Abstract
Over the past few years, extensive efforts have been made to generate in-vitro pancreatic micro-tissue, for disease modeling or cell replacement approaches in pancreatic related diseases such as diabetes mellitus. To obtain these goals, a closer look at the diverse cells participating in pancreatic development is necessary. Five major non-epithelial pancreatic (pN-Epi) cell populations namely, pancreatic endothelium, mesothelium, neural crests, pericytes, and stellate cells exist in pancreas throughout its development, and they are hypothesized to be endogenous inducers of the development. In this review, we discuss different pN-Epi cells migrating to and existing within the pancreas and their diverse effects on pancreatic epithelium during organ development mediated via associated signaling pathways, soluble factors or mechanical cell–cell interactions. In-vivo and in-vitro experiments, with a focus on N-Epi cells’ impact on pancreas endocrine development, have also been considered. Pluripotent stem cell technology and multicellular three-dimensional organoids as new approaches to generate pancreatic micro-tissues have also been discussed. Main challenges for reaching a detailed understanding of the role of pN-Epi cells in pancreas development in utilizing for in-vitro recapitulation have been summarized. Finally, various novel and innovative large-scale bioengineering approaches which may help to recapitulate cell–cell interactions and are crucial for generation of large-scale in-vitro multicellular pancreatic micro-tissues, are discussed.
Keywords: Bioengineering, Endothelium, Mesenchyme, Organoids, Pancreatic non-epithelial cells, Pancreas organogenesis
Introduction
Developing novel cell-based approaches for pancreatic degenerative diseases therapy/modeling is largely dependent upon the information from pancreases development and in-vitro co-culture/differentiation studies [1]. In-vitro multistep differentiation protocols are based on recapitulating signaling pathways involved during pancreatic epithelium development to direct human pluripotent stem cells (hPSCs) differentiation to β-cells [2]. In two-dimensional (2D) differentiation culture systems, the generated insulin-producing cells lacked important key features of β cells, had limited insulin secretion, were at a polyhormonal stage, and were more similar to fetal β-cells [3, 4]. Three-dimensional (3D) differentiation cultures improved these protocols which led to the generation of β-cells that were able to secrete insulin in response to glucose stimulation after transplantation [3, 5]. However, in these 3D cultures, challenges on dynamic insulin secretion kinetics and overall insulin secretion were observed. 3D cellular structures are created by the self-organization of tissue-specific cells. They can re-create the structure, physiology, and function of the organ in remarkable details in in-vitro conditions. These organoid-like structures are not only useful tools for basic biological research, but also provide valuable information regarding the mechanisms of human organ development and tissue regeneration [6]. In addition, in pancreas development different pancreatic non-epithelial (pN-Epi) cell types provide cues for pancreatic epithelium organogenesis by their paracrine signals and extrinsic stimuli [3, 7]. Therefore, it seems that, more knowledge on normal pancreas development [8, 9] and attention on the interactions between the pancreatic epithelium and its surrounding niche cells, can improve the in-vitro reconstruction of pancreatic cells [3, 7]. However, the more complete reconstruction of this developmental complexities in culture systems can be challenging. Therefore, Bioengineering approaches can be employed to improve these challenges by providing an appropriate cell–cell interaction and novel cell- and tissue-based modeling approaches for pancreatic degenerative diseases.
In this review, we briefly describe organogenic mechanisms of pancreas development and summarize the current knowledge about the participation of five pN-Epi cell populations, pancreatic endothelium, pancreatic mesenchyme, pancreatic neural crest (NC) cells, pancreatic pericytes and stellate cells in pancreas development, and focus on their specific roles, cell–cell interactions and associated signaling pathways during this process. It has been hypothesized that these five cell populations are endogenous inducers of pancreas development. Next, we discuss in-vitro and in-vivo studies which have used N-Epi potentials to reconstruct pancreatic tissue, to increase the differentiation rate towards islets, especially β-cells or improve islet transplantation survival. In addition, PSCs as ultimate tools for differentiating different pancreatic cell types and a medium for genetic modification of the cells have been discussed. Multicellular 3D organoids as a new approach for generation of pancreatic micro-tissues are then studied. The gaps existing in our knowledge on the effects of pN-Epi cells in human pancreas development are also mentioned. Reaching a detailed understanding of the role of pN-Epi cells in pancreas development, can help us to better recognize the pathogenesis of and therapeutic approaches for pancreatic diseases such as diabetes mellitus (DM). Ultimately, potential bioengineering approaches to improve cell–cell interactions for generating pancreatic organoids that more closely mimic pancreas tissue, are presented.
Descriptive overview of pancreas organogenesis
The pancreas is an endoderm-derived organ that controls nutrient metabolism with its endocrine and exocrine compartments. The exocrine part of adult pancreas forms more than 95–98% of the whole pancreas and is composed of acinar, centroacinar, and ductal cells. Acinar cells produce and secrete digestive enzymes and ductal cells form ductal network which has the ability of transporting these secretions into the duodenum [8]. The endocrine part is organized with highly vascularized and innervated islets of Langerhans which constitute 1–2% of the pancreas mass. The islets are composed of five different endocrine cells each secreting specific peptide hormones to regulate blood glucose levels which are α-cells (glucagon), β-cells (insulin), γ-cells (somatostatin), ε-cells (ghrelin), and PP cells (pancreatic polypeptide) [10].
Due to ethical restrictions and limited availability of human sources, few studies have so far investigated the development of human pancreas [11]. Studies of pancreas development in these sparse human studies and in rodent models have provided insight into pancreas-related diseases such as DM [9, 12]. Pancreas development initiates with the formation and growth of epithelial buds from the foregut endoderm [13]. Several inductive signals from notochord, endothelium, and mesenchyme, promote expansion of multipotent progenitor cells of the epithelial buds [9, 13]. In addition, extrinsic signals from endothelial cells (ECs) regulate dorsal bud emergence. The primary transition period, which happens around 30–40 day post-conception (dpc) in human and around embryonic day (E) 9.5–12.5 in mice, coincides with evagination of the two pancreatic buds and pancreatic progenitor’s proliferation towards the mesenchyme that surround the undifferentiated epithelium region [11, 14]. During human embryo development, the pancreatic buds start to grow and branch into the mesenchyme at 5th week post-conception (wpc) [15]. At this time, microlumens form and fuse, resulting in complex tubular network formation in the epithelium [11, 16].
During the secondary transition period (40–52 dpc in humans and E12.5–15.5 in mice), after tubulogenesis and branching morphogenesis, formation of tip and trunk domains occurs. The final fate of the tip part is mostly acinar cells, whereas the trunk has a bi-potent fate and gives rise to both ductal cells and neurogenin3 (NGN3)-positive endocrine progenitors [8, 17]. The endothelium and mesenchyme tissues affect the patterning of these tip and trunk cells. The interaction between endothelium and epithelium leads to trunk development while the mesenchymal factors and extracellular matrix (ECM) components help in tip formation [13]. The mesenchymal secreted factors also have a role in promoting differentiation of exocrine compartment and repressing endocrine cells formation [18]. Sporadic trunk cells expressing Ngn3 and turning off SRY-Box 9 (Sox9) expression, concomitantly delaminate from pancreatic epithelium into the surrounding condensed mesenchyme and start to organize endocrine clusters which finally form the islet structure [19]. Pancreatic islet formation is initiated with NGN3 expression and the initial clusters are vascularized by 10 wpc and the islets are completely apparent and contain different endocrine cells at 12–13 wpc [11]. Aberrant function of the exocrine and endocrine components can cause pancreatitis and DM, respectively [20].
Pancreatic non-epithelial (pN-Epi) tissue during development
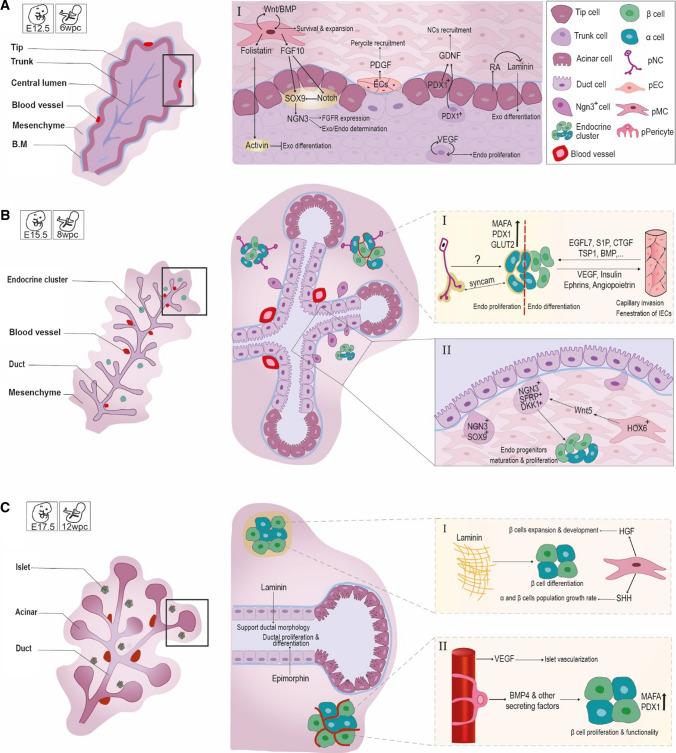
Throughout pancreatic development, in addition to the importance of the pancreatic epithelium signaling, cell–cell interactions between endodermal-, mesodermal-, and ectodermal-derived cells and the epithelium is also critical for proper pancreas development (Fig. 1). Less attention has been paid to the importance of these pN-Epi tissues in pancreatic development and their major influences on pancreatic epithelium tissue.
Fig. 1.
An overview of pancreatic organogenesis with focus on the role of pN-Epi cells interactions. The different time points of A E12.5 (6WPC), B E15.5 (8WPC) and C E17.5 (12WPC) during pancreatic organogenesis are illustrated in the left panel. The right panels are a closer view of each developmental time point; the one's which are marked in Latin numerals also show cellular interactions and effects of pN-Epi cells on the epithelium. The schematic interactions of pN-Epi cells with the pancreatic endocrine epithelium are shown with larger magnification in small dash-line boxes marked in Latin numerals. (AI) Interactions of the mesenchyme with Pdx1+ progenitor cells and with themselves and interactions of ECs with the trunk cells. (BI) Interactions of NC cells and ECs with endocrine clusters. (BII) The effect of the condensed mesenchyme on NGN3+ cell migration and increases in endocrine cluster mass. (CI) The effect of laminin and condensed mesenchyme on islet cells. (CII) Interaction of pancreatic pericyte with β-cells of the islet. Endo endocrine; Exo exocrine
Pancreatic endothelium
The ECs are mesodermal derivatives that differentiate from precursor cells called hemangioblasts [21]. The ECs are essential for viability, determination of different progenitor cell fates in a developing embryo, and maturation and patterning of tissues and organs including the pancreas and liver [22]. They organize as a heterogeneous population and, based on their location in the body, show different phenotypes and functions [23, 24].
In pancreatic bud development The ECs which originate from the dorsal aorta and vitelline veins appear in proximity to the endoderm in coincidence with pancreas formation on E8.5-9 in mice. These cells afterwards surround the pancreatic epithelial buds [25]. Pancreas-duodenum homeobox protein 1 (Pdx1) is expressed in gut tube endoderm which is in association with endothelium of dorsal and ventral veins [22, 26]. Aortic endothelium-derived signals promote development and differentiation of dorsal pancreatic progenitor cells via activating pancreas transcription factor 1 subunit alpha (PTF1a) in the dorsal pancreatic region while the ventral epithelium is separated by the mesenchyme compartment from vitelline veins [22, 27]. The ventral pancreas develops in a quite different way from the dorsal pancreas, and the evagination is not affected in the absence of aortic endothelium [28], Therefore, ECs interactions are more critical for dorsal—than ventral—pancreatic budding [21, 27].
By E11.5, the ECs locate irregularly all over the pancreatic buds. From E13.5 to 15.5, blood vessels form “honeycomb” structures around the epithelial buds, contributing to ECs localization near trunk cells and far from tip cells [25]. Epithelium-derived vascular endothelial growth factor (VEGF) is essential for recruitment of the ECs, stimulation of endocrine cell proliferation and maintenance of islet vascularization [29]. VEGF loss of function can decrease islet capillaries and insulin release, while VEGF overexpression results in hyper-vascularization and reduced β-cell proliferation with onset of DM [30]. During branching period, VEGF-A expression is heterogeneous in the epithelium whereas its expression is predominant in trunk cells but decreases in tip cells. In-vivo inactivation of VEGF-A results in vessels density reduction and significant increase in acinar differentiation [25]. As well, VEGF-B is expressed at a lower level in both pancreatic endocrine and exocrine components. VEGF-B deletion from β-cells in Vegfbfl/fl/RIP-Cre+/− mice resulted in the enhancement of insulin gene expression [31]. It has been demonstrated that pancreatic ECs enable leucocytes infiltration, leading to β-cell dysfunction and finally type 1 diabetes mellitus (T1DM) [26]. It can be concluded that embryonic pancreas development requires inductive signals from the ECs for maintenance of pdx1 expression, initiation of PTF1A, insulin and glucagon expression and pancreatic endocrine cell specification, but the exact mechanisms are not yet known [22, 27].
In pancreatic islet functions Islets are highly vascularized mini-organs in which, each β-cell is in close contact with islet ECs to form their vascular basement membrane. Islet ECs have a role in normal islet function, induce insulin gene expression and stimulate β-cells proliferation [32]. β-cells use VEGF-A to attract ECs which form vascular basement membranes adjacent to the β-cells. Laminin is one of the endothelial signals which among other basement membrane proteins (e.g., collagen IV and fibronectin), upregulates insulin gene expression and β-cell proliferation. In addition, laminin needs β1-integrin to regulate the proliferation of β-cells. The intra-islet basement membrane is not formed in VEGF-A-deficient mutant islets resulting in the reduction of insulin gene expression [33]. Islet ECs are much more permeable than the ECs in the exocrine component [21]. Islet ECs begin to associate with endocrine cell populations at the 10th wpc and fetal islet clusters containing vascular at the 14th wpc [34]. Mice with disruption of endothelial cell–specific Irs2 (ETIrs2KO mice) show a decline in their islet blood perfusion and insulin secretion, indicating that impairment in insulin response to glucose load, might be a result of impaired islet blood flow [35]. A reciprocal signaling between endothelial and endocrine cells exists to retain endocrine differentiation and finally, a functional pancreas. Studies have shown that the ECs can influence insulin expression in non-pancreatic endoderm while endocrine cells through VEGF signaling induce capillary invasion and fenestration of islet ECs [24, 36]. Inhibition of VEGFR2 contributes to a reduction in endocrine differentiation, indicating ECs role in promoting endocrine development [25]. Nevertheless, overexpression of VEGF in developing β-cells decreased endocrine mass and impaired islet structure [37].
Few endothelial factors and their external signals were revealed to be involved in various aspects of pancreas organogenesis, growth, cell proliferation and tissue organization. ECs produce multiple factors that contribute to proliferation, survival, insulin secretion and finally, proper function of β-cell which is shown in detail in Table 1.
Table 1.
Endothelial cells-derived factors involved in pancreas development
| EC-factor | Mediatory pathway | Stage | Role(s) | LOF | References | |
|---|---|---|---|---|---|---|
| EGFL7 | EGFR, AKT(PKB) | E9.0_E11.5 |
• Increase PP proliferation • Decline differentiation to endocrine cells |
• ND | [97, 203] | |
| S1P | SPHK, S1P | E10.5 | • Proliferation of PDX1+ PP |
• Declined size of pancreatic buds • Hyper-vascularization |
[204, 205] | |
| CTGF | TGFβ, BMPs, Wnts | E12.5 | • β-cell proliferation and islet morphogenesis | • Declined β-cell proliferation and islet vascularity | [206, 207] | |
| TSP1 (THBS) | TGFβ-1 | E12.5 |
• Antiangiogenic • Maintain postnatal β-cell function |
• Hyperplasia in islets | [29, 208] | |
| HGF | PI3K | E15 | • Increase β-cell proliferation and islet mass |
• Reduced islet size and insulin secretion • Glucose intolerance |
[209–211] | |
| BMP 2/4 | TGFβ-1 | ND |
• Stimulate vasculogenesis and angiogenesis • Endothelial progenitor cell differentiation • Mediate GSIS |
• ND | [78, 212] | |
| ENDOGLIN | TGFβ | ND | • Islet vascular development | • Decreased insulin secretion | [213] | |
| ENDOTHELINs | MAPK, PI3K | ND |
• Stimulate β-cell insulin secretion • Effects on native islet blood vessels |
• Vascular dysfunction | [214, 215] | |
| ECM components* | Focal adhesion kinase | ND |
• Increase Insulin gene expression • β-cell differentiation, maturation and survival |
• ND | [29, 216] | |
LOF results of loss of function, ND no data provided, EC-factor endothelial cell-factors, EGFL7 epidermal growth factor‐like domain 7, S1P sphingosine-1-phosphate, CTGF connective tissue growth factor, TSP-1 Thrombospondin-1, HGF hepatocyte growth factor, BMP bone morphogenetic protein; VEGF vascular endothelial cell growth factor
*Laminin/Collagen IV/Fibronectin
In diabetic initiation and progression The dysfunction of ECs is highly associated with microangiopathy and atherosclerosis in both type 1 and type 2 diabetes mellitus (T1DM and T2DM) patients [38]. In T1DM, the intra-islet ECs are barriers for infiltrating immune cells, and also mediate β-cell apoptosis by releasing nitric oxide which activates apoptotic caspase. The intra-islet vascular endothelium changes contribute to the onset of hyperglycemia and destruction of β-cells. The impaired endothelial function has a critical role to initiate inflammatory mechanisms via the recruitment of macrophages and leukocytes in vascular diseases of T2DM and progression of insulin resistance [39].
