Abstract
Histone H3 trimethylation on lysine 9 (H3K9me3) is a defining feature of mammalian pericentromeres, loss of which results in genome instability. Here we show that CDYL2 is recruited to pericentromeres in an H3K9me3-dependent manner and is required for faithful mitosis and genome stability. CDYL2 RNAi in MCF-7 breast cancer cells and Hela cervical cancer cells inhibited their growth, induced apoptosis, and provoked both nuclear and mitotic aberrations. Mass spectrometry analysis of CDYL2-interacting proteins identified the neurodevelopmental disease-linked mitotic regulators CHAMP1 and POGZ, which are associated with a central non-conserved region of CDYL2. RNAi rescue assays identified both the CDYL2 chromodomain and the CHAMP1-POGZ interacting region as required and, together, sufficient for CDYL2 regulation of mitosis and genome stability. CDYL2 RNAi caused loss of CHAMP1 localization at pericentromeres. We propose that CDYL2 functions as an adaptor protein that connects pericentromeric H3K9me3 with CHAMP1 and POGZ to ensure mitotic fidelity and genome stability.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04659-7.
Keywords: Epigenetics, Centromere, C13orf8, CAMP, ZNF828
Introduction
Eukaryotic mitosis is a highly regulated process involving the interplay of numerous proteins and complexes to ensure faithful segregation of the replicated genome to the daughter cells. Mitotic aberrations underlie a host of diseases, including many cancers and neurodevelopmental disorders [1–3]. However, despite significant advances, our understanding of mitosis remains incomplete, particularly in higher eukaryotes. One area of growing interest is the role of histone post-translational modifications (PTM) at centromeres and flanking pericentromeres, and the proteins that bind them. Among the most studied histone PTM is trimethylation of Histone H3 lysine 9 (H3K9me3), which is highly enriched at pericentromeres and to a lesser extent at centromeres primarily due to the actions of the SUVAR39H1/2 histone methyltransferases (HMTs) [4–6]. Loss of pericentromeric H3K9me3 enrichment in Suv39h1/2 knock-out mouse embryonic fibroblasts (MEF) results in severe mitotic defects and genome instability [7]. This appears to be at least partly due to loss of enrichment of the H3K9me3-binding HP1 proteins, which contribute to mitotic regulation via mechanisms including co-ordination of the recruitment of the mitotic kinase Haspin and the Chromosome Passenger Complex (CPC) [8, 9]. However, the full range of pericentromeric H3K9me3 functions in mitosis remains unclear.
HP1 binding to H3K9me3 depends on a triad of aromatic amino acids in its conserved N-terminal chromodomain (CD) that contribute to a binding pocket termed the aromatic cage [10]. The binding of HP1 to H3K9me3 is largely abrogated by phosphorylation of the adjacent H3 serine 10, a modification that peaks during mitosis. This results in the bulk removal of HP1 from mitotic chromosomes, though a minor fraction around centromeres is retained [9, 11]. Though less studied than HP1, the CDYL family of proteins also possesses an N-terminal CD that binds H3K9me3 with equivalently high affinity and specificity in vitro [12–14]. They are defined by the presence of a C-terminal crotonase-like domain in addition to the CD, joined together by a non-conserved linker region [15]. CDYL genes evolved recently, with the earliest copies identified in Deuterostomia. Humans possess two autosomal CDYL genes, CDYL/CDYL1 and CDYL2, in addition to several CDY genes present on the non-recombining arm of the Y chromosome [16]. CDYL and the CDY genes are highly expressed in spermatids [17, 18], and murine CDYL was shown to regulate spermatogenesis [18]. CDYL and CDYL2 are also highly expressed in other tissues including certain brain compartments, as well as in embryonic stem cells and various cancers [16, 19–22]. Regulation of murine spermatogenesis by CDYL was reported to be due at least in part to its crotonase domain, which indirectly controlled levels of histone lysine crotonylation [18]. The CDYL crotonase activity was also shown to contribute to homologous recombination-mediated DNA repair by regulating crotonylation of the single-stranded DNA-binding protein RPA1 [23]. However, the functional roles of the chromodomain and non-conserved linker region of CDYL family genes remain unclear.
We and others have shown that an important aspect of CDYL activity depends on its ability to interact with and coordinate the activity of other transcription factors (TF) and chromatin regulators. These include the TF and master regulator of neurogenesis REST/NRSF, binding of which by CDYL enables the recruitment of the H3K9 HMTs EHMT1, EHMT2, and SETDB1 to REST target genes, and promotes local enrichment of H3K9 dimethylation. We showed that this may contribute to cervical carcinoma, via the regulation of proto-oncogene NTRK3/TrkC [24]. Several other studies extended the range of CDYL functions to include neurogenesis, neuronal function, and roles in other cancer contexts including hepatocellular carcinoma [20, 25–27]. However, little remained known about CDYL2 until recently we and others showed that it was highly expressed in subsets of breast cancer patients and that at least the full-length isoform could function as a putative oncogene [21, 22]. Consistent with this, we found that CDYL2 promotes oncogenic signaling via the NF-kappaB and STAT3 pathways, downstream of CDYL2 binding to MIR124 genes and its recruitment of EHMT2 to promote H3K9 dimethylation. Although it bound EZH2 weakly compared to EHMT2, CDYL2 also affected its recruitment at MIR124 genes and H3K27 trimethylation [22]. A tumor suppressive role for CDYL2 in hepatocellular carcinoma was also described, wherein its downregulation in these tumors provoked loss of KDM5C histone demethylase recruitment to SLC7A6 regulatory elements [28]. These studies suggest that as was the case for CDYL/CDYL1, CDYL2 might mediate its functions via association with other proteins, potentially enabling it to impact diverse processes.
In the present study, we examined the effects of CDYL2 inhibition in the breast cancer cell line MCF-7 and cervical cancer Hela cells, the latter being an established model for mitosis studies. Using several independent RNAi reagents, we found that CDYL2 knockdown induced growth inhibition, apoptosis, and genome instability. Immunofluorescence analysis revealed bulk removal of CDYL2 from mitotic chromosomes, but co-localization with centromeres and enrichment on the mitotic spindle, suggesting it may interact with other proteins at these sites to mediate its mitotic regulatory effect. Using Suv39h1/2 knock-out mouse embryonic fibroblasts (MEF) we show that CDYL2 localized to pericentromeres in an H3K9me3-dependent manner. Mass spectrometry of CDYL2-binding proteins identified the spindle and kinetochore-associated protein CHAMP1 and its binding partner POGZ, both of which regulate mitosis [29–34]. Domain mapping assays revealed that CDYL2 bound to CHAMP1-POGZ via its non-conserved linker region, while RNAi-rescue assays showed that this region was required for CDYL2 regulation of mitosis as well as CHAMP1 recruitment proximal to inner centromere protein CENPB. Mutation of the CD aromatic cage residues uncovered the requirement of this CDYL2 domain for mitotic regulation. RNAi knockdown of CDYL2 resulted in diminished CHAMP1 localization proximal to centromeres. Collectively, these data provide the first insights into the regulation of mitosis and genome stability by CDYL2, with implications for our understanding of how H3K9me3 impacts these processes. It also expands our understanding of CHAMP1 and POGZ, poorly understood genome stability regulators that are recurrently mutated in neurodevelopmental disorders.
