Clin Appl. 2014;8:204–208. doi: 10.1002/prca.201300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponsioen TL, Hooymans JMMM, Los LI. Remodelling of the human vitreous and vitreoretinal interface—a dynamic process. Prog Retin Eye Res. 2010;29:580–595. doi: 10.1016/j.preteyeres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization (2019) World report on vision
- 24.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Heal. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 25.Santos AR, Ribeiro L, Bandello F, et al. Functional and structural findings of neurodegeneration in early stages of diabetic retinopathy: cross-sectional analyses of baseline data of the EUROCONDOR project. Diabetes. 2017;66:2503–2510. doi: 10.2337/db16-1453. [DOI] [PubMed] [Google Scholar]
- 26.Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2:1–13. doi: 10.1172/jci.insight.93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 29.Kusuhara S, Fukushima Y, Ogura S, et al. Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab J. 2018;42:364–376. doi: 10.4093/dmj.2018.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19:1–14. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossino MG, Dal Monte M, Casini G. Relationships between neurodegeneration and vascular damage in diabetic retinopathy. Front Neurosci. 2019;13:1–20. doi: 10.3389/fnins.2019.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gale MJ, Scruggs BA, Flaxel CJ. Diabetic eye disease: a review of screening and management recommendations. Clin Experiment Ophthalmol. 2021;49:128–145. doi: 10.1111/ceo.13894. [DOI] [PubMed] [Google Scholar]
- 33.Lechner J, O’Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vision Res. 2017;139:7–14. doi: 10.1016/j.visres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci. 2016;113:E2655–E2664. doi: 10.1073/pnas.1522014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007;117:576–586. doi: 10.1172/JCI31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kandarakis SA, Piperi C, Topouzis F, Papavassiliou AG. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog Retin Eye Res. 2014;42:85–102. doi: 10.1016/j.preteyeres.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Nawaz IM, Rezzola S, Cancarini A, et al. Human vitreous in proliferative diabetic retinopathy: characterization and translational implications. Prog Retin Eye Res. 2019;109:110–119. doi: 10.1016/j.preteyeres.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Kroll P, Rodrigues EB, Meyer CH (2014) III.L. Proliferative diabetic vitreoretinopathy. In: Vitreous. Springer New York, New York, pp 421–434
- 39.Csősz É, Deák E, Kalló G, et al. Diabetic retinopathy: proteomic approaches to help the differential diagnosis and to understand the underlying molecular mechanisms. J Proteomics. 2017;150:351–358. doi: 10.1016/j.jprot.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 40.Weber SR, Zhao Y, Gates C, et al. Proteomic analyses of vitreous in proliferative diabetic retinopathy: prior studies and future outlook. J Clin Med. 2021;10:2309. doi: 10.3390/jcm10112309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youngblood H, Robinson R, Sharma A, Sharma S. Proteomic biomarkers of retinal inflammation in diabetic retinopathy. Int J Mol Sci. 2019;20:4755. doi: 10.3390/ijms20194755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen MS, Rasmussen M, Grauslund J, et al. Proteomic analysis of vitreous humour of eyes with diabetic macular oedema: a systematic review. Acta Ophthalmol. 2022 doi: 10.1111/aos.15168. [DOI] [PubMed] [Google Scholar]
- 43.Nakanishi T, Koyama R, Ikeda T, Shimizu A. Catalogue of soluble proteins in the human vitreous humor: comparison between diabetic retinopathy and macular hole. J Chromatogr B Anal Technol Biomed Life Sci. 2002;776:89–100. doi: 10.1016/S1570-0232(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 44.Yamane K, Minamoto A, Yamashita H, et al. Proteome analysis of human vitreous proteins. Mol Cell Proteomics. 2003;2:1177–1187. doi: 10.1074/mcp.M300038-MCP200. [DOI] [PubMed] [Google Scholar]
- 45.Minamoto A, Yamane K, Yokoyama T. Proteomics of vitreous fluid. In: Thongboonkerd V, editor. Proteomics of human body fluids. 1. Totowa: Humana Press; 2007. pp. 495–507. [Google Scholar]
- 46.Shitama T, Hayashi H, Noge S, et al. Proteome profiling of vitreoretinal diseases by cluster analysis. Proteomics Clin Appl. 2008;2:1265–1280. doi: 10.1002/prca.200800017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SJ, Kim SJ, Park J, et al. Differential expression of vitreous proteins in proliferative diabetic retinopathy. Curr Eye Res. 2006;31:231–240. doi: 10.1080/02713680600557030. [DOI] [PubMed] [Google Scholar]
- 48.García-Ramírez M, Canals F, Hernández C, et al. Proteomic analysis of human vitreous fluid by fluorescence-based difference gel electrophoresis (DIGE): a new strategy for identifying potential candidates in the pathogenesis of proliferative diabetic retinopathy. Diabetologia. 2007;50:1294–1303. doi: 10.1007/s00125-007-0627-y. [DOI] [PubMed] [Google Scholar]
- 49.Simó R, Higuera M, García-Ramírez M, et al. Elevation of apolipoprotein A-I and apolipoprotein H levels in the vitreous fluid and overexpression in the retina of diabetic patients. Arch Ophthalmol. 2008;126:1076–1081. doi: 10.1001/archopht.126.8.1076. [DOI] [PubMed] [Google Scholar]
- 50.Ouchi M, West K, Crabb JW, et al. Proteomic analysis of vitreous from diabetic macular edema. Exp Eye Res. 2005;81:176–182. doi: 10.1016/j.exer.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Hernández C, García-Ramírez M, Colomé N, et al. Identification of new pathogenic candidates for diabetic macular edema using fluorescence-based difference gel electrophoresis analysis. Diabetes Metab Res Rev. 2013;29:499–506. doi: 10.1002/dmrr.2419. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Feng L, Hu J, et al. Characterisation of the vitreous proteome in proliferative diabetic retinopathy. Proteome Sci. 2012;10:1–11. doi: 10.1186/1477-5956-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao BB, Clermont A, Rook S, et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007;13:181–188. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- 54.Gao B-B, Chen X, Timothy N, et al. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res. 2008;7:2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- 55.Funatsu H, Yamashita H, Nakamura S, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2006;113:294–301. doi: 10.1016/j.ophtha.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 56.Funatsu H, Noma H, Mimura T, et al. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116:73–79. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 57.Rocha AS, Santos FM, Monteiro JP, et al. Trends in proteomic analysis of human vitreous humor samples. Electrophoresis. 2014;35:2495–2508. doi: 10.1002/elps.201400049. [DOI] [PubMed] [Google Scholar]
- 58.Santos FM, Albuquerque T, Gaspar LM, et al. Refinement of two-dimensional electrophoresis for vitreous proteome profiling using an artificial neural network. Anal Bioanal Chem. 2019;411:5115–5126. doi: 10.1007/s00216-019-01887-y. [DOI] [PubMed] [Google Scholar]
- 59.Kim K, Kim SJ, Yu HG, et al. Verification of biomarkers for diabetic retinopathy by multiple reaction monitoring. J Proteome Res. 2010;9:689–699. doi: 10.1021/pr901013d. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Feng L, Hu J, et al. Differentiating vitreous proteomes in proliferative diabetic retinopathy using high-performance liquid chromatography coupled to tandem mass spectrometry. Exp Eye Res. 2013;108:110–119. doi: 10.1016/j.exer.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Lu Q, Lu P. Quantitative proteomics analysis of vitreous body from type 2 diabetic patients with proliferative diabetic retinopathy. BMC Ophthalmol. 2018;18:151. doi: 10.1186/s12886-018-0821-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balaiya S, Zhou Z, Chalam KV. Characterization of vitreous and aqueous proteome in humans with proliferative diabetic retinopathy and its clinical correlation. Proteomics Insights. 2017;8:1–10. doi: 10.1177/1178641816686078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schori C, Trachsel C, Grossmann J, et al. The proteomic landscape in the vitreous of patients with age-related and diabetic retinal disease. Invest Ophthalmol Vis Sci. 2018;59:31–40. doi: 10.1167/iovs.18-24122. [DOI] [PubMed] [Google Scholar]
- 64.Loukovaara S, Nurkkala H, Tamene F, et al. Quantitative proteomics analysis of vitreous humor from diabetic retinopathy patients. J Proteome Res. 2015;14:5131–5143. doi: 10.1021/acs.jproteome.5b00900. [DOI] [PubMed] [Google Scholar]
- 65.Gardner TW, Sundstrom JM. A proposal for early and personalized treatment of diabetic retinopathy based on clinical pathophysiology and molecular phenotyping. Vision Res. 2017;139:153–160. doi: 10.1016/j.visres.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim T, Sang JK, Kim K, et al. Profiling of vitreous proteomes from proliferative diabetic retinopathy and nondiabetic patients. Proteomics. 2007;7:4203–4215. doi: 10.1002/pmic.200700745. [DOI] [PubMed] [Google Scholar]