Pancreatic mesenchyme
ECs of the dorsal aorta, lateral plate mesoderm, and the notochord are mesoderm-derived tissues capable of inducing the endoderm to pre-pancreatic domain. Dynamic signals from the lateral plate mesoderm, septum transversum, and cardiac mesoderm specify the ventral pancreatic bud epithelium while notochord and dorsal aorta and other transient adjacent mesoderm’s stimulate dorsal pancreatic bud [40] (reviewed in [41]). However, here, we mainly focus on the condensed pancreatic mesenchyme which surrounds the pancreatic epithelium during development.
In pancreatic bud development Fate mapping using DiI-labeling1 displayed that shortly before the evagination of pancreatic buds, the ISL1+ (insulin gene enhancer protein ISL-1) mesenchymal cells which seem to originate from a specific portion of E8.5 ventral and dorsal coelomic mesothelium, start surrounding the new forming ventral and dorsal pancreatic buds. At approximately E9.5, these cells penetrate into the dorsal pancreatic space, gather and condense around the uniform pancreatic epithelium bud [42, 43]. The condensed dorsal pancreatic mesenchyme is vital for creation of a functional pancreas. A slow infiltration and accumulation of coelomic mesenchymal cells and their patterning signals between E9.5 and 10.5 around the nascent pancreatic bud, seems to guard against premature differentiation and depletion of the progenitor pool [43]. The maximum amount of mesenchymal mass around the pancreatic area is seen around E11.5. With the progression of development, the mesenchyme mass reduces and by the end of gestation, it is only found around the islets and blood vessels [42].
Some in-vitro studies indicated that pancreatic mesenchymal cells promote exocrine differentiation and growth. Other studies observed expansion of Pdx1+ pancreatic progenitor cells which resulted in higher numbers of β-cells [44, 45]. To precisely clarify the role of condensed mesenchyme in endocrine development, genetically modified mice such as Nkx3.2-Cre;R26R-YFP and Nkx3.2-Cre;iDTR were used [42]. Genetic ablation of mouse pancreatic mesenchyme in different developmental stages has shown their supporting role in proliferation of the progenitor and differentiated cells, and precise pancreas morphogenesis [42]. The single-cell RNA sequencing technique has identified and validated underappreciated heterogeneity in the mesenchymal compartment of the pancreas; it has also provided evidence for transcriptional maturation within the mesenchyme compartment as development proceeds, providing different signals at different stages [46]. It was also demonstrated that adult pancreatic mesenchyme is a unique mesenchymal stem cell (MSC) subtype with a distinct proteome and secretome, possessing functional properties beneficial for vascular disease and DM [47]. Recently, the functional heterogeneity within E12.5 mice pancreatic mesenchyme was identified, showing that a specific NK3 Homeobox2 and NK2 Homeobox5 (Nkx3.2+/Nkx2.5+) mesenchymal microenvironment, promotes the formation of insulin- and glucagon-producing cells [48]. Nkx3.2+ cells are mostly expressed in the gastrointestinal and pancreatic mesenchyme, while the Nkx2.5+ cells are only expressed in a division of Nkx3.2+ cells placed on the left side of the dorsal pancreas. Pre-B-cell leukemia transcription factor 1 (Pbx1)-dependent molecular network within Nkx3.2+/Nkx2.5+ cell microenvironment locally regulates the epithelial-mesenchymal crosstalk underlying endocrine differentiation. The regulation of laminin/integrin a6 interaction (in mesenchyme/epithelial interface) and soluble molecules such as Slit1/3 [in the extracellular matrix (ECM)] by Pbx1, defines a pro-endocrine specialized micro-niche, particularly at the trunk domain. Pbx1 deletion in the Nkx3.2+/Nkx2.5+ pancreatic mesenchyme results in insulin+ and glucagon+ cells reduction in the dorsal portion of the pancreas [48].
Inductive mesenchymal factors Evidence provided by explant studies and genetically modified animals, highlighted several signaling molecules/pathways inside the pancreatic mesenchyme which are involved in proper development and differentiation of epithelium and for maintaining the mesenchyme itself (Table 2). Moreover, the impact of factors secreted from mesenchyme in different stages of pancreatic epithelium development has been widely investigated.
Table 2.
Selected mesenchymal intracellular signaling pathways involved in pancreas development
| Signaling pathway | SOA in pancreatic mesenchyme | SOA* in pancreatic epithelium | Function (in pancreas) | LOF (in pancreas) | Referencess |
|---|---|---|---|---|---|
| BMP | E9.5-E12.5 | ND |
• Normal development of mesenchyme • Regulation of cellular composition |
• Production of endocrines concomitant to exocrine decrease • Severe hypoplasia • Reduced epithelial branching • Anterior extension of dorsal pancreas into stomach mesenchyme • Vascular remodeling |
[217] |
| RA | ~ E11 | E11 |
• Exocrine lineage selection • Up-regulation of mesenchymal laminin-1 • Ductal differentiation • Generation of Ngn3+ and β-cells |
• Production of acinar cells in favor of ductal cells | [218–220] |
| Wnt | ~ E13 | E13.5 |
• Expansion/differentiation of epithelial progenitors • Regulation of organ growth • Survival/expansion of mesenchyme |
• Reduced PP proliferation and hypoplasia • Decrease in endocrine progenitor and islet cell mass • Reduced β-, PP-, and delta- cells but not alpha-cells |
[221, 222] |
| SHH | ≥ E13 | ≤ 2nd transition |
• Regulation of alpha and β-cell growth rate • Fine-tuning for proper development/organogenesis |
• Severe agenesis in onset of development# • Reduced endocrine and exocrine area • Disrupted islet morphology and cellular composition at E14.5# |
[223–227] |
LOF results of loss of function, SOA stage of activation, ND no data provided
*This table displays the signaling pathways activated intracellularly in the mesenchyme compartment while this column shows the time of activation of these signaling pathways in the epithelium compartment
#While this column displays the effects of LOF of SHH, these items are caused by its gain of function!
FGF10 was the first identified factor secreted by mesenchymal cells that affects the prenatal pancreas development and is crucial for normal proliferation of the progenitor cells as it activates the Notch pathway during the early stages of pancreatic development [42, 49]. FGF7 and FGF10 expression is, respectively, ten and eightfold higher in the mesenchyme compartment of 6–9 wpc compared to the epithelium compartment [50]. Explant cultures of E10.5 mouse dorsal pancreatic epithelium grown in the presence of FGF10, indicated the expansion of pancreatic precursors while their differentiation was blocked [49, 50]. It seems that a temporal competence window exists for endocrine and ductal pancreatic development regulated by the timing of FGF10 expression. Secretion of FGF10 from pancreatic mesenchyme on E8.5–10.5 is necessary for normal development of pancreatic cell lineages; nonetheless, sustained FGF10 expression onwards and until the secondary transition, results in permanent loss of endocrine and ductal development [51]. Expression of FGFR2b located on pancreatic epithelial progenitor membranes is controlled by Sox9 transcription factor (TF) which is in turn regulated by FGF10. Therefore, pancreatic identity maintenance and growth are achieved upon a gene regulatory network with Sox9 being its centerpiece. The evidence obtained from transgenic mice has shown that the activity of Sox9/Fgf10/Fgfr2b feed-forward loop in the pancreatic niche controls the progenitor cell proliferation and liver-to-pancreatic cell fate switch [52]. Fgf9, identified by pancreatic mesenchyme single-cell analysis, also regulates mesenchymal cells proliferation and vascular formation [46]. Hepatocyte growth factor (HGF) secreted from human pancreatic mesenchyme obtained at 18–24 wpc, promotes β-cells expansion and their proper development. The highest expression of HGF in pancreatic mesenchyme is observed at late embryonic developmental stages and declines until adulthood [53].
Gene expression analysis on isolated E11.5 mouse pancreatic cells by laser-captured microdissection, identified 11 genes encoding secreting factors such as FGF, Wingless (WNT), VEGF, Insulin Growth Factor (IGF), Semaphorin3A, and especially Transforming Growth Factor β (TGFβ) signaling pathway factors which were preferentially expressed at higher levels in the mesenchyme compared to the epithelium [54]. Pancreatic mesenchyme derived IGF2 is a paracrine regulator of pancreatic size and function during development [55]. Recently, another experiment using the same method identified novel signals involved in the epithelium and mesenchyme crosstalk such as Versican (Vcan) and syndecan4 (Sdc4) which are involved in pancreatic tissue repair [56]. Moreover, single-cell RNA sequencing of E14.5 pancreatic mouse showed the expression of Wnt antagonists in the mesenchyme compartment, which may regulate Wnt signaling in the developing pancreas [46]. Proteomic analysis of pancreatic mesenchyme tissue revealed the presence of two potentially important factors, α2 Laminins (LAMA2) and Galectin 1 (LGALS1), as supporters of the β-cell differentiation state and progenitor proliferation [57]. Nkx2.5+ mesenchymal cells which define pro-endocrine niche are regulated by Pbx1 TF through a non-cell-autonomous manner via the interplay of ECM-integrin and interaction of soluble factors such as Slit1/3 [48]. It has also been shown that Epimorphin, a pancreatic mesenchyme morpho-regulatory protein, affects ductal proliferation and differentiation during early and late pancreatic organogenesis [58]. In addition, Follistatin secreted by the mesenchyme binds to activin produced by the embryonic epithelium which has a repressive effect on the endocrine components development [59]. Therefore, pancreatic mesenchyme provides temporally distinct signals for proliferation and differentiation of the epithelium.
The Slit-Robo signaling pathway in the pancreas affects the islet morphogenesis via Slit1/2/3 ligands which regulate endocrine cell–cell adhesion and are important in proper formation of the unique architecture of islets [60].
The cluster of Homeobox 6 (Hox6) gene expression and function in pancreatic mesenchyme, control endocrine cell differentiation by regulating the maturation of NGN3-expressing cells into hormone-producing cells [19]. Hox6 function results in an increase in Wnt5a expression in the pancreatic mesenchyme on E12.5 and beyond, and subsequent expression of two Wnt inhibitors [Secreted Frizzled-Related Protein 3 (Sfrp3) and Dickkopf-Related Protein 1 (Dkk1)] in delaminated NGN3+/Sox9− progenitors. Therefore, a mesenchymal-epithelial crosstalk for WNT pathway is regulated by Hox6 pancreas mesenchyme genes [19].
Pancreatic pericytes and stellate cells
Pericytes and vascular smooth muscle cells, together termed ‘mural cells’, are other mesenchymal cells in the pancreas that become the major mesenchyme compartment existing in the pancreas after birth. Contractile pericytes wrap around small vessels and form a discontinuous layer while vascular smooth muscle cells surround the larger blood vessels [61]. In general, pericytes are characterized by prominent cell body, extended cytoplasmic processes, peri-endothelial location, and high expression of neural/glial antigen 2 (NG2),2 Desmin and platelet-derived growth factor receptor β (PDGFRβ) [62]. Pericytes are recruited by PDGF released by the ECs to stabilize the vascular system [63]. The pericyte-endothelial interaction is crucial for proper vascular morphology and function in organ development and tissue repair [64].
Pancreatic stellate cells build up about 4–7% of the human adult pancreas and are found in endocrine and exocrine pancreatic compartments [65, 66]. They express multiple stem cell markers and can differentiate into various cell types. Pancreatic stellate cells main function is to regulate the pathologic fibrosis in the pancreas, but they also have important roles as immune and progenitor cells for regeneration. These star-shaped cells which are woven into different mammalian organs, have two biological phenotypes: quiescent and activated states.
In physiological conditions, the quiescent stellate cells are rich in lipid droplets containing retinoid and express glial fibrillary acidic protein, nestin, desmin, and vimentin [67]. These cells proliferate rarely, influence tissue homeostasis significantly, and maintain the normal basement membrane and ECM components by secreting metalloproteinases (MMP), such as MMP-2, MMP-9, and MMP-13. They also actively supply blood flow and provide scaffolds for epithelial integrity. The quiescent phenotype is maintained by retinoid which inhibits the activation of alpha-smooth muscle actin (α-SMA) and decreases the expression of collagen synthesis [68, 69]. The activated state (myofibroblast-like cells) which promotes the formation of pancreatic fibrosis in pathological conditions, expresses α-SMA and secretes collagen I, collagen III, fibronectin, and other ECM components [67, 68]. These functional myofibroblasts are regulated by autocrine and paracrine stimulation. Stellate cells contain different cell subpopulations which express different cell surface markers. Because of this functional heterogeneity, different cell subpopulations can individually or synergistically influence the diverse function in the progression of pancreatic fibrosis which is well demonstrated in the pathogenesis of chronic pancreatitis and pancreatic ductal adenocarcinoma (PDAC) [69].
In pancreas, pericytes, along with glial cells, support the neurovascular system and are considered as micro-organ accessory cells that respond to tissue injury and inflammation in the mature pancreas [63]. Cellular responses and morphologic plasticity of pancreatic pericytes and glial cells are observed in response to islet injuries such as experimental insulitis and progressive T1DM. In streptozotocin (STZ)-treated diabetic mice, for instance, pancreatic pericytes density and NG2 marker significantly increase [70].
The appearance in pancreatic tissue In contrast to other celomic organs in which, pericytes are differentiated from the mesothelium,3 recent findings have pointed out that the multilayered pancreatic mesenchyme, which expresses the NKX3.2 TF, is the primary origin of the adult mouse pancreatic pericytes [71]. Early acquisition of pericyte fate by mesenchymal cells is indicated by high expression of PDGFRβ in a major population of cells of the mesenchyme on E13.5. From E17.5, the pancreatic mesenchyme gradually displays pericyte markers expression and pericyte characteristic [71].
Stellate cells express mesenchymal and ectodermal markers, therefore, their embryonic origin has been elusive. Recent studies have provided evidence which supports the mesodermal origin of hepatic stellate cells [72, 73]. Since pancreatic and hepatic stellate cells have significant similarities, it can be suggested that both can be evolved from a same origin but experimental evidence is still lacking. On the other hand, it has been conducted that pancreatic stellate cells have several differences with heptic stellate cells, in their characteristic features and microstructure. Though it has been shown that at least a subpopulation of pancreatic stellate cells are derived from bone marrow progenitor cells [67, 68].
In β-cell maturity and function Pericytes as pivotal components of the islet niche, cover 40% of islets microvasculature [74]. Although having a fourfold higher density in the pancreatic endocrine component, pericytes are also detected in the exocrine component [70]. Pericytes regulate islet local blood flow by adjusting islet capillary diameter via responding to active β-cell-derived adenosine signals and sympathetic noradrenaline inputs [74]. The endogenous adenosine is derived from ATP, which is transported into insulin granules and co-released with insulin upon stimulation, from active β-cells [74]. Maintenance of differentiated mature β-cells state and glucose-stimulated insulin secretion (GSIS), as an essential function of these cells, are influenced by pericytes [75]. Pancreatic pericytes depletion from the islets of DT-treated NKX3.2-Cre; iDTR mice reduces the expression levels of Musculoaponeurotic Fibrosarcoma Oncogene Family A (MafA) and Pdx1 TFs, decreases islet insulin content and impairs GSIS [75]. The pericytes-conditioned medium contains secreted factors that stimulate proliferation of primary cultured adult β-cells in a β-integrin-dependent manner implicating the contribution of basement membrane components [61]. Bone morphogenetic protein 4 (BMP4), along with other factors secreted from pericytes, is required for β-cell function [76]. In the neonatal stage, pancreatic pericytes act as regulators of neonatal β-cell proliferation and, therefore, determine the final β-cell mass [61].
In diabetic initiation and progression Pancreatic pericytes abnormal function can lead to β-cell failure and disease development. On the other hand, chronic hyperglycemia of DM can result in pancreatic pericyte loss [20]. The inactivation of Transcription Factor 7 Like 2 (Tcf7l2) which is highly expressed in pancreatic pericytes leads to impaired expression of β-cell function and maturity genes (such as Glut2, Kir6.2, Sur1, MafA, Pdx1, and NeuroD1) and glucose intolerance of transgenic mice. Genetic changes in pancreatic pericytes leading to TCF7L2 inactivity are sufficient to compromise β-cell function and loss of glycemic control suggesting that the abnormalities in pancreatic pericytes contribute to the development of genetic-associated T2DM [76, 77]. Moreover, it has been shown that pericytes lacking Tcf7l2 have a reduced expression of the BMP4 gene. Proper function and gene expression of β-cells depend on the activity of the BMP4 receptor, BMPR1A [78]. Therefore, the impaired crosstalk of the BMP4/BMPR1A signaling pathway in abnormal pericytes and β-cells can lead to T2DM progression.
