Abstract
Lipids and fatty acids play crucial roles in plant immunity, which have been highlighted over the past few decades. An increasing number of studies have shown that these molecules are pivotal in the interactions between plants and their diverse pathogens. The roles played by plant lipids fit in a wide spectrum ranging from the first physical barrier encountered by the pathogens, the cuticle, to the signalling pathways that trigger different immune responses and expression of defence-related genes, mediated by several lipid molecules. Moreover, lipids have been arising as candidate biomarkers of resistance or susceptibility to different pathogens. Studies on the apoplast and extracellular vesicles have been highlighting the possible role of lipids in the intercellular communication and the establishment of systemic acquired resistance during plant–pathogen interactions. From the pathogen perspective, lipid metabolism and specific lipid molecules play pivotal roles in the pathogen’s life cycle completion, being crucial during recognition by the plant and evasion from the host immune system, therefore potentiating infection. Studies conducted in the last years have contributed to a better understanding of the language of lipids during the cross-talk between plants and pathogens. However, it is essential to continue exploring the knowledge brought up to light by transcriptomics and proteomics studies towards the elucidation of lipid signalling processes during defence and disease. In this review, we present an updated overview on lipids associated to plant–pathogen interactions, exploiting their roles from the two sides of this battle.
Keywords: Fatty acids, Plant–pathogen interaction, Biomarkers, Signalling, Apoplast, Extracellular vesicles
Introduction
Through the different types of plant immunity, including pathogen-associated molecular patters (PAMP) triggered immunity (PTI) [1] and effector triggered immunity (ETI) [2], lipids and fatty acids (FA) play a crucial role, which has been brought to light over the past few decades [3]. The first barrier found by pathogens when encountering the host is the cuticle. This structure is mainly composed by cutin monomers and oligomers, consisting of hydroxy and epoxy-hydroxy C16 and C18 FA [4]. After entering the plant tissue, pathogens find one of the most important cellular compartments in defence, the apoplast. This compartment includes the extracellular matrix and the apoplastic fluid (APF) [5]. In plant–microbe interaction, pathogens secret molecular effectors into the apoplast, triggering a broad modulation on this compartment [6]. Protein composition alterations of the apoplast were reported to occur both qualitatively and quantitatively [7–9], but the modulation of lipids in the apoplast during plant–pathogen interaction remains a black box [10]. Nonetheless, there are a few evidences of the importance of apoplastic lipids in plant–pathogen interactions in systemic acquired resistance (SAR) establishment [11].
Considering the whole cell, it is known that upon pathogen challenge the plant’s lipidic profile may suffer alterations often associated with modification of membrane fluidity, and enzymatic and non-enzymatic synthesis of bioactive lipid mediators such as lipid and FA oxidation products, oxylipins [12]. This modulation was pointed out as a key factor to trigger plant immunity [13–15]. Lipids may be also considered as possible biomarker tools for susceptibility or resistance [16, 17] or even for disease, before the first visual symptoms appear [18]. Some lipids interact with defence-associated proteins in order to exert their role in plant–pathogen interaction, namely with lipid transfer proteins (LTP) and fibrillins [19–23]. Upon pathogen challenge, the synthesis and hydrolysis of different lipid species is necessary to trigger several defence mechanisms. These reactions are necessary so that lipids such as phosphatidic acid (PA) and free fatty acids (FFA) exert signalling roles and activate the jasmonic acid (JA) signalling pathway, programmed cell death (PCD), among others. Phospholipases A, C and D [24] are activated and contribute to release signalling lipids and FA from membranes. Recently, it was shown that pathogen-induced accumulation of nitric oxide (NO) and reactive oxygen species (ROS) promotes the production of azelaic acid (AzA), a lipid derivative that primes plants for salicylic acid (SA)-dependent defences [25]. The oxidation leads to the formation of oxylipins, which participate in a myriad of signalling pathways. These oxidation reactions can be either enzymatic or ROS mediated [26]. Most plant oxylipins are formed via enzymatic activity of the lipoxygenase (LOX) pathway. LOX enzymes catalyse the oxidation of linoleic acid (C18:2) and α-linolenic acid (C18:3) at the carbon position 9 or 13, resulting in the formation of 9- and 13-hydroperoxides, respectively [27]. Oxylipins play an important role in a variety of functions including growth, aging, development, and defence responses to environmental stimuli [28].
In the cross-talk between plants and pathogens, lipids play an important role mainly in: (1) pathogen development and life cycle completion [29, 30]; (2) pathogen recognition and defence response triggering by the host [31–33, 47] and (3) hindering host defence systems and overcoming resistance [35–38]. During plant–pathogen interaction, some lipid metabolism alterations occur also in pathogens including in FA biosynthesis, elongation and degradation and glycerophospholipid metabolism, which are necessary for the pathogen’s development and lifecycle completion [29]. In the case of pathogenic fungi and oomycete that form invasive hyphae, surrounded by an extra-invasive hyphal membrane (EIHM), the enrichment of this structure with phosphoinositides (PI) is essential to build a conductive environment [30]. There is increasing evidence that lipids are part of a language that is transversal to all life kingdoms, which opens new insights into the studies of lipid metabolism and signalling both in plant and pathogen. Understanding lipid dynamics in the field of plant–pathogen interactions is arguably essential to complete the knowledge brought up to light by proteomics and transcriptomics. The analysis of lipids and their derivatives enables the possibility of describing the cross-talk between plants and pathogens and the discovery of pathogen combined strategies targeting lipid pathways. Ultimately, a thorough and complete uncovering of the role of lipids and their signalling pathways may allow finding new control strategies and therapeutic targets for plant diseases. This review compiles the latest important discoveries that took place in the field of lipids in plant–pathogen interactions and provides a renewed look on the significance of these biomolecules in the fight against plant diseases. A different perspective, the pathogen-derived lipids, is explored, as well as virtually uncharted areas concerning lipids, including their presence in the apoplast and the extracellular vesicles lipids.
Fatty acids and lipids’ role in plant–pathogen interaction
In order to survive, plants must perceive and transduce signals to elicit appropriate responses to environmental stimuli. Plant defence responses require energy and activation of signalling molecules, primarily supplied by primary metabolism of carbohydrates, organic acids, amines, amino acids, and lipids [39].
In plant–pathogen interactions, the first barrier found by pathogens before entering the host is the cuticle. This structure protects plants against drought, extreme temperatures, UV radiation, chemical attack, mechanical injuries, and biotic stress [40]. It is mainly made up of cuticular wax and cutin (Table 1) [41], C16 (C16:0-palmitic acid) and C18 (e.g. C18:1-oleic acid) FA, produced in the chloroplast are exported to the endoplasmic reticulum (ER) [42] as Acyl-CoA esters and extended to form very-long-chain FA (VLCFA; C > 20). The acyl chain extension is catalysed by the FA elongase (FAE) complex, on the ER membrane [43]. In the process of FA elongation with the FAE complex, malonyl-CoA is the two-carbon donor [44] contrarily to plastidial FA biosynthesis where this role is played by malonyl-acyl carrier protein (ACP) [42]. The VLCFA are then converted into cuticular waxes either by deactivation of acyl-CoA thioesters to release free acids, by conversion of aliphatic esters via the condensation of an acyl moiety with a primary alcohol, or via reductive pathways that convert acyl-CoAs to primary alcohols or aldehydes. The other component of the cuticle, cutin, is a polyester of C16 and C18 diacids, and ω- and mid-chain hydroxy FA [45]. Cutin is formed by the polymerization of the hydroxy group of C16 and C18 ω-hydroxy FA [41]. Cutin biosynthesis requires the activity of FA oxidases, acyl-activating enzymes [long-chain acyl-coenzyme A synthetase (LACS)] and acyltransferases [glycerol-3-phosphate (G3P) acyltransferase (GPAT)] [41]. Plant cuticle may have multiple roles during plant–pathogen interactions, which can be affected by its thickness, permeability, or specific cuticular components in different tissues [40]. Increasing evidence indicates that the cuticle is actively involved in plant defence [46]. Xia and collaborators observed that Gibberellin-treated Arabidopsis plants respond with increased levels of cuticular wax and cutin components, in association with improved plant immunity responses against Pseudomonas syringae [47]. A number of studies have been associating the plant cuticle with PTI, including from PAMP and damage-associated molecular pattern (DAMP) and ETI. Therefore, the plant cuticle seems to have a role in the activation of both local and systemic defence [46, 48]. During plant–pathogen interactions, the composition of the plant cuticle may be affected by pathogens. Plant leaf wax components, such as very-long-chain C26 aldehydes of Zea mays could affect spore germination and penetration of Blumeria graminis f. sp. hordei in barley [49]. Upon barley inoculation with Fusarium graminearum, the causal agent of Fusarium head blight, the regulation of genes involved in FFA biosynthesis by the WAX INDUCER1 (HvWIN1) transcription factor occurs. As a result, part of the FFA are channelled to the reinforcement of the cuticle, leading to disease resistance [50]. In addition to wax and cutin, plant cuticle contains terpenoids and flavonoids, which have antifungal activities [51, 52]. Although structural lipids drawn from primary metabolism limit pathogen entry, in some situations the basal defence mechanisms are overcame by pathogens. Therefore, plants also reshape their composition of lipids in response to biotic stress to produce metabolites that function as signals or antimicrobial agents.
Table 1.
Plant lipid molecules involved in plant–pathogen interactions that contributed to major breakthroughs in the last years
| Molecule | Function | References | Example of pathosystem |
|---|---|---|---|
| Cutin | Physical barrier | [115] | Solanum lycopersicum–Botrytis cinerea |
| C16 and C18 fatty acids | Structural and signalling lipid constituents | [13] | Vitis vinifera–Plasmopara viticola |
| Signalling | |||
| α-Linolenic acid (C18:3) | JA synthesis precursor | [13] | Vitis vinifera–Plasmopara viticola |
| 7,8,9-, 9S,10S,11R-Octadecenoic acid | Pathogen growth inhibition | [116] | Boehmeria nivea–Phytophthora capsici |
| 2,13,17-Trihydroxy-octadecenoic acid | |||
| Hexadecatrienoic acid | JA synthesis precursor (C16:3 plants) | [117] | Arabidopsis thaliana–Pseudomonas syringae |
| Sphingolipids | Structure | [53] | Arabidopsis thaliana–Pseudomonas syringae |
| Regulation of membrane permeability | |||
| PCD | [53] | Arabidopsis thaliana–Botrytis cinerea | |
| [55] | Arabidopsis thaliana–Pseudomonas syringae | ||
| α-Hydroxylated ceramides | PCD | [57] | Arabidopsis thaliana–Golovinomyces cichoracearum |
| Trihydroxy-LCB | LCB-induced PCD | [57, 58] | Arabidopsis thaliana–Golovinomyces cichoracearum |
| PA | Signalling | [59] | Arabidopsis thaliana–Botrytis cinerea |
| ROS production | Triticum aestivum–Puccinia striiformis | ||
| Defence gene expression | Arabidopsis thaliana–Botrytis cinerea | ||
| PCD | Triticum aestivum–Puccinia striiformis | ||
| PI, e.g. PI 4,5-bisphosphate | Signalling | [118] | Arabidopsis thaliana–Pseudomonas syringae |
| Lysophospholipids | Signalling | [119] | Arabidopsis thaliana–Botrytis cinerea |
| PR and LOX gene expression | [78] | Nicothiana benthamiana–Phytophtora parasitica | |
| ROS production | |||
| PCD | |||
| JA, active form JA-Ile | Signalling | [120] | Arabidopsis thaliana–Pseudomonas syringae |
| Defence gene expression | |||
| DAG | Signalling | [113] | Nicothiana benthamiana–Ralstonia solanacearum |
| PA synthesis |
Sphingolipids (Table 1) are present in cell membranes and have both structural and regulatory roles [53]. These molecules are key players in signalling pathways related to development and responses to abiotic and biotic stresses and are vital for pathogen recognition [54]. These nonglycerol lipids contain a ceramide backbone and a FA attached to a long-chain amino alcohol [53]. The long-chain base (LCB) of the sphingolipid may vary in length, which is usually between 16 and 20 carbons. The balance between sphingolipid bioactive molecules, including LCB and its phosphate (LCB-P, as further discussed in this review) are determining for the regulation of the cell survival death equilibrium [54]. Ceramide is the basic component of sphingolipids and can be modified, forming more complex sphingolipids, for instance glucosyl-ceramide and inositol-phosphorylceramide [53]. Ceramide can also be converted to inositol-phosphorylceramide by transfer of inositol phosphate (IP) from PI [53]. A high degree of sphingolipid variety is also due to long-chain base and acyl chain modifications [53]. Highly hydroxylated sphingolipids increase membrane stability and decrease membrane permeability, providing a higher tolerance to fungal pathogens [53]. Sphingolipids are known to have a role in pathogen-associated PCD [55]. This defence process could be associated either with increased levels of long-chain bases or the ratio of long-chain bases to ceramides [56]. Moreover, the transfer of sphingosine between membranes also plays a key role in PCD [55]. However, König and collaborators observed that double mutants for fatty acid hydroxylase1/2 (Atfah1/Atfah2) (responsible for the hydroxylation of ceramide FA on the α position) that accumulate SA and ceramides are more tolerant to the biotrophic fungus Golovinomyces cichoracearum but do not display a PCD-like phenotype. These observations indicate that ceramides alone are not involved in the induction of PCD, being hydroxylation of the ceramides FA in the α position important for this process [57]. Moreover, a sphingoid base hydroxylase sbh1/sbh2 double mutant completely lacking trihydroxy-LCBs showed enhanced expression of PCD marker genes [58], suggesting that hydroxylated forms of LCB are primary mediators for LCB-induced PCD.