Materials and methods
Cell culture
MCF-7 cells (ATCC, HTB-22) were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, 31885-023) containing 10% heat-inactivated Fetal Bovine Serum (FBS, Gibco, 10270-106), 40 µg/mL of gentamicin (Gibco, 15710-049) and 0.6 µg/mL of insulin (NovoRapid, 3525909). Hela cells (ATCC, CRM-CCL-2), MDA-MB-436 cells (ATCC, HTB-130), and the immortalized mouse embryonic fibroblasts (i-MEF, wild-type (WT) or double knock-out Suv39h1/2 (DKO) i-MEF (generous gifts from Thomas Jenuwein, Max Planck Institute, Freiburg, Germany) were grown in DMEM (Gibco, 10566016) supplemented with 10% of FBS (Gibco, 10270-106), 1% penicillin/streptomycin (Gibco, 15140122). Hela cells carrying stably integrated H2B-GFP expression constructs were from a commercial source (Sigma, SCC117). Suspension HeLa cells (Hela S3, ATCC, CCL-2.2) were maintained and transduced as mixed adherent-suspension cultures in the same medium as normal Hela cells, but transferred to Joklik’s modified MEM (Sigma) containing 10% FBS and 1% penicillin/streptomycin for growth in spinner bottles, as previously described [24]. The siRNA used in this study were as follows: siCDYL2 #1 (Invitrogen #1299001, Assay ID HSS133715); siCDYL2 #2 (Invitrogen #1299001, Assay ID HSS133714); siCDYL2 #3 (Invitrogen #1299001, Assay ID HSS133710); Control for Invitrogen siRNA (Invitrogen #12935100); siCDYL2 targeting 3′-UTR (5′-ACGGUUCUCAGCUGUAAUA, designed using the Eurofins genomics tool (https://eurofinsgenomics.eu/en/ecom/tools/oligo-analysis/) according to default parameters, and synthesized by Eurogentec; Eurogentec siControl (#SR-CL010-005). RNAi knock-downs were performed by transfecting 5 nmol of siRNA into a 12-well of Hela or MCF-7 cells plated the day before using Interferin reagent (Polyplus), except in the case of the RNAi rescue assays, in which case 10 nmol siRNA along with 75 ng of the relevant plasmid (unless otherwise indicated) were co-transfected using JetPrime reagent (Polyplus), in both cases according to the manufacturer’s protocol except that culture media was exchanged the day after transfection. Transfected cells were analyzed 72 h post-transfection for Hela cells, and 96 h post-transfection for MCF-7 cells. MDA-MB-436 cells were transfected using JetPrime reagent, according to the manufacturer’s protocol, with the indicated quantities of pcDNA3-2HA-CDYL2, which contains a previously described full-length CDYL2 ORF [22] cloned in-frame downstream of two tandem HA tags. An empty pcDNA3-2HA plasmid was used as an empty vector (EV) control. Retroviruses and lentiviruses were produced in 293T and transduced into the relevant cell lines in the presence of 8 ug/mL polybrene (Merck) as previously described [35]. All cells were grown at 37 °C and 5% CO2 in a humidified incubator and regularly tested for mycoplasma using a commercial kit (ATCC) where relevant, antibiotic selection of infected cells was initiated 2 days after viral transduction and continued for up to 1 week. Clonal cultures of MCF-7 cells were obtained by plating 10,000 cells in a 10 cm tissue culture dish (Corning) five days after transduction and three days after initiation of selection. Cells were maintained under antibiotic selection for a further 7–10 days, at which point individual colonies were removed to separate wells of 48-well plates (Corning) by trypsinization with the aid of cloning discs (Bel-Art). The resulting clonal cultures were expanded and assayed promptly. Regular testing for CDYL2 knockdown by western blot and RT-PCR was performed.
Cell growth assays and video microscopy
Adherent colony formation assays were performed by plating 4000 cells in 12 well tissue-culture treated plates (Corning). After 12 days of growth, colonies were stained with crystal violet solution (crystal violet 0.05%, formaldehyde 1%, methanol 1%; all from Sigma) for 20 min, then excess dye was removed by washing extensively with deionized water. Colonies were counted and their size was measured using Fiji software. Real-time analysis of cell growth and mitosis was performed using the Incucyte ZOOM Live-Cell Analysis System (Essen Bioscience), according to the manufacturer’s instructions.
Flow cytometry
Propidium iodide FACS was performed on sub-confluent cell cultures as previously described using a FACS Calibur flow cytometer (BD Biosciences) [36]. Data were analyzed using Flowing Software 2 (Turku Bioscience). Flow cytometry detection of apoptotic cells was performed using a commercial kit that assays for the cell-surface phospholipid phosphatidylserine, an apoptotic marker, using fluorophore-conjugated Annexin V, in addition to the loss of membrane integrity using the vital dye 7-Amino-Actinomycin (7-AAD) (BD Biosciences). FACS was performed using a FACS Calibur flow cytometer (BD Biosciences) and the percentage of live (AV/7-AAD double negative) early-stage apoptotic (AV-positive/7-AAD negative) and end-stage apoptotic/necrotic (AV/7-AAD double-positive) cells determined according to the manufacturer’s instructions.
Antibodies
The following primary antibodies were used in these studies according to the manufacturers’ instructions: rabbit anti-CDYL2 (MyBiosource, MBS821304); rabbit anti-POGZ (Bethyl, A302-510A); rabbit anti-CHAMP1 (Bethyl, A304-217A); human CREST serum (Antibodies Incorporated, #15-234); mouse anti-alpha-tubulin (Cell Signaling Technologies, #3873); rabbit anti-beta-tubulin (Cell Signaling Technologies, #5568); rabbit anti-CENPA (Cell Signaling Technologies, #2186); rabbit anti-GFP (Sigma, G1544); rabbit anti-CENPC (ThermoFisher Scientific, PA5-66154); mouse anti-CENPB (Patrick Lomonte, INMG, Lyon); mouse anti-beta-actin (ThermoFisher Scientific, MA1-140); mouse anti-HA.11 (Biolegend, 901502); mouse anti-FLAG M2 (Sigma, F1804), mouse anti-CHAMP1 (Abnova, H00283489-B01P), mouse anti-P300 (Millipore, 05-257). Mouse anti-Gal4 (Santacruz Biotechnology, sc-577) was used for CoIP and western blotting applications, while mouse anti-Gal4 (ThermoFisher, #33-8600) was used for IF analysis.
Immunoblot
Sub-confluent cultures of cells were washed with PBS, lysed in ice-cold RIPA buffer (10 mM Tris–Cl (pH 8.0), 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl, 1 mM PMSF, all from Sigma) containing protease inhibitor cocktail (Roche) and a phosphatase inhibitors cocktail (Roche), sonicated briefly, and insoluble material removed by centrifugation at 12,000g at 4 °C for 15 min. Cleared lysates were quantified using a bicinchoninic acid assay kit (Thermo), denatured using Laemmli buffer then resolved on commercial Bis–Tris SDS-PAGE gels (Invitrogen), transferred to nitrocellulose or PVDF membranes (GE Healthcare), and probed according to the primary antibody manufacturer’s recommendations. Images were collected and analyzed using the ChemiDoc system (BioRad).
Immunofluorescence and PLA
Cells were seeded on sterile coverslips and analyzed 24–48 h later, or transfected 18 h after seeding and analyzed 48–72 h after transfection. Where indicated, enrichment of metaphase cells was performed by treating cultures with 10 μM MG-132 (Sigma) for 2 h, as previously described [29]. For the microtubule stability assays, MG-132 treated cells were incubated on ice for ten minutes in a large excess of ice-cold complete cell culture medium containing 10 μM MG-132 prior to a fixation on ice. Where indicated in the figures, pre-extraction of soluble proteins prior to cell fixation was performed by incubating cells in cytoskeletal (CSK) buffer (20 mM HEPES pH 7.4, 100 mM NaCl, 3 mM MgCl2, 300 mM Sucrose, all from Sigma) for 1 min at room temperature before replacing it gently with CSK buffer supplemented with 0.1% Triton X-100 (Sigma) for two minutes prior to fixation. Cells were fixed with freshly prepared 4% microscopy-grade paraformaldehyde (Fisher Scientific) in PBS, except in the case of analysis of CDYL2 localization to the mitotic spindle, where cells were fixed in 100% methanol at – 20 °C for 10 min. In either case, fixed cells were then permeabilized by incubating in PBS containing 0.5% NP-40 at room temperature for 15 min, then blocked with 5% FBS (Gibco) in PBS containing 0.1% NP-40 at room temperature for 1 h. Cells were then probed with the indicated primary antibodies overnight at 4 °C, washed three times using PBS, incubated with the relevant fluorescent-tagged secondary antibody for 1 h at room temperature, and washed a further three times before mounting on glass slides using Vectashield-DAPI medium (Vector Laboratories). PLA was performed on paraformaldehyde-fixed cells according to the manufacturer’s protocol (Sigma), except for the addition of an additional 2-min fixation of the stained coverslips using 1% paraformaldehyde in PBS, followed by three washes with 1:5 diluted PLA buffer B (Sigma), immediately prior to drying and mounting the coverslips. Microscopy was performed at the Centre d’imagerie Quantitative Lyon-Est de l’Université Lyon I (CIQLE) facility. A Zeiss Axioimager fitted with a 63X oil-immersion objective was used for image capture, and image analysis was performed using Zen Blue software (Zeiss). Image deconvolution was performed to facilitate analysis of CDYL2 and CREST or CENPE foci co-localization, using Zen Blue. Nuclear and mitotic phenotypes were scored in 300 interphase or 100 MG-132 synchronized metaphase cells per condition tested. Slide numbers were anonymized before analysis in a blinded manner. Data were expressed as a percentage of cells counted presenting a given phenotype, and a t-test comparison of three independent experimental replicates was performed as indicated.
Immunoprecipitation and mass spectrometry
Immunoprecipitation (IP) of FLAG-tagged CDYL2 and mass spectrometry (MS) identification of associated polypeptides were performed essentially as previously described [35]. In brief, Hela S3 cells stably expressing FLAG-tagged human CDYL2 were prepared by retroviral transduction followed by 1 μg/mL puromycin selection for two weeks. Control cells transduced with empty vectors were prepared in parallel. Nuclear extracts were prepared from 1L suspension cultures of both the FLAG-CDYL2 expressing cells and controls, incubated with anti-FLAG M2 agarose beads (Sigma) for 4 h and bound complexes eluted using triple-FLAG peptides (Sigma). Mass spectrometry of eluates and data processing was performed by The Taplin Biological Mass Spectrometry Facility (Harvard Medical School). Anti-Gal4 IP and endogenous CHAMP1 IP were performed on nuclear extracts prepared from sub-confluent endogenous Hela cells, or cells transfected with the indicated plasmids. Nuclear extracts were prepared as previously described [35], incubated with benzonase for 2 h at 4 °C, NP-40 added to a final concentration of 0.05%, pre-cleared with Protein A agarose beads (GE) for 1 h, and incubated overnight at 4 °C with the relevant antibodies (3 μg antibody added to 1 mg cleared extract, at a concentration of 5 μg/uL of extract). Immune complexes were recovered by adding 20 μL bed volume of Protein A beads (GE) for 1 h with rotation, then washed five times using IP buffer supplemented with NaCl to a final concentration of 250 mM. A final wash with IP buffer at 150 mM NaCl final concentration was performed prior to elution by boiling the beads in 50 μL of Laemmli buffer.
