Abstract
Circulating extracellular vesicles (EVs) are membrane-bound nanoparticles secreted by most cells for intracellular communication and transportation of biomolecules. EVs carry proteins, lipids, nucleic acids, and receptors that are involved in human physiology and pathology. EV cargo is variable and highly related to the type and state of the cellular origin. Three subtypes of EVs have been identified: exosomes, microvesicles, and apoptotic bodies. Exosomes are the smallest and the most well-studied class of EVs that regulate different biological processes and participate in several diseases, such as cancers and autoimmune diseases. Proteomic analysis of exosomes succeeded in profiling numerous types of proteins involved in disease development and prognosis. In rheumatoid arthritis (RA), exosomes revealed a potential function in joint inflammation. These EVs possess a unique function, as they can transfer specific autoantigens and mediators between distant cells. Current proteomic data demonstrated that exosomes could provide beneficial effects against autoimmunity and exert an immunosuppressive action, particularly in RA. Based on these observations, effective therapeutic strategies have been developed for arthritis and other inflammatory disorders.
Keywords: Proteomic analysis, Extracellular vesicles, Synovial, Exosomes, Microvesicles, Apoptotic bodies, Biogenesis, Citrullinated proteins, Antigen-presenting, Autoimmunity, Immune response, Arthritis, miRNA cytokine, FLS-derived exosomes, Urinary exosomes
Introduction to extracellular vesicles: intracellular cargo loaders
The transfer of metabolites and biological information between cells is essential for the coordination of various functions and regulation of different processes, such as cellular development, hemostasis, repair, and immunity [1]. In the past, a comprehensive analysis of intracellular communication revealed that such information could be transmitted using soluble mediators, which require specific receptors on the recipient cells or direct cell-to-cell contacts, such as in gap junctions, neurological synapses, and immunological synapses [2, 3]. Recent studies discovered a new type of cell-to-cell communication, where extracellular vesicles (EVs) serve as specific messengers between donor and recipient cells. Despite being regularly observed in the intercellular space, EVs were outside the major stream of scientific research for a long time and were typically considered as cellular junk or plasma membrane fragments with no biological or functional significance [4, 5].
The first studies specifically focused on the identification of EVs and analysis of their functional roles was conducted in the 1980s. At that time, several studies described the involvement of the plasma membrane vesicles in different physiological and pathological processes. Some of these studies recognized the formation and release of vesicles during the maturation and differentiation of blood cells, particularly reticulocytes [6, 7]. Seminal plasma analysis found the presence of membrane-surrounded particles named prostasomes that were important for the effective progression of sperm motility [8]. Another study discovered that tumors could shed a heterogeneous population of vesicles carrying coagulation factors, i.e., acting as procoagulants [9, 10]. More recently, advancements in laboratory techniques and the growing recognition of the complexity of intracellular communication helped focus the attention of the field on the need-to-solve mystery of EVs. In 2011, the International Society for Extracellular Vesicles (ISEV) was established as a reflection of the global interests, research efforts, developments, and achievements in this field [4, 11].
EVs are nanoparticles that serve as cargo carriers of molecules between body cells. EVs are membrane-bound vesicles secreted by most cells in extracellular spaces for intracellular communication and transportation of biomolecules. The cargo of EVs includes proteins, lipids, nucleic acids, small molecules, and receptors. As a result, EVs have many critical roles in the physiology, pathology, and therapy of biological systems [12]. Recently, several investigations described the involvement of EVs in many human diseases, such as cancers [10], infectious diseases [13], and autoimmune disorders, and illustrated how they participate in signaling and transferring biological information [14]. In this respect, efforts into molecular profiling of EV subsets are being made by several research groups to identify the unique molecular makers of EVs extruded by normal and abnormal cells, which may be helpful in disease prognosis, diagnosis, and therapy [15, 16].
EVs serve as mediators of the intercellular signaling and can act as biomarkers for diseases, drug delivery vehicles, or therapeutic agents [17, 18]. There are several specific classes of EVs, which depend on their origin. Some of these classes are apoptotic bodies and apoptotic microvesicles released by dying cells undergoing apoptosis [19–21], exosomes of endosomal origin [7, 22, 23], ectosomes, microparticles, microvesicles of plasma membrane origin [24], large EVs, such as oncosomes and exophers produced by cancer cells [25, 26] and large EVs generated by neurons [27], and recently discovered non-membranous nanoparticles, exomeres [28]. It was also pointed out that platelets/thrombocytes generated by megakaryocytes can be classified as EVs as well [11]. Different types of EVs are characterized by specific dimensions, where apoptotic bodies, ectosomes/microvesicles, and exosomes have sizes of 1000–5000 nm, 100–1000 nm, and 30–160 nm, respectively [22].
At the time of its origin, the term “exosome” had an exact meaning as it was introduced to describe a particular class of small EVs originating from the endosomal system of the cell [6, 7, 23, 29–36]. However, with time, the original precision was lost, and one can find in numerous literature cases when this term was used to describe not only real exosomes, which are the products of the multivesicular body of the endosomal origin, but all small EVs, and even all EVs released by various cells [37]. The situation is further complicated by the fact that although there are instances where small EVs can be unambiguously classified as exosomes following their biogenesis inside the cells with the crucial step being an observation of the multivesicular bodies, classifying small EVs in the extracellular environment as exosomes are not as straightforward and challenging [37]. In fact, a small EV cannot be defined as an “exosome” based solely on its size, as ectosomes can be just as small as exosomes. Furthermore, these two types of EVs also carry most of the same markers. Although very specific features are ascribed to the exosomes, such as the ability to be separated and isolated by particular methods, definite size range, possession of specific functions, and presence of specific cargo, often this is done without solid evidence or without appropriate comparison with other types of EVs [37]. Therefore, although PubMed has almost 20,000 papers mentioning exosome, it is likely that in many of these publications, the use of the “exosome” term is incorrect, as either faulty assumptions were used or no strong proof of the actual biogenesis pathway of the analyzed particles was provided. To resolve these issues, the ISEV has issued updated guidelines on minimal information for studies of extracellular vesicles (MISEV), where the consensus was reached on using “extracellular vesicle” as the “generic term for particles naturally released from the cell that is delimited by a lipid bilayer and cannot replicate” [11]. To follow this consensus, we use the term EV in “Introduction to extracellular vesicles: intracellular cargo loaders” and “Types, structure, and biogenesis of EVs”, where the information on the general composition, methods of study, and biogenesis are provided. However, as the subsequent sections are focused on rheumatoid arthritis (RA), where the exosomes are known as the most studied EV class contributing to the RA pathogenesis, we used there the term exosomes.
Extracellular vesicles’ proteome analysis methods
EVs are membrane-bound vehicles that are secreted extracellularly from both normal and abnormal cells into the surrounding fluids. Experimentally, EVs have been extracted from several body fluids, such as blood, urine, semen, breast milk, synovial fluid, and cerebrospinal fluid (CSF) [24, 38]. An increased interest in the EVs field led to the development of several protocols and methodologies that have been applied for the isolation of EVs and analysis of their physical, chemical, and compositional characteristics. Different methods have been used for the separation and purification of EVs from cell cultures and body fluids [38]. The conventional methods for EVs separation are ultracentrifugation and density gradient centrifugation. To date, ultracentrifugation is the standard gold method of EV isolation [39–41]. Other separation methods have been used, such as techniques based on size (e.g., ultrafiltration, sequential filtration, size exclusion chromatography (SEC), hydrostatic filtration dialysis (HFD), commercial isolation kits) [42–46], immunoaffinity capture (e.g., ELISA, magneto-immunoprecipitation) [47, 48], and precipitation (e.g., polyethylene glycol (PEG) precipitation or lectin-induced agglutination) [49, 50]. Obviously, the selection of the most appropriate separation technique for a given experiment is determined by a multitude of factors, such as sample matrix and volume, downstream analyses, and, most importantly, the peculiarities of the experimental question. The most suitable methods used to evaluate the size, morphology, and concentration of EVs include transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA) with ZetaView (that captures the Brownian motion of each particle in the video; examples of such an instrument are given by ZetaView® TWIN and ZetaView® QUATT by Analytik (UK) with two and four lasers, respectively, for enhanced fluorescence measurement capability and measuring the particle hydrodynamic size, zeta potential, and concentration), and tunable resistive pulse sensing (tRPS) [51–54]. The proteomic analyses of EVs include several techniques, such as flow cytometry, Western blotting, and mass spectrometry (MS) [38, 55, 56].
Composition of extracellular vesicles
The content or cargo of EVs is highly variable, and this dynamic variation is related to the type and state of the cellular origin, as well as to the separation and enrichment methods used for isolation of EV for analysis [57]. Using a high-throughput technology such as MS, in combination with Western blotting and electron microscopy, numerous studies have been conducted for the characterization of EV proteomes [58]. The proteome of EVs includes broad sets of proteins, with some related to EV biogenesis and others being relevant to the type and condition of the source cell [59]. The common proteomic content of EVs is raised from the cytosol and plasma membrane, and often includes proteins involved in the formation of the EVs. The proteome of a typical EV includes: (1) cytosolic proteins, such as heat shock protein 70 (HSP70), ALG-2-interacting protein X (ALIX), tumor susceptibility gene 101 (TSG101), clathrin, and ubiquitin; (2) cytoskeleton proteins, such as actin, tubulin, profilin-1, and cofilin-1; (3) adhesion and fusion-related proteins, e.g., intercellular adhesion molecules (ICAMs), Rab GTPases, integrins, and selectins; (4) membrane proteins, such as flotillins and lectins; (5) antigen-presenting proteins, such as major histocompatibility complex molecules (MHC-II) and tetraspanins (e.g., CD9, CD37, CD53, CD62, CD81, CD82); (6) transport proteins, such as annexin; (7) some metabolic enzymes, such as enolases, glyceraldehyde 3-phosphate dehydrogenase, peroxiredoxins, and pyruvate kinase; and (8) endosomal sorting complexes required for transport (ESCRT) proteins, which have a pivotal role in the EV biogenesis [24, 38, 57, 59–61].
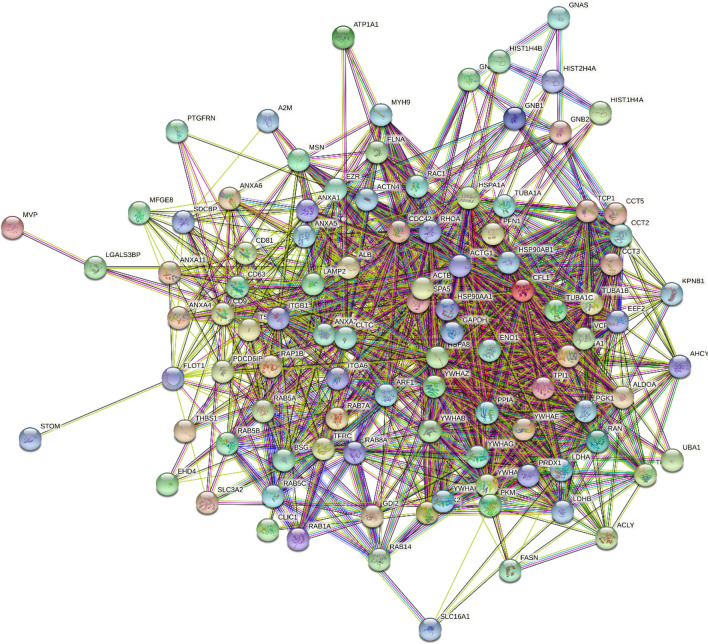
Importantly, proteins found in EVs are highly interconnected. This is illustrated by Fig. 1 representing the protein–protein interaction (PPI) network between 100 human proteins from the EV proteome. The proteins for this analysis were selected from the Web-based database of the exosomal cargo ExoCarta (http://www.exocarta.org/), which is a database of exosome protein, RNA, and lipids generated based on the results reported in 286 studies. This database contains information on 9769 proteins found in the exosomes of different species including 6514 human proteins [62]. Analyzed in our study proteins represents Top 100 human ExoCarta proteins that were identified as exosomal proteins in 44 to 98 independent experiments, and which are listed by ExoCart as exosome markers (http://www.exocarta.org/exosome_markers_new). This PPI network was generated by the Search Tool for the Retrieval of Interacting Genes (STRING, http://www.string-db.org/) [63–65], which generates a network of predicted associations based on predicted and experimentally validated information on the interaction partners of a protein of interest [66]. In the corresponding network, the nodes correspond to proteins, whereas the edges show predicted or known functional associations. Seven types of evidence are used to build the corresponding network, and are indicated by the different colored lines: a green line represents neighborhood evidence; a red line—the presence of fusion evidence; a purple line—experimental evidence; a blue line—co-occurrence evidence; a light blue line—database evidence; a yellow line—text mining evidence; and a black line—co-expression evidence [66]. In the EV-centered PPI network, there are 100 nodes (proteins) linked by 1167 edges (protein–protein interactions). Therefore, the average node degree of this network is 23.3, and its average local clustering coefficient (which defines how close its neighbors are to being a complete clique; the local clustering coefficient is equal to 1 if every neighbor connected to a given node Ni is also connected to every other node within the neighborhood, and it is equal to 0 if no node that is connected to a given node Ni connects to any other node that is connected to Ni) is 0.61. Since the expected number of interactions among proteins in a similar size set of proteins randomly selected from human proteome is equal to 337, this internal PPI network between EV-specific proteins has significantly more interactions than expected, being characterized by a PPI enrichment p value of < 10–16. Therefore, Fig. 1 shows that human proteins found within EVs can efficiently interact with each other, and the majority of EV-specific proteins are characterized by high binding promiscuity as evidenced by their average node degree of 23.3.
Fig. 1.
STRING-generated PPI network of proteins from the EV proteome. The proteins for this analysis were selected from the web-based database of exosomal cargo ExoCarta (http://www.exocarta.org/), which is the database of exosome proteins, RNAs, and lipids containing information on 9769 proteins found in the exosomes of different species including 6514 human proteins [62]. Analyzed group represents Top 100 human ExoCarta proteins that were identified as exosomal proteins in 44–98 independent experiments. This PPI network was generated by the Search Tool for the Retrieval of Interacting Genes (STRING, http://www.string-db.org/) [63–65]
Since multifunctionality and capability to be engaged in interaction with multiple partners are specific characteristics of intrinsically disordered proteins (IDPs) or hybrid proteins containing ordered domains and intrinsically disordered protein regions (IDPRs) [67–72], and since approximately 60% of proteins in eukaryotes display at least some degree of the disorder [67, 70, 73–93], we also looked at the disorder status of EV-specific proteins. Results of this analysis are summarized in Fig. 2, which clearly shows that many of these proteins are expected to have high levels of intrinsic disorder. Based on their levels of mean intrinsic disorder score, proteins are typically classified as mostly ordered (mean disorder score < 0.25), moderately disordered (mean disorder score between 0.25 and 0.5), and highly disordered (mean disorder score ≥ 0.5). Similarly, proteins can be classified based on their percent of predicted disorder, where two arbitrary cutoffs are used to classify proteins as mostly ordered (percent of predicted disorder < 10%), moderately disordered (10% ≤ percent of predicted disorder < 30%) and highly disordered (percent of predicted disorder ≥ 30%) [94]. Figure 2 shows that based on these classifications, an only very limited number of proteins (~ 3%) found within the EVs are predicted as mostly ordered, whereas 64% and 33% of these proteins are expected to be moderately or highly disordered, respectively. Therefore, proteins from the EV proteome are characterized by high levels of intrinsic disorder, suggesting the functional importance of such global disorderedness.
Fig. 2.