Glycerolipids play a critical role in plant defence against pathogens. Particularly, PA, one of the central molecules in lipid defence signalling, induces defence responses like ROS production, expression of defence genes and PCD [59]. PA can derive from several glycerophospholipids, including the hydrolysis of phosphatidylcholine (PC), phosphatidylethanolamine (PE) and PI [59]. This lipid facilitates transport of lipids across membranes [60], as binding of PA to the enzyme monogalactosyldiacylglycerol synthase 1 (MGD1) stimulates monogalactosyldiacylglycerol glycerol (MGDG) biosynthesis in the chloroplasts [61], and binding of PA to trigalactosyldiacylglycerol proteins (TGD), facilitates the import of lipids from ER into chloroplasts [62]. Due to its small head group and bulky acyl chains, PA forms a conical shape and induces negative curves in membranes [63, 64]. A local increase in membrane PA levels may impact membrane structure and charge, thereby affecting protein or cofactor docking, vesicle formation, and membrane fusion [65]. PA also presents a regulatory role in abscisic acid (ABA)-mediated stomatal closure [66]. Upon pathogen challenge in Nicotiana benthamiana, increased amounts of PA induce immune responses including programmed cell death, accumulation of ROS, and induction of PR-4 expression [67].
Phosphoinositides (Table 1) also plays an important role in plant defence. This lipid hydrolysis catalysed by PI-specific phospholipase C (PI-PLC) originates the signalling molecules IP and diacylglycerol (DAG) [68]. PI may also be processed by kinases and phosphatases, originating deferent phosphoinositide species. As an example, PI 4,5-bisphosphate [PI(4,5)P2], often called PIP2, is the principal substrate of PLC [69]. The hydrolysis of glycerophospholipids catalysed by phospholipases A (PLA) generates FFA and lysophospholipids. These molecules include, for example, lyso-PA and lyso-PC (Table 1) [68]. The signalling activity of lysophospholipids is dependent on the length and position of acyl chain, degree of saturation, and presence of the phosphate head group [70]. FFA can exert different roles in plant–pathogen interaction, from antifungal activity to signalling towards the octadecanoic pathway that leads to the formation of JA [71].
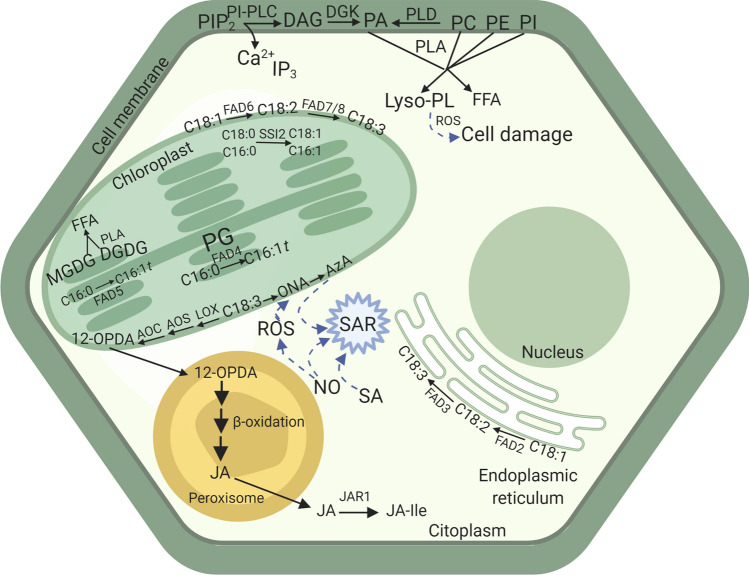
Galactolipids also play a major role in plant defence, namely in modulation of the JA pathway. An increased MGDG:DGDG ratio induces JA overproduction and changes chloroplast shape [72]. Mutations in DGD1, the major DGDG-synthesizing enzyme, severely reduce DGDG content and induce JA overproduction, resulting in stunted growth [72]. MGDG and DGDG also regulate SA levels and SAR [73]. While DGDG is responsible for NO and SA accumulation during SAR, MGDG (Table 1) regulates the biosynthesis of AzA (Fig. 2) and G3P that function downstream of NO [73].
Fig. 2.
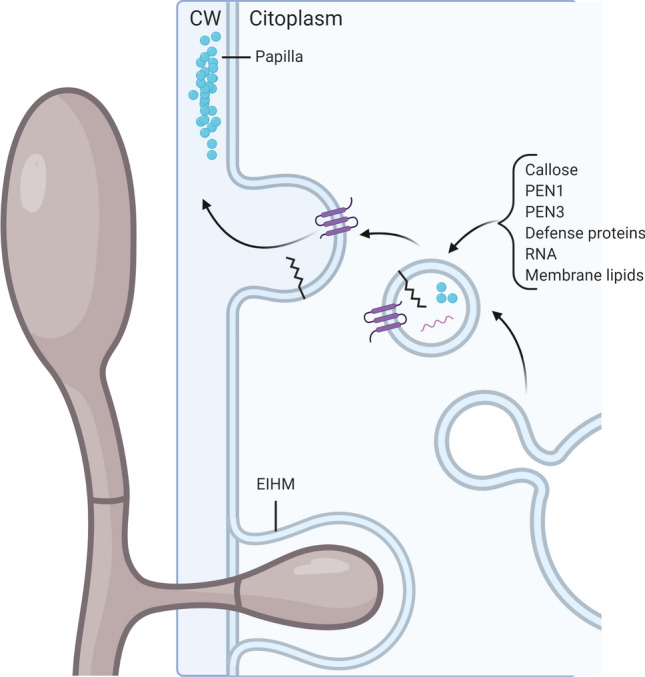
Extracellular vesicle secretion in the interaction between plant and fungal or oomycete pathogens. CW cell wall, EIHM extra-invasive haustorial membrane, PEN1/3 syntaxin/PENETRATION1/3. Blue circles indicate callose
The major players in lipid metabolism and lipid-associated signalling pathways
Upon pathogen challenge, the synthesis and hydrolysis of different lipid species is activated, which is necessary for the triggering of several defence mechanisms. These reactions are necessary so that lipid molecules such as PA (Table 1) and FFA exert signalling roles and activate defence-related genes, the JA-signalling pathway, PCD, among others. The first step to trigger lipid and FA signalling is the activation of the enzymes phospholipases A, C and D [24].
Phospholipases A (PLA), which comprehend the patatin-like, defective in anther dehiscence (DAD)-like and secretory PLA, catalyse the hydrolysis of phospholipids and glycolipids for the formation of lysophospholipids and FFA (Fig. 1) [74]. FFA, namely C18:3 (Table 1) may act as second messengers or as precursors of various oxylipins such as JA (Table 1) [75] (Fig. 1). In pepper leaves, a patatin-like PLA, CaPLP1 is strongly up-regulated during Xanthomonas campestris pv. vesicatoria infection, especially in the incompatible interactions. In this interaction, CaPLP1 is involved in PCD-mediated defence signalling in response to infective microbial pathogens [76]. Also, in Arabidopsis patatin-like PLAs were shown to be involved in pathogen response [77]. Upon inoculation of N. benthamiana with Phytophtora parasitica, higher transcription levels of PLA2 as well as higher levels of lysophosphatidylcholine (lyso-PC) are observed [78]. Upregulation of several PLA-encoding genes including patatin-like, defective in anther dehiscence (DAD)-like and secretory PLA were up-regulated in grapevine leaves infected with Plasmopara viticola [13].
Fig. 1.
Lipid signalling events in plant–pathogen interactions. PIP2 phosphatidylinositol 4,5-bisphosphate, PLA phospholipase A, PI-PLC phosphatidylinositol specific phospholipase C, DAG diacylglycerol, DGK diacylglycerol kinase, PA phosphatidic acid, PLD phospholipase D, PC phosphatidylcholine, PE phosphatidylethanolamine, PI phosphatidylinositol, IP3 inositol-3-phosphate, lyso-PL lysophospholipid, FFA free fatty acid, ROS reactive oxygen species, MGDG monogalactosyldiacylglycerol, DGDG digalactosyldiacylglycerol, FAD fatty acid desaturase, PG phosphatidylglycerol, 12-OPDA 12-oxo-phytodienoic acid, AOC allene oxide cyclase, AOS allene oxide synthase, LOX lipoxygenase, ONA 9-oxononanoic acid, AzA azelaic acid, JA jasmonic acid, JA-Ile jasmonic acid conjugated with isoleucine, JAR1 jasmonates-amide synthetase, NO nitric oxide, SA salicylic acid, SAR systemic acquired resistance, SSI2 SA-inducible 2, C16:0 palmitic acid, C16:1t trans-hexadecanoic acid, C18:0 stearic acid, C18:1 oleic acid, C18:2 linoleic acid, C18:3 α-linolenic acid. The blue arrows indicate induction
Phospholipase C catalyses, among other, the hydrolysis of PI, mainly PIP2, to produce Ca2+, inositol trisphosphate (IP3), a mobilizing second messenger, and DAG (Table 1), which is further phosphorylated in a reaction catalysed by DAG kinase (DGK) to produce PA [59, 79] (Fig. 1). In the presence of the fungal effector xylanase, there is an activation of the enzyme phosphatidylinositol-phospholipase C, SlPLC2. This enzyme is required for xylanase-induced expression of the SA-defence gene marker pathogenesis related1 (SlPR1) and the HR tomato gene marker Hypersensitive response 203 J [80]. Also, pathogen-induced lyso-PC production is mediated by PLA hydrolysis of oxidized phospholipids, which are the products of free radical damage to unsaturated acyl chains of glycerophospholipids in response to pathogen infestation. The increased levels of this lipid lead to the expression of defence-related genes, like pathogenesis-related (PR) and LOX. Moreover, this lyso-PC leads to a higher ROS production and contributes to cell death [78].
Phospholipase D (PLD) catalyses the hydrolysis mainly of PC and PE to form PA [59] (Fig. 1). Some PLD can act as positive or negative regulators of plant immunity [59, 81]. PLDs (α, β, γ, δ, ε and ζ) can be differentiated depending on their requirements and/or affinities for Ca2+, PIP2 and FFA [82]. The predominant isoenzyme is the α-type PLD, which can be detected in both the leaves and seeds of plants and is responsible for the majority of the baseline PLD activity found therein. PLDα does not require phosphoinositides for its activity when assayed in the presence of mM levels of Ca2+ ions. In contrast, the β, γ, δ and ε PLD isoenzymes from Arabidopsis show their highest activity at μM Ca2+ concentrations and require the presence of PIP2 to be fully active [83]. Recently, Schlöffel and co-workers observed that Arabidopsis knock-out mutants for the PLDγ1 (but not PLDγ2 or 3) gene showed a higher resistance to P. syringae pv. DC3000 (biotrophic) and Botrytis cinerea (necrotrophic) [84]. Since the immune response to pathogens with different infection strategies involves antagonistic signalling cascades, SA and JA pathways [85], PLDγ1 may act as a central signalling hub that modulates plant immune responses to different pathogens, working as a negative modulator of the plant immune system [84]. Upon elicitation with the flagellin flg22, mutant plants respond with a twofold increase in ROS production, which indicates that PLDγ1 acts as a negative regulator of plant immunity. This PLD functions independently of SA and JA and is not related to PA production [84].