RT-PCR, PCR, and cloning
Reverse transcription followed by polymerase chain reaction (PCR) analysis of CDYL2 expression was performed as previously described [22]. Full-length CDYL2 was cloned into Gateway pENTR-D-TOPO plasmid (Invitrogen) and its sequence was validated as previously described [22]. The various CDYL2 domain fragment constructs used in this study were derived from this clone by a PCR-based cloning strategy, using Phusion polymerase (NEB) as per the manufacturer’s instructions. The primers used were as follows: CDYL2 CD-L (Fw: 5′-CACCGCTTCTGGGGACCTTTACGA; Rv: 5′- TCAACCGACAGTTGCTTTCATTCTGG); CDYL2 CD (Fw: 5′-CACCGCTTCTGGGGACCTTTACGA; Rv: 5′-TCAGTGCAACCCATTGAATTCATCAATA); CDYL2 Linker (Fw: 5′-CACCGGGTTGCACATGTCCAAGGAC; Rv: 5′-TCACCGACAGTTGCTTTCATTCTGG). The resulting PCR products were cloned into Gateway pENTR-D-TOPO (Invitrogen) and validated by sequencing on both strands. The MutCD construct, in which all three aromatic cage residues of the CD were mutated to Alanine, was generated by gene synthesis (Invitrogen). Briefly, a sequence corresponding to the open reading frame (ORF) of CDYL2 (NCBI accession NM_152342.3) except for the omission of the first ATG, and the following nucleotide mutations: T19G; A20C; T85G; G86C; T94G; A95C, was synthetically generated and cloned into pENTR-D-TOPO. The Gal4 DNA-binding domain (DBD) fusion constructs were cloned using LR clonase (Invitrogen) to transfer inserts into a Gateway-compatible Gal4 DBD N-terminal tagging expression vector (from Yang Shi lab, Harvard Medical School). These were validated by Sanger sequencing on both strands of the inserts. Plasmids expressing CDYL2 chromodomain-CHAMP1 fusion protein (pHA-CD-CHAMP1) were constructed using the In Fusion cloning kit (Takara Bio) according to the kit protocol. Briefly, the CDYL2 chromodomain was cloned using the high fidelity In Fusion kit PCR reagent, with a previously described CDYL2 expression plasmid acting as template [22], the forward primer 5′-AACCGTCAGATCCGCTAGCGCCTCGATCCTCCCTTTAT and the reverse primer 5′- AATGCTTCCATTGAATTTCCACTGGACTGCTTCCCTGAC. The resulting PCR product was gel-purified and inserted into a pEGFP-CHAMP1 plasmid (Addgene, #159892) that was previously digested with Nhe1 and EcoR1 and gel-purified to remove the N-terminal EGFP moiety while creating linearized plasmid ends suitable for the cloning of the PCR-amplified CDYL2 chromodomain in frame with the full-length CHAMP1 open reading frame (ORF). Similarly, Flag-tagged N-terminal CDYL2 CD-POGZ fusion protein expression constructs (pFG-CD-POGZ) were cloned by PCR-amplifying the CDYL2 CD from pMSCV-CDYL2 [22] using the forward primer 5′-AAGGGTGGTAATGGATCGGCTTCTGGGGACCTTTACGA and the reverse primer 5′-GTGTCCGCCATGGATCCACTGGACTGCTTCCCTGACTTG. This PCR product was gel-purified and cloned into a double FLAG-tagged POGZ expression plasmid (Addgene #105674) that had been linearized using BamH1, such that the CDYL2 chromodomain was cloned in-frame between the double FLAG-tags and the POGZ ORF. The resulting plasmids were cloned, validated by Sanger sequencing on both strands, and western blotting of transfected cell lysates was performed to validate expression.
Results
CDYL2 RNAi inhibition diminishes cell growth
To gain insights into the effects of CDYL2 RNAi on breast cancer cells, we transiently knocked it down in the MCF-7 ER + /HER2- breast cancer cell line using a siRNA approach (Fig. 1a, b; Online Resource 1). Cells were then seeded at low density on adherent plates and cultured for 12 days, with the resulting colonies counted and analyzed for size. This revealed both reduced average colony size and number in the siCDYL2-treated cells (Fig. 1c). In a complementary approach, real-time imaging of growing cultures also revealed a severe growth-inhibitory effect of siCDYL2 treatment (Fig. 1d). In parallel, clonal cultures of MCF-7 cells transduced with lentiviral vectors expressing shCDYL2 hairpins were derived (Fig. 1e, f) and their growth compared to that of clones transduced with vectors expressing a control non-targeting shRNA. Compared to controls, the shCDYL2-treated clones formed fewer and smaller colonies when seeded at low density (Fig. 1g), and also grew poorly when plated at normal seeding density (Fig. 1h).
Fig. 1.
CDYL2 RNAi inhibits cell growth, induces apoptosis and results in genome aberrations. a RT-PCR validation of CDYL2 RNAi knock-down in MCF-7 cells. CDYL2 mRNA was normalized to GAPDH levels. Shown is the mean of three replicates and standard deviation (S.D.) b Western blot validation of CDYL2 RNAi. c Images of wells of crystal violet stained adherent MCF-7 colonies treated with control or CDYL2 RNAi (left), Colonies were counted (top chart) and measured for average size, expressed as arbitrary units (lower chart). Shown are the mean and standard deviation of the three experiments. d Live cell imaging analysis of MCF-7 growth after treatment with control (yellow) or CDYL2 (green) RNAi. e, f RT-PCR and western blot validation of CDYL2 shRNA knockdown in clonal MCF-7 cultures compared to shLuciferase (shLuc) controls was performed as in (a) and (b) respectively. g, h Adherent colony growth (g) and live cell imaging (h) analysis of the growth of MCF-7 clones carrying shCDYL2 or control shLuc was performed as in (c) and (d) respectively. i FACS analysis of the early apoptosis marker Annexin V-PE and the vital dye 7-AAD in MCF-7 cells treated with shCDYL2 or control shRNA. A representative pair of control and CDYL2 RNAi FACS plots are shown (top), along with quantitation of non-apoptotic cells (AV-/7AAD-), cells in early apoptosis (AV + /7AAD-), and cells in late or post-apoptosis (AV + /7AAD +) (lower chart). Shown are the mean and S.D. of three experiments. T test p < 0.001 (***). j Propidium iodide (PI) FACS analysis of MCF-7 cells treated with control or CDYL2 siRNA. The peaks corresponding to 2n and 4n genomic content are indicated in controls. The experiment was repeated three times with similar results. k, l RT-PCR and western blot validation of RNAi knockdown of CDYL2 in Hela cells performed as per (a) and (b) respectively. m, n Colony growth assay (m) and PI-FACS (n) was performed on Hela treated with control or CDYL2 RNAi, as per (c) and (j) respectively
Knockdown of CDYL2 results in apoptosis and genome instability
During routine phase-contrast microscopy observations of MCF-7 cells treated with CDYL2 siRNA or carrying shRNA vectors, we observed many dead cells and debris (data not shown). To further explore the effects of CDYL2 RNAi on cell viability, we performed fluorescence-activated cell sorting (FACS) analysis of the early apoptosis marker Annexin V and the vital dye 7-AAD in the clonal control or shCDYL2 cultures. This revealed that shCDYL2 increased the fraction of cells in early apoptosis (Annexin V positive, 7-AAD negative) as well as in late apoptosis (Annexin V positive, 7-AAD positive) compared to controls (Fig. 1i).
Next, we analyzed the effects of CDYL2 RNAi on the cell cycle using propidium iodide staining followed by FACS (PI-FACS). Control RNAi-treated MCF-7 cells yielded a characteristic pattern of peaks corresponding to cells in the G1 stage of the cell cycle (2n chromosomes) or G2/M stages (4n chromosomes) and an even distribution of cells between these peaks corresponding to various stages of S phase (Fig. 1j, left graph). However, this pattern was severely disrupted in the siCDYL2-treated cells, including a fraction of cells with greater than 4n chromosome content (Fig. 1j, right graph). Similar effects were observed using a panel of three additional independent siRNA sequences targeting CDYL2 (Online Resource 2). We then extended our studies to Hela cells, which are a commonly used model for studies of mitosis, cell cycle, and genome stability. We found that CDYL2 RNAi also perturbed growth, cell cycle, and genome stability in these cells (Fig. 1l–n; Online Resource 1).