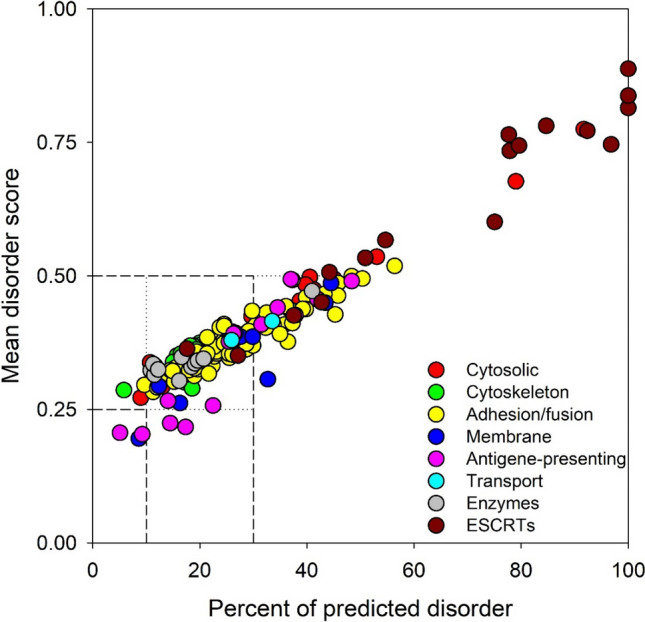
Intrinsic disorder predisposition of proteins from the human EV proteome. This plot is generated based on the evaluation of intrinsic disorder predisposition of these proteins by PONDR® VSL2 algorithm [306]. Proteins are colored according to the classification described in the text. Boundaries separating mostly ordered, moderately disordered, and highly disordered proteins are shown
This is in line with the accepted notion that IDPs and IDPRs are binding “professionals” capable of interacting with various partners via multiple binding modes [67–70] and forming static, semi-static, dynamic, or fuzzy complexes [71, 72]. The ability of IDPs/DPRs to have semi-static and dynamic polyvalent interactions [95] defines a characteristic wrapping binding mode, where multiple binding sites of one protein are simultaneously bound to multiple receptors on another protein [96]. Many IDPs/IDPRs bind partners with both high specificity and low affinity [97] and can fold at binding to their partners [98–100]. The degree of such binding-induced folding can be different in various systems, thereby forming complexes with broad structural and functional heterogeneity [71, 72]. Furthermore, some IDPs/IDPRs serve as morphing shape-changers that can adopt alternate folds because of binding to different partners [100–105]. The binding region of such a morphing IDP can assume completely different structures in the rigidified assemblies formed by binding to divergent partners [68, 106–109]. Many other IDPs/IDPRs are known to form fuzzy complexes, where significant disorder is retained, at least outside the binding interface [110–117]. Therefore, it is not surprising that many IDPs/IDPRs serve as hub proteins—nodes in complex protein–protein interaction (PPI) networks that have a very large number of connections to other nodes [105, 118–123].
In addition, proteins detected in EVs, especially microvesicles and exosomes are commonly decorated with various PTMs, such as glycosylation and phosphorylation [38, 124, 125]. Interestingly, proteomic analysis of EVs proved that some proteins were identified to be shared and fundamental components of all EV subsets regardless of the type of cell source. These most common constituents of EVs are HSP70, ESCRT proteins (e.g., TSG101 and Alix), MHC molecules, and tetraspanin (CD9, CD63, CD81, and CD82) [11, 57, 61].
It is important to note that all EVs except for apoptotic bodies contain specific membrane-bound and cytoplasmic proteins. Furthermore, there is a set of proteins that is not expected to be found in exosomes and shed microvesicles. These are proteins from the nucleus, mitochondria, endoplasmic reticulum, and Golgi complex [1]. On the contrary, apoptotic bodies have been reported to be enriched in proteins from different organelles, such as the nucleus, mitochondria, Golgi apparatus, and endoplasmic reticulum [38]. However, it was also pointed out that some EVs (e.g., EVs generated by neural stem cells) can transfer mitochondria between cells [126]. Although these EVs are not conventional, they are EVs nonetheless. Therefore, as these specialized EVs exist, one needs to take care when making statements about cargo that is included and excluded in different EVs.
In 2007, an important milestone in EV analysis was achieved as it was found that the EV-derived RNAs were analogous to the RNA profile of the source cell [127]. Evidently, many studies revealed that the RNA content of EVs mainly includes mRNA and miRNA. Further investigations discovered the existence of some non-coding RNAs, such as tRNA and structural RNA. The major challenge in identifying EV-associated RNAs was to ensure that these RNAs are not co-isolated as RNA–protein complexes [128].
Although fragments of DNA are also found incorporated in the EVs, compared to RNA, they are much less abundant and are typically limited to EVs generated under specific conditions and by specific cells [57]. Interestingly, a variety of DNAs was detected in tumor-derived EVs. These include mitochondrial DNA (mtDNA), single-stranded DNA (ssDNA), double-stranded DNA (dsDNA), and amplified oncogene sequences. Such DNAs in EVs are useful for identifying mutations in the parental cancer cells [10, 129, 130]. Altogether, these findings reflect the role of EVs in the exchange of genetic materials between the cells and suggest the involvement of these vesicles in the regulation of gene expression [24].
In terms of their stability and rigidity, EVs are highly stable vesicles when compared to the parental cells, and they are enriched in specific lipid domains and lipid rafts [131–133]. EVs are coated with a phospholipid bilayer membrane resembling that of the cell membrane, and they contain most of the lipid components from the parental cells, which play essential roles in vesicular biogenesis and protection [61]. Several lipidomic analyses using Laurdan fluorescence spectroscopy have demonstrated the great rigidity and resistance of various EVs. This stability is attributed to the increased content of specific types of lipids, such as sphingomyelin, cholesterol, di-saturated, and poly-saturated fatty acids [134]. Ceramide, sphingomyelin, prostaglandins, and lysophosphatidic acid (LBPA) are the characteristic EV lipids that are involved in the formation of EVs and help to maintain their integrity [60]. In contrast, highly abundant phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, ganglioside GM3, phosphatidylinositol, and LBPA increase the overall stability of EVs [135], and cholesterol, to a large extent, is required for the secretion of EVs [57]. Among various EVs categories, exosomes were demonstrated to be the most rigid and stable vesicles [134].
Types, structure, and biogenesis of EVs
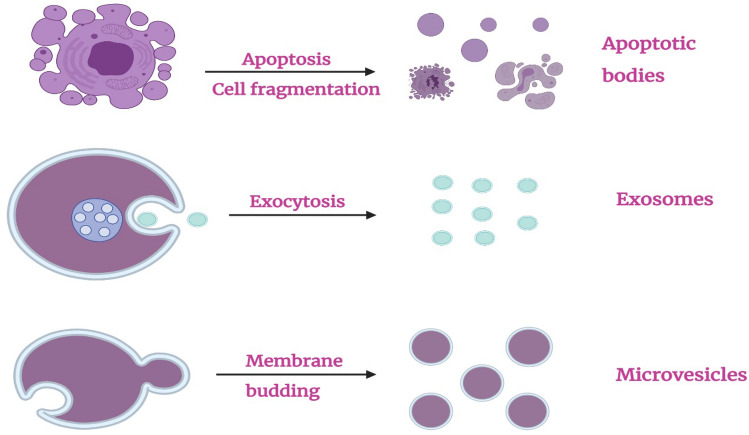
Based on the size and peculiarities of their biogenesis and releasing mechanisms, EVs are commonly classified into three subtypes [24]: exosomes, microvesicles, and apoptotic bodies. Types of the extracellular vesicles and the mechanisms leading to their generation are shown schematically in Fig. 3. EVs are vesicles encapsulated with a lipid bilayer membrane, which is more stable than the cellular membrane due to the enrichment in specific lipids [61, 136, 137]. As a result, EVs show high stability and resistance to degradation and effects of low pH. Among the three EV categories, exosomes are the most rigid and stable vesicles [134]. All types of EVs are characterized by nano-size and typically spherical morphology, and they carry biomolecules, such as cytoplasmic proteins and nucleic acids, which are surrounded by a phospholipid bilayer membrane (Table 1) [138]. Beyond that, EV subsets are heterogeneous organelles, which are variable in their size, density, content, biogenesis pathway, and secretory mechanism [139].
Fig. 3.
Types of extracellular vesicles (EVs)
Table 1.
General features of the extracellular vesicle (EVs)
| Exosomes | Microvesicles | Apoptotic bodies | |
|---|---|---|---|
| Size | 40–100 nm | 100–1000 nm | 50–5000 nm |
| Shape | Regular cup-shaped | Heterogeneous spherical | Irregular |
| Origin | MVB from living cells | Plasma membrane living cells | Cell death |
| Biogenesis |
ESCRT-I machinery ESCRT-II machinery |
Calcium-dependent ESCRT-III machinery | Apoptosis-dependent |
| Secretion | MVB exocytosis across the plasma membrane | Plasma membrane budding | Cell fragmentation |
| Content | Proteins, lipids, mRNA, and miRNA | Proteins, lipids, mRNA, and miRNA | Proteins, cell organelles, nuclear fractions, and DNA fragments |
| Marker | ALIX, CD9, CD63, CD81, TSG 101, HSP70, and HSP90 | Integrin, selectin, follitin-2, CD62, CD40 ligand, and ARF6 | Annexin V, histones, and DNA fragments |
Apoptotic bodies
These EVs are heterogeneous in size and shape. Their diameter ranges between 50 nm and 5 µm. They are characterized by an irregular morphology and mostly appear as large vesicles. Apoptotic bodies (ApoBDs) have a density of 1.24–1.28 g/ml and are released as membrane blebs from apoptotic cells during programmed cell death [38, 140]. In contrast to other EVs such as exosomes and microvesicles, which originate from living cells, apoptotic bodies arise from dying cells [12]. ApoBD formation is a physical process characterized by cellular shrinkage and an increase in hydrostatic pressure. This process, which includes bleb formation and regression, has been found to occur over persistent time during programmed cell death. ApoBD formation has a vital protection role: it prevents the leakage of toxic and immunogenic materials from dying cells to neighboring cells and tissues, and for this function, ApoBDs are regarded as “garbage bags” [141].
ApoBD biogenesis is a complex multistep process (e.g., chromatin condensation, DNA fragmentation, nuclear disruption, mitochondrial expansion, cytoskeleton disassembly, phosphatidylserine externalization, plasma membrane protrusion, or zeiosis), which is accompanied by the inhibition of several cellular functions, cell contraction, accumulation of unwanted components in the bleb lumen, and finally the release of ApoBDs extracellularly for the phagocytic clearance. A family of proteolytic enzymes known as cysteine proteases or caspases regulates the nuclear and cellular breakdown [141–143]. Interestingly, ApoBDs expose specific “eat-me” signals on their surfaces, which trigger the phagocytic cells for rapid engulfment [144]. Similar to other EVs, the cargo of ApoBDs includes components from the cytoplasm and cellular membrane. However, it is important to note that they also contain nuclear components such as histone, as well as mitochondrial DNA and variable RNAs, and protein found in ApoBDs that is typically characterized by relatively low PTM levels [140].
In integration with multi-dimensional liquid chromatography and tandem mass spectrometry (LC–MS/MS), a shotgun proteomic was implemented for the identification of the protein profile and markers of the ApoBDs isolated from human biliary epithelial cells (HBEC) [143]. The results of this proteomic profiling demonstrated that several proteins were distinctly associated with ApoBDs. The list of these proteins included annexin A6 (ANXA6), heat shock protein beta-6 (HSPβ6), low-density lipoprotein receptor-related protein 1 (LRP1), 3′-phosphoadenosine 5′-phosphosulfate synthase 2 (PAPS2), vascular endothelial growth factor receptor 3 (VGFR3), serpin H1 precursor (SERPH), secreted glypican-6 (GPC6), isomerizing enzyme (B4DN38), reductase enzyme (Q6ZR44), rab-11, and Ras-related protein Rab-11A (RAB11A) [145]. However, annexin A6 and DNA-binding histone were the most specific markers for ApoBDs [146].
Microvesicles
Microvesicles (MVs) are another class of EVs, also known as shedding vesicles or ectosomes. MVs are intermediate-to-large vesicles that are formed by the direct external budding and cleavage of the cell plasma membrane. Using NTA and TEM, MVs appear to have a spherical shape with variable sizes ranging from 100 nm to 1 µm in diameter [146]. According to their formation pathway in the cytoplasm followed by the extracellular releasing as a pinch of plasma membrane without the involvement of subcellular organelles, it is easy to trace and understand the proteome of MVs [147]. In fact, it was found that proteins from the cytosol and plasma membrane, specifically the membrane surface proteins such as tetraspanins, are the main components of the MV proteome. The amount of tetraspanins detected in these vesicles is 100-fold higher than that of the cell lysate [148]. Interestingly, the cargo of MVs was found to contain increased levels of proteins subjected to PMT, such as glycosylation and phosphorylation [38].
Different mechanisms have been suggested for MVs biogenesis and release [149]. The most common and well-studied mechanism starts with the elevation of calcium (Ca2+) levels and the accumulation of protein-degrading enzymes. Increased levels of Ca2+ stimulate the nucleation of the plasma membrane, which is associated with the clustering of lipids and tetraspanin proteins at distinct domains of the plasma membrane. Concomitantly, the degrading enzymes induce the dismantlement of cytoskeleton components and subsequent detachment from the plasma membrane. Furthermore, high Ca2+ levels activate specific enzymes known as lipid translocases on the plasma membrane, which induce the externalization of the phospholipid phosphatidylserine (PS). PS externalization promotes the outward budding of the plasma membrane. As a result of cytoskeleton disassembly, the cytosolic contents and genetic materials are sorted in the lumen of budding; tetraspanin proteins regulate the sorting of MV cargo. Recently, another mechanism of MV formation was suggested, which is dependent on the ESCRT-III complex that is transiently assembled on endosomes. TSG101 induces the translocation of ESCRT-III to the plasma membrane, which in turn promotes the conical spiral formation of the plasma membrane. After that, the cleavage of the spiral and releasing of MVs are mediated by the attachment of the Vps4 ATPase enzyme to the budding neck [143, 150]. The release of MVs is stimulated by the activation of purinergic receptors with ATP. The secretion pathways vary according to the source cells. For instance, the release of MVs from dendritic cells (DCs) is activated by lipopolysaccharides [151, 152].
As aforementioned, the route of MV formation in the cytoplasm and migration across the plasma membrane facilitates their involvement of numerous proteins. One should remember that separating MVs and exosomes is a difficult task, as the current state-of-the-art approaches are less than ideal, and the majority of related studies separate vesicles on their size and density, which cannot specifically separate MV and exosomes. In fact, although most often, differential ultracentrifugation is used to separate the more dense MVs from the less dense exosomes [16], even small variations in the isolation procedures can lead to the samples, where a mixture of exosomes and MVs is present [153]. As a result, accurate determinations of the physical properties, cargo, and functions of each type of EV are challenging [153]. However, the majority of our current knowledge on exosomes and MVs is rooted in data generated by these approaches [153], suggesting that finding novel ways for the accurate separation of MVs from exosomes is critically needed. Several studies have been carried out for the characterization of the proteome and other molecular components of MVs [154]. The most common proteins that are found to be enriched in the MVs include cytoskeleton protein (Actin), cytosolic proteins (HSPs), membrane protein (flotillin-2), transport protein (Annexin V), adhesion proteins (Integrins such as Mac-1), degradation enzymes (Matrix metallopeptidases such as MMP2), and glycoproteins (P-selectin, GPIb and GPIIb-IIIa). Recently, it was suggested that MMP2 could be used as a marker for MVs [154–160]. It is worth noting that in comparison to exosomes, MVs are markedly enriched in glycoproteins and phosphoproteins, i.e., proteins that underwent posttranslational modifications (PTMs), such as glycosylation and phosphorylation [124, 125]. Importantly, biological functions of many IDPs/IDPRs is regulated by various PTMs [161, 162]. In fact, catalytically introduced PTMs are commonly found within IDPRs, whose conformational flexibility enables accessibility of the modification sites [161–164].
Exosomes
Exosomes are the smallest and the most well-studied class of EVs, with an average diameter of 40–100 nm and density of 1.13–1.19 g/ml; they have a cup-like shape in EM images as a result of the drying process for the preparation for imaging [146], though in reality they are completely spherical, as demonstrated by cryo-TEM imaging. The process of exosome formation involves several steps and requires highly regulated machinery, while further specific mechanisms promote the secretory pathway of exosomes in the extracellular space. The first step in exosome biogenesis is the internal budding of the early endosomes, followed by the accumulation of intraluminal vesicles (ILVs) within endosomes to form microvesicle bodies (MVBs) or late endosomes [165]. ILVs contain and carry the exosomal cargo, including endosomal, cytosolic, and membranous proteins, as well as nucleic acids, such as mRNA, miRNA, and DNA fragments [166].
ESCRT machinery is a well-understood pathway for the biogenesis and secretion of exosomes. In this mechanism, the multivesicular bodies (MVBs) are formed intracellularly by the accumulation of intraluminal vesicles (ILVs). This process starts with the formation of the early endosome, followed by the inner budding of the endosomal membrane, which allows for the formation and accumulation of ILVs within the mature endosome or MVBs. ESCRT-dependent mechanisms regulate the sorting and clustering of proteins and receptors, i.e., cargo, in the ILVs. Then, the MVBs loaded with ILVs transport to fuse with the plasma membrane (PM) and release their content of exosomes in the extracellular space [165, 167–170]. According to their origin from endosomes, biogenesis in the cytoplasm, and finally fusion with plasma membrane upon releasing extracellularly, it is easy to understand why exosomes encompass a large number of proteins in their cargo, with the proteome of a typical exosome containing approximately 4400 proteins [171]. The mass spectrometry-based proteomic study of exosomes succeeded in profiling numerous types of proteins, such as ALIX, TSG101, CD9, CD63, CD81, CD82, HSP70, HSP90, annexins, flotillins, GTPases, integrins, MHC class I, MHC class II, clathrin, ubiquitin, components of the ESCRT complex, actin, tubulin, and lipid-related proteins [143, 172].