In diabetic retinopathy which is due to chronic hyperglycemia, retinal capillary pericytes are lost and the retina’s vascular system is damaged [79]. It seems that oxidative stress and also Ang-1/Tie-2 and PDGFβ/PDGFRβ signaling pathways are the cause of pericyte apoptosis and pathogenesis of diabetic retinopathy [80, 81]. Pancreatic pericytes are also sensitive to chronic hyperglycemia [74]. T2DM patients have abnormal islet-associated pericytes and vasculature systems [77]. These patients’ islet capillaries show a reduction of pericytes. With the loss of more pancreatic pericytes by the progression of the disease, a decline in proper β-cell function, insulin secretion, and further deterioration in glucose regulation are observed [74].
In pathologic conditions, stellate cells produce multiple inflammatory cytokines, inhibit proliferation, decrease β-cell function and enhance β-cell apoptosis leading to islet fibrosis and islet cell disorganization [68, 69]. It seems that islet fibrosis seen in the islets of patients with T2DM is activated through pathways that are different from those of the exocrine pancreatic fibrosis. Renin-angiotensin system (RAS) activation in islets transforms quiescent stellate cells into the activated form. Activated stellate cells proliferate and accumulate ECM proteins which consequently lead to islet fibrotic destruction [82].
Pancreatic neural crest (NC) cells
The mature pancreas is highly innervated extrinsically by the vagus nerve, splanchnic nerve, and the sympathetic nerves system [83]. NC cells migrated to the embryonic pancreas tissue on E9.5 [84, 85], eventually differentiate into Schwann glial cells, sensory neurons, and sympathetic and parasympathetic neurons. SRY-Box 10 (Sox10), Forkhead Box D3 (Foxd3), Transcription Factor AP-2 alpha (Tfap2α), and Paired Box Gene 3 (Pax3) are the key TFs required for vagal NC specification [83]. In addition to the majority population of pancreatic proliferative cells that are descended from pdx1 precursor cells, a rare population descended from the Wnt1+ pancreatic NC cells was also detected recently [86]. The NC cell derivatives enclosing adult islets and blood vessels, are presumed to have a protective influence on β-cells, especially in times of stress and islet inflammation [85, 86].
The appearance in pancreatic tissue Before the 1970s, it was proposed that the pancreatic endocrine tissue derives from the NC cells [87]. Afterwards, loss-of-function experiments such as observation of β-cells in the pancreas developed from rat embryos lacking the precursor of NC cells (the ectoderm tissue), showed that pancreatic endocrine tissue has a different origin from the pancreatic NC cell [88]. However, the NC cells do contribute indirectly to islet development by secreting factors [89]. Shortly after appearance of murine vagal NC cells in the stomach [89], they migrate into and through the surrounding mesenchyme and enter the developing pancreas region between E9.5–10.5 [84, 85]. Glial cell-derived neurotrophic factor (GDNF) signaling from epithelium seems to display the chronology of NC migration and development of the intrinsic innervation of the pancreas [90]. The peak existence of Phox2b+/Sox10+ pancreatic NC cells can be detected at the border of the pancreatic epithelial and mesenchymal cells on E12.5 [89] and intermingle with the endocrine cell clusters between E13.5–15.5. Paired-Like Homeobox 2b (Phox2b; a downstream target of Sox10) or Foxd3 loss-of-function, results in the pancreatic NC cell death and migration failure leading to the absence of NC derivatives such as glial and neuron cells in the pancreas [84, 89]. It was shown that Phox2b silencing occurs approximately 24 hours later, indicating subsequent appearance of glial cells [89]. Nkx2.2-expressing epithelial cells can mediate differentiation of NC cells towards neuronal phenotype via Phox2b silencing. Nkx2.2-expressing cells accomplish their role via secreting hormones or signaling molecules, including molecules classically involved in neural and glial differentiation such as netrin 1 [89, 91]. By E20, approximately 70% of NC-derived neurons enclosing endocrine cells are distributed in close proximity to alpha cells, a process mediated by synaptic cell adhesion molecule (SynCAM; CADM-1) expressed by both alpha cells and NC derivatives during islet development [85].
In pancreatic islet development and maturation As development proceeds, the NC derivatives which have progressively migrated towards endocrine cells, positively regulate β-cell maturation while negatively regulating their proliferation [84, 85]. NC cells' signaling regulates MafA, Pdx1, and Glucose Transporter 2 (Glut2) expression in the endocrine clusters and impedes the proliferation of endocrine cells [84]. Phox2b and Nkx2.2 form a negative feedback loop between ectoderm-derived NC cells and endoderm-derived epithelium, which impacts gene expression in both cell populations and ultimately, controls the β-cell population mass [89]. However, how β-cell proliferation is regulated by neural input is far from being understood, and further studies are needed to clarify the underlying mechanisms [90].
Using non-epithelial cells potentials to reconstruct pancreatic tissue in-vitro
Previously, we found that different pN-Epi cells which exist in the pancreatic tissue, affect differentiation, proliferation, and survival of pancreatic epithelium during organ development via soluble factors or physical cell–cell interactions. But, how can we recapitulate these interactions to reconstruct pancreatic tissue differentiation or maintenance, in-vitro? Several studies applied different pancreatic and non-pancreatic N-Epi cells co-cultured or co-transplanted with different pancreatic progenitor or islet cells, to induce further differentiation, proliferation or maintenance. These co-cultured/-transplanted supporting cells included the NC cells, ECs such as human umbilical vein endothelial cells (HUVEC) and mesenchymal cells such as bone marrow-mesenchymal stem cells (BM-MSCs).
Endothelial cells accompanying pancreatic epithelium The incorporation of ECs helps vascularization, survival and maturation of organoids through delivery and distribution of oxygen and nutrient delivery into the inner core of larger organoids [92]. Several studies showed the inductive potential of rat heart microvascular ECs or HUVEC for maturation and differentiation of human embryonic stem cells- (hESCs)-pancreatic progenitor or pancreatic endocrine cells towards insulin-producing cells via co-culture systems [93, 94]. Mouse aortic ECs promote differentiation of embryoid body cells into insulin-producing β-like cells by activating BMP signaling pathway especially at the embryoid body EC interface [95]. Culturing hESCs-pancreatic progenitor along with HUVECs and/or human neonatal dermal fibroblasts, allowed the formation of vessel-like networks in a 3D construct which provided mechanical support for the cells. It also increased the expression of specific markers, PDX1, NK6 Homeobox1 (NKX6.1), NGN3 and INSULIN, in the population of the progenitor cells [96]. The co-culture of HUVECs or mouse islet ECs with hESC-pancreatic progenitor, demonstrated that ECs by secreting Epidermal Growth Factor‐Like Domain 7 (EGFL7) maintain progenitor self-renewal and suppress further differentiation into other pancreatic endocrine cells [97]. hESC-derived β-cells and HUVECs were co-seeded on top of Matrigel to facilitate cell–cell interaction, resulting in the assembly of the islet-like structures and enhanced expression of endocrine-specific markers and functionality as investigated by GSIS [98]. Using HUVECs along with hESC-pancreatic epithelium seeded on a novel hydrogel named Amikagel, led to formation of multicellular endothelialized 3D pancreatic aggregates with enhanced expression of PDX1 and NKX6.1 markers [99]. HUVECs were also able to improve cell functionality when co-cultured with mouse insulinoma 6 (MIN6) cells. This cellular combination at 1:1 ratio (i.e., equal concentrations of HUVECs and MIN6), in non-adherent agarose microwell systems, generated aggregates of 80–100 µm in diameter and improved insulin secretion after encapsulation in a sealed device [100]. Rat aortic ECs‑coated islets embedded in polyglycolic acid (PGA) scaffold showed improvements in cell interactions, survival and revascularization in-vitro and in-vivo [101]. Heterogeneous pseudo-islet spheroids generated using HUVECs co-cultured with human cell line-based β-cells (EndoC-βH3) in a magnetic levitation system,4 facilitated HUVEC integration. Results indicated that cell–cell contacts and importantly, defined spatial distribution of the cell types, crucially affect β-cells functionality and insulin secretion [102].
Mesenchymal cells accompanying pancreatic epithelium In-vitro co-culture/co-transplantation experiments using pancreatic epithelium with heterotrophic organ mesenchyme or even soluble mesenchymal factors, demonstrated growth and differentiation of the epithelium [103, 104], or even exocrine fate differentiation [45, 59]. It has been also demonstrated that mouse pancreatic mesenchyme has a permissive effect on endocrine development and its maturity. In fact, the amount of pancreatic mesenchymal cells (cell ratio in the co-culture) and their proximity to the epithelium determines the exocrine/endocrine ratio [105]. Pancreatic mesenchyme isolated from different stages of the pancreas development might have different effects on differentiation pattern of the epithelium [106]: the recombination culture experiments using mouse E11.5 mesenchyme with E10.5 pancreatic epithelium yielded more mature epithelium when compared with contrariwise cultures. E13.5 mouse mesenchyme were found to be responsible for creating a balance between the Pdx1 progenitor pool and endocrine differentiated cells which might be also dependent on culture conditions such as the oxygen concentration [44].
Recently, it was shown that indirect co-culture of BM-MSC or its conditioned medium enhanced further proliferation/differentiation of human fetal pancreatic progenitor cells via IGF1-MEK/ERK1/2 / IGF1-PI3K/Akt signaling [107]. Pancreatic mesenchymal cells also induced proliferation/expansion and self-renewal of mouse- or hESC-derived endoderm through a 2D co-culture system. These cells, expanded on pancreatic mesenchyme, gave rise to insulin-secreting and glucose-sensing cells when transplanted in-vivo. This shows promise for large-scale production of pancreatic progenitors required for cell replacement therapies [108].
Insights into in-vivo pancreatic mesenchymal microenvironment and lineage-specific crosstalk could potentially help in defining targeted protocols for differentiating hPSCs into different pancreatic cell types. Applying factors secreted by mesenchymal cells that were identified in isolated E11.5 mouse pancreatic mesenchyme, on hESC-derived pancreatic differentiation in-vitro at the progenitor stage, promoted the induction of pancreatic progenitor while subsequently prohibited their further differentiation or maturation to insulin-producing cells [54]. The pancreatic Nkx2.5+ mesenchyme population produces a defined ECM and signaling factor which promotes endocrine differentiation. The addition of certain molecules (e.g., Slits) into pancreatic lineage differentiation protocols of human induced pluripotent stem cells (hiPSCs), enhances the endocrine differentiation and improves the functional maturation of differentiated β-like cells. Therefore, Slit molecules can be one of the mesenchymal signaling factor candidates which are able to regulate endocrine differentiation [48].
While there are significant data showing the beneficial impact of pancreatic mesenchymal-epithelial co-culture systems on endocrine development/differentiation, but the results are diverse and sometimes contradictory. Culture setups such as 2D or 3D culture, duration of culture [44, 108], oxygen level in culture [42], correct and clean separation of the mesenchyme [109], direct or indirect cell–cell interaction [107, 110], and the absence of other different cells involved in normal pancreas development may be causes for different results obtained.
Non-epithelial cells in islets survival Different N-Epi cells with different origins have been used in co-culture/-transplantation studies for pancreatic islets survival/engraftment and maintenance (Table 3). While studies include various N-Epi co-culture/-transplantation experiments, most of them focus on ECs and mesenchymal cells and their derivatives. Co-transplantation of ECs with pancreatic islets into different sites of STZ–treated diabetic mice resulted in graft revascularization, islet morphology preservation and decreased glycemia [111, 112]. Using human blood outgrowth ECs in co-transplantation with islets also led to improved graft-vessel density and decreased glycemia in diabetic mice [113]. Pancreatic islets isolated from transgenic Tie-2-GFP mouse showed that fresh islet ECs were able to increase revascularization rate and graft function for a long-time period after transplantation. This Green fluorescent protein (GFP) marked ECs formed blood vessels that integrated with the vascular system of the host’s organ. However, this approach could not increase the vascular density or metabolic function of the fresh graft compared to the cultured islet grafts [114]. Using bioengineered coating methods enabled scientists to coat the surface of mice and human islets by ECs which made a robust cell binding even after transplantation and led to greater graft capacity, higher vascular density and oxygen tension, and produced more functional chimeric blood vessels [115, 116].
Table 3.
Co-cultivation/-transplantation of non-epithelial cells with pancreatic islets
| Cell type/soluble factor | Origin | Islet type | Method of administration | Observation | Site of TX./animal model | Referencess |
|---|---|---|---|---|---|---|
| EPCs | Human | Mouse | Co-transplant |
Rapid revascularization Higher insulin level |
Kidney capsule/STZ-mice | [228] |
| BM-EPCs | Mouse | Mouse | Co-transplant |
Improve islet transplantation Revascularization Preserve islet morphology |
Kidney capsule/STZ-mice | [112] |
| Blood outgrowth ECs | Adult human | Rat | Co-transplant |
Reduce β-cells death Glycaemia improvement Increase C-peptide and graft-vessel |
Kidney capsule/NOD/SCID mice | [113] |
| EPCs | Rat | Rat | Co-transplant |
Long lasting normo-glycaemia Neovascularization Improve graft survival |
Portal vein/STZ-rat | [111] |
| Vascular ECs | Mouse | Mouse | Surface coating | Greater engraft capacity | Kidney capsule/STZ-mice | [116] |
| EPCs | Human | Human | Surface coating |
Higher vascular density Higher oxygen tension |
Kidney capsule/NOD/SCID mice | [115] |
| BM-MSCs | Rat | Rat | Direct co-culture |
Increase islet survival and viability Increase insulin secretion Improve islet functionality by preserving membrane integrity and releasing trophic factors |
– | [110, 117, 229] |
| BM-MSCs | Rat | Rat | Indirect co-culture | Increase in islet survival by trophic factors: VEGF, IL-6, Von Willebrandt | – | [110] |
| BM-MSCs |
Mouse MSC-CM |
Mouse | Co-culture |
Promote islet cell proliferation Increase in pAkt and pErk expression by islets |
– | [120] |
| BM-MSCs and dermal microvascular ECs | Human | Human | Co-culture | Promote EC proliferation, growth and sprout formation | – | [118] |
| MSCs | Human | Mouse | Co-culture |
Lower ADP/ATP ratios Less apoptosis Higher GSIS indexes Higher viability |
– | [119] |
| MSCs SFs: TGFβ, HGF, VEGF and IL-6 | Human MSC-CM | Mouse | Co-culture before transplant |
Improve islet cell viability and function Lower blood glucose levels Enhance blood vessel formation |
Diabetic mice | [119] |
| Adipose MSCs | Human | Human | Co-culture and co-transplant |
Upregulates the anti-apoptotic factor Metallopeptidase Inhibitor-1 Improve β-cell maintenance and proliferation Regulate immune response by suppression of CD4+ T helper cell-1 |
Injected intravenously in tail vein/STZ-NOD/SCID mice | [125] |
| BM-MSCs | Rat | Rat | Co-transplant |
Improvement of islet graft morphology and function Promotion of graft revascularization Enhance graft survival |
Liver/STZ-mice Kidney capsule/NOD/SCID mice and diabetic rat |
[121] [230] |
| BM-MSCs | Mouse | Mouse | Co-transplant |
Reduce ratios of T helper cell-1/2 Down-regulate of naive and memory T-cells numbers in peripheral blood Prevent islet allograft rejection Long-term normo-glycaemia MMP-2 and MMP-9 reduce CD25 expression on T-cells |
Kidney capsule/STZ-mice | [123] [126] |
| BM-MSCs | Monkey | Monkey | Co-transplant |
Enhance islet engraftment and function Increase numbers of regulatory T-cells in peripheral blood |
Intraportal | [124] |
| MSCs/ECM Pr.* | Mouse | Mouse | Co-encapsulate in silk |
Improve GSIS Enhance islet function |
– | [231] |
| MSCs | Mouse | Mouse | Co-encapsulate in silk before co-transplant |
Prompt return to euglycaemia Improves intraperitoneal glucose tolerance test Avoid host immune-attack |
Epididymal fat pad/STZ-mice | [232] |
| BM-MSCs | Rat | Rat | Co-encapsulate in synthetic PEG hydrogels | Improve islet function and GSIS in physiological environment | – | [170] |
| Adipose MSCs | Mouse | Rat | Micro Co-encapsulate in nylon mesh transplant pockets before co-transplant | Enhance the efficacy and survival of islet transplantation | Abdomen cavity/diabetic mice | [233] |
| BM-MSC derived ECM | Rat | Rat | Co-culture |
Restores the pancreatic islet basement membrane Preserves islet function Attenuates islet immunogenicity |
– | [234] |
| hESC-MSC:VEGF | Human | Mouse | Co-transplant |
Increase graft revascularization Reduction by half in islet mass |
Omental pouch of diabetic nude mice | [127] |
| Neural crest stem cell | Dorsal root ganglia of E11.5 mouse | Mouse | Co-transplant |
Enhance β-cell proliferation Increase β-cell mass Improve insulin release in normoglycaemic mice Partially restore normo-glycaemia in diabetic mice |
Kidney capsule/normoglycaemic and alloxan-induced mice | [128] |
EPC endothelial progenitor cell, EC endothelial cells, TX transplantation, PFs paracrine factors, NOD/SCID non-obese diabetic severe combined immunodeficiency, SFs secreting factors, BM-MSC bone marrow derived mesenchymal stem cell, IL-6 Interleukin-6, MSC-CM MSC derived conditioned medium, ECM pr extracellular matrix proteins, PEG poly ethylene glycol
*Laminin and Collagen IV
Studies using direct and indirect co-cultivation of MSCs with islets showed improvement in islet survival and viability. It seems that indirect co-culture of MSCs influences via their secreted trophic factors such as Von Willebrandt, VEGF, and Interleukin-6 (IL-6) [110]. Direct co-cultures of MSCs also led to higher insulin detection in culture medium probably by preserving islet membrane integrity, improvement of islet functionality or even due to differentiation of some of MSCs to insulin-releasing cells in co-culture systems [110, 117]. When MSCs are co-cultured with ECs and pancreatic islets, they promote EC proliferation, growth, and sprout formation inside islets and, therefore, enhancing islet revascularization [118]. Paracrine factors secreted from MSCs, such as TGFβ, HGF, VEGF, and IL-6, inhibit apoptosis, induce β-cell proliferation and promote islet revascularization, hence improving islet viability and functionality [119, 120]. Moreover, it has been shown that co-transplantation of MSCs and islets may enhance the chance of graft survival and reduce islet damages upon hypoxia [121, 122]. MSCs co-transplantation prevents islet allograft rejection, improves β-cell maintenance and proliferation, and maintains long-term normo-glycaemia. Co-transplantation with MSCs has also been reported to modulate immune response by down-regulation of naive and memory T-cells numbers, reduction of T helper cell-1/2 ratios and increment of regulatory T-cells numbers in peripheral blood [123, 124],and through upregulating the anti-apoptotic factor Metallopeptidase Inhibitor-1 and suppression of CD4+ T helper cell-1 [125, 126]. Therefore, an approach could be genetic modification of MSCs to positively affect the immune response of the receiver at the site of transplantation. For instance, co-transplantation of mice islets with VEGF expressing hESC-MSCs in a collagen-fibrin hydrogel increased islets function and graft revascularization, and reduced the islet mass required to reverse DM by half [127].
