Abstract
It is not often realized that the absolute protein specificity is an exception rather than a rule. Two major kinds of protein multi-specificities are promiscuity and moonlighting. This review discusses the idea of enzyme specificity and then focusses on moonlighting. Some important examples of protein moonlighting, such as crystallins, ceruloplasmin, metallothioniens, macrophage migration inhibitory factor, and enzymes of carbohydrate metabolism are discussed. How protein plasticity and intrinsic disorder enable the removing the distinction between enzymes and other biologically active proteins are outlined. Finally, information on important roles of moonlighting in human diseases is updated.
Keywords: Cancer, Ceruloplasmin, Crystallins, Glycolytic enzymes, Intrinsically disordered proteins, Metabolic reprogramming, Metamorphic proteins, Multitasking proteins, Neomorphic moonlighting proteins, Protein plasticity
Introduction
The text book level notion that enzymes are specific catalysts should be generally read in the context of comparing them with chemical catalysts. It is correct that many enzymes show power of very fine discrimination such as while being enantioselective. However, it is increasingly emerging that absolute specificity of an enzyme is an exception rather than a rule. Two important phenomena associated with non-specificity of enzymes are promiscuity and moonlighting [1–3]. Moonlighting by enzymes refers to actually what the word moonlighting means in English—doing an extra job in addition to the main job. This extra job can be at a different cellular localization or even outside the cell; it may involve differential expression in different cell types, or may be in response to change in the cellular flux of a ligand. Therefore, this kind of non-specificity depends upon the cellular context [4]. As discussed recently “three-dimensional (3D) structural features influence protein functions, in a cellular context” [5]. In 2019, Constance J. Jeffery emphasized that moonlighting is dictated by the cellular conditions [6].
It has been pointed out that “moonlighting enzymes can perform their multiple functions using distinct binding sites” [4]. It is rarely pointed out that strictly speaking, moonlighting also involves promiscuous binding; i.e., binding substrates, which are different from the normal substrates of the enzymes. However, enzyme promiscuity and moonlighting refer to different phenomena. One distinct feature of the moonlighting phenomenon is that the non-specificity of proteins cuts across the binary of enzymes and other biologically active proteins. As we will discuss below, some non-catalytic proteins turned out to moonlight as enzymes and vice versa! Furthermore, by moonlighting, proteins, which were considered just storage or structural proteins, have been shown to also possess specific catalytic activities.
The importance of moonlighting is highlighted by a continuous stream of reviews on this topic [3–24]. This review aims at (a) placing moonlighting in the broader perspectives of the idea of specificity and evolution of proteins; (b) updating information, and (c) focusing more on the emerging aspects, such as the role of intrinsic disorder in moonlighting and the importance of this phenomena in the context of pathogenesis and drug discovery and design.
Moonlighting by enzymes is an important example of the protein “multi-tasking”
As organisms became more complex, the genome size did not increase proportionately. The genetic code did not change, so the gene was expressing the same protein. Post-translational modifications (PTMs) in eukaryotes brought in some “multi-tasking”. Two main strategies, however, were protein promiscuity and moonlighting. PTMs were actually among the sub-strategies, by which promiscuity and moonlighting occurred. Protein plasticity and intrinsic disorder were other two such sub-strategies. Protein promiscuity of various kinds, substrate promiscuity, catalytic promiscuity, and promiscuous protein–protein interactions is a powerful strategy for multi-tasking [2, 25–28]. An extreme example is that of methane monooxygenase, which, besides hydroxylating methane, has 150 other substrates as well [2, 3].
These twin strategies—promiscuity and moonlighting—of multi-tasking were in place even before complexity of the organisms needed cellular economy of gene sharing. This is borne out by the fact that even Archaea had multi-functional enzymes [29]. The multi-tasking shows that the idea of “one gene–one protein–one function” was an over-simplification. However, invariably, the moonlighting form of the protein does have subtle structural changes. For example, mutations may occur, which favors the moonlighting activity over the other activity of the protein, which is being recruited. Oligomerization state may be different. The PTM status may be different. Therefore, generally the protein does differ after it gets recruited for the moonlighting function. So, while the case of intrinsically disordered proteins (IDPs) or hybrid proteins containing ordered domains and intrinsically disordered regions (IDRs) is different (please see later discussion); there is a change in the structure (even if it is not drastic) in line with the change in function. The remarkable feature is that so much functional change becomes possible with so little structural difference [4].
Joram Piatigorsky actually called this phenomenon “gene sharing” and described it as the “use of one gene encoding a protein that has two entirely different functions gene sharing [30]. This differs from the use of a single gene to generate more than one protein with different functions by alternative RNA splicing, DNA rearrangement, or posttranslational processing. It also differs from simple multifunctionality, in which different domains of a protein have different functions as the result of gene fusion or exon shuffling. Gene sharing means that a gene may acquire and maintain a second function without duplication and without loss of the primary function” [31]. Later on, the now popular term “moonlighting proteins” was coined by Constance J. Jeffery [4], who has also frequently reviewed the status of this area [6, 7, 22, 32–49].
The change in function with a change in cellular concentration of a specie is especially interesting. Aconitase at low iron concentration sheds its FeS cluster and becomes an RNA binding protein [4]. In some cases, the functions of the moonlighting proteins may be related or complementary. Some moonlighting proteins might have dual but related functions in intracellular and extracellular microenvironments [50]. However, in many cases two functions are totally unrelated. For example, a glycolytic enzyme rabbit phosphoglucose isomerase was shown to moonlight as autocrine motility factor, differentiation mediator, and neuroleukin [51]. In the following section, examples of moonlighting proteins with different features are discussed. While in some cases, original binding site is used [52–54], there are other cases, wherein moonlighting activity involves a different region of the protein [11, 21]. When an enzyme moonlights, its activity is likely to decrease. The extent of decrease may vary in different cases. For example, fumarate dehydratase gene acting as a tumor suppressor gene expresses the protein, which has practically no fumarate dehydratase activity [7].
Recent proteomic studies indicated that numerous metabolic enzymes may moonlight as RNA binding proteins [49]. Such interactions probably regulate the enzyme activity depending upon cellular dynamics. Of even broader significance are the roles of these proteins in connecting RNA metabolism and intermediate metabolism [49]. Brandon D. Moore reviewed moonlighting and bifunctional enzymes in the plant systems, indicating that the bifunctionality of the plant enzymes is typically defined by the presence of “two large structural domains whose association facilitates metabolic pathway control and/or allows more efficient substrate conversion”, whereas moonlighting enzymes have a recognized catalytic activity and “often have an alternate function in signal transduction pathways or as structural components” [9]. Furthermore, a possibility of the co-expression of the bifunctional enzymes with monofunctional forms was also pointed out [9]. The overall conclusion of this review was an important idea that “eukaryotes use multitasking activities to achieve their densely connected regulatory networks” [9]. Similar idea was depicted by Fabian M Commichau and Jörg Stülke who introduced a concept of trigger enzymes, which are defined as bifunctional proteins active in metabolism and in controlling gene expression [55].
Looking at a set of the 6799 literature-reported multifunctional proteins and their structural, functional, and evolutionary properties, Cheng et al. developed a combinatorial model of support vector machine and random forest model [56]. They found that charge, polarizability, hydrophobicity, and solvent accessibility were four important properties for predicting multifunctionality of enzymes. Using the resulting model, these authors identified 6956 potential novel multifunctional enzymes in the ENZYME database [56]. Interestingly, as Table 1 shows, bacteria have more multifunctional proteins than Archaea and Eukaryotes. What is more important, the authors indicated that on the evolutionary route from yeast to Homo sapiens, the level of multifunctional proteins in the organism showed no clear trend, with the multifunctional enzymes experiencing fluctuations of the gene gain and loss during the evolution [56]. It is worth speculating why less complex organisms would have moonlighting enzymes. Maybe moonlighting enables linkages between different biological pathways and are part of the integration and organization (maybe unknown at present). Also, while complexity may lead organisms to make enzymes multi-task, limitations and nature of the nutrients may have forced enzymes to evolve multiple activities for existing proteins. Unfortunately, there has been not much discussion on the relationship between moonlighting and promiscuity in the literature, although it is known that many moonlighting proteins are also promiscuous. As both promiscuous proteins and moonlighting proteins were included in these analyses, this study is highly relevant as a backdrop to our discussion of the moonlighting phenomenon.
Table 1.
Distribution of the multifunctional enzymes (MFEs) in four life domains of life
| Domain of life | Number of organisms | Number of enzymes | Average number of enzymes in each organism | |||
|---|---|---|---|---|---|---|
| MCD-MFEs | SMAD-MFEs | MCD-MFEs | SMAD-MFEs | MCD-MFEs | SMAD-MFEs | |
| Archaea | 40 | 36 | 71 | 66 | 1.78 (± 0.81) | 1.83 (± 1.81) |
| Bacteria | 590 | 380 | 4413 | 754 | 7.48 (± 6.18) | 1.98 (± 1.21) |
| Eukaryota | 143 | 120 | 633 | 270 | 4.43 (± 5.00) | 2.25 (± 1.74) |
| Viruses | 156 | 77 | 446 | 145 | 2.86 (± 2.50) | 1.88 (± 1.26) |
Totally, 5554 known MFEs of multiple catalytic/functional domains (MCD-MFEs) and 1274 known MFEs of single multi-activity domain (SMAD-MFEs) were included in the statistics. It was noted bacteria are superior in both total number and average number of known MCD-MFEs and SMAD-MFEs than other three domains. This Table is modified from Cheng XY, Huang WJ, Hu SC, Zhang HL, Wang H, Zhang JX, Lin HH, Chen YZ, Zou Q, Ji ZL. A global characterization and identification of multifunctional enzymes. PLoS One. 2012;7(6):e38979; https://doi.org/10.1371/journal.pone.0038979 [56], which is an open-access article distributed under the terms of the Creative Commons Attribution License that permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited
Perhaps the first report of detecting moonlighting activity by metagenomic approach was a study published in 2011 by Jiang et al. [57]. These researchers cloned a novel β-glucosidase gene that also had significant lipolytic activity through function-based screening of a metagenomic library from uncultured soil microorganisms [57]. Considering that lipases tend to be highly promiscuous class of enzyme, this also exemplifies that promiscuity and moonlighting activity are not unrelated.
As looking for moonlighting functions can be tricky, the strategies for prediction of such functions are valuable. A number of recent papers uses machine learning methods based on the available physicochemical and evolutionary data to identify novel moonlighting proteins [58, 59]. In fact, while most of the known moonlighting proteins were identified as such, the potential value of the approach was obvious as authors could predict that few proteins, which are at present are not known to be moonlighting, are likely to be moonlighting [58].
Protein specificity (or lack of it)
Our notion of enzyme specificity is rooted in the almost 130-year-old lock-and-key hypothesis [60], wherein enzymes were implied to be rigid molecules [61]. It has a bearing on the common confusion that binding affinity and protein specificity are sort of synonyms [62, 63]. To backtrack a little, enzyme specificity is commonly described in the context of selectivity between the two substrates. The enzyme selectivity is the ratio of their kcat/Km values, which itself is a measure of kinetic perfection. This assumes that the catalytic rate is diffusion limited, i.e., determined by the encounter between the substrate and the enzyme [61]. It has been pointed out that there is no straightforward correlation between binding affinity and protein specificity [62, 63]. Examples are known of high specificity, high affinity systems (e.g., some antigen–antibody interactions), high specificity, low affinity systems (e.g., repressor-regulator systems), low specificity, high affinity systems (e.g., MHC–peptide interactions), and low specificity-low affinity systems (nonspecific interactions of the DNA binding proteins with DNA). One exceedingly important example is those protein–protein interactions in signaling cascades, which are mediated by intrinsic disorder at least in one partner protein. There are couple of other considerations, when one mixes up between “selectivity” and “affinity”. Selectivity depends upon what is taken as a competing ligand. This is very relevant to moonlighting proteins, which show moonlighting by being present in different cellular localizations, where the milieu around the moonlighting proteins can be vastly different. The cases of accidental promiscuity (such as C–C bond formation catalyzed by lipase) is even more complex; by choosing a panel of substrates of very different kinds, one sees a different specificity. Neil S. Greenspan has argued that ΔG is a better measure of the inherent affinity, and ΔΔG can be taken as a parameter for selectivity [63]. Greenspan also has discussed that the often perceived direct correlation between specificity and binding affinity may have arisen from the host–guest systems in supramolecular chemistry, in which the rigidity of partners (or at least host molecules) requires that the efficient systems display both high affinity and high specificity [63]. That is how we may have misread the implications of rigid “lock-and-key” model as an analogy for the enzyme catalysis.
Furthermore, as many biological systems involve multivalent interactions, it is avidity (which is a measure of the total binding strength) and not binding affinity (which is the measure of the binding strength at a single binding site), which is important [64]. Also, the macromolecular crowding of the intracellular environments alters many biological and physicochemical properties of the biologically active proteins [65–67]. One obvious effect is the decrease in the diffusion of the substrate towards the enzyme. It has been mentioned that both translational and rotational diffusional rates of chymotrypsin inhibitor 2 were found to be lower in cell extracts as compared to the corresponding values for its solution in simple aqueous buffer [68]. A recent article discusses the complexity of how diffusion rates can influence specificity [69].
Ketudat Cairns et al. have pointed out some challenges in assessing specificity (or lack of it) of enzymes in the context of plant glucosidases but their observations are valid for all enzymes/proteins [70]. First of all, at the most, one can check activity with some related substrates (as many as readily available!). However, beyond that it often may tend to be a leap of imagination. That is why, in most of the cases, the discovery of moonlighting activities has been serendipitous. The second very important observation is about the use of synthetic substrates during purification and characterization of enzymes. While the switch to synthetic chromogenic or fluorogenic substrates has made life very convenient for enzymologists, their use can make one miss out on some highly specific enzymes. Two highly pertinent examples discussed in that work are worth mentioning [70]. Dhurrinase-1 from sorghum is highly specific for dhurrin and shows no activity for the frequently used synthetic substrate 4-nitrophenyl beta-d-glucopyronoside for β-glucosidase. Similarly, Arabidopsis scopolin β-glucosidase cannot be detected using this synthetic substrate [70]. This review also points out critical necessity to confirm that gene expression data is consistent with the function of a given protein. Another valuable insight is that solely relying on the specificity data based upon working with recombinant proteins can be sometimes misleading. Of particular relevance to moonlighting phenomenon is the discussion on challenges in correct localization of the enzymes and pitfalls associated with various techniques including those based on the computational approaches. Finally, the importance of using genetic approaches to complement assay data for assigning function is highlighted. β-Glucosidases provide a good illustration of how the active sites of different enzymes are subtly crafted to ensure the desirable catalysis, when multiple isoenzymes and multiple substrates occur together at the same place in the cell. Therefore, nature exploits both specificity and lack of it, while designing enzymes depending on the biological need [70].
A review by Alessio Peracchi deserves attention as it brings much needed clarity on several issues [71]. It points out that so many metabolic enzymes being involved in catalyzing different transformations is related to their kinetic imperfection and results from the presence or absence of selective pressure. The enzyme selectivity evolution can be due to the positive selection, negative selection, or neutral drift.
The issue of tradeoffs between selectivity and catalytic rates of an enzyme has been discussed by Dan S. Tawfik [72]. He has emphasized the importance of negative selection. The ground-state discrimination and transition-state discrimination, both seem to play a role. In a later article, Moshe Goldsmith and Dan S. Tawfik [73] have further pointed out that during evolution “the fitness peak represents the maximum possible catalytic efficiency of an enzyme for a particular reaction and substrate in a given region of sequence space” [73].