CDYL2 inhibition induces nuclear and mitotic aberrations
To better understand the apparent genomic aberrations induced by CDYL2 RNAi we performed immunofluorescence (IF) microscopy in Hela cells. This revealed numerous defects indicative of genomic instability and defective mitosis, including micronuclei (Fig. 2a, panel (ii) and (iii), white arrows), failed cytokinesis (Fig. 2a, panel (ii) and (iii), green arrows), multinuclear cells (Fig. 2a, panels (iv, v)) and fragmented nuclei (Fig. 2a, panel (vi)). These phenotypes were quantified and found to be significantly upregulated relative to control siRNA using two distinct CDYL2 siRNA (Online Resource 3). Similar phenotypes were observed in MCF-7 cells treated with siCDYL2 (Online Resource 4, panel (a)). We then repeated the analysis in cells pre-treated with the proteasome inhibitor MG-132, which induces a metaphase block, for two hours immediately before fixation. As expected, control siRNA-treated cells yielded consistent bipolar metaphases with the chromosomes aligned (Fig. 2b (i)). However, many aberrant mitotic cells were observed in the siCDYL2-treated Hela, notably with supernumerary spindle poles, misaligned chromosomes, and disorganized spindles (Fig. 2b (ii–v)).
Fig. 2.
CDYL2 RNAi induces mitotic defects and genome instability. a IF staining of beta-tubulin and DAPI staining of Hela cells treated with siControl (i) or siCDYL2 (ii–vi) revealing micronuclei and DNA bridges between daughter nuclei ((ii), and a zoom of (ii) shown in (iii)), multinuclear cells (iv, v) and fragmented nuclei (vi). Arrows added to highlight certain defects. b Hela cells treated with control siRNA showed normal metaphase (i) organization of chromosomes and the spindle. However, those treated with siCDYL2 revealed a variety of abnormal spindle organization patterns as well as misaligned chromosomes (ii–v). c Mitotic spindle stability was assayed by cold-shock of Hela cells treated with either control (i) or CDYL2 (ii, iii) siRNA. Metaphase cells were enriched by pre-treatment with MG-132 for 2 h before cold-shock and fixation. IF was performed as in (a). d Selected still images from video microscopy analysis of Hela H2B-GFP cells treated with siControl (top) or siCDYL2 (lower). The times indicated correspond to the elapsed time after the first image shown in the time-lapse series. The experiment was repeated three times with similar results
To probe the spindle defects in CDYL2 RNAi-treated cells further, we assessed the stability of the mitotic microtubules. This was done by first enriching the RNAi-treated cell cultures in metaphase cells using MG-132, then incubating the cells on ice for ten minutes to depolymerize weaker microtubules, prior to fixation and IF. As expected, in control siRNA-treated cells we observed depolymerization of microtubules in the interphase cells, while the more robust mitotic spindle microtubules were retained (Fig. 2c (i)). However, Hela cells treated with siCDYL2 showed reduced intensity of mitotic microtubule staining, along with the previously noted chromosome misalignment and spindle disorganization defects (Fig. 2c (ii, iii)). This suggests that impaired microtubule spindle stability might contribute to the siCDYL2 mitotic phenotypes.
We next performed a video microscopy analysis of the effects of siCDYL2 on Hela cells expressing GFP-tagged H2B (Hela GFP-H2B). Compared to the control siRNA-treated cells, siCDYL2-treated cells exhibited a variety of nuclear and mitotic abnormalities. These included many cells that failed to complete cytokinesis, yielding binuclear cells that in the subsequent round of mitosis produced tri- or quadriaxial metaphases (Fig. 2d and Online Resource 4 (panel b) and Online Resource 5). Taken together, our data support an important role for CDYL2 in mitotic regulation, in particular chromosome alignment and segregation, and suggest that the supernumerary spindle poles arise from segregation and cytokinesis defects in a prior round of mitosis.
Dynamic regulation of CDYL2 localization during the cell cycle
We and others have shown that CDYL2 is enriched in the nucleus of interphase cells [21, 22], but its localization during the cell cycle was not known. Using an antibody reactive with the non-conserved central linker region of CDYL2 we showed that it is largely excluded from chromosomes between metaphase and early telophase, becoming enriched once again in nuclei at late telophase (Fig. 3a and Online Resource 6). This mitotic exclusion from chromosomes is similar to that of HP1, which was shown to be due to the phosphorylation of Serine 10 residues adjacent to H3K9me3 [11], and indeed, binding of H3K9me3 peptides to CDYL2 chromodomain is similarly sensitive to H3S10 phosphorylation [12]. We also noted that CDYL2 staining was granular, and several of these granules appeared to co-localized with the centromere-reactive CREST antiserum in interphase and mitotic cells (Online Resource 7), though this could not be confirmed by intensity scans of cross sections through the foci (data not shown).
Fig. 3.
Immunofluorescence analysis of CDYL2 sub-cellular localization. a CDYL2 IF in paraformaldehyde-fixed Hela cells with DAPI, CREST and beta-tubulin co-staining. Merged images of CDYL2 (red), CREST (green), and beta-tubulin (cyan) are also shown. Representative images from interphase, prometaphase, metaphase, and both early and late anaphase and telophase are shown. The staining was repeated three times with similar results. b Deconvolution microscopy analysis of interphase Hela or MCF-7 cells, pre-extracted or not before fixation with a triton X-100-based buffer, and stained with CDYL2, CREST, and DAPI. These are colorized as red, white, and blue respectively in the merged images. Selected intensity scans of putative co-localizing foci are also shown in the rightmost three panels. The foci analyzed in each intensity scan are indicated by the yellow arrows in the CDYL2/CREST merged image. c As in (b), except metaphase cells are shown, and staining includes anti-CENPE (green). Selected intensity scans of putative co-localizing foci are shown in the rightmost three panels. The foci analyzed in each intensity scan are indicated by the yellow arrows in the CDYL2/CREST/CENPE merged image
To further investigate the putative centromeric localization of CDYL2 we first characterized the CREST serum to determine which major epitopes it recognized, by using it in a western blot of Hela whole cell lysate. This revealed a major reactive band corresponding to CENPA and a fainter band corresponding to CENPC, both of which are components of the inner kinetochore (Online Resource 8). We conclude that the CREST serum used in these studies is a reliable probe for inner kinetochore proteins. Next, we performed deconvolution microscopy on Hela or MCF-7 cells, which enabled intensity scan analysis of putative co-localizing foci. Both interphase and metaphase cells were analyzed, and we further compared cells pretreated or not with a detergent-based extraction buffer before fixation, to remove soluble proteins. These studies revealed co-localization of a subset of CDYL2 and CREST foci under all conditions tested (Fig. 3b, c; Online Resources 9–16). However, the majority of CDYL2 foci were not associated with CREST signals, suggesting additional centromere-independent functions of CDYL2. Additionally, not all CREST foci detected were associated with CDYL2, which may be due in part to technical reasons such as CDYL2 staining and imaging efficiency, but could also reflect dynamic or chromosome-specific aspects of CDYL2 association with centromeres. Co-staining of the outer kinetochore protein CENPE along with CDYL2 and CREST revealed that CDYL2 is more commonly adjacent to, rather than co-localizing with, CENPE in metaphase cells (Fig. 3c; Online Resources 13–16). Based on these analyses, we conclude that a fraction of CDYL2 is associated with both interphase and metaphase centromeres, despite its bulk removal during mitosis.