- 67.Kita T, Clermont AC, Murugesan N, et al. Plasma kallikrein-kinin system as a VEGF-independent mediator of diabetic macular edema. Diabetes. 2015;64:3588–3599. doi: 10.2337/db15-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou C, Han C, Zhao M, et al. Change of ranibizumab-induced human vitreous protein profile in patients with proliferative diabetic retinopathy based on proteomics analysis. Clin Proteomics. 2018;15:1–10. doi: 10.1186/s12014-018-9187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koskela UE, Kuusisto SM, Nissinen AE, et al. High vitreous concentration of IL-6 and IL-8, but not of adhesion molecules in relation to plasma concentrations in proliferative diabetic retinopathy. Ophthalmic Res. 2013;49:108–114. doi: 10.1159/000342977. [DOI] [PubMed] [Google Scholar]
- 70.Klaassen I, de Vries EW, Vogels IMC, et al. Identification of proteins associated with clinical and pathological features of proliferative diabetic retinopathy in vitreous and fibrovascular membranes. PLoS ONE. 2017;12:1–21. doi: 10.1371/journal.pone.0187304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Srividya G, Jain M, Mahalakshmi K, et al. A novel and less invasive technique to assess cytokine profile of vitreous in patients of diabetic macular oedema. Eye. 2018;32:820–829. doi: 10.1038/eye.2017.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Chen S, Jiang F, et al. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mesquita J, Castro-de-Sousa JP, Vaz-Pereira S, et al. Evaluation of the growth factors VEGF-a and VEGF-B in the vitreous and serum of patients with macular and retinal vascular diseases. Growth Factors. 2018;36:48–57. doi: 10.1080/08977194.2018.1477140. [DOI] [PubMed] [Google Scholar]
- 74.Mesquita J, Castro de Sousa J, Vaz-Pereira S, et al. VEGF-B levels in the vitreous of diabetic and non-diabetic patients with ocular diseases and its correlation with structural parameters. Med Sci. 2017;5:17. doi: 10.3390/medsci5030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Y, Singh RP. The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context. 2018;7:1–10. doi: 10.7573/dic.212532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arrigo A, Aragona E, Bandello F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann Med. 2022;54:1089–1111. doi: 10.1080/07853890.2022.2064541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bressler SB, Liu D, Glassman AR, et al. Change in diabetic retinopathy through 2 years secondary analysis of a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab. JAMA Ophthalmol. 2017;135:558–568. doi: 10.1001/jamaophthalmol.2017.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cai S, Bressler NM. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang W, Qu X, Chen B, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;133:48–61. doi: 10.1161/CIRCULATIONAHA.115.017472.Critical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hernández C, Burgos R, Cantón A, et al. Vitreous levels of vascular cell adhesion molecule and vascular endothelial growth factor in patients with proliferative diabetic retinopathy: a case-control study. Diabetes Care. 2001;24:516–521. doi: 10.2337/diacare.24.3.516. [DOI] [PubMed] [Google Scholar]
- 81.Chen W, Lu Q, Lu L, Guan H. Increased levels of alphaB-crystallin in vitreous fluid of patients with proliferative diabetic retinopathy and correlation with vascular endothelial growth factor. Clin Exp Ophthalmol. 2017;45:379–384. doi: 10.1111/ceo.12891. [DOI] [PubMed] [Google Scholar]
- 82.Ishizaki E, Takai S, Ueki M, et al. Correlation between angiotensin-converting enzyme, vascular endothelial growth factor, and matrix metalloproteinase-9 in the vitreous of eyes with diabetic retinopathy. Am J Ophthalmol. 2006 doi: 10.1016/j.ajo.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 83.Abu El-Asrar AM, Mohammad G, Nawaz MI, et al. Relationship between vitreous levels of matrix metalloproteinases and vascular endothelial growth factor in proliferative diabetic retinopathy. PLoS ONE. 2013;8:1–11. doi: 10.1371/journal.pone.0085857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Izuta H, Chikaraishi Y, Adachi T, et al. Extracellular SOD and VEGF are increased in vitreous bodies from proliferative diabetic retinopathy patients. Mol Vis. 2009;15:2663–2672. [PMC free article] [PubMed] [Google Scholar]
- 85.Izuta H, Matsunaga N, Shimazawa M, et al. Proliferative diabetic retinopathy and relations among antioxidant activity, oxidative stress, and VEGF in the vitreous body. Mol Vis. 2010;16:130–136. [PMC free article] [PubMed] [Google Scholar]
- 86.Brzović-Šarić V, Landeka I, Šarić B, et al. Levels of selected oxidative stress markers in the vitreous and serum of diabetic retinopathy patients. Mol Vis. 2015;21:649–664. [PMC free article] [PubMed] [Google Scholar]
- 87.Funatsu H, Yamashita H, Nakanishi Y, Hori S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Br J Ophthalmol. 2002;86:311–315. doi: 10.1136/bjo.86.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whitehead M, Osborne A, Widdowson PS, et al. Angiopoietins in diabetic retinopathy: current understanding and therapeutic potential. J Diabetes Res. 2019;2019:1–9. doi: 10.1155/2019/5140521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joussen AM, Ricci F, Paris LP, et al. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye. 2021;35:1305–1316. doi: 10.1038/s41433-020-01377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sahni J, Patel SS, Dugel PU, et al. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-a with faricimab in diabetic macular edema. Ophthalmology. 2019;126:1155–1170. doi: 10.1016/j.ophtha.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 91.Nicolò M, Ferro Desideri L, Vagge A, Traverso CE. Faricimab: an investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert Opin Investig Drugs. 2021;30:193–200. doi: 10.1080/13543784.2021.1879791. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe D, Suzuma K, Suzuma I, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005;139:476–481. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Huber M, Wachtlin J. Vitreous levels of proteins implicated in angiogenesis are modulated in patients with retinal or choroidal neovascularization. Ophthalmologica. 2012;228:188–193. doi: 10.1159/000339952. [DOI] [PubMed] [Google Scholar]
- 94.Patel JI, Hykin PG, Gregor ZJ, et al. Angiopoietin concentrations in diabetic retinopathy. Br J Ophthalmol. 2005;89:480–483. doi: 10.1136/bjo.2004.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loukovaara S, Robciuc A, Holopainen JM, et al. Ang-2 up-regulation correlates with increased levels of MMP-9, VEGF, EPO and TGFβ1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2013;91:531–539. doi: 10.1111/j.1755-3768.2012.02473.x. [DOI] [PubMed] [Google Scholar]
- 96.Simó R, Lecube A, Segura RM, et al. Free insulin growth factor-I and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2002;134:376–382. doi: 10.1016/s0002-9394(02)01538-6. [DOI] [PubMed] [Google Scholar]
- 97.Al Kahtani E, Xu Z, Al Rashaed S, et al. Vitreous levels of placental growth factor correlate with activity of proliferative diabetic retinopathy and are not influenced by bevacizumab treatment. Eye. 2017;31:529–536. doi: 10.1038/eye.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suzuki Y, Nakazawa M, Suzuki K, et al. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol. 2011;55:256–263. doi: 10.1007/s10384-011-0004-8. [DOI] [PubMed] [Google Scholar]
- 99.Kovacs K, Marra KV, Yu G, et al. Angiogenic and inflammatory vitreous biomarkers associated with increasing levels of retinal ischemia. Investig Ophthalmol Vis Sci. 2015;56:6523–6530. doi: 10.1167/iovs.15-16793. [DOI] [PubMed] [Google Scholar]
- 100.Praidou A, Klangas I, Papakonstantinou E, et al. Vitreous and serum levels of platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res. 2009;34:152–161. doi: 10.1080/02713680802585920. [DOI] [PubMed] [Google Scholar]
- 101.Patel JI, Tombran-Tink J, Hykin PG, et al. Vitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: Implications for structural differences in macular profiles. Exp Eye Res. 2006;82:798–806. doi: 10.1016/j.exer.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 102.Munk MR, Somfai GM, De Smet MD, et al. The role of intravitreal corticosteroids in the treatment of DME: predictive OCT biomarkers. Int J Mol Sci. 2022;23:7585. doi: 10.3390/ijms23147585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chawan-Saad J, Wu M, Wu A, Wu L. Corticosteroids for diabetic macular edema. Taiwan J Ophthalmol. 2019;9:233. doi: 10.4103/tjo.tjo_68_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wallsh JO, Gallemore RP. Anti-VEGF-resistant retinal diseases: a review of the latest treatment options. Cells. 2021;10:1049. doi: 10.3390/cells10051049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mysona BA, Matragoon S, Stephens M, et al. Imbalance of the nerve growth factor and its precursor as a potential biomarker for diabetic retinopathy. Biomed Res Int. 2015;2015:1–12. doi: 10.1155/2015/571456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boss JD, Singh PK, Pandya HK, et al. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Investig Opthalmology Vis Sci. 2017;58:5594. doi: 10.1167/iovs.17-21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoshida S, Kubo Y, Kobayashi Y, et al. Increased vitreous concentrations of MCP-1 and IL-6 after vitrectomy in patients with proliferative diabetic retinopathy: possible association with post-operative macular oedema. Br J Ophthalmol. 2015;99:960–966. doi: 10.1136/bjophthalmol-2014-306366. [DOI] [PubMed] [Google Scholar]