Peracchi [71] actually makes an interesting observation that as enzyme specificity is about discriminating between available substrates, moonlighting should not be considered as manifestation of non-specificity of enzymes! The enzymes at different locations do not encounter identical substrates. As an argument, this consideration is useful as it clearly distinguishes moonlighting from at least catalytic promiscuity (and promiscuity in protein–protein interactions) [71].
Another interesting point made by Peracchi is about why the selectivity of an enzyme cannot be absolute [71]. According to the transition theory, the selectivity relates to the difference between binding energies towards two substrates. The binding energies themselves are not very high, and their difference (which actually relates to the upper theoretical limit for discriminating power) also tends to be a finite number [71]. This argument, curiously, was originally given by Pauling way back in 1958 to explain the absence of absolute specificity of enzymes using the process of aminoacyl-tRNA synthesis as an example [74]. While evolution of enzymes may have turned generalists into specialists, it is almost never beyond a limit. If promiscuity (non-specificity) is desirable for fitness, it survives. Digestive enzymes, detoxifying enzyme cytochrome P450, branched chain transaminase, and transketolase are cited as examples [71]. In other cases, low level promiscuity is potentially useful for further evolution or generating new catalytic activity to deal with xenobiotics [71].
It should also be added that many techniques like protein engineering and directed evolution enable altering protein specificity. Lesser known is a technique called bioimprinting which can not only alter/ enhance protein specificity but even create moonlighting activity. Such alteration of biological activities by this technique survive only in low water systems such as nearly anhydrous organic solvents [75]. Braco et al. used “molecular imprinting” phenomenon (i.e., “when a protein is dissolved in a concentrated aqueous solution of a multifunctional organic compound, freeze-dried, and washed with an anhydrous organic solvent to remove the ligand”) to convert bovine serum albumin to a receptor for predetermined low molecular organic compounds of abiotic origin, which showed dramatic increase (up to 30 fold) in binding affinity of the template compound in anhydrous solvents than the non-imprinted protein [76]. It is important to emphasize here that this moonlighting activity is artificial and has no biological relevance. Bioimprinting, however, could enhance conditional promiscuity in several cases, such as subtilisin and lipases [77–79].
One should be cautious in not confusing broad specificity with moonlighting. Alcohol dehydrogenases can act on a range of alcohols as the binding site can accommodate substrates of various sizes. The obvious criterion is to check if the nature of biological (catalytic in this case) is different. An interesting example is that of protein kinases that may phosphorylate similar sequences of specific amino acids in many proteins. Considering that it is the same catalytic activity, this does not exemplify moonlighting activity.
Protein plasticity and its role in specificity and evolution
There are few terms related to protein conformation and protein function, which are used interchangeably, but differ subtly in their nuances. Conformational diversity implies that the old concept of a unique native structure for a protein needs revision. A protein exists in several conformations (populated differently) in equilibria. Presence of a ligand or any stress factor (such as high temperature) can shift the equilibria among these different conformations [80, 81]. It is believed that a ligand binds preferably to one or some of these conformations. In other words, substrate/ligand shifts the conformational equilibria in favor of the conformation(s) which have higher affinity towards the ligand/substrate [82]. This is sometimes called pre-equilibrium model [80]. This favors protein evolution, as one of the other conformations may be favored by a different substrate/ligand. This model links conformational diversity with functional diversity implying that promiscuity (non-specificity) or its potential inherent in most proteins to a different extent originates from this conformational diversity. Cross-reactivity is a term, which is mostly used in the context of molecules related to immune system (and vaccines) and refers to their non-specificity in binding. In a way, it is analogous to broad specificity of enzymes as binding partners (such as antigens) are structurally similar. Protein flexibility implies lack of rigidity. It increases with temperature. The induced fit mechanism for enzyme catalysis pre-supposes a flexible protein structure—unlike the rigid structure implied by the analogy of lock in the old “lock-and-key” hypothesis. It is of considerable importance in applied biocatalysis [83].
The role of enzyme flexibility/conformational dynamics in non-specificity has been discussed at several places [83, 84]. Zho et al. have mentioned that β-lactamases are actually ancient enzymes, which were there even several billion years ago [84]. The modern TEM-1 enzyme specific for penicillin has a much more rigid active site region as compared to the ancient enzymes. Therefore, pathogens are easily able to evolve more flexible and promiscuous β-lactamases to come up with antibiotic resistance [84]. This is also a good example, which supports the hypothesis that in many cases, enzymes have evolved to become specialist from generalists. As discussed, however, the potential scope for non-specificity has been retained in most of these specialists enzymes.
Another term conformational plasticity means that the protein conformation is adaptable. The twin roles of protein plasticity in exploiting the same binding site to bind to different ligands (“converging binding sites”) and allow mutations during evolution have been discussed by Eric J. Sundberg and Roy A. Mariuzza [85]. A great example of this phenomenon is given by the Fc fragment of IgG, which is believed to show most cross-reactivity/non-specificity with respect to binding to multiple ligands due to the plasticity of the binding site located between its CH2 and CH3 domains, which is capable of interaction with at least four different natural protein scaffolds [86].
In fact, the molecules of the immune system such as antibodies, receptors of T-cells and NK cells provide a good example of how nature facilitates multispecificity [87]. Contrary to general perception, “rigid adaptation” is known wherein a receptor is able to be cross-reactive without any change in the conformation (or the bonding region). Of course, induced fit is also quite common. A third strategy seems to operate in some cases. In this “differential ligand positioning”, the same antibody conformer bound to different antigenic peptides “at spatially different regions of the binding site” [87]. In the example cited, the antibody showed conformational diversity but used induced fit to a new conformer [the binding did not select any of the pre-existing conformers in the unligated form. What is interesting is that identical induced fit occurred in the case of different antigenic peptides. In as much as induced fit was involved, protein flexibility did play a role in this mode of achieving non-specificity [87].
A good discussion on exploiting protein plasticity for generating novel enzymes is available in a recent article by Crean et al. [88]. The authors emphasized that conformational dynamics plays important roles “both in the evolution of new enzymatic activities from existing enzymes and in facilitating the emergence of enzymatic activity de novo on scaffolds that were previously non-catalytic [88].
How protein dynamics and intrinsic disorder influences moonlighting?
Having discussed the role of conformational plasticity in multi-specificity of proteins, in this section, we will briefly consider its role in protein moonlighting in particular. In an insightful article, Mario A. Fares had listed protein plasticity as one of the important feature to look for in enabling moonlighting by a protein [14].
In budding yeast, long-term exposure to increased temperature was found to lead to a different conformation, subcellular re-localization, and a moonlighting function [89]. To test the changes in the Saccharomyces cerevisiae proteome associated with adaptation to the persistent high temperature, the authors compared proteomes of the yeast cells cultured at 21 °C and 35 °C. This analysis revealed that the abundance of more than 700 proteins significantly changed between these two temperatures, reflecting attenuation of a broad spectrum of biological processes [89]. Furthermore, subcellular localization of ~ 100 proteins was changed as well, reflecting a new level of functional adaptation to high temperature, as the subcellular localization is serving as a determinant of protein function. The authors also saw reduced levels of the thermolabile proteins, which likely minimizes protein misfolding/unfolding and aggregation at high temperature [89]. Analysis of the conformational changes based on the resistance of proteins to the limited proteolysis by proteinase K (PK) showed that the proteolytic susceptibility of 228 proteins noticeably changed between two temperatures [89]. Furthermore, for 61 proteins, the authors showed that the same polypeptides at two temperatures can exist in stable alternative conformations [89]. Based on these observations, the authors suggested that “conformational plasticity allows some polypeptides to acquire new biophysical properties and functions” [89]. A more focused analysis of Fet3p, which is a multicopper oxidase (MCO)2 found in Saccharomyces cerevisiae, revealed that at 21 °C, this protein was present in endoplasmic reticulum, whereas at 35 °C, it moved to plasma membrane, where it functioned as ferroxidase. It was also pointed out that Fet3p produced at different temperatures showed different conformational stability and structural properties, and the protein at two cellular locations had different levels of glycosylation [89].
The role conformational change in moonlighting also finds mention in the case of neomorphic moonlighting proteins. The term “Neomorphic moonlighting proteins” was introduced by Constance J. Jeffery, who stated that a “neomorphic moonlighting function” is a specific biochemical function (catalytic activity, binding activity, etc.) of a protein because of the mutations or changes in protein–protein interactions, or a deleterious change in the conformation of the polypeptide chain [37]. In that article, the author primarily dealt with the cases, where the mutation and the change in conformation led to a disease [37].
In recent years, the phenomenon of fold-switching has been observed, in which the same amino acid sequence can exist in two or more differently folded conformations [90]. Such proteins switch folds in “a process that involves remodeling of secondary structure in response to a few mutations (evolved fold switchers) or cellular stimuli (extant fold switchers)” [90]. An illustrative example of an extant fold switcher is given by glutathione oxidase that was shown to switch fold under the oxidizing conditions and functioned as a chloride channel [90]. The authors also pointed out that although one can expect that 0.5–4% of proteins reported in PDB can switch folds, the “extant fold-switching proteins are likely more common than the PDB reflects”, indicating that such fold-switchers might have multiple physiological and pathological functions [90]. The term “metamorphic proteins” has been used for proteins which moonlight via fold-switching [91]. Some examples of the metamorphic proteins are given by human lymphotactin, which is a member of the XC family of chemokines that undergoes a dramatic conformational rearrangement from the canonical α + β chemokine fold preferentially populated at 10 °C and 200 mM NaCl, to the dimeric all-β fold that is preferred at 40 °C in the absence of salt, with both forms being equally populated at physiological conditions [91], a caseinolytic metallopeptidase selecase that “reversibly transits between several different states of defined three-dimensional structure, which are associated with loss of enzymatic activity due to autoinhibition” [92], and HIV-1 reverse transcriptase that can adopt three different structures [93].
The intrinsic disorder is sometimes described as an extreme case of protein plasticity. That of course is an over-simplification bordering on inaccuracy. While flexibility is a trade-off trait with other properties, such as stability, catalytic rates, and enantio-selectivity [83, 94, 95], in the case of intrinsic disorder, function often depends on the lack of structure [95–106]. Flexible enzymes have pre-formed active/binding site, which may undergo induced fit in the presence of substrate/ligands. In case of intrinsically disordered proteins, there is no pre-formed binding site in the absence of the substrate/ligand. However, there is a pre-existing proneness for disorder → order transition, which favors binding [107–114].
Another important distinction between flexibility and intrinsic disorder is how the specificity/binding affinity is impacted [97, 104, 115–117]. Flexibility quite often is exploited to increase specificity, intrinsic disorder leads to faster kinetic off rates by decreasing binding affinity. As discussed earlier, the relationship between specificity and binding affinity is not quite straightforward. Therefore, so more data are needed to gain further clarity on this point. Furthermore, as mentioned elsewhere recently during a discussion of this point [95], cases are also known, where the disorder → order transition during the binding of a partner leads to the ultra-high binding in the picomolar range as enthalpic effect overcompensate the entropic factor [118].
There are proteins which are intrinsically disordered (IDPs); others have intrinsically disordered regions (IDRs). A number of reviews and books are already available on IDPs and on proteins which have IDRs [96, 97, 99, 105, 119–122]. Therefore, we will limit ourselves to the discussion if, like protein plasticity, intrinsic disorder also facilitates moonlighting activities of proteins.
Chapple et al. used protein–protein interaction network to identify what they refer to as “extreme multi-functional proteins” (EMFs), which carry out “dissimilar activities” and pointed out that the classical moonlighting proteins may be a subset of these EMFs [123]. However, only 6 out of 39 known human moonlighting proteins could be identified by their approach. This merely reflects the fact that any search for moonlighting proteins based solely on the bioinformatics approach may not be able to identify all moonlighting activities. Their conclusions are that while the EMFs are rich in eukaryotic linear motifs (which are part of the hierarchy of intrinsic disorder elements), these proteins contain less disorder as compared to hub proteins [123]. That, of course, makes sense, as hub proteins rely on the flycasting behavior (enabled by disorder) to have multiple partners, often at the same time.
Earlier, Hernandez et al. looked at a limited number of 28 proteins and found only three having long IDRs [124]. While intrinsic disorder may not be always responsible for moonlighting activity of a protein, there are increasing number of examples being reported in the literature that it does facilitates moonlighting. Many moonlighting proteins were found to be either IDPs or have IDRs [125]. These authors listed 11 moonlighting proteins which were either IDPs or had IDRs. The three mechanisms were identified, by which intrinsic disorder facilitates moonlighting. One in which IDP binds to the same ligand (with different biological consequences) in two different conformations or via two different binding sites. In second mechanism, IDP/IDR can act as a chaperone or favor a conformation of the partner, which becomes active at a location after PTMs or limited proteolysis. The third mechanism consists of two alternate conformations are possible during binding to the partner or via different though overlapping binding sites [125]. The first mechanism is exemplified by CFTR, wherein the regulatory domain was intrinsically disordered and its phosphorylation resulted in the protein becoming active chloride channel. The interaction of the enzyme inhibitor calpstatin with the protease calpain involves a structural reorganization and thus follows second mechanism. PIAS-1 moonlights via third mechanism, its moonlighting activities include transcription inhibition and activation of p53-mediated gene expression in different cell types. The authors also discussed examples, where promiscuity and moonlighting co-occur in quite a few cases [125].
The analysis of three known moonlighting proteins showed the presence of intrinsic disorder in them, GADPH, HSP60, and p53 possessing 12.24%, 36.3%, and 68.19% disordered residues, respectively [23]. In line with these observations, it was pointed out that “p53 serves as an important illustration of the protein structure–function continuum concept, where the generation of multiple proteoforms by various mechanisms defines the ability of this protein to have a multitude of structurally and functionally different states” [126]. Importantly, based on the accepted in the field approach, were two cutoffs for the levels of intrinsic disorder (as measured by the percent of predicted disordered residues (PPDR) in query proteins) are used to classify proteins as highly ordered (PPDR < 10%), moderately disordered (10% ≤ PPDR < 30%) and highly disordered (PPDR ≥ 30%) [127], even the most structured of these three moonlighting proteins, GADPH with its 12.24% of disordered residues, is still classified as moderately disordered.
The ribosomal proteins have at least 30 moonlighting activities [128–133]. The bioinformatics analysis of 3,411 ribosomal proteins from 32 different species found that many activities of these proteins, such as binding to metal ions and RNA, autoregulation, apoptosis, and protein–protein interactions exploited their IDRs [134]. A recent paper described the potential of ribosomal proteins as antimicrobials [135]. Not only ribosomal proteins, but RNA binding proteins in general have many moonlighting activities, in which their IDRs play a role [136]. These proteins use different modules to bind to the nucleic acid [137]. The linkers between these modules are IDRs that “decide” which module(s) will be involved in binding to RNA in different cases [137]. IDRs actively identified with moonlighting activities were called “disordered moonlighting regions” (DMRs) by Fanchi Meng and Lukasz Kurgan [138]. These authors developed DMRpred, a predictor based on random forest model, which can predict DMRs based upon amino acid sequence with about 82% accuracy [138].
Another important class of moonlighting proteins are 14–3–3 proteins, named so as they eluted in the 14th fraction during the ion exchange chromatography and were detected as 3.3 band on the electrophoretic gel [139, 140]. These important proteins act as can be found in all eukaryotes and are known to interact with more than 200 protein partners which include signal transduction enzymes kinases and phosphatases and many transmembrane receptors [141]. In the most cases, binding sites of the 14–3–3 partners are intrinsically disordered [141]. Curiously, seven human 14–3–3 isoforms, which are encoded by distinct genes and which recognize phosphorylated motifs within numerous human and viral proteins, bind their targets with different affinities that follow “an ordered affinity ranking with conserved relative KD ratios”, which “allows predicting proportions of 14–3–3 isoforms engaged with phosphoproteins in various tissues” [142]. It was also pointed out that 14–3–3 proteins themselves possess a hidden intrinsic disorder propensity, with their N-terminal segment being disordered in the monomeric form, but undergoing disorder-to-order transition at the formation of the homo- or heterodimers, where it exists as the first α-helix [143].