Furthermore, exosome cargo has been reported to contain RNA components such as mRNA and miRNA, which were thought to be incorporated from the cytoplasm during the invagination of ILVs into MVB [127]. Similar to MVs, exosomes are enriched in post-translationally modified proteins, particularly glycoproteins, but such modified exosomal proteins are less abundant than proteins of MVs [38]. Regardless of the type of parental cells, the most common proteins of exosomes are ALIX, Tumor susceptibility gene 101 (TSG101), heat shock protein (HSP70 and HSP90), and tetraspanins CD9, CD63, CD81, and CD82. Distinctly, these exosome-enriched proteins are highly utilized as specific markers for exosomes [173]. However, due to the imperfection of the techniques used for separation of MVs and exosomes, at least some of the proteins assumed to be specifically enriched in exosomes, and therefore, used as exosome-specific markers are not specific to exosomes and are found in MV. An example is given by CD9, CD63, and CD81 which are not only abundant in exosomes but also in MVs, questioning the usefulness of these tetraspanins with wide cellular expression as the specific means for the discrimination of these types of EVs [174–176].
Some proteins were proven to be specific for exosomes that originated from specific cell types. For example, Intercellular Adhesion Molecule 1 (ICAM-1) and CD63 are highly associated with the exosomes released by dendritic cells (DCs) and B-lymphocytes, respectively [177, 178]. Another example of a protein that accounted for cell type-specific exosomes is milk fat globule-EGF factor 8 (MFG-E8) or lactadherin, with this adhesion protein being prevalent in exosomes released from fibroblasts and immune cells [179].
The abundance of exosomes in body fluids
Several studies were conducted for the proteomic profiling of exosomes originating from different cells and extracted from different body fluids, such as blood, CSF, saliva, milk, urine, and semen. Different isolation techniques are typically applied for the purification of exosomes. Differential ultracentrifugation, density gradient centrifugation (DGC), multi-dimensional chromatography, and commercial isolation kits are the standard methods used for exosome isolation, while other technologies such as NTA, TEM, and Western blotting are frequently used for the detection of exosomes based on their size and morphology. The most common techniques used for the quantitation and proteomic profiling of exosomes are flow cytometry and coupled high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) [38, 173, 180].
Regarding the exosome concentration in body fluids, blood, i.e., plasma and serum, contains the highest number of exosomes and other EVs, followed by milk. Recent studies produced remarkable data showing that milk contains vast quantities of exosomes, larger than the exosome content of any other body fluid and cell culture [181]. Therefore, milk is considered a unique and enriched source of exosomes in humans, while blood, CSF, urine, and saliva, follow in order of exosome concentration [181–183]. Many comparative investigations used different techniques for the measurement of exosome levels in plasma, serum, and CSF, and the obtained data revealed that the quantities of plasma and serum-derived exosomes are higher than those of the CSF-derived exosomes [184]. Recently, a comprehensive proteomic analysis of the exosomes derived from seminal plasma was conducted [185]. This analysis revealed that semen is more enriched in these EVs than CSF. Urine and saliva were identified to provide inadequate exosomes for proteomic study [181, 186].
Exosomes are nanosized vehicles carrying functional biomolecules excreted from various cells in body fluids to transmit their cargo to neighboring or distant cells. The great variety of proteins and biomolecules that can be delivered by the exosomes allow them to mediate and participate in different physiological balance and pathological processes as an intracellular communicator. In the next part of the review, we will illustrate the significance of exosomes in human pathophysiology and highlight their roles in the immune system and autoimmune diseases, particularly in rheumatoid arthritis (RA).
Exosomes in normal physiology and pathology
Numerous studies revealed the significant functions of exosomes under physiological and pathological conditions. Exosomes can act as regulators of different biological processes, such as hematopoiesis [187], axonal regeneration [188], and immune response. In addition, they are involved in several pathological processes, such as infections [189], tumor progression and metastasis [190, 191], neurodegenerative diseases [192], inflammation, and autoimmune disorders [193, 194]. Exosomes are vesicles that could be released under both normal and pathological conditions from almost all types of body cells, such as DCs, lymphocytes, macrophages, platelets, mast cells, endothelial cells, neural cells, epithelial cells, mesenchymal stem cells (MSCs), muscles cells, and malignant cells [188, 190, 195, 196]. Exosomes have a crucial role in intracellular communication and disease pathogenesis, as they can transfer signals, receptors, cytokines, proteins, lipids, nucleic acids, and infectious agents from the cells of origin to local or distant cells [189, 197, 198].
Roles of exosomes in immune response modulation
Being the vehicles that mediate cell-to-cell communication, exosomes act as modulators of the immune system. The proteomic and immunological studies of immune cell-derived exosomes obtained from different body fluids revealed the significant role of these nanovesicles in the regulation and modulation of immune responses, including both immune suppression and immune stimulation. Recent studies reported that human exosomes contain a minimum of 98 immunogenic molecules in their cargo [199]. The immunological functions of exosomes are highly dependent on their membrane proteins and the cells of origin. In addition, the high stability of exosomes in the extracellular space enables them to carry their cargo far away to interact with distant cells [200]. Furthermore, the regulatory effects of exosomes involve cross-talk between multiple immune cells, such as the interaction between B-lymphocyte-derived exosomes and CD8+ cytotoxic cells, [201] and between T cells-derived exosomes and DCs [202].
Dendritic cells (DCs) derived exosomes
Exosomes secreted from immune cells, such as mature DCs, expose MHC molecules and have a unique capability to serve as antigen-presenting vesicles that can activate lymphocytes to develop innate or adaptive immune responses [203]. Interestingly, DCs are able to secrete up to one million class II MHC molecules daily through their exosomes. Such DC-derived exosomes can grab antigenic peptides either by direct capture of antigens or by indirect antigen processing through the parent DCs [204]. In the absence of antigen-presenting cells (APCs), exosomes showed an immunogenic property to activate CD8+ lymphocytes, thereby providing evidence that exosomes contain high amounts of class I MHC proteins and ICAM-1 [200]. Furthermore, DC-derived exosomes were identified to possess class II MHC proteins and mediate the activation of CD4+ helper cells via the interaction with lymphocyte function-associated antigen 1 (LFA-1) expressed on the surface of T cells [205]. In the context of antigen-presenting properties, DC-derived exosomes showed a relatively higher immunostimulatory effect than the intact DCs [206].
In contrast, immature DC-derived exosomes have an opposite impact on the immune system. The immature DCs secrete exosomes that might promote and induce the immune tolerance at the steady-state through their cargo enriched with self-antigens and anti-inflammatory factors. Furthermore, these exosomes were found to lack the class II MHC and co-stimulatory CD86+ molecules. As a result, they are unable to induce an immune response and instead produce immunosuppressive effects [194, 207]. In allograft transplantation, the immature DC-derived exosomes were shown to perpetuate the survival of the allograft by suppressing the proliferation of T cells via the secretion of anti-inflammatory IL-10 [208]. In conclusion, the DC-derived exosomes participate in the immune response modulation of both helper and cytotoxic T cells and maintain immune tolerance.
Mesenchymal stem/stromal cells (MSCs) derived exosomes
MSCs are stromal cells with the multipotent differentiation capacity in vitro that could be isolated from different tissues, such as bone marrow, adipose tissues, placenta, and skin. The regenerative capacity of MSCs reflects their important roles in immune modulation and therapeutic application [209]. Recent studies have identified the immunomodulatory effects of MSC-derived exosomes on peripheral blood mononuclear cells (PBMCs). These exosomes were obtained from the bone marrow of healthy individuals and were found to exert essential functions between MSCs and PBMCs. Such MSC-derived exosomes can modulate the activities of lymphocytes, macrophages, neutrophils, DCs, and natural killer (NK) cells [210]. The subsets of T helper (Th) cells and cytokine profiles have been analyzed using ELISA and flow cytometry techniques. One of the beneficial effects of MSC-derived exosomes that had been reported is the ability of these exosomes to inhibit the secretion of pro-inflammatory cytokines, such as interleukin-6 (IL-6), interleukin-1β (IL-1β) [211], and tumor necrosis factor-α (TNF-α), and to increase the production of anti-inflammatory factors, such as transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) [212]. In addition, under the influence of the MSC-derived exosomes, the ratio of Th1 and Th17 cells was reduced, while Th2 cells were increased. These findings showed that exosomes could induce the proliferation of CD4+ cells into Th2 cells and suppress their differentiation into Th1 and Th17 cells, which are implicated in the autoimmunity response. Furthermore, the ratio of regulatory T cells (Tregs) was elevated after the interaction with exosomes. Overall findings demonstrated that the MSC-derived exosomes have favorable immunomodulatory properties [210–213]. Recently, MSC-derived exosomes have been proposed for many therapies and are considered to be a convenient choice for the delivery of therapeutic drugs, enzymes, and genes to targeted cells [214]. New evidence showed that exosomes derived from MSC are potential tools in transplantation through their potential to treat graft-versus-host disease (GvHD) [215].
Natural killer cells (NK) derived exosomes
NK are innate immune cells that play pivotal roles in immune responses and show a natural cytotoxicity function, by which they can directly lyse malignant and virus-infected cells without the requirement of prior sensitization. In addition, activated NK cells can mediate the immune response indirectly via releasing pro-inflammatory cytokines and chemokines that modulate the adaptive cell-mediated immune response [216]. Several studies have investigated the possible role of NK-derived exosomes in the maintenance of immune hemostasis, and they found that these exosomes exert anti-tumor activity in the same manner as NK cells [217]. A recent study demonstrated that the activated NK cells released exosomes loaded with cytotoxic proteins, including perforin (PFN), granulysin (GNLY), and granzymes (Gzm-A and Gzm-B), which are known to enter the target cells and induce caspase-dependent apoptosis [218]. Exosomes formation begins with the endosomal system; early endosomes mature into late endosomes or MVBs. Cytotoxic proteins have been suggested to be transported from Golgi to early endosomes and then incorporated into the exosomes. The best-characterized trafficking machinery is the clathrin-dependent transport of lysosomal hydrolases by mannose-6-phosphate receptors (M6PRs) and lysosome-associated membrane glycoprotein 1 (LAMP1) [219, 220].
A dedicated study was designed to identify the proteomic components and cytotoxic activities of the resting NK cells and lymphokine-activated killer cells (LAK) against tumor cells. Using flow cytometry and Western blot techniques, it was shown that LAK-derived exosomes contain high levels of FasL and perforin molecules capable of inducing the cytotoxic lysis of cancer cells, especially in hematologic malignancies; i.e., leukemia and lymphoma. It is worth noting that NK-derived exosomes were not detectable in the plasma of healthy individuals [221]. Overall, several studies suggest that the understating of the molecular cytotoxic activities of NK-derived nanovesicles is useful for the development of novel immunotherapeutic intervention for cancers and viral infections [222–224].
T regulatory cells (Treg) derived exosomes
Treg cells or suppressive T cells are the subsets of T cells that play crucial roles in the modulation of immune responses through the maintenance of self-antigen tolerance and prevention of autoimmunity by inhibiting the proliferation of effector T cells, such as CD4+ and CD8+ cells [225]. Like other immune cells, Treg cells were found to release exosomes. Furthermore, the number of Treg-derived exosomes is markedly increased when compared to exosomes released by other T cell subpopulations [226]. The secretion of exosomes from Treg cells is highly dependent on hypoxia, calcium level, and IL-2 [227–229]. Recent studies have investigated the proteomic profile of Treg-derived exosomes and showed that these exosomes reflect most components of the parent cells and transfer several molecules that display significant immunomodulatory effects, such as miRNAs, CD73+, CD25+, and CD125+, also known as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [230, 231].
Recently, Treg exosomes were found to be enriched in miRNAs that are distinct from that of the parental Tregs, such as miRNA-155, Let-7b, and Let-7d. These specific miRNAs, when transferred to the conventional effector cells, can suppress the production of IFN‐γ and the expression of the effector genes and, subsequently, inhibit T cell proliferation [230]. In a related study, the analysis of Treg exosomes showed a high expression of surface enzymes CD73+, which perform an essential function in immune modulation via the enhancement of adenosine production. Adenosine is an anti-inflammatory modulator with a high capability to suppress the proliferation and function of T cells and block IFN‐γ and IL-2 production [231].
Potential functions of exosomes in autoimmune diseases
Characterization of exosomes in autoimmune diseases and inflammation is an emerging field that has been studied extensively. Many proteomic studies have been carried out to identify the components and roles of exosomes in inflammatory autoimmune diseases, such as rheumatoid arthritis (RA), osteoarthritis (OA), Sjogren’s syndrome (SS), and systemic lupus erythematosus (SLE) [232]. Although many reports have defined the role of exosomes in the enhancement of inflammation, current data also show that exosomes can provide beneficial effects against autoimmunity and exert an immunosuppressive action, particularly in RA. Based on these observations, effective therapeutic strategies have been developed for treating arthritis and other inflammatory disorders [233, 234]. In the past few years, interest in studying the exosome engagement in RA has markedly increased, with most of the corresponding studies being aimed at the identification of the proteomic components carried by exosomes and examining the immunogenic involvement of exosomes in pathological activities as either activators or suppressors of inflammation. Current reports revealed that, in the exosome samples of RA patients, variable types of miRNA with dual functions (enhancing or suppressing the inflammatory response) can be found [235].
Exosome contribution to RA pathogenesis
In the field of autoimmune diseases, particularly RA, the immunostimulatory and immunosuppressive roles of exosomes are broadly investigated. RA is a systemic autoimmune inflammatory disease in which the immune tolerance is breached, resulting in the production of autoantibodies that attack and damage joints and cartilages [236]. RA is the most widely known chronic autoimmune disease, in which the immune system attacks its own tissues. RA affects 0.5–1% of the global population, with a higher prevalence in women. RA mainly attacks synovial joints causing pain, swelling, and stiffness. In addition to joints, RA can affect other organs, such as the lung, kidney, heart, and blood vessels, which might be associated with the significant morbidity and mortality [237]. Although the etiology of RA is unknown, a bulk of evidence indicated that many factors might increase the risk of RA, including genetic, environmental, biological, and hormonal factors. Recently, it has become clear that citrullination has a direct role in RA pathogenesis. The presence of the autoantibodies against citrullinated peptides is considered as an important hallmark of RA. In addition, genetic factors, such as Shared Epitopes (SE), and bacterial infection, such as periodontitis, have been found to enhance citrullination and citrullinated peptides production [238]. Furthermore, the 2010 American College of Rheumatology/European Alliance of Associations for Rheumatology (ACR/EULAR) classification system for rheumatoid arthritis includes anticitrullinated peptides antibodies (ACPAs) as one of the essential criteria for the RA diagnosis. The diagnosis of RA depends on the combination of medical history, physical examination, laboratory testing, and radiography [237].
Recent studies demonstrated the role of exosomes in regulating the immune response of inflammatory RA through cell-to-cell communication [239, 240]. In addition, exosomes possess a unique function, as they can transfer specific autoantigens and mediators between distant cells [233]. In RA patients, immunocyte-derived exosomes have been isolated from different sources, including blood plasma and serum, synovial fluid, and synovial tissues. Interestingly, the synovial fluid of RA patients was found to be enriched in exosomes that can mediate the inflammatory response of RA either by carrying proteins or inducing the secretion of inflammatory cytokines and chemokines, which play essential roles in the initiation and progression of RA [241]. However, the proteomic analysis of exosomes revealed that they are characterized by the presence of specific proteins, which helps in RA diagnosis. Therefore, exosomes can be used as a useful diagnostic marker for RA [236].
Exosomes and citrullinated proteins: antigen processing and presentation
Antigen presentation is an immunological process through which some immunocytes or APCs, such as macrophages, DCs, and B cells, present the immunogenic peptides or autoantigens linked to MHC molecules on the cell surface to be recognized by T cells [242]. According to many reports, circulating exosomes have shown a capability to present citrullinated peptides to the effector cells in the form of an MHC-peptide complex. Proteomic analysis of APC-derived exosomes demonstrated that they possess MHC complex class I and II and other co-stimulatory molecules, such as CD80, CD86, and ICAM-1, which are involved in the formation of an immune synapse between APCs and T cells [30, 243, 244]. Furthermore, exosomes have been found to stimulate the effector T cell, regardless of whether APCs are present [245].
