Abstract
The majority of lncRNAs and a small fraction of mRNAs localize in the cell nucleus to exert their functions. A SIRLOIN RNA motif was previously reported to drive its nuclear localization by the RNA-binding protein hnRNPK. However, the underlying mechanism remains unclear. Here, we report crystal structures of hnRNPK in complex with SIRLOIN, and with the nuclear import receptor (NIR) Impα1, respectively. The protein hnRNPK bound to SIRLOIN with multiple weak interactions, and interacted Impα1 using an independent high-affinity site. Forming a complex with hnRNPK and Impα1 was essential for the nuclear import and stress granule localization of SIRLOIN in semi-permeabilized cells. Nuclear import of SIRLOIN enhanced with increasing NIR concentrations, but its stress granule localization peaked at a low NIR concentration. Collectively, we propose a mechanism of SIRLOIN localization, in which NIRs functioned as drivers/regulators, and hnRNPK as an adaptor.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-021-03992-7.
Keywords: RNA localization, KH domain, Nuclear transport, Multi-valent interaction, SILOIN motif
Introduction
Long noncoding RNAs (lncRNAs) are RNA transcripts longer than 200 nucleotides (nt) without protein-coding ability [1]. They were initially considered unimportant, but recent studies have suggested that some may be as important as protein-coding messenger RNAs (mRNAs) [2]. Many lncRNAs and hundreds of mRNAs predominantly exist in the nucleus [3]. The action of RNAs is related to their subcellular location, and dysregulation may be harmful [4–6].
Lubelsky et al. reported that RNAs with one or more stretches of 42-nt SINE-derived nuclear RNA localization (SIRLOIN) sequence were significantly more nuclear enriched [7]. They further demonstrated that hnRNPK was responsible for interaction with SIRLOIN and its nuclear localization [7]. However, the exact mechanism of hnRNPK-mediated nuclear enrichment of SIRLOIN-containing RNAs remains unknown [8].
The ubiquitously expressed RNA-binding protein (RBP) hnRNPK (or hnRNP K) regulates several cellular functions, including gene transcription, splicing, and protein translation [9]. This protein contains multiple DNA/RNA-binding motifs, including three K homology (KH) domains and an RGG domain [10]. Previous studies have suggested that hnRNPK is a nucleocytoplasmic shuttling protein, and its nuclear localization is mediated by its nuclear localization signal (NLS) and the K nuclear shuttling (KNS) domain [11, 12]. NLS binds to nuclear import receptors, such as Importin α1 (Impα1), Importin β1 (Impβ1), and Importin 4. In classical nuclear import, Impα1 functions as an adaptor that can simultaneously bind to NLS and Impβ1 [13]. If hnRNPK is tightly accompanied by SIRLOIN-containing DNA/RNAs, the nuclear import of hnRNPK may simultaneously mediate the nuclear import of the latter.
When cells are under stress, mRNAs are bound to RBPs to form stress granules (SGs), resulting in halted cellular translation [14]. Several proteomic studies have indicated that some nucleo-cytoplasmic transport factors, some lncRNAs, and hnRNPK translocate to SGs in stressed cells [15–17]. The excessive SG mislocalization of RBPs disrupts nuclear transport and participates in several neuronal diseases [18]. Whether SIRLOIN is recruited to SG by interacting with hnRNPK is unclear, and how SG localization is affected by nucleo-cytoplasmic transport factors remains unknown.
To understand how hnRNPK and nuclear import receptor may affect the localization of SIRLOIN, the following biochemical and cellular studies were performed. We obtained crystal structures showing interaction between SIRLOIN and hnRNPK, and between hnRNPK and Impα1. Using size exclusion and isothermal titration experiments, these three proteins were shown to form an oligomeric complex containing both strong interactions and weak multivalent interactions. Complex formation was essential for the nuclear import and SG accumulation of SIRLOIN in semi-permeabilized cells. Impα1 and Impβ1 promoted the nuclear import of hnRNPK and SIRLOIN but impeded their SG localization at high concentrations.
Results
SIRLOIN binds to hnRNPK and is imported into the cell nucleus
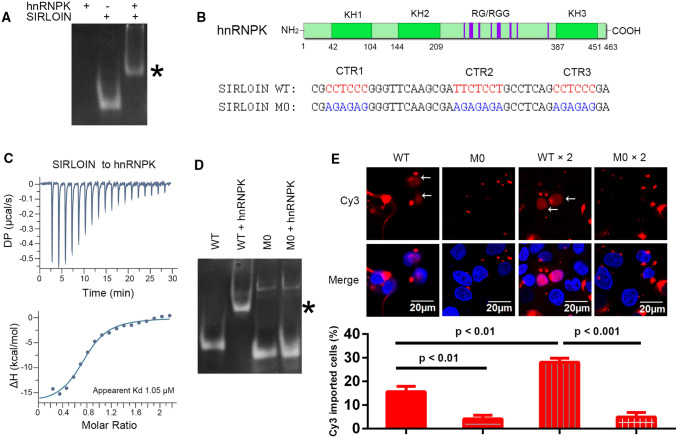
The electrophoretic mobility of SIRLOIN in the presence of hnRNPK was much slower than in the absence of hnRNPK (Fig. 1A), demonstrating that SIRLOIN bound directly to hnRNPK. There are several DNA/RNA-binding regions in hnRNPK, including three KH domains and an RGG domain (Fig. 1B). The apparent binding affinity between SIRLOIN and hnRNPK was 1.1 μM (Fig. 1C). After deleting the RGG domain, the apparent binding affinity decreased only slightly (Fig. S1, Table 1, 1.9 μM), suggesting that the KH domains are probably the main SIRLOIN-interacting sites in hnRNPK.
Fig. 1.
SIRLOIN directly binds to hnRNPK and enters the cell nucleus when transfected. A Electrophoretic mobility shift assay (EMSA) of SIRLOIN in the presence or absence of hnRNPK on an 8% (w/v) polyacrylamide gel. The protein-bound SIRLOIN band is marked with an ‘*’. B Top: domain structure of hnRNPK. KH1–KH3: three K homology (KH) domains. RG/RGG motifs are represented by purple lines. Bottom: the sequence of SIRLOIN and its mutant M0. The predicted hnRNPK binding sites are colored red. The mutations are colored blue. C Isothermal titration calorimetry of SIRLOIN (200 μM) into hnRNPK (18 μM). The bottom panel shows the integrated heat change for each injection and the fitted curve. D EMSA of SIRLOIN WT or M0 in the presence or absence of hnRNPK. E Nuclear import of Cy3-labelled SIRLOIN WT or M0. HeLa cells were transfected with 0.2 µM Cy3-SIRLOIN WT or M0 or tandem repeats of these DNA fragments. The bottom panel shows the percent of cells exhibiting nuclear Cy3 signals in each group. Error bars represent standard deviations (SD) of three independent repeats, each with at least 60 cells analyzed for each sample
Table 1.
Summary of ITC data obtained in this study
| SIRLOIN | MBP–hnRNPK | Ratioa | KDb (µM) |
|---|---|---|---|
| WT (42 bases) | FL | 3:4 | 1.05 ± 0.306 |
| WT (42 bases) | ΔRGG (Δ220–380) | 3:4 | 1.9 ± 0.34 |
| WT (42 bases) | KH1 | N.A | N.B |
| WT (42 bases) | KH1–KH2G157R | 1:4 | 4.9 ± 1.5 |
| WT (42 bases) | KH2 | 1:4 | 2.4 ± 0.9 |
| WT (42 bases) | KH2G157R | N.A | N.B |
| WT (42 bases) | KH3 | 1:4 | 2.8 ± 1.04 |
| CTR2 (GATTCTCCTGC) | KH1–KH2G157R | 1:1 | 61.4 ± 2.2 |
| CTR2 (GATTCTCCTGC) | KH2 | 1:1 | 22.2 ± 2.2 |
| CTR2 (GATTCTCCTGC) | KH3 | 1:1 | 37.5 ± 1.2 |
| CTR3 (CAGCCTCCCGA) | KH1–KH2G157R | 1:1 | 35.5 ± 2.5 |
| CTR3 (CAGCCTCCCGA) | KH2 | 1:1 | 43.8 ± 8.5 |
| CTR3 (CAGCCTCCCGA) | KH3 | 1:1 | 18.3 ± 3.0 |
| TCCC | KH3 | 1:1 | 54.2 ± 4.7 |
| CCTC | KH3 | 1:1 | 24.4 ± 5.3 |
aThe best assumed binding ratio between SIRLOIN and hnRNPK (refer to text). N.A.: not applicable
bExcept the last two rows, these values represent apparent KD, because there are multiple sites or multiple binding modes. N.B.: no binding
SIRLOIN contains three C/T-rich regions (CTRs), named CTR1, CTR2, and CTR3 (Fig. 1B), which are supposed to bind to KH domains [19]. A SIRLOIN mutant (M0), which was mutated at all three CTRs, lost binding to hnRNPK (Fig. 1D), suggesting that these CTRs are indispensable for the hnRNPK interaction. To investigate whether this interaction contributed to the subcellular localization of SIRLOIN, Cy3-labeled wild-type (WT) SIRLOIN or M0 (42-nt ssDNA) was directly incubated with HeLa cells, and subcellular localization was observed after 6 h. M0 did not display any nuclear import, but WT SIRLOIN entered the nucleus in about 16% of transfected cells (Fig. 1E). The nuclear import of WT was potentiated by fusing two SIRLOINs in tandem, both in terms of percentage of cells exhibiting nuclear import (28%) and in terms of nuclear Cy3 brightness, but a tandem M0 sequence did not enter the nucleus. In conclusion, the hnRNPK-interacting C/T-rich motifs of SIRLOIN played a role in its nuclear import.