Some interesting examples of moonlighting proteins
A comprehensive list of moonlighting proteins can be found in the MoonProt database, which was created in 2014 [144] and has been updated from time to time [145, 146]. In the latest release of the MoonProt database, there are over 500 moonlighting proteins [146]. Another database, which represents a repository of multitasking proteins found in the literature, and which was also created in 2014, is MultitaskProtDB [147]. Similar to MoonProt, this database was updated in 2017, when the number of its entries increased from 288 to 694 moonlighting proteins [148]. Analysis of the MultitaskProtDB-II revealed that 78% of its entries are involved in human diseases, and in almost 50% of these disease-related moonlighting proteins, both the canonical and moonlighting functions were shown to be linked to the molecular basis of the corresponding diseases [149]. Furthermore, it was also emphasized that 25% of the moonlighting functions ascribed to proteins in the MultitaskProtDB-II database are related to the pathogen virulence activity, with many corresponding moonlighting proteins possessing a canonical function associated with the highly conserved ancestral key functions, and with their moonlighting functions being often associated with the induction of the remodeling of the extracellular matrix (ECM) proteins [150].
Few examples of moonlighting proteins are discussed below in more details. It should be added that in spite of so much literature on moonlighting proteins, it is not always straightforward to categorically call a particular activity as moonlighting. As a careful perusal of following discussion would show, in some cases it may be difficult to say whether a second activity is completely of a different nature or is a downstream consequence of the same activity. Many biological processes, such as redox homeostasis, malignancy or inflammation are complex processes, and all the fine details are not necessarily always known in all systems.
Ceruloplasmin
Ceruloplasmin clearly deserves a brief discussion, both because probably it was the first protein whose multi-functionality was reported and also because of its considerable biological importance in many contexts [151]. For the first time, it was purified in 1948 from the alpha 2 globulin fraction of human serum, and its oxidase activities with catechol and aromatic diamines was detected [152]. It had a blue color and hence was given its descriptive name “ceruloplasmin”, which means “a blue material in plasma”. The blue color of the protein solution was due to the presence of copper. Its first physiological involvement was in acute phase protein, whose levels varied during many diseases and hormonal imbalances. Since the acute phase proteins are a part of the innate immunity, this led to considerable interest in this protein. However, as pointed out by Pamela Bielli and Lilia Calabrese, multiple roles of ceruloplasmin in various metabolic and physiological processes were rather slow to emerge [151]. Some early involvements were in “copper transport, iron homeostasis, biogenic amine metabolism, and defense against oxidative stress” [151]. Aceruloplasminaemia was discovered as a condition, in which the protein is totally absent in the blood. This is a fatal disease, wherein there is an overload of iron in tissues that leads to diabetes, retinal damage, and neurodegeneration [153, 154]. Ceruloplasmin is a multicopper oxidase converting dioxygen to two molecules of water. Multicopper oxidases are believed to have evolved from the ancient protein cupredoxin. It is believed to be the most evolved protein among those, which aerobic cells contain copper and iron in response to environments containing these reactive metals [155].
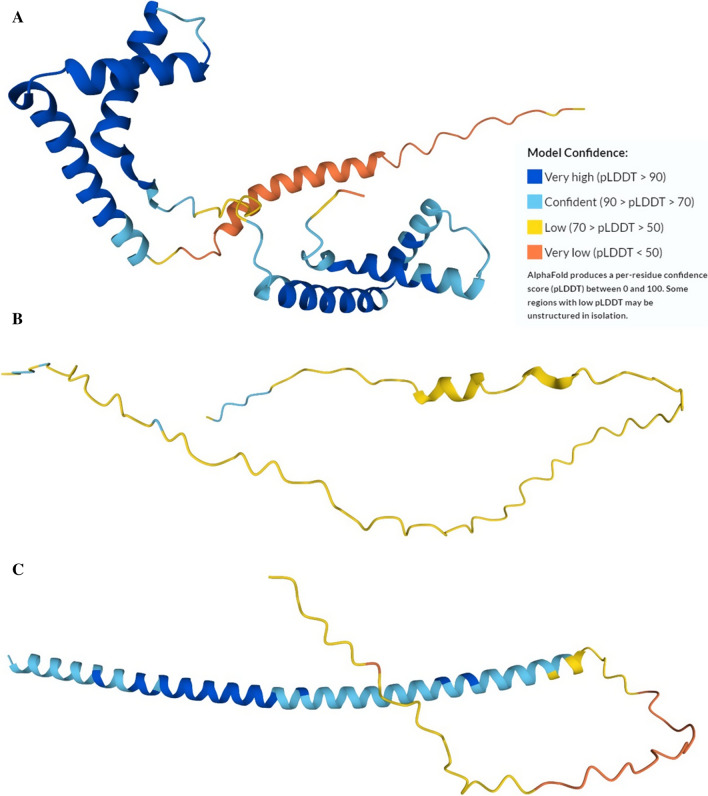
Ceruloplasmin also has ferroxidase activity and links copper and iron metabolism. Whereas other multicopper oxidases (such as ascorbate oxidase) typically have 3 domains, ceruloplasmin, a 1,065-residue-long protein has 6 compact plastocyanin-like domains (residues 20–200, 209–357, 370–560, 570–718, 730–900, and 908–1061), which are assembled in three F5/8 type modules (residues 20–357, 370–718, and 730–1061) and 6 tightly bound copper ions [151]. Figure 1A represents a 3D structure modeled for this protein by AlphaFold and shows that ceruloplasmin is characterized by mostly well-folded structure. However, there are several regions, structures of which are predicted with low or even very low confidence (e.g., residues 1–19, 358–370, 495–502, 719–730, 903–912, and 1059–1061), with most of these regions being located between the plastocyanin domain and likely serving as flexible linkers. In line with this hypothesis, Fig. 1B shows that all linkers between domains are predicted to be disordered. Thomas L. Ortel, Nobuhiro Takahashi, and Frank W. Putnam have discussed the structure of human cerluloplasmin based on the X-ray diffraction and circular dichroism (CD) data and concluded that “ceruloplasmin had approximately equal amount of β and random structure and a small α-helix content” [156]. Hence AlphaFold, which is used here to model structure of cerluloplasmin is at least not over-estimating the intrinsic disorder content of the protein.
Fig. 1.
Structure and intrinsic disorder in a moonlighting enzyme human ceruloplasmin (UniProt ID: P00450). A 3D structure modeled by AlphaFold [253]. Structure is colored by the confidence of structural prediction, where blue and cyan colors correspond to regions predicted with very high and high confidence (i.e., corresponding regions are characterized by the per-residue confidence scores (pLDDT) of pLDDT > 90 and 70 < pLDDT < 90, whereas segments colored in orange and yellow show regions with low and very low confidence (50 < pLLDT < 70 and pLDDT < 50, respectively). It was noted that the regions with low pLDDT scores may be disordered in the unbound state [253]. B Multiparametric intrinsic disorder profile generated by a high-efficiency web-based disorder predictor Rapid Intrinsic Disorder Analysis Online (RIDAO) [254]. RIDAO assembles outputs of six commonly used per-residue disorder predictors, such as PONDR® VLXT, PONDR® VL3, PONDR® VSL2, PONDR® FIT, IUPred_long, and IUPred_short [255–260]. Tool also represents a mean disorder profile (MDP) calculated by averaging of the disorder profiles of individual predictors. Light pink shade represents MDP error distribution. The thin black line at the disorder score of 0.5 is the threshold between order and disorder, where residues/regions above 0.5 are disordered and residues/regions below 0.5 are ordered. The thin dashed line at the disorder score of 0.15 is the threshold between order and flexibility, where residues/regions above 0.15 are flexible and residues/regions below 0.15 are highly ordered. Gray areas indicate positions of six plastocyanin domains
Coming to its multi-tasking, the role of this protein in copper transport is debatable since copper metabolism is normal in aceruloplasminaemia patients, though its role in the iron metabolism is more clearly established. Ceruloplasmin displays oxidase activity towards ferrous ion, diamines, amino-phenols, catechols, and 5-hydroxyindoles [151]. It has glutathione peroxidase activity, where copper ions of the protein are not involved. It participates in the lipid oxidation and oxidative damage to low density proteins. It participates in NO metabolism. All these though can be considered under broad specificity of an oxidase. The moonlighting activities of ceruloplasmin are no less noteworthy. In liver cells, it is a part of the acute phase response. In lungs, it combats hypoxia. In the case of many cancers, the levels of the ceruloplasmin production increase, and this supports malignancy. In brain, it is exclusively present as a membrane-anchored protein and is adapted to the metabolic needs of this organ, which does not have access to the protein present in rest of the body due to the blood–brain barrier. Ceruloplasmin plays important role in dealing with xenobiotics. Its moonlighting may be the oldest example, but ceruloplasmin is definitely a protein, which ticks all the boxes about the definition of moonlighting proteins [151, 157].
Crystallins
One of the earliest examples of moonlighting behavior of proteins is the eye lens proteins crystallins [31, 158–166], which account for 80–90% of the water-soluble proteins of the transparent lens and which are responsible for the optical properties of the lens. It was found that crystallins in mammals, geckos, birds, etc., were the same as some cytosolic enzymes [161]. Therefore, being expressed outside of the lens, crystallins have non-refractive roles. The crystallins from the different sources were shown to have different enzyme activities. The duck epsilon–crystallin showed lactate dehydrogenase activity (LDH), whereas turtle crystallin had enolase activity. Some other enzyme activities displayed by crystallins include arginosuccinate lyase, retinaldehyde dehydrogenase, glyceraldehyde 3-phosphate (GADP), and quinone oxidoreductase. Besides, some crystallins also were found to function as heat shock proteins [161]. It was notable that “the close relationship of taxon-specific crystallins to their respective enzymes” was observed [31]. This feature of crystallin moonlighting was confirmed later on [167]. It was also pointed out that crystallins are diverse proteins, which are currently responsible for the optical properties of the lens, but originally were recruited from the metabolic enzymes and stress proteins [159]. Furthermore, recruitment of crystallins from these enzymes and chaperones has occurred by changes in gene regulation resulting in high lens expression of these proteins [159].
Another early observation was that the duck epsilon–crystallin differs from the duck LDH in having one or two point mutations, at sites which in the LDH across the species are conserved. These mutations probably were beneficial for crystallin only. This shows that moonlighting can be accompanied by necessary changes in the gene for improving functionality [31, 158].
X-ray structure of eta-crystallin has shed further light on the molecular level details about the multi-tasking of these proteins [33]. The crystallin has retinal dehydrogenase activity although less than its activity when present in other tissues, where it’s enzymatic activity is biologically important. The X-ray crystal structure of the crystallin was similar to the tetrameric structure of other aldehyde dehydrogenase. Some structural differences included “a larger pocket for ligand binding, a large substrate tunnel cavity between the NAD-binding domain and the catalytic domain, and a smaller opening to the tunnel” [33]. While the overall protein structure was not affected by these changes, the structural flexibility was noticeably decreased, which increased the protein stability but reduced the catalytic activity. Constance J. Jeffery called this changes “adaptive conflict”, which occurs during moonlighting [33].
A recent issue of the journal Experimental Eye Research focusses on the eye lens, in which recent knowledge about crystallin gene expression has been discussed. There is a transcriptional burst when the lens epithelial cells differentiate into the fiber cells (lens cells) [168]. This results in the massive production of crystallins right at this step. Therefore, for functioning as the eye lens protein, the protein is adapted to self-crowding conditions and does not precipitate/aggregate. Point mutations and other accumulated structural changes, however, can lead to variety of aggregation; cataract is due to the formation of amorphous aggregates [169].
Macrophage migration inhibitory factor (MIF)
MIF was first reported as a cytokine secreted by T-cells and was so named as it prevented random migration of macrophages [170, 171]. Later, it was found to be secreted by several other types of cells, including macrophages. It is stored in cytosol, where it acts as a stress sensor and homeostasis regulator necessary for cell survival. When secreted, it acts as a cytokine. Its biological activities include increasing phagocytosis. The hydrogen peroxide production is increased, and in synergy with γ-interferon, this increases the production of nitric oxide. MIF mediates innate and early phase of adaptive immunity. It is involved in the inflammation during sepsis, asthma, malaria, and several other diseases and pathologies [171].
In addition to acting as cytokine, MIF-2 is also a dopachrome/phenylpyruvate tautomerase [172, 173]. It also shows thiol-protein oxidoreductase activity, and, therefore, also functions as an enzyme [174, 175]. It was also isolated from anterior pituitary cell lines [170]. It turned out that it acts on the site of inflammation and counters the inhibition of glucocorticoids on the primary immune system. In 1999, Melissa D. Swope and Elias Lois had written an excellent overview of the moonlighting actions of MIF, which include its functions as a hormone, cytokine and an enzyme [176].
Alarmins
The multi-tasking/ monlighting is also shown by a class of endogenous peptides/proteins called alarmins [171, 177]. While alarmins include a wide variety of molecules of different physico-chemical nature (e.g., proteins, ATP, uric acid crystals), here we are talking about only those, which are peptides/proteins, such as high-mobility group box-1 (HMGB1), high mobility group nucleosome binding domain 1 (HMGN1, also known as non-histone chromosomal protein HMG-14), interleukins 1α and 33 (IL-1α and IL-33), heat shock proteins (HSPs), and S100 proteins [177]. These molecules have a normal physiological functions but multitask under the stress, infection, and injury kind of situations. Alarmins are sometime also called “first responders”, since they alert the immune system in case of infection and non-programmed cell death. These proteins are of three different origins, such as granule derived (e.g., defensins, granulysin, etc.), nuclear, and cytoplasmic. In the case of severe injury, being released in excess, these proteins can result in the development of the fatal cytokine storm. Their diverse biological activities include involvement in alerting host immune system, regulation of gene expression, control of cellular homeostasis, wound healing, inflammation, allergy, and other forms of autoimmunity and oncogenesis [177]. It was pointed out that “there are probably a number of alarmins that remain to be identified and little is as yet known about the clinical role of already identified alarmins” [177]. The authors also indicated that among the most recently discovered alarmins is α-synuclein [178], a multifunctional related to the pathogenesis of Parkinson’s disease and many other synucleinopathies [179].
An important feature common to the alarmins is the presence of high levels of intrinsic disorder in these proteins, which can be attributed to the ability of these proteins to adapt to various targets and thereby possess extremely broad functional diversity. Several reviews were dedicated to the discussion of the prevalence and functional roles of disorder in some of the alarmins, such as chaperones [180–182], S100 proteins [183, 184], and α-synuclein [179]. For example, it was pointed out that the “remarkable structural, functional, and dysfunctional multifaceted nature of α-synuclein can be understood using the proteoform concept”, which is directly linked to the intrinsically disordered nature of this protein [179]. In fact, the proteoform concept suggests that instead of “one gene–one protein–one function” concept, a set of distinct protein molecules can be created from a single gene [185]. There are several natural means, by which one gene can encode a number of functionally different proteins (i.e., proteoforms) [185]. This includes “the allelic variations (i.e., single or multiple point mutations, indels, SNPs) at the DNA level, alternative splicing, and other pre-translational mechanisms affecting mRNA, complemented by a wide spectrum of various PTMs of a polypeptide chain” [179]. Furthermore, the structural and functional diversity of a given can be further increased by the presence of intrinsic disorder and structural changes induced as a result of functioning. Therefore, a correlation between protein structure and function represents a “protein structure–function continuum” [105, 121, 186–189], “where a given protein exists as a dynamic conformational ensemble containing multiple proteoforms (conformational/basic, inducible/modified, and functioning) characterized by diverse structural features and miscellaneous functions” [126].