Stellate cells co-cultivation and co-transplantation can improve the survival rate of islets, regulate the immune response, and stimulate graft vascular regeneration [69]. NC stem cells co-transplantation with islets has also shown to increase β-cell proliferation and restore normoglycemia. Therefore, neuron-islet interactions can also offer an opportunity to improve islet function after transplantation [128], however, more investigation is needed to define the exact influence of stellate and NC cells in islet co-transplantation.
Employing pluripotent stem cell technology to generate pancreatic cell types: achievements and challenges
The unique properties of PSCs make them a good candidate for large-scale production of different pancreatic cell types including islet-like cells and to generate pancreatic organoids for further co-culture studies and development simulation. Through mimicking pancreatic development cues, outstanding works from different research groups was applied to generate stem cell-derived β-cells (SC-β cells). These efforts were pioneered by scientists who generated PSC-derived definitive endoderm [129] which was subsequently further advanced towards PDX1+ pancreatic progenitors [130–132]. The insulin-positive cells which were developed from these cells, were polyhormonal, their insulin secretion was limited, and needed several months’ maturation after transplantation to observe their functionality and glucose responsiveness [4, 133, 134]. However, a breakthrough occurred by the generation of SC-β cells which were capable of secreting insulin in response to in-vitro glucose stimulation [5, 135] and also were able to cure moderate DM after transplantation into mice model. The monohormonal state observed in these protocols, was due to stimulating the sequential expression of NKX6-1 marker in PDX1+ pancreatic progenitors prior to the endocrine marker NGN3 expression [136, 137]. However, SC-β cells lacked several characteristics of mature β-cells’ such as lower overall insulin secretion and a dynamic glucose stimulation in response to insulin secretion (reviewed in [1, 3]).
Since then, different studies have attempted to improve the subsequent differentiation protocols, as well as proliferation and enrichment of monohormonal cells to generate functional and mature SC-β cells through various methods such as co-culture with mesenchymal cells or ECs [138, 139], mitogenic signal addition (e.g., EGF) [140], endocrine cell clustering [141], specific cell-surface markers identification for isolation and further differentiation of endocrine progenitors (e.g., GP2) [142], application of metabolic stimuli [143], limitation of focal adhesion kinase (FAK)-dependent activation [144], targeting and depolymerizing the cytoskeleton with latrunculin A [145], and enriching β-cells and eliminating tumorigenic by engineered PSCs with suicide genes to prevent or ablate hESC-derived tumors and select hESC-derived pancreatic β-cells in-vitro and in-vivo [146]. It must be mentioned that the stem cell fate, including quiescence, proliferation or differentiation is influenced by scaffold design properties [147]. The scaffold chemical composition, physical cues (i.e., geometry, surface topography, and degradability), and mechanical properties (i.e., bulk stiffness and stress relaxation) could be used to direct stem cell fate [148].
The first clinical trial with ESC-derived pancreatic progenitor cells (PEC-01) were conducted in 2014 [149]. Since there are no specific anatomical limitations for insulin injection, endocrine precursor or SC-β cells are usually transplanted to an environment other than the pancreas such as subcutaneous or intraperitoneal cavity. In addition to selecting the right transplant site, there are differing opinions regarding the dosage or number of cells needed to achieve better metabolic control [150]. Another challenge is to ensure adequate blood circulation and prevent or reduce immune rejection reactions in the host body. To prevent graft rejection and autoimmune damages, strategies such as enclosing cells in immunoprotective capsules, implanting cells in scaffolds or semi-permeable macro or micro physical barriers, biocompatible tools usage with angiogenic and immune system protection properties, and genetic manipulation of desired cells are examples of proposed solutions [149, 150]. A flat structure (VC-01) for macro-encapsulation of PEC-01 was developed that was transplanted subcutaneously. VC-01 consisted of a semi-permeable membrane that allowed molecules to be exchanged while preventing the transplanted cells from coming into contact with the receptor cells. While this clinical trial was stopped early due to a reaction to the implanted capsule and loss of transplant function, a new device (VC-02) was designed to allow blood vessels to enter the grafted structure for angiogenesis. Unlike the previous structure, VC-02 did not protect the recipient’s immune system and, therefore, required the use of immunosuppressive drugs. Preliminary results of the phase I clinical trial indicate successful transplantation and observation of insulin-secreting cells as well as increased levels of C-peptide in these transplants up to one year [151, 152].
PSC- and iPSC-mediated gene editing technology also can be conducted for eliminating tumorigenesis [146], resistance to immune rejection, and mutation corrections in patients with monogenic diabetes [2, 153]. CRISPR/Cas9 correction of Akita insulin gene mutation in patients derived iPSCs and differentiating them to β-like cells showed that corrected β-like cells had higher levels of insulin secretion compared to INS-mutant groups [2]. It has been reported that R(B22)E yields a bioactive insulin and R(B22)Q, causes Mutant INS-gene induced Diabetes of Youth (MIDY). When a CRISPR/Cas9-mediated was knocked into the Ins2 locus of proinsulin-R(B22)E, none of the heterozygous mice were diabetic. However, heterozygous male mice developed glucose intolerance and diabetes after exposure to a high-fat diet [153]. It has been revealed that pancreatic islet cells produced from iPSC in patients with T1DM do not differ significantly from non-diabetics in terms of expression of adult β-cell markers and response to the glucose challenge test [154, 155]. Therefore, successful production of iPSC from DM patient’s somatic cells has made it possible to study individual-based mechanisms and treatment methods through disease modeling and drug testing [156, 157].
Multi-cell type pancreatic organoids: 3D recapitulation
3D co-culture of embryonic murine E10.5 pancreatic progenitors and native mesenchyme create pancreatoids (an organotypic 3D pancreas culture) expressing markers of all the three pancreatic lineages. Pancreatoids expressed a higher number of insulin-positive and endocrine-like cells in comparison with 3D epithelium cultures without native mesenchyme. The native mesenchyme, rather than providing structural support, is essential to provide signals to the epithelium for differentiation instructions [109]. Pancreatic condensates were also created via BM-MSCs in a self-condensation culture system. Dissociated murine β-cells, isolated adult mouse/human islets or hiPSC-derived pancreatic tissues when co-cultured with ECs and MSCs, produced a dynamic condensate formation. These self-organized organ-like structures developed into large, mechanically stable, 3D tissues and when transplanted into T1DM mouse, resulted in rapid reperfusion, stimulated successful β-cell engraftment and effectively normalized blood glucose [158, 159]. Moreover, generation of pancreatic organoids from PSC-derived-ECs, -MSCs, and -PPs provided a pancreatic micro-tissue which enhanced β-cell maturation and vascularization after transplantation. The transplants developed islet-like structures with functional microvascular networks [138]. In a recent study, the effect of hESC-pancreatic progenitor’s co-cultivation with human fetal pancreatic-derived mesenchymal cells, sandwiched within two layers of Matrigel, were studied. The spheroids generated after 14 days of co-culture, had higher expression of NGN3 and INSULIN compared to hESC-pancreatic progenitors co-culture with BM-MSCs, showing an inductive impact of native mesenchyme on hESC-pancreatic progenitors further differentiation [139].
Therefore, pancreatic 3D co-cultures and organoids offer useful tools for developmental studies, disease modeling, drug testing, small molecule screening, and β-cell regeneration. They also provide evidence for mimicking in-vivo developmental processes for ESC-derived β-like cell differentiation [109, 138, 159]. Over the last decade, variety of pancreatic cancers including pancreatoblastoma, acinar cell carcinoma, cystic and mucosal cancer, PDAC, insulinoma, and tumors of other endocrine cells, as well as cystic fibrosis (CF) have been modeled in the lab [160, 161]. To produce pancreatic organoids with the CF transmembrane conductance regulator (CFTR) gene mutation, iPSCs derived from CF patients were differentiated into the pancreatic progenitors. These organoids showed the function of the duct and acinar cells and used to investigate the effect of gene mutations and new drugs on the structure and function of CFTR protein [162]. PDAC is characterized by a desmoplastic reaction that results in the formation of dense stroma and fibrosis. The co-culture of PDAC organoids with pancreatic stellate cells reconstructed the PDAC desmoplastic response by converting pancreatic stellate cells from dormant to active stroma-producing fibroblasts [163]. Co-culture of PDAC organoids and cancer-related fibroblasts can be used as a screening system for drug detection [164]. New organoid models make it possible to co-culture or simultaneously differentiate epithelial and mesenchymal components to model inflammatory diseases [165].
Bottlenecks in pancreatic 3D recapitulation To produce pancreatic/islet micro-tissues which are functionally more similar to the original organs, the knowledge gap on different unknown aspects of N-Epi cells contribution in human pancreas development should be reduced. Table 4 shows a summary of some of these known and unknown aspects. Although transcriptome and protein expression profile of pancreatic mesenchyme, their population heterogeneity during development and transcriptome similarity with their common sources are known to a large extent, little information is available for these aspects in other N-Epi cells. The cell ratio and density of each N-Epi cells during development must also be clarified. Information on arrival time of each N-Epi cells into pancreatic bud and cell ratios during development could be helpful to define the proper time point and densities for setting up the co-culture systems in-vitro. In addition, signaling crosstalks of pancreatic NC cells and pericytes with pancreatic epithelium and islets is still unclear which could be helpful to increase the efficiency of fate specific differentiation throughout 3D micro-tissues. Single-cell RNA techniques and genome-wide mRNA expression profiling on different pancreatic compartments have identified cell-surface markers, new specific cluster markers [66], and well described regulatory genes that are not yet functionally annotated in pancreas development which could be used for cell-specific sorting, different cell clustering and genetic manipulation in future studies [166]. Genetic analytical studies have helped building synthetic cellular networks to improve our understanding about the basic principles of biological behaviors of multicellular structures and better program self-organizing multicellular structures in-vitro [167].
Table 4.
Main challenges in understanding the effect of pancreatic non-epithelial cells on pancreatic development
| Challenges/lack of knowledge | Endothelial cells | Mesenchymal cells | Neural crest cells | Pericytes |
|---|---|---|---|---|
| Transcriptome/protein expression during development | – | + | – | ± |
| Heterogeneity in population | ± | + | – | – |
| Similarity of transcriptome with common sources of the same N-Epi | ± | ± | – | – |
| Cell ratio /density during development | ± | ± | – | – |
| Arrival time point of N-Epi cells in pancreatic tissue | + | + | + | ± |
| Signaling crosstalk with pEpi in early development | + | + | ± | ± |
| Signaling crosstalk with pIsl | + | + | ± | ± |
| 3D cell–cell interaction with pEpi | + | + | + | ± |
+ gained knowledge; – lack of knowledge; ±: not completely understood
N-Epi non- epithelium, pEpi pancreatic epithelium, pIsl pancreatic islets
Bioengineering approaches to recapitulate pancreatic cell–cell interactions in vitro
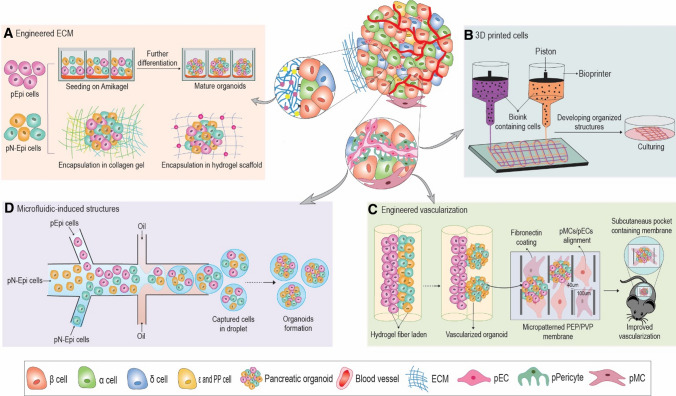
The cell patterns during development are influenced by microenvironment factors including soluble factors (i.e., small molecules, cytokines, and growth factors), ECM properties, cell–cell interactions, and mechanical forces. Cell–cell interactions are of particular importance for formation of multicellular structures. Various approaches have the potential to engineer cell–cell interactions in development of pancreatic organoids, including ECM design, vascular engineering, manipulation of signaling molecules on the cell-surface via genetic manipulation, bioprinting methods, application of remote forces, and microfluidics cell encapsulation (Fig. 2) [168, 169].
Fig. 2.
Feasible and conceivable bioengineering approaches for formation of functional multicellular pancreatic organoids. Different bioengineering approaches used to mimic mature β-cells in pIsl. A Engineered ECM: different approaches in ECM engineering for co-cultivation of Epi and Np-Epi cells including Amikagel, collagen and encapsulation in hydrogel. ECM components provide a milieu to recapitulate cell–cell interactions. B 3D printed cells: using 3D bioprinting approach to produce complex structures of β-cells and N-Epi cells with defined architecture and geometry. Using bioprinting technology a combination of proper bio-ink and pancreatic cells can be printed into a layered micro-tissue. C Engineered vascularization: hydrogel fibers and micro-patterned membranes before implantation for generating multicellular organoids and performing cell–cell interactions and vascularization. Hydrogel fibers loaded with epithelial cells, mesenchymal cells or ECs facilitating cell–cell interactions along the fiber and final assembly of a 3D tissue construct. D Microfluidic-induced structures: cell sorting and microfluidic devices for uniform multicellular organoid formation in large-scale. The microfluidic platform enables controllable cellular patterning and initial geometries of different pancreatic cell types in a combinational co-culture droplet system.
Engineered ECM components 3D multicellular constructs have been designed for co-cultivation or co-encapsulation of pancreatic epithelium cells with mesenchymal cells/endothelial cells, on polymeric or biomaterial substrates such as poly ethylene glycol (PEG) [170], Amikagel [99], fibrin [96], laminin [171], collagen [59], and matrigel [45] hydrogels. Co-encapsulations in hydrogel at defined ratios of different cell types, can facilitate the control over the proper level of each signal created by each cell in the co-culture system [172]. It was demonstrated that when epithelial cells and mesenchymal cells are placed adjacent to each other in collagen gel, the permissive 3D ECM provides a milieu for recapitulating cell–cell interactions. The use of defined synthetic matrices which can be temporary variable and cell-responsive, instead of poorly controllable and ill-defined matrices, can also improve mimicry [169]. As the N-Epi cells such as ECs and mesenchymal cells have time-sensitive interactions on epithelial cells during development, degradable two-tier hydrogel constructs may be useful for achieving proper cell interactions between cells [173]. Tunable and adjustable synthetic biomaterial can also be engineered to mimic heterotypic and homotypic cell–cell contacts and direct morphogenesis and differentiation [168]. Nanopatterned dishes have been used as a new topographical technology for co-culturing mesenchymal cells and epithelial cells and their cells growth rate are regulated by Nanopore substrates [174].
Bioengineered cell surface One of the possible approaches for in-vitro production of complex cellular structures with engineered cell–cell interactions is manipulation of synthetic cellular networks. Genetic studies revealed molecular mechanisms of cell–cell communication pathways, this opens new opportunities for directing cellular organization by programing cell interactions [167]. For instance, the engineering cell-surface signal of Notch as a cell adhesion molecule with genetic engineering recapitulate the developmental pathways in molecular scale and induced customized self-organizing similar to embryo development. Engineering of cell behavior in molecular scale may compensate limitation of in-vitro organization for formation of organoids such as pancreas which is induced by complex cell–cell interactions. Although molecular engineering is known as a biomimicking method, the techniques of molecular design and genetic engineering for obtaining a demanded structure are complex and time-consuming [167].