The multivalent interaction between hnRNPK and SIRLOIN
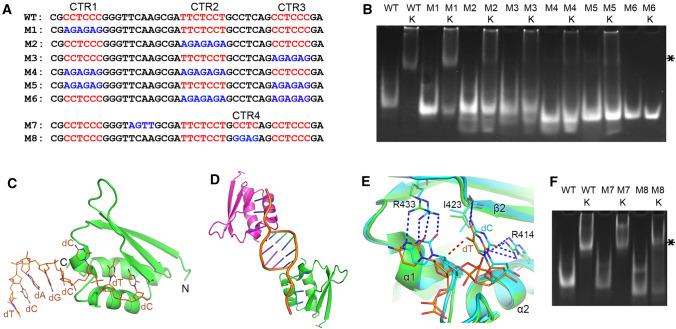
To explore the contribution of the three C/T-rich regions (CTRs) to hnRNPK binding, six mutants of SIRLOIN (M1–M6) were constructed (Fig. 2A). The EMSA showed that all CTRs are involved in hnRNPK binding, but the second and the third CTRs contributed slightly more towards hnRNPK binding (Fig. 2B). Isothermal titration calorimetry (ITC) was used to measure the binding affinity between SIRLOIN and the three KH domains. KH1, KH2, and KH3 displayed apparent binding affinities of 4.9, 2.4, and 2.5 µM, respectively (Table 1 and Fig. S1). As the CTR1 sequence is the same as CTR3, we further compared the binding affinity of the three KH domains towards CTR2 and CTR3. The binding affinities all ranged in the middle μM region for different combinations (Table 1, Fig. S2). Considering that ITC is generally less accurate when binding affinity is weaker than 10 μM, the observed small differences in affinity may not be sufficient to deduce any specific recognition pattern between CTRs and the KH domains, but was likely a weak and multivalent interaction that is often observed in membrane-less organelles (see later sections) [20, 21].
Fig. 2.
Multivalent binding mode between SIRLOIN and hnRNPK. A Sequences of SIRLOIN and its mutants. B EMSA of SIRLOIN and its mutants in the presence or absence of hnRNPK. The protein-bound SIRLOIN band is marked with an ‘*’. C Crystal structure of hnRNPK KH3 domain (green) in complex with CTR3 DNA (orange). D The imperfect double helix formed between two asymmetric unit DNA molecules. The two protein–DNA complexes are colored green and magenta, respectively. E Structural alignment with the CCCC–KH3 complex (pdb:1zzj). Only the second and the third nucleotides are displayed for clarity. The protein residues forming intermolecular hydrogen bonds (dashed lines) are displayed as sticks. The red dashed line represents mild steric hindrance (3.2 Å). F EMSA of SIRLOIN and its mutants in the presence or absence of hnRNPK
Crystal structure of KH3 in complex with CTR3
Several attempts were made to crystallize the full length or segments of hnRNPK with SIRLOIN, and a crystal structure of the KH3 domain in complex with a ssDNA spanning CTR3 was successfully obtained. The structure was solved by molecular replacement using an ssDNA:KH3 structure (PDB 1zzi). The crystal was scaled to space group P6222, containing one complex of protein–DNA per asymmetric unit (Fig. 2C). Except for four nucleotides (CCTC) that interact with KH3, four more nucleotides (TCAG) at the 5′ of CCTC have good electron densities (Fig. S3). The extra nucleotides are observed because of crystal packing, whereby TCAGC imperfectly base-paired with another TCAGC from an adjacent asymmetric-unit ssDNA (Fig. 2D).
After structural alignment with other KH3–DNA structures, a high DNA binding plasticity was noticed, although all involved four C/T nucleotides and that all backbone phosphates are oriented towards a basic patch in KH3 (Fig. S5). A comparison with the most closely related CCCC binding motif shows that this T is marginally tilted and the second C is slightly pushed away when T is present at the third position (Fig. 2E). This conformational change is likely necessary to accommodate the extra methyl group present in T. The essential hydrogen bonds between DNA and KH3 are conserved, as KH3 adapted to the base changes accordingly (Fig. 2E). These observations suggest that a T residue could be allowed at the third position. The ITC results confirmed that CCTC was able to bind to KH3, comparable to the KH3 affinities of CTR2, CTR3, and the TCCC motif in CTR3 (Table 1, Fig. S4).
A previously neglected CCTC motif between CTR2 and CTR3 was detected in the SIRLOIN sequence (Fig. 2A). To test whether this C/T rich region (CTR4) was involved in hnRNPK binding, two more mutations were introduced into SIRLOIN (Fig. 2A). The EMSA showed that a mutation in the linker between CTR1 and CTR2 (M7) did not change hnRNPK binding, but a CTR4 mutation (M8) reduced hnRNPK binding (Fig. 2F), similar to the effect of the CTR1 mutation (Fig. 2B). Thus, CTR4 was likely another KH-binding CTR motif in SIRLOIN.
Nuclear import of hnRNPK via the classical nuclear import
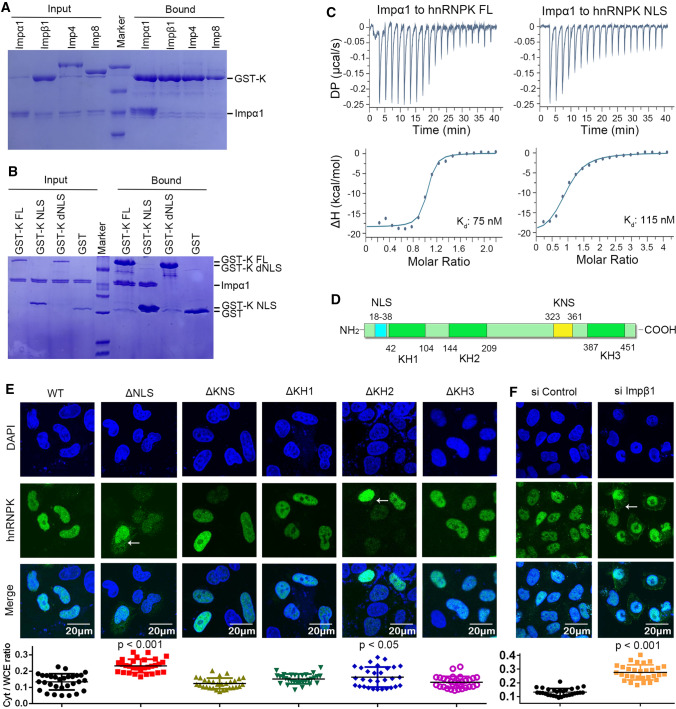
Our earlier results showed that SIRLOIN was imported into the nucleus and that it bound to hnRNPK. We hypothesized that hnRNPK, by binding to nuclear import receptors (NIRs), functioned as an import adaptor for SIRLOIN. The binding of GST–hnRNPK to several NIRs was tested by pull down assay. GST–hnRNPK specifically retained importin α1 (a.a. 70-C, Impα1), but not any of the other tested NIRs (Figs. 3A, S6). Similar to the WT protein, the isolated NLS (18–38) bound tightly to Impα1 (Fig. 3B). Moreover, deleting NLS (ΔNLS) completely abolished Impα1 binding, suggesting that NLS is solely responsible for Impα1 binding. Consistent with the pull down assay, ITC showed that FL hnRNPK and its NLS bound to Impα1 with comparable affinities (Fig. 3C, 75 and 115 nM).
Fig. 3.