FA desaturation is also a highly important process for plant defence [3]. The unsaturation of newly formed FA is carried out by the stromal enzyme SACPD (or Δ9 desaturases), which introduces a cis double bond into the acyl-ACP at C9 position [86]. The substrate specificity of the different SACPD isoforms depends on the acyl chain length and the position of the double bond [87]. Among them, the suppressor of SA-inducible (SSI2)-SACPD shows higher specific activity and preference towards C18:0 than for C16:0 [88]. Reactions catalysed by SACPD originate the monounsaturated FA C18:1 and the unsaturated palmitic acid, C16:1 (Fig. 1). Hydrolysis of unsaturated and saturated FA-ACP is preferentially catalysed by the FA acyl-ACP-thioesterases FATA and FATB, respectively [89]. FFA are activated as CoA esters by acyl-CoA synthetase and exported to the cytoplasm. These lipid species are then processed in the ER [90].
The desaturation of FA present in membrane lipids is catalysed by membrane-bound FA desaturase (FAD) enzymes present in the chloroplast or ER membranes [91]. FAD2 and FAD3 catalyse the desaturation of C18:1 and C18:2, respectively, esterified both at sn-1 and sn-2 positions of glycerolipids in the ER [92, 93] (Fig. 1). Desaturation of C18:1 and C18:2 in plastidial membranes is catalysed by FAD6 and FAD7/FAD8, respectively. The FAD6 and FAD7/FAD8 enzymes can catalyse FA desaturation in glycerolipids containing either C16 or C18 FAs at sn-1 or sn-2 positions [94] (Fig. 1). Two other plastidial desaturases, FAD4 and FAD5 specifically catalyse the synthesis of trans C16:1 or Δ7 C16:1 on phosphatidylglycerol (PG) or MGDG, respectively [73, 95] (Fig. 1). Soria-García and co-workers observed that the Arabidopsis desaturase AtFAD8 showed a JA-dependent response both at the gene expression and protein levels, suggesting that this enzyme is coordinated in defence responses [96]. Moreover, ABA induced the decreasing of AtFAD7 mRNA and protein levels, controlling AtFAD7 desaturase activity. This result suggests a higher specialization of FAD7 on biotic and defence responses (as supplier of JA biosynthesis precursors), that could be blocked antagonically by ABA [96].
In early plant–pathogen interaction, lipid and FA oxidation is one of the most important processes. The oxidation process leads to the formation of oxylipins, which participate in a myriad of signalling pathways. These oxidation reactions can be either enzymatic or ROS mediated [26]. Most plant oxylipins identified until now are formed via enzymatic activity of the LOX pathway. LOX catalyses the oxidation of C18:2 and C18:3 at the carbon position 9 or 13, resulting in the formation of 9- and 13-hydroperoxides, respectively [27]. Recently, it was shown that pathogen-induced accumulation of NO and ROS promotes the production of AzA, a lipid derivative that primes plants for SA-dependent defences [25]. Oxylipins play an important role in a variety of functions including growth, aging, development, and defence responses to environmental stimuli [28]. For instance, the 7,8,9-, 9S,10S, 11R-, and 12,13,17-trihydroxy-octadecenoic acids (Table 1) showed to inhibit the growth of the plant pathogens B. graminis, Phytophthora infestans, and B. cinerea [97]. Interestingly, the oxylipin 2(R)-hydroxy-9(Z),12(Z),15(Z)-octadecatrienoic acid (2-HOT) was also found to be produced in Arabidopsis leaf oil bodies upon inoculation with Colletotrichum higginsianum via α-dioxygenase (α-DOX) [98].
The LOX pathway has been proposed to act directly in plant defence by producing antimicrobial compounds [99] or by signalling molecules such as JA that regulates gene expression in plant defence and cell death [100]. Jasmonic acid (JA) is one of the most studied plant oxylipins. Its biosynthesis occurs through different pathways including the octadecanoid pathway starting from C18:3 and the hexadecanoid pathway starting from hexadecatrienoic acid (C16:3) (Table 1) in 16:3 plants such as Arabidopsis [101]. The sequential steps of these pathways take place in different cellular compartments: chloroplasts, peroxisome, and cytoplasm (Fig. 1). The synthesis of 12-oxo-phytodienoic acid (12-OPDA) or deoxymethylated vegetable dienic acid (dn-OPDA) from the oxidation unsaturated FA occurs in the chloroplast in reactions catalysed by LOX, allene oxide synthase (AOS) and allene oxide cyclase (AOC) (Fig. 1). JA is formed as a result of subsequent β-oxidation reactions that occur in the peroxisome. In the cytoplasm, JA is metabolized to different structures, such as methyl jasmonate (MeJA), the bioactive form of JA conjugated with isoleucine (JA-Ile), cis-jasmone (CJ), and 12-hydroxyjasmonic acid (12-OH-JA). The bioactive form of JA will then induce the expression of resistance-related genes [102]. Recent results show that JA-signalling pathway may be suppressed by uncharacterized factors derived from virulent Xanthomonas oryzae pv. oryzae [103]. A transcriptomic study on rice leaves infected with black streaked dwarf virus showed that the expression of JA synthesis-related genes, OsLOX, OsAOS and jasmonate O methyltransferase (OsJMT1) was significantly increased while the hydroperoxidelyase (OsHPL3), a competitor of AOS for the same substrate, was down-regulated [104]. Moreover, in response to Aspergillus parasiticus infection in peanut seeds, accumulation of free fatty acids and induction if LOX activity and gene expression was also observed. This signalling mechanism operates rapidly in resistant cultivars [14]. Recent results show that after wheat inoculation with pathogens of the Fusarium genus, there is a transcript accumulation mainly of TaLox2, TaJAZ9 and the putative PR genes TaPR-4b [105]. The activation of these genes showed that the JA pathway occurs in the defence responses in wheat–fusarium pathosystems. The enzyme LOXd showed to be highly up-regulated in tomato plants inoculated with Fusarium solani [106].
Also, lipid peroxidation products, such as malondialdehyde (MDA) or 4-hydroxy-2-nonenal (4-HNE), were reported to regulate stress-associated transcription factors [107]. After grapevine inoculation with P. viticola, an increase of the lipid peroxidation was observed, including an increase of the MDA levels in a resistant cultivar [21]. In contact with the bacterial effector AvrRpm1, during HR, oxidized derivatives of MGDG, DGDG, sulfoquinovosyl diacylglycerol (SQDG), PG and PI were identified in Arabidopsis [108]. Among these lipid oxidized forms were the OPDA-containing lipids. Despite the fact that the function of the OPDA-containing lipids remains uncertain, it had previously been proposed that Arabidopsis OPDA-containing galactolipids (arabidopsides) might act as chemical defensive compounds against microorganisms as well as function for delayed release of OPDA [109].
Lipid signalling is a key process for the long-distance communication of several stimuli and, therefore, for the establishment of SAR (reviewed in [107, 110]). A nonspecific LTP (nsLTP) have been described to participate in SAR through the interaction with lipid-derived molecules like JA [111]. A nsLTP from Brassica rapa displayed both antifungal and antibacterial activity [111]. Moreover, a nsLTP from Arabidopsis thaliana has been implicated in the AzA-dependent development of SAR [20]. In Arabidopsis transgenic lines expressing wheat LTP4, it was observed that this protein induced a higher resistance to the fungi B. cinerea and Alternaria solani. TdLTP4 showed to be implicated in JA signalling since it is responsive to this oxylipin and upon its expression there is downregulation of Jasmonate ZIM-domain (JAZ) encoding genes [112]. Another lipid-associated protein, fibrillin, belongs to a family called plastid lipid-associated proteins. It can be found at higher levels in plants during defence responses. Upon grapevine inoculation with P. viticola, this protein was observed at higher levels in a resistant cultivar [21]. These proteins act as scaffolds for building lipid droplets that contain FFA, pigments and other lipophilic compounds [19]. There is a correlation between the levels of fibrillin and JA synthesis. Plastoglobules may function as a specialized platform for the synthesis of early JA precursors, storing enough FA (with a prevalence of C18:3) to trigger its synthesis after local oxidative stress [22]. Moreover, upon inoculation of N. benthamiana with the bacteria Ralstonia solanacearum the protein SEC14, a phospholipid transfer protein is induced [113]. This protein exhibits phospholipid transfer activities [114], and may be involved in plant immune response via phospholipid-turnover.
Important discoveries on the role of lipids in defence and disease: an update 2013–2020
The importance of lipids and FA in plant–pathogen interactions has been previously revised [3, 121]. From 2013, an increasing number of studies have shown that plant lipids and FA play key roles in the interaction with pathogens.
Pathogen lipids may act as PAMP and PAMP recognition by the host trigger immunity responses associated with the alterations in the composition of the plant lipid membrane, modification of membrane fluidity, and enzymatic and non-enzymatic genesis of bioactive lipid mediators such as lipid and FA oxidation products, oxylipins [12]. These molecules also integrate tailored defence mechanisms against a diverse array of pathogens with different lifestyles.
Necrotrophic pathogens extract nutrients from dead cells killed prior to or during colonization [122]. On the other hand, plant biotrophic pathogens establish a long-term feeding relationship with the living cells of their hosts, rather than killing the host cells as part of the infection process [123]. Hemibiotrophic pathogens start by having a biotrophic lifestyle and then change to a necrotrophic mode [124]. Saprophytes obtain nutrients from dead and decaying organic matter [125]. Müler and co-workers observed that in the incompatible interaction between peanut seed and the necrotrophic fungus A. parasiticus there is an activation of the LOX pathway [14]. This result reinforces that the production of oxylipins is an important process in in the defence mechanisms against necrotrophs [126, 127]. Recently, several works have shown strong evidences that grapevine tolerance to the biotrophic oomycete P. viticola could also, in the first hours, be mediated by JA and lipid-associated signalling. After pathogen challenge, JA biosynthesis, JA-Ile synthesis, H2O2 accumulation, and lipid peroxidation were observed [21, 128, 129]. FA and lipids were shown to be modulated upon inoculation of the tolerant grapevine Vitis vinifera cv. Regent with P. viticola, particularly at 6 and 12hpi. Both C16:0 and C18:0 relative content decreased when compared to mock-inoculated samples, while the relative content of the unsaturated FA, C18:1, C18:2 and C18:3 increased [13]. Since C18:3 is a biosynthetic precursor of JA [130], the increase of its levels in the first stages of plant–pathogen incompatible interaction might be associated to the triggering of JA-signalling pathway. Furthermore, this alteration might be related to the protection of the photosynthetic machinery during the invasion [13]. In fact, leaf C18:3 is mostly present in the galactolipids, MGDG and DGDG, which account for more than 85% of thylakoid lipids [131]. The ability to adjust membrane lipid fluidity by changing the levels of unsaturated fatty acids is a feature of stress response, which allows to maintain the function of integral proteins, such as the photosynthetic machinery [132]. During grapevine–P. viticola incompatible interaction, a significant increase of the levels of MGDG and DGDG as well as the double bound index, which reflects membrane fluidity, occurs [13]. Leaf lipids from V. vinifera cv. Bianca, tolerant to P. viticola, showed the greatest differences among the differently accumulated metabolites. Among the accumulated lipid compounds, were arachidic acid, oleanolic acid, and uvaol [16]. Additionally, a decrease in some unsaturated fatty acids after P. viticola infection was observed, which may be linked to the activation of JA pathway [16]. JA signalling was also found to be activated in rice defence against the hemibiotrophic fungus Magnaporthe oryzae [133].