Microfluidic-induced structures Microfluidic technology involves engineered manipulation of fluids in the channels of micro- and nano-scale. Fluids show different behavior in micro-scale devices in comparison to traditional flow. The laminar flow in micro-scale allows mimicking the spatiotemporal profile of soluble factors in embryo development and tissue hemostasis [172, 175]. In addition, droplet-based microfluidics can also be used to encapsulate different cell types in a defined pattern of a multicellular structure of interest. Microfluidic encapsulation methods can produce a uniform size and shape of pancreatic organoids and help quick cell aggregation and differentiation of the desired cells [176]. The microfluidic systems also enable controllable cellular patterning and initial geometries of different cell types in a combinational cell co-culture [175]. In addition to microfluidic-based cell encapsulation, micro-patterned surfaces could induce the forced assembly of heterotypic cell constructs. In this approach without using a scaffold, arrays of a microwell with different shapes of concave and cylindrical (e.g., with 400 μm diameter) enables the production of large numbers of spheroids with controlled size and cell density. This technique has been used for the reaggregation of single β-cells and other islet cells [177]. These engineering methods have benefits compared to hanging drop due to the fast process and uniformity of aggregate size [177].
Use of 3D-printed cells to form organized structures Cell printing methods are an exciting new way to produce a complex cell structure within a biomaterial [178]. Bioprinting has been applied as a tool to engineer multicellular structures with different methods including inkjet (with a resolution of 50 µm), micro-extrusion (with a resolution of 5 µm to millimeters), and laser-assisted printing (micro-scale resolution) [179]. Current approaches in bioprinting are focused on development of new methods for bioprinting at the single-cell scale with high cell viability. Moreover, complex multicellular structures can be formed through application of remote forces such as acoustic streams [180] and magnetic fields [181]. The printing and remote force methods may pave the way for production of complex structure of β-cells and N-Epi cells with defined architecture and geometry. Bioprinting is an automated method for the construction of 3D solid tissue and organs. One of the major challenges of this method is the limitation of printable biomaterials with suitable gel time and mechanical properties. Moreover, bioprinting needs to increase the resolution for small features and enhance the processing speed for large structures [182].
Application of remote forces Remote fields have recently been used for the construction of 2D or 3D complex tissue structures by application of external forces to levitated cells without the need for ECM materials [183]. In this high-throughput approach, a multicellular organization can be guided using remote forces such as acoustic streaming, magnetic fields, and optical tweezers. In acoustic levitation, physiologically relevant structures have been generated in a form of multilayered sheets with a precise number and size of layers [184] or aggregates with a packing density similar to the native organ [185]. Another approach for remote manipulation of a cell population is using optical tweezers tailoring precise 3D position in single-cell scale [186]. Magnetic forces have been widely used for levitation and cellular assembly of complex structures. By manipulation of magnetic field patterns, multicellular structures such as spheres and rings could be formed with defined dimensions [187]. Using a magnetic field, the pseudo-islet structure of human β-cells/HUVECs in core–shell aggregates has been formed. In addition to higher stability of the formed spheroids, precise determination of spatial distribution of HUVECs in pseudo-islets promotes β-cell functionality and develops pre-vascularized transplantable islet-like structures [102].
Engineered vascularization Various engineering approaches are used to promote vascularization including engineering physical properties of ECM such as porosity, pore geometry, pore size [188], and stiffness [189]. Other methods include the manipulation of ECM chemical properties such as adhesion domains, crosslinking type [190], and density [191]; moreover, designing controlled delivery systems including micro/nanoparticles [192] and scaffold-based encapsulation [193] for delivery of growth factors, small molecules, and microRNAs [194]. Here, we further focus on engineering of the “cell–cell interactions” as a tool for enhancement of vascularization process. The processes conducted for multicellular formation are based on mixing different desired single cells for expected self-organization [159, 195]. However, some cell components may not show this preferred feature during the process. Bioengineering strategies can be applied for pancreatic organoid vascularization and desired cell interaction or condensation. Different studies developed substrates such as Star-PEG-heparin and hyaluronic acid hydrogels, to mimic EC morphogenesis for vascularization [173, 196]. Some studies also used fibrin-based hydrogels to support vascularization [197] or fibroblast-populated collagen matrix to increase islet viability in-vitro [198]. Cell-laden hydrogel fibers, as bio-structural units comprising of parallel, adjacent compartments, are each loaded with epithelial cells, mesenchymal cells or ECs and assembled in a 3D tissue construct. These multi-compartment fibers would facilitate cell–cell interactions along the fiber, leading to pancreatic vascularization in organoid-like structures [199] or numerous individual condensed pancreatic organoids [200]. Recently, a functional microvascular network with polyethersulfone/polyvinylpyrrolidone (PES/PBP) porous membrane was developed and combined with BM-MSCs. This micro-patterned membrane can be used to pre-vascularize the pancreatic organoids before transplantation [201, 202].
Conclusion
In this review, we discussed the importance and role of pN-Epi cells in proper pancreatic development and their effect on pancreatic epithelium. However, there are still remarkable gaps in our knowledge of the actual influence of N-Epi cells in human pancreas development. Human PSC technology and pancreatic multicellular organoids can be considered as a tool to provide information for developmental studies, disease modeling, drug testing, and small molecule screening for β-cell regenerative studies. Although our knowledge of pancreatic development is slowly evolving, another issue is how to translate this knowledge to produce more functional organoid structures in-vitro. Developing novel engineering platforms which help developmental biologists to generate engineered organoids would be a possible future solution. Bioengineering approaches such as ECM components, microfluidic-induced structures, engineered vascularization, and 3D-printed cells can help to recapitulate important aspects of self-organization and organoid formation.
Acknowledgements
This study was funded by Royan Institute for Stem Cell Biology and Technology (RI-SCBT) and Royan’s Lotus Charity Investment Foundation.
Abbreviations
- 2D
Two-dimensional
- 3D
Three-dimensional
- α-SMA
Alpha-smooth muscle actin
- AAT
Alpha-1 antitrypsin
- Ang-1
Angiopoietin
- BM-MSC
Bone marrow-mesenchymal stem cells
- BMP4
Bone morphogenetic protein 4
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- DM
Diabetes mellitus
- DKK1
Dickkopf-related protein 1
- DPC
Days post-conception
- EB
Embryoid bodies
- E
Embryonic day
- EC
Endothelial cell
- ECM
Extra cellular matrix
- EGFL7
Epidermal growth factor‐like domain 7
- EPC
Endothelial progenitor cell
- eNOS
Endothelial nitric-oxide synthase
- FAK
Focal adhesion kinase
- FGF10
Fibroblast growth factor 10
- FOXD3
Forkhead Box D3
- GDNF
Glial cell-derived neurotrophic factor
- GFP
Green fluorescent protein
- GLUT2
Glucose transporter 2
- GP2
Glycoprotein 2
- GSIS
Glucose -stimulated insulin secretion
- hESC
Human embryonic stem cell
- HGF
Hepatocyte growth factor
- hiPSC
Human induced pluripotent stem cell
- hPSC
Human pluripotent stem cell
- HOX
Homeobox
- HUVEC
Human umbilical vein endothelial cell
- IGF
Insulin growth factor
- IL-6
Interleukin-6
- IRS2
Insulin receptor substrate-2
- ISL-1
Insulin gene enhancer protein ISL-1
- LAMA2
α2 Laminins
- LGALS1
Galectin 1
- MAFA
Musculoaponeurotic fibrosarcoma oncogene family A.
- mESC
Mouse embryonic stem cell
- MIDY
Mutant insulin-gene-induced diabetes of youth
- MIN6
Mouse insulinoma 6
- MMP
Metalloproteinases
- MPCs
Multipotent progenitor cells
- MSCs
Mesenchymal stem cells
- NC
Neural crest
- NG2
Neural-glial Antigen 2
- NGN3
Neurogenin3
- NKX3.2
NK3 Homeobox 2 /NKX2.2: NK2 Homeobox 2 /NKX6.1: NK6 Homeobox 1
- PDAC
Pancreatic ductal adenocarcinoma
- PAX3
Paired box gene 3
- PHOX2B
Paired-like homeobox 2b
- PECAM1
Platelet and endothelial cell adhesion molecule 1
- PEG
Poly Ethylene Glycol
- PES/PBP
Poly ether sulfone/poly vinyl pyrrolidone
- PBX1
Pre-B-cell leukemia transcription factor 1
- PDX1
Pancreas-duodenum homeobox protein1
- PDGF
Platelet-derived growth factor
- PDGFRβ
Platelet-derived growth factor receptor-beta
- PGA
Poly glycolic acid
- PTF1a
Pancreas transcription factor 1 subunit alpha
- pN-Epi
Pancreatic non-epithelial
- RAS
Renin–angiotensin system
- RHMVEC
Rat heart microvascular endothelial cell
- S1P
Sphingosine-1-phosphate
- SC-β CELLS
Stem cell-derived β-cells
- SDC4
Syndecan4
- SFRP3
Secreted frizzled-related protein 3
- SOX9/10
SRY-Box 9/10
- STZ
Streptozotocin
- SynCAM
Synaptic cell adhesion molecule/CADM-1
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- TCF7L2
Transcription factor 7 like 2
- TGFβ
Transforming growth factor β
- Tie-2
Tyrosine-protein kinase receptor Tie-2
- TIMP-1
TIMP metallopeptidase inhibitor 1
- TF
Transcription factor
- TFAP2α
Transcription factor AP-2 alpha
- Vcan
Versican
- VEGF
Vascular endothelial growth factor
- vSMC
Vascular smooth muscle cell
- WPC
Weeks post-conception
- WNT
Wingless
Author contributions
The conception and design of the study was done by [YT]. Administrative support was done by [FCL]. The first draft of the manuscript was written by [ZG, MZ, MKA, and ZG], and all authors commented on previous version of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Royan Institute for Stem Cell Biology and Technology (RI-SCBT) and the Lotus Charity Investment Foundation, Royan Institute.
Declarations
Conflict of interest
The authors have no conflicts of interest.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
a Lipophilic cationic indocarbocyanine fluorescent dye for membrane staining.
A cell-surface chondroitin sulfate proteoglycan.
The epithelium lining the surface layer of the embryonic mesoderm.
Magnetic levitation method (MLM) applies growing 3D tissue by inducing cells treated with magnetic nanoparticle assemblies in spatially varying magnetic fields using neodymium magnetic drivers and promoting cell–cell interactions by levitating the cells up to the air/liquid interface of a standard petri dish.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zahra Ghezelayagh and Mahsa Zabihi contributed equally to this work.
References
- 1.Nair GG, Tzanakakis ES, Hebrok M (2020) Emerging routes to the generation of functional β-cells for diabetes mellitus cell therapy. Nat Rev Endocrinol 1–13 [DOI] [PMC free article] [PubMed]
- 2.Bourgeois S, Sawatani T, Van Mulders A, De Leu N, Heremans Y, Heimberg H, Cnop M, Staels W. Towards a functional cure for diabetes using stem cell-derived beta cells: are we there yet? Cells. 2021;10:191. doi: 10.3390/cells10010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JD. The quest to make fully functional human pancreatic beta cells from embryonic stem cells: climbing a mountain in the clouds. Diabetologia. 2016;59:2047–2057. doi: 10.1007/s00125-016-4059-4. [DOI] [PubMed] [Google Scholar]
- 4.Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O'Neil JJ, Ao Z, Warnock GL, Kieffer TJ. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 7.Abdelalim EM, Emara MM. Advances and challenges in the differentiation of pluripotent stem cells into pancreatic β cells. World J Stem Cells. 2015;7:174–181. doi: 10.4252/wjsc.v7.i1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen HL, Grapin-Botton A. The molecular and morphogenetic basis of pancreas organogenesis. Semin Cell Dev Biol. 2017;66:51–68. doi: 10.1016/j.semcdb.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Petersen MB, Gonçalves CA, Kim YH, Grapin-Botton A. Current topics in developmental biology. Amsterdam: Elsevier; 2018. Recapitulating and deciphering human pancreas development from human pluripotent stem cells in a dish; pp. 143–190. [DOI] [PubMed] [Google Scholar]
- 10.Ghani MW, Ye L, Yi Z, Ghani H, Birmani MW, Nawab A, Cun LG, Bin L, Mei X. Pancreatic β-cell replacement: advances in protocols used for differentiation of pancreatic progenitors to β-like cells. Folia Histochem Cytobiol. 2019;57:101–115. doi: 10.5603/FHC.a2019.0013. [DOI] [PubMed] [Google Scholar]
- 11.Jennings RE, Berry AA, Strutt JP, Gerrard DT, Hanley NA. Human pancreas development. Development. 2015;142:3126–3137. doi: 10.1242/dev.120063. [DOI] [PubMed] [Google Scholar]
- 12.Khosravi-Maharlooei M, Hajizadeh-Saffar E, Tahamtani Y, Basiri M, Montazeri L, Khalooghi K, Ashtiani MK. Islet transplantation for type 1 diabetes: so close and yet so far away. Eur J Endoerinol. 2015;173:R165–R183. doi: 10.1530/EJE-15-0094. [DOI] [PubMed] [Google Scholar]
- 13.Bastidas-Ponce A, Scheibner K, Lickert H, Bakhti M. Cellular and molecular mechanisms coordinating pancreas development. Development. 2017;144:2873–2888. doi: 10.1242/dev.140756. [DOI] [PubMed] [Google Scholar]
- 14.Shih HP, Wang A, Sander MJAroc Pancreas organogenesis: from lineage determination to morphogenesis. Ann Rev Cell Dev Biol. 2013;29:81–105. doi: 10.1146/annurev-cellbio-101512-122405. [DOI] [PubMed] [Google Scholar]
- 15.Yang K, Wang Y, Du Z, Zhang X. Short-reactivation of neurogenin-3 and mesenchymal microenvironment is require for β-cells differentiation during fetal pancreas development and islet regeneration. Romanian J Morphol Embryol Revue Roumaine de Morphol Embryol. 2014;55:305–311. [PubMed] [Google Scholar]
- 16.Benitez CM, Goodyer WR, Kim SK (2012) Deconstructing pancreas developmental biology. Cold Spring Harbor perspectives in biology 4:a012401 [DOI] [PMC free article] [PubMed]
- 17.Sznurkowska MK, Hannezo E, Azzarelli R, Rulands S, Nestorowa S, Hindley CJ, Nichols J, Göttgens B, Huch M, Philpott A. Defining lineage potential and fate behavior of precursors during pancreas development. Dev Cell. 2018;46:360–375365. doi: 10.1016/j.devcel.2018.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edlund H. Developmental biology of the pancreas. Diabetes. 2001;50:S5. doi: 10.2337/diabetes.50.2007.s5. [DOI] [PubMed] [Google Scholar]
- 19.Larsen BM, Hrycaj SM, Newman M, Li Y, Wellik DM. Mesenchymal Hox6 function is required for mouse pancreatic endocrine cell differentiation. Development. 2015;142:3859–3868. doi: 10.1242/dev.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakhneny L, Khalifa-Malka L, Landsman L. Pancreas organogenesis: Approaches to elucidate the role of epithelial-mesenchymal interactions. Semin Cell Dev Biol. 2019;92:89–96. doi: 10.1016/j.semcdb.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Olsson R, Carlsson P-O. The pancreatic islet endothelial cell: emerging roles in islet function and disease. Int J Biochem Cell Biol. 2006;38:710–714. doi: 10.1016/j.biocel.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Ranjan AK, Joglekar MV, Hardikar A. Endothelial cells in pancreatic islet development and function. Islets. 2009;1:2–9. doi: 10.4161/isl.1.1.9054. [DOI] [PubMed] [Google Scholar]
- 23.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 24.Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev. 2003;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 25.Pierreux CE, Cordi S, Hick A-C, Achouri Y, de Almodovar CR, Prévot P-P, Courtoy PJ, Carmeliet P, Lemaigre FPJDb Epithelial: Endothelial cross-talk regulates exocrine differentiation in developing pancreas. Dev Biol. 2010;347:216–227. doi: 10.1016/j.ydbio.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Talavera-Adame D, Dafoe DC. Endothelium-derived essential signals involved in pancreas organogenesis. World J Exp Med. 2015;5:40. doi: 10.5493/wjem.v5.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- 28.Guney MA. Gannon MJBDRPCETR. Pancreas cell fate. 2009;87:232–248. doi: 10.1002/bdrc.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogan MF, Hull RLJD. The islet endothelial cell: a novel contributor to beta cell secretory dysfunction in diabetes. Diabetologia. 2017;60:952–959. doi: 10.1007/s00125-017-4272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staels W, Heremans Y, Heimberg H, De Leu N. VEGF-A and blood vessels: a beta cell perspective. Diabetologia. 2019;62:1961–1968. doi: 10.1007/s00125-019-4969-z. [DOI] [PubMed] [Google Scholar]