Classical nuclear import pathway mediates nuclear import of hnRNPK. A GST–hnRNPK (GST-K) pull down assay of several purified nuclear import receptors (NIRs) including Impα1, Impβ1, Imp4, and Imp8. B GST-tagged hnRNPK or its fragments pull down purified Impα1. C ITC titration of Impα1 (200 μM) into hnRNPK (20 μM) or its NLS (20 μM). D Regions of hnRNPK that may be involved in its nuclear import and the three KH domains. E Subcellular localization of HA-tagged hnRNPK or its deletion mutants. Arrows indicate cytoplasmic hnRNPK. HeLa cells were stained 48 h after transfection. Quantification of the cytoplasmic ratio (Cyt/WCE) is displayed at the bottom. Error bars represent SD for at least 30 cells from each sample. The result was statistically compared with WT. F Subcellular localization of endogenous hnRNPK in the presence of siControl or siImpβ1
To compare the role of different domains of hnRNPK in its nuclear import, the subcellular localization of HA-tagged hnRNPK or its mutants was analyzed by confocal microscopy. Unlike hnRNPK which was highly nuclear-localized, a small fraction of ΔNLS was localized to the cytoplasm. In contrast, deleting KNS, KH1, or KH3 did not affect hnRNPK subcellular localization (Fig. 3E). Deleting KH2 also slightly increased its cytoplasmic localization, but this does not indicate that KH2 is involved in nuclear import, rather that deleting KH2 causes the protein to become unstable (proper folding of KH1 depends on KH2, as shown earlier). Since Impα1 has several functionally redundant homologs in cells (but they all rely on Impβ1), small interference of Impβ1 was performed to test the import dependency on classical nuclear import. Under Impβ1 knockdown, endogenous hnRNPK was localized more in the cytoplasm than the siControl (Fig. 3F).
Crystal structure of Impα1 in complex with the NLS of hnRNPK
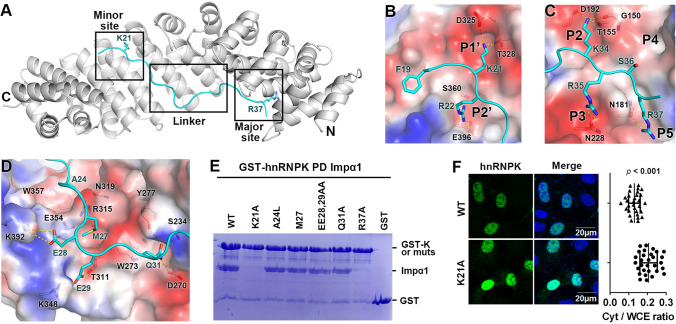
To analyze the interaction between Impα1 and the NLS of hnRNPK, their complex crystal structure was determined at 2.8 Å (Table S1). The bipartite NLS binds to the major and minor NLS binding sites of Impα1 (Fig. 4A). At the minor site, K21 and R22 are inserted in the P1’ and P2’ pockets, respectively (Fig. 4B). F19 is docked in a nearby hydrophobic pocket. At the major NLS site, K34, R35, and R37 form electrostatic interactions with residues from P2, P3, and P5, respectively (Fig. 4C). Unlike most of the other bipartite NLSs, the NLS density of hnRNPK is also strong in the middle linker region (Fig. S7). In this region, A24 and M27 form several hydrophobic contacts with Impα1 (Fig. 4D). E28 and E29 form electrostatic interactions with two lysine residues (K392 and K348) in Impα1. Q31 forms hydrogen bonds with the three polar amino acids S234, Y277, and D270.
Fig. 4.
Structural basis of hnRNPK binding to Impα1. A Crystal structure of hnRNPK NLS (cyan) in complex with Impα1 (grey). K21 and R37 are displayed as sticks. B Zoom-in view of the minor NLS site. The interacting residues are displayed as sticks. Impα1 is displayed as an electrostatic surface potential map. Main chain atoms are omitted for clarity. Yellow dashed lines represent hydrogen bonds. C Zoom-in view of the major NLS site. The main chain atoms, except G150, are omitted for clarity. D Zoom-in view of linker region and its interaction with Impα1. E GST–hnRNPK or its mutants pull down Impα1. The bound protein is stained with Coomassie Blue. F Subcellular localization of hnRNPK and its mutant K21A. Hela cells were transfected with HA–hnRNPK WT or K21A, and visualized 48 h after transfection. Right panel shows the quantification of the cytoplasmic fraction of hnRNPK (Cyt/WCE). Error bars represent SD for at least 30 cells from each sample
Based on the crystal structure, a few hnRNPK mutations were designed to evaluate the critical Impα1 binding residues. The pull down assay showed that both K21 and R37 were essential for Impα1 binding (Fig. 4E), suggesting that the interaction was dependent on the major and minor sites. None of the linker residue mutations reduced Impα1 binding dramatically, indicating that these residues were less important in the interaction. A subcellular localization analysis showed that K21A was more cytoplasmic than WT, verifying that K21 was a critical NLS element (Fig. 4F).
The oligomeric complex formed by hnRNPK, SIRLOIN, and Impα1
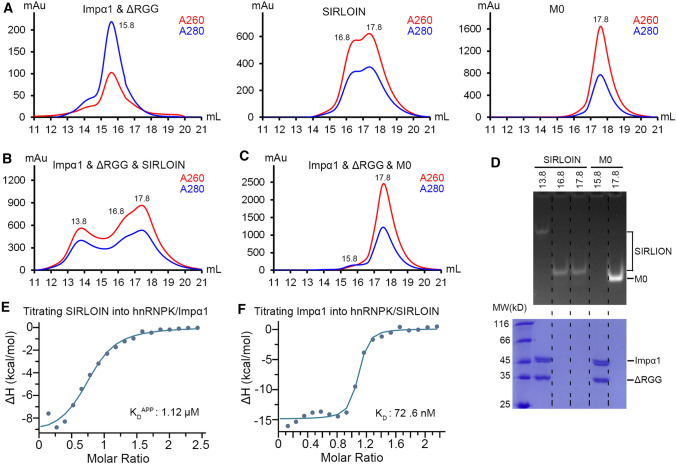
To enter the nucleus via classical nuclear import pathway, hnRNPK and SIRLOIN must be able to form a complex with Impα1. Size exclusion chromatography was used to analyze whether such complex could form in solution. The RGG region of hnRNPK is mostly disordered and often cause hnRNPK aggregation, thus a hnRNPK mutant without the RGG domain (ΔRGG) was analyzed. The ΔRGG–Impα1 complex was eluted at 15.8 mL peak volume, and SIRLOIN was eluted in two peaks at 16.8 and 17.8 mL (Fig. 5A). Co-eluting ΔRGG/Impα1 with twofold excess of SIRLOIN resulted in a new peak at 13.8 mL (Fig. 5B), but such a new peak was not observed when co-eluting ΔRGG/Impα1 and M0 (Fig. 5C). PAGE analysis showed that the 13.8 mL fraction indeed contained ΔRGG, Impα1, and SIRLOIN (Fig. 5D). In contrast, the ΔRGG–Impα1 and M0 eluted with separate peaks of protein and DNA.
Fig. 5.
Oligomeric complex formed by hnRNPK, SIRLOIN, and Impα1. A Size exclusion profile of ΔRGG (hnRNPK without a.a. 220–380)/Impα1, SIRLOIN, and M0. The number beside the elution peak indicates the elution volume. B Size exclusion elution profile of hnRNPK/Impα1 mixed with twofold molar excess of SIRLOIN. C Size exclusion elution profile of hnRNPK/Impα1 mixed with twofold molar excess of M0. D PAGE (top panel) and SDS/PAGE (bottom panel) analysis of peak fractions from B and C. E Integrated ITC heat changes and curve fitting for titrating SIRLOIN (200 µM) into a preformed complex of hnRNPK (16 µM) and Impα1 (16 µM). F Integrated ITC heat changes and curve fitting for titrating Impα1 (200 µM) into a preformed complex of hnRNPK (18 µM) and SIRLOIN (13.5 µM)
The calculated molecular weight (MW) for the Impα1/ΔRGG/SIRLOIN complex is 409 kD, which is much greater than the theoretical MW of a trimeric complex formed by one copy of each molecule (total 96 kD). Therefore, the complex formed must be a higher oligomeric state. The Impα1 to ΔRGG ratio in the complex should theoretically be one, which agreed well with the SDS-PAGE band intensities (Fig. 5D). The relative peak heights suggest that the molar ratio of Impα1/ΔRGG vs. SIRLOIN is probably greater than 1, as the height of the 13.8 mL peak is lower than the DNA peak in Fig. 5B. Our earlier analysis suggested that hnRNPK might bind to SIRLOIN at a 4:3 molar ratio (Fig. 2), with all CTR/KH sites saturated in the complex. The theoretical MW of four copies of Impα1/ΔRGG and three copies of SIRLOIN is 370 kD, largely agrees with the calculated MW of the complex. The data could not exclude the possibility of existing complexes with other molar ratios, e.g., 5:4 (468 kD) and 3:3 (288 kD), but these complexes were unlikely to be predominant events.