- 108.Deuchler S, Schubert R, Singh P, et al. Vitreous expression of cytokines and growth factors in patients with diabetic retinopathy—an investigation of their expression based on clinical diabetic retinopathy grade. PLoS ONE. 2021;16:e0248439. doi: 10.1371/journal.pone.0248439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuo CYJ, Murphy R, Rupenthal ID, Mugisho OO. Correlation between the progression of diabetic retinopathy and inflammasome biomarkers in vitreous and serum—a systematic review. BMC Ophthalmol. 2022;22:1–13. doi: 10.1186/s12886-022-02439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minaker SA, Mason RH, Lahaie Luna G, et al. Changes in aqueous and vitreous inflammatory cytokine levels in diabetic macular oedema: a systematic review and meta-analysis. Acta Ophthalmol. 2022;100:e53–e70. doi: 10.1111/aos.14891. [DOI] [PubMed] [Google Scholar]
- 111.Funatsu H, Yamashita H, Sakata K, et al. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology. 2005;112:806–816. doi: 10.1016/j.ophtha.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 112.Yenihayat F, Özkan B, Kasap M, et al. Vitreous IL-8 and VEGF levels in diabetic macular edema with or without subretinal fluid. Int Ophthalmol. 2018 doi: 10.1007/s10792-018-0874-6. [DOI] [PubMed] [Google Scholar]
- 113.Noma H, Funatsu H, Yamasaki M, et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye. 2008;22:42–48. doi: 10.1038/sj.eye.6702498. [DOI] [PubMed] [Google Scholar]
- 114.Yoshimura T, Sonoda K-HH, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS ONE. 2009;4:e8158. doi: 10.1371/journal.pone.0008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kimura K, Orita T, Kobayashi Y, et al. Concentration of acute phase factors in vitreous fluid in diabetic macular edema. Jpn J Ophthalmol. 2017;61:479–483. doi: 10.1007/s10384-017-0525-x. [DOI] [PubMed] [Google Scholar]
- 116.Ardeljan D, Chan CC. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res. 2013;37:68–89. doi: 10.1016/j.preteyeres.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 118.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Heal. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 119.Rudnicka AR, Jarrar Z, Wormald R, et al. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119:571–580. doi: 10.1016/j.ophtha.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 120.Age-Related Eye Disease Study Research Group Risk factors for the incidence of advanced age-related macular degeneration in the age-related eye disease study (AREDS) Ophthalmology. 2005;112:533–539.e1. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lambert NG, ElShelmani H, Singh MK, et al. Risk factors and biomarkers of age-related macular degeneration. Prog Retin Eye Res. 2016;54:64–102. doi: 10.1016/j.preteyeres.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Apte RS. Age-related macular degeneration. N Engl J Med. 2021;385:539–547. doi: 10.1056/NEJMcp2102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 125.Thomas CJ, Mirza RG, Gill MK. Age-related macular degeneration. Med Clin North Am. 2021;105:473–491. doi: 10.1016/j.mcna.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 126.Age-Related Eye Disease Study Research Group The age-related eye disease study severity scale for age-related macular degeneration. Arch Ophthalmol. 2005;123:1484. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Coleman HR, Chan C-C, Ferris FL, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klein ML, Ferris FL, Armstrong J, et al. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115:1026–1031. doi: 10.1016/j.ophtha.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 129.Richer S, Ulanski L, Popenko NA, et al. Age-related macular degeneration beyond the age-related eye disease study II. Adv Ophthalmol Optom. 2016;1:335–369. doi: 10.1016/j.yaoo.2016.03.018. [DOI] [Google Scholar]
- 130.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seddon JM. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 132.Feng L, Nie K, Jiang H, Fan W. Effects of lutein supplementation in age-related macular degeneration. PLoS ONE. 2019;14:1–13. doi: 10.1371/journal.pone.0227048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chew EY, Clemons TE, Agrón E, et al. Effect of omega-3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function. JAMA. 2015;314:791. doi: 10.1001/jama.2015.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lem DW, Davey PG, Gierhart DL, Rosen RB. A systematic review of carotenoids in the management of age-related macular degeneration. Antioxidants. 2021;10:1–37. doi: 10.3390/antiox10081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cabral De Guimaraes TA, Daich Varela M, Georgiou M, Michaelides M. Treatments for dry age-related macular degeneration: therapeutic avenues, clinical trials and future directions. Br J Ophthalmol. 2022;106:297–304. doi: 10.1136/bjophthalmol-2020-318452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thomas CN, Sim DA, Lee WH, et al. Emerging therapies and their delivery for treating age-related macular degeneration. Br J Pharmacol. 2022;179:1908–1937. doi: 10.1111/bph.15459. [DOI] [PubMed] [Google Scholar]
- 137.Stern JH, Tian Y, Funderburgh J, et al (2018) Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 22:834–849. DOI: 10.1016/j.stem.2018.05.013 [DOI] [PMC free article] [PubMed]
- 138.Rizzolo LJ, Nasonkin IO, Adelman RA. Retinal cell transplantation, biomaterials, and in vitro models for developing next-generation therapies of age-related macular degeneration. Stem Cells Transl Med. 2022;11:269–281. doi: 10.1093/stcltm/szac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yeong JL, Loveman E, Colquitt JL, et al. Visual cycle modulators versus placebo or observation for the prevention and treatment of geographic atrophy due to age-related macular degeneration. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD013154.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wong WT, Dresner S, Forooghian F, et al. Treatment of geographic atrophy with subconjunctival sirolimus: results of a phase I/II clinical trial. Investig Opthalmology Vis Sci. 2013;54:2941. doi: 10.1167/iovs.13-11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boyer DS, Gonzalez VH, Kunimoto DY, et al. Safety and efficacy of intravitreal risuteganib for non-exudative AMD: a multicenter, phase 2a, randomized, clinical trial. Ophthalmic Surgery, Lasers Imaging Retin. 2021;52:327–335. doi: 10.3928/23258160-20210528-05. [DOI] [PubMed] [Google Scholar]
- 142.Wu J, Sun X. Complement system and age-related macular degeneration: drugs and challenges. Drug Des Devel Ther. 2019;13:2413–2425. doi: 10.2147/DDDT.S206355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kauper K, McGovern C, Sherman S, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Investig Opthalmology Vis Sci. 2012;53:7484. doi: 10.1167/iovs.12-9970. [DOI] [PubMed] [Google Scholar]
- 144.Sharma A, Parachuri N, Kumar N, et al. Terms non-exudative and non-neovascular: awaiting entry at the doors of AMD reclassification. Graefe’s Arch Clin Exp Ophthalmol. 2021;259:1381–1383. doi: 10.1007/s00417-021-05164-6. [DOI] [PubMed] [Google Scholar]
- 145.Spaide RF, Jaffe GJ, Sarraf D, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–636. doi: 10.1016/j.ophtha.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yonekawa Y, Miller J, Kim I. Age-related macular degeneration: advances in management and diagnosis. J Clin Med. 2015;4:343–359. doi: 10.3390/jcm4020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tsai ASH, Cheung N, Gan ATL, et al. Retinal angiomatous proliferation. Surv Ophthalmol. 2017;62:462–492. doi: 10.1016/j.survophthal.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 148.Yannuzzi LA, Negrão S, Iida T, et al. Retinal angiomatous proliferation in age–related macular degeneration. Retina. 2012;32:416–434. doi: 10.1097/IAE.0b013e31823f9b3b. [DOI] [PubMed] [Google Scholar]
- 149.Sivaprasad S, Hykin P. What is new in the management of wet age-related macular degeneration? Br Med Bull. 2013;105:201–211. doi: 10.1093/bmb/ldt004. [DOI] [PubMed] [Google Scholar]
- 150.Chaili S, D. Adrean S (2020) Management strategies and visual results for the treatment of neovascular age-related macular degeneration. In: Visual impairment and blindness—what we know and what we have to know. IntechOpen, p 13
- 151.Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–740. doi: 10.1016/S0140-6736(22)00010-1. [DOI] [PubMed] [Google Scholar]
- 152.Fernandes AR, Zielińska A, Sanchez-Lopez E, et al. Exudative versus nonexudative age-related macular degeneration: physiopathology and treatment options. Int J Mol Sci. 2022;23:2592. doi: 10.3390/ijms23052592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.De Guimaraes TAC, Georgiou M, Bainbridge JWB, Michaelides M. Gene therapy for neovascular age-related macular degeneration: rationale, clinical trials and future directions. Br J Ophthalmol. 2021;105:151–157. doi: 10.1136/bjophthalmol-2020-316195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fritsche LG, Fariss RN, Stambolian D, et al. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151–171. doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hernández-Zimbrón LF, Zamora-Alvarado R, Ochoa-De la Paz L, et al. Age-related macular degeneration: new paradigms for treatment and management of AMD. Oxid Med Cell Longev. 2018;2018:1–14. doi: 10.1155/2018/8374647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koss MJ, Hoffmann J, Nguyen N, et al. Proteomics of vitreous humor of patients with exudative age-related macular degeneration. PLoS ONE. 2014;9:1–11. doi: 10.1371/journal.pone.0096895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nobl M, Reich M, Dacheva I, et al. Proteomics of vitreous in neovascular age-related macular degeneration. Exp Eye Res. 2016;146:107–117. doi: 10.1016/j.exer.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 158.Amadio M, Govoni S, Pascale A. Targeting VEGF in eye neovascularization: What’s new?: A comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol Res. 2016;103:253–269. doi: 10.1016/j.phrs.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 159.Funk M, Karl D, Georgopoulos M, et al. Neovascular age-related macular degeneration: intraocular cytokines and growth factors and the influence of therapy with ranibizumab. Ophthalmology. 2009;116:2393–2399. doi: 10.1016/j.ophtha.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 160.Holekamp NM, Bouck N, Volpert O. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am J Ophthalmol. 2002;134:220–227. doi: 10.1016/S0002-9394(02)01549-0. [DOI] [PubMed] [Google Scholar]