Dendritic cell-derived exosomes: antigen-presenting vesicles
Interestingly, some reports showed that DC-derived exosomes could directly activate CD8+ lymphocytes in the absence of DCs, which suggested that exosomes possess an adequate amount of class I MHC molecules [200]. As mentioned above, both mature and immature DCs can secrete exosomes with diverse effects. Many studies have shown that exosomes loaded with citrullinated peptides are more effective in stimulating T cells when released from mature DCs than those released from immature DCs. Furthermore, the amount of exosomes produced by mature DCs is 2–3 folds higher than that of immature DCs [246]. Mature DC-derived exosomes are enriched in MHC molecules and co-stimulatory molecules, and therefore become more efficient in stimulating RA inflammation by the induction of antigen-specific immune response through the direct activation of T cells [200]. Exosomes released from the immature DCs are less effective in immune stimulation but are rather involved in promoting immune tolerance. Therefore, they have a potent immunosuppressive role in inflammatory conditions [233]. Similar to the parent DCs, mature DC-derived exosomes can present and transfer citrullinated peptides (antigenic epitopes) by forming the MHC-peptide complex to be recognized by T cells. It is worth noting that some observations found that DC-derived exosomes can transfer the MHC-peptide complexes between DCs, thereby amplifying the number of DCs loading specific MHC-peptide complexes and subsequently increasing the chances for recognition by T cells [247, 248].
B cell-derived exosomes act as APCs
B cells, which are known as dominant APCs, have been shown to produce exosomes implicated in RA pathogenesis through the activation of T cells, especially CD4+ or T helper cells. A proteomic study of B cell-derived exosomes reported that MHC molecules class II and integrin receptors are abundant in these exosomes. B-lymphocytes were able to discard their content of MHC molecules class II via exosomes. Therefore, exosomes can perform an antigen-presenting function similar to B cells. These exosomes can capture antigenic (citrullinated) peptides forming the MHC class II-peptide complexes that induce a specific T cell response resulting in autoimmunity response and development of RA inflammation [30]. Of interest, B cell-derived exosomes, in addition to their ability to form an MHC-peptide complex, can also transfer a whole antigen independently from the MHC molecule association. Therefore, antigen-loaded exosomes produced by B cells may induce the activation of B-lymphocytes, which in turn can produce autoantibodies ACPAs or trigger an immune response via antigen-specific stimulation of T cells [249].
Synovial exosomes: potent antigen-presenting vesicles
Various proteomic studies of synovial fluid have identified the abundance of exosomes that play a crucial role in the pathogenesis of RA. This was attributed to their specific cargo of proteins and inflammatory mediators. In synovial fluid, platelets, macrophages, and fibroblast-like synoviocytes (FLS) secrete exosomes enriched in the modified proteins, such as citrullinated proteins and nuclear DNA-binding protein (DEK) [63]. Similar to other APC-derived exosomes, macrophage-derived exosomes exert an important function in antigen presentation, as they contain MHC class II molecules and co-stimulatory molecules. DEK is an autoantigen with a chemotactic function in many autoimmune diseases, such as SLE and Juvenile rheumatoid arthritis (JRA) [250]. In synovial fluid, macrophage-derived exosomes can deliver and present DEK to CD8+ cells, NK cells, and neutrophils, suggesting that they have a potential role in the attraction of immunocytes into synovial joints and progression of the inflammatory process, mainly in JRA [251]. Therefore, DEK secreted in the synovial fluid either in free form or via exosomes could stimulate the production of autoantibodies and activate the complement system, indicating that macrophage-derived exosomes are contributing to joint inflammation [251].
FLS are the most common cells that contributed to the joint destruction in RA. Proteomic investigations have reported that FLS-derived exosomes may contain citrullinated proteins [252]. FLS-derived exosomes enriched in the citrullinated peptides can induce the production of ACPAs in the synovium and drive inflammation and articular damage by triggering the pro-inflammatory responses [253]. Recent findings suggested that under certain conditions such as hypoxia, FLS with citrullinated proteins were accumulated in RA patients. Accordingly, FLS-derived exosomes showed a high probability of inducing an antigen-specific immune response and contributed to RA pathogenesis [254]. In the same context, platelet-derived exosomes may undergo citrullination and participate in the formation of the immune complex in RA synovial fluid. It was found that these exosomes can bind and express citrullinated proteins, such as vimentin and fibrinogen, on their surface, thereby showing a potential to trigger the production of ACPAs [243].
Exosomes in RA pathogenesis
Several studies assessed the role of exosomes in the pathogenesis of RA. Exosomes derived from serum and synovium were found to transfer and modulate the release of various inflammatory molecules, such as cytokines and chemokines [64, 236]. Recent data provided evidence that most cytokines in synovial fluid were detected not only in the free form but also in the exosome cargo [255], and the majority of immune complexes formed at synovium were associated with the presence of circulating exosomes that were enriched in inflammatory molecules and citrullinated proteins, such as vimentin and fibrinogen [243, 255]. Furthermore, synovium-derived exosomes can promote the destruction of bone and cartilage extracellular matrix (ECM) by inducing the degradative enzymes.
Exosomes: sources and abundance variation in RA synovial fluid
The abundance of exosomes was evaluated in different fluids and variable conditions. The number of exosomes detected in the synovial fluid of RA patients is markedly higher than that found in healthy individuals with no inflammation [256]. Multiple sources of cells were shown to secrete exosomes that participate in RA pathogenesis and synovial joint inflammation, including T cells, FLS, platelets, monocytes/macrophages, and neutrophils. Several studies have described the proteomic profiles of exosomes in RA patients and reported the most abundant cell-derived exosomes in RA serum and synovium [253]. It was observed that exosomes derived from B and T cells constitute the majority of EVs in RA synovium [257]. In the same context, an increased number of neutrophil-derived exosomes was found in the synovial fluid of RA patients [258]. Other groups reported that the platelet-derived exosomes were the most abundant and active microparticles in the RA synovium [259, 260].
Recent studies demonstrated that exosomes released by T cells, monocytes, and FLS, being the most well-characterized microparticles, are implicated in the upregulation of inflammatory cascade and production of destructive enzymes in RA [241, 253]. On the other hand, tumor necrosis factor-α (TNF-α) acts as a key cytokine that drives the inflammatory process in most RA patients, resulting in cartilage and bone damage [261]. In the same manner, the proteomic analysis of plasma exosomes from patients with active RA reported a significant increase in the exosomes derived from platelets, monocytes, B cells, and endothelial cells, which was associated with the increased levels of IL-1, IL-17, and TNF-α [262]. Although one study detected small amounts of platelet-derived exosomes present in the synovial fluid [64], another study demonstrated that these microparticles are the most abundant type of EVs in the synovium of RA and other inflammations and that they can play a significant role in RA inflammation [259]. In fact, such conflicting reports and findings can be attributed to the variation in proteomics methods and procedures utilized for the exosome analysis, such as collection, isolation, and protein preparation.
Exosomes secreted by monocytes and lymphocytes promote RA inflammation at synovium
Several studies suggested that the leukocyte-derived exosomes might have potential roles in RA pathogenesis and inflammation. As aforementioned, FLSs are among the cells implicated in joint inflammation and angiogenesis in RA patients. It is important to note that the level of T cell-, neutrophil-, and monocytes-derived exosomes is higher in synovial fluid when compared to those in plasma [258]. Some proteomic studies suggested that activated monocyte- and T cell-derived exosomes can upregulate the pro-inflammatory signals that stimulate FLS to proceed with the destructive function of joint ECM via releasing large amounts of matrix metalloproteinases (MMPs), mainly MMP-1, MMP-3, MMP-9, and MMP-13 [263, 264]. TNF-α-treated exosomes generated by T cells and monocytes have been found to induce FLS to upregulate the production of cyclooxygenase 2 (COX-2) and microsomal prostaglandin E synthase 1 (mPGES-1) to produce lipid, arachidonic acid-derived hormones, such as prostaglandin E2 (PGE2), which is an inflammatory mediator known to enhance joint pain and inflammation [265, 266]. Other proteomic studies indicated that these activated microparticles could directly induce the FLS to release inflammatory cytokines and factors, such as interleukin-6 (IL-6), granulocyte/macrophage colony-stimulating factor (GM-CSF), and vascular endothelial growth factor (VEGF) [63, 64].
Other proteomic studies showed that synovial exosomes released by activated monocytes and lymphocytes in the synovium could trigger FLS to release chemokines, such as IL-8, monocyte, chemoattractant protein-1 (MCP-1) and regulate upon activation, and express and secrete normal T cells (RANTES or CCL5). In addition, they can upregulate the expression of variable chemokines in synovial fluid, including CXCL1, CXCL2, CXCL3, CXCL5, and CXCL6. The production of these chemokines was elevated in RA synovium, and it might form a chemotactic condition that attracts other immunocytes to the joints, and consequently exacerbates the inflammation [64, 65, 264]. An interesting study assessed the role of monocyte-derived exosomes in the activation of synovial B cells, which participate in RA pathogenesis and autoimmunity. These microparticles could be secreted by THP-1 monocytic cells and were found to induce the synthesis of specific B cell activators, such as B cell activating factor (BAFF), thymic stromal lymphopoietin (TSLP), and secretory leukocyte protease inhibitor (SLPI), also known as antileukoproteinase from FLS in the synovium of RA patients [267].
Platelet-derived exosomes and RA pathogenesis
As stated above, many reports suggest that the content of the platelet-derived exosomes is higher in the synovial fluid than the plasma and other biofluids, and these exosomes constitute the majority of EVs in the synovial fluid of RA patients [65, 256, 260]. In addition, plasma can possess large quantities of platelet-derived exosomes, especially in RA patients with high disease activity [262]. Similar to their parent platelets, these microparticles contain high levels of pro-inflammatory cytokines, i.e., IL-1α and IL-1β [268]. Significant experimental work was performed on mouse arthritis to investigate the roles and mechanisms of platelet-derived exosomes in synovial RA inflammation. The presence of such exosomes was confirmed by the presence of a specific platelet marker in them, namely the CD41 signal. It was found that such exosomes can activate and trigger the FLS to produce large quantities of inflammatory cytokines (IL-6) and chemokines (IL-8). The results provide convincing evidence that platelet-derived exosomes exert an amplifying effect in inflammatory arthritis [259].
However, it is worthy of note that the platelet-derived exosomes can infiltrate from plasma and accumulate in the synovial fluid during RA inflammation [269]. In the plasma of patients with highly active RA, the platelet-derived exosomes were found to induce the inflammatory process via stimulating other autologous immune cells to release inflammatory cytokines, including IL-1, IL-17, and TNF-α [262].
FLS-derived exosomes: suppress T cells apoptosis and enhance bone and cartilage destruction
Apart from platelets, synovial FLS can release exosomes that participate in the inflammation of the synovial joint. FLS-derived exosomes possess multiple effects on the activated T-lymphocytes during the inflammatory process. These exosomes can induce the production of IFN‐γ and IL-2 from activated CD4+ T cells, inhibit caspase-3 and caspase-8 cleavage, and support the survival of these activated T cells through the activation of the Nuclear Factor Kappa-light-chain-enhancer of activated B cells (NF-κB) pathway [270, 271]. FLS-derived exosomes associated with a membrane form of TNF‐α can promote the upregulation of NF-κB and protein kinase B (PKB or AKT), which induce the resistance of activated T cells to cell death and consequently enhance the inflammatory process [270].
The proteomic and genomic studies of exosomes revealed another crucial role of exosomes produced by FLS in inflammatory arthritis. These FLS-derived exosomes have been shown to contain non-coding RNAs or miRNAs and transfer them to the recipient cells and regulate the expression of target genes, especially those involved in the inflammatory process [127]. In this context, recent studies investigated the role of exosomes in inhibiting cartilage and bone formation during RA inflammation. The findings indicated that FLS-derived exosomes upregulated several miRNAs (miRs) expressed in the RA synovial fluid and transferred them to the articular joint to inhibit the differentiation of osteoblasts and chondrocytes, subsequently causing bone erosion and cartilage damage [272].
For example, miR-221-3p is highly expressed in RA synovium. FLS-derived exosomes upregulate and deliver miR-221-3p to the target cells, where it inhibits the osteoblast differentiation and leads to bone destruction [272, 273]. Meanwhile, FLS-derived exosomes upregulate miR-155-5p, which has a significant role in the induction of osteoclastogenesis, causing bone destruction and erosive arthritis [272, 274]. On the contrary, some miRNAs, such as miR-146a, can have a protective role in the synovial joint. Synovial exosomes upregulate the expression of miR-146a in the synovium, which can inhibit osteoclastogenesis and prevent bone destruction [275]. In conclusion, these studies provided evidence supporting an important notion that exosomes are capable of promoting the development of RA inflammation and joint damage through the upregulation of miRNAs.
Proteomic profile of exosomes: unique components and interactions in RA patients
The proteomic analysis of EVs derived from different sample sources of RA patients showed distinct proteins associated with autoimmunity rather than the normal response. Several proteomic methods have been employed for protein identification, quantitation, localization, and protein–protein interactions. Gel electrophoresis, Western blot, chromatography, microarray, and mass spectrometry are the most useful tools for the proteomic analysis of the EVs [276]. Regarding exosomal RA, recent proteomic studies have been achieved by comparing the exosomal content in normal individuals and RA patients. Evidently, levels of the exosomes in inflammatory arthritis were considerably higher than those in non-inflammatory arthritis and healthy individuals [277]. The cargo of exosomes revealed significant differences between normal and patients samples. Exosomes loaded with citrullinated proteins, inflammatory cytokines, and miRNA were more abundant in RA and other arthritis [278]. Moreover, proteomic studies of exosomes allowed the specification of the candidate proteins accompanying different sample sources, i.e., SF, serum, plasma, urine, etc.
Proteomic analysis of plasma-derived exosomes in RA patients
The advancement in proteomics application helped identify exosomes and their contents. High-resolution mass spectrometry followed by bioinformatics analysis of proteomic data is a powerful method for investigating exosomal proteins, protein–protein interactions, and signaling pathways [279]. Recently, a novel proteomic study was performed on exosomes extracted from the plasma of patients with RA. Numerous proteins were detected and found to serve as new biomarkers for RA. Most of these proteins were significantly upregulated or downregulated IgGs involved in RA development (Table 2). Nano-LC–MS/MS and bioinformatics analysis elucidated the role of these proteins in RA pathogenesis. Complex proteins known as differentially expressed proteins (DEPs) were upregulated in RA patients. Exosomal DEPs can either promote the expression of specific inflammatory markers or interact with each other to form a protein network that induces the signaling pathways essential in RA onset [280].
Table 2.
Proteomic profiles of plasma-derived exosomes in rheumatoid arthritis patients revealed 37 differentially expressed exosomal proteins (32 were upregulated, and 5 were downregulated)
| Upregulated proteins | Downregulated proteins | |
|---|---|---|
| Immunoglobulin lambda-like polypeptide 1 | Immunoglobulin heavy constant gamma 2 | Alpha-actinin-1 |
| Immunoglobulin lambda variable 1–47 | Immunoglobulin heavy constant gamma 3 | Transthyretin |
| Immunoglobulin lambda variable 1–36 | Immunoglobulin gamma-1 heavy chain | Angiotensinogen |
| Immunoglobulin lambda variable 1–40 | Inter-alpha-trypsin inhibitor heavy chain H3 | Serum paraoxonase/arylesterase 1 |
| Immunoglobulin lambda variable 6–57 | Complement factor H-related protein 5 | Keratin, type I cytoskeletal 9 |
| Immunoglobulin lambda variable 3–1 | Complement C1q subcomponent subunit A | |
| Immunoglobulin lambda variable 3–19 | Monocyte differentiation antigen CD14 | |
| Immunoglobulin heavy variable 4–28 | Cartilage oligomeric matrix protein | |
| Immunoglobulin heavy variable 3–49 | Lipopolysaccharide-binding protein | |
| Immunoglobulin heavy variable 3–53 | CDKN2A-interacting protein OS | |
| Immunoglobulin heavy variable 1–8 | Prospero homeobox protein 1 | |
| Immunoglobulin heavy variable 1–58 | Serum amyloid P-component | |
| Immunoglobulin heavy variable 1–18 | Coagulation factor XI | |
| Immunoglobulin heavy variable 1–69 D | Complement factor I | |
| Immunoglobulin heavy variable 2–26 | Midasin OS | |
| Immunoglobulin heavy variable 3–53 | Tenascin | |
This analysis was conducted using 12 specimens of plasma from 6 RA patients and 6 age- and gender-matched controls from the Chinese population [280]
Cartilage oligomeric matrix protein (COMP) is a unique signature of plasma and serum exosomes. Of interest, the COMP level is highly increased in RA patients, particularly in the early stage, and may be considered as a higher predictive marker than ACPAs [280]. Moreover, many proteomic studies of RA exosomes have demonstrated the high expression of a cellular protein (annexin V), complement, and pro-inflammatory cytokine [278]. In peripheral blood, many circulating exosomes originated from the peripheral mononuclear cells. Proteomic analysis of plasma-derived exosomes from RA patients with high disease activity scores (DAS) unraveled the presence of high levels of inflammatory cytokines including, IL-1, IL-17, and TNF-α. Moreover, the abundance of complements like C1q indicated the role of circulating exosomes in inflammation spreading into synovial tissues and other biofluids [281].