Sphingolipids are key players in the induction of PCD [134]. These lipids are structurally characterized by a sphingoid base acyl chain amide linked to a FA, forming ceramide. The different physical properties of sphingolipids are due to the different structures that can present in plants. The LCB of the sphingolipid may vary in length, which is usually between 16 and 20 carbons. The amide-linked FA or VLCFA can also undergo modifications, varying in length from 16 to 30 carbons and can be hydroxylated at the C2 position and desaturated at the ω-9 position leading to high variability of sphingolipids [53]. The balance between sphingolipid bioactive molecules, including LCB and its phosphate (LCB-P) are determining for the regulation of the cell survival death equilibrium [54]. Magnin-Robert and co-workers observed in Arabidopsis that when this equilibrium was disrupted by knocking down the gene encoding for LCB-P lyase, a survival/death imbalance occurs favouring cell survival. As a result, the mutant plant showed higher susceptibility to the hemibiotrophic pathogen P. syringae pv. tomato in comparison to wild-type plants [54]. Liu and co-workers also observed that there is an activation of PCD upon N. benthamiana inoculation with P. syringae pv. maculicola. Furthermore, the authors observed that accompanying the induction of PCD there is a synergistic coordination of JA and SA signalling, which may also leave the plant less vulnerable to necrotrophic pathogens [135].
In the interaction between citrus and the bacteria Candidatus Liberibacter asiaticus, causal agent of Huanglongbing disease, metabolites including four FA and two lipid oxidation products including of C18:3 and PA were reliably decreased. In this case, the pathogen may cause the altered metabolism of long-chain fatty acids, possibly leading to the manipulation of the host FA associated defence, including the synthesis of JA [15]. The results described above show that lipids and FA not only play central roles in defence and disease, but also may be considered as candidates for resistance/susceptibility molecular biomarkers.
Untargeted metabolite analysis of tomato leaves inoculated with the biotrophic fungus Cladosporium fulvum revealed that falcarindiol, a diacetylenic FA possessing two triple bonds, is among three major metabolites present. After incubation with bacterial effectors, falcarinidol synthesis was also induced, indicating that it is involved in both bacterial and fungal interactions with tomato [136]. This unusual FA had been reported to be biosynthesized in response to pest and pathogen stress (reviewed in [129]).
Regarding saprophyte pathogens as the case of A. parasiticus, after inoculation of peanut seeds Müller and collaborators observed significant differences of FFA contents between infected and control seeds [14]. Lipids and FA were also identified as defence markers in maize grain inoculated with the fungus Fusarium verticillioides [17]. In this pathosystem, a metabolome analysis revealed that several lipid compounds correlated with the mycotoxin fumonisin accumulation. 25 discriminant metabolites, all belonging to lipid classes, have been putatively identified. Moreover, the most significantly altered pathways upon infection with F. verticillioides are involved in lipid synthesis, such as phospholipid and FA biosynthesis, glycerophospholipid metabolism, and linoleic acid metabolism [17]. Furthermore, Ludovici et al. reported a significant increase of oxylipins in maize ears after F. verticillioides infection, suggesting the triggering of defence responses [12].
The enrichment of subcellular regions in certain phospholipids, mainly phosphatidylserine (PS) may also be important for an efficient defence response against viruses. This process was observed in the interaction between A. thaliana and cucumber mosaic virus. It is vital for the formation vesicle-like membrane invaginations and the recruitment of the molecular machinery to form viral and host siRNA. Arabidopsis mutants lacking the lipid flippases ALA1 and ALA2 were not able to form vesicle-like membrane invaginations and showed an enhanced susceptibility to the cucumber mosaic virus [137].
Over the last years, the role of the plant cuticle in plant–pathogen interactions as more than just a physical barrier has been gaining attention. DAMP, such as cutin monomers have shown to serve as signals that activate plant defences against pathogens [40]. In response to infection with Colletotrichum gloeosporioides, tomato fruit cuticle was remodelled, and fruit cuticle biosynthesis was up-regulated during appressorium formation even before penetration [138]. In another study, inoculation of citrus petals with Colletotrichum acutatum, caused the epidermal cells to increase lipid synthesis, which altered the cuticle structure [139].
Pathogen lipids: a different perspective
Lipid metabolism in plant pathogens during infection plays an important role either in: (1) development and life cycle completion; (2) pathogen recognition and defence response triggering in the host; (3) hindering host defence systems and overcoming resistance.
Botero et al. (2018) observed, in the context of the hemibiotrophic P. infestans infection of potato leaves, the importance of FA biosynthesis, elongation, and degradation pathways. FA elongation pathways showed active fluxes during the early biotrophic phase and changed to a null flux at later time points. On the other hand, in the necrotrophy phase of infection, the glycerophospholipid metabolism was altered and their metabolic fluxes changed [29]. Upon plant infection, an accumulation of pathogen-specific lipids and FA molecules may also occur, which indicate that these molecules can be used as molecular biomarkers for infection. One example of this was reported by Negrel and co-workers during the interaction between grapevine and P. viticola [18]. In this work, P. viticola-specific lipids and FA were detected from very early stages of the infection process before the first external symptoms. P. viticola-specific lipids, which include eicosapentanoic acid (C20:5) and arachidonic acid (C20:4)-containing lipids and C16:1 ceramides including Cer (d16:1/16:0) were identified [18]. C20:5 and C20:4 had been previously detected in oomycetes [140]. P. viticola-specific lipid accumulation in the fully susceptible variety V. vinifera cv. Syrah was significantly higher than in V. vinifera cv. Bianca [18] which is partially resistant [141]. The pattern of lipid accumulation was modified along the infection process. At early stages, C20:4 and C20:5 were more accumulated as FFA, whereas in later stages, the triacylglycerols containing these FA, and especially trieicosapentaenoyl-glycerol (TEPG), were more accumulated. Lipid accumulation pattern may therefore be used as an indicator of the infection developmental stage [18].
Recently published reports on the mechanisms of the entry of oomycete RxLR effectors have revealed that these effectors bind to phosphoinositol-3-phosphate (PI3P) known as an intracellular molecule [142].
One of the ways by which some pathogens (mainly biotrophs) overcome plant defence is the arresting of PCD through the action of effectors. Elicitation of A. thaliana plants with the mycotoxin fumonisin B1 (FB1) resulted not only in the accumulation of LCB and of C16 FA-containing sphingolipids, but also in a decrease in the sphingolipid content containing VLCFA [143]. Furthermore, studies of Arabidopsis mutants with disruptions in gene loci governing sphingolipid metabolism confirmed a link between sphingolipid homeostasis and PCD associated with plant defence [144].
Defensins constitute an ancient and diverse set of natural antimicrobial proteins [145]. Different pathogen lipids bind to plant defensins, which causes the permeabilization of fungal membranes [146]. The engagement with specific fungal membrane phospholipids affects the ability of certain plant defensins to kill fungal cells [147, 148]. Furthermore, different phospholipids trigger the formation of discrete defensin–phospholipid complexes with unique topologies [147, 148]. Sphingolipids like glycosylceramides and mannosyl diinositolphosphoryl ceramides also bind to plant defensins forming complexes necessary for these proteins antifungal activity, although these interactions are still poorly understood [34]. Saragram and co-workers observed that a fungal PA interacts with a Medicago truncatula defensin (MtDef4) [33]. When fungal membrane lipids interact with defensin oligomers, a combination of curvature stress and lipid sequestration occurs, resulting in complete structural destabilization and subsequent permeabilization of the membrane [32].
In bacteria, PAMP are conserved cell-surface structures including flagellin, lipopeptides, peptidoglycans and lipopolysaccharides (LPS) [149]. LPS, with a major role in bacterial growth and survival [150], can trigger PTI [151]. LPS from plant pathogenic bacteria could induce a PCD in Arabidopsis leaves in a dose-dependent manner, depending on an early ROS production. Moreover, these molecules were able to induce PR1 gene expression [152]. LPS are composed of a hydrophilic heteropolysaccharide (comprising the core oligosaccharide and O-specific polysaccharide or O-chain) covalently linked to a lipophilic moiety termed lipid A, which anchors these macromolecules to the outer membrane. LPS without the O-chain are lipooligosaccharides (LOS) [153]. During Arabidopsis infection with X. campestris pv. campestris (Xcc), LOS promoted pathogen recognition. LOS induced the upregulation of the PR1 and PR2 genes in Arabidopsis leaves [154]. The LOS lipid A moiety was found to be active in a later phase of the interaction, contrarily to the oligosaccharide, which induced gene upregulation early in the interaction [154]. Although both Xcc lipid A and core oligosaccharide are active in defence gene induction, it is possible that they are recognized by different plant receptors [153]. Xcc LOS interacts with two members of the F-box protein family involved in pathogen recognition, namely F-box and F box-LRR. F box-LRR might be involved in the recognition of Xcc LOS by activating a proteasome-mediated hydrolysis of repressor proteins that negatively regulate target genes with a role in plant defence. LOS also interacts with two protein kinases involved in cellular signal transduction pathways and nsLTP1 [154]. Furthermore, LOS was found to promote the activation of ROS signalling [154].
Fungal lipases seem to play an important role in the establishment of their virulence [155]. It was reported that FFA analyses during wheat infection with the F. graminearum revealed that there was an enrichment in unsaturated FA, namely C18:1, C18:2 and C18:3 derived by the fungal secreted lipase FGL1 activity and that they could inhibit callose synthase [35]. It is likely that the FFA resulting from the fungal lipase activity have a plant lipid source. By promoting the inhibition of callose synthase, these pathogen-induced FFA inhibit the deposition of callose, allowing the fungi to overcome this layer of type II resistance. The growth of the fungus in the host implicates a challenge to lipid integrity due to the generation of ROS via mitochondrial activity [156]. The bacterial effector RipAL from R. solanacearum has lipase activity (containing a putative lipase domain that shared homology with the Arabidopsis PLA DAD1, which contributes to JA formation) and is among the type III effector proteins called Rips (Ralstonia-injected proteins) [157]. This effector induced the expression of marker genes for JA signalling in N. benthamiana and suppressed SA-mediated signalling [38]. Moreover, RipAL targets chloroplast lipids and causes chlorosis accompanied by the reduction of chlorophyll content when expressed in plant leaves [38]. Therefore, this effector might induce a disorder in chloroplasts by catalysing its lipids hydrolysis. RipAL contributes to the development of disease symptoms caused by R. solanacearum in pepper leaves through its putative lipase activity [38].
Fungal phospholipases (PL) have also been shown to counteract oxidative damage by removing oxidized fatty acids from phospholipids in membranes [37]. Corn seedlings infected with the biotrophic fungi Ustilago maydis mutant with depletion on the PL lip2 gene exhibited a reduction in the severity of disease symptoms. It is possible that lip2 plays a protective role against the oxidative stress encountered on host by removing detrimental oxidized polyunsaturated fatty acids from the cell membrane, mitigating the damage caused by plant ROS-triggered lipid peroxidation. It seems that lip2 is important for supporting lipid homeostasis during U. maydis to proliferation in the host tissue. Given the increased susceptibility of the lip2 mutant to inhibitors of respiration, it is also likely that lip2 supports mitochondrial function by influencing the integrity of the mitochondrial specific lipid cardiolipin, in the mitochondrial inner membrane [37].
Pathogenic fungi can form invasive hyphae, which are surrounded by an extra-invasive hyphal membrane (EIHM) (Fig. 2). This structure is a plant cell-derived membrane and continuous with the plant plasma membrane [158]. The fungi induces an enrichment of PI in the EIHM, which is crucial for the pathogen development [30]. During C. higginsianum infection in Arabidopsis, an enrichment of PI(4,5)P2 in the EIHM occurs. Since the exocytic factor EXO84b also accumulated at the EIHM, but not endocytic factors, the enrichment of PI(4,5)P2 may associated with an exocytic trafficking event rather than with endocytosis. The enrichment of PI(4,5)P2 in the EIHM might reflect the general importance of this phosphoinositide moiety in rapid secretion [30]. The enrichment of PI(4,5)P2 was also found in EIHM upon inoculation with Colletotrichum orbiculare, but not with Golovinomyces orontii or Hyaloperonospora arabidopsidis, which indicates a pathogen-specific strategy for the modulation of the phospholipid contents of the interfacial membrane to generate an environment conducive to the pathogen [30].