- 161.Duh EJ, Yang HS, Haller JA, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor: implications for ocular angiogenesis. Am J Ophthalmol. 2004;137:668–674. doi: 10.1016/j.ajo.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 162.Koss MJ, Pfister M, Koch FH. Inflammatory and angiogenic protein detection in the human vitreous: cytometric bead assay. J Ophthalmol. 2011;2011:1–4. doi: 10.1155/2011/459251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao M, Bai Y, Xie W, et al. Interleukin-1β level is increased in vitreous of patients with neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV) PLoS ONE. 2015;10:e0125150. doi: 10.1371/journal.pone.0125150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bai Y, Liang S, Yu W, et al. Semaphorin 3A blocks the formation of pathologic choroidal neovascularization induced by transforming growth factor beta. Mol Vis. 2014;20:1258–1270. [PMC free article] [PubMed] [Google Scholar]
- 165.Ecker SM, Pfahler SM, Hines JC, et al. Sequential in-office vitreous aspirates demonstrate vitreous matrix metalloproteinase-9 levels correlate with the amount of subretinal fluid in eyes with wet age-related macular degeneration. Mol Vis. 2012;18:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 166.Loyet KM, DeForge LE, Katschke KJ, et al. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Investig Ophthalmol Vis Sci. 2012;53:6628–6637. doi: 10.1167/iovs.12-9587. [DOI] [PubMed] [Google Scholar]
- 167.Idrees S, Sridhar J, Kuriyan AE. Proliferative vitreoretinopathy: a review. Int Ophthalmol Clin. 2019;59:221–240. doi: 10.1097/IIO.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Garweg JG, Tappeiner C, Halberstadt M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Surv Ophthalmol. 2013;58:321–329. doi: 10.1016/j.survophthal.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 169.Rodríguez De La Rúa E, Pastor JC, Aragón J, et al. Interaction between surgical procedure for repairing retinal detachment and clinical risk factors for proliferative vitreoretinopathy. Curr Eye Res. 2005;30:147–153. doi: 10.1080/02713680490904142. [DOI] [PubMed] [Google Scholar]
- 170.Kon CH, Tranos P, Aylward GW. Vitreo-retinal surgery. Berlin/Heidelberg: Springer-Verlag; 2005. Risk factors in proliferative vitreoretinopathy; pp. 121–134. [Google Scholar]
- 171.Wiedemann P, Yandiev Y, Hui Y-N, Wang Y. Pathogenesis of proliferative vitreoretinopathy. In: Ryan SJ, Hinton DR, Wiedemann P, editors. Retina. 5. Elsevier; 2013. pp. 1640–1646. [Google Scholar]
- 172.Kwon OW, Song JH, Roh MI, Song JH (2010) Retinal detachment and proliferative vitreoretinopathy. In: Retinal pharmacotherapy, 1st edn. Elsevier, pp 154–162 [DOI] [PubMed]
- 173.Tamiya S, Liu LH, Kaplan HJ. Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Investig Ophthalmol Vis Sci. 2010;51:2755–2763. doi: 10.1167/iovs.09-4725. [DOI] [PubMed] [Google Scholar]
- 174.Sethi CS, Lewis GP, Fisher SK, et al. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Investig Ophthalmol Vis Sci. 2005;46:329–342. doi: 10.1167/iovs.03-0518. [DOI] [PubMed] [Google Scholar]
- 175.Guidry C (2010) Proliferative vitreoretinopathy. In: Ocular disease, 2nd edn. Elsevier, pp 612–617
- 176.Popovic MM, Muni RH, Nichani P, Kertes PJ. Pars plana vitrectomy, scleral buckle, and pneumatic retinopexy for the management of rhegmatogenous retinal detachment: a meta-analysis. Surv Ophthalmol. 2022;67:184–196. doi: 10.1016/j.survophthal.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 177.Savur F, Aydemir O, İlhan N. The effect of infliximab and octreotide on cytokine levels experimental proliferative vitreoretinopathy. Cutan Ocul Toxicol. 2020;39:61–66. doi: 10.1080/15569527.2019.1701000. [DOI] [PubMed] [Google Scholar]
- 178.Tikhonovich MV, Erdiakov AK, Gavrilova SA. Nonsteroid anti-inflammatory therapy suppresses the development of proliferative vitreoretinopathy more effectively than a steroid one. Int Ophthalmol. 2018;38:1365–1378. doi: 10.1007/s10792-017-0594-3. [DOI] [PubMed] [Google Scholar]
- 179.Schaub F, Hoerster R, Schiller P, et al. Prophylactic intravitreal 5-fluorouracil and heparin to prevent proliferative vitreoretinopathy in high-risk patients with retinal detachment: study protocol for a randomized controlled trial. Trials. 2018;19:384. doi: 10.1186/s13063-018-2761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nourinia R, Borna F, Rahimi A, et al. Repeated injection of methotrexate into silicone oil-filled eyes for grade c proliferative vitreoretinopathy: a pilot study. Ophthalmologica. 2019;242:113–117. doi: 10.1159/000500271. [DOI] [PubMed] [Google Scholar]
- 181.Ghasemi Falavarjani K, Hashemi M, Modarres M, Hadavand Khani A. Intrasilicone oil injection of bevacizumab at the end of retinal reattachment surgery for severe proliferative vitreoretinopathy. Eye. 2014;28:576–580. doi: 10.1038/eye.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Abdullatif AM, Macky TA, Abdullatif MM, et al. Intravitreal decorin preventing proliferative vitreoretinopathy in perforating injuries: a pilot study. Graefe’s Arch Clin Exp Ophthalmol. 2018;256:2473–2481. doi: 10.1007/s00417-018-4105-7. [DOI] [PubMed] [Google Scholar]
- 183.Lei H, Velez G, Cui J, et al. N-acetylcysteine suppresses retinal detachment in an experimental model of proliferative vitreoretinopathy. Am J Pathol. 2010;177:132–140. doi: 10.2353/ajpath.2010.090604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Aldeyra Therapeutics (2022) ClinicalTrials.gov Identifier: NCT04136366. The GUARD Trial - Part 1: A Phase 3 Clinical Trial for Prevention of Proliferative Vitreoretinopathy Stress. https://clinicaltrials.gov/ct2/show/NCT04136366. Accessed 31 Jul 2022
- 185.Zahra Rabbani Khah, Beheshti S (2020) ClinicalTrials.gov Identifier: NCT04482543. Repeated Methotrexate for Proliferative Vitreoretinopathy Grade C. https://clinicaltrials.gov/ct2/show/NCT04482543. Accessed 31 Jul 2022
- 186.Roca JA, Yon-Mendoza A, Huamán N, Wu L. Adjunctive serial post-operative intravitreal methotrexate injections in the management of advanced proliferative vitreoretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2021;259:2913–2917. doi: 10.1007/s00417-021-05206-z. [DOI] [PubMed] [Google Scholar]
- 187.Chen G, Li T, Zheng Q, et al. Differential expression and significance of complement C4b and transthyretin in proliferative vitreoretinopathy. Chinese J Ophthalmol. 2011;47:726–731. [PubMed] [Google Scholar]
- 188.Yu J, Liu F, Cui SJ, et al. Vitreous proteomic analysis of proliferative vitreoretinopathy. Proteomics. 2008;8:3667–3678. doi: 10.1002/pmic.200700824. [DOI] [PubMed] [Google Scholar]
- 189.Yu J, Peng R, Chen H, et al. Elucidation of the pathogenic mechanism of rhegmatogenous retinal detachment with proliferative vitreoretinopathy by proteomic analysis. Investig Ophthalmol Vis Sci. 2012;53:8146–8153. doi: 10.1167/iovs.12-10079. [DOI] [PubMed] [Google Scholar]
- 190.Yu J, Peng R, Chen H, et al. Kininogen 1 and insulin-like growth factor binding protein 6: candidate serum biomarkers of proliferative vitreoretinopathy. Clin Exp Optom. 2014;97:72–79. doi: 10.1111/cxo.12088. [DOI] [PubMed] [Google Scholar]
- 191.Santos F, Gaspar L, Ciordia S, et al. iTRAQ quantitative proteomic analysis of vitreous from patients with retinal detachment. Int J Mol Sci. 2018;19:1–22. doi: 10.3390/ijms19041157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Öhman T, Gawriyski L, Miettinen S, et al. Molecular pathogenesis of rhegmatogenous retinal detachment. Sci Rep. 2021;11:1–15. doi: 10.1038/s41598-020-80005-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Banerjee S, Savant V, Scott RAHH, et al. Multiplex bead analysis of vitreous humor of patients with vitreoretinal disorders. Investig Ophthalmol Vis Sci. 2007;48:2203–2207. doi: 10.1167/iovs.06-1358. [DOI] [PubMed] [Google Scholar]
- 194.Roybal CN, Velez G, Toral MA, et al. Personalized proteomics in proliferative vitreoretinopathy implicate hematopoietic cell recruitment and mTOR as a therapeutic target. Am J Ophthalmol. 2018;186:152–163. doi: 10.1016/j.ajo.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wladis EJ, Falk NS, Iglesias BV, et al. Analysis of the molecular biologic milieu of the vitreous in proliferative vitreoretinopathy. Retina. 2013;33:807–811. doi: 10.1097/IAE.0b013e31826d350a. [DOI] [PubMed] [Google Scholar]
- 196.Symeonidis C, Papakonstantinou E, Androudi S, et al. Interleukin-6 and the matrix metalloproteinase response in the vitreous during proliferative vitreoretinopathy. Cytokine. 2011;54:212–217. doi: 10.1016/j.cyto.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 197.Canataroglu H, Varinli I, Ozcan AA, et al. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocul Immunol Inflamm. 2005;13:375–381. doi: 10.1080/09273940490518900. [DOI] [PubMed] [Google Scholar]