In addition to the information generated by the exosome proteomics, it is also important to detect small RNA molecules like small non-coding RNAs or miRNAs that play a role in RA severity. miRNAs are the most abundant regulatory molecules enriched in RA exosomes. Overexpression of miR-92a, miR-17, and miR-1915–3p has been detected in plasma exosomes extracted from RA patients [282]. miR-92a was implicated in the bone damage of RA patients via maintaining the FLS [283], while miR-17 was found to impair the Treg cells and immune hemostasis by inhibiting TGFBR II expression [284]. Another miRNA has been identified to be associated with RA severity; high levels of miR-1915-3p in plasma exosomes were correlated with increased DAS [285]. Therefore, the enrichment of plasma exosomes with miRNAs is a significant indicator of RA development and activity [282]. In addition, a proteomic profile of plasmatic exosomes revealed the accumulation of specific membrane glycoproteins or clusters of differentiation (CD), including CD14+, CD26+, and CD146+. High expression of CD14+ and CD26+ were correlated with DAS, while increased CD146+ was associated with disease duration [278].
Proteomic profile of synovial exosomes in RA patients
Synovial fluid is the preferred sample for investigating serological and inflammatory factors in RA patients. According to several studies on exosomal RA, the number of exosomes was higher in SF than in plasma and serum. The enrichment of EVs in SF may indicate the high impact of pro-inflammation, and the analysis of synovial EVs can be used to assess the RA severity (Fig. 4). Many studies have demonstrated the ability of exosomes to serve as APCs to T cells [286]. Synovial exosomes have been found to contain citrullinated peptides that act as antigenic determinants and participate in RA autoimmunity. Fibrin α-chain, fibrin β-chain, fibrinogen D fragment, fibrinogen β-chain, and spα receptor are the most common citrullinated proteins that have been detected in synovial exosomes [287].
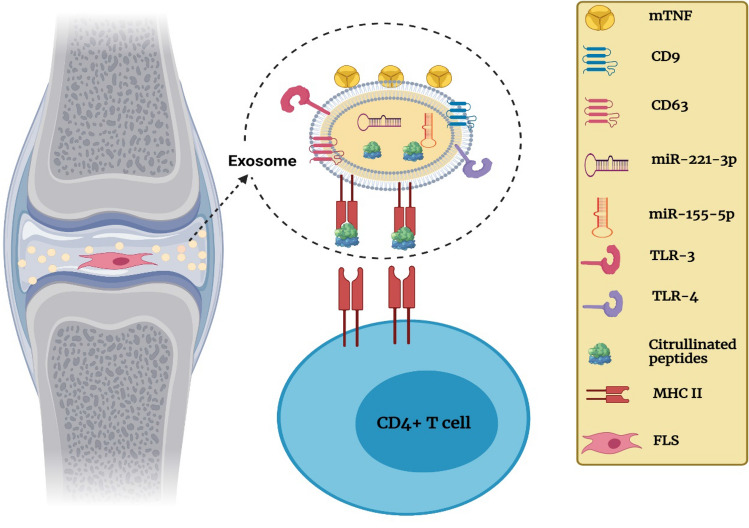
Fig. 4.
Synovial exosome cargo contributes to inflammation at RA synovial joint. Exosomes derived from the fibroblast-like cell (FLS) components: tetraspanins (CD9, CD63), Toll-like receptors (TLR-3, TLR-4), membrane form of tumor necrosis factor (mTNF), micro-RNA (miR-155-5p, miR-221-3p), citrullinated peptides, and class II major histocompatibility complex (MHC-II)
Moreover, it has been found that SF exosomes contribute to bone erosion in the inflamed joint of RA and act as an osteocalcinogenic factor. A recent proteomic study has identified the overexpression receptor activator of nuclear factor-kappa B ligand (RANKL) in synovial exosomes [277]. RANKL is a cellular receptor that induces osteoclastogenesis to form osteoclasts, key players in bone resorption [288]. The proteomics of synovial tissues demonstrated that fibroblast-derived exosomes contain a membrane form of TNF-α (mTNF) on their surface. mTNF positive synovial exosomes act as an anti-apoptotic factor that supports the resistance of activated CD4+ to apoptosis via activating AKT and NF-κB pathways [289].
Neutrophils are the most abundant cells in SF and the major source EVs of in the synovium of RA patients that participate in cartilage damage. Neutrophil-derived exosomes in RA joints with high inflammation have been found to contain a significant number of granular proteins such as CD16b, CD11b, CD18, and MMP-25 [286, 290]. These clusters play an essential role in the activation and migration of neutrophils into the inflammation site, while MMP-25 can induce the NF-κB pathways [291, 292]. Synovial fibroblasts produce exosomes loaded with pro-inflammatory cytokines involved in T cell activation, such as IL-2 and IFNγ, and cartilage degeneration, such as MMP-1 [278]. Furthermore, synovial exosomes derived from fibroblasts and macrophages displayed increased Toll-like receptors, mainly TLR-3 and TLR-4. These TLRs contribute to RA pathogenesis and the destructive phase of the disease. The ligation of these TLRs has been found to induce the secretion of IL-17, IFNγ, VEGF, and MMPs, as well as the activation of Th1 and Th17 cells [293].
Proteomic studies of synovial fibroblast-derived exosomes revealed their role in RA pathogenesis. These exosomes showed upregulation of miR-155-5p and miR-146a-5p that are known to enhance inflammation persistence. miR-155-5p downregulates the inflammatory inhibitor protein SHIP1, while miR-146a-5p suppresses the apoptosis of Jurkat CD4+ cells [289]. Besides, SFLS-derived exosomes revealed overexpression of miR-221-3p, which is known to prevent osteoblast maturation leading to bone erosion in RA joints [282]. Recently, it has been found that exosomes derived from FLS may exert positive effects on RA inflammation and alleviate disease severity. FLS-derived exosomes with a high level of miR-486-5p have been suggested to promote osteoblast maturation by inhibiting the expression of the antiproliferative protein Tob1 [294].
Proteomic profile of serum exosomes: significant predictor for disease outcome and therapy response
Serum exosomes can transfer several proteins involved in RA pathogenesis and might be predictors for therapeutic response. Similar to synovial and plasmatic exosomes, serum exosomes exhibit antigen-presenting function as they expose MHC-1 and MHC-2. However, proteomics of RA serum exosomes showed that DCs-derived exosomes could present the citrullinated peptides to effector CD8+ cells via MCH-1 manner to release TNF-α and IFN‐γ [236]. The most common finding of proteomic analysis of RA is the presence of a large amount of TLR-3 in serum exosomes, either from active or inactive RA. Exosomes loaded with TLR-3 have a critical role in RA pathogenesis by activating the NF-κB pathways and interferon regulatory factor 3 (IRF-3), which results in the secretion of IL-6, IL-8, and interferon-1 [236, 289]. A recent study of exosomes derived from serum of RA patients has identified two exosomal proteins as candidate markers for RA severity. Exosomal serum amyloid A (SAA) and lymphatic vessel endothelial hyaluronic acid receptor-1 (LYVE-1) are circulating proteins that can be used as additional markers for disease activity and RA remission [295]. Further, a membrane-bound pro-neuregulin-3, which acts as a ligand for epidermal growth factor receptor (EGFR), revealed a high expression in serum exosomes. EGFR signaling has been suggested to induce the proliferation of FLS and the secretion of cytokines in RA patients [236, 296].
In the context of RNA, several studies have detected a variable expression of many non-coding RNAs associated with RA activity and restoration. HOTAIR is a long non-coding RNA (LncRNA). HOTAIR was highly expressed in serum exosomes and has been suggested to be a diagnostic marker for RA. HOTAIR enhances the migration of macrophages into the inflamed joint and activates MMP-2 and MMP-13 in osteoclasts to promote joint damage [297, 298]. Furthermore, several forms of small non-coding RNAs (miRNA) have been identified to be implicated in RA pathogenicity and bone loss. The upregulated miR-214 was more frequent in serum exosomes. miR-214 prevents osteoblast maturation, causing increased osteoclast formation and bone destruction [299]. miR-1915-3p is one of the most deregulated non-coding RNA in various conditions. A high level of serum exosomal miR-1915-3p has been correlated with RA activity and can be used as a useful marker of disease progression in RA patients [300]. Otherwise, some kinds of miRNAs have been found upregulated in RA patients on treatment. Serum exosomes of RA patients on anti-rheumatic drugs (anti-TNF-α/DMARD) showed overexpression of miR-223-3p and mir-23-3p [301]. Moreover, high expression of miR-125b has been reported in serum exosomes of RA patients receiving rituximab [302]. Accordingly, exosomes including these miRNAs can be used as predictive markers of therapeutic response and treatment influence in RA patients [301, 303].
Proteomics of urinary EVs provide promising biomarkers for RA and other diseases
Several lines of evidence have demonstrated that the EVs found in urine are derived from urinary tract epithelial cells and other cells outside the urinary system [304]. Recently, the analysis of urinary exosomes has attracted attention as a non-invasive source that could be an alternative to tissue biopsy with a cost-effective opportunity. In recent years, the proteomic studies of urinary exosomes provided biomarkers for diagnosing and treatment of multiple diseases such as cancers, renal diseases, autoimmune disorders, and neurodegenerative diseases [305]. Regarding RA, urine samples displayed the prevalence of EVs derived from endothelial cells (CD62E), platelets (CD41+), and leukocytes including, B-lymphocytes (CD19+), T-lymphocytes (CD3+), and Monocyte/macrophages (CD14+) [278]. A recent study has demonstrated that the number of EVs in urinary samples showed a significant correlation with RA duration, activity, and immunosuppressive therapy. In RA patients with high DAS28 (disease activity score on 28 joints), urine samples were enriched with CD14+ and CD41+ EVs. Moreover, RA patients with less than 3 years duration have increased levels of urinary CD62+ EVs, while CD19+ EVs were overexpressed in established RA for more than 3 years. In the case of treated patients with immunosuppressive drugs, the levels of CD41+, CD3+, and CD14+ EVs were significantly reduced, with consistent levels of CD19+ and CD62E+ in urine samples [262].
Conclusions
Proteomics studies the complete set of proteins expressed by an organism and plays a significant role in the diagnosis and treatment of human diseases. Advances in proteomics applications and technology enhance the profiling of a wide range of proteins extracted from patient samples. Although without translational utilization, proteomics is just a technology, recently it has been applied as a guide in comprehensive analyses concerning some human diseases, such as cancers, autoimmune diseases, and infectious diseases. In the last decade, EVs, including exosomes, microvesicles, and apoptotic bodies, have drawn the attention of researchers due to their multiple roles in health and disease conditions. The proteomic studies of EVs provided strong evidence that these nanovesicles are not simply cellular debris with no biological significance, but are rather a cargo loader of biofunctional molecules, which are essential in intracellular communication and information exchange between local and distant cells.
EVs are produced by all types of body cells and could be extracted from several body fluids. Proteomic studies of various EVs types have characterized and specified common proteins that can be found in particular EVs and, therefore, could be used as markers for EV subsets. Interestingly, these microparticles participate in many human pathophysiological processes. In the context of autoimmune diseases, exosomes are the most well-known microparticles that have multiple important roles in various inflammatory diseases, particularly rheumatoid arthritis (RA). Exosomal proteomics is an emerging novel field that helps in the identification of the transferred signaling molecules and in the analysis of the changes in the exosome profiles, which could provide useful tools to prevent inflammation. Several proteomic studies were performed to identify the exosomal components and their potential functions in the development of inflammatory arthritis. In RA, most of the exosomes are produced by the leukocytes and synoviocytes, and they are loaded with inflammatory molecules and enzymes that might be implicated in RA pathogenesis and the inflammatory process.
Acknowledgements
This report is apart from the Ph.D. thesis of M. Alghamdi.
Author contributions
Conceived the idea, edited and revised the manuscript, and managed the project: EMR; wrote the primary manuscript and edited it: MA; SB and SA participated in the collection of literature data, data curation, formal analysis, and evaluation. VNU participated in manuscript writing, editing, and revision.
Funding
Not applicable.