The earlier biochemical and structural results showed that hnRNPK uses non-overlapping domains for binding to SIRLOIN or Impα1, but whether the two binding events are independent of each other is unclear. ITC revealed that the apparent binding affinity between SIRLOIN and hnRNPK was barely affected by Impα1 (compare Figs. 1C, 5E). Similarly, the binding affinity between Impα1 and hnRNPK was hardly affected by SIRLOIN (compare Figs. 3C, 5F). Hence, there was no positive or negative cooperativity in binding among the three molecules.
Forming a complex with hnRNPK and Impα1 is essential for the nuclear import of SIRLOIN
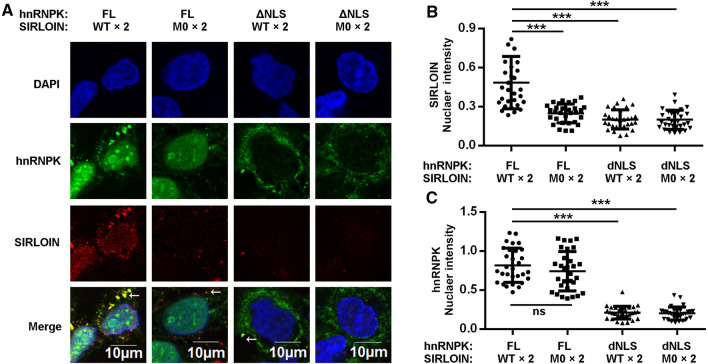
Although SIRLOIN entered the nuclei of living cells (Fig. 1E) and SIRLOIN formed a complex with hnRNPK and Impα1 (Fig. 5), it has not been demonstrated that SIRLOIN enters the nucleus via binding to hnRNPK and via classical nuclear import. Using semi-permeabilized cells, SIRLOIN WT, but not the M0 mutant, entered the nucleus in the presence of NIRs (Impα1 and Impβ1) and hnRNPK (Fig. 6A). When ΔNLS was added instead of FL hnRNPK, SIRLOIN no longer entered the nucleus, suggesting that the nuclear import of SIRLOIN is dependent on the NLS (Fig. 6B). Thus, disruption of the complex by NLS deletion or CTR mutation all inhibited the nuclear import of SIRLOIN. As expected, the nuclear import of hnRNPK required binding to Impα1, but not binding to SIRLOIN (Fig. 6C).
Fig. 6.
Nuclear import of SIRLOIN is dependent on its ability to bind hnRNPK and requires the NLS of hnRNPK. A Confocal images of semi-permeabilized HeLa cells treated with the classical NIRs (1 µM), GST–hnRNPK–HA (2 µM), and Cy3-SIRLOIN or its mutants (2 µM). GST–hnRNPK–HA was stained with HA anti-body. B Quantification of SIRLOIN nuclear intensity in the different groups, normalized by nuclear DNA intensity (DAPI). Error bars represent SD for at least 30 cells from each group. ***p < 0.001. C Quantification of hnRNPK nuclear intensity in different groups, normalized by nuclear DNA intensity (DAPI). Error bars represent SD for at least 30 cells from each group
Forming a complex with hnRNPK and Impα1 is essential for the SG localization of SIRLOIN
In addition to nuclear import, SIRLOIN and hnRNPK also formed cytoplasmic puncta resembling possible SG sites (Fig. 6A, arrows). Thus, Tia1 was transfected to mark the SGs, and the in vitro nuclear import experiment was re-performed. Interestingly, Tia1 expressing cells were slightly refractory to nuclear import of hnRNPK compared with cells not overexpressing Tia1 (Fig. S8). This inhibition of nuclear import was relieved by increasing the concentration of digitonin, a reagent used to permeabilized cell membranes, suggesting that Tia1 may have inhibited nuclear import by strengthening the integrity of the cell membrane. The Tia1 colocalization were specific for hnRNPK and SIRLOIN as another nuclear import cargo mCherry–NLSSV40 did not show such staining (Fig. S9).
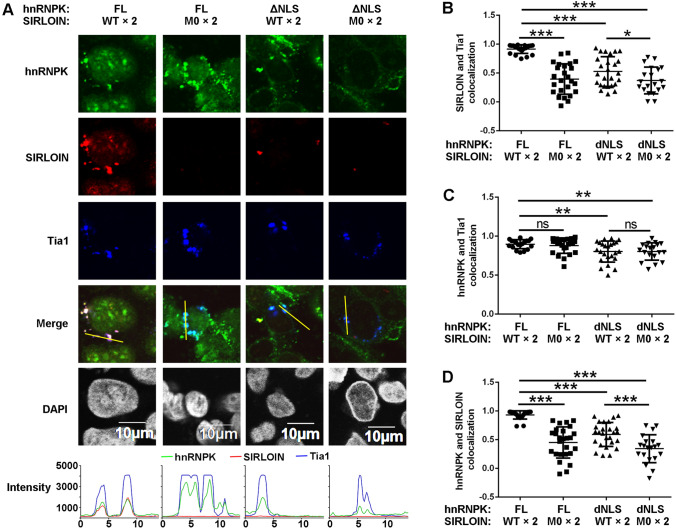
At elevated concentration of digitonin, cytoplasmic hnRNPK and SIRLOIN were highly colocalized with Tia1 (Fig. 7A), confirming that they could be incorporated into SGs. The extent of SG localization by M0 was substantially reduced, both in terms of Tia1 colocalization and intensity (Fig. 7A, B). Similarly, SIRLOIN in the presence of ΔNLS was barely localized in SGs. These results suggest that forming a complex with hnRNPK and Impα1 is required for SG deposition of SIRLOIN. In contrast, SG localization of hnRNPK was independent of binding to SIRLOIN and only slightly dependent on its NLS (Fig. 7C). The weaker colocalization of ΔNLS in SGs was likely due to the greater extent of non-SG staining (Fig. 7A). As expected, colocalization of hnRNPK and SIRLOIN decreased when the CTRs were mutated (Fig. 7D).
Fig. 7.
Stress granule localization of SIRLOIN and hnRNPK in the presence of NIRs. A Subcellular localization of GST–hnRNPK–HA (2 µM) and Cy3-SIRLOIN (2 µM) or their mutants (2 µM) in semi-permeabilized Tia1-Flag expressing HeLa cells. NIRs were added to all groups at a concentration of 1 µM. The three channel pixel intensities of the yellow lines are shown in the bottom panels. Pearson’s correlation coefficients calculated from the line intensities for SIRLOIN vs. Tia1 (B), hnRNPK vs. Tia1 (C), and hnRNPK vs. SIRLOIN (D). Error bars represent SD for at least 20 cells from each sample. ***p < 0.001, **p < 0.01, *p < 0.05
Nuclear import and SG localization of SIRLOIN and hnRNPK at different NIR concentrations
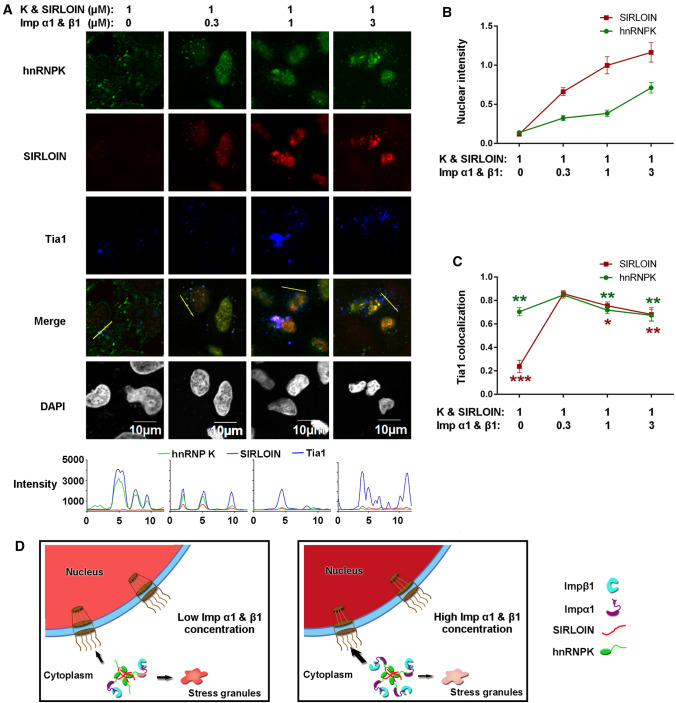
The above analysis indicated nuclear import and SG localization of SIRLOIN is dependent on the NLS of hnRNPK, but it has not been demonstrated whether the NIRs are required for this process. Thus, localization of hnRNPK and SIRLOIN at different concentrations of NIRs (Impα1 and Impβ1 at 1:1 molar ratio) was investigated. The results showed that the nuclear intensity of hnRNPK and SIRLOIN increased gradually with increasing concentrations of NIRs, as expected (Fig. 8A, B). SG localization of SIRLOIN was reduced both at a high NIR concentration and with no NIRs, suggesting that NIRs have a concentration-dependent effect (Fig. 8C). SG localization of hnRNPK was less dependent on the NIRs but also displayed a peak at a low NIR concentration (Fig. 8C). These results indicate that localization of SIRLOIN and hnRNPK indeed requires NIRs. Taken together, we propose a mechanism of SIRLOIN localization that is assisted by the adaptor hnRNPK and regulated by the concentration of NIRs (Fig. 8D).