- 198.Citirik M, Kabatas EU, Batman C, et al. Vitreous vascular endothelial growth factor concentrations in proliferative diabetic retinopathy versus proliferative vitreoretinopathy. Ophthalmic Res. 2011;47:7–12. doi: 10.1159/000324200. [DOI] [PubMed] [Google Scholar]
- 199.Sydorova M, Lee MS. Vascular endothelial growth factor levels in vitreous and serum of patients with either proliferative diabetic retinopathy or proliferative vitreoretinopathy. Ophthalmic Res. 2005;37:188–190. doi: 10.1159/000086594. [DOI] [PubMed] [Google Scholar]
- 200.Moysidis SN, Thanos A, Vavvas DG. Mechanisms of inflammation in proliferative vitreoretinopathy: from bench to bedside. Mediators Inflamm. 2012 doi: 10.1155/2012/815937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Morescalchi F, Duse S, Gambicorti E, et al. Proliferative vitreoretinopathy after eye injuries: an overexpression of growth factors and cytokines leading to a retinal keloid. Mediators Inflamm. 2013 doi: 10.1155/2013/269787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ricker LJAG, Dieudonné SC, Kessels AGH, et al. Antiangiogenic isoforms of vascular endothelial growth factor predominate in subretinal fluid of patients with rhegmatogenous retinal detachment and proliferative vitreoretinopathy. Retina. 2012;32:54–59. doi: 10.1097/IAE.0b013e31821800b9. [DOI] [PubMed] [Google Scholar]
- 203.Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:607–613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 205.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86:363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Whitcup SM, Sodhi A, Atkinson JP, et al. The role of the immune response in age-related macular degeneration. Int J Inflam. 2013 doi: 10.1155/2013/348092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chaudhary R, Scott RAH, Wallace G, et al. Inflammatory and fibrogenic factors in proliferative vitreoretinopathy development. Transl Vis Sci Technol. 2020;9:1–17. doi: 10.1167/tvst.9.3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dai Y, Dai C, Sun T. Inflammatory mediators of proliferative vitreoretinopathy: hypothesis and review. Int Ophthalmol. 2020;40:1587–1601. doi: 10.1007/s10792-020-01325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shaw PX, Stiles T, Douglas C, et al. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci. 2016;3:196–221. doi: 10.3934/molsci.2016.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Capitão M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem. 2016 doi: 10.1002/jcb.25575. [DOI] [PubMed] [Google Scholar]
- 213.Wang X, Ma W, Han S, et al. TGF-β participates choroid neovascularization through Smad2/3-VEGF/TNF-α signaling in mice with Laser-induced wet age-related macular degeneration. Sci Rep. 2017;7:9672. doi: 10.1038/s41598-017-10124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fernando N, Natoli R, Valter K, et al. The broad-spectrum chemokine inhibitor NR58-3.14.3 modulates macrophage-mediated inflammation in the diseased retina. J Neuroinflammation. 2016;13:1–14. doi: 10.1186/s12974-016-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rashid K, Akhtar-Schaefer I, Langmann T. Microglia in retinal degeneration. Front Immunol. 2019;10:1–19. doi: 10.3389/fimmu.2019.01975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cunha-Vaz J. The blood-retinal barrier in retinal disease. Eur Ophthalmic Rev. 2009;3:105. doi: 10.17925/eor.2009.03.02.105. [DOI] [Google Scholar]
- 217.Campochiaro PA, Bryan JA, Conway BP, Jaccoma EH. Intravitreal chemotactic and mitogenic activity: implication of blood-retinal barrier breakdown. Arch Ophthalmol. 1986;104:1685–1687. doi: 10.1001/archopht.1986.01050230123046. [DOI] [PubMed] [Google Scholar]
- 218.Gilbert C, Gilbert C, Unger W, et al. Inflammation and the formation of epiretinal membranes. Eye. 1988;2:S140–S156. doi: 10.1038/eye.1988.140. [DOI] [PubMed] [Google Scholar]
- 219.Taylor AW. Ocular immune privilege. Eye. 2009;23:1885–1889. doi: 10.1038/eye.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xu J, Chen LJ, Yu J, et al. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell Physiol Biochem. 2018;48:705–717. doi: 10.1159/000491897. [DOI] [PubMed] [Google Scholar]
- 221.Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008;27:622–647. doi: 10.1016/j.preteyeres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 222.Rübsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19:1–31. doi: 10.3390/ijms19040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rangasamy S, McGuire PG, Nitta CF, et al. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS ONE. 2014;9:1–10. doi: 10.1371/journal.pone.0108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Harhaj NS, Felinski EA, Wolpert EB, et al. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Investig Ophthalmol Vis Sci. 2006;47:5106–5115. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- 225.Miyamoto K, Khosrof S, Bursell SE, et al. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1) Am J Pathol. 2000;156:1733–1739. doi: 10.1016/S0002-9440(10)65044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Aveleira CA, Lin CM, Abcouwer SF, et al. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–2882. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bamforth SD, Lightman SL, Greenwood J. Interleukin-1β-induced disruption of the retinal vascular barrier of the central nervous system is mediated through leukocyte recruitment and histamine. Am J Pathol. 1997;150:329–340. [PMC free article] [PubMed] [Google Scholar]
- 228.Haurigot V, Villacampa P, Ribera A, et al. Increased intraocular insulin-like growth factor-I triggers blood-retinal barrier breakdown. J Biol Chem. 2009;284:22961–22969. doi: 10.1074/jbc.M109.014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lu M, Perez VL, Ma N, et al. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci. 1999;40:1808–1812. [PubMed] [Google Scholar]
- 230.Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes. 2007;56:2380–2387. doi: 10.2337/db06-1694. [DOI] [PubMed] [Google Scholar]
- 231.Giebel SJ, Menicucci G, McGuire PG, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alternation of the blood-retinal barrier. Lab Investig. 2005;85:597–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- 232.Da Cunha AP, Zhang Q, Prentiss M, et al. The hierarchy of proinflammatory cytokines in ocular inflammation. Curr Eye Res. 2018;43:553–565. doi: 10.1080/02713683.2017.1410180. [DOI] [PubMed] [Google Scholar]
- 233.Liu X, Ye F, Xiong H, et al. IL-1β Induces IL-6 production in retinal Müller cells predominantly through the activation of P38 MAPK/NF-κB signaling pathway. Exp Cell Res. 2015;331:223–231. doi: 10.1016/j.yexcr.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 234.Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Halle A, Hornung V, Petzold GC, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liu RT, Gao J, Cao S, et al. Inflammatory mediators induced by amyloid-beta in the retina and RPE in vivo: implications for inflammasome activation in age-related macular degeneration. Investig Ophthalmol Vis Sci. 2013;54:2225–2237. doi: 10.1167/iovs.12-10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kutty RK, Samuel W, Boyce K, et al. Proinflammatory cytokines decrease the expression of genes critical for RPE function. Mol Vis. 2016;22:1156–1168. [PMC free article] [PubMed] [Google Scholar]
- 238.Wooff Y, Man SM, Aggio-Bruce R, et al. IL-1 family members mediate cell death, inflammation and angiogenesis in retinal degenerative diseases. Front Immunol. 2019;10:1–21. doi: 10.3389/fimmu.2019.01618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Karkhur S, Hasanreisoglu M, Vigil E, et al. Interleukin-6 inhibition in the management of non-infectious uveitis and beyond. J Ophthalmic Inflamm Infect. 2019 doi: 10.1186/s12348-019-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gerhardinger C, Costa MB, Coulombe MC, et al. Expression of acute-phase response proteins in retinal Müller cells in diabetes. Investig Ophthalmol Vis Sci. 2005;46:349–357. doi: 10.1167/iovs.04-0860. [DOI] [PubMed] [Google Scholar]
- 241.Ortiz G, Salica JP, Chuluyan EH, Gallo JE. Diabetic retinopathy: could the alpha-1 antitrypsin be a therapeutic option? Biol Res. 2014;47:1–9. doi: 10.1186/0717-6287-47-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Copland DA, Theodoropoulou S, Liu J, Dick AD. A perspective of AMD through the eyes of immunology. Investig Ophthalmol Vis Sci. 2018;59:AMD83–AMD92. doi: 10.1167/iovs.18-23893. [DOI] [PubMed] [Google Scholar]
- 243.Blum A, Pastukh N, Socea D, Jabaly H. Levels of adhesion molecules in peripheral blood correlat with stages of diabetic retinopathy and may serve as bio markers for microvascular complications. Cytokine. 2018;106:76–79. doi: 10.1016/j.cyto.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 244.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 245.Bharadwaj AS, Appukuttan B, Wilmarth PA, et al. Role of the retinal vascular endothelial cell in ocular disease. Prog Retin Eye Res. 2013;32:102–180. doi: 10.1016/j.preteyeres.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Barile GR, Chang SS, Park LS, et al. Soluble cellular adhesion molecules in proliferative vitreoretinopathy and proliferative diabetic retinopathy. Curr Eye Res. 1999;19:219–227. doi: 10.1076/ceyr.19.3.219.5314. [DOI] [PubMed] [Google Scholar]