CDYL2 enrichment at pericentromeres and the mitotic spindle
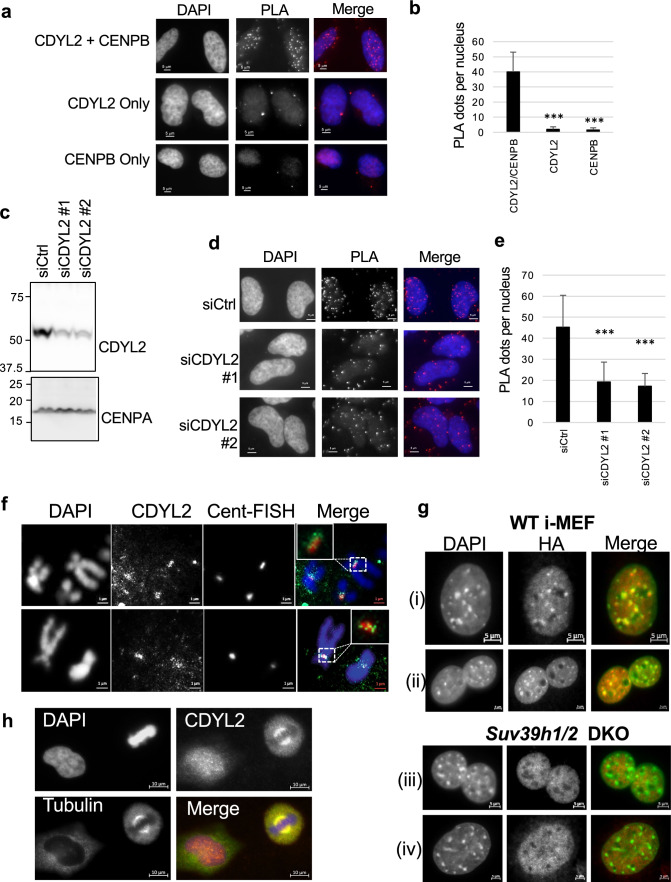
To further examine if CDYL2 was indeed associated with centromeres or pericentromeres, we performed a proximity ligation assay (PLA) for CDYL2 and CENP-B, which associates with centromeric chromatin throughout the cell cycle [37]. PLA in which fixed cells were incubated with both anti-CDYL2 and anti-CENP-B yielded many fluorescent PLA dots, whereas control PLA in which one or the other of the two antibodies was excluded yielded few dots (Fig. 4a, b). As an additional specificity control, the CENPB antibody was replaced by anti-P300, which is a nuclear-localized but not centromere-enriched histone acetyltransferase. Although the CDYL2/P300 antibody combination produced more PLA dots than the CDYL2 antibody alone, this was still significantly fewer than the CDYL2/CENPB antibody combination (Online Resource 17, panels (b) and (c). Further control was then performed, in which CDYL2/CENP-B PLA was performed on cells treated with a control siRNA (siCtrl) or two independent siRNA targeting CDYL2. The siCDYL2 significantly reduced nuclear PLA foci relative to siCtrl (Fig. 4c–e; Online Resource 18). To specifically investigate if CDYL2 is localized at pericentromeres in metaphase chromosomes, we performed CDYL2 IF and fluorescence in situ hybridization (FISH) with a centromere probe (Cent-FISH) on Hela metaphase spreads. This revealed co-localization of CDYL2 with the Cent-FISH probe (Fig. 4f), indicating that indeed it is present at centromeres in metaphase chromosomes. A control in which the CDYL2 antibody was replaced with a non-specific antibody did not yield a centromeric signal (Online Resource 19). Complementing these analyses, we investigated the localization of the murine homolog of CDYL2 at pericentromeres in immortalized murine embryonic fibroblasts (i-MEF). We found chromocenter enrichment of HA-tagged mCdyl2 in wild type (WT), but not Suv39h1/2 double knock-out (DKO) i-MEF (Fig. 4g, panel series (i) and (iii)), indicating H3K9me3-dependent mCdyl2 recruitment to pericentromeres. As a positive control, we also analyzed HA-mCdyl1, which was previously reported to colocalize with chromocenters in a Suv39h1/2-dependent manner (Fig. 4g, panel series (ii) and (iv)). In addition, CDYL2 enrichment at mitotic spindles was confirmed by performing IF in Hela cells fixed with methanol (Fig. 4h), which provides superior fixation of many proteins to microtubules compared to paraformaldehyde (as used in Fig. 3a–c). Overall, these data are consistent with the possibility that CDYL2 interacts with pericentromeric H3K9me3 and the mitotic spindle to carry out its mitotic regulatory functions.
Fig. 4.
CDYL2 colocalization with pericentromeres and the mitotic spindle. a Proximity ligation assay (PLA) of CDYL2 and CENP-B, with concomitant DAPI nuclear staining. Single antibody PLA controls are also shown. Representative images are shown. The experiment was repeated three times with similar results. b Quantification of the PLA dots in the respective conditions tested in (a). The mean number of dots per nucleus and S.D. are shown. T tests comparing single antibody controls to anti-CDYL2/CENP-B PLA were performed (***p < 0.001). c Immunoblot validation of CDYL2 siRNA knockdown corresponding to PLA in (d). d PLA of CDYL2 and CENP-B in Hela cells treated with control siRNA or two independent siCDYL2 sequences, performed as in (a). e Quantification of PLA dots from (d) was performed as in (b). f IF-FISH showing DAPI-stained metaphase chromosomes, immunostained CDYL2, and centromere repeats stained with a labeled FISH probe (Cent-FISH). Two representative images are shown. The white boxes in the merged images indicate a zoom on the co-stained regions. The staining was repeated three times with similar results. g Wild-type (WT) or Suv39h1/2 DKO i-MEF were transduced with HA-mCdyl2 expression vectors (i, iii) or HA-mCdyl1 expression vectors (ii, iv), then stained with anti-HA antibodies (red) and DAPI, shown in green to facilitate analysis of co-localization analysis in the merged images. Shown are representative images from three independent experiments. h CDYL2 and gamma-tubulin IF in Hela cells fixed with methanol. Shown is a representative image of a metaphase cell (right of frame) and interphase cell (left of frame). Staining was repeated three times with similar results
Identification of CDYL2 interaction with CHAMP1 and POGZ
An IP-MS approach was used to identify CDYL2-interacting proteins that might give insights into its regulation of mitosis. Suspension Hela cell cultures stably transduced with retroviruses carrying FLAG-tagged CDYL2 expression cassettes or empty vector controls were generated. Anti-FLAG IP was then performed on nuclear extracts prepared from both the FLAG-CDYL2 and control cultures and elution of bound immunocomplexes was performed under native conditions. The resulting eluates were subjected to tryptic digestion and tandem mass spectrometry (MS) analysis. Three independent purifications were performed. This revealed significant enrichment of the mitotic-regulatory proteins CHAMP1 and POGZ in all three purifications compared to the mock IP controls (Fig. 5a). IP-MS data for two previously validated CDYL2 interactors, EHMT1 and EHMT2 [22], are also shown for comparison (Fig. 5a). To validate the interaction of CDYL2 with CHAMP1 and POGZ, we performed an IP using an alternative tag on CDYL2, namely a Gal4 DNA binding domain. Hela or MCF-7 cells were transfected with Gal4-CDYL2 plasmids, from which nuclear extracts were prepared. These were then pre-treated with benzonase to digest residual nucleic acids, and IP was performed using either non-specific control or anti-Gal4 antibodies. This revealed specific co-immunoprecipitation (CoIP) of CHAMP1 and POGZ with Gal4-CDYL2 (Fig. 5b, c; Online Resource 20). We also performed a reciprocal CoIP using anti-CHAMP1, this time using non-transfected (endogenous) nuclear extracts prepared from Hela or MCF-7 cells, and again including a non-specific control IP. Interaction of endogenous CDYL2 and CHAMP1 was observed in both cell lines (Fig. 5d; Online Resource 20). Further validation of the interaction of CDYL2 with CHAMP1 was performed using PLA assay. This revealed abundant CDYL2/CHAMP1 PLA signals relative to single antibody controls (Fig. 5e, f), or relative to CDYL2/P300 PLA (Online Resource 17). RNAi knockdown of CDYL2 significantly reduced the number of CDYL2/CHAMP1 dots detected (Fig. 5g, h), further validating the specificity of the assay. Parallel analysis of CDYL2-POGZ interaction by both reciprocal CoIP using anti-POGZ antibodies or PLA did not reveal either CoIP of CDYL2 or enrichment of PLA foci compared to single antibody controls (data not shown). This may indicate that CDYL2 interacts more robustly with CHAMP1 than POGZ, but could also be due to the limitations of the POGZ antibody.
Fig. 5.
CDYL2 interacts with CHAMP1 and POGZ. a Number of unique CDYL2, CHAMP1, POGZ, EHMT2 and EHMT1 peptides in mass spectrometry analysis of triplicate FLAG-CDYL2 IPs and their paired mock IP controls. b, c Western blot analysis of Gal4-CDYL2 IP from transfected Hela nuclear extracts (b) or MCF7 nuclear extracts (c) along with control IgG IP. Shown are representative images from three independent assays. The positions of molecular weight markers are shown to the left of each blot (kDa). The position of bands of the expected molecular weight is indicated with an asterisk (*), and the signal from the IP antibody heavy chain is shown with a double asterisk (**). d Anti-CHAMP1 or control IgG IP in nuclear extracts prepared from non-transfected Hela cells (upper panels) or MCF-7 cells (lower panels), followed by either CHAMP1 or CDYL2 western blot. The assay was repeated three times with similar results. The position of bands of the expected molecular weight is indicated with an asterisk (*), and the signal from the IP antibody heavy chain is shown with a double asterisk (**). e PLA indicating CHAMP1 localization proximal to CDYL2 (top row). Single antibody controls are shown below. Representative images are shown. The experiment was repeated three times with similar results. f Quantification of the PLA dots from (e), expressed as the mean number of dots per nucleus and S.D. T test compared to CDYL2/CHAMP1 PLA (***p < 0.001). g CDYL2/CHAMP1 PLA was performed as in (e) but on cells treated with a control siRNA or two independent siRNA targeting CDYL2. Representative images are shown. The experiment was repeated three times with similar results. h Quantification of the nuclear PLA dots from (g) performed as in (f)
The CDYL2 chromodomain and CHAMP1-POGZ-binding linker region are necessary and sufficient for its regulation of genome stability
To determine which region of CDYL2 interacts with CHAMP1-POGZ, we expressed in Hela cells Gal4-CDYL2 full-length (FL) or various deletion mutants containing only the CD and linker region (CD + Linker, CD-L), the CD alone, or the linker alone (L). We additionally evaluated a full-length CDYL2 construct in which all three of the aromatic cage residues required for H3K9me3 binding by the chromodomain were mutated to alanine (FL-MutCD) (Fig. 6a). These constructs were transfected into HeLa cells and anti-Gal4 or control IgG IP performed on nuclear extracts. This revealed that the Mut-CD protein interacted equivalently well with CHAMP1 and POGZ as the wild-type (WT) CDYL2, indicating that the H3K9me3-binding activity of the CD was not required. Nor was the crotonase-like domain required, as the CD-L protein fragment also bound CHAMP1-POGZ. Analysis of the CD and linker regions alone revealed CHAMP1-POGZ interaction only with the latter (Fig. 6b and Online Resource 21; Gal4-CD construct was obscured by the antibody light chain signal in the upper Gal4-CDYL2 blot but was visible in a lower exposure of the same blot, as shown in the lower panel excerpt).