As discussed above, oxylipin production is a vital process in plant defence mechanisms. Nonetheless, oxylipin production showed also to be important for the development of some pathogens. In fact, in the interaction between maize and F. Verticillioides, fungal oxylipin production, including 9- and 13-hydroxyoctadecadienoic acid (HODE) showed to be important during pathogenesis [12]. In another work focusing on the same pathosystem, different fungal oxylipins, namely 9S-DOX-AOS products showed to be pathogenicity promoters by inducing the expression of maize pathogenicity-promoting LOX3 (ZmLOX3) [159]. Moreover, 10-HOME and 7,10-DiHOME also showed to play an important role in the establishment of virulence of P. aeruginosa in lettuce [160]
Apoplast: an important compartment with still much left to unveil
One of the most important cellular compartments in the first moments of plant–pathogen interaction is the apoplast. This compartment includes the extracellular matrix and the APF [5]. It is involved in several functions during normal growth and under biotic and abiotic stress conditions, including pathogen interaction, pollutants, drought, salinity and temperature [10, 161, 162]. In plant–microbe interaction, upon pathogen secretion of molecular effectors to the apoplast that trigger the host immune system, a metabolism modulation occurs [6]. It was already observed that stress conditions lead to the alteration of the protein composition of the apoplast both qualitatively and quantitatively [7, 9]. However, to this day, studies concerning the modulation of lipid in the apoplast during plant–pathogen interaction are very scarce, causing the picture of the role of lipids in the apoplast to be rather blurry [10]. Nonetheless, at a constitutive level, lipids were already identified in the grapevine leaf apoplast [163]. Moreover, different studies have been evidencing the importance of apoplastic lipids in plant–pathogen interactions.
Lipids, extremely hydrophobic molecules, need to pass through the apoplastic compartment or the highly hydrophilic cell wall. This transference process is mediated by nsLTP. As already discussed above, these proteins are associated with diverse plant functions and may be up-regulated in response to infection and exhibit antimicrobial activity [111, 112, 164]. Maldonado and collaborators identified a putative LTP protein in Arabidopsis and hypothesized that the protein may bind a lipid molecule and suggested that a plasma membrane receptor may also play a role in the LTP-mediated long-distance signalling during SAR [165].
PLA, a vital protein family in plant lipid signalling, was also described to be translocated to the apoplast during pathogen infection. Translocation of the secretory PLA2α to the apoplast was rapidly enhanced in response to inoculation of Arabidopsis leaves with P. syringae pv. tomato DC3000 carrying the effector avrRpm1. This result suggests that PLA2α secretion to apoplast and lipid signalling upon bacterial infection may play a role in host defence responses, where host cells first encounter invading pathogens [166].
Another evidence of the importance of this compartment in lipid signalling during plant–pathogen interactions is that it was previously observed that, in response to JA, a modulation of phospholipids levels of the sunflower apoplast occurs. In this study, JA treatment resulted in significant changes in the phospholipid profile, showing the accumulation of PG and a decrease in PI [11]. Considering the role of phosphoinositides in plant signalling, the modulation of its levels by JA supports the participation of apoplastic phospholipids in intercellular communication events.
Within the apoplast, extracellular vesicles (EV) contribute to innate immunity and may mediate intercellular communication in plants as well as in animals. EV can be defined as spherical particles enclosed by a phospholipid bilayer that are released from cells into their environment and are composed of bioactive molecules, including RNAs, DNAs, proteins, and lipids [167]. To this day, there are only a few studies on the EV role in plant–pathogen interactions. However, EV are reported to be mobilized in response to pathogen infection and enriched in defence-related proteins. The secretion of EV was observed to be enhanced during Arabidopsis infection with a virulent strain of the bacterial pathogen P. syringae and in response to SA treatments [168].
In the past years, a few evidences that EV may be important for lipid signalling arose. Furthermore, being EV lipid bilayer structures, their lipid composition is likely pivotal to their function.
Many fungal and oomycete pathogens enter plant cells by penetrating the host cell wall and differentiating specialized intracellular feeding structures, haustoria, by invagination of the plant plasma membrane [169]. As a result, the plant host may promote the formation of a cell wall thickening structure, the papillae and haustorial encasements in order to limit pathogen development [170] (Fig. 2). In order to form these structures, the defined components, including the proteins syntaxin AtSYP121/PENETRATION1 (PEN1) and soluble N-ethylmaleimide-sensitive factor adaptor protein 33 (SNAP33), the ATP-binding cassette (ABC) transporter PEN3, callose and membrane lipids are transported through exosomes [171] (Fig. 2). Meyer and co-workers observed that upon powdery mildew infection in Arabidopsis, not only integral membrane proteins such as PEN1, but also membrane lipids become incorporated into haustorial encasements. This phenomenon is not restricted to powdery mildew fungi, since membrane lipids were also detected in oomycete haustorial encasements [171] (Fig. 2). Recently, Regente and collaborators found that some protein families are enriched in EV upon fungal inoculation, including lipase, acyl hydrolases and LTPs [172]. Lipoxygenases were also found in the EV of Turnip mosaic virus 1-infected N. benthamiana leaves [173]. The enrichment of these proteins in EV is indicative that these particles and lipids have an important role in the establishment of SAR.
As lipid structures, it is possible that extracellular vesicles contain different lipids that confer membrane fluidity and can compress as they move through pores in the cell wall [173].
A thorough study of the apoplast, particularly concerning EV lipids may allow completely unveiling the role of this structure in plant–pathogen interactions, being a key element to uncover molecules that participate in intercellular communication and transport.
Conclusion
Lipids and FA molecules are key players in the different processes of plant–pathogen interaction. This review evidences that studying lipid metabolism and signalling both in plant and pathogen is arguably essential to completely uncover the knowledge brought up to light by proteomics and transcriptomics. The analysis of lipids and their derivatives enables the possibility of describing the cross-talk between plant and pathogen and the discovery of pathogen combined strategies targeting lipid pathways.
An increasing number of studies have described lipid modulation events that are important in defence and disease processes. Plant lipids play important roles from the first physical barrier against pathogens, the cutin, to signalling pathways that trigger different immune responses and defence-related genes. Lipids were also shown to be candidate biomarkers of resistance or susceptibility to different pathogens. Furthermore, studies on the apoplast and EV have highlighted the possible role of lipids in the intercellular communication and the establishment of SAR during plant–pathogen interactions. From the pathogen perspective, it is evidenced that lipid molecules and lipid metabolism play a pivotal role in the pathogen’s life cycle completion, triggering of recognition (e.g. bacterial LPS and LOS) and in evading the host immune system and potentiating infection.
The latest studies summarized in this review indicate that it is highly important to continue pointing the research direction towards the lipid signalling processes in plant–pathogen interaction to completely unveil the molecular mechanisms behind plant disease susceptibility and resistance. Concerning the apoplast, a deep knowledge of the lipid modulation events that take place in this compartment may allow unencrypting the first moments of pathogen perceiving and immune response.
Acknowledgements
The authors acknowledge the Portuguese Foundation for Science and Technology (FCT, Portugal) for funded fellowships and contracts to AF and ARC: IF/00819/2015 and PD/BD/131030/2017, respectively. FCT funded the Research Unit BioISI (UIDB/04046/2020 and UIDP/04046/2020) and GRAVITAS project-PTDC/BIA-BQM/28539/2017
Author contributions
ARC performed the literature search and drafted the work; ARM and AF critically revised and completed the work. ARM and AF are co-senior authors in this work.
Declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
All the authors gave their consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang P-Y, Zimmerli L. Enhancing crop innate immunity: new promising trends. Front Plant Sci. 2014 doi: 10.3389/fpls.2014.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui H, Tsuda K, Parker JE. Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev Plant Biol. 2015;66:487–511. doi: 10.1146/annurev-arplant-050213-040012. [DOI] [PubMed] [Google Scholar]
- 3.Walley JW, Kliebenstein DJ, Bostock RM, Dehesh K. Fatty acids and early detection of pathogens. Curr Opin Plant Biol. 2013;16:520–526. doi: 10.1016/j.pbi.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Bakan B, Marion D. Assembly of the cutin polyester: from cells to extracellular cell walls. Plants (Basel) 2017 doi: 10.3390/plants6040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winter H, Robinson DG, Heldt HW. Subcellular volumes and metabolite concentrations in barley leaves. Planta. 1993;191:180–190. doi: 10.1007/BF00199748. [DOI] [Google Scholar]
- 6.Toruño TY, Stergiopoulos I, Coaker G. Plant–pathogen effectors: cellular probes interfering with plant defenses in spatial and temporal manners. Annu Rev Phytopathol. 2016;54:419–441. doi: 10.1146/annurev-phyto-080615-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaunois B, Colby T, Belloy N, et al. Large-scale proteomic analysis of the grapevine leaf apoplastic fluid reveals mainly stress-related proteins and cell wall modifying enzymes. BMC Plant Biol. 2013;13:24. doi: 10.1186/1471-2229-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerra-Guimarães L, Pinheiro C, Chaves I, et al. Protein dynamics in the plant extracellular space. Proteomes. 2016;4:22. doi: 10.3390/proteomes4030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerra-Guimarães LL, Tenente RER, Pinheiro CC, et al. Proteomic analysis of apoplastic fluid of Coffea arabica leaves highlights novel biomarkers for resistance against Hemileia vastatrix. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misra BB. The black-box of plant apoplast lipidomes. Front Plant Sci. 2016 doi: 10.3389/fpls.2016.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regente M, Monzón GC, de la Canal L. Phospholipids are present in extracellular fluids of imbibing sunflower seeds and are modulated by hormonal treatments. J Exp Bot. 2008;59:553–562. doi: 10.1093/jxb/erm329. [DOI] [PubMed] [Google Scholar]
- 12.Ludovici M, Ialongo C, Reverberi M, et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis of Fusarium verticillioides and maize kernels. Food Addit Contam Part A. 2014;31:2026–2033. doi: 10.1080/19440049.2014.968810. [DOI] [PubMed] [Google Scholar]
- 13.Laureano G, Figueiredo J, Cavaco AR, et al. The interplay between membrane lipids and phospholipase a family members in grapevine resistance against Plasmopara viticola. Sci Rep. 2018;8:1–15. doi: 10.1038/s41598-018-32559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller V, Amé MV, Carrari V, et al. Lipoxygenase activation in peanut seed cultivars resistant and susceptible to Aspergillus parasiticus colonization. Phytopathology. 2014;104:1340–1348. doi: 10.1094/PHYTO-12-13-0338-R. [DOI] [PubMed] [Google Scholar]
- 15.Suh JH, Niu YS, Wang Z, et al. Metabolic analysis reveals altered long-chain fatty acid metabolism in the host by huanglongbing disease. J Agric Food Chem. 2018;66:1296–1304. doi: 10.1021/acs.jafc.7b05273. [DOI] [PubMed] [Google Scholar]