Fig. 8.
Nuclear import and SG localization of hnRNPK and SIRLOIN at different concentrations of NIRs. A Subcellular localization of GST–hnRNPK–HA and Cy3-SIRLOIN at different concentrations of NIRs in semi-permeabilized Tia1-Flag expressing HeLa cells. B Nuclear SIRLOIN or hnRNPK intensities, normalized by the respective DAPI intensities. Error bars represent standard error of the mean (SEM) for at least 20 cells from each sample. C Line intensity Pearson’s correlation coefficients for ‘SIRLOIN vs. Tia1’ and ‘hnRNPK vs. Tia1’. Error bars represent SEM for at least 20 cells from each sample. The result was statistically compared to the 0.3 µM NIR group. ***p < 0.001, **p < 0.01, *p < 0.05. D A proposed model of SIRLOIN nuclear import and stress granule localization, assisted by the hnRNPK adaptor. SIRLOIN is imported into the nucleus and SGs at low NIR concentrations, and preferably imported into the nucleus at high NIR concentrations
Localization of longer hnRNPK binding RNAs in semi-permeabilized cells
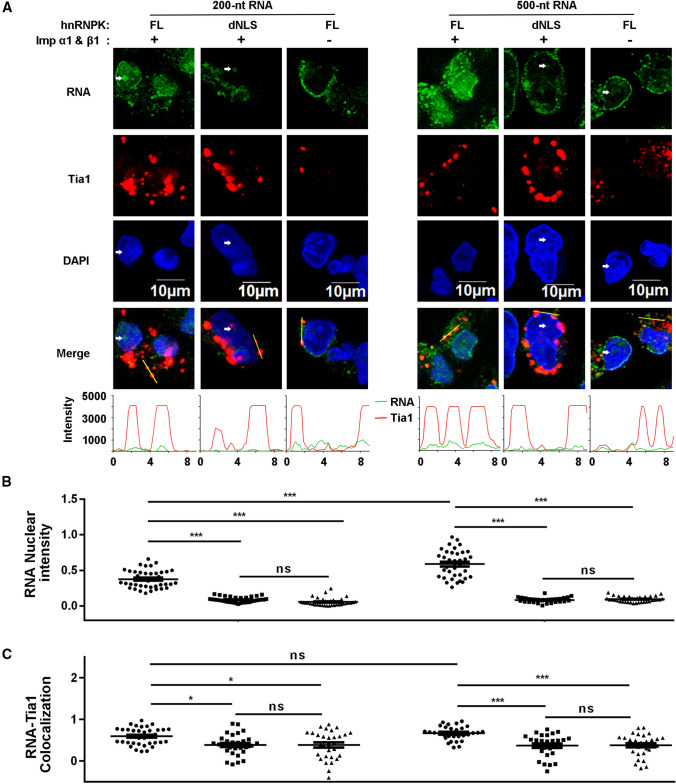
The above experiments used ssDNA of 42 or 84 nt length, and was limited to SIRLOIN sequence only, but it is unknown whether hnRNPK and NIRs could mediate the localization of longer, of RNA-type, non-SIRLOIN hnRNPK binding motifs. In this regards, two fluorescein-labelled RNA fragments (200 nt and 500 nt) encompassing the hnRNPK binding region of lncRNA MYU were in vitro transcribed and tested using in vitro nuclear import assay. Results showed that both of these two RNA fragments could enter nucleus in the presence of hnRNPK and NIRs (Fig. 9A). As expected, in the absence of NIRs, or when hnRNPK NLS was deleted, nuclear import of RNA was not observed. The intensity of 500-nt RNA was slightly stronger (1.6 fold for means) compared with that of the 200-nt RNA (Fig. 9B), but it was labelled approximately 2.7 times more with fluorescein (5.1 and 1.9 fluorescein per molecule, respectively). Therefore, the longer fragment was actually less efficient in nuclear import. Unlike SIRLOIN, the SG localization of these two RNAs were very weak (Fig. 9A, refer to line intensities), although there were statistical significances observed (Fig. 9C).
Fig. 9.
Localization of longer RNA fragments that contain hnRNPK binding motif from lncRNA MYU. A Subcellular localization of 200- and 500-nt RNAs under different protein factors in semi-permeabilized Tia1-Flag expressing HeLa cells. RNA is labelled with fluorescein. Tia1 and DAPI are stained as previous images but colored differently here to enhance visualization. NIRs, hnRNPK, and RNAs were used at 2, 1, and 0.25 µM concentration, respectively. White arrows indicate places where RNAs were enriched, but cellular DNA was largely depleted. B RNA nuclear intensities, normalized by the respective DAPI intensities. Error bars represent SEM for at least 30 cells from each sample. The quantified samples from left to right is in the same order as in panel A. C Line intensity Pearson’s correlation coefficients for ‘RNA vs. Tia1’. Error bars represent SEM for at least 30 cells from each sample. ***p < 0.001, *p < 0.05
Discussion
The KH domains of hnRNPK bind both DNA and RNA, and several studies have used DNA instead of RNA in their experiments [22, 23]. The hnRNPK–SIRLOIN affinity obtained with DNA in our study was highly similar to a recent reported affinity with RNA (1.1 vs. 0.7 µM) [24]. Furthermore, our in vitro nuclear import assays showed that hnRNPK could facilitate the nuclear import of both DNA and RNA. Thus, those binding studies obtained using DNAs should well reflect hnRNPK–RNA interactions.
It has been shown previously that KH1 does not bind to CTRs [24]. We also determined that KH1 does not bind to DNA when purified as a single domain (Table 1). However, when properly folded with the nearby KH2 domain, KH1 was active in DNA binding, similar to the other KH domains. Indeed, KH1 and KH2 fold like a single domain as predicted by AlphaFold [25]. Our work emphasizes verification of proper protein purification/folding when designing or using protein mutants.
Most reports have considered hnRNPK as a poly-C binding protein [24, 26]. A previous structural analysis proposed that KH3 binds only to TCCC or CCCC [22]. Our structural and biochemical studies showed that KH3 could bind to a CCTC motif, suggesting that the third position does not need to be a C. For the fourth position, given its high degree of flexibility (Fig. S5), substituting it with a T or U would probably not reduce KH3 binding affinity. Thus, we updated the definition of KH3 recognition (which may also work for KH1 and KH2), i.e., except the second base that is exclusively C [22], the other three bases could be any pyrimidine (C, T, or U).
This new definition can explain the partial conservation of T/U in hnRNPK-bound sequences, particularly that some of the hnRNPK binding sequences lack poly C patches [24, 26]. Furthermore, this definition predicts that the CTR2 of SIRLOIN may bind to KH domains by two overlapping motifs, being either TCTC or TCCT, but not CTCC (CTR2 contains no TCCC or CCCC motif). In solution, it is possible that due to the presence of DNA/RNA secondary structure [24] or steric hindrance only one of the motifs is selectively bound by a KH domain, just like only CCTC in CTR3 is bound by KH3 in the crystal structure (Fig. 2C). It has been previously shown that two tandem unspaced CTR motifs allow simultaneous binding to two KH domains [22]. The fourth identified CTR in SIRLOIN, a CCTC motif, is separated from CTR2 and CTR3 by one and two nucleotides, respectively, rendering SIRLOIN capable of binding four KH domains simultaneously. Together with the crystal structure, ITC, EMSA, and size exclusion results (Figs. 2C, F, 5B; Table 1), it is concluded that SIRLOIN is a tetravalent KH domain binding sequence, instead of trivalent.
Recently, Lubelsky et al. evaluated the binding affinity of different SIRLOIN mutants through hnRNPK RNA immunoprecipitation (RIP) [27]. According to their results, CTR1 and CTR3 are important for hnRNPK binding, but CTR2 and CTR4 are not. The differences between their RIP and our EMSA may be due to different experimental settings. In addition to the differences in mutation, the RIP environment contained abundant cellular factors but our EMSA contained only hnRNPK and SIRLOIN. Thus, these two experiments do not necessarily conflict each other, but may imply that CTR2 and CTR4 were occupied by other cellular factors in cells, or were modified (e.g., into incompatible secondary structure) so that hnRNPK can not bind.