Availability of data and material
All data are used within our report.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang J, Xiong J, Yang L, et al. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale. 2021;13(19):8740–8750. doi: 10.1039/d1nr01314a. [DOI] [PubMed] [Google Scholar]
- 2.Rossello RA, Kohn DH. Gap junction intercellular communication: a review of a potential platform to modulate craniofacial tissue engineering. J Biomed Mater Res B Appl Biomater. 2009;88(2):509–518. doi: 10.1002/jbm.b.31127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finetti F, Cassioli C, Baldari CT. Transcellular communication at the immunological synapse: a vesicular traffic-mediated mutual exchange. F1000Res. 2017;6:1880. doi: 10.12688/f1000research.11944.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margolis L, Sadovsky Y. The biology of extracellular vesicles: the known unknowns. PLoS Biol. 2019;17(7):e3000363. doi: 10.1371/journal.pbio.3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone RM, Adam M, Hammond JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262(19):9412–9420. [PubMed] [Google Scholar]
- 7.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 8.Stegmayr B, Ronquist G. Promotive effect on human sperm progressive motility by prostasomes. Urol Res. 1982;10(5):253–257. doi: 10.1007/BF00255932. [DOI] [PubMed] [Google Scholar]
- 9.Dvorak HF, Quay SC, Orenstein NS, et al. Tumor shedding and coagulation. Science. 1981;212(4497):923–924. doi: 10.1126/science.7195067. [DOI] [PubMed] [Google Scholar]
- 10.Jan AT, Rahman S, Badierah R, et al. Expedition into exosome biology: a perspective of progress from discovery to therapeutic development. Cancers. 2021;13(5):1157. doi: 10.3390/cancers13051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koniusz S, Andrzejewska A, Muraca M, et al. Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front Cell Neurosci. 2016;10:109. doi: 10.3389/fncel.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badierah RA, Uversky VN, Redwan EM. Dancing with Trojan horses: an interplay between the extracellular vesicles and viruses. J Biomol Struct Dynam. 2020;39(8):3034–3060. doi: 10.1080/07391102.2020.1756409. [DOI] [PubMed] [Google Scholar]
- 14.Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379(10):958–966. doi: 10.1056/NEJMra1704286. [DOI] [PubMed] [Google Scholar]
- 15.Andaloussi SEL, Mager I, Breakefield XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 16.Jia S, Zocco D, Samuels ML, et al. Emerging technologies in extracellular vesicle-based molecular diagnostics. Expert Rev Mol Diagn. 2014;14(3):307–321. doi: 10.1586/14737159.2014.893828. [DOI] [PubMed] [Google Scholar]
- 17.Andaloussi SE, Mäger I, Breakefield XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 18.Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akers JC, Gonda D, Kim R, et al. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser P, Wang S, Didenko VV. Apoptotic bodies: selective detection in extracellular vesicles. Methods Mol Biol. 2017;1554:193–200. doi: 10.1007/978-1-4939-6759-9_12. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz YS, Dolganova OM, Rudina MI, et al. Influence of apoptotic bodies and apoptotic microvesicles on no production in macrophages. Bull Exp Biol Med. 2018;165(4):453–456. doi: 10.1007/s10517-018-4192-9. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig A-K, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44(1):11–15. doi: 10.1016/j.biocel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35(2):256–263. [PubMed] [Google Scholar]
- 24.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meehan B, Rak J, Di Vizio D. Oncosomes—large and small: what are they, where they came from? J Extracell Vesicles. 2016;5:33109. doi: 10.3402/jev.v5.33109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morello M, Minciacchi VR, de Candia P, et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle. 2013;12(22):3526–3536. doi: 10.4161/cc.26539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melentijevic I, Toth ML, Arnold ML, et al. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature. 2017;542(7641):367–371. doi: 10.1038/nature21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Freitas D, Kim HS, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell P, Petfalski E, Shevchenko A, et al. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′ → 5′ exoribonucleases. Cell. 1997;91(4):457–466. doi: 10.1016/s0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 30.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidal MJ, Stahl PD. The small GTP-binding proteins Rab4 and ARF are associated with released exosomes during reticulocyte maturation. Eur J Cell Biol. 1993;60(2):261–267. [PubMed] [Google Scholar]
- 32.Johnstone RM. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol. 1992;70(3–4):179–190. doi: 10.1139/o92-028. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74(5):1844–1851. [PubMed] [Google Scholar]
- 34.Vidal M, Sainte-Marie J, Philippot JR, et al. Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: evidence precluding a role for “aminophospholipid translocase”. J Cell Physiol. 1989;140(3):455–462. doi: 10.1002/jcp.1041400308. [DOI] [PubMed] [Google Scholar]
- 35.Pan BT, Teng K, Wu C, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witwer KW, Thery C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles. 2019;8(1):1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Momen-Heravi F. Isolation of extracellular vesicles by ultracentrifugation. Methods Mol Biol. 2017;1660:25–32. doi: 10.1007/978-1-4939-7253-1_3. [DOI] [PubMed] [Google Scholar]
- 40.Onodi Z, Pelyhe C, Terezia Nagy C, et al. Isolation of high-purity extracellular vesicles by the combination of iodixanol density gradient ultracentrifugation and bind-elute chromatography from blood plasma. Front Physiol. 2018;9:1479. doi: 10.3389/fphys.2018.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Royo F, Thery C, Falcon-Perez JM, et al. Methods for separation and characterization of extracellular vesicles: results of a worldwide survey performed by the isev rigor and standardization subcommittee. Cells. 2020;9(9):1955. doi: 10.3390/cells9091955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li P, Kaslan M, Lee SH, et al. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu F, Vermesh O, Mani V, et al. The exosome total isolation chip. ACS Nano. 2017;11(11):10712–10723. doi: 10.1021/acsnano.7b04878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gamez-Valero A, Monguio-Tortajada M, Carreras-Planella L, et al. Size-Exclusion Chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci Rep. 2016;6:33641. doi: 10.1038/srep33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musante L, Tataruch D, Gu D, et al. A simplified method to recover urinary vesicles for clinical applications, and sample banking. Sci Rep. 2014;4:7532. doi: 10.1038/srep07532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heinemann ML, Ilmer M, Silva LP, et al. Benchtop isolation and characterization of functional exosomes by sequential filtration. J Chromatogr A. 2014;1371:125–135. doi: 10.1016/j.chroma.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Jeong S, Park J, Pathania D, et al. Integrated magneto-electrochemical sensor for exosome analysis. ACS Nano. 2016;10(2):1802–1809. doi: 10.1021/acsnano.5b07584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nameta M, Saijo Y, Ohmoto Y, et al. Disruption of membranes of extracellular vesicles is necessary for ELISA determination of urine AQP2: proof of disruption and epitopes of AQP2 antibodies. Int J Mol Sci. 2016;17(10):1634. doi: 10.3390/ijms17101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rider MA, Hurwitz SN, Meckes DG., Jr ExtraPEG: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep. 2016;6:23978. doi: 10.1038/srep23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samsonov R, Shtam T, Burdakov V, et al. Lectin-induced agglutination method of urinary exosomes isolation followed by mi-RNA analysis: application for prostate cancer diagnostic. Prostate. 2016;76(1):68–79. doi: 10.1002/pros.23101. [DOI] [PubMed] [Google Scholar]
- 51.Linares R, Tan S, Gounou C, et al. Imaging and quantification of extracellular vesicles by transmission electron microscopy. Methods Mol Biol. 2017;1545:43–54. doi: 10.1007/978-1-4939-6728-5_4. [DOI] [PubMed] [Google Scholar]
- 52.Dragovic RA, Gardiner C, Brooks AS, et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine. 2011;7(6):780–788. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maas SL, Broekman ML, de Vrij J. Tunable resistive pulse sensing for the characterization of extracellular vesicles. Methods Mol Biol. 2017;1545:21–33. doi: 10.1007/978-1-4939-6728-5_2. [DOI] [PubMed] [Google Scholar]
- 54.Palmieri V, Lucchetti D, Gatto I, et al. Dynamic light scattering for the characterization and counting of extracellular vesicles: a powerful noninvasive tool. J Nanopart Res. 2014;16(9):2583. [Google Scholar]
- 55.Nolte-‘t Hoen EN, van der Vlist EJ, Aalberts M, et al. Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles. Nanomedicine. 2012;8(5):712–720. doi: 10.1016/j.nano.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt C, Gronborg M, Deckert J, et al. Mass spectrometry-based relative quantification of proteins in precatalytic and catalytically active spliceosomes by metabolic labeling (SILAC), chemical labeling (iTRAQ), and label-free spectral count. RNA. 2014;20(3):406–420. doi: 10.1261/rna.041244.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raimondo F, Morosi L, Chinello C, et al. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics. 2011;11(4):709–720. doi: 10.1002/pmic.201000422. [DOI] [PubMed] [Google Scholar]
- 59.Choi DS, Kim DK, Kim YK, et al. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13(10–11):1554–1571. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- 60.Yoon YJ, Kim OY, Gho YS. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014;47(10):531–539. doi: 10.5483/BMBRep.2014.47.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaborowski MP, Balaj L, Breakefield XO, et al. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65(8):783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu H, Hu D, Zhang L, et al. Role of extracellular vesicles in rheumatoid arthritis. Mol Immunol. 2018;93:125–132. doi: 10.1016/j.molimm.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 64.Berckmans RJ, Nieuwland R, Kraan MC, et al. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res Ther. 2005;7(3):R536–R544. doi: 10.1186/ar1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reich N, Beyer C, Gelse K, et al. Microparticles stimulate angiogenesis by inducing ELR(+) CXC-chemokines in synovial fibroblasts. J Cell Mol Med. 2011;15(4):756–762. doi: 10.1111/j.1582-4934.2010.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szklarczyk D, Franceschini A, Kuhn M, et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39(Database issue):D561–D568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dunker AK, Lawson JD, Brown CJ, et al. Intrinsically disordered protein. J Mol Graph Model. 2001;19(1):26–59. doi: 10.1016/s1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 68.Oldfield CJ, Dunker AK. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu Rev Biochem. 2014;83:553–584. doi: 10.1146/annurev-biochem-072711-164947. [DOI] [PubMed] [Google Scholar]
- 69.Uversky VN. Unusual biophysics of intrinsically disordered proteins. Biochim Biophys Acta. 2013;1834(5):932–951. doi: 10.1016/j.bbapap.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Uversky VN, Dunker AK. Understanding protein non-folding. Biochim Biophys Acta. 2010;1804(6):1231–1264. doi: 10.1016/j.bbapap.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uversky VN. Multitude of binding modes attainable by intrinsically disordered proteins: a portrait gallery of disorder-based complexes. Chem Soc Rev. 2011;40(3):1623–1634. doi: 10.1039/c0cs00057d. [DOI] [PubMed] [Google Scholar]
- 72.Uversky VN. Intrinsic disorder-based protein interactions and their modulators. Curr Pharm Des. 2013;19(23):4191–4213. doi: 10.2174/1381612811319230005. [DOI] [PubMed] [Google Scholar]
- 73.Dunker AK, Obradovic Z, Romero P, et al. Intrinsic protein disorder in complete genomes. Genome Inform Ser Workshop Genome Inform. 2000;11:161–171. [PubMed] [Google Scholar]
- 74.Dunker AK, Silman I, Uversky VN, et al. Function and structure of inherently disordered proteins. Curr Opin Struct Biol. 2008;18(6):756–764. doi: 10.1016/j.sbi.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Ward JJ, Sodhi JS, McGuffin LJ, et al. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol. 2004;337(3):635–645. doi: 10.1016/j.jmb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Uversky VN. The mysterious unfoldome: structureless, underappreciated, yet vital part of any given proteome. J Biomed Biotechnol. 2010;2010:568068. doi: 10.1155/2010/568068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xue B, Dunker AK, Uversky VN. Orderly order in protein intrinsic disorder distribution: disorder in 3500 proteomes from viruses and the three domains of life. J Biomol Struct Dyn. 2012;30(2):137–149. doi: 10.1080/07391102.2012.675145. [DOI] [PubMed] [Google Scholar]
- 78.Peng Z, Yan J, Fan X, et al. Exceptionally abundant exceptions: comprehensive characterization of intrinsic disorder in all domains of life. Cell Mol Life Sci. 2015;72(1):137–151. doi: 10.1007/s00018-014-1661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tokuriki N, Oldfield CJ, Uversky VN, et al. Do viral proteins possess unique biophysical features? Trends Biochem Sci. 2009;34(2):53–59. doi: 10.1016/j.tibs.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 80.Xue B, Williams RW, Oldfield CJ, et al. Archaic chaos: intrinsically disordered proteins in Archaea. BMC Syst Biol. 2010;4(Suppl 1):S1. doi: 10.1186/1752-0509-4-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tompa P, Dosztanyi Z, Simon I. Prevalent structural disorder in E. coli and S. cerevisiae proteomes. J Proteome Res. 2006;5(8):1996–2000. doi: 10.1021/pr0600881. [DOI] [PubMed] [Google Scholar]
- 82.Krasowski MD, Reschly EJ, Ekins S. Intrinsic disorder in nuclear hormone receptors. J Proteome Res. 2008;7(10):4359–4372. doi: 10.1021/pr8003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimizu K, Toh H. Interaction between intrinsically disordered proteins frequently occurs in a human protein-protein interaction network. J Mol Biol. 2009;392(5):1253–1265. doi: 10.1016/j.jmb.2009.07.088. [DOI] [PubMed] [Google Scholar]
- 84.Pentony MM, Jones DT. Modularity of intrinsic disorder in the human proteome. Proteins. 2010;78(1):212–221. doi: 10.1002/prot.22504. [DOI] [PubMed] [Google Scholar]
- 85.Tompa P, Kalmar L. Power law distribution defines structural disorder as a structural element directly linked with function. J Mol Biol. 2010;403(3):346–350. doi: 10.1016/j.jmb.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 86.Schad E, Tompa P, Hegyi H. The relationship between proteome size, structural disorder and organism complexity. Genome Biol. 2011;12(12):R120. doi: 10.1186/gb-2011-12-12-r120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dyson HJ. Expanding the proteome: disordered and alternatively folded proteins. Q Rev Biophys. 2011;44(4):467–518. doi: 10.1017/S0033583511000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pancsa R, Tompa P. Structural disorder in eukaryotes. PLoS ONE. 2012;7(4):e34687. doi: 10.1371/journal.pone.0034687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Midic U, Obradovic Z. Intrinsic disorder in putative protein sequences. Proteome Sci. 2012;10(Suppl 1):S19. doi: 10.1186/1477-5956-10-S1-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hegyi H, Tompa P. Increased structural disorder of proteins encoded on human sex chromosomes. Mol Biosyst. 2012;8(1):229–236. doi: 10.1039/c1mb05285c. [DOI] [PubMed] [Google Scholar]
- 91.Korneta I, Bujnicki JM. Intrinsic disorder in the human spliceosomal proteome. PLoS Comput Biol. 2012;8(8):e1002641. doi: 10.1371/journal.pcbi.1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kahali B, Ghosh TC. Disorderness in Escherichia coli proteome: perception of folding fidelity and protein-protein interactions. J Biomol Struct Dyn. 2013;31(5):472–476. doi: 10.1080/07391102.2012.706071. [DOI] [PubMed] [Google Scholar]
- 93.Di Domenico T, Walsh I, Tosatto SC. Analysis and consensus of currently available intrinsic protein disorder annotation sources in the MobiDB database. BMC Bioinform. 2013;14(Suppl 7):S3. doi: 10.1186/1471-2105-14-S7-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rajagopalan K, Mooney SM, Parekh N, et al. A majority of the cancer/testis antigens are intrinsically disordered proteins. J Cell Biochem. 2011;112(11):3256–3267. doi: 10.1002/jcb.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uversky VN. The multifaceted roles of intrinsic disorder in protein complexes. FEBS Lett. 2015;589:2498–2506. doi: 10.1016/j.febslet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37(20):2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 97.Schulz GE. Nucleotide binding proteins. In: Balaban M, editor. Molecular mechanism of biological recognition. New York: Elsevier/North-Holland Biomedical Press; 1979. pp. 79–94. [Google Scholar]
- 98.Dunker AK, Brown CJ, Lawson JD, et al. Intrinsic disorder and protein function. Biochemistry. 2002;41(21):6573–6582. doi: 10.1021/bi012159+. [DOI] [PubMed] [Google Scholar]
- 99.Dunker AK, Brown CJ, Obradovic Z. Identification and functions of usefully disordered proteins. Adv Protein Chem. 2002;62:25–49. doi: 10.1016/s0065-3233(02)62004-2. [DOI] [PubMed] [Google Scholar]
- 100.Wright PE, Dyson HJ. Linking folding and binding. Curr Opin Struct Biol. 2009;19(1):31–38. doi: 10.1016/j.sbi.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meador WE, Means AR, Quiocho FA. Modulation of calmodulin plasticity in molecular recognition on the basis of X-ray structures. Science. 1993;262(5140):1718–1721. doi: 10.1126/science.8259515. [DOI] [PubMed] [Google Scholar]
- 102.Kriwacki RW, Hengst L, Tennant L, et al. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proc Natl Acad Sci USA. 1996;93(21):11504–11509. doi: 10.1073/pnas.93.21.11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dunker AK, Garner E, Guilliot S, et al. Protein disorder and the evolution of molecular recognition: theory, predictions and observations. Pac Symp Biocomput. 1998;3:473–484. [PubMed] [Google Scholar]
- 104.Uversky VN. Protein folding revisited. A polypeptide chain at the folding-misfolding-nonfolding cross-roads: which way to go? Cell Mol Life Sci. 2003;60(9):1852–1871. doi: 10.1007/s00018-003-3096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dunker AK, Cortese MS, Romero P, et al. Flexible nets: the roles of intrinsic disorder in protein interaction networks. FEBS J. 2005;272(20):5129–5148. doi: 10.1111/j.1742-4658.2005.04948.x. [DOI] [PubMed] [Google Scholar]