- 16.Chitarrini G, Soini E, Riccadonna S, et al. Identification of biomarkers for defense response to Plasmopara viticola in a resistant grape variety. Front Plant Sci. 2017 doi: 10.3389/fpls.2017.01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Righetti L, Lucini L, Giorni P, et al. Lipids as key markers in maize response to fumonisin accumulation. J Agric Food Chem. 2019;67:4064–4070. doi: 10.1021/acs.jafc.8b06316. [DOI] [PubMed] [Google Scholar]
- 18.Negrel L, Halter D, Wiedemann-Merdinoglu S, et al. Identification of lipid markers of Plasmopara viticola infection in grapevine using a non-targeted metabolomic approach. Front Plant Sci. 2018 doi: 10.3389/fpls.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bréhélin C, Kessler F, van Wijk KJ. Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci. 2007;12:260–266. doi: 10.1016/j.tplants.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Cecchini NM, Steffes K, Schläppi MR, et al. Arabidopsis AZI1 family proteins mediate signal mobilization for systemic defence priming. Nat Commun. 2015;6:1–12. doi: 10.1038/ncomms8658. [DOI] [PubMed] [Google Scholar]
- 21.Figueiredo A, Martins J, Sebastiana M, et al. Specific adjustments in grapevine leaf proteome discriminating resistant and susceptible grapevine genotypes to Plasmopara viticola. J Proteom. 2017;152:48–57. doi: 10.1016/j.jprot.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Yang J, Zhu B, Xie G. Overexpressing OsFBN1 enhances plastoglobule formation, reduces grain-filling percent and jasmonate levels under heat stress in rice. Plant Sci. 2019;285:230–238. doi: 10.1016/j.plantsci.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Salminen TA, Blomqvist K, Edqvist J. Lipid transfer proteins: classification, nomenclature, structure, and function. Planta. 2016;244:971–997. doi: 10.1007/s00425-016-2585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canonne J, Froidure-Nicolas S, Rivas S. Phospholipases in action during plant defense signaling. Plant Signal Behav. 2011;6:13–18. doi: 10.4161/psb.6.1.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C, El-Shetehy M, Shine MB, et al. Free radicals mediate systemic acquired resistance. Cell Rep. 2014;7:348–355. doi: 10.1016/j.celrep.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Niki E. Chapter 14—dual stressor effects of lipid oxidation and antioxidants. In: Sies H, editor. Oxidative stress. Cambridge: Academic Press; 2020. pp. 249–262. [Google Scholar]
- 27.Christensen SA, Nemchenko A, Park Y-S, et al. The novel monocot-specific 9-lipoxygenase ZmLOX12 is required to mount an effective jasmonate-mediated defense against Fusarium verticillioides in maize. Mol Plant Microbe Interact. 2014;27:1263–1276. doi: 10.1094/MPMI-06-13-0184-R. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths G. Biosynthesis and analysis of plant oxylipins. Free Radic Res. 2015;49:565–582. doi: 10.3109/10715762.2014.1000318. [DOI] [PubMed] [Google Scholar]
- 29.Botero D, Valdés I, Rodríguez M-J, et al. A genome-scale metabolic reconstruction of Phytophthora infestans with the integration of transcriptional data reveals the key metabolic patterns involved in the interaction of its host. Front Genet. 2018 doi: 10.3389/fgene.2018.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada TL, Betsuyaku S, Inada N, et al. Enrichment of phosphatidylinositol 4,5-bisphosphate in the extra-invasive hyphal membrane promotes Colletotrichum infection of Arabidopsisthaliana. Plant Cell Physiol. 2019;60:1514–1524. doi: 10.1093/pcp/pcz058. [DOI] [PubMed] [Google Scholar]
- 31.Iizasa S, Iizasa E, Watanabe K, Nagano Y. Transcriptome analysis reveals key roles of AtLBR-2 in LPS-induced defense responses in plants. BMC Genom. 2017;18:995. doi: 10.1186/s12864-017-4372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Järvå M, Lay FT, Phan TK, et al. X-ray structure of a carpet-like antimicrobial defensin–phospholipid membrane disruption complex. Nat Commun. 2018;9:1962. doi: 10.1038/s41467-018-04434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagaram US, El-Mounadi K, Buchko GW, et al. Structural and functional studies of a phosphatidic acid-binding antifungal plant defensin MtDef4: identification of an RGFRRR motif governing fungal cell entry. PLoS ONE. 2013;8:e82485. doi: 10.1371/journal.pone.0082485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thevissen K, François IEJA, Takemoto JY, et al. DmAMP1, an antifungal plant defensin from dahlia (Dahlia merckii), interacts with sphingolipids from Saccharomyces cerevisiae. FEMS Microbiol Lett. 2003;226:169–173. doi: 10.1016/S0378-1097(03)00590-1. [DOI] [PubMed] [Google Scholar]
- 35.Blümke A, Falter C, Herrfurth C, et al. Secreted fungal effector lipase releases free fatty acids to inhibit innate immunity-related callose formation during wheat head infection. Plant Physiol. 2014;165:346–358. doi: 10.1104/pp.114.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darwiche R, Atab OE, Baroni RM, et al. Plant pathogenesis–related proteins of the cacao fungal pathogen Moniliophthora perniciosa differ in their lipid-binding specificities. J Biol Chem. 2017;292:20558–20569. doi: 10.1074/jbc.M117.811398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambie SC, Kretschmer M, Croll D, et al. The putative phospholipase lip2 counteracts oxidative damage and influences the virulence of Ustilago maydis. Mol Plant Pathol. 2017;18:210–221. doi: 10.1111/mpp.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano M, Mukaihara T. Ralstonia solanacearum type III effector RipAL targets chloroplasts and induces jasmonic acid production to suppress salicylic acid-mediated defense responses in plants. Plant Cell Physiol. 2018;59:2576–2589. doi: 10.1093/pcp/pcy177. [DOI] [PubMed] [Google Scholar]
- 39.Rojas CM, Senthil-Kumar M, Tzin V, Mysore K. Regulation of primary plant metabolism during plant–pathogen interactions and its contribution to plant defense. Front Plant Sci. 2014 doi: 10.3389/fpls.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziv C, Zhao Z, Gao YG, Xia Y. Multifunctional roles of plant cuticle during plant–pathogen interactions. Front Plant Sci. 2018 doi: 10.3389/fpls.2018.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domínguez E, Heredia-Guerrero JA, Heredia A. Plant cutin genesis: unanswered questions. Trends Plant Sci. 2015;20:551–558. doi: 10.1016/j.tplants.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Li-Beisson Y, Shorrosh B, Beisson F, et al. Acyl-lipid metabolism. Arabidopsis Book. 2013 doi: 10.1199/tab.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morineau C, Gissot L, Bellec Y, et al. Dual Fatty acid elongase complex interactions in Arabidopsis. PLoS ONE. 2016 doi: 10.1371/journal.pone.0160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rawsthorne S. Carbon flux and fatty acid synthesis in plants. Prog Lipid Res. 2002;41:182–196. doi: 10.1016/s0163-7827(01)00023-6. [DOI] [PubMed] [Google Scholar]
- 45.Pollard M, Beisson F, Li Y, Ohlrogge JB. Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci. 2008;13:236–246. doi: 10.1016/j.tplants.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Aragón W, Reina-Pinto JJ, Serrano M. The intimate talk between plants and microorganisms at the leaf surface. J Exp Bot. 2017;68:5339–5350. doi: 10.1093/jxb/erx327. [DOI] [PubMed] [Google Scholar]
- 47.Xia Y, Yu K, Navarre D, et al. The glabra1 mutation affects cuticle formation and plant responses to microbes. Plant Physiol. 2010;154:833–846. doi: 10.1104/pp.110.161646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia Y, Gao Q-M, Yu K, et al. An intact cuticle in distal tissues is essential for the induction of systemic acquired resistance in plants. Cell Host Microbe. 2009;5:151–165. doi: 10.1016/j.chom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Hansjakob A, Riederer M, Hildebrandt U. Wax matters: absence of very-long-chain aldehydes from the leaf cuticular wax of the glossy11 mutant of maize compromises the prepenetration processes of Blumeria graminis. Plant Pathol. 2011;60:1151–1161. doi: 10.1111/j.1365-3059.2011.02467.x. [DOI] [Google Scholar]
- 50.Kumar A, Yogendra KN, Karre S, et al. WAX INDUCER1 (HvWIN1) transcription factor regulates free fatty acid biosynthetic genes to reinforce cuticle to resist Fusarium head blight in barley spikelets. J Exp Bot. 2016;67:4127–4139. doi: 10.1093/jxb/erw187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arif T, Bhosale JD, Kumar N, et al. Natural products–antifungal agents derived from plants. J Asian Nat Prod Res. 2009;11:621–638. doi: 10.1080/10286020902942350. [DOI] [PubMed] [Google Scholar]
- 52.Zacchino SA, Butassi E, Liberto MD, et al. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine. 2017;37:27–48. doi: 10.1016/j.phymed.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Michaelson LV, Napier JA, Molino D, Faure JD. Plant sphingolipids their importance in cellular organization and adaption. Biochim et Biophys Acta (BBA) Mol Cell Biol Lipids. 2016;1861:1329–1335. doi: 10.1016/j.bbalip.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magnin-Robert M, Bourse DL, Markham J, et al. Modifications of sphingolipid content affect tolerance to hemibiotrophic and necrotrophic pathogens by modulating plant defense responses in Arabidopsis. Plant Physiol. 2015;169:2255–2274. doi: 10.1104/pp.15.01126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berkey R, Bendigeri D, Xiao S. Sphingolipids and plant defense/disease: the “death” connection and beyond. Front Plant Sci. 2012 doi: 10.3389/fpls.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alden KP, Dhondt-Cordelier S, McDonald KL, et al. Sphingolipid long chain base phosphates can regulate apoptotic-like programmed cell death in plants. Biochem Biophys Res Commun. 2011;410:574–580. doi: 10.1016/j.bbrc.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 57.König S, Feussner K, Schwarz M, et al. Arabidopsis mutants of sphingolipid fatty acid α-hydroxylases accumulate ceramides and salicylates. New Phytol. 2012;196:1086–1097. doi: 10.1111/j.1469-8137.2012.04351.x. [DOI] [PubMed] [Google Scholar]
- 58.Chen M, Markham JE, Dietrich CR, et al. Sphingolipid long-chain base hydroxylation is important for growth and regulation of sphingolipid content and composition in Arabidopsis. Plant Cell. 2008;20:1862–1878. doi: 10.1105/tpc.107.057851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Wang X. Phospholipase D and phosphatidic acid in plant immunity. Plant Sci. 2019;279:45–50. doi: 10.1016/j.plantsci.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Babiychuk E, Müller F, Eubel H, et al. Arabidopsis phosphatidylglycerophosphate synthase 1 is essential for chloroplast differentiation, but is dispensable for mitochondrial function. Plant J. 2003;33:899–909. doi: 10.1046/j.1365-313X.2003.01680.x. [DOI] [PubMed] [Google Scholar]
- 61.Dubots E, Audry M, Yamaryo Y, et al. Activation of the chloroplast monogalactosyldiacylglycerol synthase mgd1 by phosphatidic acid and phosphatidylglycerol. J Biol Chem. 2010;285:6003–6011. doi: 10.1074/jbc.M109.071928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roston R, Gao J, Xu C, Benning C. Arabidopsis chloroplast lipid transport protein TGD2 disrupts membranes and is part of a large complex. Plant J. 2011;66:759–769. doi: 10.1111/j.1365-313X.2011.04536.x. [DOI] [PubMed] [Google Scholar]
- 63.Roth MG. Molecular mechanisms of PLD function in membrane traffic. Traffic. 2008;9:1233–1239. doi: 10.1111/j.1600-0854.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhukovsky MA, Filograna A, Luini A, et al. Phosphatidic acid in membrane rearrangements. FEBS Lett. 2019;593:2428–2451. doi: 10.1002/1873-3468.13563. [DOI] [PubMed] [Google Scholar]
- 65.Bargmann BOR, Munnik T. The role of phospholipase D in plant stress responses. Curr Opin Plant Biol. 2006;9:515–522. doi: 10.1016/j.pbi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Zhu H, Zhang Q, et al. Phospholipase Dα1 and phosphatidic acid regulate nadph oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell. 2009;21:2357–2377. doi: 10.1105/tpc.108.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakano M, Yoshioka H, Ohnishi K, et al. Cell death-inducing stresses are required for defense activation in DS1-phosphatidic acid phosphatase-silenced Nicotiana benthamiana. J Plant Physiol. 2015;184:15–19. doi: 10.1016/j.jplph.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Hou Q, Ufer G, Bartels D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016;39:1029–1048. doi: 10.1111/pce.12666. [DOI] [PubMed] [Google Scholar]