- 31.Ning FC, Jensen N, Mi J, Lindström W, Balan M, Muhl L, Eriksson U, Nilsson I, Nyqvist D. VEGF-B ablation in pancreatic β-cells upregulates insulin expression without affecting glucose homeostasis or islet lipid uptake. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-020-57599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson Å, Lau J, Sandberg M, Borg L, Magnusson P, Carlsson P-O. Endothelial cell signalling supports pancreatic beta cell function in the rat. Diabetologia. 2009;52:2385–2394. doi: 10.1007/s00125-009-1485-6. [DOI] [PubMed] [Google Scholar]
- 33.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber H-P, Ferrara N. The vascular basement membrane: a niche for insulin gene expression and β cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Pan FC, Brissova M. Pancreas development in humans. Curr Opin Endocrinol Diabetes Obes. 2014;21:77–82. doi: 10.1097/med.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto S, Kubota N, Sato H, Sasaki M, Takamoto I, Kubota T, Nakaya K, Noda M, Ueki K, Kadowaki T. Insulin receptor substrate-2 (Irs2) in endothelial cells plays a crucial role in insulin secretion. Diabetes. 2015;64:876–886. doi: 10.2337/db14-0432. [DOI] [PubMed] [Google Scholar]
- 36.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 37.Cai Q, Brissova M, Reinert RB, Pan FC, Brahmachary P, Jeansson M, Shostak A, Radhika A, Poffenberger G, Quaggin SE. Enhanced expression of VEGF-A in β cells increases endothelial cell number but impairs islet morphogenesis and β cell proliferation. Dev Biol. 2012;367:40–54. doi: 10.1016/j.ydbio.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadi HA, Al Suwaidi J. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853. [PMC free article] [PubMed] [Google Scholar]
- 39.Peiris H, Bonder CS, Coates PTH, Keating DJ, Jessup CF. The β-cell/EC axis: how do islet cells talk to each other? Diabetes. 2014;63:3–11. doi: 10.2337/db13-0617. [DOI] [PubMed] [Google Scholar]
- 40.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 41.Seymour PA, Serup P. Mesodermal induction of pancreatic fate commitment. Semin Cell Dev Biol. 2019;92:77–88. doi: 10.1016/j.semcdb.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Landsman L, Nijagal A, Whitchurch TJ, VanderLaan RL, Zimmer WE, MacKenzie TC, Hebrok M. Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLoS Biol. 2011;9:e1001143. doi: 10.1371/journal.pbio.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angelo JR, Tremblay KD. Identification and fate mapping of the pancreatic mesenchyme. Dev Biol. 2018;435:15–25. doi: 10.1016/j.ydbio.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Attali M, Stetsyuk V, Basmaciogullari A, Aiello V, Zanta-Boussif MA, Duvillie B, Scharfmann R. Control of β-cell differentiation by the pancreatic mesenchyme. Diabetes. 2007;56:1248–1258. doi: 10.2337/db06-1307. [DOI] [PubMed] [Google Scholar]
- 45.Duvillié B, Attali M, Bounacer A, Ravassard P, Basmaciogullari A, Scharfmann R. The mesenchyme controls the timing of pancreatic β-cell differentiation. Diabetes. 2006;55:582–589. doi: 10.2337/diabetes.55.03.06.db05-0839. [DOI] [PubMed] [Google Scholar]
- 46.Byrnes LE, Wong DM, Subramaniam M, Meyer NP, Gilchrist CL, Knox SM, Tward AD, Chun JY, Sneddon JB. Lineage dynamics of murine pancreatic development at single-cell resolution. Nat Commun. 2018;9:1–17. doi: 10.1038/s41467-018-06176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper TT, Sherman SE, Bell GI, Ma J, Kuljanin M, Jose SE, Lajoie GA, Hess DA. Characterization of a Vimentin high/Nestin high proteome and tissue regenerative secretome generated by human pancreas-derived mesenchymal stromal cells. Stem Cells. 2020;38:666–682. doi: 10.1002/stem.3143. [DOI] [PubMed] [Google Scholar]
- 48.Cozzitorto C, Mueller L, Ruzittu S, Mah N, Willnow D, Darrigrand J-F, Wilson H, Khosravinia D, Mahmoud A-A, Risolino M. A specialized niche in the pancreatic microenvironment promotes endocrine differentiation. Dev Cell. 2020;55:150–162 e156. doi: 10.1016/j.devcel.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miralles F, Lamotte L, Couton D, Joshi RL. Interplay between FGF10 and Notch signalling is required for the self-renewal of pancreatic progenitors. Int J Dev Biol. 2003;50:17–26. doi: 10.1387/ijdb.052080fm. [DOI] [PubMed] [Google Scholar]
- 50.Ye F, Duvillie B, Scharfmann R. Fibroblast growth factors 7 and 10 are expressed in the human embryonic pancreatic mesenchyme and promote the proliferation of embryonic pancreatic epithelial cells. Diabetologia. 2005;48:277–281. doi: 10.1007/s00125-004-1638-6. [DOI] [PubMed] [Google Scholar]
- 51.Kobberup S, Schmerr M, Dang M-L, Nyeng P, Jensen JN, MacDonald RJ, Jensen J. Conditional control of the differentiation competence of pancreatic endocrine and ductal cells by Fgf10. Mech Dev. 2010;127:220–234. doi: 10.1016/j.mod.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seymour PA, Shih HP, Patel NA, Freude KK, Xie R, Lim CJ, Sander M. A Sox9/Fgf feed-forward loop maintains pancreatic organ identity. Development. 2012;139:3363–3372. doi: 10.1242/dev.078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otonkoski T, Cirulli V, Beattie M, Mally MI, Soto G, Rubin JS, Hayek A. A role for hepatocyte growth factor/scatter factor in fetal mesenchyme-induced pancreatic beta-cell growth. Endocrinology. 1996;137:3131–3139. doi: 10.1210/endo.137.7.8770939. [DOI] [PubMed] [Google Scholar]
- 54.Guo T, Landsman L, Li N, Hebrok M. Factors expressed by murine embryonic pancreatic mesenchyme enhance generation of insulin-producing cells from hESCs. Diabetes. 2013;62:1581–1592. doi: 10.2337/db12-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hammerle CM, Sandovici I, Brierley GV, Smith NM, Zimmer WE, Zvetkova I, Prosser HM, Sekita Y, Lam BY, Ma M. Mesenchyme-derived IGF2 is a major paracrine regulator of pancreatic growth and function. PLoS Genet. 2020;16:e1009069. doi: 10.1371/journal.pgen.1009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerriero I, De Angelis MT, D’Angelo F, Leveque R, Savignano E, Roberto L, Lucci V, Mazzone P, Laurino S, Storto G. Exploring the molecular crosstalk between pancreatic bud and mesenchyme in embryogenesis: novel signals involved. Int J Mol Sci. 2019;20:4900. doi: 10.3390/ijms20194900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russ HA, Landsman L, Moss CL, Higdon R, Greer RL, Kaihara K, Salamon R, Kolker E, Hebrok M (2016) Dynamic proteomic analysis of pancreatic mesenchyme reveals novel factors that enhance human embryonic stem cell to pancreatic cell differentiation. Stem Cells Int 2016 [DOI] [PMC free article] [PubMed]
- 58.Tulachan SS, Doi R, Hirai Y, Kawaguchi Y, Koizumi M, Hembree M, Tei E, Crowley A, Yew H, McFall C. Mesenchymal epimorphin is important for pancreatic duct morphogenesis. Dev Growth Differ. 2006;48:65–72. doi: 10.1111/j.1440-169X.2006.00846.x. [DOI] [PubMed] [Google Scholar]
- 59.Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development. 1998;125:1017–1024. doi: 10.1242/dev.125.6.1017. [DOI] [PubMed] [Google Scholar]
- 60.Gilbert JM, Adams MT, Sharon N, Jayaraaman H, Blum B (2021) Morphogenesis of the islets of Langerhans is guided by extra-endocrine Slit2/3 signals. Molecular and cellular biology 41 [DOI] [PMC free article] [PubMed]
- 61.Epshtein A, Rachi E, Sakhneny L, Mizrachi S, Baer D, Landsman L. Neonatal pancreatic pericytes support β-cell proliferation. Mol Metab. 2017;6:1330–1338. doi: 10.1016/j.molmet.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Lin PY, Peng SJ, Shen CN, Pasricha PJ, Tang SC. PanIN-associated pericyte, glial, and islet remodeling in mice revealed by 3D pancreatic duct lesion histology. Am J Physiol Gastrointest Liver Physiol. 2016;311:412–422. doi: 10.1152/ajpgi.00071.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang S-C, Jessup CF, Campbell-Thompson M. The role of accessory cells in Islet homeostasis. Curr Diabetes Rep. 2018;18:117. doi: 10.1007/s11892-018-1096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baron M, Veres A, Wolock SL, Faust AL, Gaujoux R, Vetere A, Ryu JH, Wagner BK, Shen-Orr SS, Klein AM. A single-cell transcriptomic map of the human and mouse pancreas reveals inter-and intra-cell population structure. Cell Syst. 2016;3:346–360 e344. doi: 10.1016/j.cels.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muraro MJ, Dharmadhikari G, Grün D, Groen N, Dielen T, Jansen E, van Gurp L, Engelse MA, Carlotti F, de Koning EJ. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 2016;3:385–394 e383. doi: 10.1016/j.cels.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferdek PE, Jakubowska MA. Biology of pancreatic stellate cells—more than just pancreatic cancer. Pflügers Archiv Eur J Physiol. 2017;469:1039–1050. doi: 10.1007/s00424-017-1968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Y, Sun B, Li W, Zhou J, Gao F, Wang X, Cai M, Sun Z. Pancreatic stellate cells: a rising translational physiology star as a potential stem cell type for beta cell neogenesis. Front Physiol. 2019;10:218. doi: 10.3389/fphys.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xue R, Jia K, Wang J, Yang L, Wang Y, Gao L, Hao J. A rising star in pancreatic diseases: pancreatic stellate cells. Front Physiol. 2018;9:754. doi: 10.3389/fphys.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang S-C, Chiu Y-C, Hsu C-T, Peng S-J, Fu Y-Y. Plasticity of Schwann cells and pericytes in response to islet injury in mice. Diabetologia. 2013;56:2424–2434. doi: 10.1007/s00125-013-2977-y. [DOI] [PubMed] [Google Scholar]
- 71.Harari N, Sakhneny L, Khalifa-Malka L, Busch A, Hertel KJ, Hebrok M, Landsman L. Pancreatic pericytes originate from the embryonic pancreatic mesenchyme. Dev Biol. 2019;449:14–20. doi: 10.1016/j.ydbio.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–995. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simsek S, Zhou T, Robinson CL, Tsai S-Y, Crespo M, Amin S, Lin X, Hon J, Evans T, Chen S. Modeling cystic fibrosis using pluripotent stem cell-derived human pancreatic ductal epithelial cells. Stem Cells Transl Med. 2016;5:572–579. doi: 10.5966/sctm.2015-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almaça J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab. 2018;27:630–644634. doi: 10.1016/j.cmet.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sasson A, Rachi E, Sakhneny L, Baer D, Lisnyansky M, Epshtein A, Landsman L. Islet pericytes are required for β-cell maturity. Diabetes. 2016;65:3008–3014. doi: 10.2337/db16-0365. [DOI] [PubMed] [Google Scholar]
- 76.Sakhneny L, Rachi E, Epshtein A, Guez HC, Wald-Altman S, Lisnyansky M, Khalifa-Malka L, Hazan A, Baer D, Priel A. Pancreatic pericytes support β-cell function in a Tcf7l2-dependent manner. Diabetes. 2018;67:437–447. doi: 10.2337/db17-0697. [DOI] [PubMed] [Google Scholar]
- 77.Landsman L. Pancreatic pericytes in glucose homeostasis and diabetes. Adv Exp Med Biol. 2019;1122:27–40. doi: 10.1007/978-3-030-11093-2_2. [DOI] [PubMed] [Google Scholar]
- 78.Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in β cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5:207–219. doi: 10.1016/j.cmet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Hammes H-P. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61:29–38. doi: 10.1007/s00125-017-4435-8. [DOI] [PubMed] [Google Scholar]
- 80.Pfister F, Wang Y, Schreiter K, Vom Hagen F, Altvater K, Hoffmann S, Deutsch U, Hammes H-P, Feng Y. Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol. 2010;47:59–64. doi: 10.1007/s00592-009-0099-2. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z-S, Zhou H-N, He S-S, Xue M-Y, Li T, Liu L-M. Research advances in pericyte function and their roles in diseases. Chin J Traumatol. 2020;23:89–95. doi: 10.1016/j.cjtee.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Y, Kim JW, Park HS, Lee EY, Yoon KH. Pancreatic stellate cells in the islets as a novel target to preserve the pancreatic β-cell mass and function. J Diabetes Investig. 2020;11:268–280. doi: 10.1111/jdi.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hutchins EJ, Kunttas E, Piacentino ML, Howard AG, IV, Bronner ME, Uribe RA. Migration and diversification of the vagal neural crest. Dev Biol. 2018;444:S98–S109. doi: 10.1016/j.ydbio.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Plank JL, Mundell NA, Frist AY, LeGrone AW, Kim T, Musser MA, Walter TJ, Labosky PA. Influence and timing of arrival of murine neural crest on pancreatic beta cell development and maturation. Dev Biol. 2011;349:321–330. doi: 10.1016/j.ydbio.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimada K, Tachibana T, Fujimoto K, Sasaki T, Okabe M (2012) Temporal and spatial cellular distribution of neural crest derivatives and alpha cells during islet development. Acta Histochem et Cytochem 1202130134–1202130134 [DOI] [PMC free article] [PubMed]
- 86.Arntfield M, van der Kooy D. The adult mammalian pancreas contains separate precursors of pancreatic and neural crest developmental origins. Stem Cells Dev. 2013;22:2145–2157. doi: 10.1089/scd.2013.0027. [DOI] [PubMed] [Google Scholar]
- 87.Pearse A, Polak JM. Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut. 1971;12:783–788. doi: 10.1136/gut.12.10.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pictet RL, Rall LB, Phelps P, Rutter WJ. The neural crest and the origin of the insulin-producing and other gastrointestinal hormone-producing cells. Science. 1976;191:191–192. doi: 10.1126/science.1108195. [DOI] [PubMed] [Google Scholar]
- 89.Nekrep N, Wang J, Miyatsuka T, German MS. Signals from the neural crest regulate beta-cell mass in the pancreas. Development. 2008;135:2151–2160. doi: 10.1242/dev.015859. [DOI] [PubMed] [Google Scholar]
- 90.Muñoz-Bravo JL, Hidalgo-Figueroa M, Pascual A, López-Barneo J, Leal-Cerro A, Cano DA. GDNF is required for neural colonization of the pancreas. Development. 2013;140:3669–3679. doi: 10.1242/dev.091256. [DOI] [PubMed] [Google Scholar]
- 91.Kozlova EN, Jansson L. Differentiation and migration of neural crest stem cells are stimulated by pancreatic islets. NeuroReport. 2009;20:833–838. doi: 10.1097/WNR.0b013e32832b8e20. [DOI] [PubMed] [Google Scholar]
- 92.Vargas-Valderrama A, Messina A, Mitjavila-Garcia MT, Guenou H. The endothelium, a key actor in organ development and hPSC-derived organoid vascularization. J Biomed Sci. 2020;27:1–13. doi: 10.1186/s12929-020-00661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Banerjee I, Sharma N, Yarmush M. Impact of co-culture on pancreatic differentiation of embryonic stem cells. J Tissue Eng Regen Med. 2011;5:313–323. doi: 10.1002/term.317. [DOI] [PubMed] [Google Scholar]
- 94.Jaramillo M, Mathew S, Mamiya H, Goh SK, Banerjee I. Endothelial cells mediate islet-specific maturation of human embryonic stem cell-derived pancreatic progenitor cells. Tissue Eng Part A. 2015;21:14–25. doi: 10.1089/ten.tea.2014.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Talavera-Adame D, Wu G, He Y, Ng TT, Gupta A, Kurtovic S, Hwang JY, Farkas DL, Dafoe DC. Endothelial cells in co-culture enhance embryonic stem cell differentiation to pancreatic progenitors and insulin-producing cells through BMP signaling. Stem Cell Rev Rep. 2011;7:532–543. doi: 10.1007/s12015-011-9232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weizman A, Michael I, Wiesel-Motiuk N, Rezania A, Levenberg S. The effect of endothelial cells on hESC-derived pancreatic progenitors in a 3D environment. Biomater Sci. 2014;2:1706–1714. doi: 10.1039/c4bm00304g. [DOI] [PubMed] [Google Scholar]