- 106.Dajani R, Fraser E, Roe SM, et al. Structural basis for recruitment of glycogen synthase kinase 3beta to the axin-APC scaffold complex. Embo J. 2003;22(3):494–501. doi: 10.1093/emboj/cdg068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dyson HJ, Wright PE. Coupling of folding and binding for unstructured proteins. Curr Opin Struct Biol. 2002;12(1):54–60. doi: 10.1016/s0959-440x(02)00289-0. [DOI] [PubMed] [Google Scholar]
- 108.Hsu WL, Oldfield CJ, Xue B, et al. Exploring the binding diversity of intrinsically disordered proteins involved in one-to-many binding. Protein Sci. 2013;22(3):258–273. doi: 10.1002/pro.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oldfield CJ, Meng J, Yang JY, et al. Flexible nets: disorder and induced fit in the associations of p53 and 14-3-3 with their partners. BMC Genom. 2008;9(Suppl 1):S1. doi: 10.1186/1471-2164-9-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tompa P, Fuxreiter M. Fuzzy complexes: polymorphism and structural disorder in protein-protein interactions. Trends Biochem Sci. 2008;33(1):2–8. doi: 10.1016/j.tibs.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 111.Hazy E, Tompa P. Limitations of induced folding in molecular recognition by intrinsically disordered proteins. ChemPhysChem. 2009;10(9–10):1415–1419. doi: 10.1002/cphc.200900205. [DOI] [PubMed] [Google Scholar]
- 112.Sigalov A, Aivazian D, Stern L. Homooligomerization of the cytoplasmic domain of the T cell receptor zeta chain and of other proteins containing the immunoreceptor tyrosine-based activation motif. Biochemistry. 2004;43(7):2049–2061. doi: 10.1021/bi035900h. [DOI] [PubMed] [Google Scholar]
- 113.Sigalov AB, Zhuravleva AV, Orekhov VY. Binding of intrinsically disordered proteins is not necessarily accompanied by a structural transition to a folded form. Biochimie. 2007;89(3):419–421. doi: 10.1016/j.biochi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Permyakov SE, Millett IS, Doniach S, et al. Natively unfolded C-terminal domain of caldesmon remains substantially unstructured after the effective binding to calmodulin. Proteins. 2003;53(4):855–862. doi: 10.1002/prot.10481. [DOI] [PubMed] [Google Scholar]
- 115.Fuxreiter M. Fuzziness: linking regulation to protein dynamics. Mol Biosyst. 2012;8(1):168–177. doi: 10.1039/c1mb05234a. [DOI] [PubMed] [Google Scholar]
- 116.Fuxreiter M, Tompa P. Fuzzy complexes: a more stochastic view of protein function. Adv Exp Med Biol. 2012;725:1–14. doi: 10.1007/978-1-4614-0659-4_1. [DOI] [PubMed] [Google Scholar]
- 117.Sharma R, Raduly Z, Miskei M, et al. Fuzzy complexes: specific binding without complete folding. FEBS Lett. 2015;589:2533–2542. doi: 10.1016/j.febslet.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 118.Patil A, Nakamura H. Disordered domains and high surface charge confer hubs with the ability to interact with multiple proteins in interaction networks. FEBS Lett. 2006;580(8):2041–2045. doi: 10.1016/j.febslet.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 119.Ekman D, Light S, Bjorklund AK, et al. What properties characterize the hub proteins of the protein-protein interaction network of Saccharomyces cerevisiae? Genome Biol. 2006;7(6):R45. doi: 10.1186/gb-2006-7-6-r45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haynes C, Oldfield CJ, Ji F, et al. Intrinsic disorder is a common feature of hub proteins from four eukaryotic interactomes. PLoS Comput Biol. 2006;2(8):e100. doi: 10.1371/journal.pcbi.0020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dosztanyi Z, Chen J, Dunker AK, et al. Disorder and sequence repeats in hub proteins and their implications for network evolution. J Proteome Res. 2006;5(11):2985–2995. doi: 10.1021/pr060171o. [DOI] [PubMed] [Google Scholar]
- 122.Singh GP, Dash D. Intrinsic disorder in yeast transcriptional regulatory network. Proteins. 2007;68(3):602–605. doi: 10.1002/prot.21497. [DOI] [PubMed] [Google Scholar]
- 123.Singh GP, Ganapathi M, Dash D. Role of intrinsic disorder in transient interactions of hub proteins. Proteins. 2007;66(4):761–765. doi: 10.1002/prot.21281. [DOI] [PubMed] [Google Scholar]
- 124.Palmisano G, Jensen SS, Le Bihan MC, et al. Characterization of membrane-shed microvesicles from cytokine-stimulated beta-cells using proteomics strategies. Mol Cell Proteom. 2012;11(8):230–243. doi: 10.1074/mcp.M111.012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Palmisano G, Parker BL, Engholm-Keller K, et al. A novel method for the simultaneous enrichment, identification, and quantification of phosphopeptides and sialylated glycopeptides applied to a temporal profile of mouse brain development. Mol Cell Proteom. 2012;11(11):1191–1202. doi: 10.1074/mcp.M112.017509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peruzzotti-Jametti L, Bernstock JD, Willis CM, et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 2021;19(4):e3001166. doi: 10.1371/journal.pbio.3001166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 128.Eldh M, Lotvall J, Malmhall C, et al. Importance of RNA isolation methods for analysis of exosomal RNA: evaluation of different methods. Mol Immunol. 2012;50(4):278–286. doi: 10.1016/j.molimm.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 129.Balaj L, Lessard R, Dai L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guescini M, Genedani S, Stocchi V, et al. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna) 2010;117(1):1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 131.Pfrieger FW, Vitale N. Cholesterol and the journey of extracellular vesicles. J Lipid Res. 2018;59(12):2255–2261. doi: 10.1194/jlr.R084210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pollet H, Conrard L, Cloos AS, et al. Plasma membrane lipid domains as platforms for vesicle biogenesis and shedding? Biomolecules. 2018;8(3):94. doi: 10.3390/biom8030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Skotland T, Sagini K, Sandvig K, et al. An emerging focus on lipids in extracellular vesicles. Adv Drug Deliv Rev. 2020;159:308–321. doi: 10.1016/j.addr.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 134.Laulagnier K, Motta C, Hamdi S, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380(Pt 1):161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Record M, Carayon K, Poirot M, et al. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841(1):108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 136.Kumeda N, Ogawa Y, Akimoto Y, et al. Characterization of membrane integrity and morphological stability of human salivary exosomes. Biol Pharm Bull. 2017;40(8):1183–1191. doi: 10.1248/bpb.b16-00891. [DOI] [PubMed] [Google Scholar]
- 137.Richter M, Fuhrmann K, Fuhrmann G. Evaluation of the storage stability of extracellular vesicles. J Vis Exp. 2019;22(147):e59584. doi: 10.3791/59584. [DOI] [PubMed] [Google Scholar]
- 138.Tetta C, Ghigo E, Silengo L, et al. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44(1):11–19. doi: 10.1007/s12020-012-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2:20389. doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thery C, Boussac M, Veron P, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166(12):7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 141.Poon IK, Lucas CD, Rossi AG, et al. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kinchen JM, Doukoumetzidis K, Almendinger J, et al. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat Cell Biol. 2008;10(5):556–566. doi: 10.1038/ncb1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17(2):170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li W. Eat-me signals: keys to molecular phagocyte biology and “appetite” control. J Cell Physiol. 2012;227(4):1291–1297. doi: 10.1002/jcp.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lleo A, Zhang W, McDonald WH, et al. Shotgun proteomics: identification of unique protein profiles of apoptotic bodies from biliary epithelial cells. Hepatology. 2014;60(4):1314–1323. doi: 10.1002/hep.27230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Borges FT, Reis LA, Schor N. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res. 2013;46(10):824–830. doi: 10.1590/1414-431X20132964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stein JM, Luzio JP. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem J. 1991;274(Pt 2):381–386. doi: 10.1042/bj2740381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Escola JM, Kleijmeer MJ, Stoorvogel W, et al. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 149.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 150.Taylor J, Bebawy M. Proteins regulating microvesicle biogenesis and multidrug resistance in cancer. Proteomics. 2019;19(1–2):e1800165. doi: 10.1002/pmic.201800165. [DOI] [PubMed] [Google Scholar]
- 151.Wilson HL, Francis SE, Dower SK, et al. Secretion of intracellular IL-1 receptor antagonist (type 1) is dependent on P2X7 receptor activation. J Immunol. 2004;173(2):1202–1208. doi: 10.4049/jimmunol.173.2.1202. [DOI] [PubMed] [Google Scholar]
- 152.Obregon C, Rothen-Rutishauser B, Gitahi SK, et al. Exovesicles from human activated dendritic cells fuse with resting dendritic cells, allowing them to present alloantigens. Am J Pathol. 2006;169(6):2127–2136. doi: 10.2353/ajpath.2006.060453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Antonyak MA, Cerione RA. Emerging picture of the distinct traits and functions of microvesicles and exosomes. Proc Natl Acad Sci USA. 2015;112(12):3589–3590. doi: 10.1073/pnas.1502590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Menck K, Bleckmann A, Schulz M, et al. Isolation and characterization of microvesicles from peripheral blood. J Vis Exp. 2017;6(119):e55057. doi: 10.3791/55057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li CJ, Liu Y, Chen Y, et al. Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke. Am J Pathol. 2013;182(5):1552–1562. doi: 10.1016/j.ajpath.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Heijnen HF, Schiel AE, Fijnheer R, et al. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 157.Pluskota E, Woody NM, Szpak D, et al. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008;112(6):2327–2335. doi: 10.1182/blood-2007-12-127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102(7):2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 159.Desrochers LM, Bordeleau F, Reinhart-King CA, et al. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat Commun. 2016;7:11958. doi: 10.1038/ncomms11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12(5):671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 161.Iakoucheva LM, Radivojac P, Brown CJ, et al. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004;32(3):1037–1049. doi: 10.1093/nar/gkh253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pejaver V, Hsu WL, Xin F, et al. The structural and functional signatures of proteins that undergo multiple events of post-translational modification. Protein Sci. 2014;23(8):1077–1093. doi: 10.1002/pro.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou J, Zhao S, Dunker AK. Intrinsically disordered proteins link alternative splicing and post-translational modifications to complex cell signaling and regulation. J Mol Biol. 2018;430(16):2342–2359. doi: 10.1016/j.jmb.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 164.Niklas KJ, Bondos SE, Dunker AK, et al. Rethinking gene regulatory networks in light of alternative splicing, intrinsically disordered protein domains, and post-translational modifications. Front Cell Dev Biol. 2015;3:8. doi: 10.3389/fcell.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 166.Gusachenko ON, Zenkova MA, Vlassov VV. Nucleic acids in exosomes: disease markers and intercellular communication molecules. Biochem Mosc. 2013;78(1):1–7. doi: 10.1134/S000629791301001X. [DOI] [PubMed] [Google Scholar]
- 167.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 168.Bucci C, Thomsen P, Nicoziani P, et al. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11(2):467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464(7290):864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Babst M, Katzmann DJ, Estepa-Sabal EJ, et al. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell. 2002;3(2):271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 171.Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm. 2014;71(4):537–543. [PubMed] [Google Scholar]
- 172.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lim J, Choi M, Lee H, et al. Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires. J Nanobiotechnol. 2019;17(1):1. doi: 10.1186/s12951-018-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Crescitelli R, Lasser C, Szabo TG, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:20677. doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Willms E, Cabanas C, Mager I, et al. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:738. doi: 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leone DA, Rees AJ, Kain R. Dendritic cells and routing cargo into exosomes. Immunol Cell Biol. 2018;96:683–693. doi: 10.1111/imcb.12170. [DOI] [PubMed] [Google Scholar]
- 178.Admyre C, Bohle B, Johansson SM, et al. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120(6):1418–1424. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 179.Kooijmans SA, Vader P, van Dommelen SM, et al. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomed. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Subedi P, Schneider M, Philipp J, et al. Comparison of methods to isolate proteins from extracellular vesicles for mass spectrometry-based proteomic analyses. Anal Biochem. 2019;584:113390. doi: 10.1016/j.ab.2019.113390. [DOI] [PubMed] [Google Scholar]
- 181.Koh YQ, Peiris HN, Vaswani K, et al. Characterization of exosomes from body fluids of dairy cows. J Anim Sci. 2017;95(9):3893–3904. doi: 10.2527/jas2017.1727. [DOI] [PubMed] [Google Scholar]
- 182.Sedykh S, Kuleshova A, Nevinsky G. Milk Exosomes: Perspective Agents for Anticancer Drug Delivery. Int J Mol Sci. 2020;21(18):6646–6651. doi: 10.3390/ijms21186646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Admyre C, Johansson SM, Qazi KR, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 184.Soares Martins T, Catita J, Martins Rosa I, et al. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE. 2018;13(6):e0198820. doi: 10.1371/journal.pone.0198820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang C, Guo WB, Zhang WS, et al. Comprehensive proteomics analysis of exosomes derived from human seminal plasma. Andrology. 2017;5(5):1007–1015. doi: 10.1111/andr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Skalnikova HK, Bohuslavova B, Turnovcova K, et al. Isolation and characterization of small extracellular vesicles from porcine blood plasma, cerebrospinal fluid, and seminal plasma. Proteomes. 2019;7(2):17. doi: 10.3390/proteomes7020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Butler JT, Abdelhamed S, Kurre P. Extracellular vesicles in the hematopoietic microenvironment. Haematologica. 2018;103(3):382–394. doi: 10.3324/haematol.2017.183335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61(11):1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 189.Wang J, Yao Y, Chen X, et al. Host derived exosomes-pathogens interactions: potential functions of exosomes in pathogen infection. Biomed Pharmacother. 2018;108:1451–1459. doi: 10.1016/j.biopha.2018.09.174. [DOI] [PubMed] [Google Scholar]
- 190.Osaki M, Okada F. Exosomes and their role in cancer progression. Yonago Acta Med. 2019;62(2):182–190. doi: 10.33160/yam.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tian W, Liu S, Li B. Potential role of exosomes in cancer metastasis. Biomed Res Int. 2019;2019:4649705. doi: 10.1155/2019/4649705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jan AT, Malik MA, Rahman S, et al. Perspective insights of exosomes in neurodegenerative diseases: a critical appraisal. Front Aging Neurosci. 2017;9:317. doi: 10.3389/fnagi.2017.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Anel A, Gallego-Lleyda A, de Miguel D, et al. Role of exosomes in the regulation of T-cell mediated immune responses and in autoimmune disease. Cells. 2019;8(2):154. doi: 10.3390/cells8020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li XB, Zhang ZR, Schluesener HJ, et al. Role of exosomes in immune regulation. J Cell Mol Med. 2006;10(2):364–375. doi: 10.1111/j.1582-4934.2006.tb00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Crenshaw BJ, Sims B, Matthews QL (2018) Biological function of exosomes as diagnostic markers and therapeutic delivery vehicles in carcinogenesis and infectious diseases. In: Farrukh MA (ed) Nanomedicines. IntechOpen
- 196.Nie Y, Sato Y, Garner RT, et al. Skeletal muscle-derived exosomes regulate endothelial cell functions via reactive oxygen species-activated nuclear factor-kappaB signalling. Exp Physiol. 2019;104(8):1262–1273. doi: 10.1113/EP087396. [DOI] [PubMed] [Google Scholar]
- 197.Corrado C, Raimondo S, Chiesi A, et al. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci. 2013;14(3):5338–5366. doi: 10.3390/ijms14035338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Boriachek K, Islam MN, Moller A, et al. Biological functions and current advances in isolation and detection strategies for exosome nanovesicles. Small. 2018;14(6):1702153. doi: 10.1002/smll.201702153. [DOI] [PubMed] [Google Scholar]
- 199.Li Q, Wang H, Peng H, et al. Exosomes: versatile nano mediators of immune regulation. Cancers (Basel) 2019;11(10):1557. doi: 10.3390/cancers11101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sprent J. Direct stimulation of naive T cells by antigen-presenting cell vesicles. Blood Cells Mol Dis. 2005;35(1):17–20. doi: 10.1016/j.bcmd.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 201.Saunderson SC, McLellan AD. Role of lymphocyte subsets in the immune response to primary B cell-derived exosomes. J Immunol. 2017;199(7):2225–2235. doi: 10.4049/jimmunol.1601537. [DOI] [PubMed] [Google Scholar]
- 202.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35(2):89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 204.Morelli AE, Larregina AT, Shufesky WJ, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 205.Nolte-‘t Hoen EN, Buschow SI, Anderton SM, et al. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 206.Quah BJ, O'Neill HC. The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol Dis. 2005;35(2):94–110. doi: 10.1016/j.bcmd.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 207.Yin W, Ouyang S, Li Y, et al. Immature dendritic cell-derived exosomes: a promise subcellular vaccine for autoimmunity. Inflammation. 2013;36(1):232–240. doi: 10.1007/s10753-012-9539-1. [DOI] [PubMed] [Google Scholar]
- 208.Yang X, Meng S, Jiang H, et al. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. J Surg Res. 2011;171(2):826–832. doi: 10.1016/j.jss.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 209.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28(3):585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang B, Yin Y, Lai RC, et al. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 211.Chen W, Huang Y, Han J, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64(4):831–840. doi: 10.1007/s12026-016-8798-6. [DOI] [PubMed] [Google Scholar]