- 247.Limb GA, Chignell AH. Vitreous levels of intercellular adhesion molecule 1 (ICAM-1) as a risk indicator of proliferative vitreoretinopathy. Br J Ophthalmol. 1999;83:953–956. doi: 10.1136/bjo.83.8.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mukai R, Okunuki Y, Husain D, et al. The complement system is critical in maintaining retinal integrity during aging. Front Aging Neurosci. 2018;10:1–12. doi: 10.3389/fnagi.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Clark SJ, Bishop PN. The eye as a complement dysregulation hotspot. Semin Immunopathol. 2018;40:65–74. doi: 10.1007/s00281-017-0649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Toomey CB, Johnson LV, Bowes Rickman C. Complement factor H in AMD: bridging genetic associations and pathobiology. Prog Retin Eye Res. 2018;62:38–57. doi: 10.1016/j.preteyeres.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Whitmore SS, Sohn EH, Chirco KR, et al. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015;45:1–29. doi: 10.1016/j.preteyeres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mullins RF, Schoo DP, Sohn EH, et al. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol. 2014;184:3142–3153. doi: 10.1016/j.ajpath.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Akhtar-Schäfer I, Wang L, Krohne TU, et al. Modulation of three key innate immune pathways for the most common retinal degenerative diseases. EMBO Mol Med. 2018;10:1–27. doi: 10.15252/emmm.201708259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yang MM, Wang J, Ren H, et al. Genetic investigation of complement pathway genes in type 2 diabetic retinopathy: an inflammatory perspective. Mediators Inflamm. 2016 doi: 10.1155/2016/1313027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Shahulhameed S, Vishwakarma S, Chhablani J, et al. A systematic investigation on complement pathway activation in diabetic retinopathy. Front Immunol. 2020;11:1–14. doi: 10.3389/fimmu.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wu Z, Ding N, Yu M, et al. Identification of potential biomarkers for rhegmatogenous retinal detachment associated with choroidal detachment by vitreous iTRAQ-based proteomic profiling. Int J Mol Sci. 2016;17:1–17. doi: 10.3390/ijms17122052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sweigard JH, Matsumoto H, Smith KE, et al. Inhibition of the alternative complement pathway preserves photoreceptors after retinal injury. Sci Transl Med. 2015;7:21–24. doi: 10.1126/scitranslmed.aab1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bastiaans J, van Meurs JC, Mulder VC, et al. The role of thrombin in proliferative vitreoretinopathy. Investig Ophthalmol Vis Sci. 2014;55:4659–4666. doi: 10.1167/iovs.14-14818. [DOI] [PubMed] [Google Scholar]
- 259.Bastiaans J, Van Meurs JC, Van Holten-Neelen C, et al. Factor Xa and thrombin stimulate proinflammatory and pro-fibrotic mediator production by retinal pigment epithelial cells: a role in vitreoretinal disorders? Graefe’s Arch Clin Exp Ophthalmol. 2013;251:1723–1733. doi: 10.1007/s00417-013-2335-2. [DOI] [PubMed] [Google Scholar]
- 260.Sato K, Takeda A, Hasegawa E, et al. Interleukin-6 plays a crucial role in the development of subretinal fibrosis in a mouse model. Immunol Med. 2018;41:23–29. doi: 10.1080/09114300.2018.1451609. [DOI] [PubMed] [Google Scholar]
- 261.Fielding CA, Jones GW, McLoughlin RM, et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40:40–50. doi: 10.1016/j.immuni.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pennock S, Rheaume MA, Mukai S, Kazlauskas A. A novel strategy to develop therapeutic approaches to prevent proliferative vitreoretinopathy. Am J Pathol. 2011;179:2931–2940. doi: 10.1016/j.ajpath.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Abu El-Asrar AM, Imtiaz Nawaz M, Kangave D, et al. Osteopontin and other regulators of angiogenesis and fibrogenesis in the vitreous from patients with proliferative vitreoretinal disorders. Mediators Inflamm. 2012;2012:1–8. doi: 10.1155/2012/493043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Van Geest RJ, Klaassen I, Lesnik-Oberstein SY, et al. Vitreous TIMP-1 levels associate with neovascularization and TGF-β2 levels but not with fibrosis in the clinical course of proliferative diabetic retinopathy. J Cell Commun Signal. 2013;7:1–9. doi: 10.1007/s12079-012-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mukherjee S, Guidry C. The insulin-like growth factor system modulates retinal pigment epithelial cell tractional force generation. Investig Ophthalmol Vis Sci. 2007;48:1892–1899. doi: 10.1167/iovs.06-1095. [DOI] [PubMed] [Google Scholar]
- 266.Zhang H, Liu ZL. Transforming growth factor-β neutralizing antibodies inhibit subretinal fibrosis in a mouse model. Int J Ophthalmol. 2012;5:307–311. doi: 10.3980/j.issn.2222-3959.2012.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Miller CG, Budoff G, Prenner JL, Schwarzbauer JE. Minireview: fibronectin in retinal disease. Exp Biol Med. 2017;242:1–7. doi: 10.1177/1535370216675245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shu DY, Lovicu FJ. Myofibroblast transdifferentiation: the dark force in ocular wound healing and fibrosis. Prog Retin Eye Res. 2017;60:44–65. doi: 10.1016/j.preteyeres.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kimura K, Orita T, Liu Y, et al. Attenuation of EMT in RPE cells and subretinal fibrosis by an RAR-γ agonist. J Mol Med. 2015;93:749–758. doi: 10.1007/s00109-015-1289-8. [DOI] [PubMed] [Google Scholar]
- 270.Kita T, Hata Y, Arita R, et al. Role of TGF-β in proliferative vitreoretinal diseases and ROCK as a therapeutic target. Proc Natl Acad Sci USA. 2008;105:17504–17509. doi: 10.1073/pnas.0804054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Choi K, Lee K, Ryu SW, et al. Pirfenidone inhibits transforming growth factor-β1-induced fibrogenesis by blocking nuclear translocation of smads in human retinal pigment epithelial cell line ARPE-19. Mol Vis. 2012;18:1010–1020. [PMC free article] [PubMed] [Google Scholar]
- 272.Yao H, Ge T, Zhang Y, et al. BMP7 antagonizes proliferative vitreoretinopathy through retinal pigment epithelial fibrosis in vivo and in vitro. FASEB J. 2019;33:3212–3224. doi: 10.1096/fj.201800858RR. [DOI] [PubMed] [Google Scholar]
- 273.Fan J, Shen W, Lee SR, et al. Targeting the Notch and TGF-β signaling pathways to prevent retinal fibrosis in vitro and in vivo. Theranostics. 2020;10:7956–7973. doi: 10.7150/thno.45192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Itoh Y, Kimoto K, Imaizumi M, Nakatsuka K. Inhibition of RhoA/Rho-kinase pathway suppresses the expression of type I collagen induced by TGF-β2 in human retinal pigment epithelial cells. Exp Eye Res. 2007;84:464–472. doi: 10.1016/j.exer.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 275.Dvashi Z, Goldberg M, Adir O, et al. TGF-β1 induced transdifferentiation of RPE cells is mediated by TAK1. PLoS ONE. 2015;10:1–16. doi: 10.1371/journal.pone.0122229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Khanum BNMK, Guha R, Sur VP, et al. Pirfenidone inhibits post-traumatic proliferative vitreoretinopathy. Eye. 2017;31:1317–1328. doi: 10.1038/eye.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Dvashi Z, Ben-Yaakov K, Weinberg T, et al. OM-101 decreases the fibrotic response associated with proliferative vitreoretinopathy. J Ophthalmol. 2017 doi: 10.1155/2017/1606854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Robbins SG, Mixon RN, Wilson DJ, et al. Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal diseases. Investig Ophthalmol Vis Sci. 1994;35:3649–3663. [PubMed] [Google Scholar]
- 279.Cassidy L, Barry P, Shaw C, et al. Platelet derived growth factor and fibroblast growth factor basic levels in the vitreous of patients with vitreoretinal disorders. Br J Ophthalmol. 1998;82:181–185. doi: 10.1136/bjo.82.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Campochiaro PA, Hackett SF, Vinores SA, et al. Platelet-derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J Cell Sci. 1994;107:2459–2469. doi: 10.1242/jcs.107.9.2459. [DOI] [PubMed] [Google Scholar]
- 281.Cui J, Lei H, Samad A, et al. PDGF receptors are activated in human epiretinal membranes. Exp Eye Res. 2009;88:438–444. doi: 10.1016/j.exer.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Campochiaro PA. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol. 1997;115:237. doi: 10.1001/archopht.1997.01100150239014. [DOI] [PubMed] [Google Scholar]
- 283.Akiyama H, Kachi S, E Silva RL, et al. Intraocular injection of an aptamer that binds PDGF-B: a potential treatment for proliferative retinopathies. J Cell Physiol. 2006;207:407–412. doi: 10.1002/jcp.20583. [DOI] [PubMed] [Google Scholar]
- 284.Zheng X, Du L, Wang H, Gu Q. A novel approach to attenuate proliferative vitreoretinopathy using ultrasound-targeted microbubble destruction and recombinant adeno-associated virus-mediated RNA interference targeting transforming growth factor-β2 and platelet-derived growth factor-B. J Gene Med. 2012;14:339–347. doi: 10.1002/jgm.2629. [DOI] [PubMed] [Google Scholar]