The ITC analysis showed that increasing the number of CTRs increased the binding affinity of each KH domain. This agrees with the avidity theory, whereby tethering multiple binding sites creates ‘forced proximity’ [28]. However, increasing the number of KH domains did not increase SIRLOIN binding affinity (Table 1). Similarly, deleting KH3 did not abruptly reduce its affinity for different CTRs [24]. It has been proposed that the protein hnRNPK functions in chromatin remodeling, and that tandem KH domains in a protein can remodel the RNA structure [29, 30]. Thus, the binding energy gained through tethering multiple KH domains may be counter-balanced by the energy spent on remodeling the DNA/RNA secondary structure (to a higher energy state). In agreement with this speculation, C-rich RNAs without secondary structures have a higher affinity for hnRNPK [24].
Previous reports have shown that hnRNPK contains an NLS and a KNS domain which facilitate its nuclear import [11, 30]. Here, deleting NLS, but not KNS, partially inhibited the nuclear import of hnRNPK. However, our results do not exclude the function of KNS in nuclear import, but that NLS played a greater role than KNS for hnRNPK nuclear import. The finding that the NLS deletion mutant was also mainly nuclear localized suggests that nuclear import of hnRNPK is redundant, similar to nuclear import of many other proteins, whereby they are redundantly imported by multiple factors [31–33]. In particular, hnRNPK contains several RG/GR motifs that may be recognized by transportin 1 [34, 35]. Phosphorylated hnRNPK has been previously observed to undergo nuclear export and associate with SG [36, 37]. The localization of hnRNPK should be collectively determined by its nuclear import, phosphorylation-dependent export, and interaction with nuclear or cytoplamic structures (e.g., paraspeckels, SG). It remains unknown whether other hnRNPK import pathways can co-import SIRLOIN into the nucleus, how hnRNPK is exported, and whether the nuclear export of hnRNPK co-exports RNAs that it binds.
Lubelsky reported that SIRLOIN promotes nuclear accumulation of a mRNA when fused to that mRNA [7]. Here, it was demonstrated that SIRLOIN, but not its CTR mutant, entered the cell nucleus when directly added to cells (Fig. 1E). Tethering two SIRLOIN repeats promoted this process, which is consistent with the report that RNAs containing multiple SIRLOIN repeats are more nuclear-localized than those with one SIRLOIN [7]. In fact, hnRNPK and NIRs imported not only short SIRLOIN ssDNA fragments, but also longer RNA fragments that habours other hnRNPK binding RNA motifs (Fig. 9). Even longer nucleotides (e.g., > 1000 nt) would probably require more energy for transport (thus more NIRs), and more hnRNPK binding sites, or additional RNA binding proteins (import adaptors).
The localization mechanism of SIRLOIN-containing RNA (and more generally lncRNA) is not well understood and ‘nuclear retention’ is generally assumed [4, 38]. Different cis and transfactors are reported to promote nuclear retention for certain lncRNAs, but few bind to retaining nuclear structures, such as laminin or chromosome [38, 39]. For example, SIRLOIN-containing RNAs were depleted from chromatin and nucleolar fraction, but was localized in the nucleoplasm [7. How seemingly unrestricted nuclear lncRNAs are nuclear retained remains to be disclosed. Since most of SIRLOIN-containing RNAs (and other lncRNAs) can be detected in the cytoplasm [4], their nuclear import is not biologically irrelevant in cells. Previously, Landerer and his colleague reported that mitochondrial-encoded lncRNAs can be exported from mitochondria and relocalized to the nucleus [40]. Thus, there exists a lncRNA nuclear import mechanism in cells, and NIRs and hnRNPK may play a role in this step. More broadly, RNA nuclear import has been observed for 5S rRNA, tRNAs, snRNAs, and 890-nt viral RNA [41–44]. Furthermore, most of previous studies have not measured changes in lncRNA nuclear import upon mutagenesis, i.e., whether a mutation decreased the rate of nuclear import rather than increased the rate of nuclear export. Therefore, the possibility of nuclear import as a mechanism of nuclear localization cannot be excluded at the current stage. Nuclear import of some lncRNAs may spatial-temporally regulate the nucleo-cyto ratio and affect the function of these lncRNAs, similar to the protein functional regulation [45]. For some other lncRNAs, nuclear import may work together with nuclear retention mechanisms to scavenge cytoplasmic copies, or rectifying erroneously exported species.
A common feature of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) is the abrupt formation of SGs or cytoplasmic inclusions by different RBPs including FUS, TDP-43, and hnRNP A1 [18, 46]. These RNA-binding proteins and hnRNPK have similar characteristics, e.g., all contain RNA-binding domains (also called RNA recognition motifs) and R/G rich disordered domains, but little is known about mislocalization of hnRNPK in the cytoplasm of cells with neuronal disease [47]. Here, it was found hnRNPK had a high tendency to enter SGs, which was independent of DNA binding but partially dependent on the classical NIRs (Fig. 8). A previous report showed that the SG accumulation of TDP-43 depends on phosphorylation-mediated hnRNPK SG localization [16]. These data altogether suggest that SG localization of hnRNPK may play a role in disease.
The mechanism of hnRNPK SG localization may share some features with its localization in paraspeckles [48, 49]. The RGG domain has been proposed to non-specifically bind DNA/RNA, and to promote its SG (and possibly paraspeckle) localization by interacting with other RNAs or proteins in SGs [50]. Even without the RGG domain, hnRNPK formed an oligomeric complex with SIRLOIN in the context of size exclusion. The affinities of all CTR–KH domain pair in this complex are in the middle micromolar range (Table 1). Thus, it may not be a specifically configured complex, but rather a complex with dynamic and heterogeneous configurations, featuring a weak multivalent interaction that is often observed in membrane-less organelles [20, 21]. At higher concentrations of hnRNPK and SIRLOIN, particularly in SGs, hnRNPK and SIRLOIN may not form such a 4:3 complex, but rather a more complex network involving other CTRs and KH domains in SGs (beyond the interaction complexity of RGG-mediated networks).
The recruitment of hnRNPK into SGs co-deposited its bound SIRLOIN into SGs. This is analogous to storing mRNAs in SGs by RBPs under cellular stress [14]. A previous study suggested that P body (another cytoplasmic membrane-less organelle) association of lncRNA may affect mRNA translation by regulating the types of mRNA associated with P bodies [51], but the function of lncRNA SG localization is unknown. The 200- and 500-nt MYU RNA showed much weaker SG localization compared with SIRLOIN. We speculate that part of those RNAs formed fewer weak-multivalent interactions with SG components, thus did not retain itself in SG. It should be noted that these two RNAs were indeed imported into the nucleus, but not associated with the outer nuclear membrane, since they sometimes were enriched in places where DNA was absent (Fig. 9A). Both the details of RNA and DAPI would be blurred if focused on nuclear membrane layers. It remains to be explored how the nuclear import and SG localization of SIRLOIN is regulated, but the NIR concentration may be a factor (Fig. 8).
Several importins are demonstrated to prevent RBPs from accumulating in SGs [33, 35, 46]. Likewise, our results show that SG association of hnRNPK was reduced by a high concentration of classical NIRs (Fig. 8). However, a low concentration of NIRs enhanced its SG localization. The NIRs are able to equilibrate in and out of the membrane-less organelles including SGs [52]. It is reasoned that a low concentration of NIR promoted SG accumulation of hnRNPK via enhancing its SG accessibility, in addition to preventing its non-specific cytoplasmic staining (Fig. 6A). Unlike hnRNPK, SG accumulation of SIRLOIN was more dependent on NIRs. This warrants further investigation to understand why hnRNPK alone is inefficient at importing SIRLOIN into SGs.
Methods
Cloning, protein expression and purification
The hnRNPK gene and its mutants were cloned into a pMAL expression vector incorporating a Tobacco Etch Virus (TEV) cleavable N-terminal maltose-binding protein (MBP) tag. The plasmids were transformed into Escherichia coli BL21 (DE3) and grown in LB broth medium. Expression of MBP-tagged hnRNPK was induced by adding 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG), and the culture was grown at 37 °C for 4 h. The cells were harvested and sonicated in a buffer containing 50 mM Tris pH 8.0, 200 mM NaCl, 10% glycerol, 5 mM DTT, and 1 mM phenylmethanesulfonyl fluoride (PMSF). The proteins were purified on an MBP column and eluted in a buffer containing 50 mM Tris pH8.0, 200 mM NaCl, 10% glycerol, 5 mM DTT, and 10 mM maltose, followed by anion exchange chromatography (Hitrap Q). The eluates were further purified on a gel filtration column using Äkta Pure (GE Healthcare) in a gel filtration buffer containing 20 mM Tris pH 8.0, 200 mM NaCl, and 2 mM DTT.
Impα1 (a.a. 70-C) was cloned into pGEX-4T-1 incorporating a TEV cleavable N-terminal glutathione S-transferase (GST) tag. The plasmid was transformed into Escherichia coli BL21 (DE3) and grown in LB broth medium. Expression of GST-Impα1 was induced by adding 0.5 mM IPTG, and the culture was grown overnight at 20 °C. The cells were harvested and sonicated in a buffer containing 50 mM Tris pH 8.0, 200 mM NaCl, 10% glycerol, 2 mM DTT, and 1 mM PMSF. The protein was purified with a GST-tag and eluted after TEV cleavage in a buffer containing 50 mM Tris pH 8.0, 200 mM NaCl, 10% glycerol, and 2 mM DTT. The eluate was purified by anion exchange chromatography (Hitrap Q), and further purified by size exclusion in the gel filtration buffer.