- 69.Smrcka AV, Hepler BKO, Sternweis PC. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991;251:804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- 70.Grzelczyk A, Gendaszewska-Darmach E. Novel bioactive glycerol-based lysophospholipids: new data—new insight into their function. Biochimie. 2013;95:667–679. doi: 10.1016/j.biochi.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 71.Küpper FC, Gaquerel E, Cosse A, et al. Free fatty acids and methyl jasmonate trigger defense reactions in Laminaria digitata. Plant Cell Physiol. 2009;50:789–800. doi: 10.1093/pcp/pcp023. [DOI] [PubMed] [Google Scholar]
- 72.Yu C-W, Lin Y-T, Li H. Increased ratio of galactolipid MGDG:DGDG induces jasmonic acid overproduction and changes chloroplast shape. New Phytol. 2020 doi: 10.1111/nph.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao Q, Yu K, Xia Y, et al. Mono- and digalactosyldiacylglycerol lipids function nonredundantly to regulate systemic acquired resistance in plants. Cell Rep. 2014;9:1681–1691. doi: 10.1016/j.celrep.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 74.Matos AR, Pham-Thi A-T. Lipid deacylating enzymes in plants: old activities, new genes. Plant Physiol Biochem. 2009;47:491–503. doi: 10.1016/j.plaphy.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 75.Boudière L, Michaud M, Petroutsos D, et al. Glycerolipids in photosynthesis: composition, synthesis and trafficking. Biochim Biophys Acta (BBA) Bioenerg. 2014;1837:470–480. doi: 10.1016/j.bbabio.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 76.Kim DS, Jeun Y, Hwang BK. The pepper patatin-like phospholipase CaPLP1 functions in plant cell death and defense signaling. Plant Mol Biol. 2014;84:329–344. doi: 10.1007/s11103-013-0137-x. [DOI] [PubMed] [Google Scholar]
- 77.Scherer GFE, Ryu SB, Wang X, et al. Patatin-related phospholipase a: nomenclature, subfamilies and functions in plants. Trends Plant Sci. 2010;15:693–700. doi: 10.1016/j.tplants.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 78.Wi SJ, Seo S, yeon, Cho K,, et al. lysophosphatidylcholine enhances susceptibility in signaling pathway against pathogen infection through biphasic production of reactive oxygen species and ethylene in tobacco plants. Phytochemistry. 2014;104:48–59. doi: 10.1016/j.phytochem.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Arisz SA, Testerink C, Munnik T. Plant PA signaling via diacylglycerol kinase. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids. 2009;1791:869–875. doi: 10.1016/j.bbalip.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 80.Gonorazky G, Ramirez L, Abd-El-Haliem A, et al. The tomato phosphatidylinositol-phospholipase C2 (SlPLC2) is required for defense gene induction by the fungal elicitor xylanase. J Plant Physiol. 2014;171:959–965. doi: 10.1016/j.jplph.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 81.Zhao J. Phospholipase D and phosphatidic acid in plant defence response: from protein–protein and lipid–protein interactions to hormone signalling. J Exp Bot. 2015;66:1721–1736. doi: 10.1093/jxb/eru540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin C, Wang X. The Arabidopsis phospholipase D family. characterization of a calcium-independent and phosphatidylcholine-selective PLDζ1 with distinct regulatory domains. Plant Physiol. 2002;128:1057–1068. doi: 10.1104/pp.010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qin C, Wang C, Wang X. Kinetic analysis of Arabidopsis phospholipase Dδ substrate preference and mechanism of activation by Ca2+ and phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2002;277:49685–49690. doi: 10.1074/jbc.M209598200. [DOI] [PubMed] [Google Scholar]
- 84.Schlöffel MA, Salzer A, Wan W-L, et al. The BIR2/BIR3-associated phospholipase Dγ1 negatively regulates plant immunity. Plant Physiol. 2020;183:371–384. doi: 10.1104/pp.19.01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thaler JS, Humphrey PT, Whiteman NK. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012;17:260–270. doi: 10.1016/j.tplants.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Maximova SN, Guiltinan MJ. Characterization of a stearoyl-acyl carrier protein desaturase gene family from chocolate tree. Theobroma cacao L Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berestovoy MA, Pavlenko OS, Goldenkova-Pavlova IV. Plant fatty acid desaturases: role in the life of plants and biotechnological potential. Biol Bull Rev. 2020;10:127–139. doi: 10.1134/S2079086420020024. [DOI] [Google Scholar]
- 88.Kachroo A, Shanklin J, Whittle E, et al. The Arabidopsis stearoyl-acyl carrier protein-desaturase family and the contribution of leaf isoforms to oleic acid synthesis. Plant Mol Biol. 2006;63:257–271. doi: 10.1007/s11103-006-9086-y. [DOI] [PubMed] [Google Scholar]
- 89.Jing F, Zhao L, Yandeau-Nelson MD, Nikolau BJ. Two distinct domains contribute to the substrate acyl chain length selectivity of plant acyl-ACP thioesterase. Nat Commun. 2018 doi: 10.1038/s41467-018-03310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thelen JJ, Ohlrogge JB. Metabolic engineering of fatty acid biosynthesis in plants. Metab Eng. 2002;4:12–21. doi: 10.1006/mben.2001.0204. [DOI] [PubMed] [Google Scholar]
- 91.Dong C-J, Cao N, Zhang Z-G, Shang Q-M. Characterization of the fatty acid desaturase genes in cucumber: structure, phylogeny, and expression patterns. PLoS ONE. 2016 doi: 10.1371/journal.pone.0149917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chi X, Zhang Z, Chen N, et al. Isolation and functional analysis of fatty acid desaturase genes from peanut (Arachis hypogaea L.) PLoS ONE. 2017;12:e0189759. doi: 10.1371/journal.pone.0189759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dar AA, Choudhury AR, Kancharla PK, Arumugam N. The FAD2 gene in plants: occurrence, regulation, and role. Front Plant Sci. 2017 doi: 10.3389/fpls.2017.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He M, Qin C-X, Wang X, Ding N-Z. Plant unsaturated fatty acids: biosynthesis and regulation. Front Plant Sci. 2020 doi: 10.3389/fpls.2020.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heilmann I, Mekhedov S, King B, et al. Identification of the Arabidopsis palmitoyl-monogalactosyldiacylglycerol Δ7-desaturase gene fad5, and effects of plastidial retargeting of Arabidopsis desaturases on the fad5 mutant phenotype. Plant Physiol. 2004;136:4237–4245. doi: 10.1104/pp.104.052951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soria-García Á, Rubio MC, Lagunas B, et al. Tissue distribution and specific contribution of Arabidopsis FAD7 and FAD8 plastid desaturases to the JA- and ABA-mediated cold stress or defense responses. Plant Cell Physiol. 2019;60:1025–1040. doi: 10.1093/pcp/pcz017. [DOI] [PubMed] [Google Scholar]
- 97.An J-U, Lee I-G, Ko Y-J, Oh D-K. Microbial synthesis of linoleate 9 S-lipoxygenase derived plant C18 oxylipins from C18 polyunsaturated fatty acids. J Agric Food Chem. 2019;67:3209–3219. doi: 10.1021/acs.jafc.8b05857. [DOI] [PubMed] [Google Scholar]
- 98.Shimada TL, Takano Y, Shimada T, et al. Leaf oil body functions as a subcellular factory for the production of a phytoalexin in Arabidopsis. Plant Physiol. 2014;164:105–118. doi: 10.1104/pp.113.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry. 2008;69:2127–2132. doi: 10.1016/j.phytochem.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 100.Kombrink E. Chemical and genetic exploration of jasmonate biosynthesis and signaling paths. Planta. 2012;236:1351–1366. doi: 10.1007/s00425-012-1705-z. [DOI] [PubMed] [Google Scholar]
- 101.Chini A, Monte I, Zamarreño AM, et al. An OPR3-independent pathway uses 4,5-didehydrojasmonate for jasmonate synthesis. Nat Chem Biol. 2018;14:171–178. doi: 10.1038/nchembio.2540. [DOI] [PubMed] [Google Scholar]
- 102.Ruan J, Zhou Y, Zhou M, et al. Jasmonic acid signaling pathway in plants. Int J Mol Sci. 2019;20:2479. doi: 10.3390/ijms20102479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoshitomi K, Taniguchi S, Tanaka K, et al. Rice terpene synthase 24 (OsTPS24) encodes a jasmonate-responsive monoterpene synthase that produces an antibacterial γ-terpinene against rice pathogen. J Plant Physiol. 2016;191:120–126. doi: 10.1016/j.jplph.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 104.He Y, Zhang H, Sun Z, et al. Jasmonic acid-mediated defense suppresses brassinosteroid-mediated susceptibility to Rice black streaked dwarf virus infection in rice. New Phytol. 2017;214:388–399. doi: 10.1111/nph.14376. [DOI] [PubMed] [Google Scholar]
- 105.Wang Q, Shao B, Shaikh FI, et al. Wheat resistances to Fusarium root rot and head blight are both associated with deoxynivalenol- and jasmonate-related gene expression. Phytopathology. 2017;108:602–616. doi: 10.1094/PHYTO-05-17-0172-R. [DOI] [PubMed] [Google Scholar]
- 106.Herbel V, Sieber-Frank J, Wink M. The antimicrobial peptide snakin-2 is upregulated in the defense response of tomatoes (Solanum lycopersicum) as part of the jasmonate-dependent signaling pathway. J Plant Physiol. 2017;208:1–6. doi: 10.1016/j.jplph.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 107.Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. In: Oxidative medicine and cellular longevity. https://www.hindawi.com/journals/omcl/2014/360438/. Accessed 13 May 2020 [DOI] [PMC free article] [PubMed]
- 108.Nilsson AK, Johansson ON, Fahlberg P, et al. Formation of oxidized phosphatidylinositol and 12-oxo-phytodienoic acid containing acylated phosphatidylglycerol during the hypersensitive response in Arabidopsis. Phytochemistry. 2014;101:65–75. doi: 10.1016/j.phytochem.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 109.Andersson MX, Hamberg M, Kourtchenko O, et al. Oxylipin profiling of the hypersensitive response in Arabidopsis thaliana formation of a novel oxo-phytodienoic acid-containing galactolipid, arabidopside e. J Biol Chem. 2006;281:31528–31537. doi: 10.1074/jbc.M604820200. [DOI] [PubMed] [Google Scholar]
- 110.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 111.Schmitt AJ, Sathoff AE, Holl C, et al. The major nectar protein of Brassica rapa is a non-specific lipid transfer protein, BrLTP2.1, with strong antifungal activity. J Exp Bot. 2018;69:5587–5597. doi: 10.1093/jxb/ery319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Safi H, Saibi W, Alaoui MM, et al. A wheat lipid transfer protein (TdLTP4) promotes tolerance to abiotic and biotic stress in Arabidopsis thaliana. Plant Physiol Biochem. 2015;89:64–75. doi: 10.1016/j.plaphy.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 113.Kiba A, Galis I, Hojo Y, et al. SEC14 Phospholipid transfer protein is involved in lipid signaling-mediated plant immune responses in Nicotiana benthamiana. PLoS ONE. 2014;9:e98150. doi: 10.1371/journal.pone.0098150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kiba A, Nakano M, Vincent-Pope P, et al. A novel Sec14 phospholipid transfer protein from Nicotiana benthamiana is up-regulated in response to Ralstonia solanacearum infection, pathogen associated molecular patterns and effector molecules and involved in plant immunity. J Plant Physiol. 2012;169:1017–1022. doi: 10.1016/j.jplph.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 115.Reina-Pinto JJ, Yephremov A. Surface lipids and plant defenses. Plant Physiol Biochem. 2009;47:540–549. doi: 10.1016/j.plaphy.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 116.Xu Q-M, Liu Y-L, Li X-R, et al. Three new fatty acids from the roots of Boehmeria nivea (L.) Gaudich and their antifungal activities. Nat Prod Res. 2011;25:640–647. doi: 10.1080/14786419.2010.488230. [DOI] [PubMed] [Google Scholar]
- 117.Yaeno T, Matsuda O, Iba K. Role of chloroplast trienoic fatty acids in plant disease defense responses. Plant J. 2004;40:931–941. doi: 10.1111/j.1365-313X.2004.02260.x. [DOI] [PubMed] [Google Scholar]