Figure 2 represents AlphaFold-modeled 3D structures of HMGB, HMGN1, and α-synuclein and provides a strong visual support to the idea of a highly disordered nature of these alarmins.
Fig. 2.
Structural disorder in three human alarmins, HMGB1 (UniProt ID: P09429; A), HMGN1 (UniProt ID: P05114; B), and α-synuclein (UniProt ID: P37840; C), as evidenced by the high content of regions with low and very low confidence of predicted structure (50 < pLLDT < 70 and pLDDT < 50, respectively) in 3D structures of these proteins modeled by AlphaFold
Metallothioniens
Bert Lester Vallee (1919–2010), who laid the foundations of metalloproteins, also discovered these interesting cystein-rich proteins and gave them the name metallothioniens (MT) [190]. These proteins have been later found in both prokaryotes and eukaryotes [191]. MT1 and MT2 families occur in all cells, whereas MT3 can be found in the nervous system and MT4 in epithelial cells of mammals, wherein these proteins have been most comprehensively characterized [192]. Typically, these contain up to 7 Zn/Cd ions or 12 Cu ions. Their important role is to act as a buffer for zinc ions that serve as a cofactor of about 2000 human proteins, which gives us some idea about the important role played by MTs. Besides this function in metal homeostasis, MTs are involved in metal detoxification and protection from oxidative stress. For example, MTs are upregulated during oxidative stress. Acting as free radical scavengers (such as copper induced formation of hydroxyl radicals), these proteins display this moonlighting activity [191, 193]. It is believed that other functions evolved from the initial detoxification activity [191, 194]. Finally, the roles of MTs in carcinogenesis and their potential in both diagnosis and therapy have also emerged, with these functions being also linked to many cellular processes, such as apoptosis [195, 196]. In a recent paper, Joshua E. Kim and Paul A. Lindahl reported that the metallothionien CUP1 in S. cerevisiae was found in both cytosol and inter-membrane of the mitochondria. Its role in mitochondria can only be speculated. Presumably, it controls the concentration of copper complexes there at a low level [197].
The multitasking nature of metallothionienes has been the focus of a dedicated review in 2002, where it was pointed out that for MTs, “a primary role has not been identified, and remains elusive, as further functions continue to be discovered” [198]. Curiously, this statement is in line with the similar observations made for α-synuclein: “Many papers in the field start with an introductory sentence stating that α-synuclein is a small, highly conserved presynaptic protein with unknown function. This statement is a bit odd, taking into account all the efforts of numerous researchers working on α-synuclein. In fact, according to the PubMed database (as of 16 December 2016), there were more than 7150 papers mentioning synuclein function, many of those papers were dedicated to the detailed investigation of what this protein can do, and, as a result, many potential functions were ascribed to α-synuclein. The explanation of this contradiction (many functions are described, but function is unknown) is in the logistics of the classical structural biology relying on the influential “one gene–one enzyme–one function” hypothesis, according to which each gene encodes a single protein that has a unique biological function and is responsible for a single step in a metabolic pathway” [179].
Moonlighting by enzymes of the glycolysis and gluconeogenesis pathways
It is now fairly well established that moonlighting by enzymes involved in the glycolysis and gluconeogenesis and TCA cycle is common to many living organisms [199]. The recent review by Bian et al. updates the information on moonlighting by enzymes from the glycolysis and gluconeogenesis pathways in connecting these processes with gene expression [200]. Curiously, this phenomenon is common throughout all domains of life, as novel activities of glycolytic enzymes were described, for example, in Bacillus subtilis, where they were found to be engaged in interactions with the essential proteins involved in mRNA processing [201]. Some examples of these moonlighting members of the metabolic pathways are briefly discussed below.
Hexokinase
Hexokinase (HXK) is a key enzyme in the glycolytic pathway that catalyzes the phosphorylation of hexoses using ATP as a phosphoryl donor [202, 203] and that can be found in microorganisms, plants, and animals. Multiple HXK isoenzymes were found in yeast [204], mammals [205, 206], and plants [207]. Hexokinases from different organisms have similar structures [208, 209].
This enzyme is also known to act as a glucose sensor protein, and can moonlight due to changes in its subcellular location, oligomeric status, and conformational changes, as well as via the response to substrate/product flux and via its interaction with membranes, other proteins, and RNA. These numerous mechanisms enable hexokinase to exhibit diverse moonlighting activities which include gene repression, autophagy regulation, programmed cell death, and involvement in immune responses [210].
Enolase
Enolase converts 2-phosphoglycerate into phosphoenolate pyruvate in glycolysis. Enolase has 3 isoenzymes, out of which α-enolase (enolase-1) is present in almost all tissues. The cytoplasmic enzyme is also a heat shock protein in bacteria, yeasts, parasites and mammals. The enzyme is also located at many other parts of the cells (nucleus, mitochondrial membrane, cytoskeleton, vacuoles, extracellular space and cellular surface) and exhibits moonlighting activities depending on its localization [211]. It interact with an integral protein and supports stability of mitochondrial membrane. Its truncated form (called myc promoter binding protein) is present in nucleus and acts as tumor suppressor. The cytoskeletal enolase-1 interacts with microtubules, contractile filaments of cardiomyocytes, and centrosomes. The enzyme is overexpressed in many forms of cancer, wherein its functional involvement is in addition to via Warburg effect (icreasing glycolytic rates under hypoxic conditions of cancer cells). Its levels are also found to be altered in several diseases including Alzheimer’s disease, rheumatoid arthritis, hepatic fibrosis, and type-2 diabetes, as well as in many diseases associated with the bacterial and fungal infections. Enolase is known to bind to plasminogen and is also involved in signal transduction and RNA chaperone activity. Although the whole set of its moonlighting activities is not yet discovered, and although known moonlighting functions are not understood yet at the molecular level, it us very clear that enolase represents a truly multi-tasking protein with diverse moonlighting activities depending upon the cellular context [211].
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
An interesting example of moonlighting by glycolytic enzymes is given by the glyceraldehyde-3-phosphate dehydrogenase (GAPDH). GAPDH is an important enzyme of the glycolytic pathway, which also moonlights in being involved in initiation of apoptosis, transcription, defense against oxidative stress, and metal chaperoning [212–216]. Furthermore, GAPDH regulates various kinases, transferases, and Ca2+ release, and interacts with certain proteins such as pro-apoptotic proteins p53, seven in absentia homolog-1(Siah-1), and voltage-dependent anion-selective channel-1 (VDAC-1) binding protein [217].
One important aspect of the GAPDH moonlighting activity during oxidative stress is that it is mediated by PTMs [218]. The modifications, which include sulfenylation, S-thiolation, nitrosylation, and sulphydration alter its conformation, which leads to changes in its subcellular organization and nature of its protein–protein interactions. The modifications take place on its catalytic cysteine, thus affecting glycolysis. The shunt pathway processes the carbohydrate instead and generates NADPH which acts as the final acceptor of electrons in many reducing pathways. This helps in maintaining the redox homeostasis [218]. Obviously, these PTMs have to be reversible after the oxidative stress is abated, and cellular redox erases, such as thioredoxin, ensure this reversibility [218]. A recent review by Michael A. Sirover focusses on how PTMs are responsible for subcellular localization and consequently moonlighting activities of GAPDH [219].
More recently, there are reports about the role of GADP in the heme maturation of heme-containing enzymes [220, 221]. The non-native GAPDH species were also reported to form stable complexes with Aβ and facilitate its aggregation [222], thereby contributing to the pathogenesis of Alzheimer’s disease [217, 223, 224]. Superoxide generation during diabetes inactivates this enzyme, and signaling via protein kinase C is activated. The advanced glycation products formation occurs which are responsible for many symptoms/pathological changes associated with diabetes [218]. The role of GADPH in metastasis is discussed later. Hence, GAPDH is a good example of what Jeffery called neomorphic moonlighting proteins (see discussion in subsequent section). It also is a target for drug design for above reasons [218].
Meanwhile more and more examples of enzymes moonlighting continue to emerge. This includes fructose-1,6-bisphosphate (FBP) aldolase, which in the FBP-unoccupied form, interacts with and inhibits endoplasmic reticulum (ER)-localized transient receptor potential channel subfamily V, thereby inhibiting calcium release at the conditions of low glucose levels that links sensing of declining glucose availability to AMPK activation via the lysosomal pathway [225]. The enforced expression of the gluconeogenic isozyme fructose-1,6-bisphosphatase 2 (FBP2), which is silenced in a broad spectrum of sarcoma subtypes, inhibits sarcoma cell and tumor growth via catalytic activity in cytoplasm and being relocalized to nucleus “restrains mitochondrial biogenesis and respiration in a catalytic-activity-independent manner by inhibiting the expression of nuclear respiratory factor and mitochondrial transcription factor A (TFAM)” [226]. Finally, in human hepatocellular carcinoma (HCC) cells, AKT-driven phosphorylation of the rate-limiting enzyme in gluconeogenesis, cytosolic phosphoenolpyruvate carboxykinase 1 (PCK1), leads to the translocation of this gluconeogenic enzyme to the endoplasmic reticulum, where it phosphorylates insulin-induced gene 1 protein (INSIG1), which eventually leads to the activation of sterol regulatory element-binding proteins 1 or 2 (SREBP1 or SREBP2) and “the transcription of downstream lipogenesis-related genes, proliferation of tumor cells, and tumorigenesis” [227].
Moonlighting proteins in diseases and neomorphic moonlighting proteins
The roles of moonlighting in various diseases have been mentioned at a number of places in this review already. This aspect was also covered few years ago in a book [228]. How moonlighting impacts medical sciences was also reviewed few years ago [229]. However in view of its increasing importance, this aspect is briefly discussed below with some updated information.
A look at Multitask ProtDB-II data base reveals that about 25% moonlighting proteins act as virulence factors [149]. These are proteins whose canonical activities are associated with basic conserved pathways [12, 150, 228, 230]. In Candida, triose phosphate isomerase (TIM or TPM, an enzyme that normally catalyzes the reversible interconversion of the triose phosphate isomers dihydroxyacetone phosphate and D-glyceraldehyde 3-phosphate), occurs in fungal cell wall, where it is involved in the yeast binding to human cells. For this adhesion function, TIM uses a site different from its catalytic site [231]. Enolase (already mentioned as a moonlighting protein) of the fungus also binds to three extracellular matrix proteins of the host cells, vitronectin, fibronectin, and plasminogen [232].
In the case of M. tuberculosis, many proteins moonlight as virulence factors [229]. For example, a chaperonin Hsp65 supports the pathogen entry into macrophages, whereas other mycobacterial molecular chaperones act as secreted intercellular signaling molecules or control activities at the cell wall and even control the composition of the cell wall [233].
Another family of the moonlighting enzymes that plays crucial roles in both health and diseases is a family of phosphoinositide 3-kinases (PI3Ks) [234, 235], where, for example, mutations or dysregulation in class IA PI3Ks are associated with the abnormal development and congenital diseases [236, 237], numerous cancers [238, 239], as well as in immune disorders [240].
The role of moonlighting in parasitic diseases has been extensively reported as well [241, 242]. For example, many enzymes from parasitic protozoa moonlight in a non-enzymatic capacity in a diverse variety of cellular processes [241]. Furthermore, “unconventional protein secretion” (UPS; i.e., a process of protein translocation to, or across, the plasma membrane via non-canonical secretion pathways) is commonly used by the protist parasites from the Entamoeba, Giardia, Leishmania, Plasmodium, Trichomonas, and Trypanosoma genera to release parasite-derived virulence factors that act as a main source of pathogenicity for hosts [242].
Sometime back, Sriram et al. had reviewed the involvement of moonlighting in metabolic disorders, with special emphasis on the enzymes related to the carbohydrate metabolism [243]. These authors emphasized that if a mutation in a moonlighting enzyme is involved in a single-gene disorder, which supposed to be characterized by “simple” Mendelian inheritance, one cannot make an easy prediction of the phenotype from the genotype, since “additional functional activities of proteins could result in a lack of correlation between genotype and phenotype” [243]. The cases of phosphoglucoisomerase, deficiency of which leads to the hemolytic anemia, and GADPH, moonlighting of which is involved in several neurodegenerative diseases were discussed as illustrative examples of the complexity of related maladies [243]
Growth and proliferation of cancer cells involve increased cellular activities, though they have the constraints of limited accessibility to oxygen and nutrients [244]. The complex situation involves crosstalk between multiple signaling cascades. Aerobic glycolysis (so called “Warburg effect”) and upregulation of glutaminolysis are two main features of the metabolic reprogramming to obtain energy and material for enhanced metabolic activity under the constrained situation [244]. In non-glycolytic cancers, such as prostate cancer and B-cell lymphoma, free fatty acids serve as the alternate energy source [245, 246]. Adamo et al. have reviewed the role of moonlighting proteins in cancer immunology [247], whereas Boukouris et al. recently pointed out that metabolism-epigenetics axis, originating from the moonlighting of the metabolic enzymes in the nucleus, “facilitates adaptation to a changing environment in normal (e.g., development, stem cell differentiation) and disease states (e.g., cancer), providing a potential novel therapeutic target” [248]. Furthermore, as per Lei Lv and Qunying Lei, “the signal—moonlighting protein—metabolism axis facilitates the adaptation of tumor cells under varying environment conditions and can be therapeutically targeted for cancer treatment” [246]. In line with all these considerations, Chaoyun Pan, Bo Li, and M. Celeste Simon represented a very comprehensive review on how moonlighting by enzymes of all major pathways of carbohydrate, lipid, and amino acid metabolism are important in malignancy [249]. It is clear that targeting various disease-related moonlighting proteins represents an important strategy in finding new ways of cure different diseases. A recent review describes the potential of moonlighting proteins in overcoming antibiotic drug resistance [250].
Conclusions and future perspectives
It remains tricky to define moonlighting by proteins. Sometimes, moonlighting activity involves the same binding site as the one engaged in the canonical function, while mostly it is conducted via another site. Moonlighting functions can be independent of each other and the canonical function [246]. In some cases, moonlighting activities are related to same metabolic function. For example, cerruloplasmin plays a role in iron metabolism and acts as a ferrooxidase enzyme [157]. At one time, Constance J. Jeffery excluded PTM-generated protein variants from being considered as moonlighting proteins [4], but later discussed that PTMs are exploited by neomorphic moonlighting proteins in switching their activity [22].
Metamorphic proteins—the proteins, which have more than unique structure even in the absence of a ligand—include proteins which exploit “fold switching” for moonlighting [91]. Therefore, we have plasticity, intrinsic disorder and fold switching as parts of the strategies, which enable moonlighting by proteins.
The moonlighting and promiscuity represent twin aspects of non-specificity of proteins leading to the biochemically relevant activities. Their occurrences triggered by the pathogenic situations are bound to attract increasing attention in coming years.
Another unfinished story is the role of moonlighting in protein evolution. The paradigm of transition from generalist to specialist enzymes may turn out to be an oversimplification. Induction of moonlighting by cells under stress or during pathogenesis is like a specialist doctor having to act like a general practitioner in an emergency. Perhaps, the specialist enzymes never completely lose the potential to be promiscuous or moonlight. It is in the nature of proteins to be moonlight or/and promiscuous, or/and multifunctional. The “inherent imperfection of enzymes” is a subplot of this theme [71, 72, 251].