Pull down assay
To assess the different interactions, GST-tagged proteins were immobilized on glutathione Sepharose 4B resin and washed three times immediately after immobilization to remove unbound GST-tagged protein. Soluble proteins were incubated with the immobilized proteins in a total volume of 0.5 mL at 4 °C for 1 h. After three washes, bound proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and visualized by Coomassie Blue staining. The pull down buffer contained 20 mM Tris pH 8.0, 200 mM NaCl, 10% glycerol, 2 mM MgCl2, 0.005% Triton X-100, and 2 mM DTT.
Electrophoretic mobility shift assay (EMSA)
Single-stranded DNA (ssDNA) of SIRLOIN and its mutants were purchased from Sangon Biotech (China). SIRLOIN or its mutants (20 pmol) were incubated with or without 60 pmol of hnRNPK (Δ220–380) in a buffer containing 20 mM Tris pH 8.0, 200 mM NaCl, and 2 mM DTT. The reaction volume was 15 µL. After incubation at 4 °C for 0.5 h, the samples were resolved by 8% (w/v) PAGE, and stained with ethidium bromide.
Crystallization and data collection
The KH3 domain of hnRNPK (10 mg/mL) and the ssDNA (CTCAGCCTCCCGACTC, CTR3 is underlined) were mixed at a 1:2 molar ratio. Diffraction-quality crystals were obtained using hanging-drop vapor-diffusion (18 °C) in a solution containing 25% w/v polyethylene glycol (PEG) 3350, 100 mM Bis–Tris pH 5.5, and 200 mM ammonium acetate. The NLS of hnRNPK and Impα1 were mixed at a 2:1 molar ratio, purified by gel filtration, and concentrated to 8–9 mg/mL. Diffraction-quality crystals were obtained using hanging-drop vapor-diffusion (18 °C) in a crystallization solution containing 0.1 M ammonium acetate, 0.1 M Bis–Tris pH 5.5, and 17% w/v PEG 10,000. A 20% glycerol solution was supplemented with the crystallization conditions as a cryoprotectant for both crystals. X-ray diffraction data were collected at Shanghai Synchrotron Radiation Facility (SSRF) beamline BL17U1. The data collection statistics are given in Table S1.
Structure solution and refinement
Coordinates of KH3 (pdb code: 1zzi) were used as the search model for the KH3 and CTR3 complex. The coordinates were refined using the Refmac5 program and manually built using the COOT program. Data in the interval 50.00–3.00 Å resolution were used, and the R value was 0.257 (Rfree = 0.279) for all reflections at the end of the refinement (Table S1).
Coordinates of Impα1 (pdb code: 1pjm) were used as the search model for the NLS and Impα1 complex. The coordinates were refined using the Refmac5 program and manually built using the COOT program. Data in the interval 50.00–2.80 Å resolution were used, and the R value was 0.213 (Rfree = 0.240) for all reflections at the end of the refinement (Table S1).
Isothermal titration calorimetry (ITC)
ITC experiments were conducted at 16 °C using the ITC200 (MicroCal, USA) in a buffer containing 100 mM HEPES pH 7.5 and 200 mM NaCl. As several domains were insoluble or unstable, each fragment was fused with an N-terminal maltose-binding protein (MBP) to improve solubility/stability during protein purification. MBP–KH1 displayed a very low purification yield and no binding to SIRLOIN according to ITC (Fig. S1). However, MBP–KH1–KH2 (residues 18–199) was very stable and of high purification yield. As the G157R mutation in MBP–KH2 completely abolished its ability to bind to SIRLOIN (Fig. S1), MBP–KH1–KH2G157R was used as a surrogate of well-folded KH1. Data was analyzed with MicroCal PEAQ-ITC Analysis Software. Data were fitted using a ‘one set of sites’ model even for binding involving multiples sites, as the binding curves were mostly uniphasic and insufficient to deduce the exact binding affinities for the different sites. The (apparent) binding affinities are reported in Table 1.
Subcellular localization imaging
The pCDNA3.1 plasmid was used to express HA-hnRNPK and its mutants. HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (Hyclone, USA) supplemented with 10% (v/v) fetal bovine serum (Biological Industries, USA). The cells were transfected with TurboFect transfection reagent (ThermoFisher, USA). For the siRNA study, cells were first transfected with siKPNB1 (GAGATCGAAGACTAACAAA) using Lipo2000 (ThermoFisher, USA). After 24 h, the cells were transfected with different plasmids using TurboFect and visualized 48 h later. Antibodies against HA (Protein Tech, 1:200) and hnRNPK (Novogene, 1:100) were used to detect HA-tagged hnRNPK and endogenous hnRNPK. Images were acquired with an Olympus FV-1000 (Japan) confocal microscope, and were analyzed using NIH ImageJ and GraphPad software.
In vitro nuclear import assay in semi-permeabilized cells
HeLa cells were transfected with FLAG-tagged Tia1 plasmids, and the cell membranes were permeabilized with 0.005% digitonin. The cells were incubated with 2 µM SIRLOIN or its mutants (42-nt, ssDNA, 5’ Cy3-labeled, sequence as in Fig. 1B), 2 µM GST–hnRNPK–HA or its mutants, 1 µM Impα1, 1 µM Impβ1, 1 µM NTF2, 2 µM Ran, 1 mM ATP, and 0.005% Triton X-100. Tandem SIRLOIN (84-nt, ssDNA, 5′ Cy3-labeled, two immediately fused SIRLOIN sequences) was used in later experiments, since it gave stronger signals than single SIRLOIN. After a 1 h incubation at room temperature, the cells were washed, fixed, and visualized by immunostaining with antibodies against HA (Protein Tech, 1:200) and FLAG (Protein Tech, 1:300). SIRLOIN was visualized with its Cy3 label. Images were acquired with Olympus FV-1000 confocal microscope and were analyzed using NIH ImageJ and GraphPad software.
In vitro transcription of fluorescent RNA
LncRNA MYU 1457–1664 segment, which contains hnRNPK binding site, were cloned into pcDNA3.1 plasmid. The 200-bp DNA template containing T7 RNA Polymerase promoter and MYU 1457–1644 was amplified from the plasmid. The 500-bp DNA template includes additional 300 bps from the plasmid immediately downstream of MYU. In vitro transcriptions (20 μL) were performed with T7 Yield RNA Transcription kit (Vazyme, China) at 37 ℃ for overnight. Fluorescein–UTP (Roche, USA) was added to 0.2 mM concentration and UTP concentration was reduced to 1 mM. Post-transcription, the DNA template was degraded by incubation with DNase I at 37 ℃ for 15 min. The transcribed products were purified using RNAclean Kit (TIANGEN, China). RNA concentration was determined by A260 spectrophotometer readings. Fluorescein concentration was calculated from A493 spectrophotometer readings and a pre-generated standard curve of fluorescein.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the beamline staff from SSRF beamline BL17U1 [53] and Dr. Rundong Zhang (SKLB) for help in RNA transcription. This study was supported by the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University.
Author contributions
Conceptualization: QS, Methodology: QS, JY, YT, CS, Investigation: JY, QS, Visualization: QS, JY, Supervision: QS, QZ, HX, DJ, YT, Writing—original draft: QS, JY, Writing—review and editing: QS.
Funding
Not applicable.