- 97.Kao D-I, Lacko LA, Ding B-S, Huang C, Phung K, Gu G, Rafii S, Stuhlmann H, Chen SJScr. Endothelial cells control pancreatic cell fate at defined stages through EGFL7 signaling. Stem Cell Rep. 2015;4:181–189. doi: 10.1016/j.stemcr.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Augsornworawat P, Velazco-Cruz L, Song J, Millman JR. A hydrogel platform for in vitro three dimensional assembly of human stem cell-derived islet cells and endothelial cells. Acta Biomater. 2019;97:272–280. doi: 10.1016/j.actbio.2019.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Candiello J, Grandhi TSP, Goh SK, Vaidya V, Lemmon-Kishi M, Eliato KR, Ros R, Kumta PN, Rege K, Banerjee I. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials. 2018;177:27–39. doi: 10.1016/j.biomaterials.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 100.Skrzypek K, Barrera YB, Groth T, Stamatialis D. Endothelial and beta cell composite aggregates for improved function of a bioartificial pancreas encapsulation device. Int J Artif Organs. 2018;41:152–159. doi: 10.1177/0391398817752295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Y, Fan P, Ding X-M, Tian X-H, Feng X-S, Yan H, Pan X-M, Tian P-X, Zheng J, Ding C-G. Polyglycolic acid fibrous scaffold improving endothelial cell coating and vascularization of islet. Chin Med J. 2017;130:832. doi: 10.4103/0366-6999.202730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Urbanczyk M, Zbinden A, Layland SL, Duffy G, Schenke-Layland K. Controlled heterotypic pseudo-islet assembly of human β-cells and human umbilical vein endothelial cells using magnetic levitation. Tissue Eng Part A. 2020;26:387–399. doi: 10.1089/ten.tea.2019.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Golosow N, Grobstein C. Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev Biol. 1962;4:242–255. doi: 10.1016/0012-1606(62)90042-8. [DOI] [PubMed] [Google Scholar]
- 104.Wessells NK, Cohen JH. Early pancreas organogenesis: morphogenesis, tissue interactions, and mass effects. Dev Biol. 1967;15:237–270. doi: 10.1016/0012-1606(67)90042-5. [DOI] [PubMed] [Google Scholar]
- 105.Li Z, Manna P, Kobayashi H, Spilde T, Bhatia A, Preuett B, Prasadan K, Hembree M, Gittes GK. Multifaceted pancreatic mesenchymal control of epithelial lineage selection. Dev Biol. 2004;269:252–263. doi: 10.1016/j.ydbio.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 106.Rose MI, Crisera CA, Colen KL, Connelly PR, Longaker MT, Gittes GK. Epithelio-mesenchymal interactions in the developing mouse pancreas: morphogenesis of the adult architecture. J Pediatr Surg. 1999;34:774–780. doi: 10.1016/s0022-3468(99)90372-x. [DOI] [PubMed] [Google Scholar]
- 107.Li XY, Wu SY, Leung PS. Human fetal bone marrow-derived mesenchymal stem cells promote the proliferation and differentiation of pancreatic progenitor cells and the engraftment function of islet-like cell clusters. Int J Mol Sci. 2019;20:4083. doi: 10.3390/ijms20174083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sneddon JB, Borowiak M, Melton DA. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature. 2012;491:765–768. doi: 10.1038/nature11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scavuzzo MA, Yang D, Borowiak M. Organotypic pancreatoids with native mesenchyme develop Insulin producing endocrine cells. Sci Rep. 2017;7:10810. doi: 10.1038/s41598-017-11169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scuteri A, Donzelli E, Rodriguez-Menendez V, Ravasi M, Monfrini M, Bonandrini B, Figliuzzi M, Remuzzi A, Tredici G. A double mechanism for the mesenchymal stem cells' positive effect on pancreatic islets. PLoS ONE. 2014;9:e84309. doi: 10.1371/journal.pone.0084309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Quaranta P, Antonini S, Spiga S, Mazzanti B, Curcio M, Mulas G, Diana M, Marzola P, Mosca F, Longoni B. Co-transplantation of endothelial progenitor cells and pancreatic islets to induce long-lasting normoglycemia in streptozotocin-treated diabetic rats. PLoS ONE. 2014;9:e94783. doi: 10.1371/journal.pone.0094783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oh B, Oh S, Jin S, Suh S, Bae J, Park CG, Lee MS, Lee MK, Kim J. Co-transplantation of bone marrow-derived endothelial progenitor cells improves revascularization and organization in islet grafts. Am J Transplant. 2013;13:1429–1440. doi: 10.1111/ajt.12222. [DOI] [PubMed] [Google Scholar]
- 113.Coppens V, Heremans Y, Leuckx G, Suenens K, Jacobs-Tulleneers-Thevissen D, Verdonck K, Lahoutte T, Luttun A, Heimberg H, De Leu NJD. Human blood outgrowth endothelial cells improve islet survival and function when co-transplanted in a mouse model of diabetes. Diabetologia. 2013;56:382–390. doi: 10.1007/s00125-012-2754-3. [DOI] [PubMed] [Google Scholar]
- 114.Nyqvist D, Speier S, Rodriguez-Diaz R, Molano RD, Lipovsek S, Rupnik M, Dicker A, Ilegems E, Zahr-Akrawi E, Molina J. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes. 2011;60:2571–2577. doi: 10.2337/db10-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grapensparr L, Christoffersson G, Carlsson P-O. Bioengineering with endothelial progenitor cells improves the vascular engraftment of transplanted human islets. Cell Transplant. 2018;27:948–956. doi: 10.1177/0963689718759474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barba-Gutierrez DA, Daneri-Navarro A, Villagomez-Mendez JJA, Kanamune J, Robles-Murillo AK, Sanchez-Enriquez S, Villafan-Bernal JR, Rivas-Carrillo JD. Facilitated engraftment of isolated islets coated with expanded vascular endothelial cells for islet transplantation. Transpl Proc. 2016;48:669–672. doi: 10.1016/j.transproceed.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 117.Jung E-J, Kim S-C, Wee Y-M, Kim Y-H, Choi MY, Jeong S-H, Lee J, Lim D-G, Han D-J. Bone marrow-derived mesenchymal stromal cells support rat pancreatic islet survival and insulin secretory function in vitro. Cytotherapy. 2011;13:19–29. doi: 10.3109/14653249.2010.518608. [DOI] [PubMed] [Google Scholar]
- 118.Johansson U, Rasmusson I, Niclou SP, Forslund N, Gustavsson L, Nilsson B, Korsgren O, Magnusson PU. Formation of composite endothelial cell–mesenchymal stem cell islets: a novel approach to promote islet revascularization. Diabetes. 2008;57:2393–2401. doi: 10.2337/db07-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park K-S, Kim Y-S, Kim J-H, Choi B, Kim S-H, Tan AH-K, Lee M-S, Lee M-K, Kwon C-H, Joh J-W. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation. 2010;89:509–517. doi: 10.1097/TP.0b013e3181c7dc99. [DOI] [PubMed] [Google Scholar]
- 120.Gao X, Song L, Shen K, Wang H, Qian M, Niu W, Qin X. Bone marrow mesenchymal stem cells promote the repair of islets from diabetic mice through paracrine actions. Mol Cell Endocrinol. 2014;388:41–50. doi: 10.1016/j.mce.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 121.Figliuzzi M, Cornolti R, Perico N, Rota C, Morigi M, Remuzzi G, Remuzzi A, Benigni A. Bone marrow–derived mesenchymal stem cells improve islet graft function in diabetic rats. Transpl Proc. 2009;41:1797–1800. doi: 10.1016/j.transproceed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 122.Lu Y, Jin X, Chen Y, Li S, Yuan Y, Mai G, Tian B, Long D, Zhang J, Zeng L. Mesenchymal stem cells protect islets from hypoxia/reoxygenation-induced injury. Cell Biochem Funct. 2010;28:637–643. doi: 10.1002/cbf.1701. [DOI] [PubMed] [Google Scholar]
- 123.Li F, Wang X, Deng C, Qi H, Ren L, Zhou H. Immune modulation of co-transplantation mesenchymal stem cells with islet on T and dendritic cells. Clin Exp Immunol. 2010;161:357–363. doi: 10.1111/j.1365-2249.2010.04178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O'connor DH, Bartholomew AM. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59:2558–2568. doi: 10.2337/db10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kono TM, Sims EK, Moss DR, Yamamoto W, Ahn G, Diamond J, Tong X, Day KH, Territo PR, Hanenberg H. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: Analysis of hASC-derived paracrine effectors. Stem Cells. 2014;32:1831–1842. doi: 10.1002/stem.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and-9. Diabetes. 2009;58:1797–1806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hajizadeh-Saffar E, Tahamtani Y, Aghdami N, Azadmanesh K, Habibi-Anbouhi M, Heremans Y, De Leu N, Heimberg H, Ravassard P, Shokrgozar M. Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes. Sci Rep. 2015;5:1–10. doi: 10.1038/srep09322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olerud J, Kanaykina N, Vasilovska S, King D, Sandberg M, Jansson L, Kozlova E. Neural crest stem cells increase beta cell proliferation and improve islet function in co-transplanted murine pancreatic islets. Diabetologia. 2009;52:2594–2601. doi: 10.1007/s00125-009-1544-z. [DOI] [PubMed] [Google Scholar]
- 129.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 130.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone–expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 131.Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB, Daley GQ, Elefanty AG, Stanley EG, Keller G. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nostro MC, Keller G. Generation of beta cells from human pluripotent stem cells: potential for regenerative medicine. Semin Cell Dev Biol. 2012;23:701–710. doi: 10.1016/j.semcdb.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 134.Bruin JE, Rezania A, Xu J, Narayan K, Fox JK, O'Neil JJ, Kieffer TJ. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013;56:1987–1998. doi: 10.1007/s00125-013-2955-4. [DOI] [PubMed] [Google Scholar]
- 135.Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, Odwyer S, Quiskamp N, Mojibian M, Albrecht T. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 136.Rezania A, Bruin JE, Xu J, Narayan K, Fox JK, O'Neil JJ, Kieffer TJ. Enrichment of human embryonic stem cell-derived NKX6. 1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem cells. 2013;31:2432–2442. doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- 137.Nostro MC, Sarangi F, Yang C, Holland A, Elefanty AG, Stanley EG, Greiner DL, Keller G. Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Rep. 2015;4:591–604. doi: 10.1016/j.stemcr.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Soltanian A, Ghezelayagh Z, Mazidi Z, Halvaei M, Mardpour S, Ashtiani MK, Hajizadeh-Saffar E, Tahamtani Y, Baharvand H. Generation of functional human pancreatic organoids by transplants of embryonic stem cell derivatives in a 3D-printed tissue trapper. J Cell Physiol. 2019;234:9564–9576. doi: 10.1002/jcp.27644. [DOI] [PubMed] [Google Scholar]
- 139.Ghezelayagh Z, Zabihi M, Zarkesh I, Gonçalves CA, Larsen M, Hagh-parast N, Pakzad M, Vosough M, Arjmand B, Baharvand H (2021) Improved differentiation of hESC-derived pancreatic progenitors by using human fetal pancreatic mesenchymal cells in a micro-scalable three-dimensional co-culture system. Stem Cell Reviews and Reports. 10.1007/s12015-021-10266-z [DOI] [PubMed]
- 140.Bonfanti P, Nobecourt E, Oshima M, Albagli-Curiel O, Laurysens V, Stangé G, Sojoodi M, Heremans Y, Heimberg H, Scharfmann R. Ex vivo expansion and differentiation of human and mouse fetal pancreatic progenitors are modulated by epidermal growth factor. Stem Cells Dev Biol. 2015;24:1766–1778. doi: 10.1089/scd.2014.0550. [DOI] [PubMed] [Google Scholar]
- 141.Nair GG, Liu JS, Russ HA, Tran S, Saxton MS, Chen R, Juang C, Li M-l, Nguyen VQ, Giacometti S. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat Cell Biol. 2019;21:263–274. doi: 10.1038/s41556-018-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ameri J, Borup R, Prawiro C, Ramond C, Schachter KA, Scharfmann R, Semb H. Efficient generation of glucose-responsive beta cells from isolated GP2+ human pancreatic progenitors. Cell Rep. 2017;19:36–49. doi: 10.1016/j.celrep.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 143.Davis JC, Alves TC, Helman A, Chen JC, Kenty JH, Cardone RL, Liu DR, Kibbey RG, Melton DA. Glucose response by stem cell-derived β cells in vitro is inhibited by a bottleneck in glycolysis. Cell Rep. 2020;31:107623. doi: 10.1016/j.celrep.2020.107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu X, Qin J, Chang M, Wang S, Li Y, Pei X, Wang Y. Enhanced differentiation of human pluripotent stem cells into pancreatic endocrine cells in 3D culture by inhibition of focal adhesion kinase. Stem Cell Res Ther. 2020;11:1–12. doi: 10.1186/s13287-020-02003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hogrebe NJ, Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat Biotechnol. 2020;38:460–470. doi: 10.1038/s41587-020-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qadir MMF, Álvarez-Cubela S, Belle K, Sapir T, Messaggio F, Johnson KB, Umland O, Hardin D, Klein D, Pérez-Álvarez I. A double fail-safe approach to prevent tumorigenesis and select pancreatic β cells from human embryonic stem cells. Stem Cell Rep. 2019;12:611–623. doi: 10.1016/j.stemcr.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lutolf MP, Blau HM. Artificial stem cell niches. Adv Mater. 2009;21:3255–3268. doi: 10.1002/adma.200802582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bao M, Xie J, Huck WT. Recent advances in engineering the stem cell microniche in 3D. Adv Sci. 2018;5:1800448. doi: 10.1002/advs.201800448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Melton D (2021) The promise of stem cell-derived islet replacement therapy. Diabetologia 1–7 [DOI] [PMC free article] [PubMed]
- 150.Lanzoni G, Ricordi C. Transplantation of stem cell-derived pancreatic islet cells. Nat Rev Endocrinol. 2021;17:7–8. doi: 10.1038/s41574-020-00430-9. [DOI] [PubMed] [Google Scholar]
- 151.De Klerk E, Hebrok M (2021) Stem cell-based clinical trials for diabetes mellitus. Front Endocrinol 12 [DOI] [PMC free article] [PubMed]
- 152.Stock AA, Manzoli V, De Toni T, Abreu MM, Poh Y-C, Ye L, Roose A, Pagliuca FW, Thanos C, Ricordi C. Conformal coating of stem cell-derived islets for β cell replacement in type 1 diabetes. Stem Cell Rep. 2020;14:91–104. doi: 10.1016/j.stemcr.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alam M, Arunagiri A, Haataja L, Torres M, Larkin D, Kappler J, Jin N, Arvan P. Predisposition to proinsulin misfolding as a genetic risk to diet-induced diabetes. biorxiv. 2021;70:488. doi: 10.2337/db21-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Millman JR, Xie C, Van Dervort A, Gurtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat Commun. 2016;7:11463. doi: 10.1038/ncomms11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rajaei B, Shamsara M, Amirabad LM, Massumi M, Sanati MH. Pancreatic endoderm-derived from diabetic patient-specific induced pluripotent stem cell generates glucose-responsive insulin-secreting cells. J Cell Physiol. 2017;232:2616–2625. doi: 10.1002/jcp.25459. [DOI] [PubMed] [Google Scholar]
- 156.Jang J, Yoo J-E, Lee J-A, Lee DR, Kim JY, Huh YJ, Kim D-S, Park C-Y, Hwang D-Y, Kim H-S. Disease-specific induced pluripotent stem cells: a platform for human disease modeling and drug discovery. Exp Mol Med. 2012;44:202–213. doi: 10.3858/emm.2012.44.3.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Takahashi Y, Sekine K, Kin T, Takebe T, Taniguchi H. Self-condensation culture enables vascularization of tissue fragments for efficient therapeutic transplantation. Cell Rep. 2018;23:1620–1629. doi: 10.1016/j.celrep.2018.03.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 2015;16:556–565. doi: 10.1016/j.stem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 160.Chen H, Zhuo Q, Ye Z, Xu X, Ji S (2020) Organoid model: A new hope for pancreatic cancer treatment? Biochim et Biophys Acta (BBA)-Rev Cancer 188466 [DOI] [PubMed]
- 161.Frappart P-O, Hofmann TG. Pancreatic ductal adenocarcinoma (Pdac) organoids: The shining light at the end of the tunnel for drug response prediction and personalized medicine. Cancers. 2020;12:2750. doi: 10.3390/cancers12102750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Balak JR, Juksar J, Carlotti F, Nigro AL, de Koning EJ. Organoids from the human fetal and adult pancreas. Curr DiabRep. 2019;19:160. doi: 10.1007/s11892-019-1261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Funata M, Nio Y, Erion DM, Thompson WL, Takebe T. The promise of human organoids in the digestive system. Cell Death Differ. 2021;28:84–94. doi: 10.1038/s41418-020-00661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ouchi R, Togo S, Kimura M, Shinozawa T, Koido M, Koike H, Thompson W, Karns RA, Mayhew CN, McGrath PS. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 2019;30:374–384 e376. doi: 10.1016/j.cmet.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Enge M, Arda HE, Mignardi M, Beausang J, Bottino R, Kim SK, Quake SR. Single-cell analysis of human pancreas reveals transcriptional signatures of aging and somatic mutation patterns. Cell. 2017;171:321–330 e314. doi: 10.1016/j.cell.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Toda S, Frankel NW, Lim WA. Engineering cell–cell communication networks: programming multicellular behaviors. Curr Opin Chem Biol. 2019;52:31–38. doi: 10.1016/j.cbpa.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 168.Hunckler MD, García AJ. Engineered biomaterials for enhanced function of insulin-secreting β-cell organoids. Adv Funct Mater. 2020;30:2000134. [Google Scholar]
- 169.Woodford C, Zandstra PW. Tissue engineering 2.0: guiding self-organization during pluripotent stem cell differentiation. Curr Opin Biotechnol. 2012;23:810–819. doi: 10.1016/j.copbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bal T, Nazli C, Okcu A, Duruksu G, Karaöz E, Kizilel S. Mesenchymal stem cells and ligand incorporation in biomimetic poly (ethylene glycol) hydrogels significantly improve insulin secretion from pancreatic islets. J Tissue Eng Regenerat Med. 2017;11:694–703. doi: 10.1002/term.1965. [DOI] [PubMed] [Google Scholar]