There are a few other questions/aspects related to generalist-specialist question. When any environmental/evolutionary pressure leads to moonlighting by a protein, does that makes that protein more liable to evolve into a protein with further functions; i.e., more moonlighting or promiscuous? Is a protein, which is promiscuous, has a greater probability of becoming a moonlighting protein (or vice versa)? The basis of these questions is, of course, the few common roots for both phenomena, such as protein plasticity and intrinsic disorder.
Another unexplored aspect seems to be the moonlighting activities of less structured conformations like pre-, wet and dry molten globules [252]. Considering that intrinsic disorder is involved in moonlighting in several cases [23], what happens to the moonlighting proteins in their pre-molten and molten globular forms? It may be interesting to find the ratios of the loss in biological activities of proteins towards their canonical substrate and moonlighting activity in case of molten globules.
As we come up with tools/methods to predict moonlighting with more confidence, we may gain more clarity about these questions as well as on structure/disorder continuum–moonlighting function relationship.
Author contributions
Both authors MNG and VNU contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gupta MN, Kapoor M, Majumder AB, Singh V (2011) Isozymes, moonlighting proteins and promiscous enzymes. Curr Sci 100:1152–1162
- 2.Gupta MN, Alam A, Hasnain SE. Protein promiscuity in drug discovery, drug-repurposing and antibiotic resistance. Biochimie. 2020;175:50–57. doi: 10.1016/j.biochi.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Copley SD. Enzymes with extra talents: moonlighting functions and catalytic promiscuity. Curr Opin Chem Biol. 2003;7(2):265–272. doi: 10.1016/s1367-5931(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 4.Jeffery CJ. Moonlighting proteins. Trends Biochem Sci. 1999;24(1):8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- 5.Prabantu VM, Yazhini A, Srinivasan N. Phenotypic switching. Amsterdam: Elsevier; 2020. Manoeuvring protein functions and functional levels by structural excursions; pp. 77–104. [Google Scholar]
- 6.Jeffery CJ. An enzyme in the test tube, and a transcription factor in the cell: moonlighting proteins and cellular factors that affect their behavior. Protein Sci. 2019;28(7):1233–1238. doi: 10.1002/pro.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeffery CJ. Moonlighting proteins: old proteins learning new tricks. Trends Genet. 2003;19(8):415–417. doi: 10.1016/S0168-9525(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery CJ. Moonlighting proteins: complications and implications for proteomics research. Drug Discovery Today Targets. 2004;3(2):71–78. doi: 10.1016/S1741-8372(04)02405-3. [DOI] [Google Scholar]
- 9.Moore B. Bifunctional and moonlighting enzymes: lighting the way to regulatory control. Trends Plant Sci. 2004;9(5):221–228. doi: 10.1016/j.tplants.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Gancedo C, Flores CL. Moonlighting proteins in yeasts. Microbiol Mol Biol Rev. 2008;72(1):197–210. doi: 10.1128/MMBR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huberts DH, van der Klei IJ. Moonlighting proteins: an intriguing mode of multitasking. Biochim Biophys Acta. 2010;1803(4):520–525. doi: 10.1016/j.bbamcr.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Henderson B, Martin A. Bacterial virulence in the moonlight: multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect Immun. 2011;79(9):3476–3491. doi: 10.1128/IAI.00179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copley SD. Moonlighting is mainstream: paradigm adjustment required. BioEssays. 2012;34(7):578–588. doi: 10.1002/bies.201100191. [DOI] [PubMed] [Google Scholar]
- 14.Fares MA. The evolution of protein moonlighting: adaptive traps and promiscuity in the chaperonins. Biochem Soc Trans. 2014;42(6):1709–1714. doi: 10.1042/BST20140225. [DOI] [PubMed] [Google Scholar]
- 15.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Palkovits M, Tarakanov AO, Ciruela F, Agnati LF. Moonlighting proteins and protein-protein interactions as neurotherapeutic targets in the G protein-coupled receptor field. Neuropsychopharmacology. 2014;39(1):131–155. doi: 10.1038/npp.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginger ML. Protein moonlighting in parasitic protists. Biochem Soc Trans. 2014;42(6):1734–1739. doi: 10.1042/BST20140215. [DOI] [PubMed] [Google Scholar]
- 17.Karkowska-Kuleta J, Kozik A. Moonlighting proteins as virulence factors of pathogenic fungi, parasitic protozoa and multicellular parasites. Mol Oral Microbiol. 2014;29(6):270–283. doi: 10.1111/omi.12078. [DOI] [PubMed] [Google Scholar]
- 18.Khan IK, Kihara D. Computational characterization of moonlighting proteins. Biochem Soc Trans. 2014;42(6):1780–1785. doi: 10.1042/BST20140214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G, Xia Y, Cui J, Gu Z, Song Y, Chen YQ, Chen H, Zhang H, Chen W. The roles of moonlighting proteins in bacteria. Curr Issues Mol Biol. 2014;16:15–22. [PubMed] [Google Scholar]
- 20.Espinosa-Cantu A, Ascencio D, Barona-Gomez F, DeLuna A. Gene duplication and the evolution of moonlighting proteins. Front Genet. 2015;6:227. doi: 10.3389/fgene.2015.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min KW, Lee SH, Baek SJ. Moonlighting proteins in cancer. Cancer Lett. 2016;370(1):108–116. doi: 10.1016/j.canlet.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeffery CJ. Protein species and moonlighting proteins: Very small changes in a protein's covalent structure can change its biochemical function. J Proteomics. 2016;134:19–24. doi: 10.1016/j.jprot.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Gupta MN, Uversky VN. Structure and intrinsic disorder in enzymology. Amsterdam: Elsevier; 2023. Role of plasticity and disorder in protein moonlighting: blurring of lines between biocatalysts and other biologically active proteins; pp. 279–301. [Google Scholar]
- 24.Singh N, Bhalla N. Moonlighting proteins. Annu Rev Genet. 2020;54:265–285. doi: 10.1146/annurev-genet-030620-102906. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor M, Gupta MN. Lipase promiscuity and its biochemical applications. Process Biochem. 2012;47(4):555–569. doi: 10.1016/j.procbio.2012.01.011. [DOI] [Google Scholar]
- 26.Gupta MN, Uversky VN. Structure and intrinsic disorder in enzymology. Amsterdam: Elsevier; 2023. The various facets of protein promiscuity: not just broad specificity of proteins; pp. 241–277. [Google Scholar]
- 27.Khersonsky O, Tawfik DS. Enzyme promiscuity: a mechanistic and evolutionary perspective. Annu Rev Biochem. 2010;79:471–505. doi: 10.1146/annurev-biochem-030409-143718. [DOI] [PubMed] [Google Scholar]
- 28.Nobeli I, Favia AD, Thornton JM. Protein promiscuity and its implications for biotechnology. Nat Biotechnol. 2009;27(2):157–167. doi: 10.1038/nbt1519. [DOI] [PubMed] [Google Scholar]
- 29.Jia B, Cheong GW, Zhang S. Multifunctional enzymes in archaea: promiscuity and moonlight. Extremophiles. 2013;17(2):193–203. doi: 10.1007/s00792-012-0509-1. [DOI] [PubMed] [Google Scholar]
- 30.Piatigorsky J. Gene sharing and evolution: the diversity of protein functions. Cambridge: Harvard University Press; 2007. [Google Scholar]
- 31.Piatigorsky J, Wistow GJ. Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell. 1989;57(2):197–199. doi: 10.1016/0092-8674(89)90956-2. [DOI] [PubMed] [Google Scholar]
- 32.Jeffery CJ. Multifunctional proteins: examples of gene sharing. Ann Med. 2003;35(1):28–35. doi: 10.1080/07853890310004101. [DOI] [PubMed] [Google Scholar]
- 33.Jeffery CJ. Molecular mechanisms for multitasking: recent crystal structures of moonlighting proteins. Curr Opin Struct Biol. 2004;14(6):663–668. doi: 10.1016/j.sbi.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Jeffery CJ. Mass spectrometry and the search for moonlighting proteins. Mass Spectrom Rev. 2005;24(6):772–782. doi: 10.1002/mas.20041. [DOI] [PubMed] [Google Scholar]
- 35.Jeffery CJ. Moonlighting proteins: proteins with multiple functions. Molecular chaperones and cell signalling. Cambridge: Cambridge University Press; 2005. pp. 61–77. [Google Scholar]
- 36.Jeffery CJ. Moonlighting proteins—an update. Mol Biosyst. 2009;5(4):345–350. doi: 10.1039/b900658n. [DOI] [PubMed] [Google Scholar]
- 37.Jeffery CJ. Proteins with neomorphic moonlighting functions in disease. IUBMB Life. 2011;63(7):489–494. doi: 10.1002/iub.504. [DOI] [PubMed] [Google Scholar]
- 38.Jeffery CJ. An introduction to protein moonlighting. Biochem Soc Trans. 2014;42(6):1679–1683. doi: 10.1042/BST20140226. [DOI] [PubMed] [Google Scholar]
- 39.Amblee V, Jeffery CJ. Physical features of intracellular proteins that moonlight on the cell surface. PLoS ONE. 2015;10(6):e0130575. doi: 10.1371/journal.pone.0130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeffery CJ. Why study moonlighting proteins? Front Genet. 2015;6:211. doi: 10.3389/fgene.2015.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Jeffery CJ. An analysis of surface proteomics results reveals novel candidates for intracellular/surface moonlighting proteins in bacteria. Mol Biosyst. 2016;12(5):1420–1431. doi: 10.1039/c5mb00550g. [DOI] [PubMed] [Google Scholar]
- 42.Jeffery CJ. Moonlighting proteins—nature's Swiss army knives. Sci Prog. 2017;100(4):363–373. doi: 10.3184/003685017X15063357842574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeffery CJ. Protein moonlighting: what is it, and why is it important? Philos Trans R Soc Lond B Biol Sci. 2018 doi: 10.1098/rstb.2016.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeffery CJ. Protein moonlighting: what is it, and why is it important? Philos Trans R Soc B Biol Sci. 2018;373(1738):20160523. doi: 10.1098/rstb.2016.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeffery CJ. Multitalented actors inside and outside the cell: recent discoveries add to the number of moonlighting proteins. Biochem Soc Trans. 2019;47(6):1941–1948. doi: 10.1042/BST20190798. [DOI] [PubMed] [Google Scholar]
- 46.Jeffery CJ. Intracellular/surface moonlighting proteins that aid in the attachment of gut microbiota to the host. AIMS Microbiol. 2019;5(1):77–86. doi: 10.3934/microbiol.2019.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeffery CJ. Enzymes, pseudoenzymes, and moonlighting proteins: diversity of function in protein superfamilies. FEBS J. 2020;287(19):4141–4149. doi: 10.1111/febs.15446. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Jeffery CJ. Moonlighting proteins in the fuzzy logic of cellular metabolism. Molecules. 2020 doi: 10.3390/molecules25153440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curtis NJ, Jeffery CJ. The expanding world of metabolic enzymes moonlighting as RNA binding proteins. Biochem Soc Trans. 2021;49(3):1099–1108. doi: 10.1042/BST20200664. [DOI] [PubMed] [Google Scholar]
- 50.Radisky DC, Stallings-Mann M, Hirai Y, Bissell MJ. Single proteins might have dual but related functions in intracellular and extracellular microenvironments. Nat Rev Mol Cell Biol. 2009;10(3):228–234. doi: 10.1038/nrm2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeffery CJ, Bahnson BJ, Chien W, Ringe D, Petsko GA. Crystal structure of rabbit phosphoglucose isomerase, a glycolytic enzyme that moonlights as neuroleukin, autocrine motility factor, and differentiation mediator. Biochemistry. 2000;39(5):955–964. doi: 10.1021/bi991604m. [DOI] [PubMed] [Google Scholar]
- 52.Sirover MA. Subcellular dynamics of multifunctional protein regulation: mechanisms of GAPDH intracellular translocation. J Cell Biochem. 2012;113(7):2193–2200. doi: 10.1002/jcb.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q, Raje V, Yakovlev VA, Yacoub A, Szczepanek K, Meier J, Derecka M, Chen Q, Hu Y, Sisler J, Hamed H, Lesnefsky EJ, Valerie K, Dent P, Larner AC. Mitochondrial localized Stat3 promotes breast cancer growth via phosphorylation of serine 727. J Biol Chem. 2013;288(43):31280–31288. doi: 10.1074/jbc.M113.505057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Sampathkumar A, Kerber SM, Swart C, Hille C, Seerangan K, Graf A, Sweetlove L, Fernie AR. A moonlighting role for enzymes of glycolysis in the co-localization of mitochondria and chloroplasts. Nat Commun. 2020;11(1):4509. doi: 10.1038/s41467-020-18234-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Commichau FM, Stulke J. Trigger enzymes: bifunctional proteins active in metabolism and in controlling gene expression. Mol Microbiol. 2008;67(4):692–702. doi: 10.1111/j.1365-2958.2007.06071.x. [DOI] [PubMed] [Google Scholar]
- 56.Cheng XY, Huang WJ, Hu SC, Zhang HL, Wang H, Zhang JX, Lin HH, Chen YZ, Zou Q, Ji ZL. A global characterization and identification of multifunctional enzymes. PLoS ONE. 2012;7(6):e38979. doi: 10.1371/journal.pone.0038979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang CJ, Chen G, Huang J, Huang Q, Jin K, Shen PH, Li JF, Wu B. A novel beta-glucosidase with lipolytic activity from a soil metagenome. Folia Microbiol (Praha) 2011;56(6):563–570. doi: 10.1007/s12223-011-0083-4. [DOI] [PubMed] [Google Scholar]
- 58.Shirafkan F, Gharaghani S, Rahimian K, Sajedi RH, Zahiri J. Moonlighting protein prediction using physico-chemical and evolutional properties via machine learning methods. BMC Bioinform. 2021;22(1):261. doi: 10.1186/s12859-021-04194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Zhao J, Liu Z, Wang C, Wei L, Han S, Du W. De novo prediction of moonlighting proteins using multimodal deep ensemble learning. Front Genet. 2021;12:630379. doi: 10.3389/fgene.2021.630379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fischer E. Einfluss der configuration auf die wirkung derenzyme. Ber Dt Chem Ges. 1894;27:2985–2993. doi: 10.1002/cber.18940270364. [DOI] [Google Scholar]
- 61.Fersht AR. Enzyme structure and mechanism. 2. New York: W H Freeman and Co; 1985. [Google Scholar]
- 62.Szwajkajzer D, Carey J. Molecular and biological constraints on ligand-binding affinity and specificity. Biopolymers. 1997;44(2):181–198. doi: 10.1002/(SICI)1097-0282(1997)44:2<181::AID-BIP5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 63.Greenspan NS. Cohen's Conjecture, Howard's Hypothesis, and Ptashne's Ptruth: an exploration of the relationship between affinity and specificity. Trends Immunol. 2010;31(4):138–143. doi: 10.1016/j.it.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed Engl. 1998;37(20):2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 65.Kuznetsova IM, Zaslavsky BY, Breydo L, Turoverov KK, Uversky VN. Beyond the excluded volume effects: mechanistic complexity of the crowded milieu. Molecules. 2015;20(1):1377–1409. doi: 10.3390/molecules20011377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuznetsova IM, Turoverov KK, Uversky VN. What macromolecular crowding can do to a protein. Int J Mol Sci. 2014;15(12):23090–23140. doi: 10.3390/ijms151223090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta MN, Uversky VN. Structure and intrinsic disorder in enzymology. Amsterdam: Elsevier; 2023. Macromolecular crowding: how it affects protein structure, disorder, and catalysis; pp. 353–376. [Google Scholar]