Fig. 6.
A CHAMP1-POGZ interacting region and intact chromodomain are required for CDYL2 regulation of mitosis and genome stability. a Graphic representation of Gal4 DBD (G4)-tagged CDYL2 full-length (FL) and deletion or triple point mutants. b CHAMP1, POGZ and Gal4 immunoblot of anti-Gal4 or control IgG IP performed on nuclear extracts from Hela cells transfected with the indicated plasmids. An excerpt of the Gal4-CDYL2 blot is shown at lower exposure in the image below that of the full blot, to facilitate visualization of the Gal4-CD construct which is obscured by the antibody light chain signal in the higher exposure blot. Reactive bands of the expected molecular weight are indicated by single asterisks (*). Cross-reactive bands from the light chain of the IP antibodies in the lower panel are indicated (**). The experiment was repeated three times with similar results. c Western blot validation of CDYL2 RNAi knockdown and expression of the Gal4-CDYL2 constructs indicated above each lane. Shown are CDYL2, Gal4, and actin blots on the same membrane. Reactive bands of the expected molecular weight are indicated by single asterisks (*). d The indicated phenotypes were counted in Hela cells treated with control (siCtrl) or siCDYL2 along with an empty vector (EV) or one of the CDYL2 cDNAs indicated in (a). Cells were incubated with MG-132 for 2 h before analysis to enrich in metaphase cells. Shown is the mean of three independent assays and S.D. T test of differences between siCDYL2/EV and siCtrl/EV, or siCDYL2/EV co-transfected with one of the five rescue plasmids indicated in (a) (*p < 0.05; **p < 0.01). e PLA using CHAMP1 and CENP-B antibodies was performed on Hela cells treated with Control or CDYL2 siRNA, with co-transfection of either pcDNA3-Gal4 or Gal4-CDYL2-FL expression plasmid, as indicated. Representative images are shown. The experiment was repeated three times with similar results. f Hela cells were treated as in panels (c) and (d), fixed, and PLA assay for CHAMP1 proximity to CENP-B was performed, as in (e). Shown is the mean number of PLA dots per nucleus of three independent assays and S.D. T test compared each condition to the siCDYL2/G4 sample (***p < 0.001)
We next used the same series of Gal4-tagged CDYL2 mutants to determine which domains were required for its regulation of mitosis and genome stability. This assay was performed by co-transfecting Hela cells with siRNA targeting the 3′ untranslated region (3′ UTR) of the endogenous CDYL2 mRNA and either an empty vector or the Gal4-CDYL2 cDNA mutant series (Fig. 6c; Online Resource 21). Nuclear localization of the various Gal4-CDYL2 constructs was confirmed by anti-Gal4 IF (Online Resource 22). IF analysis was then performed on cells co-transfected with the indicated combinations of siRNA and Gal4 constructs (Fig. 6d). Cells were pre-treated for 2 h with MG132 prior to fixation. The metaphase cells were evaluated for the presence of misaligned chromosomes and supernumerary spindle poles. In addition, interphase cells were evaluated for the presence of more than one nucleus, poly-lobed nuclei, fragmented nuclei, and micronuclei. As expected, siCDYL2 increased the incidence of all of the above phenotypes relative to the control siRNA (Fig. 6d, compare siCtrl/EV to siCDYL2/EV). Significant rescue was observed by co-transfection with Gal4-CDYL2 FL (Fig. 6d, compare siCDYL2/EV to siCDYL2/CDYL2 FL) but not the full-length CDYL2 with the triple mutation on the CD. In fact, among the deletion mutants tested, only Gal4-CD-L could rescue the effects of CDYL2 knockdown (Fig. 6d). These data indicate that the CDYL2 CD and central linker are required, and together are sufficient, to mediate CDYL2 regulation of mitosis and genome stability. Along with our other findings, this is consistent with the idea that both CDYL2 recruitment to H3K9me3-rich chromatin at centromeres and pericentromeres and binding to CHAMP1-POGZ are important for its regulation of mitosis and genome stability.
CDYL2 regulates CHAMP1 localization at centromeres
To determine if CDYL2 might regulate CHAMP1 localization at centromeres, we examined the effect of CDYL2 RNAi on CHAMP1/CENP-B PLA dot abundance. Relative to control siRNA, two siRNA targeting the CDYL2 ORF significantly reduced the number of nuclear CHAMP1/CENP-B PLA dots (Online Resource 23). A third siRNA that targets the 3-prime UTR of CDYL2 mRNA also diminished the number of CHAMP1/CENP-B PLA dots relative to a negative control siRNA, and in a manner that could be rescued by co-transfection with Gal4-CDYL2-FL construct but not an empty Gal4 plasmid (Fig. 6e). We then evaluated the ability of the same series of Gal4-CDYL2 constructs used in the previous analysis (Fig. 6a–d) for their ability to rescue the effects of siCDYL2 on CHAMP1/CENP-B PLA dot abundance. We found that only the full-length, WT CDYL2 and the CD-L construct could produce rescue effects (Fig. 6f). This indicates that CDYL2 positively regulates CHAMP1 enrichment at centromeres and that the CDYL2 chromodomain and linker regions are both necessary and together sufficient to mediate this activity. Furthermore, these results are consistent with the mitotic defects observed upon CDYL2 RNAi being at least in part due to impairment of CHAMP1 enrichment proximal to centromeres.
Fusion proteins linking the CDYL2 chromodomain to the N-terminus of CHAMP1 or POGZ do not rescue siCDYL2 effects on mitosis and genome stability, but independently induce nuclear aberrations
To further probe the requirement of CDYL2 as an adaptor protein that mediates the association between centromeres and CHAMP1/POGZ in a manner that depends on an intact chromodomain, we asked if CDYL2 CD-CHAMP1 or CD-POGZ expression constructs could rescue the effects of siCDYL2 on mitosis and genome stability in Hela cells. We observed that while the CD-CHAMP1 fusion protein could suppress the occurrence of certain of the siCDYL2 effects on mitosis and genome stability, notably chromosome alignment defects (Online Resource 24), the nuclei of these cells were not restored to normal morphology. Rather, the CD-CHAMP1 construct induced severe nuclear morphology abnormalities, in particular poly-lobed nuclei, as well as multinuclear cells, even in the absence of siCDYL2. By contrast, transgenic expression of the wild-type CHAMP1 was well tolerated in both siCDYL2 and siControl cells. This indicates that rather than rescuing the effects of siCDYL2, expression of the CD-CHAMP1 fusion protein independently induces nuclear morphology defects. Broadly similar results were obtained regarding the CD-POGZ fusion construct (Online Resource 25). Overall, due to the toxicity of the CD-fusion constructs, the results of these investigations were not informative relative to the question they sought to address.
Discussion
Though identified over two decades ago [15], the cellular and molecular functions of the CDYL family remain enigmatic. This is particularly the case for CDYL2, which we identified as a candidate oncogene in breast cancer whose upregulation promoted cancer cell plasticity [22]. Here we investigated the effects of loss of function of CDYL2 in the ER + /HER2- breast cancer cell line MCF-7, and also in Hela cervical cancer cells, a common model for mitotic studies. This revealed a requirement for CDYL2 for the growth of both cell lines. Genome instability and mitotic defects were induced by CDYL2 RNAi using several independent reagents, offering a likely cause for these growth defects. This prompted us to investigate CDYL2 sub-cellular localization through the cell cycle, indicating bulk removal from chromosomes during mitosis, with a minor fraction remaining associated with pericentromeres. CDYL2 enrichment on the mitotic spindle was also observed under specific fixation conditions. H3K9me3-dependent recruitment of CDYL2 at pericentromeres was revealed using Suv39h1/2 knock-out MEF, which has drastically reduced levels of this histone modification at the major satellite repeats [7]. The localization of CDYL2 to pericentromeres and the mitotic spindle suggested that it might regulate mitosis by interacting with other proteins that are enriched at these sites, though no such candidates were known. Using an unbiased IP-MS approach, we identified two candidate CDYL2 interactors, CHAMP1 and POGZ, whose subnuclear localization overlaps with that of CDYL2 and that regulate mitosis [29, 32]. CDYL2 bound to CHAMP1 and POGZ via its central, non-conserved linker region, as revealed by domain-mapping interaction studies. Meanwhile, RNAi rescue assays demonstrated that both an intact CDYL2 chromodomain and the linker region are required, and together sufficient, to mediate both the CDYL2 mitotic regulatory function and CENPB-proximal enrichment of CHAMP1. Based on these data, we propose that CDYL2 functions as an adaptor that binds to H3K9me3 via its chromodomain, and with CHAMP1 and POGZ via its central linker region, thereby participating in the coordination of molecular interactions at pericentromeres and centromeres that ensure mitotic fidelity.