- 171.Broguiere N, Isenmann L, Hirt C, Ringel T, Placzek S, Cavalli E, Ringnalda F, Villiger L, Züllig R, Lehmann R. Growth of epithelial organoids in a defined hydrogel. Adv Mater. 2018;30:1801621. doi: 10.1002/adma.201801621. [DOI] [PubMed] [Google Scholar]
- 172.Tumarkin E, Tzadu L, Csaszar E, Seo M, Zhang H, Lee A, Peerani R, Purpura K, Zandstra PW, Kumacheva E. High-throughput combinatorial cell co-culture using microfluidics. Integr Biol. 2011;3:653–662. doi: 10.1039/c1ib00002k. [DOI] [PubMed] [Google Scholar]
- 173.Chwalek K, Levental KR, Tsurkan MV, Zieris A, Freudenberg U, Werner C. Two-tier hydrogel degradation to boost endothelial cell morphogenesis. Biomaterials. 2011;32:9649–9657. doi: 10.1016/j.biomaterials.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 174.Jung T-H, Chung E-B, Kim HW, Choi SW, Park S-J, Mukhtar AS, Chung H-M, Kim E, Huh KM, Kim DS. Application of co-culture technology of epithelial type cells and mesenchymal type cells using nanopatterned structures. PLoS ONE. 2020;15:232899. doi: 10.1371/journal.pone.0232899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Torisawa Y-s, Mosadegh B, Luker GD, Morell M, O'Shea KS, Takayama S. Microfluidic hydrodynamic cellular patterning for systematic formation of co-culture spheroids. Integr Biol. 2009;1:649–654. doi: 10.1039/b915965g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Barati G, Nadri S, Hajian R, Rahmani A, Mostafavi H, Mortazavi Y, Taromchi AH. Differentiation of microfluidic-encapsulated trabecular meshwork mesenchymal stem cells into insulin producing cells and their impact on diabetic rats. J Cell Physiol. 2019;234:6801–6809. doi: 10.1002/jcp.27426. [DOI] [PubMed] [Google Scholar]
- 177.Yu Y, Gamble A, Pawlick R, Pepper AR, Salama B, Toms D, Razian G, Ellis C, Bruni A, Gala-Lopez B. Bioengineered human pseudoislets form efficiently from donated tissue, compare favourably with native islets in vitro and restore normoglycaemia in mice. Diabetologia. 2018;61:2016–2029. doi: 10.1007/s00125-018-4672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioactive materials. 2018;3:144–156. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 180.Ma Z, Holle AW, Melde K, Qiu T, Poeppel K, Kadiri VM, Fischer P. Acoustic holographic cell patterning in a biocompatible hydrogel. Adv Mater. 2020;32:1904181. doi: 10.1002/adma.201904181. [DOI] [PubMed] [Google Scholar]
- 181.Zwi-Dantsis L, Wang B, Marijon C, Zonetti S, Ferrini A, Massi L, Stuckey DJ, Terracciano CM, Stevens MM. Remote magnetic nanoparticle manipulation enables the dynamic patterning of cardiac tissues. Adv Mater. 2020;32:1904598. doi: 10.1002/adma.201904598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mao H, Yang L, Zhu H, Wu L, Ji P, Yang J, Gu Z. Recent advances and challenges in materials for 3D bioprinting. Prog Nat Sci Mater Int. 2020;30:618–634. [Google Scholar]
- 183.Armstrong JP, Stevens MM. Using remote fields for complex tissue engineering. Trends Biotechnol. 2020;38:254–263. doi: 10.1016/j.tibtech.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bouyer C, Chen P, Güven S, Demirtaş TT, Nieland TJ, Padilla F, Demirci U. A bio-acoustic levitational (BAL) assembly method for engineering of multilayered, 3D brain-like constructs, using human embryonic stem cell derived neuro-progenitors. Adv Mater. 2016;28:161–167. doi: 10.1002/adma.201503916. [DOI] [PubMed] [Google Scholar]
- 185.Serpooshan V, Chen P, Wu H, Lee S, Sharma A, Hu DA, Venkatraman S, Ganesan AV, Usta OB, Yarmush M. Bioacoustic-enabled patterning of human iPSC-derived cardiomyocytes into 3D cardiac tissue. Biomaterials. 2017;131:47–57. doi: 10.1016/j.biomaterials.2017.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kirkham GR, Britchford E, Upton T, Ware J, Gibson GM, Devaud Y, Ehrbar M, Padgett M, Allen S, Buttery LD. Precision assembly of complex cellular microenvironments using holographic optical tweezers. Sci Rep. 2015;5:1–7. doi: 10.1038/srep08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jafari J, Han X-l, Palmer J, Tran PA, O’Connor AJ. Remote control in formation of 3D multicellular assemblies using magnetic forces. ACS Biomater Sci Eng. 2019;5:2532–2542. doi: 10.1021/acsbiomaterials.9b00297. [DOI] [PubMed] [Google Scholar]
- 188.Bobbert F, Zadpoor A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J Mater Chem B. 2017;5:6175–6192. doi: 10.1039/c7tb00741h. [DOI] [PubMed] [Google Scholar]
- 189.Zhao D, Xue C, Li Q, Liu M, Ma W, Zhou T, Lin Y. Substrate stiffness regulated migration and angiogenesis potential of A549 cells and HUVECs. J Cell Physiol. 2018;233:3407–3417. doi: 10.1002/jcp.26189. [DOI] [PubMed] [Google Scholar]
- 190.Phelps EA, Landázuri N, Thulé PM, Taylor WR, García AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci. 2010;107:3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee P-F, Bai Y, Smith R, Bayless K, Yeh A. Angiogenic responses are enhanced in mechanically and microscopically characterized, microbial transglutaminase crosslinked collagen matrices with increased stiffness. Acta Biomater. 2013;9:7178–7190. doi: 10.1016/j.actbio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou YL, Yang QQ, Zhang L, Tang JB. Nanoparticle-coated sutures providing sustained growth factor delivery to improve the healing strength of injured tendons. Acta Biomater. 2021;124:301–314. doi: 10.1016/j.actbio.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 193.Limasale YDP, Atallah P, Werner C, Freudenberg U, Zimmermann R. Tuning the local availability of VEGF within glycosaminoglycan-based hydrogels to modulate vascular endothelial cell morphogenesis. Adv Func Mater. 2020;30:2000068. [Google Scholar]
- 194.Song H-HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell. 2018;22:340–354. doi: 10.1016/j.stem.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Moeinvaziri F, Shojaei A, Haghparast N, Yakhkeshi S, Nemati S, Hassani S-N, Baharvand H. Towards maturation of human otic hair cell–like cells in pluripotent stem cell–derived organoid transplants. Cell Tissue Res. 2021;28:1–13. doi: 10.1007/s00441-021-03510-y. [DOI] [PubMed] [Google Scholar]
- 196.Hanjaya-Putra D, Bose V, Shen Y-I, Yee J, Khetan S, Fox-Talbot K, Steenbergen C, Burdick JA, Gerecht S. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood J Am Soc Hematol. 2011;118:804–815. doi: 10.1182/blood-2010-12-327338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lesman A, Koffler J, Atlas R, Blinder YJ, Kam Z, Levenberg S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials. 2011;32:7856–7869. doi: 10.1016/j.biomaterials.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 198.Jalili RB, Moeen Rezakhanlou A, Hosseini-Tabatabaei A, Ao Z, Warnock GL, Ghahary A. Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal. J Cell Physiol. 2011;226:1813–1819. doi: 10.1002/jcp.22515. [DOI] [PubMed] [Google Scholar]
- 199.Du C, Narayanan K, Leong MF, Wan AC. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials. 2014;35:6006–6014. doi: 10.1016/j.biomaterials.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 200.Lim TC, Leong MF, Lu H, Du C, Gao S, Wan AC, Ying JY. Follicular dermal papilla structures by organization of epithelial and mesenchymal cells in interfacial polyelectrolyte complex fibers. Biomaterials. 2013;34:7064–7072. doi: 10.1016/j.biomaterials.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 201.Skrzypek K, Nibbelink MG, Karbaat LP, Karperien M, van Apeldoorn A, Stamatialis D. An important step towards a prevascularized islet macroencapsulation device—effect of micropatterned membranes on development of endothelial cell network. J Mater Sci Mater Med. 2018;29:91. doi: 10.1007/s10856-018-6102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nibbelink MG, Skrzypek K, Karbaat L, Both S, Plass J, Klomphaar B, van Lente J, Henke S, Karperien M, Stamatialis D. An important step towards a prevascularized islet microencapsulation device: in vivo prevascularization by combination of mesenchymal stem cells on micropatterned membranes. J Mater Sci Mater Med. 2018;29:174. doi: 10.1007/s10856-018-6178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Luo B-H, Xiong F, Wang J-P, Li J-H, Zhong M, Liu Q-L, Luo G-Q, Yang X-J, Xiao N, Xie B. Epidermal growth factor-like domain-containing protein 7 (EGFL7) enhances EGF receptor—AKT signaling, epithelial—mesenchymal transition, and metastasis of gastric cancer cells. PLoS ONE. 2014;9:e99922. doi: 10.1371/journal.pone.0099922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sand FW, Hörnblad A, Johansson JK, Lorén C, Edsbagge J, Ståhlberg A, Magenheim J, Ilovich O, Mishani E, Dor YJDb. Growth-limiting role of endothelial cells in endoderm development. Dev Biol. 2011;352:267–277. doi: 10.1016/j.ydbio.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 205.Edsbagge J, Johansson JK, Esni F, Luo Y, Radice GL, Semb H (2005) Vascular function and sphingosine-1-phosphate regulate development of the dorsal pancreatic mesenchyme 132:1085–1092 [DOI] [PubMed]
- 206.Guney MA, Petersen CP, Boustani A, Duncan MR, Gunasekaran U, Menon R, Warfield C, Grotendorst GR, Means AL, Economides AN. Connective tissue growth factor acts within both endothelial cells and β cells to promote proliferation of developing β cells. Proc Natl Acad Sci. 2011;108:15242–15247. doi: 10.1073/pnas.1100072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Crawford LA, Guney MA, Oh YA, DeYoung RA, Valenzuela DM, Murphy AJ, Yancopoulos GD, Lyons KM, Brigstock DR, Economides A. Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and β-cell proliferation during embryogenesis. Mol Endocrinol. 2009;23:324–336. doi: 10.1210/me.2008-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Olerud J, Mokhtari D, Johansson M, Christoffersson G, Lawler J, Welsh N, Carlsson PO. Thrombospondin-1: an islet endothelial cell signal of importance for β-cell function. Diabetes. 2011;60:1946–1954. doi: 10.2337/db10-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.García-Ocaña A, Vasavada RC, Cebrian A, Reddy V, Takane KK, López-Talavera J-C, Stewart AF. Transgenic overexpression of hepatocyte growth factor in the β-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes. 2001;50:2752–2762. doi: 10.2337/diabetes.50.12.2752. [DOI] [PubMed] [Google Scholar]
- 210.Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 211.Johansson M, Mattsson GR, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic β-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 212.Yao Y, Zebboudj AF, Shao E, Perez M, Boström K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281:33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- 213.Clarkin CE, Mahmoud M, Liu B, Sobamowo EO, King A, Arthur H, Jones PM, Wheeler-Jone CPJBrn. Modulation of endoglin expression in islets of langerhans by VEGF reveals a novel regulator of islet endothelial cell function. BMC Res Notes. 2016;9:362. doi: 10.1186/s13104-016-2142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sarafidis PA, Bakris GL. Insulin and endothelin: an interplay contributing to hypertension development? J Clin Endocrinol Metab. 2006;92:379–385. doi: 10.1210/jc.2006-1819. [DOI] [PubMed] [Google Scholar]
- 215.De Carlo E, Milanesi A, Martini C, Maffei P, Sicolo N, Scandellari C. Endothelin-1 and endothelin-3 stimulate insulin release by isolated rat pancreatic islets. J Endocrinol Invest. 2000;23:240–245. doi: 10.1007/BF03343715. [DOI] [PubMed] [Google Scholar]
- 216.Narayanan S, Loganathan G, Dhanasekaran M, Tucker W, Patel A, Subhashree V, Mokshagundam S, Hughes MG, Williams SK, Balamurugan AN. Intra-islet endothelial cell and β-cell crosstalk: Implication for islet cell transplantation. World J Transplant. 2017;7:117. doi: 10.5500/wjt.v7.i2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ahnfelt-Rønne J, Ravassard P, Pardanaud-Glavieux C, Scharfmann R, Serup P. Mesenchymal bone morphogenetic protein signaling is required for normal pancreas development. Diabetes. 2010;59:1948–1956. doi: 10.2337/db09-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kobayashi H, Spilde TL, Bhatia AM, Buckingham RB, Hembree MJ, Prasadan K, Preuett BL, Imamura M, Gittes GK. Retinoid signaling controls mouse pancreatic exocrine lineage selection through epithelial–mesenchymal interactions. Gastroenterology. 2002;123:1331–1340. doi: 10.1053/gast.2002.35949. [DOI] [PubMed] [Google Scholar]
- 219.Öström M, Loffler KA, Edfalk S, Selander L, Dahl U, Ricordi C, Jeon J, Correa-Medina M, Diez J, Edlund H. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into β-cells. PLoS ONE. 2008;3:e2841. doi: 10.1371/journal.pone.0002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Arregi I, Climent M, Iliev D, Strasser J, Gouignard N, Johansson JK, Singh T, Mazur M, Semb H, Artner I. Retinol dehydrogenase-10 regulates pancreas organogenesis and endocrine cell differentiation via paracrine retinoic acid signaling. Endocrinology. 2016;157:4615–4631. doi: 10.1210/en.2016-1745. [DOI] [PubMed] [Google Scholar]
- 221.Jonckheere N, Mayes E, Shih H-P, Li B, Lioubinski O, Dai X, Sander M. Analysis of mPygo2 mutant mice suggests a requirement for mesenchymal Wnt signaling in pancreatic growth and differentiation. Dev Biol. 2008;318:224–235. doi: 10.1016/j.ydbio.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Afelik S, Pool B, Schmerr M, Penton C, Jensen J. Wnt7b is required for epithelial progenitor growth and operates during epithelial-to-mesenchymal signaling in pancreatic development. Dev Biol. 2015;399:204–217. doi: 10.1016/j.ydbio.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 223.Apelqvist Å, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997;7:801–804. doi: 10.1016/s0960-9822(06)00340-x. [DOI] [PubMed] [Google Scholar]
- 224.Kawahira H, Scheel DW, Smith SB, German MS, Hebrok M. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol. 2005;280:111–121. doi: 10.1016/j.ydbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 225.Nakayama S, Arakawa M, Uchida T, Ogihara T, Kanno R, Ikeda F, Azuma K, Hirose T, Kawamori R, Fujitani Y. Dose-dependent requirement of patched homologue 1 in mouse pancreatic beta cell mass. Diabetologia. 2008;51:1883–1892. doi: 10.1007/s00125-008-1080-2. [DOI] [PubMed] [Google Scholar]
- 226.Hibsher D, Epshtein A, Oren N, Landsman L. Pancreatic mesenchyme regulates islet cellular composition in a patched/hedgehog-dependent manner. Sci Rep. 2016;6:38008. doi: 10.1038/srep38008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yung T, Poon F, Liang M, Coquenlorge S, McGaugh EC, Hui C-c, Wilson MD, Nostro MC, Kim T-H. Sufu-and Spop-mediated downregulation of Hedgehog signaling promotes beta cell differentiation through organ-specific niche signals. Nat Commun. 2019;10:1–17. doi: 10.1038/s41467-019-12624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kang S, Park HS, Jo A, Hong SH, Lee HN, Lee YY, Park JS, Jung HS, Chung SS, Park KS. Endothelial progenitor cell cotransplantation enhances islet engraftment by rapid revascularization. Diabetes. 2012;61:866–876. doi: 10.2337/db10-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Karaoz E, Genc Z, Demircan PÇ, Aksoy A, Duruksu G. Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell death Dis. 2010;1:e36–e36. doi: 10.1038/cddis.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F, Mullen Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89:1438–1445. doi: 10.1097/tp.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 231.Davis NE, Beenken-Rothkopf LN, Mirsoian A, Kojic N, Kaplan DL, Barron AE, Fontaine MJ. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials. 2012;33:6691–6697. doi: 10.1016/j.biomaterials.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hamilton DC, Shih HH, Schubert RA, Michie SA, Staats PN, Kaplan DL, Fontaine MJ. A silk-based encapsulation platform for pancreatic islet transplantation improves islet function in vivo. J Tissue Eng Regenerat Med. 2017;11:887–895. doi: 10.1002/term.1990. [DOI] [PubMed] [Google Scholar]
- 233.Kogawa R, Nakamura K, Mochizuki Y. A new islet transplantation method combining mesenchymal stem cells with recombinant peptide pieces, microencapsulated islets, and mesh bags. Biomedicines. 2020;8:299. doi: 10.3390/biomedicines8090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang H, Li S, Dai Q, Gonzalez A, Tran ON, Sun H, DeFronzo RA, Dean DD, Yeh CK, Chen XD. Culture on a native bone marrow-derived extracellular matrix restores the pancreatic islet basement membrane, preserves islet function, and attenuates islet immunogenicity. FASEB J. 2020;34:8044–8056. doi: 10.1096/fj.201902893R. [DOI] [PMC free article] [PubMed] [Google Scholar]