- 68.Schreiber G, Keating AE. Protein binding specificity versus promiscuity. Curr Opin Struct Biol. 2011;21(1):50–61. doi: 10.1016/j.sbi.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alejo JL, Kempes CP, Adamala KP. Diffusion control in biochemical specificity. Biophys J. 2022;121(8):1541–1548. doi: 10.1016/j.bpj.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ketudat Cairns JR, Mahong B, Baiya S, Jeon JS. beta-Glucosidases: multitasking, moonlighting or simply misunderstood? Plant Sci. 2015;241:246–259. doi: 10.1016/j.plantsci.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 71.Peracchi A. The limits of enzyme specificity and the evolution of metabolism. Trends Biochem Sci. 2018;43(12):984–996. doi: 10.1016/j.tibs.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 72.Tawfik DS. Accuracy-rate tradeoffs: how do enzymes meet demands of selectivity and catalytic efficiency? Curr Opin Chem Biol. 2014;21:73–80. doi: 10.1016/j.cbpa.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Goldsmith M, Tawfik DS. Enzyme engineering: reaching the maximal catalytic efficiency peak. Curr Opin Struct Biol. 2017;47:140–150. doi: 10.1016/j.sbi.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Pauling L. Festschrift fur Prof. Dr. Arthur Stoll. Basel: Birkhauser Verlag; 1957. The probability of errors in the process of synthesis of protein molecules; pp. 597–602. [Google Scholar]
- 75.Gupta MN. Enzyme function in organic solvents. Eur J Biochem. 1992;203(1–2):25–32. doi: 10.1111/j.1432-1033.1992.tb19823.x. [DOI] [PubMed] [Google Scholar]
- 76.Braco L, Dabulis K, Klibanov AM. Production of abiotic receptors by molecular imprinting of proteins. Proc Natl Acad Sci USA. 1990;87(1):274–277. doi: 10.1073/pnas.87.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mukherjee J, Gupta MN. Dual bioimprinting of Thermomyces lanuginosus lipase for synthesis of biodiesel. Biotechnol Rep (Amst) 2016;10:38–43. doi: 10.1016/j.btre.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mukherjee J, Gupta MN. Molecular bioimprinting of lipases with surfactants and its functional consequences in low water media. Int J Biol Macromol. 2015;81:544–551. doi: 10.1016/j.ijbiomac.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 79.Mukherjee J, Gupta MN. Enhancing the catalytic efficiency of subtilisin for transesterification by dual bioimprinting. Tetrahedron Lett. 2015;56(29):4397–4401. doi: 10.1016/j.tetlet.2015.05.101. [DOI] [Google Scholar]
- 80.James LC, Tawfik DS. Conformational diversity and protein evolution–a 60-year-old hypothesis revisited. Trends Biochem Sci. 2003;28(7):361–368. doi: 10.1016/S0968-0004(03)00135-X. [DOI] [PubMed] [Google Scholar]
- 81.Tokuriki N, Tawfik DS. Protein dynamism and evolvability. Science. 2009;324(5924):203–207. doi: 10.1126/science.1169375. [DOI] [PubMed] [Google Scholar]
- 82.Plattner N, Noe F. Protein conformational plasticity and complex ligand-binding kinetics explored by atomistic simulations and Markov models. Nat Commun. 2015;6:7653. doi: 10.1038/ncomms8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mukherjee J, Gupta MN. Increasing importance of protein flexibility in designing biocatalytic processes. Biotechnol Rep (Amst) 2015;6:119–123. doi: 10.1016/j.btre.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zou T, Risso VA, Gavira JA, Sanchez-Ruiz JM, Ozkan SB. Evolution of conformational dynamics determines the conversion of a promiscuous generalist into a specialist enzyme. Mol Biol Evol. 2015;32(1):132–143. doi: 10.1093/molbev/msu281. [DOI] [PubMed] [Google Scholar]
- 85.Sundberg EJ, Mariuzza RA. Luxury accommodations: the expanding role of structural plasticity in protein-protein interactions. Structure. 2000;8(7):R137–142. doi: 10.1016/s0969-2126(00)00167-2. [DOI] [PubMed] [Google Scholar]
- 86.DeLano WL, Ultsch MH, de Vos AM, Wells JA. Convergent solutions to binding at a protein-protein interface. Science. 2000;287(5456):1279–1283. doi: 10.1126/science.287.5456.1279. [DOI] [PubMed] [Google Scholar]
- 87.Mariuzza RA. Multiple paths to multispecificity. Immunity. 2006;24(4):359–361. doi: 10.1016/j.immuni.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Crean RM, Gardner JM, Kamerlin SCL. Harnessing conformational plasticity to generate designer enzymes. J Am Chem Soc. 2020;142(26):11324–11342. doi: 10.1021/jacs.0c04924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Domnauer M, Zheng F, Li L, Zhang Y, Chang CE, Unruh JR, Conkright-Fincham J, McCroskey S, Florens L, Zhang Y, Seidel C, Fong B, Schilling B, Sharma R, Ramanathan A, Si K, Zhou C. Proteome plasticity in response to persistent environmental change. Mol Cell. 2021;81(16):3294–3309 e3212. doi: 10.1016/j.molcel.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Porter LL, Looger LL. Extant fold-switching proteins are widespread. Proc Natl Acad Sci USA. 2018;115(23):5968–5973. doi: 10.1073/pnas.1800168115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Madhurima K, Nandi B, Sekhar A. Metamorphic proteins: the Janus proteins of structural biology. Open Biol. 2021;11(4):210012. doi: 10.1098/rsob.210012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lopez-Pelegrin M, Cerda-Costa N, Cintas-Pedrola A, Herranz-Trillo F, Bernado P, Peinado JR, Arolas JL, Gomis-Ruth FX. Multiple stable conformations account for reversible concentration-dependent oligomerization and autoinhibition of a metamorphic metallopeptidase. Angew Chem Int Ed Engl. 2014;53(40):10624–10630. doi: 10.1002/anie.201405727. [DOI] [PubMed] [Google Scholar]
- 93.London RE. HIV-1 reverse transcriptase: a metamorphic protein with three stable states. Structure. 2019;27(3):420–426. doi: 10.1016/j.str.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Majumder AB, Gupta MN. Lipase-catalyzed condensation reaction of 4-nitrobenzaldehyde with acetyl acetone in aqueous-organic cosolvent mixtures and in nearly anhydrous media. Synth Commun. 2014;44(6):818–826. doi: 10.1080/00397911.2013.834059. [DOI] [Google Scholar]
- 95.Gupta MN, Uversky VN. Structure and intrinsic disorder in enzymology. Amsterdam: Elsevier; 2023. Structure and disorder: protein functions depend on this new binary transforming lock-and-key into structure-function continuum; pp. 127–148. [Google Scholar]
- 96.Uversky VN, Gillespie JR, Fink AL. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins. 2000;41(3):415–427. doi: 10.1002/1097-0134(20001115)41:3<415::aid-prot130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 97.Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ, Campen AM, Ratliff CM, Hipps KW, Ausio J, Nissen MS, Reeves R, Kang C, Kissinger CR, Bailey RW, Griswold MD, Chiu W, Garner EC, Obradovic Z. Intrinsically disordered protein. J Mol Graph Model. 2001;19(1):26–59. doi: 10.1016/s1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 98.Dunker AK, Obradovic Z. The protein trinity–linking function and disorder. Nat Biotechnol. 2001;19(9):805–806. doi: 10.1038/nbt0901-805. [DOI] [PubMed] [Google Scholar]
- 99.Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27(10):527–533. doi: 10.1016/s0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 100.Uversky VN. Natively unfolded proteins: a point where biology waits for physics. Protein Sci. 2002;11(4):739–756. doi: 10.1110/ps.4210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uversky VN. What does it mean to be natively unfolded? Eur J Biochem. 2002;269(1):2–12. doi: 10.1046/j.0014-2956.2001.02649.x. [DOI] [PubMed] [Google Scholar]
- 102.Uversky VN. Protein folding revisited. A polypeptide chain at the folding-misfolding-nonfolding cross-roads: which way to go? Cell Mol Life Sci. 2003;60(9):1852–1871. doi: 10.1007/s00018-003-3096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dunker AK, Silman I, Uversky VN, Sussman JL. Function and structure of inherently disordered proteins. Curr Opin Struct Biol. 2008;18(6):756–764. doi: 10.1016/j.sbi.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Uversky VN, Dunker AK. Understanding protein non-folding. Biochim Biophys Acta. 2010;1804(6):1231–1264. doi: 10.1016/j.bbapap.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uversky VN. Unusual biophysics of intrinsically disordered proteins. Biochim Biophys Acta. 2013;1834(5):932–951. doi: 10.1016/j.bbapap.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 106.van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, Kim PM, Kriwacki RW, Oldfield CJ, Pappu RV, Tompa P, Uversky VN, Wright PE, Babu MM. Classification of intrinsically disordered regions and proteins. Chem Rev. 2014;114(13):6589–6631. doi: 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oldfield CJ, Cheng Y, Cortese MS, Romero P, Uversky VN, Dunker AK. Coupled folding and binding with alpha-helix-forming molecular recognition elements. Biochemistry. 2005;44(37):12454–12470. doi: 10.1021/bi050736e. [DOI] [PubMed] [Google Scholar]
- 108.Mohan A, Oldfield CJ, Radivojac P, Vacic V, Cortese MS, Dunker AK, Uversky VN. Analysis of molecular recognition features (MoRFs) J Mol Biol. 2006;362(5):1043–1059. doi: 10.1016/j.jmb.2006.07.087. [DOI] [PubMed] [Google Scholar]
- 109.Cheng Y, Oldfield CJ, Meng J, Romero P, Uversky VN, Dunker AK. Mining alpha-helix-forming molecular recognition features with cross species sequence alignments. Biochemistry. 2007;46(47):13468–13477. doi: 10.1021/bi7012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vacic V, Oldfield CJ, Mohan A, Radivojac P, Cortese MS, Uversky VN, Dunker AK. Characterization of molecular recognition features, MoRFs, and their binding partners. J Proteome Res. 2007;6(6):2351–2366. doi: 10.1021/pr0701411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tompa P, Fuxreiter M, Oldfield CJ, Simon I, Dunker AK, Uversky VN. Close encounters of the third kind: disordered domains and the interactions of proteins. BioEssays. 2009;31(3):328–335. doi: 10.1002/bies.200800151. [DOI] [PubMed] [Google Scholar]
- 112.Hsu WL, Oldfield C, Meng J, Huang F, Xue B, Uversky VN, Romero P, Dunker AK (2012) Intrinsic protein disorder and protein-protein interactions. Pac Symp Biocomput:116–127 10.1142/9789814366496_0012 [DOI] [PubMed]
- 113.Uversky VN. Functional roles of transiently and intrinsically disordered regions within proteins. FEBS J. 2015;282(7):1182–1189. doi: 10.1111/febs.13202. [DOI] [PubMed] [Google Scholar]
- 114.Alterovitz WL, Faraggi E, Oldfield CJ, Meng J, Xue B, Huang F, Romero P, Kloczkowski A, Uversky VN, Dunker AK. Many-to-one binding by intrinsically disordered protein regions. Pac Symp Biocomput. 2020;25:159–170. [PubMed] [Google Scholar]
- 115.Dunker AK, Garner E, Guilliot S, Romero P, Albrecht K, Hart J, Obradovic Z, Kissinger C, Villafranca JE (1998) Protein disorder and the evolution of molecular recognition: theory, predictions and observations. Pac Symp Biocomput:473–484 [PubMed]
- 116.Wright PE, Dyson HJ. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol. 1999;293(2):321–331. doi: 10.1006/jmbi.1999.3110. [DOI] [PubMed] [Google Scholar]
- 117.Wright PE, Dyson HJ. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol. 2015;16(1):18–29. doi: 10.1038/nrm3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Borgia A, Borgia MB, Bugge K, Kissling VM, Heidarsson PO, Fernandes CB, Sottini A, Soranno A, Buholzer KJ, Nettels D, Kragelund BB, Best RB, Schuler B. Extreme disorder in an ultrahigh-affinity protein complex. Nature. 2018;555(7694):61–66. doi: 10.1038/nature25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Uversky VN. Intrinsically disordered proteins and their “mysterious”(meta) physics. Front Phys. 2019;7:10. doi: 10.3389/fphy.2019.00010. [DOI] [Google Scholar]
- 120.Uversky VN, Dunker AK. The case for intrinsically disordered proteins playing contributory roles in molecular recognition without a stable 3D structure. F1000 Biol Rep. 2013;5:1. doi: 10.3410/B5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Uversky VN. A decade and a half of protein intrinsic disorder: biology still waits for physics. Protein Sci. 2013;22(6):693–724. doi: 10.1002/pro.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jakob U, Kriwacki R, Uversky VN. Conditionally and transiently disordered proteins: awakening cryptic disorder to regulate protein function. Chem Rev. 2014;114(13):6779–6805. doi: 10.1021/cr400459c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chapple CE, Robisson B, Spinelli L, Guien C, Becker E, Brun C. Extreme multifunctional proteins identified from a human protein interaction network. Nat Commun. 2015;6:7412. doi: 10.1038/ncomms8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hernandez S, Amela I, Cedano J, Piñol J, Perez-Pons JA, Mozo-Villarias A, Querol E. Do moonlighting proteins belong to the intrinsically disordered protein class? J Proteom Bioinform. 2012;5(11):262–264. doi: 10.4172/jpb.1000247. [DOI] [Google Scholar]
- 125.Tompa P, Szasz C, Buday L. Structural disorder throws new light on moonlighting. Trends Biochem Sci. 2005;30(9):484–489. doi: 10.1016/j.tibs.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 126.Uversky VN. p53 proteoforms and intrinsic disorder: an illustration of the protein structure-function continuum concept. Int J Mol Sci. 2016 doi: 10.3390/ijms17111874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rajagopalan K, Mooney SM, Parekh N, Getzenberg RH, Kulkarni P. A majority of the cancer/testis antigens are intrinsically disordered proteins. J Cell Biochem. 2011;112(11):3256–3267. doi: 10.1002/jcb.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wool IG. Extraribosomal functions of ribosomal proteins. Trends Biochem Sci. 1996;21(5):164–165. doi: 10.1016/S0968-0004(96)20011-8. [DOI] [PubMed] [Google Scholar]
- 129.Chavez-Rios R, Arias-Romero LE, Almaraz-Barrera Mde J, Hernandez-Rivas R, Guillen N, Vargas M. L10 ribosomal protein from Entamoeba histolytica share structural and functional homologies with QM/Jif-1: proteins with extraribosomal functions. Mol Biochem Parasitol. 2003;127(2):151–160. doi: 10.1016/s0166-6851(02)00332-8. [DOI] [PubMed] [Google Scholar]
- 130.Warner JR, McIntosh KB. How common are extraribosomal functions of ribosomal proteins? Mol Cell. 2009;34(1):3–11. doi: 10.1016/j.molcel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aseev LV, Boni IV. Extraribosomal functions of bacterial ribosomal proteins. Mol Biol (Mosk) 2011;45(5):805–816. doi: 10.1134/S0026893311050025. [DOI] [PubMed] [Google Scholar]
- 132.Lu H, Zhu YF, Xiong J, Wang R, Jia Z. Potential extra-ribosomal functions of ribosomal proteins in Saccharomyces cerevisiae. Microbiol Res. 2015;177:28–33. doi: 10.1016/j.micres.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 133.Xiong W, Lan T, Mo B. Extraribosomal functions of cytosolic ribosomal proteins in plants. Front Plant Sci. 2021;12:607157. doi: 10.3389/fpls.2021.607157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peng Z, Oldfield CJ, Xue B, Mizianty MJ, Dunker AK, Kurgan L, Uversky VN. A creature with a hundred waggly tails: intrinsically disordered proteins in the ribosome. Cell Mol Life Sci. 2014;71(8):1477–1504. doi: 10.1007/s00018-013-1446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hurtado-Rios JJ, Carrasco-Navarro U, Almanza-Perez JC, Ponce-Alquicira E. Ribosomes: the new role of ribosomal proteins as natural antimicrobials. Int J Mol Sci. 2022 doi: 10.3390/ijms23169123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Balcerak A, Trebinska-Stryjewska A, Konopinski R, Wakula M, Grzybowska EA. RNA-protein interactions: disorder, moonlighting and junk contribute to eukaryotic complexity. Open Biol. 2019;9(6):190096. doi: 10.1098/rsob.190096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ottoz DSM, Berchowitz LE. The role of disorder in RNA binding affinity and specificity. Open Biol. 2020;10(12):200328. doi: 10.1098/rsob.200328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meng F, Kurgan L. High-throughput prediction of disordered moonlighting regions in protein sequences. Proteins. 2018;86(10):1097–1110. doi: 10.1002/prot.25590. [DOI] [PubMed] [Google Scholar]