- 285.Lei H, Velez G, Hovland P, et al. Growth factors outside the PDGF family drive experimental PVR. Investig Ophthalmol Vis Sci. 2009;50:3394–3403. doi: 10.1167/iovs.08-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Pennock S, Kim D, Mukai S, et al. Ranibizumab is a potential prophylaxis for proliferative vitreoretinopathy, a nonangiogenic blinding disease. Am J Pathol. 2013;182:1659–1670. doi: 10.1016/j.ajpath.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Lei H, Kazlauskas A. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor α and thereby promote proliferation and survival of cells. J Biol Chem. 2009;284:6329–6336. doi: 10.1074/jbc.M808426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lei H, Rheaume M-AA, Kazlauskas A. Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp Eye Res. 2010;90:376–381. doi: 10.1016/j.exer.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pennock S, Haddock LJ, Mukai S, Kazlauskas A. Vascular endothelial growth factor acts primarily via platelet-derived growth factor receptor α to promote proliferative vitreoretinopathy. Am J Pathol. 2014;184:3052–3068. doi: 10.1016/j.ajpath.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Van Geest RJ, Lesnik-Oberstein SY, Tan HS, et al. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96:587–590. doi: 10.1136/bjophthalmol-2011-301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, et al. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS ONE. 2008;3:1–7. doi: 10.1371/journal.pone.0002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Klaassen I, van Geest RJ, Kuiper EJ, et al. The role of CTGF in diabetic retinopathy. Exp Eye Res. 2015;133:37–48. doi: 10.1016/j.exer.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 293.Wei Q, Zhang T, Jiang R, et al. Vitreous fibronectin and fibrinogen expression increased in eyes with proliferative diabetic retinopathy after intravitreal anti-VEGF therapy. Investig Ophthalmol Vis Sci. 2017;58:5783–5791. doi: 10.1167/iovs.17-22345. [DOI] [PubMed] [Google Scholar]
- 294.Pinazo-Durán MD, Gallego-Pinazo R, García-Medina JJ, et al. Oxidative stress and its downstream signaling in aging eyes. Clin Interv Aging. 2014;9:637–652. doi: 10.2147/CIA.S52662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Berra A, Ferreira S, Stanga P, Llesuy S. Age-related antioxidant capacity of the vitreous and its possible relationship with simultaneous changes in photoreceptors, retinal pigment epithelium and Bruchs’ membrane in human donors’ eyes. Arch Gerontol Geriatr. 2002;34:371–377. doi: 10.1016/S0167-4943(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 296.Santos FM, Mesquita J, Castro-de-Sousa JP, et al. Vitreous humor proteome: targeting oxidative stress, inflammation, and neurodegeneration in vitreoretinal diseases. Antioxidants. 2022;11:505. doi: 10.3390/antiox11030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev. 2016;2016:1–23. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ankamah E, Sebag J, Ng E, Nolan JM. Vitreous antioxidants, degeneration, and vitreo-retinopathy: exploring the links. Antioxidants. 2019;9:1–20. doi: 10.3390/antiox9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012;33:399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9:315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 302.Rodríguez ML, Pérez S, Mena-Mollá S, et al. Oxidative stress and microvascular alterations in diabetic retinopathy: future therapies. Oxid Med Cell Longev. 2019;2019:1–18. doi: 10.1155/2019/4940825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:1–17. doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Datta S, Cano M, Ebrahimi K, et al. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Maugeri A, Barchitta M, Mazzone MG, et al. Complement system and age-related macular degeneration: implications of gene-environment interaction for preventive and personalized medicine. Biomed Res Int. 2018 doi: 10.1155/2018/7532507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Krohne TU, Stratmann NK, Kopitz J, Holz FG. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res. 2010;90:465–471. doi: 10.1016/j.exer.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 307.Sinha D, Valapala M, Shang P, et al. Lysosomes: regulators of autophagy in the retinal pigmented epithelium. Exp Eye Res. 2016;144:46–53. doi: 10.1016/j.exer.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kwon YH, Kim YA, Yoo YH (2017) Loss of pigment epithelial cells is prevented by autophagy. In: Autophagy: cancer, other pathologies, inflammation, immunity, infection, and aging, 11th edn. Elsevier, pp 105–117
- 309.Wang AL, Lukas TJ, Yuan M, et al. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS ONE. 2009;4:1–13. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hyttinen JMT, Błasiak J, Niittykoski M, et al. DNA damage response and autophagy in the degeneration of retinal pigment epithelial cells—implications for age-related macular degeneration (AMD) Ageing Res Rev. 2017;36:64–77. doi: 10.1016/j.arr.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 311.Marmorstein LY, Munier FL, Arsenijevic Y, et al. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc Natl Acad Sci. 2002;99:13067–13072. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Frey T, Antonetti DA. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxidants Redox Signal. 2011;15:1271–1284. doi: 10.1089/ars.2011.3906. [DOI] [PubMed] [Google Scholar]
- 313.Beatty S, Koh HH, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/S0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 314.Rivera JC, Dabouz R, Noueihed B, et al. Ischemic retinopathies: oxidative stress and inflammation. Oxid Med Cell Longev. 2017;2017:1–16. doi: 10.1155/2017/3940241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ko JA, Sotani Y, Ibrahim DG, Kiuchi Y. Role of macrophage migration inhibitory factor (MIF) in the effects of oxidative stress on human retinal pigment epithelial cells. Cell Biochem Funct. 2017;35:426–432. doi: 10.1002/cbf.3292. [DOI] [PubMed] [Google Scholar]
- 316.Yang I-H, Lee J-J, Wu P-C, et al. Oxidative stress enhanced the transforming growth factor-β2-induced epithelial-mesenchymal transition through chemokine ligand 1 on ARPE-19 cell. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-60785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Tong Y, Zhou YL, Wang YX, et al. Retinal pigment epithelium cell-derived exosomes: possible relevance to CNV in wet-age related macular degeneration. Med Hypotheses. 2016;97:98–101. doi: 10.1016/j.mehy.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 318.Atienzar-Aroca S, Flores-Bellver M, Serrano-Heras G, et al. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J Cell Mol Med. 2016;20:1457–1466. doi: 10.1111/jcmm.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kang G-Y, Bang JY, Choi AJ, et al. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. J Proteome Res. 2014;13:581–595. doi: 10.1021/pr400751k. [DOI] [PubMed] [Google Scholar]
- 320.Athanasiou D, Aguilà M, Bevilacqua D, et al. The cell stress machinery and retinal degeneration. FEBS Lett. 2013;587:2008–2017. doi: 10.1016/j.febslet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Schmidt K-G, Bergert H, Funk R. Neurodegenerative diseases of the retina and potential for protection and recovery. Curr Neuropharmacol. 2008;6:164–178. doi: 10.2174/157015908784533851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Paulus YM, Campbell JP. Neuroprotection and retinal diseases. Dev Ophthalmol. 2015;55:322–329. doi: 10.1159/000434703. [DOI] [PubMed] [Google Scholar]
- 323.Simó R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61:1902–1912. doi: 10.1007/s00125-018-4692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Barber AJ, Baccouche B. Neurodegeneration in diabetic retinopathy: potential for novel therapies. Vision Res. 2017;139:82–92. doi: 10.1016/j.visres.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 325.Zafar S, Sachdeva M, Frankfort BJ, Channa R. Retinal neurodegeneration as an early manifestation of diabetic eye disease and potential neuroprotective therapies. Curr Diab Rep. 2019 doi: 10.1007/s11892-019-1134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Nian S, C.Y. Lo A (2019) Protecting the aging retina. In: Neuroprotection. IntechOpen, p 13
- 327.Chinskey ND, Besirli CG, Zacks DN. Retinal neuroprotection in dry age-related macular degeneration. Drug Discov Today Ther Strateg. 2013;10:e21–e24. doi: 10.1016/j.ddstr.2012.07.001. [DOI] [Google Scholar]
- 328.Lo ACY, Woo TTY, Wong RLM, Wong D. Apoptosis and other cell death mechanisms after retinal detachment: Implications for photoreceptor rescue. Ophthalmologica. 2011;226:10–17. doi: 10.1159/000328206. [DOI] [PubMed] [Google Scholar]
- 329.Murakami Y, Notomi S, Hisatomi T, et al. Photoreceptor cell death and rescue in retinal detachment and degenerations. Prog Retin Eye Res. 2013;37:114–140. doi: 10.1016/j.preteyeres.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Cuenca N, Fernández-Sánchez L, Campello L, et al. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 331.Fortuny C, Flannery JG (2018) Mutation-independent gene therapies for rod-cone dystrophies. pp 75–81 [DOI] [PubMed]