Availability of data and materials
The structure factor and atomic coordinates have been deposited in the Protein Data Bank (PDB) with accession codes 7CRE and 7CRU.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have read and approved the manuscript for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kapranov P, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;361:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 2.Flynn RA, Chang HY. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell. 2014;14:752–761. doi: 10.1016/j.stem.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2020;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41:761–772. doi: 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butti Z, Patten SA. RNA dysregulation in amyotrophic lateral sclerosis. Front Genet. 2019;9:712. doi: 10.3389/fgene.2018.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubelsky Y, Ulitsky I. Sequences enriched in Alu repeats drive nuclear localization of long RNAs in human cells. Nature. 2018;555:107–111. doi: 10.1038/nature25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Y, et al. New insights into the interplay between non-coding RNAs and RNA-binding protein HnRNPK in regulating cellular functions. Cells. 2019;8:62. doi: 10.3390/cells8010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, et al. The emerging roles of hnRNPK. J Cell Physiol. 2020;235:1995–2008. doi: 10.1002/jcp.29186. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, et al. Post-translational modification control of RNA-binding protein hnRNPK function. Open Biol. 2019;9:180239. doi: 10.1098/rsob.180239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michael WM, Eder PS, Dreyfuss G. The K nuclear shuttling domain: a novel signal for nuclear import and nuclear export in the hnRNP K protein. EMBO J. 1997;16(12):3578–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchins EJ, Belrose JL, Szaro BG. A novel role for the nuclear localization signal in regulating hnRNP K protein stability in vivo. Biochem Biophys Res Commun. 2016;478:772–776. doi: 10.1016/j.bbrc.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH. Classical nuclear localization signals: definition, function, and interaction with importin alpha. J Biol Chem. 2007;282:5101–5105. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youn JY, et al. Properties of stress granule and P-body proteomes. Mol Cell. 2019;76:286–294. doi: 10.1016/j.molcel.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Chang WL, Tarn WY. A role for transportin in deposition of TTP to cytoplasmic RNA granules and mRNA decay. Nucleic Acids Res. 2009;37:6600–6612. doi: 10.1093/nar/gkp717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moujalled D, et al. Phosphorylation of hnRNP K by cyclin-dependent kinase 2 controls cytosolic accumulation of TDP-43. Hum Mol Genet. 2015;24:1655–1669. doi: 10.1093/hmg/ddu578. [DOI] [PubMed] [Google Scholar]
- 17.Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, Parker R. The stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol Cell. 2017;68:808–820 e805. doi: 10.1016/j.molcel.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang K, et al. Stress granule assembly disrupts nucleocytoplasmic transport. Cell. 2018;173:958–971 e917. doi: 10.1016/j.cell.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoga YM, et al. Contribution of the first K-homology domain of poly(C)-binding protein 1 to its affinity and specificity for C-rich oligonucleotides. Nucleic Acids Res. 2012;40:5101–5114. doi: 10.1093/nar/gks058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin EW, et al. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science. 2020;367:694–699. doi: 10.1126/science.aaw8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, et al. Multivalent m(6)A motifs promote phase separation of YTHDF proteins. Cell Res. 2019;29:767–769. doi: 10.1038/s41422-019-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backe PH, Messias AC, Ravelli RB, Sattler M, Cusack S. X-ray crystallographic and NMR studies of the third KH domain of hnRNP K in complex with single-stranded nucleic acids. Structure. 2005;13:1055–1067. doi: 10.1016/j.str.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Braddock DT, Baber JL, Levens D, Clore GM. Molecular basis of sequence-specific single-stranded DNA recognition by KH domains: solution structure of a complex between hnRNP K KH3 and single-stranded DNA. EMBO J. 2002;21:3476–3485. doi: 10.1093/emboj/cdf352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamoto MY, Lammer NC, Batey RT, Wuttke DS. hnRNPK recognition of the B motif of Xist and other biological RNAs. Nucleic Acids Res. 2020;48:9320–9335. doi: 10.1093/nar/gkaa677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jumper J, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla CJ, et al. High-throughput identification of RNA nuclear enrichment sequences. EMBO J. 2018;37:e98452. doi: 10.15252/embj.201798452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubelsky Y, Zuckerman B, Ulitsky I. High-resolution mapping of function and protein binding in an RNA nuclear enrichment sequence. EMBO J. 2021;40:e106357. doi: 10.15252/embj.2020106357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vauquelin G, Charlton SJ. Exploring avidity: understanding the potential gains in functional affinity and target residence time of bivalent and heterobivalent ligands. Br J Pharmacol. 2013;168:1771–1785. doi: 10.1111/bph.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicastro G, Taylor IA, Ramos A. KH–RNA interactions: back in the groove. Curr Opin Struct Biol. 2015;30:63–70. doi: 10.1016/j.sbi.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Bomsztyk K, Denisenko O, Ostrowski J. hnRNP K: one protein multiple processes. BioEssays. 2004;26:629–638. doi: 10.1002/bies.20048. [DOI] [PubMed] [Google Scholar]
- 31.Liang P, et al. KPNB1, XPO7 and IPO8 mediate the translocation ofNF-kappaB/p65 into the nucleus. Traffic. 2013;14:1132–1143. doi: 10.1111/tra.12097. [DOI] [PubMed] [Google Scholar]
- 32.Kimura M, Morinaka Y, Imai K, Kose S, Horton P, Imamoto N. Extensive cargo identification reveals distinct biological roles of the 12 importin pathways. Elife. 2017;6:e21184. doi: 10.7554/eLife.21184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baade I, et al. The RNA-binding protein FUS is chaperoned and imported into the nucleus by a network of import receptors. J Biol Chem. 2021;296:100659. doi: 10.1016/j.jbc.2021.100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourgeois B, et al. Nonclassical nuclear localization signals mediate nuclear import of CIRBP. Proc Natl Acad Sci USA. 2020;117:8503–8514. doi: 10.1073/pnas.1918944117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutten S, et al. Nuclear import receptors directly bind to arginine-rich dipeptide repeat proteins and suppress their pathological interactions. Cell Rep. 2020;33:108538. doi: 10.1016/j.celrep.2020.108538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habelhah H, et al. ERK phosphorylation drives cytoplasmic accumulation of hnRNP-K and inhibition of mRNA translation. Nat Cell Biol. 2001;3:325–330. doi: 10.1038/35060131. [DOI] [PubMed] [Google Scholar]
- 37.Zappa F, et al. The TRAPP complex mediates secretion arrest induced by stress granule assembly. EMBO J. 2019;38:e101704. doi: 10.15252/embj.2019101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220:e202009045. doi: 10.1083/jcb.202009045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong C, Yin Y. Localization of RNAs in the nucleus: cis- and trans-regulation. RNA Biol. 2021;8:1–14. doi: 10.1080/15476286.2021.1894025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landerer E, et al. Nuclear localization of the mitochondrial ncRNAs in normal and cancer cells. Cell Oncol (Dordr) 2011;34:297–305. doi: 10.1007/s13402-011-0018-8. [DOI] [PubMed] [Google Scholar]
- 41.Kramer EB, Hopper AK. Retrograde transfer RNA nuclear import provides a new level of tRNA quality control in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2013;110:21042–21047. doi: 10.1073/pnas.1316579110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baserga SJ, Gilmore-Hebert M, Yang XW. Distinct molecular signals for nuclear import of the nucleolar snRNA, U3. Genes Dev. 1992;6:1120–1130. doi: 10.1101/gad.6.6.1120. [DOI] [PubMed] [Google Scholar]
- 43.O'Neill RE, Jaskunas R, Blobel G, Palese P, Moroianu J. Nuclear import of influenza virus RNA can be mediated by viral nucleoprotein and transport factors required for protein import. J Biol Chem. 1995;270:22701–22704. doi: 10.1074/jbc.270.39.22701. [DOI] [PubMed] [Google Scholar]
- 44.Rudt F, Pieler T. Cytoplasmic retention and nuclear import of 5S ribosomal RNA containing RNPs. EMBO J. 1996;15:1383–1391. doi: 10.1002/j.1460-2075.1996.tb00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baade I, Kehlenbach RH. The cargo spectrum of nuclear transport receptors. Curr Opin Cell Biol. 2019;58:1–7. doi: 10.1016/j.ceb.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Guo L, et al. Nuclear-import receptors reverse aberrant phase transitions of RNA-binding proteins with prion-like domains. Cell. 2018;173:677–692 e620. doi: 10.1016/j.cell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bampton A, Gittings LM, Fratta P, Lashley T, Gatt A. The role of hnRNPs in frontotemporal dementia and amyotrophic lateral sclerosis. Acta Neuropathol. 2020;140:599–623. doi: 10.1007/s00401-020-02203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naganuma T, Nakagawa S, Tanigawa A, Sasaki YF, Goshima N, Hirose T. Alternative 3′-end processing of long noncoding RNA initiates construction of nuclear paraspeckles. EMBO J. 2012;31:4020–4034. doi: 10.1038/emboj.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuster BS, et al. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. Nat Commun. 2018;9:2985. doi: 10.1038/s41467-018-05403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loughlin FE, et al. The solution structure of FUS bound to RNA reveals a bipartite mode of RNA recognition with both sequence and shape specificity. Mol Cell. 2019;73:490–504 e496. doi: 10.1016/j.molcel.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Pitchiaya S, et al. Dynamic recruitment of single RNAs to processing bodies depends on RNA functionality. Mol Cell. 2019;74:521–533 e526. doi: 10.1016/j.molcel.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo L, Fare CM, Shorter J. Therapeutic dissolution of aberrant phases by nuclear-import receptors. Trends Cell Biol. 2019;29:308–322. doi: 10.1016/j.tcb.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang QS, et al. Upgrade of macromolecular crystallography beamline BL17U1 at SSRF. Nucl Sci Tech. 2018;29:68. doi: 10.1007/s41365-018-0398-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The structure factor and atomic coordinates have been deposited in the Protein Data Bank (PDB) with accession codes 7CRE and 7CRU.
Not applicable.