- 139.Moore BE, Perez VJ. Specific acidic proteino of the nervous system. In: Carlson FD, editor. Physiological and biochemical aspects of nervous integration. Englewood Cliffs: Prentice-Hall; 1967. pp. 343–359. [Google Scholar]
- 140.Mhawech P. 14–3-3 proteins–an update. Cell Res. 2005;15(4):228–236. doi: 10.1038/sj.cr.7290291. [DOI] [PubMed] [Google Scholar]
- 141.Bustos DM, Iglesias AA. Intrinsic disorder is a key characteristic in partners that bind 14–3-3 proteins. Proteins. 2006;63(1):35–42. doi: 10.1002/prot.20888. [DOI] [PubMed] [Google Scholar]
- 142.Gogl G, Tugaeva KV, Eberling P, Kostmann C, Trave G, Sluchanko NN. Hierarchized phosphotarget binding by the seven human 14–3-3 isoforms. Nat Commun. 2021;12(1):1677. doi: 10.1038/s41467-021-21908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sluchanko NN. Uversky VN (2015) Hidden disorder propensity of the N-terminal segment of universal adapter protein 14–3-3 is manifested in its monomeric form: novel insights into protein dimerization and multifunctionality. Biochim Biophys Acta. 1854;5:492–504. doi: 10.1016/j.bbapap.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 144.Mani M, Chen C, Amblee V, Liu H, Mathur T, Zwicke G, Zabad S, Patel B, Thakkar J, Jeffery CJ. MoonProt: a database for proteins that are known to moonlight. Nucleic Acids Res. 2015;43(Database issue):D277–282. doi: 10.1093/nar/gku954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen C, Zabad S, Liu H, Wang W, Jeffery C. MoonProt 2.0: an expansion and update of the moonlighting proteins database. Nucleic Acids Res. 2018;46(D1):D640–D644. doi: 10.1093/nar/gkx1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen C, Liu H, Zabad S, Rivera N, Rowin E, Hassan M, Gomez De Jesus SM, Llinas Santos PS, Kravchenko K, Mikhova M, Ketterer S, Shen A, Shen S, Navas E, Horan B, Raudsepp J, Jeffery C. MoonProt 3.0: an update of the moonlighting proteins database. Nucleic Acids Res. 2021;49(D1):D368–D372. doi: 10.1093/nar/gkaa1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hernandez S, Ferragut G, Amela I, Perez-Pons J, Pinol J, Mozo-Villarias A, Cedano J, Querol E. MultitaskProtDB: a database of multitasking proteins. Nucleic Acids Res. 2014;42(Database issue):D517–520. doi: 10.1093/nar/gkt1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Franco-Serrano L, Hernandez S, Calvo A, Severi MA, Ferragut G, Perez-Pons J, Pinol J, Pich O, Mozo-Villarias A, Amela I, Querol E, Cedano J. MultitaskProtDB-II: an update of a database of multitasking/moonlighting proteins. Nucleic Acids Res. 2018;46(D1):D645–D648. doi: 10.1093/nar/gkx1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Franco-Serrano L, Huerta M, Hernandez S, Cedano J, Perez-Pons J, Pinol J, Mozo-Villarias A, Amela I, Querol E. Multifunctional proteins: involvement in human diseases and targets of current drugs. Protein J. 2018;37(5):444–453. doi: 10.1007/s10930-018-9790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Franco-Serrano L, Sanchez-Redondo D, Najar-Garcia A, Hernandez S, Amela I, Perez-Pons JA, Pinol J, Mozo-Villarias A, Cedano J, Querol E. Pathogen moonlighting proteins: from ancestral key metabolic enzymes to virulence factors. Microorganisms. 2021 doi: 10.3390/microorganisms9061300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bielli P, Calabrese L. Structure to function relationships in ceruloplasmin: a 'moonlighting' protein. Cell Mol Life Sci. 2002;59(9):1413–1427. doi: 10.1007/s00018-002-8519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hotmberg CG, LaureLt CB. Investigations in serum copper. II. Isolation of the copper-containing protein and a description of some of its properties. Acta Chem Scand. 1948;2:550. doi: 10.3891/acta.chem.scand.02-0550. [DOI] [Google Scholar]
- 153.Texel SJ, Xu X, Harris ZL. Ceruloplasmin in neurodegenerative diseases. Biochem Soc Trans. 2008;36(Pt 6):1277–1281. doi: 10.1042/BST0361277. [DOI] [PubMed] [Google Scholar]
- 154.Doyle A, Rusli F, Bhathal P. Aceruloplasminaemia: a rare but important cause of iron overload. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-207541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ryden LG, Hunt LT. Evolution of protein complexity: the blue copper-containing oxidases and related proteins. J Mol Evol. 1993;36(1):41–66. doi: 10.1007/BF02407305. [DOI] [PubMed] [Google Scholar]
- 156.Ortel TL, Takahashi N, Putnam FW. Structural model of human ceruloplasmin based on internal triplication, hydrophilic/hydrophobic character, and secondary structure of domains. Proc Natl Acad Sci USA. 1984;81(15):4761–4765. doi: 10.1073/pnas.81.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- 158.Piatigorsky J. Lens crystallins and their genes: diversity and tissue-specific expression. FASEB J. 1989;3(8):1933–1940. doi: 10.1096/fasebj.3.8.2656357. [DOI] [PubMed] [Google Scholar]
- 159.Piatigorsky J. Crystallin genes: specialization by changes in gene regulation may precede gene duplication. J Struct Funct Genomics. 2003;3(1–4):131–137. doi: 10.1023/A:1022626304097. [DOI] [PubMed] [Google Scholar]
- 160.Piatigorsky J. Gene sharing in lens and cornea: facts and implications. Prog Retin Eye Res. 1998;17(2):145–174. doi: 10.1016/s1350-9462(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 161.Piatigorsky J. Multifunctional lens crystallins and corneal enzymes. More than meets the eye. Ann N Y Acad Sci. 1998;842:7–15. doi: 10.1111/j.1749-6632.1998.tb09626.x. [DOI] [PubMed] [Google Scholar]
- 162.Tomarev SI, Piatigorsky J. Lens crystallins of invertebrates–diversity and recruitment from detoxification enzymes and novel proteins. Eur J Biochem. 1996;235(3):449–465. doi: 10.1111/j.1432-1033.1996.00449.x. [DOI] [PubMed] [Google Scholar]
- 163.Piatigorsky J, Kantorow M, Gopal-Srivastava R, Tomarev SI. Recruitment of enzymes and stress proteins as lens crystallins. EXS. 1994;71:241–250. doi: 10.1007/978-3-0348-7330-7_24. [DOI] [PubMed] [Google Scholar]
- 164.Piatigorsky J. Puzzle of crystallin diversity in eye lenses. Dev Dyn. 1993;196(4):267–272. doi: 10.1002/aja.1001960408. [DOI] [PubMed] [Google Scholar]
- 165.Piatigorsky J. Lens crystallins. Innovation associated with changes in gene regulation. J Biol Chem. 1992;267(7):4277–4280. doi: 10.1016/S0021-9258(18)42826-8. [DOI] [PubMed] [Google Scholar]
- 166.Piatigorsky J. Molecular biology: recent studies on enzyme/crystallins and alpha-crystallin gene expression. Exp Eye Res. 1990;50(6):725–728. doi: 10.1016/0014-4835(90)90121-a. [DOI] [PubMed] [Google Scholar]
- 167.Wistow G, Kim H. Lens protein expression in mammals: taxon-specificity and the recruitment of crystallins. J Mol Evol. 1991;32(3):262–269. doi: 10.1007/BF02342749. [DOI] [PubMed] [Google Scholar]
- 168.Cvekl A, Eliscovich C. Crystallin gene expression: insights from studies of transcriptional bursting. Exp Eye Res. 2021;207:108564. doi: 10.1016/j.exer.2021.108564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roskamp KW, Montelongo DM, Anorma CD, Bandak DN, Chua JA, Malecha KT, Martin RW. Multiple aggregation pathways in human gammaS-crystallin and its aggregation-prone G18V variant. Invest Ophthalmol Vis Sci. 2017;58(4):2397–2405. doi: 10.1167/iovs.16-20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bucala R. MIF re-discovered: pituitary hormone and glucocorticoid-induced regulator of cytokine production. Cytokine Growth Factor Rev. 1996;7(1):19–24. doi: 10.1016/1359-6101(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 171.Kapurniotu A, Gokce O, Bernhagen J. The multitasking potential of alarmins and atypical chemokines. Front Med (Lausanne) 2019;6:3. doi: 10.3389/fmed.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rosengren E, Bucala R, Aman P, Jacobsson L, Odh G, Metz CN, Rorsman H. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996;2(1):143–149. doi: 10.1007/BF03402210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rosengren E, Aman P, Thelin S, Hansson C, Ahlfors S, Bjork P, Jacobsson L, Rorsman H. The macrophage migration inhibitory factor MIF is a phenylpyruvate tautomerase. FEBS Lett. 1997;417(1):85–88. doi: 10.1016/s0014-5793(97)01261-1. [DOI] [PubMed] [Google Scholar]
- 174.Kleemann R, Kapurniotu A, Mischke R, Held J, Bernhagen J. Characterization of catalytic centre mutants of macrophage migration inhibitory factor (MIF) and comparison to Cys81Ser MIF. Eur J Biochem. 1999;261(3):753–766. doi: 10.1046/j.1432-1327.1999.00327.x. [DOI] [PubMed] [Google Scholar]
- 175.Kleemann R, Kapurniotu A, Frank RW, Gessner A, Mischke R, Flieger O, Juttner S, Brunner H, Bernhagen J. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol. 1998;280(1):85–102. doi: 10.1006/jmbi.1998.1864. [DOI] [PubMed] [Google Scholar]
- 176.Swope MD, Lolis E. Macrophage migration inhibitory factor: cytokine, hormone, or enzyme? Rev Physiol Biochem Pharmacol. 1999;139:1–32. doi: 10.1007/BFb0033647. [DOI] [PubMed] [Google Scholar]
- 177.Yang D, Han Z, Oppenheim JJ. Alarmins and immunity. Immunol Rev. 2017;280(1):41–56. doi: 10.1111/imr.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stolzenberg E, Berry D, Yang D, Lee EY, Kroemer A, Kaufman S, Wong GCL, Oppenheim JJ, Sen S, Fishbein T, Bax A, Harris B, Barbut D, Zasloff MA. A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun. 2017;9(5):456–463. doi: 10.1159/000477990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Uversky VN. Looking at the recent advances in understanding alpha-synuclein and its aggregation through the proteoform prism. F1000Res. 2017;6:525. doi: 10.12688/f1000research.10536.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Uversky VN. Flexible nets of malleable guardians: intrinsically disordered chaperones in neurodegenerative diseases. Chem Rev. 2011;111(2):1134–1166. doi: 10.1021/cr100186d. [DOI] [PubMed] [Google Scholar]
- 181.Webster JM, Darling AL, Uversky VN, Blair LJ. Small heat shock proteins, big impact on protein aggregation in neurodegenerative disease. Front Pharmacol. 2019;10:1047. doi: 10.3389/fphar.2019.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Marzullo L, Turco MC, Uversky VN. What's in the BAGs? Intrinsic disorder angle of the multifunctionality of the members of a family of chaperone regulators. J Cell Biochem. 2022;123(1):22–42. doi: 10.1002/jcb.30123. [DOI] [PubMed] [Google Scholar]
- 183.Permyakov SE, Ismailov RG, Xue B, Denesyuk AI, Uversky VN, Permyakov EA. Intrinsic disorder in S100 proteins. Mol Biosyst. 2011;7(7):2164–2180. doi: 10.1039/c0mb00305k. [DOI] [PubMed] [Google Scholar]
- 184.Weisz J, Uversky VN. Zooming into the dark side of human annexin-S100 complexes: dynamic alliance of flexible partners. Int J Mol Sci. 2020 doi: 10.3390/ijms21165879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Smith LM, Kelleher NL, Consortium for Top Down P Proteoform: a single term describing protein complexity. Nat Methods. 2013;10(3):186–187. doi: 10.1038/nmeth.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.DeForte S, Uversky VN. Order, disorder, and everything in between. Molecules. 2016 doi: 10.3390/molecules21081090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Uversky VN. Dancing protein clouds: the strange biology and chaotic physics of intrinsically disordered proteins. J Biol Chem. 2016;291(13):6681–6688. doi: 10.1074/jbc.R115.685859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fonin AV, Darling AL, Kuznetsova IM, Turoverov KK, Uversky VN. Multi-functionality of proteins involved in GPCR and G protein signaling: making sense of structure-function continuum with intrinsic disorder-based proteoforms. Cell Mol Life Sci. 2019;76(22):4461–4492. doi: 10.1007/s00018-019-03276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Uversky VN. Protein intrinsic disorder and structure-function continuum. Prog Mol Biol Transl Sci. 2019;166:1–17. doi: 10.1016/bs.pmbts.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 190.Kagi JH, Valee BL. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960;235:3460–3465. doi: 10.1016/S0021-9258(18)64490-4. [DOI] [PubMed] [Google Scholar]
- 191.Abdin AY, Jacob C, Kastner L. The enigmatic metallothioneins: a case of upward-looking research. Int J Mol Sci. 2021 doi: 10.3390/ijms22115984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vasak M, Meloni G. Chemistry and biology of mammalian metallothioneins. J Biol Inorg Chem. 2011;16(7):1067–1078. doi: 10.1007/s00775-011-0799-2. [DOI] [PubMed] [Google Scholar]
- 193.Krezel A, Maret W. The functions of metamorphic metallothioneins in zinc and copper metabolism. Int J Mol Sci. 2017 doi: 10.3390/ijms18061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Seren N, Glaberman S, Carretero MA, Chiari Y. Molecular evolution and functional divergence of the metallothionein gene family in vertebrates. J Mol Evol. 2014;78(3–4):217–233. doi: 10.1007/s00239-014-9612-5. [DOI] [PubMed] [Google Scholar]
- 195.Si M, Lang J. The roles of metallothioneins in carcinogenesis. J Hematol Oncol. 2018;11(1):107. doi: 10.1186/s13045-018-0645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cherian MG, Jayasurya A, Bay BH. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutat Res. 2003;533(1–2):201–209. doi: 10.1016/j.mrfmmm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 197.Kim JE, Lindahl PA. CUP1 metallothionein from healthy Saccharomyces cerevisiae colocalizes to the cytosol and mitochondrial intermembrane space. Biochemistry. 2023;62(1):62–74. doi: 10.1021/acs.biochem.2c00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59(4):627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30(3):142–150. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 200.Bian X, Jiang H, Meng Y, Li YP, Fang J, Lu Z. Regulation of gene expression by glycolytic and gluconeogenic enzymes. Trends Cell Biol. 2022;32(9):786–799. doi: 10.1016/j.tcb.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 201.Commichau FM, Rothe FM, Herzberg C, Wagner E, Hellwig D, Lehnik-Habrink M, Hammer E, Volker U, Stulke J. Novel activities of glycolytic enzymes in Bacillus subtilis: interactions with essential proteins involved in mRNA processing. Mol Cell Proteomics. 2009;8(6):1350–1360. doi: 10.1074/mcp.M800546-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Meyerhof O. Über die wirkungsweise der hexokinase. Naturwissenschaften. 1935;23(50):850–851. doi: 10.1007/BF01491989. [DOI] [Google Scholar]
- 203.von Euler H, Adler E (1935) Über die Komponenten der Dehydrasesysteme. VI. Dehydrierung von Hexosen unter Mitwirkung von Adenosintriphosphorsäure.