- 332.Madeira MH, Boia R, Santos PF, et al. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediators Inflamm. 2015 doi: 10.1155/2015/673090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ponnalagu M, Subramani M, Jayadev C, et al. Retinal pigment epithelium-secretome: a diabetic retinopathy perspective. Cytokine. 2017;95:126–135. doi: 10.1016/j.cyto.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 334.Kubay OV, Charteris DG, Newland HS, Raymond GL. Retinal detachment neuropathology and potential strategies for neuroprotection. Surv Ophthalmol. 2005;50:463–475. doi: 10.1016/j.survophthal.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 335.Altmann C, Schmidt MHH. The role of microglia in diabetic retinopathy: inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci. 2018 doi: 10.3390/ijms19010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Rutar M, Valter K, Natoli R, Provis JM. Synthesis and propagation of complement C3 by microglia/monocytes in the aging retina. PLoS ONE. 2014 doi: 10.1371/journal.pone.0093343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Spooren A, Kolmus K, Laureys G, et al. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 338.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Tosi GM, Orlandini M, Galvagni F. The controversial role of TGF-β in neovascular age-related macular degeneration pathogenesis. Int J Mol Sci. 2018;19:1–16. doi: 10.3390/ijms19113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Dobolyi A, Vincze C, Pál G, Lovas G. The neuroprotective functions of transforming growth factor beta proteins. Int J Mol Sci. 2012;13:8219–8258. doi: 10.3390/ijms13078219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Calvo P, Pastor A, De La Cruz R. Vascular endothelial growth factor: an essential neurotrophic factor for motoneurons? Neural Regen Res. 2018;13:1181–1182. doi: 10.4103/1673-5374.235024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Jünemann AGM, Rejdak R, Huchzermeyer C, et al. Elevated vitreous body glial fibrillary acidic protein in retinal diseases. Graefe’s Arch Clin Exp Ophthalmol. 2015;253:2181–2186. doi: 10.1007/s00417-015-3127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lee SY, Surbeck JW, Drake M, et al. Increased glial fibrillary acid protein and vimentin in vitreous fluid as a biomarker for proliferative vitreoretinopathy. Investig Ophthalmol Vis Sci. 2020;61:1–8. doi: 10.1167/IOVS.61.5.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Eastlake K, Heywood WE, Banerjee P, et al. Comparative proteomic analysis of normal and gliotic PVR retina and contribution of Müller glia to this profile. Exp Eye Res. 2018;177:197–207. doi: 10.1016/j.exer.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Nakazawa T, Takeda M, Lewis GP, et al. Attenuated glial reactions and photoreceptor degeneration after retinal detachment in mice deficient in glial fibrillary acidic protein and vimentin. Investig Ophthalmol Vis Sci. 2007;48:2760–2768. doi: 10.1167/iovs.06-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Verardo MR, Lewis GP, Takeda M, et al. Abnormal reactivity of Müller cells after retinal detachment in mice deficient in GFAP and vimentin. Investig Ophthalmol Vis Sci. 2008;49:3659–3665. doi: 10.1167/iovs.07-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Haque A, Polcyn R, Matzelle D, Banik NL. New insights into the role of neuron-specific enolase in neuroinflammation, neurodegeneration, and neuroprotection. Brain Sci. 2018 doi: 10.3390/brainsci8020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Gold M, El Khoury J. β-amyloid, microglia, and the inflammasome in Alzheimer’s disease. Semin Immunopathol. 2015;37:607–611. doi: 10.1007/s00281-015-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Vizin T, Kos J. Gamma-enolase: a well-known tumour marker, with a less-known role in cancer. Radiol Oncol. 2015;49:217–226. doi: 10.1515/raon-2015-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ratnayaka JA, Serpell LC, Lotery AJ. Dementia of the eye: the role of amyloid beta in retinal degeneration. Eye. 2015;29:1013–1026. doi: 10.1038/eye.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Luibl V, Isas JM, Kayed R, et al. Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. J Clin Invest. 2006;116:378–385. doi: 10.1172/JCI25843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Gupta VBV, Gupta VBV, Chitranshi N, et al. One protein, multiple pathologies: multifaceted involvement of amyloid β in neurodegenerative disorders of the brain and retina. Cell Mol Life Sci. 2016;73:4279–4297. doi: 10.1007/s00018-016-2295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Zheng L, Kågedal K, Dehvari N, et al. Oxidative stress induces macroautophagy of amyloid β-protein and ensuing apoptosis. Free Radic Biol Med. 2009;46:422–429. doi: 10.1016/j.freeradbiomed.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 354.Kannan R, Sreekumar PG, Hinton DR. Novel roles for α-crystallins in retinal function and disease. Prog Retin Eye Res. 2012;31:576–604. doi: 10.1016/j.preteyeres.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Zhou P, Kannan R, Spee C, et al. Protection of retina by αB crystallin in sodium iodate induced retinal degeneration. PLoS ONE. 2014;9:1–15. doi: 10.1371/journal.pone.0098275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Böhm MRR, Pfrommer S, Chiwitt C, et al. Crystallin-β-b2-overexpressing NPCs support the survival of injured retinal ganglion cells and photoreceptors in rats. Investig Ophthalmol Vis Sci. 2012;53:8265–8279. doi: 10.1167/iovs.12-10334. [DOI] [PubMed] [Google Scholar]
- 357.Kayama M, Nakazawa T, Thanos A, et al. Heat shock protein 70 (HSP70) is critical for the photoreceptor stress response after retinal detachment via modulating anti-apoptotic Akt kinase. Am J Pathol. 2011;178:1080–1091. doi: 10.1016/j.ajpath.2010.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Golestaneh N, Chu Y, Xiao Y-Y, et al. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2018;8:1–13. doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhang M, Jiang N, Chu Y, et al. Dysregulated metabolic pathways in age-related macular degeneration. Sci Rep. 2020;10:1–14. doi: 10.1038/s41598-020-59244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Yu B, Egbejimi A, Dharmat R, et al. Phagocytosed photoreceptor outer segments activate mTORC1 in the retinal pigment epithelium. Sci Signal. 2018;11:1–14. doi: 10.1126/scisignal.aag3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zhao C, Yasumura D, Li X, et al. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J Clin Invest. 2011;121:369–383. doi: 10.1172/JCI44303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Gale J, Aiello LP, Sebag J (2014) I.E. Diabetic Vitreopathy. In: Vitreous. Springer New York, New York, pp 57–79
- 363.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058–a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Vaughan-Thomas A, Gilbert SJ, Duance VC. Elevated levels of proteolytic enzymes in the aging human vitreous. Investig Ophthalmol Vis Sci. 2000;41:3299–3304. [PubMed] [Google Scholar]
- 366.Kowluru RA, Zhong Q, Santos JM. Matrix metalloproteinases in diabetic retinopathy: potential role of MMP-9. Expert Opin Investig Drugs. 2012;21:797–805. doi: 10.1517/13543784.2012.681043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Symeonidis C, Diza E, Papakonstantinou E, et al. Correlation of the extent and duration of rhegmatogenous retinal detachment with the expression of matrix metalloproteinases in the vitreous. Retina. 2007;27:1279–1285. doi: 10.1097/IAE.0b013e3180592c00. [DOI] [PubMed] [Google Scholar]
- 368.Symeonidis C, Papakonstantinou E, Souliou E, et al. Correlation of matrix metalloproteinase levels with the grade of proliferative vitreoretinopathy in the subretinal fluid and vitreous during rhegmatogenous retinal detachment. Acta Ophthalmol. 2011;89:339–345. doi: 10.1111/j.1755-3768.2009.01701.x. [DOI] [PubMed] [Google Scholar]
- 369.Jin M, Kashiwagi K, Iizuka Y, et al. Matrix metalloproteinases in human diabetic and nondiabetic vitreous. Retina. 2001;21:28–33. doi: 10.1097/00006982-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 370.Bishop PN. The role of extracellular matrix in retinal vascular development and preretinal neovascularization. Exp Eye Res. 2015;133:30–36. doi: 10.1016/j.exer.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 371.Singh M, Tyagi SC. Metalloproteinases as mediators of inflammation and the eyes: molecular genetic underpinnings governing ocular pathophysiology. Int J Ophthalmol. 2017;10:1308–1318. doi: 10.18240/ijo.2017.08.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Ioachim E, Stefaniotou M, Gorezis S, et al. Immunohistochemical study of extracellular matrix components in epiretinal membranes of vitreoproliferative retinopathy and proliferative diabetic retinopathy. Eur J Ophthalmol. 2005;15:384–391. doi: 10.1177/112067210501500312. [DOI] [PubMed] [Google Scholar]
- 374.Hussain AA, Lee Y, Zhang JJ, Marshall J. Disturbed matrix metalloproteinase activity of Bruch’s membrane in age-related macular degeneration. Investig Ophthalmol Vis Sci. 2011;52:4459–4466. doi: 10.1167/iovs.10-6678. [DOI] [PubMed] [Google Scholar]
- 375.Lambert V, Wielockx B, Munaut C, et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003;17:2290–2292. doi: 10.1096/fj.03-0113fje. [DOI] [PubMed] [Google Scholar]
- 376.Matsuo T. TIMP-1 and TIMP-2 levels in vitreous and subretinal fluid. Jpn J Ophthalmol. 1998;42:377–380. doi: 10.1016/S0021-5155(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 377.Rodrigues M, Xin X, Jee K, et al. VEGF secreted by hypoxic Muller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes. 2013;62:3863–3873. doi: 10.2337/db13-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reviewed in this paper is available in the original papers from proteomics and multiplex studies. These revised papers include the references [43–48, 52, 54, 59, 60, 62–64, 68–71, 98] for DR/PDR, [63, 93, 156, 157] for AMD, and [46, 188–195, 256] for RRD/PVR. The reviewed data is displayed in the supplementary table.