- 212.Del Fattore A, Luciano R, Pascucci L, et al. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant. 2015;24(12):2615–2627. doi: 10.3727/096368915X687543. [DOI] [PubMed] [Google Scholar]
- 213.Duffy MM, Ritter T, Ceredig R, et al. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2(4):34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54(Suppl 2):789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 215.Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 216.Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fais S. NK cell-released exosomes: natural nanobullets against tumors. Oncoimmunology. 2013;2(1):e22337. doi: 10.4161/onci.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jong AY, Wu CH, Li J, et al. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J Extracell Vesicles. 2017;6(1):1294368. doi: 10.1080/20013078.2017.1294368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chang HF, Bzeih H, Chitirala P, et al. Preparing the lethal hit: interplay between exo- and endocytic pathways in cytotoxic T lymphocytes. Cell Mol Life Sci. 2017;74(3):399–408. doi: 10.1007/s00018-016-2350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15(6):388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- 221.Lugini L, Cecchetti S, Huber V, et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189(6):2833–2842. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 222.Zhu L, Kalimuthu S, Gangadaran P, et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7(10):2732–2745. doi: 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li S, Li S, Wu S, et al. Exosomes modulate the viral replication and host immune responses in HBV infection. Biomed Res Int. 2019;2019:2103943. doi: 10.1155/2019/2103943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang G, Hu W, Chen H, et al. Cocktail strategy based on NK cell-derived exosomes and their biomimetic nanoparticles for dual tumor therapy. Cancers (Basel) 2019;11(10):1560. doi: 10.3390/cancers11101560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Romano M, Fanelli G, Albany CJ, et al. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:43. doi: 10.3389/fimmu.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li P, Liu C, Yu Z, et al. New insights into regulatory T cells: exosome- and non-coding RNA-mediated regulation of homeostasis and resident Treg cells. Front Immunol. 2016;7:574. doi: 10.3389/fimmu.2016.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Savina A, Furlan M, Vidal M, et al. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278(22):20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 228.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fontenot JD, Rasmussen JP, Gavin MA, et al. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 230.Okoye IS, Coomes SM, Pelly VS, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41(1):89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Smyth LA, Ratnasothy K, Tsang JY, et al. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43(9):2430–2440. doi: 10.1002/eji.201242909. [DOI] [PubMed] [Google Scholar]
- 232.Tan L, Wu H, Liu Y, et al. Recent advances of exosomes in immune modulation and autoimmune diseases. Autoimmunity. 2016;49(6):357–365. doi: 10.1080/08916934.2016.1191477. [DOI] [PubMed] [Google Scholar]
- 233.Yang C, Robbins PD. Immunosuppressive exosomes: a new approach for treating arthritis. Int J Rheumatol. 2012;2012:573528. doi: 10.1155/2012/573528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tofino-Vian M, Guillen MI, Alcaraz MJ. Extracellular vesicles: a new therapeutic strategy for joint conditions. Biochem Pharmacol. 2018;153:134–146. doi: 10.1016/j.bcp.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 235.Dudics S, Venkatesha SH, Moudgil KD. The micro-RNA expression profiles of autoimmune arthritis reveal novel biomarkers of the disease and therapeutic response. Int J Mol Sci. 2018;19(8):2293. doi: 10.3390/ijms19082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tsuno H, Arito M, Suematsu N, et al. A proteomic analysis of serum-derived exosomes in rheumatoid arthritis. BMC Rheumatol. 2018;2:35. doi: 10.1186/s41927-018-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Alghamdi MF, Redwan EM. Advances in the diagnosis of autoimmune diseases based on citrullinated peptides/proteins. Expert Rev Mol Diagn. 2021;21(7):685–702. doi: 10.1080/14737159.2021.1933946. [DOI] [PubMed] [Google Scholar]
- 238.Alghamdi MA, Redwan EM. Interplay of microbiota and citrullination in the immunopathogenesis of rheumatoid arthritis. Probiotics Antimicrob Proteins. 2021 doi: 10.1007/s12602-021-09802-7. [DOI] [PubMed] [Google Scholar]
- 239.Zhang B, Zhao M, Lu Q. Extracellular vesicles in rheumatoid arthritis and systemic lupus erythematosus: functions and applications. Front Immunol. 2020;11:575712. doi: 10.3389/fimmu.2020.575712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shenoda BB, Ajit SK. Modulation of immune responses by exosomes derived from antigen-presenting cells. Clin Med Insights Pathol. 2016;9(Suppl 1):1–8. doi: 10.4137/CPath.S39925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Withrow J, Murphy C, Liu Y, et al. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18(1):286. doi: 10.1186/s13075-016-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wieczorek M, Abualrous ET, Sticht J, et al. Major histocompatibility complex (MHC) class I and MHC class II proteins: conformational plasticity in antigen presentation. Front Immunol. 2017;8:292. doi: 10.3389/fimmu.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cloutier N, Tan S, Boudreau LH, et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med. 2013;5(2):235–249. doi: 10.1002/emmm.201201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Valitutti S. Immunological synapse: center of attention again. Immunity. 2008;29(3):384–386. doi: 10.1016/j.immuni.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 245.Admyre C, Johansson SM, Paulie S, et al. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36(7):1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- 246.Segura E, Nicco C, Lombard B, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106(1):216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 247.Thery C, Duban L, Segura E, et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 248.Montecalvo A, Shufesky WJ, Stolz DB, et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008;180(5):3081–3090. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- 249.Qazi KR, Gehrmann U, Domange Jordo E, et al. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009;113(12):2673–2683. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- 250.Mor-Vaknin N, Punturieri A, Sitwala K, et al. The DEK nuclear autoantigen is a secreted chemotactic factor. Mol Cell Biol. 2006;26(24):9484–9496. doi: 10.1128/MCB.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mor-Vaknin N, Kappes F, Dick AE, et al. DEK in the synovium of patients with juvenile idiopathic arthritis: characterization of DEK antibodies and posttranslational modification of the DEK autoantigen. Arthritis Rheum. 2011;63(2):556–567. doi: 10.1002/art.30138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Skriner K, Adolph K, Jungblut PR, et al. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006;54(12):3809–3814. doi: 10.1002/art.22276. [DOI] [PubMed] [Google Scholar]
- 253.Foers AD, Cheng L, Hill AF, et al. Review: extracellular vesicles in joint inflammation. Arthritis Rheumatol. 2017;69(7):1350–1362. doi: 10.1002/art.40076. [DOI] [PubMed] [Google Scholar]
- 254.Yu R, Li C, Sun L, et al. Hypoxia induces production of citrullinated proteins in human fibroblast-like synoviocytes through regulating HIF1alpha. Scand J Immunol. 2018;87(4):e12654. doi: 10.1111/sji.12654. [DOI] [PubMed] [Google Scholar]
- 255.Gao K, Zhu W, Li H, et al. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod Rheumatol. 2019;30(4):758–764. doi: 10.1080/14397595.2019.1651445. [DOI] [PubMed] [Google Scholar]
- 256.Krajewska-Wlodarczyk M, Owczarczyk-Saczonek A, Zuber Z, et al. Role of microparticles in the pathogenesis of inflammatory joint diseases. Int J Mol Sci. 2019;20(21):5453. doi: 10.3390/ijms20215453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gyorgy B, Szabo TG, Turiak L, et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS ONE. 2012;7(11):e49726. doi: 10.1371/journal.pone.0049726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Headland SE, Jones HR, Norling LV, et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med. 2015;7(315):315ra190. doi: 10.1126/scitranslmed.aac5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Siljander PR. Platelet-derived microparticles—an updated perspective. Thromb Res. 2011;127(Suppl 2):S30–S33. doi: 10.1016/S0049-3848(10)70152-3. [DOI] [PubMed] [Google Scholar]
- 261.Manara M, Sinigaglia L. Bone and TNF in rheumatoid arthritis: clinical implications. RMD Open. 2015;1(Suppl 1):e000065. doi: 10.1136/rmdopen-2015-000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Vinuela-Berni V, Doniz-Padilla L, Figueroa-Vega N, et al. Proportions of several types of plasma and urine microparticles are increased in patients with rheumatoid arthritis with active disease. Clin Exp Immunol. 2015;180(3):442–451. doi: 10.1111/cei.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Konttinen YT, Ainola M, Valleala H, et al. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis. 1999;58(11):691–697. doi: 10.1136/ard.58.11.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Distler JH, Jungel A, Huber LC, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci USA. 2005;102(8):2892–2897. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jungel A, Distler O, Schulze-Horsel U, et al. Microparticles stimulate the synthesis of prostaglandin E(2) via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 2007;56(11):3564–3574. doi: 10.1002/art.22980. [DOI] [PubMed] [Google Scholar]
- 266.Fattahi MJ, Mirshafiey A. Prostaglandins and rheumatoid arthritis. Arthritis. 2012;2012:239310. doi: 10.1155/2012/239310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Messer L, Alsaleh G, Freyssinet JM, et al. Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Arthritis Res Ther. 2009;11(2):R40. doi: 10.1186/ar2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sedlmayr P, Blaschitz A, Wilders-Truschnig M, et al. Platelets contain interleukin-1 alpha and beta which are detectable on the cell surface after activation. Scand J Immunol. 1995;42(2):209–214. doi: 10.1111/j.1365-3083.1995.tb03647.x. [DOI] [PubMed] [Google Scholar]
- 269.Cloutier N, Pare A, Farndale RW, et al. Platelets can enhance vascular permeability. Blood. 2012;120(6):1334–1343. doi: 10.1182/blood-2012-02-413047. [DOI] [PubMed] [Google Scholar]
- 270.Zhang HG, Liu C, Su K, et al. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol. 2006;176(12):7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 271.Kelly E, Won A, Refaeli Y, et al. IL-2 and related cytokines can promote T cell survival by activating AKT. J Immunol. 2002;168(2):597–603. doi: 10.4049/jimmunol.168.2.597. [DOI] [PubMed] [Google Scholar]
- 272.Maeda Y, Farina NH, Matzelle MM, et al. Synovium-derived microRNAs regulate bone pathways in rheumatoid arthritis. J Bone Miner Res. 2017;32(3):461–472. doi: 10.1002/jbmr.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bluml S, Bonelli M, Niederreiter B, et al. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum. 2011;63(5):1281–1288. doi: 10.1002/art.30281. [DOI] [PubMed] [Google Scholar]
- 274.Pandis I, Ospelt C, Karagianni N, et al. Identification of microRNA-221/222 and microRNA-323-3p association with rheumatoid arthritis via predictions using the human tumour necrosis factor transgenic mouse model. Ann Rheum Dis. 2012;71(10):1716–1723. doi: 10.1136/annrheumdis-2011-200803. [DOI] [PubMed] [Google Scholar]
- 275.Nakasa T, Shibuya H, Nagata Y, et al. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum. 2011;63(6):1582–1590. doi: 10.1002/art.30321. [DOI] [PubMed] [Google Scholar]
- 276.Menikou S, McArdle AJ, Li MS, et al. A proteomics-based method for identifying antigens within immune complexes. PLoS ONE. 2020;15(12):e0244157. doi: 10.1371/journal.pone.0244157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Song JE, Kim JS, Shin JH, et al. Role of synovial exosomes in osteoclast differentiation in inflammatory arthritis. Cells. 2021;10(1):120. doi: 10.3390/cells10010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Schioppo T, Ubiali T, Ingegnoli F, et al. The role of extracellular vesicles in rheumatoid arthritis: a systematic review. Clin Rheumatol. 2021;40(9):3481–3497. doi: 10.1007/s10067-021-05614-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Azoulay-Alfaguter I, Mor A. Isolation and characterization of T lymphocyte-exosomes using mass spectrometry. Methods Mol Biol. 2020;2184:91–102. doi: 10.1007/978-1-0716-0802-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Qin Q, Song R, Du P, et al. Systemic proteomic analysis reveals distinct exosomal proteins profiles in rheumatoid arthritis. J Immunol Res. 2021;2021:9421720. doi: 10.1155/2021/9421720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Burbano C, Villar-Vesga J, Vasquez G, et al. Proinflammatory differentiation of macrophages through microparticles that form immune complexes leads to T- and B-Cell activation in systemic autoimmune diseases. Front Immunol. 2019;10:2058. doi: 10.3389/fimmu.2019.02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Tavasolian F, Moghaddam AS, Rohani F, et al. Exosomes: effectual players in rheumatoid arthritis. Autoimmun Rev. 2020;19(6):102511. doi: 10.1016/j.autrev.2020.102511. [DOI] [PubMed] [Google Scholar]
- 283.Xin Y, Yang Z, Fei X, et al. THU0059 plasma exosomal mir-92a are involved in the occurrence and development of bone destruction in ra patients by inhibiting apoptosis of fibroblast-like synoviocytes. London: BMJ Publishing Group Ltd; 2018. [Google Scholar]
- 284.Wang L, Wang C, Jia X, et al. Circulating exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis. Cell Physiol Biochem. 2018;50(5):1754–1763. doi: 10.1159/000494793. [DOI] [PubMed] [Google Scholar]
- 285.Lim M-K, Song J, Kim S, et al. THU0087 Microrna-1915-3p in serum exosome is associated with disease activity of rheumatoid arthritis in korea. London: BMJ Publishing Group Ltd; 2018. [Google Scholar]
- 286.Foers AD, Dagley LF, Chatfield S, et al. Proteomic analysis of extracellular vesicles reveals an immunogenic cargo in rheumatoid arthritis synovial fluid. Clin Transl Immunol. 2020;9(11):e1185. doi: 10.1002/cti2.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ni Z, Zhou S, Li S, et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Du T, Yan Z, Zhu S, et al. QKI deficiency leads to osteoporosis by promoting RANKL-induced osteoclastogenesis and disrupting bone metabolism. Cell Death Dis. 2020;11(5):330. doi: 10.1038/s41419-020-2548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hejrati A, Hasani B, Esmaili M, et al. Role of exosome in autoimmunity, with a particular emphasis on rheumatoid arthritis. Int J Rheum Dis. 2021;24(2):159–169. doi: 10.1111/1756-185X.14021. [DOI] [PubMed] [Google Scholar]
- 290.Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev. 2016;273(1):11–28. doi: 10.1111/imr.12440. [DOI] [PubMed] [Google Scholar]
- 291.Rosas EC, Correa LB, das Graças Henriques M (2017) Neutrophils in rheumatoid arthritis: a target for discovering new therapies based on natural products. In: Khajah M (ed) Role of neutrophils in disease pathogenesis. IntechOpen
- 292.Lenci E, Cosottini L, Trabocchi A. Novel matrix metalloproteinase inhibitors: an updated patent review (2014–2020) Expert Opin Ther Pat. 2021;31(6):509–523. doi: 10.1080/13543776.2021.1881481. [DOI] [PubMed] [Google Scholar]
- 293.Hu F, Li Y, Zheng L, et al. Toll-like receptors expressed by synovial fibroblasts perpetuate Th1 and th17 cell responses in rheumatoid arthritis. PLoS ONE. 2014;9(6):e100266. doi: 10.1371/journal.pone.0100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chen J, Liu M, Luo X, et al. Exosomal miRNA-486-5p derived from rheumatoid arthritis fibroblast-like synoviocytes induces osteoblast differentiation through the Tob1/BMP/Smad pathway. Biomater Sci. 2020;8(12):3430–3442. doi: 10.1039/c9bm01761e. [DOI] [PubMed] [Google Scholar]
- 295.Yoo J, Lee SK, Lim M, et al. Exosomal amyloid A and lymphatic vessel endothelial hyaluronic acid receptor-1 proteins are associated with disease activity in rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):119. doi: 10.1186/s13075-017-1334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yuan FL, Li X, Lu WG, et al. Epidermal growth factor receptor (EGFR) as a therapeutic target in rheumatoid arthritis. Clin Rheumatol. 2013;32(3):289–292. doi: 10.1007/s10067-012-2119-9. [DOI] [PubMed] [Google Scholar]
- 297.Lao MX, Xu HS. Involvement of long non-coding RNAs in the pathogenesis of rheumatoid arthritis. Chin Med J (Engl) 2020;133(8):941–950. doi: 10.1097/CM9.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Song J, Kim D, Han J, et al. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med. 2015;15(1):121–126. doi: 10.1007/s10238-013-0271-4. [DOI] [PubMed] [Google Scholar]
- 299.Dolati S, Shakouri SK, Dolatkhah N, et al. The role of exosomal non-coding RNAs in aging-related diseases. Biofactors. 2021;47(3):292–310. doi: 10.1002/biof.1715. [DOI] [PubMed] [Google Scholar]
- 300.Lim MK, Yoo J, Sheen DH, et al. Serum exosomal miRNA-1915-3p is correlated with disease activity of Korean rheumatoid arthritis. In Vivo. 2020;34(5):2941–2945. doi: 10.21873/invivo.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zakeri Z, Salmaninejad A, Hosseini N, et al. MicroRNA and exosome: key players in rheumatoid arthritis. J Cell Biochem. 2019;120:10930–10944. doi: 10.1002/jcb.28499. [DOI] [PubMed] [Google Scholar]
- 302.Cheng P, Wang J. The potential of circulating microRNA-125a and microRNA-125b as markers for inflammation and clinical response to infliximab in rheumatoid arthritis patients. J Clin Lab Anal. 2020;34(8):e23329. doi: 10.1002/jcla.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Duan W, Zhang W, Jia J, et al. Exosomal microRNA in autoimmunity. Cell Mol Immunol. 2019;16(12):932–934. doi: 10.1038/s41423-019-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Svenningsen P, Sabaratnam R, Jensen BL. Urinary extracellular vesicles: origin, role as intercellular messengers and biomarkers; efficient sorting and potential treatment options. Acta Physiol (Oxf) 2020;228(1):e13346. doi: 10.1111/apha.13346. [DOI] [PubMed] [Google Scholar]
- 305.Vitorino R, Ferreira R, Guedes S, et al. What can urinary exosomes tell us? Cell Mol Life Sci. 2021;78(7):3265–3283. doi: 10.1007/s00018-020-03739-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Peng K, Vucetic S, Radivojac P, et al. Optimizing long intrinsic disorder predictors with protein evolutionary information. J Bioinform Comput Biol. 2005;3(1):35–60. doi: 10.1142/s0219720005000886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are used within our report.