- 118.Hung C-Y, Aspesi P, Jr, Hunter MR, et al. Phosphoinositide-signaling is one component of a robust plant defense response. Front Plant Sci. 2014 doi: 10.3389/fpls.2014.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao J, Devaiah SP, Wang C, et al. Arabidopsis phospholipase Dβ1 modulates defense responses to bacterial and fungal pathogens. New Phytol. 2013;199:228–240. doi: 10.1111/nph.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gupta A, Hisano H, Hojo Y, et al. Global profiling of phytohormone dynamics during combined drought and pathogen stress in Arabidopsis thaliana reveals ABA and JA as major regulators. Sci Rep. 2017 doi: 10.1038/s41598-017-03907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kachroo A, Kachroo P. Fatty acid-derived signals in plant defense. Annu Rev Phytopathol. 2009;47:153–176. doi: 10.1146/annurev-phyto-080508-081820. [DOI] [PubMed] [Google Scholar]
- 122.Mengiste T. Plant immunity to necrotrophs. Annu Rev Phytopathol. 2012;50:267–294. doi: 10.1146/annurev-phyto-081211-172955. [DOI] [PubMed] [Google Scholar]
- 123.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 124.Block A, Alfano JR. Plant targets for Pseudomonas syringae type III effectors: virulence targets or guarded decoys? Curr Opin Microbiol. 2011;14:39–46. doi: 10.1016/j.mib.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poole P, Ramachandran V, Terpolilli J. Rhizobia: from saprophytes to endosymbionts. Nat Rev Microbiol. 2018;16:291–303. doi: 10.1038/nrmicro.2017.171. [DOI] [PubMed] [Google Scholar]
- 126.Haroth S, Feussner K, Kelly AA, et al. The glycosyltransferase UGT76E1 significantly contributes to 12-O-glucopyranosyl-jasmonic acid formation in wounded Arabidopsis thaliana leaves. J Biol Chem. 2019;294:9858–9872. doi: 10.1074/jbc.RA119.007600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wasternack C, Feussner I. The oxylipin pathways: biochemistry and function. Annu Rev Plant Biol. 2018;69:363–386. doi: 10.1146/annurev-arplant-042817-040440. [DOI] [PubMed] [Google Scholar]
- 128.Figueiredo A, Monteiro F, Sebastiana M. First clues on a jasmonic acid role in grapevine resistance against the biotrophic fungus Plasmopara viticola. Eur J Plant Pathol. 2015;142:645–652. doi: 10.1007/s10658-015-0634-7. [DOI] [Google Scholar]
- 129.Guerreiro A, Figueiredo J, Sousa Silva M, Figueiredo A. Linking jasmonic acid to grapevine resistance against the biotrophic oomycete Plasmopara viticola. Front Plant Sci. 2016 doi: 10.3389/fpls.2016.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- 131.Somerville C, Browse J. Plant lipids: metabolism, mutants, and membranes. Science. 1991;252:80–87. doi: 10.1126/science.252.5002.80. [DOI] [PubMed] [Google Scholar]
- 132.Upchurch RG. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett. 2008;30:967–977. doi: 10.1007/s10529-008-9639-z. [DOI] [PubMed] [Google Scholar]
- 133.Taniguchi S, Miyoshi S, Tamaoki D, et al. Isolation of jasmonate-induced sesquiterpene synthase of rice: product of which has an antifungal activity against Magnaporthe oryzae. J Plant Physiol. 2014;171:625–632. doi: 10.1016/j.jplph.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 134.Saucedo-García M, Gavilanes-Ruíz M, Arce-Cervantes O. Long-chain bases, phosphatidic acid, MAPKs, and reactive oxygen species as nodal signal transducers in stress responses in Arabidopsis. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu L, Sonbol F-M, Huot B, et al. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat Commun. 2016;7:13099. doi: 10.1038/ncomms13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jeon JE, Kim J-G, Fischer CR, et al. A pathogen-responsive gene cluster for highly modified fatty acids in tomato. Cell. 2020;180:176–187.e19. doi: 10.1016/j.cell.2019.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guo Z, Lu J, Wang X, et al. Lipid flippases promote antiviral silencing and the biogenesis of viral and host siRNAs in Arabidopsis. PNAS. 2017;114:1377–1382. doi: 10.1073/pnas.1614204114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alkan N, Friedlander G, Ment D, et al. Simultaneous transcriptome analysis of Colletotrichum gloeosporioides and tomato fruit pathosystem reveals novel fungal pathogenicity and fruit defense strategies. New Phytol. 2015;205:801–815. doi: 10.1111/nph.13087. [DOI] [PubMed] [Google Scholar]
- 139.Marques JPR, Amorim L, Spósito MB, Appezzato-da-Glória B. Ultrastructural changes in the epidermis of petals of the sweet orange infected by Colletotrichum acutatum. Protoplasma. 2016;253:1233–1242. doi: 10.1007/s00709-015-0877-3. [DOI] [PubMed] [Google Scholar]
- 140.Pang K-L, Lin H-J, Lin H-Y, et al. Production of arachidonic and eicosapentaenoic acids by the marine Oomycete halophytophthora. Mar Biotechnol. 2015;17:121–129. doi: 10.1007/s10126-014-9600-1. [DOI] [PubMed] [Google Scholar]
- 141.Bellin D, Peressotti E, Merdinoglu D, et al. Resistance to Plasmopara viticola in grapevine ‘Bianca’ is controlled by a major dominant gene causing localised necrosis at the infection site. Theor Appl Genet. 2009;120:163–176. doi: 10.1007/s00122-009-1167-2. [DOI] [PubMed] [Google Scholar]
- 142.Sun F, Kale SD, Azurmendi HF, et al. Structural basis for interactions of the Phytophthora sojae RxLR effector Avh5 with phosphatidylinositol 3-phosphate and for host cell entry. MPMI. 2012;26:330–344. doi: 10.1094/MPMI-07-12-0184-R. [DOI] [PubMed] [Google Scholar]
- 143.Wang W, Yang X, Tangchaiburana S, et al. An Inositolphosphorylceramide synthase is involved in regulation of plant programmed cell death associated with defense in Arabidopsis. Plant Cell. 2008;20:3163–3179. doi: 10.1105/tpc.108.060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bi F-C, Liu Z, Wu J-X, et al. Loss of ceramide kinase in Arabidopsis impairs defenses and promotes ceramide accumulation and mitochondrial H2O2 bursts. Plant Cell. 2014;26:3449–3467. doi: 10.1105/tpc.114.127050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thomma BP, Cammue BP, Thevissen K. Plant defensins. Planta. 2002;216:193–202. doi: 10.1007/s00425-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 146.Thevissen K, Ferket KKA, François IEJA, Cammue BPA. Interactions of antifungal plant defensins with fungal membrane components. Peptides. 2003;24:1705–1712. doi: 10.1016/j.peptides.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 147.Kvansakul M, Lay FT, Adda CG, et al. Binding of phosphatidic acid by NsD7 mediates the formation of helical defensin-lipid oligomeric assemblies and membrane permeabilization. Proc Natl Acad Sci USA. 2016;113:11202–11207. doi: 10.1073/pnas.1607855113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Poon IK, Baxter AA, Lay FT, et al. Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. Elife. 2014;3:e01808. doi: 10.7554/eLife.01808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Livaja M, Zeidler D, von Rad U, Durner J. Transcriptional responses of Arabidopsis thaliana to the bacteria-derived PAMPs harpin and lipopolysaccharide. Immunobiology. 2008;213:161–171. doi: 10.1016/j.imbio.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 150.Evans TJ, Ind A, Komitopoulou E, Salmond GPC. Phage-selected lipopolysaccharide mutants of Pectobacterium atrosepticum exhibit different impacts on virulence. J Appl Microbiol. 2010;109:505–514. doi: 10.1111/j.1365-2672.2010.04669.x. [DOI] [PubMed] [Google Scholar]
- 151.Sanabria NM, Dubery IA. Differential display profiling of the Nicotiana response to LPS reveals elements of plant basal resistance. Biochem Biophys Res Commun. 2006;344:1001–1007. doi: 10.1016/j.bbrc.2006.03.216. [DOI] [PubMed] [Google Scholar]
- 152.Mohamed K-H, Daniel T, Aurélien D, et al. Deciphering the dual effect of lipopolysaccharides from plant pathogenic Pectobacterium. Plant Signal Behav. 2015;10:e1000160. doi: 10.1080/15592324.2014.1000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Silipo A, Molinaro A, Sturiale L, et al. The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris. J Biol Chem. 2005;280:33660–33668. doi: 10.1074/jbc.M506254200. [DOI] [PubMed] [Google Scholar]
- 154.Proietti S, Giangrande C, Amoresano A, et al. Xanthomonas campestris lipooligosaccharides trigger innate immunity and oxidative burst in Arabidopsis. Plant Physiol Biochem. 2014;85:51–62. doi: 10.1016/j.plaphy.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 155.Gaillardin C. Lipases as pathogenicity factors of fungi. In: Timmis KN, editor. Handbook of hydrocarbon and lipid microbiology. Berlin: Springer; 2010. pp. 3259–3268. [Google Scholar]
- 156.Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. Mitochondrial reactive oxygen species. contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006;141:357–366. doi: 10.1104/pp.106.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peeters N, Carrère S, Anisimova M, et al. Repertoire, unified nomenclature and evolution of the type III effector gene set in the Ralstonia solanacearum species complex. BMC Genomics. 2013;14:859. doi: 10.1186/1471-2164-14-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Inada N, Betsuyaku S, Shimada TL, et al. Modulation of plant RAB GTPase-mediated membrane trafficking pathway at the interface between plants and obligate biotrophic pathogens. Plant Cell Physiol. 2016;57:1854–1864. doi: 10.1093/pcp/pcw107. [DOI] [PubMed] [Google Scholar]
- 159.Battilani P, Lanubile A, Scala V, et al. Oxylipins from both pathogen and host antagonize jasmonic acid-mediated defence via the 9-lipoxygenase pathway in Fusarium verticillioides infection of maize. Mol Plant Pathol. 2018;19:2162–2176. doi: 10.1111/mpp.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martínez E, Campos-Gómez J. Oxylipins produced by Pseudomonas aeruginosa promote biofilm formation and virulence. Nat Commun. 2016 doi: 10.1038/ncomms13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brune A, Urbach W, Dietz K-J. Compartmentation and transport of zinc in barley primary leaves as basic mechanisms involved in zinc tolerance. Plant Cell Environ. 1994;17:153–162. doi: 10.1111/j.1365-3040.1994.tb00278.x. [DOI] [Google Scholar]
- 162.Fecht-Christoffers MM, Braun H-P, Lemaitre-Guillier C, et al. Effect of manganese toxicity on the proteome of the leaf apoplast in cowpea. Plant Physiol. 2003;133:1935–1946. doi: 10.1104/pp.103.029215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Figueiredo J, Cavaco AR, Guerra-Guimarães L, et al. An apoplastic fluid extraction method for the characterization of grapevine leaves proteome and metabolome from a single sample. Physiol Plant. 2020 doi: 10.1111/ppl.13198. [DOI] [PubMed] [Google Scholar]
- 164.Kader J-C. Lipid-transfer proteins: a puzzling family of plant proteins. Trends Plant Sci. 1997;2:66–70. doi: 10.1016/S1360-1385(97)82565-4. [DOI] [Google Scholar]
- 165.Maldonado AM, Doerner P, Dixon RA, et al. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature. 2002;419:399–403. doi: 10.1038/nature00962. [DOI] [PubMed] [Google Scholar]
- 166.Jung J, Kumar K, Lee HY, et al. Translocation of phospholipase A2α to apoplasts is modulated by developmental stages and bacterial infection in Arabidopsis. Front Plant Sci. 2012 doi: 10.3389/fpls.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rutter BD, Innes RW. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017;173:728–741. doi: 10.1104/pp.16.01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Szabo LJ, Bushnell WR. Hidden robbers: the role of fungal haustoria in parasitism of plants. Proc Natl Acad Sci. 2001;98:7654–7655. doi: 10.1073/pnas.151262398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Škalamera D, Jibodhand S, Heath MC. Callose deposition during the interaction between cowpea (Vigna unguiculata) and the monokaryotic stage of the cowpea rust fungus (Uromyces vignae) New Phytol. 1997;136:511–524. doi: 10.1046/j.1469-8137.1997.00760.x. [DOI] [PubMed] [Google Scholar]
- 171.Meyer D, Pajonk S, Micali C, et al. Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J. 2009;57:986–999. doi: 10.1111/j.1365-313X.2008.03743.x. [DOI] [PubMed] [Google Scholar]
- 172.Regente M, Pinedo M, San Clemente H, et al. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot. 2017;68:5485–5495. doi: 10.1093/jxb/erx355. [DOI] [PubMed] [Google Scholar]
- 173.Movahed N, Cabanillas DG, Wan J, et al. Turnip mosaic virus components are released into the extracellular space by vesicles in infected leaves. Plant Physiol. 2019;180:1375–1388. doi: 10.1104/pp.19.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]