These findings extend our understanding of how pericentromeric H3K9me3-enriched chromatin contributes to mitotic regulation. Previous studies largely focused on the roles of HP1 and its orthologs, which are required for pericentromeric heterochromatin compaction and mechanical stiffness [38–40]. Pericentromeric HP1 was also shown to regulate mitosis by recruiting Haspin and the Aurora-containing CPC, kinases that regulate several aspects of mitosis [8, 9]. These and other studies illustrate that both pericentromeric H3K9me3 and HP1 proteins are pleiotropic regulators of mitosis, but the potential contributions of other H3K9me3 readers to mitotic regulation are poorly understood. Previous studies of the CDYL2 chromodomain revealed that it binds to H3K9me3 with similar affinity and specificity compared to HP1 chromodomains, and with equivalent sensitivity to phosphorylation of the adjacent H3S10 residue [11–14]. Consistent with these in vitro studies, we observed displacement of CDYL2 from mitotic chromatin that was similar to that reported for HP1, and which was shown to be due to mitotic H3S10 phosphorylation [11]. A further parallel between CDYL2 and previous reports of HP1 is the association with POGZ and CHAMP1 proteins [14]. However, our findings indicate that the roles of CDYL2 and HP1 proteins in mitotic regulation are not redundant, as CDYL2 knockdown was sufficient to induce severe mitotic phenotypes and genome instability, as well as diminished CHAMP1 recruitment proximal to centromeres.
Mitotic regulation by CDYL2 via interactions with CHAMP1 and POGZ could be conserved among chordates that possess all three genes. However, CHAMP1 and POGZ orthologs are not found in lower species. By contrast, CDYL2 orthologs containing both conserved domains are also found in echinoderms, including the purple sea urchin, implying CHAMP1/POGZ-independent functions in these species. These might include CDYL2 transcriptional regulation, which we previously showed to depend on histone methyltransferase recruitment [22]. This also indicates that CDYL2 is pleiotropic. A distant CDYL2 ortholog, HIPP1, was identified in Drosophila melanogaster [41]. However, as HIPP1 lacks the N-terminal chromodomain, and flies do not have CHAMP1 or POGZ orthologs, it is not possible that HIPP1 regulates mitosis via the mechanism we describe here.
The CDYL2 chromodomain and non-conserved linker region were found to be necessary and, together, sufficient for both CDYL2 mitotic regulation and recruitment of CHAMP1 proximal to centromeres. Because mutation of the aromatic cage residues in the CDYL2 chromodomain abrogated the ability of otherwise full-length CDYL2 to rescue RNAi mitotic phenotypes, the association of CDYL2 with pericentromeric H3K9me3 is likely required for its mitotic regulation. Surprisingly, the conserved crotonase-like domain of CDYL2 was not required for the rescue of the siCDYL2 mitotic phenotypes or CHAMP1 recruitment at centromeres. This implies that neither the previously described crotonyl CoA hydratase activity of this domain nor its potential to mediate CDYL2 homotrimerization is required for CDYL2 regulation of mitosis. Rather, we found the linker region to be required for mitotic regulation, and sufficient to mediate CHAMP1-POGZ interaction. This suggests that CDYL2 primarily functions as an adaptor protein during mitotic regulation, facilitating the recruitment of CHAMP1 at centromeres. However, given the conservation of the CDYL2 crotonase-like domain, it is likely this is required to mediate other CDYL2 activities. By analogy to CDYL/CDYL1 [18], these could include transcriptional regulation via indirect effects on histone lysine crotonylation, though this remains to be demonstrated.
CHAMP1 and POGZ both regulate mitosis and genome stability [29, 32], and both are recurrently mutated in individuals with neurodevelopmental disorders, notably intellectual disability (ID), autism, and epilepsy [30, 33, 34]. While the mitotic roles of these proteins remain poorly understood, their biochemical association with each other and the similar clinical presentations in patients containing mutant copies of either gene suggest they may co-regulate certain processes. A mitotic function for CHAMP1 was indicated by its enrichment at kinetochores and the mitotic spindle, and CHAMP1 RNAi was found to induce aberrant chromosome attachment at kinetochores and loss of mitotic spindle stability, resulting in severe chromosome misalignment during mitosis [29]. Mitotic defects, including disorganized and multipolar mitotic spindles and chromosome alignment aberrations, were also found in lymphocytes isolated from an individual with ID who had truncating mutations of CHAMP1. This study also reported that siRNA targeting CHAMP1 induced misaligned mitotic chromosomes, multipolar spindles, and mitotic catastrophe in Hela cells [42]. The shared localization of CDYL2 and CHAMP1 at centromeres and the mitotic spindle, the overlap in mitotic phenotypes resulting from their respective RNAi knockdown, and the requirement of the CHAMP1-binding linker region of CDYL2 for its mitotic regulation suggest coordination of the functions of these proteins. Similarly, at least a subset of POGZ loss-of-function cellular phenotypes are similar to those observed upon CDYL2 or CHAMP1 RNAi [32], supporting the possibility that CDYL2, CHAMP1, and POGZ co-regulate certain processes during mitosis. Our studies indicate that these include regulation of chromosome alignment at metaphase and mitotic spindle stability.
While the current study shows that loss of CDYL2 function can provoke genome instability, a known cancer hallmark, in previous work we showed that CDYL2 upregulation in breast cancer cells was associated with oncogenesis. Studies with certain other centromere-associated proteins, including CENP-A, have shown that although they are required for genome integrity, their over-expression may also produce genome instability [43, 44]. Along these lines, we found that transgenic expression of CDYL2 in the normally low-expressing MDA-MB-436 breast cancer cell line could produce genome instability, at least when expressed at high levels (Online Resource 26). Hence, cells may be sensitive to CDYL2 levels, rather than simply its loss of function, though additional studies are required to determine if CDYL2 over-expression perturbs the same genome stabilizing mechanisms as we have revealed by loss of function studies here.
In summary, this study identifies a mitotic and genome stability regulatory function for CDYL2, a gene we and others previously implicated in breast cancer. The requirement of the CDYL2 chromodomain for this activity enlarges our understanding of the molecular roles of H3K9 trimethylation. CDYL2 interaction with CHAMP1 and POGZ suggests it may also participate in the mechanisms and cellular processes deregulated in neurodevelopmental disorders characterized by mutations in these genes. Finally, our study provides a rationale for the evaluation of inhibitors of either the CDYL2 chromodomain or linker region as anti-cancer therapies whose mechanism of action might include apoptosis arising from genome instability.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Mass spectrometry analysis was performed by The Taplin Biological Mass Spectrometry Facility (Harvard Medical School). We gratefully acknowledge Thomas Jenuwein, Max Planck Institute, Freiburg, Germany, for providing the double knock-out Suv39h1/2 (DKO) i-MEF used in this study. The Gal4-CDYL2 mutant plasmids were cloned by Marine Benaissa. We thank Patrick Lomonte (INMG-PGNM, Lyon, France) for providing the CENPB antibody. We acknowledge the contribution of SFR Santé Lyon-Est (UAR3453 CNRS, US7 Inserm, UCBL) CIQLE facility (a LyMIC member), especially Denis RESSNIKOFF, for their microscopy assistance and advice. We would also like to thank the reviewers for their constructive comments.
Author contributions
MS and ADD: investigation and conceptualization. BD: investigation. PM: conceptualization, funding acquisition, project administration, supervision and mentorship of other project members, investigation, formal analysis, writing of original and updated manuscript. LS: manuscript revisions and editing, funding acquisition.
Funding
This work was funded by grant number PLBIO2016-180 from l’Institut National du Cancer, grant number AJE20131128936 from La Fondation pour la Recherche Médicale, and financial support from l’Institut national de la santé et de la recherche médicale, France (Inserm).
Availability of data and materials
All relevant data are included in the manuscript and online resources. Aliquots of unique materials generated for the completion of this study will be made available to members of the scientific community upon reasonable request and in accordance with institutional materials transfer policies.
Declarations
Conflict of interest
The authors have no competing interests to declare.
Ethical approval and consent to participate
Not applicable to this publication.