- 204.Trayser KA, Colowick SP. Properties of crystalline hexokinase from yeast. IV. Multiple forms of the enzyme. Arch Biochem Biophys. 1961;94:177–181. doi: 10.1016/0003-9861(61)90026-1. [DOI] [PubMed] [Google Scholar]
- 205.Brown J, Miller DM, Holloway MT, Leve GD. Hexokinase isoenzymes in liver and adipose tissue of man and dog. Science. 1967;155(3759):205–207. doi: 10.1126/science.155.3759.205. [DOI] [PubMed] [Google Scholar]
- 206.Gonzalez C, Ureta T, Sanchez R, Niemeyer H. Multiple molecular forms of ATP:hexose 6-phosphotransferase from rat liver. Biochem Biophys Res Commun. 1964;16(4):347–352. doi: 10.1016/0006-291x(64)90038-5. [DOI] [PubMed] [Google Scholar]
- 207.Jang JC, Leon P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9(1):5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure. 2004;12(3):429–438. doi: 10.1016/j.str.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 209.Feng J, Zhao S, Chen X, Wang W, Dong W, Chen J, Shen JR, Liu L, Kuang T. Biochemical and structural study of Arabidopsis hexokinase 1. Acta Crystallogr D Biol Crystallogr. 2015;71(Pt 2):367–375. doi: 10.1107/S1399004714026091. [DOI] [PubMed] [Google Scholar]
- 210.Rodriguez-Saavedra C, Morgado-Martinez LE, Burgos-Palacios A, King-Diaz B, Lopez-Coria M, Sanchez-Nieto S. Moonlighting proteins: the case of the hexokinases. Front Mol Biosci. 2021;8:701975. doi: 10.3389/fmolb.2021.701975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Didiasova M, Schaefer L, Wygrecka M. When place matters: shuttling of enolase-1 across cellular compartments. Front Cell Dev Biol. 2019;7:61. doi: 10.3389/fcell.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tarze A, Deniaud A, Le Bras M, Maillier E, Molle D, Larochette N, Zamzami N, Jan G, Kroemer G, Brenner C. GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene. 2007;26(18):2606–2620. doi: 10.1038/sj.onc.1210074. [DOI] [PubMed] [Google Scholar]
- 213.Boradia VM, Raje M, Raje CI. Protein moonlighting in iron metabolism: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Biochem Soc Trans. 2014;42(6):1796–1801. doi: 10.1042/BST20140220. [DOI] [PubMed] [Google Scholar]
- 214.Kosova AA, Khodyreva SN, Lavrik OI. Role of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in DNA repair. Biochemistry (Mosc) 2017;82(6):643–654. doi: 10.1134/S0006297917060013. [DOI] [PubMed] [Google Scholar]
- 215.Sweeny EA, Singh AB, Chakravarti R, Martinez-Guzman O, Saini A, Haque MM, Garee G, Dans PD, Hannibal L, Reddi AR, Stuehr DJ. Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. J Biol Chem. 2018;293(37):14557–14568. doi: 10.1074/jbc.RA118.004169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Malhotra H, Kumar M, Chauhan AS, Dhiman A, Chaudhary S, Patidar A, Jaiswal P, Sharma K, Sheokand N, Raje CI, Raje M. Moonlighting protein glyceraldehyde-3-phosphate dehydrogenase: a cellular rapid-response molecule for maintenance of iron homeostasis in hypoxia. Cell Physiol Biochem. 2019;52(3):517–531. doi: 10.33594/000000037. [DOI] [PubMed] [Google Scholar]
- 217.Ahmad I, Singh R, Pal S, Prajapati S, Sachan N, Laiq Y, Husain H. Exploring the role of glycolytic enzymes PFKFB3 and GAPDH in the modulation of Abeta and neurodegeneration and their potential of therapeutic targets in Alzheimer's disease. Appl Biochem Biotechnol. 2023 doi: 10.1007/s12010-023-04340-0. [DOI] [PubMed] [Google Scholar]
- 218.Tossounian MA, Zhang B, Gout I. The writers, readers, and erasers in redox regulation of GAPDH. Antioxidants (Basel) 2020 doi: 10.3390/antiox9121288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sirover MA. The role of posttranslational modification in moonlighting glyceraldehyde-3-phosphate dehydrogenase structure and function. Amino Acids. 2021;53(4):507–515. doi: 10.1007/s00726-021-02959-z. [DOI] [PubMed] [Google Scholar]
- 220.Dai Y, Fleischhacker AS, Liu L, Fayad S, Gunawan AL, Stuehr DJ, Ragsdale SW. Heme delivery to heme oxygenase-2 involves glyceraldehyde-3-phosphate dehydrogenase. Biol Chem. 2022;403(11–12):1043–1053. doi: 10.1515/hsz-2022-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stuehr DJ, Dai Y, Biswas P, Sweeny EA, Ghosh A. New roles for GAPDH, Hsp90, and NO in regulating heme allocation and hemeprotein function in mammals. Biol Chem. 2022;403(11–12):1005–1015. doi: 10.1515/hsz-2022-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Naletova I, Schmalhausen E, Kharitonov A, Katrukha A, Saso L, Caprioli A, Muronetz V. Non-native glyceraldehyde-3-phosphate dehydrogenase can be an intrinsic component of amyloid structures. Biochim Biophys Acta. 2008;1784(12):2052–2058. doi: 10.1016/j.bbapap.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 223.Butterfield DA, Hardas SS, Lange ML. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease: many pathways to neurodegeneration. J Alzheimers Dis. 2010;20(2):369–393. doi: 10.3233/JAD-2010-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.El Kadmiri N, Slassi I, El Moutawakil B, Nadifi S, Tadevosyan A, Hachem A, Soukri A. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease. Pathol Biol (Paris) 2014;62(6):333–336. doi: 10.1016/j.patbio.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 225.Li M, Zhang CS, Zong Y, Feng JW, Ma T, Hu M, Lin Z, Li X, Xie C, Wu Y, Jiang D, Li Y, Zhang C, Tian X, Wang W, Yang Y, Chen J, Cui J, Wu YQ, Chen X, Liu QF, Wu J, Lin SY, Ye Z, Liu Y, Piao HL, Yu L, Zhou Z, Xie XS, Hardie DG, Lin SC. Transient receptor potential V channels are essential for glucose sensing by aldolase and AMPK. Cell Metab. 2019;30(3):508–524 e512. doi: 10.1016/j.cmet.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Huangyang P, Li F, Lee P, Nissim I, Weljie AM, Mancuso A, Li B, Keith B, Yoon SS, Simon MC. Fructose-1,6-bisphosphatase 2 inhibits sarcoma progression by restraining mitochondrial biogenesis. Cell Metab. 2020;31(1):174–188 e177. doi: 10.1016/j.cmet.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Xu D, Wang Z, Xia Y, Shao F, Xia W, Wei Y, Li X, Qian X, Lee JH, Du L, Zheng Y, Lv G, Leu JS, Wang H, Xing D, Liang T, Hung MC, Lu Z. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580(7804):530–535. doi: 10.1038/s41586-020-2183-2. [DOI] [PubMed] [Google Scholar]
- 228.Henderson B, Fares MA, Martin AC. Protein moonlighting in biology and medicine. New York: Wiley; 2016. [DOI] [PubMed] [Google Scholar]
- 229.Gupta MN, Pandey S, Ehtesham NZ, Hasnain SE. Medical implications of protein moonlighting. Indian J Med Res. 2019;149(3):322–325. doi: 10.4103/ijmr.IJMR_2192_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Henderson B, Martin A. Bacterial moonlighting proteins and bacterial virulence. Curr Top Microbiol Immunol. 2013;358:155–213. doi: 10.1007/82_2011_188. [DOI] [PubMed] [Google Scholar]
- 231.Satala D, Satala G, Zawrotniak M, Kozik A. Candida albicans and Candida glabrata triosephosphate isomerase—a moonlighting protein that can be exposed on the candidal cell surface and bind to human extracellular matrix proteins. BMC Microbiol. 2021;21(1):199. doi: 10.1186/s12866-021-02235-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Satala D, Satala G, Karkowska-Kuleta J, Bukowski M, Kluza A, Rapala-Kozik M, Kozik A. Structural Insights into the Interactions of Candidal Enolase with Human Vitronectin, Fibronectin and Plasminogen. Int J Mol Sci. 2020 doi: 10.3390/ijms21217843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Henderson B, Lund PA, Coates AR. Multiple moonlighting functions of mycobacterial molecular chaperones. Tuberculosis (Edinb) 2010;90(2):119–124. doi: 10.1016/j.tube.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 234.Workman P, Clarke P. PI3 kinase in cancer: from biology to clinic. Am Soc Clin Oncol Educ Book. 2012 doi: 10.14694/EdBook_AM.2012.32.e93. [DOI] [PubMed] [Google Scholar]
- 235.Davé V, Uversky VN. Structure and intrinsic disorder in enzymology. Amsterdam: Elsevier; 2023. Roles of intrinsically disordered regions in phosphoinositide 3-kinase biocatalysis; pp. 225–240. [Google Scholar]
- 236.Madsen RR, Vanhaesebroeck B, Semple RK. Cancer-associated PIK3CA mutations in overgrowth disorders. Trends Mol Med. 2018;24(10):856–870. doi: 10.1016/j.molmed.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lindhurst MJ, Parker VE, Payne F, Sapp JC, Rudge S, Harris J, Witkowski AM, Zhang Q, Groeneveld MP, Scott CE, Daly A, Huson SM, Tosi LL, Cunningham ML, Darling TN, Geer J, Gucev Z, Sutton VR, Tziotzios C, Dixon AK, Helliwell T, O'Rahilly S, Savage DB, Wakelam MJ, Barroso I, Biesecker LG, Semple RK. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet. 2012;44(8):928–933. doi: 10.1038/ng.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Marshall JDS, Whitecross DE, Mellor P, Anderson DH. Impact of p85alpha alterations in cancer. Biomolecules. 2019 doi: 10.3390/biom9010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi: 10.1016/j.gene.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 240.Lucas CL, Tangye SG. Editorial: human disorders of PI3K biology. Front Immunol. 2020;11:617464. doi: 10.3389/fimmu.2020.617464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Collingridge PW, Brown RW, Ginger ML. Moonlighting enzymes in parasitic protozoa. Parasitology. 2010;137(9):1467–1475. doi: 10.1017/S0031182010000259. [DOI] [PubMed] [Google Scholar]
- 242.Balmer EA, Faso C. The road less traveled? Unconventional protein secretion at parasite-host interfaces. Front Cell Dev Biol. 2021;9:662711. doi: 10.3389/fcell.2021.662711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sriram G, Martinez JA, McCabe ER, Liao JC, Dipple KM. Single-gene disorders: what role could moonlighting enzymes play? Am J Hum Genet. 2005;76(6):911–924. doi: 10.1086/430799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Reina-Campos M, Moscat J, Diaz-Meco M. Metabolism shapes the tumor microenvironment. Curr Opin Cell Biol. 2017;48:47–53. doi: 10.1016/j.ceb.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lv L, Lei Q. Proteins moonlighting in tumor metabolism and epigenetics. Front Med. 2021;15(3):383–403. doi: 10.1007/s11684-020-0818-1. [DOI] [PubMed] [Google Scholar]
- 247.Adamo A, Frusteri C, Pallotta MT, Pirali T, Sartoris S, Ugel S. Moonlighting proteins are important players in cancer immunology. Front Immunol. 2020;11:613069. doi: 10.3389/fimmu.2020.613069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Boukouris AE, Zervopoulos SD, Michelakis ED. Metabolic enzymes moonlighting in the nucleus: metabolic regulation of gene transcription. Trends Biochem Sci. 2016;41(8):712–730. doi: 10.1016/j.tibs.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 249.Pan C, Li B, Simon MC. Moonlighting functions of metabolic enzymes and metabolites in cancer. Mol Cell. 2021;81(18):3760–3774. doi: 10.1016/j.molcel.2021.08.031. [DOI] [PubMed] [Google Scholar]
- 250.Yadav P, Singh R, Sur S, Bansal S, Chaudhry U, Tandon V. Moonlighting proteins: beacon of hope in era of drug resistance in bacteria. Crit Rev Microbiol. 2023;49(1):57–81. doi: 10.1080/1040841X.2022.2036695. [DOI] [PubMed] [Google Scholar]
- 251.Copley SD. An evolutionary biochemist's perspective on promiscuity. Trends Biochem Sci. 2015;40(2):72–78. doi: 10.1016/j.tibs.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gupta MN, Uversky VN. Pre-molten, wet, and dry molten globules en route to the functional state of proteins. Int J Mol Sci. 2023;24(3):2424. doi: 10.3390/ijms24032424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dayhoff GW, 2nd, Uversky VN. Rapid prediction and analysis of protein intrinsic disorder. Protein Sci. 2022;31(12):e4496. doi: 10.1002/pro.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Meszaros B, Erdos G, Dosztanyi Z. IUPred2A: context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res. 2018;46(W1):W329–W337. doi: 10.1093/nar/gky384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Obradovic Z, Peng K, Vucetic S, Radivojac P, Dunker AK. Exploiting heterogeneous sequence properties improves prediction of protein disorder. Proteins. 2005;61(Suppl 7):176–182. doi: 10.1002/prot.20735. [DOI] [PubMed] [Google Scholar]
- 257.Peng K, Radivojac P, Vucetic S, Dunker AK, Obradovic Z. Length-dependent prediction of protein intrinsic disorder. BMC Bioinform. 2006;7:208. doi: 10.1186/1471-2105-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Peng K, Vucetic S, Radivojac P, Brown CJ, Dunker AK, Obradovic Z. Optimizing long intrinsic disorder predictors with protein evolutionary information. J Bioinform Comput Biol. 2005;3(1):35–60. doi: 10.1142/s0219720005000886. [DOI] [PubMed] [Google Scholar]
- 259.Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, Dunker AK. Sequence complexity of disordered protein. Proteins. 2001;42(1):38–48. doi: 10.1002/1097-0134(20010101)42:1<38::aid-prot50>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 260.Xue B, Dunbrack RL, Williams RW, Dunker AK. Uversky VN (2010) PONDR-FIT: a meta-predictor of intrinsically disordered amino acids. Biochim Biophys Acta. 1804;4:996–1010. doi: 10.1016/j.bbapap.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.