Consent for publication
All authors have approved this publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hirano T. Chromosome dynamics during mitosis. Cold Spring Harb Perspect Biol. 2015;7:a015792. doi: 10.1101/cshperspect.a015792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews HK, Bertoli C, de Bruin RAM. Cell cycle control in cancer. Nat Rev Mol Cell Biol. 2022;23:74–88. doi: 10.1038/s41580-021-00404-3. [DOI] [PubMed] [Google Scholar]
- 3.Degrassi F, Damizia M, Lavia P. The mitotic apparatus and kinetochores in microcephaly and neurodevelopmental diseases. Cells. 2020;9:49. doi: 10.3390/cells9010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saksouk N, Simboeck E, Dejardin J. Constitutive heterochromatin formation and transcription in mammals. Epigenetics Chromatin. 2015;8:3. doi: 10.1186/1756-8935-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smurova K, De Wulf P. Centromere and pericentromere transcription: roles and regulation in sickness and in health. Front Genet. 2018 doi: 10.3389/fgene.2018.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almouzni G, Probst AV. Heterochromatin maintenance and establishment: lessons from the mouse pericentromere. Nucleus. 2011;2:332–338. doi: 10.4161/nucl.2.5.17707. [DOI] [PubMed] [Google Scholar]
- 7.Peters AH, O’Carroll D, Scherthan H, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 8.Ruppert JG, Samejima K, Platani M, et al. HP1α targets the chromosomal passenger complex for activation at heterochromatin before mitotic entry. EMBO J. 2018;37:e97677. doi: 10.15252/embj.201797677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi Q, Chen Q, Liang C, et al. HP1 links centromeric heterochromatin to centromere cohesion in mammals. EMBO Rep. 2018;19:e45484. doi: 10.15252/embr.201745484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs SA, Khorasanizadeh S. Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science. 2002;295:2080–2083. doi: 10.1126/science.1069473. [DOI] [PubMed] [Google Scholar]
- 11.Fischle W, Tseng BS, Dormann HL, et al. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–1122. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- 12.Fischle W, Franz H, Jacobs SA, et al. Specificity of the chromodomain Y chromosome family of chromodomains for lysine-methylated ARK(S/T) motifs. J Biol Chem. 2008;283:19626–35. doi: 10.1074/jbc.M802655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franz H, Mosch K, Soeroes S, et al. Multimerization and H3K9me3 binding are required for CDYL1b heterochromatin association. J Biol Chem. 2009;284:35049–59. doi: 10.1074/jbc.M109.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermeulen M, Eberl HC, Matarese F, et al. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell. 2010;142:967–980. doi: 10.1016/j.cell.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Lahn BT, Page DC. Retroposition of autosomal mRNA yielded testis-specific gene family on human Y chromosome. Nat Genet. 1999;21:429–433. doi: 10.1038/7771. [DOI] [PubMed] [Google Scholar]
- 16.Dorus S, Gilbert SL, Forster ML, et al. The CDY-related gene family: coordinated evolution in copy number, expression profile and protein sequence. Hum Mol Genet. 2003;12:1643–1650. doi: 10.1093/hmg/ddg185. [DOI] [PubMed] [Google Scholar]
- 17.Lahn BT, Tang ZL, Zhou J, et al. Previously uncharacterized histone acetyltransferases implicated in mammalian spermatogenesis. Proc Natl Acad Sci USA. 2002;99:8707–8712. doi: 10.1073/pnas.082248899082248899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Yu H, Liu Y, et al. Chromodomain protein CDYL acts as a crotonyl-CoA hydratase to regulate histone crotonylation and spermatogenesis. Mol Cell. 2017;67:853–866.e5. doi: 10.1016/j.molcel.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal. Nat Rev Mol Cell Biol. 2009;10:526–537. doi: 10.1038/nrm2727. [DOI] [PubMed] [Google Scholar]
- 20.Wu H, Zhang H, Wang P, et al. Short-Form CDYLb but not long-form CDYLa functions cooperatively with histone methyltransferase G9a in hepatocellular carcinomas. Genes Chromosom Cancer. 2013;52:644–655. doi: 10.1002/gcc.22060. [DOI] [PubMed] [Google Scholar]
- 21.Yang L-F, Yang F, Zhang F-L, et al. Discrete functional and mechanistic roles of chromodomain Y-like 2 (CDYL2) transcript variants in breast cancer growth and metastasis. Theranostics. 2020;10:5242–5258. doi: 10.7150/thno.43744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siouda M, Dujardin AD, Barbollat-Boutrand L, et al. CDYL2 epigenetically regulates MIR124 to Control NF-κB/STAT3-dependent breast cancer cell plasticity. iScience. 2020;23:101141. doi: 10.1016/j.isci.2020.101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Bu C, Liu Y, et al. Global crotonylome reveals CDYL-regulated RPA1 crotonylation in homologous recombination–mediated DNA repair. Sci Adv. 2020;6:eaay4697. doi: 10.1126/sciadv.aay4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulligan P, Westbrook TF, Ottinger M, et al. CDYL bridges REST and histone methyltransferases for gene repression and suppression of cellular transformation. Mol Cell. 2008;32:718–26. doi: 10.1016/j.molcel.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Lai S, Ma W, et al. CDYL suppresses epileptogenesis in mice through repression of axonal Nav1.6 sodium channel expression. Nat Commun. 2017;8:355. doi: 10.1038/s41467-017-00368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi C, Liu S, Qin R, et al. Coordinated regulation of dendrite arborization by epigenetic factors CDYL and EZH2. J Neurosci. 2014;34:4494–4508. doi: 10.1523/JNEUROSCI.3647-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin R, Cao S, Lyu T, et al. CDYL deficiency disrupts neuronal migration and increases susceptibility to epilepsy. Cell Rep. 2017;18:380–390. doi: 10.1016/j.celrep.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Wang Z, Zhao X, et al. STAT5A modulates CDYL2/SLC7A6 pathway to inhibit the proliferation and invasion of hepatocellular carcinoma by targeting to mTORC1. Oncogene. 2022;41:2492–2504. doi: 10.1038/s41388-022-02273-2. [DOI] [PubMed] [Google Scholar]
- 29.Itoh G, Kanno S, Uchida KSK, et al. CAMP (C13orf8, ZNF828) is a novel regulator of kinetochore–microtubule attachment. EMBO J. 2011;30:130–144. doi: 10.1038/emboj.2010.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asakura Y, Osaka H, Aoi H, et al. Intellectual disability and microcephaly associated with a novel CHAMP1 mutation. Hum Genome Var. 2021;8:1–3. doi: 10.1038/s41439-021-00165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hino M, Iemura K, Ikeda M, et al. Chromosome alignment-maintaining phosphoprotein CHAMP1 plays a role in cell survival through regulating Mcl-1 expression. Cancer Sci. 2021;112:3711–3721. doi: 10.1111/cas.15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nozawa RS, Nagao K, Masuda HT, et al. Human POGZ modulates dissociation of HP1alpha from mitotic chromosome arms through Aurora B activation. Nat Cell Biol. 2010;12:719–727. doi: 10.1038/ncb2075. [DOI] [PubMed] [Google Scholar]
- 33.Suliman-Lavie R, Title B, Cohen Y, et al. Pogz deficiency leads to transcription dysregulation and impaired cerebellar activity underlying autism-like behavior in mice. Nat Commun. 2020;11:5836. doi: 10.1038/s41467-020-19577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samanta D, Ramakrishnaiah R, Schaefer B. The neurological aspects related to POGZ mutation: case report and review of CNS malformations and epilepsy. Acta Neurol Belg. 2020;120:447–450. doi: 10.1007/s13760-019-01122-6. [DOI] [PubMed] [Google Scholar]
- 35.Mulligan P, Yang F, Di Stefano L, et al. A SIRT1-LSD1 corepressor complex regulates notch target gene expression and development. Mol Cell. 2011;42:689–99. doi: 10.1016/j.molcel.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H. Propidium iodide staining of cells for FACS analysis. Bio-Protoc. 2012;2:e195. [Google Scholar]
- 37.Uren AG, Wong L, Pakusch M, et al. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10:1319–1328. doi: 10.1016/S0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- 38.Strom AR, Biggs RJ, Banigan EJ, et al. HP1α is a chromatin crosslinker that controls nuclear and mitotic chromosome mechanics. eLife. 2021;10:e63972. doi: 10.7554/eLife.63972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canzio D, Chang EY, Shankar S, et al. Chromodomain-mediated oligomerization of hp1 suggests a nucleosome-bridging mechanism for heterochromatin assembly. Mol Cell. 2011;41:67–81. doi: 10.1016/j.molcel.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canzio D, Larson A, Narlikar GJ. Mechanisms of functional promiscuity by HP1 proteins. Trends Cell Biol. 2014;24:377–386. doi: 10.1016/j.tcb.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melnikova L, Molodina V, Erokhin M, et al. HIPP1 stabilizes the interaction between CP190 and Su(Hw) in the Drosophila insulator complex. Sci Rep. 2019;9:19102. doi: 10.1038/s41598-019-55617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamoto N, Tsuchiya Y, Kuki I, et al. Disturbed chromosome segregation and multipolar spindle formation in a patient with CHAMP1 mutation. Mol Genet Genomic Med. 2017;5:585–591. doi: 10.1002/mgg3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amato A, Schillaci T, Lentini L, Di Leonardo A. CENPA overexpression promotes genome instability in pRb-depleted human cells. Mol Cancer. 2009;8:119. doi: 10.1186/1476-4598-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeffery D, Gatto A, Podsypanina K, et al. CENP-A overexpression promotes distinct fates in human cells, depending on p53 status. Commun Biol. 2021;4:1–18. doi: 10.1038/s42003-021-01941-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included in the manuscript and online resources. Aliquots of unique materials generated for the completion of this study will be made available to members of the scientific community upon reasonable request and in accordance with institutional materials transfer policies.